- 1Regenerative Medicine Laboratory, Department of Experimental Medicine, University of Genova, Genova, Italy
- 2Centro di Ricerca E. Menni, Fondazione Poliambulanza - Istituto Ospedaliero, Brescia, Italy
- 3Ludwig Boltzmann Institute for Experimental and Clinical Traumatology, AUVA Research Center Austrian Cluster for Tissue Regeneration, Vienna, Austria
- 4Institute of Human Anatomy and Cell Biology, “A. Gemelli” Faculty of Medicine and Surgery, Catholic University of the Sacred Heart, Rome, Italy
Efficient cardiac repair and ultimate regeneration still represents one of the main challenges of modern medicine. Indeed, cardiovascular disease can derive from independent conditions upsetting heart structure and performance: myocardial ischemia and infarction (MI), pharmacological cardiotoxicity, and congenital heart defects, just to name a few. All these disorders have profound consequences on cardiac tissue, inducing the onset of heart failure over time. Since the cure is currently represented by heart transplantation, which is extremely difficult due to the shortage of donors, much effort is being dedicated to developing innovative therapeutic strategies based on stem cell exploitation. Among the broad scenario of stem/progenitor cell subpopulations, fetal and perinatal sources, namely amniotic fluid and term placenta, have gained interest due to their peculiar regenerative capacity, high self-renewal capability, and ease of collection from clinical waste material. In this review, we will provide the state-of-the-art on fetal perinatal stem cells for cardiac repair and regeneration. We will discuss different pathological conditions and the main therapeutic strategies proposed, including cell transplantation, putative paracrine therapy, reprogramming, and tissue engineering approaches.
Healing a Broken Heart: A Main Challenge in Regenerative Medicine
Cardiovascular disease and heart failure are the main killers in the Western countries representing a significant economic burden for the national health systems, as more than 1 million hospitalizations are annually reported in the EU alone. Indeed, for the majority of people suffering from heart failure, the mortality rates remains high, with 1 out of 4 patients dying within 1 year from diagnosis (Jameel and Zhang, 2009). Despite significant efforts, current therapies for cardiovascular disease have not yet fulfilled all expectations, being intrinsically non-curative and with limited improvements in reducing mortality or delaying the onset of heart failure. Currently, the ultimate therapy is still represented by organ transplantation, mainly confounded by limited supply of donor organs (Levy et al., 2002). Several different conditions can affect the cardiac tissue, from ischemic disease to drug-derived cardiotoxicity up to congenital heart defects, overall resulting in pathological disruption of heart function with the main challenge to overcome mostly represented by primary loss of cardiomyocytes.
Nonetheless, a major breakthrough for cardiac regenerative medicine has been provided by mounting evidence suggesting that the heart retains an endogenous regeneration programme, although very limited, based on cardiac progenitor cell (CPC) activation and cardiomyocyte proliferation. Unfortunately, while broadly active in the neonate, these mechanisms are quiescent and malfunctioning in the adult heart (Porrello et al., 2011; Jesty et al., 2012). Therefore, a working scheme to fully unlock and rejuvenate this potential will open new frontiers in cardiac medicine. Likewise, cardiomyocyte replacement and new vasculature formation by pluripotent-derived cell therapy, tissues engineering, and local cell reprogramming have also been widely scrutinized to identify an innovative therapeutic approach.
In particular, the optimal regenerative strategy should consider the specific patient clinical need and the pathological setting to operate within, in order to induce efficient tissue regeneration.
Myocardial infarction (MI) is the most common cause of cardiac injury in Western countries. Modern clinical interventions have substantially decreased acute mortality of MI, but have only partially diminished the number of patients who subsequently suffer from impairment of cardiac function, up to overt heart failure. The milieu of the injured heart is a battlefield for regeneration. In particular, after ischemic injury vascular supply is limited. Matrix stiffening that occurs after injury can further suppress the already limited potential for cardiomyocyte proliferation (Tzahor and Poss, 2017). Hence, prompt intervention is required to: (i) provide cardioprotection, modulate the inflammatory response, and improve wound healing, while (ii) activating resident CPC to differentiate and/or release trophic factors to support the microenvironment, and (iii) sustain proliferation of surviving cardiomyocytes, overall improving cardiac function. In such scenario, timely intervention is required and enormous interest has recently been driven toward stem/stromal cell biology for the regeneration of damaged tissues. The current endeavor of cell-based therapy in cardiac regenerative medicine is to stimulate endogenous restoration mechanisms; hence, growing interest has been directed toward speculative paracrine therapy based on exploiting the stem cell secretome, i.e., the totality of soluble paracrine factors and microRNA (miRNA)-enriched extracellular vesicles secreted by stem cells with beneficial effects on the injured myocardial tissue (Rani et al., 2015). Indeed, the MI patient presented at the hospital emergency room could be treated with the optimal drug formulation of the ideal pro-regenerative stem cell secretome to be delivered by intracoronary infusion during standard angioplasty procedure.
Cardioprotection from chemotherapy-derived cardiotoxicity represents another relevant clinical need that may be addressed by exploiting stem cell modulatory paracrine potential. Cardiotoxicity-induced cardiomyopathy leading to subclinical ventricular dysfunction or symptomatic heart failure can represent a critical complication as a side effect of oncological therapy. Notably, oncological treatments can affect both cardiomyocytes and endogenous CPC, leading to their senescence and/or apoptosis (Huang et al., 2010; Su et al., 2015). In this scenario, prompt modulation of the cardiac microenvironment could represent a crucial step to counteract chemotherapy-derived unspecific and noxious side effects and avoid long-term cardiovascular complications in cancer survivors (Hahn et al., 2014; Giza et al., 2017; Naaktgeboren et al., 2017). Indeed, a suitable preventive and complementary treatment during oncological therapy could be represented by the administration of stem cell-based secretome drug formulation via catheter-guided coronary infusion prior to chemotherapy, in order to ensure cardiac-specific delivery without jeopardizing oncological therapy.
Last, but not least, myocardial renewal for congenital heart defects is another major challenge in cardiac regenerative medicine. Congenital heart defects are common birth deficiencies often resulting in pediatric heart failure. They range from life-threatening conditions (i.e., hypo-plastic left heart syndrome) to benign defects (i.e., septal defects). CHD represent a complicated scenario with the explicit demand of reconstituting tissue that might have gone missing during development or have not properly formed (Woodward, 2011; Sun et al., 2015). Standard therapy is elective surgery within the first weeks of life to provide structural reconstruction using prosthetic implants (Ohye et al., 2016) that do not grow with the patient, thus requiring additional surgeries during life. Therefore, a suitable therapeutic approach should provide new functional cardiovascular and cardiac cells via stem cell-based therapy combined with tissue engineering strategies. Indeed, cardiovascular cells obtained by pluripotent stem cells could be loaded on biocompatible prosthetic material implanted during surgical interventions and sustain post-natal cardiac development.
In this review we will discuss the promising therapeutic role of non-embryonic fetal stem cells, from the amniotic fluid and placenta-derived progenitors in addressing relevant critical clinical needs for future cardiac regenerative medicine.
Fetal Progenitors: Amniotic Fluid Stem Cells for Cardiac Repair and Regeneration
The human amniotic fluid contains different cell subpopulations with heterogenous phenotypes (epithelioid, “amniotic” and fibroblastic type) deriving from the developing fetus, including mesenchymal broadly multipotent progenitors endowed with high self-renewal and clonogenic potential, as illustrated by several studies (In ‘t Anker et al., 2003; Prusa et al., 2003; De Coppi et al., 2007; Pozzobon et al., 2013). Immature amniotic fluid-derived stem cells can be easily isolated from leftover back-up sample for prenatal screening via amniocentesis or amnio-reduction or at term, from discarded amniotic fluid obtained as clinical waste during scheduled cesarean delivery (Schiavo et al., 2015; Loukogeorgakis and De Coppi, 2017). Human amniotic fluid stem cells can be mainly divided into two categories: mesenchymal progenitors isolated by their intrinsic capacity to adhere to the culture dish, namely amniotic fluid-derived mesenchymal stem cells (AF-MSC) and a further subpopulation which can be specifically identified by the expression of the stem marker c-KIT via FACS- or immuno-magnetic sorting (hAFSC, Ditadi et al., 2009; Pozzobon et al., 2013); moreover, amniotic fluid-derived stem cells have shown to possess osteogenic, myogenic, and adipogenic potentials similar to mesenchymal stem cells (De Coppi et al., 2007).
In recent years several independent studies have scrutinized the cardiovascular and cardiomyogenic potential of stem cells from amniotic fluid as an appealing tool for therapeutic cellular cardiomyoplasty for heart regeneration following ischemic injury (Chiavegato et al., 2007; Iop et al., 2008; Walther et al., 2009; Yeh et al., 2010; Bollini et al., 2011a). Being immature fetal progenitors, AF-MSC and hAFSC were initially considered more prone to commit to specific cardiac and cardiovascular lineages, upon suitable stimulation. Nevertheless, while amniotic fluid stem cells can easily adopt a smooth muscle and/or endothelial fate both in vitro and in vivo (Sartore et al., 2005; Iop et al., 2008; Bollini et al., 2011a; Ghionzoli et al., 2013; Schiavo et al., 2015; Tancharoen et al., 2017), a general consensus on their cardiomyogenic potential has not been reached yet. AF-MSC and hAFSC have shown to acquire cardiomyocyte-like phenotype following specific in vitro treatment (i.e., via direct co-culture with rodent neonatal cardiomyocytes or chemical induction by 5-aza-2′-deoxycytidine, with or without the addition of transforming growth factor beta-1, or by a mixture of hyaluronic, butyric and retinoic acids, up to modulation of Wnt signaling by small molecules), with evidence including immature expression of sarcomeric proteins, like cardiac troponins, along with up-regulation of early cardiac transcription factors, such as Nkx-2.5, Islet-1 and Gata-4 (Chiavegato et al., 2007; Bollini et al., 2011a; Guan et al., 2011; Maioli et al., 2013; Gao et al., 2014; Connell et al., 2015; Jiang and Zhang, 2017). However, in most cases, no organized sarcomeres were detected in the differentiated cells (Connell et al., 2015), with limited spontaneous contraction or functional maturation of their phenotype (Bollini et al., 2011a). Likewise, when transplanted into preclinical pig and rodent models of myocardial infarction, AF-MSC and AFSC maintained their disposition toward the vascular lineages via angiogenic differentiation, but almost completely failed to trans-differentiate into functionally mature cardiomyocytes, providing questionable results (Sartore et al., 2005; Chiavegato et al., 2007; Bollini et al., 2011a; Lee et al., 2011). Therefore, despite the initial enthusiasm and great expectations, it is now quite clear that amniotic fluid stem cells may require extensive ex-vivo reprogramming to be suitable for therapeutic cardiomyoplasty.
Yet, despite the low grade of in vivo engraftment and differentiation of amniotic fluid stem cells transplanted into preclinical animal models of myocardial infarction, different studies reported improvement of cardiac function with higher vascular density, increased cardiomyocyte survival and attenuation of ventricular remodeling (Bollini et al., 2011b; Lee et al., 2011). These data clearly suggest that stem cell-secreted molecules can influence in situ cell-cell interactions, establishing a regenerative milieu in the injured microenvironment by paracrine effects. Indeed, there is strong evidence that crucial cellular functions such as survival, proliferation, differentiation, communication, and migration can be specifically orchestrated by the secretome of stem cells injected into the injured cardiac tissue (Gnecchi et al., 2006, 2008; Mirotsou et al., 2011). First evidence of hAFSC paracrine cardio-protective potential came from a study in 2011 from Bollini et al. (2011b), showing that systemic injection of cells vs. their conditioned medium (hAFSC-CM) into an acute rat model of myocardial ischemia/reperfusion injury equally improved cell survival and significantly decreased infarct size by about 14% in 2 h (Bollini et al., 2011b). As well, independent studies confirmed that hAFSC can evoke a strong angiogenic response in murine recipients and promote neo-arteriogenesis in preclinical rodent models of hind-limb ischemia and ischemic fascio-cutaneous flap, due to the remarkable paracrine potential of their secretome supplemented by MCP-1, IL-8, SDF-1, and VEGF (Mirabella et al., 2011, 2012).
More recently, a preconditioning cell culture protocol has been optimized based on a short burst of hypoxia under serum-free condition to enrich the hAFSC secretome with cardio-active soluble factors. The paracrine cardio-protective potential of the hypoxic hAFSC-CM has been tested in vitro in a doxorubicin-derived cardiotoxicity model, showing to effectively antagonize premature senescence and apoptosis of murine neonatal cardiomyocytes and human cardiac progenitor cells. Such paracrine modulation was demonstrated to act on responder cells via prompt activation of the PI3K/Akt signaling cascade, resulting in decreased DNA damage, nuclear translocation of NF-kB, and upregulation of the NF-kB controlled genes, Il6 and Cxcl1, which support cardiomyocyte survival. The hypoxic hAFSC-CM also showed to instruct cardiomyocytes to up-regulate the efflux transporter, Abcb1b, thus triggering active extrusion of the drug from cardiac cells (Lazzarini et al., 2016).
The first characterization of extracellular vesicles (EV) released by hAFSC, namely hAFSC-EV, has also been recently reported (Balbi et al., 2017; Mellows et al., 2017). EV, including microvesicles and exosomes, are membrane-enclosed micro- and nanovesicles constitutively shed by every cell; in particular stem cell-derived EV have been proposed to act as biological carrier of paracrine regenerative soluble factors, including microRNA and mRNA, into target cells (Kishore and Khan, 2016; Marote et al., 2016; Shafei et al., 2017). Thus, the stem cell-derived EV being safer, immunologically inert and easier to manipulate than cell-based products (O'Loughlin et al., 2012; Lai et al., 2013), they are currently under detailed investigation as innovative promising approach for future regenerative cell-free therapy. Notably, hAFSC-EV demonstrated to be key mediators of regenerative paracrine effects, including stimulation of cell proliferation and survival, with remarkable modulatory potential in decreasing skeletal muscle inflammation in vivo. In particular, hypoxic hAFSC-EV mediated significant regenerative effects on responding cells by targeting post-transcriptional regulating mechanism by horizontal reprogramming via direct miRNA transfer, including miR-210 and miR-199a-3p, which have been previously reported to provide significant cardioprotection (Barile et al., 2014), sustain angiogenesis (Alaiti et al., 2012; Zeng et al., 2014) and the reactivation of cardiomyocyte proliferation (Eulalio et al., 2012). Therefore, human amniotic fluid stem cells represent a valuable candidate for future advanced medicinal/pharmacological product to treat both chemotherapy-related cardiotoxicity and myocardial infarction, due to their powerful paracrine potential. Yet, since optimal cardiac regeneration should be achieved by obtaining myocardial reconstitution on top of enhancing cardiac repair mechanisms, further investigation is required to assess whether the hAFSC secretome can also boost endogenous CPC reactivation and trigger resident cardiomyocyte proliferation following injury.
Another significantly relevant scenario is also represented by hAFSC as a potential therapeutic component in cardiac tissue engineering applications for congenital defect repairs. With most congenital heart defects being diagnosed by prenatal screening in the second trimester (Yagel et al., 1997), amniotic fluid may represent a convenient, exploitable, and autologous source easily available for collection during pregnancy. Previous studies from Hoerstrup and co-authors have suggested employing either freshly isolated or cryopreserved human amniotic fluid stem cells selected for CD133 expression to engineer heart valve leaflet scaffolds based on biodegradable polymers, with encouraging results in terms of endothelial tissue formation and functional behavior (Schmidt et al., 2007, 2008); likewise, ovine amniotic fluid stem cells have been used for in vitro fabrication of tri-leaflet heart valves to be implanted prenatally and orthotopically sheep fetuses, showing valvular integrity and absence of thrombus formation a week after transplantation (Weber et al., 2012). More recently, tissue engineered vascular grafts have been obtained from tubular vessel-like shaped biocompatible scaffolds seeded with ovine amniotic fluid progenitors under dynamic conditions in a flow bioreactor system, demonstrating the technical feasibility of such approach (Weber et al., 2016).
Since the major scientific breakthrough of induced pluripotent stem cells (iPS) technology in 2006 (Takahashi and Yamanaka, 2006), growing interest has been dedicated toward exploiting direct reprogramming of somatic cells into pluripotent progenitors as functional source to derive contractile autologous cardiomyocytes (Lian et al., 2012). This has been recently endorsed as a working strategy to overcome the incomplete and poor yield of somatic stem cell cardiomyogenic trans-differentiation, which may jeopardize myocardial renewal and reconstitution. Hence, the quest is now on defining the most suitable cell to efficiently induce iPS from and since amniotic fluid stem cells are immature fetal progenitors endowed with some degree of instrinsic pluripotency and active expression of embryonic genes including OCT4, NANOG, and SOX2 (Loukogeorgakis and De Coppi, 2017), they represent an ideal candidate. Recent studies have reported that murine and human AFSC can be reprogrammed into iPS more easily than adult stem cells by applying either transgene-free approaches, like chemical defined conditions via stimulation with the histone deacetylase inhibitor valproic acid (Moschidou et al., 1953), as well as non-integrating methods by episomal plasmid, transposon system, sendai virus or mRNA delivery by lipofection (Jiang et al., 2016; Slamecka et al., 2016; Bertin et al., 2017; Velasquez-Mao et al., 2017); notably the obtained AFSC-iPS have been proven capable of functional cardiac differentiation, thus providing important impact for future cardiac regenerative therapy and specific relevance for the treatment of neonatal cardiac congenital disease (Jiang et al., 2016; Velasquez-Mao et al., 2017). Indeed, hAFSC can be effortlessly harvested during prenatal diagnosis, treated by gene therapy and iPS technology to derived healthy myocardial and cardiovascular cells to be then processed by tissue engineering approaches so to provide cardiac grafts developed in parallel with gestation and promptly implanted in utero or at birth.
Notably, while II trimester fetal hAFSC have been widely investigated, little is known about the regenerative capacity of III trimester perinatal hAFSC obtained at term from discarded amniotic fluid samples from eligible cesarean delivery. Indeed, III trimester amniotic fluid might represent a more abundant source of hAFSC. While recent work has showed that III trimester hAFSC are endowed with significant pro-angiogeneic action (Schiavo et al., 2015), a detailed, comprehensive differential characterization of their cardiac and cardiovascular potential has not yet been provided.
Perinatal Progenitors: Placenta-Derived Stem Cells for Cardiac Repair
The Human Placenta
Upon attachment and invasion into the uterine wall, the embryonic trophoblast concomitant with the embryoblast start differentiating, and together with maternal endometrial transition forming the placental tissues. These tissues establish implantation, support the fetus and maintain pregnancy by orchestrating the maternal adaption. At full term, the placenta is a large discoid organ, 15–20 cm in diameter and 2–3 cm in thickness, weighing approximately 500–600 g. At this time, the placenta comprises tissues of maternal and fetal origin (Malina, 1993). At the maternal side, the basal plate, a thin layer of maternal decidua basalis is covering the cotyledons that are interspaced by grooves forming the decidua septa. The maternal plate is closely intertwined with the fetal, the chorionic plate that also comprises the vascular system of arteries and veins that feed the umbilical cord (Sadler et al., 2012). The innermost of these fetal membranes is the amnion, enclosing the amniotic fluid. Main functions of the placenta are the selective exchange of metabolic and gaseous products between maternal and fetal bloodstreams and its endocrine activity secreting more than 100 peptides and steroid hormones (Burton and Fowden, 2015). The placenta is further unique in its immuno-regulatory functions allowing maternal tolerance and support of the growing embryo/fetus throughout pregnancy (Mori et al., 2016; Vinketova et al., 2016).
This highly active organ has also been recognized as rich source of human progenitor cells, extracellular matrix and bioactive compounds. Human placenta is usually discarded after birth, and hence ethically uncontroversial, it is large in size yielding high amounts of easily accessible human tissue compounds. With the beginning of the twenty first century, researchers started to realize that cells of placental tissues show distinct stem cell qualities, such as expression of markers of pluripotency (Miki et al., 2007) and the potential to differentiate into lineages of all three germ layers (Kakishita et al., 2003; Takashima et al., 2004; Miki et al., 2005; Portmann-Lanz et al., 2006), as well as paracrine properties such as anti-inflammatory, antibacterial and anti-fibrotic activities, which recommend these cells for regenerative medicine and wound healing applications (Cargnoni et al., 2009, 2014; Lopez-Espinosa et al., 2009; Hong et al., 2010; De et al., 2011; Choi et al., 2013; Ricci et al., 2013; SantAnna et al., 2016).
Cells Isolated From Different Placental Tissues
Derivation and cultivation of several cell types with reported stem/progenitor properties from human term placenta has been described (Bailo et al., 2004; Miki et al., 2005; Soncini et al., 2007). Cell populations can be derived from specific placental tissues and the nomenclature used is based on the consensus from the First International Workshop on Placenta-Derived Stem Cells (Parolini et al., 2008): amnion to obtain human amniotic epithelial cells (hAEC) and amniotic mesenchymal stromal cells (hAMSC), and chorionic mesoderm to obtain chorionic mesenchymal stromal cells (hCMSC), chorionic villi to obtain MSC and endothelial progenitor cells (Zhang et al., 2006; Rapp et al., 2012) or placental cotyledons (Sölder et al., 2012), but also maternal cells from the decidua, to obtain decidual stromal cells (Huang et al., 2009). In addition, MSC can be obtained from umbilical cord (hUCMSC, including Wharton's Jelly) (Troyer and Weiss, 2008; La Rocca et al., 2009), from the chorionic plate/trophoblast (hCpMSC), and from placenta tissue in toto (i.e., no specific compartment selected; PDMSC). Herein other placental MSC will be discussed as well, such Placenta-derived Adherent cells (PDA-001, Celgene Therapeutics), and PLacental eXpanded (PLX) mesenchymal-like adherent stromal cells (PLX-PAD, Pluristem Therapeutics Inc.). Noteworthy, in a substantial number of studies the purity or even identity of fetal or maternal origin of cells isolated from the placenta chorionic plate and decidua has not been investigated (Heazlewood et al., 2014).
General Features of Placental Cells
Phenotype
Several studies demonstrate that the adherent cellular fraction derived from placenta express cell surface markers similar to bone marrow-derived mesenchymal stem/stromal cells such CD13, CD29, CD44, CD73, CD90, CD105, CD166, and MHC I, and are mostly negative for CD14, CD34, CD45, and MHC II at term (In ‘t Anker et al., 2003; Portmann-Lanz et al., 2006; Parolini et al., 2008; Magatti et al., 2015). A sub-fraction of these cells have further been shown to express heterogeneous degrees of markers previously identified in pluripotent stem cells including SSEA-4, Tra-160, Tra-181, octamer-binding protein 4 (Oct-4), Nanog or Sox-2 (Miki and Strom, 2006; Portmann-Lanz et al., 2006; Alviano et al., 2007; Kim et al., 2007; Miki et al., 2007), the level of which however depends on factors such as the gestational age of the placenta (Izumi et al., 2009; Barboni et al., 2014).
Differentiation Potential
It has been suggested that placenta-derived cells can be classified as an intermediate state between pluripotent stem cells and multipotent adult stem cells (reviewed by Kang et al., 2012). However, in contrast to embryonic stem cells, placental cells such as hAEC do not express substantial levels of telomerase, are not tumorigenic, and do not become aneuploid (Miki et al., 2005), thus being considered a promising source of stem cells. Indeed, several research groups have focused on the human placenta progenitor differentiation potential. Tamagawa et al. were the first to demonstrate pluripotent characteristics of cells from human amnion (Tamagawa et al., 2004). hAEC were shown to differentiate into all three germ layers, including hepatic, neural and pancreatic lineages (Miki et al., 2005; Miki and Strom, 2006). Naïve, undifferentiated cells of both amnion and chorion showed to express markers of glial and neuronal progenitor cells in vitro (Sakuragawa et al., 1996). Furthermore, cultured hAEC were found to produce acetylcholine, acetyltransferase and dopamine (Sakuragawa et al., 1997; Kakishita et al., 2000). Further, neuronal markers could be induced in vitro by exposure to retinoic acid in hAEC, hAMSC and hCMSC (Portmann-Lanz et al., 2006). Knezevic showed that ectopic in vitro transplantation of rat amnion under the kidney capsule resulted in differentiation into stratified squamous epithelium, a morphologically similar structure to skin, and derivatives, such as hair follicles and sebaceous glands (Knezevic, 1996). Mesodermal differential potential of hAMSC and hCMSC has also been demonstrated for both adipogenic and osteogenic lineages in vitro (In ‘t Anker et al., 2003). Other independent studies also reported the acquisition of chondrogenic, myogenic and cardiomyogenic fates (Wei et al., 2003; Miki et al., 2005; Portmann-Lanz et al., 2006; Ilancheran et al., 2007).
A capacity to commit to a functional phenotype has also been suggested in vivo. For example, hAEC transplanted into SCID/beige mice liver have been shown to integrate into the hepatic plate and adopt a hepatic phenotype, including secretion of albumin or α-1 antitrypsin, as well as metabolic features of functional hepatocytes (Sakuragawa et al., 2000; Marongiu et al., 2011). Differentiation of hAEC into lung epithelial cells was suggested in a bleomycin-induced lung injury model in immunodeficient mice, along with a distinct anti-fibrotic and anti-inflammatory effect was demonstrated (Moodley et al., 2010). Even pancreatic differentiation of hAEC was suggested in vivo, when transplantation of hAEC into the spleen of immunodeficient mice in a diabetic model restored normalized blood glucose levels in these animals; although also hAMSC were suggested to differentiate into the pancreatic lineage (Wei et al., 2003), the insulin production of the alleged hAMSC might actually be originating from a certain percentage of hAEC present in the culture (reviewed by Miki et al., 2005).
Immunomodulation
Together with the differentiation capabilities, different findings have demonstrated the reduced immunogenicity and multifaceted immunomodulatory capacity of placenta-derived cells. Within placenta tissues, hAMSC and their conditioned medium (hAMSC-CM) have been shown to reduce in vitro T cell proliferation induced by alloantigens, via T-cell receptor or mitogens (Magatti et al., 2008; Rossi et al., 2012). hAMSC and hAMSC-CM significantly reduce the expression of markers associated to Th1 and Th17 populations, and significantly induce the regulatory T cells compartment (Pianta et al., 2015, 2016). Amniotic cells are able to block the differentiation of monocytes into both dendritic cells (DC) and inflammatory M1-macrophages and to skew monocyte differentiation toward anti-inflammatory M2 macrophages. The macrophages generated in the presence of amniotic cells were poor inducers of T-cell proliferation, increased production of the anti-inflammatory cytokine IL-10, and reduced secretion of different pro-inflammatory factors (Magatti et al., 2009, 2015). Additionally, the macrophages generated in the presence of CM enhance wound healing in diabetic mice (Magatti et al., 2017). The therapeutic effects of amniotic cells and their secretome have been reported in other preclinical models of diseases based on inflammatory processes and with altered immune reactions, such as lung (Cargnoni et al., 2009, 2012, 2014) and liver fibrosis (Ricci et al., 2013; SantAnna et al., 2016; Cargnoni et al., 2017), sepsis, inflammatory bowel disease, autoimmune encephalomyelitis and rheumatoid arthritis (Parolini et al., 2014) cardiac ischemia (Cargnoni et al., 2009), and traumatic brain injury (Pischiutta et al., 2016). In these diseases, the modulation of inflammation seems to be a key element underlying the restoration of tissue integrity promoted by placental cells and their bioactive factors (Silini et al., 2017). Taken together, differentiation and immunomodulatory properties render placental cells, and their secretome, very interesting candidates for a variety of therapeutic applications, and in the following section their contribution to cardiac regeneration will be discussed.
Placenta-Derived Cells in Cardiac Regeneration: Mechanisms Relevant for Cardiac Repair
The placental cells used in preclinical studies discussed in this section are hAMSC, hAEC, hCMSC, and hUCMSC (including Wharton's Jelly). In addition, MSC from the chorionic plate/trophoblast (hCpMSC), and MSC isolated from placenta tissue in toto (i.e., no specific compartment described; PDMSC) will be discussed. We will also consider studies in which Placenta-derived Adherent cells (PDA-001, Celgene Therapeutics), and PLacental eXpanded (PLX) mesenchymal-like adherent stromal cells (PLX-PAD, Pluristem Therapeutics Inc.) are used.
Essentially, there are 4 mechanisms that could be held accountable for the therapeutic effects mediated by placental cells on the injured cardiac tissue, which can be mainly related to their direct cell differentiation or to specific paracrine effects, such as anti-apoptotic, pro-angiogenic, and immunomodulatory properties as the result of the administration of their derivatives (i.e., the cell secretome as represented by their cell-conditioned medium and/or extracellular vesicles), respectively. These features make placental cells worthy competitors in the field of regenerative medicine. These will be discussed in the following sections in order to provide insight onto how placental cells and derivatives are able to support cardiac regeneration.
Cardiovascular and Cardiomyogenic Differentiation
Even if nowadays it does not seem to be the major mechanism supporting the placental-cell therapeutic effects (Balbi and Bollini, 2017) there is a modest amount of groups which have provided evidence of placental-cell differentiation toward cardiovascular and cardiomyocyte-like cells. Evidence of this is shown by the expression of cardiomyocyte markers and/or acquisition of spontaneous cell beating. In vitro placental cell differentiation has been suggested for UCMSC after incubation with 5-azacytdine (Pereira et al., 2008), and after co-culture of neonatal murine cardiomyocytes with hAMSC (Tsuji et al., 2010) and PDMSC (MSC isolated from placenta tissue in toto) (Liu et al., 2015). Alviano et al. reported in vitro angiogenic differentiation of hAMSC (Alviano et al., 2007), suggesting that term placenta might also contain progenitors with endothelial commitment potential, as reported by others (König et al., 2012, 2015; Meraviglia et al., 2012; González et al., 2015; Abumaree et al., 2017).
In vivo, even if in the majority of studies presented herein placental cells are few and rarely found in preclinical models at time of sacrifice, cardiomyocyte differentiation has been reported for hAEC following transplantation in immunocompromised rats with myocardial infarction (Fang et al., 2012). Likewise, PDMSC treated with a mixed cocktail of hyaluronan, butyric and retinoic acid (HBR) as cardiogenic/vasculogenic inducer, showed partial structural differentiation into cardiomyocyte-like cells, following transplantation into infarcted rat hearts (Ventura et al., 2007).
Cardioprotection and Inhibition of Apoptosis
Inhibition of cardiomyocyte apoptosis is pivotal in rescuing the cardiac tissue. Placental cells and their derivatives have been reported to be able to counteract cardiomyocyte apoptosis both in vitro and in vivo via significant paracrine influence. For example, conditioned media of UCMSC cultured in hypoxia has been shown to reduce apoptosis of cardiomyocytes (Santos Nascimento et al., 2014; Zhao et al., 2016), in vitro; as well, cardiac cell death was further reduced when secretome of HGF-transfected UCMSC (cultured in hypoxia) was used (Zhao et al., 2016). In vivo, the same group showed that transplantation of UCMSC was able to enhance cardiac function, decrease cardiomyocyte apoptosis, increase cardiomyocyte proliferation, and increase capillary density in immunodeficient mice with MI. These parameters were further improved following treatment with HGF-transfected UCMSC (Zhao et al., 2016). The anti-apoptotic effects of UCMSC have also been described by others following implantation in immune-competent mice with MI, whereby the number of apoptotic cells in infarcted hearts was reduced by 40% compared to vehicle-treated mice (Santos Nascimento et al., 2014). In addition, extracellular vesicles and exosomes isolated from UCMSC secretome have been shown to reduce cardiac fibrosis and cell apoptosis, while increasing cell proliferation in the hearts of rats with myocardial infarction, after intravenous injection (Zhao et al., 2015). Exosomes from UCMSC have also been studied by another group who observed a decrease of apoptotic cells in exosome-treated animals compared to PBS-treated ones (Ma et al., 2016). Furthermore, in a minipig model of MI, PDMSC-treated pigs showed lower TUNEL-positive cardiomyocytes at the infarct border area, compared to vehicle-treated pigs, 8 weeks after injury (Liu et al., 2015). Cardiomyocyte proliferation has also been shown to be enhanced after transplantation of placenta-derived adherent cells (PDA-001) in a mouse model of chronic heart failure (Chen et al., 2015). To further confirm the relevance of placenta cell cardioprotective paracrine potential, an innovative hydrogel formulation obtained from placental matrix and growth factors has been recently used to improve iPS-derived cardiomyocyte culture in vitro, also showing paracrine enhancement of cardiac repair as a delivery vehicle (Francis et al., 2017).
Pro-Angiogenic Effect
Regarding the pro-angiogeneic influence of perinatal progenitors, cell secretomes from both PDMSC (Liu et al., 2015) and PLX-PAD (Pluristem's mesenchymal-like adherent cells isolated from human term placenta) (Roy et al., 2013) have been shown to stimulate HUVEC proliferation and tube formation in vitro. In addition, exosomes from UCMSC were demonstrated to stimulate proliferation (Ma et al., 2016), migration (Zhao et al., 2015; Ma et al., 2016), and tube-forming ability (Zhao et al., 2015) of EA.hy926 human endothelial cells in vitro. Similarly, different types of placental cells have been shown to promote angiogenesis within the infarcted myocardium in vivo. For example, PDMSC significantly enhanced angiogenesis when injected into the MI border area in immune-compromised mice (Liu et al., 2015), resulting in increased vascularization compared to control group (Liu et al., 2015); improved heart contractibility was also detected in minipig hearts undergoing MI when PDMSC were injected in the border zone (Liu et al., 2015). Although functional cardiomyogenic differentiation of placental cells have been generally shown to be controversial, PDMSC were retained in the minipig hearts for up to 8 weeks post treatment while co-expressing HLA-ABC and cardiac troponin T, a marker for striated cardiomyocytes (Liu et al., 2015). In contrast, in a mouse model of myocardial infarction, chorionic plate MSC (CpMSC) did not successfully engraft and survive starting from third day post-injection, and cells disappeared more rapidly after the second and third injections (i.e., disappeared after 24 h), even if at 40 days after first treatment cardiac function was improved (Passipieri et al., 2014). In line with these results, transplantation of placenta-derived adherent cells (PDA-001) in a mouse model of chronic heart failure has been shown to increase endothelial cell proliferation and capillary density when injected intramyocardially (Chen et al., 2015). Notably, capillary density improvement was observed only when a low-dose (0.5 × 104 vs. high dose 0.5 × 106) of cells was used, suggesting the latter to be more efficient in providing tissue repair (Chen et al., 2015), despite no human cells were detected in the murine heart 28 days after injection (Chen et al., 2015). PLX-PAD have also been shown to stimulate arteriogenesis after injection in the peri-infarct area, following left anterior descending artery ligation in immune-competent mice (Roy et al., 2013), resulting into more abundant and mature arterial blood vessels. In addition, PLX-PAD-treated hearts showed higher microvessel density compared to vehicle-treated hearts. The same group also reported better cardiac regeneration capability of hAEC injected in mice with MI after their specific in vitro preconditioning in order to enhance epithelial-mesenchymal transition (EMT, i.e., hAEC treated with TGF beta for 6 days in vitro prior to injection), compared to non-induced hAEC (Roy et al., 2015). Indeed, hAEC sustained local angiogenesis, with greater resident cardiomyocyte pro-survival influence as exerted by EMT-hAEC (Roy et al., 2015).
The human amniotic membrane (AM) has also been shown to provide therapeutic effects when used as a patch implant in rodent models of cardiac disease. Cargnoni et al. have demonstrated that epicardial application of the AM patch on infarcted rat hearts is able to significantly reduce post-ischemic cardiac dimensional alterations, thus improving myocardial function (Cargnoni et al., 2009). Beneficial effects were apparent 7 days after AM application and continued for up to 60 days post treatment (Cargnoni et al., 2009). In line with this, Roy et al. revealed that treatment of infarcted heart with patches of either intact or decellularized amniotic membrane reduced infarct size (Roy et al., 2016). Decellularized AM showed to be immunologically inert, suggesting that ECM components in the AM might be crucial in exerting beneficial effects on the infarcted heart (Roy et al., 2016).
The pro-angiogenic effects of UCMSC have also been described following implantation in immune-competent mice with myocardial infarction (Santos Nascimento et al., 2014). In addition, exosomes from CM obtained from UCMSC have also been shown to increase blood vessel numbers in chick allantoic membrane assay (CAM), when compared to DMEM-treated CAM (Ma et al., 2016). Interestingly, exosomes obtained from Akt-induced UCMSC had a higher efficiency in promoting angiogenesis, and this was suggested to be due to the fact that Akt-induced exosomes shuttle PDGF (a growth factor found to be enriched in this type of exosomes) to recipient cardiac and endothelial cells, thus promoting their proliferation, migration, and blood vessel formation (Ma et al., 2016). The above-mentioned angiogenic properties along with the proposed paracrine mode of action of placental cells has encouraged several groups to investigate protein and soluble factor content in the conditioned medium of cultured placental cells. Pro-angiogenic proteins such as vascular endothelial growth factor (VEGF) and angiopoietin-1 have been shown to be found in PLX-PAD cell secretome, which were found increased when cell conditioned medium was collected from cells cultured in hypoxia (Roy et al., 2013).
In addition, hypoxia was able to decrease tissue inhibitor of matrix metalloproteinase-1, an inhibitor of endothelial cells (Reed et al., 2003) and promoter of myocardial fibrosis (Takawale et al., 2017). Others have also demonstrated that PDMSC can secrete hepatocyte growth factor (HGF), interleukin (IL)-8, and growth related oncogene (GRO)-alpha (Liu et al., 2015). MSC from fetal membranes can also secrete VEGF and HGF, which is enhanced when cells are treated with hyaluronan with butyric and retinoic acid (HBR) (Ventura et al., 2007). On another note, the same group showed that HBR also enhances in vitro differentiation of MSC toward cardiomyocyte-like cells, thus suggesting that HBF encompasses both differentiating features for MSC and potential to afford growth factor-mediated paracrine regeneration (Ventura et al., 2007). Likewise, conditioned medium from UCMSC cultured in hypoxia contains angiogenic factors such as VEGF, HGF, epidermal growth factor (EGF), and basic fibroblast growth factor (bFGF), and the presence of these factors is enhanced with CM is collected from HGF-transfected UCMSC cultured in hypoxia (Zhao et al., 2016).
Immunomodulatory Potential
The inflammatory response to an injury is a critical regulator of the regenerative process. Immunomodulatory properties of placental cells have been widely demonstrated in vitro (Magatti et al., 2016) and transplantation of placental cells and their derivatives has been shown to exert in vivo therapeutic effects in a variety of diseases with altered immune responses (Magatti et al., 2016), thus supporting the notion that targeting inflammation is beneficial to the regenerative process (Silini et al., 2017).
When considering cardiac injury, placental cell immunomodulatory properties are a fertile ground for investigation and could potentially contribute to regeneration of the diseased heart. As in other immune-regulated diseases such as in lung (Cargnoni et al., 2009, 2012, 2014; Moodley et al., 2009, 2010; Murphy et al., 2011, 2012; Vosdoganes et al., 2011; Hodges et al., 2012; Chambers et al., 2014; Tan et al., 2014) and liver fibrosis (Tsai et al., 2009; Manuelpillai et al., 2010, 2012; Sant'Anna et al., 2011; SantAnna et al., 2016; Zhang et al., 2011; Ricci et al., 2013; Cargnoni et al., 2017), placental cells and their soluble derivatives have been shown to decrease fibrosis in the hearts of animals MI. Indeed, the inflammatory response after injury is critical in regulating tissue regeneration, and the inflammatory response occurs even after cardiac injury, thus what we learn from how placental cells modulate the inflammatory response to favor tissue regeneration in models of chronic fibrosis could shed light onto how placental cells could be beneficial to the regenerative process after cardiac injury. For example, transplantation of placenta-derived adherent cells (PDA-001) in a mouse model of chronic heart failure has been shown to improve cardiac performance and decrease fibrosis when injected intra-myocardially (Chen et al., 2015). Moreover, exosomes isolated from the CM of UCMSC have been shown to reduce cardiac fibrosis of rats with myocardial infarction after intravenous injection (Zhao et al., 2015). These studies instigate future ones in order to understand how the immunomodulatory properties placental cells and derivatives contribute to cardiac regeneration.
Future Perspectives in the Adult and in the Pediatric Patient
Cardiovascular disease patients need prompt therapeutic intervention, especially pediatric ones affected by congenital heart disease.
In such scenario, the ideal stem cell source should be selected upon consideration their potential, feasibility of their isolation together, with their in vitro self-renewal properties. Thus, fetal and perinatal stem cells isolated either during pregnancy from left-over samples obtained for prenatal screening, or at term from clinical waste material, can offer added value compared to somatic adult sources, given their immature and developmentally “younger” potential, ease of availability, expansion and cryopreservation for long-term use while maintaining stable karyotype and low immunogenic profile. Furthermore these cells can also offer exclusive therapeutic advantages, as illustrated in the schematic Figure 1; indeed, on top their paracrine and differentiation potential, they can be reprogrammed more efficiently into pluripotent cells for putative drug-screening, cell therapy and cardiac tissue engineering approaches, and could be also envisioned for prenatal management and treatment of cardiac congenital diseases in utero, as already suggested by preliminary testing in preclinical animal models of different disease such as gastroschisis and spina bifida (Tam et al., 1989; Ramachandra et al., 2014; Dionigi et al., 2015; Shaw et al., 2016).
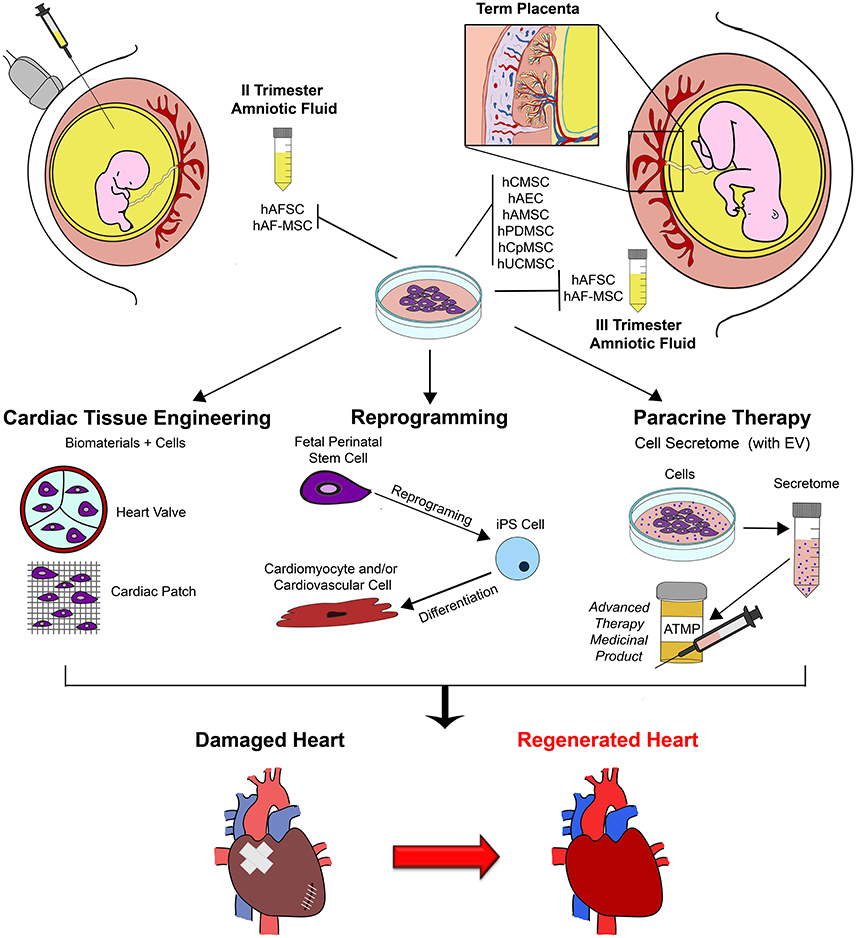
Figure 1. Schematic representation of exploitation of fetal perinatal stem cell biology for cardiac regeneration. Mesenchymal stromal progenitors can be isolated by amniotic fluid samples harvested during prenatal screening procedure (II trimester gestation) or at term, during scheduled cesarean delivery (III trimester); similarly, several population of stem cells can be isolated from term placenta at birth. Fetal perinatal stem cells can be easily isolated and cultured in vitro. Their peculiar regenerative features can be exploited to enhance cardiac repair and sustain cardiac regeneration by different approaches, including tissue engineering and cell therapy (possibly via perinatal stem cell reprogramming into more immature pluripotent cells to obtain mature cardiomyocyte and cardiovascular cells from) and paracrine therapy, via the formulation of the stem cell secretome, into a putative future advanced therapy medicinal product (ATMP). hAFSC, human c-KIT+ Amniotic Fluid Stem Cells; hAFS-MSC, human Amniotic Fluid Mesenchymal Stem Cells; EV, Extracellular Vesicles; hCMSC, human Chorionic Mesenchymal Stromal Cells; hAEC, human Amniotic Epithelial Cells; hAMSC, human Amniotic Mesenchymal Stromal Cells; hPDMSC, human Placenta-Derived Mesenchymal Stromal Cells; hCpMSC, human Chorionic plate/trophoblast Mesenchymal Stromal Cells; hUCMSC, human Umbilical Cord Mesenchymal Stromal Cells; iPS, induced Pluripotent Stem Cell.
Nevertheless, further efforts should be made to address the standardization of cell isolation protocols and cell culture conditions, and also preparation of the cell secretome, since a definitive consensus has not been obtained yet; for sure, this represents a crucial aspect in order to avoid result diversification in preclinical studies and to support opportune clinical translation for future therapeutic strategies.
Author Contributions
SB: Supervised the work and mainly contributed to the discussion on mechanisms of cardiac repair and regeneration and on the amniotic fluid stem cell biology; CB: Contributed to critical discussion and assisted with the schematic figure; AS and OP: Contributed to the discussion on the mechanisms of action of placental cells in their therapeutic applications; SW and AB: Contributed by describing the basic properties of the placenta and the cells derived thereof. All authors reviewed the manuscript and approved it.
Funding
This work was partially supported by Fondazione Poliambulanza Istituto Ospedaliero, Brescia, Italy (AS and OP).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Abumaree, M. H., Hakami, M., Abomaray, F. M., Alshabibi, M. A., Kalionis, B., Al Jumah, M. A., et al. (2017). Human chorionic villous mesenchymal stem/stromal cells modify the effects of oxidative stress on endothelial cell functions. Placenta 59, 74–86. doi: 10.1016/j.placenta.2017.05.001
Alaiti, M. A., Ishikawa, M., Masuda, H., Simon, D. I., Jain, M. K., Asahara, T., et al. (2012). Up-regulation of miR-210 by vascular endothelial growth factor in ex vivo expanded CD34+ cells enhances cell-mediated angiogenesis. J. Cell. Mol. Med. 16, 2413–2421. doi: 10.1111/j.1582-4934.2012.01557.x
Alviano, F., Fossati, V., Marchionni, C., Arpinati, M., Bonsi, L., Franchina, M., et al. (2007). Term Amniotic membrane is a high throughput source for multipotent mesenchymal stem cells with the ability to differentiate into endothelial cells in vitro. BMC Dev. Biol. 7:11. doi: 10.1186/1471-213X-7-11
Bailo, M., Soncini, M., Vertua, E., Signoroni, P. B., Sanzone, S., Lombardi, G., et al. (2004). Engraftment potential of human amnion and chorion cells derived from term placenta. Transplantation 78, 1439–1448. doi: 10.1097/01.TP.0000144606.84234.49
Balbi, C., and Bollini, S. (2017). Fetal and perinatal stem cells in cardiac regeneration: moving forward to the paracrine era. Placenta 59, 96–106. doi: 10.1016/j.placenta.2017.04.008
Balbi, C., Piccoli, M., Barile, L., Papait, A., Armirotti, A., Principi, E., et al. (2017). First characterization of human amniotic fluid stem cell extracellular vesicles as a powerful paracrine tool endowed with regenerative potential. Stem Cells Transl. Med. 6, 1340–1355. doi: 10.1002/sctm.16-0297
Barboni, B., Russo, V., Curini, V., Martelli, A., Berardinelli, P., Mauro, A., et al. (2014). Gestational stage affects amniotic epithelial cells phenotype, methylation status, immunomodulatory and stemness properties. Stem Cell Rev. 10, 725–741. doi: 10.1007/s12015-014-9519-y
Barile, L., Lionetti, V., Cervio, E., Matteucci, M., Gherghiceanu, M., Popescu, L. M., et al. (2014). Extracellular vesicles from human cardiac progenitor cells inhibit cardiomyocyte apoptosis and improve cardiac function after myocardial infarction. Cardiovasc. Res. 103, 530–541. doi: 10.1093/cvr/cvu167
Bertin, E., Piccoli, M., Franzin, C., Nagy, A., Mileikovsky, M., De Coppi, P., et al. (2017). The production of pluripotent stem cells from mouse amniotic fluid cells using a transposon system. J. Vis. Exp. doi: 10.3791/54598. [Epub ahead of print].
Bollini, S., Cheung, K. K., Riegler, J., Dong, X., Smart, N., Ghionzoli, M., et al. (2011b). Amniotic fluid stem cells are cardioprotective following acute myocardial infarction. Stem Cells Dev. 20, 1985–1994. doi: 10.1089/scd.2010.0424
Bollini, S., Pozzobon, M., Nobles, M., Riegler, J., Dong, X., Piccoli, M., et al. (2011a). In vitro and in vivo cardiomyogenic differentiation of amnioti fluid stem cells. Stem Cells Rev. 7, 364–380. doi: 10.1007/s12015-010-9200-z
Burton, G. J., and Fowden, A. L. (2015). The placenta: a multifaceted, transient organ. Philos. Trans. R. Soc. Lond. B Biol. Sci. 370:20140066. doi: 10.1098/rstb.2014.0066
Cargnoni, A., Di Marcello, M., Campagnol, M., Nassuato, C., Albertini, A., and Parolini, O. (2009). Amniotic membrane patching promotes ischemic rat heart repair. Cell Transplant. 18, 1147–1159. doi: 10.3727/096368909X12483162196764
Cargnoni, A., Farigu, S., Cotti Piccinelli, E., Bonassi Signoroni, P., Romele, P., Vanosi, G., et al. (2017). Effect of human amniotic epithelial cells on pro-fibrogenic resident hepatic cells in a rat model of liver fibrosis. J. Cell. Mol. Med. 22, 1202–1213. doi: 10.1111/jcmm.13396
Cargnoni, A., Piccinelli, E. C., Ressel, L., Rossi, D., Magatti, M., Toschi, I., et al. (2014). Conditioned medium from amniotic membrane-derived cells prevents lung fibrosis and preserves blood gas exchanges in bleomycin-injured mice-specificity of the effects and insights into possible mechanisms. Cytotherapy 16, 17–32. doi: 10.1016/j.jcyt.2013.07.002
Cargnoni, A., Ressel, L., Rossi, D., Poli, A., Arienti, D., Lombardi, G., et al. (2012). Conditioned medium from amniotic mesenchymal tissue cells reduces progression of bleomycin-induced lung fibrosis. Cytotherapy 14, 153–161. doi: 10.3109/14653249.2011.613930
Chambers, D. C., Enever, D., Ilic, N., Sparks, L., Whitelaw, K., Ayres, J., et al. (2014). A phase 1b study of placenta-derived mesenchymal stromal cells in patients with idiopathic pulmonary fibrosis. Respirology 19, 1013–1018. doi: 10.1111/resp.12343
Chen, H.-J., Chen, C.-H., Chang, M.-Y., Tsai, D.-C., Baum, E. Z., Hariri, R., et al. (2015). Human placenta-derived adherent cells improve cardiac performance in mice with chronic heart failure. Stem Cells Transl. Med. 4, 269–275. doi: 10.5966/sctm.2014-0135
Chiavegato, A., Bollini, S., Pozzobon, M., Callegari, A., Gasparotto, L., Taiani, J., et al. (2007). Human amniotic fluid-derived stem cells are rejected after transplantation in the myocardium of normal, ischemic, immuno-suppressed or immuno-deficient rat. J. Mol. Cell. Cardiol. 42, 746–759. doi: 10.1016/j.yjmcc.2006.12.008
Choi, J. S., Kim, J. D., Yoon, H. S., and Cho, Y. W. (2013). Full-thickness skin wound healing using human placenta-derived extracellular matrix containing bioactive molecules. Tissue Eng. Part A 19, 329–339. doi: 10.1089/ten.tea.2011.0738
Connell, J. P., Ruano, R., and Jacot, J. G. (2015). Amniotic fluid-derived stem cells demonstrate limited cardiac differentiation following small molecule-based modulation of Wnt signaling pathway. Biomed. Mater. 10:34103. doi: 10.1088/1748-6041/10/3/034103
De Coppi, P., Bartsch, G. Jr., Siddiqui, M. M., Xu, T., Santos, C. C., Perin, L., et al. (2007). Isolation of amniotic stem cell lines with potential for therapy. Nat. Biotechnol. 25, 100–106. doi: 10.1038/nbt1274
De, D., Chakraborty, P. D., and Bhattacharyya, D. (2011). Regulation of trypsin activity by peptide fraction of an aqueous extract of human placenta used as wound healer. J. Cell. Physiol. 226, 2033–2040. doi: 10.1002/jcp.22535
Dionigi, B., Brazzo, J. A., Ahmed, A., Feng, C., Wu, Y., Zurakowski, D., et al. (2015). Trans-amniotic stem cell therapy (TRASCET) minimizes Chiari-II malformation in experimental spina bifida. J. Pediatr. Surg. 50, 1037–1041. doi: 10.1016/j.jpedsurg.2015.03.034
Ditadi, A., de Coppi, P., Picone, O., Gautreau, L., Smati, R., Six, E., et al. (2009). Human and murine amniotic fluid c-Kit+Lin- cells display hematopoietic activity. Blood 113, 3953–3960. doi: 10.1182/blood-2008-10-182105
Eulalio, A., Mano, M., Ferro, M. D., Zentilin, L., Sinagra, G., Zacchigna, S., et al. (2012). Functional screening identifies miRNAs inducing cardiac regeneration. Nature 492, 376–381. doi: 10.1038/nature11739
Fang, C.-H., Jin, J., Joe, J.-H., Song, Y.-S., So, B.-I., Lim, S. M., et al. (2012). In vivo differentiation of human amniotic epithelial cells into cardiomyocyte-like cells and cell transplantation effect on myocardial infarction in rats: comparison with cord blood and adipose tissue-derived mesenchymal stem cells. Cell Transplant. 21, 1687–1696. doi: 10.3727/096368912X653039
Francis, M. P., Breathwaite, E., Bulysheva, A. A., Varghese, F., Rodriguez, R. U., Dutta, S., et al. (2017). Human placenta hydrogel reduces scarring in a rat model of cardiac ischemia and enhances cardiomyocyte and stem cell cultures. Acta Biomater. 52, 92–104. doi: 10.1016/j.actbio.2016.12.027
Gao, Y., Connell, J. P., Wadhwa, L., Ruano, R., and Jacot, J. G. (2014). Amniotic fluid-derived stem cells demonstrated cardiogenic potential in indirect co-culture with human cardiac cells. Ann. Biomed. Eng. 42, 2490–2500. doi: 10.1007/s10439-014-1114-5
Ghionzoli, M., Repele, A., Sartiani, L., Costanzi, G., Parenti, A., Spinelli, V., et al. (2013). Human amniotic fluid stem cell differentiation along smooth muscle lineage. FASEB J. 27, 4853–4865. doi: 10.1096/fj.12-218578
Giza, D. E., Iliescu, G., Hassan, S., Marmagkiolis, K., and Iliescu, C. (2017). Cancer as a risk factor for cardiovascular disease. Curr. Oncol. Rep. 19:39. doi: 10.1007/s11912-017-0601-x
Gnecchi, M., He, H., Noiseux, N., Liang, O. D., Zhang, L., Morello, F., et al. (2006). Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 20, 661–669. doi: 10.1096/fj.05-5211com
Gnecchi, M., Zhang, Z., Ni, A., and Dzau, V. J. (2008). Paracrine mechanisms in adult stem cell signaling and therapy. Circ. Res. 103, 1204–1219. doi: 10.1161/CIRCRESAHA.108.176826
González, P. L., Carvajal, C., Cuenca, J., Alcayaga-Miranda, F., Figueroa, F. E., Bartolucci, J., et al. (2015). Chorion mesenchymal stem cells show superior differentiation, immunosuppressive, and angiogenic potentials in comparison with haploidentical maternal placental cells. Stem Cells Transl. Med. 4, 1109–1121. doi: 10.5966/sctm.2015-0022
Guan, X., Delo, D. M., Atala, A., and Soker, S. (2011). in vitro cardiomyogenic potential of human amniotic fluid stem cells. J. Tissue Eng. Regen. Med. 5, 220–228. doi: 10.1002/term.308
Hahn, V. S., Lenihan, D. J., and Ky, B. (2014). Cancer therapy-induced cardiotoxicity: basic mechanisms and potential cardioprotective therapies. J. Am. Heart Assoc. 3:e000665. doi: 10.1161/JAHA.113.000665
Heazlewood, C. F., Sherrell, H., Ryan, J., Atkinson, K., Wells, C. A., and Fisk, N. M. (2014). High Incidence of contaminating maternal cell overgrowth in human placental mesenchymal stem/stromal cell cultures: a systematic review. Stem Cells Transl. Med. 3, 1305–1311. doi: 10.5966/sctm.2014-0051
Hodges, R. J., Jenkin, G., Hooper, S. B., Allison, B., Lim, R., Dickinson, H., et al. (2012). Human amnion epithelial cells reduce ventilation-induced preterm lung injury in fetal sheep. Am. J. Obstet. Gynecol. 206, e8–e15. doi: 10.1016/j.ajog.2012.02.038
Hong, J. W., Lee, W. J., Hahn, S. B., Kim, B. J., and Lew, D. H. (2010). The effect of human placenta extract in a wound healing model. Ann. Plast. Surg. 65, 96–100. doi: 10.1097/SAP.0b013e3181b0bb67
Huang, C., Zhang, X., Ramil, J. M., Rikka, S., Kim, L., Lee, Y., et al. (2010). Juvenile exposure to anthracyclines impairs cardiac progenitor cell function and vascularization resulting in greater susceptibility to stress-induced myocardial injury in adult mice. Circulation 121, 675–683. doi: 10.1161/CIRCULATIONAHA.109.902221
Huang, Y.-C., Yang, Z.-M., Chen, X.-H., Tan, M.-Y., Wang, J., Li, X.-Q., et al. (2009). Isolation of mesenchymal stem cells from human placental decidua basalis and resistance to hypoxia and serum deprivation. Stem Cell Rev. 5, 247–255. doi: 10.1007/s12015-009-9069-x
Ilancheran, S., Michalska, A., Peh, G., Wallace, E. M., Pera, M., and Manuelpillai, U. (2007). Stem cells derived from human fetal membranes display multilineage differentiation potential. Biol. Reprod. 77, 577–588. doi: 10.1095/biolreprod.106.055244
In ‘t Anker, P. S., Scherjon, S. A., Kleijburg-van der Keur, C., Noort, W. A., Claas, F. H., Willemze, R., et al. (2003). Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood 102, 1548–1549. doi: 10.1182/blood-2003-04-1291
Iop, L., Chiavegato, A., Callegari, A., Bollini, S., Piccoli, M., Pozzobon, M., et al. (2008). Different cardiovascular potential of adult- and fetal-type mesenchymal stem cells in a rat model of heart cryoinjury. Cell Transplant. 17, 679–694. doi: 10.3727/096368908786092739
Izumi, M., Pazin, B. J., Minervini, C. F., Gerlach, J., Ross, M. A., Stolz, D. B., et al. (2009). Quantitative comparison of stem cell marker-positive cells in fetal and term human amnion. J. Reprod. Immunol. 81, 39–43. doi: 10.1016/j.jri.2009.02.007
Jameel, M. N., and Zhang, J. (2009). Heart failure management: the present and the future. Antioxid. Redox Signal. 11, 1989–2010. doi: 10.1089/ars.2009.2488
Jesty, S. A., Steffey, M. A., Lee, F. K., Breitbach, M., Hesse, M., Reining, S., et al. (2012). c-kit+ precursors support postinfarction myogenesis in the neonatal, but not adult, heart. Proc. Natl. Acad. Sci. U.S.A. 109, 13380–13385. doi: 10.1073/pnas.1208114109
Jiang, G., Herron, T. J., Di Bernardo, J., Walker, K. A., O'Shea, K. S., and Kunisaki, S. M. (2016). Human Cardiomyocytes Prior to Birth by Integration-Free Reprogramming of Amniotic Fluid Cells. Stem Cells Transl. Med. 5, 1595–1606. doi: 10.5966/sctm.2016-0016
Jiang, S., and Zhang, S. (2017). Differentiation of cardiomyocytes from amniotic fluid-derived mesenchymal stem cells by combined induction with transforming growth factor β1 and 5-azacytidine. Mol. Med. Rep. 16, 5887–5893. doi: 10.3892/mmr.2017.7373
Kakishita, K., Elwan, M. A., Nakao, N., Itakura, T., and Sakuragawa, N. (2000). Human amniotic epithelial cells produce dopamine and survive after implantation into the striatum of a rat model of Parkinson's Disease: a potential source of donor for transplantation therapy. Exp. Neurol. 165, 27–34. doi: 10.1006/exnr.2000.7449
Kakishita, K., Nakao, N., Sakuragawa, N., and Itakura, T. (2003). Implantation of human amniotic epithelial cells prevents the degeneration of nigral dopamine neurons in rats with 6-hydroxydopamine lesions. Brain Res. 980, 48–56. doi: 10.1016/S0006-8993(03)02875-0
Kang, N.-H., Hwang, K.-A., Kim, S. U., Kim, Y.-B., Hyun, S.-H., Jeung, E.-B., et al. (2012). Potential antitumor therapeutic strategies of human amniotic membrane and amniotic fluid-derived stem cells. Cancer Gene Ther. 19, 517–522. doi: 10.1038/cgt.2012.30
Kim, J., Kang, H. M., Kim, H., Kim, M. R., Kwon, H. C., Gye, M. C., et al. (2007). Ex Vivo characteristics of human amniotic membrane-derived stem cells. Cloning Stem Cells 9, 581–594. doi: 10.1089/clo.2007.0027
Kishore, R., and Khan, M. (2016). More than tiny sacks: stem cell exosomes as cell-free modality for cardiac repair. Circ. Res. 118, 330–343. doi: 10.1161/CIRCRESAHA.115.307654
König, J., Huppertz, B., Desoye, G., Parolini, O., Fröhlich, J. D., Weiss, G., et al. (2012). Amnion-derived mesenchymal stromal cells show angiogenic properties but resist differentiation into mature endothelial cells. Stem Cells Dev. 21, 1309–1320. doi: 10.1089/scd.2011.0223
König, J., Weiss, G., Rossi, D., Wankhammer, K., Reinisch, A., Kinzer, M., et al. (2015). Placental mesenchymal stromal cells derived from blood vessels or avascular tissues: what is the better choice to support endothelial cell function? Stem Cells Dev. 24, 115–131. doi: 10.1089/scd.2014.0115
La Rocca, G., Anzalone, R., Corrao, S., Magno, F., Loria, T., Lo Iacono, M., et al. (2009). Isolation and characterization of Oct-4+/HLA-G+ mesenchymal stem cells from human umbilical cord matrix: differentiation potential and detection of new markers. Histochem. Cell Biol. 131, 267–282. doi: 10.1007/s00418-008-0519-3
Lai, R. C., Yeo, R. W., Tan, K. H., and Lim, S. K. (2013). Exosomes for drug delivery - a novel application for the mesenchymal stem cell. Biotechnol. Adv. 31, 543–551. doi: 10.1016/j.biotechadv.2012.08.008
Lazzarini, E., Balbi, C., Altieri, P., Pfeffer, U., Gambini, E., Canepa, M., et al. (2016). The human amniotic fluid stem cell secretome effectively counteracts doxorubicin-induced cardiotoxicity. Sci. Rep. 6:29994. doi: 10.1038/srep29994
Lee, W.-Y., Wei, H.-J., Lin, W.-W., Yeh, Y.-C., Hwang, S.-M., Wang, J.-J., et al. (2011). Enhancement of cell retention and functional benefits in myocardial infarction using human amniotic-fluid stem-cell bodies enriched with endogenous ECM. Biomaterials 32, 5558–5567. doi: 10.1016/j.biomaterials.2011.04.031
Levy, D., Kenchaiah, S., Larson, M. G., Benjamin, E. J., Kupka, M. J., Ho, K. K., et al. (2002). Long-term trends in the incidence of and survival with heart failure. N. Engl. J. Med. 347, 1397–1402. doi: 10.1056/NEJMoa020265
Lian, X., Zhang, J., Azarin, S. M., Zhu, K., Hazeltine, L. B., Bao, X., et al. (2012). Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/β-catenin signaling under fully defined conditions. Nat. Protoc. 8, 162–175. doi: 10.1038/nprot.2012.150
Liu, Y. H., Peng, K. Y., Chiu, Y. W., Ho, Y. L., Wang, Y. H., Shun, C. T., et al. (2015). Human placenta-derived multipotent cells (hPDMCs) modulate cardiac injury: from bench to small and large animal myocardial ischemia studies. Cell Transplant. 24, 2463–2478. doi: 10.3727/096368915X687200
Lopez-Espinosa, M.-J., Silva, E., Granada, A., Molina-Molina, J.-M., Fernandez, M. F., Aguilar-Garduño, C., et al. (2009). Assessment of the total effective xenoestrogen burden in extracts of human placentas. Biomarkers 14, 271–277. doi: 10.1080/13547500902893744
Loukogeorgakis, S. P., and De Coppi, P. (2017). Concise review: amniotic fluid stem cells: the known, the unknown, and potential regenerative medicine applications. Stem Cells 35, 1663–1673. doi: 10.1002/stem.2553
Ma, J., Zhao, Y., Sun, L., Sun, X., Zhao, X., Sun, X., et al. (2016). Exosomes derived from Akt-modified human umbilical cord mesenchymal stem cells improve cardiac regeneration and promote angiogenesis via activating platelet-derived growth factor D. Stem Cells Transl. Med. 6, 51–59. doi: 10.5966/sctm.2016-0038
Magatti, M., Caruso, M., De Munari, S., Vertua, E., De, D., Manuelpillai, U., et al. (2015). Human amniotic membrane-derived mesenchymal and epithelial cells exert different effects on monocyte-derived dendritic cell differentiation and function. Cell Transplant. 24, 1733–1752. doi: 10.3727/096368914X684033
Magatti, M., De Munari, S., Vertua, E., Gibelli, L., Wengler, G. S., and Parolini, O. (2008). Human amnion mesenchyme harbors cells with allogeneic T-cell suppression and stimulation capabilities. Stem Cells 26, 182–192. doi: 10.1634/stemcells.2007-0491
Magatti, M., De Munari, S., Vertua, E., Nassauto, C., Albertini, A., Wengler, G. S., et al. (2009). Amniotic mesenchymal tissue cells inhibit dendritic cell differentiation of peripheral blood and amnion resident monocytes. Cell Transplant. 18, 899–914. doi: 10.3727/096368909X471314
Magatti, M., Abumaree, M. H., Silini, A. R., Anzalone, R., Saieva, S., Russo, E., et al. (2016). “The immunomodulatory features of mesenchymal stromal cells derived from Wharton's jelly, amniotic membrane and chorionic villi: In vitro and In vivo Data,” in Placenta: The Tree of Life. 2, ed O. Parolini (CRC Press), 91–128.
Magatti, M., Vertua, E., De Munari, S., Caro, M., Caruso, M., Silini, A., et al. (2017). Human amnion favours tissue repair by inducing the M1-to-M2 switch and enhancing M2 macrophage features. J. Tissue Eng. Regen. Med. 11, 2895–2911. doi: 10.1002/term.2193
Maioli, M., Contini, G., Santaniello, S., Bandiera, P., Pigliaru, G., Sanna, R., et al. (2013). Amniotic fluid stem cells morph into a cardiovascular lineage: analysis of a chemically induced cardiac and vascular commitment. Drug Des. Devel. Ther. 7, 1063–1073. doi: 10.2147/DDDT.S44706
Malina, R. M. (1993). The developing human: clinically oriented embryology (5th edition). xi + 493 pp. By Keith L. Moore and T.V.N. Persaud. Philadelphia: W. B. Saunders, 1993. $31.95 (paper). Am. J. Hum. Biol. 5, 507–507.
Manuelpillai, U., Lourensz, D., Vaghjiani, V., Tchongue, J., Lacey, D., Tee, J.-Y., et al. (2012). Human amniotic epithelial cell transplantation induces markers of alternative macrophage activation and reduces established hepatic fibrosis. PLoS ONE 7:e38631. doi: 10.1371/journal.pone.0038631
Manuelpillai, U., Tchongue, J., Lourensz, D., Vaghjiani, V., Samuel, C. S., Liu, A., et al. (2010). Transplantation of human amnion epithelial cells reduces hepatic fibrosis in immunocompetent CCl 4 -treated mice. Cell Transplant. 19, 1157–1168. doi: 10.3727/096368910X504496
Marongiu, F., Gramignoli, R., Dorko, K., Miki, T., Ranade, A. R., Paola Serra, M., et al. (2011). Hepatic differentiation of amniotic epithelial cells. Hepatology 53, 1719–1729. doi: 10.1002/hep.24255
Marote, A., Teixeira, F. G., Mendes-Pinheiro, B., and Salgado, A. J. (2016). MSCs-derived exosomes: cell-secreted nanovesicles with regenerative potential. Front. Pharmacol. 7:231. doi: 10.3389/fphar.2016.00231
Mellows, B., Mitchell, R., Antonioli, M., Kretz, O., Chambers, D., Zeuner, M.-T., et al. (2017). Protein and molecular characterization of a clinically compliant amniotic fluid stem cell-derived extracellular vesicle fraction capable of accelerating muscle regeneration through enhancement of angiogenesis. Stem Cells Dev. 26, 1316–1333. doi: 10.1089/scd.2017.0089
Meraviglia, V., Vecellio, M., Grasselli, A., Baccarin, M., Farsetti, A., Capogrossi, M. C., et al. (2012). Human chorionic villus mesenchymal stromal cells reveal strong endothelial conversion properties. Differentiation 83, 260–270. doi: 10.1016/j.diff.2012.02.006
Miki, T., and Strom, S. C. (2006). Amnion-derived pluripotent/multipotent stem cells. Stem Cell Rev. 2, 133–141. doi: 10.1007/s12015-006-0020-0
Miki, T., Lehmann, T., Cai, H., Stolz, D. B., and Strom, S. C. (2005). Stem cell characteristics of amniotic epithelial cells. Stem Cells 23, 1549–1559. doi: 10.1634/stemcells.2004-0357
Miki, T., Mitamura, K., Ross, M. A., Stolz, D. B., and Strom, S. C. (2007). Identification of stem cell marker-positive cells by immunofluorescence in term human amnion. J. Reprod. Immunol. 75, 91–96. doi: 10.1016/j.jri.2007.03.017
Mirabella, T., Cilli, M., Carlone, S., Cancedda, R., and Gentili, C. (2011). Amniotic liquid derived stem cells as reservoir of secreted angiogenic factors capable of stimulating neo-arteriogenesis in an ischemic model. Biomaterials 32, 3689–3699. doi: 10.1016/j.biomaterials.2011.01.071
Mirabella, T., Hartinger, J., Lorandi, C., Gentili, C., Van Griensven, M., and Cancedda, R. (2012). Proangiogenic soluble factors from amniotic fluid stem cells mediate the recruitment of endothelial progenitors in a model of ischemic fasciocutaneous flap. Stem Cells Dev. 21, 2179–2188. doi: 10.1089/scd.2011.0639
Mirotsou, M., Jayawardena, T. M., Schmeckpeper, J., Gnecchi, M., and Dzau, V. J. (2011). Paracrine mechanisms of stem cell reparative and regenerative actions in the heart. J. Mol. Cell. Cardiol. 50, 280–289. doi: 10.1016/j.yjmcc.2010.08.005
Moodley, Y., Atienza, D., Manuelpillai, U., Samuel, C. S., Tchongue, J., Ilancheran, S., et al. (2009). Human umbilical cord mesenchymal stem cells reduce fibrosis of bleomycin-induced lung injury. Am. J. Pathol. 175, 303–313. doi: 10.2353/ajpath.2009.080629
Moodley, Y., Ilancheran, S., Samuel, C., Vaghjiani, V., Atienza, D., Williams, E. D., et al. (2010). Human amnion epithelial cell transplantation abrogates lung fibrosis and augments repair. Am. J. Respir. Crit. Care Med. 182, 643–651. doi: 10.1164/rccm.201001-0014OC
Mori, A., Nishi, H., Sasaki, T., Nagamitsu, Y., Kawaguchi, R., Okamoto, A., et al. (2016). HLA-G expression is regulated by miR-365 in trophoblasts under hypoxic conditions. Placenta 45, 37–41. doi: 10.1016/j.placenta.2016.07.004
Moschidou, D., Mukherjee, S., Blundell, M. P., Drews, K., Jones, G. N., Abdulrazzak, H., et al. (1953). Valproic acid confers functional pluripotency to human amniotic fluid stem cells in a transgene-free approach. Mol. Ther. 20, 1953–1967.
Murphy, S. V., Shiyun, S. C., Tan, J. L., Chan, S., Jenkin, G., Wallace, E. M., et al. (2012). Human amnion epithelial cells do not abrogate pulmonary fibrosis in mice with impaired macrophage function. Cell Transplant. 21, 1477–1492. doi: 10.3727/096368911X601028
Murphy, S., Lim, R., Dickinson, H., Acharya, R., Rosli, S., Jenkin, G., et al. (2011). Human amnion epithelial cells prevent bleomycin-induced lung injury and preserve lung function. Cell Transplant. 20, 909–923. doi: 10.3727/096368910X543385
Naaktgeboren, W. R., Linschoten, M., de Graeff, A. V., Rhenen, A., Cramer, M. J., Asselbergs, F. W., et al. (2017). Long-term cardiovascular health in adult cancer survivors. Maturitas 105, 37–45. doi: 10.1016/j.maturitas.2017.05.014
O'Loughlin, A. J., Woffindale, C. A., and Wood, M. J. (2012). Exosomes and the emerging field of exosome-based gene therapy. Curr. Gene Ther. 12, 262–274. doi: 10.2174/156652312802083594
Ohye, R. G., Schranz, D., and D'Udekem, Y. (2016). Current therapy for hypoplastic left heart syndrome and related single ventricle lesions. Circulation 134, 1265–1279. doi: 10.1161/CIRCULATIONAHA.116.022816
Parolini, O., Alviano, F., Bagnara, G. P., Bilic, G., Bühring, H.-J., Evangelista, M., et al. (2008). Concise review: isolation and characterization of cells from human term placenta: outcome of the first international workshop on placenta derived stem cells. Stem Cells 26, 300–311. doi: 10.1634/stemcells.2007-0594
Parolini, O., Souza-Moreira, L., O'Valle, F., Magatti, M., Hernandez-Cortes, P., Gonzalez-Rey, E., et al. (2014). Therapeutic effect of human amniotic membrane-derived cells on experimental arthritis and other inflammatory disorders. Arthritis Rheumatol. 66, 327–339. doi: 10.1002/art.38206
Passipieri, J. A., Kasai-Brunswick, T. H., Suhett, G., Martins, A. B., Brasil, G. V., Campos, D. B., et al. (2014). Improvement of cardiac function by placenta-derived mesenchymal stem cells does not require permanent engraftment and is independent of the insulin signaling pathway. Stem Cell Res. Ther. 5:102. doi: 10.1186/scrt490
Pereira, W. C., Khushnooma, I., Madkaikar, M., and Ghosh, K. (2008). Reproducible methodology for the isolation of mesenchymal stem cells from human umbilical cord and its potential for cardiomyocyte generation. J. Tissue Eng. Regen. Med. 2, 394–399. doi: 10.1002/term.107
Pianta, S., Bonassi Signoroni, P., Muradore, I., Rodrigues, M. F., Rossi, D., Silini, A., et al. (2015). Amniotic membrane mesenchymal cells-derived factors skew T cell polarization toward Treg and downregulate Th1 and Th17 cells subsets. Stem Cell Rev. 11, 394–407. doi: 10.1007/s12015-014-9558-4
Pianta, S., Magatti, M., Vertua, E., Bonassi Signoroni, P., Muradore, I., Nuzzo, A. M., et al. (2016). Amniotic mesenchymal cells from pre-eclamptic placentae maintain immunomodulatory features as healthy controls. J. Cell. Mol. Med. 20, 157–169. doi: 10.1111/jcmm.12715
Pischiutta, F., Brunelli, L., Romele, P., Silini, A., Sammali, E., Paracchini, L., et al. (2016). Protection of brain injury by amniotic mesenchymal stromal cell-secreted metabolites. Crit. Care Med. 44, e1118–e1131. doi: 10.1097/CCM.0000000000001864
Porrello, E. R., Mahmoud, A. I., Simpson, E., Hill, J. A., Richardson, J. A., Olson, E. N., et al. (2011). Transient regenerative potential of the neonatal mouse heart. Science 331, 1078–1080. doi: 10.1126/science.1200708
Portmann-Lanz, C. B., Schoeberlein, A., Huber, A., Sager, R., Malek, A., Holzgreve, W., et al. (2006). Placental mesenchymal stem cells as potential autologous graft for pre- and perinatal neuroregeneration. Am. J. Obstet. Gynecol. 194, 664–673. doi: 10.1016/j.ajog.2006.01.101
Pozzobon, M., Piccoli, M., Schiavo, A. A., Atala, A., and De Coppi, P. (2013). Isolation of c-Kit+ human amniotic fluid stem cells from second trimester. Methods Mol. Biol. 1035, 191–198. doi: 10.1007/978-1-62703-508-8_16
Prusa, A.-R., Marton, E., Rosner, M., Bernaschek, G., and Hengstschläger, M. (2003). Oct-4-expressing cells in human amniotic fluid: a new source for stem cell research? Hum. Reprod. 18, 1489–1493. doi: 10.1093/humrep/deg279
Ramachandra, D. L., Shaw, S. S. W., Shangaris, P., Loukogeorgakis, S., Guillot, P. V., Coppi, P., et al. (2014). in utero therapy for congenital disorders using amniotic fluid stem cells. Front. Pharmacol. 5:270. doi: 10.3389/fphar.2014.00270
Rani, S., Ryan, A. E., Griffin, M. D., and Ritter, T. (2015). Mesenchymal stem cell-derived extracellular vesicles: toward cell-free therapeutic applications. Mol. Ther. 23, 812–823. doi: 10.1038/mt.2015.44
Rapp, B. M., Saadatzedeh, M. R., Ofstein, R. H., Bhavsar, J. R., Tempel, Z. S., Moreno, O., et al. (2012). Resident endothelial progenitor cells from human placenta have greater vasculogenic potential than circulating endothelial progenitor cells from umbilical cord blood. Cell Med. 2, 85–96. doi: 10.3727/215517911X617888
Reed, M. J., Koike, T., Sadoun, E., Sage, E. H., and Puolakkainen, P. (2003). Inhibition of TIMP1 enhances angiogenesis in vivo and cell migration in vitro. Microvasc. Res. 65, 9–17. doi: 10.1016/S0026-2862(02)00026-2
Ricci, E., Vanosi, G., Lindenmair, A., Hennerbichler, S., Peterbauer-Scherb, A., Wolbank, S., et al. (2013). Anti-fibrotic effects of fresh and cryopreserved human amniotic membrane in a rat liver fibrosis model. Cell Tissue Bank. 14, 475–488. doi: 10.1007/s10561-012-9337-x
Rossi, D., Pianta, S., Magatti, M., Sedlmayr, P., and Parolini, O. (2012). Characterization of the conditioned medium from amniotic membrane cells: prostaglandins as key effectors of its immunomodulatory activity. PLoS ONE 7:e46956. doi: 10.1371/journal.pone.0046956
Roy, R., Brodarac, A., Kukucka, M., Kurtz, A., Becher, P. M., Jülke, K., et al. (2013). Cardioprotection by placenta-derived stromal cells in a murine myocardial infarction model. J. Surg. Res. 185, 70–83. doi: 10.1016/j.jss.2013.05.084
Roy, R., Haase, T., Ma, N., Bader, A., Becker, M., Seifert, M., et al. (2016). Decellularized amniotic membrane attenuates postinfarct left ventricular remodeling. J. Surg. Res. 200, 409–419. doi: 10.1016/j.jss.2015.08.022
Roy, R., Kukucka, M., Messroghli, D., Kunkel, D., Brodarac, A., Klose, K., et al. (2015). Epithelial-to-mesenchymal transition enhances the cardioprotective capacity of human amniotic epithelial cells. Cell Transplant. 24, 985–1002. doi: 10.3727/096368913X675151
Sadler, T. W., Thomas, W., and Langman, J. (2012). Langman's Medical Embryology. Wolters Kluwer Health/Lippincott Williams and Wilkins Available online at: https://locatorplus.gov/cgi-bin/Pwebrecon.cgi?DB=localandv1=1andti=1,1andSearch_Arg=101562744andSearch_Code=0359andCNT=20andSID=1 (Accessed February 19, 2018).
Sakuragawa, N., Enosawa, S., Ishii, T., Thangavel, R., Tashiro, T., Okuyama, T., et al. (2000). Human amniotic epithelial cells are promising transgene carriers for allogeneic cell transplantation into liver. J. Hum. Genet. 45, 171–176. doi: 10.1007/s100380050205
Sakuragawa, N., Misawa, H., Ohsugi, K., Kakishita, K., Ishii, T., Thangavel, R., et al. (1997). Evidence for active acetylcholine metabolism in human amniotic epithelial cells: applicable to intracerebral allografting for neurologic disease. Neurosci. Lett. 232, 53–56. doi: 10.1016/S0304-3940(97)00570-3
Sakuragawa, N., Thangavel, R., Mizuguchi, M., Hirasawa, M., and Kamo, I. (1996). Expression of markers for both neuronal and glial cells in human amniotic epithelial cells. Neurosci. Lett. 209, 9–12. doi: 10.1016/0304-3940(96)12599-4
Sant'Anna, L. B., Cargnoni, A., Ressel, L., Vanosi, G., and Parolini, O. (2011). Amniotic membrane application reduces liver fibrosis in a bile duct ligation rat model. Cell Transplant. 20, 441–453. doi: 10.3727/096368910X522252
SantAnna, L. B., Hage, R., Cardoso, M. A., Arisawa, E. A., Cruz, M. M., Parolini, O., et al. (2016). Antifibrotic effects of human amniotic membrane transplantation in established biliary fibrosis induced in rats. Cell Transplant. 25, 2245–2257. doi: 10.3727/096368916X692645
Santos Nascimento, D., Mosqueira, D., Sousa, L. M., Teixeira, M., Filipe, M., Resende, T. P., et al. (2014). Human umbilical cord tissue-derived mesenchymal stromal cells attenuate remodeling after myocardial infarction by proangiogenic, antiapoptotic, and endogenous cell-activation mechanisms. Stem Cell Res. Ther. 5:5. doi: 10.1186/scrt394
Sartore, S., Lenzi, M., Angelini, A., Chiavegato, A., Gasparotto, L., Coppi, P., et al. (2005). Amniotic mesenchymal cells autotransplanted in a porcine model of cardiac ischemia do not differentiate to cardiogenic phenotypes. Eur. J. Cardio Thoracic Surg. 28, 677–684. doi: 10.1016/j.ejcts.2005.07.019
Schiavo, A. A., Franzin, C., Albiero, M., Piccoli, M., Spiro, G., Bertin, E., et al. (2015). Endothelial properties of third-trimester amniotic fluid stem cells cultured in hypoxia. Stem Cell Res. Ther. 6:209. doi: 10.1186/s13287-015-0204-0
Schmidt, D., Achermann, J., Odermatt, B., Breymann, C., Mol, A., Genoni, M., et al. (2007). Prenatally fabricated autologous human living heart valves based on amniotic fluid derived progenitor cells as single cell source. Circulation 116, I-64–I-70. doi: 10.1161/CIRCULATIONAHA.106.681494
Schmidt, D., Achermann, J., Odermatt, B., Genoni, M., Zund, G., and Hoerstrup, S. P. (2008). Cryopreserved amniotic fluid-derived cells: a lifelong autologous fetal stem cell source for heart valve tissue engineering. J. Heart Valve Dis. 17, 446–455; discussion 455. doi: 10.5167/uzh-11879
Shafei, A. E., Ali, M. A., Ghanem, H. G., Shehata, A. I., Abdelgawad, A. A., Handal, H. R., et al. (2017). Mesenchymal stem cell therapy: A promising cell-based therapy for treatment of myocardial infarction. J. Gene Med. 19:e2995. doi: 10.1002/jgm.2995
Shaw, S. W. S., Bollini, S., Nader, K. A., Gastaldello, A., Mehta, V., Filppi, E., et al. (2016). autologous transplantation of amniotic fluid-derived mesenchymal stem cells into sheep fetuses. Cell Transplant. 25:615. doi: 10.3727/096368916X691006
Silini, A. R., Magatti, M., Cargnoni, A., and Parolini, O. (2017). Is immune modulation the mechanism underlying the beneficial effects of amniotic cells and their derivatives in regenerative medicine? Cell Transplant. 26, 531–539. doi: 10.3727/096368916X693699
Slamecka, J., Salimova, L., McClellan, S., van Kelle, M., Kehl, D., Laurini, J., et al. (2016). Non-integrating episomal plasmid-based reprogramming of human amniotic fluid stem cells into induced pluripotent stem cells in chemically defined conditions. Cell Cycle 15, 234–249. doi: 10.1080/15384101.2015.1121332
Sölder, E., Böckle, B. C., Nguyen, V. A., Fürhapter, C., Obexer, P., Erdel, M., et al. (2012). Isolation and characterization of CD133+CD34+VEGFR-2+CD45- fetal endothelial cells from human term placenta. Microvasc. Res. 84, 65–73. doi: 10.1016/j.mvr.2012.03.005
Soncini, M., Vertua, E., Gibelli, L., Zorzi, F., Denegri, M., Albertini, A., et al. (2007). Isolation and characterization of mesenchymal cells from human fetal membranes. J. Tissue Eng. Regen. Med. 1, 296–305. doi: 10.1002/term.40
Su, H., Gorodny, N., Gomez, L. F., Gangadharmath, U., Mu, F., Chen, G., et al. (2015). Noninvasive molecular imaging of apoptosis in a mouse model of anthracycline-induced cardiotoxicity. Circ. Cardiovasc. Imaging 8:e001952. doi: 10.1161/CIRCIMAGING.114.001952
Sun, R., Liu, M., Lu, L., Zheng, Y., and Zhang, P. (2015). Congenital heart disease: causes, diagnosis, symptoms, and treatments. Cell Biochem. Biophys. 72, 857–860. doi: 10.1007/s12013-015-0551-6
Takahashi, K., and Yamanaka, S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676. doi: 10.1016/j.cell.2006.07.024
Takashima, S., Ise, H., Zhao, P., Akaike, T., and Nikaido, T. (2004). Human amniotic epithelial cells possess hepatocyte-like characteristics and functions. Cell Struct. Funct. 29, 73–84. doi: 10.1247/csf.29.73
Takawale, A., Zhang, P., Patel, V. B., Wang, X., Oudit, G., and Kassiri, Z. (2017). Tissue inhibitor of matrix metalloproteinase-1 promotes myocardial fibrosis by mediating cd63-integrin β1 interaction. Hypertens 69, 1092–1103. doi: 10.1161/HYPERTENSIONAHA.117.09045
Tam, A. Y., Yung, R. W., and Fu, K. H. (1989). Fatal pneumonia caused by Flavobacterium meningosepticum. Pediatr. Infect. Dis. J. 8, 252–254.
Tamagawa, T., Ishiwata, I., and Saito, S. (2004). Establishment and characterization of a pluripotent stem cell line derived from human amniotic membranes and initiation of germ layers in vitro. Hum. Cell 17, 125–130. doi: 10.1111/j.1749-0774.2004.tb00028.x
Tan, J. L., Chan, S. T., Wallace, E. M., and Lim, R. (2014). Human amnion epithelial cells mediate lung repair by directly modulating macrophage recruitment and polarization. Cell Transplant. 23, 319–328. doi: 10.3727/096368912X661409
Tancharoen, W., Aungsuchawan, S., Pothacharoen, P., Markmee, R., Narakornsak, S., Kieodee, J., et al. (2017). Differentiation of mesenchymal stem cells from human amniotic fluid to vascular endothelial cells. Acta Histochem. 119, 113–121. doi: 10.1016/j.acthis.2016.11.009
Troyer, D. L., and Weiss, M. L. (2008). Concise review: wharton's jelly-derived cells are a primitive stromal cell population. Stem Cells 26, 591–599. doi: 10.1634/stemcells.2007-0439
Tsai, P.-C., Fu, T.-W., Chen, Y.-M., Ko, T.-L., Chen, T.-H., Shih, Y.-H., et al. (2009). The therapeutic potential of human umbilical mesenchymal stem cells from Wharton's jelly in the treatment of rat liver fibrosis. Liver Transpl. 15, 484–495. doi: 10.1002/lt.21715
Tsuji, H., Miyoshi, S., Ikegami, Y., Hida, N., Asada, H., Togashi, I., et al. (2010). Xenografted human amniotic membrane–derived mesenchymal stem cells are immunologically tolerated and transdifferentiated into cardiomyocytes. Circ. Res. 106, 1613–1623. doi: 10.1161/CIRCRESAHA.109.205260
Tzahor, E., and Poss, K. D. (2017). Cardiac regeneration strategies: staying young at heart. Science 356, 1035–1039. doi: 10.1126/science.aam5894
Velasquez-Mao, A. J., Tsao, C. J. M., Monroe, M. N., Legras, X., Bissig-Choisat, B., Bissig, K.-D., et al. (2017). Differentiation of spontaneously contracting cardiomyocytes from non-virally reprogrammed human amniotic fluid stem cells. PLoS ONE 12:e0177824. doi: 10.1371/journal.pone.0177824
Ventura, C., Cantoni, S., Bianchi, F., Lionetti, V., Cavallini, C., Scarlata, I., et al. (2007). Hyaluronan mixed esters of butyric and retinoic Acid drive cardiac and endothelial fate in term placenta human mesenchymal stem cells and enhance cardiac repair in infarcted rat hearts. J. Biol. Chem. 282, 14243–14252. doi: 10.1074/jbc.M609350200
Vinketova, K., Mourdjeva, M., and Oreshkova, T. (2016). Human decidual stromal cells as a component of the implantation niche and a modulator of maternal immunity. J. Pregnancy 2016:8689436. doi: 10.1155/2016/8689436
Vosdoganes, P., Hodges, R. J., Lim, R., Westover, A. J., Acharya, R. Y., Wallace, E. M., et al. (2011). Human amnion epithelial cells as a treatment for inflammation-induced fetal lung injury in sheep. Am. J. Obstet. Gynecol. 205, e26–e33. doi: 10.1016/j.ajog.2011.03.054
Walther, G., Gekas, J., and Bertrand, O. F. (2009). Amniotic stem cells for cellular cardiomyoplasty: promises and premises. Catheter. Cardiovasc. Interv. 73, 917–924. doi: 10.1002/ccd.22016
Weber, B., Emmert, M. Y., Behr, L., Schoenauer, R., Brokopp, C., Drögemüller, C., et al. (2012). Prenatally engineered autologous amniotic fluid stem cell-based heart valves in the fetal circulation. Biomaterials 33, 4031–4043. doi: 10.1016/j.biomaterials.2011.11.087
Weber, B., Kehl, D., Bleul, U., Behr, L., Sammut, S., Frese, L., et al. (2016). in vitro fabrication of autologous living tissue-engineered vascular grafts based on prenatally harvested ovine amniotic fluid-derived stem cells. J. Tissue Eng. Regen. Med. 10, 52–70. doi: 10.1002/term.1781
Wei, J. P., Zhang, T. S., Kawa, S., Aizawa, T., Ota, M., Akaike, T., et al. (2003). Human amnion-isolated cells normalize blood glucose in streptozotocin-induced diabetic mice. Cell Transplant. 12, 545–552. doi: 10.3727/000000003108747000
Woodward, C. S. (2011). Keeping children with congenital heart disease healthy. J. Pediatr. Health Care 25, 373–378. doi: 10.1016/j.pedhc.2011.03.007
Yagel, S., Weissman, A., Rotstein, Z., Manor, M., Hegesh, J., Anteby, E., et al. (1997). Congenital heart defects: natural course and in utero development. Circulation 96, 550–555. doi: 10.1161/01.CIR.96.2.550
Yeh, Y.-C., Lee, W.-Y., Yu, C.-L., Hwang, S.-M., Chung, M.-F., Hsu, L.-W., et al. (2010). Cardiac repair with injectable cell sheet fragments of human amniotic fluid stem cells in an immune-suppressed rat model. Biomaterials 31, 6444–6453. doi: 10.1016/j.biomaterials.2010.04.069
Zeng, L., He, X., Wang, Y., Tang, Y., Zheng, C., Cai, H., et al. (2014). MicroRNA-210 overexpression induces angiogenesis and neurogenesis in the normal adult mouse brain. Gene Ther. 21, 37–43. doi: 10.1038/gt.2013.55
Zhang, D., Jiang, M., and Miao, D. (2011). Transplanted human amniotic membrane-derived mesenchymal stem cells ameliorate carbon tetrachloride-induced liver cirrhosis in mouse. PLoS ONE 6:e16789. doi: 10.1371/journal.pone.0016789
Zhang, X., Mitsuru, A., Igura, K., Takahashi, K., Ichinose, S., Yamaguchi, S., et al. (2006). Mesenchymal progenitor cells derived from chorionic villi of human placenta for cartilage tissue engineering. Biochem. Biophys. Res. Commun. 340, 944–952. doi: 10.1016/j.bbrc.2005.12.091
Zhao, L., Liu, X., Zhang, Y., Liang, X., Ding, Y., Xu, Y., et al. (2016). Enhanced cell survival and paracrine effects of mesenchymal stem cells overexpressing hepatocyte growth factor promote cardioprotection in myocardial infarction. Exp. Cell Res. 344, 30–39. doi: 10.1016/j.yexcr.2016.03.024
Keywords: amniotic fluid, placenta, umbilical cord, cardiac repair, cardiomyocyte, immunomodulation, cardioprotection, paracrine effect
Citation: Bollini S, Silini AR, Banerjee A, Wolbank S, Balbi C and Parolini O (2018) Cardiac Restoration Stemming From the Placenta Tree: Insights From Fetal and Perinatal Cell Biology. Front. Physiol. 9:385. doi: 10.3389/fphys.2018.00385
Received: 19 December 2017; Accepted: 28 March 2018;
Published: 11 April 2018.
Edited by:
Alina Maloyan, Oregon Health & Science University, United StatesReviewed by:
Debora Lo Furno, Università di Catania, ItalySribalasubashini Muralimanoharan, University of Texas Southwestern Medical Center, United States
Copyright © 2018 Bollini, Silini, Banerjee, Wolbank, Balbi and Parolini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sveva Bollini, c3ZldmEuYm9sbGluaUB1bmlnZS5pdA==
 Sveva Bollini
Sveva Bollini Antonietta R. Silini
Antonietta R. Silini Asmita Banerjee3
Asmita Banerjee3 Susanne Wolbank
Susanne Wolbank Carolina Balbi
Carolina Balbi Ornella Parolini
Ornella Parolini