- 1Department of Biochemistry and Biotechnology, Vasyl Stefanyk Precarpathian National University, Ivano-Frankivsk, Ukraine
- 2D.F. Chebotarev Institute of Gerontology, National Academy of Medical Sciences (NAMS), Kyiv, Ukraine
- 3Department of Life Sciences, University of Modena and Reggio Emilia, Modena, Italy
- 4Department of Biology, Carleton University, Ottawa, ON, Canada
Anise hyssop, Agastache foeniculum, is a widely used medicinal herb with known antioxidant properties. We studied how dietary supplementation with dried A. foeniculum leaf powder affected physiological and metabolic traits as well as activities of antioxidant enzymes and markers of oxidative stress in Drosophila melanogaster. Dietary hyssop extended the lifespan in a sex and genotype independent manner over a broad range of concentrations up to 30 mg/ml. Dietary supplementation with the herb significantly increased fecundity, resistance to oxidative stress and starvation. Higher transcript levels of Drosophila insulin-like peptide (dilp2) and decreased dilp3 and dilp6 transcripts together with increased levels of glycogen and triacylglycerols support an alteration of insulin signaling by the plant extract. Increased enzymatic activities of superoxide dismutase and aconitase as well as elevated protein and low molecular mass thiols also supported an alteration of free radical process in flies treated with dietary A. foeniculum leaf powder. Thus, physiological and metabolic traits as well as free radical processed may be affected by active compounds detected in extracts of anise hyssop leaves and contribute to the increased lifespan and reproductive (egg-laying) activity observed.
Introduction
Anti-aging pharmacology is an extremely promising field as it could allow humans to substantially increase lifespan and healthspan (Vaiserman and Lushchak, 2017; Piskovatska et al., 2019). Aging is a normal physiological process that is regulated by a set of genes and signaling pathways that are evolutionarily conserved in eukaryotes. Recent research has concentrated on the influence of different naturally occurring compounds on the lifespan of model organisms. Pathways controlling lifespan and aging are partially conserved in a wide range of species, from yeast to humans (Bitto et al., 2015; Fontana and Partridge, 2015). Drosophila melanogaster is emerging as an important model to study anti-aging medications. Since D. melanogaster can be easily manipulated genetically and experimentally, it has served as a good model for examining the anti-aging properties of resveratrol (Bass et al., 2007; Wang et al., 2013), 4-phenylbutyrate (Kang et al., 2002), caffeine (Nikitin et al., 2008), curcumin (Lee et al., 2010), statin (Spindler et al., 2012), Rhodiola rosea (Gospodaryov et al., 2013), Rosa damascena (Schriner et al., 2012), blueberry extract (Peng et al., 2012), and many other natural compounds.
Longevity can be modulated by preventing age-related diseases, including cardiovascular disease, type 2 diabetes, cancer and Alzheimer’s disease. It is well-known that dietary antioxidants play potential roles in the prevention of age-related diseases (Meydani, 2001). Numerous studies have shown that plant extracts from R. rosea or Ludwigia octovalvis can extend lifespan of more than one model organism. In Drosophila, R. rosea delayed an age-related decline of locomotor activity and increased stress resistance (Gospodaryov et al., 2013), whereas Theobroma cacao increased lifespan in D. melanogaster due to its antioxidant properties (Bahadorani and Hilliker, 2008). Being known for its powerful antioxidant activity, antibacterial and hepatoprotective properties, rosemary extract (Rosmarinus officinalis L.) produced a longevity phenotype in Drosophila that was associated with increased superoxide dismutase and catalase activities (Wang et al., 2017). Extended lifespan was also observed when fruit flies were fed L. octovalvis that is a rich in antioxidants including polyphenolic compounds, phytosterols, and squalene in either regular or high-calorie diets (Lin et al., 2014).
Among herbs and spices, Agastache foeniculum (AF), known as anise hyssop, has been given much attention in particular for its high antioxidant activity and is often used for the production of essential oils. Traditional medicine applies AF for acute respiratory diseases, functional disorders of the gastrointestinal tract, and inflammatory diseases of the urinary system (Marcel et al., 2013). Three groups of compounds were identified in the essential oil of AF: monoterpenes (sylvestrene and 1-octen-3-ol acetate), phenylpropenes (methyl chavicol, eugenol, and methyl isoeugenol), and sesquiterpenes (β-caryophyllene, spathulenol, and caryophyllene oxide) (Ivanov et al., 2019). Studies have investigated the antimicrobial and antioxidant activity of hyssop essential oil (Ivanov et al., 2019), as well as antimutagenic, anti-nociceptive, anti-inflammatory and cytotoxic activity with cancer cell lines (Zielińska and Matkowski, 2014). Individual parts of the plant are used for different purposes but leaves are the most useful ones. Indeed, the leaves of hyssop can be used in herbal tea or added fresh in small quantities to a salad with other greens. The dried leaves can be used for medicinal purposes to treat coughs, fevers, wounds, and diarrhea. In this regard, we decided to evaluate the effect of AF on physiology, metabolism and free radical processes in D. melanogaster. We found that extracts of plant leaves are full of flavonoids and active compounds.
The results revealed that the lifespan of both sexes and two fly lines (Canton S and w1118) was extended significantly by dietary supplementation with different concentrations of AF dry leaves. Furthermore, AF significantly increased fertility, climbing ability, and resistance to oxidative stress and starvation of Canton S flies. Enzymatic activities of aconitase and catalase were also significantly increased when A. foeniculum was consumed and Canton S flies fed diets with AF added displayed lower body glucose content, but, higher levels of stored glycogen and triglycerides.
Materials and Methods
Analysis of Extracts by HPLC/MS
The composition of herbal extracts was analyzed using high-performance liquid chromatography (HPLC) with subsequent mass spectrometry. The high-performance liquid chromatography equipment was from Jasco (Tokyo, Japan) and included a pump (model PU-1580), UV detector (UV-1570), Rheodyne injector equipped with a 20 μL loop, and Jasco-Borwin software (rel. 1.5). Samples were separated using a 250 × 4.6-mm stainless-steel column Discovery-C18 4 μm 80 Ä (from Sigma-Aldrich). The eluates were (A) 0.5% acetic acid and (B) acetonitrile. Separations were performed at room temperature by solvent gradient elution from 0 min at 50% A/50% B to 60 min at 100% B at a flow rate of 0.8 mL/min. A UV detector set at 260 nm was also used on-line with the HPLC equipment. An Agilent 1100 VL series mass spectrometer (Agilent Technologies, Inc., Santa Clara, CA, United States) was further used on-line with the HPLC equipment. The electrospray interface was set in negative ionization mode with a capillary voltage of 3,500 V and a temperature source of 350°C in full scan spectra (200–2,200 Da, 10 full scans/s). Nitrogen was used as a drying (9 L/min) anebulizing gas (11 p.s.i.). Software versions were 4.0 LC/MSD trap control 4.2 and Data Analysis 2.2 (Agilent Technologies, Inc.).
Antioxidant Properties
Dry crushed leaves of Agastache foeniculum were purchased from a local store in Ivano-Frankivsk (Ukraine). Leaves were ground to a powder and an aqueous extraction was performed by adding the powder to hot water (85–90°C) in concentrations of 2.5, 5, 10, or 30 mg/ml. Liquid extracts were filtered and kept at 4°C for 24 h. Extracts were used the next day for experiments. This protocol of extraction was used because it closely matched the conditions for preparing food for fly experiments in which leaf powder was added into hot fly food (85–90°C) that was then poured into vials and kept at 4°C before use. To assess the antioxidant of ability, plant extracts were assessed for their ability to scavenge ABTS+ radical cations or reduce ferric ions were determined as described previously (Bayliak et al., 2016).
Resistance to Starvation and Oxidative Stress
Flies of Canton S strain were fed control or experimental food (5 or 10 mg/ml plant powder) for 30 days. Flies were separated under light CO2 anesthesia and kept overnight for recovery. Starvation resistance was measured in flies given only 1% agar as a food source. To study resistance to oxidative stress 25–30 flies of each experimental cohort were transferred into empty vials for 2 h for starvation. After starvation, flies were transferred into vials containing folded and rammed strips (2.4 × 12 cm) of 4-layer cellulose filter paper soaked with 0.8 ml of 20 mM menadione in 5% sucrose solution (Lushchak et al., 2014). Dead flies were counted at 9 a.m., 3 p.m., and 9 p.m. Stress resistance was expressed as the percentage of flies that survived over the time.
Enzyme Activities
Flies, fed control or herb-supplemented diet for 30 days were fixed by freezing in liquid nitrogen and kept at −80°C before use. For analysis were homogenized using a Potter-Elvejhem glass homogenizer (1:10 w:v) in cold 50 mM potassium phosphate buffer, pH 7.5, containing 0.5 mM EDTA and 1 mM phenylmethylsulfonyl fluoride. Centrifugation was performed at 16,000 g for 15 min at 4°C in an Eppendorf 5415R centrifuge (Germany). The supernatants were collected and used for the determination of enzyme activities.
The activities of superoxide dismutase (SOD) and catalase were measured as described previously (Lozinsky et al., 2012). Briefly, SOD activity was assayed at 406 nm by inhibition of quercetin oxidation by superoxide anion. One unit of SOD activity was defined as the amount of soluble protein that inhibited the maximum rate of quercetin oxidation by 50%. Catalase activity was determined by the rate of hydrogen peroxide decomposition at 240 nm. Enzyme activity was calculated using an extinction coefficient for hydrogen peroxide of 39.4 M–1 cm–1.
Aconitase activity was measured as a decrease in substrate concentration as described earlier (Lozinsky et al., 2013). Briefly, the decrease in absorbance at 240 nm was followed for 2 min and the extinction coefficient used for calculations was 3.701 M–1 cm–1 for cis-aconitate.
All reactions were started by addition of enzyme supernatant. Activities were measured at 25°C and expressed per milligram of soluble protein in the supernatant.
Protein and Low Molecular Mass Thiols
The content of free thiols was determined by the Ellman’s method using DTNB, as described previously (Lushchak et al., 2011). The content of protein thiols groups was calculated as the difference between total and low molecular mass thiols. The content of protein thiols was expressed as μmol per mg protein and low molecular weight thiols as mg per mg of wet weight.
Fly Stocks and Rearing
Fruit flies of Canton S (BDRC #64349) and w1118 (BDRC #3605) strains were obtained from Bloomington Stock Center (Bloomington, IN, United States). The flies were cultured in a standard molasses medium (7.5% molasses, 5% yeast, 6% corn, 1% agar, 0.18% methylparaben) in uncrowded (70–100 eggs/vial) conditions at 25°C, 60% humidity and photoperiod 12 L:12 D. Canton S flies were used throughout this study whereas flies of the w1118 strain were used only for lifespan assay.
Lifespan
Newly eclosed flies were transferred into fresh food and kept for 3 days for mating. Then flies were separated by sex under light CO2 anesthesia and kept for another day for recovery. About 150 flies of each strain and sex were gently transferred to 1.5 L demographic cages with an attached plastic vial filled with 5 ml control food or experimental food supplemented with different concentrations of anise hyssop (A. foeniculum) dry crushed leaves. The standard medium consisted of 5% of dry yeast, 5% of sucrose, 1.2% agar, and 0.18% methylparaben. Herbal powder was directly added to the experimental food (t = 70°C) in different concentrations (2.5, 5, 10, or 30 mg/ml). Food was changed every second day, and dead flies were removed and recorded. The experiment was run in two biological replicates. To study the effects of different yeast concentrations, diet with 5% yeast was defined as 1×. Yeast content was changed to 0.25% to obtain 0.05× diet, 1% to obtain 0.2× diet and 20% to get 4× diet. All experimental diets were supplemented with 5 or 10 mg/ml of herb powder.
Fecundity
To determine the impact of anise hyssop on fruit fly fecundity 20 female flies were placed into demographic cage supplemented with food vial. Amount of laid eggs was counted 24 h after fresh food was applied. The measurements were repeated every 3 days up to day 30. Four groups of flies were tested per condition.
Negative Geotaxis and Heat Shock Resistance
Flies were fed control or herb supplemented diet for 30 days. Negative geotaxis, sensitivity and recovery after heat shock were measured as described in Lushchak et al. (2012).
Glucose, Trehalose, Glycogen, and TAG
Flies were fed experimental diets for 30 days and that used for measurements of metabolites. Flies were decapitated and centrifuged 5 min at 3,000 g to extract hemolymph (Perkhulyn et al., 2015). Pre-weighted whole flies were homogenized in 50 mM sodium phosphate buffer pH 6.5 (1:10 w/v) and centrifuged. Resulted supernatants were used for the determination of glucose, glycogen and trehalose contents. Measurements were performed using a glucose assay kit (Liquick Cor-Glucose diagnostic kit, Cormay, Poland). Glycogen was converted into glucose by incubation with amyloglucosidase from Aspergillus niger at 25°C for 4 h followed by measuring glucose. Trehalose was determined in samples after incubation with porcine trehalase to digest trehalose into glucose. For TAG estimation weighed flies were homogenized in 200 mM PBST (phosphate buffered saline containing 0.05% Triton X100), boiled and centrifuged for 10 min at 13,000 g (Rovenko et al., 2015). TAG levels were measured using a diagnostic kit Liquick Cor-TG (PZ 290 Cormay S.A., Łomianki, Poland) following the manufacturer’s instructions. TAG levels were expressed as milligrams per gram of wet weight (mg/gww).
Gene Expression
Total RNA from heads or whole flies was extracted using an RNeasy Plus Mini Kit (Qiagen), concentration was measured, and 2 μg of total RNA were into converted cDNA with QuantiTect Reverse Transcription Kit (Qiagen). Expression of genes of interest was measured using an ABI Prism 7000 instrument (Applied Biosystems), a SensiFAST SYBR Hi-ROX Kit, and a QuantiTect SYBR Green PCR Kit (Qiagen) under conditions recommended by the manufacturer. Levels of mRNA were measured in heads (dilp2, 3, and 5) or whole flies (dilp6, akh, 4ebp, tobi, pepck, bmm). Each analytical and standard reaction was performed in three technical replicates. The ΔΔCt method was used with rp49 as the reference gene. All primers were as described earlier (Lushchak et al., 2015).
Statistical Analysis
Fly lifespans and survival under starvation or oxidative stress were compared by a Log Rank test. Activities of antioxidant enzymes, markers of oxidative stress and levels of metabolites were analyzed by a two-way ANOVA followed by a Dunnett’s multiple comparison test. Levels of specific mRNAs were compared by a Student’s t-test. Significant differences between groups were accepted by a p-value < 0.05. Statistical analysis was performed in Prism Graphpad 5 (GraphPad Software, San Diego, CA, United States).
Results
Active Compounds in Extract of Agastache foeniculum
Analysis of A. foeniculum extract with HPLC-MS identified the presence of many bioactive species previously described to possess beneficial properties. We detected acacetin, apigenin, 3-O-caffeoylquinic acid, calycosin, caffeic acid, 6,7-Dimethoxyquercetin 3-O-glucopyranoside, genistein, kaempferol 3-O-glucoside, rosmarinic acid, tilianin, ursolic acid, and β-sitosterol in significant amounts (Supplementary Table S1). These active compounds have been previously shown to increase the life- and healthspan of different model organisms including Drosophila. Moreover, varied additional benefits were described in relation to protection against oxidative stress.
Antioxidant Properties of A. foeniculum Extract and Fly Resistance to Oxidative Stress
The antioxidant properties of water extracts of A. foeniculum leaves were tested for their ability to scavenge ABTS+ radical cations or reduce ferric ions. An extract made of the herb at a concentration of 2.5 mg/ml had scavenging activity of ∼300 Trolox equivalents (Figure 1A). A further increase in herb concentration during extraction did not linearly increase the ability to scavenge ABTS cation. The activity was about 50% higher for the 5 mg/ml extract. Further increases of herb content to 10 and 30 mg/ml increased scavenging ability by approximately 2.2- and 3.3-fold (Figure 1A). A similar tendency was observed when the extracts were tested for the ability to reduce ferric ions (Figure 1B). Reducing ability was about 2.5 mg AAE/ml for the extract made with 2.5 mg/ml herb powder, whereas a 12-fold increase in herb content (30 mg/ml) increased the reduction ability by about 4-fold.
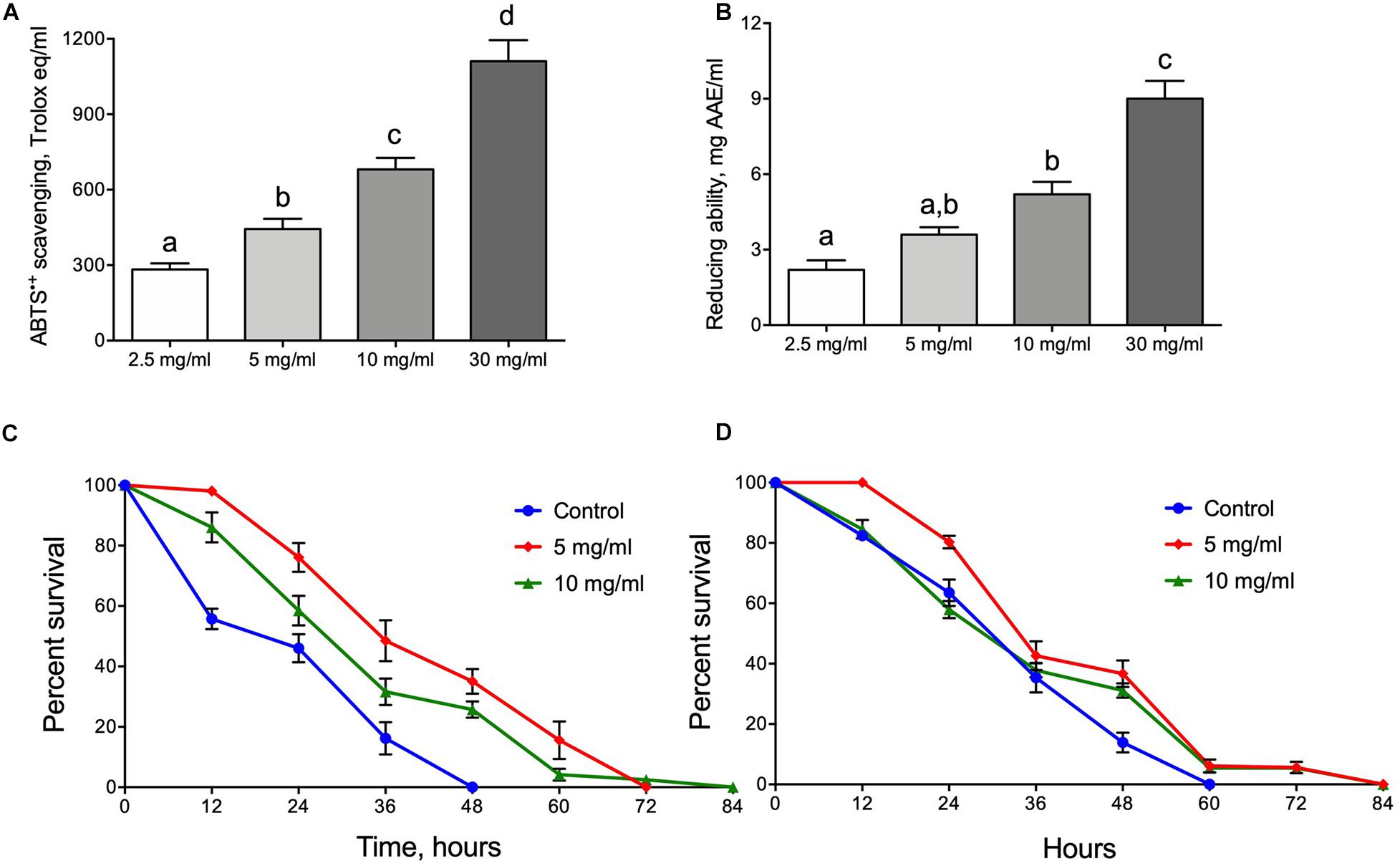
Figure 1. Antioxidant and protective properties of Hyssop extracts. ABTS+ radical scavenging activity (A) and ability to reduce ferric ions (B). Resistance of male (C) and female (D) flies to oxidative stress induced by menadione. Data shown in (A,B) represent mean ± SEM for four independent extractions. Different letters on (A,B) represent groups significantly different from each other (p < 0.05).
Since the AF extracts were identified as having significant antioxidant activities it was logical to predict that flies fed diets supplemented with AF powder would be more resistant to oxidative stress induced by the redox cycling agent menadione. Figure 1C (male) and Figure 1D (female) show that flies fed diets with AF leaf powder were lived longer under oxidative stress exposure. Male flies fed diets with 5 or 10 mg/ml of AF powder were more resistant by 40 and 75%, respectively (Log Rank test, p < 0.0001) (Figure 1C and Supplementary Table S2). AF powder in concentration of 5 mg/ml increased female resistance by 20% as compared to control (Log Rank test, p < 0.0001) (Figure 1D and Supplementary Table S2).
Antioxidant Enzymes, Aconitase, and Markers of Oxidative Stress
The antioxidant enzymes superoxide dismutase (SOD) and catalase are involved in the detoxification of the reactive oxygen species (ROS) superoxide anion and hydrogen peroxide, respectively. The activity of SOD was affected by food supplementation with AF powder (Figure 2A). Fly treatment with AF supplemented food for 30 days at a concentration of 10 mg/ml significantly increased SOD activity in flies by 1.71-fold in males (Dunnett’s test, p = 0.014) and 2.21-fold in females (Dunnett’s test, p = 0.010). However, dietary AF did not influence the activity of catalase (Figure 2B).
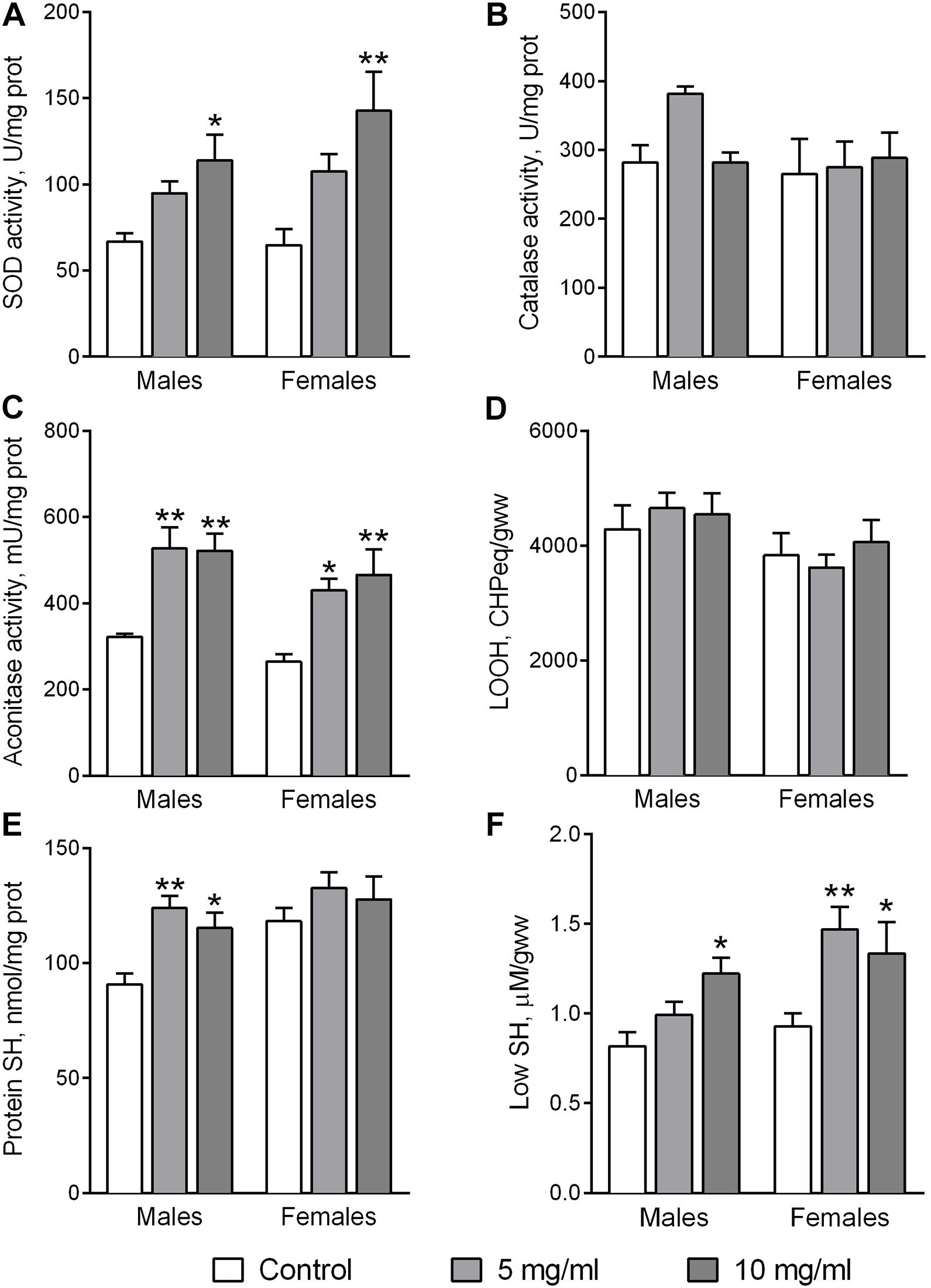
Figure 2. Activities of antioxidant enzymes SOD (A), catalase (B), aconitase (C), and the levels of lipid peroxides (D), protein (E), and low molecular weight thiols (F) in flies consuming control diet or diet supplemented with 5 or 10 mg/ml A. foeniculum. Results are presented as mean ± SEM of four replicates per group. Group comparisons were performed using Dunnett’s test. Asterisks indicate significant difference from the control flies (p < 0.05).
Increased ROS production and/or a decrease of the detoxification potential can induce oxidative stress (Lushchak, 2014). This may be reflected by specific markers such as the activity of the ROS-sensitive enzyme aconitase, protein carbonyl groups, lipid peroxides, protein and low molecular mass thiol groups (Lushchak et al., 2011). A higher activity of aconitase was observed in flies of both sexes fed by diets supplemented with 5 or 10 mg/ml AF powder (Figure 2C). Activity increased by about 1.62-fold (Dunnett’s test, p = 0.003) and 1.63-fold (Dunnett’s test, p = 0.03) in male and female flies, respectively. Interestingly, fly feeding with an AF supplemented diet did not affect the contents of lipid peroxides (LOOH) (Figure 2D) and protein carbonyl groups (not shown). However, the antioxidant properties of AF in flies were reflected in reduced protein and low molecular weight thiol (Low SH) groups (Figures 2E,F). Higher contents of protein SH groups were observed in male flies fed diets with AF in concentrations of 5 mg/ml (Dunnett’s test, p = 0.005) and 10 mg/ml (p = 0.024). Low SH contents, that are mostly represented by glutathione and cysteine, were significantly higher in flies fed diets with AF (Figure 2F). Diet supplementation with AF increased Low SH by 50% (Dunnett’s test, p = 0.011) and 45% (p = 0.05) in male and female flies, respectively (Figure 2F).
Lifespan of Different Strains
Identification of nutritional supplements that can delay aging and extend lifespan is one of the most promising ways to improve the quality of life. To directly test whether A. foeniculum elicits beneficial effects on lifespan, we raised fruit flies on diets supplemented with of dried crushed leaves added to the food medium at different concentrations. The median lifespan control group of male Canton S flies lived approximately 34 days. Lifespan was significantly increased by 15% in male Drosophila line Canton S fed diets with 5, 10 mg/ml of AF powder or by 32% at 30 mg/ml (Log Rank test, p < 0.03) (Figure 3A and Supplementary Table S3). Female flies of the Canton S strain lived longer by 37, 33, 37, and 37% when fed diet with 2.5, 5, 10, and 30 mg/ml AF powder, respectively (Log Rank test, p < 0.02) (Figure 3B and Supplementary Table S3).
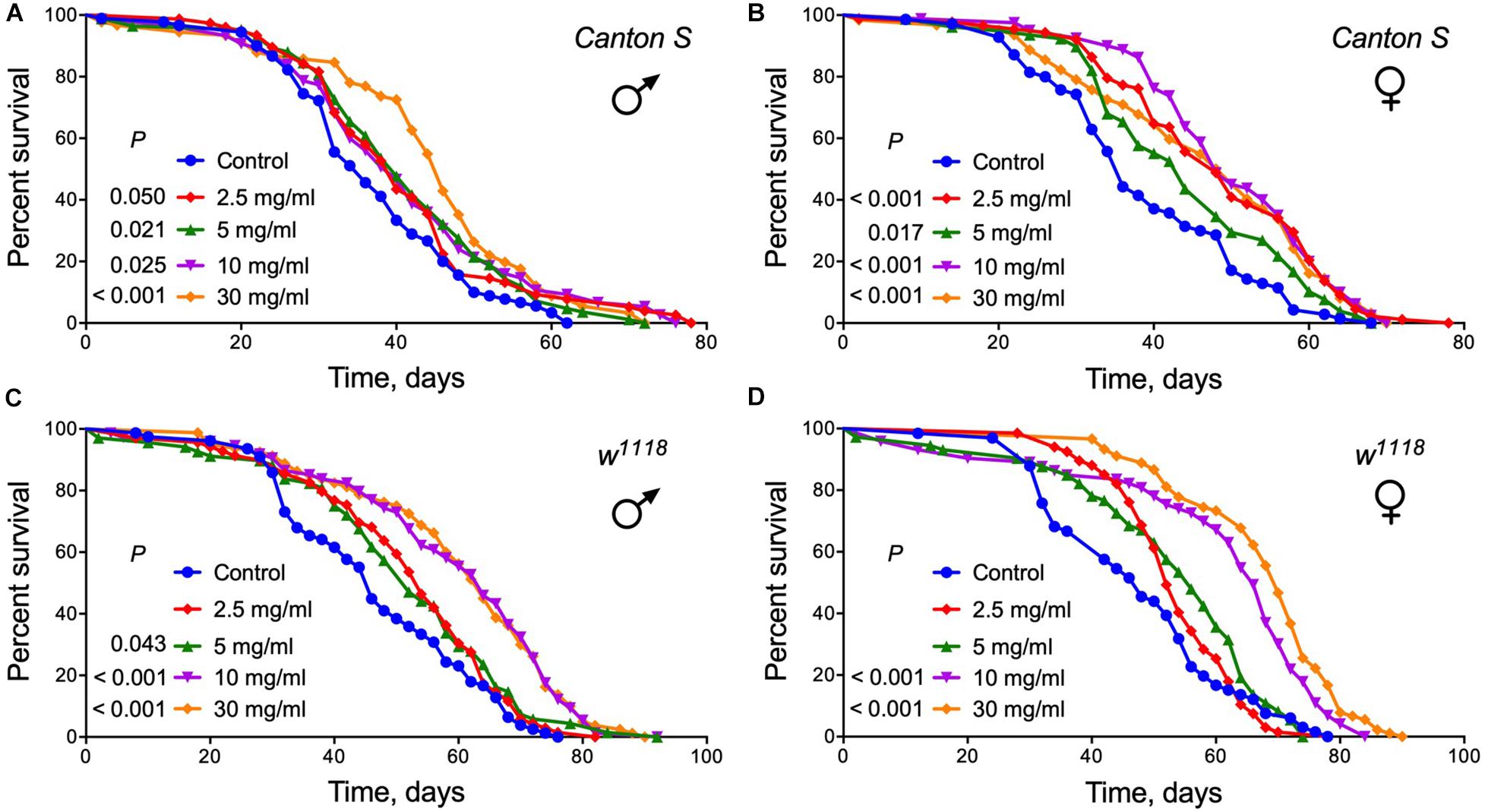
Figure 3. Survival curves for flies of two different lines fed control diet vs. diets supplemented with A. foeniculum extract at four different concentrations. (A) males of Canton S; (B) females of Canton S; (C) males of w1118; (D) females of w1118. Each curve represents the percentage of alive flies within respect time. The cohorts were compared using a Log rank test (see Supplementary Table S2 for complete statistics).
Dietary supplementation with A. foeniculum herb also extended the lifespan of male and female flies of another Drosophila strain, w1118 flies. Male flies that consumed foods with 2.5, 5, 10, or 30 mg/ml AF powder lived longer as compared to controls (Log Rank test, p < 0.05) (Figure 3C and Supplementary Table S3). We also observed longer lifespan in females, when the herb was added to the food at concentrations of 10 mg/ml (by 40%), 30 mg/ml (by 49%) (Log Rank test, p < 0.0001) (Figure 3D and Supplementary Table S3).
Lifespan on Diets With Different Yeast Contents
Dietary protein content is an important determinant of aging and lifespan that is mostly regulated by activity of TOR signaling pathway (Kapahi et al., 2004, 2017; Soultoukis and Partridge, 2016; Lushchak et al., 2017, 2019). We observed higher survival of flies of both sexes when reared on media with 0.05× yeast content and AF powder at both 5 or 10 mg/ml concentrations (Log Rank test, p ≤ 0.0001) (Figures 4A,B and Supplementary Table S4). Males and females also lived longer on 0.2× yeast (1%) containing medium with A. foeniculum (Log Rank test, p < 0.02) (Figures 4C,D and Supplementary Table S4). However, at a very high yeast content (4× or 20%) in conjunction with A. foeniculum survival was substantially lower compared with controls (Log Rank test, p < 0.04) (Figures 4E,F and Supplementary Table S4). Consequently, we detected the highest mean lifespan of both sexes at 10 mg/ml of A. foeniculum under 0.05×, 0.2×, and 1× yeast concentrations in the diet but the lowest mean lifespan occurred under 4× yeast content (Figures 4G,H).
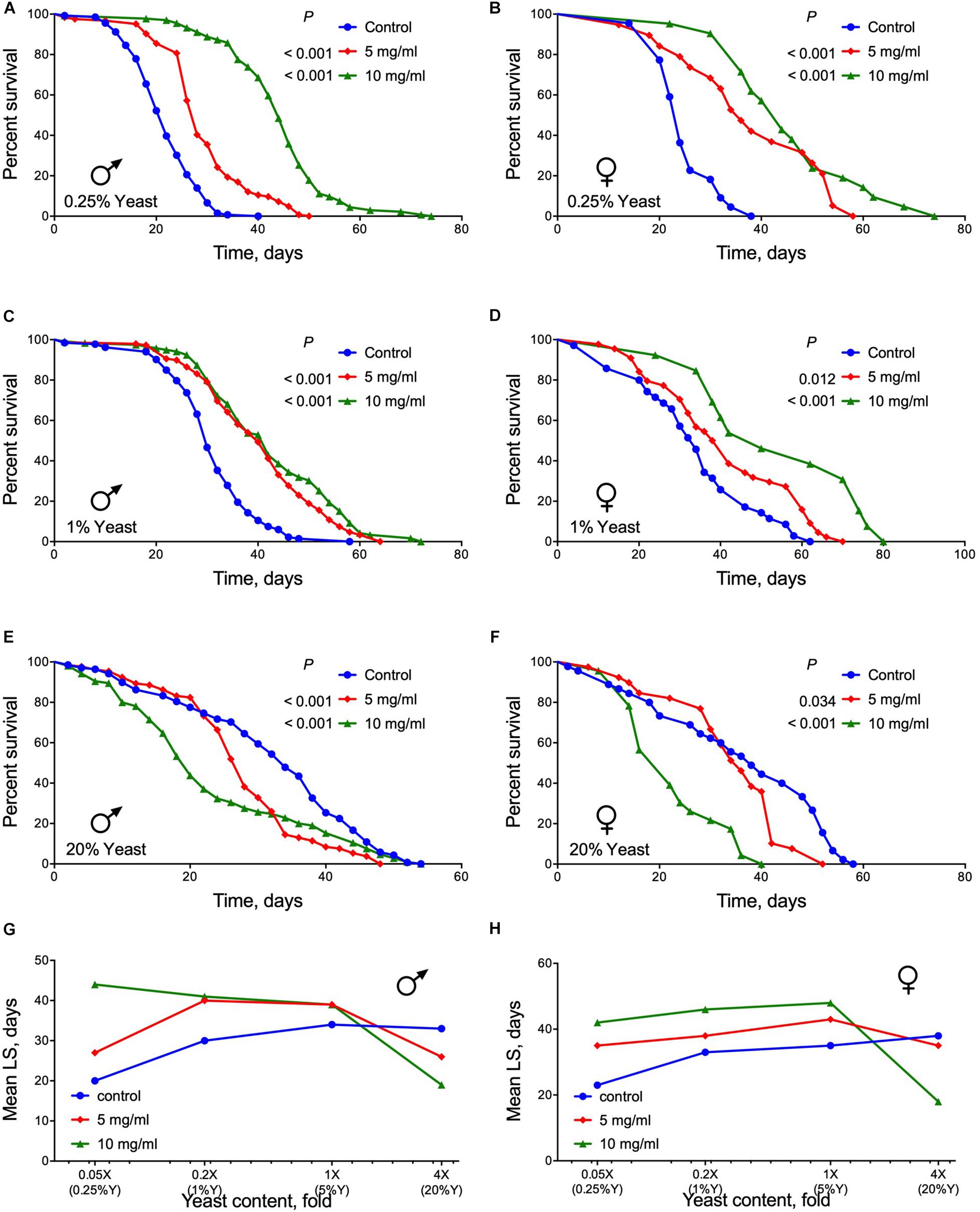
Figure 4. Lifespan of flies fed diets with different concentrations of yeast and supplemented with AF in concentrations of 5 or 10 mg/ml. (A,B) show data for yeast concentration 0.05×, (C,D) 0.2×, (E,F) 4×. Data for male flies are shown in (A,C,E) and females in (B,D,F). (G,H) show mean lifespan values for male and female flies, respectively.
Fecundity and Negative Geotaxis
Fertility and negative geotaxis are very important life history traits and are excellent indicators of overall health (Flatt, 2020). Early after eclosion, egg production rose in all three groups of adult flies tested (control, 5 and 10 mg/ml) with highest levels after about 12–15 days and a subsequent gradual decrease thereafter (Figure 5A). Flies fed with diets containing A. foeniculum (both 5 and 10 mg/ml) had significantly higher daily egg production on day 6 than controls but thereafter fecundity of the 10 mg/ml diet group dropped back by 21 days to at or below control levels. However, fecundity of flies on the 5 mg/ml diet remained significantly higher than controls from day 6 to 27. Consequently, AF has a positive impact on reproduction rate with more obvious effects at moderate concentration (5 mg/ml).
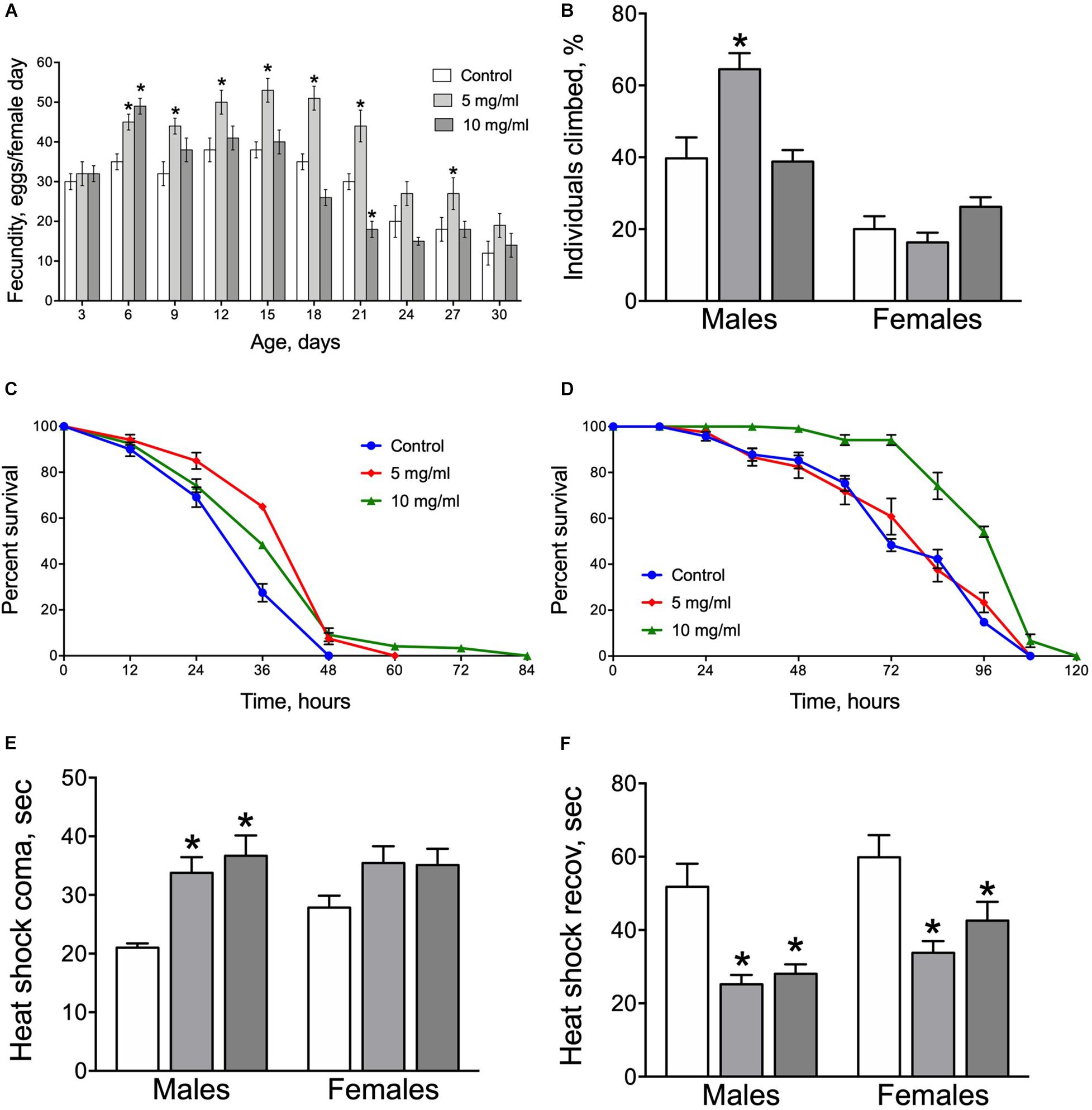
Figure 5. Fecundity (A), negative geotaxis (B), resistance to starvation (C,D) and resistance to heat stress (E) and recovery (F) in flies fed diets supplemented with A. foeniculum leaf powder. Data are presented as mean ± SEM with 10 flies tested per cohort. Group comparisons were performed using Dunnett’s test. Asterisks indicates a significant difference from control flies (p < 0.05). Color coding for figures (B,E,F) as on (A).
We observed that AF supplementation improved negative geotaxis response only in male flies (Figure 5B). Males that consumed a diet with 5 mg/ml A. foeniculum showed approximately 60% higher peroformance as compared to the control group (Dunnett’s test, p = 0.008).
Resistance to Starvation and Heat Stress
To further explore beneficial effects of A. foeniculum on lifespan, we assessed effects of this supplement on stress resistance by examining responses of flies to experimental starvation conditions and high temperature. Flies fed diets with AF powder for 30 days demonstrated a longer lifespan under complete starvation conditions with significantly higher survival of male flies fed diets with of 5 mg/ml and 10 mg/ml AF concentrations (Log Rank test, p ≤ 0.0005) (Figure 5C). Moreover, A. foeniculum supplementation to the medium led to higher starvation resistance in females fed by 10 mg/ml by 37% as compared to control (Log Rank test, p = 0.003) (Figure 5D). These data may suggest high nutritional value of the A. foeniculum.
We also examined the effect of A. foeniculum on the fly resistance to high temperature. Males, that consumed media supplemented with AF powder resisted heat shock significantly longer as compared to controls (Figure 5E). Moreover, the recovery time of flies of both sexes was shorter for the AF consuming groups (Figure 5F). Consequently, A. foeniculum in the diet enhances Drosophila resistance to the heat stress.
Metabolites
Glucose, trehalose, glycogen, and TAGs are parameters extensively used for analysis of carbohydrate and fat metabolism in Drosophila (Tennessen et al., 2014). We found that consumption of food with 10 mg/ml AF powder decreased the amount of body glucose in males by 17% (Dunnett’s test, p = 0.037), but had no impact on females (Figure 6A). Glycogen levels in flies that consumed food with AF in concentrations of 5 and 10 mg/ml was higher by 25 and 30% in males and by 21 and 29% in females, respectively (Figure 6C). TAG concentration was 43% higher in males (at 10 mg/ml AF supplementation) and by 63 and 55% in females fed diets with 5 and 10 mg/ml AF, respectively (Figure 6D). AF supplementation did not affect body trehalose levels of either sex (Figure 6B).
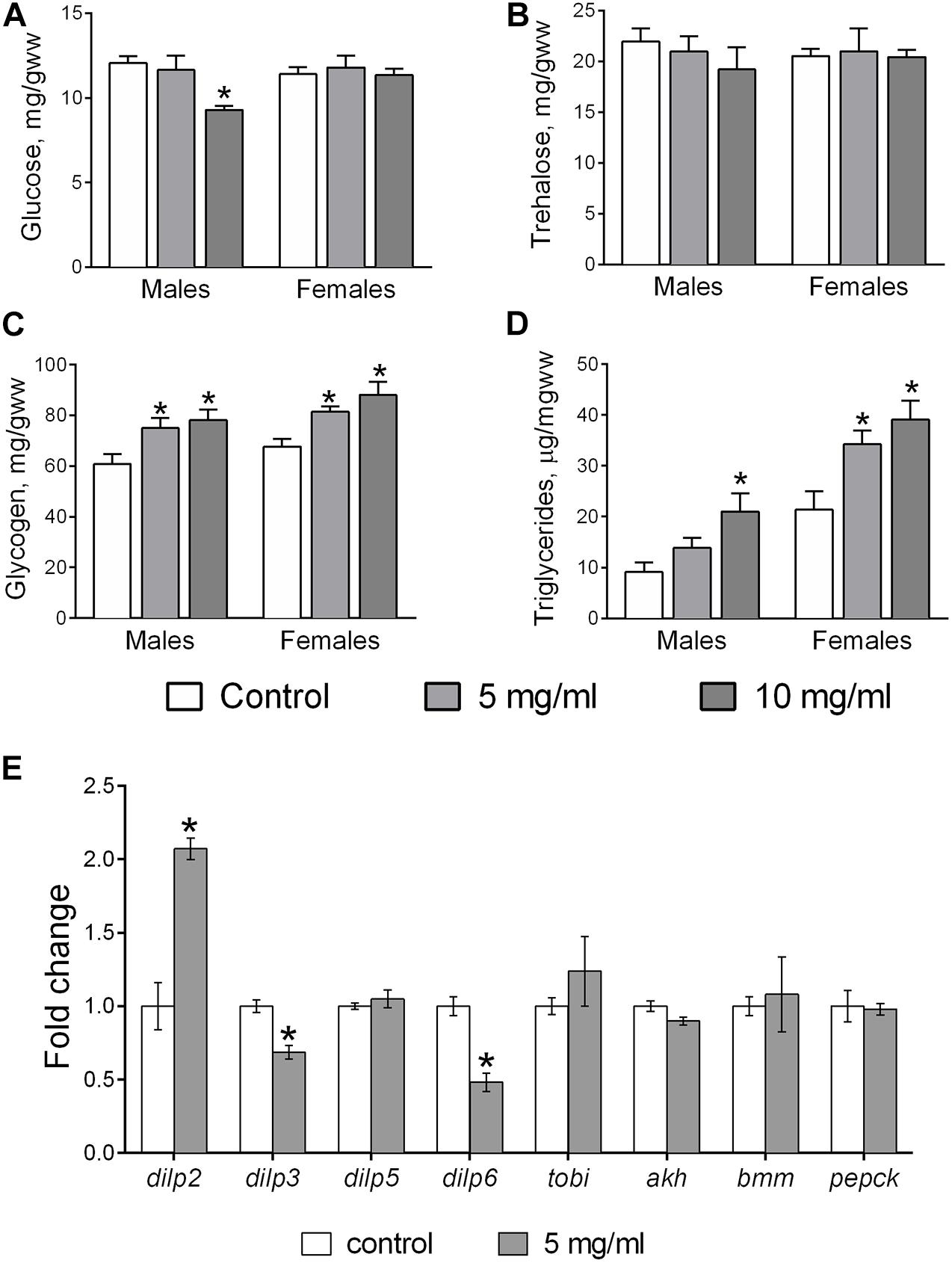
Figure 6. Content of metabolites and mRNA levels for genes involved in regulation of metabolism. Whole body glucose (A), trehalose (B), glycogen (C), TAG (D), and mRNA levels for eight metabolic genes (E) in flies treated with AF. Results are mean ± SEM of 4–5 replicates per group. Group comparisons were performed using Dunnett’s test for metabolites and Student’s t-test for mRNA. Asterisk indicates significant difference from the control flies with p < 0.05.
Gene Expression
We measured the relative expression of eight genes involved in the regulation of intermediary metabolism. The Drosophila insulin-like proteins, dilp2, 3, and 5, are co-expressed in insulin producing cells (IPCs) of the brain and hence, their transcript levels were measured in fly heads. Dilp6 is primarily expressed by fat body cells and its expression and the mRNA levels of adipokinetic hormone (akh), target of brain insulin (tobi), phosphoenolcarboxykinase (pepck), and Brummer, a triglyceride lipase (bmm) were measured in whole fly body. Flies that consumed food supplemented with 5 mg/ml AF powder had significantly higher levels of dilp2 transcripts by about 2-fold in fly heads (Figure 6E; Student’s t-test, p = 0.004). Moreover, we observed a decrease of mRNA levels for dilp3 (by 30%; Student’s t-test, p = 0.005) and dilp6 (by 50%; Student’s t-test, p = 0.003) in flies that consumed diet with A. foeniculum powder. No changes were observed in the mRNA levels of dilp5, akh, tobi, pepck, and bmm.
Discussion
Plant-derived active compounds can be used successfully to treat age-related diseases and extend life- and healthspan (Vaiserman and Lushchak, 2017). Most of the detected components in AF leaf extracts were flavonoids that are intensively studied natural compounds with antioxidant, antineoplastic, antihyperglycemic, cardioprotective, or neuroprotective properties. The HPLC-MS analysis revealed the presence of 24 bioactive compounds as shown in Supplementary Table S1. For example, 2,5-dihydroxycinnamic acid was previously shown to induce apoptosis and may impair autophagic flux in RCC4 renal cancer cells (Selka et al., 2019), whereas 3-O-caffeoylquinic acid acts as a metformin mimetic in extending Drosophila lifespan (Liu et al., 2013) and is also widely known for its anti-hyperlipidemic effect (Liu et al., 2013). 6,7-Dimethoxyquercetin 3-O-glucopyranoside has antiradical activity (de Amorim et al., 2013). The flavonoid, acacetin, is a potent inhibitory constituent and affects eclosion rate, feeding, climbing and lifespan in Drosophila (Wang et al., 2015). It is often used in humans for Alzheimer’s disease treatments. Flies that were reared on apigenin in their diet showed an increase in lifespan, glutathione, and dopamine content, as well as reduced oxidative stress and apoptosis in a transgenic Drosophila model of Parkinson’s disease (Siddique and Jyoti, 2017). Treatment with caffeic acid alleviates oxidative stress induced neurotoxicity in cells and Drosophila models (Wu et al., 2017). The naturally occurring calycosin is a known antioxidant that prevents redox imbalance in organisms. It also promotes lifespan in the nematode, Caenorhabditis elegans, because of its antioxidant action as well as its ability to enhance stress resistance and reduce ROS through insulin signaling pathway inhibition (Lu et al., 2017). The possible lifespan-extending effects of genistein were previously investigated using C. elegans (Lee et al., 2015). Protocatechuic acid similarly extended lifespan in C. elegans and increased stress resistance associated with its antioxidant properties (Kim et al., 2014). Caffeic acid was shown to increase antioxidant capacity in vivo and, by means of a lipofuscin assay, reduce oxidative damage in nematodes, which resulted in increased lifespan (Pietsch et al., 2011). Ursolic acid (UA) is a naturally occurring triterpenoid exhibiting potential antimicrobial, anti-inflammatory and antiobesity activity and it was shown that dietary UA improved health span and lifespan in male D. melanogaster (Staats et al., 2019). β-Sitosterol is capable of extending lifespan, likely via activating AMP-activated protein kinase (AMPK) (Lin et al., 2014). Consequently, all the components detected in A. foeniculum extracts have been shown to have lifespan extending effects in various animal models.
We supplemented fly food with crushed dried leaves of A. foeniculum (AF) at different concentrations and fed Drosophila of two lines (Canton S and w1118). We found strong prolongevity activity that was independent of genotype. In addition, AF considerably enhanced the survival rate of flies under both menadione-induced oxidative stress and starvation conditions. Since there is a clear correlation between lifespan-extension and stress resistance (Kenyon, 2010), the protective action against stress may be a positive factor in AF-mediated lifespan extension.
It is also important that factors extending the lifespan have no negative effects on reproduction that reflect healthspan. In this study, we found a higher fecundity rate in AF-fed flies. Although we detected an increase in reproductive ability of flies, previous studies suggested that phytoestrogen exposure negatively affects reproductive health (Jefferson et al., 2012). We also studied functional aging using a locomotion assay. Interestingly, Agastache significantly enhanced the mobility of male flies indicating that this herb provides beneficial effects on healthspan as well as lifespan.
The health- and lifespan of D. melanogaster are determined by metabolic rate, stress responses and the expression of metabolic genes (Staats et al., 2019). Our results indicate that the health-promoting effects of AF may be caused by changes in metabolism. Indeed, we observed that the prolongevity phenotype is associated with decrease in body glucose levels and increase in stored glycogen and TAG content. The level of circulating and stored metabolites is regulated by DILPs and the glucagon-like peptide AKH (Bharucha et al., 2008). Notably, a significant reduction of mRNA levels for dilp3 and dilp6 and increase dilp2 were observed in response to dietary supplementation with Agastache.
To uncover the mechanisms of how Agastache protects against oxidative stress, we analyzed antioxidant enzyme activities using fly homogenates from different treatment groups. Our results show that activities of both aconitase and catalase (in males) were significantly increased by AF in the diet, implicating an attenuation of oxidative stress. Additionally, AF-treated flies may also be more resistant to oxidative stress in part because of the various other phenolic compounds in the plant extract that exhibit antioxidant activity.
Low molecular weight (L-SH) and protein thiols are reliable markers of oxidative stress. Flies, fed with AF showed higher antioxidant potential as demonstrated by higher levels of L-SH in females and protein thiols in males, as well as higher aconitase and catalase activities. We suggest that combined together these changes could be responsible for the lower levels of oxidative damage in AF fed flies. The higher pool of L-SH in females might also be associated with a more extensive biosynthetic metabolism related to the need for egg production (Perkhulyn et al., 2017).
In conclusion, our study revealed lifespan extending effects of Agastache in D. melanogaster linked in part to elevated antioxidant activity and increased stress resistance. The health benefits of polyphenols found in Agastache extract are linked to their capacity to directly scavenge free radicals and other nitrogen species (Halliwell, 2007). However, whether dietary supplementation with AF will be beneficial to mammals and ultimately to humans will require further studies.
Data Availability Statement
The authors acknowledge that the data presented in this study must be deposited and made publicly available in an acceptable repository, prior to publication. Frontiers cannot accept a manuscript that does not adhere to our open data policies.
Author Contributions
OS: study design, experimental part, data analysis, and writing of drafts. AZ, AK, FG, NV, and AV: experiments and data analysis. KS: initial draft and critical analysis. OL: idea, study design, data analysis, manuscript preparation, and project management. All authors contributed to the article and approved the submitted version.
Funding
This work was partially supported by a grant from the National Science Foundation of Ukraine (#2020.02/0118) and a Discovery grant from the Natural Sciences and Engineering Research Council of Canada (#6793) to KS.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2020.596729/full#supplementary-material
References
Bahadorani, S., and Hilliker, A. J. (2008). Cocoa confers life span extension in Drosophila melanogaster. Nutr. Res. 28, 377–382. doi: 10.1016/j.nutres.2008.03.018
Bass, T. M., Weinkove, D., Houthoofd, K., Gems, D., and Partridge, L. (2007). Effects of resveratrol on lifespan in Drosophila melanogaster and Caenorhabditis elegans. Mech. Ageing Dev. 128, 546–552. doi: 10.1016/j.mad.2007.07.007
Bayliak, M. M., Shmihel, H. V., Lylyk, M. P., Storey, K. B., and Lushchak, V. I. (2016). Alpha-ketoglutarate reduces ethanol toxicity in Drosophila melanogaster by enhancing alcohol dehydrogenase activity and antioxidant capacity. Alcohol 55, 23–33. doi: 10.1016/j.alcohol.2016.07.009
Bharucha, K. N., Tarr, P., and Zipursky, S. L. (2008). A glucagon-like endocrine pathway in Drosophila modulates both lipid and carbohydrate homeostasis. J. Exp. Biol. 211, 3103–3110. doi: 10.1242/jeb.016451
Bitto, A., Wang, A. M., Bennett, C. F., and Kaeberlein, M. (2015). Biochemical genetic pathways that modulate aging in multiple species. Cold Spring Harb. Perspect. Med. 5:a025114. doi: 10.1101/cshperspect.a025114
de Amorim, M. R., Rinaldo, D., do Amaral, F. R., Magenta, M., Vilegas, W., and Santos, L. (2013). HPLC-DAD method for quantification of the flavonoids with antiradicalar activity in the hydroethanolic extract from Tonina fluviatilis aubl. Planta Medica 79:1348737. doi: 10.1055/s-0033-1348737
Flatt, T. (2020). Life-History Evolution and the Genetics of Fitness Components in Drosophila melanogaster. Genetics 214, 3–48. doi: 10.1534/genetics.119.300160
Fontana, L., and Partridge, L. (2015). Promoting health and longevity through diet: from model organisms to humans. Cell 161, 106–118. doi: 10.1016/j.cell.2015.02.020
Gospodaryov, D. V., Yurkevych, I. S., Jafari, M., Lushchak, V. I., and Lushchak, V. (2013). Lifespan extension and delay of age-related functional decline caused by Rhodiola rosea depends on dietary macronutrient balance. Longev. Healthspan 2:5. doi: 10.1186/2046-2395-2-5
Halliwell, B. (2007). Dietary polyphenols: good, bad, or indifferent for your health? Cardiovasc. Res. 73, 341–347. doi: 10.1016/j.cardiores.2006.10.004
Ivanov, I., Vrancheva, R., Traycheva Petkova, N., Tumbarski, Y., Nedyalkova Dincheva, I., and Badjakov, I. (2019). Phytochemical compounds of anise hyssop (Agastache foeniculum) and antibacterial, antioxidant, and acetylcholinesterase inhibitory properties of its essential oil. J. Appl. Pharmaceut. Sci. 9, 72–78. doi: 10.7324/JAPS.2019.90210
Jefferson, W. N., Patisaul, H. B., and Williams, C. J. (2012). Reproductive consequences of developmental phytoestrogen exposure. Reproduction 143, 247–260. doi: 10.1530/REP-11-0369
Kang, H. L., Benzer, S., and Min, K. T. (2002). Life extension in Drosophila by feeding a drug. Proc. Natl. Acad. Sci. U S A. 99, 838–843. doi: 10.1073/pnas.022631999
Kapahi, P., Kaeberlein, M., and Hansen, M. (2017). Dietary restriction and lifespan: Lessons from invertebrate models. Ageing Res. Rev. 39, 3–14. doi: 10.1016/j.arr.2016.12.005
Kapahi, P., Zid, B. M., Harper, T., Koslover, D., Sapin, V., and Benzer, S. (2004). Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr. Biol. 14, 885–890. doi: 10.1016/j.cub.2004.03.059
Kim, Y. S., Seo, H. W., Lee, M. H., Kim, D. K., Jeon, H., and Cha, D. S. (2014). Protocatechuic acid extends lifespan and increases stress resistance in Caenorhabditis elegans. Arch. Pharm. Res. 37, 245–252. doi: 10.1007/s12272-013-0183-6
Lee, E. B., Ahn, D., Kim, B. J., Lee, S. Y., Seo, H. W., Cha, Y. S., et al. (2015). Genistein from Vigna angularis extends lifespan in Caenorhabditis elegans. Biomol. Ther. 23, 77–83. doi: 10.4062/biomolther.2014.075
Lee, K. S., Lee, B. S., Semnani, S., Avanesian, A., Um, C.-Y., Jeon, H. J., et al. (2010). Curcumin extends life span, improves health span, and modulates the expression of age-associated aging genes in Drosophila melanogaster. Rejuvenation Res. 13, 561–570. doi: 10.1089/rej.2010.1031
Lin, W. S., Chen, J. Y., Wang, J. C., Chen, L. Y., Lin, C. H., Hsieh, T. R., et al. (2014). The anti-aging effects of Ludwigia octovalvis on Drosophila melanogaster and SAMP8 mice. Age 36, 689–703. doi: 10.1007/s11357-013-9606-z
Liu, H., Zhang, X., Wu, C., Wu, H., Guo, P., and Xu, X. (2013). Anti-hyperlipidemic caffeoylquinic acids from the fruits of Pandanus tectorius Soland. J. Appl. Pharmaceut. Sci. 3, 16–19. doi: 10.7324/JAPS.2013.3803
Lozinsky, O. V., Lushchak, O. V., Kryshchuk, N. I., Shchypanska, N. Y., Riabkina, A. H., Skarbek, S. V., et al. (2013). S-nitrosoglutathione-induced toxicity in Drosophila melanogaster: Delayed pupation and induced mild oxidative/nitrosative stress in eclosed flies. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 164, 162–170. doi: 10.1016/j.cbpa.2012.08.006
Lozinsky, O. V., Lushchak, O. V., Storey, J. M., Storey, K. B., and Lushchak, V. I. (2012). Sodium nitroprusside toxicity in Drosophila melanogaster: delayed pupation, reduced adult emergence, and induced oxidative/nitrosative stress in eclosed flies. Arch. Insect Biochem. Physiol. 80, 166–185. doi: 10.1002/arch.21033
Lu, L., Zhao, X., Zhang, J., Li, M., Qi, Y., and Zhou, L. (2017). Calycosin promotes lifespan in Caenorhabditis elegans through insulin signaling pathway via daf-16, age-1 and daf-2. J. Biosci. Bioeng. 124, 1–7. doi: 10.1016/j.jbiosc.2017.02.021
Lushchak, O. V., Carlsson, M. A., and Nässel, D. R. (2015). Food odours trigger an endocrine response that affects food ingestion and metabolism. Cell Mol. Life Sci. 72, 3143–3155. doi: 10.1007/s00018-015-1884-4
Lushchak, O. V., Gospodaryov, D. V., Rovenko, B. M., Glovyak, A. D., Yurkevych, I. S., Klyuba, V. P., et al. (2012). Balance between macronutrients affects life span and functional senescence in fruit fly Drosophila melanogaster. J. Gerontol. A Biol. Sci. Med. Sci. 67, 118–125. doi: 10.1093/gerona/glr184
Lushchak, O., Piroddi, M., Galli, F., and Lushchak, V. I. (2014). Aconitase post-translational modification as a key in linkage between Krebs cycle, iron homeostasis, redox signaling, and metabolism of reactive oxygen species. Redox Rep. 19, 8–15. doi: 10.1179/1351000213Y.0000000073
Lushchak, O., Rovenko, B., Gospodaryov, D., and Lushchak, V. (2011). Drosophila melanogaster larvae fed by glucose and fructose demonstrate difference in oxidative stress markers and antioxidant enzymes of adult flies. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 160, 27–34. doi: 10.1016/j.cbpa.2011.04.019
Lushchak, O., Strilbytska, O. M., Yurkevych, I., Vaiserman, A. M., and Storey, K. B. (2019). Implications of amino acid sensing and dietary protein to the aging process. Exp. Gerontol. 115, 69–78. doi: 10.1016/j.exger.2018.11.021
Lushchak, O., Strilbytska, O., Piskovatska, V., Storey, K. B., Koliada, A., and Vaiserman, A. (2017). The role of the TOR pathway in mediating the link between nutrition and longevity. Mech. Ageing Dev. 164, 127–138. doi: 10.1016/j.mad.2017.03.005
Lushchak, V. (2014). Free radicals, reactive oxygen species, oxidative stress and its classification. Chem. Biol. Interact. 224, 164–175. doi: 10.1016/j.cbi.2014.10.016
Marcel, D. M., Vârban, D. I., Muntean, S., Moldovan, C., and Olar, M. (2013). Use of species Agastache foeniculum (Pursh) Kuntze. Hop. Med. Plant 2, 41–42.
Meydani, M. (2001). Nutrition interventions in aging and age-associated disease. Ann. N Y. Acad. Sci. 928, 226–235. doi: 10.1111/j.1749-6632.2001.tb05652.x
Nikitin, A. G., Navitskas, S., and Gordon, L. A. (2008). Effect of varying doses of caffeine on life span of Drosophila melanogaster. J. Gerontol. A Biol. Sci. Med. Sci. 63, 149–150. doi: 10.1093/gerona/63.2.149
Peng, C., Zuo, Y., Kwan, K. M., Liang, Y., Ma, K. Y., Chan, H. Y. E., et al. (2012). Blueberry extract prolongs lifespan of Drosophila melanogaster. Exp. Gerontol. 47, 170–178. doi: 10.1016/j.exger.2011.12.001
Perkhulyn, N. V., Rovenko, B. M., Lushchak, O. V., Storey, J. M., Storey, K. B., and Lushchak, V. I. (2017). Exposure to sodium molybdate results in mild oxidative stress in Drosophila melanogaster. Redox Rep. 22, 137–146. doi: 10.1080/13510002.2017.1295898
Perkhulyn, N. V., Rovenko, B. M., Zvarych, T. V., Lushchak, O. V., Storey, J. M., Storey, K. B., et al. (2015). Sodium chromate demonstrates some insulin-mimetic properties in the fruit fly Drosophila melanogaster. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 167, 74–80. doi: 10.1016/j.cbpc.2014.08.007
Pietsch, K., Saul, N., Chakrabarti, S., Stürzenbaum, S. R., Menzel, R., and Steinberg, C. E. (2011). Hormetins, antioxidants and prooxidants: defining quercetin-, caffeic acid- and rosmarinic acid-mediated life extension in C. elegans. Biogerontology 12, 329–347. doi: 10.1007/s10522-011-9334-7
Piskovatska, V., Strilbytska, O., Koliada, A., Vaiserman, A., and Lushchak, O. (2019). Health Benefits of Anti-aging Drugs. Subcell. Biochem. 91, 339–392. doi: 10.1007/978-981-13-3681-2_13
Rovenko, B. M., Perkhulyn, N. V., Gospodaryov, D. V., Sanz, A., Lushchak, O. V., and Lushchak, V. I. (2015). High consumption of fructose rather than glucose promotes a diet-induced obese phenotype in Drosophila melanogaster. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 180, 75–85. doi: 10.1016/j.cbpa.2014.11.008
Schriner, S. E., Katoozi, N. S., Pham, K. Q., Gazarian, M., Zarban, A., and Jafari, M. (2012). Extension of Drosophila lifespan by Rosa damascena associated with an increased sensitivity to heat. Biogerontology 13, 105–117. doi: 10.1007/s10522-011-9357-0
Selka, A., Doiron, J. A., Lyons, P., Dastous, S., Chiasson, A., Cormier, M., et al. (2019). Discovery of a novel 2,5-dihydroxycinnamic acid-based 5-lipoxygenase inhibitor that induces apoptosis and may impair autophagic flux in RCC4 renal cancer cells. Eur. J. Med. Chem. 179, 347–357. doi: 10.1016/j.ejmech.2019.06.060
Siddique, Y. H., and Jyoti, S. (2017). Alteration in biochemical parameters in the brain of transgenic Drosophila melanogaster model of Parkinson’s disease exposed to apigenin. Integr. Med. Res. 6, 245–253. doi: 10.1016/j.imr.2017.04.003
Soultoukis, G. A., and Partridge, L. (2016). Dietary Protein. Metabolism, and Aging. Annu. Rev. Biochem. 2, 5–34. doi: 10.1146/annurev-biochem-060815-014422
Spindler, S. R., Li, R., Dhahbi, J. M., Yamakawa, A., Mote, P., Bodmer, R., et al. (2012). Statin treatment increases lifespan and improves cardiac health in Drosophila by decreasing specific protein prenylation. PLoS One 7:e39581. doi: 10.1371/journal.pone.0039581
Staats, S., Wagner, A. E., Lüersen, K., Künstner, A., Meyer, T., Kahns, A. K., et al. (2019). Dietary ursolic acid improves health span and life span in male Drosophila melanogaster. Biofactors 45, 169–186. doi: 10.1002/biof.1467
Tennessen, J. M., Barry, W. E., Cox, J., and Thummel, C. S. (2014). Methods for studying metabolism in Drosophila. Methods 68, 105–115. doi: 10.1016/j.ymeth.2014.02.034
Vaiserman, A., and Lushchak, O. (2017). Implementation of longevity-promoting supplements and medications in public health practice: achievements, challenges and future perspectives. J. Transl. Med. 15:160. doi: 10.1186/s12967-017-1259-8
Wang, C., Wheeler, C. T., Alberico, T., Sun, X., Seeberger, J., Laslo, M., et al. (2013). The effect of resveratrol on lifespan depends on both gender and dietary nutrient composition in Drosophila melanogaster. Age 35, 69–81. doi: 10.1007/s11357-011-9332-3
Wang, H. L., Sun, Z. O., Rehman, R. U., Wang, H., Wang, Y. F., and Wang, H. (2017). Rosemary extract-mediated lifespan extension and attenuated oxidative damage in Drosophila melanogaster fed on high-fat diet. J. Food Sci. 82, 1006–1011. doi: 10.1111/1750-3841.13656
Wang, X., Perumalsamy, H., Kwon, H. W., Na, Y. E., and Ahn, Y. J. (2015). Effects and possible mechanisms of action of acacetin on the behavior and eye morphology of Drosophila models of Alzheimer’s disease. Sci. Rep. 5:16127. doi: 10.1038/srep16127
Wu, Y. L., Chang, J. C., Lin, W. Y., Li, C. C., Hsieh, M., Chen, H. W., et al. (2017). Treatment with caffeic acid and resveratrol alleviates oxidative stress induced neurotoxicity in cell and Drosophila models of spinocerebellar ataxia type3. Sci. Rep. 7:11641. doi: 10.1038/s41598-017-11839-0
Keywords: Agastache foeniculum, lifespan, metabolism, oxidative stress, Drosophila
Citation: Strilbytska OM, Zayachkivska A, Koliada A, Galeotti F, Volpi N, Storey KB, Vaiserman A and Lushchak O (2020) Anise Hyssop Agastache foeniculum Increases Lifespan, Stress Resistance, and Metabolism by Affecting Free Radical Processes in Drosophila. Front. Physiol. 11:596729. doi: 10.3389/fphys.2020.596729
Received: 20 August 2020; Accepted: 26 November 2020;
Published: 16 December 2020.
Edited by:
Elzbieta M. Pyza, Jagiellonian University, PolandReviewed by:
Jean-René Martin, UMR 9197 Institut des Neurosciences Paris Saclay (Neuro-PSI), FrancePamela Menegazzi, Julius Maximilian University of Würzburg, Germany
Copyright © 2020 Strilbytska, Zayachkivska, Koliada, Galeotti, Volpi, Storey, Vaiserman and Lushchak. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Oleh Lushchak, b2xlaC5sdXNoY2hha0BwbnUuZWR1LnVh
 Olha M. Strilbytska1
Olha M. Strilbytska1 Alexander Koliada
Alexander Koliada Alexander Vaiserman
Alexander Vaiserman Oleh Lushchak
Oleh Lushchak