- 1Scripps Institution of Oceanography, University of California, San Diego, La Jolla, CA, United States
- 2Division of Pulmonary, Critical Care and Sleep Medicine, Department of Medicine, University of California, San Diego, La Jolla, CA, United States
- 3GAIA Antarctic Research Center, University of Magallanes, Punta Arenas, Chile
- 4Niels Bohr Institute, University of Copenhagen, Copenhagen, Denmark
- 5Department of Physiology, Anatomy, and Genetics, University of Oxford, Oxford, United Kingdom
Concern is often voiced over the ongoing loss of atmospheric O2. This loss, which is caused by fossil-fuel burning but also influenced by other processes, is likely to continue at least for the next few centuries. We argue that this loss is quite well understood, and the eventual decrease is bounded by the fossil-fuel resource base. Because the atmospheric O2 reservoir is so large, the predicted relative drop in O2 is very small even for extreme scenarios of future fossil-fuel usage which produce increases in atmospheric CO2 sufficient to cause catastrophic climate changes. At sea level, the ultimate drop in oxygen partial pressure will be less than 2.5 mm Hg out of a baseline of 159 mmHg. The drop by year 2300 is likely to be between 0.5 and 1.3 mmHg. The implications for normal human health is negligible because respiratory O2 consumption in healthy individuals is only weakly dependent on ambient partial pressure, especially at sea level. The impacts on top athlete performance, on disease, on reproduction, and on cognition, will also be very small. For people living at higher elevations, the implications of this loss will be even smaller, because of a counteracting increase in barometric pressure at higher elevations due to global warming.
Introduction
Direct observations since 1989 confirm that the atmospheric O2 abundance has been decreasing steadily year by year (Keeling and Manning, 2014). The O2 loss is the flip side of the CO2 buildup from fossil-fuel burning, and is expected to continue throughout the fossil-fuel era. The CO2 buildup is a major environmental concern, with consequences for global climate via the “greenhouse effect,” for land plants via “CO2 fertilization,” and for marine organisms via “ocean acidification” (Ciais et al., 2013). In comparison to the CO2 buildup, the O2 loss is very small in relative terms. CO2 has now risen from a preindustrial level of ∼277 ppm to a level of 410 ppm in year 2020 (Friedlingstein et al., 2020). The measured O2 loss has been of comparable magnitude in moles, but this dwarfed by the massive atmospheric store of O2, which comprises 21% of air. Still, the fact that O2 is measurably in decline raises concerns. Considering that O2 is essential for aerobic life, how sure are we that the continuing O2 decline won’t eventually have significant impacts?
We are aware of two prior reviews of this topic. The first, by Broecker (1970), makes a compelling case that the projected future O2 changes would be very small and likely insignificant. The second, by Martin et al. (2017), uses projections of much larger future O2 loss based on a parabolic model of Livina et al. (2015). Martin et al. (2017) systematically considered the major factors determining the potential impact of atmospheric oxygen (O2) depletion on human survival. They discussed the different time domains of effects of hypoxia, from acute responses, such as increased breathing and circulation, to longer-term physiological and cellular acclimatization, such as increased blood-O2 carrying capacity, and ultimately evolutionary genetic adaptations that increase reproductive success in high altitude populations. They also considered the range of responses, from relatively benign conditions such as acute mountain sickness to loss of consciousness and ultimately extinction. However, as we discuss below, the larger projected O2 losses from Livina et al. (2015) do not have a sound geochemical basis.
The purpose of this article is to reassess possible future O2 loss to address its possible importance for human physiology and health. We begin by reviewing the geochemical controls on O2 discussing likely magnitudes of changes and offering a critique of the Livina et al. (2015) prediction. Second, we provide scenarios for possible O2 trends over the next 1,000 years, accounting for impacts on O2 from fossil-fuel usage, land-use, warming, rising CO2, and changes in barometric pressure. At high elevations, global warming is predicted to increase barometric pressure (Moore and Semple, 2009), an effect which offsets the impact of O2 loss on the O2 partial pressure and which dominates above 3,000 m. To assess whether these O2 changes have potential physiological consequences, we then review the original research literature on the effects of hypoxia on human physiology. In addition to general physiological considerations, we discuss specific impacts on athletic performance, disease, altitude effects, reproduction, and evolution. In essence, we find that the physiological effects are too small to be of concern.
This article does not address a parallel question of whether the rising atmospheric CO2 might also have direct physiological impacts. This topic has been broached recently in studies focusing on cognitive impacts in indoor settings. Interested readers should consult Karnauskas et al. (2020) and references therein.
Geochemical Context
We first briefly discuss units for O2 (see also Appendix A). The current atmospheric O2 inventory corresponds to 37125 Pmol O2 (1 Pmol = 1015 mol). A common unit for physiological studies is the O2 partial pressure that would be obtained if the air were fully dried at the same total pressure, which we call . (The presence of water vapor reduces the actual O2 partial pressure slightly below ). is typically reported in mm of mercury (mmHg) (1 atm = 1013.25mb = 760.0 mm Hg) yielding a typical sea level value of 0.2094 × 760 = 159.1 mmHg. A further unit is changes in O2/N2 mole ratio, the conventional basis for reporting measured atmospheric O2 changes. These units are physically distinct but can be approximately related by simple scaling as discussed in Appendices A,B.
The main controls on atmospheric O2 are shown inFigure 1. Gains or losses of atmospheric O2 are tied to gains or losses of dissolved O2 in the oceans or gains or losses of carbon in organic reservoirs. The chemistry of photosynthesis CO2 + H2O →CH2O + O2 produces O2 and organic carbon (represented schematically as CH2O). Both on land and in the oceans, virtually all organic matter produced by photosynthesis is eventually decomposed via the reverse reaction CH2O + O2 → CO2 + H2O. The full cycle of life is therefore a do-nothing loop with respect to O2 production, as the O2 produced during photosynthetic production of organic matter is offset by O2 consumed during its eventual decomposition. The stability of atmospheric O2 therefore hinges the stability of the organic carbon reservoirs rather than on gross rates of photosynthesis and respiration. As shown in Figure 1, however, the reservoirs of organic carbon on land and in the ocean, such as vegetation, soils, permafrost, and dissolved organic matter, and the reservoir of dissolved O2 in the ocean are all very small when compared to the massive atmospheric O2 reservoir. For example, even if all photosynthesis were to cease while the decomposition continued, eventually oxidizing all tissues in vegetation and soils, including permafrost, this would consume 435 Pmol, equivalent to a 1.9 mm Hg (1.2%) drop in at sea level. Although land and marine biota can impact O2 at small detectible levels, they are not the “lungs of the planet” in the sense of ensuring global O2 supply. Similarly, wildfire does not threaten the O2 supply, not just because fire is usually followed by regrowth, but also because the impact is bounded by limited pool of carbon in vegetation. These issues are widely misunderstood in popular science.
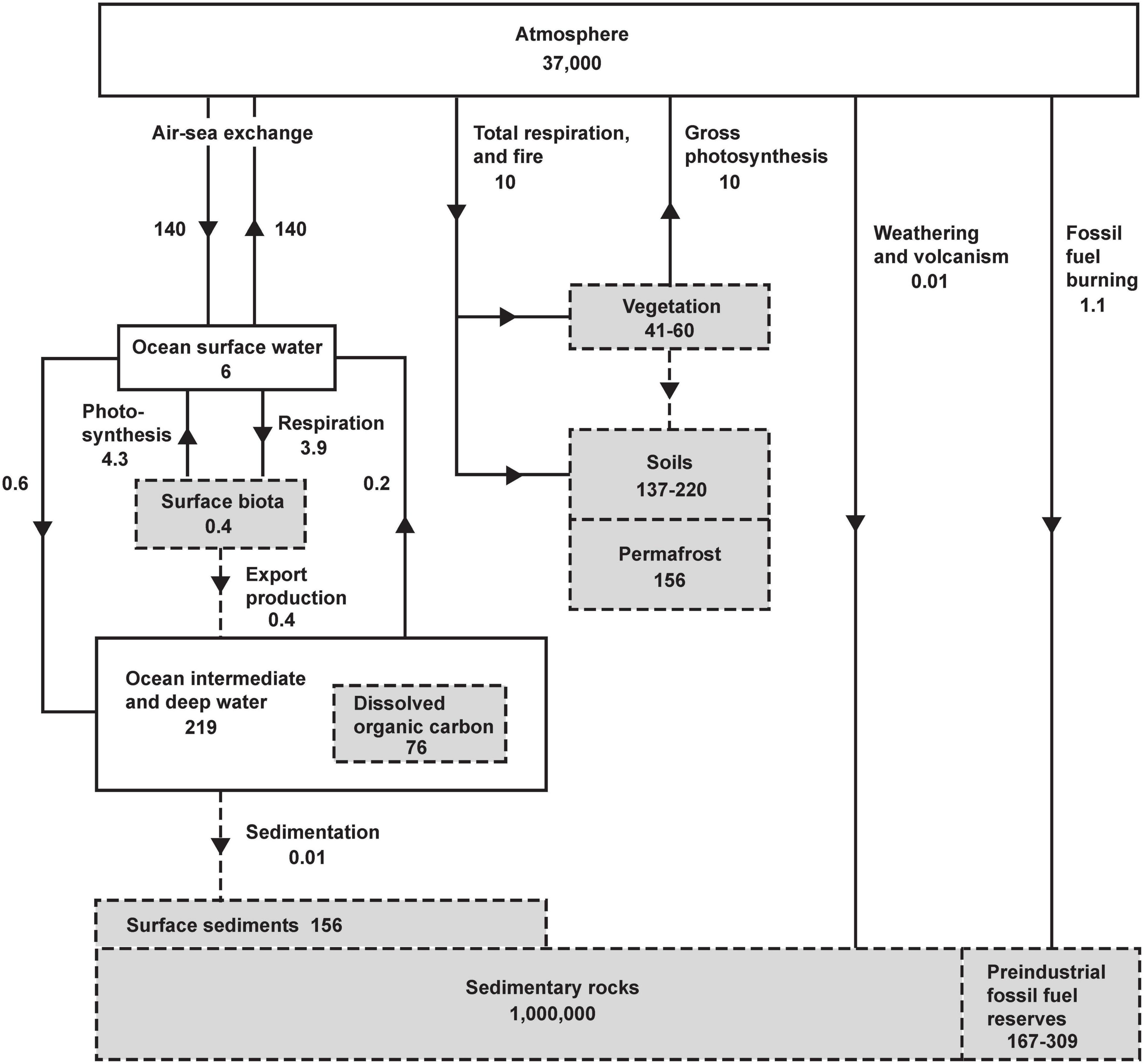
Figure 1. The global oxygen cycle, from Keeling (1988), showing short-term and long-term sources and sinks and coupling with the reservoirs of organic carbon in units of 1015 moles and 1015 moles year– 1. Oxygen fluxes and reservoirs are denoted by solid lines and solid boxes. Organic fluxes reservoirs are denoted by gray boxes with dashed perimeter, and organic fluxes with dashed lines. Organic matter is expressed in terms of O2 equivalent, i.e., the amount of O2 consumed when the material is fully oxidized. Organic reservoirs other than surface biota and sedimentary rocks have been updated using recent estimates from Ciais et al. (2013) using O2/C oxidative ratios of 1.1 (vegetation, soils, permafrost), 1.3 (dissolved organic carbon), and for fossil-fuel by fuel type from Keeling (1988). Fluxes and reservoirs other than fossil-fuel burning are notionally for a pre-industrial steady state. Fossil-fuel burning is for year 2019 (Friedlingstein et al., 2020).
Earth’s O2 supply is actually a massive geologic deposit, stored in the atmosphere rather than the solid earth and closely tied to organic matter stored in sedimentary rocks (Royer, 2014). While this deposit is a distant byproduct of photosynthesis, its size is controlled by geological processes: (1) the incorporation of organic detritus into newly forming sea-floor sediments isolated from the atmosphere, and (2) by the uplift and exposure ancient sediments and volcanic gases to atmospheric oxidation. These are very slow processes, which are capable of producing significant changes in atmospheric O2 only on time scales of millions of years (Berner, 1999). These processes also control the global amount of organic carbon stored in sedimentary formations, such as shales. Very little of this carbon has economic value, but a small component is exploitable as fossil-fuels.
At current rates, the extraction and burning of fossil fuels is equivalent to at least a 100-fold acceleration of the global exposure process, which thereby dominates humanity’s small impact on atmospheric O2 (Shaffer et al., 2008). The estimated fossil-fuel reserves in Figure 1 are based on Ciais et al. (2013). The upper estimate corresponds to an equivalent O2 loss of ∼309 Pmol or a drop of 1.3 mm Hg in from the preindustrial level. This would be achieved in ∼200 years if consumption continues at the current rate. This high estimate is not a categorically upper bound, however. If large unconventional and undiscovered fossil energy resources are ultimately exploited, an even larger decline in O2 is possible. Taking an estimate of ∼5,000 Pg C for the ultimate resource of fossil-fuels (Rogner, 1997) and assuming an oxidative ratio of 1.4 for fossil-fuels yields an upper bound on O2 loss of 580 Pmol O2 or a drop of 2.5 mmHg.
Figure 2 compares the observed changes in O2/N2 from 1991 to 2018 against simulations using a simple carbon/climate model by Shaffer et al. (2009) for the so-called A2 and B1 scenarios (Nakicenovic et al., 2000). These simulations account for O2 changes from fossil fuel burning and land use, which promotes decomposition of vegetation and soils, and account for the impact on ocean O2 and land carbon from CO2 fertilization and warming effects. Further details on these simulations are given in Appendix C. Without being explicitly tuned for O2 changes, the model for the A2 scenario accurately simulates the O2/N2 changes over the period of direct observations from 1991 to 2018. The success is partly attributable to the fact that fossil-fuel emissions from 1991 to 2018 have tracked the A2 scenario quite closely. The success also depends on quasi-realistic accounting for global influences on atmospheric O2 from land biospheric and ocean processes, which partly offset the O2 loss from fossil-fuel burning. The simulations are useful for documenting that the recent O2 changes are quite well understood. The observed drop in O2/N2 over 28 years from 1991 to 2018 period corresponds to 20 Pmol O2 or -0.09 mmHg. The model suggest that the full drop from before the industrial revolution to 2018 has been 48 Pmol or 0.21 mmHg.
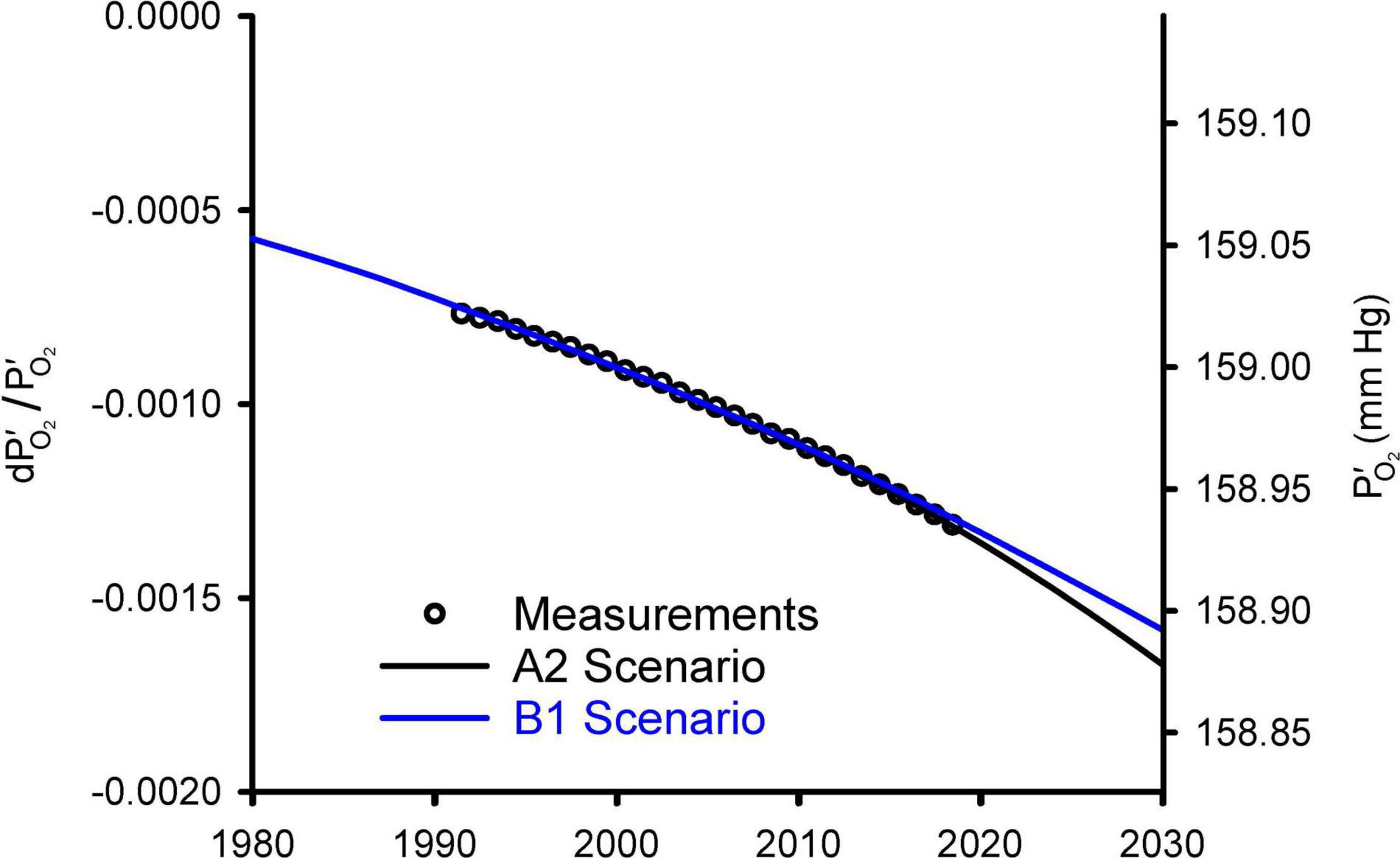
Figure 2. Modeled changes in atmospheric O2 from Shaffer et al. (2009) versus observed global averages from the Scripps O2 program (Keeling and Manning, 2014). The O2 levels are shown on left as fractional change in O2 partial pressure relative to a preindustrial reference and on right as absolute partial pressure. The observations, originally reported as changes in O2/N2 ratio in per meg units (see Appendix A), were converted to dP′O_2/ and offset with an additive constant to align with the model results. Global averages are based on the data from Alert (82.5°N), La Jolla (32.9°N), and Cape Grim (40.7°S) stations, following Keeling et al. (1996).
So what should be made of the large predicted future O2 losses of Livina et al. (2015)? Their prediction is based on an extrapolation of parabolic fit to recent atmospheric observations, an approach which lacks a geochemical basis. The success of the model simulations in Figure 2 shows that there is no evidence of a major missing process justifying such an open-ended approach. The Livina et al. loss curve is equivalent to extrapolating fossil-fuel consumption into the future based on recent fuel-use trends without accounting for eventual resource limitations.
Predicted Changes in Atmospheric O2 Over the Next 1,000 Years
Figure 3 shows the predictions of the A2 and B1 scenarios from the Shaffer et al. (2009) model extended out for the next 1,000 years. The A2 scenario is a high-end emissions scenario that assumes that fossil-fuel consumption is capped at 403 Pmol O2, which is slightly above the fossil-fuel reserves upper bound in Figure 1. The A2 scenario is similar to the more recent RCP8.5 scenario, which assumes an improbable fivefold expansion of coal use by 2100 and neglects competition from renewable energy sources, such as wind and solar (Hausfather and Peters, 2020). Warming in the A2 scenario peaks at 5.2°C, CO2 peaks at ∼1,200 ppm, and at sea level drops by 1.3 mmHg in year 2300. The B1 scenario is a middle-of-the-road scenario, allowing for modest mitigation effort, and assumes fossil-fuel consumption is capped at 159 Pmol O2. In B1, warming peaks at 2.9°C, CO2 peaks at ∼550 ppm, and at sea level drops by 0.50 mmHg in year 2300. In both scenarios, the O2 loss from fossil-fuel burning and land-use is partially offset by O2 release from the ocean due to warming and release from the land biosphere due to CO2 fertilization of plant growth. Without these offset, the O2 decline (from fossil-fuel and land use) would have amounted to 1.8 mmHg in the A2 scenario and 0.7 mmHg in the B1 scenario.
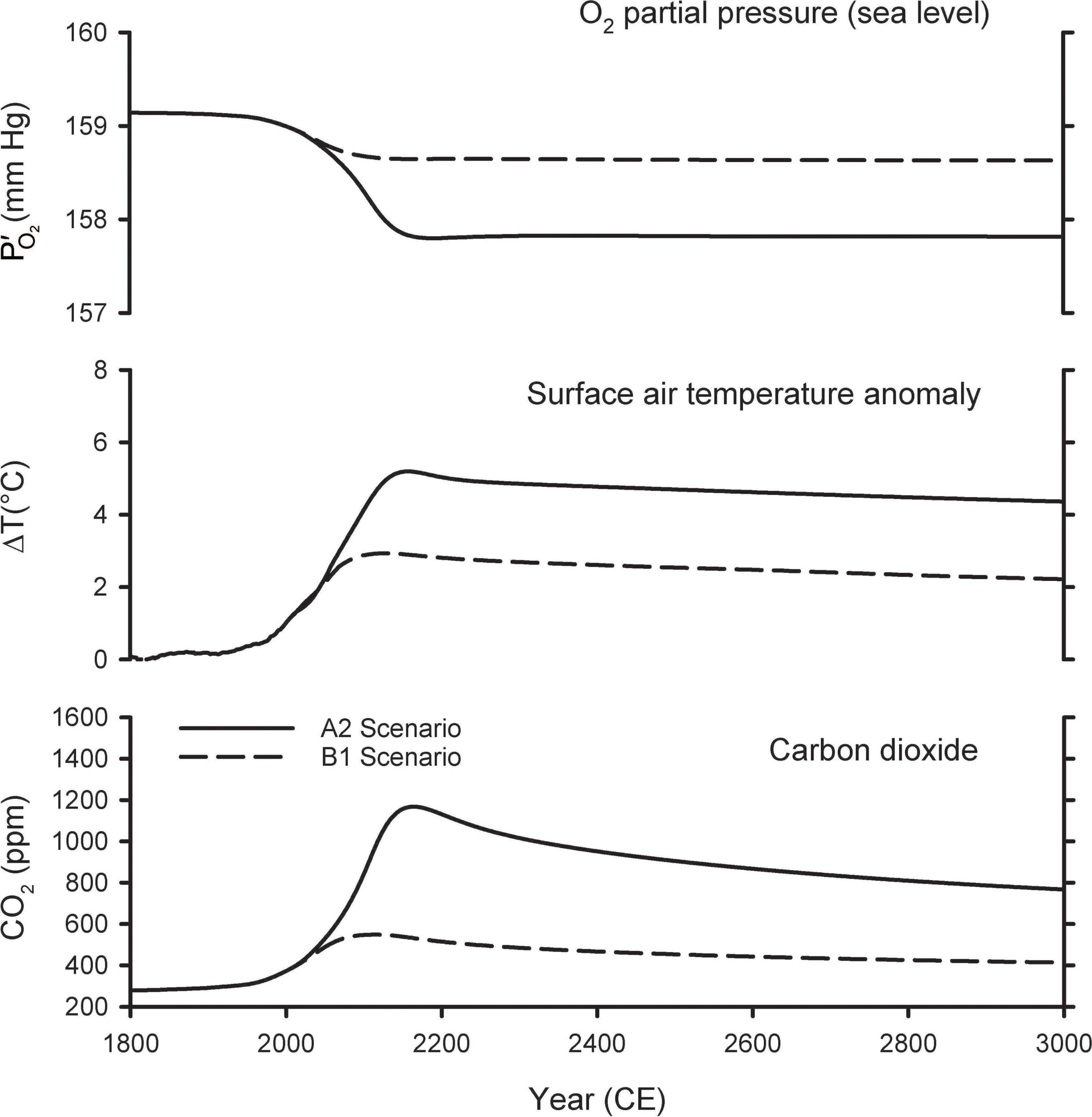
Figure 3. Predicted changes in atmospheric O2 partial pressure (), temperature, and CO2 mole fraction from model simulations of Shaffer et al. (2009).
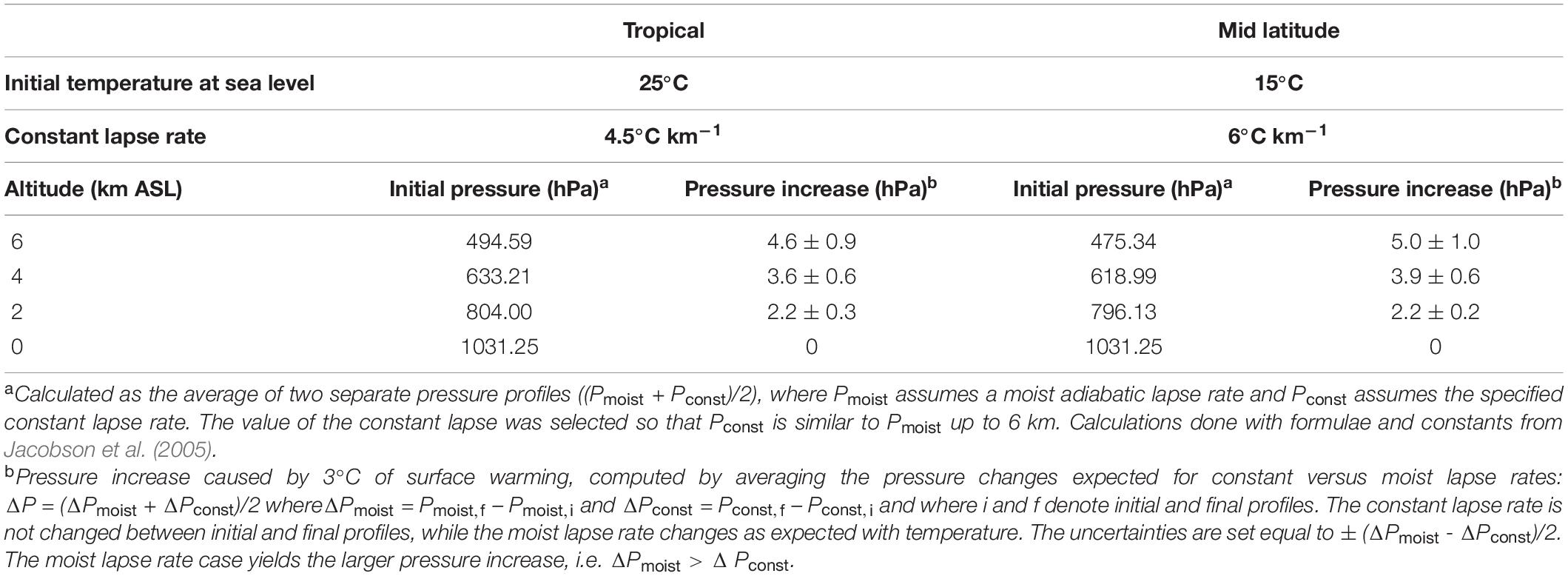
Shaffer et al. (2009) do not address changes in O2 partial pressure at higher altitudes, where barometric pressure is expected to increase due to upwards expansion of the atmosphere with warming (increasing scale height). In Table 1, we estimate the expected barometric pressure increase as a function of altitude corresponding to 3°C of surface warming for two cases, corresponding either to tropical or mid latitude atmospheric profiles. The calculations allow for uncertainty in the pressure increase associated with alternate assumptions for how the temperature lapse rate evolves, bracketed by assuming either that the lapse rate remains unchanged, or assuming it decreases in magnitude with warming as for a saturated moist adiabatic lapse rate. Current understanding suggests the lapse rate changes will lie between these limits (Bony et al., 2006). The differences between the tropical and mid latitude cases are small. In the following we consider only the tropical case.
Figure 4 shows the resulting estimates for future variations in at various altitudes. These calculations account for changes in due both to changes in the global O2 inventory from Shaffer et al. (2009), as well as the changes in barometric pressure with altitude, assuming pressure increases linearly with surface warming using the central estimates for the tropics from Table 1. At 2 km above sea level (ASL) the predicted changes are considerably smaller than those at sea level, while at 4 and 6 km, the is predicted to increase as the barometric pressure change more than offsets the O2 losses. The cross-over altitude at which there is effectively no change in is 3.6 km for A2 and 2.5 km for B1.
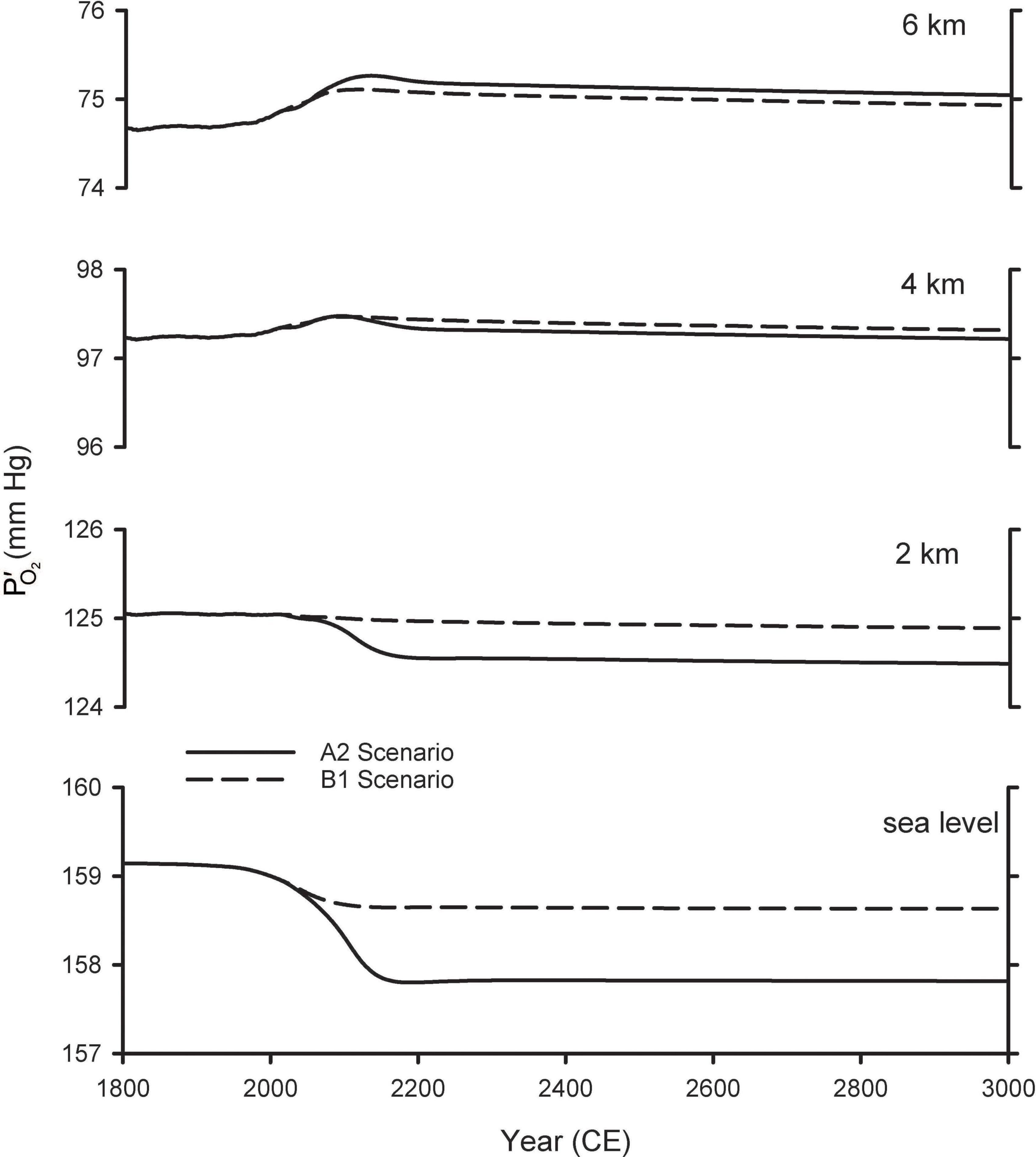
Figure 4. Predicted changes in O2 partial pressure () at different elevations. The curves for sea level are from Shaffer et al. (2009) and repeated from Figure 2. The curves for other elevations were calculated by accounting both for global O2 loss and for changes in barometric pressure with warming (Table 1), scaled by the modeled temperature changes from Shaffer et al. (2009).
Evaluation of Physiological Consequences of the Predicted O2 Declines
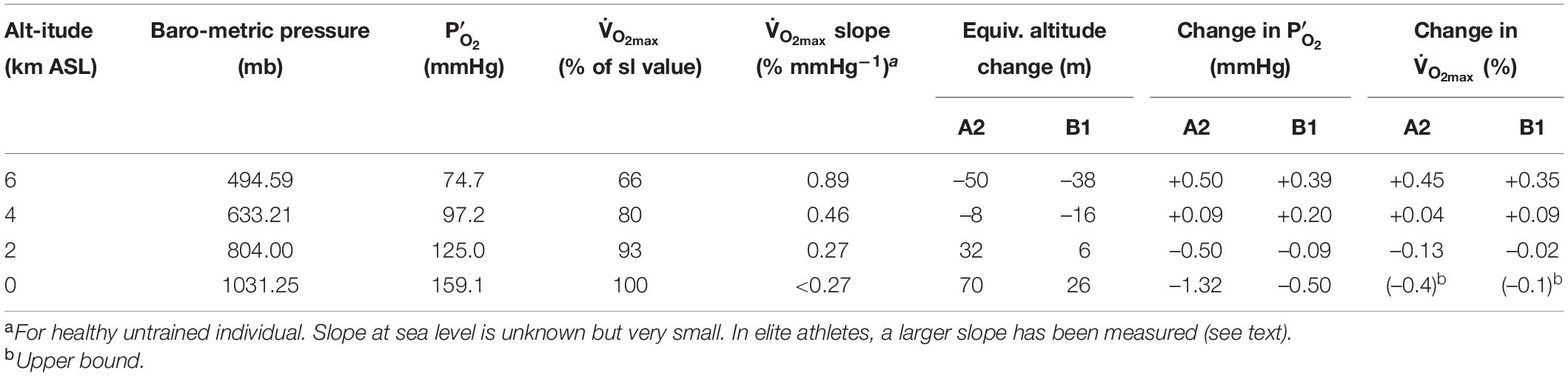
At the outset, it is clear that any physiological effects of these O2 changes must be very small because the O2 changes under consideration are very small. The physiological effect of O2 is determined by the partial pressure, which depends on the product of the O2 concentration and total barometric pressure. Whether the O2 partial pressure changes from changing barometric pressure or O2 concentration, the physiological consequences are similar, especially for small O2 changes (Richalet, 2020). Table 2 summarizes the predicted changes in for year 2300 for the A1 and B1 scenarios, as well as the equivalence of these changes in terms of altitude shift. The equivalent altitude change is consistently 70 m or less over all altitudes of human habitation (<5 km). These changes in are similar in magnitude or smaller than the changes that occur during the passage of moderately strong storm systems. We are aware of no studies that have attempted to resolve physiological impacts of such tiny changes in PO2, which would be in the noise range for physiological studies. Our analysis thus draws on inferences from studies over larger ranges in PO2 and from theoretical considerations.
Normal Healthy Individuals
To understand how changes in O2 may affect humans, we consider the basics of respiratory physiology for healthy humans (Guyton and Hall, 1996) using an approach similar to that of Martin et al. (2017) but focusing on much smaller decreases in PO2 consistent with the geochemical analysis presented above. O2 is essential for life because it provides electrons to mitochondria, cellular organelles that use these electrons to generate the molecule ATP. ATP is vital as the primary fuel for cellular ion pumps that are necessary for nerve and brain function, muscle contraction and movement, the heart beat and breathing, digestion and metabolic functions, the synthesis of reproductive hormones and all other physiological functions necessary to sustain life.
Figure 5 shows how the cellular process of generating ATP depends on the amount of O2 available. Isolated mitochondria studied in vitro can maintain a maximum rate of ATP production and maximum O2 consumption, which is abbreviated as V̇O_2max, until O2 drops to very low values (Gnaiger, 2001). O2 levels are shown as partial pressure (PO2) because this determines the “pressure head” driving diffusion for O2 into mitochondria, as well as O2 diffusion from the lungs into blood and from the blood into cells. The results shown in Figure 5 are obtained in vitro by adding metabolic substrates to a solution with mitochondria at high PO2 levels until a maximum value of O2 consumption is measured, i.e., . Then the level of substrate is maintained while PO2 is decreased. V̇O_2max is expressed as the ratio (in percent) to the value of at normal PO2 values of 159 mmHg, known as “normoxia.” Figure 5 shows that no significant change in observed in mitochondria until PO2 drops below 1–2 mmHg; decreases to 50% of the normoxic value when PO2 is <0.5 mmHg. is a reproducible and biologically meaningful way to quantify the effects of decreased PO2 on physiological processes.
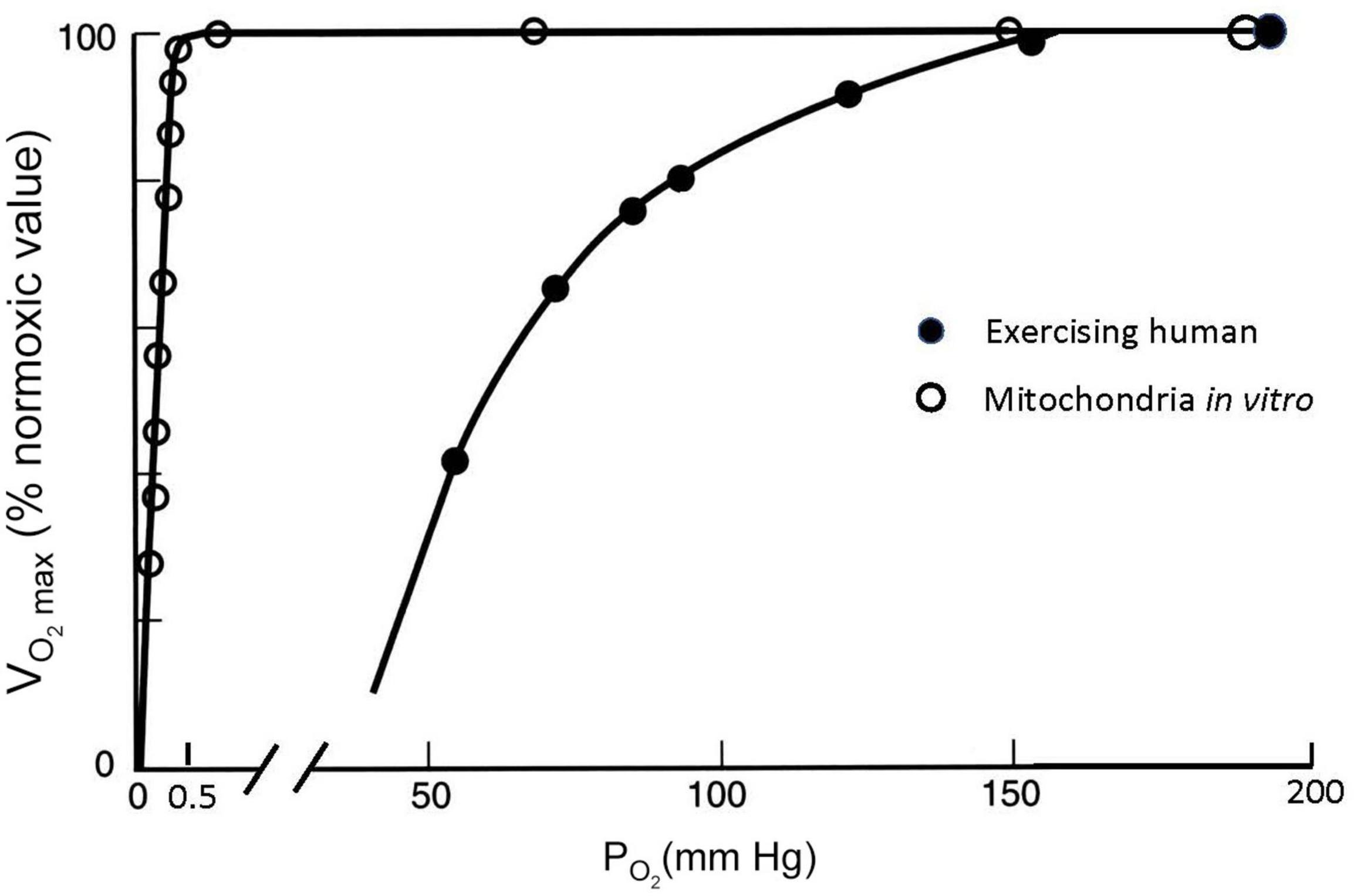
Figure 5. Maximal O2 consumption (V̇O_2max, as % of the maximum value at PO2 = 150 mmHg) measured at different ambient PO2 conditions for isolated mitochondria in a saline suspension (open symbols) and humans on a bicycle ergometer (closed symbols). V̇O_2max decreases at inspired PO2 levels in exercising humans much greater than those necessary to decrease V̇O_2max in isolated mitochondria, which can be explained by PO2 around the mitochondria in exercising humans falling to less than 1 mmHg (see text). After Gnaiger (2001) and Pugh et al. (1964).
The effects of decreasing PO2 on are very different, however, when they are measured in humans doing exercise as also illustrated in Figure 5. For this experiment data, a person is asked to pedal an exercise bike as hard as possible and the resistance or work load is increased while the subject’s O2 consumption is measured (with gas analyzers connected to a mouthpiece used to measure ventilation, Pugh et al., 1964). At some point, O2 consumption will reach a plateau as the work rate increases. This point defines the (any additional work possible beyond this is powered by anaerobic metabolism, e.g., from lactic acid). The same can be repeated at different levels of ambient PO2, establishing a curve of as a function of ambient PO2. In most healthy subjects (though not highly trained athletes as discussed below), the measured plateaus when PO2 approaches a normal sea level value of around 159 mmHg. However, decreases dramatically with modest decreases in PO2, in stark contrast to the isolated mitochondria (Figure 5). V̇O_2max falls to half the value measured at sea level when inspired PO2 is dropped to 60 mmHg. The isolated mitochondria experiment demonstrated that basic cellular function is not affected by such modest decreases in PO2 so something is different in whole organisms that makes us more sensitive to decreased PO2, i.e., hypoxia.
This difference can be understood via the “oxygen cascade,” which quantifies the fall in PO2 at sequential steps of physiological transport of O2 from the atmosphere to the mitochondria. Figure 6A shows this cascade using values typical of a healthy subject at rest (Richardson et al., 2006). Two curves are shown, one for sea level and one for 1.5 km ASL. At the first step, PO2 decreases when air is inspired from the atmosphere into the nose and upper airways. The decrease occurs because the air gains water vapor, as it saturates at a normal body temperature of 37°C, yielding a water vapor pressure 47 mmHg. The PO2 in the airway is designated PIO_2. At current O2 levels, PIO_2 is 149 mmHg at sea level and 123 mmHg at 1.5 km ASL. Because the humidity in the airway is fixed by body temperature, the inspired PO2 level is independent of ambient humidity. In contrast, the PO2 in the atmosphere will be higher if the air is drier. A suitable measure of ambient O2 which determines PIO_2 is the partial pressure that would be obtained in perfectly dry air at ambient pressure, the quantity defined in the previous section as . The drop in PO2 from dry ambient air to inspired air is ∼10 mmHg whether at sea level or 1.5 km ASL. The humidification effect is relatively larger at high altitude since it remains constant at 10 mmHg regardless of atmospheric pressure and temperature; e.g. on the summit of Mt. Everest where barometric pressure is only 1/3 that at sea level (West, 1996), PO2 decreases almost 20% with humidification versus 6% at sea level.
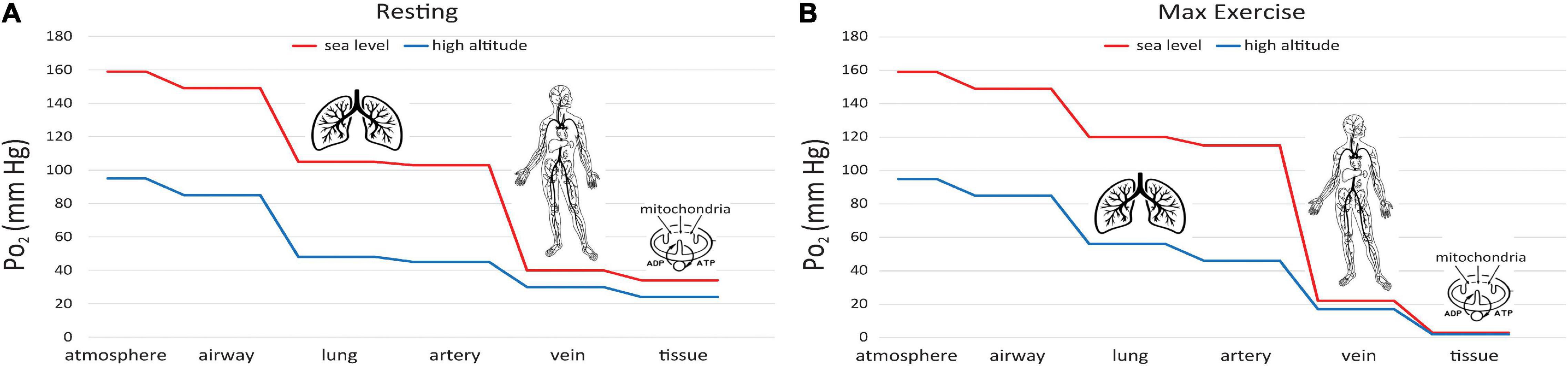
Figure 6. Oxygen cascade, showing how PO2 decreases from the atmosphere to mitochondria along the physiological O2 transport chain at sea level (red) and high altitude (blue). (A) person at rest, (B) person at maximum exercise.
A significant decrease in PO2 occurs at the step between inspired PO2 in the airways and the alveoli in the lungs where O2 exchange with blood occurs (Figure 6A). The magnitude of this decrease depends on the efficiency of convective (bulk) transport of air into the lungs by ventilation. The next step occurs as O2 diffuses from the alveoli into capillary blood in the lungs and is pumped by the heart to the tissues in arterial blood. The decrease between alveolar and arterial PO2 is very small, at least in healthy lungs that have an excess of diffusing capacity under resting conditions. There are some small differences in PO2 to this point in the oxygen cascade at moderately high altitude versus sea level, since breathing rate increases at high altitude to maintain alveolar PO2 near normal levels, at least under resting conditions.
A large decrease in PO2 occurs also along the oxygen cascade between arterial and venous PO2. The amount of O2 transported by blood flow is a non-linear function of the PO2 of the blood, rising rapidly at low PO2 but then saturating at higher PO2. The relationship is explained by O2-hemoglobin equilibrium, curve shown in Figure 7, which relates PO2 to the actual O2 concentration [O2], or the O2 saturation, SO_2, of hemoglobin available in the blood (Guyton and Hall, 1996). SO_2 is conveniently measured with a finger pulse oximeter and is the most widely used measure of arterial oxygenation. Of critical importance is the arterial to venous difference in [O2] which represents the amount of O2 that diffused out of capillaries to be consumed by the mitochondria. This difference depends on (1) the rate of O2 consumption by the tissues and (2) the blood flow to the tissues, e.g., cardiac output. Since the O2 demand and cardiac output are similar at rest at sea level and high altitude, the arterial-venous difference in [O2] is similar. Due to the non-linear shape of the O2-hemoglobin equilibrium curve, this translates into larger differences in PO2 at sea level versus altitude. As long as venous PO2 remains above a few mm Hg, as required by the mitochondrial V̇O_2max curve (cf. Figure 5), this is sufficient to satisfy mitochondrial demand.
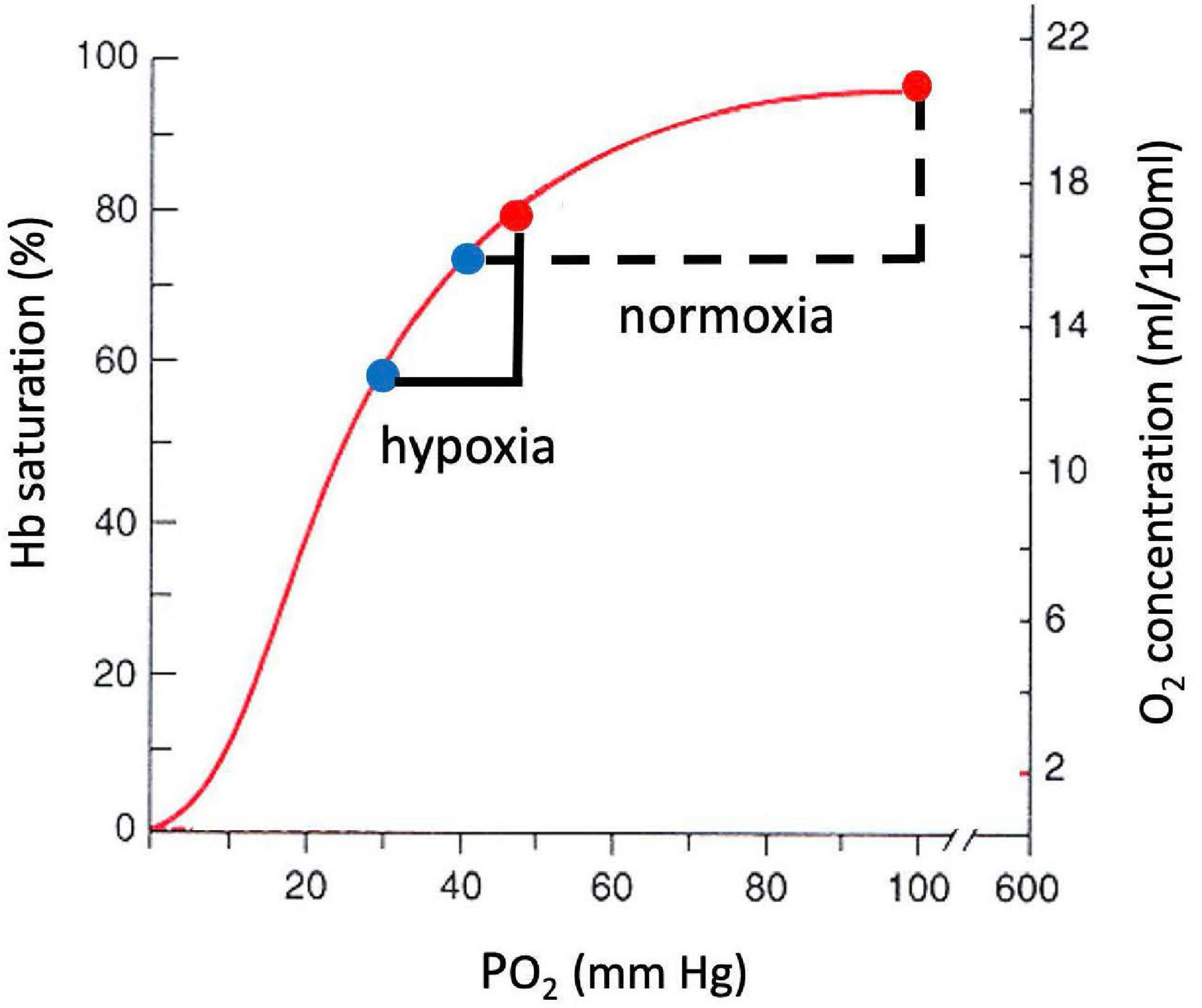
Figure 7. O2-hemoglobin equilibrium curve plotting the % hemoglobin saturation with O2 (left) or O2 concentration in blood (right) versus PO2. The sigmoidal shape of the curve allows a similar change in O2 concentration for a smaller change in PO2 in hypoxia versus normoxia.
It is also instructive to consider cases of increased O2 demand, which are illustrated in Fig. 6B. showing the effects of exercise on the oxygen cascade at sea level and high altitude (Richardson et al., 2006). As O2 consumption increases, alveolar PO2 can actually increase from hyperventilation, especially at high altitude where there is already a reflex increase in ventilation to counteract decreased O2 supply. The decrease from alveolar to arterial PO2 can increase with exercise too, especially at high altitude where diffusion may not be adequate even in healthy lungs to support complete equilibration in PO2 between the alveoli and pulmonary capillaries. Also, exercise generally decreases venous PO2 because cardiac output cannot increase as much as O2 consumption (5–7 fold versus 10–20 fold, Guyton and Hall, 1996). Finally, increased O2 demand requires greater diffusion of O2 across the capillaries into the mitochondria. Since the diffusion rate is proportional to the gradient in PO2, increased demand therefore produces an even larger drop in PO2 in the mitochondria than in the venous blood at the end of the capillaries. The ultimate limit on O2 demand is set at the point at which PO2 in the mitochondria drops below ∼1 mmHg, thus limiting ATP synthesis as for mitochondria in vitro (Figure 5). In principle, this limit (or equivalently V̇O_2max) is sensitive to all of the steps in the O2 cascade including ambient levels (Wagner, 1996).
Of critical importance to assessing the impacts of declining atmospheric O2 on normal human metabolism is the plateau of the curve near normal sea level levels. This plateau implies that small changes in can have only a very small impact on maximum O2 uptake rates. Although is ultimately determined by the combined interaction of all steps in the oxygen cascade, the existence of the plateau depends on normal humans having SO_2 close to 100%, where the SO_2 is almost independent of arterial (Wagner, 1996). This helps buffer arterial O2 supply from upstream changes in lung function or ambient .
We are not aware of measurements of slope of the curve for healthy individuals at sea level, as this slope is in any case very small. In Table 2, we adopt as an upper bound a value corresponding to the slope at 2 km, which together with the estimated change in from the A2 scenario in year 2300 yields an estimated change in of -0.4%. A best guess might be between -0.1 and -0.2%, taking a more reasonable estimate of the eventual O2 loss and a lower slope of the V̇O_2max curve. We note that a slightly larger change in is expected for elite athletes, who are more sensitive to changes in , as discussed below. However, the decrease in P’O_2 would have to be an order of magnitude greater than the ∼1.3 mm Hg predicted to have a significant effect on performance, if we consider a 10% reduction in is not observed until PO2 drops to 125 mm Hg in healthy individuals at sea level (Figure 5).
The curve as shown in Figure 5 applies to an individual exposed to short-term changes in PO2. For long-term exposure, the responses are typically smaller because of physiological acclimatization, whereby the body increases its capacity to uptake and deliver O2. Acclimatization has been observed, not just for large changes in PO2, but also for quite small changes (Donoghue et al., 2005). This is yet another reason why the changes in driven by falling are likely to be very small for a healthy individual.
Highly Trained Athletes
Highly trained athletes are likely more sensitive to changes in O2 levels than most people. For example, elite athletes actually show decreased arterial O2 saturation during maximum exercise at sea level because they have extremely high cardiac outputs and high pulmonary blood flow so there is insufficient time for O2 equilibration in the lungs, resulting in a diffusion impairment. Consequently mild increases in O2 (5% inspired, PIO2 = 149–185 mmHg) significantly increases in trained athletes with an average = 70 ml O2/kg/min but not in normal subjects with an average = 57 ml O2/kg/min (Powers et al., 1989). Such athletes do not show as pronounced a plateau in whole body versus PO2 at normal sea level PO2 as indicated in Figure 5. Trained athletes have both increased mitochondria and capillary surface area for increased O2 diffusion in tissues, in addition to increased O2 transport at other stages along the oxygen cascade, to support this increased O2 consumption.
In a study with trained athletes, Wehrlin and Hallén (2006) detected a decrease in for an increase in altitude from only 300 to 800 m ASL (Wehrlin and Hallén, 2006) associated with an 8 mmHg decrease in inspired PO2 (144–136 mm Hg). The change was consistent with measurements over a larger altitude range showing a decrease of 6.3% in for every 1 km increase in altitude ASL (Wehrlin and Hallén, 2006), corresponding to a slope of ∼0.37% per mmHg. On this basis, if at sea level drops by 2 mmHg, as might occur in an extreme scenario, this would yield a decrease of 0.75% in . The more likely scenario assuming 0.5 mmHg decrease would yield a 0.18% drop in . This study used subjects with of ∼ 66 ml O2/kg/min (Wehrlin and Hallén, 2006) compared to a normal value for a healthy individual of ∼40 ml O2/kg/min. Other reviews of the literature have found linear decreases in with inspired PO2 between 150 and 80 mmHg and predict a similar decrease in of less than 1% for the 1.5 mmHg decrease (Gonzalez and Kuwahira, 2018).
Athletic performance in aerobic sports such as long distance running has been shown to correlate with when comparing individuals with large differences in (Bassett and Howley, 2000). On this basis it is conceivable that a 0.5 mm Hg decrease in due to global O2 loss could cause a 0.18% drop in in elite athletes, and this in turn could cause a ∼0.18% increase in e.g., marathon times. But this extrapolation is fraught with uncertainty. Establishing a causal connection between small (e.g., <1%) changes in and performance is very difficult because other metabolic factors, such as lactic acid accumulation and running economy, dominate over such small ranges (Bassett and Howley, 2000). It thus seems very unlikely that the small conceivable change in performance due to dropping , occurring over centuries, could be detected in the face of changes in training, nutrition, talent recruitment, genetic changes, doping, etc.
High Altitude
The non-linearity of the curve implies greater sensitivity to PO2 changes at altitude. However, the modeling of atmospheric changes discussed earlier produces an interesting result that may actually increase above 3,000 m in 2300 CE versus today due to increasing barometric pressure with warming. One way to assess these competing effects is to calculate the expected changes at different altitudes, accounting the predicted changes in PO2 and the non-linearity of the curve. Table 2 summarizes this calculation for year 2300 at sea level, 2, 4, and 6 km ASL. At 2 km and below, is calculated to decline, but the magnitude of the changes is extremely small, less than 0.4% for both A2 and B1 scenarios. At 4 and 6 km is calculated to increase, with larger gains at the highest altitudes. Not only are these changes very small over all altitudes of normal human habitation <5 km ASL (West, 2002), but they also neglect the possibility of acclimatization, which would reduce impacts further. The calculated changes apply to an individual, living at sea level and traveling to higher altitude, who has NOT acclimatized to the small changes at sea level. The impact on at altitude after any such acclimatization would necessarily be even smaller.
Even greater gains in PO2 and can be expected above 6 km. As discussed by Moore and Semple (2009), this may have benefits for high altitude mountaineering, although Moore and Semple (2009) slightly overestimate the benefits because they consider only the impact of changes in barometric pressure and not the impact of global O2 loss.
It is interesting that the reversal of the direction of change in PO2 and versus altitude with global change occurs very near the threshold for acute mountain sickness (AMS) in today’s atmosphere. AMS symptoms include headache, insomnia, lassitude, anorexia, and/or nausea that occur above 3,000 m in most subjects, but can occur in the most sensitive individuals with rapid ascent to only 2,400 m (Hultgren, 1997). Hence, AMS could occur in the most sensitive subjects at lower altitude in the future while less sensitive subjects may be better able to tolerate higher altitude, i.e., the altitude range over which AMS symptoms appear could be expanded with global change. The altitude threshold changes are very small in any case, e.g., <50 m. Similar predictions can be made for more serious altitude illnesses such as high altitude pulmonary or cerebral edema that are extremely rare with even rapid ascent to altitude less than 2,400 or 2,700 m, respectively (Hultgren, 1997).
Disease
Lung disease reduces the efficiency of O2 uptake causing arterial PO2 levels to be lower in patients compared to normal subjects for a given inspired PO2. Such hypoxemia has a major impact on many physiological functions and provides the rationale for supplemental oxygen therapy in patients with chronic heart and lung disease. The American College of Physicians (ACP) recommends supplemental long-term oxygen therapy in all patients who have severe resting hypoxemia, defined as an arterial PO2≤59–55 mmHg (depending on the complications) or SO_2 ≤88% (Qaseem et al., 2011). The ACP does not recommend a target to which SO_2 should be restored, but there are British guidelines for 88–92% (NICE, 2016). An increase in SO_2 of 87–90% corresponds to a ∼4 mmHg increase in arterial PO2, which is about three-times the predicted changes in predicted for 2300 for the A2 scenario (Table 2). The drop in thus cannot have a significant impact on the recommended threshold for supplemental O2 usage.
High altitude studies provide another way to consider the physiological consequences of global changes in O2. Patients with emphysema living at 7,000 feet ASL show increased cardiovascular complications compared to similar patients living only 4,500 feet ASL (Moore et al., 1982). Also, the patients living at 2,133 m ASL (7,000 ft ASL) died at 68.1 years on average versus 70.1 years for the patients living at 1,372 m ASL (4,500 ft ASL). However, the decrease in PIO2 between these altitudes is from 126 to 115 mmHg, which is over sevenfold greater than the decrease in PO2 predicted at with global change by 2300 for the A2 scenario. If age at death scaled similarly with PIO2 at sea level, the change in life expectancy for year 2300 would be a few months. This is an upper bound to impacts because individuals at sea level are less sensitive to changes in , as discussed above.
Interestingly, almost the same decrease in inspired PO2 was found to be significant for another study of patients with chronic obstructive pulmonary disease (COPD) trying to determine if hypoxia during air travel proposed a risk (Gong et al., 1984). Generally, airplanes are pressurized to maintain an equivalent altitude of no higher than 8000 ft, which maintains arterial O2-hemoglobin saturation ≥85%, corresponding to a PO2 of about 55 mmHg, in healthy subjects (De La Zerda and Powell, 2014). In the patients studied by Gong et al. (1984), arterial PO2 fell from a safe level of 55 mmHg at 5000 ft altitude to 50 mmHg at 7,000 ft, a level which requires supplemental O2. The difference in inspired PO2 between these altitudes is 124–115, or 9 mmHg, which again is seven times the predicted change in for the A2 scenario. Patients at sea level are almost certainly considerably less sensitive to small changes in , which further reinforces the view that the predicted changes in are too small to be significant for these patients.
In considering the effects of global O2 decline on disease, it is important to note that changes in arterial PO2 can also exacerbate disease through processes not directly related to O2 delivery by the lungs or circulation. In fact, this significance of O2 for health and disease was recently recognized with the 2019 Nobel Prize for Physiology or Medicine being awarded to Kaelin, Ratcliffe and Semenza for their discoveries about how cells sense and adapt to O2 availability1. A key part of their discoveries related to O2-sensitive control of gene expression and specifically Hypoxia Inducible Factor-1α (HIF-1α). HIF-1α was identified by Semenza as the transcription factor for the hormone erythropoietein (EPO), which increases red blood cell production in response to hypoxemia. Originally it was a surprise that HIF-1α was expressed in essentially every type of cell tested—including cancer cells—but now this is recognized as a hallmark of the vital central role of O2 in homeostasis. Recent studies show that HIF-1α plays an important role in physiological processes as diverse as inflammatory responses (Zinkernagel et al., 2007) and neural plasticity (Moya et al., 2020), illustrating how O2 levels affect much more than just mitochondrial function. Hence, it may not be surprising that there appears to be natural selection for O2-sensitive transcription factors in high altitude populations as discussed below, although again the magnitude of O2 decreases may be greater than the global changes we are considering.
Reproduction
A major challenge to long-term habitation at high elevation is reduced birth weight impacting reproductive success. In the Rocky Mountains, Moore et al. (1998) found that birth weight decreases by ∼100 g for every km ASL, corresponding to a decrease in inspired PO2 of 13 mmHg. Assuming this relationship also holds at sea level, and assuming PO2 at sea level drops by 2 mmHg, as assumed the most extreme scenario, this would decrease birth weight by ∼15 g relative to a normal birth weight of 2,500 g. This is likely an upper bound because it is based on an extreme estimate of O2 loss and because of the non-linearities in O2 exchange discussed earlier. The impacts at higher altitude would also be small because predicted PO2 changes are also small (Table 2).
Evolution
Over geologic time, changes in O2 are known to have had a large impact on evolution of mammals and other organisms (Falkowski et al., 2005; Powell, 2010). These evolutionary changes have been in response to fairly large changes in in O2, ranging from 60 to 140% of present atmospheric levels.
Hypoxia at high altitude is also known to be a potent force of natural selection in humans. Hundreds of studies published to date provide evidence that Tibetan, Andean, and Ethiopian highland populations have genetic adaptations to high altitude that involve various hypoxia sensing and response genes, including those in the HIF pathway (Simonson, 2015; Moore, 2017). The genomes of Tibetan individuals exhibit adaptive signatures at several genes, including EPAS1, which encodes the α subunit of the HIF-2 transcription factor (HIF-2α), and EGLN1, prolyl hydroxylase 2 (PHD2) that targets HIF-α subunits for degradation under conditions of normoxia. Both are associated with relatively lower hemoglobin concentration in Tibetans resident at high altitude (Beall et al., 2010; Simonson et al., 2010; Yi et al., 2010). The genomes from Andean and Ethiopian highlanders also exhibit various adaptive signals, including some of the same gene regions identified in Tibetans, although the precise adaptive changes appear to be distinct. For example, functional variants identified at the EGLN1 locus in Tibetans are absent or found at low frequency in Andeans (Heinrich et al., 2019). The distinct population histories of continental populations (e.g., different genetic backgrounds, admixture events, and generations at altitude) are important factors that contribute to variation across and even within continental populations (Wuren et al., 2014; Simonson, 2015).
It seems possible that natural selection of populations at higher elevations is still ongoing. What is clear from our analysis, however, is that this trajectory cannot be significantly modified by the impacts of fossil-fuel burning on atmospheric O2, because these changes are tiny compared to even modest changes in elevation (Table 2).
Cognition
Brain function is critically sensitive to O2 supply, as the brain typically consumes about 20% of the body’s O2 uptake while comprising only 2% of body mass (Guyton and Hall, 1996; West, 2016). Brain impairment on exposure to high altitude has been demonstrated for both short-term and long-term exposure, including during childhood development (West, 2017). It should be noted, however, that our understanding of cognitive impacts of long-term exposure to low is informed by very few studies. Childhood development studies are especially difficult because they require comparing one group of individuals to another group, with potential confounding influences of cultural and environmental factors. Significant effects have been resolved only for large changes in altitude. The studies on young adults by Yan et al. (2011), for example, involve comparing a group raised between 2,400 and 4,200 m, with a control group raised below 400 m. These groups were thus exposed to changes in that are 25–100 times larger than the predicted drop in in sea level by year 2300. Because the future losses of O2 are equivalent to such small changes in altitude, we conclude that any cognitive impacts of this loss can be at most of very minor significance.
Discussion
From measurements and models it is well established that the background level of O2 in the atmosphere is declining slowly. The decline is mostly caused by fossil-fuel burning with smaller impacts from changes in ocean and land biogeochemistry. These processes are estimated to have caused the O2 partial pressure at sea level to drop by ∼0.21 mmHg from a preindustrial times to 2018 from a baseline of 159 mmHg. Based on results presented here, we estimate that, if no steps are taken to mitigate continued exploitation of fossil-fuels, O2 will continue to decline, eventually dropping by 1.3 mmHg over a several hundred-year time frame. If humans additionally exploit resources comprising currently uneconomic or undiscovered fossil fuels, the ultimate decrease might amount to 2.5 mmHg many centuries into the future. In either of these very extreme scenarios, atmospheric CO2 will rise above 1,200 ppm, associated with global warming of 5°C or more, triggering climate changes considered “beyond catastrophic” (Xu and Ramanathan, 2017). In a less extreme scenario, in which dependence on fossil-fuels is reduced before reserves are exhausted, capping the CO2 rise to ∼550 ppm, the O2 drop will likely be around 0.5 mmHg. Warming would then be reduced to ∼3°C, which is still considered catastrophic (Xu and Ramanathan, 2017). More aggressive measures to curtail fossil-fuel emissions and warming would likely lead to even smaller O2 changes. Above sea level, the declines in O2 partial pressure will be smaller than at sea level, due to an offsetting increase in barometric pressure from warming. In fact, above ∼3,000 m ASL, O2 partial pressure to is expected slightly increase, despite O2 loss globally.
Relatively small changes in altitude or barometric pressure are required to produce similar changes in O2 partial pressure. In the high-end A2 scenario considered here, a person living at sea level in year 2300 will experience an O2 partial pressure similar to a person living at an altitude of 70 m ASL today. A person living at 4,000 m altitude in year 2300 will experience a O2 partial pressure similar to someone living at ∼3,992 m today.
We have considered the possible impacts of these small O2 changes on human health, focusing on normal human function, athletic performance, disease, human reproduction, evolution, and cognition. In no case do we find that the changes are significant enough to raise concerns.
These conclusions are based on changes in O2 partial pressure in a clean outdoor setting, but a consideration of the additional impacts of O2 changes due to local combustion or respiration within an indoor or urban setting does not alter our conclusions. The OSHA occupational exposure limit for indoor CO2 is 5,000 ppm,2 which compares to the natural background of ∼400 ppm. To raise the CO2 concentration by respiration within a confined space from 400 to 5,000 pm, yields a drop of ∼ (0.005 - 0.0004) × 760 = ∼ 4 mmHg at sea level, which is similar to the drop that occurs on ascent of ∼220 m. Typical indoor CO2 exposures are <∼1,400 ppm (Karnauskas et al., 2020), so this limit in fact is rarely achieved. As background O2 partial pressures drop in the future, indoor partial pressures will drop in parallel from their lower starting point. Our purpose is not to analyze the physiological impact of a ∼4 mmHg indoor O2 loss (though we expect it is very small). But clearly the incremental impact of an additional loss over the next few centuries of 1.3 mmHg, for example, will be very minor.
The ongoing changes in atmospheric O2 could also potentially impact other aerobic life, such as animals, plants, fungi, bacteria, etc., a topic which remains to be carefully reviewed. We note that even in oxygen-starved aquatic systems, however, the impacts will generally be small because the low O2 levels in these systems are controlled by processes below the water surface, such as solubility, sluggish circulation and high inputs of detritus. Tiny changes in the atmospheric boundary condition will not normally have much impact on these systems. Rather, the specter of large future expansion of ocean suboxic zones with dire consequences for ocean life rests firmly on the effects of global warming itself by way of reduced O2 solubility and ocean circulation change (Shaffer et al., 2009; Oschlies et al., 2018).
The O2 loss over the next few centuries will be dominated by fossil-fuel burning. The ultimate potential loss is capped by known reserves of fossil-fuel, and future trajectory will be largely governed by actual usage trajectories. The model used here predicts that O2 loss from fossil-fuel burning will be offset at the ∼30% level by O2 sources from the land and ocean. The land source is driven by fertilization from rising CO2 leading to accumulation of land biomass. The ocean source is driven by warming-induced solubility changes of O2 in seawater and by increased ocean stratification, which increases atmospheric O2 at the expense of greater deficits of dissolved O2 in the ocean interior. These processes are known to be offsetting O2 losses today, and the model is credible because it accounts well for changes in O2 to date. Future predictions of these biogeochemical responses are clearly speculative, but they are in any case of secondary importance. We find no basis to support the higher estimate of O2 loss from Livina et al. (2015) which is based on mathematical extrapolation without geochemical underpinning.
In theory, O2 loss could be exacerbated by strategies to reduce CO2 emissions, such as carbon capture and storage (CCS), which captures CO2 and stores it underground. CCS offsets the CO2 emission from fossil-fuel burning and the associated global warming, but does not offset the O2 loss because the captured carbon is in the form of CO2, rather than biomass. Although the model simulations presented here did not consider CCS, it is easy to set bounds on O2 by considering an extreme version of the A2 scenario in which all the CO2 eventually released from fossil-fuel burning is captured and stored. Because this eliminates warming and CO2 buildup, the O2 loss is governed by fossil-fuel usage alone, yielding an ultimate drop of 1.8 mmHg or 1.1% of the initial pressure of 159 mm Hg. This fractional change would be felt throughout the atmospheric column, equivalent to a nearly uniform upwards shift in altitude of ∼90 m. The societal benefit of this outcome in terms of climate mitigation would greatly outweigh any small impacts from the changes in O2. This merely illustrates that concern over O2 loss is a very weak argument against CCS as a mitigation strategy.
More generally, there is now clearly an urgent need to curtail fossil-fuel CO2 emissions to avoid catastrophic climate change, which will certainly dominate the impact on human health. The corresponding loss of O2, which is of biogeochemical interest, is merely a physiological curiosity in comparison. The case for action rests on the consequences for CO2, not O2.
Author Contributions
RK and FP drafted the manuscript with contributions from other coauthors. RK contributed measurements of O2/N2 ratio for Figure 1 and carried out calculations of pressure O2 changes at higher elevations. GS contributed published model runs in support of Figures 2, 3. All authors contributed to the ideas and framing.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
Measurements of O2/N2 from the Scripps O2 program have been supported by a series of grants from the U.S. National Science Foundation and the National Oceanographic and Atmospheric Administration, most recently from NOAA Grant NA15OAR4320071and NSF Grant 1922922. GS was supported by ANID(Chile) FONDECYT grant 1190230 and Millennium Science Initiative/Millennium Nucleus Paleoclimate NCN17_079. Also supported by NIH National Heart, Lung, and Blood Institute (NHBLI), RO1 HL-081823 (to FP) and 1RO1HL145470 (to TS).
Acknowledgments
RK thanks the Oxford Martin School for hosting a sabbatical that helped to support this work and also thanks Myles Allen for fruitful discussions.
Footnotes
- ^ https://www.nobelprize.org/prizes/medicine/2019/summary/
- ^ https://www.osha.gov/dsg/annotated-pels/tablez-1.html
References
Bassett, D. R. Jr., and Howley, E. T. (2000). Limiting factors for maximum oxygen uptake and determinants of endurance performance. Med. Sci. Sports Exerc. 32:70.
Beall, C. M., Cavalleri, G. L., Deng, L., Elston, R. C., Gao, Y., Knight, J., et al. (2010). Natural selection on EPAS1 (HIF2α) associated with low hemoglobin concentration in Tibetan highlanders. Proc. Natl. Acad. Sci. U.S.A. 107, 11459–11464.
Berner, R. A. (1999). Atmospheric oxygen over Phanerozoic time. Proc. Natl. Acad. Sci. U.S.A. 96, 10955–10957.
Bony, S., Colman, R., Kattsov, V. M., Allan, R. P., Bretherton, C. S., Webb, M. J., et al. (2006). How well do we understand and evaluate climate change feedback processes? J. Clim. 19, 3445–3482.
Ciais, P., Sabine, C., Bala, G., Bopp, L., and Brovkin, V. (2013). “Carbon and other biogeochemical cycles,” in Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, eds T. F. Stocker, D. Qin, G.-K. Plattner, M. Tignor, and S. K. Allen (New York, NY: Cambridge University Press).
De La Zerda, D. J., and Powell, F. L. (2014). “Air travel and high-altitude medicine,” in Manual of Clinical Problems in Pulmonary Medicine, 7th Edn, eds T. Morris, A. Ries, and R. Bordow (Philidelphia: Lippincott Williams & Wilkins), 110–119.
Donoghue, S., Fatemian, M., Balanos, G. M., Crosby, A., Liu, C., O’Connor, D., et al. (2005). Ventilatory acclimatization in response to very small changes in PO2 in humans. J. Appl. Physiol. 98, 1587–1591. doi: 10.1152/japplphysiol.01019.2004
Falkowski, P. G., Katz, M. E., Milligan, A. J., Fennel, K., Cramer, B. S., and Aubry, M. P. (2005). The rise of oxygen over the past 205 million years and the evolution of large placental mammals. Science 309, 2202–2204.
Friedlingstein, P., O’Sullivan, M., Jones, M. W., Andrew, R. M., Hauck, J., Olsen, A., et al. (2020). Global carbon budget 2020. Earth Syst. Sci. Data 12, 3269–3340.
Gnaiger, E. (2001). Bioenergetics at low oxygen: dependence of respiration and phosphorylation on oxygen and adenosine diphosphate supply. Respir. Physiol. 128, 277–297.
Gong, H. Jr., Tashkin, D. P., Lee, E. Y., and Simmons, M. S. (1984). Hypoxia-altitude simulation test: evaluation of patients with chronic airway obstruction. Am. Rev. Respir. Dis. 130, 980–986.
Gonzalez, N. C., and Kuwahira, I. (2018). Systemic oxygen transport with rest, exercise, and hypoxia: a comparison of humans, rats, and mice. Compreh. Physiol. 8, 1537–1573.
Hausfather, Z., and Peters, G. P. (2020). Emissions–the ‘Business as Usual’story is Misleading. Berlin: Nature Publishing Group.
Heinrich, E. C., Wu, L., Lawrence, E. S., Cole, A. M., Anza-Ramirez, C., Villafuerte, F. C., et al. (2019). Genetic variants at the EGLN1 locus associated with high-altitude adaptation in Tibetans are absent or found at low frequency in highland Andeans. Ann. Hum. Genet. 83, 171–176.
Jacobson, M. Z. (2005). Fundamentals of Atmospheric Modeling. Cambridge: Cambridge University Press.
Karnauskas, K. B., Miller, S. L., and Schapiro, A. C. (2020). Fossil fuel combustion is driving indoor CO2 toward levels harmful to human cognition. GeoHealth 4, 1–8.
Keeling, R. F. (1988). Development of an Interferometric Oxygen Analyzer for Precise Measurement of the Atmospheric O2 Mole Fraction. Cambridge, MA: Department, Harvard University.
Keeling, R. F., Blaine, T., Paplawsky, B., Katz, L., Atwood, C., Brockwell, T., et al. (2004). Measurement of changes in atmospheric Ar/N2 ratio using a rapid-switching, single-capillary mass spectrometer system. Tellus 56B, 322–338.
Keeling, R. F., and Manning, A. C. (2014). “Studies of recent changes in atmospheric O2 content,” in Treatise on Geochemistry, eds R. F. Keeling and L. Russell (Amsterdam: Elsevier), 385–404.
Keeling, R. F., Piper, S. C., and Heimann, M. (1996). Global and hemispheric CO2 sinks deduced from changes in atmospheric O2 concentration. Nature 381, 218–221.
Livina, V. N., Martins, T. V., and Forbes, A. (2015). Tipping point analysis of atmospheric oxygen concentration. Chaos Interdiscip. J. Nonlinear Sci. 25:036403.
Martin, D., McKenna, H., and Livina, V. (2017). The human physiological impact of global deoxygenation. J. Physiol. Sci. 67, 97–106.
Moore, L. G., Rohr, A. L., Maisenbach, J. K., and Reeves, J. T. (1982). Emphysema mortality is increased in Colorado residents at high altitude. Am. Rev. Respir. Dis. 126, 225–228. doi: 10.1164/arrd.1982.126.2.225
Moore, G., and Semple, J. L. (2009). The impact of global warming on Mount Everest. High Altitude Med. Biol. 10, 383–385.
Moore, L. G., Niermeyer, S., and Zamudio, S. (1998). Human adaptation to high altitude: regional and life-cycle perspectives. Am. J. Phys. Anthropol. Off. Publ. Am. Assoc. Phys. Anthropol. 107, 25–64.
Moya, E. A., Go, A., Kim, C. B., Fu, Z., Simonson, T. S., and Powell, F. L. (2020). Neuronal HIF-1α in the nucleus tractus solitarius contributes to ventilatory acclimatization to hypoxia. J. Physiol. 598, 2021–2034.
Nakicenovic, N., Alcamo, J., Davis, G., de Vries, B., and Fenhann, J. (2000). Special Report on Emissions Scenarios. Cambridge: Cambridge University Press.
NICE (2016). National Institute for Health and Care Excellence Website. Published July, 2011. Updated February, 2016. London: NICE.
Oschlies, A., Brandt, P., Stramma, L., and Schmidtko, S. (2018). Drivers and mechanisms of ocean deoxygenation. Nat. Geosci. 11, 467–473.
Powell, F. L. (2010). Studying biological responses to global change in atmospheric oxygen. Respir. physiol. Neurobiol. 173, S6–S12.
Powers, S. K., Lawler, J., Dempsey, J. A., Dodd, S., and Landry, G. (1989). Effects of incomplete pulmonary gas exchange on . J. Appl. Physiol. 66, 2491–2495. doi: 10.1152/jappl.1989.66.6.2491
Pugh, L., Gill, M., Lahiri, S., Milledge, J., Ward, M., and West, J. (1964). Muscular exercise at great altitudes. J. Appl. Physiol. 19, 431–440.
Qaseem, A., Wilt, T. J., Weinberger, S. E., Hanania, N. A., Criner, G., van der Molen, T., et al. (2011). Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann. Intern. Med. 155, 179–191.
Richalet, J.-P. (2020). CrossTalk opposing view: barometric pressure, independent of PO_2, is not the forgotten parameter in altitude physiology and mountain medicine. J. Physiol. 598, 897–899.
Richardson, R. S., Duteil, S., Wary, C., Wray, D. W., Hoff, J., and Carlier, P. G. (2006). Human skeletal muscle intracellular oxygenation: the impact of ambient oxygen availability. J. Physiol. 571, 415–424. doi: 10.1113/jphysiol.2005.102327
Rogner, H. H. (1997). An assessment of world hydrocarbon resources. Annu. Rev. Energ. Environ. 22, 217–262.
Royer, D. L. (2014). “Atmospheric CO2 and O2 during the Phanerozoic: tools, patterns, and impacts,” in Treatise on Geochemistry, 2nd Edn, Vol. 6, The Atmosphere – History, ed. J. Farquhar (Amsterdam: Elsevier), 251–267.
Shaffer, G., Olsen, S. M., and Pedersen, J. O. P. (2008). Presentation, calibration and validation of the low-order, DCESS Earth System model. Geosci. Model Dev. 1, 17–51.
Shaffer, G., Olsen, S. M., and Pedersen, J. O. P. (2009). Long-term ocean oxygen depletion in response to carbon dioxide emissions from fossil fuels. Nat. Geosci. 2, 105–109.
Simonson, T. S. (2015). Altitude adaptation: a glimpse through various lenses. High Altitude Med. Biol. 16, 125–137.
Simonson, T. S., Yang, Y., Huff, C. D., Yun, H., Qin, G., Witherspoon, D. J., et al. (2010). Genetic evidence for high-altitude adaptation in Tibet. Science 329, 72–75.
Tohjima, Y., Machida, T., Watai, T., Akama, I., Amari, T., and Moriwaki, Y. (2005). Preparation of gravimetric standards for measurements of atmospheric oxygen and reevaluation of atmospheric oxygen concentration. J. Geophys. Res. Atmos. 110:D11302.
Trenberth, K. E., and Smith, L. (2005). The mass of the atmosphere: a constraint on global analyses. J. Clim. 18, 864–875.
Wagner, P. D. (1996). Determinants of maximal oxygen transport and utilization. Annu. Rev. Physiol. 58, 21–50.
Wehrlin, J. P., and Hallén, J. (2006). Linear decrease in and performance with increasing altitude in endurance athletes. Eur. J. Appl. Physiol. 96, 404–412.
West, J. B. (1996). Prediction of barometric pressures at high altitude with the use of model atmospheres. J. Appl. Physiol. 81, 1850–1854.
West, J. B. (2016). Barcroft’s bold assertion: all dwellers at high altitudes are persons of impaired physical and mental powers. J. Physiol. 594, 1127–1134.
West, J. B. (2017). Are permanent residents of high altitude fully adapted to their hypoxic environment? High Altitude Med. Biol. 18, 135–139.
Wuren, T., Simonson, T. S., Qin, G., Xing, J., Huff, C. D., Witherspoon, D. J., et al. (2014). Shared and unique signals of high-altitude adaptation in geographically distinct Tibetan populations. PLoS One 9:e88252. doi: 10.1371/journal.pone.0088252
Xu, Y., and Ramanathan, V. (2017). Well below 2 °C: mitigation strategies for avoiding dangerous to catastrophic climate changes. Proc. Natl. Acad. Sci. U.S.A. 114, 10315–10323.
Yan, X., Zhang, J., Gong, Q., and Weng, X. (2011). Prolonged high-altitude residence impacts verbal working memory: an fMRI study. Exp. Brain Res. 208, 437–445.
Yi, X., Liang, Y., Huerta-Sanchez, E., Jin, X., Cuo, Z. X., Pool, J. E., et al. (2010). Sequencing of 50 human exomes reveals adaptation to high altitude. Science 329, 75–78.
Zinkernagel, A. S., Johnson, R. S., and Nizet, V. (2007). Hypoxia inducible factor (HIF) function in innate immunity and infection. J. Mol. Med. 85, 1339–1346.
Appendix A. O2 Units
Total Atmospheric O2 Inventory
This can be expressed in moles or mass of O2. Using the O2 mole fraction of 0.2094 from Tohjima et al. (2005) for year 2000, a corresponding molecular weight of dry air of 28.96 g/mol, and the total mass of dry air of 5.135 × 1018 kg (Trenberth and Smith, 2005) yields an O2 inventory of 3.7125 × 1019 moles or 37125 Pmol.
O2 Mole Fraction in Dry Air, XO_2
This unit express the ratio of moles of O2 per mole of dry air in a sample, often loosely called “concentration” and typically expressed as percent or in ppm.
O2 Partial Pressure, PO2,
This unit is used to describe O2 tension in blood, for example, which is relevant to assess O2 transport to vital tissues. Typical units are atmospheres, or mmHg, Torr or kPa for physiology. For physiological applications, it is useful to quantity ambient PO2 in relation to air with the same pressure but zero humidity, which is given by = XO_2 × Ptot, where Ptot is the total barometric pressure (including water vapor), and XO_2 is the O2 dry air mole fraction. At sea level and 1 standard atmosphere this yields = (0.2094)(760 mmHg) = 159.1 mmHg.
Inspired O2 Partial Pressure, PIO_2
Another physiologically relevant measure of the ambient O2 is the partial pressure in the lung airways, which is lower than due to dilution by water vapor (as XO_2 and Ptot are unchanged). This quantity is known as the inspired O2 partial pressure, given by PIO_2 = XO_2 × (Ptot – Psat(37)) = – XO_2⋅Psat(37), where Psat(37) = 47 mmHg is the saturation vapor pressure of water at body temperature 37°C and XO_2 × Psat(37) = 10 mmHg.
Present Atmospheric Level (PAL)
This unit is used in the geochemistry community to express the abundance of O2 in the atmosphere in the distant past. The unit measures the total O2 abundance level in the atmosphere in relation to the present 21% level. An atmosphere containing 1.1 PAL has 10% more O2 molecules or 10% more O2 mass than our current atmosphere.
δ(O2/N2)
This is the conventional unit for reporting changes in atmospheric O2 abundance in the modern atmosphere. This unit expresses the relative deviation in the molar O2/N2 ratio from a standard value of this ratio:
δ(O2/N2) is typically multiplied by 106 and expressed in “per meg units,” where 1 per meg = 0.0001%. The O2/N2 ratio can be influenced both by changes in O2 or N2, but changes in N2 are typically much smaller.
Example Calculation
We start with an idealized well-mixed dry atmosphere that initially contains 21% O2, 78% N2, and 1% Ar. These figures refer to mol%. We assume the sum of the partial pressure of these gases equals 1 atm at sea level. We assume ideal gas behavior. The sea-level partial pressure of O2 is thus 0.21 atm, the dry air O2 mole fraction is 21% and the O2/N2 ratio is 21/78, which is taken as the standard ratio. The initial value of δ(O2/N2) is thus 0% or 0 per meg.
Suppose that 1% of the O2 content of the atmosphere is removed without any changes in N2 or Ar. With these assumptions, the final PAL level is 0.99 and the final O2/N2 ratio value is 0.99 × 21/79 corresponding to δ(O2/N2) = -1% or -10,000 per meg. The final dry air O2 mole fraction is 0.99 × 0.21/(0.79 + 0.99 × 0.21) = 0.208338, where the denominator (0.79 + 0.99 × 0.21) accounts for the decrease in total moles caused by the O2 loss. If CO2 is produced in association with the O2 loss, this would tend to offset the changes in the denominator. The final O2 partial pressure is given to a good approximation by 0.99 × 0.21 = 0.2079 atm, which assumes that total barometric pressure drops in proportion to total moles. Some minor limitations to this assumption are discussed in Appendix B.
Unit Conversion Factors
The above considerations suggest these conversion factors for global changes at sea level: 1 PAL = 37125 Pmol O2 = 106 per meg (δ(O2/N2)) = 159.1 mmHg (dry air partial pressure, ). Conversion based on these factors may be in error by a few percent depending also on changes in N2, CO2, and H2O, as discussed in Appendix B.
Appendix B. on the Proportionality Between and Global O2 Inventory
Figure 3 computes changes in from the results of Shaffer et al. (2009) on the assumption that is proportional to the total O2 inventory. Here we identify several corrections to this assumption, showing they are all small.
To relate to the composition of the atmosphere, we take
where Ni and mi are the number of moles and molecular mass of species i, XO_2 is the dry air O2 mole fraction (0.2094), WO_2 is the O2 mass fraction of the entire atmosphere (∼0.2314), Ptot is barometric pressure at sea level, g is gravitational acceleration, and A is Earth’s surface area. The sums in B2–B4 implicitly include contributions from all other trace gases in dry air. The sums in B3 and B4 also include the contribution from water vapor but the sum B2 for dry air does not.
To evaluate the dependence of on O2 amount, we take the logarithmic derivative of Eq. B1 with respect to NO_2 with substitutions from B2 to B4, yielding
The assumption that is proportional to NO_2 thus underestimates changes in by 2.2%. This small correction arises because barometric pressure at sea level does not scale in proportion to the number of moles of air (as would be true for an ideal gas in a constant volume) but rather with atmospheric mass. O2 has a molecular weight (32) that exceeds the average molecular weight of air (29) and thus makes a proportionally larger contribution to total mass than total moles.
is also sensitive to changes in the abundance of CO2, via the presence of NCO_2 in equations B2 and B3. Taking the logarithmic derivative of Eq. B1 with respect to NCO_2 with substitutions from B2 to B4 yields
where WCO_2 and XCO_2 are the weight and mole fractions of CO2 in air, defined similarly to B2 and B4. Assuming CO2 eventually rises to from a preindustrial level of 280 ppm to 1000 ppm (per A2 in Figure 3), this yields dP = (159)(0.516)(0.00100-0.000280) = 0.06 mmHg, which is negligible.
Atmospheric water vapor is also expected to increase with climate warming. Water vapor has no impact on the dry air mole fraction XO_2 but comprises 0.25% of the mass of the atmosphere (Trenberth and Smith, 2005), and thus contributes 0.25% to Ptot in Eq. B1. Assuming that the water content scales with warming according to the saturation vapor curve, and adopting warming estimate of 5°C from the A2 scenario (Figure 3), this yields an increase in water vapor of ∼40%. This causes barometric pressure to increase by 0.1%, corresponding to an increase in at sea level of 0.001 × (159 mmHg) = 0.16 mmHg. This compares to the drop in of 1.4 mmHg estimated under the A2 scenario due to global O2 loss (Figure 3) at year 2150, when Earth has warmed by 5C.
Warming causes the oceans to release N2 to the atmosphere due to the temperature dependence of the N2 solubility in seawater. Keeling et al. (2004) estimate a sensitivity of ∼2 × 10–6 of the atmospheric N2 inventory per 100 ZJ of ocean warming. Assuming the oceans as a whole eventually warm by an average of ∼4°C for the A2 scenario (e.g., Shaffer et al., 2009), this yields a relative increase in atmospheric N2 inventory of 0.04%, which in turn impacts both XO_2 and Ptot at the 0.04% level but in opposite directions. The impact on via Eq. B1 is less than 0.01% because the contributions from XO_2 and Ptot nearly cancel, and is thus completely negligible.
In summary, we have quantified several small corrections required to the estimated changes in in Figure 3. One correction results from changes in molecular weight of dry air with O2 loss, leading to a ∼2% correction in the direction of amplifying the decrease. A second and larger correction results from an increase in atmospheric water vapor which increases through the impact on barometric pressure. In the context of the future scenarios, this water vapor impact amounts to ∼12% of the calculated decrease in due to O2 loss, partially offsetting the overall decrease. The combined impact of both corrections is of order ∼10% in the direction of offsetting the decrease. Adjusting our calculation for these small effects would not change any conclusions. Other corrections due to changes in CO2 and N2 are even smaller.
Appendix C. Shaffer et al. (2009) Model Details
The A2 and B1 scenarios used in Shaffer et al. (2009) prescribe future fossil-fuel burning and land-use change, which drive CO2 emissions and O2 loss. The scenarios ascribe minor importance to land-use change emissions going forward. The intermediate-complexity carbon/climate model used in Shaffer et al. (2009) computes changes in CO2 and global temperature which drive additional changes in O2 from changes in ocean and land biogeochemistry (Shaffer et al., 2008). Shaffer et al. (2009) extend these scenarios past year 2100 using polynomial functions that taper emissions gradually toward zero around 2200. The A2 and B1 scenarios are often considered to bracket possible outcomes: the A2 scenario allows for emissions growth without any mitigation (“business as usual”), while B1 assumes emissions are partly curtailed to mitigate climate changes. The model assumes O2:C stoichiometric ratios of 1.391 for fossil-fuel burning and 1.1 for land biospheric exchanges.
Shaffer et al. (2009) report O2 changes in units of partial pressure, which they calculate by multiplying the estimated preindustrial sea level O2 partial pressure of 0.2094 atm by the relative change in the total atmospheric O2 inventory from their model. From the considerations Appendix B, we interpret these results as corresponding to .
The predictions shown in Figure 3 used a prescribed climate sensitivity of 3°C per doubling of CO2. Shaffer et al. (2009) also carried out simulations with a higher sensitivity of 4.8°C. The choice of climate sensitivity has very little impact on decreases in at sea level because this sensitivity impacts only the land and ocean O2 sources and not the O2 losses from fossil-fuels and land-use. The changes in at higher altitudes are somewhat sensitive to the assumed climate sensitivity. Higher climate sensitivity causes more warming and therefore larger increases in barometric pressure and at higher altitude while also lowering the cross-over altitude.
Keywords: atmospheric oxygen, fossil fuels, global change, evolution, V.O 2max, human health, hypoxia, high altitude
Citation: Keeling RF, Powell FL, Shaffer G, Robbins PA and Simonson TS (2021) Impacts of Changes in Atmospheric O2 on Human Physiology. Is There a Basis for Concern? Front. Physiol. 12:571137. doi: 10.3389/fphys.2021.571137
Received: 10 June 2020; Accepted: 11 January 2021;
Published: 02 March 2021.
Edited by:
Phil Neil Ainslie, University of British Columbia, CanadaReviewed by:
Heimo Mairbäurl, Heidelberg University Hospital, GermanyMike Tymko, University of British Columbia Okanagan, Canada
Copyright © 2021 Keeling, Powell, Shaffer, Robbins and Simonson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ralph F. Keeling, cmtlZWxpbmdAdWNzZC5lZHU=
 Ralph F. Keeling
Ralph F. Keeling Frank L. Powell
Frank L. Powell Gary Shaffer
Gary Shaffer Peter A. Robbins
Peter A. Robbins Tatum S. Simonson2
Tatum S. Simonson2