
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Physiol. , 12 November 2018
Sec. Exercise Physiology
Volume 9 - 2018 | https://doi.org/10.3389/fphys.2018.01564
This article is part of the Research Topic Exercise as a Countermeasure to Human Aging View all 33 articles
 Maamer Slimani1*
Maamer Slimani1* Rodrigo Ramirez-Campillo2
Rodrigo Ramirez-Campillo2 Armin Paravlic3
Armin Paravlic3 Lawrence D. Hayes4
Lawrence D. Hayes4 Nicola Luigi Bragazzi1
Nicola Luigi Bragazzi1 Maha Sellami5
Maha Sellami5Aim: The purposes of this meta-analysis were to quantify the effectiveness of physical training on quality of life (QoL), aerobic capacity, and cardiac functioning in older patients with heart failure (HF) and evaluate dose–response relationships of training variables (frequency, volume, and duration).
Methods: Scholarly databases (e.g., PubMed/MEDLINE, Google Scholar, and Scopus) were searched, identifying randomized controlled trials that investigated the effectiveness of different training modes on QoL (assessed by the Minnesota Living with Heart Failure Questionnaire), aerobic capacity (assessed by the 6 min walk test) and cardiac function (assessed by left ventricular ejection fraction).
Results: Twenty five studies were included with a total of 2,409 patients. Results showed that exercise training improved total QoL (small ES = −0.69; 95% CI −1.00 to 0.38; p < 0.001), aerobic capacity (small ES = 0.47; 95% CI 0.15–0.71; p = 0.002) and cardiac function (moderate ES = 0.91; 95% CI 0.37–1.45; p = 0.001). In addition, univariate analyses revealed the moderating variable ‘training mode' significantly influenced aerobic capacity (Q = 9.97; p = 0.007), whereby, resistance training had the greatest effect (ES = 1.71; 95% CI 1.03–2.39; p < 0.001), followed by aerobic training (ES = 0.51; 95% CI 0.30–0.72; p < 0.001), and combined training (ES = 0.15; 95% CI −0.24 to 0.53; p = 0.45). Meta-regression analysis showed that only the duration of an intervention predicted the effect of physical training on QoL (coefficient = −0.027; p = 0.006), with shorter training durations (12 weeks) showing larger improvements.
Conclusion: The present meta-analysis showed that physical training has positive effects on QoL, aerobic capacity, and cardiac function in older patients with HF. Practitioners should consider both training volume and mode when designing physical training programs in order to improve QoL and aerobic capacity in older patients with HF.
Heart failure (HF), a complex clinical syndrome characterized by reduced ability of the heart to pump and/or fill with blood, represents a major public health problem, with a computed prevalence of over 5.8 million in the USA, and over 23–26 million worldwide (Roger, 2013). Being a global pandemic, prevalence is still increasing and is expected to reach 8 million people in the USA by 2030, whereas up to 15 million people are living with HF in Europe (Maggioni, 2015). In Italy, the burden is especially prevalent among elderly people and in Regions such as Liguria, which is a Region with Europe's oldest residents (Marangoni et al., 2012).
As such, HF imposes high societal costs and impacts on patient quality of life (QoL) (Heo et al., 2009). QoL relates to a multitude of aspects such as physical fitness, social environment, education, employment, economic and finance conditions, and other markers such as religion and beliefs, and environment (Katschnig, 1997; Coelho et al., 2005). Among these elements, physical and mental health play an important role in management and characterization of QoL of a person (Rodríguez-Fernández et al., 2017), and especially a patient with cardiac disease (Pedersen et al., 2007). In fact, it has been shown that low pre-hospitalization health-related QoL and poor fitness are associated with lower post-hospitalization general health, increased remission rates, and mortality risk (Mendes de Leon et al., 1998; Hawkes et al., 2013).
Prevention of cardiac disease based on physical activity plays a major role in public health, and physical activity should be considered a priority in prevention of chronic-degenerative disorders (Romano-Spica et al., 2015). Regular physical activity mitigates the risk of HF hospitalization and mortality (Hegde et al., 2017). There seems to be a strong, dose-dependent association between physical activity/fitness and the risk of HF. As such, exercise can be conceived as a non-pharmacological treatment to counter the pathophysiological mechanisms leading to HF (Pandey et al., 2015).
Physical activity generally improves QoL, aerobic fitness, and cardiac function in HF patients (Beniaminovitz et al., 2002; Harris et al., 2003; Gary et al., 2004; Koukouvou et al., 2004; Davidson et al., 2010; Hassanpour Dehkordi and Khaledi Far, 2015). However, this is not always the case, as Keteyian et al. (1999) showed no significant change in QoL after 6 months' aerobic training in male HF patients.
Different modes of training (namely aerobic, resistance, and combined training) result in divergent physiological adaptations, and the most beneficial mode for HF patients remains unclear. Delagardelle et al. (2002) and Mandic et al. (2009) reported that combined aerobic and resistance training was superior to aerobic training exclusively for improving cardiorespiratory fitness (i.e., peak oxygen uptake [VO2peak]). In contrast, Haykowsky et al. (2005) reported no significant difference between combined aerobic and resistance training and aerobic training only in cardiorespiratory fitness (i.e., VO2peak), and therefore, ambiguity remains as to which modality of training is optimal for patients with HF. Moreover, most investigations report no difference between short- and long-term training for QoL and cardiorespiratory fitness (i.e., 6 min walk test [6-MWT]) improvement (Dracup et al., 2007; Jolly et al., 2009; Davidson et al., 2010).
Previous meta-analyses have shown exercise training improves cardiorespiratory fitness, ejection fraction and QoL in cardiac patients (Piepoli et al., 2004; Smart and Marwick, 2004; Haykowsky et al., 2007; Giuliano et al., 2017; Ostman et al., 2017). More specifically, Haykowsky et al. (2007) reported that aerobic training improved ejection fraction in patients with HF to a greater extent than combined aerobic and strength training. Furthermore, Ostman et al. (2017) reported that high-intensity physical training reduced total QoL score (i.e., improved QoL). However, despite the existing meta-analyses in the field, whether there is a most effective training mode and/or dose-response relationship remains unknown. The effectiveness of physical training on QoL, aerobic capacity, and left ventricular ejection fraction in older HF patients in terms of training variables (frequency, volume, and duration) is still unknown. This knowledge would allow practitioners to (i) maximize training-related health benefits and athletic performance and (ii) adequately design strength and conditioning programs for cardiac rehabilitation.
Therefore, the present meta-analysis was designed in order to fill the aforementioned void in knowledge. In particular, the aim of this meta-analysis was to establish the effects of physical training on QoL, aerobic capacity, and left ventricular ejection fraction in older HF patients. A secondary aim was to quantify dose-response relationships according to training modalities and program variables.
This meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines (Figure 1, Moher et al., 2009). A systematic literature search was conducted for randomized controlled trials (RCTs) studying the effects of physical training on QoL in older patients with HF. Studies were obtained through systematic manual and electronic searches (up to May 1st, 2018) in electronic databases (i.e., Google Scholar, MEDLINE/PubMed, and Scopus). Electronic databases were searched using the following search syntax with keywords and/or MeSH terms: [(“aerobic training” OR “resistance training” OR “power training” OR “plyometric training” OR “exercise”) AND (“elderly” OR “older”) AND “heart failure” AND (“quality of life” OR “Minnesota Living with Heart Failure Questionnaire” OR “walking test” OR “left ventricular ejection fraction”)]. Moreover, we performed manual searches of relevant journals and reference lists obtained from published articles. The present meta-analysis included studies published in journals that reported original research data from older patients with HF.
Studies were included in this meta-analysis if they met all the following Population/Intervention/Comparison/Outcome(s) (PICOS) criteria:
(1) Population: studies recruiting older patients with HF as participants from different countries (i.e., Belgium, Brazil, Canada, Greece, Iran, Italy, Netherlands, Sweden, Taiwan, United States, United Kingdom); Older patient groups includes the younger old (65–74 years), the old (75–84 years), and the older old or oldest old (>85 years) (Little et al., 2012). However, we allowed 50 years of age as a minimum reference range of age for studies on African population (World Health Organization, 2016), and from 65 years for populations from developed countries (Kowal and Dowd, 2001).
(2) Intervention or exposure:
a) Studies examining the effects of physical training on QoL, aerobic capacity, and cardiac function in older patients with HF;
b) Studies describing their training variables (e.g., volume, frequency, and duration).
(1) Comparison: Studies involving a control group against which an intervention was compared.
(2) Outcome(s): QoL, aerobic capacity, or cardiac function assessed using the Minnesota Living with Heart Failure Questionnaire (MLWHFQ), the 6-MWT (i.e., total distance covered), and left ventricular ejection fraction, respectively. In addition, we examined how moderating variables like training duration (weeks), training frequency (sessions/week), and type of training, influenced physical training related QoL, aerobic capacity and cardiac function enhancements.
(3) Study design: original research in the form of RCTs.
Studies were excluded if:
(i) They were reviews, opinion papers and commentaries, interviews, letters to the editor, editorials, posters, conference papers, abstracts, book chapters, or books. However, published review articles were examined to avoid missing relevant articles;
(ii) They did not include sufficient data to calculate standardized mean differences.
Two authors independently extracted data using a structured form. Because of the high number of variables that may affect training effectiveness, independent variables were grouped into the following areas as reported in the included studies: (i) training modes (aerobic vs. resistance vs. combined aerobic and resistance) and (ii) training variables (training duration in weeks [4–8 weeks vs. 12 weeks vs. 16 weeks vs. 5–6 months vs. 1 year], weekly training frequency [1–2 vs. 3 vs. 4 vs. 5 or more sessions per week], and session duration (20–30 min vs. 31–45 min vs. 46–65 or more min).
The main study characteristics (i.e., intervention program, training variables, relevant outcomes) were extracted into a Microsoft Excel/spreadsheet.
Data were extracted from the included studies using a standardized documentation form. Effect size (ES) and 95% confidence intervals (95% CI) were calculated for the identified studies. Meta-analyses were computed using the program Comprehensive Meta-Analysis, version 2 (Borenstein et al., 2005). Statistical heterogeneity was assessed using Q and I2. The I2 measure of inconsistency was used to examine between-study variability. Values of 25, 50, and 75% represent low, moderate, and high statistical heterogeneity, respectively (Higgins et al., 2003). Due to study heterogeneity, we applied a random-effects model for all comparisons. Potential publication bias was visually inspected with a funnel plot, looking at asymmetry of the graph. In addition, meta-regression analyses (method of moments) were applied to compute possible predictors that may have influenced training-related effects (e.g., training duration, weekly training frequency, and session duration). Effect sizes (ES) were classified as trivial (< 0.35), small (0.35–0.80), moderate (0.80–1.50), or large (>1.5) (Rhea et al., 2003) and significance level was set a priori at p < 0.05.
The applied search strategy yielded a preliminary number of 26,433 studies. After removing duplicates, 23,800 unique studies were screened. Screening of titles and abstracts resulted in 23,652 papers being discarded. This was due to the nature itself of the search strategy, which was designed to be the broadest possible in order to capture all relevant studies and was performed utilizing different scholarly databases, including the gray literature. Full texts of 148 articles were retrieved and assessed using the predetermined inclusion and exclusion criteria. After a careful review of full texts, 123 articles were excluded and the remaining 25 articles were included in this meta-analysis. A flow chart of the systematic search process is illustrated in Figure 1. Details of all included studies are depicted in Tables 1A–C.
Twenty five studies totalizing 34 ES were identified and a small effect of physical training on QoL was observed (ES = −0.69; 95% CI = −1.00 to −0.38; p < 0.001) (Figure 2). High heterogeneity was observed (I2 = 91.45%; p < 0.001). Therefore, sub-group analysis was conducted, observing a moderate QoL improvement in females (ES = −1.13; 95% CI = −2.01 to −0.24; p = 0.013), small QoL improvement in males (ES = −0.55; 95% CI = −1.29 to 0.19; p = 0.148), and small QoL improvement in males and females combined (ES = −0.69; 95% CI −1.02 to −0.36; p < 0.001), without significant difference between them (Q = 1.05; p = 0.592). When the effects of different intervention types were analyzed, QoL improvements were observed following aerobic training (moderate ES = −1.04; 95% CI = −1.67 to −0.41; p = 0.001) and combined aerobic and resistance training (small ES = −0.42; 95% CI −0.71 to −0.13; p = 0.005), with trivial effects after resistance training (ES = −0.17; 95% CI = −0.80 to 0.47; p = 0.610). However, no significant difference was observed between training modes (Q = 4.20; p = 0.123) (Table 2).
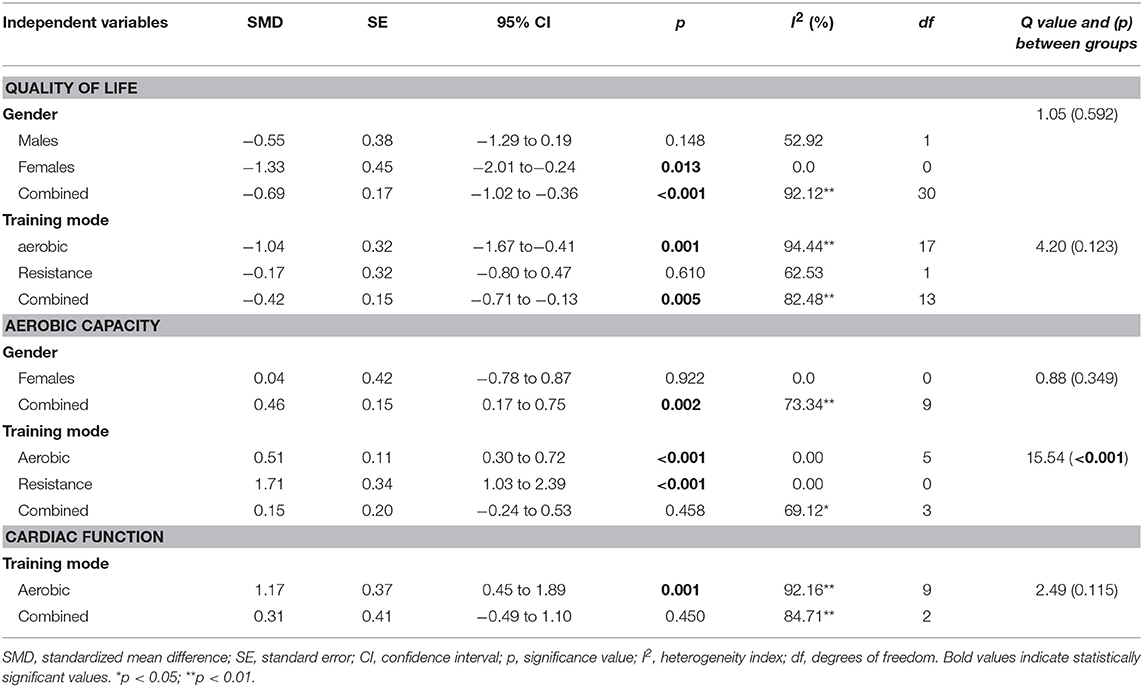
Table 2. Effects of physical training on quality of life, aerobic capacity, and cardiac function in older patients with HF patients considering different moderating variables.
Eleven studies examined the effects of physical training on aerobic capacity (i.e., total distance covered in the 6-MWT). The grouped effect (i.e., all type of physical training combined) revealed improvements following intervention (small ES = 0.43; 95% CI = 0.15 to 0.71; p = 0.002) (Figure 3). Small improvements were observed in males and females combined (ES = 0.46; 95% CI = 0.17–0.75; p = 0.002), with trivial effect on females (ES = 0.04; 95% CI = −0.78 to 0.87; p = 0.922). Due to insufficient data, the effect on males was not calculated. When different types of physical training were analyzed, large improvements were observed following resistance training (ES = 1.71; 95% CI = 1.03 to 2.39; p < 0.001), small improvements were observed following aerobic training (ES = 0.51; 95% CI = 0.30 to 0.72; p < 0.001), and trivial improvements were observed following combined aerobic and resistance training (ES = 0.15; 95% CI = −0.24 to 0.53; p = 0.458). Additionally, significant difference was observed between different training modes (Q = 15.54; p < 0.001) (Table 2).
Cardiac function (i.e., left ventricular ejection fraction) showed moderate improvements after physical training (moderate ES = 0.91; 95% CI = 0.37 to 1.45; p = 0.001) (Figure 4). When different interventions were analyzed, there were improvements following aerobic training (moderate ES = 1.17; 95% CI = 0.45 to 1.89; p = 0.001) and combined aerobic and resistance training (small ES = 0.30; 95% CI = −0.49 to 1.10; p = 0.450), without significant difference between training modes (Q = 2.49; p < 0.115) (Table 2).
Table 3 shows the results of the meta-regression for the three training variables: duration of intervention, duration of single session, and weekly frequency. Only the duration of the intervention predicted QoL changes after physical training (p = 0.006) (Figure 5). The predictive influence of the remaining training variables was p = 0.665–0.996. Therefore, none of the examined training variables predicted aerobic capacity and cardiac function adaptation (p = 0.280–0.522) (Table 3).
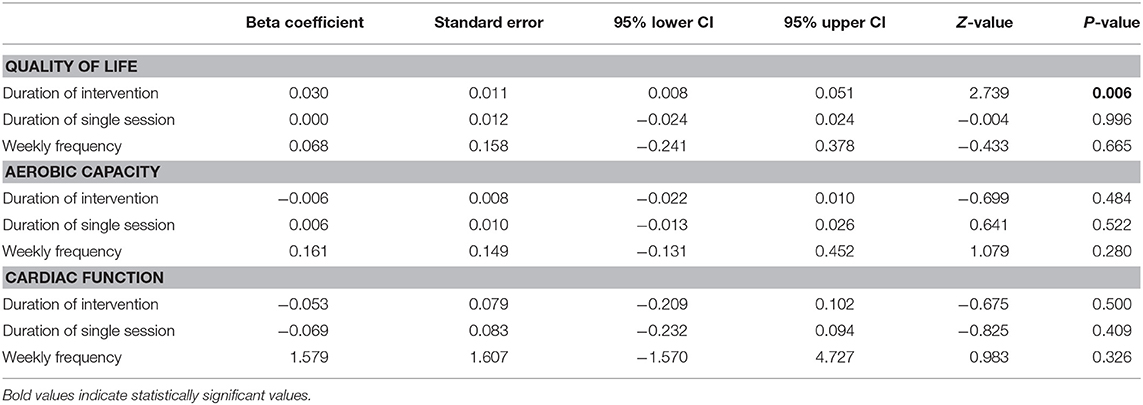
Table 3. Meta regression for training variables of different subscales to predict physical training effect on quality of life, aerobic capacity and cardiac function in in older patients with HF.
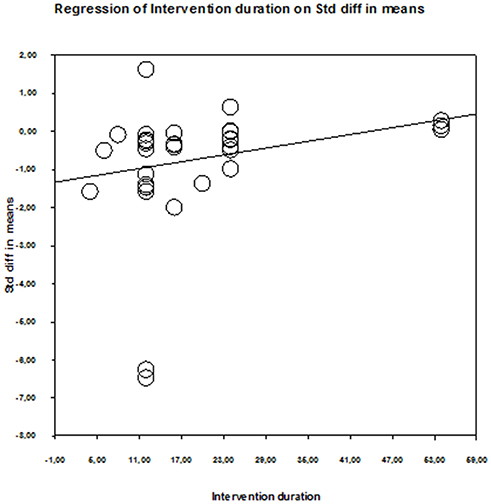
Figure 5. Scatter plot of regression analysis showing influence of intervention duration on the quality of life of older patients with HF.
Sub-analysis (Table 4) revealed that 12 weeks' training induced the greatest improvements in QoL (ES = −1.41; 95% CI = −2.20 to −0.61; p = 0.001). Regarding the frequency of training, 3–4 sessions per week induced the greatest improvements in QoL (ES = −0.98; 95% CI = −1.49 to −0.48; p < 0.001). Regarding single session duration, 31–45 min of training per session induced the greatest improvements in QoL (ES = −1.57; 95% CI = −2.53 to −0.60; p = 0.001).
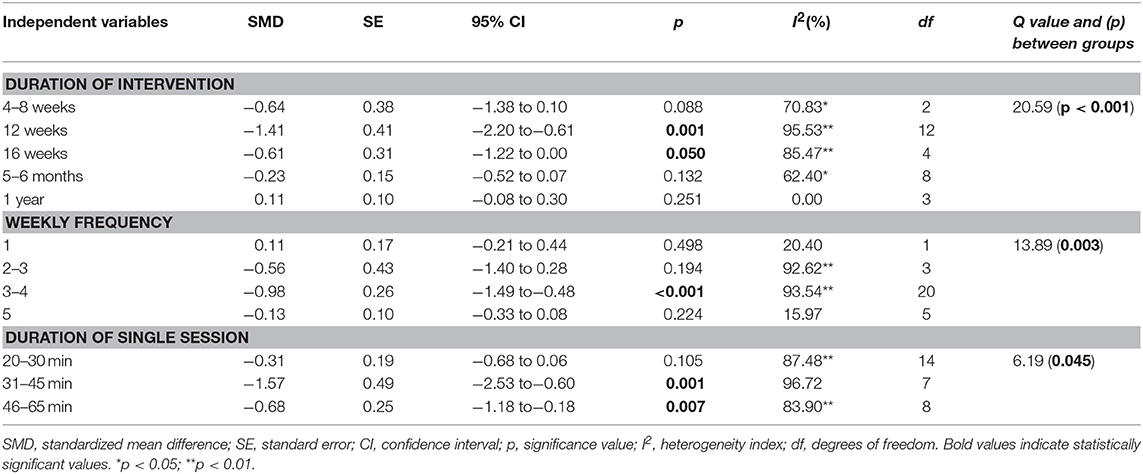
Table 4. Effects of physical training on quality of life considering different moderating variables.
The present meta-analysis summarizes evidence on the dose–response relationships between exercise (dose) and improvement of QoL, aerobic capacity, and cardiac function in (response) in older HF patients. The main findings of this meta-analysis were that (i) physical training exerted moderate effects on cardiac function, and small effects on QoL and aerobic capacity in older patients with HF and (ii) the training variable “duration” predicted the effects of physical training on QoL.
Previous meta-analyses have already examined the effect of physical training on QoL and aerobic performance and/or dose–response relationships for training variable (i.e., training intensity) in patients with HF (Pandey et al., 2015; Ostman et al., 2017). Pandey et al. (2015) showed that exercise training improves cardiorespiratory fitness (weighted mean difference = 2.72) and QoL (weighted mean difference = −3.97). These findings are in agreement with the results of Ostman et al. (2017) for QoL. However, as a novelty, our meta-analysis revealed that, aside from small improvements in QoL and aerobic capacity (i.e., 6-MWT), older patients with HF may achieve moderate improvements in cardiac function after physical training.
Current findings indicate that aerobic training provide moderate improvements in QoL, compared to only small or trivial improvements after combined aerobic and resistance training and resistance training only, respectively. Ostman et al. (2017) reported that combined aerobic and resistance training or aerobic training alone improved QoL, whilst resistance training alone did not improve QoL. Similarly, Mandic et al. (2009) compared aerobic training vs. combined aerobic and resistance training in HF patients and reported that QoL was improved following aerobic training in compliant patients only. Cardiac rehabilitation programs focusing on aerobic training have resulted in reduced symptoms (i.e., pain, lower extremity edema, coughing, and breathe problems) and enhance functional capacity (Pollentier et al., 2010) following implementation.
Concerning changes of aerobic capacity (i.e., 6-MWT), the present meta-analysis revealed that resistance training produced greater performance improvements than aerobic and combined training. This may be explained by the beneficial effects of resistance training on running economy and strength, which results in greater recruitment of type I fibers for the same submaximal load, better muscular coordination, and therefore better mechanical efficiency, whilst likely concomitantly enhancing aerobic capacity (Hartman et al., 2007; Yamamoto et al., 2008, 2010; Cadore et al., 2011). Contrastingly, Wood et al. (2001) reported greater improvements in repeated chair stand after combined training, vs. with resistance-only and endurance-only training in healthy older individuals. Furthermore, another review revealed that resistance and aerobic training have a similar effect on aerobic capacity in older adults (Liu and Latham, 2009). As such, practitioners/physicians should implement resistance training to improve 6-MWT performance, and thus functional capacity in patients with HF.
This meta-analysis showed that aerobic training had the largest effect on cardiac function and mechanisms may be related to presence of mediators such as nitric oxide, which increase cardiac vagal tone after aerobic exercise, and angiotensin II, which inhibits cardiac vagal activity, and the adaptation of the autonomic nervous system in favor of parasympathetic dominance (Kingwell, 2002; Billman and Kukielka, 2007). Previous meta-analysis has suggested that aerobic or combined exercise, but not resistance exercise, may be effective in improving cardiac function in HF patients (Haykowsky et al., 2007). Delagardelle et al. (2002) found a greater improvement of aerobic performance and systolic function after four months of combined aerobic and resistance training than aerobic training in HF patients. Of note, in the present meta-analysis, the absence of significant difference in cardiac function between aerobic and combined training may be due to the high heterogeneity among included studies.
The current meta-analysis substantially advances the literature compared to previous reviews (Piepoli et al., 2004; Smart and Marwick, 2004; Giuliano et al., 2017; Ostman et al., 2017), as we provide the dose–response relationships of physical training variables such as frequency, duration, and volume with training adaptations. Included studies showed large variation in training variables whereby training periods ranged from 4-weeks to 1-year, frequencies from one to five times/week, and duration of training sessions from 10 to 65 min. The characterization of dose–response relationships revealed that, when considered individually, and not as complete training protocol, training periods of 12 weeks, a frequency of 3–4 sessions per week, and durations of 31–45 min of a single training session, were the most effective training variable specifics for improvements in QoL.
Dose-response analyses revealed that shorter training durations are more effective at improving QoL. Specifically, sub-group analysis showed that physical training lasting 12 weeks is most effective for improving global QoL in older HF patients. As illustrated in Table 3, longer training periods produced lower ES, compared to shorter training (i.e., 4–16 weeks) periods. Davidson et al. (2010) reported significant differences between intervention and control groups in the measure of QoL (MLWHFQ) at 3 months, whilst there were no differences between groups at 12 months. Health Canada (1999), American College of Sports Medicine (ACSM) and the American Heart Association (AHA) (Nelson et al., 2007) recommended 30–60 min a day of aerobic activity of moderate-intensity for several months to promote and maintain health, and reduce the risk of chronic disease, premature mortality, functional limitations, and disability. Shorter periods of training are usually accompanied by greater adherence rates (Conraads et al., 2012; De Maeyer et al., 2013). In fact, only minor group could reach the long-term period of >6-month and could reach the 120 min per week (De Maeyer et al., 2013). Despite that, other authors relate improvements of maximal oxygen uptake to the period of training (Tabet et al., 2008) and absence of changes in this parameter is strongly correlated with cardiac risk. With regard to period of training, Lloyd-Williams et al. (2002) found that short-term physical activity is beneficial to patients with chronic heart failure and most patients experience improvement in their QoL.
Our meta-analysis has several limitations that warrant discussion. Firstly, we computed meta-regression and univariate analyses to identify effective dose-response relationships. Of note, findings from univariate analyses must be interpreted with caution because training variables were computed as single factors irrespective of potential between-variable interactions (Gebel et al., 2018). Secondly, we have not performed univariate analyses for aerobic capacity and cardiac function due to the small number of studies. Thirdly, due to limited number of studies examining the effects of physical training in female and male patients, we were not able to compare sexes.
Physical activity is an effective therapeutic method in older patients with HF, with small to moderate effects on QoL, aerobic capacity, and cardiac function, irrespective of sex and training mode. Dose–response analyses showed that none of the training variables predicted changes in aerobic capacity or cardiac function. However, for QoL, the meta-regression indicated that the training duration significantly predicted the observed improvement, with shorter training duration showing larger improvements.
MS, RR-C, AP, LH, NB, and MS study concept and design. MS, RR-C, AP, LH, NB, and MS analysis and interpretation of data. MS, RR-C, AP, LH, NB, and MS final approval of the version to be published. MS, RR-C, AP, LH, NB, and MS agreement to be accountable for all aspects of the work.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Beniaminovitz, A., Lang, C. C., LaManca, J., and Mancini, D. M. (2002). Selective low-level leg muscle training alleviates dyspnea in patients with heart failure. J. Am. Coll. Cardiol. 40, 1602–1608. doi: 10.1016/S0735-1097(02)02342-2
Billman, G. E., and Kukielka, M. (2007). Effect of endurance exercise training on the heart rate onset and heart rate recovery responses to submaximal exercise in animals susceptible to ventricular fibrillation. J. Appl. Physiol. 102, 231–240. doi: 10.1152/japplphysiol.00793.2006
Borenstein, M., Hedges, L., Higgins, J., and Rothstein, H. (2005). Comprehensive Meta-Analysis, Version 2. Englewood, NJ: Biostat Inc.
Brubaker, P. H., Moore, J. B., Stewart, K. P., Wesley, D. J., and Kitzman, D. W. (2009). Endurance exercise training in older patients with heart failure: results from a randomized, controlled, single-blind trial. J. Am. Geriatr. Soc. 57, 1982–1989. doi: 10.1111/j.1532-5415.2009.02499.x
Cadore, E. L., Pinto, R. S., Pinto, S. S., Alberton, C. L., Correa, C. S., Tartaruga, MP, et al. (2011). Effects of strength, endurance, and concurrent training on aerobic power and dynamic neuromuscular economy in elderly men. J. Strength Cond. Res. 25, 758–766. doi: 10.1519/JSC.0b013e318207ed66
Chien, C., Lee, C., Wu, Y., and Wu, Y. (2011). Home-based exercise improves the quality of life and physical function but not the psychological status of people with chronic heart failure: a randomised trial. J. Physiother. 57, 157–163. doi: 10.1016/S1836-9553(11)70036-4
Chrysohoou, C., Tsitsinakis, G., Vogiatzis, I., Cherouveim, E., Antoniou, C., Tsiantilas, A., et al. (2014). High intensity, interval exercise improves quality of life of patients with chronic heart failure: a randomized controlled trial. QJM 107, 25–32. doi: 10.1093/qjmed/hct194
Coelho, R., Ramos, S., Prata, J., Bettencourt, P., Ferreira, A., and Cerqueira-Gomes, M. (2005). Heart failure and health related quality of life. Clin Pract. Epidemiol. Mental Health 1:19. doi: 10.1186/1745-0179-1-19
Conraads, V. M., Deaton, C., Piotrowicz, E., Santaularia, N., Tierney, S., Piepoli, M. F., et al. (2012). Adherence of heart failure patients to exercise: barriers and possible solutions: a position statement of the study group on exercise training in heart failure of the heart failure association of the European society of cardiology. Eur. J. Heart Fail. 14, 451–458. doi: 10.1093/eurjhf/hfs048
Conraads, V. M., Vanderheyden, M., Paelinck, B., Verstreken, S., Blankoff, I., Miljoen, H., et al. (2007). The effect of endurance training on exercise capacity following cardiac resynchronization therapy in chronic heart failure patients: a pilot trial. Eur. J. Cardiovasc. Prev. Rehabil. 14, 99–106. doi: 10.1097/HJR.0b013e32801164b3
Davidson, P. M., Cockburn, J., Newton, P. J., Webster, J. K., Betihavas, V., Howes, L., et al. (2010). Can a heart failure-specific cardiac rehabilitation program decrease hospitalizations and improve outcomes in high-risk patients? Eur. J. Cardiovasc. Prev. Rehabil. 17, 393–402. doi: 10.1097/HJR.0b013e328334ea56
De Maeyer, C., Beckers, P., Vrints, C. J., and Conraads, V. M. (2013). Exercise training in chronic heart failure. Ther. Adv. Chronic Dis. 4, 105–117. doi: 10.1177/2040622313480382
Delagardelle, C., Feiereisen, P., Autier, P., Shita, R., Krecke, R., and Beissel, J. (2002). Strength/endurance training versus endurance training in congestive heart failure. Med. Sci. Sports Exerc. 34, 1868–1872. doi: 10.1097/00005768-200212000-00002
Dracup, K., Evangelista, L. S., Hamilton, M. A., Erickson, V., Hage, A., Moriguchi, J., et al. (2007). Effects of a home-based exercise program on clinical outcomes in heart failure. Am. Heart J. 154, 877–883. doi: 10.1016/j.ahj.2007.07.019
Edelmann, F., Gelbrich, G., Düngen, H. D., Fröhling, S., Wachter, R., Stahrenberg, R., et al. (2011). Exercise training improves exercise capacity and diastolic function in patients with heart failure with preserved ejection fraction: results of the Ex-DHF (Exercise training in Diastolic Heart Failure) pilot study. J. Am. Coll. Cardiol. 58, 1780–1791. doi: 10.1016/j.jacc.2011.06.054
Evangelista, L. S., Cacciata, M., Stromberg, A., and Dracup, K. (2017). Dose-response relationship between exercise intensity, mood states, and quality of life in patients with heart failure. J. Cardiovasc. Nurs. 32, 530–537. doi: 10.1097/JCN.0000000000000407
Fu, T. C., Yang, N. I., Wang, C. H., Cherng, W. J., Chou, S. L., Pan, T. L., et al. (2016). Aerobic interval training elicits different hemodynamic adaptations between heart failure patients with preserved and reduced ejection fraction. Am. J. Phys. Med. Rehabil. 95, 15–27. doi: 10.1097/PHM.0000000000000312
Gary, R., and Lee, S. Y. (2007). Physical function and quality of life in older women with diastolic heart failure: effects of a progressive walking program on sleep patterns. Prog. Cardiovasc. Nurs. 22, 72–80. doi: 10.1111/j.0889-7204.2007.05375.x
Gary, R. A., Sueta, C. A., Dougherty, M., Rosenberg, B., Cheek, D., Preisser, J., et al. (2004). Home-based exercise improves functional performance and quality of life in women with diastolic heart failure. Heart Lung. 33, 210–218. doi: 10.1016/j.hrtlng.2004.01.004
Gebel, A., Lesinski, M., Behm, D. G., and Granacher, U. (2018). Effects and dose-response relationship of balance training on balance performance in youth: a systematic review and meta-analysis. Sports Med. 48, 2067–2089. doi: 10.1007/s40279-018-0926-0
Giuliano, C., Karahalios, A., Neil, C., Allen, J., and Levinger, I. (2017). The effects of resistance training on muscle strength, quality of life and aerobic capacity in patients with chronic heart failure - a meta-analysis. Int. J. Cardiol. 15, 413–423. doi: 10.1016/j.ijcard.2016.11.023
Harris, S., LeMaitre, J. P., Mackenzie, G., Fox, K. A., and Denvir, M. A. (2003). A randomised study of home-based electrical stimulation of the legs and conventional bicycle exercise training for patients with chronic heart failure. Eur. Heart J. 24, 871–878. doi: 10.1016/S0195-668X(02)00822-9
Hartman, M. J., Fields, D. A., Byrne, N. M., and Hunter, G. R. (2007). Resistance training improves metabolic economy during functional tasks in older adults. J. Strength Cond. Res. 21, 91–95. doi: 10.1519/00124278-200702000-00017
Hassanpour Dehkordi, A., and Khaledi Far, A. (2015). Effect of exercise training on the quality of life and echocardiography parameter of systolic function in patients with chronic heart failure: a randomized trial. Asian J. Sports Med. 6:e22643. doi: 10.5812/asjsm.22643
Hawkes, A. L., Patrao, T. A., Ware, R., Atherton, J. J., Taylor, C. B., and Oldenburg, B. F. (2013). Predictors of physical and mental health-related quality of life outcomes among myocardial infarction patients. BMC Cardiovasc. Disord. 13:69. doi: 10.1186/1471-2261-13-69
Haykowsky, M., Vonder Muhll, I., Ezekowitz, J., and Armstrong, P. (2005). Supervised exercise training improves aerobic capacity and muscle strength in older women with heart failure. Can. J. Cardiol. 21, 1277–1280.
Haykowsky, M. J., Liang, Y., Pechter, D., Jones, L. W., McAlister, F. A., and Clark, A. M. (2007). A meta-analysis of the effect of exercise training on left ventricular remodeling in heart failure patients: the benefit depends on the type of training performed. J. Am. Coll. Cardiol. 49, 2329–2336. doi: 10.1016/j.jacc.2007.02.055
Health Canada (1999). Canada's Physical Activity Guide to Health Active Living for Older Adults. Ottawa, ON: Canada
Hegde, S. M., Claggett, B., Shah, A. M., Lewis, E. F., Anand, I., Shah, S. J., et al. (2017). Physical activity and prognosis in the TOPCAT trial (Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist). Circulation 136, 982–992. doi: 10.1161/CIRCULATIONAHA.117.028002
Heo, S., Lennie, T. A., Okoli, C., and Moser, D. K. (2009). Quality of life in patients with heart failure: ask the patients. Heart Lung. 38, 100–108. doi: 10.1016/j.hrtlng.2008.04.002
Higgins, J. P., Thompson, S. G., Deeks, J. J., and Altman, D. G. (2003). Measuring inconsistency in meta-analyses. Br. Med. J. 327, 557–660. doi: 10.1136/bmj.327.7414.557
Jolly, K., Taylor, R. S., Lip, G. Y., Davies, M., Davis, R., Mant, J., et al. (2009). A randomized trial of the addition of home-based exercise to specialist heart failure nurse care: the Birmingham Rehabilitation Uptake Maximisation study for patients with congestive heart failure (BRUM-CHF) study. Eur. J. Heart Fail. 11, 205–213. doi: 10.1093/eurjhf/hfn029
Katschnig, H. (1997). “How useful is the concept of quality of life in psychiatry?” in Quality of Life in Mental Disorders. eds H. Katschnig, H.Freeman, and N. Sartorius (Chichester: Wiley), 3–16.
Keteyian, S. J., Brawner, C. A., Schairer, J. R., Levine, T. B., Levine, A. B., Rogers, F. J., et al. (1999). Effects of exercise training on chronotropic incompetence in patients with heart failure. Am. Heart J. 138(2 Pt 1), 233–240. doi: 10.1016/S0002-8703(99)70106-7
Kingwell, B. A. (2002). Nitric oxide-mediated metabolic regulation during exercise: effects of training in health and cardiovascular disease. FASEB J. 14, 1685–1696. doi: 10.1096/fj.99-0896rev
Kitzman, D. W., Brubaker, P. H., Herrington, D. M., et al. (2013). Effect of endurance exercise training on endothelial function and arterial stiffness in older patients with heart failure and preserved ejection fraction: a randomized, controlled, single-blind trial. J. Am. Coll. Cardiol. 62, 584–592. doi: 10.1016/j.jacc.2013.04.033
Kitzman, D. W., Brubaker, P. H., Morgan, T. M., Stewart, K. P., and Little, W. C. (2010). Exercise training in older patients with heart failure and preserved ejection fraction: a randomized, controlled, single-blind trial. Circ. Heart Fail. 3, 659–667. doi: 10.1161/CIRCHEARTFAILURE.110.958785
Koukouvou, G., Kouidi, E., Iacovides, A., Konstantinidou, E., Kaprinis, G., and Deligiannis, A. (2004). Quality of life, psychological and physiological changes following exercise training in patients with chronic heart failure. J. Rehabil. Med. 36, 36–41. doi: 10.1080/11026480310015549
Kowal, P., and Dowd, J. E. (2001). Definition of an Older Person. Proposed Working Definition of an Older Person in Africa for the MDS Project. Geneva: World Health Organization, 5188–9286.
Little, W., Vyain, S., Scaramuzzo, G., Cody-Rydzewski, S., Griffiths, H., Strayer, E., et al. (2012). “Aging and the elderly,” in Introduction to Sociology-1st Canadian Edition. BC Open Textbook project.
Liu, C., and Latham, N. K. (2009). Progressive resistance strength training for improving physical function in older adults. Cochrane Database Syst. Rev. 3:CD002759. doi: 10.1002/14651858.CD002759.pub2
Lloyd-Williams, F., Mair, F. S., and Leitner, M. (2002). Exercise training and heart failure: a systematic review of current evidence. Br. J. Gen. Pract. 52, 47–55.
Maggioni, A. P. (2015). Epidemiology of Heart Failure in Europe. Heart Fail Clin. 11, 625–635. doi: 10.1016/j.hfc.2015.07.015
Mandic, S., Tymchak, W., Kim, D., Daub, B., Quinney, H. A., Taylor, D., et al. (2009). Effects of aerobic or aerobic and resistance training on cardiorespiratory and skeletal muscle function in heart failure: a randomized controlled pilot trial. Clin. Rehabil. 23, 207–216. doi: 10.1177/0269215508095362
Marangoni, E., Lissoni, F., Raimondi Cominesi, I., and Tinelli, S. (2012). Heart failure: epidemiology, costs and healthcare programs in Italy. G Ital. Cardiol. 13(10 Suppl. 2), 139S−144S. doi: 10.1714/1167.12938
Maria Sarullo, F., Gristina, T., Brusca, I., Milia, S., Raimondi, R., Sajeva, M., et al. (2006). Effect of physical training on exercise capacity, gas exchange and N-terminal pro-brain natriuretic peptide levels in patients with chronic heart failure. Eur. J. Cardiovasc. Prev. Rehabil. 13, 812–817. doi: 10.1097/01.hjr.0000238396.42718.61
McKelvie, R. S., Teo, K. K., Roberts, R., McCartney, N., Humen, D., Montague, T., et al. (2002). Effects of exercise training in patients with heart failure: the Exercise Rehabilitation Trial (EXERT). Am. Heart J. 144, 23–30. doi: 10.1067/mhj.2002.123310
Mendes de Leon, C. F., Krumholz, H. M., Vaccarino, V., Williams, C. S., Glass, T. A., Berkman, L. F., et al. (1998). A population-based perspective of changes in health-related quality of life after myocardial infarction in older men and women. J. Clin. Epidemiol. 51, 609–616. doi: 10.1016/S0895-4356(98)00037-7
Moher, D., Liberati, A., Tetzlaff, J., and Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Br. Med. J. 339:332–336. doi: 10.1136/bmj.b2535
Nelson, M. E., Rejeski, W. J., Blair, S. N., Duncan, P. W., Judge, J. O., King, A. C., et al. (2007). Physical activity and public health in olderadults: recommendation from the American College of Sports Medicine and the American Heart Association. Med. Sci. Sports Exerc. 39, 1435–1445. doi: 10.1249/mss.0b013e3180616aa2
Ostman, C., Jewiss, D., and Smart, N. A. (2017). The effect of exercise training intensity on quality of life in heart failure patients: a systematic review and meta-analysis. Cardiology 136, 79–89. doi: 10.1159/000448088
Pandey, A., Garg, S., Khunger, M., Darden, D., Ayers, C., Kumbhani, D. J., et al. (2015). Dose response relationship between physical activity and risk of heart failure: a meta-analysis. Circulation 132, 1786–1794. doi: 10.1161/CIRCULATIONAHA.115.015853
Patwala, A. Y., Woods, P. R., Sharp, L., Goldspink, D. F., Tan, L. B., and Wright, D. J. (2009). Maximizing patient benefit from cardiac resynchronization therapy with the addition of structured exercise training: a randomized controlled study. J. Am. Coll. Cardiol. 53, 2332–2339. doi: 10.1016/j.jacc.2009.02.063
Pedersen, S. S., Martens, E. J., Denollet, J., and Appels, A. (2007). Poor health-related quality of life is a predictor of early, but not late, cardiac events after percutaneous coronary intervention. Psychosom. 48, 331–337. doi: 10.1176/appi.psy.48.4.331
Piepoli, M. F., Davos, C., Francis, D. P., and Coats, A. J. (2004). Exercise training meta-analysis of trials in patients with chronic heart failure (ExTra- MATCH). BMJ 328:189. doi: 10.1136/bmj.328.7441.711-b
Pihl, E., Cider, A., Strömberg, A., Fridlund, B., and Mårtensson, J. (2011). Exercise in elderly patients with chronic heart failure in primary care: effects on physical capacity and health-related quality of life. Eur. J. Cardiovasc. Nurs. 10, 150–158. doi: 10.1016/j.ejcnurse.2011.03.002
Pollentier, B., Irons, S. L., Benedetto, C. M., DiBenedetto, A. M., Loton, D., Seyler, R. D., et al. (2010). Examination of the six minute walk test to determine functional capacity in people with chronic heart failure: a systematic review. Cardiopulm. Phys. Ther. J. 21:13.
Rhea, M. R., Alvar, B. A., Burkett, L. N., and Ball, S. D. (2003). A meta-analysis to determine the dose response for strength development. Med. Sci. Sports Exerc. 35, 456–464. doi: 10.1249/01.MSS.0000053727.63505.D4
Rodríguez-Fernández, A., Zuazagoitia-Rey-Baltar, A., and Ramos-Díaz, E. (2017). “Quality of life and physical activity: their relationship with physical and psychological well-being,” in Quality of Life and Quality of Working Life. InTech. doi: 10.5772/intechopen.69151
Roger, V. L. (2013). Epidemiology of Heart Failure. Circ. Res. 113, 646–659. doi: 10.1161/CIRCRESAHA.113.300268
Romano-Spica, V., Macini, P., Fara, G. M., and Giammanco, G. (2015). Adapted physical activity for the promotion of health and the prevention of multifactorial chronic diseases: the Erice Charter. Ann. Ig. 27, 406–414. doi: 10.7416/ai.2015.2028
Servantes, D. M., Pelcerman, A., Salvetti, X. M., Salles, A. F., de Albuquerque, P. F., de Salles, F. C., et al. (2012). Effects of home-based exercise training for patients with chronic heart failure and sleep apnoea: a randomized comparison of two different programmes. Clin. Rehabil. 26, 45–57. doi: 10.1177/0269215511403941
Smart, N., and Marwick, T. H. (2004). Exercise training for patients with heart failure: a systematic review of factors that improve mortality and morbidity. Am. J. Med. 116, 693–706. doi: 10.1016/j.amjmed.2003.11.033
Tabet, J. Y., Meurin, P., Beauvais, F., Weber, H., Renaud, N., Thabut, G., et al. (2008). The absence of exercise capacity improvement after exercise training program: a strong prognostic factor in patients with chronic heart failure. Circ. Heart. Fail. 1, 220–226. doi: 10.1161/CIRCHEARTFAILURE.108.775460
van den Berg-Emons, R., Balk, A., Bussmann, H., and Stam, H. (2004). Does aerobic training lead to a more active lifestyle and improved quality of life in patients with chronic heart failure? Eur. J. Heart Fail. 6, 95–100. doi: 10.1016/j.ejheart.2003.10.005
Wood, R. H., Reyes, R., Welsch, M. A., et al. (2001). Concurrent cardio-vascular and resistance training in healthy older adults. Med. Sci. Sports Exerc. 33, 1751–1758. doi: 10.1097/00005768-200110000-00021
World Health Organization (2016). Proposed Working Definition of an Older Person in Africa for the MDS Project.
Yamamoto, L. M., Klau, J. F., Casa, D. J., Kraemer, W. J., Armstrong, L. E., and Maresh, C. M. (2010). The effects of resistance training on road cycling performance among highly trained cyclists: a systematic review. J. Strength Cond. Res. 24, 560–566. doi: 10.1519/JSC.0b013e3181c86583
Keywords: exercise training, resistance training, physical function, health status, cardiac rehabilitation
Citation: Slimani M, Ramirez-Campillo R, Paravlic A, Hayes LD, Bragazzi NL and Sellami M (2018) The Effects of Physical Training on Quality of Life, Aerobic Capacity, and Cardiac Function in Older Patients With Heart Failure: A Meta-Analysis. Front. Physiol. 9:1564. doi: 10.3389/fphys.2018.01564
Received: 12 July 2018; Accepted: 18 October 2018;
Published: 12 November 2018.
Edited by:
Luca Paolo Ardigò, Università degli Studi di Verona, ItalyReviewed by:
Walid Bouaziz, Hôpitaux Universitaires de Strasbourg, FranceCopyright © 2018 Slimani, Ramirez-Campillo, Paravlic, Hayes, Bragazzi and Sellami. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maamer Slimani, bWFhbWVyMjAxMUBob3RtYWlsLmZy
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.