
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Physiol. , 19 December 2018
Sec. Vascular Physiology
Volume 9 - 2018 | https://doi.org/10.3389/fphys.2018.01838
This article is part of the Research Topic Vascular Dysfunction Beyond Pathological Pregnancies. An International Effort Addressed to Fill the Gaps in Latin America View all 13 articles
 Patricio Lopez-Jaramillo1,2,3*
Patricio Lopez-Jaramillo1,2,3* Juan Barajas1
Juan Barajas1 Sandra M. Rueda-Quijano1
Sandra M. Rueda-Quijano1 Cristina Lopez-Lopez4
Cristina Lopez-Lopez4 Camilo Felix3
Camilo Felix3Preeclampsia is a disorder specific of the human being that appears after 20 weeks of pregnancy, characterized by new onset of hypertension and proteinuria. Abnormal placentation and reduced placental perfusion associated to impaired trophoblast invasion and alteration in the compliance of uterine spiral arteries are the early pathological findings that are present before the clinical manifestations of preeclampsia. Later on, the endothelial and vascular dysfunction responsible of the characteristic vasoconstriction of preeclampsia appear. Different nutritional risk factors such as a maternal deficit in the intake of calcium, protein, vitamins and essential fatty acids, have been shown to play a role in the genesis of preeclampsia, but also an excess of weight gain during pregnancy or a pre-pregnancy state of obesity and overweight, which are associated to hyperinsulinism, insulin resistance and maternal systemic inflammation, are proposed as one of the mechanism that conduce to endothelial dysfunction, hypertension, proteinuria, thrombotic responses, multi-organ damage, and high maternal mortality and morbidity. Moreover, it has been demonstrated that pregnant women that suffer preeclampsia will have an increased risk of future cardiovascular disease and related mortality in their later life. In this article we will discuss the results of studies performed in different populations that have shown an interrelationship between obesity and overweight with the presence of preeclampsia. Moreover, we will review some of the common mechanisms that explain this interrelationship, particularly the alterations in the L-arginine/nitric oxide pathway as a crucial mechanism that is common to obesity, preeclampsia and cardiovascular diseases.
Obesity is considered a risk factor for preeclampsia and there are many common mechanisms that link obesity with a higher risk of developing preeclampsia (Spradley et al., 2015). Moreover, preeclampsia, similar to obesity, is associated with an increased risk of future cardiovascular diseases for the mother (Bellamy et al., 2007). Preeclampsia is a specific disease of the human being characterized by hypertension, edema of extremities, and proteinuria occurring after 20 weeks of gestation. It affects many organ systems and leads to high maternal mortality and morbidity worldwide (Lopez-Jaramillo et al., 2009). Hypertensive disorders are amongst the most common disorders that affect pregnant women and are major contributors to maternal deaths. In a systematic review conducted by the World Health Organization (WHO), 16% of maternal deaths in developed countries were attributed to hypertensive disorders, 9% in the regions of Africa and Asia, and as high as 25% in Latin America and the Caribbean (Khan et al., 2006). The WHO-review named hypertensive disorders during pregnancy the leading cause of maternal deaths in industrialized countries, responsible for 16% of maternal deaths. Regionally, hypertensive disorders are responsible of 25,000 maternal deaths in Africa, 22,000 in Asia, 3,800 in Latin America and the Caribbean, and 150 maternal deaths in industrialized countries (Khan et al., 2006). The two disorders most associated with hypertension during pregnancy are eclampsia and preeclampsia. Preeclampsia can be characterized by hypertension, proteinuria, edema of extremities, persistent severe headaches, visual disturbances, sudden onset of swelling of hands and feet, and hyperreflexia, amongst many more. It affects coagulation, the renal, respiratory, and central nervous system and can have detrimental consequences on the placenta and the baby (Duley, 2009). Most maternal hypertensive deaths are attributed to eclampsia as opposed to preeclampsia. Eclampsia occurs when preeclampsia goes untreated and the hypertensive mothers experience seizures. It is estimated that hypertensive disorders complicate 5–10% of all pregnancies and preeclampsia arises in 2–8% of them, although it is difficult to gather accurate data on the prevalence of preeclampsia worldwide because of differences in the definitions and in the symptoms that are used as diagnostic criteria (Khan et al., 2006). However, there are important variatons in the prevalence of preeclampsia between lower middle-income countries and high-income countries. For instance, preeclampsia is diagnosed in 3% of all pregnancies in the United States (Wallis et al., 2008), and 3.3% in New Zealand (Stone et al., 1995) while in Colombia it is present in 9% and in Haiti in 17% (Lopez-Jaramillo et al., 2005, 2007). In the United States, from 1987 to 2004 the average annual incidence rate of preeclampsia and gestational hypertension were 2.7 and 2.1% (Wallis et al., 2008). In the same 18-year period, the rate of preeclampsia and gestational hypertension has increased significantly. From 1987–1988 to 2003–2004, the age-adjusted rate of preeclampsia rose by 24.6%. Meanwhile, the rates for gestational hypertension increased by almost threefold from 1.07 to 3.6%. The rate for eclampsia during the same time decreased by 22% from 1.04 in 1987–1995 to 0.82 in 1996–2004, although this change was not significant. The risk of developing preeclampsia is highest amongst women <20 years of age, but women ≥35 years of age also have an increased risk of developing preeclampsia (Wallis et al., 2008).
Although the terminology and methods for classifying and diagnosing the hypertensive disorders of pregnancy differ from country to country and region to region, some common risk factors have been identified. These risk factors include, maternal age, pre-pregnancy overweight and obesity, sedentarism, insulin resistance and diabetes, subclinical infections and inflammation, and nutritional deficiencies during pregnancy as low intake of calcium and essential fatty acids (Lopez-Jaramillo et al., 1989; Lopez-Jaramillo et al., 1990b, 1997, 2005, 2008b, 2011; Lopez-Jaramillo, 1996; Otto et al., 1997, 1999; Herrera et al., 2001, 2005, 2006, 2007; Teran et al., 2001; Catov et al., 2007; García et al., 2007; Sierra-Laguado et al., 2007; Wang et al., 2008; Ramírez-Vélez et al., 2011; World Health Organization [WHO], 2011; Reyes et al., 2012,a,b).
The relationship between preeclampsia and obesity has been greatly studied. Similar to how the trend of preeclampsia has increased over the past 25 years, obesity prevalence has also been on the uprise. Over the past 30 years, the percentage of overweight or obese women in the US has increased by 60% (Wang et al., 2008). The WHO estimates that the female prevalence of overweight and obesity is 77% in the United States, 73% in Mexico, 69% in South Africa, 37% in France, 32% in China and 18% in India, with a wide variation within each continent (World Health Organization [WHO], 2011). Numerous studies have shown that obesity is associated with many complications during pregnancy, including fetal overgrowth, fetal malformations, spontaneous miscarriage, gestational diabetes, thromboembolic complications, stillbirth, preterm deliveries, cesarean section, and hypertensive complications (Yogev and Catalano, 2009). A strong direct correlation was found between an increasing body mass index (BMI) and the risk of developing preeclampsia and pregnancy induced hypertension (Fernández Alba et al., 2018). The adjusted risk of developing preeclampsia doubled for overweight mothers with a BMI of 26 kg/m2, and almost tripled for obese mothers with a BMI of 30 kg/m2 (Bodnar et al., 2005). The increased risk was found to affect not only Caucasian and African American mothers (Bodnar et al., 2007), but also mother from other ethnics around the world (Hauger et al., 2008). Not only were the early or mild forms of preeclampsia found to augment with a raise in the BMI, but also the late and severe forms, which are associated with greater perinatal morbidity and mortality (Catov et al., 2007). Further contributing to the relationship between preeclampsia and obesity, a study found that weight loss reduces the risk of developing preeclampsia (Magdaleno et al., 2012). Despite the fact that weight loss is not recommended during pregnancy, studies have found that excessive maternal weight gain is correlated with an increased risk of preeclampsia (Fortner et al., 2009), thus weight loss is recommended in women with obesity or overweight that are planning to be pregnant (Yogev and Catalano, 2009).
Many different mechanisms have been proposed as explanations of the physiopathology of preeclampsia, at a point that it is called the “disease of the theories” (Widmer et al., 2007). Table 1 shows some of the characteristics that are common to obesity and preeclampsia.
The initial phase in the development of preeclampsia is an altered invasion of the cytotrophoblast cells of fetal origin into the uterus and the spiral arterioles, situation that results in a decreased remodeling of these arterioles with a consequent lower blood flow to the placenta (Soma et al., 1982). The placenta in hypoxic conditions releases different substances into the maternal circulation, these include anti-angiogenic soluble fms-like tyrosine kinase 1 (sFlt-1) factors, and pro-inflammatory factors like tumor necrosis factor alpha (TNF-α) (Reyes et al., 2012), which are associated to maternal endothelial dysfunction (Roberts K.A. et al., 2011). As we have demonstrated, these factors are increased in the plasma of preeclamptic women (Teran et al., 2001; Reyes et al., 2012). This sequence of alterations is one of the proposed mechanisms linking obesity to the risk of preeclampsia (Kao et al., 2016), clinical and experimental evidence suggests that obesity may affect placental function and perfusion, through some of the metabolic alterations that are associated to obesity as hyperlipidemia, hyperinsulinemia, or hyperleptinemia; however, the exact mechanisms are not well-known (Hunkapiller et al., 2011). These metabolic markers are known to be elevated in plasma of obese pregnant women and even higher in women with preeclampsia. Moreover, it has been reported that the levels of total serum cholesterol in the first and second trimesters of gestation predict the onset of preeclampsia (Dey et al., 2013), and we have reported a lipid profile alterations consisting of increased levels of low-density lipoproteins (LDLs), low high-density lipoproteins levels (HDLs), and increased levels of triglycerides in women with preeclampsia (Lopez-Jaramillo et al., 1998; Reyes et al., 2012a,b). It has been reported that LDL reduces extravillous cytotrophoblast migration and promotes trophoblast apoptosis (Pavan et al., 2004). Also, high levels of triglycerides and free fatty acids, which are increased in obesity, increase the risk of preeclampsia and are elevated in preeclampsia (Hubel et al., 1996). These two conditions are known to stimulate the nuclear receptor peroxisome proliferator-activated receptor-γ (PPAR-γ). PPAR-γ expression is increased in placentas from preeclamptic pregnancies, and increased levels of this receptor inhibit the invasiveness of trophoblast cells (Fabbrini et al., 2009; Holdsworth-Carson et al., 2010).
One of the most important characteristics of obesity is insulin resistance and hyperinsulinemia, and we have shown that hyperinsulinemia and insulin resistance precede the clinic manifestation of preeclampsia (Sierra-Laguado et al., 2007). Experimental studies showed that hyperinsulinemia produces a shallower implantation site and an intrauterine growth restriction associated with an altered nitric oxide (NO) synthesis (Skarzinski et al., 2009). Moreover, the group of Granger and colleagues, reported that increasing insulin levels toward the end of pregnancy raises blood pressure in rats (Palei et al., 2013). Took together, these clinical and experimental data support the view of the crucial role played by insulin resistance as one of the common mechanisms linking obesity to preeclampsia (López-Jaramillo et al., 2006; Sierra-Laguado et al., 2007).
We have determinated the levels of cytokines that are produced in the adipose tissue in patients with diseases as metabolic syndrome and type 2 diabetes mellitus, showing that plasma adiponectin levels are decreased, while proinflammatory cytokines as TNF-α and interleukin-6 (IL-6) are elevated, developing a proinflammatory state characterized by insulin resistance and endothelial dysfunction (Teran et al., 2001; Gómez-Arbeláez et al., 2013; Lopez-Jaramillo et al., 2014; Lopez-Jaramillo, 2016). In patients with severe coronary artery disease, in who we quantify a number of dysmetabolic and inflammatory markers and test of endothelial dysfunction, it was observed that the only differences between patients with and without abdominal obesity was a decrease in plasma concentrations of adiponectin and an increase in leptin plasma levels present in patients with abdominal obesity. Moreover, when we evaluated the vascular reactivity ex vivo in rings of the internal mammary artery, we observed a low vascular response to acetylcholine and a high vasoconstriction reaction to angiotensin II (Rueda-Clausen et al., 2010). Leptin and adiponectin are both produced by adipocytes, however, while adiponectin has an anti-inflammatory activity by down-regulating the expression and release of proinflammatory cytokines, leptin has a pro-inflammatory activity. Moreover, adiponectin acts improving the sensibility to insulin but in contrast leptin increases the resistance to the action of insulin. These results suggest that in obese people the increased production of leptin and the decreased production of adiponectin are associated with the systemic low-degree inflammation and insulin resistance, observed in preeclampsia, type 2 diabetes mellitus and cardiovascular diseases, explaining the increased risk of developing these diseases present in people with obesity, particularly abdominal obesity.
In support of this proposal, it has been reported that pregnant obese women that develop preeclampsia have increased leptin levels in relation to healthy pregnant women (Hendler et al., 2005). Also, it has been described that leptin reduces cytotrophoblast proliferation (Liu et al., 2009), and as discussed above, an early alteration observed in preeclampsia is a poor cytotrophoblast proliferation, migration, and invasiveness of these cells into the uterus, it has been suggested that hyperleptinemia may play a role in placental ischemia and the consequent development of preeclampsia (Spradley et al., 2015), as shown by Mendieta Zerón et al. (2012). It was reported that chronic plasmatic leptin elevations in pregnant rats, increased blood pressure and placental factors that have a role in preeclampsia (Palei et al., 2015). Elevations in circulating leptin are associated to increased circulating levels of TNF-α in obese pregnant rats (Palei et al., 2015), and we have shown that women with preeclampsia have increased levels of TNF-α, IL-6 and C-reactive protein (CRP) (Teran et al., 2001). Experimental and clinic reports have demonstrated that TNF-α is increased in response to placental ischemia and hypoxia (Benyo et al., 2001; Peltier et al., 2011). However, other sources must contribute to the increase of circulating levels of TNF-α during preeclampsia, as peripheral blood leukocytes (Chen et al., 1996). Moreover, TNF-α mRNA levels are greater in mononuclear cells from peripheral blood and placental macrophages withdrawn from obese pregnant women (Challier et al., 2008).
We have proposed that the systemic low-degree inflammation that is present in women with preeclampsia is associated to obesity and insulin resistance, and that this low-degree inflammation state is an important mechanism that alters the endothelial function, and that it is proposed as one of the basic mechanism that precede the clinical manifestation of the disease. This proposal is supported by the results of two nested case-control studies that included Colombian pregnant women with preclampsia, demonstrating increased levels of CRP and leukocytes, a state of insulin resistance established by the homeostatic model assessment (HOMA), and a decreased flow-mediated vasodilation, as early as in the first trimester of gestation (García et al., 2007; Sierra-Laguado et al., 2007).
The results of studies that have explored the levels of plasma adiponectin in preeclampsia has been inconsistent, showing increased, decreased or not difference in relation to the observed in healthy pregnancies (Ramsay et al., 2003; Suwaki et al., 2006). Adiponectin activates the endothelial nitric oxide synthase (eNOS); increasing the levels of the vasodilator NO (Zhu et al., 2008) and some cases of preeclampsia course with reduced levels of nitrite, the stable metabolite of NO (Lopez-Jaramillo et al., 2008a). In healthy pregnancies, a positive association between circulating adiponectin concentrations and nitrite levels has been reported, but in preeclampsia there are increased levels of adiponectin with reduced nitrite levels, suggesting that for some reason, not yet determinated, in preeclampsia adiponectin has no act in the eNOS (Eleuterio et al., 2013).
Nitric oxide plays an important role in increasing blood flow by relaxing the smooth vascular muscle, it also reduces smooth muscle migration and growth, platelet aggregation and thrombosis, monocyte and macrophage adhesion, and inflammation (Caballero, 2003). Abdominal or central obesity leads to an imbalanced production of fat-derived metabolic products, hormones and adipokines that predispose to a state endothelial dysfunction (Accini et al., 2001). The different mechanisms by which obesity produces endothelial dysfunction have been studied and proven since some years ago by in vivo vascular function measurement in peripheral vessels of obese individuals (Toda and Okamura, 2013) showing that obesity reduces endothelium-dependent vasodilatation, eNOS protein expression and endothelial NO production, favoring a thicker media layer, enhancing vasoconstriction and drastically decreasing relaxation. Moreover, as discussed above, proinflammatory adipokines induces endothelial dysfunction. Recently, the Finnish Genetics of Pre-eclampsia Consortium (FINNPEC) cohort have confirmed the women that developed preeclampsia have increased pre-pregnancy BMI and altered inflammatory markers (Jääskeläinen et al., 2018). Leptin, another adipokine that is increased in obesity, induces activation of the NADPH oxidase, which impairs endothelium-dependent vasodilatation by increasing NO degradation (Fortuño et al., 2010). All this leads to a NO deficit which affects endothelial integrity and functioning, that by itself it is deleterious, but in association to pregnancy, where the endothelial function plays a fundamental role in the adequate remodeling of the uterine arteries, and in the hemodynamic adaptations, becomes determinant in the development of preeclampsia-eclampsia, diseases with a high rate of maternal and fetal morbidity and mortality (Witcher, 2018).
During a healthy pregnancy there is an increase of plasma volume, heart rate, cardiac output and on the activity of the renin-angiotensin system (Moutquin et al., 1985), however the blood pressure is lower or similar to the observed in a no pregnancy state, situation that is related with the characteristic increase in the peripheral vasodilation that is observed in the pregnant women (Lopez-Jaramillo, 1996). Nowadays there is evidence that demonstrate that this peripheral vasodilation is product of an increase in the production of NO and prostacyclin in vascular endothelial cells (Lopez-Jaramillo et al., 2008a; Félix et al., 1991). In addition, these substances suppress the leukocyte and platelet migration and adhesion to the vascular wall (Moncada et al., 1991). The NO synthases (NOSs) are a family of specialized enzymes that synthesize NO from the amino acid L-arginine. The endothelial NOS (eNOS) depends on NADPH and calcium (Moncada et al., 1991), and we have proven the critical role of concentrations of extracellular calcium in the production of endothelial NO and in the control of vascular tone via the activation of cGMP (Lopez-Jaramillo et al., 1990a) Importantly, eNOS is expressed in the human placental syncytiotrophoblasts and in the extravillous trophoblasts, suggesting that the production of NO in the placenta play an important role in the vascular adaptations that are necessary to guarantee a normal blood flow (Sladek et al., 1997). The reports showing that NO is inactivated by superoxide (O-2) and that this free radical is increased in pregnancies complicated with subclinical infections or low degree inflammation, suggest that these risk factors for preeclampsia are associated to a lower activity of NO in response to an increase oxidative stress (Lopez-Jaramillo et al., 2008a). In reality, it is now well-accepted than the production of NO in the vascular endothelium of all vascular beds play an important role in the hemodynamic adaptations that are necessary to carry on a healthy pregnancy. On the other hand, a lower production and/or an increased inactivation of NO explain the generalized peripheral vasoconstriction, one of the most important characteristics of preeclampsia (Lopez-Jaramillo et al., 2008a). However, one of the problems to determinate the role of NO in pregnancy has been the methodological approaches to quantify the production and activity of NO. We and others groups have used the measurements of NO2/NO3 levels in plasma and serum of a small group of women with preeclampsia and compared them with the levels observed in unpregnant women and/or in women with healthy. These studies have used different methods to quantify NO2/NO3, and they have report elevated, decreased, or unchanged levels of NO2/NO3 in preeclamptic women in relation to controls (Lopez-Jaramillo et al., 2008a). Others reports measuring the levels of cGMP in plasma, urine, or platelets, demonstrated consistently that women with preeclampsia have lower levels of this mediator of the action of NO (Teran et al., 2004; Baksu et al., 2005; Lopez-Jaramillo et al., 2008a). A global analysis of these results suggests that some patients with preeclampsia have a decreased production of NO demonstrated by low levels of NO2/NO3 and cGMP, but in other cases there is a normal or increased production of NO, as demonstrated by the presence of similar or higher levels of NO2/NO3 in relation to women with healthy pregnancies, but in these cases they also presented with a decreased bioactivity of NO, demonstrated by the low levels of cGMP (Lopez-Jaramillo et al., 1996). Importantly, to support the role of NO in pregnancy, there are the consistent results reporting that low mediated dilation in women with healthy pregnancies have a higher vasodilation response to hyperemia that the one observed in women with preeclampsia, even before clinical manifestations appeared (Lopez-Jaramillo et al., 2008a). The analysis of the different reports of the L-arginine-NO pathway led us to the conclusion that in face of the multicausality of this disease, there are different mechanisms that can explain the alterations in the activity of the NO (Lopez-Jaramillo, 2000; Lopez-Jaramillo et al., 2001b). For instance, we understand that in the cases of preeclampsia with low NO production (low NO2/NO3 levels) the alterations are related with a deficiency in the substrate L-arginine, a deficit in the cofactors that are needed for a normal activity of the eNOS, such as ionic calcium and BH4, build-up of the endogenous inhibitor of eNOS, the asymmetric dimethylarginine (ADMA), or by the presence of polymorphic alterations of the eNOS that result in a lower enzymatic activity (Serrano et al., 2004; Lopez-Jaramillo et al., 2008a). In the cases of preeclampsia that courses with normal or high production of NO but with a decreased NO bioactivity, there exists an increased inactivation of NO by an increased oxidative stress (Lopez-Jaramillo et al., 2008a) associated with the presence of antibodies antireceptors AT1 of angiotensin II, early abnormal placentation with placental ischemia and hypoxia, insulin resistance, subclinical infections, and overweight and obesity (Herrera et al., 2001; Herrera et al., 2007; Lopez-Jaramillo et al., 2008a). In any case, both alterations of NO explain the endothelial dysfunction observed in preeclampsia.
The presence of overweight and obesity before pregnancy, and an excessive weight gain during gestation, are causes of endothelial dysfunction and preeclampsia as demonstrated by Pardo et al reported that women with a gestational weight gain superior to 0.42 kg per week, had markers of a reduced eNOS activity and vasorelaxation in the rings of isolated umbilical veins (Pardo et al., 2015).
Other studies have proposed a role for endothelin (ET-1) in preeclampsia. ET-1 is a 21 amino acid active oligopeptide derived from a bigger precursor known as preproendothelin. This active peptide is a powerful vasoconstrictor that acts binding to the endothelin type A (ETA) receptor, located in vascular smooth muscle cells. Increased levels of ET-1 have been reported in women with preeclampsia, compared to the levels observed in a healthy pregnancy (Rust et al., 1997). Moreover, it looks like there is a correlation between the levels of ET-1 and the severity of the disease (Nova et al., 1991). It has been proposed that the reduced NO production that occurs in the placenta in conditions of hypoxia increases the production of ET-1. Some experimental evidences support this proposal, for instance it was reported that the administration of L-arginine reduces the levels of renal cortical preproET-1 mRNA expression in pregnant rats infused with sFlt-1 (Spradley et al., 2015). This result supports the view that NO regulates the synthesis of ET-1 in the kidney, and that when the production NO is decreased by an ischemic placenta, the synthesis of ET-1 increases with the consequent vasoconstrictor and hypertensive effects. Moreover, it has been reported that overweight and obese people with endothelial dysfunction have enhanced levels of ET-1 (Weil et al., 2011), and that an ETA receptor antagonist attenuated the hypertension observed in a experimental model of rats with obesity induced by a high fat diet, that also had increased leptin levels (da Silva et al., 2004). However, in the review of Spradley et al. (2015) they found only indirect evidence that ET-1 is higher in obese preeclamptic women.
Obesity and preeclampsia are diseases that result from multiple genetic and environmental factors (Spradley et al., 2015). These two conditions share many pathophysiological mechanisms, however, only 10% of obese women will develop preeclampsia (Roberts J.M. et al., 2011). As reviewed above, the women that develop preeclampsia have different metabolic alterations that precede the appearance of clinical symptoms and that are associated with lifestyles and socio-economic status (Lopez-Jaramillo et al., 2005). We have proposed that the participation of the well-known risk factors for preeclampsia is different depending of the ethnicity and the socio-economic conditions of a specific community. The socio-economic status of communities and of individuals is determining t nutritional habits and the type and quality of the health system (World Health Organization [WHO], 1988; Lopez-Jaramillo et al., 2008a).
In the last decades, the population from low and medium incomes countries is experimenting important lifestyle changes as a result of a rapid and messy urbanization characterized by an increase in the intake of processed carbohydrates and a reduction of physical activity, conditions that have increased the prevalence of obesity and insulin resistance (Lopez-Jaramillo et al., 2001a). Moreover, ethnic differences have been reported in the prevalence of insulin resistance and metabolic syndrome (Ford et al., 2002), differences that could be associated to genetic factors or socio-economic and environmental conditions. Regardless of the cause, the relationship between insulin resistance and preeclampsia is stronger in low and medium income countries. To support this proposal the Prospective Urban and Rural Epidemiology (PURE) study have demonstrated a higher prevalence of diabetes mellitus type 2 in people with lower BMI coming from low and medium income countries compared to the prevalence found in people from high-income countries (Dagenais et al., 2016).
The existence of a role of genetic factors is demonstrated by the observation that despite of the improvement of the environmental and socio-economic factors, there is still a percentage of women that develop preeclampsia. Moreover, there is evidence showing an association between preeclampsia and polymorphisms or mutations of genes related to hypertension (Ward et al., 1993; Grandone et al., 1997; Sohda et al., 1997; Kupferminc et al., 1999; Ford et al., 2002). However, it looks like environmental and socio-economic factors have a protagonic role. For instance, various studies originated in Europe have demonstrated that pregnant women with preeclampsia have increased levels of ADMA, which are associated with endothelial dysfunction (Fickling et al., 1993; Holden et al., 1998; Ellis et al., 2001; Savvidou et al., 2003). Early in our research, we showed no difference in ADMA plasma levels between a small sample of Andean women: 22 unpregnant women, 22 women with a normal pregnancy and 22 pre-eclmaptic women (Lopez-Jaramillo et al., 1996). This result was later confirmed in a study (Maas et al., 2004) that included a bigger sample of Colombian women (67 women with preeclampsia and 93 healthy controls). We have proposed some possible explanations for this unexpected finding, to finally conclude that the probable reason is that the excess of the other risk factors present in our population is more important than ADMA plasma levels in the development of preeclampsia, situation that differs from that observed in the European population, where risk factors such as nutritional deficiencies do not exist and others as subclinical infections are detected and treated early in pregnancy. In this way of reasoning it is possible that endothelial dysfunction is mainly related to nutritional deficiencies, subclinical infections, and metabolic disorders in women with preeclampsia from developing countries, while genetic and immunological alterations seem to be the principal determinants for the development of preeclampsia in developed countries (López-Jaramillo et al., 2006).
The Figure 1 resumes the proposed mechanisms that we believe are participating in the development of preeclampsia. The evidence reviewed in this article demonstrate that the process initiate with placental alterations. A decline in cytotrophoblast migration and remodeling of the uterine spiral artery conduce to placental ischemia. In this condition, soluble anti-angiogenic and inflammatory factors are released from the placenta into the maternal circulation, these factors affect the endothelial function, a critical event that conduce to the clinical manifestations of preeclampsia.
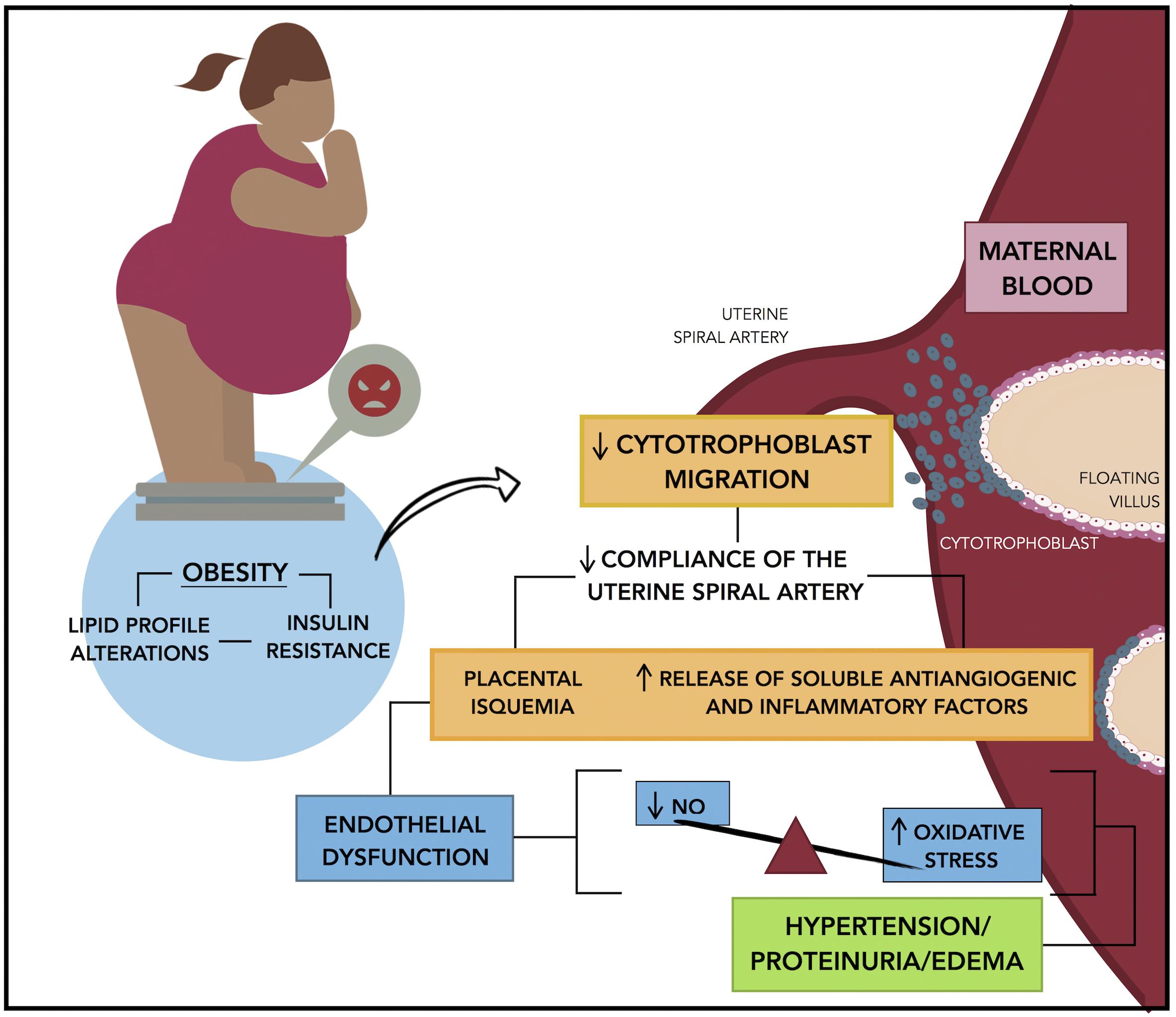
Figure 1. Mechanisms linking obesity to preeclampsia. Insulin resistance that results of pre-pregnancy obesity or by an excessive weight gain during gestation is associated with a reduced cytotrophoblast migration and uterine spiral artery remodeling, which in turn conduce to placental hypoxia and ischemia. In this condition the placenta release of soluble anti-angiogenic factors and inflammatory factors into the maternal circulation promoting the endothelial dysfunction, which is characterized by a decrease in the endothelial production of nitric oxide and an increase in the oxidative stress, that results in the characteristic symptoms of preeclampsia: hypertension, proteinuria, and edema.
Since in medium and low-income countries the prevalence of obesity is increasing, and by the fact that preeclampsia is a major cause of maternal and perinatal morbidity and mortality in these countries, it is of crucial importance to understand how obesity impacts the pathogenesis of preeclampsia.
PL-J reviewed and approved the final version. All the authors have made a substantial contribution to this manuscript and participated in the literature review, design and redaction of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Accini, L., Sotomayor, A., Trujillo, F., Barrera, J. G., Bautista, L., and López-Jaramillo, P. (2001). Colombian Study to Assess the Use of Noninvasive Determination of Endothelium-Mediated Vasodilatation (CANDEV). Normal values and factors associated. Endothelium 8, 157–166. doi: 10.3109/10623320109165324
Baksu, B., Davas, I., Baksu, A., Akyol, A., and Gulbaba, G. (2005). Plasma nitric oxide, endothelin-1 and urinary nitric oxide and cyclic guanosine monophosphate levels in hypertensive pregnant women. Int. J. Gynaecol. Obstet. 90, 112–117. doi: 10.1016/j.ijgo.2005.04.018
Bellamy, L., Casas, J. P., Hingorani, A. D., and Williams, D. J. (2007). Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ 335:974. doi: 10.1136/bmj.39335.385301.BE
Benyo, D. F., Smarason, A., Redman, C. W., Sims, C., and Conrad, K. P. (2001). Expression of inflammatory cytokines in placentas from women with preeclampsia. J. Clin. Endocrinol. Metab. 86, 2505–2512. doi: 10.1210/jcem.86.6.7585
Bodnar, L. M., Catov, J. M., Klebanoff, M. A., Ness, R. B., and Roberts, J. M. (2007). Prepregnancy body mass index and the occurrence of severe hypertensive disorders of pregnancy. Epidemiology 18, 234–239. doi: 10.1097/01.ede.0000254119.99660.e7
Bodnar, L. M., Ness, R. B., Markovic, N., and Roberts, J. M. (2005). The risk of preeclampsia rises with increasing prepregnancy body mass index. Ann. Epidemiol. 15, 475–482. doi: 10.1016/j.annepidem.2004.12.008
Caballero, A. E. (2003). Endothelial dysfunction in obesity and insulin resistance: a road to diabetes and heart disease. Obes. Res. 11, 1278–1289. doi: 10.1038/oby.2003.174
Catov, J. M., Ness, R. B., Kip, K. E., and Olsen, J. (2007). Risk of early or severe pre-eclampsia related to pre-existing conditions. Int. J. Epidemiol. 36, 412–419. doi: 10.1093/ije/dyl271
Challier, J. C., Basu, S., Bintein, T., Minium, J., Hotmire, K., Catalano, P. M., et al. (2008). Obesity in pregnancy stimulates macrophage accumulation and inflammation in the placenta. Placenta 29, 274–281. doi: 10.1016/j.placenta.2007.12.010
Chen, G., Wilson, R., Wang, S. H., Zheng, H. Z., Walker, J. J., and McKillop, J. H. (1996). Tumour necrosis factor-alpha (TNF-alpha) gene polymorphism and expression in pre-eclampsia. Clin. Exp. Immunol. 104, 154–159. doi: 10.1046/j.1365-2249.1996.d01-647.x
da Silva, A. A., Kuo, J. J., Tallam, L. S., and Hall, J. E. (2004). Role of endothelin-1 in blood pressure regulation in a rat model of visceral obesity and hypertension. Hypertension 43, 383–387. doi: 10.1161/01.HYP.0000111139.94378.74
Dagenais, G. R., Gerstein, H. C., Zhang, X., McQueen, M., Lear, S., Lopez-Jaramillo, P., et al. (2016). Variations in diabetes prevalence in low-, middle-, and high-income countries: results from the prospective urban and rural epidemiology study. Diabetes Care 39, 780–787. doi: 10.2337/dc15-2338
Dey, M., Arora, D., Narayan, N., and Kumar, R. (2013). Serum cholesterol and ceruloplasmin levels in second trimester can predict development of pre-eclampsia. N. Am. J. Med. Sci. 5, 41–46. doi: 10.4103/1947-2714.106198
Duley, L. (2009). The global impact of pre-eclampsia and eclampsia. Semin. Perinatol. 33, 130–137. doi: 10.1053/j.semperi.2009.02.010
Eleuterio, N. M., Palei, A. C., Rangel Machado, J. S., Tanus-Santos, J. E., Cavalli, R. C., and Sandrim, V. C. (2013). Relationship between adiponectin and nitrite in healthy and preeclampsia pregnancies. Clin. Chim. Acta 423, 112–115. doi: 10.1016/j.cca.2013.04.02
Ellis, J., Wennerholm, U. B., Bengtsson, A., Lilja, H., Pettersson, A., Sultan, B., et al. (2001). Levels of dimethylarginines and cytokines in mild and severe preeclampsia. Acta Obstet. Gynecol. Scand. 80, 602–608. doi: 10.1080/j.1600-0412.2001.800703.x
Fabbrini, E., deHaseth, D., Deivanayagam, S., Mohammed, B. S., Vitola, B. E., and Klein, S. (2009). Alterations in fatty acid kinetics in obese adolescents with increased intrahepatic triglyceride content. Obesity 17, 25–29. doi: 10.1038/oby.2008.494
Félix, C., Lopez, A., Delgado, F., Amores, E., Narváez, M., and Lopez-Jaramillo, P. (1991). Vascular prostacyclin production in Andean women with pregnancy-induced hypertension. Braz. J. Med. Biol. Res. 24, 59–62.
Fernández Alba, J. J., Mesa Páez, C., Vilar Sánchez,Á, Soto Pazos, E., González Macías, M. D. C., Serrano Negro, E., et al. (2018). Overweight and obesity at risk factors for hypertensive states of pregnancy: a retrospective cohort study. Nutr. Hosp. 35, 874–880. doi: 10.20960/nh.1702
Fickling, S. A., Willliams, D., Vallance, P., Nussey, S. S., and Whitley, G. S. (1993). Plasma concentrations of endogenous inhibitor of nitric oxide synthesis in normal pregnancy and pre-eclampsia. Lancet 342, 242–243. doi: 10.1016/0140-6736(93)92335-Q
Ford, E. S., Giles, W. H., and Dietz, W. H. (2002). Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA 287, 356–359. doi: 10.1001/jama.287.3.356
Fortner, R. T., Pekow, P., Solomon, C. G., Markenson, G., and Chasan-Taber, L. (2009). Prepregnancy body mass index, gestational weight gain, and risk of hypertensive pregnancy among Latina women. Am. J. Obstet. Gynecol. 200, e161–e167. doi: 10.1016/j.ajog.2008.08.021
Fortuño, A., Bidegain, J., Baltanás, A., Moreno, M. U., Montero, L., Landecho, M. F., et al. (2010). Is leptin involved in phagocytic NADPH oxidase overactivity in obesity? Potential clinical implications. J. Hypertens. 29, 1944–1950. doi: 10.1097/HJH.0b013e32833c21af
García, R. G., Celedón, J., Sierra-Laguado, J., Alarcón, M. A., Luengas, C., Silva, F., et al. (2007). Raised C-reactive protein and impaired flow-mediated vasodilation precede the development of preeclampsia. Am. J. Hypetens. 20, 98–103. doi: 10.1016/j.amjhyper.2006.06.001
Gómez-Arbeláez, D., Lahera, V., Oubiña, P., Valero-Muñoz, M., de La Heras, N., Rodríguez, Y., et al. (2013). Aged garlic extract improves adiponectin levels in subjects with metabolic syndrome: a double-blind, placebo-controlled, randomized, crossover study. Mediators Inflamm. 2013:285795. doi: 10.1155/2013/285795
Grandone, E., Margaglione, M., Colaizzo, D., Cappucci, G., Paladimi, D., Martinelli, P., et al. (1997). Factor V Leiden, C > T MTHFR polymorphism and genetic susceptibility to preeclampsia. Thromb. Haemost. 77, 1052–1054. doi: 10.1055/s-0038-1656110
Hauger, M. S., Gibbons, L., Vik, T., and Belizán, J. M. (2008). Prepregnancy weight status and the risk of adverse pregnancy outcome. Acta Obstet. Gynecol. Scand. 87, 953–959. doi: 10.1080/00016340802303349
Hendler, I., Blackwell, S. C., Mehta, S. H., Whitty, J. E., Russell, E., Sorokin, Y., et al. (2005). The levels of leptin, adiponectin, and resistin in normal weight, overweight, and obese pregnant women with and without preeclampsia. Am. J. Obstet. Gynecol. 193, 979–983. doi: 10.1016/j.ajog.2005.06.041
Herrera, J. A., Arévalo-Herrera, M., Shahabuddin, A. K. M., Ersheng, G., Herrera, S., Garcia, R. G., et al. (2006). Calcium and conjugated linoleic acid reduces pregnancy-induced hypertension and decreases intracellular calcium in lymphocytes. Am. J. Hypertens. 19, 381–387. doi: 10.1016/j.amjhyper.2005.11.004
Herrera, J. A., Chaudhuri, G., and Lopez-Jaramillo, P. (2001). Is infection a major risk factor for preeclampsia? Med. Hypotheses 57, 393–397. doi: 10.1054/mehy.2001.1378
Herrera, J. A., Parra, B., Herrera, E., Botero, J. E., Arce, R. M., Contreras, A., et al. (2007). Periodontal disease severity is related to high levels of C-reactive protein in pre-eclampsia. J. Hypertens. 25, 1459–1464. doi: 10.1097/HJH.0b013e3281139ea9
Herrera, J. A., Shahabuddin, A. K. M., Ersheng, G., Wei, Y., Garcia, R. G., and Lopez-Jaramillo, P. (2005). Calcium and linoleic acid therapy for pregnancy-induced hypertension. Int. J. Gynaecol. Obstet. 91, 221–227. doi: 10.1016/j.ijgo.2005.08.018
Holden, D. P., Fickling, S. A., Whitley, G. S., and Nussey, S. S. (1998). Plasma concentrations of asymmetric dimethylarginine, a natural inhibitor of nitric oxide synthase, in normal pregnancy and preeclampsia. Am. J. Obstet. Gynecol. 178, 551–556. doi: 10.1016/S0002-9378(98)70437-5
Holdsworth-Carson, S. J., Lim, R., Mitton, A., Whitehead, C., Rice, G. E., Permezel, M., et al. (2010). Peroxisome proliferator-activated receptors are altered in pathologies of the human placenta: gestational diabetes mellitus, intrauterine growth restriction and preeclampsia. Placenta 31, 222–229. doi: 10.1016/j.placenta.2009
Hubel, C. A., McLaughlin, M. K., Evans, R. W., Hauth, B. A., Sims, C. J., and Roberts, J. M. (1996). Fasting serum triglycerides, free fatty acids, and malondialdehyde are increased in preeclampsia, are positively correlated, and decrease within 48 hours post partum. Am. J. Obstet. Gynecol. 174, 975–982. doi: 10.1016/S0002-9378(96)70336-8
Hunkapiller, N. M., Gasperowicz, M., Kapidzic, M., Plaks, V., Maltepe, E., Kitajewski, J., et al. (2011). A role for Notch signaling in trophoblast endovascular invasion and in the pathogenesis of pre-eclampsia. Development 138, 2987–2998. doi: 10.1242/dev.066589
Jääskeläinen, T., Heinonen, S., Hämäläinen, E., Pulkki, K., Romppanen, J., and Laivuori, H. (2018). Impact of obesity on angiogenic and inflammatory markers in the Finnish Genetics of Pre-eclampsia Consortium (FINNPEC) cohort. Int. J. Obes. doi: 10.1038/s41366-018-0217-8 [Epub ahead of print].
Kao, C. K., Morton, J. S., Quon, A. L., Reyes, L. M., Lopez-Jaramillo, P., and Davidge, S. T. (2016). Mechanism of vascular dysfunction due to circulating factors in women with preeclampsia. Clin. Sci. 130, 539–549. doi: 10.1042/CS20150678
Khan, K. S., Wojdyla, D., Say, L., Gulmezoglu, A. M., and Van Look, P. F. (2006). WHO analysis of causes of maternal death: a systematic review. Lancet 367, 1066–1074. doi: 10.1016/S0140-6736(06)68397-9
Kupferminc, M. J., Eldor, A., Steinman, N., Many, A., Bar-Am, A., Jaffa, A., et al. (1999). Increased frequency of genetic thrombophilia in women with complications of pregnancy. N. Engl. J. Med. 340, 9–13. doi: 10.1056/NEJM199901073400102
Liu, H., Wu, Y., Qiao, F., and Gong, X. (2009). Effect of leptin on cytotrophoblast proliferation and invasion. J. Huazhong. Univ. Sci. Technolog. Med. Sci. 29, 631–636. doi: 10.1007/s11596-009-0519-0
Lopez-Jaramillo, P. (1996). Prevention of preeclampsia with calcium supplementation and its relation with the L-arginine:nitric oxide pathway. Braz. J. Med. Biol. Res. 29, 731–743.
Lopez-Jaramillo, P. (2000). Calcium, nitric oxide and preeclampsia. Sem. Perinatol. 24, 33–36. doi: 10.1016/S0146-0005(00)80052-X
Lopez-Jaramillo, P. (2016). The role of adiponectin in cardiometabolic diseases: effects of nutritional interventions. J. Nutr. 146, 422S–426S. doi: 10.3945/jn.114.202432
Lopez-Jaramillo, P., Casas, J. P., Bautista, L., Serrano, N. C., and Morillo, C. A. (2001a). An integrated proposal to explain the epidemia of cardiovascular disease in a developing country. From socioeconomic factors to free radicals. Cardiology 96, 1–6. doi: 10.1159/000047379
Lopez-Jaramillo, P., Casas, J. P., and Serrano, N. (2001b). Preeclampsia: from epidemiological observations to molecular mechanisms. Braz. J. Med. Biol. Res. 34, 1227–1235. doi: 10.1590/S0100-879X2001001000001
Lopez-Jaramillo, P., Delgado, F., Jácome, P., Terán, E., Ruano, C., and Rivera, J. (1997). Calcium supplementation reduces the risk of preeclampsia in ecuadorian pregnant teenagers. Obstet. Gynecol. 90, 162–167. doi: 10.1016/S0029-7844(97)00254-8
Lopez-Jaramillo, P., García, R., Reyes, L., and Ruiz, S. (2009). Appropriate prenatal care: the best way to prevent preeclampsia in Andean Countries. Colomb. Med. 40, 226–230.
Lopez-Jaramillo, P., García, R. G., and Lopez, M. (2005). Preventing pregnancy induced hypertension: are there regional differences for this global problem? J. Hypertens. 23, 1121–1129. doi: 10.1097/01.hjh.0000170371.49010.4a
Lopez-Jaramillo, P., Gómez-Arbeláez, D., Lopez-Lopez, J., Lopez-Lopez, C., Martínez-Ortega, J., Gómez-Rodríguez, A., et al. (2014). The role of leptin-adiponectin ratio in metabolic syndrome and diabetes. Horm. Mol. Biol. Clin. Investig. 18, 37–45. doi: 10.1515/hmbci-2013-0053
López-Jaramillo, P., Silva, F., Camacho, P. A., Pradilla, L. P., García, R., Rueda-Clausen, C., et al. (2006). Síndrome metabólico y preeclampsia: los aportes realizados por el Instituto de Investigaciones de la Fundación Cardiovascular de Colombia. Rev. Col. Cardiol. 13, 73–78.
Lopez-Jaramillo, P., Arenas, W. D., García, R. G., Rincon, M. Y., and Lopez, M. (2008a). The role of the L-arginine-nitric oxide pathway in preeclampsia. Ther. Adv. Cardiovas. Dis. 2, 261–275. doi: 10.1177/1753944708092277
Lopez-Jaramillo, P., Gómez–Arbeláez, D., and Lopez-Lopez, J. (2011). Periodontal disease and hypertension: the pre-eclampsia model in Hispanic population. J. Hypertens. 29, 1020–1021. doi: 10.1097/HJH.0b013e328344b6e8
Lopez-Jaramillo, P., Gonzalez, M. C., Palmer, R. M., and Moncada, S. (1990a). The crucial role of physiological Ca2+ concentrations in the productionof endothelial nitric oxide and the control of vascular tone. Br. J. Pharmacol. 101, 489–493. doi: 10.1111/j.1476-5381.1990.tb12735.x
Lopez-Jaramillo, P., Herrera, J. A., Arenas-Mantilla, M., Jáuregui, I. E., and Mendoza, M. A. (2008b). Subclinical infection as a cause of inflammation in preeclampsia. Am. J. Ther. 15, 373–376. doi: 10.1097/MJT.0b013e318164c149
Lopez-Jaramillo, P., Narváez, M., Calle, A., Rivera, J., Jácome, P., Ruano, C., et al. (1996). Cyclic guanosine 3′,5′ monophosphate concentrations in preeclampsia: effects of hydralazine. Br. J. Obstet. Gynaecol. 103, 33–38. doi: 10.1111/j.1471-0528.1996.tb09512.x
Lopez-Jaramillo, P., Narvaez, M., Felix, C., and Lopez, A. (1990b). Dietary calcium supplementation and prevention of pregnancy hypertension. Lancet 335:293. doi: 10.1016/0140-6736(90)90112-I
Lopez-Jaramillo, P., Narváez, M., Wetgel, R. M., and Yépez, R. (1989). Calcium supplementation reduces the risk of pregnancy induced hypertension in an Andean population. Br. J. Obstet. Gynaecol. 96, 648–655. doi: 10.1111/j.1471-0528.1989.tb03278.x
Lopez-Jaramillo, P., Pradilla, L. P., Castillo, V. R., and Lahera, V. (2007). Socioeconomical pathology as determinant of regional differences in the prevalence of metabolic syndrome and pregnancy-induced hypertension. Rev. Esp. Cardiol. 60, 168–178. doi: 10.1016/S1885-5857(07)60129-7
Lopez-Jaramillo, P., Terán, E., Ringqvist, A., Moya, W., Rivera, J., and Berrazueta, J. R. (1998). Oxidised low-density lipoproteins and nitric oxide during normal pregnancy and preeclampsia. Portland Press 15:322.
Maas, R., Böger, R. H., Schwedhelm, E., Casas, J. P., Lopez-Jaramillo, P., Serrano, N., et al. (2004). Plasma concentrations of Asymmetric Dimethylarginine (ADMA) in Colombian women with pre-eclampsia. JAMA 291, 823–824. doi: 10.1001/jama.291.7.823
Magdaleno, R. Jr., Pereira, B. G., Chaim, E. A., and Turato, E. R. (2012). Pregnancy after bariatric surgery: a current view of maternal, obstetrical and perinatal challenges. Arch. Gynecol. Obstet. 285, 559–566. doi: 10.1007/s00404-011-2187-0
Mendieta Zerón, H., García Solorio, V. J., Nava Díaz, P. M., Garduño Alanís, A., Santillán Benítez, J. G., Domínguez García, V., et al. (2012). Hyperleptinemia as a prognostic factor for preeclampsia: a cohort study. Acta Med. 55, 165–171. doi: 10.14712/18059694.2015.41
Moncada, S., Higgs, E. A., Hodson, H. F., Knowles, R. G., Lopez-Jaramillo, P., McCall, T., et al. (1991). The L-arginine: nitric oxide pathway. J. Cardiovasc. Pharmacol. 17, s1–s9. doi: 10.1097/00005344-199117003-00002
Moutquin, J. M., Rainville, C., Giroux, L., Raynauld, P., Amyot, G., Bilodeau, R., et al. (1985). A prospective study of blood pressure in pregnancy:prediction of preeclampsia. Am. J. Obstet. Gynecol. 151, 191–196. doi: 10.1016/0002-9378(85)90010-9
Nova, A., Sibai, B. M., Barton, J. R., Mercer, B. M., and Mitchell, M. D. (1991). Maternal plasma level of endothelin is increased in preeclampsia. Am. J. Obstet. Gynecol. 165, 724–727. doi: 10.1016/0002-9378(91)90317-K
Otto, S. J., van Houwelingen, A. C., Antal, M., Manninen, A., Godfrey, K., Lopez-Jaramillo, P., et al. (1997). Maternal and neonatal essential fatty acid status: an international comparative study. Eur. J. Clin. Nutr. 51, 232–242. doi: 10.1038/sj.ejcn.1600390
Otto, S. J., van Houwelingen, A. C., Lopez-Jaramillo, P., and Hornstra, G. (1999). Effects of pregnancy-induced hypertension on the essential fatty acid statuses of Ecuadorian and Dutch women. Am. J. Obstet. Gynecol. 180, 1185–1190. doi: 10.1016/S0002-9378(99)70614-9
Palei, A. C., Spradley, F. T., and Granger, J. P. (2013). Euglycemic hyperinsulinemia increases blood pressure in pregnant rats independent of placental anti-angiogenic and inflammatory factors. Am. J. Hypertens. 26, 1445–1451. doi: 10.1093/ajh/hpt137
Palei, A. C., Spradley, F. T., and Granger, J. P. (2015). Chronic hyperleptinemia results in the development of hypertension in pregnant rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 308, R855–R861. doi: 10.1152/ajpregu.00286.2014
Pardo, F., Silva, L., Saéz, T., Salsoso, R., Gutiérrez, J., Sanhueza, C., et al. (2015). Human supraphysiological gestational weight gain and fetoplacental vascular dysfunction. Int. J. Obes. 39, 1264–1273. doi: 10.1038/ijo.2015.57
Pavan, L., Hermouet, A., Tsatsaris, V., Thérond, P., Sawamura, T., Evain-Brion, D., et al. (2004). Lipids from oxidized low-density lipoprotein modulate human trophoblast invasion: involvement of nuclear liver X receptors. Endocrinology 145, 4583–4591. doi: 10.1210/en.2003-1747
Peltier, M. R., Gurzenda, E. M., Murthy, A., Chawala, K., Lerner, V., Kharode, I., et al. (2011). Can oxygen tension contribute to an abnormal placental cytokine milieu? Am. J. Reprod. Immunol. 66, 279–285. doi: 10.1111/j.1600-0897.2011.00998.x
Ramírez-Vélez, R., Aguilar de Plata, A. C., Escudero, M. M., Echeverry, I., Ortega, J. G., Salazar, B., et al. (2011). Influence of regular aerobic exercise on endothelium-dependent vasodilation and cardiorespiratory fitness in pregnant women. J. Obstet. Gynaecol. Res. 37, 1601–1608. doi: 10.1111/j.1447-0756.2011.01582.x
Ramsay, J. E., Jamieson, N., Greer, I. A., and Sattar, N. (2003). Paradoxical elevation in adiponectin concentrations in women with preeclampsia. Hypertension 42, 891–894. doi: 10.1161/01.HYP.0000095981.92542.F6
Reyes, L. M., García, R. G., Ruiz, S. L., Broadhurst, D., Aroca, G., Davidge, S. T., et al. (2012a). Angiogenic imbalance and plasma lipid alterations in women with preeclampsia from a developing country. Growth Fact. 30, 158–166. doi: 10.3109/08977194.2012.674035
Reyes, L. M., García, R. G., Ruiz, S. L., Camacho, P. A., Ospina, M. B., Aroca, G., et al. (2012b). Risk factors for preeclampsia in women from Colombia: a case-control study. PLoS One 7:e41622. doi: 10.1371/journal.pone.0041622
Reyes, L., Garcia, R., Ruiz, S., Dehghan, M., and Lopez-Jaramillo, P. (2012). Nutritional status among women with pre-eclampsia and healthy pregnant and not-pregnant women in Latin American country. J. Obstet. Gynecol. Res. 38, 498–504. doi: 10.1111/j.1447-0756.2011.01763.x
Roberts, J. M., Bodnar, L. M., Patrick, T. E., and Powers, R. W. (2011). The role of obesity in preeclampsia. Pregnancy Hypertens. 1, 6–16. doi: 10.1016/j.preghy.2010.10.013
Roberts, K. A., Riley, S. C., Reynolds, R. M., Barr, S., Evans, M., Statham, A., et al. (2011). Placental structure and inflammation in pregnancies associated with obesity. Placenta 32, 247–254. doi: 10.1016/j.placenta.2010
Rueda-Clausen, C. F., Lahera, V., Calderon, J., Bolivar, I. C., Castillo, V. R., Gutierrez, M., et al. (2010). The presence of abdominal obesity is associated with changes in vascular function independently of other cardiovascular risk factors. Int. J. Cardiol. 139, 32–41. doi: 10.1016/j.ijcard.2008.09.005
Rust, O. A., Bofill, J. A., Zappe, D. H., Hall, J. E., Burnett, J. C. Jr., and Martin, J. N. Jr. (1997). The origin of endothelin-1 in patients with severe preeclampsia. Obstet. Gynecol. 89, 754–757. doi: 10.1016/S0029-7844(97)00093-8
Savvidou, M. D., Hingorani, A. D., Tsikas, D., Frölich, J. C., Vallance, P., and Nicolaides, K. H. (2003). Endothelial dysfunction and raised plasma concentrations of asymmetric dimethylarginine in pregnant women who subsequently develop pre-eclampsia. Lancet 361, 1511–1517. doi: 10.1016/S0140-6736(03)13177-7
Serrano, N. C., Casas, J. P., Díaz, L. A., Páez, C., Meza, C. M., Cifuentes, R., et al. (2004). Endothelial NO synthase genotype and risk of preeclampsia: a multicenter case-control study. Hypertension 44, 702–707. doi: 10.1161/01.HYP.0000143483.66701.ec
Sierra-Laguado, J., García, R. G., Celedón, J., Arenas-Mantilla, M., Pradilla, L. P., Camacho, P. A., et al. (2007). Determination of insulin resistance using the homeostatic model assessment (HOMA) and its relation with the risk of developing pregnancy-induced hypertension. Am. J. Hypertens. 20, 437–442. doi: 10.1016/j.amjhyper.2006.10.009
Skarzinski, G., Khamaisi, M., Bursztyn, M., Mekler, J., Lan, D., Evdokimov, P., et al. (2009). Intrauterine growth restriction and shallower implantation site in rats with maternal hyperinsulinemia are associated with altered NOS expression. Placenta 30, 898–906. doi: 10.1016/j.placenta.2009.07.014
Sladek, S. M., Magness, R. R., and Conrad, K. P. (1997). Nitric oxide and pregnancy. Am. J. Physiol. 272, R441–R463. doi: 10.1152/ajpregu.1997.272.2.R441
Sohda, S., Arinami, T., Hamada, H., Yamada, N., Hamaguchi, H., and Kubo, T. (1997). Methylenetetrahydrofolate reductase polymorphism and pre-eclampsia. J. Med. Genet. 34, 525–526. doi: 10.1136/jmg.34.6.525
Soma, H., Yoshida, K., Mukaida, T., and Tabuchi, Y. (1982). Morphologic changes in the hypertensive placenta. Contrib. Gynecol. Obstet. 9, 58–75.
Spradley, F. T., Palei, A. C., and Granger, J. P. (2015). Increased risk for the development of preeclampsia in obese pregnancies: weighing in on the mechanism. Am. J. Physiol. Regul. Integr. Comp. Physiol. 309, R1326–R1343. doi: 10.1152/ajpregu.00178.2015
Stone, P., Cook, D., Hutton, J., Purdie, G., Murray, H., and Harcourt, L. (1995). Measurements of blood pressure, oedema and proteinuria in a pregnant population of New Zealand. Aust. N. Z. J. Obstet. Gynaecol. 35, 32–37. doi: 10.1111/j.1479-828X.1995.tb01826.x
Suwaki, N., Masuyama, H., Nakatsukasa, H., Masumoto, A., Sumida, Y., Takamoto, N., et al. (2006). Hypoadiponectinemia and circulating angiogenic factors in overweight patients complicated with pre-eclampsia. Am. J. Obstet. Gynecol. 195, 1687–1692. doi: 10.1016/j.ajog.2006.04.003
Teran, E., Escudero, C., Moya, W., Flores, M., Vallance, P., and Lopez-Jaramillo, P. (2001). Elevated C-reactive protein and pro-inflammatory cytokines in Andean women with pre-eclampsia. Int. J. Gynaecol. Obstet. 75, 243–249. doi: 10.1016/S0020-7292(01)00499-4
Teran, E., Escudero, C., Vivero, S., Enriquez, A., and Calle, A. (2004). Intraplatelet cyclic guanosine-3′,5′-monophosphate levels during pregnancy and preeclampsia. Hypertens. Pregnancy 23, 303–308. doi: 10.1081/PRG-200030860
Toda, N., and Okamura, T. (2013). Obesity impairs vasodilatation and blood flow. increase mediated by endothelial Nitric Oxide: an overview. Clin. Pharmacol. 53, 1228–1239. doi: 10.1002/jcph.179
Wallis, A. B., Saftlas, A. F., Hsia, J., and Atrash, H. K. (2008). Secular trends in the rates of pre-eclampsia, eclampsia, and gestational hypertension, United States, 1987–2004. Am. J. Hypertens. 21, 521–526. doi: 10.1038/ajh.2008.20
Wang, Y., Beydoun, M. A., Liang, L., Caballero, B., and Kumanyika, S. K. (2008). Will all Americans become overweight or obese? estimating the progression and cost of the US obesity epidemic. Obesity 16, 2323–2330. doi: 10.1038/oby.2008.351
Ward, K., Hata, A., Jeunemaitre, X., Helin, C., Nelson, L., Namikawa, C., et al. (1993). A molecular variant of angiotensinogen associated with preeclampsia. Nat. Genet. 4, 59–61. doi: 10.1038/ng0593-59
Weil, B. R., Westby, C. M., Van Guilder, G. P., Greiner, J. J., Stauffer, B. L., and DeSouza, C. A. (2011). Enhanced endothelin-1 system activity with overweight and obesity. Am. J. Physiol. Heart Circ. Physiol. 301, H689–H695. doi: 10.1152/ajpheart.00206.2011
Widmer, M., Villar, J., Benigni, A., Conde-Agudelo, A., Karumanchi, S. A., and Lindheimer, M. (2007). Mapping the theories of preeclampsia and the role of angiogenic factors: a systematic review. Obstet. Gynecol. 109, 168–180. doi: 10.1097/01.AOG.0000249609.04831.7c
Witcher, P. M. (2018). Preeclampsia: acute complications and management priorities. AACN Adv. Crit. Care 29, 316–326. doi: 10.4037/aacnacc2018710
World Health Organization [WHO] (1988). Geographic variation in the incidence of hypertension in pregnancy. World health organization international collaborative study of hypertensive disorders of pregnancy. Am. J. Obstet. Gynecol. 158, 80–83.
World Health Organization [WHO] (2011). Prevalence of obesity and overweight females > 15 years. Available at: https://www.who.int/gho/ncd/risk_factors/overweight_obesity/obesity_adults/en/
Yogev, Y., and Catalano, P. M. (2009). Pregnancy and obesity. Obstet. Gynecol. Clin. North Am. 36, 285–300. doi: 10.1016/j.ogc.2009.03.003
Keywords: preeclampsia, obesity, endothelial dysfunction, nitric oxide, cardiovascular risk
Citation: Lopez-Jaramillo P, Barajas J, Rueda-Quijano SM, Lopez-Lopez C and Felix C (2018) Obesity and Preeclampsia: Common Pathophysiological Mechanisms. Front. Physiol. 9:1838. doi: 10.3389/fphys.2018.01838
Received: 27 July 2018; Accepted: 06 December 2018;
Published: 19 December 2018.
Edited by:
Carlos Alonso Escudero, University of the Bío Bío, ChileReviewed by:
Nora Alicia Martínez, Instituto de Fisiología y Biofísica Bernardo Houssay (CONICET), ArgentinaCopyright © 2018 Lopez-Jaramillo, Barajas, Rueda-Quijano, Lopez-Lopez and Felix. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Patricio Lopez-Jaramillo, anBsb3BlempAZ21haWwuY29t; aW52ZXN0aWdhY2lvbmVzLmZvc2NhbEBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.