
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Physiol. , 16 August 2019
Sec. Environmental, Aviation and Space Physiology
Volume 10 - 2019 | https://doi.org/10.3389/fphys.2019.01046
This article is part of the Research Topic Optimization of Exercise Countermeasures for Human Space Flight – Lessons from Terrestrial Physiology and Operational Implementation View all 15 articles
Background: Space Agencies are planning human missions beyond Low Earth Orbit. Consideration of how physiological system adaptation with microgravity (μG) will be managed during these mission scenarios is required. Exercise countermeasures (CM) could be used more sparingly to decrease limited resource costs, including periods of no exercise. This study provides a complete overview of the current evidence, making recommendations on the length of time humans exposed to simulated μG might safely perform no exercise considering muscles only.
Methods: Electronic databases were searched for astronaut or space simulation bed rest studies, as the most valid terrestrial simulation, from start of records to July 2017. Studies were assessed with the Quality in Prognostic Studies and bed rest analog studies assessed for transferability to astronauts using the Aerospace Medicine Systematic Review Group Tool for Assessing Bed Rest Methods. Effect sizes, based on no CM groups, were used to assess muscle outcomes over time. Outcomes included were contractile work capacity, muscle cross sectional area, muscle activity, muscle thickness, muscle volume, maximal voluntary contraction force during one repetition maximum, peak power, performance based outcomes, power, and torque/strength.
Results: Seventy-five bed rest μG simulation studies were included, many with high risk of confounding factors and participation bias. Most muscle outcomes deteriorated over time with no countermeasures. Moderate effects were apparent by 7–15 days and large by 28–56 days. Moderate effects (>0.6) became apparent in the following order, power and MVC during one repetition maximum (7 days), followed by volume, cross sectional area, torques and strengths, contractile work capacity, thickness and endurance (14 days), then muscle activity (15 days). Large effects (>1.2) became apparent in the following order, volume, cross sectional area (28 days) torques and strengths, thickness (35 days) and peak power (56 days).
Conclusions: Moderate effects on a range of muscle parameters may occur within 7–14 days of unloading, with large effects within 35 days. Combined with muscle performance requirements for mission tasks, these data, may support the design of CM programmes to maximize efficiency without compromising crew safety and mission success when incorporated with data from additional physiological systems that also need consideration.
Space Agencies are planning to transition from International Space Station (ISS) missions to Lunar missions including a crewed base from which to test and develop hardware and procedures required for the longer term goal of human Mars missions (Foing, 2016). It is well documented that exposure to microgravity (μG) during spaceflight causes adaptation in response to gravitational unloading, especially in the musculoskeletal, cardiovascular and neuro-vestibular systems (Buckey, 2006; Baker et al., 2008). The risks to mission success due to potential injury or reduced function due to periods of unmanaged adaptation before arriving at a remote location such as Moon or Mars, where medical teams may not be present on landing, need to be addressed (Gernand, 2004). Bed rest is often used as a controlled Earth-based environment for simulating the effects of spaceflight on humans to enable more cost-effective, higher quality and safer research into effects and medical management of adaptation (Pavy-Le Traon et al., 2007). While bed rest fails to remove a Gx (chest-to-back) loading vector, such studies are considered the most valid simulation method for many physiological systems (Adams et al., 2003; Pavy-Le Traon et al., 2007), except for weight bearing, tissue fluid redistributions and skin surface areas of compression (Hargens and Vico, 2016), and when conducted rigorously are likely to generate results transferable to astronauts (Higgins and Green, 2011;Winnard and Nasser, 2017b).
Based on bed rest research and previous spaceflight experience, the ISS provides astronauts with 2.5 h per day for exercise (including setup, stowage and hygiene) using a treadmill, cycle ergometer and resistance exercise device designed and adapted for μG (Trappe et al., 2009; Loehr et al., 2011). Several years of refining ISS exercise countermeasures (CM) has led to astronauts completing 6-month missions with, on average, little to no change in bone mass or cardiovascular capacity, although the efficacy seems to vary widely between individuals (Moore et al., 2014; English et al., 2015; Sibonga et al., 2015), while muscle adaptation appears to have become progressively smaller as exercise devices and prescriptions have improved (Smith et al., 2012; Moore et al., 2014; Ploutz-Snyder et al., 2015). However, the exercise devices currently aboard ISS will almost certainly be too large and too numerous for, and the exercise prescriptions place too great a demand on the consumables and environmental management systems available on, the vehicles and habitats currently planned for future exploration missions. For this reason, space agencies have started designing smaller, low energy and low vibration exercise devices (Brusco, 2016). However, decisions will need to be made regarding choice and/or development of an effective exercise CM programme to manage physiological adaptation that will occur during exploration missions. Considerations may include reducing the frequency of exercise as currently performed on ISS and potentially having longer periods of not performing any exercise. The duration of any such no exercise periods needs to be evidence based to balance any increase physiological risks to crew against gains in spacecraft and consumables impact.
The objectives of this review were to provide a complete summary of and synthesize the current space-related physiological evidence base and to inform decision making processes around muscle performance requirements, regarding operational CM, for exploration human space missions. Where data is lacking for any outcomes this will be highlighted as a gap or limited area of the current evidence base and used to provide a gap analysis commentary useful for future research priority setting. The aim is to aid space agencies in designing CM programmes, provide a complete summary of what muscle groups and outcomes have been assessed in the current evidence and highlight areas of minimal data or research gaps to guide future relevant research in this area. The NASA Risk Table for the Human Research Project highlights potential risks relating to spaceflight and shows the large scope of potential physiological systems that require reviewing to cover all elements of crew health and performance (National Aeronautics and Space Administration, 2019). As the scope is too large for a single review, it is suggested that the various systems be reviewed individually. Once a series of reviews has been complete a position statement summarizing across each system can provide a holistic overview. Therefore, this specific review investigated the rate at which muscle parameters change during simulated μG exposure, when no countermeasures are taken, to inform operational decisions regarding the possibility of using exercise CM programmes more sparingly for exploration missions, including the implementation of exercise “holidays” (i.e., a period of time within the mission when no exercise CM are employed). Conclusions of this review alone must be treated in a muscle context and need considering alongside other relevant health and performance components.
At what time point do people exposed to simulated μG while not performing CM reach a moderate or large effect on muscle health outcomes?
The Cochrane Collaboration Guidebook (Higgins and Green, 2011) and preferred reporting items for systematic reviews and meta-analyses (PRISMA) were adhered to Moher et al. (2009). No external funding or research grants were received for this work.
The following inclusion criteria were employed. The target population was astronauts, however, as astronauts have taken part in space agency recommended exercise programmes to date, there was no inactive data available from this population. Therefore, healthy terrestrial adults, with no gender restrictions, taking part in μG analog bed rest studies, were included. Bed rest studies were the only terrestrial model included as they are considered the most valid ground based model for simulating human spaceflight for periods beyond a few minutes (Adams et al., 2003; Pavy-Le Traon et al., 2007). Therefore, to maintain the greatest level of transferability to astronauts and in keeping with our other systematic reviews only bed rest studies that stated they were simulating human spaceflight were considered. No clinical bed rest situations such as critical care were included as they would likely have confounding co-morbities and not transfer well to astronauts. All participants in the included bed rest studies were healthy at baseline, however, no exclusion was made relating to baseline level of physical condition beyond being healthy. Only control group data were relevant, therefore no inclusion criteria were based on interventions. Control groups had to be inactive and not undergo any type of intervention. Included studies had to report outcomes relating to muscles. For completeness of reporting the current state of the evidence base and avoid introducing selection bias, no exclusion was made based on type of outcome or amount of data. The evidence based led outcomes were determined from pre-scoping and the main review searches and were grouped for analysis as cross-sectional area, volume, shape, size, activity, power, performance and joint torque and forces at either a regional or global level. Included studies had to be randomized controlled trials (RCT), controlled clinical trials (CT), longitudinal, interrupted time series or before and after studies.
A range of relevant terms grouped by main search terms were constructed using Boolean logic (astronaut*, spaceflight, space flight, space*, weightless*, microgravity, micro gravity, bed-rest, bed rest, bed rest, dry immersion, muscle*, strength*) to search the following databases up to July 2017: Pubmed, CINAHL, Web of Science, NASA Technical Reports Server and The Cochrane Collaboration Library. No restrictions on type of bed rest or publication dates were applied, and due to the inability to use “Boolean logic” on the NASA Technical Reports Server, the strategy was adapted to keyword searches. The full search strategy is available in Table 1.
Initial screening was performed using abstracts and titles by two authors (MV and AW), blinded to each other's decisions, using Rayyan (https://rayyan.qcri.org/) (Ouzzani et al., 2016). Rayyan also automatically detects duplicate studies and data and all flagged potential duplication was assessed by agreement of three blinded authors. Where there was any disagreement whether the study met the inclusion criteria from initial screening the full text was obtained. A third author (NC) was used to resolve disagreements of included/excluded studies. An adapted version of the Aerospace Medicine Systematic Review Group (AMSRG) “Data extraction form,” version 2, July 2017 (AMSRG, 2018) was used by two authors (MV and NW) to extract data from each paper, and disagreements were discussed by three authors (AW, NW and MV) to reach consensus.
The Quality in Prognostic Studies (QUIPS) tool was used to assess risk of bias of all the included studies, with “H,” “M,” and “L” showing high, moderate and low risk, respectively, using pre-defined published definitions for each level (Hayden et al., 2013). Risk of bias results were used to comment on the current quality and completeness of the evidence base and do not change how studies were treated during analysis. As per published recommendations, only studies that were rated low risk of bias in all QUIPS domains were deemed as low risk overall (Hayden et al., 2013). The AMSRG “Tool for Assessing Bed Rest Methods” (Winnard and Nasser, 2017a,b) was used to assess the bed rest methodological quality, and transferability to astronaut populations, of all included studies, with “y” indicating the point was met, “n” not met, and “?” unclear. This is a relatively new tool, yet to be validated, that has been used in several other reviews (Richter et al., 2017; Winnard et al., 2017) and the development of the tool is explained in Winnard et al. (2017).
Effect sizes (Hedges' g) were calculated between pre and post-bed rest values for each outcome individually without an overall pooled effect. Hedges' g was used to bias correct for the typically small sample sizes, as only control group data from μG simulation studies were eventually included. The reported data set that was as close to immediate pre and the end of bed rest was used for the analysis. No exclusion or analysis variation was made based on the individual study analysis methods. The pooled standard deviation for Hedges' g was calculated using the root mean square of the pre and post-group standard deviations. This version does not specifically include the sample size (n), preventing any complications that could arise from inflating n when both group means are from the same sample. Results were first sub-grouped by outcome measure type and then by muscle group before being listed in order of ascending days spent in simulated μG. Individual effects sizes were calculated and plotted in figures for each outcome at every time point where data were available. To enable a brief overview of the large data set to also be provided, an unweighted mean effect at each common time point within each muscle group was used to provide a summary result. This was only done when more than one study assessed the same outcome at the same time point. These statistics were chosen due to data being from the same sample rather than a separate intervention and control group, thus making a traditional weighted effects meta-analysis pooling inappropriate. Traditional meta-analysis assumes two different sets of individuals in each group (Higgins and Green, 2011) meaning a violation of underlying assumptions would have occurred if applied to this review. The summary unweighted mean, while being a less robust statistic, enabled an overarching overview to be reported in addition to each individual effect size, and overlaid on the figures, without violating statistical assumptions. Ninety-five percent confidence intervals were calculated for individual and unweighted group means. Readers should note that due to varying effect sizes across the various muscles and groupings, the effect size axis scale varies accordingly throughout the figures.
The point at which effects consistently reached a magnitude of 0.6 (moderate) or 1.2 (large) was highlighted as a time point when a worthwhile mechanistic change had occurred (Hopkins et al., 2009). Plots of all individual effects and 95% confidence interval tails, in order of ascending days in simulated μG, were overlaid with the mean effect and polynomial trend line of the mean effects. A polynomial trend allowed for the trend line to curve in case of progressively worsening, or plateauing patterns. In cases where data were lacking and varied (spanning more than one effect size cut off between data points), the trend line was highlighted as likely unreliable in the results section, meaning more data should be collected before a reliable trend can be established. The limited data sets are however still included for completeness of reporting the current state of the evidence base and highlight both minimal data areas and research gaps. The mean effect summary and trend line were only used to visually highlight the time point at which the mean effects passed the 0.6 and 1.2 magnitude point. A funnel plot of all the mean effects plotted against study size was used to show potential publication bias.
Ten sub groups were created based on the measurement methods units used for each for analysis as follows (with original units measured in): (1) contractile work capacity (J), (2) cross sectional area (mm2, cm2), (3) muscle activity (μV, mV, normalized), (4) muscle thickness (mm, cm), (5) muscle volume (cm3), (6) maximal voluntary contraction force during one repetition maximum (kg, N, Nm) (7), peak power (W), (8) performance based outcomes (including endurance time, jump power, force, velocity height and acceleration, sit to stand time, center of mass variation, and sprint time) (s, mm, cm, m, W/kg), (9) power (rad·s−1, m·s−1), and (10) torques and strength (Nm, ft-lb). Within each subgroup data were further sub-grouped for analysis by major muscle groups. For completeness of reporting, any measures that did not fit within major muscle groupings were grouped for analysis and reported as either “other lower limb,” “other trunk,” or “other upper limb” outcomes, to enable every outcome measure extracted from included studies to be reported in the results. The outcomes included in the “other” groupings are listed in the text.
In total, 112 studies were included after duplicates removed, all of which were screened for inclusion into the analysis. There were 37 not included in the analysis due the reasons provided in the PRISMA flow diagram (Figure 1). Therefore, 75 studies (Table 2) were included, producing 922 individual effect sizes across all sub groups and outcomes. All studies were bed rest μG simulations as no astronaut studies to date included an inactive control group exposed to μG due to space agency recommended exercise programmes. There is no comparison descriptor column in Table 2 as we only considered control groups who had no intervention, treated as before and after simulated μG exposure comparisons. The most common bed rest duration was 60 days, with shortest and longest durations being seven and 120 days, respectively. The most common study design was RCT. Most of the studies scored four on the bed rest quality score, with the highest score being six, and the lowest score was two. Only three studies were assessed to have a low risk of bias. As only intervention studies' control group data were included and no actual prognostic studies were found and included, question three on the QUIPS about prognostic factors was rated as n/a for all the included studies. A rating for question 3 would have been provided had any actual prognostic studies been found and included. However, for this review, time in μG can be considered the prognostic factor and the quality of the μG simulation was critiqued in detail within the bed rest quality scores. There is some asymmetry in the funnel plot in Figure 2, suggesting potential publication bias toward studies reporting decreases in muscles, however there are studies, including smaller ones, that do report an increase. Fourty five studies specified a time period ahead of the bed rest period in which baseline measures were recorded ranging from 1 to 21 days. Of these, 11 (Greenleaf et al., 1983, 1989, 1994b; Dudley et al., 1989; Ellis et al., 1993; Ferrando et al., 1995; Portero et al., 1996; Muir et al., 2011; Lee et al., 2014; English et al., 2016; Schneider et al., 2016) stated utilizing a pre-bed rest ambulatory control period in their methods section. However, it was not clear in any of the studies what the control period involved or if there was any pre-bed rest deconditioning that was measured or adjusted for. One study, Mulder et al. (2008) measured baseline outcomes on day 4 of bed rest and acknowledges this could have led to underestimating the effect of bed rest, especially for time sensitive outcomes such as those associated with muscle. Full data tables for results per muscle are available in supplementary data tables as indicated in each results sub-section. The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any interested parties.
All muscle volumes decreased over time. Moderate effects were becoming apparent by 14 days and large by 28 days. Very little data were available for Hip Flexor, Gluteal, Multifidus, and Erector Spinae muscles, where a moderate or greater effect was never reached for Hip Flexors and Erector Spinae muscles and only a moderate effect was apparent by 27 and 90 days for Gluteal and Multifidus muscles, respectively. Other lower limb muscles that included Gracilis, Sartorius, Piriformis, Obturators, and Pectineus muscles, reached a moderate effect by 14 days. Other trunk muscles that included Levator Scapulae, Longus Colli, Sternocleidomastoid, and Scalene muscles never reached a moderate effect. The breakdown of individual volume effects per muscle is available in Supplementary Table 1 and associated summary plots in Figure 3.
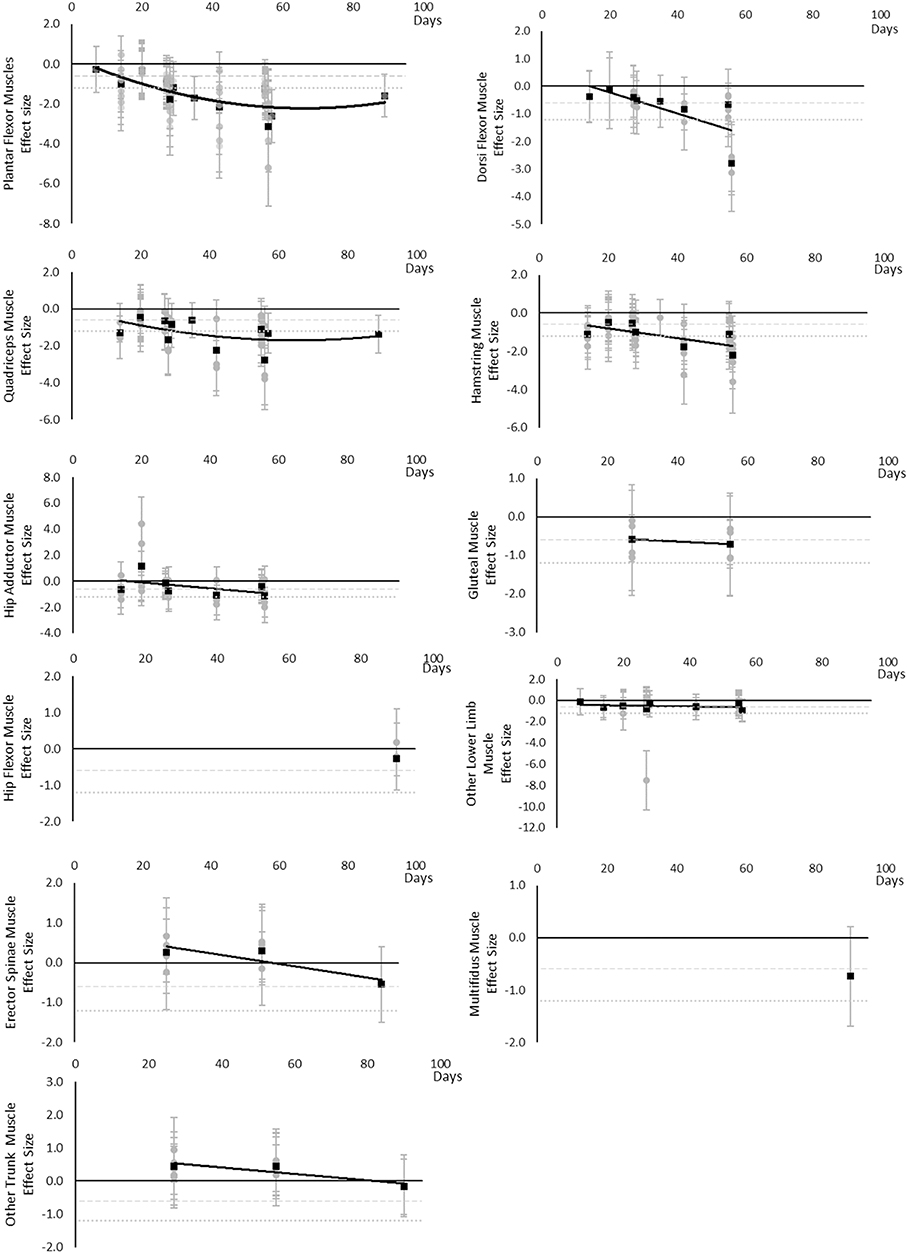
Figure 3. Effect size plots for muscle volume over time from individual (gray) and average (black) effect sizes at each time point, with 0.6 (dotted line) and 1.2 (dashed line) effect magnitudes and average effect trend line overlaid.
All muscle cross sectional areas decreased over time. Moderate effects were apparent by 14 days and large by 28 days. The same effect time points were found for other lower limb muscles that included Gracilis and Sartorius muscles and total thigh and calf cross sectional area. Very little data were available for Hip Flexor, Gluteal, Hamstring and Hip Adductor muscles where a moderate or greater effect was never reached. Multifidus and other trunk muscles, including Quadratus Lumborum and combined Multifidus and Erector Spinae cross sectional area, only reached a large effect by 60 days. Upper limb muscle outcomes consisted of forearm muscle cross sectional area which only reached a large effect after 89 days. The breakdown of individual cross sectional area effects per muscle are available in Supplementary Table 2 and associated summary plots in Figure 4. The polynomial trend for Dorsi Flexor muscles appeared to be unreliable.
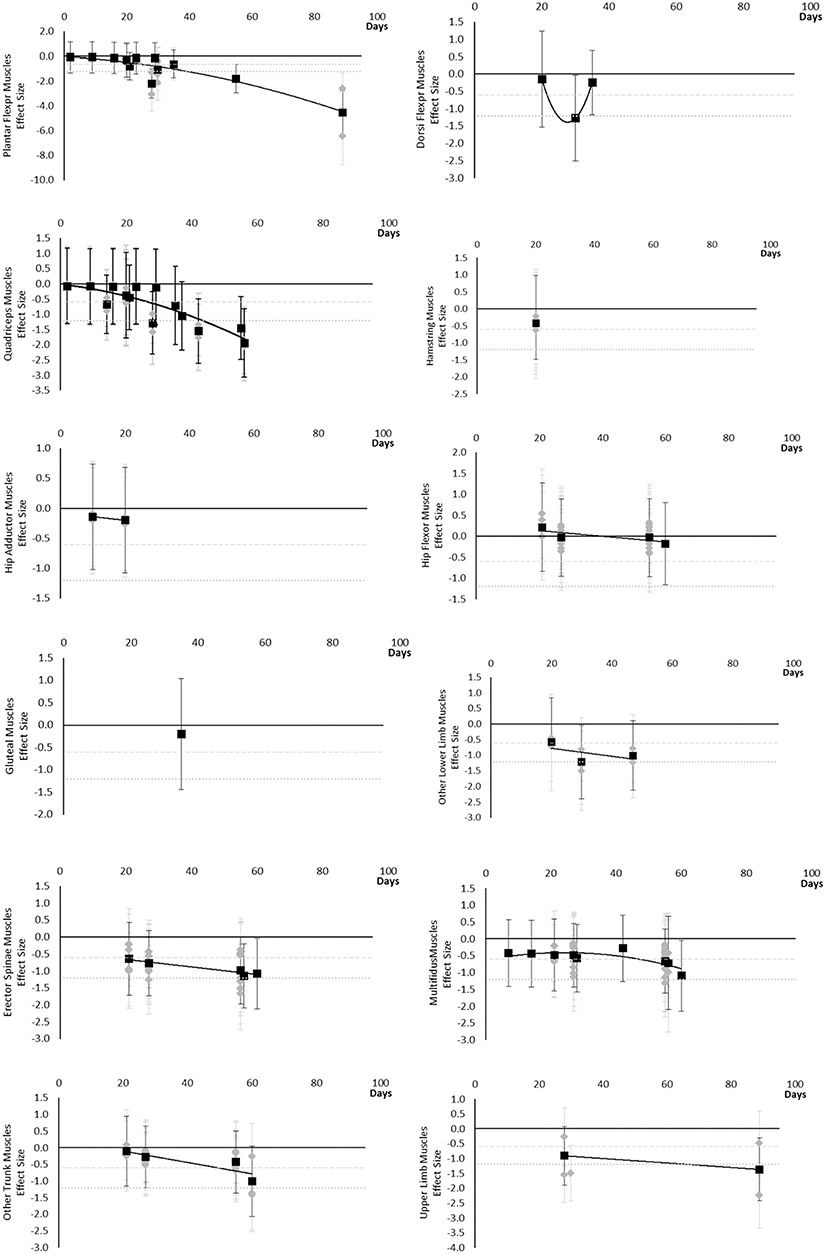
Figure 4. Effect size plots for muscle cross sectional area over time from individual (gray) and average (black) effect sizes at each time point, with 0.6 (dotted line) and 1.2 (dashed line) effect magnitudes and average effect trend line overlaid.
Torques and strengths decreased over time. Moderate effects became apparent by 14 days for Quadriceps muscles only. Additional moderate effects became apparently by 30 days and large effects by 35 days. Dorsi Flexor, Hamstring, Hip Extensor, Hip Flexor, other trunk, and other upper limb muscles never reached a large effect. Other trunk muscles included trunk flexors and extensors tested in combination within functional movements. Upper limb muscles included elbow flexor and extensor muscles and shoulder abductor and adductor muscles. The breakdown of individual torques and strength effects per muscle is available in Supplementary Table 3 and associated summary plots in Figure 5. The polynomial trend for Dorsi Flexor muscles appeared to be unreliable after 60 days.
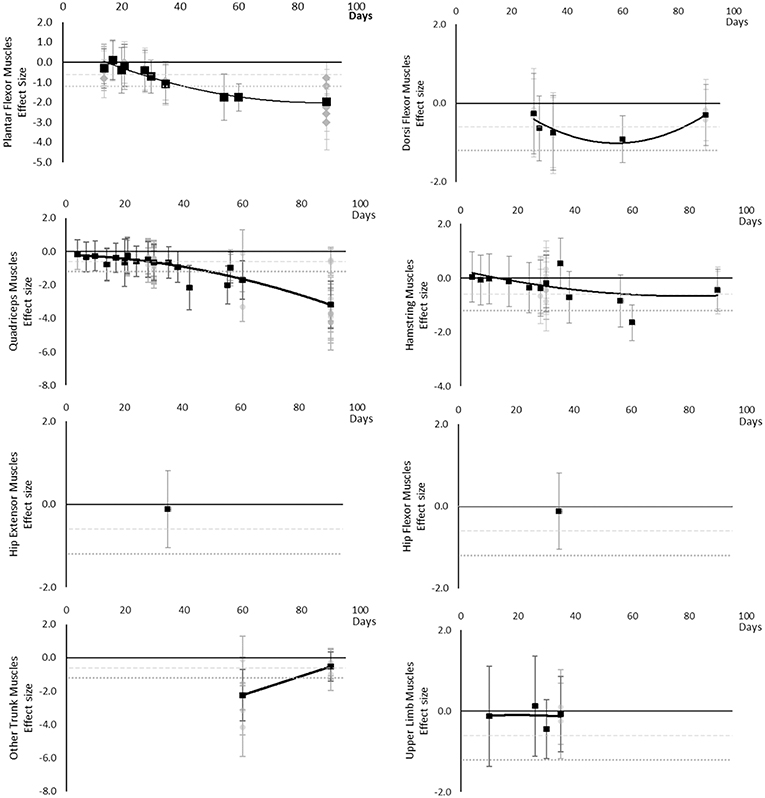
Figure 5. Effect size plots for torques and strength over time from individual (gray) and average (black) effect sizes at each time point, with 0.6 (dotted line) and 1.2 (dashed line) effect magnitudes and average effect trend line overlaid.
Although there are very little available data for contractile work capacity it appears to decrease over time. Moderate effects became apparent by 14 days in Plantar Flexor and Quadriceps muscles. However, this is based on only one study for each muscle at 14 days. The breakdown of individual contractile work capacity effects per muscle is available in Supplementary Table 4 and associated summary plots in Figure 6. Data were limited for all muscles.
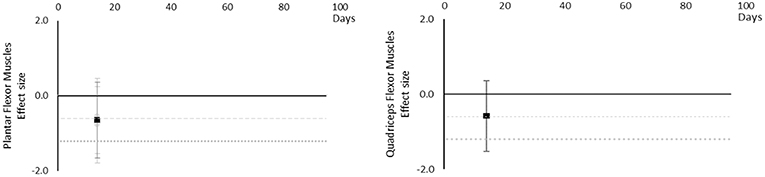
Figure 6. Effect size plots for contractile work capacity over time from individual (gray) and average (black) effect sizes at each time point, with 0.6 (dotted line) and 1.2 (dashed line) effect magnitudes and average effect trend line overlaid.
Muscle thickness decreased over time. Moderate and large effects became apparent by 14 days. There were very little data for Plantar Flexor, Dorsi Flexor and Quadriceps muscles, showing Dorsi Flexor muscles reached a moderate effect by 35 days and only Plantar Flexor and Quadriceps muscles reached a large effect by 35 days. Internal Oblique muscle reached a moderate effect at 14 days. Erector Spinae muscle was similar to Internal Oblique muscle, but only reached a borderline moderate effect within the available data. Upper limb muscles data only included Biceps Brachii muscle thickness, reaching a moderate effect by 35 days. The breakdown of individual muscle thickness effects per muscle is available in Supplementary Table 5 and associated summary plots in Figure 7.
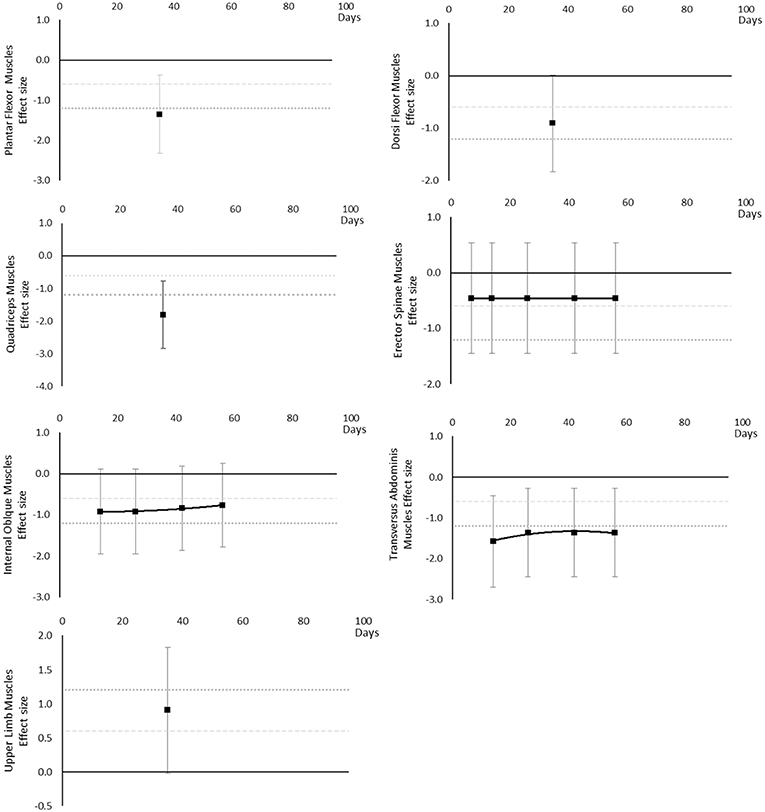
Figure 7. Effect size plots for muscle thickness over time from individual (gray) and average (black) effect sizes at each time point, with 0.6 (dotted line) and 1.2 (dashed line) effect magnitudes and average effect trend line overlaid.
Peak power decreased over time. Large effects became apparent by 56 days for jump power and 62 days for Plantar Flexor and Quadriceps muscles. There was insufficient data to determine a time point for when any moderate effects were reached. The breakdown of individual peak power effects per outcome is available in Supplementary Table 6 and associated summary plots in Figure 8.
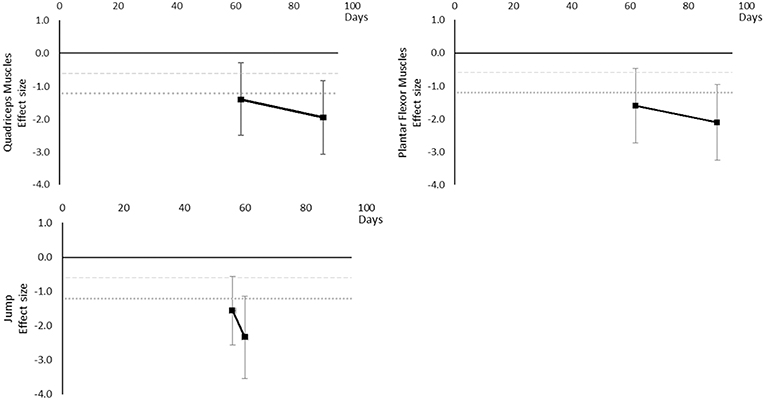
Figure 8. Effect size plots for peak power over time from individual (gray) and average (black) effect sizes at each time point, with 0.6 (dotted line) and 1.2 (dashed line) effect magnitudes and average effect trend line overlaid.
Muscle activity (via electromyography) generally decreased over time, however a transient increase was seen in Plantar Flexor, Dorsi Flexor and Quadriceps muscles and only at 20 days. In Plantar Flexor and Quadriceps muscles, muscle activity decreased again after 20 days, there were no data for Dorsi Flexor muscles beyond 20 days to establish a post 20 day trend. Moderate effects were apparent in upper limb muscle groups by 15 days but not until 90 days for Dorsi and Plantar Flexor muscles which were the only muscles with data at the 90 day point. The breakdown of individual activity effects per muscle is available in Supplementary Table 7 and associated summary plots in Figure 9.
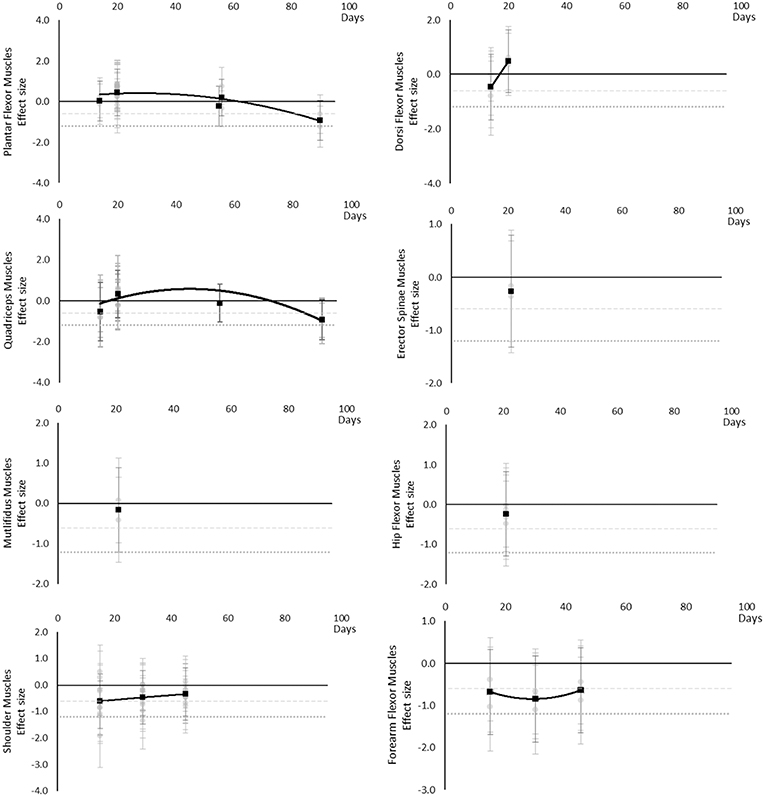
Figure 9. Effect size plots for EMG muscle activity over time from individual (gray) and average (black) effect sizes at each time point, with 0.6 (dotted line) and 1.2 (dashed line) effect magnitudes and average effect trend line overlaid.
Maximal voluntary contraction during one repetition maximum decreased over time except for other upper limb outcomes that remained mostly unchanged as far as data were available up to 45 days. Moderate effects became apparent by 7 days and large effects by 35 days. Other lower limb outcomes that included maximal isometric force during supine squat, hip extensor force and legs total work never reached a large effect, but had no data available beyond 35 days. The breakdown of individual MVC during one repetition maximum effects per muscle is available in Supplementary Table 8 and associated summary plots in Figure 10. The polynomial trend for Hamstring muscles appeared to be unsafe after 20 days.
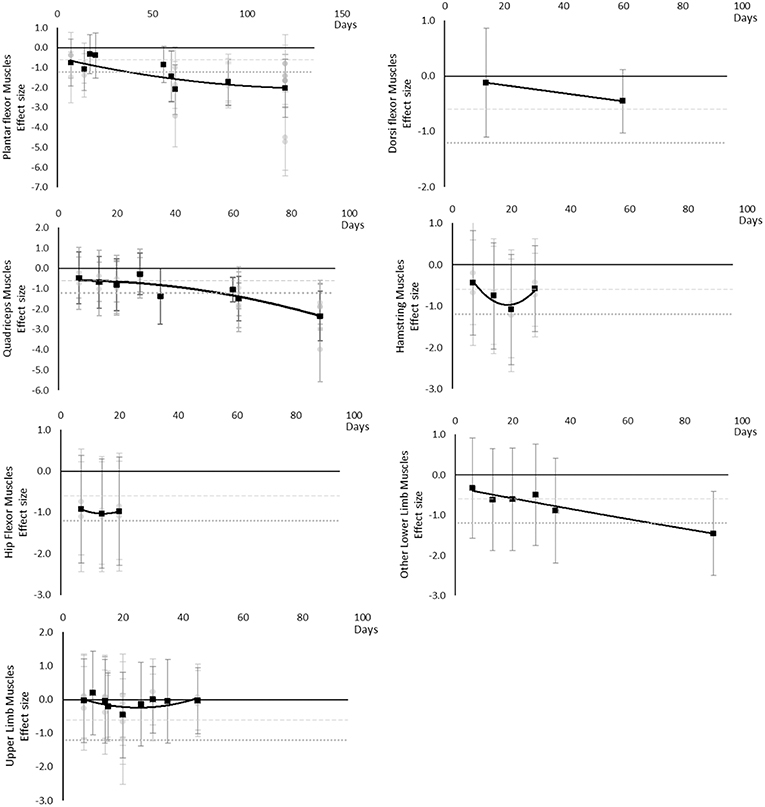
Figure 10. Effect size plots for MVC during one rep max over time from individual (gray) and average (black) effect sizes at each time point, with 0.6 (dotted line) and 1.2 (dashed line) effect magnitudes and average effect trend line overlaid.
Power decreased over time. Moderate effects became apparent by 7 days and large effects by 20 days, although these were only seen in the Quadriceps muscle data. Hamstring, Hip Flexor, and upper limb muscles that included elbow flexors and extensors reached moderate effects by 20 days. Plantar Flexors never reached a moderate effect but data were only available at 14 days. Other trunk muscles included trunk flexors and extensors tested in combination within functional movements. The breakdown of individual power effects per muscle is available in Supplementary Table 9 and associated summary plots in Figure 11.
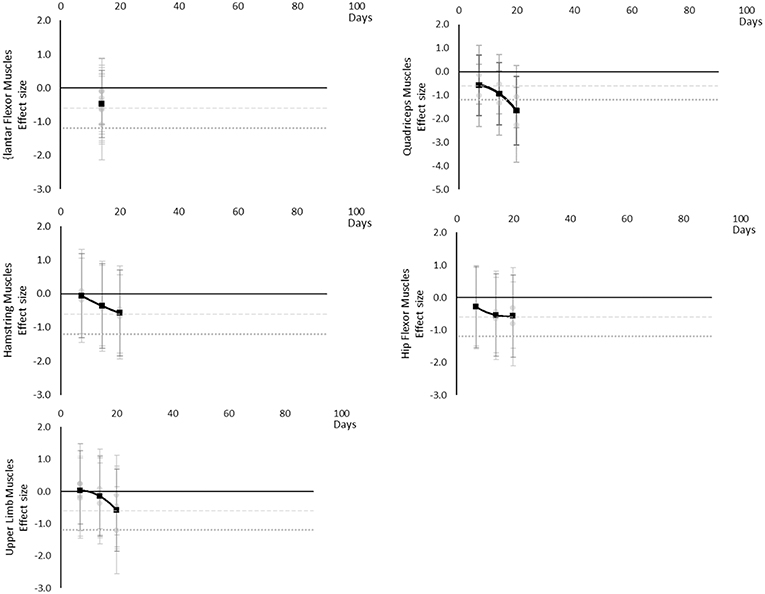
Figure 11. Effect size plots for power over time from individual (gray) and average (black) effect sizes at each time point, with 0.6 (dotted line) and 1.2 (dashed line) effect magnitudes and average effect trend line overlaid.
Performance based outcomes all worsened over time, as although sit to stand, balance, and sprint time outcomes all had positive effects, this was considered a worsening effect within these measures. Endurance reached a large effect by 14 days, jumping a moderate effect at 42 days and large by 44 days, sit to stand and balance reached large effects by 60 days and sprint time by 62 days. Data for most outcomes were only available for one time point and so trends over time for individual outcomes are not able to be determined. The breakdown of individual performance based effects per outcome is available in Supplementary Table 10 and associated summary plots in Figure 12. It should be noted that while these outcomes are grouped as being performance based for this review, they may differ and each individual measure should be considered on its own merit.
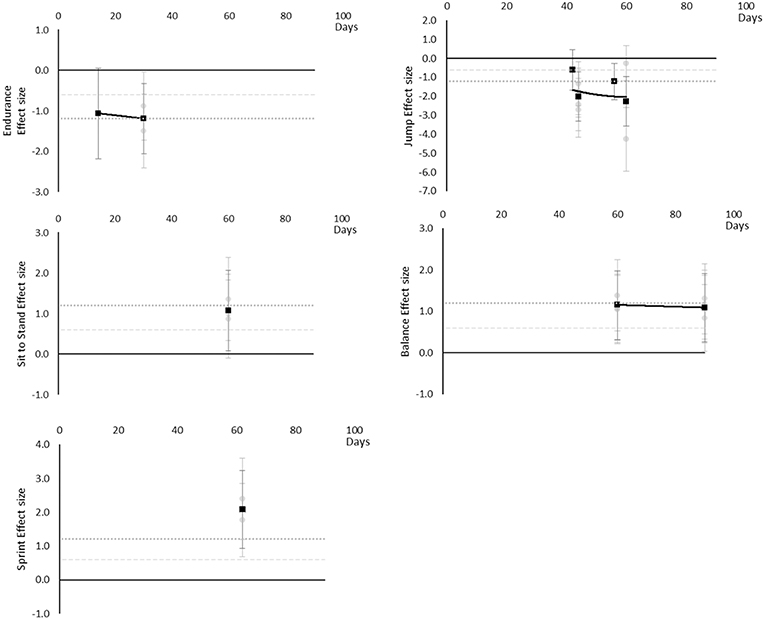
Figure 12. Effect size plots for performance based over time from individual (gray) and average (black) effect sizes at each time point, with 0.6 (dotted line) and 1.2 (dashed line) effect magnitudes and average effect trend line overlaid.
The main finding of the review was that muscle cross-sectional area, volume, shape, size, activity, power, performance, torque, and force-based outcomes, at either regional or global level, all decline over time, based on the current evidence base. Moderate effects became apparent in the following order: power and MVC during one repetition maximum (7 days), followed by volume, cross sectional area, torques and strengths, contractile work capacity, thickness and endurance (14 days), then muscle activity (15 days). Large effects became apparent in the following order: volume, cross sectional area (28 days) torques and strengths, thickness (35 days), and peak power (56 days). No large effects were found for muscle activity. There were limited data for contractile work capacity and no large effects were apparent. In general, lower limb and trunk muscles appeared to decline more rapidly than upper limb muscles. Locomotion muscles such as Plantar Flexor and Quadriceps muscles also generally appeared to decline more rapidly than other muscles groups and with larger effect sizes.
Human spaceflight missions differ in duration, so results have to be placed into the context of mission profiles and operationally important considerations. Operationally, performance-related measures such as power, MVC, torques and strengths are considered most critical. In terms of mission profiles, typical ISS missions involve approximately 180 days in μG (Bryant et al., 2017). The provision of time for exercise CM is mandated for these missions and developments have led to improved efficacy over the lifetime of ISS (Trappe et al., 2009; Ploutz-Snyder, 2013; Hackney et al., 2015). Assuming that the rate of change during bed rest is reasonably transferable to that experienced in μG, the results of this systematic review suggest that large effects would be apparent within a 180 day ISS mission if no exercise CM were employed. That ISS astronauts are able to complete missions without problems from muscle deterioration and successfully return to Earth may provide some level of evidence with which to judge current countermeasures as effective. However, the focus of this review is exploration beyond Low Earth Orbit. Lunar and Martian (exploration) mission profiles were defined in the HUMEX study (Horneck et al., 2006) that modeled exploration mission durations including transit times in μG and planetary stay times in low (<1 G) gravity (Figure 13). HUMEX defined three scenarios, including a Lunar mission with a 180 day surface stay (Horneck et al., 2003) and two Mars missions with either a 30 or 400 day surface stay (Horneck et al., 2006). In HUMEX, inter planetary transit time in μG was 5 days for Lunar missions and 203–213 days for Mars.
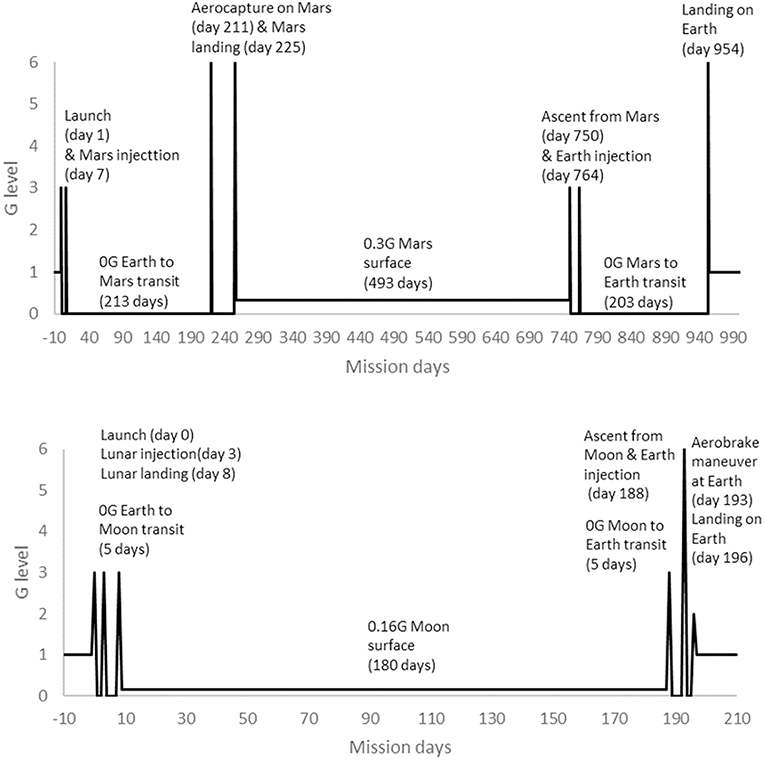
Figure 13. Mission profiles for 180 day surface stay Lunar mission (Bottom) and 493 day surface stay Mars mission (Top), adapted from HUMEX (Horneck et al., 2003, 2006).
It is clear from the findings of this review that changes in muscle outcomes, including performance related measures, with large effects would be observed if no CM were performed during a 200+ day transit to Mars. A risk assessment (Gernand, 2004) has highlighted that decreased muscle mass, strength, and endurance is likely to lead to inability to complete mission critical tasks such as exiting a spacecraft on landing, performing strenuous extra vehicular activity and being functional during increased Gz loading on non-Earth planetary surfaces where a landing support and rehabilitation team may not be available. Therefore, effective CM to prevent muscle deterioration are likely going to be required for Mars missions unless absolute strength mission requirements can be reduced or eliminated, to mitigate risks of crews being unable to perform mission critical tasks and continue to function safely on arrival at Mars. However, based on the occurrence of large effect sizes in the present results only after 28–35 days, exercise CM “holidays” might be considered during Mars transits/orbits to save resources if agencies were confident that moderate changes in muscle performance could be reversed using in-flight exercise equipment and prescriptions.
The results of this review suggest that exercise CM might not be required during a 5 day Lunar transit period, as moderate effects on muscle are not likely to be apparent until 7 days. The initial changes in power and MVC might not be functionally limiting enough to risk mission success, compared to muscle size, strength, and endurance effects that do not reach a moderate size until 14 days. Therefore, further investigation of any effects within the expected Earth-Lunar transit period, considered against minimal clinically worthwhile and mission critical magnitude changes, may be useful to confirm this finding. As a Lunar landing may occur at 8 days in the HUMEX models, the pre-flight strength of crew and the absolute strength and functional requirements of Lunar landing activities would need to be considered when deciding whether or not employ exercise CM prior to attempting a landing. While not employing exercise CM might be considered for the Earth-Lunar transit period, a recent systematic review of biomechanical responses to reduced gravity (Richter et al., 2017) showed that exercise CM would likely be needed during stays on the planetary surfaces of both Moon (0.16 g) and Mars (0.38 g). As time in both μG and low gravity accumulates over the entire mission duration (196-d in total for the HUMEX model), exercise CM are also likely to be needed during the return to Earth transit. However, not using exercise CM on the return transit might be considered if key muscle outcomes could be maintained at, or restored to, pre-mission levels by the end of a Lunar surface stay. Based on the occurrence of large effect sizes in the present results, in an off-nominal situation, such as an emergency, a longer period, possibly up to around 30 days, without exercise CM might be considered if the risks of moderate-large effects can be managed in some other way, for example, knowing support and a full rehabilitation programme are available at the destination arrival site. As with Mars missions, for long Lunar orbital missions with extended periods in μG, an exercise CM “holiday” of the same duration might be considered if agencies were confident that moderate changes in muscle performance could be reversed in-flight.
An individual with a relatively lower muscle outcome measure may be more susceptible to experiencing a negative functional impact of negative changes in these outcomes compared to someone with greater initial measures. It is expected that most missions will require an absolute (minimal) level of strength to achieve mission critical tasks such as donning/doffing and standing up/moving whilst wearing a space suit in low gravity, hatch opening, and pulling/dragging a fellow crew member wearing a space suit during an emergency. The absolute level is defined as the precise required strength outcome in raw units to achieve a task, as opposed to considering relative changes with effect size or percentage changes. A relative (%) reduction in strength will make all tasks with an absolute strength requirement more challenging for all individuals, but the biggest impact will be felt by those who have a lower initial level of absolute strength. For example, a strong individual might be able to lose 30% of their pre-flight strength and still comfortably achieve a mission critical task (and also still be stronger than a weaker individual was prior to flight), whereas a weaker individual might already be close to their physical limit during this task without any deconditioning. Operationally, having an estimate of the most rapid possible rate of change in muscle outcomes may be useful in the case of a crew member with low pre-flight absolute strength, or an individual highly susceptible to μG adaptation. In the present study, the most extreme negative value within the confidence interval for each outcome provides an estimate of the most extreme worst likely true value that might be encountered with exposure to μG. Based on this estimation, the results of this analysis suggest that the change experienced by an individual astronaut might reach a large effect size in some muscles within a 7 day lunar transit period for volume, cross sectional area, contractive work capacity, thickness, power, and MVC. However, the confidence intervals are wide due to the small sample sizes across the current evidence base, so this estimate should be treated with caution as it may be exaggerated. Individual effects are difficult to determine in a transferable way to the true population from the data currently available or from individual case studies. Ideally, a population selected for their increased susceptible to unloading/μG-induced muscular adaptation should be studied in a long-duration μG analog to produce a representable average effect that could be transferred to the true population with more reasonable confidence. Until such data are available, estimating the maximum rate of decline in an individual in response to μG exposure of such a duration will remain difficult. In addition, consideration would also be needed should an individual be selected to perform some tasks in a mission that are not considered mission critical, but are essential to other mission goals. It may be that checking for susceptibility to outcomes that are linked more strongly to mission success is checked and made part of astronaut eligibility screening, it could also be any more susceptible mission critical individuals undergo more rigorous preflight and inflight training protocols or consider use of other more removed countermeasures beyond the scope of this review.
Exercise countermeasure development may want to consider focusing on those which might best address the more susceptible outcome changes in this review, volume, cross sectional area, contractive work capacity, thickness, power, and MVC while also ensuring any proposed exercises are tailored to tasks considered critical, such as donning/doffing and standing up/moving whilst wearing a space suit in low gravity, hatch opening, and pulling/dragging a fellow crew member wearing a space suit. The impact of any chosen exercise types on future spacecraft exercise hardware would also need further consideration. Future research should consider identifying exercise countermeasures that would best address the more susceptible outcomes and be feasible with any technical constraints of new space vehicles planned for use within Moon and Mars missions.
As CM are likely to be needed on the return trip from both Moon and on the journeys to and from Mars, such CM will need developing. Countermeasure devices should support lower limb and trunk muscle exercise as these decline earlier than other body regions and are essential for locomotion and for spinal function on return to G loading (Bamman, 1996; Pavy-Le Traon et al., 2007; Evetts et al., 2014; Stokes et al., 2016; Winnard et al., 2017). Based on the results of the present study, if exercise CM are used during very short missions/transits (e.g., up to 7 days), devices should support exercise that maintains power and maximal force production, as moderate effects appeared early in these performance outcomes. Up to around 15 days, exercise CM might need only to prevent moderate size effects in muscle. Consideration could be made around if lower intensity exercise, or potentially a break in countermeasures would be safe. However, once μG exposure duration reaches around 30 days and above, large effects in muscle will likely need to be managed and this would likely require devices/prescriptions optimized within the constraints of the vehicle/habitat.
This pattern fits current European Space Agency (ESA) ISS Long Duration Mission (LDM) exercise prescriptions (Petersen et al., 2016) that include an initial 20-day familiarization phase to allow crew to adjust to exercise in μG and minimize injury risk, in which exercise intensity is moderate compared to pre-flight maximum capacity. However, as there is currently no systematic measurement of muscle performance in-flight, the impact of this period of lower intensity exercise on overall changes in muscle during an LDM is unknown. It is also unclear if crew members may have had better results at the end of a mission had they begun exercising more intensely earlier in the mission. Following the 20-day familiarization period, exercise prescriptions are increased in intensity to 80%+ maximal capacity. In the final 15–30 days of a long duration mission (>49 days) intensity is kept high, but focus on resistance and running exercises. In flight resistance exercise prescriptions for European astronauts also focus on lower limb muscles (squats, heel raises, deadlifts) ESA has found are most susceptible to μG induced changes from (non-systematic) measures that have been taken (Petersen et al., 2016). Similar exercise prescriptions, focusing on lower limb muscles and maintaining outcomes already highlighted in this review, might form a good basis for initial planning for any exercises required for Lunar and Mars missions. Additionally, research on preventing deconditioning of older adults might also be useful as preventing loss of power in functional lower limb muscles is important in this population and simple loading exercises have shown helpful in this context (Byrne et al., 2016). It should also be noted, however, that a systematic review of in-flight CM for maintaining spinal health in μG found that, while resistance based exercises helped prevent muscle changes, they did not help with non-muscle outcomes such as spinal morphology (Winnard et al., 2017). Moreover, a number of other physiological systems/organs also adapt to μG, including bone and aerobic capacity, but the efficacy of resistance exercise during gravitational unloading on them is unknown as systematic reviews similar to the present study have yet to be performed. Therefore, while the recommendations of this review are expected to help plan CM for muscle changes, additional holistic consideration of other physiological systems will likely be required. Finally, any CM development for exploration missions will also have to consider constraints of space vehicles that will be used, such as available physical space, limited number of devices that can be included in the space craft, consumables, generation of heat, carbon dioxide, and vibration, which are likely to be more restricted than the ISS (Hackney et al., 2015). Before any pause in exercise countermeasures could be taken, the results of this review would need to be validated in microgravity and ideally actual astronauts through experimental studies. No such published studies of astronauts not performing exercise to document muscle changes over the time frames considered in this review was found. Space agencies and researchers would also need to consider the ethical implications and acceptability of any such study.
There were missing and limited data across all the outcome measure subgroups, and gaps in the evidence base were clearly shown in the results tables. There was a lack of standardized time points at which measures were recorded, even across studies reporting the same outcome measures. Limited data were found repeatedly for Gluteal and Hip Flexor muscles across several outcome measure subgroups. Data were lacking for contractile work capacity, muscle thickness, and peak power outcome measures where further research is recommended to validate the trends seen over time in the current evidence base. No patient reported outcome measures have been reported across the bed rest studies, meaning it is unclear how relevant the measures are to patients (in this case astronauts) (Dawson et al., 2010; Nelson et al., 2015). In addition, only seven out of the 75 analyzed studies considered functional performance based outcomes that are more likely to be directly relevant to astronauts. While strong efforts on behalf of space agencies to standardize bed rest studies has occurred including listing required surrogate measures (Sunblad et al., 2014), patient reported outcomes such as their ability to perform a task felt of value to them, remain missing on the whole. It is recommended that the scientific and space medical operations communities agree on set times points at which outcome measures should be tested to enable easier comparisons across studies and for overall trends to be more easily identifiable. While ESA requires agency bed rest studies to be performed to set standards, it might be beneficial to consider running a specific initiative in the wider Aerospace Medicine field to establish core outcome sets relevant to space medicine operations that should then be used in all associated research. This could be based on recommending use of standard space agency developed tests such as functional and Field Test parameters developed by NASA and Russia The Core Outcome Measures in Effectiveness Trials (COMET) is an example initiative that facilitates development and application of core outcome sets and research has been published on how to reach consensus using such an approach (Prinsen et al., 2014). It is also recommended that patient reported outcome measures, and increased reporting of functional performance based outcome measures, be included in both future research and space medical operations to ensure that outcome measures are assessing phenomena that are relevant to astronauts. This recommendation echoes a recent European Space Agency topical team report that also found patient reported outcome measures not being used in space medicine research and operations (Stokes et al., 2016). The report recommended the use of such outcomes and suggested potential for development of new such outcome measures specifically for space medicine with operational space medicine input to ensure relevance across research and clinical settings. It would be of further benefit if clinically worthwhile, or concerning, changes were defined for key outcome measures, so that results can be placed into a clinically meaningful context. Reporting results based on clinically meaningful raw changes would likely be more informative to operational decisions compared to the more mechanistic null hypothesis tests, effect size or percentage change measures currently used. The high risk of bias and lack of core outcome measure sets means that the conclusions reached by this review should be treated with some caution. A bed rest study could be performed to confirm the findings of this review. If performed, the study would ideally be a randomized controlled trial comparing inactive bed rest with controls not performing bed rest but controlled for all potential confounding factors. For example, exercise and any other types of muscle interventions would need to be strictly controlled for the period of the study. The bed rest element would ideally comply with all aspects of the AMSRG bed rest quality tool to improve transferability of results to astronauts (Winnard and Nasser, 2017b). Finally, all modifiable risk of bias elements would need controlling and a risk of bias tool for randomized controlled trials, such as provided by Cochrane (Higgins et al., 2011), could be used as a guide to check what elements need to be controlled to minimize bias risks.
Most of the studies scored four on the bed rest tool, with no studies scoring a full seven points, although 13 studies scored six. The reasons for marking studies down was mostly due it being unclear if criteria had been met rather than clearly failing a point. The most common unclear criteria was related to restricted sunlight exposure followed by ensuring a fixed daily routine. The high risk of bias results were most commonly caused by not clearly showing how confounding factors were managed and providing adequate description of participation. The participation domain considers participant eligibility criteria, source of participants, baseline descriptions, description of sampling frame and recruitment, description of period and place of recruitment, and inclusion/exclusion criteria (Hayden et al., 2013). The sunlight exposure criteria has more impact on bone outcomes (Holick, 2004) due to its role in vitamin D levels within human bone homeostasis (Tarver, 2013) so might not be a large concern for the muscle outcomes presented in this review. However, it is recommended that future bed rest protocol information be clear on all the criteria assessed on the bed rest quality tool and especially on the fixed daily routine and restricted sunlight points, while also ensuring that information is provided about control of confounding factors to help reduce risk of bias and participation considerations. In addition, studies that assess time sensitive outcomes, such as muscle (in which the results of this review show effects of deconditioning can occur by 7 days), should report any potential for pre-bed rest deconditioning during familiarization and baseline measure periods and any attempts to control for this. There is potential that participants who are admitted to bed rest facilities several days in advance for control measures could decondition within this period. Some studies state including an ambulatory control period, but none report details of what this involved or if there was potential for pre-bed rest deconditioning to influence results.
There was some asymmetry in the funnel plot showing potential publication bias toward studies reporting a decrease in muscle outcomes. However, there were studies present on the increasing side of the plot, so the risk is not likely to be high. In addition, it is expected that many of the muscle outcomes would decrease during a period of inactivity such as bed rest, therefore, it not surprising most studies reported decreases. Therefore, while it appears a risk of reporting bias may exist, the presence of some studies reporting increases and the expected pattern of more decreases being reporting suggest this finding should be treated with caution and the potential risk is likely to be low.
This review only considered muscle outcomes. Spaceflight is known to affect many more human physiological systems including bone, cardiovascular and vestibular (Pavy-Le Traon et al., 2007). These results alone, therefore, only provide a muscle based perspective. As typical meta-analysis statistics assume two independent groups (Higgins and Green, 2011), a more basic effect size analysis without these assumptions had to be used due to only considering changes over time in the control group of each study. Therefore, some caution should be taken as the mean effect sizes are not weighted and heterogeneity scores are not available. However, as most studies had small sample sizes, a weighted result is not expected to produce largely different results. Additionally, the findings of this review appear to match actual spaceflight findings and patterns, such as the European Space Agency exercise prescription for long duration missions that performs lower intensity exercises for the first 20 days. While actual measures are not taken during flight, the 20 days has so far not resulted in any mission critical functional decline (Petersen et al., 2016). The 20 day period would fit with the findings of this review that only moderate effects would be expected before 28 days and gives some partial validation, from actual astronaut data, to the findings of this review. The review is also broad and, in places, the variation around the outcomes appears large suggesting heterogeneity of data may be high, although the large intervals could also be due to the small sample sizes that were a common feature of the included bed rest studies. Due to the broad data set that summarizes the entire muscle evidence base, additional data on pre-bed rest fitness of participants was not extracted for analysis. While studies were selected that had healthy adults undergoing spaceflight simulation bed rest, individual physical condition was not considered beyond this. Therefore, there may be some limitations to the transferability of astronauts who undergo training with space agencies prior to missions. However, a broad summary of the entire current evidence base with basic effect size analysis was the best way to try to address the overarching research questions, look for high level trends, and present a summary of the current state of the complete evidence base.
The results of this review suggest that moderate effects on a range of muscle function parameters may occur within 7–14 days of unloading, with large effects within 35 days. Combined with identification of muscle performance requirements for future exploration mission tasks, these data, may support the design of CM programmes to optimize their efficient use without compromising crew safety and mission success. However, the data suggests CM are likely to still be needed for longer transit/orbital periods of 14–28+ days, such as a prolonged Lunar orbit, deep space exploration, or a Mars mission, as moderate effects occur between 7–14 days and large effects by 28 days for most muscle outcomes. However, if large effect sizes occur only after 28–35 days, to save resources, space agencies might consider short missions without exercise CM, or fixed periods of abstinence during longer μG exposures, if they could be confident that moderate changes in muscle performance could be reversed in-flight. Finally, several research gaps are highlighted for future bed rest studies in which standardized time points for measurements should be used and clear information provided on sunlight exposure control, fixed daily routine, and control of any confounding factors.
1Akima et al., 2000; 2Akima et al., 2003; 3Akima et al., 2005; 4Akima et al., 2007; 5Alkner and Tesch, 2004; 6Alkner et al., 2016; 7Arbeille et al., 2009; 8Bamman et al., 1997; 9Belavy et al., 2007a; 10Belavy et al., 2008; 11Belavy et al., 2009a; 12Belavy et al., 2009b; 13Belavy et al., 2010a; 14Belavy et al., 2011a; 15Belavy et al., 2011c; 16Belavy et al., 2011b; 17Belavy et al., 2013; 18Belavy et al., 2017; 19Berg et al., 1997; 20Berg et al., 2007; 21Berry et al., 1993; 22Buehring et al., 2011; 23Caiozzo et al., 2009; 24Cescon and Gazzoni, 2010; 25Convertino et al., 1989; 26de Boer et al., 2008; 27Dudley et al., 1989; 28Duvoisin et al., 1989; 29Ellis et al., 1993; 30English et al., 2011; 31English et al., 2016; 32Ferrando et al., 1995; 33Ferretti et al., 2001; 34Fu et al., 2016; 35Funato et al., 1997; 36Gast et al., 2012; 37Germain et al., 1995; 38Greenleaf et al., 1983; 39Greenleaf et al., 1989; 40Greenleaf et al., 1994b; 41Holguin et al., 2007; 42Holt et al., 2016; 43Kawashima et al., 2004; 44Koryak, 1995a; 45Koryak, 1996; 46Koryak, 1998a; 47Koryak, 1998b; 48Koryak, 1999; 49Koryak, 2002; 50Koryak, 2010; 51Koryak, 2014; 52Kouzaki et al., 2007; 53Krainski et al., 2014; 54LeBlanc et al., 1988; 55Lee et al., 2014; 56Macias et al., 2007; 57Miokovic et al., 2011; 58Miokovic et al., 2012; 59Miokovic et al., 2014; 60Muir et al., 2011; 61Mulder et al., 2006; 62Mulder et al., 2007; 63Mulder et al., 2008; 64Mulder et al., 2009bb; 65Mulder et al., 2009a; 66Narici et al., 1997; 67Pisot et al., 2008; 68Portero et al., 1996; 69Reeves et al., 2002; 70Rittweger et al., 2005; 71Rittweger et al., 2013; 72Schneider et al., 2016; 73Shinohara et al., 2003; 74Trappe et al., 2001; 75Trappe et al., 2007.
1Belavy et al., 2007b; 2Belavy et al., 2010b; 3Belavy et al., 2012; 4Biolo et al., 2008; 5Cavanagh et al., 2016; 6Shenkman et al., 1997; 7Amorim et al., 2006; 8Rittweger and Felsenberg, 2009; 9Koriak Iu, 2010; 10Koriak Iu, 2012; 11Koriak Iu, 2013; 12Koryak, 1994; 13Bamman and Caruso, 2000; 14Bamman, 1996; 15Felsenberg et al., 2009; 16Ferretti, 1997; 17Greenleaf et al., 1994a; 18Grogor'eva and Kozlovskaia, 1987; 19Guo et al., 2001; 20Hargens et al., 2003; 21Judith Hayes et al., 1992; 22Ito et al., 1994; 23Jaweed et al., 1995; 24Koryak, 1995b; 25Kozlovskaia et al., 1984; 26LeBlanc et al., 1992; 27LeBlanc et al., 1997; 28Liu et al., 2003; 29Macias et al., 2008; 30Meuche et al., 2006; 31Meuche et al., 2005; 32Milesi et al., 1997; 33Miyoshi et al., 2001; 34Moriggi et al., 2010; 35Mulder et al., 2011; 36Netreba et al., 2004; 37Scott et al., 2017.
AW: initial concept ideas, protocol planning and drafting, search screening, analyzing, and drafting all manuscript versions. JS: methods advice, protocol drafting, and approving final draft. NW: data extraction, analysis, and drafting final version. MV: protocol planning, search screening, data analysis, drafting text, and checking final version. NC: protocol planning, search screening, methods advice, manuscript drafting, and approving final version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2019.01046/full#supplementary-material
Adams, G. R., Caiozzo, V. J., and Baldwin, K. M. (2003). Skeletal muscle unweighting: spaceflight and ground-based models. J. Appl. Physiol. 95, 2185–2201. doi: 10.1152/japplphysiol.00346.2003
Akima, H., Katayama, K., Sato, K., Ishida, K., Masuda, K., Takada, H., et al. (2005). Intensive cycle training with artificial gravity maintains muscle size during bed rest. Aviat. Space Environ. Med. 76, 923–929.
Akima, H., Kubo, K., Kanehisa, H., Suzuki, Y., Gunji, A., and Fukunaga, T. (2000). Leg-press resistance training during 20 days of 6 degrees head-down-tilt bed rest prevents muscle deconditioning. Eur. J. Appl. Physiol. 82, 30–38. doi: 10.1007/s004210050648
Akima, H., Ushiyama, J., Kubo, J., Tonosaki, S., Itoh, M., Kawakami, Y., et al. (2003). Resistance training during unweighting maintains muscle size and function in human calf. Med. Sci. Sports Exerc. 35, 655–662. doi: 10.1249/01.MSS.0000058367.66796.35
Akima, H., Ushiyama, J. I., Kubo, J., Fukuoka, H., Kanehisa, H., and Fukunaga, T. (2007). Effect of unloading on muscle volume with and without resistance training. Acta Astronaut. 60, 728–736. doi: 10.1016/j.actaastro.2006.10.006
Alkner, B. A., Norrbrand, L., and Tesch, P. A. (2016). Neuromuscular adaptations following 90 days bed rest with or without resistance exercise. Aerospace Med. Hum. Perform. 87, 610–617. doi: 10.3357/AMHP.4383.2016
Alkner, B. A., and Tesch, P. A. (2004). Knee extensor and plantar flexor muscle size and function following 90 days of bed rest with or without resistance exercise. Eur. J. Appl. Physiol. 93, 294–305. doi: 10.1007/s00421-004-1172-8
Amorim, F. T., Schneider, S. M., Lee, S. M. C., Boda, W. L., Watenpaugh, D. E., and Hargens, A. R. (2006). Twins bed rest project: LBNP/exercise minimizes changes in lean leg mass, strength and endurance. Med. Sci. Sports Exerc. 38, S389–S390. doi: 10.1249/00005768-200605001-02521
AMSRG (2018) Aerospace Medicine Systematic Review Group Data Extraction Form. Available online at: http://aerospacemed.rehab/methods-guidance (accessed August 7, 2019).
Arbeille, P., Kerbeci, P., Capri, A., Dannaud, C., Trappe, S. W., and Trappe, T. A. (2009). Quantification of muscle volume by echography: comparison with mri data on subjects in long-term bed rest. Ultrasound Med. Biol. 35, 1092–1097. doi: 10.1016/j.ultrasmedbio.2009.01.004
Baker, E. S., Barratt, M. R., and Wear, M. L. (2008). Human response to space flight, in Principles of Clinical Medicine for Space Fligh, eds Barratt, M. R. P. (New York, NY: Springer, 27–57.
Bamman, M. M. (1996). Effects of Traditional Resistance Exercise During Bed Rest on Locomotor Muscle. Gainesvill, FL: University of Florida.
Bamman, M. M., and Caruso, J. F. (2000). Resistance exercise countermeasures for space flight: implications of training specificity. J. Strength Cond. Res. 14, 45–49. doi: 10.1519/00124278-200002000-00008
Bamman, M. M., Hunter, G. R., Stevens, B. R., Guilliams, M. E., and Greenisen, M. C. (1997). Resistance exercise prevents plantar flexor deconditioning during bed rest. Med. Sci. Sports Exerc. 29, 1462–1468. doi: 10.1097/00005768-199711000-00012
Belavy, D. L., Armbrecht, G., Gast, U., Richadrson, C., Hides, J. A., and Felsenberg, D. (2010a). Countermeasures against lumbar spine deconditioning in prolonged bed rest: resistive exercise with and without whole body vibration. Appl. Physiol. 109, 1801–1811. doi: 10.1152/japplphysiol.00707.2010
Belavy, D. L., Armbrecht, G., Richardson, C. A., Felsenberg, D., and Hides, J. A. (2011a). Muscle atrophy and changes in spinal morphology: is the lumbar spine vulnerable after prolonged bed-rest? Spine. 36, 137–145. doi: 10.1097/BRS.0b013e3181cc93e8
Belavy, D. L., Bansmann, P. M., Bohme, G., Frings-Meuthen, P., Heer, M., Rittweger, J., et al. (2011b). Changes in intervertebral disc morphology persist 5 mo after 21-day bed rest. J. Appl. Physiol. 111, 1304–1314. doi: 10.1152/japplphysiol.00695.2011
Belavy, D. L., Gast, U., and Felsenberg, D. (2017). Exercise and transversus abdominis muscle atrophy after 60-d bed rest. Med. Sci. Sports Exerc. 49, 238–246. doi: 10.1249/MSS.0000000000001096
Belavy, D. L., Hides, J. A., Wilson, S. J., Stanton, W. F. C, Rittweger, J., et al. (2008). Resistive simulated weightbearing exercise with whole body vibration reduces lumbar spine deconditioning in bed-rest. Spine. 33, 121–131. doi: 10.1097/BRS.0b013e3181657f98
Belavy, D. L., Miokovic, T., Armbrecht, G., and Felsenberg, D. (2013). Hypertrophy in the cervical muscles and thoracic discs in bed rest? J. Appl. Physiol. 115, 586–596. doi: 10.1152/japplphysiol.00376.2013
Belavy, D. L., Miokovic, T., Armbrecht, G., Richardson, C. A., Rittweger, J., and Felsenberg, D. (2009a). Differential atrophy of the lower-limb musculature during prolonged bed-rest. Eur. J. Appl. Physiol. 107, 489–499. doi: 10.1007/s00421-009-1136-0
Belavy, D. L., Miokovic, T., Armbrecht, G., Rittweger, J., and Felsenberg, D. (2009b). Resistive vibration exercise reduces lower limb muscle atrophy during 56-day bed-rest. J. Musculoskelet. Neuronal Interact. 9, 225–235.
Belavy, D. L., Ng, J. K., Wilson, S. J., Armbrecht, G., Stegeman, D. F., Rittweger, J., et al. (2010b). Influence of prolonged bed-rest on spectral and temporal electromyographic motor control characteristics of the superficial lumbo-pelvic musculature. J. Electromyogr. Kinesiol. 20, 170–179. doi: 10.1016/j.jelekin.2009.03.006
Belavy, D. L., Ohshima, H., Bareille, M. P., Rittweger, J., and Felsenberg, D. (2011c). Limited effect of fly-wheel and spinal mobilization exercise countermeasures on lumbar spine deconditioning during 90 d bed-rest in the toulouse LTBR study. Acta Astronaut. 69, 406–419. doi: 10.1016/j.actaastro.2011.05.015
Belavy, D. L., Richardson, C. A., Wilson, S. J., Felsenberg, D., and Rittweger, J. (2007a). Tonic-to-phasic shift of lumbo-pelvic muscle activity during 8 weeks of bed rest and 6-months follow up. J Appl Physiol. (1985) 103, 48–54. doi: 10.1152/japplphysiol.00850.2006
Belavy, D. L., Richardson, C. A., Wilson, S. J., Rittweger, J., and Felsenberg, D. (2007b). Superficial lumbopelvic muscle overactivity and decreased cocontraction after 8 weeks of bed rest. Spine 32, E23–29. doi: 10.1097/01.brs.0000250170.53746.27
Belavy, D. L., Wilson, S. J., Armbrecht, G., Rittweger, J., Felsenberg, D., and Richardson, C. A. (2012). Resistive vibration exercise during bed-rest reduces motor control changes in the lumbo-pelvic musculature. J. Electromyogr. Kinesiol. 22, 21–30. doi: 10.1016/j.jelekin.2011.09.009
Berg, H. E., Eiken, O., Miklavcic, L., and Mekjavic, I. B. (2007). Hip, thigh and calf muscle atrophy and bone loss after 5-week bedrest inactivity. Eur. J. Appl. Physiol. 99, 283–289. doi: 10.1007/s00421-006-0346-y
Berg, H. E., Larsson, L., and Tesch, P. A. (1997). Lower limb skeletal muscle function after 6 wk of bed rest. J. Appl. Physiol. 82, 182–188. doi: 10.1152/jappl.1997.82.1.182
Berry, P., Berry, I., and Manelfe, C. (1993). Magnetic resonance imaging evaluation of lower limb muscles during bed rest–a microgravity simulation model. Aviat Space Environ. Med. 64(3 Pt 1), 212–218.
Biolo, G., Agostini, F., Simunic, B., Sturma, M., Torelli, L., Preiser, J. C., et al. (2008). Positive energy balance is associated with accelerated muscle atrophy and increased erythrocyte glutathione turnover during 5 wk of bed rest. Am. J. Clin. Nutrit. 88, 950–958. doi: 10.1093/ajcn/88.4.950
Brusco, S. (2016). NASA Built a Mini-Exercise Machine for Long, Cramped Space Missions. Medical Design Technology.
Bryant, C., Meza, D., Schoenstein, N., and Schuh, S. (2017). Understanding the international space station crew perspective following long-duration missions through data analytics & visualization of crew feedback, in 8th International Conference on Applied Human Factors and Ergonomics (Los Angeles, CA: NASA Johnson Space Center). doi: 10.1007/978-3-319-60492-3_7
Buehring, B., Belavy, D. L., Michaelis, I., Gast, U., Felsenberg, D., and Rittweger, J. (2011). Changes in lower extremity muscle function after 56 days of bed rest. J. Appl. Physiol. 111, 87–94. doi: 10.1152/japplphysiol.01294.2010
Byrne, C., Faure, C., Keene, D. J., and Lamb, S. E. (2016). Ageing, muscle power and physical function: a systematic review and implications for pragmatic training interventions. Sports Med. 46, 1311–1332. doi: 10.1007/s40279-016-0489-x
Caiozzo, V. J., Haddad, F., Lee, S., Baker, M., Paloski, W., and Baldwin, K. M. (2009). Artificial gravity as a countermeasure to microgravity: a pilot study examining the effects on knee extensor and plantar flexor muscle groups. J. Appl. Physiol. 107, 39–46. doi: 10.1152/japplphysiol.91130.2008
Cavanagh, P. R., Rice, A. J., Novotny, S. C., Genc, K. O., Englehaupt, R. K., Owings, T. M., et al. (2016). Replacement of daily load attenuates but does not prevent changes to the musculoskeletal system during bed rest. Bone Rep. 5, 299–307. doi: 10.1016/j.bonr.2016.10.001
Cescon, C., and Gazzoni, M. (2010). Short term bed-rest reduces conduction velocity of individual motor units in leg muscles. J. Electromyography Kinesiol. 20, 860–867. doi: 10.1016/j.jelekin.2010.03.008
Convertino, V. A., Doerr, D. F., Mathes, K. L., Stein, S. L., and Buchanan, P. (1989). Changes in volume, muscle compartment, and compliance of the lower-extremities in man following 30 days of exposure to simulated microgravity. Aviation Space Environ. Med. 60, 653–658.
Dawson, J., Doll, H., Fitzpatrick, R., Jenkinson, C., and Carr, A. J. (2010). The routine use of patient reported outcome measures in healthcare settings. Br. Med. J. 340:c186. doi: 10.1136/bmj.c186
de Boer, M. D., Seynnes, O. R., di Prampero, P. E., Pisot, R., Mekjavic, I. B., Biolo, G., et al. (2008). Effect of 5 weeks horizontal bed rest on human muscle thickness and architecture of weight bearing and non-weight bearing muscles. Eur. J. Appl. Physiol. 104, 401–407. doi: 10.1007/s00421-008-0703-0
Dudley, G. A., Duvoisin, M. R., Convertino, V. A., and Buchanan, P. (1989). Alterations of the in vivo torque-velocity relationship of human skeletal muscle following 30 days exposure to simulated microgravity. Aviat. Space Environ. Med. 60, 659–663.
Duvoisin, M. R., Convertino, V. A., Buchanan, P., Gollnick, P. D., and Dudley, G. A. (1989). Characteristics and preliminary observations of the influence of electromyostimulation on the size and function of human skeletal muscle during 30 days of simulated microgravity. Aviat. Space Environ. Med. 60, 671–678.
Ellis, S., Kirby, L. C., and Greenleaf, J. E. (1993). Lower extremity muscle thickness during 30-day 6 degrees head-down bed rest with isotonic and isokinetic exercise training. Aviat. Space Environ. Med. 64, 1011–1015.
English, K. L., Lee, S., Loehr, J. A., Ploutz-Snyder, R. J., and Ploutz-Snyder, L. (2015). Isokinetic Strength Changes Following Long-Duration Spaceflight on the ISS. Aerospace Med. Human Perform. 86(12, Suppl), A68–A77. doi: 10.3357/AMHP.EC09.2015
English, K. L., Mettler, J. A., Ellison, J. B., Mamerow, M. M., Arentson-Lantz, E., Pattarini, J. M., et al. (2016). Leucine partially protects muscle mass and function during bed rest in middle-aged adults. Am. J. Clin. Nutr. 103, 465–473. doi: 10.3945/ajcn.115.112359
English, K. L., Ploutz-Snyer, R. J., Crowell, J. B., Cromwell, R. L., and Ploutz-Snyder, L. L. (2011). Gender differences in isokinetic strength after 60 and 90 d bed rest. Med. Sci. Sports Exerc. 43, 822–822. doi: 10.1249/01.MSS.0000402291.80827.ae
Evetts, S. N., Caplan, N., Debuse, D., Lambrecht, G., Damann, V., Petersen, N., et al. (2014). Post space mission lumbo-pelvic neuromuscular reconditioning: a european perspective. Aviation Space Environ. Med. 85, 764–765. doi: 10.3357/ASEM.3943.2014
Felsenberg, D., Belavy, D., Miocovic, T., Armbrecht, G., Beller, G., Gast, U., et al. (2009). Changes of muscle and bone mass and bone marker during simulated weightlessness in exercise and control group- results from berlin bed rest study. Bone 44, S58–S59. doi: 10.1016/j.bone.2009.01.019
Ferrando, A. A., Stuart, C. A., Brunder, D. G., and Hillman, G. R. (1995). Magnetic resonance imaging quantitation of changes in muscle volume during 7 days of strict bed rest. Aviat. Space Environ. Med. 66, 976–981.
Ferretti, G. (1997). The effect of prolonged bed rest on maximal instantaneous muscle power and its determinants. Int. J. Sports Med. 18, S287–S289. doi: 10.1055/s-2007-972728
Ferretti, G., Berg, H. E., Minetti, A. E., Moia, C., Rampichini, S., and Narici, M. V. (2001). Maximal instantaneous muscular power after prolonged bed rest in humans. J Appl Physiol. 90, 431–435. doi: 10.1152/jappl.2001.90.2.431
Foing, B. (2016). Towards A Moon Village: Vision and 964 Opportunities. Vienna: EGU General Assembly.
Fu, A. S., Wang, C. H., Qi, H. Z., Li, F., Wang, Z., He, F., et al. (2016). Electromyography-based analysis of human upper limbs during 45-day head-down bed-rest. Acta Astronaut. 120, 260–269. doi: 10.1016/j.actaastro.2015.12.007
Funato, K., Matsuo, A., Yata, H., Akima, H., Suzuki, Y., Gunji, A., et al. (1997). Changes in force-velocity and power output of upper and lower extremity musculature in young subjects following 20 days bed rest. J. Gravit. Physiol. 4, S22–30.
Gast, U., John, S., Runge, M., Rawer, R., Felsenberg, D., and Belavy, D. L. (2012). Short-duration resistive exercise sustains neuromuscular function after bed rest. Med. Sci. Sports Exerc. 44, 1764–1772. doi: 10.1249/MSS.0b013e318256b53b
Germain, P., Guell, A., and Marini, J. F. (1995). Muscle strength during bedrest with and without muscle exercise as a countermeasure. Eur. J. Appl. Physiol. Occup. Physiol. 71, 342–348. doi: 10.1007/BF00240415
Gernand, J. M. (2004). Risk Assessment and Control Through Countermeasure System Implementation For Long-Term Crew Exposure to Microgravity. Anaheim, CA: IMECE.
Greenleaf, J. E., Bernauer, E. M., Ertl, A. C., Bulbulian, R., and Bond, M. (1994a). Isokinetic strength and endurance during 30-day 6 degrees head-down bed rest with isotonic and isokinetic exercise training. Aviat. Space Environ. Med. 65, 45–50.
Greenleaf, J. E., Bernauer, E. M., Ertl, A. C., Trowbridge, T. S., and Wade, C. E. (1989). Work capacity during 30 days of bed rest with isotonic and isokinetic exercise training. J. Appl. Physiol. 67, 1820–1826. doi: 10.1152/jappl.1989.67.5.1820
Greenleaf, J. E., Lee, L., Ellis, S., Selzer, R. H., and Ortendahl, D. A. (1994b). Leg Muscle Volume During 30-Day 6-Degree Head-Down Bed Rest With Isotonic and Isokinetic Exercise Training. NASA Technical Reports Server.
Greenleaf, J. E., Van Beaumont, W., Convertino, V. A., and Starr, J. C. (1983). Handgrip and general muscular strength and endurance during prolonged bedrest with isometric and isotonic leg exercise training. Aviat. Space Environ. Med. 54, 696–700.
Grogor'eva, L. S., and Kozlovskaia, I. B. (1987). [Effect of weightlessness and hypokinesia on the velocity-strength properties of human muscles]. Kosm. Biol. Aviakosm. Med. 21, 27–30.
Guo, L. G., Guo, Z. F., Xie, J. S., and Wang, L. J. (2001). [Effect of 7 d−6 degrees head-down tilt (HDT) on electromyogram of gastrocnemius and anterior tibialis muscles]. Space Med. Med. Eng. 14, 332–335.
Hackney, K. J., Scott, J. M., Hanson, A. M., English, K. L., Downs, M. E., and Ploutz-Snyder, L. L. (2015). The astronaut-athlete: optimizing human performance in space. J. Strength Cond. Res. 29, 3531–3545. doi: 10.1519/JSC.0000000000001191
Hargens, A. R., and Vico, L. (2016). Long-duration bed rest as an analog to microgravity. J. Appl. Physiol. 120, 891–903. doi: 10.1152/japplphysiol.00935.2015
Hargens, R., Watenpaugh, D., Lee, S., Meyer, R., Macias, B., Tanaka, K., et al. (2003). Exercise Within Lbnp as an Artificial Gravity Countermeasure. NASA Technical Reports Server, NASA.
Hayden, J. A., van der Windt, D. A., Cartwright, J. L., Cote, P., and Bombardier, C. (2013). Assessing bias in studies of prognostic factors. Ann. Intern. Med. 158, 280–286. doi: 10.7326/0003-4819-158-4-201302190-00009
Higgins, J., Altman, D., and Sterne, J. (2011). Chapter 8: Assessing risk of bias in included studies, in Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0, eds Higgins, J. P. T., and Green, S.. The Cochrane Collbaboration. Available online at: http://handbook.cochrane.org (accessed August 7, 2019).
Higgins, J. P. T., and Green, S. (Eds.). (2011). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration. Available online at: www.handbook.cochrane.org (accessed August 7, 2019).
Holguin, N., Muir, J., Evans, H. J., Qin, Y. X., Rubin, C., Wagshul, M., et al. (2007). Mechanical vibrations reduce the intervertebral disc swelling and muscle atrophy from bed rest. in 2007 IEEE 33rd Annual Northeast Bioengineering Conference (Long Island, NY).
Holick, M. F. (2004). Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am. J. Clin. Nutrit. 80, 1678s−1688s. doi: 10.1093/ajcn/80.6.1678S
Holt, J. A., Macias, B. R., Schneider, S. M., Watenpaugh, D. E., Lee, S. M., Chang, D. G., et al. (2016). WISE 2005: aerobic and resistive countermeasures prevent paraspinal muscle deconditioning during 60-day bed rest in women. J. Appl. Physiol. 120, 1215–1222. doi: 10.1152/japplphysiol.00532.2015
Hopkins, W. G., Marshall, S. W., Batterham, A. M., and Hanin, J. (2009). Progressive statistics for studies in sports medicine and exercise science. Med. Sci. Sports Exerc. 41, 3–13. doi: 10.1249/MSS.0b013e31818cb278
Horneck, G., Facius, R., Reichert, M., Rettberg, P., Seboldt, W., Manzey, D., et al. (2003). Humex a study on the survivability and adaptation of humans to long-duration exploratory missions, part I: lunar missions. Adv. Space Res. 31, 2389–2401. doi: 10.1016/S0273-1177(03)00568-4
Horneck, G., Facius, R., Reichert, M., Rettberg, P., Seboldt, W., Manzey, D., et al. (2006). HUMEX, a study on the survivability and adaptation of humans to long-duration exploratory missions, part II: Missions to Mars. Adv. Space Res. 38, 752 -759. doi: 10.1016/j.asr.2005.06.072
Ito, K., Torikoshi, S., Yokozawa, K., Nagano, J., and Suzuki, Y. (1994). Gender differences in muscle strength after 20 days bed rest. J. Gravit. Physiol. 1, P55–56.
Jaweed, M. M., Grana, E. A., Glennon, T. P., Monga, T. N., and Mirabi, B. (1995). Neuromuscular adaptations during 30 days of cast-immobilization and head-down bedrest. J. Gravit. Physiol. 2, P72–73.
Judith Hayes, C., Roper, M. J. L., Augustus Mazzocca, D., John McBrine, J., Linda Barrows, H., Bernard Harris, A., et al. (1992). Eccentric and Concentric Muscle Performance Following 7 Days of Simulated Weightlessness. NASA Technical Reports Server.
Kawashima, S., Akima, H., Kuno, S. Y., Gunji, A., and Fukunaga, T. (2004). Human adductor muscles atrophy after short duration of unweighting. Eur. J. Appl. Physiol. 92, 602–605. doi: 10.1007/s00421-004-1184-4
Koriak Iu, A. (2010). [Neuromuscular responses of the triceps surae muscle to prolonged passive stretch of the foot extensor muscles under conditions of simulated microgravity]. Fiziol Zh. 56, 62–76.
Koriak Iu, A. (2012). [Contraction properties and musculo-tendinous stiffness of the human triceps surae muscle and their change as a result of a long-term bed-rest]. Fiziol Zh. 58, 66–79.
Koriak Iu, A. (2013). [Influence of physical training under conditions of 120-day simulated microgravity on contractile properties and musculo-tendinous stiffness of the triceps surae muscle]. Fiziol Zh. 59, 71–84. doi: 10.15407/fz59.02.071
Koryak, Y. (1995a). Contractile properties of the human triceps surae muscle during simulated weightlessness. Eur. J. Appl. Physiol. Occup. Physiol. 70, 344–350. doi: 10.1007/BF00865032
Koryak, Y. (1995b). Mechanical and electrical adaptation of skeletal muscle to gravitational unloading. J. Gravit. Physiol. 2, P76–79.
Koryak, Y. (1996). Mechanical and electrical changes in human muscle after dry immersion. Eur. J. Appl. Physiol. Occup. Physiol. 74, 133–140. doi: 10.1007/BF00376505
Koryak, Y. (1998b). Effect of 120 days of bed-rest with and without countermeasures on the mechanical properties of the triceps surae muscle in young women. Eur. J. Appl. Physiol. Occup. Physiol. 78, 128–135. doi: 10.1007/s004210050397
Koryak, Y. (1999). The effects of long-term simulated microgravity on neuromuscular performance in men and women. Eur. J. Appl. Physiol. Occup. Physiol. 79, 168–175. doi: 10.1007/s004210050491
Koryak, Y. (2002). DRY immersion induces neural and contractile adaptations in the human triceps surae muscle. Environ. Med. 46, 17–27.
Koryak, Y. (2010). Mechanical responses of the human triceps surae after passive stretching training of the plantarflexors in conditions modulating weightlessness. J. Hum. Kinet. 24, 19–34. doi: 10.2478/v10078-010-0016-3
Koryak, Y. A. (1994). Contractile characteristics of the triceps surae muscle in healthy males during 120-days head-down tilt. (HDT) and countermeasures. J. Gravit. Physiol. 1, P141–143.
Koryak, Y. A. (1998a). Influence of 120-days 6 degrees head-down tilt bed rest on the functional properties of the neuromuscular system in man. Aviat. Space Environ. Med. 69, 766–770.
Koryak, Y. A. (2014). Influence of simulated microgravity on mechanical properties in the human triceps surae muscle in vivo. I: effect of 120 days of bed-rest without physical training on human muscle musculo-tendinous stiffness and contractile properties in young women. Eur. J. Appl. Physiol. 114, 1025–1036. doi: 10.1007/s00421-014-2818-9
Kouzaki, M., Masani, K., Akima, H., Shirasawa, H., Fukuoka, H., Kanehisa, H., et al. (2007). Effects of 20-day bed rest with and without strength training on postural sway during quiet standing. Acta Physiologica 189, 279–292. doi: 10.1111/j.1748-1716.2006.01642.x
Kozlovskaia, I. B., Grigor'eva, L. S., and Gevlich, G. I. (1984). [Comparative analysis of the effect of weightlessness and its model on the velocity-strength properties and tonus of human skeletal muscles]. Kosm. Biol. Aviakosm. Med. 18, 22–26.
Krainski, F., Hastings, J. L., Heinicke, K., Romain, N., Pacini, E. L., Snell, P. G., et al. (2014). The effect of rowing ergometry and resistive exercise on skeletal muscle structure and function during bed rest. J. Appl. Physiol. 116, 1569–1581. doi: 10.1152/japplphysiol.00803.2013
LeBlanc, A., Gogia, P., Schneider, V., Krebs, J., Schonfeld, E., and Evans, H. (1988). Calf muscle area and strength changes after five weeks of horizontal bed rest. Am. J. Sports Med. 16, 624–629. doi: 10.1177/036354658801600612
LeBlanc, A., Rowe, R., Evans, H., West, S., Shackelford, L., and Schneider, V. (1997). Muscle atrophy during long duration bed rest. Int. J. Sports Med. 18(Suppl 4), S283–S285. doi: 10.1055/s-2007-972726
LeBlanc, A. D., Schneider, V. S., Evans, H. J., Pientok, C., Rowe, R., and Spector, E. (1992). Regional changes in muscle mass following 17 weeks of bed rest. J. Appl. Physiol. 73, 2172–2178. doi: 10.1152/jappl.1992.73.5.2172
Lee, S. M., Schneider, S. M., Feiveson, A. H., Macias, B. R., Smith, S. M., Watenpaugh, D. E., et al. (2014). WISE-2005: countermeasures to prevent muscle deconditioning during bed rest in women. J Appl Physiol. 116, 654–667. doi: 10.1152/japplphysiol.00590.2013
Liu, Y. S., Huang, W. F., Liu, X. H., Wang, J., Zhao, D. M., and Wu, X. (2003). [Effects of exercise training during 21 d−6 degrees head down bed rest on dynamic posture equilibrium and motor coordination]. Space Med. Med. Eng. 16, 264–268. doi: 10.14712/23366052.2015.12
Loehr, J. A., Lee, S. M., English, K. L., Sibonga, J., Smith, S. M., Spiering, B. A., et al. (2011). Musculoskeletal adaptations to training with the advanced resistive exercise device. Med. Sci. Sports Exerc. 43, 146–156. doi: 10.1249/MSS.0b013e3181e4f161
Macias, B. R., Cao, P., Watenpaugh, D. E., and Hargens, A. R. (2007). LBNP treadmill exercise maintains spine function and muscle strength in identical twins during 28-day simulated microgravity. J Appl Physiol. 102, 2274–2278. doi: 10.1152/japplphysiol.00541.2006
Macias, B. R., Schneider, S., Lee, S. M. C., Guinet, P., Hughson, R., Smith, S., et al. (2008). WISE-2005: lower body negative pressure treadmill and resistive exercise countermeasures maintain physiologic function in women during 60-days of simulated microgravity. Faseb J. 22, 752.15. doi: 10.1096/fasebj.22.1_supplement.752.15
Meuche, S., Schneider, S. M., Lee, S. M. C., Macias, B. R., Smith, S. M., Watenpaugh, D. E., et al. (2005). WISE 2005: LBNP Exercise and Flywheel Resistive Exercise as an Effective Countermeasure Combination. NASA Technical Reports Server.
Meuche, S., Schneider, S. M., Lee, S. M. C., Macias, B. R., Smith, S. M., Watenpaugh, D. E., et al. (2006). Supine Treadmill Exercise in Lower Body Negative Pressure Combined with Resistive Exercise Counteracts Bone Loss, Reduced Aerobic Upright Exercise Capacity and Reduced Muscle Strength. NASA Technical Reports Server.
Milesi, S., Capelli, C., Denoth, J., Hutchinson, T., di Prampero, P. E., and Stussi, E. (1997). Effects of 17 days bed rest on the maximal isometric torque of the flexors and extensors of the ankle. J. Gravit. Physiol. 4, P125–126.
Miokovic, T., Armbrecht, G., Felsenberg, D., and Belavy, D. L. (2011). Differential atrophy of the postero-lateral hip musculature during prolonged bedrest and the influence of exercise countermeasures. J. Appl. Physiol. 110, 926–934. doi: 10.1152/japplphysiol.01105.2010
Miokovic, T., Armbrecht, G., Felsenberg, D., and Belavy, D. L. (2012). Heterogeneous atrophy occurs within individual lower limb muscles during 60 days of bed rest. J. Appl. Physiol. 113, 1545–1559. doi: 10.1152/japplphysiol.00611.2012
Miokovic, T., Armbrecht, G., Gast, U., Rawer, R., Roth, H. J., Runge, M., et al. (2014). Muscle atrophy, pain, and damage in bed rest reduced by resistive. (Vibration) Exercise. Med. Sci. Sports Exerc. 46, 1506–1516. doi: 10.1249/MSS.0000000000000279
Miyoshi, T., Sato, T., Sekiguchi, H., Yamanaka, K., Miyazaki, M., Igawa, S., et al. (2001). Effect of long-term bedrest on lower leg muscle activation patterns during quiet standing. J. Gravit. Physiol. 8, P85–P86.
Moher, D., Liberati, A., Tetzlaff, J., and Altman, D. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 6:e1000097. doi: 10.1371/journal.pmed.1000097
Moore, A. D., Downs, M. E., Lee, S. M. C., Feiveson, A. H., Knudsen, P., and Ploutz-Snyder, L. (2014). Peak exercise oxygen uptake during and following long-duration spaceflight. J. Appl. Physiol. 117, 231–238. doi: 10.1152/japplphysiol.01251.2013
Moriggi, M., Vasso, M., Fania, C., Capitanio, D., Bonifacio, G., Salanova, M., et al. (2010). Long term bed rest with and without vibration exercise countermeasures: effects on human muscle protein dysregulation. Proteomics 10, 3756–3774. doi: 10.1002/pmic.200900817
Muir, J., Judex, S., Qin, Y. X., and Rubin, C. (2011). Postural instability caused by extended bed rest is alleviated by brief daily exposure to low magnitude mechanical signals. Gait Posture 33, 429–435. doi: 10.1016/j.gaitpost.2010.12.019
Mulder, E. R., Gerrits, K. H. L., Kleine, B. U., Rittweger, J., Felsenberg, D., de Haan, A., et al. (2009a). High-density surface EMG study on the time course of central nervous and peripheral neuromuscular changes during 8 weeks of bed rest with or without resistive vibration exercise. J. Electromyography Kinesiol. 19, 208–218. doi: 10.1016/j.jelekin.2007.04.002
Mulder, E. R., Gerrits, K. H. L., Rittweger, J., Felsenberg, D., Stegeman, D. F., and de Haan, A. (2008). Characteristics of fast voluntary and electrically evoked isometric knee extensions during 56 days of bed rest with and without exercise countermeasure. Eur. J. Appl. Physiol. 103, 431–440. doi: 10.1007/s00421-008-0724-8
Mulder, E. R., Horstman, A. M., Gerrits, K., Massa, M., Kleine, B. U., de Haan, A., et al. (2011). Enhanced physiological tremor deteriorates plantar flexor torque steadiness after bed rest. J. Electromyogr. Kinesiol. 21, 384–393. doi: 10.1016/j.jelekin.2010.10.009
Mulder, E. R., Horstman, A. M., Stegeman, D. F., de Haan, A., Belavy, D. L., Miokovic, T., et al. (2009b). Influence of vibration resistance training on knee extensor and plantar flexor size, strength, and contractile speed characteristics after 60 days of bed rest. J. Appl. Physiol. 107, 1789–1798. doi: 10.1152/japplphysiol.00230.2009
Mulder, E. R., Kuebler, W. M., Gerrits, K. H. L., Rittweger, J., Felsenberg, D., Stegeman, D. F., et al. (2007). Knee extensor fatigability after bedrest for 8 weeks with and without countermeasure. Muscle. Nerve. 36, 798–806. doi: 10.1002/mus.20870
Mulder, E. R., Stegeman, D. F., Gerrits, K. H. L., Paalman, M. I., Rittweger, J., Felsenberg, D., et al. (2006). Strength, size and activation of knee extensors followed during 8 weeks of horizontal bed rest and the influence of a countermeasure. Eur. J. Appl. Physiol. 97, 706–715. doi: 10.1007/s00421-006-0241-6
Narici, M. V., Kayser, B., Barattini, P., and Cerretelli, P. (1997). Changes in electrically evoked skeletal muscle contractions during 17-day spaceflight and bed rest. Int. J. Sports Med. 18, S290–S292. doi: 10.1055/s-2007-972729
National Aeronautics and Space Administration (2019). Human Research Roadmap - List of Risks. Houston, TX: National Aeronautics and Space Administration. Available online at: https://humanresearchroadmap.nasa.gov/Risks/ (accessed August 7, 2019).
Nelson, E. C., Eftimovska, E., Lind, C., Hager, A., Wasson, J., and Lindblad, S. (2015). Patient reported outcome measures in practice. Br. Med. J. 350:7818. doi: 10.1136/bmj.g7818
Netreba, A. I., Khusnutdinova, D. R., Vinogradova, O. L., and Kozlovskaya, I. B. (2004). Effect of dry immersion in combination with stimulation of foot support zones upon muscle force-velocity characteristics. J. Gravit. Physiol. 11, P129–P130.
Ouzzani, M., Hammady, H., Fedorowicz, Z., and Elmagarmid, A. (2016). Rayyan—a web and mobile app for systematic reviews. Syst. Rev. 5:210. doi: 10.1186/s13643-016-0384-4
Pavy-Le Traon, A., Heer, M., Narici, M. V., Rittweger, J., and Vernikos, J. (2007). From space to Earth: advances in human physiology from 20 years of bed rest studies. (1986-2006). Eur. J. Appl. Physiol. 191, 143–194. doi: 10.1007/s00421-007-0474-z
Petersen, N., Jaekel, P., Rosenberger, A., Weber, T., Scott, J., Castrucci, F., et al. (2016). Exercise in space: the european space agency approach to in-flight exercise countermeasures for long-duration missions on ISS. Extrem. Physiol. Med. 5:9. doi: 10.1186/s13728-016-0050-4
Pisot, R., Narici, M. V., Simunic, B., De Boer, M., Seynnes, O., Jurdana, M., et al. (2008). Whole muscle contractile parameters and thickness loss during 35-day bed rest. Eur. J. Appl. Physiol. 104, 409–414. doi: 10.1007/s00421-008-0698-6
Ploutz-Snyder, L. (2013). An Evidence Based Approach to Exercise Prescriptions on ISS. Houston, TX: Universities Space Research Association.
Ploutz-Snyder, L., Ryder, J., English, K., Haddad, F., and Baldwin, K. (2015). Risk of Impaired Performance Due to Reduced Muscle Mass, Strength, and Endurance Human Research Program HRP-47072. NASA Evidence Report.
Portero, P., Vanhoutte, C., and Goubel, F. (1996). Surface electromyogram power spectrum changes in human leg muscles following 4 weeks of simulated microgravity. Eur. J. Appl. Physiol. Occup. Physiol. 73, 340–345. doi: 10.1007/BF02425496
Prinsen, C. A. C., Vohra, S., Rose, M. R., and Terwee, C. B. (2014). Core outcome measures in effectiveness trials. (COMET) initiative: an international delphi study to achieve consensus on how to select outcome measurement instruments for outcomes included in a ‘Core Outcome Set'. Qual. Life Res. 23, 100–101. doi: 10.1186/1745-6215-15-247
Reeves, N. J., Maganaris, C. N., Ferretti, G., and Narici, M. V. (2002). Influence of simulated microgravity on human skeletal muscle architecture and function. J. Gravit. Physiol. 9, P153–154.
Richter, C., Braustein, B., Winnard, A., Nasser, M., and Weber, T. (2017). Human biomechanical and cardiopulmonary responses to partial gravity - a systematic review. Front. Psychol. 8:583. doi: 10.3389/fphys.2017.00583
Rittweger, J., and Felsenberg, D. (2009). Recovery of muscle atrophy and bone loss from 90 days bed rest: results from a one-year follow-up. Bone 44, 214–224. doi: 10.1016/j.bone.2008.10.044
Rittweger, J., Frost, H. M., Schiessl, H., Ohshima, H., Alkner, B., Tesch, P., et al. (2005). Muscle atrophy and bone loss after 90 days' bed rest and the effects of flywheel resistive exercise and pamidronate: results from the LTBR study. Bone 36, 1019–1029. doi: 10.1016/j.bone.2004.11.014
Rittweger, J., Moller, K., Bareille, M. P., Felsenberg, D., and Zange, J. (2013). Muscle X-ray attenuation is not decreased during experimental bed rest. Muscle. Nerve. 47, 722–730. doi: 10.1002/mus.23644
Schneider, S. M., Lee, S. M. C., Feiveson, A. H., Watenpaugh, D. E., Macias, B. R., and Hargens, A. R. (2016). Treadmill exercise within lower body negative pressure protects leg lean tissue mass and extensor strength and endurance during bed rest. Physiol. Rep. 4:e12892. doi: 10.14814/phy2.12892
Scott, J. M., Martin, D. S., Ploutz-Snyder, R., Matz, T., Caine, T., Downs, M., et al. (2017). Panoramic ultrasound: a novel and valid tool for monitoring change in muscle mass. J. Cachexia Sarcopenia Muscle 8, 475–481. doi: 10.1002/jcsm.12172
Shenkman, B. S., Kozlovskaya, I. B., Nemirovskaya, T. L., and Tcheglova, I. A. (1997). Human muscle atrophy in supportlessness: effects of short-term exposure to dry immersion. J. Gravit. Physiol. 4, P137–138.
Shinohara, M., Yoshitake, Y., Kouzaki, M., Fukuoka, H., and Fukunaga, T. (2003). Strength training counteracts motor performance losses during bed rest. J Appl Physiol. 95, 1485–1492. doi: 10.1152/japplphysiol.01173.2002
Sibonga, J. D., Spector, E. R., Johnston, S. L., and Tarver, W. J. (2015). Evaluating bone loss in ISS astronauts. Aerospace Med. Human Perform. 86, A38–A44. doi: 10.3357/AMHP.EC06.2015
Smith, S. M., Heer, M., Shackelford, L. C., Sibonga, J. D., Ploutz-Snyder, L., and Zwart, S. R. (2012). Benefits for bone from resistance exercise and nutrition in long-duration spaceflight: evidence from biochemistry and densitometry. J. Bone Mineral Res. 27, 1896–1906. doi: 10.1002/jbmr.1647
Stokes, M., Evetts, S., Rittweger, J., Weber, T., Caplan, N., Danneels, L., et al. (2016). Recommendations for Future Post-mission Neuro-musculoskeletal Reconditioning Research and Practice Post-mission Exercise. (Reconditioning) Topical Team Report. European Space Agency Report.
Sunblad, P., Orlov, O., Angerer, O., Larina, I., and Cromwell, R. (2014). Guidelines for Standardization Of Bed Rest Studies In The Spaceflight Context. Paris: International Acadamy of Astronautics.
Tarver, W. J. (2013). Clinical Practice Guidelines for Vitamin D. 84th Aerospace Medical Association Annual Scientific Meeting. Chicago, IL, Aerospace Medical Association.
Trappe, S. W., Costill, D. L., Gallagher, P., Creer, A., Peters, J. R., Evans, H., et al. (2009). Exercise in space: human skeletal muscle after 6 months aboard the International space station. J. Appl. Physiol. 106, 1159–1168. doi: 10.1152/japplphysiol.91578.2008
Trappe, S. W., Trappe, T. A., Lee, G. A., Widrick, J. J., Costill, D. L., and Fitts, R. H. (2001). Comparison of a space shuttle flight (STS-78) and bed rest on human muscle function. J. Appl. Physiol. 91, 57–64. doi: 10.1152/jappl.2001.91.1.57
Trappe, T. A., Burd, N. A., Louis, E. S., Lee, G. A., and Trappe, S. W. (2007). Influence of concurrent exercise or nutrition countermeasures on thigh and calf muscle size and function during 60 days of bed rest in women. Acta Physiol. 191, 147–159. doi: 10.1111/j.1748-1716.2007.01728.x
Winnard, A., and Nasser, M. (2017b). AMSRG Bed Rest Assessment Tool v1.1. Newcastle-upon-Tyne: Aerospace Medicine Systematic Review Group.
Winnard, A., and Nasser, M. (eds.). (2017a). AMSRG Tool for Assessing Bed Rest Methods. Newcastle-upon-Tyne: Aerospace Medicine Systematic Review Group Winnard.
Winnard, A., Nasser, M., Debuse, D., Stokes, M., Evetts, S., Wilkinson, M., et al. (2017). Systematic review of countermeasures to minimise physiological changes and risk of injury to the lumbopelvic area following long-term microgravity. Musculoskelet Sci. Pract. 27, S5–S14. doi: 10.1016/j.msksp.2016.12.009
Keywords: muscle, microgravity, spaceflight, deconditioning, astronaut
Citation: Winnard A, Scott J, Waters N, Vance M and Caplan N (2019) Effect of Time on Human Muscle Outcomes During Simulated Microgravity Exposure Without Countermeasures—Systematic Review. Front. Physiol. 10:1046. doi: 10.3389/fphys.2019.01046
Received: 26 October 2018; Accepted: 30 July 2019;
Published: 16 August 2019.
Edited by:
Richard D. Boyle, National Aeronautics and Space Administration (NASA), United StatesReviewed by:
Carlo Rinaldi, University of Oxford, United KingdomCopyright © 2019 Winnard, Scott, Waters, Vance and Caplan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrew Winnard, YS53aW5uYXJkQG5vcnRodW1icmlhLmFjLnVr
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.