
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Physiol., 17 November 2020
Sec. Systems Biology Archive
Volume 11 - 2020 | https://doi.org/10.3389/fphys.2020.602844
This article is part of the Research TopicSystems Biology of Cell SignalingView all 16 articles
In pancreatic α-cells, intracellular Ca2+ ([Ca2+]i) acts as a trigger for secretion of glucagon, a hormone that plays a key role in blood glucose homeostasis. Intracellular Ca2+ dynamics in these cells are governed by the electrical activity of voltage-gated ion channels, among which ATP-sensitive K+ (KATP) channels play a crucial role. In the majority of α-cells, the global Ca2+ response to lowering external glucose occurs in the form of oscillations that are much slower than electrical activity. These Ca2+ oscillations are highly variable as far as inter-spike intervals, shapes and amplitudes are concerned. Such observations suggest that Ca2+ dynamics in α-cells are much influenced by noise. Actually, each Ca2+ increase corresponds to multiple cycles of opening/closing of voltage gated Ca2+ channels that abruptly become silent, before the occurrence of another burst of activity a few tens of seconds later. The mechanism responsible for this intermittent activity is currently unknown. In this work, we used computational modeling to investigate the mechanism of cytosolic Ca2+ oscillations in α-cells. Given the limited population of KATP channels in this cell type, we hypothesized that the stochastic activity of these channels could play a key role in the sporadic character of the action potentials. To test this assumption, we extended a previously proposed model of the α-cells electrical activity (Diderichsen and Göpel, 2006) to take Ca2+ dynamics into account. Including molecular noise on the basis of a Langevin type description as well as realistic dynamics of opening and closing of KATP channels, we found that stochasticity at the level of the activity of this channel is on its own not able to produce Ca2+ oscillations with a time scale of a few tens of seconds. However, when taking into account the intimate relation between Ca2+ and ATP changes together with the intrinsic noise at the level of the KATP channels, simulations displayed Ca2+ oscillations that are compatible with experimental observations. We analyzed the detailed mechanism and used computational simulations to identify the factors that can affect Ca2+ oscillations in α-cells.
Pancreatic islets respond to changes in blood glucose levels so that β-cells secrete insulin when blood glucose is elevated and α-cells secrete glucagon when it is low. Glucagon mobilizes glucose from the liver and when normoglycemia is reestablished, glucagon release from α-cells is suppressed. Extrinsic and intrinsic factors are involved in glucagon secretion (Briant et al., 2016; Wendt and Eliasson, 2020). Individuals with diabetes often show an impaired glucagon secretion that contributes to their hyperglycaemia (D’Alessio, 2011; Gilon, 2020). However, the detailed mechanism by which α-cells regulate glucagon secretion is not fully understood (Yu et al., 2019).
Pancreatic α-cells are electrically excitable and stimulation of glucagon secretion is secondary to repetitive action potential (AP) firing. In a low glucose medium, AP’s occur with a frequency of ∼1–3 Hz. Depolarization of the α-cell plasma membrane allows Ca2+ to enter through voltage-gated Ca2+ channels, which leads to the exocytosis of secretory granules of glucagon. In agreement with this mechanism, electrical stimulation of α-cells leads to an increase in cell membrane capacitance due to granule fusion, a well-known Ca2+ dependent process that precedes glucagon release (Barg et al., 2000; Voets, 2000; Göpel et al., 2004; González-Vélez et al., 2012).
Electrical activity in α-cells is thus accompanied by an increase in intracellular Ca2+ concentration ([Ca2+]i), which results from the activation of voltage-gated Ca2+ channels. Interestingly, this rise in [Ca2+]i occurs in the form of oscillations with an average frequency of ∼0.5 min–1, which is much lower than that of the AP’s. Parallel measurements of electrical activity and [Ca2+]i revealed that each oscillation corresponds to a burst of AP’s and that the amplitude of the Ca2+ increase correlates with the frequency of AP’s (MacDonald et al., 2007; Quoix et al., 2009; Le Marchand and Piston, 2010, 2012; Zhang et al., 2013; Kellard et al., 2020). These bursts of electrical activity are separated by quiescent periods during which [Ca2+]i is close to basal level. Ca2+ oscillations are observed in most α-cells, in low or high glucose medium, although they are much reduced in both amplitude and frequency in high glucose. A key characteristic of these oscillations is their irregularity. Their shape, frequency and amplitude are extremely variable, not only from one cell to another but also in the course of time for one individual cell (Kellard et al., 2020). In this study, we investigated the mechanism responsible for the intermittency of electrical activity and thus for the existence of slow, irregular Ca2+ oscillations.
Plasma membrane ATP-sensitive K+ channels (KATP channels) play a key role in controlling α-cells electrical activity, although the details of this control are still actively debated (Gilon, 2020; Zhang et al., 2020). When the amplitude of this current is relatively limited, voltage-gated Na+ and Ca2+ channels can indeed initiate an AP. The number of KATP channels simultaneously active is however surprisingly low, of the order of 10 (Rorsman et al., 2014). With such a low number of channels, it is expected that fluctuations of molecular origin would play a key role in the dynamical evolution of the KATP current and thus of the whole voltage and Ca2+ dynamics (Gonze et al., 2018). The low KATP channel activity in α-cells also results in a very high input resistance, meaning that small currents as those associated with openings of a few ion channels may have a drastic effect on membrane voltage and electrical activity (Rorsman et al., 2014). In agreement with this reasoning, noise-induced APs have been observed in α-cells and theoretically simulated (Diderichsen and Göpel, 2006). Here, we pushed this observation forward and investigated the possibility that fluctuations related to the small KATP current might be responsible for the intermittent character of electrical activity and hence for the noisy Ca2+ oscillations. This hypothesis, which is ideally investigated by mathematical modeling, holds with the observations that α-cells activity is highly variable even at a given external glucose concentration and that there is no “typical” α-cell signature (Kellard et al., 2020). Importantly, we here focus on the mechanism responsible for the existence of slow, irregular Ca2+ oscillations and not on the actively debated mechanism of regulation of glucagon secretion. Regulation of glucagon secretion indeed involves both intrinsic mechanisms and paracrine signals. Here, we only take into account intrinsic processes. Since intermittent electrical activity and slow [Ca2+]i oscillations have also been observed in isolated α-cells (Salehi et al., 2006; Quoix et al., 2009; Tuduri et al., 2009; Le Marchand and Piston, 2010), we indeed reasoned that paracrine signaling is not essential for their existence although it affects their characteristics (Briant et al., 2018b; Kellard et al., 2020).
Mathematical models have been developed to studyα-cells electrical activity and glucagon secretion. Diderichsen and Göpel (2006) used experimental data from patch clamp experiments onpancreatic α-cells located on the surface of intactmouse islets to develop an accurate model of plasma membraneelectrical activity. This model was extended to include firstCa2+ dynamics and secretion (Watts and Sherman, 2014), and laterglucagon-like peptide 1 (GLP-1) and adrenaline effects (Montefusco and Pedersen, 2015) as well as the α-cell heterogeneity by introducing realistic cell-to-cell variations in the values of the parameters (Montefusco et al., 2020). A functional identification of the islet cell types based on their electrophysiological characteristics allowed to improve the agreement between experiments and simulations of these models (Briant et al., 2017). Diderichsen and Göpel’s model was also re-used by Grubelnik et al. (2020) to study the link between the deformities in mitochondrial ultrastructure observed in α-cells of type 2 diabetes mellitus mice and glucagon secretion. On the other hand, Fridlyand and Philipson (2012) adapted a model initially developed to describe β-cells dynamics to propose a detailed description of α-cells electrical activity, Ca2+ changes, metabolism as well as paracrine and endocrine regulations. The effect of paracrine signaling (Watts et al., 2016; Briant et al., 2018b) on α-cells electrical activity was also investigated in models of pancreatic islets including β- and δ-cells. None of these studies have addressed the possible impact of the low number of KATP channels, nor the question of the mechanistic origin of cytosolic Ca2+ oscillations in α-cells.
The present study is based on the original Diderichsen and Göpel’s model of α-cells electrical activity. This core model is sequentially extended to take into account Ca2+ dynamics and random fluctuations of the KATP current via the Langevin formalism. We found that stochasticity in this current can indeed induce intermittent electrical activity and Ca2+ oscillations, but only for unrealistically small values of the opening and closing rates of this K+ channel. This theoretical prediction motivated us to further extend the model to take into account the variations of ATP concentrations that result from the activity of the plasma membrane Ca2+ ATPases. The resulting changes in ATP, which have been observed experimentally (Li et al., 2015), indeed slow down the dynamics of the KATP current and allow for intermittent electrical activity and Ca2+ oscillations resembling those observed experimentally. Finally, we used the model to investigate the sensitivity of calcium and electrical activities to key factors such as the number of KATP channels or the rate of Ca2+ pumping.
We base our model (Figure 1) on the mathematical description of the electrical activity of pancreatic α-cells proposed by Diderichsen and Göpel (2006), which was carefully calibrated on experimental data (see also Briant et al., 2017). The original model incorporates an ATP-sensitive K+ current (IKATP) that couples the level of external glucose to the electrical properties of the α-cell plasma membrane, through the sensitivity of the intracellular ATP/ADP ratio to external glucose concentration. It also describes a voltage-gated Na+ current (INa), a delayed rectifying (IKDR) and a A-type K+ current (IKA), an unspecific leak current (Ileak) and a L-type (ICaL) and a T-type Ca2+ current (ICaT). We also consider an additional type of Ca2+ current, because N-type (Quesada et al., 2008; González-Vélez et al., 2010) or P/Q type (Rorsman et al., 2014) Ca2+ currents have been shown to play a key role in glucagon secretion in rodents. Here, we arbitrarily chose to incorporate a N-type Ca2+ current (ICaN). Ca2+ concentrations are described differently just below the plasma membrane and in the cytoplasm. Ca2+ entry via the L-, T- and N-type Ca2+ channels increases Ca2+ concentration in a hypothetical sub-plasma membrane compartment. From this compartment, Ca2+ diffuses into the cytoplasm or leaves the cell via the plasma membrane Ca2+ ATPase (PMCA). Ca2+ efflux from the cell is thus accompanied by the hydrolysis of ATP into ADP. The resulting decrease in ATP concentration provokes an increase in the KATP conductance, thereby providing a feedback from Ca2+ changes on the electrical properties of the membrane. The main features of the model are described here below. Additional information about the equations and the values of the parameters can be found in the Supplementary Data and in the original study of Diderichsen and Göpel (2006).
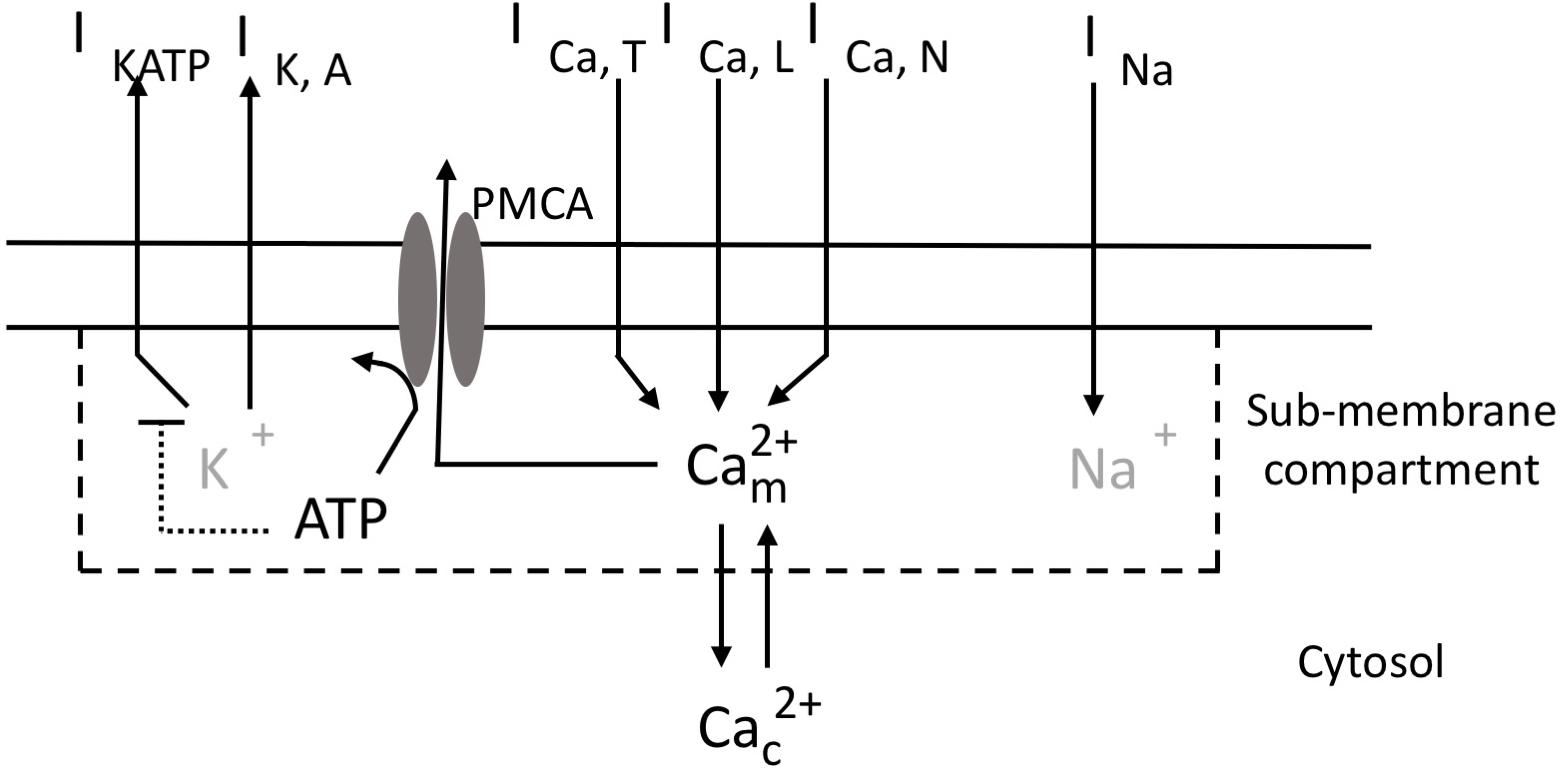
Figure 1. Schematic representation of ionic currents, Ca2+ fluxes and ATP consumption in pancreatic α-cells. Transmembrane currents are: IKATP, the ATP-sensitive K+ current; IKA, the high-voltage activated K+ current; ICaL, the high-voltage activated L-type Ca2+ current; ICaT, the low-voltage activated T-type Ca2+ current; ICaN, the high-voltage activated N-type Ca2+ current; INa, the voltage-gated Na+ current. The model describes the evolution of Ca2+ concentration in a fictitious sub-membrane compartment () and in the cytosol (). Exchanges between these 2 compartments occur by diffusion. Ca2+ is transported from the sub-membrane compartment into the extracellular medium by the ATP-consuming PMCA. ATP increases in the cytosol at a rate that depends on the concentration of extracellular glucose. ATP inhibits the ATP-sensitive K+ current (IKATP). K+ and Na+ concentrations (in gray) are not explicitly considered in the model.
Electrical activity is described by the following differential equation:
where V is the membrane voltage and Cm is the membrane capacitance set to 5 pF (Diderichsen and Göpel, 2006). For all currents, except for IKATP (see section “ATP-Sensitive K+ Current and ATP Evolution” below), we use the model proposed by Diderichsen and Göpel (2006) that is based on a Hodgkin-Huxley type description of ion channels and where the activation and inactivation functions have been fitted to experimental data. The high-voltage gated N-type Ca2+ current is described as in other studies (Csercsik et al., 2010; González-Vélez et al., 2010).
The evolution of sub-membrane Ca2+ concentration, noted Cam, is modeled as
where fr is the fraction of unbuffered Ca2+, F the Faraday constant and Vol, the volume of an α-cell. It is assumed that the sub-membrane shell represents 1/20 of the total volume of the cytoplasm. The factor 106 allows to get concentrations expressed in μM. Vb stands for a basal rate of Ca2+ entry, which ensures a ∼0.1 μM Ca2+ concentration when voltage-gated Ca2+ channels are closed. The third term represents Ca2+ pumping out of the cell through PMCA, which can be modeled by a Hill function with nH = 2 (Dupont et al., 2016). This active transport is accompanied by ATP hydrolysis. Thus,
with Ke being the Michaelis-Menten constant of ATP hydrolysis by the PMCA.
Finally, the last term of Eq. 2 represents Ca2+ diffusion into the bulk of the cytoplasm. Given a Ca2+ diffusion coefficient of 13 μm2/s (Allbritton et al., 1992) and a diameter of 8 μm for an α-cell (Zimny and Blackard, 1975) in which the nucleus occupies ∼70% of the cytoplasm, γ can be estimated to ∼0.01 ms–1 (see Supplementary Material).
The evolution of intracellular ATP follows
The first term of Eq. 4 is a phenomenological description of glycolysis in the form of Michaelis-Menten rate of ATP production from glucose. The second term represents ATP consumption by plasma membrane PMCA and is thus the same as in Eq. 2 except for the factor 2 that takes into account that one mole of ATP is hydrolyzed for two moles of Ca2+ transported. Rate constant k describes the consumption of ATP by other intracellular processes.
In agreement with Eq. 1, the evolution of cytoplasmic Ca2+ concentration is given by
In the original model (Diderichsen and Göpel, 2006), the ATP-sensitive K+ current is modeled as an ohmic ionic current with a constant conductance, i.e.,
where VK is the reversal potential for currents carried by potassium. The channel conductance, gKATP, was considered as a constant parameter and an increase in the extracellular glucose concentration was simulated by a decrease in the value of this parameter.
The main goal of the present study is to assess the effect of noise on the KATP current that arises in α-cells due to the small number of such channels. gKATP is thus not considered as a constant parameter anymore. Instead, we stochastically simulate opening and closing of these channels on the basis of the Langevin formalism. These channels can flicker between an open and a closed state, with opening and closing rate constants denoted α and β, respectively. Considering a noise term, the deterministic evolution equation for the fraction of open channels (s) becomes:
where ξ(t) is a random function of time. The last term is a noise function with 0 mean and
with NKATP the number of potentially openable KATP channels (Fall et al., 2005).
f2(ATP) takes into account that KATP channels are reversibly inhibited by ATP (Enkvetchakul et al., 2001). We chose the simple expression:
where Kinh is a constant representing the concentration of ATP leading to half-maximal inhibition.
When taking into account the stochasticity of the KATP channels, the gKATP conductance appearing in Eq. 6 is now computed as
with standing for the unitary conductance of a single ATP-sensitive K+ channel.
Together with the equations of the original model (Diderichsen and Göpel, 2006) listed in the Supplementary Data, Eqs 1–10 constitute our computational model that has been integrated in Matlab, using an Euler integration scheme with Δt = 0.012 ms. Bifurcation diagrams have been established using the AUTO package of XPPAUT (Ermentrout, 2002).
As explained in detail in the section “Results,” we consider models of increasing complexity to investigate the possible impact of the fluctuations in the KATP conductance on the existence of Ca2+ oscillations in α-cells.
Model 1 is the model proposed by Diderichsen and Göpel (2006), including N-type Ca2+ channels and two additional variables: subplasmalemmal Ca2+ (Cam, Eq. 2 with f1 = 1) and cytosolic Ca2+ (Cac, Eq. 5). It is thus defined by Eqs 1, 2, and 5.
Model 2 moreover includes stochasticity in the KATP conductance through Eqs 7, 8 and 10 that are considered in addition to the equations defining Model 1. f1 and f2 are taken equal to 1.
Model 3 allows to investigate the effect of the Ca2+-induced variations in ATP concentration. It is similar to Model 2, except that it includes Eq. 4 to describe the evolution of [ATP]. Changes in the concentration of this nucleotide impact on the evolutions of subplasmalemmal Ca2+ via f1 that is now given by Eq. 3 and of the KATP channel via f2 that is now given by Eq. 9.
When α-cells are electrically active, [Ca2+]i first rises just beneath the plasma membrane since Ca2+ is entering through voltage-gated channels located in this membrane. Sub-plasmalemmal Ca2+ is immediately buffered and diffuses in the cytosol where Ca2+ concentration is low at rest (∼100 nM). This attenuates Ca2+ increases below the membrane and transmits signaling to the rest of the cell. Additionally, sub-membrane Ca2+ is pumped out of the cell by Ca2+ ATPases. Model 1 takes these fluxes into account and combines a description of α-cell electrical activity with equations for the evolution of subplasmalemmal (Cam, Eq. 2) and cytosolic Ca2+ (Cac, Eq. 5). Simulations of Model 1 show that each AP generates one spike of Ca2+ in the subplasmalemmal space (Figures 2A,B). Considering that this fictitious compartment occupies 1/20 of the α-cell volume, corresponding to a shell thickness of ∼100 nm, Ca2+ increases up to a level between 0.5 and 1 μM depending on the value of gKATP. A supra-threshold increase in gKATP induces repetitive changes in Cam, whose frequency and amplitude are fixed by the value of this conductance. Because of the relative slowness of diffusion, cytosolic Ca2+ can only increase during repetitive AP’s (Figure 2C). When AP’s are sustained long enough, cytosolic Ca2+ remains nearly constant at a steady level (not shown). Ca2+ concentration in the cytosol does not exceed 0.4 μM. In this model, repetitive electrical activity always leads to a sustained increase in cytosolic Ca2+, which does not correspond to the slow Ca2+ oscillations observed experimentally in most α-cells.
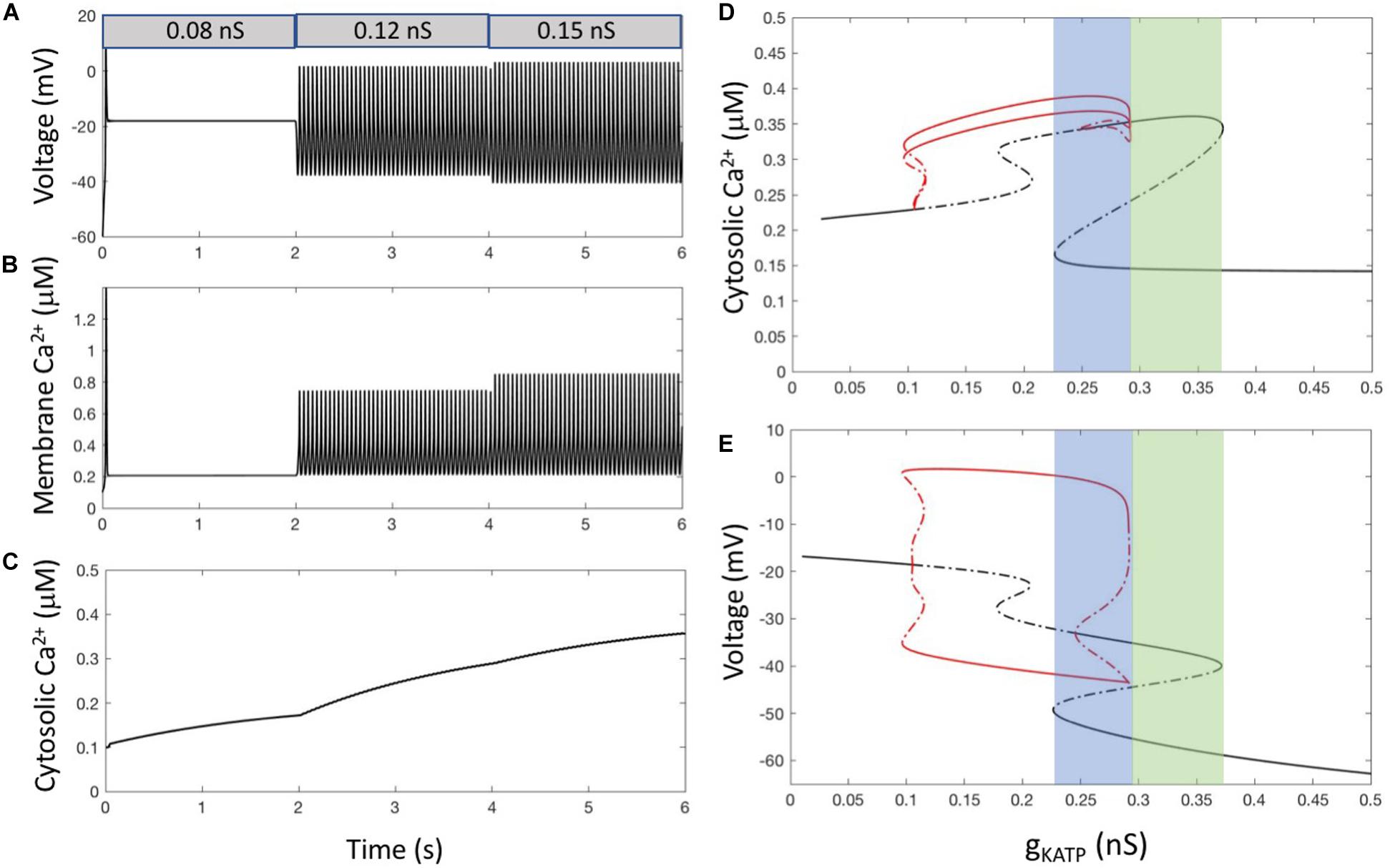
Figure 2. α-cell Ca2+ dynamics resulting from electrical activity. (A) Membrane voltage for 3 values of gKATP, simulating a decrease in external glucose. From t = 0 to 2 s, gKATP = 0.08 nS and gleak = 0.2 nS. From t = 2 to 4 s, gKATP = 0.12 nS and gleak = 0.2 nS. For t > 4 s, gKATP = 0.15 nS and gleak = 0.13 nS. The decrease in gleak that accompanies the increase in gKATP allows the model to reproduce the experimentally observed lower maximum voltage reached during repetitive AP’s at high glucose (Montefusco and Pedersen, 2015). (B) Corresponding changes in [Ca2+] in the sub-plasmalemmal compartment (Cam). (C) Corresponding changes in [Ca2+] in the cytosol (Cac). Because diffusion is much slower than electrical activity, cytosolic concentration does not follow the Ca2+ spikes occurring just below the membrane. (D,E) Bifurcation diagrams showing the evolution of Cac (D) and voltage (E) when changing gKATP. In both panels, black lines indicate steady states, while red lines show the minimum and maximum values reached during limit cycle oscillations. Plain lines indicate stable solutions and dashed lines instable ones. Bifurcation diagrams have been established using the AUTO package of xppaut (Ermentrout, 2002). For all panels, equations are those corresponding to Model 1 (see section “Outline of the Modeling Approach”) with the default values of parameters listed in Supplementary Tables 1, 2 of the Supplementary Material, except for the indicated values of gKATP and gleak. In panels (D,E), gleak = 0.2 nS.
The main characteristics of Ca2+ dynamics for different values of the KATP conductance are visible in the bifurcation diagram shown in Figure 2D. The shape of this diagram is most easily understood by looking at the companion bifurcation diagram showing how electrical activity depends on gKATP (Figure 2E). Since Ca2+ concentration does not feedback on the cell electrical activity in Model 1, Ca2+ changes can be seen as a simple output of the latter activity. Starting from large values of the KATP conductance at which cells are hyperpolarized (right part of the diagram), a decrease in this conductance leads to depolarization and electrical firing, as observed in experiments upon the addition of the KATP channel blocker tolbutamide. A further decline in the KATP conductance abolishes electrical activity and the cell remains in a constantly depolarized state. This decline in activity allows the model to reproduce the observation that α-cells are electrically inactive in the presence of large concentrations of external glucose without considering paracrine signaling.
The bifurcation analysis allows to uncover the existence of a range of values of gKATP for which multiple stable solutions can co-exist. This implies that different steady state solutions can be reached depending on the initial conditions. From 0.2265 to 0.2919 nS (blue region in Figures 2D,E), the system can potentially be in three states: a stable hyperpolarized state, an intermediate stable state (∼ −35 mV) or an electrically active state with large amplitude AP’s. However, the intermediate state, although stable from a physical point of view, is most probably of little physiological significance, as its basin of attraction is very limited. This implies that this state will rarely be reached, and that if reached, fluctuations of internal or external origin would push the system from this intermediate state to the hyperpolarized one or to the limit cycle corresponding to repetitive AP’s. For larger values of gKATP (from 0.2919 to 0.3715 nS, green region in Figures 2D,E), the labile, slightly polarized state coexist with the stable hyperpolarized one.
Figure 2C shows that this simple model predicts that when cells are in a stationary regime of repetitive AP’s, cytosolic Ca2+ tends to reach a steady state, although very small changes of concentration occur at each AP (∼20 nM). Such rapid and small changes are not expected to be visible experimentally.
In conclusion, slow Ca2+ oscillations resulting from intermittent electrical activity as observed in α-cells cannot result from repetitive AP’s controlled by a constant KATP conductance.
During electrical activity, the number of simultaneously active KATP channels is very small, of the order of 10 (Rorsman et al., 2014). Thus, the fluctuations related to internal noise cannot be neglected and the deterministic description of their contribution to membrane current (Eq. 6) must be replaced by a stochastic description. We thus considered the transitions of the channel between an open and a closed state, with a noise on this process as described above (Model 2 in section “Model of Plasma Membrane Electrical Activity”). Rate constants of channel opening and closing (α and β in Eq. 7) estimated from dwell time distributions are of the order of tenths of ms (Enkvetchakul et al., 2001). Assuming a unitary conductance () of 41 pS (Bokvist et al., 1999; Khan et al., 2001), the total number of KATP channels in the simulated cell needs to be around 60 to get an average of 5–10 simultaneously open channels during electrical activity, which corresponds to a global KATP conductance in the range 0.2–0.25 nS. This number fits in the large range of values estimated experimentally (Huang et al., 2011).
Simulations including noise at the level of KATP current display AP’s that are variable in amplitude and frequency. When the average value of gKATP is in the oscillatory range determined by the bifurcation diagram (Figures 2D,E), the simulated cell is electrically active (average cellular gKATP = 0.14 nS in Figures 3E–H), while the cell is in a fluctuating hyperpolarized state when the average value of gKATP corresponds to a stable steady state of the bifurcation diagram (average cellular gKATP = 0.47 nS in Figures 3A–D). Model simulations never result in Ca2+ oscillations whatever the values taken for the unitary conductance of the KATP channel () and the total number of such channels (NKATP) considered in the simulations. However, we observed that Ca2+ oscillations resembling experimental observations could be obtained in the simulations when ascribing to the rate constants of opening and closing of the KATP channels (parameters α and β) values at least 500 smaller than those reported from experiments (Figures 3I–L). In this case, an intermittent electrical activity generates bursts of AP’s, with each AP leading to a sharp Ca2+ increase in sub-membrane Ca2+. Sub-membrane Ca2+ diffuses in the cytoplasm, and because diffusion is slow, one burst of electrical activity involving multiple AP’s leads to one Ca2+ peak in the cytoplasm. Simulations shown in panels I–K display strong resemblance with experimental observations (MacDonald et al., 2007; Quoix et al., 2009; Le Marchand and Piston, 2010, 2012; Zhang et al., 2013; Kellard et al., 2020). The correlation between the cytosolic Ca2+ spike and the period of electrical activity has been shown in isolated mouse α-cells (see for example Quoix et al., 2009) and in α-cells from intact islets (Kellard et al., 2020).
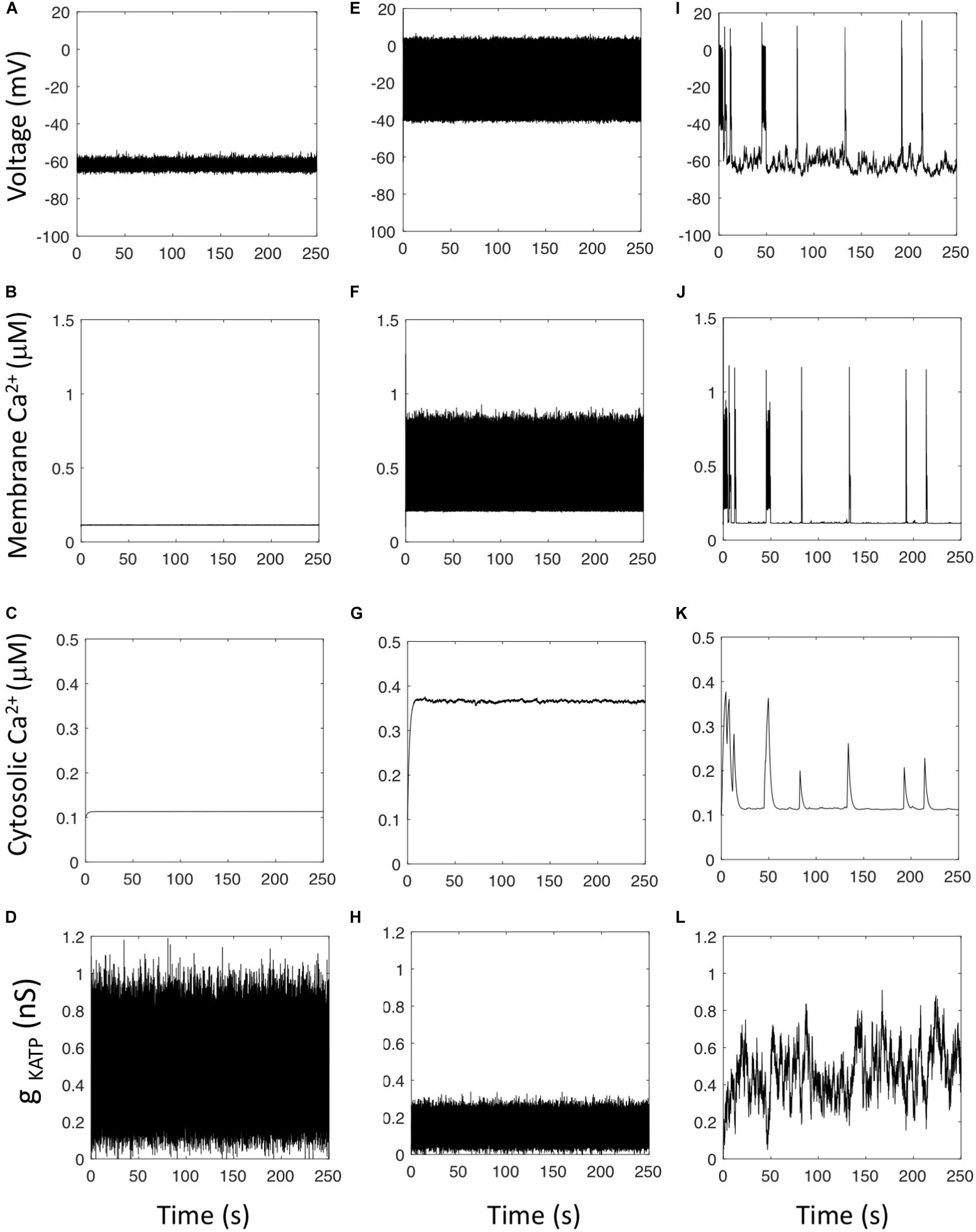
Figure 3. Effect of stochasticity in the KATP current. Panels show the evolution of the main variables of the model when the conductance of the KATP channel is described by a Langevin equation (Eqs 7 and 8 with f2 = 1) to take into account the randomness due to the small number of such channels in α-cells. (A–D) Rate constant of opening of the KATP channel, α = 0.1 ms–1, rate constant of closing of the KATP channel, β = 0.4 ms–1; unitary conductance of the KATP channel = 0.041 nS. The cell is in a quiet, hyperpolarized state despite the large variations of the total cell gKATP conductance. (E–H) When the unitary conductance of the KATP channel equals 0.012 nS, the cell is in a depolarized state resulting in a constantly high Ca2+ concentration. (I–L) When considering slow rates of opening and closing of the KATP channel, α = 1 10–4 ms–1 and β = 4 10–4 ms–1, simulations predict the occurrence of intermittent electrical activity leading to irregular Ca2+ spikes in the cytosol. Equations are those corresponding to Model 3 (see section “Outline of the Modeling Approach”) with the default values of parameters listed in the Supplementary Material, except for the indicated values of and g_leak = 0.2 nS.
From a mechanistic point of view, when the rate constants of opening and closing of the KATP channels are small, KATP current variations last long enough to induce a change in the electrical properties of the membrane: when the cell is hyperpolarized, a random decrease in gKATP of sufficient duration leads to a cell membrane depolarization that triggers electrical activity and Ca2+ entry. Similarly, when the cell membrane is electrically active, a random increase in gKATP of sufficient duration leads to a decrease in V that brings the cell in a resting state. With the experimentally reported values of channel opening and closing rates, the random changes in gKATP are too fast to induce AP’s. When simulating a stepwise decrease in gKATP from 0.47 to 0.2 nS in the absence of noise, the change in gKATP must last at least 110 ms to induce electrical activity (not shown). This agrees with the numerical observation that if the fluctuation-driven changes of gKATP have a characteristic time of a few ms as measured experimentally (Enkvetchakul et al., 2001), they will not induce AP’s (Figures 2A–C).
Thus, simulations predict that Ca2+ oscillations may in principle arise from noise-induced changes in the cell KATP current. However, the time scale of these changes must be much slower than that of the intrinsic dynamics of the voltage-gated ionic channels generating the AP’s. This does not correspond to the reported rates of opening and closing of the KATP channels and raises the possibility that some physiological process drives slow changes in the opening of the KATP channels.
KATP channels are regulated by variations of the intracellular ATP concentration (Bokvist et al., 1999). On the other hand, α-cells show oscillations in Ca2+ and ATP submembrane concentrations when observed in constant hypoglycemic conditions (Li et al., 2015). These oscillations are in opposite phase, most probably because Ca2+ transport out of the cell is an ATP-consuming process. Here, we investigate the hypothesis that even at constant external glucose, ATP/ADP changes may trigger changes in membrane electrical activity and Ca2+ entry that would thus be responsible for the observed, slow cytosolic Ca2+ oscillations. To this end, we consider [ATP] as a variable in Model 3, as well as its relationship with Ca2+ dynamics and its inhibitory effect on KATP channels conductance. For sake of simplicity, we consider that the conductance of these channels depends on [ATP] via an inhibitory function of the Michaelis-Menten type (Eq. 9). Keeping realistic values for the rates of opening and closing of the KATP channels (parameters α and β), simulations show highly variable Ca2+ oscillations that result from intermittent electrical activity (Figure 4). Each burst of electrical activity triggers a massive entry of Ca2+ in the subplasmalemmal compartment (Cam, Figure 4B), which then invades the cytoplasm (Figure 4C). Because PMCA are fully active, ATP is consumed, and the evolution of its concentration is the mirror image of that of cytosolic Ca2+ (Figure 4D). This reduced level of ATP favors a large KATP current, which in turn reduces electrical activity and Ca2+ entry, thus allowing ATP to rise again.
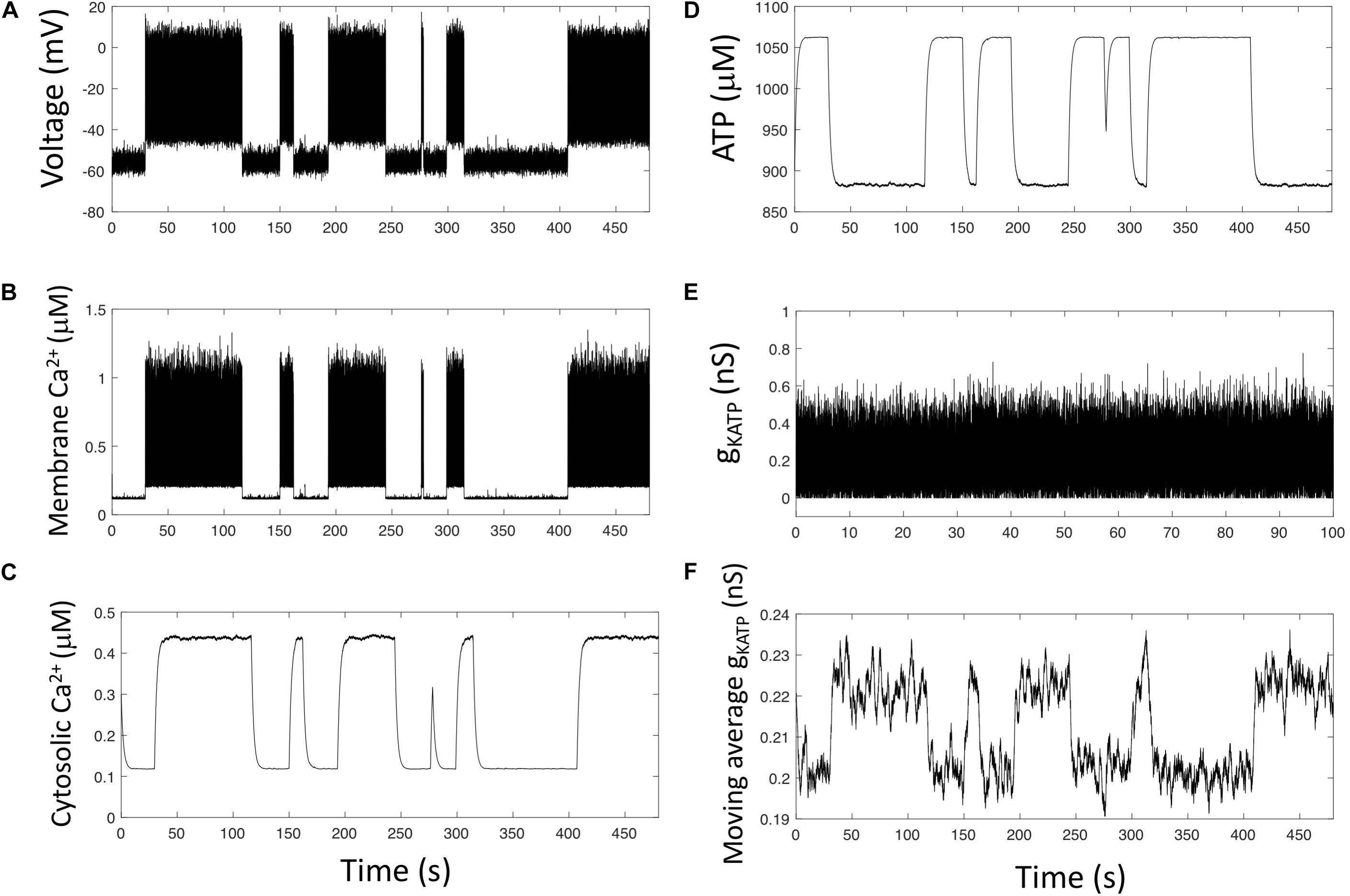
Figure 4. Simulations predict that changes in membrane ATP and stochasticity in the KATP current generate slow Ca2+ oscillations. (A–E) Numerical simulations of Model 3 (see section “Outline of the Modeling Approach”) that describes the opening and closing of the KATP channel by a Langevin stochastic equation and takes into account the consumption of ATP by PMCA as well as the inhibition of KATP channels opening by ATP. (F) The moving average of gKATP allows to visualize that, although highly random, gKATP tends to increase when ATP concentration is low and to decrease when ATP concentration is large. There are thus long-lasting trends in gKATP changes that can induce robust changes in α-cells electrical activity. Values of gKATP are averaged on a 200 s period.
Although fluctuations of the KATP conductance are very rapid (Figure 4E), gKATP is in average larger when [ATP] is low (periods of activity) than when [ATP] is high (quiescent periods) because of the inhibition of the KATP current by ATP. This is visible when computing the moving average of gKATP (Figure 4F). The trend in gKATP evolution indeed correlates with that of ATP. Such a trend in the moving average of gKATP does not appear when ATP is considered as a constant (Model 2, not shown). As a consequence of these trends, large, unlikely perturbations are necessary to switch from an inactive to an active period and vice-versa. Thus, the mutual interaction between ATP dynamics and electrical activity that occurs through the Ca2+ changes creates trends in the changes in electrical activity allowing for intermittent activity, despite the rapid fluctuations in gKATP.
The cytosolic Ca2+ oscillations shown in Figure 4C have widely different durations, from ∼15 s to ∼2 min. Because of stochasticity, these values are different from one simulation to the other. Given that glucagon secretion is triggered by Ca2+ increases above a certain threshold (González-Vélez et al., 2012), it is interesting to investigate what controls the ratio of active versus inactive periods. As randomness is required to initiate changes from an active to a quiescent period and vice-versa, it can be expected that the number of KATP channels will play a key role in controlling the number of transitions and thus the activity ratio. To investigate the effect of this factor, we performed simulations with different values of NKATP and computed the fraction of time during which cells exhibit electrical activity (Figure 5C) as well as the average number of Ca2+ spikes during a 500 s simulation (Figure 5E). In the simulations, changes in the values of NKATP were accompanied by changes in the single channel unitary conductance () in such a way that the product remains constant. Given this constraint, the global cell KATP conductance remains the same for all simulations, which allowed us to only investigate the effect of changes in randomness.
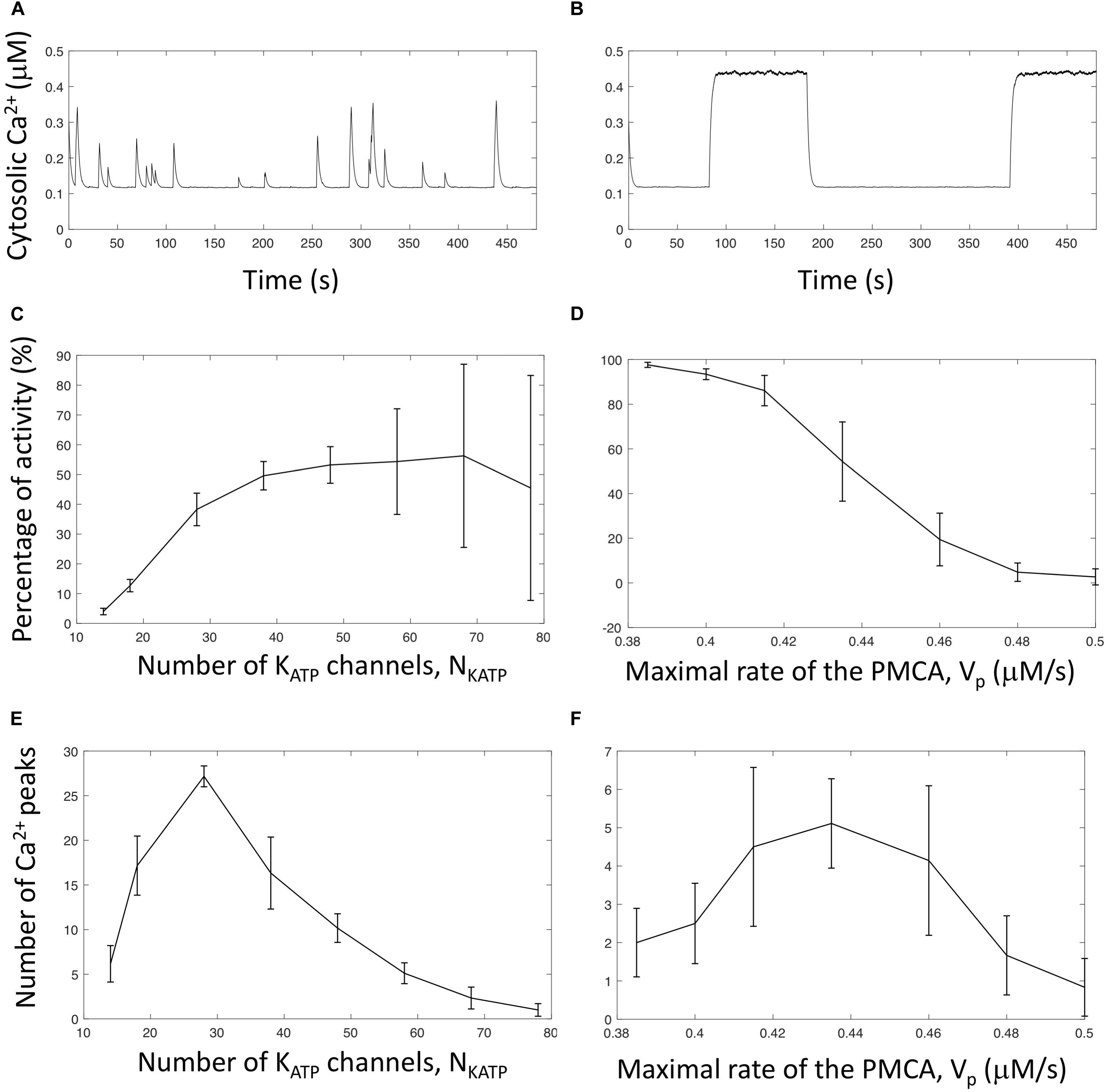
Figure 5. Effect of the number of KATP channels and rate of PMCA on the pattern of Ca2+ oscillations in α-cells. (A) Time series of cytosolic Ca2+ for NKATP = 14 and = 0.170 nS. The maximal global cellular KATP conductance () is the same as in Figure 4, but the number of participating channels is smaller. (B) Time series of cytosolic Ca2+ for NKATP = 68 and = 0.03497 nS. The maximal global cellular KATP conductance () is the same as in Figure 4, but the number of participating channels is larger. (C) Normalized duration of activity as a function of NKATP. (D) Normalized duration of activity as a function of the maximal velocity of the PMCA (Vp). (E) Number of cytosolic Ca2+ peaks in a 500 s period as a function of NKATP. (F) Number of cytosolic Ca2+ peaks in a 500 s period as a function of the maximal velocity of the PMCA (Vp). Panels (C,D) show the times during which cytosolic Ca2+ exceeds 0.3 μM, divided by the total simulation time (500 s). These ratios are expressed in percentages. Six independent simulations have been run for NKATP ≤ 58, and 9 for NKATP > 58. For panels (E,F), a peak is counted when Cac crosses 0.3 μM with a positive slope. Six independent simulations have been run for all values of Vp.
As visible in Figure 5A, when the number of channels is very small (NKATP = 14), the cell membrane is most of the time hyperpolarized and infrequent, short-duration and low-amplitudes Ca2+ spikes occur. If NKATP is smaller than 10, the cell is always in a resting state, although the average value of the cell KATP conductance (0.21 nS) is in the oscillatory domain (see Figure 2D). Fluctuations in gKATP are indeed so important that random depolarizations of the cell membrane are never long enough to initiate an AP. From NKATP = 10, infrequent Ca2+ spikes start to occur. They are very short in duration and amplitude because, once initiated, they are rapidly aborted by a fluctuation that repolarizes the cell. As a consequence, average Ca2+ and ATP concentrations are near their resting levels. These concentrations respectively increase and decrease when considering more channels in the simulations, i.e., when randomness is less pronounced. This is due to an increase in both the number of Ca2+ peaks (i.e., in the number of bursts of electrical activity, Figure 5E) and the duration of the peaks. Both changes are due to a possibly longer effect of random changes in gKATP allowing to initiate changes in electrical activity. When NKATP gets still larger, simulations exhibit a small number of Ca2+ spikes of very long duration (Figure 5B). Fluctuations indeed decrease and once in a state, active or resting, the cell tends to remain in this state. In consequence, the number of spikes on a 500 s period becomes smaller (Figure 5E), and the average time of activity larger (Figure 5C). It should be emphasized that for all simulations presented in Figures 5C and E, the average value of gKATP remains approximatively the same (∼0.21 nS). In the deterministic analysis, this value corresponds to repetitive AP’s (Figure 2D). The different behaviors observed for different values of NKATP thus exclusively rely on the characteristics of the noise. This computational observation provides an explanation for the widely different profiles of Ca2+ oscillations observed in single α-cells that probably express different number of KATP channels.
In a given cell characterized by a fixed number of KATP channels, bursts of electrical activity and Ca2+ oscillations are sensitive to factors affecting Ca2+ dynamics and particularly to the maximal rate of the PMCA, as shown in Figures 5D and F. This velocity indeed affects both Ca2+ pumping and ATP hydrolysis. When pumping is slower that in the control situation (Figure 4), Ca2+ and ATP concentrations at the membrane remain large, which tends to decrease the number of bursts of activity and increase their duration. Upon an increase in Vp, bursts become shorter. Thus, the number of spikes in a given period of time reaches a maximum value. When Vp further increases, the decrease in ATP concentration prevents this nucleotide from inducing trends in gKATP changes and Ca2+ spikes finally disappear.
Simulations thus predict that the interplay between Ca2+ and ATP dynamics that occur through PMCA activity can induce a trend in the noise-initiated changes in KATP conductance (Figure 6). Rapid fluctuations in the cell KATP conductance occur because of the small number of such channels involved in the electrical activity of α-cells. The resulting changes in membrane Ca2+ induce changes in ATP concentration, because Ca2+ and ATP levels in the submembrane space are coupled via the activity of the PMCA. ATP changes in turn feedback on the conductance of the KATP channels. As the concentration of this nucleotide evolves slowly, these changes are in average maintained on a period of time that is sufficiently long to induce or refrain electrical activity. Thus, the combination of randomness at the level of the KATP current and of ATP changes can account for the observed long-duration changes in electrical activity and thus for the Ca2+ oscillations observed in α-cells.
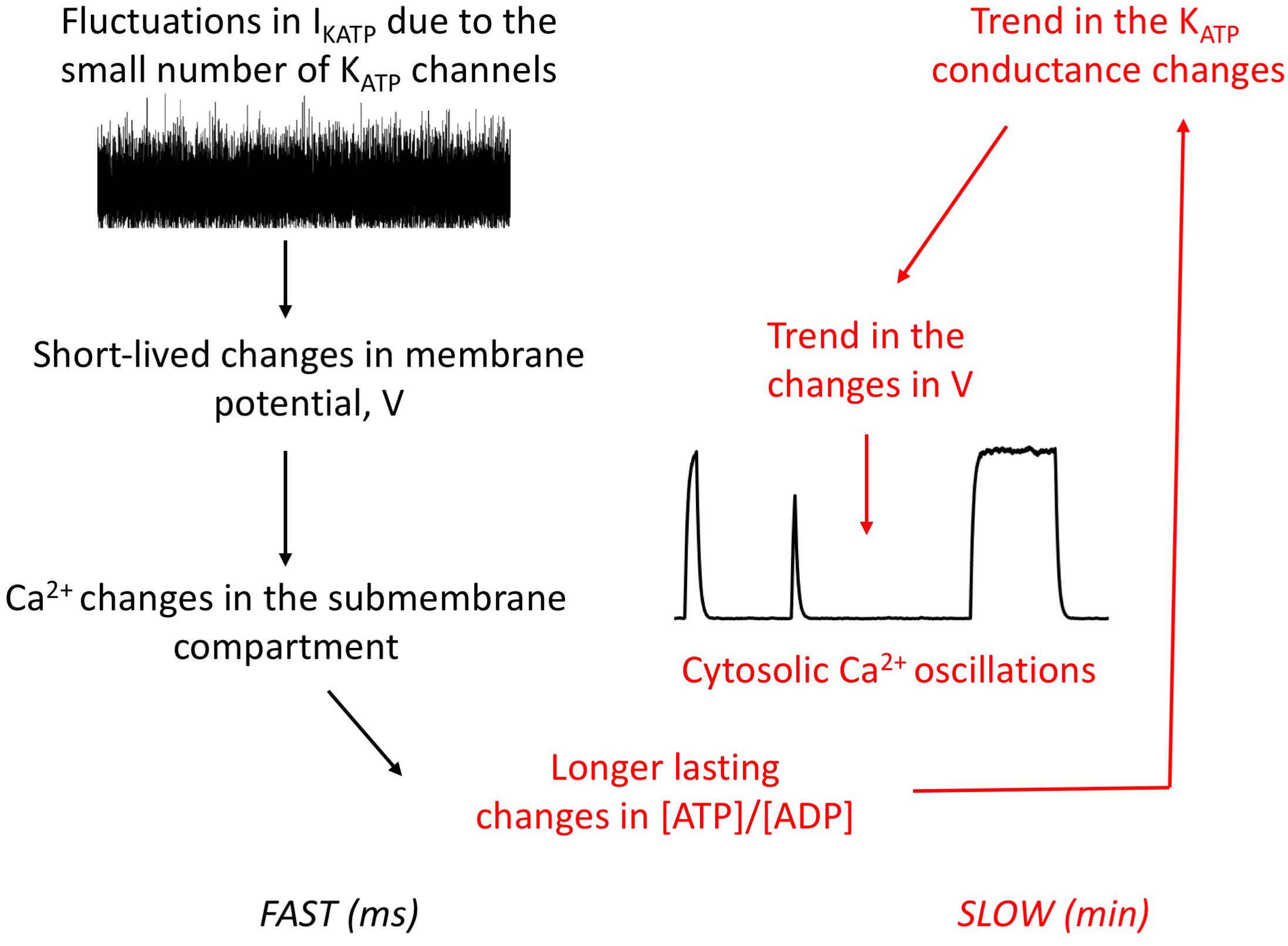
Figure 6. Schematic representation of the interplay between randomness of the KATP current and ATP changes to generate irregular, slow Ca2+ oscillations in α-cells. Rapid fluctuations in the KATP current due to the small number of channels in the α-cell plasma membrane create fluctuations in membrane voltage that can induce a short-lived change in membrane voltage, which in turn modifies submembrane Ca2+ concentration. If the dynamics of ATP is not considered, this noise-induced change in Ca2+ would last some milliseconds. If ATP is considered, large fluctuations create a change in ATP concentration of larger duration, because ATP kinetics is much slower than electrical activity. Despite the rapid noise on V that is still present, this will create a trend in the changes in V because of the feedback exerted by ATP on the KATP current. Thus, periods of quiescence and activity will alternate, which is responsible for cytosolic Ca2+ oscillations by diffusion of the membrane Ca2+.
In low glucose conditions, more than half of α-cells display Ca2+ oscillations and these oscillations can persist in high glucose, although much reduced in frequency and amplitude. These Ca2+ oscillations are highly variable in shape, duration and interspike interval, not only among different cells but also in the course of time in a given individual cell. Such a randomness strongly suggests the presence of a high level of molecular noise, which generally results from a low number of copies of one or several biochemical species (Gonze et al., 2018). In direct agreement with this theoretical concept, α-cells electrical activity involves a limited number of KATP channels that play a crucial role in their electrical activity (Rorsman et al., 2014). The aim of this study was to investigate the link between the small numbers of KATP channels and the existence of irregular, slow cytosolic Ca2+ oscillations in α-cells in the rigorous framework of a mathematical model closely based on experimental observations. As Ca2+ oscillations are observed not only in α-cells of the intact pancreas but also in isolated cells (Salehi et al., 2006; Quoix et al., 2009; Tuduri et al., 2009; Le Marchand and Piston, 2010), they most probably result from an intrinsic mechanism, modulated by paracrine signaling.
We thus built a model of increasing complexity, on the basis of a previously proposed model of the α-cells electrical activity (Diderichsen and Göpel, 2006). This sequential approach showed that the randomness of the KATP current does not per se allow for Ca2+ oscillations. Random changes in this current are indeed too fast to induce any long-term change that could induce intermittent electrical activity. However, when taking into account the triangular relationship between electrical current, Ca2+ changes and ATP consumption, the model can account for the electrical and calcium dynamics experimentally observed in α-cells. Moreover, it also accounts for the fact that these dynamics are not stereotypic as quite moderate changes in the number of KATP channels can induce significant changes in the pattern of Ca2+ oscillations. The model predicts that factors that interfere with cell Ca2+ changes – such as pumping rates, diffusion coefficients or buffering capacities – may modify the durations during which repetitive AP’s occur. This prediction is relevant for the impaired glucagon response to hypoglycemia and hyperglucagonemia observed in type 2 diabetes.
The simulations predict that the number of simultaneously active KATP channels during electrical activity is between ∼5 and 10, in agreement with experimental observations (Zhang et al., 2020). This number is much smaller than the total number of channels considered in the simulations (NKATP = 58), which are all potentially openable. Their intrinsically low open probability and their inhibition by ATP explain why only a small fraction are open simultaneously. Moreover, we expect our value of NKATP to be underestimated since we did not take into account effective fatty acid metabolism in low glucose conditions (Briant et al., 2018a) nor the spatio-temporal dynamics of ATP. In any case, that intermittency in electrical activity can be obtained with the model over a relatively large range of values of NKATP, as shown in Figure 5, indicates that the proposed mechanism for Ca2+ oscillations in α-cells is robust.
A similar role for stochasticity at the level of the KATP current has been put forward to account for the irregularity of the neuronal firing pattern in hippocampal CA3 neurons (Huang et al., 2007). However, in this case, the changes in KATP current are due to rapid, random fluctuations in the concentration of ATP, which results in an irregular frequency of AP’s and not in intermittent electrical activity. In the more general context of networks dynamics, it is known that fluctuations can propagate along the different nodes of the network with a rate of decay or enhancement that depends on the network’s structure (Maslov et al., 2007; Zhang et al., 2012).
Within this study, we did not investigate the behavior of the model when changing external glucose or considering paracrine signaling, which are left for further study. These limitations are due to the fact that the model used for electrical activity (Diderichsen and Göpel, 2006) does not reproduce one of the key characteristics of α-cells when raising external glucose, i.e., the reduction in the amplitude of the AP’s (see Ashcroft and Rorsman, 2013 for example). This reduction leads to less activation of the Ca2+ channels linked to glucagon secretion. In Figure 2, this reduction was simulated by an artificial change in the leak conductance when changing the conductance of the KATP channels to simulate the changes in external glucose. However, given the reliability of the Diderichsen and Göpel’s model and the many unknowns in the field of α-cells dynamics, we considered this unchanged model as a safe starting point to explore the impact of a stochastic KATP current and of changes in ATP concentration. However, we acknowledge that values of parameters for the ionic currents obtained with better methods of α-cell identification could improve the agreement between the simulations and the experiments (Briant et al., 2017; Montefusco et al., 2020).
The hydrolysis of ATP that parallels Ca2+ extrusion out of the cell plays a key role in the behavior predicted by the model. However, ATP synthesis is also sensitive to intracellular Ca2+ changes because mitochondrial metabolism is Ca2+-sensitive (Wacquier et al., 2019). The ATP/ADP ratio is also controlled by glycolysis and fatty acid metabolism (Briant et al., 2018a). Further studies are required to address these interrelationships in a more accurate way (Olivos-Santes et al., 2019), although the qualitative results obtained with the relatively simple model used in this study should be robust towards these numerous possible model refinements.
As another limitation, we only considered noise at the level of the KATP channel, while all molecular processes, and especially Ca2+ dynamics, are subjected to noise. This simplification was based on the experimental observation of an unusually small KATP conductance in α-cells, in particular in comparison with pancreatic β-cells that are known to be equipped with the same channels but still display very regular voltage and calcium patterns. However, the details of our conclusions may be affected by adopting a full stochastic description of the cell dynamics. In particular, it may lead to less stereotypic cytosolic Ca2+ changes, as reported in the experiments.
An interesting perspective would be to couple the model presented in this study to our previously proposed model that relates glucagon secretion to cytosolic Ca2+ changes (González-Vélez et al., 2012). The latter model is able to predict glucagon secretion using as an input experimentally obtained Ca2+ traces. It was found that glucagon secretion does not correlate with the frequency of Ca2+ oscillations. This investigation could be pursued by using Ca2+ time series obtained by simulations of the present model instead of experimental data. This could lead to identify the key elements of electrical and/or calcium activities controlling glucagon secretion.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
VG-V, AP, and GD contributed to the conceptualization, design of the study, development, simulation, and analysis of the models. GD and VG-V contributed to the preparation of the manuscript. All authors contributed to the article and approved the final version.
GD is research director at the Belgian FRS-FNRS. VG-V and GD acknowledge previous financial support of FNRS and CONACYT México through a bilateral cooperation project.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Vincent Verjans for scientific advice.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2020.602844/full#supplementary-material
Allbritton, N., Meyer, T., and Stryer, L. (1992). Range of messenger action of calcium ion and inositol 1,4,5-trisphosphate. Science 258, 1812–1815. doi: 10.1126/science.1465619
Ashcroft, F., and Rorsman, P. (2013). KATP channels and islet hormone secretion: new insights and controversies. Nat. Rev. Endocrinol. 9, 660–669. doi: 10.1038/nrendo.2013.166
Barg, S., Galvanovskis, J., Göpel, S., Rorsman, P., and Eliasson, L. (2000). Tight coupling between electrical activity and exocytosis in mouse glucagon-secreting α-cells. Diabetes Metab. Res. Rev. 49, 1500–1510. doi: 10.2337/diabetes.49.9.1500
Bokvist, K., Olsen, H., Hoy, M., Gotfredsen, C., Holmes, W., Buschard, K., et al. (1999). Characterisation of sulphonylurea and ATP-regulated Ca2+ channels in rat pancreatic α-cells. Eur. J. Physiol. 438, 428–436. doi: 10.1007/s004240051058
Briant, L., Dodd, M., Chibalina, M., Rorsman, N., Johnson, P., Carmeliet, P., et al. (2018a). CPT1a-dependent long-chain fatty acid oxidation contributes to maintaining glucagon secretion from pancreatic islets. Cell Rep. 23, 3300–3311. doi: 10.1016/j.celrep.2018.05.035
Briant, L., Reinbothe, T., Spiliotis, I., Miranda, C., Rodriguez, B., and Rorsman, P. (2018b). δ-cells and β-cells are electrically coupled and regulate α-cell activity via somatostatin. J. Physiol. 596, 197–215.
Briant, L., Salehi, A., Vergari, E., Zhang, Q., and Rorsman, P. (2016). Glucagon secretion from pancreatic α-cells. Upsala J. Med. Sci. 121, 113–119. doi: 10.3109/03009734.2016.1156789
Briant, L., Zhang, Q., Vergari, E., Kellard, J., Rodriguez, B., Ashcroft, F., et al. (2017). Functional identification of islet cell types by electrophysiological fingerprinting. J. R. Soc. Interface 14:20160999. doi: 10.1098/rsif.2016.0999
Csercsik, D., Farkas, I., Szederkenyi, G., Hrabovszky, E., Liposits, Z., and Hangos, K. (2010). Hodgkin-Huxley type modelling and parameter estimation of GnRH neurons. Biosystems 100, 198–207. doi: 10.1016/j.biosystems.2010.03.004
D’Alessio, D. (2011). The role of dysregulated glucagon secretion in type 2 diabetes. Diabetes. Obes. Metab. 13, (Suppl. 1), 126–132. doi: 10.1111/j.1463-1326.2011.01449.x
Diderichsen, P., and Göpel, S. (2006). Modelling the electrical activity of pancreatic α-cells based on experimental data from intact mouse islets. J. Biol. Phys. 32, 209–229. doi: 10.1007/s10867-006-9013-0
Dupont, G., Falcke, M., Kirk, V., and Sneyd, J. (2016). Models of Calcium Signalling. Berlin: Springer International Publishing., doi: 10.1007/978-3-319-29647-0
Enkvetchakul, D., Loussouam, G., Makhina, E., and Nichols, C. (2001). ATP interaction with the open state of the KATP channel. Biophys. J. 80, 719–728. doi: 10.1016/s0006-3495(01)76051-1
Ermentrout, B. (2002). Simulating, Analyzing, and Animating Dynamical Systems: A Guide to XPPAUT for Researchers and Students. Philadelphia, PA: SIAM.
Fall, C., Marland, E., Wagner, J., and Tyson, J. (2005). Computational Cell Biology. New York, NY: Springer.
Fridlyand, L., and Philipson, L. (2012). A computational systems analysis of factors regulating a cell glucagon secretion. Islets 4, 262–283. doi: 10.4161/isl.22193
Gilon, P. (2020). The role of α-cells in islet function and glucose homeostasis in health and type 2 diabetes. J. Mol. Biol. 432, 1367–1394. doi: 10.1016/j.jmb.2020.01.004
González-Vélez, V., Dupont, G., Gil, A., Gonzalez, A., and Quesada, I. (2012). Model for glucagon secretion by pancreatic α-cells. PLoS One 7:e32282. doi: 10.1371/journal.pone.0032282
González-Vélez, V., Gil, A., and Quesada, I. (2010). Minimal state models for ionic channels involved in glucagon secretion. Math. Biosci. Eng. 7, 793–807. doi: 10.3934/mbe.2010.7.793
Gonze, D., Gérard, C., Wacquier, B., Woller, A., Tosenberg, A., and Dupont, G. (2018). Modeling-based investigation of the effect of noise in cellular systems. Front. Mol. Biosc. 5:34. doi: 10.3389/fmolb.2018.00034
Göpel, S., Zhang, Q., Eliasson, L., Ma, X. S., Galvanovskis, J., and Kanno, T. (2004). Capacitance measurements of exocytosis in mouse pancreatic α-, β- and δ-cells within intact islets of Langerhans. J. Physiol. 556, 711–726. doi: 10.113/jphysiol.2003.059675
Grubelnik, V., Markovic, R., Lipovsek, S., Leitinger, G., Gosak, M., Dolensek, J., et al. (2020). Modelling of dysregulated glucagon secretion in type 2 diabetes by considering mitochondrial alterations in pancreatic α-cells. R. Soc. Open Sci. 7:191171. doi: 10.1098/rsos.191171
Huang, C. W., Huang, C. W., Chen, J., Tsai, J., and Wu, S. (2007). Glucose and hippocampal neuronal excitability: role of ATP-sensitive potassium channels. J. Neurosci. Res. 85, 1468–1477. doi: 10.1002/jnr.21284
Huang, Y.-C., Gaisano, H., and Leung, Y.-M. (2011). Electrophysiological identification of mouse islet -cells. From isolated single α-cells to in situ assessment within pancreas slices. Islets 3, 139–143. doi: 10.4161/isl.3.4.16166
Kellard, A., Rorsman, N., Hill, T., Armour, S., van de Bunt, M., Rorsman, P., et al. (2020). Reduced somatostatin signalling leads to hypersecretion of glucagon in mice fed a high-fat diet. Mol. Metab. 40:101021. doi: 10.1016/j.molmet.2020.101021
Khan, F., Goforth, P., Zhang, M., and Satin, L. (2001). Insulin activates ATP-sensitive K+ channels in pancreatic β-cells through a phosphatidyl-inositol 3-kinase-dependent pathway. Diabetes Metab. Res. Rev. 50, 2192–2198. doi: 10.2337/diabetes.50.10.2192
Le Marchand, S., and Piston, D. (2010). Glucose suppression of glucagon secretion. J. Biol. Chem. 285, 14389–14398.
Le Marchand, S., and Piston, D. (2012). Glucose decouples intracellular Ca2+ activity from glucagon secretion in mouse pancreatic islet alphα-cells. PLoS One 7:e47084. doi: 10.1371/journal.pone.0047084
Li, J., Yu, Q., Ahooghalandari, P., Gribble, F., Reimann, F., Tengholm, A., et al. (2015). Submembrane ATP and Ca2+ kinetics in α-cells: unexpected signaling for glucagon secretion. FASEB J. 29, 3379–3388. doi: 10.1096/fj.14-265918
MacDonald, P., De Marinis, Y. Z., Ramracheya, R., Salehi, A., Ma, X., Johnson, P., et al. (2007). A KATP channel-dependent pathway within α cells regulates glucagon release from both rodent and human islets of Langerhans. PLoS Biol. 5:e143. doi: 10.1371/journal.pbio.0050143
Maslov, S., Sneppen, K., and Ispolatov, I. (2007). Spreading out of perturbations in reversible reaction networks. New J. Phys. 9:273. doi: 10.1088/1367-2630/9/8/273
Montefusco, F., Cortese, G., and Pedersen, M. (2020). Heterogeneous alphα-cell population modeling of glucose-induced inhibition of electrical activity. J. Theor. Biol. 485:110036. doi: 10.1016/j.jtbi.2019.110036
Montefusco, F., and Pedersen, M. G. (2015). Mathematical modelling of local calcium and regulated exocytosis during inhibition and stimulation of glucagon secretion from pancreatic alphα-cells. J. Physiol. 593, 4519–4530. doi: 10.1113/jp270777
Olivos-Santes, E., Romero-Campos, H., Dupont, G., and González-Vélez, V. (2019). A modelling study of glycolytic oscillations and electrical activity in pancreatic alpha cells. Rev. Mex. De Ing. Biom. 40, 1–10.
Quesada, I., Tuduri, E., Ripoll, C., and Nadal, A. (2008). Physiology of the pancreatic α-cell and glucagon secretion: role in glucose homeostasis and diabetes. J. Endocrin. 199, 5–19. doi: 10.1677/joe-08-0290
Quoix, N., Cheng-Xue, R., Mattart, L., Zeinoun, Z., Guiot, Y., Beauvois, M., et al. (2009). Glucose and pharmacological modulators of ATP-sensitive K+ channels control [Ca2+]c by different mechanisms in isolated mouse α-cells. Diabetes Metab. Res. Rev. 58, 412–421. doi: 10.2337/db07-1298
Rorsman, P., Ramracheya, R., Rorsam, N., and Zhang, Q. (2014). ATP-regulated potassium channels in pancreatic alpha and beta cells: similar functions but reciprocal effects on secretion. Diabetologia 57, 1749–1761. doi: 10.1007/s00125-014-3279-8
Salehi, A., Vieir, E., and Gylfe, E. (2006). Paradoxical stimulation of glucagon secretion by high glucose concentrations. Diabetes Metab. Res. Rev. 55, 2318–2323. doi: 10.2337/db06-0080
Tuduri, E., Marroqui, L., Soriano, S., Ropero, A., Batista, T., Piquer, S., et al. (2009). Inhibitory effects of leptin on pancreatic α-cell function. Diabetes Metab. Res. Rev. 58, 1616–1624. doi: 10.2337/db08-1787
Voets, T. (2000). Dissection of three Ca2+-dependent steps leading to secretion in chromaffin cells from mouse adrenal slice. Neuron 28, 537–545. doi: 10.1016/s0896-6273(00)00131-8
Wacquier, B., Combettes, L., and Dupont, G. (2019). Cytoplasmic and mitochondrial calcium signaling: a two-way relationship. Cold Spring Harb. Perspect. Biol. 11:a035139. doi: 10.1101/cshperspect.a035139
Watts, M., Ha, J., Kimchi, O., and Sherman, A. (2016). Paracrine regulation of glucagon secretion: the β/α/δ model. Am. J. Physiol. Endocrinol. Metab. 310, E597–E611.
Watts, M., and Sherman, A. (2014). Modeling the pancreatic α-cell: dual mechanisms of glucose suppression of glucagon secretion. Biophys. J. 106, 741–751. doi: 10.1016/j.bpj.2013.11.4504
Wendt, A., and Eliasson, L. (2020). Pancreatic -cells – The unsung heroes in islet function. Sem. Cell and Dev. Biol. 103, 41–50. doi: 10.1016/j.semcdb.2020.01.006
Yu, A., Shuai, H., Ahooghalandari, P., Gylfe, E., and Tengholm, A. (2019). Glucose controls glucagon secretion by directly modulating cAMP in alpha cells. Diabetologia 61, 1212–1224. doi: 10.1007/s00125-019-4857-6
Zhang, H., Chen, Y., and Chen, Y. (2012). Noise propagation in gene regulation networks involving interlinked positive and negative feedback loops. PLoS One 7:e51840. doi: 10.1371/journal.pone.0051840
Zhang, Q., Dou, H., and Rorsman, P. (2020). ‘Resistance is futile?’– paradoxical inhibitory effects of KATP channel closure in glucagon-secreting α-cells. J. Physiol. 598, 4765–4780. doi: 10.1113/JP279775
Zhang, Q., Ramracheya, R., Lahmann, C., Tarasov, A., Bengtsson, M., Braha, O., et al. (2013). Role of KATP channels in glucose-regulated glucagon secretion and impaired counterregulation in Type 2 Diabetes. Cell Metab. 18, 871–882. doi: 10.1016/j.cmet.2013.10.014
Keywords: computational model, ATP-sensitive potassium channels, action potential, Langevin equation, stochastic channel, plasma-membrane Ca2+ ATPase
Citation: González-Vélez V, Piron A and Dupont G (2020) Calcium Oscillations in Pancreatic α-cells Rely on Noise and ATP-Driven Changes in Membrane Electrical Activity. Front. Physiol. 11:602844. doi: 10.3389/fphys.2020.602844
Received: 07 September 2020; Accepted: 28 October 2020;
Published: 17 November 2020.
Edited by:
Jianhua Xing, University of Pittsburgh, United StatesReviewed by:
Linford Briant, University of Oxford, United KingdomCopyright © 2020 González-Vélez, Piron and Dupont. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Geneviève Dupont, Z2R1cG9udEB1bGIuYWMuYmU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.