
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Physiol., 07 July 2017
Sec. Exercise Physiology
Volume 8 - 2017 | https://doi.org/10.3389/fphys.2017.00476
Studies have reported opposing effects of high-fat (HF) diet and mechanical stimulation on lineage commitment of the bone marrow stem cells. Yet, how bone marrow modulates its gene expression in response to the combined effects of mechanical loading and a HF diet has not been addressed. We investigated whether early-life (before onset of sexual maturity at 6 weeks of age) voluntary physical activity can modulate the effects of a HF diet on male Sprague Dawley rats. In the bone marrow, early-life HF diet resulted in adipocyte hypertrophy and a pro-inflammatory and pro-adipogenic gene expression profile. The bone marrow of the rats that undertook wheel exercise while on a HF diet retained a memory of the early-life exercise. This memory lasted at least 60 days after the cessation of the voluntary exercise. Our results are consistent with the marrow adipose tissue having a unique response to HF feeding in the presence or absence of exercise.
Childhood obesity has reached epidemic levels worldwide (WHO, 2016). The metabolic dysfunction induced by obesity in childhood persists into adulthood (Mattsson et al., 2008; Juonala et al., 2011; Schmidt et al., 2011; Lloyd et al., 2012). There is a genetic component to this complex phenotype. However, environmental factors, including excess energy intake and reduced physical activity, make a measurable contribution to the increasing obesity prevalence. As such, physical activity has long been considered as a non-pharmacological strategy to combat obesity and its associated co-morbidities (Tuomilehto et al., 2001; Misra et al., 2008).
Achieving optimal bone mass and strength during growth has biologically relevant effects on skeletal competence in both early and later life (Baxter-Jones et al., 2011). Childhood and adolescence are periods of rapid bone growth and attaining optimal bone mass during early life reduces the prevalence of orthopedic diseases such as fracture during childhood, adolescence, and later life (Ducher and Bass, 2007; Rizzoli et al., 2010). The opinion that obesity has a protective effect on bone due to the increased mechanical demand imposed by the excessive body weight on the skeleton has been challenged in recent times (Goulding et al., 2001, 2008; Skaggs et al., 2001; Sabhaney et al., 2014). Evidence from animal studies heavily favors the hypothesis that obesity may be detrimental to bone health during growth with several studies reporting an inverse relationship between excess adiposity, and the mechanical and micro-structural properties of bone (Cao et al., 2009; Woo et al., 2009; Lorincz et al., 2010; Ionova-Martin et al., 2011; Zhao, 2013; Yan et al., 2015). Understanding the effects of excessive adiposity on the growing skeleton is essential given the rising rates of childhood obesity.
Mechanical loading in the form of physical activity and/or exercise has the ability to influence body composition and bone properties. Physical activity not only suppresses the accumulation of fat mass through increased energy expenditure, but also promotes an anti-inflammatory environment, thereby helping to reduce chronic low-grade inflammation associated with excess adiposity (Gleeson et al., 2011). In the bone marrow, the adipocytes and osteoblasts share a common mesenchymal progenitor whose lineage commitment can be influenced by environmental factors (Beresford et al., 1992; Parhami et al., 2001; David et al., 2007). Studies have reported opposing effects of high-fat (HF) diet and mechanical stimulation on the lineage commitment of bone marrow stem cells. For example, an atherogenic HF diet reduced the ability of marrow stromal cells to differentiate into osteoblasts in a female mouse model (Parhami et al., 1999). By contrast, the application of high frequency-low magnitude mechanical stimuli in mice resulted in a marrow environment that favored osteogenesis (Luu et al., 2009).
Skeletal loading causes changes in the expression of genes involved in adipocyte and osteoblast differentiation and function (David et al., 2007; Menuki et al., 2008). Early studies on environmental impacts on bone and/or marrow gene expression have addressed the effects of HF diet or exercise independently (Xiao et al., 2010; Lange et al., 2013; Sontam et al., 2015a). Yet, how mechanical loading in the presence of a HF diet modulates bone marrow gene expression has not been addressed. As such, it is unclear if the beneficial effects of physical activity are retained or lost following cessation of an exercise program (Järvinen et al., 2003; Yasari et al., 2007; Sertie et al., 2013). In the present study, we determined the changes in bone marrow gene expression that were associated with early-life (before onset of sexual maturity at 6 weeks of age; Sengupta, 2013) HF diet accompanied by voluntary physical activity. In male Sprague-Dawley (SD) rats, we measured the effects of HF diet (45% Kcal from fat) and voluntary wheel running, before and after puberty, on body composition, bone mass indices, and gene expression in marrow isolated from the femur. We identified significant changes in gene expression and physiological measures. The gene expression changes were retained but did not prevent rebound obesity in previously exercised animals. Our results are consistent with the bone marrow having a unique and long-lasting response to HF feeding, depending on the presence or absence of exercise.
This study was approved by and carried out in accordance with the recommendations of the University of Auckland Animal Ethics Committee (AEC001432). Eighty male weanling rats (aged 22 days) born from normal Sprague-Dawley (SD) dams were allocated at weaning (controlling for body weight and litter of origin) into either: (A) a chow-fed group (C-SED; n = 20) which obtained 18% of total calories from fat (Diet 2018, Teklad Global 18% Protein Rodent Diet, Harlan Teklad, USA) and was allowed spontaneous movement within standard cages; or (B) a HF-fed group (n = 60) which obtained 45% of total energy intake from fat (D12451, Research Diets, USA). The HF-fed group was further divided into three sub-groups: (1) a HF sedentary (HF-SED, n = 20) which had only spontaneous movement within the cage for the duraticon of the experiment; (2) HF late exercise (HF-LEX, n = 20) group which was allowed spontaneous cage activity up to D67 and access to a running wheel from D67 to D120; and (3) a HF early exercise group (HF-EEX, n = 20) which had access to a running wheel from D22 to D60 (Model 80859, Lafayette Instrument, Lafayette, IN, USA), and only spontaneous movement for the remainder of the experiment. All rats were housed as pairs throughout the study to avoid the stressors associated with singleton housing (Lin and Scott, 2011). All animals had ad libitum access to food and water and were housed in a temperature controlled room (25°C) with a 12 h light/dark cycle. Food and water intake was recorded at regular intervals.
“Early-life” is regarded as the period before onset of sexual maturity, which is around 6 weeks of age; sexual maturity is some 3 weeks before onset of early adulthood (end of the 8th week of life; Sengupta, 2013). The EEX period was begun at weaning as physical activity before puberty results in greater and more persistent bone morphology responses than if exercise begins after puberty. Finally, the exercise periods differed (i.e., EEX, 38 days; LEX—53 days) to ensure the period of lack of exercise after cessation of EEX lasted firmly into adulthood in order to test for a memory.
Wheel exercise data were recorded at 15 min intervals using dedicated monitoring software (Model 86065). Previous studies by our group (Sontam et al., 2015b) and others (Holy and Zérath, 2000) observed a distinct circadian pattern in the wheel activity of young rats. Preliminary analyses of the exercise data confirmed that the rats in the HF-EEX group used the wheel preferentially during the dark period (18:00–6:00 h) with minimal activity during the day. For this reason, only dark period wheel exercise data were analyzed.
Animals were fasted overnight, anesthetized using sodium pentobarbitone (60 mg/kg, IP) and culled by decapitation. Five pairs of rats from each group were culled at either D60 or D120.
Dual energy x-ray absorptiometry (DXA), using dedicated small animal software (Lunar Hologic, GE, Waltham, MA, USA), was performed on animals under light isofluorane anesthesia to determine the body composition of animals in the week preceding each cull.
A sterile saw was used to partially saw the left femur at the junction of: (1) the distal and middle thirds; and (2) the middle and proximal thirds. The femur was then snapped to obtain the mid-diaphysis. Bone marrow was isolated from the diaphyseal bone by centrifugation (10,000 rpm, 4°C, 30 s), and immediately snap-frozen in liquid nitrogen and stored (−80°C) until analysis.
Measurements of adipocyte area were performed on femur tissue that had been prepared for histology, to test for evidence of between-group differences in marrow response. This was not a primary objective or hypothesis of the study and the post-hoc nature of the measurements meant that we were unable to measure total adiposity as we did not have the whole bone available to us. Thus, we were able to quantify adipocytic size in the metaphysis just inferior (distal) to the site of RNAseq sampling. Briefly, a Metasystems VSlide scanner (MetaSystems, Version 2.1.124) running Metafer4 (version 3.9.2) and coupled with MetaXpress (Molecular Devices, Version 5.3.0.4) was used to image toluidine blue-stained sections already processed to study joint cartilages. After threshold-based segmentation of the region immediately distal to the metaphyseal trabecular bone, images (x20 magnification) were stitched using the VSlide software, and viewed using Image Viewer (VSViewer, MetaSystems, Version 2.0). “Snapshot”.tiff images were processed in ImageJ calibrated to convert pixels into distance (μm), thresholded (range 228–255), and binarised for adipocyte identification. Using the “wand” tool within the ROI manager, 10 adipocytes at random were selected, and the area of each was measured.
The length of the right femur was measured (sliding caliper) from the most distal aspect of the lateral femoral condyle to the proximal extent of the major trochanter. The bone was lodged in a plastic tube filled with saline, taking care that no air bubbles were present, and scanned (XCT Research SA+ pQCT machine, StratecMedizinTechnik, Pforzheim, Germany). The reference line was positioned at the distal aspect of the condyles in the scout view before the machine made one scan at 50 and 19% of the femoral length, at the diaphysis and metaphysis respectively, voxel size 70 μm. Outcome measures were chosen to enable determination of cortical bone density and bone architecture, as previously described (Gasser, 2012).
Bone marrow total RNA was extracted using a modification of Ayturk et al. (2013). Briefly, bone marrow was ground into a fine powder using a mortar and pestle cooled in liquid nitrogen. The frozen bone marrow powder was transferred to a sterile microcentrifuge tube containing 1 mL TRIzol® (#15596-026, Life Technologies, Carlsbad, CA, USA), mixed thoroughly by shaking, homogenized by sonication (Bandelin Sonopuls HD2070, Bandelin, Berlin Germany), and extracted using TRIzol®-chloroform extraction. Total RNA was purified using an RNeasy Mini Kit (#74104, Qiagen, Hilden, Germany) and on-column DNA digestion was performed to remove traces of genomic DNA (RNase-free DNase Set, #79254, Qiagen, Hilden, Germany). RNA quantity was measured using a Qubit™ 3.0 fluorometer with the RNA High Sensitivity Assay Kit (#Q32855, ThermoFisher Scientific, Waltham, MA, USA). RNA quality was assessed using an Agilent Bioanalyser (Model 2100; Agilent Technologies, Santa Clara, CA, USA). RNA integrity numbers (RIN) for the samples ranged from 6.1 to 8.0 (Supplementary Table 3).
RNA samples were sequenced using an Illumina Hi-seq 4000 (Supplementary Table 3; 150 bp paired end; Novogene, Beijing, China). For shipping, purified total RNA samples were mixed with RNAstable® in a 96-well plate (#90220-001, Biomatrica, San Diego, CA, USA) and dried according to manufacturer's instructions.
Sequencing reads were trimmed (phred score < 30 discarded) using PRINSEQ (http://prinseq.sourceforge.net/). Processed reads that were <50 bp in length were discarded before further analyses.
Processed reads were aligned to the rat reference genome (NCBI version Rnor_5.0) using TopHat (version 2.1.0) (Supplementary Table 1; Trapnell et al., 2009). Gene model annotations were provided in a GTF file. Differential gene expression was determined using Cuffdiff (Cufflinks version 2.2.1) (Trapnell et al., 2010). Ten biological replicates from HF-SED, HF-EEX and HF-LEX conditions and seven biological replicates from the C-SED condition were sequenced and used for differential gene expression analysis.
Pathway analysis was performed using the Ingenuity pathway analysis (IPA, Ingenuity Systems Inc., Redwood City, CA, USA) software package. Data was uploaded into IPA with the ingenuity knowledge base as the reference set and a P < 0.05.
IPA was used to identify the biological functions, physiological processes and diseases associated with the differentially expressed genes. IPA downstream effects analysis was used to identify biological functions that were predicted to be up- or down-regulated based on the observed gene expression changes. An “activation z-score” of ≥2 or ≤ −2 was taken as significant for the biological functions that were predicted to be affected by the treatment (Ingenuity Systems, 2011). A regulatory effects analysis was performed to identify potential upstream regulators that explain the observed gene expression changes in the dataset.
Statistical analyses were performed using SigmaPlot 13.0 (SysStat Software Inc., CA, USA). One-way analysis of variance (ANOVA) was used to identify statistically significant differences between the groups. The Holm–Sidak method was used to correct for multiple comparison testing. Where the data failed the equal variance test, ANOVA on Ranks followed by the Tukey test for multiple comparisons was used to determine the between-group differences.
Caloric intake was significantly increased overall in the HF-fed groups compared to chow-fed controls and was not affected by exercise (Supplementary Figure 1). Similarly, body weight gain until day 60 was significantly increased in all HF-fed groups when compared to chow-fed controls (Supplementary Figure 2).
The HF-SED and HF-LEX groups were significantly heavier than C-SED, whereas the HF-EEX group was not different from C-SED (Table 1). Similarly at day 60 both the total body fat (%) (Figure 1A) and fat:lean ratio were higher in the HF-SED and HF-LEX groups compared to C-SED (Table 1). The total body fat and fat:lean ratio was not different between the C-SED and HF-EEX groups. Lean mass (%) (Figure 1C) was lower in HF-SED and HF-LEX groups than in C-SED and HF-EEX groups.
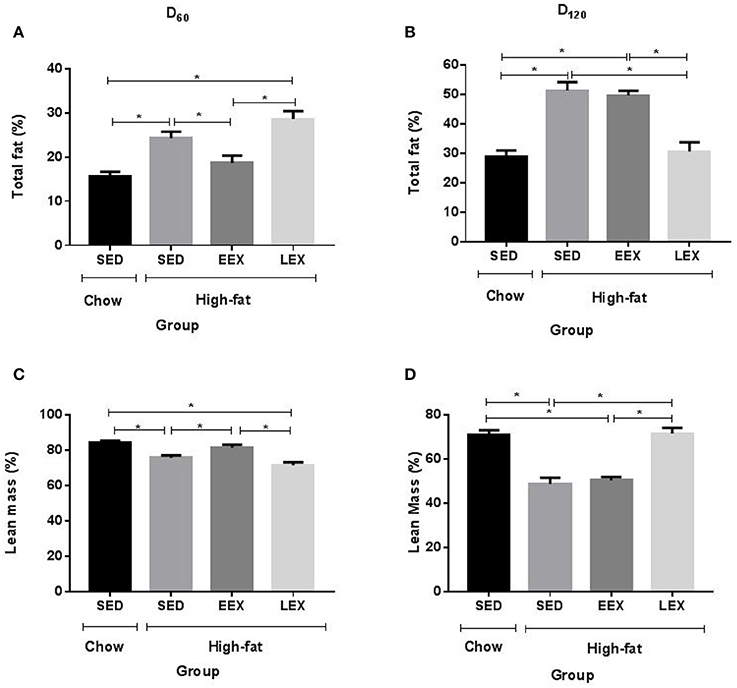
Figure 1. Total fat and lean mass percentage is affected by current but not previous physical activity. (A) Total fat percentage at D60 (B) Total fat percentage D120 (C) Lean mass percentage at D60 (D) Lean mass percentage at D120. *P < 0.05. All parameters were analyzed using one-way ANOVA followed by Holm–Sidak method for multiple comparison. SED, sedentary; EEX, early-exercise; LEX, late-exercise.
Both the HF-EEX and HF-LEX groups were fed a HF diet ad-libitum from weaning until day 120. The HF-EEX group was allowed running wheel activity from day 23, and there was a gradual increase in distance completed until day 60 (from 988.59 ± 347.05 m cage−1 night−1 increasing to 9971.97 ± 1933.34 m cage−1 night−1), at which point the running wheels were removed (Supplementary Figure 3). The HF-LEX group had wheel access between days 67 and 120. Similar to the HF-EEX animals, the HF-LEX animals exhibited a gradual increase in distance covered (from 1377 ± 254 m cage−1 night−1 increasing to a mean daily distance of 9907 ± 2550 m cage−1 night−1), followed by a gradual decrease toward the end of the study period (to 3254 ± 1169 m cage−1 night−1). As expected most activity (~98%) occurred during the dark phase of the light cycle.
Following the cessation of exercise, linear body growth in the HF-EEX group returned to match that of the animals in the HF-SED group (Supplementary Figure 2). Animals undergoing the late voluntary exercise protocol exhibited a significant decrease in body weights compared to animals in the HF-SED and HF-EEX groups, such that the HF-LEX animals were not different from those in the C-SED group by day 120. Accordingly, final body weights at day 120 were significantly higher for animals in the HF-SED and HF-EEX groups compared to those of the C-SED and HF-LEX groups (Table 1). This was reflected in a similar pattern of total body fat (%) (Figure 1B) and fat:lean ratios (Table 1) which were significantly increased in animals from the HF-SED and HF-EEX groups when compared to those from the C-SED and HF-LEX groups. Conversely, relative lean mass (%) was significantly decreased in HF-SED and HF-EEX groups compared to C-SED and HF-LEX groups (Figure 1D).
At D120, animals in the HF-SED group had the largest adipocyte area (965.76 ± 93.04 μm2). Animals in the C-SED group had the smallest adipocyte area (782.86 ± 54.89 μm2), while the adipocyte area for animals in the HF-EEX group (783.03 ± 60.07 μm2) was almost identical to that in the C-SED group. By contrast, the adipocyte area in animals in the HF-LEX exercise group (906.74 ± 34.01 μm2) was more similar to that in the HF-SED animals (Figure 2). The difference in adipocyte area between the HF-EEX and HF-SED groups approached statistical significance (p = 0.054).
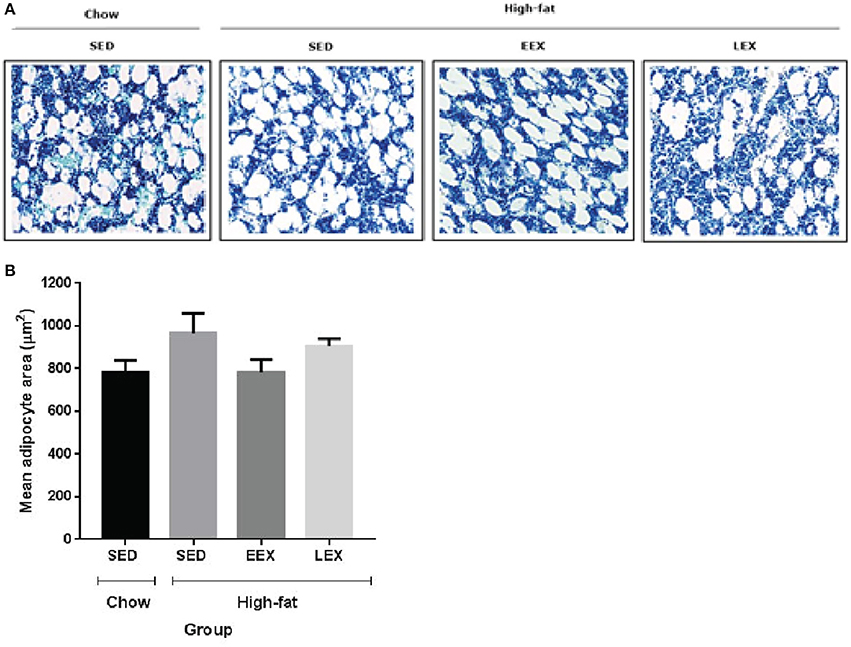
Figure 2. Mean marrow adipocyte area of the experimental groups at D120. (A) Representative images of bone marrow adipocytes in the experimental groups. (B) Graphical representation of mean adipocyte area. There were no statistically significant differences between any of the experimental groups with respect to their bone marrow adipocyte area. However, the mean marrow adipocyte area of the HF-SED group was larger than C-SED, HF-EEX, and HF-LEX groups.
There were no differences in total area, periosteal circumference or endosteal circumference within the mid-diaphysis from the femurs of any of the groups (Table 2). By contrast, animals from all of the HF-fed groups (i.e., HF-SED, HF-EEX, and HF-LEX) had greater cortical thickness and volumetric bone mineral density (vBMD) than animals from the C-SED group (Table 2). Similarly, animals from the HF-SED and HF-EEX groups had significantly higher cortical bone mineral content (BMC) than animals from the C-SED group (Table 2). Only animals from the HF-EEX group had significantly increased cortical bone area than animals from the C-SED group.
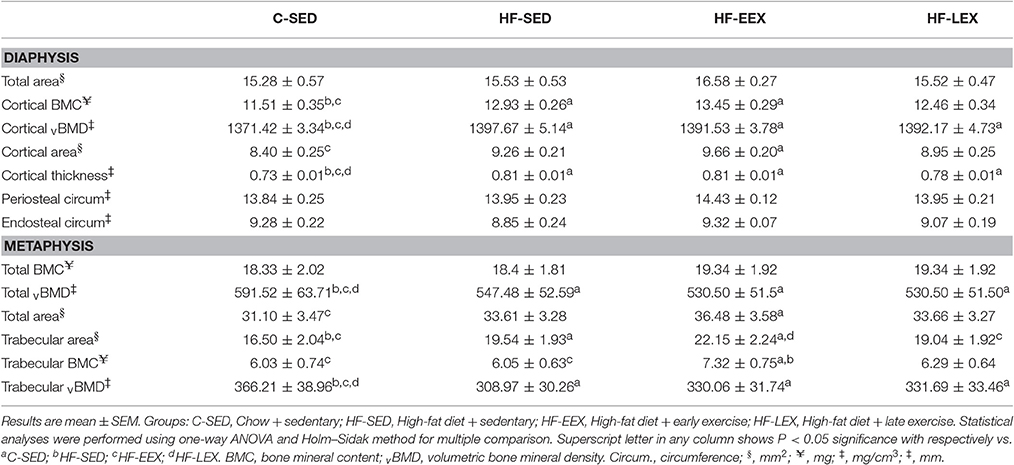
Table 2. Femoral cortical and trabecular bone properties in the experimental groups at the end of the late exercise period.
In the metaphysis, there was no statistically significant difference in total BMC between any of the groups (Table 2). Total vBMD and trabecular vBMD of animals from all three HF-fed groups (i.e., HF-SED, HF-EEX, and HF-LEX) was significantly lower than that in animals from the C-SED group (Table 2). The trabecular bone area in animals from the HF-SED and HF-EEX groups was significantly larger than that from animals in the C-SED group, which latter was not different from those in the HF-LEX group. In addition, the trabecular area of animals from the HF-EEX group was greater than that in HF-LEX animals. In the HF-EEX group total area and trabecular BMC were significantly higher than observed for animals from the C-SED group, and trabecular BMC was higher than that in the HF-SED group also.
RNA-seq identified 328 genes that were up-regulated and 210 genes that were down-regulated in the HF-SED group when compared to the C-SED group. Ingenuity Pathway Analysis (IPA) revealed that genes involved in inflammation, metabolism, and connective tissue disorders were significantly enriched within the differentially expressed genes in bone marrow from animals in the HF-SED group (Table 3). Downstream IPA effects analysis found that “quantity of adipose tissue” function was positively activated, based on the direction of changes of the genes in the dataset (z-score: +1.815; Supplementary Table 1). Genes belonging to the ontology category “morphology of bone” were also identified as significantly over-represented in the set of differentially expressed genes (Supplementary Table 1).
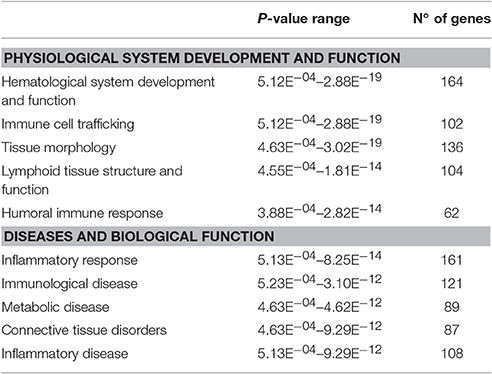
Table 3. Biological functions, physiological processes, and diseases that were over-represented by genes that were differentially regulated due to the high-fat diet.
The expression of genes coding for actin alpha 1 (Acta1), cell death inducing DFFA-like effector C (Cidec), perilipin 1 (Plin1), and phosphoenolpyruvate carboxykinase (Pck1) was up-regulated more than two-fold in the HF-SED group compared to the C-SED group. In contrast to previous reports of an inverse relationship between adiposity and Adipoq mRNA levels, we observed higher Adipoq gene expression in the HF-SED group compared to the C-SED group (Supplementary Table 1). Among the most significant regulatory networks we identified by IPA, four were associated with inflammatory pathways (Table 3). “Activation of T-lymphocytes,” “T cell homeostasis,” and “cell movement of monocytes” were all predicted to be upregulated in the bone marrow of HF-SED rats (Figure 3). In addition to their roles in inflammation, LTB, IL-1β, and CCL5 all have been shown to participate in bone metabolism (Garrett et al., 1987; Thomson et al., 1987; Boyce et al., 1989; Yano et al., 2005; Wintges et al., 2013).
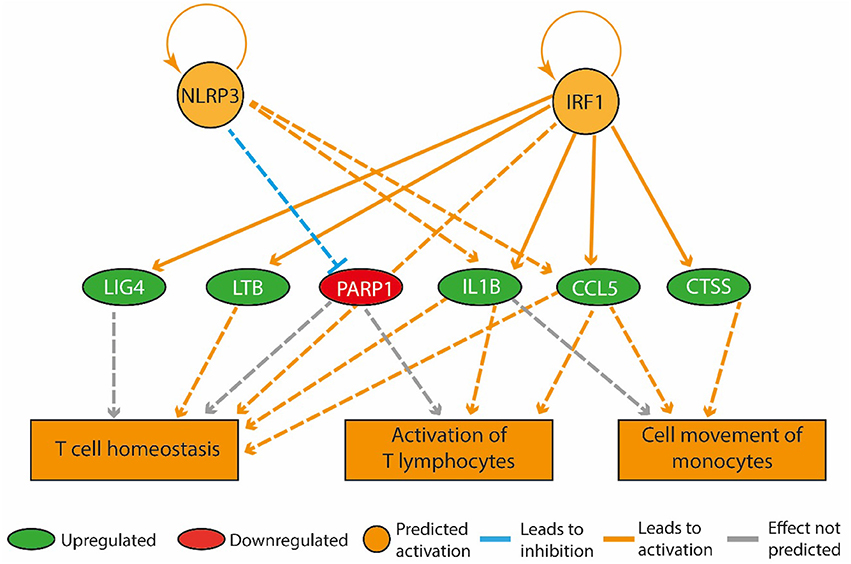
Figure 3. A high-fat diet in early life upregulated genes that promote inflammation in the bone marrow. The regulatory network pictured consists of three tiers. The top tier consists of predicted upstream regulators that might explain the gene expression changes observed in the experiment. NLRP3 and IRF1 are the upstream regulators whose predicted activated state may explain the expression changes in LIG4, LTB, PARP1, IL1B, CCL5, and CTSS (middle tier). The observed upregulation of LIG4, LTB, IL1B, CCL5, and CTSS and the downregulation of PARP1 leads to a predicted increase in T cell homeostasis, activation of T lymphocytes and cell movement of monocytes (bottom tier).
In the HF-LEX group, voluntary physical activity between D67 and D120 combined with lower food intake induced changes in the D120 bone marrow gene expression. In the D120 bone marrow samples 179 genes were differentially expressed from HF-LEX animals when compared to those from HF-SED, with 76 genes upregulated due to the exercise, and 102 genes down-regulated. Among the five most significantly affected biological functions and diseases identified by IPA, three were related to inflammation, and related ontologies (immunological disease, inflammatory response and inflammatory disease; Table 4). As observed in the HF-EEX group (Table 3), the most significantly affected regulatory networks also belonged to the “inflammation” category. Notably, most genes in the IL2, IL10 inflammatory regulatory network were down-regulated in the HF-LEX group compared to HF-SED group (Figure 4). Thus, exercise reduced the expression of genes that were involved in inflammation within HF-LEX animals while HF-SED animals exhibited up-regulation of these pro-inflammatory genes.
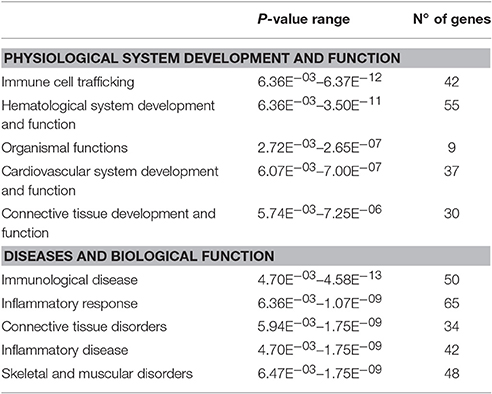
Table 4. Biological functions, physiological processes, and diseases that were over-represented by genes that were differentially expressed in HF-LEX group compared to HF-SED group.
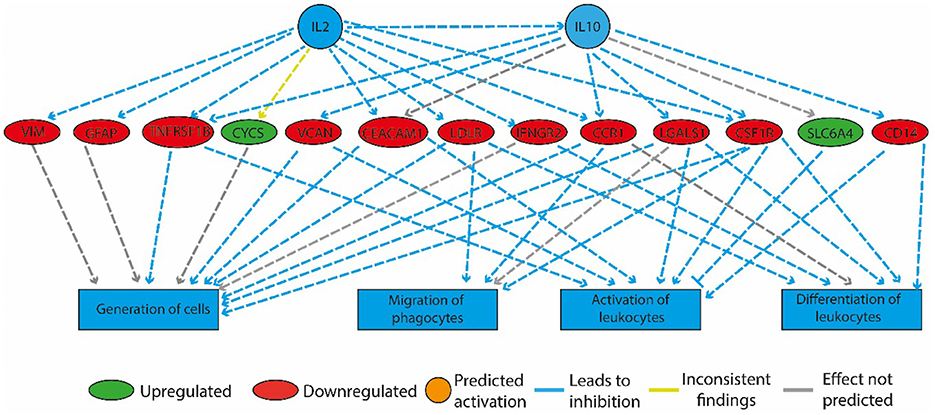
Figure 4. Physical activity in high-fat fed rats downregulated genes involved in inflammation. The regulatory network that is illustrated consists of three tiers. The top tier consists of predicted upstream regulators that might explain the gene expression changes observed in the experiment. IL2 and IL10 are the upstream regulators whose predicted activated state may explain the altered regulation of the middle tier genes. The observed gene expression changes lead to a predicted decrease in “generation of cells,” “migration of phagocytes,” “activation,” and “differentiation of leukocytes” (bottom tier).
Genes with known roles in osteoclast formation and function were down-regulated in the HF-LEX animals. RANKL-mediated induction of osteoclastogenesis requires a co-stimulatory immunoreceptor tyrosine-based activation motif (ITAM) pathway which is activated in response to ligation of osteoclast-associated receptors such as OSCAR. Moreover, in a pro-inflammatory environment, the complement C3 and C5 bind to their receptors (C3aR and C5aR) on osteoblasts and promote the expression of RANKL (Ignatius et al., 2011). We observed a down-regulation of C5aR1 and Oscar transcripts in the HF-LEX group. Notably, neuropilin 1 (NRP1), which acts with semaphorin 3A (SEMA3A) to inhibit RANKL-mediated activation of osteoclasts, was up-regulated in the HF-LEX group (Hayashi et al., 2012).
Compared to the HF-SED group, 128 genes were differentially regulated (48 up- and 80 down-regulated) in the HF-EEX group, of which 14 genes showed two-fold or greater up-regulation while 16 genes showed two-fold or greater down-regulation (Supplementary Table 2). Genes with roles in adipogenesis and adipocyte function were down-regulated, and formed the only regulatory network that was significantly differently expressed between the HF-EEX and HF-SED conditions (Figure 5).
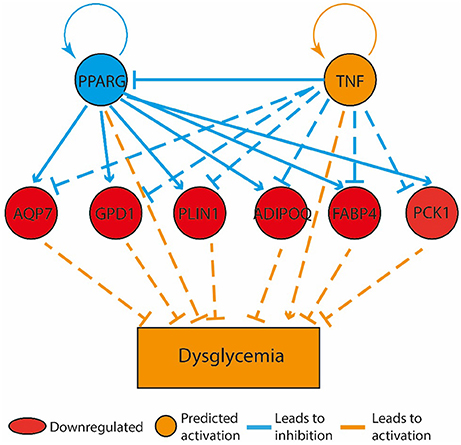
Figure 5. Bone marrow gene expression retained a memory of physical activity following 60 days of physical activity cessation. The top tier consists of predicted upstream regulators that might explain the gene expression changes observed in the experiment. PPARG and TNF are the upstream regulators whose predicted states may explain the altered regulation of the middle tier genes. The observed gene expression changes lead to a predicted increase in “dysglycemia” (bottom tier).
We also observed differential expression of genes with roles in both adipocyte and bone metabolism. Crucially, we observed significant down-regulation of Cdo1 (log ratio: −0.87) and Dlk1 (log ratio: −0.47) in the bone marrow of the HF-EEX compared to HF-SED group. Our observations thus indicate that the early-life exercise in the presence of a HF diet influenced gene expression significantly such that at D120, the gene expression profile remained different from that in animals only exposed to a HF diet (HF-SED).
How bone marrow modulates gene expression in response to diet and exercise is not known. In this study, the HF diet resulted in an obese phenotype by D60 consistent with previous observations (Zhao, 2013; Doucette et al., 2015; Reynolds et al., 2015). Continued exposure to the HF diet, through until D120 of life, was associated with changes in bone micro-architecture and bone marrow gene expression, including up-regulation of adipocyte and inflammatory genes, in adult male rats. Voluntary physical activity early in life was sufficient to cause significant changes in body composition in HF-fed rats at D60 such that the total fat percentage, lean mass percentage and fat:lean ratio resembled that of animals on the control diet. At D120, measures of bone microarchitecture and gene expression identified a pattern of gene transcription (i.e., a “transcriptional memory”) of the early-life exercise that lasted at least 60 days after cessation of wheel running activity. However, the transcriptional memory of early-life exercise did not prevent rebound to the obese phenotype, by D120, in the absence of continuing voluntary wheel running exercise.
Transcription levels for genes involved in mature adipocyte function and lipid metabolism (Adipoq, Cidec, Pck1, and Plin1) were increased in bone marrow of sedentary animals exposed to a HF diet. Both Plin1 and Cidec encode proteins that are lipid droplet-associated and predominantly expressed in adipose tissue (Konige et al., 2014) control many aspects of lipid metabolism including lipid droplet size, triglyceride storage, and lipolysis (Sun et al., 2013; Konige et al., 2014). Up-regulation of Cidec gene expression in response to a HF diet (60% of total energy from fat) has been previously reported within epididymal adipose tissue of wild-type C57B6 mice (Reynolds et al., 2015).
Bone marrow transcript levels from the Adipoq gene showed up-regulation in response to the HF diet, in contrast to previous reports of Adipoq gene expression decreasing in perigonadal white adipose tissue and epididymal fat pads in mice fed HF diets (Barnea et al., 2006; Bullen et al., 2006). This apparent contradiction may be due to differences in morphology and cellular composition of the different tissues (Trubowitz and Bathija, 1977; Bathija et al., 1979; Griffith et al., 2009), consistent with Adipoq mRNA and protein levels being significantly greater in bone marrow adipose than white adipose tissue depots in mice subjected to caloric restriction (Cawthorn et al., 2014). Therefore, we conclude that there are bone marrow adipose tissue-specific changes to Adipoq transcript levels in response to our HF diet.
Hypercaloric-induced obesity is associated with chronic low-grade inflammation, characterized by increased pro-inflammatory cytokine production (Gregor and Hotamisligil, 2011). Consistent with this, we observed a pro-inflammatory gene expression profile that included up-regulation of Il-1β, Ltb, and Ccl5 transcript levels within bone marrow of rats fed the HF diet. These inflammatory cytokines have roles in bone metabolism, and IL-1β has been shown to stimulate bone resorption (Nguyen et al., 1991; Pfeilschifter et al., 2009) in response to a HF diet (Shu et al., 2015). Similarly, LTB acts synergistically with TNF-α and IL-1β and is a potent stimulator of bone resorption (Stashenko et al., 1987). The observed increases in IL-1β, LTB and other pro-resorptive cytokines (e.g., TNF-α and RANKL) are consistent with the HF diet increasing bone resorption in our animals. However, further confirmation of this linkage requires empirical testing.
In response to the HF diet there were significant increases in cortical BMC, cortical vBMD, and trabecular vBMD, consistent with other reports that showed a positive association between HF diet and bone mass indices (Ma et al., 2010; Lecka-Czernik et al., 2015). These most likely resulted from increased mechanical loading or androgenic factors, while other reports indicate an inhibitory effect of HF diet associated with effects on the differences in bone formation and resorption (Lorincz et al., 2010; Cao and Picklo, 2015; Shu et al., 2015). There were no exercise-associated between-group differences in cortical bone mass parameters in the HF-fed animals. Critically, trabecular bone mass was significantly higher in the HF-EEX group than in either of the non-exercised groups.
Although, there were no significant phenotypic differences in the bone micro-architecture as a result of this post-puberty (D67−120) exercise in the HF animals, compared to the HF-SED group, this physical activity combined with lower caloric intake was associated with down-regulation of genes involved in inflammation, and of genes that promote osteoclastogenesis (i.e., Csf1r, Ccr1, and C5aR1). Transcript levels for Nrp1, which is osteoprotective through its enhancing effect on osteoblast differentiation, were upregulated. As such, post-puberty voluntary wheel running and lower caloric intake led to gene expression changes consistent with a reduction in the production of osteoclasts, which resorb bone, and an increase in osteoblasts, which produce it.
Voluntary pre-pubertal (D23−60) exercise affected the total fat and lean mass percentages in animals on a HF diet such that they were more similar to a control group on a standard chow diet. However, within 60 days of ceasing voluntary exercise, there was a return to an obese phenotype such that their total fat and lean mass percentages were not significantly different from sedentary animals fed a HF diet. This is consistent with the principle of training reversibility whereby the physiological adaptations induced by physical training are partially or completely lost when the training is stopped or markedly reduced (Mujika and Padilla, 2000). Similar observations of training reversibility have been observed previously in SD and Wistar rats on HF diets (Yasari et al., 2007; Sertié et al., 2015).
Pre-pubertal exercise was associated with significant differential transcript levels for 128 genes when compared to HF-fed sedentary animals. The difference is notable, as these two groups of animals received identical diets and exercise regimes between D67 and D120, and gene expression changes in responses begin hours not days or weeks after novel physical activity begins. This is consistent with a “programmed” and long-lived memory of the early-life voluntary exercise.
The mechanism of the memory remains to be determined and could be due to: (1) sustained changes to the cellular composition of the bone marrow; or (2) epigenetic changes that are affecting the program of gene expression. These mechanisms are not mutually exclusive and it is likely that the memory of the physical stimuli is retained through a combination of both compositional and epigenetic changes (Ntanasis-Stathopoulos et al., 2013; Marędziak et al., 2015). For example, reductions in bone marrow fat and increased numbers of mesenchymal stem cells have been identified in 4-week-old mice trained on a treadmill (Marędziak et al., 2015). Moreover, bone marrow mesenchymal stem cells undergo changes in their DNA methylation when stimulated by mechanical signals (Arnsdorf et al., 2010). While the transcript levels of the mouse Adipoq gene are linked to the obesity-dependent methylation status of its promoter (Kim et al., 2015), the ability of mechanical stimulation to influence the epigenetic profile of Adipoq and subsequent changes in gene expression has not been established. Future investigations should focus on the mechanisms through which Aqp7, Gpd1, Plin1, Adipoq, Fabp4, and Pck1 gene expression is modulated in response to mechanical stimulation, and for how long after cessation such modulation persists. Such studies will enable the elucidation of the mechanism(s) that lead to a memory of early-life exercise, which appears to offer possibilities to inhibit expression of some undesirable effects of HF diets on homeostasis in the longer term. Were such suppression preserved for even longer periods, it may be possible to attenuate or prevent some aspects of the early life programming that increase health risks in adulthood.
This study was designed to determine if early-life mild voluntary activity, as opposed to imposed moderate to high (and possibly aversive and/or stressful) physical activity, would have lasting effects on tissues, cells and genes. We used only male rats, because they are less active than females. Using both sexes would have increased inter-group variance in at least some outcome measures, due to sex-dependant differences in development of bone (Wang et al., 2003) and many other normal physiological processes ranging from neurodevelopment (Galea et al., 2013) feeding behavior (Fukushima et al., 2004) voluntary activity (Rosenfeld, 2017) to exercise-induced physiological cardiac hypertrophy (Foryst-Ludwig and Kintscher, 2013). Resource constraint is likely why especially initial studies cannot be conducted in both sexes concurrently, despite this obviously being desirable (Krizo and Mintz, 2015). Rats were housed as pairs, to optimize animal well-being in conformance with approval procedures, and this limited the correlation of individual rats' activity with other outcome measures. Also, we assumed spontaneous cage activity was similar across the five cages within a particular group at a particular age, since we lacked facilities to measure such activity, and this also limited ability to compare spontaneous cage activity with wheel activity. Now that we have shown a significant wheel exercise effect, further work is required to characterize the exercise undertaken in detail.
An unanticipated confounder in the current study was the reduced caloric intake in the HF-diet fed group that had access to voluntary exercise between D67 and D120. The combined effect of the reduction in caloric intake and exercise could be associated with the similarities in body weight, total fat, and lean mass percentages of the HF-LEX and chow-fed groups. However, the HF-fed late exercise group had greater whole body BMC (Rosenfeld, 2017), cortical and trabecular vBMD, cortical thickness and total metaphyseal vBMD than was observed in the chow-fed animals.
The biological significance of the study lies in the significant differences in gene expression patterns in regulatory pathways important in the understanding of obesity and its many effects. Gene expression of particular regulatory pathways was influenced by the early-life mild voluntary exercise, which was expected, but the persistence of their exercise-induced gene expression pattern into adulthood was not. The phenomenon indicates that some key aspects of the obese phenotype may be suppressed and others not. For instance, the whole body composition phenotype reverted to control values after exercise ceased, redolent of recent reports of the metabolically healthy obese phenotype (Roberson et al., 2014). This contrasts with the generally poor success of activity strategies to combat existing obesity (Marchesini et al., 2016). The nature of the world-wide obesity epidemic demands exploration of alternative strategies to reduce morbidity and soaring healthcare costs (Hammond and Levine, 2010), and assurance of early life activity is likely to be one with high chances of positive effects, even if limited to particular aspects of phenotype.
The possible translational significance in terms of human health is that our observations confirm previous data of early exercise effects in some rodent studies. The exercise was voluntary and of mild to moderate intensity. We avoided imposition of more intensive exercise since this would hardly be acceptable in strategies designed for encouraging musculo-skeletal activity in infants and young children, especially those born small for gestational age or from obese pregnancy, since such groups are predisposed to obesity and/or less than optimal bone development (Taylor and Poston, 2007; Chen et al., 2010). The opportunity for altering gene expression through increased musculoskeletal activity may be highly time (age)-sensitive within infancy and childhood, because gene and phenotype plasticity decline. Lack of detailed knowledge of when and how various levels of physical activity at young ages may affect regulation of gene expression is probably why results of exercise studies in young rodents are conflicting. The translational opportunity lies in determining which gene regulatory pathways can be effected by early life physical activity, and the specification of the physical activity regimens needed to elicit persistent effects. We conclude that some regulatory pathways related to preserving or restoring homeostasis in energy metabolism can be retained, even though whole body phenotype changes induced by such mild exercise were not retained after early-life exercise ceased. Aspects of the observed memory of early life exercise are supportive of both positive and negative consequences for later life disorders. Therefore, while there is no impact on rebound adiposity, we contend that the altered immune responses we observed will impact on future risks of inflammation-associated disease. Such impacts need to be empirically confirmed.
All sequencing data has been deposited in Gene expression omnibus (GSE97376).
DS: Investigation, writing-original draft preparation; MV, EF, JO: Conceptualization. MV, EF, and JO: writing- review and editing; MV, EF, JO: Supervision.
This work was funded by an Arthritis New Zealand grant to EF and MV. Work in JO laboratory is funded by HRC (15/604). DS received a Gravida postgraduate scholarship.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors thank Dr. Sue McGlashan, Mr. Sam Haysom, Dr. Johannes Willnecker, and Ms. Amorita Petzer for their generous discussions, technical expertise, and assistance.
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fphys.2017.00476/full#supplementary-material
Arnsdorf, E. J., Tummala, P., Castillo, A. B., Zhang, F., and Jacobs, C. R. (2010). The epigenetic mechanism of mechanically induced osteogenic differentiation. J. Biomech. 43, 2881–2886. doi: 10.1016/j.jbiomech.2010.07.033
Ayturk, U. M., Jacobsen, C. M., Christodoulou, D. C., Gorham, J., Seidman, J. G., Seidman, C. E., et al. (2013). An RNA-seq protocol to identify mRNA expression changes in mouse diaphyseal bone: applications in mice with bone property altering Lrp5 mutations. J. Bone Miner. Res. 28, 2081–2093. doi: 10.1002/jbmr.1946
Barnea, M., Shamay, A., Stark, A. H., and Madar, Z. (2006). A High-fat diet has a tissue-specific effect on adiponectin and related enzyme expression*. Obesity 14, 2145–2153. doi: 10.1038/oby.2006.251
Bathija, A., Davis, S., and Trubowitz, S. (1979). Bone marrow adipose tissue: response to acute starvation. Am. J. Hematol. 6, 191–198. doi: 10.1002/ajh.2830060303
Baxter-Jones, A. D., Faulkner, R. A., Forwood, M. R., Mirwald, R. L., and Bailey, D. A. (2011). Bone mineral accrual from 8 to 30 years of age: an estimation of peak bone mass. J. Bone Miner. Res. 26, 1729–1739. doi: 10.1002/jbmr.412
Beresford, J. N., Bennett, J. H., Devlin, C., and Owen, M. E. (1992). Evidence for an inverse relationship between the differentiation of adipocytic and osteogenic cells. Bone Miner. 17, 198. doi: 10.1016/0169-6009(92)92129-E
Boyce, B. F., Aufdemorte, T. B., Garrett, I. R., Yates, A. J. P., and Mundy, G. R. (1989). Effects of interleukin-1 on bone turnover in normal mice. Endocrinology 125, 1142–1150. doi: 10.1210/endo-125-3-1142
Bullen, J. W., Bluher, S., Kelesidis, T., and Mantzoros, C. S. (2006). Regulation of adiponectin and its receptors in response to development of diet-induced obesity in mice. AJP Endocrinol. Metab. 292, E1079–E1086. doi: 10.1152/ajpendo.00245.2006
Cao, J. J., and Picklo, M. J. (2015). Involuntary wheel running improves but does not fully reverse the deterioration of bone structure of obese rats despite decreasing adiposity. Calcif. Tissue Int. 97, 145–155. doi: 10.1007/s00223-015-9992-6
Cao, J. J., Gregoire, B. R., and Gao, H. (2009). High-fat diet decreases cancellous bone mass but has no effect on cortical bone mass in the tibia in mice. Bone 44, 1097–1104. doi: 10.1016/j.bone.2009.02.017
Cawthorn, W. P., Scheller, E. L., Learman, B. S., Parlee, S. D., Simon, B. R., Mori, H., et al. (2014). Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction. Cell Metab. 20, 368–375. doi: 10.1016/j.cmet.2014.06.003
Chen, H.-L., Lee, C.-L., Tseng, H.-I., Yang, S.-N., Yang, R.-C., and Jao, H.-C. (2010). Assisted exercise improves bone strength in very low birthweight infants by bone quantitative ultrasound. J. Paediatr. Child Health 46, 653–659. doi: 10.1111/j.1440-1754.2010.01822.x
David, V., Martin, A., Lafage-Proust, M.-H., Malaval, L., Peyroche, S., Jones, D. B., et al. (2007). Mechanical loading down-regulates peroxisome proliferator-activated receptor gamma in bone marrow stromal cells and favors osteoblastogenesis at the expense of adipogenesis. Endocrinology 148, 2553–2562. doi: 10.1210/en.2006-1704
Doucette, C. R., Horowitz, M. C., Berry, R., MacDougald, O. A., Anunciado-Koza, R., Koza, R. A., et al. (2015). A high fat diet increases bone marrow adipose tissue (MAT) but does not alter trabecular or cortical bone mass in C57BL/6J mice. J. Cell. Physiol. 230, 2032–2037. doi: 10.1002/jcp.24954
Ducher, G., and Bass, S. L. (2007). Exercise during growth: Compelling evidence for the primary prevention of osteoporosis? BoneKEy Osteovision 4, 171–180. doi: 10.1138/20070263
Foryst-Ludwig, A., and Kintscher, U. (2013). Sex differences in exercise-induced cardiac hypertrophy. Pflügers Arch. Eur. J. Physiol. 465, 731–737. doi: 10.1007/s00424-013-1225-0
Fukushima, N., Hanada, R., Teranishi, H., Fukue, Y., Tachibana, T., Ishikawa, H., et al. (2004). Ghrelin directly regulates bone formation. J. Bone Miner. Res. 20, 790–798. doi: 10.1359/JBMR.041237
Galea, L. A. M., Wainwright, S. R., Roes, M. M., Duarte-Guterman, P., Chow, C., and Hamson, D. K. (2013). Sex, hormones and neurogenesis in the hippocampus: hormonal modulation of neurogenesis and potential functional implications. J. Neuroendocrinol. 25, 1039–1061. doi: 10.1111/jne.12070
Garrett, I. R., Durie, B. G., Nedwin, G. E., Gillespie, A., Bringman, T., Sabatini, M., et al. (1987). Production of lymphotoxin, a bone-resorbing cytokine, by cultured human myeloma cells. N. Engl. J. Med. 317, 526–532. doi: 10.1056/NEJM198708273170902
Gasser, J. A. (2012). Bone measurements by peripheral quantitative computed tomography in rodents. Bone Res. Protoc. 80, 477–498. doi: 10.1007/978-1-61779-415-5_28
Gleeson, M., Bishop, N. C., Stensel, D. J., Lindley, M. R., Mastana, S. S., and Nimmo, M. A. (2011). The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 11, 607–615. doi: 10.1038/nri3041
Goulding, A., Jones, I. E., Taylor, R. W., Williams, S. M., and Manning, P. J. (2001). Bone mineral density and body composition in boys with distal forearm fractures: a dual-energy x-ray absorptiometry study. J. Pediatr. 139, 509–515. doi: 10.1067/mpd.2001.116297
Goulding, A., Taylor, R. W., Grant, A. M., Murdoch, L., Williams, S. M., and Taylor, B. J. (2008). Relationship of total body fat mass to bone area in New Zealand five-year-olds. Calcif. Tissue Int. 82, 293–299. doi: 10.1007/s00223-008-9121-x
Gregor, M. F., and Hotamisligil, G. S. (2011). Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 29, 415–445. doi: 10.1146/annurev-immunol-031210-101322
Griffith, J. F., Yeung, D. K. W., Ahuja, A. T., Choy, C. W. Y., Mei, W. Y., Lam, S. S. L., et al. (2009). A study of bone marrow and subcutaneous fatty acid composition in subjects of varying bone mineral density. Bone 44, 1092–1096. doi: 10.1016/j.bone.2009.02.022
Hammond, R., and Levine, R. (2010). The economic impact of obesity in the United States. Diabetes Metab. Syndr. Obes. Targets Ther. 3, 285–295. doi: 10.2147/dmso.s7384
Hayashi, M., Nakashima, T., Taniguchi, M., Kodama, T., Kumanogoh, A., and Takayanagi, H. (2012). Osteoprotection by semaphorin 3A. Nature 485, 69–74. doi: 10.1038/nature11000
Holy, X., and Zérath, E. (2000). bone mass increases in less than 4 wk of voluntary exercising in growing rats. Med. Sci. Sports Exerc. 32, 1562–1569. doi: 10.1097/00005768-200009000-00006
Ignatius, A., Schoengraf, P., Kreja, L., Liedert, A., Recknagel, S., Kandert, S., et al. (2011). Complement C3a and C5a modulate osteoclast formation and inflammatory response of osteoblasts in synergism with IL-1β J. Cell. Biochem. 112, 2594–2605. doi: 10.1002/jcb.23186
Ionova-Martin, S. S., Wade, J. M., Tang, S., Shahnazari, M., Ager, J. W., Lane, N. E., et al. (2011). Changes in cortical bone response to high-fat diet from adolescence to adulthood in mice. Osteoporos. Int. 22, 2283–2293. doi: 10.1007/s00198-010-1432-x
Järvinen, T. L. N., Pajamäki, I., Sievänen, H., Vuohelainen, T., Tuukkanen, J., Järvinen, M., et al. (2003). Femoral neck response to exercise and subsequent deconditioning in young and adult rats. J. Bone Miner. Res. 18, 1292–1299. doi: 10.1359/jbmr.2003.18.7.1292
Juonala, M., Magnussen, C. G., Berenson, G. S., Venn, A., Burns, T. L., Sabin, M. A., et al. (2011). Childhood adiposity, adult adiposity, and cardiovascular risk factors. N. Engl. J. Med. 365, 1876–1885. doi: 10.1056/NEJMoa1010112
Kim, A. Y., Park, Y. J., Pan, X., Shin, K. C., Kwak, S.-H., Bassas, A. F., et al. (2015). Obesity-induced DNA hypermethylation of the adiponectin gene mediates insulin resistance. Nat. Commun. 6, 7585. doi: 10.1038/ncomms8585
Konige, M., Wang, H., and Sztalryd, C. (2014). Role of adipose specific lipid droplet proteins in maintaining whole body energy homeostasis. Biochim. Biophys. Acta Mol. Basis Dis. 1842, 393–401. doi: 10.1016/j.bbadis.2013.05.007
Krizo, J. A., and Mintz, E. M. (2015). Sex differences in behavioral circadian rhythms in laboratory rodents. Front. Endocrinol. 5:234. doi: 10.3389/fendo.2014.00234
Lange, J., Barz, T., Ekkernkamp, A., Klöting, I., and Follak, N. (2013). Gene expression profile in bone of diabetes-prone BB/OK rats fed a high-fat diet. Genes Nutr. 8, 99–104. doi: 10.1007/s12263-012-0299-1
Lecka-Czernik, B., Stechschulte, L. A., Czernik, P. J., and Dowling, A. R. (2015). High bone mass in adult mice with diet-induced obesity results from a combination of initial increase in bone mass followed by attenuation in bone formation; implications for high bone mass and decreased bone quality in obesity. Mol. Cell. Endocrinol. 410, 35–41. doi: 10.1016/j.mce.2015.01.001
Lin, G. G.-H., and Scott, J. G. (2011). Investigations of the constitutive overexpression of CYP6D1 in the permethrin resistant LPR strain of house fly (Musca domestica). Pestic. Biochem. Physiol. 100, 130–134. doi: 10.1016/j.pestbp.2011.02.012
Lloyd, L. J., Langley-Evans, S. C., and McMullen, S. (2012). Childhood obesity and risk of the adult metabolic syndrome: a systematic review. Int. J. Obes. 36, 1–11. doi: 10.1038/ijo.2011.186
Lorincz, C., Reimer, R. A., Boyd, S. K., and Zernicke, R. F. (2010). High-fat, sucrose diet impairs geometrical and mechanical properties of cortical bone in mice. Br. J. Nutr. 103, 1302–1308. doi: 10.1017/S0007114509993084
Luu, Y. K., Capilla, E., Rosen, C. J., Gilsanz, V., Pessin, J. E., Judex, S., et al. (2009). Mechanical stimulation of mesenchymal stem cell proliferation and differentiation promotes osteogenesis while preventing dietary-induced obesity. J. Bone Miner. Res. 24, 50–61. doi: 10.1359/jbmr.080817
Ma, H., Torvinen, S., Silvennoinen, M., Rinnankoski-Tuikka, R., Kainulainen, H., Morko, J., et al. (2010). Effects of diet-induced obesity and voluntary wheel running on bone properties in young male C57BL/6J mice. Calcif. Tissue Int. 86, 411–419. doi: 10.1007/s00223-010-9346-3
Marchesini, G., Montesi, L., El Ghoch, M., Brodosi, L., Calugi, S., and Dalle Grave, R. (2016). Long-term weight loss maintenance for obesity: a multidisciplinary approach. Diabetes Metab. Syndr. Obes. Targets Ther. 9, 37–46. doi: 10.2147/dmso.s89836
Marędziak, M., Śmieszek, A., Chrząstek, K., Basinska, K., and Marycz, K. (2015). Physical activity increases the total number of bone-marrow-derived mesenchymal stem cells, enhances their osteogenic potential, and inhibits their adipogenic properties. Stem Cells Int. 2015, 1–11. doi: 10.1155/2015/379093
Mattsson, N., Rönnemaa, T., Juonala, M., Viikari, J. S. A., and Raitakari, O. T. (2008). Childhood predictors of the metabolic syndrome in adulthood. The cardiovascular risk in young finns study. Ann. Med. 40, 542–552. doi: 10.1080/07853890802307709
Menuki, K., Mori, T., Sakai, A., Sakuma, M., Okimoto, N., Shimizu, Y., et al. (2008). Climbing exercise enhances osteoblast differentiation and inhibits adipogenic differentiation with high expression of PTH/PTHrP receptor in bone marrow cells. Bone 43, 613–620. doi: 10.1016/j.bone.2008.04.022
Misra, A., Alappan, N. K., Vikram, N. K., Goel, K., Gupta, N., Mittal, K., et al. (2008). Effect of supervised progressive resistance-exercise training protocol on insulin sensitivity, glycemia, lipids, and body composition in Asian Indians with type 2 diabetes. Diabetes Care 31, 1282–1287. doi: 10.2337/dc07-2316
Mujika, I., and Padilla, S. (2000). Detraining: loss of training-induced physiological and performance adaptations. Part II: long term insufficient training stimulus. Sports Med. 30, 145–154. doi: 10.2165/00007256-200030030-00001
Nguyen, L., Dewhirst, F. E., Hauschka, P. V., and Stashenko, P. (1991). Interleukin-1 beta stimulates bone resorption and inhibits bone formation in vivo. Lymphokine Cytokine Res. 10, 15–21.
Ntanasis-Stathopoulos, J., Tzanninis, J. G., Philippou, A., and Koutsilieris, M. (2013). Epigenetic regulation on gene expression induced by physical exercise. J. Musculoskelet. Neuronal Interact. 13, 133–146.
Parhami, F., Jackson, S. M., Tintut, Y., Le, V., Balucan, J. P., Territo, M., et al. (1999). Atherogenic diet and minimally oxidized low density lipoprotein inhibit osteogenic and promote adipogenic differentiation of marrow stromal cells. J. Bone Miner. Res. 14, 2067–2078. doi: 10.1359/jbmr.1999.14.12.2067
Parhami, F., Tintut, Y., Beamer, W. G., Gharavi, N., Goodman, W., and Demer, L. L. (2001). Atherogenic high-fat diet reduces bone mineralization in mice. J Bone Miner. Res. 16, 182–188. doi: 10.1359/jbmr.2001.16.1.182
Pfeilschifter, J., Chenu, C., Bird, A., Mundy, G. R., and Roodman, D. G. (2009). Interleukin-1 and tumor necrosis factor stimulate the formation of human osteoclastlike cells in vitro. J. Bone Miner. Res. 4, 113–118. doi: 10.1002/jbmr.5650040116
Reynolds, T. H., Banerjee, S., Sharma, V. M., Donohue, J., Couldwell, S., Sosinsky, A., et al. (2015). Effects of a high fat diet and voluntary wheel running exercise on cidea and cidec expression in liver and adipose tissue of mice. PLoS ONE 10:e0130259. doi: 10.1371/journal.pone.0130259
Rizzoli, R., Bianchi, M. L., Garabédian, M., McKay, H. A., and Moreno, L. A. (2010). Maximizing bone mineral mass gain during growth for the prevention of fractures in the adolescents and the elderly. Bone 46, 294–305. doi: 10.1016/j.bone.2009.10.005
Roberson, L. L., Aneni, E. C., Maziak, W., Agatston, A., Feldman, T., Rouseff, M., et al. (2014). Beyond BMI: the “Metabolically healthy obese” phenotype & its association with clinical/subclinical cardiovascular disease and all-cause mortality – a systematic review. BMC Public Health 14:14. doi: 10.1186/1471-2458-14-14
Rosenfeld, C. S. (2017). Sex-dependent differences in voluntary physical activity. J. Neurosci. Res. 95, 279–290. doi: 10.1002/jnr.23896
Sabhaney, V., Boutis, K., Yang, G., Barra, L., Tripathi, R., Tran, T. T., et al. (2014). Bone fractures in children: is there an association with obesity? J. Pediatr. 165, 313.e1–318.e1. doi: 10.1016/j.jpeds.2014.04.006
Schmidt, M. D., Dwyer, T., Magnussen, C. G., and Venn, A. J. (2011). Predictive associations between alternative measures of childhood adiposity and adult cardio-metabolic health. Int. J. Obes. 35, 38–45. doi: 10.1038/ijo.2010.205
Sengupta, P. (2013). The laboratory rat: relating its age with human's. Int. J. Prev. Med. 4, 624–30.
Sertié, R. A. L., Andreotti, S., Proença, A. R. G., Campaña, A. B., and Lima, F. B. (2015). Fat gain with physical detraining is correlated with increased glucose transport and oxidation in periepididymal white adipose tissue in rats. Braz. J. Med. Biol. Res. 48, 650–653. doi: 10.1590/1414-431X20154356
Sertie, R. A. L., Andreotti, S., Proença, A. R. G., Campana, A. B., Lima-Salgado, T. M., Batista, M. L., et al. (2013). Cessation of physical exercise changes metabolism and modifies the adipocyte cellularity of the periepididymal white adipose tissue in rats. J. Appl. Physiol. 115, 394–402. doi: 10.1152/japplphysiol.01272.2012
Shu, L., Beier, E., Sheu, T., Zhang, H., Zuscik, M. J., Puzas, E. J., et al. (2015). High-fat diet causes bone loss in young mice by promoting osteoclastogenesis through alteration of the bone marrow environment. Calcif. Tissue Int. 96, 313–323. doi: 10.1007/s00223-015-9954-z
Skaggs, D. L., Loro, M. L., Pitukcheewanont, P., Tolo, V., and Gilsanz, V. (2001). Increased body weight and decreased radial cross-sectional dimensions in girls with forearm fractures. J. Bone Miner. Res. 16, 1337–1342. doi: 10.1359/jbmr.2001.16.7.1337
Sontam, D. M., Firth, E. C., Tsai, P., Vickers, M. H., and O'Sullivan, J. M. (2015a). Different exercise modalities have distinct effects on the integrin-linked kinase (ILK) and Ca2* signaling pathways in the male rat bone. Physiol. Rep. 3:e12568. doi: 10.14814/phy2.12568
Sontam, D. M., Vickers, M. H., O'Sullivan, J. M., Watson, M., and Firth, E. C. (2015b). Different short-term mild exercise modalities lead to differential effects on body composition in healthy prepubertal male rats. Biomed. Res. Int. 2015:404201. doi: 10.1155/2015/404201
Stashenko, P., Dewhirst, F. E., Peros, W. J., Kent, R. L., and Ago, J. M. (1987). Synergistic interactions between interleukin 1, tumor necrosis factor, and lymphotoxin in bone resorption. J. Immunol. 138, 1464–1468.
Sun, Z., Gong, J., Wu, H., Xu, W., Wu, L., Xu, D., et al. (2013). Perilipin1 promotes unilocular lipid droplet formation through the activation of Fsp27 in adipocytes. Nat. Commun. 4, 1594. doi: 10.1038/ncomms2581
Taylor, P. D., and Poston, L. (2007). Developmental programming of obesity in mammals. Exp. Physiol. 92, 287–298. doi: 10.1113/expphysiol.2005.032854
Thomson, B. M., Mundy, G. R., and Chambers, T. J. (1987). Tumor necrosis factors alpha and beta induce osteoblastic cells to stimulate osteoclastic bone resorption. J. Immunol. 138, 775–779.
Trapnell, C., Pachter, L., and Salzberg, S. L. (2009). TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25, 1105–1111. doi: 10.1093/bioinformatics/btp120
Trapnell, C., Williams, B. A., Pertea, G., Mortazavi, A., Kwan, G., van Baren, M. J., et al. (2010). Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 28, 511–515. doi: 10.1038/nbt.1621
Trubowitz, S., and Bathija, A. (1977). Cell size and plamitate-1-14c turnover of rabbit marrow fat. Blood 49, 599–605.
Tuomilehto, J., Lindström, J., Eriksson, J. G., Valle, T. T., Hämäläinen, H., Ilanne-Parikka, P., et al. (2001). Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N. Engl. J. Med. 344, 1343–1350. doi: 10.1056/NEJM200105033441801
Wang, L., McMahan, C. A., Banu, J., Okafor, M. C., and Kalu, D. N. (2003). Rodent model for investigating the effects of estrogen on bone and muscle relationship during growth. Calcif. Tissue Int. 72, 151–155. doi: 10.1007/s00223-001-1122-y
Wintges, K., Beil, F. T., Albers, J., Jeschke, A., Schweizer, M., Claass, B., et al. (2013). Impaired bone formation and increased osteoclastogenesis in mice lacking chemokine (C-C motif) ligand 5 (Ccl5). J. Bone Miner. Res. 28, 2070–2080. doi: 10.1002/jbmr.1937
Woo, D. G., Lee, B. Y., Lim, D., and Kim, H. S. (2009). Relationship between nutrition factors and osteopenia: effects of experimental diets on immature bone quality. J. Biomech. 42, 1102–1107. doi: 10.1016/j.jbiomech.2009.02.020
Xiao, Y., Cui, J., Li, Y.-X., Shi, Y.-H., and Le, G.-W. (2010). Expression of genes associated with bone resorption is increased and bone formation is decreased in mice fed a high-fat diet. Lipids 45, 345–355. doi: 10.1007/s11745-010-3397-0
Yan, L., Graef, G. L., Nielsen, F. H., Johnson, L. K., and Cao, J. (2015). Soy protein is beneficial but high-fat diet and voluntary running are detrimental to bone structure in mice. Nutr. Res. 35, 523–531. doi: 10.1016/j.nutres.2015.04.012
Yano, S., Mentaverri, R., Kanuparthi, D., Bandyopadhyay, S., Rivera, A., Brown, E. M., et al. (2005). Functional expression of β-chemokine receptors in osteoblasts: role of regulated upon activation, normal T cell expressed and secreted (RANTES) in osteoblasts and regulation of its secretion by osteoblasts and osteoclasts. Endocrinology 146, 2324–2335. doi: 10.1210/en.2005-0065
Yasari, S., Dufresne, E., Prud'homme, D., and Lavoie, J. M. (2007). Effect of the detraining status on high-fat diet induced fat accumulation in the adipose tissue and liver in female rats. Physiol. Behav. 91, 281–289. doi: 10.1016/j.physbeh.2007.03.012
Keywords: exercise, bone marrow, gene expression, memory
Citation: Sontam DM, Vickers MH, Firth EC and O'Sullivan JM (2017) A Memory of Early Life Physical Activity Is Retained in Bone Marrow of Male Rats Fed a High-Fat Diet. Front. Physiol. 8:476. doi: 10.3389/fphys.2017.00476
Received: 03 May 2017; Accepted: 21 June 2017;
Published: 07 July 2017.
Edited by:
Igor B. Mekjavic, Jožef Stefan Institute, SloveniaReviewed by:
Jörn Rittweger, Deutsches Zentrum für Luft- und Raumfahrt (DLR), GermanyCopyright © 2017 Sontam, Vickers, Firth and O'Sullivan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mark H. Vickers, bS52aWNrZXJzQGF1Y2tsYW5kLmFjLm56
Elwyn C. Firth, ZS5maXJ0aEBhdWNrbGFuZC5hYy5ueg==
Justin M. O'Sullivan, anVzdGluLm9zdWxsaXZhbkBhdWNrbGFuZC5hYy5ueg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.