- 1Institute of Zoology, University of Innsbruck, Innsbruck, Austria
- 2Center for Molecular Biosciences, University of Innsbruck, Innsbruck, Austria
- 3ZF-Screens, Leiden, Netherlands
Using Illumina sequencing, transcriptional changes occurring during silvering in swimbladder tissue of the European eel have been analyzed by comparison of yellow and silver eel tissue samples. Functional annotation analysis based on GO terms revealed significant expression changes in a number of genes related to the extracellular matrix, important for the control of gas permeability of the swimbladder, and to reactive oxygen species (ROS) defense, important to cope with ROS generated under hyperbaric oxygen partial pressures. Focusing on swimbladder tissue metabolism, levels of several mRNA species encoding glucose transport proteins were several-fold higher in silver eels, while enzymes of the glycolytic pathway were not affected. The significantly higher steady state level of a transcript encoding for membrane bound carbonic anhydrase, however, suggested that CO2 production in the pentose phosphate shunt and diffusion of CO2 was of particular importance in silver eel swimbladder. In addition, the mRNA level of a large number of genes related to immune response and to sexual maturation was significantly modified in the silver eel swimbladder. The modification of several processes related to protein metabolism and transport, cell cycle, and apoptosis suggested that these changes in swimbladder metabolism and permeability were achieved by increasing cell turn-over. The impact of an infection of the swimbladder with the nematode Anguillicola crassus has been assessed by comparing these expression changes with expression changes observed between uninfected yellow eel swimbladder tissue and infected silver eel swimbladder tissue. In contrast to uninfected silver eel swimbladder tissue, in infected tissue the mRNA level of several glycolytic enzymes was significantly elevated, and with respect to extracellular matrix, several mucin genes were many-fold higher in their mRNA level. Modification of many immune related genes and of the functional categories “response to DNA damage stimulus” and “cellular response to stress” illustrated the damaging effect of the nematode infection. This study has identified a range of cellular processes in the swimbladder of silver eels that appear to be altered by nematode infection. These altered cellular processes could contribute to detrimental changes in swimbladder function that, in turn, may lead to impairment of spawning migration.
Introduction
The European eel Anguilla anguilla is a catadromous fish spending most of its lifetime as yellow eel in the European freshwater system, and returning to the Sargasso Sea for reproduction. The spawning migration is a journey of about 5000–7000 km from the European coast to the Sargasso Sea, taking about 3.5–6 months. Eels do not feed during this journey and on-board fuels must be sufficient to support the journey. Recent studies revealed that migrating eels perform daily vertical migrations swimming at a depth of about 100–300 m at night time, but at a depth of 600–1000 m at day time (Aarestrup et al., 2009; Wysujack et al., 2015). The concomitant changes in hydrostatic pressure directly affect pressure and volume of the swimbladder, which is used as a buoyancy organ.
Anguillidae are physostomatous fish with a persisting ductus pneumaticus, but in the European eel the ductus pneumaticus is functionally closed, and converted to a resorbing bladder (Dorn, 1961; Pelster, 2013). Accordingly, eels cannot gulp air and the swimbladder is filled by diffusion of gas, mainly oxygen and CO2, from the blood into the swimbladder lumen. The increase in oxygen and CO2 partial pressures required to drive the diffusion of these gas molecules into the swimbladder is achieved by acidification of the blood via lactic acid and CO2 release from swimbladder gas gland cells, which reduces the oxygen carrying capacity of the hemoglobin. This so-called single concentrating effect is subsequently multiplied by countercurrent multiplication in the rete mirabile (Pelster and Randall, 1998; Pelster, 2009, 2013).
Compared with the hydrostatic pressure changes eels encounter during the diurnal vertical migrations in the ocean (21 atm at a depth of 200 m and 101 atm at a depth of 1000 m), the pressure changes eels encounter in the European freshwater system are probably only small. It therefore is not surprising that the process of silvering, which prepares the freshwater adapted yellow eel for the spawning migration in the ocean, also affects the swimbladder. During silvering the retia mirabilia are significantly enlarged, indicating an improvement of the countercurrent concentrating ability. In addition, swimbladder wall thickness and the swimbladder vascularization increase. Guanine deposition in the eel swimbladder wall is enhanced, which decreases its gas permeability and thus reduces diffusional gas loss (Kleckner, 1980a,b; Yamada et al., 2001). In the American eel Anguilla rostrata, a five-fold increase in the rate of gas deposition has been recorded in silver eels (Kleckner, 1980a). It therefore is assumed that these silvering induced changes are connected to a significant improvement in swimbladder function (Sebert et al., 2009; Righton et al., 2012).
While the metabolic processes initiating gas secretion have been repeatedly addressed (Steen, 1963; Kobayashi et al., 1990; Pelster, 1995b, 2013), the molecular changes underlying and controlling these silvering events in gas gland cells are completely unknown. Furthermore, the influence of an infection of the swimbladder with the nematode Anguillicola crassus, which has spread over Europe in the last few decades and infected most of the European eels (Lefebvre et al., 2013), on gene expression in gas gland cells has not yet been addressed. It has been suspected, however, that the infection may influence the silvering process (Fazio et al., 2012).
In this study, we therefore hypothesized that silvering was connected to large changes in the transcription of genes related to swimbladder function. We particularly focused on transcriptional changes of genes related to (1) glucose metabolism and (2) ion exchange, which are required for acid production and release in order to switch on the Root effect for gas secretion (Pelster and Randall, 1998; Pelster, 2013); (3) angiogenesis, required for appropriate blood supply to the swimbladder (Kleckner, 1980a); (4) ROS defense, required to avoid oxidative stress related to hyperbaric oxygen tensions (Morris and Albright, 1981, 1984; Pelster, 2001; Lushchak and Semchyshyn, 2012); (5) extracellular matrix, involved in reducing diffusional gas loss from the swimbladder (Kleckner, 1980a,b; Yamada et al., 2001); (6) immune response, required to defeat the nematode infection (Lefebvre et al., 2011, 2013); and (7) maturation, which occurs in silver eels during spawning migration (Dufour et al., 2003). As a second step, we hypothesized that these transcriptional changes would be modified by an infection of the swimbladder with the nematode A. crassus.
Materials and Methods
Experiments were performed with European eels A. anguilla. Yellow eels were collected by local fishermen in Lake Constance with bottom traps. They were kept in the freshwater aquarium of the Institute of Zoology at the University of Innsbruck until sampling the tissue. Eels with no or one small A. crassus in the swimbladder without visible modification of the swimbladder wall were accepted as uninfected eels (N = 5, body mass 515.0 ± 146.4 g). Silver eels were collected by local fishermen in the IJsselmeer and kept in large tanks at Leiden University until sampling the tissue. Recent studies have shown that the European eel is panmictic (Als et al., 2011; Jacobsen et al., 2014), therefore the results of our study should not be biased by the different sampling points. Table 1 summarizes the morphometrics of uninfected and infected silver eels. Body mass of uninfected silver eels was 1437.2 ± 1182.7 g (N = 5), and body mass of infected eels was 830.8 ± 150.8 g (N = 6). In infected swimbladders between 5 and 30 full grown parasites were detected, and the swimbladder wall was thickened and opaque, as described previously (Würtz and Taraschewski, 2000). Completing one infection cycle requires 3–4 month, suggesting that theses eels have been infected with A. crassus for a long time (Kirk, 2003). The silver index calculated according to Durif et al. (2005) was similar in both groups with 4.0 ± 0.8 for uninfected silver eels and 4.1 ± 0.8 for infected silver eels. Eels were killed with an overdose of neutralized MS222. The swimbladder was dissected and the swimbladder epithelium (consisting of gas gland cells) was carefully freed from connective tissue. The epithelium was shock frozen in liquid nitrogen and stored at −80°C until further use. Tissue sampling was performed in compliance with the Austrian law, the guidelines of the Austrian Federal Minister for Education, Arts and Culture, and also the Dutch law, and were approved by the animal experimental committee of Leiden University.
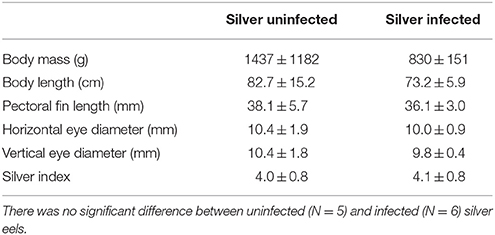
Table 1. Morphometrics of uninfected and infected silver eels including silver index, calculated according to Durif et al. (2005).
RNA Isolation and Illumina RNAseq Analysis
Total RNA was isolated from gas gland tissue using the Qiagen miRNeasy kit according to the manufacturer's instructions (Qiagen, Venlo, Netherlands). Quality and integrity of the RNA were checked on an Agilent Bioanalyzer 2100 total RNA Nano series II chip (Agilent, Amstelveen, Netherlands). Illumina RNAseq libraries were prepared from 2 μg total RNA using the Illumina TruSeq™ RNA Sample Prep Kit v2 according to the manufacturer's instructions (Illumina Inc., San Diego, CA, USA). All RNAseq libraries (150–750 bp inserts) were sequenced on an Illumina HiSeq2000 sequencer as 2 × 50 nucleotides paired-end reads according to the manufacturer's protocol. Image analysis and base calling were done using the Illumina pipeline.
Illumina Data Processing
Data processing was performed as described previously (Dirks et al., 2014; Burgerhout et al., 2016). Briefly, reads (10–20 million per sample) were aligned to the draft genome sequence of European eel (Henkel et al., 2012) using TopHat (version 2.0.5; Trapnell et al., 2009). Secondary alignments of reads were excluded by filtering the files using SAMtools (version 0.1.18; Li et al., 2009). Aligned fragments per predicted gene were counted from SAM alignment files using the Python package HTSeq (version 0.5.3p9; Anders et al., 2015). In order to make comparisons across samples possible, these fragment counts were corrected for the total amount of sequencing performed for each sample. As a correction scaling factor, library size estimates determined using the R/Bioconductor (release 2.11) package DESeq (Anders and Huber, 2010) were employed. Read counts were normalized by dividing the raw counts obtained from HTSeq by its scale factor. Detailed read coverage for individual genes was extracted from the TopHat alignments using SAMtools.
Differences in the steady state mRNA level between yellow and silver eels and also between yellow and infected silver eels were identified using DESeq, the cut-off for significance was set to P < 0.01. GO enrichment analysis was performed using Database for Annotation, Visualization and Integrated Discovery (DAVID) software tools (version 6.7; https://david.ncifcrf.gov). An EASE score of 0.05 along with standard default settings was used, and the resulting categories were considered significant at P < 0.05. Gene ontology annotations were used for a detailed pathway and biological process analysis of mRNA transcripts with different steady state level.
Results
General Observations
At the significance level of P < 0.01 in uninfected silver eels 646 genes were significantly different in their transcription level as compared with uninfected yellow eels (Figure 1). Out of these genes, 505 genes were higher in their transcript level and 141 genes were lower in their transcript level. An initial classification of differentially transcribed genes by Gene Ontology categories using DAVID revealed significant changes in 28 cellular processes in uninfected silver eels (P < 0.05), as compared with uninfected yellow eels (Table 2). Several of these categories were related to protein metabolism, translation, protein localization, and transport, but also to mitosis and cell cycle. Significantly affected were also the categories “oxidation reduction” and “ncRNA metabolic process” (Table 2).
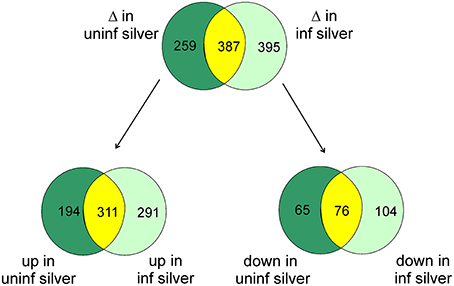
Figure 1. Venn diagram showing the total number of differentially transcribed genes participating in defined pathways expected to be involved in swimbladder function and/or silvering in uninfected silver eels (dark green) and in infected silver eels (light green), and the number of genes affected in both groups (yellow) as compared with uninfected yellow eels. The lower part shows the number of genes either up- or down-regulated.
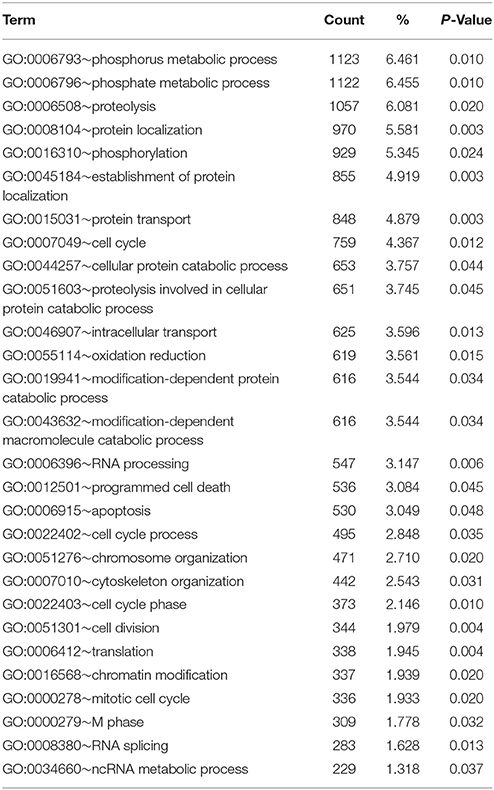
Table 2. Functional annotation analysis based on GO terms showing significantly affected cellular processes in eel gas gland tissue comparing uninfected yellow eels with uninfected silver eels (DAVID).
Transcriptional Changes in Eel Swimbladder Tissue Related to Silvering
To get more detailed insight into the affected metabolic pathways, genes with different mRNA expression level participating in defined pathways expected to be involved in swimbladder function and/or silvering were extracted from GO biological processes. In swimbladder tissue of silver eels, a number of genes involved in carbohydrate transport showed a higher mRNA expression level (Table 3). Interestingly, the mRNA level of monocarboxylate transporter 9 (mot9) was significantly reduced, and genes involved in glycolysis were not significantly modified in their expression level. Two members of the facilitated glucose transport proteins, however, were several-fold higher in their mRNA expression level, namely gtr5 and gtr6. In addition, tyrosin-protein-kinase fyn mRNA was largely elevated. Noteworthy is the almost 10-fold elevation of the carbonic anhydrase 12 (cah12) transcript, while cah6 mRNA was reduced.
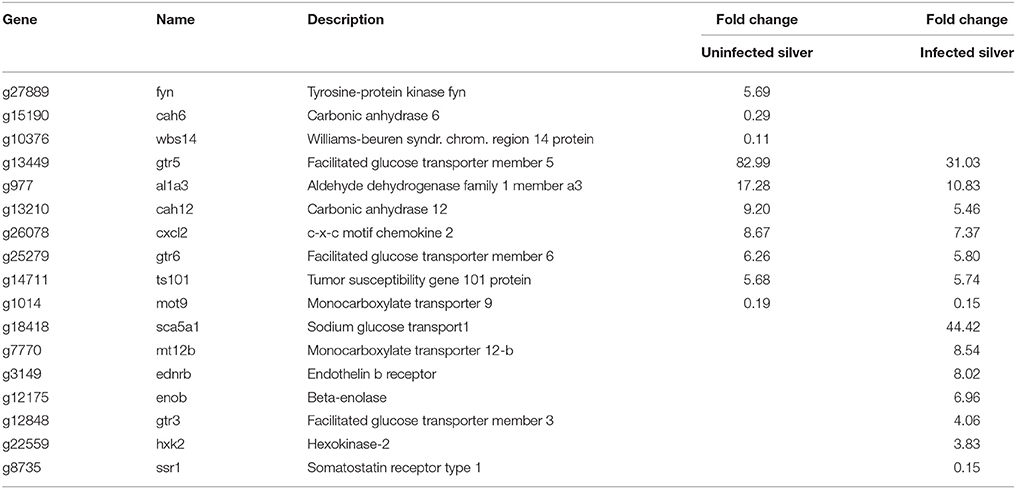
Table 3. Differentially transcribed genes based on GO terms glucose or energy metabolism in uninfected and in infected swimbladder silver eel swimbladder tissue as compared with uninfected yellow eel swimbladder tissue (P < 0.01).
In uninfected silver eel swimbladder, 28 genes connected to the metabolism of reactive oxygen species (ROS) were affected (Table 4). The mRNA species of these ROS related genes showing the largest elevation in uninfected silver eels was nadh dehydrogenase 1 (nqo1; 98-fold elevated). Among the additionally elevated genes were also cytochrome p450 1b1 (cp1b1), neuronal pentraxin (nptx1) ribonucleoside-diphosphate reductase (rir2), and glutathione peroxidase (gpx7).
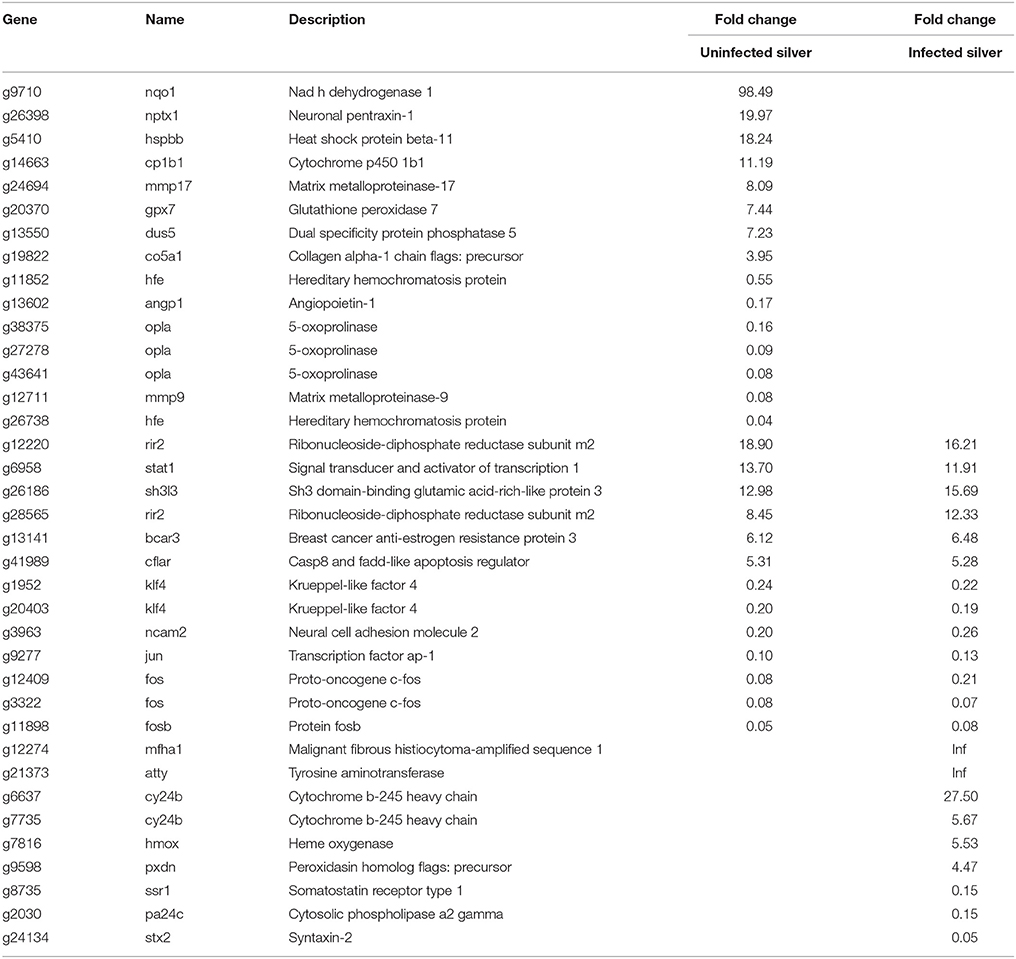
Table 4. Differentially transcribed genes based on GO terms response to oxidative stress and redox homeostasis in uninfected and in infected swimbladder silver eel swimbladder tissue as compared with uninfected yellow eel swimbladder tissue (P < 0.01).
An improvement in swimbladder function could also be achieved by a reduction in gas permeability. Therefore, genes associated to the extracellular matrix were analyzed (Table 5). Nine genes were affected in uninfected silver eels, and only two of these showed a lower steady state mRNA level. Among the genes elevated in their transcription level were mimecan (mime), collagen alpha (co5a1), collagenase 3 (mmp13), and matrix metalloproteinase-17 (mmp17), a lower level was detected for neural adhesion molecule 2 (ncam2) and metalloproteinase-9 (mmp9).
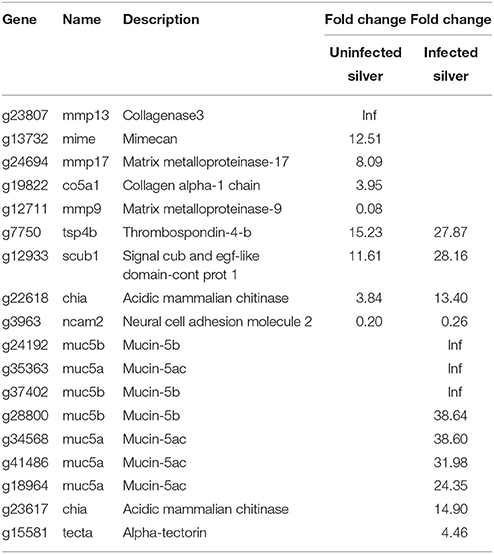
Table 5. Differentially transcribed genes based on GO terms extracellular matrix constituents in uninfected and in infected swimbladder silver eel swimbladder tissue as compared with uninfected yellow eel swimbladder tissue (P < 0.01).
Looking at genes associated with ion exchange, 28 genes were differentially expressed in uninfected eel swimbladder, out of which 12 were higher in their expression level (Supplementary Table 1). Remarkable was the elevation of NADH dehydrogenase and of sodium potassium-transporting atpase (atng). In addition, a potassium channel was elevated (kcnb1), while cystic fibrosis transmembrane conductance channel (cftr), and sodium hydrogen exchanger 1 (sl9a1) were reduced in their mRNA expression level.
An increased capillarization has been described for silver eel swimbladder, therefore the transcriptional changes observed in angiogenesis related genes were addressed (Table 6). In uninfected silver eel swimbladder tissue, mRNAs levels of hormones involved in angiogenesis like VEGF and angiopoietin (vegfc; angp1) were reduced, and several inhibitors of angiogenesis were elevated (cytc; cxl10; scub1; tsp4b; sem3f).
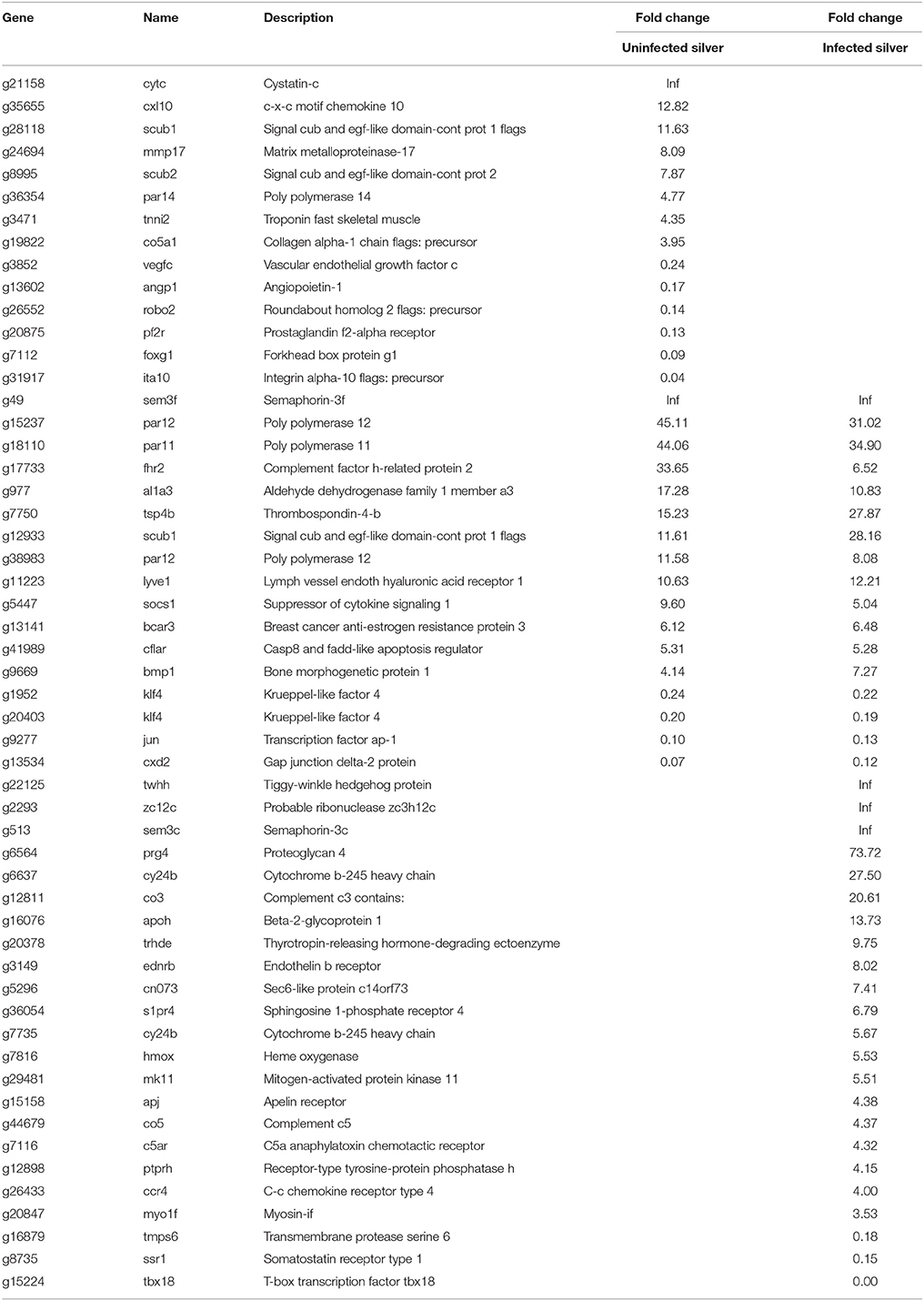
Table 6. Differentially transcribed genes based on GO terms angiogenesis or vasculogenesis in uninfected and in infected swimbladder silver eel swimbladder tissue as compared with uninfected yellow eel swimbladder tissue (P < 0.01).
Differentially transcribed immune related genes were also extracted from GO biological process. One hundred and twenty four genes were differentially transcribed in uninfected silver eel swimbladder, and only 16 out of these genes were reduced in their transcript level (Supplementary Table 2). Many of these genes were more than 10-fold elevated. Among the genes with reduced mRNA expression level were two integrins (ita2, ita10), showing a 14- and 28-fold lower expression level, respectively. Among the elevated genes were several complement proteins, stat1 and interferon induced genes [e.g., 6 mx genes (18-94-fold), 3 IF44 genes (15-23-fold), 2 gvin1 genes (eight-fold)] and interferon regulatory factors, immunoglobulin light chain, and neuronal pentraxin-1 (stat1; cfab; c1qc; in35; iigp5; nptx1).
Surprising was the large number of genes related to sexual maturation that were significantly modified in their transcription level in swimbladder tissue (Supplementary Table 3). The transcription level of 68 genes was modified in uninfected silver eel swimbladder. Among these were three zona pellucida genes, which were elevated about 200-fold (zp1, zp2, and also three copies of zp3).
Transcription Changes in Infected Silver Eel Swimbladder Tissue
To assess the influence of an A. crassus infection on the silvering related changes in swimbladder tissue we compared these silvering induced changes in the level of specific transcripts with changes observed in infected silver eel swimbladder tissue if compared with uninfected yellow eel swimbladder tissue. Compared with uninfected yellow eels in infected silver eels 782 genes were different in their mRNA expression level at the significance level of P < 0.01 (Figure 1). Of these genes, 602 were elevated, and 180 reduced. A comparison of the modified genes between uninfected yellow and silver eels and uninfected yellow and infected silver eels revealed that more than 50% of the genes that were differentially transcribed in infected silver eels (395 genes) were not affected in uninfected swimbladder tissue (Figure 1). The difference became even more obvious when looking at the genes with reduced steady state mRNA level. Of the 180 genes with reduced expression level in infected silver eel swimbladder tissue only 42% were also reduced in uninfected swimbladder tissue. Accordingly, although the total number of genes affected was similar in uninfected and infected silver eels, quite different sets of genes were affected in these two experimental groups. In none of the genes with different mRNA expression level in the two experimental groups opposite changes were detected.
Gene Ontology category analysis, performed using DAVID revealed that in infected silver eels the number of significantly modified functional categories was reduced from 28 to 17 (Table 7); and 12 of these categories were also significantly affected in uninfected silver eels. Functional categories expressed in uninfected silver eels but not in infected silver eels included, for example, “intracellular transport,” “cell cycle process,” “cell division,” “programmed cell death” (“apoptosis”), and “ncRNA metabolic process.” In turn, exclusively in infected silver eels significantly affected functional categories included “chromatin organization,” “response to DNA damage stimulus,” and “cellular response to stress.”
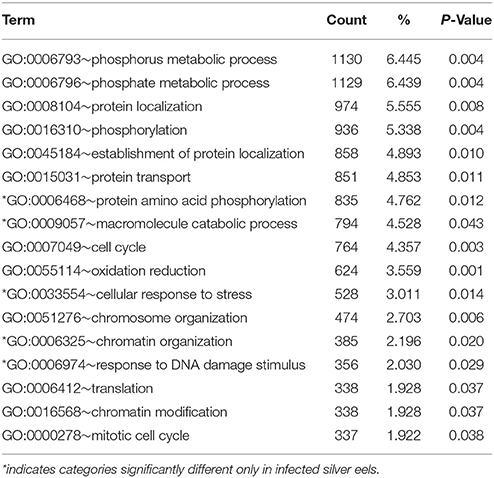
Table 7. Functional annotation analysis based on GO terms showing significantly affected cellular processes in eel gas gland tissue comparing uninfected yellow eels with infected silver eels (DAVID).
Table 8 shows an overview of changes in the transcription level detected in specific pathways that were—based on our previous experimental studies—expected to be involved in swimbladder function and/or silvering. The most obvious differences in the comparison of uninfected yellow eels with either uninfected silver eel or infected silver eels were observed in the transcription level of genes related to ion transport and extracellular matrix. In both groups < 25% of the genes showing a modified mRNA expression level were affected in both groups, meaning that more than 75% of the genes were affected either only in uninfected silver eel swimbladder tissue, or in infected eel swimbladder tissue. A higher overlap (above 40%) was observed in the transcription changes of genes related to glucose metabolism, immune response, and sexual maturation.
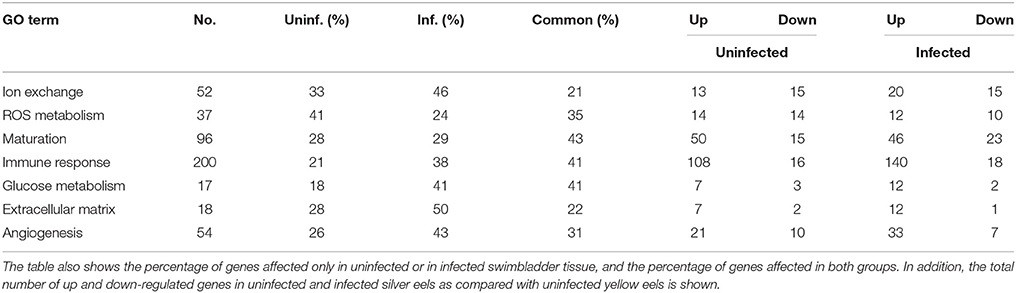
Table 8. Overview of the pathways analyzed (Tables 3–6 and Supplementary Tables 1–3) and the total number of genes affected in both experimental groups.
A detailed analysis of the pathways revealed that in contrast to uninfected silver eels in infected silver eel swimbladder glycolytic genes (enob, hxk2) were significantly elevated in their mRNA expression level (Table 3). Monocarboxylate transporter 9 (mot9) was reduced, as also seen in uninfected silver eels, while monocarboxylate transporter 12-b (mt12b) was 8.5-fold elevated. In infected swimbladders, a higher transcript level was also detected for glucose transporters (gtr3, 5, 6). Particularly noteworthy was the 44-fold elevation of the sodium dependent glucose co-transporter (sca5a1).
In infected silver eel swimbladder tissue, 22 genes connected to the metabolism of ROS were affected (Table 4), out of which 13 genes were also differentially transcribed in uninfected silver eels. Remarkable was that in contrast to uninfected silver eels glutathione peroxidase (gpx7) was not affected. Among the additionally elevated genes in infected silver eels was ribonucleoside-diphosphate reductase (rir2). Only in infected silver eels, heme oxygenase (hmox), and cytochrome b-245-heavy chain (cy24b) showed an elevated steady state mRNA level.
In infected silver eel swimbladder, 13 genes associated to the extracellular matrix were differentially transcribed, and only one of them was reduced in mRNA expression (Table 5). In contrast to uninfected silver eels, in infected eels several mucin genes were more than 20-fold elevated or even switched on (muc5a, muc5b, muc5ac). In fact, seven out of nine genes with an elevated transcript level in infected swimbladder tissue were mucin genes.
Looking at genes associated with ion exchange, 35 genes were differentially transcribed in infected silver eel swimbladder, out of which 11 were also affected in uninfected eel swimbladder (Supplementary Table 1). Sodium potassium transporting ATPase subunit g1 (at1b2) was more than seven-fold reduced, while, v-type proton ATPase subunit g1 (vatg1) was four-fold upregulated. Almost 10-fold elevated was also metalloreductase steap4 (stea4).
In infected eels, the down-regulation of the angiogenesis stimulating hormones was not observed, they remained unchanged in transcription levels. Most of the affected genes were upregulated (32), while only seven genes were down-regulated. Among the upregulated enzymes were thyrotropin-releasing hormone-degrading ectoenzyme (trhde) and MAP kinase 11 (mk11).
As to be expected in infected silver eel swimbladder tissue, 158 immune related genes were modified in the transcription level, and 76 of these genes were selectively affected in infected eel swimbladder tissue, and only nine of these genes showed a reduced mRNA level (Supplementary Table 2).
Surprising was the large number of genes related to sexual maturation that were significantly modified in their transcription level in infected eel swimbladder tissue (Supplementary Table 3). The transcription level of 69 genes was modified in infected silver eel swimbladder, and 41 of these genes were affected in both, infected and uninfected silver eels as compared with uninfected yellow eels.
Discussion
Transcription Changes Connected to Silvering
Silvering is connected to large scale transcriptional changes in swimbladder tissue, and 78% of the affected genes were elevated in their transcriptional level. A difference in steady state mRNA level can be achieved either by increasing the rate of transcription, or by decreasing the rate of mRNA break down. Thus, although we measured steady state levels of mRNA transcripts, changes in these mRNA levels were based on changes in transcription.
Cellular processes, affected by changes in steady state mRNA levels, were connected to cell cycle, cell division, and programmed cell death. In addition, a large number of genes involved in protein metabolism and transport have been modified in their transcription level. Assuming that these changes are at least partially translated into changes in translational activity (i.e., into protein synthesis), our data suggested that silvering was connected to a significant protein and cell turn-over in gas gland tissue. Intestinal epithelia are known for their high turn-over rate, and swimbladder tissue is derived from the foregut. Tissue renewal especially in connection to the silvering process, preparing the swimbladder for the spawning migration, appears quite possible.
Transcription changes observed for genes involved in the formation of the extracellular matrix supported this conclusion. In silver eel swimbladder tissue, several genes related to the formation and renewal of the extracellular matrix showed an elevated transcript level. This could result in an improvement of gas impermeability, which has been reported to occur during silvering for the American eel and the Japanese eel (Kleckner, 1980a,b; Yamada et al., 2001).
Gas secretion in the swimbladder is dependent on glucose metabolism, resulting in the production and release of lactic acid and of CO2, generated in the pentose phosphate shunt (Pelster and Scheid, 1993; Walsh and Milligan, 1993; Pelster et al., 1994; Pelster, 1995b). In uninfected swimbladder tissue, surprisingly, no significant change in the mRNA level of glycolytic enzymes was detected, while two members of the facilitated glucose transporters (gtr5, 6) were several-fold elevated. This suggested that metabolic activity of gas gland tissue is controlled by glucose uptake into the cell. Production of CO2 in the pentose phosphate shunt, subsequent release into the blood stream, and countercurrent concentration of CO2 have been identified as crucial steps in the initiation of gas secretion (Kobayashi et al., 1990; Pelster et al., 1994). Carbonic anhydrase activity including a membrane bound carbonic anhydrase (Pelster, 1995a; Würtz et al., 1999) has been shown to enhance CO2 movements in swimbladder tissue of the European eel, and the membrane bound carbonic anhydrase12 (Supuran, 2008) was nine-fold elevated in the mRNA level. The data therefore suggest that during silvering the pentose phosphate shunt gained importance with the production of CO2 for gas secretion. Membrane bound carbonic anhydrase facilitates the movements of CO2, resulting also in an acidification of blood and switching on of the Root effect (Pelster and Weber, 1991; Pelster and Randall, 1998), facilitating oxygen secretion into the swimbladder. It appears possible that during vertical migrations in the ocean glycolytic activity and lactate production may be enhanced as a second step, following the initiation of gas secretion via CO2 production in the pentose phosphate shunt.
Considering ion transport, silvering appeared to result in an enhancement of acid secretion. The mRNA level of Na+/H+ exchange regulatory cofactor (nhrf3) was 110-fold elevated, suggesting a stimulation of acid release via Na+/H+ exchange. Involvement of Na+/H+ exchange in acid secretion of gas gland cells has been shown previously (Pelster, 1995a). An increased Na+/H+ exchange activity requires an enhanced Na+/K+-ATPase activity in order to retain the sodium gradient, and Na+/K+-ATPase subunit gamma mRNA was indeed elevated. Overall, in silver eel swimbladder more ion transport protein coding genes were significantly reduced in their transcript level than elevated.
To assure appropriate gas secretion, an adequate capillarization and blood supply to the gas gland tissue must be established, but our data did not provide evidence for an improved capillarization of swimbladder tissue. mRNA levels of hormones typically involved in angiogenesis (angiopoietin-1; vascular endothelial growth factor c) were even reduced in uninfected silver eels, and inhibitors of angiogenesis showed increased transcript levels. On the other hand, Kleckner (1980a) reported an increase in the length of rete mirabile capillaries for the American eel. It therefore may be concluded that capillarization of swimbladder tissue itself is not modified during silvering in the European eel, but the capacity of the rete mirabile for countercurrent multiplication is improved by increasing the rete length in order to achieve higher oxygen partial pressures in the swimbladder (Pelster, 1997, 2013).
Due to the high oxygen partial pressures to be expected in the eel swimbladder during the spawning migration, we expected that the ROS defense pathways would be enhanced. Indeed, the results showed 28 genes with a modified transcript level in uninfected silver eel swimbladder tissue, and many of these genes were elevated. Of particular importance appeared to be the almost 100-fold elevation of NADH dehydrogenase, controlling the ratio between NADH and NAD+. Noteworthy was also the elevation of glutathione peroxidase, one of the main enzymes involved in the breakdown of ROS. In a recent study we could show that the concentration of total glutathione (GSH + GSSG) is significantly elevated in silver eel swimbladder tissue as compared with yellow eel swimbladder (Schneebauer et al., 2016). The reduction of oxidized glutathione is dependent on the presence of NADPH, and the pentose phosphate shunt, in addition to the production of CO2 (see above), generates NADPH. Taken together these results supported the conclusion that the glutathione-based ROS defense is of increasing importance in silver eels.
Somewhat surprising was that in uninfected swimbladder tissue a large number of immune related genes was elevated in the steady state mRNA level. With the changes from freshwater to seawater, eels are exposed to a different set of pathogens. It therefore appears plausible that the silvering process includes a change in the transcription pattern of immune related genes in preparation for the new environment. Another possibility would be that Dutch silver eels from the IJsselmeer are exposed to a different set of pathogens, not encountered by yellow eels in Lake Constance. In this case, the immune response might not be specifically connected to the process of silvering, but simply the results of a different environment. On the other hand, trying to identify marker genes of pectoral fin development a large number of differentially expressed immune related genes has also been detected in the European eel (Dirks et al., 2014). The immune system appears to be quite responsive to environmental changes.
Yellow eels are immature and the silvering process has always been seen in the context of maturation, although sexual maturity and gonadal development probably is completed only at the time of arrival at the Sargasso Sea (Dufour et al., 2003). Unexpected, however, was to see that a large number of maturation related genes was significantly modified in the transcription level in swimbladder tissue, which has nothing to do with reproduction. The swimbladder is an isolated tissue in the abdominal cavity of a fish, and after careful dissection and rinsing of the tissue contamination from other tissues could be excluded, except for some blood, which always will be present in dissected tissues. We therefore conclude that the changes in the mRNA level observed in our samples reflect changes in swimbladder tissue.
Particularly puzzling was the almost 200-fold elevation of three different zona pellucida genes in two out of five uninfected silver eel swimbladder tissue samples. Zona pellucida proteins are found in the extracellular matrix surrounding the egg, the so-called chorion. Expression of zona pellucida genes has been described in fish (Wang and Gong, 1999; Conner and Hughes, 2003), but so far at least ZP2 and ZP3 expression has been reported to be restricted to ovary tissue. ZP1, however, has been found in liver tissue (Conner and Hughes, 2003) or in a number other tissues including spleen and kidney (Chuang-Ju et al., 2011). The physiological meaning of transcription of all three genes in uninfected silver eel swimbladder tissue remained elusive. The severe elevation of these mRNA species in uninfected silver eels clearly suggested, however, that the silvering was connected to the onset of sexual maturation. A possible explanation for these changes in maturation related gene expression in swimbladder tissue could be that the maturation process of the gonads induced expression changes in other organs, including organs not immediately connected to sexual maturation. During maturation, an elevation in plasma steroid concentration has been reported (Burgerhout et al., 2016), and these hormones may of course induce expression changes in other tissues as well.
Transcriptional Changes Observed in Infected Eel Swimbladder Tissue
At first glance, the number of genes showing a different expression level and the number of genes showing a higher mRNA level in infected silver eels was somewhat similar to the results obtained for uninfected silver eels. A detailed analysis at the level of affected biological processes, however, revealed significant differences in the steady state mRNA expression level between the two groups. If these differences in mRNA level are at least partially translated into changes in protein concentration the silvering process may be significantly modified by the infection of the swimbladder with the nematode A. crassus.
In contrast to uninfected silver eels, in infected silver eel swimbladder tissue the GO terms “apoptosis” and “programmed cell death” were not significantly affected, and only very few GO terms related to protein metabolism or transport and to the cell cycle were significantly different. This suggested that the infection of the swimbladder reduced the tissue renewal, typically taking place during silvering. Instead, the GO terms “cellular response to stress” and “response to DNA damage” were affected. The feeding activity of the nematode causes tissue damage (Molnár et al., 1993; Beregi et al., 1998; Würtz and Taraschewski, 2000; Lefebvre et al., 2011, 2013), thereby activating genes involved in stress response and tissue repair. This finally results in a severe thickening of swimbladder tissue, and the unicellular gas gland tissue is transformed to a hyperplastic multicellular epithelium (Molnár et al., 1995; Nimeth et al., 2000; Würtz and Taraschewski, 2000).
The defense reaction of the European eel became also obvious when looking at the transcript levels of immune related genes and of extracellular matrix related genes. While in uninfected eel swimbladder tissue, various genes related to the formation and restructuring of the extracellular matrix were almost exclusively elevated, in infected swimbladder tissue various mucin genes were elevated. Cutaneous mucus is considered the first line of defense against infection (Esteban, 2012). The induction and/or the between 20- and 40-fold elevation of seven mucin genes only in infected swimbladder tissue therefore appeared to be a clear defense reaction of the host tissue. A strong defense reaction was also visible in the transcript level changes observed in immune related genes. In infected eel swimbladder tissue, 158 immune related genes were different in their mRNA level compared with uninfected yellow eels, and only 18 of these genes were reduced. More than 40% of these mRNA levels were modified exclusively in infected swimbladder tissue. Activation of the immune system has previously been indicated by presence of macrophages in infected tissue (Beregi et al., 1998; Würtz and Taraschewski, 2000). The significant elevation of the mRNA level of a number of complement proteins, of various interferon inducible genes, for example, and of several members of tripartite motive family of proteins, also inducible by interferons, supported the conclusion that the infection of the swimbladder with nematodes provoked a strong response of the immune system in the eel.
An increased mRNA level of glycolytic genes was detected in infected swimbladder tissue, including in particular hexokinase2, contributing to an initial phosphorylation of glucose taken up into the cells. In addition, in infected swimbladder tissue mRNA levels of genes encoding glucose transport proteins including a sodium dependent glucose transporter were significantly elevated. Infected swimbladder tissue becomes a multilayered epithelium (see above), and diffusion distances for the supply are significantly enlarged. Although in infected swimbladder a down-regulation of angiopoietic hormones was not observed, no evidence for an upregulation of angiopoietic factors was detected. In addition, the oxygen content of a heavily infected swimbladder is significantly lower than of an uninfected swimbladder (Würtz et al., 1996). Taken together, these data therefore suggest that oxygen supply to the thickened tissue became limiting and therefore anaerobic glycolysis with the formation of lactic acid was enhanced, increasing the demand for glucose in swimbladder tissue. Because the nematode is histophagous (Fazio et al., 2008), a proper nutrient supply to the swimbladder tissue is certainly also beneficial for the parasite. The elevated transcript level of genes encoding glucose transporters therefore may well have been for the benefit of the parasite.
These considerations raise the question, whether the nematode is able to affect the activity and gene expression in swimbladder tissue. A strong hint for this would be, if the expression of certain genes would be reversed in infected tissue as compared with uninfected tissue. At the significance level tested, this was not observed. On the other hand, the enhanced expression of heme oxygenase (hmox) and of metalloreductase (stea4) exclusively in infected tissue could indeed have been induced by the parasite. Heme oxygenase breaks down the heme of hemoglobin, resulting in Fe3+, which can be reduced to Fe2+ by metalloreductase. Previous studies indicated that the parasite may stimulate hematopoiesis by enhancing expression of the hemoglobin α-chain (Fazio et al., 2009). This could indicate that the parasite stimulated hemoglobin synthesis and subsequent degradation so that it can easily feed on these break down products.
Remarkable in this context was also the reduced level of Na+/K+-ATPase subunit beta2 (at1b2) mRNA, while in uninfected silver eels another subunit of this ATPase was elevated (see above). While in uninfected eels most of the mRNA species encoding genes involved in ion transport were reduced, in infected eels the majority was elevated. Accordingly, in terms of ion transport only 21% of the affected mRNA species were affected in uninfected as well as in infected silver eels, which was the lowest number of expression overlap. This observation underlined the severe impact of the parasite on swimbladder function.
Elevation of mRNA species of a number of ROS related genes has also been observed in infected swimbladder tissues, suggesting that the infection of the swimbladder increased the level of oxidative stress in this organ in spite of the fact, that the oxygen content of an infected swimbladder is significantly lower (Würtz et al., 1996). In infected silver eels, however, glutathione peroxidase, a crucial enzyme in ROS defense elevated in uninfected silver eels, was not elevated.
Perspectives
Our Illumina sequencing data revealed large scale changes in the steady state mRNA level in swimbladder tissue during silvering in the European eel. These changes suggest that swimbladder gas permeability and ROS defense systems are improved during silvering, and that the production and diffusion of CO2 are of particular importance. The data suggest that glucose metabolism in swimbladder tissue is mainly regulated by glucose uptake of the cells. While these data clearly support the notion that the swimbladder is of importance during spawning migration, a theoretical analysis revealed that swimbladder volume can hardly be adjusted to retain the status of neutral buoyancy during vertical migrations. Keeping swimbladder gas content constant in the face of changing hydrostatic pressure, however, significantly increases energy expenditure for swimming (Pelster, 2015). The actual contribution of the swimbladder to the spawning migration therefore remains open for further analysis. Analysis of swimbladder function under changing hydrostatic pressure probably would be very helpful to answer this question. Furthermore, the changes in the mRNA level seen in a large number of genes related to sexual maturation during silvering suggests that these studies could also be very helpful to elucidate the molecular and physiological changes connected to sexual maturation.
The data also show that an infection of the swimbladder with the nematode A. crassus significantly impairs the molecular changes observed in swimbladder tissue during silvering. Eel tissue showed a strong immune response, metabolic adjustments seen in uninfected tissue were largely abolished and the modification of extracellular matrix components was completely different. These altered cellular processes could significantly contribute to the detrimental changes in swimbladder function in infected eels as observed in a previous study (Würtz et al., 1996). This may significantly compromise a successful spawning migration. Further development of the infection status of the European eel therefore may be crucial for the development of the population.
Author Contributions
BP—designed the study, analyzed data, wrote the first draft of the paper. GS—collected tissues, analyzed the data, contributed to writing the paper. RD—collected tissues, performed illumina seq, performed original data alinement and analysis, contributed to writing the paper.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Financial support of the Austrian Science Foundation (FWF) is gratefully acknowledged (FWF P26363-B25). Bas Brittijn (ZF-screens BV) is acknowledged for preparation of silver eel gas gland samples.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fphys.2016.00175
References
Aarestrup, K., Okland, F., Hansen, M. M., Righton, D., Gargan, P., Castonguay, M., et al. (2009). Oceanic spawning migration of the european eel (Anguilla anguilla). Science 325, 1660. doi: 10.1126/science.1178120
Als, T. D., Hansen, M. M., Maes, G. E., Castonguay, M., Riemann, L., Aarestrup, K., et al. (2011). All roads lead to home: panmixia of European eel in the Sargasso Sea. Mol. Ecol. 20, 1333–1346. doi: 10.1111/j.1365-294X.2011.05011.x
Anders, S., and Huber, W. (2010). Differential expression analysis for sequence count data. Genome Biol. 11:R106. doi: 10.1186/gb-2010-11-10-r106
Anders, S., Pyl, P. T., and Huber, W. (2015). HTSeq - a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169. doi: 10.1093/bioinformatics/btu638
Beregi, A., Molnár, K., Békési, L., and Székely, C. S. (1998). Radiodiagnostic method for studying swimbladder inflammation caused by Anguillicola crassus (Nematoda: Dracunculoidea). Dis. Aquat. Org. 34, 155–160. doi: 10.3354/dao034155
Burgerhout, E., Minegishi, Y., Brittijn, S. A., de Wijze, D. L., Henkel, C. V., Jansen, H. J., et al. (2016). Changes in ovarian gene expression profiles and plasma hormone levels in maturing European eel (Anguilla anguilla); Biomarkers for broodstock selection. Gen. Comp. Endocrinol. 225, 185–196. doi: 10.1016/j.ygcen.2015.08.006
Chuang-Ju, L., Qi-Wei, W., Xi-Hua, C., Li, Z., Hong, C., Fang, G., et al. (2011). Molecular characterization and expression pattern of three zona pellucida 3 genes in the Chinese sturgeon, Acipenser sinensis. Fish Physiol. Biochem. 37, 471–484. doi: 10.1007/s10695-010-9448-x
Conner, S. J., and Hughes, D. C. (2003). Analysis of fish ZP1/ZPB homologous genes–evidence for both genome duplication and species-specific amplification models of evolution. Reproduction 126, 347–352. doi: 10.1530/rep.0.1260347
Dirks, R. P., Burgerhout, E., Brittijn, S. A., de Wijze, D. L., Ozupek, H., Tuinhof-Koelma, N., et al. (2014). Identification of molecular markers in pectoral fin to predict artificial maturation of female European eels (Anguilla anguilla). Gen. Comp. Endocrin. 204, 267–276. doi: 10.1016/j.ygcen.2014.06.023
Dorn, E. (1961). Über den feinbau der schwimmblase von Anguilla vulgaris L. Licht- und elektronenmikroskopische untersuchungen. Z. Zellforsch. 55, 849–912. doi: 10.1007/BF00381654
Dufour, S., Burzawa-Gerard, E., Le Belle, N., Sbaihi, M., and Vidal, B. (2003). “Reproductive endocrinology of the European eel, Anguilla anguilla,” in Eel Biology, eds K. Aida, K. T. Sukamoto, and K. Yamauchi (Tokyo: Springer), 373–383.
Durif, C., Dufour, S., and Elie, P. (2005). The silvering process of Anguilla anguilla: a new classification from the yellow resident to the silver migrating stage. J. Fish Biol. 66, 1025–1043. doi: 10.1111/j.0022-1112.2005.00662.x
Esteban, M. Á. (2012). An overview of the immunological defenses in fish skin. ISRN Immunol. 2012:853470. doi: 10.5402/2012/853470
Fazio, G., Monée, H., Da Silva, C., Simon-Levert, G., Allienne, J. F., Lecomte-Finiger, R., et al. (2009). Changes in gene expression in European eels (Anguilla anguilla) induced by infection with swim bladder nematodes (Anguillicola crassus). J. Parasitol. 95, 808–816. doi: 10.1645/GE-1705.1
Fazio, G., Moné, H., Lecomte-Finiger, R., and Sasal, P. (2008). Differential gene expression analysis in European eels (Anguilla anguilla, L. 1758) naturally infected by macroparasites. J. Parasitol. 94, 571–577. doi: 10.1645/GE-1316.1
Fazio, G., Sasal, P., Mouahid, G., Lecomte-Finiger, R., and Mone, H. (2012). Swim bladder nematodes (Anguillicoloides crassus) disturb silvering in European eels (Anguilla anguilla). J. Parasitol. 98, 695–705. doi: 10.1645/GE-2700.1
Henkel, C. V., Burgerhout, E., de Wijze, D. L., Dirks, R. P., Minegishi, Y., Jansen, H. J., et al. (2012). Primitive duplicate hox clusters in the European eel's genome. PLoS ONE 7:e32231. doi: 10.1371/journal.pone.0032231
Jacobsen, M. W., Pujolar, J. M., Bernatchez, L., Munch, K., Jian, J., Niu, Y., et al. (2014). Genomic footprints of speciation in Atlantic eels (Anguilla anguilla and A. rostrata). Mol. Ecol. 23, 4785–4798. doi: 10.1111/mec.12896
Kirk, R. S. (2003). The impact of Anguillicola crassus on European eels. Fish. Manag. Ecol. 10, 385–394. doi: 10.1111/j.1365-2400.2003.00355.x
Kleckner, R. C. (1980a). Swim bladder volume maintenance related to migratory depth in silver phase Anguilla rostrata. Science 208, 1481–1482. doi: 10.1126/science.7384792
Kleckner, R. C. (1980b). Swimbladder wall guanine enhancement related to migratory depth in silver phase Anguilla rostrata. Comp. Biochem. Physiol. 65A, 351–354. doi: 10.1016/0300-9629(80)90041-9
Kobayashi, H., Pelster, B., and Scheid, P. (1990). CO2 back-diffusion in the rete aids O2 secretion in the swimbladder of the eel. Respir. Physiol. 79, 231–242. doi: 10.1016/0034-5687(90)90129-M
Lefebvre, F., Fazio, G., Mounaix, B., and Crivelli, A. J. (2013). Is the continental life of the European eel Anguilla anguilla affected by the parasitic invader Anguillicoloides crassus? Proc. R. Soc. B Biol. Sci. 280:2012.2916. doi: 10.1098/rspb.2012.2916
Lefebvre, F., Fazio, G., Palstra, A. P., Székely, C., and Crivelli, A. J. (2011). An evaluation of indices of gross pathology associated with the nematode Anguillicoloides crassus in eels. J. Fish Dis. 34, 31–45. doi: 10.1111/j.1365-2761.2010.01207.x
Li, H., Handsaker, B., Wysoker, A., Fennell, T., Ruan, J., Homer, N., et al. (2009). The sequence alignment/map format and SAMtools. Bioinformatics 25, 2078–2079. doi: 10.1093/bioinformatics/btp352
Lushchak, V. I., and Semchyshyn, H. M. (2012). Oxidative Stress - Molecular Mechanisms and Biological Effects. Rijeka: InTech. doi: 10.5772/2333
Molnár, K., Baska, F., Csaba, G., Glávitis, R., and Székely, C. (1993). Pathological and histopathological studies of the swimbladder of eels Anguilla anguilla infected by Anguillicola crassus (Nematoda, Dracunculoidea). Dis. Aquat. Org. 15, 41–50. doi: 10.3354/dao015041
Molnár, K., Szakolczai, J., and Vetési, F. (1995). Histological changes in the swimbladder wall of eels due to abnormal location of adults and second stage larvae of Anguillicola crassus. Acta Vet. Hung. 43, 125–137.
Morris, S. M., and Albright, J. T. (1981). Superoxide dismutase, catalase, and glutathione peroxidase in the swim bladder of the physoclistous fish, Opsanus tau L. Cell Tiss. Res. 220, 739–752. doi: 10.1007/BF00210458
Morris, S. M., and Albright, J. T. (1984). Catalase, glutathione peroxidase, and superoxide dismutase in the rete mirabile and gas gland epithelium of six species of marine fishes. J. Exp. Zool. 232, 29–39. doi: 10.1002/jez.1402320105
Nimeth, K., Zwerger, P., Würtz, J., Salvenmoser, W., and Pelster, B. (2000). Infection of the glass-eel swimbladder with the nematode Anguillicola crassus. Parasitology 121, 75–83. doi: 10.1017/S003118209900606X
Pelster, B. (1995a). Mechanisms of acid release in isolated gas gland cells of the European eel Anguilla anguilla. Am. J. Physiol. 269, R793–R799.
Pelster, B. (1995b). Metabolism of the swimbladder tissue. Biochem. Mol. Biol. Fish. 4, 101–118. doi: 10.1016/S1873-0140(06)80008-1
Pelster, B. (1997). “Buoyancy at depth,” in Deep-Sea Fish, eds D. Randall and A. P. Farrell (San Diego, CA: Academic Press), 195–237.
Pelster, B. (2001). The generation of hyperbaric oxygen tensions in fish. News Physiol. Sci. 16, 287–291.
Pelster, B. (2009). “Buoyancy control in aquatic vertebrates,” in Cardio-Respiratory Control in Vertebrates, eds M. L. Glass and S. C. Wood (Berlin/Heidelberg: Springer Verlag), 65–98.
Pelster, B. (2013). “The swimbladder,” in Eel Physiology, eds F. Trischitta, Y. Takei, and P. Sebert (Enfield: CRC Press), 44–67.
Pelster, B. (2015). Swimbladder function and the spawning migration of the European eel Anguilla anguilla. Front. Physiol. 5:486. doi: 10.3389/fphys.2014.00486
Pelster, B., Hicks, J., and Driedzic, W. R. (1994). Contribution of the pentose phosphate shunt to the formation of CO2 in swimbladder tissue of the eel. J. Exp. Biol. 197, 119–128.
Pelster, B., and Randall, D. J. (1998). “The physiology of the root effect,” in Fish Respiration, eds S. F. Perry and B. L. Tufts (San Diego, CA: Academic Press), 113–139.
Pelster, B., and Scheid, P. (1993). Glucose metabolism of the swimbladder tissue of the European eel Anguilla anguilla. J. Exp. Biol. 185, 169–178.
Pelster, B., and Weber, R. E. (1991). The physiology of the root effect. Adv. Comp. Environ. Physiol. 8, 51–77. doi: 10.1007/978-3-642-75900-0_2
Righton, D., Aarestrup, K., Jellyman, D., Sébert, P., van den Thillart, G., and Tsukamoto, K. (2012). The Anguilla spp. migration problem: 40 million years of evolution and two millennia of speculation. J. Fish Biol. 81, 365–386. doi: 10.1111/j.1095-8649.2012.03373.x
Schneebauer, G., Hanel, R., and Pelster, B. (2016). Anguillicola crassus impairs the silvering-related enhancements of the ROS defense capacity in swimbladder tissue of the European eel (Anguilla anguilla). J. Comp. Physiol. B. doi: 10.1007/s00360-016-0994-0. [Epub ahead of print].
Sebert, P., Vettier, A., Amerand, A., and Moisan, C. (2009). “High pressure resistance and adaptation of European eels,” in Spawning Migration of the European Eel, eds G. van den Thillart, S. Dufour, and J. C. Rankin (New York, NY: Springer Verlag), 99–127.
Steen, J. B. (1963). The physiology of the swimbladder in the eel Anguilla vulgaris. III. The mechanism of gas secretion. Acta Physiol. Scand. 59, 221–241. doi: 10.1111/j.1748-1716.1963.tb02738.x
Supuran, C. T. (2008). Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat. Rev. Drug Discov. 7, 168–181. doi: 10.1038/nrd2467
Trapnell, C., Pachter, L., and Salzberg, S. L. (2009). TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25, 1105–1111. doi: 10.1093/bioinformatics/btp120
Walsh, P. J., and Milligan, C. L. (1993). Roles of buffering capacity and pentose phosphate pathway activity in the gas gland of the gulf toadfish Opsanus beta. J. Exp. Biol. 176, 311–316.
Wang, H., and Gong, Z. (1999). Characterization of two zebrafish cDNA clones encoding egg envelope proteins ZP2 and ZP3. Biochim. Biophys. Acta 1446, 156–160. doi: 10.1016/S0167-4781(99)00066-4
Würtz, J., Salvenmoser, W., and Pelster, B. (1999). Localization of carbonic anhydrase in swimbladder tissue of European eel (Anguilla anguilla) and perch (Perca fluviatilis). Acta Physiol. Scand. 165, 219–224. doi: 10.1046/j.1365-201x.1999.00501.x
Würtz, J., and Taraschewski, H. (2000). Histopathological changes in the swimbladder wall of the European eel Anguilla anguilla due to infections with Anguillicola crassus. Dis. Aquat. Org. 39, 121–134. doi: 10.3354/dao039121
Würtz, J., Taraschewski, H., and Pelster, B. (1996). Changes in gas composition in the swimbladder of the European eel (Anguilla anguilla) infected with Anguillicola crassus (Nematoda). Parasitology 112, 233–238. doi: 10.1017/S003118200008481X
Wysujack, K., Westerberg, H., Aarestrup, K., Trautner, J., Kurwie, T., Nagel, F., et al. (2015). The migration behaviour of European silver eels (Anguilla anguilla) released in open ocean conditions. Mar. Freshwater Res. 66, 145–157. doi: 10.1071/MF14023
Keywords: swimbladder function, spawning migration, nematode infections, European eel, silvering
Citation: Pelster B, Schneebauer G and Dirks RP (2016) Anguillicola crassus Infection Significantly Affects the Silvering Related Modifications in Steady State mRNA Levels in Gas Gland Tissue of the European Eel. Front. Physiol. 7:175. doi: 10.3389/fphys.2016.00175
Received: 03 February 2016; Accepted: 02 May 2016;
Published: 23 May 2016.
Edited by:
Andreas Fahlman, Texas A&M University-Corpus Christi, USAReviewed by:
Wayne R. Fitzgibbon, Medical University of South Carolina, USAVolodymyr I. Lushchak, Vasyl Stefanyk Precarpathian National University, Ukraine
Copyright © 2016 Pelster, Schneebauer and Dirks. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bernd Pelster, YmVybmQucGVsc3RlckB1aWJrLmFjLmF0
 Bernd Pelster
Bernd Pelster Gabriel Schneebauer
Gabriel Schneebauer Ron P. Dirks
Ron P. Dirks