
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Physiol. , 18 July 2019
Sec. Environmental, Aviation and Space Physiology
Volume 10 - 2019 | https://doi.org/10.3389/fphys.2019.00899
This article is part of the Research Topic The Effects of Altered Gravity on Physiology View all 11 articles
While there is a relatively good understanding of the effects of microgravity on human physiology based on five decades of experience, the physiological consequences of partial gravity remain far less well understood. Until recently, no model had been able to replicate partial gravity such as that experienced on Mars (0.38 g), which would be critical to help sustain long-term missions and ensure a safe return to Earth. Recent development of two partial weight bearing (PWB) models, one in mice and one in rats, now allows for quadrupedal partial unloading that mimics Martian gravity. Resveratrol (RSV), a polyphenol most commonly found in grapes and blueberries, has been extensively investigated for its health benefits, including its anti-inflammatory, anti-oxidative, and anti-diabetic effects. In the context of mechanical unloading, RSV has also been shown to preserve bone and muscle mass. However, there is a lack of research regarding its effect on the musculoskeletal system in partial gravity. We hypothesized that a moderate daily dose of RSV (150 mg/kg/day) would help mitigate muscle deconditioning in a Mars gravity analog. Indeed, our results demonstrate that RSV treatment during partial unloading significantly preserves muscle function (e.g., the average change in grip force after 14 days of PWB40 was of −6.18, and +10.92% when RSV was administered) and mitigates muscle atrophy (e.g., RSV supplementation led to an increase of 21.6% in soleus weight for the unloaded animals). This work suggests the potential of a nutraceutical approach to reduce musculoskeletal deconditioning on a long-term mission to Mars.
After decades of manned low earth orbit missions, space agencies are now targeting other planets for human exploration. In the upcoming years, NASA plans to send astronauts to the Moon and to Mars (National Aeronautics and Space Administration [NASA], 2018), both of which display a significantly lower gravity than Earth (0.16 and 0.38 g, respectively). While there is an extensive literature reviewing the impact of microgravity (real or simulated) on the muscular system (Fitts et al., 2000; Morey-Holton et al., 2005; Baldwin et al., 2013; Chowdhury et al., 2013; Ohira et al., 2015; Radugina et al., 2018), relatively little is known about the effects of partial gravity. Thus, mitigating strategies will be needed to prevent muscle deconditioning and allow astronauts to safely perform tasks upon arrival on Mars, especially after being reintroduced to gravity, even if at a reduced level.
Among potential medicinal interventions to prevent muscle deterioration, nutraceuticals are especially appealing since they are naturally occurring compounds that are present in the diet. Moreover, astronauts en route to Mars will not have the opportunity to exercise with the same devices currently deployed on the ISS (Petersen et al., 2016). Of potential candidates, resveratrol (RSV), a polyphenol, commonly found in grape skin, red wine, and blueberries and used as a dietary supplement, has been widely investigated (Tran et al., 2017; Yonamine et al., 2017; Zhang et al., 2017, 2018; Zhu et al., 2017; Franck et al., 2018), and declared safe for animals and humans (Williams et al., 2009). RSV has already been studied for its osteoprotective effects (Shen et al., 2012; Durbin et al., 2014; Ornstrup et al., 2014), and described as a physical exercise mimetic to prevent wasting disorders during hindlimb unloading (Momken et al., 2011). However, its mitigating effect has never been studied in a partial weight-bearing (PWB) model.
In this study, we hypothesized that daily supplementation with a moderate dose of RSV would mitigate muscle impairment in a ground-based partial gravity analog that mimics a Martian environment (0.4 g, PWB40). If correct, our results would offer proof of concept for such intervention with subsequent efforts devoted to unraveling potential underlying mechanisms of action.
Twenty-four male Wistar rats (Charles River Laboratories Inc., Wilmington, MA, United States) weighing 425 ± 6.73 g (14 weeks of age) were obtained and housed in a temperature-controlled (22 ± 2°C) room with a 12:12-hour light-dark cycle. Water and rat chow were provided ad libitum and daily food intake was monitored. Animals were exposed to normal loading (PWB100) or Martian gravity (40% of normal loading: PWB40) for 14 days. Detailed description about the control group, the housing environment and the suspension apparatus can be found in our previous articles describing this model (Mortreux et al., 2018, 2019). Half of the animals in each group received resveratrol daily (RSV, 150 mg/kg/day, Transreveratrol HPLC Purified, megaresveratrol.net, Candlewood Stars, Inc, Danbury, CT, United States) starting at baseline (day 0). RSV was prepared daily by mixing the powder with 10% sucrose water into a 150 mg/ml solution that was administered orally using a 1 mL syringe. Animals were weighed weekly, free of their suspension apparatus. All experimental protocols were approved by the Beth Israel Deaconess Medical Center Institutional Animal Care and Use Committee.
Weekly, calf circumference was measured at the left tibia mid-shaft. Animals were anesthetized with isoflurane and placed in a prone position with their left hind limb taped at a 45-degree angle. Fur was removed using a hair clipper (Braintree Scientific, Braintree, MA, United States) and suture thread was used to obtain three circumference measurements and the average was recorded.
Using a 50 N grip force meter (Chatillon, Largo, FL, United States), both front and rear paw grip force were assessed weekly by performing three gentle pulls until the animals released their grip, allowing them to rest for 30 s between trials. The peak force of each trial was recorded and the three trials averaged.
After 14 days, animals were sacrificed and the triceps surae (e.g., soleus and gastrocnemius muscles) was harvested and muscle wet mass was measured using a precision balance (Fisher Scientific, Pittsburgh, PA, United States). Muscles were fixed in 10% formalin for 48 h and embedded in paraffin. Immunohistochemical analysis was performed using anti-collagen VI (ab6588, Abcam, Cambridge, MA, United States) and anti-slow skeletal myosin heavy chain (ab11083, Abcam, Cambridge, MA, United States) antibodies. Images were obtained at 20× using an epifluorescence microscope (Zeiss Axio Imager M1) and a minimum of 100 myofibers were analyzed for each muscle with FIJI (ImageJ, NIH) using the muscle morphometry plug-in (Anthony Sinadinos using Eclipse IDE) to calculate the average myofiber cross sectional area (CSA) with the experimenter blinded for treatment and gravity level.
Longitudinal measurements were analyzed using repeated measures 2-way ANOVA followed by Tukey’s multiple comparisons test. Terminal measurements were analyzed using 1-way ANOVA followed by Tukey’s post hoc test. All results were considered significant when p < 0.05 and performed using GraphPad Prism 7.
Resveratrol supplementation did not influence body weight (Figure 1A) in any group as compared to un-supplemented animals. Calf circumference was not impacted by the RSV supplementation and animals from both PWB40 and PWB40 + RSV groups displayed a significant reduction over time compared to either their baseline values or to the two control groups (Figure 1B). After 14 days, rats undergoing PWB40 were the only group performing below baseline values for front paw grip force, and significantly lower than all other groups (Figure 1C). In the hindlimbs (Figure 1D), a significant decline in grip force was evidenced as early as 7 days. RSV supplementation totally rescued grip force in both the front and hindlimbs of the rats exposed to partial unloading (Figures 1C,D).
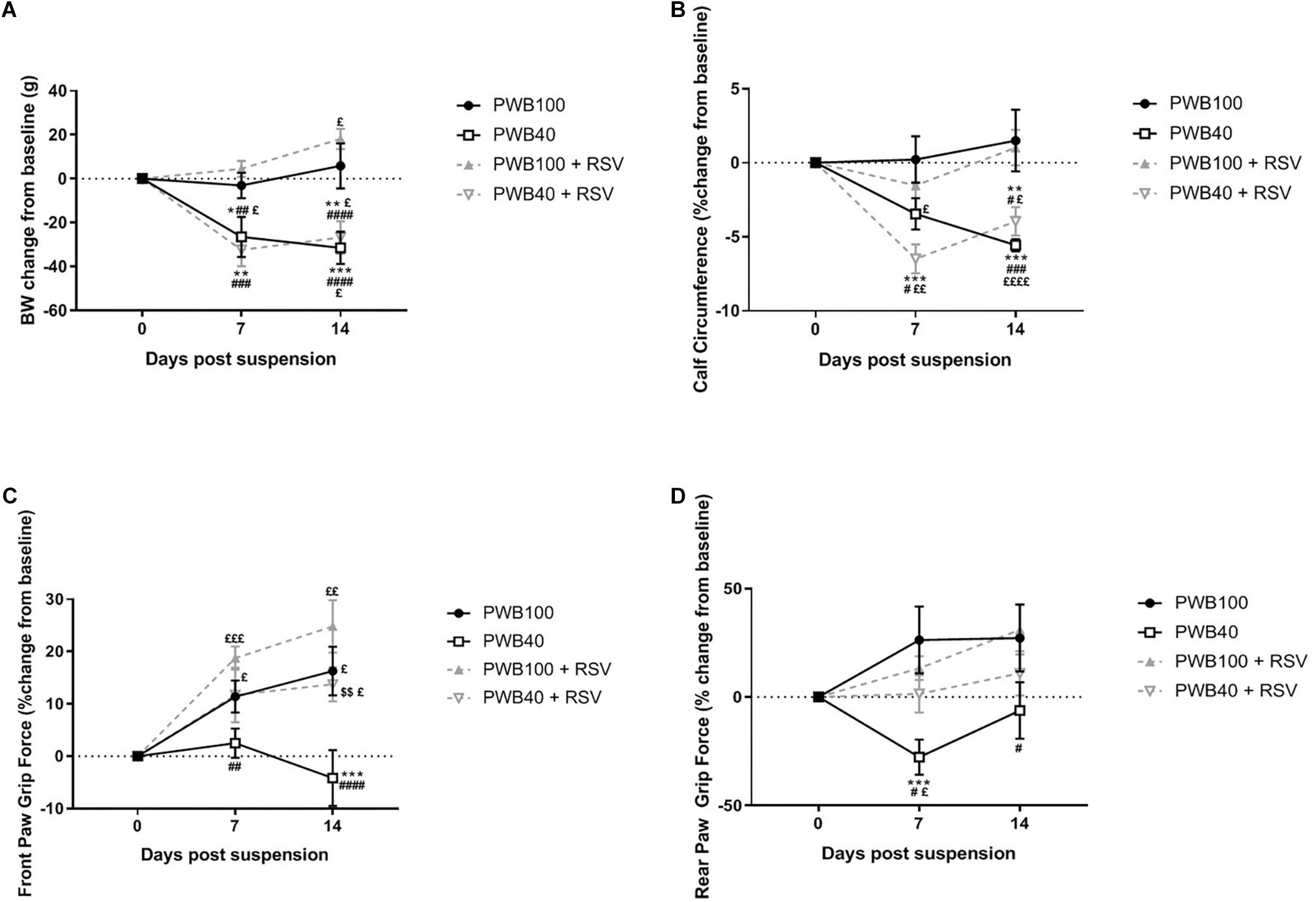
Figure 1. Weekly changes in body weight (A), calf circumference (B), front paw (C) and rear paw (D) grip force compared to pre-suspension values (day 0). Data are presented as mean ± SEM; results of the post hoc tests following the ANOVA are indicated. N = 6 per group. *p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, and ****p < 0.0001 vs. PWB100, respectively. #p < 0.05, ##p < 0.01, ###p < 0.001, and ####p < 0.0001 vs. PWB100 + RSV, respectively. $$p < 0.01 vs. PWB40. £p < 0.05, ££p < 0.01, £££p < 0.001, and ££££p < 0.0001 vs. baseline values (day 0), respectively.
Data on terminal muscle assessment are presented in Figure 2 and Supplementary Tables S1, S2. Animals exposed to PWB40 alone displayed a significantly reduced soleus mass, average CSA, and slow-twitch-CSA compared to both non-unloaded groups. In contrast, RSV supplementation in animals exposed to PWB40 prevented muscle atrophy (Figure 2A) and partially rescued myofiber CSA (Figure 2B and Supplementary Table S2), but did not induce any significant change for the group at PWB100. As visualized in the images (Figure 2C), we observed a significant reduction of the myofiber type switch in the soleus of the rats undergoing PWB40 and supplemented with RSV (p = 0.0269 vs. PWB40, Supplementary Table S1). In the gastrocnemius, PWB40 induced a significant reduction of muscle mass (Figure 2D) and CSA (Figures 2E,F). RSV supplementation successfully increased muscle wet mass compared to non-treated animals (Figure 2D), but did not fully restore the average CSA (Figure 2E). However, the fiber-specific CSA did not significantly differ compared to the two non-unloaded groups (Supplementary Table S2). While a slight increase in slow-twitch myofibers was visible in the PWB40 + RSV group (Figure 2F), it was not significantly different compared to the other groups (Supplementary Table S1).
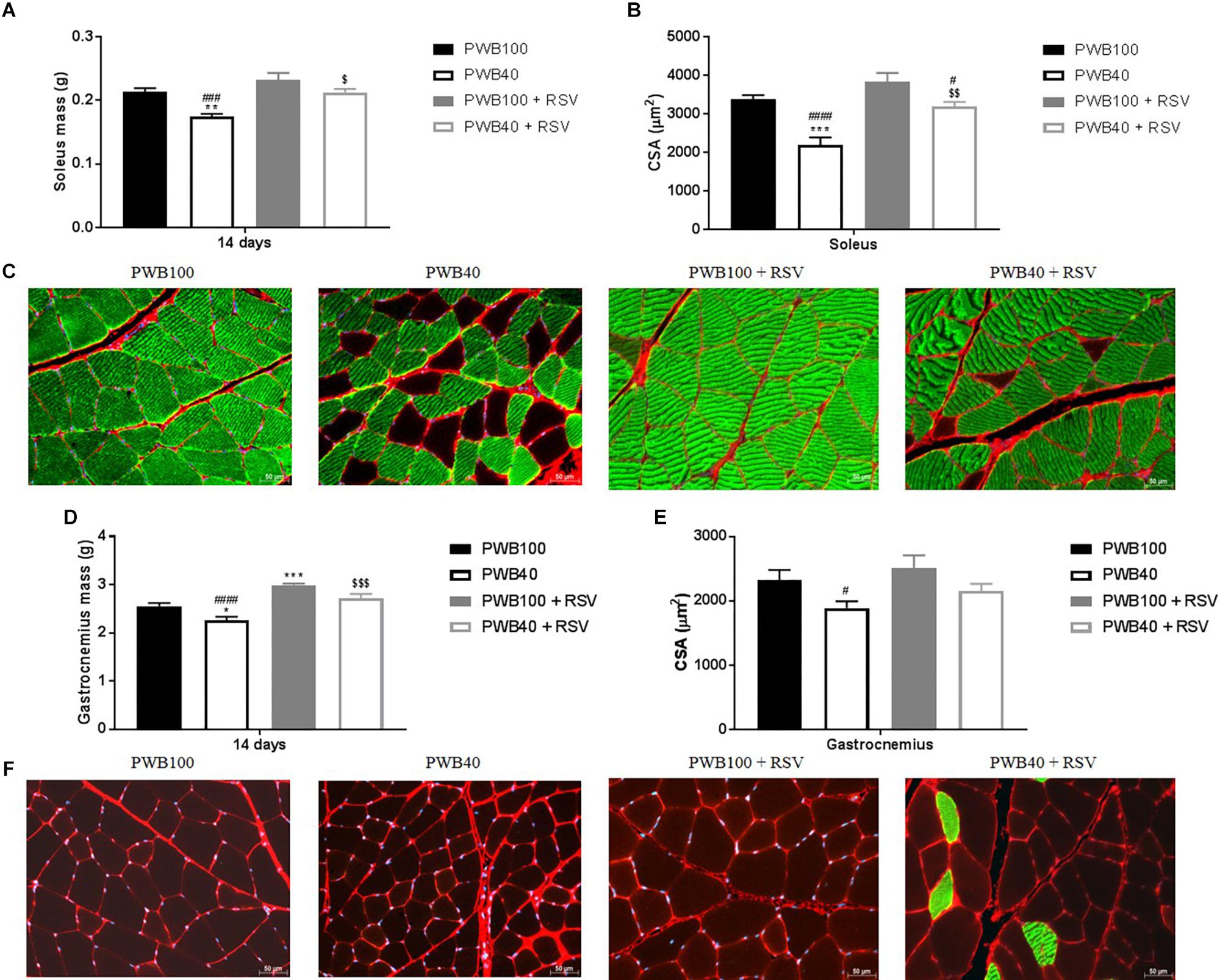
Figure 2. Terminal assessment of the muscles of the triceps surae, including soleus (A) and gastrocnemius wet mass (D), soleus (B) and gastrocnemius (E) average cross sectional area (CSA), and representative images of the soleus (C) and gastrocnemius (F) muscles stained with anti-collagen VI (red), anti-slow-skeletal myosin heavy chain (green), and DAPI (blue). N = 6 per group. The results are presented as mean ± SEM; the results of the post hoc tests following the ANOVA are indicated. *p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001 vs. PWB100, respectively. #p < 0.05, ###p < 0.001, and ####p < 0.0001 vs. PWB100 + RSV, respectively. $p < 0.05, $$p < 0.01, and $$$p < 0.001 vs. PWB40, respectively.
We previously demonstrated that our PWB system was well tolerated by the rats over a 14-day period (Mortreux et al., 2018, 2019) despite an initial body weight loss, similar to that observed in HLU models (Brooks et al., 2009) and analogous to what occurs in astronauts in flight (Matsumoto et al., 2011). As reported previously in healthy animals (Rauf et al., 2017), RSV did not affect food intake (Supplementary Table S3) or body weight (Figure 1A) in our study. In rodent models, grip force rapidly declines when animals are exposed to PWB (Wagner et al., 2010; Mortreux et al., 2018, 2019). Here we show that at all time-points, rats exposed to PWB40 + RSV did not score lower than their baseline values, or their non-unloaded counterparts. In both front and rear paw grip force experiments, the PWB40 cohort was the only group that displayed a significant decrease in grip force compared to their baseline (rear paw, Figure 1D) and that of the other groups (front paw, Figure 1C). As previously reported (Mortreux et al., 2018), we observed a slight recovery in rear paw grip force after 14 days of PWB40. The reason for this improvement is uncertain, but may be due, in part, to an adaptation to partial unloading or adult growth.
Ex vivo analysis highlighted the muscle-protective effect of RSV (Figure 2). In both the gastrocnemius and soleus muscles, we observed a significant rescue of muscle wet mass comparable to the one observed by Momken et al. (2011) after 14 days of HLU. These findings are further confirmed by the histomorphometric analyses. In the soleus, the average CSA in the PWB40 + RSV group was significantly higher than in the PWB40 group, but remained significantly lower than the PWB100 + RSV group. No differences were seen between the PWB40 + RSV group and the PWB100 controls. In the gastrocnemius, only the PWB40 group displayed a significantly lower average CSA compared to the PWB100 + RSV group. This could be due to the limited number of slides analyzed.
During spaceflight and in rodent models of mechanical unloading, a myofiber type switch has been described (Desplanches, 1997; Lomonosova et al., 2016; Shenkman, 2016; McDonald et al., 2017) in static, weight-bearing muscles such as the soleus, which predominantly consists of slow-twitch fibers, thus providing endurance over dynamic strength. Muscle deconditioning increases the proportion of type two fast twitch fibers in weight-bearing muscles, therefore making them prone to muscle fatigue. While our PWB40 animals displayed a significantly smaller percentage of type 1 fibers in the soleus compared to the normally loaded groups (Supplementary Table S1), rats undergoing PWB40 + RSV did not. This maintenance of muscle composition could explain the preservation of muscle function as demonstrated by our functional assessments. In contrast, gastrocnemius fiber type was not significantly altered by partial unloading or by RSV treatment, despite a 94.21% increase in type 1 myofiber compared to the PWB40 group.
Our initial functional study highlights the benefits of a moderate daily dose of RSV, however, several limitations are to be taken in consideration. First, our study involved only a modest number of male rats (n = 24), thus, we do not yet know if RSV supplementation would benefit females, similarly. Second, we assessed the effect of a single dosage of RSV (150 mg/kg/day). This choice was based on the literature and the known protective effects of RSV on bone (Durbin et al., 2014). Nevertheless, many dosages of RSV have been tested in animal models, up to 700 mg/kg/day (Williams et al., 2009), therefore, a more efficient dose to prevent muscle deconditioning during partial unloading could exist. Finally, our study assessed the functional outcomes of performance and composition of the main weight-bearing hindlimb muscles but did not focus on the deeper mechanisms and pathways that could be involved.
One possible explanation for our findings is that RSV could be responsible for maintaining skeletal muscle insulin sensitivity through its beneficial effect on glucose homeostasis and GLUT4 expression (Momken et al., 2011; Yonamine et al., 2017). Indeed, muscle is intrinsically linked to energy homeostasis since it is a major consumer of glucose during contraction. The transporter GLUT4 controls glucose uptake in the skeletal muscle, and when dysregulated, insulin resistance occurs (Dyar et al., 2014). This metabolic dysfunction has been observed in both astronauts and ground-based animal models of mechanical unloading (Tobin et al., 2002; Hughson et al., 2016; Gambara et al., 2017; Tascher et al., 2017). In diabetic animals, it has been shown that RSV treatment increases muscle weight and protein content by regulating GLUT4 expression in the skeletal muscle (Yonamine et al., 2017), while in healthy hypokinetic animals, RSV supplementation before, during, and after mechanical unloading, enhances insulin sensitivity and muscle recovery (Momken et al., 2011; Bennett et al., 2013), suggesting the presence of a significant link between glucose homeostasis and muscle health. Alternatively, this mitigating phenomenon could also be attributed to the anti-oxidative and anti-inflammatory effects of RSV, which could be crucial for the musculoskeletal system during a period of mechanical unloading, and are currently being investigated using other polyphenols such as dried plums (Simonavice et al., 2013; Schreurs et al., 2016). Further metabolic analyses will be required to unravel the cellular mechanisms involved in the muscle-protective effects of a moderate daily dose of RSV.
Taken together, our results highlight the therapeutic potential of RSV as a nutraceutical countermeasure to prevent muscle deconditioning in an animal model of Martian gravity. Further investigations should optimize the dose of RSV for the preservation of muscle function and explore the mechanisms involved. In addition, it will be important to confirm the lack of any potentially harmful interactions of RSV with other drugs administered to astronauts during space missions.
All studies were approved by the Institutional Animal Care and Use Committee of Beth Israel Deaconess Medical Center and conform to the National Research Council “Guide for the Care and Use of Laboratory Animals.”
MM designed the methodology. MM and DR performed the experiments, and analyzed the data. MB and SR designed the study. All authors reviewed and approved the manuscript.
This work was funded by a grant from the National Aeronautics and Space Administration (NASA: NNX16AL36G).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors would like to thank Dr. J. A. Nagy for her careful editing of the manuscript.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2019.00899/full#supplementary-material
Baldwin, K. M., Haddad, F., Pandorf, C. E., Roy, R. R., and Edgerton, V. R. (2013). Alterations in muscle mass and contractile phenotype in response to unloading models: role of transcriptional/pretranslational mechanisms. Front. Physiol. 4:284. doi: 10.3389/fphys.2013.00284
Bennett, B. T., Mohamed, J. S., and Alway, S. E. (2013). Effects of resveratrol on the recovery of muscle mass following disuse in the plantaris muscle of aged rats. PLoS One 8:e83518. doi: 10.1371/journal.pone.0083518
Brooks, N., Gj, C., Je, L., Am, F., Arbogast, S., Smith, J., et al. (2009). Countermeasures for spaceflight-induced muscle atrophy and loss of strength. Aviat. Space. Environ. Med. 80, 987–987. doi: 10.3357/asem.27009.2009
Chowdhury, P., Long, A., Harris, G., Soulsby, M. E., and Dobretsov, M. (2013). Animal model of simulated microgravity: a comparative study of hindlimb unloading via tail versus pelvic suspension. Physiol. Rep. 1:e00012. doi: 10.1002/phy2.12
Desplanches, D. (1997). Structural and functional adaptations of skeletal muscle to weightlessness. Int. J. Sports Med. 18, S259–S264. doi: 10.1055/s-2007-972722
Durbin, S. M., Jackson, J. R., Ryan, M. J., Gigliotti, J. C., Alway, S. E., and Tou, J. C. (2014). Resveratrol supplementation preserves long bone mass, microstructure, and strength in hindlimb-suspended old male rats. J. Bone Miner. Metab. 32, 38–47. doi: 10.1007/s00774-013-0469-2
Dyar, K. A., Ciciliot, S., Wright, L. E., Biensø, R. S., Tagliazucchi, G. M., Patel, V. R., et al. (2014). Muscle insulin sensitivity and glucose metabolism are controlled by the intrinsic muscle clock. Mol. Metab. 3, 29–41. doi: 10.1016/j.molmet.2013.10.005
Fitts, R. H., Riley, D. R., and Widrick, J. J. (2000). Physiology of a microgravity environment invited review: microgravity and skeletal muscle. J. Appl. Physiol. 89, 823–839. doi: 10.1152/jappl.2000.89.2.823
Franck, F. C., Benatti, B. B., Andia, D. C., Cirano, F. R., Casarin, R. C., Corrêa, M. G., et al. (2018). Impact of resveratrol on bone repair in rats exposed to cigarette smoke inhalation: histomorphometric and bone-related gene expression analysis. Int. J. Oral Maxillofac. Surg. 47, 541–548. doi: 10.1016/j.ijom.2017.08.004
Gambara, G., Salanova, M., Ciciliot, S., Furlan, S., Gutsmann, M., Schiffl, G., et al. (2017). Microgravity-induced transcriptome adaptation in mouse paraspinal longissimus dorsi muscle highlights insulin resistance-linked genes. Front. Physiol. 8:279. doi: 10.3389/fphys.2017.00279
Hughson, R. L., Robertson, A. D., Arbeille, P., Shoemaker, J. K., Rush, J. W. E., Fraser, K. S., et al. (2016). Increased postflight carotid artery stiffness and inflight insulin resistance resulting from 6-mo spaceflight in male and female astronauts. Am. J. Physiol. Circ. Physiol. 310, H628–H638. doi: 10.1152/ajpheart.00802.2015
Lomonosova, Y. N., Turtikova, O. V., and Shenkman, B. S. (2016). Reduced expression of MyHC slow isoform in rat soleus during unloading is accompanied by alterations of endogenous inhibitors of calcineurin/NFAT signaling pathway. J. Muscle Res. Cell Motil. 37, 7–16. doi: 10.1007/s10974-015-9428-y
Matsumoto, A., Storch, K. J., Stolfi, A., Mohler, S. R., Frey, M. A., and Stein, T. P. (2011). Weight loss in humans in space. Aviat. Space Environ. Med. 82, 615–621. doi: 10.3357/ASEM.2792.2011
McDonald, K. S., Blaser, C. A., and Fitts, R. H. (2017). Force-velocity and power characteristics of rat soleus muscle fibers after hindlimb suspension. J. Appl. Physiol. 77, 1609–1616. doi: 10.1152/jappl.1994.77.4.1609
Momken, I., Stevens, L., Bergouignan, A., Desplanches, D., Rudwill, F., Chery, I., et al. (2011). Resveratrol prevents the wasting disorders of mechanical unloading by acting as a physical exercise mimetic in the rat. FASEB J. 25, 3646–3660. doi: 10.1096/fj.10-177295
Morey-Holton, E., Globus, R. K., Kaplansky, A., and Durnova, G. (2005). The hindlimb unloading rat model: literature overview, technique update and comparison with space flight data. Adv. Space Biol. Med. 10, 7–40. doi: 10.1016/S1569-2574(05)10002-1
Mortreux, M., Nagy, J. A., Ko, F. C., Bouxsein, M. L., and Rutkove, S. B. (2018). A novel partial gravity ground-based analog for rats via quadrupedal unloading. J. Appl. Physiol. 125, 175–182. doi: 10.1152/japplphysiol.01083.2017
Mortreux, M., Riveros, D., Bouxsein, M. L., and Rutkove, S. B. (2019). Mimicking a space mission to mars using hindlimb unloading and partial weight bearing in rats. J. Vis. Exp. 146:e59327. doi: 10.3791/59327
National Aeronautics and Space Administration [NASA] (2018). National Space Exploration Campaign Report. Available at: https://www.nasa.gov/sites/default/files/atoms/files/nationalspaceexplorationcampaign.pdf (accessed October 24, 2018).
Ohira, T., Kawano, F., Ohira, T., Goto, K., and Ohira, Y. (2015). Responses of skeletal muscles to gravitational unloading and/or reloading. J. Physiol. Sci. 65, 293–310. doi: 10.1007/s12576-015-0375-6
Ornstrup, M. J., Harsløf, T., Kjær, T. N., Langdahl, B. L., and Pedersen, S. B. (2014). Resveratrol increases bone mineral density and bone alkaline phosphatase in obese men: a randomized placebo-controlled trial. J. Clin. Endocrinol. Metab. 99, 4720–4729. doi: 10.1210/jc.2014-2799
Petersen, N., Jaekel, P., Rosenberger, A., Weber, T., Scott, J., Castrucci, F., et al. (2016). Exercise in space: the european space agency approach to in-flight exercise countermeasures for long-duration missions on ISS. Extrem Physiol. Med. 5:9. doi: 10.1186/s13728-016-0050-4
Radugina, E. A., Almeida, E. A. C., Blaber, E., Poplinskaya, V. A., Markitantova, Y. V., and Grigoryan, E. N. (2018). Exposure to microgravity for 30 days onboard Bion M1 caused muscle atrophy and impaired regeneration in murine femoral Quadriceps. Life Sci. Space Res. 16, 18–25. doi: 10.1016/j.lssr.2017.08.005
Rauf, A., Imran, M., Sulera, H. A. R., Ahmad, B., Peters, D. G., and Mubarak, M. S. (2017). A comprehensive review of the health perspectives of resveratrol. Food Funct. 8, 4284–4305. doi: 10.1039/c7fo01300k
Schreurs, A. S., Shirazi-Fard, Y., Shahnazari, M., Alwood, J. S., Truong, T. A., Tahimic, C. G. T., et al. (2016). Dried plum diet protects from bone loss caused by ionizing radiation. Sci. Rep. 6:21343. doi: 10.1038/srep21343
Shen, C. L., von Bergen, V., Chyu, M. C., Jenkins, M. R., Mo, H., Chen, C. H., et al. (2012). Fruits and dietary phytochemicals in bone protection. Nutr. Res. 32, 897–910. doi: 10.1016/j.nutres.2012.09.018
Shenkman, B. S. (2016). From slow to fast: hypogravity-induced remodeling of muscle fiber myosin phenotype. Acta Naturae 8, 47–59. doi: 10.32607/20758251-2016-8-4-47-59
Simonavice, E., Liu, P.-Y., Ilich, J. Z., Kim, J.-S., Arjmandi, B., and Panton, L. B. (2013). The effects of a 6-month resistance training and dried plum consumption intervention on strength, body composition, blood markers of bone turnover, and inflammation in breast cancer survivors. Appl. Physiol. Nutr. Metab. 39, 730–739. doi: 10.1139/apnm-2013-0281
Tascher, G., Brioche, T., Maes, P., Chopard, A., O’Gorman, D., Gauquelin-Koch, G., et al. (2017). Proteome-wide adaptations of mouse skeletal muscles during a full month in space. J. Proteome Res. 16, 2623–2638. doi: 10.1021/acs.jproteome.7b00201
Tobin, B. W., Uchakin, P. N., and Leeper-Woodford, S. K. (2002). Insulin secretion and sensitivity in space flight. Nutrition 18, 842–848. doi: 10.1016/s0899-9007(02)00940-1
Tran, H. T., Liong, S., Lim, R., Barker, G., and Lappas, M. (2017). Resveratrol ameliorates the chemical and microbial induction of inflammation and insulin resistance in human placenta, adipose tissue and skeletal muscle. PLoS One 12:e0173373. doi: 10.1371/journal.pone.0173373
Wagner, E. B., Granzella, N. P., Saito, H., Newman, D. J., Young, L. R., and Bouxsein, M. L. (2010). Partial weight suspension: a novel murine model for investigating adaptation to reduced musculoskeletal loading. J. Appl. Physiol. 109, 350–357. doi: 10.1152/japplphysiol.00014.2009
Williams, L. D., Burdock, G. A., Edwards, J. A., Beck, M., and Bausch, J. (2009). Safety studies conducted on high-purity trans-resveratrol in experimental animals. Food Chem. Toxicol. 47, 2170–2182. doi: 10.1016/j.fct.2009.06.002
Yonamine, C. Y., Pinheiro-Machado, E., Michalani, M. L., Alves-Wagner, A. B., Esteves, J. V., Freitas, H. S., et al. (2017). Resveratrol improves glycemic control in Type 2 diabetic obese mice by regulating glucose transporter expression in skeletal muscle and liver. Molecules 22:E1180. doi: 10.3390/molecules22071180
Zhang, B., Xu, L., Zhuo, N., and Shen, J. (2017). Resveratrol protects against mitochondrial dysfunction through autophagy activation in human nucleus pulposus cells. Biochem. Biophys. Res. Commun. 493, 373–381. doi: 10.1016/j.bbrc.2017.09.015
Zhang, C., Yang, L., Zhao, X., Chen, X., Wang, L., and Geng, Z. (2018). Effect of dietary resveratrol supplementation on meat quality, muscle antioxidative capacity and mitochondrial biogenesis of broilers. J. Sci. Food Agric. 98, 1216–1221. doi: 10.1002/jsfa.8576
Keywords: partial gravity, resveratrol, rats, Mars, muscle function
Citation: Mortreux M, Riveros D, Bouxsein ML and Rutkove SB (2019) A Moderate Daily Dose of Resveratrol Mitigates Muscle Deconditioning in a Martian Gravity Analog. Front. Physiol. 10:899. doi: 10.3389/fphys.2019.00899
Received: 01 February 2019; Accepted: 27 June 2019;
Published: 18 July 2019.
Edited by:
Richard D. Boyle, National Aeronautics and Space Administration (NASA), United StatesReviewed by:
Boris Shenkman, Institute of Biomedical Problems (RAS), RussiaCopyright © 2019 Mortreux, Riveros, Bouxsein and Rutkove. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marie Mortreux, bW1vcnRyZXVAYmlkbWMuaGFydmFyZC5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.