
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Physiol. , 06 August 2020
Sec. Redox Physiology
Volume 11 - 2020 | https://doi.org/10.3389/fphys.2020.00982
This article is part of the Research Topic Smoldering Inflammation in Cardio-Immune-Metabolic Diseases View all 10 articles
 Donato Moschetta1,2
Donato Moschetta1,2 Matteo Nicola Dario Di Minno3*†
Matteo Nicola Dario Di Minno3*† Benedetta Porro4
Benedetta Porro4 Gianluca L. Perrucci5
Gianluca L. Perrucci5 Vincenza Valerio1,6
Vincenza Valerio1,6 Valentina Alfieri1
Valentina Alfieri1 Ilaria Massaiu1
Ilaria Massaiu1 Alexander N. Orekhov7
Alexander N. Orekhov7 Alessandro Di Minno8
Alessandro Di Minno8 Paola Songia1
Paola Songia1 Viviana Cavalca4
Viviana Cavalca4 Veronika A. Myasoedova1
Veronika A. Myasoedova1 Paolo Poggio1*†
Paolo Poggio1*†Introduction: Osteopontin (OPN) is involved in ectopic calcification. Its circulating form is upregulated in coronary artery disease (CAD) patients. Circulating OPN levels positively correlate with oxidative stress, one of the major triggers of endothelial dysfunction. Endothelial dysfunction is, in turn, associated with reduced nitric oxide (NO) bioavailability due to the impaired arginine pathway. The aim of this study was to better understand the correlations between OPN, oxidative stress markers, and the arginine pathway metabolites.
Methods and Results: ELISA and mass spectrometry techniques have been used to evaluate circulating OPN and arginine pathway/oxidative stress metabolites, respectively, in twenty-five control subjects and thirty-three patients with overt atherosclerosis. OPN positively correlates with 2,3-dinor-8isoPGF2a levels (p = 0.02), ornithine (p = 0.01), ADMA (p = 0.001), SDMA (p = 0.03), and citrulline (p = 0.008) levels only in CAD patients. In addition, citrulline positively correlated with ADMA (p = 0.02) levels, possibly as result of other sources of citrulline biosynthetic pathways.
Conclusion: The association between OPN and impaired arginine/NO pathway could play a role in the inhibition of endothelial NO synthase (eNOS) and/or in the arginase activation in the context of CAD patients. However, further studies are needed to verify the cause-effect relationship between OPN, oxidative stress, and arginine/NO pathway dysregulation.
Osteopontin (OPN) is a phosphoglycoprotein secreted by different cellular types (monocytes, macrophages, cardiac fibroblasts, vascular smooth muscle cells, and endothelial cells), implicated in many molecular and cellular pathophysiological processes, including ectopic calcification (Cho and Kim, 2009). It has been shown that OPN plays an important role in the atherosclerotic plaque formation as well as in coronary artery diseases (CAD) (Wolak, 2014). In particular, several studies showed that circulating OPN levels are elevated in coronary artery disease (CAD) patients (Abdel-Azeez and Al-Zaky, 2010; Tousoulis et al., 2013; Wolak, 2014; Maniatis et al., 2019) and correlated with the disease extent and severity (Ohmori et al., 2003; Momiyama et al., 2010; Wolak, 2014). Indeed, circulating OPN has been proposed as a predictor of major cardiac events, such as acute myocardial infarction and ischemic heart disease (Georgiadou et al., 2010; Okyay et al., 2011). These observations, taken together with large literature evidences, corroborate the direct link between OPN and CAD development/progression (Wolak, 2014).
In addition, the upregulation of OPN transcription is also driven by oxidative stress (Branchetti et al., 2013) that represents one of the main initial atherosclerotic triggers, leading also to endothelial dysfunction (Incalza et al., 2018). Indeed, circulating OPN positively correlates with malondialdehyde levels, a recognized biomarker of oxidative stress (Cavalca et al., 2001; Georgiadou et al., 2008).
It has also been shown that increased levels of reactive oxygen species, in patients with CAD, lead to a progressive endothelial dysfunction (Incalza et al., 2018). Furthermore, it has been shown that endothelial vascular function impairment is associated with high OPN levels (Shemyakin et al., 2012; Schreier et al., 2016; Batko et al., 2019).
The endothelial dysfunction, among other causes, is associated with the impairment of the nitric oxide (NO) pathway, where the NO synthase (NOS) plays a pivotal role (Yang and Ming, 2013). NOS, using arginine as substrate, produces NO equimolarly to citrulline (Morris, 2007). Then, NO diffuses locally and mediates endothelium-dependent vasodilatation, acting on adhesion molecules and avoiding the infiltration of inflammatory cells and subsequent detrimental effects (Tousoulis et al., 2012). Undeniably, the reduction of NO bioavailability have a crucial importance in cardiovascular diseases (Cavalca et al., 2013; Eligini et al., 2013). Thus, in this study, we investigated the link between circulating OPN, oxidative stress, and endothelial dysfunction. We, therefore, performed an association study to explore the dysregulation of the arginine pathway and different oxidative stress markers in patients with overt CAD requiring surgical myocardial revascularization.
Thirty-three patients that underwent coronary artery bypass grafting (CABG) and twenty-five control subjects were enrolled in the study between January and June 2011 at Centro Cardiologico Monzino IRCCS. Pre-operative inclusion criteria were isolated surgical myocardial revascularization, elective surgery, age more than 18 years old, ejection fraction >30% and normal sinus rhythm. Exclusion criteria were prior cardiac surgery, rheumatic heart disease, endocarditis, active malignancy, chronic liver, and kidney diseases, calcium regulation disorders (hyperparathyroidism, hyperthyroidism and hypothyroidism) and chronic or acute inflammatory states (sepsis, autoimmune disease and inflammatory bowel disease). The Institutional Review Board and Ethical Committee of Centro Cardiologico Monzino (IRCCS) approved the study. Written informed consent to participate in this prospective observational study was obtained from all enrolled patients. The study protocol was conformed to the ethical guidelines of the 1975 Declaration of Helsinki.
Whole blood: 6 mL of peripheral blood sample was drawn from patients while fasting, into tubes containing EDTA (9.3 mM, Vacutainer Systems, Becton Dickinson, Franklin Lakes NJ, United States) kept on ice. 250 μL of whole blood was immediately precipitated with 250 μL of 10% trichloroacetic acid (Sigma-Aldrich, Darmstadt, Germany) plus 1 mM EDTA solution. Samples were stored at −80°C until analysis.
Plasma EDTA: anti-coagulated EDTA blood was centrifuged at 1700 g for 10 min at 4°C within 30 min after being drawn. Plasma was separated and aliquots were stored at −80°C until analysis.
Urine: urine collection was carried out the night before surgery or the night before the visit and samples stored at −80°C until analysis.
Plasma levels of soluble OPN were measured with an enzyme-linked immunosorbent assay (ELISA) kit (Quantikine, R&D) following manufacturer instructions.
Reduced (GSH) and oxidized glutathione (GSSG) forms were determined in whole blood by liquid chromatography-tandem mass spectrometry (LC-MS/MS) method (Squellerio et al., 2012; Valerio et al., 2019). The separation of analytes was conducted on a Luna PFP analytical column (100 mm × 2.0 mm, 3 m, Phenomenex) maintained at 35°C under isocratic conditions (flow rate of 250 μL/min, mobile phase:1% methanol in 0.75 mM ammonium formate adjusted to pH 3.5 with formic acid). LC-MS/MS analysis was performed using an Accela HPLC (high performance liquid chromatography) system coupled with a triple quadrupole mass spectrometer TSQ Quantum Access (Thermo Fisher Scientific, Waltham, MA, United States) equipped with an electrospray ionization (ESI) source working in multiple reaction monitoring (MRM) and in positive ionization mode.
Data were obtained after comparison with calibration curves using GSH and GSSG pure standard solutions (Sigma-Aldrich, Darmstadt, Germany). The intra- and inter-CVs (%) obtained with standard samples were <5% for both the analytes. The limits of detection were 0.031 μmol/L for GSH and 0.008 μmol/L for GSSG. Levels of GSH and GSSG were corrected for haemoglobin (Hb) and expressed as μmol/g Hb.
Urinary 2,3-dinor-8isoPGF2a was detected by LC-MS/MS method according to Cavalca et al. (2010). The urinary concentration was calculated from the area ratio of the ion peaks of the 2,3-dinor-8isoPGF2a over the deuterated standard (8-iso-PGF2a-d4). The estimated values were corrected for the urinary creatinine levels and expressed as pg/mg of creatinine.
The assessment of arginine, ornithine, citrulline, asymmetric dimethylarginines (ADMA), and symmetric dimethylarginine (SDMA) was performed by LC-MS/MS using a target metabolomic approach (Squellerio et al., 2011). Briefly, the chromatographic analysis was conducted on a Luna HILIC (hydrophilic interaction liquid chromatography) analytical column (50 mm × 2.0 mm, 3 μm, Phenomenex, Torrance, CA, United States). The mobile phases consisted of aqueous 1.5 mM ammonium formate (pH 3.2) (A) and 1.5 mM ammonium formate in acetonitrile/methanol (95.5:0.5, v/v) (pH 3.2) (B) at a flow rate of 250 μL/min. The mobile phase gradient ran from 10% A to 70% A over 7 min, from 70% A to 94.5% A over 2 min and was held at 94.5% A for 5 min, returning to 10% A over 2 min and held at 10% A for re-equilibration. The sample injection volume was 10 μL and the column temperature was set at 30°C. Total run time per sample, including column cleaning and re-equilibration, was 25 min. The mass spectrometric analysis was performed using a TSQ Quantum Access (Thermo Fisher Scientific, Waltham, MA, United States) triple quadrupole mass spectrometer equipped with ES) interface operating in MRM and positive ionization mode. The LOQ value is 0.25 M for all compounds, making this method suitable for the analysis of samples containing relatively low concentrations of the analytes, with a satisfactory precision as documented by the intra- and inter-day CVs of less than 10%. The method is linear in a wide range of concentrations (between 0 and 20 μM), with correlation coefficients greater than 0.99 and limit of detection (LOD) around 3–10 nm for all compounds. Global arginine bioavailability (GABR) was calculated as the ratio of arginine levels and the total amount of ornithine plus citrulline levels. GABR is an index of circulating arginine bioavailability associated with markers of endothelial dysfunction and increased risk of cardiovascular mortality (Morris et al., 2005; Sourij et al., 2011).
Continuous variables were analyzed using Student’s T-test and summarized as mean ± SD, while categorical ones were analyzed using Chi-square test and summarized as frequency (n) and percentage (%). Circulating biomarkers were analyzed by the Pearson product-moment correlation coefficient (rp) and plotted using Graphpad Prism v7.0. A value of p ≤ 0.05 was deemed statistically significant.
Demographic and clinical characteristics, as well as pharmacological therapies of the study population are listed in Supplementary Table S1. As previously reported by other authors (Tousoulis et al., 2013; Wolak, 2014; Maniatis et al., 2019), circulating OPN levels were lower in controls compared to the CAD patients (57.76 ± 9.8 vs 68.37 ± 24.2 pg/ml, respectively, p = 0.04, Supplementary Figure S1).
We assess the possible relationship between OPN levels and oxidative stress status, represented by 2,3-dinor-8isoPGF2a and the ratio between the reduced (GSH) and the oxidized (GSSG) forms of glutathione, in patients before the surgical intervention.
Linear regression analysis reported that there was no significant association between OPN levels and GSH/GSSG ratio, in controls (rp = 0.002, p = 0.99, Supplementary Figure S2A), as well as in CAD patients (rp = −0.15, p = 0.42, Figure 1A). The same analysis showed that there was no association between OPN levels and 2,3-dinor-8isoPGF2a urine levels in control group (rp = −0.38, p = 0.08, Supplementary Figure S2B). However, OPN levels were directly correlated with 2,3-dinor-8isoPGF2a urine levels in CAD patients (rp = 0.42, p = 0.02, Figure 1B).
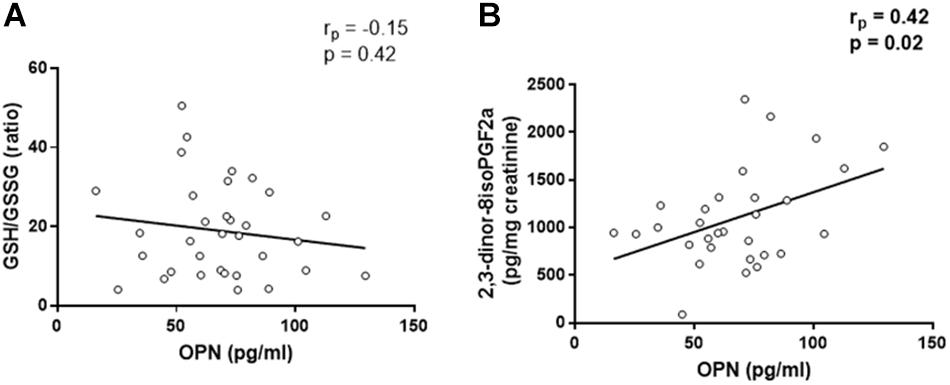
Figure 1. Linear regression analyses between osteopontin (OPN) and GSH/GSSG ratio (A) and 2,3-dinor-8isoPGF2a (B).
Since arginine is the substrate of NOS, we evaluated the metabolites involved in arginine pathway as representative molecules of NO production (Morris, 2007, 2016). In the control group, there were no correlations between the considered metabolites and circulating OPN levels (arginine, rp = −0.33, p = 0.11, ornithine, rp = −0.04, p = 0.85, citrulline, rp = 0.07, p = 0.75, ADMA, rp = −0.30, p = 0.14, SDMA, rp = −0.23, p = 0.28, GABR, rp = 0.30, p = 0.14, Supplementary Figure S3). In CAD patients, the linear regressions showed that OPN levels were not associated with arginine levels (rp = 0.20, p = 0.27, Figure 2A) and the global arginine bioavailability (GABR, rp = −0.29, p = 0.11, Figure 2F). However, OPN levels were positively correlated with ornithine (rp = 0.44, p = 0.01, Figure 2B), ADMA (rp = 0.54, p = 0.001, Figure 2D), and SDMA (rp = 0.37, p = 0.03, Figure 2E) levels.
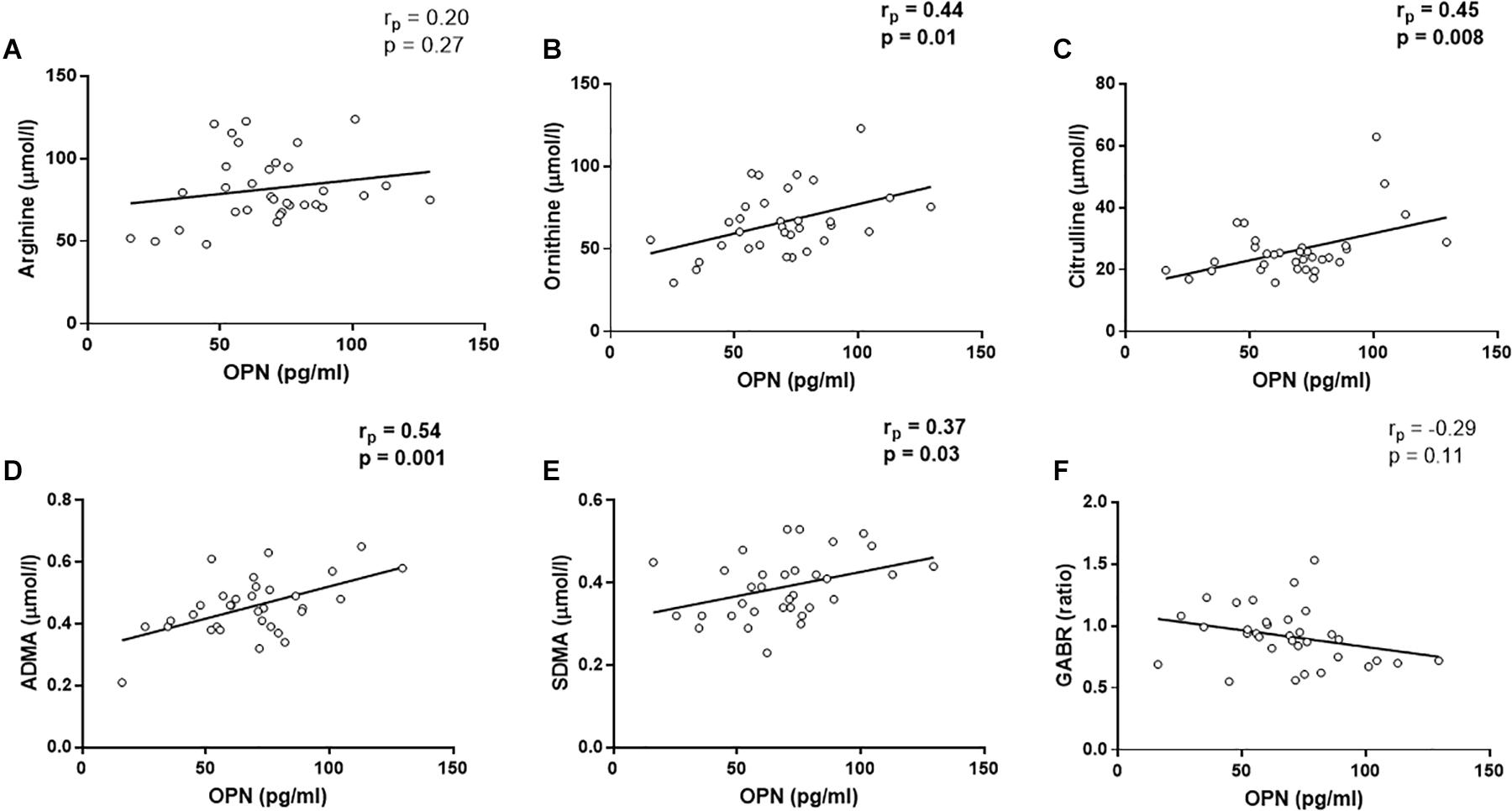
Figure 2. Linear regression analyses between osteopontin (OPN) and arginine (A), ornithine (B), Citrulline (C), asymmetric dimetilarginine [ADMA, (D)], symmetric dimethilarginine [SDMA, (E)], and global arginine bioavailability [GABR, (F)].
In addition, a positive correlation was found between OPN and citrulline (rp = 0.45, p = 0.008, Figure 2C). Citrulline is known to be produced by (i) NOS from arginine, equimolarly with NO, (ii) ornithinetranscarbamilase (OTC) form ornithine, and (iii) dimethylarginine dimethylaminohydrolase (DDAH) from ADMA. In this regard, citrulline was not associated with ornithine (rp = 0.18, p = 0.33, Figure 3A), although, we found that citrulline levels were associated with ADMA (rp = 0.40, p = 0.02, Figure 3B) levels.
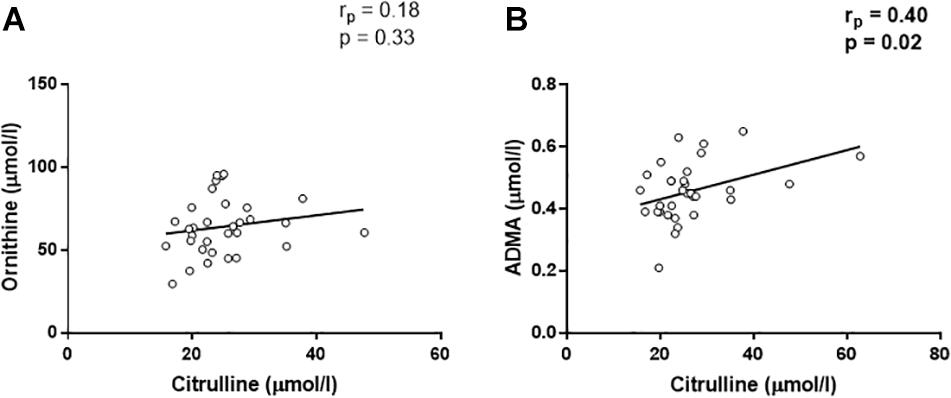
Figure 3. Linear regression analyses between citrulline and ornithine (A) and asymmetric dimetilarginine [ADMA, (B)].
To our knowledge, we show for the first time that OPN could be linked to the pathological dysregulation of the arginine pathway in CAD patients.
As largely reported before also CAD patients in our cohort showed high levels of circulating OPN. Nonetheless, CAD patients were characterized by an increased oxidative stress status and impaired endothelial function associated with a NO bioavailability reduction (Abdel-Azeez and Al-Zaky, 2010; Yang and Ming, 2013; Incalza et al., 2018). Recently some authors reported that high OPN levels could in some way interfere with vessel endothelial function (Shemyakin et al., 2012; Batko et al., 2019; Maniatis et al., 2019). – In this study, we investigated the relationship between OPN, oxidative stress, and endothelial dysfunction taking into account the arginine metabolism. In our cohort, plasma OPN levels correlated with urinary 2,3-dinor-8isoPGF2a, in agreement with literature evidences on the intensified production of OPN caused by an increased systemic oxidative stress status. However, we did not see any significant association between plasma OPN and GSH/GSSG ratio. These data suggest that lipid peroxidation may be the main process induced by the oxidative stress in the context of CAD, instead of protein oxidation. The link between OPN and lipid peroxidation is corroborated by the lack of any association between OPN and 2,3-dinor-8isoPGF2a in the control group.
It has been shown that OPN could interfere with the arginine pathway by inhibiting the inducible form of the NOS (iNOS) enzyme (Singh et al., 1995; Rabenstein et al., 2016). Thus, it is likely that the same mechanisms could cause an inhibition of endothelial NOS (eNOS) enzyme as a result of increased OPN levels. In this scenario, the reduction of NO synthesis, in combination with increased oxidative stress status, would favor the atherosclerotic milieu (Mahdi et al., 2019). For this purpose, we analyzed the metabolites of the arginine pathway both in controls and CAD patients. We found no correlation between OPN and any metabolite in control group, while in the in CAD patients, we found that OPN directly correlated with several metabolites belonging to the arginine pathway. Indeed, we found positive correlations between OPN, ADMA, SDMA, and ornithine. SDMA is not only an inhibitor of the arginine transporter CAT (Closs et al., 1997), but also a pro-inflammatory molecule (Chen et al., 2012), like OPN (Icer and Gezmen-Karadag, 2018). In the context of CAD, SDMA could play both roles acting in synergy with OPN in the development of the inflammation. However, we also observed positive correlations between citrulline and OPN. To explain this last correlation, we have to take into account that citrulline is normally produced equimolarly to NO from arginine by eNOS, but other sources of its production are known (Morris, 2007). In particular, citrulline could derive from ADMA by DDAH activity. Indeed, in our cohort we found a positive correlation between citrulline and ADMA, indicating that high levels of citrulline could be due to the activity of DDAH enzyme.
In 2012, Shemyakin et al. (2012) showed, in CAD patients, an improved endothelial functionality probably due to the inhibition of arginase. This evidence suggests that arginase activation reduces arginine bioavailability, thus NOS-mediated NO production, fundamental to maintain the endothelial function. Of notice, it has been reported a possible interaction between OPN and arginase (Partridge et al., 2008). Thus, it is likely that OPN could stimulate arginase activity in the CAD context.
We strongly believe that OPN could be directly or indirectly implicated in the decreased activity of eNOS in atherosclerosis, contributing to the endothelial dysfunction typically observed in CAD patients. We therefore propose a schematic view of the possible components that could link OPN to the arginine metabolism (Figure 4).
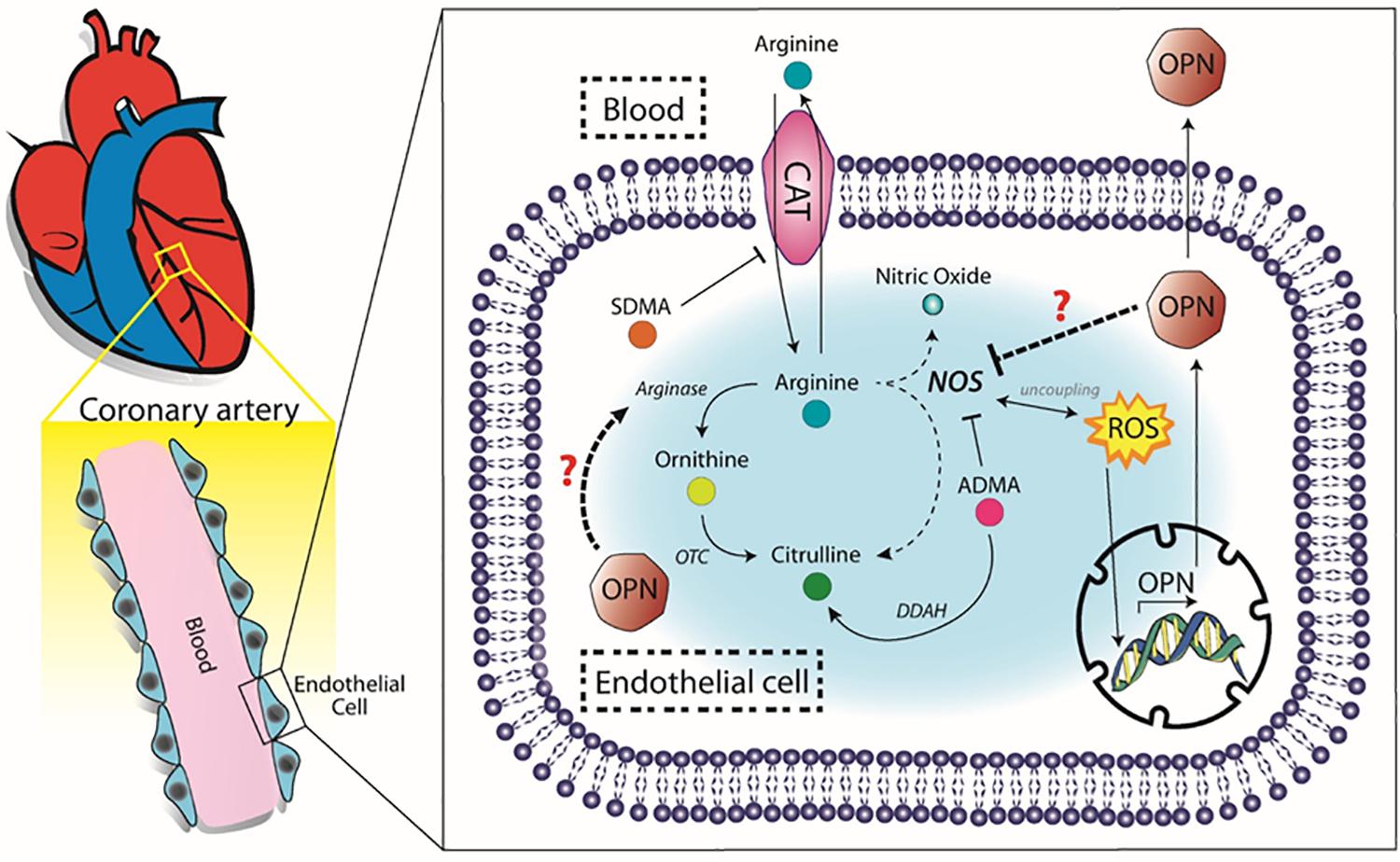
Figure 4. Proposed molecular pathway. OPN, osteopontin, SDMA, symmetric dimethilarginine, ADMA, asymmetric dimetilarginine, CAT, cationic aminoacid transporter, OTC, ornithinetranscarbamilase, DDAH, dimethylarginine dimethylaminohydrolase, ROS, reactive oxygen species.
In summary, our results showed a correlation between OPN levels, oxidative stress status, and endothelial dysfunction markers in CAD patients. Nonetheless, further studies are required to determine if OPN really drives the endothelial dysfunction by direct or indirect eNOS inhibition in CAD patients. Endothelial cells from coronary artery, genetically modified to silence or overexpress OPN, could be the appropriate in vitro model to determine the functionality of the enzymes involved in the NO/arginine pathway. While OPN knockout mice would represent the best in vivo model to evaluate the relationship between the NO/arginine pathway and the OPN (Pedersen et al., 2013).
This study has different limitations. First, we could not investigate the influence of each pharmacological treatment on the analyzed metabolites due to our small cohort. Second, we could not measure eNOS, arginase, and DDAH levels and activity. Third, although flow mediated dilation (FMD) is a recognized technique to assess endothelial dysfunction, we could not evaluate it given the status of our patients before surgery, as well as high number of drugs taken as per the 2019 European Society of Cardiology (ESC) guidelines for FMD evaluation (Thijssen et al., 2019). Lastly, we have not measured other common oxidative stress markers, such as malondialdehyde, since we wanted to investigate the glutathione system and the lipid peroxidation. Our study showed an association between OPN and endothelial dysfunction, however, further studies are necessary to prove the cause-effect relationship in CAD patients.
The datasets generated for this study are available on request to the corresponding author.
The studies involving human participants were reviewed and approved by the Institutional Review Board and Ethical Committee of Centro Cardiologico Monzino (IRCCS). The patients/participants provided their written informed consent to participate in this study.
PP and MD conceived the study. VM collected the informed consensus and the specimens. BP performed mass spectrometry evaluation. VA, VV, and IM performed the experimental evaluations. DM and PP performed statistical analyses and drafted the manuscript. GP prepared the illustration. MD, BP, GP, VV, VA, IM, AO, AD, VC, VM, and PS substantially revised the manuscript. All authors read and approved the final manuscript.
This work was supported by the Italian Ministry of Health funds (Ricerca Corrente: RC2016-BIO34-2627243 and RC2019-CA1A-2755299) and by Fondazione Gigi e Pupa Ferrari ONLUS (FPF-14).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2020.00982/full#supplementary-material
Abdel-Azeez, H. A., and Al-Zaky, M. (2010). Plasma osteopontin as a predictor of coronary artery disease: association with echocardiographic characteristics of atherosclerosis. J. Clin. Lab. Anal. 24, 201–206. doi: 10.1002/jcla.20378
Batko, K., Krzanowski, M., Gajda, M., Dumnicka, P., Fedak, D., Woziwodzka, K., et al. (2019). Endothelial injury is closely related to osteopontin and TNF receptor-mediated inflammation in end-stage renal disease. Cytokine 121:154729. doi: 10.1016/j.cyto.2019.05.016
Branchetti, E., Sainger, R., Poggio, P., Grau, J. B., Patterson-Fortin, J., Bavaria, J. E., et al. (2013). Antioxidant enzymes reduce DNA damage and early activation of valvular interstitial cells in aortic valve sclerosis. Arterioscler. Thromb. Vasc. Biol. 33, e66–e74.
Cavalca, V., Cighetti, G., Bamonti, F., Loaldi, A., Bortone, L., Novembrino, C., et al. (2001). Oxidative stress and homocysteine in coronary artery disease. Clin. Chem. 47, 887–892. doi: 10.1093/clinchem/47.5.887
Cavalca, V., Minardi, F., Scurati, S., Guidugli, F., Squellerio, I., Veglia, F., et al. (2010). Simultaneous quantification of 8-iso-prostaglandin-F(2alpha) and 11-dehydro thromboxane B(2) in human urine by liquid chromatography-tandem mass spectrometry. Anal. Biochem. 397, 168–174. doi: 10.1016/j.ab.2009.10.014
Cavalca, V., Tremoli, E., Porro, B., Veglia, F., Myasoedova, V., Squellerio, I., et al. (2013). Oxidative stress and nitric oxide pathway in adult patients who are candidates for cardiac surgery: patterns and differences. Interact. Cardiovasc. Thorac. Surg. 17, 923–930. doi: 10.1093/icvts/ivt386
Chen, S., Martens-Lobenhoffer, J., Weissenborn, K., Kielstein, J. T., Lichtinghagen, R., Deb, M., et al. (2012). Association of dimethylarginines and mediators of inflammation after acute ischemic stroke. J. Neuroinflamm. 9:251.
Cho, H. J., and Kim, H. S. (2009). Osteopontin: a multifunctional protein at the crossroads of inflammation, atherosclerosis, and vascular calcification. Curr. Atheroscler. Rep. 11, 206–213. doi: 10.1007/s11883-009-0032-8
Closs, E. I., Basha, F. Z., Habermeier, A., and Forstermann, U. (1997). Interference of L-arginine analogues with L-arginine transport mediated by the y+ carrier hCAT-2B. Nitric Oxide 1, 65–73. doi: 10.1006/niox.1996.0106
Eligini, S., Porro, B., Lualdi, A., Squellerio, I., Veglia, F., Chiorino, E., et al. (2013). Nitric oxide synthetic pathway in red blood cells is impaired in coronary artery disease. PLoS One 8:e66945. doi: 10.1371/journal.pone.0066945
Georgiadou, P., Iliodromitis, E. K., Kolokathis, F., Varounis, C., Gizas, V., Mavroidis, M., et al. (2010). Osteopontin as a novel prognostic marker in stable ischaemic heart disease: a 3-year follow-up study. Eur. J. Clin. Invest. 40, 288–293. doi: 10.1111/j.1365-2362.2010.02257.x
Georgiadou, P., Iliodromitis, E. K., Varounis, C., Mavroidis, M., Kolokathis, F., Andreadou, I., et al. (2008). Relationship between plasma osteopontin and oxidative stress in patients with coronary artery disease. Expert Opin. Ther. Targets 12, 917–920. doi: 10.1517/14728222.12.8.917
Icer, M. A., and Gezmen-Karadag, M. (2018). The multiple functions and mechanisms of osteopontin. Clin. Biochem. 59, 17–24. doi: 10.1016/j.clinbiochem.2018.07.003
Incalza, M. A., D’Oria, R., Natalicchio, A., Perrini, S., Laviola, L., and Giorgino, F. (2018). Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vasc. Pharmacol. 100, 1–19. doi: 10.1016/j.vph.2017.05.005
Mahdi, A., Kovamees, O., and Pernow, J. (2019). Improvement in endothelial function in cardiovascular disease - Is arginase the target? Int. J. Cardiol. 301, 207–214. doi: 10.1016/j.ijcard.2019.11.004
Maniatis, K., Siasos, G., Oikonomou, E., Vavuranakis, M., Zaromytidou, M., Mourouzis, K., et al. (2019). Osteoprotegerin and osteopontin serum levels are associated with vascular function and inflammation in coronary artery disease patients. Curr. Vasc. Pharmacol. doi: 10.2174/1570161117666191022095246 [Epub ahead of print].
Momiyama, Y., Ohmori, R., Fayad, Z. A., Kihara, T., Tanaka, N., Kato, R., et al. (2010). Associations between plasma osteopontin levels and the severities of coronary and aortic atherosclerosis. Atherosclerosis 210, 668–670. doi: 10.1016/j.atherosclerosis.2009.12.024
Morris, C. R., Kato, G. J., Poljakovic, M., Wang, X., Blackwelder, W. C., Sachdev, V., et al. (2005). Dysregulated arginine metabolism, hemolysis-associated pulmonary hypertension, and mortality in sickle cell disease. JAMA 294, 81–90.
Morris, S. M. Jr. (2007). Arginine metabolism: boundaries of our knowledge. J. Nutr. 137, 1602S–1609S. doi: 10.1093/jn/137.6.1602s
Morris, S. M. Jr. (2016). Arginine metabolism revisited. J. Nutr. 146, 2579S–2586S. doi: 10.3945/jn.115.226621
Ohmori, R., Momiyama, Y., Taniguchi, H., Takahashi, R., Kusuhara, M., Nakamura, H., et al. (2003). Plasma osteopontin levels are associated with the presence and extent of coronary artery disease. Atherosclerosis 170, 333–337. doi: 10.1016/s0021-9150(03)00298-3
Okyay, K., Tavil, Y., Sahinarslan, A., Tacoy, G., Turfan, M., Sen, N., et al. (2011). Plasma osteopontin levels in prediction of prognosis in acute myocardial infarction. Acta Cardiol. 66, 197–202. doi: 10.1080/ac.66.2.2071251
Partridge, C. R., He, Q., Brun, M., and Ramos, K. S. (2008). Genetic networks of cooperative redox regulation of osteopontin. Matrix Biol. 27, 462–474. doi: 10.1016/j.matbio.2008.01.009
Pedersen, T. X., Madsen, M., Junker, N., Christoffersen, C., Vikesa, J., Bro, S., et al. (2013). Osteopontin deficiency dampens the pro-atherogenic effect of uraemia. Cardiovasc. Res. 98, 352–359. doi: 10.1093/cvr/cvt049
Rabenstein, M., Vay, S. U., Flitsch, L. J., Fink, G. R., Schroeter, M., and Rueger, M. A. (2016). Osteopontin directly modulates cytokine expression of primary microglia and increases their survival. J. Neuroimmunol. 299, 130–138. doi: 10.1016/j.jneuroim.2016.09.009
Schreier, M., Schwartze, J. T., Landgraf, K., Scheuermann, K., Erbs, S., Herberth, G., et al. (2016). Osteopontin is BMI-independently related to early endothelial dysfunction in children. J. Clin. Endocrinol. Metab. 101, 4161–4169. doi: 10.1210/jc.2016-2238
Shemyakin, A., Kovamees, O., Rafnsson, A., Bohm, F., Svenarud, P., Settergren, M., et al. (2012). Arginase inhibition improves endothelial function in patients with coronary artery disease and type 2 diabetes mellitus. Circulation 126, 2943–2950. doi: 10.1161/circulationaha.112.140335
Singh, K., Balligand, J. L., Fischer, T. A., Smith, T. W., and Kelly, R. A. (1995). Glucocorticoids increase osteopontin expression in cardiac myocytes and microvascular endothelial cells. Role in regulation of inducible nitric oxide synthase. J. Biol. Chem. 270, 28471–28478.
Sourij, H., Meinitzer, A., Pilz, S., Grammer, T. B., Winkelmann, B. R., Boehm, B. O., et al. (2011). Arginine bioavailability ratios are associated with cardiovascular mortality in patients referred to coronary angiography. Atherosclerosis 218, 220–225. doi: 10.1016/j.atherosclerosis.2011.04.041
Squellerio, I., Caruso, D., Porro, B., Veglia, F., Tremoli, E., and Cavalca, V. (2012). Direct glutathione quantification in human blood by LC-MS/MS: comparison with HPLC with electrochemical detection. J. Pharm. Biomed. Anal. 71, 111–118. doi: 10.1016/j.jpba.2012.08.013
Squellerio, I., Tremoli, E., and Cavalca, V. (2011). Quantification of arginine and its metabolites in human erythrocytes using liquid chromatography-tandem mass spectrometry. Anal. Biochem. 412, 108–110. doi: 10.1016/j.ab.2011.01.018
Thijssen, D. H. J., Bruno, R. M., van Mil, A., Holder, S. M., Faita, F., Greyling, A., et al. (2019). Expert consensus and evidence-based recommendations for the assessment of flow-mediated dilation in humans. Eur. Heart J. 40, 2534–2547. doi: 10.1093/eurheartj/ehz350
Tousoulis, D., Kampoli, A. M., Tentolouris, C., Papageorgiou, N., and Stefanadis, C. (2012). The role of nitric oxide on endothelial function. Curr. Vasc. Pharmacol. 10, 4–18.
Tousoulis, D., Siasos, G., Maniatis, K., Oikonomou, E., Kioufis, S., Zaromitidou, M., et al. (2013). Serum osteoprotegerin and osteopontin levels are associated with arterial stiffness and the presence and severity of coronary artery disease. Int. J. Cardiol. 167, 1924–1928. doi: 10.1016/j.ijcard.2012.05.001
Valerio, V., Myasoedova, V. A., Moschetta, D., Porro, B., Perrucci, G. L., Cavalca, V., et al. (2019). Impact of oxidative stress and protein s-glutathionylation in aortic valve sclerosis patients with overt atherosclerosis. J Clin Med 8:552.
Wolak, T. (2014). Osteopontin - a multi-modal marker and mediator in atherosclerotic vascular disease. Atherosclerosis 236, 327–337. doi: 10.1016/j.atherosclerosis.2014.07.004
Keywords: atherosclerosis, endothelial dysfunction, OPN, nitric oxide, citrulline
Citation: Moschetta D, Di Minno MND, Porro B, Perrucci GL, Valerio V, Alfieri V, Massaiu I, Orekhov AN, Di Minno A, Songia P, Cavalca V, Myasoedova VA and Poggio P (2020) Relationship Between Plasma Osteopontin and Arginine Pathway Metabolites in Patients With Overt Coronary Artery Disease. Front. Physiol. 11:982. doi: 10.3389/fphys.2020.00982
Received: 20 December 2019; Accepted: 20 July 2020;
Published: 06 August 2020.
Edited by:
Nazareno Paolocci, Johns Hopkins University, United StatesReviewed by:
John David Horowitz, University of Adelaide, AustraliaCopyright © 2020 Moschetta, Di Minno, Porro, Perrucci, Valerio, Alfieri, Massaiu, Orekhov, Di Minno, Songia, Cavalca, Myasoedova and Poggio. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Matteo Nicola Dario Di Minno, ZGFyaW8uZGltaW5ub0Bob3RtYWlsLml0; Paolo Poggio, cGFvbG8ucG9nZ2lvQGNjZm0uaXQ=
†ORCID: Matteo Nicola Dario Di Minno, orcid.org/0000-0001-8059-3819; Paolo Poggio, orcid.org/0000-0002-7225-3379
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.