- 1Department of Cardiovascular, Tungwah Hospital of Sun Yat-sen University, Dongguan, China
- 2Department of Urology, Tungwah Hospital of Sun Yat-sen University, Dongguan, China
- 3Department of Radiation Oncology, Affiliated Cancer Hospital & Institute of Guangzhou Medical University, Guangzhou, China
- 4State Key Laboratory of Respiratory Diseases, Guangzhou Institute of Respiratory Disease, Affiliated Cancer Hospital & Institute of Guangzhou Medical University, Guangzhou, China
- 5Department of Medicine and Therapeutics, Faculty of Medicine, The Chinese University of Hong Kong, Shatin, Hong Kong
Despite advances in the treatment of coronary diseases, acute coronary syndrome (ACS) remains the leading cause of death worldwide. ACS is associated with metabolic abnormalities of lipid oxidation stress. In this study, based on liquid chromatograph mass spectrometry technique, we conducted the metabolic profiling analysis of serum samples from stable plaques (SPs) and vulnerable plaques (VPs) in ACS patients for exploring the potential biomarkers of plaque stability. The results showed that four differential metabolites were identified between the SPs and VPs, including betaine, acetylcarnitine, 1-heptadecanoyl-sn-glycero-3-phosphocholine, and isoundecylic acid. Meanwhile, the diagnostic model was identified using stepwise logistic regression and internally validated with 10-fold cross-validation. We analyzed the correlations between serum metabolic perturbations and plaque stability, and the serum betaine and ejection fraction-based model was established with a good diagnostic efficacy [area under the curve (AUC) = 0.808, sensitivity = 70.6%, and specificity = 80.0%]. In summary, we firstly illustrate the comprehensive serum metabolic profiles in ACS patients, suggesting that the combined model of serum betaine and ejection fraction seems to be used as the potential diagnostic biomarker for the vulnerability of plaque stability.
Introduction
Coronary artery disease (CAD), one of the metabolic disorder diseases, causes the most serious cardiovascular death events in the world (Terashima et al., 2000). Acute coronary syndrome (ACS), a severe category of CAD, is usually associated with the rupture of unstable plaques (VPs), formation of thrombosis, and occurrence of acute myocardial ischemia (Goodacre et al., 2009). Clinically, ACS can be distinguished into unstable angina (UA) and acute myocardial infarction (AMI). Early detection of VPs is critical to receive suitable timely therapy and inhibit the happening of AMI and heart failure (Reihani et al., 2018).
Previous studies suggested that atherosclerosis is a chronic inflammatory arterial disease and led by an imbalanced lipid metabolism, and subsequent impaired immune response in developing over decades, though a number of biomarkers related to the peripheral or coronary diseases have been found to be associated with lipid metabolism and might be useful in predicating ACS (Ridker, 2007; Wang et al., 2007; Antoniades et al., 2009). However, most of the studies on biomarkers of coronary diseases were based on retrospective study, and the predictive sensitivity and specificity of biomarkers remain to be nonconforming and inconclusive. Thus, exploration of novel circulatory biomarkers capable of predicting cardiovascular events induced by VPs is still a major challenge.
Metabolomics, a newly developed discipline, is considered to allow qualitative and quantitation analysis of low-molecular-weight metabolites or endogenous metabolic substances in physiological or pathological conditions (Calderon-Santiago et al., 2017). Metabolomics is widely used in diagnosing a variety of metabolic diseases, such as gastritis (Chen et al., 2019), liver fibrosis (Fang et al., 2017), diabetes (Klein and Shearer, 2016), and cancers (Huang et al., 2013; Chan et al., 2014; O’Keefe et al., 2015). Simultaneously, the application of metabolomics in cardiovascular diseases like hyperlipidemia (Sun et al., 2017), atherosclerosis (Wishart, 2016), and hypertension (Au et al., 2017) has also gradually attracted much more attention. However, the metabolomics change of ACS is not well known and the risk stratification role of serum metabolic biomarkers on ACS and stable CAD need to be further explored.
In the present study, we explored the metabolomics on serum samples of stable plaques (SPs) and VPs in ACS patients to analyze the metabolomics correlations between SPs and VPs and to find and identify specific metabolites that may be potentially conducted as risk stratification biomarkers of ACS patients.
Materials and Methods
Patients’ Characteristics and Sample Selection
In this study, we reviewed and identified patients with newly diagnosed ACS who were treated between November 2016 and February 2019 at the Tungwah Hospital of Sun Yat-sen University. We recruited 252 adult patients who present with acute chest pain and age >20 years old in the emergency department of the Tungwah Hospital of Sun Yat-sen University. Exclusion criteria are as follows: patients with severe liver or kidney diseases, marrow and hematological system diseases, chest pain caused by trauma, malignancy, or previously diagnosed with coronary disease in 2 months and/or were treated accordingly. All participants with ACS were enrolled based upon typical clinical features, electrocardiogram (ECG) examination, elevated cardiac troponin I (cTnI), coronary angiography (CAG), and intravenous ultrasound (IVUS) that met the criteria of ACS from guideline recommendations. This study was approved by the ethics committee of Tungwah Hospital of Sun Yat-sen University. Informed consent was obtained from all subjects before enrollment in the study. Fresh serum samples (around 800 μl) were collected after enrollment, according to the manufacturer’s protocol of Longseegen storage kit (Longsee Biomedical Corporation, Guangzhou, China), and stored at −80°C.
Chemicals
Liquid chromatograph mass spectrometer (LC-MS) grade acetonitrile, methanol, and ultrapure water were purchased from Thermo Fisher Scientific (Waltham, MA, United States). Ammonium acetate and formic acid were purchased from CNW (Shanghai, China). 1-Heptadecanoyl-sn-glycero-3-phosphocholine and betaine were from ChromaBio (Chengdu, China). Acetylcarnitine was purchased from EFFBIO (Shanghai, China).
Sample Preparation
Blood samples from ACS patients were collected and homogenized. Serum sample was collected, which was added with 800 μl methanol/acetonitrile. Then, the sample was vibrated, subjected to ultrasound, and incubated at −20°C to promote protein mixture precipitation. The serum mixtures were centrifuged, and the supernatants were collected, vacuum-dried, and re-dissolved. Lastly, the supernatants were conducted to metabolomics profiling by liquid chromatography mass spectrometry.
Untargeted Metabolomics and Measurement of Metabolites
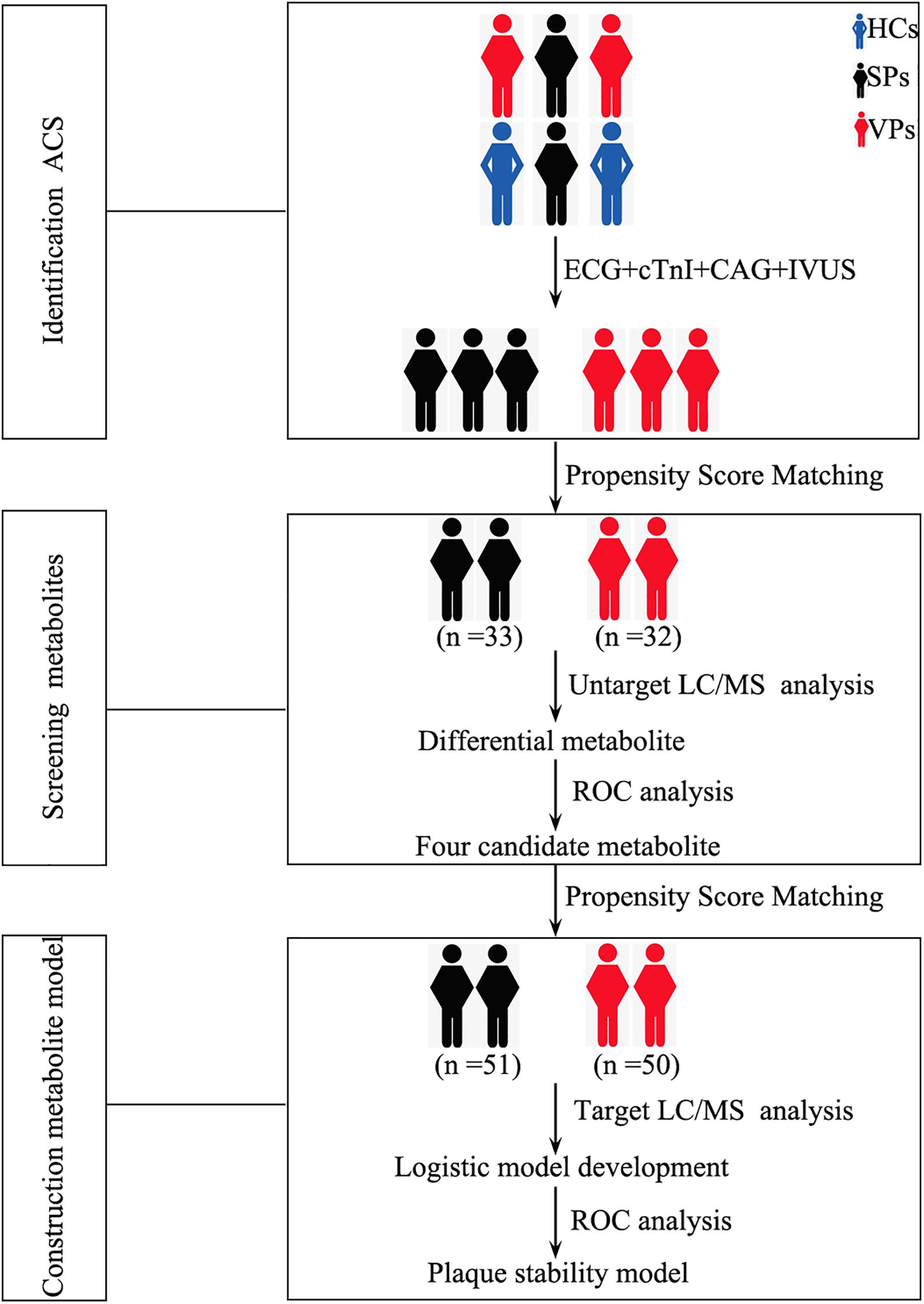
Ninety-eight serum samples from ACS patients were assigned to detect untargeted metabolomics. After the samples were screened by propensity score matching, 65 serum samples (SPs, n = 33; VPs, n = 32) were included to perform untargeted metabolomics. Followed by previous protocols with modifications (Chen et al., 2016, 2017; Shivanna et al., 2016; Zhang et al., 2019), after serum sample preparation, metabolite preprocessing and statistical analysis were performed with a Waters AcquityTM Ultra Performance.
The process was as follows: 95% A (a acetonitrile solution) and 5% B (a water solution) from 0 to 0.5 min, 90% A and 10% B from 0.5 to 2 min, 40% A and 60% B from 2 to 10 min, 5% A and 95% B from 10 to 14 min, 5% A and 95% B from 14 to 16 min, 95% A and 5% B from 16 to 18 min and 95% A and 5% B from 18 to 20 min.
Targeted Metabolomics and Measurement of Serum Biomarkers
One hundred fifty-four serum samples from ACS patients were assigned to detect targeted metabolomics. After the samples were screened by propensity score matching, 101 serum samples (SPs, n = 51; VPs, n = 50) were included to perform targeted metabolomics. The process was as follows: flow rate was kept at 0.5 ml/min with a 3-min run by isocratic elution. All samples were analyzed in positive mode. The parameters of ion spray voltage, source temperature, and the MS1 scan range and the MS2 are the same as untargeted metabolomics and the measurement of metabolites.
Metabolites Identification
As the protocol was described by the reported procedure (Zhao et al., 2012, 2013), the metabolite was analyzed. Based on the molecular mass data of metabolite, all putative identities were confirmed by matching with entries in the METLIN1, the HMDB database2, and KEGG database3. The metabolite would be identified on the condition that the mass difference between the observed value and the database value was <0.025 Da.
Metabolomics Data Analysis
Untargeted metabolomics datasets were analyzed by R with packages MetaboAnalyst. To reduce the metabolite concentration differences between samples and make the skewed distributions more symmetric, the data were normalized and non-linear conversed by log transformations.
Statistical Analysis
For univariate analysis, the statistical significance of features was analyzed between the VPs group and the SPs group using t-test. For multivariate analysis, partial-least-squares discrimination analysis (PLS-DA) was applied to reduce the effect of inter-subject variability and identify differential metabolites that significantly contributed to plaque stability. To ensure the quality of the multivariate model and avoid the risk of over-fitting, according to the variable importance in the projection (VIP) scores, the metabolites were further ranked based on the PLS-DA model. Metabolites with VIP scores >1.0 are considered as the significant contributors. *P < 0.05 and **P < 0.01 were considered to be statistically significant.
Stepwise logistic regression on dichotomized (positive/negative) data in SAS was used to narrow the number of metabolites. Based on the results of this logistic regression, the diagnostic accuracy was further performed based on the area under the curve (AUC). Models were internally validated using 10-fold cross-validation, meaning that the data were split into 10 equally sized datasets. The AUC was then calculated using each of the 10 datasets for validation in turn and the remaining 9 datasets for training. The average of the 10 results (i.e., average of the AUC calculated from the 10 validation subsets) is reported as a value for the 10-fold cross-validation.
Results
Demographic Characteristics of the Study Population
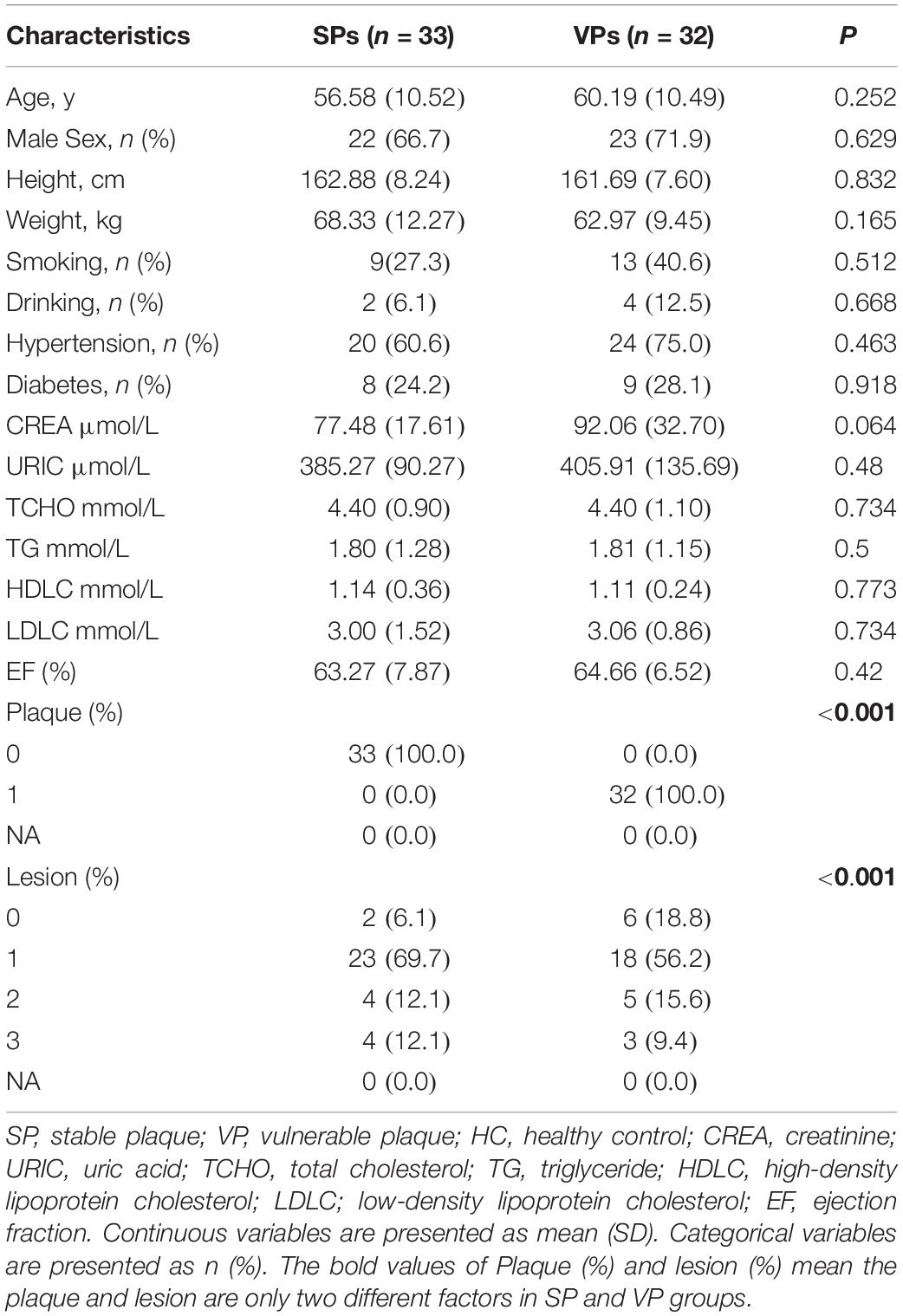
The populations are diagnosed as ACS by ECG, cTnI, and CAG. The stability of atherosclerotic plaques is detected by intravenous ultrasound (IVUS). The general clinical and demographic data on ACS patients are presented in Table 1. Atherosclerotic plaque and lesion were significantly associated with ACS. There were no significant differences in age, gender, height, weight, smoking, drinking, hypertension, diabetes, and blood biochemistry between the VPs group and SPs group by Student’s t-test. The flow diagram of the overview of study design was depicted in Figure 1.
Metabolomics Analysis of Serum Samples by Untargeted LC/MS
Representative mass spectrum images of SPs and VPs patients were shown in Figures 2A,B. Using untargeted LC/MS, we detected the variables positive ion mode and negative ion mode. Three thousand sixty-nine molecular metabolites were obtained and then subjected to statistical analysis using MetaboAnalyst (Supplementary Table S1). PLS-DA analysis found that HCs and ACS patients, especially in SPs and VPs patients, showed differential distributions with Q2 of 0.820 and R2 of 0.973, which means that the model was not only overlifting but also reliable (Figures 3A,B).
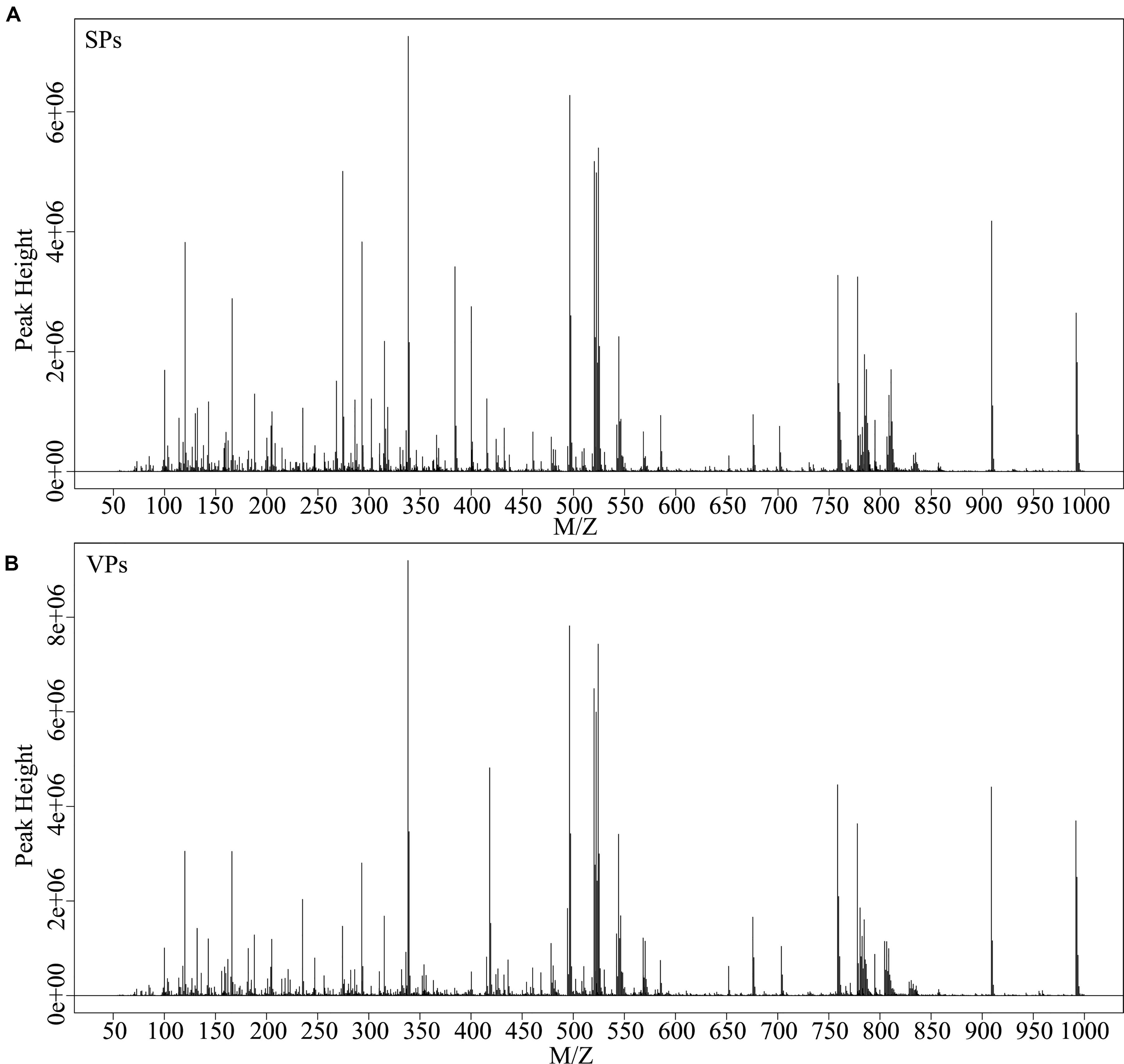
Figure 2. Typical mass spectra of the SPs group (A) and VPs group (B). SPs, stable plaque groups; VPs, vulnerable plaque groups.
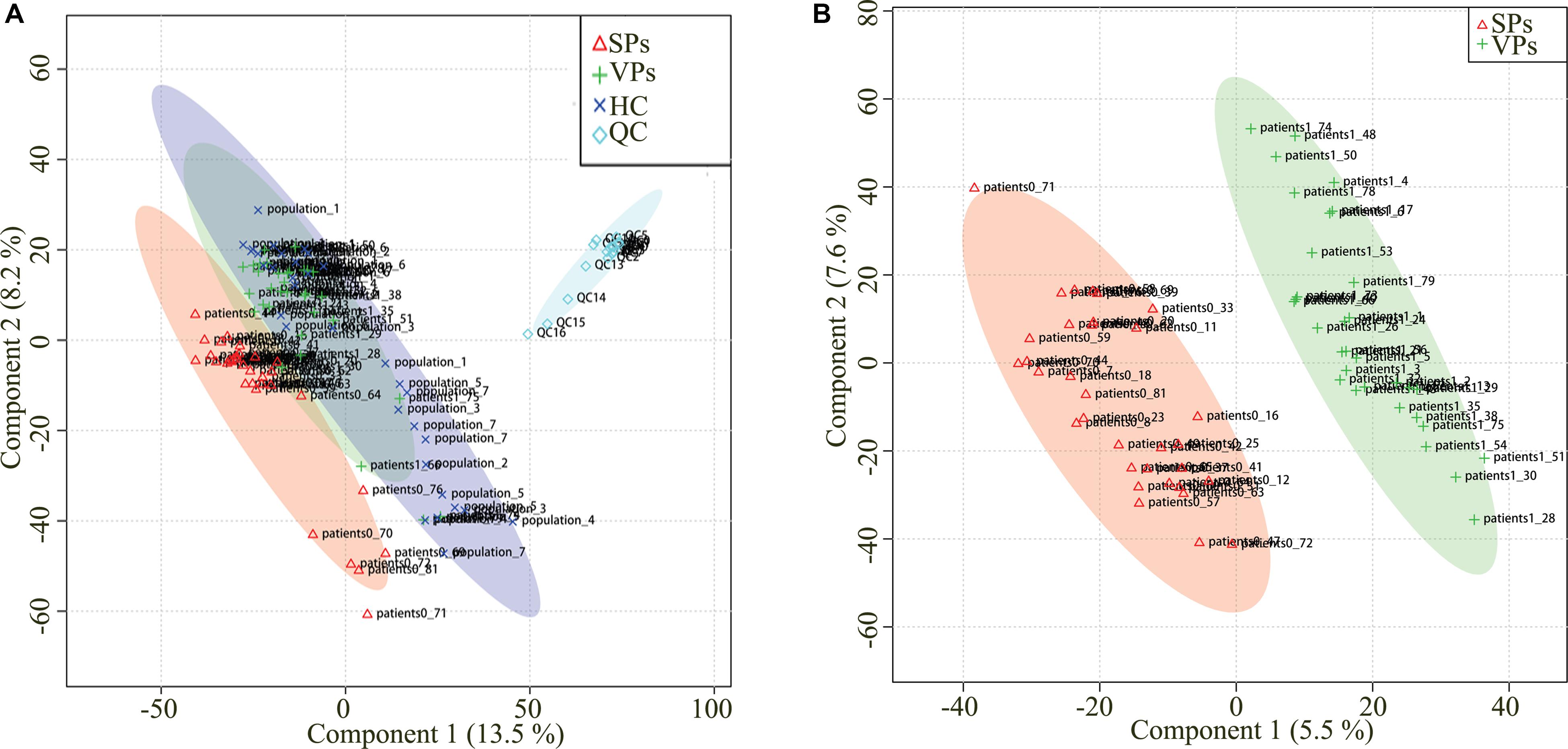
Figure 3. Partial least squares discriminant analysis (PLS-DA) of serum metabolomics data. Serum metabolomics data for quality control (A). Serum metabolites distinguished SPs group and VPs group (B). ACS, acute coronary syndrome; HC, healthy controls; VPs, vulnerable plaque groups; SPs, stable plaque groups; QC, quality control.
To explore the clinical metabolites, we screened the differential metabolites in VPs and SPs by receiver operating characteristic curve (ROC) >0.6. The four metabolites are presented in Table 2. Four metabolites including betaine, acetylcarnitine, 1-heptadecanoyl-sn-glycero-3-phosphocholine, and isoundecylic acid were significantly associated with the clinical plaque stability of ACS patients (Figures 4A–D). The curve values (AUC) of the four metabolites in VPs vs. SPs groups were 0.884, 0.689, 0.655, and 0.782, the sensitivity were 78.8, 66.7, 69.7, and 78.8%, and the specificity were 87.5, 71.9, 65.6, and 78.1% (Figures 4E–H). These results indicated that the four metabolites played important roles in the stability of plaques in ACS patients.
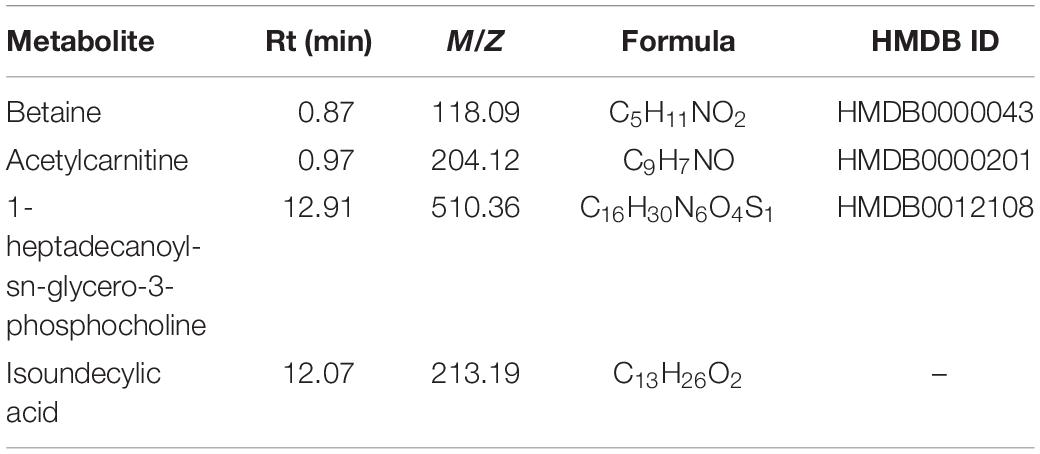
Table 2. List of Metabolite identified in combining untargeted and targeted metabolomics approaches.
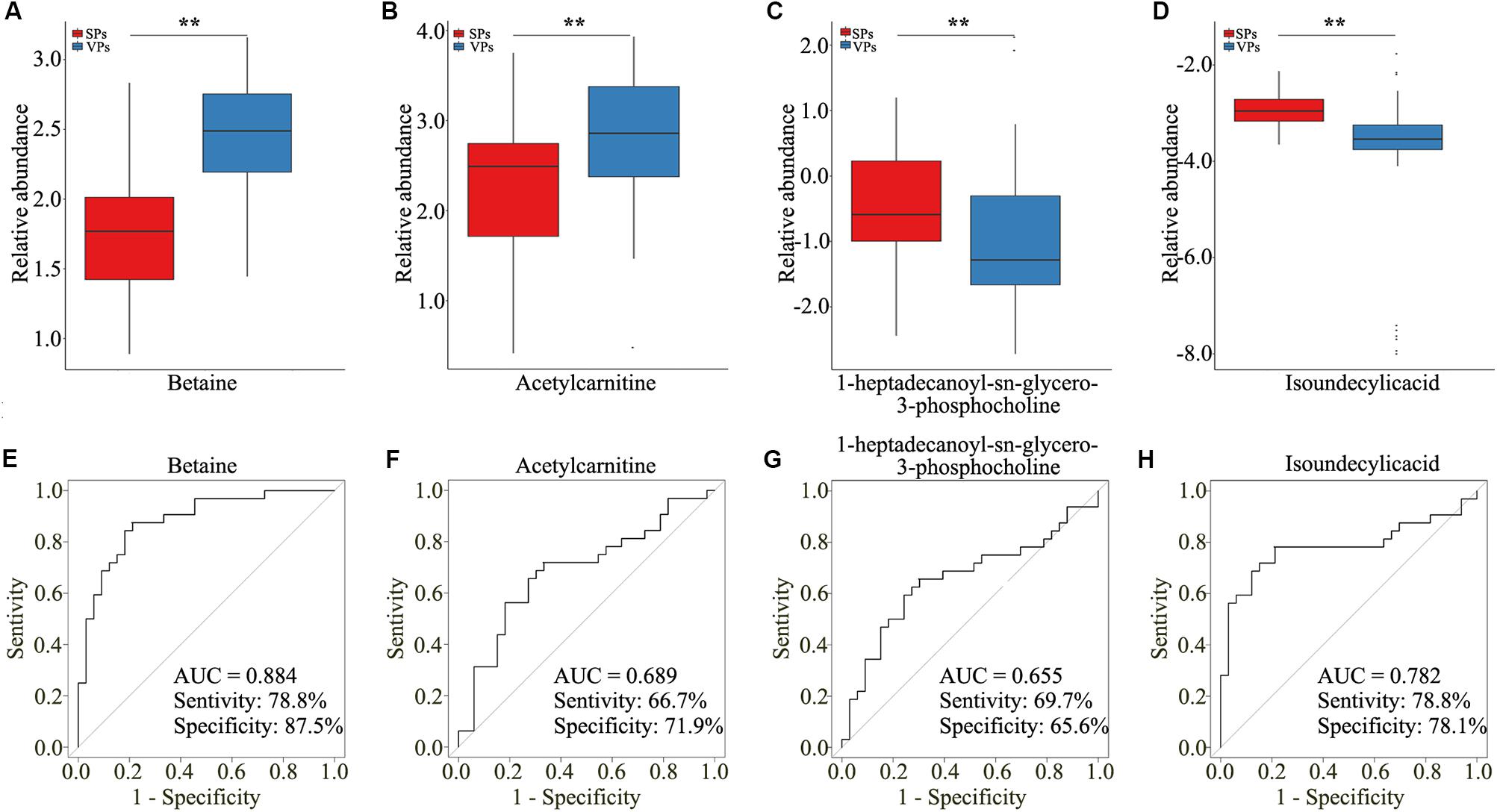
Figure 4. The four selected metabolites detected by untargeted LC/MS analysis. (A–D) Boxplots showed the relative abundance of the four metabolites in serum including betaine (A), acetylcarnitine (B), 1-heptadecanoyl-sn-glycero-3-phosphocholine (C), and isoundecylic acid (D). (E–H) Analysis of PLS-DA-based ROC curves of betaine (E), acetylcarnitine (F), 1-heptadecanoyl-sn-glycero-3-phosphocholine (G), and isoundecylic acid (H) in VPs and SPs groups. VP, vulnerable plaque groups; SP, stable plaque groups.
Metabolomics Analysis of Serum Samples by Targeted LC/MS
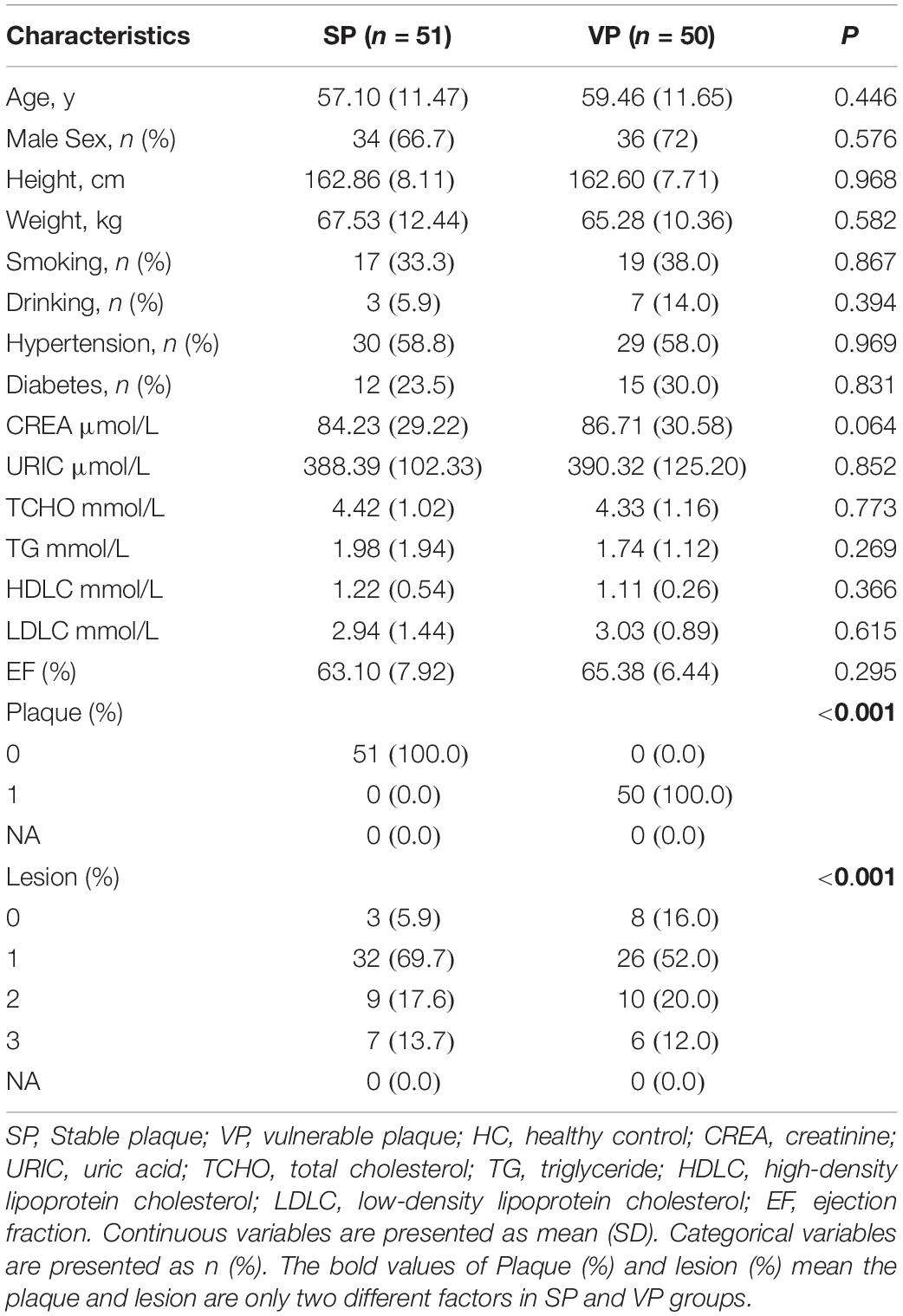
To further detect the value of candidate metabolites, we detected them by targeted LC/MS analysis. As shown in Table 3, the clinical characteristics of VPs and SPs groups were matched. As isoundecylic acid was not found in the human metabolome database (HMDB), we systematically analyzed three metabolites’ (betaine, acetylcarnitine, 1-heptadecanoyl-sn-glycero-3-phosphocholine) relative concentration and found that betaine was significantly upregulated in VPs, which was consistent with the expression of betaine in untargeted LC/MS analysis (Figures 5A–C) and indicated that betaine was significantly related to the plaque stability of ACS patients. The AUC values of the three metabolites in VPs vs. SPs groups were 0.793, 0.568, 0.518, the sensitivity in two groups were 72.5%, 72.5%, 45.1% and the specificity were 74.0, 48.0, 65.6, and 74.0% (Figures 5D–F). These results indicated that the three metabolites, especially betaine, may predict the plaque stability.
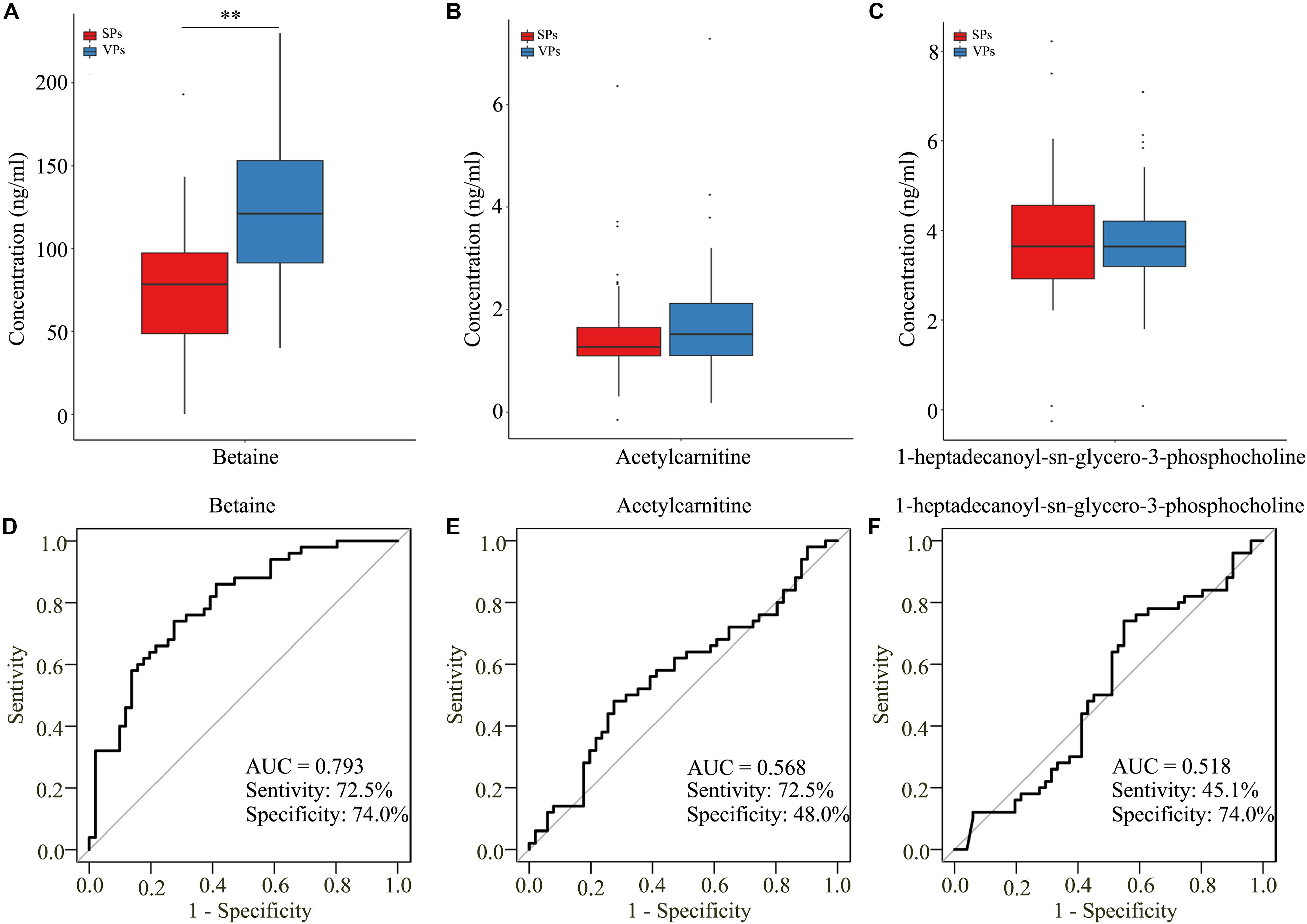
Figure 5. The three metabolites detected by target LC/MS analysis. (A–C) Boxplots showed the concentration of betaine (A), acetylcarnitine (B), and 1-heptadecanoyl-sn-glycero-3-phosphocholine (C). (D–F) Analysis of PLS-DA-based ROC curves of betaine (D), acetylcarnitine (E), and 1-heptadecanoyl-sn-glycero-3-phosphocholine (F) in VPs and SPs groups. VPs, vulnerable plaque groups; SPs, stable plaque groups.
Development of a Metabolite-Based Model
To construct a diagnostic model that could be used to identify the stability of plaque with ACS patients, the sensitivity and specificity values of the betaine, acetylcarnitine, and 1-heptadecanoyl-sn-glycero-3-phosphocholine in the diagnosis of ACS and risk stratification of plaque stability were explored by logistic regression statistical analysis. However, the metabolite-based model in VPs vs. SPs groups was developed and the AUC values of VPs vs. SPs groups were 0.793, with the corresponding sensitivity of 78.4% and specificity of 70.0% (Figure 6A).
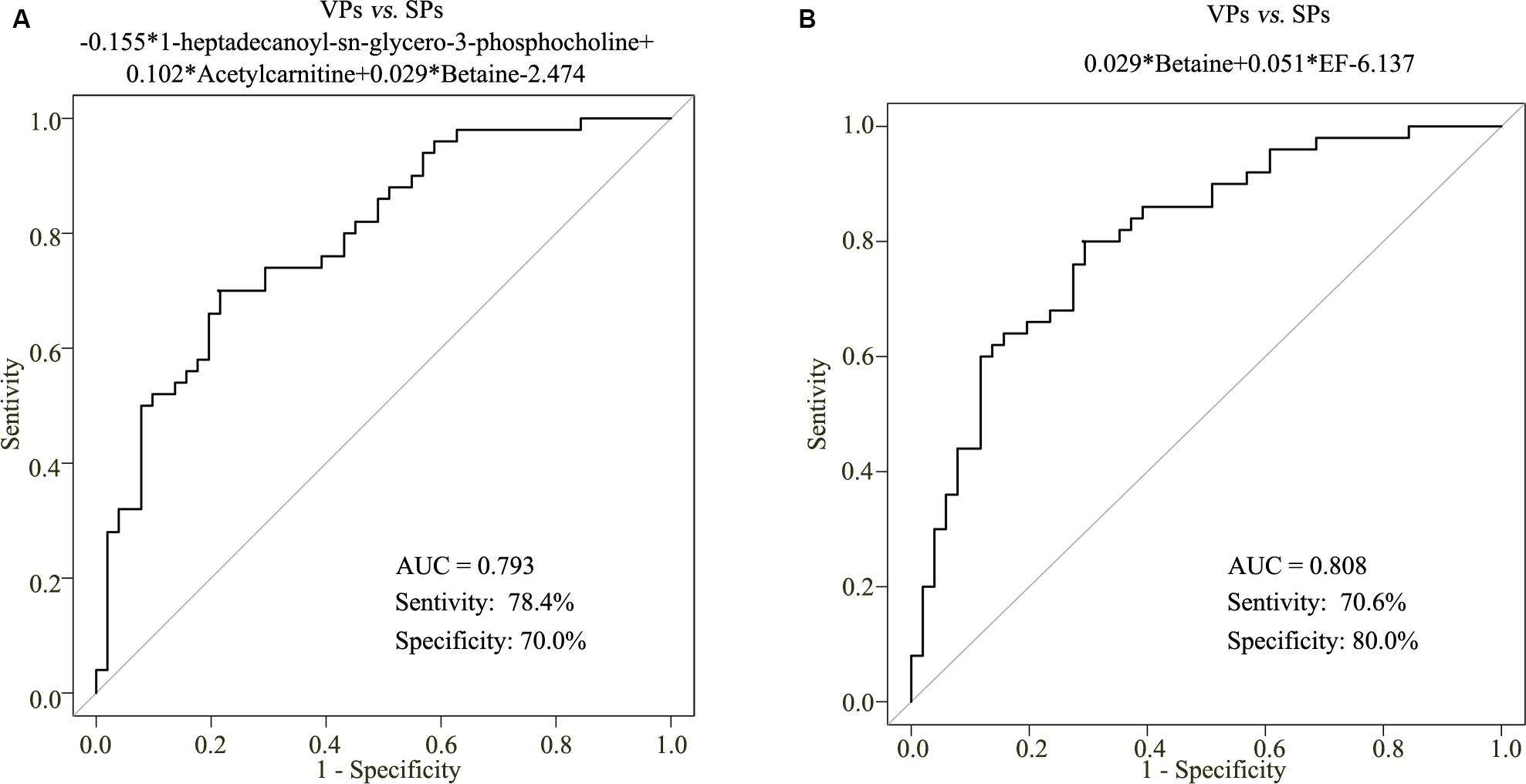
Figure 6. The metabolite-based model on plaque stability. ROC curve analysis of the three metabolite-based diagnostic models in VPs vs. SPs groups (A). ROC curve analysis of the metabolite-based diagnostic model in distinguishing VPs and SPs groups from controls in the ACS patients (B). VPs, vulnerable plaque groups; SPs, stable plaque groups.
To improve the diagnostic performance of plaque stability, step-wise backward selection analysis was further performed to determine the best model in subgroups shown in Figure 6B. The model was presented as follows: logit (P = VPs vs. SPs) = 0.029*betaine + 0.051*EF-6.137 in VPs vs. SPs group. The AUC value was 0.808, with the corresponding sensitivity of 70.6% and specificity of 80.0%. These results demonstrated that the combined model was reliable and could be applied to differentiate the stability of plaque.
Discussion
In this study, we systematically and comprehensively analyzed the serum metabolomics change on atherosclerotic plaque stability. The metabolite profiles of serum samples allowed classification of SPs and VPs patients. Four metabolites were significantly altered in ACS patients. Compared to SPs, betaine, acetylcarnitine, 1-heptadecanoyl-sn-glycero-3-phosphocholine, and isoundecylic acid were perturbed in VPs patients. Furthermore, the combined model of serum betaine and EF could be used as the potential diagnostic biomarker for distinguishing VPs patients from SPs.
Our study was firstly to identify the combination of betaine and clinical indicators that could be used for the detection of plaque stability. Betaine, a natural compound that exists in many organisms, can not only regulate cells against water osmotic or retention (Craig, 2004) but also increase energy metabolism by acting as a methyl donor (Alirezaei et al., 2012). Betaine also plays a potential antioxidant role in animals by increasing plasma glutathione peroxidase (Alirezaei et al., 2015). Oxidative stress is an important pathological process of atherosclerosis. Jiang et al. found that betaine played an important role on diabetic-induced oxidative stress-mediated p38 MAPK pathways (Jiang et al., 2019). Saeed et al. (2017) also identified that betaine could act as a natural anti-heat stress agent. Our results found that betaine was significantly upregulated in VPs patients and indicated that betaine played an important role on plaque stability, which was consistent with the protective role of betaine on organisms.
Acetylcarnitine, an index of energy substrate oxidation, could increase transportation of acetyl-CoA into the mitochondria during fatty acid oxidation. Pouralijan Amiri et al. found that acetylcarnitine was up-regulated in UA (Pouralijan Amiri et al., 2019). 1-heptadecanoyl-sn-glycero-3-phosphocholine, a lysophospholipid (LyP), originating from hydrolysis of phosphatidylcholine, can play an important role in the de-acylation/re-acylation cycle and then control molecular species composition (Lingwood and Simons, 2010). Watson identified that lipid metabolism played a physiological importance on atherosclerosis, diabetes, obesity, and Alzheimer’s disease (Watson, 2006). In our study, the level of acetylcarnitine was increased and 1-heptadecanoyl-sn-glycero-3-phosphocholine was decreased in VPs vs. SPs patients, which indicated that the two metabolites were involved in ACS. The more specific role of acetylcarnitine and 1-heptadecanoyl-sn-glycero-3-phosphocholine on the vulnerability of plaque stability needs to be further explored. And future studies should explore this relationship more fully, since it may lead to a non-invasive marker that differentiates VPs from SPs.
In this study, there are also some limitations that should be acknowledged. As isoundecylic acid was not found in the human metabolome database (HMDB), we only detect the three metabolites including in targeted LC/MS, and four metabolites including betaine, 1-heptadecanoyl-sn-glycero-3-phosphocholine, acetylcarnitine, and isoundecylic acid in untargeted LC/MS. In addition, the metabolite-based model was only validated by one center, but not validated from populations of external validation. However, the positive results from the model suggested a possible generalization of the combined model of serum betaine and EF in differentiating VPs from SPs.
In summary, the present study uncovered four metabolites which can separate patients with ACS from healthy controls. The combined model of serum betaine and EF model was proposed for the better diagnosis of the vulnerability of plaque stability in a non-invasive way.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Tungwah Hospital of Sun Yat-sen University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
JZ, BC, and HiJ conceived and designed the experiments. HiJ, HoJ, and SW performed the experiments. WC, CL, HL, and WH analyzed the data. JZ, BC, and HiJ wrote the manuscript. JZ, BC, and WH revised the manuscript for scientific content. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from the Social Science and Technology Development Key Project of Dongguan (201750715046462), Guangzhou Key Medical Discipline Construction Project Fund (B195002004042), and Open Funds of State Key Laboratory of Oncology in South China (KY013711).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank the native English-speaking scientists of Elixigen Company (Huntington Beach, CA, United States) for editing our manuscript and the data analysis assistance of Hanqi Yin (Longsee Biomedical Corporation, China). The authors also show our deepest gratitude to all the study volunteers.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2020.00776/full#supplementary-material
TABLE S1 | The 3069 metabolites detected by untargeted LC–MS.
Footnotes
References
Alirezaei, M., Khoshdel, Z., Dezfoulian, O., Rashidipour, M., and Taghadosi, V. (2015). Beneficial antioxidant properties of betaine against oxidative stress mediated by levodopa/benserazide in the brain of rats. J. Physiol. Sci. 65, 243–252. doi: 10.1007/s12576-015-0360-0
Alirezaei, M., Reza Gheisari, H., Reza Ranjbar, V., and Hajibemani, A. (2012). Betaine: a promising antioxidant agent for enhancement of broiler meat quality. Br. Poult. Sci. 53, 699–707. doi: 10.1080/00071668.2012.728283
Antoniades, C., Bakogiannis, C., Tousoulis, D., Antonopoulos, A. S., and Stefanadis, C. (2009). The CD40/CD40 ligand system: linking inflammation with atherothrombosis. J. Am. Coll. Cardiol. 54, 669–677. doi: 10.1016/j.jacc.2009.03.076
Au, A., Cheng, K. K., and Wei, L. K. (2017). Metabolomics, Lipidomics and Pharmacometabolomics of Human Hypertension. Adv. Exp. Med. Biol. 956, 599–613. doi: 10.1007/5584_2016_79
Calderon-Santiago, M., Lopez-Bascon, M. A., Peralbo-Molina, A., and Priego-Capote, F. (2017). MetaboQC: A tool for correcting untargeted metabolomics data with mass spectrometry detection using quality controls. Talanta 174, 29–37. doi: 10.1016/j.talanta.2017.05.076
Chan, A. W., Gill, R. S., Schiller, D., and Sawyer, M. B. (2014). Potential role of metabolomics in diagnosis and surveillance of gastric cancer. World J. Gastroenterol. 20, 12874–12882. doi: 10.3748/wjg.v20.i36.12874
Chen, C., Fu, Y. H., Li, M. H., Ruan, L. Y., Xu, H., Chen, J. F., et al. (2019). Nuclear magnetic resonance-based metabolomics approach to evaluate preventive and therapeutic effects of Gastrodia elata Blume on chronic atrophic gastritis. J. Pharm. Biomed. Anal. 164, 231–240. doi: 10.1016/j.jpba.2018.10.035
Chen, D. Q., Cao, G., Chen, H., Liu, D., Su, W., Yu, X. Y., et al. (2017). Gene and protein expressions and metabolomics exhibit activated redox signaling and wnt/beta-catenin pathway are associated with metabolite dysfunction in patients with chronic kidney disease. Redox Biol. 12, 505–521. doi: 10.1016/j.redox.2017.03.017
Chen, H., Cao, G., Chen, D. Q., Wang, M., Vaziri, N. D., Zhang, Z. H., et al. (2016). Metabolomics insights into activated redox signaling and lipid metabolism dysfunction in chronic kidney disease progression. Redox Biol. 10, 168–178. doi: 10.1016/j.redox.2016.09.014
Craig, S. A. (2004). Betaine in human nutrition. Am. J. Clin. Nutr. 80, 539–549. doi: 10.1093/ajcn/80.3.539
Fang, J., Wang, L., Wang, Y., Qiu, M., and Zhang, Y. (2017). Metabolomics combined with pattern recognition and bioinformatics analysis methods for the development of pharmacodynamic biomarkers on liver fibrosis. Mol. Biosyst. 13, 1575–1583. doi: 10.1039/c7mb00093f
Goodacre, S., Pett, P., Arnold, J., Chawla, A., Hollingsworth, J., Roe, D., et al. (2009). Clinical diagnosis of acute coronary syndrome in patients with chest pain and a normal or non-diagnostic electrocardiogram. Emerg. Med. J. 26, 866–870. doi: 10.1136/emj.2008.064428
Huang, Q., Tan, Y., Yin, P., Ye, G., Gao, P., Lu, X., et al. (2013). Metabolic characterization of hepatocellular carcinoma using nontargeted tissue metabolomics. Cancer Res. 73, 4992–5002. doi: 10.1158/0008-5472.CAN-13-0308
Jiang, Y. P., Yang, J. M., Ye, R. J., Liu, N., Zhang, W. J., Ma, L., et al. (2019). Protective effects of betaine on diabetic induced disruption of the male mice blood-testis barrier by regulating oxidative stress-mediated p38 MAPK pathways. Biomed. Pharmacother. 120:109474. doi: 10.1016/j.biopha.2019.109474
Klein, M. S., and Shearer, J. (2016). Metabolomics and type 2 diabetes: translating basic research into clinical application. J. Diabetes Res. 2016:3898502. doi: 10.1155/2016/3898502
Lingwood, D., and Simons, K. (2010). Lipid rafts as a membrane-organizing principle. Science 327, 46–50. doi: 10.1126/science.1174621
O’Keefe, S. J., Li, J. V., Lahti, L., Ou, J., Carbonero, F., Mohammed, K., et al. (2015). Fat, fibre and cancer risk in African Americans and rural Africans. Nat. Commun. 6:6342. doi: 10.1038/ncomms7342
Pouralijan Amiri, M., Khoshkam, M., Salek, R. M., Madadi, R., Faghanzadeh Ganji, G., and Ramazani, A. (2019). Metabolomics in early detection and prognosis of acute coronary syndrome. Clin. Chim. Acta 495, 43–53. doi: 10.1016/j.cca.2019.03.1632
Reihani, H., Sepehri Shamloo, A., and Keshmiri, A. (2018). Diagnostic value of D-Dimer in acute myocardial infarction among patients with suspected acute coronary syndrome. Cardiol. Res. 9, 17–21. doi: 10.14740/cr620w
Ridker, P. M. (2007). C-reactive protein and the prediction of cardiovascular events among those at intermediate risk: moving an inflammatory hypothesis toward consensus. J. Am. Coll. Cardiol. 49, 2129–2138. doi: 10.1016/j.jacc.2007.02.052
Saeed, M., Babazadeh, D., Naveed, M., Arain, M. A., Hassan, F. U., and Chao, S. (2017). Reconsidering betaine as a natural anti-heat stress agent in poultry industry: a review. Trop. Anim. Health Prod. 49, 1329–1338. doi: 10.1007/s11250-017-1355-z
Shivanna, S., Kolandaivelu, K., Shashar, M., Belghasim, M., Al-Rabadi, L., Balcells, M., et al. (2016). The Aryl hydrocarbon receptor is a critical regulator of tissue factor stability and an antithrombotic target in uremia. J. Am. Soc. Nephrol. 27, 189–201. doi: 10.1681/ASN.2014121241
Sun, J. H., Liu, X., Cong, L. X., Li, H., Zhang, C. Y., Chen, J. G., et al. (2017). Metabolomics study of the therapeutic mechanism of Schisandra Chinensis lignans in diet-induced hyperlipidemia mice. Lipids Health Dis. 16:145. doi: 10.1186/s12944-017-0533-3
Terashima, M., Nishimura, Y., Nakata, H., Iwai, Y., and Yokoyama, M. (2000). Spontaneous coughing up of a polyp. Respiration 67, 101–103. doi: 10.1159/000029473
Wang, Y., Li, L., Tan, H. W., Yu, G. S., Ma, Z. Y., Zhao, Y. X., et al. (2007). Transcoronary concentration gradient of sCD40L and hsCRP in patients with coronary heart disease. Clin. Cardiol. 30, 86–91. doi: 10.1002/clc.26
Watson, A. D. (2006). Thematic review series: systems biology approaches to metabolic and cardiovascular disorders. Lipidomics: a global approach to lipid analysis in biological systems. J. Lipid Res. 47, 2101–2111. doi: 10.1194/jlr.r600022-jlr200
Wishart, D. S. (2016). Emerging applications of metabolomics in drug discovery and precision medicine. Nat. Rev. Drug Discov. 15, 473–484. doi: 10.1038/nrd.2016.32
Zhang, Q., Yin, X., Wang, H., Wu, X., Li, X., Li, Y., et al. (2019). Fecal metabolomics and potential biomarkers for systemic lupus erythematosus. Front. Immunol. 10:976. doi: 10.3389/fimmu.2019.00976
Zhao, Y. Y., Cheng, X. L., Wei, F., Bai, X., Tan, X. J., Lin, R. C., et al. (2013). Intrarenal metabolomic investigation of chronic kidney disease and its TGF-beta1 mechanism in induced-adenine rats using UPLC Q-TOF/HSMS/MS(E). J. Proteome Res. 12, 692–703. doi: 10.1021/pr3007792
Keywords: serum, metabolomics, biomarkers, acute coronary syndrome, liquid chromatography, mass spectrometry
Citation: Jiang H, Jiang H, Zhang J, Chen W, Luo C, Li H, Hau W, Chen B and Wang S (2020) The Serum Metabolic Biomarkers in Early Diagnosis and Risk Stratification of Acute Coronary Syndrome. Front. Physiol. 11:776. doi: 10.3389/fphys.2020.00776
Received: 18 December 2019; Accepted: 12 June 2020;
Published: 21 July 2020.
Edited by:
Ioannis P. Androulakis, Rutgers, The State University of New Jersey, United StatesReviewed by:
Shivendra Gajraj Tewari, Biotechnology HPC Software Applications Institute (BHSAI), United StatesGeorges Nemer, American University of Beirut, Lebanon
Copyright © 2020 Jiang, Jiang, Zhang, Chen, Luo, Li, Hau, Chen and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Benfa Chen, Y2hlbmJmMDAxQDEyNi5jb20=; Shanhua Wang, MjY1OTI1OTc3MkBxcS5jb20=
†These authors have contributed equally to this work
 Huali Jiang
Huali Jiang Hualong Jiang2†
Hualong Jiang2† Weijie Chen
Weijie Chen Heng Li
Heng Li William Hau
William Hau