- 1Department of Animal Physiology and Development, Faculty of Biology, Adam Mickiewicz University, Poznań, Poland
- 2HiProMine S.A., Robakowo, Poland
The insulin-like peptide (ILP) and insulin-like growth factor (IGF) signalling pathways play a crucial role in the regulation of metabolism, growth and development, fecundity, stress resistance, and lifespan. ILPs are encoded by multigene families that are expressed in nervous and non-nervous organs, including the midgut, salivary glands, and fat body, in a tissue- and stage-specific manner. Thus, more multidirectional and more complex control of insect metabolism can occur. ILPs are not the only factors that regulate metabolism. ILPs interact in many cross-talk interactions of different factors, for example, hormones (peptide and nonpeptide), neurotransmitters and growth factors. These interactions are observed at different levels, and three interactions appear to be the most prominent/significant: (1) coinfluence of ILPs and other factors on the same target cells, (2) influence of ILPs on synthesis/secretion of other factors regulating metabolism, and (3) regulation of activity of cells producing/secreting ILPs by various factors. For example, brain insulin-producing cells co-express sulfakinins (SKs), which are cholecystokinin-like peptides, another key regulator of metabolism, and express receptors for tachykinin-related peptides, the next peptide hormones involved in the control of metabolism. It was also shown that ILPs in Drosophila melanogaster can directly and indirectly regulate AKH. This review presents an overview of the regulatory role of insulin-like peptides in insect metabolism and how these factors interact with other players involved in its regulation.
Introduction
The insulin/insulin-like peptide signalling (ILP signalling) pathway is an old and evolutionarily conserved pathway that widely regulates metabolism throughout the whole metazoan kingdom (Jin Chan and Steiner, 2000; Fernandez and Torres-Alemán, 2012; Vitali et al., 2018; Sharma et al., 2019). ILP signalling is involved in the control of metabolism sensu stricte, as well many other aspects of life, such as growth, reproduction, lifespan, resistance to stress conditions and immune activity. All these processes are directly or indirectly connected with metabolism. The best known ligand of ILP signalling pathways is mammalian insulin, probably the most deeply studied peptide hormone, but the family of insulin peptides is much larger (Veenstra, 2020). In humans, it also includes two insulin-like growth factors (IGFs), one relaxin and a number of human insulin-like peptides (INSL3-7) (Nässel and Vanden Broeck, 2016). They are synthesized as pre-propeptides consisting of a signal peptide and contiguous B-C-A peptides. The C-peptide is removed from insulin and relaxin, whereas IGFs contain a shortened C-peptide that is not excised. Thus, insulin and relaxin are heterodimeric peptides consisting of A- and B-chains linked by two to three disulphide bridges, and IGFs are single chain peptide hormones (Grönke and Partridge, 2010). In insects, the insulin peptide family is represented by numerous insulin-like peptides (ILPs) and IGF-like growth factor peptides (IGFLPs), but their number varies significantly between different species. For example, in Locusta migratoria and Schistocerca gregaria, only one ILP was identified, while there are five in Anopheles gambiae, eight in Aedes aegypti and Drosophila melanogaster, and 38 in Bombyx mori (Nässel and Vanden Broeck, 2016; Sharma et al., 2019). The target of insulin family peptides is insulin receptors (IRs). To date, several IRs belonging to the family of tyrosine kinase receptors or to G-protein coupled receptors have been identified in mammals. For a long time, only one receptor was identified in insects, but now, additional receptors have been identified, e.g., Lgr3 in D. melanogaster (Colombani et al., 2015; Van Hiel et al., 2015). The activation of IRs switches on a cascade of intracellular signalling reactions that trigger changes in cell activity. The multiplicity of ILPs, which possess various affinities to facilitate binding to different IRs, and the production and secretion of ILPs by different tissues result in signalling via ILPs that is not simple and straightforward. Signalling is more complex if it interplays with many other hormonal and nonhormonal factors, but in this way, regulation of metabolism and its coupled processes may occur with precision and in a multidirectional manner (Figures 1, 2).
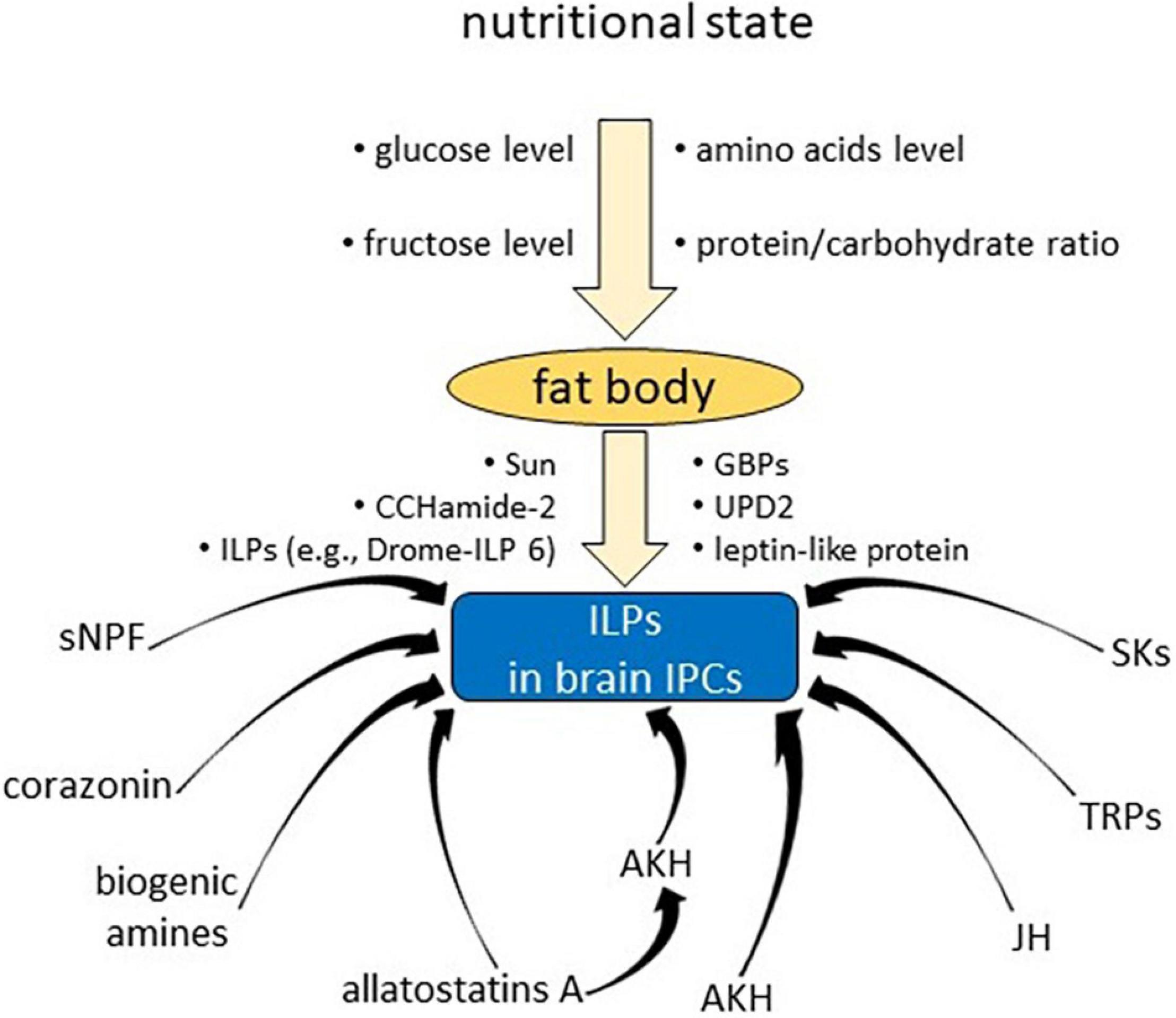
Figure 1. Examples of various factors affecting synthesis/secretion of insulin-like peptides in insulin producing cells in the brain.
In our work, we summarize knowledge how ILPs regulate the insect metabolism and known and possible interactions/cross-talk of ILPs with other agents controlling metabolic activity. In the following review, we used the nomenclature of peptide hormones according to Coast and Schooley (2011).
Genes Encoding the ILP Family
In insects, two types of insulin peptide families have been identified: insulin-like peptides (ILPs) and IGF-like growth factor peptides (IGFLPs) (Mizoguchi and Okamoto, 2013; Okamoto and Yamanaka, 2015; Fujinaga et al., 2019). Most insect ILPs are classified in the first group. ILP genes encode precursors such as vertebrate pre-proinsulin, which contain signal peptide, the B-chain, C-chain, and A-chain, starting from the N-terminus (Antonova et al., 2012; Mizoguchi and Okamoto, 2013; Okamoto and Yamanaka, 2015). The signal peptide and C-peptide are cleaved, and the mature heterodimeric peptide consists of the A-chain and B-chain, which have approximately 20 and 30 amino acids respectively, linked by disulphide bonds such as in vertebrate insulin and relaxin (Mizoguchi and Okamoto, 2013; Okamoto and Yamanaka, 2015; Nässel and Vanden Broeck, 2016). In IGF-like peptides, a short C-chain is preserved, and the peptide is a single chain with internal disulphide bonds. The number and position of cysteine residues in ILPs and IGF-like peptides are highly conserved (Mizoguchi and Okamoto, 2013; Nässel and Vanden Broeck, 2016).
The three-dimensional structures of two insect ILPs have been determined so far, Bommo-ILP2 (called bombyxin — a name used for B. mori ILPs) (Nagata et al., 1995) and Drome-ILP5 (Sajid et al., 2011). The basic folding of Bommo-ILP2 and Drome-ILP5 molecules is similar to that in mammalian insulin, distinctions in conformation and function of their C-end of the B-chains were revealed (Nagata et al., 1995; Sajid et al., 2011). It was also detected that that Drome-ILP5 has features close to mammalian insulins, as it binds and activates human IR and decreases glucose level in rats (Sajid et al., 2011).
In most insects, multiple genes encode ILPs. For example, in B. mori, approximately 38 ILP genes have been identified (Mizoguchi and Okamoto, 2013); in D. melanogaster, there are eight ILP genes (Nässel et al., 2015), as in the mosquito Ae. aegypti (Riehle et al., 2006), and four in Tribolium castaneum (Li et al., 2008), but only one ILP gene in locusts is known to date (Badisco et al., 2008; Antonova et al., 2012). In the B. mori genome, most Bommo-ILP genes are gathered in two sectors on chromosome 11 and unidentified chromosome and form gene pairs, triplets or single genes. The rest are situated singly on chromosomes 1, 9 and 11 (Aslam et al., 2011; Sharma et al., 2018). Most of these genes lack any introns; however, three of them were found to have introns and encode polypeptides similar to pre-proinsulin, while several other genes are probably pseudogenes (Aslam et al., 2011; Mizoguchi and Okamoto, 2013). In Ae. aegypti, seven of eight Aedae-ILP genes have a single intron within the C-peptide, ranging from 53 bp to at least 286000 bp. One Aedae-ILP (Aedae-ILP6) has two introns, one in the signal peptide and one in the A peptide extension (Riehle et al., 2006).
The proximity of some ILP genes in mosquitoes suggests that they form a eukaryotic operon regulated by a single promoter. In Ae. aegypti, transcription of three genes occurs in the order Aedae-ILP8-ILP1-ILP3, and all have putative poly-A-sites, suggesting that these genes are transcribed simultaneously to form polycistronic pre-mRNA, which is next processed and capped to form monocistronic mature mRNAs. It was also suggested that the ILP operon exists in A. gambiae (Riehle et al., 2006).
ILP Receptors
The ILP signalling pathway is initiated by the binding of ILPs to insulin receptors (IRs). IRs are part of the tyrosine kinase (RTK) receptor family, which contains enzymatic tyrosine kinase domains within their cytoplasmic part (Defferrari et al., 2018). In vertebrates, different distinct receptors in the IR subfamily can be distinguished (Hernández-Sánchez et al., 2008). These are the IR activated by insulin, the type 1 IGF receptor (IGF1R) activated by IGF1 or IGF2 and the type 2 IGF receptor (IGF2R) activated by IGF2. The IGF2R, as it is distinct from the others, belongs to the G protein coupled receptors (GPCRs) (Fernandez and Torres-Alemán, 2012). In invertebrates, including insects, mainly one or two IRs from the tyrosine kinase family (Defferrari et al., 2018), and two relaxin-like (LGR type C) GPCRs have been identified (Van Hiel et al., 2015). Single IRs were discovered in flies (Grönke and Partridge, 2010; Xu et al., 2015), mosquitoes (Nuss and Brown, 2018; Nuss et al., 2018), dung beetles (Lavine et al., 2013), moths (Fullbright et al., 1997), cockroaches (Abrisqueta et al., 2014) and kissing bugs (Defferrari et al., 2018), whereas two IRs were reported in honey bees (de Azevedo and Hartfelder, 2008), fire ants (Lu and Pietrantonio, 2011), brown plant hoppers (Xu et al., 2015), tenebrionid beetles (Sang et al., 2016), and aphids (Ding et al., 2017). If two receptors are present, they probably act via distinct regulatory pathways (Sang et al., 2016).
As a result of ligand stimulation, IR undergoes tyrosine phosphorylation. The IR precursor encodes A and B subunits that are connected by disulphide bridges and form a heterodimer. This dimer binds with a similar heterodimer, producing a mature and functional holoreceptor protein complex (Claeys et al., 2002). The A-chain and part of the N-terminus of the B-chain are located on the extracellular side of the plasma membrane. The remainder of the B-chain includes a single transmembrane helix, the juxtamembrane domain, and the intracellular tyrosine kinase domain. Individual subdomains that build the extracellular domain are characterized as leucine-rich, cysteine-rich and fibronectin type III domains (Scapin et al., 2018). Ligand binding specificity depends on cysteine-rich regions in the A subunits, whereas B subunits perform the tyrosine kinase activity mediating the ILP signal to downstream signalling proteins. Therefore, ILP binding is localized at the extracellular side, while interaction with downstream signalling factors occurs at the cytoplasmic side of the membrane receptor (Claeys et al., 2002). Among insects, the highest structural homology was observed in the tyrosine kinase domain (Defferrari et al., 2018).
Upon binding of insulin to its receptor, autophosphorylation of the intracellular IR subunits occurs due to the activation of their intrinsic tyrosine kinase activity. IR employs a group of adaptor molecules, known as insulin receptor substrates (IRSs), to initiate its signalling pathway (White, 1998). The interaction of IRS with the tyrosine-phosphorylated cytoplasmic tail of IR results in stimulation of the molecule by specific tyrosine residues phosphorylation in IRS molecule. Numerous tyrosine phosphorylation sites on IRS anchor molecules containing domains with Src-homology (SH2) (White, 1998). Two very important SH2-domain proteins can react with IRS, namely, Grb2 (growth factor receptor bound protein-2) and PI3K (phosphatidylinositol-3-OH kinase) (Claeys et al., 2002). Thus, IR stimulation typically activates two main intracellular pathways: the PI3K/Akt/FOXO (phosphatidylinositol-3 kinase/protein kinase B/Fork head box transcription factor) cascade and the Ras/MAPK pathway (mitogen-activated protein kinase) (Defferrari et al., 2018). The PI3K/Akt pathway regulates processes involving carbohydrates (mainly glucose) uptake and metabolism, whereas the IR-dependent MAPK pathway regulates mitogenic and cell cycle responses and is involved in control of development and reproduction (Sang et al., 2016).
The analysis of receptor or receptor expression profiles showed that in most insect species, IR is present in almost all tissues, with the highest expression level in the central nervous system and ovaries of different insect species (Defferrari et al., 2018). Moreover, in D. melanogaster, the receptor was found in imaginal discs and embryos (Garofalo and Rosen, 1988).
Developmental expression analysis showed that the highest expression of IRs was observed in adults; however, in all developmental stages (egg, larva, and pupa), a differentiated expression pattern was observed (Xu et al., 2015; Sang et al., 2016). In insects with two ILPRs, it was shown that both receptors might have different physiological significance (Lu and Pietrantonio, 2011; Sang et al., 2016) and that they functionally diverged between social and non-social insects (Sang et al., 2016).
G protein coupled receptors involved in ILP signalling thus far have been found only in D. melanogaster (Vallejo et al., 2015; Van Hiel et al., 2015). They belong to the GPCR subgroup, which contains leucine-rich repeats (LGRs), and are designated as type C (Van Hiel et al., 2015). This type of receptor also contains a very large N-terminal extracellular domain with multiple leucine-rich repeat motifs (LRRs) flanked by a cysteine-rich region and low-density lipoprotein receptor domain class A (LDLa), which are important for cyclic AMP signalling (Van Hiel et al., 2015). These receptors displayed high expression during development with higher expression levels in adult males (Van Hiel et al., 2015).
Cells Producing ILPs
Insect ILPs are mainly considered neurohormonal agents regulating life processes; thus, their expression, synthesis and secretion are studied in the context of the nervous system. Nevertheless, their expression is widespread within whole organisms. The nervous system was the first tissue where insulin-like peptide-producing cells (IPCs) were identified. Mizoguchi et al. (1987) found four pairs of large mid-dorsal neurosecretory cells of the brain and nerve fibres located peripherally to the corpora allata (CA) in B. mori, which was confirmed by Ichikawa (1991). These cells were medial neurosecretory cells (MNCs) with axons that terminated in the CA (Mizoguchi and Okamoto, 2013). In B. mori, ILPs are synthesized mainly in the brain but they are produced also in numerous other tissues at low level (Iwami et al., 1996). However, only in brain, ILP genes are expressed during whole B. mori development from the embryonic to adult stages. ILPs were also detected in the ganglia, epidermis, testis, ovary, fat body, silk gland, Malpighian tubule, midgut, and hindgut but with different stage- and age-dependent patterns of ILP gene expression (Iwami, 2000). A similar situation occurs in D. melanogaster, where ILPs are synthetized mainly by the group of MSCs in the pars intercerebralis of the brain (Nässel, 2012; Nässel and Vanden Broeck, 2016), and the secretion of ILPs occurs from axons terminated in neurohaemal areas in the corpora cardiaca (CC), anterior aorta, and foregut (Brogiolo et al., 2001; Cao and Brown, 2001; Rulifson et al., 2002). However, as suggested by Nässel (2012), probably, Drome-ILPs might be secreted into the circulation from neurohaemal sites as well as in a paracrine way from branches within the brain. The crucial role of MSCs in ILP signalling in metabolism regulation has been shown by genetic cell ablation experiments (Rulifson et al., 2002; Broughton et al., 2005). Ablated larvae showed increased haemolymph sugar levels (Rulifson et al., 2002) and increased storage of lipids and carbohydrates (Broughton et al., 2005). In some insect species, other brain cells — lateral neurosecretory cells — are also involved in ILP production, for example, in Ae. aegypti mosquitos (Cao and Brown, 2001; Riehle et al., 2006) and Anopheles stepensi mosquios (Marquez et al., 2011) and in the hemipteran bug Rhodnius prolixus (Vafopoulou and Steel, 2012). On the other hand, IGF-like ILPs, such as Drome-ILP6 in D. melanogaster or Bomme-IGFLP in B. mori, are example of ILPS mainly synthetized by the fat body (Okamoto et al., 2009a, b; Mizoguchi and Okamoto, 2013). In Table 1, examples of ILPs expressed in different tissues/cells depending on the developmental stage are presented.

Table 1. Examples of ILPs expressed in different tissues/cells depending on the developmental stage.
Factors Regulating ILP Production
Nutritional signals have been shown to be the most important factors that affect ILPs release from brain IPCs. It was demonstrated that Bommo-ILP is released from the brain of B. mori in response to availability of glucose, which is a widespread nutritional signal for releasing ILPs (Masumura et al., 2000; Mizoguchi and Okamoto, 2013). The Bommo-ILPs level in the brain decreased within 1 h after glucose injection into starved larvae of B. mori in a dose-dependent manner. It has also been demonstrated that the release of Drome-ILPs from IPCs of D. melanogaster is controlled by cell autonomous glucose sensing, comparable to mammalian pancreatic beta cells (Park et al., 2014). It was evidenced that in the glucose sensing of IPCs the glucose transporters, KATP channels and voltage-sensitive Ca2+ channels are involved as well (Fridell et al., 2009; Park et al., 2014; Nässel et al., 2015).
However, in D. melanogaster, the availability of nutrients is detected remotely by the fat body, which controls Drome-ILP releasing through humoral factors (Géminard et al., 2009; Bai et al., 2012; Rajan and Perrimon, 2012; Mizoguchi and Okamoto, 2013), and then amino acids instead of glucose become the crucial nutritional signal in the diet (Géminard et al., 2009). Amino acids do not directly impact the IPCs but rather they affect target of rapamycin (TOR) signalling pathway in fat body cells to regulate Drome-ILP release (Géminard et al., 2009). It was shown that amino acids deficit or inhibition of the TOR signalling pathway, both targeted in adipocytes, are enough to provoke Drome-ILP inhibition in IPCs and that Drome-ILP secretion is controlled by a direct humoral link between the fat body tissue and the brain (Géminard et al., 2009). It was also shown that changes in activation of the TOR signalling pathway in gut stem and/or progenitor cells caused the changes in Drome-ILP mRNA (Strilbytska et al., 2017a, b; Semaniuk et al., 2021b).
It has been detected in that dilp expression is affected also by the protein to carbohydrate ratio in the Drosophila diet and the interaction between this ratio and caloric content. For example, dilp2 expression was the highest upon ingestion of diets with a low protein to carbohydrate ratio regardless of the total caloric value. dilp5 expression increased at an approximately 1:2 protein to carbohydrate ratio and with caloric value of the diet (Post and Tatar, 2016; Semaniuk et al., 2021b).
Among detected nutrient signals that influence IPCs are unidentified factors discharged from the larval fat body cells in reaction to raised level of circulating amino acids (Géminard et al., 2009); leptin-like proteins secreted from the fat body after food intake in adults and affecting the GABAergic neurons in the brain (Rajan and Perrimon, 2012); and the brain cells that express a gustatory receptor (Gr43a) and respond to higher levels of fructose (Miyamoto and Amrein, 2014). Another fat body-derived diffusible molecule that controls the production and release of ILPs in adult Drosophila flies is Drome-ILP6. IGF-like Drome-ILP6 regulates carbohydrate and lipid storage, and its nutrient-dependent production is controlled by the FOXO transcription factor, which upregulated dilp6 transcript level in the fat body (Slaidina et al., 2009; Bai et al., 2012). Drome-ILP6 produced by Drosophila fat body cells suppresses dipl2 and dilp5 mRNA in the brain, as well as the Drome-ILP2 release from IPCs (Bai et al., 2012). In adult flies, the activity of ICPs is regulated by the fat body cells not only via Drome-ILP6, but also by the leptin-like cytokine Unpaired 2 (Upd2) (Bai et al., 2012; Rajan and Perrimon, 2012). This probably occurs with mediation of the GABAergic system in the pars intercerebralis, which appears to be inactivated by circulating Upd2 after food intake; for that reason, tonic inhibition of the IPCs is raised (via activation of the Jak/Stat signalling pathway), which facilitates Drome-ILP secretion (Rajan and Perrimon, 2012). Besides, in response to dietary amino acids, two factors released from the fat body have been detected in Drosophila: Stunted (Sun) (Delanoue et al., 2016) and growth-blocking peptides (GBPs) (Koyama and Mirth, 2016). Sun is a circulating insulinotropic peptide released by adipose tissue and acts as a ligand for Methuselah (Mth), a secretin-incretin receptor subfamily member on IPCs, inducing the secretion of ILPs and promoting organ growth. GBPs are produced in the adipocytes in reaction to amino acids and activation of TOR signalling. GBPs stimulate Drome-ILP secretion from IPCs, which results in elevated ILP signalling activity in the body cells to promote body growth.
Furthermore, the results obtained by Alfa et al. (2015) showed the presence of an orphan GPCR in IPCs, which is a limnostatin receptor whose activation by limnostatin suppresses ILPs secretion from ICPs following starvation in Drosophila. The regulation of Drome-ILP2 and Drome-ILP5 synthesis by IPCs is probably also mediated by dSir2 (the Drosophila homologue of mammalian histone deacetylase SIRT1), but independent of the FOXO transcription factor (Kannan and Fridell, 2013), and by dCbl (Casitas B-lineage lymphoma), a member of the Drosophila E3 ubiquitin ligases and adaptor proteins, which downregulates the transcript level of brain dilp genes (Yu et al., 2012).
The ILPs expression and secretion undergoes regulation not only by ingested food but also, as was shown by Lushchak et al. (2015), by odor. These authors demonstrated that exposition of D. melanogaster to vinegar odor induces increased expression of dilp2, dilp3 and dilp5 in the brain IPCs. Moreover, they also observed increased expression of dipl6 and upd2 (Lushchak et al., 2015).
Furthermore, it was proven that IPCs also receive regulatory signals from direct neuronal input as well as from hormonal factors (Figure 1; Antonova et al., 2012; Nässel and Vanden Broeck, 2016; Semaniuk et al., 2021b).
Hormones
Corazonin and sNPF
In adult Drosophila, short neuropeptide F (sNPF) and corazonin (CRZ) are expressed in a bilateral set of neurons, the so-called dorsal lateral peptidergic neurons (DLPs), localized in the pars lateralis. These sNPF-expressing nervous cells have axon terminations impinging on IPCs (Kapan et al., 2012). IPCs express the sNPF receptor 1 (sNPFR1) and probably the corazonin receptor (Crz-R) (Kapan et al., 2012). It was suggested that sNPF secreted from DLPs targets IPCs to elevate production and most likely also the secretion of Drome-ILPs, since knockdown of snpf, but not crz, in DLPs decreased the levels of mRNA for dilp2 and dilp5 in the brain. It was also shown that knockdown of either snpf or crz in DLPs prolongs survival in starved flies and changes carbohydrate and lipid metabolism, which suggests that CRZ and sNPF act via different mechanisms (Lee et al., 2008; Kapan et al., 2012).
Tachykinin-Related Peptides
In Drosophila, receptors for tachykinin-related peptides (TRPs) are expressed by IPCs, and TRP knockdown significantly affects the mRNA levels of dilp2 and dilp3 but not dilp5 in the brains of fed and exposed to starvation flies. dilp2 and dilp3 mRNA levels were elevated in fed flies, but in starved flies, dilp2 was upregulated and dilp3 was downregulated (Birse et al., 2011).
Allatostatin A
Another neuropeptide engaged in ILP signalling in Drosophila is allatostatin A (AstA). It was also shown that allotostatin A regulates AKH signalling. Expression of the AstA receptor gene Dar2 was detected in the insulin- and AKH-producing cells (Hentze et al., 2015). Knockdown of Dar2 in IPCs and AKH-producing cells (APCs) resulted in modification of expression of several genes that indicate decreased ILPs or AKH signalling. It was suggested that AstA regulates the balance between Drome-ILPs and AKH, which is believed thought to be essential to maintain the nutrient homeostasis in reaction to alternations of sugar and protein ratios in the diet (Hentze et al., 2015; Nässel and Vanden Broeck, 2016).
CCHamide-2
The CCHamide-2 (CCHa2) neuropeptide regulates IPCs and Drome-ILPs in a nutrition-dependent manner (Sano et al., 2015), and its transcription is altered, particularly in response to glucose levels. CCHa2 is produced primarily in the adipocytes and gut and directly stimulates its receptor (CCHa2R) in IPCs in the larval brain (Sano et al., 2015). In D. melanogaster mutants of both CCHa2 and CCHa2-R, the transcription of dilp5 and the secretion of both Drome-ILP2 and Drome-ILP5 were reduced, and growth during larval stages was severely retarded (Sano et al., 2015).
Octopamine and Serotonin
In IPCs of Drosophila, the expression of two monoamine receptors, the octopamine receptor OAMB and the serotonin receptor 5-HT1A, has been detected (Luo et al., 2014). Knockdown of the OAMB receptor resulted in increased dilp3 expression in the brain, whereas 5-HT1A knockdown led to elevated transcript levels of dilp2 and dilp5 (Luo et al., 2014).
Dopamine
It was shown that dopamine stimulates its receptor DopR1 which is expressed in IPCs (Andreatta et al., 2018) and in female D. melanogaster promotes ovarian dormancy (Andreatta et al., 2018; Ahmad et al., 2020). Under normal, nondormancy conditions, Drome-ILP2 and Drome-ILP5 and juvenile hormone (JH) control ovarian growth and reproduction in females, and serotonin and dopamine signalling in IPCs, CA and fat body are diminished, and the dormancy is inhibited. In contrast, under dormancy-inducing conditions (e.g., low temperature), serotonin and dopamine restrain the production and/or release of Drome-ILPs in IPCs, triggering reduction of systemic ILP signalling (and JH signalling) and thus favouring a shift into the dormancy state (Andreatta et al., 2018).
Juvenile Hormone
Juvenile hormone and 20-hydroxyecdysone (20E) were shown to influence ilp gene expression in reproducing female Ae. aegypti mosquitoes. JH and 20E modulate the production and secretion of all eight Aedae-ILPs, restricting them to appropriate amounts required during the posteclosion and postblood-meal phases of the mosquito reproductive cycle. It was shown that the JH and 20E pathways act differentially in determining the expression of ilp genes. This is achieved through a direct physical interaction of JH and 20E pathway factors with promoters of ilp genes. ilp2, ilp6 and ilp7 are positively regulated by the JH pathway. In contrast, 20E pathway factors inhibit the expression of ilp2 and ilp6 genes directly interacting with their promoters. This situation is reversed in the regulation of ilp4 and ilp5 gene expression, in which genes are downregulated by the JH pathway factor and upregulated by the 20E pathway factor. It was also found that Met, a transcription factor identified as the JH receptor, provokes fat body ilp6 expression by direct binding to this ilp gene promoter (Ling and Raikhel, 2021). Furthermore, JH elicits the expression of Trica-ILP2 and Trica-ILP3 in female T. castaneum and controls the expression of vg genes through the insulin pathway (Sheng et al., 2011). In Apis mellifera, JH works throughout Apime-ILP1 and controls metabolism of carbohydrates during the transition of worker bees from nursing to foraging (Wang et al., 2013). In B. mori, in vitro studies showed that secretion of the peptide Bomme-IGFLP from the fat body is stimulated by 20-hydroxyecdysone (20E), since both bigflp gene (encoding Bommo-IGFLP) expression and secretion of Bommo-IGFLP were significantly elevated by the supplementation of the adipocytes culture with 20E (Okamoto et al., 2009a). The expression of dilp6 in the adipose tissue of D. melanogaster is also triggered in vitro by 20E (Okamoto et al., 2009a, b; Slaidina et al., 2009; Mizoguchi and Okamoto, 2013). However, Bomme-IGFLP expression in the brain was not elicited by 20E (Okamoto et al., 2011), which indicates that the mechanisms regulating ILP and IGFLP gene expression vary in different tissues. Starvation also caused upregulation of the dilp6 expression in the larval adipocytes, through direct induction by the FOXO transcription factor, what was independent of 20E (Slaidina et al., 2009; Mizoguchi and Okamoto, 2013). Furthermore, FOXO-inducible dilp6 expression was detected in the adult fat body (Bai et al., 2012).
Other Factors
Other factors that have been demonstrated to control ILP expression in the brain and peripheral tissues in insects are microRNAs (miRNAs) expressed in IPCs. In Ae. aegypti, for lack of microRNA-277 (miR277), the mRNA levels of ilp7 and ilp8 were elevated in the head, while the mRNA levels of ilp1 and ilp3 transcript did not change, what suggests that miR277 targets the first member (ilp8) of the ilp8-ilp1-ilp3 operon (Ling et al., 2017; Sharma et al., 2019). Genetic disruption of miR-277 led to impairment of lipid storage and development of ovaries development, suggesting that miR-277 acts as an essential factor in lipid metabolism and reproduction by targeting ilp7 and ilp8 and regulating the mRNA levels of these genes (Ling et al., 2017). In D. melanogaster, different microRNAs have also been found to control the production of Drome-ILPs in direct or indirect ways. miR-14 acts in IPCs in the adult D. melanogaster brain, targeting gene sugarbabe, which encodes a predicted zinc finger protein that controls dilp gene expression in IPCs. It was also shown that removing miR-14 reduces dilp3, dilp5 and dilp2 transcript levels (Varghese et al., 2010). miR-9a has also been detected in D. melanogaster IPCs. Upregulation of miR-9a specifically in IPCs decreases ILP signalling and body size. miR-9a has been found to bind to sNPFR1 mRNA in insect cells, suggesting its role in controlling body growth by regulating sNPFR1, which modulates ILP signalling (Suh et al., 2015). miR-278, expressed predominantly in the fat body, was shown to be involved in controlling of energy homeostasis in D. melanogaster by regulating insulin responsiveness (Teleman et al., 2006). Another fat body microRNA, miR-8, acts as a regulator of ILP-signalling dilp6, and imaginal morphogenesis protein-late2 (Imp-L2), a Drome-ILP binding protein, was detected to be upregulated in the adipose tissue of miR-8 null mutant Drosophila larvae (Lee and Hyun, 2014).
Regulation of Hormone Synthesis by ILPs
The participation of ILPs in the integration of metabolism and energy utilization also involves mediating the synthesis and release of various hormones with similar or antagonistic features (Nässel and Vanden Broeck, 2016). For example, ILPs are part of a complex relationship network that leads to the control of secretion from CA and adipocytes in fat body tissue via two insect hormones: JH and 20E (Nässel and Vanden Broeck, 2016). JH might be a product of direct (ILP may stimulate CA for JH synthesis) or indirect (synthesis of JH is under neuropeptide control, and ILPs might affect the neuropeptidogenic or somatic tissues) ILP activity (Tatar et al., 2003). On the other hand, ILPs might act directly on the ovary, where they take part in ovarian ecdysteroidogenesis (Tatar et al., 2001; Nässel and Vanden Broeck, 2016). ILPs are also suspected to engage in feedback with other neuropeptide pathways, such as SKs and NPFs (Wu et al., 2005; Lingo et al., 2007; Söderberg et al., 2012; Badisco et al., 2013). Furthermore, it is believed that together with AKH, ILPs work as counterparts of glucagon and insulin loops (Birse et al., 2011). ILPs stimulate carbohydrate uptake, which causes a reduction in trehalose levels in the haemolymph, while AKH increases trehalose levels by glycolysis stimulation but elicits little or no effect on glucose levels (Birse et al., 2011; Kim and Neufeld, 2015). Research shown that in Drosophila, Akh mRNA and AKH peptide are elevated in dilp2 mutants although not in dipl1 mutants or dipl1-dilp2 double mutants, which suggests that dilp2 epistatically downstream expression of dilp1 what is required for dilp2 to modulate AKH. Thus, it was proposed that Drome-ILP2 indirectly modulate AKH by reducing dilp1 gene expression, while Drome-ILP1 otherwise activating AKH (Post et al., 2019). Notably, insulin-degrading enzyme (IDE) is also present in IPCs (Nässel and Vanden Broeck, 2016). IDE knockdown was shown to cause a reduction in carbohydrate levels in the haemolymph and increases fecundity and lifespan (Nässel and Vanden Broeck, 2016).
Metabolic Processes Regulated by ILPs
Food Intake
The neuroendocrine regulation of food intake in insects, as in other animals, is very complex (Nässel and Zandawala, 2019). It relies on the interplay of different neuropeptides, including ILPs (Nässel and Zandawala, 2020). The major neuropeptides acting as satiety and hunger signals are TKs, NPF, sNPF, SKs, AstA, hugin, leukokinins (LKs) and Upd1 (Nässel and Zandawala, 2020). The exact role of ILPs in this system is also quite complex and far from completely understood. Contrasting results regarding the role of ILPs in food intake regulation have been obtained. In general, ILPs have been shown to negatively regulate feeding and thus act as satiety signals in D. melanogaster (Söderberg et al., 2012; Pool and Scott, 2014; Semaniuk et al., 2021a). Semaniuk et al. (2021a) showed that D. melanogaster flies with knockout of different dilps ingested larger amount of carbohydrates. On the other hand, it was demonstrated that in D. melanogaster with the knockdown of insulin receptor in the progenitor and stem cells of the gut, the feeding activity was lowered as well as the glycogen and glucose content in the body (Strilbytska et al., 2020). Recent studies showed that also during starvation, blocking ILP signalling led to reduced feeding, whereas overexpression of ILP genes enhanced this process (Sudhakar et al., 2020). This shows that ILPs might be orexigenic during short periods of starvation and during extended starvation (Sudhakar et al., 2020). Moreover, upon feeding, satiety signals such as Drome-ILPs and other neuropeptides are released to terminate meal uptake (Nässel and Zandawala, 2020). Clearly, further detailed studies are needed to unravel the exact role of ILPs in this process.
In feeding regulation, ILPs interact with other neuroendocrine signals, such as SKs and sNPFs (Nässel and Zandawala, 2020). It was recently shown that ILPs form a positive feedback loop with sNPF during a short period of food deprivation. sNPF stimulates IPCs to produce ILPs, which in turn promote snpf gene expression (Sudhakar et al., 2020). sNPFs were previously shown to stimulate food intake (Fadda et al., 2019), in agreement with these studies. IPCs in D. melanogaster were shown to express, in addition to ILPs, also SKs (Söderberg et al., 2012). SKs are a satiety signal in various insects (Audsley and Weaver, 2009). Thus far, a clear mode of cooperation of SK and ILPs has not been elucidated. It was shown that knockdown of either neuropeptide affects the transcript levels of the other, suggesting possible feedback regulatory mechanism between the peptides (Söderberg et al., 2012). Recently, it was also shown that SKs influence the ILP level in the haemolymph in the Tenebrio molitor beetle, which affects circulating carbohydrate levels (Słocińska et al., 2020).
Digestion
Brain-originated ILPs directly stimulate digestion in the gut, thus they provide the nutrients used by female Ae. aegypti during egg production (Gulia-Nuss et al., 2011). Aedae-ILP3 directly stimulated late phase trypsin-like gene expression in blood-fed females. In vivo assays showed that Aedae-ILP3 return digestion to typical level in decapitated females. Moreover, in vivo knockdown of IR in Ae. aegypti retarded but did not fully excluded late phase trypsin-like gene expression and its activity in the gut as well as ecdysteroid production by ovaries and vitellogenin expression by the adipose tissue. It was also shown that amino acids do not induce the expression of late phase trypsin-like genes in the gut, but they significantly increase the ability of Aedae-ILP3 to direct stimulation of late phase trypsin-like gene expression. This indicates that ILPs released by neurosecretory cells in the brain after blood intake act as main regulators of metabolism, growth and reproduction, synchronizing blood meal digestion and amino acid availability with the production of ecdysteroids by ovaries to maximize vitellogenin expression by the fat body in Ae. aegypti (Gulia-Nuss et al., 2011).
In another mosquito species, A. gambiae, a mixture of albumin and amino acids (artificial blood) rapidly triggered the transcription of two ilps genes: ilp3 and ilp4, in the brains of starved mosquitoes, and the transcripts levels were higher than in mosquitoes fed with sucrose (Arsic and Guerin, 2008; Sharma et al., 2019). In A. stephensi, the transcription of ilps genes did not change significantly with age or after sugar or blood meal (Marquez et al., 2011; Sharma et al., 2019), which suggests differences in mosquito species.
It was also proven that ablation of IPCs in the brain or reduction of dilp gene expression reduced the expression of the target of brain insulin (tobi) gene, which encodes a highly conserved α-1,4-glucosidase in the gut and fat body in D. melanogaster (Buch et al., 2008). tobi expression was dependent on diet, as it is higher after protein ingestion and decreased after sugar meal. After IPCs ablation, diet no longer had any impact: tobi was repressed regardless of the nutrients in the meal. This pattern of the opposing regulation of tobi by protein and sugar from the diet is reminiscent of the glucagon system in mammals. It was also shown that tobi expression was totally inhibited, when the neuroendocrine cells producing AKH, an analogue of glucagon, were ablated. tobi is a target of the insulin- and glucagon-like signalling system that reacts in the opposite way to proteins and sugars in the diet (Buch et al., 2008).
Energy Homeostasis in Trophic Tissues
The major storage forms of carbohydrates in many insects are trehalose present in the haemolymph and glycogen stored in the fat body, but when energy demand increases, insects start to utilize lipids and amino acids. In some insect species, proline might be a main source of energy (Teulier et al., 2016).
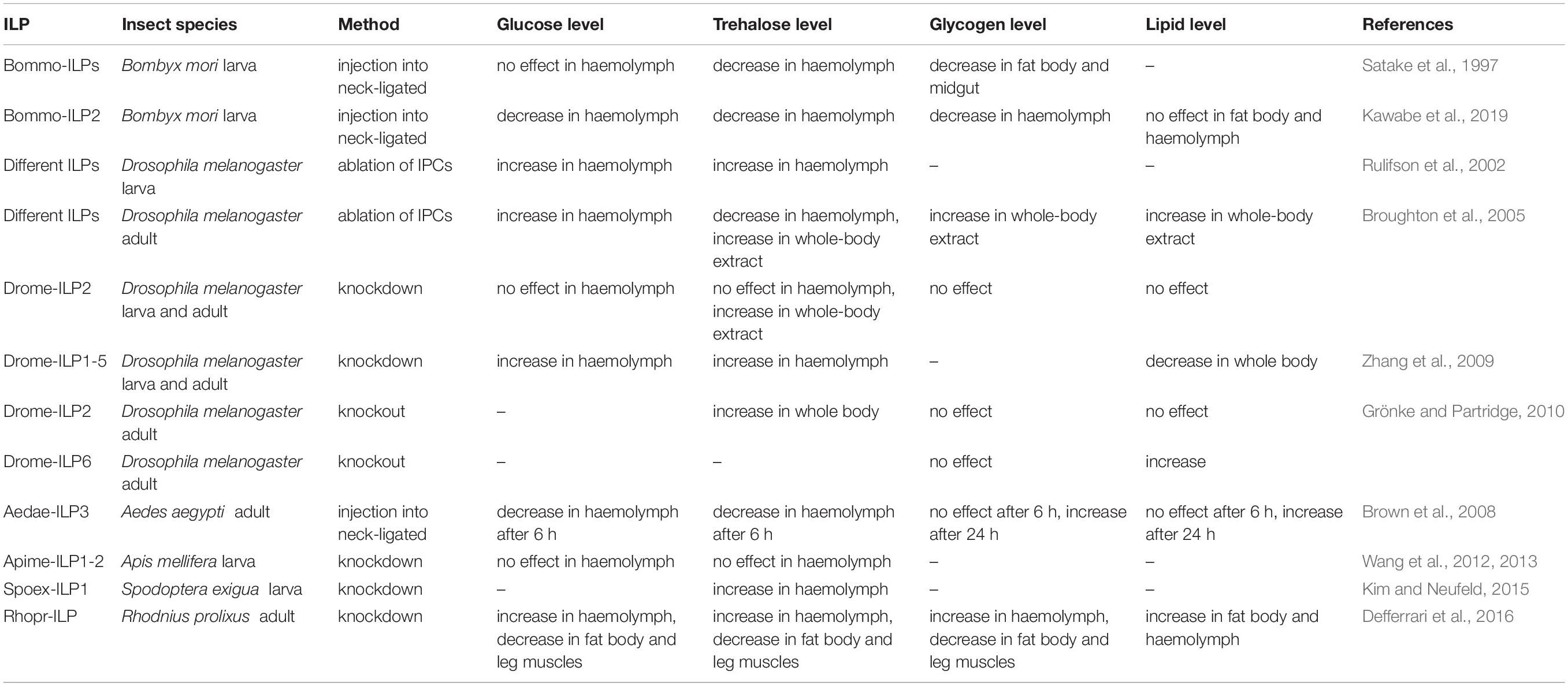
The first study investigating the impact of insect ILPs on carbohydrate metabolism included Bommo-ILPs isolated from the silkworm B. mori (Nagasawa et al., 1984). It was shown that injection of Bommo-ILP2 into neck-ligated B. mori larvae reduced the amount of one of the main haemolymph carbohydrates, trehalose, in a dose-dependent manner (Satake et al., 1997). However, the hypotrehalosemic effect of Bommo-ILP2 may be only larval stage-specific because the injection of this peptide into adult B. mori did not result in hypotrehalosemia (Satake et al., 1997; Mizoguchi and Okamoto, 2013). ILPs are thought to regulate circulating trehalose levels. But this activity relates to regulation of the trehalase activity via different molecular mechanisms which are species- and developmental-specific (Satake et al., 1997; Broughton et al., 2008; Mizoguchi and Okamoto, 2013). Research by Satake et al. (1997) and Satake et al. (1999) showed that Bommo-ILPs increased trehalase activity in the muscles of B. mori, which caused a decrease in the haemolymph trehalose level. Endogenous ILPs were able to activate fat body trehalase in vitro through a direct molecular interaction in T. molitor (Bounias et al., 1993). In T. castaneum, in the regulation of trehalase gene expression by JH, the Trica-ILP2 and ILP signalling pathways are involved. In this beetle, the ILPs role in the controlling of trehalose level is explained via regulation of expression of the gene encoding trehalase as well as it might concern influence on trehalose biosynthesis or on trehalose transporter activity (Xu et al., 2013). Lowered level of trehalase in the fat body was observed when the JH level was reduced or because of its action or when ilp2 gene expression increased. Moreover, decreased JH level and its action lowered the amount of trehalose transporter (TRET) in the gut which increase availability of trehalose in haemolymph (Xu et al., 2013).
It was also shown that Brommo-ILP2 decreased the glycogen content in the fat body and midgut and also increased the amount of active form of glycogen phosphorylase in the fat body, but any effect of Bommo-ILP2 injection on the level of glucose in haemolymph was observed compared to the control (Satake et al., 1997). A recent study showed that Bommo-ILPs facilitate cellular energy production but have no effects on lipid content in the haemolymph and fat body in B. mori larvae. Carbohydrates are probably not converted to lipids because their level is not affected by this ILP (Kawabe et al., 2019). Reduction in the trehalose concentration in the haemolymph and glycogen content in some tissues, e.g., muscles, after Bommo-ILP injection can be the result of their increased consumption for cellular energy production (Kawabe et al., 2019). Moreover, particular ILPs affect the trehalose and glucose level in haemolymph in more or less diet-dependent manner. For example, the level of trehalose in haemolymph of D. melanogaster dilp5 mutants did not change depending on diet whereas in dilp3 mutants the composition of diet strongly affected this parameter. Similar effects were observed in case of glycogen content in fly bodies. In dilp2 mutants the glycogen amount in fly body was significantly lower than in wild type, but it did not depend on diet composition what was observed in dilp3 and dilp7 mutants (Semaniuk et al., 2018).
Apart from the injection of ILPs into the insect body, the ablation of IPCs in the brain and knockout of genes encoding ILPs were applied in ILP studies. Studies have demonstrated that the ablation of IPCs in the brain of D. melanogaster causes elevated carbohydrate levels (trehalose and glucose) in the haemolymph of larvae (Rulifson et al., 2002) and elevated glucose (Broughton et al., 2005) and trehalose levels (Belgacem and Martin, 2006) in adult flies compared to the control. In addition, ablation leads to increased storage of lipids and carbohydrates. Therefore, the ablation of IPCs in the brain, which produces three Drome-ILPs, alters lipid and carbohydrate metabolism and generally causes lowered systemic ILP signalling. This study indicates that one or more Drome-ILPs, Drome-ILP2, Drome-ILP3, or Drome-ILP5, are required to stimulate glucose uptake (Broughton et al., 2005). To identify the functions of particular Drome-ILPs, many studies have been conducted using knockdown methods. Subsequent research suggested that Drome-ILP2 may solely regulate the total trehalose content because knockdown of dilp2 alone in D. melanogaster increases total trehalose correspondingly with IPC ablation but not haemolymph carbohydrate or stored glycogen levels (Broughton et al., 2008). Similar results were obtained by Grönke and Partridge (2010), in their study, and among the mutants for all 7 dilp genes in D. melanogaster, only dilp2 mutants had increased whole-body trehalose level, which suggests that it is specifically regulated by Drome-ILP2 (Grönke and Partridge, 2010). Of all the single mutants, only dilp6 mutants had slightly increased lipid levels compared to the control. Generally, knockout mutations show synergy and compensation of expression between different Drome-ILPs (Grönke and Partridge, 2010). Deletion of dilps1-5 reduces metabolic activity and decreases triacylglycerol (TAG) levels in larvae and adults of D. melanogaster. It also elevates circulating sugar levels but less so than in IPC-ablated insects, which suggests that other signals can also impact that regulation. Deletion of dilps6-7 does not lead to major metabolic defects. Most likely, dilp6 is not required for metabolic regulation in Drosophila larvae. Interestingly, insects with deletion of dilps1-5 appear relatively resistant to negative impacts of persistent hyperglycaemia (Zhang et al., 2009). In honey bee larvae, ILPs called Apime-ILP1 and Apime-ILP2 were thought to regulate energy metabolism (Wang et al., 2012); however, another study showed that neither glucose nor trehalose haemolymph concentrations were influenced by these peptides (Wang et al., 2013). In contrast, knockdown of the gene encoding Spoex-ILP1, the first reported ILP gene in the beet armyworm Spodoptera exigua, induced a significant, sevenfold increase in haemolymph trehalose levels compared to the control (Kim and Neufeld, 2015).
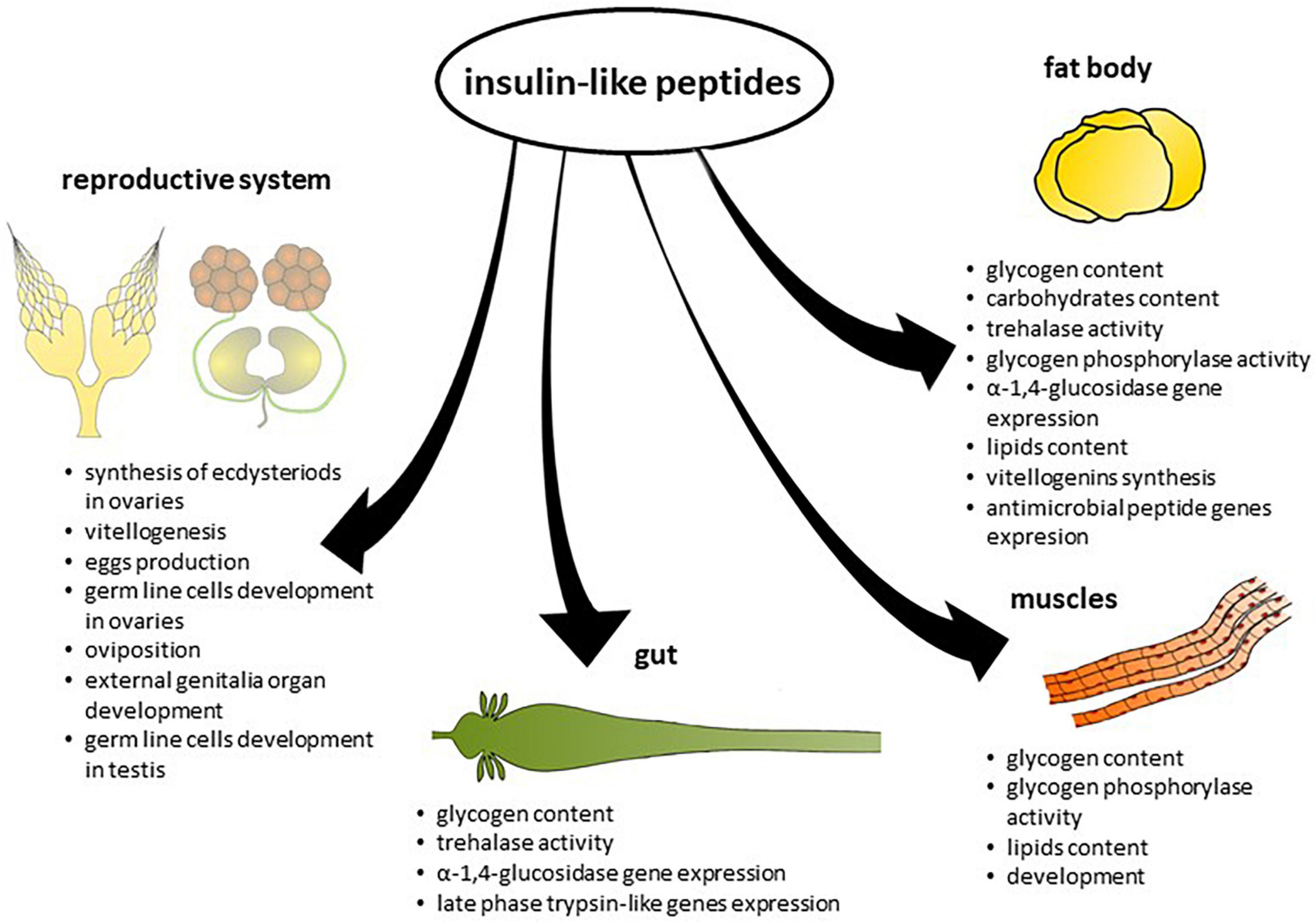
However, in some insect species, ILPs increase the amount of energy reserves. In the mosquito Ae. aegypti, Aedae-ILP3 reduced circulating sugars 6 h after injection, but it also elevated carbohydrate and lipid storage 24 h after injection (Brown et al., 2008). Knockdown of the gene encoding Rhopr-ILP, the first ILP identified in R. prolixus, resulted in an increase in carbohydrate and lipid levels in the haemolymph and decreased carbohydrate content in the fat body and leg muscles. These insects exhibited increased lipid content in the fat body and larger lipid droplets compared to the control (Defferrari et al., 2016). Thus, the effects of ILPs on insect metabolism differ between species. Insects feed with long intervals between meals; for example, R. prolixus and Ae. aegypti may have evolved mechanisms to facilitate their conversion of excess carbohydrates to glycogen or lipid reserves (Kawabe et al., 2019). The effects of ILPs on the level of energy substrates in insect tissues are presented in Table 2 and on Figure 2.
Muscle Metabolism
Insulin in vertebrates is considered as an anabolic hormone. This is related to the fact that this hormone participates in the synthesis of carbohydrates, fat, and proteins (Dimitriadis et al., 2011). Additionally, as we mentioned previously, insulin is highly involved in energy homeostasis, inter alia, by increasing the rate of glycolysis in muscles by stimulating hexokinase and 6-phosphofructokinase activity and stimulating glycogen synthesis (Dimitriadis et al., 2011). Recent reports concerning the role of ILPs in the regulation of the functioning of insect muscles showed that, similar to vertebrate insulin, these neuropeptides are also important for the regulation of growth and ageing, and the control of energy storage in the muscles (Bai et al., 2013; Bretscher and O’Connor, 2020).
Proper growth of muscles is dependent on ILP signalling. Research by Demontis and Perrimon (2009) and Kim and O’Connor (2021) showed that different components of ILP signalling may be important for the morphological properties (width, thickness, length) and ploidy of insect muscles. The results of these studies showed that the lack of ILP-dependent inhibition of the transcription factor FOXO led to a decrease in the size of D. melanogaster muscles (Demontis and Perrimon, 2009). The dependencies of FOXO activity and muscle size are related to the fact that an increase in FOXO levels causes inhibition of the gene encoding Myc. Similar to its vertebrate homologues, Myc is a central regulator of the growth and proliferation of many cell types, including myocytes (Gallant, 2013). Additionally, research carried out by Kim and O’Connor (2021) showed the importance of ILP signalling in the regulation of muscle growth in insects. The authors confirmed that Activin signalling promotes the growth of D. melanogaster muscles by positive regulation of the insulin receptor IR/TORC1 pathway and the level of Myosin heavy chain (Mhc) by increasing pdk1 and akt1 expression, genes encoding phosphoinositide-dependent kinase 1 and Akt kinase (Gallant, 2013). Moreover, Activin participates not only in controlling insect muscle growth but also in the functional ageing of this tissue. Research by Bai et al. (2013) showed that Activin is a direct, downstream target of ILPs/FOXO signalling within Drosophila muscles and may non-autonomously regulate lifespan. It should be mentioned that elevated expression of the gene encoding FOXO in muscles causes maintenance of protein homeostasis and delays the ageing-related decline in muscle activity (Bai et al., 2013).
Insulin-like peptides are important not only for muscle growth but also for muscle functioning. Research by Gorczyca et al. (1993) showed that IRs in D. melanogaster are located around synaptic boutons near the nerve branch point at each fibre of body-wall muscles. Additionally, insulin-like immunoreactivity was found in some body wall muscles (Gorczyca et al., 1993). These results are partially confirmed by Veenstra et al. (2008) because ilp3 mRNA was found in muscle cells of the midgut.
Despite the participation of ILP signalling in insect muscle growth and functioning, regulation of the availability of energy substrates stored in insect muscles by ILPs is no less important (Bretscher and O’Connor, 2020). The main energy substrate accumulated in insect muscles is glycogen. Similar to vertebrates, glycogen synthesis in insects is controlled in the muscles by ILP signalling (Yamada et al., 2018; Bretscher and O’Connor, 2020). However, ILPs participate not only in the regulation of glycogen synthesis but also in energy liberation by breaking down glycogen to glucose. Research by Post et al. (2018) showed that glycogen phosphorylase (GlyP), which is involved in this process, is negatively regulated by ILP signalling, specifically by Drome-ILP-2. Additionally, it should be mentioned that GlyP activation is related to the action of AKH, which once again presents strong relationships between these two neuropeptides (Post et al., 2018; Ahmad et al., 2020). Glycogen breakdown not only depends on GlyP activity but can also occur through autophagy. In the fat body, starvation-induced autophagy requires inhibition of the TOR pathway, which is inextricably linked with ILP signalling (Kannan and Fridell, 2013; Zirin et al., 2013). Due to some resemblances between the regulatory roles of ILPs in the fat body and muscles, we can assume that similar dependencies may also be present in insect muscles, such as a reduction in glycogen content in leg muscles of B. mori by Bommo-ILP (Kawabe et al., 2019) or reduced content of carbohydrates in R. prolixus leg muscles after knockdown of genes encoding Rhopr-ILPs (Defferrari et al., 2016) and increased trehalase activity in the muscles of B. mori. However, the results of recent research concerning the role of ILPs in energy homeostasis in insect muscles are not consistent and strongly depend on the model organism used, as mentioned before.
Interestingly, insect ILP signalling is also involved in lipid storage in the muscles. Research conducted on D. melanogaster by Zhao and Karpac (2017) showed that an increase in ILP signalling in the muscles causes the presence of TAG in this tissue. Moreover, similar to the storage of carbohydrates, this process involves the FOXO transcription factor (Zhao and Karpac, 2017).
Reproduction
It is well known that reproductive processes are energetically demanding, so it is therefore no surprise that they undergo complex pathways related to lipid and carbohydrate availability, reallocation, and metabolism (Fullbright et al., 1997; Nässel and Vanden Broeck, 2016; Leyria et al., 2021a). It was shown that ILPs and ILP signalling are necessary for correct functioning of both the female and male reproductive systems. In adult insects, disturbances in ILP levels might disrupt direct reproduction processes as well as hormone synthesis and release (Koyama et al., 2008). For example, mutations in insect ILP signalling alter JH synthesis in the CA, probably as an effect of the reduction of 3-hydroxy-3-methylglutaryl CoA reductase (HMG CoA reductase), a key enzyme in cholesterol biosynthesis necessary for JH formation (Tatar et al., 2001; Tu et al., 2005; Belgacem and Martin, 2006). Another relationship between ILPs and reproduction is ovarian ecdysteroid synthesis, which has been investigated in the mosquito (Nässel and Vanden Broeck, 2016). It was found that in adult female mosquitoes after a blood meal goes to ILPs releasing, what causes induction of ovarian ecdysteroidogenesis probably as an effect of binding of endogenous Aedae-ILP3 to the mosquito IR localized in cell membranes of follicle and nurse cells (Riehle and Brown, 2002; Brown et al., 2008; Wen et al., 2010). Additionally, in R. prolixus, a positive receptor signal was found in the tropharium, specifically in the cell membranes of nurse and follicle cells which surrounds oocytes in vitellogenic stage (Leyria et al., 2021b). Tu et al. (2002) also pointed out that in Drosophila, ecdysone is synthesized by follicle cells, and its secretion remains under the control of IR signalling.
With regard to reproduction processes, it was also shown that they undergo insulin pathway control. These neuropeptides, along with TOR, play crucial roles in acting as nutritional sensors (Badisco et al., 2013). This was confirmed by the results of Leyria et al. (2021b), who demonstrated that R. prolixus ILPs/ToR signalling in fat body tissue as well as in ovaries transduces the signal via Akt (protein kinase B) and is active only in fed insects. When considering dietary changes, it was shown that proper functioning of Drosophila ILP signalling is necessary to regulate egg production. Changes in that process might occur at different stages. First, germline cell division in Drosophila appears to be controlled in direct manner by central nervous system derived insulin (LaFever and Drummond-Barbosa, 2005). LaFever and Drummond-Barbosa (2005) showed that on a protein-poor diet, rates of division and development are decreased, and vitellogenesis processes are blocked. Moreover, it was shown that Drosophila ovarian cells require an undisturbed insulin pathway to properly function in cell proliferation and apoptosis cycling to enter vitellogenesis (Drummond-Barbosa and Spradling, 2001). The secretion of ILPs and their further activity are glucose-mediated, and it is possible that a disturbance in ILP signalling leads to deficiency of yolk protein absorbtion, which might explain the sterility (inhibited egg production as an effect of impaired development of the primary oocytes and termination of oocyte growth before relocation of the follicles despite the well-formed egg chambers) of Drosophila Chico mutants, as well as T. casteneum IR, Chico or TOR knockdown females (Drummond-Barbosa and Spradling, 2001; Richard et al., 2005; Parthasarathy and Palli, 2011). D. melanogaster female IR mutant ovaries remain stopped at the pre-stage of vitellogenesis but adding a methoprene (juvenile hormone analogue) retrieve vitellogenesis (Wu and Brown, 2006). In contrast, in late vitellogenic follicles of R. prolixus, no IR signal was found, probably since the oocytes internalized the nutrients needed for egg formation, which might prove that ILPs are engaged in the maintenance and increase of vitellogenic follicles (Leyria et al., 2021b). This is also supported by results from Silva-Oliveira et al. (2021), in which it was shown that IR-deficient R. prolixus females possess smaller ovaries and oviposition is reduced. Additionally, Al Baki et al. (2019) showed that in the reproductive period of Maruca vitrata, ILPs exhibited increased levels of gene expression. Observation of the terminal region of ovarioles shows that after treatment with dsRNAs specific to Marvi-ILP1 or Marvi-ILP2, the number of diving cells was decreased (Al Baki et al., 2019). This proves that in M. vitrata, ILPs also play crucial roles in mediating cell proliferation and triggering vitellogenesis (Al Baki et al., 2019). Similar effects were observed in Ae. aegypti (Riehle et al., 2006; Gulia-Nuss et al., 2011). One hour after blood feeding, the endocrine cascade starts, and ILPs and different neuroendocrine agents activate ovaries to secrete ecdysteroid hormones (ECDs) into the haemolymph, which is regarded as a first step for egg growth and development, as an effect of ECD signalling in the activation of vitellogenesis (Riehle et al., 2006; Wu and Brown, 2006; Gulia-Nuss et al., 2011).
As it has become clear that ILP signalling pathways are key factors in reproductive physiology, no wonder that ILP signalling is one of the basic mechanisms that controls diapause (Badisco et al., 2013). Reduced juvenile hormone level, observed in diapausing insects during diapause is potentially the eliciting factor and presumably the result of reduced ILP signalling (Tatar et al., 2001). There are also reports that FOXO plays a key role, which remains under the control of ILPs, since knockdown of this molecule inhibited Culex pipiens from entering diapause in addition to the data that FOXO seems to be present in the fat body of mosquitoes during diapause in high levels (Sim and Denlinger, 2008).
The lack of literature data on ILP signalling function in female insects in relation to male reproductive physiology shows that little attention has been conferred upon this issue thus far. Despite less documented data, insect ILP signalling has been studied as a coordinator of several aspects of male reproductive physiology when considered together with nutritional state. ILP signalling regulates spermatogenesis in male Drosophila insects, directly influencing the maintenance and proliferation of germline stem cells in testes (Ueishi et al., 2009; McLeod et al., 2010). According to Masly et al. (2011), ILP signalling also affects the growth of both male external genitalia, as well as horns, used to compete with rivals, in horn beetles such as Onthophagus nigriventris (Emlen et al., 2012; Lavine et al., 2013). Similar observations were made on Trypoxylus dichotomus and manifested in a 16% reduction in horn length after IR knockdown (Emlen et al., 2012).
There are reports that larval growth also operates under ILP coordination (Nagasawa et al., 1984; Emlen et al., 2012). The expression level of Bombyx ILP signalling pathway genes (such as InR, IRS or PDK) in fat body tissue was upregulated during moulting and pupation (Liu et al., 2010). Additionally, in Drosophila, Drome-ILP6 is produced in larger amounts during metamorphosis (Slaidina et al., 2009). Furthermore, it was shown that M. vitrata larvae treated with Marvi-ILP1 and Marvi-ILP2 RNAi exhibited significant growth retardation, which in some cases manifested with higher mortality (Al Baki et al., 2019).
Immune Activity
Insulin-like peptides, similar to other insect neuropeptides, probably exert direct immunotropic activities on immune-related cells. This supposition is supported, for example, by research conducted on Ae. aegypti. The results obtained by Castillo et al. (2011) showed that the expression of the gene encoding IR was found in phagocytic granulocytes and oenocytoids. Also, ILP singling could be involved in the regulation of antimicrobial peptides (AMPs) genes expression (Becker et al., 2010; McCormack et al., 2016; Urbański and Rosiński, 2018). In addition, recent results strongly suggest that ILP signalling is strictly connected with immune-related pathways and participates in the regulation of metabolic changes related to the activation of immune mechanisms (Dolezal et al., 2019).
Generally, activation of immune cells leads to suppression of systematic metabolism (Dolezal et al., 2019). Additionally, some elements switch their functions. A perfect example is apolipophorin III, which participates in the pathogen recognition process and increases lysozyme activity, but during the stress response apolipophorin III, is mainly involved in lipid transport (Adamo et al., 2008; Zdybicka-Barabas et al., 2013). Many of these metabolic changes are related to ILPs, both at the molecular level by blocking ILP signalling and at the cellular level by affecting ILP release (Dolezal et al., 2019). Interestingly, recent research clearly indicates that activation of all the main immune-related pathways (Toll, Imd, JAK/STAT) elicits effects associated with ILP signalling.
The Toll pathway is crucial for the response of insect organisms to infection by various pathogens, including bacteria, fungi, and viruses (Vigneron et al., 2019). Activation of this pathway causes AMP synthesis and modulates the activity of the cellular response (Johnston et al., 2014; Shafeeq et al., 2018). Research by DiAngelo et al. (2009) showed that activation of the Toll pathway in D. melanogaster may also suppress ILP signalling in the fat body, which results in a reduction in nutrient storage. These results are supported by Suzawa et al. (2019), which demonstrated that activation of the Toll receptor suppresses animal growth. The observed effect of Toll activation is related to the reduction in the level of circulating Drome-ILP6. Interestingly, restoring the expression of ilp in the fat body upon activation of the Toll pathway rescued the growth of tested fruit flies (Suzawa et al., 2019). It should also be highlighted that activation of the Toll pathway depends on the PGRP-SA receptor (peptidoglycan-recognition protein SA) and cytokine Spätzle. Transcripts of genes encoding these proteins are also found in insect haemocytes; for this reason, we can assume that haemocytes play a role in the activation of humoral immunity associated with a metabolic switch (Dolezal et al., 2019).
The second, but no less important, set of molecular pathways involved in the regulation of immune system functions and ILP signalling is the Imd (immunodeficiency) pathway (Zhai et al., 2018). Imd signalling plays a pivotal role in insect defence against microorganisms, especially Gram-negative bacteria (Kleino and Silverman, 2014). Interestingly, despite dependencies between the functioning of the Toll and Imd pathways, activation of the Imd pathway does not antagonize ILP signalling (DiAngelo et al., 2009). However, Eiger (an orthologue of tumour necrosis factor α, TNF-α), one of the cytokines important for activation of the Imd pathway, may also modulate ILP signalling. A recent study showed that Eiger can bind to the Grindewald receptor in IPCs, which may result in the inhibition of D. melanogaster growth by reducing the expression of genes encoding ILPs (Agrawal et al., 2016).
Similar to the Toll and Imd pathways, JAK/STAT activation modulates the activity of immune mechanisms and insect metabolism via ILP signalling. JAK/STAT is a highly conserved molecular pathway in insects that participates in the regulation of immune system activity as well as cell growth, differentiation, and apoptosis (Bang, 2019). JAK-STAT signalling is activated by the Unpaired 3 (Upd3) cytokine. Recent studies have shown that the appearance of this cytokine may have some implications not only for immune system functioning but also for muscle metabolism (Bretscher and O’Connor, 2020; Kierdorf et al., 2020). Releasing Upd3 from haemocytes during infection could reduce ILP sensitivity in muscles by activating JAK-STAT. This results in inhibition of glucose consumption by muscles and the redirection of available energy to haemocytes, which is required during defence against pathogen infection (Gallant, 2013; Zhao and Karpac, 2017). The results obtained by Lourido et al. (2021) also support supposition about the close relationships between JAK/STAT and ILPs in the regulation of insect metabolism. These authors showed that loss of the Domless receptor (part of JAK/STAT pathway) in the fat body of D. melanogaster reverses hyperglycaemia and increases the expression level of the insulin resistance marker nlaz in larvae on a high sugar diet.
Summary
Analysis of data concerning the role of ILPs in the regulation of insect physiology shows that this peptide family is one of the crucial groups of peptide hormones that control insect life, which is in line with situations occurring in mammals or more generally in vertebrates. The comprehensive role of ILPs results from their metabotropic activity. They regulate the insect’s nutritional status at various levels, and the intake and utilization of nutrients underlie all other life processes (Figure 2). Their multidirectional activity produces outcomes at several levels: (1) multiplicity of ILP family members, (2) ubiquity of production by various cells, (3) commonality of prevalence of IR receptors in different tissues, and (4) interplay of ILP signalling pathways with signalling pathways of other hormonal and nonhormonal factors. Because of the above, as well as the fact that ILPs affect cells/tissues/organs both in a direct and indirect manner, studies concerning the role of these peptides, their mode of action, and the mechanism regulating their production and secretion are not simple, and the obtained data are not easy to interpret. Moreover, it has to be borne in mind that the interpretations and comparison of different results about ILPs activity is all the more hard to interpret because of many various techniques and approaches used in research on ILPs. Even, if studies concern the same aspect, the used techniques do not allow for direct comparison or interpretation. Thus, although knowledge about ILPs increases from year to year, many aspects of their activity remain unclear and unknown. On the other hand, one of the most ancient regulatory systems is widespread in the animal kingdom, and the high similarity of insect and mammalian ILP signalling systems allows the use of insects as models for many human disorders and illnesses, e.g., obesity or diabetes.
As was mentioned above, ILPs are involved in regulation of almost all life processes in insects and their activity and mode of action is highly complexed and, in many points, crosses with other hormonal and non-hormonal systems regulating insect physiology. Because of that, still many of their activities are unclear and remain unknown. Nevertheless, that gives possibility for the next research with many perspectives. All the time the number of studies with knockdown of genes encoding ILPs (with single or multigene knockdown) increases. But still, the research about cross-talks with other factors is scanty. For example, simultaneous knockdown of ilps gene and genes encoding other hormonal factors like AKH, sNPFs or SKs. Also studies with application of ILPs together with other factors are not numerous. This research approach with e.g., pre-treating with SKs or sNPFs might show which signal dominates over another and “is more important”. Moreover, the role of nervous system in regulation of ILPs synthesis or secretion is not well explored. It is known that muscarinic receptors are involved in secretion of Bommo-ILPs, but how they interplay with other factors? Since the ILPs are a crucial controllers of metabolism, it is interesting what is their role in response to stress, e.g., cold stress or whether and how signalization via ILPs regulates mitochondria activity.
Despite that insulin-like peptides are one of the most explored group of peptide hormones in insects, and not only in insect, there are also still a lot of to do and discover.
Author Contributions
SC and JP-B contributed conception of the manuscript. SC, KW-N, MW, PM, AU, and JP-B wrote and edited the manuscript. SC coordinated the preparation of manuscript. All authors contributed to the article and approved the submitted version.
Funding
The project was partially supported by program Initiative of Excellence – Research University (Grant number: 011/08/POB2/0013).
Conflict of Interest
AU is employed by the company HiProMine S.A.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Abrisqueta, M., Süren-Castillo, S., and Maestro, J. L. (2014). Insulin receptor-mediated nutritional signalling regulates juvenile hormone biosynthesis and vitellogenin production in the German cockroach. Insect Biochem. Mol. Biol. 49, 14–23. doi: 10.1016/j.ibmb.2014.03.005
Adamo, S. A., Roberts, J. L., Easy, R. H., and Ross, N. W. (2008). Competition between immune function and lipid transport for the protein apolipophorin III leads to stress-induced immunosuppression in crickets. J. Exp. Biol. 211, 531–538. doi: 10.1242/jeb.013136
Agrawal, N., Delanoue, R., Mauri, A., Basco, D., Pasco, M., Thorens, B., et al. (2016). The Drosophila TNF Eiger is an adipokine that acts on insulin-producing cells to mediate nutrient response. Cell Metab. 23, 675–684. doi: 10.1016/j.cmet.2016.03.003
Ahmad, M., He, L., and Perrimon, N. (2020). Regulation of insulin and adipokinetic hormone/glucagon production in flies. WIREs Dev. Biol. 9:e360.
Al Baki, M. A., Lee, D.-W., Jung, J. K., and Kim, Y. (2019). Insulin signaling mediates previtellogenic development and enhances juvenile hormone-mediated vitellogenesis in a lepidopteran insect, Maruca vitrata. BMC Dev. Biol. 19:14. doi: 10.1186/s12861-019-0194-8
Alfa, R. W., Park, S., Skelly, K. R., Poffenberger, G., Jain, N., Gu, X., et al. (2015). Suppression of insulin production and secretion by a decretin hormone. Cell Metab. 21, 323–334. doi: 10.1016/j.cmet.2015.01.006
Andreatta, G., Kyriacou, C. P., Flatt, T., and Costa, R. (2018). Aminergic signaling controls ovarian dormancy in Drosophila. Sci. Rep. 8:2030.
Antonova, Y., Arik, A. J., Moore, W., Riehle, M., and Brown, M. R. (2012). “Insulin-like peptides: structure, signaling, and function”, in Insect Endocrinology, ed. L. I. Gilbert, Amsterdam Elsevier, 63–92. doi: 10.1016/b978-0-12-384749-2.10002-0
Arsic, D., and Guerin, P. (2008). Molecular Functional Characterization of Appetence Maturation and Its Nutrient-Dependent Control in the African Malaria Mosquito Anopheles Gambiae. Faculté des sciences, PhD. Thesis, Université de Neuchâtel, Neuchâtel.
Aslam, A. F. M., Kiya, T., Mita, K., and Iwami, M. (2011). Identification of novel bombyxin genes from the genome of the silkmoth Bombyx mori and analysis of their expression. Zoolog. Sci. 28:608.
Audsley, N., and Weaver, R. J. (2009). Neuropeptides associated with the regulation of feeding in insects. Gen. Comp. Endocrinol. 162, 93–104. doi: 10.1016/j.ygcen.2008.08.003
Badisco, L., Claeys, I., Van Hiel, M., Clynen, E., Huybrechts, J., Vandersmissen, T., et al. (2008). Purification and characterization of an insulin-related peptide in the desert locust, Schistocerca gregaria: immunolocalization, cDNA cloning, transcript profiling and interaction with neuroparsin. J. Mol. Endocrinol. 40, 137–150. doi: 10.1677/jme-07-0161
Badisco, L., Van Wielendaele, P., and Vanden Broeck, J. (2013). Eat to reproduce: a key role for the insulin signaling pathway in adult insects. Front. Physiol. 4:202. doi: 10.3389/fphys.2013.00202
Bai, H., Kang, P., Hernandez, A. M., and Tatar, M. (2013). Activin signaling targeted by insulin/dFOXO regulates aging and muscle proteostasis in Drosophila. PLoS Genet. 9:e1003941. doi: 10.1371/journal.pgen.1003941
Bai, H., Kang, P., and Tatar, M. (2012). Drosophila insulin-like peptide-6 (dilp6) expression from fat body extends lifespan and represses secretion of Drosophila insulin-like peptide-2 from the brain. Aging Cell. 11, 978–985. doi: 10.1111/acel.12000
Bang, I. S. (2019). JAK/STAT signaling in insect innate immunity. Entomol. Res. 49, 339–353. doi: 10.1111/1748-5967.12384
Becker, T., Loch, G., Beyer, M., Zinke, I., Aschenbrenner, A. C., Carrera, P., et al. (2010). FOXO-dependent regulation of innate immune homeostasis. Nature 463, 369–373. doi: 10.1038/nature08698
Belgacem, Y. H., and Martin, J.-R. (2006). Disruption of insulin pathways alters trehalose level and abolishes sexual dimorphism in locomotor activity in Drosophila. J. Neurobiol. 66, 19–32. doi: 10.1002/neu.20193
Birse, R. T., Söderberg, J. A. E., Luo, J., Winther, ÅM. E., and Nässel, D. R. (2011). Regulation of insulin-producing cells in the adult Drosophila brain via the tachykinin peptide receptor DTKR. J. Exp. Biol. 214, 4201–4208. doi: 10.1242/jeb.062091
Bounias, M., Bahjou, A., Gourdoux, L., and Moreau, R. (1993). Molecular activation of a trehalase purified from the fat body of a coleopteran insect (Tenebrio molitor), by an endogenous insulin-like peptide. Biochem. Mol. Biol. Int. 31, 249–266.
Bretscher, H., and O’Connor, M. B. (2020). The role of muscle in insect energy homeostasis. Front. Physiol. 11:580687. doi: 10.3389/fphys.2020.580687
Brogiolo, W., Stocker, H., Ikeya, T., Rintelen, F., Fernandez, R., and Hafen, E. (2001). An evolutionarily conserved function of the Drosophila insulin receptor and insulin-like peptides in growth control. Curr. Biol. 11, 213–221. doi: 10.1016/s0960-9822(01)00068-9
Broughton, S., Alic, N., Slack, C., Bass, T., Ikeya, T., Vinti, G., et al. (2008). Reduction of DILP2 in Drosophila triages a metabolic phenotype from lifespan revealing redundancy and compensation among DILPs. PLoS One 3:e3721. doi: 10.1371/journal.pone.0003721
Broughton, S. J., Piper, M. D., Ikeya, T., Bass, T. M., Jacobson, J., Driege, Y., et al. (2005). Longer lifespan, altered metabolism, and stress resistance in Drosophila from ablation of cells making insulin-like ligands. Proc. Natl. Acad. Sci. U.S.A. 102, 3105–3110. doi: 10.1073/pnas.0405775102
Brown, M. R., Clark, K. D., Gulia, M., Zhao, Z., Garczynski, S. F., Crim, J. W., et al. (2008). An insulin-like peptide regulates egg maturation and metabolism in the mosquito Aedes aegypti. Proc. Natl. Acad. Sci. U.S.A. 105, 5716–5721. doi: 10.1073/pnas.0800478105
Buch, S., Melcher, C., Bauer, M., Katzenberger, J., and Pankratz, M. J. (2008). Opposing effects of dietary protein and sugar regulate a transcriptional target of Drosophila insulin-like peptide signaling. Cell Metab. 7, 321–332. doi: 10.1016/j.cmet.2008.02.012
Cao, C., and Brown, M. R. (2001). Localization of an insulin-like peptide in brains of two flies. Cell Tissue Res. 304, 317–321. doi: 10.1007/s004410100367
Castillo, J., Brown, M. R., and Strand, M. R. (2011). Blood feeding and insulin-like peptide 3 stimulate proliferation of hemocytes in the mosquito Aedes aegypti. PLoS Pathog. 7:e1002274. doi: 10.1371/journal.ppat.1002274
Castro-Arnau, J., Marín, A., Castells, M., Ferrer, I., and Maestro, J. L. (2019). The expression of cockroach insulin-like peptides is differentially regulated by physiological conditions and affected by compensatory regulation. J. Insect Physiol. 114, 57–67. doi: 10.1016/j.jinsphys.2019.02.010
Claeys, I., Simonet, G., Poels, J., Van Loy, T., Vercammen, L., De Loof, A., et al. (2002). Insulin-related peptides and their conserved signal transduction pathway. Peptides 23, 807–816. doi: 10.1016/s0196-9781(01)00666-0
Coast, G. M., and Schooley, D. A. (2011). Toward a consensus nomenclature for insect neuropeptides and peptide hormones. Peptides 32, 620–631. doi: 10.1016/j.peptides.2010.11.006
Colombani, J., Andersen, D. S., Boulan, L., Boone, E., Romero, N., Virolle, V., et al. (2015). Drosophila Lgr3 couples organ growth with maturation and ensures developmental stability. Curr. Biol. 25, 2723–2729. doi: 10.1016/j.cub.2015.09.020
de Azevedo, S. V., and Hartfelder, K. (2008). The insulin signaling pathway in honey bee (Apis mellifera) caste development — differential expression of insulin-like peptides and insulin receptors in queen and worker larvae. J. Insect Physiol. 54, 1064–1071. doi: 10.1016/j.jinsphys.2008.04.009
Defferrari, M. S., Da Silva, S. R., Orchard, I., and Lange, A. B. (2018). A Rhodnius prolixus insulin receptor and its conserved intracellular signaling pathway and regulation of metabolism. Front. Endocrinol. (Lausanne) 9:745. doi: 10.3389/fendo.2018.00745
Defferrari, M. S., Orchard, I., and Lange, A. B. (2016). Identification of the first insulin-like peptide in the disease vector Rhodnius prolixus: involvement in metabolic homeostasis of lipids and carbohydrates. Insect Biochem. Mol. Biol. 70, 148–159. doi: 10.1016/j.ibmb.2015.12.009
Delanoue, R., Meschi, E., Agrawal, N., Mauri, A., Tsatskis, Y., McNeill, H., et al. (2016). Drosophila insulin release is triggered by adipose Stunted ligand to brain Methuselah receptor. Science 353, 1553–1556. doi: 10.1126/science.aaf8430
Demontis, F., and Perrimon, N. (2009). Integration of Insulin receptor/Foxo signaling and dMyc activity during muscle growth regulates body size in Drosophila. Development 136, 983–993. doi: 10.1242/dev.027466
DiAngelo, J. R., Bland, M. L., Bambina, S., Cherry, S., and Birnbaum, M. J. (2009). The immune response attenuates growth and nutrient storage in Drosophila by reducing insulin signaling. Proc. Natl. Acad. Sci.U.S.A. 106, 20853–20858. doi: 10.1073/pnas.0906749106
Dimitriadis, G., Mitrou, P., Lambadiari, V., Maratou, E., and Raptis, S. A. (2011). Insulin effects in muscle and adipose tissue. Diabetes Res. Clin. Pract. 93, S52–S59.
Ding, B.-Y., Shang, F., Zhang, Q., Xiong, Y., Yang, Q., Niu, J.-Z., et al. (2017). Silencing of two insulin receptor genes disrupts nymph-adult transition of alate brown citrus aphid. Int. J. Mol. Sci. 18:357. doi: 10.3390/ijms18020357
Dolezal, T., Krejcova, G., Bajgar, A., Nedbalova, P., and Strasser, P. (2019). Molecular regulations of metabolism during immune response in insects. Insect Biochem. Mol. Biol. 109, 31–42. doi: 10.1016/j.ibmb.2019.04.005
Drummond-Barbosa, D., and Spradling, A. C. (2001). Stem cells and their progeny respond to nutritional changes during Drosophila oogenesis. Dev. Biol. 231, 265–278. doi: 10.1006/dbio.2000.0135
Emlen, D. J., Warren, I. A., Johns, A., Dworkin, I., and Lavine, L. C. (2012). A mechanism of extreme growth and reliable signaling in sexually selected ornaments and weapons. Science. 337, 860–864. doi: 10.1126/science.1224286
Fadda, M., Hasakiogullari, I., Temmerman, L., Beets, I., Zels, S., and Schoofs, L. (2019). Regulation of feeding and metabolism by neuropeptide F and short neuropeptide F in invertebrates. Front. Endocrinol. (Lausanne) 10:64. doi: 10.3389/fendo.2019.00064
Fernandez, A. M., and Torres-Alemán, I. (2012). The many faces of insulin-like peptide signalling in the brain. Nat. Rev. Neurosci. 13, 225–239. doi: 10.1038/nrn3209
Fridell, Y.-W. C., Hoh, M., Kréneisz, O., Hosier, S., Chang, C., Scantling, D., et al. (2009). Increased uncoupling protein (UCP) activity in Drosophila insulin-producing neurons attenuates insulin signaling and extends lifespan. Aging (Albany NY) 1, 699–713. doi: 10.18632/aging.100067
Fujinaga, D., Shiomi, K., Yagi, Y., Kataoka, H., and Mizoguchi, A. (2019). An insulin-like growth factor-like peptide promotes ovarian development in the silkmoth Bombyx mori. Sci. Rep. 9:18446.
Fullbright, G., Lacy, E. R., and Büllesbach, E. E. (1997). Bombyxin: an insect neurohormone targets the ovaries in Lepidoptera. SAAS Bull. Biochem. Biotechnol. 10, 37–41.
Gallant, P. (2013). Myc function in Drosophila. Cold Spring Harb. Perspect. Med. 3:a014324. doi: 10.1101/cshperspect.a014324
Garelli, A., Gontijo, A. M., Miguela, V., Caparros, E., and Dominguez, M. (2012). Imaginal discs secrete insulin-like peptide 8 to mediate plasticity of growth and maturation. Science 336, 579–582. doi: 10.1126/science.1216735
Garofalo, R. S., and Rosen, O. M. (1988). Tissue localization of Drosophila melanogaster insulin receptor transcripts during development. Mol. Cell. Biol. 8, 1638–1647. doi: 10.1128/mcb.8.4.1638
Géminard, C., Rulifson, E. J., and Léopold, P. (2009). Remote control of insulin secretion by fat cells in Drosophila. Cell Metab. 10, 199–207. doi: 10.1016/j.cmet.2009.08.002
Gorczyca, M., Augart, C., and Budnik, V. (1993). Insulin-like receptor and insulin-like peptide are localized at neuromuscular junctions in Drosophila. J. Neurosci. 13, 3692–3704. doi: 10.1523/jneurosci.13-09-03692.1993
Grönke, S., and Partridge, L. (2010). “The functions of insulin-like peptides in insects,” in IGFs:Local Repair and Survival Factors Throughout Life Span, eds D. Clemmons, I. C. A. F. Robinson, and Y. Christen (Berlin, Heidelberg: Springer Berlin Heidelberg), 105–124. doi: 10.1007/978-3-642-04302-4_9
Gulia-Nuss, M., Robertson, A. E., Brown, M. R., and Strand, M. R. (2011). Insulin-like peptides and the target of rapamycin pathway coordinately regulate blood digestion and egg maturation in the mosquito Aedes aegypti. PLoS One 6:e20401. doi: 10.1371/journal.pone.0020401
Hentze, J. L., Carlsson, M. A., Kondo, S., Nässel, D. R., and Rewitz, K. F. (2015). The neuropeptide allatostatin A regulates metabolism and feeding decisions in Drosophila. Sci. Rep. 5:11680.
Hernández-Sánchez, C., Mansilla, A., de Pablo, F., and Zardoya, R. (2008). Evolution of the insulin receptor family and receptor isoform expression in vertebrates. Mol. Biol. Evol. 25, 1043–1053. doi: 10.1093/molbev/msn036
Ichikawa, T. (1991). Architecture of cerebral neurosecretory cell systems in the silkworm Bombyx Mori. J. Exp. Biol. 161, 217–237. doi: 10.1242/jeb.161.1.217
Iga, M., and Smagghe, G. (2011). Relationship between larval-pupal metamorphosis and transcript expression of insulin-like peptide and insulin receptor in Spodoptera littoralis. Peptides 32, 531–538. doi: 10.1016/j.peptides.2010.10.033
Ikeya, T., Galic, M., Belawat, P., Nairz, K., and Hafen, E. (2002). Nutrient-dependent expression of insulin-like peptides from neuroendocrine cells in the CNS contributes to growth regulation in Drosophila. Curr. Biol. 12, 1293–1300. doi: 10.1016/s0960-9822(02)01043-6
Iwami, M. (2000). Bombyxin: an insect brain peptide that belongs to the insulin family. Zoolog. Sci. 17, 1035–1044. doi: 10.2108/zsj.17.1035
Iwami, M., Tanaka, A., Hano, N., and Sakurai, S. (1996). Bombyxin gene expression in tissues other than brain detected by reverse transcription-polymerase chain reaction (RT-PCR) and in situ hybridization. Experientia 52, 882–887. doi: 10.1007/bf01938875
Jin Chan, S., and Steiner, D. F. (2000). Insulin through the ages: phylogeny of a growth promoting and metabolic regulatory hormone. Am. Zool. 40:210.
Johnston, P. R., Makarova, O., and Rolff, J. (2014). Inducible defenses stay up late: temporal patterns of immune gene expression in Tenebrio molitor. G3 Genes Genomes Genet. 4, 947–955. doi: 10.1534/g3.113.008516
Kannan, K., and Fridell, Y. W. (2013). Functional implications of Drosophila insulin-like peptides in metabolism, aging, and dietary restriction. Front. Physiol. 4:288. doi: 10.3389/fphys.2013.00288
Kapan, N., Lushchak, O. V., Luo, J., and Nässel, D. R. (2012). Identified peptidergic neurons in the Drosophila brain regulate insulin-producing cells, stress responses and metabolism by coexpressed short neuropeptide F and corazonin. Cell. Mol. Life Sci. 69, 4051–4066. doi: 10.1007/s00018-012-1097-z
Kawabe, Y., Waterson, H., and Mizoguchi, A. (2019). Bombyxin (Bombyx insulin-like peptide) increases the respiration rate through facilitation of carbohydrate catabolism in Bombyx mori. Front. Endocrinol. (Lausanne) 10:150. doi: 10.3389/fendo.2019.00150
Kierdorf, K., Hersperger, F., Sharrock, J., Vincent, C. M., Ustaoglu, P., Dou, J., et al. (2020). Muscle function and homeostasis require cytokine inhibition of AKT activity in Drosophila. eLife 9:e51595.
Kim, J., and Neufeld, T. P. (2015). Dietary sugar promotes systemic TOR activation in Drosophila through AKH-dependent selective secretion of Dilp3. Nat. Commun. 6:6846.
Kim, M.-J., and O’Connor, M. B. (2021). Drosophila activin signaling promotes muscle growth through InR/TORC1-dependent and -independent processes. Development 148:dev190868.
Kim, Y., and Hong, Y. (2015). Regulation of hemolymph trehalose level by an insulin-like peptide through diel feeding rhythm of the beet armyworm, Spodoptera exigua. Peptides 68, 91–98. doi: 10.1016/j.peptides.2015.02.003
Kleino, A., and Silverman, N. (2014). The Drosophila IMD pathway in the activation of the humoral immune response. Dev. Comp. Immunol. 42, 25–35. doi: 10.1016/j.dci.2013.05.014
Koyama, T., and Mirth, C. K. (2016). Growth-blocking peptides as nutrition-sensitive signals for insulin secretion and body size regulation. PLoS Biol. 14:e1002392. doi: 10.1371/journal.pbio.1002392
Koyama, T., Syropyatova, M. O., and Riddiford, L. M. (2008). Insulin/IGF signaling regulates the change in commitment in imaginal discs and primordia by overriding the effect of juvenile hormone. Dev. Biol. 324, 258–265. doi: 10.1016/j.ydbio.2008.09.017
LaFever, L., and Drummond-Barbosa, D. (2005). Direct control of germline stem cell division and cyst growth by neural insulin in Drosophila. Science 309, 1071–1073. doi: 10.1126/science.1111410
Lavine, L. C., Hahn, L. L., Warren, I. A., Garczynski, S. F., Dworkin, I., and Emlen, D. J. (2013). Cloning and characterization of an mrna encoding an insulin receptor from the horned scarab beetle Onthophagus nigriventris (Coleoptera: Scarabaeidae). Arch. Insect Biochem. Physiol. 82, 43–57. doi: 10.1002/arch.21072
Lee, G. J., and Hyun, S. (2014). Multiple targets of the microRNA miR-8 contribute to immune homeostasis in Drosophila. Dev. Comp. Immunol. 45, 245–251. doi: 10.1016/j.dci.2014.03.015
Lee, K.-S., Kwon, O. Y., Lee, J. H., Kwon, K., Min, K.-J., Jung, S.-A., et al. (2008). Drosophila short neuropeptide F signalling regulates growth by ERK-mediated insulin signalling. Nat. Cell Biol. 10, 468–475. doi: 10.1038/ncb1710
Leyria, J., El-Mawed, H., Orchard, I., and Lange, A. B. (2021a). Regulation of a trehalose-specific facilitated transporter (TRET) by insulin and adipokinetic hormone in Rhodnius prolixus, a vector of Chagas disease. Front. Physiol. 12:624165. doi: 10.3389/fphys.2021.624165
Leyria, J., Orchard, I., and Lange, A. B. (2021b). The involvement of insulin/ToR signaling pathway in reproductive performance of Rhodnius prolixus. Insect Biochem. Mol. Biol. 130:103526. doi: 10.1016/j.ibmb.2021.103526
Li, B., Predel, R., Neupert, S., Hauser, F., Tanaka, Y., Cazzamali, G., et al. (2008). Genomics, transcriptomics, and peptidomics of neuropeptides and protein hormones in the red flour beetle Tribolium castaneum. Genome Res. 18, 113–122. doi: 10.1101/gr.6714008
Ling, L., Kokoza, V. A., Zhang, C., Aksoy, E., and Raikhel, A. S. (2017). MicroRNA-277 targets insulin-like peptides 7 and 8 to control lipid metabolism and reproduction in Aedes aegypti mosquitoes. Proc. Natl. Acad. Sci. U.S.A. 114, E8017–E8024.
Ling, L., and Raikhel, A. S. (2021). Cross-talk of insulin-like peptides, juvenile hormone, and 20-hydroxyecdysone in regulation of metabolism in the mosquito Aedes aegypti. Proc. Natl. Acad. Sci. U.S.A. 118:e2023470118. doi: 10.1073/pnas.2023470118
Lingo, P. R., Zhao, Z., and Shen, P. (2007). Co-regulation of cold-resistant food acquisition by insulin- and neuropeptide Y-like systems in Drosophila melanogaster. Neuroscience 148, 371–374. doi: 10.1016/j.neuroscience.2007.06.010
Liu, Y., Liao, S., Veenstra, J. A., and Nässel, D. R. (2016). Drosophila insulin-like peptide 1 (DILP1) is transiently expressed during non-feeding stages and reproductive dormancy. Sci. Rep. 6:26620.
Liu, Y., Zhou, S., Ma, L., Tian, L., Wang, S., Sheng, Z., et al. (2010). Transcriptional regulation of the insulin signaling pathway genes by starvation and 20-hydroxyecdysone in the Bombyx fat body. J. Insect Physiol. 56, 1436–1444. doi: 10.1016/j.jinsphys.2010.02.011
Lourido, F., Quenti, D., Salgado-Canales, D., and Tobar, N. (2021). Domeless receptor loss in fat body tissue reverts insulin resistance induced by a high-sugar diet in Drosophila melanogaster. Sci. Rep. 11:3263.
Lu, H.-L., and Pietrantonio, P. V. (2011). Insect insulin receptors: insights from sequence and caste expression analyses of two cloned hymenopteran insulin receptor cDNAs from the fire ant. Insect Mol. Biol. 20, 637–649. doi: 10.1111/j.1365-2583.2011.01094.x
Lu, K., Chen, X., Li, W., Li, Y., Zhang, Z., and Zhou, Q. (2018). Insulin-like peptides and DNA/tRNA methyltransferases are involved in the nutritional regulation of female reproduction in Nilaparvata lugens (Stål). Gene 639, 96–105. doi: 10.1016/j.gene.2017.10.011
Lushchak, O. V., Carlsson, M. A., and Nässel, D. R. (2015). Food odors trigger an endocrine response that affects food ingestion and metabolism. Cell Mol. Life Sci. 72, 3143–3155. doi: 10.1007/s00018-015-1884-4
Luo, J., Lushchak, O. V., Goergen, P., Williams, M. J., and Nässel, D. R. (2014). Drosophila insulin-producing cells are differentially modulated by serotonin and octopamine receptors and affect social behavior. PLoS One 9:e99732. doi: 10.1371/journal.pone.0099732
Marquez, A. G., Pietri, J. E., Smithers, H. M., Nuss, A., Antonova, Y., Drexler, A. L., et al. (2011). Insulin-like peptides in the mosquito Anopheles stephensi: identification and expression in response to diet and infection with Plasmodium falciparum. Gen. Comp. Endocrinol. 173, 303–312. doi: 10.1016/j.ygcen.2011.06.005
Masly, J. P., Dalton, J. E., Srivastava, S., Chen, L., and Arbeitman, M. N. (2011). The genetic basis of rapidly evolving male genital morphology in Drosophila. Genetics 189, 357–374. doi: 10.1534/genetics.111.130815
Masumura, M., Satake, S. I., Saegusa, H., and Mizoguchi, A. (2000). Glucose stimulates the release of bombyxin, an insulin-related peptide of the silkworm Bombyx mori. Gen. Comp. Endocrinol. 118, 393–399. doi: 10.1006/gcen.1999.7438
McCormack, S., Yadav, S., Shokal, U., Kenney, E., Cooper, D., and Eleftherianos, I. (2016). The insulin receptor substrate Chico regulates antibacterial immune function in Drosophila. Immun. Ageing 13:15.
McLeod, C. J., Wang, L., Wong, C., and Jones, D. L. (2010). Stem cell dynamics in response to nutrient availability. Curr. Biol. 20, 2100–2105. doi: 10.1016/j.cub.2010.10.038
Miyamoto, T., and Amrein, H. (2014). Diverse roles for the Drosophila fructose sensor Gr43a. Fly 8, 19–25. doi: 10.4161/fly.27241
Mizoguchi, A., Ishizaki, H., Nagasawa, H., Kataoka, H., Isogai, A., Tamura, S., et al. (1987). A monoclonal antibody against a synthetic fragment of bombyxin (4K-prothoracicotropic hormone) from the silkmoth, Bombyx mori: characterization and immunohistochemistry. Mol. Cell. Endocrinol. 51, 227–235. doi: 10.1016/0303-7207(87)90032-3
Mizoguchi, A., and Okamoto, N. (2013). Insulin-like and IGF-like peptides in the silkmoth Bombyx mori: discovery, structure, secretion, and function. Front. Physiol. 4:217. doi: 10.3389/fphys.2013.00217
Nagasawa, H., Kataoka, H., Isogai, A., Tamura, S., Suzuki, A., Ishizaki, H., et al. (1984). Amino-terminal amino acid sequence of the silkworm prothoracicotropic hormone: homology with insulin. Science 226, 1344–1346. doi: 10.1126/science.226.4680.1344
Nagata, K., Hatanaka, H., Kohda, D., Kataoka, H., Nagasawa, H., Isogai, A., et al. (1995). Three-dimensional solution structure of bombyxin-II an insulin-like peptide of the silkmoth Bombyx mori: structural comparison with insulin and relaxin. J. Mol. Biol. 253, 749–758. doi: 10.1006/jmbi.1995.0588
Nässel, D. R. (2012). Insulin-producing cells and their regulation in physiology and behavior of Drosophila. Can. J. Zool. 90, 476–488.
Nässel, D. R., Liu, Y., and Luo, J. (2015). Insulin/IGF signaling and its regulation in Drosophila. Gen. Comp. Endocrinol. 221, 255–266. doi: 10.1016/j.ygcen.2014.11.021
Nässel, D. R., and Vanden Broeck, J. (2016). Insulin/IGF signaling in Drosophila and other insects: factors that regulate production, release and post-release action of the insulin-like peptides. Cell. Mol. Life Sci. 73, 271–290. doi: 10.1007/s00018-015-2063-3
Nässel, D. R., and Zandawala, M. (2019). Recent advances in neuropeptide signaling in Drosophila, from genes to physiology and behavior. Prog. Neurobiol. 179:101607. doi: 10.1016/j.pneurobio.2019.02.003
Nässel, D. R., and Zandawala, M. (2020). Hormonal axes in Drosophila: regulation of hormone release and multiplicity of actions. Cell Tissue Res. 382, 233–266. doi: 10.1007/s00441-020-03264-z
Nuss, A. B., and Brown, M. R. (2018). Isolation of an insulin-like peptide from the Asian malaria mosquito, Anopheles stephensi, that acts as a steroidogenic gonadotropin across diverse mosquito taxa. Gen. Comp. Endocrinol. 258, 140–148. doi: 10.1016/j.ygcen.2017.05.007
Nuss, A. B., Brown, M. R., Murty, U. S., and Gulia-Nuss, M. (2018). Insulin receptor knockdown blocks filarial parasite development and alters egg production in the southern house mosquito, Culex quinquefasciatus. PLoS Negl. Trop. Dis. 12:e0006413. doi: 10.1371/journal.pntd.0006413
Ohhara, Y., Kobayashi, S., Yamakawa-Kobayashi, K., and Yamanaka, N. (2018). Adult-specific insulin-producing neurons in Drosophila melanogaster. J. Comp. Neurol. 526, 1351–1367. doi: 10.1002/cne.24410
Okamoto, N., and Yamanaka, N. (2015). Nutrition-dependent control of insect development by insulin-like peptides. Curr. Opin. Insect Sci. 11, 21–30. doi: 10.1016/j.cois.2015.08.001
Okamoto, N., Yamanaka, N., Endo, Y., Kataoka, H., and Mizoguchi, A. (2011). Spatiotemporal patterns of IGF-like peptide expression in the silkmoth Bombyx mori predict its pleiotropic actions. Gen. Comp. Endocrinol. 173, 171–182. doi: 10.1016/j.ygcen.2011.05.009
Okamoto, N., Yamanaka, N., Satake, H., Saegusa, H., Kataoka, H., and Mizoguchi, A. (2009a). An ecdysteroid−inducible insulin−like growth factor−like peptide regulates adult development of the silkmoth Bombyx mori. Febs J. 276, 1221–1232. doi: 10.1111/j.1742-4658.2008.06859.x
Okamoto, N., Yamanaka, N., Yagi, Y., Nishida, Y., Kataoka, H., O’Connor, M. B., et al. (2009b). A fat body-derived IGF-like peptide regulates postfeeding growth in Drosophila. Dev. Cell. 17, 885–891. doi: 10.1016/j.devcel.2009.10.008
Park, S., Alfa, R. W., Topper, S. M., Kim, G. E., Kockel, L., and Kim, S. K. (2014). A genetic strategy to measure circulating Drosophila insulin reveals genes regulating insulin production and secretion. PLoS Genet. 10:e1004555. doi: 10.1371/journal.pgen.1004555
Parthasarathy, R., and Palli, S. R. (2011). Molecular analysis of nutritional and hormonal regulation of female reproduction in the red flour beetle, Tribolium castaneum. Insect Biochem. Mol. Biol. 41, 294–305. doi: 10.1016/j.ibmb.2011.01.006
Pool, A.-H., and Scott, K. (2014). Feeding regulation in Drosophila. Curr. Opin. Neurobiol. 29, 57–63. doi: 10.1016/j.conb.2014.05.008
Post, S., Karashchuk, G., Wade, J. D., Sajid, W., De Meyts, P., and Tatar, M. (2018). Drosophila insulin-like peptides DILP2 and DILP5 differentially stimulate cell signaling and glycogen phosphorylase to regulate longevity. Front. Endocrinol. (Lausanne) 9:245. doi: 10.3389/fendo.2018.00245
Post, S., Liao, S., Yamamoto, R., Veenstra, J. A., Nässel, D. R., and Tatar, M. (2019). Drosophila insulin-like peptide dilp1 increases lifespan and glucagon-like Akh expression epistatic to dilp2. Aging Cell 18:e12863. doi: 10.1111/acel.12863
Post, S., and Tatar, M. (2016). Nutritional geometric profiles of insulin/IGF expression in Drosophila melanogaster. PLoS One 11:e0155628. doi: 10.1371/journal.pone.0155628
Rajan, A., and Perrimon, N. (2012). Drosophila cytokine unpaired 2 regulates physiological homeostasis by remotely controlling insulin secretion. Cell 151, 123–137. doi: 10.1016/j.cell.2012.08.019
Richard, D. S., Rybczynski, R., Wilson, T. G., Wang, Y., Wayne, M. L., Zhou, Y., et al. (2005). Insulin signaling is necessary for vitellogenesis in Drosophila melanogaster independent of the roles of juvenile hormone and ecdysteroids: female sterility of the chico1 insulin signaling mutation is autonomous to the ovary. J. Insect Physiol. 51, 455–464. doi: 10.1016/j.jinsphys.2004.12.013
Riehle, M. A., and Brown, M. R. (2002). Insulin receptor expression during development and a reproductive cycle in the ovary of the mosquito Aedes aegypti. Cell Tissue Res. 308, 409–420. doi: 10.1007/s00441-002-0561-8
Riehle, M. A., Fan, Y., Cao, C., and Brown, M. R. (2006). Molecular characterization of insulin-like peptides in the yellow fever mosquito, Aedes aegypti: expression, cellular localization, and phylogeny. Peptides 27, 2547–2560. doi: 10.1016/j.peptides.2006.07.016
Rulifson, E. J., Kim, S. K., and Nusse, R. (2002). Ablation of insulin-producing neurons in flies: growth and diabetic phenotypes. Science 296, 1118–1120. doi: 10.1126/science.1070058
Sajid, W., Kulahin, N., Schluckebier, G., Ribel, U., Henderson, H. R., Tatar, M., et al. (2011). Structural and biological properties of the Drosophila insulin-like peptide 5 show evolutionary conservation. J. Biol. Chem. 286, 661–673. doi: 10.1074/jbc.m110.156018
Sang, M., Li, C., Wu, W., and Li, B. (2016). Identification and evolution of two insulin receptor genes involved in Tribolium castaneum development and reproduction. Gene 585, 196–204. doi: 10.1016/j.gene.2016.02.034
Sano, H., Nakamura, A., Texada, M. J., Truman, J. W., Ishimoto, H., Kamikouchi, A., et al. (2015). The nutrient-responsive hormone CCHamide-2 controls growth by regulating insulin-like peptides in the brain of Drosophila melanogaster. PLoS Genet. 11:e1005209. doi: 10.1371/journal.pgen.1005209
Satake, S., Masumura, M., Ishizaki, H., Nagata, K., Kataoka, H., Suzuki, A., et al. (1997). Bombyxin, an insulin-related peptide of insects, reduces the major storage carbohydrates in the silkworm Bombyx mori. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 118, 349–357. doi: 10.1016/s0305-0491(97)00166-1
Satake, S. I., Nagata, K., Kataoka, H., and Mizoguchi, A. (1999). Bombyxin secretion in the adult silkmoth Bombyx mori: sex-specificity and its correlation with metabolism. J. Insect Physiol. 45, 939–945. doi: 10.1016/s0022-1910(99)00074-8
Scapin, G., Dandey, V. P., Zhang, Z., Prosise, W., Hruza, A., Kelly, T., et al. (2018). Structure of the insulin receptor–insulin complex by single-particle cryo-EM analysis. Nature 556, 122–125. doi: 10.1038/nature26153
Semaniuk, U., Gospodaryov, D., Mishchanyn, K., Storey, K., and Lushchak, O. (2021a). Drosophila insulin-like peptides regulate concentration-dependent changes of appetite to different carbohydrates. Zoology 146:125927. doi: 10.1016/j.zool.2021.125927
Semaniuk, U., Piskovatska, V., Strilbytska, O., Strutynska, T., Burdyliuk, N., Vaiserman, A., et al. (2021b). Drosophila insulin-like peptides: from expression to functions – a review. Entomol. Exp. Appl. 169, 195–208. doi: 10.1111/eea.12981
Semaniuk, U. V., Gospodaryov, D. V., Feden’ko, K. M., Yurkevych, I. S., Vaiserman, A. M., Storey, K. B., et al. (2018). Insulin-like peptides regulate feeding preference and metabolism in Drosophila. Front. Physiol. 9:1083. doi: 10.3389/fphys.2018.01083
Shafeeq, T., Ahmed, S., and Kim, Y. (2018). Toll immune signal activates cellular immune response via eicosanoids. Dev. Comp. Immunol. 84, 408–419. doi: 10.1016/j.dci.2018.03.015
Sharma, A., Nuss, A. B., and Gulia-Nuss, M. (2019). Insulin-like peptide signaling in mosquitoes: the road behind and the road ahead. Front. Endocrinol. (Lausanne) 10:166. doi: 10.3389/fendo.2019.00166
Sharma, P., Aslam Khan, I., and Singh, R. (2018). Curcumin and quercetin ameliorated cypermethrin and deltamethrin-induced reproductive system impairment in male wistar rats by upregulating the activity of pituitary-gonadal hormones and steroidogenic enzymes. Int. J. Fertil. Steril. 12, 72–80.
Sheng, Z., Xu, J., Bai, H., Zhu, F., and Palli, S. R. (2011). Juvenile hormone regulates vitellogenin gene expression through insulin-like peptide signaling pathway in the red flour beetle, Tribolium castaneum. J. Biol. Chem. 286, 41924–41936. doi: 10.1074/jbc.m111.269845
Silva-Oliveira, G., De Paula, I. F., Medina, J. M., Alves-Bezerra, M., and Gondim, K. C. (2021). Insulin receptor deficiency reduces lipid synthesis and reproductive function in the insect Rhodnius prolixus. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 1866:158851. doi: 10.1016/j.bbalip.2020.158851
Sim, C., and Denlinger, D. L. (2008). Insulin signaling and FOXO regulate the overwintering diapause of the mosquito Culex pipiens. Proc. Natl. Acad. Sci. U.S.A. 105, 6777–6781. doi: 10.1073/pnas.0802067105
Slaidina, M., Delanoue, R., Gronke, S., Partridge, L., and Léopold, P. (2009). A Drosophila insulin-like peptide promotes growth during nonfeeding states. Dev. Cell 17, 874–884. doi: 10.1016/j.devcel.2009.10.009
Słocińska, M., Chowański, S., and Marciniak, P. (2020). Identification of sulfakinin receptors (SKR) in Tenebrio molitor beetle and the influence of sulfakinins on carbohydrates metabolism. J. Comp. Physiol. B 190, 669–679. doi: 10.1007/s00360-020-01300-6
Söderberg, J. A., Carlsson, M. A., and Nässel, D. R. (2012). Insulin-producing cells in the Drosophila brain also express satiety-inducing cholecystokinin-like peptide, drosulfakinin. Front. Endocrinol. (Lausanne) 3:109. doi: 10.3389/fendo.2012.00109
Strilbytska, O. M., Koliada, A. K., Storey, K. B., Mudra, O., Vaiserman, A. M., and Lushchak, O. (2017a). Longevity and stress resistance are affected by activation of TOR/Myc in progenitor cells of Drosophila gut. Open Life Sci. 12, 429–442. doi: 10.1515/biol-2017-0051
Strilbytska, O. M., Semaniuk, U. V., Storey, K. B., Edgar, B. A., and Lushchak, O. V. (2017b). Activation of the Tor/Myc signaling axis in intestinal stem and progenitor cells affects longevity, stress resistance and metabolism in Drosophila. Compar. Biochem. Physiol. Part B Biochem. Mol. Biol. 203, 92–99. doi: 10.1016/j.cbpb.2016.09.008
Strilbytska, O. M., Semaniuk, U. V., Storey, K. B., Yurkevych, I. S., and Lushchak, O. (2020). Insulin signaling in intestinal stem and progenitor cells as an important determinant of physiological and metabolic traits in Drosophila. Cells 9:803. doi: 10.3390/cells9040803
Sudhakar, S. R., Pathak, H., Rehman, N., Fernandes, J., Vishnu, S., and Varghese, J. (2020). Insulin signalling elicits hunger-induced feeding in Drosophila. Dev. Biol. 459, 87–99. doi: 10.1016/j.ydbio.2019.11.013
Suh, Y. S., Bhat, S., Hong, S.-H., Shin, M., Bahk, S., Cho, K. S., et al. (2015). Genome-wide microRNA screening reveals that the evolutionary conserved miR-9a regulates body growth by targeting sNPFR1/NPYR. Nat. Commun. 6:7693.
Suzawa, M., Muhammad, N. M., Joseph, B. S., and Bland, M. L. (2019). The Toll signaling pathway targets the insulin-like peptide Dilp6 to inhibit growth in Drosophila. Cell Rep. 28, 1439.e–1446.e.
Tatar, M., Bartke, A., and Antebi, A. (2003). The endocrine regulation of aging by insulin-like signals. Science 299, 1346–1351. doi: 10.1126/science.1081447
Tatar, M., Kopelman, A., Epstein, D., Tu, M.-P., Yin, C.-M., and Garofalo, R. (2001). A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science 292, 107–110. doi: 10.1126/science.1057987
Teleman, A. A., Maitra, S., and Cohen, S. M. (2006). Drosophila lacking microRNA miR-278 are defective in energy homeostasis. Genes Dev. 20, 417–422. doi: 10.1101/gad.374406
Teulier, L., Weber, J. M., Crevier, J., and Darveau, C. A. (2016). Proline as a fuel for insect flight: enhancing carbohydrate oxidation in hymenopterans. Proc. Biol. Sci. 283:20160333. doi: 10.1098/rspb.2016.0333
Tu, M.-P., Epstein, D., and Tatar, M. (2002). The demography of slow aging in male and female Drosophila mutant for the insulin-receptor substrate homologue chico. Aging Cell. 1, 75–80. doi: 10.1046/j.1474-9728.2002.00010.x
Tu, M.-P., Yin, C.-M., and Tatar, M. (2005). Mutations in insulin signaling pathway alter juvenile hormone synthesis in Drosophila melanogaster. Gen. Comp. Endocrinol. 142, 347–356. doi: 10.1016/j.ygcen.2005.02.009
Ueishi, S., Shimizu, H., and H Inoue, Y. (2009). Male germline stem cell division and spermatocyte growth require insulin signaling in Drosophila. Cell Struct. Funct. 34, 61–69. doi: 10.1247/csf.08042
Urbański, A., and Rosiński, G. (2018). Role of neuropeptides in the regulation of the insect immune system - current knowledge and perspectives. Curr. Protein Pept. Sci. 19, 1201–1213. doi: 10.2174/1389203719666180809113706
Vafopoulou, X., and Steel, C. G. (2012). Insulin-like and testis ecdysiotropin neuropeptides are regulated by the circadian timing system in the brain during larval-adult development in the insect Rhodnius prolixus (Hemiptera). Gen. Comp. Endocrinol. 179, 277–288. doi: 10.1016/j.ygcen.2012.08.018
Vallejo, D. M., Juarez-Carreńo, S., Bolivar, J., Morante, J., and Dominguez, M. (2015). A brain circuit that synchronizes growth and maturation revealed through Dilp8 binding to Lgr3. Science 350:aac6767. doi: 10.1126/science.aac6767
Van de Velde, S., Badisco, L., Claeys, I., Verleyen, P., Chen, X., Bosch, L. V., et al. (2007). Insulin-like peptides in Spodoptera littoralis (Lepidoptera): Detection, localization and identification. Gen. Comp. Endocrinol. 153, 72–79. doi: 10.1016/j.ygcen.2007.05.001
Van Hiel, M. B., Vandersmissen, H. P., Proost, P., and Vanden Broeck, J. (2015). Cloning, constitutive activity and expression profiling of two receptors related to relaxin receptors in Drosophila melanogaster. Peptides 68, 83–90. doi: 10.1016/j.peptides.2014.07.014
Varghese, J., Lim, S. F., and Cohen, S. M. (2010). Drosophila miR-14 regulates insulin production and metabolism through its target, sugarbabe. Genes Dev. 24, 2748–2753. doi: 10.1101/gad.1995910
Veenstra, J. A. (2020). Arthropod IGF, relaxin and gonadulin, putative orthologs of Drosophila insulin-like peptides 6, 7 and 8, likely originated from an ancient gene triplication. PeerJ. 8:e9534. doi: 10.7717/peerj.9534
Veenstra, J. A., Agricola, H.-J., and Sellami, A. (2008). Regulatory peptides in fruit fly midgut. Cell Tissue Res. 334, 499–516. doi: 10.1007/s00441-008-0708-3
Vigneron, A., Jehan, C., Rigaud, T., and Moret, Y. (2019). Immune defenses of a beneficial pest: the mealworm beetle, Tenebrio molitor. Front. Physiol. 10:138. doi: 10.3389/fphys.2019.00138
Vitali, V., Horn, F., and Catania, F. (2018). Insulin-like signaling within and beyond metazoans. Biol. Chem. 399, 851–857. doi: 10.1515/hsz-2018-0135
Wang, Y., Azevedo, S. V., Hartfelder, K., and Amdam, G. V. (2013). Insulin-like peptides (AmILP1 and AmILP2) differentially affect female caste development in the honey bee (Apis mellifera L.). J. Exp. Biol. 216, 4347–4357.
Wang, Y., Brent, C. S., Fennern, E., and Amdam, G. V. (2012). Gustatory perception and fat body energy metabolism are jointly affected by vitellogenin and juvenile hormone in honey bees. PLoS Genet. 8:e1002779. doi: 10.1371/journal.pgen.1002779
Wen, Z., Gulia, M., Clark, K. D., Dhara, A., Crim, J. W., Strand, M. R., et al. (2010). Two insulin-like peptide family members from the mosquito Aedes aegypti exhibit differential biological and receptor binding activities. Mol. Cell. Endocrinol. 328, 47–55. doi: 10.1016/j.mce.2010.07.003
White, M. F. (1998). The IRS-signalling system: a network of docking proteins that mediate insulin action. Mol. Cell. Biochem. 182, 3–11. doi: 10.1007/978-1-4615-5647-3_1
Wu, Q., and Brown, M. R. (2006). Signaling and function of insulin-like peptides in insects. Annu. Rev. Entomol. 51, 1–24. doi: 10.1146/annurev.ento.51.110104.151011
Wu, Q., Zhao, Z., and Shen, P. (2005). Regulation of aversion to noxious food by Drosophila neuropeptide Y–and insulin-like systems. Nat. Neurosci. 8, 1350–1355. doi: 10.1038/nn1540
Xu, H. J., Xue, J., Lu, B., Zhang, X. C., Zhuo, J. C., He, S. F., et al. (2015). Two insulin receptors determine alternative wing morphs in planthoppers. Nature 519, 464–467. doi: 10.1038/nature14286
Xu, J., Sheng, Z., and Palli, S. R. (2013). Juvenile hormone and insulin regulate trehalose homeostasis in the red flour beetle, Tribolium castaneum. PLoS Genet. 9:e1003535. doi: 10.1371/journal.pgen.1003535
Xue, W.-H., Liu, Y.-L., Jiang, Y.-Q., He, S.-F., Wang, Q.-Q., Yang, Z.-N., et al. (2020). Molecular characterization of insulin-like peptides in the brown planthopper, Nilaparvata lugens (Hemiptera: Delphacidae). Insect Mol. Biol. 29, 309–319. doi: 10.1111/imb.12636
Yamada, T., Habara, O., Kubo, H., and Nishimura, T. (2018). Fat body glycogen serves as a metabolic safeguard for the maintenance of sugar levels in Drosophila. Development 145:dev158865.
Yang, C.-H., Belawat, P., Hafen, E., Jan, L. Y., and Jan, Y.-N. (2008). Drosophila egg-laying site selection as a system to study simple decision-making processes. Science 319, 1679–1683. doi: 10.1126/science.1151842
Yu, Y., Sun, Y., He, S., Yan, C., Rui, L., Li, W., et al. (2012). Neuronal Cbl controls biosynthesis of insulin-like peptides in Drosophila melanogaster. Mol. Cell. Biol. 32, 3610–3623. doi: 10.1128/mcb.00592-12
Zdybicka-Barabas, A., Stączek, S., Mak, P., Skrzypiec, K., Mendyk, E., and Cytryńska, M. (2013). Synergistic action of Galleria mellonella apolipophorin III and lysozyme against Gram-negative bacteria. Biochim. Biophys. Acta (BBA) Biomembranes 1828, 1449–1456. doi: 10.1016/j.bbamem.2013.02.004
Zhai, Z., Boquete, J.-P., and Lemaitre, B. (2018). Cell-specific Imd-NF-κB responses enable simultaneous antibacterial immunity and intestinal epithelial cell shedding upon bacterial infection. Immunity 48, 897.e–910.e.
Zhang, H., Liu, J., Li, C. R., Momen, B., Kohanski, R. A., and Pick, L. (2009). Deletion of Drosophila insulin-like peptides causes growth defects and metabolic abnormalities. Proc. Natl. Acad. Sci. U.S.A. 106, 19617–19622. doi: 10.1073/pnas.0905083106
Zhao, X., and Karpac, J. (2017). Muscle directs diurnal energy homeostasis through a myokine-dependent hormone module in Drosophila. Curr. Biol. 27, 1941.e–1955.e.
Keywords: insulin-like peptides (ILPs), insulin-like growth factors (ILGFs), neuropeptides, metabolism, insects, cross-talk
Citation: Chowański S, Walkowiak-Nowicka K, Winkiel M, Marciniak P, Urbański A and Pacholska-Bogalska J (2021) Insulin-Like Peptides and Cross-Talk With Other Factors in the Regulation of Insect Metabolism. Front. Physiol. 12:701203. doi: 10.3389/fphys.2021.701203
Received: 27 April 2021; Accepted: 04 June 2021;
Published: 29 June 2021.
Edited by:
Dov Borovsky, University of Colorado Anschutz Medical Campus, United StatesReviewed by:
Hua Bai, Iowa State University, United StatesTakashi Adachi-Yamada, Gakushuin University, Japan
Oleh Lushchak, Vasyl Stefanyk Precarpathian National University, Ukraine
Copyright © 2021 Chowański, Walkowiak-Nowicka, Winkiel, Marciniak, Urbański and Pacholska-Bogalska. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Szymon Chowański, c3p5eW1vbkBhbXUuZWR1LnBs; Joanna Pacholska-Bogalska, cGFjaG9sc2tAYW11LmVkdS5wbA==
†ORCID: Szymon Chowański, orcid.org/0000-0002-5667-1781; Karolina Walkowiak-Nowicka, orcid.org/0000-0002-2490-3576; Magdalena Winkiel, orcid.org/0000-0002-5983-8997; Pawel Marciniak, orcid.org/0000-0002-4790-001X; Arkadiusz Urbański, orcid.org/0000-0003-4875-4541; Joanna Pacholska-Bogalska, orcid.org/0000-0001-9752-4705
 Szymon Chowański
Szymon Chowański Karolina Walkowiak-Nowicka
Karolina Walkowiak-Nowicka Magdalena Winkiel
Magdalena Winkiel Pawel Marciniak
Pawel Marciniak Arkadiusz Urbański
Arkadiusz Urbański Joanna Pacholska-Bogalska
Joanna Pacholska-Bogalska