
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Physiol., 28 January 2019
Sec. Integrative Physiology
Volume 10 - 2019 | https://doi.org/10.3389/fphys.2019.00022
This article is part of the Research TopicInsights into Brown Adipose Tissue Functions and Browning PhenomenonView all 19 articles
Autophagy, lipophagy, and mitophagy are considered to be the major recycling processes for protein aggregates, excess fat, and damaged mitochondria in adipose tissues in response to nutrient status-associated stress, oxidative stress, and genotoxic stress in the human body. Obesity with increased body weight is often associated with white adipose tissue (WAT) hypertrophy and hyperplasia and/or beige/brown adipose tissue atrophy and aplasia, which significantly contribute to the imbalance in lipid metabolism, adipocytokine secretion, free fatty acid release, and mitochondria function. In recent studies, hyperactive autophagy in WAT was observed in obese and diabetic patients, and inhibition of adipose autophagy through targeted deletion of autophagy genes in mice improved anti-obesity phenotypes. In addition, active mitochondria clearance through activation of autophagy was required for beige/brown fat whitening – that is, conversion to white fat. However, inhibition of autophagy seemed detrimental in hypermetabolic conditions such as hepatic steatosis, atherosclerosis, thermal injury, sepsis, and cachexia through an increase in free fatty acid and glycerol release from WAT. The emerging concept of white fat browning–conversion to beige/brown fat–has been controversial in its anti-obesity effect through facilitation of weight loss and improving metabolic health. Thus, proper regulation of autophagy activity fit to an individual metabolic profile is necessary to ensure balance in adipose tissue metabolism and function, and to further prevent metabolic disorders such as obesity and diabetes. In this review, we summarize the effect of autophagy in adipose tissue browning in the context of obesity prevention and its potential as a promising target for the development of anti-obesity drugs.
Macroautophagy, generally referred to as autophagy, is a cytosolic degradation and recycling process of damaged organelles and unwanted components in the cell (Singh et al., 2009b; Zhang et al., 2012; Cairo et al., 2016). When the cells or tissues are limited in their nutrient supply or exposed to a substantial amount of environmental, oxidative, or genotoxic stresses, autophagy as a cellular survival and defense mechanism can be activated (Zhang et al., 2012; Bluher, 2013; Choi et al., 2013; Cairo et al., 2016). Autophagy can be induced in the cell through inhibition of either the nutrient-sensing kinase, mechanistic target of rapamycin complex 1 (mTORC1), or the activating-stress-sensing kinase, 5′-AMP activated protein kinase (AMPK) (Jung et al., 2010; Kim et al., 2011; Stienstra et al., 2014). On the other hand, when cells or tissues are supplied with excessive nutrients, the autophagy process is not necessary and is attenuated through mTORC1 activation and AMPK inhibition (Jung et al., 2010; Kim et al., 2011; Stienstra et al., 2014). When autophagy is suppressed for an extended period of time from continuous overnutrition, as with a high fat and/or fructose diet, the accumulation of unwanted proteins and organelles in the major metabolic tissues – such as adipose, liver, muscle, and pancreas – can become detrimental and eventually induce metabolic dysfunction and diseases such as obesity and diabetes (Zhang et al., 2012; Bluher, 2013; Rocchi and He, 2015). However, contradictory to findings of previous studies, autophagy seems hyperactivated in an effort to generate more fats from recycled energy in adipose tissues of obese patients (Kovsan et al., 2011; Jansen et al., 2012). A few reports suggest that autophagy inhibition can be a protective mechanism against high-fat diet-induced metabolic dysfunction by converting white adipose tissue (WAT) to brown adipose tissue (BAT) (Armani et al., 2014; Parray and Yun, 2017). Throughout the last decade, the therapeutics of modulating autophagy activity have drawn much attention; however, their clinical effectiveness in improving the metabolic profiles of humans with adipocyte metabolic dysfunctions linked to overweight, obesity, and diabetes has not been ascertained (Shoji-Kawata et al., 2013; Galluzzi et al., 2017).
Adipogenesis is a unique adipocyte differentiation process that generates lipid droplets with triglycerides and fatty acids inside the lipid vacuoles (Rosen and Spiegelman, 2006; Ro et al., 2013; Ahmed et al., 2018). Autophagy for non-selective bulk degradation of proteins and lipids through the fusion of autophagosomes and lysosomes is suggested as one of the major types of autophagy in adipocytes (Singh et al., 2009b; Singh and Cuervo, 2012; Ro et al., 2013). The relationship between adipogenesis and autophagy has drawn much attention over the past decade regarding the potential link to metabolic diseases such as obesity. Autophagy is necessary and activated when white adipocyte undergoes differentiation (Singh et al., 2009b; Singh and Cuervo, 2012; Zhang et al., 2012; Ahmed et al., 2018). Chloroquine treatment and autophagy-related protein (ATG) 5 knockdown decreases adipogenesis of mouse embryonic fibroblast (MEF) cells. Targeted deletion of ATG5 in mice leads to a dramatically reduced mass of Perilipin A-positive white adipocytes in late-stage embryos and neonatal pups (Baerga et al., 2009; Singh et al., 2009b). Singh et al. and other research groups also observed decreased levels of microtubule-associated protein 1A/1B-light chain 3 (LC3), peroxisome proliferator-activated receptor (PPAR)- γ, and triglyceride. This finding indicates that adipocyte differentiation and lipid accumulation are blocked by inhibition of autophagy when ATG7 is knocked down in adipocytes or in adipose-specific deletion in mice (Singh et al., 2009b; Zhang et al., 2009; Singh and Cuervo, 2012). When ULK1, the mammalian homolog of ATG1 and the downstream autophagy kinase target of mTORC1, is knocked down in 3T3-L1 white adipocytes, adipogenesis increases, although autophagy is inhibited. However, the ULK2 knockdown in white adipocytes blocks both autophagy and adipogenesis (Ro et al., 2013). An increase in autophagy has been reported in adipose tissues derived from obese humans and mice, supporting adipogenesis in forming and storing more fat depots in the face of overnutrition (Kovsan et al., 2011; Cummins et al., 2014; Kosacka et al., 2015). Both an increased level of the lipidated form of LC3 (LC3-II) as an autophagosome marker and a decreased level of ubiquitin-binding scaffold protein p62, also called sequestosome 1(SQSTM1), were observed in obese humans and mice, the combination of which would appear to be consistent with an increase in autophagy activity during adipogenesis (Klionsky et al., 2016; Yoshii and Mizushima, 2017). The level of autophagy activity seems to differ or at least to fluctuate depending on the adipose tissue type, external stimuli, and tissue age (Bluher, 2013; Kosacka et al., 2015; Li and Ding, 2017). Autophagy is activated when white adipocyte is undergoing differentiation to form lipid droplets. However, in an opposite way, autophagy also can be inhibited when brown adipocyte is activated with UCP1 and PPAR-γ, increasing thermogenesis and browning, respectively, under cold exposure (Singh and Cuervo, 2012; Cairo et al., 2016; Ferhat et al., 2018). Based on previous studies on autophagy in different types of adipocytes, the inhibition of autophagy seems to be better for obesity prevention by reducing the formation of lipid droplets in white adipocytes and promoting energy expenditure in beige or brown adipocytes (Singh et al., 2009b; Kovsan et al., 2011; Ro et al., 2013; Cairo et al., 2016).
Adipocytes are the main type of cells found in both white and brown adipose tissues (Lo and Sun, 2013). White adipocytes contain a single lipid droplet and a small number of mitochondria. Brown adipocytes contain multiple small lipid droplets, enriched amounts of mitochondria, and exhibit a unique thermogenesis function through uncoupling protein 1 (UCP1). Beige adipocytes are the brown-like cells located within WAT, and they have a higher expression level of UCP1 than white adipocytes (Wu et al., 2012; Lo and Sun, 2013; Armani et al., 2014; Cummins et al., 2014). Browning is a process of dynamic conversion or modification of white adipocytes into beige/brown adipocytes upon activation by exposure to physiological, pharmacological, or hormonal stimuli (Wu et al., 2013; Abdullahi and Jeschke, 2016). Browning of white adipocytes is generally induced under cold exposure and exercise (Wu et al., 2012; Lo and Sun, 2013; Aldiss et al., 2018). However, this process does not completely transform or transdifferentiate white adipocytes into brown adipocytes. The white adipocytes become only a brown adipocyte-like phenotype, which is also called beige, inducible brown, brown-in-white, or brite adipocyte (Bartelt and Heeren, 2014; Scheele and Nielsen, 2017). In mouse studies, more beige cells have been detected in WAT of lean, compared to obese mice (Rachid et al., 2015). However in human studies, they only observed higher native BAT activity but not active beige cells in lean subjects (Vijgen et al., 2011). Conventional methods to increase UCP1 in beige/brown fat cells, such as cold acclimation in humans, have not revealed any inducible browning fat depots in addition to the constitutively present depots and have not also proven enough to mediate browning of white fat depots (Scheele and Nielsen, 2017). There indeed are a few reports on cold-induced browning of human perirenal fat, but whether this fat depot is a good representation of visceral fat still remains controversial (Betz et al., 2013). In the study of human patients with pheochromocytoma disease who had performed both 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) and plasma total metanephrine (TMN) measurements in China, browning of human visceral fat has been observed and reduces whole body fat mass by burning more fats through increased UCP1 in beige cells or BAT (Wang et al., 2011). Additionally, recent studies suggest that browning can increase the basal metabolism by burning fat through UCP1 and has been proposed as a potential approach for reducing body fat or treating obesity (Wu et al., 2012; Cummins et al., 2014).
Adipocytes undergo three major types of autophagy: macroautophagy, macrolipophagy (generally referred as lipophagy), and mitophagy. These occur dynamically depending on browning status (Baerga et al., 2009; Singh et al., 2009b; Zhang et al., 2009; Singh and Cuervo, 2012; Li and Ding, 2017; Ghosh et al., 2018). Both autophagy malfunction and adipocyte dysfunction are clearly connected with the causes of metabolic disorders such as obesity and diabetes (Baerga et al., 2009; Singh et al., 2009b; Zhang et al., 2009; Bjorndal et al., 2011; Singh and Cuervo, 2012; Bluher, 2013; Scheele and Nielsen, 2017; Ghosh et al., 2018). To examine this, both mechanistic and clinical studies have investigated the significant relationship between autophagy and browning (Armani et al., 2014; Stienstra et al., 2014). Here, we summarize the following: 1) the three major types of autophagy and their significance in regulating adipose lipid and energy metabolism; and 2) autophagy manipulations through direct autophagy gene knockdown or chemical/drug administration affecting the browning process in humans and mice from previous publications.
Lipophagy is the selective removal of lipid droplets in cytosolic organelles by lysosomes, which are derived from stimulated autophagy markers such as LC3 and p62 (Singh and Cuervo, 2012; Ward et al., 2016). Lipogenesis, often considered identical to adipogenesis, is focused on the formation of lipid droplets during white adipocyte differentiation with autophagy activation; lipolysis, on the other hand, is the secretion of glycerol and fatty acid partially resulting from the degradation of lipid droplets by autophagy (Cingolani and Czaja, 2016; Ahmed et al., 2018). The balance between lipogenesis and lipolysis plays a vital role in regulating the lipid metabolism in white and brown adipocytes (Singh and Cuervo, 2012; Martinez-Lopez et al., 2016; Zechner et al., 2017). ULK1 activates lipolysis by activating autophagy in 3T3-L1 adipocytes. However, ULK1 inhibits fatty acid synthesis and uptake and activates fatty acid oxidation in the mitochondria independent of autophagy in adipocytes (Ro et al., 2013). In an in vivo study of POMC neurons using C57BL/6 WT mice, lipophagy in BAT and liver was activated by both cold exposure and rapamycin administration via the specific surface protein of lipid droplets, adipose triglyceride lipase (ATGL), and LC3 association (Martinez-Lopez et al., 2016). Although both liver and adipose tissue are important tissues in regulating lipid metabolism (Martinez-Lopez et al., 2016), when lipophagy was blocked in liver-specific ATG7 knockout mice, the lipid droplets accumulated in the liver and showed a steatosis-like phenotype (Singh and Cuervo, 2012; Liu and Czaja, 2013). However, in the case of adipose-specific ATG7 knockout mice, white adipocytes showed more brown adipocyte phenotypes with decreased lipids, increased number of mitochondria and beta oxidation (Singh et al., 2009b; Zhang et al., 2009).
The mechanism underlying different tissue specificity is still unclear (Singh and Cuervo, 2012; Martinez-Lopez et al., 2016). When basal lipophagy is inhibited by hyperactivation of mTORC1 due to overnutrition in the human body, lipid droplets are rapidly accumulated in BAT and liver (Singh et al., 2009a). By contrast, when inducible lipophagy is enhanced by inhibition of mTORC1 and activation of AMPK under starvation, lipophagy actively degrades lipid droplets in WAT and releases them as free fatty acids so that other metabolic tissues such as liver and muscle can utilize them as an energy source (Rosen and Spiegelman, 2006; Liu and Czaja, 2013; Ward et al., 2016). Thus, the balance between basal lipophagy and inducible lipophagy, as well as the balance between lipogenesis and lipolysis, is important and seems to be a possible mechanism explaining tissue specificity. BAT and liver tissue would be more prone to the balance between the basal and inducible status of lipophagy, whereas WAT would be more prone to the balance between lipogenesis and lipolysis. These different sensitivities and availability of lipophagy according to the type of tissues and stimuli may create advantages by allowing it to quickly adapt to the different levels of nutrient status in the human body (Martinez-Lopez et al., 2016; Ward et al., 2016). In future studies, transgenic mice with an inducible lipophagy system may serve as a very plausible model for identifying lipophagy specificity and its effect on lipid contents depending on nutrient availability (Singh and Cuervo, 2012).
Mitophagy is the process of actively removing excess mitochondria through selective autophagy when mitochondria have accumulated during differentiation or have been damaged by oxidative stress such as ROS (Zhang et al., 2012; Ashrafi and Schwarz, 2013; Li et al., 2015; Taylor and Gottlieb, 2017). Mitophagy can be induced by ULK1 upon AMPK activation or mTORC1 inhibition under cellular maturation or nutrient deprivation (Kundu et al., 2008; Egan et al., 2011; Kim et al., 2011). The main mitophagy process, the association between mitochondria and autophagolysosomes, is mediated by the ubiquitin-dependent PINK1-Parkin pathway (Narendra et al., 2010; Vincow et al., 2013; Bingol and Sheng, 2016). Alternatively, mitochondria can be degraded by selective autophagy via LC3 and p62 protein independent of ubiquitin in adipose tissue (Altshuler-Keylin and Kajimura, 2017; Taylor and Gottlieb, 2017; Lu et al., 2018). Mitochondria can also be degraded and decreased in number through mitophagy to form more lipid droplets in white adipocyte tissue during differentiation by limiting fatty acid oxidation (Gospodarska et al., 2015; Altshuler-Keylin and Kajimura, 2017). Mitophagy at least in part contributes to whitening of beige adipocytes, turning them into white adipocytes by removing mitochondria after the withdrawal of cold exposure (Altshuler-Keylin et al., 2016; Altshuler-Keylin and Kajimura, 2017; Lu et al., 2018). Therefore, when mitophagy is blocked in white adipocytes, mitochondria cannot be degraded and accumulated while inhibiting adipogenesis, which results in a beige/brown adipocyte phenotype (Altshuler-Keylin and Kajimura, 2017). Consistent with cell culture studies, when mitophagy is inhibited in mice either by autophagy gene deficiency or chemical administration, WAT shows accumulation of mitochondria with decreased fat mass and changes into a phenotype like the beige or brown adipocytes (Singh and Cuervo, 2012; Altshuler-Keylin et al., 2016; Taylor and Gottlieb, 2017; Lu et al., 2018). Clinical researchers have observed more accumulation of dysfunctional or metabolically impaired mitochondria in obese people compared to a lean control group (Kraunsoe et al., 2010; Chattopadhyay et al., 2015). These observations possibly suggest that mitophagy would be negatively regulated by excessive fat accumulation or in obese condition. In conditions of overnutrition, mTORC1 activation and mitophagy inhibition resulted in greater accumulation of impaired mitochondria (Altshuler-Keylin and Kajimura, 2017). Studies using autophagy-related gene knockout mice fed with a high-fat diet suggest that when autophagy and mitophagy in adipocytes are impaired by overnutrition, inhibition of lipogenesis and activation of lipophagy can occur as a compensatory mechanism (Zhang et al., 2009; Altshuler-Keylin and Kajimura, 2017). To our surprise, the browning of WAT was observed in skeletal muscle-specific Atg7 knockout mice that were resistant to obesity induced by a high-fat diet (Kim et al., 2013). This ambivalence of mitophagy in adipocyte turnover and the existence of compensation mechanisms with other selective autophagy may be for purposes of more effectively maintaining mitochondrial integrity and mass in adipocytes (Lu et al., 2018). Although mitophagy is suggested as a positive regulator of white adipogenesis and a negative regulator of beige and brown adipogenesis, the level of mitophagy necessary for browning seems controversial due to the complicated regulation of activity dependent on nutrition status (Altshuler-Keylin and Kajimura, 2017). Therefore, the proper modulation of mitophagy in adipocytes in humans and mice seems necessary for the timely turnover between white, beige, and brown adipocytes, dependent on nutrition level.
Although the distribution of adipose tissue is distinct in humans and mice, both share common characteristics (Seale et al., 2009; Zhang et al., 2018). Anatomically in male mice, interscapular brown adipose tissue (iBAT) contains classic brown adipocytes, whereas epididymal white adipose tissue (eWAT) and subcutaneous white adipose tissue (sWAT) contain classic white adipocytes (Sanchez-Gurmaches and Guertin, 2014; Gospodarska et al., 2015). In humans, most classic brown adipocytes develop mainly around the neck and supraclavicular area through infancy, but gradually decrease until adulthood (Wu et al., 2012; Zhang et al., 2018). Several studies using positron emission tomography (PET)-CT have demonstrated that, in addition to size reduction in aging, BAT activity is reduced in obese and diabetic patients (Lee et al., 2010; Leitner et al., 2017). A few other clinical research groups have suggested that stimulating browning in WAT would be beneficial in slowing obesity, diabetes, and even the aging process (Scheele and Nielsen, 2017). Therefore, the existence of active turnover from WAT to beige fat to BAT in humans and mice has been recognized as a potential therapeutic target for prevention and treatment of obesity and related metabolic diseases (Kajimura et al., 2015; Schrauwen et al., 2015; Giordano et al., 2016). Even whole tissue switching of WAT to BAT through surgical transplantation or implantation of mesenchymal stem cells, brown adipocytes, or BAT into WAT areas in humans and mice is gaining a new spotlight as a novel method to prevent or treat obesity and diabetes (Liu et al., 2013; Soler-Vazquez et al., 2018). However, the activity and selectivity of autophagy after the transplantation or implantation still needs further investigation.
Indeed, autophagy plays an important role in the browning of WAT and beige adipocytes. A recent study has reported that autophagy is needed to convert beige adipocytes to WAT upon removal of β3-AR agonists or recovery from cold exposure (Altshuler-Keylin et al., 2016). Cairo et al. (2016) reported that thermogenic activation through cold exposure inhibits autophagy, which leads to increased UCP1 level in BAT. Although we have selected only a few significant factors to discuss in our review, numerous factors are involved in the manipulation of the autophagy pathway, which regulate the browning of WAT and beige adipocyte (Table 1).
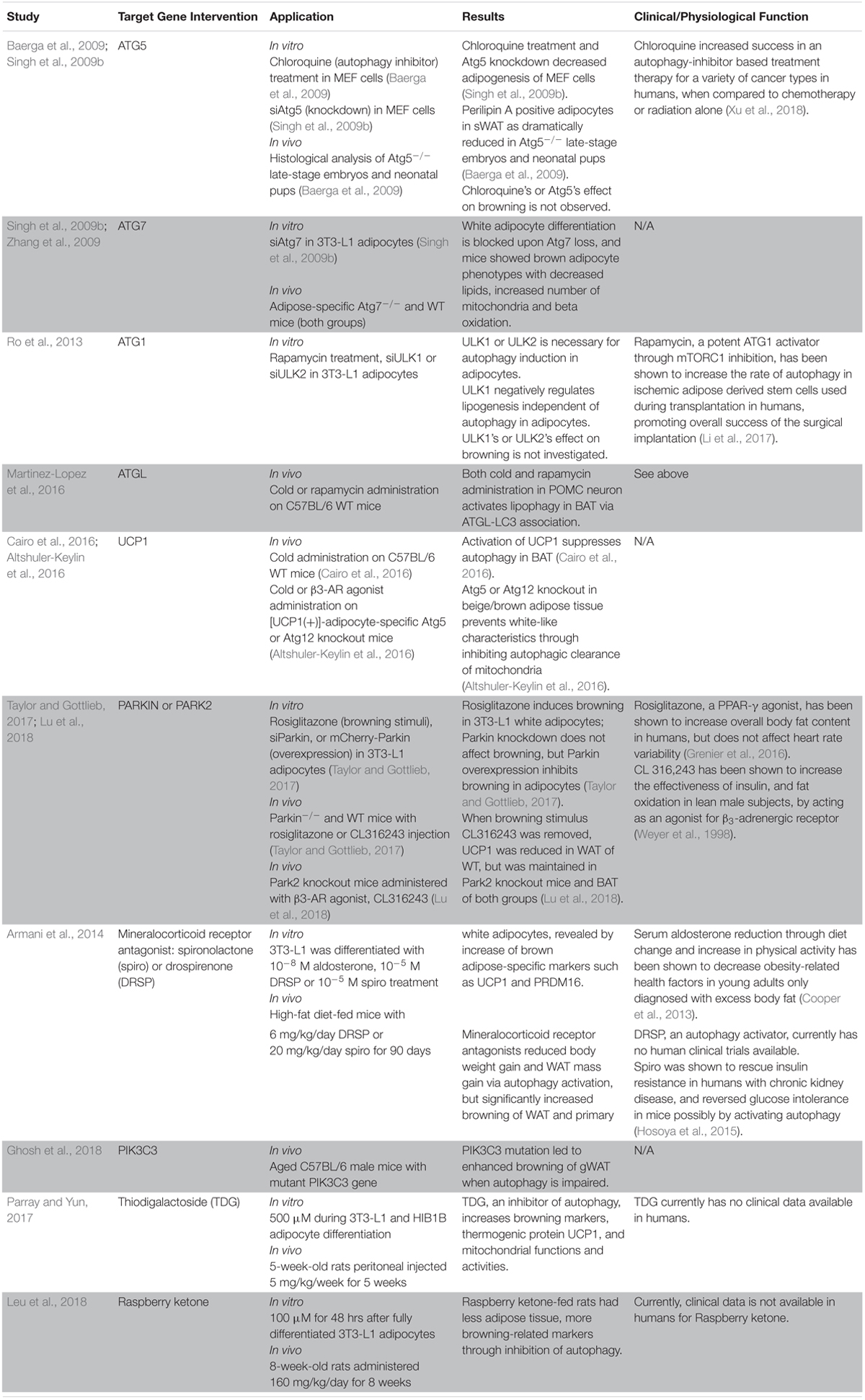
Table 1. Summary of recent studies about the effect of direct autophagy gene manipulation or autophagy-related regulators on adipocyte browning.
Parkin (gene name: Park2) is a E3 ubiquitin ligase that plays a critical role in ubiquitination as a mitophagy-associated degradation signal (Geisler et al., 2010; Jin and Youle, 2012; Pickrell and Youle, 2015). The role of Parkin in browning of WAT has been studied in 3T3-L1 adipocytes and the Parkin-deficient C57BL/6 mice model. Parkin expression increases during 3T3-L1 adipocyte differentiation, while its expression decreases in rosiglitazone-treated 3T3-L1 adipocytes, which have phenotypes of beige adipocytes due to enhanced UCP1 expression. Inhibition of the Parkin gene does not affect browning, but overexpression of Parkin significantly reduces browning in adipocytes (Taylor and Gottlieb, 2017). Furthermore, Parkin is highly expressed during beige adipocyte differentiation (Lu et al., 2018). The Kajimura group has shown that Parkin is required to maintain beige adipocytes in WAT. When CL316243, a β3-AR agonist, is removed, UCP1 expression is significantly reduced in WAT of wild type (WT) mice, but still expressed in WAT of Park2 knockout mice. In contrast, UCP1 expression in BAT is not changed in both WT and Park2 knockout mice after CL316243 is removed (Lu et al., 2018).
The Yan group has shown that autophagy is regulated by mineralocorticoid receptor (MR) antagonism (Li et al., 2016). Spironolacton induces LC3 and ATG5 expression and reduces PI3K/AKT/mTOR pathways in injured human podocytes (Li et al., 2016). Previous research has reported the role of MR in adipocyte differentiation. Drospirenone (DRSP) significantly reduces 3T3-L1 and 3T3-F442A adipocyte differentiation without cell cytotoxicity (Caprio et al., 2011). MR also regulates browning of WAT though autophagy. Additionally, it has been determined that MR antagonists fully prevent aldosterone-induced autophagy in white adipocytes along with an increase of UCP1 expression. MR antagonists significantly enhance browning of WAT in diet-induced obese mice as well as brown adipose-specific markers in primary adipocytes isolated from WAT (Armani et al., 2014).
PIK3C3 is a subunit of class III phosphoinositide 3-kindase (PI3K) that phosphorylates phosphatidylinositol to generate phosphatidylinositol 3-phosphate. The PIK3C3-ATG14 complex induces autophagy especially in nutrient-stress conditions such as starvation (Yuan et al., 2013). Recently, it has been shown that in aged mice with a PIK3C3 mutation, compared to fl/fl control mice, adipogenesis markers, such as AP2 and C/EBP-α, are reduced, but brown adipose-specific markers, such as UCP1 and PPAR- γ coactivator (PGC)1α, are enhanced in both mRNA and protein levels in the gonadal WAT (gWAT), possibly through blocking of autophagy (Ghosh et al., 2018).
Thiodigalactoside (TDG), an inhibitor of galectin 1 and autophagy, has recently been studied in obesity research by the Yun group (Mukherjee et al., 2015; Parray and Yun, 2015; 2017). They have reported that TDG-treated adipocytes significantly inhibit lipid accumulation, and TDG also reduces body weight in high-fat diet-fed rats (Mukherjee et al., 2015). Their second study has shown proteomic identification of TDG in WAT of rats with high-fat diet-induced obesity. Specifically, proteins involved in carbohydrate metabolism and the tricarboxylic acid cycle remarkably increased in WAT of TDG-injected obese rats (Parray and Yun, 2015). A most recent study has reported that TDG plays an important role in browning of white adipocytes and WAT in obese rats (Parray and Yun, 2017). Dose-dependent TDG treatment reduces galectin 1 and ATG 5 gene expression, but enhances brown-specific markers, UCP1 and PGC1α, in 3T3-L1 adipocytes. Moreover, UCP1 and PGC1α gene and protein expressions are upregulated by PDG injection in iWAT, eWAT, and BAT of diet-induced obese rats, possibly through inhibition of ATG5/LC3-II and increase of p62 expression (Parray and Yun, 2017).
Raspberry ketone, 4-(4-hydroxyphenyl) butan-2-one, a phenolic compound found in red raspberry, has emerged as a dietary bioactive compound with beneficial effects on obesity (Cotten et al., 2017; Tsai et al., 2017). Evidence suggests that raspberry ketone reduces body weight and food intake in high-fat diet-fed mice (Cotten et al., 2017) and ovariectomy-induced obese rats (Leu et al., 2017). In addition, raspberry ketone inhibits 3T3-L1 adipogenesis, revealed by inhibition of expression of adipogenesis markers such as PPAR-γ, C/EBP-α, FAS, and AP2, possibly via inhibition of autophagy, confirmed by decrease of ATG12 and LC3B levels, as well as increase of p62 and mTORC1 levels (Leu et al., 2017; Tsai et al., 2017). It has been recently reported that high concentration of raspberry ketone (100 μM) significantly increases browning of 3T3-L1 adipocytes, revealed by an increase of browning-specific markers, including UCP1 and PGC1α, and lipolysis markers such as hormone-sensitive lipase and triglyceride lipase (Leu et al., 2018). Moreover, expression of brown adipose markers is increased in ovariectomy-induced obese rats that have been administered raspberry, compared to control groups mediated by inhibition of ATG12 and an increase of p62 expression (Leu et al., 2018).
Adipose metabolism is closely linked with metabolic dysfunctions such as obesity and diabetes when fat distribution and energy balance through mitochondria are not strictly maintained (Bjorndal et al., 2011; Bluher, 2013; Stienstra et al., 2014; Scheele and Nielsen, 2017). The relationship between adipose metabolism and autophagy has become an increasingly intriguing topic since the dawn of the discovery of selective autophagy, including lipophagy and mitophagy, which can also actively occur in adipose tissue (Rocchi and He, 2015). Autophagy has previously been shown to be increased in adipocytes from obese humans and mice (Ost et al., 2010; Kovsan et al., 2011; Jansen et al., 2012). Previous reports indicate that excess free fatty acid (FFA) – particularly saturated FFA like palmitic acid (PA), but not unsaturated FFA such as oleic acid (OA) – formed by a high-fat diet can activate autophagy through JNK2 or PKC activation (Tan et al., 2012; Tu et al., 2014). Conversely, lipophagy seems beneficial for degrading excess fats from WAT and generating more intracellular space for the expansion of the mitochondrial contents from BAT when mitophagy is inhibited or mitochondrial biogenesis is activated, thus protecting the human body from nutrient oversupply which, can occur in obesity conditions (Singh and Cuervo, 2012; Cummins et al., 2014). Mitophagy seems much more controversial because basal activity can be beneficial through elimination of damaged mitochondria from accumulated ROS in obesity; however, hyperactive inducible mitophagy can convert BAT or beige fat to white during differentiation, which is called “reverse browning or whitening,” and subsequently cause systemic change in adipocytokine release and lipid metabolism (Hill et al., 2012; Gospodarska et al., 2015). Overall, inhibition or deficiency of autophagy, activation of lipophagy rather than lipolysis, and a basal or moderate level of mitophagy seems the most optimal combination for the prevention of obesity so far (Figure 1).
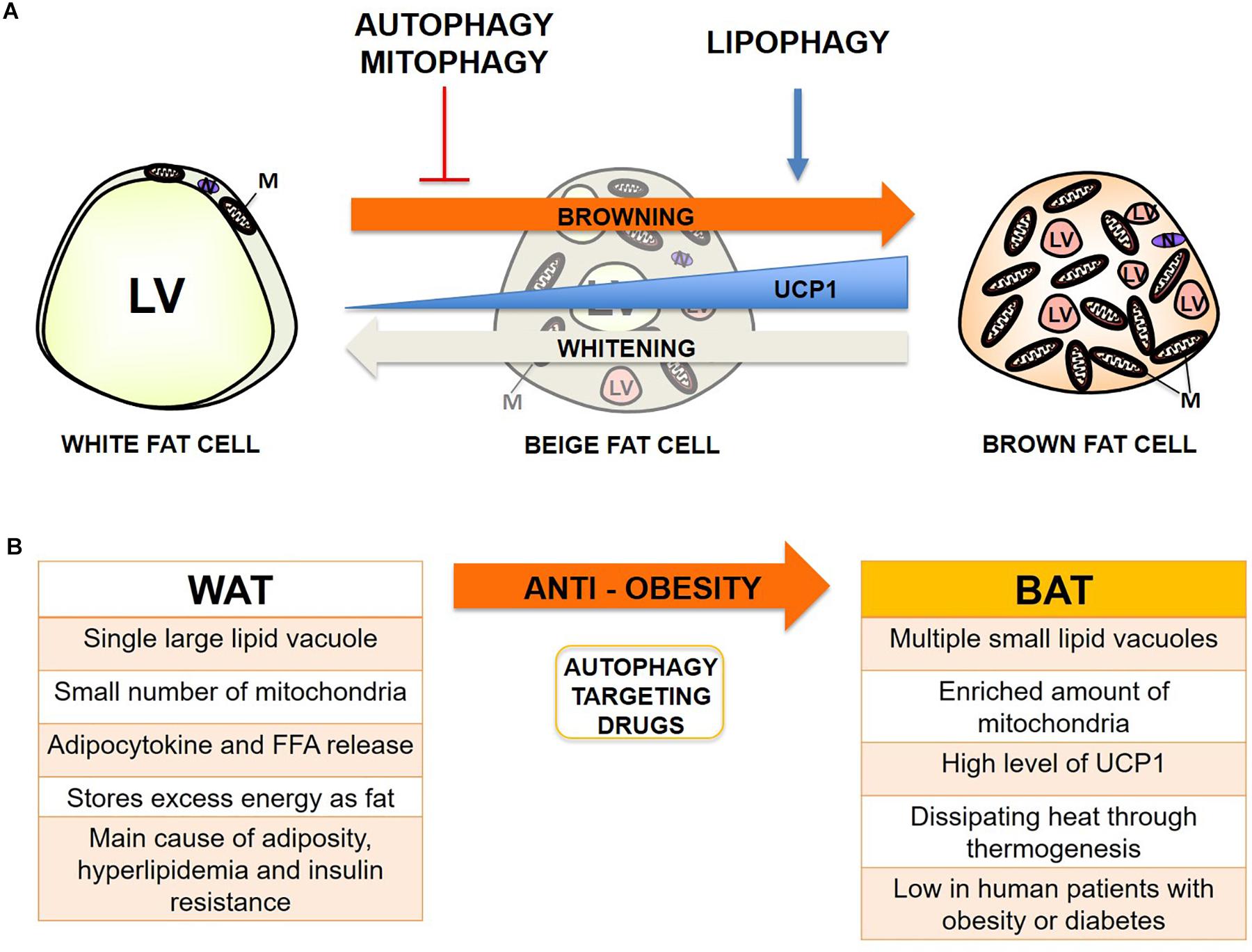
Figure 1. Autophagy effect on adipocyte browning and potential therapeutic target for prevention of obesity. (A) Summary of the proposed effect of autophagy, lipophagy, and mitophagy on adipocyte browning from recent studies. LV, lipid vacuoles; M, mitochondria; N, Nucleus. (B) Summary of the main characteristics of WAT and BAT in obesity, and the induction of browning through manipulation of autophagy as a promising target for anti-obesity therapy.
However, we have also encountered a few exceptional cases from previous reports noting that hyperactive autophagy can be beneficial during hypermetabolic conditions such as hepatic steatosis, atherosclerosis, injuries from burning, sepsis, and cachexia (Volzke et al., 2005; Penna et al., 2014; Pravda, 2014; Song et al., 2014; Abdullahi and Jeschke, 2016). Normally, hyperactivation of autophagy leads to apoptotic cell death (Lum et al., 2005), but highly autophagic cells under hypermetabolic conditions such as post-thermal injury may survive better by efficiently regulating energy metabolism (Auger et al., 2017). Paradoxically, autophagy activation in WAT can be beneficial for obese or diabetic patients with a hypermetabolic profile or complications, because it decreases FFA and glycerol release from hypertrophic and hyperplasic WAT by actively degrading lipid vacuoles (LV) as an energy source. We have summarized previously reported autophagy and selective autophagy manipulations and their effect on adipocyte browning (Table 1). Since the combined delicate manipulation of autophagy, lipophagy, and mitophagy seems necessary for the timely turnover between white, beige, and brown adipocytes dependent on nutrition levels in humans and mice, the direct manipulation of autophagy and selective autophagy or the administration of autophagy-targeting drugs should be cautiously performed. Finally, summarizing autophagy regulation and its implications in browning could help give insights for the development of autophagy-targeting drugs in the prevention of obesity.
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
This work was supported by NSF-REU site: Training in Redox Biology (DBI-1757951) to ZS, Undergraduate Creative Activities and Research Experience (UCARE) program-scholarship from the Pepsi Quasi Endowment and Union Bank & Trust to CS, University of Nebraska-Lincoln ARD/ORED/BIOC grants, Layman award, Nebraska Tobacco Settlement-Biomedical research enhancement funds, and Nebraska Center for the Prevention of Obesity Diseases (NPOD) seed grant from NIH (P20GM104320) to S-HR.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank every present and past Ro lab member for helpful discussion and comments. We apologize that we could not include many original publications due to limited space.
Abdullahi, A., and Jeschke, M. G. (2016). White adipose tissue browning: a double-edged sword. Trends Endocrinol. Metab. 27, 542–552. doi: 10.1016/j.tem.2016.06.006
Ahmed, M., Nguyen, H. Q., Hwang, J. S., Zada, S., Lai, T. H., Kang, S. S., et al. (2018). Systematic characterization of autophagy-related genes during the adipocyte differentiation using public-access data. Oncotarget 9, 15526–15541. doi: 10.18632/oncotarget.24506
Aldiss, P., Betts, J., Sale, C., Pope, M., Budge, H., and Symonds, M. E. (2018). Exercise-induced ’browning’ of adipose tissues. Metabolism 81, 63–70. doi: 10.1016/j.metabol.2017.11.009
Altshuler-Keylin, S., and Kajimura, S. (2017). Mitochondrial homeostasis in adipose tissue remodeling. Sci. Signal. 10:eaai9248. doi: 10.1126/scisignal.aai9248
Altshuler-Keylin, S., Shinoda, K., Hasegawa, Y., Ikeda, K., Hong, H., Kang, Q., et al. (2016). Beige adipocyte maintenance is regulated by autophagy-induced mitochondrial clearance. Cell Metab. 24, 402–419. doi: 10.1016/j.cmet.2016.08.002
Armani, A., Cinti, F., Marzolla, V., Morgan, J., Cranston, G. A., Antelmi, A., et al. (2014). Mineralocorticoid receptor antagonism induces browning of white adipose tissue through impairment of autophagy and prevents adipocyte dysfunction in high-fat-diet-fed mice. FASEB J. 28, 3745–3757. doi: 10.1096/fj.13-245415
Ashrafi, G., and Schwarz, T. L. (2013). The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ. 20, 31–42. doi: 10.1038/cdd.2012.81
Auger, C., Samadi, O., and Jeschke, M. G. (2017). The biochemical alterations underlying post-burn hypermetabolism. Biochim. Biophys. Acta 1863(10 Pt B), 2633–2644. doi: 10.1016/j.bbadis.2017.02.019
Baerga, R., Zhang, Y., Chen, P. H., Goldman, S., and Jin, S. (2009). Targeted deletion of autophagy-related 5 (atg5) impairs adipogenesis in a cellular model and in mice. Autophagy 5, 1118–1130. doi: 10.4161/auto.5.8.9991
Bartelt, A., and Heeren, J. (2014). Adipose tissue browning and metabolic health. Nat. Rev. Endocrinol. 10, 24–36. doi: 10.1038/nrendo.2013.204
Betz, M. J., Slawik, M., Lidell, M. E., Osswald, A., Heglind, M., Nilsson, D., et al. (2013). Presence of brown adipocytes in retroperitoneal fat from patients with benign adrenal tumors: relationship with outdoor temperature. J. Clin. Endocrinol. Metab. 98, 4097–4104. doi: 10.1210/jc.2012-3535
Bingol, B., and Sheng, M. (2016). Mechanisms of mitophagy: PINK1, Parkin, USP30 and beyond. Free Radic. Biol. Med. 100, 210–222. doi: 10.1016/j.freeradbiomed.2016.04.015
Bjorndal, B., Burri, L., Staalesen, V., Skorve, J., and Berge, R. K. (2011). Different adipose depots: their role in the development of metabolic syndrome and mitochondrial response to hypolipidemic agents. J. Obes. 2011, 490650. doi: 10.1155/2011/490650
Bluher, M. (2013). Adipose tissue dysfunction contributes to obesity related metabolic diseases. Best Pract. Res. Clin. Endocrinol. Metab. 27, 163–177. doi: 10.1016/j.beem.2013.02.005
Cairo, M., Villarroya, J., Cereijo, R., Campderros, L., Giralt, M., and Villarroya, F. (2016). Thermogenic activation represses autophagy in brown adipose tissue. Int. J. Obes. (Lond.) 40, 1591–1599. doi: 10.1038/ijo.2016.115
Caprio, M., Antelmi, A., Chetrite, G., Muscat, A., Mammi, C., Marzolla, V., et al. (2011). Antiadipogenic effects of the mineralocorticoid receptor antagonist drospirenone: potential implications for the treatment of metabolic syndrome. Endocrinology 152, 113–125. doi: 10.1210/en.2010-0674
Chattopadhyay, M., Khemka, V. K., Chatterjee, G., Ganguly, A., Mukhopadhyay, S., and Chakrabarti, S. (2015). Enhanced ROS production and oxidative damage in subcutaneous white adipose tissue mitochondria in obese and type 2 diabetes subjects. Mol. Cell Biochem. 399, 95–103. doi: 10.1007/s11010-014-2236-7
Choi, A. M., Ryter, S. W., and Levine, B. (2013). Autophagy in human health and disease. N. Engl. J. Med. 368, 651–662. doi: 10.1056/NEJMra1205406
Cingolani, F., and Czaja, M. J. (2016). Regulation and functions of autophagic lipolysis. Trends Endocrinol. Metab. 27, 696–705. doi: 10.1016/j.tem.2016.06.003
Cooper, J. N., Fried, L., Tepper, P., Barinas-Mitchell, E., Conroy, M. B., Evans, R. W., et al. (2013). Changes in serum aldosterone are associated with changes in obesity-related factors in normotensive overweight and obese young adults. Hypertens. Res. 36, 895–901. doi: 10.1038/hr.2013.45
Cotten, B. M., Diamond, S. A., Banh, T., Hsiao, Y. H., Cole, R. M., Li, J., et al. (2017). Raspberry ketone fails to reduce adiposity beyond decreasing food intake in C57BL/6 mice fed a high-fat diet. Food Funct. 8, 1512–1518. doi: 10.1039/c6fo01831a
Cummins, T. D., Holden, C. R., Sansbury, B. E., Gibb, A. A., Shah, J., Zafar, N., et al. (2014). Metabolic remodeling of white adipose tissue in obesity. Am. J. Physiol. Endocrinol. Metab. 307, E262–E277. doi: 10.1152/ajpendo.00271.2013
Egan, D. F., Shackelford, D. B., Mihaylova, M. M., Gelino, S., Kohnz, R. A., Mair, W., et al. (2011). Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science 331, 456–461. doi: 10.1126/science.1196371
Ferhat, M., Funai, K., and Boudina, S. (2018). Autophagy in adipose tissue physiology and pathophysiology. Antioxid. Redox Signal. doi: 10.1089/ars.2018.7626 [Epub ahead of print].
Galluzzi, L., Bravo-San Pedro, J. M., Levine, B., Green, D. R., and Kroemer, G. (2017). Pharmacological modulation of autophagy: therapeutic potential and persisting obstacles. Nat. Rev. Drug Discov. 16, 487–511. doi: 10.1038/nrd.2017.22
Geisler, S., Holmstrom, K. M., Skujat, D., Fiesel, F. C., Rothfuss, O. C., Kahle, P. J., et al. (2010). PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat. Cell Biol. 12, 119–131. doi: 10.1038/ncb2012
Ghosh, A. K., Mau, T., O’Brien, M., and Yung, R. (2018). Novel role of autophagy-associated Pik3c3 gene in gonadal white adipose tissue browning in aged C57/Bl6 male mice. Aging (Albany NY) 10, 764–774. doi: 10.18632/aging.101426
Giordano, A., Frontini, A., and Cinti, S. (2016). Convertible visceral fat as a therapeutic target to curb obesity. Nat. Rev. Drug Discov. 15, 405–424. doi: 10.1038/nrd.2016.31
Gospodarska, E., Nowialis, P., and Kozak, L. P. (2015). Mitochondrial turnover: a phenotype distinguishing brown adipocytes from interscapular brown adipose tissue and white adipose tissue. J. Biol. Chem. 290, 8243–8255. doi: 10.1074/jbc.M115.637785
Grenier, A., Brassard, P., Bertrand, O. F., Despres, J. P., Costerousse, O., Almeras, N., et al. (2016). Rosiglitazone influences adipose tissue distribution without deleterious impact on heart rate variability in coronary heart disease patients with type 2 diabetes. Clin. Auton Res. 26, 407–414. doi: 10.1007/s10286-016-0373-7
Hill, B. G., Benavides, G. A., Lancaster, J. R., Ballinger, S., Dell’Italia, L., Jianhua, Z., et al. (2012). Integration of cellular bioenergetics with mitochondrial quality control and autophagy. Biol. Chem. 393, 1485–1512. doi: 10.1515/hsz-2012-0198
Hosoya, K., Minakuchi, H., Wakino, S., Fujimura, K., Hasegawa, K., Komatsu, M., et al. (2015). Insulin resistance in chronic kidney disease is ameliorated by spironolactone in rats and humans. Kidney Int. 87, 749–760. doi: 10.1038/ki.2014.348
Jansen, H. J., van Essen, P., Koenen, T., Joosten, L. A., Netea, M. G., Tack, C. J., et al. (2012). Autophagy activity is up-regulated in adipose tissue of obese individuals and modulates proinflammatory cytokine expression. Endocrinology 153, 5866–5874. doi: 10.1210/en.2012-1625
Jin, S. M., and Youle, R. J. (2012). PINK1- and Parkin-mediated mitophagy at a glance. J. Cell Sci. 125(Pt 4), 795–799. doi: 10.1242/jcs.093849
Jung, C. H., Ro, S. H., Cao, J., Otto, N. M., and Kim, D. H. (2010). mTOR regulation of autophagy. FEBS Lett. 584, 1287–1295. doi: 10.1016/j.febslet.2010.01.017
Kajimura, S., Spiegelman, B. M., and Seale, P. (2015). Brown and beige fat: physiological roles beyond heat generation. Cell Metab. 22, 546–559. doi: 10.1016/j.cmet.2015.09.007
Kim, J., Kundu, M., Viollet, B., and Guan, K. L. (2011). AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 13, 132–141. doi: 10.1038/ncb2152
Kim, K. H., Jeong, Y. T., Oh, H., Kim, S. H., Cho, J. M., Kim, Y. N., et al. (2013). Autophagy deficiency leads to protection from obesity and insulin resistance by inducing Fgf21 as a mitokine. Nat. Med. 19, 83–92. doi: 10.1038/nm.3014
Klionsky, D. J., Abdelmohsen, K., Abe, A., Abedin, M. J., Abeliovich, H., Acevedo Arozena, A., et al. (2016). Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 12, 1–222. doi: 10.1080/15548627.2015.1100356
Kosacka, J., Kern, M., Kloting, N., Paeschke, S., Rudich, A., Haim, Y., et al. (2015). Autophagy in adipose tissue of patients with obesity and type 2 diabetes. Mol. Cell. Endocrinol. 409, 21–32. doi: 10.1016/j.mce.2015.03.015
Kovsan, J., Bluher, M., Tarnovscki, T., Kloting, N., Kirshtein, B., Madar, L., et al. (2011). Altered autophagy in human adipose tissues in obesity. J. Clin. Endocrinol. Metab. 96, E268–E277. doi: 10.1210/jc.2010-1681
Kraunsoe, R., Boushel, R., Hansen, C. N., Schjerling, P., Qvortrup, K., Stockel, M., et al. (2010). Mitochondrial respiration in subcutaneous and visceral adipose tissue from patients with morbid obesity. J. Physiol. 588(Pt 12), 2023–2032. doi: 10.1113/jphysiol.2009.184754
Kundu, M., Lindsten, T., Yang, C. Y., Wu, J., Zhao, F., Zhang, J., et al. (2008). Ulk1 plays a critical role in the autophagic clearance of mitochondria and ribosomes during reticulocyte maturation. Blood 112, 1493–1502. doi: 10.1182/blood-2008-02-137398
Lee, P., Greenfield, J. R., Ho, K. K., and Fulham, M. J. (2010). A critical appraisal of the prevalence and metabolic significance of brown adipose tissue in adult humans. Am. J. Physiol. Endocrinol. Metab. 299, E601–E606. doi: 10.1152/ajpendo.00298.2010
Leitner, B. P., Huang, S., Brychta, R. J., Duckworth, C. J., Baskin, A. S., McGehee, S., et al. (2017). Mapping of human brown adipose tissue in lean and obese young men. Proc. Natl. Acad. Sci. U.S.A. 114, 8649–8654. doi: 10.1073/pnas.1705287114
Leu, S. Y., Chen, Y. C., Tsai, Y. C., Hung, Y. W., Hsu, C. H., Lee, Y. M., et al. (2017). Raspberry ketone reduced lipid accumulation in 3T3-L1 cells and ovariectomy-induced obesity in wistar rats by regulating autophagy mechanisms. J. Agric. Food Chem. 65, 10907–10914. doi: 10.1021/acs.jafc.7b03831
Leu, S. Y., Tsai, Y. C., Chen, W. C., Hsu, C. H., Lee, Y. M., and Cheng, P. Y. (2018). Raspberry ketone induces brown-like adipocyte formation through suppression of autophagy in adipocytes and adipose tissue. J. Nutr. Biochem. 56, 116–125. doi: 10.1016/j.jnutbio.2018.01.017
Li, C., Ye, L., Yang, L., Yu, X., He, Y., Chen, Z., et al. (2017). Rapamycin promotes the survival and adipogenesis of ischemia-challenged adipose derived stem cells by improving autophagy. Cell Physiol. Biochem. 44, 1762–1774. doi: 10.1159/000485783
Li, D., Lu, Z., Xu, Z., Ji, J., Zheng, Z., Lin, S., et al. (2016). Spironolactone promotes autophagy via inhibiting PI3K/AKT/mTOR signalling pathway and reduce adhesive capacity damage in podocytes under mechanical stress. Biosci. Rep. 36:e00355. doi: 10.1042/BSR20160086
Li, L., Tan, J., Miao, Y., Lei, P., and Zhang, Q. (2015). ROS and autophagy: interactions and molecular regulatory mechanisms. Cell. Mol. Neurobiol. 35, 615–621. doi: 10.1007/s10571-015-0166-x
Li, Y., and Ding, W. X. (2017). Adipose tissue autophagy and homeostasis in alcohol-induced liver injury. Liver Res. 1, 54–62. doi: 10.1016/j.livres.2017.03.004
Liu, K., and Czaja, M. J. (2013). Regulation of lipid stores and metabolism by lipophagy. Cell Death Differ. 20, 3–11. doi: 10.1038/cdd.2012.63
Liu, X., Zheng, Z., Zhu, X., Meng, M., Li, L., Shen, Y., et al. (2013). Brown adipose tissue transplantation improves whole-body energy metabolism. Cell Res. 23, 851–854. doi: 10.1038/cr.2013.64
Lo, K. A., and Sun, L. (2013). Turning WAT into BAT: a review on regulators controlling the browning of white adipocytes. Biosci. Rep. 33:e00065. doi: 10.1042/bsr20130046
Lu, X., Altshuler-Keylin, S., Wang, Q., Chen, Y., Henrique Sponton, C., Ikeda, K., et al. (2018). Mitophagy controls beige adipocyte maintenance through a Parkin-dependent and UCP1-independent mechanism. Sci. Signal. 11:eaap8526. doi: 10.1126/scisignal.aap8526
Lum, J. J., Bauer, D. E., Kong, M., Harris, M. H., Li, C., Lindsten, T., et al. (2005). Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell 120, 237–248. doi: 10.1016/j.cell.2004.11.046
Martinez-Lopez, N., Garcia-Macia, M., Sahu, S., Athonvarangkul, D., Liebling, E., Merlo, P., et al. (2016). Autophagy in the CNS and periphery coordinate lipophagy and lipolysis in the brown adipose tissue and liver. Cell Metab. 23, 113–127. doi: 10.1016/j.cmet.2015.10.008
Mukherjee, R., Kim, S. W., Park, T., Choi, M. S., and Yun, J. W. (2015). Targeted inhibition of galectin 1 by thiodigalactoside dramatically reduces body weight gain in diet-induced obese rats. Int. J. Obes. (Lond.) 39, 1349–1358. doi: 10.1038/ijo.2015.74
Narendra, D. P., Jin, S. M., Tanaka, A., Suen, D. F., Gautier, C. A., Shen, J., et al. (2010). PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 8:e1000298. doi: 10.1371/journal.pbio.1000298
Ost, A., Svensson, K., Ruishalme, I., Brannmark, C., Franck, N., Krook, H., et al. (2010). Attenuated mTOR signaling and enhanced autophagy in adipocytes from obese patients with type 2 diabetes. Mol. Med. 16, 235–246. doi: 10.2119/molmed.2010.00023
Parray, H. A., and Yun, J. W. (2015). Proteomic identification of target proteins of thiodigalactoside in white adipose tissue from diet-induced obese rats. Int. J. Mol. Sci. 16, 14441–14463. doi: 10.3390/ijms160714441
Parray, H. A., and Yun, J. W. (2017). Combined inhibition of autophagy protein 5 and galectin-1 by thiodigalactoside reduces diet-induced obesity through induction of white fat browning. IUBMB Life 69, 510–521. doi: 10.1002/iub.1634
Penna, F., Baccino, F. M., and Costelli, P. (2014). Coming back: autophagy in cachexia. Curr. Opin. Clin. Nutr. Metab Care 17, 241–246. doi: 10.1097/MCO.0000000000000048
Pickrell, A. M., and Youle, R. J. (2015). The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron 85, 257–273. doi: 10.1016/j.neuron.2014.12.007
Pravda, J. (2014). Metabolic theory of septic shock. World J. Crit. Care Med. 3, 45–54. doi: 10.5492/wjccm.v3.i2.45
Rachid, T. L., Penna-de-Carvalho, A., Bringhenti, I., Aguila, M. B., Mandarim-de-Lacerda, C. A., and Souza-Mello, V. (2015). Fenofibrate (PPARalpha agonist) induces beige cell formation in subcutaneous white adipose tissue from diet-induced male obese mice. Mol. Cell Endocrinol. 402, 86–94. doi: 10.1016/j.mce.2014.12.027
Ro, S. H., Jung, C. H., Hahn, W. S., Xu, X., Kim, Y. M., Yun, Y. S., et al. (2013). Distinct functions of Ulk1 and Ulk2 in the regulation of lipid metabolism in adipocytes. Autophagy 9, 2103–2114. doi: 10.4161/auto.26563
Rocchi, A., and He, C. (2015). Emerging roles of autophagy in metabolism and metabolic disorders. Front. Biol. (Beijing) 10:154–164. doi: 10.1007/s11515-015-1354-2
Rosen, E. D., and Spiegelman, B. M. (2006). Adipocytes as regulators of energy balance and glucose homeostasis. Nature 444, 847–853. doi: 10.1038/nature05483
Sanchez-Gurmaches, J., and Guertin, D. A. (2014). Adipocyte lineages: tracing back the origins of fat. Biochim. Biophys. Acta 1842, 340–351. doi: 10.1016/j.bbadis.2013.05.027
Scheele, C., and Nielsen, S. (2017). Metabolic regulation and the anti-obesity perspectives of human brown fat. Redox. Biol. 12, 770–775. doi: 10.1016/j.redox.2017.04.011
Schrauwen, P., van Marken Lichtenbelt, W. D., and Spiegelman, B. M. (2015). The future of brown adipose tissues in the treatment of type 2 diabetes. Diabetologia 58, 1704–1707. doi: 10.1007/s00125-015-3611-y
Seale, P., Kajimura, S., and Spiegelman, B. M. (2009). Transcriptional control of brown adipocyte development and physiological function–of mice and men. Genes Dev. 23, 788–797. doi: 10.1101/gad.1779209
Shoji-Kawata, S., Sumpter, R., Leveno, M., Campbell, G. R., Zou, Z., Kinch, L., et al. (2013). Identification of a candidate therapeutic autophagy-inducing peptide. Nature 494, 201–206. doi: 10.1038/nature11866
Singh, R., and Cuervo, A. M. (2012). Lipophagy: connecting autophagy and lipid metabolism. Int. J. Cell. Biol. 2012:282041. doi: 10.1155/2012/282041
Singh, R., Kaushik, S., Wang, Y., Xiang, Y., Novak, I., Komatsu, M., et al. (2009a). Autophagy regulates lipid metabolism. Nature 458, 1131–1135. doi: 10.1038/nature07976
Singh, R., Xiang, Y., Wang, Y., Baikati, K., Cuervo, A. M., Luu, Y. K., et al. (2009b). Autophagy regulates adipose mass and differentiation in mice. J. Clin. Invest. 119, 3329–3339. doi: 10.1172/jci39228
Soler-Vazquez, M. C., Mera, P., Zagmutt, S., Serra, D., and Herrero, L. (2018). New approaches targeting brown adipose tissue transplantation as a therapy in obesity. Biochem. Pharmacol. 155, 346–355. doi: 10.1016/j.bcp.2018.07.022
Song, J., de Libero, J., and Wolf, S. E. (2014). Hepatic autophagy after severe burn in response to endoplasmic reticulum stress. J. Surg. Res. 187, 128–133. doi: 10.1016/j.jss.2013.09.042
Stienstra, R., Haim, Y., Riahi, Y., Netea, M., Rudich, A., and Leibowitz, G. (2014). Autophagy in adipose tissue and the beta cell: implications for obesity and diabetes. Diabetologia 57, 1505–1516. doi: 10.1007/s00125-014-3255-3
Tan, S. H., Shui, G., Zhou, J., Li, J. J., Bay, B. H., Wenk, M. R., et al. (2012). Induction of autophagy by palmitic acid via protein kinase C-mediated signaling pathway independent of mTOR (mammalian target of rapamycin). J. Biol. Chem. 287, 14364–14376. doi: 10.1074/jbc.M111.294157
Taylor, D., and Gottlieb, R. A. (2017). Parkin-mediated mitophagy is downregulated in browning of white adipose tissue. Obesity (Silver Spring) 25, 704–712. doi: 10.1002/oby.21786
Tsai, Y. C., Yang, B. C., Peng, W. H., Lee, Y. M., Yen, M. H., and Cheng, P. Y. (2017). Heme oxygenase-1 mediates anti-adipogenesis effect of raspberry ketone in 3T3-L1 cells. Phytomedicine 31, 11–17. doi: 10.1016/j.phymed.2017.05.005
Tu, Q. Q., Zheng, R. Y., Li, J., Hu, L., Chang, Y. X., Li, L., et al. (2014). Palmitic acid induces autophagy in hepatocytes via JNK2 activation. Acta Pharmacol. Sin. 35, 504–512. doi: 10.1038/aps.2013.170
Vijgen, G. H., Bouvy, N. D., Teule, G. J., Brans, B., Schrauwen, P., and van Marken Lichtenbelt, W. D. (2011). Brown adipose tissue in morbidly obese subjects. PLoS One 6:e17247. doi: 10.1371/journal.pone.0017247
Vincow, E. S., Merrihew, G., Thomas, R. E., Shulman, N. J., Beyer, R. P., MacCoss, M. J., et al. (2013). The PINK1-Parkin pathway promotes both mitophagy and selective respiratory chain turnover in vivo. Proc. Natl. Acad. Sci. U.S.A. 110, 6400–6405. doi: 10.1073/pnas.1221132110
Volzke, H., Robinson, D. M., Kleine, V., Deutscher, R., Hoffmann, W., Ludemann, J., et al. (2005). Hepatic steatosis is associated with an increased risk of carotid atherosclerosis. World J. Gastroenterol. 11, 1848–1853. doi: 10.3748/wjg.v11.i12.1848
Wang, Q., Zhang, M., Ning, G., Gu, W., Su, T., Xu, M., et al. (2011). Brown adipose tissue in humans is activated by elevated plasma catecholamines levels and is inversely related to central obesity. PLoS One 6:e21006. doi: 10.1371/journal.pone.0021006
Ward, C., Martinez-Lopez, N., Otten, E. G., Carroll, B., Maetzel, D., Singh, R., et al. (2016). Autophagy, lipophagy and lysosomal lipid storage disorders. Biochim. Biophys. Acta 1861, 269–284. doi: 10.1016/j.bbalip.2016.01.006
Weyer, C., Tataranni, P. A., Snitker, S., Danforth, E. Jr., and Ravussin, E. (1998). Increase in insulin action and fat oxidation after treatment with CL 316,243, a highly selective beta3-adrenoceptor agonist in humans. Diabetes 47, 1555–1561. doi: 10.2337/diabetes.47.10.1555
Wu, J., Bostrom, P., Sparks, L. M., Ye, L., Choi, J. H., Giang, A. H., et al. (2012). Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150, 366–376. doi: 10.1016/j.cell.2012.05.016
Wu, J., Cohen, P., and Spiegelman, B. M. (2013). Adaptive thermogenesis in adipocytes: is beige the new brown? Genes Dev. 27, 234–250. doi: 10.1101/gad.211649.112
Xu, R., Ji, Z., Xu, C., and Zhu, J. (2018). The clinical value of using chloroquine or hydroxychloroquine as autophagy inhibitors in the treatment of cancers: a systematic review and meta-analysis. Medicine (Baltimore) 97:e12912. doi: 10.1097/MD.0000000000012912
Yoshii, S. R., and Mizushima, N. (2017). Monitoring and measuring autophagy. Int. J. Mol. Sci. 18:E1865. doi: 10.3390/ijms18091865
Yuan, H. X., Russell, R. C., and Guan, K. L. (2013). Regulation of PIK3C3/VPS34 complexes by MTOR in nutrient stress-induced autophagy. Autophagy 9, 1983–1995. doi: 10.4161/auto.26058
Zechner, R., Madeo, F., and Kratky, D. (2017). Cytosolic lipolysis and lipophagy: two sides of the same coin. Nat. Rev. Mol. Cell. Biol. 18, 671–684. doi: 10.1038/nrm.2017.76
Zhang, F., Hao, G., Shao, M., Nham, K., An, Y., Wang, Q., et al. (2018). An adipose tissue Atlas: an image-guided identification of human-like BAT and beige depots in rodents. Cell Metab. 252.e3–262.e3. doi: 10.1016/j.cmet.2017.12.004
Zhang, Y., Goldman, S., Baerga, R., Zhao, Y., Komatsu, M., and Jin, S. (2009). Adipose-specific deletion of autophagy-related gene 7 (atg7) in mice reveals a role in adipogenesis. Proc. Natl. Acad. Sci. U.S.A. 106, 19860–19865. doi: 10.1073/pnas.0906048106
Keywords: autophagy, lipophagy, mitophagy, beige/brown adipose tissue, browning, white adipose tissue, whitening, obesity
Citation: Ro S-H, Jang Y, Bae J, Kim IM, Schaecher C and Shomo ZD (2019) Autophagy in Adipocyte Browning: Emerging Drug Target for Intervention in Obesity. Front. Physiol. 10:22. doi: 10.3389/fphys.2019.00022
Received: 06 October 2018; Accepted: 10 January 2019;
Published: 28 January 2019.
Edited by:
Rita De Matteis, University of Urbino Carlo Bo, ItalyReviewed by:
Xiaohu Huang, Children’s National Health System, United StatesCopyright © 2019 Ro, Jang, Bae, Kim, Schaecher and Shomo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Seung-Hyun Ro, c2hyb0B1bmwuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.