- 1Univ Lyon, Université Claude Bernard Lyon1, Centre de Recherche en Neurosciences de Lyon (CRNL), INSERM U1028/Centre National de la Recherche Scientifique UMR5292 Team Olfaction: From Coding to Memory, Lyon, France
- 2Department of Biological Science, Florida State University, Tallahassee, FL, United States
- 3Program in Neuroscience, Florida State University, Tallahassee, FL, United States
- 4Institute of Molecular Biophysics, Florida State University, Tallahassee, FL, United States
Olfaction is a major sensory modality involved in real time perception of the chemical composition of the external environment. Olfaction favors anticipation and rapid adaptation of behavioral responses necessary for animal survival. Furthermore, recent studies have demonstrated that there is a direct action of metabolic peptides on the olfactory network. Orexigenic peptides such as ghrelin and orexin increase olfactory sensitivity, which in turn, is decreased by anorexigenic hormones such as insulin and leptin. In addition to peptides, nutrients can play a key role on neuronal activity. Very little is known about nutrient sensing in olfactory areas. Nutrients, such as carbohydrates, amino acids, and lipids, could play a key role in modulating olfactory sensitivity to adjust feeding behavior according to metabolic need. Here we summarize recent findings on nutrient-sensing neurons in olfactory areas and delineate the limits of our knowledge on this topic. The present review opens new lines of investigations on the relationship between olfaction and food intake, which could contribute to determining the etiology of metabolic disorders.
The Olfactory System is An Interface
According to its anatomical location, the olfactory system is well poised to be an interface, with the ability to gather and process information simultaneously from the external and internal environment.
Interaction with the External Environment
The traditional function of the olfactory system is to sense the external chemical world. Odors are inhaled directly into the nose following an orthonasal pathway, or come from the back part of the mouth following a retronasal pathway. Both pathways lead odors to the posterior part of the nasal cavity. Odors bind to protein receptors located in the ciliary membrane of olfactory sensory neurons (OSNs) within the olfactory epithelium (OE). Each OSN expresses only one type of olfactory receptor (Malnic et al., 1999; Serizawa et al., 2003). Odor/receptor association selectively activates OSNs in the OE. All OSNs expressing the same odorant receptor project their axons to one or two olfactory bulb (OB) glomeruli where OSN axons synapse with the dendrites of mitral cells (MCs); the second order olfactory neurons (Ressler et al., 1994; Vassar et al., 1994; Breer et al., 2006). The electrical signal is then transmitted to neuronal networks in the piriform cortex (PC). Olfaction thereby informs the central nervous system in real time about the chemical composition of the external environment prior to any visual or tactile information. This event allows the animal to anticipate and rapidly adapt its behavior when seeking food or when engaging in social or sexual behavior.
Interaction with the Internal Environment
The hypothalamus is the main central actor in food intake regulation. Internal signals carried by the blood inform various central areas about the body's fuel availability, which in turn implement appropriate behavioral and metabolic responses to physiological requirements. Orexigenic and anorexigenic signals, respectively, stimulate or inhibit food intake by modulating neuronal activity of hypothalamic nuclei. During fasting, the hypothalamus induces food intake in response to nutrient scarcity and high level of ghrelin released by the stomach. Alternatively, the hypothalamus suppresses feeding behavior when it detects insulin secretion from the pancreas, leptin secretion from the adipose tissue, and nutrient abundance (Blouet and Schwartz, 2010; Berthoud, 2011). Interestingly, the olfactory system is also becoming widely considered as an active sensor of internal signaling (hormones, micronutrients availability). Olfactory structures like the OE, OB, and PC (Palouzier-Paulignan et al., 2012) express high levels of various hormone receptors (insulin, leptin, ghrelin, CCK) similar to that of the hypothalamus (Figure 1). When targeting their receptors, metabolic hormones modulate the electrical activity of olfactory networks (Fadool et al., 2000, 2011; Apelbaum et al., 2005; Hardy et al., 2005; Lacroix et al., 2008; Savigner et al., 2009; Kuczewski et al., 2014). OB neurons respond not only to peptides, but they also respond to glucose and express molecular hallmarks of glucose sensing cells (Tucker et al., 2010, 2013; Aimé et al., 2014; Al Koborssy et al., 2014; Kovach et al., 2016).
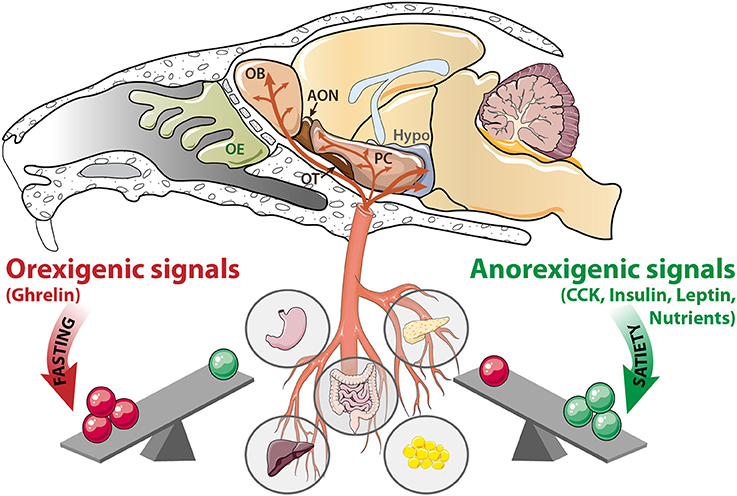
Figure 1. The olfactory system is a metabolic sensor like the hypothalamus. According to the nutritional status, a balance exists between peripheral signals delivered by the stomach, intestine, liver, pancreas, and adipose tissue. During fasting, orexigenic signals (ghrelin, and nutrients scarcity) prevail. In contrast, during satiation, anorexigenic signals (CCK, insulin, leptin and nutrients abundance) are predominant. These signaling molecules reach the central nervous system via the blood flow, where they target the hypothalamus (Hypo) as well as a variety of olfactory structures: OE, olfactory epithelium; OB, olfactory bulb; AON, anterior olfactory nucleus, OT, olfactory tubercle; PC: piriform cortex; CCK, cholecystokinin.
The metabolic sensing function of the OB is consistent with its high density of capillary network (Chaigneau et al., 2007) and its vascular properties. The blood brain barrier of the OB is not as tight as it is in the cerebral cortex or other brain regions (Ueno et al., 1991, 1996), indicating that blood-borne metabolic signals can enter the OB more easily than other brain regions. The permeable blood barrier facilitates transport of intravascular macromolecules, including nutrients and peripheral hormones, and their direct action on the OB. This enhanced permeability allows adaptation of olfactory perception to the physiological state: highly sensitive when the animal is fasted and needs to find food, and slightly sensitive when the animal is satiated (Aimé et al., 2007, 2012; Julliard et al., 2007; Prud'homme et al., 2009; Tong et al., 2011). Based upon its sensitivity to metabolic hormones and glucose availability, the olfactory system is proposed to be a metabolic sensor.
The present review provides an updated outlook of nutrient sensing in olfactory structures. We argue that in addition to being glucose-sensitive (Tucker et al., 2010, 2013; Aimé et al., 2014; Al Koborssy et al., 2014; Kovach et al., 2016) olfactory structures are sensors of amino acids (AAs) and potentially of fatty acid (FA) content of the internal medium.
Transmembrane Protein Families Involved in Nutrient Sensing
In contrast to unicellular organisms, most eukaryotic cells are not directly exposed to changes in environmental nutrients. Nevertheless, nutrient homeostasis is essential for all living organisms to maintain constant fuel supply despite discontinuity in food intake. Nutrient scarcity and abundance exert a strong pressure on the selection of efficient mechanisms for nutrient sensing in mammalian cells including central neurons. However, the molecular nature of brain nutrient sensors has only recently started to be deciphered. The present review focuses on sensors that are present in olfactory areas. In particular, we present two major sensing mechanisms that involve either the family of solute carrier (SLC) transporters (called T in Figure 2) or receptors having seven or two transmembrane domains (called R in Figure 2).
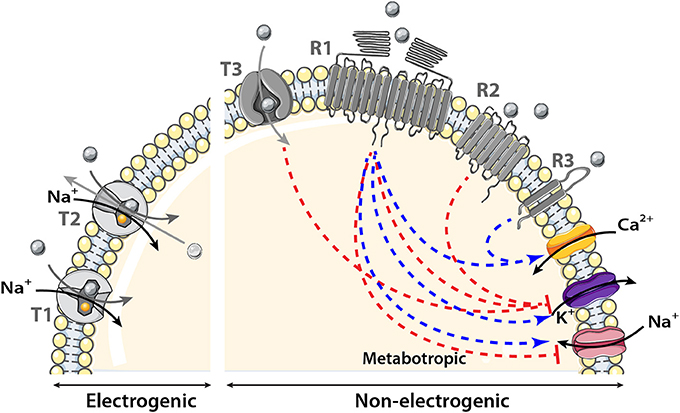
Figure 2. Schematic model showing the transmembrane proteins involved in nutrient sensing. The main transporter (T) family involved in nutrient sensing is the solute carrier (SLC) transporter family. It couples the movement of the nutrient (gray circle) to that of another molecule or ion crossing the membrane either in the same (symporter) named T1 in the figure or opposite direction (antiporter or exchanger) named T2 in the model. Nutrient influx down SLC transporters is called electrogenic when associated with a net inward of ion of Na+ of sufficient magnitude to cause direct membrane depolarization. Transport is non-electrogenic when it activates intracellular cascades that in turn depolarizes the membrane for example via K+ conductance inhibition. The two receptor (R) families involved in nutrient sensing are: the large receptor family of seven transmembrane domains (7TM) named R1 and R2 in the figure and the smaller family of two transmembrane domains (2TM) named R3 in the schematic model. The main receptor family is composed of 7TM it could be observed as heterodimer, homodimer (R1) or monomer (R2). Nutrients binding to their receptors activate an intracellular cascade which induces membrane depolarization by activating (blue arrow) a Na+ influx or by inhibiting (red line) K+ conductance or hyperpolarization by the reverse events. Metabotropic (via intracellular cascades) activation and inhibition of ion channels induced by nutrients are represented by the blue and red dotted lines respectively.
In the first mechanism, the sensed molecule is transported intracellularly. Numerous transmembrane protein transporters belonging to the SLC superfamily have been associated with nutrient sensing that control feeding, energy expenditure, and counterregulation (Marty et al., 2007; Gonzalez et al., 2009; Routh, 2010; Broer, 2014). The SLC superfamily mediates passage of nutrients across the phospholipid bilayer via passive transport, in which the nutrient moves down its concentration gradient, or via active transport (or co-transport) that couples the movement of the nutrient to that of another molecule or ion crossing the membrane either in the same (symporter) or opposite direction (antiporter or exchanger). As a result, the membrane potential can be modulated directly when the sensed molecule is co-transported with ions (electrogenic transport) or indirectly when the sensed molecule activates an intracellular cascade which, in turn, modulates ion channel permeability (non-electrogenic transport).
In the second sensing mechanism, the sensed molecule binds to its transmembrane receptor and activates an intracellular cascade to depolarize the membrane through activation of Na+ and/or Ca2+ inflow or inhibition of K+ conductance (Lindemann, 2001; Chaudhari and Roper, 2010). In nutrient sensing, the most important transmembrane receptors belong to the seven transmembrane (7TM) G protein–coupled receptors (GPCRs) family and are activated by glucose, AAs, or FAs. These 7TM receptors are expressed in central nervous areas involved in energy homeostasis regulation (Wellendorph et al., 2010). The 7TM receptors exist across the phospholipid bilayer as homodimers, heterodimers, or monomers. It is noteworthy that a 2TM receptor called cluster of differentiation 36 (CD36), is often associated with FAs transporters in the hypothalamus (Doege and Stahl, 2006; Magnan et al., 2015).
Glucose Sensing
Physiological Role of Glucose Supply to the Brain
Glucose is the primary metabolic substrate for the brain and a continuous supply of glucose is required for normal neuronal function (Mergenthaler et al., 2013). The brain accounts for 2% of the total body mass but requires 10 times more energy in the resting state compared to other energy consumption needs of the body (Mink et al., 1981; Molina and DiMaio, 2012). Glucose metabolism provides the fuel for physiological brain function through the generation of ATP that serves for the basic maintenance of cellular processes such as cytoskeletal dynamics, DNA repair, protein turnover, and growth. More specifically, during neuronal activation, the brain consumes a lot of energy in order to maintain the turnover of glutamate through metabolic neuron-astrocyte interactions (Magistretti and Allaman, 2015). Furthermore, 80% of total energy consumption fuels the Na+/K+ ATPase pump but <10% is used to recycle second messengers and neurotransmitters (Laughlin, 2001).
Glucose supply is critical for physiology, therefore a tight regulation between supply and demand is required. Several brain areas, such as the hypothalamus, brainstem, amygdala, septum, hippocampus, cortex, and OB contain glucose sensing neurons (Anand et al., 1964; Oomura et al., 1969; Ritter et al., 1981; Nakano et al., 1986; Shoji, 1992; Balfour et al., 2006; Tucker et al., 2013). These specialized neurons respond to local fluctuations in extracellular glucose levels, and modulate their mean firing rate accordingly. Glucose sensing neurons have been classified as “glucose-excited” (GE) or “glucose-inhibited” (GI) depending on whether they increase or decrease action potential frequency in response to extracellular glucose variations (McCrimmon, 2008; Gonzalez et al., 2009). GE and GI neurons integrate fluctuations in whole-body metabolic signals related to feeding behavior (Routh et al., 2007).
Several transporters, receptors, and ion channels are expressed in glucose sensing neurons of olfactory structures. Our laboratories and others have studied the role of the sodium-dependent glucose transporters (SGLTs), glucose transporters (GLUTs), potassium channels, and the insulin receptor (IR) in sensing glucose.
Sensing Role of Glucose in Olfactory Structures: Molecular Hallmarks
Glucose Transporters Expressed in Olfactory Structures
Electrogenic solute carrier transporter (SGLT1)
The family of sodium-dependent glucose transporters (SGLTs), also named SLC5, belongs to the SLC super family and uses a Na+ gradient to transport glucose against its concentration gradient into the cell. To date, six SGLTs isoforms have been identified (Wright and Turk, 2004). SGLT1 can modify its conformation to first release the two Na+ ions intracellularly while transporting glucose against its concentration gradient albeit in a symport orientation (Figure 3).
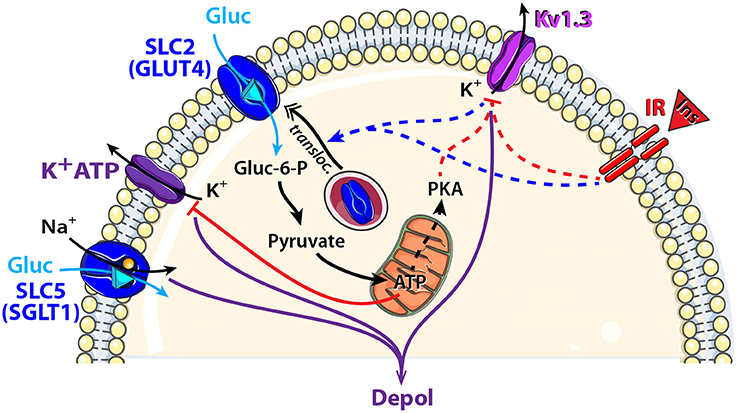
Figure 3. Schematic model showing glucose sensing signaling pathways that might modulate neuronal activity of central olfactory areas. Two types of glucose transporters and their associated downstream cellular processes are observed in central olfactory areas. SGLT1, located in the OB, is electrogenic and combines glucose (Gluc: blue triangle) translocation with an influx of Na+. GLUT4, located mainly in the OB and PC, is non-electrogenic and is associated with the insulin pathway. Indeed, insulin (Ins, red triangle) binding to its receptor (IR: insulin receptor) depolarizes MCs through Kv1.3 channel closure and induces GLUT4 translocation to the membrane. Glucose intake increases as well as the mitochondrial production of ATP and the cytosolic protein kinase A (PKA). Activation: blue arrow, inhibition: red line. Direct and indirect action of one molecule: full and dotted line respectively.
In the brain, SGLT1 has been found mainly in the hypothalamus, hippocampus, amygdala and OB (Kang et al., 2004; Yu et al., 2010; Aimé et al., 2014; Al Koborssy et al., 2014). In the OB, we found strong staining of SGLT1 in the inner part of the external plexiform layer (iEPL), in some mitral cells (MCs) and in some glomeruli (Al Koborssy et al., 2014). The iEPL is the site of reciprocal dendro-dendritic synapses between the secondary dendrites of MCs and the dendritic spines of inhibitory granule cells; this inhibitory interaction modulates odor information including olfactory discrimination (Yokoi et al., 1995; Lledo et al., 2005; Abraham et al., 2010). The availability of inhibitory control over MCs combined with the presence of rapidly activating SGLTs in the iEPL could explain the inhibitory response to glucose observed in the GI class of MCs (Tucker et al., 2013).
Non-electrogenic solute carrier transporter (GLUT4)
The Na+-independent GLUTs family (gene family slc2a) transports glucose across biological membranes. GLUTs comprise 14 family members and exhibit diverse substrate and tissue specificity resulting in distinct functional characteristics. GLUT1 exists as two isoforms in the brain and is exclusively expressed in endothelial cells and astrocytes. GLUT3 is localized to the neuropil and is largely absent from neuronal cell bodies (McCall et al., 1994; Gerhart et al., 1995) while GLUT4 exhibits a somato-dendritic labeling. The more discrete presence of GLUT4 compared with other GLUTs suggests that GLUT4 may be involved in highly specialized activities in the central nervous system. GLUT4 is consistently colocalized with IR and glucose transport through GLUT4 is the rate-limiting step in insulin-stimulated glucose uptake in the brain including olfactory areas (Alquier et al., 2006). Interestingly, 75% of GE neurons in the central nervous system coexpress GLUT4 and the IR mRNA (Kang et al., 2004).
The olfactory system has been found to express GLUT1 in the OE (Nunez-Parra et al., 2011), whereas GLUT1, GLUT3, and GLUT4 have been reported in the central olfactory areas (Brant et al., 1993; Leloup et al., 1996; El Messari et al., 1998, 2002; Vannucci et al., 1998; Dobrogowska and Vorbrodt, 1999; Choeiri et al., 2002; Al Koborssy et al., 2014). GLUT4 and IR are found to be localized in the main central olfactory areas such as the OB, PC, anterior olfactory nucleus (AON), and olfactory tubercle (OT) (Unger et al., 1989; Marks et al., 1990; El Messari et al., 1998; Schulingkamp et al., 2000; Alquier et al., 2006; Aimé et al., 2012, 2014). In a previous study, we have shown that GLUT4 is co-localized with IR in MCs and glomeruli of the OB. Interestingly, subcellular localization of GLUT4 is modulated by the feeding state. During the postprandial period when glucose levels in the blood are high, GLUT4 is found on the plasma membrane of dendritic processes. Following a fast however, it becomes internalized into the cytoplasm (Al Koborssy et al., 2014).
The dynamic expression of GLUT4 within MCs can be regulated by two complementary mechanisms (Figure 3). First, we observed that the feeding state-dependent modulation of GLUT4 subcellular localization in the OB correlates with the feeding state-dependent fluctuations of insulin levels in the OB as insulin was 2 fold higher in fed rats compared to fasted rats (Aimé et al., 2012). We infer that insulin levels increase in the OB during satiety to stimulate translocation of GLUT4 storage vesicles to the plasma membrane thereby increasing glucose uptake. Second, subcellular expression of GLUT4 can be regulated by the voltage-dependent potassium channel, Kv1.3 (Xu et al., 2004; Kovach et al., 2016). Blocking Kv1.3 conductance by applying a specific inhibitor (margatoxin) to cultured adipocytes or by co-transfecting GLUT4 and a non-conducting pore form of the channel in human embryonic kidney cells, increases plasma membrane expression of GLUT4 (Xu et al., 2004; Kovach et al., 2016). Gene-targeted deletion of Kv1.3 channel renders glucose-sensitive MCs non-responsive to glucose modulation in terms of action potential firing frequency (Tucker et al., 2013). Kv1.3 was further hypothesized to act as an insulin receptor substrate in MCs whereby IR activation phosphorylates the channel and suppresses its peak current (Fadool et al., 2000). It results that insulin-dependent or -independent blockade of Kv1.3 increases glucose translocation to the membrane.
While GLUT4 is highly expressed in MCs, and these neurons are clearly able to sense changes in glucose concentration either experimentally or evoked by nutritional state in vivo, the steps linking glucose entry to the change in firing pattern of MCs are yet unknown. We speculate that glucose sensing of MCs might use similar molecular means as reported for glucose sensing of the hypothalamus (Ashford et al., 1990; Spanswick et al., 1997; Ashcroft and Gribble, 1999; Song et al., 2001). In addition to KATP, other transporters like the Na+/K+ ATPase pump (Oomura, 1983; Silver and Erecinska, 1998), and the cystic fibrosis transmembrane conductance regulator chloride channel (Hwang and Sheppard, 1999; Song et al., 2001) could elicit either depolarization or hyperpolarization of a neuron during extracellular glucose fluctuation.
Further studies are required to elucidate (i) if glucose transport across MCs recruits an electrogenic symport of Na+, (ii) if the metabolic product of glucose (ATP) acts on downstream ion channels similar to mechanisms observed in the hypothalamus or (iii) if byproducts of glucose metabolism could phosphorylate Kv1.3 through ATP, cAMP, or PKA (Lewis and Cahalan, 1995; Dalle et al., 2013).
Metabolic Dysfunction and Glucose Sensors in Olfactory Areas
A variety of functions have been suggested for central glucose sensing neurons. Glucose sensing neurons are involved (i) in maintaining local energy requirements for synaptic transmission and (ii) in regulating whole body energy and glucose homeostasis. Glucose not only serves as a metabolic substrate but also alters neuronal activity linked to metabolism. Therefore, it's suggested that correct functioning of glucose sensing neurons would be essential to prevent metabolic disorders such as obesity and Type 2 diabetes mellitus but also stroke and other neurodegenerative disorders where neuronal energy supply is disrupted (Routh et al., 2007).
Central olfactory areas such as the OB and PC, have an expensive energy budget in terms of glucose metabolism, which is high during odor stimulation and increases further during coding and processing of olfactory information (Nawroth et al., 2007; Gire et al., 2013; Litaudon et al., 2017). Given that, we previously established a link between feeding states and olfactory performance, and adding the dynamic changes in GLUT4 expression, insulin levels, and the numerous metabolic hormones present in the OB, we suggest that glucose sensing neurons are keys regulators of metabolic-dependent olfactory behavior.
In rodents, the concentration, expression, and activity of several molecules involved in glucose-sensing in olfactory areas are not only modified with feeding behavior but they are also altered by metabolic pathologies and their subsequent nutritional imbalance. In the OB, insulin concentration and GLUT4 expression are feeding-dependent but SGLT1 and IR expression are not (Aimé et al., 2012; Al Koborssy et al., 2014). In commonly used rodent models of obesity and type 2 diabetes, insulin concentration is elevated and SGLT1 is upregulated in the OB. Moreover, IR expression is down regulated but GLUT4 remained affected in both the OB and PC (Livingston et al., 1993; Vannucci et al., 1998; Aimé et al., 2014). Rodent models of obesity further display increased olfactory sensitivity and discrimination (Aimé et al., 2014; Chelminski et al., 2017).
We propose that dysregulation of glucose sensing markers could induce an increase in olfactory sensitivity which could lead to hyperphagia and metabolic disorders. These results suggest that dysfunctional glucose sensing neurons in the OB could alter olfactory pathways crucial to the regulation of food intake.
Amino Acid Sensing
Physiological Role of Amino Acid Supply to the Brain
Amino acids (AAs) play a key physiological role as building blocks of proteins. Proteins not only play a structural role in the organism but they are involved in various metabolic processes, including enzymatic reactions. Among the 20 AAs that serve for protein synthesis, 10 are referred to as the essential AAs because they are acquired only from the diet and cannot be stored in the body. AA supply requires numerous membrane transporters and receptors that are tissue specific. Each carrier recognizes several AAs having structural similarities. In this manner, one AA is transported inside cells through multiple carriers with overlapping specificities (Taylor, 2014).
AAs are key regulators of metabolism (Wu, 2009). Homeostatic regulation of AA level is necessary to adapt AA concentration (essential and non-essential AAs) to physiological body requirements. In order to maintain an adequate AA supply, the hypothalamus senses AA notably through leucine detection that signals AA abundance and directly regulates food intake. Leucine intake activates the mammalian target of rapamycin complex 1 (mTORC1) and inhibits AMP-activated protein kinase (AMPK) in order to regulate protein translation and to reduce food intake (Cota et al., 2006; Ropelle et al., 2008). Indeed, central injection of leucine in the ventromedial hypothalamic nucleus has an anorectic effect through activation of a hypothalamic-brainstem circuit (Cota et al., 2006; Blouet et al., 2009; Haissaguerre et al., 2014). The nature of ingested AAs is also a very important parameter. Animals reject diet imbalanced in essential AAs, and forage for food with adequate AA content (Morrison et al., 2012; Anthony and Gietzen, 2013).
In the brain, AAs sensing could also implicate membrane receptors of GPCR family including the taste heterodimer receptor family (T1R1/T1R3) (Hoon et al., 1999; Li et al., 2002; Nelson et al., 2002) and CasR receptors (Conigrave et al., 2002).
The olfactory system plays a major role in AA sensing. The most studied mechanism uses SLC transporters but some receptors might also be implicated.
Sensing Role of Amino Acids in Olfactory Structures: Molecular Hallmarks
Amino Acid Transporters Expressed in Olfactory Structures
This chapter will focus attention on selected transporters that are known to be involved in metabolic regulation and are expressed in olfactory areas: the electrogenic transporters encoded by the slc6a15, slc38a2, and slc1a5 genes and the non-electrogenic transporters encoded by slc7a5 (Figure 4A).
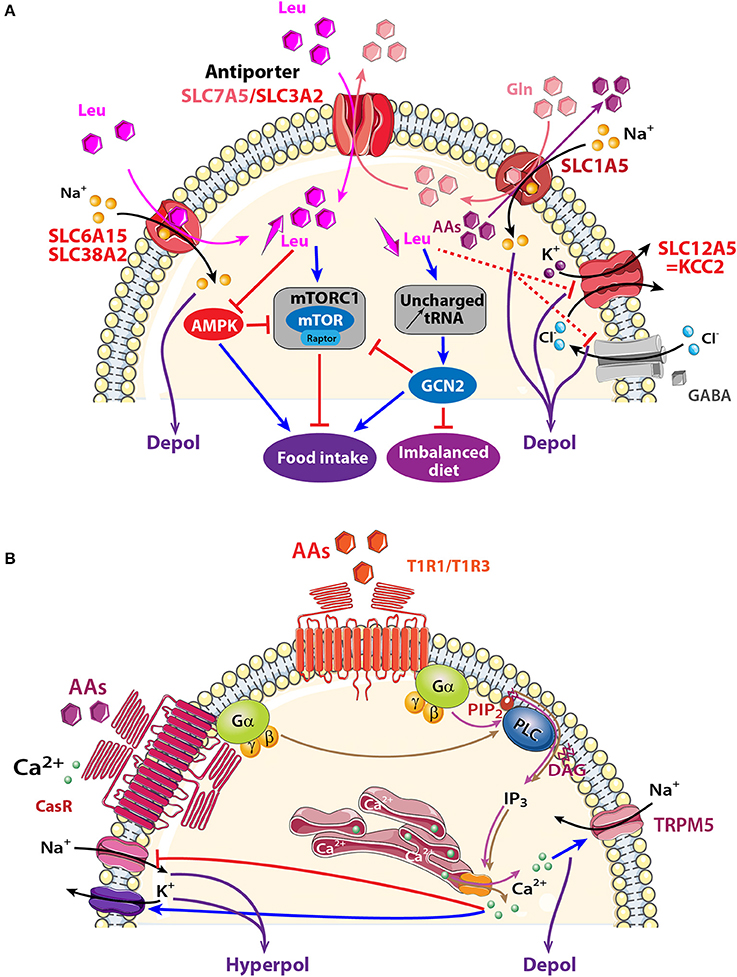
Figure 4. Schematic model showing AA sensing signaling pathways that might modulate neuronal activity of central olfactory areas. (A) Three electrogenic transporters (SLC6A15, SLC38A2, and SLC1A5) and one non-electrogenic antiporter SLC7A5/SLC3A2 are observed in the OB and the PC. AAs fluxes depend on physiological needs, on the importance of transported AAs (essential or non-essential), and on the cellular gradient of AAs. When leucine (Leu) and glutamine (Gln) are highly available, they are co-transported with sodium inside the cell through SLC6A15, SLC38A2 or SLC1A5. Intracellular Gln is in turn co-exchanged with Leu via the bidirectional antiporter SLC7A5/SLC3A2. The anterior PC (APC) detects essential AA deficiency that increases uncharged tRNA and activates the general amino acid control non-derepressible 2 (GCN2) pathway. The concomitant down regulation of GABAA receptor and KCC2 transporter disinhibits the APC that send messages to nutritional brain areas in order to stop eating the imbalanced diet. Signaling proteins of the mammalian target of rapamycin complex1 (mTORC1) and AMP-activated protein kinase (AMPK) pathways are also present in olfactory areas, which suggests that these structures could also be implicated in detecting AA abundancy or scarcity and indirectly modulating food intake. (B) Two AA receptors are described: T1R1/T1R3, and CasR receptors. Both are G-protein-coupled receptors and AA binding activates heterotrimeric GTP-binding proteins composed of α-gustducin (Gα) and Gβγ subunits (brown and pink arrows). Gαpromotes phosphatidylinositol phosphate 2 (PIP2) activation of phospholipase C (PLC), leading to the production of inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (D). IP3 opens ion channels on the endoplasmic reticulum, releasing Ca2+ into the cytosol of cells. Depending on the specific ion channels present on the membrane, a cell could be depolarized after melastatin-related transient receptor potential (TRPM5) channel opening or could be hyperpolarized after Na+ channel closure (red line) or Ca2+-dependent-K+ channel opening (blue arrow). AAs: hexagons; activation: blue arrow, inhibition: red line. Direct and indirect action of one molecule: full and dotted line respectively.
Electrogenic solute carrier transporters (SLC6A15, SLC38A2, SLC1A5)
At least three electrogenic AA transporters are observed in olfactory areas (Figure 4A). They displace AAs together with Na+ and induce a subsequent depolarization.
Two of them, SLC6A15 and SLC38A2 transport small neutral AAs like leucine, isoleucine, and valine together with Na+ in a 1:1 stoichiometry (Yao et al., 2000; Mackenzie and Erickson, 2004; Broer et al., 2006; Hagglund et al., 2013). SLC6A15 is present in the OB, AON, and endopiriform and piriform cortices (Inoue et al., 1996; Masson et al., 1996; Drgonova et al., 2013; Hagglund et al., 2013; Allen Institute for Brain Science, 2015). SLC38A2 mRNA is three times higher in the OB than other brain areas like the hippocampus, hypothalamus, cortex, or PC (Sundberg et al., 2008; Allen Institute for Brain Science, 2015). SLC38A2 is associated with the general amino acid control non-derepressible 2 (GCN2) pathway (Blais et al., 2003; Palii et al., 2006; Gietzen and Aja, 2012; Taylor, 2014). This pathway is activated when essential AAs are deficient causing accumulation of uncharged tRNA (Zhang et al., 2002; Maurin et al., 2005; Gietzen and Aja, 2012). One or two hours after AA reduction, SLC38A2 synthesis is upregulated in order to increase AA uptake (Blais et al., 2003; Palii et al., 2006; Gietzen and Aja, 2012; Taylor, 2014). Deficiency in essential AAs affects the PC where it causes downregulation of GABAA receptors and the K+/Cl− co-transporter (KCC2), also known as SLC12A5 (Sharp et al., 2013). KCC2 is localized in GABAergic neurons in the OB and PC (Wang et al., 2005; Sharp et al., 2013). The PC is thus identified as the central structure that detects imbalanced diet lacking essential AAs. PC activation interrupts protein synthesis in 20 min and stops food intake in animals to promote foraging for a more appropriate diet (Leung et al., 1968; Koehnle et al., 2003; Gietzen and Aja, 2012; Morrison et al., 2012).
The third transporter, SLC1A5, is an antiport that exchanges one Na+ and glutamine against neutral AAs in a 1:1 stoichiometry (Kanai and Hediger, 2004; Nicklin et al., 2009; Pochini et al., 2014). SLC1A5 has long been considered an electroneutral transporter (Utsunomiya-Tate et al., 1996) but recently Scalise and collaborators suggested that more than one Na+ might be transported (Scalise et al., 2014). A wide distribution of the slc1a5 is shown in MCs and the glomerular layer of the OB, and in the PC (Allen Institute for Brain Science, 2015). Glutamine and leucine intake through SLC1A5, together with SLC7A5/SLC3A2 (described in the next section), are proposed to be upstream steps of mTORC1 activation (Nicklin et al., 2009). The presence of these transporters in olfactory structures together with molecules involved in the mTORC1 pathway, such as raptor (Bar-Peled and Sabatini, 2014; Haissaguerre et al., 2014) makes it compelling to look for looking for AAs sensing through activation of the mTORC1 pathway in the olfactory system.
Non-electrogenic solute carrier transporter (SCL7A5/SLC3A2)
SLC7A5 is associated covalently with the glycoprotein SLC3A2. Both SLC7A5 and SLC3A2 are expressed in the OB, hippocampus, and hypothalamus (Kageyama et al., 2000; Allen Institute for Brain Science, 2015). SLC7A5/SLC3A2 is an AA exchanger that combines efflux of glutamine to influx of large neutral AAs like leucine with a 1:1 stoichiometry. Intracellular AA availability limits its transport rate given the low affinity of the intracellular domain of the transporter compared with its extracellular domain (Meier et al., 2002; Verrey, 2003). The net transport of AAs through SLC7A5/SLC3A2 is linked with electrogenic AA transporters like SLC1A5 that provides intracellular AAs for SLC7A5/SLC3A2 functioning. As a consequence, a reduced influx of glutamine through electrogenic transporters could limit leucine influx through SLC7A5 and consequently block the mTORC1 pathway (Verrey, 2003; Nicklin et al., 2009; Taylor, 2014).
Amino Acid Receptors Expressed in Olfactory Structures
Taste Receptor Family (T1R1/T1R3) Expressed in Olfactory Structures
Taste buds of the tongue express the heterodimer receptor (T1R1/T1R3) belonging to a GPCR family that detects essential AAs (Hoon et al., 1999; Li et al., 2002; Nelson et al., 2002). Tas1r1 and Tas1r3 genes encoding for this receptor, and their associated G-proteins are found in a variety of central areas including the OB, hypothalamus and hippocampus, (Ren et al., 2009; Allen Institute for Brain Science, 2015; Voigt et al., 2015). Most members of the IP3 transduction pathway triggered by T1R1/T1R3 activation in the taste buds and the cation channel TRPM5 (Chaudhari et al., 2009; Chaudhari and Roper, 2010) are present in the OE, OB, and PC (Ross et al., 1989; Lin et al., 2007; Rolen et al., 2014; Allen Institute for Brain Science, 2015; Pyrski et al., 2017). In the future, studying the role played by T1R1/T1R3 in olfactory areas will be interesting in the context of AAs sensing (Figure 4B).
Calcium Receptor Family (CasR) Expressed in Olfactory Structures
The localization and function of CasR in olfactory structures is species variant. In the OE of fish, CasR has the capacity to detect environmental Ca2+ and nutrients (Loretz, 2008). In rats, CasR transcript is expressed in the OB, AON and PC (Rogers et al., 1997; Ferry et al., 2000; Yano et al., 2004; Mudo et al., 2009). CasR is a multimodal receptor and it has been proposed to contribute to Ca2+ homeostasis and AA transport in neurons (Conigrave et al., 2002). When extracellular Ca2+ concentration reaches a threshold, CasR cooperatively binds to Ca2+ and to aromatic, aliphatic, or polar AAs (Conigrave et al., 2002; Conigrave and Hampson, 2006). Various intracellular pathways, including the downstream IP3 pathway, are activated to release internally stored Ca2+ (Hofer, 2005; Zhang et al., 2015). Excitability is reduced by opening Ca2+-dependent potassium channels and closing sodium channels (Han et al., 2015; Jones and Smith, 2016). The presence of CasR in olfactory structures together with components of IP3 pathway are good cues to investigate in the future if this transport allows olfactory structures to sense AAs.
Metabolic Dysfunction and Amino Acid Sensors in Olfactory Areas
Taken together, the fact that olfactory areas express transporters, receptors and intracellular molecules implicated in the regulation of AA content, strongly suggests that the OB and PC could play an important role in AAs sensing.
When it comes to AA sensing via transporter activation, two mechanisms coexist: one involves the mTORC1/AMPK pathway that detects AA availability and the second one involves GCN2 that specifically alerts when one or more essential AAs are insufficiently ingested. The hypothalamus is proposed to be the center for mTORC1/AMPK signaling (Cota et al., 2006; Ropelle et al., 2008; Hagglund et al., 2013) while the anterior part of PC (APC) utilizes GCN2. Leung's and Gietzen's teams have collected convergent data showing that the APC is a sensor of AAs imbalanced diet. Briefly, deficiency in one essential AA induces rapid rejection of the imbalanced diet (Leung et al., 1968; Koehnle et al., 2003; Gietzen and Aja, 2012; Morrison et al., 2012). This aversion disappears after APC lesion (Leung and Rogers, 1971) and persists after hypothalamus or OB injury (Leung and Rogers, 1970; Leung et al., 1972), which identifies the APC as the sensor of an AA imbalanced diet. Moreover, local injection of the deficient AA in the APC reduces food aversion by maintaining consumption of the imbalanced diet (Beverly et al., 1990; Russell et al., 2003). Accumulation of uncharged tRNA caused by AAs deficiency activates the GCN2 pathway (Hao et al., 2005; Rudell et al., 2011) and disinhibits the APC mainly through downregulation of GABAA receptor and KCC2, also known as SLC12A5 transporter (Sharp et al., 2013). KCC2 is localized in GABAergic neurons in the OB and PC (Wang et al., 2005; Sharp et al., 2013). Glutamatergic pyramidal neurons in the APC would then send messages to feeding circuits, including the hypothalamus, in order to stop food intake (Gietzen and Magrum, 2001). Noteworthy is that mTORC1 is not involved here because behavioral rejection of the improper diet remains in the presence of rapamycin (Hao et al., 2010) (Figure 4A).
The role played by the APC in sensing AA deficiency is thus clear. However, sensing AA abundance via other olfactory structures has not been explored yet. It would be interesting to explore the possible implication of OB and/or PC in detecting AA abundancy and scarcity through mTORC1/AMPK pathways and through AA receptor activation.
Another sensor of AAs, Tas1R1, seems to be dependent on the feeding state when expressed in the hypothalamus. Tas1r1 levels increase following a 24-h food deprivation (Ren et al., 2009). Tas1r1 is highly expressed in the hypothalamus of obese and hyperglycemic ob/ob mice. The similarities between the nutrient sensing properties of the hypothalamus and that of the OB (Figure 1) prompt further investigation of the role of T1R1 or the gene it encodes Tas1r1, in sensing AAs in olfactory structures.
Fatty Acid Sensing
Physiological Role of Fatty Acid Supply to the Brain
The brain is roughly 50% fatty acids (FAs) by weight making it the organ with the second highest lipid content after that of adipose tissue (Watkins et al., 2001). Cerebral lipids are uptaken from the blood or synthesized locally (Rapoport et al., 2001; Smith and Nagura, 2001). Indeed, brain neurons express enzymes for both intracellular metabolism and de novo synthesis of FAs (Le Foll et al., 2009). In the human brain, the main source of polyunsaturated fatty acids (PUFAs) such as docosahexaenoic acid, eicosapentaenoic acid, and arachidonic acid, is dietary. Even though free FAs are not the primary metabolic fuel for neurons, they are key components of membranes and intracellular signaling pathways. PUFAs are of great importance in neurobiology because they are essential for neurogenesis, memory, learning, and play a key role in modulating ion channels and neurotransmitter receptors. In fact, an adequate lipid environment is vital for the normal functioning of neuronal membrane proteins such as ion channels, enzymes, ion pumps, and receptors. Long-term nutritional PUFA deficiency impairs brain functioning (Khan and He, 2017). FA sensing in neurons was first reported by Oomura et al. (1975). Since then, a growing body of evidence has established the importance of brain FA sensing in the regulation of food intake (Loftus et al., 2000; Lam et al., 2005; Levin et al., 2011). Specific areas of the central nervous system including the hypothalamus, brainstem, and hippocampus (Gao and Lane, 2003; Lam et al., 2005; Picard et al., 2014) have been shown to use FAs as cellular messengers to inform “FA-sensitive neurons” about the energy status of the body (Migrenne et al., 2011). Similar to glucose sensing and AAs sensing described previously, lipid sensing is involved in the control of feeding behavior (Obici and Rossetti, 2003; Cruciani-Guglielmacci et al., 2004). Hypothalamic lipid sensing mechanisms are disrupted during conditions of prolonged fasting (Yue and Lam, 2012). The molecular mechanisms involved in FA sensing by the brain are still a matter of debate.
The FA transporter proteins (FATP also called SLC27), is a protein family of six isoforms. SLC27A4 (FATP4) is the major FATP expressed in the brain (Fitscher et al., 1998). In hypothalamic neurons, FAs are transported inside cells through FATPs. FAs are then oxidized to generate ATP that can modulate the activity of a wide variety of ATP-dependent ion channels including K+ channels, and the Na+-K+ ATPase pump. The resulting change in neuronal firing rate suggests that FAs metabolism play a role in the regulation of energy balance (Migrenne et al., 2011; Picard et al., 2014).
In the brain, membrane receptors mediating FAs sensing consist of two GPCRs (GPR40 and GPR120) and CD36, often associated to fatty acid translocase (FAT) to make a translocator/receptor complex FAT/CD36. CD36 has been reported to be involved in FA sensing in taste buds (Fukuwatari et al., 1997; Laugerette et al., 2005) and in hypothalamic neurons (Le Foll et al., 2009). Hypothalamic CD36 expression induced by fasting or following high-fat diet, could modulate lipid signaling in the brain and participate in the regulation of energy homeostasis (Moulle et al., 2012, 2014). All together, these findings strongly suggest that lipid sensing by CD36 is responsible for basic physiological functions in relation to behavior and energy balance (Martin et al., 2011). In the hypothalamus, it has been postulated that binding of FAs to CD36 alters neuronal activity in a manner analogous to that utilized for fat perception by taste receptor cells (Le Foll et al., 2009). This causes phosphorylation of protein tyrosine kinases, leading to generation of IP3, recruitment of Ca2+ from the endoplasmic reticulum, followed by influx of calcium via opening of store-operated calcium channels, membrane depolarization via TRPM5 channel activation, and ultimately neurotransmitter release (El Yassimi et al., 2008).
In this review, only FA transporters (FATP/SLC27) and the FA receptors GPR40 and CD36 will be detailed. Intracellular proteins including long-chain fatty acyl-coenzyme A (CoA) synthetases and FA oxidative proteins are largely involved in neuronal FA sensing but are beyond the scope of this review (Picard et al., 2014).
Sensing Role of Fatty Acids in Olfactory Structures: Molecular Hallmarks
Fatty Acid Solute Carrier Transporters Expressed in Olfactory Structures (SLC27)
According to the Allen Mouse Brain Atlas, SLC27A1 and SLC27A4 are expressed in the OB, AON, and PC. In the OB, SLC27A4 is mainly expressed in MCs (Allen Institute for Brain Science, 2015). While no previous study has investigated lipid sensing in central olfactory structures, many molecular cues seem to suggest that free FAs could be used as a messenger in these olfactory areas neurons to inform about the energy status of the whole body (Figure 5).
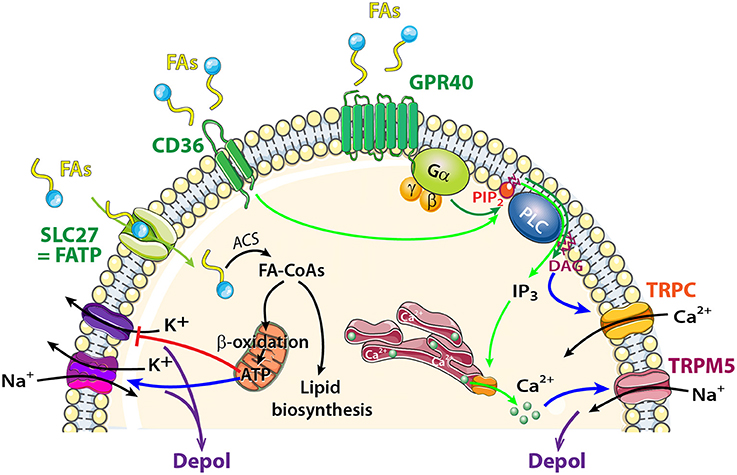
Figure 5. Schematic model showing FA sensing signaling pathways that might modulate neuronal activity of central olfactory areas. The transporter SLC27 induces influx of FAs, and acyl-CoA synthetase (ACS) to esterify FAs to fatty acyl-CoAs (FA-CoAs). Following mitochondrial β oxidation of FA-CoAs, production of ATP induces depolarization by acting on a wide variety of ATP dependent ion channels. FAs Receptors: Activation of CD36 by FA binding (light green arrows) causes phosphorylation of protein tyrosine kinases, leading to generation of inositol 1,4,5-trisphosphate (IP3) that induces Ca2+ release from the endoplasmic reticulum. [Ca2+]Iincrease depolarizes the membrane via TRPM5 channel. FAs receptors 7TM GPR40 receptor signaling (dark green arrows) acts through heterotrimeric G-proteins and produces IP3 and diacylglycerol (DAG). Phospholipase C (PLC) and DAG activate transient receptor potential cation channel subfamily C (TRPC).
Fatty Acid Receptors Expressed in Olfactory Structures
GPR40 (but not GPR120) is highly expressed in the OB (Nakamoto et al., 2012; Khan and He, 2017). Like all GPCRs, GPR40 is coupled to an intracellular heterotrimeric G protein (Gα) that activates the phospholipase C (PLC) located on the plasma membrane. PLC hydrolyzes phosphatidylinositol 4,5-bisphosphate (PIP2) into 2 s messengers: IP3 and diacyglycerol (DAG) (Figure 5). The generation of PLC facilitates transport of PKC from the cytosol to the plasma membrane. PLC, PKC, and DAG were described as activators of the TRP subfamily C (Khan and He, 2017). In the OB, MCs and external tufted cells extensively express TRPC3, C4, and C5 whereas neurons of the granule cell layer express TRPC1 and C4 only (Otsuka et al., 1998; Philipp et al., 1998; Dong et al., 2012). Studying modulation in MCs firing in response to fluctuations in extracellular FA concentration would be interesting in the context food intake and/or food choice.
In addition to GPRs, CD36 is a well described receptor for FAs. In the peripheral olfactory system, CD36 has been identified in insect and rodent OSNs (Benton et al., 2007; Lee et al., 2015). In recent studies, CD36 has been localized in the cilia, dendrites, and soma of a subset of OSNs in young rodents (Lee et al., 2015; Oberland et al., 2015). The CD36-positive OSNs respond in an age-dependent manner to oleic acid, a major milk component. This suggests that CD36 is involved in FA detection by the peripheral olfactory system during the suckling period (Oberland et al., 2015). CD36 was also found in central olfactory areas such as the glomerular layer of the OB (Oberland et al., 2015), PC and nucleus of the lateral olfactory tract (Glezer et al., 2009). The role of CD36 in these central olfactory areas has been raised whereby similar to taste buds, CD36 would sense FAs. TRPM5 channel is present in the OE, OB, and PC (Lin et al., 2007; Rolen et al., 2014; Allen Institute for Brain Science, 2015; Pyrski et al., 2017) and can serve as a downstream member of FA sensing where it is activated by an increase in Ca2+; the latter resulting from FA intake. CD36 activation would be investigated in the context of FAs sensing of olfactory areas.
Metabolic Dysfunction and Lipid Sensors in Olfactory Areas
In contrast to glucose and AAs sensing, only one study has explored the neuron lipid sensing in peripheral olfactory structures (Oberland et al., 2015). The fact that CD36, GPR40 and molecules involved in their intracellular pathways, are expressed in neurons of olfactory structures raises the question of their role(s) in lipid olfactory perception, central FA sensing, and regulation of energy balance. Indeed, lipid sensing is described as an important contributor to the regulation of energy balance (Magnan et al., 2015). In circumvallate taste buds, a decrease in CD36 expression induced by high-fat diet causes obesity and reduced sensitivity to fat taste, which in turn increased the intake of fatty foods as a compensatory response (Zhang et al., 2011). In the same way, reduction in hypothalamic CD36 expression induced redistribution of fat from visceral to subcutaneous deposits and markedly impaired insulin sensitivity (Le Foll et al., 2009, 2013, 2015). Growing evidence shows that dysregulation of brain FA sensing may contribute to energy imbalance and development of obesity, associated with type 2 diabetes or not (Yue and Lam, 2012; Picard et al., 2014). It will be interesting in future studies to investigate if olfactory dysfunction caused by altered energy balance (Thiebaud et al., 2014) could be linked to a change in expression of GPR40 and/or CD36.
Conclusion
In order to regulate nutrient homeostasis, the body initiates multiple and redundant mechanisms in response to modulation in internal nutrient levels. In addition to the hypothalamic regulatory center, olfactory structures are proposed to detect both odors and nutrients. In this manner, the olfactory system contributes, through foraging and food, selection in maintaining metabolic homeostasis. In particular, mounting evidence indicates that the OB and the PC are involved in food intake, via regulation of choice of food with the appropriate nutrient content. This review presents a new approach to the problem of energy balance by suggesting that the nature of ingested nutrients could act on subpopulations of nutrient sensing neurons discreetly located in key brain areas including olfactory areas. In spite of numerous arguments described in this review (see Table 1), our understanding of the mechanisms implicated in nutrient sensing in olfactory areas is far from complete. The links between hormones involved in food intake regulation and that of nutrient sensing have to be deciphered. In the hypothalamus the mTORC1 is known to be a key component of the intracellular path integrating all these internal signals (i.e., nutrients and hormones) (Wullschleger et al., 2006; Wiczer and Thomas, 2010; Haissaguerre et al., 2014). We suggest that nutrient sensing in olfactory areas, could involve mTORC1 signaling. However, GCN2, and not mTORC1, is necessary for the detection of AA imbalance in the PC (Hao et al., 2010). The role of mTORC1 in detecting over consumption of nutrients in the PC, is a separate question to investigate. In addition to these and other unanswered questions, we still lack an integrative view of the presumably coordinated role played by olfactory areas and the hypothalamus regarding their metabolic homeostasis. Deciphering these aspects might offer new solutions in mitigating metabolic dysfunctions such as obesity and/or diabetes and provide new approaches to investigate physiological functions such as memory, and sleep that exhibit reciprocal relationships with homeostasis regulation and olfactory function (Barnes and Wilson, 2014).
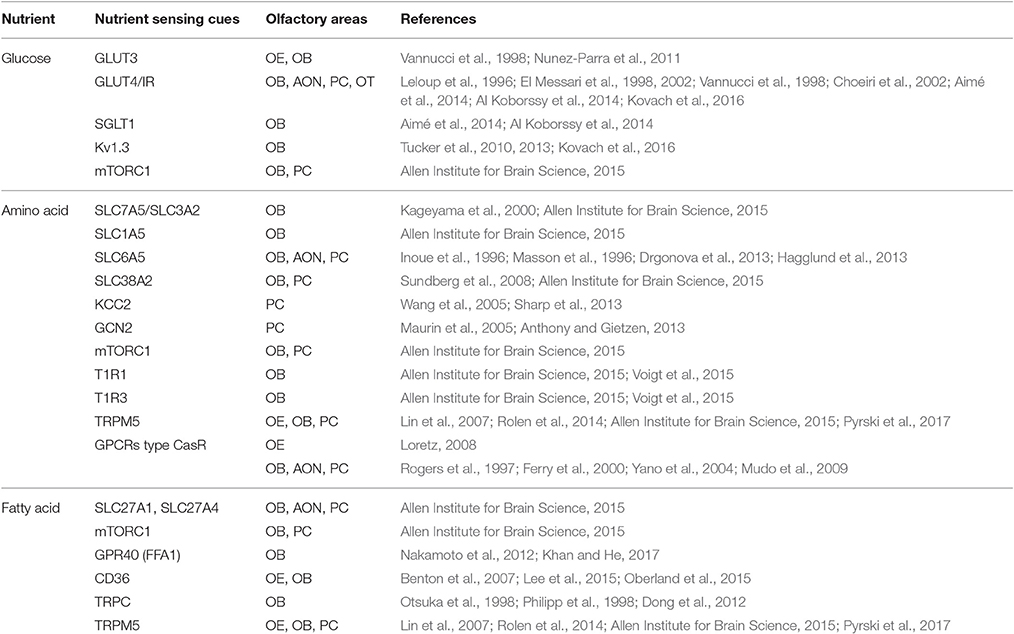
Table 1. Overview of nutrient sensing molecular cues and their corresponding nutrients, present in olfactory structures.
Author Contributions
AJ and BP were responsible for the conception and design of the review; DK, BP, and AJ drafted the review; All authors revised the manuscript critically for important intellectual content and approved the final version of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by Centre National de la Recherche Scientifique, University Lyon 1, the Laboratoire d'Excellence Cortex (ANR-11-LABX-0042), NIH grant R01 DC013080 from the National Institutes of Deafness and Communication Disorders (NIDCD), and a PALSE grant (Programme Avenir Lyon St Etienne) from the University of Lyon. This work was additionally supported by the Robinson Foundation at the Tallahassee Memorial Hospital.
Abbreviations
CoA, Acyl-coenzyme A; AA, Amino acid; AON, Anterior olfactory nucleus; APC, Anterior piriform cortex; CD36, Cluster of Differentiation 36; DAG, Diacylglycerol; iEPL, Internal External plexiform layer; FA, Fatty acid; FAT, Fatty acid translocase; FATP, Fatty acid transport proteins; GCN2, General amino acid control non-derepressible 2; GE, Glucose-excited; GI, Glucose-inhibited; GLUT, Glucose transporter; GPCR or GPR, G-protein-coupled receptor; iEPL, Inner part of the external plexiform layer; IP3, Inositol 1,4,5-trisphosphate; IR, Insulin receptor; KCC2, K+/Cl- co-transporter; mTORC1, Mammalian target of rapamycin complex 1; MCs, Mitral cells; OB, Olfactory bulb; OE, Olfactory epithelium; OSN, Olfactory sensory neuron; OT, Olfactory tubercle; PIP2, Phosphatidylinositol 4,5-bisphosphate; PLC, Phospholipase C; PC, Piriform cortex; PUFA, Polyunsaturated fatty acid; 7TM, Seven transmembrane domains; SGLT, Sodium-dependent glucose transporter; SLC, Solute carrier; TRPC, Transient receptor potential cation channel subfamily C; TRPM, Transient receptor potential cation channel subfamily M.
References
Abraham, N. M., Egger, V., Shimshek, D. R., Renden, R., Fukunaga, I., Sprengel, R., et al. (2010). Synaptic inhibition in the olfactory bulb accelerates odor discrimination in mice. Neuron 65, 399–411. doi: 10.1016/j.neuron.2010.01.009
Aimé, P., Duchamp-Viret, P., Chaput, M. A., Savigner, A., Mahfouz, M., and Julliard, A. K. (2007). Fasting increases and satiation decreases olfactory detection for a neutral odor in rats. Behav. Brain Res. 179, 258–264. doi: 10.1016/j.bbr.2007.02.012
Aimé, P., Hegoburu, C., Jaillard, T., Degletagne, C., Garcia, S., Messaoudi, B., et al. (2012). A physiological increase of insulin in the olfactory bulb decreases detection of a learned aversive odor and abolishes food odor-induced sniffing behavior in rats. PLoS ONE 7:e51227. doi: 10.1371/journal.pone.0051227
Aimé, P., Palouzier-Paulignan, B., Salem, R., Al Koborssy, D., Garcia, S., Duchamp, C., et al. (2014). Modulation of olfactory sensitivity and glucose-sensing by the feeding state in obese Zucker rats. Front. Behav. Neurosci. 8:326. doi: 10.3389/fnbeh.2014.00326
Al Koborssy, D., Palouzier-Paulignan, B., Salem, R., Thevenet, M., Romestaing, C., and Julliard, A. K. (2014). Cellular and molecular cues of glucose sensing in the rat olfactory bulb. Front. Neurosci. 8:333. doi: 10.3389/fnins.2014.00333
Allen Institute for Brain Science (2015). Allen Brain Atlas API. Available online at: http://brain-map.org/api/index.html
Alquier, T., Leloup, C., Lorsignol, A., and Pénicaud, L. (2006). Translocable glucose transporters in the brain: where are we in 2006? Diabetes 55, S131–S138. doi: 10.2337/db06-S021
Anand, B. K., Chhina, G. S., Sharma, K. N., Dua, S., and Singh, B. (1964). Activity of single neurons in the hypothalamic feeding centers: effect of glucose. Am. J. Physiol. 207, 1146–1154.
Anthony, T. G., and Gietzen, D. W. (2013). Detection of amino acid deprivation in the central nervous system. Curr. Opin. Clin. Nutr. Metab. Care 16, 96–101. doi: 10.1097/MCO.0b013e32835b618b
Apelbaum, A. F., Perrut, A., and Chaput, M. (2005). Orexin A effects on the olfactory bulb spontaneous activity and odor responsiveness in freely breathing rats. Regul. Pept. 129, 49–61. doi: 10.1016/j.regpep.2005.01.003
Ashcroft, F. M., and Gribble, F. M. (1999). ATP-sensitive K+ channels and insulin secretion: their role in health and disease. Diabetologia 42, 903–919. doi: 10.1007/s001250051247
Ashford, M. L., Boden, P. R., and Treherne, J. M. (1990). Glucose-induced excitation of hypothalamic neurones is mediated by ATP-sensitive K+ channels. Pflugers Arch. 415, 479–483. doi: 10.1007/BF00373626
Balfour, R. H., Hansen, A. M., and Trapp, S. (2006). Neuronal responses to transient hypoglycaemia in the dorsal vagal complex of the rat brainstem. J. Physiol. 570, 469–484. doi: 10.1113/jphysiol.2005.098822
Barnes, D. C., and Wilson, D. A. (2014). Sleep and olfactory cortical plasticity. Front. Behav. Neurosci. 8:134. doi: 10.3389/fnbeh.2014.00134
Bar-Peled, L., and Sabatini, D. M. (2014). Regulation of mTORC1 by amino acids. Trends Cell Biol. 24, 400–406. doi: 10.1016/j.tcb.2014.03.003
Benton, R., Vannice, K. S., and Vosshall, L. B. (2007). An essential role for a CD36-related receptor in pheromone detection in Drosophila. Nature 450, 289–293. doi: 10.1038/nature06328
Berthoud, H. R. (2011). Metabolic and hedonic drives in the neural control of appetite: who is the boss? Curr. Opin. Neurobiol. 21, 888–896. doi: 10.1016/j.conb.2011.09.004
Beverly, J. L., Gietzen, D. W., and Rogers, Q. R. (1990). Effect of dietary limiting amino acid in prepyriform cortex on food intake. Am. J. Physiol. 259, R709–R715.
Blais, A., Huneau, J. F., Magrum, L. J., Koehnle, T. J., Sharp, J. W., Tome, D., et al. (2003). Threonine deprivation rapidly activates the system A amino acid transporter in primary cultures of rat neurons from the essential amino acid sensor in the anterior piriform cortex. J. Nutr. 133, 2156–2164.
Blouet, C., Jo, Y. H., Li, X., and Schwartz, G. J. (2009). Mediobasal hypothalamic leucine sensing regulates food intake through activation of a hypothalamus-brainstem circuit. J. Neurosci. 29, 8302–8311. doi: 10.1523/JNEUROSCI.1668-09.2009
Blouet, C., and Schwartz, G. J. (2010). Hypothalamic nutrient sensing in the control of energy homeostasis. Behav. Brain Res. 209, 1–12. doi: 10.1016/j.bbr.2009.12.024
Brant, A. M., Jess, T. J., Milligan, G., Brown, C. M., and Gould, G. W. (1993). Immunological analysis of glucose transporters expressed in different regions of the rat brain and central nervous system. Biochem. Biophys. Res. Commun. 192, 1297–1302. doi: 10.1006/bbrc.1993.1557
Breer, H., Fleischer, J., and Strotmann, J. (2006). The sense of smell: multiple olfactory subsystems. Cell. Mol. Life Sci. 63, 1465–1475. doi: 10.1007/s00018-006-6108-5
Broer, A., Tietze, N., Kowalczuk, S., Chubb, S., Munzinger, M., Bak, L. K., et al. (2006). The orphan transporter v7-3 (slc6a15) is a Na+-dependent neutral amino acid transporter (B0AT2). Biochem. J. 393, 421–430. doi: 10.1042/BJ20051273
Broer, S. (2014). The SLC38 family of sodium-amino acid co-transporters. Pflugers Arch. 466, 155–172. doi: 10.1007/s00424-013-1393-y
Chaigneau, E., Tiret, P., Lecoq, J., Ducros, M., Knopfel, T., and Charpak, S. (2007). The relationship between blood flow and neuronal activity in the rodent olfactory bulb. J. Neurosci. 27, 6452–6460. doi: 10.1523/JNEUROSCI.3141-06.2007
Chaudhari, N., Pereira, E., and Roper, S. D. (2009). Taste receptors for umami: the case for multiple receptors. Am. J. Clin. Nutr. 90, 738S–742S. doi: 10.3945/ajcn.2009.27462H
Chaudhari, N., and Roper, S. D. (2010). The cell biology of taste. J. Cell Biol. 190, 285–296. doi: 10.1083/jcb.201003144
Chelminski, Y., Magnan, C., Luquet, S. H., Everard, A., Meunier, N., Gurden, H., et al. (2017). Odor-induced neuronal rhythms in the olfactory bulb are profoundly modified in ob/ob obese mice. Front. Physiol. 8:2. doi: 10.3389/fphys.2017.00002
Choeiri, C., Staines, W., and Messier, C. (2002). Immunohistochemical localization and quantification of glucose transporters in the mouse brain. Neurosci 111, 19–34. doi: 10.1016/S0306-4522(01)00619-4
Conigrave, A. D., Franks, A. H., Brown, E. M., and Quinn, S. J. (2002). L-amino acid sensing by the calcium-sensing receptor: a general mechanism for coupling protein and calcium metabolism? Eur. J. Clin. Nutr. 56, 1072–1080. doi: 10.1038/sj.ejcn.1601463
Conigrave, A. D., and Hampson, D. R. (2006). Broad-spectrum L-amino acid sensing by class 3 G-protein-coupled receptors. Trends Endocrinol. Metab. 17, 398–407. doi: 10.1016/j.tem.2006.10.012
Cota, D., Proulx, K., Smith, K. A., Kozma, S. C., Thomas, G., Woods, S. C., et al. (2006). Hypothalamic mTOR signaling regulates food intake. Science 312, 927–930. doi: 10.1126/science.1124147
Cruciani-Guglielmacci, C., Hervalet, A., Douared, L., Sanders, N. M., Levin, B. E., Ktorza, A., et al. (2004). Beta oxidation in the brain is required for the effects of non-esterified fatty acids on glucose-induced insulin secretion in rats. Diabetologia 47, 2032–2038. doi: 10.1007/s00125-004-1569-2
Dalle, S., Burcelin, R., and Gourdy, P. (2013). Specific actions of GLP-1 receptor agonists and DPP4 inhibitors for the treatment of pancreatic beta-cell impairments in type 2 diabetes. Cell. Signal. 25, 570–579. doi: 10.1016/j.cellsig.2012.11.009
Dobrogowska, D. H., and Vorbrodt, A. W. (1999). Quantitative immunocytochemical study of blood-brain barrier glucose transporter (GLUT-1) in four regions of mouse brain. J. Histochem. Cytochem. 47, 1021–1030. doi: 10.1177/002215549904700806
Doege, H., and Stahl, A. (2006). Protein-mediated fatty acid uptake: novel insights from in vivo models. Physiology (Bethesda) 21, 259–268. doi: 10.1152/physiol.00014.2006
Dong, H. W., Davis, J. C., Ding, S., Nai, Q., Zhou, F. M., and Ennis, M. (2012). Expression of transient receptor potential (TRP) channel mRNAs in the mouse olfactory bulb. Neurosci. Lett. 524, 49–54. doi: 10.1016/j.neulet.2012.07.013
Drgonova, J., Jacobsson, J. A., Han, J. C., Yanovski, J. A., Fredriksson, R., Marcus, C., et al. (2013). Involvement of the neutral amino acid transporter SLC6A15 and leucine in obesity-related phenotypes. PLoS ONE 8:e68245. doi: 10.1371/journal.pone.0068245
El Messari, S., Ait-Ikhlef, A., Ambroise, D. H., Penicaud, L., and Arluison, M. (2002). Expression of insulin-responsive glucose transporter GLUT4 mRNA in the rat brain and spinal cord: an in situ hybridization study. J. Chem. Neuroanat. 24, 225–242. doi: 10.1016/S0891-0618(02)00058-3
El Messari, S., Leloup, C., Quignon, M., Brisorgueil, M. J., Penicaud, L., and Arluison, M. (1998). Immunocytochemical localization of the insulin-responsive glucose transporter 4 (Glut4) in the rat central nervous system. J. Comp. Neurol. 399, 492–512. doi: 10.1002/(SICI)1096-9861(19981005)399:4<492::AID-CNE4>3.0.CO;2-X
El Yassimi, A., Hichami, A., Besnard, P., and Khan, N. A. (2008). Linoleic acid induces calcium signaling, Src kinase phosphorylation, and neurotransmitter release in mouse CD36-positive gustatory cells. J. Biol. Chem. 283, 12949–12959. doi: 10.1074/jbc.M707478200
Fadool, D. A., Tucker, K., and Pedarzani, P. (2011). Mitral cells of the olfactory bulb perform metabolic sensing and are disrupted by obesity at the level of the Kv1.3 ion channel. PLoS ONE 6:e24921. doi: 10.1371/journal.pone.0024921
Fadool, D. A., Tucker, K., Phillips, J. J., and Simmen, J. A. (2000). Brain insulin receptor causes activity-dependent current suppression in the olfactory bulb through multiple phosphorylation of Kv1.3. J. Neurophysiol. 83, 2332–2348.
Ferry, S., Traiffort, E., Stinnakre, J., and Ruat, M. (2000). Developmental and adult expression of rat calcium-sensing receptor transcripts in neurons and oligodendrocytes. Eur. J. Neurosci. 12, 872–884. doi: 10.1046/j.1460-9568.2000.00980.x
Fitscher, B. A., Riedel, H. D., Young, K. C., and Stremmel, W. (1998). Tissue distribution and cDNA cloning of a human fatty acid transport protein (hsFATP4). Biochim. Biophys. Acta 1443, 381–385. doi: 10.1016/S0167-4781(98)00231-0
Fukuwatari, T., Kawada, T., Tsuruta, M., Hiraoka, T., Iwanaga, T., Sugimoto, E., et al. (1997). Expression of the putative membrane fatty acid transporter (FAT) in taste buds of the circumvallate papillae in rats. FEBS Lett. 414, 461–464. doi: 10.1016/S0014-5793(97)01055-7
Gao, S., and Lane, M. D. (2003). Effect of the anorectic fatty acid synthase inhibitor C75 on neuronal activity in the hypothalamus and brainstem. Proc. Natl. Acad. Sci. U.S.A. 100, 5628–5633. doi: 10.1073/pnas.1031698100
Gerhart, D. Z., Leino, R. L., Borson, N. D., Taylor, W. E., Gronlund, K. M., McCall, A. L., et al. (1995). Localization of glucose transporter GLUT 3 in brain: comparison of rodent and dog using species-specific carboxyl-terminal antisera. Neuroscience 66, 237–246. doi: 10.1016/0306-4522(94)00544-F
Gietzen, D. W., and Aja, S. M. (2012). The brain's response to an essential amino acid-deficient diet and the circuitous route to a better meal. Mol. Neurobiol. 46, 332–348. doi: 10.1007/s12035-012-8283-8
Gietzen, D. W., and Magrum, L. J. (2001). Molecular mechanisms in the brain involved in the anorexia of branched-chain amino acid deficiency. J. Nutr. 131, 851S–855S.
Gire, D. H., Restrepo, D., Sejnowski, T. J., Greer, C., De Carlos, J. A., and Lopez-Mascaraque, L. (2013). Temporal processing in the olfactory system: can we see a smell? Neuron 78, 416–432. doi: 10.1016/j.neuron.2013.04.033
Glezer, I., Bittencourt, J. C., and Rivest, S. (2009). Neuronal expression of Cd36, Cd44, and Cd83 antigen transcripts maps to distinct and specific murine brain circuits. J. Comp. Neurol. 517, 906–924. doi: 10.1002/cne.22185
Gonzalez, J. A., Reimann, F., and Burdakov, D. (2009). Dissociation between sensing and metabolism of glucose in sugar sensing neurones. J. Physiol. 587, 41–48. doi: 10.1113/jphysiol.2008.163410
Hagglund, M. G., Roshanbin, S., Lofqvist, E., Hellsten, S. V., Nilsson, V. C., Todkar, A., et al. (2013). B(0)AT2 (SLC6A15) is localized to neurons and astrocytes, and is involved in mediating the effect of leucine in the brain. PLoS ONE 8:e58651. doi: 10.1371/journal.pone.0058651
Haissaguerre, M., Saucisse, N., and Cota, D. (2014). Influence of mTOR in energy and metabolic homeostasis. Mol. Cell. Endocrinol. 397, 67–77. doi: 10.1016/j.mce.2014.07.015
Han, P., Trinidad, B. J., and Shi, J. (2015). Hypocalcemia-induced seizure: demystifying the calcium paradox. ASN Neuro 7:1759091415578050. doi: 10.1177/1759091415578050
Hao, S., Ross-Inta, C. M., and Gietzen, D. W. (2010). The sensing of essential amino acid deficiency in the anterior piriform cortex, that requires the uncharged tRNA/GCN2 pathway, is sensitive to wortmannin but not rapamycin. Pharmacol. Biochem. Behav. 94, 333–340. doi: 10.1016/j.pbb.2009.09.014
Hao, S., Sharp, J. W., Ross-Inta, C. M., McDaniel, B. J., Anthony, T. G., Wek, R. C., et al. (2005). Uncharged tRNA and sensing of amino acid deficiency in mammalian piriform cortex. Science 307, 1776–1778. doi: 10.1126/science.1104882
Hardy, A. B., Aioun, J., Baly, C., Julliard, K. A., Caillol, M., Salesse, R., et al. (2005). Orexin A modulates mitral cell activity in the rat olfactory bulb: patch-clamp study on slices and immunocytochemical localization of orexin receptors. Endocrinology 146, 4042–4053. doi: 10.1210/en.2005-0020
Hofer, A. M. (2005). Another dimension to calcium signaling: a look at extracellular calcium. J. Cell Sci. 118, 855–862. doi: 10.1242/jcs.01705
Hoon, M. A., Adler, E., Lindemeier, J., Battey, J. F., Ryba, N. J., and Zuker, C. S. (1999). Putative mammalian taste receptors: a class of taste-specific GPCRs with distinct topographic selectivity. Cell 96, 541–551. doi: 10.1016/S0092-8674(00)80658-3
Hwang, T. C., and Sheppard, D. N. (1999). Molecular pharmacology of the CFTR Cl- channel. Trends Pharmacol. Sci. 20, 448–453. doi: 10.1016/S0165-6147(99)01386-3
Inoue, K., Sato, K., Tohyama, M., Shimada, S., and Uhl, G. R. (1996). Widespread brain distribution of mRNA encoding the orphan neurotransmitter transporter v7-3. Brain Res. Mol. Brain Res. 37, 217–223. doi: 10.1016/0169-328X(95)00298-7
Jones, B. L., and Smith, S. M. (2016). Calcium-Sensing receptor: a key target for extracellular calcium signaling in neurons. Front. Physiol. 7:116. doi: 10.3389/fphys.2016.00116
Julliard, A. K., Chaput, M. A., Apelbaum, A., Aime, P., Mahfouz, M., and Duchamp-Viret, P. (2007). Changes in rat olfactory detection performance induced by orexin and leptin mimicking fasting and satiation. Behav. Brain Res. 183, 123–129. doi: 10.1016/j.bbr.2007.05.033
Kageyama, T., Imura, T., Matsuo, A., Minato, N., and Shimohama, S. (2000). Distribution of the 4F2 light chain, LAT1, in the mouse brain. Neuroreport 11, 3663–3666. doi: 10.1097/00001756-200011270-00015
Kanai, Y., and Hediger, M. A. (2004). The glutamate/neutral amino acid transporter family SLC1: molecular, physiological and pharmacological aspects. Pflugers Arch. 447, 469–479. doi: 10.1007/s00424-003-1146-4
Kang, L., Routh, V. H., Kuzhikandathil, E. V., Gaspers, L. D., and Levin, B. E. (2004). Physiological and molecular characteristics of rat hypothalamic ventromedial nucleus glucosensing neurons. Diabetes 53, 549–559. doi: 10.2337/diabetes.53.3.549
Khan, M. Z., and He, L. (2017). The role of polyunsaturated fatty acids and GPR40 receptor in brain. Neuropharmacology 113(Pt B), 639–651. doi: 10.1016/j.neuropharm.2015.05.013
Koehnle, T. J., Russell, M. C., and Gietzen, D. W. (2003). Rats rapidly reject diets deficient in essential amino acids. J. Nutr. 133, 2331–2335.
Kovach, C. P., Al Koborssy, D., Huang, Z., Chelette, B. M., Fadool, J. M., and Fadool, D. A. (2016). Mitochondrial ultrastructure and glucose signaling pathways attributed to the Kv1.3 ion channel. Front. Physiol. 7:178. doi: 10.3389/fphys.2016.00178
Kuczewski, N., Fourcaud-Trocme, N., Savigner, A., Thevenet, M., Aime, P., Garcia, S., et al. (2014). Insulin modulates network activity in olfactory bulb slices: impact on odour processing. J. Physiol. (Lond). 592, 2751–2769. doi: 10.1113/jphysiol.2013.269639
Lacroix, M. C., Badonnel, K., Meunier, N., Tan, F., Schlegel-Le Poupon, C., Durieux, D., et al. (2008). Expression of insulin system in the olfactory epithelium: first approaches to its role and regulation. J. Neuroendocrinol. 20, 1176–1190. doi: 10.1111/j.1365-2826.2008.01777.x
Lam, T. K., Schwartz, G. J., and Rossetti, L. (2005). Hypothalamic sensing of fatty acids. Nat. Neurosci. 8, 579–584. doi: 10.1038/nn1456
Laugerette, F., Passilly-Degrace, P., Patris, B., Niot, I., Febbraio, M., Montmayeur, J. P., et al. (2005). CD36 involvement in orosensory detection of dietary lipids, spontaneous fat preference, and digestive secretions. J. Clin. Invest. 115, 3177–3184. doi: 10.1172/JCI25299
Laughlin, S. B. (2001). Energy as a constraint on the coding and processing of sensory information. Curr. Opin. Neurobiol. 11, 475–480. doi: 10.1016/S0959-4388(00)00237-3
Lee, S., Eguchi, A., Tsuzuki, S., Matsumura, S., Inoue, K., Iwanaga, T., et al. (2015). Expression of CD36 by olfactory receptor cells and its abundance on the epithelial surface in mice. PLoS ONE 10:e0133412. doi: 10.1371/journal.pone.0133412
Le Foll, C., Dunn-Meynell, A. A., and Levin, B. E. (2015). Role of FAT/CD36 in fatty acid sensing, energy, and glucose homeostasis regulation in DIO and DR rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 308, R188–R198. doi: 10.1152/ajpregu.00367.2014
Le Foll, C., Dunn-Meynell, A., Musatov, S., Magnan, C., and Levin, B. E. (2013). FAT/CD36: a major regulator of neuronal fatty acid sensing and energy homeostasis in rats and mice. Diabetes 62, 2709–2716. doi: 10.2337/db12-1689
Le Foll, C., Irani, B. G., Magnan, C., Dunn-Meynell, A. A., and Levin, B. E. (2009). Characteristics and mechanisms of hypothalamic neuronal fatty acid sensing. Am. J. Physiol. Regul. Integr. Comp. Physiol. 297, R655–R664. doi: 10.1152/ajpregu.00223.2009
Leloup, C., Arluison, M., Kassis, N., Lepetit, N., Cartier, N., Ferre, P., et al. (1996). Discrete brain areas express the insulin-responsive glucose transporter GLUT4. Brain Res. Mol. Brain Res. 38, 45–53. doi: 10.1016/0169-328X(95)00306-D
Leung, P. M. B., and Rogers, Q. R. (1970). Effect of amino acid imbalance and deficiency on food intake of rats with hypothalamic lesions. Nutr. Rep. Int. 1, 1–10.
Leung, P. M., Larson, D. M., and Rogers, Q. R. (1972). Food intake and preference of olfactory bulbectomized rats fed amino acid imbalanced or deficient diets. Physiol. Behav. 9, 553–557. doi: 10.1016/0031-9384(72)90011-X
Leung, P. M., and Rogers, Q. R. (1971). Importance of prepyriform cortex in food-intake response of rats to amino acids. Am. J. Physiol. 221, 929–935.
Leung, P. M., Rogers, Q. R., and Harper, A. E. (1968). Effect of amino acid imbalance on dietary choice in the rat. J. Nutr. 95, 483–492.
Levin, B. E., Magnan, C., Dunn-Meynell, A., and Le Foll, C. (2011). Metabolic sensing and the brain: who, what, where, and how? Endocrinology 152, 2552–2557. doi: 10.1210/en.2011-0194
Lewis, R. S., and Cahalan, M. D. (1995). Potassium and calcium channels in lymphocytes. Annu. Rev. Immunol. 13, 623–653. doi: 10.1146/annurev.iy.13.040195.003203
Li, X., Staszewski, L., Xu, H., Durick, K., Zoller, M., and Adler, E. (2002). Human receptors for sweet and umami taste. Proc. Natl. Acad. Sci. U.S.A. 99, 4692–4696. doi: 10.1073/pnas.072090199
Lin, W., Margolskee, R., Donnert, G., Hell, S. W., and Restrepo, D. (2007). Olfactory neurons expressing transient receptor potential channel M5 (TRPM5) are involved in sensing semiochemicals. Proc. Natl. Acad. Sci. U.S.A. 104, 2471–2474. doi: 10.1073/pnas.0610201104
Lindemann, B. (2001). Receptors and transduction in taste. Nature 413, 219–225. doi: 10.1038/35093032
Litaudon, P., Bouillot, C., Zimmer, L., Costes, N., and Ravel, N. (2017). Activity in the rat olfactory cortex is correlated with behavioral response to odor: a microPET study. Brain Struct. Funct. 222, 577–586. doi: 10.1007/s00429-016-1235-8
Livingston, J. N., Unger, J. W., Moxley, R. T., and Moss, A. (1993). Phosphotyrosine-containing proteins in the CNS of obese Zucker rats are decreased in the absence of changes in the insulin receptor. Neuroendocrinology 57, 481–488. doi: 10.1159/000126395
Lledo, P. M., Gheusi, G., and Vincent, J. D. (2005). Information processing in the mammalian olfactory system. Physiol. Rev. 85, 281–317. doi: 10.1152/physrev.00008.2004
Loftus, T. M., Jaworsky, D. E., Frehywot, G. L., Townsend, C. A., Ronnett, G. V., Lane, M. D., et al. (2000). Reduced food intake and body weight in mice treated with fatty acid synthase inhibitors. Science 288, 2379–2381. doi: 10.1126/science.288.5475.2379
Loretz, C. A. (2008). Extracellular calcium-sensing receptors in fishes. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 149, 225–245. doi: 10.1016/j.cbpa.2008.01.037
Mackenzie, B., and Erickson, J. D. (2004). Sodium-coupled neutral amino acid (System N/A) transporters of the SLC38 gene family. Pflugers Arch. 447, 784–795. doi: 10.1007/s00424-003-1117-9
Magistretti, P. J., and Allaman, I. (2015). A cellular perspective on brain energy metabolism and functional imaging. Neuron 86, 883–901. doi: 10.1016/j.neuron.2015.03.035
Magnan, C., Levin, B. E., and Luquet, S. (2015). Brain lipid sensing and the neural control of energy balance. Mol. Cell. Endocrinol. 418(Pt 1), 3–8. doi: 10.1016/j.mce.2015.09.019
Malnic, B., Hirono, J., Sato, T., and Buck, L. B. (1999). Combinatorial receptor codes for odors. Cell 96, 713–723. doi: 10.1016/S0092-8674(00)80581-4
Marks, J. L., Porte, D. Jr., Stahl, W. L., and Baskin, D. G. (1990). Localization of insulin receptor mRNA in rat brain by in situ hybridization. Endocrinology 127, 3234–3236. doi: 10.1210/endo-127-6-3234
Martin, C., Chevrot, M., Poirier, H., Passilly-Degrace, P., Niot, I., and Besnard, P. (2011). CD36 as a lipid sensor. Physiol. Behav. 105, 36–42. doi: 10.1016/j.physbeh.2011.02.029
Marty, N., Dallaporta, M., and Thorens, B. (2007). Brain glucose sensing, counterregulation, and energy homeostasis. Physiology (Bethesda) 22, 241–251. doi: 10.1152/physiol.00010.2007
Masson, J., Pohl, M., Aidouni, Z., Giros, B., Hamon, M., and el Mestikawy, S. (1996). The two orphan Na+/Cl(-)-dependent transporters Rxt1 and V-7-3-2 have an overlapping expression pattern in the rat central nervous system. Recept. Channels 4, 227–242.
Maurin, A. C., Jousse, C., Averous, J., Parry, L., Bruhat, A., Cherasse, Y., et al. (2005). The GCN2 kinase biases feeding behavior to maintain amino acid homeostasis in omnivores. Cell Metab. 1, 273–277. doi: 10.1016/j.cmet.2005.03.004
McCall, A. L., Van Bueren, A. M., Moholt-Siebert, M., Cherry, N. J., and Woodward, W. R. (1994). Immunohistochemical localization of the neuron-specific glucose transporter (GLUT3) to neuropil in adult rat brain. Brain Res. 659, 292–297. doi: 10.1016/0006-8993(94)90896-6
McCrimmon, R. (2008). The mechanisms that underlie glucose sensing during hypoglycaemia in diabetes. Diabet. Med. 25, 513–522. doi: 10.1111/j.1464-5491.2008.02376.x
Meier, C., Ristic, Z., Klauser, S., and Verrey, F. (2002). Activation of system L heterodimeric amino acid exchangers by intracellular substrates. EMBO J. 21, 580–589. doi: 10.1093/emboj/21.4.580
Mergenthaler, P., Lindauer, U., Dienel, G. A., and Meisel, A. (2013). Sugar for the brain: the role of glucose in physiological and pathological brain function. Trends Neurosci. 36, 587–597. doi: 10.1016/j.tins.2013.07.001
Migrenne, S., Le Foll, C., Levin, B. E., and Magnan, C. (2011). Brain lipid sensing and nervous control of energy balance. Diabetes Metab. 37, 83–88. doi: 10.1016/j.diabet.2010.11.001
Mink, J. W., Blumenschine, R. J., and Adams, D. B. (1981). Ratio of central nervous system to body metabolism in vertebrates: its constancy and functional basis. Am. J. Physiol. 241, R203–R212.
Molina, D. K., and DiMaio, V. J. (2012). Normal organ weights in men: part II- the brain, lungs, liver, spleen, and kidneys. Am. J. Forensic Med. Pathol. 33, 368–372. doi: 10.1097/PAF.0b013e31823d29ad
Morrison, C. D., Reed, S. D., and Henagan, T. M. (2012). Homeostatic regulation of protein intake: in search of a mechanism. Am. J. Physiol. Regul. Integr. Comp. Physiol. 302, R917–R928. doi: 10.1152/ajpregu.00609.2011
Moulle, V. S., Cansell, C., Luquet, S., and Cruciani-Guglielmacci, C. (2012). The multiple roles of fatty acid handling proteins in brain. Front. Physiol. 3:385. doi: 10.3389/fphys.2012.00385
Moulle, V. S., Picard, A., Le Foll, C., Levin, B. E., and Magnan, C. (2014). Lipid sensing in the brain and regulation of energy balance. Diabetes Metab. 40, 29–33. doi: 10.1016/j.diabet.2013.10.001
Mudo, G., Trovato-Salinaro, A., Barresi, V., Belluardo, N., and Condorelli, D. F. (2009). Identification of calcium sensing receptor (CaSR) mRNA-expressing cells in normal and injured rat brain. Brain Res. 1298, 24–36. doi: 10.1016/j.brainres.2009.08.074
Nakamoto, K., Nishinaka, T., Matsumoto, K., Kasuya, F., Mankura, M., Koyama, Y., et al. (2012). Involvement of the long-chain fatty acid receptor GPR40 as a novel pain regulatory system. Brain Res. 1432, 74–83. doi: 10.1016/j.brainres.2011.11.012
Nakano, Y., Oomura, Y., Lenard, L., Nishino, H., Aou, S., Yamamoto, T., et al. (1986). Feeding-related activity of glucose- and morphine-sensitive neurons in the monkey amygdala. Brain Res. 399, 167–172. doi: 10.1016/0006-8993(86)90613-X
Nawroth, J. C., Greer, C. A., Chen, W. R., Laughlin, S. B., and Shepherd, G. M. (2007). An energy budget for the olfactory glomerulus. J. Neurosci. 27, 9790–9800. doi: 10.1523/JNEUROSCI.1415-07.2007
Nelson, G., Chandrashekar, J., Hoon, M. A., Feng, L., Zhao, G., Ryba, N. J., et al. (2002). An amino-acid taste receptor. Nature 416, 199–202. doi: 10.1038/nature726
Nicklin, P., Bergman, P., Zhang, B., Triantafellow, E., Wang, H., Nyfeler, B., et al. (2009). Bidirectional transport of amino acids regulates mTOR and autophagy. Cell 136, 521–534. doi: 10.1016/j.cell.2008.11.044
Nunez-Parra, A., Cortes-Campos, C., Bacigalupo, J., Garcia Mde, L., Nualart, F., and Reyes, J. G. (2011). Expression and distribution of facilitative glucose (GLUTs) and monocarboxylate/H+ (MCTs) transporters in rat olfactory epithelia. Chem. Senses 36, 771–780. doi: 10.1093/chemse/bjr052
Oberland, S., Ackels, T., Gaab, S., Pelz, T., Spehr, J., Spehr, M., et al. (2015). CD36 is involved in oleic acid detection by the murine olfactory system. Front. Cell. Neurosci. 9:366. doi: 10.3389/fncel.2015.00366
Obici, S., and Rossetti, L. (2003). Minireview: nutrient sensing and the regulation of insulin action and energy balance. Endocrinology 144, 5172–5178. doi: 10.1210/en.2003-0999
Oomura, Y. (1983). Glucose as a regulator of neuronal activity. Adv. Metab. Disord. 10, 31–65. doi: 10.1016/B978-0-12-027310-2.50008-6
Oomura, Y., Nakamura, T., Sugimori, M., and Yamada, Y. (1975). Effect of free fatty acid on the rat lateral hypothalamic neurons. Physiol. Behav. 14, 483–486. doi: 10.1016/0031-9384(75)90015-3
Oomura, Y., Ono, T., Ooyama, H., and Wayner, M. J. (1969). Glucose and osmosensitive neurones of the rat hypothalamus. Nature 222, 282–284. doi: 10.1038/222282a0
Otsuka, Y., Sakagami, H., Owada, Y., and Kondo, H. (1998). Differential localization of mRNAs for mammalian trps, presumptive capacitative calcium entry channels, in the adult mouse brain. Tohoku J. Exp. Med. 185, 139–146. doi: 10.1620/tjem.185.139
Palii, S. S., Thiaville, M. M., Pan, Y. X., Zhong, C., and Kilberg, M. S. (2006). Characterization of the amino acid response element within the human sodium-coupled neutral amino acid transporter 2 (SNAT2) System A transporter gene. Biochem. J. 395, 517–527. doi: 10.1042/BJ20051867
Palouzier-Paulignan, B., Lacroix, M. C., Aime, P., Baly, C., Caillol, M., Congar, P., et al. (2012). Olfaction under metabolic influences. Chem. Senses 37, 769–797. doi: 10.1093/chemse/bjs059
Philipp, S., Hambrecht, J., Braslavski, L., Schroth, G., Freichel, M., Murakami, M., et al. (1998). A novel capacitative calcium entry channel expressed in excitable cells. EMBO J. 17, 4274–4282. doi: 10.1093/emboj/17.15.4274
Picard, A., Moulle, V. S., Le Foll, C., Cansell, C., Veret, J., Coant, N., et al. (2014). Physiological and pathophysiological implications of lipid sensing in the brain. Diabetes Obes. Metab. 16(Suppl. 1), 49–55. doi: 10.1111/dom.12335
Pochini, L., Scalise, M., Galluccio, M., and Indiveri, C. (2014). Membrane transporters for the special amino acid glutamine: structure/function relationships and relevance to human health. Front. Chem. 2:61. doi: 10.3389/fchem.2014.00061
Prud'homme, M. J., Lacroix, M. C., Badonnel, K., Gougis, S., Baly, C., Salesse, R., et al. (2009). Nutritional status modulates behavioural and olfactory bulb Fos responses to isoamyl acetate or food odour in rats: roles of orexins and leptin. Neuroscience 162, 1287–1298. doi: 10.1016/j.neuroscience.2009.05.043
Pyrski, M., Eckstein, E., Schmid, A., Bufe, B., Weiss, J., Chubanov, V., et al. (2017). Trpm5 expression in the olfactory epithelium. Mol. Cell. Neurosci. 80, 75–88. doi: 10.1016/j.mcn.2017.02.002
Rapoport, S. I., Chang, M. C., and Spector, A. A. (2001). Delivery and turnover of plasma-derived essential PUFAs in mammalian brain. J. Lipid Res. 42, 678–685.
Ren, X., Zhou, L., Terwilliger, R., Newton, S. S., and de Araujo, I. E. (2009). Sweet taste signaling functions as a hypothalamic glucose sensor. Front. Integr. Neurosci. 3:12. doi: 10.3389/neuro.07.012.2009
Ressler, K. J., Sullivan, S. L., and Buck, L. B. (1994). Information coding in the olfactory system: evidence for a stereotyped and highly organized epitope map in the olfactory bulb. Cell 79, 1245–1255. doi: 10.1016/0092-8674(94)90015-9
Ritter, R. C., Slusser, P. G., and Stone, S. (1981). Glucoreceptors controlling feeding and blood glucose: location in the hindbrain. Science 213, 451–452. doi: 10.1126/science.6264602
Rogers, K. V., Dunn, C. K., Hebert, S. C., and Brown, E. M. (1997). Localization of calcium receptor mRNA in the adult rat central nervous system by in situ hybridization. Brain Res. 744, 47–56. doi: 10.1016/S0006-8993(96)01070-0
Rolen, S. H., Salcedo, E., Restrepo, D., and Finger, T. E. (2014). Differential localization of NT-3 and TrpM5 in glomeruli of the olfactory bulb of mice. J. Comp. Neurol. 522, 1929–1940. doi: 10.1002/cne.23512
Ropelle, E. R., Pauli, J. R., Fernandes, M. F., Rocco, S. A., Marin, R. M., Morari, J., et al. (2008). A central role for neuronal AMP-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR) in high-protein diet-induced weight loss. Diabetes 57, 594–605. doi: 10.2337/db07-0573
Ross, C. A., MacCumber, M. W., Glatt, C. E., and Snyder, S. H. (1989). Brain phospholipase C isozymes: differential mRNA localizations by in situ hybridization. Proc. Natl. Acad. Sci. U.S.A. 86, 2923–2927. doi: 10.1073/pnas.86.8.2923
Routh, V. H. (2010). Glucose sensing neurons in the ventromedial hypothalamus. Sensors (Basel) 10, 9002–9025. doi: 10.3390/s101009002
Routh, V. H., McArdle, J. J., Sanders, N. M., Song, Z., and Wang, R. (2007). “Glucose sensing neurons,” in Handbook of Neurochemistry and Molecular Neurobiology, eds A. Lajtha and D. Johnson (New York, NY: Springer), 205–228.
Rudell, J. B., Rechs, A. J., Kelman, T. J., Ross-Inta, C. M., Hao, S., and Gietzen, D. W. (2011). The anterior piriform cortex is sufficient for detecting depletion of an indispensable amino acid, showing independent cortical sensory function. J. Neurosci. 31, 1583–1590. doi: 10.1523/JNEUROSCI.4934-10.2011
Russell, M. C., Koehnle, T. J., Barrett, J. A., Blevins, J. E., and Gietzen, D. W. (2003). The rapid anorectic response to a threonine imbalanced diet is decreased by injection of threonine into the anterior piriform cortex of rats. Nutr. Neurosci. 6, 247–251. doi: 10.1080/1028415031000151567
Savigner, A., Duchamp-Viret, P., Grosmaitre, X., Chaput, M., Garcia, S., Ma, M., et al. (2009). Modulation of spontaneous and odorant-evoked activity of rat olfactory sensory neurons by two anorectic peptides, insulin and leptin. J. Neurophysiol. 101, 2898–2906. doi: 10.1152/jn.91169.2008
Scalise, M., Pochini, L., Panni, S., Pingitore, P., Hedfalk, K., and Indiveri, C. (2014). Transport mechanism and regulatory properties of the human amino acid transporter ASCT2 (SLC1A5). Amino Acids 46, 2463–2475. doi: 10.1007/s00726-014-1808-x
Schulingkamp, R. J., Pagano, T. C., Hung, D., and Raffa, R. B. (2000). Insulin receptors and insulin action in the brain: review and clinical implications. Neurosci. Biobehav. Rev. 24, 855–872. doi: 10.1016/S0149-7634(00)00040-3
Serizawa, S., Miyamichi, K., Nakatani, H., Suzuki, M., Saito, M., Yoshihara, Y., et al. (2003). Negative feedback regulation ensures the one receptor-one olfactory neuron rule in mouse. Science 302, 2088–2094. doi: 10.1126/science.1089122
Sharp, J. W., Ross-Inta, C. M., Baccelli, I., Payne, J. A., Rudell, J. B., and Gietzen, D. W. (2013). Effects of essential amino acid deficiency: down-regulation of KCC2 and the GABAA receptor; disinhibition in the anterior piriform cortex. J. Neurochem. 127, 520–530. doi: 10.1111/jnc.12403
Shoji, S. (1992). Glucose regulation of synaptic transmission in the dorsolateral septal nucleus of the rat. Synapse 12, 322–332. doi: 10.1002/syn.890120409
Silver, I. A., and Erecinska, M. (1998). Glucose-induced intracellular ion changes in sugar-sensitive hypothalamic neurons. J. Neurophysiol. 79, 1733–1745.
Smith, Q. R., and Nagura, H. (2001). Fatty acid uptake and incorporation in brain: studies with the perfusion model. J. Mol. Neurosci. 16, 167–172. discussion: 215–221. doi: 10.1385/jmn:16:2-3:167
Song, Z., Levin, B. E., McArdle, J. J., Bakhos, N., and Routh, V. H. (2001). Convergence of pre- and postsynaptic influences on glucosensing neurons in the ventromedial hypothalamic nucleus. Diabetes 50, 2673–2681. doi: 10.2337/diabetes.50.12.2673
Spanswick, D., Smith, M. A., Groppi, V. E., Logan, S. D., and Ashford, M. L. (1997). Leptin inhibits hypothalamic neurons by activation of ATP-sensitive potassium channels. Nature 390, 521–525. doi: 10.1038/37379
Sundberg, B. E., Waag, E., Jacobsson, J. A., Stephansson, O., Rumaks, J., Svirskis, S., et al. (2008). The evolutionary history and tissue mapping of amino acid transporters belonging to solute carrier families SLC32, SLC36, and SLC38. J. Mol. Neurosci. 35, 179–193. doi: 10.1007/s12031-008-9046-x
Taylor, P. M. (2014). Role of amino acid transporters in amino acid sensing. Am. J. Clin. Nutr. 99, 223S–230S. doi: 10.3945/ajcn.113.070086
Thiebaud, N., Johnson, M. C., Butler, J. L., Bell, G. A., Ferguson, K. L., Fadool, A. R., et al. (2014). Hyperlipidemic diet causes loss of olfactory sensory neurons, reduces olfactory discrimination, and disrupts odor-reversal learning. J. Neurosci. 34, 6970–6984. doi: 10.1523/JNEUROSCI.3366-13.2014
Tong, J., Mannea, E., Aime, P., Pfluger, P. T., Yi, C. X., Castaneda, T. R., et al. (2011). Ghrelin enhances olfactory sensitivity and exploratory sniffing in rodents and humans. J. Neurosci. 31, 5841–5846. doi: 10.1523/JNEUROSCI.5680-10.2011
Tucker, K., Cavallin, M. A., Jean-Baptiste, P., Biju, K. C., Overton, J. M., Pedarzani, P., et al. (2010). The olfactory bulb: a metabolic sensor of brain insulin and glucose concentrations via a voltage-gated potassium channel. Results Probl. Cell Differ. 52, 147–157. doi: 10.1007/978-3-642-14426-4_12
Tucker, K., Cho, S., Thiebaud, N., Henderson, M. X., and Fadool, D. A. (2013). Glucose sensitivity of mouse olfactory bulb neurons is conveyed by a voltage-gated potassium channel. J. Physiol. 591, 2541–2561. doi: 10.1113/jphysiol.2013.254086
Ueno, M., Akiguchi, I., Naiki, H., Fujibayashi, Y., Fukuyama, H., Kimura, J., et al. (1991). The persistence of high uptake of serum albumin in the olfactory bulbs of mice throughout their adult lives. Arch. Gerontol. Geriatr. 13, 201–209. doi: 10.1016/0167-4943(91)90062-U
Ueno, M., Dobrogowska, D. H., and Vorbrodt, A. W. (1996). Immunocytochemical evaluation of the blood-brain barrier to endogenous albumin in the olfactory bulb and pons of senescence-accelerated mice (SAM). Histochem. Cell Biol. 105, 203–212. doi: 10.1007/BF01462293
Unger, J., McNeill, T. H., Moxley, R. T. III., White, M., Moss, A., and Livingston, J. N. (1989). Distribution of insulin receptor-like immunoreactivity in the rat forebrain. Neuroscience 31, 143–157. doi: 10.1016/0306-4522(89)90036-5
Utsunomiya-Tate, N., Endou, H., and Kanai, Y. (1996). Cloning and functional characterization of a system ASC-like Na+-dependent neutral amino acid transporter. J. Biol. Chem. 271, 14883–14890. doi: 10.1074/jbc.271.25.14883
Vannucci, S. J., Koehler-Stec, E. M., Li, K., Reynolds, T. H., Clark, R., and Simpson, I. A. (1998). GLUT4 glucose transporter expression in rodent brain: effect of diabetes. Brain Res. 797, 1–11. doi: 10.1016/S0006-8993(98)00103-6
Vassar, R., Chao, S. K., Sitcheran, R., Nunez, J. M., Vosshall, L. B., and Axel, R. (1994). Topographic organization of sensory projections to the olfactory bulb. Cell 79, 981–991. doi: 10.1016/0092-8674(94)90029-9
Verrey, F. (2003). System L: heteromeric exchangers of large, neutral amino acids involved in directional transport. Pflugers Arch. 445, 529–533. doi: 10.1007/s00424-002-0973-z
Voigt, A., Bojahr, J., Narukawa, M., Hubner, S., Boehm, U., and Meyerhof, W. (2015). Transsynaptic tracing from taste receptor cells reveals local taste receptor gene expression in gustatory ganglia and brain. J. Neurosci. 35, 9717–9729. doi: 10.1523/JNEUROSCI.0381-15.2015
Wang, C., Ohno, K., Furukawa, T., Ueki, T., Ikeda, M., Fukuda, A., et al. (2005). Differential expression of KCC2 accounts for the differential GABA responses between relay and intrinsic neurons in the early postnatal rat olfactory bulb. Eur. J. Neurosci. 21, 1449–1455. doi: 10.1111/j.1460-9568.2005.03975.x
Watkins, P. A., Hamilton, J. A., Leaf, A., Spector, A. A., Moore, S. A., Anderson, R. E., et al. (2001). Brain uptake and utilization of fatty acids: applications to peroxisomal biogenesis diseases. J. Mol. Neurosci. 16, 87–92. discussion: 151–157. doi: 10.1385/JMN:16:2-3:87
Wellendorph, P., Johansen, L. D., and Brauner-Osborne, H. (2010). The emerging role of promiscuous 7TM receptors as chemosensors for food intake. Vitam. Horm. 84, 151–184. doi: 10.1016/B978-0-12-381517-0.00005-9
Wiczer, B. M., and Thomas, G. (2010). The role of the mTOR pathway in regulating food intake. Curr. Opin. Drug Discov. Devel. 13, 604–612.
Wright, E. M., and Turk, E. (2004). The sodium/glucose cotransport family SLC5. Pflugers Arch. 447, 510–518. doi: 10.1007/s00424-003-1202-0
Wu, G. (2009). Amino acids: metabolism, functions, and nutrition. Amino Acids 37, 1–17. doi: 10.1007/s00726-009-0269-0
Wullschleger, S., Loewith, R., and Hall, M. N. (2006). TOR signaling in growth and metabolism. Cell 124, 471–484. doi: 10.1016/j.cell.2006.01.016
Xu, J., Wang, P., Li, Y., Li, G., Kaczmarek, L. K., Wu, Y., et al. (2004). The voltage-gated potassium channel Kv1.3 regulates peripheral insulin sensitivity. Proc. Natl. Acad. Sci. U.S.A. 101, 3112–3117. doi: 10.1073/pnas.0308450100
Yano, S., Brown, E. M., and Chattopadhyay, N. (2004). Calcium-sensing receptor in the brain. Cell Calcium 35, 257–264. doi: 10.1016/j.ceca.2003.10.008
Yao, D., Mackenzie, B., Ming, H., Varoqui, H., Zhu, H., Hediger, M. A., et al. (2000). A novel system A isoform mediating Na+/neutral amino acid cotransport. J. Biol. Chem. 275, 22790–22797. doi: 10.1074/jbc.M002965200
Yokoi, M., Mori, K., and Nakanishi, S. (1995). Refinement of odor molecule tuning by dendrodendritic synaptic inhibition in the olfactory bulb. Proc. Natl. Acad. Sci. U.S.A. 92, 3371–3375. doi: 10.1073/pnas.92.8.3371
Yu, A. S., Hirayama, B. A., Timbol, G., Liu, J., Basarah, E., Kepe, V., et al. (2010). Functional expression of SGLTs in rat brain. Am. J. Physiol. Cell Physiol. 299, C1277–C1284. doi: 10.1152/ajpcell.00296.2010
Yue, J. T., and Lam, T. K. (2012). Lipid sensing and insulin resistance in the brain. Cell Metab. 15, 646–655. doi: 10.1016/j.cmet.2012.01.013
Zhang, C., Miller, C. L., Brown, E. M., and Yang, J. J. (2015). The calcium sensing receptor: from calcium sensing to signaling. Sci. China Life Sci. 58, 14–27. doi: 10.1007/s11427-014-4779-y
Zhang, P., McGrath, B. C., Reinert, J., Olsen, D. S., Lei, L., Gill, S., et al. (2002). The GCN2 eIF2alpha kinase is required for adaptation to amino acid deprivation in mice. Mol. Cell. Biol. 22, 6681–6688. doi: 10.1128/MCB.22.19.6681-6688.2002
Keywords: nutrient sensing, olfaction, piriform cortex, transporter, receptor, food intake, obesity, type 2 diabetes
Citation: Julliard A-K, Al Koborssy D, Fadool DA and Palouzier-Paulignan B (2017) Nutrient Sensing: Another Chemosensitivity of the Olfactory System. Front. Physiol. 8:468. doi: 10.3389/fphys.2017.00468
Received: 30 March 2017; Accepted: 19 June 2017;
Published: 12 July 2017.
Edited by:
Xavier Fioramonti, Laboratoire NutriNeurO, Université de Bordeaux, FranceReviewed by:
Claire Martin, Centre National de la Recherche Scientifique (CNRS), FranceXavier Grosmaitre, Centre National de la Recherche Scientifique (CNRS), France
Copyright © 2017 Julliard, Al Koborssy, Fadool and Palouzier-Paulignan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: A-Karyn Julliard, a2FyeW4uanVsbGlhcmRAdW5pdi1seW9uMS5mcg==
 A-Karyn Julliard
A-Karyn Julliard Dolly Al Koborssy
Dolly Al Koborssy Debra A. Fadool
Debra A. Fadool Brigitte Palouzier-Paulignan
Brigitte Palouzier-Paulignan