
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Physiol., 27 January 2022
Sec. Cardiac Electrophysiology
Volume 12 - 2021 | https://doi.org/10.3389/fphys.2021.730127
This article is part of the Research TopicAcquired and Inherited Cardiac ArrhythmiasView all 5 articles
 Luca Sala1*
Luca Sala1* Vladislav Leonov1,2
Vladislav Leonov1,2 Manuela Mura3
Manuela Mura3 Federica Giannetti1
Federica Giannetti1 Aleksandr Khudiakov1
Aleksandr Khudiakov1 Alessandra Moretti4,5
Alessandra Moretti4,5 Lia Crotti1,6,7
Lia Crotti1,6,7 Massimiliano Gnecchi3,8
Massimiliano Gnecchi3,8 Peter J. Schwartz1*
Peter J. Schwartz1*
In the early phases of the COVID-19 pandemic, drug repurposing was widely used to identify compounds that could improve the prognosis of symptomatic patients infected by SARS-CoV-2. Hydroxychloroquine (HCQ) was one of the first drugs used to treat COVID-19 due to its supposed capacity of inhibiting SARS-CoV-2 infection and replication in vitro. While its efficacy is debated, HCQ has been associated with QT interval prolongation and potentially Torsades de Pointes, especially in patients predisposed to developing drug-induced Long QT Syndrome (LQTS) as silent carriers of variants associated with congenital LQTS. If confirmed, these effects represent a limitation to the at-home use of HCQ for COVID-19 infection as adequate ECG monitoring is challenging. We investigated the proarrhythmic profile of HCQ with Multi-Electrode Arrays after exposure of human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) from two healthy donors, one asymptomatic and two symptomatic LQTS patients. We demonstrated that: I) HCQ induced a concentration-dependent Field Potential Duration (FPD) prolongation and halted the beating at high concentration due to the combined effect of HCQ on multiple ion currents. II) hiPSC-CMs from healthy or asymptomatic carriers tolerated higher concentrations of HCQ and showed lower susceptibility to HCQ-induced electrical abnormalities regardless of baseline FPD. These findings agree with the clinical safety records of HCQ and demonstrated that hiPSC-CMs potentially discriminates symptomatic vs. asymptomatic mutation carriers through pharmacological interventions. Disease-specific cohorts of hiPSC-CMs may be a valid preliminary addition to assess drug safety in vulnerable populations, offering rapid preclinical results with valuable translational relevance for precision medicine.
Drug repurposing is a key strategy aimed to identify new applications for compounds that have already been approved by regulatory authorities. The known safety profiles and toxicology of the repurposed compounds reduce the chances of failure and drug attrition, shorten the time frame for drug development and lower the costs associated with drug discovery and screening (Pushpakom et al., 2019). It had been a key process during the early phases of the COVID-19 pandemic to promote the identification of marketed compounds that could improve prognosis and therapy for symptomatic patients infected by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Hydroxychloroquine (HCQ), an antimalarial drug successfully used for the treatment of systemic lupus erythematosus and rheumatoid arthritis, was identified as a compound capable of inhibiting SARS-CoV-2 infection and replication in vitro (Liu et al., 2020; Touret et al., 2020; Yao et al., 2020); its off-label use was thus proposed for the treatment but also the prevention of COVID-19 in multiple clinical trials.
However, HCQ carries known pharmacological side-effects which include the block of the rapid delayed-rectifier potassium current (IKr) and the inward delayed-rectifier potassium current (IK1) at therapeutic concentrations, with peak sodium current (INa) and L-type calcium current (ICaL) blocked at higher concentrations (Wang et al., 2020).
At the clinical level, HCQ prolongs the QT interval (Saleh et al., 2020), with effects particularly exacerbated in the presence of factors such as plasma electrolyte imbalance (e.g., hypokalemia) and fever, both frequently present in patients with COVID-19, or secondary organ dysfunction. For these reasons, HCQ is classified by the CredibleMeds® database (Woosley et al., 2021) as a drug with a known risk of causing Torsades de Pointes (TdP) and which has to be avoided in patients with the congenital Long QT Syndrome (cLQTS) (Schwartz and Ackerman, 2013). The proarrhythmic potential of HCQ seems further enhanced by its administration in combination with Azhithromycin, a macrolide antibiotic also proposed for the treatment of COVID-19 which can also cause TdPs (Mercuro et al., 2020; Fiolet et al., 2021).
Consequently, the administration of HCQ to a broad and heterogeneous population of patients, particularly at dosages higher than those already approved or while being proposed as at-home therapy regardless of any information on the patients’ genetic background (Procter et al., 2020), may create safety risks without adequate QT monitoring (Derwand et al., 2020); the susceptibility to QT-prolonging drugs is indeed exacerbated in case of silent cLQTS mutation carriers, i.e., patients carrying variant(s) in LQTS gene(s) who do not have the clinical hallmarks of LQTS despite having a compromised-, in case of loss-of-function variants in K+ channels, or insufficient, as in case of gain-of-function variants in depolarizing currents, repolarization reserve (O’Hara and Rudy, 2012; Schwartz and Woosley, 2016). These effects were correctly recapitulated in hiPSC-CMs (Braam et al., 2013). The potential proarrhythmic effect of HCQ has highlighted the complications of assessing the safety of pharmacological therapies, particularly on how to predict and quantify the risks of drugs used at doses not previously tested. A strategy to identify patients at risk of developing drug-induced QT prolongation and arrhythmias is still lacking, but there is a strong need for valid models which reproduce or predict the genotype-specific consequences of drugs on silent cLQTS mutation carriers; this would be particularly relevant for individuals with borderline QT intervals whose pathogenic variants often go undiagnosed in routine ECGs but which may significantly increase the arrhythmogenic susceptibility to QT prolonging drugs. This also applies for individuals carrying variants in protective/detrimental genetic modifiers (Itoh et al., 2016; Chai et al., 2018; Lee et al., 2021).
Despite the promising in vitro results, HCQ did not confirm its efficacy in vivo neither in preventing SARS-CoV-2 infection nor in treating the severe consequences of the infection, with findings confirmed by several independent studies (Graham et al., 2020; Axfors et al., 2021; Jorge, 2021; Macías et al., 2021; Rentsch et al., 2021; WHO Solidarity Trial Consortium et al., 2021). Conclusive evidence of its efficacy will come as several clinical trials are still recruiting (288 studies, 29 not yet recruiting, 77 recruiting or enrolling by invitation; 24 Active, not recruiting; 7 suspended, 34 terminated; 80 completed; 37 withdrawn ClinicalTrials.gov, accessed on 19/12/2021). We believe that more information is required to understand the risks and boundaries of HCQ when administered in a potentially susceptible population of patients in which asymptomatic individuals carry a higher predisposition for TdP.
Here we used MultiElectrode Arrays (MEAs), one of the platforms of choice from the Comprehensive In vitro Proarrhythmia Assay (CiPA) consortium (Millard et al., 2018), to report the consequences of subchronic exposure to different concentrations of HCQ in a heterogeneous subset of three LQTS subjects and two unrelated healthy controls.
We aimed to assess whether: (i) hiPSC-CMs can identify potential proarrhythmic effects by HCQ in vitro; (ii) a genotype-specific response to HCQ can be reproduced with hiPSC-CMs and whether it correlates with clinical observations; and (iii) hiPSC-CMs from homogeneous disease-specific cohorts could provide quick preclinical readouts before repurposing drugs to patients in large trials.
Three patients affected by LQTS were studied. One carries the KCNQ1-p.R594Q variant (Mura et al., 2018), associated with the most common form of LQTS (LQT1). One was affected by the Jervell and Lange-Nielsen syndrome (JLNS) carrying the KCNQ1-p.R594Q & KCNQ1-p.R190W variants in compound heterozygosity (Mura et al., 2018); JLNS is one of the most severe forms of LQTS, caused by the presence of homozygous or compound heterozygous variants on KCNQ1 or on KCNE1 associated to congenital deafness in addition to the typical cardiac features (Schwartz et al., 2006). Another patient was affected by the CALM1-p.F142L variant (Crotti et al., 2013) associated with CALM-LQTS, which manifests in infants, is extremely severe, and responds poorly to therapy. These hiPSC-CMs were previously characterized (Rocchetti et al., 2017; Crotti et al., 2019).
hiPSCs from healthy subjects: one was an unrelated bona fide healthy donor (WT (Lee et al., 2021) while hiPSCs from the second donor (WT2) were provided by the Coriell Institute for Medical Research (WTC-11 line, catalog No. GM25256). The electrophysiological maturation state of WT2, JLNS and CALM-LQTS hiPSC-CMs was confirmed with patch clamp in this study and it can be observed by the hyperpolarized resting membrane potential and the prominent upstroke velocity, indirectly indicating a robust presence of IK1 and INa, respectively (Supplementary Figure 1).
hiPSCs were cultured on recombinant human vitronectin (rhVTN) in E8 Flex medium and differentiated on cell culture-grade Matrigel. hiPSCs were differentiated to hiPSC-CMs following a protocol based on the modulation of the Wnt-signaling pathway (Lian et al., 2012), purified through glucose starvation (> 90% CMs) and cryopreserved at days 17–20. Cryopreserved hiPSC-CMs were thawed before each experiment and maintained in culture for 7 days in RPMI medium supplemented with B27 Supplement (RPMI + B27). Data were obtained from 3 independent differentiations for each line. Details on reagents are provided in Supplementary Table 1.
Multiwell MEAs (MultiChannel Systems) were coated with 40 μg/mL bovine fibronectin and processed as previously described (Sala et al., 2017b). hiPSC-CMs were dissociated with TrypLE Select and replated on Multiwell MEAs as confluent monolayers. RPMI + B27 was refreshed every 48–72 h. The recordings started after 1 week and were made at Baseline and at 2, 24, and 48 h after the treatment with HCQ (Figure 1).
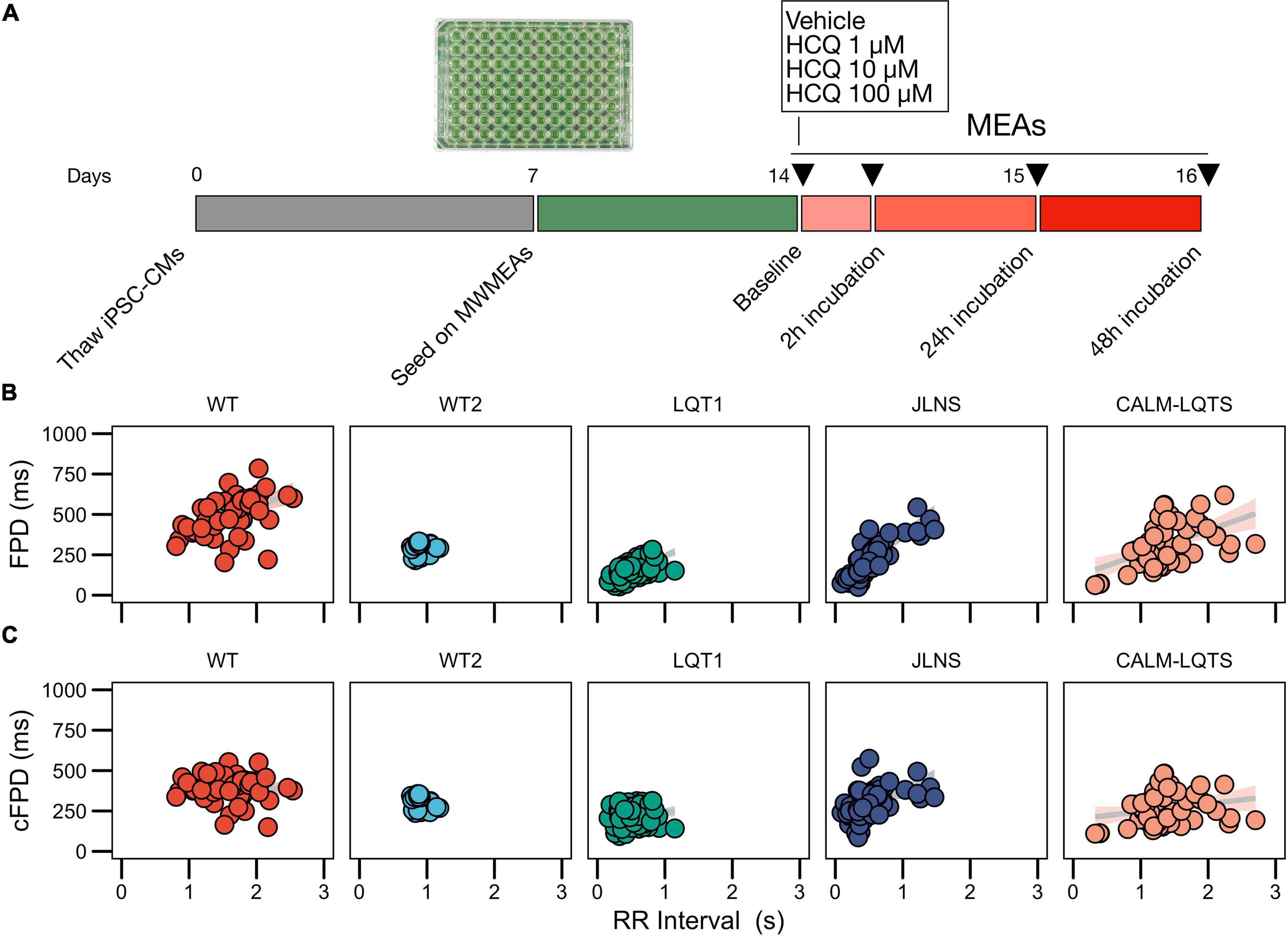
Figure 1. (A) Study protocol. (B) Relationships between FPD and RR for the iPSC lines used in this study measured with MWMEA at Baseline. Data were fitted with a linear model. N of MEA at Baseline: WT: 58; WT2: 48; LQT1: 106; JLNS: 62; CALM-LQTS: 56. (C) Relationships between cFPD and RR for the iPSC lines used in this study measured with MWMEA at Baseline. Data were fitted with a linear model. N of MEA at Baseline: WT: 58; WT2: 48; LQT1: 106; JLNS: 62; CALM-LQTS: 56.
HCQ was dissolved in water to obtain a stock solution of 50 mM. After having recorded Field Potentials (FP) values at Baseline, RPMI + B27 in Multiwell MEAs was refreshed with working concentrations of HCQ prepared in RPMI + B27 (1, 10, 100 μM). Vehicle was balanced accordingly and used as a control in each experiment and each Multiwell MEA plate.
A 6-point Field Potential (FP) scoring system was implemented to evaluate the quality of FPs and to classify potential detrimental effects of the drug treatment on FP quality (Figure 3B).
Score 5: Assigned to FPs with both markedly pronounced positive and negative upstrokes, a unambiguous repolarization wave and a large FP amplitude; noise and signal oscillations at baseline must not be present and the RR interval must be regular.
Score 4: Assigned to FPs with either a clear positive or negative depolarization peak, a visible repolarization wave and a large FP amplitude; noise and signal oscillations at baseline must not be present and the RR interval must be regular.
Score 3: Assigned to FPs similar to the previous condition but with a smaller FP amplitude, a less markedly pronounced repolarization peak and a potentially ambiguous repolarization peak. Signal oscillations at baseline can be present and RR must be stable.
Score 2: Assigned to FPs characterized by a small positive or negative depolarization, a small FP amplitude, a small and potentially ambiguous repolarization peak. Signal oscillations at baseline can be present and RR interval can be irregular.
Score 1: Assigned to FPs characterized by a very irregular pattern(s), with small/absent depolarization and a small/absent repolarization peak; noise is present in terms of oscillations at baseline and electrical interference from neighboring electrodes; RR interval can be unstable.
Score 0: Assigned to electrically inactive wells.
The analysis of FPs was performed as previously described (Sala et al., 2017b) and automated through custom R scripts (R v.4.0.4) to accommodate a large number of data points. In total, data from 1320 individual MEA recordings were analyzed.
Comparisons of FP Durations (FPD), RR, corrected FPD (cFPD) were performed for each line with Dunnett’s test using Baseline as reference values. Statistical significance was defined as p < 0.05 and is indicated in text and figures with an asterisk. Data are presented in the text as mean, mean ± standard error of the mean (sem) and scatter points where relevant.
To exclude any potential bias carried by the endogenous FPD of healthy hiPSC lines, we chose two healthy hiPSC lines characterized by different baseline FPD. Baseline FPD is a reliable parameter for comparisons only in case of isogenic lines or at least in case of lines sharing part of the genetic background (i.e., from relatives, as in case of LQT1 and JLNS lines in this paper) while it might often provide misleading results in case of cross-line comparisons (Sala et al., 2016, 2017a).
Given the significant non-zero slope of the FPD-RR linear fit for the majority of the lines, data were corrected with the clinically used Bazett’s QT correction formula, validated across all ages (Stramba-Badiale et al., 2018). As previously shown, corrected FPDs (cFPD) nullified in the majority of the cases (Sala et al., 2016) the positive correlation with RR when present (Figures 1B,C), normalizing the rate-dependency of FPD. Data are indicated in Supplementary Table 2.
Signs of spontaneous arrhythmias and electrical instability were detected at baseline in MEAs from the JLNS and CALM-LQTS (Supplementary Figure 4).
We first investigated the presence of potential detrimental effects of the exposure to HCQ on the spontaneous activity of hiPSC-CMs monolayers.
We observed a dose-dependent effect of HCQ quantified as an increasing number of wells that ceased to beat with higher concentrations (Figures 2, 3A). Here, a genotype-dependent effect was observed and it became particularly evident in two conditions: after the acute (2 h) exposure to 10 μM HCQ, hiPSC-CMs from the two controls showed no effect on the spontaneous beating while ∼20% of the monolayers from the LQT1, ∼35% of the JLNS and ∼50% of the monolayers from the very severe CALM-LQTS temporarily halted the beating, which only partially recovered at longer timepoints (Figure 2). Similarly, 100 μM HCQ induced a larger termination of the spontaneous beating in monolayers from JLNS and CALM-LQTS, while asymptomatic LQT1 and the two WT seems more resistant to higher concentrations of HCQ (Figures 2, 3A). A similar trend was also observed in isolated hiPSC-CMs paced at 1 Hz, with cells from JLNS and CALM-LQTS exhibiting a lower tolerance to 10 μM HCQ than those from WT2 (Supplementary Figure 1). Next, we developed a 6-point scoring system to incorporate information regarding the FP quality degradation due to HCQ into the classification of the drug effect. This is particularly relevant to provide differential weights to MEAs in which the main temporal FP parameters (i.e., FPD, RR, cFPD, etc.) could still be tracked and quantified but were spoiled by the drug treatment. We observed that HCQ had more severe effects on the FP quality of hiPSC-CMs from the two symptomatic subjects, with minor or modest effects in hiPSC-CMs from the asymptomatic LQT1 and from the healthy controls. These differences could be appreciated already at 1 μM and were particularly relevant at 10 μM. At 100 μM, only hiPSC-CMs from one of the two healthy donors maintained a higher proportion of good FPs despite their FP shapes being significantly modified. The deterioration of the FP signal quality has to be attributed to a significant decrease in action potential (AP) amplitude, AP peak, upstroke velocity and to a trend toward depolarization of the Ediast (Supplementary Figures 1, 2).

Figure 2. Cessation of beating, intended as the proportion of wells with a quantifiable signal at each timepoint.
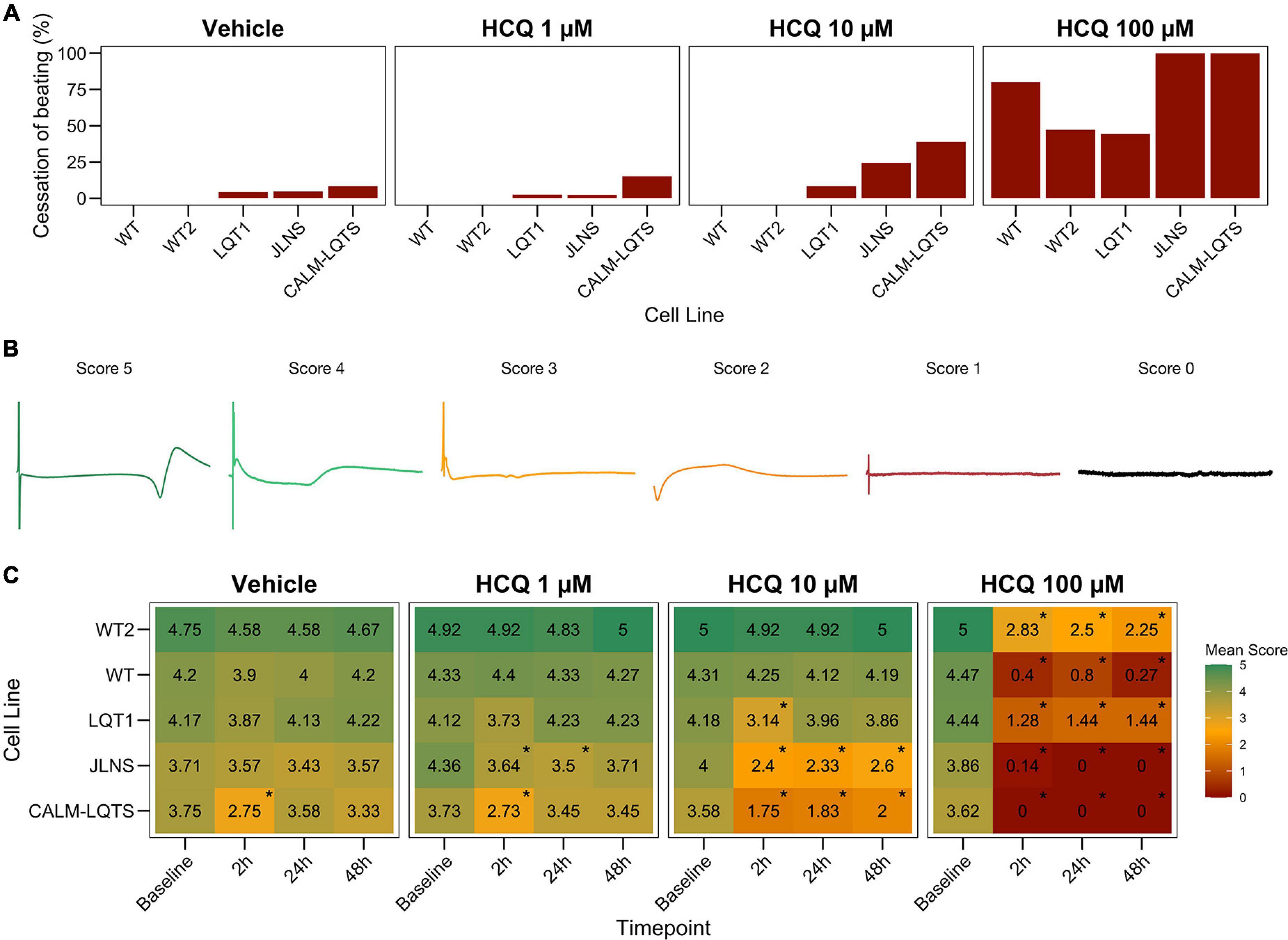
Figure 3. (A) Percentage of MEAs that ceased to beat in at least one recording timepoint at different HCQ concentrations. (B) Representative examples of FPs with the associated score that were used to quantify the effect of HCQ on FP quality. (C) Mean FP scores at different HCQ concentrations and different timepoints in all the iPSC lines. The color code indicates the mean FP score.
After having assessed the detrimental effects of HCQ on FP quality, we investigated the effect of different concentrations of HCQ on MEA temporal parameters to verify whether HCQ could trigger a differential FPD prolongation based on the donor’s genotype. Higher susceptibility to media changes has been observed in the CALM-LQTS at all timepoints, even with vehicle, with spontaneous arrhythmic events often present (Supplementary Figure 4). Treatment with vehicle induced changes (shortening) in the cFPD of WT at 2 h, while it prolonged cFPD in CALM-LQTS at all the timepoints.
HCQ 1 μM induced prolongation in the cFPD of CALM-LQTS at all the timepoints and increased the number of MEAs without electrical activity.
HCQ 10 μM induced shortening in the cFPD of WT only at 2 h, while it significantly increased cFPD in WT2, LQT1, JLNS and CALM-LQTS at all the time points and was paired with a cessation of beating only affecting LQT1, JLNS, and CALM-LQTS.
HCQ 100 μM induced cFPD prolongation in WT, WT2, LQT1 while monolayers from JLNS and CALM-LQTS did not tolerate such high dosage and no electrical activity was observed at all timepoints.
Our findings provide novel evidence that hiPSC-CMs from subjects with a different propensity toward life-threatening arrhythmias, largely driven by their genetic background, may respond differently to drugs with the potential of blocking repolarizing currents. As drug screening moves progressively toward more sophisticated approaches, not unmindful of precision medicine, the incorporation of disease-specific cohorts of hiPSC-CMs should be considered as rational step in the assessment of drug repurposing strategies in vulnerable populations and possibly also in the safety screening of new drugs.
Although the concentrations and the modality of administration (acute vs. cumulative) are different among in vitro experiments and many clinical trials, results for the WT subjects were similar to results from early timepoints published by other investigators with MEAs (TeBay et al., 2021) or in a more sophisticated organ-on-a-chip system (Charrez et al., 2021). Our study further extends these results including a susceptible population of patients affected by cLQTS that may be at higher risk of exhibiting diLQTS and developing TdP. The mean HCQ plasma concentration in subjects treated with HCQ has been measured as 50.3 ng/mL following a single 200 mg dose of HCQ (Plaquenil®) and a mean peak blood concentration of 129.6 ng/mL (Plaquenil label indication); based on these data, it is reasonable to estimate an in vitro concentration of 0.15–0.387 μM.
Inhibition of SARS-CoV-2 infection in vitro was achieved at concentrations larger than 4 μM (EC50 4.06 μM or higher) regardless of the multiplicity of infection (MOI) used for SARS-CoV-2, Liu et al. (2020), while the cytotoxic concentration (CC50) in ATCC-1586 cells was around 250 μM. Other studies identified a similar range of concentrations in Vero/VeroE6 cells, with an EC50 of ∼6 μM after 24 h of treatment (Yao et al., 2020) or an IC50 of 2–4 μM at 48 or 72, h respectively (Maisonnasse et al., 2020).
In the RECOVERY trial, patients were treated with 800 mg administered at zero and 6 h, followed by 400 mg starting at 12 h after the initial dose and subsequently every 12 h for the following 9 days or until discharge (RECOVERY Collaborative Group et al., 2020). The simulated whole-blood concentration-time plots derived the theoretical whole-blood concentration of HCQ to span from 1 to 6 μM, with an upper safety bound for whole-blood of ∼10 μM (3 μM at plasma concentration) (White et al., 2020).
The concentrations used in this study appropriately covered clinically relevant concentrations as well as the in vitro effective dosages for SARS-CoV-2 inhibition, but also take into consideration a potential higher tolerance to drugs by hiPSC-CMs and the unknown effect of serum-free cell culture media on HCQ bioavailability.
Concentration-dependent effects were recorded for HCQ in all the lines, with the main modifications being changes in the repolarization peak as well as in the FP amplitude and quality. We could detect indications of proarrhythmic events in many of the MEAs treated with high HCQ concentrations, with the main events being abnormal shape of the repolarization wave, an irregular beat rate, a variable amplitude and presence of multiple depolarization peaks among consecutive beats. Some of these features were already visible at baseline for hiPSC-CMs from the two most severe lines used in this study (i.e., JLNS, CALM-LQTS).
A genotype-specific drug response pattern could also be identified and it aligned with the severity of the underlying genotypes. Our data indicate that hiPSC-CMs from the healthy donors or the asymptomatic LQT1 mutation carrier tolerated higher concentrations of HCQ and showed lower susceptibility to HCQ regardless of baseline in vitro FPD values.
Importantly and in agreement with previous observations (Sala et al., 2016, 2017a), the genotype-specific effects were present and predominant over the different baseline FPD parameters, further confirming that absolute FPD (or AP duration, APD) values alone might provide misleading information when classifying drug effects in different cell lines, with the relative drug effects and a more comprehensive analysis of all FP parameters to take into account the underlying genotypes may provide more relevant results.
Healthy controls showed a higher tolerance for QTc prolongation and higher resistance to high HCQ concentrations. This might have impacted directly the patients: the asymptomatic LQT1 carrier (Female, Basal QTc = 458 ms), for example, was identified in our center only through a family screening of the sibling (JLNS, Female, Basal QTc = 578 ms, symptomatic) and might have been included in one of the COVID-19 clinical trials with HCQ because in most of them the exclusion criteria for QTc were a cutoff of 480 ms. According to our MEA data, this would have meant a significantly higher chance for this patient to experience QTc prolongation, a degradation of the electrical signal (Figure 3C) and a potentially pathological FPD prolongation (Figure 4).
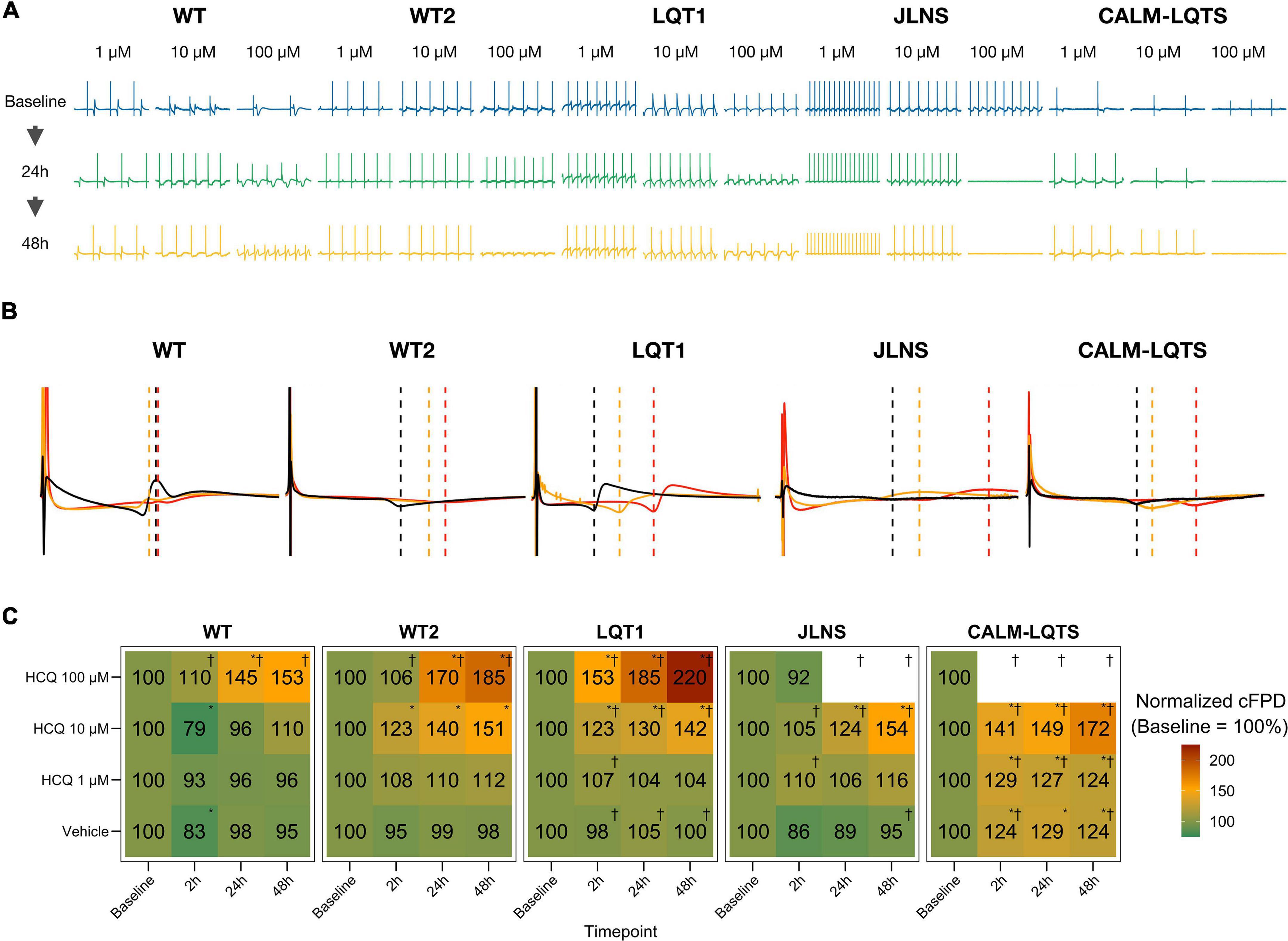
Figure 4. (A) Representative examples of HCQ effect on MEA recordings. (B) Representative mean FP profiles of MEAs treated with 10 μM HCQ at Baseline (black), after 24 h (yellow) and 48 h (red) exposure. (C) Mean FPD change, expressed as% and normalized to the respective Baseline value. Color code indicates the relative cFPD change compared to Baseline. * Indicates p < 0.05 vs. Baseline. †Indicates that the treatment caused the cessation of beating at a specific combination of Dosage and Timepoint.
Similarly to Chloroquine (Sánchez-Chapula et al., 2001), also HCQ blocks IK1, and this could explain the increased beating frequency observed particularly in hiPSC-CMs from WT despite HCQ should inhibit also the funny current (If) (Capel et al., 2015); in our experimental settings, this effect did not clearly emerge in hiPSC-CMs from LQTS subjects; however, a drug-induced IK1 blockade may generate an additive effect on the underlying LQTS, especially on spontaneously beating cells, with the effect on the beating frequency potentially being masked by an additive and abnormal FPD prolongation due to an already compromised repolarization reserve. Single-cell patch clamp data on hiPSC-CMs from WT2, LQTS and CALM-LQTS was performed and confirmed the trend toward depolarization (n.s. in all the lines), a large decrease in the upstroke velocity and a decrease in the plateau amplitude (p < 0.05 in all the lines) that overall could be linked to the decreased FP quality observed in Figure 3. These effects are consistent with patch clamp data in heterologous systems indicating HCQ as a blocker of multiple ion currents (Thomet et al., 2021). Some of the cells in all groups did not tolerate the acute exposure to 10 μM HCQ (Supplementary Figure 1), with a larger effects on cells from JLNS and CALM-LQTS groups; this confirms that isolated hiPSC-CMs have higher sensitivity to drugs than those included in monolayers, and that data from these two models may be interpreted and considered differently in terms of translational relevance (Sala et al., 2016). A trend toward APD shortening (p < 0.05 for WT2, n.s. for LQTS and CALM-LQTS) was observed and, for WT2, was similar to the results at 2 h in Figure 4 and Supplementary Figure 3. For JLNS and CALM-LQTS, the likely higher sensitivity to 10 μM HCQ exhibited by isolated hiPSC-CMs compared to monolayers may have contributed to exacerbate the effects on INa and dV/dtmax and thus the trend toward APD shortening.
Drug discovery and drug approval pipelines are already designed to prevent or minimize compounds that show a proarrhythmic potential due to a hERG blocking activity to be approved and commercialized (International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use, 2005). Even though important results have been achieved in the predictive potential of in vitro and in silico systems (Blinova et al., 2017; Li et al., 2017; Passini et al., 2017), comprehensive experimental models capable of recapitulating the complexity of human physiology are lacking and thus several compounds were withdrawn from the market after their approval [e.g., cisapride (Henney, 2000), astemizole (Gottlieb, 1999), terfenadine (Fung et al., 2001), etc.], with cardiovascular toxicity still being a major cause for molecules to be discarded in the preclinical phases of drug development (Ferri et al., 2013). Platforms to guide clinical decision making and orient research efforts are required, particularly in critical situations when there is a compelling need for repurposed compounds or when therapies are being recommended for home use. In recent years, the use of hiPSC-CMs for safety pharmacology has progressively gained momentum and multiple validation strategies have been attempted to generate experimental models with enhanced predictive potential, and recently the CiPA approach or similar strategies have been used to evaluate the proarrhythmic risk of HCQ on hiPSC-CMs from commercial hiPSC lines or healthy individuals (Delaunois et al., 2021). Platforms based on hiPSCs can offer rapid and relevant readouts for safety pharmacology (Sala et al., 2017a) but the possibility of incorporating patient-specific drug responses in addition to those from healthy individuals can increase the predictive capacity of these platforms in conditions where prompt actions are required.
Despite the remarkable progress in the strategies to mature hiPSC-CMs (Kamakura et al., 2013; Parikh et al., 2017; Feyen et al., 2020; Fukushima et al., 2020; Giacomelli et al., 2020), their electrophysiological phenotype still does not resemble that of adult cardiomyocytes and the relative ion channel distribution and drug responses might reflect this partial maturation; we anticipate that baseline absolute phenotypes as well as raw dose-response curves might potentially differ in case other differentiation, purification or maturation strategies are used (da Rocha et al., 2017). Nevertheless, given the early expression of the main ion channel targeted by HCQ (i.e., Kv11.1), a consistent expression of IKs (Kv7.1) in WT and CALM-LQTS (Rocchetti et al., 2017; Lee et al., 2021) and robust electrophysiological baseline parameters in isolated hiPSC-CMs (Supplementary Figure 1), we expect the effects to be reproduced in other experimental settings; this work is in agreement with the longstanding clinical safety profile of HCQ in healthy individuals and also allowed solid drug-mediated discrimination of the underlying genotypes.
The intrinsic technical aspects of MEAs do not allow the comparison of absolute FP voltages as it would have been possible in single cells with patch clamp; this cost is repaid in terms of throughput. However, we cannot assume that the monolayers of hiPSC-CMs tested here, despite being constituted by purified populations, exhibited comparable resting membrane potentials and, thus, drug effects may be influenced by the inherent baseline electrophysiological properties of each line. We confirmed that isolated hiPSC-CMs from three lines had Ediast with similar polarization levels (Supplementary Figure 1). This study provides information on the short-term (≤48 h) arrhythmogenic potential of HCQ and this timeframe was sufficient to discriminate genotype-specific responses; this study does not provide information on cumulative effects caused by repetitive doses or long-term treatments.
We have demonstrated that HCQ can induce cFPD prolongation, with effects becoming particularly important in susceptible subjects, at concentrations similar to those used in COVID-19 trials. More modest or negligible effects are instead evoked in hiPSC-CMs from healthy donors, particularly at concentrations close to those used to treat rheumatic disease; this was consistent with data from clinical studies (Abella et al., 2021).
Despite the highly controversial and debated (lack of) effectiveness for the prevention or treatment of COVID-19 (Axfors et al., 2021), the use of HCQ in a population of healthy individuals should not be stigmatized, as this compound has been successfully used for decades for the treatment of malaria, systemic lupus erythematosus and rheumatoid arthritis with more benefits than harm for patients (Wallace et al., 2012; Pareek et al., 2020), without significant increases in TdP risk at standard concentrations used in rheumatology (Taylor and White, 2004) and mainly only in association with co-prescribed QT-prolonging drugs and very high HCQ dosages (causal association) (Saint-Gerons and Tabarés-Seisdedos, 2021). Our report confirms this evidence.
Conversely, caution should be used for subjects who may be particularly susceptible to QT prolongation, and a particular focus should be put on silent or asymptomatic carriers of cLQTS variants and especially when genotype is additive to other detrimental pathophysiological factors or prescribed medications, as frequently occurs in patients with COVID-19 infection. Indeed, in a worldwide survey of COVID-19 associated arrhythmias, with almost 60% of the patients treated with hydroxychloroquine and 50% with azithromycin, those who developed cardiac arrhythmias already had borderline-prolonged QTc at the time of hospital admission and were more frequently affected by comorbidities and by severe pulmonary impairment (40% were mechanically ventilated) (Coromilas et al., 2021). On this issue, recommendations to further minimize risks of QT prolongation with Chloroquine or with HCQ in susceptible subjects have been recently drafted (Giudicessi et al., 2020; Offerhaus et al., 2020).
This work confirms that disease-specific hiPSC-CMs may provide additional information to drug screenings performed solely on hiPSC lines from healthy donors and may thus represent a viable option for preclinical drug repurposing screenings aiming for a higher translational readout. Larger homogeneous cohorts of hiPSC-CMs, with the ideal involvement of multiple facilities and including multiple disease-causing variants, genetic backgrounds and clinical manifestations, may represent a solid preliminary validation to assess the proarrhythmic effect of drugs, particularly in situations requiring rapid decisions. The combination of gene-editing and robust commercial iPSC lines from industry may further contribute to enhance and refine this aspect. These results should warn investigators interested in enrolling patients in clinical trials aimed to study the effects of HCQ in the prevention or treatment of COVID-19, of the existence of potentially important cardiac side-effects at concentrations far from those approved for rheumatoid arthritis, which may impact asymptomatic LQTS-mutation carriers whose underlying condition might go unnoticed at admission due to short or borderline QTc intervals. The most critical task indeed remains the identification of who might be a susceptible subject and how a patient or a disease-cohort might respond to a new or repurposed therapy, and in this regard the contribution provided by subject-specific hiPSC-CMs combined with genome sequencing and in silico predictive approaches can provide insights with clinical implications for precision medicine (Gnecchi et al., 2021).
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
LS: study design, hiPSC-CMs culture, MEA, patch clamp, data analysis, and wrote the manuscript. VL: CALM1-p.F142L hiPSC-CMs culture, critical analysis of data and manuscript. MM: LQT1-p.R594Q and JLNS-p.R594Q/p.R190W hiPSC line generation. FG: patch clamp data collection and analysis, critical analysis of the manuscript. AK: iPSCs and iPSC-CMs culture and differentiation, critical analysis of the manuscript. AM: CALM1-p.F142L hiPSC line generation. LC, MG, and PS: critical analysis of the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the Marie Skłodowska-Curie Individual Fellowship (H2020-MSCA-IF-2017 No. 795209) to LS, the Fondazione CARIPLO, “Biomedical Research Conducted by Young Researchers” (Grant No. 2019-1691) to LS, the Leducq Foundation for Cardiovascular Research [18CVD05] “Toward Precision Medicine with Human iPSCs for Cardiac Channelopathies” to PS, MG, LC, LS, MM, the Ph.D. fellowship from the University of Verona to VL, the DZHK and the German Research Foundation Transregio Research Units 152 and 267 to AM.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thank Michela Morano, Ph.D. for assisting in hiPSCs culture and differentiation.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2021.730127/full#supplementary-material
Abella, B. S., Jolkovsky, E. L., Biney, B. T., Uspal, J. E., Hyman, M. C., Frank, I., et al. (2021). Efficacy and safety of Hydroxychloroquine vs Placebo for Pre-exposure SARS-CoV-2 prophylaxis among health care workers: a randomized clinical trial. JAMA Intern. Med. 181, 195–202. doi: 10.1001/jamainternmed.2020.6319
Axfors, C., Schmitt, A. M., Janiaud, P., Van’t Hooft, J., Abd-Elsalam, S., Abdo, E. F., et al. (2021). Mortality outcomes with hydroxychloroquine and chloroquine in COVID-19 from an international collaborative meta-analysis of randomized trials. Nat. Commun. 12:2349. doi: 10.1038/s41467-021-22446-z
Blinova, K., Stohlman, J., Vicente, J., Chan, D., Johannesen, L., Hortigon-Vinagre, M. P., et al. (2017). Comprehensive translational assessment of human-induced pluripotent stem cell derived cardiomyocytes for evaluating drug-induced arrhythmias. Toxicol. Sci. 155, 234–247. doi: 10.1093/toxsci/kfw200
Braam, S. R., Tertoolen, L., Casini, S., Matsa, E., Lu, H. R., Teisman, A., et al. (2013). Repolarization reserve determines drug responses in human pluripotent stem cell derived cardiomyocytes. Stem Cell Res. 10, 48–56. doi: 10.1016/j.scr.2012.08.007
Capel, R. A., Herring, N., Kalla, M., Yavari, A., Mirams, G. R., Douglas, G., et al. (2015). Hydroxychloroquine reduces heart rate by modulating the hyperpolarization-activated current If: novel electrophysiological insights and therapeutic potential. Heart Rhythm 12, 2186–2194. doi: 10.1016/j.hrthm.2015.05.027
Chai, S., Wan, X., Ramirez-Navarro, A., Tesar, P. J., Kaufman, E. S., Ficker, E., et al. (2018). Physiological genomics identifies genetic modifiers of long QT syndrome type 2 severity. J. Clin. Invest. 128, 1043–1056. doi: 10.1172/JCI94996
Charrez, B., Charwat, V., Siemons, B. A., Goswami, I., Sakolish, C., Luo, Y.-S., et al. (2021). Heart muscle microphysiological system for cardiac liability prediction of repurposed COVID-19 therapeutics. Front. Pharmacol. 12:684252. doi: 10.3389/fphar.2021.684252
Coromilas, E. J., Kochav, S., Goldenthal, I., Biviano, A., Garan, H., Goldbarg, S., et al. (2021). Worldwide survey of COVID-19-associated arrhythmias. Circ. Arrhythm. Electrophysiol. 14:e009458. doi: 10.1161/CIRCEP.120.009458
Crotti, L., Johnson, C. N., Graf, E., De Ferrari, G. M., Cuneo, B. F., Ovadia, M., et al. (2013). Calmodulin mutations associated with recurrent cardiac arrest in infants. Circulation 127, 1009–1017. doi: 10.1161/CIRCULATIONAHA.112.001216
Crotti, L., Spazzolini, C., Tester, D. J., Ghidoni, A., Baruteau, A.-E., Beckmann, B.-M., et al. (2019). Calmodulin mutations and life-threatening cardiac arrhythmias: insights from the International Calmodulinopathy Registry. Eur. Heart J. 40, 2964–2975. doi: 10.1093/eurheartj/ehz311
da Rocha, A. M., Campbell, K., Mironov, S., Jiang, J., Mundada, L., Guerrero-Serna, G., et al. (2017). hiPSC-CM monolayer maturation state determines drug responsiveness in high throughput pro-arrhythmia screen. Sci. Rep. 7:13834. doi: 10.1038/s41598-017-13590-y
Delaunois, A., Abernathy, M., Anderson, W. D., Beattie, K. A., Chaudhary, K. W., Coulot, J., et al. (2021). Applying the CiPA approach to evaluate cardiac proarrhythmia risk of some antimalarials used off-label in the first wave of COVID-19. Clin. Transl. Sci. 14, 1133–1146. doi: 10.1111/cts.13011
Derwand, R., Scholz, M., and Zelenko, V. (2020). COVID-19 outpatients: early risk-stratified treatment with zinc plus low-dose hydroxychloroquine and azithromycin: a retrospective case series study. Int. J. Antimicrob. Agents 56:106214. doi: 10.1016/j.ijantimicag.2020.106214
Ferri, N., Siegl, P., Corsini, A., Herrmann, J., Lerman, A., and Benghozi, R. (2013). Drug attrition during pre-clinical and clinical development: understanding and managing drug-induced cardiotoxicity. Pharmacol. Ther. 138, 470–484. doi: 10.1016/j.pharmthera.2013.03.005
Feyen, D. A. M., McKeithan, W. L., Bruyneel, A. A. N., Spiering, S., Hörmann, L., Ulmer, B., et al. (2020). Metabolic maturation media improve physiological function of human iPSC-Derived cardiomyocytes. Cell Rep. 32:107925. doi: 10.1016/j.celrep.2020.107925
Fiolet, T., Guihur, A., Rebeaud, M. E., Mulot, M., Peiffer-Smadja, N., and Mahamat-Saleh, Y. (2021). Effect of hydroxychloroquine with or without azithromycin on the mortality of coronavirus disease 2019 (COVID-19) patients: a systematic review and meta-analysis. Clin. Microbiol. Infect. 27, 19–27. doi: 10.1016/j.cmi.2020.08.022
Fukushima, H., Yoshioka, M., Kawatou, M., López-Dávila, V., Takeda, M., Kanda, Y., et al. (2020). Specific induction and long-term maintenance of high purity ventricular cardiomyocytes from human induced pluripotent stem cells. PLoS One 15:e0241287. doi: 10.1371/journal.pone.0241287
Fung, M., Thornton, A., Mybeck, K., Wu, J. H.-H., Hornbuckle, K., and Muniz, E. (2001). Evaluation of the characteristics of safety withdrawal of prescription drugs from worldwide pharmaceutical Markets-1960 to 1999. Drug Inf. J. 35, 293–317. doi: 10.1177/009286150103500134
Giacomelli, E., Meraviglia, V., Campostrini, G., Cochrane, A., Cao, X., van Helden, R. W. J., et al. (2020). Human-iPSC-Derived cardiac stromal cells enhance maturation in 3D cardiac microtissues and reveal non-cardiomyocyte contributions to heart disease. Cell Stem Cell 26, 862.e11–879.e11. doi: 10.1016/j.stem.2020.05.004
Giudicessi, J. R., Noseworthy, P. A., Friedman, P. A., and Ackerman, M. J. (2020). Urgent Guidance for navigating and circumventing the QTc-Prolonging and torsadogenic potential of possible pharmacotherapies for coronavirus disease 19 (COVID-19). Mayo Clin. Proc. 95, 1213–1221. doi: 10.1016/j.mayocp.2020.03.024
Gnecchi, M., Sala, L., and Schwartz, P. J. (2021). Precision medicine and cardiac channelopathies: when dreams meet reality. Eur. Heart J. 42, 1661–1675. doi: 10.1093/eurheartj/ehab007
Gottlieb, S. (1999). Antihistamine drug withdrawn by manufacturer. BMJ 319:7. doi: 10.1136/bmj.319.7201.7a
Graham, F., Castelvecchi, D., and Phillips, N. (2020). Daily briefing: the evidence is not stacking up for hydroxychloroquine as a treatment for COVID-19. Nature [Online ahead of print] doi: 10.1038/d41586-020-01053-w
Henney, J. E. (2000). Withdrawal of troglitazone and cisapride. JAMA 283, 2228–2228. doi: 10.1001/jama.283.17.2228
International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (2005). The Non-Clinical Evaluation of the Potential for Delayed Ventricular Repolarization (Qt Interval Prolongation) by Human Pharmaceuticals. Geneva: International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use.
Itoh, H., Crotti, L., Aiba, T., Spazzolini, C., Denjoy, I., Fressart, V., et al. (2016). The genetics underlying acquired long QT syndrome: impact for genetic screening. Eur. Heart J. 37, 1456–1464. doi: 10.1093/eurheartj/ehv695
Jorge, A. (2021). Hydroxychloroquine in the prevention of COVID-19 mortality. Lancet Rheumatol. 3, e2–e3. doi: 10.1016/S2665-9913(20)30390-8
Kamakura, T., Makiyama, T., Sasaki, K., Yoshida, Y., Wuriyanghai, Y., Chen, J., et al. (2013). Ultrastructural maturation of human-induced pluripotent stem cell-derived cardiomyocytes in a long-term culture. Circ. J. 77, 1307–1314. doi: 10.1253/circj.cj-12-0987
Lee, Y.-K., Sala, L., Mura, M., Rocchetti, M., Pedrazzini, M., Ran, X., et al. (2021). MTMR4 SNVs modulate ion channel degradation and clinical severity in congenital long QT syndrome: insights in the mechanism of action of protective modifier genes. Cardiovasc. Res. 117, 767–779. doi: 10.1093/cvr/cvaa019
Li, Z., Dutta, S., Sheng, J., Tran, P. N., Wu, W., Chang, K., et al. (2017). Improving the In Silico Assessment Of Proarrhythmia Risk By Combining hERG (Human Ether-à-go-go-Related Gene) channel-drug binding kinetics and multichannel pharmacology. Circ. Arrhythm. Electrophysiol. 10:e004628. doi: 10.1161/CIRCEP.116.004628
Lian, X., Hsiao, C., Wilson, G., Zhu, K., Hazeltine, L. B., Azarin, S. M., et al. (2012). Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc. Natl. Acad. Sci. U.S.A. 109, E1848–E1857. doi: 10.1073/pnas.1200250109
Liu, J., Cao, R., Xu, M., Wang, X., Zhang, H., Hu, H., et al. (2020). Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 6:16. doi: 10.1038/s41421-020-0156-0
Macías, J., González-Moreno, P., Sánchez-García, E., Morillo-Verdugo, R., Pérez-Venegas, J. J., Pinilla, A., et al. (2021). Similar incidence of coronavirus disease 2019 (COVID-19) in patients with rheumatic diseases with and without hydroxychloroquine therapy. PLoS One 16:e0249036. doi: 10.1371/journal.pone.0249036
Maisonnasse, P., Guedj, J., Contreras, V., Behillil, S., Solas, C., Marlin, R., et al. (2020). Hydroxychloroquine use against SARS-CoV-2 infection in non-human primates. Nature 585, 584–587. doi: 10.1038/s41586-020-2558-4
Mercuro, N. J., Yen, C. F., Shim, D. J., Maher, T. R., McCoy, C. M., Zimetbaum, P. J., et al. (2020). Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus disease 2019 (COVID-19). JAMA Cardiol. 5, 1036–1041. doi: 10.1001/jamacardio.2020.1834
Millard, D., Dang, Q., Shi, H., Zhang, X., Strock, C., Kraushaar, U., et al. (2018). Cross-site reliability of human induced pluripotent stem cell-derived cardiomyocyte based safety assays using microelectrode arrays: results from a blinded CiPA pilot study. Toxicol. Sci. 164, 550–562. doi: 10.1093/toxsci/kfy110
Mura, M., Lee, Y.-K., Ginevrino, M., Zappatore, R., Pisano, F., Boni, M., et al. (2018). Generation of the human induced pluripotent stem cell (hiPSC) line PSMi002-A from a patient affected by the Jervell and Lange-Nielsen syndrome and carrier of two compound heterozygous mutations on the KCNQ1 gene. Stem Cell Res. 29, 157–161. doi: 10.1016/j.scr.2018.04.002
Offerhaus, J. A., Wilde, A. A. M., and Remme, C. A. (2020). Prophylactic (hydroxy)chloroquine in COVID-19: potential relevance for cardiac arrhythmia risk. Heart Rhythm 17, 1480–1486. doi: 10.1016/j.hrthm.2020.07.001
O’Hara, T., and Rudy, Y. (2012). Arrhythmia formation in subclinical (“silent”) long QT syndrome requires multiple insults: quantitative mechanistic study using the KCNQ1 mutation Q357R as example. Heart Rhythm 9, 275–282. doi: 10.1016/j.hrthm.2011.09.066
Pareek, A., Sharma, T. S., and Mehta, R. T. (2020). Hydroxychloroquine and QT prolongation: reassuring data in approved indications. Rheumatol. Adv. Pract. 4:rkaa044. doi: 10.1093/rap/rkaa044
Parikh, S. S., Blackwell, D. J., Gomez-Hurtado, N., Frisk, M., Wang, L., Kim, K., et al. (2017). Thyroid and glucocorticoid hormones promote functional T-Tubule development in human-induced pluripotent stem cell-derived cardiomyocytes. Circ. Res. 121, 1323–1330. doi: 10.1161/CIRCRESAHA.117.311920
Passini, E., Britton, O. J., Lu, H. R., Rohrbacher, J., Hermans, A. N., Gallacher, D. J., et al. (2017). Human in silico drug trials demonstrate higher accuracy than animal models in predicting clinical pro-arrhythmic cardiotoxicity. Front. Physiol. 8:668. doi: 10.3389/fphys.2017.00668
Procter, B. C., Ross, C., Pickard, V., Smith, E., Hanson, C., and McCullough, P. A. (2020). Clinical outcomes after early ambulatory multidrug therapy for high-risk SARS-CoV-2 (COVID-19) infection. Rev. Cardiovasc. Med. 21, 611–614. doi: 10.31083/j.rcm.2020.04.260
Pushpakom, S., Iorio, F., Eyers, P. A., Escott, K. J., Hopper, S., Wells, A., et al. (2019). Drug repurposing: progress, challenges and recommendations. Nat. Rev. Drug Discov. 18, 41–58. doi: 10.1038/nrd.2018.168
RECOVERY Collaborative Group, Horby, P., Mafham, M., Linsell, L., Bell, J. L., Staplin, N., et al. (2020). Effect of hydroxychloroquine in hospitalized patients with Covid-19. N. Engl. J. Med. 383, 2030–2040. doi: 10.1056/NEJMoa2022926
Rentsch, C. T., DeVito, N. J., MacKenna, B., Morton, C. E., Bhaskaran, K., Brown, J. P., et al. (2021). Effect of pre-exposure use of hydroxychloroquine on COVID-19 mortality: a population-based cohort study in patients with rheumatoid arthritis or systemic lupus erythematosus using the OpenSAFELY platform. Lancet Rheumatol. 3, e19–e27. doi: 10.1016/S2665-9913(20)30378-7
Rocchetti, M., Sala, L., Dreizehnter, L., Crotti, L., Sinnecker, D., Mura, M., et al. (2017). Elucidating arrhythmogenic mechanisms of long-QT syndrome CALM1-F142L mutation in patient-specific induced pluripotent stem cell-derived cardiomyocytes. Cardiovasc. Res. 113, 531–541. doi: 10.1093/cvr/cvx006
Saint-Gerons, D. M., and Tabarés-Seisdedos, R. (2021). Torsade de pointes associated with chloroquine, hydroxychloroquine, and azithromycin: a retrospective analysis of individual case safety reports from VigiBase. Eur. J. Clin. Pharmacol. 77, 1513–1521. doi: 10.1007/s00228-021-03133-w
Sala, L., Ward-van Oostwaard, D., Tertoolen, L. G. J., Mummery, C. L., and Bellin, M. (2017b). Electrophysiological analysis of human pluripotent stem cell-derived cardiomyocytes (hPSC-CMs) using multi-electrode arrays (MEAs). J. Vis. Exp. 123:55587. doi: 10.3791/55587
Sala, L., Bellin, M., and Mummery, C. L. (2017a). Integrating cardiomyocytes from human pluripotent stem cells in safety pharmacology: has the time come? Br. J. Pharmacol. 174, 3749–3765. doi: 10.1111/bph.13577
Sala, L., Yu, Z., Ward-van Oostwaard, D., van Veldhoven, J. P., Moretti, A., Laugwitz, K.-L., et al. (2016). A new hERG allosteric modulator rescues genetic and drug-induced long-QT syndrome phenotypes in cardiomyocytes from isogenic pairs of patient induced pluripotent stem cells. EMBO Mol. Med. 8, 1065–1081. doi: 10.15252/emmm.201606260
Saleh, M., Gabriels, J., Chang, D., Soo Kim, B., Mansoor, A., Mahmood, E., et al. (2020). Effect of chloroquine, hydroxychloroquine, and azithromycin on the corrected QT interval in patients with SARS-CoV-2 infection. Circ. Arrhythm. Electrophysiol. 13:e008662. doi: 10.1161/CIRCEP.120.008662
Sánchez-Chapula, J. A., Salinas-Stefanon, E., Torres-Jácome, J., Benavides-Haro, D. E., and Navarro-Polanco, R. A. (2001). Blockade of currents by the antimalarial drug chloroquine in feline ventricular myocytes. J. Pharmacol. Exp. Ther. 297, 437–445.
Schwartz, P. J., and Ackerman, M. J. (2013). The long QT syndrome: a transatlantic clinical approach to diagnosis and therapy. Eur. Heart J. 34, 3109–3116. doi: 10.1093/eurheartj/eht089
Schwartz, P. J., Spazzolini, C., Crotti, L., Bathen, J., Amlie, J. P., Timothy, K., et al. (2006). The Jervell and Lange-Nielsen syndrome: natural history, molecular basis, and clinical outcome. Circulation 113, 783–790. doi: 10.1161/CIRCULATIONAHA.105.592899
Schwartz, P. J., and Woosley, R. L. (2016). Predicting the unpredictable: drug-induced QT prolongation and torsades de pointes. J. Am. Coll. Cardiol. 67, 1639–1650. doi: 10.1016/j.jacc.2015.12.063
Stramba-Badiale, M., Karnad, D. R., Goulene, K. M., Panicker, G. K., Dagradi, F., Spazzolini, C., et al. (2018). For neonatal ECG screening there is no reason to relinquish old Bazett’s correction. Eur. Heart J. 39, 2888–2895. doi: 10.1093/eurheartj/ehy284
Taylor, W. R. J., and White, N. J. (2004). Antimalarial drug toxicity: a review. Drug Saf. 27, 25–61. doi: 10.2165/00002018-200427010-00003
TeBay, C., McArthur, J., Mangala, M., Kerr, N., Heitmann, S., Perry, M., et al. (2021). Comprehensive preclinical evaluation of how cardiac safety profiles of potential COVID-19 drugs are modified by disease associated factors. Authorea [Preprints] doi: 10.22541/au.161429217.72966648/v1
Thomet, U., Amuzescu, B., Knott, T., Mann, S. A., Mubagwa, K., and Radu, B. M. (2021). Assessment of proarrhythmogenic risk for chloroquine and hydroxychloroquine using the CiPA concept. Eur. J. Pharmacol. 913:174632. doi: 10.1016/j.ejphar.2021.174632
Touret, F., Gilles, M., Barral, K., Nougairède, A., van Helden, J., Decroly, E., et al. (2020). In vitro screening of a FDA approved chemical library reveals potential inhibitors of SARS-CoV-2 replication. Sci. Rep. 10:13093. doi: 10.1038/s41598-020-70143-6
Wallace, D. J., Gudsoorkar, V. S., Weisman, M. H., and Venuturupalli, S. R. (2012). New insights into mechanisms of therapeutic effects of antimalarial agents in SLE. Nat. Rev. Rheumatol. 8, 522–533. doi: 10.1038/nrrheum.2012.106
Wang, G., Lu, C.-J., Trafford, A. W., Tian, X., Flores, H. M., Maj, P., et al. (2020). Mechanistic insights into ventricular arrhythmogenesis of hydroxychloroquine and azithromycin for the treatment of COVID-19. bioRxiv [preprint] 2020.05.21.108605. doi: 10.1101/2020.05.21.108605
White, N. J., Watson, J. A., Hoglund, R. M., Chan, X. H. S., Cheah, P. Y., and Tarning, J. (2020). COVID-19 prevention and treatment: a critical analysis of chloroquine and hydroxychloroquine clinical pharmacology. PLoS Med. 17:e1003252. doi: 10.1371/journal.pmed.1003252
WHO Solidarity Trial Consortium, Pan, H., Peto, R., Henao-Restrepo, A.-M., Preziosi, M.-P., Sathiyamoorthy, V., et al. (2021). Repurposed antiviral drugs for Covid-19 - interim WHO solidarity trial results. N. Engl. J. Med. 384, 497–511. doi: 10.1056/NEJMoa2023184
Woosley, R. L., Heise, C. W., and Romero, K. A. (2021). QTdrugs List. CredibleMeds. Available online at: www.Crediblemeds.org (accessed April 20, 2021).
Yao, X., Ye, F., Zhang, M., Cui, C., Huang, B., Niu, P., et al. (2020). In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin. Infect. Dis. 71, 732–739. doi: 10.1093/cid/ciaa237
Keywords: Long QT Syndrome, COVID-19, hydroxychloroquine, induced pluripotent stem cells, safety pharmacology, precision medicine
Citation: Sala L, Leonov V, Mura M, Giannetti F, Khudiakov A, Moretti A, Crotti L, Gnecchi M and Schwartz PJ (2022) Use of hiPSC-Derived Cardiomyocytes to Rule Out Proarrhythmic Effects of Drugs: The Case of Hydroxychloroquine in COVID-19. Front. Physiol. 12:730127. doi: 10.3389/fphys.2021.730127
Received: 24 June 2021; Accepted: 31 December 2021;
Published: 27 January 2022.
Edited by:
Jose Jalife, Centro Nacional de Investigaciones Cardiovasculares (CNIC), SpainReviewed by:
Larisa G. Tereshchenko, Oregon Health and Science University, United StatesCopyright © 2022 Sala, Leonov, Mura, Giannetti, Khudiakov, Moretti, Crotti, Gnecchi and Schwartz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Luca Sala, bHVjYS5zYWxhQGF1eG9sb2dpY28uaXQ=; Peter J. Schwartz, cGV0ZXIuc2Nod2FydHpAdW5pcHYuaXQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.