- Department of Automation, Biocybernetics and Robotics, Jozef Stefan Institute, Ljubljana, Slovenia
Obesity is associated with numerous chronic ailments and represents one of the major health and economic issues in the modernized societies. Accordingly, there is an obvious need for novel treatment approaches. Recently, based on the reports of reduced appetite and subsequent weight loss following high-altitude sojourns, exposure to hypoxia has been proposed as a viable weight-reduction strategy. While altitude-related appetite modulation is complex and not entirely clear, hypoxia-induced alterations in hormonal appetite modulation might be among the key underlying mechanisms. The present paper summarizes the up-to-date research on hypoxia/altitude-induced changes in the gut and adipose tissue derived peptides related to appetite regulation. Orexigenic hormone ghrelin and anorexigenic peptides leptin, glucagon-like peptide-1, peptide YY, and cholecystokinin have to-date been investigated as potential modulators of hypoxia-driven appetite alterations. Current evidence suggests that hypoxia can, especially acutely, lead to decreased appetite, most probably via reduction of acylated ghrelin concentration. Hypoxia-related short and long-term changes in other hormonal markers are more unclear although hypoxia seems to importantly modulate leptin levels, especially following prolonged hypoxic exposures. Limited evidence also suggests that different activity levels during exposures to hypoxia do not additively affect hormonal appetite markers. Although very few studies have been performed in obese/overweight individuals, the available data indicate that hypoxia/altitude exposures do not seem to differentially affect appetite regulation via hormonal pathways in this cohort. Given the lack of experimental data, future well-controlled acute and prolonged studies are warranted to expand our understanding of hypoxia-induced hormonal appetite modulation and its kinetics in health and disease.
Introduction
Obesity represents one of the key health issues in many western societies and is associated with numerous chronic ailments (Ng et al., 2014). Given that, over one billion of world population is currently overweight and at least 300 million people obese, there is an obvious need for new therapeutic strategies (Ng et al., 2014). Sojourns to high altitude and/or exposures to hypoxia have recently been proposed as a potential novel weight-loss strategy (Netzer et al., 2008; Quintero et al., 2010; Kayser and Verges, 2013). The rationale is based on anecdotal as well as scientific observations of significant body mass decreases following high altitude sojourns. Reductions in body mass observed at high altitudes seem to be a consequence of blunted appetite resulting in decreased energy intakes (Westerterp and Kayser, 2006; Benso et al., 2007; Kalson et al., 2010). This phenomenon, also termed “Altitude anorexia” was initially thought to be associated with altitude-related environmental factors (cold, dehydration, etc.) and medical conditions (acute mountain sickness) (Hackett and Roach, 2001). However, growing body of literature from well-controlled laboratory investigations indicates that hypoxia per se underlies the observed appetite changes (Westerterp-Plantenga et al., 1999; Wasse et al., 2012; Bailey et al., 2015).
While hypoxia-related appetite modulation is not completely understood, alterations in hormonal appetite regulation might be one of the key underlying mechanisms (Quintero et al., 2010; Kayser and Verges, 2013). The purpose of the present paper is to summarize to-date research on hypoxia/altitude-induced changes in the gut and adipose tissue derived peptides related to appetite regulation. Contemporary studies suggest that orexigenic hormone ghrelin and anorexigenic agents leptin, glucagon-like peptide-1 (GLP-1), peptide YY (PYY), and cholecystokinin (CCK) are among the main potential hormonal modulators of hypoxia-driven appetite changes. In addition to reviewing the current evidence regarding these hormonal markers, the review will also address the potential influence of different activity levels on hormonal appetite regulation under hypoxic conditions. Finally, considerations regarding future research directions are also discussed.
Hormonal Appetite Regulation
Energy balance is dynamically maintained by a complex integration of afferent and efferent metabolic and neural signals orchestrated by the central nervous system. It is indeed fascinating that most mammals are capable of maintaining relatively stable body mass over their lifespan despite significant fluctuations in energy expenditure on the one hand, and energy intake on the other (Strader and Woods, 2005). This indicates that long-term appetite control is tightly controlled and closely related to energy expenditure. Hypothalamus plays one of the main roles in the central appetite control (Schwartz, 2006). In particular, the efferent signals from the gut, pancreas, liver, and adipose tissue get integrated within the hypothalamus which then modulates appetite in order to maintain energy homeostasis (Hussain and Bloom, 2013). Several hormonal markers are involved in both, long (days-years) and short-term (meal-to-meal) appetite regulation. Leptin is one of the crucial peptides modulating long-term energy balance and constantly provides tonic signals from the body fat stores (Strader and Woods, 2005). On the other hand, various peptides, originating mostly from the gastrointestinal tract, modulate appetite in the short-term. These peptides signal meal-to-meal fluctuations in immediate energy availability and thereby regulate short-term changes in satiety and hunger control (Perry and Wang, 2012). While the key hormonal markers are briefly summarized below, the interested readers are referred to a number of comprehensive reviews on the topic (Neary et al., 2004; Strader and Woods, 2005; Huda et al., 2006; Coll et al., 2007; Stensel, 2010; Perry and Wang, 2012).
As mentioned above leptin is the key long-term satiety signaling peptide produced almost exclusively within the adipocytes (Zhang et al., 1994). The systemic circulating leptin levels closely mirror the amount of adipose tissue (Shimizu et al., 1997) and tend to decrease and increase in response to starvation and overfeeding, respectively. While the critical importance of leptin in maintaining energy balance through direct anorexigenic signaling to the hypothalamus is clearly established, it is of note that the ability of leptin to inhibit appetite can be blunted (Enriori et al., 2006). This phenomenon, also termed “leptin resistance” is commonly observed in obese and overweight individuals. Another adipose tissue derived peptide proposed to play important role in energy balance is adiponectin (Trujillo and Scherer, 2005). In contrast to leptin, adiponectin concentration is inversely related to adipose tissue mass and seems to augment appetite via adenosine monophosphate-activated protein kinase stimulation within the hypothalamus (Steinberg and Kemp, 2007).
Peptide GLP-1 has been shown to promote satiety through its direct effect on central appetite regulation (Naslund et al., 1999) and also indirectly by reducing gastric emptying and suppressing gastric acid secretion (Verdich et al., 2001). GLP-1 is released from the endocrine L-cells within the small and large intestine in response to feeding and is one of the most powerful incretin hormones (Drucker, 2006), significantly influencing insulin secretion and biosynthesis within the pancreas (Baggio and Drucker, 2007). It is of note, that peripherally the GLP-1 exists in its active (GLP-17–36) and inactive (GLP-19–37) form. Concurrently with the GLP-1, the endocrine L-cells of the intestines release the PYY which is also a potent short-term appetite modulating peptide with an anorexogenic effect (Chaudhri et al., 2006). While the circulating PYY levels in fasting state are low they increase rapidly in response to food intake when PYY1–36 and PYY3–36 forms are released into the circulation, with the latter being the most abundant and active form. PYY mostly mediates its appetite effects through neuropeptide Y receptors and is additionally involved in gastric motility inhibition and electrolyte absorption augmentation in the gut (Batterham and Bloom, 2003). Another gut-derived peptide implicated in satiety signaling is CCK. It was the first discovered gut peptide demonstrated to be an important modulator of postprandial satiety (Kissileff et al., 1981). The CCK is released in the circulation, in response to fatty acids, from the I cells of the small intestine and exerts its action via the influence of CCK1 receptor on the hypothalamus and the brainstem (Moran et al., 1992). Besides its appetite-related effects, the CCK also promotes fat and protein digestion (Liddle et al., 1985).
Ghrelin, also known as the “hunger hormone,” is an enteric peptide involved in short-term energy balance maintenance. Interestingly, it is the only orexigenic appetite-related gut signal discovered to date (Williams and Cummings, 2005) and is produced exclusively within the oxyntic glands of the stomach. It is well established that ghrelin levels decrease postprandially and are increased prior to meals (Cummings et al., 2001). Augmented ghrelin levels promote gastric motility, growth hormone release and attenuate fat utilization (Kojima and Kangawa, 2005). It is important to note that ghrelin tends to exert the orexigenic effect only in its acylated form (Ghigo et al., 2005). Given that the acylated ghrelin form represents only 10–20% of the total circulating ghrelin, this notion needs to be taken into account when interpreting the outcomes of the studies investigating its appetite-related function.
Obviously, there are also other peptides that have been implicated in the general energy balance maintenance. In particular, the pancreas derived amylin (Roth, 2013) and pancreatic polypeptide (Batterham et al., 2003), interleukins from the adipose tissue (Zorrilla et al., 2007) as well as the oxyntomodulin (Cohen et al., 2003), obestatin (Beasley et al., 2009), and insulin-like peptide 5 (Grosse et al., 2014) produced within the gut have all been shown to influence appetite in a complex interplay with the other incretin and previously discussed hormones. However, given the limited scope, the present paper is focused solely on the appetite-signaling peptides that have already been investigated in regards to their role in hypoxia-related appetite modulation. The main findings of the up-to-date studies on the effects of altitude/hypoxia on these select markers is summarized in the following section.
Hypoxia-Related Changes in Hormonal Regulation
As noted above, appetite reductions are consistently reported in individuals acutely exposed to higher terrestrial altitudes (Westerterp-Plantenga et al., 1999; Westerterp and Kayser, 2006). While this was initially thought to be a consequence of other altitude-related factors (Hackett and Roach, 2001), growing body of literature indicates that altitude-related hypoxia seems to be the main driver of the observed appetite alterations. Indeed, hypoxia has been shown to affect a number of hormonal markers involved in appetite regulation. The outcomes of the key well-controlled short-term and long-term investigations on the effects of hypobaric (terrestrial) and/or normobaric (simulated altitude) hypoxia are presented in Tables 1, 2, respectively. Within the present review, the term acute (short-term) and prolonged (long-term) exposures are used to define hypoxic exposures of ≤ 24-h and >24-h duration, respectively.
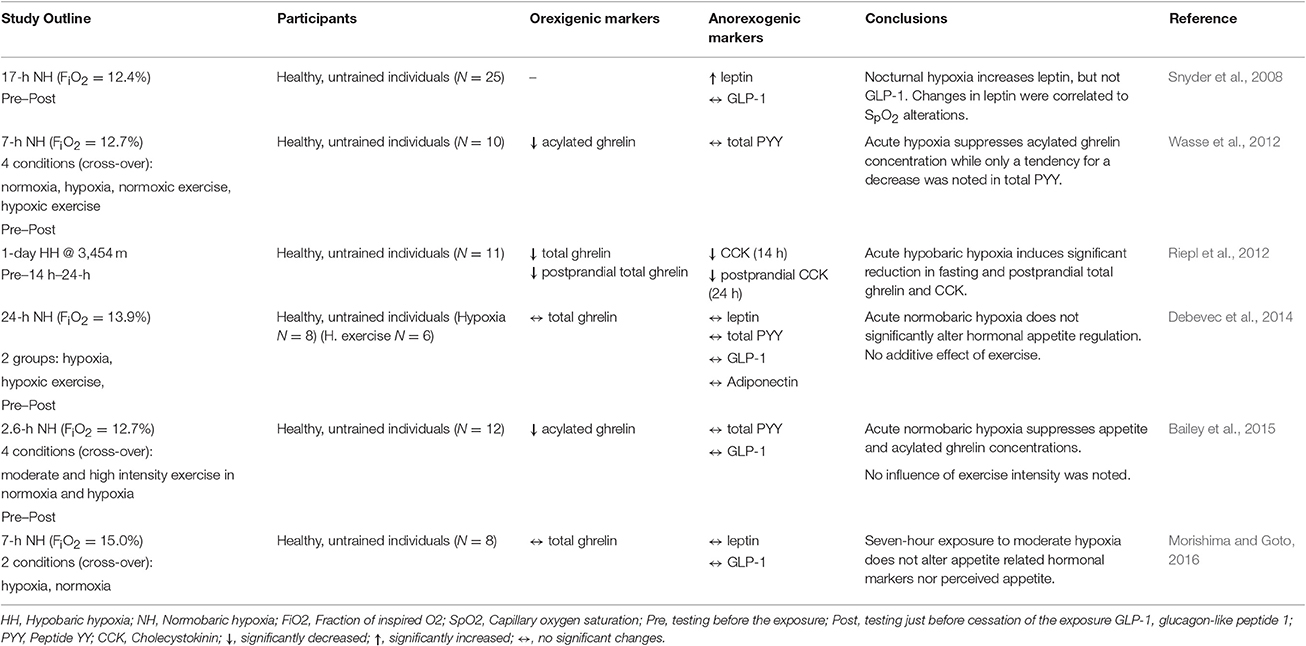
Table 1. Key findings from the controlled studies investigating the effects of acute and short-term hypoxic/altitude exposures on select orexigenic and anorexigenic hormonal markers.
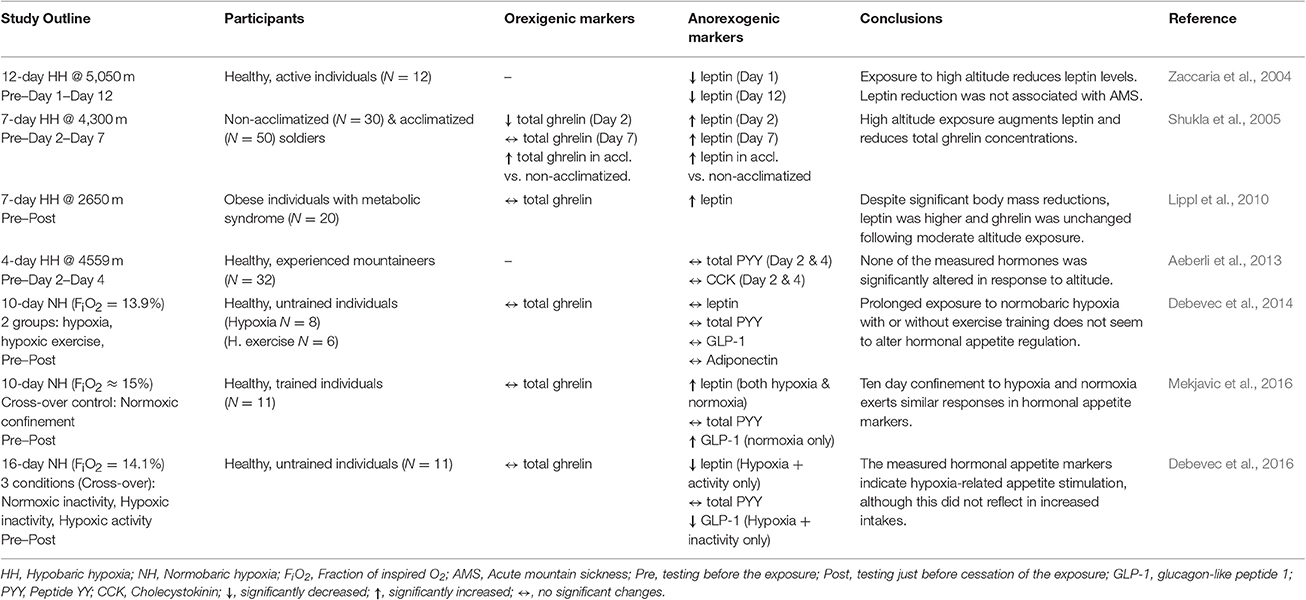
Table 2. Key findings from the controlled studies investigating the effects of long-term hypoxic/altitude exposures on select orexigenic and anorexigenic hormonal markers.
Leptin has been one of the first hormones implicated in altitude-related anorexia (Tschop et al., 1998). Mechanistically, hypoxia-induced stimulation of transcription factor HIF-1α, a key regulator of cellular responses to reduced O2 availability, can also effect circulating leptin via HIF-1α-dependent expression of the leptin gene expression (Grosfeld et al., 2002). However, albeit the fact that both acute (Tschop et al., 1998; Snyder et al., 2008) and prolonged (Shukla et al., 2005; Lippl et al., 2010; Mekjavic et al., 2016) exposures to hypoxia have demonstrated increases in circulating leptin levels its role in hypoxia-related appetite modulation is unclear. In particular, numerous studies have also demonstrated unaltered (Benso et al., 2007; Debevec et al., 2014; Morishima and Goto, 2016) or even decreased (Zaccaria et al., 2004; Debevec et al., 2016) leptin levels in response to hypoxic exposure. Interestingly, the only to-date study investigating the effects of altitude exposure on leptin levels in obese individuals showed a significant increase following 7-day exposure to 2,650 m (Lippl et al., 2010). It is important to note that the outcomes of field, or not suitably controlled, studies might have been confounded by factors such as environmental influences (i.e., temperature, humidity), diet, activity levels, as well as changes in body composition, all known to importantly modulate leptin release (Sierra-Johnson et al., 2008). In addition, the pulsatile manner of leptin secretion and its circadian variation (Park and Ahima, 2015) might have also explain some of the discrepancies. However, even when taking into account only the outcomes of the laboratory based, strictly-controlled studies, both short (Snyder et al., 2008; Morishima and Goto, 2016) and long-term (Debevec et al., 2014, 2016) exposures led to contrasting leptin responses. Although the effect of hypoxia on leptin release, as well as its influence on hypoxia-related appetite modulation, needs to be clarified it seems that hypoxia does influence leptin concentration, at least in response to long-term exposures.
To-date, hypoxia-related GLP-1 modulation received little attention. Snyder et al. (2008) were the first to assess the influence of acute (17-h) exposure to simulated hypoxia on fasting GLP-1 levels and did not find any independent hypoxic effect. These finding were further corroborated by subsequent acute (Bailey et al., 2015; Morishima and Goto, 2016) and also prolonged (Debevec et al., 2014; Mekjavic et al., 2016) hypoxic exposures. All of these studies did not show any significant effect of different hypoxia levels (fraction of inspired O2 (FiO2) from 12 to 15%) on fasting as well as postprandial GLP-1 levels. Interestingly, postprandial GLP-1 plasma concentration was shown to decrease following 16 days of hypoxic confinement when combined with bed rest-induced inactivity (Debevec et al., 2016). While this might suggest an effect of different activity levels on hypoxia-related GLP-1 modulation, previous two studies on the topic did not elucidate any significant influence of exercise (Debevec et al., 2014; Bailey et al., 2015). Based on the current studies, GLP-1 does not seem to be particularly influenced by environmental hypoxia and thus its potential role and contribution to the complex altitude-related appetite reduction is questionable.
In contrast to GLP-1, a tendency for a decrease in PYY was noted following acute (7-h) hypoxic exposure (Wasse et al., 2012). The study by Wasse et al. (2012) was the first to assess the potential contribution of PYY to hypoxia-provoked appetite changes. Few recent studies also investigated the hypoxia-induced alterations in PYY. However, all of them failed to demonstrate any significant effect of hypoxia per se either following acute (Bailey et al., 2015) and prolonged (Aeberli et al., 2013; Debevec et al., 2014, 2016; Mekjavic et al., 2016) hypoxic exposures. The fact that the changes in total PYY instead of its active and most potent form (PYY3–36) were assessed in all of the above investigations might underlie the lack of changes. Nevertheless, based on the available data the PYY does not seem to be affected by hypoxia and thus, might not play a role in altitude-related appetite modulation.
Bailey et al. (2000) initially demonstrated that high altitude trekking (up to ~5,100 m) can augment CCK levels in lowlanders and suggested that this increase might underlie the observed food intake reduction. Interestingly they also noted higher CCK responses in those suffering from AMS symptoms than those without. Albeit this initial findings, subsequent study from the same group conversely suggested that hypoxia might reduce CCK levels if applied during acute exercise (Bailey et al., 2001). This is congruent with the only two other remaining studies on the topic suggesting that acute exposure to ~4,500 m does not alter CCK levels (Aeberli et al., 2013) and furthermore that fasting and postprandial CCK levels might be reduced as a consequence of 24-h exposure to ~3,500 m (Riepl et al., 2012). Regardless of the fact that, except for the study by Bailey et al. (2001), all investigations were performed in the field scenarios and might therefore be confounded by other terrestrial altitude-related environmental factors, current evidence does not support the notion that CCK plays an important role in hypoxia-related appetite modulation.
Ghrelin is the only orexigenic gastrointestinal peptide that was implicated in hypoxia-related appetite regulation. More than a decade ago, Shukla et al. (2005) initially demonstrated that acute exposure to terrestrial altitude (~4,300 m) reduces fasting ghrelin although this decrease was not observed following 7 days of altitude residence. Although the exact mechanism of hypoxia-related changes in ghrelin is unclear, the reduction of the liver blood flow, due to reduced O2 availability-induced blood redistribution, and subsequent reduction in ghrelin acylation might play a role (Bailey et al., 2015). Subsequent terrestrial investigations provided further support for the altitude-induced acute ghrelin reduction (Riepl et al., 2012) as well as for the lack of changes following long-term altitude residence (Benso et al., 2007). Two recent well-controlled studies performed in normobaric hypoxia further showed that hypoxia per se can significantly blunt circulating acylated ghrelin concentration regardless of activity levels (Wasse et al., 2012; Bailey et al., 2015). In contrast, total ghrelin concentration did not seem to change in response to seven (Morishima and Goto, 2016) or twenty-four (Debevec et al., 2014) hour exposures to moderate (FiO2 = 15.0%) or high (FiO2 = 13.9%) simulated altitudes, respectively. As suggested by Bailey et al, the discrepancies in the acute findings are most probably a consequence of measuring total in some and acylated ghrelin form in the other studies. Regardless of the above, studies indicate that ghrelin levels do not seem to be reduced following prolonged exposures to normobaric hypoxia (Debevec et al., 2014, 2016; Mekjavic et al., 2016). No changes in total ghrelin were also observed in obese individuals following 7-day exposure to 2,600 m (Lippl et al., 2010). Taken together, hypoxia seems to, at least acutely, blunt ghrelin levels and thereby directly induce appetite reduction. However, the kinetics and long-term hypoxia-induced ghrelin modulation remains currently ambiguous.
Hypoxia, Exercise, and Hormonal Appetite Regulation
Very few studies to date examined the combined effects of hypoxia and exercise on hormonal appetite regulation although this approach seems promising for obesity treatment (Netzer et al., 2008; Urdampilleta et al., 2012; Millet et al., 2016). Besides the already mentioned acute investigations (Bailey et al., 2001; Wasse et al., 2012), only two studies to date scrutinized the influence of concomitant hypoxia and exercise training (4 weeks, three session per week) on select hormonal appetite markers (Haufe et al., 2008; Morishima et al., 2014). Haufe et al. (2008) did not find any additional effect of hypoxia following 4-week training period on both leptin and adiponectin concentrations although their data suggests that hypoxic as compared to normoxic training can induce superior overall metabolic adaptations. Similarly, Morishima and Goto (2016) did not elucidate any hypoxia-dependent effect on fasting or postprandial total ghrelin or leptin concentrations, while the GLP-1 concentration tended to be lower following hypoxic as compared to normoxic training. Also, no additional effect of daily moderate intensity exercise training during 10-day hypoxic confinement was observed on postprandial total ghrelin, PYY and GLP-1 concentrations (Debevec et al., 2014). Collectively, the data from acute and prolonged investigations combining hypoxia and activity do not provide support for importance of activity levels in hypoxia-related hormonal appetite modulation.
Conclusions
The reviewed data, pertinent to altitude/hypoxia-related hormonal appetite regulation suggests that acutely, exposure to hypoxia can reduce acylated ghrelin concentration and thereby decrease appetite. Hypoxia-related short and long-term changes in other hormonal markers are more unclear although hypoxia seems to importantly modulate leptin levels, especially following prolonged hypoxic exposures. The lack of any significant effects of hypoxia on both total PYY and GLP-1 might suggest that they are not crucial in hypoxia-related appetite regulation. Limited investigations regarding the combined effects of hypoxia and exercise also indicate that different activity levels during hypoxic exposures, do not seem to additively affect hormonal appetite markers.
Given the complexity of hormonal appetite regulation (Coll et al., 2007) and the potential influence of other (environmental) factors, future studies should aim at appropriate standardization of the procedures and strict control of potential confounding factors. This is especially important for long-term investigations which are crucial for expanding our understanding of the time and hypoxic-dose dependent changes in appetite hormonal markers. Indeed, while up-to-date studies provided some evidence regarding both acute and prolonged effects of hypoxia on select markers the time course and kinetics (i.e., acclimatization effect) of these markers has yet to be investigated. Furthermore, potential differences in hormonal appetite modulation responses to repeated acute hypoxic exposures (e.g., few hypoxic sessions per week), as opposed to continuous prolonged exposures are currently unclear and worthy of a study. It is also important to note that hypobaric and normobaric hypoxia have previously been shown to differentially affect select cardiorespiratory and hematological parameters (Faiss et al., 2013). Accordingly, potential independent effect of hypobaria and hypoxia on hormonal appetite markers should be considered and further explored. Collectively, future well-controlled acute and prolonged studies are warranted to expand our understanding of hypoxia-induced hormonal appetite modulation and its kinetics in health and disease.
Author Contributions
TD collected the data, drafted and revised the manuscript and approved the final version.
Funding
The work was supported by Automation, Biocybernetics and Robotics program group grant (P2-0076), Jozef Stefan Institute.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Aeberli, I., Erb, A., Spliethoff, K., Meier, D., Gotze, O., Fruhauf, H., et al. (2013). Disturbed eating at high altitude: influence of food preferences, acute mountain sickness and satiation hormones. Eur. J. Nutr. 52, 625–635. doi: 10.1007/s00394-012-0366-9
Baggio, L. L., and Drucker, D. J. (2007). Biology of incretins: GLP-1 and GIP. Gastroenterology 132, 2131–2157. doi: 10.1053/j.gastro.2007.03.054
Bailey, D. M., Davies, B., Castell, L. M., Newsholme, E. A., and Calam, J. (2001). Physical exercise and normobaric hypoxia: independent modulators of peripheral cholecystokinin metabolism in man. J. Appl. Physiol. 90, 105–113.
Bailey, D. M., Davies, B., Milledge, J. S., Richards, M., Williams, S. R., Jordinson, M., et al. (2000). Elevated plasma cholecystokinin at high altitude: metabolic implications for the anorexia of acute mountain sickness. High Alt. Med. Biol. 1, 9–23. doi: 10.1089/152702900320649
Bailey, D. P., Smith, L. R., Chrismas, B. C., Taylor, L., Stensel, D. J., Deighton, K., et al. (2015). Appetite and gut hormone responses to moderate-intensity continuous exercise versus high-intensity interval exercise, in normoxic and hypoxic conditions. Appetite 89, 237–245. doi: 10.1016/j.appet.2015.02.019
Batterham, R. L., and Bloom, S. R. (2003). The gut hormone peptide YY regulates appetite. Ann. N.Y. Acad. Sci. 994, 162–168. doi: 10.1111/j.1749-6632.2003.tb03176.x
Batterham, R. L., Le Roux, C. W., Cohen, M. A., Park, A. J., Ellis, S. M., Patterson, M., et al. (2003). Pancreatic polypeptide reduces appetite and food intake in humans. J. Clin. Endocrinol. Metab. 88, 3989–3992. doi: 10.1210/jc.2003-030630
Beasley, J. M., Ange, B. A., Anderson, C. A., Miller Iii, E. R., Holbrook, J. T., and Appel, L. J. (2009). Characteristics associated with fasting appetite hormones (obestatin, ghrelin, and leptin). Obesity 17, 349–354. doi: 10.1038/oby.2008.551
Benso, A., Broglio, F., Aimaretti, G., Lucatello, B., Lanfranco, F., Ghigo, E., et al. (2007). Endocrine and metabolic responses to extreme altitude and physical exercise in climbers. Eur. J. Endocrinol. 157, 733–740. doi: 10.1530/EJE-07-0355
Chaudhri, O., Small, C., and Bloom, S. (2006). Gastrointestinal hormones regulating appetite. Philos. Trans. R. Soc. Lond. B Biol. Sci. 361, 1187–1209. doi: 10.1098/rstb.2006.1856
Cohen, M. A., Ellis, S. M., Le Roux, C. W., Batterham, R. L., Park, A., Patterson, M., et al. (2003). Oxyntomodulin suppresses appetite and reduces food intake in humans. J. Clin. Endocrinol. Metab. 88, 4696–4701. doi: 10.1210/jc.2003-030421
Coll, A. P., Farooqi, I. S., and O'Rahilly, S. (2007). The hormonal control of food intake. Cell 129, 251–262. doi: 10.1016/j.cell.2007.04.001
Cummings, D. E., Purnell, J. Q., Frayo, R. S., Schmidova, K., Wisse, B. E., and Weigle, D. S. (2001). A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 50, 1714–1719. doi: 10.2337/diabetes.50.8.1714
Debevec, T., Simpson, E. J., Macdonald, I. A., Eiken, O., and Mekjavic, I. B. (2014). Exercise training during normobaric hypoxic confinement does not alter hormonal appetite regulation. PLoS ONE 9:e98874. doi: 10.1371/journal.pone.0098874
Debevec, T., Simpson, E. J., Mekjavic, I. B., Eiken, O., and Macdonald, I. A. (2016). Effects of prolonged hypoxia and bed rest on appetite and appetite-related hormones. Appetite 107, 28–37. doi: 10.1016/j.appet.2016.07.005
Drucker, D. J. (2006). The biology of incretin hormones. Cell Metab. 3, 153–165. doi: 10.1016/j.cmet.2006.01.004
Enriori, P. J., Evans, A. E., Sinnayah, P., and Cowley, M. A. (2006). Leptin resistance and obesity. Obesity 14(Suppl. 5), 254S–258S. doi: 10.1038/oby.2006.319
Faiss, R., Pialoux, V., Sartori, C., Faes, C., Deriaz, O., and Millet, G. P. (2013). Ventilation, oxidative stress and nitric oxide in hypobaric vs. normobaric hypoxia. Med. Sci. Sports Exerc. 45, 253–260. doi: 10.1249/MSS.0b013e31826d5aa2
Ghigo, E., Broglio, F., Arvat, E., Maccario, M., Papotti, M., and Muccioli, G. (2005). Ghrelin: more than a natural GH secretagogue and/or an orexigenic factor. Clin. Endocrinol. 62, 1–17. doi: 10.1111/j.1365-2265.2004.02160.x
Grosfeld, A., Andre, J., Hauguel-De Mouzon, S., Berra, E., Pouyssegur, J., and Guerre-Millo, M. (2002). Hypoxia-inducible factor 1 transactivates the human leptin gene promoter. J. Biol. Chem. 277, 42953–42957. doi: 10.1074/jbc.M206775200
Grosse, J., Heffron, H. A., Burling, K., Hossain, M. A., Habib, A. M., Rogers, G. J., et al. (2014). Insulin-like peptide 5 is an orexigenic gastrointestinal hormone. Proc. Natl. Acad. Sci. U.S.A. 111, 11133–11138. doi: 10.1073/pnas.1411413111
Hackett, P. H., and Roach, R. C. (2001). High-altitude illness. N. Engl. J. Med. 345, 107–114. doi: 10.1056/NEJM200107123450206
Haufe, S., Wiesner, S., Engeli, S., Luft, F. C., and Jordan, J. (2008). Influences of normobaric hypoxia training on metabolic risk markers in human subjects. Med. Sci. Sports Exerc. 40, 1939–1944. doi: 10.1249/MSS.0b013e31817f1988
Huda, M. S., Wilding, J. P., and Pinkney, J. H. (2006). Gut peptides and the regulation of appetite. Obes. Rev. 7, 163–182. doi: 10.1111/j.1467-789X.2006.00245.x
Hussain, S. S., and Bloom, S. R. (2013). The regulation of food intake by the gut-brain axis: implications for obesity. Int. J. Obes. 37, 625–633. doi: 10.1038/ijo.2012.93
Kalson, N. S., Hext, F., Davies, A. J., Chan, C. W., Wright, A. D., Imray, C. H., et al. (2010). Do changes in gastro-intestinal blood flow explain high-altitude anorexia? Eur. J. Clin. Invest. 40, 735–741. doi: 10.1111/j.1365-2362.2010.02324.x
Kayser, B., and Verges, S. (2013). Hypoxia, energy balance and obesity: from pathophysiological mechanisms to new treatment strategies. Obes. Rev. 14, 579–592. doi: 10.1111/obr.12034
Kissileff, H. R., Pi-Sunyer, F. X., Thornton, J., and Smith, G. P. (1981). C-terminal octapeptide of cholecystokinin decreases food intake in man. Am. J. Clin. Nutr. 34, 154–160.
Kojima, M., and Kangawa, K. (2005). Ghrelin: structure and function. Physiol. Rev. 85, 495–522. doi: 10.1152/physrev.00012.2004
Liddle, R. A., Goldfine, I. D., Rosen, M. S., Taplitz, R. A., and Williams, J. A. (1985). Cholecystokinin bioactivity in human plasma. Molecular forms, responses to feeding, and relationship to gallbladder contraction. J. Clin. Invest. 75, 1144–1152. doi: 10.1172/JCI111809
Lippl, F. J., Neubauer, S., Schipfer, S., Lichter, N., Tufman, A., Otto, B., et al. (2010). Hypobaric hypoxia causes body weight reduction in obese subjects. Obesity 18, 675–681. doi: 10.1038/oby.2009.509
Mekjavic, I. B., Amon, M., Kölegård, R., Kounalakis, S. N., Simpson, E., Eiken, O., et al. (2016). The effect of normobaric hypoxic confinement on metabolism, gut hormones and body composition. Front. Physiol. 7:202. doi: 10.3389/fphys.2016.00202
Millet, G. P., Debevec, T., Brocherie, F., Malatesta, D., and Girard, O. (2016). Therapeutic use of exercising in hypoxia: promises and limitations. Front. Physiol. 7:224. doi: 10.3389/fphys.2016.00224
Moran, T. H., Ameglio, P. J., Schwartz, G. J., and McHugh, P. R. (1992). Blockade of type A, not type B, CCK receptors attenuates satiety actions of exogenous and endogenous CCK. Am. J. Physiol. 262, R46–R50.
Morishima, T., and Goto, K. (2016). Ghrelin, GLP-1, and leptin responses during exposure to moderate hypoxia. Appl. Physiol. Nutr. Metab. 41, 375–381. doi: 10.1139/apnm-2015-0311
Morishima, T., Kurihara, T., Hamaoka, T., and Goto, K. (2014). Whole body, regional fat accumulation, and appetite-related hormonal response after hypoxic training. Clin. Physiol. Funct. Imaging 34, 90–97. doi: 10.1111/cpf.12069
Naslund, E., Barkeling, B., King, N., Gutniak, M., Blundell, J. E., Holst, J. J., et al. (1999). Energy intake and appetite are suppressed by glucagon-like peptide-1 (GLP-1) in obese men. Int. J. Obes. Relat. Metab. Disord. 23, 304–311. doi: 10.1038/sj.ijo.0800818
Neary, N. M., Goldstone, A. P., and Bloom, S. R. (2004). Appetite regulation: from the gut to the hypothalamus. Clin. Endocrinol. 60, 153–160. doi: 10.1046/j.1365-2265.2003.01839.x
Netzer, N. C., Chytra, R., and Kupper, T. (2008). Low intense physical exercise in normobaric hypoxia leads to more weight loss in obese people than low intense physical exercise in normobaric sham hypoxia. Sleep Breath. 12, 129–134. doi: 10.1007/s11325-007-0149-3
Ng, M., Fleming, T., Robinson, M., Thomson, B., Graetz, N., Margono, C., et al. (2014). Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the global burden of disease study 2013. Lancet 384, 766–781. doi: 10.1016/S0140-6736(14)60460-8
Park, H. K., and Ahima, R. S. (2015). Physiology of leptin: energy homeostasis, neuroendocrine function and metabolism. Metab. Clin. Exp. 64, 24–34. doi: 10.1016/j.metabol.2014.08.004
Perry, B., and Wang, Y. (2012). Appetite regulation and weight control: the role of gut hormones. Nutr. Diabetes 2:e26. doi: 10.1038/nutd.2011.21
Quintero, P., Milagro, F. I., Campion, J., and Martinez, J. A. (2010). Impact of oxygen availability on body weight management. Med. Hypotheses 74, 901–907. doi: 10.1016/j.mehy.2009.10.022
Riepl, R. L., Fischer, R., Hautmann, H., Hartmann, G., Muller, T. D., Tschop, M., et al. (2012). Influence of acute exposure to high altitude on basal and postprandial plasma levels of gastroenteropancreatic peptides. PLoS ONE 7:e44445. doi: 10.1371/journal.pone.0044445
Roth, J. D. (2013). Amylin and the regulation of appetite and adiposity: recent advances in receptor signaling, neurobiology and pharmacology. Curr. Opin. Endocrinol. Diabetes Obes. 20, 8–13. doi: 10.1097/MED.0b013e32835b896f
Schwartz, M. W. (2006). Central nervous system regulation of food intake. Obesity 14(Suppl. 1), 1S–8S. doi: 10.1038/oby.2006.275
Shimizu, H., Shimomura, Y., Hayashi, R., Ohtani, K., Sato, N., Futawatari, T., et al. (1997). Serum leptin concentration is associated with total body fat mass, but not abdominal fat distribution. Int. J. Obes. Relat. Metab. Disord. 21, 536–541. doi: 10.1038/sj.ijo.0800437
Shukla, V., Singh, S. N., Vats, P., Singh, V. K., Singh, S. B., and Banerjee, P. K. (2005). Ghrelin and leptin levels of sojourners and acclimatized lowlanders at high altitude. Nutr. Neurosci. 8, 161–165. doi: 10.1080/10284150500132823
Sierra-Johnson, J., Romero-Corral, A., Somers, V. K., and Johnson, B. D. (2008). Effect of altitude on leptin levels, does it go up or down? J. Appl. Physiol. 105, 1684–1685. doi: 10.1152/japplphysiol.01284.2007
Snyder, E. M., Carr, R. D., Deacon, C. F., and Johnson, B. D. (2008). Overnight hypoxic exposure and glucagon-like peptide-1 and leptin levels in humans. Appl. Physiol. Nutr. Metab. 33, 929–935. doi: 10.1139/H08-079
Steinberg, G. R., and Kemp, B. E. (2007). Adiponectin: starving for attention. Cell Metab. 6, 3–4. doi: 10.1016/j.cmet.2007.06.008
Stensel, D. (2010). Exercise, appetite and appetite-regulating hormones: implications for food intake and weight control. Ann. Nutr. Metab. 57(Suppl. 2), 36–42. doi: 10.1159/000322702
Strader, A. D., and Woods, S. C. (2005). Gastrointestinal hormones and food intake. Gastroenterology 128, 175–191. doi: 10.1053/j.gastro.2004.10.043
Trujillo, M. E., and Scherer, P. E. (2005). Adiponectin–journey from an adipocyte secretory protein to biomarker of the metabolic syndrome. J. Intern. Med. 257, 167–175. doi: 10.1111/j.1365-2796.2004.01426.x
Tschop, M., Strasburger, C. J., Hartmann, G., Biollaz, J., and Bartsch, P. (1998). Raised leptin concentrations at high altitude associated with loss of appetite. Lancet 352, 1119–1120. doi: 10.1016/S0140-6736(05)79760-9
Urdampilleta, A., Gonzalez-Muniesa, P., Portillo, M. P., and Martinez, J. A. (2012). Usefulness of combining intermittent hypoxia and physical exercise in the treatment of obesity. J. Physiol. Biochem. 68, 289–304. doi: 10.1007/s13105-011-0115-1
Verdich, C., Flint, A., Gutzwiller, J. P., Naslund, E., Beglinger, C., Hellstrom, P. M., et al. (2001). A meta-analysis of the effect of glucagon-like peptide-1 (7-36) amide on ad libitum energy intake in humans. J. Clin. Endocrinol. Metab. 86, 4382–4389. doi: 10.1210/jc.86.9.4382
Wasse, L. K., Sunderland, C., King, J. A., Batterham, R. L., and Stensel, D. J. (2012). Influence of rest and exercise at a simulated altitude of 4,000 m on appetite, energy intake, and plasma concentrations of acylated ghrelin and peptide YY. J. Appl. Physiol. 112, 552–559. doi: 10.1152/japplphysiol.00090.2011
Westerterp, K. R., and Kayser, B. (2006). Body mass regulation at altitude. Eur. J. Gastroenterol. Hepatol. 18, 1–3. doi: 10.1097/00042737-200601000-00001
Westerterp-Plantenga, M. S., Westerterp, K. R., Rubbens, M., Verwegen, C. R., Richelet, J. P., and Gardette, B. (1999). Appetite at “high altitude” [Operation Everest III (Comex-′97)]: a simulated ascent of Mount Everest. J. Appl. Physiol. 87, 391–399.
Williams, D. L., and Cummings, D. E. (2005). Regulation of ghrelin in physiologic and pathophysiologic states. J. Nutr. 135, 1320–1325.
Zaccaria, M., Ermolao, A., Bonvicini, P., Travain, G., and Varnier, M. (2004). Decreased serum leptin levels during prolonged high altitude exposure. Eur. J. Appl. Physiol. 92, 249–253. doi: 10.1007/s00421-004-1070-0
Zhang, Y., Proenca, R., Maffei, M., Barone, M., Leopold, L., and Friedman, J. M. (1994). Positional cloning of the mouse obese gene and its human homologue. Nature 372, 425–432. doi: 10.1038/372425a0
Keywords: altitude, hypoxemia, satiety, hunger, regulation
Citation: Debevec T (2017) Hypoxia-Related Hormonal Appetite Modulation in Humans during Rest and Exercise: Mini Review. Front. Physiol. 8:366. doi: 10.3389/fphys.2017.00366
Received: 13 October 2016; Accepted: 17 May 2017;
Published: 30 May 2017.
Edited by:
Niels H. Secher, University of Copenhagen, DenmarkReviewed by:
Samuel Verges, Institut National pour la Science et la Recherche en Médecine—Université Joseph Fourier, FranceFabien Andre Basset, Memorial University, Canada
Copyright © 2017 Debevec. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tadej Debevec, dGFkZWouZGViZXZlY0BpanMuc2k=
 Tadej Debevec
Tadej Debevec