
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Physiol., 17 May 2018
Sec. Redox Physiology
Volume 9 - 2018 | https://doi.org/10.3389/fphys.2018.00477
 Zewen Liu1,2†
Zewen Liu1,2† Zhangpin Ren3†
Zhangpin Ren3† Jun Zhang4†
Jun Zhang4† Chia-Chen Chuang1,5
Chia-Chen Chuang1,5 Eswar Kandaswamy1
Eswar Kandaswamy1 Tingyang Zhou1,5
Tingyang Zhou1,5 Li Zuo1,5*
Li Zuo1,5*The overproduction of reactive oxygen species (ROS) has been implicated in the development of various chronic and degenerative diseases such as cancer, respiratory, neurodegenerative, and digestive diseases. Under physiological conditions, the concentrations of ROS are subtlety regulated by antioxidants, which can be either generated endogenously or externally supplemented. A combination of antioxidant-deficiency and malnutrition may render individuals more vulnerable to oxidative stress, thereby increasing the risk of cancer occurrence. In addition, antioxidant defense can be overwhelmed during sustained inflammation such as in chronic obstructive pulmonary diseases, inflammatory bowel disease, and neurodegenerative disorders, cardiovascular diseases, and aging. Certain antioxidant vitamins, such as vitamin D, are essential in regulating biochemical pathways that lead to the proper functioning of the organs. Antioxidant supplementation has been shown to attenuate endogenous antioxidant depletion thus alleviating associated oxidative damage in some clinical research. However, some results indicate that antioxidants exert no favorable effects on disease control. Thus, more studies are warranted to investigate the complicated interactions between ROS and different types of antioxidants for restoration of the redox balance under pathologic conditions. This review highlights the potential roles of ROS and nutritional antioxidants in the pathogenesis of several redox imbalance-related diseases and the attenuation of oxidative stress-induced damages.
Malnutrition is a poor prognostic sign in various diseases, and it is considered a major health concern in developing countries (Muller and Krawinkel, 2005). Reactive oxygen species (ROS) are involved in many important cellular activities including gene transcription, signaling transduction, and immune response. Common ROS include hydroxyl radical (•OH), superoxide (O2•–) and hydrogen peroxide (H2O2) (Rogers et al., 2014; Zuo et al., 2015b). An overproduction of ROS can result in oxidative damage to biomolecules such as lipids, proteins, and DNA, which has been implicated in the development of aging as well as various ailments including cancer, respiratory, cardiovascular, neurodegenerative, and digestive diseases. It is reported that the deleterious effects of excess ROS, or oxidative stress (OS), eventually lead to cell death [71]. The body has equipped several mechanisms to counteract the detrimental effects of OS. Antioxidants, either endogenously generated or externally supplied, are capable of scavenging ROS and reducing the oxidation of cellular molecules, thus alleviating OS (Gilgun-Sherki et al., 2001). Antioxidants obtained from the diet are essential in supplying endogenous antioxidants for the neutralization of OS. Indeed, malnutrition and certain antioxidant deficiencies have been correlated with diseases such as chronic obstructive pulmonary disease (COPD) and Crohn’s disease (CD) (King et al., 2008; Alzoghaibi, 2013). A disturbed nutritional and redox balance is frequently observed in these patients. Malnutrition-induced antioxidant deficiency may contribute to increased risks of disease occurrence and poor treatment outcomes (Ames and Wakimoto, 2002; Evatt et al., 2008; Schols et al., 2014). Currently, the clinical awareness of nutritional balance in disease occurrence, progression, and outcomes is limited. An update on the literature review that focuses on the relationship between patients’ nutritional status and disease development is needed. In this review, we will outline the roles of ROS in common OS-associated diseases and aging as well as discuss the effects of nutritional antioxidants as treatments or adjuvants.
Respiratory diseases such as COPD and asthma have been identified as major health problems due to increased prevalence and mortality worldwide (Masoli et al., 2004; Pauwels and Rabe, 2004). Environmental exposures to air pollutants and cigarette smoke contribute greatly to an increase in OS in COPD (Figure 1). The toxicity of oxidants directly damages alveoli and connective tissues of the lungs, exacerbating the development of COPD (van Eeden and Sin, 2013). Excessive ROS formation can also activate inflammatory cells, which in turn generate more ROS in the lungs. This process initiates a vicious cycle of chronic inflammation and OS, as seen in COPD (van Eeden and Sin, 2013). OS is also implicated in the pathophysiology of asthma (Comhair and Erzurum, 2010). Although it remains inconclusive regarding whether increased OS in asthma is a causative factor of the disease or a consequence of inflammation, OS is suggested to play a pivotal role in asthma progression (Cho and Moon, 2010). In bronchial asthma, OS aggravates airway inflammation by activating transcription factors such as nuclear factor-kappa B (NF-κB), mitogen-activated protein kinase (MAPK), activator protein-1 (AP-1), as well as pro-inflammatory mediators (Figure 1). Moreover, it enhances airway hyper-responsiveness and stimulates mucin secretion, both of which are associated with severe asthma (Fitzpatrick et al., 2009; Cho and Moon, 2010; Zuo et al., 2016). OS-induced damages in the respiratory system and the reduced antioxidant defenses further lead to an increase in endogenous ROS formation (Jiang et al., 2014).
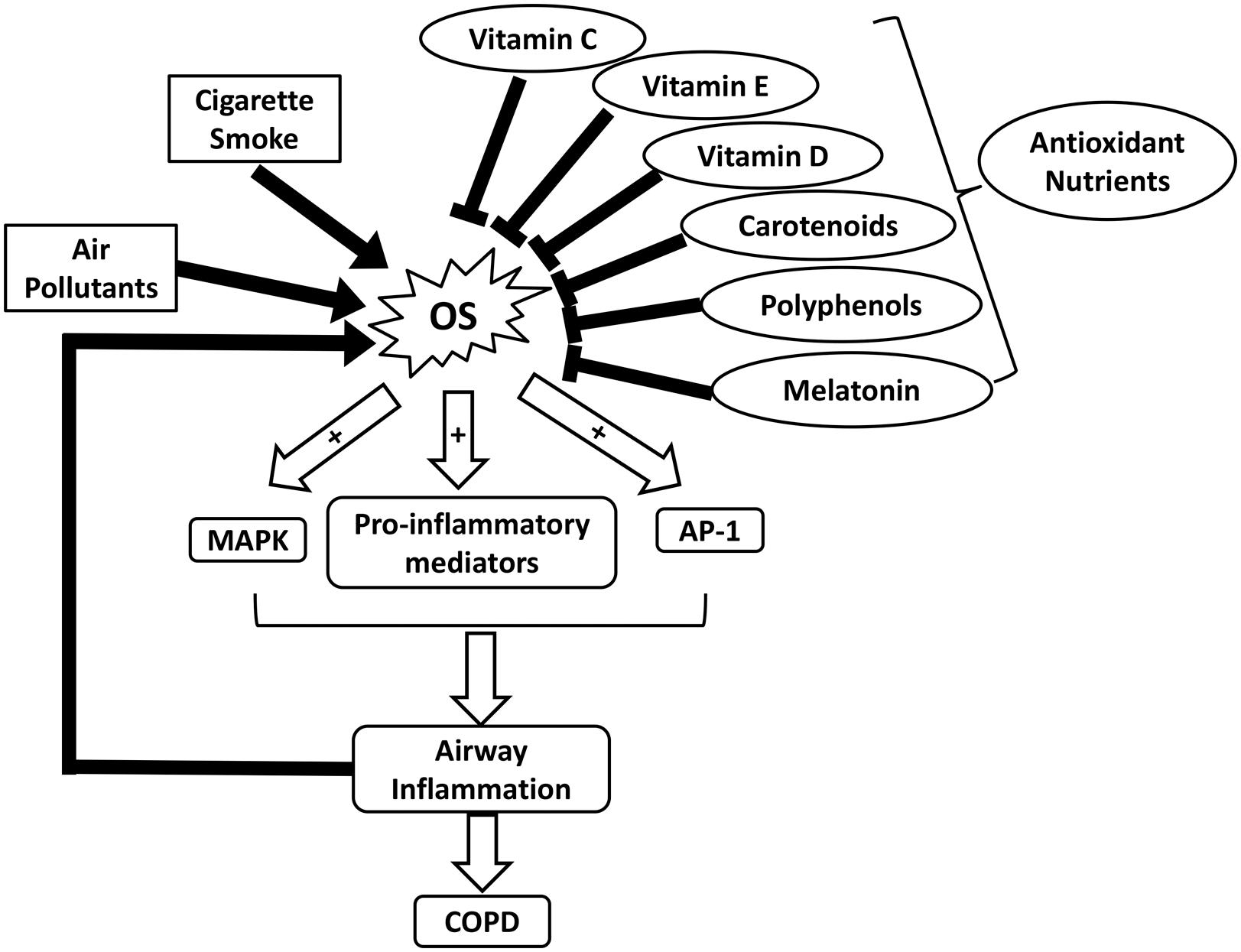
FIGURE 1. Schematic illustrating the roles of OS and nutrient antioxidants in COPD. AP-1, activator protein-1; COPD, chronic obstructive pulmonary diseases; MAPK, mitogen-activated protein kinase; OS, oxidative stress.
In addition to OS, low body mass index (BMI) and malnutrition are suggested to correlate with the severity of COPD (King et al., 2008). Underweight COPD patients tend to experience more pulmonary damage, exercise intolerance, and increased mortality rates, in comparison to individuals with normal weights (King et al., 2008; Ferreira et al., 2012). Malnutrition can lead to respiratory muscle mass reduction, which lowers the strength and endurance of these muscles (Ferreira et al., 2012). In addition, decreased intake or availability of dietary antioxidants such as vitamins C and E, carotenoids, and polyphenols, can weaken the antioxidant system and exacerbate disease progression (Figure 1 and Table 1) (Sies et al., 2005; King et al., 2008). A dietary pattern that is rich in vegetables, fruits, fish, and whole grains has been associated with improved pulmonary function and a lower risk of COPD (Varraso et al., 2015). It is suggested that nutritional supplementation enhances respiratory muscle function in malnourished COPD patients, thereby improving their quality of life (Ferreira et al., 1998). For example, Hornikx et al. (2012) reported that high doses of vitamin D supplementation strengthen respiratory muscle function and exercise capacity in individuals with COPD. As the most well-known nutritional antioxidant, vitamin C is capable of reducing oxidative damages and inflammation in the pulmonary system by scavenging excess ROS and activating NF-κB pathway, respectively (Tecklenburg et al., 2007). Furthermore, melatonin, a powerful antioxidant and a regulator of the sleep-wake cycle, can also attenuate OS-related lung deterioration (Figure 1 and Table 1) (Gumral et al., 2009). These findings support the potential use of nutritional antioxidants as an adjuvant to COPD treatment. Similarly, several observational studies suggest that nutritional antioxidants from diets or supplements can improve asthma control and lung function in asthmatic patients (Moreno-Macias and Romieu, 2014). A systematic review has proposed that there is an inverse association between dietary intake of vitamins A and C and incidence of asthma (Table 1) (Allen et al., 2009). Vitamin C functions in conjunction with vitamin E to stimulate the regeneration of membrane-bound α-tocopherol from its oxidized states (Moreno-Macias and Romieu, 2014). In addition, dietary carotenoids have been shown to correlate with improved asthma outcomes and lung function (Wood et al., 2012).
The linkage between OS and the development of respiratory diseases suggests a pivotal role of nutritional antioxidants (Romieu, 2005). Vulnerable populations include those with deficiency in dietary antioxidants, increased exposure to environmental sources of oxidants, and poor access to nutritional antioxidants (Moreno-Macias and Romieu, 2014). It is important to note that although antioxidants may help to mitigate the progression of respiratory diseases, antioxidant supplements can act as pro-oxidants or OS inducers if consumed at levels that significantly surpass the recommended dietary intake (Pham-Huy et al., 2008). The potential benefits and risks of nutritional antioxidant supplementation trials in respiratory diseases should be considered on a case-by-case basis. Furthermore, it remains unknown whether OS is a consequence or the causative factor for some pulmonary diseases. Therefore, antioxidant treatment may not be an effective approach to modify disease progression although it may be able to alleviate OS-related symptoms (Margaritelis, 2016).
Cardiovascular diseases (CVD) are the leading cause of mortality in the United States, resulting in nearly one million deaths each year (Madamanchi et al., 2005; Heidenreich et al., 2011). The majority of CVD is correlated with atherosclerosis development, in which OS play a causal role (Madamanchi et al., 2005). Excessive ROS can be generated in vascular cells from NAD(P)H oxidase (Nox), nitric oxide synthases (NOS) uncoupling, and mitochondria, which cause oxidative modifications of low density lipoprotein (LDL) (Azumi et al., 2002; Ambrose and Barua, 2004; Madamanchi et al., 2005). The oxidized LDL (ox-LDL) transported through the arterial lumens induces apoptosis of endothelial cells and smooth muscle cells (SMCs). By taking up ox-LDL, macrophages may transform into foam cells, which secrete growth mediators to attract SMCs into the intima. SMCs can secret extracellular matrix that forms a thin fibrous cap surrounding the fatty streak (Madamanchi et al., 2005; Cachofeiro et al., 2008). With the continuous propagation of SMCs, monocytes, and macrophages, fatty streaks are ultimately converted into more advanced fibrous plaque (Madamanchi et al., 2005), potentially leading to vessel occlusion (Cachofeiro et al., 2008). Further, OS has also been implicated in the development cardiac hypertrophy, ischemic-reperfusion injury, and myocyte apoptosis, all of which may contribute to heart failure (Madamanchi et al., 2005; Zhou et al., 2018).
Considering the implications of ROS in CVD development, numerous studies have been performed to evaluate the effects of nutritional antioxidants in CVD patients. Consumption of fruit and vegetable is found to increase the levels of antioxidants such as carotene and vitamin C in the blood as well as decrease the cholesterol oxidation (Zino et al., 1997; Asplund, 2002). Therefore, the potential benefits of fruits and vegetables in CVD have been broadly investigated. In a meta-analysis consisting of 16 prospective cohort studies and 833,234 participants, CVD-related mortality was found to be inversely correlated with fruit and vegetable consumption (Wang et al., 2014a). Another study involving 2002 patients with coronary atherosclerosis showed that supplementation of natural α-tocopherol (RRR-AT) can significantly reduce the incidence of CVD-related death and non-fatal myocardial infarction (Table 1) (Stephens et al., 1996). However, different results are present suggesting no beneficial effect of vitamin supplementation on CVD mortality or morbidity (Kris-Etherton et al., 2004). For example, a meta-analysis study, which involved 81,788 participants, reported that daily supplementation of either vitamin E at a dose of 50–800 IU or β carotene at a dose of 15–80 mg did not decrease the mortality associated with CVD (Vivekananthan et al., 2003). Therefore, vitamin E and β carotene may not be the only active constituent of fruits and vegetables that exert cardiovascular protective effects. Instead, other antioxidant compounds such as lycopene and polyphenols could play a more important role in the protection against CVD as will be discussed below (Ignarro et al., 2007). Furthermore, the inconsistency in treatment outcomes are likely associated with antioxidant formulation. Most of the trials reporting inefficacy of vitamin E have used all-racemic α tocopherol, which is a major constituent of synthetic vitamin E (Hoppe and Krennrich, 2000; Madamanchi et al., 2005). By contrast, RRR-AT, the natural form of vitamin E has been associated with better treatment effects (Hoppe and Krennrich, 2000; Madamanchi et al., 2005). Thus, further research is needed to address the difference between RRR-AT and all-racemic α tocopherol in terms of their therapeutic efficacy. Additionally, the complicated redox mechanisms of antioxidant are far from clear now. Some of the antioxidant such as vitamin C may exhibit prooxidant properties when administrated at high doses (Madamanchi et al., 2005). This could partially explain why some of the trials using antioxidant supplementation failed to show any protective effect.
Lycopene is a natural dietary antioxidant most abundant in tomatoes. An inverse association was found between CVD incidence and consumption of either tomatoes or lycopene (Kohlmeier and Hastings, 1995; Arab and Steck, 2000; Rao and Agarwal, 2000). This could be attributed to the protective effects of lycopene against LDL oxidation by inhibiting cholesterol synthesis and improving LDL degradation (Table 1) (Ignarro et al., 2007). An early population-based study was conducted to evaluate the relationship between the risk of myocardial infarction and the status of three types of carotenoids including lycopene, α carotene, and β carotene, respectively. It was found that only lycopene had significant protective effects (Kohlmeier et al., 1997). Therefore, lycopene may be one of the primary contributors that underlie the protective mechanisms of vegetable consumption against CVD (Kohlmeier et al., 1997). Additionally, polyphenols are the most abundant antioxidants in human diet (∼1 g/d), widespread in fruit, vegetables, coffee, tea, and cereals (Ignarro et al., 2007). Epidemiologic studies found a significantly reduced risk for CVD with higher polyphenol intake (Table 1) (Vita, 2005). Beverages rich in flavonoid such as tea can markedly improve endothelial function. However, tea consumption did not reduce the oxidative markers in the blood. So it remains elusive whether this beneficial effect of tea is elicited by its antioxidant effects (Vita, 2005). Indeed, increasing evidence has suggested that the protective effects of polyphenols are not solely contributed by their antioxidant ability but more likely correlated with their anti-inflammatory effects as well as the regulation of vasodilation and apoptosis of endothelial cells (Quinones et al., 2013).
Neurons are particularly vulnerable to OS-induced damage due to their weakened antioxidant defense system, high demand for oxygen consumption, and abundant polyunsaturated fatty acid content in their cell membranes (Rego and Oliveira, 2003). A growing number of studies indicate that ROS may be generated via different mechanisms and play complex roles in the development of neurodegenerative diseases such as Alzheimer’s disease (AD), Huntington’s disease (HD), amyotrophic lateral sclerosis (ALS), Parkinson’s disease (PD), and spinocerebellar ataxia (SCA) (Davila and Torres-Aleman, 2008; Hakonen et al., 2008; Patten et al., 2010; Blesa et al., 2015; Covarrubias-Pinto et al., 2015; Zuo et al., 2015b). AD is a major cause of dementia in elderly (Harman, 2006). Although the exact pathogenesis of AD remains elusive, aging-related progressive increase in OS has been considered a chief contributor to the formation of AD lesions (Harman, 2006; Pimplikar et al., 2010). Evidence has suggested that oxidative events occur prior to the onset of plaque pathology and amyloid-β (Aβ) accumulation, which further supports the critical roles of OS in the initiating stage of AD (Lin and Beal, 2006; Wang et al., 2014b). In AD, OS modulates JNK/p38 MAPK pathways, leading to the accumulation of Aβ and the hyper-phosphorylation of tau proteins (Patten et al., 2010). HD is an autosomal dominant inherited disease, which is caused by a mutated expansion of CAG repeat in exon 1 of HD gene and its resulted mutant protein product “huntingtin” (mHtt) (Browne and Beal, 2006). OS is not the initiation factor of HD. However, severe OS is a typical feature of HD and may contribute to the increased DNA oxidation in the HD brain (Browne and Beal, 2006). OS-induced mitochondrial dysfunction is commonly observed in HD and the impairment of respiratory chain can exacerbate ROS formation (Browne and Beal, 2006). Furthermore, mitochondrial aconitase, an important tricarboxylic acid (TCA)-cycle enzyme, is significantly impaired in HD. The decline in aconitase activity is thought to be caused by ROS-induced oxidation of Fe-S cluster within aconitase (Browne and Beal, 2006). As a result, OS is responsible for the metabolic defects seen in HD (Browne and Beal, 2006). In HD, OS is also related to decreased expression of glucose transporter (GLUT)-3, which results in the inhibition of glucose uptake and the over-accumulation of lactate (Covarrubias-Pinto et al., 2015). It remains inconclusive whether OS is an initiator or consequence of neurodegeneration in PD. However, excessive ROS production is a critical component of the mechanisms underlying PD progression (Jenner, 2003). Loss of antioxidant defense, especially glutathione (GSH) content is found early in PD although the cause remains unknown (Jenner, 2003). High levels of oxidation of protein, DNA, and lipids are observed in PD. The toxic products from the oxidative damage may lead to neural cell death (Jenner, 2003). In the substantia nigra pars compacta (SNc) of PD patients, reduced activity of Complex I in the mitochondrial respiratory chain contributes to excessive ROS generation and consequently induces the apoptosis of dopaminergic neurons (Blesa et al., 2015). In ALS, superoxide dismutase (SOD) 1 mutation and mitochondrial degeneration represent one of the major mechanisms underlying ALS pathology (Rotunno and Bosco, 2013). Specifically, significant vacuolar degeneration of mitochondria was observed just before the death of neuron in SOD1 mutant mice, indicating that mitochondrial dysfunction initiates the onset of ALS (Lin and Beal, 2006). Mutant SOD1 has been shown to abnormally interact with mitochondria, leading to cytochrome c release and activation of apoptosis (Lin and Beal, 2006). A decline in antioxidant capability due to SOD1 mutation is potentially associated with motor neuron degeneration (Zuo et al., 2015b). In addition, elevated OS can inhibit neuroprotective IGF-I/AKT pathways, resulting in neuron cell dysfunction (Davila and Torres-Aleman, 2008). Furthermore, marked mitochondrial alterations caused by OS have been suggested to be involved in the development of SCA (Stucki et al., 2016).
Considering the complex roles of OS in neurodegenerative disorders, the regulation of cellular ROS levels may represent a potential treatment to impede neurodegeneration and alleviate associated symptoms (Uttara et al., 2009). Clinical evidence indicates that neurodegenerations can be ameliorated upon proper intake of natural or supplementary antioxidants (Zandi et al., 2004). On the other hand, a lack of major antioxidants due to malnutrition, which is implicated in various neurodegenerative diseases, can worsen the progress of neurological conditions (Brambilla et al., 2008; Tsagalioti et al., 2016). For example, vitamin D deficiency has recently emerged as one of the contributing factors leading to aberrant neurological development. Vitamin D is an essential antioxidant that regulates calcium-mediated neuronal excitotoxicity and the induction of neurotransmitters and synaptic structural proteins (Mpandzou et al., 2016; Wang et al., 2016). Wang et al. (2016) suggested that inadequate vitamin D in serum is highly associated with the loss of dopaminergic neurons in PD brains and increased risk of PD. Neurological impairments have also been manifested in individuals with a vitamin B deficiency. Multiple vitamin B (e.g., B1, B3, and folate) deficiencies are implicated in the pathophysiology of numerous neurodegenerative diseases such as PD and AD (Sechi et al., 2016).
Rutin, resveratrol, and vitamin E, which target ROS-mediated cascades such as JNK and NF-κB, have yielded some positive outcomes in improving neurodegeneration both in vitro and in vivo (Zuo et al., 2015a). In a rat brain, vitamin E was found to be more effective in modulating OS than vitamins A and C (Zaidi and Banu, 2004). Accordingly, a 2-year administration of vitamin E at a dose of 2000 IU per day has been shown to reduce the functional decline associated with AD (Sano et al., 1997). The combination of vitamin E and coenzyme Q10 improves energy generation in some cases of Friedreich ataxia by attenuating OS and restoring mitochondrial function (Lodi et al., 2001). In addition to vitamins, phytochemicals, another type of bioactive compounds that can be found in fruits and vegetables, exhibit high antioxidant capacity with potential neuroprotective effects against PD (Mazo et al., 2017). Anthocyanin derived from strawberries possesses anti-oxidative, anti-inflammatory, and anti-apoptotic abilities. It has been reported to alleviate astrogliosis and preserve neuromuscular junctions and muscle function, serving as a possible therapeutic agent for ALS and other neurodegenerative diseases (Winter et al., 2017). During AD progression, resveratrol’s potential in protecting neurons from Aβ and OS-induced toxicity shows promising therapeutic applications (Anekonda, 2006; Bellaver et al., 2014). Lipoic acid (LA) is shown to enhance GSH generation and deplete lipid peroxide, thus protecting neurons against OS-induced mitochondrial dysfunction (Table 1) (Moreira et al., 2010; Zuo and Motherwell, 2013). Long-term administration of MitoQ, a mitochondria-target antioxidant, also significantly restores mitochondrial functions in Purkinje cells and alleviates SCA1-related symptoms such as motor incoordination (Stucki et al., 2016).
Numerous studies have been performed to investigate the therapeutic effects of natural antioxidants on neurodegenerative disorders; however, mixed results have been yielded (Dias et al., 2013; Yan et al., 2013). For instance, despite the seeming effectiveness of vitamin E, a study has showed that vitamin E intake for 5 months failed to elevate vitamin E levels in ventricular cerebrospinal fluid of PD patients (Pappert et al., 1996). ROS formation is subtly regulated by antioxidant defense systems within the human body (Zuo et al., 2015b). Hence, single antioxidant intake could not be sufficient to resist OS under pathophysiological conditions and could result in cellular damage (Murphy, 2014). In this regard, a combined use of various nutritional antioxidants should be considered. Importantly, the simple dichotomy in redox biology comprised of good antioxidants and bad ROS is regarded as untenable. It is now well accepted that a small amount of ROS is essential to activate redox-sensitive signaling pathways, while excessive ROS can lead to detrimental effects (Margaritelis et al., 2016). The different characteristics and sources of ROS may define their specific roles in regulating cellular activities (Winterbourn and Hampton, 2008; Margaritelis et al., 2016). Numerous studies have stressed the need for a more precise description of the metabolism of ROS in aspects of quantity, reactivity, location, and reaction kinetics (Winterbourn and Hampton, 2008; Forman et al., 2014; Margaritelis et al., 2016). However, most of the exogenously administrated antioxidants are non-selective and distributed uniformly across various parts of the cells or tissues (Margaritelis et al., 2016). The lack of specificity of antioxidants may account for their inefficacy in treating OS-related diseases. It is thus imperative that researchers focus on developing novel and targeted antioxidants such as mitoQ and Nox inhibitors to improve the precise therapeutic effects of antioxidants in future studies (Altenhofer et al., 2015; Margaritelis et al., 2016).
ROS are involved in all three stages of cancer development, namely initiation, promotion, and progression (Khandrika et al., 2009; Wells et al., 2009; Katakwar et al., 2016). In the initiation stage, ROS-induced DNA mutations can accumulate if they are not repaired in cancerous tissues (Poulsen et al., 1998). Excessive ROS production may lead to oncogenic mutation of DNA, potentially contributing to the onset of cancer (Valko et al., 2006). In addition, cancer cells are characterized by more ROS production than normal cells due to an altered metabolism and increased energy demand (Sosa et al., 2013). ROS-induced OS in carcinoma cells may promote cancer growth by triggering cell growth signaling, enhancing tumor resistance to therapies, increasing blood supply to tumors, and promoting metastasis (Brown and Bicknell, 2001). ROS promote the expansion of cancerous cells by modifying the genes related to apoptosis, cell proliferation and transcription factors (Trueba et al., 2004). ROS also upregulate antiapoptotic genes and downregulate proapoptotic proteins via PI3K/AKT and ERK/MEK pathways (McCubrey et al., 2007). In the progression stage of cancer development, ROS contribute to the upregulation of matrix metalloproteinases, inhibiting the action of anti-proteases and angiogenesis, eventually leading to metastasis (Maulik, 2002; Mori et al., 2004; Shinohara et al., 2010).
A depletion of endogenous antioxidants or a disruption of redox equilibrium may lead to cancer development. Research has shown that 35% of cancer can be prevented by dietary modifications (Doll and Peto, 1981; Rayman, 2005). Fruits and vegetables, which are rich in antioxidants, exert a protective effect against several different types of cancers (Soerjomataram et al., 2010; Turati et al., 2015). Plant foods that contain polyphenols have proven to be effective antioxidant agents for the body (Barrajon-Catalan et al., 2010; Paredes-Lopez et al., 2010; Sreelatha et al., 2012; Cordero-Herrera et al., 2013). They have been shown to possess anti-cancer activity which is effective against lung, breast, tongue, gastric, larynx, colon, and prostate cancers (Table 1) (Manikandan et al., 2012; Sak, 2014). Fruits containing higher phenolic content have stronger antioxidant properties since they can induce hydroxyl group substitution in the aromatic rings of phenol compounds (Sun et al., 2002; Rokayya et al., 2014). Polyphenols induce apoptosis of cancer cells, inhibit proliferation of mutated cells, reduce production of cyclooxygenase-2 (COX-2), and downregulate cancer gene expression (Gloria et al., 2014; Li et al., 2014; Xie et al., 2014; Zhang et al., 2015). Moreover, nutrients such as vitamins and minerals can reduce cancer risk by eliciting antioxidant action, inhibiting proliferation of cancerous cells, maintaining DNA methylation, and promoting cell-cycle arrest (Pathak et al., 2003; Rayman, 2005). In individuals previously treated for cancer, a healthy diet rich in fruits and vegetables can modify biologic markers of cancer progression (Jones and Demark-Wahnefried, 2006). Healthy plant foods have shown to reduce the risk of death after being diagnosed with breast (Vrieling et al., 2013; George et al., 2014), head and neck (Arthur et al., 2013), and rectal cancers (Pelser et al., 2014). A high vegetable diet has been shown to be effective in reducing breast cancer recurrence for patients on tamoxifen (Gold et al., 2009; Thomson et al., 2011).
Vitamins such as Vitamin A and E have a preventive effect against oral cancer (Garewal, 1995). Selected micronutrients (vitamin D, carnitine, and selenium) have been shown to improve compliance and prognosis, patients’ quality of life, and reduced adverse effects of cancer treatments (Block et al., 2008; Grober et al., 2015). However, limited evidence supports the effectiveness of vitamins and minerals in cancer prevention (Fortmann et al., 2013), and such nutritional regimens are not currently recommended for practice in healthy individuals (World Cancer Research Fund/American Institute for Cancer Research, 2007). Additionally, there is a lack of randomized control trials investigating diets and cancer due to difficulty in whole diet interventions as well as ethical issues in the proposed research (Norat et al., 2015). Hence, current recommendations are based on the effectiveness of a healthy diet (rich in fruits, vegetables, and grains, and low on red meat and alcohol) and lifestyle on reducing cancer risk (Norat et al., 2015).
It is well established that intestinal inflammation-associated OS plays an essential role in the pathophysiology of various gastrointestinal (GI) diseases, such as inflammatory bowel diseases (IBD) (Balmus et al., 2016). Although the exact etiology of IBD remains unclear, the underlying pathologies can be partially attributed to excess ROS formation (Zhu and Li, 2012; Bhattacharyya et al., 2014). Due to the presence of food particles, pathogens, or microbiota imbalance, the GI tract may become irritated, generating excess ROS and compromising endogenous antioxidant defenses (Moura et al., 2015). OS disrupts the intestinal epithelial barrier and increases intestinal permeability, further exacerbating inflammation (Figure 2) (Balmus et al., 2016). IBD, which is comprised of CD and ulcerative colitis (UC), is characterized by chronic and prominent inflammation associated with OS in the GI tract (Balmus et al., 2016). Elevated levels of pro-inflammatory mediators such as platelet activating factor (PAF) and leukotriene B4 (LTB4) observed in the mucosal samples from active IBD patients have been shown to trigger the release of cytotoxic reactive oxygen metabolites by overstimulating phagocytes (Ingraham et al., 1982; Sharon and Stenson, 1984; Wallace and Chin, 1997). Moreover, myeloperoxidases are released during the massive infiltration of polymorphonuclear neutrophils and macrophages into the inflamed mucosa, producing hypochlorous acid, a potent oxidizing agent, via the metabolism of H2O2. Other sources of ROS include enzymes such as cyclooxygenase, xanthine oxidase, and 5-lipoxygenase that reside in the intestinal mucosa (Alzoghaibi, 2013).
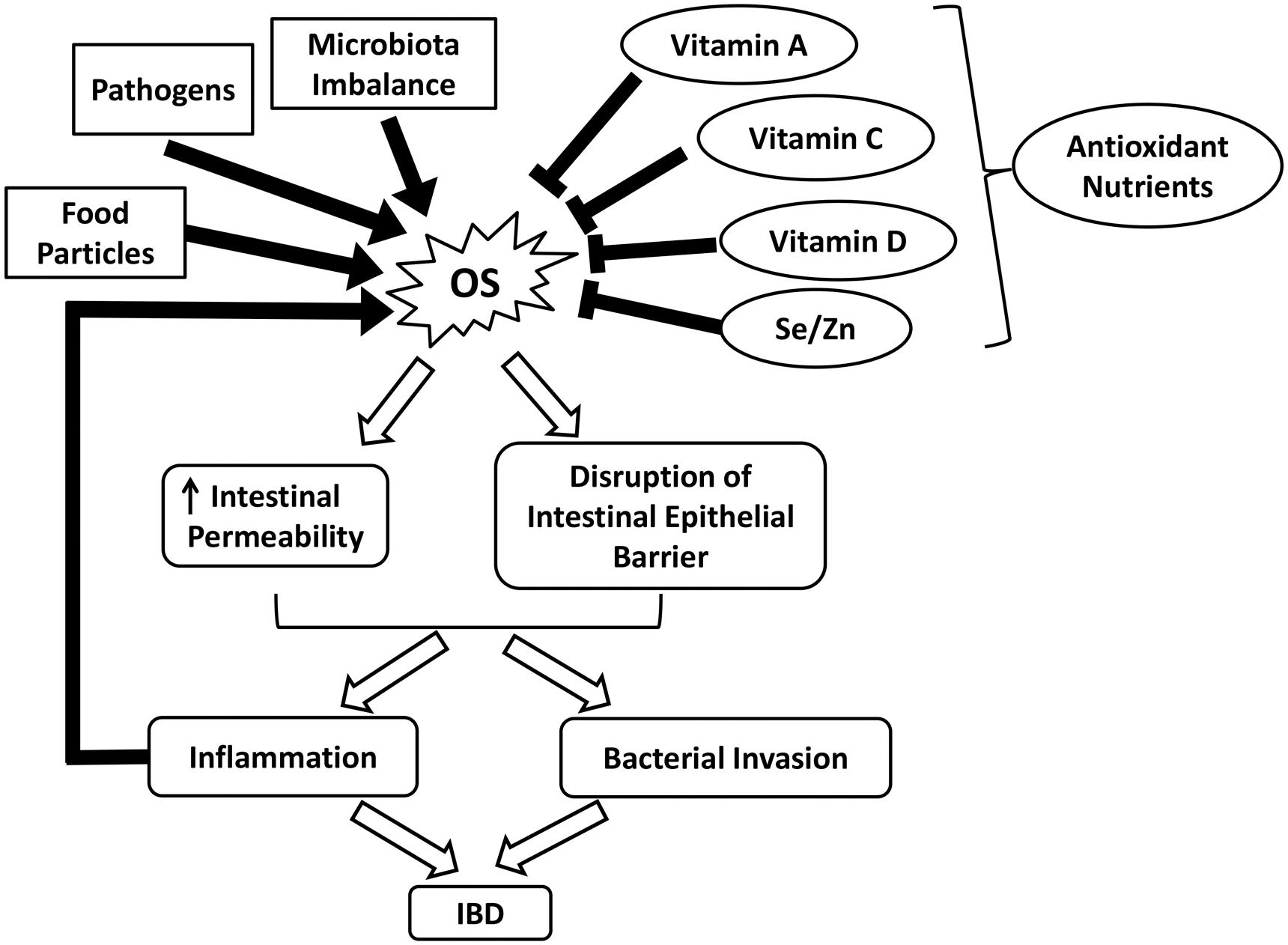
FIGURE 2. Schematic illustrating the roles of OS and nutrient antioxidants in IBD. IBD, inflammatory bowel diseases; OS, oxidative stress; Se, selenium; Zn, zinc.
Despite ROS overproduction, a deficiency in dietary and enzymatic antioxidants also contributes to the development of OS (Alzoghaibi, 2013). For example, low levels of enzymatic antioxidants and vitamins have been observed in patients with CD, which is partly due to malnutrition (Buffinton and Doe, 1995; Alzoghaibi, 2013). In malnourished IBD patients, the reduced dietary intakes of fruits and vegetables greatly influence the concentration of carotenoid (vitamin A) (Balmus et al., 2016). Vitamin C, which helps to repair and protect mucosal lining against detrimental insults, is depleted in peptic ulcers and gastritis (Aditi and Graham, 2012). Notably, the increased incidence of vitamin D deficiency in CD patients is highly associated with skeletal morbidity and a worsened quality of life (Figure 2) (van Hogezand and Hamdy, 2006; Alastair et al., 2011). Persistent OS can damage the intestinal barrier and increase the permeability of GI epithelium via lipid peroxidation and tight junction disruption. This alters the composition of commensal microbiota in the GI tract and interrupts their ability to establish colonization resistance, thus promoting the invasion of pathogenic bacteria (Buffie and Pamer, 2013; Moura et al., 2015). Such infections further aggravate ROS production and inflammation and potentially increase the risk of inflammatory bowel syndrome (Zhu and Li, 2012).
Considering a strong indication of ROS elevation in IBD and other GI diseases, the adjuvant or treatment potential of antioxidants are largely investigated. Antioxidant applications have been shown to restore redox balance, thereby attenuating intestinal damages and maintaining GI health (Bhattacharyya et al., 2014). For example, studies have shown that CuZn-SOD and 5-aminosalicylic acid effectively alleviate mucosal injuries in CD by scavenging or inducing rapid decomposition of ROS (Emerit et al., 1989; Couto et al., 2010; Alzoghaibi, 2013). In a randomized placebo-controlled study, 3 months of oral antioxidant supplementation markedly improved the serum antioxidant status in CD patients in remission. The combination of antioxidants with n-3 fatty acids further attenuated pro-inflammatory activities, thus serving as a potential treatment for CD (Geerling et al., 2000). Compared to supplements, dietary intakes of antioxidants from natural fruits and vegetables may be a safer approach to avoid overconsumption. Inappropriate antioxidant application can be harmful by scavenging of physiological ROS (Bjelakovic et al., 2004; Poljsak et al., 2013). Foods rich in micronutrients such as α-tocopherol (vitamin E) and minerals have been reported to be beneficial in alleviating ROS damage. For example, selenium and zinc interact with GPx and SOD, respectively, to combat OS. The combination of selenium and vitamin E has demonstrated protective effects against oxidative damage in the colon of UC rats (Figure 2 and Table 1) (Bitiren et al., 2010). Several functional foods may be beneficial for IBD without undesirable effects.
Free radical theory, which was first proposed by Harman in 1956, suggests that aging is process related with progressive and irreversible accumulation of oxidative damage in the cells (Harman, 1956; Mariani et al., 2005). A shift of redox balance toward a more oxidized status is noted in aging cells, as indicated by decreased GSH/GSSG ratio. This alteration of redox profile may blunt cellular capability of buffering ROS produced both under physiological conditions and in response to external stress (Kregel and Zhang, 2007). Excessive ROS accumulation can directly damage DNA, protein, and lipids, which disturbs normal cellular function (Zuo et al., 2015b). Mitochondrial DNA (mtDNA) is particularly susceptible to OS and the mutation of mtDNA has been closely linked with the aging process (Trifunovic et al., 2004). It was reported that mice with somatic mtDNA mutation exhibited an earlier onset of aging-related features such as hair loss, osteoporosis, and decreased subcutaneous fat as well as a shorter lifespan (Trifunovic et al., 2004). Exposure to high levels of ROS can also accelerate telomere shortening, which ultimately triggers cellular senescence (Kregel and Zhang, 2007). For example, fibroblast cells cultured under high OS showed increased rate of telomere shortening and a reduced lifespan (Vonzglinicki et al., 1995). Additionally, aging-associated OS could be responsible for the chronic systematic inflammation as commonly seen in the elderly via the activation of NF-κB (Chung et al., 2009). NF-κB is a key regulator for inflammatory factors such as tumor necrosis factor-alpha (TNF-α), interleukin (IL)-1β, and IL-6 (Chung et al., 2009). OS-induced NF-κB signaling is short-lived under normal conditions in contrast to chronic activation during aging (Chung et al., 2009). The persistent low-level inflammation could be responsible for the development of age-related diseases such as atherosclerosis, cancer, and dementia (Chung et al., 2009).
Aging population are at a higher risk of suffering from malnutrition due to a general decline in body function including decreased metabolic rate, digestive and absorptive capability (Brownie, 2006). Therefore, the elderly are more likely to be affected by diseases associated with nutritional inadequacy. For example, aging-related vitamin D deficiency has been shown to result in bone loss, susceptibility to fracture, and hyperparathyroidism (Lips, 2001). Therefore, appropriate supplementation with vitamin D can reduce the risk of hip and other fractures in housebound elderly (Table 1) (Lips, 2001). In recent years, focus on the diet has increased due to the diet being an essential source of exogenously obtained antioxidants. It appears that dietary antioxidants have the anti-aging activity by their ability to suppress the generation of free radicals (Kandola et al., 2015). Cognitive decline represents a major health concern in aging population (Kang et al., 2005). A key study by Kang et al. (2005) followed over ten thousand women from 1984–2003 to investigate the relationship between their dietary pattern and cognitive function. It was found that women who consumed more green leafy or cruciferous vegetables demonstrated the lowest cognitive decline; while fruit consumption did not affect their cognitive function (Kang et al., 2005). Interestingly, higher intake of green and yellow vegetables was also correlated with a slower rate of skin aging in Japanese women after adjustment for age, BMI, smoking status, and sun exposure (Nagata et al., 2010).
Energy restriction (ER) has recently been put up as a potential way to extend life expectancy. This was partially due to the favorable effects of ER on redox management. In fruit flies, ER diet significantly increased the expression of SOD1 and SOD2 as well as extended lifespan by 16% (Peng et al., 2014). Various natural antioxidants, nutraceuticals, and functional foods have been identified as free radical or progressive oxygen hunters. Therefore, functional foods and nutraceuticals which control the antioxidant activity may represent an important role in slowing the aging process (Peng et al., 2014). A diet rich in antioxidant has been shown to increase lifespan in animal models (Miquel, 2002; Peng et al., 2014). For instance, a diet supplemented blueberry extract was found to markedly improve the lifespan in fruit flies and Caenorhabditis elegans (Wilson et al., 2006; Peng et al., 2014). This was accompanied by an increased expression of SOD and catalase. The prolongevity induced by blueberry extract was not observed in SOD or catalase-mutated fruit flies. These results suggest that the beneficial effects of blueberry to extend lifespan are potentially linked with boosted endogenous antioxidant system (Peng et al., 2014). Other nutritional antioxidants including apple polyphenols, black rice anthocyanin extract, and black tea theaflavins all demonstrated prominent prolongevity effects by upregulating the endogenous antioxidant levels in animal models (Table 1) (Peng et al., 2014). Further research is needed to evaluate the potential effects of natural antioxidants on life expectancy in human beings.
The implication of OS in the etiology of several chronic and inflammatory diseases indicates that antioxidant-based therapy could be promising for these disorders. A therapeutic strategy that increases an individual’s antioxidant capacity may be useful for a long-term treatment. However, many problems remain elusive regarding antioxidant supplements in disease prevention. It remains to be elucidated about the precise roles of ROS in the pathogenesis of various diseases. Current recommendations are based on the intake of a healthy diet (rich in fruits, vegetables, and grains and low on red meat and alcohol) and healthy lifestyle, which has demonstrated the ability to reduce the risk for diseases. Further research is warranted before using antioxidant supplements as an adjuvant therapy. In the meantime, avoiding oxidant sources such as cigarette smoke and alcohol must be considered when taking dietary antioxidants.
LZ conceptualized and designed the review. ZL, ZR, and JZ summarized the literature and wrote the manuscript. LZ, EK, C-CC, and TZ critically revised the manuscript. TZ prepared the figures and abstract. All authors agreed to be accountable for the content of this work.
This study was supported by 2016 American Physiology Society S&R Foundation Ryuji Ueno Award.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Paige Henry, Alicia Simpson, and Denethi Wijegunawardana for their assistance during the manuscript preparation.
Aditi, A., and Graham, D. Y. (2012). Vitamin C, gastritis, and gastric disease: a historical review and update. Dig. Dis. Sci. 57, 2504–2515. doi: 10.1007/s10620-012-2203-7
Alastair, F., Emma, G., and Emma, P. (2011). Nutrition in inflammatory bowel disease. JPEN J. Parenter. Enteral Nutr. 35, 571–580. doi: 10.1177/0148607111413599
Allen, S., Britton, J. R., and Leonardi-Bee, J. A. (2009). Association between antioxidant vitamins and asthma outcome measures: systematic review and meta-analysis. Thorax 64, 610–619. doi: 10.1136/thx.2008.101469
Altenhofer, S., Radermacher, K. A., Kleikers, P. W., Wingler, K., and Schmidt, H. H. (2015). Evolution of NADPH oxidase inhibitors: selectivity and mechanisms for target engagement. Antioxid. Redox Signal. 23, 406–427. doi: 10.1089/ars.2013.5814
Alzoghaibi, M. A. (2013). Concepts of oxidative stress and antioxidant defense in Crohn’s disease. World J. Gastroenterol. 19, 6540–6547. doi: 10.3748/wjg.v19.i39.6540
Ambrose, J. A., and Barua, R. S. (2004). The pathophysiology of cigarette smoking and cardiovascular disease: an update. J. Am. Coll. Cardiol. 43, 1731–1737. doi: 10.1016/j.jacc.2003.12.047
Ames, B. N., and Wakimoto, P. (2002). Are vitamin and mineral deficiencies a major cancer risk? Nat. Rev. Cancer 2, 694–704. doi: 10.1038/Nrc886
Anekonda, T. S. (2006). Resveratrol–a boon for treating Alzheimer’s disease? Brain Res. Rev. 52, 316–326. doi: 10.1016/j.brainresrev.2006.04.004
Arab, L., and Steck, S. (2000). Lycopene and cardiovascular disease. Am. J. Clin. Nutr. 71, 1691s–1695s. doi: 10.1093/ajcn/71.6.1691S
Arthur, A. E., Peterson, K. E., Rozek, L. S., Taylor, J. M., Light, E., Chepeha, D. B., et al. (2013). Pretreatment dietary patterns, weight status, and head and neck squamous cell carcinoma prognosis. Am. J. Clin. Nutr. 97, 360–368. doi: 10.3945/ajcn.112.044859
Asplund, K. (2002). Antioxidant vitamins in the prevention of cardiovascular disease: a systematic review. J. Intern. Med. 251, 372–392. doi: 10.1046/j.1365-2796.2002.00973.x
Azumi, H., Inoue, N., Ohashi, Y., Terashima, M., Mori, T., Fujita, H., et al. (2002). Superoxide generation in directional coronary atherectomy specimens of patients with angina pectoris - Important role of NAD(P)H oxidase. Arterioscler. Thromb. Vasc. Biol. 22, 1838–1844. doi: 10.1161/01.Atv.0000037101.40667.62
Balmus, I. M., Ciobica, A., Trifan, A., and Stanciu, C. (2016). The implications of oxidative stress and antioxidant therapies in inflammatory bowel disease: clinical aspects and animal models. Saudi J. Gastroenterol. 22, 3–17. doi: 10.4103/1319-3767.173753
Barrajon-Catalan, E., Fernandez-Arroyo, S., Saura, D., Guillen, E., Fernandez-Gutierrez, A., Segura-Carretero, A., et al. (2010). Cistaceae aqueous extracts containing ellagitannins show antioxidant and antimicrobial capacity, and cytotoxic activity against human cancer cells. Food Chem. Toxicol. 48, 2273–2282. doi: 10.1016/j.fct.2010.05.060
Bellaver, B., Souza, D. G., Souza, D. O., and Quincozes-Santos, A. (2014). Resveratrol increases antioxidant defenses and decreases proinflammatory cytokines in hippocampal astrocyte cultures from newborn, adult and aged Wistar rats. Toxicol. In Vitro 28, 479–484. doi: 10.1016/j.tiv.2014.01.006
Bhattacharyya, A., Chattopadhyay, R., Mitra, S., and Crowe, S. E. (2014). Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 94, 329–354. doi: 10.1152/physrev.00040.2012
Bitiren, M., Karakilcik, A. Z., Zerin, M., Ozardali, I., Selek, S., Nazligul, Y., et al. (2010). Protective effects of selenium and vitamin E combination on experimental colitis in blood plasma and colon of rats. Biol. Trace Elem. Res. 136, 87–95. doi: 10.1007/s12011-009-8518-3
Bjelakovic, G., Nikolova, D., Simonetti, R. G., and Gluud, C. (2004). Antioxidant supplements for prevention of gastrointestinal cancers: a systematic review and meta-analysis. Lancet 364, 1219–1228. doi: 10.1016/S0140-6736(04)17138-9
Blesa, J., Trigo-Damas, I., Quiroga-Varela, A., and Jackson-Lewis, V. R. (2015). Oxidative stress and Parkinson’s disease. Front. Neuroanat. 9:91. doi: 10.3389/fnana.2015.00091
Block, K. I., Koch, A. C., Mead, M. N., Tothy, P. K., Newman, R. A., and Gyllenhaal, C. (2008). Impact of antioxidant supplementation on chemotherapeutic toxicity: a systematic review of the evidence from randomized controlled trials. Int. J. Cancer 123, 1227–1239. doi: 10.1002/ijc.23754
Bonnefont-Rousselot, D., and Collin, F. (2010). Melatonin: action as antioxidant and potential applications in human disease and aging. Toxicology 278, 55–67. doi: 10.1016/j.tox.2010.04.008
Brambilla, D., Mancuso, C., Scuderi, M. R., Bosco, P., Cantarella, G., Lempereur, L., et al. (2008). The role of antioxidant supplement in immune system, neoplastic, and neurodegenerative disorders: a point of view for an assessment of the risk/benefit profile. Nutr. J. 7:29. doi: 10.1186/1475-2891-7-29
Brown, N. S., and Bicknell, R. (2001). Hypoxia and oxidative stress in breast cancer - Oxidative stress: its effects on the growth, metastatic potential and response to therapy of breast cancer. Breast Cancer Res. 3, 323–327. doi: 10.1186/Bcr315
Browne, S. E., and Beal, M. F. (2006). Oxidative damage in Huntington’s disease pathogenesis. Antioxid. Redox Signal. 8, 2061–2073. doi: 10.1089/ars.2006.8.2061
Brownie, S. (2006). Why are elderly individuals at risk of nutritional deficiency? Int. J. Nurs. Pract. 12, 110–118. doi: 10.1111/j.1440-172X.2006.00557.x
Buffie, C. G., and Pamer, E. G. (2013). Microbiota-mediated colonization resistance against intestinal pathogens. Nat. Rev. Immunol. 13, 790–801. doi: 10.1038/nri3535
Buffinton, G. D., and Doe, W. F. (1995). Altered ascorbic acid status in the mucosa from inflammatory bowel disease patients. Free Radic. Res. 22, 131–143. doi: 10.3109/10715769509147535
Cachofeiro, V., Goicochea, M., De Vinuesa, S. G., Oubina, P., Lahera, V., and Luno, J. (2008). Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease. Kidney Int. 74, S4–S9. doi: 10.1038/ki.2008.516
Cho, Y. S., and Moon, H. B. (2010). The role of oxidative stress in the pathogenesis of asthma. Allergy Asthma Immunol. Res. 2, 183–187. doi: 10.4168/aair.2010.2.3.183
Chung, H. Y., Cesari, M., Anton, S., Marzetti, E., Giovannini, S., Seo, A. Y., et al. (2009). Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res. Rev. 8, 18–30. doi: 10.1016/j.arr.2008.07.002
Comhair, S. A., and Erzurum, S. C. (2010). Redox control of asthma: molecular mechanisms and therapeutic opportunities. Antioxid. Redox Signal. 12, 93–124. doi: 10.1089/ARS.2008.2425
Cordero-Herrera, I., Martin, M. A., Bravo, L., Goya, L., and Ramos, S. (2013). Epicatechin gallate induces cell death via p53 activation and stimulation of p38 and JNK in human colon cancer SW480 cells. Nutr. Cancer 65, 718–728. doi: 10.1080/01635581.2013.795981
Couto, D., Ribeiro, D., Freitas, M., Gomes, A., Lima, J. L., and Fernandes, E. (2010). Scavenging of reactive oxygen and nitrogen species by the prodrug sulfasalazine and its metabolites 5-aminosalicylic acid and sulfapyridine. Redox Rep. 15, 259–267. doi: 10.1179/135100010X12826446921707
Covarrubias-Pinto, A., Moll, P., Solis-Maldonado, M., Acuna, A. I., Riveros, A., Miro, M. P., et al. (2015). Beyond the redox imbalance: oxidative stress contributes to an impaired GLUT3 modulation in Huntington’s disease. Free Radic. Biol. Med. 89, 1085–1096. doi: 10.1016/j.freeradbiomed.2015.09.024
Davila, D., and Torres-Aleman, I. (2008). Neuronal death by oxidative stress involves activation of FOXO3 through a two-arm pathway that activates stress kinases and attenuates insulin-like growth factor I signaling. Mol. Biol. Cell 19, 2014–2025. doi: 10.1091/mbc.E07-08-0811
Dias, V., Junn, E., and Mouradian, M. M. (2013). The role of oxidative stress in Parkinson’s disease. J. Parkinsons Dis. 3, 461–491. doi: 10.3233/JPD-130230
Doll, R., and Peto, R. (1981). The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today. J. Natl. Cancer Inst. 66, 1191–1308. doi: 10.1093/jnci/66.6.1192
Emerit, J., Pelletier, S., Tosoni-Verlignue, D., and Mollet, M. (1989). Phase II trial of copper zinc superoxide dismutase (CuZnSOD) in treatment of Crohn’s disease. Free Radic. Biol. Med. 7, 145–149. doi: 10.1016/0891-5849(89)90005-1
Evatt, M. L., Delong, M. R., Khazai, N., Rosen, A., Triche, S., and Tangpricha, V. (2008). Prevalence of vitamin D insufficiency in patients with Parkinson disease and Alzheimer disease. Arch. Neurol. 65, 1348–1352. doi: 10.1001/archneur.65.10.1348
Ferreira, I. M., Brooks, D., White, J., and Goldstein, R. (2012). Nutritional supplementation for stable chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 12:CD000998. doi: 10.1002/14651858.CD000998.pub3
Ferreira, I. M., Verreschi, I. T., Nery, L. E., Goldstein, R. S., Zamel, N., Brooks, D., et al. (1998). The influence of 6 months of oral anabolic steroids on body mass and respiratory muscles in undernourished COPD patients. Chest 114, 19–28. doi: 10.1378/chest.114.1.19
Fitzpatrick, A. M., Teague, W. G., Holguin, F., Yeh, M., Brown, L. A., and Severe Asthma Research Program (2009). Airway glutathione homeostasis is altered in children with severe asthma: evidence for oxidant stress. J. Allergy Clin. Immunol. 123, 146.e8–152.e8. doi: 10.1016/j.jaci.2008.10.047
Forman, H. J., Ursini, F., and Maiorino, M. (2014). An overview of mechanisms of redox signaling. J. Mol. Cell Cardiol. 73, 2–9. doi: 10.1016/j.yjmcc.2014.01.018
Fortmann, S. P., Burda, B. U., Senger, C. A., Lin, J. S., and Whitlock, E. P. (2013). Vitamin and mineral supplements in the primary prevention of cardiovascular disease and cancer: an updated systematic evidence review for the U.S. Preventive Services Task Force. Ann. Intern. Med. 159, 824–834. doi: 10.7326/0003-4819-159-12-201312170-00729
Garewal, H. (1995). Antioxidants in oral cancer prevention. Am. J. Clin. Nutr. 62, 1410S–1416S. doi: 10.1093/ajcn/62.6.1410S
Geerling, B. J., Badart-Smook, A., Van Deursen, C., Van Houwelingen, A. C., Russel, M. G., Stockbrugger, R. W., et al. (2000). Nutritional supplementation with N-3 fatty acids and antioxidants in patients with Crohn’s disease in remission: effects on antioxidant status and fatty acid profile. Inflamm. Bowel Dis. 6, 77–84. doi: 10.1097/00054725-200005000-00002
George, S. M., Ballard-Barbash, R., Shikany, J. M., Caan, B. J., Freudenheim, J. L., Kroenke, C. H., et al. (2014). Better postdiagnosis diet quality is associated with reduced risk of death among postmenopausal women with invasive breast cancer in the women’s health initiative. Cancer Epidemiol. Biomarkers Prev. 23, 575–583. doi: 10.1158/1055-9965.EPI-13-1162
Gilgun-Sherki, Y., Melamed, E., and Offen, D. (2001). Oxidative stress induced-neurodegenerative diseases: the need for antioxidants that penetrate the blood brain barrier. Neuropharmacology 40, 959–975. doi: 10.1016/S0028-3908(01)00019-3
Gloria, N. F., Soares, N., Brand, C., Oliveira, F. L., Borojevic, R., and Teodoro, A. J. (2014). Lycopene and beta-carotene induce cell-cycle arrest and apoptosis in human breast cancer cell lines. Anticancer Res. 34, 1377–1386.
Gold, E. B., Pierce, J. P., Natarajan, L., Stefanick, M. L., Laughlin, G. A., Caan, B. J., et al. (2009). Dietary pattern influences breast cancer prognosis in women without hot flashes: the women’s healthy eating and living trial. J. Clin. Oncol. 27, 352–359. doi: 10.1200/JCO.2008.16.1067
Goodman, M., Bostick, R. M., Kucuk, O., and Jones, D. P. (2011). Clinical trials of antioxidants as cancer prevention agents: past, present, and future. Free Radic. Biol. Med. 51, 1068–1084. doi: 10.1016/j.freeradbiomed.2011.05.018
Grober, U., Kisters, K., and Adamietz, I. A. (2015). Vitamin D in oncology: update 2015. Med. Monatsschr. Pharm. 38, 512–516.
Gumral, N., Naziroglu, M., Ongel, K., Beydilli, E. D., Ozguner, F., Sutcu, R., et al. (2009). Antioxidant enzymes and melatonin levels in patients with bronchial asthma and chronic obstructive pulmonary disease during stable and exacerbation periods. Cell Biochem. Funct. 27, 276–283. doi: 10.1002/cbf.1569
Hakonen, A. H., Goffart, S., Marjavaara, S., Paetau, A., Cooper, H., Mattila, K., et al. (2008). Infantile-onset spinocerebellar ataxia and mitochondrial recessive ataxia syndrome are associated with neuronal complex I defect and mtDNA depletion. Hum. Mol. Genet. 17, 3822–3835. doi: 10.1093/hmg/ddn280
Harman, D. (1956). Aging - a theory based on free-radical and radiation-chemistry. J. Gerontol. 11, 298–300. doi: 10.1093/geronj/11.3.298
Harman, D. (2006). Alzheimer’s disease pathogenesis: role of aging. Ann. N. Y. Acad. Sci. 1067, 454–460. doi: 10.1196/annals.1354.065
Heidenreich, P. A., Trogdon, J. G., Khavjou, O. A., Butler, J., Dracup, K., Ezekowitz, M. D., et al. (2011). Forecasting the future of cardiovascular disease in the United States a policy statement from the American heart association. Circulation 123, 933–944. doi: 10.1161/CIR.0b013e31820a55f5
Holick, M. F., Binkley, N. C., Bischoff-Ferrari, H. A., Gordon, C. M., Hanley, D. A., Heaney, R. P., et al. (2011). Evaluation, treatment, and prevention of vitamin D deficiency: an endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 96, 1911–1930. doi: 10.1210/jc.2011-0385
Hoppe, P. P., and Krennrich, G. (2000). Bioavailability and potency of natural-source and all-racemic alpha-tocopherol in the human: a dispute. Eur. J. Nutr. 39, 183–193. doi: 10.1007/s003940070010
Hornikx, M., Van Remoortel, H., Lehouck, A., Mathieu, C., Maes, K., Gayan-Ramirez, G., et al. (2012). Vitamin D supplementation during rehabilitation in COPD: a secondary analysis of a randomized trial. Respir. Res. 13:84. doi: 10.1186/1465-9921-13-84
Ignarro, L. J., Balestrieri, M. L., and Napoli, C. (2007). Nutrition, physical activity, and cardiovascular disease: an update. Cardiovasc. Res. 73, 326–340. doi: 10.1016/j.cardiores.2006.06.030
Ingraham, L. M., Coates, T. D., Allen, J. M., Higgins, C. P., Baehner, R. L., and Boxer, L. A. (1982). Metabolic, membrane, and functional responses of human polymorphonuclear leukocytes to platelet-activating factor. Blood 59, 1259–1266.
Jenner, P. (2003). Oxidative stress in Parkinson’s disease. Ann. Neurol. 53, S26–S36. doi: 10.1002/ana.10483
Jiang, L., Diaz, P. T., Best, T. M., Stimpfl, J. N., He, F., and Zuo, L. (2014). Molecular characterization of redox mechanisms in allergic asthma. Ann. Allergy Asthma Immunol. 113, 137–142. doi: 10.1016/j.anai.2014.05.030
Jones, L. W., and Demark-Wahnefried, W. (2006). Diet, exercise, and complementary therapies after primary treatment for cancer. Lancet Oncol. 7, 1017–1026. doi: 10.1016/S1470-2045(06)70976-7
Kandola, K., Bowman, A., and Birch-Machin, M. A. (2015). Oxidative stress–a key emerging impact factor in health, ageing, lifestyle and aesthetics. Int. J. Cosmet Sci. 37(Suppl. 2), 1–8. doi: 10.1111/ics.12287
Kang, J. H., Ascherio, A., and Grodstein, F. (2005). Fruit and vegetable consumption and cognitive decline in aging women. Ann. Neurol. 57, 713–720. doi: 10.1002/ana.20476
Katakwar, P., Metgud, R., Naik, S., and Mittal, R. (2016). Oxidative stress marker in oral cancer: a review. J. Cancer Res. Ther. 12, 438–446. doi: 10.4103/0973-1482.151935
Khandrika, L., Kumar, B., Koul, S., Maroni, P., and Koul, H. K. (2009). Oxidative stress in prostate cancer. Cancer Lett. 282, 125–136. doi: 10.1016/j.canlet.2008.12.011
King, D. A., Cordova, F., and Scharf, S. M. (2008). Nutritional aspects of chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 5, 519–523. doi: 10.1513/pats.200707-092ET
Kohlmeier, L., and Hastings, S. B. (1995). Epidemiologic evidence of a role of carotenoids in cardiovascular-disease prevention. Am. J. Clin. Nutr. 62, 1370–1376. doi: 10.1093/ajcn/62.6.1370S
Kohlmeier, L., Kark, J. D., Gomezgracia, E., Martin, B. C., Steck, S. E., Kardinaal, A. F., et al. (1997). Lycopene and myocardial infarction risk in the EURAMIC Study. Am. J. Epidemiol. 146, 618–626. doi: 10.1093/oxfordjournals.aje.a009327
Kregel, K. C., and Zhang, H. J. (2007). An integrated view of oxidative stress in aging: basic mechanisms, functional effects, and pathological considerations. Am. J. Physiol. Regul. Integr. Comp. Physiol. 292, R18–R36. doi: 10.1152/ajpregu.00327.2006
Kris-Etherton, P. M., Lichtenstein, A. H., Howard, B. V., Steinberg, D., Witztum, J. L., et al. (2004). Antioxidant vitamin supplements and cardiovascular disease. Circulation 110, 637–641. doi: 10.1161/01.Cir.0000137822.39831.F1
Li, Y., Ma, C., Qian, M., Wen, Z., Jing, H., and Qian, D. (2014). Butein induces cell apoptosis and inhibition of cyclooxygenase2 expression in A549 lung cancer cells. Mol. Med. Rep. 9, 763–767. doi: 10.3892/mmr.2013.1850
Lin, M. T., and Beal, M. F. (2006). Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443, 787–795. doi: 10.1038/nature05292
Lips, P. (2001). Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr. Rev. 22, 477–501. doi: 10.1210/edrv.22.4.0437
Lodi, R., Hart, P. E., Rajagopalan, B., Taylor, D. J., Crilley, J. G., Bradley, J. L., et al. (2001). Antioxidant treatment improves in vivo cardiac and skeletal muscle bioenergetics in patients with Friedreich’s ataxia. Ann. Neurol. 49, 590–596. doi: 10.1002/ana.1001
Madamanchi, N. R., Vendrov, A., and Runge, M. S. (2005). Oxidative stress and vascular disease. Arterioscler. Thromb. Vasc. Biol. 25, 29–38. doi: 10.1161/01.ATV.0000150649.39934.13
Manikandan, R., Beulaja, M., Arulvasu, C., Sellamuthu, S., Dinesh, D., Prabhu, D., et al. (2012). Synergistic anticancer activity of curcumin and catechin: an in vitro study using human cancer cell lines. Microsc. Res. Tech. 75, 112–116. doi: 10.1002/jemt.21032
Margaritelis, N. V. (2016). Antioxidants as therapeutics in the intensive care unit: Have we ticked the redox boxes? Pharmacol. Res. 111, 126–132. doi: 10.1016/j.phrs.2016.06.004
Margaritelis, N. V., Cobley, J. N., Paschalis, V., Veskoukis, A. S., Theodorou, A. A., Kyparos, A., et al. (2016). Principles for integrating reactive species into in vivo biological processes: examples from exercise physiology. Cell. Signal. 28, 256–271. doi: 10.1016/j.cellsig.2015.12.011
Mariani, E., Polidori, M. C., Cherubini, A., and Mecocci, P. (2005). Oxidative stress in brain aging, neurodegenerative and vascular diseases: an overview. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 827, 65–75. doi: 10.1016/j.jchromb.2005.04.023
Masoli, M., Fabian, D., Holt, S., Beasley, R., and Global Initiative for Asthma (GINA) Program (2004). The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy 59, 469–478. doi: 10.1111/j.1398-9995.2004.00526.x
Maulik, N. (2002). Redox signaling of angiogenesis. Antioxid. Redox Signal. 4, 805–815. doi: 10.1089/152308602760598963
Mazo, N. A., Echeverria, V., Cabezas, R., Avila-Rodriguez, M., Aliev, G., Leszek, J., et al. (2017). Medicinal plants as protective strategies against Parkinson’s Disease. Curr. Pharm. Des. 23, 4180–4188 doi: 10.2174/1381612823666170316142803
McCubrey, J. A., Steelman, L. S., Chappell, W. H., Abrams, S. L., Wong, E. W., Chang, F., et al. (2007). Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim. Biophys. Acta 1773, 1263–1284. doi: 10.1016/j.bbamcr.2006.10.001
Miquel, J. (2002). Can antioxidant diet supplementation protect against age-related mitochondrial damage? Ann. N. Y. Acad. Sci. 959, 508–516. doi: 10.1111/j.1749-6632.2002.tb02120.x
Moreira, P. I., Zhu, X., Wang, X., Lee, H. G., Nunomura, A., Petersen, R. B., et al. (2010). Mitochondria: a therapeutic target in neurodegeneration. Biochim. Biophys. Acta 1802, 212–220. doi: 10.1016/j.bbadis.2009.10.007
Moreno-Macias, H., and Romieu, I. (2014). Effects of antioxidant supplements and nutrients on patients with asthma and allergies. J. Allergy Clin. Immunol. 133, 1237–1244; quiz 1245. doi: 10.1016/j.jaci.2014.03.020
Mori, K., Shibanuma, M., and Nose, K. (2004). Invasive potential induced under long-term oxidative stress in mammary epithelial cells. Cancer Res. 64, 7464–7472. doi: 10.1158/0008-5472.CAN-04-1725
Moura, F. A., De Andrade, K. Q., Dos Santos, J. C., Araujo, O. R., and Goulart, M. O. (2015). Antioxidant therapy for treatment of inflammatory bowel disease: Does it work? Redox Biol. 6, 617–639. doi: 10.1016/j.redox.2015.10.006
Mpandzou, G., Ait Ben Haddou, E., Regragui, W., Benomar, A., and Yahyaoui, M. (2016). Vitamin D deficiency and its role in neurological conditions: a review. Rev. Neurol. 172, 109–122. doi: 10.1016/j.neurol.2015.11.005
Muller, O., and Krawinkel, M. (2005). Malnutrition and health in developing countries. CMAJ 173, 279–286. doi: 10.1503/cmaj.050342
Murphy, M. P. (2014). Antioxidants as therapies: can we improve on nature? Free Radic. Biol. Med. 66, 20–23. doi: 10.1016/j.freeradbiomed.2013.04.010
Nagata, C., Nakamura, K., Wada, K., Oba, S., Hayashi, M., Takeda, N., et al. (2010). Association of dietary fat, vegetables and antioxidant micronutrients with skin ageing in Japanese women. Br. J. Nutr. 103, 1493–1498. doi: 10.1017/S0007114509993461
Navarro-Alarcon, M., and Cabrera-Vique, C. (2008). Selenium in food and the human body: a review. Sci. Total Environ. 400, 115–141. doi: 10.1016/j.scitotenv.2008.06.024
Norat, T., Scoccianti, C., Boutron-Ruault, M. C., Anderson, A., Berrino, F., Cecchini, M., et al. (2015). European code against cancer 4th edition: diet and cancer. Cancer Epidemiol. 39(Suppl. 1), S56–S66. doi: 10.1016/j.canep.2014.12.016
Pappert, E. J., Tangney, C. C., Goetz, C. G., Ling, Z. D., Lipton, J. W., Stebbins, G. T., et al. (1996). Alpha-tocopherol in the ventricular cerebrospinal fluid of Parkinson’s disease patients: dose-response study and correlations with plasma levels. Neurology 47, 1037–1042. doi: 10.1212/WNL.47.4.1037
Paredes-Lopez, O., Cervantes-Ceja, M. L., Vigna-Perez, M., and Hernandez-Perez, T. (2010). Berries: improving human health and healthy aging, and promoting quality life–a review. Plant Foods Hum. Nutr. 65, 299–308. doi: 10.1007/s11130-010-0177-1
Pathak, S. K., Sharma, R. A., and Mellon, J. K. (2003). Chemoprevention of prostate cancer by diet-derived antioxidant agents and hormonal manipulation (Review). Int. J. Oncol. 22, 5–13. doi: 10.3892/ijo.22.1.5
Patten, D. A., Germain, M., Kelly, M. A., and Slack, R. S. (2010). Reactive oxygen species: stuck in the middle of neurodegeneration. J. Alzheimers Dis. 20(Suppl. 2), S357–S367. doi: 10.3233/JAD-2010-100498
Pauwels, R. A., and Rabe, K. F. (2004). Burden and clinical features of chronic obstructive pulmonary disease (COPD). Lancet 364, 613–620. doi: 10.1016/S0140-6736(04)16855-4
Pelser, C., Arem, H., Pfeiffer, R. M., Elena, J. W., Alfano, C. M., Hollenbeck, A. R., et al. (2014). Prediagnostic lifestyle factors and survival after colon and rectal cancer diagnosis in the National Institutes of Health (NIH)-AARP Diet and Health Study. Cancer 120, 1540–1547. doi: 10.1002/cncr.28573
Peng, C., Wang, X., Chen, J., Jiao, R., Wang, L., Li, Y. M., et al. (2014). Biology of ageing and role of dietary antioxidants. Biomed Res. Int. 2014:831841. doi: 10.1155/2014/831841
Pham-Huy, L. A., He, H., and Pham-Huy, C. (2008). Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 4, 89–96.
Pimplikar, S. W., Nixon, R. A., Robakis, N. K., Shen, J., and Tsai, L. H. (2010). Amyloid-independent mechanisms in Alzheimer’s disease pathogenesis. J. Neurosci. 30, 14946–14954. doi: 10.1523/Jneurosci.4305-10.2010
Poljsak, B., Suput, D., and Milisav, I. (2013). Achieving the balance between ROS and antioxidants: when to use the synthetic antioxidants. Oxid. Med. Cell. Longev. 2013:956792. doi: 10.1155/2013/956792
Poulsen, H. E., Prieme, H., and Loft, S. (1998). Role of oxidative DNA damage in cancer initiation and promotion. Eur. J. Cancer Prev. 7, 9–16.
Proteggente, A. R., Pannala, A. S., Paganga, G., Van Buren, L., Wagner, E., Wiseman, S., et al. (2002). The antioxidant activity of regularly consumed fruit and vegetables reflects their phenolic and vitamin C composition. Free Radic. Res. 36, 217–233. doi: 10.1080/10715760290006484
Quinones, M., Miguel, M., and Aleixandre, A. (2013). Beneficial effects of polyphenols on cardiovascular disease. Pharmacol. Res. 68, 125–131. doi: 10.1016/j.phrs.2012.10.018
Rao, A. V., and Agarwal, S. (2000). Role of antioxidant lycopene in cancer and heart disease. J. Am. Coll. Nutr. 19, 563–569. doi: 10.1080/07315724.2000.1071895
Rayman, M. P. (2005). Selenium in cancer prevention: a review of the evidence and mechanism of action. Proc. Nutr. Soc. 64, 527–542. doi: 10.1079/Pns2005467
Reboul, E., Richelle, M., Perrot, E., Desmoulins-Malezet, C., Pirisi, V., and Borel, P. (2006). Bioaccessibility of carotenoids and vitamin E from their main dietary sources. J. Agric. Food Chem. 54, 8749–8755. doi: 10.1021/jf061818s
Rego, A. C., and Oliveira, C. R. (2003). Mitochondrial dysfunction and reactive oxygen species in excitotoxicity and apoptosis: implications for the pathogenesis of neurodegenerative diseases. Neurochem. Res. 28, 1563–1574. doi: 10.1023/A:1025682611389
Rogers, N. M., Seeger, F., Garcin, E. D., Roberts, D. D., and Isenberg, J. S. (2014). Regulation of soluble guanylate cyclase by matricellular thrombospondins: implications for blood flow. Front. Physiol. 5:134. doi: 10.3389/fphys.2014.00134
Rokayya, S., Li, C. J., Zhao, Y., Li, Y., and Sun, C. H. (2014). Cabbage (Brassica oleracea L. var. capitata) phytochemicals with antioxidant and anti-inflammatory potential. Asian Pac. J. Cancer Prev. 14, 6657–6662. doi: 10.7314/APJCP.2013.14.11.6657
Rotunno, M. S., and Bosco, D. A. (2013). An emerging role for misfolded wild-type SOD1 in sporadic ALS pathogenesis. Front. Cell. Neurosci. 7:253. doi: 10.3389/fncel.2013.00253
Sak, K. (2014). Site-specific anticancer effects of dietary flavonoid quercetin. Nutr. Cancer 66, 177–193. doi: 10.1080/01635581.2014.864418
Sano, M., Ernesto, C., Thomas, R. G., Klauber, M. R., Schafer, K., Grundman, M., et al. (1997). A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer’s disease. N. Engl. J. Med. 336, 1216–1222. doi: 10.1056/NEJM199704243361704
Schols, A. M., Ferreira, I. M., Franssen, F. M., Gosker, H. R., Janssens, W., Muscaritoli, M., et al. (2014). Nutritional assessment and therapy in COPD: a European respiratory society statement. Eur. Respir. J. 44, 1504–1520. doi: 10.1183/09031936.00070914
Sechi, G., Sechi, E., Fois, C., and Kumar, N. (2016). Advances in clinical determinants and neurological manifestations of B vitamin deficiency in adults. Nutr. Rev. 74, 281–300. doi: 10.1093/nutrit/nuv107
Sesso, H. D., Buring, J. E., Zhang, S. M., Norkus, E. P., and Gaziano, J. M. (2005). Dietary and plasma lycopene and the risk of breast cancer. Cancer Epidemiol. Biomarkers Prev. 14, 1074–1081. doi: 10.1158/1055-9965.EPI-04-0683
Sharon, P., and Stenson, W. F. (1984). Enhanced synthesis of leukotriene B4 by colonic mucosa in inflammatory bowel disease. Gastroenterology 86, 453–460.
Shay, K. P., Moreau, R. F., Smith, E. J., Smith, A. R., and Hagen, T. M. (2009). Alpha-lipoic acid as a dietary supplement: molecular mechanisms and therapeutic potential. Biochim. Biophys. Acta 1790, 1149–1160. doi: 10.1016/j.bbagen.2009.07.026
Shinohara, M., Adachi, Y., Mitsushita, J., Kuwabara, M., Nagasawa, A., Harada, S., et al. (2010). Reactive oxygen generated by NADPH oxidase 1 (Nox1) contributes to cell invasion by regulating matrix metalloprotease-9 production and cell migration. J. Biol. Chem. 285, 4481–4488. doi: 10.1074/jbc.M109.071779
Sies, H., Stahl, W., and Sevanian, A. (2005). Nutritional, dietary and postprandial oxidative stress. J. Nutr. 135, 969–972. doi: 10.1093/jn/135.5.969
Soerjomataram, I., Oomen, D., Lemmens, V., Oenema, A., Benetou, V., Trichopoulou, A., et al. (2010). Increased consumption of fruit and vegetables and future cancer incidence in selected European countries. Eur. J. Cancer 46, 2563–2580. doi: 10.1016/j.ejca.2010.07.026
Sosa, V., Moline, T., Somoza, R., Paciucci, R., Kondoh, H., and Me, L. L. (2013). Oxidative stress and cancer: an overview. Ageing Res. Rev. 12, 376–390. doi: 10.1016/j.arr.2012.10.004
Sreelatha, S., Dinesh, E., and Uma, C. (2012). Antioxidant properties of Rajgira (Amaranthus paniculatus) leaves and potential synergy in chemoprevention. Asian Pac. J. Cancer Prev. 13, 2775–2780. doi: 10.7314/APJCP.2012.13.6.2775
Stephens, N. G., Parsons, A., Schofield, P. M., Kelly, F., Cheeseman, K., and Mitchinson, M. J. (1996). Randomised controlled trial of vitamin E in patients with coronary disease: Cambridge heart antioxidant study (CHAOS). Lancet 347, 781–786. doi: 10.1016/S0140-6736(96)90866-1
Stucki, D. M., Ruegsegger, C., Steiner, S., Radecke, J., Murphy, M. P., Zuber, B., et al. (2016). Mitochondrial impairments contribute to Spinocerebellar ataxia type 1 progression and can be ameliorated by the mitochondria-targeted antioxidant MitoQ. Free Radic. Biol. Med. 97, 427–440. doi: 10.1016/j.freeradbiomed.2016.07.005
Sun, J., Chu, Y. F., Wu, X., and Liu, R. H. (2002). Antioxidant and antiproliferative activities of common fruits. J. Agric. Food Chem. 50, 7449–7454. doi: 10.1021/jf0207530
Tang, G. W. (2010). Bioconversion of dietary provitamin A carotenoids to vitamin A in humans. Am. J. Clin. Nutr. 91, 1468s–1473s. doi: 10.3945/ajcn.2010.28674G
Tecklenburg, S. L., Mickleborough, T. D., Fly, A. D., Bai, Y., and Stager, J. M. (2007). Ascorbic acid supplementation attenuates exercise-induced bronchoconstriction in patients with asthma. Respir. Med. 101, 1770–1778. doi: 10.1016/j.rmed.2007.02.014
Thomson, C. A., Rock, C. L., Thompson, P. A., Caan, B. J., Cussler, E., Flatt, S. W., et al. (2011). Vegetable intake is associated with reduced breast cancer recurrence in tamoxifen users: a secondary analysis from the Women’s Healthy Eating and Living Study. Breast Cancer Res. Treat. 125, 519–527. doi: 10.1007/s10549-010-1014-9
Trifunovic, A., Wredenberg, A., Falkenberg, M., Spelbrink, J. N., Rovio, A. T., Bruder, C. E., et al. (2004). Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 429, 417–423. doi: 10.1038/nature02517
Trueba, G. P., Sanchez, G. M., and Giuliani, A. (2004). Oxygen free radical and antioxidant defense mechanism in cancer. Front. Biosci. 9, 2029–2044. doi: 10.2741/1335
Tsagalioti, E., Trifonos, C., Morari, A., Vadikolias, K., and Giaginis, C. (2016). Clinical value of nutritional status in neurodegenerative diseases: What is its impact and how it affects disease progression and management? Nutr. Neurosci. 21, 162–175. doi: 10.1080/1028415X.2016.1261529
Turati, F., Rossi, M., Pelucchi, C., Levi, F., and La Vecchia, C. (2015). Fruit and vegetables and cancer risk: a review of southern European studies. Br. J. Nutr. 113(Suppl. 2), S102–S110. doi: 10.1017/S0007114515000148
Uttara, B., Singh, A. V., Zamboni, P., and Mahajan, R. T. (2009). Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 7, 65–74. doi: 10.2174/157015909787602823
Valko, M., Rhodes, C. J., Moncol, J., Izakovic, M., and Mazur, M. (2006). Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 160, 1–40. doi: 10.1016/j.cbi.2005.12.009
van Eeden, S. F., and Sin, D. D. (2013). Oxidative stress in chronic obstructive pulmonary disease: a lung and systemic process. Can. Respir. J. 20, 27–29. doi: 10.1155/2013/509130
van Hogezand, R. A., and Hamdy, N. A. (2006). Skeletal morbidity in inflammatory bowel disease. Scand. J. Gastroenterol. Suppl. 41, 59–64. doi: 10.1080/00365520600664276
Varraso, R., Chiuve, S. E., Fung, T. T., Barr, R. G., Hu, F. B., Willett, W. C., et al. (2015). Alternate Healthy Eating Index 2010 and risk of chronic obstructive pulmonary disease among US women and men: prospective study. BMJ 350:h286. doi: 10.1136/bmj.h286
Vita, J. A. (2005). Polyphenols and cardiovascular disease: effects on endothelial and platelet function. Am. J. Clin. Nutr. 81, 292s–297s. doi: 10.1093/ajcn/81.1.292S
Vivekananthan, D. P., Penn, M. S., Sapp, S. K., Hsu, A., and Topol, E. J. (2003). Use of antioxidant vitamins for the prevention of cardiovascular disease: meta-analysis of randomised trials. Lancet 362, 922–922. doi: 10.1016/S0140-6736(03)13637-9
Vonzglinicki, T., Saretzki, G., Docke, W., and Lotze, C. (1995). Mild hyperoxia shortens telomeres and inhibits proliferation of fibroblasts - a model for senescence. Exp. Cell Res. 220, 186–193. doi: 10.1006/excr.1995.1305
Vrieling, A., Buck, K., Seibold, P., Heinz, J., Obi, N., Flesch-Janys, D., et al. (2013). Dietary patterns and survival in German postmenopausal breast cancer survivors. Br. J. Cancer 108, 188–192. doi: 10.1038/bjc.2012.521
Wallace, J. L., and Chin, B. C. (1997). Inflammatory mediators in gastrointestinal defense and injury. Proc. Soc. Exp. Biol. Med. 214, 192–203. doi: 10.3181/00379727-214-44087
Wang, J., Yang, D., Yu, Y., Shao, G., and Wang, Q. (2016). Vitamin D and sunlight exposure in newly-diagnosed Parkinson’s disease. Nutrients 8:142. doi: 10.3390/nu8030142
Wang, X., Ouyang, Y. Y., Liu, J., Zhu, M. M., Zhao, G., Bao, W., et al. (2014a). Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: systematic review and dose-response meta-analysis of prospective cohort studies. BMJ 349:g4490. doi: 10.1136/bmj.g4490
Wang, X., Wang, W., Li, L., Perry, G., Lee, H. G., and Zhu, X. W. (2014b). Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. Biochim. Biophys. Acta 1842, 1240–1247. doi: 10.1016/j.bbadis.2013.10.015
Wells, P. G., Mccallum, G. P., Chen, C. S., Henderson, J. T., Lee, C. J., Perstin, J., et al. (2009). Oxidative stress in developmental origins of disease: teratogenesis, neurodevelopmental deficits, and cancer. Toxicol. Sci. 108, 4–18. doi: 10.1093/toxsci/kfn263
Wilson, M. A., Shukitt-Hale, B., Kalt, W., Ingram, D. K., Joseph, J. A., and Wolkow, C. A. (2006). Blueberry polyphenols increase lifespan and thermotolerance in Caenorhabditis elegans. Aging Cell 5, 59–68. doi: 10.1111/j.1474-9726.2006.00192.x
Winter, A. N., Ross, E. K., Wilkins, H. M., Stankiewicz, T. R., Wallace, T., Miller, K., et al. (2017). An anthocyanin-enriched extract from strawberries delays disease onset and extends survival in the hSOD1G93A mouse model of amyotrophic lateral sclerosis. Nutr. Neurosci. doi: 10.1080/1028415X.2017.1297023 [Epub ahead of print].
Winterbourn, C. C., and Hampton, M. B. (2008). Thiol chemistry and specificity in redox signaling. Free Radic. Biol. Med. 45, 549–561. doi: 10.1016/j.freeradbiomed.2008.05.004
Wood, L. G., Garg, M. L., Smart, J. M., Scott, H. A., Barker, D., and Gibson, P. G. (2012). Manipulating antioxidant intake in asthma: a randomized controlled trial. Am. J. Clin. Nutr. 96, 534–543. doi: 10.3945/ajcn.111.032623
World Cancer Research Fund/American Institute for Cancer Research (2007). Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. Washington, DC: AICR.
Xie, Q., Bai, Q., Zou, L. Y., Zhang, Q. Y., Zhou, Y., Chang, H., et al. (2014). Genistein inhibits DNA methylation and increases expression of tumor suppressor genes in human breast cancer cells. Genes Chromosomes Cancer 53, 422–431. doi: 10.1002/gcc.22154
Yan, M. H., Wang, X., and Zhu, X. (2013). Mitochondrial defects and oxidative stress in Alzheimer disease and Parkinson disease. Free Radic. Biol. Med. 62, 90–101. doi: 10.1016/j.freeradbiomed.2012.11.014
Zaidi, S. M., and Banu, N. (2004). Antioxidant potential of vitamins A, E and C in modulating oxidative stress in rat brain. Clin. Chim. Acta 340, 229–233. doi: 10.1016/j.cccn.2003.11.003
Zandi, P. P., Anthony, J. C., Khachaturian, A. S., Stone, S. V., Gustafson, D., Tschanz, J. T., et al. (2004). Reduced risk of Alzheimer disease in users of antioxidant vitamin supplements: the Cache County Study. Arch. Neurol. 61, 82–88. doi: 10.1001/archneur.61.1.82
Zhang, Y. J., Gan, R. Y., Li, S., Zhou, Y., Li, A. N., Xu, D. P., et al. (2015). Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules 20, 21138–21156. doi: 10.3390/molecules201219753
Zhou, T., Prather, E. R., Garrison, D. E., and Zuo, L. (2018). Interplay between ROS and antioxidants during ischemia-reperfusion injuries in cardiac and skeletal muscle. Int. J. Mol. Sci. 19:E417. doi: 10.3390/ijms19020417
Zhu, H., and Li, Y. R. (2012). Oxidative stress and redox signaling mechanisms of inflammatory bowel disease: updated experimental and clinical evidence. Exp. Biol. Med. 237, 474–480. doi: 10.1258/ebm.2011.011358
Zino, S., Skeaff, M., Williams, S., and Mann, J. (1997). Randomised controlled trial of effect of fruit and vegetable consumption on plasma concentrations of lipids and antioxidants. BMJ 314, 1787–1791. doi: 10.1136/bmj.314.7097.1787
Zuo, L., Hemmelgarn, B. T., Chuang, C. C., and Best, T. M. (2015a). The role of oxidative stress-induced epigenetic alterations in amyloid-beta production in Alzheimer’s disease. Oxid. Med. Cell. Longev. 2015:604658. doi: 10.1155/2015/604658
Zuo, L., and Motherwell, M. S. (2013). The impact of reactive oxygen species and genetic mitochondrial mutations in Parkinson’s disease. Gene 532, 18–23. doi: 10.1016/j.gene.2013.07.085
Zuo, L., Pannell, B. K., and Liu, Z. (2016). Characterization and redox mechanism of asthma in the elderly. Oncotarget 7, 25010–25021. doi: 10.18632/oncotarget.7075
Keywords: antioxidants, cancer, GI diseases, neurodegenerative diseases, oxidative stress, respiratory diseases, vitamins
Citation: Liu Z, Ren Z, Zhang J, Chuang C-C, Kandaswamy E, Zhou T and Zuo L (2018) Role of ROS and Nutritional Antioxidants in Human Diseases. Front. Physiol. 9:477. doi: 10.3389/fphys.2018.00477
Received: 02 February 2018; Accepted: 16 April 2018;
Published: 17 May 2018.
Edited by:
Murugesan Velayutham, University of Pittsburgh, United StatesReviewed by:
Michalis G. Nikolaidis, Aristotle University of Thessaloniki, GreeceCopyright © 2018 Liu, Ren, Zhang, Chuang, Kandaswamy, Zhou and Zuo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Zuo, enVvLjRAb3N1LmVkdQ==
†These authors have contributed equally to this work.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.