
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Physiol. , 28 October 2021
Sec. Aquatic Physiology
Volume 12 - 2021 | https://doi.org/10.3389/fphys.2021.768907
This article is part of the Research Topic Genetic Adaption and Metabolic Response of Aquatic Animals to Diverse Water Environment Parameters View all 13 articles
Non-nutritional stress during early life period has been reported to promote the metabolic programming in fish induced by nutritional stimulus. Sodium chloride (NaCl) and hydrogen peroxide (H2O2) have been widely applied during fish egg hatching, but the influences on health and metabolism of fish in their later life remain unknown. In the present study, H2O2 treatment at 400mg/L but not 200mg/L significantly increased the loach hatchability and decreased the egg mortality, while NaCl treatment at 1,000 and 3,000mg/L showed no significant influences on the loach hatchability nor egg mortality. Further studies indicated that 400mg/L H2O2 pre-treatment significantly enhanced the antioxidant capacity and the mRNA expression of genes involved in immune response of loach larvae, accompanied by the increased expression of genes involved in fish early development. However, the expression of most genes involved in lipid metabolism, including catabolism and anabolism of loach larvae, was significantly upregulated after 200mg/L H2O2 pre-treatment. NaCl pre-treatment also increased the expression of antioxidant enzymes; however, only the expression of C1q within the detected immune-related genes was upregulated in loach larvae. One thousand milligram per liter NaCl pre-treatment significantly increased the expression of LPL and genes involved in fish early development. Thus, our results suggested the programming roles of 400mg/L H2O2 pre-treatment during egg hatching in enhancing antioxidant capacity and immune response of fish larvae via promoting fish early development.
The environmental and trophic conditions encountered at the early developmental period of animals have been confirmed to perform profound effects on the metabolism and physiology of individuals later in life, which is termed metabolic programming (when modifying metabolism; Lucas, 1998). Long-lasting modification in gene expression patterns is one of the most important biological mechanisms described in such case of adaptions, and it may persist later in life in the absence of the environmental stimulus that initiated them (George et al., 2012; Kongsted et al., 2014). In aquatic animals, including fish and shrimp, the concept of metabolic programming has been tested as well. Preliminary study in rainbow trout (Oncorhynchus mykiss) showed that only a strict nutritional stimulus had a minor programming effect on hepatic glucose metabolism (Geurden et al., 2007, 2014). Later studies indicated that an acute exposure to hypoxia alone (Liu et al., 2017a) or combined with an early nutritional stimulus, such as high-carbohydrate diet (Liu et al., 2017b), high dietary carbohydrate:protein ratios (Hu et al., 2018) induced obvious programming in the liver of juvenile rainbow trout. The hypoxic conditions resulted in the higher expression of HIF-1α which has been reported to modulate the nutrient metabolism (Menendez-Montes et al., 2021), antioxidant capacity (Lacher et al., 2018), and immune responses (Ni et al., 2020). However, the hypoxia may easily result in high mortality, and it is important to explore other non-nutritional stress. Due to the safety and friendly to human health and environment ecology, sodium chloride (NaCl) and hydrogen peroxide (H2O2) have been tested in the fry hatch of many fish species (Magondu et al., 2011). NaCl has been used effectively in aquaculture as antiparasitic agent (Schelkle et al., 2011; Dewi et al., 2018), growth-promoting agent in Carassius auratus (Imanpoor et al., 2012) and Mugil liza (Lisboa et al., 2015), and survival enhancing agent in Pelecus cultratus larvae (Kujawa et al., 2017), Ictalurus punctatus, C. auratus, Morone saxatilis, and Acipenser oxyrinchus (Altinok and Grizzle, 2001). Moreover, NaCl affects the embryonic development and larval vigor of Epinephelus akaara (Wang et al., 2002) and Rhombosolea tapirina (Hart and Purser, 1995). In one plateau species of loach, Triplophysa (Hedinichthys) yarkandensis, NaCl application with salinity at 4% resulted in the lowest deformity rate (Chen et al., 2016). H2O2 has received attention for its control of several fish pathogens and is recommended as a general disinfectant in aquaculture for treating aquaculture water and surface of tanks before introduction of fish (Avendaño-Herrera et al., 2006). H2O2 has been shown to promote the egg hatching rate of rainbow trout (Schreier et al., 1996; Barnes et al., 1998), channel catfish (I. punctatus; Small and Wolters, 2003), and C. gariepinus (Rasowo et al., 2007).
During multiple environmental challenges, free radical would be released, but the over-production of O2− would cause oxidative damage to proteins, nucleic acids, and lipids (Kurien and Scofield, 2003). Thus, cellular antioxidant defenses system in fish and other animals are developed to scavenge the excessive reactive oxygen species (ROS; Pisoschi and Pop, 2015; Klein et al., 2017). Like hypoxia, H2O2 and NaCl treatment have also been proved to affect antioxidant capacity. Salinity or NaCl treatment significantly affected the mRNA expression and activity of antioxidant enzymes, including superoxide dismutase (SOD), glutathione S-transferase (GST), and glutathione (GSH) in multiple tissues of olive flounder (Paralichthys olivaceus; Kim et al., 2021), European seabass (Dicentrarchus labrax; Islam et al., 2020), D. labrax, and Chanos Chanos (Chang et al., 2021). Similarly, H2O2 exposure has also been reported to affect antioxidant capacity in common carp (Cyprinus carpio; Jia et al., 2020) and largemouth bass (Micropterus salmoides; Sinha et al., 2020). Besides antioxidant system, fish remains the first bony vertebrate to develop both innate and adaptive immunity which help themselves to defend against infected pathogens or other environmental challenges (Wang et al., 2019). The immune responses of European seabass and common carp (C. carpio) were significantly affected by different salinities (Islam et al., 2020) and H2O2 exposure (Jia et al., 2021), respectively. The programming effects on individuals of later life by environmental treatment or nutritional stimulus at early life stage mainly result from an alteration of the functional development of crucial organs (Pittman et al., 2013). It is well known that fish larvae along with the fertilized eggs grow very fast and experience significant changes in physiology; thus, they are very fragile and most susceptible to environmental stressors during fish ontogeny (Fuiman, 1983; Alvarez et al., 2021). The organs in the newly hatched fish larva are not well developed, and thus, it is not easy to do histological evaluation in fish larvae (Fuiman et al., 1999). The molecular methods via evaluating the relative mRNA expression levels of early development-related genes are useful and effective to systematically evaluate the influences of pre-treatment on the fry (Hu et al., 2018).
Dojo loach Misgurnus anguillicaudatus (Cantor 1842) is one of the important freshwater aquaculture species in China whose production has reached 367,428 tons by 2020 (Ministry of Agriculture and Rural Affairs of the People’s Republic of China, 2021) and can be used as a Chinese medicine for the treatment of hepatitis, carbuncles, inflammations, and cancers (Qin et al., 2002). The sustainable development of loach aquaculture industry relies on the stable loach fry supply, whose artificial breeding has been successfully overcome in recent years (Gao et al., 2014; Huang et al., 2015). However, the diseases resulting from microorganism infection or other environmental factors during fish hatchery have threatened the production of larval loach (Shamsi et al., 2021). The applications of antibiotics and insecticides have been seriously restricted in many countries (Holmström et al., 2003; Cabello, 2006; Shao et al., 2021), while no specific fish vaccine nor mature vaccination route is available for fish fry (Rojo-Cebreros et al., 2018; Wang et al., 2020), which seriously restricts the stable fish fry stocks. In the present study, NaCl and H2O2 were applied during loach egg hatching and the effects on the antioxidant capacity, immunity and lipid metabolism of fish larvae were evaluated as well as monitoring the early development-related genes.
Mature broodstock fish (average weight 18±2.1g), obtained from broodstock ponds, were selected and transferred to the hatchery. All fish were then acclimated in hatching tanks for 1day without feeding. To induce spawning, the selected female fish were injected with DOM (4mg/kg fish) and LRH-A2 (35μg/kg fish), and the male fish were injected with same reagents but half dosage. After 12h, the eggs were stripped into a dry bowl and fertilized with milt from a ripe male. After fertilization, the fertilized eggs were randomly counted into bottles with 100 eggs each. The individual hatching bottles were randomly assigned in triplicate to static bath treatments of given concentrations of either NaCl (1,000 and 3,000mg/L), H2O2 (200 and 400mg/L), and a control (nothing added) for 60-min exposure before being transferred to randomized compartments of the incubation tank for further incubation. The water temperature was controlled at 24–26°C and dissolved oxygen (DO) controlled at 7.5–7.8mg/L, which were monitored using an oxygen-temperature meter (model 55, YSI, Yellow Springs Ohio, United States).
Loach larvae came out of the membrane after 24-h fertilization. Then, the hatching bottles were removed from the incubation tank. The numbers of live hatched larvae, dead hatched larvae, total dead eggs, and fungi-infected dead eggs were counted for the calculation of following parameters and then sent back to the incubation tank.
Loach larvae showed feed-hunting behavior at 4days after rupture, and then, larvae in all groups were fed with artemia for another 7days. At the end of feeding, all the loach larvae were collected and immediately frozen in liquid nitrogen and stored at −80°C before analysis.
The whole loach larvae were homogenized in TRIzol reagent (Invitrogen, Carlsbad, CA, United States) for RNA extraction according to the manufacturer’s recommendations. After RNA extraction procedures, the purity and concentration of RNA were monitored by NanoDrop 2000 spectrophotometer (Thermo scientific, United States), with their 260:280 ratios between 1.8 and 2.0. Additionally, 1.0% agarose gel electrophoresis was adopted to determine the integrity of RNA. The quantified RNA samples were then used for cDNA synthesis (Invitrogen, Carlsbad, CA, United States). Briefly, the potential existing genomic DNA was removed from the RNA samples with same amount using DNase. Then, 1μg of treated RNA was used for the synthesis of cDNA using the reverse transcriptase kit with oligo dT primers following manufacturer’s instructions.
The synthesized cDNA was used for the quantitative real-time PCR (qPCR) analysis using the Eva Green 2×qPCR Master mix (ABM, Canada). qPCR was conducted on 7500 Real-time PCR system (Applied Biosystems, United States), with each PCR performed with triplicate samples and the cycling conditions set with 30s at 95°C, 1s at 95°C, and 10s at 58°C for 40cycles. In addition, a melt curve analysis was performed after amplification to verify the accuracy of each amplicon.
The relative quantification of the target genes involved in the antioxidant system [SOD, catalase (CAT), glutathione peroxidase (GPx), and metallothionein (Mt)], genes related to immune responses [C1q, C3-1, C8b, mannose-binding lectin-associated serine protease-1 (MASP-1), interleukin 15 receptor subunit alpha (IL15Rα), and heat shock protein 70 (Hsp70)], genes involved in lipid metabolism [carnitine palmitoyltransferase 1alpha (Cpt1α), lipoprotein lipase (LPL), fatty acid desaturase 2 (Fads2), and proliferator-activated receptor gamma (PPARγ)] and early development-related genes [spondin 1b (spon1b), intraflagellar transport protein 22 (IFT22), vascular endothelial growth factor Aa (VEGFAa), glutamate dehydrogenase (gdh), annexin A1a (anxa1a), vasoactive intestinal peptide (VIP), protein phosphatase 1 (PP1), and protein phosphatase 2A catalytic subunit beta isoform (PP2AB)] were determined via normalized against elongation factor 1-alpha (EF1α). Then, relative abundance of target genes was calculated by using the 2−ΔΔCt method. All primers used in the present study are shown in Table 1.
All statistical analyses were performed using SPSS 17.0. Data were analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s multiple range tests to determine the effects of NaCl and H2O2 on egg hatching and gene expression. Differences were considered significant when p<0.05. All data were expressed as mean±standard deviation of the mean (SD), except the specific statement.
The hatching performances including hatchability, larval mortality, egg mortality including fungi-induced mortality and other-induced mortality of loach after H2O2 and NaCl treatment are shown in Figure 1. Four hundred milligram per liter H2O2 treatment significantly increased larvae hatchability, while the larvae mortality showed no significant differences after H2O2 treatment. Additionally, the egg mortality was also significantly decreased after 400mg/L H2O2 treatment. However, the decreased egg mortality after 400mg/L H2O2 treatment was not due to fungi, but by other factors, as the fungi-induced egg mortality was even higher in 400mg/L H2O2 treatment group.
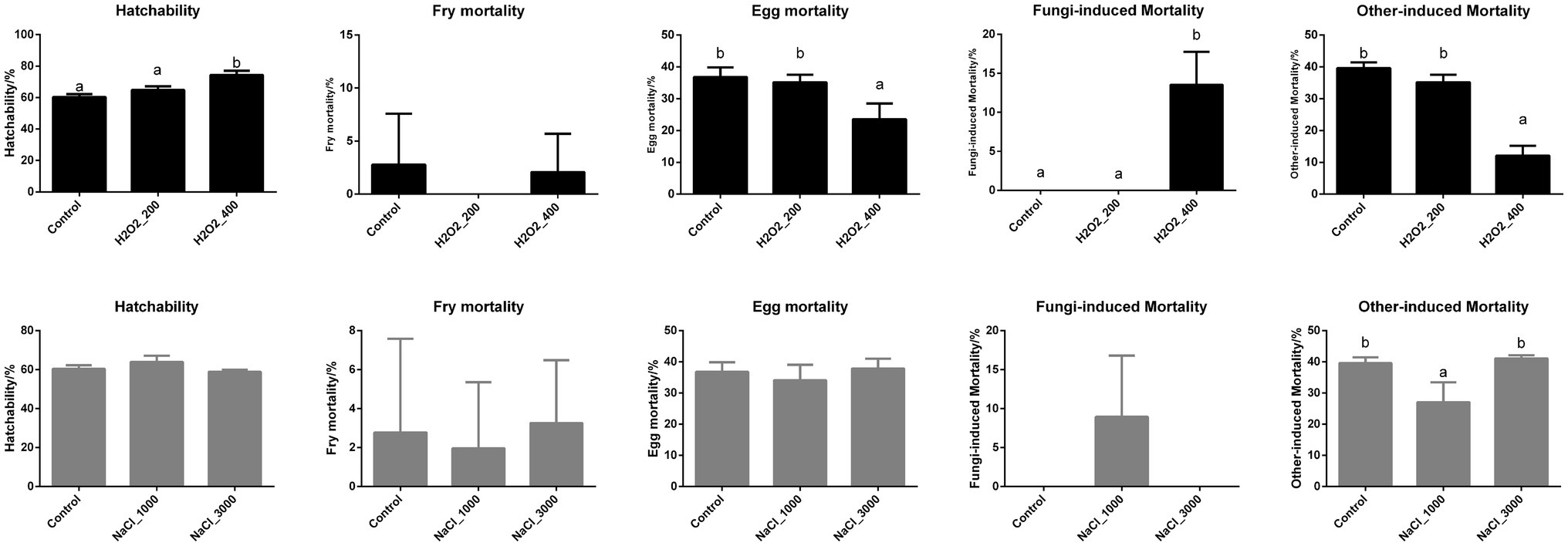
Figure 1. Effects of H2O2 (200, 400mg/ml) and NaCl (1,000, 3,000mg/ml) pre-treatment during egg hatching on the hatchability, fry mortality, egg mortality including fungi-induced mortality and other factor-induced mortality of loach.
NaCl treatment showed no significant effects on larval hatchability nor fry mortality. Similarly, the total egg mortality along with the fungi-induced egg mortality was not affected by NaCl treatment. However, the other factor-induced egg mortality was decreased during 1,000mg/L NaCl treatment.
Figure 2 indicated the influences of H2O2 and NaCl pre-treatment during egg hatching on the expression of early development-related genes of loach larvae. The expression of spon1b, IFT22, VEGFAa, and PP2AB was significantly upregulated after 400mg/L H2O2 pre-treatment. The expression of gdh, VIP, and PP1 was significantly upregulated with the increased dosage of H2O2, and highest expression level was detected at 400mg/L H2O2 pre-treatment. No significant effects of H2O2 pre-treatment were detected on the expression of anxa1a.
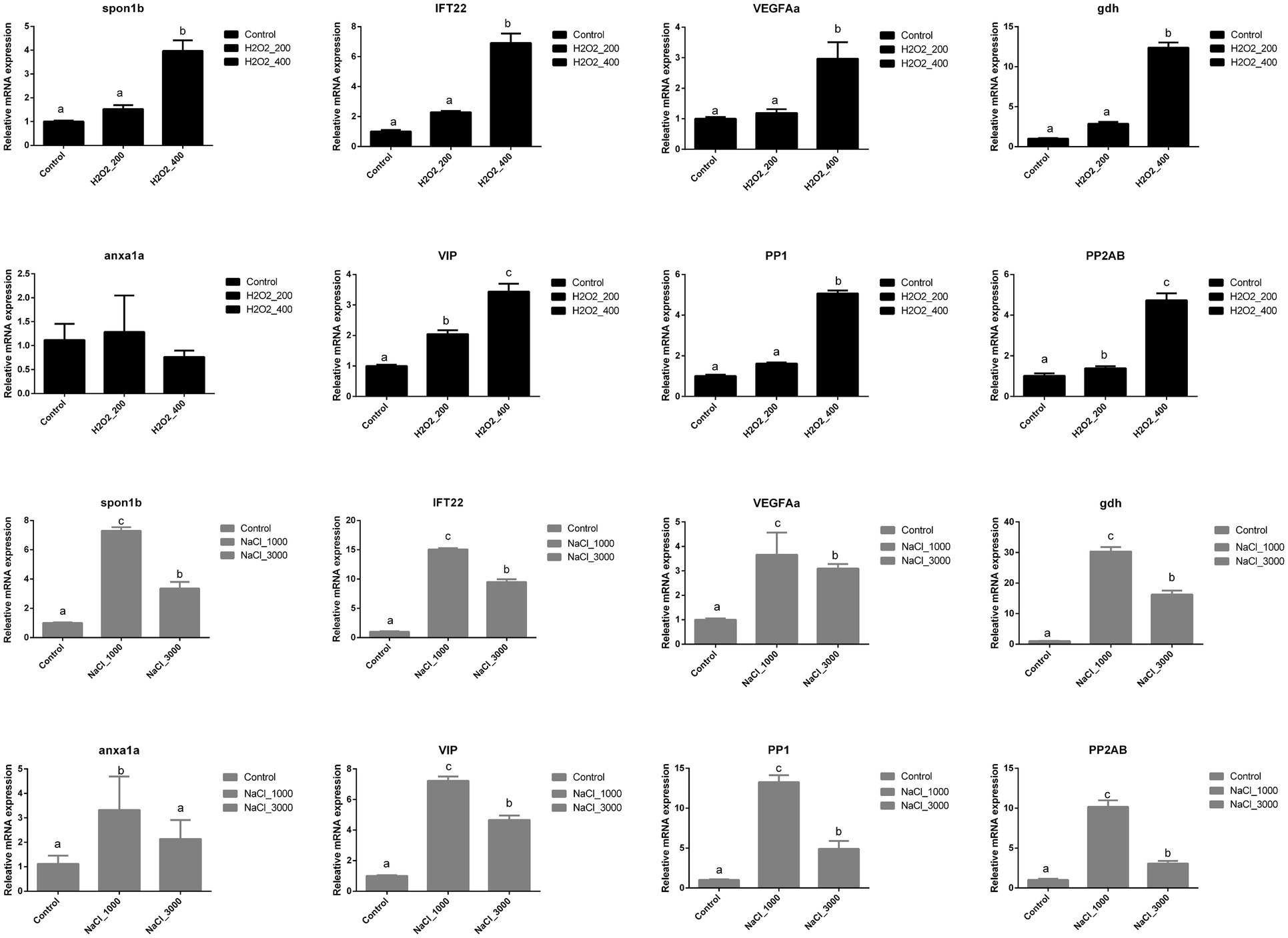
Figure 2. Effects of H2O2 (200, 400mg/ml) and NaCl (1,000, 3,000mg/ml) pre-treatment during egg hatching on the relative expression levels of genes involving early development including spon1b, IFT22, VEGFAa, gdh, anxa1a, VIP, PP1, and PP2AB in loach larvae.
The expression of spon1b, IFT22, gdh, VIP, VEGFAa, PP1, and PP2AB in loach larvae was significantly upregulated after NaCl pre-treatment; however, their expression levels were significantly higher at 1,000mg/L NaCl pre-treatment than those at 3,000mg/L NaCl pre-treatment. The expression of anxa1a was also significantly upregulated after 1,000mg/L NaCl pre-treatment but back to normal after 3,000mg/L NaCl pre-treatment.
The mRNA expression levels of genes involved in the antioxidant capacity of loach larvae after H2O2 and NaCl pre-treatment are shown in Figure 3. The expression levels of SOD and Mt were significantly upregulated with the increased dosage of H2O2, and the highest expression levels were both detected at 400mg/L H2O2 pre-treatment. The expression of GPx was also significantly upregulated after H2O2 pre-treatment; however, no significant differences were detected between two dosages. Additionally, the expression of CAT was not significantly affected by H2O2 pre-treatment.
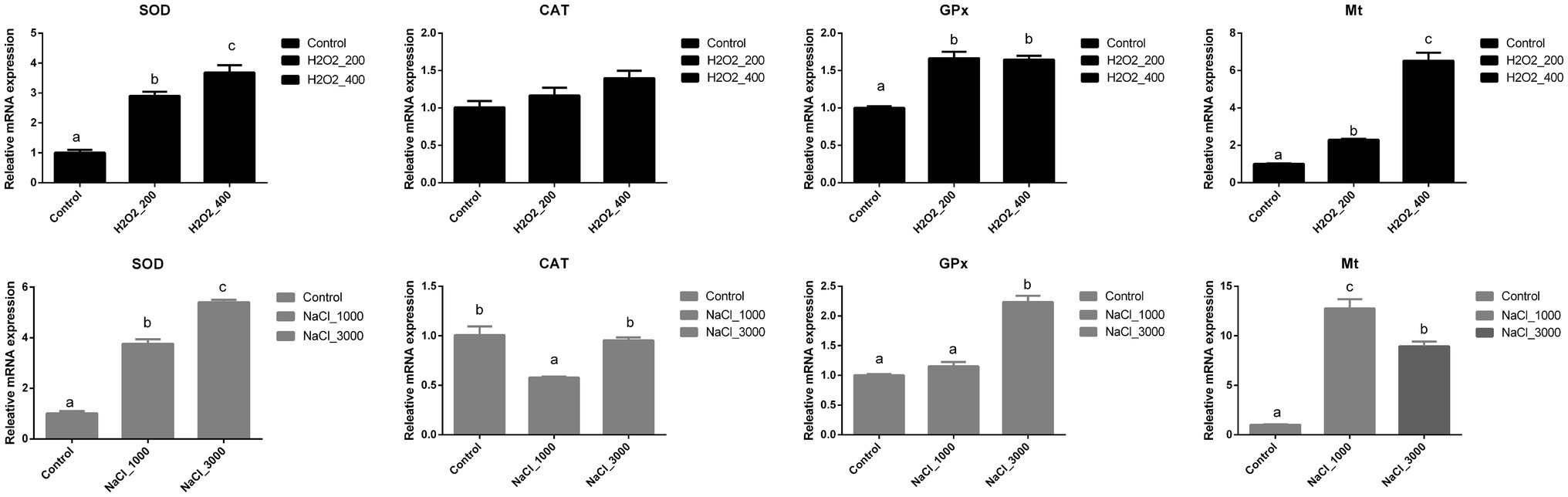
Figure 3. Effects of H2O2 (200, 400mg/ml) and NaCl (1,000, 3,000mg/ml) pre-treatment during egg hatching on the relative expression levels of genes involving antioxidant capacity, including SOD, CAT, GPx, and Mt in loach larvae.
The expression of SOD was significantly upregulated with the increased dosage of NaCl, and highest expression level was detected at 3,000mg/L NaCl pre-treatment. The expression of GPx was only significantly upregulated after 3,000mg/L NaCl pre-treatment. The expression of Mt was significantly upregulated by H2O2 pre-treatment, but the highest expression level was detected at 1,000mg/L NaCl pre-treatment. Additionally, 1,000mg/L NaCl pre-treatment significantly decreased the expression of CAT.
Figure 4 indicated the different expression levels of genes involved in the immune response of loach larvae after H2O2 and NaCl pre-treatment during egg hatching. The expression of C1q was also significantly upregulated after H2O2 pre-treatment; however, no significant differences were detected between two dosages. The expression of C3-1 and Hsp70 was significantly upregulated after 400mg/L H2O2 pre-treatment. The expression of IL15Rα was significantly higher in loach larvae after 200mg/L H2O2 pre-treatment than that after 400mg/L H2O2 pre-treatment. No significant influences of H2O2 pre-treatment were detected on the expression of C8b nor MASP-1.
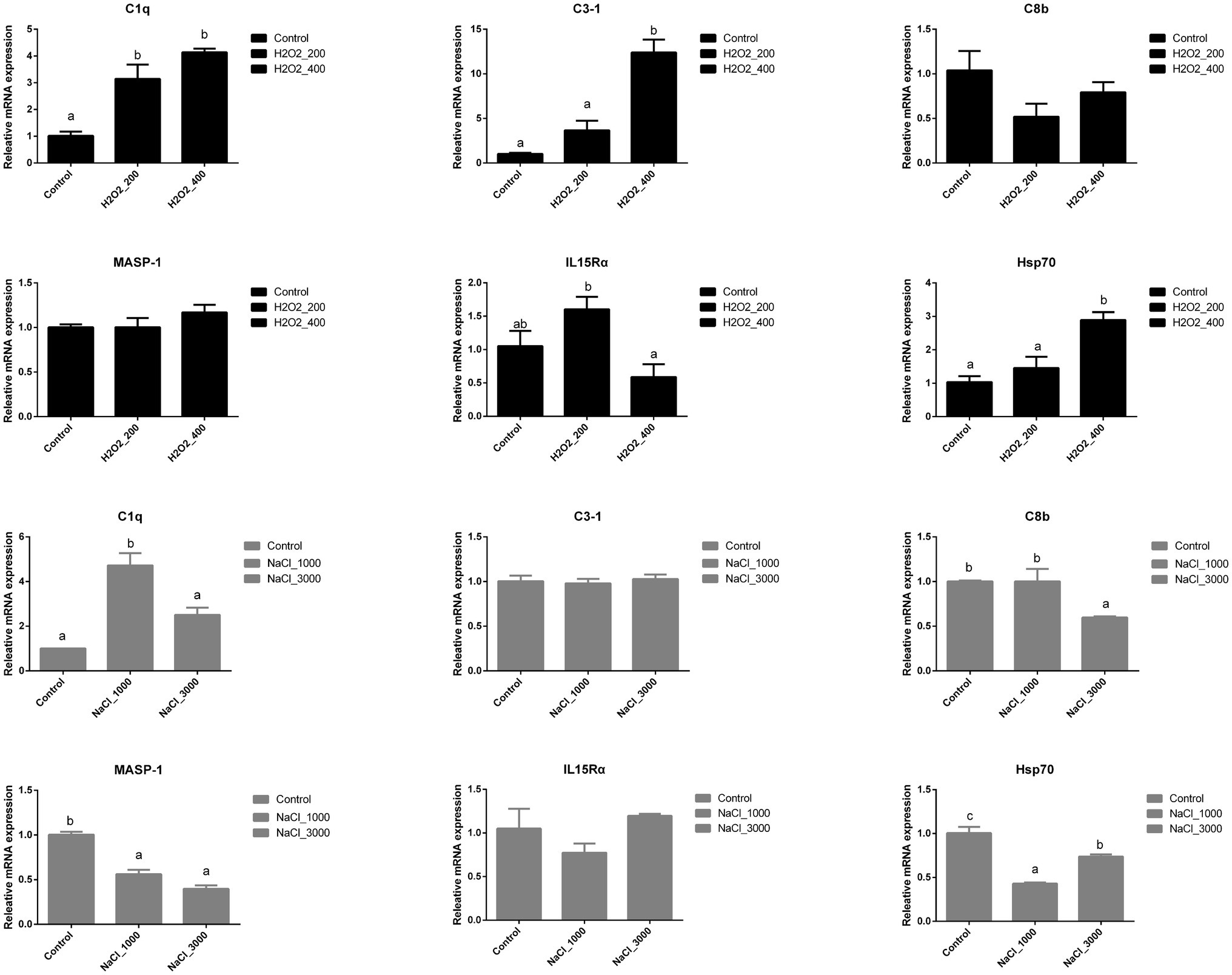
Figure 4. Effects of H2O2 (200, 400mg/ml) and NaCl (1,000, 3,000mg/ml) pre-treatment during egg hatching on the relative expression levels of genes involving immunity, including C1q, C3-1, C8b, MASP-1, IL15Rα, and Hsp70 in loach larvae.
The expression of C1q was also significantly upregulated after 1,000mg/L NaCl pre-treatment but back to normal after 3,000mg/L NaCl pre-treatment. The expression of C8b was significantly downregulated after 3,000mg/L NaCl pre-treatment. The expression of MASP-1 was significantly downregulated after NaCl pre-treatment but no significant differences were detected between two dosages. The expression of Hsp70 was significantly downregulated after NaCl pre-treatment, and the lowest expression level was detected after 1,000mg/L NaCl pre-treatment. No significant influences were detected on the expression of C3-1 nor IL15Rα in loach larvae after NaCl pre-treatment.
H2O2 and NaCl pre-treatment during egg hatching also significantly affected the expression of genes involved in the lipid metabolism of loach larvae (Figure 5). The expression of Cpt1α, LPL, and Fads2 was significantly upregulated after 200mg/L H2O2 pre-treatment. The mRNA expression levels of Cpt1α and LPL went back to normal after 400mg/L H2O2 pre-treatment, while the expression of Fads2 was even decreased after 400mg/L H2O2 pre-treatment. The expression of PPARγ was significantly higher in loach larvae after 200mg/L H2O2 pre-treatment than that after 400mg/L H2O2 pre-treatment.
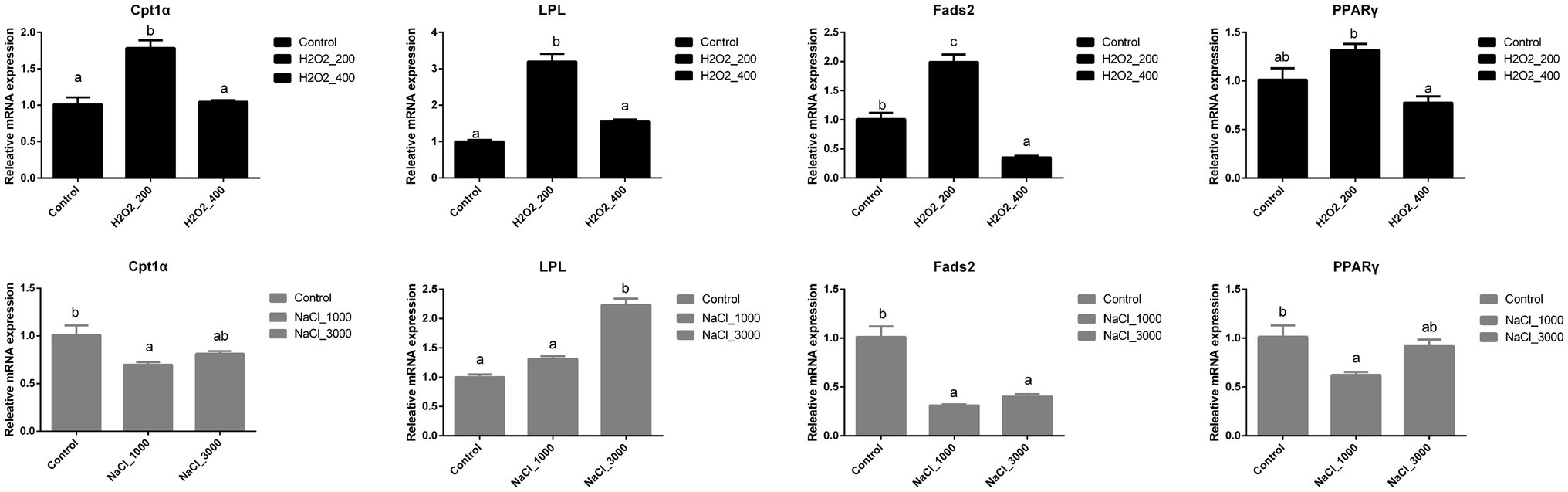
Figure 5. Effects of H2O2 (200, 400mg/ml) and NaCl (1,000, 3,000mg/ml) pre-treatment during egg hatching on the relative expression levels of genes involving lipid metabolism, including Cpt1α, LPL, Fads2, and PPARγ in loach larvae.
The expression of Cpt1α and PPARγ was significantly downregulated after 1,000mg/L NaCl pre-treatment, but back to normal after 3,000mg/L NaCl pre-treatment. The genes expression level of LPL was significantly upregulated after 3,000mg/L NaCl pre-treatment. The expression of Fads2 was significantly downregulated after NaCl pre-treatment, while no significant differences were detected between two NaCl dosages.
The disease prevention or control is of great importance to keep the healthy fish fry stocks (Subasinghe et al., 2000), so antibiotics and insecticides were traditionally applied during fish fry breeding. However, the applications of antibiotics and insecticides have been seriously restricted in many countries, including China, because they are not only highly toxic to humans and fish and not easy to be degraded in the environment, but also lead to the potential development of antibiotic resistance (Holmström et al., 2003; Cabello, 2006; Zhou et al., 2019; Shao et al., 2021). Fish vaccine is of great potential in the prevention of disease outbreaks; however, only eight fish vaccines have been licensed in China, which is far more from enough to main the continual development of aquaculture production in China (Wang et al., 2020). Especially in fish fry, there is no available fish vaccine nor mature vaccination route, which seriously restricts the stable fish fry stocks (Rojo-Cebreros et al., 2018). The safe and environmental-friendly drugs, including H2O2 and NaCl, have been tested in the fry hatching of many fish species (Magondu et al., 2011). H2O2 has been proved to promote the hatching rate of eggs in multiple fish species. For example, H2O2 treatment at 500–1,000ppm significantly increased hatching rates and controlled fungi in rainbow trout eggs (Schreier et al., 1996; Barnes et al., 1998). In channel catfish (I. punctatus), H2O2 treatment at low concentrations of 70–250mg/L significantly increased percent hatching of fish eggs (Small and Wolters, 2003), and later studies indicated that higher dosage of H2O2 (500 or 750mg/L) could also improve the percent hatching of channel catfish eggs (Rach et al., 2004). One study also compared the effects of H2O2 on eight species of warm- and cool-water fish eggs which identified the concentration of 1,000mg/L to be most effective in improving hatching rate (Rach et al., 1998). The unfertilized fish eggs are especially vulnerable to fungal infection from the family Saprolegniaceae (Post, 1987), which produces mycelia to facilitate spreading from the nonviable to the healthy eggs and cause egg mortality (Teresa Vega-Ramírez et al., 2013). In the present study, H2O2 treatment at 400mg/L significantly improved the hatchability and also decreased the egg mortality. However, the decreased egg mortality after 400mg/L treatment was not due to the inhibition of fungi as the fungi-induced egg mortality was even increased in 400mg/L H2O2 treatment. Four hundred milligram per liter H2O2 treatment might contribute to the other factors, including bacterial inhibition or water parameters protection. This was in accordance with studies in salmon (Salmo salar) as H2O2 concentration strongly affected salmon mortality, but did not alter mucous cell area or density, pre-adult lice removal efficiency, or the re-infection success of lice copepodids (Overton et al., 2018). Besides H2O2, NaCl has also been reported to affect the hatching rate of fish eggs; however, the effects varied depending on the dosage of NaCl and the fish species. Schnick (1988) reported that the 3,000ppm NaCl dip effectively removed protozoa from fish egg surfaces and limited any mycelial production that may lower egg hatching. Salt treatment at 0–5,000mg/L significantly improved egg hatching in channel catfish (Froelich and Engelhardt, 1996), and NaCl significantly improved the hatching rate of koi carp (C. carpio haematopterus) at 1,000 and 2,500mg/L for a 60min exposure duration but even toxic to the eggs at 5,000mg/L (Phelps and Walser, 1993). In the present study, NaCl treatment at 1,000 and 3,000mg/L did not significantly affect fish egg hatching rate nor the fry mortality. Moreover, the total mortality and fungi-induced mortality of loach eggs were also not significantly affected after NaCl treatment. However, 1,000mg/L NaCl treatment significantly inhibited the other factor-induced egg mortality excepting fungi. NaCl treatment showing no effects on the hatching performance of loach in the present study may be due to the test dosages of NaCl and the fish species. A much wider dosage range of NaCl during loach hatching could be tested in the future study.
Although plenty of studies have evaluated the influences of NaCl and H2O2 pre-treatment on the egg hatching of many fish species, little information is known about the influences of these pre-treatments on fish larvae health and nutrient metabolism. Recent studies have indicated that nutritional stimuli (quantity or quality of nutrients) and non-nutritional environmental stress experienced at critical periods of an organism’s life can result in permanent changes in postnatal growth potential, health, and metabolic status in animals including fish and shrimp (Burdge and Lillycrop, 2010; Hu et al., 2018). Moreover, temperature has also been reported to affect the liver transcriptome response of spotted seabass (Lateolabrax maculatus) induced by dietary protein level (Cai et al., 2020). Thus, the effects of NaCl and H2O2 during egg hatching on antioxidant capacity, immune responses, and lipid metabolism of loach larvae were systematically evaluated. In previous studies, the metabolic programming in aquatic has been mainly focused on carbohydrate metabolism due to the desired protein-sparing effects (Hu et al., 2018); however, lipid metabolism is also important and also serves the protein-sparing effect (Peng et al., 2019). Especially, fish larvae require much higher energy consumption for the rapid growth (Abi-Ayad and Kestemont, 1994; Gaon et al., 2021) and lipid serves as the most efficient nutrient for energy supply (Kupriyanova et al., 2021). In the present study, both the genes involved in lipid catabolism, such as Cpt1α and LPL, and genes involved in lipid anabolism, such as Fads2, along with the regulatory factor, PPARγ, were significantly upregulated in 200mg/L H2O2 pre-treatment group, but back to normal level at 400mg/L. However, most genes were downregulated by NaCl pre-treatment excepting LPL which was significantly upregulated after 3,000mg/L NaCl pre-treatment. This phenomenon has been reported in earlier studies which suggested that nutritional programming by dietary carbohydrates in European seabass larvae may not always be as expected (Zambonino-Infante et al., 2019). These differential results may result from different fish species, different stimulus patterns, and/or dosages.
In animals, the ROS play important roles in tissue homeostasis, cellular signaling, differentiation (Harris and DeNicola, 2020), and their levels are tightly regulated by cellular antioxidant system to prevent unwanted consequences (Pérez-Jiménez et al., 2017). However, oxidative stress will be generated when the balance between the production and neutralization of ROS is broken to favor the former, thus causing oxidative damage to proteins, nucleic acids and lipids, destroying important cellular processes and increasing mutations (Loro et al., 2012). Like in mammals, the cellular antioxidant defenses system in fish has been identified and proven to be functional during multiple situations which include ROS scavenging, oxidative stress protection, and attenuation of membrane lipid peroxidation (Hermans et al., 2007). Consequently, the major front-line antioxidant enzymes, such as SOD (neutralizes superoxide radicals to H2O2), CAT, and GPx (neutralizes H2O2 to water), and small non-protein antioxidants (scavenges all active oxygen species directly) work in a cascade to protect cells from oxidative stress (Ighodaro and Akinloye, 2018). Oxidative responses of both invertebrates and vertebrates under salinity challenges have been emphatically discussed. In juvenile olive flounders, the activities of SOD, GST, and GSH in the liver and gill were significantly affected by salinity (Kim et al., 2021). The activities of serum antioxidants, including SOD, GPx, CAT, and glutathione reductase (GR) in the spleen of European seabass after cold stress, were affected by salinity (Islam et al., 2020). Early studies have indicated the influences of environmental parameters including seawater acidification and cadmium on the antioxidant defense of flounder P. olivaceus larvae (Cui et al., 2020). In the present study, NaCl pre-treatment significantly induced the higher expression levels of SOD, GPx, and Mt, which is similar to previous studies in other juvenile fish and fish larvae. However, the expression level of CAT was not significantly upregulated but even decreased after 1,000mg/L NaCl pre-treatment. This is similar to earlier reports that, unlike SOD, no significant changes were observed in the mRNA expression or activity of CAT in the livers of D. labrax and Chanos Chanos under different salinity (Chang et al., 2021). H2O2, as a strong oxidant, can increase the intracellular ROS level and induce oxidative stress. However, the effects of H2O2 on fish antioxidant defense, including the levels of antioxidant enzymes (e.g., SOD and CAT) and nonenzymatic antioxidants (e.g., GSH), varied depending on the duration and dosage of H2O2 treatment. It has been reported that short and moderate H2O2 treatment stimulated the levels of the antioxidant enzymes, while chronic and severe H2O2 treatment impaired antioxidant defense system (Jia et al., 2021). In common carp (C. carpio), the oxidative stress-related genes, including nrf2, gstα, sod, cat, and/or gpx1, were upregulated in liver, gills, muscle, intestines, and/or kidney, but downregulated in heart after H2O2 exposure (Jia et al., 2020). In the brain and liver tissue of largemouth bass, 2.5mg/L sodium carbonate peroxyhydrate containing H2O2 as the active ingredient resulted in an increase of SOD, CAT, GPX, GR, and GST activity (Sinha et al., 2020). In the present study, the expression of SOD, GPx, and Mt in loach larvae was significantly increased after H2O2 pre-treatment. However, like the unaffected CAT expression during NaCl treatment, no significant changes were found on the expression of CAT in loach larvae after H2O2 pre-treatment. Thus, H2O2 (200 and 400mg/L) and NaCl (1,000 and 3,000mg/L) pre-treatment during egg hatching significantly stimulated the antioxidant defense system in loach larvae.
Besides antioxidant defense system, the immune system also protects fish against environmental stress and teleost is the first bony vertebrate to develop both innate and adaptive immunity. Salinity and H2O2 have been shown to affect the fish immune responses, for example, the immune responses of European seabass acclimatized after extreme ambient cold stress were significantly affected by different salinities (Islam et al., 2020) and transcriptome analysis also identified 100 differentially expressed genes involved in the immune system of common carp (C. carpio) after H2O2 exposure (Jia et al., 2021). Especially, the complement system, which is composed of more than 35 soluble plasma proteins, plays an essential role in alerting and clearing of potential pathogens and also contributes to the development of an acquired immune response (Ferreira and Cortes, 2021). The complement system of teleost fish, like that of higher vertebrates, can be activated through all three pathways of complement (Nakao et al., 2011). Complement 3 (C3), the key component in teleost, is present in several isoforms that are the products of different genes (Sunyer et al., 1996). The lectin pathway is initiated through the interaction of MBL (like C1q) and ficolins with sugar moieties expressed on the surface of many microorganisms. C1q has been cloned in multiple fish species, such as channel catfish I. punctatus (Li et al., 2012) and killifish F. heteroclitus (Kocabas et al., 2002). Moreover, MASP1 has such a broad specificity and has significant substrates other than complement proteins (Hajela et al., 2002). Besides, C8 is responsible for the formation of membrane attack complex (Liyanage et al., 2018). In the present study, H2O2 pre-treatment during egg hatching significantly induced the higher mRNA expression level of C3-1 and C1q in loach larvae, but did not affect the mRNA expression level of C8b nor MASP-1. This was similar to previous study that the expression levels of complement C3, C4, and C7 in the Atlantic salmon skin were significantly upregulated by 24-h exposure to H2O2 (Karlsen et al., 2021). NaCl pre-treatment only increased the mRNA expression level of C1q, but decreased the mRNA expression levels of C8b and MASP-1. The mRNA expression level of C3-1 was not significantly affected during NaCl pre-treatment. Under the stimulation of inflammatory mediators, activation signals, and pathogenic infection, interleukin 15 (IL15) could transfer from the endoplasmic reticulum to cell membrane after binding with its receptor (IL15R) and control multiple process, including cell proliferation and inhibition of apoptosis (Chen et al., 2018). Additionally, Hsps has been shown to be an integral part of the cellular stress response pathways in fishes (Metzger et al., 2016) and widely used as biomarkers of exposure to environmental stressors (Mitra et al., 2018). In the present study, H2O2 pre-treatment induced the mRNA expression of IL15Rα at 200mg/L and Hsp70 at 400mg/L, while NaCl pre-treatment decreased Hsp70 expression at two dosages but did not affect the expression of IL15Rα.
Fish fry is rather fragile at the early development period and can be easily affected by the surrounding environment. As reported earlier, the newly hatched loach larva had a long straight intestinal tube with a very simple structure (Luo et al., 2016), and the effectiveness of drug pre-treatment on the fry could be monitored by evaluating the relative mRNA expression levels of early development-related genes. Spon1b was originally isolated from the developing embryonic floor plate of vertebrates and performs a positive function in nervous system development. A study in Japanese flounder showed that spon1b was maternally expressed with transcripts present from one-cell stage to hatching stage, peaking at tailbud stage (Hu et al., 2016). IFT sculpts the proteome of cilia and flagella and plays critical roles in cilia biogenesis, quality control, and signal transduction by delivering proteins to the growing ciliary tip and selectively transporting signaling molecules (Webb et al., 2020). VEGFA is required for the differentiation of endothelial cells (vasculogenesis) and for the sprouting of new capillaries (angiogenesis), and duplicated VEGFA in the zebrafish has been reported to mediate vascular development (Bahary et al., 2007). Gdh in the Antarctic fish Chaenocephalus aceratus has been reported to have relationship with cold adaptation (Ciardiello et al., 2000). Anxa1a also play a significant role in epimorphic regeneration of zebrafish caudal fin tissue (Quoseena et al., 2020). In zebrafish, VIP-like immunoreactive cells exist in the olfactory pit, the retina, and several regions of the brain at 24h post-fertilization (hpf) embryos (Mathieu et al., 2001). PP1 and PP2A are proteins with major Ser/Thr protein phosphatase activity in eukaryotic cells and always interact with multiple proteins of diverse structure (regulatory subunits) with little substrate specificity; thus, they are a key regulator of cell development and oncogenic transformation (Dzulko et al., 2020). In the present study, excepting anxa1a, the expression levels of early development-related genes, including spon1b, IFT22, VEGFAa, gdh, VIP, PP1, and PP2AB, were significantly increased after 400mg/L H2O2 pre-treatment, which agrees well with the higher hatching rate in this group. However, although NaCl treatment increased their expression in loach larvae especially at 1,000mg/L, the hatching rate of loach was not significantly affected.
In all, our study indicated the long-time effects of H2O2 and NaCl pre-treatment during fish egg hatching on the health and metabolism of fish larvae. Besides the role in promoting egg hatchability, H2O2 pre-treatment at appropriate dosage also stimulated the antioxidant system and immune system of fish larvae, which could be linked to the good performance in fish early development.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The animal study was reviewed and approved by Animal Experiment Committee of Huazhong Agricultural University.
QW designed and wrote the main context. MW conducted most experimental protocol. WX wrote the manuscript. JZ, SL, and ZS conducted the experimental analysis. FZ, WJ, and ZX supplied the relevant materials. All authors contributed to the article and approved the submitted version.
This article was funded by National Natural Science Foundation of China (Grant Nos. 31802317 and 32172996).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abi-Ayad, A., and Kestemont, P. (1994). Comparison of the nutritional status of goldfish (Carassius auratus) larvae fed with live, mixed or dry diet. Aquaculture 128, 163–176. doi: 10.1016/0044-8486(94)90111-2
Altinok, I., and Grizzle, J. M. (2001). Effects of low salinities on Flavobacterium columnare infection of euryhaline and freshwater stenohaline fish. J. Fish Dis. 24, 361–367. doi: 10.1046/j.1365-2761.2001.00306.x
Alvarez, P., Cotano, U., Estensoro, I., Etxebeste, E., and Irigoien, X. (2021). Assessment of larval growth patterns: a comparison across five fish species in the Bay of Biscay. Reg. Stud. Mar. Sci. 47:101958. doi: 10.1016/j.rsma.2021.101958
Avendaño-Herrera, R., Magariños, B., Irgang, R., and Toranzo, A. E. (2006). Use of hydrogen peroxide against the fish pathogen Tenacibaculum maritimum and its effect on infected turbot (Scophthalmus maximus). Aquaculture 257, 104–110. doi: 10.1016/j.aquaculture.2006.02.043
Bahary, N., Goishi, K., Stuckenholz, C., Weber, G., Leblanc, J., Schafer, C. A., et al. (2007). Duplicate VegfA genes and orthologues of the KDR receptor tyrosine kinase family mediate vascular development in the zebrafish. Blood 110, 3627–3636. doi: 10.1182/blood-2006-04-016378
Barnes, M. E., Ewing, D. E., Cordes, R. J., and Young, G. L. (1998). Observations on hydrogen peroxide control of Saprolegnia spp. during rainbow trout eggs incubation. Prog. Fish Cult. 60, 67–70. doi: 10.1577/1548-8640(1998)060<0067:OOHPCO>2.0.CO;2
Burdge, G. C., and Lillycrop, K. A. (2010). Nutrition, epigenetics, and developmental plasticity: implications for understanding human disease. Annu. Rev. Nutr. 30, 315–339. doi: 10.1146/annurev.nutr.012809.104751
Cabello, F. C. (2006). Heavy use of prophylactic antibiotics in aquaculture: a growing problem for human and animal health and for the environment. Environ. Microbiol. 8, 1137–1144. doi: 10.1111/j.1462-2920.2006.01054.x
Cai, L. S., Wang, L., Song, K., Lu, K. L., Zhang, C. X., and Rahimnejad, S. (2020). Evaluation of protein requirement of spotted seabass (Lateolabrax maculatus) under two temperatures, and the liver transcriptome response to thermal stress. Aquaculture 516:734615. doi: 10.1016/j.aquaculture.2019.734615
Chang, C. H., Mayer, M., Rivera-Ingraham, G., Blondeau-Bidet, E., Wu, W. Y., Lorin-Nebel, C., et al. (2021). Effects of temperature and salinity on antioxidant responses in livers of temperate (Dicentrarchus labrax) and tropical (Chanos Chanos) marine euryhaline fish. J. Therm. Biol. 99:103016. doi: 10.1016/j.jtherbio.2021.103016
Chen, X., Kong, W., Yu, Y., Dong, S., Huang, Z., Yu, W., et al. (2018). Molecular characterization and expression analysis of interleukin 15 (IL15) and interleukin-15 receptor subunit alpha (IL15Rα) in Dojo loach (Misgurnus anguillicaudatus): their salient roles during bacterial, parasitic and fungal infection. Mol. Immunol. 103, 293–305. doi: 10.1016/j.molimm.2018.10.012
Chen, S. G., Li, D. P., Xie, C. X., Yao, N., Wang, S., and Ren, D. Q. (2016). “Influence of salinity on hatching rate and larval vitality in Triplophysa (Hedinichthys) yarkandensis (Day).” in The Annual Conference of Chinese Fisheries Association; November 2–4, 2016.
Ciardiello, M. A., Camardella, L., Carratore, V., and Prisco, G. D. (2000). L-glutamate dehydrogenase from the Antarctic fish Chaenocephalus aceratus: primary structure, function and thermodynamic characterisation: relationship with cold adaptation. Biochim. Biophys. Acta 1543, 11–23. doi: 10.1016/s0167-4838(00)00186-2
Cui, W., Cao, L., Liu, J., Ren, Z., Zhao, B., and Dou, S. (2020). Effects of seawater acidification and cadmium on the antioxidant defense of flounder Paralichthys olivaceus larvae. Sci. Total Environ. 718:137234. doi: 10.1016/j.scitotenv.2020.137234
Dewi, R. R., Siallagan, W., and Suryanto, D. (2018). The efficacy of sodium chloride application in the control of fish lice (Argulus sp.) infection on tilapia (Oreochromis niloticus). Acta Aquat. Sci. J. 5, 4–7. doi: 10.29103/aa.v5i1.584
Dzulko, M., Pons, M., Henke, A., Schneider, G., and Krämer, O. H. (2020). The PP2A subunit PR130 is a key regulator of cell development and oncogenic transformation. Biochim. Biophys. Acta Rev. Cancer 1874:188453. doi: 10.1016/j.bbcan.2020.188453
Ferreira, V. P., and Cortes, C. (2021). The Complement System, Reference Module in Biomedical Sciences. Main St. Salt Lake City, UT 84111, USA: Elsevier.
Froelich, S. L., and Engelhardt, T. (1996). Comparative effects of formalin and salt treatments on hatch rate of koi carp eggs. Prog. Fish Cult. 58, 209–211. doi: 10.1577/1548-8640(1996)058<0209:CEOFAS>2.3.CO;2
Fuiman, L. A. (1983). Growth gradients in fish larvae. J. Fish Biol. 23, 117–123. doi: 10.1111/j.1095-8649.1983.tb02886.x
Fuiman, L. A., Smith, M. E., and Malley, V. N. (1999). Ontogeny of routine swimming speed and startle responses in red drum, with a comparison of responses to acoustic and visual stimuli. J. Fish Biol. 55, 215–226. doi: 10.1111/j.1095-8649.1999.tb01057.x
Gao, J., Koshio, S., Wang, W., Li, Y., Huang, S., and Cao, X. (2014). Effects of dietary phospholipid levels on growth performance, fatty acid composition and antioxidant responses of Dojo loach Misgurnus anguillicaudatus larvae. Aquaculture 426–427, 304–309. doi: 10.1016/j.aquaculture.2014.02.022
Gaon, A., Tandler, A., Nixon, O., El Sadin, S., Allon, G., and Koven, W. (2021). The combined DHA and taurine effect on vision, prey capture and growth in different age larvae of gilthead sea bream (Sparus aurata). Aquaculture 545:737181. doi: 10.1016/j.aquaculture.2021.737181
George, L. A., Zhang, L., Tuersunjiang, N., Ma, Y., Long, N. M., Uthlaut, A. B., et al. (2012). Early maternal undernutrition programs increased feed intake, altered glucose metabolism and insulin secretion, and liver function in aged female offspring. Am. J. Physiol. Regul. Integr. Comp. Physiol. 302, R795–R804. doi: 10.1152/ajpregu.00241.2011
Geurden, I., Aramendi, M., Zambonino-Infante, J., and Panserat, S. (2007). Early feeding of carnivorous rainbow trout (Oncorhynchus mykiss) with a hyperglucidic diet during a short period: effect on dietary glucose utilization in juveniles. Am. J. Physiol. Regul. Integr. Comp. Physiol. 292, R2275–R2283. doi: 10.1152/ajpregu.00444.2006
Geurden, I., Mennigen, J., Plagnes-Juan, E., Veron, V., Cerezo, T., Mazurais, D., et al. (2014). High or low dietary carbohydrate:protein ratios during first-feeding affect glucose metabolism and intestinal microbiota in juvenile rainbow trout. J. Exp. Biol. 217, 3396–3406. doi: 10.1242/jeb.106062
Hajela, K., Kojima, M., Ambrus, G., Wong, K. H., Moffatt, B. E., Ferluga, J., et al. (2002). The biological functions of MBL-associated serine proteases (MASPs). Immunobiology 205, 467–475. doi: 10.1078/0171-2985-00147
Harris, I. S., and DeNicola, G. M. (2020). The complex interplay between antioxidants and ROS in cancer. Trends Cell Biol. 30, 440–451. doi: 10.1016/j.tcb.2020.03.002
Hart, P. R., and Purser, G. J. (1995). Effects of salinity and temperature on eggs and yolk sac larvae of the greenback flounder (Rhombosolea tapirina Günther, 1862). Aquaculture 136, 221–230. doi: 10.1016/0044-8486(95)01061-0
Hermans, N., Cos, P., Maes, L., De Bruyne, T., Vanden Berghe, D., Vlietinck, A. J., et al. (2007). Challenges and pitfalls in antioxidant research. Curr. Med. Chem. 14, 417–430. doi: 10.2174/092986707779941005
Holmström, K., Gräslund, S., Wahlström, A., Poungshompoo, S., Bengtsson, B. E., and Kautsky, N. (2003). Antibiotic use in shrimp farming and implications for environmental impacts and human health. Int. J. Food Sci. Technol. 38, 255–266. doi: 10.1046/j.1365-2621.2003.00671.x
Hu, H., Liu, J., Plagnes-Juan, E., Herman, A., Leguen, I., Goardon, L., et al. (2018). Programming of the glucose metabolism in rainbow trout juveniles after chronic hypoxia at hatching stage combined with a high dietary carbohydrate: protein ratios intake at first-feeding. Aquaculture 488, 1–8. doi: 10.1016/j.aquaculture.2018.01.015
Hu, H., Xin, N., Liu, J., Liu, M., Wang, Z., Wang, W., et al. (2016). Characterization of F-spondin in Japanese flounder (Paralichthys olivaceus) and its role in the nervous system development of teleosts. Gene 575, 623–631. doi: 10.1016/j.gene.2015.09.037
Huang, S., Cao, X., Tian, X., Luo, W., and Wang, W. (2015). Production of tetraploid gynogenetic loach using diploid eggs of natural tetraploid loach, Misgurnus anguillicaudatus, fertilized with UV-irradiated sperm of Megalobrama amblycephala without treatments for chromosome doubling. Cytogenet. Genome Res. 147, 260–267. doi: 10.1159/000444384
Ighodaro, O. M., and Akinloye, O. A. (2018). First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 54, 287–293. doi: 10.1016/j.ajme.2017.09.001
Imanpoor, M. R., Najafi, E., and Kabir, M. (2012). Effects of different salinity and temperatures on the growth, survival, haematocrit and blood biochemistry of goldfish (Carassius auratus). Aquac. Res. 43, 332–338. doi: 10.1111/j.1365-2109.2011.02832.x
Islam, M. J., Kunzmann, A., Bögner, M., Meyer, A., Thiele, R., and James Slater, M. (2020). Metabolic and molecular stress responses of European seabass, Dicentrarchus labrax at low and high temperature extremes. Ecol. Indic. 112:106118. doi: 10.1016/j.ecolind.2020.106118
Jia, R., Du, J., Cao, L., Feng, W., He, Q., Xu, P., et al. (2020). Chronic exposure of hydrogen peroxide alters redox state, apoptosis and endoplasmic reticulum stress in common carp (Cyprinus carpio). Aquat. Toxicol. 229:105657. doi: 10.1016/j.aquatox.2020.105657
Jia, R., Du, J., Cao, L., Feng, W., He, Q., Xu, P., et al. (2021). Application of transcriptome analysis to understand the adverse effects of hydrogen peroxide exposure on brain function in common carp (Cyprinus carpio). Environ. Pollut. 286:117240. doi: 10.1016/j.envpol.2021.117240
Karlsen, C., Bogevik, A. S., Krasnov, A., and Ytteborg, E. (2021). In vivo and in vitro assessment of Atlantic salmon skin exposed to hydrogen peroxide. Aquaculture 540:736660. doi: 10.1016/j.aquaculture.2021.736660
Kim, J. H., Jeong, E. H., Jeon, Y. H., Kim, S. K., and Hur, Y. B. (2021). Salinity-mediated changes in hematological parameters, stress, antioxidant responses, acetylcholinesterase of juvenile olive flounders (Paralichthys olivaceus). Environ. Toxicol. Pharmacol. 83:103597. doi: 10.1016/j.etap.2021.103597
Klein, R. D., Rosa, C. E., Colares, E. P., Robaldo, R. B., Martinez, P. E., and Bianchini, A. (2017). Antioxidant defense system and oxidative status in Antarctic fishes: the sluggish rockcod Notothenia coriiceps versus the active marbled notothen Notothenia rossii. J. Therm. Biol. 68, 119–127. doi: 10.1016/j.jtherbio.2017.02.013
Kocabas, A. M., Li, P., Cao, D., Karsi, A., He, C., Patterson, A., et al. (2002). Expression profile of the channel catfish spleen: analysis of genes involved in immune functions. Mar. Biotechnol. 4, 526–536. doi: 10.1007/s10126-002-0067-0
Kongsted, A. H., Tygesen, M. P., Husted, S. V., Oliver, M. H., Tolver, A., Christensen, V. G., et al. (2014). Programming of glucose-insulin homoeostasis: long-term consequences of pre-natal versus early post-natal nutrition insults. Evidence from a sheep model. Acta Physiol. 210, 84–98. doi: 10.1111/apha.12080
Kujawa, R., Lach, M., Pol, P., Ptaszkowski, M., and Kucharczyk, D. (2017). Influence of water salinity on the survival of embryos and growth of the sichel larvae Pelecus cultratus (L.) under controlled conditions. Aquac. Res. 48, 1302–1314. doi: 10.1111/are.12972
Kupriyanova, Y., Zaharia, O. P., Bobrov, P., Karusheva, Y., Burkart, V., Szendroedi, J., et al. (2021). Early changes in hepatic energy metabolism and lipid content in recent-onset type 1 and 2 diabetes mellitus. J. Hepatol. 74, 1028–1037. doi: 10.1016/j.jhep.2020.11.030
Kurien, B. T., and Scofield, R. H. (2003). Free radical mediated peroxidative damage in systemic lupus erythematosus. Life Sci. 73, 1655–1666. doi: 10.1016/S0024-3205(03)00475-2
Lacher, S. E., Levings, D. C., Freeman, S., and Slattery, M. (2018). Identification of a functional antioxidant response element at the HIF1A locus. Redox Biol. 19, 401–411. doi: 10.1016/j.redox.2018.08.014
Li, C., Zhang, Y., Wang, R., Lu, J., Nandi, S., Mohanty, S., et al. (2012). RNA-seq analysis of mucosal immune responses reveals signatures of intestinal barrier disruption and pathogen entry following Edwardsiella ictaluri infection in channel catfish, Ictalurus punctatus. Fish Shellfish Immunol. 32, 816–827. doi: 10.1016/j.fsi.2012.02.004
Lisboa, V., Barcarolli, I. F., Sampaio, L. A., and Bianchini, A. (2015). Acclimation of juvenile Mugil liza Valenciennes, 1836 (Mugiliformes: Mugilidae) to different environmental salinities. Neotrop. Ichthyol. 13, 591–598. doi: 10.1590/1982-0224-20140123
Liu, J., Plagnes-Juan, E., Geurden, I., Panserat, S., and Marandel, L. (2017a). Exposure to an acute hypoxic stimulus during early life affects the expression of glucose metabolism-related genes at first-feeding in trout. Sci. Rep. 7:363. doi: 10.1038/s41598-017-00458-4
Liu, J., Dias, K., Plagnes-Juan, E., Veron, V., Panserat, S., and Marandel, L. (2017b). Long-term programming effect of embryonic hypoxia exposure and high-carbohydrate diet at first feeding on glucose metabolism in juvenile rainbow trout. J. Exp. Biol. 220, 3686–3694. doi: 10.1242/jeb.161406
Liyanage, D. S., Omeka, W. K. M., Godahewa, G. I., Lee, S., Nam, B. H., and Lee, J. (2018). Membrane attack complex-associated molecules from redlip mullet (Liza haematocheila): molecular characterization and transcriptional evidence of C6, C7, C8β, and C9 in innate immunity. Fish Shellfish Immunol. 81, 1–9. doi: 10.1016/j.fsi.2018.07.006
Loro, V. L., Jorge, M. B., Silva, K. R., and Wood, C. M. (2012). Oxidative stress parameters and antioxidant response to sublethal waterborne zinc in a euryhaline teleost Fundulus heteroclitus: protective effects of salinity. Aquat. Toxicol. 110–111, 187–193. doi: 10.1016/j.aquatox.2012.01.012
Lucas, A. (1998). Programming by early nutrition: an experimental approach. J. Nutr. 128, 401s–406s.
Luo, W., Cao, X., Xu, X., Huang, S., Liu, C., and Tomljanovic, T. (2016). Developmental transcriptome analysis and identification of genes involved in formation of intestinal air-breathing function of Dojo loach, Misgurnus anguillicaudatus. Sci. Rep. 6:31845. doi: 10.1038/srep39108
Magondu, E. W., Rasowo, J., Oyoo-Okoth, E., and Charo-Karisa, H. (2011). Evaluation of sodium chloride (NaCl) for potential prophylactic treatment and its short-term toxicity to African catfish Clarias gariepinus (Burchell 1822) yolk-sac and swim-up fry. Aquaculture 319, 307–310. doi: 10.1016/j.aquaculture.2011.06.038
Mathieu, M., Tagliafierro, G., Angelini, C., and Vallarino, M. (2001). Organization of vasoactive intestinal peptide-like immunoreactive system in the brain, olfactory organ and retina of the zebrafish, Danio rerio, during development. Brain Res. 888, 235–247. doi: 10.1016/S0006-8993(00)03065-1
Menendez-Montes, I., Escobar, B., Gomez, M. J., Albendea-Gomez, T., Palacios, B., Bonzon-Kulichenko, E., et al. (2021). Activation of amino acid metabolic program in cardiac HIF1-alpha-deficient mice. iScience 24:102124. doi: 10.1016/j.isci.2021.102124
Metzger, D. C., Hemmer-Hansen, J., and Schulte, P. M. (2016). Conserved structure and expression of hsp70 paralogs in teleost fishes. Comp. Biochem. Physiol. Part D Genomics Proteomics 18, 10–20. doi: 10.1016/j.cbd.2016.01.007
Ministry of Agriculture and Rural Affairs of the People’s Republic of China (2021). China Fishery Statistical Yearbook. Beijing: China Agriculture Press.
Mitra, T., Mahanty, A., Ganguly, S., Purohit, G. K., Mohanty, S., Parida, P. K., et al. (2018). Expression patterns of heat shock protein genes in Rita rita from natural riverine habitat as biomarker response against environmental pollution. Chemosphere 211, 535–546. doi: 10.1016/j.chemosphere.2018.07.093
Nakao, M., Tsujikura, M., Ichiki, S., Vo, T. K., and Somamoto, T. (2011). The complement system in teleost fish: progress of post-homolog-hunting researches. Dev. Comp. Immunol. 35, 1296–1308. doi: 10.1016/j.dci.2011.03.003
Ni, J., Wang, X., Stojanovic, A., Zhang, Q., Wincher, M., Bühler, L., et al. (2020). Single-cell RNA sequencing of tumor-infiltrating NK cells reveals that inhibition of transcription factor HIF-1α unleashes NK cell activity. Immunity 52, 1075–1087. doi: 10.1016/j.immuni.2020.05.001
Overton, K., Samsing, F., Oppedal, F., Dalvin, S., Stien, L. H., and Dempster, T. (2018). The use and effects of hydrogen peroxide on salmon lice and post-smolt Atlantic salmon. Aquaculture 486, 246–252. doi: 10.1016/j.aquaculture.2017.12.041
Peng, X. R., Feng, L., Jiang, W. D., Wu, P., Liu, Y., Jiang, J., et al. (2019). Supplementation exogenous bile acid improved growth and intestinal immune function associated with NF-κB and TOR signalling pathways in on-growing grass carp (Ctenopharyngodon idella): enhancement the effect of protein-sparing by dietary lipid. Fish Shellfish Immunol. 92, 552–569. doi: 10.1016/j.fsi.2019.06.047
Pérez-Jiménez, A., Abellán, E., Arizcun, M., Cardenete, G., Morales, A. E., and Hidalgo, M. C. (2017). Dietary carbohydrates improve oxidative status of common dentex (Dentex dentex) juveniles, a carnivorous fish species. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 203, 17–23. doi: 10.1016/j.cbpa.2016.08.014
Phelps, R. P., and Walser, C. A. (1993). Effect of sea salt on the hatching of channel catfish eggs. J. Aquat. Anim. Health 5, 205–207. doi: 10.1577/1548-8667(1993)005<0205:EOSSOT>2.3.CO;2
Pisoschi, A. M., and Pop, A. (2015). The role of antioxidants in the chemistry of oxidative stress: a review. Eur. J. Med. Chem. 97, 55–74. doi: 10.1016/j.ejmech.2015.04.040
Pittman, K., Yúfera, M., Pavlidis, M., Geffen, A. J., Koven, W., Ribeiro, L., et al. (2013). Fantastically plastic: fish larvae equipped for a new world. Rev. Aquac. 5, S224–S267. doi: 10.1111/raq.12034
Post, G. (1987). Textbook of Fish Health. Revised and Expanded Edition. Neptune City, NJ: T.F.H. Publications.
Qin, C., Huang, K., and Xu, H. (2002). Protective effect of polysaccharide from the loach on the in vitro and in vivo peroxidative damage of hepatocyte. J. Nutr. Biochem. 13, 592–597. doi: 10.1016/S0955-2863(02)00193-6
Quoseena, M., Vuppaladadium, S., Hussain, S., Banu, S., Bharathi, S., and Idris, M. M. (2020). Functional role of annexins in zebrafish caudal fin regeneration – a gene knockdown approach in regenerating tissue. Biochimie 175, 125–131. doi: 10.1016/j.biochi.2020.05.014
Rach, J. J., Gaikowski, M. P., Howe, G. E., and Schreier, T. M. (1998). Evaluation of the toxicity and efficacy of hydrogen peroxide treatments on eggs of warm- and coolwater fishes. Aquaculture 165, 11–25. doi: 10.1016/S0044-8486(98)00248-8
Rach, J. J., Valentine, J. J., Schreier, T. M., Gaikowski, M. P., and Crawford, T. G. (2004). Efficacy of hydrogen peroxide to control saprolegniasis on channel catfish (Ictalurus punctatus) eggs. Aquaculture 238, 135–142. doi: 10.1016/j.aquaculture.2004.06.007
Rasowo, J., Okoth, O. E., and Ngugi, C. C. (2007). Effects of formaldehyde, sodium chloride, potassium permanganate and hydrogen peroxide on hatch rate of African catfish Clarias gariepinus eggs. Aquaculture 269, 271–277. doi: 10.1016/j.aquaculture.2007.04.087
Rojo-Cebreros, A. H., Ibarra-Castro, L., and Martínez-Brown, J. M. (2018). Immunostimulation and trained immunity in marine fish larvae. Fish Shellfish Immunol. 80, 15–21. doi: 10.1016/j.fsi.2018.05.044
Schelkle, B., Doetjes, R., and Cable, J. (2011). The salt myth revealed: treatment of gyrodactylid infections on ornamental guppies, Poecilia reticulata. Aquaculture 311, 74–79. doi: 10.1016/j.aquaculture.2010.11.036
Schnick, R. A. (1988). The impetus to register new therapeutants for aquaculture. Prog. Fish Cult. 50, 190–196. doi: 10.1577/1548-8640(1988)050<0190:TITRNT>2.3.CO;2
Schreier, T. M., Rach, J. J., and Howe, G. E. (1996). Efficacy of formalin, hydrogen peroxide, and sodium chloride on fungal-infected rainbow trout eggs. Aquaculture 140, 323–331. doi: 10.1016/0044-8486(95)01182-X
Shamsi, S., Steller, E., and Zhu, X. (2021). The occurrence and clinical importance of infectious stage of Echinocephalus (Nematoda: Gnathostomidae) larvae in selected Australian edible fish. Parasitol. Int. 83:102333. doi: 10.1016/j.parint.2021.102333
Shao, Y., Wang, Y., Yuan, Y., and Xie, Y. (2021). A systematic review on antibiotics misuse in livestock and aquaculture and regulation implications in China. Sci. Total Environ. 798:149205. doi: 10.1016/j.scitotenv.2021.149205
Sinha, A. K., Romano, N., Shrivastava, J., Monico, J., and Bishop, W. M. (2020). Oxidative stress, histopathological alterations and anti-oxidant capacity in different tissues of largemouth bass (Micropterus salmoides) exposed to a newly developed sodium carbonate peroxyhydrate granular algaecide formulated with hydrogen peroxide. Aquat. Toxicol. 218:105348. doi: 10.1016/j.aquatox.2019.105348
Small, B. C., and Wolters, W. R. (2003). Hydrogen peroxide treatment during egg incubation improves channel catfish hatching success. N. Am. J. Aquac. 65, 314–317. doi: 10.1577/C02-048
Subasinghe, R. P., Bueno, P. B., Phillips, M. J., Hough, C., McGladdery, S. E., and Arthur, J. (2000). “Aquaculture in the third millennium.” in Technical Proceedings of the Conference on Aquaculture in the Third Millennium; February 20–25, 2000. NACA, Bangkok and FAO, 167–191.
Sunyer, J. O., Zarkadis, I. K., Sahu, A., and Lambris, J. D. (1996). Multiple forms of complement C3 in trout that differ in binding to complement activators. Proc. Natl. Acad. Sci. U. S. A. 93, 8546–8551.
Teresa Vega-Ramírez, M., Moreno-Lafont, M. C., Valenzuela, R., Cervantes-Olivares, R., Miguel Aller-Gancedo, J., Fregeneda-Grandes, J. M., et al. (2013). New records of Saprolegniaceae isolated from rainbow trout, from their eggs, and water in a fish farm from the state of México. Rev. Mex. Biodivers. 84, 637–649. doi: 10.7550/rmb.28627
Wang, H. S., Fang, Q. S., and Zheng, L. Y. (2002). Effects of salinity on hatching rates and survival activity index of the larvae of Epinephelus akaara. J. Fish. China 26, 344–350.
Wang, Q., Ji, W., and Xu, Z. (2020). Current use and development of fish vaccines in China. Fish Shellfish Immunol. 96, 223–234. doi: 10.1016/j.fsi.2019.12.010
Wang, Q., Yu, Y., Zhang, X., and Xu, Z. (2019). Immune responses of fish to Ichthyophthirius multifiliis (Ich): a model for understanding immunity against protozoan parasites. Dev. Comp. Immunol. 93, 93–102. doi: 10.1016/j.dci.2019.01.002
Webb, S., Mukhopadhyay, A. G., and Roberts, A. J. (2020). Intraflagellar transport trains and motors: insights from structure. Semin. Cell Dev. Biol. 107, 82–90. doi: 10.1016/j.semcdb.2020.05.021
Zambonino-Infante, J. L., Panserat, S., Servili, A., Mouchel, O., Madec, L., and Mazurais, D. (2019). Nutritional programming by dietary carbohydrates in European sea bass larvae: not always what expected at juvenile stage. Aquaculture 501, 441–447. doi: 10.1016/j.aquaculture.2018.11.056
Keywords: fish egg hatching, hydrogen peroxide, sodium chloride, programming, antioxidant capacity
Citation: Wang M, Xu W, Zou J, Li S, Song Z, Zheng F, Ji W, Xu Z and Wang Q (2021) The Programming of Antioxidant Capacity, Immunity, and Lipid Metabolism in Dojo Loach (Misgurnus anguillicaudatus) Larvae Linked to Sodium Chloride and Hydrogen Peroxide Pre-treatment During Egg Hatching. Front. Physiol. 12:768907. doi: 10.3389/fphys.2021.768907
Received: 01 September 2021; Accepted: 11 October 2021;
Published: 28 October 2021.
Edited by:
Youji Wang, Shanghai Ocean University, ChinaCopyright © 2021 Wang, Xu, Zou, Li, Song, Zheng, Ji, Xu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qingchao Wang, cWN3YW5nQG1haWwuaHphdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.