Countergradient Variation in Reptiles: Thermal Sensitivity of Developmental and Metabolic Rates Across Locally Adapted Populations
A Corrigendum on
Countergradient Variation in Reptiles: Thermal Sensitivity of Developmental and Metabolic Rates Across Locally Adapted Populations
by Pettersen, A. K. (2020). Front. Physiol. 11:547. doi: 10.3389/fphys.2020.00547
In the original article, there was a misinterpretation of the results of Tiatragul et al., 2017 and Hall and Warner, 2018, where population-level differences in development time for A. cristatellus and A. sagrei were incorrectly stated.
These studies did not find any significant effect of habitat of origin on development time – mean incubation times between forested and urban wild populations were similar across temperature treatments. Tiatragul et al. (2017) showed slight differences in incubation duration between forested and urban populations (Figure 2), however these were not significant (Table 1). The “Forest” and “City” headings of Table 1 in Hall and Warner (2018) refer to the incubation treatments (forest or city thermal profile), not the population – since no population x incubation treatment interactions were found, data across populations were pooled to estimate mean incubation period for each treatment.
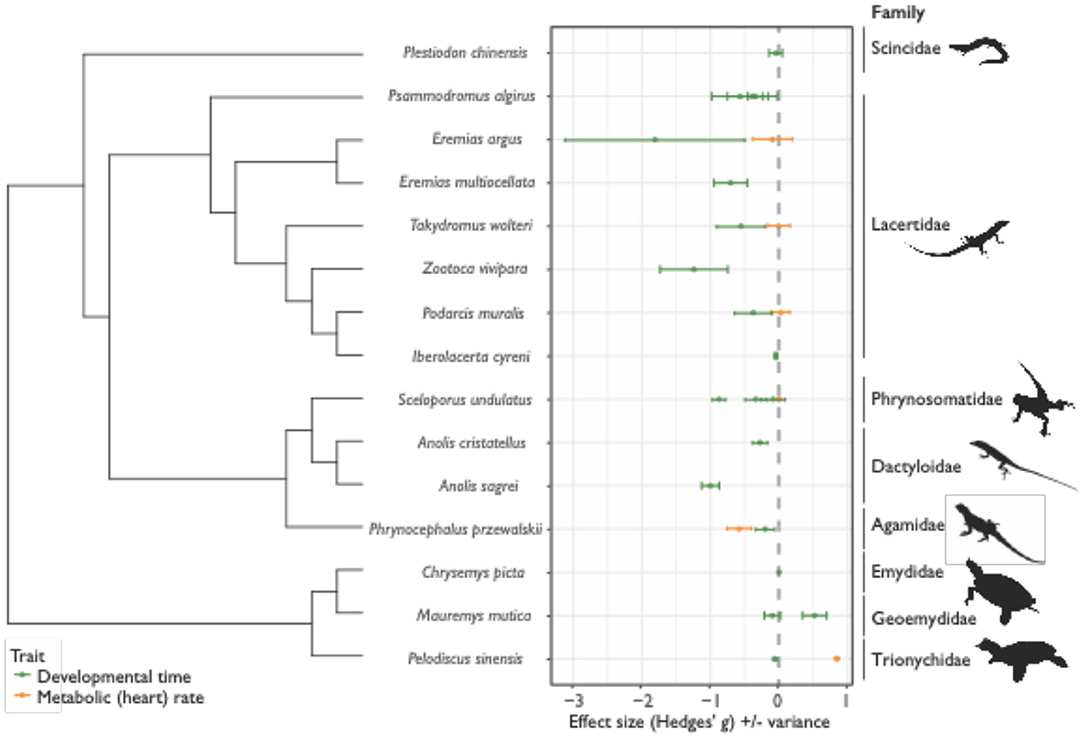
Figure 2. Effect sizes (Hedges' g) for differences in the thermal sensitivity of development time (time from oviposition until hatching) and metabolic (heart) rate across cold and warm-adapted populations for 15 species of reptiles across 8 families (± variance). For development time (D; green data points and variance bars), positive Hedges' g values indicate positive Cov(G,E), or cogradient variation, where cold-adapted populations have longer D, relative to warm-adapted populations. Negative values of D indicate negative Cov(G,E), or countergradient variation, where genotypic differences oppose environmental temperature effects – in these instances, cold-adapted populations develop faster than warm-adapted populations. For metabolic rates (MR; orange data points and variance bars), negative Hedges' g values indicate positive Cov(G,E), or cogradient variation, where cold-adapted populations have lower MR, relative to warm-adapted populations, while positive values of MR indicate negative Cov(G,E), or countergradient variation – here cold-adapted populations maintain higher MR, relative to warm-adapted populations.
A correction has been made to “Consequences of Countergradient Adaptation: When and Why Is Thermal Countergradient Adaptation Absent?”:
Despite the prevalence of CnGV in development time, there are studies that do not show this trend, for example evidence for CnGV was absent across native-non-native ranges for species adapting to hot temperatures. Differences in development time were absent when comparing forested (cool) vs. urban (hot) populations of Anolis cristatellus and Anolis sagrei under common garden conditions (Tiatragul et al., 2017; Hall and Warner, 2018). Further measures of the relative temperature dependencies of D and MR in other species are needed to elucidate the temperature-dependent costs of development as a potentially general mechanism for local thermal adaptation to extreme high temperatures.
In the original article, there was a mistake in Figure 2 as published. Due to the misinterpretation of results by Hall and Warner, 2018 (as per above), effect sizes for development time of Anolis cristatellus were incorrect. Since data across populations were pooled for this study, effect sizes were unable to be recalculated. The corrected Figure 2 appears below.
The authors apologize for this error and state that this does not change the scientific conclusions of the article in any way. The original article has been updated.
References
Hall, J. M., and Warner, D. A. (2018). Thermal spikes from the urban heat island increase mortality and alter physiology of lizard embryos. J. Exp. Biol. 221:jeb181552. doi: 10.1242/jeb.181552
Keywords: temperature, climate, adaptation, cogradient, incubation, embryo, maternal investment
Citation: Pettersen AK (2021) Corrigendum: Countergradient Variation in Reptiles: Thermal Sensitivity of Developmental and Metabolic Rates Across Locally Adapted Populations. Front. Physiol. 12:652269. doi: 10.3389/fphys.2021.652269
Received: 12 January 2021; Accepted: 28 January 2021;
Published: 18 February 2021.
Edited and reviewed by: Julia Nowack, Liverpool John Moores University, United Kingdom
Copyright © 2021 Pettersen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amanda K. Pettersen, amanda.pettersen@biol.lu.se
 Amanda K. Pettersen
Amanda K. Pettersen