
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Physiol., 17 June 2021
Sec. Exercise Physiology
Volume 12 - 2021 | https://doi.org/10.3389/fphys.2021.686176
 Matteo Beretta-Piccoli1,2
Matteo Beretta-Piccoli1,2 Massimo Negro1
Massimo Negro1 Luca Calanni1
Luca Calanni1 Angela Berardinelli3
Angela Berardinelli3 Gabriele Siciliano4
Gabriele Siciliano4 Rossella Tupler5
Rossella Tupler5 Emiliano Soldini6
Emiliano Soldini6 Corrado Cescon2
Corrado Cescon2 Giuseppe D’Antona1,7*
Giuseppe D’Antona1,7*A majority of patients with facioscapulohumeral muscular dystrophy (FSHD) report severe fatigue. The aim of this study was to explore whether fatigability during a performance task is related to the main clinical features of the disease in mildly affected patients. A total of 19 individuals with a molecular genetic-based diagnosis of FSHD (median D4Z4 deletion length of 27 kb) performed two isometric flexions of the dominant biceps brachii at 20% of their maximal voluntary contraction (MVC) for 2 min, and then at 60% MVC until exhaustion. Fatigability indices (average rectified value, mean frequency, conduction velocity, and fractal dimension) were extracted from the surface electromyogram (sEMG) signal, and their correlations with age, age at onset, disease duration, D4Z4 contraction length, perceived fatigability, and clinical disability score were analyzed. The conduction velocity during the low level contraction showed a significant negative correlation with the age at onset (p < 0.05). This finding suggest the assessment of conduction velocity at low isometric contraction intensities, as a potential useful tool to highlight differences in muscle involvement in FSHD patients.
Facioscapulohumeral dystrophy (FSHD) is the third most common hereditary myopathy worldwide, with an estimated prevalence of 1/15,000–1/20,000 (Mostacciuolo et al., 2009). The clinical heterogeneity of FSHD makes it difficult to predict the disease course; diagnosis depends on a combination of genetic and clinical features (Lemmers et al., 2012) and eventually, exclusion of other similar disorders. Diagnosis is facilitated by the fact that in most patients there is contraction in the DNA fragment on chromosome 4q35 containing the 3.3-kb D4Z4 repeat (Wijmenga et al., 1992; van Deutekom et al., 1993). The classical clinical presentation of FSHD includes asymmetric sequential face and shoulder girdle weakness followed by weakness in the foot extensor, abdominal, and hip girdle muscles caused by atrophy and fatty infiltration (Zampatti et al., 2019). The clinical features of FSHD were shown to be correlated with sex and age of onset (Zernov and Skoblov, 2019). Moreover, several genotype–phenotype correlation studies reported a rough inverse correlation between the number of D4Z4 repeats and the severity of FSHD (Lunt et al., 1995; Tawil et al., 1996; Ricci et al., 1999). However, more recent publications did not find satisfactory correlations, even using a large number of patients (Ruggiero et al., 2020; Salort-Campana et al., 2020; Vercelli et al., 2021).
In addition to muscle deterioration, severe fatigue is reported by a majority of FSHD patients (Hamel et al., 2019) and is an early manifestation of the disease that constrains the performance of common daily activities (Schipper et al., 2017). Despite its prevalence, fatigue in FSHD has not been adequately investigated and the extent to which performance and perceived fatigability limit cognitive and physical functions in patients is unknown (Kluger et al., 2013). Performance fatigability refers to a decline in objective measures of performance such as the production of maximal voluntary force, ability to provide an adequate signal to voluntarily activated muscle, and involuntary twitch response to stimulation (Enoka and Duchateau, 2016); it can be assessed by surface electromyography (sEMG) using spectral parameters, muscle fiber conduction velocity (CV), or nonlinear parameters (Rampichini et al., 2020). Fatigue during isometric constant force contractions is reflected by a decrease in CV mainly related to a change in intracellular pH (Komi and Tesch, 1979). A reduction in the fractal dimension (FD) of the sEMG signal has been linked to fatigability, aging, and disease (Goldberger et al., 2002; Arjunan and Kumar, 2013; Beretta-Piccoli et al., 2021), and is a useful index for assessing performance fatigability.
To date there have been no studies on the relationship between performance fatigability and genetic (e.g., D4Z4 allele size) and clinical (e.g., FSHD score) aspects of the disease. To address this issue, the present study investigated whether indices of performance fatigability extracted from the sEMG signal can predict the genetic and clinical features of patients with mild FSHD.
The study was part of a crowdfunded project (#Sport4therapy) carried out at the CRIAMS-Sport Medicine Centre Voghera supported by the University of Pavia that was aimed at identifying the correct sports therapy approach for patients affected by FSHD (Berardinelli and D’Antona, 2019). Data collection started in 2013 and was completed in 2019 (Beretta-Piccoli et al., 2021). Inclusion criteria were as follows: age of ≥ 16 years; clinical or genetic diagnosis of FSHD; and enrollment in the Italian National Registry for FSHD. Exclusion criteria were as follows wheelchair-bound at selection; use of corticosteroids; severe cardiac and respiratory dysfunction; and psychological or psychiatric disorders.
A diagnosis of FSHD was confirmed by DNA testing (Lemmers et al., 2012) at the University of Modena and Reggio Emilia (Modena, Italy). Disease severity was determined by FSHD clinical score (Lamperti et al., 2010), which ranges from 0 (no sign of functional impairment) to 15 (severe impairment of all tested muscle groups and wheelchair dependency). The scale is divided into six independent sections that assess the strength and functionality of facial (score 0–2), scapular girdle (score 0–3), upper limb (score 0–2), distal leg (score 0–2), pelvic girdle (score 0–5), and abdominal (score 0–1) muscles.
Patients were allocated to the four clinical categories of the Comprehensive Clinical Evaluation Form (Ricci et al., 2016), which classifies subjects presenting facial and scapular girdle muscle weakness (category A); subjects with muscle weakness limited to the scapular girdle or facial muscles (category B); asymptomatic or healthy subjects (category C); and subjects with a myopathic phenotype presenting clinical features not consistent with the canonical FSHD phenotype (category D).
Asymmetry of muscle involvement was clinically evaluated and patients with predominantly right or left side involvement were compared to determine whether side involvement was correlated with disease severity. Significant asymmetry (right-side predominance) in upper extremity muscle involvement was previously observed that was independent of handedness (Rijken et al., 2014). The participants’ characteristics are listed in Table 1.
All subjects provided written, informed consent to participate in this study, which was conducted according to the Declaration of Helsinki with approval from the local Ethics Committee of the University of Pisa.
The fatigue severity subscale of the Checklist Individual Strength (CIS-fatigue) was used to assess trait levels of perceived fatigability before task performance. The CIS-fatigue consists of eight questions regarding fatigue experienced in the previous 2 weeks; each question was scored on a 7-point Likert scale (Vercoulen et al., 1994), with a total score ≥ 35 indicating severe fatigue (Vercoulen et al., 1996). The CIS-fatigue has good internal consistency (Cronbach’s α = 0.83–0.92), high discriminant validity, and high sensitivity to change in patients with FSHD (Kalkman et al., 2007).
Performance fatigability was evaluated in FSHD patients as previously described (Beretta-Piccoli et al., 2017, 2020, 2021). Briefly, participants performed two maximal voluntary contractions (MVC) separated by 2 min rest, followed by a 20% MVC contraction lasting 2 min and a 60% MVC held until the force level decreased to < 90% of the target (endurance time). The two submaximal contractions were separated by 5 min rest. Because arm muscles show early disability in FSHD (Derry et al., 2012; Rijken et al., 2014; Tawil, 2018), the biceps brachii was selected for testing. Submaximal contractions were analyzed as their intensity is more representative of that associated with the performance of activities of daily living.
Myoelectric signals in the dominant biceps brachii were detected in monopolar configuration. Participants were seated on a height-adjustable chair with their arm positioned on an isometric ergometer (MUC1; OT-Bioelettronica, Turin, Italy) equipped with a load cell (Model TF022; CCT Transducers, Turin, Italy). The wrist was fastened to the ergometer, with the elbow flexed at 120°. A bidimensional array of 64 electrodes (3 mm diameter, 8 × 8 grid, 10 mm interelectrode distance) (model ELSCH064NM3; OT-Bioelettronica) was positioned on the biceps brachii as previously described (Beretta-Piccoli et al., 2020). The ground electrode was placed on the contralateral wrist.
Elbow torque was assessed using a torque meter operating linearly in the range of 0–1,000 Nm. The torque signal was amplified (MISO II; OT-Bioelettronica) and saved on a computer. EMG signals were amplified by a variable factor ranging from 2,000 to 5,000 (EMG-USB2+; OT-Bioelettronica), filtered with the hardware filter (10–500 Hz bandpass) followed by an offline Butterworth anticausal bandpass filter, and sampled together with the torque signal at 2,048 Hz using a 16-bit A/D converter with 5-V dynamic range and saved on a computer. The torque signal was displayed on a screen as real-time biofeedback.
The channels used for CV estimation were selected based on visual inspection of single differential signals along one of the array columns as previously described (Beretta-Piccoli et al., 2017); numbers typically ranged from 4 to 7 (Farina et al., 2004). CV was estimated using a multichannel algorithm (Farina and Merletti, 2003) on single differential signals using non-overlapping signal epochs of 1 s on the selected channels. Each of the selected signal epochs was used to estimate average rectified value (ARV), mean frequency of the power spectrum (MNF), and FD of the sEMG signal; these variables were averaged across all selected channels. ARV (a measure of the amplitude) and MNF (a parameter used to quantify changes in the spectral content of the sEMG signal based on the Fourier transform) were computed offline with numerical algorithms (Merletti et al., 1990). FD was estimated using the box-counting method (Gitter and Czerniecki, 1995). Performance fatigability was indirectly quantified as the slope of sEMG variables during the contractions.
Linear regression over time was applied to ARV, MNF, CV, and FD and the slopes were extracted and normalized with respect to their initial values. A Shapiro–Wilk test revealed that the variable distributions deviated from normality; consequently, the analyses were conducted using nonparametric statistical indicators. Relationships between continuous variables were investigated with Spearman’s correlation coefficient. Differences in fatigability parameters in relation to sex, FSHD categories, asymmetry of muscle involvement, and scapular girdle involvement score were assessed with the Mann–Whitney U-test. The statistical significance was set at α = 0.05. All statistical analyses were carried out with Stata/IC v16.0 (StataCorp, College Station, Texas, United States), and the results are reported as median and interquartile range (IQR).
Of the 19 patients, 14 belonged to category A and presented facial and scapular girdle muscle weakness (Ricci et al., 2016). Muscle weakness was more pronounced on the right side in 10 patients, 9 of whom were right-handed (Table 1); in 3 patients the weakness was greater on the left side and in 6, both sides showed equal weakness. The number of patients per D4Z4 contraction/number of repeats is shown in Table 1. The median FSHD clinical score was 4 (IQR = 6.25), indicating mild involvement of facial, scapular girdle, and upper limb muscles. The median scapular girdle involvement score was 2 (IQR = 1), suggesting moderate involvement with limitations in arm abduction (>45° but <180°) (Lamperti et al., 2010). Perceived fatigability as measured with the CIS-fatigue was mild (26 [IQR = 15]). The median MVC at 60° of isometric elbow flexion was 20.28 kg [IQR = 18.59].
No significant correlation was observed between age, age at onset, disease duration, D4Z4 deletion length, CIS-fatigue score, clinical severity score, and rates of change in ARV, MNF, and FD. On the contrary, the rate of change of CV during the 20% MVC contraction significantly correlated with the age at onset (rho = −0.51, p < 0.05). When patients with borderline length of D4Z4 contraction (9–10 repeats) were excluded from the analysis, the significance dramatically increased (rho = −0.81; p < 0.001; Figure 1).
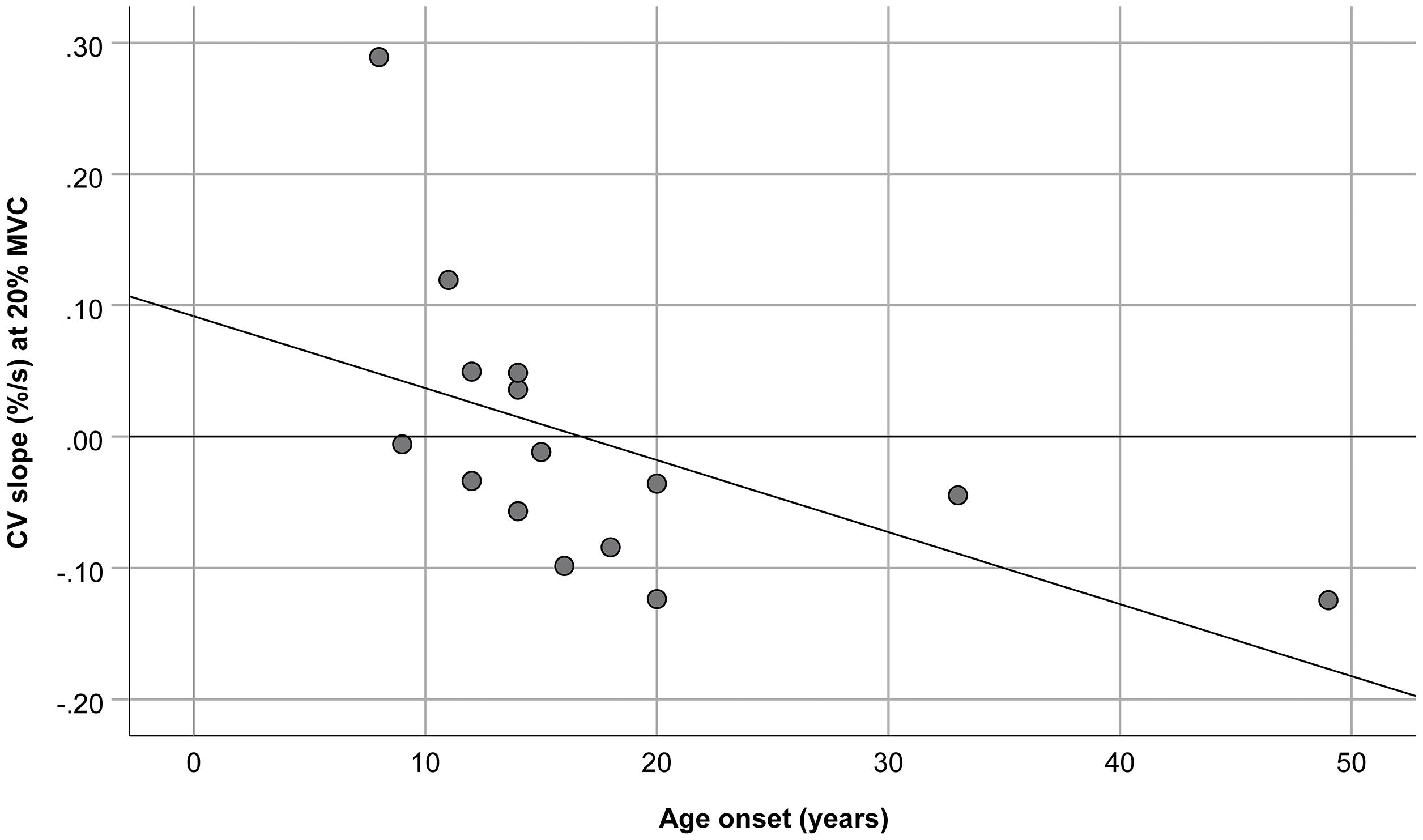
Figure 1. Relationship between the age at onset and muscle fiber conduction velocity (CV) slope during the 20% maximal voluntary contraction (MVC) of the biceps brachii. The slope was normalized with respect to the initial values. R2 = 0.29, y = 0.09–5.48E-3*x.
Within patients with a minimal D4Z4 contraction (1–5 alleles, n = 9), the following significant correlations were observed: (1) age with CV during the 60% MVC (rho = 0.86; p < 0.05); (2) age with MVC (rho = −0.77; p < 0.05); (3) disease duration with MVC (rho = −0.85; p < 0.01; Table 2).
Because of the small sample size, in order to facilitate the assessment of differences in clinical variables we recoded FSHD category, asymmetry, and scapular girdle involvement score as binary variables [“A” and “Not A”; “Right > Left” and “Not right > Left”; and “normal abduction” (score 0–1) and “impaired abduction” (score 2–3), respectively]. There were no statistically significant differences in the performance fatigability parameters and maximal force according to sex, FSHD asymmetry, or scapular girdle involvement score. However, the median slope of MNF and FD during the 60% MVC contraction differed significantly between the A and Not A categories of FSHD (p = 0.026; Figure 2).
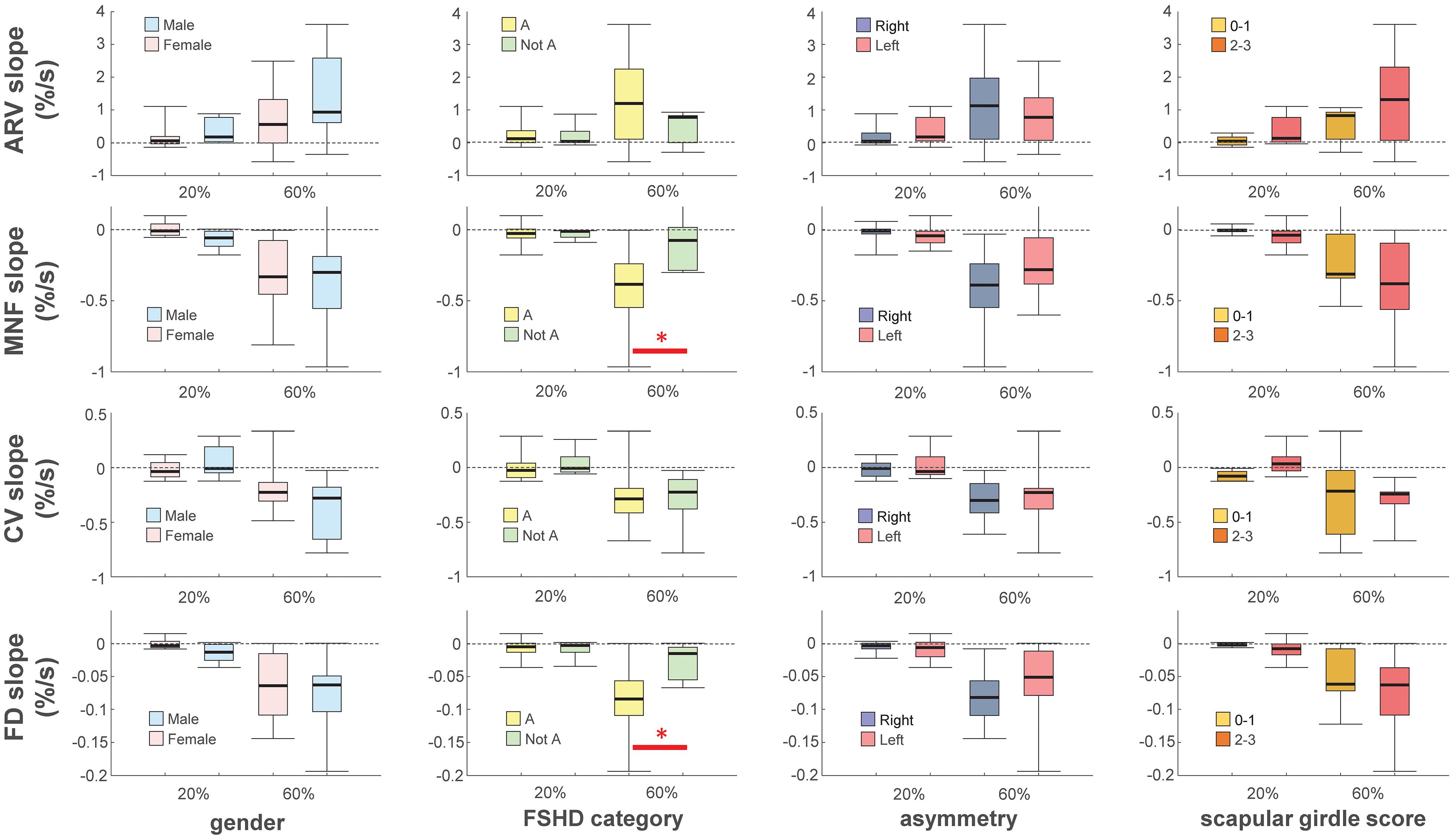
Figure 2. Box-and-whisker plots of slopes of average rectified value (ARV), mean power spectrum frequency (MNF), conduction velocity (CV) and fractal dimension (FD) during the 20 and 60% maximal voluntary contractions. Slopes were normalized with respect to their initial values. Asterisks denote statistical significance at ∗p < 0.05. Categories definition see text.
This study investigated (1) whether sEMG parameters that are indirect indices of performance fatigability are correlated with clinical variables of FSHD patients; and (2) the relationship between fatigability parameters and sex, FSHD category, asymmetry of muscle involvement, and scapular girdle involvement score. The results showed a significant correlation between muscle fiber CV and the age at onset. Moreover, a decrease in the slopes of MNF and FD during endurance contraction was observed in patients in category A compared to those in categories B and D (Not A).
In this study, we established that a late age at onset was associated with a more negative slope of muscle fiber CV, during the 20% MVC isometric elbow flexion, particularly in patients with 1–8 alleles. Patients with borderline D4Z4 contractions may have more mild phenotypes and older age at onset (Statland et al., 2015). In a previous publication on the same patients, we showed that this contraction level was not fatiguing, as the slope of CV was not different from zero (Beretta-Piccoli et al., 2021). Considering that at low level of muscle contraction graded recruitment prevails on rate coding to maintain the force (Milner-Brown et al., 1973), the observed negative correlation may mirror the overall lower deterioration of fast twitch fibers and/or fast to slow switch in biceps brachii when the disease clinical onset occurs later in life. Indeed, this finding identifies the assessment of CV at low % MVC isometric contraction as a potential useful tool to highlight differences in muscle involvement in FSHD patients.
No significant correlation was observed between D4Z4 contraction length and clinical variables or performance fatigability parameters. Although this has not been previously investigated, a negative correlation between muscle computed tomography grade and D4Z4 fragment size has been reported (Wang et al., 2012). Radiologic severity was shown to be unrelated to the size of the D4Z4 array (Olsen et al., 2006), but a correlation between the number of D4Z4 repeats and isokinetic peak torque of trunk extensors and flexors has been demonstrated (Esnault et al., 2018). Moreover, D4Z4 repeat size was shown to be correlated with total score for a manual muscle test (Sacconi et al., 2019). The observed variability in the correlation results may be related to the mean deletion length (Scionti et al., 2012). In fact, a borderline number of D4Z4 repeats (between 8 and 10) showed no association with the severity of clinical manifestations (Zernov and Skoblov, 2019).
Interestingly, within minimal D4Z4 contraction (1–5 repeats), the age of the patients showed significant correlations with the slope of the muscle fiber CV during the 60% MVC contraction, as well as with the MVC, suggesting that elderly patients are less prone to get fatigued, but at the same time generate lower levels of maximal force. Similarly, disease duration was negatively correlated to the exerted maximal force. In healthy subjects it is well known that a correlation between muscle fiber CV and age, characterized by a tendency of delay due to age, both during MVC and submaximal contractions, exists (Merletti et al., 1992; Hara et al., 1998; Bilodeau et al., 2001; Yamada et al., 2002; Bazzucchi et al., 2005; Mase et al., 2006). Differences between younger and older subjects become more apparent as motor units (MUs) recruitment becomes complete. In fact, the fast twitch, rapidly fatiguing fibers, being the later recruited MUs, are those more affected by the aging process. This is in agreement with the hypothesis that in older individuals, slow twitch fibers are predominant (D’Antona et al., 2003). Accordingly, reduction in maximal force in adult aging may be associated with alterations to the neuromuscular system, that affect the muscle fiber, the neuromuscular junction and the innervating motor neurons (Rice and Cunningham, 2002; Klass et al., 2007; Hunter et al., 2016). Data from the present investigation appear to corroborate the hypothesis that duration of the disease is a critical factor late deterioration of muscle force and fibers composition transition toward a slower phenotype and that this phenomenon appears to sum to the expected physiological change due to the aging process.
In line with previous findings in patients with FSHD (Schillings et al., 2007) or other neuromuscular disorders such as multiple sclerosis (e.g., Dodd et al., 2011; Wolkorte et al., 2015a; Severijns et al., 2016; Beretta-Piccoli et al., 2020), performance fatigability showed no correlation with clinical parameters. This may be because the patients in our study were not severely fatigued (CIS-fatigue < 35). Age and MVC values were shown to have positive and negative effects, respectively, on performance fatigability (Wolkorte et al., 2015b); these variables interact with each other and can interfere with the association between perceived and performance fatigability (Romani et al., 2004).
Performance fatigability parameters did not differ significantly according to most of the considered variables (sex, asymmetry, and scapular girdle involvement score). However, the slopes of MNF and FD during the endurance contraction differed between the two categories of FSHD (A and Not A), suggesting that patients with facial and scapular girdle muscle weakness experienced greater fatigue during isometric elbow flexion compared to the other patients. The two categories share pathophysiologic traits including impaired abduction of the upper limb with winged scapula and varying degrees of facial weakness (Ricci et al., 2016), which reflect a worsening phenotype (Vercelli et al., 2021). Importantly, the greater declines in the slopes of MNF (which is related to decreased muscle fiber CV and increased MU firing rate and synchronization (Bigland-Ritchie and Woods, 1984; Brody et al., 1991; Gabriel and Kamen, 2009) and FD (which is related to increased MU firing rate and synchronization Rampichini et al., 2020) suggest that the difference in fatigability between A and Not A is mainly due to central factors. This is supported by the results obtained for ARV and CV, which were altered during submaximal isometric contractions mostly as a result of peripheral factors (Dimitrova and Dimitrov, 2002, 2003; Dimitrov et al., 2006).
This study had some limitations. Firstly, the statistical power was low because of the small sample size. Therefore, the results should be considered as exploratory and confirmed in a larger cohort. Secondly, as the assessment of fatigability is task-dependent (Enoka, 1995), protocol specifications can affect the findings regarding the mechanisms underlying fatigue. Thirdly, we evaluated fatigability in the dominant biceps brachii only, which may not be representative of the disease as a whole and lastly, perceived fatigability is attributed to the whole body and does not necessarily correspond to fatigue in specific muscles.
The length of D4Z4 contraction was not associated with disease severity or fatigability, indicating that unknown environmental factors have a significant impact on the genotype–phenotype interactions underlying clinical manifestations. However, trends in the fatigability parameters suggest more prominent fatigue in patients with a typical phenotype (category A) compared to other patient subsets.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by University of Pisa. The patients/participants provided their written informed consent to participate in this study.
MB-P analyzed and interpreted the data and wrote the first and final drafts of the manuscript. MN supervised functional measurements. LC performed functional experiments. AB and GS contributed to medical examination of the patients and to writing and revision of the manuscript for intellectual content. RT participated in testing and medical tracking of the patients and contributed to writing and revision of the manuscript for intellectual content. ES performed statistical analyses. CC interpreted the data. GD’A developed the study concept, interpreted the data, and wrote the first and final drafts of the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by the Crowdfunding #sport4therapy and AFM (Grant No. 17191 to GD’A).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank the patients and their families for their support.
Arjunan, S. P., and Kumar, D. K. (2013). Age-associated changes in muscle activity during isometric contraction. Muscle Nerve 47, 545–549. doi: 10.1002/mus.23619
Bazzucchi, I., Marchetti, M., Rosponi, A., Fattorini, L., Castellano, V., Sbriccoli, P., and Felici, F. (2005). Differences in the force/endurance relationship between young and older men. Eur. J. ApplPhysiol. 93, 390–397. doi: 10.1007/s00421-004-1277-0
Berardinelli, A., and D’Antona, G. (2019). Physiological aspects of muscular adaptations to training translated to neuromuscular diseases. Acta Myol. 38, 197–206.
Beretta-Piccoli, M., Calanni, L., Negro, M., Ricci, G., Bettio, C., Barbero, M., et al. (2021). Increased resistance towards fatigability in patients with facioscapulohumeral muscular dystrophy. Eur. J. Appl. Physiol. 121, 1617–1629. doi: 10.1007/s00421-021-04650-3
Beretta-Piccoli, M., Cescon, C., Barbero, M., Villiger, M., Clijsen, R., Kool, J., et al. (2020). Upper and lower limb performance fatigability in people with multiple sclerosis investigated through surface electromyography: a pilot study. Physiol. Meas. 41:025002. doi: 10.1088/1361-6579/ab6f54
Beretta-Piccoli, M., D’Antona, G., Zampella, C., Barbero, M., Clijsen, R., and Cescon, C. (2017). Test-retest reliability of muscle fiber conduction velocity and fractal dimension of surface EMG during isometric contractions. Physiol. Meas. 38, 616–630. doi: 10.1088/1361-6579/aa614c
Bigland-Ritchie, B., and Woods, J. J. (1984). Changes in muscle contractile properties and neural control during human muscular fatigue. Muscle Nerve 7, 691–699. doi: 10.1002/mus.880070902
Bilodeau, M., Erb, M. D., Nichols, J. M., Joiner, K. L., and Weeks, J. B. (2001). Fatigue of elbow flexor muscles in younger and older adults. Muscle Nerve 24, 98–106. doi: 10.1002/1097-4598(200101)24:1<98::aid-mus11>3.0.co;2-d
Brody, L. R., Pollock, M. T., Roy, S. H., De Luca, C. J., and Celli, B. (1991). pH-induced effects on median frequency and conduction velocity of the myoelectric signal. J. Appl. Physiol. 71, 1878–1885. doi: 10.1152/jappl.1991.71.5.1878
D’Antona, G., Pellegrino, M. A., Adami, R., Rossi, R., Carlizzi, C. N., Canepari, M., et al. (2003). The effect of ageing and immobilization on structure and function of human skeletal muscle fibres. J. Physiol. 552, 499–511. doi: 10.1113/jphysiol.2003.046276
Derry, K. L., Venance, S. L., and Doherty, T. J. (2012). Decomposition-based quantitative electromyography in the evaluation of muscular dystrophy severity. Muscle Nerve 45, 507–513. doi: 10.1002/mus.22341
Dimitrov, G. V., Arabadzhiev, T. I., Mileva, K. N., Bowtell, J. L., Crichton, N., and Dimitrova, N. A. (2006). Muscle fatigue during dynamic contractions assessed by new spectral indices. Med. Sci. Sports Exerc. 38, 1971–1979. doi: 10.1249/01.mss.0000233794.31659.6d
Dimitrova, N. A., and Dimitrov, G. V. (2002). Amplitude-related characteristics of motor unit and M-wave potentials during fatigue. A simulation study using literature data on intracellular potential changes found in vitro. J. Electromyogr. Kinesiol. 12, 339–349. doi: 10.1016/s1050-6411(02)00046-9
Dimitrova, N. A., and Dimitrov, G. V. (2003). Interpretation of EMG changes with fatigue: facts, pitfalls, and fallacies. J. Electromyogr. Kinesiol. 13, 13–36. doi: 10.1016/s1050-6411(02)00083-4
Dodd, K. J., Taylor, N. F., Shields, N., Prasad, D., Mcdonald, E., and Gillon, A. (2011). Progressive resistance training did not improve walking but can improve muscle performance, quality of life and fatigue in adults with multiple sclerosis: a randomized controlled trial. Mult. Scler. 17, 1362–1374. doi: 10.1177/1352458511409084
Enoka, R. M. (1995). Mechanisms of muscle fatigue: central factors and task dependency. J. Electromyogr. Kinesiol. 5, 141–149. doi: 10.1016/1050-6411(95)00010-w
Enoka, R. M., and Duchateau, J. (2016). Translating fatigue to human performance. Med. Sci. Sports Exerc. 48, 2228–2238. doi: 10.1249/mss.0000000000000929
Esnault, J., Missaoui, B., Bendaya, S., Mane, M., Eymard, B., Laforet, P., et al. (2018). Isokinetic assessment of trunk muscles in facioscapulohumeral muscular dystrophy type 1 patients. Neuromuscul. Disord. 28, 996–1002. doi: 10.1016/j.nmd.2018.09.007
Farina, D., and Merletti, R. (2003). A novel approach for estimating muscle fiber conduction velocity by spatial and temporal filtering of surface EMG signals. IEEE Trans. Biomed. Eng. 50, 1340–1351. doi: 10.1109/TBME.2003.819847
Farina, D., Merletti, R., and Enoka, R. M. (2004). The extraction of neural strategies from the surface EMG. J. Appl. Physiol. 96, 1486–1495. doi: 10.1152/japplphysiol.01070.2003
Gabriel, D. A., and Kamen, G. (2009). Experimental and modeling investigation of spectral compression of biceps brachii SEMG activity with increasing force levels. J. Electromyogr. Kinesiol. 19, 437–448. doi: 10.1016/j.jelekin.2007.10.009
Gitter, J. A., and Czerniecki, M. J. (1995). Fractal analysis of the electromyographic interference pattern. J. Neurosci. Methods 58, 103–108. doi: 10.1016/0165-0270(94)00164-c
Goldberger, A. L., Amaral, L. A., Hausdorff, J. M., Ivanov, P., Peng, C. K., and Stanley, H. E. (2002). Fractal dynamics in physiology: alterations with disease and aging. Proc. Natl. Acad. Sci. U.S.A. 99(Suppl. 1), 2466–2472. doi: 10.1073/pnas.012579499
Hamel, J., Johnson, N., Tawil, R., Martens, W. B., Dilek, N., Mcdermott, M. P., et al. (2019). Patient-reported symptoms in facioscapulohumeral muscular dystrophy (PRISM-FSHD). Neurology 93, e1180–e1192. doi: 10.1212/WNL.0000000000008123
Hara, Y., Findley, T. W., Sugimoto, A., and Hanayama, K. (1998). Muscle fiber conduction velocity (MFCV) after fatigue in elderly subjects. Electromyogr. Clin. Neurophysiol. 38, 427–435.
Hunter, S. K., Pereira, H. M., and Keenan, K. G. (2016). The aging neuromuscular system and motor performance. J. Appl. Physiol. 121, 982–995. doi: 10.1152/japplphysiol.00475.2016
Kalkman, J. S., Schillings, M. L., Zwarts, M. J., Van Engelen, B. G., and Bleijenberg, G. (2007). The development of a model of fatigue in neuromuscular disorders: a longitudinal study. J. Psychosom. Res. 62, 571–579. doi: 10.1016/j.jpsychores.2006.11.014
Klass, M., Baudry, S., and Duchateau, J. (2007). Voluntary activation during maximal contraction with advancing age: a brief review. Eur. J. Appl. Physiol. 100, 543–551. doi: 10.1007/s00421-006-0205-x
Kluger, B. M., Krupp, L. B., and Enoka, R. M. (2013). Fatigue and fatigability in neurologic illnesses: proposal for a unified taxonomy. Neurology 80, 409–416. doi: 10.1212/WNL.0b013e31827f07be
Komi, P. V., and Tesch, P. (1979). EMG frequency spectrum, muscle structure, and fatigue during dynamic contractions in man. Eur. J. Appl. Physiol. Occup. Physiol. 42, 41–50. doi: 10.1007/BF00421103
Lamperti, C., Fabbri, G., Vercelli, L., D’Amico, R., Frusciante, R., Bonifazi, E., et al. (2010). A standardized clinical evaluation of patients affected by facioscapulohumeral muscular dystrophy: the FSHD clinical score. Muscle Nerve 42, 213–217. doi: 10.1002/mus.21671
Lemmers, R. J., Tawil, R., Petek, L. M., Balog, J., Block, G. J., Santen, G. W., et al. (2012). Digenic inheritance of an SMCHD1 mutation and an FSHD-permissive D4Z4 allele causes facioscapulohumeral muscular dystrophy type 2. Nat. Genet. 44, 1370–1374. doi: 10.1038/ng.2454
Lunt, P. W., Jardine, P. E., Koch, M., Maynard, J., Osborn, M., Williams, M., et al. (1995). Phenotypic–genotypic correlation will assist genetic counseling in 4q35-facioscapulohumeral muscular dystrophy. Muscle Nerve 18, S103–S109. doi: 10.1002/mus.880181319
Mase, K., Kamimura, H., Imura, S., and Kitagawa, K. (2006). Effect of age and gender on muscle function-analysis by muscle fiber conduction velocity. J. Phys. Ther. Sci. 18, 81–87. doi: 10.1589/jpts.18.81
Merletti, R., Knaflitz, M., and De Luca, C. J. (1990). Myoelectric manifestations of fatigue in voluntary and electrically elicited contractions. J. Appl. Physiol. 69, 1810–1820. doi: 10.1152/jappl.1990.69.5.1810
Merletti, R., Lo Conte, L. R., Cisari, C., and Actis, M. V. (1992). Age related changes in surface myoelectric signals. Scand. J. Rehabil. Med. 24, 25–36.
Milner-Brown, H. S., Stein, R. B., and Yemm, R. (1973). Changes in firing rate of human motor units during linearly changing voluntary contractions. J. Physiol. 230, 371–390. doi: 10.1113/jphysiol.1973.sp010193
Mostacciuolo, M. L., Pastorello, E., Vazza, G., Miorin, M., Angelini, C., Tomelleri, G., et al. (2009). Facioscapulohumeral muscular dystrophy: epidemiological and molecular study in a north-east Italian population sample. Clin. Genet. 75, 550–555. doi: 10.1111/j.1399-0004.2009.01158.x
Olsen, D. B., Gideon, P., Jeppesen, T. D., and Vissing, J. (2006). Leg muscle involvement in facioscapulohumeral muscular dystrophy assessed by MRI. J. Neurol. 253, 1437–1441. doi: 10.1007/s00415-006-0230-z
Rampichini, S., Vieira, T. M., Castiglioni, P., and Merati, G. (2020). Complexity analysis of surface electromyography for assessing the myoelectric manifestation of muscle fatigue: a review. Entropy 22:529. doi: 10.3390/e22050529
Ricci, E., Galluzzi, G., Deidda, G., Cacurri, S., Colantoni, L., Merico, B., et al. (1999). Progress in the molecular diagnosis of facioscapulohumeral muscular dystrophy and correlation between the number of KpnI repeats at the 4q35 locus and clinical phenotype. Ann. Neurol. 45, 751–757. doi: 10.1002/1531-8249(199906)45:6<751::aid-ana9>3.0.co;2-m
Ricci, G., Ruggiero, L., Vercelli, L., Sera, F., Nikolic, A., Govi, M., et al. (2016). A novel clinical tool to classify facioscapulohumeral muscular dystrophy phenotypes. J. Neurol. 263, 1204–1214. doi: 10.1007/s00415-016-8123-2
Rice, C. L., and Cunningham, D. A. (2002). “Aging of the neuromuscular system: influences of gender and physical activity,” in Gender, Physical Activity, and Aging, ed. R. J. Shephard (Boca Raton, FL: CRC Press), 121–150.
Rijken, N. H., Van Der Kooi, E. L., Hendriks, J. C., Van Asseldonk, R. J., Padberg, G. W., Geurts, A. C., et al. (2014). Skeletal muscle imaging in facioscapulohumeral muscular dystrophy, pattern and asymmetry of individual muscle involvement. Neuromuscul. Disord. 24, 1087–1096. doi: 10.1016/j.nmd.2014.05.012
Romani, A., Bergamaschi, R., Candeloro, E., Alfonsi, E., Callieco, R., and Cosi, V. (2004). Fatigue in multiple sclerosis: multidimensional assessment and response to symptomatic treatment. Mult. Scler. 10, 462–468. doi: 10.1191/1352458504ms1051oa
Ruggiero, L., Mele, F., Manganelli, F., Bruzzese, D., Ricci, G., Vercelli, L., et al. (2020). Phenotypic variability among patients with D4Z4 reduced allele facioscapulohumeral muscular dystrophy. JAMA Netw. Open 3:e204040. doi: 10.1001/jamanetworkopen.2020.4040
Sacconi, S., Briand-Suleau, A., Gros, M., Baudoin, C., Lemmers, R., Rondeau, S., et al. (2019). FSHD1 and FSHD2 form a disease continuum. Neurology 92, e2273–e2285. doi: 10.1212/WNL.0000000000007456
Salort-Campana, E., Fatehi, F., Beloribi-Djefaflia, S., Roche, S., Nguyen, K., Bernard, R., et al. (2020). Type 1 FSHD with 6–10 repeated units: factors underlying severity in index cases and disease penetrance in their relatives attention. Int. J. Mol. Sci. 21:2221. doi: 10.3390/ijms21062221
Schillings, M. L., Kalkman, J. S., Janssen, H. M., Van Engelen, B. G., Bleijenberg, G., and Zwarts, M. J. (2007). Experienced and physiological fatigue in neuromuscular disorders. Clin. Neurophysiol. 118, 292–300. doi: 10.1016/j.clinph.2006.10.018
Schipper, K., Bakker, M., and Abma, T. (2017). Fatigue in facioscapulohumeral muscular dystrophy: a qualitative study of people’s experiences. Disabil. Rehabil. 39, 1840–1846. doi: 10.1080/09638288.2016.1212109
Scionti, I., Greco, F., Ricci, G., Govi, M., Arashiro, P., Vercelli, L., et al. (2012). Large-scale population analysis challenges the current criteria for the molecular diagnosis of fascioscapulohumeral muscular dystrophy. Am. J. Hum. Genet. 90, 628–635. doi: 10.1016/j.ajhg.2012.02.019
Severijns, D., Lemmens, M., Thoelen, R., and Feys, P. (2016). Motor fatigability after low-intensity hand grip exercises in persons with multiple sclerosis. Mult. Scler. Relat. Disord. 10, 7–13. doi: 10.1016/j.msard.2016.08.007
Statland, J. M., Donlin-Smith, C. M., Tapscott, S. J., Lemmers, R. J., van der Maarel, S. M., and Tawil, R. (2015). Milder phenotype in facioscapulohumeral dystrophy with 7-10 residual D4Z4 repeats. Neurology 85, 2147–2150. doi: 10.1212/WNL.0000000000002217
Tawil, R. (2018). Facioscapulohumeral muscular dystrophy. Handb. Clin. Neurol. 148, 541–548. doi: 10.1016/B978-0-444-64076-5.00035-1
Tawil, R., Forrester, J., Griggs, R. C., Mendell, J., Kissel, J., McDermott, M., et al. (1996). Evidence for anticipation and association of deletion size with severity in facioscapulohumerd muscular dystrophy. Ann. Neurol. 39, 744–748. doi: 10.1002/ana.410390610
van Deutekom, J. C., Wijmenga, C., Van Tienhoven, E. A., Gruter, A. M., Hewitt, J. E., Padberg, G. W., et al. (1993). FSHD associated DNA rearrangements are due to deletions of integral copies of a 3.2 kb tandemly repeated unit. Hum. Mol. Genet. 2, 2037–2042. doi: 10.1093/hmg/2.12.2037
Vercelli, L., Mele, F., Ruggiero, L., Sera, F., Tripodi, S., Ricci, G., et al. (2021). A 5-year clinical follow-up study from the Italian National Registry for FSHD. J. Neurol. 268, 356–366. doi: 10.1007/s00415-020-10144-7
Vercoulen, J. H., Hommes, O. R., Swanink, C. M., Jongen, P. J., Fennis, J. F., Galama, J. M., et al. (1996). The measurement of fatigue in patients with multiple sclerosis. A multidimensional comparison with patients with chronic fatigue syndrome and healthy subjects. Arch. Neurol. 53, 642–649. doi: 10.1001/archneur.1996.00550070080014
Vercoulen, J. H., Swanink, C. M., Fennis, J. F., Galama, J. M., Van Der Meer, J. W., and Bleijenberg, G. (1994). Dimensional assessment of chronic fatigue syndrome. J. Psychosom. Res. 38, 383–392. doi: 10.1016/0022-3999(94)90099-x
Wang, C. H., Leung, M., Liang, W. C., Hsieh, T. J., Chen, T. H., and Jong, Y. J. (2012). Correlation between muscle involvement, phenotype and D4Z4 fragment size in facioscapulohumeral muscular dystrophy. Neuromuscul. Disord. 22, 331–338. doi: 10.1016/j.nmd.2011.10.018
Wijmenga, C., Hewitt, J. E., Sandkuijl, L. A., Clark, L. N., Wright, T. J., Dauwerse, H. G., et al. (1992). Chromosome 4q DNA rearrangements associated with facioscapulohumeral muscular dystrophy. Nat. Genet. 2, 26–30. doi: 10.1038/ng0992-26
Wolkorte, R., Heersema, D. J., and Zijdewind, I. (2015a). Muscle fatigability during a sustained index finger abduction and depression scores are associated with perceived fatigue in patients with relapsing-remitting multiple sclerosis. Neurorehabil. Neural Repair 29, 796–802. doi: 10.1177/1545968314567151
Wolkorte, R., Heersema, D. J., and Zijdewind, I. (2015b). Reduced dual-task performance in MS patients is further decreased by muscle fatigue. Neurorehabil. Neural Repair 29, 424–435. doi: 10.1177/1545968314552529
Yamada, H., Masuda, T., and Okada, M. (2002). Age-related EMG variables during maximum voluntary contraction. Percept. Mot. Skills 95, 10–14. doi: 10.2466/pms.2002.95.1.10
Zampatti, S., Colantoni, L., Strafella, C., Galota, R. M., Caputo, V., Campoli, G., et al. (2019). Facioscapulohumeral muscular dystrophy (FSHD) molecular diagnosis: from traditional technology to the NGS era. Neurogenetics 20, 57–64. doi: 10.1007/s10048-019-00575-4
Keywords: neuromuscular disease, dystrophy, electromyography, fatigability, D4Z4, correlation, muscle fiber conduction velocity
Citation: Beretta-Piccoli M, Negro M, Calanni L, Berardinelli A, Siciliano G, Tupler R, Soldini E, Cescon C and D’Antona G (2021) Muscle Fiber Conduction Velocity Correlates With the Age at Onset in Mild FSHD Cases. Front. Physiol. 12:686176. doi: 10.3389/fphys.2021.686176
Received: 26 March 2021; Accepted: 14 May 2021;
Published: 17 June 2021.
Edited by:
Emiliano Cè, University of Milan, ItalyReviewed by:
Jerôme D. Robin, INSERM U1251 Centre de Génétique Médicale de Marseille (MMG), FranceCopyright © 2021 Beretta-Piccoli, Negro, Calanni, Berardinelli, Siciliano, Tupler, Soldini, Cescon and D’Antona. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giuseppe D’Antona, Z2RhbnRvbmFAdW5pcHYuaXQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.