
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Physiol., 08 June 2021
Sec. Integrative Physiology
Volume 12 - 2021 | https://doi.org/10.3389/fphys.2021.686251
This article is part of the Research TopicFunctional Profile of the Lipocalin Protein FamilyView all 12 articles
α1-acid glycoprotein (AGP), also known as Orosomucoid (ORM), belongs to the Lipocalin protein family and it is well-known for being a positive acute-phase protein. AGP is mostly found in plasma, with the liver as main contributor, but it is also expressed in other tissues such as the brain or the adipose tissue. Despite the vast literature on AGP, the physiological functions of the protein remain to be elucidated. A large number of activities mostly related to protection and immune system modulation have been described. Recently created AGP-knockout models have suggested novel physiological roles of AGP, including regulation of metabolism. AGP has an outstanding ability to efficiently bind endogenous and exogenous small molecules that together with the complex and variable glycosylation patterns, determine AGP functions. This review summarizes and discusses the recent findings on AGP structure (including glycans), ligand-binding ability, regulation, and physiological functions of AGP. Moreover, this review explores possible molecular and functional connections between AGP and other members of the Lipocalin protein family.
α1-acid glycoprotein (AGP), also known as Orosomucoid (ORM), is member of the Lipocalin protein family and well-known for being a positive acute-phase protein. Approximately 70 years have passed since the discovery of AGP (Schmid, 1950; Weimer et al., 1950) and thousands of studies have been performed since then. In the big picture, AGP is commonly defined as a transport protein in plasma whose main function is to modulate the immune system, including cytokine secretion (Hochepied et al., 2003). Numerous in vitro and in vivo activities such as the inhibition of platelet aggregation (Costello et al., 1979), modulation of cell proliferation/differentiation (Chiu et al., 1977; Qin et al., 2017; Lee et al., 2021; Shi et al., 2021), and drug transport have been reported (Israili and Dayton, 2001). However, the exact molecular mechanism of AGP function remains to be elucidated. AGP is mostly found in plasma from hepatic origin, but other tissues/cells such as the adipose tissue, the nervous system, endothelial cells, and immune cells also express AGP, especially during inflammatory conditions (Dente et al., 1988; Sorensson et al., 1999; Hochepied et al., 2003). Indeed, a large number of pathological conditions (including many types of cancers, infection, obesity, and cardiovascular diseases) raise AGP levels in plasma (Israili and Dayton, 2001).
This work represents an overview of the multiple faces of AGP and focuses on its physiological roles. Furthermore, this review explores possible molecular and functional connections between AGP and other members of the Lipocalin protein family (summarized in Table 1).
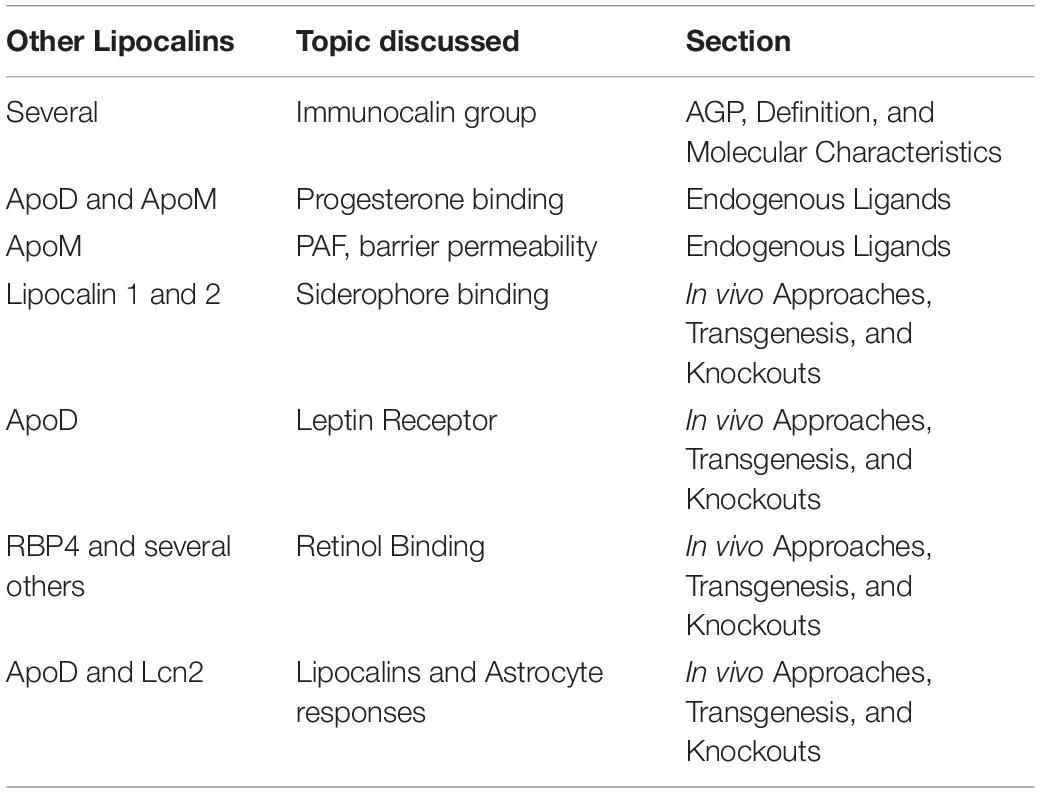
Table 1. Summary of the associations/common features of AGP and other members of the Lipocalin protein family discussed in this mini-review. Note many other examples might exit.
Human AGP (hAGP) is actually not a single unique protein. Instead, two main forms of AGP coexists in humans. They are encoded by a cluster of genes: AGP1 is encoded by the ORM1 gene and AGP2 by the ORM2 gene. Both genes have identical structures with 5 introns (Sanchez et al., 2003), and AGP1/2 sequences only differ in 22 amino acids. Besides this complexity, ORM1 gene has three common variants: F1, F2, and S (collectively referred as F∗S). Equally, AGP2 is sometimes referred as variant A. Interestingly, other mammals have a different number of Orm genes. For instance, AGP is coded by three Orm genes in mouse, whereas rats have a single gene. The different number of “AGP-genes” in laboratory animal models could be turned into a research advantage, though this has not been much exploited yet.
The crystals of unglycosylated AGP1/2 (produced in E. coli) revealed a typical Lipocalin fold comprising an eight-stranded β-barrel which is flanked by a C-terminal α-helix (Figure 1A; Schonfeld et al., 2008; Nishi et al., 2011). Four loops connect the β-sheets and a tryptophan is buried inside of the cavity. This is one of the few, but key, amino acid mostly conserved across the Lipocalin family (Flower et al., 2000; Ganfornina et al., 2000; Schiefner and Skerra, 2015). AGP2 ligand-binding region is narrower than that in AGP1, explaining the different compound-binding affinities reported for AGP1/2 (several examples can be found in Herve et al., 1993; Herve et al., 1996, 1998; Kuroda et al., 2003; Zsila et al., 2008; Nishi et al., 2011). Two disulfide bridges stabilize the structure of hAGPs (Schonfeld et al., 2008; Nishi et al., 2011). Interestingly, hAGP2 has an extra free Cys that could form a covalent binding with other proteins or participate in redox reactions, though this is merely a speculation.
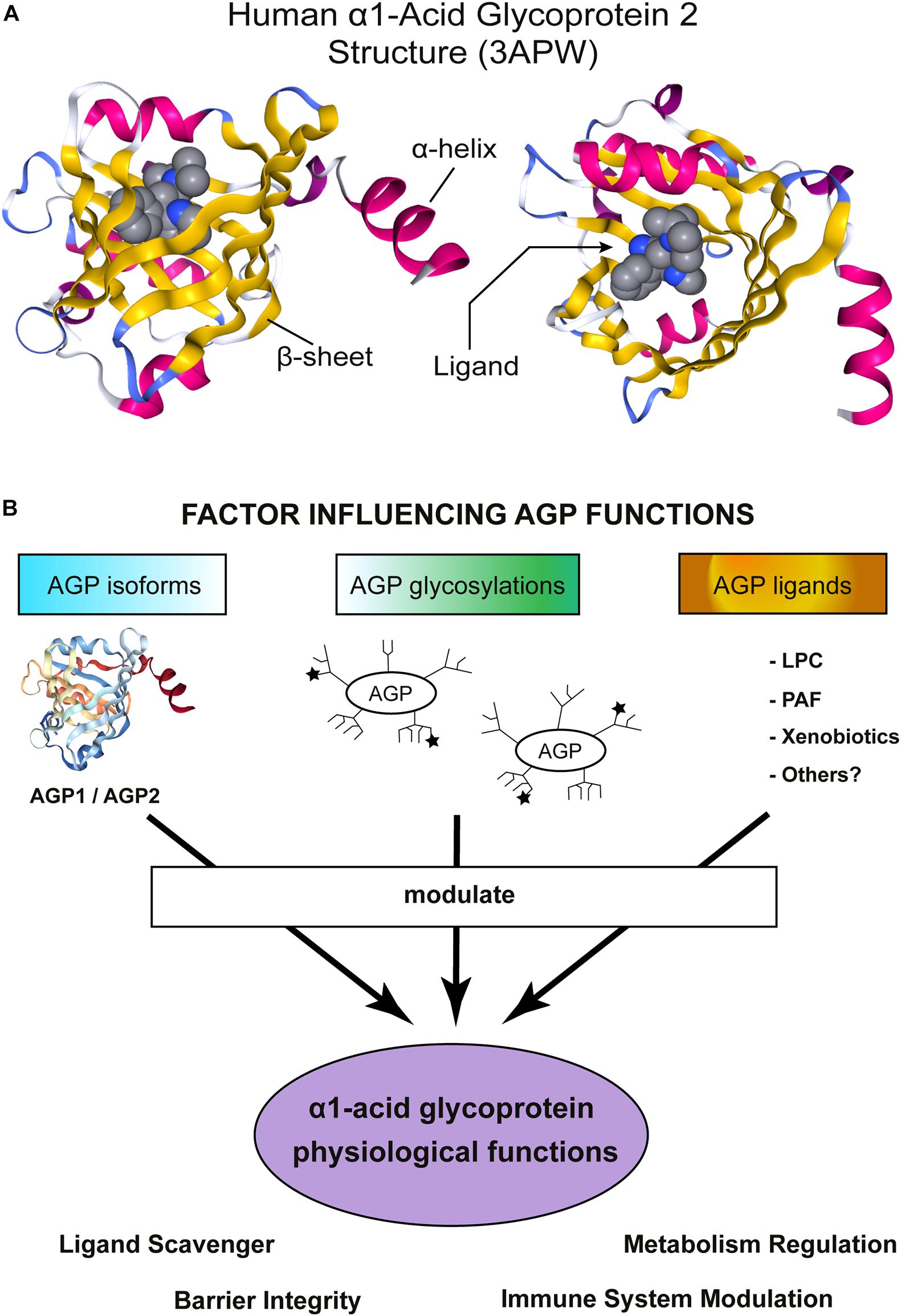
Figure 1. α1-Acid Glycoprotein (AGP) structure and functions. (A) Schematic representation of hAGP2 structure in complex with a drug in the Lipocalin cavity. In this case the ligand is the antiarrhythmic medication disopyramide (3APW) [originaly published by Nishi et al. (2011)]. The left model is a lateral view of AGP2 and the right model show the view from the top, the open side of AGP β-barrel. The secondary structures are colored: β-sheets in yellow and α-helix in magenta. The ligand (disopyramide) is represented in a bulky format to highlight the cavity inside of AGP2. The figure was generating using NGL viewer (Rose et al., 2018). (B) Schematic representation showing important factors influencing AGP functions. AGP is highly heterogenic and numerous activities in vitro and in vivo have been reported. AGP is an acute-phase protein that modulates the immune system and, recently, AGP has also been shown to regulate the metabolism. AGP tissue/cell type of expression determines which isoform is expressed: AGP1 or AGP2. Then, each tissue counts with a specific set of enzymes to glycosylate proteins and, indeed, multiple glycosylation patterns have been detected in AGP. The ★ symbols represent the possible presence of sLex groups. Note that the schematic cartoon of the glycosylation does not represent the actual complex and heterogeneous AGP glycosylation pattern. More detailed information can be found in Baerenfaenger and Meyer (2018), Keser et al. (2021). Finally, the environment where AGP is secreted allows to bind/scavenger a different set of ligands, Indeed, an enormous number of compounds are efficiently bound by AGP. The sum of all the above-mentioned factors would contribute to AGP physiological functions.
α1-acid glycoprotein is heavily glycosylated, five N-linked glycans are present in hAGPs. These glycans represent around 45% of the AGP molecular weight and contain a high proportion of sialic acid, giving AGP its characteristic acid isoelectric point (pI = 2.7–3.2). In contrast, the unglycosylated AGP pI was calculated to be 4.97. Glycosylation increases AGP solubility but, importantly, increases its molecular weight such that it escapes glomerular filtration in the kidneys. AGP glycosylation pattern is rather complex and heterogeneous [reviewed in detail by Fournier et al. (2000); and further studied by Fernandes et al., 2015]. Multiple glycan combinations have been detected in the plasma of healthy humans (Treuheit et al., 1992; Ongay and Neususs, 2010; Baerenfaenger and Meyer, 2018; Keser et al., 2021) and changes under pathological states have been reported (De Graaf et al., 1993; Liang et al., 2019; Keser et al., 2021). More specifically, branches with sialic acid are also fucosilated creating highly biologically active sialyl-Lewis X epitopes (sLex). It is not fully clear how glycosylation affects AGP binding toward endogenous ligands, but the fact that Asn-75 localizes near to the entrance of the binding pocket should be considered (Nishi et al., 2011). Additionally, there are documented examples where branching and fucosylation do limit drug-binding affinity (i.e., Wu et al., 2018).
Evolutionary, Lipocalins were classified in fourteen clades (I-XIV), where AGP clusters among the modern ones and included in clade XII (Gutierrez et al., 2000). AGP is only found in vertebrates and classified as an “outlier Lipocalin” because it contains only one of the three Lipocalin structurally conserved regions (SCRs) (Flower et al., 2000). Besides the traditional way of classifying Lipocalins, the term Immunocalins was proposed some time ago (Logdberg and Wester, 2000). Immunocalins would be a group of proteins sharing the Lipocalin fold and involved in immune system regulation. The founder group included: AGP, α1-Microglobulin, Glycodelin, and Lipocalin 2 (Lcn2 and also known as Siderocalin, NGAL, or 24p3), Complement Factor 8, γ-subunit, Tear Lipocalin (also known as Lcn1 or Von Ebner’s gland protein) and Lipocalin Prostaglandin D Synthase (L-PGDS). Even though the concept is still valid, we now know that many other Lipocalins modulate the immune response. Examples include Apolipoprotein D (ApoD) (Dassati et al., 2014) and Apolipoprotein M (ApoM) (Frej et al., 2017; Ruiz et al., 2017). Altogether then, there is a mounting evidence that the first ancestral Lipocalin may have had general defensive functions.
A powerful approach to investigate a Lipocalin’s physiological function(s) is often to identify its ligand(s). Such an approach would be suitable for some Lipocalins, such as RBP (Retinol Binding Protein 4), L-PGDS or ApoM. However, AGP is much more complex: it has a promiscuous ligand-binding behavior and is capable of binding hundreds of molecules from endogenous or exogenous origin. AGP has one primary high-affinity binding-site -“the classical Lipocalin binding-site”- but other sites with different capacities and lower affinity exists. Binding data for more than 300 drugs and endogenous substances were compiled several years ago and the list of compounds keeps growing (Israili and Dayton, 2001; Wishart et al., 2006; Zsila et al., 2006; di Masi et al., 2016; Smith and Waters, 2018). AGP binds mainly to basic molecules, given its highly acidic nature, but it is also able to bind neutral and acidic drugs (Taguchi et al., 2013).
Several endogenous molecules bind to AGP. Catecholamines are long-known but are low-affinity ligands of AGP (Sager et al., 1987). Similarly, the ability of AGP to bind progesterone with low affinity was documented earlier (Westphal et al., 1961; Ganguly et al., 1967), and further confirmed with modern methods (Albani, 1997; Ojala et al., 2006; Huang and Hudgens, 2013). It was suggested that progesterone sequestration by AGP would represent a buffer system (Westphal et al., 1961), but not much have been experimentally demonstrated. Curiously, plasma AGP is not the only Lipocalin able to bind progesterone. ApoD was isolated as a progesterone-binding protein from mammary cystic fluid (Pearlman et al., 1973; Rassart et al., 2000) and even the complex crystalized (Eichinger et al., 2007). Additionally, recombinant ApoM showed certain ability to interact with progesterone in vitro (Ahnstrom et al., 2007), but no evidences of progesterone being an endogenous ApoM ligand have been presented so far.
Another report strongly argued for biliverdin as the endogenous ligand of hAGP1. Even though convincing in vitro data supports the binding, no evidences of biliverdin as endogenous ligand were presented (Zsila and Mady, 2008). The authors speculated that inside of the β -barrel, biliverdin might be transiently protected from enzymatic oxidation, thereby preventing accumulation of toxic bilirubin (Zsila and Mady, 2008).
In the search of physiological AGP ligand(s), a big effort was implement in Finland a few years ago (Ojala et al., 2006). Large amounts of AGP were isolated from plasma, followed by lipid extraction and mass spectrometry analysis. Significant amounts of lysophospholipids, and more specifically lysophosphatidylcholines (LPC) with unsaturated acyl chains, were identified. Further in vitro ligand-binding assays confirmed the highest affinity for LPC20:4, LPC18:3, and LPC18:1. However, AGP was also able to efficiently bind free fatty acids and platelet activated factor [(PAF), 1-alkyl-2-acetyl-sn-glycero-3-phosphocholine, (as also previously reported in McNamara et al., 1986)]. Finally, to highlight the biological relevance of AGP-LPC and AGP-PAF complexes, it was shown that AGP prevented LPC-induced priming and PAF-induced activation of human granulocytes (Ojala et al., 2006). Systemically, several studies have shown that AGP contributes to maintain cellular barriers in the kidneys, lungs, brain and vessels (i.e., Haraldsson et al., 1992; Johnsson and Haraldsson, 1993; Muchitsch et al., 1996, 1998, 2000; Sorensson et al., 1999) whereas LPC and PAF induce permeability (Huang et al., 2005; Hudry-Clergeon et al., 2005); suggesting that AGP could be a LPC/PAF scavenger. Interestingly, the expression of another Lipocalin, ApoM, is induced by PAF (Xu et al., 2002) and ApoM is fundamental to maintain barrier function (Christoffersen et al., 2011; Ruiz et al., 2017; Mathiesen Janiurek et al., 2019). In conclusion, the work by Ojala et al. (2006) could potentially explain the anti-inflammatory/protective effects of AGP and it probably has been the best attempt to explain the physiological relevance of AGP ligand(s).
Many compounds show potential therapeutic capacity when examined in vitro or in animal models. However, the impressive ability of AGP to bind drugs sometimes represents a limitation for their clinical use (as discussed on Smith and Waters, 2018; Bteich, 2019; Bteich et al., 2021). UCN-01 (7-hydroxystaurosporine) is an anti-cancer drug and its sequestration by AGP is a classic example of AGP affecting drug pharmacokinetics and pharmacodynamics. hAGP displays an unexpected high affinity for UCN-01 and hence increases drug plasma concentration, while blocking its distribution and elimination. In contrast, canine AGP has lower affinity for UCN-01 and therefore has little effect on the pharmacodynamics of UCN-01 and rat AGP exhibited only weak and nonspecific binding to UCN-01 (Fuse et al., 1998, 1999). Interestingly, encapsulation of UCN-01 in liposomes has been proposed to reduce the impact of AGP on UCN-01 pharmacodynamics (Yamauchi et al., 2005, 2008).
Examples of recent studies on drugs bound by AGP include: Warfarin (Hanada, 2017), Pinometostat (Smith et al., 2017), Aripiprazole (Nishi et al., 2019), Imatinib (Mic et al., 2020), Voriconazole (Yuan et al., 2020), ONO-2160 (Kono et al., 2019, 2021), SCO-272 (Ebihara et al., 2018, 2019), and Brigatinib (Wang et al., 2020).
The paragraphs above discussed the binding capabilities of AGP. But it is unclear if AGP is a passive scavenger protein or, alternatively, whether AGP delivers its cargos to particular receptors. Several membrane proteins have been reported to interact with AGP. For instance, AGP binds to the C-C chemokine receptor type 5 (CCR5) in the plasma membrane of macrophages and skeletal muscle cells (Atemezem et al., 2001; Lei et al., 2016). Both, AGP polypeptide and glycans are important for AGP-CCR5 interaction. Speculatively, the authors suggested that AGP-CCR5 association could block the infection of macrophages by the HIV-1 virus (Seddiki et al., 1997).
The presence of numerous sLex groups in AGP have led to depict AGP as an interacting partner of the endothelial adhesion molecules P-selectin and E-selectin. In this way, AGP binding would block the adhesion of circulating leukocytes to the endothelium upon inflammatory stimuli (Jorgensen et al., 1998; Hochepied et al., 2000). A structural model of AGP and P-selectin interaction have been calculated in silico (Fernandes et al., 2015). Furthermore, AGP sLex groups mediate the interaction of AGP with immunoglobulin-like lectins (Siglecs) (Gunnarsson et al., 2007) and modulate reactive oxygen species (ROS) generation in neutrophils (Gunnarsson et al., 2010).
Finally, the liver-expressed asialoglycoprotein receptor binds molecules of AGP in which the terminal sialic groups are missing and efficiently clears asialo-AGP from circulation (Kindberg et al., 1990; Matsumoto et al., 2010). It has been suggested that another, yet unknown, receptor would mediate AGP uptake (with sialic acid residues) (Matsumoto et al., 2010; Taguchi et al., 2013). However, it is not known if AGP is simply targeted for degradation or has also intracellular functions.
Remarkably, AGP binds to membranes and undergoes a pH-induced conformational change (a unique transition from a β-sheet-rich structure to an α-helix-rich structure) which caused a decrease in AGP affinity for progesterone (Nishi et al., 2002, 2004, 2006). This has been interpreted as a mechanism to release molecules inside of the cell (illustrated in Taguchi et al., 2013). However, this has also been interpreted in the opposite way: as a mechanism to sequester LPC/PAF from the plasma membranes where they are generated (Ojala et al., 2006). Interestingly, this unique β-sheet to α-helix transition has also been reported for Tear Lipocalin (Gasymov et al., 1998). Follow-up investigations to address the relevance of AGP β-sheet to α-helix transition in vivo would be highly valuable.
α1-acid glycoprotein being an acute-phase protein, most of the early in vivo studies were related to inflammatory insults. For that, different transgenic mouse models were initially created to study AGP. Bacterial lipopolysaccharide (LPS) strongly induced expression and liver secretion of hAGP1 in mice carrying the whole hAGP gene cluster (ORM1, ORM2, and ORM3 genes) or a fragment with only the ORM1 gene (Dente et al., 1988). Later, another transgenic mouse in which the rat Orm gene was over-expressed was made. LPS, IL-1, IL-6, or glucocorticoids were used to trigger the inflammatory response and this boosted rated AGP expression several folds (Dewey et al., 1990). In general, AGP has shown to be protective in vivo against inflammatory insults (as summarized in Hochepied et al., 2003). One example is that the intraperitoneal injection of hAGP (but also rat and bovine) protected against lethal shock induced by TNFα (Libert et al., 1994). However, the overexpression of rat AGP led to a more aggressive development of acute colitis (Hochepied et al., 2002).
Another example of a protective effect is that pre-administration of exogenous bovine or hAGP or transgenic over-expression of rat AGP in mice, significantly increased survival against a lethal infection with the Gram-negative bacteria Klebsiella pneumoniae (Hochepied et al., 2000) or Bacillus anthracis (Shemyakin et al., 2005). However, the molecular mechanism involved is unknown. One explanation could be the reported capacity of AGP to form complexes with LPS (Moore et al., 1997). However, AGP-LPS complexes cannot explain the documented protection against the Gram-positive Bacillus anthracis. Alternatively, a recent paper proposed a direct action of AGP on bacterial growth. Siderophores are small molecules secreted by bacteria to secure their iron supply (a scarce and essential micronutrient) and growth. The authors reasoned that many bacteria secrete stealth siderophores that escape Lcn2 recognition (the archetype Siderocalin), and suggested that AGP may be a “Siderocalin” and hence able to bind siderophores (Samsonov et al., 2021). Interestingly, Tear Lipocalin, a highly abundant Lipocalin in secretions, interferes with microbial growth by scavenging of siderophores (Fluckinger et al., 2004), Tear Lipocalin is, however, not present in plasma. Given the similarities between Lcn2, Tear Lipocalin and AGP, one may speculate that AGP can also neutralize siderophores escaping Lcn2 entrapment and inhibit K. pneumoniae or B. anthracis growth. Unfortunately, AGP computational experiments were inconclusive for K. pneumoniae siderophores (Samsonov et al., 2021). However, the results were more positive about petrobactin (one of the siderophores secreted by B. anthracis) being a candidate ligand for AGP (Samsonov et al., 2021). In any case, the ability of AGP to bind siderophores and inhibit bacterial growth needs to be experimentally demonstrated.
AGP1 is highly abundant in plasma (∼0.075 g/dl; ∼15 μM) (Kremer et al., 1988; Gannon et al., 2019; McDonald et al., 2020), easy to purify and represents a relatively affordable commercial source of AGP protein for in vitro and in vivo experiments. However, there are some limitations that can complicate the interpretations of the results. First, different batches of protein come from different donors and likely have distinct glycosylation patterns. Importantly, mouse and human livers possess a different set of fucosyltransferases and hAGP produced in mice lacks sLex (Havenaar et al., 1998). sLex groups can be important to efficiently modulate hAGP function. Thus, hAGP might be not fully functional in murine experimental models. Additionally, isolated AGP will likely come with uncharacterized ligand(s) in its cavity and variations in the purification protocols may impact their presence and nature.
The absence of AGP-KO animal model was a strong limitation to understand AGP functions, until, for the first time, an Orm1-KO mouse was published in 2016 (Lei et al., 2016). The newly created mouse mutants were first used to demonstrate that AGP1 binds to CCR5 on skeletal muscle cells to increase muscle endurance (Lei et al., 2016). Later, the same group showed that AGP1-CCR5 increased the activity of glycogen synthase (the rate-limiting enzyme in the glycogen synthesis pathway) via AMPKα2 (Qin et al., 2016). Further, they identified estrogens (as a negative) and erythromycin (as a positive) regulators of the AGP1-CCR5 pathway (Sun et al., 2018; Wan et al., 2020).
Interestingly, Orm1-KO mice show altered metabolic parameters, such as increased levels of insulin and leptin together with impaired glucose tolerance (Sun et al., 2016) and AGP1 deficiency increases the expression of genes related to fibrosis in adipose tissue (Wang et al., 2021). Previously, and in agreement with the Orm1-KO mouse model, the continuous systemic infusion of hAGP1 improved glucose and insulin tolerance in obese/diabetic mice (Lee et al., 2010). Further explorations in the Orm1-KO mouse led to the discovery that AGP inhibits food intake. Mechanistically, AGP1 interacts with the leptin receptor (Lepr) in the hypothalamus and activates the JAK2-STAT3 pathway to inhibited food intake (Sun et al., 2016). However, it is not clear how AGP1, the main isoform in circulation, crosses the blood brain barrier to interact with the Lepr in the hypothalamus. Additionally, the main isoform in the brain is AGP2 and its levels do not change under metabolic stress (Sun et al., 2016). Even though, the nature of the AGP-Lepr interaction model is not completely understood, its existence is certainly an interesting observation. ApoD, the most ancestral Lipocalin in vertebrates, has also been shown to interact with the Lepr (Liu et al., 2001). ApoD interaction is thought to take place with the cytosolic domain of the Lepr (Liu et al., 2001), whereas AGP interaction was modeled to occur via the leptin-binding domain of the Lepr (Sun et al., 2016).
The first complete AGP-KO (Orm1, Orm2 and Orm3-KO) was finally published last year, 2020 (Watanabe et al., 2020). The AGP-KO mice did not show any obvious defects in appearance or growth. However, the AGP-KO animals had exacerbated fibrosis, inflammatory response and macrophage infiltration in a model of renal fibrosis (Watanabe et al., 2020, 2021). Accordingly, AGP administration reduced renal fibrosis and inflammation (Bi et al., 2018). Interestingly, all-trans retinoic acid treatment boosted AGP serum concentration in plasma and required AGP to protect against renal fibrosis. So, how do all-trans retinoic acid and AGP damper renal fibrosis and the immune response? It is noteworthy that all-trans retinoic acid is a classical Lipocalin ligand and binds to AGP with micromolar affinity (Breustedt et al., 2006; Ruiz et al., 2013). Therefore, all-trans retinoic acid might just induce AGP expression that then transports it to the damaged area? Interestingly, the major transporter of retinol in plasma, the Lipocalin RBP4, is a negative acute-phase protein (Rosales et al., 1996). Thus, AGP could take the place of RBP4 and transport retinols during inflammation.
Interestingly, AGP1-KO did not affect the infarct area in a model of ischemic stroke (even when the blood brain barrier was compromised). Instead, the expression of AGP2 was induced in the ischemic tissue (Wan et al., 2016, 2019). Unfortunately, an AGP2-KO model was not available at that time. Therefore, the availability of a full AGP-KO is now a great tool to explore anew the role of AGP in the central nervous system. Expression of AGP2 in the brain is induced upon systemic inflammation, astrocytes being the main source of AGP. Mechanistically, AGP2 inhibited CCL4-induced microglial activation by blocking the interaction of AGP with CCR5 and reduced microglia-mediated neurotoxicity (Jo et al., 2017). Noteworthy, other Lipocalins are also expressed in glial cells. For instance, astrocytes express ApoD upon stress conditions to promote neuronal survival (Bajo-Graneras et al., 2011; Pascua-Maestro et al., 2018). Oppositely to AGP and ApoD, Lcn2 is an autocrine mediator of astrocytosis and renders astrocytes more sensitive to cell-death signals (Lee et al., 2009). To add one extra level of complexity, only apo-Lcn2 (no-ligand bound) sensitized activated astrocytes to cell-death (Lee et al., 2009). Therefore, it would be relevant to investigate if any ligand mediates the protection by AGP in the brain upon inflammation.
α1-acid glycoprotein expression is strongly up-regulated during the acute-phase response probably as a counter-balance to damper an excessive inflammatory response. Thus, AGP is typically associated with protection. Interestingly, AGP investigations are not limited to inflammation and new studies reported an active role of AGP in metabolic regulation. One of the most interesting features of AGP is its heterogeneity, from the amino acid sequence to the glycosylation pattern (Figure 1B). Multiple AGP forms are possible which suggests the existence of fine-tuned mechanisms to regulate AGP functions and highlights AGP versatility to participate in multiple process. The best example of AGP versatility is its ability to bind hundreds of small molecules (Figure 1B). Despite thousands of publications about AGP, its molecular functions are not fully understood. Hopefully, the newly created AGP-KO mice will help to shed light on AGP physiological roles.
The author confirms being the sole contributor of this work and has approved it for publication.
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author thank Prof. Marc Pilon for its support, critical comments on the manuscript, and for giving me the time to write this review; Profs. Diego Sánchez and Maria D. Ganfornina for introducing me in the Lipocalin world and encouraging me to contribute to this special issue on Lipocalins; and Drs. Beatriz Martinez-Abad and Ranjan Devkota for their critical and constructive comments.
Ahnstrom, J., Faber, K., Axler, O., and Dahlback, B. (2007). Hydrophobic ligand binding properties of the human lipocalin apolipoprotein M. J Lipid Res 48, 1754–1762. doi: 10.1194/jlr.m700103-jlr200
Albani, J. R. (1997). Binding effect of progesterone on the dynamics of alpha1-acid glycoprotein. Biochim Biophys Acta 1336, 349–359. doi: 10.1016/s0304-4165(97)00043-3
Atemezem, A., Mbemba, E., Vassy, R., Slimani, H., Saffar, L., and Gattegno, L. (2001). Human alpha1-acid glycoprotein binds to CCR5 expressed on the plasma membrane of human primary macrophages. Biochem J 356, 121–128. doi: 10.1042/0264-6021:3560121
Baerenfaenger, M., and Meyer, B. (2018). Intact Human Alpha-Acid Glycoprotein Analyzed by ESI-qTOF-MS: Simultaneous Determination of the Glycan Composition of Multiple Glycosylation Sites. J Proteome Res 17, 3693–3703. doi: 10.1021/acs.jproteome.8b00309
Bajo-Graneras, R., Sanchez, D., Gutierrez, G., Gonzalez, C., Do Carmo, S., Rassart, E., et al. (2011). Apolipoprotein D alters the early transcriptional response to oxidative stress in the adult cerebellum. J Neurochem 117, 949–960. doi: 10.1111/j.1471-4159.2011.07266.x
Bi, J., Watanabe, H., Fujimura, R., Nishida, K., Nakamura, R., Oshiro, S., et al. (2018). A downstream molecule of 1,25-dihydroxyvitamin D3, alpha-1-acid glycoprotein, protects against mouse model of renal fibrosis. Sci Rep 8, 17329.
Breustedt, D. A., Schonfeld, D. L., and Skerra, A. (2006). Comparative ligand-binding analysis of ten human lipocalins. Biochim Biophys Acta 1764, 161–173. doi: 10.1016/j.bbapap.2005.12.006
Bteich, M. (2019). An overview of albumin and alpha-1-acid glycoprotein main characteristics: highlighting the roles of amino acids in binding kinetics and molecular interactions. Heliyon 5, e02879. doi: 10.1016/j.heliyon.2019.e02879
Bteich, M., Poulin, P., and Haddad, S. (2021). Comparative Assessment of Extrapolation Methods Based on the Conventional Free Drug Hypothesis and Plasma Protein-Mediated Hepatic Uptake Theory for the Hepatic Clearance Predictions of Two Drugs Extensively Bound to Both the Albumin And Alpha-1-Acid Glycoprotein. J Pharm Sci 110, 1385–1391. doi: 10.1016/j.xphs.2020.11.009
Chiu, K. M., Mortensen, R. F., Osmand, A. P., and Gewurz, H. (1977). Interactions of alpha1-acid glycoprotein with the immune system. I. Purification and effects upon lymphocyte responsiveness. Immunology 32, 997–1005.
Christoffersen, C., Obinata, H., Kumaraswamy, S. B., Galvani, S., Ahnstrom, J., Sevvana, M., et al. (2011). Endothelium-protective sphingosine-1-phosphate provided by HDL-associated apolipoprotein M. Proc Natl Acad Sci U S A 108, 9613–9618. doi: 10.1073/pnas.1103187108
Costello, M., Fiedel, B. A., and Gewurz, H. (1979). Inhibition of platelet aggregation by native and desialised alpha-1 acid glycoprotein. Nature 281, 677–678. doi: 10.1038/281677a0
Dassati, S., Waldner, A., and Schweigreiter, R. (2014). Apolipoprotein D takes center stage in the stress response of the aging and degenerative brain. Neurobiol Aging 35, 1632–1642. doi: 10.1016/j.neurobiolaging.2014.01.148
De Graaf, T. W., Van Der Stelt, M. E., Anbergen, M. G., and Van Dijk, W. (1993). Inflammation-induced expression of sialyl Lewis X-containing glycan structures on alpha 1-acid glycoprotein (orosomucoid) in human sera. J Exp Med 177, 657–666. doi: 10.1084/jem.177.3.657
Dente, L., Ruther, U., Tripodi, M., Wagner, E. F., and Cortese, R. (1988). Expression of human alpha 1-acid glycoprotein genes in cultured cells and in transgenic mice. Genes Dev 2, 259–266. doi: 10.1101/gad.2.2.259
Dewey, M. J., Rheaume, C., Berger, F. G., and Baumann, H. (1990). Inducible and tissue-specific expression of rat alpha-1-acid glycoprotein in transgenic mice. J Immunol 144, 4392–4398.
di Masi, A., Trezza, V., Leboffe, L., and Ascenzi, P. (2016). Human plasma lipocalins and serum albumin: Plasma alternative carriers? J Control Release 228, 191–205. doi: 10.1016/j.jconrel.2016.02.049
Ebihara, T., Nishihara, M., Takahashi, J., Jinno, F., and Tagawa, Y. (2018). Differences in nonclinical pharmacokinetics between species and prediction of human pharmacokinetics of TAK-272 (SCO-272), a novel orally active renin inhibitor. Biopharm Drug Dispos 39, 175–183. doi: 10.1002/bdd.2124
Ebihara, T., Shimizu, H., Yamamoto, M., Higuchi, T., Jinno, F., and Tagawa, Y. (2019). The effect of elevated alpha1-acid glycoprotein on the pharmacokinetics of TAK-272 (SCO-272), an orally active renin inhibitor, in rats. Xenobiotica 49, 584–590. doi: 10.1080/00498254.2018.1480817
Eichinger, A., Nasreen, A., Kim, H. J., and Skerra, A. (2007). Structural insight into the dual ligand specificity and mode of high density lipoprotein association of apolipoprotein D. J Biol Chem 282, 31068–31075. doi: 10.1074/jbc.m703552200
Fernandes, C. L., Ligabue-Braun, R., and Verli, H. (2015). Structural glycobiology of human alpha1-acid glycoprotein and its implications for pharmacokinetics and inflammation. Glycobiology 25, 1125–1133. doi: 10.1093/glycob/cwv041
Flower, D. R., North, A. C., and Sansom, C. E. (2000). The lipocalin protein family: structural and sequence overview. Biochim Biophys Acta 1482, 9–24. doi: 10.1016/s0167-4838(00)00148-5
Fluckinger, M., Haas, H., Merschak, P., Glasgow, B. J., and Redl, B. (2004). Human tear lipocalin exhibits antimicrobial activity by scavenging microbial siderophores. Antimicrob Agents Chemother 48, 3367–3372. doi: 10.1128/aac.48.9.3367-3372.2004
Fournier, T., Medjoubi, N. N., and Porquet, D. (2000). Alpha-1-acid glycoprotein. Biochim Biophys Acta 1482, 157–171.
Frej, C., Mendez, A. J., Ruiz, M., Castillo, M., Hughes, T. A., Dahlback, B., et al. (2017). A Shift in ApoM/S1P Between HDL-Particles in Women With Type 1 Diabetes Mellitus Is Associated With Impaired Anti-Inflammatory Effects of the ApoM/S1P Complex. Arterioscler Thromb Vasc Biol 37, 1194–1205. doi: 10.1161/atvbaha.117.309275
Fuse, E., Tanii, H., Kurata, N., Kobayashi, H., Shimada, Y., Tamura, T., et al. (1998). Unpredicted clinical pharmacology of UCN-01 caused by specific binding to human alpha1-acid glycoprotein. Cancer Res 58, 3248–3253.
Fuse, E., Tanii, H., Takai, K., Asanome, K., Kurata, N., Kobayashi, H., et al. (1999). Altered pharmacokinetics of a novel anticancer drug, UCN-01, caused by specific high affinity binding to alpha1-acid glycoprotein in humans. Cancer Res 59, 1054–1060.
Ganfornina, M. D., Gutierrez, G., Bastiani, M., and Sanchez, D. (2000). A phylogenetic analysis of the lipocalin protein family. Mol Biol Evol 17, 114–126. doi: 10.1093/oxfordjournals.molbev.a026224
Ganguly, M., Carnighan, R. H., and Westphal, U. (1967). Steroid-protein interactions. XIV. Interaction between human alpha 1-acid glycoprotein and progesterone. Biochemistry 6, 2803–2814. doi: 10.1021/bi00861a022
Gannon, B. M., Glesby, M. J., Finkelstein, J. L., Raj, T., Erickson, D., and Mehta, S. (2019). A point-of-care assay for alpha-1-acid glycoprotein as a diagnostic tool for rapid, mobile-based determination of inflammation. Curr Res Biotechnol 1, 41–48. doi: 10.1016/j.crbiot.2019.09.002
Gasymov, O. K., Abduragimov, A. R., Yusifov, T. N., and Glasgow, B. J. (1998). Structural changes in human tear lipocalins associated with lipid binding. Biochim Biophys Acta 1386, 145–156. doi: 10.1016/s0167-4838(98)00092-2
Gunnarsson, P., Fornander, L., Pahlsson, P., and Grenegard, M. (2010). Sialic acid residues play a pivotal role in alpha(1)-acid glycoprotein (AGP)-induced generation of reactive oxygen species in chemotactic peptide pre-activated neutrophil granulocytes. Inflamm Res 59, 89–95. doi: 10.1007/s00011-009-0071-1
Gunnarsson, P., Levander, L., Pahlsson, P., and Grenegard, M. (2007). The acute-phase protein alpha 1-acid glycoprotein (AGP) induces rises in cytosolic Ca2+ in neutrophil granulocytes via sialic acid binding immunoglobulin-like lectins (siglecs). FASEB J 21, 4059–4069. doi: 10.1096/fj.07-8534com
Gutierrez, G., Ganfornina, M. D., and Sanchez, D. (2000). Evolution of the lipocalin family as inferred from a protein sequence phylogeny. Biochim Biophys Acta 1482, 35–45. doi: 10.1016/s0167-4838(00)00151-5
Hanada, K. (2017). Lipophilicity Influences Drug Binding to alpha1-Acid Glycoprotein F1/S Variants But Not to the A Variant. Drugs R D 17, 475–480. doi: 10.1007/s40268-017-0193-9
Haraldsson, B. S., Johnsson, E. K., and Rippe, B. (1992). Glomerular permselectivity is dependent on adequate serum concentrations of orosomucoid. Kidney Int 41, 310–316. doi: 10.1038/ki.1992.43
Havenaar, E. C., Hoff, R. C., Van Den Eijnden, D. H., and Van Dijk, W. (1998). Sialyl Lewis(x) epitopes do not occur on acute phase proteins in mice: relationship to the absence of alpha3-fucosyltransferase in the liver. Glycoconj J 15, 389–395.
Herve, F., Caron, G., Duche, J. C., Gaillard, P., Abd Rahman, N., Tsantili-Kakoulidou, A., et al. (1998). Ligand specificity of the genetic variants of human alpha1-acid glycoprotein: generation of a three-dimensional quantitative structure-activity relationship model for drug binding to the A variant. Mol Pharmacol 54, 129–138. doi: 10.1124/mol.54.1.129
Herve, F., Duche, J. C., D’athis, P., Marche, C., Barre, J., and Tillement, J. P. (1996). Binding of disopyramide, methadone, dipyridamole, chlorpromazine, lignocaine and progesterone to the two main genetic variants of human alpha 1-acid glycoprotein: evidence for drug-binding differences between the variants and for the presence of two separate drug-binding sites on alpha 1-acid glycoprotein. Pharmacogenetics 6, 403–415. doi: 10.1097/00008571-199610000-00004
Herve, F., Gomas, E., Duche, J. C., and Tillement, J. P. (1993). Evidence for differences in the binding of drugs to the two main genetic variants of human alpha 1-acid glycoprotein. Br J Clin Pharmacol 36, 241–249. doi: 10.1111/j.1365-2125.1993.tb04224.x
Hochepied, T., Berger, F. G., Baumann, H., and Libert, C. (2003). Alpha(1)-acid glycoprotein: an acute phase protein with inflammatory and immunomodulating properties. Cytokine Growth Factor Rev 14, 25–34. doi: 10.1016/s1359-6101(02)00054-0
Hochepied, T., Van Molle, W., Berger, F. G., Baumann, H., and Libert, C. (2000). Involvement of the acute phase protein alpha 1-acid glycoprotein in nonspecific resistance to a lethal gram-negative infection. J Biol Chem 275, 14903–14909. doi: 10.1074/jbc.275.20.14903
Hochepied, T., Wullaert, A., Berger, F. G., Baumann, H., Brouckaert, P., Steidler, L., et al. (2002). Overexpression of alpha(1)-acid glycoprotein in transgenic mice leads to sensitisation to acute colitis. Gut 51, 398–404. doi: 10.1136/gut.51.3.398
Huang, F., Subbaiah, P. V., Holian, O., Zhang, J., Johnson, A., Gertzberg, N., et al. (2005). Lysophosphatidylcholine increases endothelial permeability: role of PKCalpha and RhoA cross talk. Am J Physiol Lung Cell Mol Physiol 289, L176–L185.
Huang, R. Y., and Hudgens, J. W. (2013). Effects of desialylation on human alpha1-acid glycoprotein-ligand interactions. Biochemistry 52, 7127–7136. doi: 10.1021/bi4011094
Hudry-Clergeon, H., Stengel, D., Ninio, E., and Vilgrain, I. (2005). Platelet-activating factor increases VE-cadherin tyrosine phosphorylation in mouse endothelial cells and its association with the PtdIns3’-kinase. FASEB J 19, 512–520.
Israili, Z. H., and Dayton, P. G. (2001). Human alpha-1-glycoprotein and its interactions with drugs. Drug Metab Rev 33, 161–235. doi: 10.1081/dmr-100104402
Jo, M., Kim, J. H., Song, G. J., Seo, M., Hwang, E. M., and Suk, K. (2017). Astrocytic Orosomucoid-2 Modulates Microglial Activation and Neuroinflammation. J Neurosci 37, 2878–2894. doi: 10.1523/jneurosci.2534-16.2017
Johnsson, E., and Haraldsson, B. (1993). Addition of purified orosomucoid preserves the glomerular permeability for albumin in isolated perfused rat kidneys. Acta Physiol Scand 147, 1–8. doi: 10.1111/j.1748-1716.1993.tb09466.x
Jorgensen, H. G., Elliott, M. A., Priest, R., and Smith, K. D. (1998). Modulation of sialyl Lewis X dependent binding to E-selectin by glycoforms of alpha-1-acid glycoprotein expressed in rheumatoid arthritis. Biomed Chromatogr 12, 343–349. doi: 10.1002/(sici)1099-0801(199811/12)12:6<343::aid-bmc760>3.0.co;2-6
Keser, T., Tijardovic, M., Gornik, I., Lukic, E., Lauc, G., Gornik, O., et al. (2021). High-throughput and site-specific N-glycosylation analysis of human alpha-1-acid glycoprotein offers a great potential for new biomarker discovery. Mol Cell Proteomics 20, 100044. doi: 10.1074/mcp.ra120.002433
Kindberg, G. M., Gudmundsen, O., and Berg, T. (1990). The effect of vanadate on receptor-mediated endocytosis of asialoorosomucoid in rat liver parenchymal cells. J Biol Chem 265, 8999–9005. doi: 10.1016/s0021-9258(19)38802-7
Kono, K., Fukuchi, Y., Okawa, H., Nunoya, K. I., Imawaka, H., Watanabe, H., et al. (2019). Unique Hydrolysis of an Ester-Type Prodrug of Levodopa in Human Plasma: Relay-Type Role Sharing between Alpha-1 Acid Glycoprotein and Human Serum Albumin. Mol Pharm 16, 4131–4138. doi: 10.1021/acs.molpharmaceut.9b00435
Kono, K., Nunoya, K. I., Nakamura, Y., Bi, J., Mukunoki, A., Takeo, T., et al. (2021). Species Difference in Hydrolysis of an Ester-type Prodrug of Levodopa in Human and Animal Plasma: Different Contributions of Alpha-1 Acid Glycoprotein. Mol Pharm 18, 1985–1991. doi: 10.1021/acs.molpharmaceut.0c01134
Kremer, J. M., Wilting, J., and Janssen, L. H. (1988). Drug binding to human alpha-1-acid glycoprotein in health and disease. Pharmacol Rev 40, 1–47.
Kuroda, Y., Matsumoto, S., Shibukawa, A., and Nakagawa, T. (2003). Capillary electrophoretic study on pH dependence of enantioselective disopyramide binding to genetic variants of human alpha1-acid glycoprotein. Analyst 128, 1023–1027. doi: 10.1039/b212850k
Lee, S., Park, J. Y., Lee, W. H., Kim, H., Park, H. C., Mori, K., et al. (2009). Lipocalin-2 is an autocrine mediator of reactive astrocytosis. J Neurosci 29, 234–249. doi: 10.1523/jneurosci.5273-08.2009
Lee, S. H., Choi, J. M., Jung, S. Y., Cox, A. R., Hartig, S. M., Moore, D. D., et al. (2021). The bile acid induced hepatokine orosomucoid suppresses adipocyte differentiation. Biochem Biophys Res Commun 534, 864–870. doi: 10.1016/j.bbrc.2020.10.086
Lee, Y. S., Choi, J. W., Hwang, I., Lee, J. W., Lee, J. H., Kim, A. Y., et al. (2010). Adipocytokine orosomucoid integrates inflammatory and metabolic signals to preserve energy homeostasis by resolving immoderate inflammation. J Biol Chem 285, 22174–22185. doi: 10.1074/jbc.m109.085464
Lei, H., Sun, Y., Luo, Z., Yourek, G., Gui, H., Yang, Y., et al. (2016). Fatigue-induced Orosomucoid 1 Acts on C-C Chemokine Receptor Type 5 to Enhance Muscle Endurance. Sci Rep 6, 18839.
Liang, J., Zhu, J., Wang, M., Singal, A. G., Odewole, M., Kagan, S., et al. (2019). Evaluation of AGP Fucosylation as a Marker for Hepatocellular Carcinoma of Three Different Etiologies. Sci Rep 9, 11580.
Libert, C., Brouckaert, P., and Fiers, W. (1994). Protection by alpha 1-acid glycoprotein against tumor necrosis factor-induced lethality. J Exp Med 180, 1571–1575. doi: 10.1084/jem.180.4.1571
Liu, Z., Chang, G. Q., and Leibowitz, S. F. (2001). Apolipoprotein D interacts with the long-form leptin receptor: a hypothalamic function in the control of energy homeostasis. FASEB J 15, 1329–1331. doi: 10.1096/fj.00-0530fje
Logdberg, L., and Wester, L. (2000). Immunocalins: a lipocalin subfamily that modulates immune and inflammatory responses. Biochim Biophys Acta 1482, 284–297. doi: 10.1016/s0167-4838(00)00164-3
Mathiesen Janiurek, M., Soylu-Kucharz, R., Christoffersen, C., Kucharz, K., and Lauritzen, M. (2019). Apolipoprotein M-bound sphingosine-1-phosphate regulates blood-brain barrier paracellular permeability and transcytosis. Elife 8, e49405.
Matsumoto, K., Nishi, K., Kikuchi, M., Watanabe, H., Nakajou, K., Komori, H., et al. (2010). Receptor-mediated uptake of human alpha1-acid glycoprotein into liver parenchymal cells in mice. Drug Metab Pharmacokinet 25, 101–107. doi: 10.2133/dmpk.25.101
McDonald, C. M., Suchdev, P. S., Krebs, N. F., Hess, S. Y., Wessells, K. R., Ismaily, S., et al. (2020). Adjusting plasma or serum zinc concentrations for inflammation: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am J Clin Nutr 111, 927–937. doi: 10.1093/ajcn/nqz304
McNamara, P. J., Brouwer, K. R., and Gillespie, M. N. (1986). Autacoid binding to serum proteins. Interaction of platelet activating factor (PAF) with human serum alpha-1-acid glycoprotein (AAG). Biochem Pharmacol 35, 621–624.
Mic, M., Pirnau, A., Floare, C. G., and Bogdan, M. (2020). Study of the binding affinity between imatinib and alpha-1 glycoprotein using nuclear spin relaxation and isothermal titration calorimetry. Int J Biol Macromol 147, 326–332. doi: 10.1016/j.ijbiomac.2020.01.077
Moore, D. F., Rosenfeld, M. R., Gribbon, P. M., Winlove, C. P., and Tsai, C. M. (1997). Alpha-1-acid (AAG, orosomucoid) glycoprotein: interaction with bacterial lipopolysaccharide and protection from sepsis. Inflammation 21, 69–82.
Muchitsch, E. M., Auer, W., and Pichler, L. (1998). Effects of alpha 1-acid glycoprotein in different rodent models of shock. Fundam Clin Pharmacol 12, 173–181. doi: 10.1111/j.1472-8206.1998.tb00938.x
Muchitsch, E. M., Teschner, W., Linnau, Y., and Pichler, L. (1996). In vivo effect of alpha 1-acid glycoprotein on experimentally enhanced capillary permeability in guinea-pig skin. Arch Int Pharmacodyn Ther 331, 313–321.
Muchitsch, E. M., Varadi, K., and Pichler, L. (2000). Effects of alpha 1-acid glycoprotein on acute pancreatitis and acute lung injury in rats. Arzneimittelforschung 50, 987–994. doi: 10.1055/s-0031-1300322
Nishi, K., Komine, Y., Fukunaga, N., Maruyama, T., Suenaga, A., and Otagiri, M. (2006). Involvement of disulfide bonds and histidine 172 in a unique beta-sheet to alpha-helix transition of alpha 1-acid glycoprotein at the biomembrane interface. Proteins 63, 611–620. doi: 10.1002/prot.20923
Nishi, K., Maruyama, T., Halsall, H. B., Handa, T., and Otagiri, M. (2004). Binding of alpha1-acid glycoprotein to membrane results in a unique structural change and ligand release. Biochemistry 43, 10513–10519. doi: 10.1021/bi0400204
Nishi, K., Ono, T., Nakamura, T., Fukunaga, N., Izumi, M., Watanabe, H., et al. (2011). Structural insights into differences in drug-binding selectivity between two forms of human alpha1-acid glycoprotein genetic variants, the A and F1∗S forms. J Biol Chem 286, 14427–14434. doi: 10.1074/jbc.m110.208926
Nishi, K., Sakai, N., Komine, Y., Maruyama, T., Halsall, H. B., and Otagiri, M. (2002). Structural and drug-binding properties of alpha(1)-acid glycoprotein in reverse micelles. Biochim Biophys Acta 1601, 185–191. doi: 10.1016/s1570-9639(02)00465-x
Nishi, K., Sakurama, K., Kobashigawa, Y., Morioka, H., Udo, N., Hashimoto, M., et al. (2019). Interaction of Aripiprazole With Human alpha1-Acid Glycoprotein. J Pharm Sci 108, 3911–3916.
Ojala, P. J., Hermansson, M., Tolvanen, M., Polvinen, K., Hirvonen, T., Impola, U., et al. (2006). Identification of alpha-1 acid glycoprotein as a lysophospholipid binding protein: a complementary role to albumin in the scavenging of lysophosphatidylcholine. Biochemistry 45, 14021–14031. doi: 10.1021/bi061657l
Ongay, S., and Neususs, C. (2010). Isoform differentiation of intact AGP from human serum by capillary electrophoresis-mass spectrometry. Anal Bioanal Chem 398, 845–855. doi: 10.1007/s00216-010-3948-5
Pascua-Maestro, R., Gonzalez, E., Lillo, C., Ganfornina, M. D., Falcon-Perez, J. M., and Sanchez, D. (2018). Extracellular Vesicles Secreted by Astroglial Cells Transport Apolipoprotein D to Neurons and Mediate Neuronal Survival Upon Oxidative Stress. Front Cell Neurosci 12:526.
Pearlman, W. H., Gueriguian, J. L., and Sawyer, M. E. (1973). A specific progesterone-binding component of human breast cyst fluid. J Biol Chem 248, 5736–5741. doi: 10.1016/s0021-9258(19)43566-7
Qin, X. Y., Hara, M., Arner, E., Kawaguchi, Y., Inoue, I., Tatsukawa, H., et al. (2017). Transcriptome Analysis Uncovers a Growth-Promoting Activity of Orosomucoid-1 on Hepatocytes. EBioMedicine 24, 257–266. doi: 10.1016/j.ebiom.2017.09.008
Qin, Z., Wan, J. J., Sun, Y., Wang, P. Y., Su, D. F., Lei, H., et al. (2016). ORM Promotes Skeletal Muscle Glycogen Accumulation via CCR5-Activated AMPK Pathway in Mice. Front Pharmacol 7:302.
Rassart, E., Bedirian, A., Do Carmo, S., Guinard, O., Sirois, J., Terrisse, L., et al. (2000). Apolipoprotein D. Biochim Biophys Acta 1482, 185–198.
Rosales, F. J., Ritter, S. J., Zolfaghari, R., Smith, J. E., and Ross, A. C. (1996). Effects of acute inflammation on plasma retinol, retinol-binding protein, and its mRNA in the liver and kidneys of vitamin A-sufficient rats. J Lipid Res 37, 962–971. doi: 10.1016/s0022-2275(20)42007-3
Rose, A. S., Bradley, A. R., Valasatava, Y., Duarte, J. M., Prlic, A., and Rose, P. W. (2018). NGL viewer: web-based molecular graphics for large complexes. Bioinformatics 34, 3755–3758. doi: 10.1093/bioinformatics/bty419
Ruiz, M., Frej, C., Holmer, A., Guo, L. J., Tran, S., and Dahlback, B. (2017). High-Density Lipoprotein-Associated Apolipoprotein M Limits Endothelial Inflammation by Delivering Sphingosine-1-Phosphate to the Sphingosine-1-Phosphate Receptor 1. Arterioscler Thromb Vasc Biol 37, 118–129. doi: 10.1161/atvbaha.116.308435
Ruiz, M., Sanchez, D., Correnti, C., Strong, R. K., and Ganfornina, M. D. (2013). Lipid-binding properties of human ApoD and Lazarillo-related lipocalins: functional implications for cell differentiation. FEBS J 280, 3928–3943. doi: 10.1111/febs.12394
Sager, G., Bratlid, H., and Little, C. (1987). Binding of catecholamines to alpha-1 acid glycoprotein, albumin and lipoproteins in human serum. Biochem Pharmacol 36, 3607–3612. doi: 10.1016/0006-2952(87)90009-8
Samsonov, S. A., Zsila, F., and Maszota-Zieleniak, M. (2021). Acute phase alpha1-acid glycoprotein as a siderophore-capturing component of the human plasma: A molecular modeling study. J Mol Graph Model 105, 107861. doi: 10.1016/j.jmgm.2021.107861
Sanchez, D., Ganfornina, M. D., Gutierrez, G., and Marin, A. (2003). Exon-intron structure and evolution of the Lipocalin gene family. Mol Biol Evol 20, 775–783. doi: 10.1093/molbev/msg079
Schiefner, A., and Skerra, A. (2015). The menagerie of human lipocalins: a natural protein scaffold for molecular recognition of physiological compounds. Acc Chem Res 48, 976–985. doi: 10.1021/ar5003973
Schmid, K. (1950). Preparation and Properties of an Acid Glycoprotein Prepared from Human Plasma. J. Am. Chem. Soc 72, 2816. doi: 10.1021/ja01162a553
Schonfeld, D. L., Ravelli, R. B., Mueller, U., and Skerra, A. (2008). The 1.8-A crystal structure of alpha1-acid glycoprotein (Orosomucoid) solved by UV RIP reveals the broad drug-binding activity of this human plasma lipocalin. J Mol Biol 384, 393–405. doi: 10.1016/j.jmb.2008.09.020
Seddiki, N., Rabehi, L., Benjouad, A., Saffar, L., Ferriere, F., Gluckman, J. C., et al. (1997). Effect of mannosylated derivatives on HIV-1 infection of macrophages and lymphocytes. Glycobiology 7, 1229–1236. doi: 10.1093/glycob/7.8.1229
Shemyakin, I. G., Pukhalsky, A. L., Stepanshina, V. N., Shmarina, G. V., Aleshkin, V. A., and Afanas’ev, S. S. (2005). Preventive and therapeutic effects of alpha-acid glycoprotein in mice infected with B. anthracis. Bull Exp Biol Med 140, 439–444. doi: 10.1007/s10517-005-0514-9
Shi, M., Ma, X., Yang, Q., Wang, W., Li, X., Song, X., et al. (2021). miR-362-3p Targets Orosomucoid 1 to Promote Cell Proliferation, Restrain Cell Apoptosis and Thereby Mitigate Hypoxia/Reoxygenation-Induced Cardiomyocytes Injury. Cardiovasc Toxicol 21, 387–398. doi: 10.1007/s12012-020-09631-0
Smith, S. A., Gagnon, S., and Waters, N. J. (2017). Mechanistic investigations into the species differences in pinometostat clearance: impact of binding to alpha-1-acid glycoprotein and permeability-limited hepatic uptake. Xenobiotica 47, 185–193. doi: 10.3109/00498254.2016.1173265
Smith, S. A., and Waters, N. J. (2018). Pharmacokinetic and Pharmacodynamic Considerations for Drugs Binding to Alpha-1-Acid Glycoprotein. Pharm Res 36, 30.
Sorensson, J., Matejka, G. L., Ohlson, M., and Haraldsson, B. (1999). Human endothelial cells produce orosomucoid, an important component of the capillary barrier. Am J Physiol 276, H530–H534.
Sun, Y., Qin, Z., Wan, J. J., Wang, P. Y., Yang, Y. L., Yu, J. G., et al. (2018). Estrogen weakens muscle endurance via estrogen receptor-p38 MAPK-mediated orosomucoid (ORM) suppression. Exp Mol Med 50, e463. doi: 10.1038/emm.2017.307
Sun, Y., Yang, Y., Qin, Z., Cai, J., Guo, X., Tang, Y., et al. (2016). The Acute-Phase Protein Orosomucoid Regulates Food Intake and Energy Homeostasis via Leptin Receptor Signaling Pathway. Diabetes 65, 1630–1641. doi: 10.2337/db15-1193
Taguchi, K., Nishi, K., Giam Chuang, V. T., Maruyama, T., and Otagiri, M. (2013). “Molecular Aspects of Human Alpha-1 Acid Glycoprotein — Structure and Function,” in Acute Phase Proteins, ed. S. Janciauskiene 139–162.
Treuheit, M. J., Costello, C. E., and Halsall, H. B. (1992). Analysis of the five glycosylation sites of human alpha 1-acid glycoprotein. Biochem J 283(Pt 1), 105–112. doi: 10.1042/bj2830105
Wan, J., Qin, Z., Lei, H., Wang, P., Zhang, Y., Feng, J., et al. (2020). Erythromycin has therapeutic efficacy on muscle fatigue acting specifically on orosomucoid to increase muscle bioenergetics and physiological parameters of endurance. Pharmacol Res 161, 105118. doi: 10.1016/j.phrs.2020.105118
Wan, J. J., Qin, Z., and Liu, X. (2016). ORM Elevation in Response to Cognitive Impairment Is an Accompanying Phenomenon. CNS Neurosci Ther 22, 723–724. doi: 10.1111/cns.12586
Wan, J. J., Wang, P. Y., Zhang, Y., Qin, Z., Sun, Y., Hu, B. H., et al. (2019). Role of acute-phase protein ORM in a mice model of ischemic stroke. J Cell Physiol 234, 20533–20545. doi: 10.1002/jcp.28653
Wang, B. L., Kou, S. B., Lin, Z. Y., Shi, J. H., and Liu, Y. X. (2020). Insights on the interaction mechanism of brigatinib to human alpha-1-acid glycoprotein: Experimental and computational approaches. Int J Biol Macromol 157, 340–349. doi: 10.1016/j.ijbiomac.2020.04.151
Wang, P. Y., Feng, J. Y., Zhang, Z., Chen, Y., Qin, Z., Dai, X. M., et al. (2021). The adipokine orosomucoid alleviates adipose tissue fibrosis via the AMPK pathway. Acta Pharmacol Sin 33875797. doi: 10.1038/s41401-021-00666-9
Watanabe, H., Bi, J., Murata, R., Fujimura, R., Nishida, K., Imafuku, T., et al. (2020). A synthetic retinoic acid receptor agonist Am80 ameliorates renal fibrosis via inducing the production of alpha-1-acid glycoprotein. Sci Rep 10, 11424.
Watanabe, H., Fujimura, R., Hiramoto, Y., Murata, R., Nishida, K., Bi, J., et al. (2021). An acute phase protein alpha1-acid glycoprotein mitigates AKI and its progression to CKD through its anti-inflammatory action. Sci Rep 11, 7953.
Weimer, H. E., Mehl, J. W., and Winzler, R. J. (1950). Studies on the mucoproteins of human plasma. V. Isolation and characterization of a homogeneous mucoprotein. J Biol Chem 185, 561–568.
Westphal, U., Ashley, B. D., and Selden, G. L. (1961). Steroid-protein interactions. VII. Interactions of progesterone and corticosteroids with human plasma proteins determined by multiple equilibrium dialysis. Arch Biochem Biophys 92, 441–448.
Wishart, D. S., Knox, C., Guo, A. C., Shrivastava, S., Hassanali, M., Stothard, P., et al. (2006). DrugBank: a comprehensive resource for in silico drug discovery and exploration. Nucleic Acids Res 34, D668–D672.
Wu, D., Struwe, W. B., Harvey, D. J., Ferguson, M. A. J., and Robinson, C. V. (2018). N-glycan microheterogeneity regulates interactions of plasma proteins. Proc Natl Acad Sci U S A 115, 8763–8768. doi: 10.1073/pnas.1807439115
Xu, N., Zhang, X. Y., Dong, X., Ekstrom, U., Ye, Q., and Nilsson-Ehle, P. (2002). Effects of platelet-activating factor, tumor necrosis factor, and interleukin-1alpha on the expression of apolipoprotein M in HepG2 cells. Biochem Biophys Res Commun 292, 944–950. doi: 10.1006/bbrc.2002.6755
Yamauchi, M., Kusano, H., Nakakura, M., and Kato, Y. (2005). Reducing the impact of binding of UCN-01 to human alpha1-acid glycoprotein by encapsulation in liposomes. Biol Pharm Bull 28, 1259–1264. doi: 10.1248/bpb.28.1259
Yamauchi, M., Kusano, H., Saito, E., Abe, M., Tsutsumi, K., Uosaki, Y., et al. (2008). Controlled release of a protein kinase inhibitor UCN-01 from liposomes influenced by the particle size. Int J Pharm 351, 250–258. doi: 10.1016/j.ijpharm.2007.08.021
Yuan, Z. Q., Qiao, C., Yang, Z. C., Yu, L., Sun, L. N., Qian, Y., et al. (2020). The Impact of Plasma Protein Binding Characteristics and Unbound Concentration of Voriconazole on Its Adverse Drug Reactions. Front Pharmacol 11:505.
Zsila, F., and Mady, G. (2008). Biliverdin is the endogenous ligand of human serum alpha1-acid glycoprotein. Biochem Biophys Res Commun 372, 503–507. doi: 10.1016/j.bbrc.2008.05.090
Zsila, F., Matsunaga, H., Bikadi, Z., and Haginaka, J. (2006). Multiple ligand-binding properties of the lipocalin member chicken alpha1-acid glycoprotein studied by circular dichroism and electronic absorption spectroscopy: the essential role of the conserved tryptophan residue. Biochim Biophys Acta 1760, 1248–1273. doi: 10.1016/j.bbagen.2006.04.006
Keywords: α1-acid glycoprotein, orosomucoid, inflammation, metabolism, ligand-binding, Lipocalin
Citation: Ruiz M (2021) Into the Labyrinth of the Lipocalin α1-Acid Glycoprotein. Front. Physiol. 12:686251. doi: 10.3389/fphys.2021.686251
Received: 26 March 2021; Accepted: 17 May 2021;
Published: 08 June 2021.
Edited by:
Belma Turan, Ankara University, TurkeyReviewed by:
Miroslawa Ferens-Sieczkowska, Wrocław Medical University, PolandCopyright © 2021 Ruiz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mario Ruiz, bWFyaW8ucnVpei5nYXJjaWFAZ3Uuc2U=; orcid.org/0000-0002-7149-6600
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.