
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Physiol., 04 June 2021
Sec. Striated Muscle Physiology
Volume 12 - 2021 | https://doi.org/10.3389/fphys.2021.625044
This article is part of the Research TopicModulators of Skeletal Muscle Hypertrophy: Mechanisms to Lifestyle StrategiesView all 8 articles
A correction has been applied to this article in:
Corrigendum: Myofibre Hypertrophy in the Absence of Changes to Satellite Cell Content Following Concurrent Exercise Training in Young Healthy Men
Concurrent exercise training has been suggested to create an ‘interference effect,’ attenuating resistance training-based skeletal muscle adaptations, including myofibre hypertrophy. Satellite cells support myofibre hypertrophy and are influenced by exercise mode. To determine whether satellite cells contribute to the ‘interference effect’ changes in satellite cell and myonuclear content were assessed following a period of training in 32 recreationally active males (age: 25 ± 5 year; body mass index: 24 ± 3 kg⋅m–2; mean ± SD) who undertook 12-week of either isolated (3 d⋅w–1) resistance (RES; n = 10), endurance (END; n = 10), or alternate day (6 d⋅w–1) concurrent (CET, n = 12) training. Skeletal muscle biopsies were obtained pre-intervention and after 2, 8, and 12 weeks of training to determine fibre type-specific cross-sectional area (CSA), satellite cell content (Pax7+DAPI+), and myonuclei (DAPI+) using immunofluorescence microscopy. After 12 weeks, myofibre CSA increased in all training conditions in type II (P = 0.0149) and mixed fibres (P = 0.0102), with no difference between conditions. Satellite cell content remained unchanged after training in both type I and type II fibres. Significant correlations were observed between increases in fibre type-specific myonuclear content and CSA of Type I (r = 0.63, P < 0.0001), Type II (r = 0.69, P < 0.0001), and mixed fibres (r = 0.72, P < 0.0001). Resistance, endurance, and concurrent training induce similar myofibre hypertrophy in the absence of satellite cell and myonuclear pool expansion. These findings suggest that myonuclear accretion via satellite cell fusion is positively correlated with hypertrophy after 12 weeks of concurrent training, and that individuals with more myonuclear content displayed greater myofibre hypertrophy.
Combining resistance- and endurance-based exercise training, or ‘concurrent exercise training,’ has previously been shown to impair strength and power adaptations compared to resistance training undertaken in isolation (Hickson, 1980; Craig et al., 1991; Hennessy and Watson, 1994; Kraemer et al., 1995; Dolezal and Potteiger, 1998; Bell et al., 2000; Häkkinen et al., 2003; Mikkola et al., 2012; Fyfe et al., 2016, 2018) and is referred to as the ‘interference effect.’ Notably, the result of concurrent exercise training on ‘interferences’ to lean mass gains relative to resistance training alone appear equivocal, with some studies showing greater gains in lean mass compared to resistance training alone (Kraemer et al., 1995; Bell et al., 2000; Rønnestad et al., 2012; Lundberg et al., 2013, 2014; Tomiya et al., 2017; Fyfe et al., 2018), while others have observed comparable (de Souza et al., 2013) or smaller gains in lean mass compared to resistance training alone (Sale et al., 1990; Timmons et al., 2018; Spiliopoulou et al., 2019). As such, understanding the ability of skeletal muscle to simultaneously adapt to divergent training stimuli is a topic that has received considerable attention (Nader, 2006; Wilson et al., 2012; Hamilton and Philp, 2013; Baar, 2014; Fyfe et al., 2014; Perez-Schindler et al., 2015; Murach and Bagley, 2016; Varela-Sanz et al., 2016; Coffey and Hawley, 2017; Doma et al., 2017; Berryman et al., 2018; Eddens et al., 2018; Fyfe and Loenneke, 2018; Hughes et al., 2018). Though the underlying cause of discrepancies in the degree of muscle hypertrophy achieved with concurrent versus resistance training remains unclear, it has recently been proposed that the potential for myofibre hypertrophy in response to chronic concurrent exercise training may be limited by satellite cell content (Babcock et al., 2012).
Satellite cells are myogenic precursor cells that reside between the sarcolemma and basal lamina (Mauro, 1961). In adult skeletal muscle, satellite cells exist in a quiescent state and are activated in response to various stimuli, such as exercise-induced mechanical stress, growth factors, and nutrients (Shamim et al., 2018b). Once activated, satellite cells can differentiate to form new myonuclei and increase transcriptional capacity, or return to quiescence to replenish the satellite cell pool through self-renewal (Kadi et al., 2005). Both satellite cell (Petrella et al., 2008; Verdijk et al., 2014; Moore et al., 2018) and myonuclear (Petrella et al., 2006, 2008) content have been shown to positively correlate with changes in myofibre cross-sectional area, suggesting an important relationship between myonuclear accretion and myofibre hypertrophy.
The degree of satellite cell activation and proliferation appears to be influenced by the mode of exercise performed. While evidence of satellite cell proliferation following resistance exercise is well documented (Crameri et al., 2004, 2007; Babcock et al., 2012; Farup et al., 2014; Snijders et al., 2014; Nederveen et al., 2015, 2017; Reidy et al., 2017a; Damas et al., 2018; Pugh et al., 2018), the capacity for acute endurance exercise to expand the satellite cell pool is less apparent (Snijders et al., 2011; Babcock et al., 2012; Nederveen et al., 2015), and may be dependent on type of exercise performed (Mackey et al., 2007) or intensity (Nederveen et al., 2015; McKenzie et al., 2016). Nonetheless, increases in satellite cell content have been reported following prolonged endurance training both in the presence (Charifi et al., 2003; Verney et al., 2008; Murach et al., 2016) and absence (Joanisse et al., 2013) of myofibre hypertrophy. In contrast, satellite cell activation and proliferation in response to concurrent exercise remains poorly understood.
To date, only two studies have evaluated changes in satellite cell content in response to concurrent exercise compared to isolated resistance exercise, with mixed results. Following a single bout of unilateral concurrent exercise incorporating moderate intensity cycling, Babcock et al. (2012) demonstrated that satellite cell proliferation was impaired in young, healthy males compared to resistance exercise performed alone in the contralateral leg. Based on this observation, the authors hypothesised that concurrent exercise impairs satellite cell responses and may contribute to limitations in myofibre hypertrophy observed with chronic concurrent exercise training. However, baseline satellite cell content prior to exercise was elevated in the leg that performed concurrent exercise compared to the one that performed resistance exercise only. Thus, the stable satellite cell content in response to concurrent exercise may have been indicative of a reduced need for proliferation rather than an inhibition of satellite cell activation per se. Recently, Pugh et al. (2018) demonstrated that a single bout of resistance or concurrent exercise incorporating high-intensity interval cycling results in comparable increases in satellite cell content in sedentary, overweight and obese middle-aged individuals, suggesting that concurrent exercise does not prevent satellite cell expansion if a high intensity endurance exercise stimulus is provided. While exercise order was the same in both studies (i.e., resistance exercise followed by endurance exercise), differences in the intensity of endurance exercise make direct comparisons between investigations cumbersome (Babcock et al., 2012; Pugh et al., 2018). Despite disparities in exercise protocols, to date, no studies have directly investigated the effect of chronic concurrent exercise training on changes in satellite cell content compared to isolated resistance or endurance training.
We have previously demonstrated that 12 weeks of concurrent exercise training resulted in similar lean mass gains as isolated resistance and endurance training, despite performing a greater volume of work (Shamim et al., 2018a). As satellite cell content appears to be associated with changes in myofibre cross-sectional area (Petrella et al., 2008; Verdijk et al., 2014; Moore et al., 2018), the aim of the present investigation was to evaluate whether changes in fibre type-specific satellite cell abundance are associated with the magnitude of hypertrophy achieved during concurrent training. Given the findings that performing a greater volume of work during acute concurrent exercise does not augment satellite cell proliferation compared to resistance or endurance exercise alone (Babcock et al., 2012; Pugh et al., 2018), it was hypothesised that chronic exercise training would result in similar increases in satellite cell content across all groups.
Using a parallel-groups design, participants were stratified according to lean body mass and allocated to either a resistance only (RES; n = 10), endurance only (END; n = 10) or concurrent resistance and endurance exercise training (CET; n = 12) group for 12 weeks. Consistent with previous recommendations to potentiate hypertrophy with concurrent training (Murach and Bagley, 2016), participants were given longer recovery periods (i.e., 24 h) between exercise sessions, cycling rather than running was selected for endurance exercise, and this was limited to ≤3 days per week. Measures of maximal strength, aerobic capacity, and anaerobic power, as well as body composition were performed pre- and post-intervention and have been previously reported (Shamim et al., 2018a; Timmins et al., 2020). Resting skeletal muscle biopsies were taken from the vastus lateralis at baseline (pre-intervention), and after 2, 8, and 12 weeks of training. The study was approved by the Australian Catholic University Human Research Ethics Committee and was carried out in accordance with the latest revision of the Declaration of Helsinki. This trial was registered with the Australian New Zealand Clinical Trials Registry (ACTRN12617001229369).
Thirty-two young, healthy, and recreationally active males (age: 25 ± 5 year, body mass index: 24 ± 3 kg⋅m–2; mean ± SD) who had not participated in a structured exercise programme for ≥6 months preceding the study volunteered to participate. Participants were deemed healthy and eligible to participate based on their responses to a cardiovascular risk-factor questionnaire. All experimental procedures and risks associated with the study were explained to participants prior to providing written informed consent.
A detailed outline of the training programmes has been reported elsewhere (Shamim et al., 2018a). Briefly, for the duration of the intervention, participants in the RES and END group performed three non-consecutive days of training each week. Participants in the CET group trained 6 d⋅wk–1 and performed identical resistance and endurance programmes on alternating days as those in the RES and END groups, respectively. All training sessions were performed under the supervision of a member of the research team. Resistance training consisted of whole-body exercises with a focus on the leg press, knee extension and bench press movements, with these exercises performed at an intensity of ∼60–98% of 1RM. For example, leg press was progressed from participants doing 5 sets of 10 to 15 repetitions at 70% of 1RM in the first week of training to 2 sets of 2 repetitions at 97.5% of 1RM in the final week. If the participant was unable to achieve the prescribed number of repetitions, the weight was lowered by ∼5–10% for the following set to uphold the repetition scheme. Endurance cycle training was performed on Lode cycle ergometers and consisted of a mixture of a hill simulation ride of varying intensity (25–110% of maximum aerobic power (MAP), moderate-intensity continuous training at 50% MAP, moderate-intensity interval training at 70% MAP, and high-intensity interval training at 100% MAP. Moderate-intensity intervals were separated by a 60 s recovery period at ∼40% MAP, to establish a 2.5:1 or 5:1 work-to-rest ratio. High-intensity intervals were separated by 20–60 s recovery periods, completed at ∼40% MAP, to establish a 1:5, 1:2, or 1:1 work-to-rest ratio. All sessions were preceded by a standardised warm up for the respective training modality. Progressive overload was applied by periodically manipulating the number of sets and repetitions (resistance training), number of intervals (endurance training), and relative intensity of load the 12-week programme. All training programmes have been previously described in full detail and published elsewhere (Shamim et al., 2018a).
A free-living, high-protein (2 g⋅kg–1⋅d–1) eating plan was implemented over the 12-week intervention. Participants completed daily food records and attended consultations with an Accredited Practising Dietitian on a fortnightly basis to monitor protein and energy intakes. All participants consumed ∼34 g of whey protein upon cessation of every training session to promote muscle protein synthesis (Macnaughton et al., 2016). Diet records were analysed for energy (kJ⋅kg–1), protein, carbohydrate, and fat (g⋅kg–1 for all macronutrients) to provide a daily average for the entire 12-week intervention. Daily averages of these dietary parameters have been previously published (Shamim et al., 2018a).
Resting skeletal muscle biopsies were collected after an overnight fast pre-intervention and 72 h after the last exercise session of weeks 2, 8, and 12 from a distal portion of the vastus lateralis using a Bergstrom needle modified for manual suction under local anaesthesia (2% Xylocaine). Each muscle biopsy was obtained from a separate site 2–3 cm proximal to the previous biopsy site on the same leg. Biopsies in the CET condition were taken 72 h after resistance exercise to determine the effects of endurance exercise performed on alternate days on satellite cell expansion (Figure 1). Samples were immediately frozen in liquid nitrogen or embedded in optimum cutting temperature compound (Scigen) and frozen in liquid nitrogen-cooled isopentane. Samples were stored at −80oC for subsequent analysis.
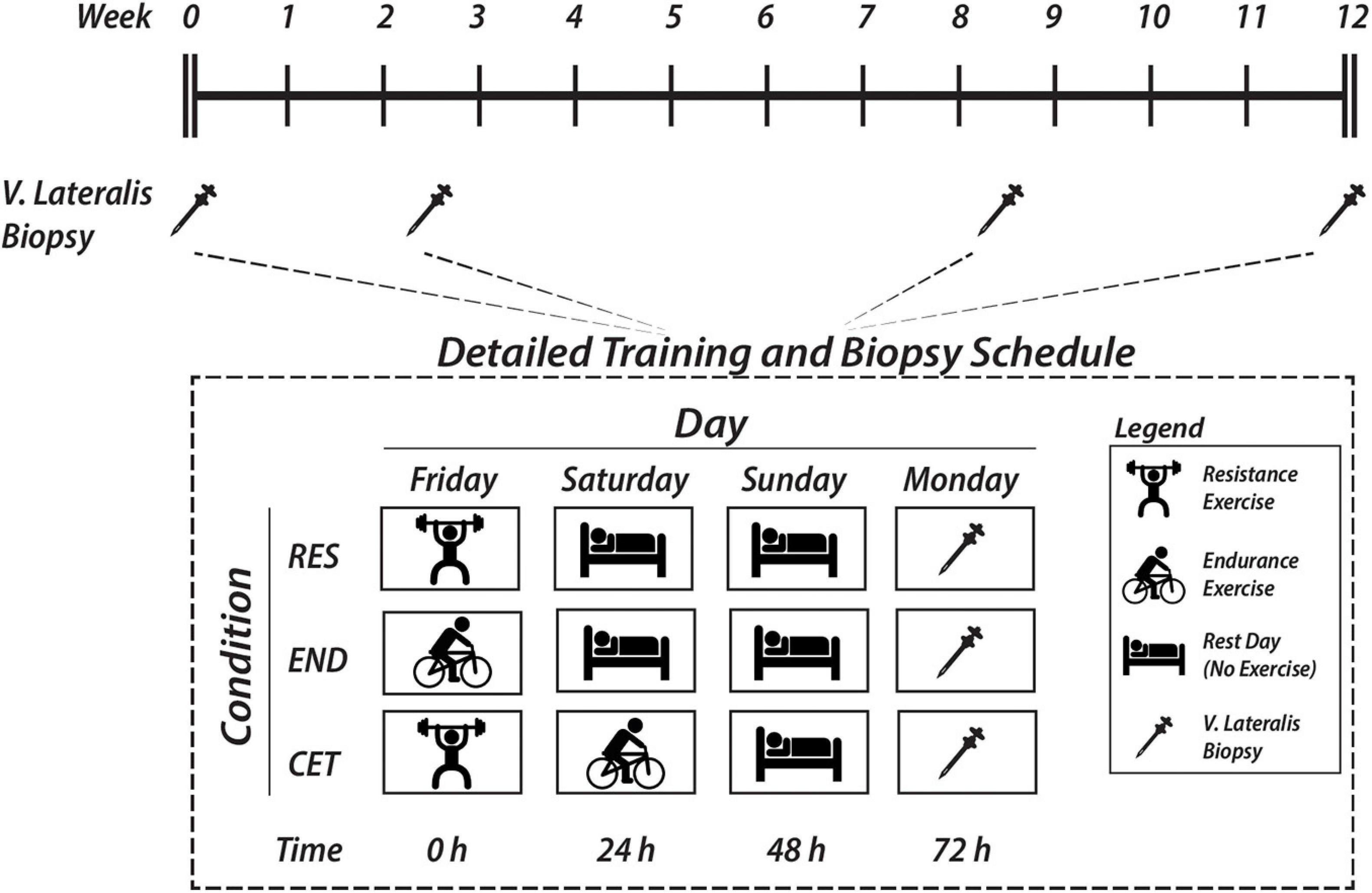
Figure 1. Schematic overview of study timeline and detailed vastus lateralis muscle biopsy sampling times following endurance (END), resistance (RES), or concurrent (CET) training.
Muscle cross-sections (7 μm) were stained for cross-sectional area (CSA), fibre type, myonuclei, and satellite cells. Cross-sections were fixed in 2% formaldehyde, washed, blocked for 90 min (1X phosphate-buffered saline (PBS) containing 2% bovine serum albumin (BSA), 5% fetal bovine serum (FBS), 0.2% Triton X-100, 2% goat serum (GS), and 0.02% NaN3), and then incubated in primary antibodies for Pax7 (1:4, DSHB) and laminin (1:250, ab11575, Abcam) overnight at 4oC. Sections were washed, then incubated for 2 h in secondary antibodies for Alexa Fluor 488 (#A32731) and 594 (#A11032; Invitrogen). Sections were re-fixed, washed, blocked for 2 h (5% GS containing 0.01% Triton x-100 and 0.05% NaN3), and then incubated in primary antibody for MHCI (1:4, A.4.951, DSHB) overnight at 4oC. Sections were washed then incubated for 2 h in secondary antibody (Alexa Fluor 488, #A11029, Invitrogen), nuclei were labelled with 4’,6-diamidino-2-phenylindole (DAPI, 1:20,000, Life Technologies), and cover slips affixed with ProLongTM Diamond Antifade Mountant (Invitrogen).
Staining for fibre CSA and fibre-typing was undertaken following fixation by blocking sections for 2 h (5% GS containing 0.01% Triton X-100 and 0.05% NaN3), then incubating in primary antibodies for MHCI and laminin overnight at 4oC. Sections were washed, incubated in appropriate secondary antibodies, washed again, and then affixed with cover slips. As an antibody for MHCIIa was not used, fibres that were negative for MHCI are referred to as MHCII, which includes MHCIIa and MHCIIx fibre types (Babcock et al., 2012).
All antibodies were diluted in 1% BSA, and secondary antibodies diluted 1:500. Images were observed under an EVOSTM FL Auto 2 microscope (Invitrogen) at 20X (nuclei) and 10X (CSA) objectives. An average of 218 ± 30 (90 ± 31 Type I, 128 ± 35 Type II) and 230 ± 44 (98 ± 24 Type I, 132 ± 38 Type II) fibres were assessed per participant at each time point for CSA and satellite cell enumeration, respectively. Peripheral muscle fibres that displayed irregular edge staining patterns or disrupted cell membranes and longitudinal fibres (circularity < 0.6) were excluded from analyses. Images were analysed using ImageJ-Fiji.
The present study is a secondary analysis from previously published work powered to detect a medium effect (Cohen’s f = 0.25) with a significance level of α = 0.05 and 80% power for change in lean body mass (Shamim et al., 2018a). Thus, outcome measures including change in fibre CSA, satellite cell content, and myonuclear content are exploratory. Linear mixed-effect (LME) model, fit by restricted maximum likelihood estimate with random intercept for subject was used to test the effect of training condition on myofibre CSA, myofibre-type distribution, myonuclear number, and satellite cell content. Interactions for training condition × time were tested by the same LME. Where LME revealed significance, a Bonferroni post hoc test for pair-wise comparisons was performed. The relationships between baseline satellite cell content and change in fibre CSA as well as change in myonuclear content and change in fibre CSA were determined by calculating Pearson correlation coefficients (r). Statistical significance was set at P < 0.05. Data are presented as mean ± standard deviation. Statistical analysis was performed using R (v3.5.2).
Following 12 weeks of training, there was a main effect of condition (P = 0.0317), but not time or condition by time for an increase in Type I fibre CSA. However, when corrected for multiple comparisons, post hoc analysis revealed no significant differences in Type I fibre CSA for condition (Figure 2A). Despite employing different training modalities, only a main effect of time (P = 0.0474), but not condition or condition by time was observed for an increase in Type II fibre CSA. Post hoc analysis revealed an increase in Type II fibre CSA at week 12 compared to Pre (P = 0.0149; Figure 2B). Similarly, when the mean CSA of both Type I and II fibres was assessed, there was a main effect of time (P = 0.0487), but no main effect for condition or condition by time. Post hoc analysis revealed an increase in mixed fibres CSA at week 12 compared to Pre (P = 0.0102; Figure 2C). Fibre-type distribution was unaffected by the training intervention, and displayed no main effect of condition, time or condition by time (Figures 3A,B).

Figure 2. Changes to myosin heavy chain type I (A), myosin heavy chain type II (B), and mixed (C) myofibre cross-sectional area (CSA) in response to endurance (END; n = 10), resistance (RES; n = 10), or concurrent (CET; n = 12) training. a, significantly different from Pre time point (P < 0.05). Values are presented as Mean ± SD.
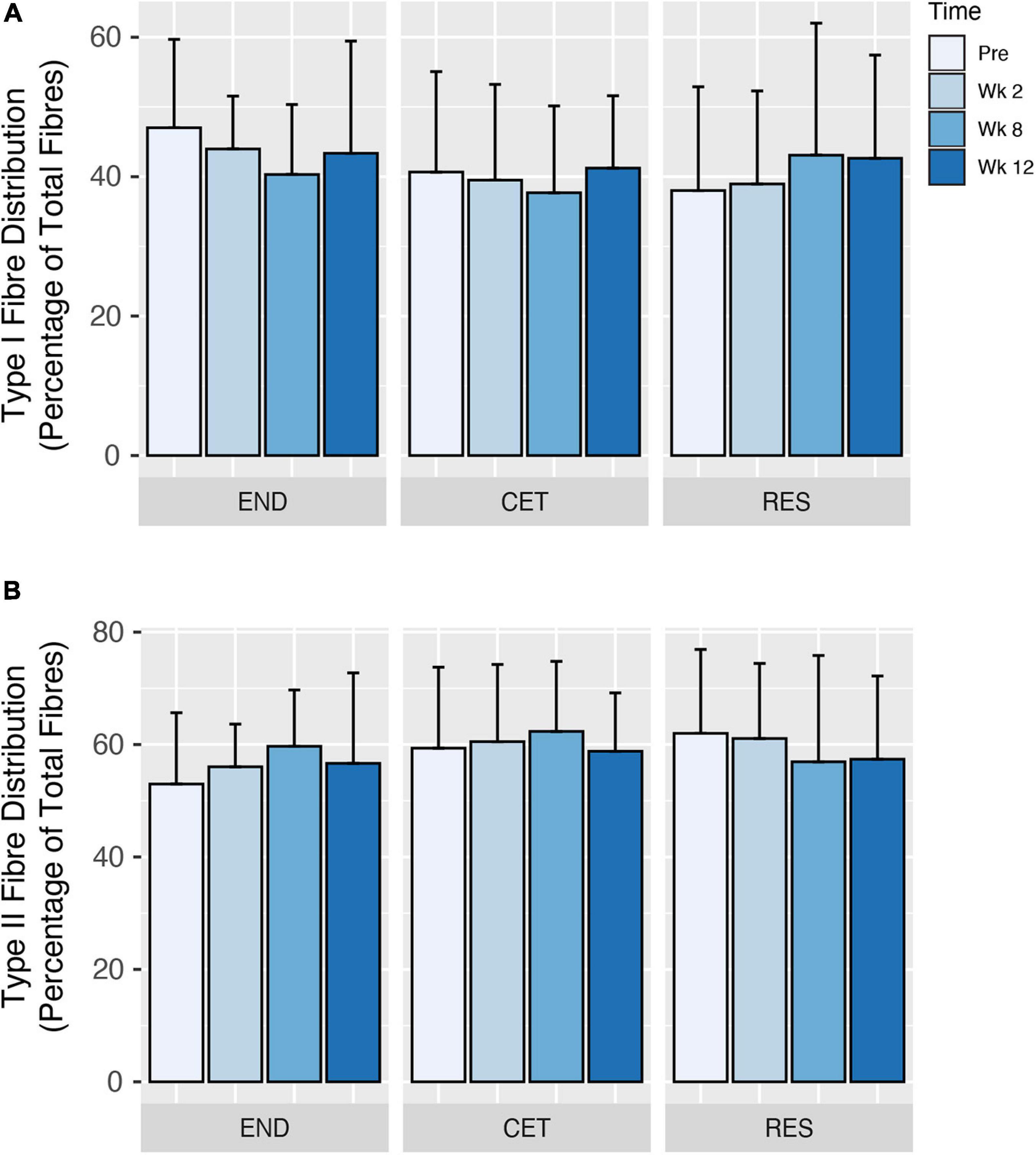
Figure 3. Changes to type I (A) and type II (B) fibre distribution in response to endurance (END; n = 10), resistance (RES; n = 10), or concurrent (CET; n = 12) training. Values are presented as Mean ± SD.
Satellite cells were determined as staining positive for both DAPI and Pax7 and having a location between the basal lamina and the plasma membrane of myofibres (see Figure 4 for representative stain). In response to 12 weeks of exercise training there was no main effect of condition, time or condition by time for change in Type I satellite cell content (Figure 5A). Similarly, there was no main effect of condition, time or condition by time for change in Type II satellite cell content (Figure 5B). There was no main effect of condition, time or condition by time for change in mixed fibre-type satellite cell content (Figure 5C).
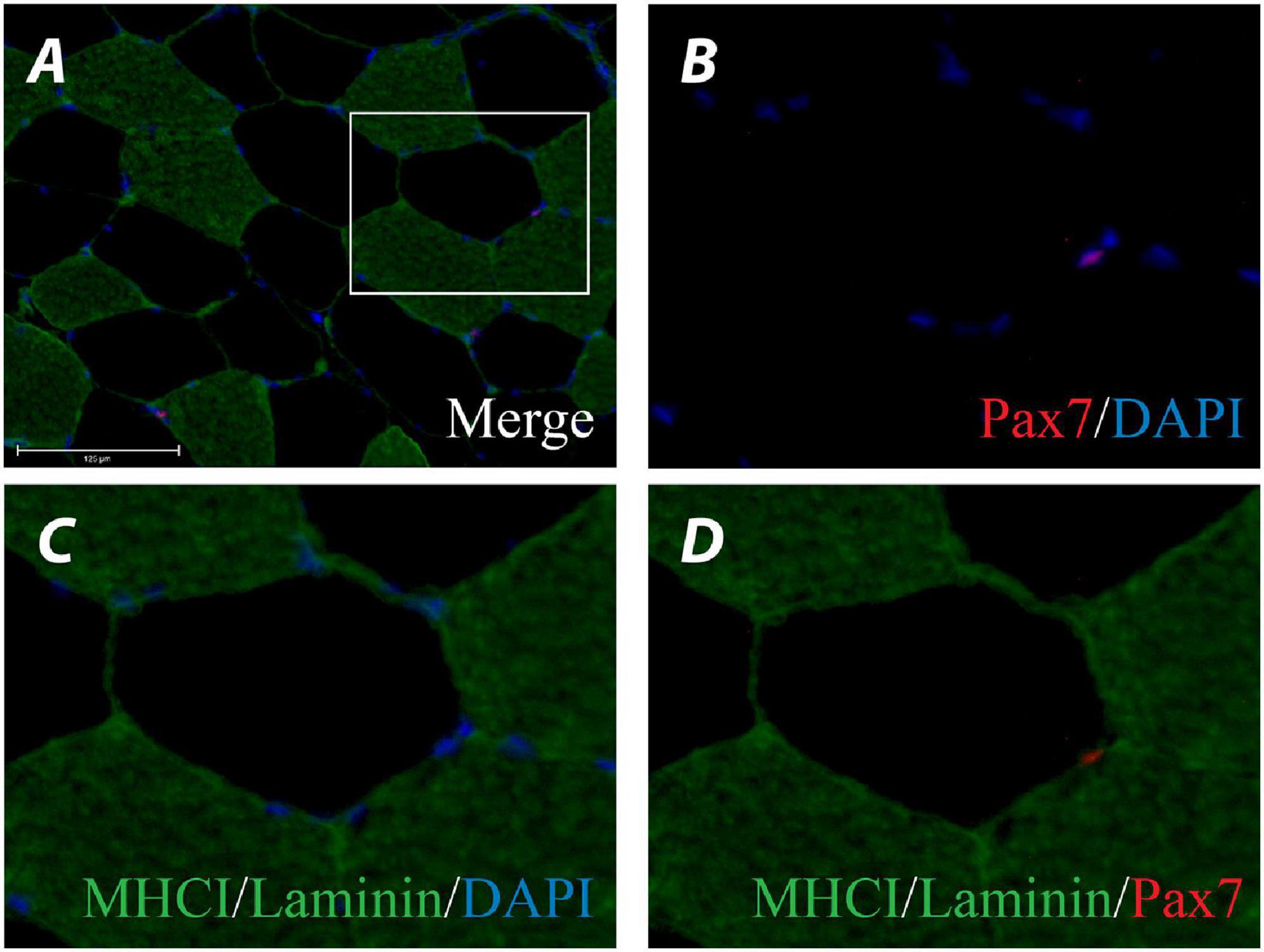
Figure 4. Representative image of a myosin heavy chain (MHC) I/Laminin/Pax7/DAPI stain of a muscle cross-section (A). Channel view of Pax7/DAPI (B), MHCI/Laminin/DAPI (C), and MHCI/Laminin/Pax7 (D).
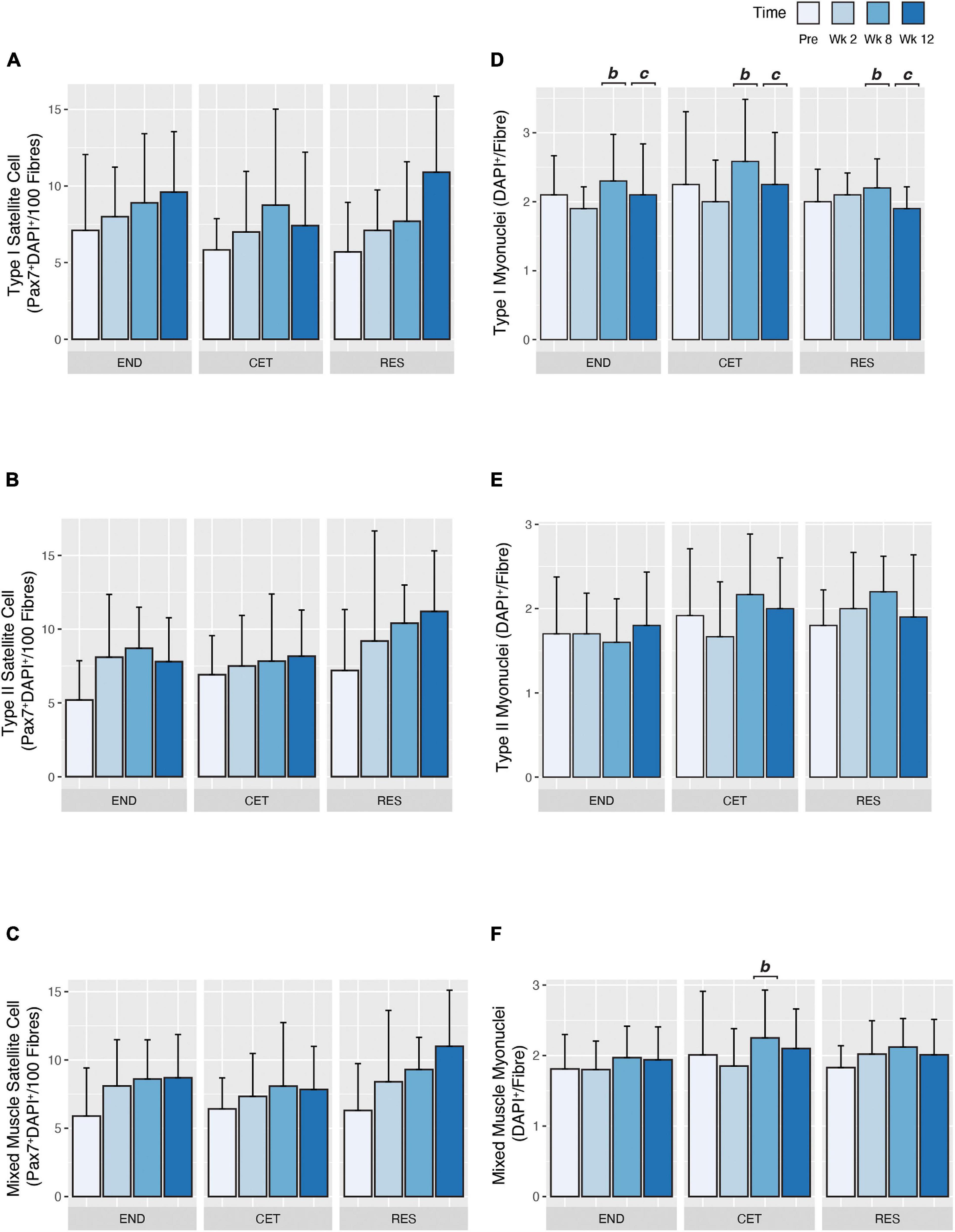
Figure 5. Fibre type-specific satellite cell (A–C) and myonuclear (D–F) expansion in response to endurance (END, n = 10), resistance (RES; n = 10), or concurrent (CET; n = 12) training. Change in myosin heavy chain type I (A), myosin heavy chain type II (B), and mixed (C) myofibre satellite cell content. Change in myosin heavy chain type I (D), myosin heavy chain type II (E), and mixed (F) myofibre myonuclear content. b, significantly different from week 2 time point (P < 0.05); c, significantly different from week 8 time point (P < 0.05). Values are presented as Mean ± SD.
In response to training, there was a main effect of time (P = 0.044), but not condition or condition by time for an increase in Type I myonuclear content. Post hoc analysis revealed that myonuclear content was greater at week 8 compared to week 2 f (P = 0.0031) and week 12 (P = 0.0410; Figure 5D). Conversely, training did not alter Type II myonuclear content as no main effect of condition, time or condition by time was observed (Figure 5E). When the mean myonuclear content of both Type I and II fibres was assessed, a main effect of time (P = 0.0302), and condition by time (P = 0.0350), but not condition was observed. Post hoc analysis revealed mixed fibre myonuclear content was greater at week 8 (2.25 ± 0.679 DAPI+/Fibre) compared to week 2 (1.85 ± 0.532 DAPI+/Fibre; P = 0.0297; Figure 5F).
Despite observing an increase in Type I myonuclear content, no main effect of condition, time or condition by time was seen for Type I myonuclear domain. Conversely, Type II myonuclear domain increased in response to training as a main effect of time (P = 0.0341), but not condition or condition by time. However, when corrected for multiple comparisons, post hoc analysis revealed no significant differences in Type II myonuclear domain with time. Likewise, when the mean myonuclear domain of both Type I and II fibres was assessed, no main effect of condition, time or condition by time was present (data not shown).
There were significant correlations between increases in fibre type-specific myonuclear content and increases in fibre CSA of Type I (r = 0.63, P < 0.0001; Figure 6A), Type II (r = 0.69, P < 0.0001; Figure 6B), and mixed fibres (r = 0.72, P < 0.0001; Figure 6C). Conversely, there was no relationship between pre-intervention fibre type-specific satellite cell content and increases in fibre CSA of Type I (r = −0.073, P = 0.69; Figure 6D), Type II (r = 0.048, P = 0.8; Figure 6E), or mixed fibres (r = −0.08, P = 0.66; Figure 6F). There was no relationship between change in fibre type-specific satellite cell content and increase in fibre CSA for Type I (r = 0.22, P = 0.23), Type II (r = 0.24, P = 0.19), or mixed fibres (r = 0.23, P = 0.22).
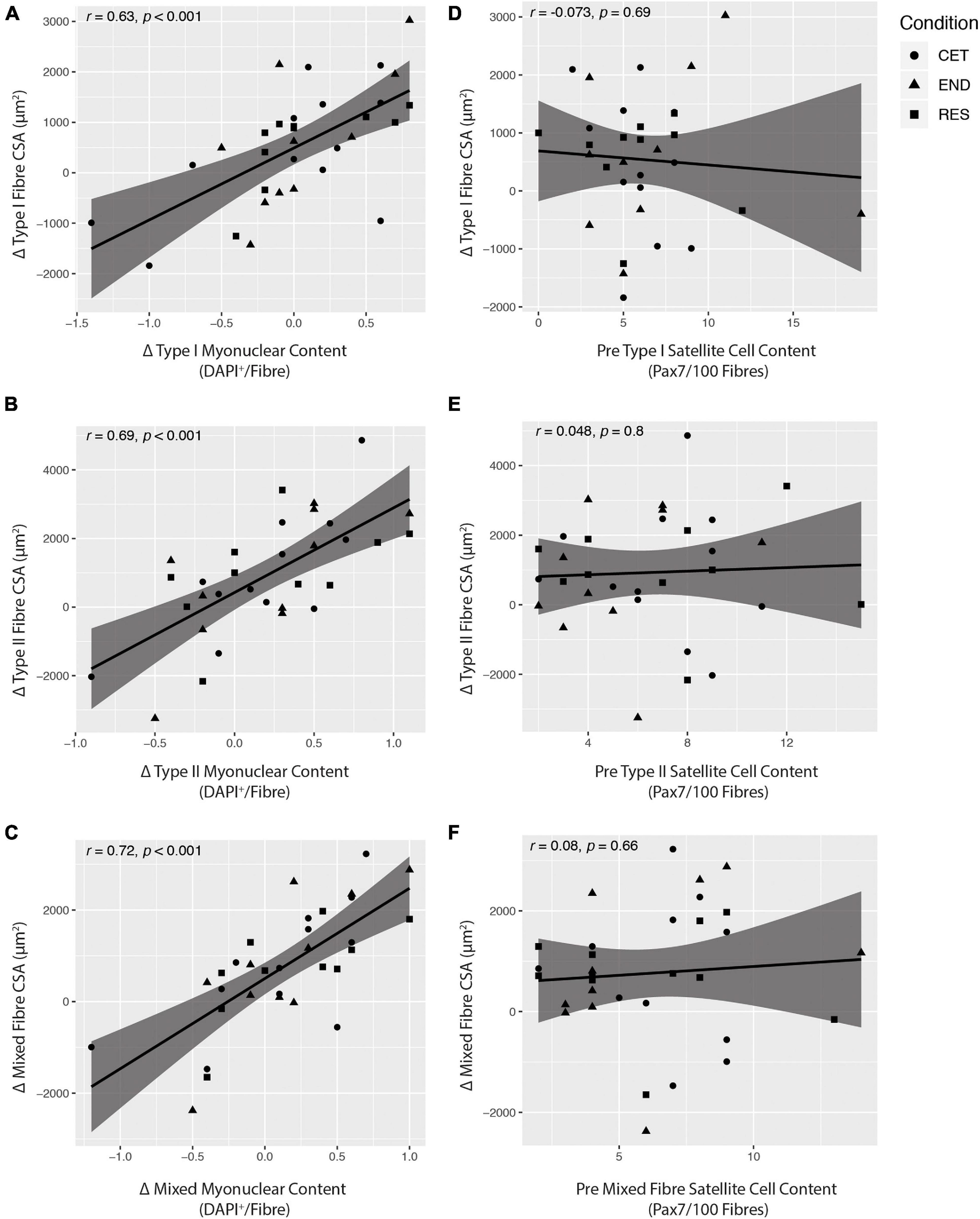
Figure 6. Pearson correlation coefficients (r) showing the relationship between fibre type-specific changes in cross-sectional area (CSA) and myonuclear content (A–C) and fibre type-specific changes in CSA and pre-intervention baseline satellite cell content (D–F). Statistical significance was set at P < 0.05. CET, concurrent training; END, endurance training; RES, resistance training.
The aim of the present investigation was to evaluate fibre type-specific changes in satellite cell abundance are associated with the magnitude of hypertrophy achieved during concurrent training. The present study shows that in response to 12 weeks of endurance, resistance, and concurrent training, Type II and mixed fibre CSA and Type I myonuclear content are increased without a significant change in total satellite cell content, with no differences across training modalities. This is consistent with the changes in lean mass previously reported following the intervention, as the increase in lean mass was similar across CET, RES, and END, despite CET completing a greater overall training volume than RES and END (Shamim et al., 2018a). It has been suggested concurrent exercise training may augment the hypertrophic response to resistance exercise training in some circumstances as aerobic exercise training alone can induce hypertrophy (Murach and Bagley, 2016). However, despite implementing longer between-session recovery and limiting endurance exercise to three sessions per week in the form of cycling in line with recommendations to augment hypertrophy with concurrent training (Murach and Bagley, 2016), no differences in the magnitude of myofibre hypertrophy achieved were observed between modalities. Notably, previous reports of augmented hypertrophy (e.g., increased myofibre CSA) with concurrent training compared to resistance training alone have been observed during shorter (i.e., ≤7 weeks) interventions (Lundberg et al., 2013; Kazior et al., 2016). However, longer training interventions (i.e., ≥20 weeks), have failed to observe any differences in myofibre CSA hypertrophy compared to resistance training (Sale et al., 1990; Häkkinen et al., 2003). While the exact timeline of muscle hypertrophy remains ambiguous (Damas et al., 2015), it is possible that disparities in the magnitude of myofibre hypertrophy observed with concurrent training between previous work (Lundberg et al., 2013; Kazior et al., 2016) and the present study are due to differences in the length of the intervention, training variables (e.g., frequency, intensity, and duration), as well as training and nutritional status of the participants. Nevertheless, while the findings in the present study support the recent recommendations for concurrent exercise training prescription to promote hypertrophy (Murach and Bagley, 2016), they do not appear to facilitate an greater hypertrophic response compared to resistance or endurance training alone over 12 weeks at the myofibre CSA level.
It is unclear why the greater volume of work completed by participants in the concurrent training group did not induce a greater increase in myofibre CSA. It has been hypothesised that the potential for myofibre hypertrophy to occur with chronic concurrent training may be limited by satellite cells (Babcock et al., 2012). Evidence for impaired satellite cell responses with concurrent exercise compared to resistance exercise alone has been observed when a bout of moderate intensity resistance exercise is followed immediately by moderate intensity continuous cycling in young, healthy males (Babcock et al., 2012). However, performing moderate intensity resistance exercise followed immediately by high-intensity interval cycling results in an increase in Type I fibre satellite cell content comparable to resistance exercise alone in sedentary, overweight and obese, middle-aged individuals (Pugh et al., 2018). In the present study, both moderate intensity continuous and high intensity interval cycling was implemented, however, contrary to the initial hypothesis, no significant increase in satellite cell content was detected in response to any training modality.
Activation of fibre type-specific satellite cells has also been shown to be attenuated following concurrent exercise compared to resistance exercise (Pugh et al., 2018). As satellite cell activation can generate both progeny for self-renewal of the satellite cell pool and myogenic precursors to undergo terminal differentiation (Kuang et al., 2007), this blunted response may underlie the limited satellite cell expansion previously observed following concurrent exercise (Babcock et al., 2012; Pugh et al., 2018). Increases in satellite cell activation have been observed as soon as 9 h after a single bout of concurrent exercise in young, healthy males (Snijders et al., 2012), with subsequent increases in satellite cell content peaking ∼72 h post-exercise (Snijders et al., 2015). Given the transient nature of this response, it is possible that the absence of active satellite cells at 96 h after concurrent exercise previously reported (Babcock et al., 2012; Pugh et al., 2018) may be due to post-exercise biopsy timing. In the present study, muscle biopsies were collected 48–72 h post-exercise in order to capture peak increases in satellite cell content. Though satellite cell activation was not directly assessed in the present study, a stable number of satellite cells and increased number of myonuclei was observed after 8 weeks in Type I fibres in all training conditions. This finding suggests that satellite cell activation and proliferation occurred in order to maintain a stable satellite cell pool. Furthermore, training-induced increases in myonuclear content in response to concurrent training were not different from isolated endurance or resistance training and occurred without fibre-type specific hypertrophy. Accordingly, concurrent training does not prevent satellite cell differentiation and myonuclear accretion. Collectively, these observations suggest that chronic concurrent training does not inhibit satellite cell activation or myogenesis.
While differences in exercise intensity, training status of participants, and baseline satellite cell content may underlie disparities in satellite cell expansion previously observed with concurrent exercise (Babcock et al., 2012; Pugh et al., 2018), it is difficult to reconcile why no increase in satellite cell content was observed in response to either resistance or endurance training alone. Given the hypertrophy observed in Type II and mixed fibre CSA in the absence of myonuclear addition, it is possible that the existing myonuclei were able to support the degree of hypertrophy achieved and that a large magnitude of expansion to the satellite cell pool to promote myogenesis was not needed. It has been suggested that myonuclear content may regulate the capacity for myofibre hypertrophy. According to the myonuclear domain theory, a single myonucleus can only provide sufficient transcriptional capacity over a finite amount of cytoplasm (Cheek, 1985; Allen et al., 1999). During periods of extensive myofibre hypertrophy, increases in myofibre CSA are accompanied by an increase in cell volume, which results in a strain on the myonuclear domain (i.e., μm2 fibre area/myonucleus). In turn, myofibre hypertrophy can occur as a result of increasing the size of existing myonuclear domains or by increasing the absolute number of domains within the myofibre (Edgerton and Roy, 1991; Kadi and Thornell, 2000). Contrary to this idea, Kirby et al. (2016) demonstrated that existing myonuclei are able to increase transcriptional capacity to support hypertrophy in the absence of satellite cell-dependent myonuclear accretion. Thus, it is possible that the hypertrophy observed in Type II and mixed fibre CSA may be influenced by enhanced transcriptional responses of existing myonuclei.
It has been hypothesised that myonuclear addition only occurs when the myonuclear domain exceeds an absolute ‘ceiling’ (∼2,250 μm2) (Petrella et al., 2006, 2008), or myofibre hypertrophy exceeds a relative magnitude (∼26%) (Kadi et al., 2004). While the average Type II myonuclear domain (∼4,200 μm2) in the present study exceeds the aforementioned theoretical myonuclear domain ‘ceiling,’ this value is comparable with others previously reported (Karlsen et al., 2015). Additionally, despite a lack of hypertrophy, there was an increase in myonuclear number in Type I fibres observed in the present study, which has recently been demonstrated following 12 weeks of resistance training in older adults (Moro et al., 2020). Collectively, these findings do not support the rationale that hypertrophy must exceed a relative threshold to permit myonuclear addition. Likewise, the hypertrophy observed in Type II fibres occurred without a prior increase in myonuclear domain and demonstrates that changes in myonuclear domain size do not precede myofibre hypertrophy. These findings are supported by previous work demonstrating that myofibre hypertrophy in response to 12 weeks of resistance training occurs without prior changes in myonuclear domain size in young, healthy men (Snijders et al., 2016). In contrast, in the present study, the average Type II and mixed fibre-type hypertrophy observed was ∼15% and ∼13%, respectively, which occurred in the absence of myonuclear addition or significant expansion in the myonuclear domain. Taken in isolation, this observation would be in agreement with the notion that a ∼26% increase in CSA is needed to evoke myonuclear addition (Kadi et al., 2004). However, collectively the changes in myonuclear numeration and domain sizes across fibre-types are equivocal as it pertains to the threshold hypothesis. Indeed, the concept of a universal myonuclear threshold has recently been challenged (Conceição et al., 2018), which is consistent with the equivocal responses found in the current study across fibre-types. These observations therefore highlight that the myonuclear domain does not dictate increases to myonuclear content and does not appear to be a limiting factor in the degree of myofibre hypertrophy achieved with concurrent training.
While there is considerable debate around the notion that satellite cells are required to facilitate overload-induced myofibre hypertrophy (Petrella et al., 2006, 2008; Verdijk et al., 2009, 2014; Bellamy et al., 2014; Karlsen et al., 2015; Dirks et al., 2017; McCarthy et al., 2017; Murach et al., 2017; Reidy et al., 2017b), current evidence indicates that a positive correlation exists between satellite cell-mediated myonuclear accumulation and myofibre hypertrophy (Petrella et al., 2006, 2008; Verdijk et al., 2010, 2014; Bellamy et al., 2014; Reidy et al., 2017b). In accordance, higher baseline satellite cell content has been associated with a greater magnitude of myofibre hypertrophy achieved after a period of resistance training (Petrella et al., 2008). In the current study, there was no relationship between baseline satellite cell content and increases in Type I, Type II, or mixed myofibre CSA. However, a positive correlation was observed for increases in fibre type-specific myonuclear content and increases in myofibre CSA. These observations are consistent with previous works (Petrella et al., 2006, 2008; Verdijk et al., 2010, 2014; Bellamy et al., 2014; Reidy et al., 2017b) demonstrating that increases in myonuclear number are tightly coupled to increases in myofibre CSA. As myonuclear accretion is dependent on the fusogenic capacity of satellite cells (McCarthy et al., 2011; Fry et al., 2014), the present findings suggest that satellite cells directly support enhanced hypertrophy through myonuclear accretion following resistance, endurance, and concurrent exercise training.
Previous work has demonstrated that satellite cell proliferation is dependent on exercise mode (Mackey et al., 2007; Babcock et al., 2012; Nederveen et al., 2015) and contraction type (Farup et al., 2014), with resistance-type exercise contractions stimulating the greatest response (Snijders et al., 2015). It is therefore unclear why exercise modality did not influence satellite cell proliferation in the current study. One possible explanation is the finding that the relative intensity of exercise is also critical factor in provoking a satellite cell response (Nederveen et al., 2015; McKenzie et al., 2016). Given the recreationally active training status of the participants, it is possible that the training protocols were not strenuous enough to elicit a differential satellite cell response between training modalities. Recent evidence has shown that exercise promotes myogenesis in a load-dependent manner in mice (Masschelein et al., 2020), suggesting that higher loads performed during exercise may also illicit greater satellite cell responses in human skeletal muscle. Likewise, as resistance exercise was not performed to volitional muscle failure in the present study, the relative intensity of training may not have been sufficient to maximally stimulate muscle hypertrophy (Mitchell et al., 2012), but, instead, only produced modest hypertrophy comparable to endurance cycle training. While expansions in the satellite cell pool have been observed in the absence of hypertrophy (Joanisse et al., 2013), whether or not a greater degree of myofibre hypertrophy would have promoted an increased demand for satellite cell proliferation to support myogenesis remains unresolved. Thus, future studies evaluating relative load and exercise intensity (i.e., training to volitional muscle failure) of concurrent training are needed to fully appreciate the contribution of modulating training variables (such as load or intensity) on satellite cell responses.
We acknowledge there are several limitations in the present study. As the present study is a follow up analysis to a study investigating changes in lean body mass to concurrent exercise training (Shamim et al., 2018a) and the first to employ a chronic, parallel groups design incorporating resistance, endurance, and concurrent exercise training, it is possible that the current sample size may not be large enough to detect modest changes to sensitive variables, such as satellite cells. However, to the best of our knowledge, no chronic training study has utilised satellite cell enumeration as a primary outcome. It is therefore difficult to accurately calculate the minimum required sample size in order to be sufficiently powered to detect significantly meaningful changes in this outcome. Similarly, due to the relatively small sample size analysed in the current study, large variations in changes to fibre CSA may have masked the detection of significant differences in the magnitude of muscle hypertrophy achieved between training conditions. Next, biopsies were collected 48 h after the last bout of endurance exercise in the CET condition and 72 h after the last bout of exercise in RES and END. As the number of satellite cells has been shown to peak ∼72 h after exercise (Snijders et al., 2015), consideration must be given to discrepancies in biopsy sampling time between CET and END conditions. Thus, whether CET alters satellite cell and/or myonuclear content 72 h after endurance exercise in cannot be distinguished with the selected biopsy sampling time. Likewise, as no pre-exercise biopsies were collected during weeks 2, 8, or 12, it is not possible to determine whether training altered satellite cell activation. It has recently been shown that chronic resistance training enhances the activation of satellite cells in response to an acute bout of exercise (Nederveen et al., 2017). Thus, in the present study only static measures of satellite cell abundance were assessed, making it difficult to determine specific satellite cell responses to exercise. Future investigations assessing markers of satellite cell activation (i.e., MyoD, Myf5, and Myogenin) are required to understand if chronic concurrent training alters satellite cell activation in response to an acute exercise stimulus. Likewise, previous training history may alter satellite cell activation and myonuclear accretion in response to exercise stimuli (Nederveen et al., 2017). While myonuclear density does not appear to be sustained after periods of detraining (Dungan et al., 2019), it is possible that epigenetic modifications to within myonuclei may contribute to growth responses to future exercise training (Seaborne et al., 2018). Thus, future investigations examining long-term training history will be prudent for understanding mechanisms that mediate satellite cell-dependent hypertrophy.
This is the first investigation to assess changes in satellite cell and myonuclear content following a period of chronic concurrent exercise training compared to isolated resistance and endurance training. The findings demonstrate that resistance, endurance, and concurrent training induce myofibre hypertrophy in the absence of significant expansion to the satellite cell, however, myonuclear accretion via satellite cell fusion is positively correlated with hypertrophy after 12 weeks of exercise training. Despite clear differences between training modalities with regards to physiological adaptations (i.e., one-repetition maximum strength, maximum anaerobic power, and VO2peak) (Shamim et al., 2018a), implementing the current recommended strategies to maximise hypertrophic potential with chronic concurrent training do not result in greater myofibre hypertrophy, satellite cell pool expansion, or myonuclear accretion compared to endurance or resistance training alone. Likewise, myonuclear domain size remains stable throughout chronic endurance, resistance, and concurrent training, and, as such, does not appear to be a critical mediator in myonuclear accretion or limit the degree of hypertrophy achieved with concurrent training. The current data suggest that changes in myonuclear content are not prerequisite to changes in myofibre hypertrophy but do appear to be associated with the magnitude of myofibre hypertrophy achieved in young, healthy males.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved the Australian Catholic University Human Research Ethics Committee (2016-54H). The patients/participants provided their written informed consent to participate in this study.
BS and DC conceptualised and designed the study. BS performed the experiments and analysed the data. BS and JW interpreted the results and drafted and edited the manuscript. All authors approved the final version of the manuscript.
BS was supported by an Australian Government Research Training Program (RTP). This work was supported by an Australian Catholic University Research Fund Grant awarded to DC (#36331). JW was also supported by a Natural Sciences and Engineering Research Council of Canada (NSERC) Postdoctoral Fellowship.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Allen, D. L., Roy, R. R., and Edgerton, V. R. (1999). Myonuclear domains in muscle adaptation and disease. Muscle Nerve 22, 1350–1360. doi: 10.1002/(sici)1097-4598(199910)22:10<1350::aid-mus3>3.0.co;2-8
Baar, K. (2014). Using Molecular Biology to Maximize Concurrent Training. Sports Med Auckl Nz 44, 117–125. doi: 10.1007/s40279-014-0252-0
Babcock, L., Escano, M., D’Lugos, A., Todd, K., Murach, K., and Luden, N. (2012). Concurrent aerobic exercise interferes with the satellite cell response to acute resistance exercise. AJP Regul Integr Comp Physiol 302, R1458–R1465.
Bell, G. J., Syrotuik, D., Martin, T. P., Burnham, R., and Quinney, H. A. (2000). Effect of concurrent strength and endurance training on skeletal muscle properties and hormone concentrations in humans. Eur J Appl Physiol 81, 418–427. doi: 10.1007/s004210050063
Bellamy, L. M., Joanisse, S., Grubb, A., Mitchell, C. J., McKay, B. R., Phillips, S. M., et al. (2014). The Acute Satellite Cell Response and Skeletal Muscle Hypertrophy following Resistance Training ed. Asakura A. PLoS ONE 9:e109739. doi: 10.1371/journal.pone.0109739
Berryman, N., Mujika, I., and Bosquet, L. (2018). Concurrent Training for Sports Performance: The Two Sides of the Medal. Int J Sports Physiol Perform. 14, 1–22.
Charifi, N., Kadi, F., Féasson, L., and Denis, C. (2003). Effects of endurance training on satellite cell frequency in skeletal muscle of old men. Muscle Nerve 28, 87–92. doi: 10.1002/mus.10394
Cheek, D. B. (1985). The control of cell mass and replication. The DNA unit - a personal 20-year study. Early Hum Dev 12, 211–239. doi: 10.1016/0378-3782(85)90144-6
Coffey, V. G., and Hawley, J. A. (2017). Concurrent exercise training: do opposites distract? J Physiol 595, 2883–2896. doi: 10.1113/jp272270
Conceição, M. S., Vechin, F. C., Lixandrão, M., Damas, F., Libardi, C. A., Tricoli, V., et al. (2018). Muscle Fiber Hypertrophy and Myonuclei Addition: A Systematic Review and Meta-analysis. Med Sci Sports Exerc 50, 1385–1393. doi: 10.1249/mss.0000000000001593
Craig, B. W., Lucas, J., Pohlman, R., and Stelling, H. (1991). The Effects of Running, Weightlifting and a Combination of Both on Growth Hormone Release. J Strength Cond Res. 5, 198–203. doi: 10.1519/00124278-199111000-00005
Crameri, R. M., Aagaard, P., Qvortrup, K., Langberg, H., Olesen, J., and Kjær, M. (2007). Myofibre damage in human skeletal muscle: effects of electrical stimulation versus voluntary contraction. J Physiol 583, 365–380. doi: 10.1113/jphysiol.2007.128827
Crameri, R. M., Langberg, H., Magnusson, P., Jensen, C. H., Schrøder, H. D., Olesen, J. L., et al. (2004). Changes in satellite cells in human skeletal muscle after a single bout of high intensity exercise. J Physiol 558, 333–340. doi: 10.1113/jphysiol.2004.061846
Damas, F., Libardi, C. A., Ugrinowitsch, C., Vechin, F. C., Lixandrão, M. E., Snijders, T., et al. (2018). Early- and later-phases satellite cell responses and myonuclear content with resistance training in young men. PLoS One 13:e0191039. doi: 10.1371/journal.pone.0191039
Damas, F., Phillips, S. M., Lixandrão, M. E., Vechin, F. C., Libardi, C. A., Roschel, H., et al. (2015). Early resistance training-induced increases in muscle cross-sectional area are concomitant with edema-induced muscle swelling. Eur J Appl Physiol 116, 49–56. doi: 10.1007/s00421-015-3243-4
Dirks, M. L., Tieland, M., Verdijk, L. B., Losen, M., Nilwik, R., Mensink, M., et al. (2017). Protein Supplementation Augments Muscle Fiber Hypertrophy but Does Not Modulate Satellite Cell Content During Prolonged Resistance-Type Exercise Training in Frail Elderly. J Am Med Dir Assoc 18, 608–615. doi: 10.1016/j.jamda.2017.02.006
Dolezal, B. A., and Potteiger, J. A. (1998). Concurrent resistance and endurance training influence basal metabolic rate in nondieting individuals. J Appl Physiol 85, 695–700. doi: 10.1152/jappl.1998.85.2.695
Doma, K., Deakin, G. B., and Bentley, D. J. (2017). Implications of Impaired Endurance Performance following Single Bouts of Resistance Training: An Alternate Concurrent Training Perspective. Sports Med. 47, 2187–2200. doi: 10.1007/s40279-017-0758-3
Dungan, C. M., Murach, K. A., Frick, K. K., Jones, S. R., Crow, S. E., Englund, D. A., et al. (2019). Elevated myonuclear density during skeletal muscle hypertrophy in response to training is reversed during detraining. Am J Physiol Cell Physiol 316, C649–C654.
Eddens, L., van Someren, K., and Howatson, G. (2018). The Role of Intra-Session Exercise Sequence in the Interference Effect: A Systematic Review with Meta-Analysis. Sports Med Auckl NZ 48, 177–188. doi: 10.1007/s40279-017-0784-1
Edgerton, V. R., and Roy, R. R. (1991). Regulation of skeletal muscle fiber size, shape and function. J Biomech 24, (Suppl. 1), 123–133. doi: 10.1016/0021-9290(91)90383-x
Farup, J., Rahbek, S. K., Riis, S., Vendelbo, M. H., Paoli, F. D., and Vissing, K. (2014). Influence of exercise contraction mode and protein supplementation on human skeletal muscle satellite cell content and muscle fiber growth. J Appl Physiol 117, 898–909. doi: 10.1152/japplphysiol.00261.2014
Fry, C. S., Lee, J. D., Jackson, J. R., Kirby, T. J., Stasko, S. A., Liu, H., et al. (2014). Regulation of the muscle fiber microenvironment by activated satellite cells during hypertrophy. FASEB J 28, 1654–1665. doi: 10.1096/fj.13-239426
Fyfe, J. J., Bartlett, J. D., Hanson, E. D., Stepto, N. K., and Bishop, D. J. (2016). Endurance Training Intensity Does Not Mediate Interference to Maximal Lower-Body Strength Gain during Short-Term Concurrent Training. Front. Physiol 7:487.
Fyfe, J. J., Bishop, D. J., Bartlett, J. D., Hanson, E. D., Anderson, M. J., Garnham, A. P., et al. (2018). Enhanced skeletal muscle ribosome biogenesis, yet attenuated mTORC1 and ribosome biogenesis-related signalling, following short-term concurrent versus single-mode resistance training. Sci Rep 8, 560.
Fyfe, J. J., Bishop, D. J., and Stepto, N. K. (2014). Interference between Concurrent Resistance and Endurance Exercise: Molecular Bases and the Role of Individual Training Variables. Sports Med 44, 743–762. doi: 10.1007/s40279-014-0162-1
Fyfe, J. J., and Loenneke, J. P. (2018). Interpreting Adaptation to Concurrent Compared with Single-Mode Exercise Training: Some Methodological Considerations. Sports Med 48, 289–297. doi: 10.1007/s40279-017-0812-1
Häkkinen, K., Alen, M., Kraemer, W. J., Gorostiaga, E., Izquierdo, M., Rusko, H., et al. (2003). Neuromuscular adaptations during concurrent strength and endurance training versus strength training. Eur J Appl Physiol 89, 42–52. doi: 10.1007/s00421-002-0751-9
Hamilton, D. L., and Philp, A. (2013). Can AMPK mediated suppression of mTORC1 explain the concurrent training effect? Cell Mol Exerc Physiol 2, e4.
Hennessy, L. C., and Watson, A. W. S. (1994). The Interference Effects of Training for Strength and Endurance Simultaneously. J Strength Cond Res 8, 12. doi: 10.1519/00124278-199402000-00003
Hickson, R. C. (1980). Interference of strength development by simultaneously training for strength and endurance. Eur J Appl Physiol 45, 255–263. doi: 10.1007/bf00421333
Hughes, D. C., Ellefsen, S., and Baar, K. (2018). Adaptations to Endurance and Strength Training. Cold Spring Harb Perspect Med 8, a029769.
Joanisse, S., Gillen, J. B., Bellamy, L. M., McKay, B. R., Tarnopolsky, M. A., Gibala, M. J., et al. (2013). Evidence for the contribution of muscle stem cells to nonhypertrophic skeletal muscle remodeling in humans. FASEB J 27, 4596–4605. doi: 10.1096/fj.13-229799
Kadi, F., Charifi, N., Denis, C., Lexell, J., Andersen, J. L., Schjerling, P., et al. (2005). The behaviour of satellite cells in response to exercise: what have we learned from human studies? Pflüg Arch - Eur J Physiol 451, 319–327. doi: 10.1007/s00424-005-1406-6
Kadi, F., Schjerling, P., Andersen, L. L., Charifi, N., Madsen, J. L., Christensen, L. R., et al. (2004). The effects of heavy resistance training and detraining on satellite cells in human skeletal muscles. J Physiol 558, 1005–1012. doi: 10.1113/jphysiol.2004.065904
Kadi, F., and Thornell, L.-E. (2000). Concomitant increases in myonuclear and satellite cell content in female trapezius muscle following strength training. Histochem Cell Biol 113, 99–103. doi: 10.1007/s004180050012
Karlsen, A., Couppé, C., Andersen, J. L., Mikkelsen, U. R., Nielsen, R. H., Magnusson, S. P., et al. (2015). Matters of fiber size and myonuclear domain: Does size matter more than age? Muscle Nerve 52, 1040–1046. doi: 10.1002/mus.24669
Kazior, Z., Willis, S. J., Moberg, M., Apró, W., Calbet, J. A. L., Holmberg, H.-C., et al. (2016). Endurance Exercise Enhances the Effect of Strength Training on Muscle Fiber Size and Protein Expression of Akt and mTOR. PLoS One 11:e0149082. doi: 10.1371/journal.pone.0149082
Kirby, T. J., Patel, R. M., McClintock, T. S., Dupont-Versteegden, E. E., Peterson, C. A., and McCarthy, J. J. (2016). Myonuclear transcription is responsive to mechanical load and DNA content but uncoupled from cell size during hypertrophy. Mol Biol Cell 27, 788–798. doi: 10.1091/mbc.e15-08-0585
Kraemer, W. J., Patton, J. F., Gordon, S. E., Harman, E. A., Deschenes, M. R., Reynolds, K., et al. (1995). Compatibility of high-intensity strength and endurance training on hormonal and skeletal muscle adaptations. J Appl Physiol. 78, 976–989. doi: 10.1152/jappl.1995.78.3.976
Kuang, S., Kuroda, K., Le Grand, F., and Rudnicki, M. A. (2007). Asymmetric Self-Renewal and Commitment of Satellite Stem Cells in Muscle. Cell 129, 999–1010. doi: 10.1016/j.cell.2007.03.044
Lundberg, T. R., Fernandez-Gonzalo, R., Gustafsson, T., and Tesch, P. A. (2013). Aerobic exercise does not compromise muscle hypertrophy response to short-term resistance training. J Appl Physiol 114, 81–89. doi: 10.1152/japplphysiol.01013.2012
Lundberg, T. R., Fernandez-Gonzalo, R., and Tesch, P. A. (2014). Exercise-induced AMPK activation does not interfere with muscle hypertrophy in response to resistance training in men. J Appl Physiol 116, 611–620. doi: 10.1152/japplphysiol.01082.2013
Mackey, A. L., Kjaer, M., Dandanell, S., Mikkelsen, K. H., Holm, L., Dossing, S., et al. (2007). The influence of anti-inflammatory medication on exercise-induced myogenic precursor cell responses in humans. J Appl Physiol 103, 425–431. doi: 10.1152/japplphysiol.00157.2007
Macnaughton, L. S., Wardle, S. L., Witard, O. C., McGlory, C., Hamilton, D. L., Jeromson, S., et al. (2016). The response of muscle protein synthesis following whole-body resistance exercise is greater following 40 g than 20 g of ingested whey protein. Physiol Rep 4, e12893. doi: 10.14814/phy2.12893
Masschelein, E., D’Hulst, G., Zvick, J., Hinte, L., Soro-Arnaiz, I., Gorski, T., et al. (2020). Exercise promotes satellite cell contribution to myofibers in a load-dependent manner. Skelet Muscle 10, 21. doi: 10.1186/s13395-020-00237-2
Mauro, A. (1961). Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol 9, 493–495. doi: 10.1083/jcb.9.2.493
McCarthy, J. J., Dupont-Versteegden, E. E., Fry, C. S., Murach, K. A., and Peterson, C. A. (2017). Methodological issues limit interpretation of negative effects of satellite cell depletion on adult muscle hypertrophy. Development 144, 1363–1365. doi: 10.1242/dev.145797
McCarthy, J. J., Mula, J., Miyazaki, M., Erfani, R., Garrison, K., Farooqui, A. B., et al. (2011). Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development 138, 3657–3666. doi: 10.1242/dev.068858
McKenzie, A. I., D’Lugos, A. C., Saunders, M. J., Gworek, K. D., and Luden, N. D. (2016). Fiber Type-Specific Satellite Cell Content in Cyclists Following Heavy Training with Carbohydrate and Carbohydrate-Protein Supplementation. Front Physiol 7:550.
Mikkola, J., Rusko, H., Izquierdo, M., Gorostiaga, E., and Häkkinen, K. (2012). Neuromuscular and Cardiovascular Adaptations During Concurrent Strength and Endurance Training in Untrained Men. Int J Sports Med 33, 702–710. doi: 10.1055/s-0031-1295475
Mitchell, C. J., Churchward-Venne, T. A., West, D. W. D., Burd, N. A., Breen, L., Baker, S. K., et al. (2012). Resistance exercise load does not determine training-mediated hypertrophic gains in young men. J Appl Physiol 113, 71–77. doi: 10.1152/japplphysiol.00307.2012
Moore, D. R., Kelly, R. P., Devries, M. C., Churchward-Venne, T. A., Phillips, S. M., Parise, G., et al. (2018). Low-load resistance exercise during inactivity is associated with greater fibre area and satellite cell expression in older skeletal muscle. J. Cachexia Sarcopenia Muscle 9, 747–754. doi: 10.1002/jcsm.12306
Moro, T., Brightwell, C. R., Volpi, E., Rasmussen, B. B., and Fry, C. S. (2020). Resistance exercise training promotes fiber type-specific myonuclear adaptations in older adults. J Appl Physiol. 128, 795–804. doi: 10.1152/japplphysiol.00723.2019
Murach, K. A., and Bagley, J. R. (2016). Skeletal Muscle Hypertrophy with Concurrent Exercise Training: Contrary Evidence for an Interference Effect. Sports Med Auckl NZ 46, 1029–1039. doi: 10.1007/s40279-016-0496-y
Murach, K. A., Walton, R. G., Fry, C. S., Michaelis, S. L., Groshong, J. S., Finlin, B. S., et al. (2016). Cycle training modulates satellite cell and transcriptional responses to a bout of resistance exercise. Physiol Rep 4, e12973. doi: 10.14814/phy2.12973
Murach, K. A., White, S. H., Wen, Y., Ho, A., Dupont-Versteegden, E. E., McCarthy, J. J., et al. (2017). Differential requirement for satellite cells during overload-induced muscle hypertrophy in growing versus mature mice. Skelet Muscle 7, 14.
Nader, G. A. (2006). Concurrent strength and endurance training: from molecules to man. Med Sci Sports Exerc 38, 1965–1970. doi: 10.1249/01.mss.0000233795.39282.33
Nederveen, J. P., Joanisse, S., Séguin, C. M. L., Bell, K. E., Baker, S. K., Phillips, S. M., et al. (2015). The effect of exercise mode on the acute response of satellite cells in old men. Acta Physiol 215, 177–190. doi: 10.1111/apha.12601
Nederveen, J. P., Snijders, T., Joanisse, S., Wavell, C. G., Mitchell, C. J., Johnston, L. M., et al. (2017). Altered muscle satellite cell activation following 16 wk of resistance training in young men. Am J Physiol - Regul Integr Comp Physiol 312, R85–R92.
Perez-Schindler, J., Hamilton, D. L., Moore, D. R., Baar, K., and Philp, A. (2015). Nutritional strategies to support concurrent training. Eur J Sport Sci 15, 41–52. doi: 10.1080/17461391.2014.950345
Petrella, J. K., Kim, J., Cross, J. M., Kosek, D. J., and Bamman, M. M. (2006). Efficacy of myonuclear addition may explain differential myofiber growth among resistance-trained young and older men and women. Am J Physiol - Endocrinol Metab 291, E937–E946.
Petrella, J. K., Kim, J., Mayhew, D. L., Cross, J. M., and Bamman, M. M. (2008). Potent myofiber hypertrophy during resistance training in humans is associated with satellite cell-mediated myonuclear addition: a cluster analysis. J Appl Physiol 104, 1736–1742. doi: 10.1152/japplphysiol.01215.2007
Pugh, J. K., Faulkner, S. H., Turner, M. C., and Nimmo, M. A. (2018). Satellite cell response to concurrent resistance exercise and high-intensity interval training in sedentary, overweight/obese, middle-aged individuals. Eur J Appl Physiol 118, 225–238. doi: 10.1007/s00421-017-3721-y
Reidy, P. T., Fry, C. S., Dickinson, J. M., Drummond, M. J., and Rasmussen, B. B. (2017a). Postexercise essential amino acid supplementation amplifies skeletal muscle satellite cell proliferation in older men 24 hours postexercise. Physiol Rep 5, e13269. doi: 10.14814/phy2.13269
Reidy, P. T., Fry, C. S., Igbinigie, S., Deer, R. R., Jennings, K., Cope, M. B., et al. (2017b). Protein Supplementation Does Not Affect Myogenic Adaptations to Resistance Training. Med Sci Sports Exerc 49, 1197–1208. doi: 10.1249/MSS.0000000000001224
Rønnestad, B. R., Hansen, E. A., and Raastad, T. (2012). High volume of endurance training impairs adaptations to 12 weeks of strength training in well-trained endurance athletes. Eur J Appl Physiol 112, 1457–1466. doi: 10.1007/s00421-011-2112-z
Sale, D. G., Jacobs, I., MacDougall, J. D., and Garner, S. (1990). Comparison of two regimens of concurrent strength and endurance training. Med Sci Sports Exerc 22, 348–356.
Seaborne, R. A., Strauss, J., Cocks, M., Shepherd, S., O’Brien, T. D., Someren, K. A., et al. (2018). Human Skeletal Muscle Possesses an Epigenetic Memory of Hypertrophy. Sci Rep 8, 1898.
Shamim, B., Devlin, B. L., Timmins, R. G., Tofari, P., Lee Dow, C., Coffey, V. G., et al. (2018a). Adaptations to Concurrent Training in Combination with High Protein Availability: A Comparative Trial in Healthy, Recreationally Active Men. Sports Med 48, 2869–2883. doi: 10.1007/s40279-018-0999-9
Shamim, B., Hawley, J. A., and Camera, D. M. (2018b). Protein Availability and Satellite Cell Dynamics in Skeletal Muscle. Sports Med 48, 1329–1343. doi: 10.1007/s40279-018-0883-7
Snijders, T., Nederveen, J. P., McKay, B. R., Joanisse, S., Verdijk, L. B., van Loon, L. J. C., et al. (2015). Satellite cells in human skeletal muscle plasticity. Front Physiol 6:283.
Snijders, T., Smeets, J. S. J., van Kranenburg, J., Kies, A. K., van Loon, L. J. C., and Verdijk, L. B. (2016). Changes in myonuclear domain size do not precede muscle hypertrophy during prolonged resistance-type exercise training. Acta Physiol 216, 231–239. doi: 10.1111/apha.12609
Snijders, T., Verdijk, L. B., Beelen, M., McKay, B. R., Parise, G., Kadi, F., et al. (2012). A single bout of exercise activates skeletal muscle satellite cells during subsequent overnight recovery. Exp Physiol 97, 762–773. doi: 10.1113/expphysiol.2011.063313
Snijders, T., Verdijk, L. B., Hansen, D., Dendale, P., and van Loon, L. J. C. (2011). Continuous endurance-type exercise training does not modulate satellite cell content in obese type 2 diabetes patients. Muscle Nerve 43, 393–401. doi: 10.1002/mus.21891
Snijders, T., Verdijk, L. B., Smeets, J. S. J., McKay, B. R., Senden, J. M. G., Hartgens, F., et al. (2014). The skeletal muscle satellite cell response to a single bout of resistance-type exercise is delayed with aging in men. Age Dordr Neth 36, 9699.
de Souza, E. O., Tricoli, V., Roschel, H., Brum, P. C., Bacurau, A. V. N., Ferreira, J. C. B., et al. (2013). Molecular adaptations to concurrent training. Int J Sports Med 34, 207–213.
Spiliopoulou, P., Zaras, N., Methenitis, S., Papadimas, G., Papadopoulos, C., Bogdanis, G. C., et al. (2019). Effect of Concurrent Power Training and High-Intensity Interval Cycling on Muscle Morphology and Performance. J Strength Cond Res doi: 10.1519/JSC.0000000000003172 Online ahead of print.
Timmins, R. G., Shamim, B., Tofari, P. J., Hickey, J. T., and Camera, D. M. (2020). Differences in Lower Limb Strength and Structure After 12 Weeks of Resistance, Endurance, and Concurrent Training. Int J Sports Physiol Perform 1, 1–8.
Timmons, J. F., Minnock, D., Hone, M., Cogan, K. E., Murphy, J. C., and Egan, B. (2018). Comparison of time-matched aerobic, resistance, or concurrent exercise training in older adults. Scand J Med Sci Sports 28, 2272–2283. doi: 10.1111/sms.13254
Tomiya, S., Kikuchi, N., and Nakazato, K. (2017). Moderate Intensity Cycling Exercise after Upper Extremity Resistance Training Interferes Response to Muscle Hypertrophy but Not Strength Gains. J Sports Sci Med 16, 391–395.
Varela-Sanz, A., Tuimil, J. L., Abreu, L., and Boullosa, D. A. (2016). Does concurrent training intensity distribution matter? J Strength Cond Res Natl Strength Cond Assoc. 31, 181–195. doi: 10.1519/JSC.0000000000001474
Verdijk, L. B., Gleeson, B. G., Jonkers, R. A. M., Meijer, K., Savelberg, H. H. C. M., Dendale, P., et al. (2009). Skeletal Muscle Hypertrophy Following Resistance Training Is Accompanied by a Fiber Type-Specific Increase in Satellite Cell Content in Elderly Men. J Gerontol A Biol Sci Med Sci 64A, 332–339. doi: 10.1093/gerona/gln050
Verdijk, L. B., Snijders, T., Beelen, M., Savelberg, H. H. C. M., Meijer, K., Kuipers, H., et al. (2010). Characteristics of Muscle Fiber Type Are Predictive of Skeletal Muscle Mass and Strength in Elderly Men. J Am Geriatr Soc 58, 2069–2075. doi: 10.1111/j.1532-5415.2010.03150.x
Verdijk, L. B., Snijders, T., Drost, M., Delhaas, T., Kadi, F., and van Loon, L. J. C. (2014). Satellite cells in human skeletal muscle; from birth to old age. AGE 36, 545–557.
Verney, J., Kadi, F., Charifi, N., Féasson, L., Saafi, M. A., Castells, J., et al. (2008). Effects of combined lower body endurance and upper body resistance training on the satellite cell pool in elderly subjects. Muscle Nerve 38, 1147–1154. doi: 10.1002/mus.21054
Keywords: concurrent exercise, resistance exercise, endurance exercise, skeletal muscle, satellite cells
Citation: Shamim B, Camera DM and Whitfield J (2021) Myofibre Hypertrophy in the Absence of Changes to Satellite Cell Content Following Concurrent Exercise Training in Young Healthy Men. Front. Physiol. 12:625044. doi: 10.3389/fphys.2021.625044
Received: 02 November 2020; Accepted: 11 May 2021;
Published: 04 June 2021.
Edited by:
David Cameron-Smith, Singapore Institute for Clinical Sciences (A∗STAR), SingaporeReviewed by:
Christopher S. Fry, University of Kentucky, United StatesCopyright © 2021 Shamim, Camera and Whitfield. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jamie Whitfield, SmFtaWUuV2hpdGZpZWxkQGFjdS5lZHUuYXU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.