
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CORRECTION article
Front. Physiol. , 06 December 2017
Sec. Computational Physiology and Medicine
Volume 8 - 2017 | https://doi.org/10.3389/fphys.2017.01025
This article is part of the Research Topic Safety Pharmacology - Risk Assessment QT Interval Prolongation and Beyond View all 28 articles
This article is a correction to:
Optimization of an In silico Cardiac Cell Model for Proarrhythmia Risk Assessment
 Sara Dutta1
Sara Dutta1 Kelly C. Chang1
Kelly C. Chang1 Kylie A. Beattie1
Kylie A. Beattie1 Jiansong Sheng1
Jiansong Sheng1 Phu N. Tran1
Phu N. Tran1 Wendy W. Wu1
Wendy W. Wu1 Min Wu1
Min Wu1 David G. Strauss1
David G. Strauss1 Thomas Colatsky2
Thomas Colatsky2 Zhihua Li1*
Zhihua Li1*A corrigendum on
Optimization of an In silico Cardiac Cell Model for Proarrhythmia Risk Assessment
by Dutta, S., Chang, K. C., Beattie, K. A., Sheng, J., Tran, P. N., Wu, W. W., et al. (2017). Front. Physiol. 8:616. doi: 10.3389/fphys.2017.00616
In the original article, there was a mistake in Figure 6 as published. In the top left panel the qNet gray value should be 0.109 instead of 0.011. The corrected Figure 6 appears below. The authors apologize for this error and state that this does not change the scientific conclusions of the article in any way.
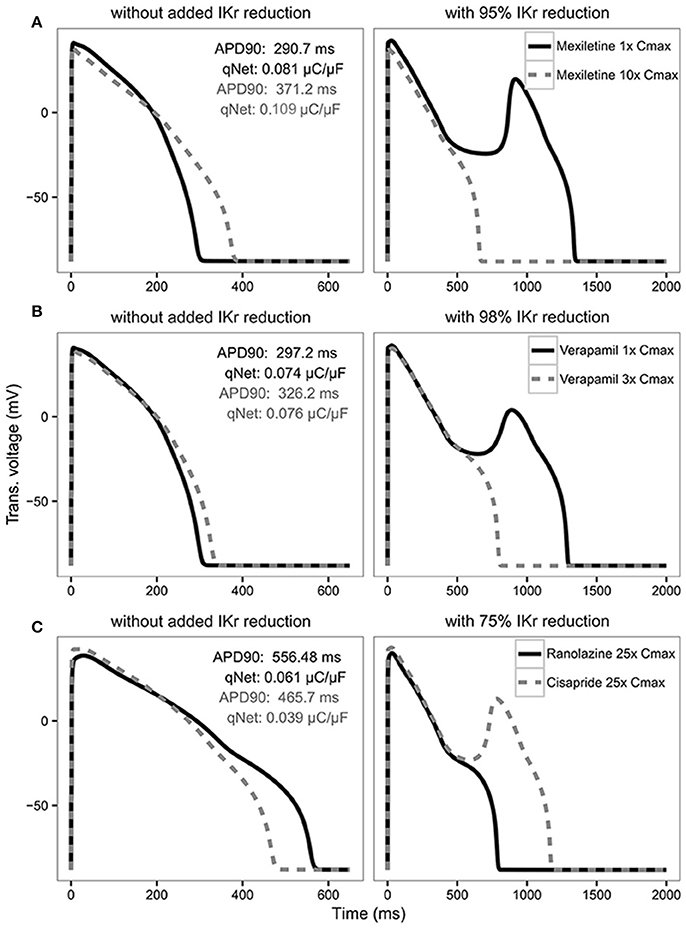
Figure 6. AP traces for mexiletine (A) at 1x Cmax (black solid line) and 10x Cmax (gray dashed line) without (left panel) and with 95% IKr reduction (right panel); verapamil (B) at 1x Cmax (black solid line) and 3x Cmax (gray dashed line) without (left panel) and with 98% IKr reduction (right panel); and ranolazine (black solid line) and cisapride (dashed gray line) (C) at 25x Cmax without (left panel) and with 75% IKr reduction (right panel) for a CL of 2,000 ms. Corresponding APD90 (ms) and qNet (μC/μF) values are reported in black for mexiletine 1x Cmax, verapamil 1x Cmax and ranolazine 25x Cmax and in gray for mexiletine 10x Cmax, verapamil 3x Cmax and cisapride 25x Cmax. Note the IKr reduction (simulated by scaling the IKr maximum conductance) is applied in addition to the drug block effect and is used to assess the system's robustness against EADs (see Results section).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Keywords: Torsade-de-Pointes (TdP), Comprehensive in vitro Proarrhythmia Assay (CiPA), rapid delayed rectifier potassium current (IKr), in silico cardiac cell model, drug block, proarrythmia risk, model optimization
Citation: Dutta S, Chang KC, Beattie KA, Sheng J, Tran PN, Wu WW, Wu M, Strauss DG, Colatsky T and Li Z (2017) Corrigendum: Optimization of an In silico Cardiac Cell Model for Proarrhythmia Risk Assessment. Front. Physiol. 8:1025. doi: 10.3389/fphys.2017.01025
Received: 22 November 2017; Accepted: 27 November 2017;
Published: 06 December 2017.
Edited and reviewed by: Eleonora Grandi, University of California, Davis, United States
Copyright © 2017 Dutta, Chang, Beattie, Sheng, Tran, Wu, Wu, Strauss, Colatsky and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhihua Li, emhpaHVhLmxpQGZkYS5oaHMuZ292
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.