- Department of Cardiology, Yonsei University Health System, Seoul, South Korea
Catheter ablation is the most effective rhythm control method for patients with atrial fibrillation (AF); however, it inevitably causes atrial tissue damage. We previously reported that AF catheter ablation (AFCA) increases left atrial (LA) pressure without changes in symptom scores. We hypothesized that extensive LA ablation increased the risk of stiff LA physiology. We included 1,720 patients (69.1% male, 60.0 [53.0–68.0] years old, 66.2% with paroxysmal AF) who underwent de novo AFCA and echocardiography before and 1-year after the procedure. Stiff LA physiology was defined, when the amount of the estimated pulmonary arterial pressure increase between the pre-procedural and the 1-year post-procedural follow-up echocardiography was >10 mmHg and when right ventricular systolic pressure (RVSP) was >35 mmHg at 1-year follow-up echocardiography. The failed rhythm control within 1 year was defined as recurrent AF despite using anti-arrhythmic drugs or cardioversion within a year of AFCA. We explored the incidence and risk factors for stiff LA physiology and the rhythm outcome of AFCA. Among the 1,720 patients, 64 (3.7%) had stiff LA physiology 1 year after AFCA. Stiff LA physiology was independently associated with diabetes (odds ratio [OR], 2.36 [95% CI, 1.14–4.87], p = 0.020), the ratio of the peak mitral flow velocity of the early rapid filling to the early diastolic velocity of the mitral annulus (E/Em; OR, 1.04 [95% CI, 1.00–1.10], p = 0.049), LA pulse pressure (Model 2: OR, 1.05 [95% CI, 1.00–1.11], p = 0.049), low LA voltage (OR, 0.36 [95% CI, 0.18–0.74], p = 0.005), empirical extra-pulmonary vein (PV) LA ablation (OR, 2.60 [95% CI, 1.17–5.74], p = 0.018), and radiofrequency (RF) ablation duration (Model 2: OR, 1.02 [95% CI, 1.01–1.03], p = 0.003). Although the incidence of post-AFCA stiff LA physiology was 3.7% and most of the cases were subclinical, the empirical extra-PV ablation was associated with this undesirable condition. In addition, patients who had low mean LA voltage before AFCA could be susceptible to stiff LA physiology.
Introduction
Catheter ablation is the most effective rhythm control method for patients with atrial fibrillation (AF), and various clinical benefits of catheter ablation have been reported, including a reduction in the mortality rate in patients with heart failure (Marrouche et al., 2018). However, it is a destructive procedure involving heating or freezing as the energy source for AF catheter ablation (AFCA); this inevitably causes atrial tissue damage, resulting in desiccation necrosis, fibrosis, and scar generation (Nath et al., 1994; Cesario et al., 2007). In particular, atrial substrate modification, such as an empirical or targeted extra-pulmonary vein (PV) ablation, has been performed in patients with advanced AF and significant atrial structural remodeling. Park et al. reported an elevated left atrial (LA) pressure and stiffness at the time of repeat ablation as compared to de novo procedures (Park et al., 2019). They found that the level of the increase in LA pressure was more significant in patients who underwent an empirical extra-PV LA ablation than in those who underwent a circumferential PV isolation (CPVI) alone. However, the symptom score did not differ in that study (Park et al., 2019). In addition, patients with AF with higher LA pressure, stiffness, or wall stress had a higher recurrence rate after AFCA (Park et al., 2014, 2015; Lee et al., 2021a). Stiff LA syndrome is a form of symptomatic pulmonary arterial (PA) hypertension, which is caused by a decreased LA function after mitral valve surgery (Pilote et al., 1988). Recently, it has been reported that stiff LA syndrome can occur after extensive AFCA. However, little is known about the frequency and mechanism of the post-AFCA stiff LA physiology (Gibson et al., 2011; Witt et al., 2014). As previous studies that compared the LA pressure before and after AFCA measured the invasive LA pressure only in patients who underwent repeat ablation, there might be a selection bias (Park et al., 2019). Therefore, in this study, we explored the incidence and clinical features of stiff LA physiology in all patients who underwent de novo AFCA using 1-year follow-up echocardiographic parameters. The purpose of this study was to identify the incidence and clinical predictors associated with the development of stiff LA physiology after AFCA and to evaluate whether it was particularly related to the empirical extra-PV LA ablation. We applied the previously reported echocardiographic definition of stiff LA physiology (Witt et al., 2014).
Materials and Methods
Study Population
The study protocol adhered to the principles of the Declaration of Helsinki and was approved by the Institutional Review Board of the Yonsei University Health System. All patients provided written informed consent for inclusion in the Yonsei AF Ablation cohort. A total of 1,720 consecutive patients who underwent de novo AFCA between March 2009 and January 2020 in a single center were prospectively enrolled in this study. The comorbidities were gathered from the medical records at the time of AFCA. Heart failure was defined according to the guidelines (Writing Committee et al., 2016). In all patients, LA pressure was measured during the procedure, and echocardiography with measurement of the right ventricular systolic pressure (RVSP) was conducted before and 1 year after AFCA. The patients were divided into two groups based on the occurrence of stiff LA physiology using pre- and post-AFCA echocardiographic estimation of PA pressure. The exclusion criteria were as follows: (1) AF refractory to electrical cardioversion; (2) no available data on RVSP on echocardiography before or after AFCA; (3) RVSP >40 mmHg on the echocardiography conducted before the AFCA; (4) repeat ablation within a year after de novo procedure; (5) AF with rheumatic valvular disease; (6) patients who had PV stenosis; and (7) prior AF ablation or cardiac surgery. All patients stopped all anti-arrhythmic drugs (AADs) for a period corresponding to at least five half-lives before AFCA.
Echocardiography Follow-Up and Definition of Stiff LA Physiology
Transthoracic echocardiography (TTE) was conducted within 3 months prior to the procedure and at the 1-year follow-up. PA systolic pressure was estimated using RVSP on echocardiography. RVSP was calculated from the peak tricuspid regurgitant jet velocity (V) using the modified Bernoulli's equation (RVSP = 4V2 + right atrial pressure). Stiff LA physiology was defined, when the amount of the estimated increase in PA pressure between the pre-procedural and the 1-year post-procedural follow-up echocardiography was >10 mmHg and when RVSP was >35 mmHg at 1-year follow-up echocardiography (Witt et al., 2014). The interobserver and intraobserver reliability for the RVSP on echocardiography were 92 and 95%, respectively.
Measurement of LA Pressure, LA Wall Thickness, and LA Wall Stress
During the AFCA procedure, LA pressure was measured during sinus rhythm and AF immediately after a transseptal puncture, as described in the previous studies (Park et al., 2014, 2019). If the initial rhythm was AF, we measured LA pressure during sinus rhythm after terminating AF by internal cardioversion, followed by a waiting period of at least 3 min to allow for recovery from atrial stunning from cardioversion (Park et al., 2014, 2019). We excluded patients in whom LA pressure during sinus rhythm could not be measured due to frequent reinitiation of AF after electrical cardioversion.
We developed a customized software (AMBER, Laonmed Inc., Seoul, Korea) that measured the LA wall thickness by applying Laplace's equation in the cardiac CT images (Kwon et al., 2020; Lee et al., 2021b). The CT scan was conducted within a month before the AFCA. The spatial resolutions of the CT images were within 0.3–0.55 mm for the x- and y-axes, and the slice thickness of the z-axis was 0.5 mm. The spatial resolution of the CT was set to the normalized vector in the 3D Euclidean space. The methods and principles of the customized software (AMBER) were previously described in detail, and the results have been well validated with a 3D printed phantom model in 120 patients (Kwon et al., 2020; Lee et al., 2021b). In brief, the endocardium of the LA was semiautomatically divided on the cardiac CT using an edge detector. Then, the LA wall was extracted with an overlapped area by the morphological operations after separation from other tissues using the multi-Otsu threshold algorithm in a histogram of Hounsfield units. The LA wall thickness was measured by applying Laplace's equation and Euler's method in 3D space.
The LA wall stress (LAW-stress) (dyn/cm2) was calculated using the Law of Laplace [σ = (P × r)/2h (σ, wall stress; P, pressure; r, radius; h, wall thickness)] (Falsetti et al., 1970; Wang et al., 2011). The peak LA pressure during sinus rhythm was directly measured during the AF procedure, and the LA radius was defined as half of the LA anterior-posterior (AP) diameter with TTE. Therefore, LAW-stress was calculated using the following equation: LAW-stress = (peak LA pressure × LA AP diameter)/(4 × LA wall thickness). LAW-stress was expressed as dyn/cm2 (1 mmHg = 1,333 dyn/cm2). We previously reported that the LAW-stress calculated using the abovementioned equation is a useful prognostic parameter for AF recurrence after AFCA (Lee et al., 2021a).
Electrophysiological Studies and Catheter Ablation
The electrophysiological mapping method and the AFCA technique/strategy used during the study period were consistently performed as described in a previous study (Yu et al., 2017). In brief, an open irrigated-tip catheter (Celsius, ThermoCool SF [Johnson & Johnson Inc., Diamond Bar, CA, USA] or CoolFlex [St. Jude Medical Inc., Minnetonka, MN, USA]; 30–35 W, 45°C) was used to deliver radiofrequency (RF) energy for ablation under 3D electroanatomical mapping (NavX [St. Jude Medical, Minnetonka, MN, USA] or CARTO3 [Johnson & Johnson Inc.]) merged with 3D spiral CT. For high-quality voltage maps, LA electrogram voltage maps were generated using a circumferential mapping catheter during high right atrial pacing at 500 ms before CPVI. However, in a minority of the patients with recurrent AF at the beginning of the procedure, we acquired voltage maps during sinus rhythm after the completion of CPVI. To avoid any false detection of inadequate voltages, the Automap module, which is the system setting for a high-quality voltage map, was used during the map acquisition. In brief, we set the criteria of components, such as the morphology of original template beat, cycle length, catheter moving speed, and signal-to-noise threshold, for adequate point in the Automap module, and the system could discriminate adequate contact electrogram from inadequate mapping point, such as noise. We obtained the peak-to-peak amplitude of contact bipolar electrograms from 500 to 1,000 points on the LA endocardium, and the mean LA electrogram voltage was calculated. If frequently recurring AF persisted after three attempts at cardioversion, no further efforts were made to generate an LA voltage map. All patients initially underwent a CPVI. For patients with persistent AF, roof line, posterior-inferior line, anterior line, cavotricuspid isthmus line, superior vena cava to the septal line, or complex fractionated atrial electrogram-guided ablation, were added at the discretion of the operator. The procedure was considered complete when there was no immediate recurrence of AF after cardioversion with isoproterenol infusion (5–10 μg/min; target heart rate, 120 bpm). In the case of mappable AF triggers, extra-PV foci were mapped and ablated as much as possible. Although we have kept consistent ablation protocol used by experienced operators, the catheter technology and mapping technologies kept changing during the long period of enrollment (Park JW, CircJ2019). We used contact force catheters in 11.6% of the patients enrolled, and extra-PV ablation was dependent on the study protocol or discretion of the operators. Systemic anticoagulation was achieved with intravenous heparin while maintaining an activated clotting time of 350–400 s during the procedure. Representative images of the bipolar voltage map and usual ablation lesion set are presented in Figure 1.
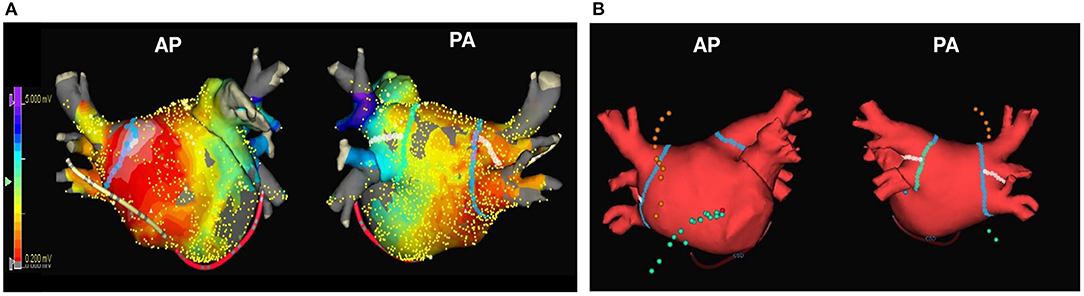
Figure 1. The representative images of the LA voltage map and usual ablation lesion set. The representative images of LA voltage map (A) and usual ablation lesion set that include CPVI, SVC-right septal line, and CTI line (B). CPVI, circumferential pulmonary vein isolation; CTI, cavo-tricuspid isthmus; LA, left atrium; SVC, superior vena cava.
Post-ablation Management and Follow-Up
All patients visited the scheduled outpatient clinic at 1, 3, 6, and 12 months after AFCA and every 6 months thereafter or whenever symptoms occurred. All patients underwent electrocardiography at every visit, as well as 24 h Holter recording at 3 and 6 months, then every 6 months for 2 years, annually for 2–5 years, and then biannually after 5 years, following the modified 2012 HRS/EHRA/ECAS expert consensus statement guidelines (Calkins et al., 2012). Whenever patients reported palpitations, Holter monitor or event monitor recordings were obtained and evaluated to check for the recurrence of arrhythmias. AF recurrence was defined as any episodes of AF or atrial tachycardia lasting for at least 30 s. Any electrocardiographic documentation of AF recurrence 3 months after the blanking period was identified as clinical recurrence. We defined a “failed rhythm control” as recurrent AF despite AAD or cardioversion within a year of AFCA.
Statistical Analysis
Continuous variables were expressed as the mean ± SD for normally distributed variables and as the median with the interquartile range for non-normally distributed variables; they were compared using the Student's t-test and the Wilcoxon rank-sum test, respectively. The categorical variables were reported as counts (percentages) and were compared using the chi-square or Fisher's exact test. The echocardiographic parameters before and 1 year after the procedure were compared using a paired t-test. The logistic regression analysis was used to identify risk factors for stiff LA physiology after AFCA and to estimate the odds ratios (ORs), 95% CIs, and p-values. The variables selected for the multivariate analysis were those with a p-value < 0.05 on the univariate analysis. Since there were variables that had multicollinearity, two logistic regression models were investigated separately. The Kaplan-Meier analysis with log-rank test was used to analyze the probability of freedom from AF recurrences after AFCA. The Statistical Package for the Social Sciences (SPSS) version 25.0 for Windows (IBM Corporation, Armonk, NY, USA) and the R software version 3.6.2 (The R Foundation for Statistical Computing, Vienna, Austria) were used for the data analysis.
Results
Incidence of Stiff LA Physiology and Clinical Characteristics
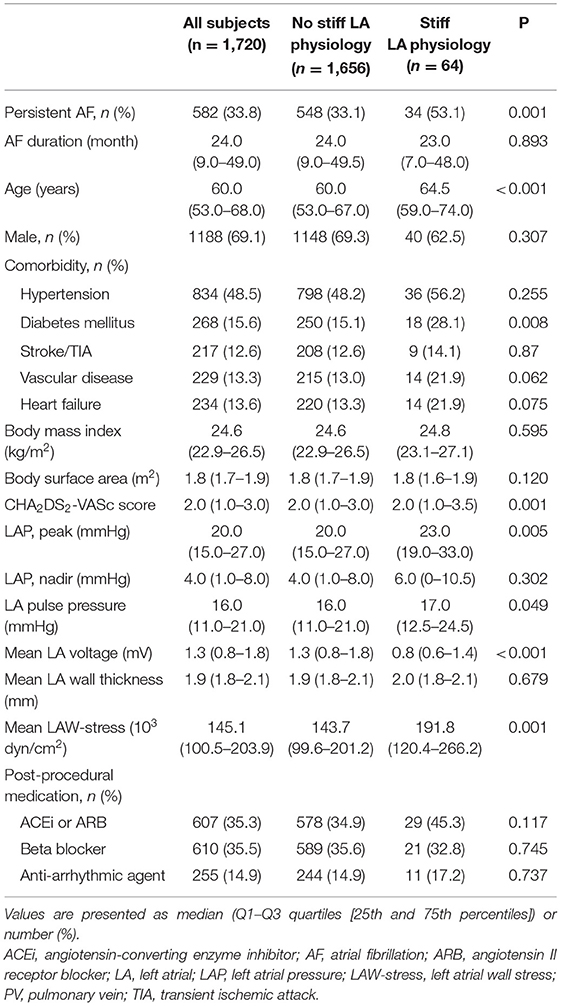
A total of 1,720 patients (69.1% male, 60.0 [53.0–68.0] years old, 66.2% with paroxysmal AF) who underwent de novo AFCA and echocardiography before and 1 year after the procedure were enrolled in this study. We found stiff LA physiology in 64 (3.7%) out of the 1,720 patients 1 year after the de novo procedure (Figure 2). The patients who had stiff LA physiology after ablation were older (p < 0.001) and had diabetes (p = 0.008), higher proportion of persistent AF (p = 0.001), and higher CHA2DS2-VASc score (p = 0.001) than their counterparts. LA peak pressure (p = 0.005) and LAW-stress (p = 0.001) were higher, and mean LA voltage was lower (p = 0.001) in patients with stiff LA physiology (Table 1).
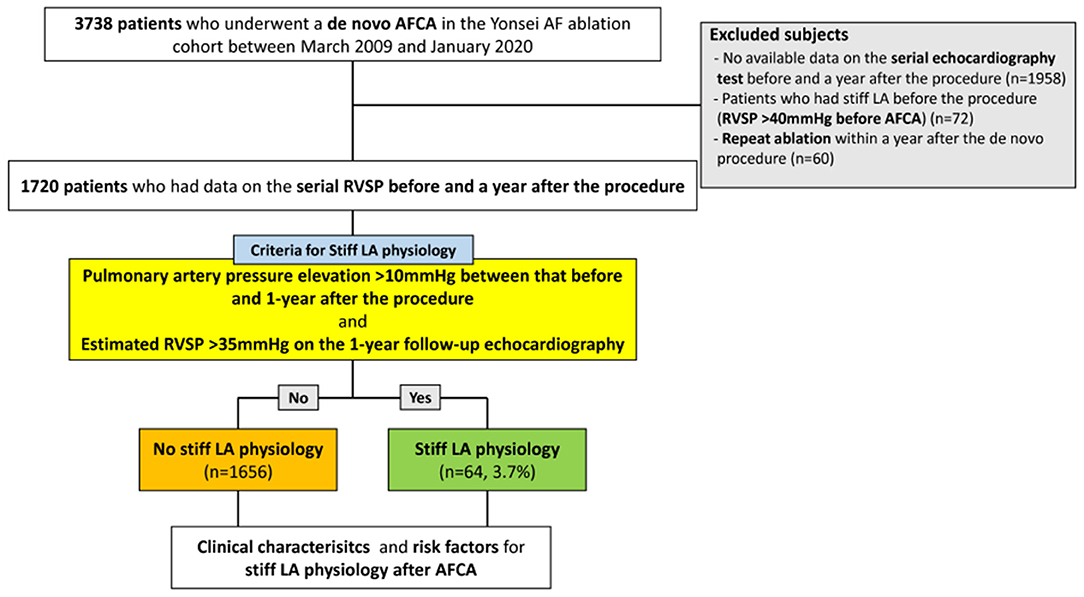
Figure 2. Flow chart of the study. AFCA, atrial fibrillation catheter ablation; LA, left atrial or left atrium; RVSP, right ventricular systolic pressure.
Echocardiographic and Procedural Characteristics in Patients With Stiff LA Physiology
Patients who had stiff LA physiology after AFCA had higher LA dimension (p < 0.001), LA volume index (p < 0.001), and the ratio of the peak mitral flow velocity of the early rapid filling to the early diastolic velocity of the mitral annulus (E/Em) (p < 0.001) than those without stiff LA physiology at both the pre-procedural and follow-up TTE (Table 2). Figure 3 presents changes in echocardiographic parameters 1 year after the procedures, depending on the development of stiff LA physiology. While RVSP (p < 0.001, Figure 3A), E/Em (p < 0.001, Figure 3B), and LA dimension (p < 0.001, Figure 3C) significantly decreased in the majority of patients without stiff LA physiology, RVSP (p < 0.001, Figure 3D) and E/Em (p < 0.001, Figure 3E) increased, and LA dimension (p = 0.430, Figure 3F) did not change in the stiff LA physiology group. The stiff LA physiology group had a longer procedure (p < 0.001) and RF ablation (p < 0.001) times and underwent the empirical extra-PV LA ablations more often (34.8% vs. 57.8%, p < 0.001, Table 2).
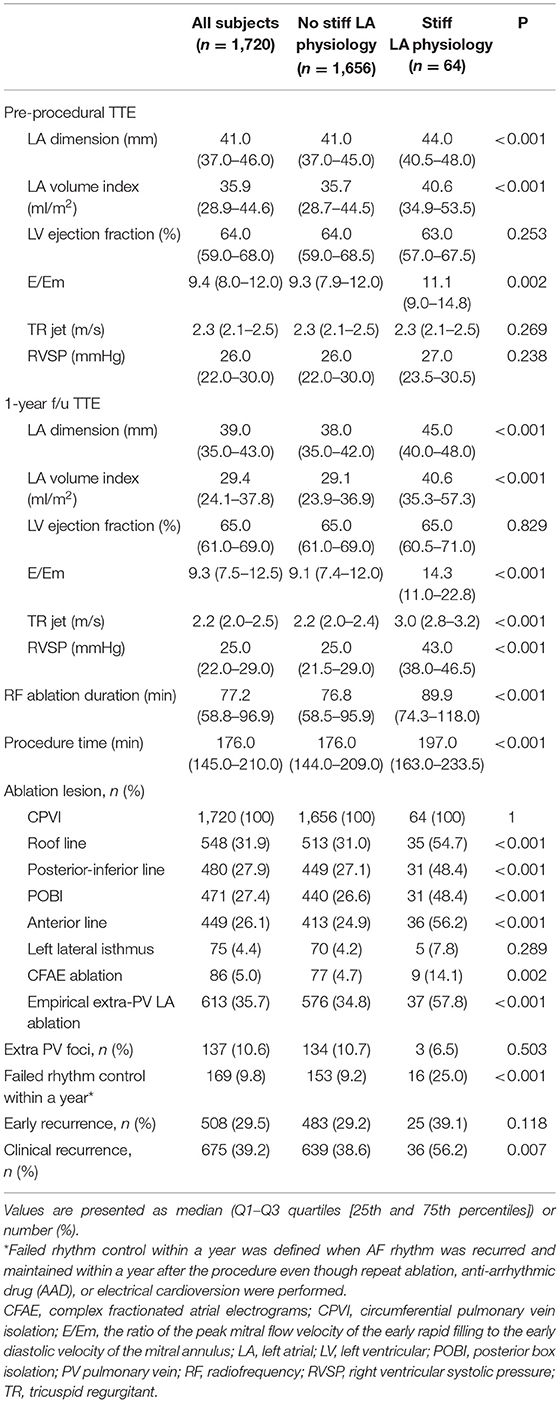
Table 2. Echocardiographic and procedural characteristics according to the occurrence of stiff LA physiology.
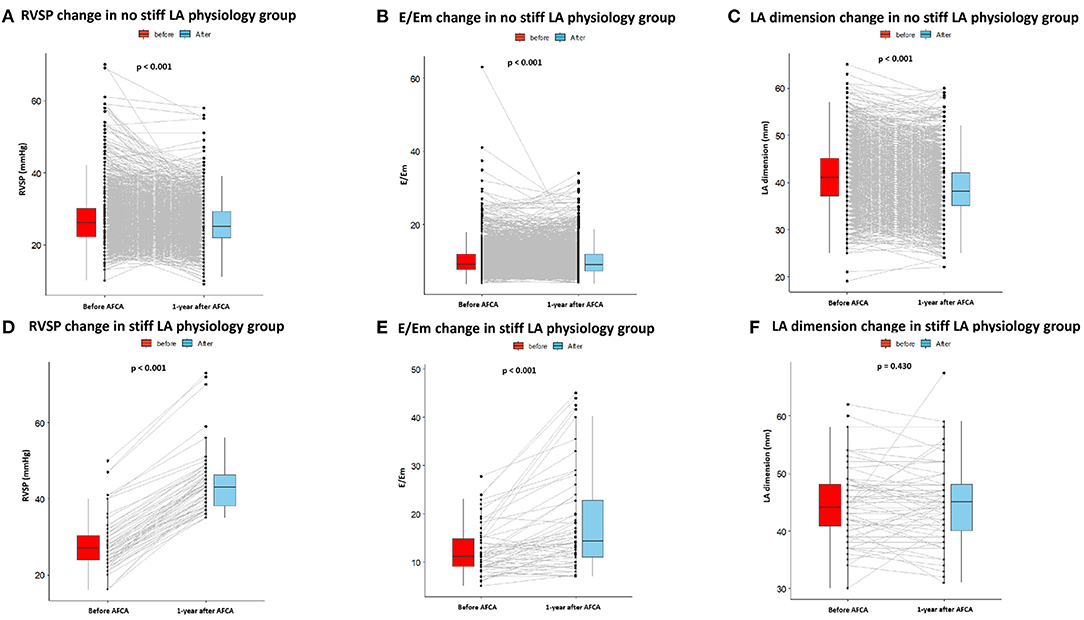
Figure 3. Changes in echocardiography parameters before and 1 year after AFCA depending on the development of stiff LA physiology. In patients without stiff LA physiology, RVSP (A), E/Em (B), and LA dimension (C) significantly decreased 1 year after AFCA. In contrast, in patients with stiff LA physiology, RVSP (D) and E/Em (E) increased, and LA dimension (F) did not change. E/Em, the ratio of the peak mitral flow velocity of the early rapid filling to the early diastolic velocity of the mitral annulus; LA, left atrial or left atrium; RVSP, right ventricular systolic pressure.
Risk Factors of Stiff LA Physiology After AFCA and Rhythm Outcome
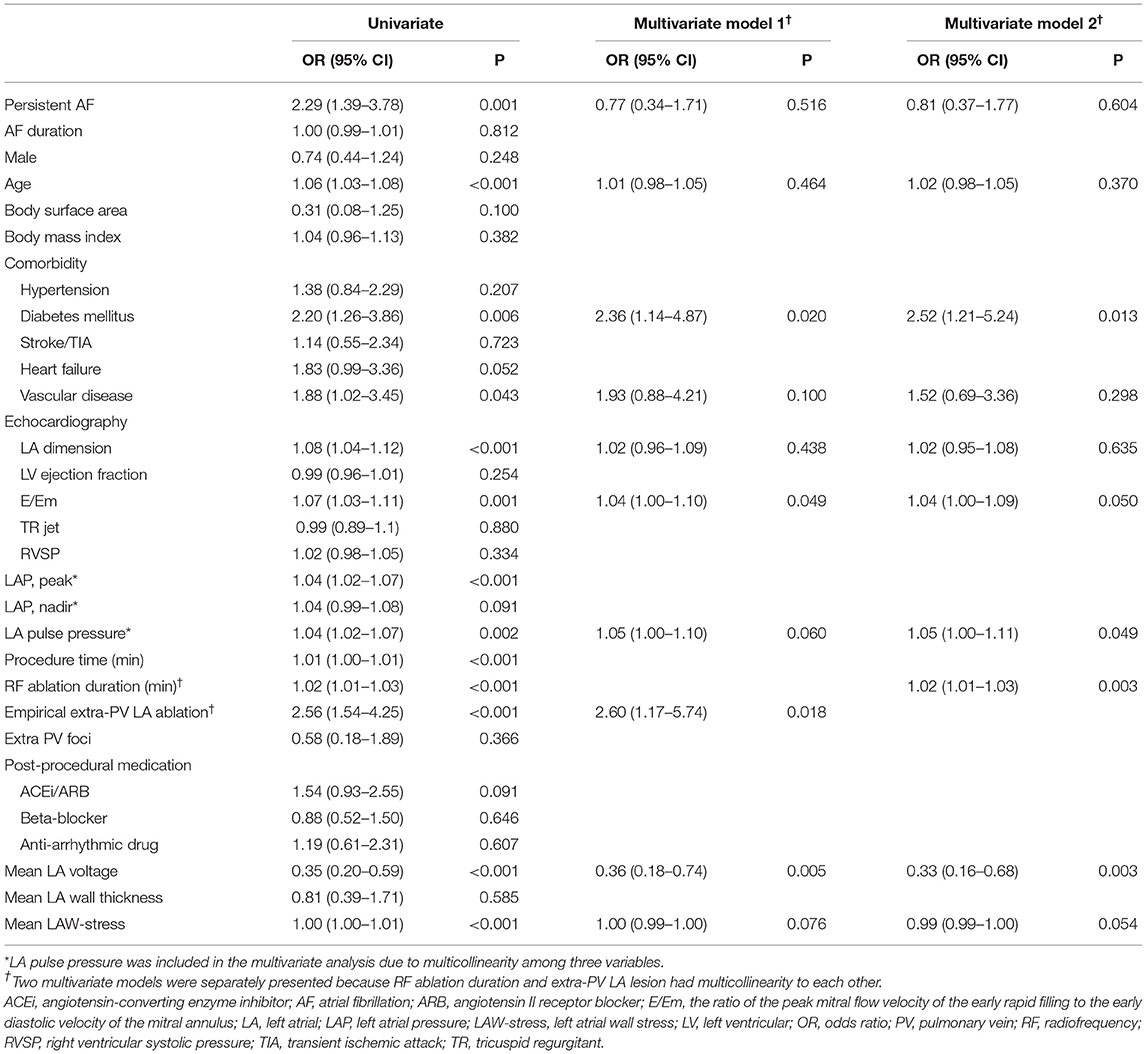
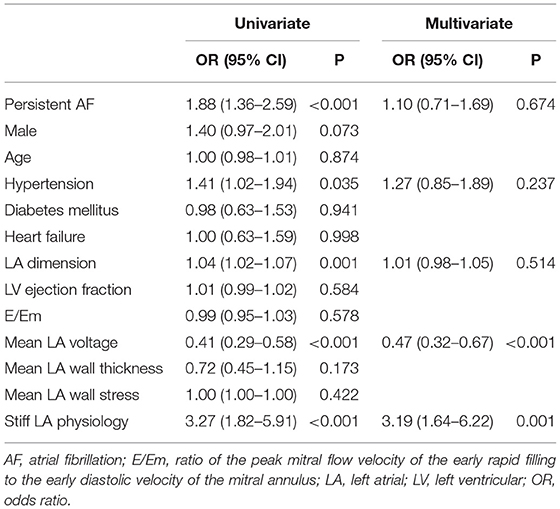
We investigated the factors associated with stiff LA physiology using the multivariate logistic regression analysis (Table 3). We used two different models because RF ablation duration and empirical extra-PV LA ablation had multicollinearity with each other as variables for adjustment. Stiff LA physiology was independently associated with diabetes (OR, 2.36 [95% CI, 1.14–4.87], p = 0.020), E/Em (OR, 1.04 [95% CI, 1.00–1.10], p = 0.049), LA pulse pressure (Model 2: OR, 1.05 [95% CI, 1.00–1.11], p = 0.049), low LA voltage (OR, 0.36 [95% CI 0.18–0.74], p = 0.005), empirical extra-PV LA ablation (OR, 2.60 [95% CI, 1.17–5.74], p = 0.018), and RF ablation duration (Model 2: OR, 1.02 [95% CI, 1.01–1.03], p = 0.003). Furthermore, the low mean LA voltage (OR, 0.47 [95% CI, 0.32–0.67], p < 0.001) and the presence of stiff LA physiology (OR, 3.19 [95% CI, 1.64–6.22], p = 0.001) were independently associated with failed rhythm control within 1 year (Table 4).
During 28 (14.0–56.0) months of follow-up, the clinical recurrence (p = 0.007) rates were significantly higher in patients who had stiff LA physiology 1 year after AFCA (Table 2). On the Kaplan-Meier analysis, rhythm outcome was worse in patients with stiff LA physiology (log-rank p = 0.002) (Figure 4).
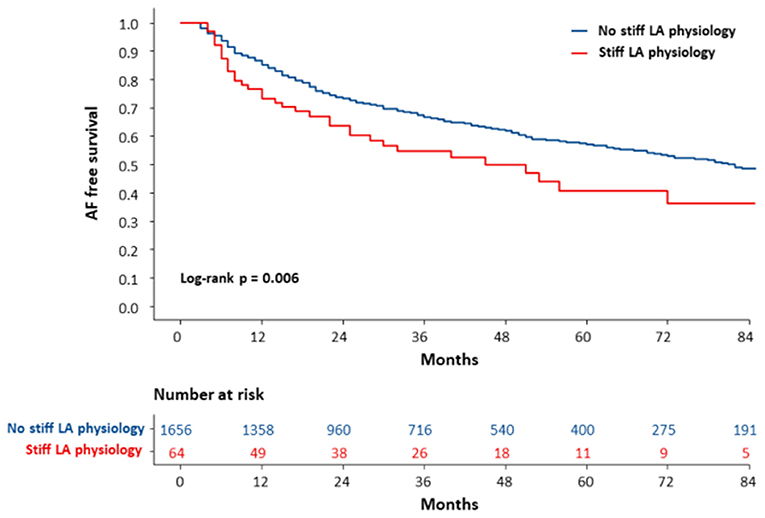
Figure 4. The Kaplan-Meier curve for clinical recurrence of AF according to the development of stiff LA physiology. The clinical recurrence of AF was worse in patients with stiff LA physiology (log-rank p = 0.006). AF, atrial fibrillation; LA, left atrial or left atrium; RVSP, right ventricular systolic pressure.
Discussion
Main Findings
In this study, we evaluated the incidence and risk factors of stiff LA physiology 1 year after AFCA using echocardiographic estimated RVSP and PA pressure. Stiff LA physiology occurred in 3.7% of patients after de novo AFCA. Stiff LA physiology was independently associated with diabetes, left ventricular (LV) diastolic function, and low LA voltage. Empirical extra-PV ablation was an independent predictor of stiff LA physiology. In addition, rhythm outcomes were worse in patients with stiff LA physiology. Although the incidence of post-AFCA stiff LA physiology was low and most of the cases were subclinical, the empirical extra-PV ablation contributed to this undesirable condition.
Stiff LA Physiology After Cardiovascular Interventions
Stiff LA syndrome was first described in patients who had developed pulmonary hypertension after undergoing mitral valve surgery, and this concept has recently been applied to patients after AFCA procedures (Pilote et al., 1988; Gibson et al., 2011). Park et al. (2014, 2015) previously reported that increased LA pressure and reduced compliance after AFCA were associated with advanced LA substrate remodeling and worse rhythm outcomes. Although empirical extra-PV ablation increases LA pressure more significantly than CPVI alone, the symptom score did not differ in our recent study (Park et al., 2019). Small LA diameter, diabetes, and obstructive sleep apnea were also reported as risk factors for stiff LA syndrome (Gibson et al., 2011). A previous study reported that a severe LA scar, defined as an area with low voltage, was associated with stiff LA syndrome (Gibson et al., 2011). This study also showed that low mean LA voltage at baseline was independently associated with stiff LA physiology. It might be explained that patients who had more advanced structural remodeling before the procedure would be susceptible to stiff LA physiology after AFCA. Although the mechanism is unclear, diabetes which accompanies inflammation and fibrosis was an independent predictor of stiff LA physiology in this study. The prevalence of failed rhythm control within a year was higher in the stiff LA physiology group (p < 0.001, Table 2), and the stiff LA physiology (OR, 3.19 [1.64–6.22], p = 0.001) was also the independent factor of failed rhythm control within a year (Table 4). However, it was hard to conclude a causal-result relationship between stiff LA physiology and failed rhythm control within a year because we determined both parameters simultaneously 1 year after the procedure. We found E/Em elevation, which reflected worsening of LV diastolic function, among patients with stiff LA physiology. LV diastolic dysfunction increases PA pressure and RVSP and is attributed to stiff LA physiology (Shoemaker et al., 2011; Witt et al., 2014). A reduced reservoir or pump function in stiff LA physiology inversely has a negative effect on the LV filling pressure, resulting in increased E/Em (Appleton et al., 1988).
Empirical Extra-PV LA Ablations in AFCA
Although AFCA is an effective AF rhythm control method, various extra-PV substrate modifications have been attempted to reduce the substantial and continuous recurrence rate, especially after persistent AF ablation (Jais et al., 2004; Haissaguerre et al., 2005; Willems et al., 2006; Knecht et al., 2008). However, the recently attempted randomized clinical trials have failed to prove the benefits of empirical extra-PV ablation in terms of the AFCA rhythm outcome (Verma et al., 2015; Lee et al., 2019). In addition, the current guidelines do not recommend routine empirical extra-PV ablation (Calkins et al., 2017). The untargeted empirical substrate ablation may generate new scars that are correlated with LA function (Wylie et al., 2008): the more the touches, the more the scars. We previously reported that empirical extra-PV LA ablation increased LA pressure and stiffness without the aggravation of symptom scores compared with CPVI alone (Park et al., 2019). In this study, the empirical extra-PV LA ablation and the long duration of RF ablation were consistently associated with stiff LA physiology on follow-up echocardiography. Therefore, appropriate mapping and ablating extra-PV foci, which is the main cause of the long-term AF recurrence after AFCA, are still challenging issues.
Study Limitations
This study had several limitations. First, this was an observational prospective cohort study of a highly selective group of patients who underwent AFCA. Second, the exact definition of the stiff LA syndrome included symptoms of the patients, such as dyspnea on exertion; however, since we could not obtain the data on symptoms, we designated stiff LA physiology. Furthermore, we estimated stiff LA physiology according to the RVSP change on echocardiography. However, several studies have defined LA stiffness using various parameters, such as LA peak pressure, pulmonary hypertension with large v-wave, and LA pulse pressure indicating LA compliance (Gibson et al., 2011; Witt et al., 2013; Park et al., 2015, 2019). Since there has been no gold-standard method for the LA stiffness, the associated results of each study could be different. In addition, applying the same criteria for stiff LA physiology in patients who already had diseased LA would be limited; therefore, the generalization of the results should be considered with circumspection. Third, patients with a lack of pre-procedural and appropriate follow-up echocardiography data were excluded. Thus, there could be a possibility of selection bias. In addition, since the post-ablation echocardiography was conducted 1 year after the procedure, other potential confounding factors, such as age, baseline comorbidities, prescribed medications, and inadequate rate control, could contribute to the progression of heart failure and eventually LA stiffness. Fourth, the echocardiography measurements could be inaccurate when measured during AF due to beat-to-beat variability. However, since all of the patients underwent serial echocardiography before and 1 year after the procedure, we could evaluate the changes in the parameters according to the development of stiff LA physiology. Fifth, in 6.7% of all the subjects, voltage mapping was performed after CPVI ablation, and it could have affected the mean LA voltage. Finally, although we waited for LA pressure to stabilize for at least 3 min in each patient, the mechanical stunning of LA after cardioversion may have affected the LA pressure.
Conclusion
Although the incidence of post-AFCA stiff LA physiology was 3.7% and most of the cases were subclinical, the empirical extra-PV ablation was associated with this undesirable condition. In addition, patients who had low mean LA voltage before AFCA could be susceptible to stiff LA physiology.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by The institutional Review Board of the Yonsei University Health System. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
J-HL and H-NP conceived and designed the study, performed the statistical analysis, and drafted manuscript. HY, T-HK, J-SU, BJ, M-HL, and H-NP recruited study subjects. O-SK developed customized software for calculating left atrial wall thickness and performed technical support. All authors have read and approved the final manuscript.
Funding
This study was supported by the Ministry of Health and Welfare (Grant Nos. HI19C0114 and HI21C0011), and the National Research Foundation of Korea through the Basic Science Research Program (Grant No. NRF-2020R1A2B01001695), which is funded by the Ministry of Science, ICT, and Future Planning.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank Mr. John Martin for his linguistic assistance.
References
Appleton, C. P., Hatle, L. K., and Popp, R. L. (1988). Relation of transmitral flow velocity patterns to left ventricular diastolic function: new insights from a combined hemodynamic and Doppler echocardiographic study. J. Am. Coll. Cardiol. 12, 426–440. doi: 10.1016/0735-1097(88)90416-0
Calkins, H., Hindricks, G., Cappato, R., Kim, Y. H., Saad, E. B., Aguinaga, L., et al. (2017). HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm 14, e275–e444. doi: 10.1016/j.hrthm.2017.05.012
Calkins, H., Kuck, K. H., Cappato, R., Brugada, J., Camm, A. J., Chen, S. A., et al. (2012). HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Heart Rhythm 9, 632–696. doi: 10.1093/europace/eum120
Cesario, D. A., Mahajan, A., and Shivkumar, K. (2007). Lesion-forming technologies for catheter ablation of atrial fibrillation. Heart Rhythm 4, S44–50. doi: 10.1016/j.hrthm.2006.12.025
Falsetti, H. L., Mates, R. E., Grant, C., Greene, D. G., and Bunnell, I. L. (1970). Left ventricular wall stress calculated from one-plane cineangiography. Circ. Res. 26, 71–83. doi: 10.1161/01.RES.26.1.71
Gibson, D. N., Di Biase, L., Mohanty, P., Patel, J. D., Bai, R., Sanchez, J., et al. (2011). Stiff left atrial syndrome after catheter ablation for atrial fibrillation: clinical characterization, prevalence, and predictors. Heart Rhythm 8, 1364–1371. doi: 10.1016/j.hrthm.2011.02.026
Haissaguerre, M., Hocini, M., Sanders, P., Sacher, F., Rotter, M., Takahashi, Y., et al. (2005). Catheter ablation of long-lasting persistent atrial fibrillation: clinical outcome and mechanisms of subsequent arrhythmias. J. Cardiovasc. Electrophysiol. 16, 1138–1147. doi: 10.1111/j.1540-8167.2005.00308.x
Jais, P., Hocini, M., Hsu, L. F., Sanders, P., Scavee, C., Weerasooriya, R., et al. (2004). Technique and results of linear ablation at the mitral isthmus. Circulation 110, 2996–3002. doi: 10.1161/01.CIR.0000146917.75041.58
Knecht, S., Hocini, M., Wright, M., Lellouche, N., O'neill, M. D., Matsuo, S., et al. (2008). Left atrial linear lesions are required for successful treatment of persistent atrial fibrillation. Eur. Heart J. 29, 2359–2366. doi: 10.1093/eurheartj/ehn302
Kwon, O. S., Lee, J., Lim, S., Park, J. W., Han, H. J., Yang, S. H., et al. (2020). Accuracy and clinical feasibility of 3D-myocardial thickness map measured by cardiac computed tomogram. Int. J. Arrhythmia 21, 1–11. doi: 10.1186/s42444-020-00020-w
Lee, J. H., Kwon, O. S., Shim, J., Lee, J., Han, H. J., Yu, H. T., et al. (2021a). Left atrial wall stress and the long-term outcome of catheter ablation of atrial fibrillation: an artificial intelligence-based prediction of atrial wall stress. Front. Physiol. 12:686507. doi: 10.3389/fphys.2021.686507
Lee, J. H., Yu, H. T., Kwon, O. S., Han, H. J., Kim, T. H., Uhm, J. S., et al. (2021b). Atrial wall thickness and risk of hemopericardium in elderly women after catheter ablation for atrial fibrillation. Circ. Arrhythm. Electrophysiol. 14:e009368. doi: 10.1161/CIRCEP.120.009368
Lee, J. M., Shim, J., Park, J., Yu, H. T., Kim, T. H., Park, J. K., et al. (2019). The electrical isolation of the left atrial posterior wall in catheter ablation of persistent atrial fibrillation. JACC Clin. Electrophysiol. 5, 1253–1261. doi: 10.1016/j.jacep.2019.08.021
Marrouche, N. F., Brachmann, J., Andresen, D., Siebels, J., Boersma, L., Jordaens, L., et al. (2018). Catheter ablation for atrial fibrillation with heart failure. N. Engl. J. Med. 378, 417–427. doi: 10.1056/NEJMoa1707855
Nath, S., Redick, J. A., Whayne, J. G., and Haines, D. E. (1994). Ultrastructural observations in the myocardium beyond the region of acute coagulation necrosis following radiofrequency catheter ablation. J. Cardiovasc. Electrophysiol. 5, 838–845. doi: 10.1111/j.1540-8167.1994.tb01122.x
Park, J., Joung, B., Uhm, J. S., Young Shim, C., Hwang, C., Hyoung Lee, M., et al. (2014). High left atrial pressures are associated with advanced electroanatomical remodeling of left atrium and independent predictors for clinical recurrence of atrial fibrillation after catheter ablation. Heart Rhythm 11, 953–960. doi: 10.1016/j.hrthm.2014.03.009
Park, J., Yang, P. S., Kim, T. H., Uhm, J. S., Kim, J. Y., Joung, B., et al. (2015). Low left atrial compliance contributes to the clinical recurrence of atrial fibrillation after catheter ablation in patients with structurally and functionally normal heart. PLoS ONE 10:e0143853. doi: 10.1371/journal.pone.0143853
Park, J. W., Yu, H. T., Kim, T. H., Uhm, J. S., Joung, B., Lee, M. H., et al. (2019). Atrial fibrillation catheter ablation increases the left atrial pressure. Circ. Arrhythm. Electrophysiol. 12:e007073. doi: 10.1161/CIRCEP.118.007073
Pilote, L., Huttner, I., Marpole, D., and Sniderman, A. (1988). Stiff left atrial syndrome. Can. J. Cardiol. 4, 255–257.
Shoemaker, M. B., Hemnes, A. R., Robbins, I. M., Langberg, J. J., Ellis, C. R., Aznaurov, S. G., et al. (2011). Left atrial hypertension after repeated catheter ablations for atrial fibrillation. J. Am. Coll. Cardiol. 57, 1918–1919. doi: 10.1016/j.jacc.2011.01.021
Verma, A., Jiang, C. Y., Betts, T. R., Chen, J., Deisenhofer, I., Mantovan, R., et al. (2015). Approaches to catheter ablation for persistent atrial fibrillation. N. Engl. J. Med. 372, 1812–1822. doi: 10.1056/NEJMoa1408288
Wang, W., Buehler, D., Martland, A. M., Feng, X. D., and Wang, Y. J. (2011). Left atrial wall tension directly affects the restoration of sinus rhythm after Maze procedure. Eur. J. Cardiothorac. Surg. 40, 77–82. doi: 10.1016/j.ejcts.2010.10.022
Willems, S., Klemm, H., Rostock, T., Brandstrup, B., Ventura, R., Steven, D., et al. (2006). Substrate modification combined with pulmonary vein isolation improves outcome of catheter ablation in patients with persistent atrial fibrillation: a prospective randomized comparison. Eur. Heart J. 27, 2871–2878. doi: 10.1093/eurheartj/ehl093
Witt, C., Powell, B., Holmes, D., and Alli, O. (2013). Recurrent dyspnea following multiple ablations for atrial fibrillation explained by the “stiff left atrial syndrome. Catheter. Cardiovasc. Interv. 82, E747–749. doi: 10.1002/ccd.24556
Witt, C. M., Fenstad, E. R., Cha, Y. M., Kane, G. C., Kushwaha, S. S., Hodge, D. O., et al. (2014). Increase in pulmonary arterial pressure after atrial fibrillation ablation: incidence and associated findings. J. Interv. Card. Electrophysiol. 40, 47–52. doi: 10.1007/s10840-014-9875-1
Writing Committee, M., Yancy, C. W., Jessup, M., Bozkurt, B., Butler, J., Casey, D. E. Jr., et al. (2016). ACC/AHA/HFSA focused update on new pharmacological therapy for heart failure: an update of the accf/aha guideline for the management of heart failure: a report of the american college of cardiology/american heart association task force on clinical practice guidelines and the heart failure society of America. Circulation 134, e282–293. doi: 10.1161/CIR.0000000000000460
Wylie, J. V. Jr., Peters, D. C., Essebag, V., Manning, W. J., Josephson, M. E., and Hauser, T. H. (2008). Left atrial function and scar after catheter ablation of atrial fibrillation. Heart Rhythm 5, 656–662. doi: 10.1016/j.hrthm.2008.02.008
Yu, H. T., Shim, J., Park, J., Kim, I. S., Kim, T. H., Uhm, J. S., et al. (2017). Pulmonary vein isolation alone versus additional linear ablation in patients with persistent atrial fibrillation converted to paroxysmal type with antiarrhythmic drug therapy: a multicenter, prospective, randomized study. Circ. Arrhythm. Electrophysiol. 10:4915. doi: 10.1161/CIRCEP.116.004915
Keywords: atrial fibrillation, catheter ablation, stiff left atrium, extensive ablation, rhythm outcome
Citation: Lee J-H, Kwon O-S, Yu HT, Kim T-H, Uhm J-S, Joung B, Lee M-H and Pak H-N (2021) Risk Factors for Stiff Left Atrial Physiology 1 Year After Catheter Ablation of Atrial Fibrillation. Front. Physiol. 12:740600. doi: 10.3389/fphys.2021.740600
Received: 13 July 2021; Accepted: 23 August 2021;
Published: 20 September 2021.
Edited by:
Vijay S. Chauhan, Peter Munk Cardiac Center, CanadaReviewed by:
Sheldon Singh, University of Toronto, CanadaSusanna Mak, University of Toronto, Canada
Copyright © 2021 Lee, Kwon, Yu, Kim, Uhm, Joung, Lee and Pak. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui-Nam Pak, aG5wYWtAeXVocy5hYw==
 Jae-Hyuk Lee
Jae-Hyuk Lee Oh-Seok Kwon
Oh-Seok Kwon Hee Tae Yu
Hee Tae Yu Jae-Sun Uhm
Jae-Sun Uhm Hui-Nam Pak
Hui-Nam Pak