
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Physiol. , 03 March 2021
Sec. Redox Physiology
Volume 12 - 2021 | https://doi.org/10.3389/fphys.2021.634842
This article is part of the Research Topic Cigarette Smoke, E-Cigarette/E-Vaping and COVID-19: Risks and Implications in This New Era View all 13 articles
Background: Cigarette smoking has been proven to be a risk factor in the development of many diseases. However, it remains controversial with respect to the relationship of smoking with COVID-19. The purpose of this study was to explore the role of smoking in COVID-19.
Methods: A total of 622 patients with COVID-19 in China were enrolled in the study. Corresponding clinical and laboratory data were collected and analyzed. Meanwhile, Kaplan-Meier curve and Cox regression analysis were employed to analyze the association of smoking with survival in patients with COVID-19.
Results: Smoking was statistically significant comparing non-survivors and survivors of patients with COVID-19 (P = 0.007). Males had higher proportion of smoking than females (91.9% vs. 8.1%, P < 0.001). Compared with the non-smoker, there was significant statistical difference in the incidence of cerebrovascular disease in smoking patients with COVID-19 (9.7% vs. 3.4%, P = 0.017). White blood cell count (6.3 vs. 5.4; P = 0.037), hemoglobin level (139.0 vs. 127.0; P < 0.001), and creatinine level (77.3 vs. 61.0; P < 0.001) were significantly increased in COVID-19 patients who smoked. Moreover, smoking patients showed a worse survival compared with non-smoking patients (Log Rank P = 0.045). After adjustment for age, gender and underlying diseases, patients with smoking still had higher risk of mortality than that of non-smoking patients (hazard ratio[HR] 1.897, 95% confidence interval [CI]1.058–3.402, P = 0.032).
Conclusion: Smoking was thought to be a risk factor in predicting the prognosis of COVID-19 and smoking patients might have a higher risk of mortality than that of the non-smoking patients.
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) was firstly reported in Wuhan, China in late 2019 and has already become an evolution of pandemic (Cucinotta and Vanelli, 2020). The World Health Organization has declared it as a “Public Health Emergency of International Concern.” SARS-CoV-2 belongs to the same family of RNA virus as SARS and Middle East respiratory syndrome (Zhou et al., 2020) and has a higher risk of human-to-human transmission (Chan et al., 2020). Until now, more than sixty-one million cases of COVID-19 as well as 1440000 deaths have been identified across the world (World Health Organization, 2020).
Smoking history is defined as a history of continuous or cumulative smoking at least 6 months during the whole life (World Health Organisation, 1997), and cigarette smoking is quite prevalent all over the world. It kills approximately 50% of users and 8 million people are died from it every year, 1.2 million of which are exposed to the second-hand smoking (Lippi et al., 2020). The mechanisms of smoking in inducing the occurrence of respiratory diseases are altering airway architecture, enhancing mucosal permeability, disrupting respiratory epithelium and inhibiting ciliary clearance (Arcavi and Benowitz, 2004). It was reported that smoking played an important role in chronic obstructive pulmonary disease (COPD) in developed countries which was the fourth leading cause of death (Agarwal et al., 2020), and smokers were also more likely to have increased incidence of cancer, influenza, tuberculosis and pneumonia relative to non-smokers (Warren et al., 2014; Brake et al., 2020). However, the relationship of smoking and COVID-19 remains controversial. The purpose of this study was to explore the role of smoking in COVID-19.
This case series was subjected to the approval by the institutional ethics board of the Second Xiangya Hospital of Central South University (No. 2020001). The objects of study were laboratory-confirmed adult COVID-19 patients using real-time polymerase chain reaction who were admitted to the Public Health Treatment Center of Changsha and Tongji Medical College of Huazhong University of Science and Technology, China, by March 26th 2020. The patients older than 18 years were included in the study and were divided into two groups according to the survival and smoking statuses, including survivors and non-survivors, as well as the smokers and non-smokers.
Two members of our team carefully collected and reviewed the medical records of enrolled patients individually. The detailed information of those patients were recorded, including the demographic data, underlying diseases, symptoms throughout the course of the disease, blood test parameters, and results of chest computed tomography (CT) scans. The date of disease onset was defined as the day when the symptoms were noticed.
According to the criteria of severe cases of COVID-19 (National health commission and National administration of traditional Chinese medicine, 2020), the following criteria was used to determine severe COVID-19: (1) respiratory rate ≥ 30/min; (2) oxygen saturation ≤ 93%; (3) arterial partial pressure of oxygen(PaO2)/fraction of inspiration oxygen(FiO2) ≤ 300 mmHg; (4) progression of lung lesions progressed >50% within 24–48 h; (5) implementation of mechanical ventilation; (6) shock; and (7) intensive care unit admission. The primary endpoint was the mortality of COVID-19 patients.
All continuous variables were depicted using Median with interquartile range, and Mann-Whitney test was used to analyze all continuous variables because of their non-normal distributions. The χ2 test or Fisher’s exact test was used to analyze the categorical variables. The Kaplan-Meier (KM) curve with Log Rank tests were applied to estimate the survival of smoking patients. Finally, the risk of mortality was estimated using Cox regression model with adjustment for the gender, age, and underlying diseases. All analyses were carried out by using IBM SPSS version 26 software.
Baseline characteristics of the included patients grouped according to the survival status(survivors and non-survivors) were summarized in Table 1. A total of 622 adult patients with laboratory-confirmed COVID-19 were included in our study, including 547 survivors and 75 non-survivors. Patients of non-survivors were further classified into three groups based on ages, i.e., ≥65 years (68%), 45 years ≤ age < 65 years (32%), <45 years (0%), respectively. Males who smoked cigarettes were found to have a higher rate of mortality than females (65.3% vs. 34.7%, P = 0.011). In addition, statistically significant difference were shown in the symptoms of fatigue, ground glass changes indicated by chest CT, comorbidities (hypertension, cardiovascular disease, and cancer), and smoking between non-survivors and survivors with COVID-19 (P < 0.05).
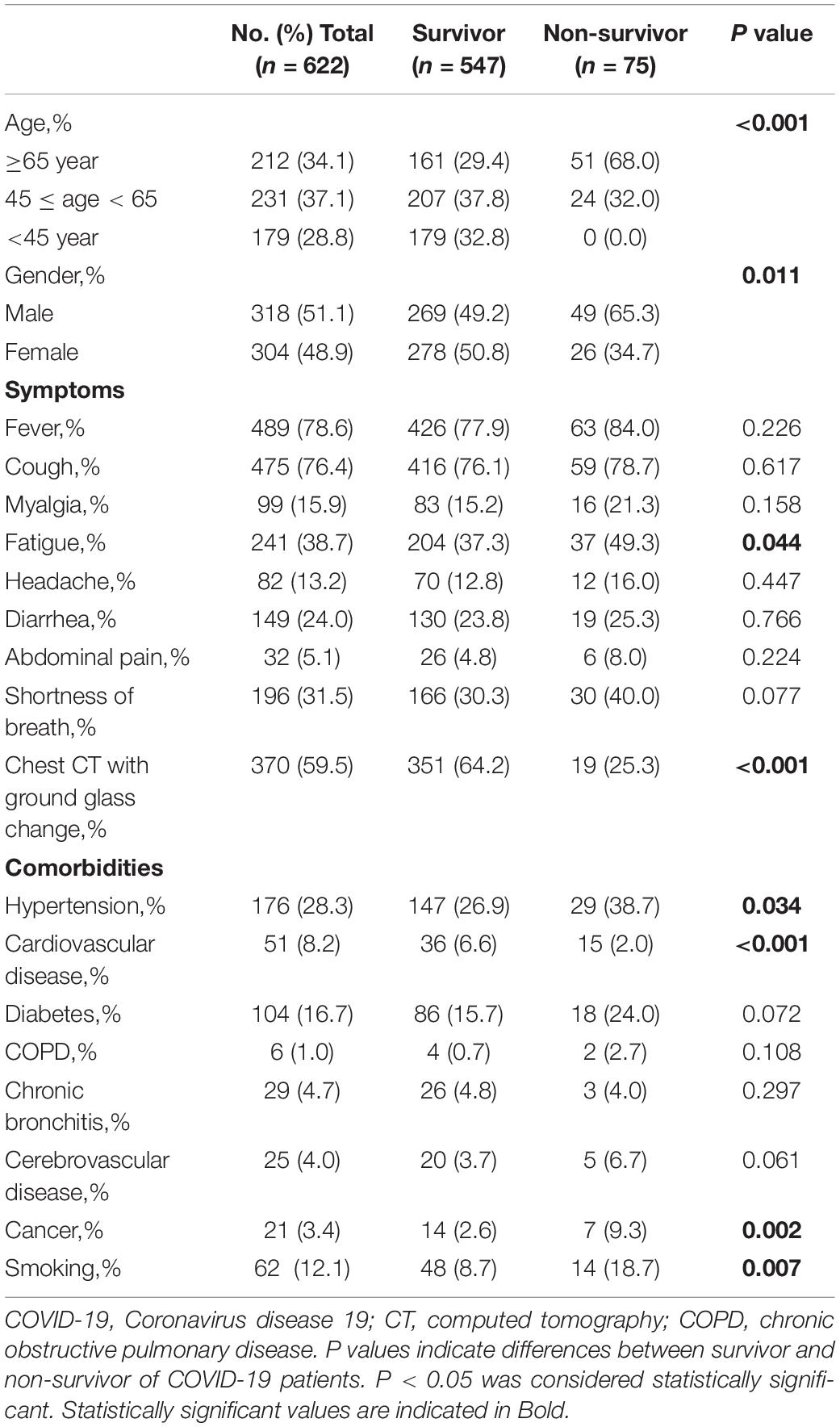
Table 1. Demographics and baseline characteristics of survivor and non-survivor of COVID-19 patients.
White blood cells (10.5 vs. 5.2; P < 0.001), neutrophils (8.8 vs. 3.3; P < 0.001), alanine aminotransferase (29.0 vs. 20.0; P < 0.001), aspartate aminotransferase (43.0 vs. 24.9; P < 0.001), total bilirubin (13.8 vs. 10.0; P < 0.001), creatinine (88.0 vs. 60.0; P < 0.001), creatine kinase (135.5 vs. 69.0; P < 0.001), D-Dimer (2.6 vs. 0.4; P < 0.001), C-reactive protein (88.0 vs. 13.7; P < 0.001) were significantly increased in COVID-19 patients who died; However, lymphocytes (0.6 vs. 1.1; P < 0.001), platelets (161.0 vs. 183.5; P < 0.001), albumin (30.8 vs. 37.3; P < 0.001), prothrombin time (11.6 vs. 13.0; P < 0.001), and activated partial thromboplastin time (15.3 vs. 35.3; P < 0.001) were significantly decreased in survivors than that of non- survivors (Table 2).
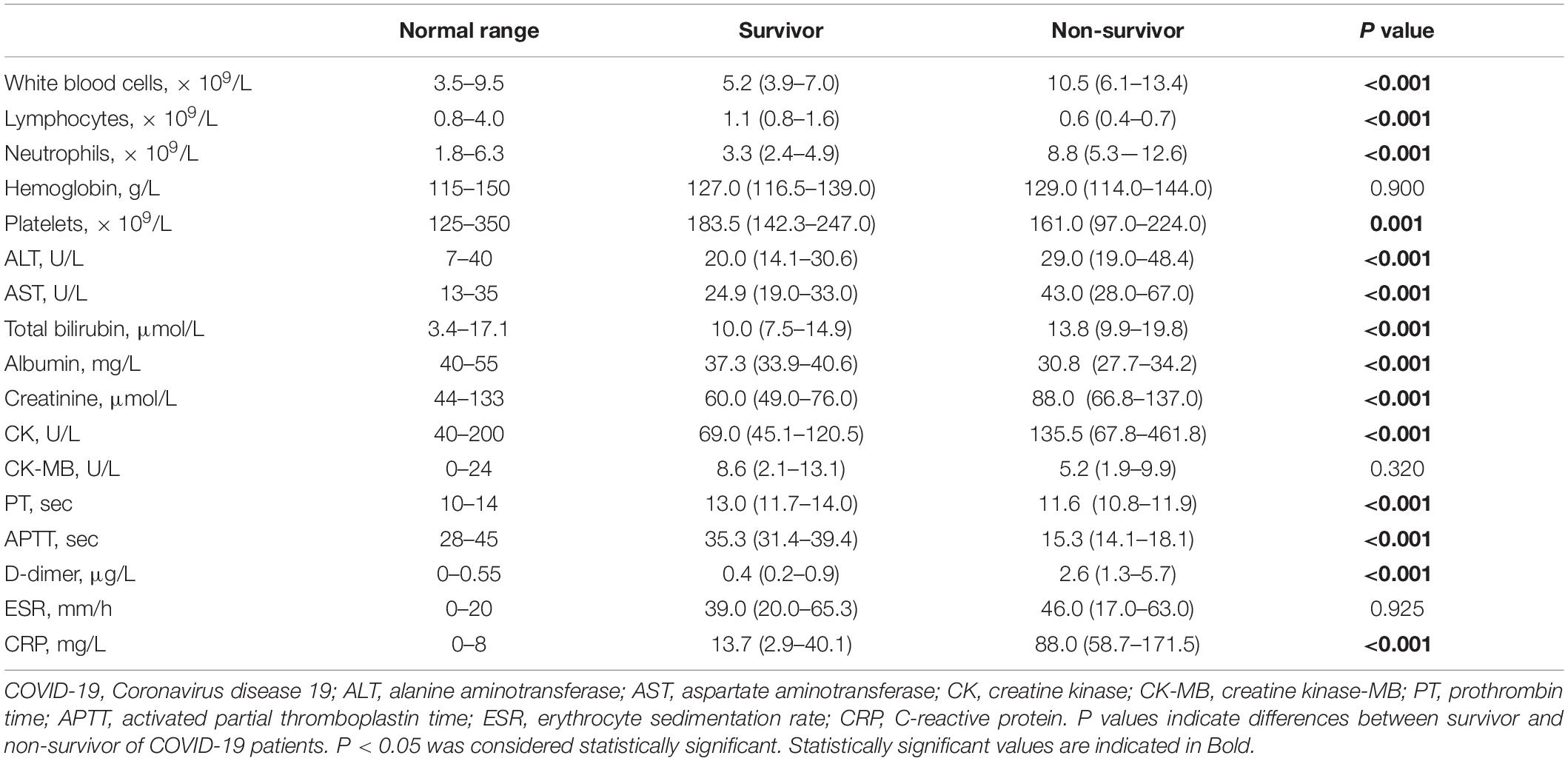
Table 2. Comparison of laboratory parameters between the survivor and non-survivor of COVID-19 patients.
Baseline characteristics of the smoking and non-smoking patients were summarized in Table 3. There were 560 non-smokers and 62 smokers. The ratios of smokers who aged ≥ 65 years, 45 years ≤ age < 65 years and <45 years were 38.7%, 33.9%, 27.4%, respectively. The proportion of male smokers was higher than that of female smokers (91.9% vs. 8.1%, P < 0.001). Besides, there was statistical difference in the incidence of cerebrovascular disease between non-smoking and smoking patients with COVID-19 (9.7% vs. 3.4%, P = 0.017). However, the severity of the patients and smoking were not statistically related (40.8% vs. 59.2%, P = 0.105), to further confirm the relationship, we did logistic regression and found that smoking was not a risk factor for the severity of COVID-19 (Supplementary Tables 1–4).
White blood cells (6.3 vs. 5.4; P = 0.037), hemoglobin (139.0 vs. 127.0; P < 0.001), creatinine (77.3 vs. 61.0; P < 0.001), and activated partial thromboplastin time (37.4 vs. 35.4; P < 0.001) were significantly increased in COVID-19 patients who smoked, but erythrocyte sedimentation rate (28.0 vs. 40.0; P = 0.016) was significantly decreased than that in non-smokers with COVID-19 (Table 4).
Moreover, the association between smoking and survival were analyzed by KM curve and Cox regression analysis in non-survivors after admission (Table 5 and Figure 1). Smoking patients showed a worse survival compared with non-smoking patients (Log Rank P = 0.045). After adjusting for age, gender and underlying diseases, patients with smoking still had higher risk of mortality than non-smoking patients (hazard ratio [HR] 1.897, 95% confidence interval [CI] 1.058–3.402, P = 0.032).
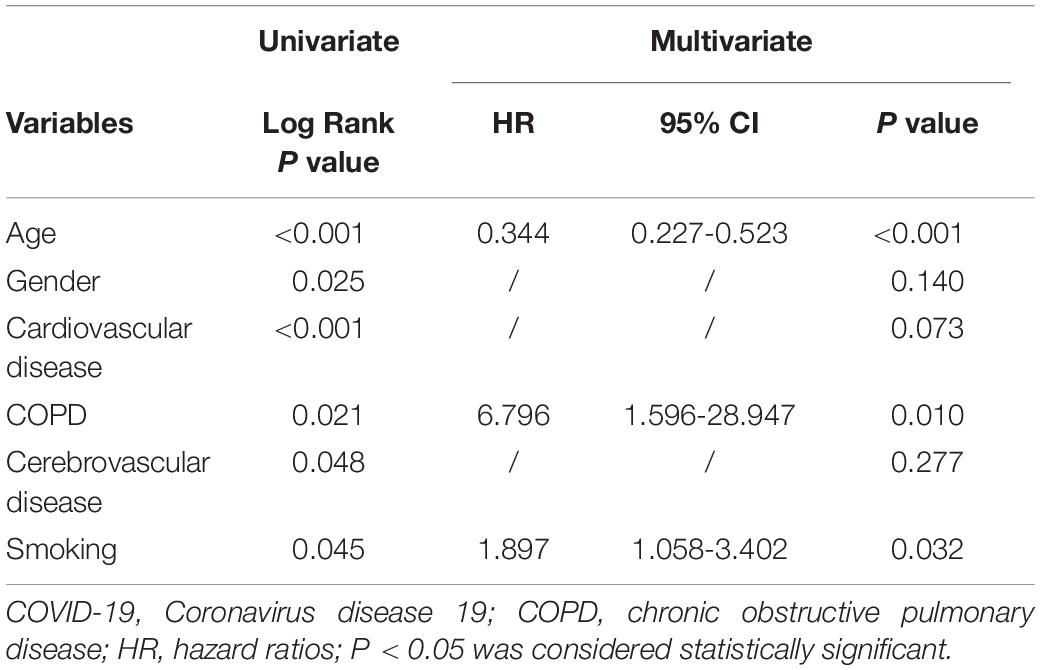
Table 5. Univariate and multivariate Cox regression analysis for the mortality of COVID-19 patients.
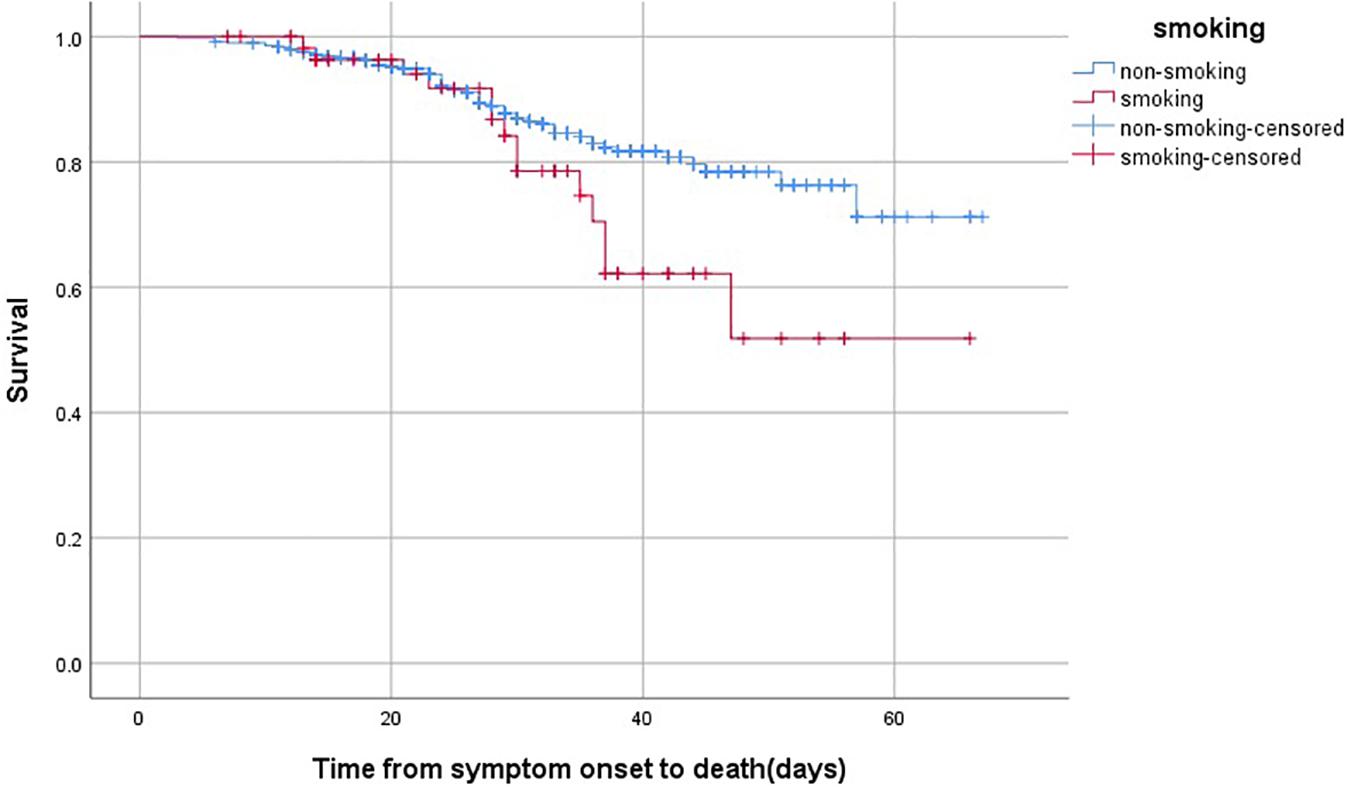
Figure 1. The time-dependent risk of death in COVID-19 patients who smoked using Kaplan-Meier curve. Smoking patients showed a worse survival compared with those with non-smoking (Log Rank P = 0.045).
So far, there is still no definitively effective vaccine for COVID-19. Besides, it is still controversial concerning the relationship between smoking and COVID-19. Some findings supported that smoking patients with COVID-19 had greater severity of illness (Ahmed et al., 2020; Brake et al., 2020; Kaur et al., 2020; van Zyl-Smit et al., 2020). However, others suggested that the risk of infection was lower among smokers for the reason of nicotine (Tindle et al., 2020). Farsalinos et al. pointed out that nicotine might have protective effect against acute inflammatory lung injury caused by cholinergic mediated COVID-19 (Panigrahi et al., 2020). Lippi and Henry even claimed that active smoking had no relationship with the severity of COVID-19 (Lippi and Henry, 2020). Subsequently, Silvano et al. argued that there were several mistakes in the study and concluded that smoking did play a role in the severity of COVID-19 (Gallus et al., 2020). In the present study, smoking was thought to have a statistically significant influence in the prognosis of COVID-19, and smoking patients had higher risk of mortality than non-smokers.
Robust evidences supported smoking to be a significant risk factor during the development of human diseases. Smoking is thought to play an important role in the progression of cancers and respiratory distress such as COPD and pulmonary fibrosis (Hikichi et al., 2019). Smoking seriously affects vascular system including fatal cardiovascular diseases and neurological diseases, abnormal brain development, ischemic stroke and Alzheimer’s diseases (West, 2017). Smoking can lead to lung injury and structural changes thus develop minimal or no resistance to virus attack (Arcavi and Benowitz, 2004), increase the patients’ susceptibility to viral and bacterial infections (Archie and Cucullo, 2020). Besides, the vulnerability to influenza infection increased to a five-hold enhancement in smokers when compared with non-smokers (van Zyl-Smit et al., 2020).
As for prevention of COVID-19, owing to the requirements of social isolation and stay-at-home, the stress on potentially fatal condition, possibility of unemployment and feeling of confinement could stimulate people’s desire to smoke (van Zyl-Smit et al., 2020). Smoking implies repeated exposure among fingers, cigarettes shafts and lips, which will in turn increase the risk of COVID-19 transmission (Sherman, 1991; Sabino-Silva et al., 2020). Exhaled smoke, coughing or sneezing caused by tobacco smoking may produce aerosols containing SARS-CoV-2 which can survive for several hours to days in the surroundings and contaminating surfaces. It has also reported that secondhand smoke had the same damage caused by smoking (Moritsugu, 2007) which may suggest that passive smokers are equally possible to suffer from COVID-19 (Benjamin, 2011; Ma et al., 2020). Furthermore, compared with non-smoking, smoking, which can regulate both the immune and adaptive response, weakens the normal defective system of body (Qiu et al., 2017). It was a strange phenomenon that the proportion was extremely low among smokers of hospitalized COVID-19 patients constituting only 6.5% in China and 1.3% in America (Cai, 2020; Lange et al., 2020). Similarly, our investigation suggested that smokers comprised a proportion of 10% in all COVID-19 hospital admissions. Scholars explained that part of older smokers progressed too fast to be sent to hospital for treatment, and hence their death data were not captured (Appleby, 2020; Simons et al., 2020); Moreover, there was no detailed information of patients if they were exposed to second-hand smoke (Silva et al., 2020).
Coronaviruses have large type 1 transmembrane spike (S) glycoproteins, including two quite different functional domains S1 and S2. To be specific, S1 contains binding site for angiotensin-converting enzyme-2 (ACE2) (Li et al., 2005), while S2 promotes viral and host-cell membrane fusion which is necessary for cellular infiltration (Coutard et al., 2020). S proteins can be enzymatically modified and then fusion sites related to cellular adhesion are exposed (Coutard et al., 2020), which play important roles in virus attack and transmission (Archie and Cucullo, 2020). ACE2 receptors have been verified to be the site of access for the SARS-CoV2 virus entry. A significantly higher affinity was observed between modified S protein of SARS-CoV-2 and ACE2, almost 10 to 20 folds compared with S protein of the previous SARS-CoV (Di Filippo et al., 2020; Wrapp et al., 2020), which might explain the high susceptibility of human-to-human transmission in the spread of COVID-19. It was reported that ACE2 was upregulated in the resected lung tissue of smokers, but no ACE2 was found in non-smokers, and smokers were more likely to have a significant elevation of ACE2 in respiratory epithelial cells (Brake et al., 2020; Leung et al., 2020). In addition, cumulative cigarette smoke was associated strongly with human ACE2 expression, and subjects with the longest pack-years of smoking had the highest ACE2 levels (Smith et al., 2020) indicating that the older were more vulnerable and easier to have poor outcomes. Besides, ACE2 also was thought to have higher expression in germ cells of males when compared with females (Sama et al., 2020), and smoking males accounted for nearly 50% in rural areas of China while approximately 44.8% overall (Zhi et al., 2019), suggesting possibly that males were more prone than females to be at the risk of COVID-19, which were consistent with our study.
Lung macrophages contribute importantly to the development and resolution of human lung inflammation. Macrophages can exert anti-inflammatory effects, downregulate acquired immune responses and suppress inflammation response under normal physiological conditions. Nevertheless, when encountering the attack of pathogenic fungi, bacteria or viruses, macrophages differentiate into pro-inflammatory phenotype, release a large number of inflammatory cytokines and recruit other types of inflammatory cells to the sites of inflammation simultaneously (Hussell and Bell, 2014; Joshi et al., 2018; Hu and Christman, 2019). It was reported that alveolar cavities were filled with amounts of macrophages, which could express ACE2 receptors and recognize SARS-CoV-2 viruses, resulting in cytokine storm (Kloc et al., 2020). Significantly, cytokine storm signifies the failure of restoring homeostasis due to inflammatory response and has become a well-established phenomenon in the viral and bacterial infections. It may lead to acute lung injury and further develop into acute respiratory distress syndrome (Tisoncik et al., 2012).
The aforementioned interpretation supports the importance of ACE2in the process. While, serine protease TMPRSS2 also acts as a crucial role for S protein priming to promote the entry of SARS-CoV2 (Hoffmann et al., 2020b). It was observed that only a small number of ACE2 + cells express TMPRSS2 by single cell RNA sequencing analyses, demonstrating that there were other proteases exerted the same effects (Sungnak et al., 2020). Interesting, through the stimulation of smoking, the level of Cathepsin B was increased and the activity of furin (cleave the spike protein of SARS-CoV-2 on the S1/S2 site) was preserved, which raised the likelihood of COVID-19 infection (Hoffmann et al., 2020a; Kaur et al., 2020).
Besides, in the lung autopsy of COVID-19 patients, there were an infiltration of neutrophil in pulmonary capillaries, along with fibrin deposition and neutrophil extravasation into the alveolar space, which indicated that the formation of Neutrophil Extracellular Traps might lead to organ damage and mortality of COVID-19 patients (Barnes et al., 2020). Evidence suggested that smoking might exert impact on the formation of Neutrophil Extracellular Traps, neutrophil trafficking and mediating both humoral and cell immune responses (Kaur et al., 2020), which was tightly associated with the acute respiratory distress syndrome development or even death (Barnes et al., 2020).
In conclusion, smoking might play a significant role in the prognosis of COVID-19, and smoking patients might have a higher risk of mortality than the non-smokers.
This study has some limitations. Firstly, there was no information to determine whether non-smoking patients were in the environment of secondhand smoke before the onset of COVID-19, which might show a presence of observation bias. Secondly, for the reason of the medical records which had no specific distinction if patients were former, active or never smokers, so we couldn’t elaborate on the role of smoking status more in detail. Finally, the small total sample size and the number of patients who smoked which might also affect the current results. If it’s possible, we would enlarge the sample size in the future to make the results more convincing.
The original contributions presented in the study are included in the manuscript/Supplementary Material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by Second Xiangya Hospital of Central South University (No.2020001). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
YZ and SW contributed to the study design, implementation, and critical revision. FP contributed to methodology, software, and writing original draft preparation. SL and QZ collected and interpreted the data. All authors read and approved the final manuscript.
This study was funded by Emergency Project of Prevention and Control for COVID-19 of Central South University: 160260005.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2021.634842/full#supplementary-material
Agarwal, A. K., Raja, A., and Brown, B. D. (2020). Chronic Obstructive Pulmonary Disease. Treasure Island, FL: Statpearls.
Ahmed, N., Maqsood, A., Abduljabbar, T., and Vohra, F. (2020). Tobacco smoking a potential risk factor in transmission of covid-19 infection. Pak. J. Med. Sci. 36, S104–S107. doi: 10.12669/pjms.36.COVID19-S4.2739
Arcavi, L., and Benowitz, N. L. (2004). Cigarette smoking and infection. Arch. Intern. Med. 164, 2206–2216. doi: 10.1001/archinte.164.20.2206
Archie, S. R., and Cucullo, L. (2020). Cerebrovascular and neurological dysfunction under the threat of covid-19: is there a comorbid role for smoking and vaping? Int. J. Mol. Sci. 21:3916. doi: 10.3390/ijms21113916
Barnes, B. J., Adrover, J. M., Baxter-Stoltzfus, A., Borczuk, A., Cools-Lartigue, J., Crawford, J. M., et al. (2020). Targeting potential drivers of covid-19: neutrophil extracellular traps. J. Exp. Med. 217:e20200652. doi: 10.1084/jem.20200652
Benjamin, R. M. (2011). Exposure to tobacco smoke causes immediate damage: a report of the surgeon general. Public Health Rep. 126, 158–159. doi: 10.1177/003335491112600202
Brake, S. J., Barnsley, K., Lu, W., McAlinden, K. D., Eapen, M. S., and Sohal, S. S. (2020). Smoking upregulates angiotensin-converting enzyme-2 receptor: a potential adhesion site for novel coronavirus sars-cov-2 (covid-19). J. Clin. Med. 9:841. doi: 10.3390/jcm9030841
Cai, H. (2020). Sex difference and smoking predisposition in patients with covid-19. Lancet Respir. Med. 8:e20. doi: 10.1016/s2213-2600(20)30117-x
Chan, J. F., Yuan, S., Kok, K. H., To, K. K., Chu, H., Yang, J., et al. (2020). A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet 95, 514–523. doi: 10.1016/s0140-6736(20)30154-9
Coutard, B., Valle, C., de Lamballerie, X., Canard, B., Seidah, N. G., and Decroly, E. (2020). The spike glycoprotein of the new coronavirus 2019-ncov contains a furin-like cleavage site absent in cov of the same clade. Antiviral Res. 176:104742. doi: 10.1016/j.antiviral.2020.104742
Di Filippo, S., Servadio, A., Bellucci, P., Fabbrini, G., Niolu, C., De Santis, R., et al. (2020). Validation and cross-cultural adaptation of the volitional questionnaire in an italian population with psychiatric disorders: a cross-sectional study. Occup. Ther. Health Care 34, 19–31. doi: 10.1080/07380577.2019.1703237
Gallus, S., Lugo, A., and Gorini, G. (2020). No double-edged sword and no doubt about the relation between smoking and covid-19 severity. Eur. J. Intern. Med. 77, 33–35. doi: 10.1016/j.ejim.2020.06.014
Hikichi, M., Mizumura, K., Maruoka, S., and Gon, Y. (2019). Pathogenesis of chronic obstructive pulmonary disease (copd) induced by cigarette smoke. J. Thorac. Dis. 11, S2129–S2140. doi: 10.21037/jtd.2019.10.43
Hoffmann, M., Kleine-Weber, H., and Pohlmann, S. (2020a). A multibasic cleavage site in the spike protein of sars-cov-2 is essential for infection of human lung cells. Mol. Cell. 78, 779.e5–784.e5. doi: 10.1016/j.molcel.2020.04.022
Hoffmann, M., Kleine-Weber, H., Schroeder, S., Kruger, N., Herrler, T., Erichsen, S., et al. (2020b). Sars-cov-2 cell entry depends on ace2 and tmprss2 and is blocked by a clinically proven protease inhibitor. Cell 181, 271.e8–280.e8. doi: 10.1016/j.cell.2020.02.052
Hu, G., and Christman, J. W. (2019). Editorial: alveolar macrophages in lung inflammation and resolution. Front. Immunol. 10:2275. doi: 10.3389/fimmu.2019.02275
Hussell, T., and Bell, T. J. (2014). Alveolar macrophages: plasticity in a tissue-specific context. Nat. Rev. Immunol. 14, 81–93. doi: 10.1038/nri3600
Joshi, N., Walter, J. M., and Misharin, A. V. (2018). Alveolar macrophages. Cell. Immunol. 330, 86–90. doi: 10.1016/j.cellimm.2018.01.005
Kaur, G., Lungarella, G., and Rahman, I. (2020). Sars-cov-2 covid-19 susceptibility and lung inflammatory storm by smoking and vaping. J. Inflamm. 17;21. doi: 10.1186/s12950-020-00250-8
Kloc, M., Ghobrial, R. M., and Kubiak, J. Z. (2020). How nicotine can inhibit cytokine storm in the lungs and prevent or lessen the severity of covid-19 infection? Immunol. Lett. 224, 28–29. doi: 10.1016/j.imlet.2020.06.002
Lange, S. J., Ritchey, M. D., Goodman, A. B., Dias, T., Twentyman, E., Fuld, J., et al. (2020). Potential indirect effects of the covid-19 pandemic on use of emergency departments for acute life-threatening conditions - united states, january-may 2020. MMWR Morb. Mortal Wkly. Rep. 69, 795–800. doi: 10.15585/mmwr.mm6925e2
Leung, J. M., Yang, C. X., Tam, A., Shaipanich, T., Hackett, T. L., Singhera, G. K., et al. (2020). Ace-2 expression in the small airway epithelia of smokers and copd patients: implications for covid-19. Eur. Respir. J. 55:2000688. doi: 10.1183/13993003.00688-2020
Li, F., Li, W., Farzan, M., and Harrison, S. C. (2005). Structure of sars coronavirus spike receptor-binding domain complexed with receptor. Science 309, 1864–1868. doi: 10.1126/science.1116480
Lippi, G., and Henry, B. M. (2020). Active smoking is not associated with severity of coronavirus disease 2019 (covid-19). Eur. J. Intern. Med. 75, 107–108. doi: 10.1016/j.ejim.2020.03.014
Lippi, G., Sanchis-Gomar, F., and Henry, B. M. (2020). Active smoking and covid-19: a double-edged sword. Eur. J. Intern. Med. 77, 123–124. doi: 10.1016/j.ejim.2020.04.060
Ma, J., Qi, X., Chen, H., Li, X., Zhang, Z., Wang, H., et al. (2020). Covid-19 patients in earlier stages exhaled millions of sars-cov-2 per hour. Clin. Infect. Dis. [Epub ahead of print].
Moritsugu, K. P. (2007). The 2006 report of the surgeon general: the health consequences of involuntary exposure to tobacco smoke. Am. J. Prev. Med. 32, 542–543. doi: 10.1016/j.amepre.2007.02.026
National health commission, and National administration of traditional Chinese medicine (2020). Diagnosis and treatment of new coronavirus pneumonia (trial sixth edition). Chin. J. Viral Dis. 10, 1–5.
Panigrahi, D. P., Bhol, C. S., Nivetha, R., Nagini, S., Patil, S., Maiti, T. K., et al. (2020). Abrus agglutinin inhibits oral carcinogenesis through inactivation of nrf2 signaling pathway. Int. J. Biol. Macromol. 155, 1123–1132. doi: 10.1016/j.ijbiomac.2019.11.079
Qiu, F., Liang, C. L., Liu, H., Zeng, Y. Q., Hou, S., Huang, S., et al. (2017). Impacts of cigarette smoking on immune responsiveness: up and down or upside down? Oncotarget 8, 268–284. doi: 10.18632/oncotarget.13613
Sabino-Silva, R., Jardim, A. C. G., and Siqueira, W. L. (2020). Coronavirus covid-19 impacts to dentistry and potential salivary diagnosis. Clin. Oral Investig. 24, 1619–1621. doi: 10.1007/s00784-020-03248-x
Sama, I. E., Ravera, A., Santema, B. T., van Goor, H., Ter Maaten, J. M., Cleland, J. G. F., et al. (2020). Circulating plasma concentrations of angiotensin-converting enzyme 2 in men and women with heart failure and effects of renin-angiotensin-aldosterone inhibitors. Eur. Heart J. 41, 1810–1817. doi: 10.1093/eurheartj/ehaa373
Silva, A., Moreira, J. C., and Martins, S. R. (2020). Covid-19 and smoking: a high-risk association. Cad. Saude Publica 36:e00072020.
Simons, D., Shahab, L., Brown, J., and Perski, O. (2020). The Association of Smoking Status with Sars-Cov-2 Infection, Hospitalization and Mortality from Covid-19: A Living Rapid Evidence Review with Bayesian Meta-Analyses (version 7). doi: 10.32388/UJR2AW.8
Smith, J. C., Sausville, E. L., Girish, V., Yuan, M. L., Vasudevan, A., John, K. M., et al. (2020). Cigarette smoke exposure and inflammatory signaling increase the expression of the sars-cov-2 receptor ace2 in the respiratory tract. Dev. Cell. 53, 514.e3–529.e3. doi: 10.1016/j.devcel.2020.05.012
Sungnak, W., Huang, N., Becavin, C., Berg, M., Queen, R., Litvinukova, M., et al. (2020). Sars-cov-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat. Med. 26, 681–687. doi: 10.1038/s41591-020-0868-6
Tindle, H. A., Newhouse, P. A., and Freiberg, M. S. (2020). Beyond smoking cessation: investigating medicinal nicotine to prevent and treat covid-19. Nicotine Tob. Res. 22, 1669–1670. doi: 10.1093/ntr/ntaa077
Tisoncik, J. R., Korth, M. J., Simmons, C. P., Farrar, J., Martin, T. R., and Katze, M. G. (2012). Into the eye of the cytokine storm. Microbiol. Mol. Biol. Rev. 76, 16–32. doi: 10.1128/MMBR.05015-11
van Zyl-Smit, R. N., Richards, G., and Leone, F. T. (2020). Tobacco smoking and covid-19 infection. Lancet Respir. Med. 8, 664–665. doi: 10.1016/s2213-2600(20)30239-3
Warren, G. W., Alberg, A. J., Kraft, A. S., and Cummings, K. M. (2014). The 2014 surgeon general’s report: “The health consequences of smoking–50 years of progress”: a paradigm shift in cancer care. Cancer 120, 1914–1916. doi: 10.1002/cncr.28695
West, R. (2017). Tobacco smoking: health impact, prevalence, correlates and interventions. Psychol. Health 32, 1018–1036. doi: 10.1080/08870446.2017.1325890
World Health Organisation (1997). Guidelines for Controlling and Monitoring the Tobacco Epidemic. Geneva: WHO.
World Health Organization (2020). WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19. Geneva: WHO.
Wrapp, D., Wang, N., Corbett, K. S., Goldsmith, J. A., Hsieh, C. L., Abiona, O., et al. (2020). Cryo-em structure of the 2019-ncov spike in the prefusion conformation. Science 367, 1260–1263. doi: 10.1126/science.abb2507
Zhi, K., Wang, L., Han, Y., Vaughn, M. G., Qian, Z., Chen, Y., et al. (2019). Trends in cigarette smoking among older male adults in china: an urban-rural comparison. J. Appl. Gerontol. 38, 884–901. doi: 10.1177/0733464817716967
Keywords: COVID-19, SARS-CoV-2, cigarette, smoking, ACE2, inflammation, prognosis
Citation: Peng F, Lei S, Zhang Q, Zhong Y and Wu S (2021) Smoking Is Correlated With the Prognosis of Coronavirus Disease 2019 (COVID-19) Patients: An Observational Study. Front. Physiol. 12:634842. doi: 10.3389/fphys.2021.634842
Received: 29 November 2020; Accepted: 08 February 2021;
Published: 03 March 2021.
Edited by:
Zhi Tian, University of South Florida, United StatesReviewed by:
Xingping Qin, Renmin Hospital of Wuhan University, ChinaCopyright © 2021 Peng, Lei, Zhang, Zhong and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanjun Zhong, emhvbmd5YW5qdW5AY3N1LmVkdS5jbg==; Shangjie Wu, d3VzaGFuZ2ppZUBjc3UuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.