- 1Institute for Theoretical Biology, Charité – Universitätsmedizin Berlin, Berlin, Germany
- 2Leiden University Medical Center, Leiden University, Leiden, Netherlands
The suprachiasmatic nucleus (SCN), which serves as the central pacemaker in mammals, regulates the 24-h rhythm in behavioral activity. However, it is currently unclear whether and how bouts of activity and rest are regulated within the 24-h cycle (i.e., over ultradian time scales). Therefore, we used passive infrared sensors to measure temporal behavior in mice housed under either a light–dark (LD) cycle or continuous darkness (DD). We found that a probabilistic Markov model captures the ultradian changes in the behavioral state over a 24-h cycle. In this model, the animal’s behavioral state in the next time interval is determined solely by the animal’s current behavioral state and by the “toss” of a proverbial “biased coin.” We found that the bias of this “coin” is regulated by light input and by the phase of the clock. Moreover, the bias of this “coin” for an animal is related to the average length of rest and activity bouts in that animal. In LD conditions, the average length of rest bouts was greater during the day compared to during the night, whereas the average length of activity bouts was greater during the night compared to during the day. Importantly, we also found that day-night changes in the rest bout lengths were significantly greater than day-night changes in the activity bout lengths. Finally, in DD conditions, the activity and rest bouts also differed between subjective night and subjective day, albeit to a lesser extent compared to LD conditions. The ultradian regulation represented by the model does not result in ultradian rhythms, although some weak ultradian rhythms are present in the data. The persistent differences in bout length over the circadian cycle following loss of the external LD cycle indicate that the central pacemaker plays a role in regulating rest and activity bouts on an ultradian time scale.
Introduction
In most organisms, the circadian clock facilitates adaptation to the natural periodic light cycle. This clock regulates a wide range of physiological processes, including behavior (Herzog, 2007). Therefore, behavior has been used to determine the state of the clock in vivo since the early days of the field of chronobiology (Pittendrigh, 1960; Pittendrigh and Daan, 1976). In mammals, the circadian clock is located in the suprachiasmatic nucleus (SCN) at the base of the hypothalamus (Ralph et al., 1990). The neurons in the SCN have near 24-h oscillations in both protein expression and neuronal firing (Nakamura et al., 2002; Quintero et al., 2003; Schaap et al., 2003; Yamaguchi et al., 2003; Hastings et al., 2018).
Recording the frequency of action potential firing in the SCN of freely moving animals has allowed researchers to measure the degree of correspondence between SCN firing and behavioral activity (Houben et al., 2009, 2014). These studies showed that the onset and offset of behavioral activity are regulated probabilistically by differences in firing between high levels of firing activity during the day and low levels during the night (Houben et al., 2009). Moreover, the 24-h rhythmic waveform of SCN firing is correlated with the distribution of behavioral activity within the active phase (Houben et al., 2009, 2014). Does the circadian clock then also regulate temporal behavior within the circadian cycle (i.e., over ultradian time scales)? If so, how is temporal behavior organized and by what law, if any, can the alteration of activity and rest at the ultradian time scale be described?
Suprachiasmatic nucleus lesions resulted in the loss of ultradian rhythms in rats (Wollnik and Turek, 1989), almost no disruption of ultradian rhythms in voles (Gerkema et al., 1990) and low power or unstable ultradian rhythms in mice (Schwartz and Zimmerman, 1991) and hamsters (Rusak, 1977). Furthermore, genetic manipulation of the molecular clocks in the entire mouse (including the SCN) still retained arrhythmic ultradian bouts of behavior (Vitaterna et al., 1994; Horst et al., 1999). Moreover, scale-invariant patterns of behavior are disrupted by SCN lesions at time scales from 4 h to 24 h, but not at time scales below 4 h (Hu et al., 2007). In summary, whether – and to what extent – the SCN regulates temporal behavior over ultradian time scales is largely unknown. In our study, we use “ultradian” to indicate a scale much smaller than 24 h, but do not imply rhythmicity.
As a first step in addressing this question, we examined whether bouts of activity and rest (i.e., prolonged stretches of activity and rest, respectively) are regulated at ultradian time scales in mice. We present a simple probabilistic model of the transitions between behavioral states fit to behavioral data collected under a light–dark (LD) cycle or continuous darkness (DD). Our model shows how bouts of rest and activity are regulated on a scale of seconds to minutes. This time scale is far below the scale that has been explored and detected by detrended fluctuation analysis (Hu et al., 2007). In addition, the model shows that changes in the duration of rest bouts, rather than changes in the duration of activity bouts, determine the differences in activity between day and night.
Materials and Methods
Ethics Statement
All animal experiments were performed in accordance with Dutch law and were approved by the Animal Ethics Committee at the Leiden University Medical Center (Leiden, Netherlands).
Animals
Wild-type C57BL6 mice were purchased from Harlan (Harlan, Horst, Netherlands). All mice were between 12 and 24 weeks of age.
Behavioral Data
Each animal’s behavioral activity was recorded using passive IR (PIR) motion detection sensors (Hygrosens Instruments) mounted under the lid of the cage and connected to a ClockLab data collection system (Actimetrics Software), which recorded sensor activity in 10-sec bins.
Mice were housed under either continuous darkness (DD) or an LD cycle with a 22-, 24-, or 26-h period with equal duration of light and dark (also termed T-cycles); for example, a 22-h T-cycle consisted of 11 h of light and 11 h of darkness. Only recordings of mice with at least four circadian cycles in DD or four cycles in an LD cycle were included in our analysis; the lengths of the activity recordings are presented in Supplementary Figure 1. Furthermore, all mice housed under an LD cycle were entrained to the external Zeitgeber. In this study, a total of 16 mice were housed under DD conditions, and 32 mice were housed under a 22-h (N = 8 mice), 24-h (N = 16 mice), or a 26-h (N = 8 mice) T-cycle.
The data consist of the start time of the 10 s bin, with lights “on” or “off” marked as “L” and “D,” respectively (Figure 1); in addition, behavioral activity was counted in 10-s intervals. For this study, activity counts were converted to either “A” (active; activity counts > 0) or “R” (rest; activity counts = 0); thus, we studied the duration of activity and rest, not the intensity of activity.
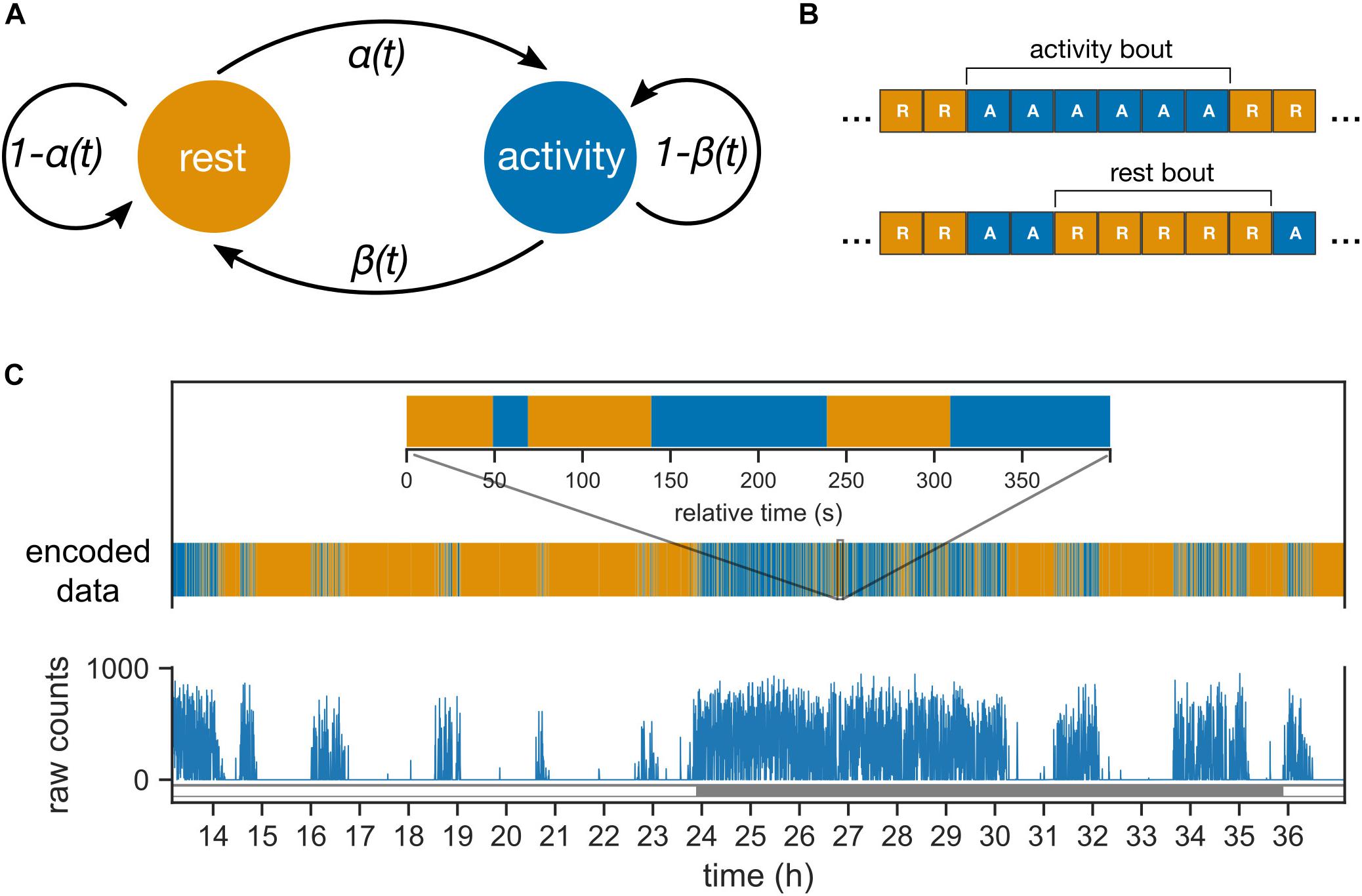
Figure 1. Probabilistic model of mouse behavior. (A) The transitions between the two behavioral states “rest” and “activity” are controlled by the two probability parameters, α and β. The parameters can vary between day and night (or subjective day and night in constant darkness). (B) The behavioral activity data is encoded into sequences of “activity” (behavioral activity > 0) and “rest” (behavioral activity = 0) bins. The encoded sequences are inputs to the model in panel (A). One or more contiguous occurrences of “activity” and “rest” bins are termed activity and rest bouts, respectively. (C) The behavioral activity of an animal is recorded as raw counts in 10 s bins (shown here for a mouse under 24 h light–dark cycles). The corresponding encoded sequence is shown with the same color-coding as in panel (B) for rest and activity. Inset zooms into a 400 s interval during the night (active phase).
Description of the Probabilistic Model
The probabilistic model describes the transitions in the animal’s behavioral state between “rest” and “activity” (Figure 1A). The animal’s behavioral state in the next 10-sec bin Sn+1 is determined solely by the behavioral state in the current 10-sec bin (Sn) and the probability of transition; such a property defines a Markov model. The transition probability from rest to activity is defined as α, and the transition probability from activity to rest is defined as β. Probabilities were allowed to change between day and night under LD conditions and between subjective day and subjective night under DD conditions. Under an LD cycle, both the central clock and the external LD cycle contribute to the behavioral state; in contrast, under DD, the effect of the external LD cycle is absent.
For mice in LD, α and β were fit separately for day and night using the following equations:
and
where Ln represents the lighting condition during state Sn and #(.) is the number of occurrences of the condition within the parenthesis. Thus, the data obtained from each animal in LD yields four probabilities, two for the day phase and two for night phase.
For mice housed in DD, cosine curves were fit to the raw behavioral activity counts in order to identify the subjective day and subjective night phases. α and β were computed for the subjective day and subjective night using the equations shown above, producing a similar set of four probabilities.
We define bouts of activity and rest to be one or more adjacent bins of activity and rest, respectively (Figure 1B). Bouts of activity and rest (Figure 1C) are easier to interpret and identify in the data compared to the probability parameters. The Markov model leads to a geometric distribution of the bout durations, and the mean bout durations are conveniently dependent only upon α and β. The mean activity bouts and rest bouts (expressed in min) are 1/6β and 1/6α. In addition, the average activity during a phase of the clock is defined using the following formula: α/α + β.
Statistical Analysis
The average activity of the mice was analyzed using the analysis of variance (aov) function in R (version 3.5.1). The duration of bouts estimated using the model were first transformed in order to ensure uniform variance across groups; because each animal contributed multiple estimates, they were then analyzed using linear mixed models with the lmer function in the R package (“lme4” version 1.1-21).
Results
Model Fits Are Robust and Consistent With the Assumptions
First, we determined the ability of our model to produce reasonable parameter estimates that are consistent with the model’s assumptions.
The estimates of transition probability obtained from the behavioral data were stable over the course of acquisition. The estimates of α and β obtained from the first half of each acquisition were highly correlated with the estimates obtained from the second half (Supplementary Figure 2A, α: r(96) = 0.85, p < 0.001; β: r(96) = 0.69, p < 0.001). Thus, the data can be considered stationary for the purposes of this model.
The data also support the Markov assumption made in the probabilistic model, which can be paraphrased as the “the future is independent of the past, given the present.” Estimates derived from the data regarding dependence (via mutual information) between the next state (Sn+1) and the previous state (Sn–1), given the current state (Sn), were all close to zero (Supplementary Figure 2B), indicating near independence. In summary, our model provides a consistent representation of the data and produces robust estimates of the average duration of activity and rest bouts.
Activity and Rest in Mice Are Not Restricted to Night and Day, Respectively
Next, we examined the distribution of activity during the day and night under 22-, 24-, and 26-h T-cycles (i.e., LD cycles consisting of 11 h light/11 h dark, 12 h light/12 h dark, and 13 h light/13 h dark, respectively).
We defined average activity as the average fraction of time an animal was active in an interval; the interval is the length of the day for the average activity during the day. The average activity across day and night (the interval here is the T-cycle period) was similar between the 22-h and the 24-h T-cycles, but was significantly higher in the 26-h T-cycle compared to the 24-h T-cycle (Supplementary Figure 3, F(2, 29) = 4.95, p = 0.01, Tukey post hoc test). The mice were more active at night than during the day, consistent with their nocturnal nature (Figure 2A). The mice spent about ∼30% of the night and about ∼10% of the day being active. Thus, the mice were active for a minority of the time not only in their rest phase (day), but also in their active phase (night). Moreover, in the rest phase, the mice were not inactive, but rather active for 10% of the time.
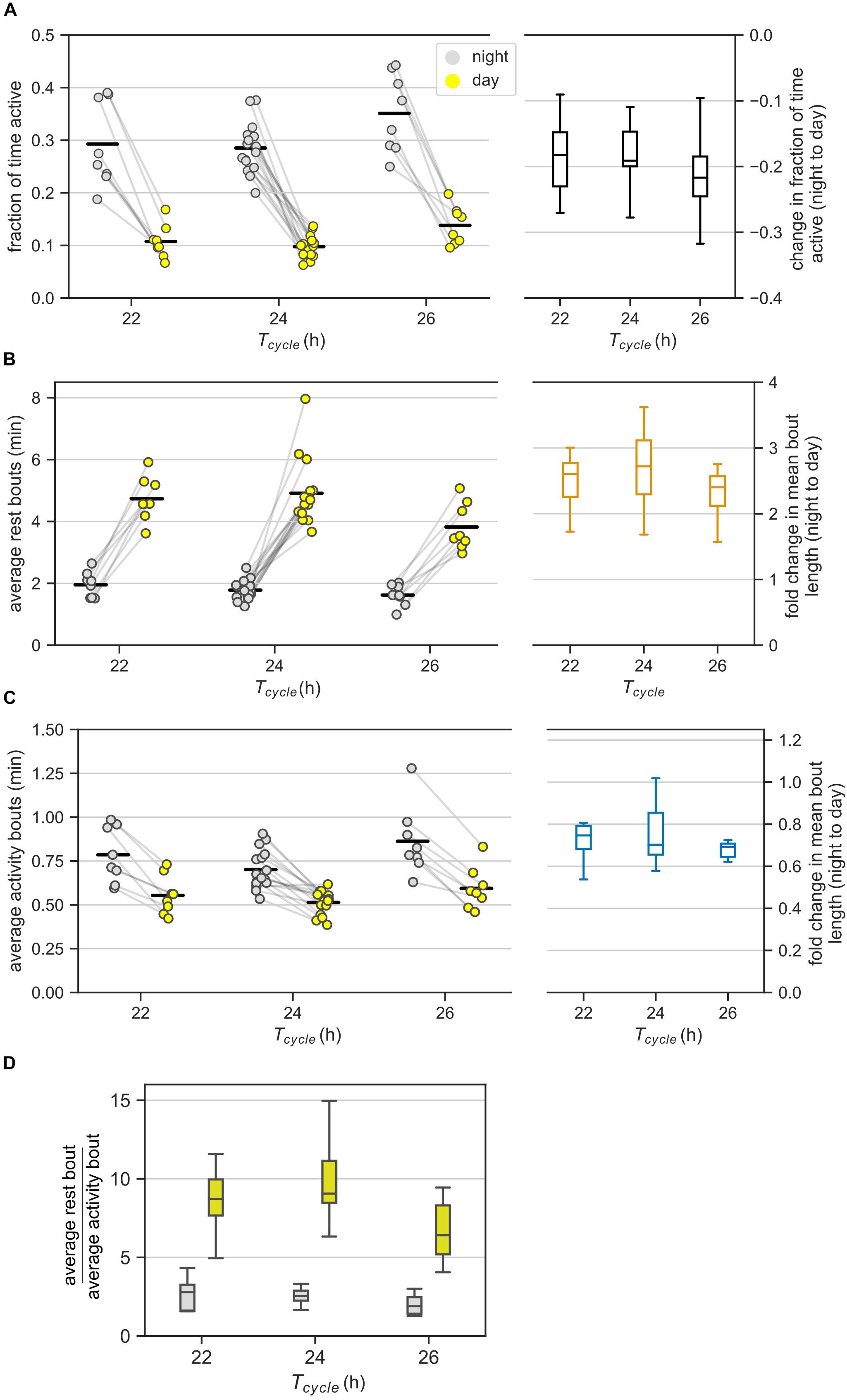
Figure 2. Ultradian behavior under LD cycles: (A) Average activity of mice in the day and night under different LD cycles. Average activity is the fraction of bins with activity during an interval of day or night. Each animal contributed two points (one each for the day and the night) that are connected. The changes in the average activity from night to day for different T-cycles are shown in the boxplot. (B,C) The average bout lengths for rest (B) and activity (C) estimated by the model in the day and the night under different T-cycles. The pair of points (one each for day and night) contributed by each animal is connected. The fold-change in mean bout length from night to day is provided as boxplots on the right. (D) The relative lengths of mean rest and mean activity bouts in the night and day for different T-cycles. The same data in panels (B) and (C) are visualized differently in panel (D). Color-coding is maintained throughout the figure. Horizontal black bars in the scatter plots represent mean of the values in that column.
The night to day change in average activity was similar among the three T-cycles. Specifically, the average activity during the day was one-third of the levels during the night for all three T-cycles (Figure 2A). The fold reduction of 0.38, 0.35, and 0.40 for the 22-, 24-, and 26-h T-cycles had 95% confidence intervals (CI) of [0.31, 0.45], [0.31, 0.40], and [0.33, 0.47], respectively. Despite the higher average activity under the 26-h T-cycle, the night to day change in the 26-h T-cycle was indistinguishable from the night to day change in the other two T-cycles.
Activity and Rest Bouts Are Inversely Regulated During the Day and Night
Next, we examined whether the average duration of the activity and rest bouts were different between the day (i.e., the resting phase) and the night (i.e., the active phase) for the three different T-cycles.
We observed higher activity during the night than during the day (Figure 2A). This could result from three different scenarios: (i) rest bouts are longer during the day than during the night, but activity bouts are unchanged between day and night (ii) activity bouts are longer during the night than during the day, but rest bouts are unchanged between day and night (iii) rest bouts are shortened and activity bouts are lengthened from day to night. In this section, we identify the scenario that is most consistent with the data.
According to our analysis, on average, the rest bouts were longer during the day compared to during the night under all three T-cycles (Figure 2B). Average bouts of rest during the day were 2.75-fold longer during the night (CI: [2.44, 3.09]) with no significant differences across the three T-cycles; fold-change for the 22-h and the 26-h T-cycles relative to the 24-h T-cycle had CIs of [0.94, 1.27] and [0.78, 1.05].
On the other hand, the activity bouts on average were shorter during the day compared to during the night under all three T-cycles (Figure 2C). Average activity bouts were 0.74-fold shorter during the day compared to during the night with only 26-h T-cycle having a slightly larger decrease in the activity bout length; fold-change for 22-h and 26-h T-cycles relative to 24-h T-cycle had CIs of [0.96, 1.29] and [1.05, 1.41].
Thus, both rest and activity bouts are indeed regulated reciprocally between day and night.
Day-Night Changes in Rest Bouts Dominate Day-Night Changes in Activity Bouts
This section compares the relative durations of rest and activity bouts during the day and during the night.
We observed that the average rest bout was always longer than the average activity bout (Figures 2B,C, ratiorest/activity = 2.53, CI: [2.25, 2.86]). This agrees with mice having more rest than activity (average activity < 0.5) during both day and night (Figure 2A). The average rest bout was about twice as long as the average activity bout in the night (ratiorest/activity = 2.28, CI: [2.08, 2.49], Figure 2D). The ratio increased to about eight-fold in the day (ratiorest/activity = 8.06, CI: [7.37, 8.81]) and was significantly greater under the 24-h T-cycle (ratio = 1.17, CI: [1.05, 1.31]).
It appears therefore that the average rest bout is always longer than the average activity bout and the absolute day-night change in the rest bouts is also greater than the absolute day-night change in the activity bouts (Figures 2B–D). We therefore hypothesize that the day-night changes in rest bouts dominate the day-night changes in activity bouts. Quantifying the day-night change in the number of bouts can help test this hypothesis.
Given that “rest” is defined as the lack of activity, bouts of rest and bouts of activity always alternate (Figures 1B,C); therefore, the number of rest and activity bouts in any given time interval is equal (or differs by no more than one). As a result, we only report the total number of bouts in an interval. If rest bouts were to dominate the day-night change, then the number of bouts would be expected to be higher during the night than during the day (rest bouts are shorter during the night). If, on the other hand, the activity bouts dominate, then the number of bouts would be expected to be lower during the night than during the day. Since the total number of bouts during the night was higher than during the day (Supplementary Figure 4A), we conclude that rest bouts rather than activity bouts dominate the day-night change in activity.
Mice in DD Are Less Active Than in LD Due to Reduced Activity During the Subjective Night
In LD cycles, both light and the central pacemaker influence behavioral activity, while DD conditions allow us to study behavior without the influence of light. This section compares ultradian behavior in DD with LD in order to distinguish between the effect of the circadian system and light.
In DD, mice had lower average activity overall, particularly during the subjective night. Mice in DD were about 20% less active than mice in LD (Supplementary Figure 3, F(1, 46) = 11.68, p = 0.0013, ratioDD/LD = 0.79); we pooled data from all three T-cycles in Figure 2 into the LD group. In DD, mice were active for about 20% of the time during the subjective night and about 12% of the time during the subjective day. Thus, a difference in activity between night and day also existed in DD (Figure 3A, ratiolight/dark = 0.61, CI: [0.54, 0.69])), but was considerably smaller than in LD (ratioDD/LD = 0.59, CI: [0.51, 0.68]). Interestingly, this difference resulted only from reduced activity during the subjective night in DD (ratioDD/LD during the subjective day = 1.13, CI: [0.97, 1.31]; ratioDD/LD during the subjective night = 0.66, CI: [0.57, 0.77]).
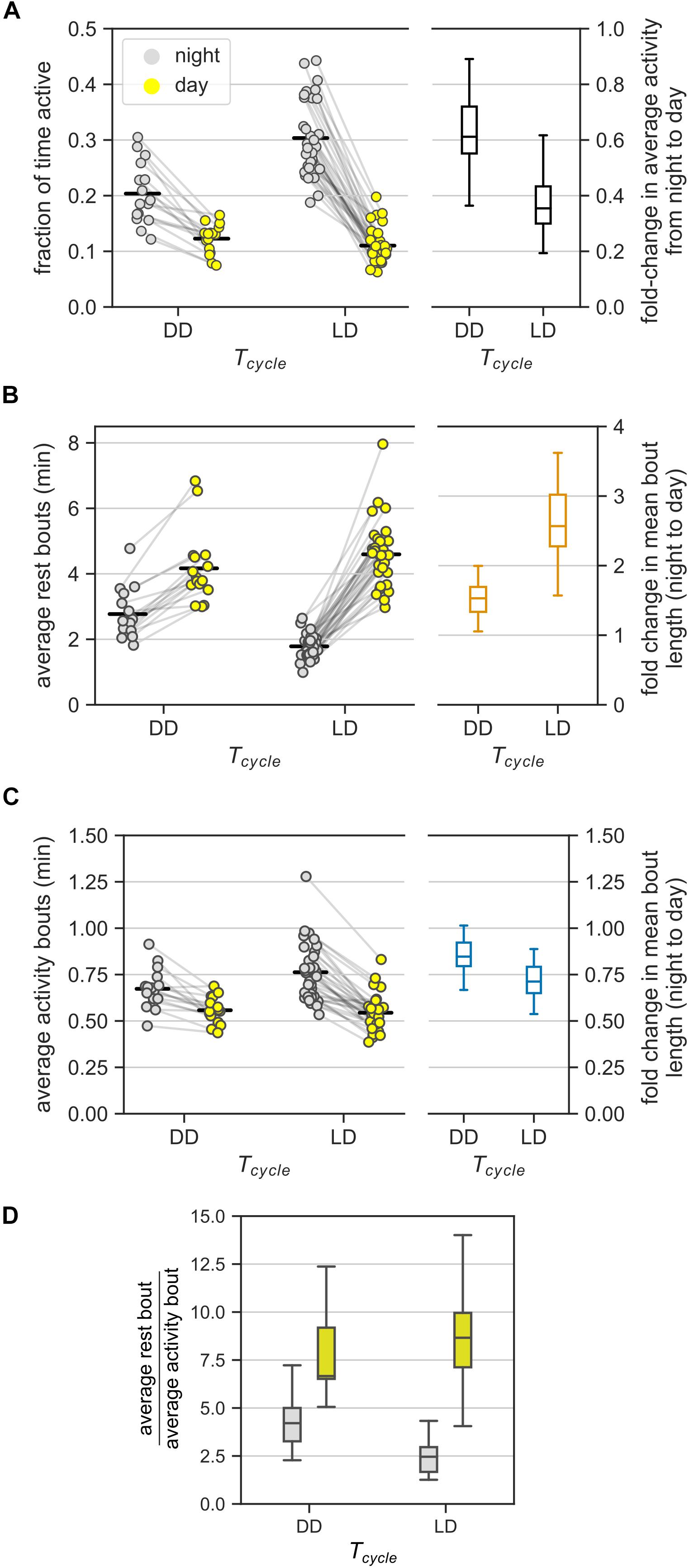
Figure 3. Ultradian behavior under constant darkness (DD). (A) Average activity of mice (measured as the fraction of active bins) during the day and during the night under DD and LD cycles. In DD, day and night refer to subjective day and subjective night, respectively. The LD group consists of data from the three T-cycles. Each animal contributed two points, one each to the day and the night groups – pairing is denoted by gray lines. Fold-change in the average activity between night and day is presented as a boxplot. (B,C) The model-based estimates of the mean rest (B) and mean activity (C) bout lengths in the (subjective) night and (subjective) day in DD and LD. Data points from the same mice are connected by gray lines. The bout length averaged over all individuals is marked with black bars. Boxplots show the fold-change in bout lengths from night to day. (D) The relative lengths of the mean rest and mean activity bouts in the (subjective) day and (subjective) night in DD and LD [a different representation of data in panels (B) and (C)].
Day-Night Changes in Bout Lengths Persisted in DD, but Were Moderated
This section continues the comparison between DD and LD cycles with a focus on the model-based estimates of mean bout lengths.
Mean rest bout and mean activity bout lengths changed inversely between subjective day and subjective night also in DD (Figures 3B,C). The mean length of the rest bouts increased 1.5-fold from the subjective night to the subjective day (Figure 3B and Table 1), whereas the mean length of the activity bouts decreased by 20% from the subjective night to the subjective day (Figure 3C and Table 1).
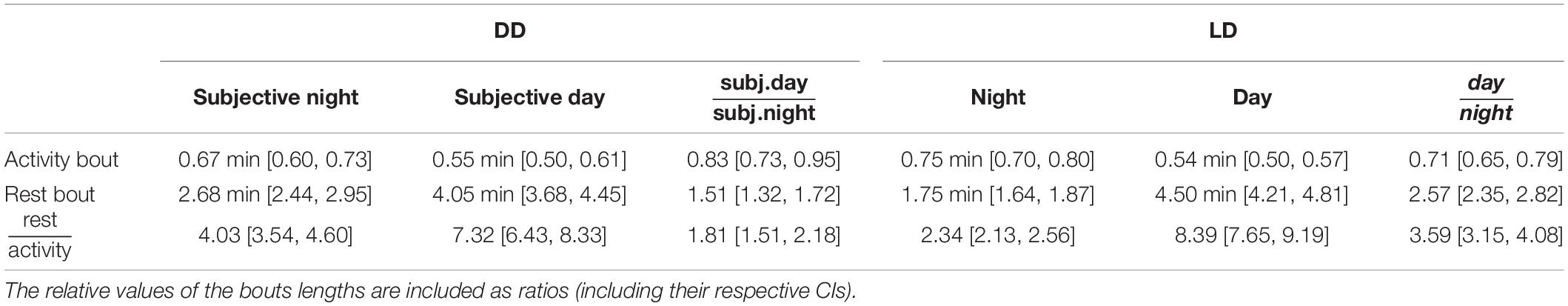
Table 1. Mean and 95% confidence intervals (CI) for the average bout lengths under DD and LD cycles.
This day-night change in the mean length of the rest bouts in DD was smaller in comparison to the corresponding change in LD (Figure 3B, ratioDD/LD = 0.59, CI: [0.50, 0.69]). However, the day-night change in the mean length of the activity bouts was statistically indistinguishable between LD and DD conditions (Figure 3C, ratioDD/LD = 1.16, CI: [0.99, 1.36]). Thus, day-night changes in the rest bout lengths, but not in the activity bout lengths, were moderated in DD.
The mean rest bouts were longer than the mean activity bouts during both the subjective day and the subjective night in DD (Figure 3D). The day-night changes in the rest bout lengths and the activity bout lengths result in different ratios of mean rest bout and mean activity bout lengths between subjective day and subjective night. The ratio of rest bout lengths to activity bout lengths during the night was significantly greater under DD than under a LD cycle (Figure 3D and Table 1). However, the ratio of rest bout lengths to activity bout lengths during the day was similar in DD and LD (Table 1). As a result, the ratio of rest bout lengths to activity bout lengths varied less in DD than in LD.
Day-night changes in the rest bouts rather than in the activity bouts predominantly contributed to the day-night changes in activity. The number of total bouts was higher during the subjective night compared to the subjective day, which coincides with the shorter rest bouts during the subjective night (Figure 3B). However, the day-night difference in the total number of bouts was smaller in DD than in LD (Supplementary Figure 4B).
Model Underestimates the Number of Very Short and Very Long Rest Bouts
The probabilistic model fitting ensures that the mean bout lengths in the behavioral data and the model are identical (fitting probability parameters is tantamount to fitting the means). In this section, the observed distribution of the rest and activity bout lengths is contrasted against the distribution predicted by the model.
The model-derived rest bout length distribution deviates from the observed distribution. Under the probabilistic model (Figure 1A), bout lengths follow a geometric distribution with a mean given by the model parameters. The model predicts fewer extremely short and extremely long rest bouts than those observed in the data (Figure 4). Nevertheless, the activity bout distribution in the data closely matched the predicted distribution. This predicted rest bout distribution consistently differed from the data across the three T-cycles and constant darkness.
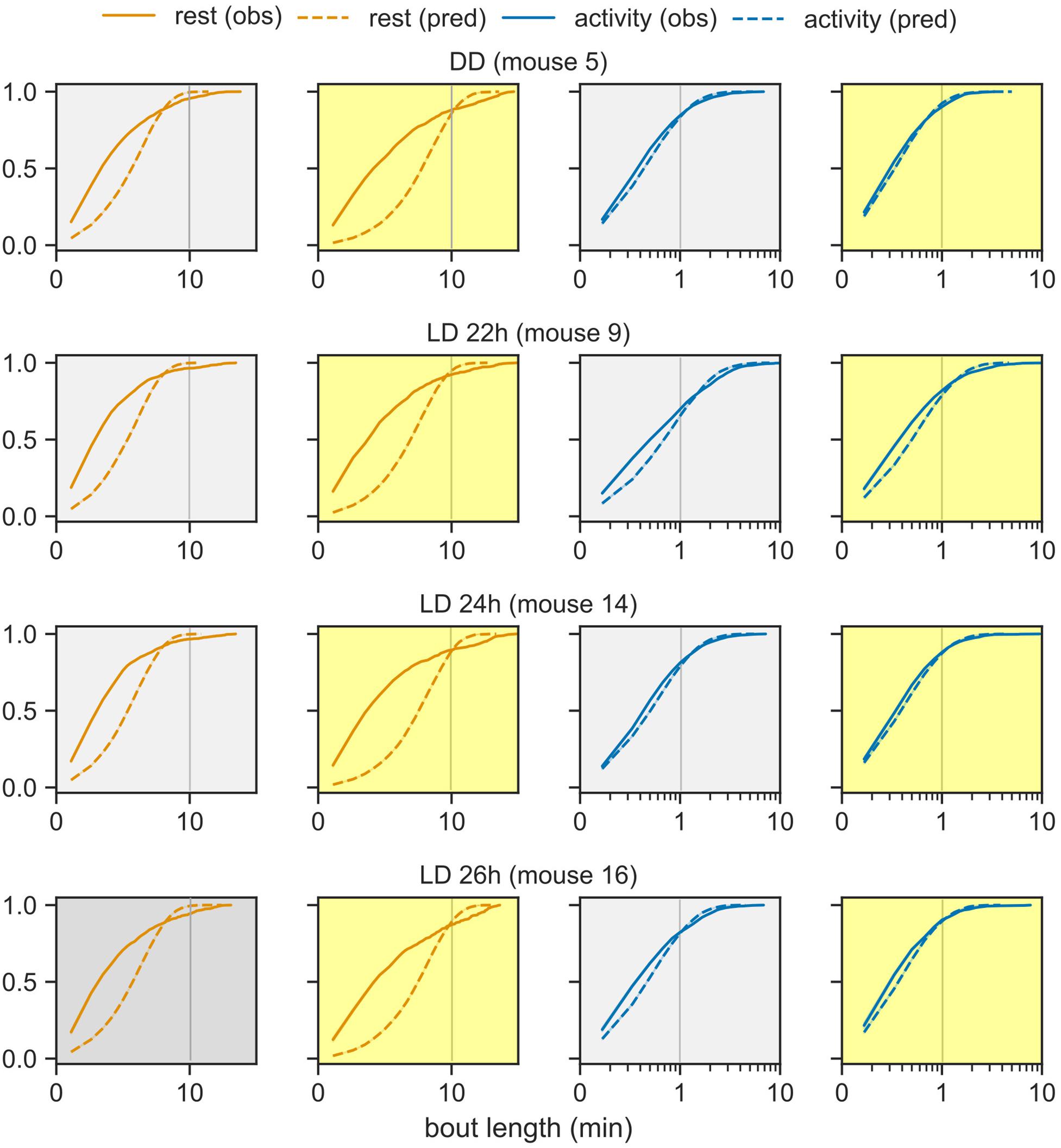
Figure 4. Distribution of bout lengths of activity and rest. The observed distribution of bout lengths (solid lines) is compared against the distribution predicted by the model (dashed lines). The rest and activity bouts in the day and in the night are plotted in separate panels for one representative animal from each group: LD cycles of 22-, 24-, and 26- h and DD. The x-axis is plotted in logarithmic scale in order to see the very short and very long bouts in one graph. The color-coding from earlier figures is maintained.
Ultradian Rhythms in Behavior Are Absent Under This Model
The utility of a model is its ability to make predictions. We found ultradian regulation in bout lengths. Can the ultradian regulation (represented by the model) also result in ultradian rhythms? In the probabilistic model, (i) rest and activity bouts alternate, (ii) the duration of rest and activity bouts are independent of the preceding bout (Markov property) in both light and dark, and (iii) bouts have random durations. If there is a rhythmic pattern of bouts, then the repeating sequence of bouts must be of approximately constant length (the period). Here, the duration of a sequence of bouts is also random (as each bout has a random length) and has a variance that grows with the length of the sequence. Thus, ultradian rhythms cannot occur under this simple model.
To confirm this expectation, we first simulated behavioral data using our fitted models and produced an artificial dataset with the same composition of animals at each T-cycle and the same length of behavioral recording. We then applied the χ2-periodogram (Sokolove and Bushell, 1978) to this artificial data to check for ultradian rhythms in the range 1 min-2 h. As expected, we did not observe any rhythms in this time scale at the 0.05 level (Figure 5A).
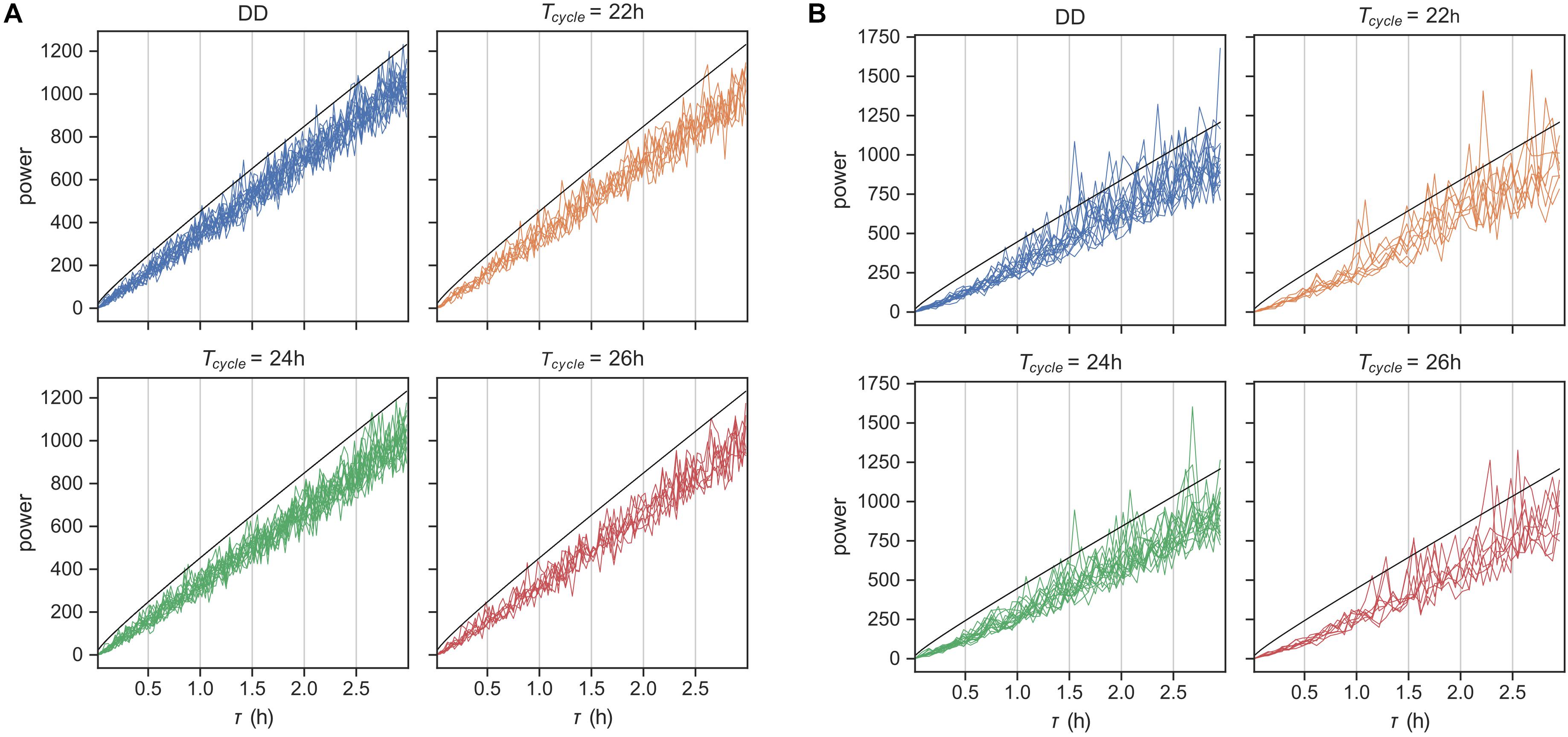
Figure 5. Rhythms in behavior at ultradian time scales. χ2-periodogram analysis of behavioral data simulated using the probabilistic model in Figure 1 in panel (A) and for the original encoded data in panel (B). Simulated data matched the original data in number of replicates and length of data record. The periodograms are grouped according to the Zeitgeber input. The black line is the significance threshold for rhythmicity at the tested period with Bonferroni-adjusted p-value < 0.05.
We applied the same analysis next to the original encoded behavioral data (Figure 5B). Although none of the animals showed any ultradian rhythms on the order of the average bout length (time scale of minutes), many animals showed noisy weak ultradian rhythms in the range 1.5–3 h. However, the mice showed circadian rhythmicities, with a few mice also showing weak harmonic frequencies (Supplementary Figure 5). The model prediction was thus partially confirmed.
Discussion
The central pacemaker in mammals contributes to daily rhythmic patterns of behavioral activity. This paper set out to test whether behavioral activity is also regulated within the 24 h circadian cycle. Using data on spontaneous behavioral activity of mice under LD and DD, we quantified activity in terms of rest and activity bouts in the day and in the night using a probabilistic model. We observed day-night differences in the average bouts lengths of rest and activity under LD that persisted also under DD. The probabilistic model was able to exploit the structure in the behavioral activity to accurately capture the distribution of bouts (with the exception of extremely short and extremely long rest bouts). The model is evidence that (a) behavioral activity is indeed regulated at the level of bouts and (b) these bouts are under the control of both light input and the circadian system.
The main analytical contribution of this work is the probabilistic model of mouse behavior. A probabilistic, as opposed to a deterministic, model is necessary, since there is clearly large intra-individual (across the length of the recordings) and inter-individual variability even in isogenic mice kept under identical conditions. A simpler model would have a single probability parameter defining the switch between rest and activity and vice-versa (i.e., α = β). But, the stark difference in activity between the day and night makes this simpler model inadequate. The model we propose is thus the simplest (non-trivial) model of mouse behavior in the ultradian timescale. Such Markov models are well established in many fields including genomics (Pardoux, 2010) and sleep research (Kemp and Kamphuisen, 1986; Stephenson et al., 2013).
The model has several benefits over traditional spectral analysis methods, such as MESA, Lomb-Scargle and Enright periodograms, to study behavior at different time scales. Spectral analysis only identifies statistically significant patterns in the behavioral data. However, the model is a mechanistic description, albeit abstracted and simplified, of the biological mechanism driving spontaneous behavior. In that sense, the model is generative, i.e., the model can simulate the behavioral activity of a mouse. We used simulation to predict the lack of ultradian rhythms under our probabilistic model. Enright periodogram analysis of behavior did not find ultradian rhythms at the time scale of the bouts. But, noisy weak ultradian rhythms in 1.5–3 h time scale were identified in many mice. This is likely the consequence of unaccounted features in the model, such as very short and very long rest bouts.
Conveniently, the model directly relates to behavior of mice at the level of bouts. Early studies qualitatively described the organization of behavior in small mammals into characteristic bouts (Kavanau and Rischer, 1968; Davis and Menaker, 1980). Penev et al. (1997) studied temporal patterns of behavior in hamsters and showed increased fragmentation with age. Farajnia et al. (2012) showed similar fragmentation in aged mice. Ultradian periodicity of behavior in mammals also manifests as rhythmic consolidated bouts of activity. Ultradian rhythms are observed under natural conditions in the common vole (Gerkema and van der Leest, 1991) and mice (del Pozo et al., 1978; Poon et al., 1997; Dowse et al., 2010), and after surgical or genetic manipulation of the clock in rats and mice (Vitaterna et al., 1994; Horst et al., 1999; Blum et al., 2014).
Mice were inactive for a majority of the day and the night, but with significant activity even during the day. Nevertheless, the mice showed more activity in the night versus the day. Spontaneous behavior (measured using passive IR sensors) is not as clearly segregated into an active phase and a rest phase as is wheel-running activity (Schwartz and Zimmerman, 1990). The mean rest bouts were shortened and the mean activity bouts lengthened in the night relative to the day in the 22-, 24-, and 26-h T-cycles. The different T-cycles affect clock function under the entrained conditions studied here. Since the day-night differences in bout lengths were unaltered across T-cycles, we conclude that light regulates the length of rest and activity bouts independent of the central clock. To fully support the latter conclusion, we may study ultradian behavioral regulation in animals under short and long photoperiod, as an additional modifier of clock function.
We observed that, on average, rest bouts were always longer than activity bouts. Moreover, the day-night changes in mean rest bout lengths were about two-fold larger than the changes in mean activity bout lengths in LD. Taken together, the day-night changes in rest bouts (in minutes) is significantly larger than day-night changes in activity bouts. Therefore, we conclude that regulation of rest bouts predominantly underlies the differences between the active and rest phases. If this were true, we would expect higher number of bouts (rest and activity) in the night compared to the day. This is indeed the case. Thus, the LD environment regulates rest bouts rather than activity bouts over the 24 h cycle.
Under a LD cycle, both light and the central clock affect behavioral activity. To determine the effects of the central clock on behavior, animals are routinely exposed to constant darkness in the absence of all potential time cues. In DD, mice continued to show the same qualitative changes in mean bout lengths between subjective day and subjective night. The persistence of bout regulation in DD between subjective day and night suggests that the central clock also regulates bout length.
The day-night difference in activity and rest bout duration was larger in LD cycles than in DD. Given the enhanced difference between the day and night under LD cycles, we conclude that environmental light cycles reinforce the SCN effect on bout regulation. In other words, exposure to LD cycles increases the “amplitude” of circadian regulation of bouts. Interestingly, the amplitude of the circadian rhythm is also increased under LD as compared to DD conditions. This is the case both at the level of behavioral activity and also at the level of SCN electrical discharge rate (Coomans et al., 2013). It is possible therefore, that even the influence of light on ultradian behavior involves the SCN. At least, and given our results obtained from DD conditions, we suggest that the SCN is a node in the central regulation of ultradian behavioral activity, and is a regulator of the duration of ultradian bouts. Thus, in the absence of the SCN, ultradian bouts will still be present, but the day-night difference in their duration will be completely lost. This interpretation is in line with the ongoing presence of ultradian behavioral rhythmicity in transgenic clockless animals (Vitaterna et al., 1994; Horst et al., 1999; Blum et al., 2014), as well as in voles with SCN lesions (Gerkema et al., 1990).
Surprising, the Markov model predicted well the behavior at the next 10 s bin based on the current bin and a coin toss. We also confirmed explicitly the validity of the Markov assumption for our behavioral data. The rest and activity states in the Markov model have positive (auto-enforcing) feedback loops (Figure 1). When the strength of this positive feedback is large (>0.5, which is the case for all fits in this study), then the Markov model shows inertia, i.e., a tendency to remain at the state it is in. Occasionally, behavior breaks out of this state and switches to the alternative state. We found that this principle applies well to the ultradian regulation of rest and activity. The parameters describing the duration in a state are apparently under the control of environmental light and the central clock. The model is analogous to the proposed “flip-flop” switch between sleep and wakefulness, where various neuronal inputs regulate the balance between the two states (Saper et al., 2001). It is very likely that other brain areas are also involved in the underlying circuitry, and for instance, there is good evidence for the role of dopamine (Blum et al., 2014).
The Markov property is manifested as a geometric distribution of bout lengths, where short bouts are more common than long bouts. While the model predicted the distribution of activity bouts accurately, it underestimated both the number of very short and very long rest bouts. Nevertheless, our model is elegant in its simplicity in that it captures most features of murine behavior with some exceptions. In fact, there are multiple reports showing that especially rest bouts do not follow an exponential distribution. The first quantitative analysis of bouts (Penev et al., 1997) already proposed that rest bouts were of two types: short bouts within an activity bout or long bouts between activity bouts. More generally, rest bout lengths appear to follow a power-law (heavy-tailed) distribution in mice, humans and fruit flies (Nakamura et al., 2008; Cascallares et al., 2018) that breakdown under certain pathologies. The rest bout distribution in this study did not show power-law characteristics (not shown). The deviation of the rest bout distribution might be due to the presence of different types of rest bouts, such as a “pause” in activity explaining the very short bouts, and the existence of other processes regulating rest bouts, such as homeostatic sleep drive.
Our conclusions must be viewed in the context of our analysis methodology. The model treats behavior within the dark and light phases as homogeneous, which is not often the case (Houben et al., 2014). The simplified data capture the duration of activity, but ignore the intensity of activity. Thus, there could be differences in intensity of activity across LD cycles or between DD and LD, which our analysis overlooked. All these limitations provide interesting avenues for further study. Finally, the behavioral activity was collected in 10 s bins and although bout lengths were of the order of minutes, the effect of bin size on the results cannot be excluded.
Data Availability Statement
Requests to access the datasets from this study should be directed to JM, ai5oLm1laWplckBsdW1jLm5s.
Ethics Statement
The animal study was reviewed and approved by the Animal Ethics Committee at the Leiden University Medical Center (Leiden, Netherlands).
Author Contributions
BA conceived and designed the study, performed the analysis, and wrote the manuscript. JM acquired the data and critically revised the manuscript. Both authors interpreted the data, contributed to the manuscript revision, and read and approved the submitted version.
Funding
BA acknowledges support from the BMBF grant 01GQ1503 and Deutsche Forschungsgemeinschaft (DFG) grant HE2168/11-1 (SPP 2041). JM acknowledges support from the Swiss VELUX Foundation (project 1131), Dutch Research Council (NWO) grant ALW.OP.389, and European Research Council grant 854513 ERC-2018-ADG. We acknowledge support from the German Research Foundation (DFG) and the Open Access Publication Fund of Charité – Universitätsmedizin Berlin.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Hanspeter Herzel and Achim Kramer for useful discussions and Jos H. T. Rohling for compiling data from prior studies.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2020.00268/full#supplementary-material
References
Blum, I. D., Zhu, L., Moquin, L., Kokoeva, M. V., Gratton, A., Giros, B., et al. (2014). A highly tunable dopaminergic oscillator generates ultradian rhythms of behavioral arousal. eLife 3:e05105. doi: 10.7554/eLife.05105
Cascallares, G., Riva, S., Franco, D. L., Risau-Gusman, S., and Gleiser, P. M. (2018). Role of the circadian clock in the statistics of locomotor activity in Drosophila. PLoS One 13:e0202505. doi: 10.1371/journal.pone.0202505
Coomans, C. P., van den Berg, S. A. A., Houben, T., van Klinken, J.-B., van den Berg, R., Pronk, A. C. M., et al. (2013). Detrimental effects of constant light exposure and high-fat diet on circadian energy metabolism and insulin sensitivity. FASEB J. 27, 1721–1732. doi: 10.1096/fj.12-210898
Davis, F. C., and Menaker, M. (1980). Hamsters through time’s window: temporal structure of hamster locomotor rhythmicity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 239, R149–R155. doi: 10.1152/ajpregu.1980.239.1.R149
del Pozo, F., DeFeudis, F. V., and Jimenez, J. M. (1978). Motilities of isolated and aggregated mice; A difference in ultradian rhythmicity. Experientia 34, 1302–1304. doi: 10.1007/BF01981433
Dowse, H., Umemori, J., and Koide, T. (2010). Ultradian components in the locomotor activity rhythms of the genetically normal mouse, Mus musculus. J. Exp. Biol. 213, 1788–1795. doi: 10.1242/jeb.038877
Farajnia, S., Michel, S., Deboer, T., vanderLeest, H. T., Houben, T., Rohling, J. H. T., et al. (2012). Evidence for neuronal desynchrony in the aged suprachiasmatic nucleus clock. J. Neurosci. 32, 5891–5899. doi: 10.1523/JNEUROSCI.0469-12.2012
Gerkema, M. P., Groos, G. A., and Daan, S. (1990). Differential elimination of circadian and ultradian rhythmicity by hypothalamic lesions in the common vole, Microtus arvalis. J. Biol. Rhythms 5, 81–95. doi: 10.1177/074873049000500201
Gerkema, M. P., and van der Leest, F. (1991). Ongoing ultradian activity rhythms in the common vole. Microtus arvalis, during deprivations of food, water and rest. J. Comp. Physiol. A 168, 591–597. doi: 10.1007/BF00215081
Hastings, M. H., Maywood, E. S., and Brancaccio, M. (2018). Generation of circadian rhythms in the suprachiasmatic nucleus. Nat. Rev. Neurosci. 19, 453–469. doi: 10.1038/s41583-018-0026-z
Herzog, E. D. (2007). Neurons and networks in daily rhythms. Nat. Rev. Neurosci. 8, 790–802. doi: 10.1038/nrn2215
Houben, T., Coomans, C. P., and Meijer, J. H. (2014). Regulation of circadian and acute activity levels by the murine suprachiasmatic nuclei. PLoS One 9:e110172. doi: 10.1371/journal.pone.0110172
Houben, T., Deboer, T., van Oosterhout, F., and Meijer, J. H. (2009). Correlation with behavioral activity and rest implies circadian regulation by SCN neuronal activity levels. J. Biol. Rhythms 24, 477–487. doi: 10.1177/0748730409349895
Hu, K., Scheer, F. A., Ivanov, P. Ch, Buijs, R. M., and Shea, S. A. (2007). The suprachiasmatic nucleus functions beyond circadian rhythm generation. Neuroscience 149, 508–517. doi: 10.1016/j.neuroscience.2007.03.058
Kavanau, J. L., and Rischer, C. E. (1968). Program clocks in smail mammals. Science 161, 1256–1259. doi: 10.1126/science.161.3847.1256
Kemp, B., and Kamphuisen, H. A. (1986). Simulation of human hypnograms using a Markov chain model. Sleep 9, 405–414. doi: 10.1093/sleep/9.3.405
Nakamura, T., Takumi, T., Takano, A., Aoyagi, N., Yoshiuchi, K., Struzik, Z. R., et al. (2008). Of mice and men – universality and breakdown of behavioral organization. PLoS One 3:e2050. doi: 10.1371/journal.pone.0002050
Nakamura, W., Honma, S., Shirakawa, T., and Honma, K. (2002). Clock mutation lengthens the circadian period without damping rhythms in individual SCN neurons. Nat. Neurosci. 5, 399–400. doi: 10.1038/nn843
Pardoux, E. (2010). Markov Processes and Applications: Algorithms, Networks, Genome and Finance. Hoboken, NJ: Wiley.
Penev, P. D., Zee, P. C., and Turek, F. W. (1997). Quantitative analysis of the age-related fragmentation of hamster 24-h activity rhythms. Am. J. Physiol. Regul. Integr. Comp. Physiol. 273, R2132–R2137. doi: 10.1152/ajpregu.1997.273.6.R2132
Pittendrigh, C. S. (1960). Circadian rhythms and the circadian organization of living systems. Cold. Spring Harb. Symp. Quant. Biol. 25, 159–184. doi: 10.1101/SQB.1960.025.01.015
Pittendrigh, C. S., and Daan, S. (1976). A functional analysis of circadian pacemakers in nocturnal rodents. J. Comp. Physiol. 106, 291–331. doi: 10.1007/BF01417859
Poon, A. M. S., Wu, B. M., Poon, P. W. F., Cheung, E. P. W., Chan, F. H. Y., and Lam, F. K. (1997). Effect of cage size on ultradian locomotor rhythms of laboratory mice. Physiol. Behav. 62, 1253–1258. doi: 10.1016/s0031-9384(97)00305-3
Quintero, J. E., Kuhlman, S. J., and McMahon, D. G. (2003). The biological clock nucleus: a multiphasic oscillator network regulated by light. J. Neurosci. 23, 8070–8076. doi: 10.1523/JNEUROSCI.23-22-08070.2003
Ralph, M., Foster, R., Davis, F., and Menaker, M. (1990). Transplanted suprachiasmatic nucleus determines circadian period. Science 247, 975–978. doi: 10.1126/science.2305266
Rusak, B. (1977). The role of the suprachiasmatic nuclei in the generation of circadian rhythms in the golden hamster, Mesocricetus auratus. J. Comp. Physiol. 118, 145–164. doi: 10.1007/BF00611819
Saper, C. B., Chou, T. C., and Scammell, T. E. (2001). The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 24, 726–731. doi: 10.1016/s0166-2236(00)02002-6
Schaap, J., Albus, H., vanderLeest, H. T., Eilers, P. H. C., Detari, L., and Meijer, J. H. (2003). Heterogeneity of rhythmic suprachiasmatic nucleus neurons: implications for circadian waveform and photoperiodic encoding. Proc. Natl. Acad. Sci. U.S.A. 100, 15994–15999. doi: 10.1073/pnas.2436298100
Schwartz, W., and Zimmerman, P. (1990). Circadian timekeeping in BALB/c and C57BL/6 inbred mouse strains. J. Neurosci. 10, 3685–3694. doi: 10.1523/JNEUROSCI.10-11-03685.1990
Schwartz, W. J., and Zimmerman, P. (1991). Lesions of the suprachiasmatic nucleus disrupt circadian locomotor rhythms in the mouse. Physiol. Behav. 49, 1283–1287. doi: 10.1016/0031-9384(91)90364-T
Sokolove, P. G., and Bushell, W. N. (1978). The chi square periodogram: its utility for analysis of circadian rhythms. J. Theor. Biol. 72, 131–160. doi: 10.1016/0022-5193(78)90022-X
Stephenson, R., Famina, S., Caron, A. M., and Lim, J. (2013). Statistical properties of sleep-wake behavior in the rat and their relation to circadian and ultradian phases. Sleep 36, 1377–1390. doi: 10.5665/sleep.2970
Horst, G. T. J. van der, Muijtjens, M., Kobayashi, K., Takano, R., Kanno, S., Takao, M., et al. (1999). Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature 398, 627–630. doi: 10.1038/19323
Vitaterna, M., King, D., Chang, A., Kornhauser, J., Lowrey, P., McDonald, J., et al. (1994). Mutagenesis and mapping of a mouse gene, clock, essential for circadian behavior. Science 264, 719–725. doi: 10.1126/science.8171325
Wollnik, F., and Turek, F. W. (1989). SCN lesions abolish ultradian and circadian components of activity rhythms in LEW/Ztm rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 256, R1027–R1039. doi: 10.1152/ajpregu.1989.256.5.R1027
Keywords: spontaneous behavior, probabilistic model, light cycles, circadian clock, activity duration
Citation: Ananthasubramaniam B and Meijer JH (2020) Regulation of Rest, Rather Than Activity, Underlies Day-Night Activity Differences in Mice. Front. Physiol. 11:268. doi: 10.3389/fphys.2020.00268
Received: 08 November 2019; Accepted: 09 March 2020;
Published: 31 March 2020.
Edited by:
Rodolfo Costa, University of Padua, ItalyReviewed by:
Charalambos P. Kyriacou, University of Leicester, United KingdomMarco Brancaccio, Imperial College London, United Kingdom
Copyright © 2020 Ananthasubramaniam and Meijer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bharath Ananthasubramaniam, YmhhcmF0aC5hbmFudGhAY2hhcml0ZS5kZQ==
 Bharath Ananthasubramaniam
Bharath Ananthasubramaniam Johanna H. Meijer
Johanna H. Meijer