
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Physiol. , 22 March 2021
Sec. Invertebrate Physiology
Volume 12 - 2021 | https://doi.org/10.3389/fphys.2021.642893
This article is part of the Research Topic Entomopathogenic Fungi for the Control of Arthropod Pests View all 12 articles
In order to explore the synergistic control effect of crude extracts of Artemisia sieversiana and Metarhizium anisopliae on Oedaleus asiaticus, we used different doses of M. anisopliae and crude extracts of A. sieversiana singly and in combination, to determine their toxicities to fourth instar O. asiaticus. The results showed that the combination of 10% crude extract of A. sieversiana with 107 and 108 spores/g M. anisopliae concentrations and the combination of 20% crude extract of A. sieversiana with 107 and 108 spores/g M. anisopliae concentrations had significant effects on the mortality, body weight gain, body length gain, growth rate, and overall performance of O. asiaticus than those of the crude extract of A. sieversiana and M. anisopliae alone. Among them, the 20% A. sieversiana crude extract mixed with 108 spores/g M. anisopliae and 10% A. sieversiana crude extract combined with 107 spores/g M. anisopliae, had the best control efficacy. In order to clarify the biochemical mechanism underlying the immune responses of O. asiaticus to the pesticide treatments, we monitored the activities of four enzymes: superoxidase dismutase (SOD), peroxidase (POD), catalase (CAT), and polyphenol oxidase (PPO). The results showed that the activities of three enzymes (SOD, CAT, and PPO) were significantly increased from the treatment with the combination of M. anisopliae mixed with crude extract of A. sieversiana. Interestingly, compared to the crude extract, the combination treatment did not significantly induce the expression of POD enzyme activity, which may be a biochemical factor for increasing the control effect of the combination treatment. Our results showed that the combination treatment had synergistic and antagonistic effects on host mortality, growth, development, and enzyme activities in O. asiaticus.
As important biological control agents, botanical pesticides have received extensive research attention due to characteristics such as their low toxicities, easy degradation, and miscibility with other pesticides and against which insects are yet to develop resistance (Dubey et al., 2010). Crude plant extracts, the most notable of botanical pesticides, contain secondary metabolites such as alkaloids, terpenoids, phenols (mainly including flavonoids), tannins, lignin, nitrogenous organic compounds, etc., which have shown to be effective against insects (Wink, 1988). They have been extracted from plants species such as Melilotus suavcolen, Peganum harmala (zygophylllaceae), Ajuga iva (labiateae), Aristolochia baetica (Aristolochiaceae), Raphanus raphanistrum (Brassicaceae), etc. (Jbilou et al., 2006). These secondary metabolites are chemical compounds which have evolved in plants, to have specialized functions such as adaptation, survival of competition, defense against herbivorous insects, etc. They deter herbivorous insects from feeding on plants by poisoning them or interfering with digestion and the absorption of nutrients in herbivorous insects (Rattan, 2010). For instance, flavonoids can inhibit many insect species from feeding and oviposition (Simmonds, 2001). In addition, these substances also induce a rise in the detoxification mechanism of foreign compounds in insects (Bi and Felton, 1995). They can also stimulate and induce insects to feed or to produce sex hormones. Thus, they have mutually beneficial and co-evolutionary relationships (Kergoat et al., 2017).
China is rich in plant resources. There are more than 220 plant species that can be used as botanical insecticides. Compositae, one of the plant families which accounts for more than 20% of these species, is characterized by the most complex and diverse chemical compositions (Shang et al., 1997). Artemisia, a dominant genus in grasslands in Inner Mongolia, has shown promising insecticidal activities. In recent years, it has increasingly become one of the common plants, with the intensification of degradation of the grasslands in Inner Mongolia. The main chemical components of Artemisia are flavonoids, terpenoids, sesquiterpene lactones, cyanogenic glycides, flavonal, coumarins, etc. (Vander et al., 2008). Extensive studies have been conducted on the insecticidal activity of Artemisia plants (da Silva et al., 2005). For example, the crude extract of Anthemideae was shown to have strong anti-feedant properties against Tribolium castaneum Herbst, Tetranychus cinnabarinus, and Maruca testulalis. Also, Artemisia mongolica, Artemisia lavandulaefolia, and Acacia vestita showed excellent insecticidal effects against Sitophilus zeamais (Wu, 2015).
Artemisia sieversiana, a typical Artemisia plant, also known as Artemisia, A. alba, A. scoparia, etc., is one of the dominant species in grasslands in China. It is widely distributed in India, Pakistan, Afghanistan, Russia, Mongolia, and China’s Heilongjiang, Shanxi, Hebei, Ningxia, Gansu, Xinjiang, Inner Mongolia, and other regions (Cui et al., 2020). A. sieversiana contains a variety of insecticidal and antibacterial chemical components, such as flavonoids, lignins, sesquiterpenes, and volatile oils (Liu et al., 2010; Cui et al., 2020). In addition, its essential oil was shown to have fumigation and killing activity on Callosobruchus chinensis, grubs, and other insects (Ayub et al., 2019). Further, the ethanol extract of A. sieversiana was shown to have significantly inhibited and killed the growth of human colon cancer cells (HT-29, HCT-15, and Colo-205; Tang et al., 2015).
Entomopathogenic fungi are another group of important biological control agents with low environmental risk, used in pest management. Insect pests are not easily resistant to entomopathogenic fungi. They have great potential for pest control in integrated pest management programs (Blanford et al., 2005; Sabbour and Sahab, 2005; Zimmermann, 2007). Metarhizium anisopliae, one of such entomopathogenic fungal species, is widely used globally, and has been studied for more than 100 years in China. It has been used on more than 200 insect species and has shown significant control effects against the larvae of Curculio chinensis Chevrolat, Monochamus alternatus, Polyphylla laticollis Lewis, Locusta migratoria manilensis, Plutella xylostella, O. asiaticus, etc. (Peng et al., 2008; Kaaya et al., 2011).
M. anisopliae infects insects through the insect cuticle or when insects feed (stomach poison; Mannino et al. 2019). M. anisopliae directly enters the midgut through the feeding of insects, and the mycelium propagates rapidly in the midgut cells, resulting in the loss of cellular functions, directly affecting cellular metabolism, and ultimately destroying the midgut tissue. These result in the death of insects. Mortality rates of insects from midgut infections are significantly higher than that from cuticle infections. M. anisopliae infections are very easy to spread in their colonies. However, the long lethal time of fungal insecticides lowers their efficacy (MeCoy, 1981; Dimbi et al., 2009).
All groups of insecticides, including botanical insecticides, fungicides, and chemical insecticides, etc., have advantages and disadvantages (Marican and Durán-Lara, 2018). For example, botanical insecticides are environment friendly, but also have unstable formulations, easy photodegradation and their active ingredients are difficult to extract (Pavela, 2016). Insects do not easily develop resistance against fungal insecticides, but their lethal times are longer, and their prevention and control of pest outbreaks is slower. Chemical insecticides show rapid effectiveness and good control effects, but their excessive usage generates residues which pollute the environment and also have negative impact on food safety for humans and animals (Sabarwal et al., 2018). However, the use of a combination of these insecticides can effectively solve these challenges by producing a synergistic efficacy to achieve better control of insect pest. It can also solve the challenge of excessive use of chemical agents (Gibson et al., 2014). For example, the use of a combination of M. anisopliae V275 and imidacloprid or fipronil had a significantly higher control effect on Otiorhynchus sulcatus than that from each insecticide alone (Shah et al., 2007). Also, neem oil effectively maintained the toxicity of the entomopathogenic fungus M. anisopliae, protected it from UV radiation, and increased the control effect on Aedes aegypti larvae (Paula et al., 2019). The use of a combination of the insecticide chlorantraniliprole and M. anisopliae showed excellent synergistic control on locusts, scarabaeidea, Empoasca pirisuga Matumura, rice planthoppers, and other agricultural pest (Farenhorst et al., 2010; Jia et al., 2016).
Oedaleus decorus asiaticus (Bey-Bienko), is a major pest in the grasslands of northern China. In recent years, its frequent large-scale outbreaks are partly responsible for the degradation and desertification of grasslands in Inner Mongolia, in China (Zhang et al., 2013). At present, the main control of O. asiaticus is based on biological control methods such as the use of poxvirus, M. anisopliae, Beauveria bassiana, and the introduction of insect natural enemies. Among them, M. anisopliae has shown a very significant control effect on O. asiaticus. It has the advantage of being green, safe, and has long duration, but has a slow effect on insects. Quercetin (a kind of flavonoids) was shown to reduce the survival rate, inhibit the growth and development of O. asiaticus (Cui et al., 2019). The crude extract of A. sieversiana contains flavonoids, which has been shown to have a toxic effect on insects, but its efficacy lasted for a short period of time. For practical applications, the simultaneous use of fungi and chemical agents for insect pest control, may result in higher mortality than from the use of each alone (Duarte et al., 2016; Jia et al., 2016). Therefore, we evaluated the synergetic control effect of the crude extract of A. sieversiana and M. anisopliae on O. asiaticus in this study, to provide a new direction for the research and development of new biological agents for the effective control of locust or grasshopper pest.
O. asiaticus were caught from the wild, in the grasslands of Xilinhot City, Inner Mongolia, China. They were reared on their host plant, Stipa krylovii, in a cage (1 m × 1 m × 1 m) at room temperature (about 27°C) for 1 week.
A. sieversiana was collected from the Scientific Observing and Experimental Station of Pests in Xilingol Rangeland, Ministry of Agriculture, P.R. China (E116°36'', N43.57'12''), located in the Xilingol League, Inner Mongolia, northeast China.
M. anisopliae IMI330-189 was provided by the Grassland Pest Group, Institute of Plant Protection, Chinese Academy of Agricultural Sciences (IPPCAAS), which was introduced from the International Centre for Biological Control, United Kingdom, and preserved in the IPPCAAS.
Insect net (mesh diameter: 5 mm, mesh bag diameter: 40 cm, and mesh length: 80–130 cm), insect cage (1 m × 1 m × 1 m), insect rearing frame (26 × 13 × 7 cm), glass cover plate (30 × 50 × 0.3 cm), conical flask (100 ml), test tube (10 ml), petri dish, writing brush, stirring rod, glass beads (dm = 3 mm), hemocytometer (25 × 16 mm), microscope (OLYMPUS-SZX16), oscillator MX-F, oven DHG-9123A, autoclave YXQ-LS-SII, and electronic balance PL-403.
Liquid nitrogen, distilled water, soil temperature 80 (dosage was 0.001), vegetable oil, and carrier (wheat bran).
The crude plant extract was extracted with water and two concentrations (10 and 20%) of the extracts were prepared (Table 1). Briefly, the fresh plant materials of A. sieversiana were cut into small pieces of about 1 cm and put into a conical flask. Distilled water was added and then autoclaved at 120°C for 90 min. They were taken out after heating and cooled to room temperature to filter plant residues.

Table 1. Proportion of components in the different concentrations of the prepared crude extract from Artemisia sieversiana.
Healthy 4th-instar O. asiaticus were selected from the insect cage in the laboratory, and raised in a 26 × 13 × 7 cm3 breeding frame with a glass cover (30 × 50 × 0.3 cm). Each cage contained 10 females and 10 males (20 heads in each frame). One frame served as a replicate, and five repetitions were treated as a group. In the A. sieversiana extract treatment, the 10 and 20% A. sieversiana extracts were spread separately on leaves of S. krylovii with a brush and fed to O. asiaticus in the cages. Leaves on which distilled water was spread, served as the control. The experiment lasted for 7 days and data on mortality were recorded.
In the M. anisopliae treatment, 1 g each of the three concentrations of M. anisopliae (106, 107, and 108 spores/g) was weighed and separately mixed with a carrier (10 g vegetable oil and 100 g wheat bran) as poison bait. The mixture was divided into five petri dishes (dm = 7 cm) and placed in the insect rearing frame containing 20 healthy 4th instar O. asiaticus which had been prior fed with fresh S. kirnii for 24 h. The experiment lasted for 7 days and data on mortality were recorded.
In this treatment, mixtures which were made up of each concentration of A. sieversiana extract and each concentration of M. anisopliae were prepared (Table 2). These combinations were then added to a mixture made up of 10 g vegetable oil mixed into 100 g wheat bran. They were mixed evenly, and then dispensed into glass culture dishes to feed O. asiaticus in the cages. After feeding for 24 h, the diet was changed to fresh S. kirnii for continuous feeding for 7 days. Data on mortality were recorded during this period.
Female and male 4th instar O. asiaticus caught from the Xilinguole grassland were used to determine the effects of the treatments on body weight. We randomly selected 30 healthy 4th females and 4th males, respectively, from the captured 4th instar O. asiaticus to detect their initial body weight and body length. We use a vernier caliper to measure these selected females and males, and then put them in the oven at 90°C for 24 h after which the dry weights were measured using an electronic balance.
We also selected enough healthy 4th instar females and 4th instar males from the captured O. asiaticus for experiments. We conducted the experiment twice, each with five biological replicates, each cage contained 10 females and 10 males (20 heads in each frame). The survival and developmental time of O. asiatica in the cages were recorded every day to ensure that there were enough males and females for body weight and body length measurement at the end of the experiment. Dead individuals were removed in time when we change fresh S. krylovii. At the end of 7 days, the body lengths of 30 locusts were measured using the vernier caliper. They were then dried in the oven at 90°C for 24 h after which the dry weights were measured using an electronic balance.
The effect of the pesticide treatments on activities of four antioxidant enzymes [superoxidase dismutase (SOD), peroxidase (POD), catalase (CAT), and polyphenol oxidase (PPO)] in O. asiaticus were measured. To extract crude enzyme solutions, about 0.1 g of insect tissue was weighed, to which 1 ml of an extraction solution was added (purchased from Suzhou Keming Biotechnology Co., Ltd.). They were then placed in an ice bath for homogenization. The supernatant was then centrifuged at 8000 g at 4°C for 10 min and placed on ice for the measurement of enzyme activities with detection kits of each antioxidant enzymes (Suzhou Keming Biotechnology Co., Ltd.). Preparation of the test samples followed the manufacturer’s instructions. The absorbance wavelength of each enzyme was measured on a microplate reader (DNM-9602G).
For POD, the wavelength was adjusted to 470 nm, and that of distilled water adjusted to zero. The initial absorbance value, A1, was recorded at 470 nm at 1 min and that of A2 at 2 min. The final absorbance value was given by ΔA = A2−A1. For CAT, the wavelength was adjusted to 240 nm, and that of distilled water was adjusted to zero. The initial absorbance value A1 was recorded at 240 nm and the 2nd absorbance value A2, was recorded after 1 min. The final absorbance value was given by ΔA = A1−A2. For PPO, the wavelength was adjusted to 525 nm, and that of distilled water was adjusted to zero. The absorbance of the determination tube and reference tube was detected at 525 nm. The final absorbance value was given by ΔA = A measured-A control. For SOD, the absorbance was measured at 560 nm. The final absorbance value was given by the formula, Inhibition percentage = (A control tube-control tube) ÷ A control tube × 100%.
(1) The mortality rate (%) was calculated as the ratio of the number of O. asiaticus deaths in each frame to the number of O. asiaticus in the initial 4th instar (N = 20). (2) Weight gain (increased body dry mass, mg) = dry weight of O. asiaticus after 7 days (mg) − 4th instar O. asiaticus weight (mg). (3) Increase in body length (mm) = 7 days later O. asiaticus length (mm) − the length of the first 4th instar O. asiaticus (mm). (4) The developmental period was the experimental period of 7 days. (5) Growth rate (mg/day) = weight gain ÷ developmental duration. (6) Overall performance = survival rate × growth rate (Cease et al., 2012; Cui et al., 2019).
The activities of the enzymes were determined using the following formulae;
Paragraph text.
(Vtotal reaction: total volume of reaction; Vsample: volume of added sample; Vsample total: volume of added extract; T: reaction time; W: sample mass; ε: molar extinction coefficient of H2O2, 4.36 × 104 L/mol/cm)
The experimental data were all analyzed using Excel 2010, the means were evaluated via the one-way ANOVAs at the 5% significance level, and differences among means were compared by using the Turkey at p < 0.05 (Jia et al., 2016). And Prism6 was used for making the figure, and the error bars is the SE.
The 10% crude extract of A. sieversiana had significant inhibitory effect on the body weight of female (Figure 1A) and male 4th instar (Figure 1B) O. asiatica. However, the different concentrations (106, 107, and 108 spores/g) of M. anisopliae promoted weight gain in female (Figure 1A) and male 4th instar (Figure 1B) O. asiatica. Among them, the 107 and 108 spores/g M. anisopliae concentrations had the strongest effect on the weight gain of female (29.5 ± 1.63 g) and male O. asiatica (26.9 ± 2.00 g), respectively. The combined treatment of 10% crude extract of A. sieversiana and each of the different concentration of M. anisopliae, had a significant inhibitory effect on the weight gain of O. asiatica. Among them, the combination of 10% A. sieversiana crude extract + 107 spores/g of M. anisopliae had the most significant inhibitory effect on the weight gain of the female (Figure 1A) and male 4th instar (Figure 1B) O. asiatica (F = 233.829, DF = 7, p = 0), with the weight gain of 129.73 ± 1.97 g and −7.2 ± 1.07, respectively.
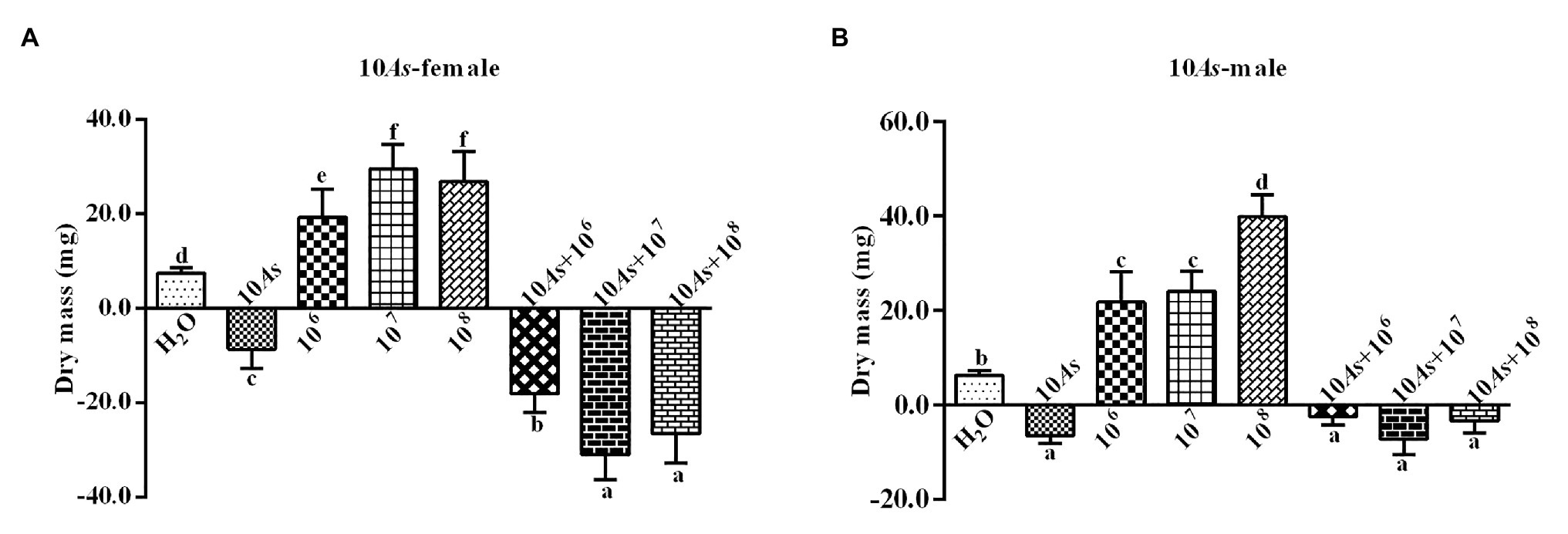
Figure 1. Body weight gain and loss of (A) female and (B) male 4th instar Oedaleus asiatica from treatments with 10% A. sieversiana (10% As) crude extract, different concentrations of M. anisopliae and their combinations. Different lowercase letters indicate significant differences in the growth rate of the O. asiatica in the same year.
The 10% crude extract of A. sieversiana and M. anisopliae alone or in combination had effects on the body length of female (Figure 2A) and male (Figure 2B) O. asiatica. The 10% crude extract of A. sieversiana had an inhibitory effect on the growth of the body length of the O. asiatica. The different concentration (106, 107, and 108 spores/g) of M. anisopliae all increased the body length of the O. asiatica, of which the 108 spores/g M. anisopliae concentration had the most obvious effect on male (10.78 ± 0.36 mm) and female (9.28 ± 0.31 mm) O. asiatica. The combined treatment of 10% crude extract of A. sieversiana and the different concentrations of M. anisopliae (106, 107, and 108 spores/g) had an inhibitory effect on the body length of O. asiatica. The combined treatment of 10% of A. sieversiana crude extract + 108 spores/g of M. anisopliae, had the most significant inhibitory effect on the increase in body length of O. asiatica (Female: 3.66 ± 0.23 mm; male: 2.87 ± 0.18 mm).
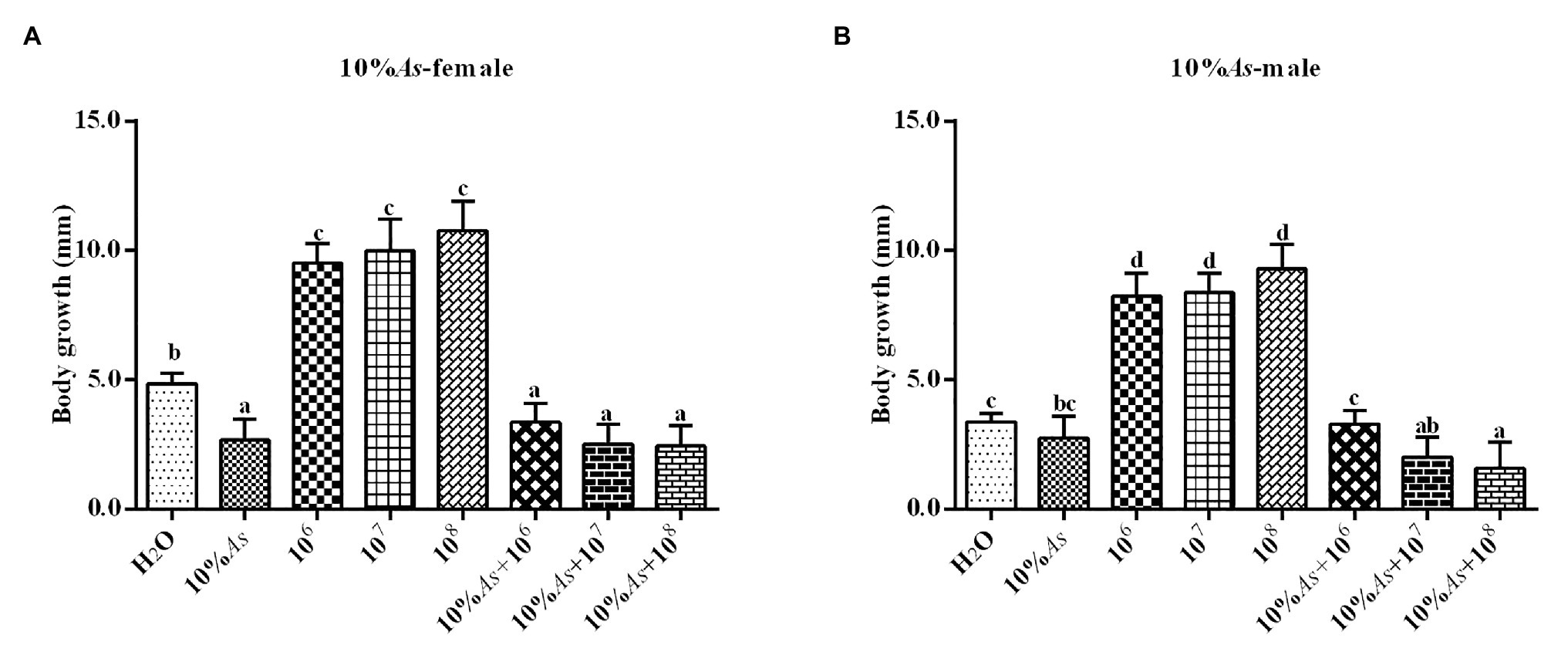
Figure 2. Increase in body length of the (A) female and (B) male 4th instar O. asiatica from the treatment with 10% A. sieversiana (10% As) crude extract, different concentrations of M. anisopliae and their combinations. Different lowercase letters indicate significant differences in the growth rate of the O. asiatica in the same year.
The 10% crude extract of A. sieversiana and M. anisopliae alone or in combination had effects on the growth rate of female (Figure 3A) and male (Figure 3B) O. asiatica. The growth rate of O. asiatica was significantly inhibited by the 10% crude extract of A. sieversiana, However, all the concentrations of M. anisopliae (106,107, and 108 spores/g) promoted the growth rate of female and male grasshoppers. For female, the 107 spores/g concentration had the highest effect on growth rate. For male, the 108 spores/g concentration had the highest effect. The results showed that the combination of the 10% crude extract of A. sieversiana and the different concentrations of M. anisopliae (106, 107, and 108 spores/g) had significant inhibitory effect on the growth rate of O. asiatica. The combination of 10% crude extract + 108 spores/g M. anisopliae and 10% crude extract + 107 spores/g M. anisopliae had the most significant inhibitory effect on the growth rate of female and male O. asiatica, respectively.
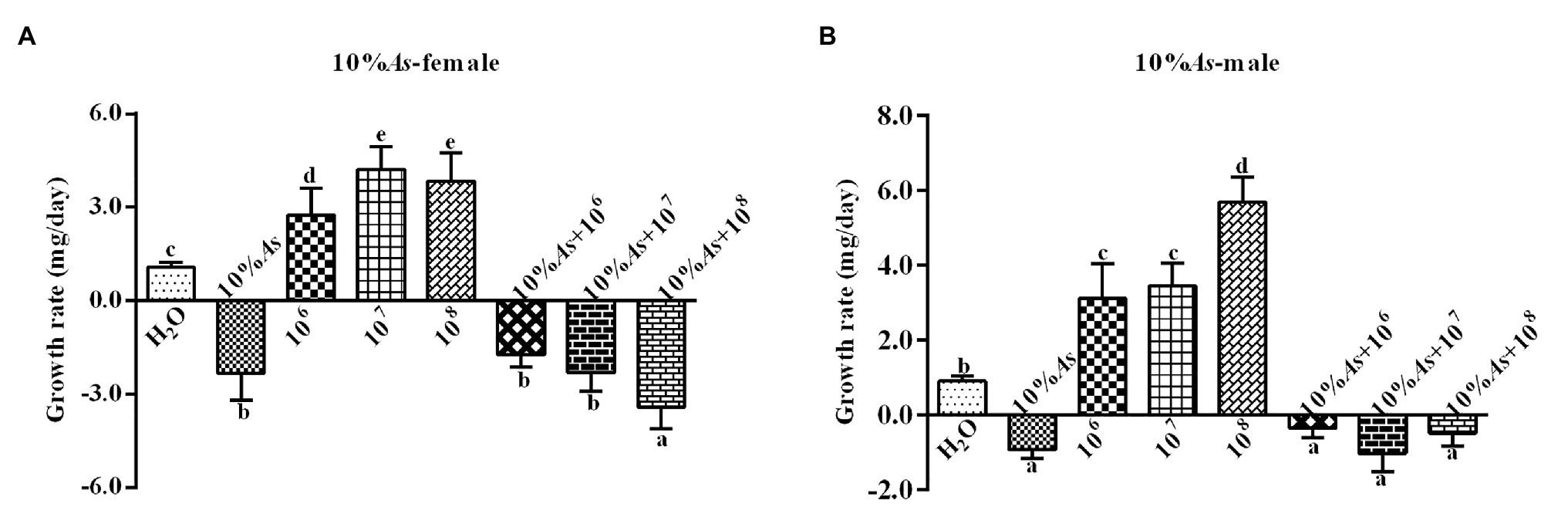
Figure 3. Growth rates of (A) female and (B) male 4th instar O. asiatica from the treatments with 10% A. sieversiana (10% As) crude extract, different concentrations of M. anisopliae and their combinations. Different lowercase letters indicate significant differences in the growth rate of the O. asiatica in the same year.
The 10% A. sieversiana crude extract and M. anisopliae alone or in combination had effect on the overall performance of female (Figure 4A) and male (Figure 4B) O. asiatica. The 10% crude extract of A. sieversiana had an extremely significant effect on the overall performance. All concentrations of M. anisopliae (106, 107, and 108 spores/g) promoted the growth and development of O. asiatica. Among them, the 107 spores/g M. anisopliae concentration had the most significant effect on the growth and development of male (1.13 ± 0.37) and female (0.96 ± 0.08) O. asiatica. The combined treatment of 10% crude extract of A. sieversiana + each of the concentration of M. anisopliae (106, 107, and 108 spores/g), had an effection on the growth of O. asiatica. The combined treatment of 10% A. sieversiana crude extract + 106 spores/g M. anisopliae and 10% crude extract of A. sieversiana + 107 spores/g M. anisopliae had the best inhibition on the overall performance of female O. asiatica (−0.95 ± 0.15) and male O. asiatica (−0.21 ± 0.07), respectively.
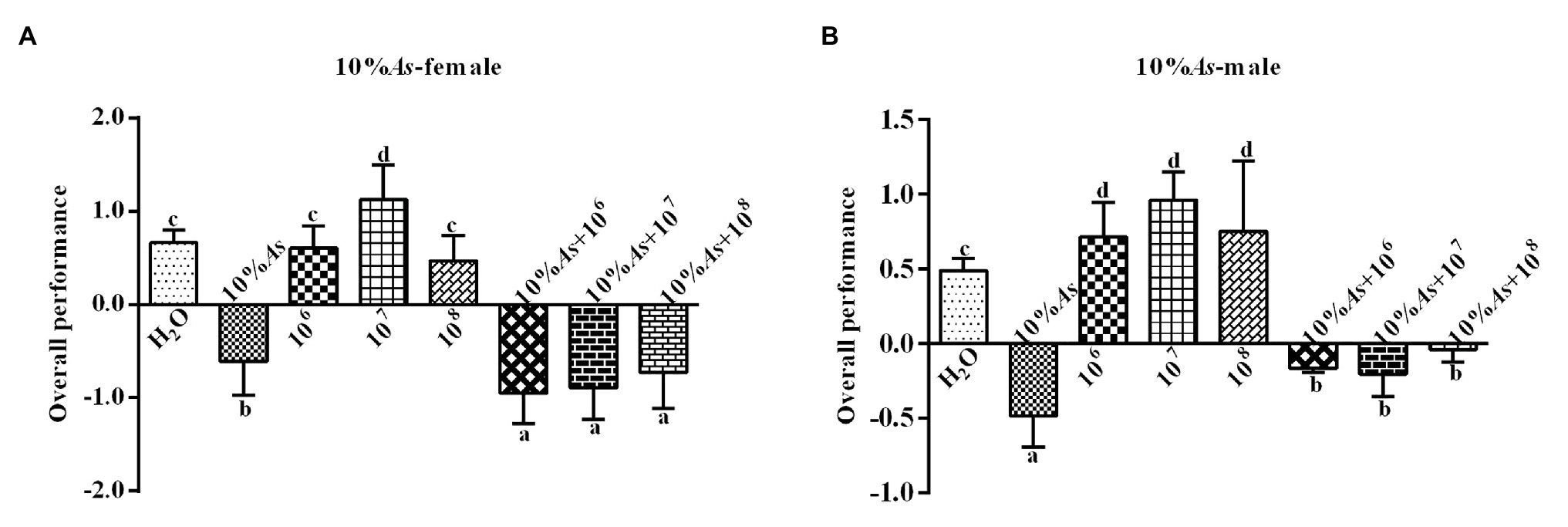
Figure 4. Overall performance of (A) female and (B) male 4th instar O. asiatica from the treatments with 10% A. sieversiana (20% As) crude extract, different concentrations of M. anisopliae and their combinations. Different lowercase letters indicate significant differences in the overall performance of the O. asiatica in the same year.
The 20% crude extract of A. sieversiana had a significant inhibitory effect on the body weight of female (Figure 5A) and male (Figure 5B) 4th instar O. asiatica. However, the different concentrations of M. anisopliae (106, 107, and 108 spores/g) promoted weight gain in the female and male O. asiatica. Among them, the 107 spores/g M. anisopliae concentration had the strongest effect on the weight gain of female O. asiatica (19.3 ± 1.88 mg). The combined treatment of 20% A. sieversiana crude extract and the different concentrations of M. anisopliae (106, 107, 108 spores/g) had a significant inhibitory effect on the weight gain of the O. asiatica. Among them, the combined treatment of 20% crude extract of A. sieversiana + 108 spores/g of M. anisopliae and 20% A. sieversiana crude extract + 107 spores/g of M. anisopliae had the strongest inhibitory effect on the weight gain of the male (−23.9 ± 1.05 mg) and female (−4.3 ± 0.52 mg) O. asiatica, respectively.
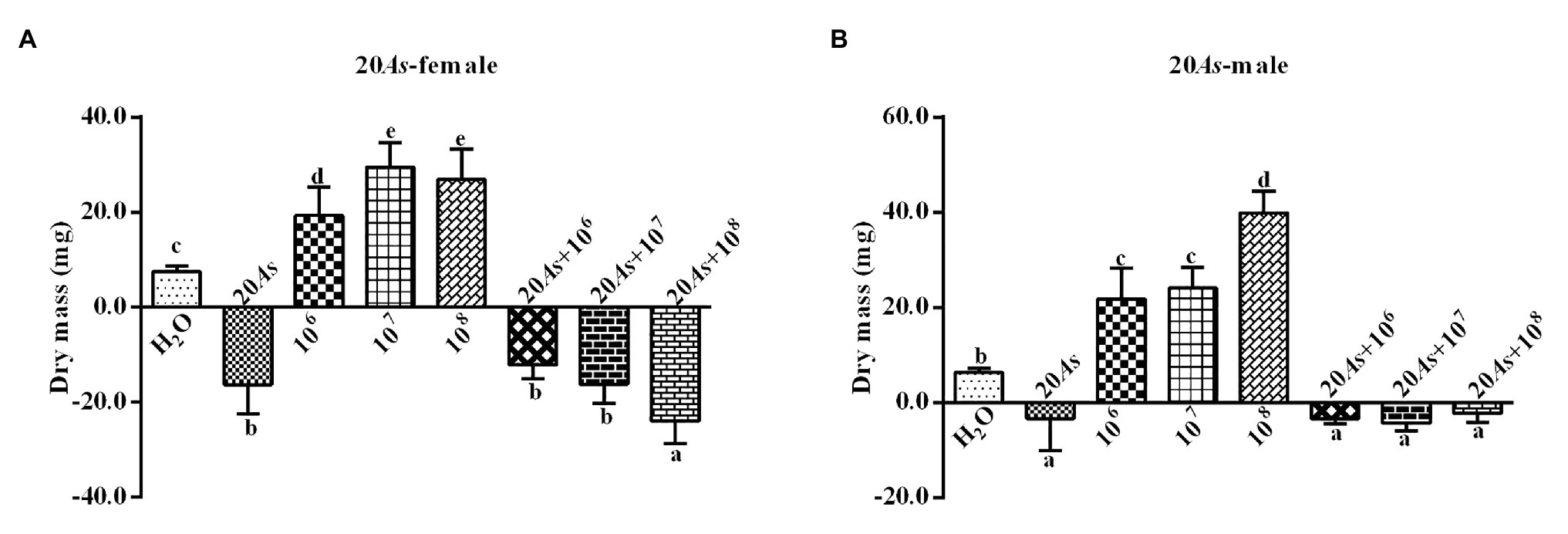
Figure 5. Weight gain and loss of the (A) female and (B) male 4th instar O. asiatica from the treatments with 20% A. sieversiana (20% As) crude extract, different concentrations of M. anisopliae and their combinations. Different lowercase letters indicate significant differences in the weight gain of the O. asiatica in the same year.
The 20% crude extract of A. sieversiana and M. anisopliae alone or in combination had effect on the body length of the female (Figure 6A) and male (Figure 6B) O. asiatica. The 20% crude extract of A. sieversiana had a relatively inhibitory effect on the body length of the O. asiatica. The different concentrations of M. anisopliae (106, 107, and 108 spores/g) increased the body length of O. asiatica. Among them, the 108 spores/g M. anisopliae concentration had the most significant effect on the body length. The combined treatment of 20% crude extract of A. sieversiana and different concentrations of M. anisopliae (106, 107, and 108 spores/g) had an inhibitory effect on the body length of female and male O. asiatica. The combined treatment of 20% crude extract of A. sieversiana + 108 spores/g M. anisopliae had the most significant inhibitory effect on the body length of O. asiatica (female 2.44 ± 2.52 mm; male: 1.58 ± 0.32 mm).
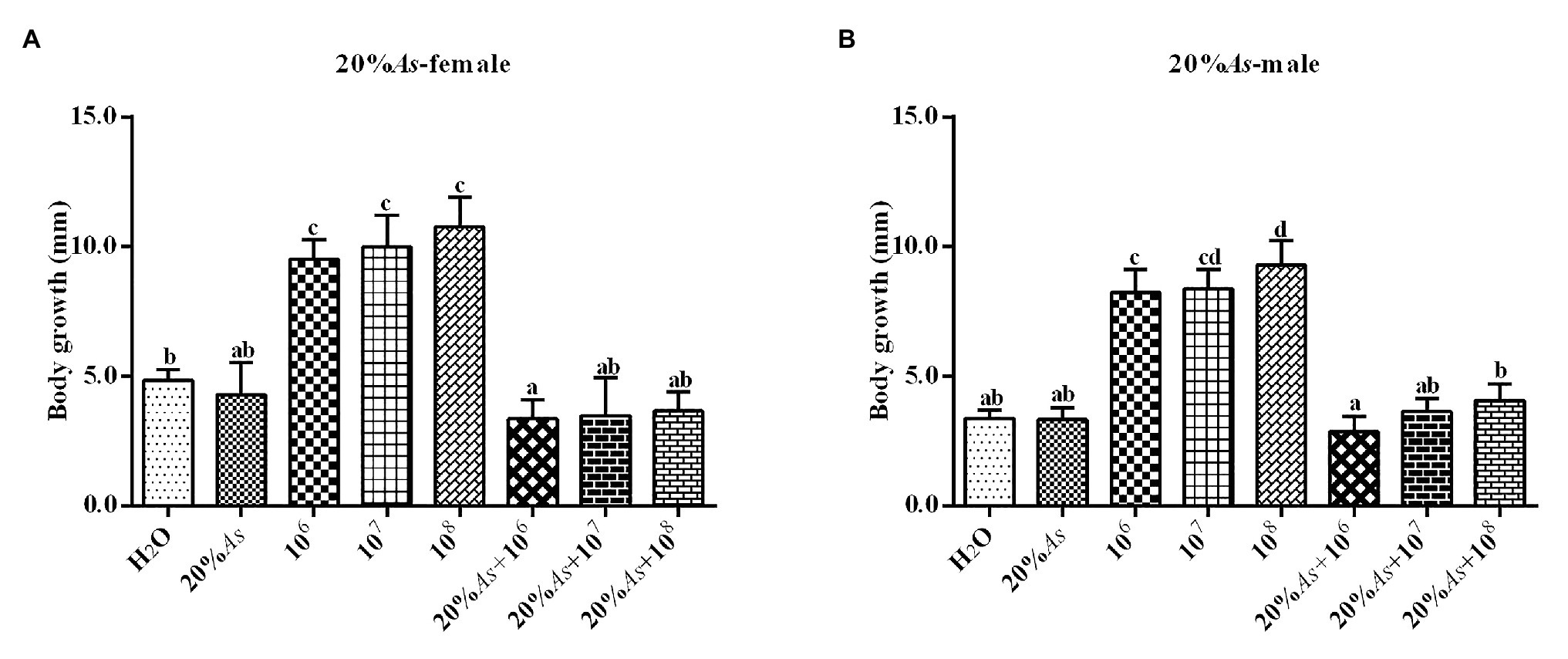
Figure 6. Increase in body length of (A) female and (B) male 4th instar O. asiatica from treatments with 20% A. sieversiana (20% As) crude extract, different concentrations of M. anisopliae and their combinations. Different lowercase letters indicate significant differences in the body length of the O. asiatica in the same year.
The 20% A. sieversiana crude extract and M. anisopliae alone or in combination had effects on the growth rate of female (Figure 7A) and male (Figure 7B) O. asiatica. The 20% crude extract of A. sieversiana had inhibitory effects on the growth rates of O. asiatica. The results also showed that the concentration of 107 spores/g M. anisopliae and 108 spores/g of M. anisopliae had a significant effect on the growth rate of female (F = 185.11, DF = 7, p = 0), and male (F = 161.59, DF = 7, p = 0) O. asiatica. The combination treatment of 20% crude extract + 108 spores/g M. anisopliae had the most significant inhibitory effect on the growth rate of female (−3.41 ± 0.22 mg/d) and combination treatment of 20% crude extract + 107 spores/g M. anisopliae had the most significant inhibitory effect on the growth rate of male (−0.61 ± −0.78 mg/d) O. asiatica.
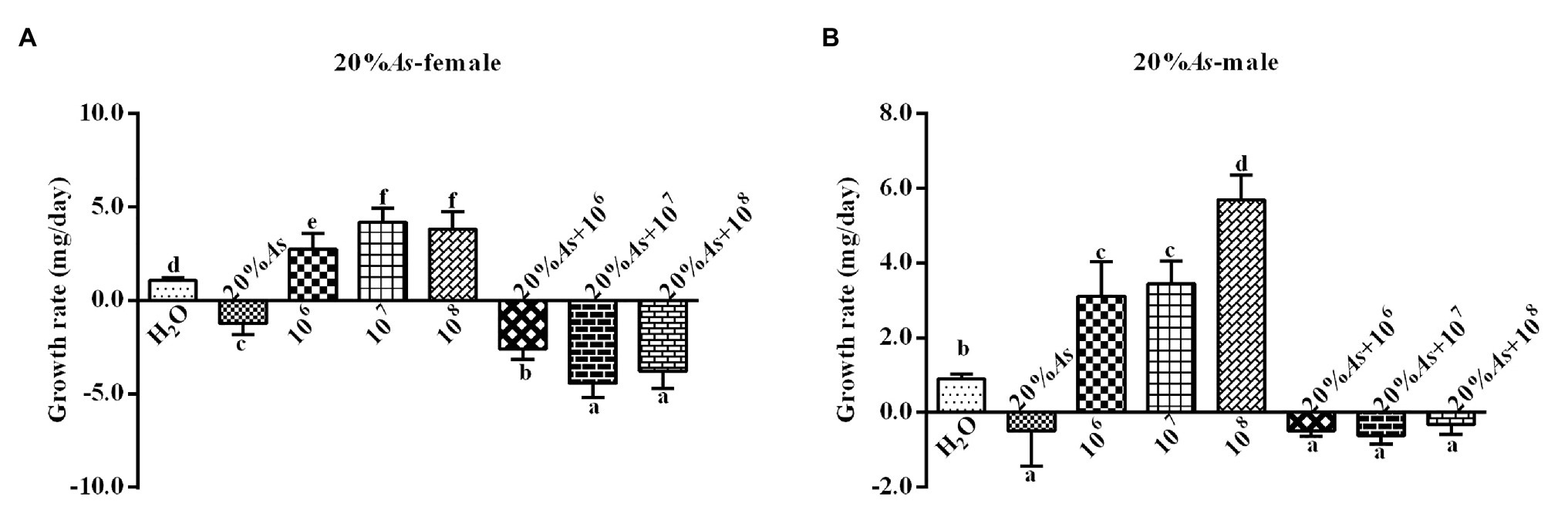
Figure 7. Growth rates of (A) female and (B) male 4th instar O. asiatica, from treatments with 20% A. sieversiana (20% As) crude extract, different concentrations of M. anisopliae and their combinations. Different lowercase letters indicate significant differences in the growth rate of the O. asiatica in the same year.
The 20% A. sieversiana crude extract and M. anisopliae alone or in combination had effects on the overall performance of female (Figure 8A) and male (Figure 8B) O. asiatica. The 20% crude extract of A. sieversiana had an inhibition on the overall performance of female (−0.93 ± 0.10) and male (−0.44 ± 0.03). The 107 spores/g M. anisopliae concentration had a promoting effect on the growth and development of female (1.13 ± 0.16) and male (0.96 ± 0.08) O. asiatica compared to the control. The combination of the 20% A. sieversiana crude extract and different concentration of M. anisopliae had a significant inhibitory effect on the overall performance of O. asiatica. Among them, the combination of 20% A. sieversiana crude extract + 106 spores/g had the strongest inhibition on the overall performance of female (−0.71 ± 0.09; Figure 8A) and male (−0.16 ± 0.03) O. asiatica (Figure 8B).
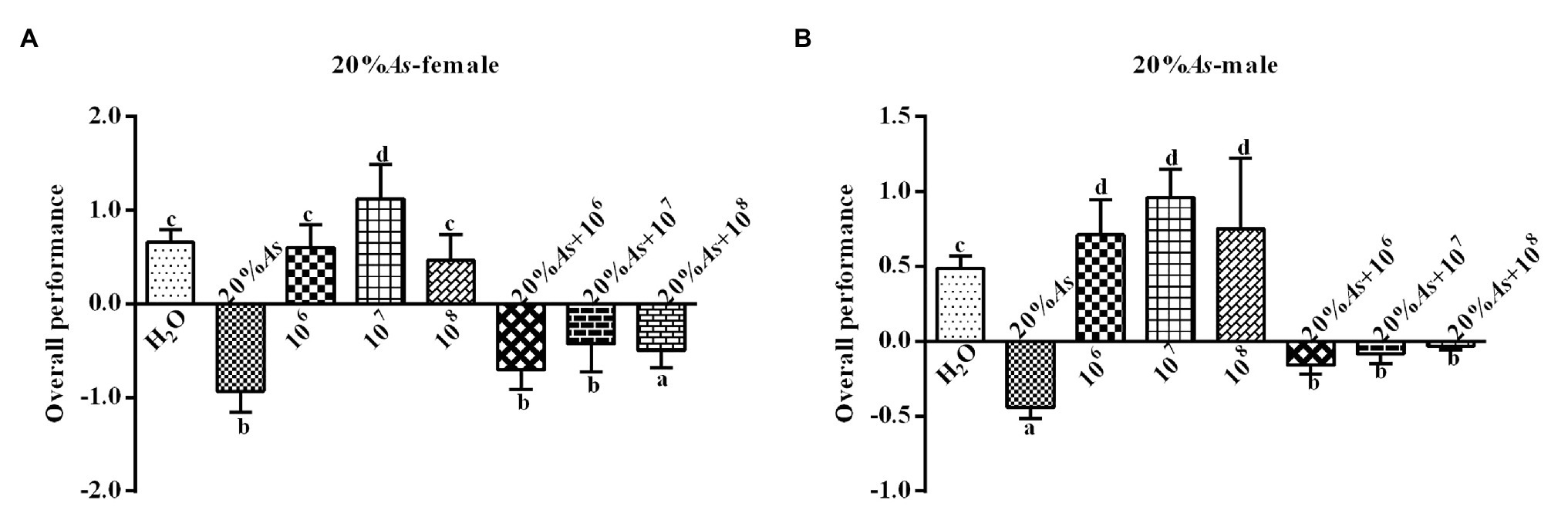
Figure 8. Overall performance of (A) female and (B) male 4th instar O. asiatica from treatments with 20% A. sieversiana (20% As) crude extract, different concentrations of M. anisopliae and their combinations. Different lowercase letters indicate significant differences in the overall performance of the O. asiatica in the same year.
When A. sieversiana was used alone, the lethal effect of 20% crude extract was greater than that of 10% crude extract. The lethal effect of the different concentrations of M. anisopliae on O. asiatica followed a decreasing order as follows: 108 spores/g > 106 spores/g > 107 spores/g, but there was no significant difference between the 106 and 107 spores/g concentration. The lethal effect of combined treatment on O. asiatica also followed a decreasing order as follows: 20% As + 108 spores/g M. anisopliae > 20% As + 107 spores/g M. anisopliae > 10% As + 107 spores/g M. anisopliae > 10% As + 106 spores/g M. anisopliae > 20% As + 106 spores/g M. anisopliae. Among them, 108 spores/g M. anisopliae had the best efficacy, resulting into a mortality rate of 85 ± 5.08%. The results showed that the combination of 10% crude extract of A. sieversiana + 107 and 108 spores/g M. anisopliae, the combination of 20% crude extract + 107 and 108 spores/g M. anisopliae had significantly better control effect on O. asiatica than that of using only crude extract of A. sieversiana (F = 14.23, DF = 11, p = 0; Figure 9).
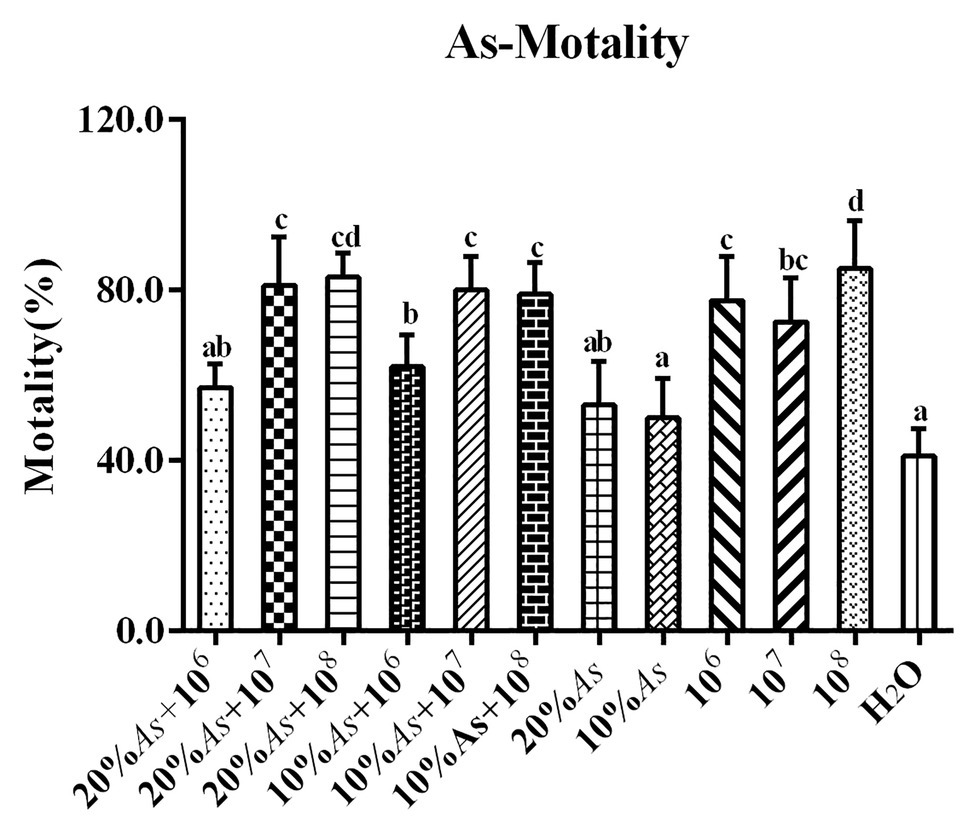
Figure 9. Mortalities of O. asiatica from treatments with 10 and 20% crude extract A. sieversiana and M. anisopliae alone or in combination. Different lowercase letters indicate significant differences in the mortality of the O. asiatica in the same year.
There was no significant difference in the lethal effect of the 20% As + 107 spores/g M. anisopliae and 10% As + 107 spores/g M. anisopliae on O. asiatica (Figure 9). Likewise the effect of the increase in concentration of A. sieversiana extracts, on the mortality of O. asiatica was not significantly different. Therefore, in this experiment, we tested the activities of antioxidant enzymes in O. asiatica from the following treatments: 10% A. sieversiana extract + 107 spores/g M. anisopliae, 10% A. sieversiana extract alone, 107 spores/g M. anisopliae alone, and distilled water (control). The activities of catalase (CAT; 414.94 ± 16.94 U/g) and polyphenol oxidase (PPO; 88.20 ± 5.49 U/g) were significantly increased by the 10% A. sieversiana extract + 107 spores/g M. anisopliae (Figure 10). The activities of SOD and POD were also activated by this treatment. The highest activity of SOD (2564.10 ± 218.08 U/g) was recorded under the 107 spores/g M. anisopliae treatment, and that of POD (368.40 ± 47.45 U/g) was recorded under the 10% A. sieversiana extract treatment (Figure 10).
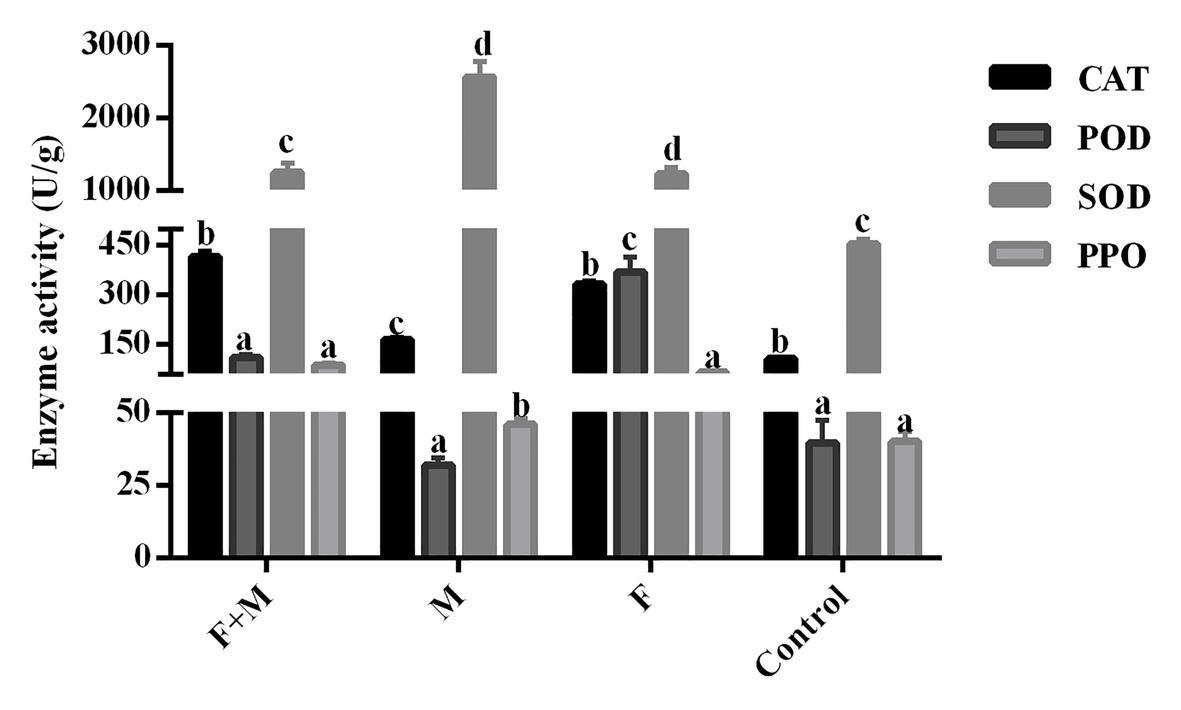
Figure 10. Activities of antioxidant enzymes in O. asiatica from the treatment with 10% crude extract A. sieversiana and M. anisopliae alone or their combined use. 1. F, M. anisopliae alone; M, crude extract of 10% A. sieversiana alone; F+M, combination of M. anisopliae and the 10% crude extract of A. sieversiana; Control, distilled water; CAT, catalase; SOD, superoxide dismutase; POD, peroxidase; PPO, polyphenol oxidase.
The frequent large-scale outbreaks of O. asiatica in the grasslands of northern China, are partly responsible for the recent degradation of grasslands and desertification in Inner Mongolia in China. The entomopathogenic fungus M. anisopliae, a recognized non-polluting and non-toxic pesticide, has been widely studied for pest control and has shown potential as a chemical substitute for locust and grasshopper control (Gillespie et al., 2000; Peng et al., 2008). However, insect pathogenic fungi have shortcomings such as a longer time of attacking pest and unstable control effect (Pilz et al., 2009). In addition, external conditions such as temperature, humidity, density, and height of vegetation also affect the control efficacy of M. anisopliae on locust. In dense vegetation areas, the reduced infectivity of M. anisopliae conidia on the vegetation surface also reduces its efficacy (Hunter et al., 2001).
Therefore, the use of appropriate adjuvants can increase the persistence of fungi. Many entomopathogenic fungi are compatible with a range of chemical and natural insecticides (Santos et al., 2007; Pelizza et al., 2015). One possible candidate is crude plant extracts. For example, the combination of neem oil and M. anisopliae significantly increased the mortalities of A. aegypti and locusts. By this, the efficacy of this biological control agent was increased (Haroona et al., 2011; Paula et al., 2019). However, neem oil inhibited the vegetative growth of Beauveria bassiana and reduced the yield and viability of conidia (Depieri et al., 2005).
The secondary metabolites of Artemisia plants include terpenoids, flavonoids, coumarins, glycosides, steroids, and polyacetylenes (Tan et al., 1998). The crude extract of A. sieversiana was shown to have insecticidal activity (Liu et al., 2010). In this regard, we also found that the treatment with A. sieversiana alone significantly reduced the mortality of O. asiatica. Correspondingly, it caused a series of immune and physiological responses in the host insect after M. anisopliae infected the host (Wang et al., 2013; Ling et al., 2016). Therefore, the high concentration of conidia may enhance host fungal infections and cause high mortality in a relatively short time (Kirkland et al., 2004; Shrestha et al., 2015). This study also indirectly confirmed that a higher concentration of M. anisopliae enhances its lethality.
Fungal insecticides have good potential for pest control, as there is currently no record of development of insect resistance against them (Behie and Bidochka, 2013; Erler and Ates, 2015). In order to reduce the risk of resistance, the use of fungal agents should be minimized. The efficacy of a combination of fungi and chemicals was shown to be higher under laboratory or field conditions, and has been reported in several studies involving Anomala cuprea Hope and Locusta migratoria (Hiromori and Nishigaki, 1998; Jia et al., 2016). Although there was no significant difference between the mortality of O. asiatica treated with 20% crude extract of A. sieversiana combined with 108 spores/g M. anisopliae and 108 spores/g M. anisopliae alone in this study, the overall performance of O. asiatica was decreased. This indicates that the efficacy of the crude extract of A. sieversiana was significantly improved.
The death time of pest is an important factor affecting the control efficacy of insecticides for pest management (Asi et al., 2008; Gupta et al., 2009). The results of a field application of 6 × 107 spore M. anisopliae bait on locusts, showed that the peak infection of the locust population was reached on the 15th day after infection, with a mortality rate of 6.5%, and the mortality rate increased with time (Dong et al., 2011). Compared to this experiment, the mortality rate was much lower than that in the treatment with crude extract of A. sieversiana and M. anisopliae, and the peak time of infection was three times longer than that under the mixture of crude extract of A. sieversiana and M. anisopliae. The results showed that treatment with the combination of the crude extract of A. sieversiana and M. anisopliae significantly shortened the lethal time, and its efficacy was significantly better than that of other groups and any single agent. This shows that the synergetic control effect from the combination treatment was faster and better than the control effect of each pesticide alone. The effect of 10% A. sieversiana crude extract mixed with 107 spores/g M. anisopliae on the mortality of O. asiatica reached the effect of 108 spores/g M. anisopliae alone in this study; therefore, the dosage of M. anisopliae was reduced, and may largely prevent the development of resistance in insects against it.
Although it has not been reported that insects resistance to M. anisopliae (Dubovskiy et al., 2013), the risk of resistance to M. anisopliae can be reduced by observing the effects of A. sieversiana and M. anisopliae on the protective enzyme activities of O. asiatica. We found that the enzymatic activities of catalase and polyphenol oxidase of O. asiatica were over-expressed under the mixed treatment of the combination of the crude extract of A. sieversiana and M. anisopliae. And some studies have found that the high expression of catalase could shorten the germination time and increase the pathogenicity. of M. anisopliae (Hernandez et al., 2010). Therefore, we found that the crude extract of A. sieversiana can be used as a synergist of M. anisopliae to improve its pathogenicity. Under the conditions of external factors, such as plants and biological pesticides, the slower growth and development of insects are often associated with the expression of enzyme activity in their bodies (Serebrov et al., 2006). Our results also confirmed the conclusion that the mortality, body weight, growth rate, and overall performance of O. asiatica were significantly reduced under the treatment with crude extract of A. sieversiana and M. anisopliae, while the activity of its protective enzymes was significantly increased.
The use of M. anisopliae in combination with chemical agents have caused varied mortality rates of locusts, and even showed antagonistic effect (Jia et al., 2016). In our research, similar results of different concentration ratios causing varied mortality of O. asiatica were recorded. Therefore, in the considerations for the use of a combination of new biopesticides and fungal preparations for pest control, the compatibility of the two should be carefully evaluated. A number of studies have comprehensively evaluated the compatibility of fungi and chemicals agents under laboratory and field conditions (Cuthbertson et al., 2005; Asi et al., 2010).
We found an interesting phenomenon in this study. Metarhizium anisopliae can promote the increase of body weight and body length of O. asiatica. However, when M. anisopliae was mixed with the crude extract of A. sieversiana, it inhibited the growth of body weight and length, especially the growth of body weight, and the effect of the mixture of M. anisopliae and the crude extract of A. sieversiana on the development of female was greater than that of male. However, the reasons for this phenomenon need to be further studied. Our work was carried out under laboratory conditions. We, therefore, propose that further studies under field conditions characterized by many complicated and uncontrollable factors, be carried out to determine the best compound formula which shows promising efficacy. Additionally, other parameters such as the toxicity of fungi, the influence of the mixture of crude extract of A. sieversiana and M. anisopliae on natural enemies and non-target organisms, etc. should be considered in the evaluation of the combination of fungi and new biopesticides.
In summary, our research showed that M. anisopliae was a good biological control agent for on O. asiatica. The crude extract of A. sieversiana increased the virulence of M. anisopliae and shortened the mortality time of the pest. These results indicate that the crude extract of A. sieversiana could be used as a synergist for M. anisopliae, and this compound formulation can become an alternative biological control agent to control insect pest.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
ZZ and XT designed the experiments. SL and XT performed the experiments. SL and ZZ wrote the manuscript. SL, CX, and GD collected the data and analyzed it. ZZ and GW provided technical and material support. All authors contributed to the article and approved the submitted version.
This work was supported by the Special funds for Basic Scientific Research of Chinese Academy of Agricultural Sciences (Y2020YJ02) and the Earmarked Fund for China Agriculture Research System (CARS-34-07).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank the Grassland Pest Group, Institute of Plant Protection, Chinese Academy of Agricultural Sciences to provide the M. anisopliae IMI330-189. We also would like to thank DBM editing for professional English language editing.
Asi, M. R., Afzal, M., Anwar, S. A., and Bashir, M. H. (2008). Comparative efficacy of insecticides against sucking insect pests of cotton. Pakistan J. Life Soc. Sci. 15, 631–638.
Asi, M. R., Bashir, M. H., Afzal, M., Ashfaq, M., and Sahi, S. T. (2010). Compatibility of entomopathogenic fungi, Metarhizium anisopliae and Paecilomyces fumosoroseus with selective insecticides. Pak. J. Bot. 42, 4207–4214.
Ayub, M., Hussain, S., Rasool, A., Hussain, I., Khan, F., and Sultan, A. (2019). Insecticidal activity of Artemisia sieversiana Ehrh. plant extract against white grub (Scarabaeidae: Coleoptera). J. Entomo. Zool. Studies 7, 304–308.
Behie, S. W., and Bidochka, M. J. (2013). Potential agricultural benefits through biotechnological manipulation of plant fungal associations. Bioessays 35, 328–331. doi: 10.1002/bies.201200147
Bi, J. L., and Felton, G. W. (1995). Foliar oxidative stress and insect herbivory: primary compounds, secondary metabolites, and reactive oxygen species as components of induced resistance. J. Chem. Ecol. 21, 1511–1530. doi: 10.1007/BF02035149
Blanford, S., Chan, B. H. K., Jenkins, N., Sim, D., Turner, R. J., Read, A. F., et al. (2005). Fungal pathogen reduces potential for malaria transmission. Science 308, 1638–1641. doi: 10.1126/science.1108423
Cease, A. J., Elser, J. J., Ford, C. F., Hao, S. G., Kang, L., and Harrison, J. F. (2012). Heavy livestock grazing promotes locust outbreaks by lowering plant nitrogen content. Science 335, 467–469. doi: 10.1126/science.1214433
Cui, B. Y., Huang, X. b., Li, S., Hao, K., Chang, B. H., Tu, X. B., et al. (2019). Quercetin affects the growth and development of the grasshopper Oedaleus asiaticus (Orthoptera: Acrididae). J. Econ. Entomol. 112, 1175–1182. doi: 10.1093/jee/toz050
Cui, J., Nuermaimaiti, M., Zou, G., and Xin, X. (2020). Chemical composition of Artemisia sieversiana. Chem. Nat. Compd. 56, 1120–1121. doi: 10.1007/s10600-020-03241-6
Cuthbertson, A. G., Walters, K. F., and Deppe, C. (2005). Compatibility of the entomopathogenic fungus Lecanicillium muscarium and insecticides for eradication of sweetpotato whitefly, Bemisia tabaci. Mycopathologia 160, 35–41. doi: 10.1007/s11046-005-6835-4
da Silva, J. A. T., Yonekura, L., Kaganda, J., Mookdasanit, J., Nhut, D. T., and Afach, G. (2005). Important secondary metabolites and essential oils of species within the anthemideae (Asteraceae). Int. J. Geogr. Inf. Syst. 11, 1–46. doi: 10.1300/J044v11n01_01
Depieri, R. A., Martinez, S. S., and Menezes, J. (2005). Compatibility of the fungus Beauveria bassiana (Bals.) Vuill. (Deuteromycetes) with extracts of neem seeds and leaves and the emulsible oil. Neotrop. Entomol. 34, 601–606. doi: 10.1590/S1519-566X2005000400010
Dimbi, S., Maniania, N. K., and Ekesi, S. (2009). Effect of Metarhizium anisopliae inoculation on the mating behavior of three species of African Tephritid fruit flies, Ceratitis capitata, Ceratitis cosyra and Ceratitis fasciventris. Biol. Control 50, 111–116. doi: 10.1016/j.biocontrol.2009.04.006
Dong, H., Gao, S., Nong, X. Q., Cong, B., Zhang, Z. H., and Sun, D. Y. (2011). The prevailing of Metarhizium anisopliae in rangeland and its interaction with parasitic flies for biological control of grasshoppers. Chin. J. Biol. Control 2011, 52–56.
Duarte, R. T., Goncalves, K. C., Espinosa, D. J., Moreira, L. F., De Bortoli, S. A., Humber, R. A., et al. (2016). Potential of entomopathogenic fungi as biological control agents of Diamondback Moth (Lepidoptera: Plutellidae) and compatibility with chemical insecticides. J. Econ. Entomol. 109, 594–601. doi: 10.1093/jee/tow008
Dubey, N. K., Shukla, R., Kumar, A., Singh, P., and Prakash, B. (2010). Prospects of botanical pesticides in sustainable agriculture. Curr. Sci. 98, 479–480. doi: 10.1126/science.1183962
Dubovskiy, I. M., Whitten, M. M. A., Yaroslavtseva, O. N., Greig, C., Kryukov, V. Y., Grizanova, E. V., et al. (2013). Can insects develop resistance to insect pathogenic fungi? PLoS One 8:e60248. doi: 10.1371/journal.pone.0060248
Erler, F., and Ates, A. O. (2015). Potential of two entomopathogenic fungi, Beauveria bassiana and Metarhizium anisopliae (Coleoptera: scarabaeidae), as biological control agents against the June beetle. J. Insect Sci. 15:44. doi: 10.1093/jisesa/iev029
Farenhorst, M., Knols, B. G. J., Thomas, M. B., Howard, A. F. V., Takken, W., Rowland, M., et al. (2010). Synergy in efficacy of fungal entomopathogens and permethrin against west african insecticide-resistant anopheles gambiae mosquitoes. PLoS One 5:e12081. doi: 10.1371/journal.pone.0012081
Gibson, D. M., Donzelli, B. G., Krasnoff, S. B., and Keyhani, N. O. (2014). Discovering the secondary metabolite potential encoded within entomopathogenic fungi. Nat. Prod. Rep. 31, 1287–1305. doi: 10.1039/C4NP00054D
Gillespie, J. P., Burnett, C., and Charnley, A. K. (2000). The immune response of the desert locust Schistocerca gregaria during mycosis of the entomopathogenic fungus, Metarhizium anisopliae var acridum. J. Insect Physiol. 46, 429–437. doi: 10.1016/S0022-1910(99)00128-6
Gupta, S., Sharma, R. K., Gupta, R. K., Sinha, S. R., Singh, R., and Gajbhiye, V. T. (2009). Persistence of new insecticides and their efficacy against insect pests of okra. Bull. Environ. Contam. Toxicol. 82, 243–247. doi: 10.1007/s00128-008-9581-8
Haroona, W. M., Pagesb, C., Vassalb, J. M., Abdallaa, A. M., Luong-Skovmandb, M., and Lecoq, M. (2011). Laboratory and field investigation of a mixture of Metarhizium acridum and neem seed oil against the tree locust Anacridium melanorhodon melanorhodon (Orthoptera: Acrididae). Biocontrol Sci. Tech. 21, 353–366. doi: 10.1080/09583157.2010.550678
Hernandez, C. E. M., Guerrero, I. E. P., Hernandez, G. A. G., Solis, E. S., and Guzman, J. C. T. (2010). Catalase overexpression reduces the germination time and increases the pathogenicity of the fungus Metarhizium anisopliae. Appl. Microbiol. Biotechnol. 87, 1033–1044. doi: 10.1007/s00253-010-2517-3
Hiromori, H., and Nishigaki, J. (1998). Joint action of an entomopathogenic fungus (Metarhizium anisopliae) with synthetic insecticides against the scarab beetle, Anomala cuprea (Coleoptera: Scarabaeidae) Iarvae. Appl. Entomol. Zool. 33, 77–84. doi: 10.1303/aez.33.77
Hunter, D. M., Milner, R. J., and Spurgin, P. A. (2001). Aerial treatment of the Australian plague locust, Chortoicetes terinifera (Orthoptera: Acrididae) with Metarhizium anisopliae (Deuteromycotina: Hyphomycetes). Bull. Entomol. Res. 91, 93–99.
Jbilou, R., Ennabili, A., and Sayah, F. (2006). Insecticidal activity of four medicinal plant extracts against Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). Afr. J. Biotechnol. 5, 936–940.
Jia, M., Cao, G., Li, Y., Tu, X. B., Wang, G. J., Nong, X. Q., et al. (2016). Biochemical basis of synergism between pathogenic fungus Metarhizium anisopliae and insecticide chlorantraniliprole in Locusta migratoria (Meyen). Sci. Rep. 6:28424. doi: 10.1038/srep28424
Kaaya, G. P., Samish, M., Hedimbi, M., Gindin, G., and Glazer, I. (2011). Control of tick populations by spraying Metarhizium anisopliae conidia on cattle under field conditions. Exp. Appl. Acarol. 55, 273–281. doi: 10.1007/s10493-011-9471-3
Kergoat, G. J., Meseguer, A. S., and Jousselin, E. (2017). Evolution of plant–insect interactions: insights from macroevolutionary approaches in plants and herbivorous insects. Adv. Bot. Res. 81, 25–53. doi: 10.1016/bs.abr.2016.09.005
Kirkland, B. H., Cho, E. M., and Keyhani, N. O. (2004). Differential susceptibility of Amblyomma maculatum and Amblyomma americanum (Acari: ixodidea) to the entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae. Biol. Control 31, 414–421. doi: 10.1016/j.biocontrol.2004.07.007
Ling, E., Cao, G. C., Jia, M., Zhao, X., Wang, L., Tu, X. B., et al. (2016). Different effects of Metarhizium anisopliae strains IMI330189 and IBC200614 on enzymes activities and hemocytes of Locusta migratoria. PLoS One 11:e0155257. doi: 10.1371/journal.pone.0167914
Liu, Z. L., Liu, Q. R., Chu, S. S., and Jiang, G. H. (2010). Insecticidal activity and chemical composition of the essential oils of Artemisia lavandulaefolia and Artemisia sieversiana from China. Chem. Biodivers. 7, 2040–2045. doi: 10.1002/cbdv.200900410
Mannino, M. C., Huarte-Bonnet, C., Davyt-Colo, B., and Nicolás, P. (2019). Is the insect cuticle the only entry gate for fungal infection? Insights into alternative modes of action of entomopathogenic fungi. J. Fungi 5:33. doi: 10.3390/jof5020033
Marican, A., and Durán-Lara, E. F. (2018). A review on pesticide removal through different processes. Environ. Sci. Pollut. Res. 25, 2051–2064. doi: 10.1007/s11356-017-0796-2
MeCoy, C. W. (1981). “Pest control by the fungus Hirsutell thompsonii//Burges” in Microbial control of pests and plant disease 1970–1980. eds. H. D. Burges and N. W. Hussey (London: Academic Press), 499–512.
Paula, A. R., Ribeiro, A., Lemos, F. J. A., Silva, C. P., and Samuels, R. I. (2019). Neem oil increases the persistence of the entomopathogenic fungus Metarhizium anisopliae for the control of Aedes aegypti (Diptera: Culicidae) larvae. Parasit. Vectors 12:163. doi: 10.1186/s13071-019-3415-x
Pavela, R. (2016). History, presence and perspective of using plant extracts as commercial botanical insecticides and farm products for protection against insects–a review. Plant Prot. Sci. 52, 229–241. doi: 10.17221/31/2016-pps
Pelizza, S. A., Scorsetti, A. C., Fogel, M. N., Pacheco-Marino, S. G., Stenglein, S. A., Cabello, M. N., et al. (2015). Compatibility between entomopathogenic fungi and biorational insecticides in toxicity against Ronderosia bergi under laboratory conditions. BioControl 60, 81–91. doi: 10.1007/s10526-014-9606-7
Peng, G., Wang, Z., Yin, Y., Zeng, D. Y., and Xia, Y. X. (2008). Field trials of Metarhizium anisopliae var. acridum (Ascomycota: Hypocreales) against oriental migratory locusts, Locusta migratoria manilensis (Meyen) in Northern China. Crop Prot. 27, 1244–1250. doi: 10.1016/j.cropro.2008.03.007
Pilz, C., Keller, S., Kuhlmann, U., and Toepfer, S. (2009). Comparative efficacy assessment of fungi, nematodes and insecticides to control western corn rootworm larvae in maize. BioControl 54, 671–684. doi: 10.1007/s10526-009-9209-x
Rattan, R. S. (2010). Mechanism of action of insecticidal secondary metabolites of plant origin. Crop Prot. 29, 913–920. doi: 10.1016/j.cropro.2010.05.008
Sabarwal, A., Kumar, K., and Singh, R. P. (2018). Hazardous effects of chemical pesticides on human health-Cancer and other associated disorders. Environ. Toxicol. Pharmacol. 63, 103–114. doi: 10.1016/j.etap.2018.08.018
Sabbour, M. M., and Sahab, A. F. (2005). Efficacy of some microbial control agents against cabbage pests in Egypt. Pak. J. Biol. Sci. 8, 1351–1356. doi: 10.3923/pjbs.2005.1351.1356
Santos, A., Oliveira, B. L., and Samuels, R. I. (2007). Selection of entomopathogenic fungi for use in combination with sub-lethal doses of imidacloprid: perspectives for the control of the leaf-cutting ant Atta sexdens rubropilosa Forel (Hymenoptera: Formicidae). Mycopathology 163, 233–240. doi: 10.1007/s11046-007-9009-8
Serebrov, V. V., Gerber, O. N., Malyarchuk, A. A., Martemyanova, V. V., Alekseevc, A. A., and Glupova, V. V. (2006). Effect of Entomopathogenic fungi on detoxification enzyme activity in greater wax moth galleria mellonella L. (Lepidoptera, Pyralidae) and role of detoxification enzymes in development of insect resistance to Entomopathogenic Fungi. Biol. Bull. 33, 581–586. doi: 10.1134/S1062359006060082
Shah, F. A., Ansari, M. A., Prasad, M., and Butta, T. M. (2007). Evaluation of black vine weevil (Otiorhynchus sulcatus) control strategies using Metarhizium anisopliae with sublethal doses of insecticides in disparate horticultural growing media. Biol. Control 40, 246–252. doi: 10.1016/j.biocontrol.2006.10.005
Shang, F. J., Du, X. Q., and Liu, G. J. (1997). Resources and utilization of medicinal plants of Eupatorium in Northeast China. For. By-Prod. Spec. China 3:42.
Shrestha, G., Enkegaard, A., and Steenberg, T. (2015). Laboratory and semi-field evaluation of Beauveria bassiana (Ascomycota: Hypocreales) against the lettuce aphid, Nasonovia ribisnigri (Hemiptera: aphididae). Biol. Control 85, 37–45. doi: 10.1016/j.biocontrol.2015.03.005
Simmonds, M. S. J. (2001). Importance of flavonoids in insect-plant interactions: feeding and oviposition. Phytochemistry 56, 245–252. doi: 10.1016/S0031-9422(00)00453-2
Tan, R. X., Zheng, W. F., and Tang, H. Q. (1998). Biologically active substances from the genus Artemisia. Planta Med. 64, 295–302. doi: 10.1055/s-2006-957438
Tang, J., Zhao, J. J., and Li, Z. H. (2015). Ethanol extract of Artemisia sieversiana exhibits anticancer effects and induces apoptosis through a mitochondrial pathway involving DNA damage in COLO-205 colon carcinoma cells. Bangladesh J. Pharmacol. 10:518. doi: 10.3329/bjp.v10i3.23196
Vander, K. F., Verpoorte, R., and Meyer, J. J. M. (2008). Metabolomic quality control of claimed anti-malarial Artemisia afra herbal remedy and A. afra and A. annua plant extracts. S. Afr. J. Bot. 74, 186–189. doi: 10.1016/j.sajb.2007.10.004
Wang, Y., Yang, P., Cui, F., and Kang, L. (2013). Altered immunity in crowded locust reduced fungal (Metarhizium anisopliae) pathogenesis. PLoS Pathog. 9:e1003102. doi: 10.1371/journal.ppat.1003102
Wink, M. (1988). Plant breeding: importance of plant secondary metabolites for protection against pathogens and herbivores. Theor. Appl. Genet. 75, 225–233. doi: 10.1007/BF00303957
Wu, H. Q. (2015). Research progress on insecticidal effect of Artemisia in Compositae. Bulletin Agric. Sci. Technol. 5, 221–223.
Zhang, W. Z., He, B., Cao, G. C., Zhang, Z. H., Wu, Y. H., Liu, S. C., et al. (2013). Quantitative analysis of the effects of Stipa krylovii and Leymus chinensis on the factors of vitiality of Oedaleus decorus asiaticus. Acta Pratacul. Sin. 22, 302–309. doi: 10.13268/j.cnki.fbsic.1997.03.037
Keywords: Artemisia, Metarhizium anisopliae, Oedaleus asiaticus, synergetic control, biopesticide
Citation: Li S, Xu CM, Du GL, Wang GJ, Tu XB and Zhang ZH (2021) Synergy in Efficacy of Artemisia sieversiana Crude Extract and Metarhizium anisopliae on Resistant Oedaleus asiaticus. Front. Physiol. 12:642893. doi: 10.3389/fphys.2021.642893
Received: 17 December 2020; Accepted: 26 February 2021;
Published: 22 March 2021.
Edited by:
Patricia Golo, Federal Rural University of Rio de Janeiro, BrazilReviewed by:
Xunbing Huang, Linyi University, ChinaCopyright © 2021 Li, Xu, Du, Wang, Tu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiongbing Tu, eGJ0dUBpcHBjYWFzLmNu; dHhiMTIwOEAxNjMuY29t; Zehua Zhang, emhhbmd6ZWh1YUBjYWFzLmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.