- 1Institute of Metrology and Biomedical Engineering, Faculty of Mechatronics, Warsaw University of Technology, Warsaw, Poland
- 2Department of Applied Physiology, Mossakowski Medical Research Centre, Polish Academy of Sciences, Warsaw, Poland
Training of elite athletes requires regular physiological and medical monitoring to plan the schedule, intensity and volume of training, and subsequent recovery. In sports medicine, ECG-based analyses are well-established. However, they rarely consider the correspondence of respiratory and cardiac activity. Given such mutual influence, we hypothesize that athlete monitoring might be developed with causal inference and that detailed, time-related techniques should be preceded by a more general, time-independent approach that considers the whole group of participants and parameters describing whole signals. The aim of this study was to discover general causal paths among cardiac and respiratory variables in elite athletes in two body positions (supine and standing), at rest. ECG and impedance pneumography signals were obtained from 100 elite athletes. The mean heart rate, the root-mean-square difference of successive RR intervals (RMSSD), its natural logarithm (lnRMSSD), the mean respiratory rate (RR), the breathing activity coefficients, and the resulting breathing regularity (BR) were estimated. Several causal discovery frameworks were applied, comprising Generalized Correlations (GC), Causal Additive Modeling (CAM), Fast Greedy Equivalence Search (FGES), Greedy Fast Causal Inference (GFCI), and two score-based Bayesian network learning algorithms: Hill-Climbing (HC) and Tabu Search. The discovery of cardiorespiratory paths appears ambiguous. The main, still mild, rules best supported by data are: for supine - tidal volume causes heart activity variation, which causes average heart activity, which causes respiratory timing; and for standing - normalized respiratory activity variation causes average heart activity. The presented approach allows data-driven and time-independent analysis of elite athletes as a particular population, without considering prior knowledge. However, the results seem to be consistent with the medical background. Causality inference is an interesting mathematical approach to the analysis of biological responses, which are complex. One can use it to profile athletes and plan appropriate training. In the next step, we plan to expand the study using time-related causality analyses.
1. Introduction
Elite athletes require regular physiological and medical evaluation and monitoring for proper planning of the schedule, intensity, and volume of training (Meeusen et al., 2013). Therefore, exercise scientists and sports physicians seek convenient biomarkers to evaluate the state of an athletes body during training to monitor homeostasis, maximize effect, and avoid over-training (Wiewelhove et al., 2015).
Some of the most commonly used parameters are related to cardiac function. Heart rate monitoring is popular in sport and recreational activity, and widely used thanks to easy access to sophisticated tools, enabling beat-by-beat registration of electrocardiographic (ECG) signals and evaluation of heart rate variability (HRV) (Buchheit, 2014; Schmitt et al., 2015; Bellenger et al., 2016; Duking et al., 2016; Giles et al., 2016; Plews et al., 2017).
Methodical acquisition of RR intervals, performed during different training periods, provides a chance to discern the proper course of HRV changes under the influence of exercise training, and possibly to recognize anomalous patterns indicating poor post-exercise recovery, sustained fatigue, impaired adaptation, and development of over-training syndrome.
Despite wide access, practical application of HRV parameters in sports training monitoring remains limited. The seemingly simple phenomenon, related to autonomic nervous system activity, defies simple evaluation, because of many modifying factors. An additional problem is the selection of optimal HRV parameters. There is no clear consensus as to which are best in training response evaluation. Is a single parameter enough, or will a set be more effective? Should more advanced mathematical methods be used for optimal modeling? There is growing interest in this field and recent studies have identified new directions (Sala et al., 2016, 2017).
Because there is a complex relationship between heart rate and breathing, taking breathing activity into account seems relevant and necessary for proper analysis. (Grossman and Taylor, 2007; Gasior et al., 2016; Sobiech et al., 2017). The influence of inspiration and expiration is usually apparent in resting ECG as sinus respiratory arrhythmia (Larsen et al., 2010; Shaffer et al., 2014; McCraty and Shaffer, 2015). Bidirectional neural relationships between cardiac functioning and the respiratory processes have previously been presented. The cardiorespiratory coupling effect, where heartbeats seem to coincide with specific respiratory phases, has been tested recently (Penzel et al., 2016; Sobiech et al., 2017). The possible physiological mechanisms behind it appear to include increased sympathetic nervous activity, as well as changes in arterial blood pressure. The effect of baroreflex, with baroreceptors playing a crucial role in the adjustment of neural responses, was also extensively described (Reyes del Paso et al., 2013).
The studies present concerns about whether respiratory control should be conducted in the HRV analysis. For example, Saboul et al. (2013) found that the RMSSD index is uninfluenced by respiratory patterns, for both spontaneous and controlled breathing. Nevertheless, the relations are more evident when different mathematical analyses are performed.
Therefore, the identification of an optimal mathematical method has an essential role in the daily practice. Usually, in sports science research methodology, a parameter's value is a dependent variable of exercise stimuli (top-down approach). However, in practical applications, the strategy can be reversed, with the optimal parameter as the one which best indicates and describes adaptation status (bottom-up approach). The relation between perception and performance outcomes can be “correlated” with the results of the objective analysis. For instance, an over-training syndrome is a major issue that negatively affects an athlete's performance, but it is not always subjectively felt in the same way.
In that context, one should consider evaluating cross-dependencies or even causalities in recorded signals and calculated parameters. Causality is then the way to describe the relationships, also specifying the direction and the structure. Causal relations can be established as a directed acyclic graph (DAG), the type of Bayesian network, in which each node can represent a parameter, and each directed link has a probability measure. Also, the graph may be rewritten into structural equation models (SEM) to get coefficients, which express the strength of connections. For such DAGs, the do-calculus rules theorem was introduced to analyze the effects of interventions (Pearl, 1995).
Other frameworks to analyze different types of causalities in physiological signals have been also proposed (Javorka et al., 2016; Müller et al., 2016; Penzel et al., 2016). Non-linear approaches, assuming cardiorespiratory interactions, were developed (Jamsek et al., 2004; Lopes et al., 2011). Relatively newly recognized phenomena—phase synchronization between heartbeats and ventilatory signal, inverse respiratory sinus arrhythmia—have also been used (Bartsch et al., 2015; Kuhnhold et al., 2017; Mazzucco et al., 2017). Information domain applications for more than three signals were presented (Wejer et al., 2017). Granger-based causality was employed (Porta et al., 2017), and also tested along with coherence measure and cross-sample entropy (Radovanović et al., 2018). Cardiorespiratory coordination, proposed by Moser et al. (1995), defines the mutual influence of the onsets of cardiac and respiratory cycles on each other. Various methods were proposed to analyze this phenomenon (Riedl et al., 2014; Sobiech et al., 2017; Valenza et al., 2018).
We hypothesize, that the main athletic rationale for causal path discovery is to profile athletes within a new causal domain, plan training modifications—considered to be interventions done to found cause variables (Pearl, 2010), and track changes in objectivecardiorespiratory responses. However, the methods mentioned in the previous paragraph are specifically intended to describe the systems' mutual temporal activity, not to propose directly the possible changes to training and causal-related parameters. We think that such approaches should be preceded by a more general one, which takes into account the parameters describing the entire segment of data, and tries to search for the structure of directional relationships.
Therefore, in this paper, we seek general time-independent causal paths between basic cardiac and respiratory variables in certain population, elite athletes, in two body positions (supine and standing) at rest.
2. Materials and Methods
2.1. Subjects and Device
A group of 116 elite athletes (38 female; ages 24.4 ± 6.3) participated. Due to artifacts in signals gathered during examination, resulting from body movement and imprecise electrode mounting, data from 16 athletes could not be reasonably analyzed and were therefore excluded. The final study group consisted of 100 athletes (32 female; ages 24.6 ± 6.4).
The study was carried out at the National Centre for Sports Medicine in Warsaw during the routine periodic health evaluation and medical monitoring program, 3–4 months before the 2016 Olympic Games in Rio de Janeiro. The study group comprised athletes in sports of differing type and intensity. Data on sex, height, and body mass in specific sports are presented in Table 1.
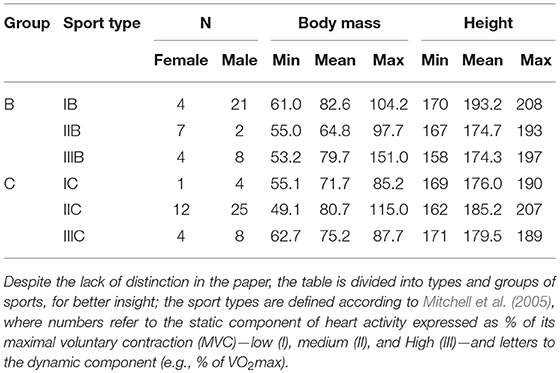
Table 1. The information of the set of participants evaluated after excluding those with too much signal distortion.
The study, including the consent procedure, was approved by the Ethics Committee of Warsaw Medical University (permission AKBE/74/17). All participants were informed about the general aim of the measurements, though not about the importance of breathing activity (Mortola et al., 2016). Each subject had previously signed a consent form for the routine medical monitoring, which includes a statement of acceptance of the use of the results for scientific purposes.
Pneumonitor 2 was used to collect single-lead ECG signals (Lead 2), along with impedance pneumography (IP), which is related to respiratory activity (Młyńczak et al., 2017). The IP signal was measured using the tetrapolar method, with the specified electrode configuration (Seppa et al., 2013). Receiving electrodes were placed on the mid-axillary line at about 5th-rib level. Application electrodes were positioned on the same level on the insides of the arms. Standard Holter-type, disposable ECG electrodes were used. The sampling frequency was 250 Hz, sufficient in terms of heart rate variability analysis and over-sampled from a respiratory perspective (Task Force, 1996).
2.2. Protocol and Preprocessing
The measurements were performed in a diagnostic room designated for cardiological examinations. Since the periodic health evaluation and medical monitoring are performed frequently for Olympic-level athletes, the diagnostic room and the measurement procedure (very similar to that for resting ECG) were familiar to them.
Each athlete was asked to lie down on the diagnostic (ECG) couch. After attachment of the electrodes and passage of a 10-min stabilization phase, they were asked to remain supine and breathe freely (spontaneous breathing), and recording began. After 6 min, the athlete was asked to stand and again breathe freely while standing for another 6 min (Gilder and Ramsbottom, 2008; Sala et al., 2016). The duration of analysis (about 6 min for each body position) seems appropriate for characterizing cardiac and respiratory activities in a “single” measurement. This is consistent with the method used in other studies, with HRV measurement in the supine position followed by the standing position (Gilder and Ramsbottom, 2008; Sala et al., 2016). While this resembles the orthostatic maneuver, we took the latter only as an inspiration, performing the analysis for the entire supine and standing periods, i.e., without consideration of adaptation, recovery, etc. Physical data (height, body mass, and sex) were registered during the routine medical examination performed the same day.
Pre-processing of the obtained ECG signal consisted of non-linear detrending for baseline alignment and finding R peaks based on the Pan-Tompkins algorithm. Raw IP signals were pre-processed by removing the cardiac component from the IP signal by subtracting the noise component derived from least mean square adaptive filtration, then smoothing with a 400 ms averaging window (Młyńczak and Cybulski, 2017). Then, we detected and delimited breathing phases by applying an adaptive algorithm to the differentiated, flow-related signal. We did not carry out the calibration procedure to transform impedance values into volumes, instead assuming that impedance changes had reproduced the tidal volume signal in terms of shape since linear fitting provides the best agreement between IP and the reference, pneumotachometry (PNT) (Młyńczak et al., 2015).
We finally considered 10 cardiac and respiratory parameters, estimated for each participant, for the entire recording, separately for supine and standing:
• Mean heart rate (HR);
• Root-mean-square difference of successive RR intervals (RMSSD);
• Natural logarithm thereof (lnRMSSD);
• Mean respiratory rate (RR);
• ciRR—coefficient of variation of instantaneous breathing rate (iRR, calculated between inspiratory onsets);
• cInsT—coefficient of variation of the durations of the inspiratory phases (InsT);
• cExpT—coefficient of variation of the durations of the expiratory phases (ExpT);
• cInsV—coefficient of variation of the amplitudes of the inspiratory phases (InsV);
• cExpV—coefficient of variation of the amplitudes of the expiratory phases (ExpV); and
• Breathing regularity (BR), as described in formula (1) - tanh operations are added to ensure that a range of 0–100% is preserved).
The differences between body positions were assessed using the paired T or Wilcoxon rank tests (depending on the normality of the parameters, checked using the Shapiro-Wilk test; all with a significance level of α = 0.05). Signal processing was performed using MATLAB software. Graphics and statistical inference were obtained using R software (R Core Team, 2018). The dataset of parameters and the R script are provided as Data Sheet 1 and Data Sheet 2, respectively, to ensure reproducibility.
2.3. Time-Independent Causal Path Discovery
We started with the assumption that the general time-independent causality can be revealed only when the correlation appears meaningful. Therefore, we calculated the Bayesian correlation coefficient as a result of the multiplication of the slope coefficient from the linear model and the ratio of standard deviations of both vectors. Assuming
estimated using Bayesian approach, then
The significance was assumed when the maximum probability of effect MPE > 0.9 (from the estimation of the linear model) (Makowski, 2018).
Then, we studied all pairs using six techniques:
• The generalized correlations and , with suggesting that y is more likely to be the “kernel cause” of x (though only when the p-value is significant) - equation (4) below implements the generalized correlation (Vinod, 2017) using the generalCorr R package (Vinod, 2018);
• Causal additive modeling, with selGAM pruning (Buhlmann et al., 2014), using the CAM R package (Peters and Ernest, 2015);
• Fast Greedy Equivalence Search for continuous variables (Ramsey, 2015) using the rcausal R package (Wongchokprasitti, 2017);
• Greedy Fast Causal Inference for continuous variables (Ogarrio et al., 2016) using the rcausal R package (Wongchokprasitti, 2017);
• Hill-Climbing—score-based Bayesian network learning algorithms—using the bnlearn R packaged (Scutari and Lebre, 2013); and
• Tabu Search, a modified hill-climbing algorithm able to escape local optima, using the bnlearn R package (Scutari and Lebre, 2013).
where rxy is the Pearson's correlation coefficient, var is variance, and the expression inside the square root is a generalized measure of correlation (GMC) defined in Zheng et al. (2012).
Finally, where possible, exploratory mediation analyses were conducted using the medmod R package (Selker, 2017). Sobel tests were performed to evaluate the significance of also considering the mediation effect, using the powerMediation R package (Qiu, 2018).
3. Results
3.1. Statistics and Impact of Body Position
Figures 1–10 provide exploratory summaries (using violin and box plots) across body positions for all considered parameters, along with the paired test results. All parameters have statistically significant differences between body positions. As expected, HR was greater when standing; the reverse was true for RMSSD along with all respiratory parameters.
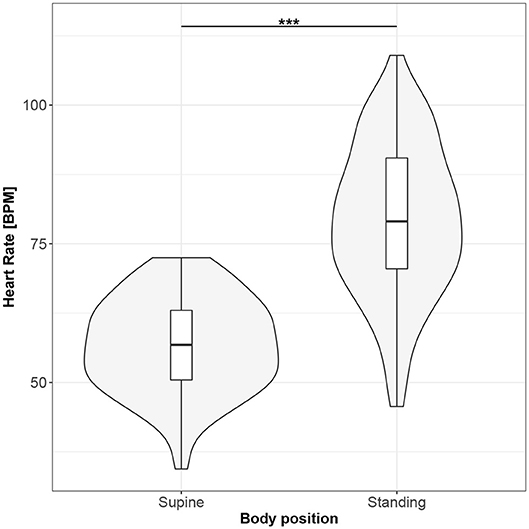
Figure 1. The exploratory statistic of mean heart rate (HR) for the supine and standing body positions, along with the T paired test result. ***means statistical significance at the level p < 0.001.
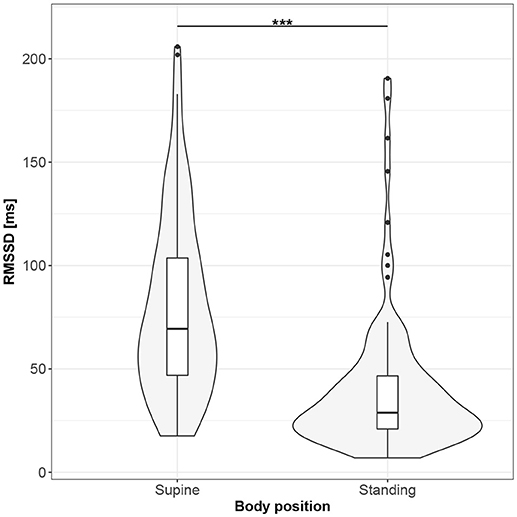
Figure 2. The exploratory statistic of root-mean-square difference of successive RR intervals (RMSSD) for the supine and standing body positions, along with the T paired test result. ***means statistical significance at the level p < 0.001.
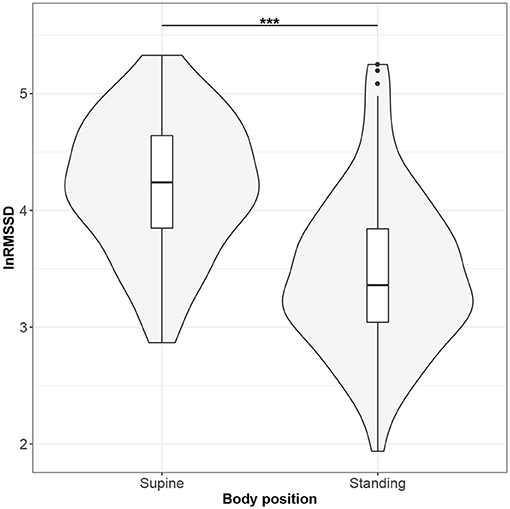
Figure 3. The exploratory statistic of natural logarithm of root-mean-square difference of successive RR intervals (lnRMSSD) for the supine and standing body positions, along with the Wilcoxon rank paired test result. ***means statistical significance at the level p < 0.001.
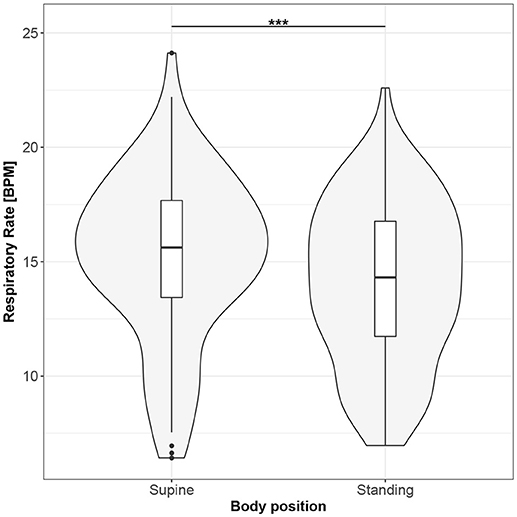
Figure 4. The exploratory statistic of mean respiratory rate (RR) for the supine and standing body positions, along with the T paired test result. ***means statistical significance at the level p < 0.001.
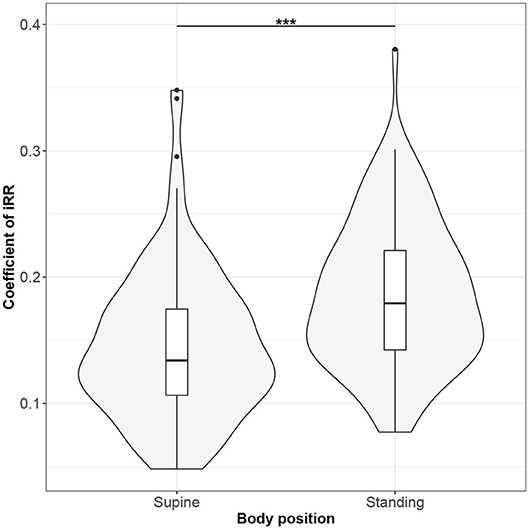
Figure 5. The exploratory statistic of standard deviation of instantaneous breathing rate (calculated between inspiratory onsets - iRR), normalized to mean iRR, for the supine and standing body positions, along with the Wilcoxon rank paired test result. ***means statistical significance at the level p < 0.001.
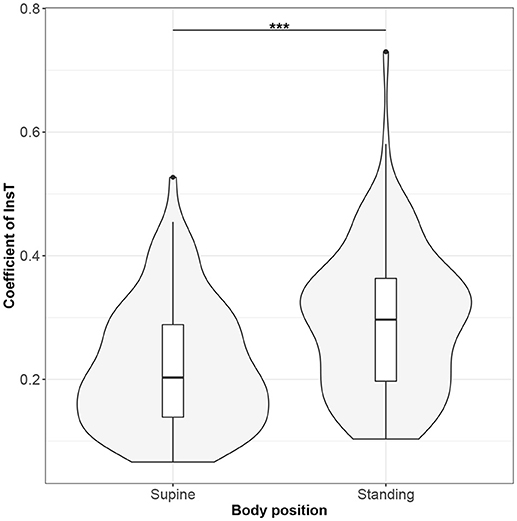
Figure 6. The exploratory statistic of standard deviation of the durations of inspiratory phases (InsT), normalized to mean InsT, for the supine and standing body positions, along with the Wilcoxon rank paired test result. ***means statistical significance at the level p < 0.001.
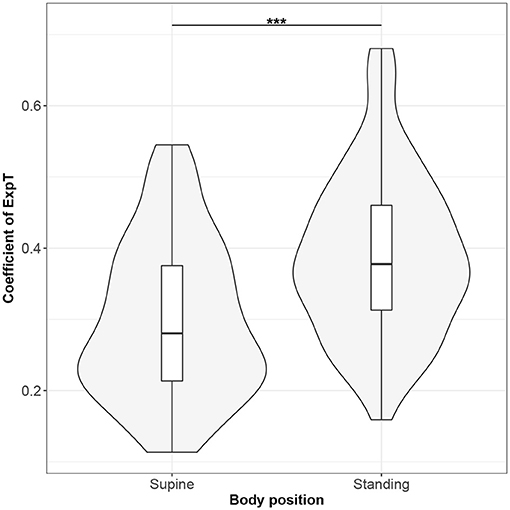
Figure 7. The exploratory statistic of standard deviation of the durations of expiratory phases (ExpT), normalized to mean ExpT, for the supine and standing body positions, along with the Wilcoxon rank paired test result. ***means statistical significance at the level p < 0.001.
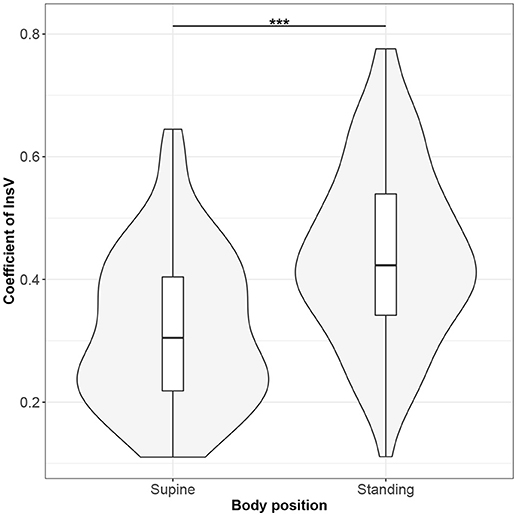
Figure 8. The exploratory statistic of standard deviation of the amplitudes of expiratory phases (InsV), normalized to mean InsV, for the supine and standing body positions, along with the Wilcoxon rank paired test result. ***means statistical significance at the level p < 0.001.
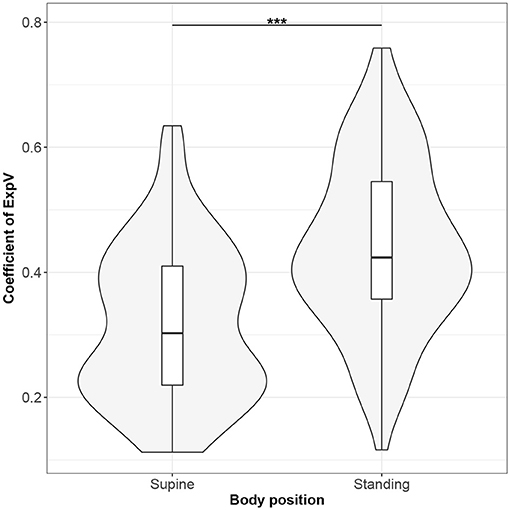
Figure 9. The exploratory statistic of standard deviation of the amplitudes of expiratory phases (ExpV), normalized to mean ExpV, for the supine and standing body positions, along with the Wilcoxon rank paired test result. ***means statistical significance at the level p < 0.001.
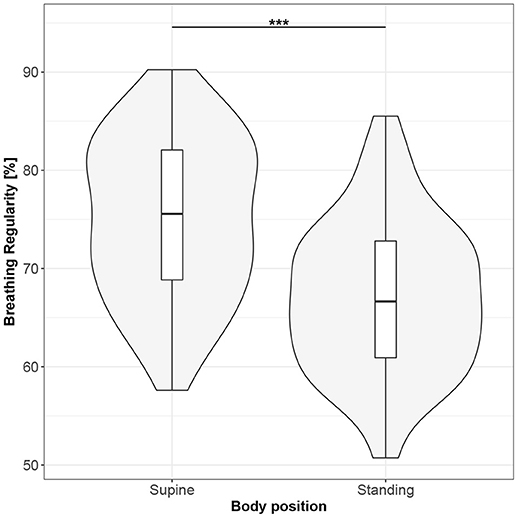
Figure 10. The exploratory statistic of breathing regularity (BR) for the supine and standing body positions, along with the Wilcoxon rank paired test result. ***means statistical significance at the level p < 0.001.
The significant Bayesian correlation coefficients are presented in Table 2. Results for supine are above the diagonal; for standing - below. Low correlations between cardiac and respiratory parameters (bottom left and top right corners of the table) suggested moderate connections between the analyzed parameters.
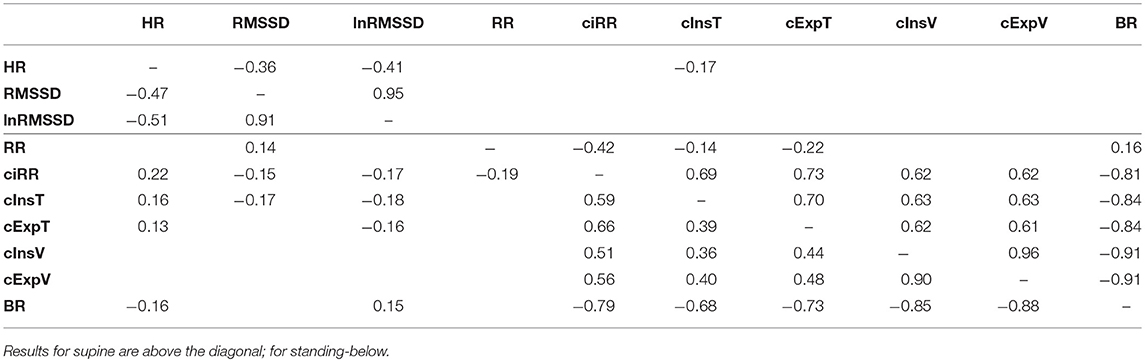
Table 2. Bayesian correlation coefficients calculated between parameters and presented when significant.
3.2. Causal Paths Discovery and Mediation Analysis
The discovered causal paths (ignoring the relationships between RMSSD and lnRMSSD, and between BR and its input coefficients) for the supine and standing positions are presented in Figures 11–16, separately for each of the considered methods.
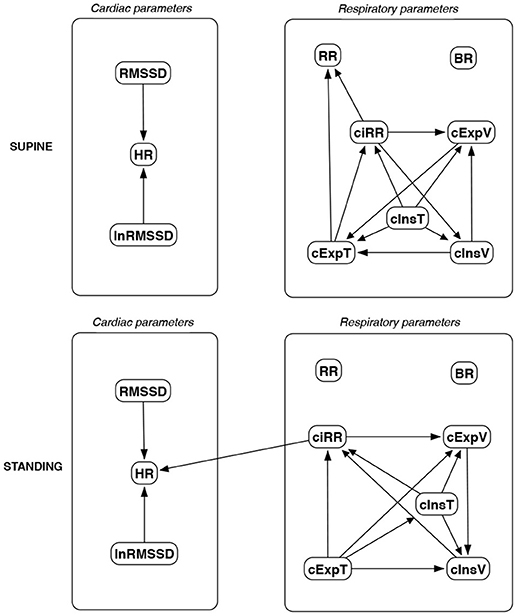
Figure 11. Causal paths discovered for supine and standing body positions using generalized correlations (GC); relationships between RMSSD and lnRMSSD, and between BR and its input coefficients, are ignored.
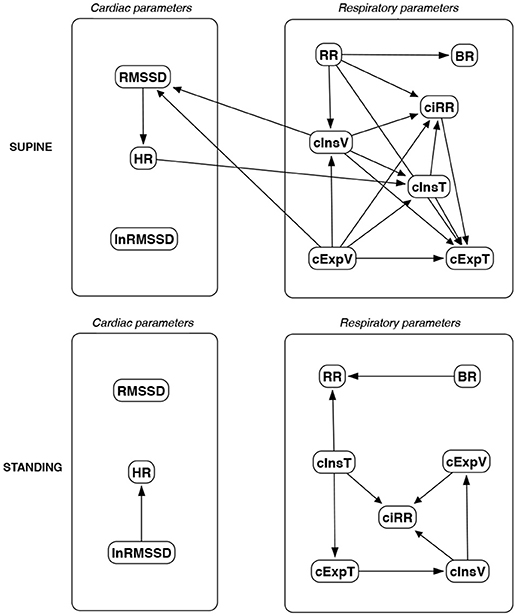
Figure 12. Causal paths discovered for supine and standing body positions using causal additive modeling (CAM); relationships between RMSSD and lnRMSSD, and between BR and its input coefficients, are ignored.
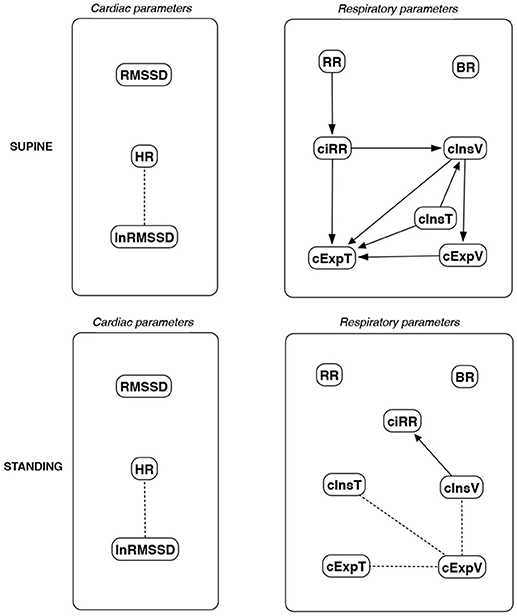
Figure 13. Causal paths discovered for supine and standing body positions using fast greedy equivalence search (FGES); relationships between RMSSD and lnRMSSD, and between BR and its input coefficients, are ignored.
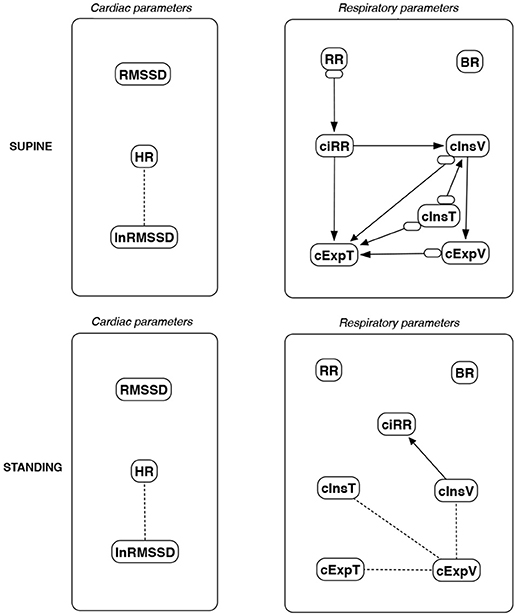
Figure 14. Causal paths discovered for supine and standing body positions using greedy fast causal inference (GFCI); relationships between RMSSD and lnRMSSD, and between BR and its input coefficients, are ignored. Inclined circles at the beginnings of arrows indicate either a presented direction, an unmeasured confounder, or both.
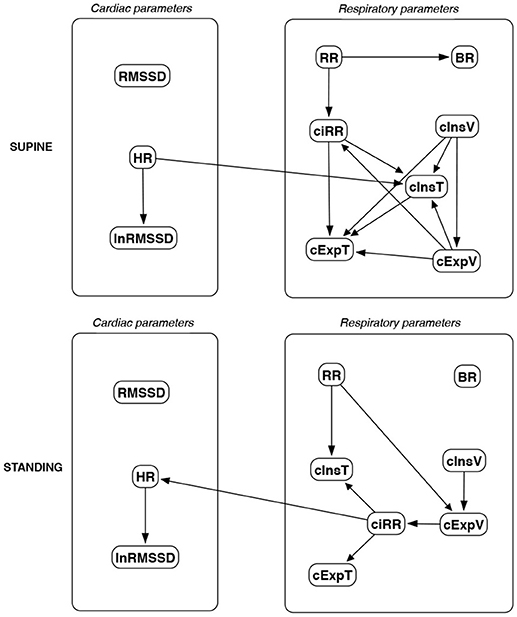
Figure 15. Causal paths discovered for supine and standing body positions using the Hill Climbing Bayesian network learning algorithm (HC); relationships between RMSSD and lnRMSSD, and between BR and its input coefficients, are ignored.
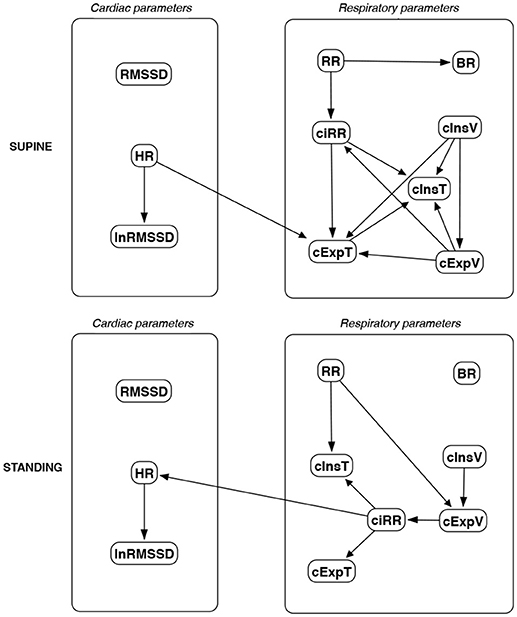
Figure 16. Causal paths discovered for supine and standing body positions using Tabu Search (TS); relationships between RMSSD and lnRMSSD, and between BR and its input coefficients, are ignored.
Th connections between cardiac parameters seem equivocal because GC and CAM suggested the direction from RMSSD to HR for both body positions, the greedy algorithms (FGES and GFCI) could not determine the direction, while the Bayesian network methods (HC and Tabu) recommended assuming that the right direction is the opposite.
For respiratory parameters and supine body positions, 5 of the 6 methods showed that cInsT causes cExpT, and 5 out of 6 also that cInsV causes cExpV. Furthermore, all methods indicate that cExpV causes cExpT, and that cInsV causes cExpT.
The findings for the standing body position were different. Only 3 of the 6 methods confirmed the direction from cInsV to cExpV (2 were ambiguous). InsT seems to be connected much more weakly with ExpT. cInsV and cExpV appear not to cause cExpT to the same extent as for the supine position. Several loops are present between cInsV, cExpV, and ciRR. One should note another connection, in which cInsV causes ciRR, indirectly or directly, via cExpV.
Three methods propose four connections between cardiac and respiratory parameters in supine body positions:
• cInsV → RMSSD (CAM),
• cExpV → RMSSD (CAM),
• HR → cInsT (CAM and HC), and
• HR → cExpT (Tabu).
These all indicate the occurrence of a relatively complex connection even in the most static cases. The relationships appear to be weak. Several paths can be created (in parallel); however, we think one of them may suggest the general direction for cardiorespiratory data during supine rest:
Tidal Volume →Heart Activity Variation → Average Heart Activity → Respiratory Timing
Three methods (GC, HC, and Tabu) indicate the connection ciRR → HR for the standing body position, implying the general rule for standing to be:
Normalized Respiratory Activity Variation → Average Heart Activity
Finally, we set several paths to test for mediation effects:
• (1) RMSSD → HR → cInsT (supine),
• (2) HR → cInsT → cExpT (supine),
• (3) HR → cInsT → cInsV (supine),
• (4) cInsT → ciRR → HR (standing), and
• (5) cInsV → ciRR → HR (standing).
The Sobel p-values for the analyzed mediations are presented in Table 3. While none of the considered connections have a statistically significant mediation effect, the p-values suggest tendency. It appears that the nature of these links is more non-linear, still being very light in terms of mutual correlations.
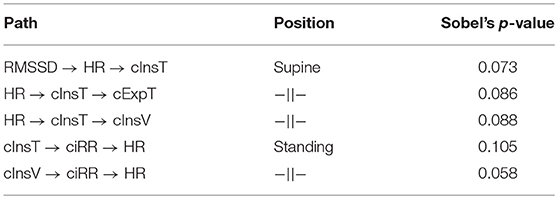
Table 3. The Sobel's p-values assessing the significance of the mediation effect for the chosen causal path connections discovered for the supine and standing body positions.
4. Discussion
The main finding from our analysis is that, for the supine body position and in the elite athletes group, tidal volume seems to cause heart activity variation, then the latter causes average heart activity, which appears to affect the timing of inspiratory and expiratory phases. The relations are mild and this statement is not supported by all methods, which is not to say that any oppose it. For the standing body position, the causal relations are weaker. The most important remains that in which normalized respiratory activity variation causes average heart activity. On the other hand, for these conditions, more of the cross-correlations between cardiac and respiratory parameters were statistically significant.
This suggests the need to consider activity measures from both systems; however, in the common practice, only ECG analyses are usually carried out. The simplest, but still very informative, parameters of heart activity are mean heart rate and root-mean-square difference of successive RR intervals. The first enables study of the average value of the rhythm, while the other shows its diversity.
The concept of using HRV-related data in sports analysis has been already proposed in many applications, e.g.:
• Quantitative assessment of training load (Saboul et al., 2016),
• Monitoring of weekly HRV in futsal players during the preseason to evaluate high vagal activity (Nakamura et al., 2016),
• Progressive sympathetic predominance at peak training load as a performance prediction factor in recreational marathon runners (Triposkiadis et al., 2009),
• Parasympathetic modulation elevation over a 24 h recording period induced by endurance and athletic activities (Vanderlei et al., 2008),
• Assessment of the parasympathetic tone resulting from training (Berkoff et al., 2007),
• Analysis of over-training syndrome (Dong, 2016), and
• Analysis of training adaptation (Plews et al., 2013).
However, in the presented works, the authors did not consider adding breathing activity to the analysis. Therefore, we performed the study with a device allowing recording of ventilation with minimal disruption of said activity. The Pneumonitor 2 was used to measure changes in thoracic impedance, which is related to changes in the amount of air in the lungs (Młyńczak et al., 2017). From that information, we estimated the average respiratory rate, along with five coefficients specifying the deviation of the respiratory rate, inspiratory and expiratory phase durations and amplitudes (related to volumes), allowing creation of a novel index, breathing regularity. Consequently, we parameterized cardiac and respiratory activity with indexes, which estimate a mean value and variation.
This approach connected with findings for supine body position create an interesting cardiorespiratory loop between systems. Several studies proposed to consider multi-directionality in the coupling of the cardiovascular and respiratory systems (Porta et al., 2013; Platisa et al., 2016; Radovanović et al., 2018). More importantly, the relation seems to combine (in terms of a specific mathematical framework) several physiological mechanisms. The first “arrow” indirectly describes the RSA phenomenon (Shaffer et al., 2014; McCraty and Shaffer, 2015). Some have already observed that the respiratory centers can modulate the frequency of the heart through the vagal sinus node intervention (Eckberg, 2009).
The last connection appears related to cardiorespiratory coupling, described by Sobiech et al. (2017) as a relation between the histograms of R peaks appearing before the inspiratory onset and of peaks appearing just after.
The lack of a direct “arrow” from heart activity variation, namely RMSSD in this study, to breathing corresponds with the findings of Saboul et al. (2013) and Sala et al. (2016), wherein this index did not correlate with breathing, neither spontaneous nor controlled.
In that context, causality inference is a promising mathematical tool to expand the current analytical framework. Complex biological responses appear to be the right input, even when results are quite diverse.
All the techniques mentioned in the Introduction are based on pre-processed time series or beat-by-beat sequences. They allow evaluation of different time resolutions, in order to focus on the specific condition and subject. We believe that this is the right approach, and that for a better grasp of how to prepare a sport-sensitive biomarker, it should be preceded by a more general approach. The approach would be time-independent, more holistic (considering a whole group of participants), and not based on prior medical knowledge. As the causality frameworks were originally introduced to deal with interventional variables, they can be even used for prediction, e.g., when several changes in a training program can be interpreted as changes to the system. As this is a retrospective study, it is also assumed that interventions are impossible, and the networks are created with no prior knowledge. Causal search relies on passive observation.
It appears debatable whether correct causal explanations can be chosen only by looking at observed data. On the other hand prior knowledge would enable acquisition of answers to causal questions without performing interventions (still difficult to manage from physiological perspective). Here, the context is different. We believe that causal analysis and cardiorespiratory relation can be a relevant supplement to already-established techniques and play the role of a biomarker (or its part) for establishing the state of the athlete. In this introductory approach, we assumed the single parameters describe specific subjects, and the analysis collects them all together.
The proposed protocol is inspired by the orthostatic maneuver, but the analysis for each positions is independent. Moreover, we hold that, from a causal discovery perspective, one can consider the entire segment of the signal, instead of subsections like the adaptation after standing up. Looking at the entire segment makes the analysis simpler from an operators perspective.
As the estimated causal structures seem mild, analyzing supine-to-standing changes would make the method more robust (by considering changes in intrathoracic pressure or differences in venous return characteristics). Radovanović et al. (2018) reported that even slight change of body position may change the direction of the relationships. Sobiech et al. (2017) also suggested that the results can be reliably analyzed only during static conditions and that the effect is the strongest in a resting state. However, in our opinion, the differences between body positions may have a significant impact on the analysis of cardiorespiratory data and should not be ignored. These differences, established for two body positions, may serve as an additional input for determination of the adaptation profile.
The effect observed for standing body position has already been studied by Sala et al. (2016), who analyzed on a basic level the effectiveness of assessment of the balance change in the autonomic system during active verticalization. They observed that this change should be stronger in athletes and assumed that it is associated with improved physical capacity. Apart from that, increased coupling between heart period and arterial pressure in response to postural changes was reported in Silvani et al. (2017).
4.1. Use of Causal Discovery Framework
The findings presented in this study open up a novel domain of possible parameterization of physiological connections. We think the framework can be used to profile athletes, analyzing trends throughout the training schedule and testing changes in relations' direction and strength relative to improved adaptation, etc.
Causal inference, even if used to search for graphs between analyzed parameters, was originally proposed to evaluate the effects of interventions, dividing variables into interventional and observational sets. In both sports medicine and daily practice, modification of the training can be regarded as such an intervention.
The paths discovered by the various algorithms should be reconciled with medical background knowledge and, even more importantly, should be verified further in a prospective study. With so many possible interventions, the paths may reduce the complexity of test protocols: one might expect to influence heart rate and its variability by changing the depth, not the frequency, of breathing.
4.2. Limitations of the Study
There were only 116 participants, of whom 100 were ultimately considered. The study group appears to be heterogeneous, apart from the fact that the studies were conducted in the “hot period” 3–4 months before the Olympic Games, which may suggest a state of over-training (neither questionnaires nor objective data about it were collected). Also, the device was new for all subjects. These factors might influence the results, particularly the relatively high supine respiratory rates and standing heart rate. The existence of differences between sports would also affect the conclusions. The collection of only one observation per athlete precludes reproducibility analysis.
There were no control variables assumed in the protocol. One may consider a respiratory activity to be controlled during the measurement. However, the primary objective of the registration was to measure cardiorespiratory data without any restrictions to the respiratory activity. As this is a retrospective study, we could not affect it during the analysis. This may be one of the reasons why the studied relationships were so mild.
Measurements were carried out only once for each subject, and only at rest, not in a natural environment. It is necessary to perform comparative registrations on a unified group of athletes under laboratory conditions, during the preparatory period and just before the season, in combination with a psychometric questionnaire. The results of a study in which the registration could be performed outside the laboratory, during normal training, or even with 24 h Holter-based tracking of natural functioning, would yield more condensed findings.
Notably, the methods used to discover causal paths sense different aspects (the algorithms having different starting points) and can produce more liberal or conservative results. As only the “significant” pairs of causal-effect links were presented, possible bias cannot be evaluated directly. As the relevant analysis could not be performed for a few participants, we decided not to divide participants by sex or sport type.
4.3. Further Considerations
In the presented work, we use a data-driven and time-independent approach (on a large inter-individual scale). It is considered as a starting point. As stated in the introduction, the presented approach seems to be an overview, to be performed before time-dependent methods which may allow the separate assessment of a single person, with finer time resolution. Therefore, as a next step, we plan to discover various non-linear parameters and techniques to confirm, expand and enhance the findings presented in this paper. Still, the main outcomes are coherent with the knowledge-based predictions and results reported, e.g., in Sobiech et al. (2017).
Eckberg showed that the RSA phenomenon is not only the effect of respiration on RR intervals (or heart rate in general) but might be also treated as the response of heart rate to the respiratory modulations resulting from arterial pressure changes mediated by baroreflex (Eckberg, 2009). Sobiech et al. (2017) suggested that arterial blood pressure is probably the driver (cause) of both cardiac and respiratory function. This requires further research with more modalities included in the analysis (Zhang et al., 2017).
Other important questions include: Are the cardiorespiratory connections (direction and strength) the same in different sports, or specific to each? What other factors, like sex, height, or body mass, confound the results? These questions are especially important not only for elite athletes, but also for subjects with abnormalities, and will be studied (Sharma et al., 2018).
5. Conclusions
Adding respiratory data to cardiac signals in sports medicine would provide better monitoring and evaluation of athletic training.
In the presented paper, we pose the hypothesis that average or diversity cardiac and respiratory parameters, describing overall characteristics of RR intervals and tidal volume curves, without consideration of the time resolution, are linked by causal paths. Furthermore, such an approach can precede more detailed, individual, time-series-related analysis.
Based on the data gathered from 100 elite athletes at rest in two body positions, the applied causal discovery frameworks suggested moderate connections. For supine, the general path led from tidal volume, through heart activity variation and average heart activity, to respiratory timing. For standing - from normalized respiratory activity variation to average heart activity. Different graphical structures and directions were observed for the two body positions, which may improve the resolution of the findings.
We think posterior-style causal inference and characterization may develop descriptions of cardiorespiratory connections and possibly distinguish between various groups of athletes. The method can be used to profile athletes, elaborate on modifications of their training schedules, and find objective ways to improve their competitive performance.
Author Contributions
MM and HK worked on the conceptualization, investigation, methodology, project administration, validation, and writing and reviewing. MM worked on data curation, formal analysis, and visualization. HK provided the resources.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Martin Berka for linguistic adjustments. MM gratefully acknowledges the Institute of Metrology and Biomedical Engineering of the Faculty of Mechatronics, Warsaw University of Technology, for supporting this work.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2018.01455/full#supplementary-material
Data Sheet 1. The data file with cardiac and respiratory parameters calculated from all participants for the two body positions.
Data Sheet 2. The R script comprising all algorithms mentioned in the text, from loading of the above data to delivery of final outcomes.
Refereces
Bartsch, R. P., Liu, K. K. L., Bashan, A., and Ivanov, P. C. (2015). Network physiology: how organ systems dynamically interact. PLoS ONE 10:e0142143. doi: 10.1371/journal.pone.0142143
Bellenger, C. R., Fuller, J. T., Thomson, R. L., Davison, K., Robertson, E. Y., and Buckley, J. D. (2016). Monitoring athletic training status through autonomic heart rate regulation: a systematic review and meta-analysis. Sports Med. 46, 1461–1486. doi: 10.1007/s40279-016-0484-2
Berkoff, D. J., Cairns, C. B., Sanchez, L. D., and Moorman, C. T. (2007). Heart rate variability in elite American track-and-field athletes. J. Strength Condition. Res. 21:227. doi: 10.1519/00124278-200702000-00041
Buchheit, M. (2014). Monitoring training status with hr measures: do all roads lead to Rome? Front. Physiol. 5:73. doi: 10.3389/fphys.2014.00073
Buhlmann, P., Peters, J., Ernest, J. (2014). Cam: Causal additive models, high-dimensional order search and penalized regression. Ann. Stat. 42, 2526–2556. doi: 10.1214/14-AOS1260
Dong, J. -G. (2016). The role of heart rate variability in sports physiology. Exp. Therapeut. Med. 11, 1531–1536. doi: 10.3892/etm.2016.3104
Duking, P., Hotho, A., Holmberg, H. -C., Fuss, F. K., and Sperlich, B. (2016). Comparison of non-invasive individual monitoring of the training and health of athletes with commercially available wearable technologies. Front. Physiol. 7:71. doi: 10.3389/fphys.2016.00071
Eckberg, D. L. (2009). Point: counterpoint: respiratory sinus arrhythmia is due to a central mechanism vs. respiratory sinus arrhythmia is due to the baroreflex mechanism. J. Appl. Physiol. 106, 1740–1742. doi: 10.1152/japplphysiol.91107.2008
Gasior, J. S., Sacha, J., Jelen, P. J., Zielinski, J., and Przybylski, J. (2016). Heart rate and respiratory rate influence on heart rate variability repeatability: effects of the correction for the prevailing heart rate. Front. Physiol. 7:356. doi: 10.3389/fphys.2016.00356
Gilder, M. and Ramsbottom, R. (2008). Change in heart rate variability following orthostasis relates to volume of exercise in healthy women. Auton. Neurosci. 143, 73–76. doi: 10.1016/j.autneu.2008.06.002
Giles, D., Draper, N., and Neil, W. (2016). Validity of the polar v800 heart rate monitor to measure RR intervals at rest. Eur. J. Appl. Physiol. 116, 563–571. doi: 10.1007/s00421-015-3303-9
Grossman, P. and Taylor, E. W. (2007). Toward understanding respiratory sinus arrhythmia: relations to cardiac vagal tone, evolution and biobehavioral functions. Biol. Psychol. 74, 263–285. doi: 10.1016/j.biopsycho.2005.11.014
Jamsek, J., Stefanovska, A., and McClintock, P. V. E. (2004). Nonlinear cardio-respiratory interactions revealed by time-phase bispectral analysis. Phys. Med. Biol. 49:4407. doi: 10.1088/0031-9155/49/18/015
Javorka, M., Czippelova, B., Turianikova, Z., Lazarova, Z., Tonhajzerova, I., and Faes, L. (2016). Causal analysis of short-term cardiovascular variability: state-dependent contribution of feedback and feedforward mechanisms. Med. Biol. Eng. Comput. 55, 179–190. doi: 10.1007/s11517-016-1492-y
Kuhnhold, A., Schumann, A. Y., Bartsch, R. P., Ubrich, R., Barthel, P., Schmidt, G., et al. (2017). Quantifying cardio-respiratory phase synchronizationa comparison of five methods using ECGs of post-infarction patients. Physiol. Meas. 38:925. doi: 10.1088/1361-6579/aa5dd3
Larsen, P., Tzeng, Y., Sin, P., and Galletly, D. (2010). Respiratory sinus arrhythmia in conscious humans during spontaneous respiration. Respir. Physiol. Neurobiol. 174, 111–118. doi: 10.1016/j.resp.2010.04.021
Lopes, T. C., Beda, A., Granja-Filho, P. C. N., Jandre, F. C., and Giannella-Neto, A. (2011). Cardio-respiratory interactions and relocation of heartbeats within the respiratory cycle during spontaneous and paced breathing. Physiol. Meas. 32:1389. doi: 10.1088/0967-3334/32/9/003
Makowski, D. (2018). The psycho package: an efficient and publishing-oriented workflow for psychological science. J. Open Source Softw. 3:470. doi: 10.21105/joss.00470
Mazzucco, C. E., Marchi, A., Bari, V., De Maria, B. D., Guzzetti, S., Raimondi, F., et al. (2017). Mechanical ventilatory modes and cardioventilatory phase synchronization in acute respiratory failure patients. Physiol. Meas. 38, 895–911. doi: 10.1088/1361-6579/aa56ae
McCraty, R., and Shaffer, F. (2015). Heart rate variability: new perspectives on physiological mechanisms, assessment of self-regulatory capacity, and health risk. Glob. Adv. Health Med. 4, 46–61. doi: 10.7453/gahmj.2014.073
Meeusen, R., Duclos, M., Foster, C., Fry, A., Gleeson, M., Nieman, D., et al. (2013). Prevention, diagnosis, and treatment of the overtraining syndrome: joint consensus statement of the european college of sport science and the American College of Sports Medicine. Med. Sci. Sports Exerc. 45, 186–205. doi: 10.1249/MSS.0b013e318279a10a
Mitchell, J. H., Haskell, W., Snell, P., and Van Camp, S. P. (2005). Task force 8: classification of sports. J. Am. Coll. Cardiol. 45, 1364–1367. doi: 10.1016/j.jacc.2005.02.015
Młyńczak, M. and Cybulski, G. (2017). “Decomposition of the cardiac and respiratory components from impedance pneumography signals,” in Proceedings of the 10th International Joint Conference on Biomedical Engineering Systems and Technologies (Porto: SciTePress), 26–33. doi: 10.5220/0006107200260033
Młyńczak, M., Niewiadomski, W., Żyliński, M., and Cybulski, G. (2015). Assessment of calibration methods on impedance pneumography accuracy. Biomed. Eng. 61, 587–593. doi: 10.1515/bmt-2015-0125
Młyńczak, M., Niewiadomski, W., Żyliński, M., and Cybulski, G. (2017). “Ambulatory devices measuring cardiorespiratory activity with motion,” in Proceedings of the 10th International Joint Conference on Biomedical Engineering Systems and Technologies (Porto: SciTePress), 91–97. doi: 10.5220/0006111700910097
Mortola, J. P., Marghescu, D., and Siegrist-Johnstone, R. (2016). Thinking about breathing: effects on respiratory sinus arrhythmia. Respir. Physiol. Neurobiol. 223, 28–36. doi: 10.1016/j.resp.2015.12.004
Moser, M., Lehofer, M., Hildebrandt, G., Voica, M., Egner, S., and Kenner, T. (1995). Phase-and frequency coordination of cardiac and respiratory function. Biol. Rhythm Res. 26, 100–111. doi: 10.1080/09291019509360328
Müller, A., Kraemer, J. F., Penzel, T., Bonnemeier, H., Kurths, J., and Wessel, N. (2016). Causality in physiological signals. Physiol. Meas. 37:R46. doi: 10.1088/0967-3334/37/5/R46
Nakamura, F. Y., Pereira, L. A., Rabelo, F. N., Flatt, A. A., Esco, M. R., Bertollo, M., et al. (2016). Monitoring weekly heart rate variability in futsal players during the preseason: the importance of maintaining high vagal activity. J. Sports Sci. 34, 2262–2268. doi: 10.1080/02640414.2016.1186282
Ogarrio, J. M., Spirtes, P., and Ramsey, J. (2016). “A hybrid causal search algorithm for latent variable models,” in Conference on Probabilistic Graphical Models, 368–379.
Pearl, J. (1995). Causal diagrams for empirical research. Biometrika 82, 669–688. doi: 10.1093/biomet/82.4.669
Pearl, J. (2010). The foundations of causal inference. Sociol. Methodol. 40, 75–149. doi: 10.1111/j.1467-9531.2010.01228.x
Penzel, T., Kantelhardt, J. W., Bartsch, R. P., Riedl, M., Kraemer, J. F., Wessel, N., et al. (2016). Modulations of heart rate, ecg, and cardio-respiratory coupling observed in polysomnography. Front. Physiol. 7:460. doi: 10.3389/fphys.2016.00460
Peters, J., and Ernest, J. (2015). CAM: Causal Additive Model (CAM). R package version 1.0. Available online at: https://cran.r-project.org/web/packages/CAM/CAM.pdf
Platisa, M. M., Bojic, T., Pavlovic, S. U., Radovanovic, N. N., and Kalauzi, A. (2016). Uncoupling of cardiac and respiratory rhythm in atrial fibrillation. Biomed. Eng. 61, 657–663. doi: 10.1515/bmt-2016-0057
Plews, D. J., Laursen, P. B., Stanley, J., Kilding, A. E., and Buchheit, M. (2013). Training adaptation and heart rate variability in elite endurance athletes: opening the door to effective monitoring. Sports Med. 43, 773–781. doi: 10.1007/s40279-013-0071-8
Plews, D. J., Scott, B., Altini, M., Wood, M., Kilding, A. E., and Laursen, P. B. (2017). Comparison of heart rate variability recording with smart phone photoplethysmographic, polar h7 chest strap and electrocardiogram methods. Int. J. Sports Physiol. Perform. 12, 1324–1328. doi: 10.1123/ijspp.2016-0668
Porta, A., Bari, V., Maria, B. D., Perseguini, N. M., Milan, J., Rehder-Santos, P., et al. (2017). Assessing the evolution of redundancy/synergy of spontaneous variability regulation with age. Physiol. Meas. 38:940. doi: 10.1088/1361-6579/aa5908
Porta, A., Castiglioni, P., Di Rienzo, M., Bassani, T., Bari, V., Faes, L., et al. (2013). Cardiovascular control and time domain granger causality: insights from selective autonomic blockade. Philos. Trans. R. Soc. A 371:20120161. doi: 10.1098/rsta.2012.0161
Qiu, W. (2018). powerMediation: Power/Sample Size Calculation for Mediation Analysis. R package version 0.2.9. Available online at: https://cran.r-project.org/web/packages/powerMediation/powerMediation.pdf
R Core Team (2018). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Radovanović, N. N., Pavlović, S. U., Milasinović, G., Kirćanski, B., and Platisa, M. M. (2018). Bidirectional cardio-respiratory interactions in heart failure. Front. Physiol. 9:165. doi: 10.3389/fphys.2018.00165
Ramsey, J. D. (2015). Scaling up greedy causal search for continuous variables. arXiv [preprint] arXiv:1507.07749. Available online at: https://arxiv.org/abs/1507.07749
Reyes del Paso, G. A., Langewitz, W., Mulder, L. J., Van Roon, A., and Duschek, S. (2013). The utility of low frequency heart rate variability as an index of sympathetic cardiac tone: a review with emphasis on a reanalysis of previous studies. Psychophysiology 50, 477–487. doi: 10.1111/psyp.12027
Riedl, M., Müller, A., Kraemer, J. F., Penzel, T., Kurths, J., and Wessel, N. (2014). Cardio-respiratory coordination increases during sleep apnea. PLoS ONE 9:e93866. doi: 10.1371/journal.pone.0093866
Saboul, D., Balducci, P., Millet, G., Pialoux, V., and Hautier, C. (2016). A pilot study on quantification of training load: The use of HRV in training practice. Eur. J. Sport Sci. 16, 172–181. doi: 10.1080/17461391.2015.1004373
Saboul, D., Pialoux, V., and Hautier, C. (2013). The impact of breathing on HRV measurements: implications for the longitudinal follow-up of athletes. Eur. J. Sport Sci. 13, 534–542. doi: 10.1080/17461391.2013.767947
Sala, R., Malacarne, M., Solaro, N., Pagani, M., and Lucini, D. (2017). A composite autonomic index as unitary metric for heart rate variability: a proof of concept. Eur. J. Clin. Invest. 47, 241–249. doi: 10.1111/eci.12730
Sala, R., Spataro, A., Malacarne, M., Vigo, C., Tamorri, S., Benzi, M., et al. (2016). Discriminating between two autonomic profiles related to posture in olympic athletes. Eur. J. Appl. Physiol. 116, 815–822. doi: 10.1007/s00421-016-3337-7
Schmitt, L., Regnard, J., and Millet, G. P. (2015). Monitoring fatigue status with hrv measures in elite athletes: an avenue beyond RMSSD? Front. Physiol. 6:343. doi: 10.3389/fphys.2015.00343
Scutari, M., and Lebre, S. (2013). Bayesian Networks in R: With Applications in Systems Biology. New York, NY: Springer Verlag. Available online at: https://www.springer.com/us/book/9781461464457
Selker, R. (2017). medmod: Simple Mediation and Moderation Analysis. R package version 1.0.0. Available online at: https://cran.r-project.org/web/packages/medmod/medmod.pdf
Seppa, V. P., Hyttinen, J., Uitto, M., Chrapek, W., and Viik, J. (2013). Novel electrode configuration for highly linear impedance pneumography. Biomed. Eng. 58, 35–38. doi: 10.1515/bmt-2012-0068
Shaffer, F., McCraty, R., and Zerr, C. L. (2014). A healthy heart is not a metronome: an integrative review of the heart's anatomy and heart rate variability. Front. Psychol. 5:1040. doi: 10.3389/fpsyg.2014.01040
Sharma, S., Drezner, J. A., Baggish, A., Papadakis, M., Wilson, M. G., Prutkin, J. M., et al. (2018). International recommendations for electrocardiographic interpretation in athletes. Eur. Heart J. 39, 1466–1480. doi: 10.1093/eurheartj/ehw631
Silvani, A., Calandra-Buonaura, G., Johnson, B. D., von Helmond, N., Barletta, G., Cecere, A. G., et al. (2017). Physiological mechanisms mediating the coupling between heart period and arterial pressure in response to postural changes in humans. Front. Physiol. 8:163. doi: 10.3389/fphys.2017.00163
Sobiech, T., Buchner, T., Krzesiski, P., and Gielerak, G. (2017). Cardiorespiratory coupling in young healthy subjects. Physiol. Meas. 38, 2186–2202. doi: 10.1088/1361-6579/aa9693
Task Force, E. S. C. (1996). Heart rate variability standards of measurement, physiological interpretation, and clinical use. Eur. Heart J. 17, 354–381.
Triposkiadis, F., Karayannis, G., Giamouzis, G., Skoularigis, J., Louridas, G., and Butler, J. (2009). The sympathetic nervous system in heart failure: physiology, pathophysiology, and clinical implications. J. Am. Coll. Cardiol. 54, 1747–1762. doi: 10.1016/j.jacc.2009.05.015
Valenza, G., Faes, L., Citi, L., Orini, M., and Barbieri, R. (2018). Instantaneous transfer entropy for the study of cardiovascular and cardiorespiratory nonstationary dynamics. IEEE Trans. Biomed. Eng. 65, 1077–1085. doi: 10.1109/TBME.2017.2740259
Vanderlei, L., Silva, R., Pastre, C., Azevedo, F. M. d., and Godoy, M. (2008). Comparison of the polar s810i monitor and the ECG for the analysis of heart rate variability in the time and frequency domains. Braz. J. Med. Biol. Res. 41, 854–859. doi: 10.1590/S0100-879X2008005000039
Vinod, H. D. (2017). Generalized correlation and kernel causality with applications in development economics. Communications in Statistics-Simulation and Computation 46, 4513–4534. doi: 10.1080/03610918.2015.1122048
Vinod, H. D. (2018). generalCorr: Generalized Correlations and Initial Causal Path. R package version 1.1.1. Available online at: https://cran.r-project.org/web/packages/generalCorr/generalCorr.pdf
Wejer, D., Graff, B., Makowiec, D., Budrejko, S., and Struzik, Z. R. (2017). Complexity of cardiovascular rhythms during head-up tilt test by entropy of patterns. Physiol. Meas. 38, 819–832. doi: 10.1088/1361-6579/aa64a8
Wiewelhove, T., Raeder, C., Meyer, T., Kellmann, M., Pfeiffer, M., and Ferrauti, A. (2015). Markers for routine assessment of fatigue and recovery in male and female team sport athletes during high-intensity interval training. PLoS ONE 10:e0139801. doi: 10.1371/journal.pone.0139801
Zhang, Z., Wang, B., Wu, H., Chai, X., Wang, W., and Peng, C. -K. (2017). Effects of slow and regular breathing exercise on cardiopulmonary coupling and blood pressure. Med. Biol. Eng. Comput. 55, 327–347. doi: 10.1007/s11517-016-1517-6
Keywords: athlete training adaptation biomarker, cardiac function, tidal volume, cardiorespiratory causality, elite athletes
Citation: Młyńczak M and Krysztofiak H (2018) Discovery of Causal Paths in Cardiorespiratory Parameters: A Time-Independent Approach in Elite Athletes. Front. Physiol. 9:1455. doi: 10.3389/fphys.2018.01455
Received: 09 July 2018; Accepted: 25 September 2018;
Published: 30 October 2018.
Edited by:
Toby Mündel, College of Health, Massey University, New ZealandReviewed by:
Jui-Lin Fan, University of Otago, New ZealandCristina Blasco-Lafarga, Universitat de Valencia, Spain
Anabel Forte Deltell, Universitat de Valencia, Spain
Copyright © 2018 Młyńczak and Krysztofiak. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marcel Młyńczak, bWFyY2VsLm1seW5jemFrQHB3LmVkdS5wbA==
 Marcel Młyńczak
Marcel Młyńczak Hubert Krysztofiak
Hubert Krysztofiak