
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Physiol., 20 March 2018
Sec. Integrative Physiology
Volume 9 - 2018 | https://doi.org/10.3389/fphys.2018.00235
This article is part of the Research TopicGravitational Physiology, Aging and MedicineView all 37 articles
Prolonged periods of skeletal muscle inactivity or mechanical unloading (bed rest, hindlimb unloading, immobilization, spaceflight and reduced step) can result in a significant loss of musculoskeletal mass, size and strength which ultimately lead to muscle atrophy. With advancement in understanding of the molecular and cellular mechanisms involved in disuse skeletal muscle atrophy, several different signaling pathways have been studied to understand their regulatory role in this process. However, substantial gaps exist in our understanding of the regulatory mechanisms involved, as well as their functional significance. This review aims to update the current state of knowledge and the underlying cellular mechanisms related to skeletal muscle loss during a variety of unloading conditions, both in humans and animals. Recent advancements in understanding of cellular and molecular mechanisms, including IGF1-Akt-mTOR, MuRF1/MAFbx, FOXO, and potential triggers of disuse atrophy, such as calcium overload and ROS overproduction, as well as their role in skeletal muscle protein adaptation to disuse is emphasized. We have also elaborated potential therapeutic countermeasures that have shown promising results in preventing and restoring disuse-induced muscle loss. Finally, identified are the key challenges in this field as well as some future prospectives.
Skeletal muscle is composed of muscular fibers and fascicles. It possesses a variety of functions in an organism's body and plays a vital role in the regulation of body metabolism. Skeletal muscles may differ significantly in mass, size, shape, and arrangement, depending upon their location and physical function in the organism. Elevated physical activity, like exercise, leads to increase the muscle mass (Bogdanis, 2012). On the other hand, decreased or limited use of skeletal muscle is one of the greatest contributing factors leading to muscle atrophy. Muscle atrophy may occur in a variety of conditions in unhealthy and healthy individuals, e.g., many common illnesses (Evans, 2010) including diabetes (Bonaldo and Sandri, 2013), cancers (Stephens et al., 2010), renal/heart failure, sepsis (Gordon et al., 2013), muscle genetic diseases (Sandri, 2010) and neurodegenerative disorders (Verdijk et al., 2012) cause significant loss of muscle mass. While in healthy individuals, muscle atrophy can also occur during conditions like spaceflight, bed rest, reduced step, hindlimb unloading (HLU) and immobilization. Furthermore, aging is also associated with muscle loss (Keller and Engelhardt, 2013; Hughes et al., 2017). Over the years, disuse skeletal muscle atrophy has been widely studied in humans as well as in animals.
Low skeletal muscle mass, decreased muscle fiber cross-sectional area (mCSA), muscle fiber transition from slow to fast and the change of functional properties have been observed in different muscle types under disuse conditions (Booth and Gollnick, 1983; Baldwin, 1996; Baldwin and Haddad, 2001; Fitts et al., 2001; Ohira et al., 2006). Several studies indicated that bed rest and immobilization cause disturbances in protein turnover (Goldspink, 1977; Janssen et al., 2000; Phillips et al., 2009; Rennie, 2009). It is commonly believed that a disproportion in the rate of protein synthesis and protein degradation is the main cause of muscle loss (Boonyarom and Inui, 2006; Bialek et al., 2011).
This review provides a summary of disuse muscle atrophy. In the first section, the functional and structural adaptations or alterations of skeletal muscle to disuse are discussed in different unloading models such as spaceflight, head down bed rest (HDBR), immobilization and reduced step in humans as well as HLU and immobilization in animals. The second section of the review provides a detailed discussion of anabolic and catabolic pathways and potential triggers involved in muscle protein synthesis and degradation under disuse-induced muscle atrophy. These include insulin-like growth factor-1-protein kinase B-mammalian target of rapamycin (IGF-1-Akt/PKB-mTOR), muscle ring finger 1/muscle atrophy F-box (MuRF1/MAFbx) and forkhead family of transcription factors (FOXO) pathways. The third section discusses the efficiency of potential countermeasures (antioxidants, resistance exercises and protein supplements) to counteract the loss of skeletal muscle.
Spaceflight, bed rest, immobilization (cast/leg brace), step reduction and HLU are the key models extensively used to study the muscle loss in humans as well as in animals. These models provide deeper insights into the molecular and cellular mechanisms underlying disuse-induced muscle atrophy.
The primary models of disuse in human research include microgravity induced muscle changes during spaceflight (Fitts et al., 2010; Goswami, 2017), bed rest immobilization (Spector et al., 2009), as well as other forms of immobilization (cast or leg brace) (Wall et al., 2013) and step reduction (Breen et al., 2013; McGlory et al., 2017). The majority of the published results of spaceflight experiments indicate that significant declines in skeletal muscle size, volume, CSA and strength occur after exposure to microgravity. It appear that longer sojourns in space lead to further atrophy and weakening of the muscles: 115–197 days in space were associated with significant decreases in muscle volume of gastrocnemius 17%, soleus 17%, and quadriceps 10% (LeBlanc et al., 2000). Another study also reported that prolonged spaceflight (approximately 180 days) significantly reduced force and fibers size in the gastrocnemius and soleus muscles. Specifically, the atrophy order (greatest-least) was: atrophy in soleus type I > soleus type II > gastrocnemius type I > gastrocnemius type II (Fitts et al., 2010). Furthermore, two long term spaceflight studies (140 and 175 days) reported a considerable decrease in characteristics of gastrocnemius muscle strength (Kozlovskaya et al., 1981). Similarly, 6 months Mir-mission in space found that isometric maximal voluntary contractions of the triceps surae muscle and peak tetanic force (Po) decreased by 42 and 25%, respectively (Koryak, 2001). In addition, short term spaceflight studies have also been reported to cause muscle weakness. For instance, volume changes in the knee extensor, knee flexor and plantar flexor muscle ranged from −15.4 to −5.5, −14.1 to −5.6 and −8.8 to −15.9, respectively, after 2 weeks in spaceflight (Akima et al., 2000). Edgerton and colleagues found that the size of all muscle fiber types of the vastus lateralis (VL) muscle decreased after 5–11 days of spaceflight (e.g., type I 16%, IIa 23%, and IIb 36%) and the percentage of type I myofibers decreased 6–8% (Edgerton et al., 1995). For instance, up to 8% decrease in CSA of the knee extensor and the gluteal muscles as well as 10% decrease of strength were observed after 17 days of spaceflight (Tesch et al., 2005).
In addition, differential rates of muscle atrophy have also been observed in response to disuse induced by HDBR in different muscle and fiber types. For example, prolonged bed rest with a 6° HDBR is one of the commonly used methods to mimic the effects of microgravity on muscle and bone turnover (Pavy-Le Traon et al., 2007; Spector et al., 2009). It was repeatedly demonstrated that HDBR leads to significant reduction in muscle strength and mass. Using magnetic resonance imaging (MRI), up to 17 and 40% loss in VL muscle volume and function were seen following 84-day HDBR (Trappe et al., 2004). Another 90-day study of HDBR reported up to 26% reduction in mCSA in young, healthy males (Rittweger et al., 2005). Indeed, similar results were obtained following 35 days of bed rest study (e.g., type I fiber CSA of VL showed greater loss than type II fiber) (Brocca et al., 2012). Similarly, a short duration HDBR study of 17–20 days also showed a 10–12% decrease in muscle size (Akima et al., 1997, 2001). On the other hand, Miokovic and associates observed that during prolonged bed rest, intramuscular differential atrophy did occur in most muscles, but some muscles of the lower limb remained unaffected (Miokovic et al., 2012).
In addition to bed rest, various other forms of immobilization have been used in ground-based studies of disuse muscle atrophy. Two week limb immobilization (casting) studies on young males reported reduction in quadriceps muscle volume, mCSA and strength by 9, 5–8, and 23%, respectively (Glover et al., 2008; Suetta et al., 2009). Even some shorter duration disuse studies have also reported significant reductions in muscle size (Wall et al., 2014). A five day study reported a 9% reduction in strength and 4% in quadriceps CSA (Dirks et al., 2014). Furthermore, immobilization appeared to impact the knee extensors to a greater degree than knee flexors (Veldhuizen et al., 1993; Deschenes et al., 2002). In addition to the above models of disuse, reduced step model has been demonstrated to influence muscle functions (Olsen et al., 2008; Knudsen et al., 2012; Breen et al., 2013). For example, reduced daily ambulatory activity has shown to lead to 2.8% loss in lean leg mass (Krogh-Madsen et al., 2010). Further data have shown that loss in muscle mass through lower limb disuse is more pronounced in older individuals (as they show increased vulnerability to loss of muscle size and strength) compared to younger individuals (Trappe, 2009; Degens and Korhonen, 2012; Tanner et al., 2015). It was observed in older participants (aged 68 ± 5 years) that 10-day bed rest leads to 7% reductions in muscle mass (lean tissue) (Kortebein et al., 2006). These findings were consistent with the results from a 7-day bed rest study involving six 60–73-year old participants, which reported 3.0 and 4.1% loss in total lean mass and lean leg mass, respectively (Drummond et al., 2012). Moreover, it has been observed that both contractile rate of force (torque) development and maximal isometric muscle strength are reduced significantly in the elderly as compared to younger men after 2 weeks of unilateral leg casting (Hvid et al., 2010). The discussed differences between young and old subjects are correlated with the effects of age-associated muscle atrophy (sarcopenia). Sarcopenia has been defined specifically as related to a subgroup of older persons with muscle-mass depletion, whose appendicular skeletal muscle mass (kg)/height2 (m2) is less than two standard deviations below the mean of a young reference group (Baumgartner et al., 1998). The muscle loss of sarcopenia has been attributed to the reduction in muscle fiber size (predominately in type II) and the muscle fiber number (Nilwik et al., 2013). Further detailed characteristics, mechanisms and functional significance of sarcopenia have been discussed in previous reviews (Evans, 2010; Narici and Maffulli, 2010).
From the foregoing discussion, it can be seen that the effects of several models of disuse (spaceflight, HDBR, immobilization and reduced step) on loss of muscle mass and strength have been widely investigated. Not all the studies have, however, reported consistent findings. This could be due to the fact that in most of these studies, either the study was restricted to investigation of only one muscle type or the immobilization was of short duration (≤14 days). Therefore, it is difficult to determine the details regarding differential rate of atrophy from these studies. Additionally, an important limiting factor in human studies is that most of the available data on atrophy and fiber CSA are based on a small biopsy sample taken from a single site.
Among all models of disuse-induced muscle atrophy, HLU and immobilization are, by far, the most widely employed animal models to study skeletal muscle atrophy in small mammals (Fitts et al., 1989; Morey-Holton and Globus, 2002; Caron et al., 2009). Generally, disuse muscle atrophy in rodents results in a rapid loss of muscle mass as well as in fiber CSA and function (within 14 days of unloading; Bodine, 2013). In the following section, we will attempt to evaluate the effectiveness of these two models with specific regards to the adaptations that are thought to occur in skeletal muscle.
The rodent HLU model is extensively used to simulate the physiological effects of microgravity (Morey-Holton and Globus, 2002; Lawler et al., 2003; Baehr et al., 2016). Most rodent studies focused on the slow soleus muscle, as it showed rapid atrophy (Wang et al., 2006; Sandonà et al., 2012). After 14 days of HLU 34-50% decrease of soleus muscle wet weight and 49% CSA were observed in rats (Ohira et al., 1992; Zhang et al., 2017). Similarly, another study indicated that electromyography of soleus was reduced at the start of unloading but then recovered fully within a week but muscle atrophy continued to increase (Ohira et al., 2015). Furthermore, fast type muscles (including gastrocnemius, plantaris and tibialis anterior but not extensor digitorum longus) also showed significant reduction in muscle mass following HLU (Tsika et al., 1987; Kyparos et al., 2005).
Another key model is limb immobilization, in which the desired part of the animal is covered with a plaster bandage or with a spiral wire and surgical skin staplers, which helps to maintain the joint in a particular position (Caron et al., 2009; Du et al., 2011). It is now well documented in rodents that soleus muscle wet weight and muscle fiber diameters for type I and II significantly decreased after 4 weeks of immobilization (Okita et al., 2001). It should be noted that muscle atrophy varies significantly under different conditions of immobilization. For example, it depends upon the position in which the joint is fixed/immobilized. Additionally, it was reported that muscles immobilized in short positions favor atrophy (Onda et al., 2016). Some studies also reported that the extensor muscles atrophy more than flexor muscles during ankle-joint immobilization (Roy et al., 1991; Adams et al., 2003; Ohira et al., 2006). The degree of skeletal muscles atrophy also showed differential responses in regards to the types of fiber (Jozsa et al., 1988). For example, 4 week hindlimb immobilization studies on male rats reported that type I fibers of the soleus muscle undergo greater reductions than type II fibers (Booth and Kelso, 1973; Thomason and Booth, 1990) and similar results have also been obtained during ankle-joint immobilization (Thomason and Booth, 1990; Ohira et al., 2006).
Taken together, varying results among different studies in rodent models of HLU and limb immobilization are related to the muscle type, muscle fiber type and conditions of immobilization. It has been shown that the amount of muscle loss is greater in the extensor muscles of the ankle (soleus and gastrocnemius) as compared to the flexor muscles (tibialis anterior and extensor digitorum longus) (Ohira et al., 2002; Adams et al., 2003; Zhong et al., 2005). Additionally, muscle atrophy appears to be different across muscle types. For example, slow-twitch (type I) fibers are more vulnerable and therefore, show a greater loss in the amount of protein than fast twitch (type II) fibers (Tsika et al., 1987; Thomason and Booth, 1990; Zhang et al., 2015), and the muscles immobilized in short positions showed more sensitivity to disuse (Desaphy et al., 2010). These observations suggest that the rate and extent of muscle loss appear to depend on the degree of unloading, the extent of physical inactivity and the muscle type.
Alterations and underlying mechanisms of muscle protein synthesis and degradation have been investigated extensively in different disuse models (Bodine, 2013; Bonaldo and Sandri, 2013; Rudrappa et al., 2016). In addition, some new insights and findings also have been reported recently (Mirzoev et al., 2016; Baehr et al., 2017). In this section, we provide a comprehensive summary of the molecular basis of disuse atrophy including potential triggers to signaling pathways and their ultimate effects on the myofibrillar apparatus.
It has been well recognized that decreased protein synthesis in muscle seems to be the major contributor in disuse muscle atrophy (Bodine, 2013). The decline of the basal protein synthesis rate in the early period of unloading has been investigated and confirmed in human and animal models (Booth and Seider, 1979; de Boer et al., 2007; Mirzoev et al., 2016; Baehr et al., 2017). Thus, the underlying mechanisms of decreased protein synthesis in disused skeletal muscle have been a main focus research area the field for the past few decades. Although many problems have not been solved yet, recent research has confirmed that decreased activation of the Akt-mTOR pathway is involved in the mechanisms of the attenuated protein synthesis under disuse conditions. In the following section, we give the details about this pathway both in normal and unloading conditions.
Under normal physiological condition, the IGF-1-Akt-mTOR pathway acts as a key regulator in the translation initiation step of protein synthesis in skeletal muscle (Erbay et al., 2003; Han et al., 2008; Schiaffino and Mammucari, 2011). Firstly, the IGF-1-Akt-mTOR pathway is initiated by binding of the IGF-1 to its specific IGF-1 receptor (IGF-1R), which triggers a signaling cascade mainly stimulating the intrinsic tyrosine kinase activity through insulin receptor substrate-1 (IRS-1). Subsequent activation of phosphatidylinositol 3 kinase (PI3K) may be achieved by binding p85 regulatory subunit with phosphorylated IRS-1 (Jheng et al., 2012). The membrane phospholipid-phosphatidylinositol-4,5-bisphosphate (PIP2), is phosphorylated into phosphatidylinositol-3,4,5-triphosphate (PIP3) by PI3K. Then PIP3 recruits phosphoinositide-dependent protein kinase-1 (PDK1) to phosphorylate and activate Akt. Phosphorylated Akt further activates mTORC1 (Inoki et al., 2002; Goodman et al., 2010; Miyazaki et al., 2011). Consequently, activated mTORC1 phosphorylates both 4E-BP1 and S6K1 which finally leads to protein synthesis (Gordon et al., 2013). Besides the indirect pathway by mTORC1, activated Akt can also phosphorylate glycogen synthase kinase 3β (GSK-3β), which directly leads to the increase of global protein synthesis through an increased activity of eukaryotic initiation factor 2B (eIF2B) (Welsh et al., 1998). The simplified version of the regulating mechanism underlying IGF-1-Akt-mTOR pathway on muscle protein synthesis is shown in Figure 1.
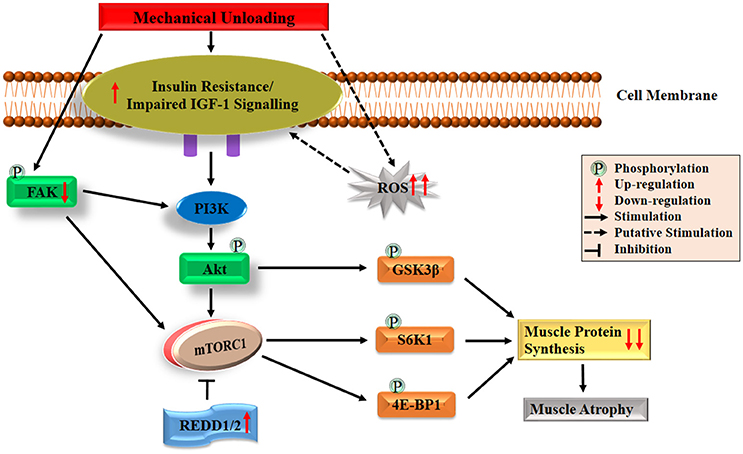
Figure 1. Diagrammatic representation of the protein synthesis signaling mechanisms responsible of skeletal muscle atrophy following mechanical unloading. IGF-1, insulin-like growth factor-1; PI3K, phosphatidylinositol 3 kinase; Akt/PKB, protein kinase B; FAK, focal adhesion kinase; mTORC1, mechanistic target of rapamycin in complex 1; REDD, regulated in DNA damage and development; GSK-3β, glycogen synthase kinase 3β; S6K1, 70 kDa ribosomal protein S6 kinase 1; 4E-BP1, eIF4E binding protein 1; ROS, reactive oxygen species. The black arrow and inhibition symbol show the association of molecules under loading condition, red arrow shows the up/down-regulation of molecules under unloading condition. For more details see text.
Under unloading conditions, insulin resistance plays an important role in driving depression of protein synthesis. This has been widely observed in humans subjected to bed rest confinement (Shangraw et al., 1988; Stuart et al., 1988; Hamburg et al., 2007), immobilization (Richter et al., 1989) and HLU in animals (Allen et al., 1997). Research on disuse models of rodents (e.g., HLU, immobilization and denervation) showed that insulin resistance induced attenuation of Akt-mTORC1 pathway may provide a mechanism for decreased protein synthesis (Gordon et al., 2013). Reduced activation of this pathway which characterized as decreased phosphorylation of Akt, S6K1, and 4E-BP1 has been shown in the soleus and medial gastrocnemius muscles (Bodine et al., 2001b; Hornberger et al., 2001; Sugiura et al., 2005; Haddad et al., 2006; Kelleher et al., 2013). Liu and colleagues also found that the binding of 4E-BP1 and eIF4E altered in rat gastrocnemius muscle during the early period of HLU (Liu et al., 2012). In addition, decreased phosphorylation of GSK3β has been observed in HLU rats (Stevenson et al., 2003; Mirzoev et al., 2016). Recent studies have also reported that an important mTOR signaling repressors such as mRNA expressions of regulated in DNA damage and development 1 and 2 (REDD1/2), significantly elevated following unloading in rats (Kelleher et al., 2013, 2015). Moreover, it has been reported that both Akt-null and mTOR knockout mice exhibited significant skeletal muscle atrophy as well as growth deficiency, which also proved the essential role of Akt-mTOR pathway on muscle maintenance (Peng et al., 2003; Risson et al., 2009). All the above discussion demonstrates the essential role of Akt-mTOR pathway in animals, but its role in controlling muscle protein synthesis in humans unloading models remains unclear. For example, human immobilization studies reported decrease in protein synthesis rate, but no changes were observed in Akt-mTORC1 signaling pathway (de Boer et al., 2007; Glover et al., 2008; Marimuthu et al., 2011). This suggests that decreased protein synthesis rate could also be regulated through other signaling pathways in human. Among other possible pathways, the most noteworthy one is focal adhesion kinase (FAK), a mechanosensitive non-receptor protein tyrosine kinase, located in the costamere region of skeletal muscle fibers and is sensitive to changes in mechanical loading (Bloch and Gonzalez-Serratos, 2003; Anastasi et al., 2008). Some crosstalk has been reported between FAK and PI3K-Akt-mTOR pathway. On the one hand, phosphorylation of tyrosine 397 of FAK results in the binding of FAK to the SH2 domain of the 85 kDa subunit of PI3K, which can lead to the increase in PI3K activity and subsequently activate Akt-mTOR pathway (Chen et al., 1996). On the other hand, activated FAK may upregulate mTOR through inhibiting TSC2 (a negative regulator of mTOR) by phosphorylation (Graham et al., 2015). Under physical inactivity conditions, reduced phosphorylation of FAK was discovered in humans and animals, which suggests that attenuated activation of FAK-Akt-mTOR is another key contributor to the decreased protein synthesis during atrophy condition (de Boer et al., 2007; Glover et al., 2008; Graham et al., 2015). Collectively, both the declined activation of IGF1–Akt–mTOR pathway induced by impaired IGF-1 signaling/insulin resistance and the decreased activation of FAK-Akt-mTOR pathway caused by reduced FAK phosphorylation play essential roles in regulating skeletal muscle protein synthesis during atrophy conditions. The possible mechanisms are also summarized briefly in Figure 1.
In addition to the regulation of translation initiation, it has also been reported that eukaryotic elongation factor 2 (eEF2) plays an important role in the regulation of protein synthesis at the level of elongation of mRNA translation process (Redpath et al., 1996). Recently it was observed that the level of eEF2 phosphorylation (inactive form) in soleus muscle elevated significantly after 14 days of HLU in rats (Lomonosova et al., 2017). Besides, protein synthesis also depends on translational capacity, the main component of which is the number of ribosomes (McCarthy and Esser, 2010; Chaillou et al., 2014). Decreases in the content of both total RNA and 28S rRNA (one of the key markers of ribosome content) were observed after 1, 3, 6, and 7 days of HLU in rat soleus (Bajotto et al., 2011; Mirzoev et al., 2016). Although great progress has been made as described above, there is still a lot of work that needs to be done. For example, studies with frequent biopsy sampling are required to comprehensively understand the role of mTORC1 signaling in regulating the depression in postprandial and post-absorptive muscle protein synthesis, especially in human models. In addition, more research is needed to clarify the relative downstream genes for regulation of ribosome assembly in the Akt-mTOR pathway.
In contrast to the recognized deficits in muscle protein synthesis during disuse conditions, the role of protein breakdown in disuse-induced muscle atrophy is less clear. This is partly due to the lack of direct measurements of muscle protein degradation in studies. Instead, indirect studies of protein degradation pathways have to be employed to measure molecular markers of muscle proteolysis. The major protein degradation pathways in skeletal muscle include the Ca2+-dependent proteases, lysosomal system, caspases and ubiquitin proteasome pathways (Scicchitano et al., 2015). It was proposed that Ca2+-dependent proteases (calpains) act as a promoter of muscle protein degradation, and might be responsible for the discharge of myofilaments from the surface of myofibrils (Dayton et al., 1976). Subsequently, the myofilaments were ubiquitinated and degraded to amino acids by proteasome intracellular peptidase cathepsins (Figure 2; Huang and Zhu, 2016). Emerging evidence suggests that calpains (Huang and Zhu, 2016), caspase-3 (Talbert et al., 2013), autophagy-lysosomal system (Sandri, 2010) and ubiquitin proteasome pathway (Bodine and Baehr, 2014), all are involved in disuse-induced muscle atrophy. However, the ubiquitin proteasome system is often considered as the most important proteolytic system during disuse conditions that promotes muscle wasting (Scicchitano et al., 2015; Baehr et al., 2017). The breakdown of protein via the ubiquitin proteasome system requires three distinct enzymatic components of the ubiquitin proteasome pathway: E1 (ubiquitin-activating enzyme), E2 (ubiquitin-conjugating enzyme) and E3 (ubiquitin ligase, key enzyme which regulates proteolysis as it recognizes multiple target protein substrates). Two muscle specific classes of E3s (MuRF1 and MAFbx/atrogin-1) have been studied widely and play an essential role during skeletal muscle atrophy (Mitch and Goldberg, 1996; Foletta et al., 2011; Bodine and Baehr, 2014). In various animal models of disuse muscle atrophy (HLU, immobilization, spaceflight and denervation), mRNA levels of both genes (MuRF1/MAFbx) were rapidly increasing and thought to play a crucial role in the initiation of the atrophy process (Bodine et al., 2001a; Lecker et al., 2004; Murton et al., 2008; Allen et al., 2009; Baehr et al., 2017; Gambara et al., 2017). In addition, it has been reported that the degree and the time course of the upregulation of these two genes is not uniform among different muscles. For example, a more significant increase in the expression of MuRF1/MAFbx genes occurred in ankle plantar flexors (soleus and medial gastrocnemius) than dorsi flexors (tibialis anterior), and there was a longer duration of expression in ankle plantar flexors than dorsi flexors in response to unloading (Bodine et al., 2001a; Lecker et al., 2004). Moreover, studies using MAFbx and MuRF1-deficient mouse models further supported a potential contributing role of these two genes in the development of muscle atrophy. For instance, mice deficient in either MAFbx or MuRF1 were found to be resistant to atrophy (Bodine et al., 2001a). Another study also indicated remarkable protection of the soleus muscle in the MuRF1-KO mice during 10 days of HLU (Labeit et al., 2010). It should be noted that various reports have been shown about MuRF1 and MAFbx during disuse-induced atrophy in human models (Murton et al., 2008). For example, both the MuRF1 and MAFbx mRNAs increased significantly after 2 days (Abadi et al., 2009) and 5 days of immobilization (Dirks et al., 2014) or after 3 days of unilateral lower limb suspension (ULLS) (Gustafsson et al., 2010). However, in a 20-day bed rest study, elevated MAFbx but not MuRF1 mRNA were observed in VL (Ogawa et al., 2006), same results were observed during 14 days of leg immobilization (Jones et al., 2004). On the other hand, de Boer and colleagues reported that increased MuRF1, mRNA expression, but not MAFbx were observed during 0–10 days of immobilization (de Boer et al., 2007). In addition, it has been reported that MuRF1 protein expression increased in soleus, but not in VL after 60 days of bed rest (Salanova et al., 2008).
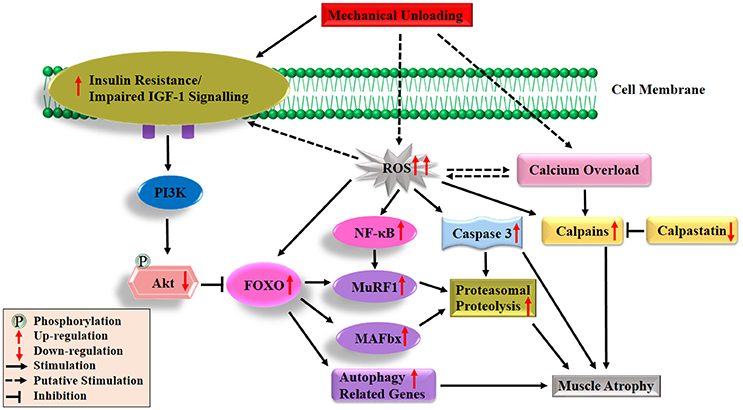
Figure 2. Diagrammatic representation of the protein degradation signaling mechanisms responsible of skeletal muscle atrophy following mechanical unloading. IGF-1, insulin-like growth factor-1; PI3K, phosphatidylinositol 3 kinase; Akt/PKB, protein kinase B; FOXO, forkhead family of transcription factors; NF-κB, nuclear factor kappa-B; MuRF1, muscle ring finger 1; MAFbx, muscle atrophy F-box 1; ROS, reactive oxygen species. The black arrow and inhibition symbol show the association of molecules under loading condition, red arrow shows the up/down-regulation of molecules under unloading condition. For more details see text.
Under disuse conditions, it has been reported that expression of E3 ubiquitin ligases MuRF1 and MAFbx are regulated by various upstream factors or signaling pathways (Bodine and Baehr, 2014). FOXO transcription factors (FOXO1 and FOXO3) have been stated as the major transcription factors regulating both the MuRF1 and MAFbx expressions (Sandri et al., 2004; Stitt et al., 2004). It is noteworthy that the functional aspects of FOXO are determined by their cellular location and predominantly regulated by the IGF-1-PI3K-Akt pathways (Brunet et al., 1999; Zhao et al., 2007). Under normal physiological conditions, Akt phosphorylates FOXO on specific threonine and serine residues, which results in the retention of FOXO in the cytosol instead of translocating to the nucleus (Brunet et al., 1999). Under muscle disuse conditions, dephosphorylated FOXO translocates to the nucleus and up-regulates several different types of atrogenes (E3 ligases) or autophagy related genes as shown in Figure 2 (Stitt et al., 2004). Several studies reported that mRNA and protein levels of FOXO1 and/or FOXO3 expression in the slow-twitch soleus muscle, mixed fiber type gastrocnemius muscle and fast twitch plantaris muscle are upregulated following different muscle atrophy condition (Giresi et al., 2005; Sacheck et al., 2007; Allen et al., 2009; Levine et al., 2011; Okamoto and Machida, 2017). Sandri and colleagues found that constitutively active FOXO3 acts on the MAFbx promoter to cause MAFbx transcription and dramatic atrophy of myotubes and muscle fiber. It further suggested that stimulation of the two main proteolytic pathways (MuRF1/MAFbx) via overexpression of FOXO1 and FOXO3 leads to a reduction in muscle mass and strength during disuse inactivity (Sandri et al., 2004). Direct or indirect inhibition of FOXO transcriptional activity or suppressed expression of co-factors (MuRF1/MAFbx) and interaction with other transcription factors lead to the attenuation of disuse-induced muscle atrophy (Senf et al., 2008; Reed et al., 2012; Brocca et al., 2017). But these two transcription factors mRNA are not always linked and that changes in the expression levels of both genes (MuRF1/MAFbx) are dependent on the muscle and the time after unloading (Atherton et al., 2016). Interestingly, it was observed that constitutively active FOXO3 controls the stimulation of autophagic/lysosomal proteolysis pathway, thus leading to muscle wasting in fasting and denervation models (Mammucari et al., 2007). Put-together, these findings strongly support the notion that most of these transcription factors and signaling pathways play important roles in the progression of muscle disuse atrophy. Remarkably, increased mRNA levels of both genes (MuRF1/MAFbx) were observed in animals following disuse conditions as shown in Figure 2. While, data were inconsistent in human models, a previous review showed that activation of these two genes mainly occurs in muscle wasting caused by inflammation (e.g., cancer, chronic obstructive pulmonary disease, severe head trauma, amyotrophic lateral sclerosis, critical illness, AIDS) with the data in non-inflammatory muscle atrophy is inconsistent (Narici and Maffulli, 2010). Only a few studies have measured the changes in ubiquitinated protein content and proteasomal proteolysis following disuse atrophy (Helliwell et al., 1998; Ogawa et al., 2006; Slimani et al., 2012; Baehr et al., 2016). There is a need for additional studies on human biopsies to explore the activity of ubiquitin-proteosome pathway components.
Besides the above-mentioned pathways regarding muscle protein synthesis and degradation following disuse atrophy, there are two important factors contributing to protein turnover. The following discussion gives a review regarding the possible triggers reported during muscle disuse.
Oxidative stress, characterized by the increased reactive oxygen species (ROS) production and impairment of antioxidant defense systems, has frequently been observed in disuse and other pathological conditions. It is now widely considered as a major trigger of the imbalance between protein synthesis and degradation leading to muscle atrophy (Powers et al., 2005, 2007; Moylan and Reid, 2007; Powers, 2014; Zuo and Pannell, 2015). Production of ROS results in the inhibition of insulin action and acts as a putative mediator in the development of insulin resistance (Bashan et al., 2009; Di Meo et al., 2017). Furthermore, growing evidence indicates that oxidative stress can promote muscle protein breakdown through the following aspects. Firstly, oxidative stress, promotes expression of proteins involved in proteolytic pathways, such as autophagy, calpain and the ubiquitin–proteasome system of proteolysis. Secondly, oxidative stress results in the activation of two important proteases, calpain and caspase-3. Thirdly, increased ROS production in muscle fibers can also promote proteolysis by oxidative modification of myofibrillar proteins, which enhances their susceptibility to proteolytic processing. The details about the three aspects have been discussed in a previous published review (Powers, 2014). ROS also might be the upstream activators of nuclear factor kappa-B (NF-κB) and FOXO pathways in skeletal muscle atrophy (Dodd et al., 2010). The roles played by ROS in protein synthesis and degradation are depicted in Figures 1, 2, respectively.
Calcium (Ca2+) is necessary to carry out many important body functions such as cell metabolism, cardiac and skeletal muscle contraction, tissue differentiation and neurotransmission (Zhou et al., 2013). Ca2+ and endogenous inhibitor calpastatin are the two major regulators on calpains activation during disuse conditions (Figure 2; Huang and Zhu, 2016). Previous studies reported highly elevated cytosolic free Ca2+ concentration in soleus and gastrocnemius muscles during disuse conditions (Ingalls et al., 2001; Xu et al., 2012; Hu et al., 2017). Two ubiquitous calpains, calpain1 and calpain2 (also called u- and m-) are activated by elevated intracellular Ca2+ in HLU rats (Matsumoto et al., 2014; Zhang et al., 2017). In addition, caspase-3-dependent apoptosis, another major signaling pathway involved in disuse muscle atrophy (Talbert et al., 2013), is also activated by intracellular Ca2+ overload through two distinct pathways. On the one hand, the intracellular Ca2+ overload leads to the activation of caspase-12 which then activates caspase-3 (Primeau et al., 2002). On the other hand, increasing Ca2+ levels induces activation of pro-apoptotic protein Bax, which translocated and inserted into the outer membrane of mitochondria via forming Bax/Bax-homo-oligomerization. Bcl-2, another Bcl-2 family protein, could inhibit the formation of Bax/Bax-homo-oligomerization. The decline of the ratio of Bax/Bcl-2 leads to the release of pro-apoptotic factors from the mitochondria, which subsequently activates caspase-9 and caspase-3 (Zha et al., 1996; Antonsson et al., 1997; Chen et al., 2002; Garrido et al., 2006). In one of our previous studies, the increase of Bax/Bcl-2 and cytochrome C release were observed in gastrocnemius in rats following 14-day HLU (Hu et al., 2017). Collectively, both the overproduction of ROS and Ca2+ overload play an essential role in regulation of protein synthesis and degradation following disuse conditions as shown in Figure 2. However, the relationship between ROS and Ca2+ remains unclear and more research is needed to clarify this relationship.
In addition to the anabolic and catabolic pathways mentioned above, recently emerging evidence indicates some other key factors, such as P53, activating transcription factor 4 (ATF4), growth arrest and DNA damage-inducible 45a protein (Gadd45a) and P21, are significantly elevated under disuse conditions (Ebert et al., 2012; Fox et al., 2014; Bullard et al., 2016). Therefore, we recommend that the reader consult the most recently published reviews on the topic (Brooks and Myburgh, 2014; Adams et al., 2017). Taken together, during disuse conditions, both the protein synthesis and degradation play an essential role during muscle atrophy, and in particular, suppressed protein synthesis has been confirmed. However, more research has to be carried out to clarify the details mechanisms of protein degradation and insulin resistance in driving disuse-induced muscle atrophy.
Various therapeutic interventions, pharmaceutical options and rehabilitation programs have been used to prevent and limit the loss of skeletal muscle. These therapeutic countermeasures can be grouped into three categories: antioxidant and anti-inflammatory compounds, nutritional supplements and physical training and exercise.
It has been demonstrated that muscle damage, oxidative stress and inflammation have a negative impact on protein turnover of skeletal muscle, predominantly via decreases in protein synthesis (Peterson et al., 2011; Powers et al., 2012). Oxidative stress is thought to be one of the major factors leading to many health-related disorders including skeletal muscle dysfunction (Powers et al., 2012). In addition, increased mitochondria ROS production, as well as endoplasmic reticulum stress and decrease of antioxidant capacity, are three major factors that play key roles in triggering sarcopenia with aging (Drew et al., 2003; Short et al., 2005; Narici and Maffulli, 2010). Thus, antioxidants have been shown to prevent oxidative stress associated damage and have been proven to be effective countermeasures against muscle atrophy to maintain skeletal muscle mass and strength, especially in elders (Servais et al., 2007; Cornetti, 2009; Rieu et al., 2009; Stojiljkovic et al., 2016).
In recent years, polyphenols, a well-recognized antioxidants, have been studied extensively with regard to their roles in the prevention of neurodegenerative diseases/skeletal muscle atrophy. Resveratrol, is one of the naturally occurring polyphenols, well known for its great health benefits, and frequently found in berries, grapes, red wine and some other fruits and vegetables (Brito et al., 2008; Das et al., 2008). It has been suggested that resveratrol plays an important role in the transcription of two important antioxidant enzymes, i.e., Mn superoxide dismutase (SOD) and catalase (Dani et al., 2008; Kode et al., 2008; Robb et al., 2008; Ryan et al., 2010). Six month old adult rats were treated with resveratrol (12.5 mg/kg/day) for 5 weeks (including 2 weeks of muscle immobilization) with results indicating that it reduced the functional decrements and the oxidative stress level (Jackson et al., 2010). In another study, rats were supplemented with resveratrol at a dose (400 mg/kg/day) before unloading and 2 weeks of muscle immobilization. During treatment with resveratrol, muscle disuse atrophy was significantly reduced (Momken et al., 2011). These two studies strongly suggest that metabolic and muscle deconditioning in response to mechanical unloading can be prevented by the use of high dosage of the antioxidant, resveratrol. Similarly, in another study, mice were treated with tea catechins (comprising of up to 81% polyphenols) at a dosage of 46–50 mg/kg. The antioxidant diet was consumed before and during immobilization of 14 and 10 days, respectively. This study showed that the antioxidant (tea catechins) did not suppress muscle atrophy completely, but it helped in the maintenance of tetanic force observed in the soleus muscle in response to immobilization. This study also suggested that tea catechins have positive effects on skeletal muscle function rather than skeletal mass, and they help to improve muscle strength (Ota et al., 2011).
In addition to the above findings, several studies have reported the use of several other antioxidants [e.g., vitamin E or SS-31 (D-Arg-2′6′dimethylTyr-Lys-Phe-NH2) for the prevention of disuse muscle atrophy, (Servais et al., 2007; Powers, 2014)]. For instance, administration of vitamin E was shown to significantly reduce soleus muscle atrophy during 14-day HLU. The results of this research also demonstrated that the protective role of vitamin E does not depend on its antioxidant activity, but it might be due to alteration in muscle protein degradation (Servais et al., 2007). While, Koesterer and colleagues reported that vitamin E supplementation has no effect on HLU induced soleus and gastrocnemius muscle atrophy (Koesterer et al., 2002). In addition, it has been reported that SS-31, one of the essential mitochondrial-targeted antioxidant, could protect against HLU induced soleus and plantaris muscles atrophy both in rats and mice (Min et al., 2011; Talbert et al., 2013). Additionally, some authors reported that another two widely used antioxidants, either curcumin or N-acetylcysteine treatment could protect the diaphragm against ventilator-induced muscle wasting (Agten et al., 2011; Smuder et al., 2012), but did not prevent against inactivity-induced limb muscle atrophy (Farid et al., 2005).
In addition to the widely used antioxidant to prevent disuse atrophy, some anti-inflammatory compounds and pharmaceutical options were also adopted in this field. Some studies recommended the use of dietary fish oil to prevent immobilization-induced atrophy. Fish oils are well known for their anti-inflammatory properties as they contain different fatty acids (long chain n-3 fatty acid; Fetterman and Zdanowicz, 2009). N-3 fatty acids facilitate insulin-sensitive protein anabolism through the Akt-mTOR-S6K1 pathway, which prevents anabolic resistance and leads to decreased muscle atrophy induced by disuse (Gingras et al., 2007). Furthermore, another study reported that ingestion of 5% fish oil reduces disuse muscle atrophy via Akt pathway through E3 ubiquitin ligases and S6K1 pathway (You et al., 2010). Chromium (Cr) is also believed to preserve muscle mass by inhibiting the elevation of ubiquitin proteasome system pathway and restoring the impaired Akt signal through elevating the Akt phosphorylation (Dong et al., 2009).
Studies carried out in our laboratory show that tetramethylpyrazine is a dietary supplement that could effectively alleviate muscle atrophy in HLU rats (Gao et al., 2005; Zhang et al., 2007; Li et al., 2012). It was demonstrated that attenuating disuse-induced Ca2+ overload and activation of calpains system might be involved in the underlying mechanism of tetramethylpyrazine to counteract disuse-induced muscle atrophy (Wu et al., 2012; Hu et al., 2017; Zhang et al., 2017). Furthermore, various Chinese herbal medicines have also been used to reduce and prevent muscle loss caused by different unloading models. For example, Sijunzi Decoction (Hu et al., 2009), Angelica Sinensis (Qin et al., 2009; Du and Gao, 2014), Ligusticum (Qin et al., 2009), and Radix Astragali (Gao et al., 2005, 2008; Zhang et al., 2007) have been tested under different muscle atrophy conditions by our laboratory.
The majority of these traditional Chinese medicines have positive effects on blood circulation and/or are blood tonics have been reported to attenuate disuse-induced muscle atrophy (Gao et al., 2005; Zhang et al., 2007, 2017; Wu et al., 2012; Hu et al., 2017). Due to numerous effective components in these herbs, the major effective ingredients and the mechanisms involved against muscle atrophy remain unclear. Thus, future research should be carried out to identify specific and effective components of these herbs, thus leading to wider applications of these herbs to counter muscle atrophy.
Exercise is one of the best countermeasure against disuse atrophy. During physical inactivity conditions such as HDBR and HLU, exercise has been shown to be the most efficient countermeasure to address the deficits in muscle structure and function, as well as for maintenance of balance between muscle protein synthesis and protein breakdown (Herbert et al., 1988; Widrick et al., 1996; Shinohara et al., 2003). Loss in muscle mass during physical inactivity is more challenging in aged people compared to younger persons and, therefore, to maintain muscle protein synthesis rate, older individuals required more resistance exercise than younger individuals (Kumar et al., 2012). For instance, after 2 weeks of ULLS following 4 weeks of resistive exercise employed in younger and older individuals suggested that recovery of strength and muscle size was much more reduced in older adults (Suetta et al., 2009; Hvid et al., 2010). These findings were further supported by a study of older women in which resistance training of 12 weeks was performed (at a frequency of 3 times per week). These investigators observed an increase in quadriceps muscle volume by up to 6% in young women and only 3% increase in older women (Greig et al., 2011). Hvid and co-workers observed marked decrements in knee extensor muscle function in young and old individuals after 4-day lower limb disuse. Following 7-day recovery, knee extensor (isometric or isokinetic) strength was recovered in young individuals, while an impaired ability to fully recover was observed in older individuals (Hvid et al., 2014).
Moreover, it has been documented that different types of resistance exercises play a key role in the maintenance of muscle mass in disuse models (e.g., bed rest, HLU) by improving muscle protein synthesis via activation of the PI3K-Akt-mTOR pathway (Ferrando et al., 1997; Fluckey et al., 2004; Hornberger et al., 2004; Philp et al., 2011). It has been proposed that short period of resistance exercise can activate IGF-1 gene expression in healthy individuals (Chesley et al., 1992). For instance, full restoration of quadriceps muscle mass following 2 weeks of single leg immobilization in humans can be achieved via high-resistance training (Oates et al., 2010). Additionally, combination of endurance and resistance exercise is an effective modality to counter the muscle loss associated with disuse or inactivity in HLU mice (Adams et al., 2007). In fact, resistance exercise partly rescued the loss in cytoskeletal and dystrophin-associated glycoprotein in VL and soleus muscles at protein level for the duration of extended bed rest (84 days) in human beings (Chopard et al., 2005). It was also demonstrated that resistance exercises associated with a ~50% decrease in the stimulation of the ubiquitin-proteasome system (MuRF1/MAFbx) and alleviate muscle atrophy caused by 14 days of HLU (Dupont-Versteegden et al., 2006).
Moreover, a number of other published literatures opine that the increased mechanical load can activate cellular signaling that initiates the protein synthesis independent of a traditionally described functioning IGF-1 receptor (Bickel et al., 2005; Hornberger et al., 2006; Spangenburg et al., 2008; Hamilton et al., 2010; West et al., 2010; Witkowski et al., 2010; Gabriel et al., 2016). Noticeably, these experiments pave the way for future investigations regarding Akt-mediated signaling in response to mechanical loading and other growth stimuli, as well as provide new insights for the prevention of disuse-induced muscle atrophy.
In general, applying exercise and physical training is one of the most widely used, effective and with minimal side effects in all countermeasures, but exercise and physical training cannot always be applied to injured patients with fractures and is often problematic for bed rest patients. In addition, the acceptance of this countermeasure is also difficult for those who do not want to exercise. Thus, exercises that possess minimum load or minimum exercise time against disuse muscle atrophy needs further investigation.
Exercise alone cannot avert the disuse-induced muscle loss under different unloading conditions, including bed rest confinement during hospitalizations and sedentary lifestyle. Hence, several other approaches, such as nutritional supplementation (essential amino acid and protein), should be used in conjunction with exercise to rescue or counteract better against catabolic processes during chronic disuse. Specifically, muscle mass and strength can be more effectively enhanced by combining the nutritional regulation (dietary carbohydrates and amino acid) with an adaptive exercise regimen than by application of either treatment approach alone (Dreyer et al., 2008; Pasiakos et al., 2011). The use of protein supplement along with heavy resistance training can significantly improve muscle strength and mass in very old individuals (Bechshøft et al., 2017). Generally speaking, it appears that during immobilization and bed rest confinement, protein synthesis can significantly be improved by using amino acid supplementation, but it can only partially avert muscle atrophy (Glover et al., 2008). Amino acids are very well known for their important role in the regulation of protein synthesis and metabolism by accelerating the initiation step of peptide chain formation in skeletal muscle (Biolo et al., 1997; Bohé et al., 2001; Rennie et al., 2004; Stipanuk, 2007). It has consistently been shown that essential amino acid supplementation strengthens the response of muscle protein synthesis and partially rescues skeletal muscle loss experienced during bed rest (Stuart et al., 1990; Paddon-Jones, 2006). Furthermore, ingestion of amino acid supplements has been shown to enhance the fractional synthesis rate of protein in the soleus muscle (Carroll et al., 2005).
Leucine has been widely studied among all nutritional supplementations that were effective to prevent disuse muscle atrophy. It was documented that leucine, as an anabolic factor among all essential amino acids, has potential to affect muscle protein metabolism in several ways and is considered as a strong stimulator of protein synthesis (Katsanos et al., 2006). However, it is still unclear how leucine is sensed by the processes that regulate protein synthesis? Several in vivo and in vitro studies described that leucine plays an important role in improvement of protein synthesis via the IGF1-mTOR-Akt pathway (Kimball et al., 1999; Anthony et al., 2000b; Drummond et al., 2017). Anthony and co-workers reported that leucine is implicated in the stimulation of the eIF4E complex (that is, mRNA binding step in translation initiation; Anthony et al., 2000a). Branched-chain amino acids supplementation was observed to have an anabolic effect on human muscles during 14 days of bed rest, specifically, a slight improvement of protein synthesis in the early recovery period was observed (Stein et al., 1999, 2003). In addition, resistance exercise and essential amino acid supplementation were also shown to lead to preserve skeletal muscle mass and strength during 28-day bed rest (Brooks et al., 2008). Physical inactivity appears to inhibit mTORC1 signaling associated with reduced amino acid transporter protein contents thus suggesting that a blunted response in essential amino acid stimulation could be the underlying basis of muscle loss in older individuals (Hvid et al., 2010; Drummond et al., 2012). However, another study reported that supplementation of protein (0.8–1.5 g/kg/day) throughout 10 days of bed rest in older subjects promoted muscle strength but had no impact on muscle mass loss (Dillon et al., 2009). Discussions related to the efficiency of amino acid countermeasures in preventing protein mass losses during inactivity can be seen in the detailed review of Stein and associates (Stein and Blanc, 2011). In their conclusion, it is mentioned that various nutrition and protein supplements have different degrees of prevention and treatment of muscular atrophy, but the effect of these treatments is limited as a result of the blunted post absorptive and postprandial muscle protein synthesis after disuse atrophy. Therefore, searching for nutritional supplements with less synthetic metabolism resistance is undoubtedly an important research direction for the treatment of muscle atrophy.
Over the past few decades, our current understanding of the cellular and molecular mechanisms involved in disuse muscle atrophy has significantly increased. However, this understanding remains incomplete with numerous unanswered questions. So far, the importance of protein turnover in driving disuse-induced muscle atrophy has been widely recognized. When the rate of protein synthesis becomes slower than the rate of protein breakdown, muscle atrophy begins. From a growing number of clinical and preclinical experiments, it is now clear that blunted or suppressed muscle protein synthesis seems to be the major drivers of disuse atrophy rather than increased muscle protein breakdown. However, further investigations are still needed to completely understand that how and why the activation of MuRF1/MAFbx, FOXO and other genes affect both protein synthesis and protein breakdown, which strongly suggests that increased proteolysis occurred somewhere. Additionally, it has been suggested frequently that during prolonged periods of disuse, oxidative stress can influence biochemical pathways and gene expression that regulates both muscle protein synthesis and breakdown. There are many factors leading to oxidative stress, such as low temperature, hypoxia, tissue damage, inflammation and calcium overload, etc., but which one among these factors contributing to oxidative stress in disuse-induced muscle atrophy need to be further elucidated.
On the other hand, to date, many studies have demonstrated beneficial effects of therapeutics countermeasure in disuse-induced muscle loss in model organisms. But we still lack of appropriate treatment strategies and have limited pharmaceutical options. The major hindrance, of course, is lack of knowledge regarding the cellular and molecular mechanisms involved in disuse muscle atrophy, which is far more complicated than we have been led to believe. There is much more to learn about how to prevent muscle atrophy as it is evidently not limited to muscle protein synthesis and breakdown only. Even as far as protein turnover is concerned, more accurate and targeted studies are needed to be done to unlock the secrets of abnormal or unbalanced protein metabolism underlying disuse muscle atrophy. More effective therapeutic agents or measures will emerge and develop along with the deepening of the research on the detailed mechanism in future.
Conceived and designed by: YG and YA; Critical Analysis provided by: YG, YA, HW, and NG; Proof reading and edited the manuscript: YG, YA, HW, and NG; Wrote the paper: YG and YA; Figures drawn by: YA.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The critical reading of Kashif Majeed is gratefully acknowledged. We apologize to colleagues whose studies were not cited owing to space limitations.
Our work is supported by grants from the National Natural Science Foundation of China (No. 31772459) and the Post-doctoral Science Foundation of China (No. 2016M592831).
Abadi, A., Glover, E. I., Isfort, R. J., Raha, S., Safdar, A., Yasuda, N., et al. (2009). Limb immobilization induces a coordinate down-regulation of mitochondrial and other metabolic pathways in men and women. PLoS ONE 4:e6518. doi: 10.1371/journal.pone.0006518
Adams, C. M., Ebert, S. M., and Dyle, M. C. (2017). Role of ATF4 in skeletal muscle atrophy. Curr. Opin. Clin. Nutr. Metab. Care 20, 164–168. doi: 10.1097/MCO.0000000000000362
Adams, G. R., Caiozzo, V. J., and Baldwin, K. M. (2003). Skeletal muscle unweighting: spaceflight and ground-based models. J. Appl. Physiol. (1985) 95, 2185–2201. doi: 10.1152/japplphysiol.00346.2003
Adams, G. R., Haddad, F., Bodell, P. W., Tran, P. D., and Baldwin, K. M. (2007). Combined isometric, concentric, and eccentric resistance exercise prevents unloading-induced muscle atrophy in rats. J. Appl. Physiol. (1985) 103, 1644–1654. doi: 10.1152/japplphysiol.00669.2007
Agten, A., Maes, K., Smuder, A., Powers, S. K., Decramer, M., and Gayan-Ramirez, G. (2011). N-Acetylcysteine protects the rat diaphragm from the decreased contractility associated with controlled mechanical ventilation. Crit. Care Med. 39, 777–782. doi: 10.1097/CCM.0b013e318206cca9
Akima, H., Kawakami, Y., Kubo, K., Sekiguchi, C., Ohshima, H., Miyamoto, A., et al. (2000). Effect of short-duration spaceflight on thigh and leg muscle volume. Med. Sci. Sports Exerc. 32, 1743–1747. doi: 10.1097/00005768-200010000-00013
Akima, H., Kubo, K., Imai, M., Kanehisa, H., Suzuki, Y., Gunji, A., et al. (2001). Inactivity and muscle: effect of resistance training during bed rest on muscle size in the lower limb. Acta Physiol. Scand. 172, 269–278. doi: 10.1046/j.1365-201x.2001.00869.x
Akima, H., Kuno, S., Suzuki, Y., Gunji, A., and Fukunaga, T. (1997). Effects of 20 days of bed rest on physiological cross-sectional area of human thigh and leg muscles evaluated by magnetic resonance imaging. J. Gravit. Physiol. 4, S15–21.
Allen, D. L., Bandstra, E. R., Harrison, B. C., Thorng, S., Stodieck, L. S., Kostenuik, P. J., et al. (2009). Effects of spaceflight on murine skeletal muscle gene expression. J. Appl. Physiol. 106, 582–595. doi: 10.1152/japplphysiol.90780.2008
Allen, D. L., Linderman, J. K., Roy, R. R., Grindeland, R. E., Mukku, V., and Edgerton, V. R. (1997). Growth hormone/IGF-I and/or resistive exercise maintains myonuclear number in hindlimb unweighted muscles. J. Appl. Physiol. (1985) 83, 1857–1861. doi: 10.1152/jappl.1997.83.6.1857
Anastasi, G., Cutroneo, G., Santoro, G., Arco, A., Rizzo, G., Bramanti, P., et al. (2008). Costameric proteins in human skeletal muscle during muscular inactivity. J. Anat. 213, 284–295. doi: 10.1111/j.1469-7580.2008.00921.x
Anthony, J. C., Anthony, T. G., Kimball, S. R., Vary, T. C., and Jefferson, L. S. (2000a). Orally administered leucine stimulates protein synthesis in skeletal muscle of postabsorptive rats in association with increased elF4F formation. J. Nutr. 130, 139–145. doi: 10.1093/jn/130.2.139
Anthony, J. C., Yoshizawa, F., Anthony, T. G., Vary, T. C., Jefferson, L. S., and Kimball, S. R. (2000b). Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J. Nutr. 130, 2413–2419. doi: 10.1093/jn/130.10.2413
Antonsson, B., Conti, F., Ciavatta, A., Montessuit, S., Lewis, S., Martinou, I., et al. (1997). Inhibition of Bax channel-forming activity by Bcl-2. Science 277, 370–372. doi: 10.1126/science.277.5324.370
Atherton, P. J., Greenhaff, P. L., Phillips, S. M., Bodine, S. C., Adams, C. M., and Lang, C. H. (2016). Control of skeletal muscle atrophy in response to disuse: clinical/preclinical contentions and fallacies of evidence. Am. J. Physiol. Endocrinol. Metab. 311, E594–E604. doi: 10.1152/ajpendo.00257.2016
Baehr, L. M., West, D. W. D., Marshall, A. G., Marcotte, G. R., Baar, K., and Bodine, S. C. (2017). Muscle-specific and age-related changes in protein synthesis and protein degradation in response to hindlimb unloading in rats. J. Appl. Physiol. 122, 1336–1350. doi: 10.1152/japplphysiol.00703.2016
Baehr, L. M., West, D. W., Marcotte, G., Marshall, A. G., De Sousa, L. G., Baar, K., et al. (2016). Age-related deficits in skeletal muscle recovery following disuse are associated with neuromuscular junction instability and ER stress, not impaired protein synthesis. Aging (Albany. NY). 8, 127–146. doi: 10.18632/aging.100879
Bajotto, G., Sato, Y., Kitaura, Y., and Shimomura, Y. (2011). Effect of branched-chain amino acid supplementation during unloading on regulatory components of protein synthesis in atrophied soleus muscles. Eur. J. Appl. Physiol. 111, 1815–1828. doi: 10.1007/s00421-010-1825-8
Baldwin, K. M. (1996). Effect of spaceflight on the functional, biochemical, and metabolic properties of skeletal muscle. Med. Sci. Sports Exerc. 28, 983–987. doi: 10.1097/00005768-199608000-00008
Baldwin, K. M., and Haddad, F. (2001). Effects of different activity and inactivity paradigms on myosin heavy chain gene expression in striated muscle. J. Appl. Physiol. (1985) 90, 345–357. doi: 10.1152/jappl.2001.90.1.345
Bashan, N., Kovsan, J., Kachko, I., Ovadia, H., and Rudich, A. (2009). Positive and negative regulation of insulin signaling by reactive oxygen and nitrogen species. Physiol. Rev. 89, 27–71. doi: 10.1152/physrev.00014.2008
Baumgartner, R. N., Koehler, K. M., Gallagher, D., Romero, L., Heymsfield, S. B., Ross, R. R., et al. (1998). Epidemiology of sarcopenia among the elderly in New Mexico. Am. J. Epidemiol. 147, 755–763. doi: 10.1093/oxfordjournals.aje.a009520
Bechshøft, R. L., Malmgaard-Clausen, N. M., Gliese, B., Beyer, N., Mackey, A. L., Andersen, J. L., et al. (2017). Improved skeletal muscle mass and strength after heavy strength training in very old individuals. Exp. Gerontol. 92, 96–105. doi: 10.1016/j.exger.2017.03.014
Bialek, P., Morris, C., Parkington, J., St Andre, M., Owens, J., Yaworsky, P., et al. (2011). Distinct protein degradation profiles are induced by different disuse models of skeletal muscle atrophy. Physiol. Genomics 43, 1075–1086. doi: 10.1152/physiolgenomics.00247.2010
Bickel, C. S., Slade, J., Mahoney, E., Haddad, F., Dudley, G. A., and Adams, G. R. (2005). Time course of molecular responses of human skeletal muscle to acute bouts of resistance exercise. J. Appl. Physiol. (1985) 98, 482–488. doi: 10.1152/japplphysiol.00895.2004
Biolo, G., Tipton, K. D., Klein, S., and Wolfe, R. R. (1997). An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am. J. Physiol. 273(1 Pt 1), E122–129. doi: 10.1152/ajpendo.1997.273.1.E122
Bloch, R. J., and Gonzalez-Serratos, H. (2003). Lateral force transmission across costameres in skeletal muscle. Exerc. Sport Sci. Rev. 31, 73–78. doi: 10.1097/00003677-200304000-00004
Bodine, S. C. (2013). Disuse-induced muscle wasting. Int. J. Biochem. Cell Biol. 45, 2200–2208. doi: 10.1016/j.biocel.2013.06.011
Bodine, S. C., and Baehr, L. M. (2014). Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am. J. Physiol. Endocrinol. Metab. 307, E469–E484. doi: 10.1152/ajpendo.00204.2014
Bodine, S. C., Latres, E., Baumhueter, S., Lai, V. K., Nunez, L., Clarke, B. A., et al. (2001a). Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294, 1704–1708. doi: 10.1126/science.1065874
Bodine, S. C., Stitt, T. N., Gonzalez, M., Kline, W. O., Stover, G. L., Bauerlein, R., et al. (2001b). Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat. Cell Biol. 3, 1014–1019. doi: 10.1038/ncb1101-1014
Bogdanis, G. C. (2012). Effects of physical activity and inactivity on muscle fatigue. Front. Physiol. 3:142. doi: 10.3389/fphys.2012.00142
Bohé, J., Low, J. F., Wolfe, R. R., and Rennie, M. J. (2001). Latency and duration of stimulation of human muscle protein synthesis during continuous infusion of amino acids. J. Physiol. 532(Pt 2), 575–579. doi: 10.1111/j.1469-7793.2001.0575f.x
Bonaldo, P., and Sandri, M. (2013). Cellular and molecular mechanisms of muscle atrophy. Dis. Model. Mech. 6, 25–39. doi: 10.1242/dmm.010389
Boonyarom, O., and Inui, K. (2006). Atrophy and hypertrophy of skeletal muscles: structural and functional aspects. Acta Physiol. (Oxf). 188, 77–89. doi: 10.1111/j.1748-1716.2006.01613.x
Booth, F. W., and Gollnick, P. D. (1983). Effects of disuse on the structure and function of skeletal muscle. Med. Sci. Sports Exerc. 15, 415–420. doi: 10.1249/00005768-198315050-00013
Booth, F. W., and Kelso, J. R. (1973). Effect of hind-limb immobilization on contractile and histochemical properties of skeletal muscle. Pflugers Arch. 342, 231–238. doi: 10.1007/BF00591371
Booth, F. W., and Seider, M. J. (1979). Early change in skeletal muscle protein synthesis after limb immobilization of rats. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 47, 974–977. doi: 10.1152/jappl.1979.47.5.974
Breen, L., Stokes, K. A., Churchward-Venne, T. A., Moore, D. R., Baker, S. K., Smith, K., et al. (2013). Two weeks of reduced activity decreases leg lean mass and induces “anabolic resistance” of myofibrillar protein synthesis in healthy elderly. J. Clin. Endocrinol. Metab. 98, 2604–2612. doi: 10.1210/jc.2013-1502
Brito, P. M., Simões, N. F., Almeida, L. M., and Dinis, T. C. (2008). Resveratrol disrupts peroxynitrite-triggered mitochondrial apoptotic pathway: a role for Bcl-2. Apoptosis 13, 1043–1053. doi: 10.1007/s10495-008-0235-4
Brocca, L., Cannavino, J., Coletto, L., Biolo, G., Sandri, M., Bottinelli, R., et al. (2012). The time course of the adaptations of human muscle proteome to bed rest and the underlying mechanisms. J. Physiol. (Lond). 590, 5211–5230. doi: 10.1113/jphysiol.2012.240267
Brocca, L., Toniolo, L., Reggiani, C., Bottinelli, R., Sandri, M., and Pellegrino, M. A. (2017). FoxO-dependent atrogenes vary among catabolic conditions and play a key role in muscle atrophy induced by hindlimb suspension. J. Physiol. (Lond). 595, 1143–1158. doi: 10.1113/JP273097
Brooks, N., Cloutier, G. J., Cadena, S. M., Layne, J. E., Nelsen, C. A., Freed, A. M., et al. (2008). Resistance training and timed essential amino acids protect against the loss of muscle mass and strength during 28 days of bed rest and energy deficit. J. Appl. Physiol. (1985) 105, 241–248. doi: 10.1152/japplphysiol.01346.2007
Brooks, N. E., and Myburgh, K. H. (2014). Skeletal muscle wasting with disuse atrophy is multi-dimensional: the response and interaction of myonuclei, satellite cells and signaling pathways. Front. Physiol. 5:99. doi: 10.3389/fphys.2014.00099
Brunet, A., Bonni, A., Zigmond, M. J., Lin, M. Z., Juo, P., Hu, L. S., et al. (1999). Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96, 857–868. doi: 10.1016/S0092-8674(00)80595-4
Bullard, S. A., Seo, S., Schilling, B., Dyle, M. C., Dierdorff, J. M., Ebert, S. M., et al. (2016). Gadd45a Protein Promotes Skeletal Muscle Atrophy by Forming a Complex with the Protein Kinase MEKK4. J. Biol. Chem. 291, 17496–17509. doi: 10.1074/jbc.M116.740308
Caron, A. Z., Drouin, G., Desrosiers, J., Trensz, F., and Grenier, G. (2009). A novel hindlimb immobilization procedure for studying skeletal muscle atrophy and recovery in mouse. J. Appl. Physiol. (1985) 106, 2049–2059. doi: 10.1152/japplphysiol.91505.2008
Carroll, C. C., Fluckey, J. D., Williams, R. H., Sullivan, D. H., and Trappe, T. A. (2005). Human soleus and vastus lateralis muscle protein metabolism with an amino acid infusion. Am. J. Physiol. Endocrinol. Metab. 288, E479–E485. doi: 10.1152/ajpendo.00393.2004
Chaillou, T., Kirby, T. J., and McCarthy, J. J. (2014). Ribosome biogenesis: emerging evidence for a central role in the regulation of skeletal muscle mass. J. Cell. Physiol. 229, 1584–1594. doi: 10.1002/jcp.24604
Chen, H. C., Appeddu, P. A., Isoda, H., and Guan, J. L. (1996). Phosphorylation of tyrosine 397 in focal adhesion kinase is required for binding phosphatidylinositol 3-kinase. J. Biol. Chem. 271, 26329–26334. doi: 10.1074/jbc.271.42.26329
Chen, M., Won, D. J., Krajewski, S., and Gottlieb, R. A. (2002). Calpain and mitochondria in ischemia/reperfusion injury. J. Biol. Chem. 277, 29181–29186. doi: 10.1074/jbc.M204951200
Chesley, A., MacDougall, J. D., Tarnopolsky, M. A., Atkinson, S. A., and Smith, K. (1992). Changes in human muscle protein synthesis after resistance exercise. J. Appl. Physiol. (1985) 73, 1383–1388. doi: 10.1152/jappl.1992.73.4.1383
Chopard, A., Arrighi, N., Carnino, A., and Marini, J. F. (2005). Changes in dysferlin, proteins from dystrophin glycoprotein complex, costameres, and cytoskeleton in human soleus and vastus lateralis muscles after a long-term bedrest with or without exercise. FASEB J. 19, 1722–1724. doi: 10.1096/fj.04-3336fje
Cornetti, U. (2009). Antioxidant use in nutraceuticals. Clin. Dermatol. 27, 175–194. doi: 10.1016/j.clindermatol.2008.01.010
Dani, C., Bonatto, D., Salvador, M., Pereira, M. D., Henriques, J. A., and Eleutherio, E. (2008). Antioxidant protection of resveratrol and catechin in Saccharomyces cerevisiae. J. Agric. Food Chem. 56, 4268–4272. doi: 10.1021/jf800752s
Das, S., Khan, N., Mukherjee, S., Bagchi, D., Gurusamy, N., Swartz, H., et al. (2008). Redox regulation of resveratrol-mediated switching of death signal into survival signal. Free Radic. Biol. Med. 44, 82–90. doi: 10.1016/j.freeradbiomed.2007.09.008
Dayton, W. R., Goll, D. E., Zeece, M. G., Robson, R. M., and Reville, W. J. (1976). A Ca2+-activated protease possibly involved in myofibrillar protein turnover. Purification from porcine muscle. Biochemistry 15, 2150–2158. doi: 10.1021/bi00655a019
de Boer, M. D., Selby, A., Atherton, P., Smith, K., Seynnes, O. R., Maganaris, C. N., et al. (2007). The temporal responses of protein synthesis, gene expression and cell signalling in human quadriceps muscle and patellar tendon to disuse. J. Physiol. 585(Pt 1), 241–251. doi: 10.1113/jphysiol.2007.142828
Degens, H., and Korhonen, M. T. (2012). Factors contributing to the variability in muscle ageing. Maturitas 73, 197–201. doi: 10.1016/j.maturitas.2012.07.015
Desaphy, J. F., Pierno, S., Liantonio, A., Giannuzzi, V., Digennaro, C., Dinardo, M. M., et al. (2010). Antioxidant treatment of hindlimb-unloaded mouse counteracts fiber type transition but not atrophy of disused muscles. Pharmacol. Res. 61, 553–563. doi: 10.1016/j.phrs.2010.01.012
Deschenes, M. R., Giles, J. A., McCoy, R. W., Volek, J. S., Gomez, A. L., and Kraemer, W. J. (2002). Neural factors account for strength decrements observed after short-term muscle unloading. Am. J. Physiol. Regul. Integr. Comp. Physiol. 282, R578–R583. doi: 10.1152/ajpregu.00386.2001
Dillon, E. L., Sheffield-Moore, M., Paddon-Jones, D., Gilkison, C., Sanford, A. P., Casperson, S. L., et al. (2009). Amino acid supplementation increases lean body mass, basal muscle protein synthesis, and insulin-like growth factor-I expression in older women. J. Clin. Endocrinol. Metab. 94, 1630–1637. doi: 10.1210/jc.2008-1564
Di Meo, S., Iossa, S., and Venditti, P. (2017). Skeletal muscle insulin resistance: role of mitochondria and other ROS sources. J. Endocrinol. 233, R15–R42. doi: 10.1530/JOE-16-0598
Dirks, M. L., Wall, B. T., Snijders, T., Ottenbros, C. L., Verdijk, L. B., and van Loon, L. J. (2014). Neuromuscular electrical stimulation prevents muscle disuse atrophy during leg immobilization in humans. Acta Physiol. (Oxf). 210, 628–641. doi: 10.1111/apha.12200
Dodd, S. L., Gagnon, B. J., Senf, S. M., Hain, B. A., and Judge, A. R. (2010). Ros-mediated activation of NF-kappaB and Foxo during muscle disuse. Muscle Nerve 41, 110–113. doi: 10.1002/mus.21526
Dong, F., Hua, Y., Zhao, P., Ren, J., Du, M., and Sreejayan, N. (2009). Chromium supplement inhibits skeletal muscle atrophy in hindlimb-suspended mice. J. Nutr. Biochem. 20, 992–999. doi: 10.1016/j.jnutbio.2008.09.006
Drew, B., Phaneuf, S., Dirks, A., Selman, C., Gredilla, R., Lezza, A., et al. (2003). Effects of aging and caloric restriction on mitochondrial energy production in gastrocnemius muscle and heart. Am. J. Physiol. Regul. Integr. Comp. Physiol. 284, R474–R480. doi: 10.1152/ajpregu.00455.2002
Dreyer, H. C., Drummond, M. J., Pennings, B., Fujita, S., Glynn, E. L., Chinkes, D. L., et al. (2008). Leucine-enriched essential amino acid and carbohydrate ingestion following resistance exercise enhances mTOR signaling and protein synthesis in human muscle. Am. J. Physiol. Endocrinol. Metab. 294, E392–E400. doi: 10.1152/ajpendo.00582.2007
Drummond, M. J., Dickinson, J. M., Fry, C. S., Walker, D. K., Gundermann, D. M., Reidy, P. T., et al. (2012). Bed rest impairs skeletal muscle amino acid transporter expression, mTORC1 signaling, and protein synthesis in response to essential amino acids in older adults. Am. J. Physiol. Endocrinol. Metab. 302, E1113–E1122. doi: 10.1152/ajpendo.00603.2011
Drummond, M. J., Reidy, P. T., Baird, L. M., Dalley, B. K., and Howard, M. T. (2017). Leucine differentially regulates gene-specific translation in mouse skeletal muscle. J. Nutr. 147, 1616–1623. doi: 10.3945/jn.117.251181
Du, B., and Gao, Y. (2014). Angelica and the contractile properties of soleus muscle in tail-suspended rats. J. Northwest Univ. 44, 588–594. doi: 10.16152/j.cnki.xdxbzr.2014.04.063
Du, F., Wang, J., Gao, Y., Wang, H., Wang, Q., Jiang, S., et al. (2011). A hind limb disuse model inducing extensor digitorum longus atrophy in rats: tail suspension-immobilization. Aviat. Space Environ. Med. 82, 689–693. doi: 10.3357/ASEM.2984.2011
Dupont-Versteegden, E. E., Fluckey, J. D., Knox, M., Gaddy, D., and Peterson, C. A. (2006). Effect of flywheel-based resistance exercise on processes contributing to muscle atrophy during unloading in adult rats. J. Appl. Physiol. (1985) 101, 202–212. doi: 10.1152/japplphysiol.01540.2005
Ebert, S. M., Dyle, M. C., Kunkel, S. D., Bullard, S. A., Bongers, K. S., Fox, D. K., et al. (2012). Stress-induced skeletal muscle Gadd45a expression reprograms myonuclei and causes muscle atrophy. J. Biol. Chem. 287, 27290–27301. doi: 10.1074/jbc.M112.374777
Edgerton, V. R., Zhou, M. Y., Ohira, Y., Klitgaard, H., Jiang, B., Bell, G., et al. (1995). Human fiber size and enzymatic properties after 5 and 11 days of spaceflight. J. Appl. Physiol. (1985) 78, 1733–1739. doi: 10.1152/jappl.1995.78.5.1733
Erbay, E., Park, I. H., Nuzzi, P. D., Schoenherr, C. J., and Chen, J. (2003). IGF-II transcription in skeletal myogenesis is controlled by mTOR and nutrients. J. Cell Biol. 163, 931–936. doi: 10.1083/jcb.200307158
Evans, W. J. (2010). Skeletal muscle loss: cachexia, sarcopenia, and inactivity. Am. J. Clin. Nutr. 91, 1123S–1127S. doi: 10.3945/ajcn.2010.28608A
Farid, M., Reid, M. B., Li, Y. P., Gerken, E., and Durham, W. J. (2005). Effects of dietary curcumin or N-acetylcysteine on NF-kappaB activity and contractile performance in ambulatory and unloaded murine soleus. Nutr. Metab. (Lond). 2:20. doi: 10.1186/1743-7075-2-20
Ferrando, A. A., Tipton, K. D., Bamman, M. M., and Wolfe, R. R. (1997). Resistance exercise maintains skeletal muscle protein synthesis during bed rest. J. Appl. Physiol. (1985) 82, 807–810. doi: 10.1152/jappl.1997.82.3.807
Fetterman, J. W. Jr., and Zdanowicz, M. M. (2009). Therapeutic potential of n-3 polyunsaturated fatty acids in disease. Am. J. Health Syst. Pharm. 66, 1169–1179. doi: 10.2146/ajhp080411
Fitts, R. H., Brimmer, C. J., Heywood-Cooksey, A., and Timmerman, R. J. (1989). Single muscle fiber enzyme shifts with hindlimb suspension and immobilization. Am. J. Physiol. 256(5 Pt 1), C1082–C1091. doi: 10.1152/ajpcell.1989.256.5.C1082
Fitts, R. H., Riley, D. R., and Widrick, J. J. (2001). Functional and structural adaptations of skeletal muscle to microgravity. J. Exp. Biol. 204(Pt 18), 3201–3208.
Fitts, R. H., Trappe, S. W., Costill, D. L., Gallagher, P. M., Creer, A. C., Colloton, P. A., et al. (2010). Prolonged space flight-induced alterations in the structure and function of human skeletal muscle fibres. J. Physiol. 588(Pt 18), 3567–3592. doi: 10.1113/jphysiol.2010.188508
Fluckey, J. D., Dupont-Versteegden, E. E., Knox, M., Gaddy, D., Tesch, P. A., and Peterson, C. A. (2004). Insulin facilitation of muscle protein synthesis following resistance exercise in hindlimb-suspended rats is independent of a rapamycin-sensitive pathway. Am. J. Physiol. Endocrinol. Metab. 287, E1070–E1075. doi: 10.1152/ajpendo.00329.2004
Foletta, V. C., White, L. J., Larsen, A. E., Léger, B., and Russell, A. P. (2011). The role and regulation of MAFbx/atrogin-1 and MuRF1 in skeletal muscle atrophy. Pflugers Arch. 461, 325–335. doi: 10.1007/s00424-010-0919-9
Fox, D. K., Ebert, S. M., Bongers, K. S., Dyle, M. C., Bullard, S. A., Dierdorff, J. M., et al. (2014). p53 and ATF4 mediate distinct and additive pathways to skeletal muscle atrophy during limb immobilization. Am. J. Physiol. Endocrinol. Metab. 307, E245–E261. doi: 10.1152/ajpendo.00010.2014
Gabriel, B. M., Hamilton, D. L., Tremblay, A. M., and Wackerhage, H. (2016). The Hippo signal transduction network for exercise physiologists. J. Appl. Physiol. (1985) 120, 1105–1117. doi: 10.1152/japplphysiol.01076.2015
Gambara, G., Salanova, M., Ciciliot, S., Furlan, S., Gutsmann, M., Schiffl, G., et al. (2017). Microgravity-induced transcriptome adaptation in mouse paraspinal longissimus dorsi muscle highlights insulin resistance-linked genes. Front. Physiol. 8:279. doi: 10.3389/fphys.2017.00279
Gao, Y. F., Fan, X. L., He, Z. X., Wu, S. D., and Song, X. A. (2005). [Effects of Ligustrazine and Radix Astragali on activities of myosin adenosine triphosphatase of soleus muscle and muscle atrophy in tail-suspended rats]. Space Med. Med. Eng. (Beijing). 18, 262–266. doi: 10.16289/j.cnki.1002-0837.2005.04.007
Gao, Y., Goswami, N., Grasser, E., Rössler, A., Stöger, E., Schwaberger, G., et al. (2008). Radix astragali and orthostatic response: a double-masked crossover study. Aviat. Space Environ. Med. 79, 94–98. doi: 10.3357/ASEM.2193.2008
Garrido, C., Galluzzi, L., Brunet, M., Puig, P. E., Didelot, C., and Kroemer, G. (2006). Mechanisms of cytochrome c release from mitochondria. Cell Death Differ. 13, 1423–1433. doi: 10.1038/sj.cdd.4401950
Gingras, A. A., White, P. J., Chouinard, P. Y., Julien, P., Davis, T. A., Dombrowski, L., et al. (2007). Long-chain omega-3 fatty acids regulate bovine whole-body protein metabolism by promoting muscle insulin signalling to the Akt-mTOR-S6K1 pathway and insulin sensitivity. J. Physiol. 579(Pt 1), 269–284. doi: 10.1113/jphysiol.2006.121079
Giresi, P. G., Stevenson, E. J., Theilhaber, J., Koncarevic, A., Parkington, J., Fielding, R. A., et al. (2005). Identification of a molecular signature of sarcopenia. Physiol. Genomics 21, 253–263. doi: 10.1152/physiolgenomics.00249.2004
Glover, E. I., Phillips, S. M., Oates, B. R., Tang, J. E., Tarnopolsky, M. A., Selby, A., et al. (2008). Immobilization induces anabolic resistance in human myofibrillar protein synthesis with low and high dose amino acid infusion. J. Physiol. (Lond). 586, 6049–6061. doi: 10.1113/jphysiol.2008.160333
Goldspink, D. F. (1977). The influence of immobilization and stretch on protein turnover of rat skeletal muscle. J. Physiol. (Lond). 264, 267–282. doi: 10.1113/jphysiol.1977.sp011667
Goodman, C. A., Miu, M. H., Frey, J. W., Mabrey, D. M., Lincoln, H. C., Ge, Y., et al. (2010). A phosphatidylinositol 3-kinase/protein kinase B-independent activation of mammalian target of rapamycin signaling is sufficient to induce skeletal muscle hypertrophy. Mol. Biol. Cell 21, 3258–3268. doi: 10.1091/mbc.E10-05-0454
Gordon, B. S., Kelleher, A. R., and Kimball, S. R. (2013). Regulation of muscle protein synthesis and the effects of catabolic states. Int. J. Biochem. Cell Biol. 45, 2147–2157. doi: 10.1016/j.biocel.2013.05.039
Goswami, N. (2017). Falls and fall-prevention in older persons: geriatrics meets spaceflight! Front Physiol. 8:603. doi: 10.3389/fphys.2017.00603
Graham, Z. A., Gallagher, P. M., and Cardozo, C. P. (2015). Focal adhesion kinase and its role in skeletal muscle. J. Muscle Res. Cell Motil. 36, 305–315. doi: 10.1007/s10974-015-9415-3
Greig, C. A., Gray, C., Rankin, D., Young, A., Mann, V., Noble, B., et al. (2011). Blunting of adaptive responses to resistance exercise training in women over 75y. Exp. Gerontol. 46, 884–890. doi: 10.1016/j.exger.2011.07.010
Gustafsson, T., Osterlund, T., Flanagan, J. N., von Waldén, F., Trappe, T. A., Linnehan, R. M., et al. (2010). Effects of 3 days unloading on molecular regulators of muscle size in humans. J. Appl. Physiol. (1985) 109, 721–727. doi: 10.1152/japplphysiol.00110.2009
Haddad, F., Adams, G. R., Bodell, P. W., and Baldwin, K. M. (2006). Isometric resistance exercise fails to counteract skeletal muscle atrophy processes during the initial stages of unloading. J. Appl. Physiol. (1985) 100, 433–441. doi: 10.1152/japplphysiol.01203.2005
Hamburg, N. M., McMackin, C. J., Huang, A. L., Shenouda, S. M., Widlansky, M. E., Schulz, E., et al. (2007). Physical inactivity rapidly induces insulin resistance and microvascular dysfunction in healthy volunteers. Arterioscler. Thromb. Vasc. Biol. 27, 2650–2656. doi: 10.1161/ATVBAHA.107.153288
Hamilton, D. L., Philp, A., MacKenzie, M. G., and Baar, K. (2010). A limited role for PI(3,4,5)P3 regulation in controlling skeletal muscle mass in response to resistance exercise. PLoS ONE 5:e11624. doi: 10.1371/journal.pone.0011624
Han, B., Tong, J., Zhu, M. J., Ma, C., and Du, M. (2008). Insulin-like growth factor-1 (IGF-1) and leucine activate pig myogenic satellite cells through mammalian target of rapamycin (mTOR) pathway. Mol. Reprod. Dev. 75, 810–817. doi: 10.1002/mrd.20832
Helliwell, T. R., Wilkinson, A., Griffiths, R. D., McClelland, P., Palmer, T. E., and Bone, J. M. (1998). Muscle fibre atrophy in critically ill patients is associated with the loss of myosin filaments and the presence of lysosomal enzymes and ubiquitin. Neuropathol. Appl. Neurobiol. 24, 507–517. doi: 10.1046/j.1365-2990.1998.00144.x
Herbert, M. E., Roy, R. R., and Edgerton, V. R. (1988). Influence of one-week hindlimb suspension and intermittent high load exercise on rat muscles. Exp. Neurol. 102, 190–198. doi: 10.1016/0014-4886(88)90093-3
Hornberger, T. A., Hunter, R. B., Kandarian, S. C., and Esser, K. A. (2001). Regulation of translation factors during hindlimb unloading and denervation of skeletal muscle in rats. Am. J. Physiol. Cell Physiol. 281, C179–C187. doi: 10.1152/ajpcell.2001.281.1.C179
Hornberger, T. A., Stuppard, R., Conley, K. E., Fedele, M. J., Fiorotto, M. L., Chin, E. R., et al. (2004). Mechanical stimuli regulate rapamycin-sensitive signalling by a phosphoinositide 3-kinase-, protein kinase B- and growth factor-independent mechanism. Biochem. J. 380(Pt 3), 795–804. doi: 10.1042/bj20040274
Hornberger, T. A., Sukhija, K. B., and Chien, S. (2006). Regulation of mTOR by mechanically induced signaling events in skeletal muscle. Cell Cycle 5, 1391–1396. doi: 10.4161/cc.5.13.2921
Hu, N. F., Chang, H., Du, B., Zhang, Q. W., Arfat, Y., Dang, K., et al. (2017). Tetramethylpyrazine ameliorated disuse-induced gastrocnemius muscle atrophy in hindlimb unloading rats through suppression of Ca2+/ROS-mediated apoptosis. Appl. Physiol. Nutr. Metab. 42, 117–127. doi: 10.1139/apnm-2016-0363
Hu, Q., Gao, Y., and Cao, J. (2009). Effects of different doses of Sijunzi Decoction on soleus atrophy in tail-suspmended rats. Space Med. Med. Eng. 22, 9–12. doi: 10.16289/j.cnki.1002-0837.2009.01.006
Huang, J., and Zhu, X. (2016). The molecular mechanisms of calpains action on skeletal muscle atrophy. Physiol. Res. 65, 547–560.
Hughes, D. C., Marcotte, G. R., Marshall, A. G., West, D. W. D., Baehr, L. M., Wallace, M. A., et al. (2017). Age-related differences in dystrophin: impact on force transfer proteins, membrane integrity, and neuromuscular junction stability. J. Gerontol. A Biol. Sci. Med. Sci. 72, 640–648. doi: 10.1093/gerona/glw109
Hvid, L., Aagaard, P., Justesen, L., Bayer, M. L., Andersen, J. L., Ørtenblad, N., et al. (2010). Effects of aging on muscle mechanical function and muscle fiber morphology during short-term immobilization and subsequent retraining. J. Appl. Physiol. (1985) 109, 1628–1634. doi: 10.1152/japplphysiol.00637.2010
Hvid, L. G., Suetta, C., Nielsen, J. H., Jensen, M. M., Frandsen, U., Ørtenblad, N., et al. (2014). Aging impairs the recovery in mechanical muscle function following 4 days of disuse. Exp. Gerontol. 52, 1–8. doi: 10.1016/j.exger.2014.01.012
Ingalls, C. P., Wenke, J. C., and Armstrong, R. B. (2001). Time course changes in [Ca2+]i, force, and protein content in hindlimb-suspended mouse soleus muscles. Aviat. Space Environ. Med. 72, 471–476.
Inoki, K., Li, Y., Zhu, T. Q., Wu, J., and Guan, K. L. (2002). TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat. Cell Biol. 4, 648–657. doi: 10.1038/ncb839
Jackson, J. R., Ryan, M. J., Hao, Y., and Alway, S. E. (2010). Mediation of endogenous antioxidant enzymes and apoptotic signaling by resveratrol following muscle disuse in the gastrocnemius muscles of young and old rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 299, R1572–R1581. doi: 10.1152/ajpregu.00489.2010
Janssen, I., Heymsfield, S. B., Wang, Z. M., and Ross, R. (2000). Skeletal muscle mass and distribution in 468 men and women aged 18-88 yr. J. Appl. Physiol. (1985) 89, 81–88. doi: 10.1152/jappl.2000.89.1.81
Jheng, H. F., Tsai, P. J., Guo, S. M., Kuo, L. H., Chang, C. S., Su, I. J., et al. (2012). Mitochondrial fission contributes to mitochondrial dysfunction and insulin resistance in skeletal muscle. Mol. Cell. Biol. 32, 309–319. doi: 10.1128/MCB.05603-11
Jones, S. W., Hill, R. J., Krasney, P. A., O'Conner, B., Peirce, N., and Greenhaff, P. L. (2004). Disuse atrophy and exercise rehabilitation in humans profoundly affects the expression of genes associated with the regulation of skeletal muscle mass. FASEB J. 18, 1025–1027. doi: 10.1096/fj.03-1228fje
Jozsa, L., Thöring, J., Järvinen, M., Kannus, P., Lehto, M., and Kvist, M. (1988). Quantitative alterations in intramuscular connective tissue following immobilization: an experimental study in the rat calf muscles. Exp. Mol. Pathol. 49, 267–278. doi: 10.1016/0014-4800(88)90039-1
Katsanos, C. S., Kobayashi, H., Sheffield-Moore, M., Aarsland, A., and Wolfe, R. R. (2006). A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am. J. Physiol. Endocrinol. Metab. 291, E381–E387. doi: 10.1152/ajpendo.00488.2005
Kelleher, A. R., Kimball, S. R., Dennis, M. D., Schilder, R. J., and Jefferson, L. S. (2013). The mTORC1 signaling repressors REDD1/2 are rapidly induced and activation of p70S6K1 by leucine is defective in skeletal muscle of an immobilized rat hindlimb. Am. J. Physiol. Endocrinol. Metab. 304, E229–E236. doi: 10.1152/ajpendo.00409.2012
Kelleher, A. R., Pereira, S. L., Jefferson, L. S., and Kimball, S. R. (2015). REDD2 expression in rat skeletal muscle correlates with nutrient-induced activation of mTORC1: responses to aging, immobilization, and remobilization. Am. J. Physiol. Endocrinol. Metab. 308, E122–E129. doi: 10.1152/ajpendo.00341.2014
Keller, K., and Engelhardt, M. (2013). Strength and muscle mass loss with aging process. Age and strength loss. Muscles Ligaments Tendons J. 3, 346–350.
Kimball, S. R., Shantz, L. M., Horetsky, R. L., and Jefferson, L. S. (1999). Leucine regulates translation of specific mRNAs in L6 myoblasts through mTOR-mediated changes in availability of eIF4E and phosphorylation of ribosomal protein S6. J. Biol. Chem. 274, 11647–11652. doi: 10.1074/jbc.274.17.11647
Knudsen, S. H., Hansen, L. S., Pedersen, M., Dejgaard, T., Hansen, J., Hall, G. V., et al. (2012). Changes in insulin sensitivity precede changes in body composition during 14 days of step reduction combined with overfeeding in healthy young men. J. Appl. Physiol. (1985) 113, 7–15. doi: 10.1152/japplphysiol.00189.2011
Kode, A., Rajendrasozhan, S., Caito, S., Yang, S. R., Megson, I. L., and Rahman, I. (2008). Resveratrol induces glutathione synthesis by activation of Nrf2 and protects against cigarette smoke-mediated oxidative stress in human lung epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 294, L478–L488. doi: 10.1152/ajplung.00361.2007
Koesterer, T. J., Dodd, S. L., and Powers, S. (2002). Increased antioxidant capacity does not attenuate muscle atrophy caused by unweighting. J. Appl. Physiol. (1985) 93, 1959–1965. doi: 10.1152/japplphysiol.00511.2002
Kortebein, P., Paddon-Jones, D., Ronsen, O., Trappe, T., Lombeida, J., Ferrando, A., et al. (2006). Effects of 10 days of bedrest on body composition and the rate of muscle protein synthesis in older men and women. FASEB J. 20, A159–A159.
Koryak, Y. U. (2001). Electrically evoked and voluntary properties of the human triceps surae muscle: effects of long-term spaceflights. Acta Physiol. Pharmacol. Bulg. 26, 21–27.
Kozlovskaya, I. B., Kreidich Yu, V., Oganov, V. S., and Koserenko, O. P. (1981). Pathophysiology of motor functions in prolonged manned space flights. Acta Astronaut. 8, 1059–1072. doi: 10.1016/0094-5765(81)90079-5
Krogh-Madsen, R., Thyfault, J. P., Broholm, C., Mortensen, O. H., Olsen, R. H., Mounier, R., et al. (2010). A 2-wk reduction of ambulatory activity attenuates peripheral insulin sensitivity. J. Appl. Physiol. (1985) 108, 1034–1040. doi: 10.1152/japplphysiol.00977.2009
Kumar, V., Atherton, P. J., Selby, A., Rankin, D., Williams, J., Smith, K., et al. (2012). Muscle protein synthetic responses to exercise: effects of age, volume, and intensity. J. Gerontol. A Biol. Sci. Med. Sci. 67, 1170–1177. doi: 10.1093/gerona/gls141
Kyparos, A., Feeback, D. L., Layne, C. S., Martinez, D. A., and Clarke, M. S. (2005). Mechanical stimulation of the plantar foot surface attenuates soleus muscle atrophy induced by hindlimb unloading in rats. J. Appl. Physiol. (1985) 99, 739–746. doi: 10.1152/japplphysiol.00771.2004
Labeit, S., Kohl, C. H., Witt, C. C., Labeit, D., Jung, J., and Granzier, H. (2010). Modulation of muscle atrophy, fatigue and MLC phosphorylation by MuRF1 as indicated by hindlimb suspension studies on MuRF1-KO mice. J. Biomed. Biotechnol. 2010:693741. doi: 10.1155/2010/693741
Lawler, J. M., Song, W., and Demaree, S. R. (2003). Hindlimb unloading increases oxidative stress and disrupts antioxidant capacity in skeletal muscle. Free Radic. Biol. Med. 35, 9–16. doi: 10.1016/S0891-5849(03)00186-2
LeBlanc, A., Lin, C., Shackelford, L., Sinitsyn, V., Evans, H., Belichenko, O., et al. (2000). Muscle volume, MRI relaxation times (T2), and body composition after spaceflight. J. Appl. Physiol. (1985) 89, 2158–2164. doi: 10.1152/jappl.2000.89.6.2158
Lecker, S. H., Jagoe, R. T., Gilbert, A., Gomes, M., Baracos, V., Bailey, J., et al. (2004). Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J. 18, 39–51. doi: 10.1096/fj.03-0610com
Levine, S., Biswas, C., Dierov, J., Barsotti, R., Shrager, J. B., Nguyen, T., et al. (2011). Increased proteolysis, myosin depletion, and atrophic AKT-FOXO signaling in human diaphragm disuse. Am. J. Respir. Crit. Care Med. 183, 483–490. doi: 10.1164/rccm.200910-1487OC
Li, S., Yang, Z., Gao, Y., Li, G., Wang, H., and Hinghofer-Szalkay, H. G. (2012). Ligustrazine and the contractile properties of soleus muscle in hindlimb-unloaded rats. Aviat. Space Environ. Med. 83, 1049–1054. doi: 10.3357/ASEM.3249.2012
Liu, H., Blough, E. R., Arvapalli, R., Wang, Y., Reiser, P. J., Paturi, S., et al. (2012). Regulation of contractile proteins and protein translational signaling in disused muscle. Cell. Physiol. Biochem. 30, 1202–1214. doi: 10.1159/000343310
Lomonosova, Y. N., Belova, S. P., Mirzoev, T. M., Kozlovskaya, I. B., and Shenkman, B. S. (2017). Eukaryotic elongation factor 2 kinase activation in M. soleus under 14-day hindlimb unloading of rats. Dokl. Biochem. Biophys. 474, 165–167. doi: 10.1134/S1607672917030048
Mammucari, C., Milan, G., Romanello, V., Masiero, E., Rudolf, R., Del Piccolo, P., et al. (2007). FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab. 6, 458–471. doi: 10.1016/j.cmet.2007.11.001
Marimuthu, K., Murton, A. J., and Greenhaff, P. L. (2011). Mechanisms regulating muscle mass during disuse atrophy and rehabilitation in humans. J. Appl. Physiol. (1985) 110, 555–560. doi: 10.1152/japplphysiol.00962.2010
Matsumoto, A., Fujita, N., Arakawa, T., Fujino, H., and Miki, A. (2014). Influence of electrical stimulation on calpain and ubiquitin-proteasome systems in the denervated and unloaded rat tibialis anterior muscles. Acta Histochem. 116, 936–942. doi: 10.1016/j.acthis.2014.03.006
McCarthy, J. J., and Esser, K. A. (2010). Anabolic and catabolic pathways regulating skeletal muscle mass. Curr. Opin. Clin. Nutr. Metab. Care 13, 230–235. doi: 10.1097/MCO.0b013e32833781b5
McGlory, C., von Allmen, M. T., Stokes, T., Morton, R. G., Hector, A. J., Lago, B. A., et al. (2017). Failed recovery of glycemic control and myofibrillar protein synthesis with two weeks of physical inactivity in overweight, pre-diabetic older adults. J. Gerontol. A Biol. Sci. Med. Sci. doi: 10.1093/gerona/glx203. [Epub ahead of print].
Min, K., Smuder, A. J., Kwon, O. S., Kavazis, A. N., Szeto, H. H., and Powers, S. K. (2011). Mitochondrial-targeted antioxidants protect skeletal muscle against immobilization-induced muscle atrophy. J. Appl. Physiol. (1985) 111, 1459–1466. doi: 10.1152/japplphysiol.00591.2011
Miokovic, T., Armbrecht, G., Felsenberg, D., and Belavý, D. L. (2012). Heterogeneous atrophy occurs within individual lower limb muscles during 60 days of bed rest. J. Appl. Physiol. 113, 1545–1559. doi: 10.1152/japplphysiol.00611.2012
Mirzoev, T., Tyganov, S., Vilchinskaya, N., Lomonosova, Y., and Shenkman, B. (2016). Key markers of mTORC1-dependent and mTORC1-independent signaling pathways regulating protein synthesis in rat soleus muscle during early stages of hindlimb unloading. Cell Physiol. Biochem. 39, 1011–1020. doi: 10.1159/000447808
Mitch, W. E., and Goldberg, A. L. (1996). Mechanisms of muscle wasting. The role of the ubiquitin-proteasome pathway. N. Engl. J. Med. 335, 1897–1905. doi: 10.1056/NEJM199612193352507
Miyazaki, M., McCarthy, J. J., Fedele, M. J., and Esser, K. A. (2011). Early activation of mTORC1 signalling in response to mechanical overload is independent of phosphoinositide 3-kinase/Akt signalling. J. Physiol. 589(Pt 7), 1831–1846. doi: 10.1113/jphysiol.2011.205658
Momken, I., Stevens, L., Bergouignan, A., Desplanches, D., Rudwill, F., Chery, I., et al. (2011). Resveratrol prevents the wasting disorders of mechanical unloading by acting as a physical exercise mimetic in the rat. FASEB J. 25, 3646–3660. doi: 10.1096/fj.10-177295
Morey-Holton, E. R., and Globus, R. K. (2002). Hindlimb unloading rodent model: technical aspects. J. Appl. Physiol. (1985) 92, 1367–1377. doi: 10.1152/japplphysiol.00969.2001
Moylan, J. S., and Reid, M. B. (2007). Oxidative stress, chronic disease, and muscle wasting. Muscle Nerve 35, 411–429. doi: 10.1002/mus.20743
Murton, A. J., Constantin, D., and Greenhaff, P. L. (2008). The involvement of the ubiquitin proteasome system in human skeletal muscle remodelling and atrophy. Biochim. Biophys. Acta 1782, 730–743. doi: 10.1016/j.bbadis.2008.10.011
Narici, M. V., and Maffulli, N. (2010). Sarcopenia: characteristics, mechanisms and functional significance. Br. Med. Bull. 95, 139–159. doi: 10.1093/bmb/ldq008
Nilwik, R., Snijders, T., Leenders, M., Groen, B. B., van Kranenburg, J., Verdijk, L. B., et al. (2013). The decline in skeletal muscle mass with aging is mainly attributed to a reduction in type II muscle fiber size. Exp. Gerontol. 48, 492–498. doi: 10.1016/j.exger.2013.02.012
Oates, B. R., Glover, E. I., West, D. W., Fry, J. L., Tarnopolsky, M. A., and Phillips, S. M. (2010). Low-volume resistance exercise attenuates the decline in strength and muscle mass associated with immobilization. Muscle Nerve 42, 539–546. doi: 10.1002/mus.21721
Ogawa, T., Furochi, H., Mameoka, M., Hirasaka, K., Onishi, Y., Suzue, N., et al. (2006). Ubiquitin ligase gene expression in healthy volunteers with 20-day bedrest. Muscle Nerve 34, 463–469. doi: 10.1002/mus.20611
Ohira, T., Kawano, F., Ohira, T., Goto, K., and Ohira, Y. (2015). Responses of skeletal muscles to gravitational unloading and/or reloading. J. Physiol. Sci. 65, 293–310. doi: 10.1007/s12576-015-0375-6
Ohira, Y., Jiang, B., Roy, R. R., Oganov, V., Ilyina-Kakueva, E., Marini, J. F., et al. (1992). Rat soleus muscle fiber responses to 14 days of spaceflight and hindlimb suspension. J. Appl. Physiol. (1985) 73(2 Suppl), 51S–57S. doi: 10.1152/jappl.1992.73.2.S51
Ohira, Y., Yoshinaga, T., Nomura, T., Kawano, F., Ishihara, A., Nonaka, I., et al. (2002). Gravitational unloading effects on muscle fiber size, phenotype and myonuclear number. Adv. Space Res. 30, 777–781. doi: 10.1016/S0273-1177(02)00395-2
Ohira, Y., Yoshinaga, T., Ohara, M., Kawano, F., Wang, X. D., Higo, Y., et al. (2006). The role of neural and mechanical influences in maintaining normal fast and slow muscle properties. Cells Tissues Organs (Print). 182, 129–142. doi: 10.1159/000093963
Okamoto, T., and Machida, S. (2017). Changes in FOXO and proinflammatory cytokines in the late stage of immobilized fast and slow muscle atrophy. Biomed. Res. 38, 331–342. doi: 10.2220/biomedres.38.331
Okita, M., Yoshimura, T., Nakano, J., Saeki, A., Uehara, A., Mineshita, A., et al. (2001). Effects of short duration stretching on disuse muscle atrophy in immobilized rat soleus muscles. J. Jpn. Phys. Ther. Assoc. 4, 1–5. doi: 10.1298/jjpta.4.1
Olsen, R. H., Krogh-Madsen, R., Thomsen, C., Booth, F. W., and Pedersen, B. K. (2008). Metabolic responses to reduced daily steps in healthy nonexercising men. JAMA 299, 1261–1263. doi: 10.1001/jama.299.11.1259
Onda, A., Kono, H., Jiao, Q. B., Akimoto, T., Miyamoto, T., Sawada, Y., et al. (2016). New mouse model of skeletal muscle atrophy using spiral wire immobilization. Muscle Nerve 54, 788–791. doi: 10.1002/mus.25202
Ota, N., Soga, S., Haramizu, S., Yokoi, Y., Hase, T., and Murase, T. (2011). Tea catechins prevent contractile dysfunction in unloaded murine soleus muscle: a pilot study. Nutrition 27, 955–959. doi: 10.1016/j.nut.2010.10.008
Paddon-Jones, D. (2006). Interplay of stress and physical inactivity on muscle loss: nutritional countermeasures. J. Nutr. 136, 2123–2126. doi: 10.1093/jn/136.8.2123
Pasiakos, S. M., McClung, H. L., McClung, J. P., Margolis, L. M., Andersen, N. E., Cloutier, G. J., et al. (2011). Leucine-enriched essential amino acid supplementation during moderate steady state exercise enhances postexercise muscle protein synthesis. Am. J. Clin. Nutr. 94, 809–818. doi: 10.3945/ajcn.111.017061
Pavy-Le Traon, A., Heer, M., Narici, M. V., Rittweger, J., and Vernikos, J. (2007). From space to Earth: advances in human physiology from 20 years of bed rest studies (1986-2006). Eur. J. Appl. Physiol. 101, 143–194. doi: 10.1007/s00421-007-0474-z
Peng, X. D., Xu, P. Z., Chen, M. L., Hahn-Windgassen, A., Skeen, J., Jacobs, J., et al. (2003). Dwarfism, impaired skin development, skeletal muscle atrophy, delayed bone development, and impeded adipogenesis in mice lacking Akt1 and Akt2. Genes Dev. 17, 1352–1365. doi: 10.1101/gad.1089403
Peterson, J. M., Bakkar, N., and Guttridge, D. C. (2011). NF-kappaB signaling in skeletal muscle health and disease. Curr. Top. Dev. Biol. 96, 85–119. doi: 10.1016/B978-0-12-385940-2.00004-8
Phillips, S. M., Glover, E. I., and Rennie, M. J. (2009). Alterations of protein turnover underlying disuse atrophy in human skeletal muscle. J. Appl. Physiol. (1985) 107, 645–654. doi: 10.1152/japplphysiol.00452.2009
Philp, A., Hamilton, D. L., and Baar, K. (2011). Signals mediating skeletal muscle remodeling by resistance exercise: PI3-kinase independent activation of mTORC1. J. Appl. Physiol. (1985) 110, 561–568. doi: 10.1152/japplphysiol.00941.2010
Powers, S. K. (2014). Can antioxidants protect against disuse muscle atrophy? Sports Med. 44 (Suppl 2), S155–S165. doi: 10.1007/s40279-014-0255-x
Powers, S. K., Kavazis, A. N., and DeRuisseau, K. C. (2005). Mechanisms of disuse muscle atrophy: role of oxidative stress. Am. J. Physiol. Regul. Integr. Comp. Physiol. 288, R337–R344. doi: 10.1152/ajpregu.00469.2004
Powers, S. K., Kavazis, A. N., and McClung, J. M. (2007). Oxidative stress and disuse muscle atrophy. J. Appl. Physiol. (1985) 102, 2389–2397. doi: 10.1152/japplphysiol.01202.2006
Powers, S. K., Smuder, A. J., and Judge, A. R. (2012). Oxidative stress and disuse muscle atrophy: cause or consequence? Curr. Opin. Clin. Nutr. Metab. Care 15, 240–245. doi: 10.1097/MCO.0b013e328352b4c2
Primeau, A. J., Adhihetty, P. J., and Hood, D. A. (2002). Apoptosis in heart and skeletal muscle. Can. J. Appl. Physiol. 27, 349–395. doi: 10.1139/h02-020
Qin, W. X., Gao, Y., Xue, W., Zhang, H., and Wang, H. (2009). Effects of two chinese herbs on muscle composition after 14 d of hind-limb unloading in rats. Space Med. Med. Eng. 22, 235–239. doi: 10.16289/j.cnki.1002-0837.2009.04.004
Redpath, N. T., Foulstone, E. J., and Proud, C. G. (1996). Regulation of translation elongation factor-2 by insulin via a rapamycin-sensitive signalling pathway. EMBO J. 15, 2291–2297.
Reed, S. A., Sandesara, P. B., Senf, S. M., and Judge, A. R. (2012). Inhibition of FoxO transcriptional activity prevents muscle fiber atrophy during cachexia and induces hypertrophy. FASEB J. 26, 987–1000. doi: 10.1096/fj.11-189977
Rennie, M. J. (2009). Anabolic resistance: the effects of aging, sexual dimorphism, and immobilization on human muscle protein turnover. Appl. Physiol. Nutr. Metab. 34, 377–381. doi: 10.1139/H09-012
Rennie, M. J., Wackerhage, H., Spangenburg, E. E., and Booth, F. W. (2004). Control of the size of the human muscle mass. Annu. Rev. Physiol. 66, 799–828. doi: 10.1146/annurev.physiol.66.052102.134444
Richter, E. A., Kiens, B., Mizuno, M., and Strange, S. (1989). Insulin action in human thighs after one-legged immobilization. J. Appl. Physiol. (1985) 67, 19–23. doi: 10.1152/jappl.1989.67.1.19
Rieu, I., Magne, H., Savary-Auzeloux, I., Averous, J., Bos, C., Peyron, M. A., et al. (2009). Reduction of low grade inflammation restores blunting of postprandial muscle anabolism and limits sarcopenia in old rats. J. Physiol. 587(Pt 22), 5483–5492. doi: 10.1113/jphysiol.2009.178319
Risson, V., Mazelin, L., Roceri, M., Sanchez, H., Moncollin, V., Corneloup, C., et al. (2009). Muscle inactivation of mTOR causes metabolic and dystrophin defects leading to severe myopathy. J. Cell Biol. 187, 859–874. doi: 10.1083/jcb.200903131
Rittweger, J., Frost, H. M., Schiessl, H., Ohshima, H., Alkner, B., Tesch, P., et al. (2005). Muscle atrophy and bone loss after 90 days' bed rest and the effects of flywheel resistive exercise and pamidronate: results from the LTBR study. Bone 36, 1019–1029. doi: 10.1016/j.bone.2004.11.014
Robb, E. L., Winkelmolen, L., Visanji, N., Brotchie, J., and Stuart, J. A. (2008). Dietary resveratrol administration increases MnSOD expression and activity in mouse brain. Biochem. Biophys. Res. Commun. 372, 254–259. doi: 10.1016/j.bbrc.2008.05.028
Roy, R. R., Baldwin, K. M., and Edgerton, V. R. (1991). The plasticity of skeletal muscle: effects of neuromuscular activity. Exerc. Sport Sci. Rev. 19, 269–312. doi: 10.1249/00003677-199101000-00008
Rudrappa, S. S., Wilkinson, D. J., Greenhaff, P. L., Smith, K., Idris, I., and Atherton, P. J. (2016). Human skeletal muscle disuse atrophy: effects on muscle protein synthesis, breakdown, and insulin resistance-a qualitative review. Front. Physiol. 7:361. doi: 10.3389/fphys.2016.00361
Ryan, M. J., Jackson, J. R., Hao, Y. L., Williamson, C. L., Dabkowski, E. R., Hollander, J. M., et al. (2010). Suppression of oxidative stress by resveratrol after isometric contractions in gastrocnemius muscles of aged mice. J. Gerontol. A Biol. Sci. Med. Sci. 65, 815–831. doi: 10.1093/gerona/glq080
Sacheck, J. M., Hyatt, J. P., Raffaello, A., Jagoe, R. T., Roy, R. R., Edgerton, V. R., et al. (2007). Rapid disuse and denervation atrophy involve transcriptional changes similar to those of muscle wasting during systemic diseases. FASEB J. 21, 140–155. doi: 10.1096/fj.06-6604com
Salanova, M., Schiffl, G., Püttmann, B., Schoser, B. G., and Blottner, D. (2008). Molecular biomarkers monitoring human skeletal muscle fibres and microvasculature following long-term bed rest with and without countermeasures. J. Anat. 212, 306–318. doi: 10.1111/j.1469-7580.2008.00854.x
Sandonà, D., Desaphy, J. F., Camerino, G. M., Bianchini, E., Ciciliot, S., Danieli-Betto, D., et al. (2012). Adaptation of mouse skeletal muscle to long-term microgravity in the MDS mission. PLoS ONE 7:e33232. doi: 10.1371/journal.pone.0033232
Sandri, M. (2010). Autophagy in skeletal muscle. FEBS Lett. 584, 1411–1416. doi: 10.1016/j.febslet.2010.01.056
Sandri, M., Sandri, C., Gilbert, A., Skurk, C., Calabria, E., Picard, A., et al. (2004). Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 117, 399–412. doi: 10.1016/S0092-8674(04)00400-3
Schiaffino, S., and Mammucari, C. (2011). Regulation of skeletal muscle growth by the IGF1-Akt/PKB pathway: insights from genetic models. Skelet. Muscle 1:4. doi: 10.1186/2044-5040-1-4
Scicchitano, B. M., Faraldi, M., and Musarò, A. (2015). The proteolytic systems of muscle wasting. Recent Adv. DNA Gene Seq. 9, 26–35. doi: 10.2174/2352092209999150911121502
Senf, S. M., Dodd, S. L., McClung, J. M., and Judge, A. R. (2008). Hsp70 overexpression inhibits NF-kappaB and Foxo3a transcriptional activities and prevents skeletal muscle atrophy. FASEB J. 22, 3836–3845. doi: 10.1096/fj.08-110163
Servais, S., Letexier, D., Favier, R., Duchamp, C., and Desplanches, D. (2007). Prevention of unloading-induced atrophy by vitamin E supplementation: links between oxidative stress and soleus muscle proteolysis? Free Radic. Biol. Med. 42, 627–635. doi: 10.1016/j.freeradbiomed.2006.12.001
Shangraw, R. E., Stuart, C. A., Prince, M. J., Peters, E. J., and Wolfe, R. R. (1988). Insulin responsiveness of protein metabolism in vivo following bedrest in humans. Am. J. Physiol. 255(4 Pt 1), E548–E558. doi: 10.1152/ajpendo.1988.255.4.E548
Shinohara, M., Yoshitake, Y., Kouzaki, M., Fukuoka, H., and Fukunaga, T. (2003). Strength training counteracts motor performance losses during bed rest. J. Appl. Physiol. (1985) 95, 1485–1492. doi: 10.1152/japplphysiol.01173.2002
Short, K. R., Bigelow, M. L., Kahl, J., Singh, R., Coenen-Schimke, J., Raghavakaimal, S., et al. (2005). Decline in skeletal muscle mitochondrial function with aging in humans. Proc. Natl. Acad. Sci. U.S.A. 102, 5618–5623. doi: 10.1073/pnas.0501559102
Slimani, L., Micol, D., Amat, J., Delcros, G., Meunier, B., Taillandier, D., et al. (2012). The worsening of tibialis anterior muscle atrophy during recovery post-immobilization correlates with enhanced connective tissue area, proteolysis, and apoptosis. Am. J. Physiol. Endocrinol. Metab. 303, E1335–E1347. doi: 10.1152/ajpendo.00379.2012
Smuder, A. J., Hudson, M. B., Nelson, W. B., Kavazis, A. N., and Powers, S. K. (2012). Nuclear factor-kappaB signaling contributes to mechanical ventilation-induced diaphragm weakness*. Crit. Care Med. 40, 927–934. doi: 10.1097/CCM.0b013e3182374a84
Spangenburg, E. E., Le Roith, D., Ward, C. W., and Bodine, S. C. (2008). A functional insulin-like growth factor receptor is not necessary for load-induced skeletal muscle hypertrophy. J. Physiol. Lond. 586, 283–291. doi: 10.1113/jphysiol.2007.141507
Spector, E. R., Smith, S. M., and Sibonga, J. D. (2009). Skeletal effects of long-duration head-down bed rest. Aviat. Space Environ. Med. 80, A23–A28. doi: 10.3357/ASEM.BR02.2009
Stein, T. P., and Blanc, S. (2011). Does protein supplementation prevent muscle disuse atrophy and loss of strength? Crit. Rev. Food Sci. Nutr. 51, 828–834. doi: 10.1080/10408398.2010.482679
Stein, T. P., Donaldson, M. R., Leskiw, M. J., Schluter, M. D., Baggett, D. W., and Boden, G. (2003). Branched-chain amino acid supplementation during bed rest: effect on recovery. J. Appl. Physiol. (1985) 94, 1345–1352. doi: 10.1152/japplphysiol.00481.2002
Stein, T. P., Leskiw, M. J., Schluter, M. D., Donaldson, M. R., and Larina, I. (1999). Protein kinetics during and after long-duration spaceflight on MIR. Am. J. Physiol. 276(6 Pt 1), E1014–E1021. doi: 10.1152/ajpendo.1999.276.6.E1014
Stephens, N. A., Gallagher, I. J., Rooyackers, O., Skipworth, R. J., Tan, B. H., Marstrand, T., et al. (2010). Using transcriptomics to identify and validate novel biomarkers of human skeletal muscle cancer cachexia. Genome Med. 2:1. doi: 10.1186/gm122
Stevenson, E. J., Giresi, P. G., Koncarevic, A., and Kandarian, S. C. (2003). Global analysis of gene expression patterns during disuse atrophy in rat skeletal muscle. J. Physiol. 551(Pt 1), 33–48. doi: 10.1113/jphysiol.2003.044701
Stipanuk, M. H. (2007). Leucine and protein synthesis: mTOR and beyond. Nutr. Rev. 65, 122–129. doi: 10.1111/j.1753-4887.2007.tb00289.x
Stitt, T. N., Drujan, D., Clarke, B. A., Panaro, F., Timofeyva, Y., Kline, W. O., et al. (2004). The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol. Cell 14, 395–403. doi: 10.1016/S1097-2765(04)00211-4
Stojiljkovic, D., Arsic, I., and Tadic, V. (2016). Extracts of wild apple fruit (Malus sylvestris (L.) Mill., Rosaceae) as a source of antioxidant substances for use in production of nutraceuticals and cosmeceuticals. Ind. Crops Prod. 80, 165–176. doi: 10.1016/j.indcrop.2015.11.023
Stuart, C. A., Shangraw, R. E., Peters, E. J., and Wolfe, R. R. (1990). Effect of dietary protein on bed-rest-related changes in whole-body-protein synthesis. Am. J. Clin. Nutr. 52, 509–514. doi: 10.1093/ajcn/52.3.509
Stuart, C. A., Shangraw, R. E., Prince, M. J., Peters, E. J., and Wolfe, R. R. (1988). Bed-rest-induced insulin resistance occurs primarily in muscle. Metab. Clin. Exp. 37, 802–806. doi: 10.1016/0026-0495(88)90018-2
Suetta, C., Hvid, L. G., Justesen, L., Christensen, U., Neergaard, K., Simonsen, L., et al. (2009). Effects of aging on human skeletal muscle after immobilization and retraining. J. Appl. Physiol. (1985) 107, 1172–1180. doi: 10.1152/japplphysiol.00290.2009
Sugiura, T., Abe, N., Nagano, M., Goto, K., Sakuma, K., Naito, H., et al. (2005). Changes in PKB/Akt and calcineurin signaling during recovery in atrophied soleus muscle induced by unloading. Am. J. Physiol. Regul. Integr. Comp. Physiol. 288, R1273–R1278. doi: 10.1152/ajpregu.00688.2004
Talbert, E. E., Smuder, A. J., Min, K., Kwon, O. S., and Powers, S. K. (2013). Calpain and caspase-3 play required roles in immobilization-induced limb muscle atrophy. J. Appl. Physiol. (1985) 114, 1482–1489. doi: 10.1152/japplphysiol.00925.2012
Tanner, R. E., Brunker, L. B., Agergaard, J., Barrows, K. M., Briggs, R. A., Kwon, O. S., et al. (2015). Age-related differences in lean mass, protein synthesis and skeletal muscle markers of proteolysis after bed rest and exercise rehabilitation. J. Physiol. (Lond). 593, 4259–4273. doi: 10.1113/JP270699
Tesch, P. A., Berg, H. E., Bring, D., Evans, H. J., and LeBlanc, A. D. (2005). Effects of 17-day spaceflight on knee extensor muscle function and size. Eur. J. Appl. Physiol. 93, 463–468. doi: 10.1007/s00421-004-1236-9
Thomason, D. B., and Booth, F. W. (1990). Atrophy of the soleus muscle by hindlimb unweighting. J. Appl. Physiol. (1985) 68, 1–12. doi: 10.1152/jappl.1990.68.1.1
Trappe, S., Trappe, T., Gallagher, P., Harber, M., Alkner, B., and Tesch, P. (2004). Human single muscle fibre function with 84 day bed-rest and resistance exercise. J. Physiol. 557(Pt 2), 501–513. doi: 10.1113/jphysiol.2004.062166
Trappe, T. (2009). Influence of aging and long-term unloading on the structure and function of human skeletal muscle. Appl. Physiol. Nutr. Metab. 34, 459–464. doi: 10.1139/H09-041
Tsika, R. W., Herrick, R. E., and Baldwin, K. M. (1987). Effect of anabolic steroids on skeletal muscle mass during hindlimb suspension. J. Appl. Physiol. (1985) 63, 2122–2127. doi: 10.1152/jappl.1987.63.5.2122
Veldhuizen, J. W., Verstappen, F. T., Vroemen, J. P., Kuipers, H., and Greep, J. M. (1993). Functional and morphological adaptations following four weeks of knee immobilization. Int. J. Sports Med. 14, 283–287. doi: 10.1055/s-2007-1021178
Verdijk, L. B., Dirks, M. L., Snijders, T., Prompers, J. J., Beelen, M., Jonkers, R. A., et al. (2012). Reduced satellite cell numbers with spinal cord injury and aging in humans. Med. Sci. Sports Exerc. 44, 2322–2330. doi: 10.1249/MSS.0b013e3182667c2e
Wall, B. T., Dirks, M. L., Snijders, T., Senden, J. M., Dolmans, J., and van Loon, L. J. (2014). Substantial skeletal muscle loss occurs during only 5 days of disuse. Acta Physiol. (Oxf). 210, 600–611. doi: 10.1111/apha.12190
Wall, B. T., Snijders, T., Senden, J. M., Ottenbros, C. L., Gijsen, A. P., Verdijk, L. B., et al. (2013). Disuse impairs the muscle protein synthetic response to protein ingestion in healthy men. J. Clin. Endocrinol. Metab. 98, 4872–4881. doi: 10.1210/jc.2013-2098
Wang, X. D., Kawano, F., Matsuoka, Y., Fukunaga, K., Terada, M., Sudoh, M., et al. (2006). Mechanical load-dependent regulation of satellite cell and fiber size in rat soleus muscle. Am. J. Physiol. Cell Physiol. 290, C981–C989. doi: 10.1152/ajpcell.00298.2005
Welsh, G. I., Miller, C. M., Loughlin, A. J., Price, N. T., and Proud, C. G. (1998). Regulation of eukaryotic initiation factor eIF2B: glycogen synthase kinase-3 phosphorylates a conserved serine which undergoes dephosphorylation in response to insulin. FEBS Lett. 421, 125–130. doi: 10.1016/S0014-5793(97)01548-2
West, D. W., Burd, N. A., Tang, J. E., Moore, D. R., Staples, A. W., Holwerda, A. M., et al. (2010). Elevations in ostensibly anabolic hormones with resistance exercise enhance neither training-induced muscle hypertrophy nor strength of the elbow flexors. J. Appl. Physiol. (1985) 108, 60–67. doi: 10.1152/japplphysiol.01147.2009
Widrick, J. J., Bangart, J. J., Karhanek, M., and Fitts, R. H. (1996). Soleus fiber force and maximal shortening velocity after non-weight bearing with intermittent activity. J. Appl. Physiol. (1985) 80, 981–987. doi: 10.1152/jappl.1996.80.3.981
Witkowski, S., Lovering, R. M., and Spangenburg, E. E. (2010). High-frequency electrically stimulated skeletal muscle contractions increase p70(s6k) phosphorylation independent of known IGF-I sensitive signaling pathways. FEBS Lett. 584, 2891–2895. doi: 10.1016/j.febslet.2010.05.003
Wu, X., Gao, Y. F., Zhao, X. H., and Cui, J. H. (2012). [Effects of tetramethylpyrazine on nitric oxide synthase activity and calcium ion concentration of skeletal muscle in hindlimb unloading rats]. Zhonghua Yi Xue Za Zhi 92, 2075–2077. doi: 10.3760/cma.j.issn.0376-2491.2012.29.016
Xu, P. T., Li, Q., Sheng, J. J., Chang, H., Song, Z., and Yu, Z. B. (2012). Passive stretch reduces calpain activity through nitric oxide pathway in unloaded soleus muscles. Mol. Cell. Biochem. 367, 113–124. doi: 10.1007/s11010-012-1325-8
You, J. S., Park, M. N., Song, W., and Lee, Y. S. (2010). Dietary fish oil alleviates soleus atrophy during immobilization in association with Akt signaling to p70s6k and E3 ubiquitin ligases in rats. Appl. Physiol. Nutr. Metab. 35, 310–318. doi: 10.1139/H10-022
Zha, H., Aimé-Sempé, C., Sato, T., and Reed, J. C. (1996). Proapoptotic protein Bax heterodimerizes with Bcl-2 and homodimerizes with Bax via a novel domain (BH3) distinct from BH1 and BH2. J. Biol. Chem. 271, 7440–7444. doi: 10.1074/jbc.271.13.7440
Zhang, H., He, Z., Gao, Y., Hinghofer-Szalkay, H. G., and Fan, X. (2007). Muscle composition after 14-day hindlimb unloading in rats: effects of two herbal compounds. Aviat. Space Environ. Med. 78, 926–931. doi: 10.3357/ASEM.2072.2007
Zhang, J., Li, Y., Li, G., Ma, X., Wang, H., Goswami, N., et al. (2017). Identification of the optimal dose and calpain system regulation of tetramethylpyrazine on the prevention of skeletal muscle atrophy in hindlimb unloading rats. Biomed. Pharmacother. 96, 513–523. doi: 10.1016/j.biopha.2017.10.012
Zhang, Q. W., Li, G. Y., Ren, Y. P., and Gao, Y. F. (2015). [Effect of two Pi deficiency syndrome models on the configuration and function of the skeletal muscle in mice]. Zhongguo Zhong Xi Yi Jie He Za Zhi 35, 71–75. doi: 10.7661/CJIM.2015.01.0071
Zhao, J., Brault, J. J., Schild, A., Cao, P., Sandri, M., Schiaffino, S., et al. (2007). FoxO3 coordinately activates protein degradation by the autophagic/lysosomal and proteasomal pathways in atrophying muscle cells. Cell Metab. 6, 472–483. doi: 10.1016/j.cmet.2007.11.004
Zhong, H., Roy, R. R., Siengthai, B., and Edgerton, V. R. (2005). Effects of inactivity on fiber size and myonuclear number in rat soleus muscle. J. Appl. Physiol. (1985) 99, 1494–1499. doi: 10.1152/japplphysiol.00394.2005
Zhou, Y., Xue, S., and Yang, J. J. (2013). Calciomics: integrative studies of Ca2+-binding proteins and their interactomes in biological systems. Metallomics 5, 29–42. doi: 10.1039/C2MT20009K
Keywords: disuse muscle atrophy, mechanical unloading, protein synthesis, protein degradation, molecular and cellular pathways, therapeutic countermeasures
Citation: Gao Y, Arfat Y, Wang H and Goswami N (2018) Muscle Atrophy Induced by Mechanical Unloading: Mechanisms and Potential Countermeasures. Front. Physiol. 9:235. doi: 10.3389/fphys.2018.00235
Received: 26 October 2017; Accepted: 02 March 2018;
Published: 20 March 2018.
Edited by:
Kevin I. Watt, Baker Heart and Diabetes Institute, AustraliaReviewed by:
Andrew Philp, University of Birmingham, United KingdomCopyright © 2018 Gao, Arfat, Wang and Goswami. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yunfang Gao, Z2FveXVuZkBud3UuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.