
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Physiol. , 07 September 2017
Sec. Computational Physiology and Medicine
Volume 8 - 2017 | https://doi.org/10.3389/fphys.2017.00664
This article is part of the Research Topic Modern Measures of Heart Rate Complexity View all 22 articles
Background: Ecosystems with seasonal fluctuations in climate and food availability present physiological challenges to resident mammals and may cause “stress.” The two predominant physiological responses to stressors are (1) the activation of the hypothalamic-pituitary-adrenal axis and (2) the modulation of the autonomic nervous system. To date, the primary indicator for “stress” in wildlife- and zoo animal research are glucocorticoid levels. By measuring the autonomic regulation of cardiac activity, particularly the vagal tone, heart rate variability (HRV) is presently emerging as a suitable indicator of “stress” in farm- and domestic animal research.
Objective: The aim of this study was to use HRV, a novel method in wildlife research, to assess seasonal patterns of “stress” in a group of free-ranging Przewalski's horses (Equus ferus przewalskii).
Methods: Six pregnant Przewalski's horses from one harem within the Hortobágy National Park in Hungary were subjected to the study. We used a dedicated telemetry system consisting of a subcutaneously implanted transmitter and a receiver and storage unit in a collar to record HRV, heart rate (HR), subcutaneous body temperature, and activity throughout a one-year study period—climate data was also collected. We defined “stress” as a decrease in parasympathetic nervous system tone and calculated RMSSD (root mean square of successive differences) as a measure of HRV. Linear mixed effects models with random intercept per individual were used for statistical analysis.
Results: HRV and HR varied considerably throughout the year. Similar to temperate ruminants and hibernating mammals, Przewalski's horses experienced lower HR and HRV during winter, when resources are limited indicating decreased metabolic rates coupled with “stress.” In spring, we observed a drop of HRV along with a peak in HR indicating an increase of allostatic load that is most likely associated with increased energy demands during pregnancy and/or seasonal routines such as the adjustment of the gastrointestinal system to better quality diet.
Conclusion: Measuring telemetric HRV is a proven method to study undisturbed reactions of wild animals to their changing environment over the long term. Przewalski's horses experience a loss of complexity in cardiovascular dynamics over the winter and particularly during spring, indicating seasonal “stress.”
In seasonal environments, free-ranging organisms experience regular patterns of physiological and behavioral responses that allow the individual to cope with predictable environmental changes (Romero, 2002; McEwen and Wingfield, 2003; Boonstra, 2004; Reeder and Kramer, 2005). Measuring “stress” allows us to quantify these responses and to investigate how organisms integrate their life cycles in the natural world—knowledge critical for conservation physiology (Wikelski and Cooke, 2006; Tarlow and Blumstein, 2007; Busch and Hayward, 2009; Dantzer et al., 2014).
“Stress” was first described by Hans Selye in 1936, who defined it as a non-specific response of the body to any demand for change (Selye, 1936). Later, the concept has been refined by introducing the terms “stressor” and “stress response.” A stressor is a stimulus that threatens homeostasis (stability of physiological variables), whereas the stress response is the reaction of the organism to regain homeostasis (Chrousos, 2009). “Stress” would then be a state in which homeostasis is threatened. The hypothalamic-pituitary-adrenocortical (HPA) axis and the autonomic nervous system are the two key-players of the stress response (McEwen and Wingfield, 2003; Reeder and Kramer, 2005; Koolhaas et al., 2011). These systems respond to unpredictable stressful events in daily life, but also to predictable challenges throughout the day and year.
To date, measuring “stress” in wildlife has mainly focused on the quantification of glucocorticoid steroid hormones (i.e., cortisol and corticosterone), the primary mediators of the HPA axis (Möstl and Palme, 2002; Millspaugh and Washburn, 2004; Schwarzenberger, 2007; Busch and Hayward, 2009; Sheriff et al., 2011; Dantzer et al., 2014). In human medicine and farm- and companion animal research, heart rate variability (HRV) has been used increasingly over the past decades to evaluate physical and psychological stress (Mohr et al., 2002; Pumprla et al., 2002; VonBorell et al., 2007; Stucke et al., 2015). HRV quantifies the constantly changing time intervals between consecutive heartbeats (NN-intervals). This means that it is based on the antagonistic oscillatory influences of the parasympathetic- and sympathetic-nervous system on the heart and thus reflects the functioning of the autonomic nervous system (Malik et al., 1996; Pumprla et al., 2002; VonBorell et al., 2007).
Whilst the parasympathetic nervous system promotes homeostasis and optimizes the function of internal viscera, the sympathetic nervous system responds to challenges from outside the body. In an attempt to redefine “stress,” Porges (1995a) proposed a model based on the tradeoff between internal and external needs—when internal needs (homeostasis) are no longer being adequately serviced by the parasympathetic nervous system, the organism is experiencing stress (Porges, 1995a). Thus, “stress” can be investigated by measuring parasympathetic nervous system function.
Compared to the sympathetic nervous system, the parasympathetic modulation of the heart is much faster (Pumprla et al., 2002). Various HRV measures that reflect these rapid changes, and hence cardiac vagal activity, have recently been reviewed in order to determine the best method to characterize parasympathetic nervous system function (Laborde et al., 2017). Of all measures, root mean square of successive differences (RMSSD) has been considered the preferred index under free-running conditions, as it is relatively free from respiratory influences (Penttila et al., 2001; Hill and Siebenbrock, 2009; Saboul et al., 2013) and fairly easy to calculate and interpret (Malik et al., 1996; VonBorell et al., 2007; Plews et al., 2013).
In this study, we investigated seasonal patterns of “stress” in a group of free-ranging Przewalski's horses (Equus ferus przewalskii) within the Hortobágy National Park in Hungary by measuring HRV—a novel method in wildlife research. We employed RMSSD as an indicator for parasympathetic nervous system tone and measure of “stress.”
The Przewalski's horse (Equus ferus przewalskii Poljakov, 1881) is a typical steppe herbivore whose distribution once covered the entire Eurasian steppe belt (Wakefield et al., 2002). Pasture competition with livestock and over-hunting led to the horses moving east to Asia, and eventually becoming extinct in the wild in the 1960s (Boyd and Houpt, 1994; Wakefield et al., 2002). The species has been saved from total extinction by breeding in captivity (Ryder and Wedemeyer, 1982; Ryder, 1993) and reintroduction into its formal range in Mongolia (Wakefield et al., 2002; King et al., 2015). There, temperatures range from −40° to +40°C and water resources are limited (Boyd and Houpt, 1994). Semi reserves, such as the Hortobágy National Park in Hungary, were created to prepare horses for release into the wild and to offer opportunities for research (Zimmermann, 2005)—understanding the species' physiological capabilities and seasonal adjustments is critical for the successful management of reintroduced animals and hence, conservation of the Przewalski's horse.
In order to observe undisturbed reactions of free-ranging Przewalski's horses to their changing environment over the long term, we recorded cardiac activity with a transmitter-implant under the skin of the horses. We hypothesized that HRV would be highest during the summer and lowest during the winter, indicating seasonal “stress” due to energetic bottlenecks.
The study was performed in the Pentezug region of the semi reserve Hortobágy National Park in Hungary. Pentezug is a steppe region of 2,388 ha (27 ha forest, 2,361 ha meadow) that is under the highest level of protection. It is closed to the public and any agricultural activity that might cause damage to the sensitive fauna and flora is prohibited. At present, ~300 Przewalski's horses freely roam the grasslands of the park representing the largest population in the world. The horses live in harem units, consisting of one mature breeding stallion and up to eight adult mares with their offspring. Female horses frequently give birth to one foal per year and, as there are no predators in the park, the population is increasing. In fact, more than 50 foals are currently being born annually (Zimmermann et al., 2009b; Makra, 2016). Average temperature within the park ranges around 21°C in the summer and −2.5°C in the winter. The average annual rainfall is ~500 mm, and snowfall ~2–10 cm, distributed throughout 40–45 days per year (Zimmermann et al., 2009a). The horses depend entirely on the natural vegetation and are fully exposed to the changing climatic conditions.
The study and all procedures were performed 2008 in Hungary. The responsible veterinary administration authorities in the Hajdú-Bihar County approved the captures and subsequent procedures according to the Act on Animal Protection 1998. In 2008, there was no provision for the Animal Welfare and Ethics Committee of the Vetmeduni Vienna to review or approve projects carried out outside of Austria.
In October 2008, we captured six adult Przewalski's horse mares (aged 3–11 years old; mean ± SD age, 6 ± 3 years; mean estimated weight, 295 ± SD 15 kg; range, 270–320 kg) by remote darting from foot or vehicle. Only female horses from one harem were selected for two reasons: (1) their behavior is synchronized reducing interindividual variability (Souris et al., 2007), (2) stallions tend to break valuable monitoring equipment due to their aggressive behavior (Kolter and Zimmermann, 2001). All of the six mares were pregnant and gave birth to healthy foals during the study period (6th May–7th June 2009; mean 23rd May ± SD 5 days).
A combination of 10 mg butorphanol (Torbugesic, Fort Dodge Animal Health, Fort Dodge, Iowa 50501, USA), 10 mg detomidine (Domosedan, Orion Corp. Farmos Finland), and 0.7–1.4 mg ethorphine (M99, C-Vet Veterinary Products, Lancs, UK) was used to induce anesthesia. Anesthesia was maintained with an intravenous infusion of guaifenesin-ketamine-xylazine [1 L of 5% guaifenesin (Myolaxin, Vétoquinol UK Ltd, Buckingham, MK18 1PA, UK) 1,000 mg ketamine (Ketamidor, Richter Pharma, 4600 Wels, Austria) and 500 mg xylazine (Rompun, Bayer Austria Ges.m.b.H, 1160 Vienna, Austria)]. For further details regarding anesthesia, see Walzer et al. (2009).
The horses were fitted with collars, which included a dual-axis motion sensor to monitor activity, and very high frequency (VHF) transmitters for tracking purposes. In order to measure cardiac activity, we used a system consisting of an implantable transmitter unit with two electrodes (Figure 1), and a receiver and storage unit located in the collar. The microprocessor-controlled transmitter (50 g, 65 × 35 × 11 mm) was encapsulated in physiologically inert medical-grade silicone rubber and housed a thermistor. Two electrode plates (surgical steel, 8 mm diameter) were connected to the transmitter with a coiled silicone rubber-insulated wire of multi-stranded stainless steel fitted in silicone rubber tubing. We surgically implanted the transmitter between the muscle and subcutaneous fat on the ventro-lateral aspect on the left side of the neck. Two small caudo-ventral incisions served to pull the electrodes subcutaneously from the transmitter to a peristernal position. Once instrumentation was in place, we affixed the transmitter and the electrodes to the subcutaneous tissue and closed the skin incisions with absorbable suture material. The implantable unit recorded heart rate (HR) and subcutaneous body temperature at intervals of 1 min. Every 15 min, cardiac activity was recorded on a beat-to-beat basis for a time window of 3 min. Resolution of body temperature was 0.01°C, and of cardiac measurements beats per minute (bpm).
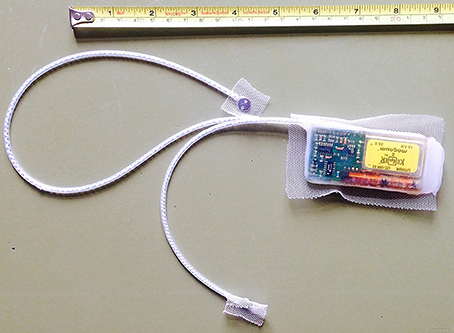
Figure 1. Photograph showing the self-constructed transmitter with the two electrode plates. We implanted the transmitter under the skin of the left side of the neck. Electrodes were placed next to the sternum.
The parameters were transmitted at 100 kHz via a short-range telemetry data link to the receiver and storage unit in the collar. We obtained hourly climate data (outdoor temperature, wind, rain, and humidity) from a weather station located in the study area.
Data was retrieved from the collars 18 months after instrumentation. One horse lost its collar 3 months after instrumentation (it was therefore excluded from the study) and two horses at ~10 months.
The raw, telemetrically obtained, data contained obviously erroneous values due to electronic noise and disturbance during transmission. We removed all HR-values outside the described physiological range for horses of 10–250 bpm from the database. We computed the corresponding NN-intervals and converted the values into milliseconds (i.e., divided 60 through the HR, and multiplied the resulting values with 1,000). In order to clean the data from transmission errors and other noise, we removed sessions with <30 measurements and removed NN-interval values that deviated more than two standard deviations from the respective session mean. To avoid a bias resulting from handling and surgery, the first 2 weeks after anesthesia and surgery were discarded from statistical analysis. As the duration of recordings varied between the individuals, data of no longer than 12 months were included.
We determined HRV by calculating the square root of the mean of the sum of the squares of differences between adjacent NN intervals (RMSSD). It is recommended to perform basal cardiac measurements in horses only under resting conditions, when parasympathetic activity is high (Parker et al., 2010; Stucke et al., 2015). Therefore, we first evaluated RMSSD throughout the 24-h period of the day and used only data from the 6-h period of the day with the highest values for further analysis.
Statistical tests were performed using R 3.3.1 for Windows (The R Foundation, Vienna, Austria). We used a linear mixed-effects model to assess the effect of two continuous independent climate variables (outdoor temperature and humidity), two binary independent climate variables (rain and wind), two continuous independent physiological variables (HR and body temperature), and one binary independent physiological variable (activity) on one continuous dependent variable (RMSSD) for all Przewalski's mares. The mixed-effects model allows repeated measures by including a random intercept for each subject (each horse). Standardized scores were used for continuous variables, and RMSSD was log-transformed to account for its heavily skewed distribution. We fit multiple nested models including (1) random intercepts for each horse and a fixed effect for HR and month. We then stepwise added fixed effects for (2) activity and body temperature, (3) climate data, and (4) a quadratic effect of outdoor temperature. Akaike's Information Criterion (AIC) was used to compare the goodness of fit for the set of models.
Mean values of RMSSD for each hour of the day are plotted in Figure 2—RMSSD was highest between 12 p.m. and 6 p.m., the third quarter of the day, and lowest at around midnight.
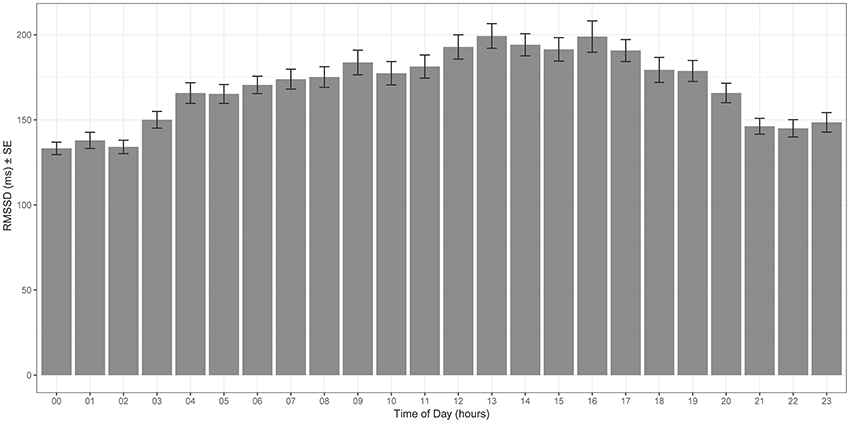
Figure 2. Hourly mean RMSSD throughout the 12 months study period [x-axis time of the day (hour), y-axis RMSSD (ms)]. RMSSD is highest during the third quarter of the day (12 p.m. to 6 p.m.).
Figures 3, 4 represent mean values of RMSSD (Figure 3) and HR (Figure 4) for the third quarter of each day of the year (individual values are represented in Supplementary information: Figures 1, 2). Cardiac activity varied considerably throughout the year. RMSSD and HR showed lower values during the winter than during the summer. RMSSD dropped in April along with a concurrent peak in HR.
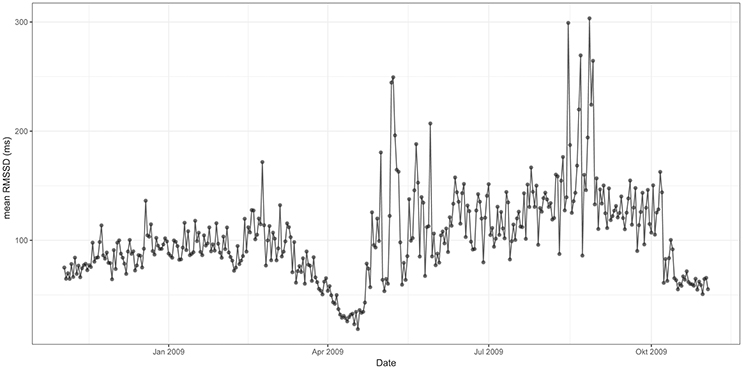
Figure 3. Mean RMSSD from 12 p.m. to 6 p.m. throughout the 12 months study period [x-axis date (month), y-axis RMSSD (ms)]. RMSSD is lower during the winter than during the summer—it experiences a drop in spring.
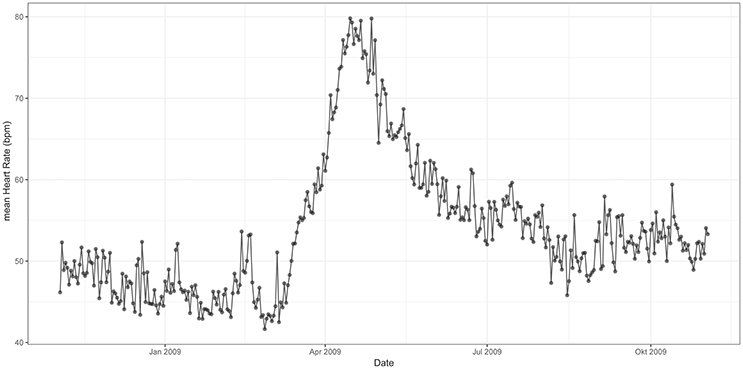
Figure 4. Mean HR from 12 p.m. to 6 p.m. throughout the 12 months study period [x-axis date (month), y-axis HR (bpm)]. HR is lower during the winter than during the summer—it experiences a peak in spring.
When compared to January, RMSSD experienced a pronounced drop in April. It increased rapidly thereafter and remained on significantly higher levels from June to October (mean 120 ms) whereby highest values were observed in August. RMSSD decreased in fall, and remained lower (mean 87 ms) during the winter-months when compared to the summer months (Figure 3).
In contrast, HR increased considerably during March and experienced a pronounced peak in April. This peak was concurrent with the observed drop in RMSSD. After a rapid decrease, HR remained on an intermediate level (mean 50 bpm) during summer and further declined during autumn to reach a mean of ~45 bpm during the winter. The total annual change was approximately twofold, with a maximum of 80 bpm in spring (Figure 4).
RMSSD correlated negatively with HR and body temperature, and positively with activity. Regarding climatic effects, RMSSD correlated positively with rain. Humidity showed a small positive effect on RMSSD, whereas wind had no significant effect. Outdoor temperature had a negative effect on RMSSD—this effect was quadratic: temperatures of around 18°C were associated with minimum RMSSD values. The model including the quadratic effect of outdoor temperature showed the lowest AIC (i.e., the best fit, see Supplementary Table 1). In summary, the energetic consequences of thermoregulation and weather conditions contributed significantly to the seasonal changes in RMSSD (Table 1). Of all physiological and climatic parameters, activity had the strongest effect on RMSSD. HR only accounted for ~2.5% of variation.
This is the first study using HRV to measure seasonal patterns of “stress” in free-ranging wildlife. During the past decades, measuring fecal glucocorticoid metabolite levels has become the method of choice to non-invasively index “stress” in zoo- and wild animal research (Möstl and Palme, 2002; Touma and Palme, 2005; Schwarzenberger, 2007; Sheriff et al., 2011; Dantzer et al., 2014; Hadinger et al., 2015). However, long-term recordings with short sampling intervals are often logistically difficult. Samples collected under field conditions cannot always be preserved immediately, the length of time between defecation and collection is often unknown, and assignment of the sample to an individual might be difficult, all factors influencing final results (Huber et al., 2003; Sheriff et al., 2011; Hadinger et al., 2015). HR is another parameter that has been used to study physiological reactions of free-ranging wildlife to environmental stressors (MacArthur et al., 1979; Harlow et al., 1987; Weisenberger et al., 1996; Ackerman et al., 2004; Theil et al., 2004; Arnold et al., 2006; Laske et al., 2011). With advancements in technology, transmitters that include cardiac monitors have become smaller and less invasive, while the monitoring period has increased from days to months to years (White and Garrott, 1990; Rutz and Hays, 2009). Although, HR measurement has been applied to assess short-term effects of “stress,” it more exactly represents an indicator for metabolic rate and energy expenditure (Weimerskirch et al., 2002; Butler et al., 2004; Groscolas et al., 2010; Green, 2011). By measuring the autonomic regulation of cardiac activity, particularly the vagal tone, HRV is now progressively emerging as a suitable indicator of “stress” in farm- and domestic animal research (VonBorell et al., 2007). In wildlife research, it is a fairly new approach, and only few previous studies have applied it (Theil et al., 2004; Mentaberre et al., 2010; Støen et al., 2015; Evans et al., 2016). All of these studies used SDANN (standard deviation of the average NN interval calculated over short periods, usually 5 min) as a measure of HRV. In contrast, we applied RMSSD (the square root of the mean squared differences of successive NN intervals) as a measure of HRV. Our aim was to assess seasonal variations in parasympathetic nervous system activity and hence, “stress” in free ranging Przewalski's horses. Whereas SDANN represents a general measurement of vagal and sympathetic influences on the heart, RMSSD specifically reflects vagally mediated alterations in the autonomic nervous system (Malik et al., 1996; VonBorell et al., 2007). SDANN is easier to calculate, but RMSSD has superior statistical properties (Malik et al., 1996). In our study, HRV was recorded in sequences of 3 min. The Task Force of The European Society of Cardiology and The North American Society of Pacing and Electrophysiology recommends recordings of at least 5-min-intervals to generate accurate HRV measurements (Malik et al., 1996). However, it has been shown that RMSSD, but not SDANN, can reliably be assessed from shorter recordings (Nussinovitch et al., 2011; Plews et al., 2013; Esco and Flatt, 2014; Munoz et al., 2015). Given our hypothesis and the experimental setting, RMSSD was the better parameter to choose. Parker et al. (2010) compared an automatic RR-detector (Polar®S810) with a gold standard ECG in horses and described failure to correctly identify one or more R-peaks. Different electrode types might explain the different signal quality between the two systems. In our study, electrodes were implanted under the skin of the horses, providing greater contact than conventional RR-detectors. To date, there are no studies comparing insertable cardiac monitors with conventional ECG in horses or other animals. The assessment of sensitivity of the current method was outside the scope of the study, but would be of great future interest.
In domestic horses, it is recommended to perform basal cardiac measurements only under resting conditions (Parker et al., 2010; Stucke et al., 2015). We overcame this problem by documenting diurnal variations in RMSSD. We identified the quarter of the day with the highest RMSSD values and included only data from that respective quarter of the day in statistical analysis.
We found that free-ranging Przewalski's horses exhibited diurnal variations of the autonomic nervous system function. RMSSD values were highest during the third quarter of the day (12 p.m.–6 p.m.), indicating an increased parasympathetic tone. At around midnight, parasympathetic tone was lowest. Accordingly, a peak in resting behavior in the middle of the day and a more vigilant and reactive behavior during the night has been observed in Przewalski's horses under natural conditions (Berger et al., 1999; Souris et al., 2007; King et al., 2016). Circadian patterns of HRV have been described in only a few domestic animal species. In contrast to our study, parasympathetic tone was higher during the night and lower during the day in most domestic species, possibly reflecting adaptation to human diurnality (Kuwahara et al., 1999a,b; Murphy, 2010; Gehrke et al., 2011; Kovács et al., 2016). Photoperiod is known to strongly influence daily and seasonal cycles in autonomic nervous system function (Buijs et al., 2003; Hastings et al., 2007; Vandewalle, 2007). However, the examination of the possible association between day-length and HRV was beyond the scope of this study.
RMSSD and HR varied considerably throughout the year. Both parameters showed lower values during the winter than during the summer. In spring, RMSSD dropped significantly along with a concurrent peak in HR.
The lower levels of RMSSD during winter and particularly the decrease during spring indicate a considerable reduction of the vagal tone. This is in agreement with the main assumptions of the polyvagal theory of Porges (1995a,b, 2007), who considered the vagal nerve as a mediator in reaction to stress. According to the theory, the vagal nerve is distinct with two different branches: (1) the myelinated vagus that is associated with attention, motion, and emotion, and (2) the unmyelinated vagus that mediates the reflexive regulation of visceral function. Both branches may have different outputs to the heart, but both branches can cause bradycardia. Upon reduction of the myelinated vagal tone, HR increases. At the same time, cardiac pacemakers are still prone to neurogenic bradycardia mediated by the unmyelinated vagus (Porges, 1995b, 2007). This means that, even though HR is lower during the winter, the organism can still experience “stress.”
HR correlates with cardiac output and oxygen consumption. Therefore, it can be used as an indicator for energy expenditure (Butler et al., 2004; Green, 2011). The Przewalski's horses in our study had a lower metabolic rate during the winter than during the summer. In spring, there was a peak in energy expenditure. A low metabolic rate during the winter and resumption of high metabolic activity in spring are well known from hibernators and species exhibiting daily torpor (Heldmaier et al., 2004; Evans et al., 2016). This phenomenon has also been previously described in a number of other non-hibernating mammal species (Weiner, 1977; Moen, 1978; Mesteig et al., 2000; Arnold et al., 2004; Theil et al., 2004), including the Przewalski's horse in the National Park Neusiedlersee-Seewinkel in Austria (Arnold et al., 2006). It is believed that these mammals, reduce body mass and organ size during the winter, and decrease endogenous heat production to be able to cope with predictable energetic bottlenecks (Ruf and Heldmaier, 1992; Arnold et al., 2004; Heldmaier et al., 2004).
In a recent study, Evans et al. (2016) investigated the drivers of hibernation in the Scandinavian brown bear. Amongst others, they examined HR and HRV (by measuring SDANN) throughout the year and found, similar to our study, that both parameters were significantly lower during the winter than during the summer. In our study, HRV correlated negatively with HR and body temperature. This is not surprising as an increase in HR results, in most cases, from a combination of reduced vagal activity (measured as a decrease in RMSSD) and increased sympathetic activity (VonBorell et al., 2007; Battipaglia and Lanza, 2015). The associated increase in metabolic heat production leads to an elevation of body temperature (Buller et al., 2013; Sim et al., 2014). In our (linear) model with log-transformed RMSSD, HR only accounted for ~2.5% of the observed variance. The primary reason to log-transform the raw RMSSD scores was to account for its skewed distribution, and this could have influenced the relationship between our HRV and HR measures. It would be interesting to explore this further with different operationalization of HRV in future research.
Of all physiologic parameters measured in our study, activity had the highest effect on HRV. When horses were active, RMSSD increased indicating that horses were more relaxed when moving as compared to standing still. Accordingly, Theil et al. (2004) described highest HRV values for locomotive behavior in European roe deer. In domestic horses in contrast, HRV increased from rest to walk, but decreased from walk to trot and gallop (Physick-Sheard et al., 2000; Kinnunen et al., 2006).
Theil et al. (2004) further described a positive effect of wind speed on HRV in European roe deer. Of all climatic parameters measured in this study, wind had no significant effect on RMSSD. Rain in contrast, was associated with a significant increase in RMSSD. Precipitation is the single most important climatic variable controlling the ecology of semiarid steppe-regions, the Przewalski's horse's habitat (Boyd and Houpt, 1994; Zimmermann et al., 2009a; Werger and VanStaalduinen, 2012). Hence, rainfall highly influences vegetation quality and water availability (Lauenroth and Sala, 1992; Kuntz et al., 2006), and might therefore indirectly affect HRV (Singh et al., 2000).
Outdoor temperature in contrast had a negative effect on HRV indicating that horses might be more vulnerable to heat-stress than to cold-stress. However, the effect of outdoor temperature was quadratic: minimum values of RMSSD were associated with temperatures ranging at around 18°C. This is the case during spring, when we observed a pronounced drop in HRV along with a peak in HR. A peak in metabolic rate during May has been described in European roe deer (Theil et al., 2004) and free-ranging Przewalski's horses within the National Park Neusiedlersee-Seewinkel in Austria (Arnold et al., 2006). Theil et al. (2004) suggested that this peak probably reflects the increased energetic costs of gestation and preparation for lactation. As all horses in our study were pregnant, reproduction might be a possible cause for the increased energy demands observed in April. Additional load on the cardiovascular system, hormonal control and induction of insulin resistance to provide energy to the growing fetus result in metabolic stress (Fowden et al., 1984; Pashen, 1984; Boyd and Houpt, 1994; Satué and Domingo, 2011). Interestingly, the horses of our study did not give birth until 1 month after the observed drop in HRV and peak in HR. Likewise, the roe deer in Theil et al. (2004) study only gave birth in early June whereas the peak took already place in May. This could be explained by a pronounced increase in fetal size during this stage of pregnancy in both species (Robbins and Robbins, 1979; Platt, 1984). However, domestic horses have been shown to only exhibit a slight increase in HR and no significant changes in RMSSD toward the end of pregnancy and postpartum (Nagel et al., 2011, 2012). Furthermore, Arnold et al. (2006) described a May peak in HR in a mare that only foaled in August. Thus, higher energy expenditure for gestation does not seem to entirely explain the drop of parasympathetic nervous system activity and peak of metabolic rate during spring. Kuntz et al. (2006) argued that seasonal changes in forage composition might be the driver of respective physiological changes in the Przewalski's horses. As crude protein increases during the vegetative period in spring, dry matter intake increases and hence, energy intake. After being in a katabolic state over the winter, horses have to adapt, amongst others, their gastrointestinal system to the higher quality forage and increase food intake to restore energy balance (Arnold et al., 2006; Kuntz et al., 2006). These metabolic changes have been found to occur prior to seasonal changes in plant phenology, suggesting an endogenous control mechanism preparing the organism in advance to the predictable seasonal changes in food quality (Arnold et al., 2006). In order to understand the role of pregnancy within these metabolic changes, seasonal variations in HR and HRV need to be studied in male and non-pregnant female horses.
For decades, demands associated with predictable seasonal variations have been considered stressful and have not been differentiated from “stress” associated with life-threatening events (Landys et al., 2006). The “concept of allostasis,” maintaining stability through change, allows us to distinguish between these two types of stress (McEwen and Wingfield, 2003). Daily and seasonal adjustments that maintain homeostasis within narrow life-sustaining ranges represent “allostatic state.” If environmental changes or changes in life history make the animal work harder to maintain homeostasis, an additional cost (allostatic load) is incurred. This increase in workload can be measured with overall energy expenditure. The animal enters life-threatening “allostatic overload” when energy expenditure exceeds energy intake (McEwen and Wingfield, 2003).
The combined measurement of RMSSD as a parameter for stress, and HR as an indicator for energy expenditure allowed us to assess allostatic load in our study. The decrease of HRV and HR during the winter indicate the adjustment of homeostatic set points and “allostatic state.” The decrease of HRV during spring with the associated increase in energy expenditure indicate that horses engendered an “allostatic load.”
A major limitation of our study was the small number of study-animals and the relatively high inter-individual variation in HRV. We dealt with this issue by implementing a longitudinal design that allows heterogeneity between individuals. Addressing effects of behavior and social interactions on HRV in free ranging Przewalski's horses would be of interest for future studies. Applying HRV in several wildlife species would allow for investigation of interspecific variations.
Here, we demonstrate that measuring telemetric HRV is a proven method to study undisturbed reactions of wild animals to their changing environment over the long term. Seasonal “stress” can be identified by using HRV measures, such as RMSSD, that indicate parasympathetic nervous system activity. We recommend to measure HR on a beat-to-beat basis, when collecting physiological data in future studies, in order to allow the additional assessment of HRV. We recorded cardiac activity in 3-min sequences with a self-constructed telemetric system. For future studies, the length of sequences should be increased to at least 5 min and more than one parameter for HRV should be calculated (Malik et al., 1996) i.e., estimates of overall HRV (such as SDNN, standard deviation of NN interval), long term components of HRV (e.g., SDANN), and short term components of HRV (RMSSD). Simultaneous evaluation of fecal corticoid metabolites would be of interest for method validation and comparison.
In conclusion, measuring HRV represents a promising tool to understand how animals integrate their life cycles in an ever-changing environment and potentially identify anthropogenic influences that cause stress. The combined measurement of HRV as an indicator for autonomic nervous system function and HR as an indicator for energy expenditure enables the valuation of allostatic load and “stress” in individual free-ranging mammals over a prolonged period of time.
Conceived and designed the experiments: KB, CW. Designed and build transmitter: GF. Performed the experiments: KB, GS, TP, CW. Analyzed the data: FP. Wrote paper: FP and CW. Edited manuscript: KB, GF, GS, TP.
This research was supported by the Hortobágy National Park and Zoo Cologne.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors thank Mag. Radoslaw Karpienko for statistical analysis and Dr. Franz Schober and Dr. Waltraut Zimmermann for logistic and management support in setting up and implementing this project. This manuscript represents a portion of an MSc-thesis submitted by FP to the University of Natural Resources and Life Sciences, Vienna. Presented partially in abstract form at the 12th Conference of the European Wildlife Disease Association (EWDA), Berlin, Germany, August 2016, and the Veterinary Management of African Wildlife Conference, Pretoria, South Africa, February 2017.
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fphys.2017.00664/full#supplementary-material
Ackerman, J. T., Takekawa, J. Y., Kruse, K. L., Orthmeyer, D. L., Yee, J. L., Ely, C. R., et al. (2004). Using radiotelemetry to monitor cardiac response of free-living tule greater white-fronted geese (Anser albifrons elgasi) to human disturbance. Wilson Bull. 116, 146–151. doi: 10.1676/03-110
Arnold, W., Ruf, T., and Kuntz, R. (2006). Seasonal adjustment of energy budget in a large wild mammal, the Przewalski horse (Equus ferus przewalskii) II. Energy expenditure. J. Exp. Biol. 209, 4566–4573. doi: 10.1242/jeb.02536
Arnold, W., Ruf, T., Reimoser, S., Tataruch, F., Onderscheka, K., and Schober, F. (2004). Nocturnal hypometabolism as an overwintering strategy of red deer (Cervus elaphus). Am. J. Physiol. Regul. Integr. Compar. Physiol. 286, R174–R181. doi: 10.1152/ajpregu.00593.2002
Battipaglia, I., and Lanza, G. A. (2015). “The autonomic nervous system of the heart in slart,” Autonomic Innervation of the Heart. Role of Molecular Imaging, 1st Edn, eds H. J. A. Riemer, R. A. Tio, P. H. Elsinga and M. Schwaiger (Berlin; Heidelberg: Springer), 1–12. doi: 10.1007/978-3-662-45074-1_1
Berger, A., Scheibe, K. M., Eichhorn, K., Scheibe, A., and Streich, J. (1999). Diurnal and ultradian rhythms of behaviour in a mare group of Przewalski horse (Equus ferus przewalskii), measured through one year under semi-reserve conditions. Appl. Anim. Behav. Sci. 64, 1–17. doi: 10.1016/S0168-1591(99)00026-X
Boonstra, R. (2004). Coping with changing northern environments: the role of the stress axis in birds and mammals. Integr. Comp. Biol. 44, 95–108. doi: 10.1093/icb/44.2.95
Boyd, L., and Houpt, K. (1994). Przewalski's Horse: The History and Biology of an Endangered Species. Albany, NY: State University of New York Press.
Buijs, R. M., La Fleur, S. E., Wortel, J., Van Heyningen, C., Zuiddam, L., Mettenleiter, T. C., et al. (2003). The suprachiasmatic nucleus balances sympathetic and parasympathetic output to peripheral organs through separate preautonomic neurons. J. Comp. Neurol. 464, 36–48. doi: 10.1002/cne.10765
Buller, M. J., Tharion, W. J., Cheuvront, S. N., Montain, S. J., Kenefick, R. W., Castellani, J., et al. (2013). Estimation of human core temperature from sequential heart rate observations. Physiol. Meas. 34, 781–798. doi: 10.1088/0967-3334/34/7/781
Busch, D. S., and Hayward, L. S. (2009). Stress in a conservation context: a discussion of glucocorticoid actions and how levels change with conservation-relevant variables. Biol. Conserv. 142, 2844–2853. doi: 10.1016/j.biocon.2009.08.013
Butler, P. J., Green, J. A., Boyd, I. L., and Speakman, J. R. (2004). Measuring meatabolic rate in the fiels: the pros and cons of the doubly labeled water and heart rate methods. Funct. Ecol. 18, 168–183. doi: 10.1111/j.0269-8463.2004.00821.x
Chrousos, G. P. (2009). Stress and disorders of the stress system. Nat. Rev. Endocrinol. 5, 374–381. doi: 10.1038/nrendo.2009.106
Dantzer, B., Fletcher, Q. E., Boonstra, R., and Sheriff, M. J. (2014). Measures of physiological stress: a transparent or opaque window into the status, management and conservation of species? Conserv. Physiol. 2, 1–18. doi: 10.1093/conphys/cou023
Esco, M. R., and Flatt, A. A. (2014). Ultra-short-term heart rate variability indexes at rest and post-exercise in athletes: evaluating the agreement with accepted recommendations. J. Sports Sci. Med. 13, 535–541.
Evans, A. L., Singh, N. J., Friebe, A., Arnemo, J. M., Laske, T. G., Fröbert, O., et al. (2016). Drivers of hibernation in the brown bear. Front. Zool. 13:7. doi: 10.1186/s12983-016-0140-6
Fowden, A. L., Comline, R. S., and Silver, M. (1984). Insulin secretion and carbohydrate metabolism during pregnancy in the mare. Equine Vet. J. 16, 239–246. doi: 10.1111/j.2042-3306.1984.tb01919.x
Gehrke, E. K., Baldwin, A., and Schiltz, P. M. (2011). Heart rate variability in horses engaged in equine-assisted activities. J. Equine Vet. Sci. 31, 78–84. doi: 10.1016/j.jevs.2010.12.007
Green, J. A. (2011). The heart rate method for estimating metabolic rate: review and recommendations. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 158, 287–304. doi: 10.1016/j.cbpa.2010.09.011
Groscolas, R., Viera, V. M., Guerin, N., Handrich, Y., and Côté, S. D. (2010). Heart rate as a predictor of energy expenditure in undisturbed fasting and incubating penguins. J. Exp. Biol. 213, 153–160. doi: 10.1242/jeb.033720
Hadinger, U., Haymerle, A., Knauer, F., Schwarzenberger, F., and Walzer, C. (2015). Faecal cortisol metabolites to assess stress in wildlife: evaluation of a field method in free-ranging chamois. Methods Ecol. Evol. 6, 1349–1357. doi: 10.1111/2041-210X.12422
Harlow, H. J., Thorne, E. T., Williams, E. S., Belden, E. L., and Gern, W. A. (1987). Cardiac frequency: a potential predictor of blood cortisol levels during acute and chronic stress exposure in Rocky Mountain bighorn sheep (Ovis canadensis canadensis). Can. J. Zool. 65, 2028–2034. doi: 10.1139/z87-308
Hastings, M., O'Neill, J. S., and Maywood, E. S. (2007). Circadian clocks: regulators of endocrine and metabolic rhythms. J. Endocrinol. 195, 187–198. doi: 10.1677/JOE-07-0378
Heldmaier, G., Ortmann, S., and Elvert, R. (2004). Natural hypometabolism during hibernation and daily torpor in mammals. Respir. Physiol. Neurobiol. 141, 317–329. doi: 10.1016/j.resp.2004.03.014
Hill, L. K., and Siebenbrock, A. (2009). Are all measures created equal? Heart rate variability and respiration - biomed 2009. Biomed. Sci. Instrum. 45, 71–76.
Huber, S., Palme, R., and Arnold, G. W. (2003). Effects of season sex and Sample collection on Concentrations of faecal cortisol metabolites in red deer(Cervus elaphus). Gen. Comp. Endocrinol. 130, 48–54. doi: 10.1016/S0016-6480(02)00535-X
King, S. R. B., Asa, C., Pluhacek, J., Houpt, K., and Ransom, J. I. (2016). “Behaviour of horses, zebras, and asses,” in Wild Equids: Ecology, Management, and Conservation, eds J. I. Ransom and P. Kacenczky (Baltimore, MA: Johns Hopkins University Press), 23–40.
King, S. R. B., Boyd, L., Zimmermann, W., and Kendall, B. E. (2015). Equus ferus ssp. przewalskii The IUCN Red List of Threatened Species. e.T7961AT97. doi: 10.2305/IUCN.UK.2015-2.RLTS.T7961A45172099.en
Kinnunen, S., Laukkanen, R., Haldi, J., Hanninen, O., and Atalay, M. (2006). Heart rate variability in trotters during different training periods. Equine Vet. J. 38(Suppl. 36), 214–217. doi: 10.1111/j.2042-3306.2006.tb05542.x
Kolter, L., and Zimmermann, W. (2001). Die Haltung von junggesellengruppen für das EEP-Przewalskipferd – Hengste in Gehegen und Reservaten. Z. Kölner Zoo 44, 135–151. Available online at: http://www.koelner-zoo.de/takhi/PDF/Junggesellen_Przewalskipferde.pdf
Koolhaas, J. M., Bartolomucci, A., Buwalda, B., de Boer, S. F., Flügge, G., Korte, S. M., et al. (2011). Stress revisited: a critical evaluation of the stress concept. Neurosci. Biobehav. Rev. 35, 1291–1301. doi: 10.1016/j.neubiorev.2011.02.003
Kovács, L., Luca Kézér, F., Ruff, F., and Szenci, O. (2016). Cardiac autonomic activity has a circadian rhythm in summer but not in winter in non-lactating pregnant dairy cows. Physiol. Behav. 155, 56–65. doi: 10.1016/j.physbeh.2015.11.031
Kuntz, R., Kubalek, C., Ruf, T., Tataruch, F., and Arnold, W. (2006). Seasonal adjustment of energy budget in a large wild mammal, the Przewalski horse (Equus ferus przewalskii) I. Energy intake. J. Exp. Biol. 209, 4557–4565. doi: 10.1242/jeb.02535
Kuwahara, M., Hiraga, A., Kai, M., Tsubone, H., and Sugano, S. (1999a). Influence of training on autonomic nervous function in horses: evaluation by power spectral analysis of heart rate variability. Equine Vet. J. 21, 178–180. doi: 10.1111/j.2042-3306.1999.tb05213.x
Kuwahara, M., Suzuki, A., Tsutsumi, H., Tanigawa, M., Tsubone, H., and Sugano, S. (1999b). Power spectral analysis of heart rate variability for assessment of diurnal variation of autonomic nervous activity in miniature swine. Lab. Anim. Sci. 49, 202–208.
Laborde, S., Mosley, E., and Thayer, J. F. (2017). Heart rate variability and cardiac vagal tone in psychophysiological research – recommendations for experiment planning, data analysis, and data reporting. Front. Psychol. 8:213. doi: 10.3389/fpsyg.2017.00213
Landys, M. M., Ramenofsky, M., and Wingfield, J. C. (2006). Actions of glucocorticoids at a seasonal baseline as compared to stress-related levels in the regulation of periodic life processes. Gen. Comp. Endocrinol. 148, 132–149. doi: 10.1016/j.ygcen.2006.02.013
Laske, T. G., Garshelis, D. L., and Iaizzo, P. (2011). Monitoring the wild black bear's reaction to human and environmental stressors. BMC Physiol. 11:13. doi: 10.1186/1472-6793-11-13
Lauenroth, W. K., and Sala, O. E. (1992). Long-term forage production of North American shortgrass steppe. Ecol. Appl. 2, 397–403. doi: 10.2307/1941874
MacArthur, R. A., Johnston, R. H., and Geist, V. (1979). Factors influencing heart rate in free-ranging bighorn sheep: a physiological approach to the study of wildlife harassment. Can. J. Zool. 57, 2010–2021. doi: 10.1139/z79-265
Makra, Z. (2016). Przewalski's Horse Populations in Hungary, World Equine Veterinary Association - The Horse Magazine. Available online at: http://www.thehorse.com/articles/38236/przewalskis-horse-populations-in-hungary
Malik, M., Bigger, T. J., Camm, A. J., Kleiger, R. E., Malliani, A., Moss, A. J., et al. (1996). Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Eur. Heart J. 17, 354–381. doi: 10.1093/oxfordjournals.eurheartj.a014868
McEwen, B. S., and Wingfield, J. C. (2003). The concept of allostasis in biology and biomedicine. Horm. Behav. 43, 2–15. doi: 10.1016/S0018-506X(02)00024-7
Mentaberre, G., López-Olvera, J. R., Casas-Díaz, E., Fernández-Sirera, L., Marco, I., and Lavín, S. (2010). Effects of azaperone and haloperidol on the stress response of drive-net captured Iberian ibexes (Capra pyrenaica). Eur. J. Wildlife Res. 56, 757–764. doi: 10.1007/s10344-010-0371-3
Mesteig, K., Tyler, N. J. C., and Blix, A. S. (2000). Seasonal changes in heart rate and food intake in reindeer (Rangifer tarandus tarandus). Acta Physiol. Scand. 170, 145–151. doi: 10.1046/j.1365-201x.2000.00767.x
Millspaugh, J. J., and Washburn, B. E. (2004). Use of fecal glucocorticoid metabolite measures in conservation biology research: considerations for application and interpretation. Gen. Comp. Endocrinol. 138, 189–199. doi: 10.1016/j.ygcen.2004.07.002
Moen, A. N. (1978). Seasonal changes in heart rates, activity, metabolism, and forage intake of white-tailed deer. J. Wildlife Manage. 42, 715–738. doi: 10.2307/3800763
Mohr, E., Langbein, J., and Nurnber, G. (2002). Heart rate variability: a non -invasive approach to measure stress in calves. Physiol. Behav. 75, 1–15. doi: 10.1016/S0031-9384(01)00651-5
Möstl, E., and Palme, R. (2002). Hormones as indicators of stress. Domest. Anim. Endocrinol. 23, 67–74. doi: 10.1016/S0739-7240(02)00146-7
Munoz, M. L., Van Roon, A., Riese, H., Thio, C., Oostenbroek, E., Westrik, I., et al. (2015). Validity of (Ultra-)Short recordings for heart rate variability measurements. PLoS ONE 10:e0138921. doi: 10.1371/journal.pone.0138921
Murphy, B. A. (2010). Chronobiology and the horse: recent revelations and future directions. Vet. J. 185, 105–114. doi: 10.1016/j.tvjl.2009.04.013
Nagel, C., Aurich, J., and Aurich, C. (2011). Heart rate and heart rate variability in the pregnant mare and its foetus. Reprod. Domest. Anim. 46, 990–993. doi: 10.1111/j.1439-0531.2011.01772.x
Nagel, C., Erber, R., Bergmaier, C., Wulf, M., Aurich, J., Möstl, E., et al. (2012). Cortisol and progestin release, heart rate and heart rate variability in the pregnant and postpartum mare, fetus and newborn foal. Theriogenology 78, 759–767. doi: 10.1016/j.theriogenology.2012.03.023
Nussinovitch, U., Politi Elishkevitz, K., Katz, K., Nussinovitch, M., Segev, S., Volovitz, B., et al. (2011). Reliability of ultra-short ECG Indices for heart rate variability. Ann. Noninvasive Electrocardiol. 16, 117–122. doi: 10.1111/j.1542-474X.2011.00417.x
Parker, M., Goodwin, D., Eager, R. A., Redhead, E. S., and Marlin, D. J. (2010). Comparison of Polar heart rate interval data with simultaneously recorded ECG signals in horses. Comp. Exerc. Physiol. 6, 137–142. doi: 10.1017/S1755254010000024
Pashen, R. L. (1984). Maternal and fetal endocrinology muring late pregnancy and parturition in the mare. Equine Vet. J. 16, 233–238. doi: 10.1111/j.2042-3306.1984.tb01918.x
Penttila, J., Helminen, A., Jartti, T., and Kuusela, T. (2001). Time domain, geometrical and frequency domain analysis of cardiac vagal outflow: effects of various respiratory patterns. Clin. Physiol. 21, 365–376. doi: 10.1046/j.1365-2281.2001.00337.x
Physick-Sheard, P. W., Marlin, D. J., Thornhill, R., and Schroter, R. C. (2000). Frequency domain analysis of heart rate variability in horses at rest and during exercise. Equine Vet. J. 32, 253–262. doi: 10.2746/042516400776563572
Platt, H. (1984). Growth of the equine foetus. Equine Vet. J. 16, 247–252. doi: 10.1111/j.2042-3306.1984.tb01920.x
Plews, D. J., Laursen, P. B., Stanley, J., Kilding, A. E., and Buchheit, M. (2013). Training adaptation and heart rate variability in elite endurance athletes: opening the door to effective monitoring. Sports Med. 43, 773–781. doi: 10.1007/s40279-013-0071-8
Poljakov, I. S. (1881). Przewalski's horse (Equus przewalskii n. sp.). Isvestia Russki Geograph. Obs. St Petersburg 17, 1–20.
Porges, S. W. (1995a). Cardiac Vagal Tone A physiological index of stress. Neurosci. Biobehav. Rev. 19, 225–233. doi: 10.1016/0149-7634(94)00066-A
Porges, S. W. (1995b). Orienting in a defensive world: mammalian modifications of our evolutionary heritage. A polyvagal theory. Psychophysiology 32, 301–318.
Porges, S. W. (2007). The polyvagal perspective. Biol. Psychol. 74, 116–143. doi: 10.1016/j.biopsycho.2006.06.009
Pumprla, J., Pumprla, J., Howorka, K., Howorka, K., Groves, D., Groves, D., et al. (2002). Functional assessment of heart rate variability: physiological basis and practical applications. Int. J. Cardiol. 84, 1–14. doi: 10.1016/S0167-5273(02)00057-8
Reeder, D. M., and Kramer, K. M. (2005). Stress in free-ranging mammals: integrating physiology, ecology, and natural history. J. Mammal. 86, 225–235. doi: 10.1644/BHE-003.1
Robbins, C. T., and Robbins, B. L. (1979). Fetal and neonatal growth patterns and maternal reproductive effort in ungulates and subungulates Am. Nat. 114, 101–116.
Romero, L. M. (2002). Seasonal changes in plasma glococorticoid concentrations in free-living vertebrates. Gen. Comp. Endocrinol. 128, 1–24. doi: 10.1016/S0016-6480(02)00064-3
Ruf, T., and Heldmaier, G. (1992). The impact of daily torpor on energy requirements in the Djungarian hamster, Phodopus sungorus. Physiol. Zool. 65, 994–1010. doi: 10.1086/physzool.65.5.30158554
Rutz, C., and Hays, G. C. (2009). New frontiers in biologging science. Biol. Lett. 5, 289–292. doi: 10.1098/rsbl.2009.0089
Ryder, O. A. (1993). Przewalski's horse: prospects for reintroduction into the wild. Conserv. Biol. 7, 13–15. doi: 10.1046/j.1523-1739.1993.07010013.x
Ryder, O. A., and Wedemeyer, E. A. (1982). A cooperative breeding programme for the Mongolian wild horse Equus przewalskii in the United States. Biol. Conserv. 22, 259–271.
Saboul, D., Pialoux, V., and Hautier, C. (2013). The impact of breathing on HRV measurements: implications for the longitudinal follow-up of athletes. Eur. J. Sport Sci. 13, 534–542. doi: 10.1080/17461391.2013.767947
Satué, K., and Domingo, R. (2011). Longitudinal study of the renin angiotensin aldosterone system in purebred Spanish broodmares during pregnancy. Theriogenology 75, 1185–1194. doi: 10.1016/j.theriogenology.2010.11.029
Schwarzenberger, F. (2007). The many uses of non-invasive faecal steroid monitoring in zoo and wildlife species. Int. Zoo Yearbook 41, 52–74. doi: 10.1111/j.1748-1090.2007.00017.x
Selye, H. (1936). A syndrome produced by diverse nocuous agents. J. Neuropsychiatry Clin. Neurosci. 10, 230–231.
Sheriff, M. J., Dantzer, B., Delehanty, B., Palme, R., and Boonstra, R. (2011). Measuring stress in wildlife: techniques for quantifying glucocorticoids. Oecologia 166, 869–887. doi: 10.1007/s00442-011-1943-y
Sim, S., Yoon, H., Ryou, H., and Park, K. (2014). “Estimation of body temperature rhythm based on heart activity parameters in daily life,” in 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (Chicago, IL), 2245–2248. doi: 10.1109/EMBC.2014.6944066
Singh, R. B., Weydahl, A., Otsuka, K., Watanabe, Y., Yano, S., Mori, H., et al. (2000). Can nutrition influence circadian rhythm and heart rate variability ? Biomed. Pharmacother. 55(Suppl. 1), 115–124. doi: 10.1016/S0753-3322(01)90016-2
Souris, A. C., Kaczensky, P., Julliard, R., and Walzer, C. (2007). Time budget-, behavioral synchrony- and body score development of a newly released Przewalski's horse group Equus ferus przewalskii, in the Great Gobi B strictly protected area in SW Mongolia. Appl. Anim. Behav. Sci. 107, 307–321. doi: 10.1016/j.applanim.2006.09.023
Støen, O. G., Ordiz, A., Evans, A. L., Laske, T. G., Kindberg, J., Fröbert, O., et al. (2015). Physiological evidence for a human-induced landscape of fear in brown bears (Ursus arctos). Physiol. Behav. 152, 244–248. doi: 10.1016/j.physbeh.2015.09.030
Stucke, D., Große Ruse, M., and Lebelt, D. (2015). Measuring heart rate variability in horses to investigate the autonomic nervous system activity – Pros and cons of different methods. Appl. Anim. Behav. Sci. 166, 1–10. doi: 10.1016/j.applanim.2015.02.007
Tarlow, E. M., and Blumstein, D. T. (2007). Evaluating methods to quantify anthropogenic stressors on wild animals. Appl. Anim. Behav. Sci. 102, 429–451. doi: 10.1016/j.applanim.2006.05.040
Theil, P. K., Coutant, A. E., and Olesen, C. R. (2004). Seasonal changes and activity-dependent variation in heart rate of Roe Deer. J. Mammal. 85, 245–325. doi: 10.1644/1545-1542(2004)085<0245:SCAAVI>2.0.CO;2
Touma, C., and Palme, R. (2005). Measuring fecal glucocorticoid metabolites in mammals and birds: the importance of validation. Ann. N.Y. Acad. Sci. 1046, 54–74. doi: 10.1196/annals.1343.006
Vandewalle, G. (2007). Robust circadian rhythm in heart rate and its variability : influence of exogenous melatonin and photoperiod. J. Sleep Res. 16, 148–155. doi: 10.1111/j.1365-2869.2007.00581.x
vonBorell, E., Langbein, J., Després, G., Hansen, S., Leterrier, C., Marchant-Forde, J., et al. (2007). Heart rate variability as a measure of autonomic regulation of cardiac activity for assessing stress and welfare in farm animals - A review. Physiol. Behav. 92, 293–316. doi: 10.1016/j.physbeh.2007.01.007
Wakefield, S., Zimmermann, W., and Van Dierendonck, M. C. (2002). “Status and action plan for the przewalski's horse (Equus ferus przewalskii),” in Equids: Zebras, Asses and Horses. IUCN/SCC Equid Specialist Group, ed P. Moehlman (Cambridge: IUCN Publication Services Unit), 82–92.
Walzer, C., Stalder, G., Petit, T., Sos, E., Molnar, V., Brabender, K., et al. (2009). “Surgical field anesthesia in Przewalski's horse (Equus ferus przewalskii) in Hortobagy national park,” in Hungary AAZV Annual Conference. Available online at: https://vetdoc.vu-wien.ac.at/vetdoc/suche.publikationen_mug_autoren?sprache_in=de&menue_id_in=&id_in=&publikation_id_in=66288
Weimerskirch, H., Shaffer, S. A., Mabille, G., Martin, J., Boutard, O., and Rouanet, J. L. (2002). Heart rate and energy expenditure of incubating wandering albatrosses: basallevels, natural variation, and the effects of human disturbance. J. Exp. Biol. 205, 475–483. doi: 10.1385/1592591639
Weiner, J. (1977). Energy metabolism of the roe deer. Acta Theriol. 22, 3–24. doi: 10.4098/AT.arch.77-1
Weisenberger, M. E., Krausman, P. R., Wallace, M. C., DeYoung, D. W., and Maughan, O. E. (1996). Effects of simulated jet aircraft noise on heart rate and behavior of desert ungulates. J. Wildlife Manage. 60, 52–61. doi: 10.2307/3802039
Werger, M. J. A., and VanStaalduinen, M. A. (2012). Eurasian Steppes. Ecological Problems and Livelihoods in a Changing World. Dordrecht: Springer Netherlands.
White, G. C., and Garrott, R. A. (1990). Analysis of Wildlife Radio- Tracking Data. San Diego, CA: Academic Press.
Wikelski, M., and Cooke, S. J. (2006). Conservation physiology. Trends Ecol. Evol. 21, 38–46. doi: 10.1016/j.tree.2005.10.018
Zimmermann, W. (2005). Przewalski's horses on the track to reintroduction- various projects compared. Z. Kölner Zoo 48, 183–209. Available online at: http://www.koelner-zoo.de/takhi/PDF/Heft4_2005_Reservate.pdf; http://lpv-augsburg.de/files/downloads/Przewalskis_horse_Reserves_EN.pdf
Zimmermann, W., Brabender, K., and Kolter, L. (2009a). A Przewalski's Horse Population in a Unique European Steppe Reserve – the Hortobágy Nationalpark in Hungary, Symposium proceedings Equus. Praha Zoo 257–288. Available online at: http://www.savethewildhorse.org/media/forschung/2009Monitoringofagonisticbehaviorandfoalmortalityinfree-rangingPrzewalskishorseharemsintheMongolianGobi.TaniaHoeslietal..pdf
Zimmermann, W., Sandor, I., and Brabender, K. (2009b). Naturschutzprojekt Hortobágy – Jahresbericht 2008. Z. Kölner Zoo 52, 47–60. Available online at: https://www.koelnerzoo.de/images/pdf/Zeitschriften/Zeitschrift_2009_01.pdf
Keywords: adaptation, allostatic load, Equus ferus przewalskii, heart rate variability, parasympathetic nervous system, Przewalski's horse, seasonal variations, stress
Citation: Pohlin F, Brabender K, Fluch G, Stalder G, Petit T and Walzer C (2017) Seasonal Variations in Heart Rate Variability as an Indicator of Stress in Free-Ranging Pregnant Przewalski's Horses (E. ferus przewalskii) within the Hortobágy National Park in Hungary. Front. Physiol. 8:664. doi: 10.3389/fphys.2017.00664
Received: 30 May 2017; Accepted: 22 August 2017;
Published: 07 September 2017.
Edited by:
Zbigniew R. Struzik, The University of Tokyo, JapanReviewed by:
Kathleen M. Gustafson, University of Kansas Medical Center, United StatesCopyright © 2017 Pohlin, Brabender, Fluch, Stalder, Petit and Walzer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Friederike Pohlin, ZnJpZWRlcmlrZS5wb2hsaW5AZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.