- 1Sport Science Program, College of Arts and Science, Qatar University, Doha, Qatar
- 2Athlete Health and Performance Research Centre, ASPETAR, Qatar Orthopaedic and Sports Medicine Hospital, Doha, Qatar
- 3School of Sport, Exercise and Health Sciences, Loughborough University, Loughborough, United Kingdom
- 4Exercise is Medicine, ASPETAR, Qatar Orthopaedic and Sports Medicine Hospital, Doha, Qatar
- 5Biomedical Sciences, College of Health Sciences, Qatar University, Doha, Qatar
- 6Department of Medical Imaging, ASPETAR, Qatar Orthopaedic and Sports Medicine Hospital, Doha, Qatar
- 7School of Sport Science and Physical Activity, Institute for Sport and Physical Activity Research, University of Bedfordshire, Bedford, United Kingdom
Background: Cultural, environmental and logistical factors challenge the Qatari population, particularly females, to engage in physical activity, and there is a high prevalence of diabetes in this population. Sedentary behavior is associated with increased cardiometabolic disease risk and early mortality and breaking up sitting can attenuate postprandial cardiometabolic risk markers. However, no studies have evaluated the cardiometabolic response to breaking up sitting in a Qatari population.
Purpose: To examine the effects of breaking up sitting with moderate-intensity walking breaks on cardiometabolic disease markers in Qatari females.
Methods: Eleven sedentary (sitting ≥ 7 h/day) females completed two experimental conditions in a cross-over randomized design. The two conditions were identical, except participants either remained seated for 5-h (SIT), or interrupted their sitting every 30-min with a 3-min walk (WALK) on a motorized treadmill (rating of perceived exertion 12–14). A fasting venous blood sample was obtained at baseline (-10-min) followed by samples at 0.5-, 1-, 2-, 3-, 3.5-, 4-, and 5-h. Postprandial cardiometabolic variables (insulin, glucose, triglycerides) were calculated as derivatives of total area under the curve [AUC; total (tAUC), net incremental (iAUC) and positive AUC].
Results: Data is reported as effect size; ±90% confidence limit. There was a most likely “moderate” lower tAUC (-0.92 ± 0.26), iAUC (-0.96 ± 0.33), and positive AUC (-0.96 ± 0.33) for insulin in WALK compared to SIT. Additionally, there was a most likely “moderate” lower tAUC (-0.63 ± 0.37), iAUC (-0.91 ± 0.49), and positive AUC (-0.91 ± 0.49) for triglycerides in WALK compared to SIT. Glucose did not differ between conditions.
Conclusion: Breaking up prolonged sitting with moderate-intensity walking offers a culturally compatible intervention to acutely improve cardiometabolic risk markers in sedentary Qatari females. Whilst the data offers promise, the long-term chronic effects of breaking up sitting in Qatari adults requires investigation before population level and/or policy recommendations can be made.
Introduction
Within Qatar, >70% of the population is either “overweight” or “obese” and 83% participate in little or no physical activity (PA) (Qatar Biobank, 2016). Furthermore, there are concerning levels of clinically high cholesterol (44% prevalence), 13% have impaired glucose tolerance; a strong predictor of future diabetes (Unwin et al., 2002; Bener et al., 2009); and diabetes is prevalent in 17% of Qatari adults (Bener et al., 2009). This may be due to lifestyle behaviors that promote poor regulation of postprandial glucose, insulin and triglycerides, which increase cardiometabolic disease risk (O’Keefe and Bell, 2007). Interventions to improve postprandial metabolism are thus required.
Physical activity levels are low in Qatar with 63% of the population engaging in no recreational PA whatsoever (Qatar National Physical Activity Guidelines, 2014). Particular cultural barriers, beliefs, values and practices, as well as the climate (i.e., hot and humid desert climate), and PA infrastructure, challenge this population to engage with and obtain sufficient PA. Indeed, Islamic traditional clothing (i.e., Abaya and Hijab), adopted widely by Qatari females in public places, has been considered an additional barrier regarding engagement in PA (Klautzer et al., 2014) contributing to 44% of Qatari females achieving <5,000 steps per day (Sayegh et al., 2016). Recent systematic review data shows high levels of physical inactivity within Qatar, which may be due to physical exertion being associated with lower status occupations (Sharara et al., 2018), car-dependent transportation and limited opportunities for PA while at work (Mabry et al., 2016). The yearly direct and indirect costs associated with physical inactivity in Qatar is estimated at $60.7 million (Ding et al., 2016). As part of Qatar’s National Health Strategy (Supreme Council of Health, 2013) and the Nutrition and PA Plan to “reduce morbidity and mortality attributable to chronic non-communicable diseases” (Al Thani et al., 2018), Qatar developed its first edition of Qatar’s National PA guidelines (Qatar National Physical Activity Guidelines, 2014). However, these guidelines do not contain any recommendations for reducing or breaking up sedentary time, which could help improve postprandial metabolic risk markers (Healy et al., 2011). Sedentary behavior measurement within the Arab countries (including Qatar) is very limited, with the few studies conducted reporting relatively low levels of daily sedentary time (Mabry et al., 2016). However, these studies used self-report measures which typically underestimate sitting by ≥2 to 3 h/day (Chastin et al., 2014). Given the very low levels of PA in this population, it is highly likely that Qatari females engage in high amounts of sitting that could increase their risk of chronic disease and mortality (Stamatakis et al., 2019). Indeed, the association between sitting and all-cause and cardiovascular-mortality risk is greatest among the least physically active adults (Stamatakis et al., 2019). Cross-sectional evidence has shown that an increased number of breaks in sedentary time is beneficially associated with cardiometabolic risk markers (Healy et al., 2011). Therefore, breaking up sitting time could be a practical and potentially more achievable intervention to improve cardiometabolic risk markers, which can be performed while wearing Islamic clothing. The effects of such an intervention thus requires evaluation in this population.
Several acute laboratory studies have shown improvements in postprandial glucose, insulin, and triglycerides with short-duration (i.e., 2–5-min) and frequent (i.e., every 20–30-min) light or moderate-intensity walking breaks (Peddie et al., 2013; Miyashita et al., 2016; Bailey et al., 2017; Duvivier et al., 2017). However, no study has examined this in a female Arab population. This type of intervention may prove particularly beneficial for Qatari females, who are likely to be highly sedentary (Sayegh et al., 2016), consume a diet that is associated with undesirable postprandial metabolic responses (Al-Thani et al., 2017), and have or are at increased risk of impaired glucose tolerance and diabetes (Bener et al., 2009). Furthermore, females with type 2 diabetes have exhibited a greater attenuation in postprandial glucose in response to breaking up sitting compared to males (Dempsey et al., 2016a), which makes Qatari females an important population to study. Therefore, the aim of this study was to examine the effects of breaking up sitting with short-duration and frequent, moderate-intensity walking breaks on cardiometabolic risk markers in Qatari females. It was hypothesized that glucose, insulin, and triglyceride concentrations would be significantly attenuated in response to breaking up sitting compared within uninterrupted sitting.
Materials and Methods
Experimental Design
This was a randomized crossover design trial. Randomization was completed using GraphPad online QuickCalcs. All experimental procedures were conducted within a temperature controlled laboratory (24 ± 0.3°C). The intervention utilized to break up sitting (see Figure 1 for experimental protocol schematic) has been employed elsewhere (Dunstan et al., 2012; Bailey and Locke, 2015; Bailey et al., 2016). All participants completed a familiarization session followed by two experimental conditions, with temporality indicated on Figure 1. Participants were recruited from February 01, 2017 to November 01, 2017, and were blinded to the condition until arrival on the morning of the first experimental condition. Due to the influence of the female hormonal cycle on glucose metabolism (Bennal and Kerure, 2016), participants completed experimental conditions only in the follicular phase of their menstrual cycle (days 1–10). Participants refrained from exercise for 48-h prior to each condition and recorded volume and timings of all food and liquids consumed in the 24-h prior to the first condition in a food diary and were asked to replicate this intake exactly the day before the subsequent condition. Ethical approval was received from the Anti-Doping Laboratory Qatar Institutional Review Board (IRB# F2016000196). Prior to any experimental procedure occurring, written informed consent was obtained in the spirit of the World Medical Association (2013) and the SPIRIT checklist (Chan et al., 2015).
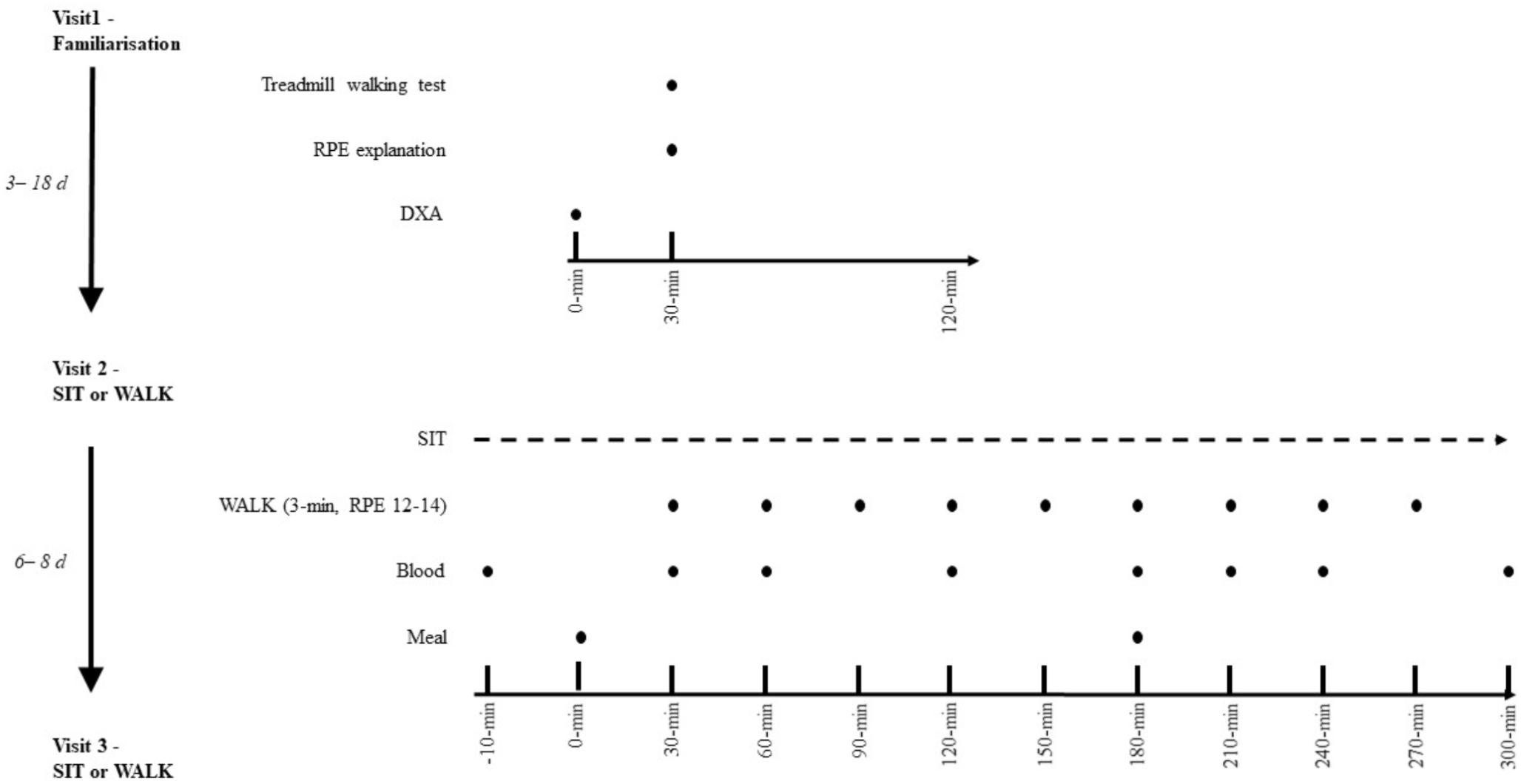
Figure 1. Experimental schematic. SIT, uninterrupted sitting; WALK, breaking up sitting with walking. Both conditions are identical except in SIT, no walking breaks occurred.
Participants
A total of eleven sedentary (sitting ≥ 7 h/day; average 198 MET min/week) Qatari females [median (minimum–maximum) age 27 (21–44) years; height 1.64 (1.57–1.74) meters; body mass 57.8 (47.0–87.4) kg; body fat 36 (24–45) %] completed all experimental procedures. Self-report sitting time was measured using the domain specific sitting time questionnaire (Marshall et al., 2010) and PA (MET min/week) was measured using the International Physical Activity Questionnaire (Craig et al., 2003). Inclusion criteria was premenopausal females aged 18–45 years sitting ≥ 7 h/day. Participants were excluded if they met any of the criteria stated in Table 1. Exclusion criteria was assessed using self-report via completion of an enrolment medical questionnaire. An a priori power calculation based on previous research (Bailey and Locke, 2015) estimated that nine participants would be required to detect an effect size of d = 1.47 with an SD of 2.37, a within-person correlation of 0.5, 95% power, and an alpha of 0.05.
Familiarization Visit
Body composition was analyzed using dual-energy X-ray (DXA) absorptiometry (Lunar Idxa, GE Health Care), and stature was measured using a stadiometer (Holtain Ltd., Crymych, Wales). Participants were familiarized, using standardized language, by the same researcher to the Borg 6–20 (“no exertion at all” – “maximum exertion”) rating of perceived exertion (RPE) scale (Borg, 1982) and completed walking on a motorized treadmill (Pulsar, h/p/cosmos, Nussdorf-Traunstein, Germany) at a 1% gradient to determine a self-perceived moderate-intensity speed (RPE 12–14, “somewhat hard”). In order to determine this self-perceived moderate-intensity speed, participants were asked to walk on the treadmill at a speed that corresponded to an RPE 12–14 for 3-min. They were clearly instructed that it must be a walk, and not a run, and were free to adjust the speed of the treadmill until the appropriate RPE was attained. Each minute, participants were asked for their RPE. After a 5-min rest the participants repeated the walking test to confirm the moderate-intensity walking speed. The speed identified was used during the walking breaks in the relevant experimental condition. Treadmill speed was blinded to the participants throughout this test.
Experimental Conditions
Participants attended the laboratory in the morning (∼08:00 h) following an overnight fast (minimum of 9 h) and were asked to minimize PA during their travel to the laboratory, such as traveling by car. Upon arrival at Aspetar Orthopedic and Sports Medicine Hospital, participants were transported via a wheelchair to the laboratory (∼08:00 h). Participants then transferred to a chair and rested for ∼10 min until the nurse arrived to insert the cannula. Time between arrival and the start of the experimental period was 30–45 min. The two experimental conditions are shown in Figure 1 and were as follows:
Uninterrupted Sitting (SIT)
During this condition, participants remained seated throughout the 5-h experimental period.
Sitting interrupted with walking breaks (WALK): sitting was interrupted every 30-min with 3-min of moderate-intensity walking at the speed that corresponded to an RPE of 12–14 identified during the familiarization session. The mean treadmill belt speed (minimum–maximum) was 6.0 (5.0–8.3) km.h-1. The nine walking break bouts resulted in a total of 27-min walking. Frequent walking breaks (i.e., 3-min) performed for a total time of 27-min at a moderate-intensity walking speed was used in line with the Qatar PA guidelines that focus on increasing moderate-intensity PA for at least 30-min per day, five times per week (Qatar National Physical Activity Guidelines, 2014). The frequency of the breaks was based on previous research demonstrating beneficial glucose responses to breaking up sitting every 30-min (Dunstan et al., 2012; Bailey and Locke, 2015; Bailey et al., 2016).
At 0-h a standardized mixed breakfast meal was consumed. A further snack meal was consumed at 3-h to ensure sufficient glucose and insulin stimulus throughout the experimental period. The composition of the breakfast and snack can be seen in Table 2. The food provided are culturally accepted and representative of a typical Qatari diet which is now largely westernized (Al Thani et al., 2018). The breakfast and snack provided 30 and 20%, respectively, of estimated daily energy needs for each participant. Participant energy requirements were calculated using validated equations with a PA factor of 1.4 applied to represent a sedentary day (Mifflin et al., 1990). The glycaemic index of the breakfast and snack was 70 and 67, respectively, which was calculated as described previously (Bailey et al., 2017). Throughout each condition participants were supervised by a researcher to ensure protocol adherence and they were permitted to watched DVDs, read, talk, or work on a laptop when seated (e.g., to simulate a sedentary “office” environment). Participants were asked to minimize excessive movement while seated. During the conditions, participants were able to void when needed and were transported in a wheelchair to the toilets so that they remained inactive. Water was provided ad libitum during the first condition and the volume consumed was recorded and then replicated during the subsequent condition.
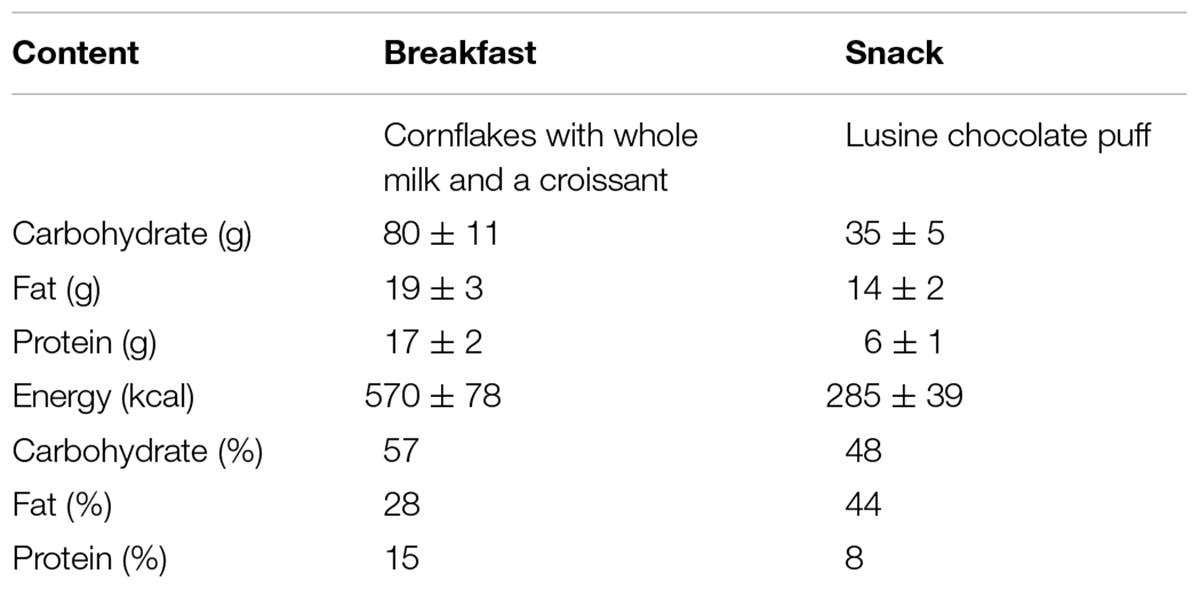
Table 2. Standardized breakfast and snack composition and details. Data is presented as mean ± standard deviation.
Blood Collection and Biochemistry
A fasting venous blood sample was obtained at -10-min (baseline), followed by samples at 0.5-, 1-, 2-, 3-, 3.5-, 4-, and 5-h. A cannula (20 GA 1.25 1.1 mm × 32 mm, BD Nexiva, United States) was inserted into the antecubital fossa by a registered and licensed nurse (Qatar Council for Healthcare Practitioners) following standardized procedures. Blood samples were collected into vacutainers in the following order; serum separator vacutainer (4 mL), EDTA (6 mL), and fluoride tube (4 mL) for the determination of triglycerides, insulin and glucose, respectively. Blood samples were centrifuged at 3000-rpm for 10-min (Multifuge® 1S/1S-R). The resulting serum was decanted in triplicate and stored in Eppendorf tubes (Eppendorf, Hamburg, Germany) at -80°C for subsequent analysis. Glucose and triglyceride concentrations were measured using a clinical chemistry analyzer (Medica EasyRA, Medica Corporation, Bedford, MA, United States). Insulin was measured using a commercially available enzyme linked immunoassay kit (Mercodia AB, Uppsala, Sweden, cat# 10-1113-10) with absorbance read using a microplate photometer (Multiskan FC, Thermo Fisher Scientific, Waltham, MA, United States). All samples were run in duplicate, and the intra-assay coefficient of variance was 12.3% for glucose, 4.5% for insulin, and 3.8% for triglycerides.
Calculation of Outcome Variables
For each cardiometabolic risk marker, total area under the curve (tAUC) was first calculated using the trapezoidal rule. Net incremental area under the curve (iAUC) was calculated by subtracting the baseline area from tAUC, and positive iAUC was calculated whereby any value below baseline was treated as a baseline value. These area under the curve variables were calculated for the total 5-h experimental period in addition to separately for the breakfast and snack postprandial periods. The primary outcome was net iAUC for postprandial glucose across the 5-h experimental period as this is suggested as the most appropriate method for describing postprandial glycaemic responses (Le Floch et al., 1990).
Statistical Analyses
Statistical analyses were performed using the statistical package for the social sciences (SPSS) version 24 (IBM, SPSS Inc, Chicago, IL, United States) and magnitude-based inferences (MBIs) customizable spreadsheets, using the raw data (Hopkins et al., 2009). Prior to analyses, data were checked and confirmed for assumptions of normality using quantile-quantile (Q-Q) plots (Grafen and Hails, 2002). Descriptive statistics are reported as mean ± standard deviation (SD) and range (minimum–maximum). Differences between conditions (SIT and WALK) for each of the AUC variables (adjusted for baseline blood values, age, and body fat%) were examined using linear mixed models (LMM). Fixed (i.e., condition, baseline values, age, and body fat%) and random (i.e., participants) effects for the LMM were fit for each dependent variable (West et al., 2014). Normality and homogeneity of variance of the residuals were checked using Q-Q plots and scatter plots, respectively, and deemed plausible in each instance. The smallest Hurvich and Tsai criterion (AICC) was used to determine the most appropriate model (Hurvich and Tsai, 1995) in accordance with the principle of parsimony. The least squares mean test provided pairwise comparisons between the conditions. Significant effects were further assessed using Cohen’s d effect sizes (ES), and 90% confidence limits (CLs) using the MBI spreadsheets, and categorized using standardized thresholds of; <0.2 trivial, 0.21–0.60 small, 0.61–1.20 moderate, 1.21–2.0 large, and >2.0 very large (Hopkins et al., 2009) only when the LMM results showed a significant p value. A magnitude-based approach was adopted, where differences of >75% likelihood of being greater than the smallest worthwhile effect (0.20 × between subject SD), were reported using the following qualitative descriptions: 75–95% likely, 95–99.5% very likely, and >99.5% most likely (Hopkins et al., 2009). Data is reported as ES; ± 90% CL. Significance was accepted as p ≤ 0.05.
Results
Sixteen participants were recruited for this study. Three participants withdrew from the study prior to the familiarization visit, and two participants withdrew prior to completion of the experimental conditions. The cardiometabolic results for SIT and WALK are shown in Table 3. Baseline (fasting) glucose, insulin and triglycerides did not differ between conditions. Fasting glucose values on average were considered normal (i.e., <6.1 mmol/L) (World Health Organization, 2006) with only one individual presenting a value consistent with impaired fasting glucose. Fasting triglycerides levels for the participants were considered “optimal” or “normal” (Miller et al., 2011). The average body fat in the present study was 36% [minimum–maximum (24–45 %)] suggesting that overall the sample was obese; only n = 2 participants were considered “non-obese.”
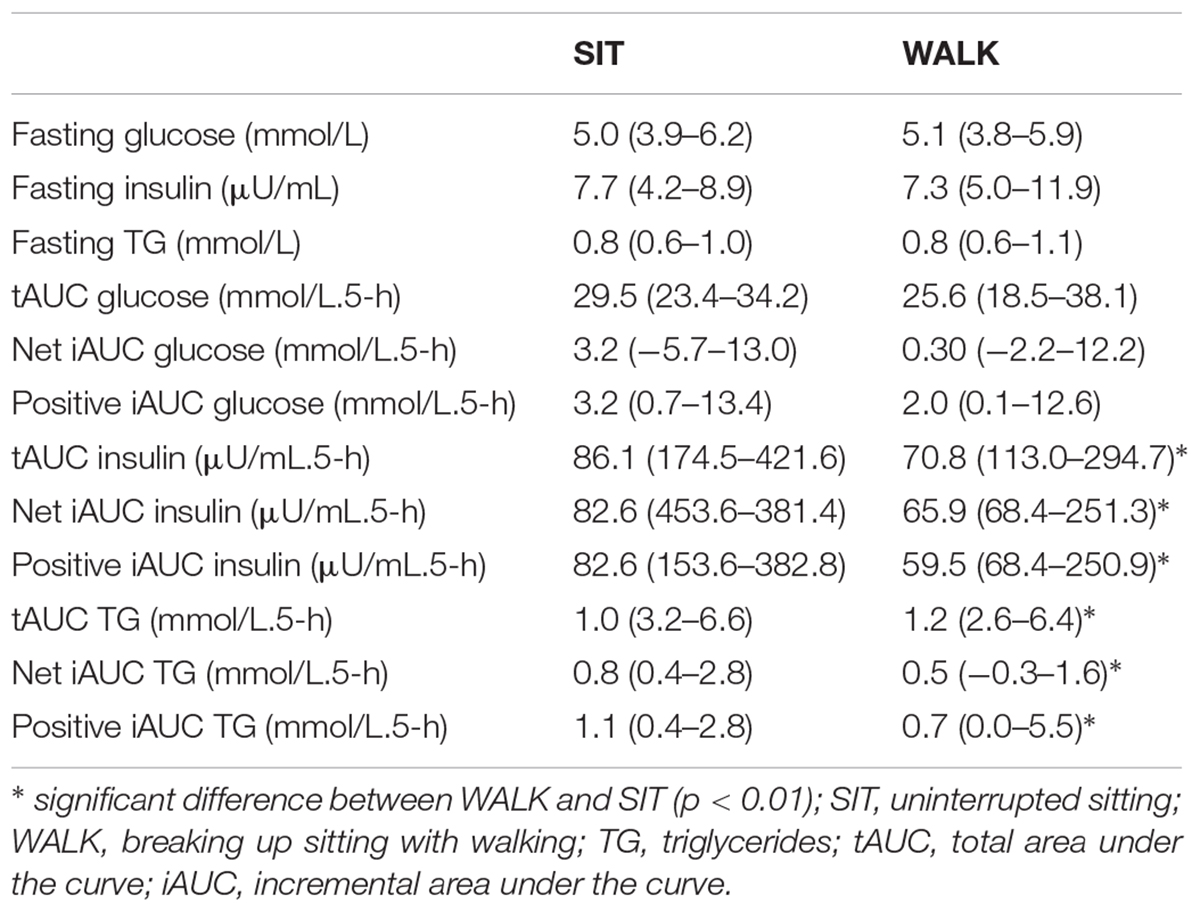
Table 3. Cardiometabolic risk marker values in SIT and WALK. Data are presented as median (minimum–maximum).
For the total 5-h experimental period, there was a significant main effect of condition for insulin tAUC (p < 0.001), iAUC (p < 0.001), and positive iAUC (p < 0.001). Specifically, there was a most likely “moderate” lower tAUC (-0.92 ± 0.26), iAUC (-0.96 ± 0.33), and positive iAUC (-0.96 ± 0.33) for insulin in WALK compared to SIT. Additionally, there was a significant main effect of condition for triglyceride tAUC (p = 0.009), iAUC (p = 0.005), and positive iAUC (p = 0.009). Compared with SIT, there was a most likely “moderate” lower tAUC (-0.63 ± 0.37), iAUC (-0.91 ± 0.49), and positive iAUC (-0.91 ± 0.49) for triglycerides during WALK. Glucose tAUC, iAUC and positive iAUC did not differ significantly between conditions (p ≥ 0.14). Time course data for changes in glucose, insulin and triglycerides is shown in Figure 2.
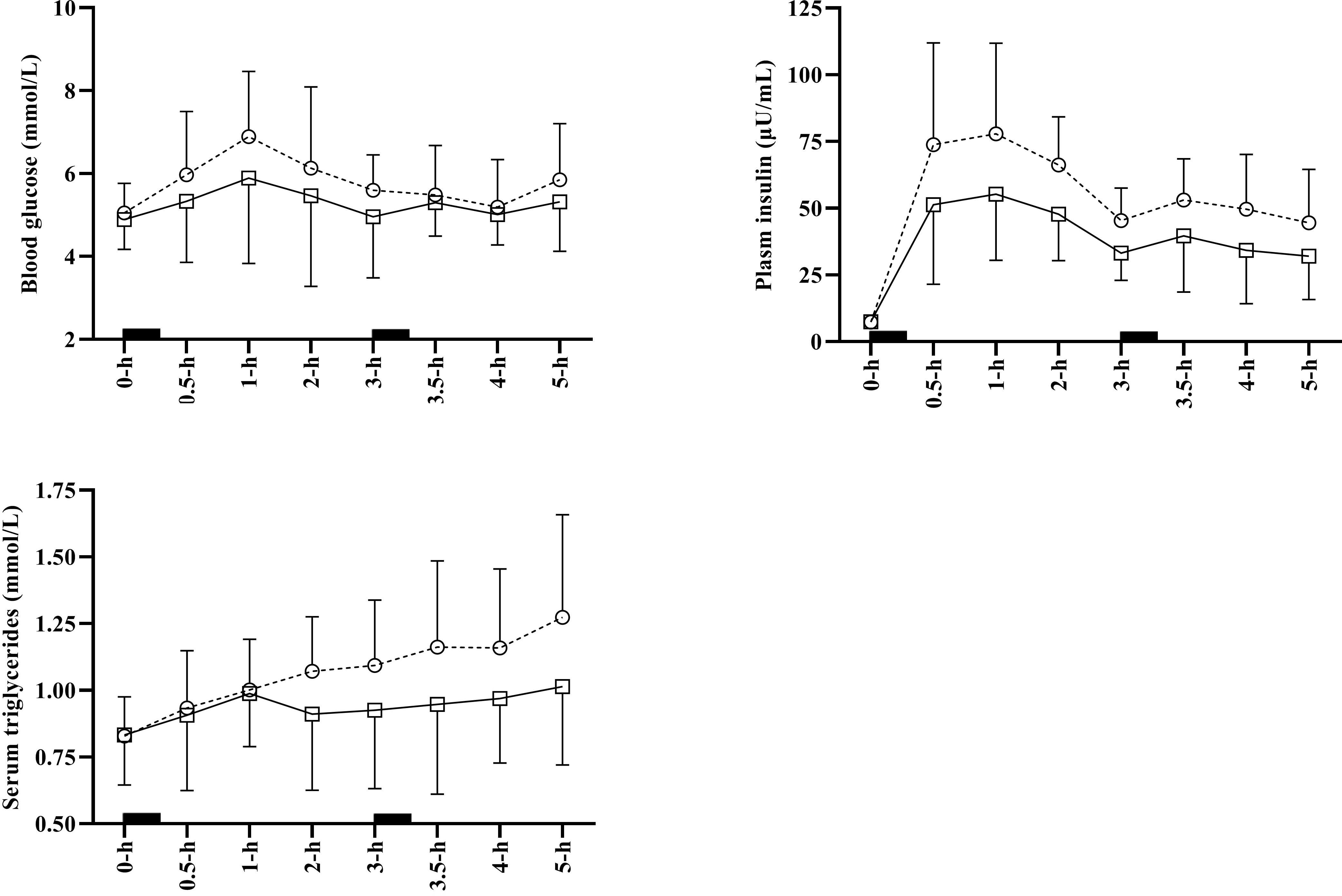
Figure 2. Average time course data for glucose, insulin and triglycerides for SIT (open circles and dashed line), and WALK (open squares and sold line). Error bars indicate standard deviation and solid black squares indicate meal consumption. Some error bars have been omitted to aid clarity. SIT, uninterrupted sitting; WALK, breaking up sitting with walking.
When evaluating the breakfast postprandial period, the findings were consistent with the total 5-h experimental period with none of the glucose AUC variables being different between conditions (all p > 0.220; see Supplementary Table 1). There was a significant main effect of condition for insulin tAUC (p = 0.047), iAUC (p = 0.001), and positive iAUC (p = 0.004). Specifically, there was a most likely “moderate” lower tAUC (-0.89; ± 0.39) and iAUC (-0.93; ± 0.41), and a very likely “moderate” lower positive iAUC in WALK compared to SIT. For triglycerides, there was a significant main effect of condition for tAUC (p = 0.049), and iAUC (p = 0.007). Within WALK there was a likely “small” lower tAUC (-0.42 ± 0.39) and very likely “moderate” lower iAUC (-0.82 ± 0.47) compared to SIT. For the snack postprandial period, there was a significant main effect of condition for insulin tAUC (p = 0.017), and triglyceride tAUC (p = 0.031). Within WALK there was a very likely “moderate” lower insulin tAUC (-0.80 ± 0.54) and likely “moderate” lower triglyceride tAUC (-0.69 ± 0.51) compared with SIT (see Supplementary Table 2). There were no significant differences for the snack postprandial period for any other AUC variables (p > 0.33).
Discussion
The main findings in the present study were that breaking up sitting with 3-min of moderate-intensity walking every 30-min improved postprandial triglycerides and insulin over a 5-h period compared to uninterrupted sitting.
In comparison to findings in the present study, previous acute studies examining the effects of regularly breaking up sitting time on triglycerides have shown mixed results (Altenburg et al., 2013; Peddie et al., 2013; Bailey and Locke, 2015; Miyashita et al., 2016; Maylor et al., 2018). In agreement with the present findings, previous research has found that interrupted sitting every 30 min with brisk walking significantly reduced and triglyceride concentrations (Miyashita et al., 2016). It appears from previous studies that frequent short light or moderate-intensity PA breaks (Peddie et al., 2013; Miyashita et al., 2016), rather than longer duration less frequent PA breaks (Altenburg et al., 2013; Maylor et al., 2018), may be superior relative to improving postprandial triglycerides. These frequency dependent responses may be a result of enhanced lipoprotein lipase enzyme activity due to greater stimuli (e.g., more frequent and higher volume of activity bouts) (Peddie et al., 2013). The present study supports the efficacy of frequent moderate-intensity PA breaks in for suppressing postprandial triglycerides in Qatari females. However, studies that directly compare different frequencies and intensities of PA breaks are needed to confirm a dose-response effect. As hypertriglyceridemia (elevated postprandial triglycerides) significantly increases the risk of cardiovascular disease (O’Keefe and Bell, 2007; Watson and Wiesner, 2016), breaking up sitting in Qatari adults could be an important public health target given the concerning high prevalence of cardiometabolic disease in this population (Qatar Biobank, 2016). Long-term intervention studies are thus required to establish the potential chronic effects of breaking up siting time.
The postprandial glucose and insulin response to breaking up sitting is mixed within the literature (Dunstan et al., 2012; Peddie et al., 2013; Bailey et al., 2016, 2017; Hansen et al., 2016; Miyashita et al., 2016). In agreement with the present study, short-duration and frequent walking breaks have been shown to attenuate insulin concentrations (Dunstan et al., 2012; Peddie et al., 2013). Conversely, no effect on insulin concentration has also been reported in response to breaking up sitting with light or moderate-intensity walking (Bailey et al., 2016, 2017; Hansen et al., 2016; Miyashita et al., 2016). However, it should be noted that none of these studies were powered to detect changes in postprandial insulin and this may thus lead to null findings. Also in contradiction to findings in the present study, reduced glucose concentrations have been reported in response to moderate-intensity walking breaks (Dunstan et al., 2012; Peddie et al., 2013; Miyashita et al., 2016; Bailey et al., 2017). Variation in the findings from these studies may be due to composition of the test meals provided (i.e., carbohydrate content, glycaemic index, and load), individual differences (i.e., genetics), and duration and intensity of PA used to break up sitting. Furthermore, the cardiometabolic health profile of the participants studied (e.g., normal vs. impaired glycaemia) may affect glucose and insulin responses to PA breaks (Benatti and Ried-Larsen, 2015). Although the body fat % of the participants in the present study was relatively high (i.e., >30%), only one participant reached the criteria for impaired fasting glucose levels. Therefore, overall the participants in the present study can be categorized as overweight but metabolically healthy. Studies in people with high adiposity (Dunstan et al., 2012), impaired glucose levels (Henson et al., 2016), and Type 2 diabetes (Dempsey et al., 2016a) have reported greater attenuation of postprandial insulin and glucose in response to breaking up sitting (Dempsey et al., 2016a) compared with studies in “healthy” samples (Bailey and Locke, 2015; Bailey et al., 2016; Maylor et al., 2018). Individuals with high body fat who are metabolically impaired may thus benefit more from breaking up sitting time than those who are healthy and non-overweight. However, further studies adequately powered to detect the interacting effects of health status with breaking up sitting are required before definitive conclusions can be made in this respect.
In the present sample, it appears that breaking up sitting enhances insulin-stimulated glucose disposal in light of the unchanged postprandial glucose levels despite a reduction in insulin concentrations. It has been postulated that breaking up sitting may improve insulin sensitivity via upregulation of insulin signaling pathways (e.g., the Akt-mediated insulin-sensitive glucose uptake pathway) (Bergouignan et al., 2016), increases in muscle insulin sensitivity, or changes in sympathetic nervous system activity (Dempsey et al., 2016b). However, it is not possible to determine which of these potential mechanisms are responsible for the reductions in postprandial insulin in the present study and future research should evaluate such mechanisms to elucidate the reasons for cardiometabolic benefits observed in response to breaking up sitting.
The present data and associated discussion above centers on acute studies, and evidently longer term interventions produce more robust evidence, from which PA policy adoption evolves. Currently, such longer-term evidence from experimental designs using a comparative intervention and outcome measures to the present study is not available. As aforementioned, >70% of the Qatari population is either “overweight” or “obese” (Qatar Biobank, 2016). Furthermore, 86% of Qatari females report engaging in no recreational PA whatsoever (Qatar National Physical Activity Guidelines, 2014) The participants in the current study thus appear to represent the general female Qatari population and future research evaluating the long-term chronic response to breaking up sitting should be conducted in order to corroborate the findings from this acute study and establish evidence based guidelines for reducing sedentary behavior in this population.
This study is limited as it was a laboratory controlled study, and therefore, results are not generalisable to a workplace or domestic environment. Light-intensity walking (as opposed to the moderate-intensity walking employed) could have greater ecologically validity. Future work should examine the effects of light-intensity walking breaks on cardiometabolic responses in Qatari females within the workplace and during leisure time.
Conclusion
Breaking up prolonged sitting with short-duration frequent moderate-intensity walking improves postprandial triglyceride and insulin responses in Qatari females. Given the low PA levels and undesirable diet (despite country specific recommendations for both), this population predisposes itself to a high risk of cardiometabolic disease and early mortality. Therefore, breaking up sitting time, which may be a more achievable target than increasing moderate-to-vigorous PA levels, could be recommended for reducing cardiometabolic disease risk in this population. However, before population wide recommendations or policy progression is initiated, it is important to examine the long-term feasibility and efficacy of breaking up sitting in the Qatari population.
Ethics Statement
Ethical approval was received from the Anti-Doping Laboratory Qatar Institutional Review Board (IRB# F2016000196). Prior to any experimental procedure occurring, written informed consent was obtained in the spirit of the World Medical Association (2013) and the SPIRIT checklist.
Author Contributions
BC, DB, and LT contributed to study conception, interpretation, and manuscript writing. BC, AC, SS, NR, AE-G, and SA contributed to data collection, participant recruitment, and analyses. All authors edited and approved the final manuscript.
Funding
Funding for this study was received from Qatar University Centre for Health Sciences SEED interdisciplinary grant (CHSS-SF-16-2). The publication of this article was funded by the Qatar National Library.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling Editor declared a past co-authorship with authors BC and LT.
Acknowledgments
We thank the participants for their time. We also thank Aspetar Orthopaedic and Sports Medicine Hospital for providing the following expertise, Tessa Smith and Helen Lloyd (Department of Medical Imaging) for their performances in DXA scans, Fabricio Forchino [Exercise and Sport Science Department (ESSD)] for analyzing the DXA results, and Dhouha Ben Soltane (Anesthesia and Recovery Department clinical nurse) and Elena Curatenie [National Sports Medicine Program (NSMP) nurse] for their cannulation and blood sampling.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2019.00706/full#supplementary-material
References
Al Thani, M., Al Thani, A. A., Al-Chetachi, W., Al Malki, B., Khalifa, S. A. H., Bakri, A. H., et al. (2018). Adherence to the Qatar dietary guidelines: a cross-sectional study of the gaps, determinants and association with cardiometabolic risk amongst adults. BMC Public Health 18:503. doi: 10.1186/s12889-018-5400-2
Altenburg, T. M., Rotteveel, J., Dunstan, D. W., Salmon, J., and Chinapaw, M. J. (2013). The effect of interrupting prolonged sitting time with short, hourly, moderate-intensity cycling bouts on cardiometabolic risk factors in healthy, young adults. J. Appl. Physiol. 115, 1751–1756. doi: 10.1152/japplphysiol.00662.2013
Al-Thani, M., Al-Thani, A.-A., Al-Mahdi, N., Al-Kareem, H., Barakat, D., Al-Chetachi, W., et al. (2017). An overview of food patterns and diet quality in qatar: findings from the national household income expenditure survey. Cureus 9:e1249. doi: 10.7759/cureus.1249
Bailey, D. P., Broom, D. R., Chrismas, B. C., Taylor, L., Flynn, E., and Hough, J. (2016). Breaking up prolonged sitting time with walking does not affect appetite or gut hormone concentrations but does induce an energy deficit and suppresses postprandial glycaemia in sedentary adults. Appl. Physiol. Nutr. Metab. 41, 324–331. doi: 10.1139/apnm-2015-0462
Bailey, D. P., and Locke, C. D. (2015). Breaking up prolonged sitting with light-intensity walking improves postprandial glycemia, but breaking up sitting with standing does not. J. Sci. Med. Sport 18, 294–298. doi: 10.1016/j.jsams.2014.03.008
Bailey, D. P., Maylor, B. D., Orton, C. J., and Zakrzewski-Fruer, J. K. (2017). Effects of breaking up prolonged sitting following low and high glycaemic index breakfast consumption on glucose and insulin concentrations. Eur. J. Appl. Physiol. 117, 1299–1307. doi: 10.1007/s00421-017-3610-4
Benatti, F. B., and Ried-Larsen, M. (2015). The effects of breaking up prolonged sitting time: a review of experimental studies. Med. Sci. Sports Exerc. 47, 2053–2061. doi: 10.1249/mss.0000000000000654
Bener, A., Zirie, M., Janahi, I. M., Al-Hamaq, A. O., Musallam, M., and Wareham, N. J. (2009). Prevalence of diagnosed and undiagnosed diabetes mellitus and its risk factors in a population-based study of Qatar. Diabetes Res. Clin. Pract. 84, 99–106. doi: 10.1016/j.diabres.2009.02.003
Bennal, A. S., and Kerure, S. B. (2016). Glucose handling during menstrual cycle. Int. J. Reprod. Contracept. Obstet. Gyneco. 2, 284–287. Available at: https://www.ijrcog.org/index.php/ijrcog/article/view/94 (accessed June 01, 2019).
Bergouignan, A., Latouche, C., Heywood, S., Grace, M. S., Reddy-Luthmoodoo, M., Natoli, A. K., et al. (2016). Frequent interruptions of sedentary time modulates contraction- and insulin-stimulated glucose uptake pathways in muscle: ancillary analysis from randomized clinical trials. Sci. Rep. 6:32044. doi: 10.1038/srep32044
Borg, G. A. (1982). Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 14, 377–381.
Chan, A. W., Tetzlaff, J. M., Altman, D. G., Laupacis, A., Gotzsche, P. C., Krle, A. J. K., et al. (2015). SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann. Intern. Med. 38, 506–514.
Chastin, S. F., Culhane, B., and Dall, P. M. (2014). Comparison of self-reported measure of sitting time (IPAQ) with objective measurement (activPAL). Physiol. Meas. 35, 2319–2328. doi: 10.1088/0967-3334/35/11/2319
Craig, C. L., Marshall, A. L., Sjostrom, M., Bauman, A. E., Booth, M. L., Ainsworth, B. E., et al. (2003). International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 35, 1381–1395. doi: 10.1249/01.mss.0000078924.61453.fb
Dempsey, P. C., Larsen, R. N., Sethi, P., Sacre, J. W., Straznicky, N. E., Cohen, N. D., et al. (2016a). Benefits for type 2 diabetes of interrupting prolonged sitting with brief bouts of light walking or simple resistance activities. Diabetes Care 39, 964–972. doi: 10.2337/dc15-2336
Dempsey, P. C., Owen, N., Yates, T. E., Kingwell, B. A., and Dunstan, D. W. (2016b). Sitting less and moving more: improved glycaemic control for type 2 diabetes prevention and management. Curr. Diab. Rep. 16:114. doi: 10.1007/s11892-016-0797-4
Ding, D., Lawson, K. D., Kolbe-Alexander, T. L., Finkelstein, E. A., Katzmarzyk, P. T., van Mechelen, W., et al. (2016). The economic burden of physical inactivity: a global analysis of major non-communicable diseases. Lancet 388, 1311–1324. doi: 10.1016/s0140-6736(16)30383-x
Dunstan, D. W., Kingwell, B. A., Larsen, R., Healy, G. N., Cerin, E., Hamilton, M. T., et al. (2012). Breaking up prolonged sitting reduces postprandial glucose and insulin responses. Diabetes Care 35, 976–983. doi: 10.2337/dc11-1931
Duvivier, B. M. F. M., Schaper, N. C., Hesselink, M. K. C., van Kan, L., Stienen, N., Winkens, B., et al. (2017). Breaking sitting with light activities vs structured exercise: a randomised crossover study demonstrating benefits for glycaemic control and insulin sensitivity in type 2 diabetes. Diabetologia 60, 490–498. doi: 10.1007/s00125-016-4161-7
Grafen, G., and Hails, R. (2002). Modern Statistics for the Life Sciences. New York, NY: Oxford University Press.
Hansen, R. K., Andersen, J. B., Vinther, A. S., Pielmeier, U., and Larsen, R. G. (2016). Breaking up prolonged sitting does not alter postprandial glycemia in young, normal-weight men and women. Int. J. Sports Med. 37, 1097–1102. doi: 10.1055/s-0042-113466
Healy, G. N., Matthews, C. E., Dunstan, D. W., Winkler, E. A., and Owen, N. (2011). Sedentary time and cardio-metabolic biomarkers in US adults: NHANES 2003-06. Eur. Heart J. 32, 590–597. doi: 10.1093/eurheartj/ehq451
Henson, J., Davies, M. J., Bodicoat, D. H., Edwardson, C. L., Gill, J. M., Stensel, D. J., et al. (2016). Breaking up prolonged sitting with standing or walking attenuates the postprandial metabolic response in postmenopausal women: a randomized acute study. Diabetes Care 39, 130–138. doi: 10.2337/dc15-1240
Hopkins, W. G., Marshall, S. W., Batterham, A. M., and Hanin, J. (2009). Progressive statistics for studies in sports medicine and exercise science. Med. Sci. Sports Exerc. 41, 3–13. doi: 10.1249/MSS.0b013e31818cb278
Hurvich, C. M., and Tsai, C. L. (1995). Model selection for extended quasi-likelihood models in small samples. Biometrics 51, 1077–1084.
Klautzer, L., Becker, J., and Mattke, S. (2014). The curse of wealth - middle eastern countries need to address the rapidly rising burden of diabetes. Int. J. Health Policy Manag. 2, 109–114. doi: 10.15171/ijhpm.2014.33
Le Floch, J. P., Escuyer, P., Baudin, E., Baudon, D., and Perlemuter, L. (1990). Blood glucose area under the curve. Methodological aspects. Diabetes Care 13, 172–175. doi: 10.2337/diacare.13.2.172
Mabry, R., Koohsari, M. J., Bull, F., and Owen, N. (2016). A systematic review of physical activity and sedentary behaviour research in the oil-producing countries of the Arabian Peninsula. BMC Public Health 16:1003. doi: 10.1186/s12889-016-3642-4
Marshall, A. L., Miller, Y. D., Burton, N. W., and Brown, W. J. (2010). Measuring total and domain-specific sitting: a study of reliability and validity. Med. Sci. Sports Exerc. 42, 1094–1102. doi: 10.1249/MSS.0b013e3181c5ec18
Maylor, B. D., Zakrzewski-Fruer, J. K., Orton, C. J., and Bailey, D. P. (2018). Beneficial postprandial lipaemic effects of interrupting sedentary time with high-intensity physical activity versus a continuous moderate-intensity physical activity bout: a randomised crossover trial. J. Sci. Med. Sport 21, 1250–1255. doi: 10.1016/j.jsams.2018.05.022
Mifflin, M. D., St Jeor, S. T., Hill, L. A., Scott, B. J., Daugherty, S. A., and Koh, Y. O. (1990). A new predictive equation for resting energy expenditure in healthy individuals. Am. J. Clin. Nutr. 51, 241–247. doi: 10.1093/ajcn/51.2.241
Miller, M., Stone Neil, J., Ballantyne, C., Bittner, V., Criqui Michael, H., Ginsberg Henry, N., et al. (2011). Triglycerides and cardiovascular disease. Circulation 123, 2292–2333. doi: 10.1161/CIR.0b013e3182160726
Miyashita, M., Edamoto, K., Kidokoro, T., Yanaoka, T., Kashiwabara, K., Takahashi, M., et al. (2016). Interrupting sitting time with regular walks attenuates postprandial triglycerides. Int. J. Sports Med. 37, 97–103. doi: 10.1055/s-0035-1559791
O’Keefe, J. H., and Bell, D. S. H. (2007). Postprandial hyperglycemia/hyperlipidemia (Postprandial Dysmetabolism) is a cardiovascular risk factor. Am. J. Cardiol. 100, 899–904. doi: 10.1016/j.amjcard.2007.03.107
Peddie, M. C., Bone, J. L., Rehrer, N. J., Skeaff, C. M., Gray, A. R., and Perry, T. L. (2013). Breaking prolonged sitting reduces postprandial glycemia in healthy, normal-weight adults: a randomized crossover trial. Am. J. Clin. Nutr. 98, 358–366. doi: 10.3945/ajcn.112.051763
Qatar Biobank (2016). Qatar Qatar Biobank, 2016; Qatar National Physical Activity Guidelines, 2014; Supreme Council of Health, 2013; World Health Organization, 2006. Biobank Report. Qatar. Available: https://www.qatarbiobank.org.qa/app/media/1730 (accessed November 15, 2018).
Qatar National Physical Activity Guidelines (2014). Qatar National Physical Activity Guidelines. Qatar: Aspetar Orthopaedic & Sports Medicine Hospital. Available: https://www.namat.qa/NamatImages/Publications/75/QATAR%20PA%20GUIDLINE%20ENGLISH.PDF (accessed September 6, 2018).
Sayegh, S., Van Der Walt, M., and Al-Kuwari, M. G. (2016). One-year assessment of physical activity level in adult Qatari females: a pedometer-based longitudinal study. Int. J. Womens Health 8, 287–293. doi: 10.2147/IJWH.S99943
Sharara, E., Akik, C., Ghattas, H., and Makhlouf Obermeyer, C. (2018). Physical inactivity, gender and culture in Arab countries: a systematic assessment of the literature. BMC Public Health 18:639. doi: 10.1186/s12889-018-5472-z
Stamatakis, E., Gale, J., Bauman, A., Ekelund, U., Hamer, M., and Ding, D. (2019). Sitting time, physical activity, and risk of mortality in adults. J. Am. Coll. Cardiol. 73, 2062–2072. doi: 10.1016/j.jacc.2019.02.031
Supreme Council of Health (2013). National Health Strategy 2011-2016 Caring for the Future: Project Implementation Plans Update 2013. Qatar. Available: http://www.nationalplanningcycles.org/sites/default/files/country_docs/Qatar/nhp_qatar.pdf (accessed April 25, 2018).
Unwin, N., Shaw, J., Zimmet, P., and Alberti, K. G. (2002). Impaired glucose tolerance and impaired fasting glycaemia: the current status on definition and intervention. Diabet Med. 19, 708–723. doi: 10.1046/j.1464-5491.2002.00835.x
Watson, K. E., and Wiesner, P. (2016). Slippery slope of triglycerides: they are associated with risk, but in the statin era, does targeting them confer benefit? Circ. Cardiovasc. Qual. Outcomes 9, 97–99. doi: 10.1161/circoutcomes.116.002675
West, B. T., Welch, K. B., and Galecki, A. T. (2014). Linear Mixed Models: A Practical Guide Using Statistical Software. Didcot: Taylor and Francis.
World Health Organization (2006). Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycaemia. Available: http://www.who.int/diabetes/publications/Definition%20and%20diagnosis%20of%20diabetes_new.pdf (accessed November 15, 2018).
Keywords: sedentary behavior, cardiometabolic markers, physical inactivity, cardiovascular disease, diabetes
Citation: Chrismas BCR, Taylor L, Cherif A, Sayegh S, Rizk N, El-Gamal A, Allenjawi SH and Bailey DP (2019) Postprandial Insulin and Triglyceride Concentrations Are Suppressed in Response to Breaking Up Prolonged Sitting in Qatari Females. Front. Physiol. 10:706. doi: 10.3389/fphys.2019.00706
Received: 07 January 2019; Accepted: 20 May 2019;
Published: 11 June 2019.
Edited by:
David C. Hughes, The University of Iowa, United StatesReviewed by:
Ryan Godsk Larsen, Aalborg University, DenmarkSophie Carter, York St John University, United Kingdom
Copyright © 2019 Chrismas, Taylor, Cherif, Sayegh, Rizk, El-Gamal, Allenjawi and Bailey. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bryna Chrismas, YmNocmlzbWFzQHF1LmVkdS5xYQ==
 Bryna C. R. Chrismas
Bryna C. R. Chrismas Lee Taylor
Lee Taylor Anissa Cherif2
Anissa Cherif2 Nasser Rizk
Nasser Rizk Abdelrahman El-Gamal
Abdelrahman El-Gamal Daniel P. Bailey
Daniel P. Bailey