
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Physiol. , 26 July 2018
Sec. Gastrointestinal Sciences
Volume 9 - 2018 | https://doi.org/10.3389/fphys.2018.00997
This article is part of the Research Topic NU AGE: MedDiet for the Health of the Elderly Across Europe View all 10 articles
 Monica Maijo1
Monica Maijo1 Kamal Ivory1
Kamal Ivory1 Sarah J. Clements1
Sarah J. Clements1 Jack R. Dainty2
Jack R. Dainty2 Amy Jennings2
Amy Jennings2 Rachel Gillings2
Rachel Gillings2 Susan Fairweather-Tait2
Susan Fairweather-Tait2 Massimo Gulisano3
Massimo Gulisano3 Aurelia Santoro4,5
Aurelia Santoro4,5 Claudio Franceschi5
Claudio Franceschi5 Simon R. Carding1,2
Simon R. Carding1,2 Claudio Nicoletti1,3*
Claudio Nicoletti1,3*Amongst the major features of aging are chronic low grade inflammation and a decline in immune function. The Mediterranean diet (MedDiet) is considered to be a valuable tool to improve health status, and although beneficial effects have been reported, to date, immunological outcomes have not been extensively studied. We aimed to test the hypothesis that 1 year of a tailored intervention based on the MedDiet with vitamin D (10 μg/day) would improve innate immune responses in healthy elderly subjects (65–79 years) from the English cohort (272 subjects recruited) of the NU-AGE randomized, controlled study (clinicaltrials.gov, NCT01754012). Of the 272 subjects forming the United Kingdom cohort a subgroup of 122 subjects (61 in the intervention group and 61 in the control group) was used to evaluate ex vivo innate immune response, phenotype of circulating immune cells, and levels of pro- and anti-inflammatory markers. Odds Ratio (OR) was calculated for all the parameters analyzed. After adjustment by gender, MedDiet-females with a BMI < 31 kg/m2 had a significant upregulation of circulating CD40+CD86+ cells (OR 3.44, 95% CI 1.01–11.75, P = 0.0437). Furthermore, in all MedDiet subjects, regardless of gender, we observed a MedDiet-dependent changes, although not statistically significant of immune-critical parameters including T cell degranulation, cytokine production and co-receptor expression. Overall, our study showed that adherence to an individually tailored Mediterranean-like dietary pattern with a daily low dose of vitamin D3 supplements for 1 year modified a large variety of parameters of immune function in healthy, elderly subjects. We interpreted these data as showing that the MedDiet in later life could improve aspects of innate immunity and thus it could aid the design of strategies to counteract age-associated disturbances.
Clinical Trial Registration: clinicaltrials.gov, NCT01754012.
Aging is an irreversible process that is associated with a significant decline of the immune system that leads to increased susceptibility to infection, reduced efficiency of vaccination protocols and significantly higher incidence of autoimmune disorders and cancer (Castle, 2000; Dewan et al., 2012). Chronological age may also result in vascular aging with deterioration in arterial function (Jani and Rajkumar, 2006) associated with cardiovascular diseases (CVD) that are the most common world-wide cause of morbidity and mortality in persons aged over 65 years, particularly those with diabetes mellitus. The negative impact on vascular function compromises the ability to resist oxidative stress and inflammation. Aging is characterized by a chronic, low grade inflammatory status termed “inflammaging” (Franceschi et al., 2000, 2007; Monti et al., 2017), which plays a critical role in the pathogenesis of major age-related chronic diseases and in important geriatric conditions (De Martinis et al., 2006; Pedersen, 2009; Lencel and Magne, 2011; Franceschi and Campisi, 2014) and in so doing contributes to both a poor quality of life and elderly mortality (Cevenini et al., 2013; Varadhan et al., 2014). Increased production of inflammatory mediators and the simultaneous down-regulation of anti-inflammatory ones takes place in a variety of tissues, organs and systems including adipose tissue, muscles, brain, and contribute to the onset and progression of such a systemic inflammatory state (Cevenini et al., 2010, 2013).
Immunosenescence is a hallmark of the aging organism and both the innate and adaptive arms of the mucosal and systemic immune systems are affected (Maijó et al., 2014; Man et al., 2015), with alterations in constituent cells linked to the onset of chronic diseases and it is generally accepted that immunosenescence is accelerated in those subjects who present inflammaging. The design of interventional strategies to delay, halt or even possibly reverse immunosenescence requires a detailed understanding of the aging process at cellular level.
Although the individual genetic make-up that dictates immune responses cannot be modified, other factors contributing to aging such as lifestyle and diet (Pae et al., 2012) could be changed in the attempt to halt/reverse the progression of the detrimental effects of aging on immune effectiveness (Franceschi et al., 2017). To this end, the adoption of nutritional regimen to improve immune function in the elderly is a potentially important approach. Indeed, a diet rich in fruit, vegetables, legumes, unrefined cereals and olive oil and accompanied by low intake of meat, dairy products and alcohol consumption typical of the Mediterranean is effective in decreasing the incidence of cardiovascular and other chronic diseases and increased longevity (Mena et al., 2009; Vasto et al., 2012; Casas et al., 2014, 2018; Martinez-Gonzalez et al., 2015; Santangelo et al., 2018).
The recognition of nutrition as a possible determinant of successful aging was acknowledged by the European Union by funding a dietary intervention study, the NU-AGE project 1. A multidisciplinary European consortium developed a tailored dietary strategy to address the needs of the elderly (65–79 years) for promoting healthy aging in Europe. The dietary strategy included the daily supplementation of a low dose of vitamin D (10 μg or 400 IU/day) to minimize differences between study sites part of the whole NuAge project across different areas of Europe that differed in the diet and exposure to sunlight, two very critical factors for determining the vitamin D status. The aim of the study was to examine the effect of consuming a Mediterranean-like dietary pattern (NU-AGE diet) with vitamin D3 for 1 year on a large variety of parameters of immunity that included immune cell phenotype, co-receptor expression and innate immune responses.
This study was a part of the larger multi-national NU-AGE study, a parallel-group, multi-centric, randomized, controlled clinical trial of 1-year duration (clinicaltrials.gov, NCT01754012) aimed at assessing the effects of the MedDiet in healthy aging [European seven framework project (FP7); Grant Agreement no. 2664862]. Full details of the study protocol have been described elsewhere (Santoro et al., 2014). It was not possible to blind the study to the participants or scientists responsible for managing the dietary intervention, but all the sample analysis was blinded. All subjects provided written informed consent prior to their inclusion in the study. This study reports data from 272 subjects recruited at baseline in the NU-AGE English cohort (134 control, 138 MedDiet). One arm of the study aimed to study the effects of dietary intervention on a variety of parameters of the innate immune system. To this end, 122/272 subjects of the United Kingdom cohort were utilized to study immune responses, (61 control, 61 MedDiet). After 1 year, 115/122 subjects, among those studied for immune responses, completed the intervention. Participants were independently living in their own home. Specific inclusion criteria were as follows: men or women aged 65–79 years; with no chronic disease, such as cancer, organ failures for the last 2 years; responsible for own meal choices and preparation; willing to make changes to their habitual diet for 1 year. Exclusion criteria were: current disease (e.g., cancer or dementia); organ failure or organ failure requiring a special diet; the following chronic diseases: severe heart disease, kidney disease, respiratory insufficiency, liver cirrhosis; diabetes Mellitus type 1. Also, exclusion criteria included long-term use of corticosteroids, use of non-steroidal anti-inflammatory drugs, use of antibiotics within the previous 2 months, change in habitual medication use (e.g., statins, thyroxin) within 3 months prior the beginning of the study, participation in another current study involving either dietary interventions or sampling/donation of blood, malnutrition (BMI lower than 18.5 kg/m2); body weight loss of more than 10% within 6 months. Participants were assigned randomly (1:1) to the control or intervention groups, after stratification by sex, age (65–72 or >72–79 years), frailty status (pre-frail or non-frail), and body mass index (<25 or ≥25 kg/m2). Randomization was done by computer-generated allocation and ran on Python interpreter (version 2.6 or 2.7). The majority of the subjects (81%) were non-frail; the remainder were pre-frail (19%).
The NU-AGE dietary intervention has been previously described (Berendsen et al., 2014). In brief, all participants (control and intervention groups) completed a 7-day food record diary within 2 weeks after the baseline assessment. This was followed by an interview with a specialized team member to review the records. The consumed food recorded in the food diary was coded according to standardized coding procedures (Berendsen et al., 2014). The control group, following a habitual diet, received only a leaflet with national dietary guidelines. The intervention group was given individually tailored Mediterranean diet advice plus a daily vitamin D3 supplement of 10 μg. The combination of the Mediterranean dietary pattern and vitamin D is henceforth referred to solely as MedDiet. The nutritional intervention was from the Food Based Dietary Guidelines for older adults (Berendsen et al., 2014) conducted by a dietician/nutritionist using individual education using a Motivational Intervention technique. Participants were advised to consume on average 15–20% of their daily energy from protein, 50–60% from carbohydrate, 25–30% from fat (<10% from saturated fat, <1% from trans fatty acids, <12% from polyunsaturated fatty acids, and 8–28% from monounsaturated fatty acids). In addition, subjects were recommended to consume 30–40 g/day of fiber, <10–20 g/day of preferably red wine (1 serving/day for women and 2 for men), 2 g/day sodium, 1.3 g/day potassium, 10 mg/day iron, 15 μg/day of vitamin D3, 400 μg/day of folate, and 5 μg/day of vitamin B12. The MedDiet cohort (n = 61) received individual, tailored dietary advice (summary in Table 1) based on dietary reference values in the United Kingdom3 and recommendations from bodies including the modified My Pyramid for Older Adults (Peregrin, 2008; Shelnutt et al., 2009), European Commission (Commission of the European Communities and Nutrient and Energy Intakes for European Community, 1993), and Institute of Medicine (Institute of Medicine of the National Academies and Dietary Reference Intakes, 2006). Subjects in the control group (n = 61) were given a standard healthy-living advice leaflet from the British Dietetic Association and asked to maintain their normal dietary habits over the course of the year.
Blood collected in vacutainers (Becton Dickinson, Oxford, United Kingdom) containing ethylenediaminetetraacetic acid (EDTA) was centrifuged at 2200 rpm to separate plasma from the pelleted cells. Plasma was collected and stored at -80°C for future analyses. Cell pellets were diluted 1:3 with Hank’s Balanced Salt Solution (HBSS; Sigma-Aldrich, Dorset, United Kingdom) and peripheral blood mononuclear cell (PBMCs) were separated using Leucosep tubes according to the manufacturer instructions (Greiner Bio-one). PBMCs were collected from the interface, washed and cell counts were adjusted to 4 × 106 viable cells/mL with ‘complete’ DMEM/F12 (Life Technologies Ltd., Paisley, United Kingdom) supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin, and 50 mL of FBS (Sigma, United Kingdom).
Peripheral blood mononuclear cells at 1 × 106 viable cells/mL (final concentration) in complete DMEM/F12 were cultured with 50 μg/mL Poly A:U, 2.5 μg/mL Imiquimod-R837, 25 μg/mL ODN-M362, 5 μg/mL LPS-ED and 50 ng/mL Pam2CSK4 (Invivogen, San Diego, CA, United States) or without for 22 h in 24-well flat-bottom plates at 37°C in an atmosphere of 5% CO2 in air. Cell-free supernatants were collected and stored at -80°C until analysis. Freshly collected cells were washed and stained for expression of costimulatory molecules by incubation for 20 min at 20°C with fluorochrome-labeled monoclonal antibodies (mAbs): anti-CD154 Brilliant Violet (BV) 421 (Biolegend, San Diego, CA, United States), anti-CD80 Alexa Fluor (AF488) (Biolegend), anti-CD86 Allophycocyanin (APC) (Biolegend), anti-HLA-Dr AF700 (Biolegend), anti-CD152 Phycoerythrin (PE) (Biolegend), anti-CD40 Phycoerythrin-Cyanine 7 (PE/Cy7) (Biolegend), and anti-CD1d APC (Biolegend). Negative control cells were incubated with the corresponding isotype-matched negative control mAbs conjugated with BV421, AF488, APC, AF700, PE/Cy7, and PE (Biolegend). Cells were washed, then fixed with Formalin 1X, before analysis by flow cytometry (EC800 Sony Biotechnology Inc., Surrey, United Kingdom). Analysis was performed using FlowJo software (FlowJo, Oregon, United States).
Culture supernatants were collected following culture with TLR agonists as described above. Quantitative determination of IFN-α, IFN-β, IFN-γ, IL-12p40, IL-12p70 present in the culture supernatants was performed by ProcartaPlexTM Immunoassay (eBioscience, United Kingdom) according to the manufacturer’s instructions and analysis using Luminex 200 instrumentation (Luminex Corporation, Netherlands). The immunoassay kits were specific for human cytokines. Assay sensitivities were 0.6 pg/mL for IFN-α, 8.54 pg/mL for IFN-β, 8.89 pg/mL for IFN-γ, 1.81 pg/mL for IL-12p40, and 6.30 pg/mL IL-12p70. The soluble expression of SOCS3, IL-12Rβ1, and IL-12Rβ2 in the supernatants was performed using enzyme-linked immunosorbent assay (ELISA) kits (Antibodies-online, Atlanta, GA, United States) according to the manufacturer’s instructions. The assay sensitivities were 15.6 pg/mL for SOCS3, 0.156 ng/mL for IL-12Rβ1, and 0.156 ng/mL IL-12Rβ2 and ELISA plates were read in a microplate reader at 450 nm (Benchmark Plus Bio-Rad, Hercules, CA, United States).
Peripheral blood mononuclear cells at 2 × 106 viable cells/mL (final concentration) in complete DMEM/F12 were cultured with 5 μg/mL of Staphylococcal enterotoxin B (SEB; Sigma-Aldrich, United Kingdom) for 6 days in 24-well flat-bottom plates at 37°C and 5% CO2. Cells were stained with anti-CD3 BV421 (Biolegend), and anti-CD8 AF488 (Biolegend) for 20 min at 20°C. T cell proliferation was measured using the Coulter DNA Prep Reagent Kit (Beckman Coulter Ltd., High Wycombe, United Kingdom) according to the manufacturer’s instructions. Data were acquired by Flow cytometry (EC800 Sony). Analysis was performed using FlowJo software (FlowJo, United States).
DCs were enumerated in heparinized blood using a Blood Dendritic Cell Enumeration Kit (Miltenyi Biotec Ltd., Surrey, United Kingdom) according to the manufacturer’s instructions. The anti-BDCA cocktail contains: anti CD303 (BDCA-2) Fluorescein (FITC), anti-CD1c (BDCA-1) PE, anti-CD14 Phycoerythrin-Cyanine 5 (PE-Cy5), and anti-CD19 PE-Cy5 for the determination of plasmacytoid (pDC) and myeloid (mDC) DC. Antibodies to CD14 and CD19 antigens were used for exclusion of monocytes and B cells, respectively. To enable quantification in terms of absolute counts/μL blood, Flow count fluorospheres (Beckman Coulter) were added to each sample prior to flow cytometric (EC800 Sony) data acquisition. Analysis was performed using FlowJo software (FlowJo, United States). The ratio of mDC to pDC were calculated by applying the following formula DC = number of DC/number count beads × concentration).
Peripheral blood mononuclear cells at 1 × 106 viable cells/ml (final concentration) in complete DMEM/F12 were placed into each of two sterile tubes labeled ‘test’ or ‘control.’ Cells in the ‘test’ tube were incubated with anti-CD107a AF647 (Biolegend) for 15 min, before addition of 1.1 μg/mL SEB (Sigma-Aldrich, United Kingdom) as stimulant and 5 μL ethanol. The ‘control’ sample was not stimulated but had 5 μL (1 μM) colchicine (Sigma, Missouri, United States) in ethanol added to minimize spontaneous degranulation. In both tubes the final volume was adjusted 1 mL with tissue media culture. Tubes were incubated for 5 h at 37°C and 5% CO2, then washed and stained with anti-CD3 AF488 (Biolegend) and anti-CD8 PE (Biolegend). After further washing and fixation with 1% formalin, data were acquired by flow cytometry (EC800 Sony) and analyzed using FlowJo software.
The quantitative determination of leukocytes oxidative burst was performed in whole blood using the PhagoburstTM kit (Glycotope Biotechnology, Heidelberg, Germany) according to the manufacturer’s instructions. Burst activity in leukocytes was evaluated after stimulation with N-formyl-methionineleucine-phenylalanine (fMLP), a moderate stimulus. The generation of ROS was measured by assessing the oxidative conversion of the fluorogenic substrate dihydorohomdamine-123 into rhodamine. Data were acquired by flow cytometry (EC800 Sony) and analyzed using FlowJo software.
Plasma samples stored at -80°C were thawed for the quantitative determination of IL-2, IL-9, IL-22, IL-23 by ProcartaPlexTM Immunoassay (eBioscience, Hatfield, United Kingdom) according to the manufacturer’s instructions. Analysis was performed using Luminex 200 instrumentation (Luminex Corporation, Netherlands). Assay sensitivities were 4.44 pg/ml for IL-2, 11 pg/ml for IL-9, 33 pg/ml for IL-22, 19 pg/ml for IL-23.
Peripheral blood mononuclear cells at 1 × 106 viable cells/mL (final concentration) in complete DMEM/F12 were incubated with anti-CD3 AF700 (Biolegend), anti-CD28 Pacific blue (PB) (Biolegend), anti-CD161 PE-Cy7 (Biolegend), anti-CD158d PE (Biolegend), and anti-TCR Vα24-Jα18 FITC (Biolegend) or the corresponding isotype controls. All antibodies were purchased from Biolegend (United Kingdom). After 20 min at 20°C cells were washed, and fixed with 1% formalin. Data were acquired by flow cytometry (EC800 Sony) and analyzed using FlowJo software.
Primary endpoints of the NU-AGE study were changes in immune responses; and a variety of secondary endpoints (ClinicalTrials.gov, NCT01754012). This work focuses on the effects of MedDiet on parameters of immune function. Samples from all volunteers were run in triplicate for each assay. Associations were investigated based on an adjusted Mantel–Haenszel odds ratio (OR), for a post-study change in the intervention group compared to the controls, some analysis were stratified by gender. Associations were evaluated using a Mantel–Haenszel chi-squared test. Confidence intervals were calculated using the method of Robins–Breslow–Greenland. Sex interactions were evaluated using Tarone’s test of homogeneity. Odds ratios were calculated such that a value less than 1 corresponds to a reduction in the intervention arm compared to the control arm. P-values were considered significant at less than 0.05.
Figure 1 shows the recruitment and retention of the volunteers. Quantitative dietary guidelines given to study participants and physical features of the cohort, such as age, body mass index (BMI) at baseline and at the end of the study, gender and percentage of pre-frail individuals of the cohort studied are detailed in Tables 1, 2, respectively.
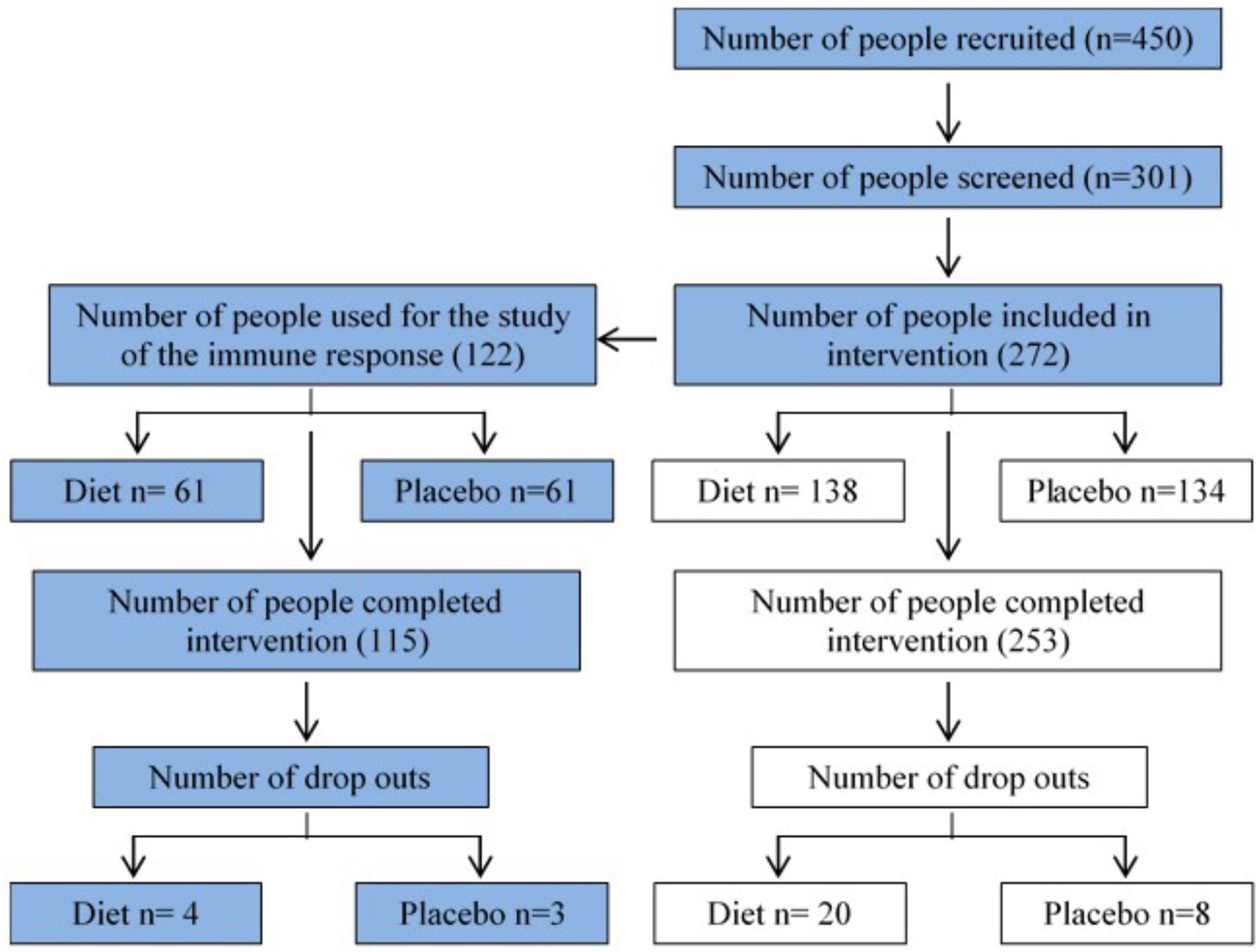
FIGURE 1. Flow chart summarizing recruitment and retention of study participants. 272 volunteers were recruited at baseline in the NU-AGE English (Norwich) cohort (134 control, 138 MedDiet) for the dietary intervention. This study (blue boxes) focuses on the effects of dietary intervention on a variety of parameters of the immune responses.
The expression of a large panel of costimulatory molecules comprising CD154, CD80, CD86, HLA-Dr, CD40, CD152, and CD1d after in vitro challenge with TLR agonists was studied in lymphocyte and monocyte/DC populations. Expression of selected co-receptors monitored after stimulation is expressed as percentage (%) upregulation compared to expression on unstimulated resting cells. A gender-specific effect of the MedDiet was observed within the lymphocyte population with a significant upregulation of CD40+CD86+ cells (OR 3.44, 95% CI 1.01–11.75, P = 0.0437) observed in female study participants. Interestingly, this was restricted to individuals with BMI < 31 kg/m2 (Figure 2).
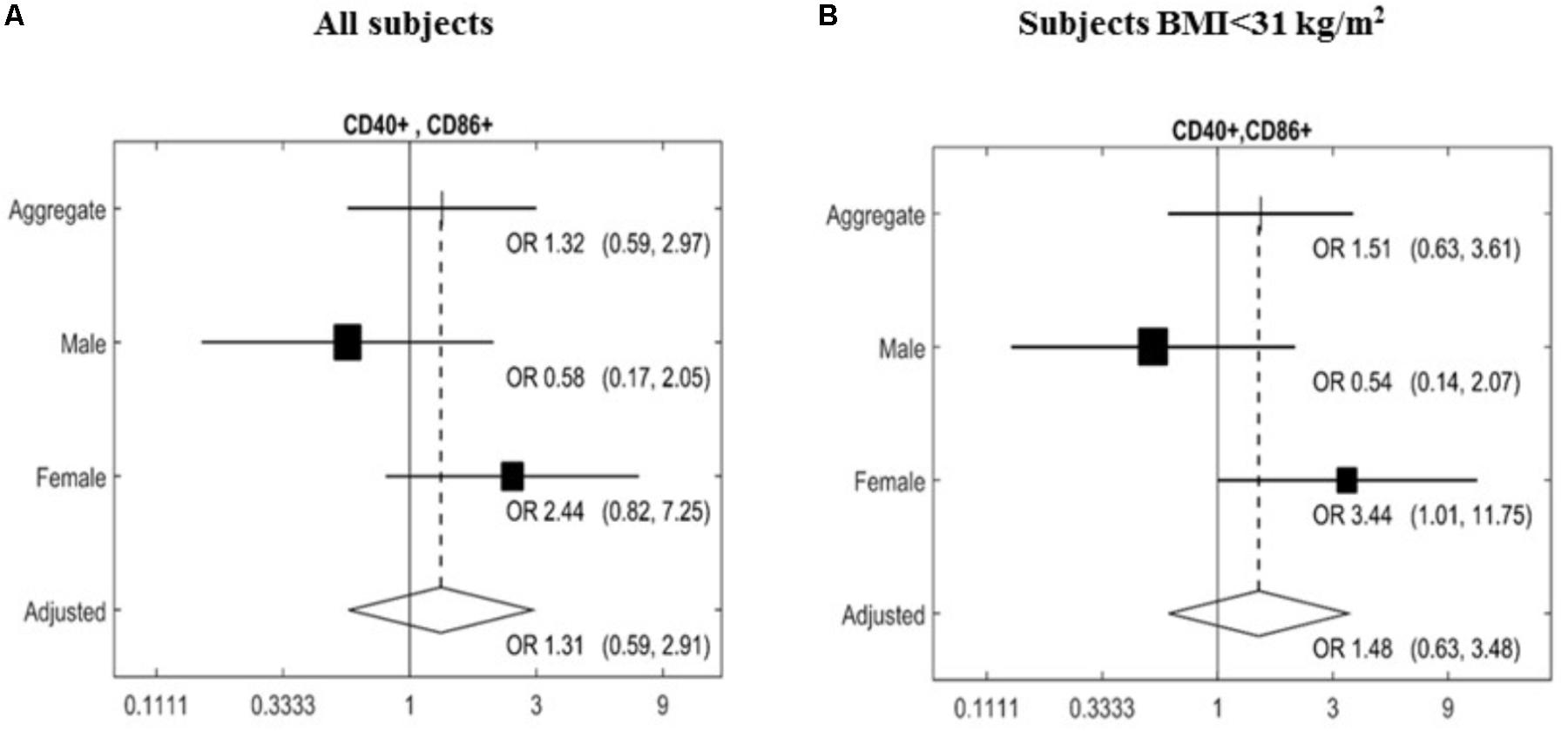
FIGURE 2. Effects of MedDiet on CD40+CD86+ expression following in vitro challenge with TLR agonists. PBMC were cultured with a cocktail of TLRs agonists (PolyA:U, Imiquimod, ODN-M362, LPS, Pam2CSK4) for 22 h. Forest plot of the study of the CD40+CD86+ lymphocytes comparing the association between Mediterranean diet and normal diet after 1 year of intervention with the random effect model. Associations were investigated based on an adjusted Mantel–Haenszel odds ratio (OR), stratified by gender (A) or BMI > 31 kg/m2 (B). OR and 95%Cl were calculated. Cl, confidence interval.
From all the other markers studied (CD154, CD80, CD86, HLA-Dr, CD40, CD152, and CD1d) no significant changes were found in the monocyte/DC populations (Table 3).
Following in vitro TLR stimulation, levels of selected cytokines were assessed in the culture supernatants. Overall, the MedDiet intervention did not significantly affected TLR-induced cytokine production (Table 4). On the other hand there were trends toward increased production of IFN-β (OR 1.98, 95%CI 0.86–4.53, P = 0.1056), and decreased production of IL-12Rβ1 (OR 0.50, 95%CI 0.22–1.13, P = 0.0929), SOCS3 (OR 0.50, 95%CI 0.21–1.18, P = 0.1108) and the ratio between IL-12p40/IL-12p70 (OR 0.54, 95%CI 0.26–1.16, P = 0.1122) (Figures 3, 4). Gender stratification did not reveal any differences in cytokine production. Taken together these data suggested that a prolonged period of exposure to the MedDiet might result in more profound changes in the immunological parameters evaluated.
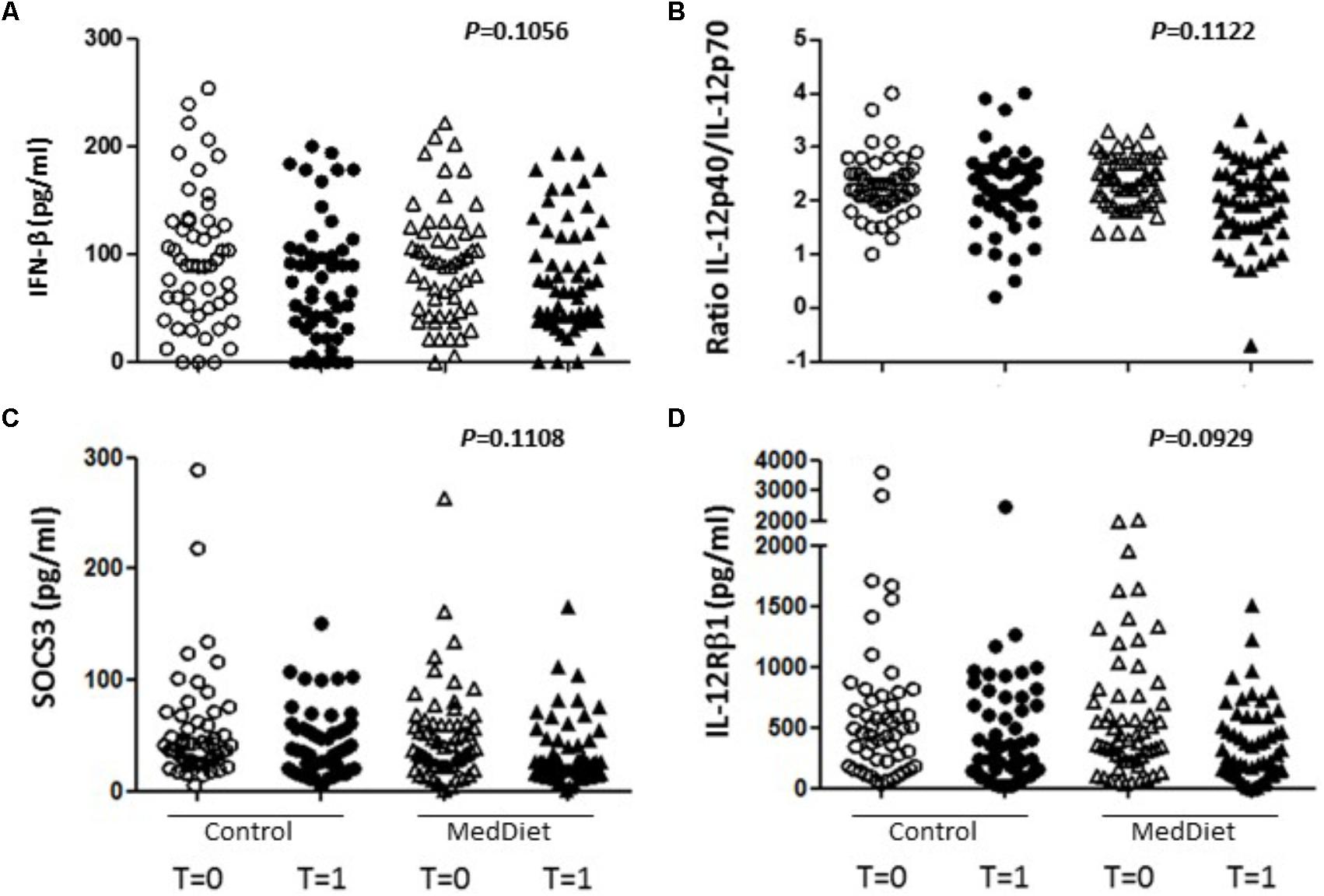
FIGURE 3. Effects of the MedDiet on cytokine expression following in vitro challenge with TLR agonists-all individual values. PBMC were cultured with a cocktail of TLRs agonists (PolyA:U, Imiquimod, ODN-M362, LPS, Pam2CSK4) for 22 h. Levels of cytokines were determined in culture supernatants. Representation of the individual values for IFN-β (A), IL-12p40/70 ratio (B), SOCS3 (C) and IL-12Rβ1 (D) concentration in all the volunteers at baseline and after 1 year of intervention. Results showed a tendency toward increased production of IFN-β (OR 1.98, 95%CI 0.86–4.53, P = 0.1056), and decreased production of IL-12Rβ1 (OR 0.50, 95%CI 0.22–1.13, P = 0.0929), SOCS3 (OR 0.50, 95%CI 0.21–1.18, P = 0.1108) and the ratio between IL-12p40/IL-12p70 (OR 0.54, 95%CI 0.26–1.16, P = 0.1122. OR = odds ratio), CI, confidence intervals.
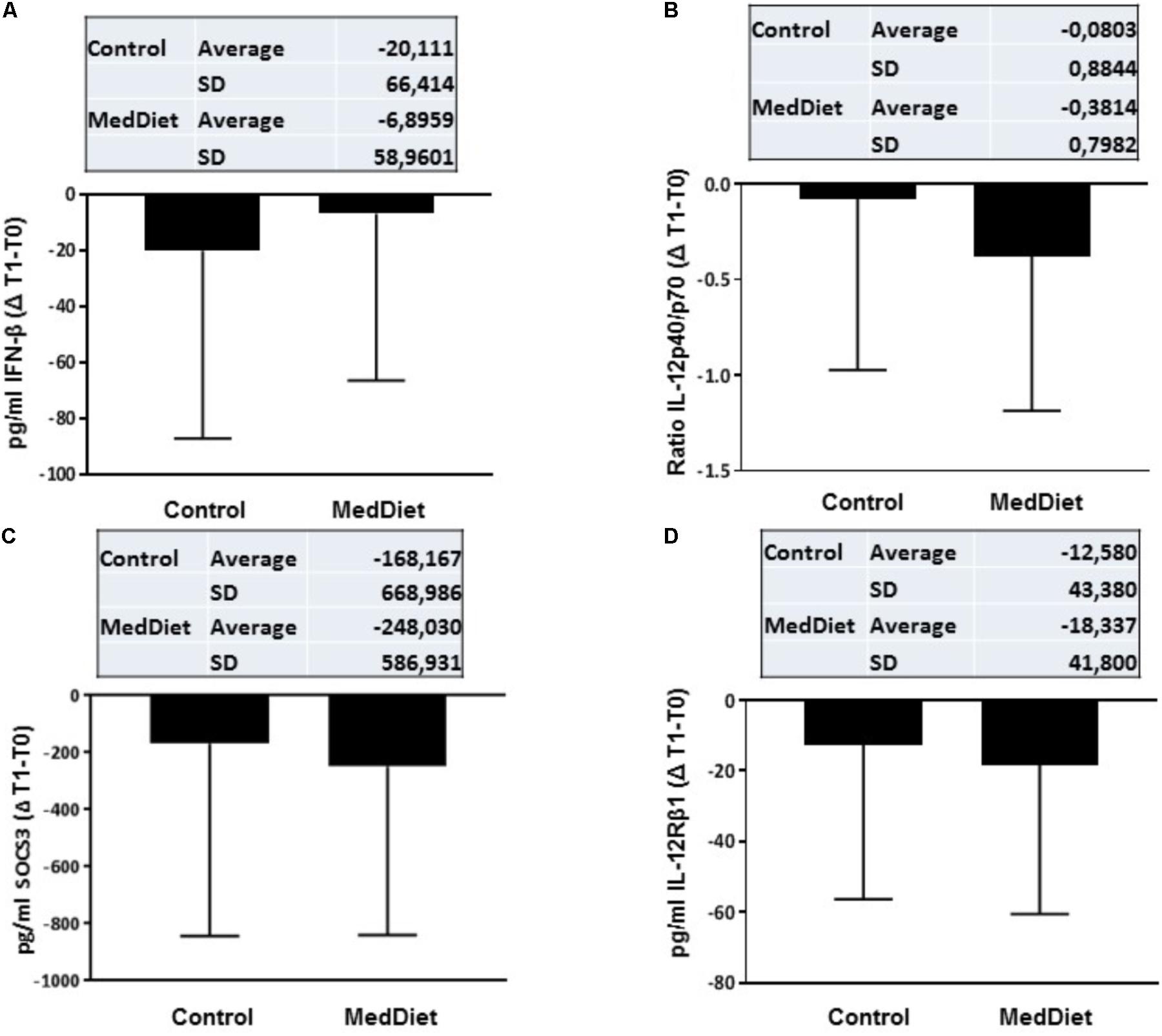
FIGURE 4. Cytokine expression following in vitro challenge with TLR agonists- Changes (Δ) of the parameter values in MedDiet and control diet at baseline and after 1-year of intervention. Δ of the parameters at baseline (T0) and after 1-year (T1) for IFN-β (A), IL-12p40/70 ratio (B), SOCS3 (C), and IL-12Rβ1 (D) concentration were calculated and compared. For each parameter, the mean ± SD of the Δ at T0 and T1 for the control group and MedDiet group is shown in the relevant inset.
First, the T cell proliferation response to 6-day SEB stimulation was compared to cells cultured in the absence of any stimulation. There were no significant differences between the control and MedDiet intervention groups, when comparing the change of T cell proliferation after 1 year of intervention (Table 5). Also, CD8 T cell degranulation was measured as % of CD107a expression on CD3+CD8+ cells induced by SEB stimulation, and the oxidative burst activity of granulocytes measured as % oxidizing cells within the granulocyte population; the MedDiet had no effect in altering both parameters after 1 year of intervention (Table 5), although a trend toward increased degranulation in the MedDiet intervention group was observed. No gender-specific differences were noted.
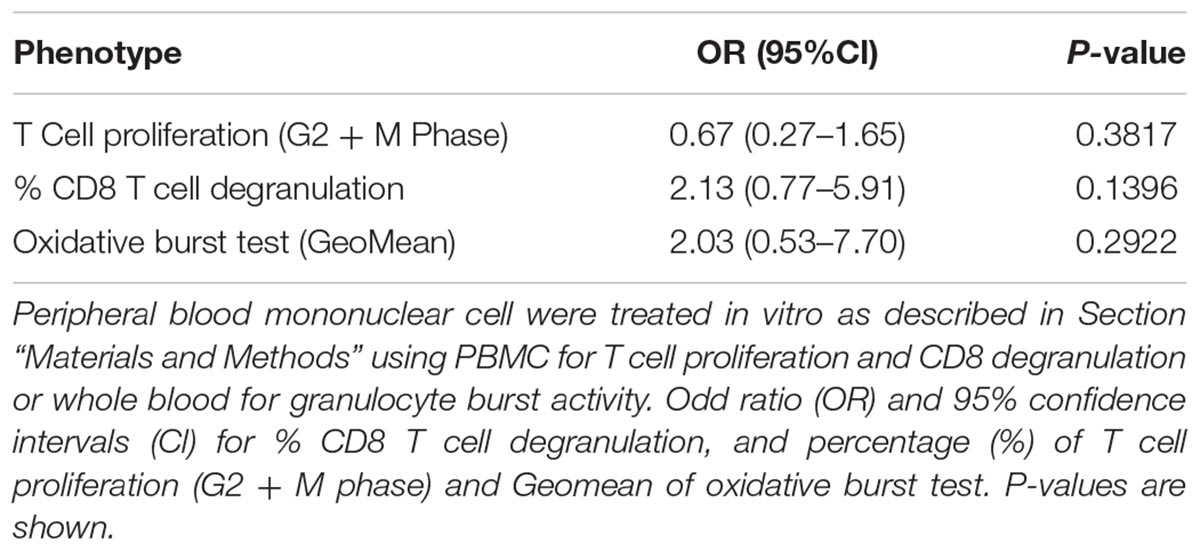
TABLE 5. Effects of MedDiet on T cell proliferation, CD8 T cell degranulation and granulocyte oxidative burst activity.
It has been shown that DC populations are affected by the aging process, and we have previously shown that the MedDiet had no effect on the numbers of mDCs or pDCs (Jing et al., 2009; Clements et al., 2017). Here we determined whether the MedDiet intervention had an impact on the ratio between mDCs and pDCs. DC subpopulations were identified and enumerated by using four-color flow cytometry in whole blood. Figure 5 shows a trend toward decrease, but not statistically significant, in the ratio of mDC/pDC (P = 0.0536).
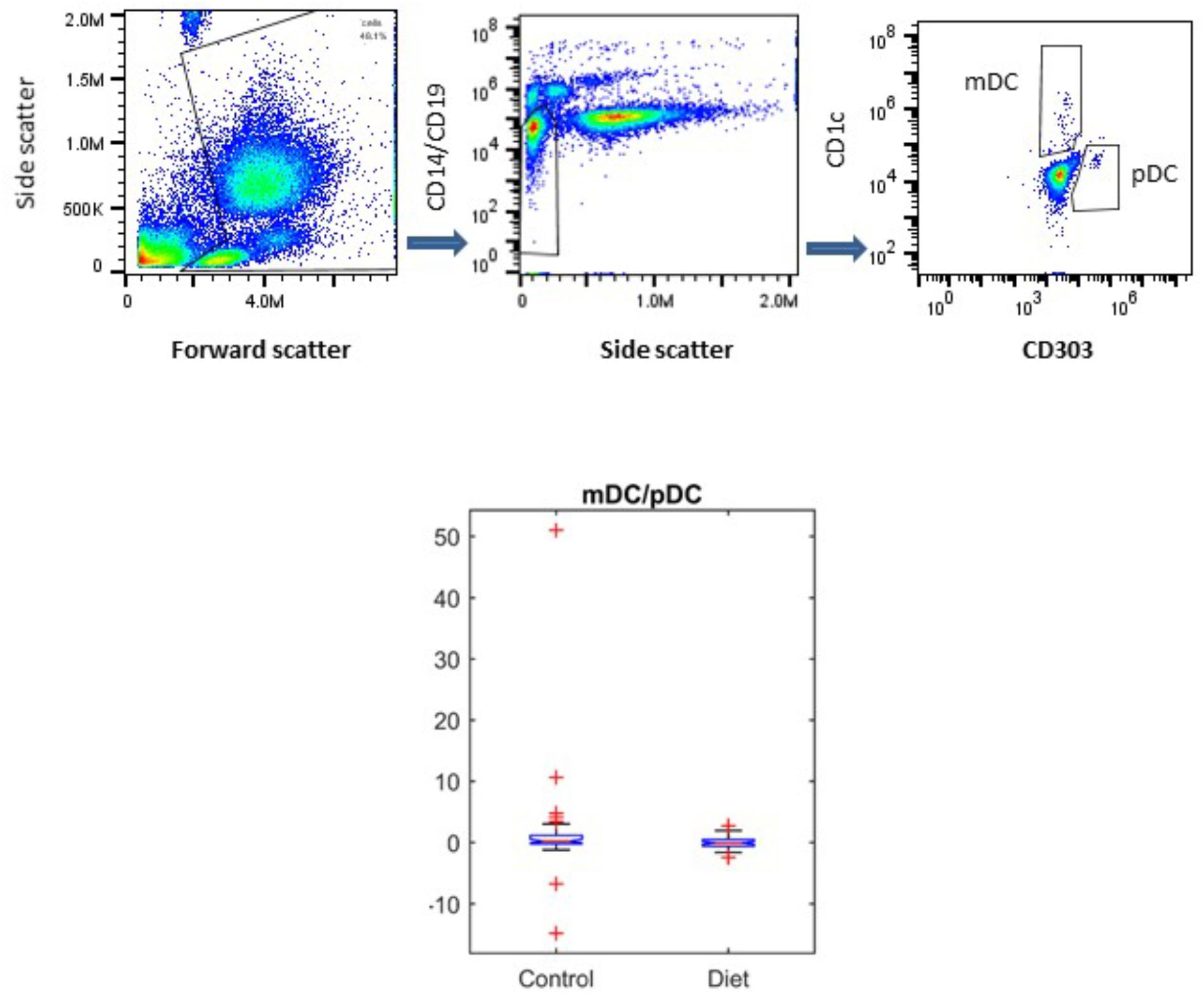
FIGURE 5. Effects of MedDiet on dendritic cell (DC) subpopulations. Whole blood was used at baseline and at the end of the 1-year dietary intervention to determine myeloid (m)DC and plasmacytoid (p)DC ratio. Representation of the gating strategy for the DCs enumeration is shown in (A–C). (D) Boxplot showing the difference in the control and diet groups for mDC/pDC ratio right panel. A decrease, but not statistically significant of the ratio of mDC/pDC (P = 0.0536) was observed.
We then sought to investigate the effects of the MedDiet on the expression of the co-stimulatory molecules CD28, KIRDL4 (CD158d), CD161 and TCR Vα24-Jα18 (NKT) on freshly isolated PBMCs. We observed that 1-year intervention had no effect on the expression of these immunoregulatory molecules between the different study groups (Table 6). However, a trend toward higher frequency of CD3+CD28+ cells in the MedDiet intervention group was seen in both genders (OR 2.19, 95%CI 0.92–5.21, P = 0.1174) (Figure 6).
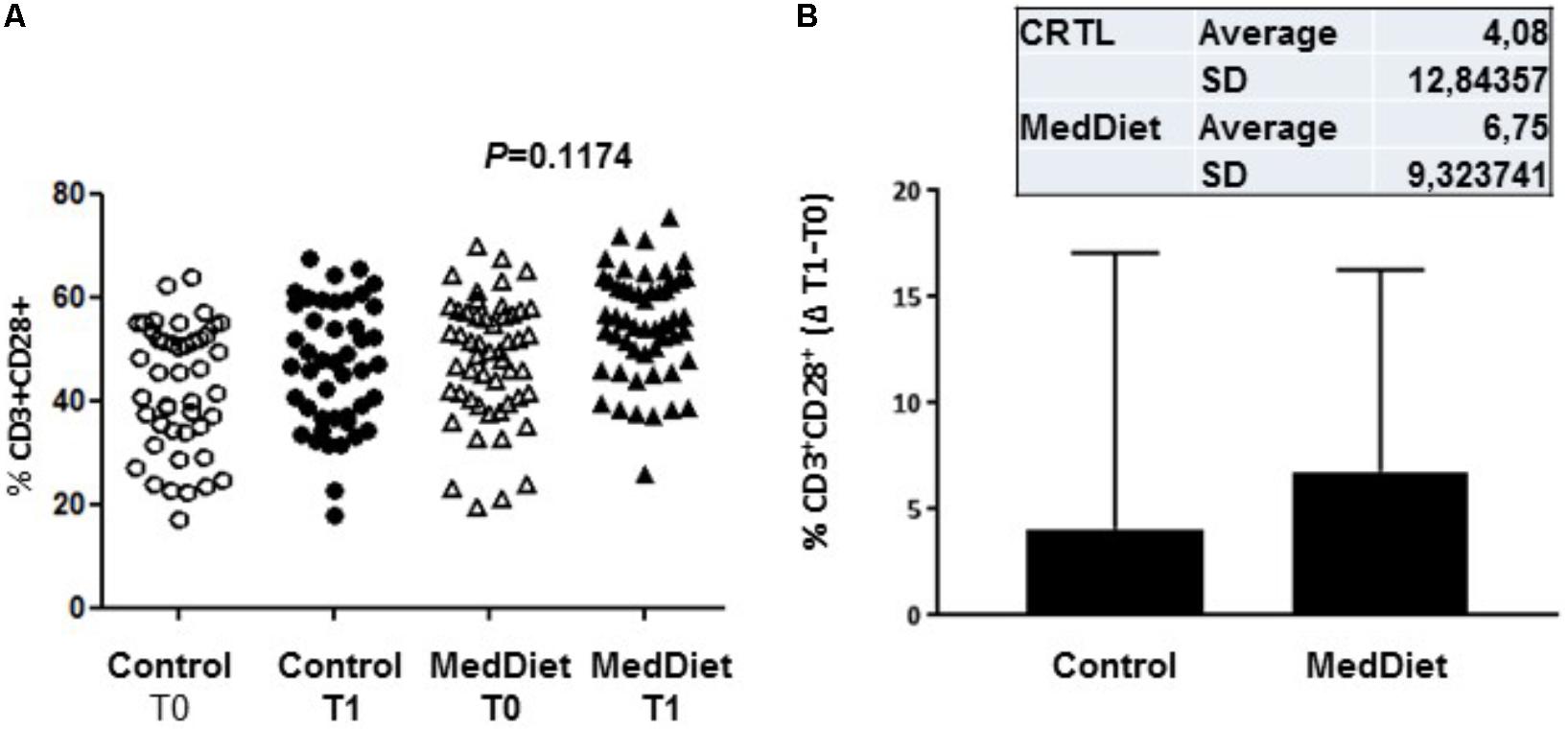
FIGURE 6. Expression of co-stimulatory molecules on freshly isolated PBMC. PBMCs were stained with CD3 and CD28 antibodies. All individual values for both the MedDiet and control group at T0 and T1 are shown (A). A trend toward higher frequency of CD3+CD28+ cells in the MedDiet intervention group was seen in both genders (OR 2.19, 95%CI 0.92–5.21, P = 0.1174). Also, changes (Δ) of parameter values in MedDiet and control diet at baseline and after 1-year of intervention were determined (mean ± SD in inset) and compared (B).
Plasma was used to monitor the levels of the regulatory cytokines IL-9, IL-23, IL-2, IL-22 prior and after the 1-year dietary intervention. The cytokines IL-9 and IL-23 in plasma were above the lower limit of detection in only 4 of the 115 volunteers analyzed. IL-2 was present in more than 90% and IL-22 in more than 60% of the plasma samples analyzed. The statistical analysis of IL-2 and IL-22 did not show any significant MedDiet-associated change (Table 7).
The MedDiet has been shown to down-regulate cellular and soluble inflammatory biomarkers related to the atherogenic process in subjects at high risk of CVD (Mena et al., 2009; Casas et al., 2014) and metabolic syndrome (Esposito et al., 2004; Fragopoulou et al., 2010). However, the immunological outcomes have not been extensively studied. It is important to highlight that the MedDiet was supplemented with Vitamin D3 (10 μg/day, 400 IU). The reason for giving a low dose vitamin D supplement was to minimize differences between study sites part of the whole NuAge project, which covered North, South, East and West Europe in which habitual diet and sunlight exposure affect vitamin D status. This combined intervention design does not allow defining the relative influence of the MedDiet and/or vitamin D on innate immune parameters. However, it has to be stressed that previous study showed that higher daily dose of vitamin D supplementation appeared to be effective on innate immunity and T cell reactivity (Prietl et al., 2013; Bashir et al., 2016). In our volunteers, adherence to a MedDiet diet for 1 year led to significant increase in the expression of CD40+CD86+ lymphocytes in women with a BMI < 31. The initiation of an adaptive immune response, requires multiple signals, including the expression of costimulatory molecules. CD40 is expressed in B cells and APCs and contribute to activate T cells through the CD40 ligand, in the absence of CD40 many cellular and immune functions are defective (Quezada et al., 2004). CD86 is also necessary for T cell activation and survival, is the ligand for CD28 and CTLA-4 and works in tandem with CD80. As these costimulatory molecules are important in immune activation, an association with gender may be related to the fact that women exhibit stronger cellular- and humoral-mediated immune responses compared to men, with a higher risk of autoimmune disease.
Within the MedDiet group we observed that the ratio of IL-12p40/IL-12p70 tended to decline. IL-12 up-regulates the expression of its own receptor, and elderly individuals have increased production of IL-12. The biological activity of the IL-12 is driven by the heterodimeric molecule IL-12p70, while IL-12p40 may antagonize IL-12. Indeed, total IL-12, IL-12p40, and the ratio IL-12p40/IL-12p70 increase significantly in aging (Rea et al., 2000). So, a decrease of the ratio IL-12p40/IL-12p70 may improve IL-12 functionality in elderly people. If this was the case, it is plausible that increased functionality of IL-12 might contribute to inflammageing. Also, a trend of decreased secretion of IL-12Rβ1 in the intervention group was observed. IL-12Rβ1 is a type 1 transmembrane receptor physically associated with the p40-domain common to both IL-12/IL23 and promotes their respective signaling pathways (Robinson, 2015). Regarding the trend in higher IFN-β but lower SOCS3 production in the MedDiet intervention group, IL-12 and IFN- β both signal through STAT4 phosphorylation, and a defective type I IFN response is described in T cells from elderly people (Li et al., 2015). Higher production of type I IFN may improve the immune response of elderly people against infection. Upregulation of TGF-β would therefore result in suppression of pro-inflammatory IL-12 and, also SOCS3 that down-regulates the STAT4-mediated IL-12 signaling by binding to the STAT4 docking site in IL-12Rβ2 (Yamamoto et al., 2003). SOCS3 proteins are constitutively expressed in naïve T helper cells and tends to decrease after cell stimulation. In aging, expression of SOCS3 is higher than in young people and does not decrease after stimulation (Tortorella et al., 2006). Our results showing a trend toward lower expression of SOCS3 after TLR stimulation would suggest the potential of MedDiet to exert beneficial effects.
DCs in human peripheral blood are divided in two subsets. Myeloid (m)DC are characterized by the production of high levels of IL-12 when stimulated with tumor necrosis factor-α or CD40 ligand and trigger a Th1-polarized immune response. Plasmacytoid (p)DCs drive an IL-4-independent Th2 polarization of naive T cells and secrete high levels of type I IFN, and IL-12 in response to certain bacteria (Stout-Delgado et al., 2008). In the elderly people, a decrease in pDCs but not mDCs (Shodell and Siegal, 2002; Pérez-Cabezas et al., 2007; Jing et al., 2009) was reported although others have reported conflicting results (Della Bella et al., 2007). However, it appeared that in frail individuals both populations are affected (Jing et al., 2009; Clements et al., 2017). Our data did not show any significant change in the mDCs and pDC populations in the control group compared to the intervention group, but it would appear that the mDC/pDC ratio tend to decrease. This finding suggests a TH2 predisposition of immune responses in the MedDiet group related to a polarized mDC:pDC balance toward pDCs, but a prolonged intervention study would be needed to confirm and possibly extend this observation.
The number, phenotype, and functions of NK cells are affected by the aging process in humans (Gayoso et al., 2011) and NKT cell numbers are increased (Peralbo et al., 2007). T cells from aging individuals showed increased expression of KIR whose expression is mostly found on senescent memory T cells that have lost CD28 expression (Li et al., 2008). Cells that produce IL-17 express CD161, and in the elderly, there is a decrease in expression of CD161+CD4 T cells (Lee et al., 2011). Our study shows no change in the expression of these markers following MedDiet intervention.
The majority of studies on aging to date have involved at-risk populations such as, for example individuals with pre-existing hypertension or metabolic syndrome. Instead our study focused on healthy elderly that did not have any signs of chronic disease or significantly impaired immune function; it is likely that this approach might have made more difficult to observe striking beneficial effects of the MedDiet.
After 1 year of adherence to a Mediterranean-like dietary pattern with vitamin D3 supplements the only significant changes observed were in female participants with a BMI < 31. However, it is important to stress there was a tendency toward improvement of certain importance in some of the inflammaging-related parameters measured, such as a greater T cell response after TLRs stimulation. For the other parameters studied, it is likely that in the absence of overt risk factors or overly compromised immune function overt benefits cannot be detected after a relatively short-term adoption of the MedDiet.
This study was approved by the National Research Ethics Committee East of England (12/EE/0109). All subjects gave written informed consent in accordance with the Declaration of Helsinki.
CN, KI, SRC, AS, SF-T, and CF designed the study. MM, KI, SJC, and JD performed the experimental work and data analysis. AJ and RG coordinated recruitment of volunteers and collected biological specimens. KI, CN, MG, and MM wrote the manuscript. CN supervised research. All authors participated to the finalization of the manuscript.
This study was supported by the European Union’s Seventh Framework Program ‘NU-AGE: New dietary strategies addressing the specific needs of the elderly population for healthy aging in Europe’ (to CF, SRC, and CN) (Agreement No. 266486) and the Biotechnology and Biological Sciences Research Council (BBSRC), United Kingdom, Institute Strategic Programme Grant (BB/J004529/1) (to SRC and CN).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Bashir, M., Prietl, B., Tauschmann, M., Mautner, S. I., Kump, P. K., Treiber, G., et al. (2016). Effects of high doses of vitamin D3 on mucosa-associated gut microbiome vary between regions of the human gastrointestinal tract. Eur. J. Nutr. 55, 1479–1489. doi: 10.1007/s00394-015-0966-2
Berendsen, A., Santoro, A., Pini, E., Cevenini, E., Ostan, R., Pietruszka, B., et al. (2014). A parallel randomized trial on the effect of a healthful diet on inflammaging and its consequences in European elderly people: design of the Nu-AGE dietary intervention study. Mech. Ageing Dev. 13, 14–21. doi: 10.1016/j.mad.2014.03.001
Casas, R., Estruch, R., and Sacanella, E. (2018). The protective effects of extra virgin olive oil on immune-mediated inflammatory responses. Endocr. Metab. Immune Disord. Drug Targets 18, 23–25. doi: 10.2174/1871530317666171114115632
Casas, R., Sacanella, E., Urpí-Sardà, M., Chiva-Blanch, G., Ros, E., Martínez-González, M. A., et al. (2014). The effects of the mediterranean diet on biomarkers of vascular wall inflammation and plaque vulnerability in subjects with high risk for cardiovascular disease. A randomized trial. PLoS One 9:e100084. doi: 10.1371/journal.pone.0100084
Castle, S. C. (2000). Impact of age-related immune dysfunction on risk of infections. Z. Gerontol. Geriatr. 33, 341–349. doi: 10.1007/s003910070030
Cevenini, E., Caruso, C., Candore, G., Capri, M., Nuzzo, D., Duro, G., et al. (2010). Age-related inflammation: the contribution of different organs, tissues and systems. How to face it for therapeutic approaches? Curr. Pharm. Des. 16, 609–618. doi: 10.2174/138161210790883840
Cevenini, E., Monti, D., and Franceschi, C. (2013). Inflammaging. Curr. Opin. Clin. Nutr. Metab. Care 16, 14–20. doi: 10.1097/MCO.0b013e32835ada13
Clements, S. J., Maijó, M., Ivory, K., Nicoletti, C., and Carding, S. R. (2017). Age-associated decline in dendritic cell function and the impact of Mediterranean diet intervention in elderly subjects. Front. Nutr. 4:65. doi: 10.3389/fnut.2017.00065
Commission of the European Communities and Nutrient and Energy Intakes for European Community (1993). Reports of the Scientific Committee for Food. Luxemburg: European Food Safety Aurhority (EFSA).
De Martinis, M., Franceschi, C., Monti, D., and Ginaldi, L. (2006). Inflammation markers predicting frailty and mortality in the elderly. Exp. Mol. Pathol. 80, 219–227. doi: 10.1016/j.yexmp.2005.11.004
Della Bella, S., Bierti, L., Presicce, P., Arienti, R., Valenti, M., Saresella, M., et al. (2007). Peripheral blood dendritic cells and monocytes are differently regulated in the elderly. Clin. Immunol. 122, 220–228. doi: 10.1016/j.clim.2006.09.012
Dewan, S. K., Zheng, S. B., Xia, S. J., and Bill, K. (2012). Senescent remodeling of the immune system and its contribution to the predisposition of the elderly to infections. Chin. Med. J. 125, 3325–3331.
Esposito, K., Marfella, R., Ciotola, M., Di Palo, C., Giugliano, F., Giugliano, G., et al. (2004). Effect of a mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA 292, 1440–1446. doi: 10.1001/jama.292.12.1440
Fragopoulou, E., Panagiotakos, D. B., Pitsavos, C., Tampourlou, M., Chrysohoou, C., Nomikos, T., et al. (2010). The association between adherence to the Mediterranean diet and adiponectin levels among healthy adults: the ATTICA study. J. Nutr. Biochem. 21, 285–289. doi: 10.1016/j.jnutbio.2008.12.013
Franceschi, C., Bonafè, M., Valensin, S., Olivieri, F., De Luca, M., Ottaviani, E., et al. (2000). Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 908, 244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x
Franceschi, C., and Campisi, J. (2014). Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. A Biol. Sci. Med. Sci. 69(Suppl. 1), S4–S9. doi: 10.1093/gerona/glu057
Franceschi, C., Capri, M., Monti, D., Giunta, S., Olivieri, F., Sevini, F., et al. (2007). Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech. Ageing Dev. 128, 92–105. doi: 10.1016/j.mad.2006.11.016
Franceschi, C., Salvioli, S., Garagnani, P., de Eguileor, M., Monti, D., and Capri, M. (2017). Immunobiography and the heterogeneity of immune responses in the elderly: a focus on inflammaging and trained immunity. Front. Immunol. 8:982. doi: 10.3389/fimmu.2017.00982
Gayoso, I., Sanchez-Correa, B., Campos, C., Alonso, C., Pera, A., Casado, J. G., et al. (2011). Immunosenescence of human natural killer cells. J. Innate Immun. 3, 337–343. doi: 10.1159/000328005
Institute of Medicine of the National Academies and Dietary Reference Intakes (2006). The Essential Guide to Nutrient Requirements. Washington, DC: The National Academies Press.
Jani, B., and Rajkumar, C. (2006). Ageing and vascular ageing. Postgrad. Med. J. 82, 357–362. doi: 10.1136/pgmj.2005.036053
Jing, Y., Shaheen, E., Drake, R. R., Chen, N., Gravenstein, D., and Deng, Y. (2009). Aging is associated with a numerical and functional decline in plasmacytoid dendritic cells, whereas myeloid dendritic cells are relatively unaltered in human peripheral blood. Hum. Immunol. 70, 777–784. doi: 10.1016/j.humimm.2009.07.005
Lee, J. S., Lee, W. W., Kim, S. H., Kang, Y., Lee, N., Shin, M. S., et al. (2011). Age-associated alteration in naive and memory Th17 cell response in humans. Clin. Immunol. 140, 84–91. doi: 10.1016/j.clim.2011.03.018
Lencel, P., and Magne, D. (2011). Inflammaging: the driving force in osteoporosis? Med. Hypotheses 76, 317–321. doi: 10.1016/j.mehy.2010.09.023
Li, G., Ju, J., Weyand, C. M., and Goronzy, J. J. (2015). Age-associated failure to adjust type I IFN receptor signalling thresholds after T cell activation. J. Immunol. 195, 865–874. doi: 10.4049/jimmunol.1402389
Li, G., Weyand, C. M., and Goronzy, J. J. (2008). Epigenetic mechanisms of age-dependent of KIR2DL4 expression in T cells. J. Leukoc. Biol. 84, 824–838. doi: 10.1189/jlb.0807583
Maijó, M., Clements, S. J., Ivory, K., Nicoletti, C., and Carding, S. R. (2014). Nutrition, diet and immunosenescence. Mech. Ageing Dev. 13, 116–128. doi: 10.1016/j.mad.2013.12.003
Man, A. L., Bertelli, E., Rentini, S., Regoli, M., Briars, G., Marini, M., et al. (2015). Age-associated modifications of intestinal permeability and innate immunity in human small intestine. Clin. Sci. 129, 515–527. doi: 10.1042/CS20150046
Martinez-Gonzalez, M. A., Salas-Salvadó, J., Estruch, R., Corella, D., Fitó, M., and Ros, E. (2015). Predimd invesigators. benefits of the Mediterranean diet: insights from the predimed study. Prog. Cardiovasc. Dis. 58, 50–60.
Mena, M. P., Sacanella, E., Vazquez-Agell, M., Morales, M., Fitó, M., Escoda, R., et al. (2009). Inhibition of circulating immune cell activation: a molecular antiinflammatory effect of the Mediterranean diet. Am. J. Clin. Nutr. 89, 248–256. doi: 10.3945/ajcn.2008.26094
Monti, D., Ostan, R., Borelli, V., Castellani, G., and Franceschi, C. (2017). Inflammaging and human longetivity in the omics era. Mech. Ageing Dev. 165, 129–138. doi: 10.1016/j.mad.2016.12.008
Pae, M., Meydani, S. N., and Wu, D. (2012). The role of nutrition in enhancing immunity in aging. Aging Dis. 3, 91–129.
Pedersen, B. K. (2009). The diseasome of physical inactivity – and the role of myokines in muscle – fat cross talk. J. Physiol. 587, 5559–5568. doi: 10.1113/jphysiol.2009.179515
Peralbo, E., Alonso, C., and Solana, R. (2007). Invariant NKT and NKT-like lymphocytes: two different T cell subsets that are differentially affected by ageing. Exp. Gerontol. 42, 703–708. doi: 10.1016/j.exger.2007.05.002
Peregrin, T. (2008). Getting to know the modified MyPyramid for older adults. J. Am. Diet Assoc. 108, 937–938. doi: 10.1016/j.jada.2008.04.006
Pérez-Cabezas, B., Naranjo-Gómez, M., Fernández, M. A., Grífols, J. R., Pujol-Borrell, R., and Borràs, F. E. (2007). Reduced numbers o plasmacytoid dendritic cells in aged blood donors. Exp. Gerontol. 42, 1033–1038. doi: 10.1016/j.exger.2007.05.010
Prietl, B., Treiber, G., Pieber, T. R., and Amrein, K. (2013). Vitamin D and Immune Function. Nutrients 5, 2502–2521. doi: 10.3390/nu5072502
Quezada, S. A., Jarvinen, L. Z., Lind, E. F., and Noelle, R. J. (2004). CD40/CD154 interactions at the interface of tolerance and immunity. Annu. Rev. Immunol. 22, 307–328. doi: 10.1146/annurev.immunol.22.012703.104533
Rea, I. M., McNerlan, S. E., and Alexander, H. D. (2000). Total serum IL-12 and IL-12p40, but not IL-12p70, are increased in the serum of older subjects; relationship to CD3+ and NK subsets. Cytokine 12, 156–169. doi: 10.1006/cyto.1999.0537
Robinson, R. T. (2015). IL12Rβ1: the cytokine receptor that we used to know. Cytokine 71, 348–359. doi: 10.1016/j.cyto.2014.11.018
Santangelo, C., Vari, R., Scazzocchio, B., De Sanctis, P., Giovannini, C., D’Archivio, M., et al. (2018). Anti-inflammatory activity of extra virgin olive oil polyphenols: which role in the prevention and treatment of immune-mediated inflammatory diseases? Endocr. Metab. Immune Disord. Drug Targets 18, 36–50. doi: 10.2174/1871530317666171114114321
Santoro, A., Pini, E., Scurti, M., Palmas, G., Berendsen, A., Brzozowska, A., et al. (2014). Combating inflammaging through a Mediterranean whole diet approach: the NU-AGE project’s conceptual framework and design. Mech. Ageing Dev. 136-137, 3–13. doi: 10.1016/j.mad.2013.12.001
Shelnutt, K. P., Bobroff, L. B., and Diehl, D. C. (2009). MyPyramid for older adults. J. Nutr. Educ. Behav. 41, 300–302. doi: 10.1016/j.jneb.2008.07.007
Shodell, M., and Siegal, F. P. (2002). Circulating, interferon-producing plasmacytoid dendritic cells decline during human ageing. Scand. J. Immunol. 56, 518–521. doi: 10.1046/j.1365-3083.2002.01148.x
Stout-Delgado, H. W., Yang, X., Walker, W. E., Tesar, B. M., and Goldstein, D. R. (2008). Aging impairs IFN regulatory factor 7 up-regulation in plasmacytoid dendritic cells during TLR9 activation. J. Immunol. 181, 6747–6756. doi: 10.4049/jimmunol.181.10.6747
Tortorella, C., Stella, I., Piazzolla, G., Cappiello, V., Simone, O., Pisconti, A., et al. (2006). Impaired interleukin-12-dependent T-cell functions during aging: role of signal transducer and activator of transcription 4 (STAT4) and suppressor of cytokine signalling 3 (SOCS3). J. Gerontol. A Biol. Sci. Med. Sci. 61, 125–135. doi: 10.1093/gerona/61.2.125
Varadhan, R., Yao, W., Matteini, A., Beamer, B. A., Xue, Q. L., Yang, H., et al. (2014). Simple biologically informed inflammatory index of two serum cytokines predicts 10year all-cause mortality in older adults. J. Gerontol. A Biol. Sci. Med. Sci. 69, 165–173. doi: 10.1093/gerona/glt023
Vasto, S., Scapagnini, G., Rizzo, C., Monastero, R., Marchese, A., and Caruso, C. (2012). Mediterranean diet and longevity in Sicily: survey in a Sicani Mountains population. Rejuvenation Res. 15, 184–188. doi: 10.1089/rej.2011.1280
Keywords: aging, dietary intervention, nutrition, inflammation, elderly, NU-AGE
Citation: Maijo M, Ivory K, Clements SJ, Dainty JR, Jennings A, Gillings R, Fairweather-Tait S, Gulisano M, Santoro A, Franceschi C, Carding SR and Nicoletti C (2018) One-Year Consumption of a Mediterranean-Like Dietary Pattern With Vitamin D3 Supplements Induced Small Scale but Extensive Changes of Immune Cell Phenotype, Co-receptor Expression and Innate Immune Responses in Healthy Elderly Subjects: Results From the United Kingdom Arm of the NU-AGE Trial. Front. Physiol. 9:997. doi: 10.3389/fphys.2018.00997
Received: 01 March 2018; Accepted: 09 July 2018;
Published: 26 July 2018.
Edited by:
Stephen J. Pandol, Cedars-Sinai Medical Center, United StatesReviewed by:
Hiroshi Ishiguro, Nagoya University, JapanCopyright © 2018 Maijo, Ivory, Clements, Dainty, Jennings, Gillings, Fairweather-Tait, Gulisano, Santoro, Franceschi, Carding and Nicoletti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Claudio Nicoletti, Y2xhdWRpby5uaWNvbGV0dGlAdW5pZmkuaXQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.