- 1Department of Nephrology, The Second Xiangya Hospital, Central South University, Changsha, China
- 2Hunan Key Laboratory of Kidney Disease and Blood Purification, Changsha, China
- 3Department of Cardiovascular Surgery, The Second Xiangya Hospital of Central South University, Changsha, China
The incidence of chronic kidney disease (CKD) has rapidly increased in the past decades. A progressive loss of kidney function characterizes a part of CKD even with intensive supportive treatment. Irrespective of its etiology, CKD progression is generally accompanied with the development of chronic kidney inflammation that is pathologically featured by the low-grade but chronic activation of recruited immune cells. Cumulative evidence support that aberrant DNA methylation pattern of diverse peripheral immune cells, including T cells and monocytes, is closely associated with CKD development in many chronic disease settings. The change of DNA methylation profile can sustain for a long time and affect the future genes expression in the circulating immune cells even after they migrate from the circulation into the involved kidney. It is of clinical interest to reveal the underlying mechanism of how altered DNA methylation regulates the intensity and the time length of the inflammatory response in the recruited effector cells. We and others recently demonstrated that altered DNA methylation occurs in peripheral immune cells and profoundly contributes to CKD development in systemic chronic diseases, such as diabetes and hypertension. This review will summarize the current findings about the influence of aberrant DNA methylation on circulating immune cells and how it potentially determines the outcome of CKD.
Introduction
Over the past decades, the incidence of chronic kidney disease (CKD) has rapidly increased worldwide (GBD Chronic Kidney Disease Collaboration, 2020), likely due to the huge changes in human living habits and the environment. A subset of CKD is characterized by a gradual loss of kidney function over time even with intensive supportive treatment and thereby irreversibly progresses to end-stage renal disease (ESRD). Epidemiological studies have revealed that all stages of CKD are correlated with greater risks of cardiovascular morbidity, premature death rates, declined quality of life, and tremendous economic burden (Cockwell and Fisher, 2020). In 2017, the number of deaths caused by CKD reached 1.2 million, known as the 12th leading causes of global death (DALYs GBD and Collaborators H, 2018). Undoubtedly, CKD is one of the biggest threats to global health as well as one of the top challenges to limited medical resources in most countries. Because multiple factors contribute to the disease progression, current therapeutic strategies to manage CKD mostly rely on the control of the detectable abnormalities, like proteinuria, hyperglycemia, hypertension, and so on. However, a proportion of CKD still progresses to ESRD even when these mentioned disadvantages are fully under control. For example, compelling evidence from multiple large-scale clinical trials remains insufficient to definitively conclude a relative risk reduction by intensive glycemic control for long-term diabetic kidney disease (DKD) exposures, which are generally accompanied by chronic hyperglycemia (Hemmingsen et al., 2011). A more in-depth understanding of the underlying molecular mechanisms implicated in the pathogenesis of CKD remains necessary for the development of novel therapeutic strategies.
Chronic kidney inflammation in the process of CKD is featured by the diffusive interstitial infiltration of various immunocytes, including T lymphocytes, B lymphocytes, neutrophils, and monocytes. In general, the function of leukocytes trafficking to the kidney is to eliminate pathogens, remove necrotic cells and tissue debris from the original insult, and finally facilitate kidney tissue repair. The infiltrated leukocytes produce abundant cytokines and growth factors to establish an inflammatory milieu. Meanwhile, they also secrete anti-inflammatory and pro-regenerative cytokines to promote inflammation resolution as well as tissue repair (Peiseler and Kubes, 2019). Usually, transient activation of kidney recruited immune cells is beneficial for tissue repair and functional recovery because they are helpful in removing the pathogenic factors of kidney injury. However, the accumulation of recruited leukocytes in the renal interstitial compartment promotes chronic inflammation and ultimately leads to renal fibrosis (Gieseck et al., 2018). Emerging evidence has identified altered the trafficking of pathogenic immune cells as crucial drivers of tubulointerstitial inflammation and tissue destruction in the progression of CKD (Schnaper, 2017; Tang and Yiu, 2020). Therefore, the recruited leukocytes might facilitate or undermine the kidney repair process under different conditions. An intriguing issue is which underlying mechanism determines the role of recruited immune cells in the kidney.
CKD Is an Inflammatory Disease
Chronic inflammation is generally characterized by persistent production of pro-inflammatory cytokines from both circulating and resident effector cells (Anderton et al., 2020). Emerging evidence has demonstrated that systemic chronic inflammation (SCI) is a major pathological event implicated in the development of most chronic diseases or pathological conditions (e.g., chronic heart disease, diabetes mellitus, and CKD; Furman et al., 2017, 2019; Bennett et al., 2018). Under SCI, the low-grade but persistent activation of effector immune cells consistently compromise the normal tissue at the cellular level by direct contact or paracrine of pro-inflammatory cytokines (Kotas and Medzhitov, 2015). Of note, a gradual loss of renal function per se can initiate SCI in disease progression, which is commonly mixed with some other inflammatory conditions, including diabetes mellitus, hypertension, and obesity. For example, DKD is the leading cause of CKD, which has also been considered as an inflammatory disease (Tuttle, 2005). In the condition of DKD, hyperglycemia-induced oxidative stress pathologically activates circulating immune cells, which infiltrate into the involved kidney and aggravate tissue inflammation by abundant production of pro-inflammatory cytokines and chemokines (Donate-Correa et al., 2020). The accumulation of macrophages in the kidney has been correlated to a decline of renal function in DKD patients (Klessens et al., 2017). Furthermore, these infiltrated cells account for the huge release of cytokines, growth factors, reactive oxygen species (ROS), and metalloproteinases, which initiate and amplify the irreversible process of renal fibrogenesis (Matoba et al., 2019). Another common cause of CKD is hypertension that is likewise featured by progressive SCI (Harrison et al., 2011; Chen et al., 2019b). In the progression of hypertension-associated kidney involvements, predominant accumulation of different immune cells, including antigen-presenting cells and T cells, can be detected at the early stage of kidney inflammatory response (Loperena et al., 2018; Norlander et al., 2018). In the pathogenesis, hypertension-associated influence initially activates dendritic cells (DCs) in the kidney largely by promoting the exuberant formation of isoketals. The activated DCs produce abundant cytokines, including interleukin (IL)-6, IL-1β, and IL-23, to recruit T cells from secondary lymphoid organs to the kidney (Kirabo et al., 2014). Meanwhile, hypertension per se can promote T cell infiltration into the kidney by increasing glomerular perfusion pressure (Evans et al., 2017). As a vicious cycle, infiltrated T cells enhance the production of angiotensin (ANG) II and further aggravate hypertension-associated kidney involvements (De Miguel et al., 2010). Collectively, regardless of its pathogenesis, SCI plays a detrimental role in the progression of CKD by promoting renal infiltration of circulating immune cell and aggravating chronic kidney inflammation. It is of clinical significance to further understand the regulatory mechanism of immune cells recruitment in the context of CKD progression.
Aberrant DNA Methylation Participates in CKD Development
DNA methylation is a common type of epigenetic modification that reversibly affects gene expression without changes in the sequence of nucleotides (Berger et al., 2009; Chen and Riggs, 2011). This process of adding a methyl group to the cytosine is catalyzed by DNA methyltransferases (DNMT), including DNMT1, DNMT3A, and DNMT3B. Generally, DNMT3A and DNMT3B are the major de novo DNA methyltransferases, whereas DNMT1 acts as a maintenance enzyme, restoring hemi-methylated DNA to full methylation after replication (Jones and Liang, 2009; Jones, 2012). In the course of cell division, DNA demethylation occurs in the absence of DNMT1 activation. On the other hand, active DNA demethylation can be induced by the mammalian ten-eleven translocation (TET) family, which catalyzes the stepwise oxidation of 5-methylcytosine in DNA to 5-hydroxymethylcytosine (5hmC; Ambrosi et al., 2017). In somatic cells, functional DNA methylation mostly occurs in clusters of CpG dinucleotides (termed CpG islands), and approximately 60–70% of human gene promoters contain a CpG island (Saxonov et al., 2006; Illingworth et al., 2010). DNA methylation is generally believed to induce transcriptional downregulation, either by impairing the interaction between transcription factors and their targets or by recruiting transcriptional repressors with specific affinity for the methylated DNA. At present, known transcriptional repressors can be classified into three families: the methyl-CpG binding domain (MBD) proteins (Hendrich and Bird, 1998; Defossez and Stancheva, 2011), the UHRF proteins (Hashimoto et al., 2008), and the zinc finger proteins (Hudson and Buck-Koehntop, 2018). In brief, DNA methylation, by altering DNA accessibility to gene promoters, induces transcriptional suppression while demethylation is associated with transcriptional activation.
In recent decades, a surge in epigenome-wide association studies (EWAS) has highlighted that DNA methylation can be markedly influenced by environmental exposures, like CKD and SCI (Ligthart et al., 2016; Heintze, 2018). A Renal Insufficiency Cohort (CRIC) identifies enhanced DNA methylation in genes of IQ motif and Sec7 domain 1 (IQSEC1), nephronophthisis 4 (NPHP4), and transcription factor 3 (TCF3) in participants with stable renal function while compared to those with rapid loss of eGFR (Wing et al., 2014). Meanwhile, differential DNA methylation profiles between the two groups can also be detected in the genes associated with oxidative stress and inflammation. Using whole blood DNA, recent EWAS on a large CKD cohort demonstrated that abnormal DNA methylation of 19 CpG sites is significantly associated with CKD development. Importantly, five of these differential methylated sites are also associated with fibrosis in renal biopsies of CKD patients (Chu et al., 2017). The concordant DNA methylation changes can be further identified in the kidney cortex. In animal studies, targeting DNA methylation, either global or gene-specific, can effectively attenuate renal inflammation and fibrosis in progressive CKD (Tampe et al., 2014, 2015; Yin et al., 2017). For example, low-dose hydralazine induces promoter demethylation in the gene of RAS protein activator like 1 (RASAL1), and subsequently attenuates renal fibrosis in the context of AKI to CKD (Tampe et al., 2017). Although hydralazine is an anti-hypertensive medication, the optimum demethylating activity seems to be independent of its blood pressure-lowering effect. Consistently, altered DNA methylation patterns in the renal outer medulla have been shown to induce differential gene expression regulating metabolism and inflammation in the hypertension animal model (Liu et al., 2018), further supporting that DNA methylation is involved in chronic kidney inflammation and a subsequent loss of kidney function. A number of studies have also highlighted the importance of DNA methylation in the pathogenesis of polycystic kidney disease (PKD; Li, 2020). For instance, downregulation of PKD1 in kidney tissue by hypermethylation may contribute to cyst formation and progression (Woo et al., 2014). Given its relevance to environmental influences, DNA methylation has been intensively explored in DKD. Cumulative evidence suggests that progressive loss of renal function is closely correlated to abnormal DNA methylation in DKD subjects (Swan et al., 2015; Qiu et al., 2018; Gluck et al., 2019; Gu, 2019; Kim and Park, 2019; Park et al., 2019). A recent genome-wide analysis of DNA methylation on 500 DKD subjects reveals that DNA methylation-mediated gene expression likely determines the disease phenotypes, including glycemic control, albuminuria, and kidney function decline. Importantly, further functional annotation analysis indicates that distinct DNA methylation patterns are involved in the pathogenesis of DKD-associated inflammation (Sheng et al., 2020). Collectively, DNA methylation participates in the development of CKD and chronic kidney inflammation in particular.
DNA Methylation in Peripheral Immune Cells
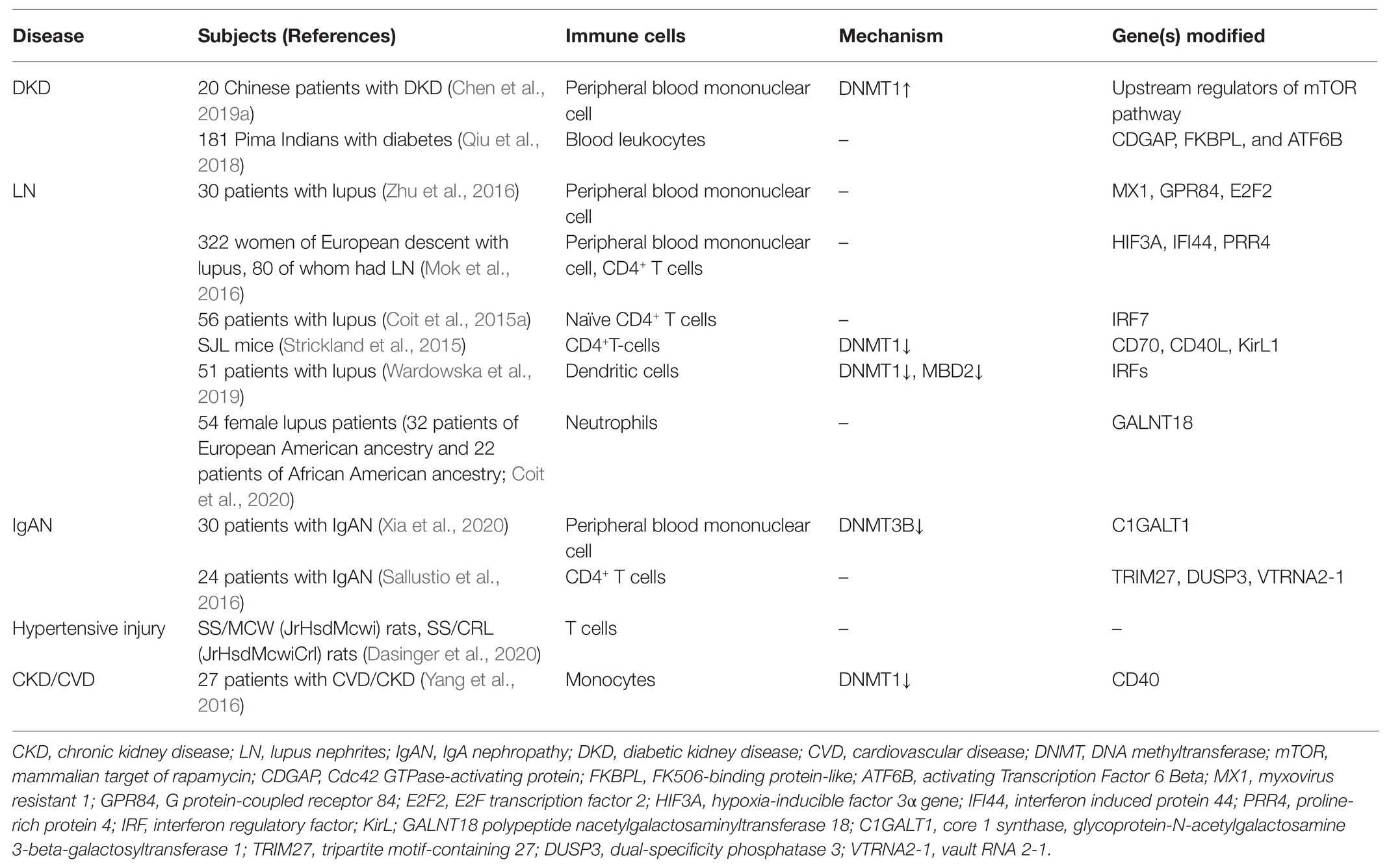
Chronic kidney inflammation occurs in the process of CKD development regardless of its pathogenesis. Pathologically, it is featured in the cumulative infiltration of diverse immune cells from the circulation into the tubulointerstitial compartment. The recruited immune cells are major participants in the progression of chronic kidney inflammation. Upon infiltration, these cells produce abundant chemokines to establish a pro-inflammatory milieu; meanwhile, they also secrete anti-inflammatory cytokines and pro-regenerative growth factors to promote inflammation resolution as well as tissue fibrosis (Gieseck et al., 2018; Tang and Yiu, 2020). It is of clinical interest to understand the underlying mechanism that regulates the intensity and the time length of the inflammatory response in these circulating immune cells. The current status of epigenetic research acknowledges that altered DNA methylation induces permissive or negative expressions of target genes, which result in pathogenic activation of effector immune cells and the consequential loss of inflammatory homeostasis (Stylianou, 2019). Compelling evidence has revealed that circulating immune cells experience dynamic epigenetic changes in their response to the challenge of either acute insult or chronic pathogenic factors (Keating et al., 2016). The epigenetic “memory” of the previous stimuli can sustain for a long time and affect the future gene expression profile even after their migration from the circulation into the involved kidney. Recent emerging findings support the fact that an aberrant DNA methylation pattern of diverse peripheral immune cells is closely associated with CKD development in multiple disease settings (summarized in Table 1).
Firstly, we have recently reported that chronic hyperglycemia induces over-expression of DNMT1 and subsequent aberrant DNA methylation of multiple regulator genes of the mechanistic target of rapamycin (mTOR) in peripheral blood mononuclear cells (PBMCs). These effector cells in turn activate and migrate into the involved kidney with the abundant secretion of inflammatory cytokines, resulting in persistent kidney inflammatory injuries and progressive fibrosis (Chen et al., 2019a). By adoptive transfer, we confirm that circulating PBMCs with “inflammatory memory” can aggravate DKD progression in the recipient animals. Of clinical importance, we demonstrate that the inhibition of DNA methylation by targeting DNMT1 promotes the regulatory phenotype of circulating immune cells and improves the diabetic inflammatory state and the long-term outcome of DKD. Aberrant DNA methylation is also observed in PBMCs from lupus nephritis (LN) patients. Hypomethylated CpG sites can be detected in the promoter region of interferon (IFN)- and toll-like receptor (TLR)-related genes, which are highly associated with the pathogenic inflammatory condition of LN progression (Mok et al., 2016; Zhu et al., 2016). These findings highly support the fact that the differential methylation of genes regulating the inflammatory activity of PBMCs has a causal role in the pathogenesis of LN. In addition, we have observed that mRNA expression of DNMT3B is notably increased in PBMCs isolated from immunoglobulin A nephropathy (IgAN) patients (Xia et al., 2020).
Based on these findings, we propose that SCI occurs and progresses in the condition of CKD derived from multiple primary and secondary diseases, such as hyperglycemia, hypertension, autoimmune disorder, and chronic infection. These chronic stimuli substantially alter the DNA methylation profile of circulating immune cells, leading to enhanced activities of pro-inflammatory genes and a cell-type switch toward inflammatory effectors. The altered DNA methylation might act as “epigenetic memory” and sustain in circulating immune cells for a long time. It thereby pathologically and persistently activates the inflammatory response of immune cells, which continue to participate in chronic tissue injury after their kidney recruitments. It might partly explain why a subset of CKD is characterized by ongoing kidney inflammation and irreversibly progresses to ESRD even when treatment targets have been achieved, like glycemic recovery and blood pressure control (Figure 1). Of note, leukocytes are composed of a variety of circulating immune cells and DNA methylation affects genes transcription activity by a cell type-specific manner. Although emerging evidence has revealed abnormal DNA methylation in both B cells (Absher et al., 2013; Fali et al., 2014; Scharer et al., 2019; Breitbach et al., 2020; Wardowska, 2020) and neutrophils (Lande et al., 2011; Coit et al., 2015b, 2020) in the condition of SLE, there is a lack of data derived from studies with kidney involvements by far. Therefore, we next discuss the potential role of DNA methylation in CKD development with a focus on T cell and monocyte lineages.
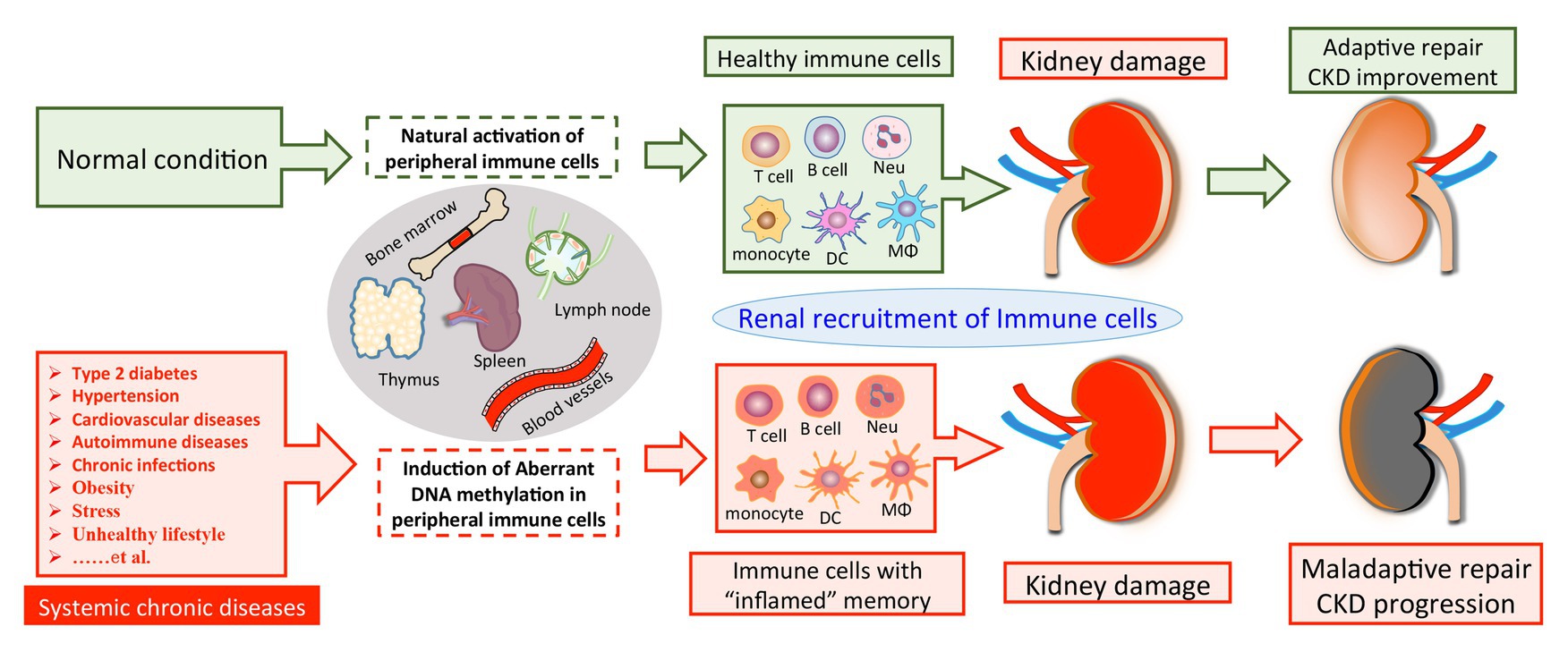
Figure 1. A model of DNA methylation in peripheral immune cells in the pathogenesis of CKD development. Chronic pathogenic conditions induce aberrant DNA methylation in peripheral immune cells, leading to enhanced activities of pro-inflammatory genes and a cell-type switch toward “inflamed” effectors. Normally, renal recruitment of circulating immune cells can facilitate adaptive repair and improve the outcome of kidney damage. On the other hand, peripheral immune cells with “inflamed” DNA methylation profile may constantly migrate into the diseased kidney and overact tissue inflammation, which consequentially results in maladaptive repair and CKD progression. CKD, chronic kidney disease; Neu, neutrophil; DC, dendritic cell; Mϕ, macrophage.
DNA Methylation in T Cell Lineages
Upon antigen stimulation, naïve T cells differentiate into several lineages, including T helper (Th)1, Th2, Th17, and regulatory T (Treg) cells. Th1 cells control intracellular bacterial infection, while Th2 cells initiate antibody response against the extracellular pathogens. During the polarization of CD4+ T cells toward Th1, DNA hypomethylation occurs in Th1 cytokine genes (such as interferon gamma, IFNγ) whereas Th2 cytokine genes achieve DNA methylation, and vice versa in the polarization of Th2 cells. Evidence showed that the imbalance of Th1/Th2 cytokine profiles play a crucial role in the pathogenesis of IgAN (Suzuki and Suzuki, 2018). In the early stage of IgAN studied in ddY mice, strong polarization toward Th1 can be observed (Suzuki et al., 2007). A genome-wide screening for DNA methylation shows that the ratio of IL-2 to IL-5 is significantly elevated, indicating a Th1 shift of CD4+ T cells in IgAN (Sallustio et al., 2016). In brief, this Th1/Th2 polarization is associated with three specific aberrantly methylated DNA regions in peripheral CD4+T cells from IgAN patients. Low methylation levels are observed in genes involved in T cell receptor (TCR) signaling, including tripartite motif-containing 27 (TRIM27) and dual-specificity phosphatase 3 (DUSP3). Meanwhile, a hypermethylated region can be detected in the miR-886 precursor and is associated with decreased CD4+ T cell proliferation following TCR stimulation. Therefore, aberrant DNA methylation causes reduced TCR signal strength and the low activation of CD4+ T cells in the pathogenesis of IgAN.
Th17 cells are characterized by the signature production of cytokines such as IL-17A and IL-17F and the expression of the key transcription factor retinoic orphan receptor γt (RORγt; Cua et al., 2003). Due to their pro-inflammatory phenotype, Th17 cells are capable of protecting against infections on mucosal surfaces (Park et al., 2005) but contribute to the development of renal inflammatory diseases (Turner et al., 2010). On the other hand, Treg cells are characterized by the expression of forkhead box P3 (Foxp3) and the production of anti-inflammatory cytokines (e.g., IL-10 and transforming growth factor-β; Lu et al., 2017) and usually have a pivotal role in dampening chronic kidney inflammation (Chen et al., 2016; Sharma and Kinsey, 2018). Changes in epigenetic status at the Foxp3 and IL17 gene loci are essential for the polarization of CD4+ T cells toward the Treg or Th17 cells (Yang et al., 2015; Lu et al., 2016). Peripheral CD4+ T cells from SLE patients were presented with decreased expression of regulatory factor X 1 (RFX1), which causes DNA demethylation in the IL17A locus of CD4+ T cells and thereby promotes Th17 cell differentiation (Zhao et al., 2018). On the other hand, abnormal epigenetic regulation of Foxp3 in Treg cells has been documented in SLE patients, which suggests that hypermethylation of the Foxp3+ promoter region is associated with a decreased proportion of Treg cells and increased disease activity (Zhao et al., 2012). Of clinical significance, DNA methylation levels of the Foxp3 promoter region can be markedly suppressed by effective treatment, which consequently downregulates Foxp3 expression and promotes CD4+CD25+ Treg cells.
In addition, recent EWAS has revealed that unique DNA methylation patterns in CD4+ T cells are closely related to disease activity. In SLE, the DNA methylation state in peripheral naïve CD4+ T cells is significantly different between patients with and without renal involvement (Coit et al., 2015a). Increased DNA methylation in multiple IFN-regulated genes is closely associated with the onset of LN. Moreover, a lupus susceptibility gene, the type-I interferon master regulator gene (IRF7), is specifically demethylated as shown in patients with LN. Consistently, the modification of DNA methylation, by targeting DNMT1 expression in CD4+ T cells, contributes to the development of LN-like glomerulonephritis in animals (Strickland et al., 2015).
As described above, abnormal epigenetics is implicated in the pathogenesis of hypertensive renal injury due to its influence on immune homeostasis. It is known that high salt intake is the major cause of hypertension and intriguingly associated with obesity, independent of energy intake (Ma et al., 2015). An intriguing question is whether and how environmental influences, like unhealthy diet, might induce aberrant epigenetic changes in immune cells that subsequently participate in hypertension-associated kidney inflammatory involvement. The Dahl salt-sensitive (SS) rat is a genetic model of hypertension and renal disease that is accompanied with immune cell activation in response to a high-salt diet (Mattson et al., 2006). In SS rats, a high-salt diet induced increasing global methylation rate in circulating and kidney T cells, of which differentially methylated regions (DMRs) are more prominent in animals with a pronounced hypertensive phenotype. Importantly, the application of decitabine, a hypomethylating agent, significantly attenuates hypertension and renal inflammatory injury in SS rats (Dasinger et al., 2020). In-depth RNA-seq analysis on kidney T cells has revealed the upregulation of multiple inflammatory and oxidative genes in response to a high-salt diet, which are inversely correlated with DNA methylation levels. These genes are known to play an important role in the development of salt sensitivity in the SS rat (Zheleznova et al., 2016). Collectively, these findings thereby highlight the important role of DNA methylation in linking the influence of abnormal environment/diet to the clinical manifestations of hypertension-associated involvements, which might be at least partly mediated by pathologically activated T cells.
DNA Methylation in Monocyte Lineages
Monocytes, representing the mononuclear phagocyte system, are the largest type of circulating immune cells and can differentiate into macrophages (Mϕ) and myeloid lineage dendritic cells (DCs). Multiple lines of evidence have confirmed the fundamental roles of monocyte lineage in the inflammatory progression of CKD (Heine et al., 2012; Kinsey, 2014; Bowe et al., 2017). Generally, Mϕ can be divided into two subsets, classically activated Mϕ (M1) and alternatively activated Mϕ (M2), depending on their activation paradigm and cellular functions. The classic M1 macrophages commonly produce pro-inflammatory cytokines and cytotoxic mediators contributing to acute and chronic tissue inflammation. On the other hand, M2 macrophages are mostly implicated in inflammation resolution, tissue remodeling, and fibrogenesis by secreting various anti-inflammatory cytokines, growth factors, and proangiogenic cytokines (Wang et al., 2014; Tian and Chen, 2015). In the context of DKD, Mϕ constitutes a major part of infiltrated leucocytes and their accumulation is associated with the progression of diabetic status and renal pathological changes (Chow et al., 2004; Tesch, 2010). Importantly, M1/M2 ratio is positively associated with the progression of chronic inflammation into pathogenic fibrosis during CKD development (Tang et al., 2019; Zhang et al., 2019). Recent studies have revealed an essential role of epigenetic regulation in the phenotype switch of M1 and M2. For example, DNMT3B plays an important role in regulating macrophage polarization and is expressed relatively less in M2 compared to M1 (Yang et al., 2014). Deletion/inhibition of DNMT1, either pharmacologically or genetically, contributes to M2 alternative activation in obesity (Wang et al., 2016), which is known as a typical type of SCI. Under the pathological conditions of hyperlipidemia and type 2 diabetes mellitus, DNA methylation alterations steer the Mϕ phenotype toward pro-inflammatory M1 as opposed to the tissue repairing M2 phenotype by differentially methylating gene promoters of M1 and M2 (Babu et al., 2015).
Besides differentiation into Mϕ, monocytes can be classified into various subsets with diverse inflammatory phenotypes based on their cell surface markers expression (Zawada et al., 2012), which similarly can be interfered with in the stage of CKD. Accumulation of uremic toxins during CKD progression induces aberrant DNA methylation that affects some transcription regulators that are important for monocyte differentiation (Zawada et al., 2016). Similar to other chronic diseases, CKD can promote a pro-inflammatory phenotype of monocytes via the DNA hypomethylation of CD40, which activates and contributes to inflammatory involvements and disease progression (Yang et al., 2016). DCs can be generally divided into two groups, myeloid dendritic cells (mDCs) and plasmacytoid dendritic cells (pDCs; Kitching and Ooi, 2018). Although the majority of DCs within the kidney are cDCs, active pDCs can migrate and contribute to tissue inflammation in nephritic kidneys (Fiore et al., 2008; Tucci et al., 2008). Myeloid DCs (mDCs, BDCA1+ or BDCA3+ DCs) are also shown to increase in the renal tubulointerstitium of patients with LN (Fiore et al., 2008). DNA methylome of peripheral DCs reveals that global DNA hypermethylation in LN patients is associated with severe kidney involvement (Wardowska et al., 2019). Taken together, current evidence supports the fact that aberrant DNA methylation induces an inflammatory switch of monocyte lineage, which contributes to the development of chronic kidney inflammation in multiple chronic disease settings, like obesity, hypertension, diabetes, lupus, and CKD.
Summary and Perspectives
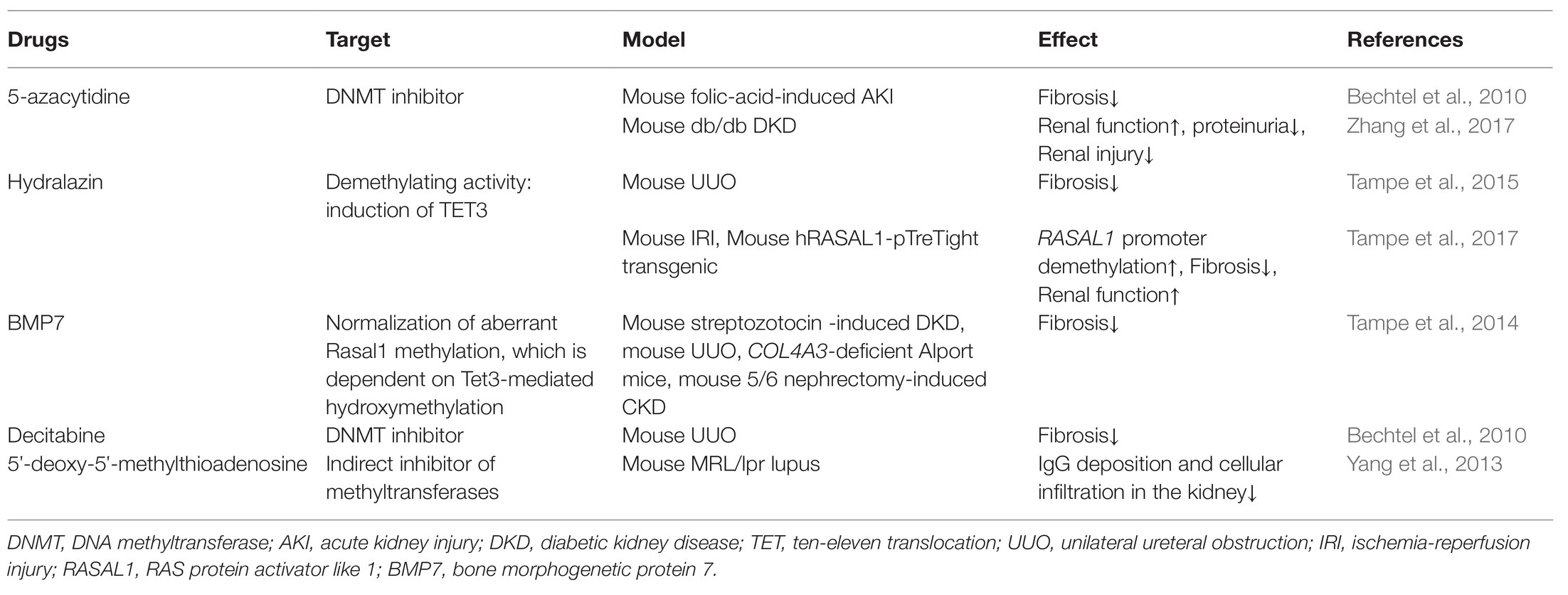
In summary, a variety of pathological conditions induce an aberrant DNA methylation profile in circulating immune cells with a cell-type specific manner, leading to a phenotype switch toward the inflammatory side (Figure 2). These “inflamed” immune cells sustain enhanced inflammatory activity upon the recruitment into diseased kidneys and consequentially participate in chronic kidney inflammation and CKD progression. DNA methylation-targeted treatment by either inhibiting methylation (e.g., 5-azacytidine) or activating demethylation (e.g., hydralazine) have been explored to ameliorate kidney injury in several preclinical studies (Table 2), though some of the interventions have nephrotoxic potential in the clinical setting. A series of novel therapeutic methods, such as modified oligonucleotide inhibitors and small RNA molecules targeting DNMTs, have yet to be tested in the setting of kidney disease (Xu et al., 2016). Meanwhile, there is a lack of intervention strategies specifically targeting immune cells. Given its complex roles in cell biology, clinicians should comprehensively assess the therapeutic value, as well as the potential risk of targeting DNA methylation in immune cells. An in-depth understanding of DNMTs functions in different scenarios might help to develop effective strategies to restore immune homeostasis with consideration of the timing, the signaling intensity, and the disease settings. In future mechanistic research, it remains necessary to clarify the causal relationship between DNA methylation and CKD development, since it is technically hard to separate “driver” events from “passenger” events in the setting of SCI. A combined application of current cutting-edge technologies, like single-cell epigenomic methods of ATAC-seq (Mezger et al., 2018) and single-cell RNA-seq (Kolodziejczyk et al., 2015), may be able to provide a solution to this problem.
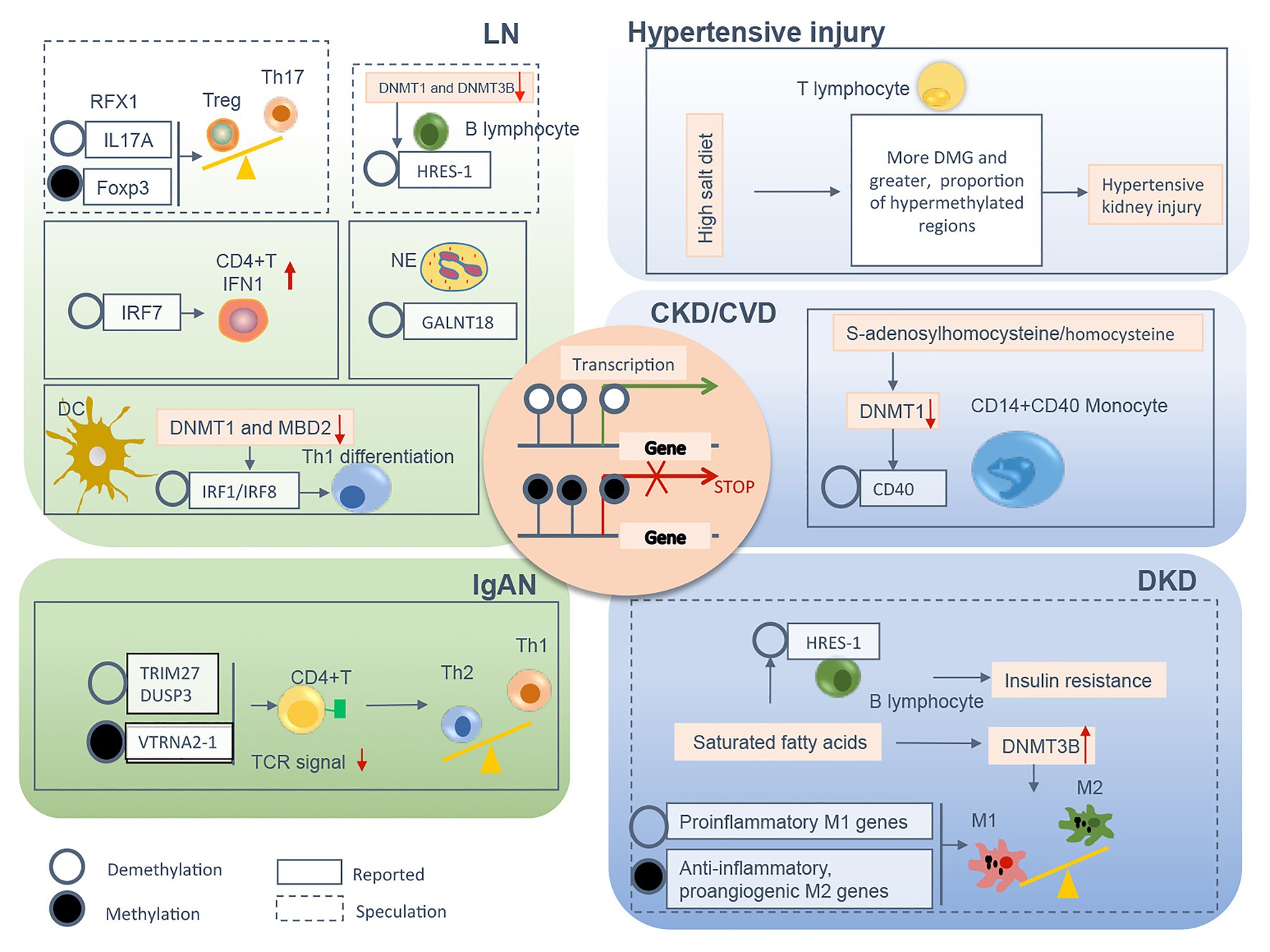
Figure 2. The relevant DNA methylation profiles in immune cells from CKD patients are summarized by different chronic pathogenic conditions, including LN, IgAN, hypertensive kidney injury, DKD, and uremia. Demethylation or methylation of certain genes regulates immune cell phenotype shift/differentiation, or pro/anti-inflammation signal, therefore contributes to uncontrolled kidney inflammation and CKD progression. The mechanism boxed off with solid lines is documented in CKD with different etiology, whereas the one with dashed lines is speculated to relate to the development of kidney diseases founded on circumstantial evidence. CKD, chronic kidney disease; LN, lupus nephrites; IgAN, IgA nephropathy; DKD, diabetic kidney disease; CVD, cardiovascular disease; Treg, regulatory T; Th, T helper; NE, neutrophil; DC, dendritic cell; TCR, T cell receptor; M1, classically activated macrophage; M2, alternatively activated macrophage; IFN, interferon; Foxp3, forkhead box P3; DNMT, DNA methyltransferase; MBD, methyl-CpG binding domain; RFX, regulatory factor X; HRES-1, human T cell lymphotropic virus-related endogenous sequence-1; IRF, interferon regulatory factor; GALNT18, polypeptide nacetylgalactosaminyltransferase 18; TRIM27, tripartite motif-containing 27; DUSP3, dual-specificity phosphatase 3; VTRNA2-1, vault RNA 2-1.
Author Contributions
GC conceived the review. X-JC and HZ collected literature data, interpreted literature, and wrote the manuscript. FY and YL created and revised the figures and tables. GC oversaw the work and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from the National Natural Science Foundation of China to Dr. Guochun Chen (81770691, 81300566).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Absher, D. M., Li, X., Waite, L. L., Gibson, A., Roberts, K., Edberg, J., et al. (2013). Genome-wide DNA methylation analysis of systemic lupus erythematosus reveals persistent hypomethylation of interferon genes and compositional changes to CD4+ T-cell populations. PLoS Genet. 9:e1003678. doi: 10.1371/journal.pgen.1003678
Ambrosi, C., Manzo, M., and Baubec, T. (2017). Dynamics and context-dependent roles of DNA methylation. J. Mol. Biol. 429, 1459–1475. doi: 10.1016/j.jmb.2017.02.008
Anderton, H., Wicks, I. P., and Silke, J. (2020). Cell death in chronic inflammation: breaking the cycle to treat rheumatic disease. Nat. Rev. Rheumatol. 16, 496–513. doi: 10.1038/s41584-020-0455-8
Babu, M., Durga Devi, T., Makinen, P., Kaikkonen, M., Lesch, H. P., Junttila, S., et al. (2015). Differential promoter methylation of macrophage genes is associated with impaired vascular growth in ischemic muscles of hyperlipidemic and Type 2 diabetic mice: genome-wide promoter methylation study. Circ. Res. 117, 289–299. doi: 10.1161/CIRCRESAHA.115.306424
Bechtel, W., McGoohan, S., Zeisberg, E. M., Muller, G. A., Kalbacher, H., Salant, D. J., et al. (2010). Methylation determines fibroblast activation and fibrogenesis in the kidney. Nat. Med. 16, 544–550. doi: 10.1038/nm.2135
Bennett, J. M., Reeves, G., Billman, G. E., and Sturmberg, J. P. (2018). Inflammation-nature’s way to efficiently respond to all types of challenges: implications for understanding and managing “the epidemic” of chronic diseases. Front. Med. 5:316. doi: 10.3389/fmed.2018.00316
Berger, S. L., Kouzarides, T., Shiekhattar, R., and Shilatifard, A. (2009). An operational definition of epigenetics. Genes Dev. 23, 781–783. doi: 10.1101/gad.1787609
Bowe, B., Xie, Y., Xian, H., Li, T., and Al-Aly, Z. (2017). Association between monocyte count and risk of incident CKD and progression to ESRD. Clin. J. Am. Soc. Nephrol. 12, 603–613. doi: 10.2215/CJN.09710916
Breitbach, M. E., Ramaker, R. C., Roberts, K., Kimberly, R. P., and Absher, D. (2020). Population-specific patterns of epigenetic defects in the B cell lineage in patients with systemic lupus erythematosus. Arthritis Rheum. 72, 282–291. doi: 10.1002/art.41083
Chen, J., Bundy, J. D., Hamm, L. L., Hsu, C. Y., Lash, J., Miller, E. R. 3rd., et al. (2019b). Inflammation and apparent treatment-resistant hypertension in patients with chronic kidney disease. Hypertension 73, 785–793. doi: 10.1161/HYPERTENSIONAHA.118.12358
Chen, G., Chen, H., Ren, S., Xia, M., Zhu, J., Liu, Y., et al. (2019a). Aberrant DNA methylation of mTOR pathway genes promotes inflammatory activation of immune cells in diabetic kidney disease. Kidney Int. 96, 409–420. doi: 10.1016/j.kint.2019.02.020
Chen, G., Dong, Z., Liu, H., Liu, Y., Duan, S., Liu, Y., et al. (2016). mTOR signaling regulates protective activity of transferred CD4+Foxp3+ T cells in repair of acute kidney injury. J. Immunol. 197, 3917–3926. doi: 10.4049/jimmunol.1601251
Chen, Z. X., and Riggs, A. D. (2011). DNA methylation and demethylation in mammals. J. Biol. Chem. 286, 18347–18353. doi: 10.1074/jbc.R110.205286
Chow, F., Ozols, E., Nikolic-Paterson, D. J., Atkins, R. C., and Tesch, G. H. (2004). Macrophages in mouse type 2 diabetic nephropathy: correlation with diabetic state and progressive renal injury. Kidney Int. 65, 116–128. doi: 10.1111/j.1523-1755.2004.00367.x
Chu, A. Y., Tin, A., Schlosser, P., Ko, Y. A., Qiu, C., Yao, C., et al. (2017). Epigenome-wide association studies identify DNA methylation associated with kidney function. Nat. Commun. 8:1286. doi: 10.1038/s41467-017-01297-7
Cockwell, P., and Fisher, L. A. (2020). The global burden of chronic kidney disease. Lancet 395, 662–664. doi: 10.1016/S0140-6736(19)32977-0
Coit, P., Ortiz-Fernandez, L., Lewis, E. E., McCune, W. J., Maksimowicz-McKinnon, K., and Sawalha, A. H. (2020). A longitudinal and transancestral analysis of DNA methylation patterns and disease activity in lupus patients. JCI Insight 5:e143654. doi: 10.1172/jci.insight.143654
Coit, P., Renauer, P., Jeffries, M. A., Merrill, J. T., McCune, W. J., Maksimowicz-McKinnon, K., et al. (2015a). Renal involvement in lupus is characterized by unique DNA methylation changes in naive CD4+ T cells. J. Autoimmun. 61, 29–35. doi: 10.1016/j.jaut.2015.05.003
Coit, P., Yalavarthi, S., Ognenovski, M., Zhao, W., Hasni, S., Wren, J. D., et al. (2015b). Epigenome profiling reveals significant DNA demethylation of interferon signature genes in lupus neutrophils. J. Autoimmun. 58, 59–66. doi: 10.1016/j.jaut.2015.01.004
Cua, D. J., Sherlock, J., Chen, Y., Murphy, C. A., Joyce, B., Seymour, B., et al. (2003). Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 421, 744–748. doi: 10.1038/nature01355
DALYs GBD and Collaborators H (2018). Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392, 1859–1922. doi: 10.1016/S0140-6736(18)32335-3
Dasinger, J. H., Alsheikh, A. J., Abais-Battad, J. M., Pan, X., Fehrenbach, D. J., Lund, H., et al. (2020). Epigenetic modifications in T cells: the role of DNA methylation in salt-sensitive hypertension. Hypertension 75, 372–382. doi: 10.1161/HYPERTENSIONAHA.119.13716
De Miguel, C., Das, S., Lund, H., and Mattson, D. L. (2010). T lymphocytes mediate hypertension and kidney damage in Dahl salt-sensitive rats. Am. J. Phys. Regul. Integr. Comp. Phys. 298, R1136–R1142. doi: 10.1152/ajpregu.00298.2009
Defossez, P. A., and Stancheva, I. (2011). Biological functions of methyl-CpG-binding proteins. Prog. Mol. Biol. Transl. Sci. 101, 377–398. doi: 10.1016/B978-0-12-387685-0.00012-3
Donate-Correa, J., Luis-Rodriguez, D., Martin-Nunez, E., Tagua, V. G., Hernandez-Carballo, C., Ferri, C., et al. (2020). Inflammatory targets in diabetic nephropathy. J. Clin. Med. 9:458. doi: 10.3390/jcm9020458
Evans, L. C., Petrova, G., Kurth, T., Yang, C., Bukowy, J. D., Mattson, D. L., et al. (2017). Increased perfusion pressure drives renal T-cell infiltration in the Dahl salt-sensitive rat. Hypertension 70, 543–551. doi: 10.1161/HYPERTENSIONAHA.117.09208
Fali, T., Le Dantec, C., Thabet, Y., Jousse, S., Hanrotel, C., Youinou, P., et al. (2014). DNA methylation modulates HRES1/p28 expression in B cells from patients with lupus. Autoimmunity 47, 265–271. doi: 10.3109/08916934.2013.826207
Fiore, N., Castellano, G., Blasi, A., Capobianco, C., Loverre, A., Montinaro, V., et al. (2008). Immature myeloid and plasmacytoid dendritic cells infiltrate renal tubulointerstitium in patients with lupus nephritis. Mol. Immunol. 45, 259–265. doi: 10.1016/j.molimm.2007.04.029
Furman, D., Campisi, J., Verdin, E., Carrera-Bastos, P., Targ, S., Franceschi, C., et al. (2019). Chronic inflammation in the etiology of disease across the life span. Nat. Med. 25, 1822–1832. doi: 10.1038/s41591-019-0675-0
Furman, D., Chang, J., Lartigue, L., Bolen, C. R., Haddad, F., Gaudilliere, B., et al. (2017). Expression of specific inflammasome gene modules stratifies older individuals into two extreme clinical and immunological states. Nat. Med. 23, 174–184. doi: 10.1038/nm.4267
GBD Chronic Kidney Disease Collaboration (2020). Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 395, 709–733. doi: 10.1016/S0140-6736(20)30045-3
Gieseck, R. L. 3rd., Wilson, M. S., and Wynn, T. A. (2018). Type 2 immunity in tissue repair and fibrosis. Nat. Rev. Immunol. 18, 62–76. doi: 10.1038/nri.2017.90
Gluck, C., Qiu, C., Han, S. Y., Palmer, M., Park, J., Ko, Y. A., et al. (2019). Kidney cytosine methylation changes improve renal function decline estimation in patients with diabetic kidney disease. Nat. Commun. 10:2461. doi: 10.1038/s41467-019-10378-8
Gu, H. F. (2019). Genetic and epigenetic studies in diabetic kidney disease. Front. Genet. 10:507. doi: 10.3389/fgene.2019.00507
Harrison, D. G., Guzik, T. J., Lob, H. E., Madhur, M. S., Marvar, P. J., Thabet, S. R., et al. (2011). Inflammation, immunity, and hypertension. Hypertension 57, 132–140. doi: 10.1161/HYPERTENSIONAHA.110.163576
Hashimoto, H., Horton, J. R., Zhang, X., Bostick, M., Jacobsen, S. E., and Cheng, X. (2008). The SRA domain of UHRF1 flips 5-methylcytosine out of the DNA helix. Nature 455, 826–829. doi: 10.1038/nature07280
Heine, G. H., Ortiz, A., Massy, Z. A., Lindholm, B., Wiecek, A., Martinez-Castelao, A., et al. (2012). Monocyte subpopulations and cardiovascular risk in chronic kidney disease. Nat. Rev. Nephrol. 8, 362–369. doi: 10.1038/nrneph.2012.41
Heintze, J. M. (2018). Epigenetics: EWAS of kidney function. Nat. Rev. Nephrol. 14:3. doi: 10.1038/nrneph.2017.164
Hemmingsen, B., Lund, S. S., Gluud, C., Vaag, A., Almdal, T., Hemmingsen, C., et al. (2011). Intensive glycaemic control for patients with type 2 diabetes: systematic review with meta-analysis and trial sequential analysis of randomised clinical trials. BMJ 343:d6898. doi: 10.1136/bmj.d6898
Hendrich, B., and Bird, A. (1998). Identification and characterization of a family of mammalian methyl-CpG binding proteins. Mol. Cell. Biol. 18, 6538–6547. doi: 10.1128/MCB.18.11.6538
Hudson, N. O., and Buck-Koehntop, B. A. (2018). Zinc finger readers of methylated DNA. Molecules 23:2555. doi: 10.3390/molecules23102555
Illingworth, R. S., Gruenewald-Schneider, U., Webb, S., Kerr, A. R., James, K. D., Turner, D. J., et al. (2010). Orphan CpG islands identify numerous conserved promoters in the mammalian genome. PLoS Genet. 6:e1001134. doi: 10.1371/journal.pgen.1001134
Jones, P. A. (2012). Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat. Rev. Genet. 13, 484–492. doi: 10.1038/nrg3230
Jones, P. A., and Liang, G. (2009). Rethinking how DNA methylation patterns are maintained. Nat. Rev. Genet. 10, 805–811. doi: 10.1038/nrg2651
Keating, S. T., Plutzky, J., and El-Osta, A. (2016). Epigenetic changes in diabetes and cardiovascular risk. Circ. Res. 118, 1706–1722. doi: 10.1161/CIRCRESAHA.116.306819
Kim, Y., and Park, C. W. (2019). Mechanisms of Adiponectin action: implication of Adiponectin receptor agonism in diabetic kidney disease. Int. J. Mol. Sci. 20:1782. doi: 10.3390/ijms20071782
Kinsey, G. R. (2014). Macrophage dynamics in AKI to CKD progression. J. Am. Soc. Nephrol. 25, 209–211. doi: 10.1681/ASN.2013101110
Kirabo, A., Fontana, V., de Faria, A. P., Loperena, R., Galindo, C. L., Wu, J., et al. (2014). DC isoketal-modified proteins activate T cells and promote hypertension. J. Clin. Invest. 124, 4642–4656. doi: 10.1172/JCI74084
Kitching, A. R., and Ooi, J. D. (2018). Renal dendritic cells: the long and winding road. J. Am. Soc. Nephrol. 29, 4–7. doi: 10.1681/ASN.2017101145
Klessens, C. Q. F., Zandbergen, M., Wolterbeek, R., Bruijn, J. A., Rabelink, T. J., Bajema, I. M., et al. (2017). Macrophages in diabetic nephropathy in patients with type 2 diabetes. Nephrol. Dial. Transplant. 32, 1322–1329. doi: 10.1093/ndt/gfw260
Kolodziejczyk, A. A., Kim, J. K., Svensson, V., Marioni, J. C., and Teichmann, S. A. (2015). The technology and biology of single-cell RNA sequencing. Mol. Cell 58, 610–620. doi: 10.1016/j.molcel.2015.04.005
Kotas, M. E., and Medzhitov, R. (2015). Homeostasis, inflammation, and disease susceptibility. Cell 160, 816–827. doi: 10.1016/j.cell.2015.02.010
Lande, R., Ganguly, D., Facchinetti, V., Frasca, L., Conrad, C., Gregorio, J., et al. (2011). Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci. Transl. Med. 3:73ra19. doi: 10.1126/scitranslmed.3001180
Li, X. (2020). Epigenetics and cell cycle regulation in cystogenesis. Cell. Signal. 68:109509. doi: 10.1016/j.cellsig.2019.109509
Ligthart, S., Marzi, C., Aslibekyan, S., Mendelson, M. M., Conneely, K. N., Tanaka, T., et al. (2016). DNA methylation signatures of chronic low-grade inflammation are associated with complex diseases. Genome Biol. 17:255. doi: 10.1186/s13059-016-1119-5
Liu, P., Liu, Y., Liu, H., Pan, X., Li, Y., Usa, K., et al. (2018). Role of DNA De novo (De)methylation in the kidney in salt-induced hypertension. Hypertension 72, 1160–1171. doi: 10.1161/HYPERTENSIONAHA.118.11650
Loperena, R., Van Beusecum, J. P., Itani, H. A., Engel, N., Laroumanie, F., Xiao, L., et al. (2018). Hypertension and increased endothelial mechanical stretch promote monocyte differentiation and activation: roles of STAT3, interleukin 6 and hydrogen peroxide. Cardiovasc. Res. 114, 1547–1563. doi: 10.1093/cvr/cvy112
Lu, L., Barbi, J., and Pan, F. (2017). The regulation of immune tolerance by FOXP3. Nat. Rev. Immunol. 17, 703–717. doi: 10.1038/nri.2017.75
Lu, C. H., Wu, C. J., Chan, C. C., Nguyen, D. T., Lin, K. R., Lin, S. J., et al. (2016). DNA methyltransferase inhibitor promotes human CD4+CD25hFOXP3+ regulatory T lymphocyte induction under suboptimal TCR stimulation. Front. Immunol. 7:488. doi: 10.3389/fimmu.2016.00488
Ma, Y., He, F. J., and MacGregor, G. A. (2015). High salt intake: independent risk factor for obesity? Hypertension 66, 843–849. doi: 10.1161/HYPERTENSIONAHA.115.05948
Matoba, K., Takeda, Y., Nagai, Y., Kawanami, D., Utsunomiya, K., and Nishimura, R. (2019). Unraveling the role of inflammation in the pathogenesis of diabetic kidney disease. Int. J. Mol. Sci. 20:3393. doi: 10.3390/ijms20143393
Mattson, D. L., James, L., Berdan, E. A., and Meister, C. J. (2006). Immune suppression attenuates hypertension and renal disease in the Dahl salt-sensitive rat. Hypertension 48, 149–156. doi: 10.1161/01.HYP.0000228320.23697.29
Mezger, A., Klemm, S., Mann, I., Brower, K., Mir, A., Bostick, M., et al. (2018). High-throughput chromatin accessibility profiling at single-cell resolution. Nat. Commun. 9:3647. doi: 10.1038/s41467-018-05887-x
Mok, A., Solomon, O., Nayak, R. R., Coit, P., Quach, H. L., Nititham, J., et al. (2016). Genome-wide profiling identifies associations between lupus nephritis and differential methylation of genes regulating tissue hypoxia and type 1 interferon responses. Lupus Sci. Med. 3:e000183. doi: 10.1136/lupus-2016-000183
Norlander, A. E., Madhur, M. S., and Harrison, D. G. (2018). The immunology of hypertension. J. Exp. Med. 215, 21–33. doi: 10.1084/jem.20171773
Park, J., Guan, Y., Sheng, X., Gluck, C., Seasock, M. J., Hakimi, A. A., et al. (2019). Functional methylome analysis of human diabetic kidney disease. JCI Insight 4:e128886. doi: 10.1172/jci.insight.128886
Park, H., Li, Z., Yang, X. O., Chang, S. H., Nurieva, R., Wang, Y. H., et al. (2005). A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 6, 1133–1141. doi: 10.1038/ni1261
Peiseler, M., and Kubes, P. (2019). More friend than foe: the emerging role of neutrophils in tissue repair. J. Clin. Invest. 129, 2629–2639. doi: 10.1172/JCI124616
Qiu, C., Hanson, R. L., Fufaa, G., Kobes, S., Gluck, C., Huang, J., et al. (2018). Cytosine methylation predicts renal function decline in American Indians. Kidney Int. 93, 1417–1431. doi: 10.1016/j.kint.2018.01.036
Sallustio, F., Serino, G., Cox, S. N., Dalla Gassa, A., Curci, C., De Palma, G., et al. (2016). Aberrantly methylated DNA regions lead to low activation of CD4+ T-cells in IgA nephropathy. Clin. Sci. 130, 733–746. doi: 10.1042/CS20150711
Saxonov, S., Berg, P., and Brutlag, D. L. (2006). A genome-wide analysis of CpG dinucleotides in the human genome distinguishes two distinct classes of promoters. Proc. Natl. Acad. Sci. U. S. A. 103, 1412–1417. doi: 10.1073/pnas.0510310103
Scharer, C. D., Blalock, E. L., Mi, T., Barwick, B. G., Jenks, S. A., Deguchi, T., et al. (2019). Epigenetic programming underpins B cell dysfunction in human SLE. Nat. Immunol. 20, 1071–1082. doi: 10.1038/s41590-019-0419-9
Schnaper, H. W. (2017). The tubulointerstitial pathophysiology of progressive kidney disease. Adv. Chronic Kidney Dis. 24, 107–116. doi: 10.1053/j.ackd.2016.11.011
Sharma, R., and Kinsey, G. R. (2018). Regulatory T cells in acute and chronic kidney diseases. Am. J. Physiol. Ren. Physiol. 314, F679–F698. doi: 10.1152/ajprenal.00236.2017
Sheng, X., Qiu, C., Liu, H., Gluck, C., Hsu, J. Y., He, J., et al. (2020). Systematic integrated analysis of genetic and epigenetic variation in diabetic kidney disease. Proc. Natl. Acad. Sci. U. S. A. 117, 29013–29024. doi: 10.1073/pnas.2005905117
Strickland, F. M., Li, Y., Johnson, K., Sun, Z., and Richardson, B. C. (2015). CD4+ T cells epigenetically modified by oxidative stress cause lupus-like autoimmunity in mice. J. Autoimmun. 62, 75–80. doi: 10.1016/j.jaut.2015.06.004
Stylianou, E. (2019). Epigenetics of chronic inflammatory diseases. J. Inflamm. Res. 12, 1–14. doi: 10.2147/JIR.S129027
Suzuki, H., and Suzuki, Y. (2018). Murine models of human IgA nephropathy. Semin. Nephrol. 38, 513–520. doi: 10.1016/j.semnephrol.2018.05.021
Suzuki, H., Suzuki, Y., Aizawa, M., Yamanaka, T., Kihara, M., Pang, H., et al. (2007). Th1 polarization in murine IgA nephropathy directed by bone marrow-derived cells. Kidney Int. 72, 319–327. doi: 10.1038/sj.ki.5002300
Swan, E. J., Maxwell, A. P., and McKnight, A. J. (2015). Distinct methylation patterns in genes that affect mitochondrial function are associated with kidney disease in blood-derived DNA from individuals with Type 1 diabetes. Diabet. Med. 32, 1110–1115. doi: 10.1111/dme.12775
Tampe, B., Steinle, U., Tampe, D., Carstens, J. L., Korsten, P., Zeisberg, E. M., et al. (2017). Low-dose hydralazine prevents fibrosis in a murine model of acute kidney injury-to-chronic kidney disease progression. Kidney Int. 91, 157–176. doi: 10.1016/j.kint.2016.07.042
Tampe, B., Tampe, D., Muller, C. A., Sugimoto, H., LeBleu, V., Xu, X., et al. (2014). Tet3-mediated hydroxymethylation of epigenetically silenced genes contributes to bone morphogenic protein 7-induced reversal of kidney fibrosis. J. Am. Soc. Nephrol. 25, 905–912. doi: 10.1681/ASN.2013070723
Tampe, B., Tampe, D., Zeisberg, E. M., Muller, G. A., Bechtel-Walz, W., Koziolek, M., et al. (2015). Induction of Tet3-dependent epigenetic remodeling by low-dose hydralazine attenuates progression of chronic kidney disease. EBioMedicine 2, 19–36. doi: 10.1016/j.ebiom.2014.11.005
Tang, P. M., Nikolic-Paterson, D. J., and Lan, H. Y. (2019). Macrophages: versatile players in renal inflammation and fibrosis. Nat. Rev. Nephrol. 15, 144–158. doi: 10.1038/s41581-019-0110-2
Tang, S. C. W., and Yiu, W. H. (2020). Innate immunity in diabetic kidney disease. Nat. Rev. Nephrol. 16, 206–222. doi: 10.1038/s41581-019-0234-4
Tesch, G. H. (2010). Macrophages and diabetic nephropathy. Semin. Nephrol. 30, 290–301. doi: 10.1016/j.semnephrol.2010.03.007
Tian, S., and Chen, S. Y. (2015). Macrophage polarization in kidney diseases. Macrophage 2:e679. doi: 10.14800/macrophage.679
Tucci, M., Quatraro, C., Lombardi, L., Pellegrino, C., Dammacco, F., and Silvestris, F. (2008). Glomerular accumulation of plasmacytoid dendritic cells in active lupus nephritis: role of interleukin-18. Arthritis Rheum. 58, 251–262. doi: 10.1002/art.23186
Turner, J. E., Paust, H. J., Steinmetz, O. M., and Panzer, U. (2010). The Th17 immune response in renal inflammation. Kidney Int. 77, 1070–1075. doi: 10.1038/ki.2010.102
Tuttle, K. R. (2005). Linking metabolism and immunology: diabetic nephropathy is an inflammatory disease. J. Am. Soc. Nephrol. 16, 1537–1538. doi: 10.1681/ASN.2005040393
Wang, X., Cao, Q., Yu, L., Shi, H., Xue, B., and Shi, H. (2016). Epigenetic regulation of macrophage polarization and inflammation by DNA methylation in obesity. JCI Insight 1:e87748. doi: 10.1172/jci.insight.87748
Wang, N., Liang, H., and Zen, K. (2014). Molecular mechanisms that influence the macrophage m1-m2 polarization balance. Front. Immunol. 5:614. doi: 10.3389/fimmu.2014.00614
Wardowska, A. (2020). The epigenetic face of lupus: focus on antigen-presenting cells. Int. Immunopharmacol. 81:106262. doi: 10.1016/j.intimp.2020.106262
Wardowska, A., Komorniczak, M., Bullo-Piontecka, B., Debska-Slizien, M. A., and Pikula, M. (2019). Transcriptomic and epigenetic alterations in dendritic cells correspond with chronic kidney disease in lupus nephritis. Front. Immunol. 10:2026. doi: 10.3389/fimmu.2019.02026
Wing, M. R., Devaney, J. M., Joffe, M. M., Xie, D., Feldman, H. I., Dominic, E. A., et al. (2014). DNA methylation profile associated with rapid decline in kidney function: findings from the CRIC study. Nephrol. Dial. Transplant. 29, 864–872. doi: 10.1093/ndt/gft537
Woo, Y. M., Bae, J. B., Oh, Y. H., Lee, Y. G., Lee, M. J., Park, E. Y., et al. (2014). Genome-wide methylation profiling of ADPKD identified epigenetically regulated genes associated with renal cyst development. Hum. Genet. 133, 281–297. doi: 10.1007/s00439-013-1378-0
Xia, M., Chen, G., Liu, D., Tang, X., Liu, Y., Wu, L., et al. (2020). Association analysis of DNA methyltransferases in IgA nephropathy. Int. Immunopharmacol. 80:106147. doi: 10.1016/j.intimp.2019.106147
Xu, P., Hu, G., Luo, C., and Liang, Z. (2016). DNA methyltransferase inhibitors: an updated patent review (2012–2015). Expert Opin. Ther. Pat. 26, 1017–1030. doi: 10.1080/13543776.2016.1209488
Yang, J., Fang, P., Yu, D., Zhang, L., Zhang, D., Jiang, X., et al. (2016). Chronic kidney disease induces inflammatory CD40+ monocyte differentiation via homocysteine elevation and DNA hypomethylation. Circ. Res. 119, 1226–1241. doi: 10.1161/CIRCRESAHA.116.308750
Yang, B. H., Floess, S., Hagemann, S., Deyneko, I. V., Groebe, L., Pezoldt, J., et al. (2015). Development of a unique epigenetic signature during in vivo Th17 differentiation. Nucleic Acids Res. 43, 1537–1548. doi: 10.1093/nar/gkv014
Yang, M. L., Gee, A. J., Gee, R. J., Zurita-Lopez, C. I., Khare, S., Clarke, S. G., et al. (2013). Lupus autoimmunity altered by cellular methylation metabolism. Autoimmunity 46, 21–31. doi: 10.3109/08916934.2012.732133
Yang, X., Wang, X., Liu, D., Yu, L., Xue, B., and Shi, H. (2014). Epigenetic regulation of macrophage polarization by DNA methyltransferase 3b. Mol. Endocrinol. 28, 565–574. doi: 10.1210/me.2013-1293
Yin, S., Zhang, Q., Yang, J., Lin, W., Li, Y., Chen, F., et al. (2017). TGFbeta-incurred epigenetic aberrations of miRNA and DNA methyltransferase suppress Klotho and potentiate renal fibrosis. Biochim. Biophys. Acta Mol. Cell Res. 1864, 1207–1216. doi: 10.1016/j.bbamcr.2017.03.002
Zawada, A. M., Rogacev, K. S., Schirmer, S. H., Sester, M., Bohm, M., Fliser, D., et al. (2012). Monocyte heterogeneity in human cardiovascular disease. Immunobiology 217, 1273–1284. doi: 10.1016/j.imbio.2012.07.001
Zawada, A. M., Schneider, J. S., Michel, A. I., Rogacev, K. S., Hummel, B., Krezdorn, N., et al. (2016). DNA methylation profiling reveals differences in the 3 human monocyte subsets and identifies uremia to induce DNA methylation changes during differentiation. Epigenetics 11, 259–272. doi: 10.1080/15592294.2016.1158363
Zhang, X., Yang, Y., and Zhao, Y. (2019). Macrophage phenotype and its relationship with renal function in human diabetic nephropathy. PLoS One 14:e0221991. doi: 10.1371/journal.pone.0221991
Zhang, L., Zhang, Q., Liu, S., Chen, Y., Li, R., Lin, T., et al. (2017). DNA methyltransferase 1 may be a therapy target for attenuating diabetic nephropathy and podocyte injury. Kidney Int. 92, 140–153. doi: 10.1016/j.kint.2017.01.010
Zhao, M., Liang, G. P., Tang, M. N., Luo, S. Y., Zhang, J., Cheng, W. J., et al. (2012). Total glucosides of paeony induces regulatory CD4+ CD25+ T cells by increasing Foxp3 demethylation in lupus CD4+ T cells. Clin. Immunol. 143, 180–187. doi: 10.1016/j.clim.2012.02.002
Zhao, M., Tan, Y., Peng, Q., Huang, C., Guo, Y., Liang, G., et al. (2018). IL-6/STAT3 pathway induced deficiency of RFX1 contributes to Th17-dependent autoimmune diseases via epigenetic regulation. Nat. Commun. 9:583. doi: 10.1038/s41467-018-02890-0
Zheleznova, N. N., Yang, C., and Cowley, A. W. Jr. (2016). Role of Nox4 and p67phox subunit of Nox2 in ROS production in response to increased tubular flow in the mTAL of Dahl salt-sensitive rats. Am. J. Physiol. Ren. Physiol. 311, F450–F458. doi: 10.1152/ajprenal.00187.2016
Zhu, H., Mi, W., Luo, H., Chen, T., Liu, S., Raman, I., et al. (2016). Whole-genome transcription and DNA methylation analysis of peripheral blood mononuclear cells identified aberrant gene regulation pathways in systemic lupus erythematosus. Arthritis Res. Ther. 18:162. doi: 10.1186/s13075-016-1050-x
Keywords: chronic kidney disease, DNA methylation, inflammation, peripheral immune cells, epigenetic memory
Citation: Chen X-J, Zhang H, Yang F, Liu Y and Chen G (2021) DNA Methylation Sustains “Inflamed” Memory of Peripheral Immune Cells Aggravating Kidney Inflammatory Response in Chronic Kidney Disease. Front. Physiol. 12:637480. doi: 10.3389/fphys.2021.637480
Edited by:
Xiao-ming Meng, Anhui Medical University, ChinaReviewed by:
Yanggang Yuan, Nanjing Medical University, ChinaRui Zeng, Huazhong University of Science and Technology, China
Copyright © 2021 Chen, Zhang, Yang, Liu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guochun Chen, Y2hlbmd1b2NodW5AaG90bWFpbC5jb20=; Z3VvY2h1bmNoZW5AY3N1LmVkdS5jbg==
†These authors have contributed equally to this work
 Xiao-Jun Chen
Xiao-Jun Chen Hong Zhang3†
Hong Zhang3† Guochun Chen
Guochun Chen