
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Physiol., 06 February 2017
Sec. Membrane Physiology and Membrane Biophysics
Volume 8 - 2017 | https://doi.org/10.3389/fphys.2017.00043
This article is part of the Research TopicModulation of Ion Channels and Ionic Pumps by Fatty Acids: Implications in Physiology and PathologyView all 11 articles
Polyunsaturated fatty acids (PUFAs) act on most ion channels, thereby having significant physiological and pharmacological effects. In this review we summarize data from numerous PUFAs on voltage-gated ion channels containing one or several voltage-sensor domains, such as voltage-gated sodium (NaV), potassium (KV), calcium (CaV), and proton (HV) channels, as well as calcium-activated potassium (KCa), and transient receptor potential (TRP) channels. Some effects of fatty acids appear to be channel specific, whereas others seem to be more general. Common features for the fatty acids to act on the ion channels are at least two double bonds in cis geometry and a charged carboxyl group. In total we identify and label five different sites for the PUFAs. PUFA site 1: The intracellular cavity. Binding of PUFA reduces the current, sometimes as a time-dependent block, inducing an apparent inactivation. PUFA site 2: The extracellular entrance to the pore. Binding leads to a block of the channel. PUFA site 3: The intracellular gate. Binding to this site can bend the gate open and increase the current. PUFA site 4: The interface between the extracellular leaflet of the lipid bilayer and the voltage-sensor domain. Binding to this site leads to an opening of the channel via an electrostatic attraction between the negatively charged PUFA and the positively charged voltage sensor. PUFA site 5: The interface between the extracellular leaflet of the lipid bilayer and the pore domain. Binding to this site affects slow inactivation. This mapping of functional PUFA sites can form the basis for physiological and pharmacological modifications of voltage-gated ion channels.
Fish, fish oils, and polyunsaturated fatty acids (PUFAs; which are major components of fish oils) have beneficial effects on cardiac-, brain-, and muscle-related disorders. This has been shown in a number of studies at different levels:
1. Anthropological studies suggest that the Eskimo and Mediterranean diets, rich in mono- and PUFAs, lower the risk of heart disease and early death (Keys, 1970; Bang et al., 1971) (but see Fodor et al., 2014).
2. Large clinical trials show beneficial effects of dietary fish oil or PUFAs with decreased risk of sudden cardiac death (Burr et al., 1989; de Lorgeril et al., 1994; GISSI-Prevenzione Investigators, 1999; Albert et al., 2002; Marchioli et al., 2002).
3. In vivo animal models show that both intraperitoneal and intravenous administration of fish oil or isolated PUFAs prevent induced fatal ventricular arrhythmias (McLennan et al., 1988; McLennan, 1993; Billman et al., 1994, 1997, 1999).
4. In vitro models show that PUFAs applied directly to cardiomyocytes terminate arrhythmia and arrhythmia resumes upon removal of PUFAs (Kang and Leaf, 1994).
The last point suggests that PUFAs merely need to partition into the phospholipid cell membrane to exert their antiarrhythmic effect, probably via ion channels, which are responsible for electrical excitability of cells. Despite intense research, the molecular details of the action of PUFAs on ion channels and on excitability are largely unknown. In this review we will summarize what is known about the interaction between PUFAs and one superfamily of ion channels, the voltage-gated ion channels.
Voltage-gated ion channel are pore-forming molecules in the lipid bilayer of most cells, which open in response to alterations in the cell's transmembrane electrical potential (Hille, 2001). Opening of these channels allows the passage of specific types of ion across the cell membrane, thereby initiating and altering essential processes such as, signaling via nervous impulses, or movement via muscle contractions. Ion channels can be regulated by endogenous or exogenous compounds like hormones, pharmaceutical drugs, or toxins. Some compounds, such as PUFAs, can be both endogenous and exogenous.
PUFA effects on ion channels have been reviewed in several excellent papers (Ordway et al., 1991; Meves, 1994; Leaf and Xiao, 2001; Boland and Drzewiecki, 2008) but few, if any, have tried to outline the molecular sites of action and the molecular mechanism of the effects. Even fewer have tried to search for common mechanisms across the channel families. These two aspects are the focus of the present review. We will start with brief overviews of voltage-gated ion channels and of PUFAs. Then, we will summarize the current literature concerning PUFA effects on voltage-gated ion channels. This will be followed by an attempt to explain the data in molecular terms. Finally, we will briefly discuss relevant physiological and therapeutic implications.
The general structure of voltage-gated ion channels has been described in many extensive reviews (e.g., Tombola et al., 2006; Catterall et al., 2007; Bezanilla, 2008; Börjesson and Elinder, 2008). Therefore, we will only briefly describe core features that are pertinent to the subsequent discussion.
The human genome contains 144 genes coding for members of the superfamily of voltage-gated ion channels (http://guidetopharmacology.org/GRAC/ReceptorFamiliesForward?type=IC). Figure 1 shows an overview of how these 144 channels are classified into families.
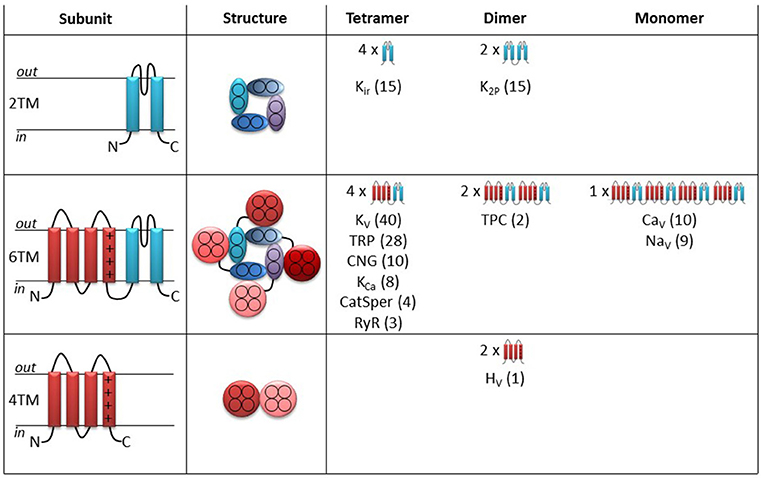
Figure 1. Topology and cartoons on the different ion channels in the superfamily of voltage-gated ion channels. Left column illustrates side view of the topology of a single subunit. Pore forming segments in blue and voltage-sensor domain segments in red. Middle column illustrates top view of the functional ion channel. Right column provides an overview of the different subfamilies and their topology. The numbers in parentheses denote the number of ion channels within each subfamily.
Thirty of the channels (upper row in Figure 1) only contain pore-forming subunits (blue in Figure 1). Each pore-forming subunit has two transmembrane (2TM) segments with a pore-lining segment in-between (left column in Figure 1). Four pore-forming subunits fused together make up a functional channel with a central ion-conducting pore (middle column). This tetrameric structure is referred to as the pore domain. The potassium-selective inward rectifiers (Kir) are examples of such channels (Figure 1, right column). Also the two-pore potassium (K2P) channels have a similar 3D architecture but are instead formed as dimer-of-dimers (each K2P gene is coding for two linked pore-forming subunits). Channels that contain only the pore domain are not intrinsically voltage sensitive but belong to the superfamily of voltage-gated ion channels because of molecular kinship. These channels are, instead, regulated by mechanical forces or ligands (Kim, 2003; Honoré, 2007).
113 channels in the superfamily of voltage-gated ion channels are composed of pore-forming segments, as described above, linked to voltage sensing segments (red in Figure 1) in a six transmembrane (6TM) architecture (Figure 1, middle row). These types of channels have a central pore domain surrounded by four voltage-sensor domains (VSDs) (Figure 1, middle column, middle row). In most cases, the VSD confers voltage dependence to these channels. Molecular details about the voltage-sensing mechanism will be described below when we discuss the molecular mechanism for PUFA action on voltage-gated ion channels. Six families are arranged as tetramers of 6TM subunits (Figure 1, right column, middle row): Voltage-gated K (KV) channels, transient receptor potential (TRP) channels, cyclic nucleotide activated (CNG) channels (including the hyperpolarization and cyclic nucleotide-activated (HCN) channels), calcium-activated K (KCa) channels, ryanodine receptors (RyR), and cation channels of sperm (CatSper). In contrast, two-pore (TPC) channels are formed as dimers of two linked 6TM subunits, while voltage-gated calcium (CaV) and sodium (NaV) channels are formed as monomers of four linked 6TM subunits.
Finally, one channel, the voltage-gated proton (HV1) channel is a dimer of 4TM-VSD motifs (Figure 1, lower row). This channel lacks the pore domain but allows protons to pass through the center of each VSD (Koch et al., 2008; Tombola et al., 2008).
The present review focuses on PUFA effects on intrinsically voltage-gated ion channels. We will therefore mainly summarize and discuss data from the VSD-containing channels (6TM and 4TM channels in Figure 1, the middle and lower rows). Effects on the channels in the upper row will not be covered. However, some of the 2TM channels are highly sensitive to PUFAs, such that some of them have names reflecting regulation by PUFAs. For example, the K2P4.1 channel is also referred to as the TWIK-related arachidonic-acid activated K (TRAAK) channel. Some of the described PUFA effects on these channels will be briefly mentioned later in this review, when we discuss the molecular mechanism of PUFA effects on intrinsically voltage sensitive ion channels. It should also be noted that some early studies were performed before the molecular identity was known. In these cases we have assigned channels to different families based of their functional characteristics.
Fatty acids are important messengers in cell signaling and critical components of the phospholipids that constitute the plasma membrane. The general structure of most naturally occurring fatty acids is a carboxylic acid with an unbranched aliphatic hydrocarbon tail. These fatty acids can be classified according to the number of carbon-carbon double bonds in the tail (Figure 2A):
– Saturated fatty acids (SFAs) such as stearic acid lack double bonds.
– Monounsaturated fatty acids (MUFAs) such as oleic acid have one double bond.
– Polyunsaturated fatty acids (PUFAs) such as linoleic acid, arachidonic acid (AA), and docosahexaenoic acid (DHA) have two or more double bonds.
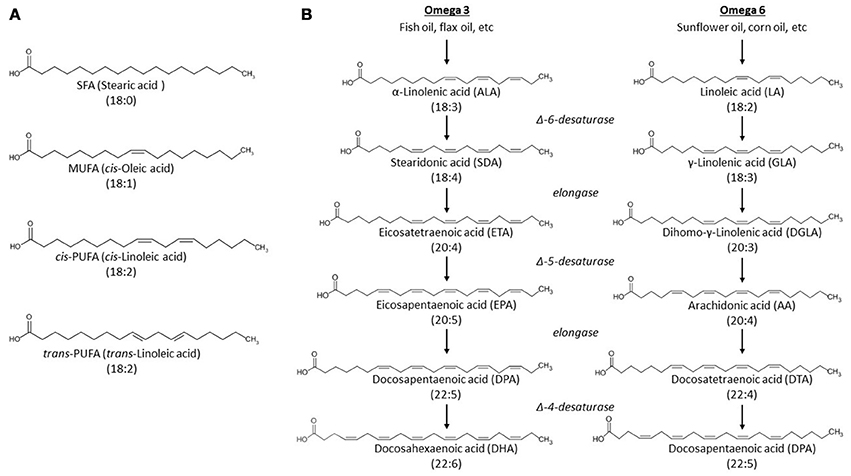
Figure 2. Structures of unesterified fatty acids. (A) Unesterified fatty acids are classified according to the presence, number and geometry of double bonds in the acyl tail. Abbreviations: MUFA, monounsaturated fatty acid; SFA, saturated fatty acid; PUFA, polyunsaturated fatty acid; The PUFA is shown in both cis and trans geometry. (B) Metabolic pathways of n-3 and n-6 fatty acid synthesis. α-linolenic acid and linoleic acid are the precursors of n-3 and 6 PUFAs, respectively. Different desaturases and elongases convert these precursors to different long-chain PUFAs.
A common way to name fatty acids is by the number of carbons and double bonds. For example, DHA is also called 22:6 (22 carbons and six double bonds). Moreover, double bonds can display cis geometry (the adjacent carbons are on the same side of the carbon chain) or trans geometry (the adjacent carbons are on opposite sides of the carbon chain). Cis geometry is most common among naturally occurring unsaturated fatty acids, while trans is usually caused by industrial processing of fatty acids (Micha and Mozaffarian, 2009) (Figure 2A).
Certain fatty acids, in particular SFAs and MUFAs, can be synthesized de novo in the human body (Mullen and Yet, 2015). Others, especially PUFAs, must instead be acquired through the diet (Jakobsson et al., 2006; Kihara, 2012). Dietary intake of α-linolenic acid and linoleic acid (obtained from fish oil or sunflower oil, respectively) is a vital source for PUFAs (Figure 2B). The first double bond in α-linolenic acid is located at the third carbon, counting from the methyl end of the tail, and is therefore an n-3 (or ω-3) fatty acid. Linoleic acid, on the other hand, has its first double bond located at the sixth carbon, and is therefore an n-6 (or ω-6) fatty acid. These dietary PUFAs function as precursors in the synthesis of longer PUFAs like the n-3 docosahexaenoic acid (DHA) or the n-6 arachidonic acid (AA) (Figure 2B). Non-esterified fatty acids can circulate in the plasma bound to transport proteins such as albumin. These non-esterified free fatty acids are directly available to dissociate from albumin and interact with membrane-bound ion channels (as will be discussed later) or be metabolized by various enzymatic systems (described below).
The phospholipids that constitute the plasma membrane are another important source for fatty acids. Each phospholipid is composed of two fatty acids and a head-group bound to a glycerol backbone (Figure 3). SFAs are generally esterified to the first carbon of the glycerol backbone (sn1) while PUFAs, or (less commonly) MUFAs, are esterified to the second carbon (sn2). The polarity and charge of different phospholipids are determined by the properties of the head group bound to the third carbon of the glycerol backbone (sn3). Esterified fatty acids in the plasma membrane can be hydrolyzed to non-esterified free fatty acids, which are then available to interact with ion channels and other cellular proteins. The hydrolysis of esterified AA has been most extensively studied. It is primarily mediated by four different phospholipases that act at four distinct sites in the phospholipid (Figure 3) (Dennis et al., 1991; Siddiqui et al., 2008); Phospholipase A2 (PLA2) -mediated hydrolysis of the sn2 linkage directly releases AA. In contrast, Phospholipase A1 (PLA1), phospholipase C (PLC), or phospholipase D (PLD) -mediated hydrolysis yield precursors of AA (such as 1, 2 diacylglycerol and phosphatidic acid) that require additional enzymatic conversions before non-esterified AA is released. AA and DHA are the most common PUFAs to be found in sn2 position in mammalian phospholipids. Release of DHA (or other unsaturated fatty acids) from phospholipids follows the same overall pathway as AA release, although the chemical intermediates formed are different due to differences in the fatty acid acyl tail.
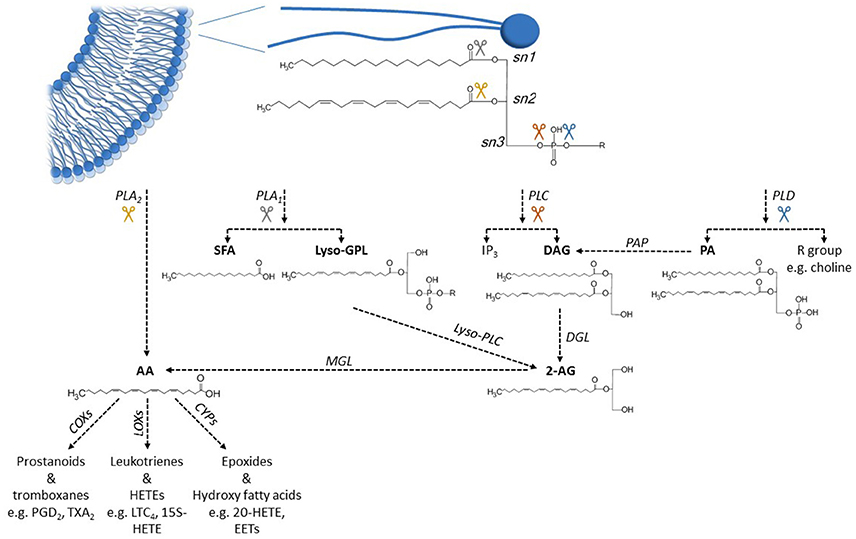
Figure 3. Metabolic pathways of arachidonic acid hydrolysis and oxidation. Phospholipids in the cell membranes commonly have a SFA esterified to sn1 position and a PUFA, such as arachidonic acid (AA) esterified to sn2 position. Activation of different phospholipases releases AA from phospholipids, either in one enzymatic step (PLA2) or through several enzymatic steps (PLA1, PLC, PLD). Unesterified AA can be further metabolized to various eicosanoid metabolites by different COX, LOX, and CYP enzymes. Abbreviations: 2-AG, 2-arachidonoylglycerol; AA, arachidonic acid; COX, cyclooxygenase; CYP, cytochrome P450 enzyme; DAG, 1,2-diacylglycerol; DGL, DAG lipase; EET, epoxyeicosatrienoic acid; HETE, hydroxyeicosatrienoic acid; IP3, inositol 1,4,5-trisphosphate; LOX, lipooxygenase; LTC4, leucotriene C4; Lyso-GPL, lyso-glycerolphospholipid; Lyso-PLC, lysophospholipase C; MGL, monoacylglycerol lipase; PA, phosphatidic acid; PAP, PA phosphatase; PGD2, prostaglandin D2; PLA1, phospholipase A1; PLA2, phospholipase A2; PLC, phospholipase C; PLD, phospholipase D; SFA, saturated fatty acid; TXA2, thromboxane A2.
Once released from the plasma membrane, these non-esterified fatty acids may diffuse to and interact with membrane-bound ion channels, take part in intracellular signaling, or be further metabolized by various oxygenases. Metabolism of non-esterified fatty acids is mediated by three main types of oxygenases (Figure 3) (Siddiqui et al., 2008; Jenkins et al., 2009): Cyclooxygenases (COX), lipoxygenases (LOX), and cytochrome P450 epoxygenases (CYP). These enzymes produce a family of fatty-acid metabolites named eicosanoids, which includes prostaglandins, leukotrienes, thromboxanes, and epoxides (Siddiqui et al., 2008; Jenkins et al., 2009). Again, the structures of these metabolites depend on the structure of the specific fatty acid that is substrate for oxygenation.
In this review we will focus on the effect of non-esterified PUFAs on voltage-gated ion channels. Several fatty acid metabolites and intermediates formed during phospholipid hydrolysis are also known to modulate the activity of voltage-gated ion channels. However, we will not discuss these interactions here.
To collect papers describing the effects of PUFA on the VSD-containing channels we searched PubMed for various combinations of voltage-gated ion channels and fatty acids, and extended the list when relevant articles were found during the work. In total we identified, read and analyzed data from 295 original papers containing voltage-clamp data from voltage-gated ion channels published between 1987 and June 2016 (Table 1). In addition, we read and analyzed about 400 papers concerning PUFA effects on non-VSD containing channels, review papers, or papers describing PUFA effects on excitability in general.
In 1981, Takenaka et al. reported that fatty acids with chain lengths exceeding eight carbons, in the concentration range of 0.2–2.2 mM, decreased the voltage-gated Na current in squid giant axons while leaving the delayed-rectifier K current unaffected. Cis-2-decenoic acid, which has ten carbons and a double bond between carbon 2 and 3 was the most effective fatty acid in their experiments (Takenaka et al., 1981). In 1987, the same group reported that both saturated and unsaturated medium-chain fatty acids (8–13 carbons) reversibly attenuated voltage-dependent Na currents in squid giant axons by shifting the conductance-vs.-voltage, G(V), curve in a positive direction along the voltage axis (Takenaka et al., 1987). The effect developed much faster upon intracellular application, suggesting an intracellular site of action. The fatty acid concentration needed for 50% reduction of the peak Na current decreased by a factor of 1/3 for each extra carbon. The presence of a carboxyl or hydroxyl group at the ω end of the fatty acid abolished the effect completely. These findings suggested that a hydrophobic interaction between the fatty acid and Na channel could be an important factor for the effect.
Longer chain fatty acids like palmitic acid (16:0), linoleic acid (18:2), and linolenic acid (18:3) decreased both Na and K currents, but the effects were irreversible, probably because of high concentrations tested would result in micelle formation. Finally, in 1988, by using α-cyclodextrin to dissolve the fatty acids, this group reported that long-chain PUFAs produced effects similar to medium-chain fatty acids (Takenaka et al., 1988). Intracellularly applied AA (20:4) reversibly suppressed the Na current of the squid giant axon with little effect on the K current. 180 μM AA reduced the Na current by 50%, which is a concentration almost ten times lower than required for the medium-chain fatty acid, 2-decenoic acid. Longer PUFAs, Docosatetraenoic (22:4) and DHA (22:6), had effects quantitatively similar to AA. Shorter PUFAs, linoleic acid (18:2) and linolenic acid (18:3), had smaller effects than AA, while the effects of the MUFA oleic acid (18:1) were even smaller, and the SFA stearic acid (18:0) had almost no effect.
In 1989, Bregetovski et al. reported that 2-decenoic acid increased the open probability of KCa channels up to 10-fold in the membrane of smooth muscle cells from the human aorta (Bregestovski et al., 1989). They suggested that 2-decenoic acid alters the Ca2+-binding mechanism of the channel. The same year Linden and Routtenberg reported that low concentrations (1–50 μM) of the MUFA oleic acid (18:1), the PUFAs linoleic acid (18:2), and linolenic acid (18:3), but not the SFA stearic acid (18:0) or the trans-isomer of oleic acid blocked the Na current in N1E-115 neuroblastoma cells (Linden and Routtenberg, 1989); 5 μM oleic acid decreased the peak Na current by 36%. K currents were not affected while both T-type and L-type Ca currents were blocked. This study also excluded the possible explanation that fatty acid effects were produced by increased fluidization of the membrane.
In 1991, Rouzaire-Dubois et al. showed that several MUFAs and PUFAs induced or accelerated inactivation of KV channels via a direct mechanism (not activation of protein kinase C). For instance, 5 μM of oleic acid accelerated the inactivation by a factor of about 10. Among the 18-carbon fatty acids, linoleic acid (18:2) was the most potent inactivator (50-fold acceleration at 5 μM), followed by oleic acid (18:1), linolenic acid (18:3), elaidic acid (18:1, trans), and stearic acid (18:0) which did not affect the inactivation time course at all.
In 1992 several papers on different ion channels established that low μM concentrations of PUFAs affect voltage-gated ion channels, opening as well as closing (Béhé et al., 1992; Finkel et al., 1992; Hallaq et al., 1992; Huang et al., 1992; Kirber et al., 1992; Ling et al., 1992; Shimada and Somlyo, 1992; Wieland et al., 1992).
At about the same time several influential studies were published suggesting that PUFA or PUFA-metabolites had direct effects on other, non-voltage-gated, ion channels (Buttner et al., 1989; Giaume et al., 1989; Kim and Clapham, 1989; Kurachi et al., 1989; Ordway et al., 1989; Anderson and Welsh, 1990; Cantiello et al., 1990; Hwang et al., 1990; Kim and Duff, 1990).
Despite the multiple different types of ion channels and PUFAs included in this review, the effects PUFAs have on voltage-gated ion channels are surprisingly general and can be summarized in a few points (Table 1). However, it should be noted that quantitative differences do exist.
i. Alteration in voltage dependence of ion channels: A common finding is that PUFAs shift the G(V) and/or the steady-state inactivation curves in a negative direction along the voltage axis (Figures 4A,B). Such a shift of the G(V) curve opens the channel, while this shift of the steady-state inactivation curve closes (inactivates) the channel. For NaV and CaV channels, shifts of the steady-state inactivation curve tend to be larger than shifts of the G(V) curves. As a consequence, NaV and CaV channels are generally inhibited by PUFAs. In contrast, KV channels which in many cases are less affected by steady-state inactivation at resting voltage are typically activated by PUFAs.
ii. Alteration in maximal conductance of ion channels: PUFAs are also able to increase or decrease the conductance at positive voltages (either open probability or the single-channel conductance), where the conductance is not affected as a consequence of the G(V) shift (Figure 4C). In many cases, there is a combination of effect i and ii (Figure 4D). Despite these combined effects it is relatively easy to distinguish them without curve fitting. Increased conductance can be measured directly at voltages where the conductance has saturated while a G(V) shift can be measured at the foot of the curve (e.g. at 10% of maximal conductance in control–the error for the G(V) curve shown in Figure 4D is only 1.7 mV if the maximal conductance is increased by 50%).
iii. Alteration in the time course of ion channel kinetics: Consistent with the negative shift of the channel's voltage dependence in negative direction along the voltage axis, the opening kinetics are sometimes faster (Figure 4E) and the closing kinetics slower (Figure 4F) in the presence of PUFAs. There are also multiple reports of a PUFA-induced acceleration of channel inactivation (Figure 4G).
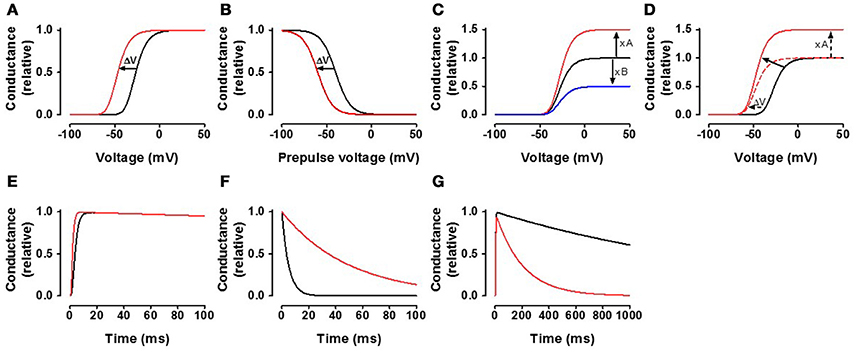
Figure 4. General effects of the fatty acids on the channels. (A) The black curve represents a typical control conductance-vs.-voltage curve [G(V) = 1/(1+exp((V-V)/s))n, where V is the membrane voltage, s = 8 mV, V = −40 mV, n = 4] for a voltage-gated ion channel. The red curve is the control curve shifted by −20 mV. (B) The black curve represents a typical steady-state inactivation curve [G(VPP) = 1/(1+exp((V-V)/s)), s = −8 mV, V = −40 mV]. The red curve is the control curve shifted by −20 mV. (C) The black curve represents a typical control curve as in (A). The red curve is the control curve increased by a factor 1.5. The blue curve is the control curve decreased by multiplying by 0.5. (D) The black curve represents a typical control curve as in (A). The red continuous curve is an example where the curve is both shifted in negative direction along the voltage axis and increased. The amplitude increase can reliably be measured at high voltages where the conductance levels out. The shift can reliably be measured at the foot of the conductance curve (at 10% of the max value of the control curve) without normalization of the curve. The shift of the curve is −20 mV. Measured at the foot, when the maximum conductance is increased by 50%, the shift is over-estimated by 1.7 mV (–21.7 mV instead of –20 mV). (E) The black curve represents a typical activation time course (τ = 2 ms, n = 4, τinact = 2 s). The red curve is a two fold increase in opening rate. (F) The black curve represents a typical single exponential channel closure (τ = 5 ms). The red curve is 10 times slower. (G) The black curve represents a typical channel inactivation (τ = 2 s), while the red inactivates 10 times faster.
Although many of the PUFA effects are general for the voltage-gated ion channels, there are quantitative and qualitative differences. We will therefore briefly describe the specific PUFA effects on different sub-families of voltage-gated ion channels. Table 1 describes the general effects for the specific families and lists the references.
The largest and most studied family when it comes to PUFA effects on voltage-gated ion channels is the family of voltage-gated K (KV) channels. Because of the size and diversity of this family, we will divide this family into three groups of subfamilies. Subfamilies that are not included in our description below have not, to our knowledge, been studied with respect to PUFAs.
KV1–4: KV channels within these subfamilies open rapidly and thereby cause fairly fast repolarization of the action potential. Therefore, these channels have special importance for neurons that fire with high frequency. Some of these channels [such as KV4 channels which generate transient outward (Ito) neuronal and cardiac K currents] also inactivate rapidly and are thus sometimes referred to as A-type KV channels. Other members within this subfamily, such as Kv2.1, inactivate slowly generating persistent K currents, in the physiological time frame. Some studies describe PUFA-induced increases in native K currents of unclear molecular identity (e.g., Horimoto et al., 1997; Ferroni et al., 2003; Fioretti et al., 2004), however the most commonly observed PUFA effect on fast native K currents (Lynch and Voss, 1994) and heterologously expressed KV1–4 channels is inhibition (by 20–100% at ~10 μM PUFA). This inhibition is commonly associated with an acceleration of the time course of channel inactivation. PUFA effects on channel voltage dependence are less consistent, but the most commonly described are negative voltage shifts of G(V) and/or steady-state inactivation curves. The overall effect is typically a reduced current, but a few exceptions describe PUFA-induced activation of KV1–4 channels (Zhao et al., 2007; Börjesson et al., 2008, 2010; Zhang M. et al., 2008; Börjesson and Elinder, 2011).
KV7: KV channels within this subfamily open slowly and are referred to as slow delayed rectifiers. KV7 channels underlie the neuronal M current, which contributes to the negative resting membrane potential in neurons, and the cardiac IKs current, which contributes to the repolarization in cardiomyocytes. PUFAs are reported to activate both natively and heterologously expressed KV7 channels. PUFA-induced increases of KV7 current amplitudes are associated with a small negative shift in the G(V) curve (roughly −5 to −10 mV by 10 μM PUFA). There are, however, some inconsistencies concerning the role of the auxiliary subunit KCNE1 during PUFA exposure. The cardiac IKs channel is a complex between KV7.1 and KCNE1. Doolan et al. find that PUFA effects on the IKs channel require the presence of KCNE1 (Doolan et al., 2002). In contrast, we describe that KCNE1 causes reduced PUFA sensitivity of the IKs channel compared to KV7.1 alone (Liin et al., 2015). Moreover, Moreno et al. show that PUFA effects on the IKs channel vary over time (Moreno et al., 2015).
KV10–12: These subfamilies contain the KV10.1 channel (= EAG1) and the KV11.1 channel (= hERG or ERG1). KV11.1 forms the major portion of the rapid delayed rectifier current (IKr), which is critical in correctly timing the repolarization of cardiac action potentials. Mutations in KV11.1 and compounds targeting IKr channels can cause long QT syndrome and subsequent lethal ventricular fibrillation. Most PUFA studies on this group have been performed on the KV11.1 channel, with a single study performed on KV10.1. The effects in this small group are mixed. Both current reductions and current increases have been reported. The G(V) curve is negatively shifted in most studies. This shift is rather large for KV10.1, around −30 mV at 10 μM for all PUFAs studied (Gavrilova-Ruch et al., 2007). Several studies also suggest that PUFAs speed up closure (inactivation) of these channels.
The family of Ca-activated K channels contains three types of channels: Big, intermediate, and small conductance channels. Only the KCa1.1 (BK) family is clearly voltage dependent as it is opened by alterations in membrane voltage in addition to increases in the intracellular Ca2+ concentration. Almost all studies of PUFA effects on KCa channels have been performed on KCa1.1 channel. This channel is essential for the regulation of smooth muscle tone and neuronal excitability. PUFAs, even at submicromolar concentrations, increase the maximum conductance and shift the G(V) curve in negative direction along the voltage axis. In addition, the KCa1.1 channel is quite sensitive to PUFA metabolites (Meves, 2008). Recent studies have mapped the binding site for PUFAs to a region near the intracellular gate (Hoshi et al., 2013d; Tian et al., 2016).
The transient receptor potential (TRP) channels form a large family, consisting of 28 channels divided in six subfamilies. TRP channels are for example involved in mediating the sensations of cold, heat, and pain. These channels are fairly non-selective and therefore conduct several types of cations (e.g., Na+, Ca2+). TRP channels are generally described as being activated by PUFAs. However, many of these studies measured TRP channel activity indirectly using fluorescence-based calcium imaging, which provides limited information about TRP channel voltage dependence and the time course of TRP currents. In studies that include electrophysiological recordings (primarily from TRPVs, TRPCs, TRPAs, and drosophila TRPs), the amplitude of TRP currents are found to increase many-fold following application of >10 μM PUFA. Moreover, Shimizu et al. describe a PUFA-induced negative shift in the G(V) curve of TRPP3 channels (Shimizu et al., 2009). However, TRPM channels are an exception among TRP channels, as they are almost completely inhibited by PUFAs (Andersson et al., 2007; Parnas et al., 2009b; Bavencoffe et al., 2011).
The family of voltage-gated Na channels contains the first ion channel to be discovered and explored electrophysiologically (Hodgkin and Huxley, 1952a,b), and later, cloned and sequenced (Noda et al., 1984). NaV channels generate action potentials in neurons, the heart, and other muscles. Thus, they are important targets for the regulation of excitability. With few exceptions, PUFAs reduce NaV currents. However, PUFAs also shift the G(V) and steady-state inactivation curves of most NaV channels in a negative direction along the voltage axis. In general, the steady-state inactivation curve is shifted more than the G(V) curve. These shifts have conflicting results; the G(V)-curve shift opens channels and thereby increase excitability, while the steady-state inactivation curve shift inactivates/closes channels and thereby decrease excitability. Altogether, these mixed effects result in reduced excitability.
Voltage-gated Ca channels have two critical functions: Generating (or boosting) action potentials, and conducting extracellular Ca2+ ions into the cell where they can act as a second messenger. PUFA effects on CaV channels have been studied rather extensively. The effects are very similar to the effects on NaV channels, that is, the maximal conductance is decreased, and G(V) and steady-state inactivation curves are shifted in a negative direction along the voltage axis, with the steady-state inactivation shift being larger than the G(V) shift. In addition, the inactivation time course is in some cases accelerated. Altogether, these mixed effects result in reduced excitability.
The proton channel, which was cloned only 10 years ago (Ramsey et al., 2006; Sasaki et al., 2006), deviates from all other ion channels in lacking the conventional ion-conducting pore domain. However, the voltage sensing mechanism is similar to the other voltage-gated ion channels; the difference is that two VSDs act together as a dimer (Koch et al., 2008; Lee et al., 2008). The effects of PUFAs on the HV channels are reminiscent of the effects on the other channels, suggesting that at least some of the effects are conferred by the VSD. PUFAs increase the maximal current of HV channels–for most other channels the maximal current is decreased. The shift of the G(V) is in the negative direction along the voltage axis, but the size is smaller than for most other channels. One surprising finding is that the PUFA carboxyl charge is not important for this effect (Kawanabe and Okamura, 2016).
Several other ion channels belonging to the superfamily of voltage-gated ion channels have been explored with respect to PUFA effects, but many of them are difficult to study in biophysical detail. For several of the families only few studies have been performed, often with mixed data, making it difficult to draw general conclusions. These families are briefly mentioned here and the references are found in Table 1. The family of cyclic-nucleotide gated (CNG) ion channels contains two types of channels–hyperpolarization-activated cyclic nucleotide gated (HCN) channels, which are highly voltage dependent (even though the polarity is opposite to most other ion channels), and the non-voltage dependent CNG channels. HCN channel have an important role as pacemaker channels in the sino-atrial node of the heart. AA has been found to directly facilitate HCN channel opening, and rats fed a diet enriched with fish oil show reduced pacemaker currents and consequently reduced heart rate (see Table 1). The ryanodine receptor (RyR) family is an intracellular cation channel critical for the regulation of intracellular levels of Ca2+. PUFAs have been reported both to increase and decrease the RyR current. CatSper channels and TCP channels are molecularly related. CatSper channels are found in the plasma membrane of sperm while TCP channels are found in intracellular endolysosomes. Here the effects of PUFAs are also mixed.
There are some general properties of fatty acids that are often described as being required to induce the PUFA effects described above (e.g., Xiao et al., 1997, 1998; Danthi et al., 2003, 2005; Börjesson et al., 2008; Liin et al., 2015):
(a) At least two double bonds in the acyl tail are required. Therefore, PUFAs induce these effects while SFAs and MUFAs generally do not. However, there is usually no clear difference between n-3 and n-6 PUFAs. Also, there is no large or systematic difference between PUFAs with respect to chain lengths from 16 to 24 carbons.
(b) Cis-geometry of the double bonds in the acyl tail is required. Trans-geometry renders the PUFAs ineffective.
(c) The negative charge of the carboxyl group is required. Uncharged methyl esters of PUFAs generally lack effects.
In addition, PUFAs need to remain in their intact form. Experiments conducted with non-metabolizable PUFA analogs (such as ETYA) and cyclooxygenase inhibitors (that prevent PUFA metabolism) show that the PUFAs themselves, and not their metabolites, induce these general effects. Some exceptions, however, have been reported (Twitchell et al., 1997; Lee et al., 2002; Judé et al., 2003).
Despite the large number of studies published (Table 1), only a few PUFA sites of action have been described and little has been described concerning the mechanism by which PUFAs interact with voltage-gated ion channels.
The first major question is whether the reported effects of PUFAs on the voltage-gated ions channels are direct channel effects or if they are mediated via non-specific membrane effects. In general, the concentrations needed for the PUFA effects are relatively low (1–10 μM), ruling out unspecific membrane fluidizing effects (Pound et al., 2001). Moreover, there is no correlation between a PUFA's propensity to fluidize the membrane and their effects on voltage-gated ion channels (Villarroel and Schwarz, 1996). Alterations of the lipid membrane by soaking out cholesterol affect ion channel function but do not affect acute PUFA effects (Moreno et al., 2015). Further, the onset and washout of the effect on KV channels is very rapid (2–3 s), suggesting a direct channel effect (Poling et al., 1996). An early suggestion that PUFAs may bind directly to voltage-gated ion channels came from experiments on NaV channels in which the PUFA eicosapentaenoic acid (EPA) inhibited the binding of a radio-labeled toxin to cardiac NaV channels (Kang et al., 1995; Kang and Leaf, 1996). Further evidence that PUFAs have direct ion channel effects is provided by the demonstration that single point mutations in various voltage-gated ion channels also affects the ability of PUFAs to modulate those channels (e.g., Xiao et al., 2001; Börjesson and Elinder, 2011; Ottosson et al., 2014; Liin et al., 2015).
Secondly, we may ask on which side of the membrane the PUFAs act. Whereas, most studies have used extracellular application of PUFAs, one study made a direct comparison of PUFA-induced effects upon PUFA application from either side of the membrane. They found no difference in PUFA effects on KV channels based on the side of application (Oliver et al., 2004). In contrast, some studies have demonstrated ion channel modulation when PUFAs are applied extracellularly but fail to observe modulation when PUFAs are added intracellularly (Honoré et al., 1994; Poling et al., 1995, 1996; Garratt et al., 1996; Kehl, 2001; McKay and Jennings, 2001; Guizy et al., 2008). Yet other studies primarily observe effects when the PUFAs are applied to the intracellular side (Boland et al., 2009; Decher et al., 2010). These differences in the side of action may be explained by differences in the predominant PUFA sites of action in different types of ion channels.
From our analysis of PUFA publications in the field we have identified five sites of actions (Figures 5A,B). The first two sites are located in the ion-conducting pore, one at the intracellular entrance (PUFA site 1), and the other at the extracellular entrance (PUFA site 2). The third is located at the VSD-to-pore domain linker close to the intracellular gate (PUFA site 3). The last two are located at the interface between the extracellular part of the ion channel and the outer leaflet of the lipid bilayer from which PUFAs electrostatically interact with the VSD (PUFA site 4) or the pore domain (PUFA site 5).
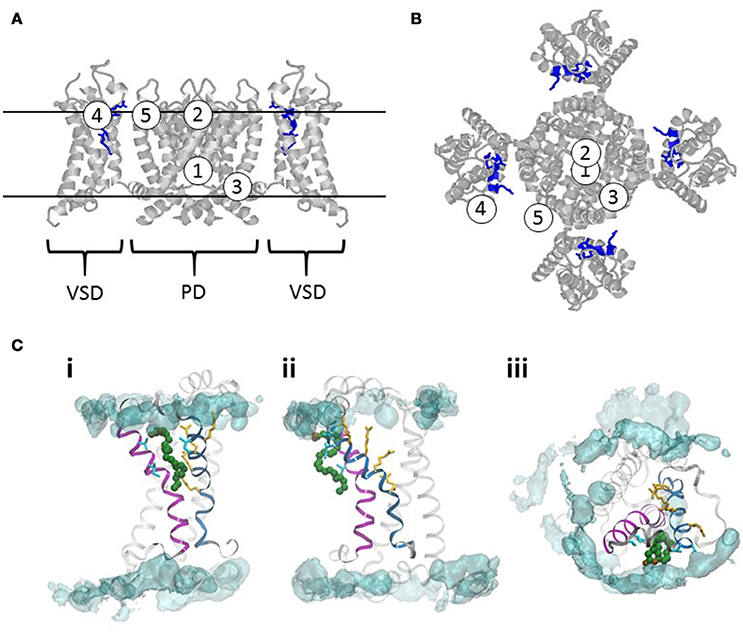
Figure 5. Sites of actions of PUFAs on voltage-gated ion channels. (A) A homology model of the Shaker KV channel based on the structure of the KV2.1/1.2 chimera (Long et al., 2007; Henrion et al., 2012). Side view. VSD denote one voltage-sensor domain. PD denotes the pore domain. For clarity, the VSD in the front and the back are removed. The long loop between S3 and S4 are removed (residues 337–353). The two continuous lines delineate the approximate outer and inner borders of the lipid bilayer. The Figures 1–5 denote five proposed sites of actions of PUFA. (B) Top view of the channel in (A). (C) Interaction site for a DHA molecule with the VSD of the Shaker KV channel. The helix in magenta is S3 and the helix in blue is S4. The four yellow amino acid residues are the four gating charges [R362 (in the top), R365, R368, and R371]. The four residues in cyan (two in S3, residues 325 and 329; two in S4, 359 and 360) are the residues identified to be close to the PUFA binding site (Börjesson and Elinder, 2011). A typical binding pose for a DHA molecule in green is from Yazdi et al. (2016). The POPC lipid bilayer is represented by a cyan iso-density surface corresponding to the positions of lipid nitrogens in the simulation at 5% occupancy. The left and middle panels are the VSD viewed along the membrane from two different angles. The right panel is the VSD viewed from the extracellular side.
Several studies have identified the intracellular part of the pore lining S6, with residues facing the intracellular cavity, as critical for the PUFA effects. A common mechanism is an open-channel block causing a time dependent current reduction–an inactivation.
A single point mutation of domain I of the cardiac NaV1.5 channel (N406K) clearly reduces the inhibitory effect of DHA (Xiao et al., 2001). The negative shift of the steady-state inactivation curve is also attenuated. The identified amino-acid residue is located in the middle of S6, facing the intracellular cavity, in a similar position where local anesthetics bind to domain IV of a rat brain NaV channel (Ragsdale et al., 1994). However, the molecular detail why the steady-state inactivation curve is shifted by DHA has not been described.
In KV1.1 channels, DHA and AA, but also the uncharged anandamide induces inactivation by interacting with hydrophobic residues lining the inner cavity of the pore (Decher et al., 2010). The inactivation was suggested to be caused by open-channel block by PUFA binding to the cavity of the channel. KV1.5 has been proposed to be inactivated via a similar mechanism. Point mutations combined with computer docking support PUFA binding in the cavity (Bai et al., 2015).
In the Ca-activated KCa3.1 (= SK4 or IK1) channel, which is not voltage sensitive despite having VSDs, AA inhibits the current. This inhibition is completely prevented by the T250S mutation at the inner end of the pore loop, together with the V275A mutation in the middle of S6, close to residue 250 (Hamilton et al., 2003). Furthermore, introducing the threonine and the valine in the equivalent positions of the AA-insensitive KCa2.2 (= SK2) channel makes this channel sensitive to AA. Thus, AA interacts with the pore-lining amino acids of KCa3.1 to inhibit the channel.
Thus, several studies on different ion channels have identified the middle of S6, in the cavity, as a major determinant for PUFA interactions.
Another type of channel-inactivating pore-interacting mechanism has been described for AA on KV3.1 (Oliver et al., 2004). AA is equally effective from either side of the membrane. AA-induced inactivation was not affected by the presence of TEA at the extracellular or intracellular side of the channel protein. These results rule out open-channel block as the mechanism underlying AA-induced inactivation, but suggest a lipid-induced closure of the “pore gate”.
KV1.1 (Garratt et al., 1996), KV1.2 (Garratt et al., 1996; Poling et al., 1996), KV1.5 (Honoré et al., 1994; Bai et al., 2015), and KV3.1a (Poling et al., 1996) are inactivated by PUFAs via a proposed open-channel block where the pore is accessed from the extracellular side. Point mutations combined with computer-guided docking support a PUFA binding site at the extracellular entrance of the pore (Bai et al., 2015).
Some studies have identified a PUFA site at the inner end of S6 or in the S4–S5 linker, which are close to each other and form the intracellular gate of the channel (Long et al., 2005). In the absence of detailed data we have brought them together to a single site. The difference from PUFA site 1 and 2 is that this site is outside the central axis of the channel and that this site thus can host PUFA molecules to open the channel by bending the gate open.
The Ca2+-activated KCa1.1 (= BK) channel is, in contrast to the NaV1.5 and the KCa3.1 channels described above, opened by several PUFAs such as DHA, AA and α-linolenic acid. Hoshi and collaborators have identified Y318 near the cytoplasmic end of S6 in the KCa1.1 channel as a critical determinant of the stimulatory action of DHA (Hoshi et al., 2013d; Tian et al., 2016). The Y318S mutation greatly diminishes the channel's response to DHA, but not to AA or α-linolenic acid.
KV4.2 inactivates very quickly upon application of AA, while the inactivation of the Shaker KV channel is fairly unaffected. Transplanting the Shaker S4–S5 linker to KV4.2 attenuates the effect of AA on the KV4.2 channel, and conversely, transplanting the KV4.2 S4–S5 linker to the Shaker KV channel makes the Shaker KV channel more sensitive to AA (Villarroel and Schwarz, 1996). Molecular docking approaches using a KV4.2 homology model predicted a membrane-embedded binding pocket for AA comprised of the S4–S5 linker on one subunit and several hydrophobic residues within S3, S5, and S6 from an adjacent subunit (Heler et al., 2013). The pocket is conserved among KV4 channels.
It is well-known that the lipid environment is important for the function of voltage-gated ion channels. Crystal structures show that phospholipids are making close and specific contacts with the channel (Long et al., 2007). Molecular dynamics simulations suggest that the negatively charged phosphate group of phospholipids make electrostatic interactions with the positive charges of the voltage sensor (Freites et al., 2005; Sansom et al., 2005). Experiments altering the charge of the phospholipids show that the charge of the phospholipids is necessary for proper function of voltage-gated ion channels (Schmidt et al., 2006). Free PUFA molecules can also affect ion-channel gating. PUFA molecules in the extracellular solution can quickly incorporate in the extracellular leaflet of the phospholipid bilayer; the hydrophobic tail is tucked into the hydrophobic part of the bilayer and the carboxyl group is facing the extracellular water (Feller et al., 2002; Yazdi et al., 2016). The PUFA molecules are most likely everywhere in the lipid bilayer but they could potentially be clustered around ion channels (Yazdi et al., 2016).
In studies of the Shaker KV channel and several KV7 channels we have identified a site between the extracellular leaflet of the lipid bilayer and S4 of the VSD. Mutational analysis and molecular dynamics simulations have suggested that the PUFA molecules interact between the transmembrane segments S3 and S4 and the lipid bilayer (Figure 5C) (Börjesson and Elinder, 2011; Yazdi et al., 2016). The electric charge of free PUFA molecules in the lipid bilayer affects the gating machinery of the VSD (Börjesson et al., 2008, 2010; Börjesson and Elinder, 2011; Ottosson et al., 2014; Liin et al., 2015, 2016b; Yazdi et al., 2016). Because lipophilicity and electrostatic forces are central in this model, we have called this the lipoelectric mechanism.
PUFAs modulate the KV1.4 channel inactivation. It has been suggested that the PUFA molecule partition in the membrane as has been suggested for PUFA site 4. The difference is that the negatively charged PUFA molecule line up outside the pore domain and from this position the acidic head group of the PUFAs raises the pKa of H508 in the pore domain. This raised pKa of the histidine reduces the K+ occupancy of the selectivity filter, stabilizing the C-type inactivated state (Farag et al., 2016).
Of all five sites described above, the mechanism by which PUFAs affect KV channels via PUFA site 4 has been studied in most detail. In the remaining part of this section we will focus on this PUFA mechanism. The mechanism by which voltage-gated ion channels sense membrane voltage is central for this effect (reviewed for instance in Armstrong, 1981; Keynes and Elinder, 1999; Bezanilla, 2000; Swartz, 2004; Börjesson and Elinder, 2008). Therefore, we will here, in brief, describe the mechanism for voltage sensing.
The four VSDs connected to a central ion-conducting pore domain make, in most cases, the channel voltage sensitive. Each VSD has four transmembrane segments labeled S1 to S4. The fourth transmembrane segment, S4, has several positively charged amino-acid residues (blue sticks in Figure 6) interspaced by two hydrophobic residues. The transmembrane segments S1 to S3 host negative counter charges (red sticks in Figure 6) that neutralize the positive S4 charges in the transmembrane section of the VSD. The positive charges of S4 can change partners and thereby slide along the rest of the VSD (from the deepest state C4 to the open state O in Figure 6). At negative membrane voltages, S4 is close to the intracellular side (the down state) and at positive membrane voltages S4 is close to the extracellular side (the up state) of the membrane. At resting states C4 and C3 most S4 charges are below the hydrophobic barrier (Tao et al., 2010) (the green phenylalanine in Figure 6). Upon activation three to four charges of each S4 move across the barrier, in three to four discrete steps. The total movement is around 13 Å, even though distances from 7 to 15 Å have been reported (e.g., Ruta et al., 2005; Campos et al., 2007; Delemotte et al., 2011; Henrion et al., 2012).
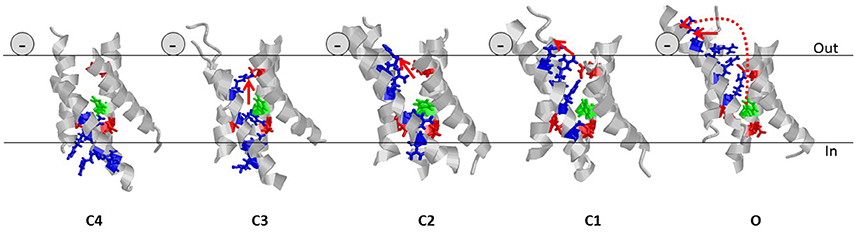
Figure 6. Helical screw and the lipoelectric effect. Five states of the VSD of the Shaker KV channel are shown (Henrion et al., 2012). For clarity, only the transmembrane segments (224–246, 278–300, 311–332, 354–377) are shown and the intra- and extracellular loops are removed. Gating charges (residues R362, R365, R368, and R371) of S4 are shown as blue sticks. Negative counter charges (E283, E293, and D316) are shown as red sticks. The hydrophobic barrier in S2 is shown in green (F290). The continuous red arrows indicated the movement of the charge of R362 in each step. The dotted red arrow in state O denotes the complete movement of R362, from state C4 to state O. The negative sign denotes the position of the carboxyl group of the PUFA molecule.
S4 not only slides along S1–S3 during activation but also rotates around its longitudinal axis because the positive charges are spiraling around S4 (Figure 6). This means that the top positive charge in S4 (R1) moves in a spiral from the center of the channel to the extracellular surface and then along the surface (arrows in Figure 6). Thus, fixed negative charges at or close to the extracellular surface of the channel can electrostatically “pull” S4 to open the channel, while fixed positive charges could do the opposite. For instance, charged residues in the extracellular linkers connecting the transmembrane segments of a voltage-gated ion channel can control the voltage dependence of the channel (Elinder et al., 2016).
Our data are consistent with one (or several) PUFA molecules interacting with the VSD close to a cleft between the extracellular ends of S3 and S4 (Börjesson and Elinder, 2011). Experimental data from the Shaker KV channel suggests that it is mainly the C1 → O transition that is affected by the PUFA molecules and that the top charge of S4, which moves horizontally along the lipid bilayer during this last step, is the most important charge for the effect.
Here we list experimental support for the proposed lipoelectric model. Most of the experiments have been performed on the Shaker KV channel. Some experiments have also been performed on KV7.1 and KV7.2/3 channels:
(1) The sign and size of the PUFA charge is critical for the effect. (i) A PUFA molecule, expected to be at least partially negatively charged at neutral pH, increases the current (Figure 7A, red curve) by shifting the G(V) curve in negative direction along the voltage axis (Figure 7B, red curve), as expected from an electrostatic mechanism. (ii) If the PUFA molecule is not permanently charged at neutral pH, alterations in pH are expected to affect the PUFA effect. In fact, pH has a pronounced effect on the G(V) shift for PUFAs (Figure 7C, red symbols). At pH 6.5 there is no shift as if the PUFA molecule is uncharged. At pH 9 or 10 the shift is saturated as if the PUFA molecule is fully negatively charged. The midpoint value of the curve is at pH 7.9 for the Shaker K channel. This surprisingly high value compared to the predicted pKa value in solution of pH 4.9 suggests that the local pH at the surface is radically different from the bulk solution. Similar effects have been described for KV7.1 (pKa = 7.7) and KV7.2/3 (pKa = 7.5). Alteration of the charge of amino acids close to the binding site can alter the apparent pKa value of PUFAs (Börjesson and Elinder, 2011). Interestingly the auxiliary subunit KCNE1 alters the pKa value of KV7.1 to pKa = 8.6 to render the channel essentially insensitive to PUFA at neutral pH (Liin et al., 2015). (iii) If the charge is essential, an uncharged molecule should not shift the G(V) and a permanently charged should shift the G(V) as much the PUFA molecule at high pH. In fact, uncharged methyl esters of the PUFAs do not shift the G(V) despite competing with PUFAs for the same site (Liin et al., 2015). Designed PUFAs with a shifted pKa value, for instance docosahexaenoyl glycine (DHA-Gly), shifts the G(V) much more than a PUFA molecule at neutral pH (Figure 7D) (Liin et al., 2015, 2016b). Most importantly, a positively charged “PUFA” should shift the G(V) in positive direction along the voltage axis and reduce the current. This is in fact the case (Figures 7A,C blue trace and symbols) (Börjesson et al., 2010; Liin et al., 2015). Also these positively charged PUFA analogs show pH dependence, but now the effect is in opposite direction (Figure 7C).
(2) The positions and valence of the charges on S4 are critical. To investigate the PUFA interaction with S4, in closer detail, we decorated the extracellular end of S4 in the Shaker KV channel with positively charged residues in different positions (Ottosson et al., 2014). The major findings were the following: (i) moving the top charge R1 in S4 from position 362 to 359 (by constructing the A359R/R362Q mutant) increased the effect by DHA by a factor of about two (Figure 8A). (ii) Adding more arginines than just one sometimes increased the effect; adding two extra charges (356R and 359R) to the existing top charge of S4 (R362) increased the PUFA-induced G(V) shift by a factor of three (Figure 8A). Because there are three (positively charged) arginines in the sequence 356–362 in this construct, we have called this the 3R channel. (iii) A positively charged residue on the opposite side to R359 (the most influential charge) of the α-helical S4 (i.e., R361) abolished the PUFA-induced G(V) shift (Figure 8B), supporting the idea that S4 rotates and that R1 is moved along the bilayer surface (at least in its last step). (iv) Negatively charged residues introduced at these specific positions in S4 had opposite effects to positive charges supporting electrostatic effects.
(3) PUFA mainly act on the final channel-opening step. A voltage-gated ion channel undergoes several voltage dependent transitions between closed (C) states before it enters into the open (O) state (Figure 8C). PUFAs act on the voltage-sensor transitions and can theoretically act on any of the transitions. It is possible to differentiate effects on the early voltage-dependent transitions, before the channel reaches the open state, and the final voltage-dependent transition, which open the channel, in the Shaker KV channel by introducing a set of mutations in S4 (the ILT mutation) (Smith-Maxwell et al., 1998). We found that DHA only has a minor effect on the early transitions, and that almost all effects of DHA are on the last step (Börjesson and Elinder, 2011). This means that the critical, PUFA-sensitive step, is when R1 moves from a position close to the pore domain to a position close to the lipid bilayer (C1 to O in Figure 6). In KV7.1 channels, both early S4 movements and S4 movements associated with channel opening are affected by PUFA (Liin et al., 2016b). However, the relative PUFA effect on these different gating transitions in the KV7.1 channel remains to be quantified.
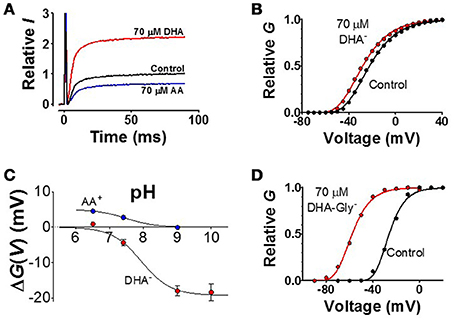
Figure 7. Examples of experimental data. (A) K current though Shaker KV channels at a voltage step to V = −40 from a holding voltage of −80 mV. The negatively charged DHA increases the current (red curve), while the positively charged arachidonoyl amine (AA+) decreases the current (Börjesson et al., 2010). (B) The normalized conductance-vs.- voltage curve for control and 70 μM DHA (Börjesson et al., 2010). (C) pH dependent shift of the G(V) curves (Börjesson et al., 2008, 2010). (D) Decreasing the pKa value of the DHA molecule, and thereby charging the molecule, by adding a glycine motif increases the shift (Liin et al., 2015) (data on the KV7.1 channel).
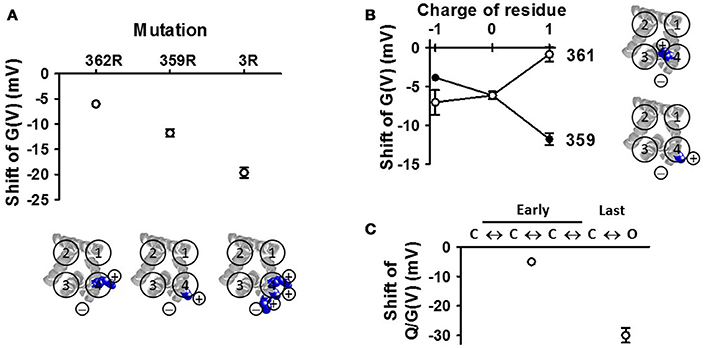
Figure 8. VSD mutations affect the DHA effect on the Shaker KV channel (A) The number and positions of the arginines in the top of S4 affects the DHA-induced G(V) shift (Ottosson et al., 2014). The 3R channel contains the positive charges R356, R359, and R362. Only the transmembrane parts are shown (residues 224–246, 278–300, 311–332, 354–377) (B) Altering the valence or altering the side of S4 for the charge alters the effect of DHA (Ottosson et al., 2014). (C) Effects on the early and late transitions are measured on the conducting and non-conducting ILT-mutant (Börjesson and Elinder, 2011).
The lipolelectric mechanism described above clearly explains why the charge of the PUFA molecule plays such an important role. However, we have less information about why multiple double bonds in cis geometry in the tail are required, and we have no information about the length of the tail. Double bonds restrict the conformational freedom in C = C bonds of the fatty acid but double bonds in cis geometry (Figure 2), causes the chain to bend and explore conformations not found for saturated fatty acids–the more cis double bonds the more curved the molecule is. The curvedness goes from a kink for one double bond to hairpin shapes for five or six double bonds. DHA, a PUFA with a 22-carbon chain and six cis double bonds, undergoes fast conformational changes and has a highly flexible structure (Eldho et al., 2003). In contrast, double bonds in trans geometry (Figure 2) do not cause the chain to bend much, having a shape similar to straight saturated fatty acids. Thus, it is not surprising that saturated fatty acids and trans PUFAs lack effects on ion channels if a curved shape is required.
The same pattern for the effective molecules is not restricted to voltage-gated ion channels but also to fatty-acid activation of K2P channels where fatty acid-induced stimulation requires at least one C = C bond and the anionic (COO-) form of the fatty acid (Lotshaw, 2007). Unesterified DHA molecules are predicted to infiltrate certain spaces between the transmembrane helices of rhodopsin (Grossfield et al., 2006). The dynamic changes in the protein during gating would thus be influenced by the packing of DHA within these spaces, which could explain how DHA facilitates conformational changes in rhodopsin upon activation (Feller and Gawrisch, 2005). This flexibility can explain the promiscuity of the PUFAs, why they act on so many channels and sites.
The concentration of unesterified PUFAs available to affect voltage-gated ion channels in different tissues is largely unknown. It is therefore difficult to assess the physiological relevance of the PUFA effects described in this paper. A concentration range of 1–30 μM of PUFA is often effective for experimental modulation of voltage-gated ion channels. The concentration of unesterified PUFA in plasma has been reported to be roughly 10–50 μM (Burtis and Ashwood, 1998; De Caterina et al., 2000; Fraser et al., 2003; Siddiqui et al., 2008). This plasma concentration of PUFA can dramatically increase to 130–400 μM during consumption of certain diets (Kuriki et al., 2002; Fraser et al., 2003; Siddiqui et al., 2008). Moreover, the local PUFA concentration in specific tissues may be increased during pathological conditions such as ischemia and epileptic seizures (Hochachka, 1986; Siesjö et al., 1989). Based on these reported PUFA concentrations it seems plausible that most voltage-gated ion channels that are PUFA sensitive would experience some degree of PUFA modulation under physiological as well as pathological conditions. The outcome of different synergistic and opposing PUFA modulations in terms of, for instance, neuronal and cardiac excitability is hard to predict and would depend on the PUFA sensitivity and relative importance of each type of ion channel.
In the present review, we have suggested five different PUFA-binding sites. Two of the sites (PUFA site 1 and PUFA site 2) are located in the ion conducting pore and binding to these sites reduce the current. Two of the sites (PUFA site 3 and PUFA site 4) can either increase or decrease the open probability of the channel by either affecting the gate (PUFA site 3) or the voltage sensor (PUFA site 4). Finally, one site in the periphery of the pore domain (PUFA site 5) can regulate slow inactivation by acting on distance. We suggest that all five sites can exist in a single ion channel and the overall effect is determined by the relative contributions of the five sites.
FE and SL designed the study, analyzed data, and wrote the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Sammy Yazdi for making Figure 5C, Sarah Lindström for linguistic advice, and Johan Brask for comments on the text. This work was supported by grants from the Swedish Research Council, the Swedish Brain Foundation, the Swedish Society for Medical Research, and the Swedish Heart-Lung Foundation.
Ahn, D. S., Kim, Y. B., Lee, Y. H., Kang, B. S., and Kang, D. H. (1994). Fatty acids directly increase the activity of Ca(2+)-activated K+ channels in rabbit coronary smooth muscle cells. Yonsei Med. J. 35, 10–24. doi: 10.3349/ymj.1994.35.1.10
Albert, C. M., Campos, H., Stampfer, M. J., Ridker, P. M., Manson, J. E., Willett, W. C., et al. (2002). Blood levels of long-chain n-3 fatty acids and the risk of sudden death. N. Engl. J. Med. 346, 1113–1118. doi: 10.1056/NEJMoa012918
Anderson, M. P., and Welsh, M. J. (1990). Fatty acids inhibit apical membrane chloride channels in airway epithelia. Proc. Natl. Acad. Sci. U.S.A. 87, 7334–7338. doi: 10.1073/pnas.87.18.7334
Andersson, D. A., Nash, M., and Bevan, S. (2007). Modulation of the cold-activated channel TRPM8 by lysophospholipids and polyunsaturated fatty acids. J. Neurosci. 27, 3347–3355. doi: 10.1523/JNEUROSCI.4846-06.2007
Angelova, P. R., and Müller, W. S. (2009). Arachidonic acid potently inhibits both postsynaptic-type Kv4.2 and presynaptic-type Kv1.4 IA potassium channels. Eur. J. Neurosci. 29, 1943–1950. doi: 10.1111/j.1460-9568.2009.06737.x
Angelova, P., and Müller, W. (2006). Oxidative modulation of the transient potassium current IA by intracellular arachidonic acid in rat CA1 pyramidal neurons. Eur. J. Neurosci. 23, 2375–2384. doi: 10.1111/j.1460-9568.2006.04767.x
Asano, M., Nakajima, T., Iwasawa, K., Hazama, H., Omata, M., Soma, M., et al. (1997). Inhibitory effects of omega-3 polyunsaturated fatty acids on receptor-mediated non-selective cation currents in rat A7r5 vascular smooth muscle cells. Br. J. Pharmacol. 120, 1367–1375. doi: 10.1038/sj.bjp.0701047
Bai, J. Y., Ding, W. G., Kojima, A., Seto, T., and Matsuura, H. (2015). Putative binding sites for arachidonic acid on the human cardiac Kv 1.5 channel. Br. J. Pharmacol. 172, 5281–5292. doi: 10.1111/bph.13314
Bang, H. O., Dyerberg, J., and Nielsen, A. B. (1971). Plasma lipid and lipoprotein pattern in Greenlandic West-coast Eskimos. Lancet 1, 1143–1145. doi: 10.1016/S0140-6736(71)91658-8
Barbara, G., Alloui, A., Nargeot, J., Lory, P., Eschalier, A., Bourinet, E., et al. (2009). T-type calcium channel inhibition underlies the analgesic effects of the endogenous lipoamino acids. J. Neurosci. 29, 13106–13114. doi: 10.1523/JNEUROSCI.2919-09.2009
Barlow, R. S., El-Mowafy, A. M., and White, R. E. (2000). H(2)O(2) opens BK(Ca) channels via the PLA(2)-arachidonic acid signaling cascade in coronary artery smooth muscle. Am. J. Physiol. Heart Circ. Physiol. 279, H475–H483.
Barrett, C. F., Liu, L., and Rittenhouse, A. R. (2001). Arachidonic acid reversibly enhances N-type calcium current at an extracellular site. Am. J. Physiol. Cell Physiol. 280, C1306–C1318.
Bavencoffe, A., Kondratskyi, A., Gkika, D., Mauroy, B., Shuba, Y., Prevarskaya, N., et al. (2011). Complex regulation of the TRPM8 cold receptor channel: role of arachidonic acid release following M3 muscarinic receptor stimulation. J. Biol. Chem. 286, 9849–9855. doi: 10.1074/jbc.M110.162016
Béhé, P., Sandmeier, K., and Meves, H. (1992). The effect of arachidonic acid on the M current of NG108-15 neuroblastoma x glioma hybrid cells. Pflugers Arch. 422, 120–128. doi: 10.1007/BF00370411
Bendahhou, S., Cummins, T. R., and Agnew, W. S. (1997). Mechanism of modulation of the voltage-gated skeletal and cardiac muscle sodium channels by fatty acids. Am. J. Physiol. 272, C592–C600.
Bezanilla, F. (2000). The voltage sensor in voltage-dependent ion channels. Physiol. Rev. 80, 555–592.
Bezanilla, F. (2008). How membrane proteins sense voltage. Nat. Rev. Mol. Cell Biol. 9, 323–332. doi: 10.1038/nrm2376
Billman, G. E., Hallaq, H., and Leaf, A. (1994). Prevention of ischemia-induced ventricular fibrillation by omega 3 fatty acids. Proc. Natl. Acad. Sci. U.S.A. 91, 4427–4430. doi: 10.1073/pnas.91.10.4427
Billman, G. E., Kang, J. X., and Leaf, A. (1997). Prevention of ischemia-induced cardiac sudden death by n-3 polyunsaturated fatty acids in dogs. Lipids 32, 1161–1168. doi: 10.1007/s11745-997-0149-2
Billman, G. E., Kang, J. X., and Leaf, A. (1999). Prevention of sudden cardiac death by dietary pure omega-3 polyunsaturated fatty acids in dogs. Circulation 99, 2452–2457. doi: 10.1161/01.CIR.99.18.2452
Bittner, K., and Müller, W. (1999). Oxidative downmodulation of the transient K-current IA by intracellular arachidonic acid in rat hippocampal neurons. J. Neurophysiol. 82, 508–511.
Bogdanov, K. Y., Spurgeon, H. A., Vinogradova, T. M., and Lakatta, E. G. (1998). Modulation of the transient outward current in adult rat ventricular myocytes by polyunsaturated fatty acids. Am. J. Physiol. 274, H571–H579.
Boland, L. M., and Drzewiecki, M. M. (2008). Polyunsaturated fatty acid modulation of voltage-gated ion channels. Cell Biochem. Biophys. 52, 59–84. doi: 10.1007/s12013-008-9027-2
Boland, L. M., Drzewiecki, M. M., Timoney, G., and Casey, E. (2009). Inhibitory effects of polyunsaturated fatty acids on Kv4/KChIP potassium channels. Am. J. Physiol. Cell Physiol. 296, C1003–C1014. doi: 10.1152/ajpcell.00474.2008
Börjesson, S. I., and Elinder, F. (2008). Structure, function, and modification of the voltage sensor in voltage-gated ion channels. Cell Biochem. Biophys. 52, 149–174. doi: 10.1007/s12013-008-9032-5
Börjesson, S. I., and Elinder, F. (2011). An electrostatic potassium channel opener targeting the final voltage sensor transition. J. Gen. Physiol. 137, 563–577. doi: 10.1085/jgp.201110599
Börjesson, S. I., Hammarström, S., and Elinder, F. (2008). Lipoelectric modification of ion channel voltage gating by polyunsaturated fatty acids. Biophys. J. 95, 2242–2253. doi: 10.1529/biophysj.108.130757
Börjesson, S. I., Parkkari, T., Hammarström, S., and Elinder, F. (2010). Electrostatic tuning of cellular excitability. Biophys. J. 98, 396–403. doi: 10.1016/j.bpj.2009.10.026
Bregestovski, P. D., Bolotina, V. M., and Serebryakov, V. N. (1989). Fatty acid modifies Ca2+-dependent potassium channel activity in smooth muscle cells from the human aorta. Proc. R Soc. Lond. B Biol. Sci. 237, 259–266. doi: 10.1098/rspb.1989.0048
Bringmann, A., Schopf, S., Faude, F., and Reichenbach, A. (2001). Arachidonic acid-induced inhibition of Ca2+ channel currents in retinal glial (Muller) cells. Graefes. Arch. Clin. Exp. Ophthalmol. 239, 859–864. doi: 10.1007/s004170100372
Bringmann, A., Skatchkov, S. N., Biedermann, B., Faude, F., and Reichenbach, A. (1998). Alterations of potassium channel activity in retinal Muller glial cells induced by arachidonic acid. Neuroscience 86, 1291–1306. doi: 10.1016/S0306-4522(98)00079-7
Burr, M. L., Fehily, A. M., Gilbert, J. F., Rogers, S., Holliday, R. M., Sweetnam, P. M., et al. (1989). Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART). Lancet 2, 757–761. doi: 10.1016/S0140-6736(89)90828-3
Burtis, C. A., and Ashwood, E. R. (1998). Tietz Fundamentals of Clinical Chemistry. Philadelphia: WB Saunders Company.
Buttner, N., Siegelbaum, S. A., and Volterra, A. (1989). Direct modulation of Aplysia S-K+ channels by a 12-lipoxygenase metabolite of arachidonic acid. Nature 342, 553–555. doi: 10.1038/342553a0
Campos, F. V., Chanda, B., Roux, B., and Bezanilla, F. (2007). Two atomic constraints unambiguously position the S4 segment relative to S1 and S2 segments in the closed state of Shaker K channel. Proc. Natl. Acad. Sci. U.S.A. 104, 7904–7909. doi: 10.1073/pnas.0702638104
Cantiello, H. F., Patenaude, C. R., Codina, J., Birnbaumer, L., and Ausiello, D. A. (1990). G alpha i-3 regulates epithelial Na+ channels by activation of phospholipase A2 and lipoxygenase pathways. J. Biol. Chem. 265, 21624–21628.
Carta, M., Lanore, F., Rebola, N., Szabo, Z., Da Silva, S. V., Lourenco, J., et al. (2014). Membrane lipids tune synaptic transmission by direct modulation of presynaptic potassium channels. Neuron 81, 787–799. doi: 10.1016/j.neuron.2013.12.028
Casavant, R. H., Xu, Z., and Dryer, S. E. (2000). Fatty acid-activated K+ channels in autonomic neurons: activation by an endogenous source of free fatty acids. J. Neurochem. 74, 1026–1033. doi: 10.1046/j.1471-4159.2000.0741026.x
Catterall, W. A., Cestèle, S., Yarov-Yarovoy, V., Yu, F. H., Konoki, K., and Scheuer, T. (2007). Voltage-gated ion channels and gating modifier toxins. Toxicon 49, 124–141. doi: 10.1016/j.toxicon.2006.09.022
Cazade, M., Bidaud, I., Hansen, P. B., Lory, P., and Chemin, J. (2014). 5,6-EET potently inhibits T-type calcium channels: implication in the regulation of the vascular tone. Pflugers Arch. 466, 1759–1768. doi: 10.1007/s00424-013-1411-0
Charpentier, G., Béhue, N., and Fournier, F. (1995). Phospholipase C activates protein kinase C during induction of slow Na current in Xenopus oocytes. Pflugers Arch. 429, 825–831. doi: 10.1007/BF00374807
Chemin, J., Nargeot, J., and Lory, P. (2007). Chemical determinants involved in anandamide-induced inhibition of T-type calcium channels. J. Biol. Chem. 282, 2314–2323. doi: 10.1074/jbc.M610033200
Chen, J., Capdevila, J. H., Zeldin, D. C., and Rosenberg, R. L. (1999). Inhibition of cardiac L-type calcium channels by epoxyeicosatrienoic acids. Mol. Pharmacol. 55, 288–295.
Chen, W. H., Chen, C. R., Yang, K. T., Chang, W. L., Su, M. J., Wu, C. C., et al. (2001). Arachidonic acid-induced H+ and Ca2+ increases in both the cytoplasm and nucleoplasm of rat cerebellar granule cells. J. Physiol. 537, 497–510. doi: 10.1111/j.1469-7793.2001.00497.x
Chesnoy-Marchais, D., and Fritsch, J. (1994). Concentration-dependent modulations of potassium and calcium currents of rat osteoblastic cells by arachidonic acid. J. Membr. Biol. 138, 159–170. doi: 10.1007/BF00232644
Chyb, S., Raghu, P., and Hardie, R. C. (1999). Polyunsaturated fatty acids activate the Drosophila light-sensitive channels TRP and TRPL. Nature 397, 255–259. doi: 10.1038/16703
Clarke, A. L., Petrou, S., Walsh, J. V. Jr., and Singer, J. J. (2002). Modulation of BK(Ca) channel activity by fatty acids: structural requirements and mechanism of action. Am. J. Physiol. Cell Physiol. 283, C1441–C1453. doi: 10.1152/ajpcell.00035.2002
Clarke, A. L., Petrou, S., Walsh, J. V. Jr., and Singer, J. J. (2003). Site of action of fatty acids and other charged lipids on BKCa channels from arterial smooth muscle cells. Am. J. Physiol. Cell Physiol. 284, C607–C619. doi: 10.1152/ajpcell.00364.2002
Colbert, C. M., and Pan, E. (1999). Arachidonic acid reciprocally alters the availability of transient and sustained dendritic K(+) channels in hippocampal CA1 pyramidal neurons. J. Neurosci. 19, 8163–8171.
Cui, Y., Liu, X., Yang, T., Mei, Y. A., and Hu, C. (2014). Exposure to extremely low-frequency electromagnetic fields inhibits T-type calcium channels via AA/LTE4 signaling pathway. Cell Calcium 55, 48–58. doi: 10.1016/j.ceca.2013.11.002
Damron, D. S., and Bond, M. (1993). Modulation of Ca2+ cycling in cardiac myocytes by arachidonic acid. Circ. Res. 72, 376–386. doi: 10.1161/01.RES.72.2.376
Damron, D. S., and Summers, B. A. (1997). Arachidonic acid enhances contraction and intracellular Ca2+ transients in individual rat ventricular myocytes. Am. J. Physiol. 272, H350–H359.
Damron, D. S., Van Wagoner, D. R., Moravec, C. S., and Bond, M. (1993). Arachidonic acid and endothelin potentiate Ca2+ transients in rat cardiac myocytes via inhibition of distinct K+ channels. J. Biol. Chem. 268, 27335–27344.
Danthi, S. J., Enyeart, J. A., and Enyeart, J. J. (2005). Modulation of native T-type calcium channels by omega-3 fatty acids. Biochem. Biophys. Res. Commun. 327, 485–493. doi: 10.1016/j.bbrc.2004.12.033
Danthi, S., Enyeart, J. A., and Enyeart, J. J. (2003). Modulation of native TREK-1 and Kv1.4 K+ channels by polyunsaturated fatty acids and lysophospholipids. J. Membr. Biol. 195, 147–164. doi: 10.1007/s00232-003-0616-0
De Caterina, R., Liao, J. K., and Libby, P. (2000). Fatty acid modulation of endothelial activation. Am. J. Clin. Nutr. 71, 213S-223S.
Decher, N., Streit, A. K., Rapedius, M., Netter, M. F., Marzian, S., Ehling, P., et al. (2010). RNA editing modulates the binding of drugs and highly unsaturated fatty acids to the open pore of Kv potassium channels. EMBO J. 29, 2101–2113. doi: 10.1038/emboj.2010.88
DeCostanzo, A. J., Voloshyna, I., Rosen, Z. B., Feinmark, S. J., and Siegelbaum, S. A. (2010). 12-Lipoxygenase regulates hippocampal long-term potentiation by modulating L-type Ca2+ channels. J. Neurosci. 30, 1822–1831. doi: 10.1523/JNEUROSCI.2168-09.2010
DeCoursey, T. E., and Cherny, V. V. (1993). Potential, pH, and arachidonate gate hydrogen ion currents in human neutrophils. Biophys. J. 65, 1590–1598. doi: 10.1016/S0006-3495(93)81198-6
Delemotte, L., Tarek, M., Klein, M. L., Amaral, C., and Treptow, W. (2011). Intermediate states of the Kv1.2 voltage sensor from atomistic molecular dynamics simulations. Proc. Natl. Acad. Sci. U.S.A. 108, 6109–6114. doi: 10.1073/pnas.1102724108
Delgado, R., and Bacigalupo, J. (2009). Unitary recordings of TRP and TRPL channels from isolated Drosophila retinal photoreceptor rhabdomeres: activation by light and lipids. J. Neurophysiol. 101, 2372–2379. doi: 10.1152/jn.90578.2008
de Lorgeril, M., Renaud, S., Mamelle, N., Salen, P., Martin, J. L., Monjaud, I., et al. (1994). Mediterranean alpha-linolenic acid-rich diet in secondary prevention of coronary heart disease. Lancet 343, 1454–1459. doi: 10.1016/S0140-6736(94)92580-1
Dennis, E. A., Rhee, S. G., Billah, M. M., and Hannun, Y. A. (1991). Role of phospholipase in generating lipid second messengers in signal transduction. FASEB J. 5, 2068–2077.
Denson, D. D., Li, J., and Eaton, D. C. (2006). Co-localization of the alpha-subunit of BK-channels and c-PLA2 in GH3 cells. Biochem. Biophys. Res. Commun. 350, 39–49. doi: 10.1016/j.bbrc.2006.08.193
Denson, D. D., Li, J., Wang, X., and Eaton, D. C. (2005). Activation of BK channels in GH3 cells by a c-PLA2-dependent G-protein signaling pathway. J. Neurophysiol. 93, 3146–3156. doi: 10.1152/jn.00865.2004
Denson, D. D., Wang, X., Worrell, R. T., and Eaton, D. C. (2000). Effects of fatty acids on BK channels in GH(3) cells. Am. J. Physiol. Cell Physiol. 279, C1211–C1219.
Denson, D. D., Worrell, R. T., Middleton, P., and Eaton, D. C. (1999). Ca2+ sensitivity of BK channels in GH3 cells involves cytosolic phospholipase A2. Am. J. Physiol. 276, C201–C209.
Dettbarn, C., and Palade, P. (1993). Arachidonic acid-induced Ca2+ release from isolated sarcoplasmic reticulum. Biochem. Pharmacol. 45, 1301–1309. doi: 10.1016/0006-2952(93)90283-3
Devor, D. C., and Frizzell, R. A. (1998). Modulation of K+ channels by arachidonic acid in T84 cells. II. Activation of a Ca(2+)-independent K+ channel. Am. J. Physiol. 274, C149–C160.
Ding, G. L., Hu, D., Liu, X., Li, J., Zhang, H., and Li, C. (2000). Suppression of sodium current by arachidonic acid in atrial myocytes from patients with coronary heart disease. Pacing Clin. Electrophysiol. 23, 1820–1822. doi: 10.1111/j.1540-8159.2000.tb07028.x
Doolan, G. K., Panchal, R. G., Fonnes, E. L., Clarke, A. L., Williams, D. A., and Petrou, S. (2002). Fatty acid augmentation of the cardiac slowly activating delayed rectifier current (IKs) is conferred by hminK. FASEB J. 16, 1662–1664. doi: 10.1096/fj.02-0084fje
Dryer, L., Xu, Z., and Dryer, S. E. (1998). Arachidonic acid-sensitive A-currents and multiple Kv4 transcripts are expressed in chick ciliary ganglion neurons. Brain Res. 789, 162–166. doi: 10.1016/S0006-8993(98)00077-8
Duan, Y., Liao, C., Jain, S., and Nicholson, R. A. (2008). The cannabinoid receptor agonist CP-55,940 and ethyl arachidonate interfere with [(3)H]batrachotoxinin A 20 alpha-benzoate binding to sodium channels and inhibit sodium channel function. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 148, 244–249. doi: 10.1016/j.cbpc.2008.06.002
Duerson, K., White, R. E., Jiang, F., Schonbrunn, A., and Armstrong, D. L. (1996). Somatostatin stimulates BKCa channels in rat pituitary tumor cells through lipoxygenase metabolites of arachidonic acid. Neuropharmacology 35, 949–961. doi: 10.1016/0028-3908(96)00131-1
Dujardin, K. S., Dumotier, B., David, M., Guizy, M., Valenzuela, C., and Hondeghem, L. M. (2008). Ultrafast sodium channel block by dietary fish oil prevents dofetilide-induced ventricular arrhythmias in rabbit hearts. Am. J. Physiol. Heart Circ. Physiol. 295, H1414–H1421. doi: 10.1152/ajpheart.01219.2007
Eldho, N. V., Feller, S. E., Tristram-Nagle, S., Polozov, I. V., and Gawrisch, K. (2003). Polyunsaturated docosahexaenoic vs docosapentaenoic acid-differences in lipid matrix properties from the loss of one double bond. J. Am. Chem. Soc. 125, 6409–6421. doi: 10.1021/ja029029o
Elinder, F., Madeja, M., Zeberg, H., and Århem, P. (2016). Extracellular Linkers Completely Transplant the Voltage Dependence from Kv1.2 Ion Channels to Kv2.1. Biophys. J. 111, 1679–1691. doi: 10.1016/j.bpj.2016.08.043
Enyeart, J. J., and Enyeart, J. A. (2013). Ca2+ and K+ channels of normal human adrenal zona fasciculata cells: properties and modulation by ACTH and AngII. J. Gen. Physiol. 142, 137–155. doi: 10.1085/jgp.201310964
Erichsen, D., Xu, X.-P., and Elinder, F. (2002). Docosahexaenoic acid induced opening of voltage-gated K channels prevents repetitive firing in neurons. Brain Dev. 24, 399.
Erriquez, J., Gilardino, A., Ariano, P., Munaron, L., Lovisolo, D., and Distasi, C. (2005). Calcium signals activated by arachidonic acid in embryonic chick ciliary ganglion neurons. Neurosignals. 14, 244–254. doi: 10.1159/000088640
Fang, X., Weintraub, N. L., Stoll, L. L., and Spector, A. A. (1999). Epoxyeicosatrienoic acids increase intracellular calcium concentration in vascular smooth muscle cells. Hypertension 34, 1242–1246. doi: 10.1161/01.HYP.34.6.1242
Fang, Y. J., Zhou, M. H., Gao, X. F., Gu, H., and Mei, Y. A. (2011). Arachidonic acid modulates Na+ currents by non-metabolic and metabolic pathways in rat cerebellar granule cells. Biochem. J. 438, 203–215. doi: 10.1042/BJ20110569
Farag, N. E., Jeong, D., Claydon, T., Warwicker, J., and Boyett, M. R. (2016). Polyunsaturated fatty acids inhibit Kv1.4 by interacting with positively charged extracellular pore residues. Am. J. Physiol. Cell Physiol. 311, C255–C268. doi: 10.1152/ajpcell.00277.2015
Feller, S. E., and Gawrisch, K. (2005). Properties of docosahexaenoic-acid-containing lipids and their influence on the function of rhodopsin. Curr. Opin. Struct. Biol. 15, 416–422. doi: 10.1016/j.sbi.2005.07.002
Feller, S. E., Gawrisch, K., and MacKerell, A. D. Jr. (2002). Polyunsaturated fatty acids in lipid bilayers: intrinsic and environmental contributions to their unique physical properties. J. Am. Chem. Soc. 124, 318–326. doi: 10.1021/ja0118340
Feng, D. D., Luo, Z., Roh, S. G., Hernandez, M., Tawadros, N., Keating, D. J., et al. (2006). Reduction in voltage-gated K+ currents in primary cultured rat pancreatic beta-cells by linoleic acids. Endocrinology 147, 674–682. doi: 10.1210/en.2005-0225
Feng, D. D., Zhao, Y. F., Luo, Z. Q., Keating, D. J., and Chen, C. (2008). Linoleic acid induces Ca2+-induced inactivation of voltage-dependent Ca2+ currents in rat pancreatic beta-cells. J. Endocrinol. 196, 377–384. doi: 10.1677/JOE-07-0426
Ferrier, G. R., Redondo, I., Zhu, J., and Murphy, M. G. (2002). Differential effects of docosahexaenoic acid on contractions and L-type Ca2+ current in adult cardiac myocytes. Cardiovasc. Res. 54, 601–610. doi: 10.1016/S0008-6363(02)00275-4
Ferroni, S., Valente, P., Caprini, M., Nobile, M., Schubert, P., and Rapisarda, C. (2003). Arachidonic acid activates an open rectifier potassium channel in cultured rat cortical astrocytes. J. Neurosci. Res. 72, 363–372. doi: 10.1002/jnr.10580
Finkel, M. S., Romeo, R. C., Hartsell, T. L., and Oddis, C. V. (1992). Bay K 8644 is a negative inotrope in the presence of arachidonic acid but not eicosapentaenoic acid. J. Cardiovasc. Pharmacol. 20, 563–571. doi: 10.1097/00005344-199210000-00009
Fioretti, B., Catacuzzeno, L., Tata, A. M., and Franciolini, F. (2004). Histamine activates a background, arachidonic acid-sensitive K channel in embryonic chick dorsal root ganglion neurons. Neuroscience 125, 119–127. doi: 10.1016/j.neuroscience.2004.01.028
Fiorio Pla, A., and Munaron, L. (2001). Calcium influx, arachidonic acid, and control of endothelial cell proliferation. Cell Calcium 30, 235–244. doi: 10.1054/ceca.2001.0234
Fodor, J. G., Helis, E., Yazdekhasti, N., and Vohnout, B. (2014). “Fishing” for the origins of the “Eskimos and heart disease” story: facts or wishful thinking? Can. J. Cardiol. 30, 864–868. doi: 10.1016/j.cjca.2014.04.007
Fogle, K. J., Lyashchenko, A. K., Turbendian, H. K., and Tibbs, G. R. (2007). HCN pacemaker channel activation is controlled by acidic lipids downstream of diacylglycerol kinase and phospholipase A2. J. Neurosci. 27, 2802–2814. doi: 10.1523/JNEUROSCI.4376-06.2007
Fraser, D. D., Hoehn, K., Weiss, S., and MacVicar, B. A. (1993). Arachidonic acid inhibits sodium currents and synaptic transmission in cultured striatal neurons. Neuron 11, 633–644. doi: 10.1016/0896-6273(93)90075-3
Fraser, D. D., Whiting, S., Andrew, R. D., Macdonald, E. A., Musa-Veloso, K., and Cunnane, S. C. (2003). Elevated polyunsaturated fatty acids in blood serum obtained from children on the ketogenic diet. Neurology 60, 1026–1029. doi: 10.1212/01.WNL.0000049974.74242.C6
Freites, J. A., Tobias, D. J., von Heijne, G., and White, S. H. (2005). Interface connections of a transmembrane voltage sensor. Proc. Natl. Acad. Sci. U.S.A. 102, 15059–15064. doi: 10.1073/pnas.0507618102
Fukao, M., Mason, H. S., Kenyon, J. L., Horowitz, B., and Keef, K. D. (2001). Regulation of BKCa channels expressed in human embryonic kidney 293 cells by epoxyeicosatrienoic acid. Mol. Pharmacol. 59, 16–23. doi: 10.1124/mol.59.1.16
Fyfe, G. K., Kemp, P. J., and Olver, R. E. (1997). Conductive Na+ transport in fetal lung alveolar apical membrane vesicles is regulated by fatty acids and G proteins. Biochim. Biophys. Acta 1355, 33–42. doi: 10.1016/S0167-4889(96)00114-0
GISSI-Prevenzione Investigators (1999). Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico. Lancet 354, 447–455. doi: 10.1016/S0140-6736(99)07072-5
Garratt, J. C., McEvoy, M. P., and Owen, D. G. (1996). Blockade of two voltage-dependent potassium channels, mKv1.1 and mKv1.2, by docosahexaenoic acid. Eur. J. Pharmacol. 314, 393–396. doi: 10.1016/S0014-2999(96)00722-4
Gauthier, K. M., Campbell, W. B., and McNeish, A. J. (2014). Regulation of KCa2.3 and endothelium-dependent hyperpolarization (EDH) in the rat middle cerebral artery: the role of lipoxygenase metabolites and isoprostanes. PeerJ 2:e414. doi: 10.7717/peerj.414
Gauthier, K. M., Spitzbarth, N., Edwards, E. M., and Campbell, W. B. (2004). Apamin-sensitive K+ currents mediate arachidonic acid-induced relaxations of rabbit aorta. Hypertension 43, 413–419. doi: 10.1161/01.HYP.0000110945.84443.d2
Gavrilova-Ruch, O., Schönherr, R., and Heinemann, S. H. (2007). Activation of hEAG1 potassium channels by arachidonic acid. Pflugers Arch. 453, 891–903. doi: 10.1007/s00424-006-0173-3
Gebremedhin, D., Yamaura, K., and Harder, D. R. (2008). Role of 20-HETE in the hypoxia-induced activation of Ca2+-activated K+ channel currents in rat cerebral arterial muscle cells. Am. J. Physiol. Heart Circ. Physiol. 294, H107–H120. doi: 10.1152/ajpheart.01416.2006
Giaume, C., Randriamampita, C., and Trautmann, A. (1989). Arachidonic acid closes gap junction channels in rat lacrimal glands. Pflugers Arch. 413, 273–279. doi: 10.1007/BF00583541
Gilbertson, T. A., Fontenot, D. T., Liu, L., Zhang, H., and Monroe, W. T. (1997). Fatty acid modulation of K+ channels in taste receptor cells: gustatory cues for dietary fat. Am. J. Physiol. 272, C1203-C1210.
Godlewski, G., Offertáler, L., Osei-Hyiaman, D., Mo, F. M., Harvey-White, J., Liu, J., et al. (2009). The endogenous brain constituent N-arachidonoyl L-serine is an activator of large conductance Ca2+-activated K+ channels. J. Pharmacol. Exp. Ther. 328, 351–361. doi: 10.1124/jpet.108.144717
Gordienko, D. V., Tare, M., Parveen, S., Fenech, C. J., Robinson, C., and Bolton, T. B. (1996). Voltage-activated proton current in eosinophils from human blood. J. Physiol. 496(Pt 2), 299–316. doi: 10.1113/jphysiol.1996.sp021686
Grossfield, A., Feller, S. E., and Pitman, M. C. (2006). A role for direct interactions in the modulation of rhodopsin by omega-3 polyunsaturated lipids. Proc. Natl. Acad. Sci. U.S.A. 103, 4888–4893. doi: 10.1073/pnas.0508352103
Gu, H., Fang, Y. J., He, Y. L., Sun, J., Zhu, J., and Mei, Y. A. (2009). Modulation of muscle rNaV1.4 Na+ channel isoform by arachidonic acid and its non-metabolized analog. J. Cell. Physiol. 219, 173–182. doi: 10.1002/jcp.21664
Gu, H., Fang, Y. J., Liu, D. D., Chen, P., and Mei, Y. A. (2015). cAMP/PKA pathways and S56 phosphorylation are involved in AA/PGE2-induced increases in rNaV1.4 current. PLoS ONE 10:e0140715. doi: 10.1371/journal.pone.0140715
Gubitosi-Klug, R. A., Yu, S. P., Choi, D. W., and Gross, R. W. (1995). Concomitant acceleration of the activation and inactivation kinetics of the human delayed rectifier K+ channel (Kv1.1) by Ca(2+)-independent phospholipase A2. J. Biol. Chem. 270, 2885–2888. doi: 10.1074/jbc.270.7.2885
Guermouche, B., Yessoufou, A., Soulimane, N., Merzouk, H., Moutairou, K., Hichami, A., et al. (2004). n-3 fatty acids modulate T-cell calcium signaling in obese macrosomic rats. Obes. Res. 12, 1744–1753. doi: 10.1038/oby.2004.216
Guibert, C., Marthan, R., and Savineau, J. P. (2004). 5-HT induces an arachidonic acid-sensitive calcium influx in rat small intrapulmonary artery. Am. J. Physiol. Lung Cell. Mol. Physiol. 286, L1228–L1236. doi: 10.1152/ajplung.00265.2003
Guizy, M., Arias, C., David, M., González, T., and Valenzuela, C. (2005). {Omega}-3 and {omega}-6 polyunsaturated fatty acids block HERG channels. Am. J. Physiol. Cell Physiol. 289, C1251–1260. doi: 10.1152/ajpcell.00036.2005
Guizy, M., David, M., Arias, C., Zhang, L., Cofán, M., Ruiz-Gutiérrez, V., et al. (2008). Modulation of the atrial specific Kv1.5 channel by the n-3 polyunsaturated fatty acid, alpha-linolenic acid. J. Mol. Cell. Cardiol. 44, 323–335. doi: 10.1016/j.yjmcc.2007.11.004
Guo, D., Xiang, W., Seebahn, A., Becker, C. M., and Strauss, O. (2012). Modulation of TTX-sensitive voltage-dependent Na+ channels by beta-bungarotoxin in rat cerebellar neurons. BMC Neurosci. 13:36. doi: 10.1186/1471-2202-13-36
Gutla, P. V., Boccaccio, A., De Angeli, A., Gambale, F., and Carpaneto, A. (2012). Modulation of plant TPC channels by polyunsaturated fatty acids. J. Exp. Bot. 63, 6187–6197. doi: 10.1093/jxb/ers272
Hallaq, H., Smith, T. W., and Leaf, A. (1992). Modulation of dihydropyridine-sensitive calcium channels in heart cells by fish oil fatty acids. Proc. Natl. Acad. Sci. U.S.A. 89, 1760–1764. doi: 10.1073/pnas.89.5.1760
Hamilton, K. L., Syme, C. A., and Devor, D. C. (2003). Molecular localization of the inhibitory arachidonic acid binding site to the pore of hIK1. J. Biol. Chem. 278, 16690–16697. doi: 10.1074/jbc.M212959200
Harrell, M. D., and Stimers, J. R. (2002). Differential effects of linoleic Acid metabolites on cardiac sodium current. J. Pharmacol. Exp. Ther. 303, 347–355. doi: 10.1124/jpet.102.038166
Harris, W. S., De Caterina, R., and Marik, P. E. (2013). Docosahexaenoic acid ethyl esters ineffective? Proc. Natl. Acad. Sci. U.S.A. 110, E2259. doi: 10.1073/pnas.1305038110
Hartmannsgruber, V., Heyken, W. T., Kacik, M., Kaistha, A., Grgic, I., Harteneck, C., et al. (2007). Arterial response to shear stress critically depends on endothelial TRPV4 expression. PLoS ONE 2:e827. doi: 10.1371/journal.pone.0000827
Hatton, C. J., and Peers, C. (1998). Arachidonic acid inhibits both K+ and Ca2+ currents in isolated type I cells of the rat carotid body. Brain Res. 787, 315–320. doi: 10.1016/S0006-8993(97)01486-8
Hazama, H., Nakajima, T., Asano, M., Iwasawa, K., Morita, T., Igarashi, K., et al. (1998). Omega-3 polyunsaturated fatty acids–modulation of voltage-dependent L-type Ca2+ current in guinea-pig tracheal smooth muscle cells. Eur. J. Pharmacol. 355, 257–266. doi: 10.1016/S0014-2999(98)00484-1
Heler, R., Bell, J. K., and Boland, L. M. (2013). Homology model and targeted mutagenesis identify critical residues for arachidonic acid inhibition of Kv4 channels. Channels 7, 74–84. doi: 10.4161/chan.23453
Heneghan, J. F., Mitra-Ganguli, T., Stanish, L. F., Liu, L., Zhao, R., and Rittenhouse, A. R. (2009). The Ca2+ channel beta subunit determines whether stimulation of Gq-coupled receptors enhances or inhibits N current. J. Gen. Physiol. 134, 369–384. doi: 10.1085/jgp.200910203
Henrion, U., Renhorn, J., Börjesson, S. I., Nelson, E. M., Schwaiger, C. S., Bjelkmar, P., et al. (2012). Tracking a complete voltage-sensor cycle with metal-ion bridges. Proc. Natl. Acad. Sci. U.S.A. 109, 8552–8557. doi: 10.1073/pnas.1116938109
Hirafuji, M., Ebihara, T., Kawahara, F., Hamaue, N., Endo, T., and Minami, M. (2001). Inhibition by docosahexaenoic acid of receptor-mediated Ca(2+) influx in rat vascular smooth muscle cells stimulated with 5-hydroxytryptamine. Eur. J. Pharmacol. 427, 195–201. doi: 10.1016/S0014-2999(01)01274-2
Hochachka, P. W. (1986). Defense strategies against hypoxia and hypothermia. Science 231, 234–241. doi: 10.1126/science.2417316
Hodgkin, A. L., and Huxley, A. F. (1952a). The dual effect of membrane potential on sodium conductance in the giant axon of loligo. J. Physiol. 116, 497–506. doi: 10.1113/jphysiol.1952.sp004719
Hodgkin, A. L., and Huxley, A. F. (1952b). A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 117, 500–544. doi: 10.1113/jphysiol.1952.sp004764
Holmes, A. M., Roderick, H. L., McDonald, F., and Bootman, M. D. (2007). Interaction between store-operated and arachidonate-activated calcium entry. Cell Calcium 41, 1–12. doi: 10.1016/j.ceca.2006.04.005
Holmqvist, M. H., Cao, J., Knoppers, M. H., Jurman, M. E., Distefano, P. S., Rhodes, K. J., et al. (2001). Kinetic modulation of Kv4-mediated A-current by arachidonic acid is dependent on potassium channel interacting proteins. J. Neurosci. 21, 4154–4161.
Honen, B. N., Saint, D. A., and Laver, D. R. (2003). Suppression of calcium sparks in rat ventricular myocytes and direct inhibition of sheep cardiac RyR channels by EPA, DHA and oleic acid. J. Membr. Biol. 196, 95–103. doi: 10.1007/s00232-003-0628-9
Hong, M. P., Kim, H. I., Shin, Y. K., Lee, C. S., Park, M., and Song, J. H. (2004). Effects of free fatty acids on sodium currents in rat dorsal root ganglion neurons. Brain Res. 1008, 81–91. doi: 10.1016/j.brainres.2004.02.033
Honoré, E. (2007). The neuronal background K2P channels: focus on TREK1. Nat. Rev. Neurosci. 8, 251–261. doi: 10.1038/nrn2117
Honoré, E., Barhanin, J., Attali, B., Lesage, F., and Lazdunski, M. (1994). External blockade of the major cardiac delayed-rectifier K+ channel (Kv1.5) by polyunsaturated fatty acids. Proc. Natl. Acad. Sci. U.S.A. 91, 1937–1941. doi: 10.1073/pnas.91.5.1937
Horimoto, N., Nabekura, J., and Ogawa, T. (1997). Arachidonic acid activation of potassium channels in rat visual cortex neurons. Neuroscience 77, 661–671. doi: 10.1016/S0306-4522(96)00490-3
Hoshi, T., Tian, Y., Xu, R., Heinemann, S. H., and Hou, S. (2013a). Mechanism of the modulation of BK potassium channel complexes with different auxiliary subunit compositions by the omega-3 fatty acid DHA. Proc. Natl. Acad. Sci. U.S.A. 110, 4822–4827. doi: 10.1073/pnas.1222003110
Hoshi, T., Wissuwa, B., Tian, Y., Tajima, N., Xu, R., Bauer, M., et al. (2013b). Omega-3 fatty acids lower blood pressure by directly activating large-conductance Ca(2)(+)-dependent K(+) channels. Proc. Natl. Acad. Sci. U.S.A. 110, 4816–4821. doi: 10.1073/pnas.1221997110
Hoshi, T., Wissuwa, B., Tian, Y., Tajima, N., Xu, R., Bauer, M., et al. (2013c). Reply to Harris et al.: differential impacts of omega-3 fatty acids and their derivatives on blood pressure. Proc. Natl. Acad. Sci. U.S.A. 110, E2260. doi: 10.1073/pnas.1305790110
Hoshi, T., Xu, R., Hou, S., Heinemann, S. H., and Tian, Y. (2013d). A point mutation in the human Slo1 channel that impairs its sensitivity to omega-3 docosahexaenoic acid. J. Gen. Physiol. 142, 507–522. doi: 10.1085/jgp.201311061
Hourton-Cabassa, C., Mesneau, A., Miroux, B., Roussaux, J., Ricquier, D., Zachowski, A., et al. (2002). Alteration of plant mitochondrial proton conductance by free fatty acids. Uncoupling protein involvement. J. Biol. Chem. 277, 41533–41538. doi: 10.1074/jbc.M202805200
Hu, H. Z., Xiao, R., Wang, C., Gao, N., Colton, C. K., Wood, J. D., et al. (2006). Potentiation of TRPV3 channel function by unsaturated fatty acids. J. Cell. Physiol. 208, 201–212. doi: 10.1002/jcp.20648
Huang, J. M., Xian, H., and Bacaner, M. (1992). Long-chain fatty acids activate calcium channels in ventricular myocytes. Proc. Natl. Acad. Sci. U.S.A. 89, 6452–6456. doi: 10.1073/pnas.89.14.6452
Hwang, T. C., Guggino, S. E., and Guggino, W. B. (1990). Direct modulation of secretory chloride channels by arachidonic and other cis unsaturated fatty acids. Proc. Natl. Acad. Sci. U.S.A. 87, 5706–5709. doi: 10.1073/pnas.87.15.5706
Isbilen, B., Fraser, S. P., and Djamgoz, M. B. (2006). Docosahexaenoic acid (omega-3) blocks voltage-gated sodium channel activity and migration of MDA-MB-231 human breast cancer cells. Int. J. Biochem. Cell Biol. 38, 2173–2182. doi: 10.1016/j.biocel.2006.06.014
Jacobson, D. A., Weber, C. R., Bao, S., Turk, J., and Philipson, L. H. (2007). Modulation of the pancreatic islet beta-cell-delayed rectifier potassium channel Kv2.1 by the polyunsaturated fatty acid arachidonate. J. Biol. Chem. 282, 7442–7449. doi: 10.1074/jbc.M607858200
Jakobsson, A., Westerberg, R., and Jacobsson, A. (2006). Fatty acid elongases in mammals: their regulation and roles in metabolism. Prog. Lipid Res. 45, 237–249. doi: 10.1016/j.plipres.2006.01.004
Jenkins, C. M., Cedars, A., and Gross, R. W. (2009). Eicosanoid signalling pathways in the heart. Cardiovasc. Res. 82, 240–249. doi: 10.1093/cvr/cvn346
Jo, T., Iida, H., Kishida, S., Imuta, H., Oonuma, H., Nagata, T., et al. (2005). Acute and chronic effects of eicosapentaenoic acid on voltage-gated sodium channel expressed in cultured human bronchial smooth muscle cells. Biochem. Biophys. Res. Commun. 331, 1452–1459. doi: 10.1016/j.bbrc.2005.04.062
Jörs, S., Kazanski, V., Foik, A., Krautwurst, D., and Harteneck, C. (2006). Receptor-induced activation of Drosophila TRP gamma by polyunsaturated fatty acids. J. Biol. Chem. 281, 29693–29702. doi: 10.1074/jbc.M602215200
Judé, S., Bedut, S., Roger, S., Pinault, M., Champeroux, P., White, E., et al. (2003). Peroxidation of docosahexaenoic acid is responsible for its effects on I TO and I SS in rat ventricular myocytes. Br. J. Pharmacol. 139, 816–822. doi: 10.1038/sj.bjp.0705308
Kacik, M., Oliván-Viguera, A., and Kohler, R. (2014). Modulation of K(Ca)3.1 channels by eicosanoids, omega-3 fatty acids, and molecular determinants. PLoS ONE 9:e112081. doi: 10.1371/journal.pone.0112081
Kahn-Kirby, A. H., Dantzker, J. L., Apicella, A. J., Schafer, W. R., Browse, J., Bargmann, C. I., et al. (2004). Specific polyunsaturated fatty acids drive TRPV-dependent sensory signaling in vivo. Cell 119, 889–900. doi: 10.1016/j.cell.2004.11.005
Kang, D., La, J. H., Kim, E. J., Park, J. Y., Hong, S. G., and Han, J. (2006). An endogenous acid-sensitive K+ channel expressed in COS-7 cells. Biochem. Biophys. Res. Commun. 341, 1231–1236. doi: 10.1016/j.bbrc.2006.01.082
Kang, J. X., and Leaf, A. (1994). Effects of long-chain polyunsaturated fatty acids on the contraction of neonatal rat cardiac myocytes. Proc. Natl. Acad. Sci. U.S.A. 91, 9886–9890. doi: 10.1073/pnas.91.21.9886
Kang, J. X., and Leaf, A. (1996). Evidence that free polyunsaturated fatty acids modify Na+ channels by directly binding to the channel proteins. Proc. Natl. Acad. Sci. U.S.A. 93, 3542–3546. doi: 10.1073/pnas.93.8.3542
Kang, J. X., Li, Y., and Leaf, A. (1997). Regulation of sodium channel gene expression by class I antiarrhythmic drugs and n-3 polyunsaturated fatty acids in cultured neonatal rat cardiac myocytes. Proc. Natl. Acad. Sci. U.S.A. 94, 2724–2728. doi: 10.1073/pnas.94.6.2724
Kang, J. X., Xiao, Y. F., and Leaf, A. (1995). Free, long-chain, polyunsaturated fatty acids reduce membrane electrical excitability in neonatal rat cardiac myocytes. Proc. Natl. Acad. Sci. U.S.A. 92, 3997–4001. doi: 10.1073/pnas.92.9.3997
Kapus, A., Romanek, R., and Grinstein, S. (1994). Arachidonic acid stimulates the plasma membrane H+ conductance of macrophages. J. Biol. Chem. 269, 4736–4745.
Kawanabe, A., and Okamura, Y. (2016). Effects of unsaturated fatty acids on the kinetics of voltage-gated proton channels heterologously expressed in cultured cells. J. Physiol. 594, 595–610. doi: 10.1113/JP271274
Kehl, S. J. (2001). Eicosatetraynoic acid (ETYA), a non-metabolizable analogue of arachidonic acid, blocks the fast-inactivating potassium current of rat pituitary melanotrophs. Can. J. Physiol. Pharmacol. 79, 338–345. doi: 10.1139/y01-001
Keros, S., and McBain, C. J. (1997). Arachidonic acid inhibits transient potassium currents and broadens action potentials during electrographic seizures in hippocampal pyramidal and inhibitory interneurons. J. Neurosci. 17, 3476–3487.
Keynes, R. D., and Elinder, F. (1999). The screw-helical voltage gating of ion channels. Proc. Biol. Sci. 266, 843–852. doi: 10.1098/rspb.1999.0714
Keyser, D. O., and Alger, B. E. (1990). Arachidonic acid modulates hippocampal calcium current via protein kinase C and oxygen radicals. Neuron 5, 545–553. doi: 10.1016/0896-6273(90)90092-T
Kihara, A. (2012). Very long-chain fatty acids: elongation, physiology and related disorders. J. Biochem. 152, 387–395. doi: 10.1093/jb/mvs105
Kim, D. (2003). Fatty acid-sensitive two-pore domain K+ channels. Trends Pharmacol. Sci. 24, 648–654. doi: 10.1016/j.tips.2003.10.008
Kim, D., and Clapham, D. E. (1989). Potassium channels in cardiac cells activated by arachidonic acid and phospholipids. Science 244, 1174–1176. doi: 10.1126/science.2727703
Kim, D., and Duff, R. A. (1990). Regulation of K+ channels in cardiac myocytes by free fatty acids. Circ. Res. 67, 1040–1046. doi: 10.1161/01.RES.67.4.1040
Kim, H. I., Kim, T. H., Shin, Y. K., Lee, C. S., Park, M., and Song, J. H. (2005). Anandamide suppression of Na+ currents in rat dorsal root ganglion neurons. Brain Res. 1062, 39–47. doi: 10.1016/j.brainres.2005.09.004
Kirber, M. T., Ordway, R. W., Clapp, L. H., Walsh, J. V. Jr., and Singer, J. J. (1992). Both membrane stretch and fatty acids directly activate large conductance Ca(2+)-activated K+ channels in vascular smooth muscle cells. FEBS Lett. 297, 24–28. doi: 10.1016/0014-5793(92)80319-C
Koch, H. P., Kurokawa, T., Okochi, Y., Sasaki, M., Okamura, Y., and Larsson, H. P. (2008). Multimeric nature of voltage-gated proton channels. Proc. Natl. Acad. Sci. U.S.A. 105, 9111–9116. doi: 10.1073/pnas.0801553105
Kong, S., Zhang, C., Li, W., Wang, L., Luan, H., Wang, W. H., et al. (2012). Stimulation of Ca2+-sensing receptor inhibits the basolateral 50-pS K channels in the thick ascending limb of rat kidney. Biochim. Biophys. Acta 1823, 273–281. doi: 10.1016/j.bbamcr.2011.10.007
Koshida, S., Kurata, Y., Notsu, T., Hirota, Y., Kuang, T. Y., Li, P., et al. (2009). Stabilizing effects of eicosapentaenoic acid on Kv1.5 channel protein expressed in mammalian cells. Eur. J. Pharmacol. 604, 93–102. doi: 10.1016/j.ejphar.2008.12.016
Krutetskaia, Z. I., Lebedev, O. E., Krutetskaia, N. I., and Kurilova, L. S. (2001). [Effect of arachidonic and other fatty acids on the intracellular calcium concentration and calcium signaling in peritoneal macrophages]. Tsitologiia 43, 1051–1060.
Kurachi, Y., Ito, H., Sugimoto, T., Shimizu, T., Miki, I., and Ui, M. (1989). Arachidonic acid metabolites as intracellular modulators of the G protein-gated cardiac K+ channel. Nature 337, 555–557. doi: 10.1038/337555a0
Kuriki, K., Nagaya, T., Imaeda, N., Tokudome, Y., Fujiwara, N., Sato, J., et al. (2002). Discrepancies in dietary intakes and plasma concentrations of fatty acids according to age among Japanese female dietitians. Eur. J. Clin. Nutr. 56, 524–531. doi: 10.1038/sj.ejcn.1601344
Lai, L. H., Dong, P. S., Li, Z. Z., Li, Z. J., Wang, R. X., and Jiang, W. P. (2011). [Effects of docosahexaenoic acid on sodium channel current and transient outward potassium channel current in rat ventricular myocytes]. Zhonghua Xin Xue Guan Bing Za Zhi 39, 451–456. doi: 10.3760/cma.j.issn.0253-3758.2011.05.015
Lai, L. H., Wang, R. X., Jiang, W. P., Yang, X. J., Song, J. P., Li, X. R., et al. (2009). Effects of docosahexaenoic acid on large-conductance Ca2+-activated K+ channels and voltage-dependent K+ channels in rat coronary artery smooth muscle cells. Acta Pharmacol. Sin. 30, 314–320. doi: 10.1038/aps.2009.7
Latorre, R., and Contreras, G. (2013). Keeping you healthy: BK channel activation by omega-3 fatty acids. J. Gen. Physiol. 142, 487–491. doi: 10.1085/jgp.201311100
Lauterbach, B., Barbosa-Sicard, E., Wang, M. H., Honeck, H., Kärgel, E., Theuer, J., et al. (2002). Cytochrome P450-dependent eicosapentaenoic acid metabolites are novel BK channel activators. Hypertension 39, 609–613. doi: 10.1161/hy0202.103293
Leaf, A., and Xiao, Y. F. (2001). The modulation of ionic currents in excitable tissues by n-3 polyunsaturated fatty acids. J. Membr. Biol. 184, 263–271. doi: 10.1007/s00232-001-0095-0
Leaf, A., Xiao, Y. F., and Kang, J. X. (2002). Interactions of n-3 fatty acids with ion channels in excitable tissues. Prostaglandins Leukot. Essent. Fatty Acids 67, 113–120. doi: 10.1054/plef.2002.0407
Lee, G. Y., Shin, Y. K., Lee, C. S., and Song, J. H. (2002). Effects of arachidonic acid on sodium currents in rat dorsal root ganglion neurons. Brain Res. 950, 95–102. doi: 10.1016/S0006-8993(02)03008-1
Lee, H. C., Lu, T., Weintraub, N. L., VanRollins, M., Spector, A. A., and Shibata, E. F. (1999). Effects of epoxyeicosatrienoic acids on the cardiac sodium channels in isolated rat ventricular myocytes. J. Physiol. 519(Pt 1), 153–168. doi: 10.1111/j.1469-7793.1999.0153o.x
Lee, S. Y., Letts, J. A., and Mackinnon, R. (2008). Dimeric subunit stoichiometry of the human voltage-dependent proton channel Hv1. Proc. Natl. Acad. Sci. U.S.A. 105, 7692–7695. doi: 10.1073/pnas.0803277105
Lee, Y., Lee, H. J., Crain, R. C., Lee, A., and Korn, S. J. (1994). Polyunsaturated fatty acids modulate stomatal aperture and two distinct K+ channel currents in guard cells. Cell. Signal. 6, 181–186. doi: 10.1016/0898-6568(94)90075-2
Leifert, W. R., McMurchie, E. J., and Saint, D. A. (1999). Inhibition of cardiac sodium currents in adult rat myocytes by n-3 polyunsaturated fatty acids. J. Physiol. 520, 671–679. doi: 10.1111/j.1469-7793.1999.00671.x
Li, G. R., Sun, H. Y., Zhang, X. H., Cheng, L. C., Chiu, S. W., Tse, H. F., et al. (2009). Omega-3 polyunsaturated fatty acids inhibit transient outward and ultra-rapid delayed rectifier K+currents and Na+current in human atrial myocytes. Cardiovasc. Res. 81, 286–293. doi: 10.1093/cvr/cvn322
Li, J., Al-Khalili, O., Ramosevac, S., Eaton, D. C., and Denson, D. D. (2010). Protein-protein interaction between cPLA2 and splice variants of alpha-subunit of BK channels. Am. J. Physiol. Cell Physiol. 298, C251–C262. doi: 10.1152/ajpcell.00221.2009
Li, P. L., Zhang, D. X., Ge, Z. D., and Campbell, W. B. (2002). Role of ADP-ribose in 11,12-EET-induced activation of K(Ca) channels in coronary arterial smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol. 282, H1229–H1236. doi: 10.1152/ajpheart.00736.2001
Liin, S. I., Karlsson, U., Bentzen, B. H., Schmitt, N., and Elinder, F. (2016a). Polyunsaturated fatty acids are potent openers of human M-channels expressed in Xenopus laevis oocytes. Acta Physiol. 218, 28–37. doi: 10.1111/apha.12663
Liin, S. I., Larsson, J. E., Barro-Soria, R., Bentzen, B. H., and Larsson, H. P. (2016b). Fatty acid analogue N-arachidonoyl taurine restores function of IKs channels with diverse long QT mutations. eLife 5:e20272. doi: 10.7554/eLife.20272
Liin, S. I., Silverå Ejneby, M., Barro-Soria, R., Skarsfeldt, M. A., Larsson, J. E., Starck Harlin, F., et al. (2015). Polyunsaturated fatty acid analogs act antiarrhythmically on the cardiac IKs channel. Proc. Natl. Acad. Sci. U.S.A. 112, 5714–5719. doi: 10.1073/pnas.1503488112
Linden, D. J., and Routtenberg, A. (1989). cis-Fatty acids, which activate protein kinase C, attenuate Na+ and Ca2+ currents in mouse neuroblastoma cells. J. Physiol. 419, 95–119. doi: 10.1113/jphysiol.1989.sp017863
Ling, B. N., Webster, C. L., and Eaton, D. C. (1992). Eicosanoids modulate apical Ca(2+)-dependent K+ channels in cultured rabbit principal cells. Am. J. Physiol. 263, F116–F126.
Liu, L., and Rittenhouse, A. R. (2000). Effects of arachidonic acid on unitary calcium currents in rat sympathetic neurons. J. Physiol. 525 (Pt 2), 391–404. doi: 10.1111/j.1469-7793.2000.00391.x
Liu, L., and Rittenhouse, A. R. (2003). Arachidonic acid mediates muscarinic inhibition and enhancement of N-type Ca2+ current in sympathetic neurons. Proc. Natl. Acad. Sci. U.S.A. 100, 295–300. doi: 10.1073/pnas.0136826100
Liu, L., Barrett, C. F., and Rittenhouse, A. R. (2001). Arachidonic acid both inhibits and enhances whole cell calcium currents in rat sympathetic neurons. Am. J. Physiol. Cell Physiol. 280, C1293–C1305.
Liu, L., Heneghan, J. F., Michael, G. J., Stanish, L. F., Egertova, M., and Rittenhouse, A. R. (2008). L- and N-current but not M-current inhibition by M1 muscarinic receptors requires DAG lipase activity. J. Cell. Physiol. 216, 91–100. doi: 10.1002/jcp.21378
Liu, L., Roberts, M. L., and Rittenhouse, A. R. (2004). Phospholipid metabolism is required for M1 muscarinic inhibition of N-type calcium current in sympathetic neurons. Eur. Biophys. J. 33, 255–264. doi: 10.1007/s00249-003-0387-7
Liu, S. J. (2007). Inhibition of L-type Ca2+ channel current and negative inotropy induced by arachidonic acid in adult rat ventricular myocytes. Am. J. Physiol. Cell Physiol. 293, C1594–C1604. doi: 10.1152/ajpcell.00284.2007
Liu, X., Yang, T., Miao, L., Mei, Y. A., and Hu, C. (2015). Leukotriene B4 inhibits L-type calcium channels via p38 signaling pathway in vascular smooth muscle cells. Cell. Physiol. Biochem. 37, 1903–1913. doi: 10.1159/000438551
Liu, X., Zhu, P., and Freedman, B. D. (2006). Multiple eicosanoid-activated nonselective cation channels regulate B-lymphocyte adhesion to integrin ligands. Am. J. Physiol. Cell Physiol. 290, C873–C882. doi: 10.1152/ajpcell.00229.2005
Liu, Y. C., and Wu, S. N. (2003). Block of erg current by linoleoylamide, a sleep-inducing agent, in pituitary GH3 cells. Eur. J. Pharmacol. 458, 37–47. doi: 10.1016/S0014-2999(02)02728-0
Long, S. B., Campbell, E. B., and Mackinnon, R. (2005). Voltage sensor of Kv1.2: structural basis of electromechanical coupling. Science 309, 903–908. doi: 10.1126/science.1116270
Long, S. B., Tao, X., Campbell, E. B., and MacKinnon, R. (2007). Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment. Nature 450, 376–382. doi: 10.1038/nature06265
Lotshaw, D. P. (2007). Biophysical, pharmacological, and functional characteristics of cloned and native mammalian two-pore domain K+ channels. Cell Biochem. Biophys. 47, 209–256. doi: 10.1007/s12013-007-0007-8
Lowenthal, A., and Levy, R. (1999). Essential requirement of cytosolic phospholipase A(2) for activation of the H(+) channel in phagocyte-like cells. J. Biol. Chem. 274, 21603–21608. doi: 10.1074/jbc.274.31.21603
Lu, T., Katakam, P. V., VanRollins, M., Weintraub, N. L., Spector, A. A., and Lee, H. C. (2001). Dihydroxyeicosatrienoic acids are potent activators of Ca(2+)-activated K(+) channels in isolated rat coronary arterial myocytes. J. Physiol. 534, 651–667. doi: 10.1111/j.1469-7793.2001.t01-1-00651.x
Lu, T., Wang, X. L., He, T., Zhou, W., Kaduce, T. L., Katusic, Z. S., et al. (2005). Impaired arachidonic acid-mediated activation of large-conductance Ca2+-activated K+ channels in coronary arterial smooth muscle cells in Zucker Diabetic Fatty rats. Diabetes 54, 2155–2163. doi: 10.2337/diabetes.54.7.2155
Luo, D., Broad, L. M., Bird, G. S., and Putney, J. W. Jr. (2001). Mutual antagonism of calcium entry by capacitative and arachidonic acid-mediated calcium entry pathways. J. Biol. Chem. 276, 20186–20189. doi: 10.1074/jbc.M100327200
Lynch, M. A., and Voss, K. L. (1994). Membrane arachidonic acid concentration correlates with age and induction of long-term potentiation in the dentate gyrus in the rat. Eur. J. Neurosci. 6, 1008–1014. doi: 10.1111/j.1460-9568.1994.tb00595.x
Macleod, J. C., Macknight, A. D., and Rodrigo, G. C. (1998). The electrical and mechanical response of adult guinea pig and rat ventricular myocytes to omega3 polyunsaturated fatty acids. Eur. J. Pharmacol. 356, 261–270. doi: 10.1016/S0014-2999(98)00528-7
Marchioli, R., Barzi, F., Bomba, E., Chieffo, C., Di Gregorio, D., Di Mascio, R., et al. (2002). Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico (GISSI)-Prevenzione. Circulation 105, 1897–1903. doi: 10.1161/01.CIR.0000014682.14181.F2
Martín, P., Moncada, M., Enrique, N., Asuaje, A., Valdez Capuccino, J. M., Gonzalez, C., et al. (2014). Arachidonic acid activation of BKCa (Slo1) channels associated to the beta1-subunit in human vascular smooth muscle cells. Pflugers Arch. 466, 1779–1792. doi: 10.1007/s00424-013-1422-x
Matta, J. A., Miyares, R. L., and Ahern, G. P. (2007). TRPV1 is a novel target for omega-3 polyunsaturated fatty acids. J. Physiol. 578, 397–411. doi: 10.1113/jphysiol.2006.121988
McKay, M. C., and Jennings, F. W. (2001). Linoleic acid both enhances activation and blocks Kv1.5 and Kv2.1 channels by two separate mechanisms. Am. J. Physiol. 281, 1277–1284.
McLennan, P. L. (1993). Relative effects of dietary saturated, monounsaturated, and polyunsaturated fatty acids on cardiac arrhythmias in rats. Am. J. Clin. Nutr. 57, 207–212.
McLennan, P. L., Abeywardena, M. Y., and Charnock, J. S. (1988). Dietary fish oil prevents ventricular fibrillation following coronary artery occlusion and reperfusion. Am. Heart J. 116, 709–717. doi: 10.1016/0002-8703(88)90328-6
Meves, H. (1994). Modulation of ion channels by arachidonic acid. Prog. Neurobiol. 43, 175–186. doi: 10.1016/0301-0082(94)90012-4
Meves, H. (2008). Arachidonic acid and ion channels: an update. Br. J. Pharmacol. 155, 4–16. doi: 10.1038/bjp.2008.216
Micha, R., and Mozaffarian, D. (2009). Trans fatty acids: effects on metabolic syndrome, heart disease and diabetes. Nat. Rev. Endocrinol. 5, 335–344. doi: 10.1038/nrendo.2009.79
Mignen, O., and Shuttleworth, T. J. (2001). Permeation of monovalent cations through the non-capacitative arachidonate-regulated Ca2+ channels in HEK293 cells. Comparison with endogenous store-operated channels. J. Biol. Chem. 276, 21365–21374. doi: 10.1074/jbc.M102311200
Milberg, P., Frommeyer, G., Kleideiter, A., Fischer, A., Osada, N., Breithardt, G., et al. (2011). Antiarrhythmic effects of free polyunsaturated fatty acids in an experimental model of LQT2 and LQT3 due to suppression of early afterdepolarizations and reduction of spatial and temporal dispersion of repolarization. Heart Rhythm 8, 1492–1500. doi: 10.1016/j.hrthm.2011.03.058
Mitra-Ganguli, T., Vitko, I., Perez-Reyes, E., and Rittenhouse, A. R. (2009). Orientation of palmitoylated CaVbeta2a relative to CaV2.2 is critical for slow pathway modulation of N-type Ca2+ current by tachykinin receptor activation. J. Gen. Physiol. 134, 385–396. doi: 10.1085/jgp.200910204
Mochizuki-Oda, N., Negishi, M., Mori, K., and Ito, S. (1993). Arachidonic acid activates cation channels in bovine adrenal chromaffin cells. J. Neurochem. 61, 1882–1890. doi: 10.1111/j.1471-4159.1993.tb09830.x
Moreno, C., de la Cruz, A., Oliveras, A., Kharche, S. R., Guizy, M., Comes, N., et al. (2015). Marine n-3 PUFAs modulate IKs gating, channel expression, and location in membrane microdomains. Cardiovasc. Res. 105, 223–232. doi: 10.1093/cvr/cvu250
Morgan, D., Cherny, V. V., Finnegan, A., Bollinger, J., Gelb, M. H., and DeCoursey, T. E. (2007). Sustained activation of proton channels and NADPH oxidase in human eosinophils and murine granulocytes requires PKC but not cPLA2 alpha activity. J. Physiol. 579, 327–344. doi: 10.1113/jphysiol.2006.124248
Morgan, D., Cherny, V. V., Price, M. O., Dinauer, M. C., and DeCoursey, T. E. (2002). Absence of proton channels in COS-7 cells expressing functional NADPH oxidase components. J. Gen. Physiol. 119, 571–580. doi: 10.1085/jgp.20018544
Morin, C., Sirois, M., Echave, V., Gomes, M. M., and Rousseau, E. (2007a). Epoxyeicosatrienoic acid relaxing effects involve Ca2+-activated K+ channel activation and CPI-17 dephosphorylation in human bronchi. Am. J. Respir. Cell Mol. Biol. 36, 633–641. doi: 10.1165/rcmb.2006-0281OC
Morin, C., Sirois, M., Echave, V., Gomes, M. M., and Rousseau, E. (2007b). Functional effects of 20-HETE on human bronchi: hyperpolarization and relaxation due to BKCa channel activation. Am. J. Physiol. Lung Cell. Mol. Physiol. 293, L1037–L1044. doi: 10.1152/ajplung.00145.2007
Morin, C., Sirois, M., Echave, V., Gomes, M. M., and Rousseau, E. (2007c). Relaxing effects of 5-oxo-ETE on human bronchi involve BK Ca channel activation. Prostaglandins Other Lipid Mediat. 83, 311–319. doi: 10.1016/j.prostaglandins.2007.03.001
Motter, A. L., and Ahern, G. P. (2012). TRPA1 is a polyunsaturated fatty acid sensor in mammals. PLoS ONE 7:e38439. doi: 10.1371/journal.pone.0038439
Mullen, G. E., and Yet, L. (2015). Progress in the development of fatty acid synthase inhibitors as anticancer targets. Bioorg. Med. Chem. Lett. 25, 4363–4369. doi: 10.1016/j.bmcl.2015.08.087
Müller, W., and Bittner, K. (2002). Differential oxidative modulation of voltage-dependent K+ currents in rat hippocampal neurons. J. Neurophysiol. 87, 2990–2995.
Munaron, L., Antoniotti, S., Distasi, C., and Lovisolo, D. (1997). Arachidonic acid mediates calcium influx induced by basic fibroblast growth factor in Balb-c 3T3 fibroblasts. Cell Calcium 22, 179–188. doi: 10.1016/S0143-4160(97)90011-7
Muslikhov, E. R., Sukhanova, I. F., and Avdonin, P. V. (2014). Arachidonic acid activates release of calcium ions from reticulum via ryanodine receptor channels in C2C12 skeletal myotubes. Biochemistry Mosc. 79, 435–439. doi: 10.1134/S0006297914050071
Nagano, N., Imaizumi, H., and Watanabe, M. (1995a). [Modulation of A-type calcium independent transient outward potassium current by fatty acids in guinea pig single smooth muscle cell]. J. Smooth Muscle Res. 31, 403–404.
Nagano, N., Imaizumi, Y., and Watanabe, M. (1995b). Modulation of calcium channel currents by arachidonic acid in single smooth muscle cells from vas deferens of the guinea-pig. Br. J. Pharmacol. 116, 1887–1893. doi: 10.1111/j.1476-5381.1995.tb16678.x
Nagano, N., Imaizumi, Y., and Watanabe, M. (1997). Effects of arachidonic acid on A-type potassium currents in smooth muscle cells of the guinea pig. Am. J. Physiol. 272, C860–C869.
Nakajima, T., Davies, S. S., Matafonova, E., Potet, F., Amarnath, V., Tallman, K. A., et al. (2010). Selective gamma-ketoaldehyde scavengers protect Nav1.5 from oxidant-induced inactivation. J. Mol. Cell. Cardiol. 48, 352–359. doi: 10.1016/j.yjmcc.2009.11.016
Nakajima, T., Kubota, N., Tsutsumi, T., Oguri, A., Imuta, H., Jo, T., et al. (2009). Eicosapentaenoic acid inhibits voltage-gated sodium channels and invasiveness in prostate cancer cells. Br. J. Pharmacol. 156, 420–431. doi: 10.1111/j.1476-5381.2008.00059.x
Noda, M., Shimizu, S., Tanabe, T., Takai, T., Kayano, T., Ikeda, T., et al. (1984). Primary structure of Electrophorus electricus sodium channel deduced from cDNA sequence. Nature 312, 121–127. doi: 10.1038/312121a0
Oike, H., Wakamori, M., Mori, Y., Nakanishi, H., Taguchi, R., Misaka, T., et al. (2006). Arachidonic acid can function as a signaling modulator by activating the TRPM5 cation channel in taste receptor cells. Biochim. Biophys. Acta 1761, 1078–1084. doi: 10.1016/j.bbalip.2006.07.005
Oliver, D., Lien, C. C., Soom, M., Baukrowitz, T., Jonas, P., and Fakler, B. (2004). Functional conversion between A-type and delayed rectifier K+ channels by membrane lipids. Science 304, 265–270. doi: 10.1126/science.1094113
Olszewska, A., Bednarczyk, P., Siemen, D., and Szewczyk, A. (2014). Modulation of the mitochondrial large-conductance calcium-regulated potassium channel by polyunsaturated fatty acids. Biochim. Biophys. Acta 1837, 1602–1610. doi: 10.1016/j.bbabio.2014.07.010
Ordway, R. W., Singer, J. J., and Walsh, J. V. Jr. (1991). Direct regulation of ion channels by fatty acids. Trends Neurosci. 14, 96–100. doi: 10.1016/0166-2236(91)90069-7
Ordway, R. W., Walsh, J. V. Jr., and Singer, J. J. (1989). Arachidonic acid and other fatty acids directly activate potassium channels in smooth muscle cells. Science 244, 1176–1179. doi: 10.1126/science.2471269
Ottosson, N. E., Liin, S. I., and Elinder, F. (2014). Drug-induced ion channel opening tuned by the voltage sensor charge profile. J. Gen. Physiol. 143, 173–182. doi: 10.1085/jgp.201311087
Oz, M., Tchugunova, Y., and Dinc, M. (2004). Differential effects of endogenous and synthetic cannabinoids on voltage-dependent calcium fluxes in rabbit T-tubule membranes: comparison with fatty acids. Eur. J. Pharmacol. 502, 47–58. doi: 10.1016/j.ejphar.2004.08.052
Parnas, M., Katz, B., Lev, S., Tzarfaty, V., Dadon, D., Gordon-Shaag, A., et al. (2009a). Membrane lipid modulations remove divalent open channel block from TRP-like and NMDA channels. J. Neurosci. 29, 2371–2383. doi: 10.1523/JNEUROSCI.4280-08.2009
Parnas, M., Peters, M., and Minke, B. (2009b). Linoleic acid inhibits TRP channels with intrinsic voltage sensitivity: implications on the mechanism of linoleic acid action. Channels 3, 164–166. doi: 10.4161/chan.3.3.8873
Pepe, S., Bogdanov, K., Hallaq, H., Spurgeon, H., Leaf, A., and Lakatta, E. (1994). Omega 3 polyunsaturated fatty acid modulates dihydropyridine effects on L-type Ca2+ channels, cytosolic Ca2+, and contraction in adult rat cardiac myocytes. Proc. Natl. Acad. Sci. U.S.A. 91, 8832–8836. doi: 10.1073/pnas.91.19.8832
Petit-Jacques, J., and Hartzell, H. C. (1996). Effect of arachidonic acid on the L-type calcium current in frog cardiac myocytes. J. Physiol. 493 (Pt. 1), 67–81. doi: 10.1113/jphysiol.1996.sp021365
Pignier, C., Revenaz, C., Rauly-Lestienne, I., Cussac, D., Delhon, A., Gardette, J., et al. (2007). Direct protective effects of poly-unsaturated fatty acids, DHA and EPA, against activation of cardiac late sodium current: a mechanism for ischemia selectivity. Basic Res. Cardiol. 102, 553–564. doi: 10.1007/s00395-007-0676-x
Poling, J. S., Karanian, J. W., Salem, N. Jr., and Vicini, S. (1995). Time- and voltage-dependent block of delayed rectifier potassium channels by docosahexaenoic acid. Mol. Pharmacol. 47, 381–390.
Poling, J. S., Vicini, S., Rogawski, M. A., and Salem, N. Jr. (1996). Docosahexaenoic acid block of neuronal voltage-gated K+ channels: subunit selective antagonism by zinc. Neuropharmacology 35, 969–982. doi: 10.1016/0028-3908(96)00127-X
Pound, E. M., Kang, J. X., and Leaf, A. (2001). Partitioning of polyunsaturated fatty acids, which prevent cardiac arrhythmias, into phospholipid cell membranes. J. Lipid Res. 42, 346–351.
Premkumar, L. S., Gage, P. W., and Chung, S. H. (1990). Coupled potassium channels induced by arachidonic acid in cultured neurons. Proc. Biol. Sci. 242, 17–22. doi: 10.1098/rspb.1990.0097
Ragsdale, D. S., McPhee, J. C., Scheuer, T., and Catterall, W. A. (1994). Molecular determinants of state-dependent block of Na+ channels by local anesthetics. Science 265, 1724–1728. doi: 10.1126/science.8085162
Ramakers, G. M., and Storm, J. F. (2002). A postsynaptic transient K(+) current modulated by arachidonic acid regulates synaptic integration and threshold for LTP induction in hippocampal pyramidal cells. Proc. Natl. Acad. Sci. U.S.A. 99, 10144–10149. doi: 10.1073/pnas.152620399
Ramsey, I. S., Moran, M. M., Chong, J. A., and Clapham, D. E. (2006). A voltage-gated proton-selective channel lacking the pore domain. Nature 440, 1213–1216. doi: 10.1038/nature04700
Redmond, W. J., Gu, L., Camo, M., McIntyre, P., and Connor, M. (2014). Ligand determinants of fatty acid activation of the pronociceptive ion channel TRPA1. PeerJ 2:e248. doi: 10.7717/peerj.248
Reiter, B., Kraft, R., Gunzel, D., Zeissig, S., Schulzke, J. D., Fromm, M., et al. (2006). TRPV4-mediated regulation of epithelial permeability. FASEB J. 20, 1802–1812. doi: 10.1096/fj.06-5772com
Rimmerman, N., Bradshaw, H. B., Hughes, H. V., Chen, J. S., Hu, S. S., McHugh, D., et al. (2008). N-palmitoyl glycine, a novel endogenous lipid that acts as a modulator of calcium influx and nitric oxide production in sensory neurons. Mol. Pharmacol. 74, 213–224. doi: 10.1124/mol.108.045997
Roberts-Crowley, M. L., and Rittenhouse, A. R. (2009). Arachidonic acid inhibition of L-type calcium (CaV1.3b) channels varies with accessory CaVbeta subunits. J. Gen. Physiol. 133, 387–403. doi: 10.1085/jgp.200810047
Roberts-Crowley, M. L., and Rittenhouse, A. R. (2015). Characterization of ST14A Cells for Studying Modulation of Voltage-Gated Calcium Channels. PLoS ONE 10:e0132469. doi: 10.1371/journal.pone.0132469
Rocha, M. L., and Bendhack, L. M. (2009). Relaxation evoked by extracellular Ca2+ in rat aorta is nerve-independent and involves sarcoplasmic reticulum and L-type Ca2+ channel. Vascul. Pharmacol. 50, 98–103. doi: 10.1016/j.vph.2008.11.004
Rock, M. J., Prenen, J., Funari, V. A., Funari, T. L., Merriman, B., Nelson, S. F., et al. (2008). Gain-of-function mutations in TRPV4 cause autosomal dominant brachyolmia. Nat. Genet. 40, 999–1003. doi: 10.1038/ng.166
Roudbaraki, M. M., Vacher, P., and Drouhault, R. (1995). Arachidonic acid increases cytosolic calcium and stimulates hormone release in rat lactotrophs. Am. J. Physiol. 268, E1215–E1223.
Rouzaire-Dubois, B., Gerard, V., and Dubois, J. M. (1991). Modification of K+ channel properties induced by fatty acids in neuroblastoma cells. Pflugers Arch. 419, 467–471. doi: 10.1007/BF00370790
Ruparel, S., Bendele, M., Wallace, A., and Green, D. (2015). Released lipids regulate transient receptor potential channel (TRP)-dependent oral cancer pain. Mol. Pain 11, 30. doi: 10.1186/s12990-015-0016-3
Ruta, V., Chen, J., and MacKinnon, R. (2005). Calibrated measurement of gating-charge arginine displacement in the KvAP voltage-dependent K+ channel. Cell 123, 463–475. doi: 10.1016/j.cell.2005.08.041
Rychkov, G. Y., Litjens, T., Roberts, M. L., and Barritt, G. J. (2005). Arachidonic acid inhibits the store-operated Ca2+ current in rat liver cells. Biochem. J. 385, 551–556. doi: 10.1042/BJ20041604
Safrany-Fark, A., Petrovszki, Z., Kekesi, G., Liszli, P., Benedek, G., Keresztes, C., et al. (2015). In vivo potency of different ligands on voltage-gated sodium channels. Eur. J. Pharmacol. 762, 158–164. doi: 10.1016/j.ejphar.2015.05.053
Sansom, M. S., Bond, P. J., Deol, S. S., Grottesi, A., Haider, S., and Sands, Z. A. (2005). Molecular simulations and lipid-protein interactions: potassium channels and other membrane proteins. Biochem. Soc. Trans. 33, 916–920. doi: 10.1042/BST0330916
Sasaki, M., Takagi, M., and Okamura, Y. (2006). A voltage sensor-domain protein is a voltage-gated proton channel. Science 312, 589–592. doi: 10.1126/science.1122352
Schledermann, W., Wulfsen, I., Schwarz, J. R., and Bauer, C. K. (2001). Modulation of rat erg1, erg2, erg3 and HERG K+ currents by thyrotropin-releasing hormone in anterior pituitary cells via the native signal cascade. J. Physiol. 532, 143–163. doi: 10.1111/j.1469-7793.2001.0143g.x
Schmidt, D., Jiang, Q. X., and MacKinnon, R. (2006). Phospholipids and the origin of cationic gating charges in voltage sensors. Nature 444, 775–779. doi: 10.1038/nature05416
Schmitt, H., and Meves, H. (1995). Modulation of neuronal calcium channels by arachidonic acid and related substances. J. Membr. Biol. 145, 233–244. doi: 10.1007/BF00232715
Seebungkert, B., and Lynch, J. W. (2002). Effects of polyunsaturated fatty acids on voltage-gated K+ and Na+ channels in rat olfactory receptor neurons. Eur. J. Neurosci. 16, 2085–2094. doi: 10.1046/j.1460-9568.2002.02288.x
Shah, B. P., Liu, P., Yu, T., Hansen, D. R., and Gilbertson, T. A. (2012). TRPM5 is critical for linoleic acid-induced CCK secretion from the enteroendocrine cell line, STC-1. Am. J. Physiol. Cell Physiol. 302, C210–C219. doi: 10.1152/ajpcell.00209.2011
Shimada, T., and Somlyo, A. P. (1992). Modulation of voltage-dependent Ca channel current by arachidonic acid and other long-chain fatty acids in rabbit intestinal smooth muscle. J. Gen. Physiol. 100, 27–44. doi: 10.1085/jgp.100.1.27
Shimasue, K., Urushidani, T., Hagiwara, M., and Nagao, T. (1996). Effects of anandamide and arachidonic acid on specific binding of (+) -PN200-110, diltiazem and (-) -desmethoxyverapamil to L-type Ca2+ channel. Eur. J. Pharmacol. 296, 347–350. doi: 10.1016/0014-2999(95)00826-8
Shimizu, T., Janssens, A., Voets, T., and Nilius, B. (2009). Regulation of the murine TRPP3 channel by voltage, pH, and changes in cell volume. Pflugers Arch. 457, 795–807. doi: 10.1007/s00424-008-0558-6
Shuttleworth, T. J. (1996). Arachidonic acid activates the noncapacitative entry of Ca2+ during [Ca2+]i oscillations. J. Biol. Chem. 271, 21720–21725.
Siddiqui, R. A., Harvey, K. A., and Zaloga, G. P. (2008). Modulation of enzymatic activities by n-3 polyunsaturated fatty acids to support cardiovascular health. J. Nutr. Biochem. 19, 417–437. doi: 10.1016/j.jnutbio.2007.07.001
Siesjö, B. K., Agardh, C. D., Bengtsson, F., and Smith, M. L. (1989). Arachidonic acid metabolism in seizures. Ann. N. Y. Acad. Sci. 559, 323–339. doi: 10.1111/j.1749-6632.1989.tb22619.x
Singleton, C. B., Valenzuela, S. M., Walker, B. D., Tie, H., Wyse, K. R., Bursill, J. A., et al. (1999). Blockade by N-3 polyunsaturated fatty acid of the Kv4.3 current stably expressed in Chinese hamster ovary cells. Br. J. Pharmacol. 127, 941–948. doi: 10.1038/sj.bjp.0702638
Smirnov, S. V., and Aaronson, P. I. (1996). Modulatory effects of arachidonic acid on the delayed rectifier K+ current in rat pulmonary arterial myocytes. Structural aspects and involvement of protein kinase C. Circ Res 79, 20–31. doi: 10.1161/01.RES.79.1.20
Smith-Maxwell, C. J., Ledwell, J. L., and Aldrich, R. W. (1998). Uncharged S4 residues and cooperativity in voltage-dependent potassium channel activation. J. Gen. Physiol. 111, 421–439. doi: 10.1085/jgp.111.3.421
Sokolowski, B. H., Sakai, Y., Harvey, M. C., and Duzhyy, D. E. (2004). Identification and localization of an arachidonic acid-sensitive potassium channel in the cochlea. J. Neurosci. 24, 6265–6276. doi: 10.1523/JNEUROSCI.1291-04.2004
Soldati, L., Lombardi, C., Adamo, D., Terranegra, A., Bianchin, C., Bianchi, G., et al. (2002). Arachidonic acid increases intracellular calcium in erythrocytes. Biochem. Biophys. Res. Commun. 293, 974–978. doi: 10.1016/S0006-291X(02)00327-3
Soliven, B., and Wang, N. (1995). Arachidonic acid inhibits potassium conductances in cultured rat oligodendrocytes. Am. J. Physiol. 269, C341–C348.
Stockand, J. D., Silverman, M., Hall, D., Derr, T., Kubacak, B., and Sansom, S. C. (1998). Arachidonic acid potentiates the feedback response of mesangial BKCa channels to angiotensin II. Am. J. Physiol. 274, F658–F664.
Striggow, F., and Ehrlich, B. E. (1997). Regulation of intracellular calcium release channel function by arachidonic acid and leukotriene B4. Biochem. Biophys. Res. Commun. 237, 413–418. doi: 10.1006/bbrc.1997.7152
Sukumar, P., Sedo, A., Li, J., Wilson, L. A., O'Regan, D., Lippiat, J. D., et al. (2012). Constitutively active TRPC channels of adipocytes confer a mechanism for sensing dietary fatty acids and regulating adiponectin. Circ. Res. 111, 191–200. ddoi: 10.1161/CIRCRESAHA.112.270751
Sun, P., Liu, W., Lin, D. H., Yue, P., Kemp, R., Satlin, L. M., et al. (2009). Epoxyeicosatrienoic acid activates BK channels in the cortical collecting duct. J. Am. Soc. Nephrol. 20, 513–523. doi: 10.1681/ASN.2008040427
Sun, X., Zhou, D., Zhang, P., Moczydlowski, E. G., and Haddad, G. G. (2007). Beta-subunit-dependent modulation of hSlo BK current by arachidonic acid. J. Neurophysiol. 97, 62–69. doi: 10.1152/jn.00700.2006
Swan, J. S., Dibb, K., Negretti, N., O'Neill, S. C., and Sitsapesan, R. (2003). Effects of eicosapentaenoic acid on cardiac SR Ca(2+)-release and ryanodine receptor function. Cardiovasc. Res. 60, 337–346. doi: 10.1016/S0008-6363(03)00545-5
Swartz, K. J. (2004). Towards a structural view of gating in potassium channels. Nat. Rev. Neurosci. 5, 905–916. doi: 10.1038/nrn1559
Szekely, A., Kitajka, K., Panyi, G., Márián, T., Gáspár, R., and Krasznai, Z. (2007). Nutrition and immune system: certain fatty acids differently modify membrane composition and consequently kinetics of KV1.3 channels of human peripheral lymphocytes. Immunobiology 212, 213–227. doi: 10.1016/j.imbio.2007.01.005
Takahira, M., Sakurada, N., Segawa, Y., and Shirao, Y. (2001). Two types of K+ currents modulated by arachidonic acid in bovine corneal epithelial cells. Invest. Ophthalmol. Vis. Sci. 42, 1847–1854.
Takenaka, T., Horie, H., and Hori, H. (1987). Effects of fatty acids on membrane currents in the squid giant axon. J. Membr. Biol. 95, 113–120. doi: 10.1007/BF01869156
Takenaka, T., Horie, H., Hori, H., and Kawakami, T. (1988). Effects of arachidonic acid and the other long-chain fatty acids on the membrane currents in the squid giant axon. J. Membr. Biol. 106, 141–147. doi: 10.1007/BF01871396
Takenaka, T., Horie, H., Hori, H., Yoshioka, T., and Iwanami, Y. (1981). Inhibitory effects of myrmicacin on the sodium channel in the squid giant axon. Proc. Jpn. Acad. 57, 314–317. doi: 10.2183/pjab.57.314
Talavera, K., Staes, M., Janssens, A., Droogmans, G., and Nilius, B. (2004). Mechanism of arachidonic acid modulation of the T-type Ca2+ channel alpha1G. J. Gen. Physiol. 124, 225–238. doi: 10.1085/jgp.200409050
Tao, X., Lee, A., Limapichat, W., Dougherty, D. A., and MacKinnon, R. (2010). A gating charge transfer center in voltage sensors. Science 328, 67–73. doi: 10.1126/science.1185954
Thompson, M. A., Prakash, Y. S., and Pabelick, C. M. (2014). Arachidonate-regulated Ca(2+) influx in human airway smooth muscle. Am. J. Respir. Cell Mol. Biol. 51, 68–76. doi: 10.1165/rcmb.2013-0144OC
Tian, Y., Aursnes, M., Hansen, T. V., Tungen, J. E., Galpin, J. D., Leisle, L., et al. (2016). Atomic determinants of BK channel activation by polyunsaturated fatty acids. Proc. Natl. Acad. Sci. U.S.A. 113, 13905–13910. doi: 10.1073/pnas.1615562113
Tombola, F., Pathak, M. M., and Isacoff, E. Y. (2006). How does voltage open an ion channel? Annu. Rev. Cell Dev. Biol. 22, 23–52. doi: 10.1146/annurev.cellbio.21.020404.145837
Tombola, F., Ulbrich, M. H., and Isacoff, E. Y. (2008). The voltage-gated proton channel Hv1 has two pores, each controlled by one voltage sensor. Neuron 58, 546–556. doi: 10.1016/j.neuron.2008.03.026
Törnquist, K., Ekokoski, E., and Forss, L. (1994). TRH-evoked entry of extracellular calcium in GH4C1 cells: possible importance of arachidonic acid metabolites. Mol. Cell. Endocrinol. 102, 103–110. doi: 10.1016/0303-7207(94)90103-1
Twitchell, W. A., Peña, T. L., and Rane, S. G. (1997). Ca2+-dependent K+ channels in bovine adrenal chromaffin cells are modulated by lipoxygenase metabolites of arachidonic acid. J. Membr. Biol. 158, 69–75. doi: 10.1007/s002329900244
Uehara, A., Yasukochi, M., and Imanaga, I. (1996). Modulation of ryanodine binding to the cardiac Ca2+ release channel by arachidonic acid. J. Mol. Cell. Cardiol. 28, 43–51. doi: 10.1006/jmcc.1996.0005
Unno, T., Komori, S., and Ohashi, H. (1996). Some evidence against the involvement of arachidonic acid in muscarinic suppression of voltage-gated calcium channel current in guinea-pig ileal smooth muscle cells. Br. J. Pharmacol. 119, 213–222. doi: 10.1111/j.1476-5381.1996.tb15973.x
van der Zee, L., Nelemans, A., and den Hertog, A. (1995). Arachidonic acid is functioning as a second messenger in activating the Ca2+ entry process on H1-histaminoceptor stimulation in DDT1 MF-2 cells. Biochem. J. 305 (Pt 3), 859–864. doi: 10.1042/bj3050859
Vellani, V., Reynolds, A. M., and McNaughton, P. A. (2000). Modulation of the synaptic Ca2+ current in salamander photoreceptors by polyunsaturated fatty acids and retinoids. J. Physiol. 529(Pt 2), 333–344. doi: 10.1111/j.1469-7793.2000.00333.x
Verkerk, A. O., den Ruijter, H. M., Bourier, J., Boukens, B. J., Brouwer, I. A., Wilders, R., et al. (2009). Dietary fish oil reduces pacemaker current and heart rate in rabbit. Heart Rhythm 6, 1485–1492. doi: 10.1016/j.hrthm.2009.07.024
Villarroel, A. (1993). Suppression of neuronal potassium A-current by arachidonic acid. FEBS Lett. 335, 184–188. doi: 10.1016/0014-5793(93)80726-B
Villarroel, A. (1994). On the role of arachidonic acid in M-current modulation by muscarine in bullfrog sympathetic neurons. J. Neurosci. 14, 7053–7066.
Villarroel, A., and Schwarz, T. L. (1996). Inhibition of the Kv4 (Shal) family of transient K+ currents by arachidonic acid. J. Neurosci. 16, 1016–1025.
Visentin, S., and Levi, G. (1998). Arachidonic acid-induced inhibition of microglial outward-rectifying K+ current. Glia 22, 1–10. doi: 10.1002/(SICI)1098-1136(199801)22:1<1::AID-GLIA1>3.0.CO;2-C
Vreugdenhil, M., Bruehl, C., Voskuyl, R. A., Kang, J. X., Leaf, A., and Wadman, W. J. (1996). Polyunsaturated fatty acids modulate sodium and calcium currents in CA1 neurons. Proc. Natl. Acad. Sci. U.S.A. 93, 12559–12563. doi: 10.1073/pnas.93.22.12559
Vriens, J., Owsianik, G., Janssens, A., Voets, T., and Nilius, B. (2007). Determinants of 4 alpha-phorbol sensitivity in transmembrane domains 3 and 4 of the cation channel TRPV4. J. Biol. Chem. 282, 12796–12803. doi: 10.1074/jbc.M610485200
Wang, J., Zhang, Y., Wang, H., Han, H., Nattel, S., Yang, B., et al. (2004). Potential mechanisms for the enhancement of HERG K+ channel function by phospholipid metabolites. Br. J. Pharmacol. 141, 586–599. doi: 10.1038/sj.bjp.0705646
Wang, R. X., Chai, Q., Lu, T., and Lee, H. C. (2011a). Activation of vascular BK channels by docosahexaenoic acid is dependent on cytochrome P450 epoxygenase activity. Cardiovasc. Res. 90, 344–352. doi: 10.1093/cvr/cvq411
Wang, R. X., Li, K. L., Zhang, C. Y., Zheng, J., Guo, S. X., Wu, Y., et al. (2011b). [Mechanism related to docosahexaenoic acid induced large conductance calcium-activated potassium channel currents increase in coronary smooth muscle cells]. Zhonghua Xin Xue Guan Bing Za Zhi 39, 348–352. doi: 10.3760/cma.j.issn.0253-3758.2011.04.014
Wang, R. X., Li, X. R., Lai, L. H., Wu, X. Q., Chen, Y. F., Song, J. P., et al. (2009). [Effects of docosahexaenoic acid on action potential and transient outward potassium current on ventricular myocytes of Sprague-Dawley rat]. Zhonghua Xin Xue Guan Bing Za Zhi 37, 108–111. doi: 10.3760/cma.j.issn.0253-3758.2009.02.004
Wang, W., and Lu, M. (1995). Effect of arachidonic acid on activity of the apical K+ channel in the thick ascending limb of the rat kidney. J. Gen. Physiol. 106, 727–743. doi: 10.1085/jgp.106.4.727
Wannous, R., Bon, E., Gillet, L., Chamouton, J., Weber, G., Brisson, L., et al. (2015). Suppression of PPARbeta, and DHA treatment, inhibit NaV1.5 and NHE-1 pro-invasive activities. Pflugers Arch. 467, 1249–1259. doi: 10.1007/s00424-014-1573-4
Watanabe, H., Vriens, J., Prenen, J., Droogmans, G., Voets, T., and Nilius, B. (2003). Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature 424, 434–438. doi: 10.1038/nature01807
Wieland, S. J., Fletcher, J. E., and Gong, Q. H. (1992). Differential modulation of a sodium conductance in skeletal muscle by intracellular and extracellular fatty acids. Am. J. Physiol. 263, C308–C312.
Wieland, S. J., Gong, Q., Poblete, H., Fletcher, J. E., Chen, L. Q., and Kallen, R. G. (1996). Modulation of human muscle sodium channels by intracellular fatty acids is dependent on the channel isoform. J. Biol. Chem. 271, 19037–19041. doi: 10.1074/jbc.271.32.19037
Williams, E. J., Walsh, F. S., and Doherty, P. (1994). The production of arachidonic acid can account for calcium channel activation in the second messenger pathway underlying neurite outgrowth stimulated by NCAM, N-cadherin, and L1. J. Neurochem. 62, 1231–1234. doi: 10.1046/j.1471-4159.1994.62031231.x
Wilson, S. M., Lee, S. C., Shook, S., and Pappone, P. A. (2000). ATP and beta-adrenergic stimulation enhance voltage-gated K current inactivation in brown adipocytes. Am. J. Physiol. Cell Physiol. 279, C1847–C1858.
Wolkowicz, P., Umeda, P. K., Sharifov, O. F., White, C. R., Huang, J., Mahtani, H., et al. (2014). Inhibitors of arachidonate-regulated calcium channel signaling suppress triggered activity induced by the late sodium current. Eur. J. Pharmacol. 724, 92–101. doi: 10.1016/j.ejphar.2013.12.020
Woolcott, O. O., Gustafsson, A. J., Dzabic, M., Pierro, C., Tedeschi, P., Sandgren, J., et al. (2006). Arachidonic acid is a physiological activator of the ryanodine receptor in pancreatic beta-cells. Cell Calcium 39, 529–537. doi: 10.1016/j.ceca.2006.02.003
Wu, S. N., Li, H. F., and Chiang, H. T. (2000). Actions of epoxyeicosatrienoic acid on large-conductance Ca(2+)-activated K(+) channels in pituitary GH(3) cells. Biochem. Pharmacol. 60, 251–262. doi: 10.1016/S0006-2952(00)00317-8
Xiao, Y. F., Gomez, A. M., Morgan, J. P., Lederer, W. J., and Leaf, A. (1997). Suppression of voltage-gated L-type Ca2+ currents by polyunsaturated fatty acids in adult and neonatal rat ventricular myocytes. Proc. Natl. Acad. Sci. U.S.A. 94, 4182–4187. doi: 10.1073/pnas.94.8.4182
Xiao, Y. F., Kang, J. X., Morgan, J. P., and Leaf, A. (1995). Blocking effects of polyunsaturated fatty acids on Na+ channels of neonatal rat ventricular myocytes. Proc. Natl. Acad. Sci. U.S.A. 92, 11000–11004. doi: 10.1073/pnas.92.24.11000
Xiao, Y. F., Ke, Q., Wang, S. Y., Auktor, K., Yang, Y., Wang, G. K., et al. (2001). Single point mutations affect fatty acid block of human myocardial sodium channel alpha subunit Na+ channels. Proc. Natl. Acad. Sci. U.S.A. 98, 3606–3611. doi: 10.1073/pnas.061003798
Xiao, Y. F., Ke, Q., Wang, S. Y., Yang, Y., Chen, Y., Wang, G. K., et al. (2004). Electrophysiologic properties of lidocaine, cocaine, and n-3 fatty-acids block of cardiac Na+ channels. Eur. J. Pharmacol. 485, 31–41. doi: 10.1016/j.ejphar.2003.11.042
Xiao, Y. F., Ma, L., Wang, S. Y., Josephson, M. E., Wang, G. K., Morgan, J. P., et al. (2006). Potent block of inactivation-deficient Na+ channels by n-3 polyunsaturated fatty acids. Am. J. Physiol. Cell Physiol. 290, C362–C370. doi: 10.1152/ajpcell.00296.2005
Xiao, Y. F., Morgan, J. P., and Leaf, A. (2002). Effects of polyunsaturated fatty acids on cardiac voltage-activated K(+) currents in adult ferret cardiomyocytes. Sheng Li Xue Bao 54, 271–281.
Xiao, Y. F., Sigg, D. C., and Leaf, A. (2005). The antiarrhythmic effect of n-3 polyunsaturated fatty acids: modulation of cardiac ion channels as a potential mechanism. J. Membr. Biol. 206, 141–154. doi: 10.1007/s00232-005-0786-z
Xiao, Y. F., Wright, S. N., Wang, G. K., Morgan, J. P., and Leaf, A. (1998). Fatty acids suppress voltage-gated Na+ currents in HEK293t cells transfected with the alpha-subunit of the human cardiac Na+ channel. Proc. Natl. Acad. Sci. U.S.A. 95, 2680–2685. doi: 10.1073/pnas.95.5.2680
Xiao, Y. F., Wright, S. N., Wang, G. K., Morgan, J. P., and Leaf, A. (2000). Coexpression with beta(1)-subunit modifies the kinetics and fatty acid block of hH1(alpha) Na(+) channels. Am. J. Physiol. Heart Circ. Physiol. 279, H35–H46.
Xu, X. P., Erichsen, D., Börjesson, S. I., Dahlin, M., Åmark, P., and Elinder, F. (2008). Polyunsaturated fatty acids and cerebrospinal fluid from children on the ketogenic diet open a voltage-gated K channel: a putative mechanism of antiseizure action. Epilepsy Res. 80, 57–66. doi: 10.1016/j.eplepsyres.2008.03.013
Yagami, T., Ueda, K., Asakura, K., Nakazato, H., Hata, S., Kuroda, T., et al. (2003). Human group IIA secretory phospholipase A2 potentiates Ca2+ influx through L-type voltage-sensitive Ca2+ channels in cultured rat cortical neurons. J. Neurochem. 85, 749–758. doi: 10.1046/j.1471-4159.2003.01712.x
Yan, J., Chen, R., Liu, P., and Gu, Y. (2014). Docosahexaenoic acid attenuates hypoxic pulmonary vasoconstriction by activating the large conductance Ca2+-activated K+ currents in pulmonary artery smooth muscle cells. Pulm. Pharmacol. Ther. 28, 9–16. doi: 10.1016/j.pupt.2013.11.004
Yang, K. T., Chen, W. P., Chang, W. L., Su, M. J., and Tsai, K. L. (2005). Arachidonic acid inhibits capacitative Ca2+ entry and activates non-capacitative Ca2+ entry in cultured astrocytes. Biochem. Biophys. Res. Commun. 331, 603–613. doi: 10.1016/j.bbrc.2005.03.221
Yang, M., Li, X. L., Xu, H. Y., Sun, J. B., Mei, B., Zheng, H. F., et al. (2005). Role of arachidonic acid in hyposmotic membrane stretch-induced increase in calcium-activated potassium currents in gastric myocytes. Acta Pharmacol. Sin. 26, 1233–1242. doi: 10.1111/j.1745-7254.2005.00201.x
Yazdi, S., Stein, M., Elinder, F., Andersson, M., and Lindahl, E. (2016). The molecular basis of polyunsaturated fatty acid interactions with the shaker voltage-gated potassium channel. PLoS Comput. Biol. 12:e1004704. doi: 10.1371/journal.pcbi.1004704
Ye, D., Zhang, D., Oltman, C., Dellsperger, K., Lee, H. C., and VanRollins, M. (2002). Cytochrome p-450 epoxygenase metabolites of docosahexaenoate potently dilate coronary arterioles by activating large-conductance calcium-activated potassium channels. J. Pharmacol. Exp. Ther. 303, 768–776. doi: 10.1124/jpet.303.2.768
Yu, D., Feng, C., and Guo, A. (1999). Altered outward K(+) currents in Drosophila larval neurons of memory mutants rutabaga and amnesiac. J. Neurobiol. 40, 158–170. doi: 10.1002/(sici)1097-4695(199908)40:2<158::aid-neu3>3.0.co;2-#
Yu, S. P. (1995). Roles of arachidonic acid, lipoxygenases and phosphatases in calcium-dependent modulation of M-current in bullfrog sympathetic neurons. J. Physiol. 487 (Pt. 3), 797–811. doi: 10.1113/jphysiol.1995.sp020919
Zhang, M., Shi, W. J., Fei, X. W., Liu, Y. R., Zeng, X. M., and Mei, Y. A. (2008). Mefenamic acid bi-directionally modulates the transient outward K+ current in rat cerebellar granule cells. Toxicol. Appl. Pharmacol. 226, 225–235. doi: 10.1016/j.taap.2007.09.008
Zhang, P., Yang, C., and Delay, R. J. (2008). Urine stimulation activates BK channels in mouse vomeronasal neurons. J. Neurophysiol. 100, 1824–1834. doi: 10.1152/jn.90555.2008
Zhang, P., Yang, C., and Delay, R. J. (2010). Odors activate dual pathways, a TRPC2 and a AA-dependent pathway, in mouse vomeronasal neurons. Am. J. Physiol. Cell Physiol. 298, C1253–C1264. doi: 10.1152/ajpcell.00271.2009
Zhang, Y., Cribbs, L. L., and Satin, J. (2000). Arachidonic acid modulation of alpha1H, a cloned human T-type calcium channel. Am. J. Physiol. Heart Circ. Physiol. 278, H184–H193.
Zhang, Y., Oltman, C. L., Lu, T., Lee, H. C., Dellsperger, K. C., and VanRollins, M. (2001). EET homologs potently dilate coronary microvessels and activate BK(Ca) channels. Am. J. Physiol. Heart Circ. Physiol. 280, H2430–H2440.
Zhao, Z. G., Zhang, M., Zeng, X. M., Fei, X. W., Liu, L. Y., Zhang, Z. H., et al. (2007). Flufenamic acid bi-directionally modulates the transient outward K(+) current in rat cerebellar granule cells. J. Pharmacol. Exp. Ther. 322, 195–204. doi: 10.1124/jpet.106.117556
Zheng, H. F., Li, X. L., Jin, Z. Y., Sun, J. B., Li, Z. L., and Xu, W. X. (2005). Effects of unsaturated fatty acids on calcium-activated potassium current in gastric myocytes of guinea pigs. World J. Gastroenterol. 11, 672–675. doi: 10.3748/wjg.v11.i5.672
Zheng, H., Nam, J. H., Nguen, Y. H., Kang, T. M., Kim, T. J., Earm, Y. E., et al. (2008). Arachidonic acid-induced activation of large-conductance potassium channels and membrane hyperpolarization in mouse B cells. Pflugers Arch. 456, 867–881. doi: 10.1007/s00424-008-0445-1
Zheng, X., Zinkevich, N. S., Gebremedhin, D., Gauthier, K. M., Nishijima, Y., Fang, J., et al. (2013). Arachidonic acid-induced dilation in human coronary arterioles: convergence of signaling mechanisms on endothelial TRPV4-mediated Ca2+ entry. J. Am. Heart Assoc. 2:e000080. doi: 10.1161/JAHA.113.000080
Keywords: voltage-gated ion channels, polyunsaturated fatty acids, voltage sensor domain, S4, Excitability disorders
Citation: Elinder F and Liin SI (2017) Actions and Mechanisms of Polyunsaturated Fatty Acids on Voltage-Gated Ion Channels. Front. Physiol. 8:43. doi: 10.3389/fphys.2017.00043
Received: 17 November 2016; Accepted: 16 January 2017;
Published: 06 February 2017.
Edited by:
Mario Diaz, University of La Laguna, SpainReviewed by:
John Cuppoletti, University of Cincinnati, USACopyright © 2017 Elinder and Liin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fredrik Elinder, ZnJlZHJpay5lbGluZGVyQGxpdS5zZQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.