- 1Department of Traditional Chinese Medicine, Nanjing Drum Tower Hospital, The Affiliated Hospital of Nanjing University Medical School, Nanjing, China
- 2Department of General Surgery, Affiliated Hospital of Nanjing Medical University, Changzhou Second People's Hospital, Changzhou, China
- 3Department of General Surgery, Wuxi Third People's Hospital, Wuxi, China
Tumor necrosis factor (TNF)-α, a major part in inflammatory, infectious and tumor processes, and is pivotal at the early stages of gastric cancer. Relationship between its risk and TNF-α rs361525 polymorphism has been demonstrated, but remains conflicting and controversial. Therefore, a meta-analysis was conducted to more precisely estimate this relationship. PubMed, Web of Science, EMBASE and CNKI were comprehensively searched to find out relevant articles through October 5, 2017. The strength of the relationship was assessed using pooled odds ratios and 95% confidence intervals. Totally 20 articles were included involving 4,084 cases and 7,010 controls. No significant relationship between TNF-α rs361525 polymorphism and increased GC risk was found in the whole populations. Subgroup analyses uncovered TNF-α rs361525 polymorphism intensified the risk of GC among Asians under five models, but decreased the risk of GC among Caucasiansin the allelic and dominant models. Subgroup analysis by genotyping methods revealed increased risk for other methods. In conclusion, this meta-analysis suggests TNF-α rs361525 polymorphism is related to the risk of GC, especially for Asians.
Introduction
Gastric cancer (GC) is the fourth major malignancy and the second dominant cause of cancer-induced death in the world (de Martel et al., 2012). In 2017, 28,000 new cases and 10,960 deaths of GC were projected to occur in the United States (Siegel et al., 2017). Helicobacter pylori infection contributes to causing the progression of chronic inflammation to GC (Fox and Wang, 2007). Some studies demonstrated the extremely low risk of developing GC in H. pylori- negative subjects (Uemura et al., 2002). However, nearly all H. pylori-positive subjects have chronic gastritis, and only 1–2% develop to GC. Therefore, other factors such as genetic factors and lifestyle may play important roles in the gastric tumorigenesis (Carcas, 2014).
Tumor necrosis factor (TNF)-α belonging to the TNF/TNF receptor cytokine superfamily can be found in plasma or serum of healthy people, as well as some cancer patients (Balkwill, 2006). TNF-α production by tumors is related with hormone irresponsiveness, poor prognosis, and cachexia/asthenia (Szlosarek and Balkwill, 2003; Tisdale, 2004). The TNF-α-blocked experimental mice were resistant to skin and colorectal cancer occurrence (Moore et al., 1999; Popivanova et al., 2008). (Oshima et al., 2014). The TNF-α/TNFR1 signaling could promote GC occurrence by inducing NADPH oxidase organizer 1 (Noxo1) and G protein subunit alpha 14 (GNA14), which are crucial in the tumorigenicity and stemness of GC cells, in tumor cells (Oshima et al., 2014). The above observations suggest TNF-α plays important roles in the etiology of GC.
TNF-α located in chromosome 2 has four exon counts. The gene contains several polymorphic sites, including the widely-studied TNF-α-238 (rs361525) and−308 (rs1800629). Some studies demonstrated the relationship between TNF-α rs361525 polymorphism and GC risk (Zambon et al., 2005; Xing et al., 2006; Zeng et al., 2007; Bai et al., 2009; Yin et al., 2012), but other studies have found no such relationship (Jang et al., 2001; Wu et al., 2002, 2003, 2004; Glas et al., 2004; Lee et al., 2004; Lu et al., 2005; Kamangar et al., 2006; Garcia-Gonzalez et al., 2007; Hou et al., 2007; Crusius et al., 2008; Yang et al., 2009; Whiteman et al., 2010; Essadik et al., 2015; Xu et al., 2017). This inconsistency may be attributed to weak statistical power, small sample size, and clinical heterogeneity. Therefore, a meta-analysis was conducted to overcome the limitations of individual studies and clarify whether TNF-α rs361525 polymorphism conferred susceptibility to GC.
Materials and Methods
Literature Search
Two investigators systematically searched PubMed, Elsevier, EMBASE, and CNKI through Oct 5, 2017 to identify relevant studies using the following search terms: “Gastric Neoplasm,” “Stomach Cancer,” “Gastric Cancer,” “Gastric Carcinoma,” “Gastric Adenocarcinoma,” “tumor necrosis factor alpha,” “TNF-α,” “polymorphism,” “SNP,” and “variant.” In addition, all cited references were reviewed to find out studies that were not included in the above electronic databases. When two studies overlapped, we chose the latest study or the one with larger sample size. There was no restriction on language, ethnicity or region of study population. GC was diagnosed according to classification criteria.
Inclusion and Exclusion Criteria
Inclusion criteria were: (1) evaluation of relationship between GC risk and TNF-α rs361525 polymorphism; (2) study on humans; (3) provision of enough data for computation of odds ratios (ORs) and 95% confidence intervals (CIs); (4) case- control study. Exclusion criteria were: (1) duplication; (2) case report or review; (3) lack of genotype data; (4) irrelevant topic.
Data Extraction and Quality Assessment
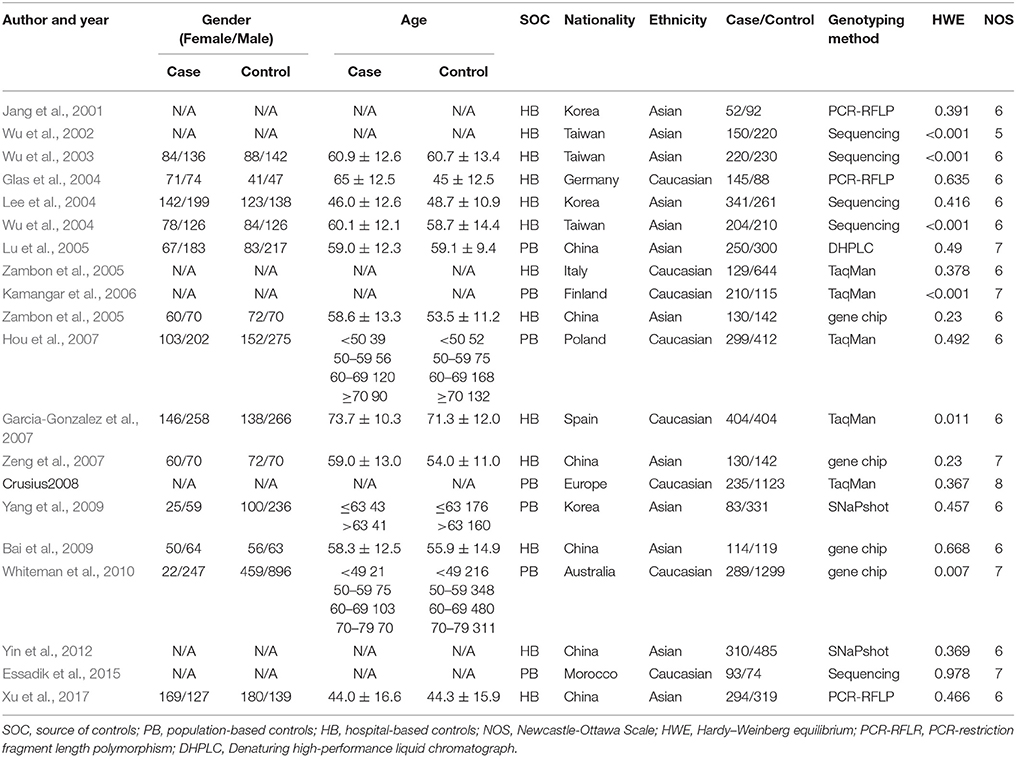
From each included study, data including name of first author, country of origin, publication year, ethnicity, age, and genotype numbers in cases and controls was extracted. When more than one ethnicity were involved, genotype data was processed separately. Data extraction and study quality assessment based on the Newcastle-Ottawa Scale (NOS) (Stang, 2010) were conducted by two investigators independently. The NOS score varies from 0 up to 9: high-quality study: >7; medium-quality study: 4–6; poor-quality study: <4. All conflicting information was discussed and resolved with consensus.
Genotype and Gene Expression Correlation Analysis
Genotype data of TNF-α rs361525 polymorphism and its mRNA expression data were available from the International HapMap Project and GTex portal (https://www.gtexportal.org/home/), respectively (Gong et al., 2017).
Statistical Analysis
The strength of relationship between GC risk and TNF-α rs361525 polymorphism was investigated by using crude ORs and 95%CIs. The following comparisons for this relationship were made: the dominant model (AA+GA vs. GG), the recessive model (AA vs. GA+GG), the heterozygote model (GA vs. GG), the homozygote model (AA vs. GG), and the allele model (A vs. G). Stratification analyses were carried out by ethnicity, source of control (SOC), Hardy-Weinberg equilibrium (HWE), genotyping method and NOS score (He et al., 2014). The null hypothesis that all studies evaluated the same effect was tested by Cochran's Q-statistics. When significant heterogeneity was found (P > 0.10 or I2 > 50%), a random-effect model was used; otherwise, a fixed-effect model was applied (Higgins and Thompson, 2002). Sensitivity analysis was conducted by omitting one study at a time to test the relative influence on the pooled estimate. The significant findings were evaluated by calculating false-positive report probability (FPRP). An FPRP threshold of 0.2 and a prior probability of 0.1 were set to detect an OR for a correlation with the tested genotype. FPRP <0.2 implied a significant relationship (He et al., 2013). χ2 test was carried out to clarify whether the observed genotype frequencies conformed to the HWE. Potential publication bias was examined by Begger's and Egger's linear regression tests (Wassen and Jertborn, 2006), with the significant level at P < 0.05. All statistical analyses were conducted on Stata 11.0 (StataCorp, College Station, USA).
Results
Study Characteristics
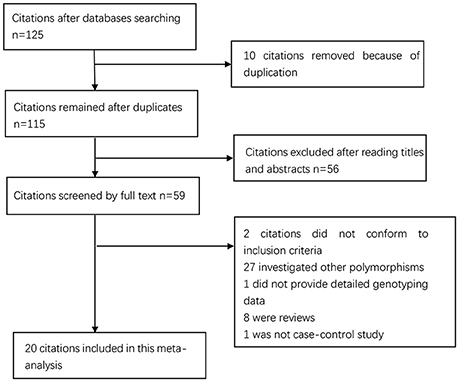
The process of study selection is shown in Figure 1. The initial search returned 125 studies. Of them, 10 duplicates and 56 papers unrelated to the topic based on their abstracts and titles were excluded. Among the remaining 59 papers: 2 papers did not meet inclusion criteria; 27 papers investigated other polymorphisms; 1 paper 1 paper was not case-control study; 1 paper did not provide enough data. Finally, 20 4,084 cases and 7,010 controls were included. Of them, 12 articles were from Asian populations (Jang et al., 2001; Wu et al., 2002, 2003, 2004; Lee et al., 2004; Lu et al., 2005; Xing et al., 2006; Zeng et al., 2007; Bai et al., 2009; Yang et al., 2009; Yin et al., 2012; Xu et al., 2017) and 8 from Caucasian populations (Glas et al., 2004; Zambon et al., 2005; Kamangar et al., 2006; Garcia-Gonzalez et al., 2007; Hou et al., 2007; Crusius et al., 2008; Whiteman et al., 2010; Essadik et al., 2015). Six studies failed to obey HWE (Wu et al., 2002, 2003, 2004; Kamangar et al., 2006; Garcia-Gonzalez et al., 2007; Whiteman et al., 2010). The details of the included studies are presented in Table 1. The year of publication ranged from 2001 to 2012. The numbers of cases and controls ranged from 52 to 404 and from 74 to 1,299, respectively.
Quantitative Analysis
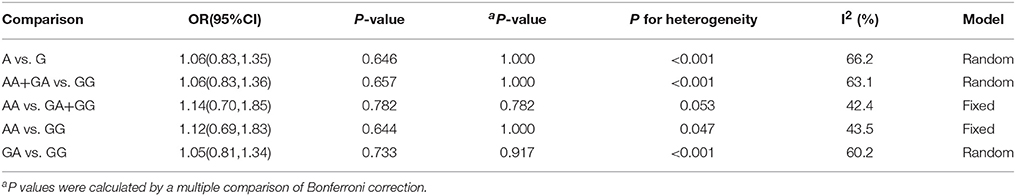
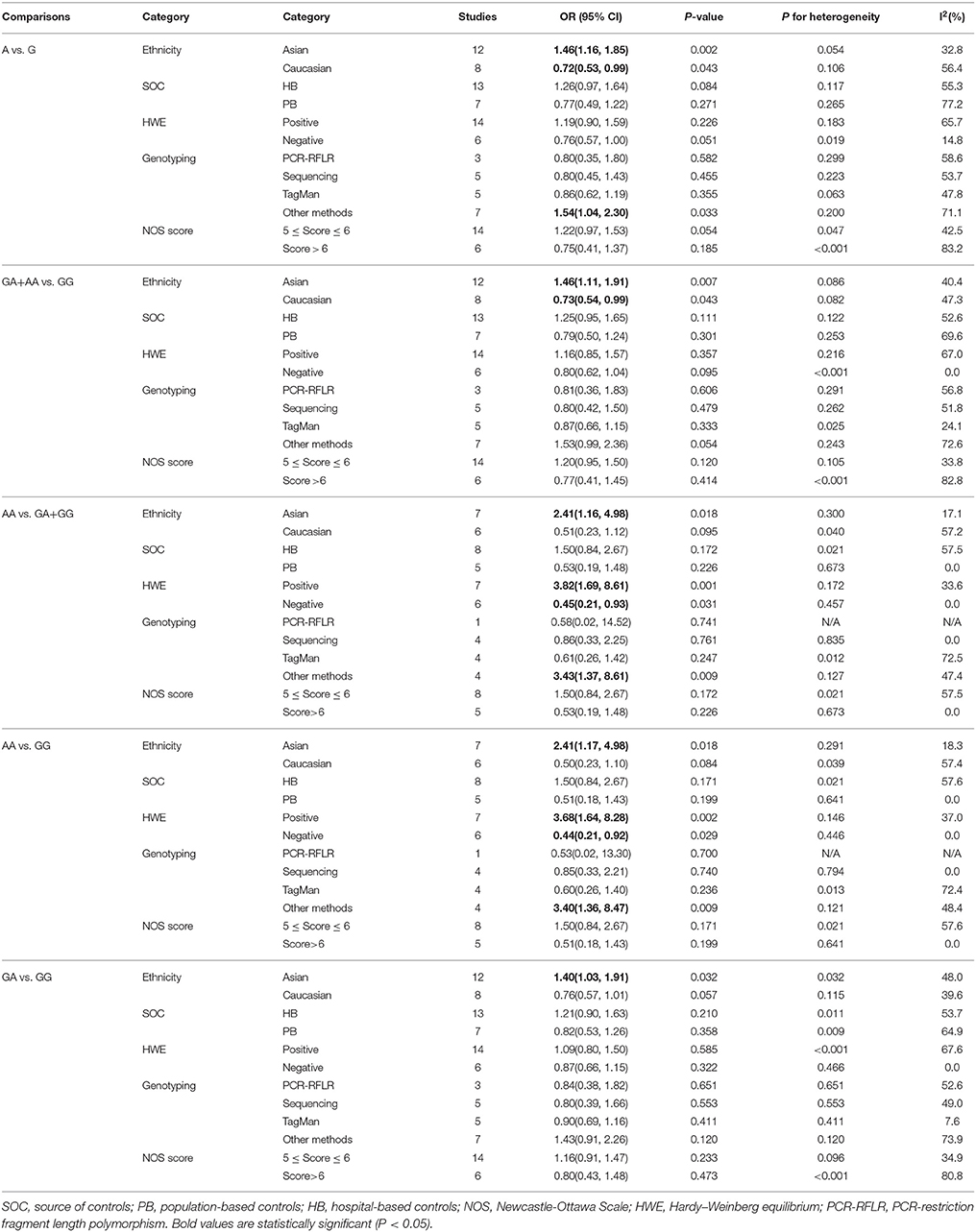
The results concerning the relationship between TNF-α rs361525 polymorphism and GC risk were summarized in Tables 2, 3. This relationship was insignificant in the overall population (A vs. G: OR, 1.06; 95%CI, 0.83–1.35, P = 0.646, Figure 2). Stratification analyses of ethnicity indicated TNF-α rs361525 polymorphism intensified the risk of GC among Asians in most of the comparisons (AA+GA vs. GG: OR, 1.46; 95%CI, 1.11–1.91, P = 0.007, Figure 3), but decreased the risk among Caucasians in the allele and dominant models (AA+GA vs. GG: OR, 0.73; 95% CI, 0.54–0.99, P = 0.043, Table 3). Subgroup analyses by genotyping methods revealed increased risk for other methods (A vs. G: OR, 1.54; 95%CI, 1.04–2.30, P = 0.033, Figure 4) and this relationship also held true in the HWE-positive studies. Stratified analysis by SOC did not find significant correlation in the hospital- or population-based studies (Table 3). Similar results were observed in the subgroup analysis of NOS score.
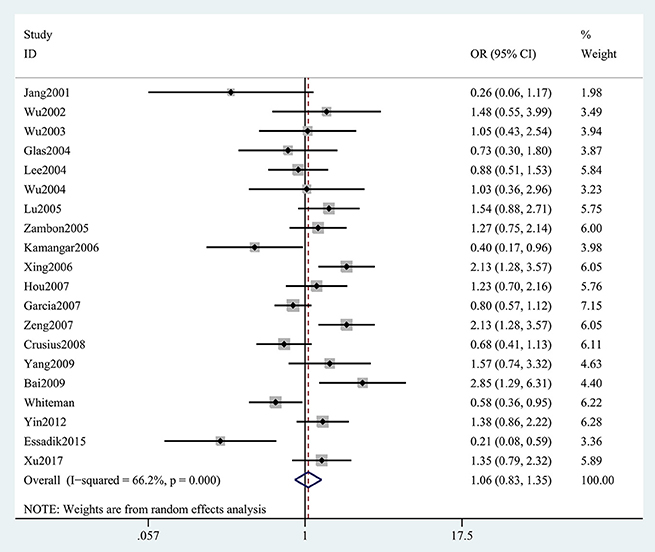
Figure 2. Forest plot shows odds ratio for the association between TNF-α rs361525 polymorphism and GC risk (A vs. G).
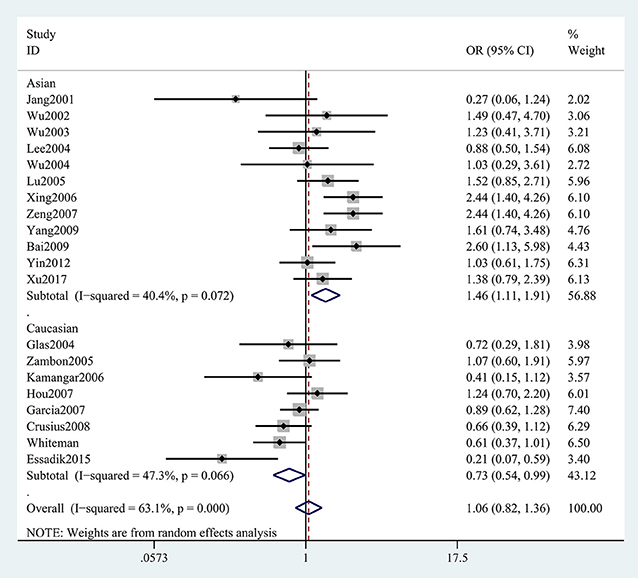
Figure 3. Stratification analyses of ethnicity between TNF-α rs361525 polymorphism and GC risk (AA+GA vs. GG).
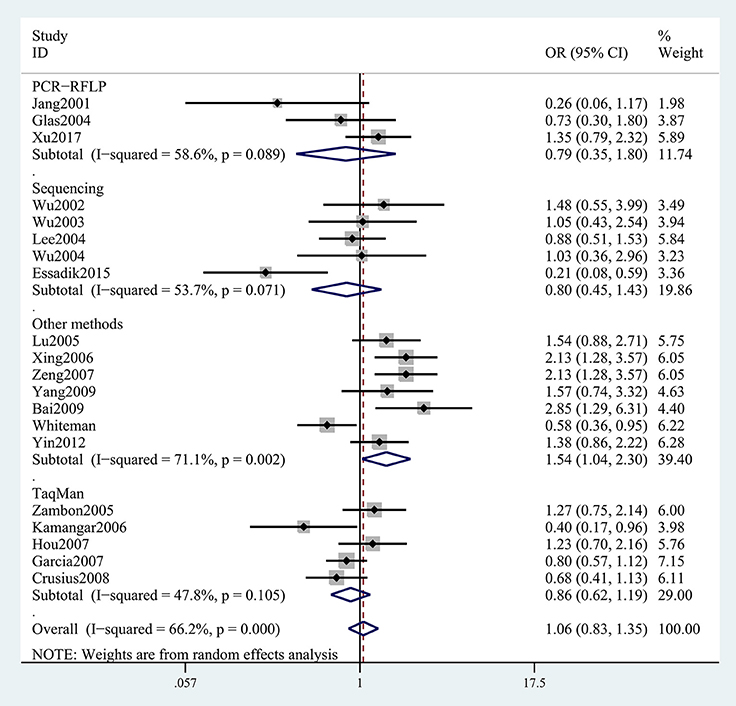
Figure 4. Stratification analyses of genotyping methods between TNF-α rs361525 polymorphism and GC risk (A vs. G).
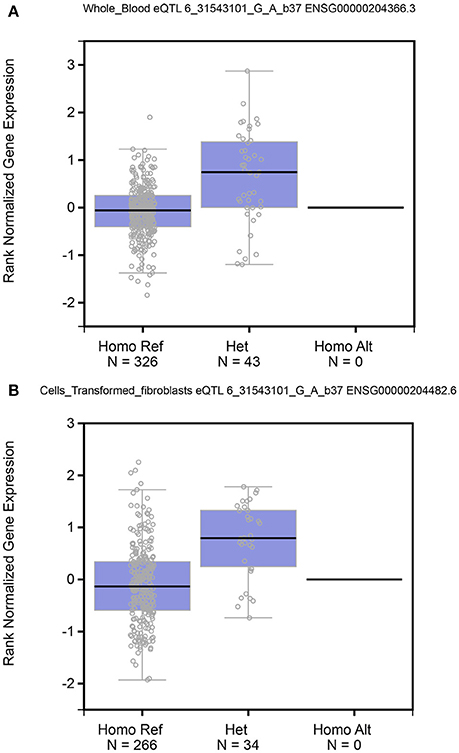
TNF-α mRNA Expression by Genotypes
The TNF-α mRNA expression levels by the genotypes of rs361525 polymorphism were significantly different for the whole blood (P = 5.10 × 10−14) and transformed fibroblasts (P = 6.65 × 10−10) (Figure 5).
Sensitivity and Publication Bias
In the sensitivity analysis, no overall significant change was found when any single study was removed, suggesting our results are statistically robust. Neither Egger's nor Begg's tests (GA vs. GG, Figure 6) showed any evidence of publication bias in this meta-analysis.
Figure 6. Begg's tests for publication bias between TNF-α rs361525 polymorphism and risk of GC (GA vs. GG).
FPRP Analyses
The FPRPs for significant results at different p levels are shown in Table 4. At the level of 0.1, some FPRPs were all <0.20, indicating the significant associations between TNF-α rs361525 polymorphism and GC risk were noteworthy (Table 4). However, the FPRPs for other significant associations were larger, suggesting some possible bias due to sample size reduction existed in some subgroups, which should be validated by larger-size studies in the future.
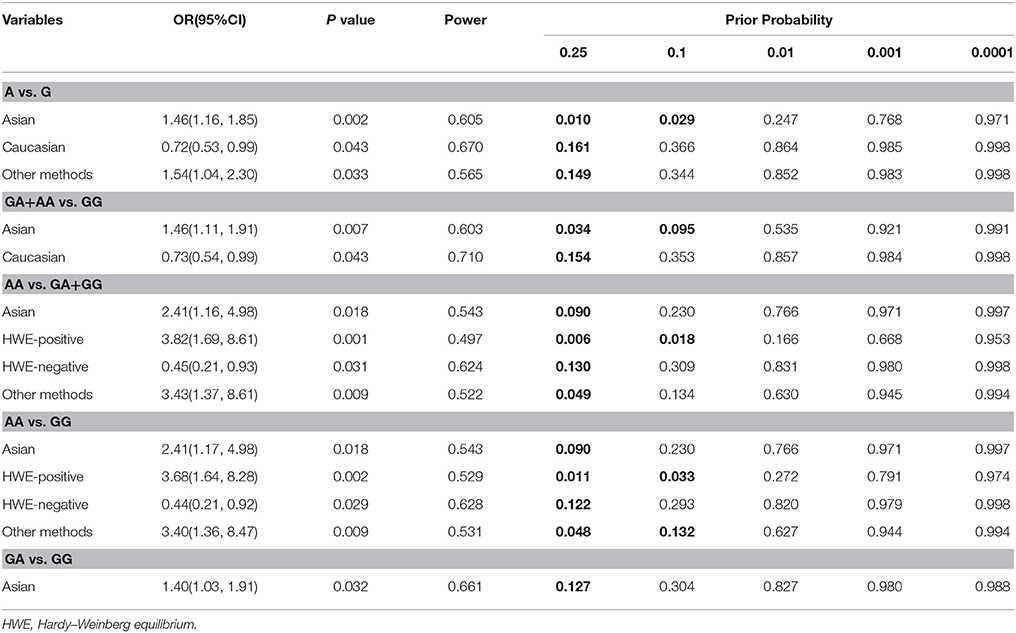
Table 4. False-positive report probability values for associations between the TNF-α−238 polymorphism and gastric cancer risk.
Discussion
It is hypothesized chronic inflammation plays a crucial role in the etiology of GC and other cancers. Epplein et al. found the upregulated circulating levels of inflammation-related cytokines such as TNF-α may intensify the risk of GC (Epplein et al., 2013). TNF-α-induced protein secretion from H. pylori is involved in the development of GC (Suganuma et al., 2012). It is assumed the stimulation of the TNF-α/TNFR1 signaling in the tumor microenvironment enhances GC progression by inducing Noxo1 and GNA14 (Oshima et al., 2014). TNF-α is a promising effective target for GC therapy. The TNF-α rs361525 polymorphism could change TNF-α gene transcription and adjust TNF-α generation (Kaluza et al., 2000).
Many studies have reported the relationship between TNF-α rs361525 polymorphism and GC risk (Jang et al., 2001; Wu et al., 2002, 2003, 2004; Glas et al., 2004; Lee et al., 2004; Lu et al., 2005; Zambon et al., 2005; Kamangar et al., 2006; Xing et al., 2006; Garcia-Gonzalez et al., 2007; Hou et al., 2007; Zeng et al., 2007; Crusius et al., 2008; Bai et al., 2009; Yang et al., 2009; Whiteman et al., 2010; Yin et al., 2012; Essadik et al., 2015; Xu et al., 2017), but with conflicting findings. Given such conflicts, several meta-analyses in this field have been conducted (Zhou et al., 2011; Yu et al., 2013; Rokkas et al., 2014; Hui et al., 2016). Zhou et al. firstly meta-analyzed the association between TNF-α rs361525 polymorphism and cancer risk (Zhou et al., 2011), but found no significant result in the general populations (Zhou et al., 2011) or in the subgroups of cancer type including GC (Zhou et al., 2011). The two subsequent meta-analyses also did not observe an association between TNF-α rs361525 polymorphism and GC risk (Rokkas et al., 2014; Hui et al., 2016). Noticeably, Hui et al. revealed TNF-α rs361525 polymorphism could significantly increase the risk of digestive system cancer, but not GC (Hui et al., 2016). However, Yu et al. uncovered a significant relationship between TNF-α rs361525 polymorphism and increased GC risk in Asians, but not in Caucasians (Yu et al., 2013). Recently, new studies in this field have emerged, which further necessitates a new comprehensive meta-analysis. Here our data are consistent with Yu et al. It is worth noting that we found a significant association of TNF-α rs361525 polymorphism with GC among both Asians and Caucasians in the stratified analysis by ethnicity, while Yu et al. did not find any association among Caucasians (Yu et al., 2013). We found TNF-α rs361525 polymorphism increased the risk of GC among Asians, while this SNP seemed to protect Caucasians from GC. There are several possible interpretations for the different findings between Asians and Caucasians. Firstly, GC may be genetically and clinically heterogeneous among different populations. Secondly, varying sample sizes of Asians and Caucasians may also account. The third reason may be the differences in genotyping methods and random errors. The fourth reason may be the varying prevalence of H. pylori among different populations. Last but not least, different geographical environments and dietary pattern may also be influential (such as Asians eat more pickled and fried food). We believe this meta-analysis has several strengths over previous meta-analyses. Firstly, our sample size was larger. Secondly, sensitivity analysis proved the reliability and stability of our data. Thirdly, we conducted subgroup analyses of ethnicity, HWE, SOC, NOS score, and genotyping methods, and explored the potential sources of heterogeneity. Finally, we calculated false-positive report probability and statistical power.
However, this study also has some limitations. Firstly, no subgroup analyses of confounding factors such as age, sex, smoking or H. pylori infection were conducted. Secondly, potential gene-gene or gene-environment interactions were not assessed. Thirdly, only one SNP of TNF-α gene was investigated. Finally, the relationship between this SNP and clinical manifestations of GC was not examined.
In conclusion, this meta-analysis indicates the TNF-α rs361525 polymorphism increases the risk of GC among Asians, but decreases the risk of GC among Caucasians. This finding should be validated by larger case-control studies in other ethnicities.
Author Contributions
ZK conceived the entire study. TX and HZ analyzed the data. TX and ZK performed statistical analysis. ZK and HZ wrote the paper. All authors read and agreed with the final version of this paper.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Bai, J., Guo, Y., Chen, R., Shen, Y., Zhang, P., and Su, H. (2009). Study of gastric cancer susceptibility genes in heliothrephans inhales in yunnan province. Gastroenterology 14, 669–673. doi: 10.3969/j.issn.1008-7125.2009.11.010
Balkwill, F. (2006). TNF-alpha in promotion and progression of cancer. Cancer Metastasis Rev. 25, 409–416. doi: 10.1007/s10555-006-9005-3
Crusius, J. B., Canzian, F., Capella, G., Pena, A. S., Pera, G., Sala, N., et al. (2008). Cytokine gene polymorphisms and the risk of adenocarcinoma of the stomach in the European prospective investigation into cancer and nutrition (EPIC-EURGAST). Ann. Oncol. 19, 1894–1902. doi: 10.1093/annonc/mdn400
de Martel, C., Ferlay, J., Franceschi, S., Vignat, J., Bray, F., Forman, D., et al. (2012). Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 13, 607–615. doi: 10.1016/S1470-2045(12)70137-7
Epplein, M., Xiang, Y. B., Cai, Q., Peek, R. M. Jr., Li, H., Correa, P., et al. (2013). Circulating cytokines and gastric cancer risk. Cancer Causes Control 24, 2245–2250. doi: 10.1007/s10552-013-0284-z
Essadik, A., Jouhadi, H., Rhouda, T., Nadifiyine, S., Kettani, A., and Maachi, F. (2015). Polymorphisms of tumor necrosis factor alpha in moroccan patients with gastric pathology: new single-nucleotide polymorphisms in TNF-alpha(-193) (G/A). Mediat. Inflamm. 2015:143941. doi: 10.1155/2015/143941
Fox, J. G., and Wang, T. C. (2007). Inflammation, atrophy, and gastric cancer. J. Clin. Invest. 117, 60–69. doi: 10.1172/JCI30111
Garcia-Gonzalez, M. A., Lanas, A., Quintero, E., Nicolas, D., Parra-Blanco, A., Strunk, M., et al. (2007). Gastric cancer susceptibility is not linked to pro-and anti-inflammatory cytokine gene polymorphisms in whites: a Nationwide Multicenter Study in Spain. Am. J. Gastroenterol. 102, 1878–1892. doi: 10.1111/j.1572-0241.2007.01423.x
Glas, J., Torok, H. P., Schneider, A., Brunnler, G., Kopp, R., Albert, E. D., et al. (2004). Allele 2 of the interleukin-1 receptor antagonist gene is associated with early gastric cancer. J. Clin. Oncol. 22, 4746–4752. doi: 10.1200/JCO.2004.03.034
Gong, J., Mei, S., Liu, C., Xiang, Y., Ye, Y., Zhang, Z., et al. (2017). PancanQTL: systematic identification of cis-eQTLs and trans-eQTLs in 33 cancer types. Nucleic Acids Res. 46, D971–D976. doi: 10.1093/nar/gkx861
He, J., Liao, X. Y., Zhu, J. H., Xue, W. Q., Shen, G. P., Huang, S. Y., et al. (2014). Association of MTHFR C677T and A1298C polymorphisms with non-Hodgkin lymphoma susceptibility: evidence from a meta-analysis. Sci. Rep. 4:6159. doi: 10.1038/srep06159
He, J., Wang, M. Y., Qiu, L. X., Zhu, M. L., Shi, T. Y., Zhou, X. Y., et al. (2013). Genetic variations of mTORC1 genes and risk of gastric cancer in an Eastern Chinese population. Mol. Carcinog. 52(Suppl. 1), E70–E79. doi: 10.1002/mc.22013
Higgins, J. P., and Thompson, S. G. (2002). Quantifying heterogeneity in a meta-analysis. Stat. Med. 21, 1539–1558. doi: 10.1002/sim.1186
Hou, L., El-Omar, E. M., Chen, J., Grillo, P., Rabkin, C. S., Baccarelli, A., et al. (2007). Polymorphisms in Th1-type cell-mediated response genes and risk of gastric cancer. Carcinogenesis 28, 118–123. doi: 10.1093/carcin/bgl130
Hui, M., Yan, X., and Jiang, Y. (2016). The tumor necrosis factor-alpha-238 polymorphism and digestive system cancer risk: a meta-analysis. Clin. Exp. Med. 16, 367–374. doi: 10.1007/s10238-015-0363-4
Jang, W. H., Yang, Y. I., Yea, S. S., Lee, Y. J., Chun, J. H., Kim, H. I., et al. (2001). The−238 tumor necrosis factor-alpha promoter polymorphism is associated with decreased susceptibility to cancers. Cancer Lett. 166, 41–46. doi: 10.1016/S0304-3835(01)00438-4
Kaluza, W., Reuss, E., Grossmann, S., Hug, R., Schopf, R. E., Galle, P. R., et al. (2000). Different transcriptional activity and in vitro TNF-alpha production in psoriasis patients carrying the TNF-alpha 238A promoter polymorphism. J. Invest. Dermatol. 114, 1180–1183. doi: 10.1046/j.1523-1747.2000.00001.x
Kamangar, F., Abnet, C. C., Hutchinson, A. A., Newschaffer, C. J., Helzlsouer, K., Shugart, Y. Y., et al. (2006). Polymorphisms in inflammation-related genes and risk of gastric cancer (Finland). Cancer Causes Control 17, 117–125. doi: 10.1007/s10552-005-0439-7
Lee, S. G., Kim, B., Yook, J. H., Oh, S. T., Lee, I., and Song, K. (2004). TNF/LTA polymorphisms and risk for gastric cancer/duodenal ulcer in the Korean population. Cytokine 28, 75–82. doi: 10.1016/j.cyto.2004.06.009
Lu, W., Pan, K., Zhang, L., Lin, D., Miao, X., and You, W. (2005). Genetic polymorphisms of interleukin (IL)-1B, IL-1RN, IL-8, IL-10 and tumor necrosis factor {alpha} and risk of gastric cancer in a Chinese population. Carcinogenesis 26, 631–636. doi: 10.1093/carcin/bgh349
Moore, R. J., Owens, D. M., Stamp, G., Arnott, C., Burke, F., East, N., et al. (1999). Mice deficient in tumor necrosis factor-alpha are resistant to skin carcinogenesis. Nat. Med. 5, 828–831. doi: 10.1038/10552
Oshima, H., Ishikawa, T., Yoshida, G. J., Naoi, K., Maeda, Y., Naka, K., et al. (2014). TNF-alpha/TNFR1 signaling promotes gastric tumorigenesis through induction of Noxo1 and Gna14 in tumor cells. Oncogene 33, 3820–3829. doi: 10.1038/onc.2013.356
Popivanova, B. K., Kitamura, K., Wu, Y., Kondo, T., Kagaya, T., Kaneko, S., et al. (2008). Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J. Clin. Invest. 118, 560–570. doi: 10.1172/JCI32453
Rokkas, T., Sechopoulos, P., Pistiolas, D., Kothonas, F., Margantinis, G., and Koukoulis, G. (2014). Population differences concerning TNF-alpha gene polymorphisms in gastric carcinogenesis based on meta-analysis. Ann. Gastroenterol. 27, 139–148.
Siegel, R. L., Miller, K. D., and Jemal, A. (2017). Cancer Statistics, 2017. CA Cancer J. Clin. 67, 7–30. doi: 10.3322/caac.21387
Stang, A. (2010). Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 25, 603–605. doi: 10.1007/s10654-010-9491-z
Suganuma, M., Watanabe, T., Yamaguchi, K., Takahashi, A., and Fujiki, H. (2012). Human gastric cancer development with TNF-alpha-inducing protein secreted from Helicobacter pylori. Cancer Lett. 322, 133–138. doi: 10.1016/j.canlet.2012.03.027
Szlosarek, P. W., and Balkwill, F. R. (2003). Tumour necrosis factor alpha: a potential target for the therapy of solid tumours. Lancet Oncol. 4, 565–573. doi: 10.1016/S1470-2045(03)01196-3
Tisdale, M. J. (2004). Cancer cachexia. Langenbecks. Arch. Surg. 389, 299–305. doi: 10.1007/s00423-004-0486-7
Uemura, N., Okamoto, S., and Yamamoto, S. (2002). H. pylori infection and the development of gastric cancer. Keio J. Med. 51(Suppl. 2), 63–68.
Wassen, L., and Jertborn, M. (2006). Influence of exogenous reproductive hormones on specific antibody production in genital secretions after vaginal vaccination with recombinant cholera toxin B subunit in humans. Clin. Vaccine Immunol. 13, 202–207. doi: 10.1128/CVI.13.2.202-207.2006
Whiteman, D. C., Parmar, P., Fahey, P., Moore, S. P., Stark, M., Zhao, Z. Z., et al. (2010). Association of Helicobacter pylori infection with reduced risk for esophageal cancer is independent of environmental and genetic modifiers. Gastroenterology 139, 73–83; quiz e11–72. doi: 10.1053/j.gastro.2010.04.009
Wu, M. S., Chen, L. T., Shun, C. T., Huang, S. P., Chiu, H. M., Wang, H. P., et al. (2004). Promoter polymorphisms of tumor necrosis factor-alpha are associated with risk of gastric mucosa-associated lymphoid tissue lymphoma. Int. J. Cancer 110, 695–700. doi: 10.1002/ijc.20199
Wu, M. S., Huang, S. P., Chang, Y. T., Shun, C. T., Chang, M. C., Lin, M. T., et al. (2002). Tumor necrosis factor-alpha and interleukin-10 promoter polymorphisms in Epstein-Barr virus-associated gastric carcinoma. J. Infect. Dis. 185, 106–109. doi: 10.1086/324771
Wu, M. S., Wu, C. Y., Chen, C. J., Lin, M. T., Shun, C. T., and Lin, J. T. (2003). Interleukin-10 genotypes associate with the risk of gastric carcinoma in Taiwanese Chinese. Int. J. Cancer 104, 617–623. doi: 10.1002/ijc.10987
Xing, P. X. D. Z. Q., and Gao, W. (2006). Relationship between cytokine gene polymorphisms on development and clinical characteristics of gastric adenocarcinoma. Chin. J. General Surg. 15, 659–663.
Xu, Y., Cao, X., Jiang, J., Chen, Y., and Wang, K. (2017). TNF-alpha-308/-238 polymorphisms are associated with gastric cancer: a case-control family study in China. Clin. Res. Hepatol. Gastroenterol. 41, 103–109. doi: 10.1016/j.clinre.2016.05.014
Yang, J. J., Ko, K. P., Cho, L. Y., Shin, A., Gwack, J., Chang, S. H., et al. (2009). The role of TNF genetic variants and the interaction with cigarette smoking for gastric cancer risk: a nested case-control study. BMC Cancer 9:238. doi: 10.1186/1471-2407-9-238
Yin, D., Wang, Q., Wang, F., Meng, T., Paerhati, S., Ge, L., et al. (2012). Relationship between tagSNPs and haplotype of TNF-A gene and gastric cancer in Uygur and Han ethnic groups in Xinjiang. Carcinogenesis Teratogenesis Mutagenesis 24, 261–265.
Yu, J. Y., Li, L., Ma, H., Liu, K., Cheng, X., Li, Y. L., et al. (2013). Tumor necrosis factor-alpha 238 G/A polymorphism and gastric cancer risk: a meta-analysis. Tumour Biol. 34, 3859–3863. doi: 10.1007/s13277-013-0972-z
Zambon, C. F., Basso, D., Navaglia, F., Belluco, C., Falda, A., Fogar, P., et al. (2005). Pro- and anti-inflammatory cytokines gene polymorphisms and Helicobacter pylori infection: interactions influence outcome. Cytokine 29, 141–152. doi: 10.1016/j.cyto.2004.10.013
Zeng, Q. D., Lu, L. H., Xing, P. X., Lu, B., and Wang, Y. S. (2007). [Relationship between cytokine gene polymorphism and development of gastric adenocarcinoma]. Zhonghua Yi Xue Za Zhi 87, 1037–1039.
Keywords: tumor necrosis factor α, gene polymorphism, meta-analysis, gastric cancer, false-positive report probability
Citation: Xu T, Kong Z and Zhao H (2018) Relationship Between Tumor Necrosis Factor-α rs361525 Polymorphism and Gastric Cancer Risk: A Meta-Analysis. Front. Physiol. 9:469. doi: 10.3389/fphys.2018.00469
Received: 01 February 2018; Accepted: 13 April 2018;
Published: 15 May 2018.
Edited by:
Stephen J Pandol, Cedars-Sinai Medical Center, United StatesReviewed by:
Savio George Barreto, Medanta The Medicity, IndiaMeghit Boumediene Khaled, University of Sidi-Bel-Abbès, Algeria
Copyright © 2018 Xu, Kong and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhijun Kong, a29uZ196aGlqdW5Ac2luYS5jb20=
Hui Zhao, aHVpXzEwMDdAMTYzLmNvbQ==
 Tianshu Xu
Tianshu Xu Zhijun Kong
Zhijun Kong Hui Zhao
Hui Zhao