
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Physiol., 08 February 2019
Sec. Integrative Physiology
Volume 10 - 2019 | https://doi.org/10.3389/fphys.2019.00083
This article is part of the Research TopicInsights into Brown Adipose Tissue Functions and Browning PhenomenonView all 19 articles
 Madhu Asnani-Kishnani1
Madhu Asnani-Kishnani1 Ana M. Rodríguez1,2,3
Ana M. Rodríguez1,2,3 Alba Serrano1,2
Alba Serrano1,2 Andreu Palou1,2,3
Andreu Palou1,2,3 M. Luisa Bonet1,2,3*
M. Luisa Bonet1,2,3* Joan Ribot1,2,3
Joan Ribot1,2,3Nutritional programming of the thermogenic and fuel oxidation capacity of white adipose tissue (WAT) through dietary interventions in early life is a potential strategy to enhance future metabolic health. We previously showed that mild neonatal supplementations with the polyphenol resveratrol (RSV) and the vitamin B3 form nicotinamide riboside (NR) have sex-dependent, long-term effects on the thermogenic/oxidative phenotype of WAT of mice in adulthood, enhancing this phenotype selectively in male animals. Here, we tested the hypothesis that these dietary interventions may impact the commitment of progenitor cells resident in the developing WAT toward brown-like (beige) adipogenesis. NMRI mice received orally from postnatal day 2–20 (P2–20) a mild dose of RSV or NR, in independent experiments; control littermates received the vehicle. Sex-separated primary cultures were established at P35 from the stromovascular fraction of inguinal WAT (iWAT) and of brown adipose tissue (BAT). Expression of genes related to thermogenesis and oxidative metabolism was assessed in the differentiated cultures, and in vivo in the iWAT depot of young (P35) animals. Neonatal RSV and NR treatments had little impact on the animals’ growth during early postnatal life and the expression of thermogenesis- and oxidative metabolism-related genes in the iWAT depot of young mice. However, the expression of brown/beige adipocyte marker genes was upregulated in the iWAT primary cultures from RSV supplemented and NR supplemented male mice, and downregulated in those from supplemented female mice, as compared to cultures derived from sex-matched control littermates. RSV supplementation had similar sex-dependent effects on the expression of thermogenesis-related genes in the BAT primary cultures. A link between the sex-dependent short-term effects of neonatal RSV and NR supplementations on primary iWAT preadipocyte differentiation observed herein and their previously reported sex-dependent long-term effects on the thermogenic/oxidative capacity of adult iWAT is suggested. The results provide proof-of-concept that the fate of preadipocytes resident in WAT of young animals toward the beige adipogenesis transcriptional program can be modulated by specific food bioactives/micronutrients received in early postnatal life.
Brown adipocytes in canonical BAT depots in mammals express uncoupling protein 1 (UCP1) and a high oxidative capacity and mitochondria content, which distinguishes them metabolically from white adipocytes in typical WAT depots. A third type of adipocytes, beige adipocytes (also called brite [brown-in-white]), are brown-like adipocytes found in WAT depots. Beige adipocytes share with brown adipocytes a common core thermogenesis gene signature including inducible UCP1 expression, which allows for the regulated dissipation as heat of the energy contained in fatty acid and glucose fuel molecules (Bartelt and Heeren, 2014). Additionally, transcripts of several markers have been identified that appear to be specific for beige adipocytes (Wu et al., 2012). The emergence of beige adipocytes in WAT is termed WAT browning or beigeing. BAT activation and WAT browning are both viewed as potential therapeutic targets in the management of obesity and related metabolic disorders such as hyperlipidemia and diabetes (Bonet et al., 2013; Harms and Seale, 2013).
The recruitment of brown and beige adipocytes responds to a variety of hormonal and diet-related stimulus including the intake of specific food bioactives (Bonet et al., 2013, 2017). This is well established in adult rodents and appears to hold for humans as well (Rossato et al., 2014; Fleckenstein-Elsen et al., 2016; Palmeri et al., 2016; Saito et al., 2016). Besides contemporary dietary intake in adulthood, exposure to food bioactives in prenatal and early postnatal life might be important. Epidemiological and experimental evidence indicates that early life nutrition can influence metabolic health in adulthood through programming effects on the developing tissues, with consequences in the long-term (Cottrell and Ozanne, 2008). The browning potential of WAT might be nutritionally programmed (Palou et al., 2013), as well as other aspects such as WAT cellularity (Lecoutre and Breton, 2014), though these aspects are still largely unexplored.
The B3 vitamin and NAD+ precursor NR and the polyphenol resveratrol (RSV; 3,5,4′-trihydroxy-trans-stilbene) are two dietary compounds that enhance oxidative metabolism in adipose and other tissues when supplemented to adult rodents, possibly through their shared ability to activate sirtuin 1 (SIRT1) protein deacetylase (Lagouge et al., 2006; Canto et al., 2012; Alberdi et al., 2013; Andrade et al., 2014; Wang et al., 2015). Nutritional programming by RSV and NR has recently begun to be addressed (Zou et al., 2017; Ros et al., 2018; Serrano et al., 2018). We have shown that direct treatment of newborn mouse with mild doses of RSV or NR throughout lactation leads to sex-dependent long-term effects on the WAT thermogenic/oxidative phenotype. Signs of white-to-brown fat remodeling of iWAT in adulthood including smaller adipocyte size, enrichment in multilocular adipocytes, increased immunostaining against UCP1 and the respiratory chain protein component COXIV, and higher expression levels of genes related to brown fat determination, mitochondrial oxidative metabolism and beige markers (e.g., Prdm16, Ppargc1b, Pparg, Cpt1b, Mfn2, and Slc27a1) were found selectively in the treated male mice relative to sex-matched controls (Serrano et al., 2018). In parallel with these changes in WAT, RSV, and NR treated male mice displayed in adulthood better responses to an obesogenic high-fat diet than controls, such as a delayed body weight gain, a blunted increase in the plasma leptin-to-adiponectin ratio and a decreased lipolytic response (Serrano et al., 2018). However, the mechanisms behind the early programming of WAT energy metabolism related features by neonatal RSV and NR supplementations remain unknown.
Adipose tissue is a reservoir of immature progenitor cells which are part of the tissue stromovascular fraction (SVF). These cells undergo proliferation and differentiation (adipogenesis) in vivo to allow continuous renewal of the adipocytes in the fat depots throughout life, as well as hyperplasic adipose tissue expansion (i.e., increased adipocyte number) under conditions of positive energy balance, and are thus very important for adipose tissue homeostasis. Importantly, primary cultures established from the SVF of WAT can be used to unveil beige adipocytes (Petrovic et al., 2010), and these cultures reflect genetic differences in the capacity for brown-like adipogenesis in animals in vivo (Petrov et al., 2016). It is conceivable that nutritional influences during critical windows of WAT development condition the fate of adipogenesis from adipose tissue progenitor cells and, consequently, the metabolic features of adult WAT. The early postnatal period might be especially important for WAT programming. Subcutaneous (inguinal) WAT, in particular, basically forms in rodents coincident with the suckling period, after BAT (that develops mainly during the late fetal stage), and prior to visceral WAT (Cryer and Jones, 1979; Kozak, 2011).
In this work, we tested the hypothesis that mild supplementations with RSV or NR in early postnatal life can impact the programming of beige adipogenesis in progenitor cells resident in iWAT. To this end, gene expression related to thermogenesis and oxidative metabolism was studied in corresponding primary cultures established from the tissue SVF of young mice. The studies were conducted both in male and female mice, in view of previous reports of sexual dimorphism in thermogenic responses (Rodriguez and Palou, 2004; Valencak et al., 2017) and our previous characterization of RSV and NR treated offspring in adulthood, which pointed to sex-dependence of treatments effects (Serrano et al., 2018).
Study protocols were approved by the Bioethical Committee of the University of the Balearic Islands (Ref. 3513, 26/03/2012). International standards for the use and care of laboratory animals were followed. Animals were housed at 22°C, with a 12 h light-dark cycle (lights on at 08:00), and free access to tap water and standard chow diet (type A03; 3.3 kcal/g, 8% calories from fat; Panlab, Barcelona, Spain). Virgin female NMRI mice (Charles River Laboratories, Barcelona, Spain) were mated. Each female was single-caged after mating. The morning in which newborn litters were found was designated as day 0. At day 2, litter size was adjusted to 12 pups per dam. Pups in four litters were randomly assigned to control or RSV group; in a separate experiment, pups in four additional litters were assigned to control or NR group (six pups per litter per group in both experiments). From postnatal day 2 (P2) to P20, the pups received daily orally 10–15 μL of either vehicle (water, control groups), a solution of RSV (Sigma, Madrid, Spain) or a solution of NR (Chemical Point, Diessenhofen, Germany), using a pipette. The amount of supplemented RSV was adjusted along the suckling period to meet a daily dose of 2 mg/kg body weight. RSV at 10–30 mg/kg animal/day decreases adiposity in adult rodents (Rivera et al., 2009; Alberdi et al., 2013) and we further applied a security factor considering the young age of the animals. The NR supplied ranged from 24 μg on P2 to 45 μg on P20, and was equivalent to ∼15-fold the total vitamin B3 ingested daily from maternal milk, considering the vitamin B3 content found in milk of in-home mouse dams [2378 ppb (Serrano et al., 2018)] and the daily milk intake of pups throughout lactation (Kojima et al., 1998). Animals were weaned and separated by sex on P21. The studies were performed separately in male and female mice. Body weight was periodically recorded from birth. Body composition was analyzed at P34, using an Echo MRI body composition analyzer (EchoMRI, LCC, Houston, TX, United States). Naso-anal length was also measured on P34, and used to calculate Lee’s obesity index (body weight in g0.33 × 1000/naso-anal length in cm). Energy intake from weaning until euthanization was estimated on a per-cage basis, from the actual amount of food consumed by the animals and its caloric equivalence. The animals were euthanized on P35, by decapitation, under fed conditions, within the first 2 h of the light cycle; half of the animals in each sex and treatment group (n = 5–6, from four different litters) were used to establish sex-separated adipose tissue primary cultures (see below), while the other half were used for tissue sampling for molecular analyses. Interscapular BAT and WAT depots (inguinal, epididymal, and retroperitoneal) were dissected in their entirety, weighed, snap-frozen in liquid nitrogen and stored at -80°C until processed. Glucose was measured from trunk blood using the Accu-Chek Aviva system (Roche Diagnostics). Adiposity index was calculated as the sum of all WAT depot mass divided per body weight and per cent.
The SVF containing preadipocytes was obtained from iWAT depot and pooled (interscapular, cervical, and axillary) BAT depots of 35-day-old mice, following a previously described protocol (Petrovic et al., 2010). The cell pellet was suspended in culture medium (0.5 mL/animal for brown preadipocytes, 0.4 mL/animal for white preadipocytes) and seeded onto 6-well culture plates (2 mL culture medium and 0.2 mL of cell suspension per well). The culture medium was Dulbecco’s modified Eagle’s medium with 10% (v/v) newborn calf serum (Gibco, Thermo Fisher Scientific, Waltham, MA, United States), 4 nM insulin, 25 μg/mL sodium ascorbate, 10 mM HEPES, 4 mM glutamine, 50 units/mL penicillin, and 50 μg/mL streptomycin. From day 0 of culture, all wells were supplemented with 1 μM rosiglitazone (BioVision, Milpitas, CA, United States) to favor the recruitment of brown-like adipocytes (Petrovic et al., 2010). Medium was changed on day 1 and then every second day, except the day the cells were harvested. The cells were grown for 7 days at 37°C in an atmosphere of 8% CO2 in air. Adipogenic differentiation of the cells was regularly monitored through phase contrast microscopical examination. At harvesting, the percentage of cells showing intracellular lipid accumulation was ∼80% and 60% for the primary cultures established from, respectively, BAT and iWAT, as in previous reports from our group using the same primary culture protocol (Petrov et al., 2016) and without apparent differences between neonatal treatments (see representative microphotographs in Supplementary Figures S1, S2). Half of the cultures were exposed to 1 μM NA for the 2 h prior harvesting, to stimulate thermogenically competent cells and UCP1 expression (Petrovic et al., 2010).
Total RNA was extracted using Tripure Reagent (Roche, Barcelona, Spain) according to the manufacturer’s instructions, followed by a sodium acetate/ethanol precipitation to purify nucleic acids. Isolated RNA was quantified using NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies Inc., Wilmington, United States) and its integrity confirmed by agarose gel electrophoresis. Total RNA (0.25 μg/reaction) was reverse-transcribed using MuLV Reverse Transcriptase and random hexamers priming (Applied Biosystems, Madrid, Spain). Diluted cDNA template was used to perform PCR amplification of selected genes, along with specific forward and reverse primers (Sigma, Madrid, Spain), and Power SYBER Green PCR Master Mix (Applied Biosystems). Primers sequences are available on request. For Real-Time PCR the Applied Biosystems StepOnePlus Real-Time PCR Systems (Applied Biosystems) was used, with the following profile: 10 min at 95°C, followed by a total of 40 two-temperature cycles (15 s at 95°C and 1 min at 60°C). To verify the purity of the products, a melting curve was produced after each run. The threshold cycle was calculated by the instrument’s software (StepOne Software v2.2.2, Applied Biosystem) and the relative expression of each mRNA was calculated according to the 2-ΔCt method, using 18S rRNA as reference gene.
Data are expressed as mean ± SEM. Statistical analyses were conducted separately by treatment (RSV and NR supplementation) and sex (male and female mice). Student’s t-test was used for single comparisons. Two-way ANOVA was used for analysis of treatments effects in primary adipocytes under basal and NA-stimulated conditions; post hoc comparisons were included when two-way ANOVA indicated an interactive effect between neonatal treatment and NA exposure of cultures. Threshold of significance was set at p < 0.05. IBM SPSS Statistics for Windows, version 19.0 (IBM Corp., Armonk, NY, United States) was used for the analyses.
To study the influence of treatments on the commitment of WAT preadipocytes toward the beige adipogenesis transcriptional program, expression levels of markers of brown/beige adipocyte determination and thermogenic function (Ucp1, Cpt1b, Prdm16, Ppargc1a, Ppargc1b, Ppara), and of purported beige adipocyte-selective transcriptional markers (Tmem26, Hoxc9, Slc27a1) were compared in differentiated iWAT primary cultures from RSV and NR mice and their respective controls. Pparg expression was also assessed, since the encoded product, PPARγ, is a master adipogenic factor that is also central to WAT browning (Ohno et al., 2012).
Adipocytes differentiated from the iWAT SVF of RSV female mice showed a generalized decreased expression of transcripts of brown and beige adipocyte marker genes (namely: Ucp1, Cpt1, Prdm16, Ppargc1b, Ppara, Tmem26, Slc27a1), as well as of Pparg, when compared to primary adipocytes from control female mice (Figure 1A). On the contrary, primary adipocytes from RSV male mice showed increased expression levels of Ucp1 and Slc27a1 compared with corresponding controls (Figure 1B). Ucp1 expression is the hallmark of the brown and beige adipocyte phenotype, and Slc27a1 encodes a fatty acid transport protein (FATP1) that is highly expressed in BAT and other tissues actively oxidizing fatty acids and that is required for BAT thermogenesis (Wu et al., 2006). Furthermore, Slc27a1 has been related to mitochondrial function in clonal white (3T3-L1) adipocytes (Wiczer and Bernlohr, 2009), and it is the only beige adipocyte transcriptional marker (out of 6 studied) found to be induced together with classical brown adipocyte marker genes in mouse WAT primary cultures following rosiglitazone exposure (Petrov et al., 2016). Additionally, trends to increased expression levels of most other genes related to oxidative metabolism/fatty acid oxidation assayed were apparent in the primary adipocytes derived from RSV male mice vs. controls under NA stimulated conditions (Figure 1B).
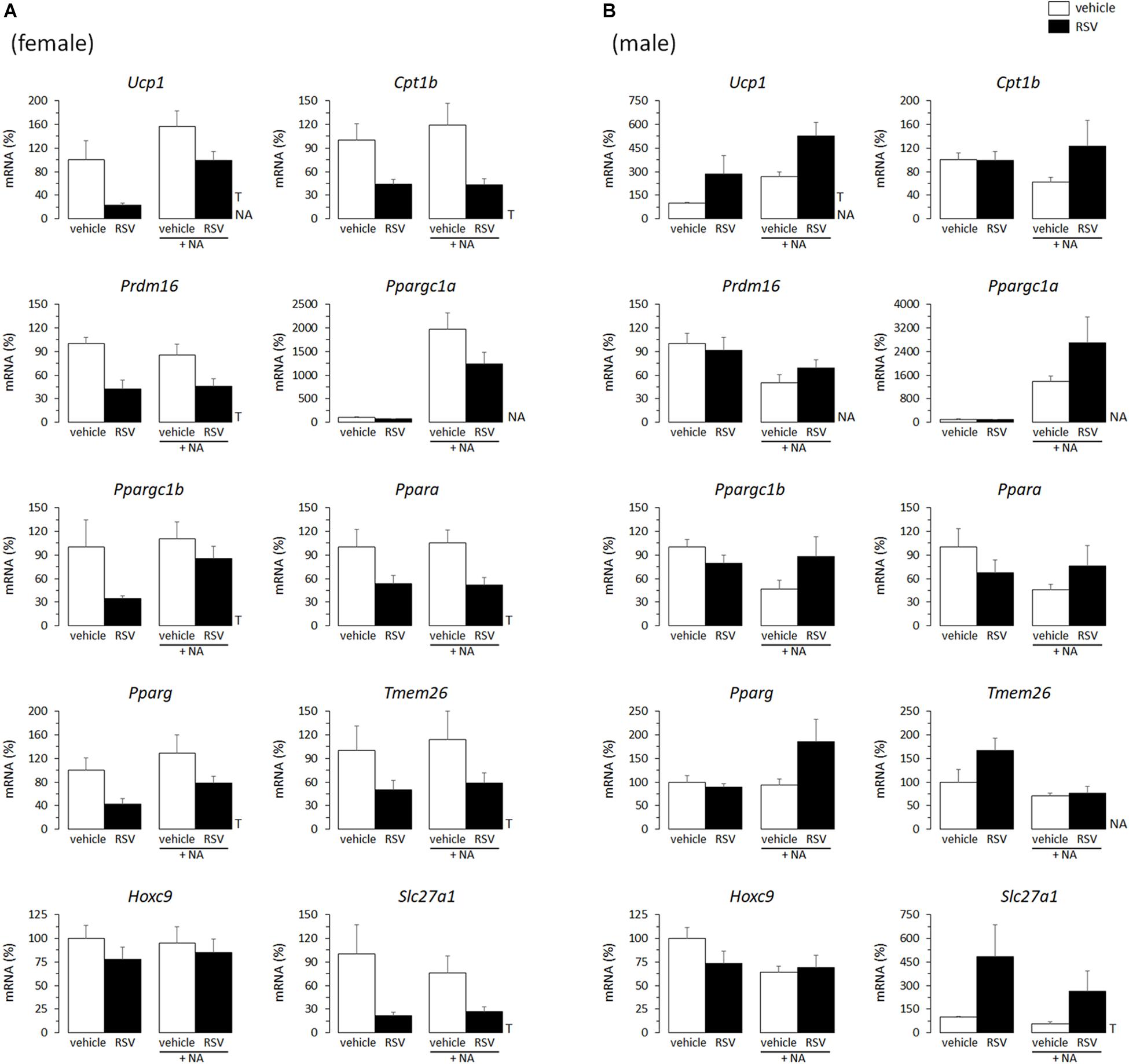
Figure 1. Gene expression (mRNA levels) of the indicated genes in primary adipocyte cultures established from inguinal white adipose tissue (iWAT) of female (A) and male (B) mice neonatally supplemented throughout the suckling period with resveratrol (RSV) and their corresponding controls given the vehicle (water). Cells were harvested on day 7 of culture, under basal conditions and after 2 h incubation with noradrenaline (NA). Data are the mean ± SEM of 5–6 animals/group; mean value of controls under basal conditions used as reference and set to 100%. Statistics (p < 0.05): T, treatment; NA, noradrenaline (two-way ANOVA).
The overall picture was similar for the NR treatment. Ucp1 mRNA levels were increased in primary iWAT adipocytes derived from NR male mice but not female mice (Figure 2). Moreover, primary iWAT adipocytes from NR female mice showed a downregulated expression of several of the brown and beige transcriptional markers assessed (Ppargc1a, Ppargc1b, Tmem26, Hoxc9), whereas those from NR male mice showed an upregulated expression of Slc27a1 – as for the RSV treatment – and also of Prdm16 and Pparg (the latter with p = 0.073, two-way ANOVA) compared to corresponding controls (Figure 2). Prdm16 encodes a transcriptional coactivator critical for WAT browning in postnatal life (Seale et al., 2011; Cohen et al., 2014), and agonism of PPARγ favors WAT browning in vivo and beige adipogenesis in primary adipose cultures (Fukui et al., 2000; Petrovic et al., 2010), possibly through stabilization of the PRDM16 protein (Ohno et al., 2012).
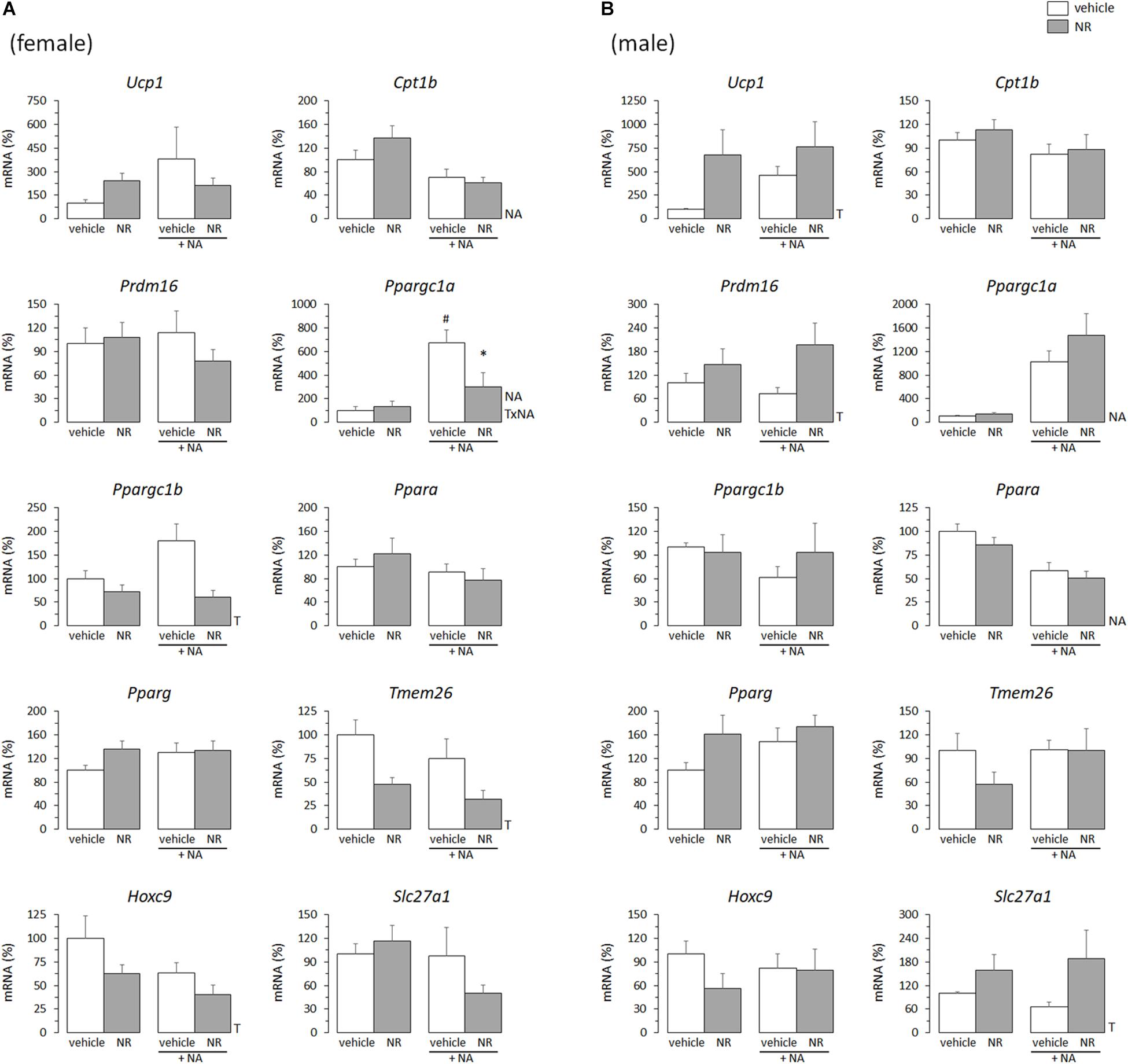
Figure 2. Gene expression (mRNA levels) of the indicated genes in primary adipocyte cultures established from inguinal white adipose tissue (iWAT) of female (A) and male (B) mice neonatally supplemented throughout the suckling period with nicotinamide riboside (NR) and their corresponding controls given the vehicle (water). Cells were harvested on day 7 of culture, under basal conditions and after 2 h incubation with noradrenaline (NA). Data are the mean ± SEM of 5–6 animals/group; mean value of controls under basal conditions used as reference and set to 100%. Statistics (p < 0.05): T, treatment; NA, noradrenaline; TxNA, interactive effect (two-way ANOVA); ∗NR vs. control, #NA vs. basal (t-test).
Half of the cultures were adrenergically stimulated prior harvesting to potentiate the emergence of thermogenically competent cells (Petrovic et al., 2010). Expected transcriptional responses to NA stimulation were found. In particular, Ppargc1a expression (encoding PGC1α) was induced by NA exposure in all iWAT primary cultures, regardless of sex of origin or neonatal treatment (Figures 1, 2). Ucp1 expression was induced by NA exposure in the iWAT cultures from control and RSV mice regardless of sex, but not further induced by NA in the iWAT cultures from NR mice of either sex, which already overexpressed Ucp1 compared to controls under the basal, non-stimulated, conditions (Figure 2). Apart from Ppargc1a and Ucp1, other brown/beige marker genes analyzed in iWAT primary cultures were not induced following NA exposure, but were rather unaffected or downregulated (Figures 1, 2). Down-regulation of genes in this class upon NA exposure was especially evident in the cultures derived from control male mice, and was attenuated in the cultures derived from RSV male mice (see results for Cpt1b, Prdm16, Ppargc1b, Ppara, Hoxc9, and Slc27a1 expression in Figure 1B).
Altogether, these results indicated that the neonatal RSV and NR treatments had sex-dependent effects on the brown/beige transcriptional signature of iWAT primary cultures from young mice.
In adult animals, it is common that the same stimuli that induce WAT browning also induce BAT recruitment (Bonet et al., 2013, 2017). Thus, effects of the in vivo neonatal treatments on thermogenic gene expression in primary BAT adipocytes from young mice were studied, to check for potential parallelisms between results in iWAT- and BAT-derived cultures. Compared with levels in cultures from sex-matched controls, the mRNA levels of Ucp1, Cpt1b, Ppargc1b, and Ppara were downregulated in the BAT primary cultures from RSV female mice, but not (except for Ppargc1b expression under basal conditions) in those from RSV male mice (Figures 3A,B). On the contrary, BAT cultures from RSV male mice more readily induced Ucp1 and Ppargc1a mRNA levels following NA stimulation, and showed increased Ppargc1a and Ppara mRNA levels under NA-stimulated conditions compared with cultures from control male mice. Furthermore, upon NA stimulation, Ppargc1b expression levels decreased in the BAT cultures from control male mice but not in those from RSV male mice. Thus, the overall effect of neonatal in vivo RSV treatment on BAT primary adipocyte cultures from young mice resembled that in iWAT primary adipocyte cultures, with thermogenic gene expression being potentiated in the cultures derived from male mice and attenuated in the cultures derived from female mice. Somewhat oppositely, neonatal NR treatment associated with higher Ucp1 expression selectively in the female mice-derived BAT cultures, whereas expression of other genes assayed (Cpt1b, Prdm16, Ppargc1a, Ppargc1b, Ppara, Pparg) was not significantly affected by neonatal NR treatment in either sex (Figures 4A,B). Expected responses to NA, namely induction of Ucp1 and Ppargc1a expression, were present in all BAT primary cultures regardless of sex of origin or treatment (Figures 3, 4).
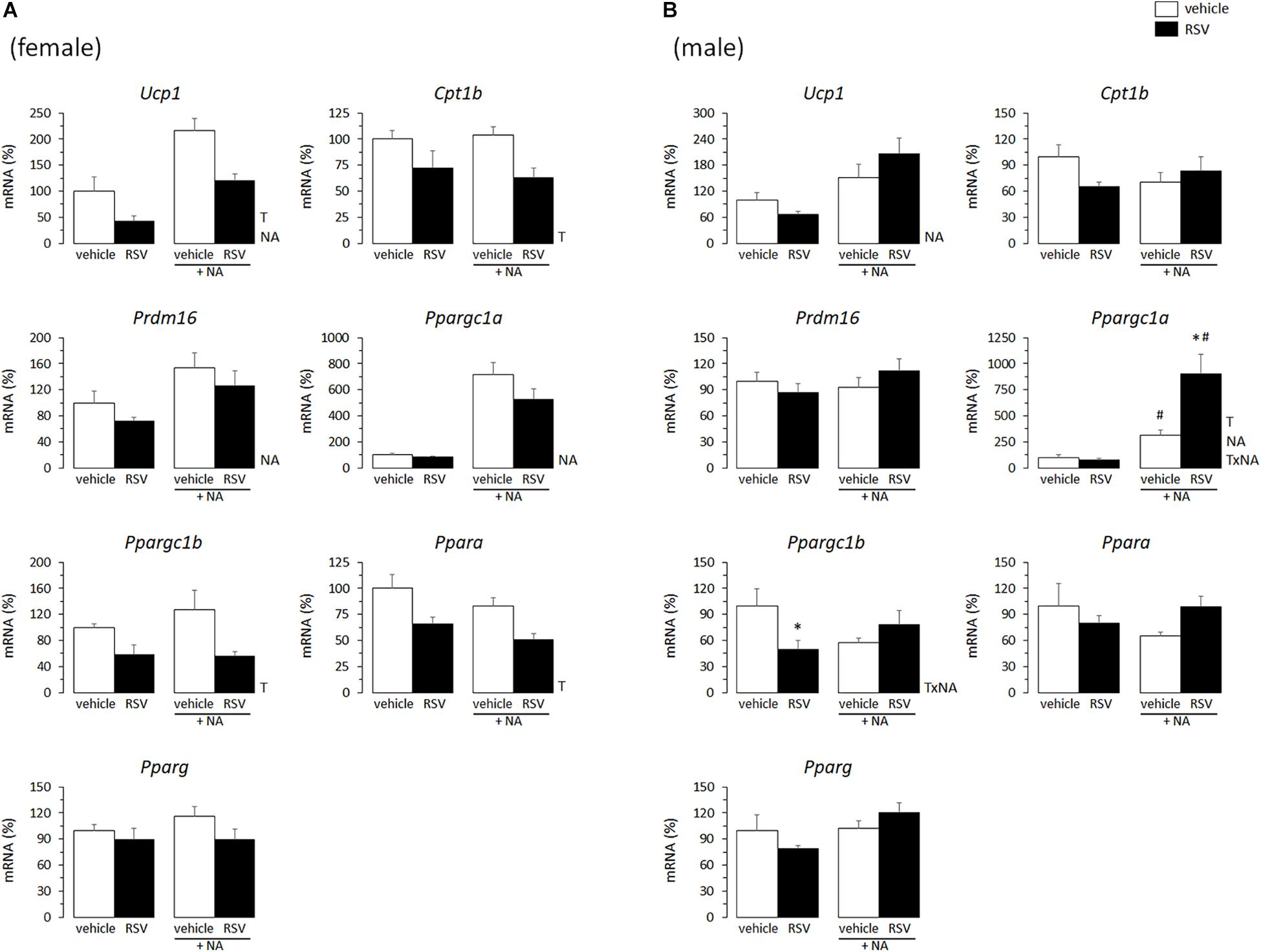
Figure 3. Gene expression (mRNA levels) of the indicated genes in primary adipocyte cultures established from brown adipose tissue (BAT) of female (A) and male (B) mice neonatally supplemented throughout the suckling period with resveratrol (RSV) and their corresponding controls given the vehicle (water). Cells were harvested on day 7 of culture, under basal conditions and after 2 h incubation with noradrenaline (NA). Data are the mean ± SEM of 5–6 animals/group; mean value of controls under basal conditions used as reference and set to 100%. Statistics (p < 0.05): T, treatment; NA, noradrenaline; TxNA, interactive effect (two-way ANOVA); ∗RSV vs. control, #NA vs. basal (t-test).
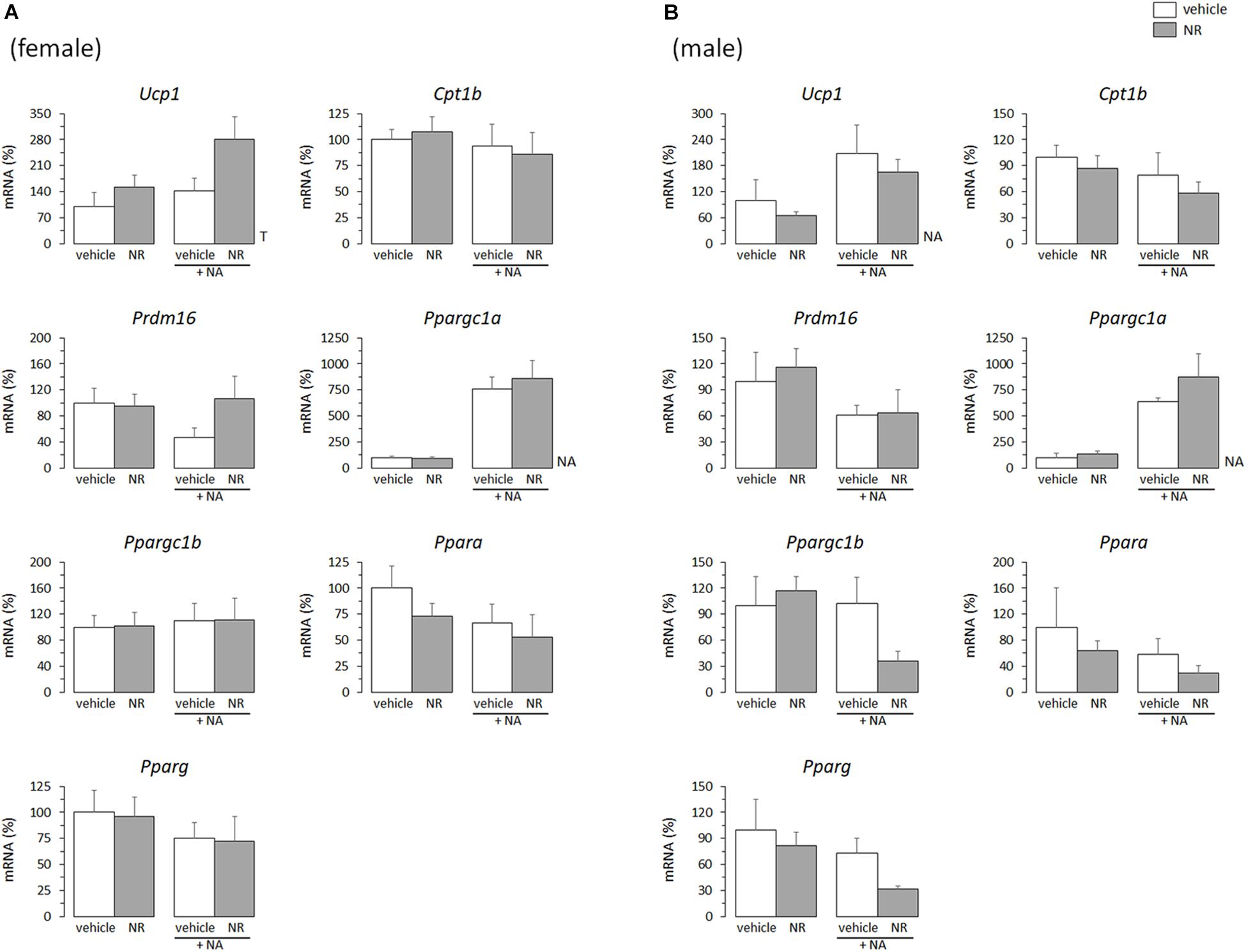
Figure 4. Gene expression (mRNA levels) of the indicated genes in primary adipocyte cultures established from brown adipose tissue (BAT) of female (A) and male (B) mice neonatally supplemented throughout the suckling period with nicotinamide riboside (NR) and their corresponding controls given the vehicle (water). Cells were harvested on day 7 of culture, under basal conditions and after 2 h incubation with noradrenaline (NA). Data are the mean ± SEM of 5–6 animals/group; mean value of controls under basal conditions used as reference and set to 100%. Statistics (p < 0.05): T, treatment; NA, noradrenaline (two-way ANOVA).
Eventual effects of treatments on early postnatal growth were evaluated by monitoring body weight evolution from birth; body length (nasal-to-anal distance), Lee index and body composition (by EchoMRI) at P34; and tissue/organ weights at euthanization at P35. RSV treatment had no significant effect on any of these parameters, neither in male nor in female animals (Table 1). As for the NR treatment, treated male mice, but not females, were slightly but significantly heavier (by ∼6%) and had higher (by ∼20%) body fat content and fat/lean ratio at P34 compared to sex-matched controls (Table 2). Consistent with these body composition results, NR male mice, but not females, displayed after dissection a trend to higher gonadal WAT mass (p = 0.090) (Table 2). Cumulative energy intake from weaning until P30 was significantly lower (by ∼4%) in the NR animals of both sexes relative to sex-matched controls (Table 2), and showed a trend to be lower (also by ∼4%, p = 0.076) in the RSV male mice, but not the RSV female mice. Fed blood glucose levels at P35 were unaffected by treatments in either sex (not shown). Collectively, these results indicate that the neonatal RSV and NR supplementations applied did not compromise the animals’ growth during early postnatal life, and had only a minor impact on it.
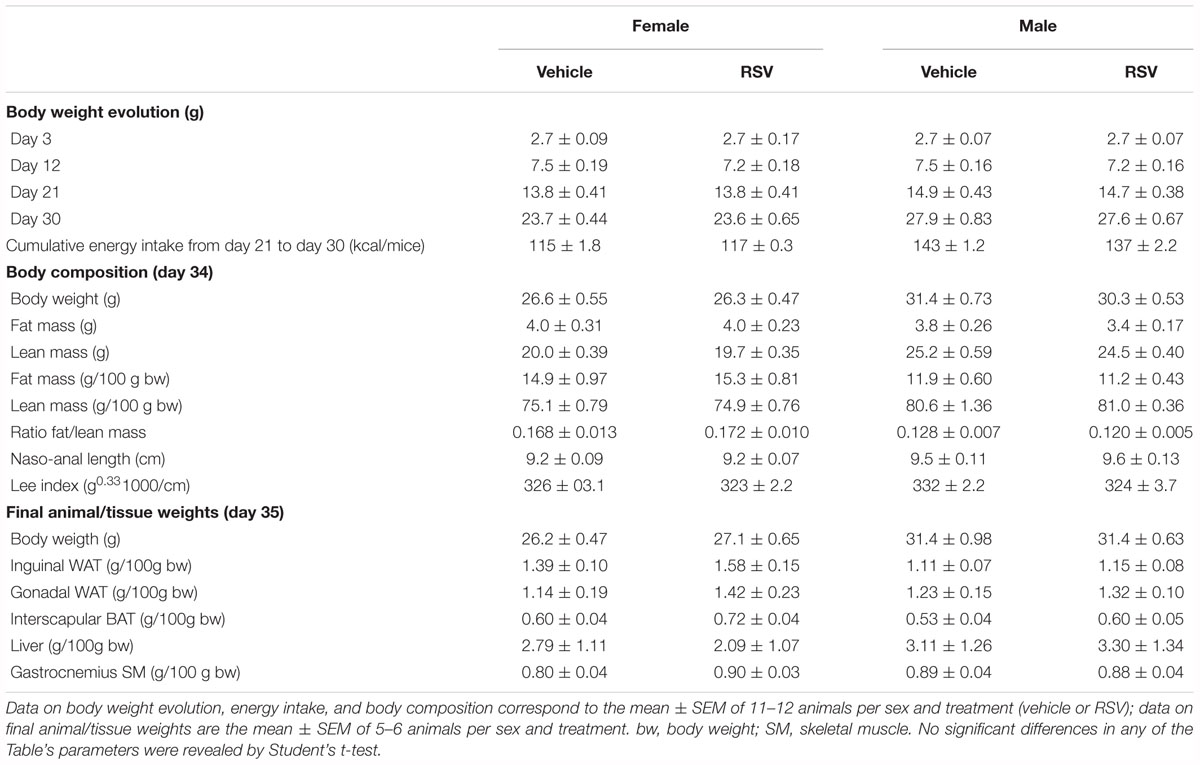
Table 1. Biometric parameters in mice receiving resveratrol (RSV) supplementation throughout lactation and their corresponding control littermates.
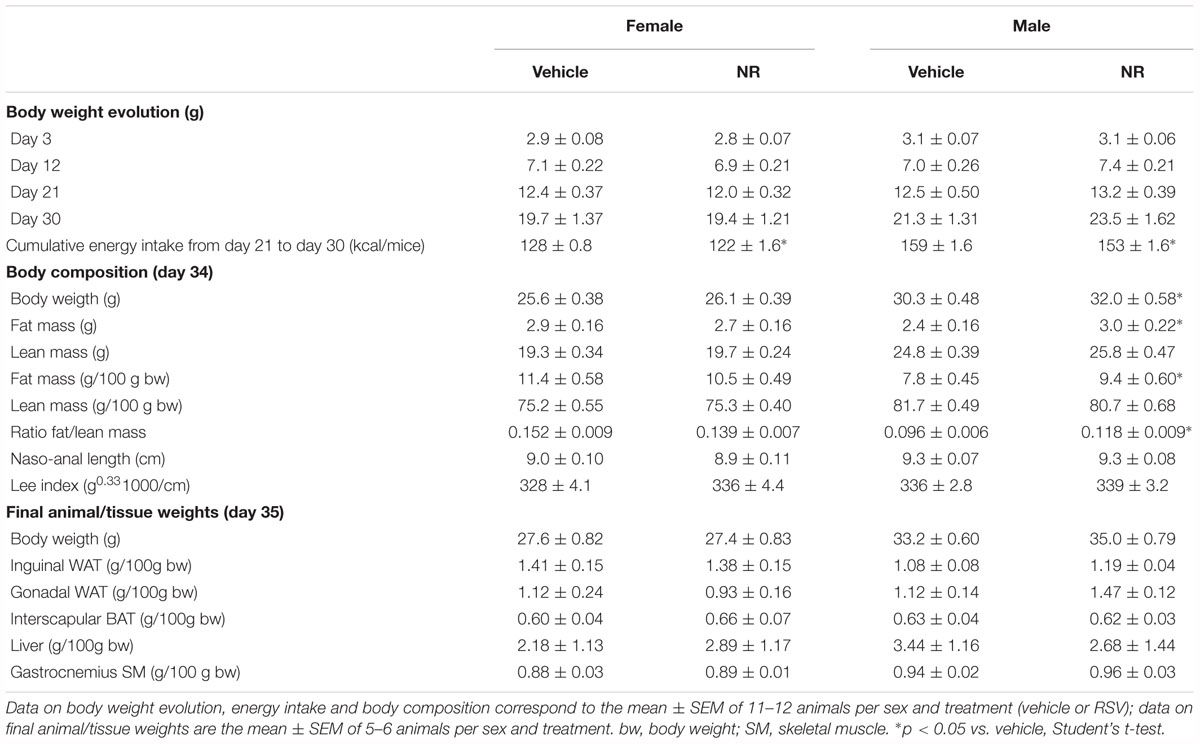
Table 2. Biometric parameters in mice receiving nicotinamide riboside (NR) supplementation throughout lactation and their corresponding control littermates.
The same panel of brown and beige adipocyte transcriptional markers analyzed in the iWAT primary cultures was analyzed in the iWAT depot of 35-day-old RSV and NR male mice and their respective control littermates (Figure 5). Genes related to mitochondria biogenesis and function were additionally analyzed in the iWAT tissue, since expression of genes in this category (Nrf1, Nrf2, Tfam, Tfb2m, Mfn2) is increased at adult age in iWAT of RSV and NR neonatally treated male mice compared to control littermates (Serrano et al., 2018). Of the genes analyzed, the only significant difference from expression levels in controls was a greater than 2-fold increase in Prdm16 mRNA levels in the iWAT of young NR male mice (Figure 5). Lep, Mest, and Lpl mRNA levels in iWAT, which were used as indicators of adipose tissue mass and expandability, were unaffected by treatments (Figure 5), in good concordance with the lack of effect of treatments on iWAT depot mass (Tables 1, 2). mRNA expression levels of a selection of genes in the above categories (Ucp1, Slc27a1, Mfn2, Lpl) were analyzed in iWAT of young (P35) female mice and found to be unaffected by treatments (data not shown).
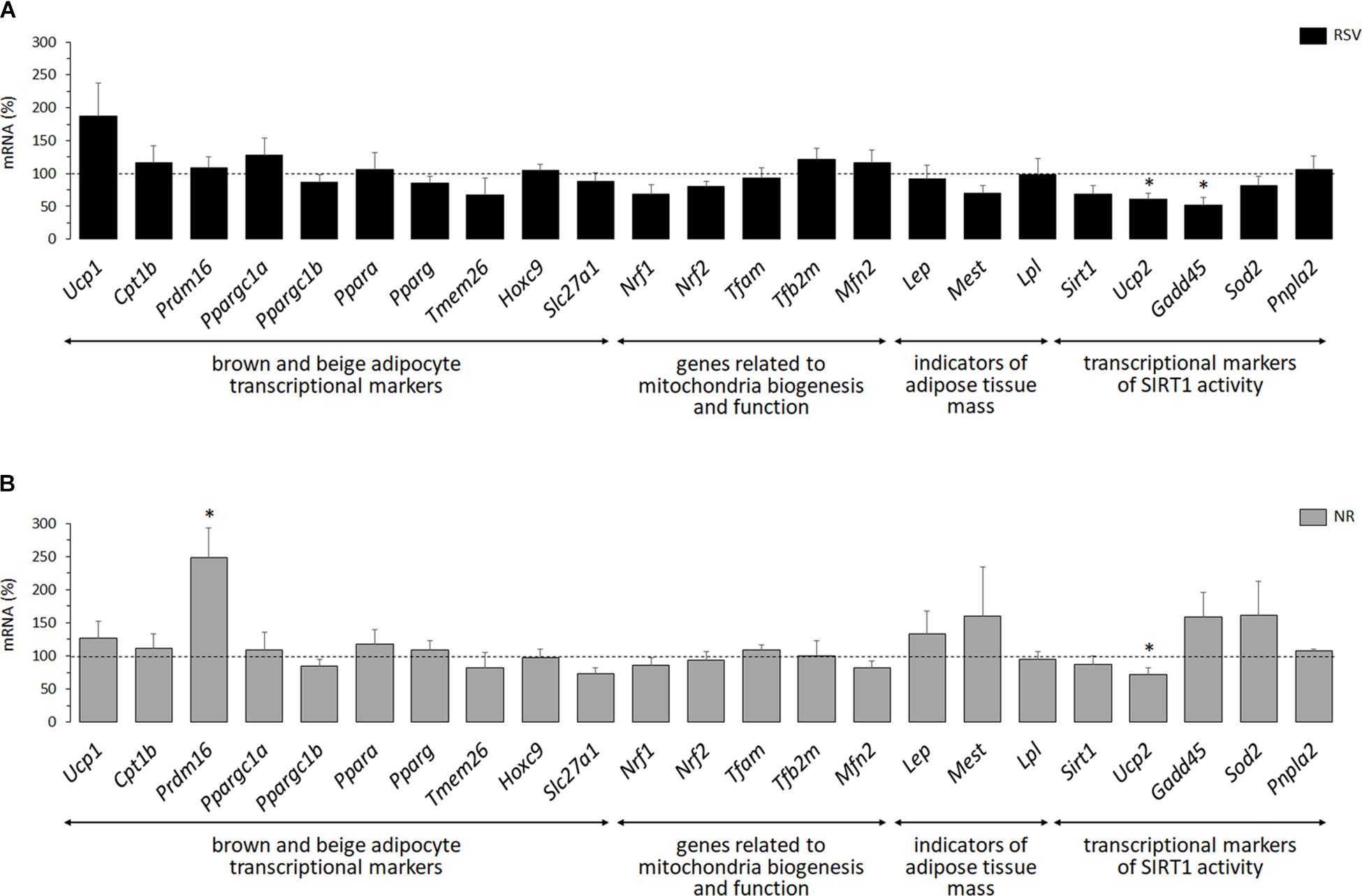
Figure 5. Gene expression (mRNA levels) of the indicated genes in inguinal white adipose tissue (iWAT) of 35-day-old male mice neonatally supplemented throughout the suckling period with resveratrol (RSV, A) or nicotinamide riboside (NR, B) relative to corresponding controls given the vehicle (water; dotted line). Data are the mean ± SEM of 5–6 animals/group. Statistics (p < 0.05): ∗RSV or NR vs. control (t-test).
Finally, because RSV and NR can both enhance energy metabolism through the activation of SIRT1 (Canto et al., 2012; Park et al., 2012; Price et al., 2012), transcriptional markers of SIRT1 activity were assessed in iWAT of young animals. SIRT1 represses Ucp2 gene transcription (Bordone et al., 2006) and deacetylates FOXO1 transcription factor, resulting in higher expression levels in cells of FOXO1 target genes such as Gadd45, Sod2, and Atgl (Calnan and Brunet, 2008; Chakrabarti et al., 2011). Consistent with neonatal RSV and NR treatments activating SIRT1 specifically in iWAT of male mice, Ucp2 mRNA levels were significantly decreased in iWAT of both groups of treated male mice relative to controls (Figure 5), but not in the iWAT depot of treated female mice (controls, 100 ± 14 and 100 ± 21; treated 134 ± 63 and 92 ± 21, for the RSV and NR treatments, respectively). However, none of the three FOXO1 target genes analyzed was significantly induced by the experimental treatments in iWAT of young male mice, and expression of Gadd45 was even significantly downregulated in the RSV male mice. Sirt1 expression was also measured, and a trend to down-regulation of its mRNA levels was apparent in the RSV male mice (p = 0.053). Overall, from the transcriptional analysis performed, we conclude that there is little evidence of iWAT browning or SIRT1 activation in iWAT of young male mice receiving neonatal RSV or NR supplementation.
Modulation of the browning potential of adipose tissues through the intake of food bioactives in early life is a potential, yet still largely unexplored, strategy to enhance metabolic health in later life (Palou et al., 2013). Adipose progenitor cells resident in fat depots are likely to be natural depositors of programming information from dietary and other cues in critical life stages, making the primary culture an attractive model for studies in the field of nutritional programming of adipose tissue cellular and metabolic features. Using this model, we show here that the intake of mild supraphysiological doses of RSV or NR during the suckling period impacts adipocyte precursor cells resident in subcutaneous WAT of young mice and affects the commitment of these cells to the beige adipogenesis transcriptional program in a sex-dependent manner, down-regulating it in the female mice and up-regulating it in the male mice. As expected considering the rather mild dietary treatments applied, gene expression changes observed were relatively small, yet they affected different functionally related genes concertedly. Interestingly, long-term consequences of neonatal RSV and NR treatments under the same conditions as those used here include signs of iWAT-to-BAT remodeling and better responses to an obesogenic high-fat diet in adulthood selectively in the male mice (Serrano et al., 2018). A causal link between the sex-dependent effects on iWAT preadipocyte differentiation revealed here in the primary cultures and the sex-dependent long-term effects on iWAT features and benefits against metabolic challenge found in vivo in adulthood is thus suggested.
Our studies are first to examine nutritional programming of adipose tissue metabolic features by NR and RSV supplements given directly to the lactating pups. Programming effects of supplemental NR has not previously been addressed, to our knowledge. In the case of RSV, maternal supplementation with this compound was previously shown to enhance brown/beige adipocyte function in the male offspring of high-fat diet-fed dams, as indicated by WAT and BAT analysis and systemic measurements (Zou et al., 2017). However, in this previous study RSV was included in the dam’s diet at a rather high dose (0.2% in the diet, ∼200 mg/kg body weight per day) and throughout pregnancy and lactation, whereas we here used a mild, precise dose of RSV (2 mg/kg body weight per day) supplied directly to the pups. Additionally, only effects on the male offspring of RSV-supplemented dams were previously reported (Zou et al., 2017), whereas our study included both male and female offspring, thus allowing to unveil sex-dependent effects.
Differences in WAT browning capacity due to early metabolic imprinting might be better revealed in young animals through the establishment of WAT primary cultures, which can be readily stimulated with rosiglitazone and NA in order to recruit and activate beige adipocytes in them, than through direct WAT depot analysis (especially in the absence of extra in vivo challenges, such as cold-exposure). In fact, whereas brown/beige gene expression was enhanced in the derived primary cultures, iWAT of young (P35) RSV and NR treated male mice did not show an obvious brown fat-like gene expression signature: out of the fifteen brown/beige markers and mitochondria-related genes assayed, only Prdm16 in the NR-treated male group was found to be upregulated at P35 compared to levels in controls. However, up-regulation of many of these genes in the iWAT of mice neonatally treated with RSV or NR is apparent at adult age (P164), after regular diet feeding and/or high-fat diet feeding (Serrano et al., 2018). Taken together, we interpret these results as indicating that, with time–after rounds of preadipocyte proliferation and de novo adipogenesis in vivo, linked to aging and eventual obesogenic diet feeding–the greater commitment to brown-like adipogenesis translates into a greater appearance of BAT-like properties in the iWAT of treated male mice. In contrast, the decreased capacity for beige adipogenesis found here in iWAT primary cultures from young RSV and NR treated female mice does not translate into a generalized lower thermogenic/oxidative gene expression in the adult female iWAT, in which most of these markers are unaffected (Serrano et al., 2018). Challenging the adult animals with a strong acute pro-browning stimulus, such as cold-exposure, may be required to reveal inhibitory programming of WAT browning in vivo.
Whereas for iWAT of male animals there is a good concordance between observed effects of neonatal RSV and NR treatments on primary adipose cultures established at young age and observed adipose depot features at adult age, this is not the case for BAT. For instance, neonatal RSV supplementation enhanced the expression of brown marker genes in the BAT primary cultures (this work) but not in BAT of male mice in adulthood, where if anything there was decreased expression (Serrano et al., 2018). The difference may relate to the fact that our treatment period is coincident with iWAT rather than BAT development during ontogeny (Kozak, 2011). Thus, we suggest that observed effects of neonatal RSV and NR treatments on BAT preadipocyte adipogenesis in primary culture are likely a transient reflect of recent treatment, whereas effects on iWAT preadipocyte adipogenesis are likely to be persistent and of programming nature, when the treatment is performed in the specific developmental time window of lactation.
Previous studies on the nutritional programming of adipose tissue features have mainly focused on the influence of maternal diet during pregnancy and lactation, studying factors such as maternal total energy intake (Garcia et al., 2011; Palou et al., 2015), diet macronutrient composition (Dumortier et al., 2017) or dietary fat quality (Priego et al., 2013; Kodde et al., 2017). Specific nutrients/bioactives such as RSV itself (Zou et al., 2017; Ros et al., 2018), grape seed procyanidins (del Bas et al., 2015), or leptin (Konieczna et al., 2015; Szostaczuk et al., 2017), among others, have been tested in this context mainly for their ability to counteract detrimental effects of maternal malnutrition, as in calorie restricted or high-fat diet fed dams. Early postnatal nutritional programming by specific nutrients/bioactives independently of maternal diet has been much less studied, despite its biological interest and its translational interest for the baby food market. There are studies of this kind for vitamin A (Granados et al., 2013; Musinovic et al., 2014), n-3 long-chain polyunsaturated fatty acids (Oosting et al., 2010), and leptin (Priego et al., 2010), for instance, but those studies generally focused on effects on WAT expansion, and did not specifically address effects on the WAT browning potential. Our findings are among first proof-of-concept that the fate of preadipocytes in WAT toward the beige adipogenesis transcriptional program can be influenced by the intake of specific nutrients and food bioactives in the early postnatal life, independently of maternal diet. Another novel aspect of the present work is the use of the primary culture model, which so far has been little exploited in the field of nutritional programming, despite its potential relevance for this field. To our knowledge, only one recent report has used a similar approach, to demonstrate that neonatal overfeeding affects the differentiation capacities of adipose tissue mesenchymal stem cells (Dias et al., 2018).
Sexual dimorphism regarding the BAT thermogenic system is well-known (reviewed in Rodriguez and Palou, 2004; Valencak et al., 2017). Female rats possess more BAT of higher thermogenic capacity compared to male rats, and the same appears to be the case in humans (Valencak et al., 2017). BAT thermogenesis is more easily activated by cold in female rats (Rodriguez and Palou, 2004) and in women (van den Beukel et al., 2015) than in male counterparts, but less efficiently activated by excess palatable food intake in female than in male rats (Rodriguez and Palou, 2004). Sex differences in the capabilities for browning of WAT have also been described, with greater levels of WAT browning in female than male mice (Kim et al., 2016) and in women than in men (Barquissau et al., 2018). Our results extend sexual dimorphism of the WAT browning phenomenon to its developmental programming in response to dietary factors. Additionally, from an operational point of view, results herein underscore that sex-dependent responses can be unveiled in primary cultures, a fact often neglected in studies using this model system.
Programming effects of RSV and NR neonatal treatments observed in this work may result from epigenetic changes in adipocyte precursor cells subsequent to treatment effects at the iWAT level and/or the central level, on neuronal circuitries impinging on the developing adipose tissue. Such actions may involve SIRT1 activation, since (i) RSV and NR can both activate SIRT1, albeit through distinct mechanisms (Canto et al., 2012; Park et al., 2012; Price et al., 2012), (ii) the activity of SIRT1 favors BAT thermogenesis and WAT browning [(Boutant and Canto, 2016) and references therein], and (iii) SIRT1 interacts with epigenetic mechanisms including DNA methylation (Ions et al., 2013). However, besides SIRT1, both RSV and NR have distinct, non-overlapping biological targets for interaction, and it is to be noted that observed effects of neonatal NR and RSV treatments, though similar, were not identical, neither in the short-term (this work) nor in the long-term (Serrano et al., 2018). Moreover, we found little evidence of activation of SIRT1 in iWAT of young treated mice, where of four transcriptional markers of SIRT1 activity assayed, only changes in Ucp2 expression in the expected sense (Bordone et al., 2006) relative to controls were present. Further studies on the mechanisms behind programming by neonatal RSV and NR treatments of energy metabolism in adipose tissues and other key tissues in whole body energy homeostasis are warranted.
MLB, JR, AMR, and AP conceived and designed the work. MA-K managed the animals. MA-K and AR established the primary cultures. MA-K and AS acquired the data. MLB and JR wrote the article. All authors analyzed and interpreted the data, revised the manuscript critically, and gave final approval of the version to be published.
This work was sponsored by grants from the Spanish Government (AGL2015-67019-P to AP; Agencia Estatal de Investigación, MINECO/FEDER, EU) and Fundación Ramón Areces (to MLB). Our group is a member of the European Nutrigenomics Organization (NuGO). CIBER de Fisiopatología de la Obesidad y Nutrición (CIBEROBN) is an initiative of the ISCIII (Spanish Government).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2019.00083/full#supplementary-material
BAT, brown adipose tissue; iWAT, inguinal WAT; NA, noradrenaline; NR, nicotinamide riboside; RSV, resveratrol; WAT, white adipose tissue.
Alberdi, G., Rodriguez, V. M., Miranda, J., Macarulla, M. T., Churruca, I., and Portillo, M. P. (2013). Thermogenesis is involved in the body-fat lowering effects of resveratrol in rats. Food Chem. 141, 1530–1535. doi: 10.1016/j.foodchem.2013.03.085
Andrade, J. M., Frade, A. C., Guimaraes, J. B., Freitas, K. M., Lopes, M. T., Guimaraes, A. L., et al. (2014). Resveratrol increases brown adipose tissue thermogenesis markers by increasing SIRT1 and energy expenditure and decreasing fat accumulation in adipose tissue of mice fed a standard diet. Eur. J. Nutr. 53, 1503–1510. doi: 10.1007/s00394-014-0655-6
Barquissau, V., Leger, B., Beuzelin, D., Martins, F., Amri, E. Z., Pisani, D. F., et al. (2018). Caloric restriction and diet-induced weight loss do not induce browning of human subcutaneous white adipose tissue in women and men with obesity. Cell Rep. 22, 1079–1089. doi: 10.1016/j.celrep.2017.12.102
Bartelt, A., and Heeren, J. (2014). Adipose tissue browning and metabolic health. Nat. Rev. Endocrinol. 10, 24–36. doi: 10.1038/nrendo.2013.204
Bonet, M. L., Mercader, J., and Palou, A. (2017). A nutritional perspective on UCP1-dependent thermogenesis. Biochimie 134, 99–117. doi: 10.1016/j.biochi.2016.12.014
Bonet, M. L., Oliver, P., and Palou, A. (2013). Pharmacological and nutritional agents promoting browning of white adipose tissue. Biochim. Biophys. Acta 1831, 969–985. doi: 10.1016/j.bbalip.2012.12.002
Bordone, L., Motta, M. C., Picard, F., Robinson, A., Jhala, U. S., Apfeld, J., et al. (2006). Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic beta cells. PLoS Biol. 4:e31. doi: 10.1371/journal.pbio.0040031
Boutant, M., and Canto, C. (2016). SIRT1: a novel guardian of brown fat against metabolic damage. Obesity 24:554. doi: 10.1002/oby.21432
Calnan, D. R., and Brunet, A. (2008). The FoxO code. Oncogene 27, 2276–2288. doi: 10.1038/onc.2008.21
Canto, C., Houtkooper, R. H., Pirinen, E., Youn, D. Y., Oosterveer, M. H., Cen, Y., et al. (2012). The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. 15, 838–847. doi: 10.1016/j.cmet.2012.04.022
Chakrabarti, P., English, T., Karki, S., Qiang, L., Tao, R., Kim, J., et al. (2011). SIRT1 controls lipolysis in adipocytes via FOXO1-mediated expression of ATGL. J. Lipid Res. 52, 1693–1701. doi: 10.1194/jlr.M014647
Cohen, P., Levy, J. D., Zhang, Y., Frontini, A., Kolodin, D. P., Svensson, K. J., et al. (2014). Ablation of PRDM16 and beige adipose causes metabolic dysfunction and a subcutaneous to visceral fat switch. Cell 156, 304–316. doi: 10.1016/j.cell.2013.12.021
Cottrell, E. C., and Ozanne, S. E. (2008). Early life programming of obesity and metabolic disease. Physiol. Behav. 94, 17–28. doi: 10.1016/j.physbeh.2007.11.017
Cryer, A., and Jones, H. M. (1979). The early development of white adipose tissue. Effects of litter size on the lipoprotein lipase activity of four adipose-tissue depots, serum immunoreactive insulin and tissue cellularity during the first four weeks of life in the rat. Biochem. J. 178, 711–724. doi: 10.1042/bj1780711
del Bas, J. M., Crescenti, A., Arola-Arnal, A., Oms-Oliu, G., Arola, L., and Caimari, A. (2015). Grape seed procyanidin supplementation to rats fed a high-fat diet during pregnancy and lactation increases the body fat content and modulates the inflammatory response and the adipose tissue metabolism of the male offspring in youth. Int. J. Obes. 39, 7–15. doi: 10.1038/ijo.2014.159
Dias, I., Salviano, I., Mencalha, A., de Carvalho, S. N., Thole, A. A., Carvalho, L., et al. (2018). Neonatal overfeeding impairs differentiation potential of mice subcutaneous adipose mesenchymal stem cells. Stem Cell Rev. 14, 535–545. doi: 10.1007/s12015-018-9812-2
Dumortier, O., Roger, E., Pisani, D. F., Casamento, V., Gautier, N., Lebrun, P., et al. (2017). Age-dependent control of energy homeostasis by brown adipose tissue in progeny subjected to maternal diet-induced fetal programming. Diabetes Metab. Res. Rev. 66, 627–639. doi: 10.2337/db16-0956
Fleckenstein-Elsen, M., Dinnies, D., Jelenik, T., Roden, M., Romacho, T., and Eckel, J. (2016). Eicosapentaenoic acid and arachidonic acid differentially regulate adipogenesis, acquisition of a brite phenotype and mitochondrial function in primary human adipocytes. Mol. Nutr. Food Res. 60, 2065–2075. doi: 10.1002/mnfr.201500892
Fukui, Y., Masui, S., Osada, S., Umesono, K., and Motojima, K. (2000). A new thiazolidinedione, NC-2100, which is a weak PPAR-gamma activator, exhibits potent antidiabetic effects and induces uncoupling protein 1 in white adipose tissue of KKAy obese mice. Diabetes Metab. Res. Rev. 49, 759–767. doi: 10.2337/diabetes.49.5.759
Garcia, A. P., Palou, M., Sanchez, J., Priego, T., Palou, A., and Pico, C. (2011). Moderate caloric restriction during gestation in rats alters adipose tissue sympathetic innervation and later adiposity in offspring. PLoS One 6:e17313. doi: 10.1371/journal.pone.0017313
Granados, N., Amengual, J., Ribot, J., Musinovic, H., Ceresi, E., von Lintig, J., et al. (2013). Vitamin A supplementation in early life affects later response to an obesogenic diet in rats. Int. J. Obes. 37, 1169–1176. doi: 10.1038/ijo.2012.190
Harms, M., and Seale, P. (2013). Brown and beige fat: development, function and therapeutic potential. Nat. Med. 19, 1252–1263. doi: 10.1038/nm.3361
Ions, L. J., Wakeling, L. A., Bosomworth, H. J., Hardyman, J. E., Escolme, S. M., Swan, D. C., et al. (2013). Effects of Sirt1 on DNA methylation and expression of genes affected by dietary restriction. Age 35, 1835–1849. doi: 10.1007/s11357-012-9485-8
Kim, S. N., Jung, Y. S., Kwon, H. J., Seong, J. K., Granneman, J. G., and Lee, Y. H. (2016). Sex differences in sympathetic innervation and browning of white adipose tissue of mice. Biol. Sex Differ. 7:67. doi: 10.1186/s13293-016-0121-7
Kodde, A., van der Beek, E. M., Phielix, E., Engels, E., Schipper, L., and Oosting, A. (2017). Supramolecular structure of dietary fat in early life modulates expression of markers for mitochondrial content and capacity in adipose tissue of adult mice. Nutr. Metab. 14:37. doi: 10.1186/s12986-017-0191-5
Kojima, T., Nishimura, M., Yajima, T., Kuwata, T., Suzuki, Y., Goda, T., et al. (1998). Effect of intermittent feeding on the development of disaccharidase activities in artificially reared rat pups. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 121, 289–297. doi: 10.1016/S1095-6433(98)10133-2
Konieczna, J., Palou, M., Sanchez, J., Pico, C., and Palou, A. (2015). Leptin intake in suckling rats restores altered T3 levels and markers of adipose tissue sympathetic drive and function caused by gestational calorie restriction. Int. J. Obes. 39, 959–966. doi: 10.1038/ijo.2015.22
Kozak, L. P. (2011). The genetics of brown adipocyte induction in white fat depots. Front. Endocrinol. 2:64. doi: 10.3389/fendo.2011.00064
Lagouge, M., Argmann, C., Gerhart-Hines, Z., Meziane, H., Lerin, C., Daussin, F., et al. (2006). Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 127, 1109–1122. doi: 10.1016/j.cell.2006.11.013
Lecoutre, S., and Breton, C. (2014). The cellularity of offspring’s adipose tissue is programmed by maternal nutritional manipulations. Adipocyte 3, 256–262. doi: 10.4161/adip.29806
Musinovic, H., Bonet, M. L., Granados, N., Amengual, J., von Lintig, J., Ribot, J., et al. (2014). beta-Carotene during the suckling period is absorbed intact and induces retinoic acid dependent responses similar to preformed vitamin A in intestine and liver, but not adipose tissue of young rats. Mol. Nutr. Food Res. 58, 2157–2165. doi: 10.1002/mnfr.201400457
Ohno, H., Shinoda, K., Spiegelman, B. M., and Kajimura, S. (2012). PPARgamma agonists induce a white-to-brown fat conversion through stabilization of PRDM16 protein. Cell Metab. 15, 395–404. doi: 10.1016/j.cmet.2012.01.019
Oosting, A., Kegler, D., Boehm, G., Jansen, H. T., van de Heijning, B. J., and van der Beek, E. M. (2010). N-3 long-chain polyunsaturated fatty acids prevent excessive fat deposition in adulthood in a mouse model of postnatal nutritional programming. Pediatr. Res. 68, 494–499. doi: 10.1203/PDR.0b013e3181f74940
Palmeri, R., Monteleone, J. I., Spagna, G., Restuccia, C., Raffaele, M., Vanella, L., et al. (2016). Olive leaf extract from sicilian cultivar reduced lipid accumulation by inducing thermogenic pathway during adipogenesis. Front. Pharmacol. 7:143. doi: 10.3389/fphar.2016.00143
Palou, A., Pico, C., and Bonet, M. L. (2013). Nutritional potential of metabolic remodelling of white adipose tissue. Curr. Opin. Clin. Nutr. Metab. Care 16, 650–656. doi: 10.1097/MCO.0b013e328365980f
Palou, M., Priego, T., Romero, M., Szostaczuk, N., Konieczna, J., Cabrer, C., et al. (2015). Moderate calorie restriction during gestation programs offspring for lower BAT thermogenic capacity driven by thyroid and sympathetic signaling. Int. J. Obes. 39, 339–345. doi: 10.1038/ijo.2014.56
Park, S. J., Ahmad, F., Philp, A., Baar, K., Williams, T., Luo, H., et al. (2012). Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell 148, 421–433. doi: 10.1016/j.cell.2012.01.017
Petrov, P. D., Palou, A., Bonet, M. L., and Ribot, J. (2016). Cell-autonomous brown-like adipogenesis of preadipocytes from retinoblastoma haploinsufficient mice. J. Cell. Physiol. 231, 1941–1952. doi: 10.1002/jcp.25299
Petrovic, N., Walden, T. B., Shabalina, I. G., Timmons, J. A., Cannon, B., and Nedergaard, J. (2010). Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J. Biol. Chem. 285, 7153–7164. doi: 10.1074/jbc.M109.053942
Price, N. L., Gomes, A. P., Ling, A. J., Duarte, F. V., Martin-Montalvo, A., North, B. J., et al. (2012). SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 15, 675–690. doi: 10.1016/j.cmet.2012.04.003
Priego, T., Sanchez, J., Garcia, A. P., Palou, A., and Pico, C. (2013). Maternal dietary fat affects milk Fatty Acid profile and impacts on weight gain and thermogenic capacity of suckling rats. Lipids 48, 481–495. doi: 10.1007/s11745-013-3764-8
Priego, T., Sanchez, J., Palou, A., and Pico, C. (2010). Leptin intake during the suckling period improves the metabolic response of adipose tissue to a high-fat diet. Int. J. Obes. 34, 809–819. doi: 10.1038/ijo.2010.18
Rivera, L., Moron, R., Zarzuelo, A., and Galisteo, M. (2009). Long-term resveratrol administration reduces metabolic disturbances and lowers blood pressure in obese Zucker rats. Biochem. Pharmacol. 77, 1053–1063. doi: 10.1016/j.bcp.2008.11.027
Rodriguez, A. M., and Palou, A. (2004). Uncoupling proteins: gender-dependence and their relation to body weight control. Int. J. Obes. Relat. Metab. Disord. 28, 327–329. doi: 10.1038/sj.ijo.0802579
Ros, P., Diaz, F., Freire-Regatillo, A., Argente-Arizon, P., Barrios, V., Argente, J., et al. (2018). Resveratrol intake during pregnancy and lactation modulates the early metabolic effects of maternal nutrition differently in male and female offspring. Endocrinology 159, 810–825. doi: 10.1210/en.2017-00610
Rossato, M., Granzotto, M., Macchi, V., Porzionato, A., Petrelli, L., Calcagno, A., et al. (2014). Human white adipocytes express the cold receptor TRPM8 which activation induces UCP1 expression, mitochondrial activation and heat production. Mol. Cell. Endocrinol. 383, 137–146. doi: 10.1016/j.mce.2013.12.005
Saito, M., Yoneshiro, T., and Matsushita, M. (2016). Activation and recruitment of brown adipose tissue by cold exposure and food ingredients in humans. Best Pract. Res. Clin. Endocrinol. Metab. 30, 537–547. doi: 10.1016/j.beem.2016.08.003
Seale, P., Conroe, H. M., Estall, J., Kajimura, S., Frontini, A., Ishibashi, J., et al. (2011). Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J. Clin. Invest. 121, 96–105. doi: 10.1172/JCI44271
Serrano, A., Asnani-Kishnani, M., Rodriguez, A. M., Palou, A., Ribot, J., and Bonet, M. L. (2018). Programming of the beige phenotype in white adipose tissue of adult mice by mild resveratrol and nicotinamide riboside supplementations in early postnatal life. Mol. Nutr. Food Res. 62:e1800463. doi: 10.1002/mnfr.201800463
Szostaczuk, N., Priego, T., Palou, M., Palou, A., and Pico, C. (2017). Oral leptin supplementation throughout lactation in rats prevents later metabolic alterations caused by gestational calorie restriction. Int. J. Obes. 41, 360–371. doi: 10.1038/ijo.2016.241
Valencak, T. G., Osterrieder, A., and Schulz, T. J. (2017). Sex matters: the effects of biological sex on adipose tissue biology and energy metabolism. Redox Biol. 12, 806–813. doi: 10.1016/j.redox.2017.04.012
van den Beukel, J. C., Grefhorst, A., Hoogduijn, M. J., Steenbergen, J., Mastroberardino, P. G., Dor, F. J., et al. (2015). Women have more potential to induce browning of perirenal adipose tissue than men. Obesity 23, 1671–1679. doi: 10.1002/oby.21166
Wang, S., Liang, X., Yang, Q., Fu, X., Rogers, C. J., Zhu, M., et al. (2015). Resveratrol induces brown-like adipocyte formation in white fat through activation of AMP-activated protein kinase (AMPK) alpha1. Int. J. Obes. 39, 967–976. doi: 10.1038/ijo.2015.23
Wiczer, B. M., and Bernlohr, D. A. (2009). A novel role for fatty acid transport protein 1 in the regulation of tricarboxylic acid cycle and mitochondrial function in 3T3-L1 adipocytes. J. Lipid Res. 50, 2502–2513. doi: 10.1194/jlr.M900218-JLR200
Wu, J., Bostrom, P., Sparks, L. M., Ye, L., Choi, J. H., Giang, A. H., et al. (2012). Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150, 366–376. doi: 10.1016/j.cell.2012.05.016
Wu, Q., Kazantzis, M., Doege, H., Ortegon, A. M., Tsang, B., Falcon, A., et al. (2006). Fatty acid transport protein 1 is required for nonshivering thermogenesis in brown adipose tissue. Diabetes Metab. Res. Rev. 55, 3229–3237. doi: 10.2337/db06-0749
Keywords: adipose tissue, beigeing, browning, metabolic programming, sex differences, food bioactives, primary culture
Citation: Asnani-Kishnani M, Rodríguez AM, Serrano A, Palou A, Bonet ML and Ribot J (2019) Neonatal Resveratrol and Nicotinamide Riboside Supplementations Sex-Dependently Affect Beige Transcriptional Programming of Preadipocytes in Mouse Adipose Tissue. Front. Physiol. 10:83. doi: 10.3389/fphys.2019.00083
Received: 05 October 2018; Accepted: 24 January 2019;
Published: 08 February 2019.
Edited by:
Rita De Matteis, University of Urbino Carlo Bo, ItalyReviewed by:
Paul Thomas Pfluger, Helmholtz Center Munich – German Research Center for Environmental Health, GermanyCopyright © 2019 Asnani-Kishnani, Rodríguez, Serrano, Palou, Bonet and Ribot. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: M. Luisa Bonet, bHVpc2Fib25ldEB1aWIuZXM=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.