- 1State Key Laboratory of Turbulence and Complex Systems and Department of Mechanics and Engineering Science, College of Engineering, Peking University, Beijing, China
- 2PKU-HKUST Shenzhen-Hong Kong Institution, Shenzhen, China
- 3Shenzhen Graduate School, Peking University, Shenzhen, China
Ca2+ waves in cardiac myocytes can lead to arrhythmias owing to delayed after-depolarisations. Based on Ca2+ regulation from the junctional sarcoplasmic reticulum (JSR), a mathematical model was developed to investigate the interplay of clustered and rogue RyRs on Ca2+ waves. The model successfully reproduces Ca2+ waves in cardiac myocytes, which are in agreement with experimental results. A new wave propagation mode of “spark-diffusion-quark-spark” is put forward. It is found that rogue RyRs greatly increase the initiation of Ca2+ sparks, further contribute to the formation and propagation of Ca2+ waves when the free Ca2+ concentration in JSR lumen ([Ca2+]lumen) is higher than a threshold value of 0.7 mM. Computational results show an exponential increase in the velocity of Ca2+ waves with [Ca2+]lumen. In addition, more CRUs of rogue RyRs and Ca2+ release from rogue RyRs result in higher velocity and amplitude of Ca2+ waves. Distance between CRUs significantly affects the velocity of Ca2+ waves, but not the amplitude. This work could improve understanding the mechanism of Ca2+ waves in cardiac myocytes.
Introduction
Ca2+ sparks due to the opening of clustered RyRs are the elementary Ca2+ release events in normal cardiac myocytes (Cheng et al., 1993; Cheng and Lederer, 2008), which could occur in self-propagating succession along the length, and contribute to waves of elevated Ca2+ concentration under some pathological conditions (López-López et al., 1995). Ca2+ waves have been observed in a diversity of cells (Ridgway et al., 1977; Fabiato, 1983; Cornellbell and Finkbeiner, 1991) and studied experimentally and theoretically (Fabiato and Fabiato, 1972; Fabiato, 1985; Backx et al., 1989; Swietach et al., 2010). Generating Ca2+ waves in myocytes is associated with RyRs gating and sarcoplasmic reticulum Ca2+ overload (Petrovic et al., 2015; Williams et al., 2017). Quarky Ca2+ release (QCR or Ca2+ quark) with a small amplitude and a long duration arising from rogue RyRs is another significant Ca2+ release mechanism (Wang et al., 2001; Cheng and Wang, 2002; Brochet et al., 2011; Shang et al., 2014). Hence, Ca2+ waves are a natural consequence of regenerative Ca2+ releases of both Ca2+ sparks and quarks. There is, however, lack of studies to relate Ca2+ waves to the interplay of Ca2+ sparks and quarks from the junctional sarcoplasmic reticulum (JSR). Based on the Fickian diffusion of cytoplasmic Ca2+, a computational model was developed to show the effects of rogue RyRs on Ca2+ waves under heart failure (Lu et al., 2010). Given the spark-width paradox from the Fickian diffusion models (Walker et al., 2014), the anomalous diffusion model can solve the problem and look more deeply into the mechanism of diffusion (Sato and Bers, 2011).
On the other hand, one of the challenges in developing models for Ca2+ waves is the inconsistence between computational and experimental free Ca2+ concentration in the cytoplasm ([Ca2+]cyto) (Izu et al., 2013). The computational results of [Ca2+]cyto were ~20 μM (Chen et al., 2013) under physiological conditions or even as high as ~100 μM (Izu et al., 2001; Chen et al., 2014) under pathological conditions, which disagrees with the measured [Ca2+]cyto of ~1 μM (Williams et al., 1985; Takamatsu and Wier, 1990). Although a “wave front sensitization” model showed [Ca2+]cyto of ~1 μM (Keller et al., 2007), Sobie et al. indicated that elevated JSR Ca2+ level is a critical factor to raise RyRs open probability (Sobie et al., 2017). Hence, JSR Ca2+ regulation should be incorporated into the computational models of Ca2+ waves to show the decrease of Ca2+ flux through rogue and clustered RyRs as the JSR depletes (Sobie et al., 2004; Picht et al., 2011; Izu et al., 2013).
The objective of the study is to quantify the interplay of rogue and clustered RyRs on regulating Ca2+ waves in cardiac myocytes. A two-dimensional (2D) model of Ca2+ waves in the cytoplasm was proposed with considering the distribution of clustered and rogue RyRs on the JSR membrane. The anomalous subdiffusion of Ca2+ in the cytoplasm and JSR Ca2+ regulation were also included. The stochastic opening Ca2+ release units (CRUs) of rogue and clustered RyRs was regulated by free Ca2+ concentrations in both cytoplasm and JSR lumen. With these features, we showed the importance of rogue RyRs on the initiation and propagation of Ca2+ waves.
Materials and Methods
Geometrical Model
Considering the quasi-isotropic diffusion of Ca2+ in the cytoplasm (Izu et al., 2001), we adopted a 2D model to mimic Ca2+ waves. Figure 1A shows the geometrical model of a cardiac myocyte, the x- and y-directions of which refer to the longitudinal axis and z-line, respectively. Baddeley et al. have experimentally observed the RyR distribution on the JSR membrane (Baddeley et al., 2010). Most RyR channels form regular arrays, defined as “clustered RyRs.” Others are rogue RyRs uncoupled from the clustered RyRs. Clustered and rogue RyRs are randomly distributed. Figure 1B shows schematic representative of the CRU distribution on JSRs, which includes CRUs of clustered RyRs (~22 RyR channels in a CRU) and CRUs of rogue RyRs (~3 RyR channels in a CRU). CRUs of clustered RyRs (~2 CRUs in a JSR) are surrounded by randomly distributed CRUs of rogue RyRs (~8 CRUs in a JSR).
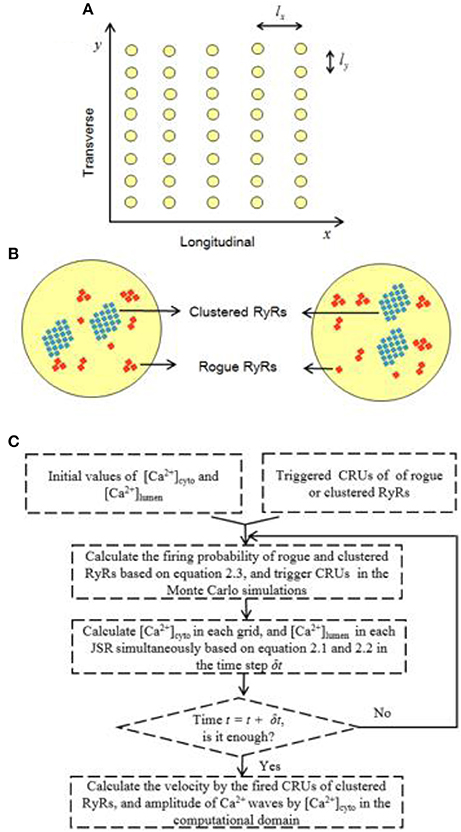
Figure 1. Schematic illustration of the 2D model for Ca2+ waves. (A) Schematic representative of the 2D geometrical model of a cardiac myocyte. The yellow circles denote JSRs. The spacing intervals between JSRs: lx = 2 μm and ly = 0.8 μm. (B) Schematic representative of two JSRs, which include randomly distributed clustered and rogue RyRs (l = 0.1 μm). (C) A flow diagram for the Monte Carlo simulations.
Governing Equations
Ca2+ release events are simulated synchronously by a hybrid model, which consists of two parts: a model of Ca2+ waves in the cytoplasm and a model of Ca2+ blinks in JSRs. The reaction-diffusion system for Ca2+ waves in the cytoplasm based on the anomalous subdiffusion model, including the distribution of clustered and rogue RyRs, is described as follows:
where [Ca2+]cyto is the free Ca2+ concentration in the cytoplasm, t is time, x and y are the spatial coordinates, Dx(= 300 μm2s−1) and Dy (= 150 μm2s−1) denote the Ca2+ diffusion coefficients for anisotropic diffusion. The anomalous subdiffusion order β is 2.25. Jdye is the flux due to the Ca2+ fluorescent indicator dye, Fluo-4-AM, in the cytoplasm. Jbuffer−cyto is the flux due to the endogenous stationary buffers. Jpump is the pumping rate of SR Ca2+-ATPase. SR pumps will be started when exceeds the resting Ca2+ concentration level (0.1 μM). The detailed description is in the Appendix A in Supplementary Material. On the other hand, the balance equation for Ca2+ blinks in each JSR is written as:
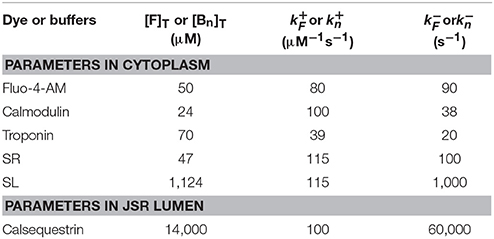
where [Ca2+]lumen is the free Ca2+ concentration in the lumen of a JSR. Jrelease−lumen denotes the Ca2+ release flux caused by opening of clustered RyRs (Jclustered) and rogue RyRs (Jrogue). Jbuffer−lumen is the Ca2+ flux due to the buffer, calsequestrin, in the JSR. Jrefill is the refilled Ca2+ flux to the JSR. The detailed description is in the Appendix B in Supplementary Material. Various parameters of the dye and buffers in the cytoplasm and JSR lumen are listed in Table 1, similar to previous studies (Chen et al., 2013; Kong et al., 2013).
Firing Probability of Rogue and Clustered RyRs
The firing probability per unit time for CRUs of rogue or clustered RyRs is determined by Ca2+ concentrations in the cytoplasm and JSR (Györke and Gyorke, 1998; Qin et al., 2008, 2009), which can be expressed as:
where Pcyto and Φlumen refer to the firing probability per unit time of Ca2+ release events controlled by and [Ca2+]lumen, respectively. The detailed description is in the Appendix C in Supplementary Material.
Numerical Solutions
The 2D computational domain of a cardiac cytoplasm (20 × 20 μm2) was meshed with squares of 0.1 × 0.1 μm to simulate Ca2+ release events from multiple JSRs. JSRs (i.e., yellow circles with radius of 0.3 μm in Figure 1A) are uniformly distributed in the computational domain with lx (2 μm) along x-axis and ly (0.8 μm) along y-axis. Moreover, CRUs are stochastically distributed at nodes within each JSR, which includes 8 CRUs of rogue RyRs (Nrogue = 8) and 2 CRUs of clustered RyRs (Nclustered = 2). As shown in Figure 1C, Equations (1–3) were solved using a FORTRAN-developed program similar to a recent study (Chen et al., 2018). The shifted Grünwald formula of center difference (Tadjeran and Meerschaert, 2007) was used to discretize the fractional differential term in Equation (1) as:
where , Γ denotes the Gamma function. α = β−1 = 1.25, k is an integer with α < k < α+1, and h is the mesh size. Free Ca2+ concentrations in the cytoplasm and JSR were calculated simultaneously. The variable time step algorithm was used. The zero-flux boundary condition was set to the 2D computational domain of a cardiac cytoplasm for the Monte Carlo simulations.
Results and Discussion
Interplay of Rogue and Clustered RyRs in Neighbor JSRs
We have recently studied the effects of rogue RyRs on single Ca2+ sparks and quarks using the model in Equations (1–5), which was validated against the experimental measurements in rat cardiac myocytes (Chen et al., 2018). Here, we used the experimentally-validated numerical model to investigate whether a Ca2+ spark could trigger rogue and clustered RyRs in neighbor JSRs or not. Snapshots of Ca2+ release events in a computational domain of 20 × 20 μm2 were taken at 10, 20, and 40 ms when a CRU of clustered RyRs was fired. Figures 2A,B show rogue RyRs at point (11.9, 9.6) and clustered RyRs at point (12.0, 9.6), respectively, activated by the Ca2+ spark at point (10.0, 9.6). Moreover, the release of clustered RyRs could trigger other CRUs of clustered RyRs in neighbor JSRs with the help of rogue RyRs, as shown in Figure 2C. The results demonstrate that Ca2+ sparks from the opening CRUs of clustered RyRs could activate CRUs of clustered RyRs and rogue RyRs in neighbor JSRs to increase [Ca2+]cyto.
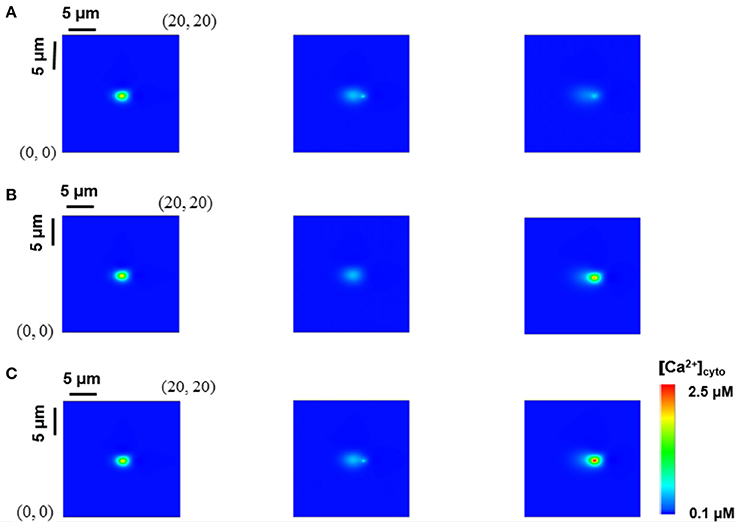
Figure 2. Interplay of rogue and clustered RyRs in neighbor JSRs. Snapshots of Ca2+ release events in a computational region of 20 × 20 μm2 were taken at 10, 20, and 40 ms from left to right when a CRU of clustered RyRs was fired. (A) Rogue RyRs in a neighbor JSR are activated. (B) Clustered RyRs in a neighbor JSR are initiated by a Ca2+ spark directly. (C) Clustered RyRs are triggered with the help of rogue RyRs.
Initiation of Ca2+ Waves
Ca2+ waves could be triggered in a domain where [Ca2+]cyto is higher than the resting Ca2+ concentration level (Lu et al., 2010; Izu et al., 2013). A Ca2+ spark with a large current or several neighbor Ca2+ sparks could also trigger Ca2+ waves (Chen et al., 2014). Here, several neighbor Ca2+ sparks are used to activate Ca2+ waves in the simulation. The lowest propagation velocity of Ca2+ waves was detected in the range of 40–110 μm/s (Cheng et al., 1996). Since the disappearance of Ca2+ waves is related to a progressive decline of the wave propagation velocity, the longitudinal velocity is assumed to be higher than 40 μm/s. Figure 3A shows the probability of inducing Ca2+ waves as a function of the number of Ca2+ sparks initiating from a corner in a square of 20 × 20 μm2 for 100 ms. The beginning level of [Ca2+]lumen is 1.0 mM. The initial Ca2+ sparks arise from one CRU of clustered RyRs. Since Ca2+ quarks increase Ca2+ concentration in the cytoplasm, they enhance the probability of inducing Ca2+ waves in myocytes. Accordingly, Figure 3B shows the number of triggered Ca2+ sparks in 100 ms. A single Ca2+ spark could not form Ca2+ waves while four neighbor Ca2+ sparks with the help of rogue RyRs guarantee the formation of Ca2+ waves.
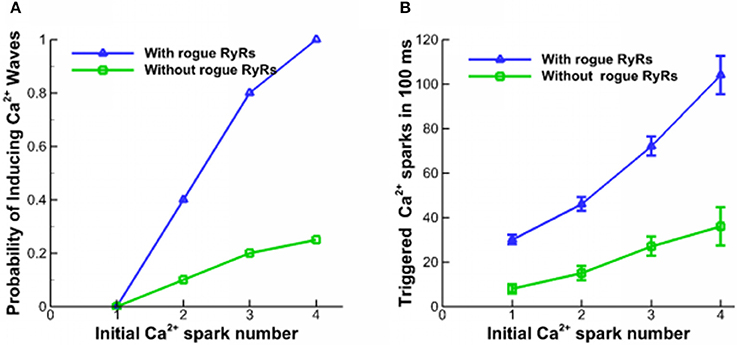
Figure 3. Properties of Ca2+ waves. (A) The probability of inducing Ca2+ waves triggered by different numbers of Ca2+ sparks. (B) The number of triggered Ca2+ sparks in 100 ms.
Propagation of Ca2+ Waves
Ca2+ waves in Figure 4 were generated in a computational domain of 20 × 20 μm2 and recorded at 10, 50, 100, and 150 ms from left to right when the beginning level of [Ca2+]lumen is 1.0 mM. Four Ca2+ sparks were initiated at points (18, 19.2), (18, 18.4), (18, 17.6), and (18, 16.8) with considering rogue RyRs. A comparison of Figures 4A,B indicates much faster Ca2+ waves and higher amplitude when the effects of rogue RyRs are included in the computational model. Moreover, we found the “spark-diffusion-quark-spark” mode. Ca2+ released from clustered RyRs diffuses to a neighbor JSR, rogue RyRs are firstly activated in a stochastic manner to form Ca2+ quarks, and subsequently they make activation of clustered RyRs to produce a Ca2+ spark. The CRUs on the next z-line repeat the process to release Ca2+ in the cytoplasm.
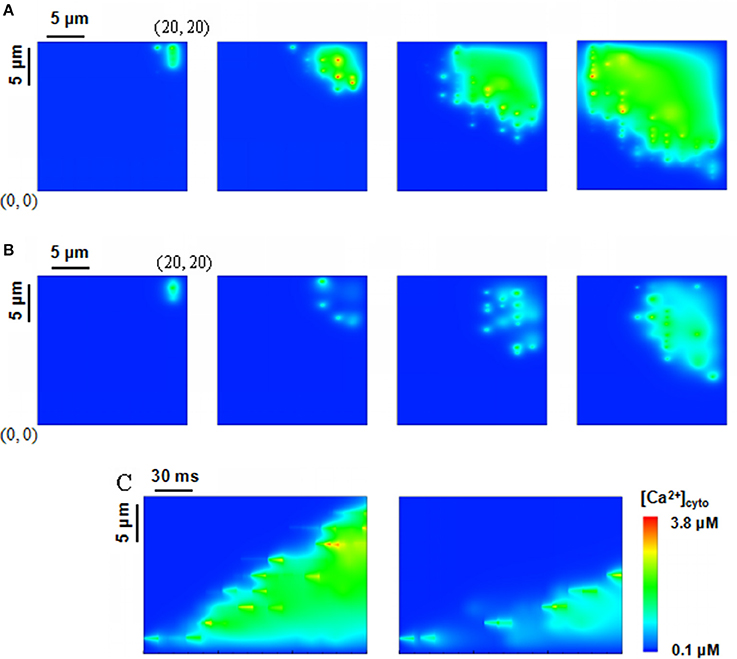
Figure 4. Ca2+ waves triggered by four sparks at the corner of a computational domain, i.e., (18, 19.2), (18, 18.4), (18, 17.6), (18, 16.8) in the region of 20 × 20 μm2. The beginning level of [Ca2+]lumen is 1.0 mM. (A,B) Snapshots of Ca2+ waves initially triggered by Ca2+ sparks with rogue RyRs (A) and without considering rogue RyRs (B) at 10, 50, 100, 150 ms from left to right. (C) Computational results for line-scan images of [Ca2+]cyto with and without rogue RyRs at the line of y = 16.8.
Figure 4C shows the computational results for line-scan images of Ca2+ concentration with and without considering rogue RyRs at the line of y = 16.8. The results reveal that rogue RyRs accelerate the propagation of waves by triggering more Ca2+ sparks. The longitudinal propagating velocity has mean ± SD value of 95.9 ± 8.0 μm/s, which agrees with the experimental records (typically 100 μm/s) (Takamatsu and Wier, 1990; Wier and Blatter, 1991). Furthermore, Ca2+ concentration in the simulation is in the range of 0.1–3.8 μM. The computational predictions are close to the experimental measurements (0.5–1.2 μM; Williams et al., 1985), and less than previous simulations (Izu et al., 2001; Chen et al., 2013).
Importance of [Ca2+]lumen-Dependent Regulation
In previous mathematical models of Ca2+ waves, [Ca2+]lumen-dependent regulation was not considered, and Ca2+ fluxes from CRUs were related to the current and duration of Ca2+ sparks and quarks only. We determined the effects of [Ca2+]lumen-dependent regulation on properties of Ca2+ waves when the beginning level of [Ca2+]lumen was set to 0.3, 0.6, and 0.9 mM. Local variation in the waves is shown in Figure 5A. Ca2+ waves are very sensitive to [Ca2+]lumen. There is an exponential increase in the velocity of Ca2+ waves with the increase of [Ca2+]lumen, as shown in Figure 5B. Moreover, a threshold value of [Ca2+]lumen (i.e., >0.7 mM) exists for generation of steady Ca2+ waves. Figure 5C shows the amplitude of Ca2+ waves linearly increase with [Ca2+]lumen because of the large driving force ([Ca2+]lumen-[Ca2+]cyto) of Ca2+ sparks and quarks.
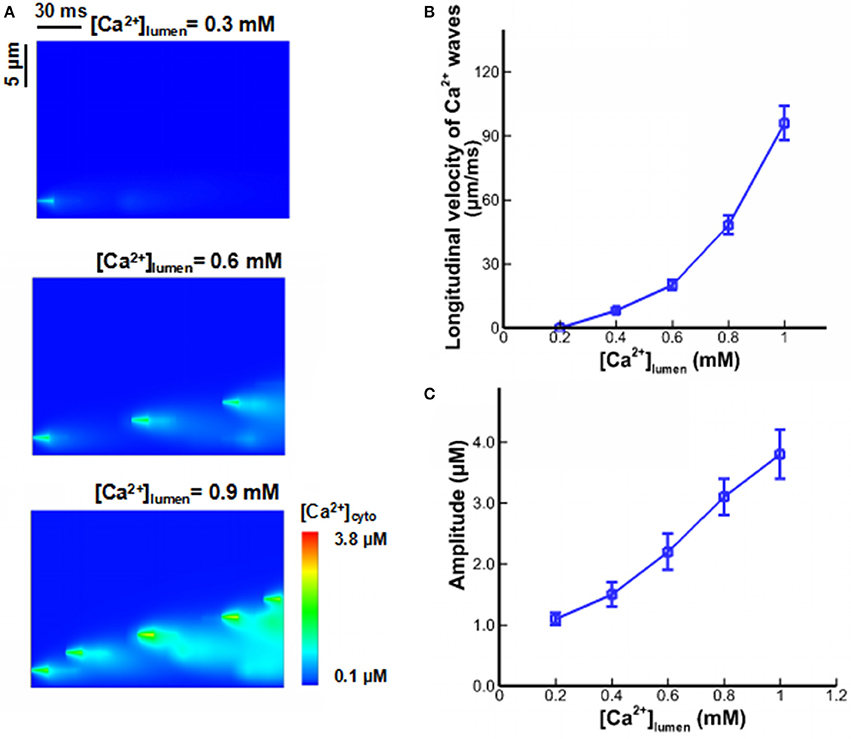
Figure 5. Ca2+ waves under different [Ca2+]lumen. (A) Computational results for line-scan images of [Ca2+]cyto at the line of y = 16.8 when [Ca2+]lumen = 0.3, 0.6, and 0.9 mM. (B) The longitudinal velocity of Ca2+ waves affected by [Ca2+]lumen. (C) The amplitude of Ca2+ waves affected by [Ca2+]lumen.
Effects of Parameters of Rogue RyRs
There are a large number of randomly-distributed rogue RyRs near clustered RyRs. Sensitivity analysis on Ca2+ waves was performed with respect to the changes in CRU number of rogue RyRs in a JSR (Nrogue). Figure 6A shows computational results for line-scan images of [Ca2+]cyto at the line of y = 16.8 when CRU number of rogue RyRs in a JSR are 2, 8, and 14. As shown in Figure 6B, the amplitude and velocity increase with the increased CRU number of rogue RyRs because of high Ca2+ quarks and Ca2+ sparks.
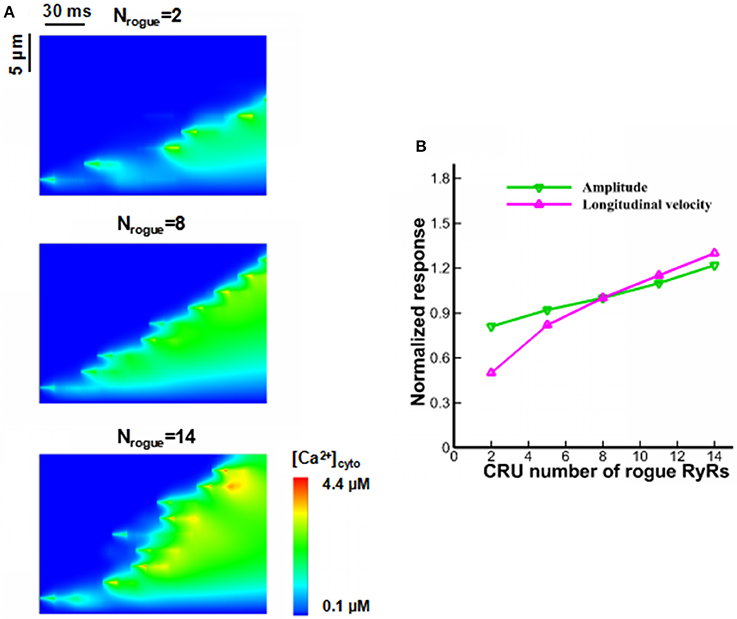
Figure 6. Effects of CRU number of rogue RyRs in a JSR on wave propagation. (A) Computational results for line-scan images of [Ca2+]cyto at the line of y = 16.8 when CRU number of rogue RyRs has values of 2, 8, and 14. (B) The amplitude and longitudinal velocity of Ca2+ waves when Nrogue varies from 2 to 14.
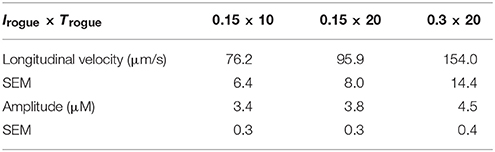
The Ca2+ release per CRU of rogue RyRs is mainly determined by the current through rogue RyRs (Irogue) and the duration of current flow (Trogue). The current and duration of rogue RyRs are 0.15 pA and 20 ms in Figures 2–6. Table 2 presents properties of Ca2+ waves for different values of (Irogue × Trogue). There is a strong correlation between Ca2+ release through rogue RyRs and wave properties. When the release time of rogue RyRs decreases by half to 10 ms with the current of 0.15 pA, the longitudinal velocity and amplitude of waves decrease. When the current increases from 0.15 to 0.3 pA with the duration of 20 ms, Ca2+ waves have higher values of amplitude and longitudinal velocity. Hence, the release amount of rogue RyRs characterized by (Irogue × Trogue) is a significant parameter to affect wave properties.
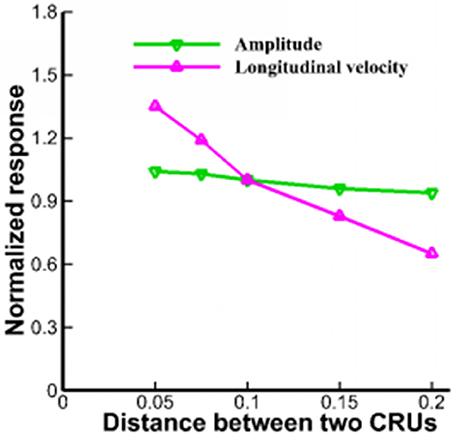
Lu et al. pointed out that rogue RyRs were scattered over the 2D plane randomly (Lu et al., 2010). However, fluorescent imaging showed that QCR events were detected almost at the same site as Ca2+ sparks (Brochet et al., 2011). The stochastic gating of a cluster contains 10–100 RyRs in a JSR and the mean number of RyRs is ~21.6 (Soeller et al., 2007; Baddeley et al., 2009). Figure 7 shows that the distance between CRUs in the range of 0.05–0.2 μm has slight effects on the amplitude of Ca2+ waves. However, the longitudinal velocities are 144.3 ± 13.1 and 63.6 ± 7.8 μm/s, respectively with respect to the distance of 0.05 and 0.2 μm. Therefore, the distance between CRUs of rogue RyRs and clustered RyRs should be taken into consideration in the propagation of Ca2+ waves.
Effects on Activities From Subcellular to Cellar Levels
When a myocyte is under paced, several spontaneous Ca2+ sparks or quarks can occur in the cytoplasm. It is, however, difficult to induce Ca2+ waves that require abundant currents to trigger cardiac action potentials (Izu et al., 2013). In some pathological conditions (e.g., arrhythmias), Ca2+ waves occur in cardiac myocytes and affect the heart's normal function (Lakatta and Guarnieri, 1993). Ten Tusscher and Panfilov developed a ventricular cell model including subspace calcium dynamics that controls L-type calcium current and calcium-induced calcium release (CICR) (ten Tusscher and Panfilov, 2006). The model was used to study the effects of INa recovery dynamics in combination with action potential duration (ADP) restitution on alternans and spiral breakup. On the other hand, the present results suggest that Ca2+ release from JSRs is prone to be initiated to cause Ca2+ waves with the help of rogue RyRs when [Ca2+]lumen is large enough. Moreover, when the CRU number of rogue RyRs in a JSR or Ca2+ release per CRU of rogue RyRs is large enough, or the distance between CRUs is small, the membrane potential would be elevated significantly to trigger action potential in single myocytes.
Potential Implications
This study shows some implications to heart diseases relevant to Ca2+ waves in cardiac myocytes. According to our simulations, JSR Ca2+ overload could increase RyR opening probability and generate Ca2+ waves in heart (Williams et al., 2017). Mutation in RyRs could trigger ventricular tachycardia and sudden cardiac death (Lehnart et al., 2006). Moreover, the amplitude and velocity of Ca2+ waves are significantly affected by the parameters of rogue RyRs, which may contribute to the formation of fibrillation (Macquaide et al., 2015) and arrhythmias (Ter Keurs and Boyden, 2007). A reduction of CRU number, the averaged current and releasing time of rogue RyRs resulted in an inhibition of Ca2+ waves or dyssynchronous Ca2+ transients in myocytes of congestive heart failure (Louch et al., 2013). Therefore, inhibition of Ca2+ quarks through rogue RyRs may be a promising therapeutic target to prevent fibrillation in congestive heart failure.
Critique of the Study
The present study showed Ca2+ waves relevant to the interplay of rogue and clustered RyRs. It addressed the importance of rogue RyRs to increase the initiation of Ca2+ sparks, the incidence and propagation of Ca2+ waves when [Ca2+]lumen is large. The amplitude and velocity of Ca2+ waves are in agreement with the experimental measurements. However, the model should be improved such that more parameters are added to investigate the mechanisms of Ca2+ waves. This study used the fixed release time of rogue and clustered RyRs. It should be a random variable depending on JSR regulation. The detailed structure of clustered RyRs should be taken into consideration because it can influence the frequency of Ca2+ sparks (Walker et al., 2014). Moreover, the present study comes from the assumption of a 2D model in healthy myocytes. A 3D model should be developed to investigate Ca2+ waves in future studies.
Conclusion
We developed a mathematical model to investigate the interplay of rogue and clustered RyRs on regulating Ca2+ waves in the cytoplasm. The computational results on Ca2+ waves agree with experimental measurements in cardiac myocytes. It shows that four neighbor Ca2+ sparks at the corner of a cardiac myocyte could induce Ca2+ waves. Ca2+ quarks increase the probabilities of triggering Ca2+ sparks and speed up the propagation of Ca2+ waves at high [Ca2+]lumen. A new wave propagation mode of “spark-diffusion-quark-spark” is put forward. Particularly, Ca2+ waves could occur only when [Ca2+]lumen is higher than a threshold value of 0.7 mM. More rogue RyRs in a JSR result in more opening CRUs of rogue and clustered RyRs. Besides, Ca2+ release from CRUs of rogue RyRs is a strong factor of wave properties. The velocity of Ca2+ waves is affected significantly by the distance between CRUs. This study helps to understand basic mechanisms of Ca2+ waves in cardiac myocytes.
Author Contributions
XC, YH, and WT designed and performed the numerical calculation; YF analyzed and interpreted data; XC, YH, and WT wrote the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (11732001 and 11328201) and the Leading Talents of Guangdong Province Program. We would like to thank Xi Chen for valuable discussions about simulation and revision of the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2018.00393/full#supplementary-material
References
Backx, P. H., Detombe, P. P., Vandeen, J. H. K., Mulder, B. J. M., and Terkeurs, H. (1989). A model of propagating calcium-induced calcium release mediated by calcium diffusion. J. Gen. Physiol. 93, 963–977. doi: 10.1085/jgp.93.5.963
Baddeley, D., Jayasinghe, I. D., Lam, L., Rossberger, S., Cannell, M. B., and Soeller, C. (2009). Optical single-channel resolution imaging of the ryanodine receptor distribution in rat cardiac myocytes. Proc. Natl. Acad. Sci. U.S.A. 106, 22275–22280. doi: 10.1073/pnas.0908971106
Baddeley, D., Jayasinghe, I. D., Lam, L., Rossberger, S., Cannell, M. B., and Soeller, C. (2010). Imaging of the ryanodine receptor distribution in rat cardiac myocytes with optical single channel resolution. Biophys. J. 98:296A. doi: 10.1016/j.bpj.2009.12.1611
Brochet, D. X. P., Xie, W. J., Yang, D. M., Cheng, H. P., and Lederer, W. J. (2011). Quarky calcium release in the heart. Circ. Res. 108, 210–218. doi: 10.1161/CIRCRESAHA.110.231258
Cheng, H., Lederer, M. R., Lederer, W. J., and Cannell, M. B. (1996). Calcium sparks and Ca2+ (i) waves in cardiac myocytes. Am. J. Physiol. Cell Physiol. 270, C148–C159. doi: 10.1152/ajpcell.1996.270.1.C148
Cheng, H., Lederer, W. J., and Cannell, M. B. (1993). Calcium sparks - elementary events underlying excitation-contraction coupling in heart-muscle. Science 262, 740–744. doi: 10.1126/science.8235594
Cheng, H. P., and Lederer, W. J. (2008). Calcium sparks. Physiol. Rev. 88, 1491–1545. doi: 10.1152/physrev.00030.2007
Cheng, H. P., and Wang, S. Q. (2002). Calcium signaling between sarcolemmal calcium channels and ryanodine receptors in heart cells. Front. Biosci. 7, d1867–d1878. doi: 10.2741/A885
Chen, X., Feng, Y., Huo, Y., and Tan, W. (2018). Effects of rogue ryanodine receptors on Ca2+ sparks in cardiac myocytes. R. Soc. Open Sci. 5:171462. doi: 10.1098/rsos.171462
Chen, X., Guo, L., Kang, J. H., Huo, Y. L., Wang, S. Q., and Tan, W. C. (2014). Calcium waves initiating from the anomalous subdiffusive calcium sparks. J. R. Soc. Interface 11:20130934. doi: 10.1098/rsif.2013.0934
Chen, X., Kang, J. H., Fu, C. J., and Tan, W. C. (2013). Modeling calcium wave based on anomalous subdiffusion of calcium sparks in cardiac myocytes. PLoS ONE 8:e57093. doi: 10.1371/journal.pone.0057093
Cornellbell, A. H., and Finkbeiner, S. M. (1991). Ca2+ waves in astrocyte cells. Calcium 12, 185–204.
Fabiato, A. (1983). Calcium-induced release of calcium from the cardiac sarcoplasmic-reticulum. Am. J. Physiol. 245, C1–C14. doi: 10.1152/ajpcell.1983.245.1.C1
Fabiato, A. (1985). Time and calcium dependence of activation and inactivation of calcium-induced release of calcium from the sarcoplasmic-reticulum of a skinned canine cardiac purkinje-cell. J. Gen. Physiol. 85, 247–289. doi: 10.1085/jgp.85.2.247
Fabiato, A., and Fabiato, F. (1972). Excitation-contraction coupling of isolated cardiac fibers with disrupted or closed sarcolemmas - calcium-dependent cyclic and tonic contractions. Circ. Res. 31, 293–307.
Györke, I., and Gyorke, S. (1998). Regulation of the cardiac ryanodine receptor channel by luminal Ca2+ involves luminal Ca2+ sensing sites. Biophys. J. 75, 2801–2810. doi: 10.1016/S0006-3495(98)77723-9
Izu, L. T., Wier, W. G., and Balke, C. W. (2001). Evolution of cardiac calcium waves from stochastic calcium sparks. Biophys. J. 80, 103–120. doi: 10.1016/S0006-3495(01)75998-X
Izu, L. T., Xie, Y. F., Sato, D., Banyasz, T., and Chen-Izu, Y. (2013). Ca2+ waves in the heart. J. Mol. Cell. Cardiol. 58, 118–124. doi: 10.1016/j.yjmcc.2012.11.014
Keller, M., Kao, J. P. Y., Egger, M., and Niggli, E. (2007). Calcium waves driven by “sensitization” wave-fronts. Cardiovasc. Res. 74, 39–45. doi: 10.1016/j.cardiores.2007.02.006
Kong, C. H. T., Laver, D. R., and Cannell, M. B. (2013). Extraction of sub-microscopic Ca fluxes from blurred and noisy fluorescent indicator images with a detailed model fitting approach. PLoS Comput. Biol. 9:e1002931. doi: 10.1371/journal.pcbi.1002931
Lakatta, E. G., and Guarnieri, T. (1993). Spontaneous myocardial calcium oscillations - are they linked to ventricular-fibrillation. J. Cardiovasc. Electrophysiol. 4, 473–489. doi: 10.1111/j.1540-8167.1993.tb01285.x
Lehnart, S. E., Terrenoire, C., Reiken, S., Wehrens, X. H. T., Song, L. S., Tillman, E. J., et al. (2006). Stabilization of cardiac ryanodine receptor prevents intracellular calcium leak and arrhythmias. Proc. Natl. Acad. Sci. U.S.A. 103, 7906–7910. doi: 10.1073/pnas.0602133103
Li, J. H., Xie, W. J., Chen, X., Huo, Y. L., Cheng, H. P., and Tan, W. C. (2017). A novel stochastic reaction-diffusion model of Ca2+ blink in cardiac myocytes. Sci. Bull. 62, 5–8. doi: 10.1016/j.scib.2016.12.001
López-López, J. R., Shacklock, P. S., Balke, C. W., and Wier, W. G. (1995). Local calcium transients triggered by single L-type Calcium-Channel Currents In Cardiac-Cells. Science 268, 1042–1045.
Louch, W. E., Hake, J., Mork, H. K., Hougen, K., Skrbic, B., Ursu, D., et al. (2013). Slow Ca2+ sparks de-synchronize Ca2+ release in failing cardiomyocytes: evidence for altered configuration of Ca2+ release units? J. Mol. Cell. Cardiol. 58, 41–52. doi: 10.1016/j.yjmcc.2013.01.014
Lu, L. Y., Xia, L., Ye, X. S., and Cheng, H. P. (2010). Simulation of the effect of rogue ryanodine receptors on a calcium wave in ventricular myocytes with heart failure. Phys. Biol. 7:026005. doi: 10.1088/1478-3975/7/2/026005
Macquaide, N., Tuan, H. T. M., Hotta, J. I., Sempels, W., Lenaerts, I., Holemans, P., et al. (2015). Ryanodine receptor cluster fragmentation and redistribution in persistent atrial fibrillation enhance calcium release. Cardiovasc. Res. 108, 387–398. doi: 10.1093/cvr/cvv231
Metzler, R., and Klafter, J. (2000). The random walk's guide to anomalous diffusion: a fractional dynamics approach. Phys. Rep. Rev. Sect. Phys. Lett. 339, 1–77. doi: 10.1016/S0370-1573(00)00070-3
Petrovic, P., Valent, I., Cocherova, E., Pavelkova, J., and Zahradnikova, A. (2015). Ryanodine receptor gating controls generation of diastolic calcium waves in cardiac myocytes. J. Gen. Physiol. 145, 489–511. doi: 10.1085/jgp.201411281
Picht, E., Zima, A. V., Shannon, T. R., Duncan, A. M., Blatter, L. A., and Bers, D. M. (2011). Dynamic calcium movement inside cardiac sarcoplasmic reticulum during release. Circ. Res. 108, 847–856. doi: 10.1161/CIRCRESAHA.111.240234
Qin, J., Valle, G., Nani, A., Chen, H., Ramos-Franco, J., Nori, A., et al. (2009). Ryanodine receptor luminal Ca2+ regulation: swapping calsequestrin and channel isoforms. Biophys. J. 97, 1961–1970. doi: 10.1016/j.bpj.2009.07.030
Qin, J., Valle, G., Nani, A., Nori, A., Rizzi, N., Priori, S., et al. (2008). Luminal Ca2+ regulation of single cardiac ryanodine receptors: insights provided by calsequestrin and its mutants. J. Gen. Physiol. 131, 325–334. doi: 10.1085/jgp.200709907
Ridgway, E. B., Gilkey, J. C., and Jaffe, L. F. (1977). Free calcium increases explosively in activating medaka eggs. Proc. Natl. Acad. Sci. U.S.A. 74, 623–627. doi: 10.1073/pnas.74.2.623
Sato, D., and Bers, D. M. (2011). How does stochastic ryanodine receptor-mediated Ca leak fail to initiate a Ca spark? Biophys. J. 101, 2370–2379. doi: 10.1016/j.bpj.2011.10.017
Shang, W., Lu, F. J., Sun, T., Xu, J. J., Li, L. L., Wang, Y. R., et al. (2014). Imaging Ca2+ nanosparks in heart with a new targeted biosensor. Circ. Res. 114, 412–420. doi: 10.1161/CIRCRESAHA.114.302938
Smith, G. D., Keizer, J. E., Stern, M. D., Lederer, W. J., and Cheng, H. P. (1998). A simple numerical model of calcium spark formation and detection in cardiac myocytes. Biophys. J. 75, 15–32. doi: 10.1016/S0006-3495(98)77491-0
Sobie, E. A., Dilly, K. W., Cruz, J. D., Lederer, W. J., and Jafri, M. S. (2004). Termination of cardiac of Ca2+ sparks: an investigative mathematical model of calcium-induced calcium release. Biophys. J. 83, 59–78. doi: 10.1016/S0006-3495(02)75149-7
Sobie, E. A., Williams, G. S. B., and Lederer, W. J. (2017). Ambiguous interactions between diastolic and SR Ca2+ in the regulation of cardiac Ca2+ release. J. Gen. Physiol. 149, 847–855. doi: 10.1085/jgp.201711814
Soeller, C., Crossman, D., Gilbert, R., and Cannell, M. B. (2007). Analysis of ryanodine receptor clusters in rat and human cardiac myocytes. Proc. Natl. Acad. Sci. U.S.A. 104, 14958–14963. doi: 10.1073/pnas.0703016104
Swietach, P., Spitzer, K. W., and Vaughan-Jones, R. D. (2010). Modeling calcium waves in cardiac myocytes: importance of calcium diffusion. Front. Biosci. 15, 661–680. doi: 10.2741/3639
Tadjeran, C., and Meerschaert, M. M. (2007). A second-order accurate numerical method for the two-dimensional fractional diffusion equation. J. Comput. Phys. 220, 813–823. doi: 10.1016/j.jcp.2006.05.030
Takamatsu, T., and Wier, W. G. (1990). Calcium waves in mammalian heart - quantification of origin, magnitude, wave-form, and velocity. FASEB J. 4, 1519–1525.
ten Tusscher, K., and Panfilov, A. V. (2006). Alternans and spiral breakup in a human ventricular tissue model. Am. J. Physiol. Heart Circ. Physiol. 291, H1088–H1100. doi: 10.1152/ajpheart.00109.2006
Ter Keurs, H., and Boyden, P. A. (2007). Calcium and arrhythmogenesis. Physiol. Rev. 87, 457–506. doi: 10.1152/physrev.00011.2006
Walker, M. A., Williams, G. S. B., Kohl, T., Lehnart, S. E., Jafri, M. S., Greenstein, J. L., et al. (2014). Superresolution modeling of calcium release in the heart. Biophys. J. 107, 3009–3020. doi: 10.1016/j.bpj.2014.11.003
Wang, S. Q., Song, L. S., Lakatta, E. G., and Cheng, H. P. (2001). Ca2+ signalling between single L-type Ca2+ channels and ryanodine receptors in heart cells. Nature 410, 592–596. doi: 10.1038/35069083
Wier, W. G., and Blatter, L. A. (1991). Ca2+-oscillations And Ca2+-waves in mammalian cardiac and vascular smooth-muscle cells. Cell Calcium 12, 241–254.
Williams, D. A., Fogarty, K. E., Tsien, R. Y., and Fay, F. S. (1985). Calcium gradients in single smooth-muscle cells revealed by the digital imaging microscope using FURA-2. Nature 318, 558–561. doi: 10.1038/318558a0
Williams, G. S. B., Wescott, A. P., Lehnart, S. E., and Lederer, W. J. (2017). How does calcium overload generate calcium waves in heart? Biophys. J. 112, 541A–541A. doi: 10.1016/j.bpj.2016.11.2923
Keywords: Ca2+ wave, Ca2+ quark, anomalous subdiffusion, rogue ryanodine receptors, clustered ryanodine receptors
Citation: Chen X, Feng Y, Huo Y and Tan W (2018) The Interplay of Rogue and Clustered Ryanodine Receptors Regulates Ca2+ Waves in Cardiac Myocytes. Front. Physiol. 9:393. doi: 10.3389/fphys.2018.00393
Received: 05 December 2017; Accepted: 03 April 2018;
Published: 26 April 2018.
Edited by:
Ghassan S. Kassab, California Medical Innovations Institute, United StatesReviewed by:
Vijay Rajagopal, University of Melbourne, AustraliaAlok Ranjan Nayak, International Institute of Information Technology, India
Copyright © 2018 Chen, Feng, Huo and Tan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yunlong Huo, eWh1b0Bwa3UuZWR1LmNu
Wenchang Tan, dGFud2NoQHBrdS5lZHUuY24=
 Xudong Chen1
Xudong Chen1 Yunlong Huo
Yunlong Huo