Voltage-Activated Ion Channels in Non-excitable Cells—A Viewpoint Regarding Their Physiological Justification
- 1Theoretical Medicine and Biosciences, Saarland University, Homburg, Germany
- 2Experimental Physics, Saarland University, Saarbrücken, Germany
- 3Sorbonne Université, CNRS, UMR 8227, Integrative Biology of Marine Models, Station Biologique de Roscoff, Roscoff, France
- 4Laboratoire d'Excellence GR-Ex, Paris, France
A Commentary on
Voltage Gating of Mechanosensitive PIEZO Channels
by Moroni, M., Servin-Vences, M. R., Fleischer, R., Sánchez-Carranza, O., and Lewin, G. R. (2018). Nat. Commun. 9:1096. doi: 10.1038/s41467-018-03502-7
This is a commentary about a recently published paper, elucidating the voltage dependent properties of a channel that was primarily regarded as a mechanosensitive channel: Piezo1 (Moroni et al., 2018). Here we consider the importance of this report in the red blood cell field and provide a link to previously reported data on functional channel activity in red blood cells.
Piezo1 is an ion channel that is believed to be present in red blood cells. Although data from molecular biology are limited (Kaestner, 2015) mutations in Piezo1 cause the red blood cell-related disease Hereditary Xerocytosis (Zarychanski et al., 2012; Bae et al., 2013), which provides convincing evidence. Beside this pathophysiological scenario, the interplay of Piezo1 and the Gardos channel appear to have a vital physiological function in volume adaptation when red blood cells pass constrictions in the narrowest of the capillaries or the spleen slits (Faucherre et al., 2014; Cahalan et al., 2015; Danielczok et al., 2017).
Among the ion channels in red blood cells there are reports about a non-selective voltage-dependent cation channel (Christophersen and Bennekou, 1991; Kaestner et al., 1999; Rodighiero et al., 2004). This channel is also permeable to Ca2+ and comprises a rather unique hysteresis like open probability (Kaestner et al., 2000). Although the physiological function of voltage activated ion channels in non-excitable cells, such as red blood cells is everything else but obvious, a proposal of their regulation was recently published (Kaestner et al., 2018). However, so far the molecular identity of this channel remained obscure (Kaestner, 2011; Bouyer et al., 2012).
The very recent report about voltage-gating of Piezo channels (Moroni et al., 2018) provides evidence that the molecular identity of the non-selective voltage-dependent cation channel in red blood cells might be Piezo1. Figure 1 shows single channel currents of the non-selective voltage-dependent cation channel recorded in red blood cells (Figure 1A) and of Piezo1 overexpressed in Neuro2A cells (Figure 1B). There is quite some similarity in the channel properties: The single channel conductance in Figures 1A,B is 21 ± 5 and 27.1 ± 1.2 pS, respectively. The general dynamic behavior is similar and in both recordings, substates of the channel activity can be seen. The recordings show a slightly different gating, which very well could be caused by differences in the bilayer composition of the plasma membrane between red blood cells and Neuro2A cells. Beside the different cell types one should consider that both recordings (Figure 1A vs. Figure 1B) originate from different laboratories (although performed in the same city) with different equipment and vastly different experimental protocols (including recording solution, patch-clamp configuration and voltage protocols). Furthermore, we like to mention that the different appearance of the traces is more due to different sampling frequencies and different filtering in both recordings than due to channel properties. More importantly, the hysteresis like behavior of the open probability of the non-selective voltage-dependent cation channel recorded in red blood cells is depicted in Figure 1C. A similar pattern could be achieved when Piezo1 was measured with or without a previous conditioning step (Figure 1D). Although the hysteresis like gating is a unusual property, mathematical simulation can well explain the phenomenon (Andersson, 2010).
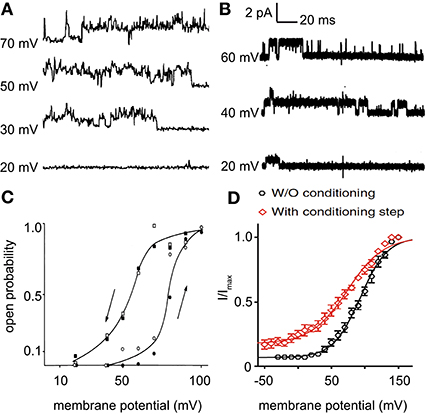
Figure 1. Comparison of the non-selective voltage activated cation channel recorded in red blood cells (A,C) and Piezo1 recorded in overexpressing Neuro2A cells (B,D). (A) Current traces of the non-selective voltage-dependent cation channel in inside-out patches of red blood cells in Na-tartrate-solution in mM (bath solution: 70 Na-tartrate, 2.5 BaCl2, 10 MOPS, 10 glucose, 75 saccharose, pH = 7.4; pipette solution: 20 Na-tartrate, 2.5 BaCl2, 10 MOPS, 10 glucose, 225 saccharose, pH = 7.4). (B) Current traces of Piezo1 in outside-out patches of overexpressing Neuro2A cells in symmetrical NaCl-solution in mM (140 NaCl, 10 HEPES, 5 EGTA, pH = 7.4). The conductance of the channels presented in (A,B) is 21 ± 5 and 27.1 ± 1.2 pS, respectively. (C) The open state probability as function of the membrane potential. In both series (open symbols and filled symbols), the open probability was calculated from 3 min of continuous recording at each potential. The curves were drawn by eye. (D) Tail currents from individual cells were normalized to their maximum and fitted to a Boltzmann relationship. Pooled data are shown as mean ± SEM. (A,C) are reproduced from Kaestner et al. (1999, 2000), respectively and (B,D) from Moroni et al. (2018).
Although current traces have a certain fingerprint, comparison of Figure 1A with Figure 1B does not provide evidence that the non-selective voltage-dependent cation channel in red blood cells is Piezo1 but it is compatible with this hypothesis. More interesting is the unusual hysteresis like behavior plotted in Figures 1C,D. In this respect Moroni et al. provide the explanation that positive voltage together with outward permeation may hold the channel in a fully active conformation preventing the desensitized state (Moroni et al., 2018). This statement is 100% compatible with the recordings performed on red blood cells, where the direction of the membrane potential change (indicated by the arrows in Figure 1C) provides rational explanation to the difference in the open probability. This direction of the membrane potential change goes hand in hand with the amount of outward permeating ions. Under physiological conditions in red blood cells, the non-selective voltage-dependent cation channel is thought to be closed at membrane potentials below +40 to +30 mV. This may imply that regarding very fast closing of the channel in patch-clamp may be accounted for by the mechanosensitivity and voltage-dependence since in red blood cells deactivation has a time constant of below 15 ms (Bennekou et al., 2003).
With the recent paper reporting voltage gating of mechanosensitive Piezo channels, we got a new and very plausible indication for the molecular identity of the non-selective voltage-dependent cation channel in red blood cells. This may explain why sudden calcium entry through Ca2+ permeating mechanosensitive channel may induce longer permeation and thus enhanced subsequent Gárdos channel activity that eventually lead to phenotypic xerocytosis (Dyrda et al., 2010) since Ca2+ entry will lead to transient depolarisation.
A further detailed study on red blood cells is required to confirm the observations performed in overexpressing cells (Moroni et al., 2018) also in the primary red blood cells. The application of planar patch-clamp chips may technically favor the investigation of the rather difficult to patch red blood cells (Minetti et al., 2013; Rotordam et al., 2018).
To know about the voltage sensitivity of Piezo1, will allow (independent whether identical with the non-selective voltage-dependent cation channel in red blood cells or not) a differential view of the role of Piezo1 and its interaction with other ion channels especially in red blood cells in health and disease (Thomas et al., 2011; Kaestner et al., 2018). This is of particular importance since an increasing number of Piezo1 mutations have been reported (Zarychanski et al., 2012; Albuisson et al., 2013; Andolfo et al., 2013; Bae et al., 2013) and also other channelopathies, e.g., involving the Gardos channel may interact with the non-selective voltage-dependent cation channel/Piezo1 (Glogowska et al., 2015; Rapetti-Mauss et al., 2015, 2016; Fermo et al., 2017; Kaestner et al., 2018).
Author Contributions
Both authors have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We acknowledge the funding from the European Union's Horizon 2020 research and innovation programme under grant agreement No. 675115 (RELEVANCE).
References
Albuisson, J., Murthy, S. E., Bandell, M., Coste, B., Louis-Dit-Picard, H., Mathur, J., et al. (2013). Dehydrated hereditary stomatocytosis linked to gain-of-function mutations in mechanically activated PIEZO1 ion channels. Nat. Commun. 4:1884. doi: 10.1038/ncomms2899
Andersson, T. (2010). Exploring voltage-dependent ion channels in silico by hysteretic conductance. Math. Biosci. 226, 16–27. doi: 10.1016/j.mbs.2010.03.004
Andolfo, I., Alper, S. L., De Franceschi, L., Auriemma, C., Russo, R., De Falco, L., et al. (2013). Multiple clinical forms of dehydrated hereditary stomatocytosis arise from mutations in PIEZO1. Blood 121, 3925–3935. doi: 10.1182/blood-2013-02-482489
Bae, C., Gnanasambandam, R., Nicolai, C., Sachs, F., and Gottlieb, P. A. (2013). Xerocytosis is caused by mutations that alter the kinetics of the mechanosensitive channel PIEZO1. Proc. Natl. Acad. Sci. U.S.A. 110, E1162–E1168. doi: 10.1073/pnas.1219777110
Bennekou, P., Kristensen, B. I., and Christophersen, P. (2003). The human red cell voltage-regulated cation channel. The interplay with the chloride conductance, the Ca(2+)-activated K(+) channel and the Ca(2+) pump. J. Membr. Biol. 195, 1–8. doi: 10.1007/s00232-003-2036-6
Bouyer, G., Thomas, S., and Egée, S. (2012). “Patch-clamp analysis of membrane transport in erythrocytes,” in Patch Clamp Technique, ed F. S. Kaneez (Rijeka: InTech), 171–202. doi: 10.5772/37279
Cahalan, S. M., Lukacs, V., Ranade, S. S., Chien, S., Bandell, M., and Patapoutian, A. (2015). Piezo1 links mechanical forces to red blood cell volume. Elife 4:e07370. doi: 10.7554/eLife.07370
Christophersen, P., and Bennekou, P. (1991). Evidence for a voltage-gated, non-selective cation channel in the human red cell membrane. Biochim. Biophys. Acta 1065, 103–106. doi: 10.1016/0005-2736(91)90017-3
Danielczok, J. G., Terriac, E., Hertz, L., Petkova-Kirova, P., Lautenschläger, F., Laschke, M. W., et al. (2017). Red blood cell passage of small capillaries is associated with transient Ca2+-mediated adaptations. Front. Physiol. 8:979. doi: 10.3389/fphys.2017.00979
Dyrda, A., Cytlak, U., Ciuraszkiewicz, A., Lipinska, A., Cueff, A., Bouyer, G., et al. (2010). Local membrane deformations activate Ca2+-dependent K+ and anionic currents in intact human red blood cells. PLoS ONE 5:e9447. doi: 10.1371/journal.pone.0009447
Faucherre, A., Kissa, K., Nargeot, J., Mangoni, M. E., and Jopling, C. (2014). Piezo1 plays a role in erythrocyte volume homeostasis. Haematologica 99, 70–75. doi: 10.3324/haematol.2013.086090
Fermo, E., Bogdanova, A., Petkova-Kirova, P., Zaninoni, A., Marcello, A. P., Makhro, A., et al. (2017). “Gardos Channelopathy”: a variant of hereditary Stomatocytosis with complex molecular regulation. Sci. Rep. 7:1744. doi: 10.1038/s41598-017-01591-w
Glogowska, E., Lezon-Geyda, K., Maksimova, Y., Schulz, V. P., and Gallagher, P. G. (2015). Mutations in the Gardos channel (KCNN4) are associated with hereditary xerocytosis. Blood 126, 1281–1284. doi: 10.1182/blood-2015-07-657957
Kaestner, L. (2011). Cation Channels in erythrocytes - historical and future perspective. Open Biol. J. 4, 27–34. doi: 10.2174/1874196701104010027
Kaestner, L. (2015). Channelizing the red blood cell: molecular biology competes with patch-clamp. Front. Mol. Biosci. 2:46. doi: 10.3389/fmolb.2015.00046
Kaestner, L., Bollensdorff, C., and Bernhardt, I. (1999). Non-selective voltage-activated cation channel in the human red blood cell membrane. Biochim. Biophys. Acta 1417, 9–15. doi: 10.1016/S0005-2736(98)00240-5
Kaestner, L., Christophersen, P., Bernhardt, I., and Bennekou, P. (2000). The non-selective voltage-activated cation channel in the human red blood cell membrane: reconciliation between two conflicting reports and further characterisation. Bioelectrochemistry 52, 117–125. doi: 10.1016/S0302-4598(00)00110-0
Kaestner, L., Wang, X., Hertz, L., and Bernhardt, I. (2018). Voltage-activated ion channels in non-excitable cells - a viewpoint regarding their physiological justification. Front. Physiol. 9:450. doi: 10.3389/fphys.2018.00450
Minetti, G., Egée, S., Mörsdorf, D., Steffen, P., Makhro, A., Achilli, C., et al. (2013). Red cell investigations: art and artefacts. Blood Rev. 27, 91–101. doi: 10.1016/j.blre.2013.02.002
Moroni, M., Servin-Vences, M. R., Fleischer, R., Sánchez-Carranza, O., and Lewin, G. R. (2018). Voltage gating of mechanosensitive PIEZO channels. Nat. Commun. 9:1096. doi: 10.1038/s41467-018-03502-7
Rapetti-Mauss, R., Lacoste, C., Picard, V., Guitton, C., Lombard, E., Loosveld, M., et al. (2015). A mutation in the Gardos channel is associated with hereditary xerocytosis. Blood 126, 1273–1280. doi: 10.1182/blood-2015-04-642496
Rapetti-Mauss, R., Soriani, O., Vinti, H., Badens, C., and Guizouarn, H. (2016). Senicapoc: a potent candidate for the treatment of a subset of Hereditary Xerocytosis caused by mutations in the Gardos channel. Haematologica 2016:149104. doi: 10.3324/haematol.2016.149104
Rodighiero, S., De Simoni, A., and Formenti, A. (2004). The voltage-dependent nonselective cation current in human red blood cells studied by means of whole-cell and nystatin-perforated patch-clamp techniques. Biochim. Biophys. Acta 1660, 164–170. doi: 10.1016/j.bbamem.2003.11.011
Rotordam, G. M., Fermo, E., Becker, N., Barcellini, W., Brüggemann, A., Fertig, N., et al. (2018). A novel gain-of-function mutation of Piezo1 is functionally affirmed in red blood cells by high-throughput patch clamp. Haematologica doi: 10.3324/haematol.2018.201160. [Epub ahead of print].
Thomas, S. L. Y., Bouyer, G., Cueff, A., Egée, S., Glogowska, E., and Ollivaux, C. (2011). Ion channels in human red blood cell membrane: actors or relics? Blood Cells Mol. Dis. 46, 261–265. doi: 10.1016/j.bcmd.2011.02.007
Keywords: erythrocyte, non-selective voltage-dependent cation channel, Piezo1, Hereditary Xerocytosis, patch-clamp
Citation: Kaestner L and Egee S (2018) Commentary: Voltage Gating of Mechanosensitive PIEZO Channels. Front. Physiol. 9:1565. doi: 10.3389/fphys.2018.01565
Received: 25 June 2018; Accepted: 18 October 2018;
Published: 20 November 2018.
Edited by:
Helene Guizouarn, Centre National de la Recherche Scientifique (CNRS), FranceReviewed by:
Remi Peyronnet, Albert-Ludwigs-Universität Freiburg, GermanyCopyright © 2018 Kaestner and Egee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lars Kaestner, lars_kaestner@me.com
 Lars Kaestner
Lars Kaestner Stephane Egee3,4
Stephane Egee3,4