
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Physiol., 24 November 2021
Sec. Vascular Physiology
Volume 12 - 2021 | https://doi.org/10.3389/fphys.2021.762586
This article is part of the Research TopicWidely Used and Novel Approaches of the Measurement of Arterial Stiffness and Central Hemodynamic Parameters: is There a Consensus on the Horizon?View all 10 articles
Background: Antihypertensive treatment may have different effects on central arterial hemodynamics. The extent of the difference in effects between various antihypertensive drugs remains undefined.
Methods: We conducted a systematic review and meta-analysis of randomized controlled trials that explored the effects of antihypertensive agents on both central and peripheral systolic blood pressure (SBP) and pulse pressure (PP) or central augmentation index, with a special focus on the comparison between newer [renin-angiotensin-aldosterone system (RAS) inhibitors and calcium-channel blockers (CCBs)] and older antihypertensive agents (diuretics and β- and α-blockers).
Results: In total, 20 studies (n = 2,498) were included. Compared with diuretics (10 studies), β-blockers (16 studies), or an α-blocker (1 study), RAS inhibitors (21 studies), and CCBs (6 studies) more efficaciously (P < 0.001) reduced both central and peripheral SBP by a weighted mean difference of −5.63 (−6.50 to −4.76 mmHg) and −1.97 mmHg (−2.99 to −0.95 mmHg), respectively. Compared with older agents, the newer agents also more efficaciously (P < 0.001) reduced central PP (−3.27 mmHg; −4.95 to −1.59 mmHg), augmentation index (−6.11%; −7.94 to −4.29) and augmentation (−3.35 mmHg; −5.28 to –1.42 mmHg) but not peripheral PP (p ≥ 0.09). Accordingly, the newer agents reduced central-to-peripheral PP amplification significantly less than the older agents (0.11 mmHg; 0.05 to 0.17 mmHg; P < 0.001).
Conclusion: Newer agents, such as RAS inhibitors and CCBs, were significantly more efficacious than older agents in their effects on central hemodynamics.
When the blood flows from the central large elastic aorta to the peripheral smaller muscular arteries, systolic blood pressure (SBP) increases, without significant changes in diastolic blood pressure and mean arterial pressure, resulting in widened pulse pressure (PP). Although brachial blood pressure highly correlates with central blood pressure, substantial individual discrepancies between the central and peripheral blood pressure exist. Several studies have shown that the association between target-organ damage and SBP and PP is stronger for the central arteries than the brachial arteries (Kollias et al., 2016). Indeed, in a meta-analysis of 11 studies that included 5,648 subjects followed up for 3.8 years, central PP showed borderline superiority to brachial PP in the prediction of cardiovascular events (Vlachopoulos et al., 2010). Similar results were obtained in at least two recent systematic reviews and meta-analyses (Li et al., 2019; Vieceli et al., 2021).
Previous studies have shown that various classes of antihypertensive drugs may have different treatment effects between the central and peripheral arterial sites and that the newer antihypertensive agents, such as renin-angiotensin-aldosterone (RAS) inhibitors and calcium-channel blockers (CCBs), might be more efficacious than the older ones, such as diuretics, β-blockers, and α-blockers in the effect on central hemodynamics. The Conduit Artery Function Evaluation (CAFE) study, a substudy of the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT), showed that treatment with amlodipine/perindopril was more efficacious than with atenolol/bendroflume thiazide in reducing central SBP and PP by 4.3 and 3.0 mmHg, respectively, despite similar reductions in the brachial arteries (Williams et al., 2006). The cardiovascular benefits of treatment with amlodipine and perindopril observed in ASCOT (Dahlöf et al., 2005) might have been resulted at least in part from the lowering of central blood pressure, although other hemodynamic effects, such as reduced blood pressure variability, might have also played a part (Rothwell et al., 2010). In fact, there is a growing interest in central blood pressure as a target of treatment in hypertension.
In this comparative meta-analysis of randomized controlled trials, we investigated the effects of the newer agents vs. older antihypertensive agents on various central hemodynamic measurements.
Our meta-analysis strictly followed the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis: The PRISMA Statement (Moher et al., 2009). A total of 5,158 abstracts and full-text articles were retrieved systematically from electronic databases (PubMed, Embase, and Cochrane Central Register of Controlled Trials) and searched manually on September 15, 2020. The search key terms included “central pressure,” “aortic pressure,” “carotid pressure,” “pulse amplification,” “central-to-peripheral pulse pressure ratio,” “augmentation index,” “antihypertensive drug,” “antihypertensive treatment,” and “antihypertensive agent.” We limited our search to studies published in peer-reviewed journals in English. We checked the reference lists of review and original articles identified by the electronic search to find other potentially eligible studies.
The selection criteria for the inclusion of clinical trials in this meta-analysis were as follows: parallel-group randomized actively controlled trials in humans, the duration of treatment was no less than 4 weeks, and peripheral and central SBP or augmentation index after intervention were reported in a published article. Studies were excluded if the intervention was not an antihypertensive drug, or if the comparison was within the same drug class, between two newer (RAS inhibitors and CCBs) or older agents (diuretics and β- or α-blockers) or with a combination antihypertensive therapy.
Data extraction was performed using predefined data fields. Variables included author name, year of publication, study design, study population, number of patients, study intervention, duration of treatment and specifications of the blood pressure measuring device and other methods for blood pressure measurement, arterial sites, and the algorithm for augmentation index estimation. Baseline and post-intervention mean values of central and peripheral hemodynamic measurements for the experimental and control groups were extracted with standard deviation (SD), standard error of mean (SEM), or 95% confidence intervals (CIs) separately.
Central hemodynamic measurements included central systolic and diastolic blood pressure (mmHg), central PP (mmHg), augmentation pressure (mmHg), and augmentation index (%). Central augmentation pressure was the absolute difference between the second peak (P2) and the first peak (P1) of the central blood pressure wave. The central augmentation index was calculated either as the ratio of the P2 to the P1, or as augmentation pressure (P2−P1) divided by PP, expressed in percent. Peripheral measurements were brachial SBP and diastolic blood pressure and PP. Central-to-peripheral PP amplification was calculated as the ratio of the central PP to the peripheral PP.
When multiple usable groups were available within an individual study, the data were counted as another study in the meta-analysis. Methodological quality was assessed using the Jadad scores (Jadad et al., 1996). Study selection, quality assessment, and data extraction were performed independently by two investigators (Y-BC and J-HX) in an unblinded standardized manner. Disagreements were resolved by negotiation or consensus with a third authoritative investigator (J-GW).
For each comparison within each trial, we calculated the absolute differences between the experimental and control groups. If significant between-group differences in any outcome measure were reported at baseline, we calculated the absolute difference in the mean changes over time. The pooled effect for each grouping of trials was derived from the point estimate for each separate trial weighted by the inverse of the variance (1/SE2). Heterogeneity of effect sizes was tested across trials using the χ2 test. If trials were homogeneous (p < 0.10), a fixed-effects model was used to calculate pooled effect sizes. Otherwise, a random-effects model was applied to calculate overall differences. Net treatment effects on central pressure and augmentation index were determined by subtracting the mean change in the experimental group from the corresponding mean change in the control group. We performed all aforementioned computations and statistical analyses in Stata version 15 (Stata Corp LP, College Station, TX, United States). SEM was converted to SD [SE = SD/√(sample size)], and CIs were calculated [CI = mean difference ± (SEM × 1.96)], as appropriate.
Publication bias was assessed using the Egger’s statistic and visual inspection of funnel plots. Potential heterogeneity was further inspected by visual inspection of the data and by subgroup and sensitivity analyses. We performed subgroup analyses based on the classes of drugs, sensitivity analyses by limiting to studies with a Jadad score of ≥3, central blood pressure via the radial approach with the SphygmoCor device (AtCor Medical, Sydney, NSW, Australia), and a primary diagnosis of hypertension. All p-values were calculated from two-tailed tests of statistical significance with a type 1 error of 5%.
Figure 1 shows the flow diagram of the selection procedure for studies. The initial literature search retrieved 5,158 potentially eligible articles, and 4,007 records remained after removing duplicates. After having reviewed the title and abstract, 3,845 were excluded. Of the 162 full-text articles retrieved, 142 original articles were excluded for various reasons (Figure 1), leaving 20 eligible original articles in the analysis (Chen et al., 1995; Klingbeil et al., 2002; Ariff et al., 2006; Dart et al., 2007; Jiang et al., 2007; Schneider et al., 2008; Mackenzie et al., 2009; Matsui et al., 2009; Boutouyrie et al., 2010; Doi et al., 2010; Vitale et al., 2012; Kubota et al., 2013; Radchenko et al., 2013; Kim et al., 2014; Koumaras et al., 2014; Ghiadoni et al., 2017; Jekell et al., 2017; Miyoshi et al., 2017; Webster et al., 2017; Choi et al., 2018), comparing RAS inhibitors (n = 21) or CCBs (n = 6) with diuretics, β-blockers, or an α-blocker (Table 1).
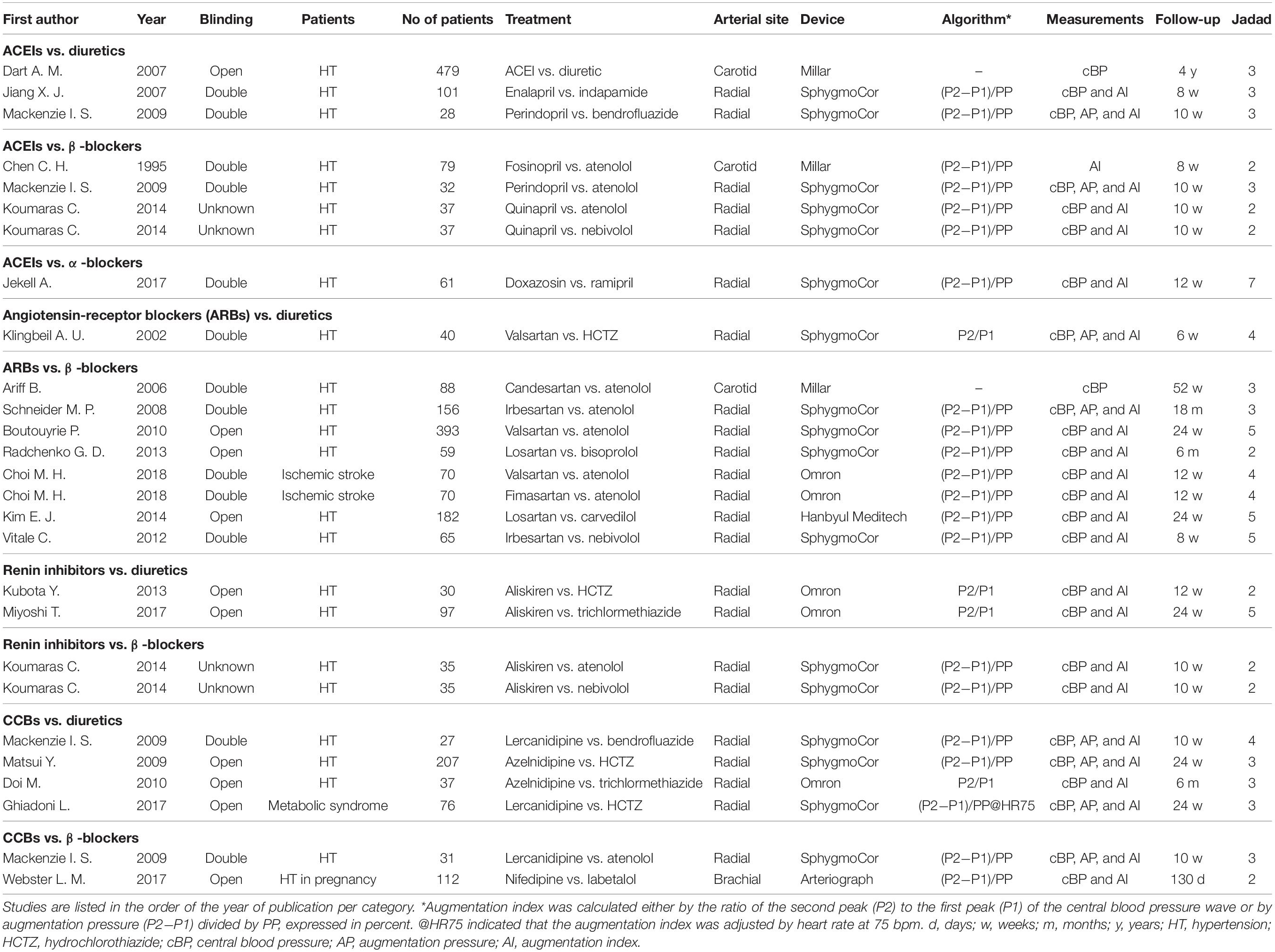
Table 1. Trials of the renin-angiotensin-aldosterone system (RAS) inhibitors and calcium-channel blockers (CCBs) vs. diuretics, β-blockers, and α-blockers.
These 20 trials included a total of 2,498 participants. The mean age of the study participants ranged from 35.5 (Webster et al., 2017) to 71.6 years (Dart et al., 2007), the proportion of women from 21.0% (Ghiadoni et al., 2017) to 100% (Webster et al., 2017), and the mean follow-up time from 6 (Klingbeil et al., 2002) to 52 weeks (Ariff et al., 2006). The study design was double-blinded in 10 studies, open in 9 studies, and not reported in 1 study. Central hemodynamics was estimated non-invasively from radial, carotid, and brachial applanation tonometry in 16 studies, 3 studies, and 1 study, respectively. Radial tonometry was performed using the SphygmoCor device (n = 11), (Omron Healthcare, Kyoto, Japan) (n = 4), or (Hanbyul Meditech, Jeonju, Korea) (n = 1).
In total, we performed analyses in 20 trials with 1,250 and 1,248 participants in the treatment groups of newer and older antihypertensive drugs, respectively (Table 1). Newer antihypertensive agents consisted of an ACEI in eight trials (n = 854), an ARB in nine trials (n = 1,123), a renin inhibitor in four trials (n = 197), and a CCB in six trials (n = 490).
Compared with diuretics (n = 10), β-blockers (n = 16), or an α-blocker (n = 1), the weighted mean differences in the central SBP were statistically significant for ACEIs (n = 7) by −3.39 mmHg (−5.91 to −0.89, p = 0.008), for ARBs (n = 9) by −4.12 mmHg (−6.02 to −2.21, p < 0.001), for renin inhibitors (n = 4) by −6.67 mmHg (−7.83 to −5.50, p < 0.001), and for CCBs (n = 6) by −5.60 mmHg (−8.21 to −2.99, p < 0.001). No significant heterogeneity was noticed within all four classes of newer drugs (p ≥ 0.24). The overall weighted mean difference in the central SBP across all 20 trials was −5.63 mmHg (−6.50 to −4.76, p < 0.001; I2 = 15.1%, P for heterogeneity = 0.25). The weighted mean differences in central PP were statistically significant for ARBs (n = 7) by −5.52 mmHg (−8.56 to −2.48, p = 0.006) but not significant for ACEIs (n = 6), renin inhibitors (n = 2), or CCBs (n = 4, p ≥ 0.07). The overall weighted mean difference in central PP across 12 studies with data was −3.27 mmHg (−4.95 to −1.59, p < 0.001; I2 = 55.8%, P for heterogeneity = 0.002, Figure 2).
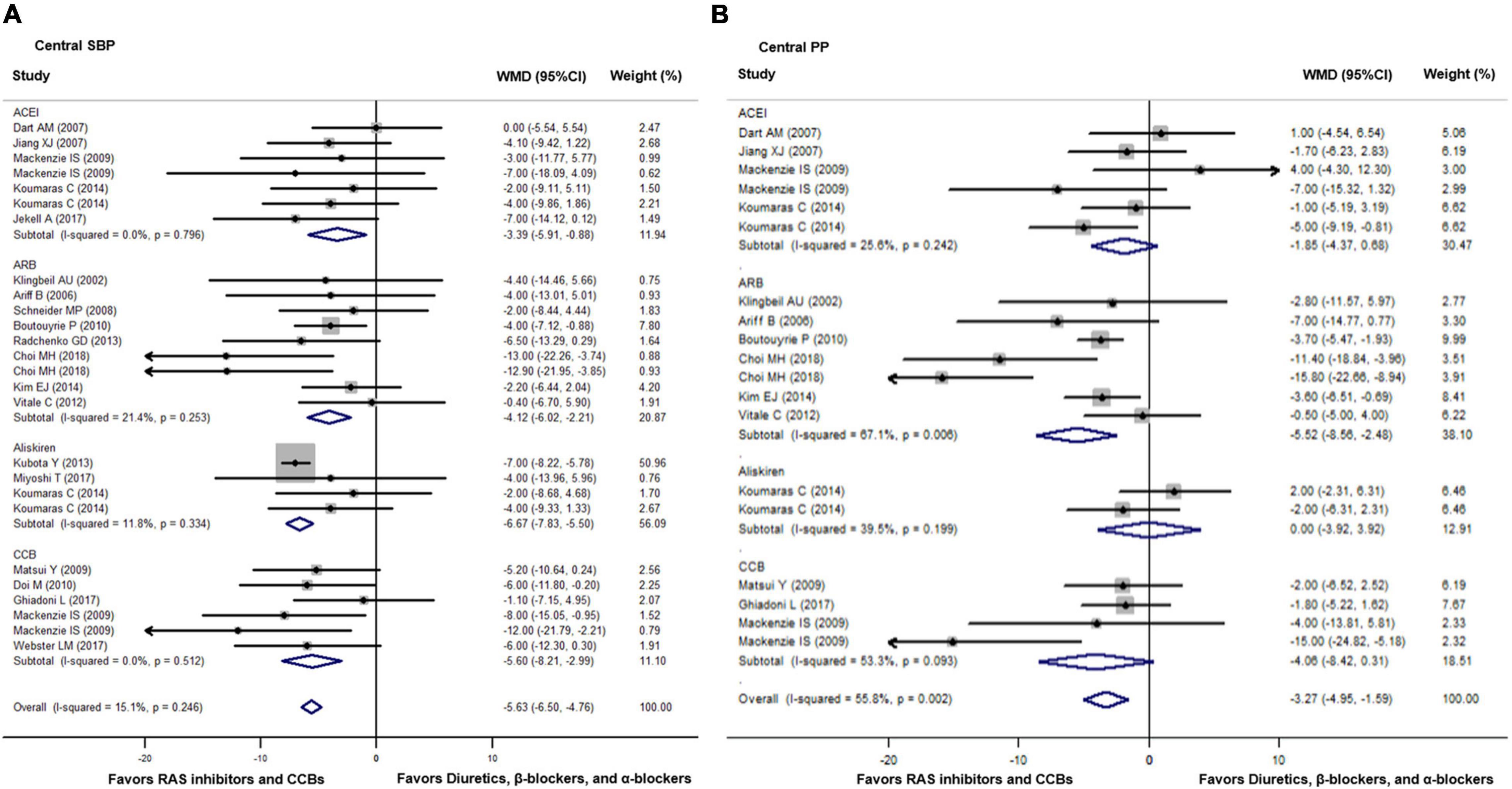
Figure 2. Effect of angiotensin-converting enzyme inhibitors (ACEIs), angiotensin-receptor blockers (ARBs), renin inhibitors, and calcium-channel blockers (CCBs) vs. diuretics, β-blockers, and α-blockers on central systolic blood pressure (SBP, A) and pulse pressure (PP, B). Weights are from the fixed (A) and random (B) effects analyses. RAS indicates renin-angiotensin-aldosterone system. Dots represent mean difference of each study. The size of the squares is proportional to the sample size. Horizontal lines represent the 95% confidence intervals (CI). Open diamonds represent the weighted mean difference (WMD) with 95% CI.
Compared with diuretics (n = 9), β-blockers (n = 15), or an α-blocker (n = 1), the weighted mean differences in the central augmentation index were statistically significant for ACEIs (n = 7) by −4.89% (−7.28 to −2.50, p = 0.001), for ARBs (n = 8) by −9.20% (−12.54 to −5.86, p < 0.001), and for CCBs (n = 6) by −5.27% (−9.14 to −1.40, p = 0.008). The overall weighted mean difference in the central augmentation index across 18 studies with data was −6.11% (−7.94 to −4.29, p < 0.001; I2 = 67.5%, P for heterogeneity < 0.001, Figure 3). In addition, across five trials with data, the overall weighted mean difference in the central augmentation pressure was −3.35 mmHg (−5.28 to −1.42, p < 0.001; I2 = 55.7%, P for heterogeneity = 0.03, Figure 4).
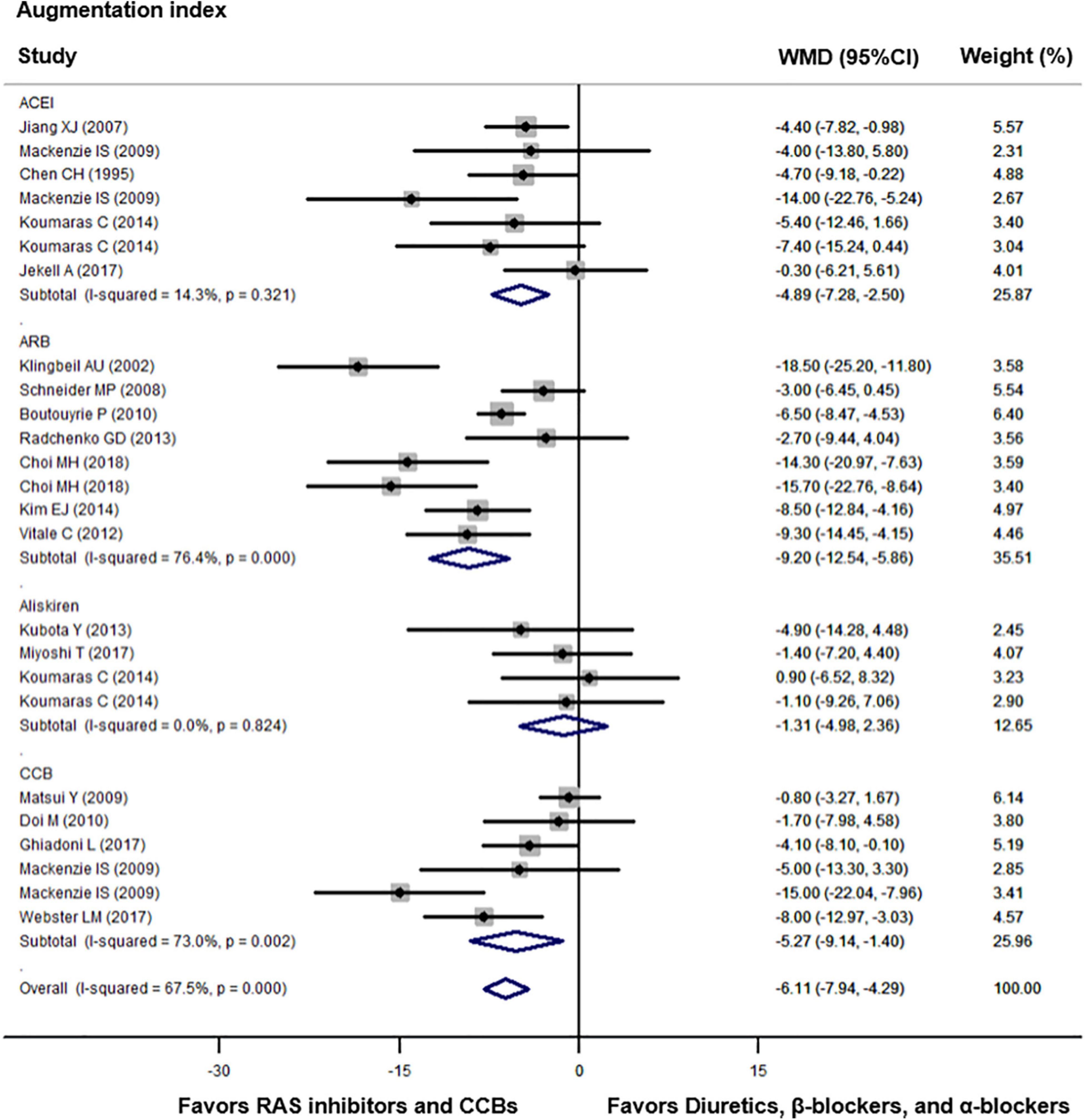
Figure 3. Effect of angiotensin-converting enzyme inhibitors (ACEIs), angiotensin-receptor blockers (ARBs), renin inhibitors, and calcium-channel blockers (CCBs) vs. diuretics, β-blockers, and α-blockers on central augmentation index. Weights are from the random-effects analysis. For further details, see legends in Figure 2.
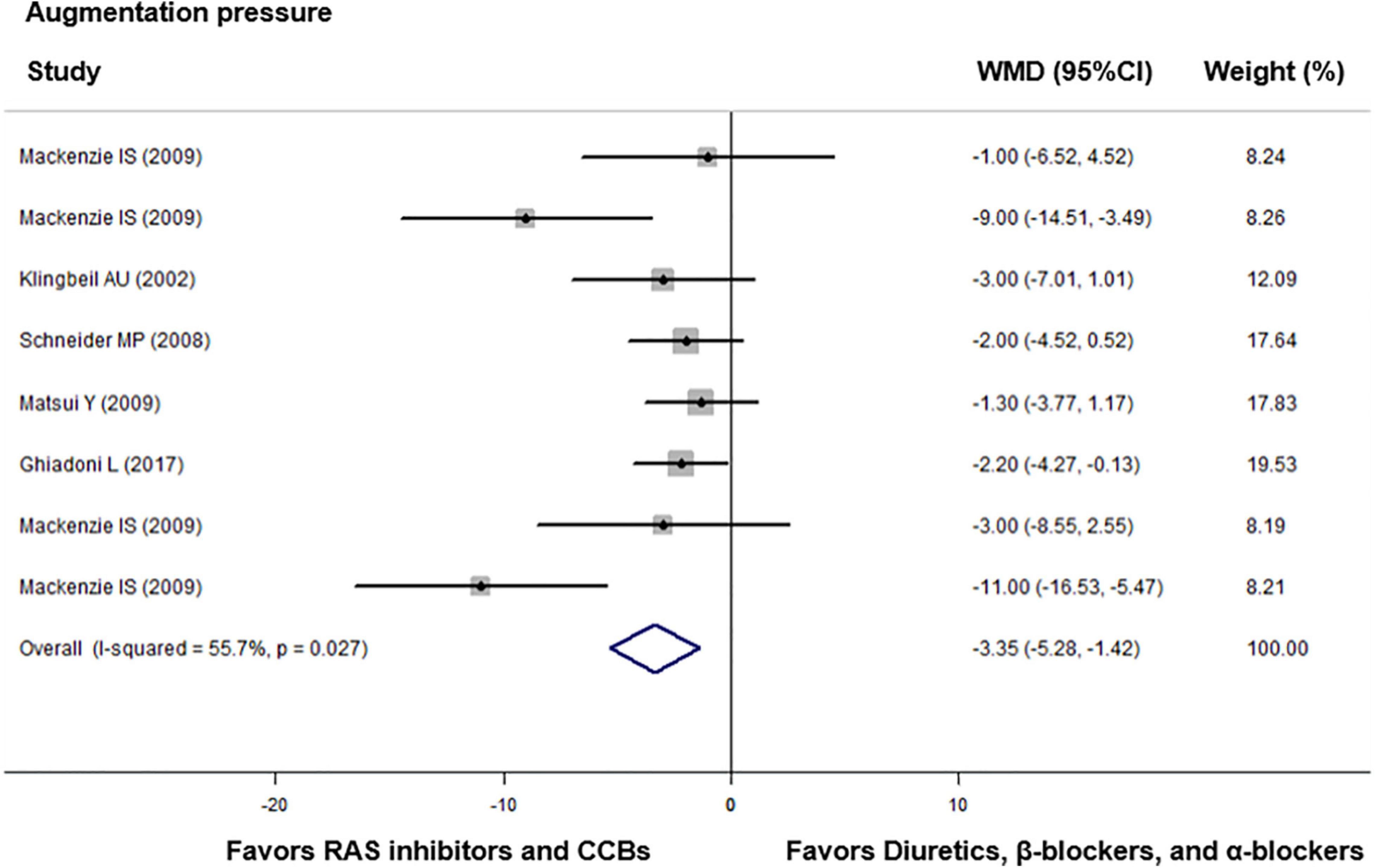
Figure 4. Effect of angiotensin-converting enzyme inhibitors (ACEIs), angiotensin-receptor blockers (ARBs), renin inhibitors, and calcium-channel blockers (CCBs) vs. diuretics, β-blockers, and α-blockers on central augmentation pressure. Weights are from the fixed-effects analysis. For further details, see legends in Figure 2.
With regard to peripheral measurements, the weighted mean difference was significant for SBP across 19 trials by −1.97 mmHg (−2.99 to −0.95, p < 0.001; I2 = 0.0%, P for heterogeneity = 0.67), but not for PP across 9 studies [−0.90 mmHg (−1.92 to 0.13 mmHg), p = 0.09; I2 = 25.8%, P for heterogeneity = 0.16, Supplementary Figure 1].
Compared with diuretics (n = 3) or β-blockers (n = 4), the overall weighted mean differences in central-to-peripheral PP amplification were significantly smaller across four trials with either ACEIs (n = 2), ARBs (n = 2), or CCBs (n = 3) by 0.11 mmHg (0.05 to 0.17, p < 0.001; I2 = 54.3%, P for heterogeneity = 0.041, Figure 5).
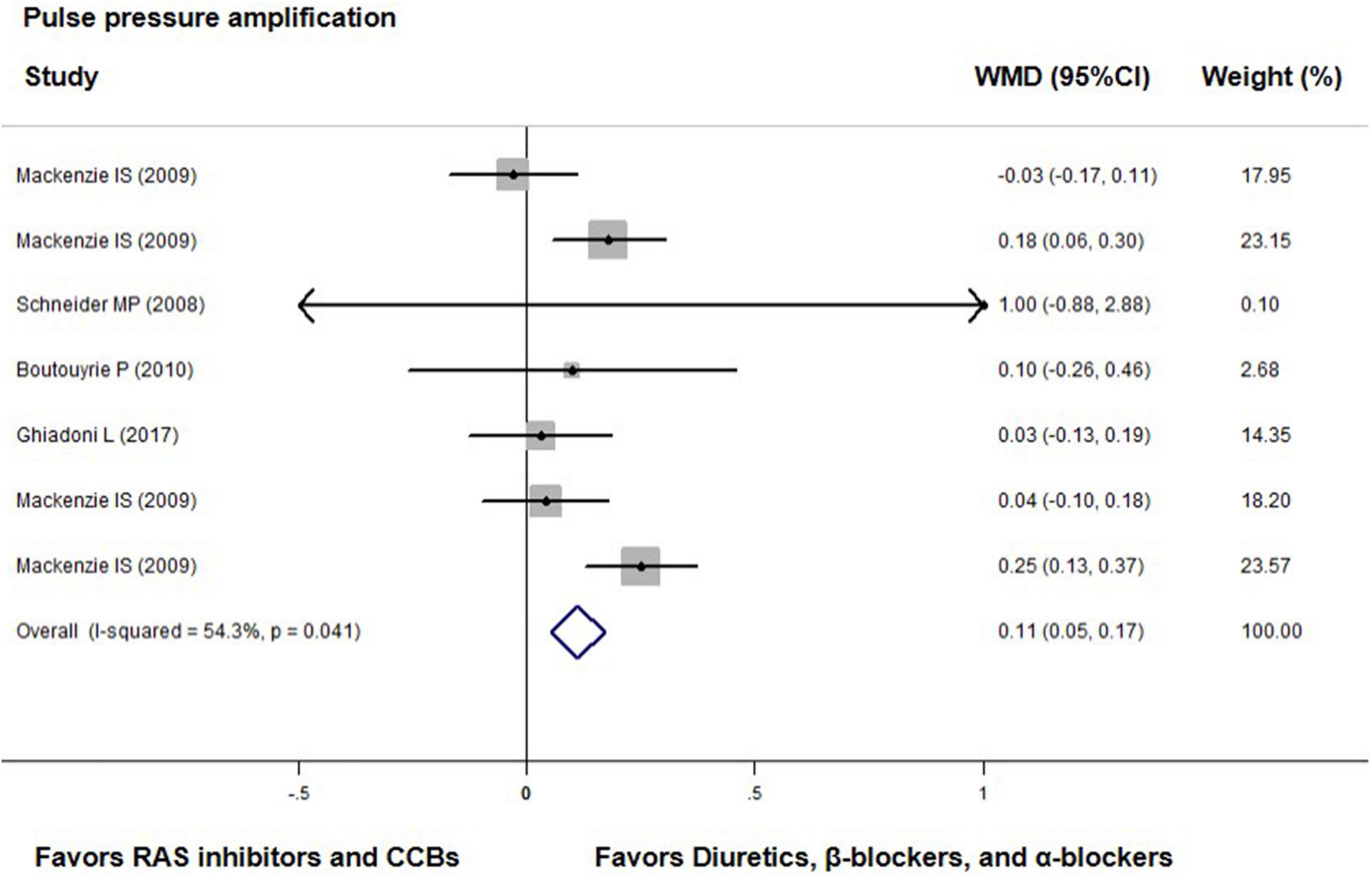
Figure 5. Effect of angiotensin-converting enzyme inhibitors (ACEIs), angiotensin-receptor blockers (ARBs), renin inhibitors, and calcium-channel blockers (CCBs) vs. diuretics, β-blockers, and α-blockers on central-to-peripheral pulse pressure amplification. Weights are from the fixed-effects analysis. For further details, see legends in Figure 2.
No publication bias was suggested by visual inspection of funnel plot in reporting changes in central SBP (Figure 6, the Egger’s test, p ≥ 0.08).
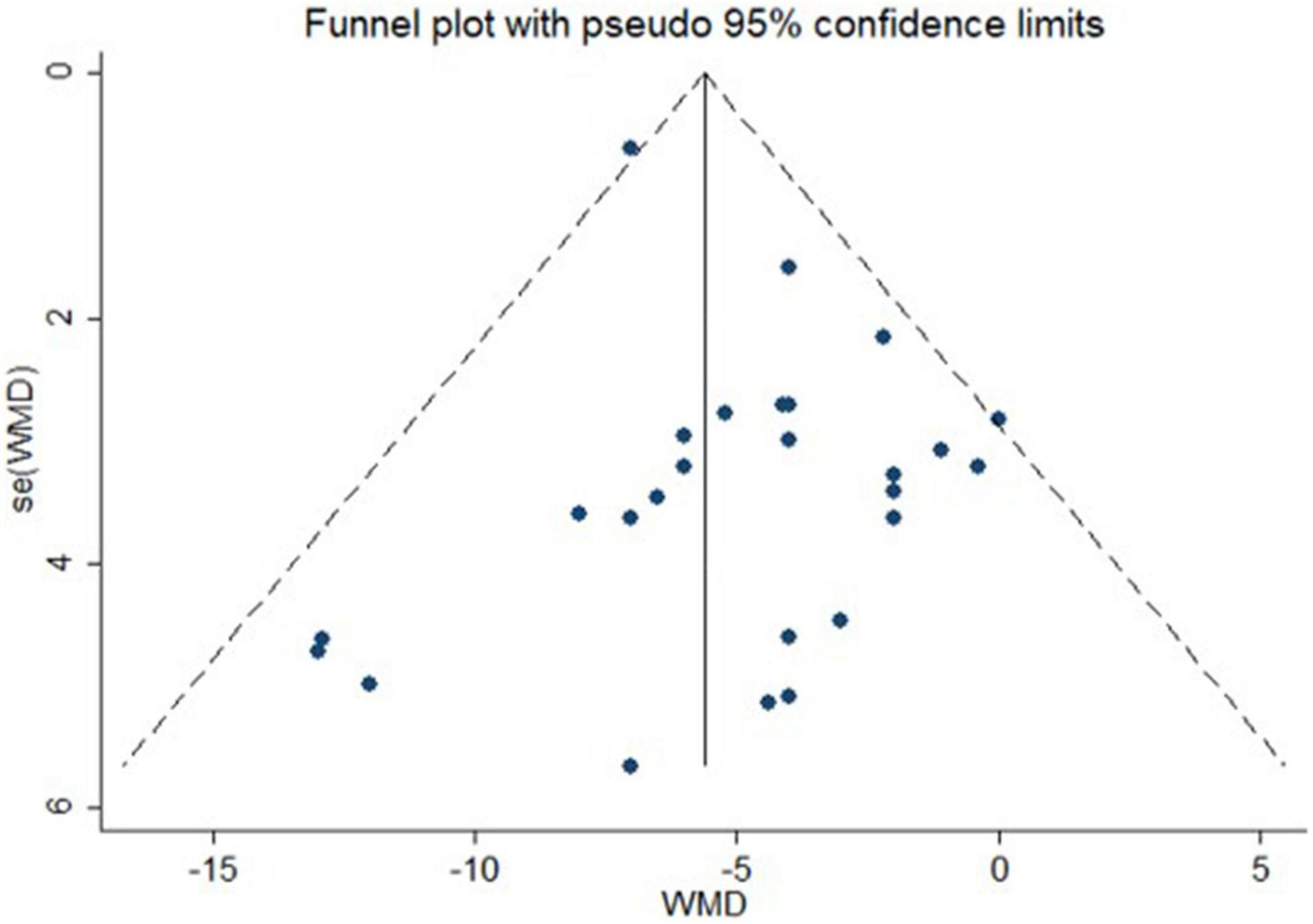
Figure 6. Funnel plot with pseudo 95% confidence limits for publication bias of actively controlled studies on central systolic blood pressure. WMD indicates weighted mean difference.
We also repeated analyses in subgroups according to the prespecified characteristics. In these subgroup analyses, the weighted mean differences were in agreement with the overall results (Supplementary Table 1).
When the trials of diuretics (n = 10) and β-blockers (n = 16) were compared, these older antihypertensive drugs behaved similar to the effects on the central SBP (n = 25) and peripheral SBP and PP (n = 24, p ≥ 0.17), but different to the effects on central PP (n = 19), central-to-peripheral PP amplification (n = 7), and central augmentation index (n = 24) in favor of β-blockers (p ≤ 0.002, Supplementary Table 2). Furthermore, when the trials of vasodilating (n = 4) and non-vasodilating β-blockers (n = 12) were compared, these two classes of β-blockers did not show significant difference in the effects on central and peripheral arterial hemodynamics (p ≥ 0.15, Supplementary Table 3).
This meta-analysis showed the differential effects of various antihypertensive drug classes on central hemodynamics. These results might help explain why some antihypertensive drugs, such as the β-blocker atenolol, were less efficacious in reducing the risk of stroke and cardiovascular mortality (Carlberg et al., 2004; McEniery, 2009), although it is generally believed that blood pressure reduction per se matters more than the choice of antihypertensive agents.
London et al. (1994) first conducted a controlled, blinded study to compare perindopril and nitrendipine in patients on chronic hemodialysis with a focus on central hemodynamic effects of vasoactive antihypertensive agents. The results showed that 12 months of treatment with ACEI and CCB had similar effects on augmentation index and carotid blood pressure. The following REASON study (Asmar et al., 2001) revealed in 471 hypertensive participants who were followed for 12 months that the combination of indapamide and perindopril decreased brachial SBP and PP more significantly than atenolol, with an adjusted between-group difference of −6.02 (95% CI, −8.90 to −3.14) and −5.57 mmHg (95% CI, −7.70 to −3.44), respectively. Similar adjusted between-group differences were observed for central SBP [−12.52 mmHg (95% CI, −17.97 to −7.08)] and PP [−10.34 mmHg (95% CI, −14.12 to −6.56)], and for carotid [−5.57% (95% CI, −10.77 to −0.36)] and aortic augmentation indices [−5.17% (95% CI, −7.74 to −2.61)].
A series of subsequent studies revealed a discrepancy in antihypertensive treatment on central and peripheral blood pressure. In a meta-analyses of 24 trials, Manisty and Hughes (2013) reported that treatment with β-blockers and diuretics posed a significantly less reduction in the central SBP than the brachial SBP by 6.9 and 6.8 mmHg, respectively, whereas other agents of monotherapy similarly lowered central and brachial SBP. Similar results were confirmed by McGaughey et al. (2016) in another meta-analyses of 52 studies with 4,381 participants and 58 studies with 3,716 participants for central SBP and augmentation index, respectively. Fifteen of the included studies had a crossover design, and 46 studies had a parallel group comparison design. Overall, antihypertensive drugs reduced brachial SBP more than central SBP by 2.52 mmHg, which was mainly attributed to the 5.19 mmHg greater reduction in the central-to-brachial amplification observed in β-blockers. Moreover, a significant reduction in the augmentation index was seen with RAS inhibitors, CCBs, and diuretics, but not β-blockers or α-blockers.
Both of the aforementioned meta-analyses were based on summary statistics instead of individual-subject data. The calculated differences in brachial and central SBP were less standardized in statistical analysis, such as adjustment for confounding factors. We tabulated head-to-head comparisons of various antihypertensive drugs regarding the effect on arterial hemodynamics. Despite similar reductions in peripheral PP, RAS inhibitors, and CCBs were more effective in reducing central PP than diuretics, β-blockers, and α-blockers. Newer antihypertensive agents significantly reduced more central blood pressure and augmentation than older agents.
The mechanisms for these differential treatment effects on central hemodynamics remain under investigation. As non-vasodilating β-blockers showed much less central blood pressure-lowering effect than the other classes of antihypertensive drugs, heart rate and vascular dilation or constriction must play a major role in the regulation of central hemodynamics. Indeed, in a meta-regression analysis (Ding et al., 2013), we previously found that slowing heart rate may to a large extent explain the less efficacy of β-blockers vs. the other classes of antihypertensive drugs. Although not shown in our present meta-analysis probably because of a limited number of trials, the vasoactive property must also play an important part in the central hemodynamic regulation. Indeed, a previous head-to-head comparison study showed divergent effects between vasodilating (nebivolol) and non-vasodilating (atenolol) β-blockers (Redón et al., 2014). Studies on the If inhibitor ivabradine provided further evidence. In a randomized, double-blind placebo-controlled, crossover study (Dillinger et al., 2015) in 12 patients with stable coronary artery disease, normal blood pressure, a sinus heart rate ≥70 beats per minute and β-blocker therapy, ivabradine treatment for 3 weeks reduced heart rate (−15.8 ± 7.7 vs. 0.3 ± 5.8 beats per minute, p = 0.001) and increased left ventricular ejection time (18.5 ± 17.8 vs. 2.8 ± 19.3 ms, p = 0.074) and diastolic perfusion time (215.6 ± 105.3 vs. −3.0 ± 55.8 ms, p = 0.0005), but did not significantly increase central SBP (−4.0 ± 9.6 vs. 2.4 ± 12.0 mmHg, p = 0.13) or augmentation index (−0.8% ± 10.0% vs. 0.3% ± 7.6%, p = 0.87). Taken together, it is probably the interaction between heart rate and vasoactive property that determines the extent of central pressure augmentation from wave reflections. This hypothesis may be tested in future animal experiments as well as human research. In addition, thiazide diuretics might be different in the central hemodynamic effects, for instance, between the so-called thiazide-type and thiazide-like diuretics. However, the present analysis did not allow us to perform this comparison because the thiazide-like diuretic was only used in one of the nine studies.
A major limitation of our meta-analysis was that two recent studies on an even newer antihypertensive drug class, i.e., angiotensin receptor neprilysin inhibitor (ARNI), were not included, because the comparative drug was an ARB (Schmieder et al., 2017; Williams et al., 2017), which was defined as a newer agent in the present analysis. In the PARAMETER (The Prospective comparison of Angiotensin Receptor neprilysin inhibitor with Angiotensin receptor blocker MEasuring arterial sTiffness in the eldERly) study, sacubitril/valsartan reduced central aortic systolic pressure (primary outcome) greater than olmesartan [between-treatment difference: −3.7 mmHg (95% CI, −6.4 to −0.9), p = 0.01] after 12 weeks of treatment but not after 52 weeks of treatment, probably because more subjects in the olmesartan group required add-on antihypertensive therapy than in the sacubitril/valsartan group (47% vs. 32%, p < 0.002). Indeed, Schmieder found that sacubitril/valsartan reduced central aortic PP to a greater extent than olmesartan (−3.5 mmHg, p = 0.01) after 52 weeks of treatment, with similar add-on treatment of amlodipine in the two groups (17.5% vs. 29.8%, p = 0.12). These observations shed some light on the potential beneficial effect of novel antihypertensive agents on central hemodynamics.
Antihypertensive drug treatment with RAS inhibitors and CCBs was more efficacious than that with diuretics, β-blockers, and α-blockers in the central hemodynamic effects. At present, there is still no direct evidence regarding the clinical relevance of central hemodynamics for decision-making in the management of hypertension and cardiovascular prevention. Therefore, it is imperative to run adequately powered outcome trials to investigate whether central hemodynamic measurements are clinically useful in guiding antihypertensive treatment and other cardiovascular therapeutic approaches for the prevention of cardiovascular events.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
J-GW and Y-BC conceived the study, performed the statistical analyses, and prepared the first draft of the manuscript. Y-BC and J-HX coordinated the data extraction. All authors participated in the interpretation of the data and approved the final version of the manuscript.
This study investigators were financially supported by grants from the National Natural Science Foundation of China (91639203, 81770455, 82070432, and 82070435), Ministry of Science and Technology (2018YFC1704902), and Ministry of Health (2016YFC0900902), Beijing, China, from the Shanghai Commissions of Science and Technology (grant 19DZ2340200 and “Sailing Program” 19YF1441000), and Health (“Three-Year Action Program of Shanghai Municipality for Strengthening the Construction of Public Health System” GWV-10.1-XK05 and a special grant for “leading academics”), Shanghai, China, and from the Clinical Research Program, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine (grant 2018CR010), Shanghai, China.
J-GW reports receiving lecture and consulting fees from Novartis, Omron, and Viatris.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2021.762586/full#supplementary-material
Ariff, B., Zambanini, A., Vamadeva, S., Barratt, D., Xu, Y., Sever, P., et al. (2006). Candesartan- and atenolol-based treatments induce different patterns of carotid artery and left ventricular remodeling in hypertension. Stroke 37, 2381–2384. doi: 10.1161/01.STR.0000236839.69658.c5
Asmar, R. G., London, G. M., O’Rourke, M. E., Mallion, J. M., Romero, R., Rahn, K. H., et al. (2001). Amelioration of arterial properties with a perindopril-indapamide very-low-dose combination. J. Hypertens. Suppl. 19, S15–S20.
Boutouyrie, P., Achouba, A., Trunet, P., Laurent, S., and Group, E. T. (2010). Amlodipine-valsartan combination decreases central systolic blood pressure more effectively than the amlodipine-atenolol combination: the explor study. Hypertension 55, 1314–1322. doi: 10.1161/HYPERTENSIONAHA.109.148999
Carlberg, B., Samuelsson, O., and Lindholm, L. H. (2004). Atenolol in hypertension: is it a wise choice? Lancet 364, 1684–1689. doi: 10.1016/S0140-6736(04)17355-8
Chen, C. H., Ting, C. T., Lin, S. J., Hsu, T. L., Yin, F. C., Siu, C. O., et al. (1995). Different effects of fosinopril and atenolol on wave reflections in hypertensive patients. Hypertension 25, 1034–1041. doi: 10.1161/01.hyp.25.5.1034
Choi, M. H., Lee, J. S., Lee, S. E., Lee, S. J., Yoon, D., Park, R. W., et al. (2018). Central and cerebral haemodynamic changes after antihypertensive therapy in ischaemic stroke patients: a double-blind randomised trial. Sci. Rep. 8:1556. doi: 10.1038/s41598-018-19998-4
Dahlöf, B., Sever, P. S., Poulter, N. R., Wedel, H., Beevers, D. G., Caulfield, M., et al. (2005). Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the anglo-scandinavian cardiac outcomes trial-blood pressure lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet 366, 895–906. doi: 10.1016/S0140-6736(05)67185-1
Dart, A. M., Cameron, J. D., Gatzka, C. D., Willson, K., Liang, Y. L., Berry, K. L., et al. (2007). Similar effects of treatment on central and brachial blood pressures in older hypertensive subjects in the second australian national blood pressure trial. Hypertension 49, 1242–1247. doi: 10.1161/HYPERTENSIONAHA.106.085803
Dillinger, J. G., Maher, V., Vitale, C., Henry, P., Logeart, D., Manzo Silberman, S., et al. (2015). Impact of ivabradine on central aortic blood pressure and myocardial perfusion in patients with stable coronary artery disease. Hypertension 66, 1138–1144. doi: 10.1161/HYPERTENSIONAHA.115.06091
Ding, F. H., Li, Y., Li, L. H., and Wang, J. G. (2013). Impact of heart rate on central hemodynamics and stroke: a meta-analysis of beta-blocker trials. Am. J. Hypertens. 26, 118–125. doi: 10.1093/ajh/hps003
Doi, M., Miyoshi, T., Hirohata, S., Kamikawa, S., Usui, S., Kaji, Y., et al. (2010). Combination therapy of calcium channel blocker and angiotensin II receptor blocker reduces augmentation index in hypertensive patients. Am. J. Med. Sci. 339, 433–439. doi: 10.1097/MAJ.0b013e3181d658c4
Ghiadoni, L., Bruno, R. M., Cartoni, G., Stea, F., Magagna, A., Virdis, A., et al. (2017). Combination therapy with lercanidipine and enalapril reduced central blood pressure augmentation in hypertensive patients with metabolic syndrome. Vascul. Pharmacol. 92, 16–21. doi: 10.1016/j.vph.2015.06.004
Jadad, A. R., Moore, R. A., Carroll, D., Jenkinson, C., Reynolds, D. J., Gavaghan, D. J., et al. (1996). Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin. Trials 17, 1–12. doi: 10.1016/0197-2456(95)00134-4
Jekell, A., Kalani, M., and Kahan, T. (2017). The effects of alpha 1-adrenoceptor blockade and angiotensin converting enzyme inhibition on central and brachial blood pressure and vascular reactivity: the doxazosin-ramipril study. Heart Vessels 32, 674–684. doi: 10.1007/s00380-016-0924-9
Jiang, X. J., O’Rourke, M. F., Zhang, Y. Q., He, X. Y., and Liu, L. S. (2007). Superior effect of an angiotensin-converting enzyme inhibitor over a diuretic for reducing aortic systolic pressure. J. Hypertens. 25, 1095–1099. doi: 10.1097/HJH.0b013e3280ac1533
Kim, E. J., Song, W. H., Lee, J. U., Shin, M. S., Lee, S., Kim, B. O., et al. (2014). Efficacy of losartan and carvedilol on central hemodynamics in hypertensives: a prospective, randomized, open, blinded end point, multicenter study. Hypertens. Res. 37, 50–56. doi: 10.1038/hr.2013.112
Klingbeil, A. U., John, S., Schneider, M. P., Jacobi, J., Weidinger, G., and Schmieder, R. E. (2002). AT1-receptor blockade improves augmentation index: a double-blind, randomized, controlled study. J. Hypertens. 20, 2423–2428. doi: 10.1097/00004872-200212000-00022
Kollias, A., Lagou, S., Zeniodi, M. E., Boubouchairopoulou, N., and Stergiou, G. S. (2016). Association of central versus brachial blood pressure with target-organ damage: systematic review and meta-analysis. Hypertension 67, 183–190. doi: 10.1161/HYPERTENSIONAHA.115.06066
Koumaras, C., Tziomalos, K., Stavrinou, E., Katsiki, N., Athyros, V. G., Mikhailidis, D. P., et al. (2014). Effects of renin-angiotensin-aldosterone system inhibitors and beta-blockers on markers of arterial stiffness. J. Am. Soc. Hypertens. 8, 74–82. doi: 10.1016/j.jash.2013.09.001
Kubota, Y., Takahashi, H., Asai, K., Yasutake, M., and Mizuno, K. (2013). The influence of a direct renin inhibitor on the central blood pressure. J. Nippon Med. Sch. 80, 25–33. doi: 10.1272/jnms.80.25
Li, W. F., Huang, Y. Q., and Feng, Y. Q. (2019). Association between central haemodynamics and risk of all-cause mortality and cardiovascular disease: a systematic review and meta-analysis. J. Hum. Hypertens. 33, 531–541. doi: 10.1038/s41371-019-0187-x
London, G. M., Pannier, B., Guerin, A. P., Marchais, S. J., Safar, M. E., and Cuche, J. L. (1994). Cardiac hypertrophy, aortic compliance, peripheral resistance, and wave reflection in end-stage renal disease. Comparative effects of ACE inhibition and calcium channel blockade. Circulation 90, 2786–2796. doi: 10.1161/01.cir.90.6.2786
Mackenzie, I. S., McEniery, C. M., Dhakam, Z., Brown, M. J., Cockcroft, J. R., and Wilkinson, I. B. (2009). Comparison of the effects of antihypertensive agents on central blood pressure and arterial stiffness in isolated systolic hypertension. Hypertension 54, 409–413. doi: 10.1161/HYPERTENSIONAHA.109.133801
Manisty, C. H., and Hughes, A. D. (2013). Meta-analysis of the comparative effects of different classes of antihypertensive agents on brachial and central systolic blood pressure, and augmentation index. Br. J. Clin. Pharmacol. 75, 79–92. doi: 10.1111/j.1365-2125.2012.04342.x
Matsui, Y., Eguchi, K., O’Rourke, M. F., Ishikawa, J., Miyashita, H., Shimada, K., et al. (2009). Differential effects between a calcium channel blocker and a diuretic when used in combination with angiotensin II receptor blocker on central aortic pressure in hypertensive patients. Hypertension 54, 716–723. doi: 10.1161/HYPERTENSIONAHA.109.131466
McEniery, C. M. (2009). Antihypertensive drugs and central blood pressure. Curr. Hypertens. Rep. 11, 253–259. doi: 10.1007/s11906-009-0043-4
McGaughey, T. J., Fletcher, E. A., and Shah, S. A. (2016). Impact of antihypertensive agents on central systolic blood pressure and augmentation index: a meta-analysis. Am. J. Hypertens. 29, 448–457. doi: 10.1093/ajh/hpv134
Miyoshi, T., Murakami, T., Sakuragi, S., Doi, M., Nanba, S., Mima, A., et al. (2017). Comparable effect of aliskiren or a diuretic added on an angiotensin II receptor blocker on augmentation index in hypertension: a multicentre, prospective, randomised study. Open Heart 4:e000591. doi: 10.1136/openhrt-2017-000591
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., and Group, P. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann. Intern. Med. 151, 264–9, W64. doi: 10.7326/0003-4819-151-4-200908180-00135
Radchenko, G. D., Sirenko, Y. M., Kushnir, S. M., Torbas, O. O., and Dobrokhod, A. S. (2013). Comparative effectiveness of a fixed-dose combination of losartan + HCTZ versus bisoprolol + HCTZ in patients with moderate-to-severe hypertension: results of the 6-month ELIZA trial. Vasc. Health Risk Manag. 9, 535–549. doi: 10.2147/VHRM.S44568
Redón, J., Pascual-Izuel, J. M., Rodilla, E., Vicente, A., Oliván, J., Bonet, J., et al. (2014). Effects of nebivolol and atenolol on central aortic pressure in hypertensive patients: a multicenter, randomized, double-blind study. Blood Press 23, 181–188. doi: 10.3109/08037051.2013.840421
Rothwell, P. M., Howard, S. C., Dolan, E., O’Brien, E., Dobson, J. E., Dahlöf, B., et al. (2010). Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet 375, 895–905. doi: 10.1016/S0140-6736(10)60308-X
Schmieder, R. E., Wagner, F., Mayr, M., Delles, C., Ott, C., Keicher, C., et al. (2017). The effect of sacubitril/valsartan compared to olmesartan on cardiovascular remodelling in subjects with essential hypertension: the results of a randomized, double-blind, active-controlled study. Eur. Heart J. 38, 3308–3317. doi: 10.1093/eurheartj/ehx525
Schneider, M. P., Delles, C., Klingbeil, A. U., Ludwig, M., Kolloch, R. E., Krekler, M., et al. (2008). Effect of angiotensin receptor blockade on central haemodynamics in essential hypertension: results of a randomised trial. J. Renin Angiotensin Aldosterone Syst. 9, 49–56. doi: 10.3317/jraas.2008.003
Vieceli, T., Brambilla, B., Pereira, R. Q., Dellamea, B. S., Stein, A. T., and Grezzana, G. B. (2021). Prediction of all-cause and cardiovascular mortality using central hemodynamic indices among elderly people: systematic review and meta-analysis. Sao Paulo Med. J. 139, 123–126. doi: 10.1590/1516-3180.2020.0364.R1.0412020
Vitale, C., Marazzi, G., Iellamo, F., Spoletini, I., Dall’Armi, V., Fini, M., et al. (2012). Effects of nebivolol or irbesartan in combination with hydrochlorothiazide on vascular functions in newly-diagnosed hypertensive patients: the NINFE (Nebivololo, Irbesartan Nella Funzione Endoteliale) study. Int. J. Cardiol. 155, 279–284. doi: 10.1016/j.ijcard.2011.10.099
Vlachopoulos, C., Aznaouridis, K., and Stefanadis, C. (2010). Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J. Am. Coll. Cardiol. 55, 1318–1327. doi: 10.1016/j.jacc.2009.10.061
Webster, L. M., Myers, J. E., Nelson-Piercy, C., Harding, K., Cruickshank, J. K., Watt-Coote, I., et al. (2017). Labetalol versus nifedipine as antihypertensive treatment for chronic hypertension in pregnancy: a randomized controlled trial. Hypertension 70, 915–922. doi: 10.1161/HYPERTENSIONAHA.117.09972
Williams, B., Cockcroft, J. R., Kario, K., Zappe, D. H., Brunel, P. C., Wang, Q., et al. (2017). Effects of sacubitril/valsartan versus olmesartan on central hemodynamics in the elderly with systolic hypertension: the parameter study. Hypertension 69, 411–420. doi: 10.1161/HYPERTENSIONAHA.116.08556
Williams, B., Lacy, P. S., Thom, S. M., Cruickshank, K., Stanton, A., Collier, D., et al. (2006). Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the conduit artery function evaluation (CAFE) study. Circulation 113, 1213–1225. doi: 10.1161/CIRCULATIONAHA.105.595496
Keywords: antihypertensive treatment, central blood pressure, augmentation index, randomized controlled trial, drug
Citation: Cheng Y-B, Xia J-H, Li Y and Wang J-G (2021) Antihypertensive Treatment and Central Arterial Hemodynamics: A Meta-Analysis of Randomized Controlled Trials. Front. Physiol. 12:762586. doi: 10.3389/fphys.2021.762586
Received: 22 August 2021; Accepted: 22 October 2021;
Published: 24 November 2021.
Edited by:
Christopher Clemens Mayer, Austrian Institute of Technology (AIT), AustriaReviewed by:
Stefano Omboni, Istituto Italiano di Telemedicina, ItalyCopyright © 2021 Cheng, Xia, Li and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ji-Guang Wang, amlndWFuZ3dhbmdAYWltLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.