
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Physiol. , 23 November 2021
Sec. Vascular Physiology
Volume 12 - 2021 | https://doi.org/10.3389/fphys.2021.742338
This article is part of the Research Topic Sport Activity: From Beneficial Effects to Cardiac Disease View all 7 articles
 Amilia Aminuddin1
Amilia Aminuddin1 Muhammad Fakhrurrazi Noor Hashim1
Muhammad Fakhrurrazi Noor Hashim1 Nur Aina Syazana Mohd Zaberi1
Nur Aina Syazana Mohd Zaberi1 Lee Zheng Wei1
Lee Zheng Wei1 Beh Ching Chu1
Beh Ching Chu1 Nur Amalina Jamaludin1
Nur Amalina Jamaludin1 Norizam Salamt1
Norizam Salamt1 Nur Aishah Che Roos2
Nur Aishah Che Roos2 Azizah Ugusman1*
Azizah Ugusman1*Skeletal muscle is one of the major tissues in the body and is important for performing daily physical activity. Previous studies suggest that vascular dysfunction contributes to reduced skeletal muscle mass. However, the association between vascular dysfunction and muscle mass, muscle strength and muscle flexibility are less established. Therefore, the focus of this review was to investigate the association between arterial stiffness (AS) which is a marker of vascular function, and muscle indices among healthy and those with cardiovascular risk factors. Three databases were used to search for relevant studies. These keywords were used: “arterial stiffness” OR “vascular stiffness” OR “aortic stiffness” OR “pulse wave velocity” OR “carotid femoral pulse wave velocity” OR “pulse wave analysis” AND “muscle” OR “skeletal” OR “flexibility” OR “range of motion” OR “articular” OR “arthrometry” OR “strength” OR “hand strength” OR “pinch strength” OR “mass” OR “lean” OR “body composition.” The criteria were; (1) original, full-text articles, (2) articles written in English language, (3) human studies involving healthy adults and/or adults with cardiovascular disease (CVD) or CVD risk factors (4) articles that reported the relationship between AS (measured as carotid-femoral pulse wave velocity or brachial-ankle pulse wave velocity) and muscle indices (measured as muscle mass, muscle flexibility and muscle strength) after adjusting for relevant confounders. The search identified 2295 articles published between 1971 and June 2021. Only 17 articles fulfilled the criteria. Two studies showed an inverse association between AS and muscle strength in healthy subjects, whereas in subjects with CVD risk factors, five out of seven studies found an inverse correlation between the two parameters. Eleven studies showed an inverse association between AS and muscle mass in subjects with CVD and CVD risk factors. The association between AS and muscle flexibility was not studied in any of the articles reviewed. In conclusion, there is an inverse correlation between muscle indices and AS in healthy adults and those with CVD or CVD risk factors. However, most of the studies were cross-sectional studies, hence the need for future prospective studies to address this issue.
Skeletal muscle comprises about 40% of the body weight and is important for performing daily physical activity (Sherwood, 2008). A good muscle strength and flexibility may help to reduce the risk of injury and fall which could lead to physical disabilities, poor quality of life and mortality. Muscle function is also related with muscle mass (Reed et al., 1991). Muscle receives about 15% of cardiac output at rest, and the need for blood supply increases during exercise (Sherwood, 2008). Thus, a good blood supply is important for muscle to function efficiently. The role of vascular function in the development of muscle mass was addressed in a recent study by Jeon et al. (2021). It was observed that poor vascular function may impair oxygen and nutrient delivery to the muscles, hence causing impairment of muscle protein synthesis and alteration in mitochondrial function (Groen et al., 2014; Jeon et al., 2021).
In human research, one of the non-invasive methods to assess vascular function is by measuring arterial stiffness (AS). AS is described as an elastic resistance to deformation that involves complex interactions between the extracellular matrix components such as elastin, collagen, glycoproteins and proteoglycans, and vascular smooth muscle cells in the arterial wall (Safar et al., 2003). AS is accelerated by aging and classic CVD risk factor such as atherosclerosis, thus reducing the normal arterial compliance. Stiffness of the aorta is frequently studied and the gold standard measurement of aortic stiffness is carotid-femoral pulse wave velocity (cfPWV), which measures the speed of the pressure wave that travels from the aorta to the femoral artery (Laurent et al., 2006). Another marker of AS is brachial-ankle PWV (baPWV) which indicates central and peripheral arterial stiffness (Katakami et al., 2014). PWV was independently associated with cardiovascular events as highlighted by the European Society of Cardiology/European Society of Hypertension Guidelines (Mancia et al., 2013).
Previous studies have investigated the association between PWV and muscle indices (Watson et al., 2011; van Dijk et al., 2015; Lima-Junior et al., 2019). A prospective study showed that increased PWV was associated with poor muscular function in people with altered blood flow (Watson et al., 2011). It was also revealed that reduction in muscle flexibility and strength was an indicator of increased arterial stiffness (AS) (Yamamoto et al., 2009; Fahs et al., 2010). However, several studies showed no significant association (van Dijk et al., 2015; Lima-Junior et al., 2019). This might be contributed by different methods of measurements and low sample sizes. Hence, the objective of this study was to investigate the relationship between AS and muscle indices by systematically reviewing the relevant studies involving healthy adults and those with established CVD or CVD risk factors, which were known to affect vascular function.
The literature search was conducted up to June 2021 based on three databases: Web of Science, PubMed and Scopus. The following keywords were used as search strategy: (“aortic stiffness”) OR (“arterial stiffness”) OR (“vascular stiffness”) OR (“pulse wave velocity”) OR (“carotid femoral pulse wave velocity”) OR (“pulse wave analysis”) AND (“skeletal”) OR (“muscle”) OR (“range of motion”) OR (“flexibility”) OR (“arthrometry”) OR (“articular”) OR (“strength”) OR (“pinch strength”) OR (“hand strength”) OR (“body composition”) OR (“lean”) OR (“mass”).
Articles that that have been extracted using the keywords were screened by two authors (BC and NJ). The criteria used were (1) original, full-text articles, (2) articles written in English language, (3) human studies involving healthy adults and/or adults with established CVD or CVD risk factors (4) articles that reported the relationship between AS (measured as cfPWV or baPWV) and muscle indices (measured as muscle mass, muscle flexibility and muscle strength) after adjusting for relevant covariates or confounders. Adjustment for covariates is necessary since there are several factors that influence PWV such as age, blood pressure, heart rate and CVD risk factors such as hypertension, dyslipidemia and diabetes mellitus. Studies involving subjects with chronic lung, kidney, inflammatory diseases and malignancies were excluded. We also excluded studies that used simple correlation for the associations.
In this study, the articles were screened in three phases. Initially, the articles were omitted in view of their title and keywords. Next, after reviewing the abstracts, the articles that did not follow the criteria were omitted. In the final phase, articles that were not related to the association between AS and muscle indices were omitted after reading the full text. The details of the studies were summarized in a table which included the design of the study, subjects’ characteristic, mean age, male percentage, method of measurement and the findings. The selected studies were divided into two categories; (1) studies involving healthy subjects or (2) studies involving subjects with established CVD or CVD risk factors.
From the three databases, a total of 2295 articles were obtained. These included 561 articles from PubMed, 1089 articles from Scopus, and 645 articles from Web of Science. The articles were published between the years 1971 and June 2021. A total of 56 articles written in languages other than English were omitted. After reading the titles or abstracts, 2204 articles were further omitted. The remaining 35 articles were obtained and reviewed thoroughly by fully reading the whole text. Out of these 35 articles, only 17 articles fulfilled the selection criteria, hence included in this review. The process of article selection is shown in Figure 1.
Table 1 summarized the two studies related to the association between muscle strength and AS in healthy subjects, while Table 2 summarized the seven studies related to the association between muscle strength and AS in subjects with established CVD and CVD risk factors. Table 3 summarized 11 studies related to the association between muscle mass and AS in subjects with CVD risk factors. There were no studies related to the association between muscle mass and AS in healthy subjects. The details of the parameters measured in each study were included in Tables 4, 5, respectively.
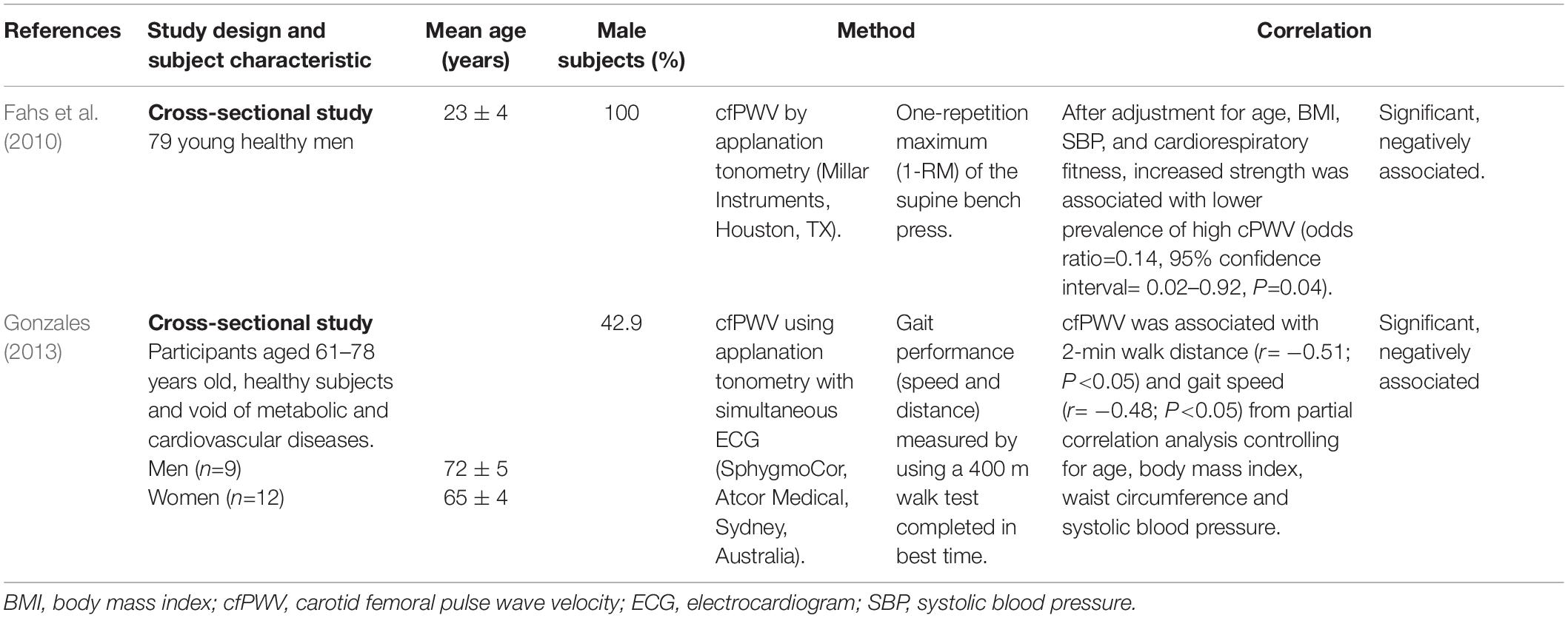
Table 1. Selected studies focusing on the association between arterial stiffness and muscle strength in healthy subjects.
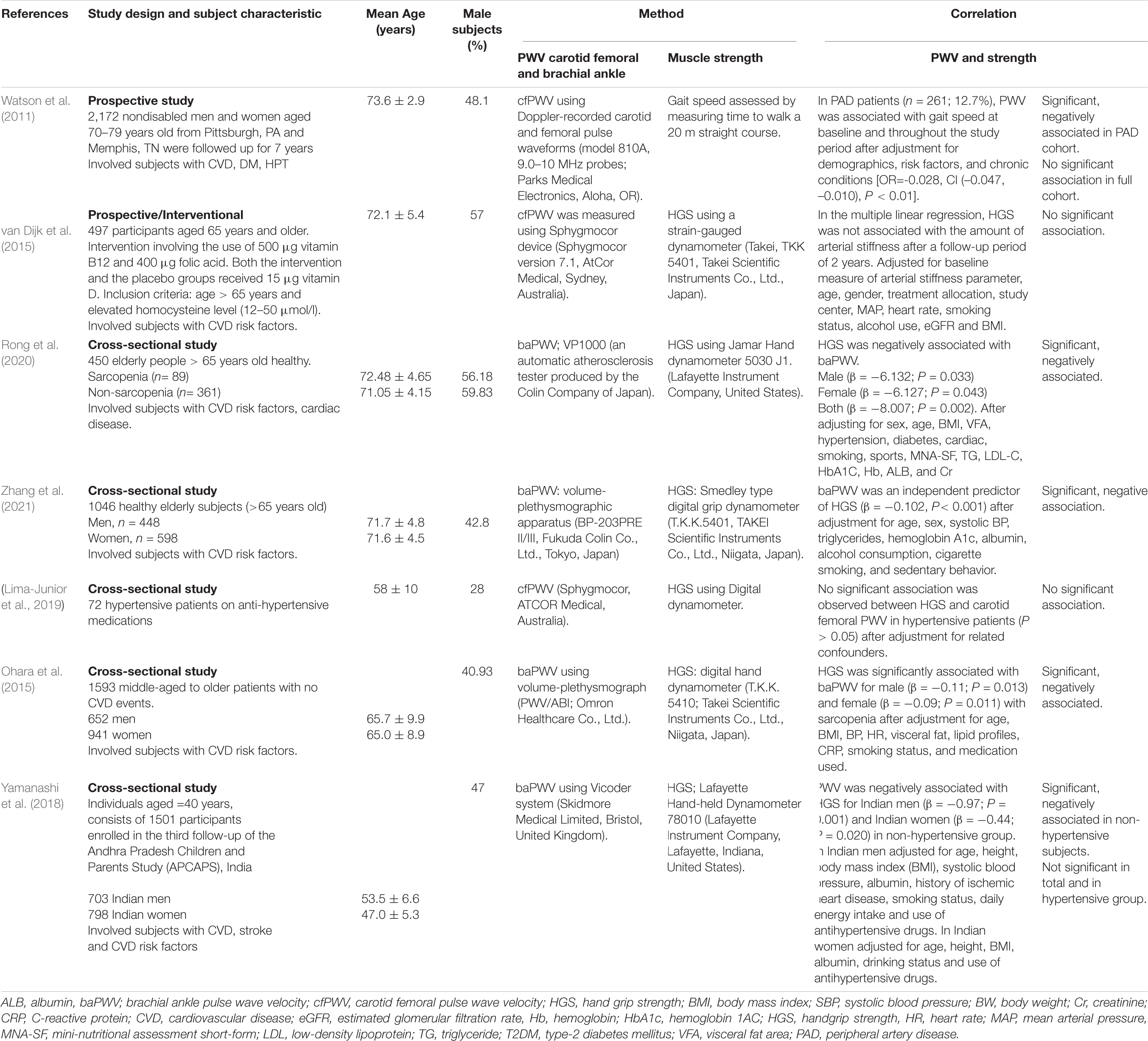
Table 2. Selected studies focusing on the association between arterial stiffness and muscle strength in subjects with cardiovascular diseases or cardiovascular diseases risk factors.
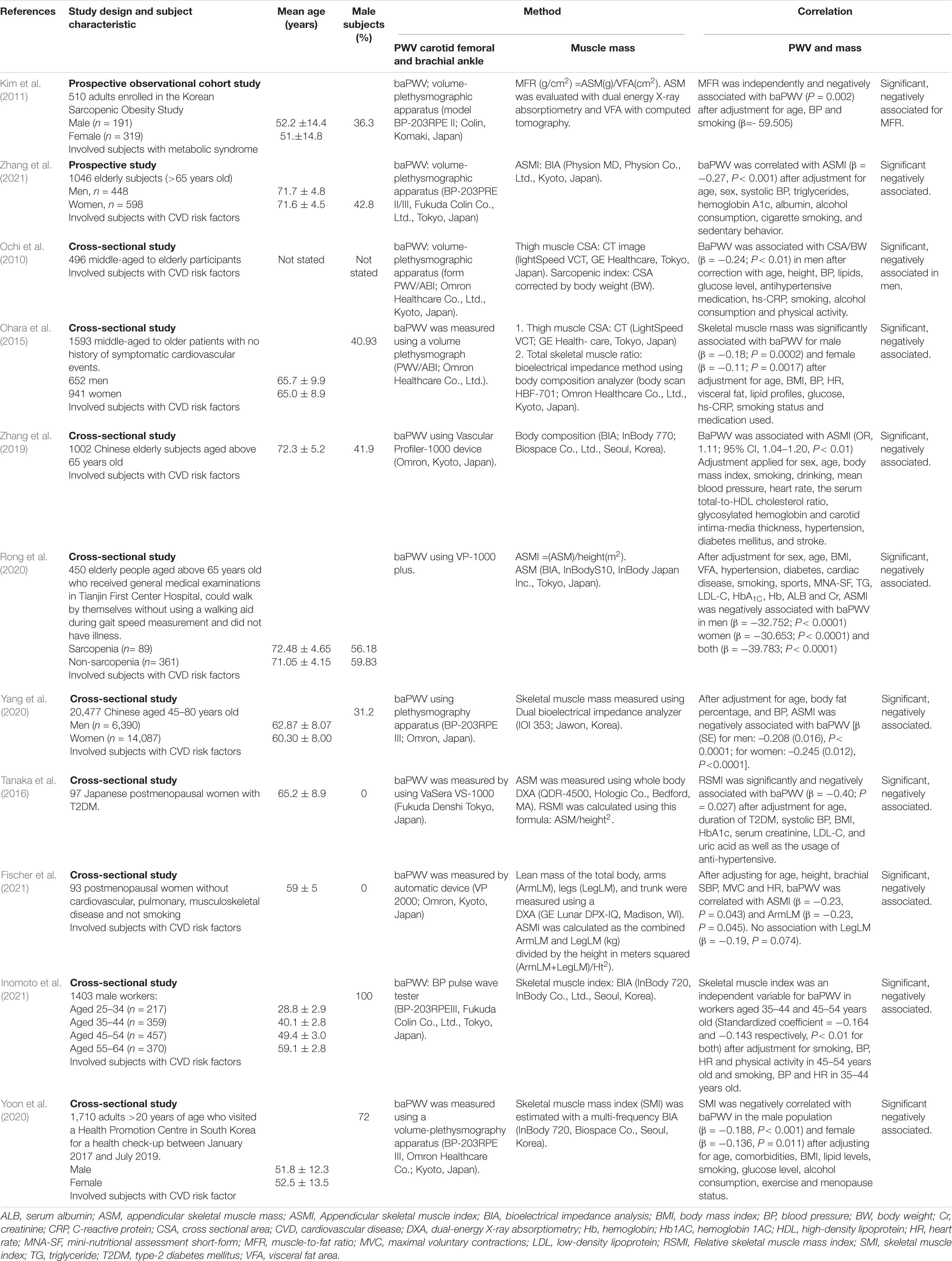
Table 3. Selected studies focusing on the association between arterial stiffness and muscle mass in subjects with cardiovascular diseases or cardiovascular diseases risk factors.
For the association between muscle strength and AS in healthy subjects (Table 1), all studies found that muscle strength was inversely correlated with arterial stiffness as measured by cfPWV (Fahs et al., 2010; Gonzales, 2013). A cross-sectional study showed that increased muscle strength was associated with lower prevalence of high cfPWV in young healthy men (odds ratio = 0.14, 95% confidence interval = 0.02–0.92, P = 0.04) (Fahs et al., 2010). Another study by Gonzales (2013) demonstrated that cfPWV was associated with the gait distance in healthy, older subjects (r = −0.51; P < 0.05) (Gonzales, 2013).
In subjects with CVD or CVD risk factors (Table 2), five out of seven studies found significant associations (Watson et al., 2011; Ohara et al., 2015; Yamanashi et al., 2018; Rong et al., 2020; Zhang et al., 2021). For example, the study by Rong et al. (2020) found that muscle strength as measured by handgrip dynamometer was negatively associated with baPWV in elderly subjects (Rong et al., 2020). Whereas the study by Ohara et al. (2015) found that muscle strength as measured by handgrip dynamometer was negatively associated with baPWV in middle-aged and older subjects (Ohara et al., 2015). A prospective study found a significant association between aortic PWV and gait speed in peripheral arterial disease (PAD) patients (OR = −0.028, CI (−0.047, −0.010), P < 0.01), but the association was not significant in total cohort population (Watson et al., 2011). Similarly, the study by Yamanashi et al. (2018) found an inverse association between handgrip strength and PWV in non-hypertensive adults [men (β = −0.97; P = 0.001), women (β = −0.44; P = 0.020)], but not in the hypertensive group and the total cohort (Yamanashi et al., 2018). Another two studies did not observe any significant association between AS and muscle strength (van Dijk et al., 2015; Lima-Junior et al., 2019).
As for the association between muscle mass and PWV, all 11 relevant studies involved subjects with CVD or CVD risk factors. Most of the studies were cross-sectional studies. All the eleven studies found a negative association between muscle mass and PWV (Ochi et al., 2010; Kim et al., 2011; Ohara et al., 2015; Tanaka et al., 2016; Zhang et al., 2019, 2021; Rong et al., 2020; Yang et al., 2020; Yoon et al., 2020; Fischer et al., 2021; Inomoto et al., 2021). Five studies that measured the appendicular skeletal muscle index (ASMI) as the muscle mass marker showed a negative association between ASMI and baPWV (Zhang et al., 2019, 2021; Rong et al., 2020; Yang et al., 2020; Fischer et al., 2021). For example, the study by Rong et al. (2020) found that ASMI was negatively associated with baPWV in elderly Chinese after adjustment of potential confounders [(β = −32.752; P < 0.0001 (men); β = −39.783; P < 0.0001) (women)] (Rong et al., 2020).
Yang et al. (2020) found that low muscle mass was associated with increased risk of AS in Chinese nationals aged 45 years old and older (men, P ≤ 0.0001, β = −0.208, women, P ≤ 0.0001, β = −0.245) (Yang et al., 2020). In Japan, subjects older than 65 years old showed a negative association between baPWV and ASMI (β = −0.27; P < 0.001) (Zhang et al., 2021). A study on post-menopausal women also revealed similar finding whereby ASMI and arm leg mass (armLM) were negatively associated with baPWV with β = −0.23 (P = 0.043) and β = −0.23 (P = 0.045), respectively (Fischer et al., 2021). A cross-sectional study by Kim et al. (2011) observed that appendicular skeletal muscle mass (ASM) was not associated with baPWV. However, there was a significant association between ASM and visceral fat area ratio (MFR) (β = −59.505, P = 0.002) (Kim et al., 2011).
Relative skeletal muscle mass (calculated using the formula ASM/height2) had a negative association with baPWV in Japanese postmenopausal women with type 2 diabetes mellitus (T2DM) (Tanaka et al., 2016). Ohara et al. (2015) also found that skeletal muscle mass was inversely associated with baPWV in male (β = −0.18; P = 0.0002) and female subjects (β = −0.11; P = 0.0017) (Ohara et al., 2015). Skeletal muscle index is another measurement of muscle mass used in two studies. South Korean men and women showed a negative association between their skeletal muscle index and baPWV [(β = −0.188; P < 0.001 (men); β = −0.136; P = 0.011) (women)], whereas working men with low muscle mass in Japan (aged 35–44 and 45–54 years old) had a higher risk of AS (Yoon et al., 2020; Inomoto et al., 2021).
Arterial compliance represents the capacity of the artery to expand and recoil following heart contraction and relaxation, which permits blood flow from pulsatile and intermittent form to a steadier, laminar flow (Sherwood, 2008). Increased AS give more resistant to the blood flow and higher workload for the left ventricle. This led to increased blood pressure and enhanced atherosclerosis development (Oren et al., 2003; Mitchell et al., 2005).
The main factors contributing to AS are aging and atherosclerosis (Greenland et al., 2010). The artery becomes stiff when the collagen in the arterial wall increased and the elastin tissue decreased (Zieman et al., 2005). Besides structural changes, increase in local vasoconstrictor such as endothelin-1 (ET-1) or reduction in vasodilator such as nitric oxide (NO) may contribute to AS (Bellien et al., 2010; Guo et al., 2018). These modulators are released from vascular endothelial cells and has a significant role in the control of vascular activity (Santos-Parker et al., 2017; Trindade et al., 2017). Poor NO production is a cardinal feature of endothelial dysfunction (ED) (Sun et al., 2020). Several CAD risk factors such as hypertension, dyslipidemia and diabetes mellitus were known to increase AS through underlying ED (Rider et al., 2012; Aminuddin et al., 2020; Ji et al., 2020). Thus, having CAD risk factors would accelerate AS on top of the aging process.
In our review, it was found that in most of the studies, muscle indices had negative association with AS as measured by PWV. This association is evident in both healthy subjects and subjects with CVD or CVD risk factors. However, most of the studies are cross-sectional studies, hence the studies were unable to determine the cause-effect association between muscular functions and AS. There were only two prospective studies that determined the direct relationship between AS and muscle indices (Watson et al., 2011; van Dijk et al., 2015). Watson et al. (2011) showed that in PAD patients, higher PWV was independently associated with slower gait speed, thus suggesting that increased PWV leads to reduction in muscle strength. In contrast, van Dijk et al. (2015) showed that hand-grip strength was not associated with AS after a follow-up duration of 2 years. The author proposed that the lack of an association might be due to the difference between short- and long-term alteration of the arteries. It has been shown that structural adaptations of the artery such as changes in elastin and collagen need a longer time to develop (van Dijk et al., 2015). Another cross-sectional study by Lima-Junior et al. (2019) also showed no significant association between PWV and muscle indices. Small sample size might account for such discrepancy (Lima-Junior et al., 2019).
Regular exercise is an important modality in improving cardiovascular health and endothelial function (Francescomarino et al., 2009; Aminuddin et al., 2011), thus improving AS (Kollet et al., 2021). Exercise also increases muscle strength (Chen et al., 2017; Sanian et al., 2019; Otsuki et al., 2020). Increased muscle strength and reduced AS were inversely related with CVD risk factors such as increased levels of low-density lipoprotein, body fat and inflammation, decreased lean tissue mass and insulin resistance (Fahs et al., 2010; Nishitani et al., 2011; Rider et al., 2012; Farias et al., 2013; Aminuddin et al., 2020; Ji et al., 2020).
In terms of the association between AS and muscle mass, previous studies showed that there were negative association between low muscle mass and AS. There are several mechanisms that can explain such relationship. Firstly, an increase in AS may reduce basal limb blood flow, leading to decreased delivery of nutrients and oxygen to the muscle tissues and lower muscle mass (Suzuki et al., 2001; Abbatecola et al., 2012; Rong et al., 2020). AS is also associated with increased reflected wave, systolic blood pressure and pulse pressure along the arterial system that cause small vessel injury (Safar et al., 2003). Secondly, the muscle mass itself may exert an effect on AS. When there is muscle death due to muscle disuse or aging, maladaptive muscle remodeling may occur if there is impaired removal of the apoptotic cells (Siu et al., 2005; Sciorati et al., 2016). This includes fatty infiltration within the muscles that leads to the release of inflammatory cytokines (Sciorati et al., 2016). Inflammatory cytokines such as tumor necrosis factor-α, interleukin (IL)-1β and IL-6 activate several inflammatory signaling pathways that promote insulin resistance (Chen et al., 2015). Inflammation also causes proteolysis which leads to reduced muscle mass (Goodman, 1991). In addition, reduced muscle mass is also associated with insulin resistance, since skeletal muscle is the major site of glucose utilization (Yamanashi et al., 2018). Insulin resistance has been linked to AS (Ikonomidis et al., 2015), which could be explained by the underlying reduction in NO bioavailability (Sonne et al., 2009), increased endothelin levels and increased proliferation of vascular smooth muscle cells (Trovati and Anfossi, 2002).
Another dynamic that links muscle mass and AS is through increased oxidative stress. In this case, muscle mass may not directly affect AS and vice versa, but rather a distinct complication of a common factor which is oxidative stress. Oxidative stress happens when there is an imbalance between the antioxidant and free radicals in the body that leads to damage to the cellular protein, lipid and nucleic acids (Wu et al., 2013). Oxidative stress is related to sarcopenia (Bellanti et al., 2018) through modulations of transcription factors and inflammatory mediators such as nuclear factor-κB, Forkhead box (FOXO) and mitogen-activated protein kinase that lead to muscle apoptosis and reduced protein synthesis. Besides, oxidative stress causes myocardial DNA damage and dysfunction which later leads to muscle apoptosis and sarcopenia (Meng and Yu, 2010). Additionally, oxidative stress is related to the formation of AS through reduction in NO (Zhao et al., 2011; Förstermann et al., 2017; Guzik and Touyz, 2017). The proposed, complex mechanisms that explain the association between AS and muscle indices are summarized in Figure 2.
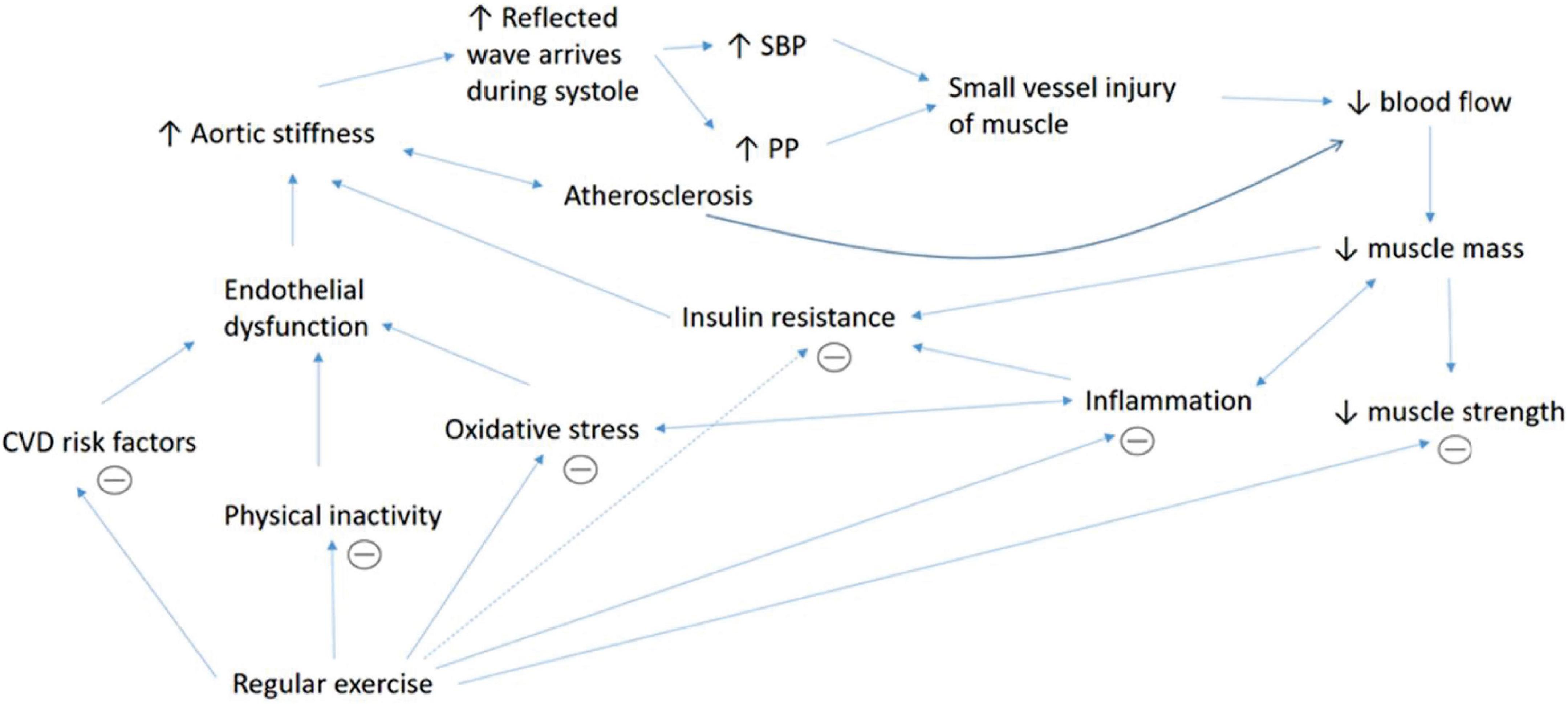
Figure 2. Summary of the underlying mechanisms that explain the association between AS and muscle indices. The association between aortic stiffness and muscular indices is complex, involves multiple intermediators and may act in a vicious cycle. Increased aortic stiffness leads to increased wave reflection and augmentation of systolic blood pressure (SBP) and pulse pressure (PP), which causes injury to small vessels in the organ such as muscle. Aortic stiffness which is also associated with atherosclerosis causes reduced blood supply to the muscle that impairs nutrient supplementation. This contributes to muscle death and reduced muscle mass. Reduced muscle mass also contributes to lower muscle strength. Besides, lower muscle mass causes less glucose intake into the muscle cells, which leads to insulin resistance. Poor muscle removal after cell death leads to fat infiltration and inflammation. Increased inflammation itself may cause proteolysis, reduced muscle mass, insulin resistance and increased oxidative stress. Oxidative stress causes proteolysis by causing myocardial DNA damage and stimulating the release of various inflammatory mediators. Inflammation, oxidative stress, various cardiovascular diseases (CVD) risk factors and physical inactivity are linked to endothelial dysfunction, which leads to increased aortic stiffness. Regular physical activity increases muscle mass and strength and improves endothelial function, oxidative stress, CVD risk factors, insulin resistance and inflammation which subsequently reduces aortic stiffness.
The strength of this study is that we focused on the established markers of PWV which are cfPWV and baPWV. However, there are certain limitations of this study that include (1) the cross-sectional design of the selected studies, in which cause-effect associations between muscle indices and arterial stiffness could not be determined, and (2) the absence of studies related to AS and muscle flexibility, which is one of important markers for muscular functions. On the other hand, we also excluded several studies that reported significant associations between AS and muscle indices that were derived from simple correlations without adjustment for confounders (Yamamoto et al., 2009; Komatsu et al., 2017; Logan et al., 2018). Thus, future studies should be conducted with a detail analysis adjusted for the covariates to address such associations.
There is an inverse association between muscle indices and AS in healthy subjects and subjects with established CVD and CVD risk factors. However, most of the studies reviewed are cross-sectional studies, hence no causal relationship or explanatory capacity between muscle indices and AS could be established. Therefore, more prospective studies should be conducted in the future to determine the interaction between muscle indices and AS.
BC and NJ performed the screening of articles. AA, MN, NM, and LZ drafted the manuscript. AA and AU finalized the manuscript. NC and NS contributed to the revision and editing of the manuscript. All authors read and approved the final manuscript.
This review was funded by the Universiti Kebangsaan Malaysia under the grant FF-2020-302.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thank Hafizah Abd Hamid for her kind contribution on technical aspects of the manuscript.
Abbatecola, A. M., Chiodini, P., Gallo, C., Lakatta, E., Sutton-Tyrrell, K., Tylavsky, F. A., et al. (2012). Pulse wave velocity is associated with muscle mass decline: health abc study. Age (Dordr.) 34, 469–478. doi: 10.1007/s11357-011-9238-0
Aminuddin, A., Lazim, M. L. M., Hamid, A. A., Hui, C. K., Yunus, M. H. M., Kumar, J., et al. (2020). The association between inflammation and pulse wave velocity in dyslipidemia: an evidence-based review. Mediators Inflamm. 2020, 1–11. doi: 10.1155/2020/4732987
Aminuddin, A., Zakaria, Z., Nordin, N. A. M. N., Karim, A. A. H., Maskon, O., Pei, T. S., et al. (2011). Two months aerobic exercise improves endothelial function and reduces oxidative dna damage in women with elevated blood pressure. New Iraqi J. Med. 7, 52–59.
Bellanti, F., Romano, A. D., Buglio, A. L., Castriotta, V., Guglielmi, G., Greco, A., et al. (2018). Oxidative stress is increased in sarcopenia and associated with cardiovascular disease risk in sarcopenic obesity. Maturitas 109, 6–12. doi: 10.1016/j.maturitas.2017.12.002
Bellien, J., Favre, J., Iacob, M., Gao, J., Thuillez, C., Richard, V., et al. (2010). Arterial stiffness is regulated by nitric oxide and endothelium-derived hyperpolarizing factor during changes in blood flow in humans. Hypertension 55, 674–680. doi: 10.1161/HYPERTENSIONAHA.109.142190
Chen, H. T., Chung, Y. C., Chen, Y. J., Ho, S. Y., and Wu, H. J. (2017). Effects of different types of exercise on body composition, muscle strength, and igf-1 in the elderly with sarcopenic obesity. J. Am. Geriatr. Soc. 65, 827–832. doi: 10.1111/jgs.14722
Chen, L., Chen, R., Wang, H., and Liang, F. (2015). Mechanisms linking inflammation to insulin resistance. Int. J. Endocrinol. 2015:508409. doi: 10.1155/2015/508409
van Dijk, S. C., Swart, K. M. A., Ham, A. C., Enneman, A. W., Wijngaarden, J. P., van Feskens, E. J., et al. (2015). Physical fitness, activity and hand-grip strength are not associated with arterial stiffness in older individuals. J. Nutr. Health Aging 19, 779–784. doi: 10.1007/s12603-015-0519-7
Fahs, C. A., Heffernan, K. S., Ranadive, S., Jae, S. Y., and Fernhall, B. (2010). Muscular strength is inversely associated with aortic stiffness in young men. Med. Sci. Sports Exerc. 42, 1619–1624. doi: 10.1249/MSS.0b013e3181d8d834
Farias, D. L., Ramires, A. T., Tatiane, G. T., Denis César, L. V., Vitor, T., Dahan, d. C. N., et al. (2013). Elderly women with metabolic syndrome present higher cardiovascular risk and lower relative muscle strength. Einstein (São Paulo, Brazil) 11, 174–79. doi: 10.1590/S1679-45082013000200007
Fischer, S. M., Wong, A., Maharaj, A., Jaime, S. J., and Figueroa, A. (2021). Impaired pulse pressure amplification, augmentation index, and arterial stiffness are associated with reduced limb lean mass in overweight and obese postmenopausal women. Exp. Gerontol. 145:111194. doi: 10.1016/j.exger.2020.111194
Förstermann, U., Xia, N., and Li, H. (2017). Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Circ. Res. 120, 713–735. doi: 10.1161/CIRCRESAHA.116.309326
Francescomarino, S. D., Sciartilli, A., Valerio, V. D., Baldassarre, A. D., and Gallina, S. (2009). The effect of physical exercise on endothelial function. Sports Med. 39, 797–812. doi: 10.2165/11317750-000000000-00000
Gonzales, J. U. (2013). Gait performance in relation to aortic pulse wave velocity, carotid artery elasticity and peripheral perfusion in healthy older adults. Clin. Physiol. Funct. Imag. 33, 245–251. doi: 10.1111/cpf.12019
Goodman, M. N. (1991). Tumor necrosis factor induces skeletal muscle protein breakdown in rats. Am. J. Physiol. Endocrinol. Metab. 260, 727–730. doi: 10.1152/ajpendo.1991.260.5.e727
Greenland, P., Alpert, J. S., Beller, G. A., Benjamin, E. J., Budoff, M. J., Fayad, Z. A., et al. (2010). 2010 ACCF/AHA Guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the american college of cardiology foundation/american heart association task force on practice guidelines. Circulation 122, e584–e636.
Groen, B. B. L., Hamer, H. M., Snijders, T., Kranenburg, J. V., Frijns, D., Vink, H., et al. (2014). Skeletal muscle capillary density and microvascular function are compromised with aging and type 2 diabetes. J. Appl. Physiol. 116, 998–1005. doi: 10.1152/japplphysiol.00919.2013
Guo, X., Chen, H., Han, L., Haulon, S., and Kassab, G. S. (2018). Chronic ETA antagonist reverses hypertension and impairment of structure and function of peripheral small arteries in aortic stiffening. Sci. Rep. 8:3076. doi: 10.1038/s41598-018-20439-5
Guzik, T. J., and Touyz, R. M. (2017). Oxidative stress, inflammation, and vascular aging in hypertension. Hypertension 70, 660–667. doi: 10.1161/HYPERTENSIONAHA.117.07802
Ikonomidis, I., Lambadiari, V., Pavlidis, G., Koukoulis, C., Kousathana, F., Varoudi, M., et al. (2015). Insulin resistance and acute glucose changes determine arterial elastic properties and coronary flow reserve in dysglycaemic and first-degree relatives of diabetic patients. Atherosclerosis 241, 455–462. doi: 10.1016/j.atherosclerosis.2015.06.006
Inomoto, A., Deguchi, J., Fukuda, R., Yotsumoto, T., and Toyonaga, T. (2021). Age-specific determinants of brachial-ankle pulse wave velocity among male Japanese workers. Tohoku J. Exp. Med. 253, 135–141. doi: 10.1620/tjem.253.135
Jeon, Y. K., Shin, M. J., Saini, S. K., Custodero, C., Aggarwal, M., Anton, S. D., et al. (2021). Vascular dysfunction as a potential culprit of Sarcopenia. Exp. Gerontol. 145:111220. doi: 10.1016/j.exger.2020.111220
Ji, X., Zhao, H., Wang, M., Li, Y., Zhang, C., and Wang, X. (2020). Study of Correlations between Metabolic Risk Factors, PWV and Hypertension in College Students. Clin. Exp. Hypertens. 42, 376–380. doi: 10.1080/10641963.2020.1723617
Katakami, N., Osonoi, T., Takahara, M., Saitou, M., Matsuoka, T., Yamasaki, Y., et al. (2014). Clinical utility of brachial-ankle pulse wave velocity in the prediction of cardiovascular events in diabetic patients. Cardiovasc. Diabetol. 13:128. doi: 10.1186/s12933-014-0128-5
Kim, T. N., Park, M. S., Lim, K. I., Yang, S. J., Yoo, H. J., Kang, H. J., et al. (2011). Skeletal muscle mass to visceral fat area ratio is associated with metabolic syndrome and arterial stiffness: the korean sarcopenic obesity study (KSOS). Diab. Res. Clin. Pract. 93, 285–291. doi: 10.1016/j.diabres.2011.06.013
Kollet, D. P., Marenco, A. B., Bellé, N. L., Barbosa, E., Boll, L., Eibel, B., et al. (2021). Aerobic exercise, but not isometric handgrip exercise, improves endothelial function and arterial stiffness in patients with myocardial infarction undergoing coronary intervention: a randomized pilot study. BMC Cardiovasc. Disord. 21:101. doi: 10.1186/s12872-021-01849-2
Komatsu, M., Akazawa, N., Tanahashi, K., Kumagai, H., Yoshikawa, T., Kosaki, K., et al. (2017). Central blood pressure is associated with trunk flexibility in older adults. Artery Res. 19, 91–96. doi: 10.1016/j.artres.2017.07.002
Laurent, S., Cockcroft, J., Bortel, L. V., Boutouyrie, P., Giannattasio, C., Hayoz, D., et al. (2006). Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur. Heart J. 27, 2588–2605. doi: 10.1093/eurheartj/ehl254
Lima-Junior, D., Farah, B. Q., Germano-Soares, A. H., Andrade-Lima, A., Silva, G. O., Rodrigues, S. L. C., et al. (2019). Association between handgrip strength and vascular function in patients with hypertension. J. Hypertens. 41, 692–695. doi: 10.1080/10641963.2018.1539096
Logan, J. G., Kim, S. S., Lee, M., Byon, H. D., and Yeo, S. (2018). Effects of static stretching exercise on lumbar flexibility and central arterial stiffness. J. Cardiovasc. Nurs. 33, 322–328. doi: 10.1097/JCN.0000000000000460
Mancia, G., Fagard, R., Narkiewicz, K., Redon, J., Zanchetti, A., Bohm, M., et al. (2013). The task force for the management of arterial hypertension of the European Society Ofhypertension (Esh) and of the European Society of Cardiology (ESC). J. Hypertens. 31, 1281–1357. doi: 10.1097/01.hjh.0000431740.32696.cc
Meng, S. J., and Yu, L. J. (2010). Oxidative Stress, Molecular Inflammation and Sarcopenia. Int. J. Mol. Sci. 11, 1509–1526. doi: 10.3390/ijms11041509
Mitchell, G. F., Vita, J. A., Larson, M. G., Parise, H., Keyes, M. J., Warner, E., et al. (2005). Cross-Sectional Relations of Peripheral Microvascular Function, Cardiovascular Disease Risk Factors, and Aortic Stiffness: the Framingham heart study. Circulation 112, 3722–3728. doi: 10.1161/CIRCULATIONAHA.105.551168
Nishitani, M., Shimada, K., Sunayama, S., Masaki, Y., Kume, A., Fukao, K., et al. (2011). Impact of diabetes on muscle mass, muscle strength, and exercise tolerance in patients after coronary artery bypass grafting. J. Cardiol 58, 173–180. doi: 10.1016/j.jjcc.2011.05.001
Ochi, M., Kohara, K., Tabara, Y., Kido, T., Uetani, E., Ochi, N., et al. (2010). Arterial stiffness is associated with low thigh muscle mass in middle-aged to elderly men. Atherosclerosis 212, 327–332. doi: 10.1016/j.atherosclerosis.2010.05.026
Ohara, M., Kohara, K., Tabara, Y., Igase, M., and Miki, T. (2015). Portable Indices for Sarcopenia Are Associated with Pressure Wave Reflection and Central Pulse Pressure: the J-SHIPP Study. J. Hypertens. 33, 314–322. doi: 10.1097/HJH.0000000000000394
Oren, A., Vos, L. E., Uiterwaal, C., Grobbee, D. E., and Bots, M. L. (2003). Aortic stiffness and carotid intima-media thickness: two independent markers of subclinical vascular damage in young adults? Eur. J. Clin. Invest. 33, 949–954. doi: 10.1046/j.1365-2362.2003.01259.x
Otsuki, T., Namatame, H., Yoshikawa, T., and Zempo-Miyaki, A. (2020). Combined Aerobic and Low-Intensity Resistance Exercise Training Increases Basal Nitric Oxide Production and Decreases Arterial Stiffness in Healthy Older Adults. J. Clin. Biochem. Nutr. 66, 62–66. doi: 10.3164/jcbn.19-81
Reed, R. L., Pearlmutter, L., Yochum, K., Meredith, K. E., and Mooradian, A. D. (1991). The Relationship between muscle mass and muscle strength in the elderly. J. Am. Geriatr. Soc. 39, 555–561. doi: 10.1111/j.1532-5415.1991.tb03592.x
Rider, O. J., Holloway, C. J., Emmanuel, Y., Bloch, E., Clarke, K., and Neubauer, S. (2012). Increasing plasma free fatty acids in healthy subjects induces aortic distensibility changes seen in obesity. Circ. Cardiovasc. Imag. 5, 367–375. doi: 10.1161/CIRCIMAGING.111.971804
Rong, Y. D., Bian, A. L., Hu, H. Y., Ma, Y., and Zhou, X. Z. (2020). A cross-sectional study of the relationships between different components of sarcopenia and brachial ankle pulse wave velocity in community-dwelling elderly. BMC Geriatrics 20:115. doi: 10.1186/s12877-020-01525-8
Safar, M. E., Levy, B. I., and Struijker-Boudier, H. (2003). Current perspectives on arterial stiffness and pulse pressure in hypertension and cardiovascular diseases. Circulation 107, 2864–2869. doi: 10.1161/01.CIR.0000069826.36125.B4
Sanian, H., Matinhomaee, H., and Peeri, M. (2019). The Effect of Circuit Resistance Training on Plasma Concentration of Endothelin-1, Nitric Oxide and Vascular Diameter in Elderly Men. J. Clin. Res. Paramed. Sci. 8, 1–6. doi: 10.5812/jcrps.80932
Santos-Parker, J. R., Strahler, T. R., Bassett, C. J., Bispham, N. Z., Chonchol, M. B., and Seals, D. R. (2017). Curcumin older adults by increasing nitric oxide bioavailability and reducing oxidative stress. Aging Us 9, 187–208.
Sciorati, C., Rigamonti, E., Manfredi, A. A., and Rovere-Querini, P. (2016). Cell Death, Clearance and Immunity in the Skeletal Muscle. Cell Death Differ. 23, 927–937. doi: 10.1038/cdd.2015.171
Siu, P. M., Pistilli, E. E., Butler, D. C., and Always, S. E. (2005). Aging influences cellular and molecular responses of apoptosis to skeletal muscle unloading. Am. J. Physiol. Cell Physiol. 288, 338–349. doi: 10.1152/ajpcell.00239.2004
Sonne, M. P., Højbjerre, L., Alibegovic, A. C., Vaag, A., Stallknecht, B., and Dela, F. (2009). Impaired endothelial function and insulin action in first-degree relatives of patients with type 2 diabetes mellitus. Metab. Clin. Exp. 58, 93–101. doi: 10.1016/j.metabol.2008.08.011
Sun, H. J., Wu, Z. Y., Nie, X. W., and Bian, J. S. (2020). Role of endothelial dysfunction in cardiovascular diseases: the link between inflammation and hydrogen sulfide. Front. Pharmacol. 10:1568. doi: 10.3389/fphar.2019.01568
Suzuki, E., Kashiwagi, A., Nishio, Y., Egawa, K., Shimizu, S., Maegawa, H., et al. (2001). Increased arterial wall stiffness limits flow volume in the lower extremities in type 2 diabetic patients. Diabetes Care 24, 2107–2114. doi: 10.2337/diacare.24.12.2107
Tanaka, K., Kanazawa, I., and Sugimoto, T. (2016). Reduced muscle mass and accumulation of visceral fat are independently associated with increased arterial stiffness in postmenopausal women with type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 122, 141–147. doi: 10.1016/j.diabres.2016.10.014
Trindade, M., Oigman, W., and Neves, M. F. (2017). Potential Role of Endothelin in Early Vascular Aging. Curr. Hypertens. Rev. 13, 33–40. doi: 10.2174/1573402113666170414165735
Trovati, M., and Anfossi, G. (2002). Influence of insulin and of insulin resistance on platelet and vascular smooth muscle cell function. J. Diabetes Its Complic. 16, 35–40. doi: 10.1016/S1056-8727(01)00196-9
Watson, N. L., Sutton-Tyrrell, K., Youk, A. O., Boudreau, R. M., MacKey, R. H., Simonsick, E. M., et al. (2011). Arterial stiffness and gait speed in older adults with and without peripheral arterial disease. Am. J. Hypertens. 24, 90–95. doi: 10.1038/ajh.2010.193
Wu, J. Q., Kosten, T. R., and Zhang, X. Y. (2013). Free Radicals, Antioxidant Defense Systems, and Schizophrenia. Progr. Neuro Psychopharmacol. Biol. Psychiatry 46, 200–206. doi: 10.1016/j.pnpbp.2013.02.015
Yamamoto, K., Kawano, H., Gando, Y., Iemitsu, M., Murakami, H., Sanada, K., et al. (2009). Poor Trunk Flexibility Is Associated with Arterial Stiffening. Am. J. Physiol. Heart Circ. Physiol. 297, 1314–1318. doi: 10.1152/ajpheart.00061.2009
Yamanashi, H., Kulkarni, B., Edwards, T., Kinra, S., Koyamatsu, J., Nagayoshi, M., et al. (2018). Association between atherosclerosis and handgrip strength in non-hypertensive populations in India and Japan. Geriatr. Gerontol. Int. 18, 1071–1078. doi: 10.1111/ggi.13312
Yang, M., Zhang, X., Ding, Z., Wang, F., Wang, Y., Jiao, C., et al. (2020). Low skeletal muscle mass is associated with arterial stiffness in community-dwelling Chinese aged 45 years and older. BMC Public Health 20:226. doi: 10.1186/s12889-020-8323-7
Yoon, S. K., Kim, H. N., and Song, S. W. (2020). Associations of skeletal muscle mass with atherosclerosis and inflammatory markers in Korean adults. Arch. Gerontol. Geriatr. 90:104163. doi: 10.1016/j.archger.2020.104163
Zhang, L., Guo, Q., Feng, B. L., Wang, C. Y., Han, P. P., Hu, J., et al. (2019). A cross-sectional study of the association between arterial stiffness and sarcopenia in Chinese community-dwelling elderly using the asian working group for sarcopenia criteria. J. Nutr. Health Aging 23, 195–201. doi: 10.1007/s12603-018-1147-9
Zhang, Y., Miyai, N., Abe, K., Utsumi, M., Uematsu, Y., Terada, K., et al. (2021). Muscle mass reduction, low muscle strength, and their combination are associated with arterial stiffness in community-dwelling elderly population: the Wakayama study. J. Hum. Hypertens. 35, 446–454. doi: 10.1038/s41371-020-0355-z
Zhao, M. M., Xu, M. J., Cai, Y., Zhao, G., Guan, Y., Kong, W., et al. (2011). Mitochondrial Reactive oxygen species promote P65 nuclear translocation mediating high-Phosphate-induced vascular calcification in Vitro and in Vivo. Kidney Int. 79, 1071–1079. doi: 10.1038/ki.2011.18
Keywords: arterial stiffness, pulse wave velocity, muscle mass, muscle strength, muscle flexibility, cardiovascular
Citation: Aminuddin A, Noor Hashim MF, Mohd Zaberi NAS, Zheng Wei L, Ching Chu B, Jamaludin NA, Salamt N, Che Roos NA and Ugusman A (2021) The Association Between Arterial Stiffness and Muscle Indices Among Healthy Subjects and Subjects With Cardiovascular Risk Factors: An Evidence-Based Review. Front. Physiol. 12:742338. doi: 10.3389/fphys.2021.742338
Received: 16 July 2021; Accepted: 08 October 2021;
Published: 23 November 2021.
Edited by:
Antonio Crisafulli, University of Cagliari, ItalyReviewed by:
Davide Agnoletti, Sacro Cuore Don Calabria Hospital, ItalyCopyright © 2021 Aminuddin, Noor Hashim, Mohd Zaberi, Zheng Wei, Ching Chu, Jamaludin, Salamt, Che Roos and Ugusman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Azizah Ugusman, ZHIuYXppemFoQHBwdWttLnVrbS5lZHUubXk=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.