- Department of Gastrointestinal Colorectal and Anal Surgery, China-Japan Union Hospital of Jilin University, Changchun, China
In China, majority of the mortality in gastric cancer are associated with peritoneal metastasis. Since most gastric tumors are metastatic at initial diagnosis, the treatment of gastric cancer is limited to radical resection. Therefore, it is imperative to identify diagnostic and prognostic biomarkers. From 2014 to 2015, 20 patients were enrolled in the study. To search translationally upregulated genes in the context of epithelial to mesenchymal transition (EMT), polysome profiling was performed. The MTT, migration, and invasion assay were conducted to determine cell proliferation, migration, and invasion ability respectively. Experiments of gain and loss of function were performed using the overexpression plasmid, siRNA, and shRNA. Xenograft assay was established using nude mice to explore the role of targets translationally upregulated gene in vivo. Polysome profiling defined the landscape of translationally regulated gene products with differential expression between non-metastatic and metastatic cohorts. Six-transmembrane epithelial antigen of the prostate 1 (STEAP1) was found to be the most translationally upregulated gene product in either experimental groups. STEAP1 was found to be required for cell proliferation, in vitro migration and invasion, and in vivo tumorigenesis. RNAi-mediated silencing of STEAP1 potentiated chemosensitivity of the MKN45 cells to docetaxel treatment, highlighting the importance of STEAP1 as a novel biomarker in gastric cancer patients with peritoneal metastasis. STEAP1 is thus induced translationally and its expression promotes proliferation, migration, invasiveness, and tumorigenicity of gastric cancer. STEAP1 can be a potent candidate for designing of targeted therapy.
Introduction
In China, 300,000 patients are projected to die annually from gastric cancer (Chen et al., 2013). An alarming 50% of this mortality is associated with peritoneal metastasis (Cho et al., 2014). Radical resection remains the standard mode of management, but it cannot prevent peritoneal metastasis (Boku et al., 1990; Tanaka et al., 2000; Yoo et al., 2000; Hippo et al., 2001). Like most other tumorigenic conditions, discovery and validation of both diagnostic and prognostic markers are thus important for optimal management of gastric cancer patients with peritoneal metastasis (Poste and Fidler, 1980; Crosby et al., 2001).
Previous work have shown that legumain might be an important marker since it is universally overexpressed in metastatic gastric cancer patients (Dando et al., 1999; Barrett et al., 2001; Guo et al., 2013; Li et al., 2013; Zhang et al., 2016). In addition, our earlier work has shown that the RNA binding protein, poly r(C) binding protein 1 (PCBP1)-mediated regulation of microRNA-3978 (miR-3978) inhibits the expression of legumain in normal peritoneum (Zhang et al., 2016; Ji et al., 2017). Expression of both PCBP1 and miR-3978 is downregulated whereas expression of legumain is upregulated in gastric cancer patients with peritoneal metastasis (Zhang et al., 2016; Ji et al., 2017). Legumain can potentiate metastatic progression by proteolytic activation of other zymogens or by augmenting epithelial to mesenchymal transition (EMT) via activation of Akt and MAPK signaling pathways (Murthy et al., 2005; Bajjuri et al., 2011; Zhen et al., 2015; Cui et al., 2016).
In ovarian, pancreatic, and breast cancer, PCBP1 can regulate the pro-oncogenic p63 transcript stability (Cho et al., 2013). In addition, suppression of PCBP1 expression or post-translational modification can increase the translation of genes and long non-coding RNAs (LncRNAs) which were required for EMT and metastasis in different cancers, including gastric cancer (Chaudhury et al., 2010; Wang et al., 2010; Hussey et al., 2011; Liu et al., 2015; Zhang et al., 2015). In addition, inactivating mutations in PCBP1 has been identified in Burkitt lymphoma (Wagener et al., 2015). Thus it might be possible that downregulation of PCBP1 represents a central point, inhibition of which is a common mechanism to increase stemness and mesenchymal in a context dependent mode (Chaudhury et al., 2010; Wang et al., 2010; Hussey et al., 2011; Cho et al., 2013; Liu et al., 2015; Wagener et al., 2015; Zhang et al., 2015; Grelet et al., 2017; Howley and Howe, 2018). Cumulatively, this indicates that EMT might be an important part of the puzzle that regulates both peritoneal metastases in gastric cancer patients as well as induction of chemoresistance in this cohort of patients.
However, not much is known beyond expression pattern of legumain and PCBP1 during peritoneal metastasis of gastric cancer patients. And given that expression of legumain is post-transcriptionally regulated by miR-3978 and loss of PCBP1 is known to translationally induce gene products that are EMT facilitators, our objective in the current study was to define the post transcriptional landscape of EMT regulators in gastric cancer patients with peritoneal metastasis. Our analysis revealed that the membrane bound mesenchymal cell marker, six-transmembrane epithelial antigen of the prostate 1 (STEAP1) is translationally induced during peritoneal metastasis and can potentially drive both tumorigenesis and chemoresistance to docetaxel.
Materials and Methods
Patient Samples
From 2014 to 2015, 20 patients were enrolled in the current study. These patients received surgery because of gastric cancer at the China-Japan Union Hospital of Jilin University. There were 12 male and 8 female patients. The age of the 20 patients ranged from 39 to 78 years (mean age was 61.34 years). The inclusion criteria were presence of peritoneal metastasis at the time of initial presentation, which was independently confirmed by two pathologists. Tumor tissue and tumor-adjacent normal gastric tissue samples were obtained from all patients during surgery. Ethics Committee of the China-Japan Union Hospital of Jilin University approved the protocol of the study. Written informed consents were obtained from all patients.
Cell Culture and Treatment
The HMrSV5 and MKN45 cell lines were obtained from BeNa Culture Collection (Beijing, China). The cells were cultured using RPMI 1640 medium (Life Technologies, Shanghai, China) containing 20% FBS (Lonza, Germany) and maintained in a 37°C incubator with 5% CO2. Where indicated cells were treated with indicated dose of docetaxel.
Plasmids and Transfection
STEAP1 overexpression plasmid, siRNA, and shRNA were obtained from Open Biosystems. The indicated cells were either transfected with STEAP1 plasmids or Firefly luciferase overexpression constructs, or siRNAs targeting either STEAP1 or Renilla luciferase using Lipofectamine 3000 (Life Technologies, Shanghai, China).
Polysome Profiling
Cells and tissue samples were treated with cycloheximide (50 μg/mL; Sigma-Aldrich, Shanghai, China) for 30 min at 37°C. Then the cells were washed by cold PBS which contained 50 μg/mL cycloheximide. Cells were lysed and processed for polysome profiling as described before (Chaudhury et al., 2010).
RNA Isolation From Polysomal Fractions and Quantitative Real Time Polymerase Chain Reaction (qRT-PCR)
The RNA from the various polysome fraction and total lysate aliquots were extracted using the TRIzol LS reagent (Life Technologies, Shanghai, China) according to the instruction of manufacturers. Complementary DNA (cDNA) were generated from the RNA samples in order to act as the template of the Human EMT RT2 Profiler PCR Array (Qiagen, Beijing, China) which contained 84 key genes that either change their expression during EMT or regulate the expression of those genes. Data was normalized to GAPDH expression.
MTT Assay
To measure the rates of cell proliferation, the experiment was performed using the MTT assay kit (Sigma-Aldrich, Shanghai, China). Cells (106) were seeded in triplicates on day 0 and proliferation ere measured after 24, 48, and 72 h, respectively. The relative optical density (OD) was measured at 570 nm and data was represented as mean ± standard deviation from at least three independent experiments.
Cells Migration and Invasion Assays
The analyzes of cell migration and invasion ability were performed using cell migration assay kits (R&D Systems) following manufacturer’s protocols. BME was used as the matrix in the invasion assay.
Xenograft Assay
SPF grade male six-week old BALB/c nude mice were purchased from the Charles River Laboratories (Beijing, China). The animal experiments were proved by the Institute’s Animal Care and Use Committee., the parental MKN45 cells or MKN45 cells which were stably transfected with two different shRNAs targeting STEAP1 (shRNA-1 and shRNA-2) were used to establish the tumor model (n = 4 in each group) as previously described (Zhang et al., 2016). Cells stably expressed Firefly luciferase to aid in the in vivo luciferase imaging to track tumor formation over indicated time frame. Weights of animals were measured on alternative days until the end of the experiment.
Statistical Analyses
SPSS statistical software program 18.0 (IBM Corporation, NY, United States) was chosen to conduct the data analyzes. A p-value < 0.05 was considered as statistically significant.
Results
Since we earlier showed that legumain expression is post-transcriptionally downregulated by miR-3978 in normal peritoneum and is deregulated during metastatic progression (Zhang et al., 2016), we were interested in defining the post-transcriptional landscape during peritoneal metastasis progression of gastric cancer. Legumain has been shown to promote mesenchymal markers in these patients (Zhang et al., 2016). Hence, we performed polysome profiling to investigate the differentially translated genes in the context of EMT. Tissue specimens from peritoneal metastasis patients and tumor-adjacent normal controls were subjected to polysome profiling. We have earlier shown that miR-3978 expression is high in the normal human mesothelial cell line, HMrSV5, whereas it is low in well-differentiated cell line mimicking peritoneal metastasis, MKN45 (Zhang et al., 2016; Ji et al., 2017). To understand the EMT effector genes that are potentially being impacted by the expression of miR-3978, we also performed polysome profiling in the HMrSV5 cells, either mock transfected or transfected with anti-miR-3978 antagomir. HMrSV5 cells transfected with anti-miR-3978 antagomir has been previously shown to mimic properties of the highly metastatic MKN45 cells (Zhang et al., 2016). Total RNA was isolated from non-translating low molecular weight non-polysomal fractions and actively-translating high molecular weight polysome fractions from triplicate set of tissue specimens or cell lines and then used to template a qRT-PCR reaction using the Human EMT RT2 Profiler PCR Array that comprised of 84 key genes which either can change their expression during EMT or regulate the expression of those genes (Supplementary Tables S1, S2). Of the 49 differentially translated genes in the tumor versus normal tissue control and the 59 differentially translated genes in the mock treated versus anti-miR-3978 antagomir treated MKN45 cell, 41 were common (Figure 1), indicating the similarity between the two chosen experimental models. Well-differentiated cell line mimicking peritoneal metastasis, MKN45, and the human mesothelial cell line, HMrSV5.
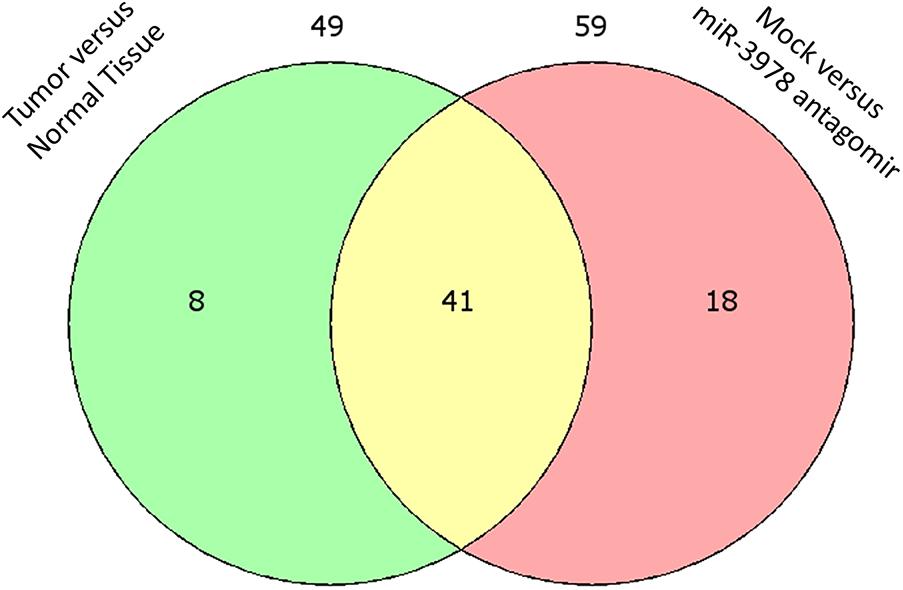
FIGURE 1. Differential translation of EMT effector genes in metastatic gastric cancer. Venn diagram showing overlap of differentially translated genes when peritoneal gastric cancer and adjacent normal control tissue specimens were compared to differential translation of same genes in mock transfected or miR-3978 antagomir transfected normal human mesothelial cell line HMrSV5 (also refer Supplementary Tables S1, S2).
Among the differentially translated genes in both groups, STEAP1 (encoding six-transmembrane epithelial antigen of prostate) was the most enriched in the polysome. Polysome profiling results were validated by immunoblotting for STEAP1 (data not shown).
We next wanted to determine the role of STEAP1 in the pathogenesis of peritoneal metastasis. To determine effect of STEAP1 expression on intrinsic changes in cell proliferation ability, the assays of cell viability were performed in mock or MKN45 cells which were transfected by siRNA targeting STEAP1. RNAi-mediated silencing of STEAP1 in MKN45 cells was verified by qRT-PCR (Figure 2A). RNAi-mediated silencing of STEAP1 in MKN45 cells (Mimic: Day 1 – 0.49 ± 0.04, Day 2 – 0.91 ± 0.04; Day 3 – 2.04 ± 0.06 / siRNA-STEAP1: Day 1 – 0.29 ± 0.05, Day 2 – 0.43 ± 0.09; Day 3 – 1.04 ± 0.09) significantly inhibited cell proliferation compare to the controls (p < 0.05 in each case) (Figure 2B).
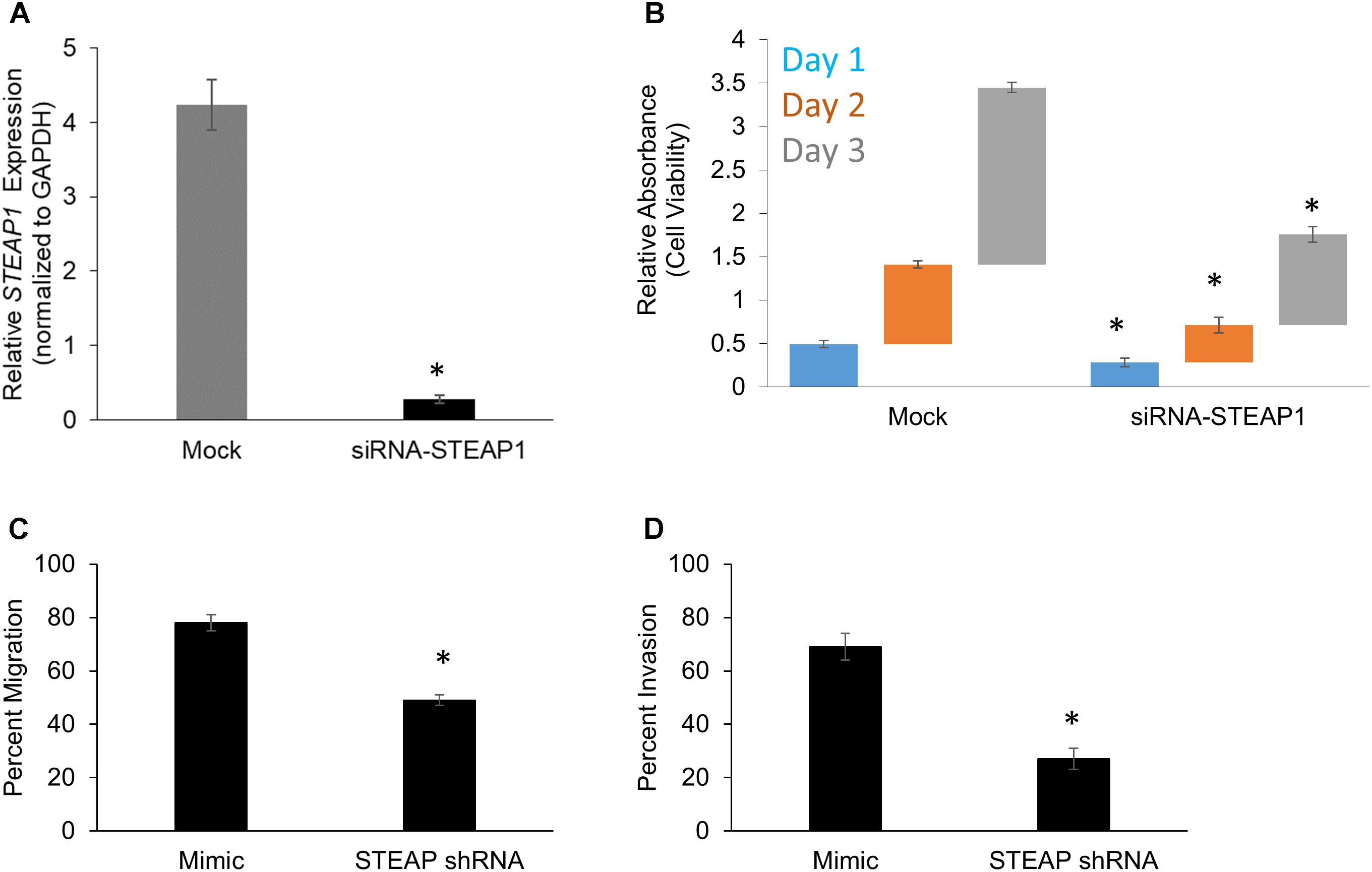
FIGURE 2. STEAP potentiates cell growth and in vitro metastatic properties in gastric cancer. (A) STEAP1 mRNA expression was significantly downregulated in MKN45 cells transfected with siRNA targeting STEAP1. Relative mRNA expression of STEAP1, normalized to GAPDH expression, in mock and siRNA-STEAP1 are shown. Data represents mean ± standard deviation of three independent replicates. MTT assay-based determination of cell proliferation (B), in vitro migration (C), and in vitro invasion (D) in mock transfected MKN45 cells and MKN45 cells transduced with siRNA targeting STEAP1. In each case, data shown are mean ± standard deviation of three independent replicates. ∗p < 0.05.
Each of the aforementioned transfectants in MKN45 cells were also subjected to in vitro migration (Figure 2C) and invasion (Figure 2D) assay in standard transwell assays. Silencing of STEAP1 expression suppressed migration (78 ± 3% versus 49 ± 2%; p < 0.001) and invasion (69 ± 5% versus 27 ± 4%) in MKN45 cells based on these criteria. Taken together, these results suggested that STEAP1 expression is associated to proliferative capacity of MKN45 cells and also functions associated with mesenchymal cells, namely migration and invasion.
Since our results indicated that STEAP1 can dictate cell proliferation, we investigated the role of STEAP1 expression on tumorigenesis per se. MKN45 cells stably expressing two independent shRNAs targeting STEAP1 were generated and used for xenograft studies. Successful knockdown of STEAP1 was confirmed by qRT-PCR (Supplementary Figure S1). shRNA-1 was significantly more potent in silencing STEAP1 expression, compared to shRNA-2. Compared with mock transduced MKN45 cells, STEAP1 silencing completely inhibited (shRNA-1) or significantly attenuated (shRNA-1) the growth of tumor (Figures 3A–C). There were no significant changes in body weights of the animals among the different experimental groups (data not shown). Tumor growth rate measured by in vivo luminescence showed a similar trend (Figures 3D–F), with shRNA-1 showing a better effect than shRNA-2. The differences observed with the two shRNAs can be attributed to the degree of STEAP1 knockdown achieved by the respective shRNA (Supplementary Figure S1). Taken together, these data suggest that high expression of STEAP1 is required for gastric cancer formation and progression.
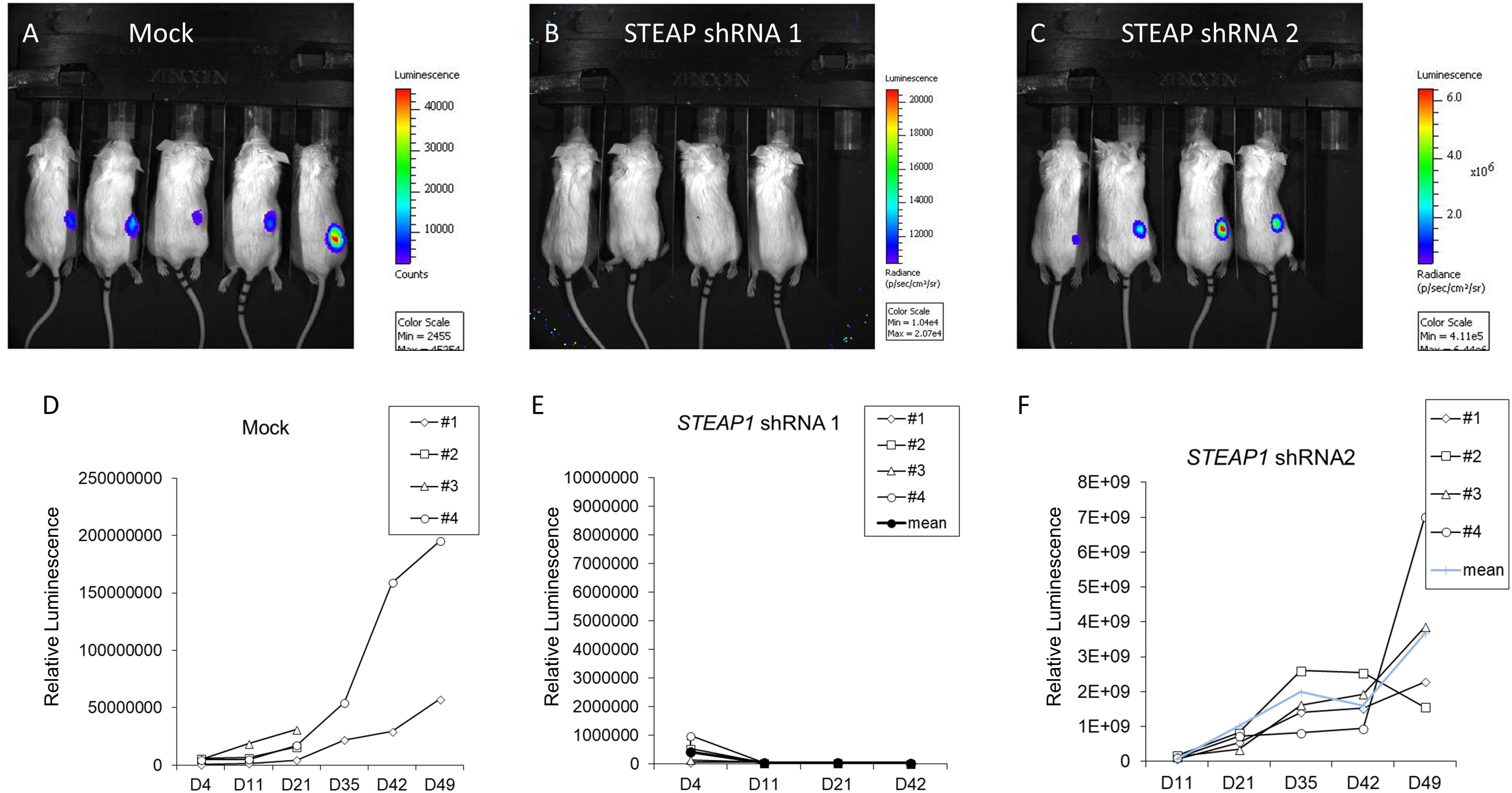
FIGURE 3. STEAP potentiates tumor growth in vivo. Panels (A–C) Bioluminescence images obtained in mice 6 weeks after implantation with either mock-transduced MKN45 cells, or MKN45 cells transduced with two independent shRNAs targeting STEAP1. Please note the intensity axis on the right of each panel. (D–F) Corresponding quantification of tumor growth patterns for images shown in A–C. ∗p < 0.05 (also refer Supplementary Figure S1).
Docetaxel-based therapy has emerged as the treatment of choice in gastric cancer patients (Tetzlaff et al., 2008). Thus, to explore the potential effects of STEAP1 expression on chemosensitivity to docetaxel we either overexpressed STEAP1 in the mesothelial cell line HMrSV5 or silenced its expression in the metastatic MKN45 cells before treatment with docetaxel. Overexpression of STEAP1 made HMrSV5 cells significantly resistant to docetaxel treatment (p < 0.05) (Figure 4A). Conversely, downregulation of STEAP1 significantly increased chemosensitivity in MKN45 cells to docetaxel treatment (p < 0.05) (Figure 4B).
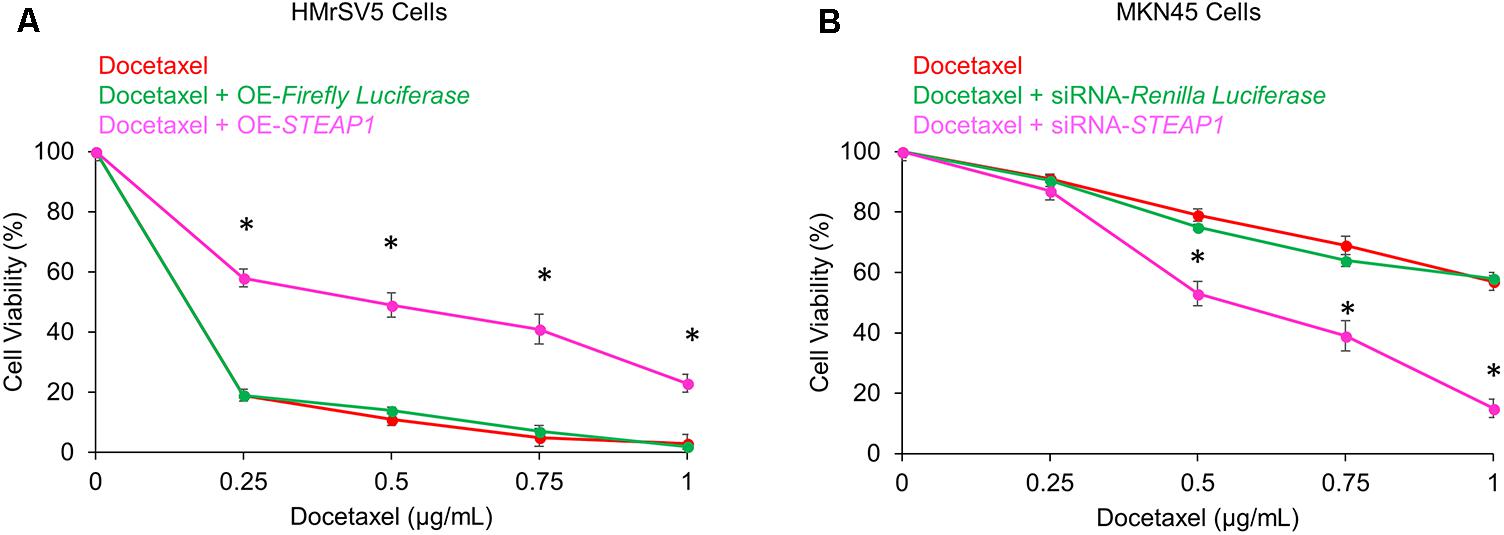
FIGURE 4. Modulation of STEAP1 expression changes susceptibility to docetaxel treatment. (A) Firefly luciferase or STEAP1 overexpressing HMrSV5 cells were treated with indicated doses of docetaxel 72 h. (B) MKN45 cells transiently transfected with siRNA targeting Renilla luciferase or STEAP1 were treated with indicated doses of docetaxel for 72 h. MTT assay was performed to assess the cell viability after 72 h. Data was obtained from three independent replicates experiments and was represented as mean ± standard deviation (SD). ∗p < 0.05.
Discussion
The profound induction in relative expression of STEAP1 by translational regulatory mechanisms in metastatic gastric cancer patients along with its capacity to promote mesenchymal traits and chemoresistance in vitro and tumorigenesis in vivo suggest that STEAP1 is a critical determinant that drives EMT like programs during peritoneal metastasis of gastric cancer.
Our findings corroborate recently reported finding that STEAP1 potentiates oxidative properties and invasiveness in Ewing tumor cells (Grunewald et al., 2012). STEAP1 encodes for the six-transmembrane epithelial antigen of the prostate 1, which is a membrane-bound protein functioning in mitochondrial transmembrane electron transfer (Hubert et al., 1999; Sendamarai et al., 2008). STEAP1 is overexpressed in wide variety of cancers including prostate and bladder cancer (Hubert et al., 1999; Ohgami et al., 2006; Moreaux et al., 2012). STEAP1 has also been classified as a mesenchymal stem cell marker (Vaghjiani et al., 2009), supporting the relationship we observed with peritoneal metastasis of gastric cancer.
STEAP1 mRNA is hardly expressed in benign tissues (Hubert et al., 1999; Valenti et al., 2009). Our results here show that STEAP1 expression is upregulated at the translational level. Thus, given its high tumor specificity and membrane-bound localization, STEAP1 is an attractive candidate for targeted therapy in gastric cancer patients with peritoneal metastasis (Hubert et al., 1999; Cheung et al., 2007; Valenti et al., 2009). In fact, it has been shown that monoclonal antibodies designed against STEAP1 can inhibit bladder and prostate cancer in mice models (Challita-Eid et al., 2007). It will be interesting and vital to determine if these or similar monoclonal antibodies will be able to prevent onset and progression of gastric cancer.
In summary, we have shown that in the present study the putative oncogenic and pro-metastatic function of STEAP1. We prove that STEAP1 is induced translationally and that its expression promotes proliferation, migration, invasiveness, and tumorigenicity of gastric cancer. The regulatory mechanisms dictating translational upregulation of STEAP1 and the benefit of targeted therapy against STEAP1 alone and in the context of chemoresistance in patients with peritoneal metastasis will be extensive areas of future investigation.
Author Contributions
Y-YW conceived and designed the study, and acquired the data. J-NJ analyzed and interpreted the data, and drafted the manuscript. X-DF revised the manuscript for intellectual content. F-JJ approved the final manuscript.
Funding
The study was funded by the Construction Project of University Engineering Research Center of Jilin Province Education Department of Jilin Province, Item number: Kyrgyzstan textbook word [2015] No. 39 and the Health Special Items of the Finance Department of Jilin Province, Item number: Sczsy201503 and Sczsy201603.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2018.01132/full#supplementary-material
FIGURE S1 | Efficiency of shRNA-1 and 2 targeting STEAP1 to downregulate STEAP1 expression. Relative mRNA expression of STEAP1, normalized to GAPDH expression, in parental MKN45 cells and MK N45 cells stably transduced with two different shRNAs (shRNA-1 and shRNA-2) targeting STEAP1. Data represents mean ± standard deviation of three independent replicates. ∗p < 0.05.
TABLE S1 | Genes showing 2-fold changes in polysome occupancy between tumor and tumor adjacent normal gastric cancer tissue with peritoneal metastasis.
TABLE S2 | Genes showing 2-fold changes in polysome occupancy in the normal human mesothelial cell line, HMrSV5, either mock transfected or transfected with anti-miR-3978 antagomir.
References
Bajjuri, K. M., Liu, Y., Liu, C., and Sinha, S. C. (2011). The legumain protease-activated auristatin prodrugs suppress tumor growth and metastasis without toxicity. ChemMedChem 6, 54–59. doi: 10.1002/cmdc.201000478
Barrett, A. J., Rawlings, N. D., and O’brien, E. A. (2001). The MEROPS database as a protease information system. J. Struct. Biol. 134, 95–102. doi: 10.1006/jsbi.2000.4332
Boku, T., Nakane, Y., Minoura, T., Takada, H., Yamamura, M., Hioki, K., et al. (1990). Prognostic significance of serosal invasion and free intraperitoneal cancer cells in gastric cancer. Br. J. Surg. 77, 436–439. doi: 10.1002/bjs.1800770425
Challita-Eid, P. M., Morrison, K., Etessami, S., An, Z., Morrison, K. J., Perez-Villar, J. J., et al. (2007). Monoclonal antibodies to six-transmembrane epithelial antigen of the prostate-1 inhibit intercellular communication in vitro and growth of human tumor xenografts in vivo. Cancer Res. 67, 5798–5805. doi: 10.1158/0008-5472.CAN-06-3849
Chaudhury, A., Hussey, G. S., Ray, P. S., Jin, G., Fox, P. L., and Howe, P. H. (2010). TGF-beta-mediated phosphorylation of hnRNP E1 induces EMT via transcript-selective translational induction of Dab2 and ILEI. Nat. Cell Biol. 12, 286–293. doi: 10.1038/ncb2029
Chen, W., Zheng, R., Zhang, S., Zhao, P., Zeng, H., and Zou, X. (2013). Report of cancer incidence and mortality in China, 2010. Ann. Transl. Med. 2:61.
Cheung, I. Y., Feng, Y., Danis, K., Shukla, N., Meyers, P., Ladanyi, M., et al. (2007). Novel markers of subclinical disease for ewing family tumors from gene expression profiling. Clin. Cancer Res. 13, 6978–6983. doi: 10.1158/1078-0432.CCR-07-1417
Cho, J. M., Jang, Y. J., Kim, J. H., Park, S. S., Park, S. H., and Mok, Y. J. (2014). Pattern, timing and survival in patients with recurrent gastric cancer. Hepatogastroenterology 61, 1148–1153.
Cho, S. J., Jung, Y. S., and Chen, X. (2013). Poly (C)-binding protein 1 regulates p63 expression through mRNA stability. PLoS One 8:e71724. doi: 10.1371/journal.pone.0071724
Crosby, J. A., Catton, C. N., Davis, A., Couture, J., O’sullivan, B., Kandel, R., et al. (2001). Malignant gastrointestinal stromal tumors of the small intestine: a review of 50 cases from a prospective database. Ann. Surg. Oncol. 8, 50–59. doi: 10.1007/s10434-001-0050-4
Cui, Y., Wang, Y., Li, H., Li, Q., Yu, Y., Xu, X., et al. (2016). Asparaginyl endopeptidase promotes the invasion and metastasis of gastric cancer through modulating epithelial-to-mesenchymal transition and analysis of their phosphorylation signaling pathways. Oncotarget 7, 34356–34370. doi: 10.18632/oncotarget.8879
Dando, P. M., Fortunato, M., Smith, L., Knight, C. G., Mckendrick, J. E., and Barrett, A. J. (1999). Pig kidney legumain: an asparaginyl endopeptidase with restricted specificity. Biochem. J. 339(Pt 3), 743–749. doi: 10.1042/bj3390743
Grelet, S., Link, L. A., Howley, B., Obellianne, C., Palanisamy, V., Gangaraju, V. K., et al. (2017). A regulated PNUTS mRNA to lncRNA splice switch mediates EMT and tumour progression. Nat. Cell Biol. 19, 1105–1115. doi: 10.1038/ncb3595
Grunewald, T. G., Diebold, I., Esposito, I., Plehm, S., Hauer, K., Thiel, U., et al. (2012). STEAP1 is associated with the invasive and oxidative stress phenotype of Ewing tumors. Mol. Cancer Res. 10, 52–65. doi: 10.1158/1541-7786.MCR-11-0524
Guo, P., Zhu, Z., Sun, Z., Wang, Z., Zheng, X., and Xu, H. (2013). Expression of legumain correlates with prognosis and metastasis in gastric carcinoma. PLoS One 8:e73090. doi: 10.1371/journal.pone.0073090
Hippo, Y., Yashiro, M., Ishii, M., Taniguchi, H., Tsutsumi, S., Hirakawa, K., et al. (2001). Differential gene expression profiles of scirrhous gastric cancer cells with high metastatic potential to peritoneum or lymph nodes. Cancer Res. 61, 889–895.
Howley, B., and Howe, P. H. (2018). TGF-beta signaling in cancer: post-transcriptional regulation of EMT via hnRNP E1. Cytokine doi: 10.1016/j.cyto.2017.12.032 [Epub ahead of print].
Hubert, R. S., Vivanco, I., Chen, E., Rastegar, S., Leong, K., Mitchell, S. C., et al. (1999). STEAP: a prostate-specific cell-surface antigen highly expressed in human prostate tumors. Proc. Natl. Acad. Sci. U.S.A. 96, 14523–14528. doi: 10.1073/pnas.96.25.14523
Hussey, G. S., Chaudhury, A., Dawson, A. E., Lindner, D. J., Knudsen, C. R., Wilce, M. C., et al. (2011). Identification of an mRNP complex regulating tumorigenesis at the translational elongation step. Mol. Cell 41, 419–431. doi: 10.1016/j.molcel.2011.02.003
Ji, F. J., Wu, Y. Y., An, Z., Liu, X. S., Jiang, J. N., Chen, F. F., et al. (2017). Expression of both poly r(C) binding protein 1 (PCBP1) and miRNA-3978 is suppressed in peritoneal gastric cancer metastasis. Sci. Rep. 7:15488. doi: 10.1038/s41598-017-15448-9
Li, N., Liu, Q., Su, Q., Wei, C., Lan, B., Wang, J., et al. (2013). Effects of legumain as a potential prognostic factor on gastric cancers. Med. Oncol. 30:621. doi: 10.1007/s12032-013-0621-9
Liu, Y., Gai, L., Liu, J., Cui, Y., Zhang, Y., and Feng, J. (2015). Expression of poly(C)-binding protein 1 (PCBP1) in NSCLC as a negative regulator of EMT and its clinical value. Int. J. Clin. Exp. Pathol. 8, 7165–7172.
Moreaux, J., Kassambara, A., Hose, D., and Klein, B. (2012). STEAP1 is overexpressed in cancers: a promising therapeutic target. Biochem. Biophys. Res. Commun. 429, 148–155. doi: 10.1016/j.bbrc.2012.10.123
Murthy, R. V., Arbman, G., Gao, J., Roodman, G. D., and Sun, X. F. (2005). Legumain expression in relation to clinicopathologic and biological variables in colorectal cancer. Clin. Cancer Res. 11, 2293–2299. doi: 10.1158/1078-0432.CCR-04-1642
Ohgami, R. S., Campagna, D. R., Mcdonald, A., and Fleming, M. D. (2006). The steap proteins are metalloreductases. Blood 108, 1388–1394. doi: 10.1182/blood-2006-02-003681
Poste, G., and Fidler, I. J. (1980). The pathogenesis of cancer metastasis. Nature 283, 139–146. doi: 10.1038/283139a0
Sendamarai, A. K., Ohgami, R. S., Fleming, M. D., and Lawrence, C. M. (2008). Structure of the membrane proximal oxidoreductase domain of human Steap3, the dominant ferrireductase of the erythroid transferrin cycle. Proc. Natl. Acad. Sci. U.S.A. 105, 7410–7415. doi: 10.1073/pnas.0801318105
Tanaka, T., Kumagai, K., Shimizu, K., Masuo, K., and Yamagata, K. (2000). Peritoneal metastasis in gastric cancer with particular reference to lymphatic advancement; extranodal invasion is a significant risk factor for peritoneal metastasis. J. Surg. Oncol. 75, 165–171. doi: 10.1002/1096-9098(200011)75:3<165::AID-JSO3>3.0.CO;2-5
Tetzlaff, E. D., Cheng, J. D., and Ajani, J. A. (2008). Review of docetaxel in the treatment of gastric cancer. Ther. Clin. Risk Manag. 4, 999–1007. doi: 10.2147/TCRM.S3226
Vaghjiani, R. J., Talma, S., and Murphy, C. L. (2009). Six-transmembrane epithelial antigen of the prostate (STEAP1 and STEAP2)-differentially expressed by murine and human mesenchymal stem cells. Tissue Eng. Part A 15, 2073–2083. doi: 10.1089/ten.tea.2008.0519
Valenti, M. T., Dalle Carbonare, L., Donatelli, L., Bertoldo, F., Giovanazzi, B., Caliari, F., et al. (2009). STEAP mRNA detection in serum of patients with solid tumours. Cancer Lett. 273, 122–126. doi: 10.1016/j.canlet.2008.07.037
Wagener, R., Aukema, S. M., Schlesner, M., Haake, A., Burkhardt, B., Claviez, A., et al. (2015). The PCBP1 gene encoding poly(rC) binding protein I is recurrently mutated in burkitt lymphoma. Genes Chromosomes Cancer 54, 555–564. doi: 10.1002/gcc.22268
Wang, H., Vardy, L. A., Tan, C. P., Loo, J. M., Guo, K., Li, J., et al. (2010). PCBP1 suppresses the translation of metastasis-associated PRL-3 phosphatase. Cancer Cell 18, 52–62. doi: 10.1016/j.ccr.2010.04.028
Yoo, C. H., Noh, S. H., Shin, D. W., Choi, S. H., and Min, J. S. (2000). Recurrence following curative resection for gastric carcinoma. Br. J. Surg. 87, 236–242. doi: 10.1046/j.1365-2168.2000.01360.x
Zhang, Y., Wu, Y. Y., Jiang, J. N., Liu, X. S., Ji, F. J., and Fang, X. D. (2016). MiRNA-3978 regulates peritoneal gastric cancer metastasis by targeting legumain. Oncotarget 7, 83223–83230. doi: 10.18632/oncotarget.12917
Zhang, Z. Z., Shen, Z. Y., Shen, Y. Y., Zhao, E. H., Wang, M., Wang, C. J., et al. (2015). HOTAIR long noncoding RNA promotes gastric cancer metastasis through suppression of poly r(c)-binding protein (PCBP) 1. Mol. Cancer Ther. 14, 1162–1170. doi: 10.1158/1535-7163.MCT-14-0695
Keywords: STEAP1, tumorigenesis, chemoresistance, gastric cancer, peritoneal metastasis
Citation: Wu Y-Y, Jiang J-N, Fang X-D and Ji F-J (2018) STEAP1 Regulates Tumorigenesis and Chemoresistance During Peritoneal Metastasis of Gastric Cancer. Front. Physiol. 9:1132. doi: 10.3389/fphys.2018.01132
Received: 09 March 2018; Accepted: 30 July 2018;
Published: 21 August 2018.
Edited by:
Atsushi Masamune, Tohoku University, JapanReviewed by:
Manlio Vinciguerra, International Clinical Research Center (FNUSA-ICRC), CzechiaShikha Prasad, Northwestern University, United States
Copyright © 2018 Wu, Jiang, Fang and Ji. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xue-Dong Fang, ZmFuZ3hkQGpsdS5lZHUuY24= Fu-Jian Ji, amlmakBqbHUuZWR1LmNu
 Yuan-Yu Wu
Yuan-Yu Wu Jun-Nan Jiang
Jun-Nan Jiang Xue-Dong Fang
Xue-Dong Fang Fu-Jian Ji
Fu-Jian Ji