- Department of Physiology, Development and Neuroscience, Centre for Trophoblast Research, University of Cambridge, Cambridge, United Kingdom
During pregnancy, the mother must adapt her body systems to support nutrient and oxygen supply for growth of the baby in utero and during the subsequent lactation. These include changes in the cardiovascular, pulmonary, immune and metabolic systems of the mother. Failure to appropriately adjust maternal physiology to the pregnant state may result in pregnancy complications, including gestational diabetes and abnormal birth weight, which can further lead to a range of medically significant complications for the mother and baby. The placenta, which forms the functional interface separating the maternal and fetal circulations, is important for mediating adaptations in maternal physiology. It secretes a plethora of hormones into the maternal circulation which modulate her physiology and transfers the oxygen and nutrients available to the fetus for growth. Among these placental hormones, the prolactin-growth hormone family, steroids and neuropeptides play critical roles in driving maternal physiological adaptations during pregnancy. This review examines the changes that occur in maternal physiology in response to pregnancy and the significance of placental hormone production in mediating such changes.
Introduction
Pregnancy is a dynamic and precisely coordinated process involving systemic and local changes in the mother that support the supply of nutrients and oxygen to the baby for growth in utero and in the subsequent lactation. Inappropriate adaptation of maternal physiology may lead to complications of pregnancy, such as gestational diabetes, preeclampsia, fetal growth restriction, fetal overgrowth and pre-term birth; which can have immediate consequences for fetal and maternal health. Furthermore, these pregnancy complications can also lead to long-term health consequences for the mother and infant. Altered fetal growth is associated with an increased risk of the offspring developing obesity, type-2 diabetes and cardiovascular disease in adulthood (Hales and Barker, 2001; Barker, 2004; Fowden et al., 2006). Moreover, women who develop gestational diabetes or preeclampsia are more likely to develop type-2 diabetes or cardiovascular disease in later life (Kim et al., 2002; Petry et al., 2007). Maternal adaptations to pregnancy are largely mediated by the placenta; the functional interface between the mother and fetus that secretes hormones and growth factors into the mother with physiological effects. This review aims to provide an overview of the physiological changes that occur in the mother in response to pregnancy and to discuss the role of key placental hormones in mediating such adaptations. In particular, this review focuses on the importance of the prolactin-growth hormone family (e.g., prolactin, placental lactogen and growth hormone), steroids (estrogens and progesterone) and neuropeptides (serotonin, melatonin and oxytocin) in adaptations of maternal physiology during pregnancy. Where possible, this review draws upon findings in women and animal models, including rodents and sheep. However, differences exist between species in the specific hormones produced by the placenta, the access of these hormones to the maternal circulation, and the relative proportion of conceptus mass to maternal size (hence constraint on the mother to provide resources for fetal growth; Haig, 2008; Carter, 2012; Fowden and Moore, 2012). Where such differences between species exist, these have been highlighted and discussed as necessary in the relevant sections. Nevertheless, although some effects described may not be applicable to all species, the different animal models of pregnancy still provide novel insight into the fundamental mechanisms of maternal adaptation during gestation.
Adaptations in Maternal Physiology During Pregnancy and Lactation
Most tissues and organs in the mother respond to the pregnant state. Changes include alterations in size, morphology, function and responsiveness of tissues and organs to hormonal and metabolic cues. These changes arise in the cardiovascular, pulmonary, immune, and metabolic systems of the mother (Figure 1). Some of these changes are seen from very early in pregnancy, prior to the establishment of a fully functional placenta, highlighting that non-placental factors may also be important (Paller et al., 1989; Drynda et al., 2015). The specific nature of changes in maternal physiology depends on the stage of the pregnancy and appears to track with alterations in the metabolic requirements of the mother versus the developing fetus.
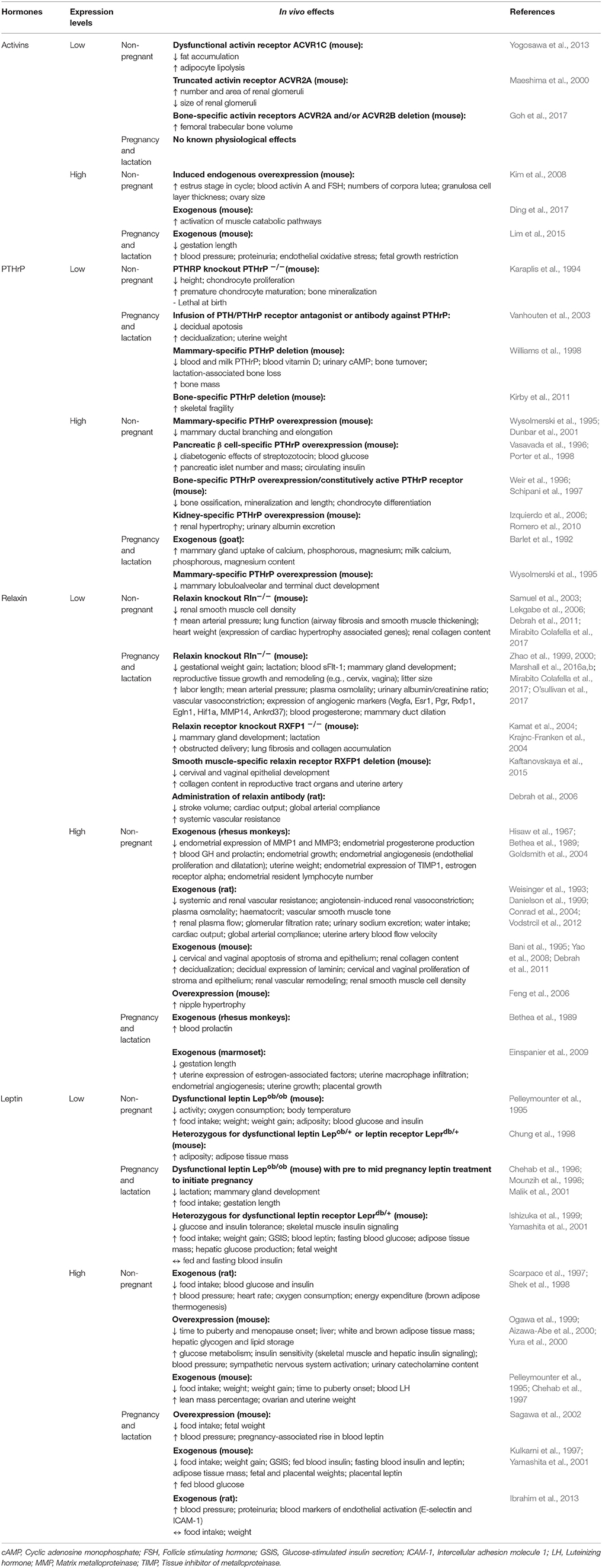
Figure 1. Schematic diagram highlighting the main physiological modifications in the maternal physiology in response to pregnancy. Many of the changes described in the figure for women during pregnancy also occur in other species, including mice. Respiratory system (Macrae and Palavradji, 1967; Weinberger et al., 1980; Contreras et al., 1991; Hegewald and Crapo, 2011; Frise et al., 2013; Lomauro and Aliverti, 2015; Soma-Pillay et al., 2016); cardiovascular system (Adamova et al., 2009; Li et al., 2012; Pieper, 2015; Soma-Pillay et al., 2016); hematological system (Shakhmatova et al., 2000; Chang and Streitman, 2012; Rodger et al., 2015; Soma-Pillay et al., 2016); spleen (Maroni and De Sousa, 1973; Sasaki et al., 1981; Norton et al., 2009); renal system (Davison and Dunlop, 1980; Atherton et al., 1982; Krutzén et al., 1992; Elsheikh et al., 2001; Cheung and Lafayette, 2013; Lumbers and Pringle, 2014; Pieper, 2015; Soma-Pillay et al., 2016); pancreas (Ziegler et al., 1985; Ernst et al., 2011; Ohara-Imaizumi et al., 2013; Baeyens et al., 2016); adipose tissue (Catalano et al., 2006; Hauguel-De Mouzon et al., 2006; Lain and Catalano, 2007; Nien et al., 2007; Hadden and Mclaughlin, 2009; Valsamakis et al., 2010; Musial et al., 2016); skeletal muscle (Alperin et al., 2015, 2016; Musial et al., 2016); bone (Shahtaheri et al., 1999; Ulrich et al., 2003; Hellmeyer et al., 2006; Salles, 2016); digestive tract (Everson, 1992; Fudge and Kovacs, 2010; Pieper, 2015); liver (Munnell and Taylor, 1947; Van Bodegraven et al., 1998; Lain and Catalano, 2007; Bacq, 2013); mammary tissue (Elling and Powell, 1997; Neville et al., 2002; Sternlicht, 2006; Pang and Hartmann, 2007); immune system (Clarke and Kendall, 1994; Kendall and Clarke, 2000; Veenstra Van Nieuwenhoven et al., 2002; Norton et al., 2009; Mor and Cardenas, 2010; Saito et al., 2010; Racicot et al., 2014; Groen et al., 2015; Zöllner et al., 2017; Edey et al., 2018); nervous system (Shingo et al., 2003; Gregg, 2009; Roos et al., 2011; Hoekzema et al., 2017).
Alterations in the maternal cardiovascular system begin very early in gestation (Chapman et al., 1998) and ultimately lead to systemic vasodilation and increased blood perfusion of maternal organs, including the gravid uterus. Systemic vascular resistance is reduced by 25–30% and accompanied by a 40% increase in cardiac output during human pregnancy; while in mice, blood pressure decreases by 15% and cardiac output is increased by 48% (Bader et al., 1955; Kulandavelu et al., 2006; Soma-Pillay et al., 2016). Renal blood flow and glomerular filtration rates are also increased (Davison and Dunlop, 1980; Soma-Pillay et al., 2016). The renin-angiotensin-aldosterone system (RAAS) which is a major determinant for sodium balance during gestation, is progressively upregulated toward term with associated plasma volume expansion (Elsheikh et al., 2001; Tkachenko et al., 2014). This rise in blood volume, which is required to cope with the oxygen requirements of the maternal organs and the conceptus growth, plateaus by the late gestation, resulting in an increase in total blood volume by approximately 30% at the end of pregnancy (Chang and Streitman, 2012). There is also an increase in the numbers of red blood cells in the mother during pregnancy, due to proliferation of erythroid progenitors in the spleen (Bustamante et al., 2008). Pulmonary function is also altered and encompasses changes in ventilation rates and blood gases. For instance, lung tidal volume and minute ventilation increases by 30–50% (Hegewald and Crapo, 2011). As a result of increased oxygen consumption during hyperventilation, there is greater carbon dioxide production, which leads to chronic respiratory alkalosis that is compensated by an increased renal excretion of bicarbonate (Weinberger et al., 1980). Overall, these adaptations ensure the well-being of the mother, while also providing an adequate blood flow to the placenta for fetal nutrition, oxygenation and maturation.
There are also alterations in maternal metabolic and endocrine state during gestation. In early pregnancy, the maternal pancreatic β-cell mass expands due to both hyperplasia and hypertrophy of islets, which for example in rats, results in a >50% increase (Ackermann and Gannon, 2007; Rieck and Kaestner, 2010). The threshold for glucose-stimulated insulin production is also lowered and maternal circulating insulin concentration is greater compared to the non-pregnant state. In early pregnancy, when fetal demands are relatively low, whole body maternal insulin sensitivity is unchanged or increased and there is accumulation of energy reserves in the mother. In particular, early pregnancy is associated with adipocyte hypertrophy, increased lipogenesis and lipid storage and relates to improved insulin sensitivity of white adipose tissue in the mother (Hadden and Mclaughlin, 2009; Mcilvride et al., 2017). Interestingly, in pregnant mice, brown adipose stores of the dam also switch to a white adipose tissue-like phenotype in early gestation (Mcilvride et al., 2017). Additionally, glycogen accumulates in the liver, which also increases in size from early gestation (Bustamante et al., 2010). In contrast, late pregnancy is associated with diminished maternal tissue insulin sensitivity and a concomitant increase in lipolysis and hepatic gluconeogenesis (Freemark et al., 2002; Lain and Catalano, 2007; Musial et al., 2016). Despite the pregnancy-related rise in leptin and insulin concentrations, maternal appetite increases in pregnancy (Villar et al., 1992; Douglas et al., 2007; Hadden and Mclaughlin, 2009; Díaz et al., 2014). Together, these metabolic and endocrine alterations increase lipid and glucose availability for the rapidly growing fetus in late gestation. Intriguingly in rodents, whole body responsiveness to insulin starts to improve near term, which may be important for conserving nutrients for maternal use, as parturition and lactation approach (Musial et al., 2016). There are also notable changes in maternal bone metabolism during pregnancy. In particular, intestinal calcium absorption is enhanced in the mother during pregnancy via upregulation of 1,25-dihydroxyvitamin D levels, improved renal conservation and increased calcium mobilization from the maternal skeleton (Hellmeyer et al., 2006). These processes support the supply of calcium for the formation, growth and mineralization of the fetal skeleton (King, 2000; Kalkwarf and Specker, 2002).
The immune system of the mother during pregnancy is tightly regulated to prevent an unwanted immune response against the paternal antigens present in the developing conceptus (Racicot et al., 2014; Groen et al., 2015; Zöllner et al., 2017). As gestation progresses, there is suppression of the pro-inflammatory Th1 type of immunity and a shift toward a more anti-inflammatory, Th2 immune state in the mother (Saito et al., 2010), which supports fetal growth and maternal well-being (Mor and Cardenas, 2010). In particular, the total abundance of circulating leukocytes, monocytes, granulocytes and T lymphocytes increase in the mother in response to pregnancy (Groen et al., 2015). However, expression of major histocompatibility complex class II by circulating monocytes is reduced in the mother, which would decrease antigen presentation and stimulation of T cells during pregnancy and prevent the maternal immune system from mounting an unwanted response against fetal antigens (Groen et al., 2015). The total number of circulating natural killer cells and secretion of pro-inflammatory cytokines (IFN-gamma) is also reduced in the pregnant state (Veenstra Van Nieuwenhoven et al., 2002). However, close to parturition, the maternal immune system shifts to a pro-inflammatory state, particularly locally within the uterus, to promote labor (Mor and Cardenas, 2010; Edey et al., 2018). There are also specific changes in the numbers of different leukocyte populations in the maternal thymus and spleen during pregnancy (Clarke and Kendall, 1994; Kendall and Clarke, 2000; Norton et al., 2009). The spleen, which also has functions in hematopoiesis, enlarges due to an expansion of the splenic red pulp during pregnancy (Maroni and De Sousa, 1973; Norton et al., 2009). Neurological changes must also occur during pregnancy to increase maternal nursing behavior and enable the mother to properly care for her newborn infant (Bridges et al., 1997; Bridges, 2015; Kim, 2016; Kim et al., 2016). For instance, there is increased activation of the prefrontal cortex and neurogenesis of the forebrain olfactory bulb (Shingo et al., 2003), which are important in regulating behavior. In addition, formation of lobulo-alveolar units in the mammary gland commences during pregnancy, in preparation for lactational support of the neonate.
Placental Hormones that Mediate Maternal Adaptations to Pregnancy, Parturition and Lactation
The placenta is a highly active endocrine organ during gestation; secreting a variety of hormones with physiological effects in the mother. Placental hormones include members of the prolactin and growth hormone family, steroid hormones and neuroactive hormones. The function of these hormones in driving physiological changes during pregnancy has been assessed in two main ways. First, the expression and activity of the hormones have been manipulated in vivo by either exogenously administering or genetically manipulating the expression of hormones and hormone receptors to study the physiological consequences for the animal. Secondly, hormones have been manipulated similarly in cultured cells and tissue explants to inform on the cellular and molecular mechanisms by which they modulate function. The effects of hormones in non-pregnant animals have been included as they provide information on the baseline of physiological changes that occur in the absence of hormone expression/activity, which is especially important in the case of some placental-derived hormones, where analyses in the pregnant state have not been conducted.
Prolactin (PRL)-Growth Hormone (GH) Family
The PRL-GH family is one of the main families of hormones secreted by the placenta during gestation. Members of this family consist of prolactin (PRL) (Handwerger et al., 1992), placental lactogens (PLs) (Wiemers et al., 2003), PRL-like hormones (Wiemers et al., 2003), proliferins (PLF) (Lee et al., 1988), proliferin-related proteins (PRP) (Jackson et al., 1994) and growth hormone (GH). Between mammalian species, there are differences in the number and type of family members expressed by the placenta [reviewed elsewhere (Linzer and Fisher, 1999; Soares, 2004; Soares et al., 2007)]. For instance, in the mouse and rat, the placenta expresses all these members except for PRL and GH whereas the human placenta only expresses GH and PL genes. In mice and rats, expression of the individual PRL-GH family members vary spatially and temporally in the placenta (Dai et al., 2002; Simmons et al., 2008; Urbanek et al., 2015). The anterior pituitary also produces PRL and GH; however this is diminished by mid-pregnancy, when placental hormone production predominates (Bridges, 2015). In several species including rodents and humans, PRL is additionally produced by the decidua during pregnancy. The family members share structural similarity to one another and may bind, with varying affinity to PRL and GH receptors (PRLR and GHR, respectively), which are widely expressed by tissues in the body (Haig, 2008; Trott et al., 2008; Ben-Jonathan and Hugo, 2015). As the PRL-GH members also exert similar functions, these have been presented in a grouped fashion in the text and tables (Tables 1, 2). However, where possible, the roles of individual family members of the PRL-GH in physiological changes have been described.
Studies performed both in vivo and in vitro support a role for the PRL-GH family in mediating maternal metabolic adaptations to pregnancy (Tables 1, 2). PRL, PRL-like proteins and PL, principally via the PRL receptor, induce β-cell mass expansion by both increasing β-cell proliferation and reducing apoptosis of islets in vivo and in vitro (Table 2; PRL/PL/GH; Brelje et al., 1993; Huang et al., 2009). PRL and PL also increase insulin secretion during pregnancy, particularly in response to glucose, by enhancing the expression of glucose sensors (glucokinase, hexokinase and glucose transporter-2) and activating the serotonin biosynthesis pathway in pancreatic islets (Table 2; PRL/PL/GH; Nielsen, 1982; Brelje et al., 1989, 1993; Weinhaus et al., 1996; Sorenson and Brelje, 1997; Arumugam et al., 2014). Moreover, PL protects β-cells against streptozotocin-induced cell death in mice (Fujinaka et al., 2004). GH may also be important for modulating pancreatic insulin production (Billestrup and Nielsen, 1991; Brelje et al., 1993). However, GH from the placenta appears to be primarily important in the acquisition of insulin resistance and shifting metabolic fuel use from glucose to lipid in the mother during pregnancy (Table 1; PRL/PL/GH; Horber and Haymond, 1990; Goodman et al., 1991; Galosy and Talamantes, 1995; Barbour et al., 2002; Dominici et al., 2005; Boparai et al., 2010; Liao et al., 2016b; Sairenji et al., 2017). Placental GH reduces insulin receptor expression and signaling, as well as, diminishes the abundance of the insulin-sensitive glucose-transporter, GLUT-4, in the skeletal muscle (Barbour et al., 2004; Kirwan et al., 2004). Insulin receptor abundance and signaling in the liver is also reduced in response to increased GH abundance in transgenic mice (Dominici et al., 1999). In white adipose tissue, GH also disrupts the insulin signaling pathway, and inhibits insulin action on glucose uptake and lipid accumulation (Del Rincon et al., 2007). In part, the effects of GH may be mediated through insulin-like growth factor-1 (IGF1), which is primarily secreted from the liver in response to GH and exerts lipolytic effects during pregnancy (Randle, 1998; Sferruzzi-Perri et al., 2006; Del Rincon et al., 2007). Insulin-like growth factor-2 (IGF2), which is not directly regulated by GH, but is secreted by the placenta is also important for modulating the sensitivity of β cells to glucose (Tables 1, 2; IGF2; Casellas et al., 2015; Modi et al., 2015) and maternal insulin and glucose concentrations during pregnancy (Petry et al., 2010; Sferruzzi-Perri et al., 2011). Polymorphisms/mutations in the PRL-GH family of genes and receptors have been reported in human pregnancies associated with gestational diabetes and fetal growth restriction (Rygaard et al., 1998; Le et al., 2013). Moreover, loss of PRLR signaling in β-cells causes gestational diabetes mellitus (GDM) in mice (Banerjee et al., 2016). Taken together, the production of PRL-GH family of hormones by the placenta appears to be important in regulating both insulin production and sensitivity of the mother in response to pregnancy.
The PRL-GH family is also implicated in the regulation of appetite and body weight. For instance, exogenous PRL increases food intake through inhibiting the action of leptin in non-pregnant rats (Table 1; PRL/PL/GH; Sorenson et al., 1987; Farmer et al., 1991, 1992; Ladyman et al., 2010). In contrast, GH appears to decrease food intake in rodents through reducing ghrelin production and hypothalamic expression of appetite-stimulating neuropeptides, AgRP and NPY (Table 1; PRL/PL/GH; Farmer et al., 1991, 1992). In non-pregnant animals, GH is important for controlling body weight and composition (such as adiposity; Farmer et al., 1991, 1992; Zhou et al., 1997). However, in pregnancy, exogenous GH or GH releasing hormone (GHRH) does not appear to affect maternal weight gain in mice, although increases it in pigs (Table 1; PRL/PL/GH; Brown et al., 2012). The effect of PRL on weight gain and body adiposity is even less clear; with both no effect and an increase reported for non-pregnant and pregnant rodents.
The PRL-GH family also plays an important role in lactation and maternal behavior. In mice, a deficiency in PRLR or inhibition of PRL secretion in vivo compromises mammary gland development, differentiation and milk production; the latter of which is associated with loss of STAT5 signaling and fewer leaky tight junctions (Table 1; PRL/PL/GH; Weinhaus et al., 1996; Zhou et al., 1997). In contrast, exogenous GHRH in sheep and cows increases mammary gland milk production (Hart et al., 1985; Enright et al., 1988). There is also evidence that PRL induces maternal behaviors, such as nurturing, nursing and pup retrieval in non-pregnant rodents (Table 1; PRL/PL/GH; Bridges and Millard, 1988). Taken together, members of the PRL-GH family appear to promote changes in maternal glucose metabolism, behavior and mammary gland function which are expected to be important for supporting the growth of offspring during pregnancy and lactation.
Steroid Hormones
The placenta is a primary source of steroid hormones during pregnancy. Placental steroid hormones include estrogens and progesterone (Costa, 2016; Edey et al., 2018). In species like rodents, the corpus luteum continues to contribute to the circulating pool of steroid hormones during pregnancy, whereas in other species such as humans and ruminants, the placenta serves as the main source (Costa, 2016). Physiological effects of progesterone are mediated predominately by nuclear receptors (PR-A, PR-B) although membrane bound-type receptors (mPR) enable non-genomic actions. Steroid hormones are implicated in pregnancy complications such as gestational diabetes and preeclampsia. High progesterone and estrogen concentrations have been reported for women with gestational diabetes (Branisteanu and Mathieu, 2003; Qi et al., 2017). Moreover, placental estrogen and progesterone levels are reduced in preeclamptic patients compared with healthy pregnant women (Açikgöz et al., 2013).
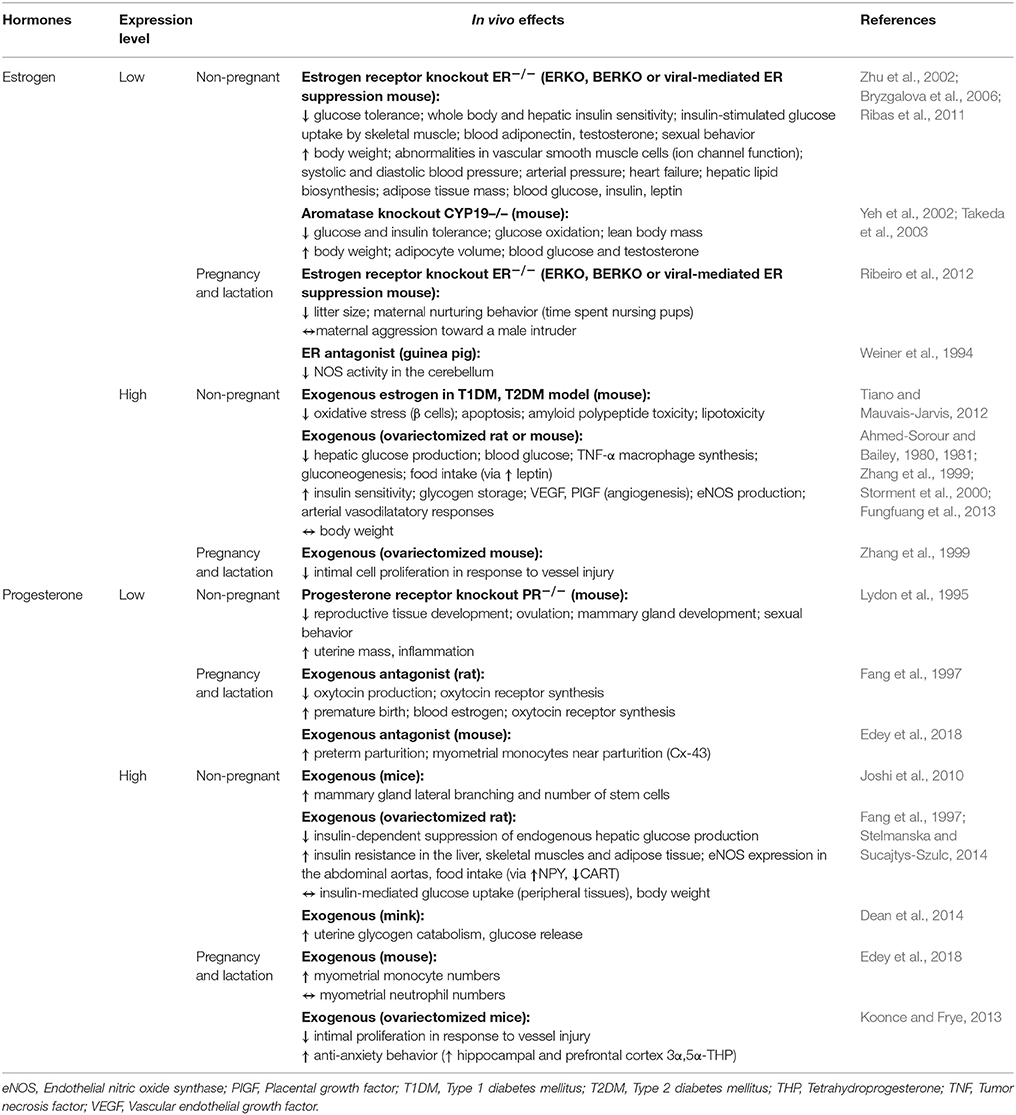
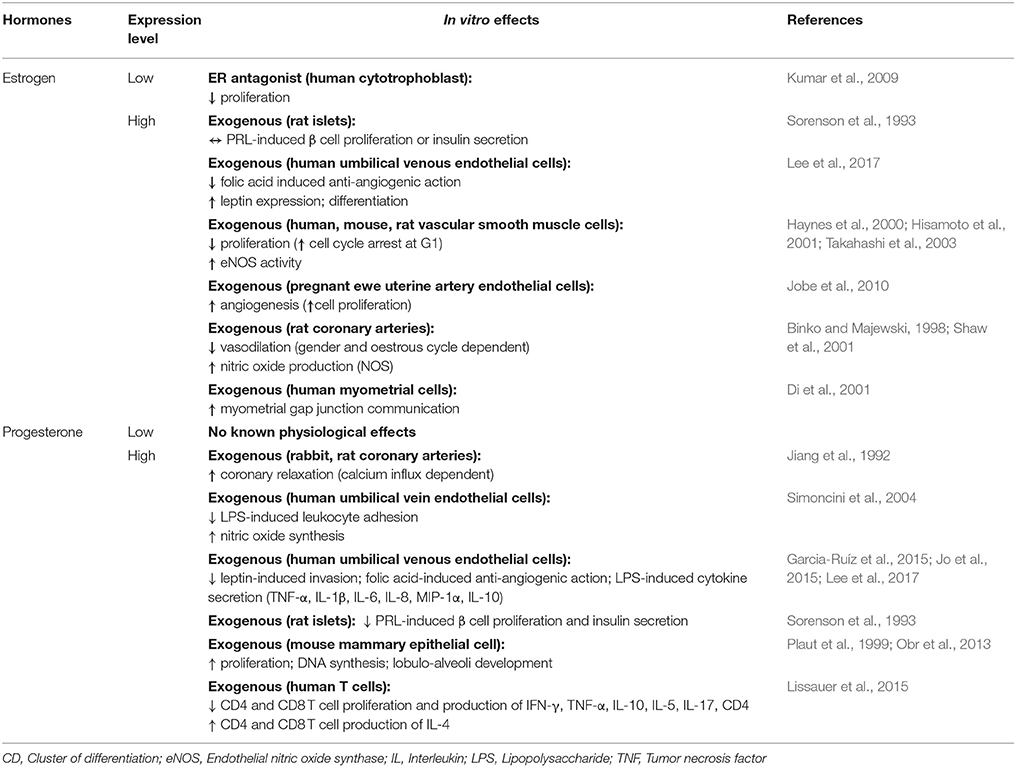
Studies performed in vivo, suggest placental steroid hormones may be important in driving the changes in insulin sensitivity and glucose metabolism of the mother during pregnancy (Table 3). Hyperinsulinemic-euglycemic clamp studies in women and rodents highlight a role for progesterone in reducing maternal insulin sensitivity during pregnancy. Progesterone administration decreases the ability of insulin to inhibit glucose production by the liver, and diminishes insulin-stimulated glucose uptake by skeletal muscle and to a lesser extent in the adipose tissue of non-pregnant animals (Table 3; Progesterone; Leturque et al., 1984; Ryan et al., 1985; Kim, 2009). In contrast, exogenous estrogen increases whole body insulin sensitivity in non-pregnant state (Table 3; Estrogen; Ahmed-Sorour and Bailey, 1980). Similarly, genetic deficiency of ERα or aromatase (Cyp19), which is involved in estrogen production, reduces hepatic and whole body insulin sensitivity and impairs glucose tolerance in non-pregnant mice (Takeda et al., 2003; Bryzgalova et al., 2006). Loss of the estrogen receptor or estrogen production is also associated with increased body weight, adiposity and hepatic lipogenesis (Table 3; Estrogen; Takeda et al., 2003; Bryzgalova et al., 2006). Progesterone and estrogen also exert opposite effects on food intake in vivo (Table 3). In particular, estrogen depresses food intake in part via induction of leptin production by adipose tissue, whereas progesterone increases food intake by enhancing NPY and reducing CART expression by the hypothalamus (Table 3; Fungfuang et al., 2013; Stelmanska and Sucajtys-Szulc, 2014). Estrogen and progesterone however seem to have similar effects on the pancreas; they both appear to induce islet hypertrophy and/or increase pancreatic insulin levels and glucose-stimulated secretion in vivo (Table 3; Costrini and Kalkhoff, 1971; Bailey and Ahmed-Sorour, 1980). Nevertheless, there is some evidence that progesterone may inhibit the PRL-induced proliferation and insulin secretion of β cells in vitro (Table 4; Progesterone; Sorenson et al., 1993). Furthermore, in rodent models of type 1 and 2 diabetes mellitus, estrogen supplementation protects pancreatic β-cells from oxidative stress, lipotoxicity and apoptosis (Table 3; Estrogen; Tiano and Mauvais-Jarvis, 2012). Therefore, both estrogen and progesterone play roles in regulating insulin and glucose homeostasis, lipid handling and appetite regulation, which may be important in promoting metabolic changes in the mother during pregnancy.
Work conducted both in vitro and in vivo indicate that estrogen and progesterone may also facilitate some of the cardiovascular changes that accompany pregnancy (Tables 3, 4). Estrogen attenuates the vasoconstrictor responses of blood vessels, impairs vascular smooth muscle cell proliferation and calcium influx, and increases vasodilatory nitric oxide synthase activity in vitro (Table 4; Estrogen; Takahashi et al., 2003). It also increases uterine artery angiogenesis and amplifies the vasodilatory impact of vascular endothelial growth factor on isolated rat uterine vessels (Storment et al., 2000; Jobe et al., 2010). In non-pregnant mice, deficiency of the ERβ gene leads to defects in vascular smooth muscle function, hypertension and signs of heart failure (Table 4; Estrogen; Zhu et al., 2002; Fliegner et al., 2010). Conversely, estrogen supplementation appears to protect the heart and vasculature from pressure overload or vessel injury (Zhang et al., 1999; Zhu et al., 2002; Fliegner et al., 2010). Progesterone also exerts cardiovascular effects. It stimulates nitric oxide synthesis by human umbilical vein endothelial cells in vitro and by rat abdominal aorta and mesenteric arteries in vivo (Tables 3, 4; Progesterone; Chataigneau et al., 2004; Simoncini et al., 2004). It also decreases blood pressure, when infused into ovariectomised ewes and protects against vascular injury in non-pregnant mice (Pecins-Thompson and Keller-Wood, 1997; Zhang et al., 1999). In culture, progesterone induces hypertrophy and inhibits apoptosis of rodent cardiomyocytes (Morrissy et al., 2010; Chung et al., 2012). Thus, via its impacts on cardiomyocytes, progesterone may mediate the pregnancy-induced growth of the mother's heart in vivo. In late pregnancy, the murine heart shifts to use fatty acids, rather than glucose and lactate, as a metabolic fuel. In part, this metabolic shift is proposed to be mediated by progesterone during pregnancy, which inhibits pyruvate dehydrogenase activity in ventricular myocytes (Liu et al., 2017). Thus, placental-derived progesterone and estrogen may mediate part of the changes in the maternal cardiovascular system during pregnancy.
In many mammalian species, progesterone levels decline just before parturition and this is associated with the initiation of labor. Indeed, in rodents, inhibition of progesterone synthesis or administration of a progesterone antagonist results in premature delivery of the neonate (Table 3; Progesterone; Fang et al., 1997; Kota et al., 2013). In humans, circulating progesterone levels continue to be high until birth. Commencement of labor is therefore proposed to be related to a functional withdrawal of progesterone activity in the myometrium of women (Brown A. G. et al., 2004; Norwitz and Caughey, 2011). In experimental animals, progesterone reduces the production of prostaglandins and decreases the expression of contraction-associated genes including oxytocin and prostaglandin receptors, gap junction proteins and ion channels in the myometrium (Table 3; Progesterone; Fang et al., 1997; Soloff et al., 2011; Edey et al., 2018). Together, these progesterone-mediated actions decrease contractility of uterine smooth muscle cells and maintain uterine quiescence until term. In contrast to progesterone, estrogen levels rise prior to term and estrogen promotes the expression of contraction-associated genes and contraction of the myometrium (Table 4; Estrogen; Nathanielsz et al., 1998; Di et al., 2001; Chandran et al., 2014). Therefore, in many species, the high ratio of estrogen to progesterone in the maternal circulation is thought to contribute the onset of labor. Parturition is associated with an influx of inflammatory cells and release of pro-inflammatory cytokines, including interleukin (IL)-1β and tumor necrosis factor (TNF)-α, in the myometrium, cervix and fetal membranes (Golightly et al., 2011). In mice, progesterone reduces the expression of pro-inflammatory cytokines, including IL-1β and IL-6 by the uterus and trophoblast and may modulate the abundance of myometrial monocytes (Table 3; Estrogen; Edey et al., 2018). Progesterone also decreases the ability of LPS to induce pro-inflammatory cytokine secretion by human myometrium and placental explants (Youssef et al., 2009; Garcia-Ruíz et al., 2015). It also diminishes the ability of estrogen to induce the infiltration of macrophages and neutrophils into the uterus, and decreases LPS-induced leukocyte adhesion to human umbilical vein cells (Simoncini et al., 2004). Thus, it is perhaps not surprising that progesterone receptor null mice demonstrate chronic uterine inflammation, particularly in response to estrogen treatment (Table 3; Estrogen; Lydon et al., 1995). There is also evidence that placental steroids participate in cervical softening, by regulating the expression of matrix remodeling enzymes as well as leukocyte infiltration and function (Chinnathambi et al., 2014; Gopalakrishnan et al., 2016; Berkane et al., 2017). In addition to regulating the events leading to parturition, recent data suggest that during the course of pregnancy, both estrogen and progesterone contribute to the maternal tolerance of the fetus by modulating proliferation and cytokine expression of CD4 and CD8 T cells and enhancing the suppressive function of T-regulatory cells (Mao et al., 2010; Robinson and Klein, 2012; Lissauer et al., 2015).
Additionally, both estrogen and progesterone are key stimulators of mammary gland development. For instance, progesterone stimulates proliferation of mammary stem cells and mammary epithelium (Tables 3, 4; Progesterone; Joshi et al., 2010; Lee et al., 2013). In mice, deficiency of the progesterone receptor restricts mammary gland development, whereas exogenous progesterone induces ductal side branching and lobuloalveolar differentiation and development (Table 3; Progesterone; Plaut et al., 1999; Joshi et al., 2010). In addition, both estrogen and progesterone may have indirect effects on mammary gland development by regulating prolactin secretion from the pituitary gland (Rezaei et al., 2016).
Maternal behavior during and after birth are regulated by the steroid hormones. Estrogen stimulates maternal nurturing behavior in numerous species, including rats, mice, sheep and primates (Bridges, 2015). In particular, maternal care is induced by estrogen treatment, whereas the converse happens when ERα expression is suppressed; deficiency of ERα increases the latency to pup retrieval and reduces the length of time dams spend nursing and licking their pups (Table 3; Estrogen; Ribeiro et al., 2012). Findings from animal models suggest that progesterone plays a role in regulating anxiety and depression-related behavior. For instance, exogenous progesterone stimulates anti-anxiety and anti-depressive actions in mouse dams (Table 3; Progesterone; Koonce and Frye, 2013). In contrast, progesterone withdrawal increases these types of behaviors (Gulinello et al., 2002). Thus, placental-derived steroids may modulate several aspects of maternal physiology which are beneficial to both pregnancy and post-partum support of the offspring.
Neuroactive Hormones
One major target of placental hormones is the maternal brain and related neuroendocrine organs such as the hypothalamus and pituitary glands. These neuroendocrine effects enable the mother to respond and adapt accordingly to her environment, so as to mitigate the adverse effects of stress and maintain homeostasis (Voltolini and Petraglia, 2014). Neuroactive hormones also prepare and enable the future mother to adequately care for her young (Lévy, 2016). In addition to their impact on the maternal neuroendocrine system, these hormones have additional functions in vivo and in vitro functions as well, which are detailed in Tables 5, 6, respectively.
Melatonin and Serotonin
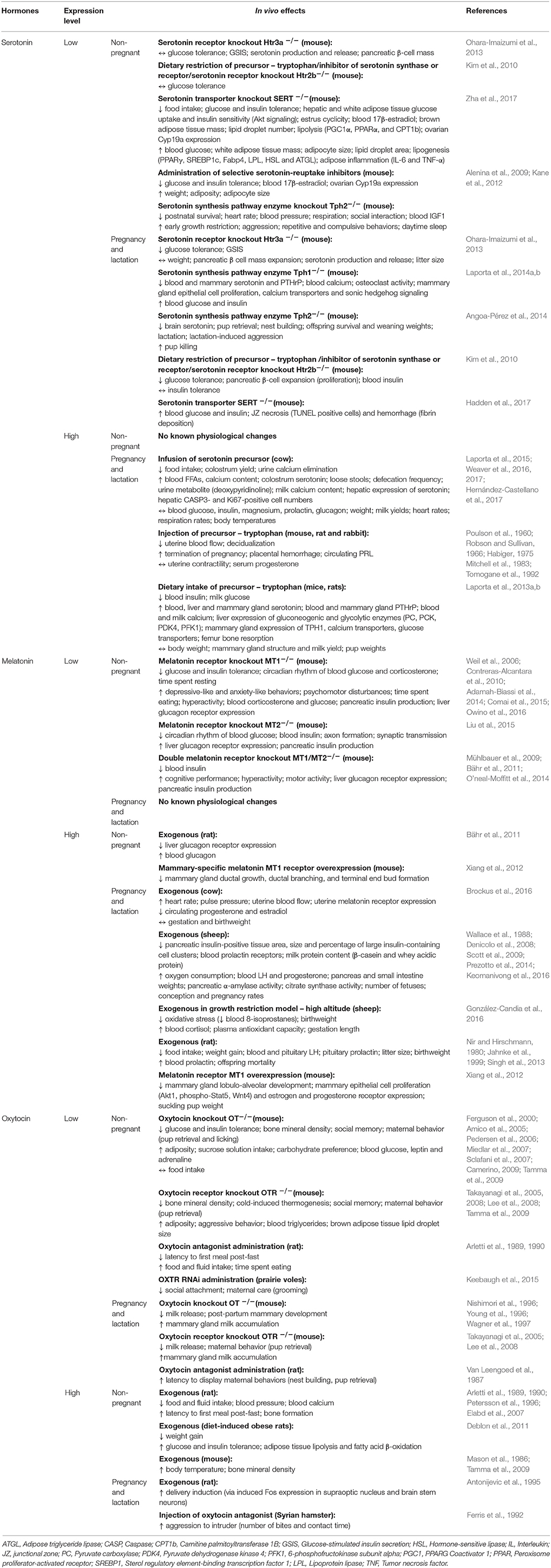
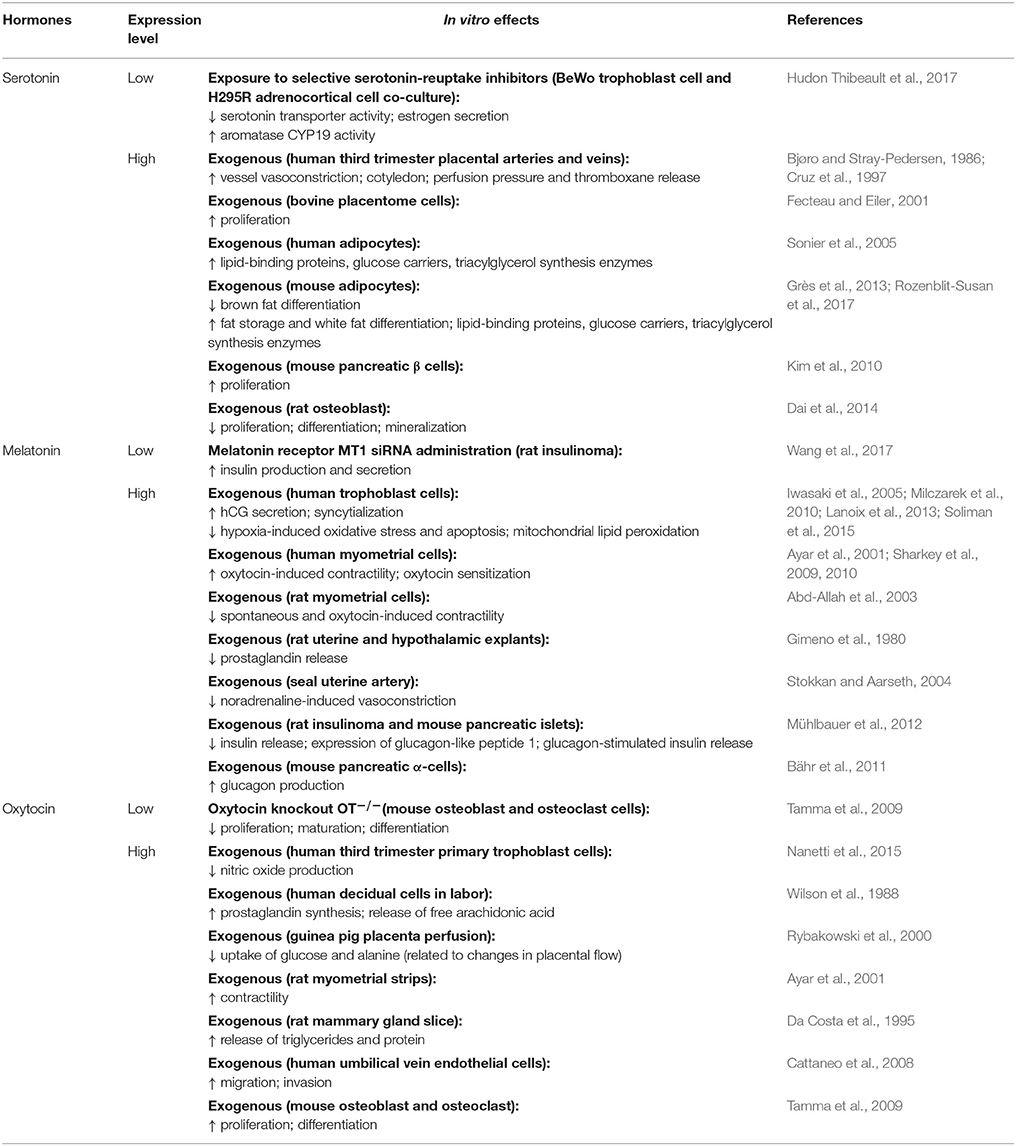
Melatonin and its precursor, serotonin, are tryptophan-derived hormones with well-known neuroendocrine impacts. In humans, circulating concentrations of melatonin and serotonin increase as pregnancy advances (Lin et al., 1996; Nakamura et al., 2001). In the non-pregnant state, melatonin and serotonin are primarily produced by the pineal gland and the brain, respectively. However, the enzymes involved in melatonin and serotonin biosynthesis are also expressed by the human placenta throughout gestation (Iwasaki et al., 2005; Soliman et al., 2015; Laurent et al., 2017). The mouse placenta similarly expresses the enzymes needed for serotonin synthesis (Wu et al., 2016), although work is required to assess if melatonin synthesizing enzymes are also expressed. The rat placenta does not produce melatonin de novo due to the lack of synthesizing enzymes (Tamura et al., 2008). However, the same study demonstrated that conditioned medium from cultured term rat placentas stimulated melatonin release by the maternal pineal gland (Tamura et al., 2008). These findings suggest that placental-derived factors may indirectly regulate melatonin levels by the mother during pregnancy. Placental expression of melatonin, serotonin and their respective enzymes, also remains to be investigated in other species such as rabbits and sheep, which are commonly used in pregnancy-related studies. Mouse models that result in deficiencies or reduced bioactivity of these hormones demonstrate altered sleep patterns, melancholic behavior, hyperactivity and aggression in the non-pregnant state (Table 5; Serotonin and Melatonin; Weil et al., 2006; Alenina et al., 2009; Kane et al., 2012; Adamah-Biassi et al., 2014; O'neal-Moffitt et al., 2014; Comai et al., 2015). Serotonin is thus a major regulator of maternal mood and behavior (Angoa-Pérez and Kuhn, 2015). For instance, genetically-induced serotonin deficiency leads to increased maternal aggression, lower pup retrieval and greater pup cannibalization, which reduces postnatal survival of offspring in mice (Angoa-Pérez et al., 2014). There is some evidence that serotonin and melatonin may also impact maternal feeding behavior. For example, increased serotonin signaling reduces food intake in pregnant cows (Laporta et al., 2015; Weaver et al., 2016, 2017; Hernández-Castellano et al., 2017). Similarly, exogenous melatonin lowers food intake in pregnant rats (Nir and Hirschmann, 1980; Jahnke et al., 1999; Singh et al., 2013). These negative effects on maternal food intake suggest that peak serotonin and melatonin concentrations in late pregnancy may serve to control the maternal appetite and prevent excessive weight gain.
Another key function of melatonin and serotonin is glucose homeostasis and the regulation of steroid synthesis (Table 5; Serotonin and Melatonin). In mice, loss of melatonin or serotonin signaling leads to glucose intolerance and insulin resistance, with consequences for blood glucose and insulin concentrations in both the non-pregnant and pregnant state (Contreras-Alcantara et al., 2010; Kim et al., 2010; Owino et al., 2016). However, these neuroactive hormones appear to have differential effects on the pancreas (Table 6; Serotonin and Melatonin). Serotonin promotes pancreatic β-cell proliferation in vitro (Kim et al., 2010), and is thus important for pancreatic β-cell mass expansion during pregnancy in mice (Goyvaerts et al., 2016). In contrast, melatonin reduces insulin release by rodent pancreatic islets in vitro (Mühlbauer et al., 2012). Non-pregnant mice with deficient serotonin signaling have impaired lipid handling and excessive lipid accumulation in association with reduced adipose aromatase expression and circulating estrogen (Zha et al., 2017). Similarly, treating placental-derived trophoblast cells with norfluoxetine, a selective serotonin-reuptake inhibitor, inhibits aromatase activity and estrogen secretion in vitro (Hudon Thibeault et al., 2017). Supplementation of melatonin in non-pregnant humans reduces circulating triglycerides and cholesterol levels, but effects of lipid handling in pregnancy are unknown (Mohammadi-Sartang et al., 2017). Melatonin also modulates steroid production. For instance, melatonin treatment in pregnant cows reduces circulating estrogen and progesterone (Brockus et al., 2016), while lack of melatonin signaling raises blood corticosterone in mice (Comai et al., 2015).
Given melatonin's additional effects on regulating the circadian rhythm (Mühlbauer et al., 2009), there is some weak evidence for its role in the timing of parturition (Yellon and Longo, 1988; González-Candia et al., 2016). Melatonin can either enhance or reduce uterine myometrial contractility depending on the species (Table 6; Melatonin; Ayar et al., 2001; Sharkey et al., 2009, 2010). Both melatonin and serotonin are also important for lactation, specifically for mammary gland development and milk nutrient content (Okatani et al., 2001; Xiang et al., 2012; Laporta et al., 2014a,b). For instance, mammary gland proliferation and calcium transport is impaired in pregnant mice with genetically-induced serotonin deficiency (Laporta et al., 2014a,b). Conversely, supplementation of a serotonin precursor increases mammary calcium transporter expression and milk calcium content in lactating mice and cows (Laporta et al., 2013a,b, 2015; Weaver et al., 2016, 2017; Hernández-Castellano et al., 2017). In contrast to serotonin, increased melatonin signaling is associated with reduced ductal growth and branching, as well as impaired terminal end bud formation in the non-pregnant state (Xiang et al., 2012). Thus, during lactation, these mice with increased melatonin signaling have impaired mammary gland lobulo-alveolar development and reduced milk protein content, which reduces the weight of suckling pups (Xiang et al., 2012). Indeed, a recent study showed antenatal melatonin supplementation further exacerbated the growth restriction of offspring and raised circulating maternal cortisol in a sheep model of fetal growth restriction (González-Candia et al., 2016). Nevertheless, melatonin supplementation during pregnancy confers significant beneficial neuroprotective effects on the fetus and enhances maternal antioxidant capacity (Miller et al., 2014; González-Candia et al., 2016; Castillo-Melendez et al., 2017). Therefore, while melatonin supplementation shows promise for use in the clinic, particularly for enhancing the neurodevelopmental outcomes of offspring in growth compromised pregnancies, the potential adverse outcomes for both mother and child must also be considered and should be assessed in further studies.
Oxytocin
Another key neuroendocrine factor is oxytocin. Oxytocin is widely known for its role in triggering maternal nursing behavior (Bosch and Neumann, 2012). This is mediated by oxytocin's actions on the maternal brain, as well as, the mammary glands. Indeed, a greater rise in circulating oxytocin concentrations from early to late pregnancy in pregnant women, is associated with a stronger bond between a mother and her infant (Levine et al., 2007). Concurrently, placental expression of oxytocin also peaks at term in humans (Kim S. C. et al., 2017). The rat placenta also produces oxytocin (Lefebvre et al., 1992), while placental expression in other species remains unclear. Reduced oxytocin signaling decreases maternal nurturing behavior such as pup retrieval in rats (Van Leengoed et al., 1987). It also decreases the willingness of female voles to care for, groom and lick unrelated pups (Keebaugh et al., 2015). Low oxytocin signaling can additionally impair social bonding in voles and mice (Ferguson et al., 2000; Takayanagi et al., 2005; Lee et al., 2008; Keebaugh et al., 2015), while high levels builds trust and cooperation in a group setting to facilitate group survival in humans (Declerck et al., 2010; De Dreu et al., 2010). Moreover, a lack of oxytocin disrupts mammary gland proliferation and lobuloalveolar development, which impairs milk release from the mammary tissues in mice (Nishimori et al., 1996; Wagner et al., 1997). Therefore, high oxytocin levels enable the mother to bond better and protect her newborn, when it is most vulnerable.
Oxytocin is also important in the process of parturition (Table 6; Oxytocin); it stimulates the contraction of smooth muscle cells in the myometrium (Ayar et al., 2001; Arrowsmith and Wray, 2014), by inducing calcium influx and stimulating prostaglandin release (Wilson et al., 1988; Voltolini and Petraglia, 2014; Kim S. H. et al., 2017). Cardiovascular effects of oxytocin include its ability to significantly lower blood pressure in non-pregnant rats (Petersson et al., 1996). There is also some evidence that oxytocin induces anti-inflammatory and antioxidant effects in the heart under hypoxic conditions in non-pregnant rats (Gutkowska and Jankowski, 2012). Nevertheless, the specific cardiovascular effects of oxytocin in pregnancy remain to be explored.
Studies performed in non-pregnant rodents show that oxytocin also affects metabolic function in vivo (Table 5; Oxytocin). In particular, loss of oxytocin reduces glucose and insulin tolerance and increases adiposity (Camerino, 2009), whereas exogenous oxytocin has the reverse effect (Deblon et al., 2011). Studies are however, required to determine whether the rise in oxytocin in late pregnancy (Levine et al., 2007) may serve to improve insulin sensitivity in the mother in preparation for the metabolic requirements of delivery and lactation. There is some evidence that oxytocin may additionally play a role in controlling energy expenditure and thermoregulation during pregnancy. Even with a similar diet and activity level to control mice, oxytocin-deficient mice become obese due to reduced energy expenditure from poor thermoregulation in the non-pregnant state (Chaves et al., 2013). Furthermore, exogenous oxytocin in non-pregnant mice causes a rise in body temperature (Mason et al., 1986; Tamma et al., 2009). Nevertheless, whether oxytocin may play a role in controlling heat dissipation due to the increased maternal energy expenditure during pregnancy requires exploration. Exogenous oxytocin also reduces food intake in non-pregnant rats (Arletti et al., 1989, 1990). However, the role of oxytocin in appetite regulation during pregnancy remains to be explored. There is also evidence for oxytocin's possible involvement in maternal bone metabolism and calcium homeostasis during pregnancy and lactation. For instance, oxytocin stimulates both bone resorption and bone formation by osteoclasts and osteoblasts respectively in vitro (Tamma et al., 2009). Moreover, oxytocin administration in rats reduces circulating calcium with an overall skew toward bone formation (Elabd et al., 2007). These findings may suggest that the peak in circulating oxytocin toward term promote the restoration of depleted maternal skeletal calcium stores.
Other Neuroactive Hormones
In addition to the aforementioned melatonin, serotonin and oxytocin, the human placenta also produces neuroactive hormones such as kisspeptin and thyrotropin-releasing hormone (TRH), which may function in adapting maternal physiology to support pregnancy (Bajoria and Babawale, 1998; De Pedro et al., 2015). In humans, circulating kisspeptin rises throughout pregnancy to concentrations 10,000-fold that of the non-pregnant state, with the placenta speculated as a major source (Horikoshi et al., 2003). In the non-pregnant state, kisspeptin can both stimulate and impede glucose stimulated insulin secretion in mice (Bowe et al., 2009; Song et al., 2014). The nature of the effect may partly relate to differences in the actions of kisspeptin isoforms on pancreatic islets (Bowe et al., 2012). Kisspeptin may also have effects on the maternal cardiovascular system, given its reported vasoconstrictive effects on vascular smooth muscle cells and fibrotic effects on the heart in non-pregnant rats (Mead et al., 2007; Zhang et al., 2017). Studies in humans highlight the importance of regulating kisspeptin production during gestation; increased placental kisspeptin is associated with pre-eclampsia (Whitehead et al., 2013; Matjila et al., 2016) and reduced circulating kisspeptin is observed in women with hypertension and diabetes during pregnancy (Cetković et al., 2012; Matjila et al., 2016). Like the human, the murine placenta produces kisspeptin. Although a kisspeptin-deficient mouse has been established, previous work has been focused on feto-placental outcomes, with no examination of maternal physiology (Herreboudt et al., 2015). Studies are required to determine the consequences of abnormal placental kisspeptin on the maternal physiology during pregnancy.
In the non-pregnant state, hypothalamic TRH stimulates release of thyroid-stimulating hormone and PRL from the pituitary (Hershman et al., 1973; Vale et al., 1973; Askew and Ramsden, 1984). However, during pregnancy, the placenta serves as an additional source of TRH (Bajoria and Babawale, 1998). Excess TRH in pregnancy raises blood concentrations of thyroid-stimulating hormone and PRL in humans, rhesus monkeys, sheep and rats (Thomas et al., 1975; Azukizawa et al., 1976; Roti et al., 1981; Moya et al., 1986; Lu et al., 1998). Conversely, a lack of TRH reduces blood PRL in mice (Rabeler et al., 2004; Yamada et al., 2006). Thyroid hormones are necessary for optimal brain development as well as thyroid function (Miranda and Sousa, 2018). Impaired TRH signaling is associated with anxiety-like and depressive-like behavior in non-pregnant mice (Zeng et al., 2007; Sun et al., 2009) and there is some evidence which suggests a link between thyroid dysfunction and poor maternal mood during pregnancy in humans (Basraon and Costantine, 2011). However, whether any direct causal relationship between placental hormones, like TRH and perinatal depression remains unclear. Additionally, TRH is implicated in glucose homeostasis and appetite regulation. For example, mice with TRH deficiency are hyperglycaemic, due to an impaired insulin response to glucose (Yamada et al., 1997). Reduced TRH signaling also impedes leptin production and ghrelin acylation, which results in less energy conservation during fasting and a lower body mass in the non-pregnant state (Groba et al., 2013; Mayerl et al., 2015). Investigations are warranted to identify whether TRH may contribute to the regulation of glucose handling and appetite in the mother during pregnancy.
Additional Hormones
The placenta also produces numerous other hormones with pleiotropic effects. Several key ones, which have been implicated in pregnancy failure or disorders of pregnancy such as hypertension, hyperglycemia and hypercalcemia, are discussed here. The hormones presented here are by no means exhaustive and were selected primarily on their major associations with abnormal maternal physiology during pregnancy. The gonadotropin, chorionic gonadotropin (CG); transforming growth factor β (TGF β) family member, activin; angiogenic factor, relaxin; bone metabolism-associated parathyroid hormone-related protein (PTHrP) and energy homeostasis regulator, leptin are reviewed (Tables 7, 8).
Chorionic Gonadotropin (CG)
CG, is secreted by the human (hCG) and equine (eCG) placenta, although hCG has been more extensively studied. hCG is a large glycoprotein composed of α and β subunits, of which the α subunit identical to luteinizing hormone (LH), follicle stimulating hormone (FSH) and thyroid stimulating hormone (TSH). As a result, hCG can interact with LH, FSH and TSH receptors. In women, hCG is secreted from the trophoblast from very early in gestation and is thought to be the first placental hormone to act on the mother (Ogueh et al., 2011). Indeed, maternal circulating hCG concentrations peak in the first trimester and then decline toward term (Ogueh et al., 2011). In early pregnancy, hCG maintains corpus luteum allowing the continued secretion of ovarian progesterone and estrogens until the steroidogenic activity of the fetal-placental unit can compensate for maternal ovarian function (Fournier et al., 2015). In particular, hCG increases the abundance of low-density lipoprotein receptor and thus uptake of cholesterol for steroidogenesis. It also enhances the expression and/or activity of steroidogenic enzymes including 3β-hydroxysteroid and aromatase. There is also some evidence which suggests hCG may inhibit factors that promote luteal demise, such as the prostaglandins. The high levels of hCG in early pregnancy are also sufficient to bind to the TSH receptor and may act to increase maternal thyroid hormone production, which as mentioned previously, may exert effects in the mother and fetus.
CG may also play important autocrine and paracrine roles at the maternal-fetal interface. Administration of hCG antisera prevents implantation in marmoset in vivo (Hearn et al., 1988). Recent proteomic analysis of estrogen and hCG treated human endometrial epithelial cells demonstrates that hCG targets pathways involved in metabolism, basement membrane and cell connectivity, proliferation and differentiation, cellular adhesion, extracellular-matrix organization, developmental growth, growth factor regulation and cell signaling (Greening et al., 2016). Such pathways are likely to be important for placental development, as attenuating hCG signaling disrupts trophoblast differentiation in vitro (Shi et al., 1993). In contrast, supplementing human trophoblast cells with hCG increases their differentiation, migration, invasion and adhesion to uterine epithelial cells, and decreases their leptin secretion in vitro (Table 8; hCG; Shi et al., 1993; Prast et al., 2008; Lee C. L. et al., 2013; Chen et al., 2015). hCG also promotes angiogenic vascular endothelial growth factor secretion by both trophoblast and endometrial epithelial cells (Islami et al., 2003a; Berndt et al., 2006) and enhances endothelial tube formation and migration (Zygmunt et al., 2002). Furthermore, hCG is key in suppressing the maternal immune system from mounting a response against paternal antigens carried by the allogenic conceptus. Administration of hCG in a mouse model of spontaneous abortion significantly reduces the number of fetal resorptions due to improved immune tolerance of the fetus (Schumacher et al., 2013). In vitro, hCG enhances proliferation of immunosuppressive uterine natural killer cells (Kane et al., 2009), and the production of immunosuppressing IL-10 by B cells (Fettke et al., 2016). hCG can also modulate the immune system even in a non-pregnant state, as shown by its efficacy in preventing the development of autoimmune diabetes in a mouse model (Khil et al., 2007). In pregnancy, hCG additionally inhibits the contractile function of smooth muscle cells in the uterus to help sustain myometrial quiescence (Ambrus and Rao, 1994; Eta et al., 1994), so as to prevent premature expulsion of the fetus. Glycosylation of hCG affects its biological activity and half-life (Fournier et al., 2015). Given its involvement with multiple systems, it is perhaps unsurprising that abnormal concentrations of hCG and hCG glycoforms have been linked with pregnancy complications such as fetal growth restriction and preeclampsia (Chen et al., 2012). However, whether the abnormal concentrations of hCG are cause or consequence of the disorders remains to be determined.
Activins
Activins are members of the TGFβ family and were first discovered for their role in stimulating FSH production and determining estrus cyclicity and fertility in mice (Ahn et al., 2004; Sandoval-Guzmán et al., 2012). Activin signaling promotes the decidualization, as well as, apoptosis of endometrial stroma cells (Table 8; Activins; Tessier et al., 2003; Clementi et al., 2013; Yong et al., 2017); processes that accommodate implantation and conceptus development (Peng et al., 2015). Additionally, activin A enhances steroid production, invasion and apoptosis of human trophoblast in vitro (Ni et al., 2000; Yu et al., 2012; Li et al., 2015). However, activins may also be of importance in modulating the physiology of the mother during pregnancy (Table 7; Activins). In normal human pregnancy, activin A concentrations gradually rise during gestation and peak at term (Fowler et al., 1998). The placenta is thought to be the main source of activin A in the maternal circulation during pregnancy, given the rapid clearance after delivery of the placenta (Muttukrishna et al., 1997; Fowler et al., 1998). A similar rise of activin in the maternal circulation is observed in pregnant ewes (Jenkin et al., 2001), while the circulating profiles in other species remain undetermined. Nevertheless, in mice, impaired activin signaling leads to poor pregnancy outcomes such as fewer viable pups (Clementi et al., 2013; Peng et al., 2015). However, there is evidence that an increase in activin may also be pathological and detrimental to pregnancy outcome. For instance in pregnant mice, infusion of activin A or plasmid overexpression of activin A results in the development of a preeclamptic phenotype; dams display hypertension and proteinuria, in addition to growth restriction and greater in utero deaths (Kim et al., 2008; Lim et al., 2015). The maternal hypertension observed likely results from pathological concentrations of activin A inducing vascular endothelial dysfunction (Yong et al., 2015). In the non-pregnant state, activins are also important for renal glomeruli development (Maeshima et al., 2000), as well as, for bone, fat and muscle metabolism (Yogosawa et al., 2013; Ding et al., 2017; Goh et al., 2017). The possible contributions of activin to these latter functions in pregnancy are currently unclear. Therefore, the impact of activin signaling on these other body systems during pregnancy remains to be determined.
Relaxin
Relaxin is a potent vasodilator (Danielson et al., 1999), and regulates hemodynamics in both the non-pregnant and pregnant state (Table 7; Relaxin; Conrad et al., 2004). In pregnant women, circulating relaxin concentration peaks in the first trimester, declines in the second trimester and is maintained until delivery in the third trimester (Quagliarello et al., 1979; Seki et al., 1985). In contrast, circulating relaxin peaks toward term in mice, rats, guinea pigs and hamsters (O'byrne and Steinetz, 1976; O'byrne et al., 1976; Renegar and Owens, 2002). In pregnant mice, relaxin deficiency leads to proteinuria, suggesting a particular role of relaxin in modulating renal function during pregnancy (O'sullivan et al., 2017). In addition, relaxin-deficient mice remain sensitive to vasoconstrictors such as angiotensin and endothelin, and are hypertensive during pregnancy (Marshall et al., 2016a; Mirabito Colafella et al., 2017). During pregnancy, relaxin-deficient mice also display stiffer uterine vessels and fetal growth is retarded (Gooi et al., 2013). Relaxin also enhances capillarisation and glucose uptake of skeletal muscles in non-pregnant mice (Bonner et al., 2013). Taken together, these data highlight the importance of relaxin in mediating changes in maternal vascular function that serve to promote blood flow to the gravid uterus during pregnancy.
Relaxin may play additional roles within the uterus that are important for implantation, placentation and pregnancy maintenance (Tables 7, 8; Relaxin). In vitro, relaxin increases decidual cell insulin-like growth factor binding protein-1 expression, a marker of decidualization (Mazella et al., 2004). It also enhances survival and proliferation of cultured human trophoblast cells (Lodhi et al., 2013; Astuti et al., 2015). During early mouse pregnancy, relaxin modulates the uterine expression of genes involved in angiogenesis, steroid hormone action and remodeling (Marshall et al., 2016b). Indeed in pregnant marmosets, exogenous relaxin improves uterine and placental growth (Einspanier et al., 2009). Relaxin infusion also alters the endometrial lymphocyte number in vivo (Goldsmith et al., 2004), which suggests a possible role of relaxin in achieving immune tolerance of the allogenic conceptus. Relaxin impedes spontaneous contractility of myometrium in humans, rats and pigs (Maclennan and Grant, 1991; Longo et al., 2003), and is thus thought to play a role in regulating the onset of parturition (Vannuccini et al., 2016). In mice with a deficiency in relaxin signaling, obstructed deliveries occur at a higher rate due to poor maturation of the cervix (Zhao et al., 1999; Kamat et al., 2004; Krajnc-Franken et al., 2004; Kaftanovskaya et al., 2015). Conversely in hamsters, the rise in circulating relaxin toward term coincides with cervical ripening in preparation for delivery (O'byrne et al., 1976). Insufficient relaxin signaling also impedes mammary development through excessive duct dilation and reduces the nursing of offspring in mice (Zhao et al., 1999; Kamat et al., 2004; Krajnc-Franken et al., 2004). Conversely, overexpression leads to hypertrophy of the nipples in non-pregnant mice (Feng et al., 2006). Hence, relaxin is important in driving changes at the maternal-fetal interface that establish pregnancy, adapts the cardiovascular system of the mother to support the pregnancy and prepares the mother for lactation post-partum.
Parathyroid Hormone-Related Protein (PTHrP)
During pregnancy, the placenta serves as an additional source of PTHrP (Bowden et al., 1994; Emly et al., 1994), a key hormone involved in bone metabolism (Table 7; PTHrP). PTHrP concentrations in the maternal blood rise throughout gestation in humans (Gallacher et al., 1994; Ardawi et al., 1997; Hirota et al., 1997) and correlate with the rise in maternal circulating calcium during pregnancy (Bertelloni et al., 1994). However, excessively high circulating PTHrP can lead to hypercalcaemia during pregnancy (Winter and Appelman-Dijkstra, 2017). PTHrP increases maternal bone resorption, thereby enabling calcium transfer from mother to fetus for bone development (Salles, 2016). Thus, it is perhaps not surprising that complete knockout of PTHrP in mice is lethal at birth in association with abnormal bone development (Karaplis et al., 1994). Carrying one defective PTHrP copy is enough to also impede bone development and reduce snout length in mice (Amizuka et al., 1996). Mammary-specific PTHrP deletion increases maternal bone mass and protects against lactation-associated bone loss by reducing bone turnover in mice (Williams et al., 1998; Vanhouten et al., 2003). However, deleting bone-specific PTHrP increases skeletal fragility, both in the non-pregnant and pregnant state (Kirby et al., 2011). PTHrP infusion of lactating goats increases mammary gland uptake calcium, phosphorous and magnesium for transfer in milk to the neonate (Barlet et al., 1992). These findings imply that a fine balance of PTHrP production by gestational and maternal tissues must be achieved for appropriate regulation of maternal bone metabolism and offspring calcium requirements during pregnancy and lactation.
Placental-derived PTHrP may also exert additional effects on the placenta and the mother which are beneficial for offspring development and growth. PTHrP stimulates the proliferation, differentiation, outgrowth and calcium uptake of trophoblast in vitro (Table 8; PTHrP; Hershberger and Tuan, 1998; El-Hashash and Kimber, 2006). In vivo, blocking PTHrP signaling during mouse pregnancy leads to excessive uterine growth and decidualization in association with a decrease in decidual cell apoptosis (Williams et al., 1998; Vanhouten et al., 2003). Moreover, over-expression of PTHrP impairs mammary gland branching morphogenesis (Wysolmerski et al., 1995; Dunbar et al., 2001). These studies highlight a possible important regulatory role of PTHrP in the control of decidualization and mammary gland development in vivo. In non-pregnant mice, PTHrP enhances pancreatic β-cells proliferation and insulin secretion whilst it inhibits islet cell apoptosis (Vasavada et al., 1996; Porter et al., 1998; Cebrian et al., 2002; Fujinaka et al., 2004). It also increases renal plasma flow and glomerular filtration rate, and exerts proliferative effects on renal glomerular and tubule cells in rodents (Izquierdo et al., 2006; Romero et al., 2010). Additionally, in vitro studies show PTHrP can induce relaxation of uterine arteries (Meziani et al., 2005). However, the significance of PTHrP on glucose-insulin dynamics and renal and vascular function of the mother during pregnancy remains to be investigated.
Leptin
Leptin is an abundant circulating hormone involved in regulating appetite. In the non-pregnant state, the adipose tissue is the exclusive source of circulating leptin. During pregnancy in humans, baboons and mice, concentrations of leptin rapidly rise throughout gestation, peaking toward term (Highman et al., 1998; Henson et al., 1999; Malik et al., 2005). The rise in leptin positively correlates with increases in maternal body fat (Highman et al., 1998). In humans, blood leptin rapidly falls to non-pregnant concentrations within 24 h of delivery, indicating that the placenta contributes to the main rise of leptin in pregnancy (Masuzaki et al., 1997). In particular, leptin is produced by the human placental trophoblast cells (Masuzaki et al., 1997). A similar post-pregnancy decline and placental trophoblast expression is seen in baboons (Henson et al., 1999). However, this is not the case for mice, as the murine placenta does not produce leptin (Malik et al., 2005). Nevertheless, leptin studies in mice still provide useful knowledge about pregnancy-related effects of leptin (Table 7; Leptin). For instance, leptin in pregnancy helps prepare the mother for lactation, as a deficiency results in impaired mammary gland development, which is detrimental for lactation post-delivery (Mounzih et al., 1998; Malik et al., 2001). Another significant effect of leptin in pregnancy observed through mouse studies is leptin resistance, whereby the dam increases her food intake in mid-pregnancy to meet increased energy demands despite an increase in circulating leptin, which in the non-pregnant state would lead to satiety (Mounzih et al., 1998). In contrast, excessive leptin significantly decreases maternal food intake and restricts feto-placental growth (Yamashita et al., 2001). Leptin exposure of rat and human islets and cultured insulinoma cells significantly decreases insulin production in vitro, demonstrating that leptin may be directly involved in glucose metabolism (Table 8; Leptin; Kulkarni et al., 1997). Indeed dysfunctional leptin signaling in pregnancy leads to the spontaneous development of a gestational diabetic phenotype in db/+ mice, who are heterozygous for the leptin receptor (Table 7; Leptin; Yamashita et al., 2001). Further in vitro studies on placental explants or trophoblast cultures highlight a potential for leptin to be involved in immune modulation and placental hormone production, given its stimulatory effects on HLA-G and hCG expression (Table 8; Leptin; Chardonnens et al., 1999; Islami et al., 2003a,b; Barrientos et al., 2015). Additional effects of leptin on the placenta are thoroughly reviewed elsewhere (Schanton et al., 2018). Therefore, placental leptin can have systemic effects on the mother in pregnancy.
Conclusion
Pregnancy represents a unique physiological paradigm; there are dynamic and reversible changes in the function of many organ systems in the mother that are designed to support offspring development. In part, these changes are signaled via the placental secretion of hormones, which in turn, alter in abundance, interact with one another and exert wide effects on maternal tissues during pregnancy. For instance, steroid hormones modulate most systems of the mother throughout pregnancy. However, they also alter the production of other hormones, such as prolactin and placental lactogens, which in turn, may contribute to the physiological changes in the mother (Figure 2). However, further work is required to better define how placental hormones elicit their actions in the mother, as well as, identify the extent to which they interplay with hormones produced by maternal tissues. As the endocrine and metabolic state of the mother is also influenced by her environment, maternal conditions such as poor nutrition and obesity may modulate placental hormone production and pregnancy adaptations. Indeed, previous work has shown that an obesogenic diet during pregnancy alters the expression of PRL/PL genes in the placenta in association with mal-adaptations of maternal metabolism in mice (Musial et al., 2017). Further studies are nonetheless needed to assess the interaction of the maternal environment with placental endocrine function. Placental hormones are also released into the fetal circulation, where they may have direct impacts on fetal growth and development (Freemark, 2010). Investigations exploring the importance of placental endocrine function on fetal growth, independent of the mother, will require future examination. Collectively, further studies on the nature and role of placental endocrine function in maternal adaptations and fetal growth will undoubtedly provide novel insights into understanding of the potential causes of obstetrical syndromes such as gestational diabetes and preeclampsia that are marked by maternal physiological maladaptation.
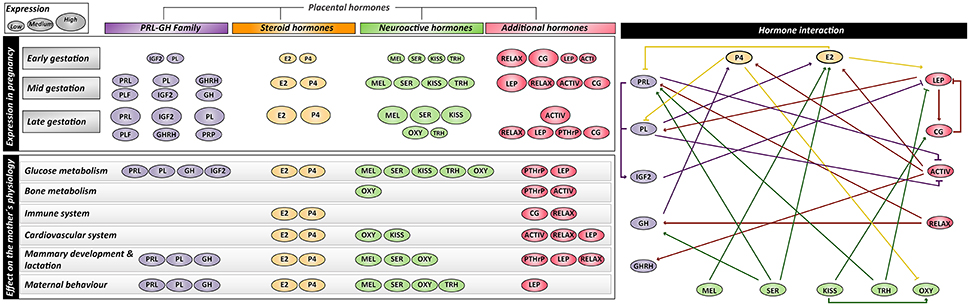
Figure 2. Summary of expression profiles, interactions and maternal physiological effects of placental-derived hormones. PRL, prolactin; PL, placental lactogen; PLF, proliferins; PRP, proliferin-related proteins; GH, growth hormone; GHRH, growth hormone releasing hormone; IGF1/2, insulin-like growth factor-1/2; E2, estrogen; P4, progesterone; MEL, melatonin; SER, serotonin; KISS, kisspeptin; OXY, Oxytocin; TRH, thyrotropin-releasing hormone; RELAX, relaxin; ACTIV, activin; CG, chorionic gonadotropin; LEP, leptin; PTHrP, parathyroid hormone-related protein.
Author Contributions
TN and HY substantially contributed to the conception of the work, drafting and revision of the manuscript, preparation of the tables and approved of the final version. JL-T substantially contributed to the conception of the work, drafting and revision of the manuscript, preparation of the figures and approved of the final version. AS-P substantially contributed to the conception of the work, critical revision of the manuscript for intellectual content and approved of the final version.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
TN was supported by the Marie Skłodowska-Curie Individual Fellowship from the European Union; HY was supported by an A*STAR International Fellowship from the Agency for Science, Technology and Research; JL-T was supported by the Newton International Fellowship from the Royal Society; AS-P was supported by the Dorothy Hodgkin Research Fellowship from the Royal Society.
References
Abd-Allah, A. R., El-Sayed El, S. M., Abdel-Wahab, M. H., and Hamada, F. M. (2003). Effect of melatonin on estrogen and progesterone receptors in relation to uterine contraction in rats. Pharmacol. Res. 47, 349–354. doi: 10.1016/S1043-6618(03)00014-8
Abribat, T., Lapierre, H., Dubreuil, P., Pelletier, G., Gaudreau, P., Brazeau, P., et al. (1990). Insulin-like growth factor-I concentration in Holstein female cattle: variations with age, stage of lactation and growth hormone-releasing factor administration. Domest. Anim. Endocrinol. 7, 93–102. doi: 10.1016/0739-7240(90)90058-8
Açikgöz, S., Bayar, U. O., Can, M., Güven, B., Mungan, G., Dogan, S., et al. (2013). Levels of oxidized LDL, estrogens, and progesterone in placenta tissues and serum paraoxonase activity in preeclampsia. Mediators Inflamm. 2013:862982. doi: 10.1155/2013/862982
Ackermann, A. M., and Gannon, M. (2007). Molecular regulation of pancreatic beta-cell mass development, maintenance, and expansion. J. Mol. Endocrinol. 38, 193–206. doi: 10.1677/JME-06-0053
Adamah-Biassi, E. B., Hudson, R. L., and Dubocovich, M. L. (2014). Genetic deletion of MT1 melatonin receptors alters spontaneous behavioral rhythms in male and female C57BL/6 mice. Horm. Behav. 66, 619–627. doi: 10.1016/j.yhbeh.2014.08.012
Adamova, Z., Ozkan, S., and Khalil, R. A. (2009). Vascular and cellular calcium in normal and hypertensive pregnancy. Curr. Clin. Pharmacol. 4, 172–190. doi: 10.2174/157488409789375320
Ahmed-Sorour, H., and Bailey, C. J. (1980). Role of ovarian hormones in the long-term control of glucose homeostasis. Interaction with insulin, glucagon and epinephrine. Horm. Res. 13, 396–403. doi: 10.1159/000179307
Ahmed-Sorour, H., and Bailey, C. J. (1981). Role of ovarian hormones in the long-term control of glucose homeostasis, glycogen formation and gluconeogenesis. Ann. Nutr. Metab. 25, 208–212. doi: 10.1159/000176496
Ahn, J. M., Jung, H. K., Cho, C., Choi, D., Mayo, K. E., and Cho, B. N. (2004). Changes in the reproductive functions of mice due to injection of a plasmid expressing an inhibin alpha-subunit into muscle: a transient transgenic model. Mol. Cells 18, 79–86.
Ahumada-Solórzano, S. M., Martínez-Moreno, C. G., Carranza, M., Ávila-Mendoza, J., Luna-Acosta, J. L., Harvey, S., et al. (2016). Autocrine/paracrine proliferative effect of ovarian GH and IGF-I in chicken granulosa cell cultures. Gen. Comp. Endocrinol. 234, 47–56. doi: 10.1016/j.ygcen.2016.05.008
Aizawa-Abe, M., Ogawa, Y., Masuzaki, H., Ebihara, K., Satoh, N., Iwai, H., et al. (2000). Pathophysiological role of leptin in obesity-related hypertension. J. Clin. Invest. 105, 1243–1252. doi: 10.1172/JCI8341
Alenina, N., Kikic, D., Todiras, M., Mosienko, V., Qadri, F., Plehm, R., et al. (2009). Growth retardation and altered autonomic control in mice lacking brain serotonin. Proc. Natl. Acad. Sci. U.S.A. 106, 10332–10337. doi: 10.1073/pnas.0810793106
Alperin, M., Kaddis, T., Pichika, R., Esparza, M. C., and Lieber, R. L. (2016). Pregnancy-induced adaptations in intramuscular extracellular matrix of rat pelvic floor muscles. Am. J. Obstet. Gynecol. 215, 210 e211–210 e217. doi: 10.1016/j.ajog.2016.02.018
Alperin, M., Lawley, D. M., Esparza, M. C., and Lieber, R. L. (2015). Pregnancy-induced adaptations in the intrinsic structure of rat pelvic floor muscles. Am. J. Obstet. Gynecol. 213, 191 e191–191 e197. doi: 10.1016/j.ajog.2015.05.012
Ambrus, G., and Rao, C. V. (1994). Novel regulation of pregnant human myometrial smooth muscle cell gap junctions by human chorionic gonadotropin. Endocrinology 135, 2772–2779. doi: 10.1210/endo.135.6.7988470
Amico, J. A., Vollmer, R. R., Cai, H. M., Miedlar, J. A., and Rinaman, L. (2005). Enhanced initial and sustained intake of sucrose solution in mice with an oxytocin gene deletion. Am. J. Physiol. Regul. Integr. Comp. Physiol. 289, R1798–R1806. doi: 10.1152/ajpregu.00558.2005
Amizuka, N., Karaplis, A. C., Henderson, J. E., Warshawsky, H., Lipman, M. L., Matsuki, Y., et al. (1996). Haploinsufficiency of parathyroid hormone-related peptide (PTHrP) results in abnormal postnatal bone development. Dev. Biol. 175, 166–176. doi: 10.1006/dbio.1996.0104
Angoa-Pérez, M., Kane, M. J., Sykes, C. E., Perrine, S. A., Church, M. W., and Kuhn, D. M. (2014). Brain serotonin determines maternal behavior and offspring survival. Genes Brain Behav. 13, 579–591. doi: 10.1111/gbb.12159
Angoa-Pérez, M., and Kuhn, D. M. (2015). Neuronal serotonin in the regulation of maternal behavior in rodents. Neurotransmitter (Houst) 2:e615. doi: 10.14800/nt.615
Antonijevic, I. A., Leng, G., Luckman, S. M., Douglas, A. J., Bicknell, R. J., and Russell, J. A. (1995). Induction of uterine activity with oxytocin in late pregnant rats replicates the expression of c-fos in neuroendocrine and brain stem neurons as seen during parturition. Endocrinology 136, 154–163. doi: 10.1210/endo.136.1.7828526
Apa, R., Lanzone, A., Miceli, F., Mastrandrea, M., Macchione, E., Caruso, A., et al. (1995). Growth hormone-releasing factor stimulates meiotic maturation in follicle- and cumulus-enclosed rat oocyte. Mol. Cell. Endocrinol. 112, 195–201. doi: 10.1016/0303-7207(95)03599-3
Ardawi, M. S., Nasrat, H. A., and BA'Aqueel, H. S. (1997). Calcium-regulating hormones and parathyroid hormone-related peptide in normal human pregnancy and postpartum: a longitudinal study. Eur. J. Endocrinol. 137, 402–409. doi: 10.1530/eje.0.1370402
Arletti, R., Benelli, A., and Bertolini, A. (1989). Influence of oxytocin on feeding behavior in the rat. Peptides 10, 89–93. doi: 10.1016/0196-9781(89)90082-X
Arletti, R., Benelli, A., and Bertolini, A. (1990). Oxytocin inhibits food and fluid intake in rats. Physiol. Behav. 48, 825–830. doi: 10.1016/0031-9384(90)90234-U
Arrowsmith, S., and Wray, S. (2014). Oxytocin: its mechanism of action and receptor signalling in the myometrium. J. Neuroendocrinol. 26, 356–369. doi: 10.1111/jne.12154
Arumugam, R., Fleenor, D., and Freemark, M. (2014). Knockdown of prolactin receptors in a pancreatic beta cell line: effects on DNA synthesis, apoptosis, and gene expression. Endocrine 46, 568–576. doi: 10.1007/s12020-013-0073-1
Askew, R. D., and Ramsden, D. B. (1984). Effect of repeated stimulation by thyrotropin-releasing hormone (TRH) on thyrotropin and prolactin secretion in perfused euthyroid and hypothyroid rat pituitary fragments. Horm. Res. 20, 269–276. doi: 10.1159/000180007
Astuti, Y., Nakabayashi, K., Deguchi, M., Ebina, Y., and Yamada, H. (2015). Human recombinant H2 relaxin induces AKT and GSK3beta phosphorylation and HTR-8/SVneo cell proliferation. Kobe J. Med. Sci. 61, E1–8. doi: 10.24546/81008925
Atherton, J. C., Dark, J. M., Garland, H. O., Morgan, M. R., Pidgeon, J., and Soni, S. (1982). Changes in water and electrolyte balance, plasma volume and composition during pregnancy in the rat. J. Physiol. (Lond). 330, 81–93. doi: 10.1113/jphysiol.1982.sp014330
Ayar, A., Kutlu, S., Yilmaz, B., and Kelestimur, H. (2001). Melatonin inhibits spontaneous and oxytocin-induced contractions of rat myometrium in vitro. Neuro Endocrinol. Lett. 22, 199–207.
Azukizawa, M., Murata, Y., Ikenoue, T., Martin, C. B. Jr., and Hershman, J. M. (1976). Effect of thyrotropin-releasing hormone on secretion of thyrotropin, prolactin, thyroxine, and triiodothyronine in pregnant and fetal rhesus monkeys. J. Clin. Endocrinol. Metab. 43, 1020–1028. doi: 10.1210/jcem-43-5-1020
Bacq, Y. (2013). “The liver in normal pregnancy,” in Madame Curie Bioscience Database. (Austin, TX: Landes Bioscience).
Bader, R. A., Bader, M. E., Rose, D. F., and Braunwald, E. (1955). Hemodynamics at rest and during exercise in normal pregnancy as studies by cardiac catheterization. J. Clin. Invest. 34, 1524–1536. doi: 10.1172/JCI103205
Bae, M. H., Lee, M. J., Bae, S. K., Lee, O. H., Lee, Y. M., Park, B. C., et al. (1998). Insulin-like growth factor II (IGF-II) secreted from HepG2 human hepatocellular carcinoma cells shows angiogenic activity. Cancer Lett. 128, 41–46. doi: 10.1016/S0304-3835(98)00044-5
Baeyens, L., Hindi, S., Sorenson, R. L., and German, M. S. (2016). beta-Cell adaptation in pregnancy. Diabetes Obes. Metab. 18(Suppl. 1), 63–70. doi: 10.1111/dom.12716
Bähr, I., Mühlbauer, E., Schucht, H., and Peschke, E. (2011). Melatonin stimulates glucagon secretion in vitro and in vivo. J. Pineal Res. 50, 336–344. doi: 10.1111/j.1600-079X.2010.00848.x
Bailey, C. J., and Ahmed-Sorour, H. (1980). Role of ovarian hormones in the long-term control of glucose homeostasis. Effects of insulin secretion. Diabetologia 19, 475–481. doi: 10.1007/BF00281829
Bajoria, R., and Babawale, M. (1998). Ontogeny of endogenous secretion of immunoreactive-thyrotropin releasing hormone by the human placenta. J. Clin. Endocrinol. Metab. 83, 4148–4155. doi: 10.1210/jcem.83.11.5216
Banerjee, R. R., Cyphert, H. A., Walker, E. M., Chakravarthy, H., Peiris, H., Gu, X., et al. (2016). Gestational diabetes mellitus from inactivation of prolactin receptor and mafb in islet beta-cells. Diabetes 65, 2331–2341. doi: 10.2337/db15-1527
Bani, G., Maurizi, M., Bigazzi, M., and Bani Sacchi, T. (1995). Effects of relaxin on the endometrial stroma. Studies in mice. Biol. Reprod 53, 253–262. doi: 10.1095/biolreprod53.2.253
Barbour, L. A., Shao, J., Qiao, L., Leitner, W., Anderson, M., Friedman, J. E., et al. (2004). Human placental growth hormone increases expression of the p85 regulatory unit of phosphatidylinositol 3-kinase and triggers severe insulin resistance in skeletal muscle. Endocrinology 145, 1144–1150. doi: 10.1210/en.2003-1297
Barbour, L. A., Shao, J., Qiao, L., Pulawa, L. K., Jensen, D. R., Bartke, A., et al. (2002). Human placental growth hormone causes severe insulin resistance in transgenic mice. Am. J. Obstet. Gynecol. 186, 512–517. doi: 10.1067/mob.2002.121256
Barker, D. J. (2004). The developmental origins of well-being. Philos. Trans. R. Soc. Lond. B Biol. Sci. 359, 1359–1366. doi: 10.1098/rstb.2004.1518
Barlet, J. P., Champredon, C., Coxam, V., Davicco, M. J., and Tressol, J. C. (1992). Parathyroid hormone-related peptide might stimulate calcium secretion into the milk of goats. J. Endocrinol. 132, 353–359. doi: 10.1677/joe.0.1320353
Barrichon, M., Hadi, T., Wendremaire, M., Ptasinski, C., Seigneuric, R., Marcion, G., et al. (2015). Dose-dependent biphasic leptin-induced proliferation is caused by non-specific IL-6/NF-kappaB pathway activation in human myometrial cells. Br. J. Pharmacol. 172, 2974–2990. doi: 10.1111/bph.13100
Barrientos, G., Toro, A., Moschansky, P., Cohen, M., Garcia, M. G., Rose, M., et al. (2015). Leptin promotes HLA-G expression on placental trophoblasts via the MEK/Erk and PI3K signaling pathways. Placenta 36, 419–426. doi: 10.1016/j.placenta.2015.01.006
Basraon, S., and Costantine, M. M. (2011). Mood disorders in pregnant women with thyroid dysfunction. Clin. Obstet. Gynecol. 54, 506–514. doi: 10.1097/GRF.0b013e3182273089
Bearfield, C., Jauniaux, E., Groome, N., Sargent, I. L., and Muttukrishna, S. (2005). The secretion and effect of inhibin A, activin A and follistatin on first-trimester trophoblasts in vitro. Eur. J. Endocrinol. 152, 909–916. doi: 10.1530/eje.1.01928
Ben-Jonathan, N., and Hugo, E. (2015). Prolactin (PRL) in adipose tissue: regulation and functions. Adv. Exp. Med. Biol. 846, 1–35. doi: 10.1007/978-3-319-12114-7_1
Berkane, N., Liere, P., Oudinet, J. P., Hertig, A., Lefèvre, G., Pluchino, N., et al. (2017). From pregnancy to preeclampsia: a key role for estrogens. Endocr. Rev. 38, 123–144. doi: 10.1210/er.2016-1065
Berndt, S., Perrier D'hauterive, S., Blacher, S., Péqueux, C., Lorquet, S., Munaut, C., et al. (2006). Angiogenic activity of human chorionic gonadotropin through LH receptor activation on endothelial and epithelial cells of the endometrium. FASEB J. 20, 2630–2632. doi: 10.1096/fj.06-5885fje
Bertelloni, S., Baroncelli, G. I., Pelletti, A., Battini, R., and Saggese, G. (1994). Parathyroid hormone-related protein in healthy pregnant women. Calcif. Tissue Int. 54, 195–197. doi: 10.1007/BF00301677
Bethea, C. L., Cronin, M. J., Haluska, G. J., and Novy, M. J. (1989). The effect of relaxin infusion on prolactin and growth hormone secretion in monkeys. J. Clin. Endocrinol. Metab. 69, 956–962. doi: 10.1210/jcem-69-5-956
Billestrup, N., and Nielsen, J. H. (1991). The stimulatory effect of growth hormone, prolactin, and placental lactogen on beta-cell proliferation is not mediated by insulin-like growth factor-I. Endocrinology 129, 883–888. doi: 10.1210/endo-129-2-883
Binart, N., Helloco, C., Ormandy, C. J., Barra, J., Clément-Lacroix, P., Baran, N., et al. (2000). Rescue of preimplantatory egg development and embryo implantation in prolactin receptor-deficient mice after progesterone administration. Endocrinology 141, 2691–2697. doi: 10.1210/endo.141.7.7568
Binko, J., and Majewski, H. (1998). 17β-Estradiol reduces vasoconstriction in endothelium-denuded rat aortas through inducible NOS. Am. J. Physiol. Heart Circ. Physiol. 274, H853–H859. doi: 10.1152/ajpheart.1998.274.3.H853
Bittorf, T., Jaster, R., Soares, M. J., Seiler, J., Brock, J., Friese, K., et al. (2000). Induction of erythroid proliferation and differentiation by a trophoblast-specific cytokine involves activation of the JAK/STAT pathway. J. Mol. Endocrinol. 25, 253–262. doi: 10.1677/jme.0.0250253
Bjøro, K., and Stray-Pedersen, S. (1986). Effects of vasoactive autacoids on different segments of human umbilicoplacental vessels. Gynecol. Obstet. Invest. 22, 1–6. doi: 10.1159/000298881
Blanchard, M. M., Goodyer, C. G., Charrier, J., Kann, G., Garcia-Villar, R., Bousquet-Melou, A., et al. (1991). GRF treatment of late pregnant ewes alters maternal and fetal somatotropic axis activity. Am. J. Physiol. 260, E575–580. doi: 10.1152/ajpendo.1991.260.4.E575
Bonner, J. S., Lantier, L., Hocking, K. M., Kang, L., Owolabi, M., James, F. D., et al. (2013). Relaxin treatment reverses insulin resistance in mice fed a high-fat diet. Diabetes 62, 3251–3260. doi: 10.2337/db13-0033
Boparai, R. K., Arum, O., Khardori, R., and Bartke, A. (2010). Glucose homeostasis and insulin sensitivity in growth hormone-transgenic mice: a cross-sectional analysis. Biol. Chem. 391, 1149–1155. doi: 10.1515/bc.2010.124
Bosch, O. J., and Neumann, I. D. (2012). Both oxytocin and vasopressin are mediators of maternal care and aggression in rodents: from central release to sites of action. Horm. Behav. 61, 293–303. doi: 10.1016/j.yhbeh.2011.11.002
Bowden, S. J., Emly, J. F., Hughes, S. V., Powell, G., Ahmed, A., Whittle, M. J., et al. (1994). Parathyroid hormone-related protein in human term placenta and membranes. J. Endocrinol. 142, 217–224. doi: 10.1677/joe.0.1420217
Bowe, J. E., Foot, V. L., Amiel, S. A., Huang, G. C., Lamb, M. W., Lakey, J., et al. (2012). GPR54 peptide agonists stimulate insulin secretion from murine, porcine and human islets. Islets 4, 20–23. doi: 10.4161/isl.18261
Bowe, J. E., King, A. J., Kinsey-Jones, J. S., Foot, V. L., Li, X. F., O'byrne, K. T., et al. (2009). Kisspeptin stimulation of insulin secretion: mechanisms of action in mouse islets and rats. Diabetologia 52, 855–862. doi: 10.1007/s00125-009-1283-1
Branisteanu, D. D., and Mathieu, C. (2003). Progesterone in gestational diabetes mellitus: guilty or not guilty? Trends Endocrinol. Metab. 14, 54–56. doi: 10.1016/S1043-2760(03)00003-1
Brelje, T. C., Allaire, P., Hegre, O., and Sorenson, R. L. (1989). Effect of prolactin versus growth hormone on islet function and the importance of using homologous mammosomatotropic hormones. Endocrinology 125, 2392–2399. doi: 10.1210/endo-125-5-2392
Brelje, T. C., Scharp, D. W., Lacy, P. E., Ogren, L., Talamantes, F., Robertson, M., et al. (1993). Effect of homologous placental lactogens, prolactins, and growth hormones on islet B-cell division and insulin secretion in rat, mouse, and human islets: implication for placental lactogen regulation of islet function during pregnancy. Endocrinology 132, 879–887. doi: 10.1210/endo.132.2.8425500
Bridges, R. S. (2015). Neuroendocrine regulation of maternal behavior. Front. Neuroendocrinol. 36, 178–196. doi: 10.1016/j.yfrne.2014.11.007
Bridges, R. S., and Millard, W. J. (1988). Growth hormone is secreted by ectopic pituitary grafts and stimulates maternal behavior in rats. Horm. Behav. 22, 194–206. doi: 10.1016/0018-506X(88)90066-9
Bridges, R. S., Robertson, M. C., Shiu, R. P., Sturgis, J. D., Henriquez, B. M., and Mann, P. E. (1997). Central lactogenic regulation of maternal behavior in rats: steroid dependence, hormone specificity, and behavioral potencies of rat prolactin and rat placental lactogen I. Endocrinology 138, 756–763. doi: 10.1210/endo.138.2.4921
Brockus, K. E., Hart, C. G., Gilfeather, C. L., Fleming, B. O., and Lemley, C. O. (2016). Dietary melatonin alters uterine artery hemodynamics in pregnant Holstein heifers. Domest. Anim. Endocrinol. 55, 1–10. doi: 10.1016/j.domaniend.2015.10.006
Brown, A. G., Leite, R. S., and Strauss, J. F. III. (2004). Mechanisms underlying “functional” progesterone withdrawal at parturition. Ann. N. Y. Acad. Sci. 1034, 36–49. doi: 10.1196/annals.1335.004
Brown, P. A., Davis, W. C., and Draghia-Akli, R. (2004). Immune-enhancing effects of growth hormone-releasing hormone delivered by plasmid injection and electroporation. Mol. Ther. 10, 644–651. doi: 10.1016/j.ymthe.2004.06.1015
Brown, P. A., Khan, A. S., Draghia-Akli, R., Pope, M. A., Bodles-Brakhop, A. M., and Kern, D. R. (2012). Effects of administration of two growth hormone-releasing hormone plasmids to gilts on sow and litter performance for the subsequent three gestations. Am. J. Vet. Res. 73, 1428–1434. doi: 10.2460/ajvr.73.9.1428
Bryant-Greenwood, G. D., Yamamoto, S. Y., Sadowsky, D. W., Gravett, M. G., and Novy, M. J. (2009). Relaxin stimulates interleukin-6 and interleukin-8 secretion from the extraplacental chorionic cytotrophoblast. Placenta 30, 599–606. doi: 10.1016/j.placenta.2009.04.009
Bryzgalova, G., Gao, H., Ahren, B., Zierath, J. R., Galuska, D., Steiler, T. L., et al. (2006). Evidence that oestrogen receptor-alpha plays an important role in the regulation of glucose homeostasis in mice: insulin sensitivity in the liver. Diabetologia 49, 588–597. doi: 10.1007/s00125-005-0105-3
Bustamante, J. J., Copple, B. L., Soares, M. J., and Dai, G. (2010). Gene profiling of maternal hepatic adaptations to pregnancy. Liver Int. 30, 406–415. doi: 10.1111/j.1478-3231.2009.02183.x
Bustamante, J. J., Dai, G., and Soares, M. J. (2008). Pregnancy and lactation modulate maternal splenic growth and development of the erythroid lineage in the rat and mouse. Reprod. Fertil. Dev. 20, 303–310. doi: 10.1071/RD07106
Cameo, P., Bischof, P., and Calvo, J. C. (2003). Effect of leptin on progesterone, human chorionic gonadotropin, and interleukin-6 secretion by human term trophoblast cells in culture. Biol. Reprod. 68, 472–477. doi: 10.1095/biolreprod.102.006122
Camerino, C. (2009). Low sympathetic tone and obese phenotype in oxytocin-deficient mice. Obesity (Silver. Spring). 17, 980–984. doi: 10.1038/oby.2009.12
Carter, A. M. (2012). Evolution of placental function in mammals: the molecular basis of gas and nutrient transfer, hormone secretion, and immune responses. Physiol. Rev. 92, 1543–1576. doi: 10.1152/physrev.00040.2011
Casellas, A., Mallol, C., Salavert, A., Jimenez, V., Garcia, M., Agudo, J., et al. (2015). Insulin-like growth factor 2 overexpression induces beta-cell dysfunction and increases beta-cell susceptibility to damage. J. Biol. Chem. 290, 16772–16785. doi: 10.1074/jbc.M115.642041
Castellucci, M., De Matteis, R., Meisser, A., Cancello, R., Monsurrò, V., Islami, D., et al. (2000). Leptin modulates extracellular matrix molecules and metalloproteinases: possible implications for trophoblast invasion. Mol. Hum. Reprod. 6, 951–958. doi: 10.1093/molehr/6.10.951
Castillo-Melendez, M., Yawno, T., Sutherland, A., Jenkin, G., Wallace, E. M., and Miller, S. L. (2017). effects of antenatal melatonin treatment on the cerebral vasculature in an ovine model of fetal growth restriction. Dev. Neurosci. 39, 323–337. doi: 10.1159/000471797
Catalano, P. M., Hoegh, M., Minium, J., Huston-Presley, L., Bernard, S., Kalhan, S., et al. (2006). Adiponectin in human pregnancy: implications for regulation of glucose and lipid metabolism. Diabetologia 49, 1677–1685. doi: 10.1007/s00125-006-0264-x
Cattaneo, M. G., Chini, B., and Vicentini, L. M. (2008). Oxytocin stimulates migration and invasion in human endothelial cells. Br. J. Pharmacol. 153, 728–736. doi: 10.1038/sj.bjp.0707609
Cebrian, A., García-Ocaña, A., Takane, K. K., Sipula, D., Stewart, A. F., and Vasavada, R. C. (2002). Overexpression of parathyroid hormone-related protein inhibits pancreatic beta-cell death in vivo and in vitro. Diabetes 51, 3003–3013. doi: 10.2337/diabetes.51.10.3003
Cetković, A., Miljic, D., Ljubić, A., Patterson, M., Ghatei, M., Stamenkovic, J., et al. (2012). Plasma kisspeptin levels in pregnancies with diabetes and hypertensive disease as a potential marker of placental dysfunction and adverse perinatal outcome. Endocr. Res. 37, 78–88. doi: 10.3109/07435800.2011.639319
Chandran, S., Cairns, M. T., O'brien, M., and Smith, T. J. (2014). Transcriptomic effects of estradiol treatment on cultured human uterine smooth muscle cells. Mol. Cell. Endocrinol. 393, 16–23. doi: 10.1016/j.mce.2014.05.020
Chang, J., and Streitman, D. (2012). Physiologic adaptations to pregnancy. Neurol. Clin. 30, 781–789. doi: 10.1016/j.ncl.2012.05.001
Chapman, A. B., Abraham, W. T., Zamudio, S., Coffin, C., Merouani, A., Young, D., et al. (1998). Temporal relationships between hormonal and hemodynamic changes in early human pregnancy. Kidney Int. 54, 2056–2063. doi: 10.1046/j.1523-1755.1998.00217.x
Chardonnens, D., Cameo, P., Aubert, M. L., Pralong, F. P., Islami, D., Campana, A., et al. (1999). Modulation of human cytotrophoblastic leptin secretion by interleukin-1α and 17β-oestradiol and its effect on HCG secretion. Mol. Hum. Reprod. 5, 1077–1082. doi: 10.1093/molehr/5.11.1077
Chataigneau, T., Zerr, M., Chataigneau, M., Hudlett, F., Hirn, C., Pernot, F., et al. (2004). Chronic treatment with progesterone but not medroxyprogesterone acetate restores the endothelial control of vascular tone in the mesenteric artery of ovariectomized rats. Menopause 11, 255–263. doi: 10.1097/01.GME.0000097847.95550.E3
Chaves, V. E., Tilelli, C. Q., Brito, N. A., and Brito, M. N. (2013). Role of oxytocin in energy metabolism. Peptides 45, 9–14. doi: 10.1016/j.peptides.2013.04.010
Chehab, F. F., Lim, M. E., and Lu, R. (1996). Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nat. Genet. 12, 318–320. doi: 10.1038/ng0396-318
Chehab, F. F., Mounzih, K., Lu, R., and Lim, M. E. (1997). Early onset of reproductive function in normal female mice treated with leptin. Science 275, 88–90. doi: 10.1126/science.275.5296.88
Chen, J. Z., Sheehan, P. M., Brennecke, S. P., and Keogh, R. J. (2012). Vessel remodelling, pregnancy hormones and extravillous trophoblast function. Mol. Cell. Endocrinol. 349, 138–144. doi: 10.1016/j.mce.2011.10.014
Chen, L., Xie, Y., Fan, J., Sui, L., Xu, Y., Zhang, N., et al. (2015). HCG induces beta1,4-GalT I expression and promotes embryo implantation. Int. J. Clin. Exp. Pathol. 8, 4673–4683.
Cheung, K. L., and Lafayette, R. A. (2013). Renal physiology of pregnancy. Adv. Chronic Kidney Dis. 20, 209–214. doi: 10.1053/j.ackd.2013.01.012
Chinnathambi, V., Blesson, C. S., Vincent, K. L., Saade, G. R., Hankins, G. D., Yallampalli, C., et al. (2014). Elevated testosterone levels during rat pregnancy cause hypersensitivity to angiotensin II and attenuation of endothelium-dependent vasodilation in uterine arteries. Hypertension 64, 405–414. doi: 10.1161/HYPERTENSIONAHA.114.03283
Chung, E., Yeung, F., and Leinwand, L. A. (2012). Akt and MAPK signaling mediate pregnancy-induced cardiac adaptation. J Appl. Physiol. (1985) 112, 1564–1575. doi: 10.1152/japplphysiol.00027.2012
Chung, W. K., Belfi, K., Chua, M., Wiley, J., Mackintosh, R., Nicolson, M., et al. (1998). Heterozygosity for Lep(ob) or Lep(rdb) affects body composition and leptin homeostasis in adult mice. Am. J. Physiol. 274, R985–R990.
Clarke, A. G., and Kendall, M. D. (1994). The thymus in pregnancy: the interplay of neural, endocrine and immune influences. Immunol. Today 15, 545–551. doi: 10.1016/0167-5699(94)90212-7
Clementi, C., Tripurani, S. K., Large, M. J., Edson, M. A., Creighton, C. J., Hawkins, S. M., et al. (2013). Activin-like kinase 2 functions in peri-implantation uterine signaling in mice and humans. PLoS Genet. 9:e1003863. doi: 10.1371/journal.pgen.1003863
Comai, S., Ochoa-Sanchez, R., Dominguez-Lopez, S., Bambico, F. R., and Gobbi, G. (2015). Melancholic-Like behaviors and circadian neurobiological abnormalities in melatonin MT1 receptor knockout mice. Int. J. Neuropsychopharmacol. 18. doi: 10.1093/ijnp/pyu075
Conrad, K. P., Debrah, D. O., Novak, J., Danielson, L. A., and Shroff, S. G. (2004). Relaxin modifies systemic arterial resistance and compliance in conscious, nonpregnant rats. Endocrinology 145, 3289–3296. doi: 10.1210/en.2003-1612
Contreras-Alcantara, S., Baba, K., and Tosini, G. (2010). Removal of melatonin receptor type 1 induces insulin resistance in the mouse. Obesity (Silver. Spring). 18, 1861–1863. doi: 10.1038/oby.2010.24
Contreras, G., Gutiérrez, M., Beroíza, T., Fantín, A., Oddó, H., Villarroel, L., et al. (1991). Ventilatory drive and respiratory muscle function in pregnancy. Am. Rev. Respir. Dis. 144, 837–841. doi: 10.1164/ajrccm/144.4.837
Costa, M. A. (2016). The endocrine function of human placenta: an overview. Reprod. Biomed. Online 32, 14–43. doi: 10.1016/j.rbmo.2015.10.005
Costrini, N. V., and Kalkhoff, R. K. (1971). Relative effects of pregnancy, estradiol, and progesterone on plasma insulin and pancreatic islet insulin secretion. J. Clin. Invest. 50, 992–999. doi: 10.1172/JCI106593
Coya, R., Martul, P., Algorta, J., Aniel-Quiroga, M. A., Busturia, M. A., and Señarís, R. (2006). Effect of leptin on the regulation of placental hormone secretion in cultured human placental cells. Gynecol. Endocrinol. 22, 620–626. doi: 10.1080/09513590601012587
Crocker, I., Kaur, M., Hosking, D. J., and Baker, P. N. (2002). Rescue of trophoblast apoptosis by parathyroid hormone-related protein. BJOG 109, 218–220. doi: 10.1111/j.1471-0528.2002.01033.x
Cruz, M. A., Gallardo, V., Miguel, P., Carrasco, G., and Gonzalez, C. (1997). Serotonin-induced vasoconstriction is mediated by thromboxane release and action in the human fetal-placental circulation. Placenta 18, 197–204. doi: 10.1016/S0143-4004(97)90093-X
Díaz, P., Powell, T. L., and Jansson, T. (2014). The role of placental nutrient sensing in maternal-fetal resource allocation. Biol. Reprod. 91:82. doi: 10.1095/biolreprod.114.121798
Da Costa, T. H., Taylor, K., Ilic, V., and Williamson, D. H. (1995). Regulation of milk lipid secretion: effects of oxytocin, prolactin and ionomycin on triacylglycerol release from rat mammary gland slices. Biochem. J. 308(Pt 3), 975–981. doi: 10.1042/bj3080975
Dai, G., Lu, L., Tang, S., Peal, M. J., and Soares, M. J. (2002). Prolactin family miniarray: a tool for evaluating uteroplacental-trophoblast endocrine cell phenotypes. Reproduction 124, 755–765. doi: 10.1530/rep.0.1240755
Dai, S. Q., Yu, L. P., Shi, X., Wu, H., Shao, P., Yin, G. Y., et al. (2014). Serotonin regulates osteoblast proliferation and function in vitro. Braz. J. Med. Biol. Res. 47, 759–765. doi: 10.1590/1414-431X20143565
Danielson, L. A., Sherwood, O. D., and Conrad, K. P. (1999). Relaxin is a potent renal vasodilator in conscious rats. J. Clin. Invest. 103, 525–533. doi: 10.1172/JCI5630
Datta, N. S., Chen, C., Berry, J. E., and Mccauley, L. K. (2005). PTHrP signaling targets cyclin D1 and induces osteoblastic cell growth arrest. J. Bone Miner. Res. 20, 1051–1064. doi: 10.1359/JBMR.050106
Davison, J. M., and Dunlop, W. (1980). Renal hemodynamics and tubular function normal human pregnancy. Kidney Int. 18, 152–161. doi: 10.1038/ki.1980.124
Dean, M., Hunt, J., Mcdougall, L., and Rose, J. (2014). Uterine glycogen metabolism in mink during estrus, embryonic diapause and pregnancy. J. Reprod. Dev. 60, 438–446. doi: 10.1262/jrd.2014-013
Deblon, N., Veyrat-Durebex, C., Bourgoin, L., Caillon, A., Bussier, A. L., Petrosino, S., et al. (2011). Mechanisms of the anti-obesity effects of oxytocin in diet-induced obese rats. PLoS ONE 6:e25565. doi: 10.1371/journal.pone.0025565
Debrah, D. O., Debrah, J. E., Haney, J. L., Mcguane, J. T., Sacks, M. S., Conrad, K. P., et al. (2011). Relaxin regulates vascular wall remodeling and passive mechanical properties in mice. J. Appl. Physiol. (1985) 111, 260–271. doi: 10.1152/japplphysiol.00845.2010
Debrah, D. O., Novak, J., Matthews, J. E., Ramirez, R. J., Shroff, S. G., and Conrad, K. P. (2006). Relaxin is essential for systemic vasodilation and increased global arterial compliance during early pregnancy in conscious rats. Endocrinology 147, 5126–5131. doi: 10.1210/en.2006-0567
Declerck, C. H., Boone, C., and Kiyonari, T. (2010). Oxytocin and cooperation under conditions of uncertainty: the modulating role of incentives and social information. Horm. Behav. 57, 368–374. doi: 10.1016/j.yhbeh.2010.01.006
De Dreu, C. K., Greer, L. L., Handgraaf, M. J., Shalvi, S., Van Kleef, G. A., Baas, M., et al. (2010). The neuropeptide oxytocin regulates parochial altruism in intergroup conflict among humans. Science 328, 1408–1411. doi: 10.1126/science.1189047
Del Rincon, J. P., Iida, K., Gaylinn, B. D., Mccurdy, C. E., Leitner, J. W., Barbour, L. A., et al. (2007). Growth hormone regulation of p85alpha expression and phosphoinositide 3-kinase activity in adipose tissue: mechanism for growth hormone-mediated insulin resistance. Diabetes 56, 1638–1646. doi: 10.2337/db06-0299
Denicolo, G., Morris, S. T., Kenyon, P. R., Morel, P. C., and Parkinson, T. J. (2008). Melatonin-improved reproductive performance in sheep bred out of season. Anim. Reprod. Sci. 109, 124–133. doi: 10.1016/j.anireprosci.2007.10.012
De Pedro, M. A., Morán, J., Díaz, I., Murias, L., Fernández-Plaza, C., Gonzále, C., et al. (2015). Circadian Kisspeptin expression in human term placenta. Placenta 36, 1337–1339. doi: 10.1016/j.placenta.2015.09.009
Dill, R., and Walker, A. M. (2017). Role of prolactin in promotion of immune cell migration into the mammary gland. J. Mammary Gland Biol. Neoplasia 22, 13–26. doi: 10.1007/s10911-016-9369-0
Ding, H., Zhang, G., Sin, K. W., Liu, Z., Lin, R. K., Li, M., et al. (2017). Activin A induces skeletal muscle catabolism via p38beta mitogen-activated protein kinase. J. Cachexia Sarcopenia Muscle 8, 202–212. doi: 10.1002/jcsm.12145
Di, W. L., Lachelin, G. C., Mcgarrigle, H. H., Thomas, N. S., and Becker, D. L. (2001). Oestriol and oestradiol increase cell to cell communication and connexin43 protein expression in human myometrium. Mol. Hum. Reprod. 7, 671–679. doi: 10.1093/molehr/7.7.671
Dominici, F. P., Argentino, D. P., Muñoz, M. C., Miquet, J. G., Sotelo, A. I., and Turyn, D. (2005). Influence of the crosstalk between growth hormone and insulin signalling on the modulation of insulin sensitivity. Growth Horm. IGF Res. 15, 324–336. doi: 10.1016/j.ghir.2005.07.001
Dominici, F. P., Cifone, D., Bartke, A., and Turyn, D. (1999). Loss of sensitivity to insulin at early events of the insulin signaling pathway in the liver of growth hormone-transgenic mice. J. Endocrinol. 161, 383–392. doi: 10.1677/joe.0.1610383
Douglas, A. J., Johnstone, L. E., and Leng, G. (2007). Neuroendocrine mechanisms of change in food intake during pregnancy: a potential role for brain oxytocin. Physiol. Behav. 91, 352–365. doi: 10.1016/j.physbeh.2007.04.012
Drynda, R., Peters, C. J., Jones, P. M., and Bowe, J. E. (2015). The role of non-placental signals in the adaptation of islets to pregnancy. Horm. Metab. Res. 47, 64–71. doi: 10.1055/s-0034-1395691
Dunbar, M. E., Dann, P., Brown, C. W., Van Houton, J., Dreyer, B., Philbrick, W. P., et al. (2001). Temporally regulated overexpression of parathyroid hormone-related protein in the mammary gland reveals distinct fetal and pubertal phenotypes. J. Endocrinol. 171, 403–416. doi: 10.1677/joe.0.1710403
Duval, C., Dilworth, M. R., Tunster, S. J., Kimber, S. J., and Glazier, J. D. (2017). PTHrP is essential for normal morphogenetic and functional development of the murine placenta. Dev. Biol. 430, 325–336. doi: 10.1016/j.ydbio.2017.08.033
Edey, L. F., Georgiou, H., O'dea, K. P., Mesiano, S., Herbert, B. R., Lei, K., et al. (2018). Progesterone, the maternal immune system and the onset of parturition in the mouse. Biol. Reprod. 98, 376–395. doi: 10.1093/biolre/iox146
Einspanier, A., Lieder, K., Husen, B., Ebert, K., Lier, S., Einspanier, R., et al. (2009). Relaxin supports implantation and early pregnancy in the marmoset monkey. Ann. N. Y. Acad. Sci. 1160, 140–146. doi: 10.1111/j.1749-6632.2009.03947.x
Elabd, S. K., Sabry, I., Hassan, W. B., Nour, H., and Zaky, K. (2007). Possible neuroendocrine role for oxytocin in bone remodeling. Endocr. Regul. 41, 131–141.
El-Hashash, A. H., and Kimber, S. J. (2006). PTHrP induces changes in cell cytoskeleton and E-cadherin and regulates Eph/Ephrin kinases and RhoGTPases in murine secondary trophoblast cells. Dev. Biol. 290, 13–31. doi: 10.1016/j.ydbio.2005.10.010
Elling, S. V., and Powell, F. C. (1997). Physiological changes in the skin during pregnancy. Clin. Dermatol. 15, 35–43. doi: 10.1016/S0738-081X(96)00108-3
Elsheikh, A., Creatsas, G., Mastorakos, G., Milingos, S., Loutradis, D., and Michalas, S. (2001). The renin-aldosterone system during normal and hypertensive pregnancy. Arch. Gynecol. Obstet. 264, 182–185. doi: 10.1007/s004040000104
Emly, J. F., Gregory, J., Bowden, S. J., Ahmed, A., Whittle, M. J., Rushton, D. I., et al. (1994). Immunohistochemical localization of parathyroid hormone-related protein (PTHrP) in human term placenta and membranes. Placenta 15, 653–660. doi: 10.1016/S0143-4004(05)80411-4
Enright, W. J., Chapin, L. T., Moseley, W. M., and Tucker, H. A. (1988). Effects of infusions of various doses of bovine growth hormone-releasing factor on growth hormone and lactation in Holstein cows. J. Dairy Sci. 71, 99–108. doi: 10.3168/jds.S0022-0302(88)79530-2
Enright, W. J., Chapin, L. T., Moseley, W. M., Zinn, S. A., Kamdar, M. B., Krabill, L. F., et al. (1989). Effects of infusions of various doses of bovine growth hormone-releasing factor on blood hormones and metabolites in lactating Holstein cows. J. Endocrinol. 122, 671–679. doi: 10.1677/joe.0.1220671
Enright, W. J., Chapin, L. T., Moseley, W. M., Zinn, S. A., and Tucker, H. A. (1986). Growth hormone-releasing factor stimulates milk production and sustains growth hormone release in Holstein cows. J. Dairy Sci. 69, 344–351. doi: 10.3168/jds.S0022-0302(86)80412-X
Ernst, S., Demirci, C., Valle, S., Velazquez-Garcia, S., and Garcia-Ocaña, A. (2011). Mechanisms in the adaptation of maternal beta-cells during pregnancy. Diabetes Manag. (Lond). 1, 239–248. doi: 10.2217/dmt.10.24
Eta, E., Ambrus, G., and Rao, C. V. (1994). Direct regulation of human myometrial contractions by human chorionic gonadotropin. J. Clin. Endocrinol. Metab. 79, 1582–1586.
Etienne, M., Bonneau, M., Kann, G., and Deletang, F. (1992). Effects of administration of growth hormone-releasing factor to sows during late gestation on growth hormone secretion, reproductive traits, and performance of progeny from birth to 100 kilograms live weight. J. Anim. Sci. 70, 2212–2220. doi: 10.2527/1992.7072212x
Everson, G. T. (1992). Gastrointestinal motility in pregnancy. Gastroenterol. Clin. North Am. 21, 751–776.
Fang, X., Wong, S., and Mitchell, B. F. (1997). Effects of RU486 on estrogen, progesterone, oxytocin, and their receptors in the rat uterus during late gestation. Endocrinology 138, 2763–2768. doi: 10.1210/endo.138.7.5247
Farmer, C., Dubreuil, P., Pelletier, G., Petitclerc, D., Gaudreau, P., and Brazeau, P. (1991). Effects of active immunization against somatostatin (SRIF) and/or injections of growth hormone-releasing factor (GRF) during gestation on hormonal and metabolic profiles in sows. Domest. Anim. Endocrinol. 8, 415–422. doi: 10.1016/0739-7240(91)90009-9
Farmer, C., Petitclerc, D., Pelletier, G., and Brazeau, P. (1992). Lactation performance of sows injected with growth hormone-releasing factor during gestation and(or) lactation. J. Anim. Sci. 70, 2636–2642. doi: 10.2527/1992.7092636x
Farmer, C., Robert, S., and Matte, J. J. (1996). Lactation performance of sows fed a bulky diet during gestation and receiving growth hormone-releasing factor during lactation. J. Anim. Sci. 74, 1298–1306. doi: 10.2527/1996.7461298x
Fecteau, K. A., and Eiler, H. (2001). Placenta detachment: unexpected high concentrations of 5-hydroxytryptamine (serotonin) in fetal blood and its mitogenic effect on placental cells in bovine. Placenta 22, 103–110. doi: 10.1053/plac.2000.0596
Feng, S., Bogatcheva, N. V., Kamat, A. A., Truong, A., and Agoulnik, A. I. (2006). Endocrine effects of relaxin overexpression in mice. Endocrinology 147, 407–414. doi: 10.1210/en.2005-0626
Ferguson, J. N., Young, L. J., Hearn, E. F., Matzuk, M. M., Insel, T. R., and Winslow, J. T. (2000). Social amnesia in mice lacking the oxytocin gene. Nat. Genet. 25, 284–288. doi: 10.1038/77040
Ferris, C. F., Foote, K. B., Meltser, H. M., Plenby, M. G., Smith, K. L., and Insel, T. R. (1992). Oxytocin in the amygdala facilitates maternal aggression. Ann. N. Y. Acad. Sci. 652, 456–457. doi: 10.1111/j.1749-6632.1992.tb34382.x
Fettke, F., Schumacher, A., Canellada, A., Toledo, N., Bekeredjian-Ding, I., Bondt, A., et al. (2016). Maternal and fetal mechanisms of B cell regulation during pregnancy: human chorionic gonadotropin stimulates B cells to Produce IL-10 while alpha-fetoprotein drives them into apoptosis. Front. Immunol. 7:495. doi: 10.3389/fimmu.2016.00495
Fliegner, D., Schubert, C., Penkalla, A., Witt, H., Kararigas, G., Dworatzek, E., et al. (2010). Female sex and estrogen receptor-beta attenuate cardiac remodeling and apoptosis in pressure overload. Am. J. Physiol. Regul. Integr. Comp. Physiol. 298, R1597–R1606. doi: 10.1152/ajpregu.00825.2009
Flores-Espinosa, P., Preciado-Martínez, E., Mejía-Salvador, A., Sedano-González, G., Bermejo-Martínez, L., Parra-Covarruvias, A., et al. (2017). Selective immuno-modulatory effect of prolactin upon pro-inflammatory response in human fetal membranes. J. Reprod. Immunol. 123, 58–64. doi: 10.1016/j.jri.2017.09.004
Florio, P., Lombardo, M., Gallo, R., Di Carlo, C., Sutton, S., Genazzani, A. R., et al. (1996). Activin A, corticotropin-releasing factor and prostaglandin F2 alpha increase immunoreactive oxytocin release from cultured human placental cells. Placenta 17, 307–311. doi: 10.1016/S0143-4004(96)90054-5
Fournier, T., Guibourdenche, J., and Evain-Brion, D. (2015). Review: hCGs: different sources of production, different glycoforms and functions. Placenta 36(Suppl. 1), S60–S65. doi: 10.1016/j.placenta.2015.02.002
Fowden, A. L., Giussani, D. A., and Forhead, A. J. (2006). Intrauterine programming of physiological systems: causes and consequences. Physiology (Bethesda). 21, 29–37. doi: 10.1152/physiol.00050.2005
Fowden, A. L., and Moore, T. (2012). Maternal-fetal resource allocation: co-operation and conflict. Placenta 33(Suppl. 2), e11–e15. doi: 10.1016/j.placenta.2012.05.002
Fowler, P. A., Evans, L. W., Groome, N. P., Templeton, A., and Knight, P. G. (1998). A longitudinal study of maternal serum inhibin-A, inhibin-B, activin-A, activin-AB, pro-alphaC and follistatin during pregnancy. Hum. Reprod. 13, 3530–3536. doi: 10.1093/humrep/13.12.3530
Freemark, M. (2010). Placental hormones and the control of fetal growth. J. Clin. Endocrinol. Metab. 95, 2054–2057. doi: 10.1210/jc.2010-0517
Freemark, M., Avril, I., Fleenor, D., Driscoll, P., Petro, A., Opara, E., et al. (2002). Targeted deletion of the PRL receptor: effects on islet development, insulin production, and glucose tolerance. Endocrinology 143, 1378–1385. doi: 10.1210/endo.143.4.8722
Freemark, M., Fleenor, D., Driscoll, P., Binart, N., and Kelly, P. (2001). Body weight and fat deposition in prolactin receptor-deficient mice. Endocrinology 142, 532–537. doi: 10.1210/endo.142.2.7979
Frise, C., Noori, M., and Williamson, C. (2013). Severe metabolic alkalosis in pregnancy. Obstet. Med. 6, 138–140. doi: 10.1258/om.2012.120030
Fudge, N. J., and Kovacs, C. S. (2010). Pregnancy up-regulates intestinal calcium absorption and skeletal mineralization independently of the vitamin D receptor. Endocrinology 151, 886–895. doi: 10.1210/en.2009-1010
Fujinaka, Y., Sipula, D., Garcia-Ocaña, A., and Vasavada, R. C. (2004). Characterization of mice doubly transgenic for parathyroid hormone-related protein and murine placental lactogen: a novel role for placental lactogen in pancreatic beta-cell survival. Diabetes 53, 3120–3130. doi: 10.2337/diabetes.53.12.3120
Fungfuang, W., Terada, M., Komatsu, N., Moon, C., and Saito, T. R. (2013). Effects of estrogen on food intake, serum leptin levels and leptin mRNA expression in adipose tissue of female rats. Lab. Anim. Res. 29, 168–173. doi: 10.5625/lar.2013.29.3.168
Gallacher, S. J., Fraser, W. D., Owens, O. J., Dryburgh, F. J., Logue, F. C., Jenkins, A., et al. (1994). Changes in calciotrophic hormones and biochemical markers of bone turnover in normal human pregnancy. Eur. J. Endocrinol. 131, 369–374. doi: 10.1530/eje.0.1310369
Gallego, M. I., Binart, N., Robinson, G. W., Okagaki, R., Coschigano, K. T., Perry, J., et al. (2001). Prolactin, growth hormone, and epidermal growth factor activate Stat5 in different compartments of mammary tissue and exert different and overlapping developmental effects. Dev. Biol. 229, 163–175. doi: 10.1006/dbio.2000.9961
Galosy, S. S., and Talamantes, F. (1995). Luteotropic actions of placental lactogens at midpregnancy in the mouse. Endocrinology 136, 3993–4003. doi: 10.1210/endo.136.9.7649108
Garcia-Ruíz, G., Flores-Espinosa, P., Preciado-Martínez, E., Bermejo-Martínez, L., Espejel-Nuñez, A., Estrada-Gutierrez, G., et al. (2015). In vitro progesterone modulation on bacterial endotoxin-induced production of IL-1beta, TNFalpha, IL-6, IL-8, IL-10, MIP-1alpha, and MMP-9 in pre-labor human term placenta. Reprod. Biol. Endocrinol. 13:115. doi: 10.1186/s12958-015-0111-3
Gimeno, M. F., Landa, A., Sterin-Speziale, N., Cardinali, D. P., and Gimeno, A. L. (1980). Melatonin blocks in vitro generation of prostaglandin by the uterus and hypothalamus. Eur. J. Pharmacol. 62, 309–317. doi: 10.1016/0014-2999(80)90098-9
Goh, B. C., Singhal, V., Herrera, A. J., Tomlinson, R. E., Kim, S., Faugere, M. C., et al. (2017). Activin receptor type 2A (ACVR2A) functions directly in osteoblasts as a negative regulator of bone mass. J. Biol. Chem. 292, 13809–13822. doi: 10.1074/jbc.M117.782128
Goldsmith, L. T., Weiss, G., Palejwala, S., Plant, T. M., Wojtczuk, A., Lambert, W. C., et al. (2004). Relaxin regulation of endometrial structure and function in the rhesus monkey. Proc. Natl. Acad. Sci. U.S.A. 101, 4685–4689. doi: 10.1073/pnas.0400776101
Golightly, E., Jabbour, H. N., and Norman, J. E. (2011). Endocrine immune interactions in human parturition. Mol. Cell. Endocrinol. 335, 52–59. doi: 10.1016/j.mce.2010.08.005
González-Candia, A., Veliz, M., Araya, C., Quezada, S., Ebensperger, G., Seron-Ferre, M., et al. (2016). Potential adverse effects of antenatal melatonin as a treatment for intrauterine growth restriction: findings in pregnant sheep. Am J Obstet Gynecol 215, 245 e241–245 e247. doi: 10.1016/j.ajog.2016.02.040
Goodman, H. M., Tai, L. R., Ray, J., Cooke, N. E., and Liebhaber, S. A. (1991). Human growth hormone variant produces insulin-like and lipolytic responses in rat adipose tissue. Endocrinology 129, 1779–1783. doi: 10.1210/endo-129-4-1779
Gooi, J. H., Richardson, M. L., Jelinic, M., Girling, J. E., Wlodek, M. E., Tare, M., et al. (2013). Enhanced uterine artery stiffness in aged pregnant relaxin mutant mice is reversed with exogenous relaxin treatment. Biol. Reprod. 89:18. doi: 10.1095/biolreprod.113.108118
Gopalakrishnan, K., Mishra, J. S., Chinnathambi, V., Vincent, K. L., Patrikeev, I., Motamedi, M., et al. (2016). Elevated testosterone reduces uterine blood flow, spiral artery elongation, and placental oxygenation in pregnant rats. Hypertension 67, 630–639. doi: 10.1161/HYPERTENSIONAHA.115.06946
Goyvaerts, L., Schraenen, A., and Schuit, F. (2016). Serotonin competence of mouse beta cells during pregnancy. Diabetologia 59, 1356–1363. doi: 10.1007/s00125-016-3951-2
Greening, D. W., Nguyen, H. P., Evans, J., Simpson, R. J., and Salamonsen, L. A. (2016). Modulating the endometrial epithelial proteome and secretome in preparation for pregnancy: the role of ovarian steroid and pregnancy hormones. J. Proteomics 144, 99–112. doi: 10.1016/j.jprot.2016.05.026
Gregg, C. (2009). Pregnancy, prolactin and white matter regeneration. J. Neurol. Sci. 285, 22–27. doi: 10.1016/j.jns.2009.06.040
Grès, S., Canteiro, S., Mercader, J., and Carpene, C. (2013). Oxidation of high doses of serotonin favors lipid accumulation in mouse and human fat cells. Mol. Nutr. Food Res. 57, 1089–1099. doi: 10.1002/mnfr.201200681
Groba, C., Mayerl, S., Van Mullem, A. A., Visser, T. J., Darras, V. M., Habenicht, A. J., et al. (2013). Hypothyroidism compromises hypothalamic leptin signaling in mice. Mol. Endocrinol. 27, 586–597. doi: 10.1210/me.2012-1311
Groen, B., Van Der Wijk, A. E., Van Den Berg, P. P., Lefrandt, J. D., Van Den Berg, G., Sollie, K. M., et al. (2015). Immunological Adaptations to Pregnancy in Women with Type 1 Diabetes. Sci. Rep. 5:13618. doi: 10.1038/srep13618
Groskopf, J. C., Syu, L. J., Saltiel, A. R., and Linzer, D. I. (1997). Proliferin induces endothelial cell chemotaxis through a G protein-coupled, mitogen-activated protein kinase-dependent pathway. Endocrinology 138, 2835–2840. doi: 10.1210/endo.138.7.5276
Gulinello, M., Gong, Q. H., and Smith, S. S. (2002). Progesterone withdrawal increases the alpha4 subunit of the GABA(A) receptor in male rats in association with anxiety and altered pharmacology-a comparison with female rats. Neuropharmacology 43, 701–714. doi: 10.1016/S0028-3908(02)00171-5
Gutkowska, J., and Jankowski, M. (2012). Oxytocin revisited: its role in cardiovascular regulation. J. Neuroendocrinol. 24, 599–608. doi: 10.1111/j.1365-2826.2011.02235.x
Habiger, V. W. (1975). Serotonin effect on the fetus and the feto-maternal relationship in the rat. Arzneimittelforschung. 25, 626–632.
Hadden, C., Fahmi, T., Cooper, A., Savenka, A. V., Lupashin, V. V., Roberts, D. J., et al. (2017). Serotonin transporter protects the placental cells against apoptosis in caspase 3-independent pathway. J. Cell. Physiol. 232, 3520–3529. doi: 10.1002/jcp.25812
Hadden, D. R., and Mclaughlin, C. (2009). Normal and abnormal maternal metabolism during pregnancy. Semin. Fetal Neonatal Med. 14, 66–71. doi: 10.1016/j.siny.2008.09.004
Haig, D. (2008). Placental growth hormone-related proteins and prolactin-related proteins. Placenta 29(Suppl. A), S36–S41. doi: 10.1016/j.placenta.2007.09.010
Hales, C. N., and Barker, D. J. (2001). The thrifty phenotype hypothesis. Br. Med. Bull. 60, 5–20. doi: 10.1093/bmb/60.1.5
Handwerger, S., Richards, R. G., and Markoff, E. (1992). The physiology of decidual prolactin and other decidual protein hormones. Trends Endocrinol. Metab. 3, 91–95. doi: 10.1016/1043-2760(92)90019-W
Harris, L. K., Crocker, I. P., Baker, P. N., Aplin, J. D., and Westwood, M. (2011). IGF2 actions on trophoblast in human placenta are regulated by the insulin-like growth factor 2 receptor, which can function as both a signaling and clearance receptor. Biol. Reprod. 84, 440–446. doi: 10.1095/biolreprod.110.088195
Hart, I. C., Chadwick, P. M., James, S., and Simmonds, A. D. (1985). Effect of intravenous bovine growth hormone or human pancreatic growth hormone-releasing factor on milk production and plasma hormones and metabolites in sheep. J. Endocrinol. 105, 189–196. doi: 10.1677/joe.0.1050189
Hauguel-De Mouzon, S., Lepercq, J., and Catalano, P. (2006). The known and unknown of leptin in pregnancy. Am. J. Obstet. Gynecol. 194, 1537–1545. doi: 10.1016/j.ajog.2005.06.064
Haynes, M. P., Sinha, D., Russell, K. S., Collinge, M., Fulton, D., Morales-Ruiz, M., et al. (2000). Membrane estrogen receptor engagement activates endothelial nitric oxide synthase via the PI3-kinase-Akt pathway in human endothelial cells. Circ. Res. 87, 677–682. doi: 10.1161/01.RES.87.8.677
Hearn, J. P., Gidley-Baird, A. A., Hodges, J. K., Summers, P. M., and Webley, G. E. (1988). Embryonic signals during the peri-implantation period in primates. J. Reprod. Fertil. Suppl. 36, 49–58.
Hegewald, M. J., and Crapo, R. O. (2011). Respiratory physiology in pregnancy. Clin. Chest Med. 32, 1-13, vii. doi: 10.1016/j.ccm.2010.11.001
Hellmeyer, L., Ziller, V., Anderer, G., Ossendorf, A., Schmidt, S., and Hadji, P. (2006). Biochemical markers of bone turnover during pregnancy: a longitudinal study. Exp. Clin. Endocrinol. Diabetes 114, 506–510. doi: 10.1055/s-2006-951627
Henson, M. C., Castracane, V. D., O'neil, J. S., Gimpel, T., Swan, K. F., Green, A. E., et al. (1999). Serum leptin concentrations and expression of leptin transcripts in placental trophoblast with advancing baboon pregnancy. J. Clin. Endocrinol. Metab. 84, 2543–2549. doi: 10.1210/jc.84.7.2543
Hernández-Castellano, L. E., Hernandez, L. L., Weaver, S., and Bruckmaier, R. M. (2017). Increased serum serotonin improves parturient calcium homeostasis in dairy cows. J. Dairy Sci. 100, 1580–1587. doi: 10.3168/jds.2016-11638
Herreboudt, A. M., Kyle, V. R., Lawrence, J., Doran, J., and Colledge, W. H. (2015). Kiss1 mutant placentas show normal structure and function in the mouse. Placenta 36, 52–58. doi: 10.1016/j.placenta.2014.10.016
Hershberger, M. E., and Tuan, R. S. (1998). Placental 57-kDa Ca(2+)-binding protein: regulation of expression and function in trophoblast calcium transport. Dev. Biol. 199, 80–92. doi: 10.1006/dbio.1998.8926
Hershman, J. M., Kojima, A., and Friesen, H. G. (1973). Effect of thyrotropin-releasing hormone on human pituitary thyrotropin, prolactin, placental lactogen, and chorionic thyrotropin. J. Clin. Endocrinol. Metab. 36, 497–501. doi: 10.1210/jcem-36-3-497
Highman, T. J., Friedman, J. E., Huston, L. P., Wong, W. W., and Catalano, P. M. (1998). Longitudinal changes in maternal serum leptin concentrations, body composition, and resting metabolic rate in pregnancy. Am. J. Obstet. Gynecol. 178, 1010–1015. doi: 10.1016/S0002-9378(98)70540-X
Hirota, Y., Anai, T., and Miyakawa, I. (1997). Parathyroid hormone-related protein levels in maternal and cord blood. Am. J. Obstet. Gynecol. 177, 702–706. doi: 10.1016/S0002-9378(97)70167-4
Hisamoto, K., Ohmichi, M., Kurachi, H., Hayakawa, J., Kanda, Y., Nishio, Y., et al. (2001). Estrogen induces the Akt-dependent activation of endothelial nitric-oxide synthase in vascular endothelial cells. J. Biol. Chem. 276, 3459–3467. doi: 10.1074/jbc.M005036200
Hisaw, F. L., Hisaw, F. L. Jr., and Dawson, A. B. (1967). Effects of relaxin on the endothelium of endometrial blood vessels in monkeys (Macaca mulatta). Endocrinology 81, 375–385. doi: 10.1210/endo-81-2-375
Hoekzema, E., Barba-Müller, E., Pozzobon, C., Picado, M., Lucco, F., García-García, D., et al. (2017). Pregnancy leads to long-lasting changes in human brain structure. Nat. Neurosci. 20, 287–296. doi: 10.1038/nn.4458
Horber, F. F., and Haymond, M. W. (1990). Human growth hormone prevents the protein catabolic side effects of prednisone in humans. J. Clin. Invest. 86, 265–272. doi: 10.1172/JCI114694
Horikoshi, Y., Matsumoto, H., Takatsu, Y., Ohtaki, T., Kitada, C., Usuki, S., et al. (2003). Dramatic elevation of plasma metastin concentrations in human pregnancy: metastin as a novel placenta-derived hormone in humans. J. Clin. Endocrinol. Metab. 88, 914–919. doi: 10.1210/jc.2002-021235
Horseman, N. D., Zhao, W., Montecino-Rodriguez, E., Tanaka, M., Nakashima, K., Engle, S. J., et al. (1997). Defective mammopoiesis, but normal hematopoiesis, in mice with a targeted disruption of the prolactin gene. EMBO J. 16, 6926–6935. doi: 10.1093/emboj/16.23.6926
Huang, C., Snider, F., and Cross, J. C. (2009). Prolactin receptor is required for normal glucose homeostasis and modulation of beta-cell mass during pregnancy. Endocrinology 150, 1618–1626. doi: 10.1210/en.2008-1003
Hudon Thibeault, A. A., Laurent, L., Vo Duy, S., Sauve, S., Caron, P., Guillemette, C., et al. (2017). Fluoxetine and its active metabolite norfluoxetine disrupt estrogen synthesis in a co-culture model of the feto-placental unit. Mol. Cell. Endocrinol. 442, 32–39. doi: 10.1016/j.mce.2016.11.021
Hughes, C. K., Xie, M. M., Mccoski, S. R., and Ealy, A. D. (2017). Activities for leptin in bovine trophoblast cells. Domest. Anim. Endocrinol. 58, 84–89. doi: 10.1016/j.domaniend.2016.09.001
Ibrahim, H. S., Omar, E., Froemming, G. R., and Singh, H. J. (2013). Leptin increases blood pressure and markers of endothelial activation during pregnancy in rats. Biomed Res. Int. 2013, 298401. doi: 10.1155/2013/298401
Ishizuka, T., Klepcyk, P., Liu, S., Panko, L., Liu, S., Gibbs, E. M., et al. (1999). Effects of overexpression of human GLUT4 gene on maternal diabetes and fetal growth in spontaneous gestational diabetic C57BLKS/J Lepr(db/+) mice. Diabetes 48, 1061–1069. doi: 10.2337/diabetes.48.5.1061
Islami, D., Bischof, P., and Chardonnens, D. (2003a). Modulation of placental vascular endothelial growth factor by leptin and hCG. Mol. Hum. Reprod. 9, 395–398. doi: 10.1093/molehr/gag053
Islami, D., Bischof, P., and Chardonnens, D. (2003b). Possible interactions between leptin, gonadotrophin-releasing hormone (GnRH-I and II) and human chorionic gonadotrophin (hCG). Eur. J. Obstet. Gynecol. Reprod. Biol. 110, 169–175. doi: 10.1016/S0301-2115(03)00185-4
Islam, M. S., Morton, N. M., Hansson, A., and Emilsson, V. (1997). Rat insulinoma-derived pancreatic beta-cells express a functional leptin receptor that mediates a proliferative response. Biochem. Biophys. Res. Commun. 238, 851–855. doi: 10.1006/bbrc.1997.7399
Iwasaki, S., Nakazawa, K., Sakai, J., Kometani, K., Iwashita, M., Yoshimura, Y., et al. (2005). Melatonin as a local regulator of human placental function. J. Pineal Res. 39, 261–265. doi: 10.1111/j.1600-079X.2005.00244.x
Izquierdo, A., López-Luna, P., Ortega, A., Romero, M., Guitiérrez-Tarrés, M. A., Arribas, I., et al. (2006). The parathyroid hormone-related protein system and diabetic nephropathy outcome in streptozotocin-induced diabetes. Kidney Int. 69, 2171–2177. doi: 10.1038/sj.ki.5000195
Jackson, D., Volpert, O. V., Bouck, N., and Linzer, D. I. (1994). Stimulation and inhibition of angiogenesis by placental proliferin and proliferin-related protein. Science 266, 1581–1584. doi: 10.1126/science.7527157
Jahnke, G., Marr, M., Myers, C., Wilson, R., Travlos, G., and Price, C. (1999). Maternal and developmental toxicity evaluation of melatonin administered orally to pregnant Sprague-Dawley rats. Toxicol. Sci. 50, 271–279. doi: 10.1093/toxsci/50.2.271
Jenkin, G., Ward, J., Loose, J., Schneider-Kolsky, M., Young, R., Canny, B., et al. (2001). Physiological and regulatory roles of activin A in late pregnancy. Mol. Cell. Endocrinol. 180, 131–138. doi: 10.1016/S0303-7207(01)00504-4
Jiang, C. W., Sarrel, P. M., Lindsay, D. C., Poole-Wilson, P. A., and Collins, P. (1992). Progesterone induces endothelium-independent relaxation of rabbit coronary artery in vitro. Eur. J. Pharmacol. 211, 163–167. doi: 10.1016/0014-2999(92)90524-8
Jobe, S. O., Ramadoss, J., Koch, J. M., Jiang, Y., Zheng, J., and Magness, R. R. (2010). Estradiol-17beta and its cytochrome P450- and catechol-O-methyltransferase-derived metabolites stimulate proliferation in uterine artery endothelial cells: role of estrogen receptor-alpha versus estrogen receptor-beta. Hypertension 55, 1005–1011. doi: 10.1161/HYPERTENSIONAHA.109.146399
Jones, R. L., Findlay, J. K., Farnworth, P. G., Robertson, D. M., Wallace, E., and Salamonsen, L. A. (2006). Activin A and inhibin A differentially regulate human uterine matrix metalloproteinases: potential interactions during decidualization and trophoblast invasion. Endocrinology 147, 724–732. doi: 10.1210/en.2005-1183
Joshi, P. A., Jackson, H. W., Beristain, A. G., Di Grappa, M. A., Mote, P. A., Clarke, C. L., et al. (2010). Progesterone induces adult mammary stem cell expansion. Nature 465, 803–807. doi: 10.1038/nature09091
Jo, Y. S., Lee, G. S., Nam, S. Y., and Kim, S. J. (2015). Progesterone inhibits leptin-induced invasiveness of BeWo cells. Int. J. Med. Sci. 12, 773–779. doi: 10.7150/ijms.11610
Kaftanovskaya, E. M., Huang, Z., Lopez, C., Conrad, K., and Agoulnik, A. I. (2015). Conditional deletion of the relaxin receptor gene in cells of smooth muscle lineage affects lower reproductive tract in pregnant mice. Biol. Reprod. 92:91. doi: 10.1095/biolreprod.114.127209
Kalkwarf, H. J., and Specker, B. L. (2002). Bone mineral changes during pregnancy and lactation. Endocrine 17, 49–53. doi: 10.1385/ENDO:17:1:49
Kamat, A. A., Feng, S., Bogatcheva, N. V., Truong, A., Bishop, C. E., and Agoulnik, A. I. (2004). Genetic targeting of relaxin and insulin-like factor 3 receptors in mice. Endocrinology 145, 4712–4720. doi: 10.1210/en.2004-0515
Kane, M. J., Angoa-Peréz, M., Briggs, D. I., Sykes, C. E., Francescutti, D. M., Rosenberg, D. R., et al. (2012). Mice genetically depleted of brain serotonin display social impairments, communication deficits and repetitive behaviors: possible relevance to autism. PLoS ONE 7:e48975. doi: 10.1371/journal.pone.0048975
Kane, N., Kelly, R., Saunders, P. T., and Critchley, H. O. (2009). Proliferation of uterine natural killer cells is induced by human chorionic gonadotropin and mediated via the mannose receptor. Endocrinology 150, 2882–2888. doi: 10.1210/en.2008-1309
Karaplis, A. C., Luz, A., Glowacki, J., Bronson, R. T., Tybulewicz, V. L., Kronenberg, H. M., et al. (1994). Lethal skeletal dysplasia from targeted disruption of the parathyroid hormone-related peptide gene. Genes Dev. 8, 277–289. doi: 10.1101/gad.8.3.277
Keebaugh, A. C., Barrett, C. E., Laprairie, J. L., Jenkins, J. J., and Young, L. J. (2015). RNAi knockdown of oxytocin receptor in the nucleus accumbens inhibits social attachment and parental care in monogamous female prairie voles. Soc. Neurosci. 10, 561–570. doi: 10.1080/17470919.2015.1040893
Kendall, M. D., and Clarke, A. G. (2000). The thymus in the mouse changes its activity during pregnancy: a study of the microenvironment. J. Anat. 197(Pt 3), 393–411. doi: 10.1046/j.1469-7580.2000.19730393.x
Keomanivong, F. E., Lemley, C. O., Camacho, L. E., Yunusova, R., Borowicz, P. P., Caton, J. S., et al. (2016). Influence of nutrient restriction and melatonin supplementation of pregnant ewes on maternal and fetal pancreatic digestive enzymes and insulin-containing clusters. Animal 10, 440–448. doi: 10.1017/S1751731115002219
Khil, L. Y., Jun, H. S., Kwon, H., Yoo, J. K., Kim, S., Notkins, A. L., et al. (2007). Human chorionic gonadotropin is an immune modulator and can prevent autoimmune diabetes in NOD mice. Diabetologia 50, 2147–2155. doi: 10.1007/s00125-007-0769-y
Kim, C., Newton, K. M., and Knopp, R. H. (2002). Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care 25, 1862–1868. doi: 10.2337/diacare.25.10.1862
Kim, H., Toyofuku, Y., Lynn, F. C., Chak, E., Uchida, T., Mizukami, H., et al. (2010). Serotonin regulates pancreatic beta cell mass during pregnancy. Nat. Med. 16, 804–808. doi: 10.1038/nm.2173
Kim, J. K. (2009). Hyperinsulinemic-euglycemic clamp to assess insulin sensitivity in vivo. Methods Mol. Biol. 560, 221–238. doi: 10.1007/978-1-59745-448-3_15
Kim, M. N., Park, M. N., Jung, H. K., Cho, C., Mayo, K. E., and Cho, B. N. (2008). Changes in the reproductive function and developmental phenotypes in mice following intramuscular injection of an activin betaA-expressing plasmid. Reprod. Biol. Endocrinol. 6:63. doi: 10.1186/1477-7827-6-63
Kim, P. (2016). Human maternal brain plasticity: adaptation to parenting. New Dir. Child Adolesc. Dev. 2016, 47–58. doi: 10.1002/cad.20168
Kim, P., Strathearn, L., and Swain, J. E. (2016). The maternal brain and its plasticity in humans. Horm. Behav. 77, 113–123. doi: 10.1016/j.yhbeh.2015.08.001
Kim, S. C., Lee, J. E., Kang, S. S., Yang, H. S., Kim, S. S., and An, B. S. (2017). The regulation of oxytocin and oxytocin receptor in human placenta according to gestational age. J. Mol. Endocrinol. 59, 235–243. doi: 10.1530/JME-16-0223
Kim, S. H., Bennett, P. R., and Terzidou, V. (2017). Advances in the role of oxytocin receptors in human parturition. Mol. Cell. Endocrinol. 449, 56–63. doi: 10.1016/j.mce.2017.01.034
King, J. C. (2000). Physiology of pregnancy and nutrient metabolism. Am. J. Clin. Nutr 71, 1218S–1225S. doi: 10.1093/ajcn/71.5.1218s
Kirby, B. J., Ardeshirpour, L., Woodrow, J. P., Wysolmerski, J. J., Sims, N. A., Karaplis, A. C., et al. (2011). Skeletal recovery after weaning does not require PTHrP. J. Bone Miner. Res. 26, 1242–1251. doi: 10.1002/jbmr.339
Kirwan, J. P., Varastehpour, A., Jing, M., Presley, L., Shao, J., Friedman, J. E., et al. (2004). Reversal of insulin resistance postpartum is linked to enhanced skeletal muscle insulin signaling. J. Clin. Endocrinol. Metab. 89, 4678–4684. doi: 10.1210/jc.2004-0749
Kleiman, A., Keats, E. C., Chan, N. G., and Khan, Z. A. (2013). Elevated IGF2 prevents leptin induction and terminal adipocyte differentiation in hemangioma stem cells. Exp. Mol. Pathol. 94, 126–136. doi: 10.1016/j.yexmp.2012.09.023
Kobayashi, K., Tsugami, Y., Matsunaga, K., Oyama, S., Kuki, C., and Kumura, H. (2016). Prolactin and glucocorticoid signaling induces lactation-specific tight junctions concurrent with beta-casein expression in mammary epithelial cells. Biochim. Biophys. Acta 1863, 2006–2016. doi: 10.1016/j.bbamcr.2016.04.023
Koonce, C. J., and Frye, C. A. (2013). Progesterone facilitates exploration, affective and social behaviors among wildtype, but not 5alpha-reductase Type 1 mutant, mice. Behav. Brain Res. 253, 232–239. doi: 10.1016/j.bbr.2013.07.025
Kota, S. K., Gayatri, K., Jammula, S., Kota, S. K., Krishna, S. V., Meher, L. K., et al. (2013). Endocrinology of parturition. Indian J. Endocrinol. Metab. 17, 50–59. doi: 10.4103/2230-8210.107841
Krajnc-Franken, M. A., Van Disseldorp, A. J., Koenders, J. E., Mosselman, S., Van Duin, M., and Gossen, J. A. (2004). Impaired nipple development and parturition in LGR7 knockout mice. Mol. Cell. Biol. 24, 687–696. doi: 10.1128/MCB.24.2.687-696.2004
Krutzén, E., Olofsson, P., Bäck, S. E., and Nilsson-Ehle, P. (1992). Glomerular filtration rate in pregnancy: a study in normal subjects and in patients with hypertension, preeclampsia and diabetes. Scand. J. Clin. Lab. Invest. 52, 387–392. doi: 10.3109/00365519209088374
Kulandavelu, S., Qu, D., and Adamson, S. L. (2006). Cardiovascular function in mice during normal pregnancy and in the absence of endothelial NO synthase. Hypertension 47, 1175–1182. doi: 10.1161/01.HYP.0000218440.71846.db
Kulkarni, R. N., Wang, Z. L., Wang, R. M., Hurley, J. D., Smith, D. M., Ghatei, M. A., et al. (1997). Leptin rapidly suppresses insulin release from insulinoma cells, rat and human islets and, in vivo, in mice. J. Clin. Invest. 100, 2729–2736. doi: 10.1172/JCI119818
Kumar, P., Kamat, A., and Mendelson, C. R. (2009). Estrogen receptor alpha (ERalpha) mediates stimulatory effects of estrogen on aromatase (CYP19) gene expression in human placenta. Mol. Endocrinol. 23, 784–793. doi: 10.1210/me.2008-0371
Ladyman, S. R., Augustine, R. A., and Grattan, D. R. (2010). Hormone interactions regulating energy balance during pregnancy. J. Neuroendocrinol. 22, 805–817. doi: 10.1111/j.1365-2826.2010.02017.x
Lain, K. Y., and Catalano, P. M. (2007). Metabolic changes in pregnancy. Clin. Obstet. Gynecol. 50, 938–948. doi: 10.1097/GRF.0b013e31815a5494
Lanoix, D., Lacasse, A. A., Reiter, R. J., and Vaillancourt, C. (2013). Melatonin: the watchdog of villous trophoblast homeostasis against hypoxia/reoxygenation-induced oxidative stress and apoptosis. Mol. Cell. Endocrinol. 381, 35–45. doi: 10.1016/j.mce.2013.07.010
Lapensee, C. R., Horseman, N. D., Tso, P., Brandebourg, T. D., Hugo, E. R., and Ben-Jonathan, N. (2006). The prolactin-deficient mouse has an unaltered metabolic phenotype. Endocrinology 147, 4638–4645. doi: 10.1210/en.2006-0487
Lapierre, H., Pelletier, G., Petitclerc, D., Dubreuil, P., Morisset, J., Gaudreau, P., et al. (1988). Effect of human growth hormone-releasing factor (1-29)NH2 on growth hormone release and milk production in dairy cows. J. Dairy Sci. 71, 92–98. doi: 10.3168/jds.S0022-0302(88)79529-6
Laporta, J., Keil, K. P., Vezina, C. M., and Hernandez, L. L. (2014a). Peripheral serotonin regulates maternal calcium trafficking in mammary epithelial cells during lactation in mice. PLoS ONE 9:e110190. doi: 10.1371/journal.pone.0110190
Laporta, J., Keil, K. P., Weaver, S. R., Cronick, C. M., Prichard, A. P., Crenshaw, T. D., et al. (2014b). Serotonin regulates calcium homeostasis in lactation by epigenetic activation of hedgehog signaling. Mol. Endocrinol. 28, 1866–1874. doi: 10.1210/me.2014-1204
Laporta, J., Moore, S. A., Weaver, S. R., Cronick, C. M., Olsen, M., Prichard, A. P., et al. (2015). Increasing serotonin concentrations alter calcium and energy metabolism in dairy cows. J. Endocrinol. 226, 43–55. doi: 10.1530/JOE-14-0693
Laporta, J., Peters, T. L., Merriman, K. E., Vezina, C. M., and Hernandez, L. L. (2013a). Serotonin (5-HT) affects expression of liver metabolic enzymes and mammary gland glucose transporters during the transition from pregnancy to lactation. PLoS ONE 8:e57847. doi: 10.1371/journal.pone.0057847
Laporta, J., Peters, T. L., Weaver, S. R., Merriman, K. E., and Hernandez, L. L. (2013b). Feeding 5-hydroxy-l-tryptophan during the transition from pregnancy to lactation increases calcium mobilization from bone in rats. Domest. Anim. Endocrinol. 44, 176–184. doi: 10.1016/j.domaniend.2013.01.005
Laurent, L., Deroy, K., St-Pierre, J., Côté, F., Sanderson, J. T., and Vaillancourt, C. (2017). Human placenta expresses both peripheral and neuronal isoform of tryptophan hydroxylase. Biochimie 140, 159–165. doi: 10.1016/j.biochi.2017.07.008
Lee, C. L., Chiu, P. C., Hautala, L., Salo, T., Yeung, W. S., Stenman, U. H., et al. (2013). Human chorionic gonadotropin and its free beta-subunit stimulate trophoblast invasion independent of LH/hCG receptor. Mol. Cell. Endocrinol. 375, 43–52. doi: 10.1016/j.mce.2013.05.009
Lee, H. J., Caldwell, H. K., Macbeth, A. H., Tolu, S. G., and Young, W. S. III. (2008). A conditional knockout mouse line of the oxytocin receptor. Endocrinology 149, 3256–3263. doi: 10.1210/en.2007-1710
Lee, H. J., Gallego-Ortega, D., Ledger, A., Schramek, D., Joshi, P., Szwarc, M. M., et al. (2013). Progesterone drives mammary secretory differentiation via RankL-mediated induction of Elf5 in luminal progenitor cells. Development 140, 1397–1401. doi: 10.1242/dev.088948
Lee, O. H., Bae, S. K., Bae, M. H., Lee, Y. M., Moon, E. J., Cha, H. J., et al. (2000). Identification of angiogenic properties of insulin-like growth factor II in in vitro angiogenesis models. Br. J. Cancer 82, 385–391. doi: 10.1054/bjoc.1999.0931
Lee, S. J., Talamantes, F., Wilder, E., Linzer, D. I., and Nathans, D. (1988). Trophoblastic giant cells of the mouse placenta as the site of proliferin synthesis. Endocrinology 122, 1761–1768. doi: 10.1210/endo-122-5-1761
Lee, W. S., Lu, Y. C., Kuo, C. T., Chen, C. T., and Tang, P. H. (2017). Effects of female sex hormones on folic acid-induced anti-angiogenesis. Acta Physiol. (Oxf). 222:e13001. doi: 10.1111/apha.13001
Lefebvre, D. L., Giaid, A., and Zingg, H. H. (1992). Expression of the oxytocin gene in rat placenta. Endocrinology 130, 1185–1192.
Lekgabe, E. D., Royce, S. G., Hewitson, T. D., Tang, M. L., Zhao, C., Moore, X. L., et al. (2006). The effects of relaxin and estrogen deficiency on collagen deposition and hypertrophy of nonreproductive organs. Endocrinology 147, 5575–5583. doi: 10.1210/en.2006-0533
Le, T. N., Elsea, S. H., Romero, R., Chaiworapongsa, T., and Francis, G. L. (2013). Prolactin receptor gene polymorphisms are associated with gestational diabetes. Genet. Test. Mol. Biomarkers 17, 567–571. doi: 10.1089/gtmb.2013.0009
Leturque, A., Burnol, A. F., Ferré, P., and Girard, J. (1984). Pregnancy-induced insulin resistance in the rat: assessment by glucose clamp technique. Am. J. Physiol. 246, E25–31. doi: 10.1152/ajpendo.1984.246.1.E25
Levine, A., Zagoory-Sharon, O., Feldman, R., and Weller, A. (2007). Oxytocin during pregnancy and early postpartum: individual patterns and maternal-fetal attachment. Peptides 28, 1162–1169. doi: 10.1016/j.peptides.2007.04.016
Lévy, F. (2016). Neuroendocrine control of maternal behavior in non-human and human mammals. Ann. Endocrinol. (Paris). 77, 114–125. doi: 10.1016/j.ando.2016.04.002
Liao, S., Vickers, M. H., Evans, A., Stanley, J. L., Baker, P. N., and Perry, J. K. (2016a). Comparison of pulsatile vs. continuous administration of human placental growth hormone in female C57BL/6J mice. Endocrine 54, 169–181. doi: 10.1007/s12020-016-1060-0
Liao, S., Vickers, M. H., Stanley, J. L., Ponnampalam, A. P., Baker, P. N., and Perry, J. K. (2016b). The placental variant of human growth hormone reduces maternal insulin sensitivity in a dose-dependent manner in C57BL/6J Mice. Endocrinology 157, 1175–1186. doi: 10.1210/en.2015-1718
Li, J., Umar, S., Amjedi, M., Iorga, A., Sharma, S., Nadadur, R. D., et al. (2012). New frontiers in heart hypertrophy during pregnancy. Am. J. Cardiovasc. Dis. 2, 192–207.
Lim, R., Acharya, R., Delpachitra, P., Hobson, S., Sobey, C. G., Drummond, G. R., et al. (2015). Activin and NADPH-oxidase in preeclampsia: insights from in vitro and murine studies. Am. J. Obstet. Gynecol. 212, 86 e81–86 e12. doi: 10.1016/j.ajog.2014.07.021
Lin, B., Zhu, S., and Shao, B. (1996). Changes of plasma levels of monoamines in normal pregnancy and pregnancy-induced hypertension women and their significance. Zhonghua Fu Chan Ke Za Zhi 31, 670–672.
Linzer, D. I., and Fisher, S. J. (1999). The placenta and the prolactin family of hormones: regulation of the physiology of pregnancy. Mol. Endocrinol. 13, 837–840. doi: 10.1210/mend.13.6.0286
Lissauer, D., Eldershaw, S. A., Inman, C. F., Coomarasamy, A., Moss, P. A., and Kilby, M. D. (2015). Progesterone promotes maternal-fetal tolerance by reducing human maternal T-cell polyfunctionality and inducing a specific cytokine profile. Eur. J. Immunol. 45, 2858–2872. doi: 10.1002/eji.201445404
Liu, D., Wei, N., Man, H. Y., Lu, Y., Zhu, L. Q., and Wang, J. Z. (2015). The MT2 receptor stimulates axonogenesis and enhances synaptic transmission by activating Akt signaling. Cell Death Differ. 22, 583–596. doi: 10.1038/cdd.2014.195
Liu, H., Wu, Y., Qiao, F., and Gong, X. (2009). Effect of leptin on cytotrophoblast proliferation and invasion. J. Huazhong Univ. Sci. Technol. Med. Sci. 29, 631–636. doi: 10.1007/s11596-009-0519-0
Liu, L. X., Rowe, G. C., Yang, S., Li, J., Damilano, F., Chan, M. C., et al. (2017). PDK4 inhibits cardiac pyruvate oxidation in late pregnancy. Circ. Res. 121, 1370–1378. doi: 10.1161/CIRCRESAHA.117.311456
Li, Y., Klausen, C., Cheng, J. C., Zhu, H., and Leung, P. C. (2014). Activin A, B, and AB increase human trophoblast cell invasion by up-regulating N-cadherin. J. Clin. Endocrinol. Metab. 99, E2216–2225. doi: 10.1210/jc.2014-2118
Li, Y., Klausen, C., Zhu, H., and Leung, P. C. (2015). Activin A Increases human trophoblast invasion by inducing SNAIL-mediated MMP2 up-regulation through ALK4. J. Clin. Endocrinol. Metab. 100, E1415–1427. doi: 10.1210/jc.2015-2134
Lodhi, R. S., Nakabayashi, K., Suzuki, K., Yamada, A. Y., Hazama, R., Ebina, Y., et al. (2013). Relaxin has anti-apoptotic effects on human trophoblast-derived HTR-8/SV neo cells. Gynecol. Endocrinol. 29, 1051–1054. doi: 10.3109/09513590.2013.829444
Lomauro, A., and Aliverti, A. (2015). Respiratory physiology of pregnancy: physiology masterclass. Breathe (Sheff) 11, 297–301. doi: 10.1183/20734735.008615
Longo, M., Jain, V., Vedernikov, Y. P., Garfield, R. E., and Saade, G. R. (2003). Effects of recombinant human relaxin on pregnant rat uterine artery and myometrium in vitro. Am. J. Obstet. Gynecol. 188, 1468–1474; discussion 1474-1466. doi: 10.1067/mob.2003.454
Lucas, B. K., Ormandy, C. J., Binart, N., Bridges, R. S., and Kelly, P. A. (1998). Null mutation of the prolactin receptor gene produces a defect in maternal behavior. Endocrinology 139, 4102–4107. doi: 10.1210/endo.139.10.6243
Lu, C. C., Chen, J. J., Tsai, S. C., Chien, E. J., Chien, C. H., and Wang, P. S. (1998). Increase of thyrotropin response to thyrotropin-releasing hormone (TRH) and TRH release in rats during pregnancy. Chin. J. Physiol. 41, 211–216.
Lumbers, E. R., and Pringle, K. G. (2014). Roles of the circulating renin-angiotensin-aldosterone system in human pregnancy. Am. J. Physiol. Regul. Integr. Comp. Physiol. 306, R91–101. doi: 10.1152/ajpregu.00034.2013
Lydon, J. P., Demayo, F. J., Funk, C. R., Mani, S. K., Hughes, A. R., Montgomery, C. A. Jr., et al. (1995). Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 9, 2266–2278. doi: 10.1101/gad.9.18.2266
Maclennan, A. H., and Grant, P. (1991). Human relaxin. In vitro response of human and pig myometrium. J. Reprod. Med. 36, 630–634.
Macrae, D. J., and Palavradji, D. (1967). Maternal acid-base changes in pregnancy. J. Obstet. Gynaecol. Br. Commonw. 74, 11–16. doi: 10.1111/j.1471-0528.1967.tb03925.x
Maeshima, A., Shiozaki, S., Tajima, T., Nakazato, Y., Naruse, T., and Kojima, I. (2000). Number of glomeruli is increased in the kidney of transgenic mice expressing the truncated type II activin receptor. Biochem. Biophys. Res. Commun. 268, 445–449. doi: 10.1006/bbrc.2000.2171
Magariños, M. P., Sánchez-Margalet, V., Kotler, M., Calvo, J. C., and Varone, C. L. (2007). Leptin promotes cell proliferation and survival of trophoblastic cells. Biol. Reprod. 76, 203–210. doi: 10.1095/biolreprod.106.051391
Malik, N. M., Carter, N. D., Murray, J. F., Scaramuzzi, R. J., Wilson, C. A., and Stock, M. J. (2001). Leptin requirement for conception, implantation, and gestation in the mouse. Endocrinology 142, 5198–5202. doi: 10.1210/endo.142.12.8535
Malik, N. M., Carter, N. D., Wilson, C. A., Scaramuzzi, R. J., Stock, M. J., and Murray, J. F. (2005). Leptin expression in the fetus and placenta during mouse pregnancy. Placenta 26, 47–52. doi: 10.1016/j.placenta.2004.03.009
Mao, G., Wang, J., Kang, Y., Tai, P., Wen, J., Zou, Q., et al. (2010). Progesterone increases systemic and local uterine proportions of CD4+CD25+ Treg cells during midterm pregnancy in mice. Endocrinology 151, 5477–5488. doi: 10.1210/en.2010-0426
Maroni, E. S., and De Sousa, M. A. (1973). The lymphoid organs during pregnancy in the mouse. A comparison between a syngeneic and an allogeneic mating. Clin. Exp. Immunol. 13, 107–124.
Marshall, S. A., Leo, C. H., Senadheera, S. N., Girling, J. E., Tare, M., and Parry, L. J. (2016a). Relaxin deficiency attenuates pregnancy-induced adaptation of the mesenteric artery to angiotensin II in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 310, R847–R857. doi: 10.1152/ajpregu.00506.2015
Marshall, S. A., Ng, L., Unemori, E. N., Girling, J. E., and Parry, L. J. (2016b). Relaxin deficiency results in increased expression of angiogenesis- and remodelling-related genes in the uterus of early pregnant mice but does not affect endometrial angiogenesis prior to implantation. Reprod. Biol. Endocrinol. 14:11. doi: 10.1186/s12958-016-0148-y
Maruo, N., Nakabayashi, K., Wakahashi, S., Yata, A., and Maruo, T. (2007). Effects of recombinant H2 relaxin on the expression of matrix metalloproteinases and tissue inhibitor metalloproteinase in cultured early placental extravillous trophoblasts. Endocrine 32, 303–310. doi: 10.1007/s12020-008-9034-5
Mason, G. A., Caldwell, J. D., Stanley, D. A., Hatley, O. L., Prange, A. J. Jr., and Pedersen, C. A. (1986). Interactive effects of intracisternal oxytocin and other centrally active substances on colonic temperatures of mice. Regul. Pept. 14, 253–260. doi: 10.1016/0167-0115(86)90008-X
Masuzaki, H., Ogawa, Y., Sagawa, N., Hosoda, K., Matsumoto, T., Mise, H., et al. (1997). Nonadipose tissue production of leptin: leptin as a novel placenta-derived hormone in humans. Nat. Med. 3, 1029–1033. doi: 10.1038/nm0997-1029
Matjila, M., Millar, R., Van Der Spuy, Z., and Katz, A. (2016). Elevated placental expression at the maternal-fetal interface but diminished maternal circulatory kisspeptin in preeclamptic pregnancies. Pregnancy Hypertens. 6, 79–87. doi: 10.1016/j.preghy.2015.11.001
Mayerl, S., Liebsch, C., Visser, T. J., and Heuer, H. (2015). Absence of TRH receptor 1 in male mice affects gastric ghrelin production. Endocrinology 156, 755–767. doi: 10.1210/en.2014-1395
Mazella, J., Tang, M., and Tseng, L. (2004). Disparate effects of relaxin and TGFbeta1: relaxin increases, but TGFbeta1 inhibits, the relaxin receptor and the production of IGFBP-1 in human endometrial stromal/decidual cells. Hum. Reprod. 19, 1513–1518. doi: 10.1093/humrep/deh274
Mcilvride, S., Mushtaq, A., Papacleovoulou, G., Hurling, C., Steel, J., Jansen, E., et al. (2017). A progesterone-brown fat axis is involved in regulating fetal growth. Sci. Rep. 7:10671. doi: 10.1038/s41598-017-10979-7
Mead, E. J., Maguire, J. J., Kuc, R. E., and Davenport, A. P. (2007). Kisspeptins: a multifunctional peptide system with a role in reproduction, cancer and the cardiovascular system. Br. J. Pharmacol. 151, 1143–1153. doi: 10.1038/sj.bjp.0707295
Meziani, F., Van Overloop, B., Schneider, F., and Gairard, A. (2005). Parathyroid hormone-related protein-induced relaxation of rat uterine arteries: influence of the endothelium during gestation. J. Soc. Gynecol. Investig. 12, 14–19. doi: 10.1016/j.jsgi.2004.07.005
Miedlar, J. A., Rinaman, L., Vollmer, R. R., and Amico, J. A. (2007). Oxytocin gene deletion mice overconsume palatable sucrose solution but not palatable lipid emulsions. Am. J. Physiol. Regul. Integr. Comp. Physiol. 293, R1063–R1068. doi: 10.1152/ajpregu.00228.2007
Mikaelsson, M. A., Constância, M., Dent, C. L., Wilkinson, L. S., and Humby, T. (2013). Placental programming of anxiety in adulthood revealed by Igf2-null models. Nat. Commun. 4:2311. doi: 10.1038/ncomms3311
Milczarek, R., Hallmann, A., Sokołowska, E., Kaletha, K., and Klimek, J. (2010). Melatonin enhances antioxidant action of alpha-tocopherol and ascorbate against NADPH- and iron-dependent lipid peroxidation in human placental mitochondria. J. Pineal Res. 49, 149–155. doi: 10.1111/j.1600-079X.2010.00779.x
Miller, S. L., Yawno, T., Alers, N. O., Castillo-Melendez, M., Supramaniam, V. G., Vanzyl, N., et al. (2014). Antenatal antioxidant treatment with melatonin to decrease newborn neurodevelopmental deficits and brain injury caused by fetal growth restriction. J. Pineal Res. 56, 283–294. doi: 10.1111/jpi.12121
Mirabito Colafella, K. M., Samuel, C. S., and Denton, K. M. (2017). Relaxin contributes to the regulation of arterial pressure in adult female mice. Clin. Sci. 131, 2795–2805. doi: 10.1042/CS20171225
Miranda, A., and Sousa, N. (2018). Maternal hormonal milieu influence on fetal brain development. Brain Behav. 8:e00920. doi: 10.1002/brb3.920
Mitchell, J. A., Hammer, R. E., and Goldman, H. (1983). Serotonin-induced disruption of implantation in the rat: II. Suppression of decidualization. Biol. Reprod 29, 151–156. doi: 10.1095/biolreprod29.1.151
Modi, H., Jacovetti, C., Tarussio, D., Metref, S., Madsen, O. D., Zhang, F. P., et al. (2015). Autocrine action of IGF2 regulates adult beta-cell mass and function. Diabetes 64, 4148–4157. doi: 10.2337/db14-1735
Mohammadi-Sartang, M., Ghorbani, M., and Mazloom, Z. (2017). Effects of melatonin supplementation on blood lipid concentrations: a systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. doi: 10.1016/j.clnu.2017.11.003. [Epub ahead of print].
Mor, G., and Cardenas, I. (2010). The immune system in pregnancy: a unique complexity. Am J Reprod Immunol 63, 425–433. doi: 10.1111/j.1600-0897.2010.00836.x
Morrissy, S., Xu, B., Aguilar, D., Zhang, J., and Chen, Q. M. (2010). Inhibition of apoptosis by progesterone in cardiomyocytes. Aging Cell 9, 799–809. doi: 10.1111/j.1474-9726.2010.00619.x
Mounzih, K., Qiu, J., Ewart-Toland, A., and Chehab, F. F. (1998). Leptin is not necessary for gestation and parturition but regulates maternal nutrition via a leptin resistance state. Endocrinology 139, 5259–5262. doi: 10.1210/endo.139.12.6523
Moya, F., Mena, P., Heusser, F., Foradori, A., Paiva, E., Yazigi, R., et al. (1986). Response of the maternal, fetal, and neonatal pituitary-thyroid axis to thyrotropin-releasing hormone. Pediatr. Res. 20, 982–986. doi: 10.1203/00006450-198610000-00018
Mühlbauer, E., Albrecht, E., Bazwinsky-Wutschke, I., and Peschke, E. (2012). Melatonin influences insulin secretion primarily via MT(1) receptors in rat insulinoma cells (INS-1) and mouse pancreatic islets. J. Pineal Res. 52, 446–459. doi: 10.1111/j.1600-079X.2012.00959.x
Mühlbauer, E., Gross, E., Labucay, K., Wolgast, S., and Peschke, E. (2009). Loss of melatonin signalling and its impact on circadian rhythms in mouse organs regulating blood glucose. Eur. J. Pharmacol. 606, 61–71. doi: 10.1016/j.ejphar.2009.01.029
Müller, H., Liu, B., Croy, B. A., Head, J. R., Hunt, J. S., Dai, G., et al. (1999). Uterine natural killer cells are targets for a trophoblast cell-specific cytokine, prolactin-like protein A. Endocrinology 140, 2711–2720. doi: 10.1210/endo.140.6.6828
Munnell, E. W., and Taylor, H. C. (1947). Liver Blood Flow in Pregnancy-Hepatic Vein Catheterization. J. Clin. Invest. 26, 952–956. doi: 10.1172/JCI101890
Musial, B., Fernandez-Twinn, D. S., Vaughan, O. R., Ozanne, S. E., Voshol, P., Sferruzzi-Perri, A. N., et al. (2016). Proximity to Delivery Alters Insulin Sensitivity and Glucose Metabolism in Pregnant Mice. Diabetes 65, 851–860. doi: 10.2337/db15-1531
Musial, B., Vaughan, O. R., Fernandez-Twinn, D. S., Voshol, P., Ozanne, S. E., Fowden, A. L., et al. (2017). A Western-style obesogenic diet alters maternal metabolic physiology with consequences for fetal nutrient acquisition in mice. J. Physiol. (Lond). 595, 4875–4892. doi: 10.1113/JP273684
Muttukrishna, S., Child, T. J., Groome, N. P., and Ledger, W. L. (1997). Source of circulating levels of inhibin A, pro alpha C-containing inhibins and activin A in early pregnancy. Hum. Reprod. 12, 1089–1093. doi: 10.1093/humrep/12.5.1089
Nakamura, Y., Tamura, H., Kashida, S., Takayama, H., Yamagata, Y., Karube, A., et al. (2001). Changes of serum melatonin level and its relationship to feto-placental unit during pregnancy. J. Pineal Res. 30, 29–33. doi: 10.1034/j.1600-079X.2001.300104.x
Nanetti, L., Raffaelli, F., Giulietti, A., Sforza, G., Raffaele Giannubilo, S., Ciavattini, A., et al. (2015). Oxytocin, its antagonist Atosiban, and preterm labor: a role for placental nitric oxide. J. Matern. Fetal Neonatal Med. 28, 611–616. doi: 10.3109/14767058.2014.927859
Nathanielsz, P. W., Jenkins, S. L., Tame, J. D., Winter, J. A., Guller, S., and Giussani, D. A. (1998). Local paracrine effects of estradiol are central to parturition in the rhesus monkey. Nat. Med. 4, 456–459. doi: 10.1038/nm0498-456
Neville, M. C., Mcfadden, T. B., and Forsyth, I. (2002). Hormonal regulation of mammary differentiation and milk secretion. J. Mammary Gland Biol. Neoplasia 7, 49–66. doi: 10.1023/A:1015770423167
Nielsen, J. H. (1982). Effects of growth hormone, prolactin, and placental lactogen on insulin content and release, and deoxyribonucleic acid synthesis in cultured pancreatic islets. Endocrinology 110, 600–606. doi: 10.1210/endo-110-2-600
Nien, J. K., Mazaki-Tovi, S., Romero, R., Erez, O., Kusanovic, J. P., Gotsch, F., et al. (2007). Plasma adiponectin concentrations in non-pregnant, normal and overweight pregnant women. J. Perinat. Med. 35, 522–531. doi: 10.1515/JPM.2007.123
Nir, I., and Hirschmann, N. (1980). Melatonin-induced changes in blood and pituitary luteinizing hormone and prolactin levels during the perinatal period in rat dams. J Neural Transm 49, 219–228. doi: 10.1007/BF01252127
Nishimori, K., Young, L. J., Guo, Q., Wang, Z., Insel, T. R., and Matzuk, M. M. (1996). Oxytocin is required for nursing but is not essential for parturition or reproductive behavior. Proc. Natl. Acad. Sci. U.S.A. 93, 11699–11704. doi: 10.1073/pnas.93.21.11699
Ni, X., Luo, S., Minegishi, T., and Peng, C. (2000). Activin A in JEG-3 cells: potential role as an autocrine regulator of steroidogenesis in humans. Biol. Reprod. 62, 1224–1230. doi: 10.1095/biolreprod62.5.1224
Norton, M. T., Fortner, K. A., Bizargity, P., and Bonney, E. A. (2009). Pregnancy alters the proliferation and apoptosis of mouse splenic erythroid lineage cells and leukocytes. Biol. Reprod. 81, 457–464. doi: 10.1095/biolreprod.109.076976
Norwitz, E. R., and Caughey, A. B. (2011). Progesterone supplementation and the prevention of preterm birth. Rev. Obstet. Gynecol. 4, 60–72.
Obr, A. E., Grimm, S. L., Bishop, K. A., Pike, J. W., Lydon, J. P., and Edwards, D. P. (2013). Progesterone receptor and Stat5 signaling cross talk through RANKL in mammary epithelial cells. Mol. Endocrinol. 27, 1808–1824. doi: 10.1210/me.2013-1077
O'byrne, E. M., Sawyer, W. K., Butler, M. C., and Steinetz, B. G. (1976). Serum immunoreactive relaxin and softening of the uterine cervix in pregnant hamsters. Endocrinology 99, 1333–1335. doi: 10.1210/endo-99-5-1333
O'byrne, E. M., and Steinetz, B. G. (1976). Radioimmunoassay (RIA) of relaxin in sera of various species using an antiserum to porcine relaxin. Proc. Soc. Exp. Biol. Med. 152, 272–276. doi: 10.3181/00379727-152-39377
Ogawa, Y., Masuzaki, H., Hosoda, K., Aizawa-Abe, M., Suga, J., Suda, M., et al. (1999). Increased glucose metabolism and insulin sensitivity in transgenic skinny mice overexpressing leptin. Diabetes 48, 1822–1829. doi: 10.2337/diabetes.48.9.1822
Ogueh, O., Clough, A., Hancock, M., and Johnson, M. R. (2011). A longitudinal study of the control of renal and uterine hemodynamic changes of pregnancy. Hypertens. Pregnancy 30, 243–259. doi: 10.3109/10641955.2010.484079
Ohara-Imaizumi, M., Kim, H., Yoshida, M., Fujiwara, T., Aoyagi, K., Toyofuku, Y., et al. (2013). Serotonin regulates glucose-stimulated insulin secretion from pancreatic beta cells during pregnancy. Proc. Natl. Acad. Sci. U.S.A. 110, 19420–19425. doi: 10.1073/pnas.1310953110
Okatani, Y., Wakatsuki, A., Shinohara, K., Taniguchi, K., and Fukaya, T. (2001). Melatonin protects against oxidative mitochondrial damage induced in rat placenta by ischemia and reperfusion. J. Pineal Res. 31, 173–178. doi: 10.1034/j.1600-079x.2001.310212.x
O'neal-Moffitt, G., Pilli, J., Kumar, S. S., and Olcese, J. (2014). Genetic deletion of MT(1)/MT(2) melatonin receptors enhances murine cognitive and motor performance. Neuroscience 277, 506–521. doi: 10.1016/j.neuroscience.2014.07.018
O'sullivan, K. P., Marshall, S. A., Cullen, S., Saunders, T., Hannan, N. J., Senadheera, S. N., et al. (2017). Evidence of proteinuria, but no other characteristics of pre-eclampsia, in relaxin-deficient mice. Reprod. Fertil. Dev. 29, 1477–1485. doi: 10.1071/RD16056
Owino, S., Contreras-Alcantara, S., Baba, K., and Tosini, G. (2016). Melatonin signaling controls the daily rhythm in blood glucose levels independent of peripheral clocks. PLoS ONE 11:e0148214. doi: 10.1371/journal.pone.0148214
Palejwala, S., Stein, D. E., Weiss, G., Monia, B. P., Tortoriello, D., and Goldsmith, L. T. (2001). Relaxin positively regulates matrix metalloproteinase expression in human lower uterine segment fibroblasts using a tyrosine kinase signaling pathway. Endocrinology 142, 3405–3413. doi: 10.1210/endo.142.8.8295
Paller, M. S., Gregorini, G., and Ferris, T. F. (1989). Pressor responsiveness in pseudopregnant and pregnant rats: role of maternal factors. Am. J. Physiol. 257, R866–R871. doi: 10.1152/ajpregu.1989.257.4.R866
Pang, W. W., and Hartmann, P. E. (2007). Initiation of human lactation: secretory differentiation and secretory activation. J. Mammary Gland Biol. Neoplasia 12, 211–221. doi: 10.1007/s10911-007-9054-4
Pecins-Thompson, M., and Keller-Wood, M. (1997). Effects of progesterone on blood pressure, plasma volume, and responses to hypotension. Am. J. Physiol. 272, R377–385. doi: 10.1152/ajpregu.1997.272.1.R377
Pedersen, C. A., Vadlamudi, S. V., Boccia, M. L., and Amico, J. A. (2006). Maternal behavior deficits in nulliparous oxytocin knockout mice. Genes Brain Behav. 5, 274–281. doi: 10.1111/j.1601-183X.2005.00162.x
Pelletier, G., Petitclerc, D., Lapierre, H., Bernier-Cardou, M., Morisset, J., Gaudreau, P., et al. (1987). Injection of synthetic human growth hormone-releasing factors in dairy cows. 1. Effect on feed intake and milk yield and composition. J. Dairy Sci. 70, 2511–2517. doi: 10.3168/jds.S0022-0302(87)80319-3
Pelleymounter, M. A., Cullen, M. J., Baker, M. B., Hecht, R., Winters, D., Boone, T., et al. (1995). Effects of the obese gene product on body weight regulation in ob/ob mice. Science 269, 540–543. doi: 10.1126/science.7624776
Peng, J., Fullerton, P. T. Jr., Monsivais, D., Clementi, C., Su, G. H., and Matzuk, M. M. (2015). Uterine activin-like kinase 4 regulates trophoblast development during mouse placentation. Mol. Endocrinol. 29, 1684–1693. doi: 10.1210/me.2015-1048
Petersson, M., Alster, P., Lundeberg, T., and Uvnäs-Moberg, K. (1996). Oxytocin causes a long-term decrease of blood pressure in female and male rats. Physiol. Behav. 60, 1311–1315. doi: 10.1016/S0031-9384(96)00261-2
Petry, C. J., Evans, M. L., Wingate, D. L., Ong, K. K., Reik, W., Constância, M., et al. (2010). Raised late pregnancy glucose concentrations in mice carrying pups with targeted disruption of H19delta13. Diabetes 59, 282–286. doi: 10.2337/db09-0757
Petry, C. J., Ong, K. K., and Dunger, D. B. (2007). Does the fetal genotype affect maternal physiology during pregnancy? Trends Mol. Med. 13, 414–421. doi: 10.1016/j.molmed.2007.07.007
Pieper, P. G. (2015). Use of medication for cardiovascular disease during pregnancy. Nat. Rev. Cardiol. 12, 718–729. doi: 10.1038/nrcardio.2015.172
Pitera, A. E., Smith, G. C., Wentworth, R. A., and Nathanielsz, P. W. (1998). Parathyroid hormone-related peptide (1 to 34) inhibits in vitro oxytocin-stimulated activity of pregnant baboon myometrium. Am. J. Obstet. Gynecol. 179, 492–496. doi: 10.1016/S0002-9378(98)70385-0
Plaut, K., Maple, R., Ginsburg, E., and Vonderhaar, B. (1999). Progesterone stimulates DNA synthesis and lobulo-alveolar development in mammary glands in ovariectomized mice. J. Cell Physiol. 180, 298–304. doi: 10.1002/(SICI)1097-4652(199908)180:2<298::AID-JCP17>3.0.CO;2-V
Porter, S. E., Sorenson, R. L., Dann, P., Garcia-Ocana, A., Stewart, A. F., and Vasavada, R. C. (1998). Progressive pancreatic islet hyperplasia in the islet-targeted, parathyroid hormone-related protein-overexpressing mouse. Endocrinology 139, 3743–3751. doi: 10.1210/endo.139.9.6212
Poulson, E., Botros, M., and Robson, J. M. (1960). Effect of 5-hydroxytryptamine and iproniazid on pregnancy. Science 131, 1101–1102. doi: 10.1126/science.131.3407.1101
Prast, J., Saleh, L., Husslein, H., Sonderegger, S., Helmer, H., and Knöfler, M. (2008). Human chorionic gonadotropin stimulates trophoblast invasion through extracellularly regulated kinase and AKT signaling. Endocrinology 149, 979–987. doi: 10.1210/en.2007-1282
Prezotto, L. D., Lemley, C. O., Camacho, L. E., Doscher, F. E., Meyer, A. M., Caton, J. S., et al. (2014). Effects of nutrient restriction and melatonin supplementation on maternal and foetal hepatic and small intestinal energy utilization. J. Anim. Physiol. Anim. Nutr. (Berl). 98, 797–807. doi: 10.1111/jpn.12142
Prigent-Tessier, A., Pageaux, J. F., Fayard, J. M., Lagarde, M., Laugier, C., and Cohen, H. (1996). Prolactin up-regulates prostaglandin E2 production through increased expression of pancreatic-type phospholipase A2 (type I) and prostaglandin G/H synthase 2 in uterine cells. Mol. Cell. Endocrinol. 122, 101–108. doi: 10.1016/0303-7207(96)03888-9
Qi, X., Gong, B., Yu, J., Shen, L., Jin, W., Wu, Z., et al. (2017). Decreased cord blood estradiol levels in related to mothers with gestational diabetes. Medicine (Baltimore). 96:e6962. doi: 10.1097/MD.0000000000006962
Quagliarello, J., Szlachter, N., Steinetz, B. G., Goldsmith, L. T., and Weiss, G. (1979). Serial relaxin concentrations in human pregnancy. Am. J. Obstet. Gynecol. 135, 43–44.
Qu, J., and Thomas, K. (1993). Regulation of inhibin secretion in human placental cell culture by epidermal growth factor, transforming growth factors, and activin. J. Clin. Endocrinol. Metab. 77, 925–931.
Rabeler, R., Mittag, J., Geffers, L., Rüther, U., Leitges, M., Parlow, A. F., et al. (2004). Generation of thyrotropin-releasing hormone receptor 1-deficient mice as an animal model of central hypothyroidism. Mol. Endocrinol. 18, 1450–1460. doi: 10.1210/me.2004-0017
Racicot, K., Kwon, J. Y., Aldo, P., Silasi, M., and Mor, G. (2014). Understanding the complexity of the immune system during pregnancy. Am J Reprod Immunol 72, 107–116. doi: 10.1111/aji.12289
Randle, P. J. (1998). Regulatory interactions between lipids and carbohydrates: the glucose fatty acid cycle after 35 years. Diabetes Metab. Rev. 14, 263–283. doi: 10.1002/(sici)1099-0895(199812)14:4<263::aid-dmr233>3.0.co;2-c
Rawn, S. M., Huang, C., Hughes, M., Shaykhutdinov, R., Vogel, H. J., and Cross, J. C. (2015). Pregnancy hyperglycemia in prolactin receptor mutant, but not prolactin mutant, mice and feeding-responsive regulation of placental lactogen genes implies placental control of maternal glucose homeostasis. Biol. Reprod. 93:75. doi: 10.1095/biolreprod.115.132431
Renegar, R. H., and Owens, C. R. III. (2002). Measurement of plasma and tissue relaxin concentrations in the pregnant hamster and fetus using a homologous radioimmunoassay. Biol. Reprod. 67, 500–505. doi: 10.1095/biolreprod67.2.500
Rezaei, R., Wu, Z., Hou, Y., Bazer, F. W., and Wu, G. (2016). Amino acids and mammary gland development: nutritional implications for milk production and neonatal growth. J. Anim. Sci. Biotechnol. 7:20. doi: 10.1186/s40104-016-0078-8
Ribas, V., Drew, B. G., Le, J. A., Soleymani, T., Daraei, P., Sitz, D., et al. (2011). Myeloid-specific estrogen receptor alpha deficiency impairs metabolic homeostasis and accelerates atherosclerotic lesion development. Proc. Natl. Acad. Sci. U.S.A. 108, 16457–16462. doi: 10.1073/pnas.1104533108
Ribeiro, A. C., Musatov, S., Shteyler, A., Simanduyev, S., Arrieta-Cruz, I., Ogawa, S., et al. (2012). siRNA silencing of estrogen receptor-alpha expression specifically in medial preoptic area neurons abolishes maternal care in female mice. Proc. Natl. Acad. Sci. U.S.A. 109, 16324–16329. doi: 10.1073/pnas.1214094109
Rieck, S., and Kaestner, K. H. (2010). Expansion of beta-cell mass in response to pregnancy. Trends Endocrinol. Metab. 21, 151–158. doi: 10.1016/j.tem.2009.11.001
Robinson, D. P., and Klein, S. L. (2012). Pregnancy and pregnancy-associated hormones alter immune responses and disease pathogenesis. Horm. Behav. 62, 263–271. doi: 10.1016/j.yhbeh.2012.02.023
Robson, J. M., and Sullivan, F. M. (1966). Analysis of actions of 5-hydroxytryptamine in pregnancy. J. Physiol. (Lond). 184, 717–732. doi: 10.1113/jphysiol.1966.sp007943
Rodger, M., Sheppard, D., Gándara, E., and Tinmouth, A. (2015). Haematological problems in obstetrics. Best Pract. Res. Clin. Obstet. Gynaecol. 29, 671–684. doi: 10.1016/j.bpobgyn.2015.02.004
Romero, M., Ortega, A., Izquierdo, A., López-Luna, P., and Bosch, R. J. (2010). Parathyroid hormone-related protein induces hypertrophy in podocytes via TGF-beta(1) and p27(Kip1): implications for diabetic nephropathy. Nephrol. Dial. Transplant 25, 2447–2457. doi: 10.1093/ndt/gfq104
Roos, A., Robertson, F., Lochner, C., Vythilingum, B., and Stein, D. J. (2011). Altered prefrontal cortical function during processing of fear-relevant stimuli in pregnancy. Behav. Brain Res. 222, 200–205. doi: 10.1016/j.bbr.2011.03.055
Roti, E., Gnudi, A., Braverman, L. E., Robuschi, G., Emanuele, R., Bandini, P., et al. (1981). Human cord blood concentrations of thyrotropin, thyroglobulin, and iodothyronines after maternal administration of thyrotropin-releasing hormone. J. Clin. Endocrinol. Metab. 53, 813–817. doi: 10.1210/jcem-53-4-813
Rozenblit-Susan, S., Chapnik, N., and Froy, O. (2017). Serotonin prevents differentiation into brown adipocytes and induces transdifferentiation into white adipocytes. Int. J. Obes (Lond). 42, 704–710. doi: 10.1038/ijo.2017.261
Ryan, E. A., O'sullivan, M. J., and Skyler, J. S. (1985). Insulin action during pregnancy. Studies with the euglycemic clamp technique. Diabetes 34, 380–389. doi: 10.2337/diab.34.4.380
Rybakowski, C., Niemax, K., Goepel, E., and Schröder, H. J. (2000). The effect of oxytocin, prostaglandin E2 and acetylsalicylic acid on flow distribution and on the transfer of alanine, glucose and water in isolated perfused guinea pig placentae. Placenta 21, 126–131. doi: 10.1053/plac.1999.0459
Rygaard, K., Revol, A., Esquivel-Escobedo, D., Beck, B. L., and Barrera-Saldana, H. A. (1998). Absence of human placental lactogen and placental growth hormone (HGH-V) during pregnancy: PCR analysis of the deletion. Hum. Genet. 102, 87–92. doi: 10.1007/s004390050658
Sagawa, N., Yura, S., Itoh, H., Mise, H., Kakui, K., Korita, D., et al. (2002). Role of leptin in pregnancy–a review. Placenta 23(Suppl. A), S80–S86. doi: 10.1053/plac.2002.0814
Sairenji, T. J., Ikezawa, J., Kaneko, R., Masuda, S., Uchida, K., Takanashi, Y., et al. (2017). Maternal prolactin during late pregnancy is important in generating nurturing behavior in the offspring. Proc. Natl. Acad. Sci. U.S.A. 114, 13042–13047. doi: 10.1073/pnas.1621196114
Saito, S., Nakashima, A., Shima, T., and Ito, M. (2010). Th1/Th2/Th17 and regulatory T-cell paradigm in pregnancy. Am. J. Reprod Immunol. 63, 601–610. doi: 10.1111/j.1600-0897.2010.00852.x
Salles, J. P. (2016). Bone metabolism during pregnancy. Ann. Endocrinol. (Paris). 77, 163–168. doi: 10.1016/j.ando.2016.04.004
Samuel, C. S., Zhao, C., Bathgate, R. A., Bond, C. P., Burton, M. D., Parry, L. J., et al. (2003). Relaxin deficiency in mice is associated with an age-related progression of pulmonary fibrosis. FASEB J. 17, 121–123. doi: 10.1096/fj.02-0449fje
Sandoval-Guzmán, T., Gongrich, C., Moliner, A., Guo, T., Wu, H., Broberger, C., et al. (2012). Neuroendocrine control of female reproductive function by the activin receptor ALK7. FASEB J. 26, 4966–4976. doi: 10.1096/fj.11-199059
Sasaki, K., Matsumura, G., and Ito, T. (1981). Effects of pregnancy on erythropoiesis in the splenic red pulp of the mouse: a quantitative electron microscopic study. Arch. Histol. Jpn. 44, 429–438. doi: 10.1679/aohc1950.44.429
Sasaki, Y., Morimoto, T., Saito, H., Suzuki, M., Ichizuka, K., and Yanaihara, T. (2000). The role of parathyroid hormone-related protein in intra-tracheal fluid. Endocr. J. 47, 169–175. doi: 10.1507/endocrj.47.169
Scarpace, P. J., Matheny, M., Pollock, B. H., and Tümer, N. (1997). Leptin increases uncoupling protein expression and energy expenditure. Am. J. Physiol. 273, E226–230. doi: 10.1152/ajpendo.1997.273.1.E226
Schanton, M., Maymó, J. L., Pérez-Pérez, A., Sánchez-Margalet, V., and Varone, C. L. (2018). Involvement of leptin in the molecular physiology of the placenta. Reproduction 155, R1–R12. doi: 10.1530/REP-17-0512
Schipani, E., Lanske, B., Hunzelman, J., Luz, A., Kovacs, C. S., Lee, K., et al. (1997). Targeted expression of constitutively active receptors for parathyroid hormone and parathyroid hormone-related peptide delays endochondral bone formation and rescues mice that lack parathyroid hormone-related peptide. Proc. Natl. Acad. Sci. U.S.A. 94, 13689–13694. doi: 10.1073/pnas.94.25.13689
Schulz, L. C., and Widmaier, E. P. (2004). The effect of leptin on mouse trophoblast cell invasion. Biol. Reprod. 71, 1963–1967. doi: 10.1095/biolreprod.104.032722
Schumacher, A., Heinze, K., Witte, J., Poloski, E., Linzke, N., Woidacki, K., et al. (2013). Human chorionic gonadotropin as a central regulator of pregnancy immune tolerance. J. Immunol. 190, 2650–2658. doi: 10.4049/jimmunol.1202698
Sclafani, A., Rinaman, L., Vollmer, R. R., and Amico, J. A. (2007). Oxytocin knockout mice demonstrate enhanced intake of sweet and nonsweet carbohydrate solutions. Am. J. Physiol. Regul. Integr. Comp. Physiol. 292, R1828–1833. doi: 10.1152/ajpregu.00826.2006
Scott, P. R., Sargison, N. D., Macrae, A. I., and Gough, M. R. (2009). Melatonin treatment prior to the normal breeding season increases fetal number in United Kingdom sheep flocks. Vet. J. 182, 198–202. doi: 10.1016/j.tvjl.2008.07.010
Seki, K., Uesato, T., Tabei, T., and Kato, K. (1985). The secretory patterns of relaxin and human chorionic gonadotropin in human pregnancy. Endocrinol. Jpn. 32, 741–744. doi: 10.1507/endocrj1954.32.741
Seufert, J., Kieffer, T. J., Leech, C. A., Holz, G. G., Moritz, W., Ricordi, C., et al. (1999). Leptin suppression of insulin secretion and gene expression in human pancreatic islets: implications for the development of adipogenic diabetes mellitus. J. Clin. Endocrinol. Metab. 84, 670–676. doi: 10.1210/jc.84.2.670
Sferruzzi-Perri, A. N., Owens, J. A., Pringle, K. G., Robinson, J. S., and Roberts, C. T. (2006). Maternal insulin-like growth factors-I and -II act via different pathways to promote fetal growth. Endocrinology 147, 3344–3355. doi: 10.1210/en.2005-1328
Sferruzzi-Perri, A. N., Owens, J. A., Standen, P., Taylor, R. L., Heinemann, G. K., Robinson, J. S., et al. (2007). Early treatment of the pregnant guinea pig with IGFs promotes placental transport and nutrient partitioning near term. Am. J. Physiol. Endocrinol. Metab. 292, E668–676. doi: 10.1152/ajpendo.00320.2006
Sferruzzi-Perri, A. N., Vaughan, O. R., Coan, P. M., Suciu, M. C., Darbyshire, R., Constancia, M., et al. (2011). Placental-specific Igf2 deficiency alters developmental adaptations to undernutrition in mice. Endocrinology 152, 3202–3212. doi: 10.1210/en.2011-0240
Shahtaheri, S. M., Aaron, J. E., Johnson, D. R., and Purdie, D. W. (1999). Changes in trabecular bone architecture in women during pregnancy. Br. J. Obstet. Gynaecol. 106, 432–438. doi: 10.1111/j.1471-0528.1999.tb08296.x
Shakhmatova, E. I., Osipova, N. A., and Natochin, Y. V. (2000). Changes in osmolality and blood serum ion concentrations in pregnancy. Hum. Physiol. 26, 92–95. doi: 10.1007/BF02760724
Sharkey, J. T., Cable, C., and Olcese, J. (2010). Melatonin sensitizes human myometrial cells to oxytocin in a protein kinase C alpha/extracellular-signal regulated kinase-dependent manner. J. Clin. Endocrinol. Metab. 95, 2902–2908. doi: 10.1210/jc.2009-2137
Sharkey, J. T., Puttaramu, R., Word, R. A., and Olcese, J. (2009). Melatonin synergizes with oxytocin to enhance contractility of human myometrial smooth muscle cells. J. Clin. Endocrinol. Metab. 94, 421–427. doi: 10.1210/jc.2008-1723
Shaw, L., Taggart, M., and Austin, C. (2001). Effects of the oestrous cycle and gender on acute vasodilatory responses of isolated pressurized rat mesenteric arteries to 17 beta-oestradiol. Br. J. Pharmacol. 132, 1055–1062. doi: 10.1038/sj.bjp.0703908
Shek, E. W., Brands, M. W., and Hall, J. E. (1998). Chronic leptin infusion increases arterial pressure. Hypertension 31, 409–414. doi: 10.1161/01.HYP.31.1.409
Shingo, T., Gregg, C., Enwere, E., Fujikawa, H., Hassam, R., Geary, C., et al. (2003). Pregnancy-stimulated neurogenesis in the adult female forebrain mediated by prolactin. Science 299, 117–120. doi: 10.1126/science.1076647
Shi, Q. J., Lei, Z. M., Rao, C. V., and Lin, J. (1993). Novel role of human chorionic gonadotropin in differentiation of human cytotrophoblasts. Endocrinology 132, 1387–1395. doi: 10.1210/endo.132.3.7679981
Sierra-Honigmann, M. R., Nath, A. K., Murakami, C., García-Cardeña G, G., Papapetropoulos, A., Sessa, W. C., et al. (1998). Biological action of leptin as an angiogenic factor. Science 281, 1683–1686. doi: 10.1126/science.281.5383.1683
Simmons, D. G., Rawn, S., Davies, A., Hughes, M., and Cross, J. C. (2008). Spatial and temporal expression of the 23 murine Prolactin/Placental Lactogen-related genes is not associated with their position in the locus. BMC Genomics 9:352. doi: 10.1186/1471-2164-9-352
Simoncini, T., Mannella, P., Fornari, L., Caruso, A., Willis, M. Y., Garibaldi, S., et al. (2004). Differential signal transduction of progesterone and medroxyprogesterone acetate in human endothelial cells. Endocrinology 145, 5745–5756. doi: 10.1210/en.2004-0510
Singh, H. J., Saleh, H. I., Gupalo, S., and Omar, E. (2013). Effect of melatonin supplementation on pregnancy outcome in Wistar-Kyoto and Sprague-Dawley rats. Sheng Li Xue Bao 65, 149–157.
Slattery, M. M., O'leary, M. J., and Morrison, J. J. (2001). Effect of parathyroid hormone-related peptide on human and rat myometrial contractility in vitro. Am. J. Obstet. Gynecol. 184, 625–629. doi: 10.1067/mob.2001.110695
Soares, M. J. (2004). The prolactin and growth hormone families: pregnancy-specific hormones/cytokines at the maternal-fetal interface. Reprod. Biol. Endocrinol. 2:51. doi: 10.1186/1477-7827-2-51
Soares, M. J., Konno, T., and Alam, S. M. (2007). The prolactin family: effectors of pregnancy-dependent adaptations. Trends Endocrinol. Metab. 18, 114–121. doi: 10.1016/j.tem.2007.02.005
Soliman, A., Lacasse, A. A., Lanoix, D., Sagrillo-Fagundes, L., Boulard, V., and Vaillancourt, C. (2015). Placental melatonin system is present throughout pregnancy and regulates villous trophoblast differentiation. J. Pineal Res. 59, 38–46. doi: 10.1111/jpi.12236
Soloff, M. S., Jeng, Y. J., Izban, M. G., Sinha, M., Luxon, B. A., Stamnes, S. J., et al. (2011). Effects of progesterone treatment on expression of genes involved in uterine quiescence. Reprod. Sci. 18, 781–797. doi: 10.1177/1933719111398150
Soma-Pillay, P., Nelson-Piercy, C., Tolppanen, H., and Mebazaa, A. (2016). Physiological changes in pregnancy. Cardiovasc. J. Afr. 27, 89–94. doi: 10.5830/CVJA-2016-021
Song, G. J., Fiaschi-Taesch, N., and Bisello, A. (2009). Endogenous parathyroid hormone-related protein regulates the expression of PTH type 1 receptor and proliferation of vascular smooth muscle cells. Mol. Endocrinol. 23, 1681–1690. doi: 10.1210/me.2009-0098
Song, W. J., Mondal, P., Wolfe, A., Alonso, L. C., Stamateris, R., Ong, B. W., et al. (2014). Glucagon regulates hepatic kisspeptin to impair insulin secretion. Cell Metab. 19, 667–681. doi: 10.1016/j.cmet.2014.03.005
Song, Y., Keelan, J., and France, J. T. (1996). Activin-A stimulates, while transforming growth factor beta 1 inhibits, chorionic gonadotrophin production and aromatase activity in cultured human placental trophoblasts. Placenta 17, 603–610. doi: 10.1016/S0143-4004(96)80078-6
Sonier, B., Lavigne, C., Arseneault, M., Ouellette, R., and Vaillancourt, C. (2005). Expression of the 5-HT2A serotoninergic receptor in human placenta and choriocarcinoma cells: mitogenic implications of serotonin. Placenta 26, 484–490. doi: 10.1016/j.placenta.2004.08.003
Sorenson, R. L., and Brelje, T. C. (1997). Adaptation of islets of Langerhans to pregnancy: beta-cell growth, enhanced insulin secretion and the role of lactogenic hormones. Horm. Metab. Res. 29, 301–307. doi: 10.1055/s-2007-979040
Sorenson, R. L., Brelje, T. C., and Roth, C. (1993). Effects of steroid and lactogenic hormones on islets of Langerhans: a new hypothesis for the role of pregnancy steroids in the adaptation of islets to pregnancy. Endocrinology 133, 2227–2234. doi: 10.1210/endo.133.5.8404674
Sorenson, R. L., Johnson, M. G., Parsons, J. A., and Sheridan, J. D. (1987). Decreased glucose stimulation threshold, enhanced insulin secretion, and increased beta cell coupling in islets of prolactin-treated rats. Pancreas 2, 283–288. doi: 10.1097/00006676-198705000-00006
Spicer, L. J., and Aad, P. Y. (2007). Insulin-like growth factor (IGF) 2 stimulates steroidogenesis and mitosis of bovine granulosa cells through the IGF1 receptor: role of follicle-stimulating hormone and IGF2 receptor. Biol. Reprod. 77, 18–27. doi: 10.1095/biolreprod.106.058230
Steele, G. L., Currie, W. D., Yuen, B. H., Jia, X. C., Perlas, E., and Leung, P. C. (1993). Acute stimulation of human chorionic gonadotropin secretion by recombinant human activin-A in first trimester human trophoblast. Endocrinology 133, 297–303. doi: 10.1210/endo.133.1.8319577
Stelmanska, E., and Sucajtys-Szulc, E. (2014). Enhanced food intake by progesterone-treated female rats is related to changes in neuropeptide genes expression in hypothalamus. Endokrynol. Pol. 65, 46–56. doi: 10.5603/EP.2014.0007
Sternlicht, M. D. (2006). Key stages in mammary gland development: the cues that regulate ductal branching morphogenesis. Breast Cancer Res. 8:201. doi: 10.1186/bcr1368
Stokkan, K. A., and Aarseth, J. J. (2004). Melatonin reduces noradrenaline-induced vasoconstriction in the uterine artery of pregnant hooded seals (Cystophora cristata). Pflugers Arch. 447, 405–407. doi: 10.1007/s00424-003-1198-5
Storment, J. M., Meyer, M., and Osol, G. (2000). Estrogen augments the vasodilatory effects of vascular endothelial growth factor in the uterine circulation of the rat. Am. J. Obstet. Gynecol. 183, 449–453. doi: 10.1067/mob.2000.105910
Sun, Y., Zupan, B., Raaka, B. M., Toth, M., and Gershengorn, M. C. (2009). TRH-receptor-type-2-deficient mice are euthyroid and exhibit increased depression and reduced anxiety phenotypes. Neuropsychopharmacology 34, 1601–1608. doi: 10.1038/npp.2008.217
Takahashi, K., Ohmichi, M., Yoshida, M., Hisamoto, K., Mabuchi, S., Arimoto-Ishida, E., et al. (2003). Both estrogen and raloxifene cause G1 arrest of vascular smooth muscle cells. J. Endocrinol. 178, 319–329. doi: 10.1677/joe.0.1780319
Takayanagi, Y., Kasahara, Y., Onaka, T., Takahashi, N., Kawada, T., and Nishimori, K. (2008). Oxytocin receptor-deficient mice developed late-onset obesity. Neuroreport 19, 951–955. doi: 10.1097/WNR.0b013e3283021ca9
Takayanagi, Y., Yoshida, M., Bielsky, I. F., Ross, H. E., Kawamata, M., Onaka, T., et al. (2005). Pervasive social deficits, but normal parturition, in oxytocin receptor-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 102, 16096–16101. doi: 10.1073/pnas.0505312102
Takeda, K., Toda, K., Saibara, T., Nakagawa, M., Saika, K., Onishi, T., et al. (2003). Progressive development of insulin resistance phenotype in male mice with complete aromatase (CYP19) deficiency. J. Endocrinol. 176, 237–246. doi: 10.1677/joe.0.1760237
Tamma, R., Colaianni, G., Zhu, L. L., Dibenedetto, A., Greco, G., Montemurro, G., et al. (2009). Oxytocin is an anabolic bone hormone. Proc. Natl. Acad. Sci. U.S.A. 106, 7149–7154. doi: 10.1073/pnas.0901890106
Tamura, H., Takayama, H., Nakamura, Y., Reiter, R. J., and Sugino, N. (2008). Fetal/placental regulation of maternal melatonin in rats. J. Pineal Res. 44, 335–340. doi: 10.1111/j.1600-079X.2007.00537.x
Tessier, C., Prigent-Tessier, A., Bao, L., Telleria, C. M., Ferguson-Gottschall, S., Gibori, G. B., et al. (2003). Decidual activin: its role in the apoptotic process and its regulation by prolactin. Biol. Reprod. 68, 1687–1694. doi: 10.1095/biolreprod.102.011684
Thomas, A. L., Jack, P. M., Manns, J. G., and Nathanielsz, P. W. (1975). Effect of synthetic thyrotrophin releasing hormone on thyrotrophin and prolactin concentractions in the peripheral plasma of the pregnant ewe, lamb fetus and neonatal lamb. Biol. Neonate 26, 109–116. doi: 10.1159/000240722
Tiano, J. P., and Mauvais-Jarvis, F. (2012). Importance of oestrogen receptors to preserve functional beta-cell mass in diabetes. Nat. Rev. Endocrinol. 8, 342–351. doi: 10.1038/nrendo.2011.242
Tkachenko, O., Shchekochikhin, D., and Schrier, R. W. (2014). Hormones and hemodynamics in pregnancy. Int J Endocrinol Metab 12:e14098. doi: 10.5812/ijem.14098
Tomogane, H., Mistry, A. M., and Voogt, J. L. (1992). Late pregnancy and rat choriocarcinoma cells inhibit nocturnal prolactin surges and serotonin-induced prolactin release. Endocrinology 130, 23–28. doi: 10.1210/endo.130.1.1727699
Toro, A. R., Maymó, J. L., Ibarbalz, F. M., Pérez-Pérez, A., Maskin, B., Faletti, A. G., et al. (2014). Leptin is an anti-apoptotic effector in placental cells involving p53 downregulation. PLoS ONE 9:e99187. doi: 10.1371/journal.pone.0099187
Trott, J. F., Vonderhaar, B. K., and Hovey, R. C. (2008). Historical perspectives of prolactin and growth hormone as mammogens, lactogens and galactagogues—agog for the future!. J. Mammary Gland Biol. Neoplasia 13, 3–11. doi: 10.1007/s10911-008-9064-x
Ulrich, U., Miller, P. B., Eyre, D. R., Chesnut, C. H. III., Schlebusch, H., and Soules, M. R. (2003). Bone remodeling and bone mineral density during pregnancy. Arch. Gynecol. Obstet. 268, 309–316. doi: 10.1007/s00404-002-0410-8
Unemori, E. N., Erikson, M. E., Rocco, S. E., Sutherland, K. M., Parsell, D. A., Mak, J., et al. (1999). Relaxin stimulates expression of vascular endothelial growth factor in normal human endometrial cells in vitro and is associated with menometrorrhagia in women. Hum. Reprod. 14, 800–806. doi: 10.1093/humrep/14.3.800
Urbanek, M. O., Nawrocka, A. U., and Krzyzosiak, W. J. (2015). Small RNA Detection by in Situ Hybridization Methods. Int. J. Mol. Sci. 16, 13259–13286. doi: 10.3390/ijms160613259
Vaccarello, M. A., Diamond, F. B. Jr., Guevara-Aguirre, J., Rosenbloom, A. L., Fielder, P. J., Gargosky, S., et al. (1993). Hormonal and metabolic effects and pharmacokinetics of recombinant insulin-like growth factor-I in growth hormone receptor deficiency/Laron syndrome. J. Clin. Endocrinol. Metab. 77, 273–280.
Vale, W., Blackwell, R., Grant, G., and Guillemin, R. (1973). TRF and thyroid hormones on prolactin secretion by rat anterior pituitary cells in vitro. Endocrinology 93, 26–33. doi: 10.1210/endo-93-1-26
Valsamakis, G., Kumar, S., Creatsas, G., and Mastorakos, G. (2010). The effects of adipose tissue and adipocytokines in human pregnancy. Ann. N. Y. Acad. Sci. 1205, 76–81. doi: 10.1111/j.1749-6632.2010.05667.x
Van Bodegraven, A. A., Böhmer, C. J., Manoliu, R. A., Paalman, E., Van Der Klis, A. H., Roex, A. J., et al. (1998). Gallbladder contents and fasting gallbladder volumes during and after pregnancy. Scand. J. Gastroenterol. 33, 993–997. doi: 10.1080/003655298750027047
Vanhouten, J. N., Dann, P., Stewart, A. F., Watson, C. J., Pollak, M., Karaplis, A. C., et al. (2003). Mammary-specific deletion of parathyroid hormone-related protein preserves bone mass during lactation. J. Clin. Invest. 112, 1429–1436. doi: 10.1172/JCI200319504
Van Leengoed, E., Kerker, E., and Swanson, H. H. (1987). Inhibition of post-partum maternal behaviour in the rat by injecting an oxytocin antagonist into the cerebral ventricles. J. Endocrinol. 112, 275–282. doi: 10.1677/joe.0.1120275
Vannuccini, S., Bocchi, C., Severi, F. M., Challis, J. R., and Petraglia, F. (2016). Endocrinology of human parturition. Ann. Endocrinol. (Paris). 77, 105–113. doi: 10.1016/j.ando.2016.04.025
Vasavada, R. C., Cavaliere, C., D'ercole, A. J., Dann, P., Burtis, W. J., Madlener, A. L., et al. (1996). Overexpression of parathyroid hormone-related protein in the pancreatic islets of transgenic mice causes islet hyperplasia, hyperinsulinemia, and hypoglycemia. J. Biol. Chem. 271, 1200–1208. doi: 10.1074/jbc.271.2.1200
Vasavada, R. C., Garcia-Ocaña, A., Zawalich, W. S., Sorenson, R. L., Dann, P., Syed, M., et al. (2000). Targeted expression of placental lactogen in the beta cells of transgenic mice results in beta cell proliferation, islet mass augmentation, and hypoglycemia. J. Biol. Chem. 275, 15399–15406. doi: 10.1074/jbc.275.20.15399
Veenstra Van Nieuwenhoven, A. L., Bouman, A., Moes, H., Heineman, M. J., De Leij, L. F., Santema, J., et al. (2002). Cytokine production in natural killer cells and lymphocytes in pregnant women compared with women in the follicular phase of the ovarian cycle. Fertil. Steril. 77, 1032–1037. doi: 10.1016/S0015-0282(02)02976-X
Villar, J., Cogswell, M., Kestler, E., Castillo, P., Menendez, R., and Repke, J. T. (1992). Effect of fat and fat-free mass deposition during pregnancy on birth weight. Am. J. Obstet. Gynecol. 167, 1344–1352. doi: 10.1016/S0002-9378(11)91714-1
Vodstrcil, L. A., Tare, M., Novak, J., Dragomir, N., Ramirez, R. J., Wlodek, M. E., et al. (2012). Relaxin mediates uterine artery compliance during pregnancy and increases uterine blood flow. FASEB J. 26, 4035–4044. doi: 10.1096/fj.12-210567
Voltolini, C., and Petraglia, F. (2014). Neuroendocrinology of pregnancy and parturition. Handb. Clin. Neurol. 124, 17–36. doi: 10.1016/B978-0-444-59602-4.00002-2
Wagner, K. U., Young, W. S. III., Liu, X., Ginns, E. I., Li, M., Furth, P. A., et al. (1997). Oxytocin and milk removal are required for post-partum mammary-gland development. Genes Funct. 1, 233–244. doi: 10.1046/j.1365-4624.1997.00024.x
Wallace, J. M., Robinson, J. J., Wigzell, S., and Aitken, R. P. (1988). Effect of melatonin on the peripheral concentrations of LH and progesterone after oestrus, and on conception rate in ewes. J. Endocrinol. 119, 523–530. doi: 10.1677/joe.0.1190523
Wang, J. W., Jiang, Y. N., Huang, C. Y., Huang, P. Y., Huang, M. C., Cheng, W. T., et al. (2006). Proliferin enhances microvilli formation and cell growth of neuroblastoma cells. Neurosci. Res. 56, 80–90. doi: 10.1016/j.neures.2006.05.011
Wang, S. J., Liu, W. J., Wang, L. K., Pang, X. S., and Yang, L. G. (2017). The role of Melatonin receptor MTNR1A in the action of Melatonin on bovine granulosa cells. Mol. Reprod. Dev. 84, 1140–1154. doi: 10.1002/mrd.22877
Weaver, S. R., Prichard, A. P., Endres, E. L., Newhouse, S. A., Peters, T. L., Crump, P. M., et al. (2016). Elevation of circulating serotonin improves calcium dynamics in the peripartum dairy cow. J. Endocrinol. 230, 105–123. doi: 10.1530/JOE-16-0038
Weaver, S. R., Prichard, A. S., Maerz, N. L., Prichard, A. P., Endres, E. L., Hernández-Castellano, L. E., et al. (2017). Elevating serotonin pre-partum alters the Holstein dairy cow hepatic adaptation to lactation. PLoS ONE 12:e0184939. doi: 10.1371/journal.pone.0184939
Weil, Z. M., Hotchkiss, A. K., Gatien, M. L., Pieke-Dahl, S., and Nelson, R. J. (2006). Melatonin receptor (MT1) knockout mice display depression-like behaviors and deficits in sensorimotor gating. Brain Res. Bull. 68, 425–429. doi: 10.1016/j.brainresbull.2005.09.016
Weinberger, S. E., Weiss, S. T., Cohen, W. R., Weiss, J. W., and Johnson, T. S. (1980). Pregnancy and the lung. Am. Rev. Respir. Dis. 121, 559–581. doi: 10.1164/arrd.1980.121.3.559
Weiner, C. P., Lizasoain, I., Baylis, S. A., Knowles, R. G., Charles, I. G., and Moncada, S. (1994). Induction of calcium-dependent nitric oxide synthases by sex hormones. Proc. Natl. Acad. Sci. USA. 91, 5212–5216. doi: 10.1073/pnas.91.11.5212
Weinhaus, A. J., Stout, L. E., and Sorenson, R. L. (1996). Glucokinase, hexokinase, glucose transporter 2, and glucose metabolism in islets during pregnancy and prolactin-treated islets in vitro: mechanisms for long term up-regulation of islets. Endocrinology 137, 1640–1649. doi: 10.1210/endo.137.5.8612496
Weir, E. C., Philbrick, W. M., Amling, M., Neff, L. A., Baron, R., and Broadus, A. E. (1996). Targeted overexpression of parathyroid hormone-related peptide in chondrocytes causes chondrodysplasia and delayed endochondral bone formation. Proc. Natl. Acad. Sci. U.S.A. 93, 10240–10245. doi: 10.1073/pnas.93.19.10240
Weisinger, R. S., Burns, P., Eddie, L. W., and Wintour, E. M. (1993). Relaxin alters the plasma osmolality-arginine vasopressin relationship in the rat. J. Endocrinol. 137, 505–510. doi: 10.1677/joe.0.1370505
Wennbo, H., Kindblom, J., Isaksson, O. G., and Törnell, J. (1997). Transgenic mice overexpressing the prolactin gene develop dramatic enlargement of the prostate gland. Endocrinology 138, 4410–4415. doi: 10.1210/endo.138.10.5461
Whitehead, C. L., Walker, S. P., Ye, L., Mendis, S., Kaitu'u-Lino, T. J., Lappas, M., et al. (2013). Placental specific mRNA in the maternal circulation are globally dysregulated in pregnancies complicated by fetal growth restriction. J. Clin. Endocrinol. Metab. 98, E429–436. doi: 10.1210/jc.2012-2468
White, V., González, E., Capobianco, E., Pustovrh, C., Martínez, N., Higa, R., et al. (2006). Leptin modulates nitric oxide production and lipid metabolism in human placenta. Reprod. Fertil. Dev. 18, 425–432. doi: 10.1071/RD05105
Wiemers, D. O., Shao, L.-J., Ain, R., Dai, G., and Soares, M. J. (2003). The mouse prolactin gene family locus. Endocrinology 144, 313–325. doi: 10.1210/en.2002-220724
Williams, E. D., Leaver, D. D., Danks, J. A., Moseley, J. M., and Martin, T. J. (1994). Effect of parathyroid hormone-related protein (PTHrP) on the contractility of the myometrium and localization of PTHrP in the uterus of pregnant rats. J. Reprod. Fertil. 102, 209–214. doi: 10.1530/jrf.0.1020209
Williams, E. D., Major, B. J., Martin, T. J., Moseley, J. M., and Leaver, D. D. (1998). Effect of antagonism of the parathyroid hormone (PTH)/PTH-related protein receptor on decidualization in rat uterus. J. Reprod. Fertil. 112, 59–67. doi: 10.1530/jrf.0.1120059
Wilson, T., Liggins, G. C., and Whittaker, D. J. (1988). Oxytocin stimulates the release of arachidonic acid and prostaglandin F2 alpha from human decidual cells. Prostaglandins 35, 771–780. doi: 10.1016/0090-6980(88)90149-9
Winter, E. M., and Appelman-Dijkstra, N. M. (2017). Parathyroid hormone-related protein-induced hypercalcemia of pregnancy successfully reversed by a dopamine agonist. J. Clin. Endocrinol. Metab. 102, 4417–4420. doi: 10.1210/jc.2017-01617
Wu, H. H., Choi, S., and Levitt, P. (2016). Differential patterning of genes involved in serotonin metabolism and transport in extra-embryonic tissues of the mouse. Placenta 42, 74–83. doi: 10.1016/j.placenta.2016.03.013
Wysolmerski, J. J., Mccaughern-Carucci, J. F., Daifotis, A. G., Broadus, A. E., and Philbrick, W. M. (1995). Overexpression of parathyroid hormone-related protein or parathyroid hormone in transgenic mice impairs branching morphogenesis during mammary gland development. Development 121, 3539–3547.
Xiang, S., Mao, L., Yuan, L., Duplessis, T., Jones, F., Hoyle, G. W., et al. (2012). Impaired mouse mammary gland growth and development is mediated by melatonin and its MT1G protein-coupled receptor via repression of ERalpha, Akt1, and Stat5. J. Pineal Res. 53, 307–318. doi: 10.1111/j.1600-079X.2012.01000.x
Yamada, M., Saga, Y., Shibusawa, N., Hirato, J., Murakami, M., Iwasaki, T., et al. (1997). Tertiary hypothyroidism and hyperglycemia in mice with targeted disruption of the thyrotropin-releasing hormone gene. Proc. Natl. Acad. Sci. U.S.A. 94, 10862–10867. doi: 10.1073/pnas.94.20.10862
Yamada, M., Shibusawa, N., Ishii, S., Horiguchi, K., Umezawa, R., Hashimoto, K., et al. (2006). Prolactin secretion in mice with thyrotropin-releasing hormone deficiency. Endocrinology 147, 2591–2596. doi: 10.1210/en.2005-1326
Yamaguchi, M., Endo, H., Tasaka, K., and Miyake, A. (1995). Mouse growth hormone-releasing factor secretion is activated by inhibin and inhibited by activin in placenta. Biol. Reprod. 53, 368–372. doi: 10.1095/biolreprod53.2.368
Yamashita, H., Shao, J., Ishizuka, T., Klepcyk, P. J., Muhlenkamp, P., Qiao, L., et al. (2001). Leptin administration prevents spontaneous gestational diabetes in heterozygous Lepr(db/+) mice: effects on placental leptin and fetal growth. Endocrinology 142, 2888–2897. doi: 10.1210/endo.142.7.8227
Yao, L., Agoulnik, A. I., Cooke, P. S., Meling, D. D., and Sherwood, O. D. (2008). Relaxin acts on stromal cells to promote epithelial and stromal proliferation and inhibit apoptosis in the mouse cervix and vagina. Endocrinology 149, 2072–2079. doi: 10.1210/en.2007-1176
Yeh, S., Tsai, M. Y., Xu, Q., Mu, X. M., Lardy, H., Huang, K. E., et al. (2002). Generation and characterization of androgen receptor knockout (ARKO) mice: an in vivo model for the study of androgen functions in selective tissues. Proc. Natl. Acad. Sci. U.S.A. 99, 13498–13503. doi: 10.1073/pnas.212474399
Yellon, S. M., and Longo, L. D. (1988). Effect of maternal pinealectomy and reverse photoperiod on the circadian melatonin rhythm in the sheep and fetus during the last trimester of pregnancy. Biol. Reprod. 39, 1093–1099. doi: 10.1095/biolreprod39.5.1093
Yogosawa, S., Mizutani, S., Ogawa, Y., and Izumi, T. (2013). Activin receptor-like kinase 7 suppresses lipolysis to accumulate fat in obesity through downregulation of peroxisome proliferator-activated receptor gamma and C/EBPalpha. Diabetes 62, 115–123. doi: 10.2337/db12-0295
Yong, H. E. J., Murthi, P., Kalionis, B., Keogh, R. J., and Brennecke, S. P. (2017). Decidual ACVR2A regulates extravillous trophoblast functions of adhesion, proliferation, migration and invasion in vitro. Pregnancy Hypertens. 12, 189–193. doi: 10.1016/j.preghy.2017.11.002
Yong, H. E., Murthi, P., Wong, M. H., Kalionis, B., Cartwright, J. E., Brennecke, S. P., et al. (2015). Effects of normal and high circulating concentrations of activin A on vascular endothelial cell functions and vasoactive factor production. Pregnancy Hypertens. 5, 346–353. doi: 10.1016/j.preghy.2015.09.006
Young, W. S. III., Shepard, E., Amico, J., Hennighausen, L., Wagner, K. U., Lamarca, M. E., et al. (1996). Deficiency in mouse oxytocin prevents milk ejection, but not fertility or parturition. J. Neuroendocrinol. 8, 847–853. doi: 10.1046/j.1365-2826.1996.05266.x
Youssef, R. E., Ledingham, M. A., Bollapragada, S. S., O'gorman, N., Jordan, F., Young, A., et al. (2009). The role of toll-like receptors (TLR-2 and−4) and triggering receptor expressed on myeloid cells 1 (TREM-1) in human term and preterm labor. Reprod. Sci. 16, 843–856. doi: 10.1177/1933719109336621
Yu, L., Li, D., Liao, Q. P., Yang, H. X., Cao, B., Fu, G., et al. (2012). High levels of activin A detected in preeclamptic placenta induce trophoblast cell apoptosis by promoting nodal signaling. J. Clin. Endocrinol. Metab. 97, E1370–E1379. doi: 10.1210/jc.2011-2729
Yura, S., Ogawa, Y., Sagawa, N., Masuzaki, H., Itoh, H., Ebihara, K., et al. (2000). Accelerated puberty and late-onset hypothalamic hypogonadism in female transgenic skinny mice overexpressing leptin. J. Clin. Invest. 105, 749–755. doi: 10.1172/JCI8353
Zeng, H., Schimpf, B. A., Rohde, A. D., Pavlova, M. N., Gragerov, A., and Bergmann, J. E. (2007). Thyrotropin-releasing hormone receptor 1-deficient mice display increased depression and anxiety-like behavior. Mol. Endocrinol. 21, 2795–2804. doi: 10.1210/me.2007-0048
Zhang, L., Fishman, M. C., and Huang, P. L. (1999). Estrogen mediates the protective effects of pregnancy and chorionic gonadotropin in a mouse model of vascular injury. Arterioscler. Thromb. Vasc. Biol. 19, 2059–2065. doi: 10.1161/01.ATV.19.9.2059
Zhang, Y., Hou, Y., Wang, X., Ping, J., Ma, Z., Suo, C., et al. (2017). The effects of kisspeptin-10 on serum metabolism and myocardium in rats. PLoS ONE 12:e0179164. doi: 10.1371/journal.pone.0179164
Zhao, L., Roche, P. J., Gunnersen, J. M., Hammond, V. E., Tregear, G. W., Wintour, E. M., et al. (1999). Mice without a functional relaxin gene are unable to deliver milk to their pups. Endocrinology 140, 445–453. doi: 10.1210/endo.140.1.6404
Zhao, L., Samuel, C. S., Tregear, G. W., Beck, F., and Wintour, E. M. (2000). Collagen studies in late pregnant relaxin null mice. Biol. Reprod. 63, 697–703. doi: 10.1095/biolreprod63.3.697
Zha, W., Ho, H. T. B., Hu, T., Hebert, M. F., and Wang, J. (2017). Serotonin transporter deficiency drives estrogen-dependent obesity and glucose intolerance. Sci. Rep. 7:1137. doi: 10.1038/s41598-017-01291-5
Zhou, B., Kong, X., and Linzer, D. I. (2005). Enhanced recovery from thrombocytopenia and neutropenia in mice constitutively expressing a placental hematopoietic cytokine. Endocrinology 146, 64–70. doi: 10.1210/en.2004-1011
Zhou, B., Lum, H. E., Lin, J., and Linzer, D. I. (2002). Two placental hormones are agonists in stimulating megakaryocyte growth and differentiation. Endocrinology 143, 4281–4286. doi: 10.1210/en.2002-220447
Zhou, Y., Xu, B. C., Maheshwari, H. G., He, L., Reed, M., Lozykowski, M., et al. (1997). A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Laron mouse). Proc. Natl. Acad. Sci. U.S.A. 94, 13215–13220. doi: 10.1073/pnas.94.24.13215
Zhu, Y., Bian, Z., Lu, P., Karas, R. H., Bao, L., Cox, D., et al. (2002). Abnormal vascular function and hypertension in mice deficient in estrogen receptor beta. Science 295, 505–508. doi: 10.1126/science.1065250
Ziegler, B., Lucke, S., Besch, W., and Hahn, H. J. (1985). Pregnancy-associated changes in the endocrine pancreas of normoglycaemic streptozotocin-treated Wistar rats. Diabetologia 28, 172–175.
Zöllner, J., Howe, L. G., Edey, L. F., O'dea, K. P., Takata, M., Gordon, F., et al. (2017). The response of the innate immune and cardiovascular systems to LPS in pregnant and nonpregnant mice. Biol. Reprod. 97, 258–272. doi: 10.1093/biolre/iox076
Keywords: pregnancy, placenta, hormones, maternal adaptations, metabolism, fetal growth, endocrine, cardiovascular
Citation: Napso T, Yong HEJ, Lopez-Tello J and Sferruzzi-Perri AN (2018) The Role of Placental Hormones in Mediating Maternal Adaptations to Support Pregnancy and Lactation. Front. Physiol. 9:1091. doi: 10.3389/fphys.2018.01091
Received: 20 April 2018; Accepted: 23 July 2018;
Published: 17 August 2018.
Edited by:
Emilio A. Herrera, Universidad de Chile, ChileReviewed by:
Carina Mallard, University of Gothenburg, SwedenCharles Andrew Ducsay, Loma Linda University School of Medicine, United States
Paola Casanello, Pontificia Universidad Católica de Chile, Chile
Copyright © 2018 Napso, Yong, Lopez-Tello and Sferruzzi-Perri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amanda N. Sferruzzi-Perri, YW5zNDhAY2FtLmFjLnVr
†These authors have contributed equally to this work
 Tina Napso
Tina Napso Hannah E. J. Yong†
Hannah E. J. Yong† Jorge Lopez-Tello
Jorge Lopez-Tello Amanda N. Sferruzzi-Perri
Amanda N. Sferruzzi-Perri