- 1Institute of Cellular and Organismic Biology, Academia Sinica, Taipei, Taiwan
- 2Institute of Physiology, Christian-Albrechts University Kiel, Kiel, Germany
- 3Institute of Biological Chemistry, Academia Sinica, Taipei, Taiwan
- 4Institute of Marine Biology, National Taiwan Ocean University, Keelung, Taiwan
- 5Department of Life Science, National Taiwan Normal University, Taipei, Taiwan
- 6Biodiversity Research Center, Academia Sinica, Taipei, Taiwan
Hydrothermal vent organisms have evolved physiological adaptations to cope with extreme abiotic conditions including temperature and pH. To date, acid-base regulatory abilities of vent organisms are poorly investigated, although this physiological feature is essential for survival in low pH environments. We report the acid-base regulatory mechanisms of a hydrothermal vent crab, Xenograpsus testudinatus, endemic to highly acidic shallow-water vent habitats with average environment pH-values ranging between 5.4 and 6.6. Within a few hours, X. testudinatus restores extracellular pH (pHe) in response to environmental acidification of pH 6.5 (1.78 kPa pCO2) accompanied by an increase in blood levels from 8.8 ± 0.3 to 31 ± 6 mM. Branchial Na+/K+-ATPase (NKA) and V-type H+-ATPase (VHA), the major ion pumps involved in branchial acid-base regulation, showed dynamic increases in response to acidified conditions on the mRNA, protein and activity level. Immunohistochemical analyses demonstrate the presence of NKA in basolateral membranes, whereas the VHA is predominantly localized in cytoplasmic vesicles of branchial epithelial- and pillar-cells. X. testudinatus is closely related to other strong osmo-regulating brachyurans, which is also reflected in the phylogeny of the NKA. Accordingly, our results suggest that the evolution of strong ion regulatory abilities in brachyuran crabs that allowed the occupation of ecological niches in euryhaline, freshwater, and terrestrial habitats are probably also linked to substantial acid-base regulatory abilities. This physiological trait allowed X. testudinatus to successfully inhabit one of the world's most acidic marine environments.
Introduction
Deep sea hydrothermal vent systems support ecosystems with an enormous biomass, and reveal a rich biodiversity ranging from microbes to vertebrates (Tunnicliffe, 1992). To survive in these extreme habitats, vent associated organisms show a range of morphological and physiological adaptations to cope with challenging environmental conditions including temperature, metallic sulfides, anoxia, hypercapnia, and low pH (Goffredi et al., 1997; Ramirez-Llodra et al., 2007). Highly acidified conditions due to the release of HCl and CO2 are a characteristic of most seafloor vent systems including the shallow-water hydrothermal vent system of Kueishan Island (24°50′N, 121°57′E), off the coast of Taiwan (Han et al., 2014). This shallow water hydrothermal vent system has been described as one of the most acidic vents in the world, discharging water with a high content of elemental sulfur particles, having temperatures ranging between 76 and 116°C and a minimum pH of 1.52 (Chen et al., 2005; Figure 1A). The gas composition released by the underwater volcano is mainly CO2 (<92%; Han et al., 2014). Even in the surrounding areas with depths between 2 and 14 m, the seawater is highly acidic ranging from pH 6.6 to 5.4 (Han et al., 2014). This challenging hydrothermal vent habitat is inhabited by Xenograpsus testudinatus, a crab species that is endemic to shallow-water (<200 m) vent systems (Ng et al., 2000). X. testudinatus is the only metazoan species found in the direct surroundings of the vents, and individuals congregate in large numbers in vent crevices with an average of 364 individuals per m2 (Figure 1B). These crabs have evolved a unique feeding behavior by feeding on dead zooplankton killed by the toxic vent discharges (Jeng et al., 2004). During slack water conditions, when there are no currents, the crabs swarm out of their crevices (Figures 1C,D) to rapidly feed on this “marine snow” of dead zooplankton (Jeng et al., 2004).
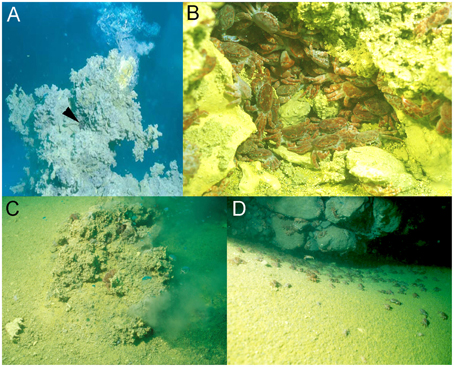
Figure 1. Xenograpsus testudinats crabs in the hydrothermal vent habitat at Kueishan island located off Taiwan's east coast. Underwater photographs of the hydrothermal vent system at Kueishan island, showing an active chimney discharging acidic, CO2 rich water (note the X. testudinatus crab in the direct vicinity of the vent opening) (A). High densities of X. testudinatus crabs can be found in the direct surrounding of the vents hiding in sulfur-rich crevices (B). Whilst feeding on zooplankton killed by the toxic discharges of the vents crabs swarm out of their shelters, and are directly exposed to highly acidic waters (C,D). All images were obtained and authorized from the co-author Dr. Ming-Shiou Jeng.
Crustaceans are probably one of the most successful invertebrate group that occupy ecological niches in marine systems ranging from polar regions (Frederich et al., 2001) to hydrothermal vents (Joel and Haney, 2005; Dittel et al., 2008) and have radiated from seawater to freshwater and even into terrestrial habitats (Schubart et al., 1997). While those found in stenohaline marine habitats are more likely to be weak osmo-regulators or even osmo-conformers crustaceans from euryhaline or intertidal habitats are moderate to strong osmo-regulators, a physiological feature that is beneficial for adaptation to habitats with fluctuating salinities (Charmantier and Charmantier-Daures, 2001; Henry et al., 2012). The gills are the major organ involved in extracellular ion homeostasis, equipped with an efficient ion regulatory machinery that shows evolutionary conserved features comparable to the ion regulatory epithelia in cephalopods and fish (Henry et al., 2012). Similar to the situation in most vertebrate and invertebrate systems, the ubiquitous Na+/K+-ATPase localized in basolateral membranes creates the electro-chemical gradient that is used by secondary active transporters such as apical Na+/H+-exchangers or anion exchangers that can mediate ionic and pH homeostasis in crustaceans (Cameron, 1978; Heisler, 1986; Pörtner et al., 1991; Gutowska et al., 2010; Henry et al., 2012). The V-type H+-ATPase (VHA) has also been described in gill epithelia of crustaceans, demonstrating a cytosolic or apical localization of this enzyme (Weihrauch et al., 2001; Tsai and Lin, 2007). The VHA has been shown to be involved in the acidification of intracellular organelles, and in the secretion of protons across the plasma membrane of specialized cells located in ion-regulatory epithelia (Weihrauch et al., 2002; Tresguerres et al., 2006; Hwang, 2009; Hu et al., 2014). Based on the structure of the VHA with the catalytic (V1 complex) site facing the cytoplasm, the VHA is restricted to transport protons out of the cytoplasm (Beyenbach, 2006).
In euryhaline crabs changes in environmental salinity were demonstrated to directly affect the extracellular acid-base status of these animals (Truchot, 1981, 1992). The fact that a reduction in environmental salinity induces a metabolic alkalosis while an increase in salinity leads to a metabolic acidosis demonstrated that osmo-regulation and the maintenance of acid-base homeostasis are directly linked (Whiteley et al., 2001). This connection between osmo and acid-base regulation may explain why most osmo-regulating crustaceans are relatively tolerant to environmental acid-base disturbances (Henry and Cameron, 1982; Spicer et al., 2007).
X. testudinatus is a brachyuran crab species that has a phylogenetic position close to other crab species that are characterized as strong osmo-regulators including the euryhaline crab Eriocheir sinensis and crabs of the genus Hemigrapsus spp. (Hicks, 1973; Bedford and Leader, 1977; Onken and Graszynski, 1989; Ki et al., 2009). The potential link between acid-base and osmo-regulation in many euryhaline decapod crustaceans prompted us to formulate the hypothesis that X. testudinatus utilizes conserved ion pumps to regulate extracellular pH. To test this hypothesis we exposed X. testudinatus to CO2-induced seawater acidification (pH 6.5), and monitored changes in extracellular acid-base status as well as expression, protein concentrations and activities of the branchial Na+/K+-ATPase and V-type H+-ATPase over a time course of 48 h. These results will demonstrate that evolution of strong osmo-regulatory abilities in this phylogenetic group may represent a requisite that is accompanied by substantial acid-base regulatory abilities that allowed X. testudinatus to inhabit one of the most acidic marine habitats.
Materials and Methods
Acidification Experiments
X. testudinatus with carapace width ranging between 3 to 4 cm were obtained by SCUBA divers from Kueishan Island, Taiwan (ROC) in July 2013. Crabs were collected from depth ranging between 5 and 10 m and held in recirculating natural seawater systems (500 l total volume, nitrification filter, salinity 31–32‰, temperature 28°C, constant 12 h dark:12 h light cycle) at the Institute of Cellular and Organismic Biology, Academia Sinica. Animals were fed ad libitum twice per day with tilapia meat. CO2 perturbation experiments were carried our using a total of 48 animals that were distributed into six 20 l tanks (8 animals per tank). The six tanks, with three replicate tanks for each pH treatment were connected to a flow through system providing filtered (0.2 μm), natural seawater. Water was exchanged at a flow rate of ~3 l h−1 to guarantee high water quality inside the test aquaria. The pH (8.0 and 6.5) in the experimental tanks was continuously adjusted by the addition of the appropriate gas mixtures using a continuous pH-stat system (pH controller, MACRO) that controlled the addition of pure CO2 into the seawater. The experimental tanks were additionally aerated with air (O2 saturation > 90%) to assure sufficient seawater pO2 during the experiment. Specific seawater physicochemical conditions for the different incubations are shown in Table S1. Animals were not fed during the 48 h experimental period in order to minimize physiological artifacts caused by feeding activity. pHSW was measured using a WTW 340i meter equipped with a WTW SenTix 81 electrode that was calibrated daily with Tris and AMP buffers at a salinity of 31 to monitor the experiment. Alkalinity in seawater samples was determined spectrophotometrically according to Sarazin et al. (1999). The carbonate system of seawater was calculated from AT and pHSW using the software CO2SYS (Lewis and Wallace, 1998), including the dissociation constants of Mehrbach et al. (1973) as refitted by Dickson and Millero (1987). Along the experimental duration of 48 h, extracellular acid-base parameters were measured, and gill tissue samples were collected for gene expression, protein, and enzyme analyses. Tissue and hemolymph sampling was carried out at six time points (0, 1, 4, 12, 24, and 48 h) along the time course of 48 h. At each sampling time point, one to two animals were sampled from the three experimental replicate tanks, leading to a biological replication of n = 4. Animals were anesthetized by cooling on ice and were killed by an incision of the frontal region of the carapace. The carapace was removed to access the branchial chamber where the gills are located. X. testudinatus has six gill pairs (Figures S2A,B), of which the anterior-most gill 1 (G1), is minute (length 1–2 mm); G6 is the posterior-most gill with a total length of ~10 mm. G1–G3 are designated as anterior gills, while G4–6 are considered posterior gills. G3–G6 are similar in size while G2 is smaller (~5 mm long). The gill formula of X. testudinatus is: G1,G2 podobranch, G3 and G4 arthrobranchs with a common insertion point, G5 and G6 arthrobranchs with a common insertion point. Gills from the left side were sampled for gene expression studies while those from the right side were sampled for protein and enzyme analyses. Pooled samples of posterior gills (4,5,6) were used for gene expression, activity and protein analyses. The experimental protocols for the present study were approved by the National Taiwan Normal University Institutional Animal Care and Utilization Committee (approval no.: 101005).
Extracellular Acid-Base Status
Hemolymph samples were taken from the coxa using a gas-tight Hamilton syringe. Determination of pHe in venous hemolymph was performed in 100 μl samples using a microelectrode (WTW Mic-D) and a WTW 340 pH meter (precision ± 0.01 units) that was calibrated with Radiometer precision buffers 7 and 10 (S11M44, S11 M007). Measurements were performed inside a temperature controlled water bath adjusted to 28°C. Due to low hemolymph sample volumes for total dissolved inorganic carbon (CT) determinations (~100 μl) samples were diluted 1:1 with de-ionized water prior to measurements. After hemolymph sampling, the dilution and measurement of samples was carried out within less than 30 s to guarantee negligible changes in the carbonate system of hemolymph samples. CT was determined in duplicates (100 μl each) via a Corning 965 carbon dioxide analyzer (precision ± 0.1 mmol l−1; Olympic Analytical Service, England) that was calibrated using a fresh dilution series of 40, 20, 10, 5, and 2.5 mM bicarbonate in distilled water to generate a sodium bicarbonate standard curve. Carbonate system speciation (i.e., pCO2, []) of hemolymph samples of X. testudinatus was calculated from extracellular pH (pHe) and CT using the Henderson–Hasselbalch equation with dissociation constants and solubility coefficients as previously described for the shore crab Carcinus maenas (Truchot, 1976).
Immunohistochemistry and Western Blot Analyses
For immunohistochemistry gill tissues from control animals were fixed and mounted to slides as previously described (Hu et al., 2014). The primary antibodies, a mouse monoclonal antibody α5, raised against the avian α subunit of the Na+/K+-ATPase (Hybridoma Bank) and a polyclonal antibody raised against part the subunit A region (SYSKYTRALDEFYDK) of the molluscan V-type-H+-ATPase (VHA; for more detail see Hu et al., 2013) were diluted in PBS (1:100) and placed in droplets of 200 μl onto the sections, and incubated over night at 4°C inside a wet chamber. Sections were then washed (3 × 5 min) with PBS and incubated for 1 h with the secondary antibody, anti-mouse Alexa Fluor 488, or anti-rabbit Alexa Fluor 568 (Invitrogen) (dilution 1:250). After rinses in PBS (3 × 5 min), sections were examined and photographed using a fluorescence microscope (Zeiss imager A1) equipped with an appropriate filter set. Negative controls were performed several times for every antibody by omitting the primary antibody.
Immunoblotting was essentially performed as previously described (Hu et al., 2014) using 15 μL of gill crude extracts. Proteins were fractionated by SDS-PAGE on 10% polyacrylamide gels, and transferred to PVDF membranes (Millipore), using a tank blotting system (Bio-Rad). Blots were exposed to the primary antibody (see previous section) diluted 1:250–500 and incubated at 4°C overnight. After washing with PBS-T (phosphate buffered saline containing 0.1% Tween20), blots were incubated for 2 h with horseradish conjugated goat anti-rabbit IgG antibody (diluted 1:1000–2000, at room temperature; Amersham Pharmacia Biotech). Protein signals were visualized using the enhanced chemiluminescence system (ECL, Amersham Pharmacia Biotech) and recorded using Biospectrum 600 imaging system (UVP, Upland, CA, USA). Signal intensities were calculated using the free software “Image J” (e.g., Schneider et al., 2012).
Enzyme Activity
ATPase activity was measured in crude extracts of the three posterior gills (4,5,6). The measurement is based on a coupled enzyme assay containing pyruvate kinase (PK) and lactate dehydrogenase (LDH) as previously described (Hu et al., 2014). Crude extracts were obtained by quickly homogenizing the tissue samples using a pestle followed by complete homogenization in a tissue lyzer (Qiagen) in five volumes of ice-cold imidazole buffer (Hu et al., 2014). After centrifugation for 10 min at 1000 g and 4°C, cell debris was removed and the supernatant was used as a crude extract. The reaction was started by adding 1.5 μl of the sample homogenate to the reaction buffer. The coupled to The hydrolysis of ATP reflected by the oxidation of NADH was measured photometrically at 30°C in a temperature controlled plate reader (Molecular Device, Spectra Max, M5), over a period of 15 min, with the decrease of extinction being measured at λ = 339 nm. Addition of 2 μl ouabain (5 mM final concentration) or bafilomycin (Bafilomycin A1, Sigma-Aldrich) (1 μM final concentration) to the assay was used to determine the fraction of Na+/K+-ATPase or H+-ATPase activity from the total ATPase (TA) activity. The concentrations of inhibitors used in this enzyme assay are sufficient to fully inhibit the NKA (Morris et al., 1997) and VHA (Dröse and Altendorf, 1997), respectively. Six measurement replicates were performed for each sample (three with inhibitor dissolved in DMSO and three with DMSO). Enzyme activities were calculated by using the extinction coefficient for NADH of ε = 6.31 mM−1·cm−1 and given as micromoles of ATP consumed per gram tissue fresh mass (gFM) per hour.
Preparation of mRNA
Separated gills (1–6) were homogenized in Trizol reagent (Invitrogen, Carlsbad, CA, USA) using a Tissue lyser (Quiagen). Chloroform was added to the Trizol homogenates, and total RNA was extracted from the aqueous phase and purified by addition of isopropanol. Genomic DNA contaminations were removed by DNase I (Promega, Madison, WI, USA) treatment. The mRNA for the RT-PCR was obtained using a QuickPrep Micro mRNA Purification Kit (Amersham Pharmacia, Piscataway, NJ, USA) according to the supplier protocol. Extracted mRNA concentrations were determined by spectrophotometry (ND-2000, NanoDrop Technol, Wilmington, DE), and the integrity of the mRNA was controlled by electrophoresis in RNA gels. All mRNA pellets were stored at −80°C.
Cloning of xtNKA Fragment
Fragments of the X. testudinatus Na+/K+-ATPase (NKA) and V-Type H+-ATPase (VHA) genes were amplified from gill tissue by means of reverse transcription followed by PCR (RT–PCR) using primers based on highly conserved regions of the NKA and VHA from the green shore crab C. maenas. Reverse transcription was performed as previously described (Hu et al., 2013) and the primer pair 5′-CAGTCACTTCATCCACATCA-3′ and 5′-CACATCTCCAATAGCCAGTT-3′ resulted in a 540 bp fragment of the NKA. PCR fragments were separated by electrophoresis in 1.5% agarose gels. Extraction, purification, and cloning of the PCR fragments from the gel was accomplished as previously described (Hu et al., 2013). Plasmids were sequenced and sequence analysis was performed using the BLASTx program (NCBI, http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Real-Time Quantitative PCR (qPCR)
The mRNA expressions of selected candidate genes were measured by qPCR using the Roche LightCycler® 480 System (Roche Applied Science, Mannheim, Germany). Primers for the Na+/K+-ATPase, V-type H+-ATPase and the reference gene arginine kinase were designed using Primer Premier software (vers. 5.0; PREMIER Biosoft International, Palo Alto, CA) and are provided in Table S2. PCR reactions were performed as previously described (Hu et al., 2013, 2014) and PCR products were subjected to a melting-curve analysis. Primer efficiencies were >96% and control reactions were performed using nuclease-free water to determine background levels. Additionally, DNAse I treated RNA samples served as a control, demonstrating that no PCR product was obtained, and thus the success of the DNase I treatment. The standard curve of each gene was in a linear range with arginine kinase (AK) that served as reference gene. The expression of this reference gene has been demonstrated to be stable in the green shore crab C. maenas during CO2 treatments (Fehsenfeld et al., 2011).
Statistical Analyses
Statistical analyses were performed using Sigma Stat 3.0 (Systat) software. Statistical differences between pH treatments within one time point were analyzed using a Student's t-test. The significance levels were set to p < 0.05* and p < 0.01**.
Results
Extracellular Acid-Base Status
Mean extracellular pH (pHe) measured in hemolymph samples along the time series of 48 h ranged between pH 7.50 ± 0.02 to 7.59 ± 0.01 in control animals (Figure 2A; Figure S1). In response to acidified conditions of pH 6.5, pHe dropped (p < 0.05) by ~0.25 pH units compared to control animals after 1 h. pHe was partially restored after 4 h and remained stable at levels of ~0.1 pH units below control pHe. Mean blood levels were found to range from 6.4 ± 0.5 to 8.2 ± 1.1 mM in control animals along the incubation period of 48 h (Figure 2B). In response to acidified conditions of pH 6.5, hemolymph levels progressively increased from 8.8 ± 0.3 to 31 ± 6 mM during the 48 h incubation period and significant differences (p < 0.05 and 0.01) to control animals were observed after 4 h incubation until the end of the experiment. Hemolymph pCO2 levels ranged from 0.41 to 0.55 kPa in control animals along the incubation period of 48 h (Figure 2C). In response to acidified conditions, hemolymph pCO2 levels increased to peak values of 2.7 ± 0.3 kPa (Figure 2C).
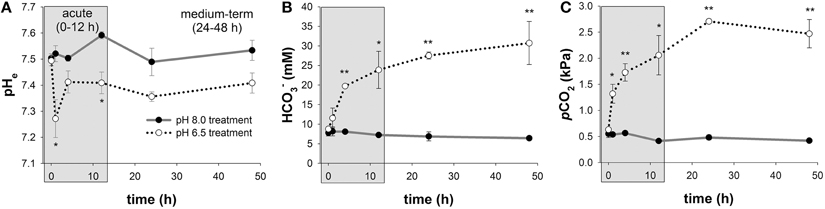
Figure 2. Extracellular acid-base parameters during exposure to acidified conditions. Time series measurements of in vivo extracellular pH (pHe) (A), hemolymph levels (B), and pCO2 (C) along the experimental period of 48 h. The exposure period is separated into a short-term (gray) and medium-term (white) acclimation period. Extracellular acid-base parameters are additionally presented in a Davenport diagram in Figure S1. Asterisks indicate significant differences between pH treatments (*p < 0.05 and **p < 0.001). Bars represent mean ± SE (n = 4).
Regulation of Branchial Na+/K+-ATPase and V-Type H+-ATPase upon Low pH Exposure
Comparisons of routine mRNA levels of NKA and VHA did not reveal any significant differences between anterior (1–3) and posterior gills (4–6; Figure S2). In response to acidified conditions, mRNA levels of the NKA and VHA measured in the posterior gills (4,5,6) increased rapidly within 1 h by 2.3- and 11.4-fold, respectively (Figures 3A,B). While NKA mRNA levels returned to control levels within the acute acclimation phase of 4 h, VHA mRNA levels rapidly increased upon low pH exposure for 1 h and then decreased back to control levels within 24 h (Figures 3A,B). The increase in mRNA concentrations is paralleled by an increase in NKA and VHA enzyme activities (Figures 3C,D). Compared to control conditions where maximum NKA enzyme activities ranged from 335 ± 60 to 456 ± 9 μmolATP h−1 , branchial NKA activities in crabs exposed to acidified conditions increased within 1 h to a maximum of 719 ± 115 μmolATP h−1 . Along the period of 24 h, NKA activities decreased back to control levels (Figure 3C). Branchial VHA enzyme activities increased in response to acidified conditions with an activity peak after 12 h reaching 1.7-fold increased enzyme activities compared to control animals (Figure 3D). After 24 h of low pH exposure, branchial VHA activities returned back to control levels and remained slightly above control levels for the experimental period of 48 h. When comparing relative changes of NKA mRNA and activity levels along the entire experimental period a slight shift by ~3 h in peak NKA activities can be found in comparison to mRNA levels. Moreover, while NKA mRNA levels returned to control conditions after 4 h, NKA enzyme activities remain elevated by 40% to 30% along the experimental duration of 48 h (Figure 3E). A more pronounced shift between peak mRNA and enzyme activity levels of ~9 h has been observed for the branchial VHA (Figure 3F). After this acute phase, VHA enzyme activities decreased back to control levels after 48 h exposure to acidified conditions.
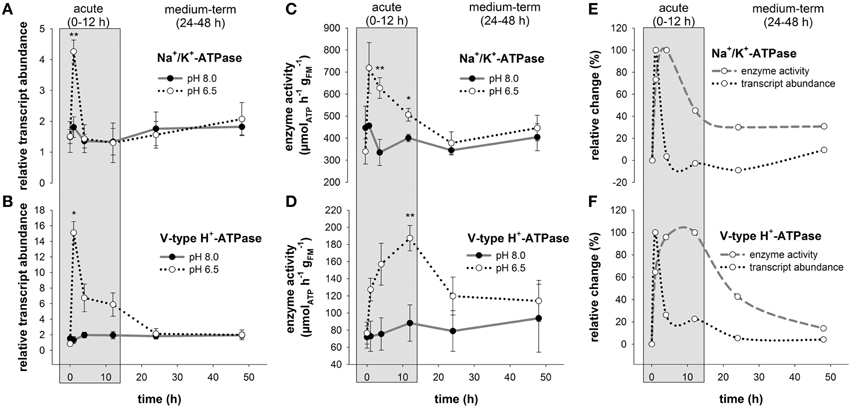
Figure 3. Branchial Na+/K+-ATPase and V-type H+-ATPase transcript abundance and enzyme activities during acclimation to acidified conditions. Relative changes in Na+/K+-ATPase (NKA) (A) and V-type H+-ATPase (VHA) (B) transcript levels under control and acidified conditions. Transcript levels were normalized to arginine kinase (AK) as an internal control. NKA (C) and VHA (D) enzyme activities in control and low pH treated animals along the experimental period of 48 h. Combined presentation of relative changes in NKA (E) and VHA (F) mRNA levels and enzyme activities normalized to control animals. The exposure period is separated into a short-term (gray) and medium-term (white) acclimation period. Asterisks indicate significant differences between pH treatments (*p < 0.05 and **p < 0.001). Bars represent mean ± SE (n = 3–4).
Localization of Na+/K+-ATPase and V-Type H+-ATPase in Gill Epithelia
Immunohistochemical analyses demonstrate the sub-cellular localization of Na+/K+-ATPase and V-type H+-ATPase in gill epithelia of posterior gills from control animals (Figure 4A). Using double staining, high concentrations of Na+/K+-ATPase were detected in basolateral membranes of the entire gill epithelium as well as in pillar cells spanning between the two epithelial layers (Figure 4A). V-type H+-ATPase was predominantly located in the cytoplasm of epithelial- and pillar- cells. In contrast to the distribution of NKA in cells of the entire gill lamella, VHA immunoreactivity is only observed in single cells, predominantly pillar cells. Negative controls performed by omitting the primary antibody, did not show any signal in posterior gill lamellae, supporting the specificity of the primary antibodies used (Figure S3). Western blot analyses demonstrate specific immunoreactivity of antibodies with proteins including Na+/K+-ATPase (≈110 kDa) and V-type H+-ATPase (≈70 kDa; Figure 4B). Western blot analyses of posterior gills of pH 8.0 and 6.5 treated crabs demonstrates increased NKA as well as VHA protein concentrations in low pH treated animals during the acute low pH acclimation phase (Figure 4C). NKA protein concentrations in the acute acclimation phase (12 h) are 1.4-fold higher whereas VHA protein concentrations of posterior gills are increased by 3-fold in low pH treated animals compared to pH 8.0 acclimated animals.
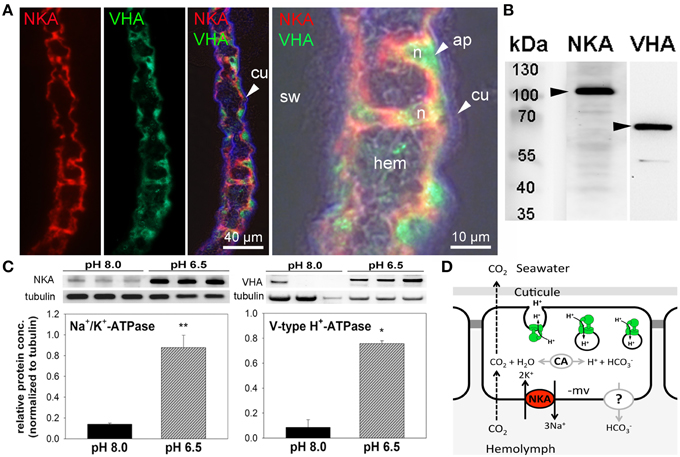
Figure 4. Immuno-histochemical localization of Na+/K+-ATPase and V-type H+-ATPase in branchial epithelia. Positive Na+/K+-ATPase (NKA) (red) immunoreactivity in basolateral membranes of the 5th gill pair of a control (pH 8) animal (A). Positive immunoreactivity of the V-type H+-ATPase (VHA) (green) antibody is mainly located in the cytoplasm of single epithelial as well as pillar cells. Background fluorescence (blue), showing the lining of the cuticule along the gill lamellae. Larger magnification of one branchial lamella demonstrating the sub cellular localization of the NKA and VHA including a bright field overlay to visualize the histology of this tissue (A). Western blot analyses using gill homogenates, indicating specific immunoreactivity of the different antibodies with proteins in the predicted size range (B). Determination of NKA and VHA protein concentrations normalized to tubulin in posterior gills after 12 h exposure to pH 8.0 and 6.5 conditions (*p < 0.05; **p < 0.01) (C). Hypothetical model of acid-base regulation in epithelial cells of posterior gills in X. testudinatus (D). NKA located in basolateral membranes energizes the import of bicarbonate via a putative basolateral transporter. CO2 diffuses across membranes along concentration gradients. Intracellular carbonic anhydrase (CAc) facilitates the formation of and protons. Based on the findings of the present work it is speculated that cytoplasmic VHA is involved in the acidification of vesicles and protons are exocytosed across the apical membrane. Cu, cuticula; hem, hemolymph; sw, sea water; n, nucleus; ap, apical.
Discussion
Acid-Base Regulation in the Vent Crab Xenograpsus testudinatus
Substantial acid-base regulatory abilities are a characteristic of many active marine organisms including fish, cephalopods, and crustaceans (Cameron, 1978; Heisler, 1986; Pörtner et al., 1991; Melzner et al., 2009; Gutowska et al., 2010; Henry et al., 2012). In these taxa, buffering of extracellular pH (pHe) is associated with an increase in blood levels, which is a conserved and efficient mechanism to counter respiratory acidosis (Heisler, 1986; Pörtner et al., 1991; Gutowska et al., 2010; Henry et al., 2012). The present work demonstrates powerful extracellular acid-base regulatory abilities of X. testudinatus that are beneficial for this species to inhabit highly acidic hydrothermal vent habitats over long time scales. The hyperbolic increase in blood levels of X. testudinatus in response to acidified conditions is in general accordance with findings for other strong acid-base regulators (Heisler, 1986). For example, most teleosts can rapidly and fully compensate pHe during moderate to strong hypercapnia, accompanied by an increase in blood [HCO3−] in excess of 20 to 30 mM (Larsen et al., 1997; Perry et al., 2010). While cephalopods were characterized to have moderate acid-base regulatory abilities (Gutowska et al., 2010; Hu et al., 2014), most brachyuran crabs were described to be moderate to strong acid-base regulators (Cameron, 1978; Truchot, 1984; Pane and Barry, 2007; Spicer et al., 2007). Detailed investigations of extracellular acid-base parameters during hypercapnic exposure in crustaceans are restricted to a few species, including the European shore crab, C. maenas (Truchot, 1984), the blue crab, Callinectes sapidus (Cameron, 1978), Cancer magister, Chionoecetes tanneri (Pane and Barry, 2007), and the velvet swimming crab, Necora puber (Spicer et al., 2007). All species showed an initial depression of extracellular pH over the course of 4 h, which was partially restored over the next 24–48 h through an active accumulation of hemolymph . Such a marked acidosis in the acute acclimation phase (0–12 h) was not observed in X. testudinatus exposed to pH 6.5 (1.78 kPa pCO2), a level greatly in excess of those experimental conditions under which C. maenas (0.57 kPa CO2; ≈pH 7.2), C. sapidus (1.0 kPa CO2; pH 7.08), C. magister and C. tanneri (1.0 kPa CO2; pH 7.08) were examined. As gills were demonstrated to be the major site for ion and acid-base regulation in decapod crustaceans, (Henry et al., 2012) the following paragraph focuses on the branchial mechanisms that mediate extracellular acid-base balance in the hydrothermal vent crab X. testudinatus.
Branchial Acid-Base Regulatory Mechanisms
Immunohistochemical analyses demonstrate the presence of Na+/K+-ATPase and V-type H+-ATPase in branchial epithelia of the hydrothermal vent crab X. testudinatus. These primary active ion-transporters are key players for intra- and extra-cellular regulation of ion and pH homeostasis in all animals (Emery et al., 1998; Tresguerres et al., 2005; Colina et al., 2007; Horng et al., 2009). The ubiquitous NKA located in basolateral membranes creates an electrochemical gradient that fuels secondary active transporters. For example, blocking of NKA in perfused gills of the crab Neohelice granulata inhibited secretion and H+ reabsorption indicating a central role in fueling secondary active transport mechanisms relevant for acid-base regulation (Tresguerres et al., 2008).
The sub cellular localization of VHA in branchial epithelia of marine organisms seems to be less conserved compared to the NKA. For example, in most teleosts, the VHA is located in apical membranes where it is believed to mediate the direct secretion of protons (Horng et al., 2007; Tsai and Lin, 2007; Hwang et al., 2011). However, in marine species like the teleost, Oryzias latipes (Lin et al., 2012) and the squid Sepioteuthis lessoniana the VHA is located in basolateral membranes of ion-regulatory epithelia where it is involved in acid-base regulatory processes as well (Hu et al., 2013). In most crustaceans, the VHA has been demonstrated to be localized in apical membranes and/or in the cytoplasm of branchial epithelial cells (Weihrauch et al., 2001; Tsai and Lin, 2007). The strong cytosolic but weak apical abundance of the VHA in branchial cells of X. testudinatus is in accordance with observations made on other marine and intertidal brachyuran crabs including Ocypodid crabs, Uca laceta, and Macrophthalmus spp. as well as the brachyuran crabs, Hemigrapsus sanguineus, H. penicillatus, Perisesarma bidens, and Chiromantes dehaani (Tsai and Lin, 2007). The cytoplasmic localization of the VHA has been hypothesized to be involved in the trapping of within acidified vesicles and subsequent exocytosis across the apical membrane (Weihrauch et al., 2002). As secretion of also results in a net export of protons, it is likely that excretion of nitrogenous waste products and acid-base regulation are linked processes.
Gene expression analyses and enzyme activity measurements in the present study indicate that branchial NKA and VHA are important players of acid-base regulation that mediate bicarbonate accumulation as well as H+ secretion in this hydrothermal vent species. In fish, cephalopods and crustaceans, the compensation of an extracellular acidosis by an accumulation of is always associated with a significant net export of protons (Heisler, 1984, 1986; Cameron, 1986). As formation through the hydration of CO2 is always associated with the generation of H+, H+ export pathways represent an essential feature in animals that compensate an extracellular acidosis. These observations are in line with the results of the present study, demonstrating that environmental acidification stimulates expression levels of branchial VHA that is involved in the secretion of proton equivalents. Moreover, basolateral NKA has been hypothesized to energize transport in crustacean branchial epithelia (Tresguerres et al., 2008). In this context a Na+/ co-transporter (NBC) located in basolateral membranes has been proposed for the branchial epithelium of Neohelice granulate based on pharmacological observations (Tresguerres et al., 2008). Other studies suggested that basolateral anion exchangers are involved in the re-absorption in crustacean gills (Freire et al., 2008; Harms et al., 2014). Although the existence, function and cellular localization of transporters from the SLC 4 family are not confirmed for X. testudinatus, this work strongly suggests that active re-absorption significantly contributes to extracellular pH homeostasis during environmental acidification. Active transport mechanism can be considered essential for the buffering since dissolution of the carapace has been demonstrated to represent only a minor contribution to extracellular accumulation in crustaceans (Cameron, 1985). According to this information, we propose a first model for acid-base regulatory mechanisms in gill epithelia of the hydrothermal vent crab, X. testudinatus (Figure 4D). The NKA energizes uptake via a currently not identified basolateral transporter and cytosolic carbonic anhydrase (CA). Based on a study by Weihrauch et al. (2002) we hypothesize that also in X. testudinatus cytoplasmic VHA is involved in countering an acidosis by pumping H+ ions into vesicles that exocytose protons or proton equivalents (e.g., ) across the apical membrane. Accordingly, during vesicle fusion at the apical membrane a certain fraction of the cellular VHA must be temporarily transferred to the apical plasma membrane as well. In order to support this hypothesis of vesicular proton excretion in this species future studies will compare the subcellular distribution of VHA-rich vesicles between control and low pH acclimated animals.
Based on the findings of the present work this model suggests that NKA seems to be involved in acid-base compensatory mechanisms by fueling additional secondary active ion-transporters. The electroneutral transport of acid-base equivalents (e.g., and H+) via anion and cation exchangers is directly connected to the transport of counter ions (e.g., Na+ and Cl−) that supports the link between acid-base and osmoregulation found in marine euryhaline decapod crustaceans (Truchot, 1981; Whiteley et al., 2001). Accordingly it is very likely that X. testudinatus utilizes Na+- and Cl−-dependent acid-base regulatory pathways via Na+/H+-exchangers (NHEs), and maybe also NBCs or anion exchangers that are fueled by the NKA. Using X. testudinatus as a model for a strong acid-base regulating crustacean further studies regarding the functional expression, enzyme abundance and subcellular localization of these secondary active transporters will provide important insights to the mechanistic connection between acid-base and osmotic regulation in decapod crustaceans.
Interestingly, the increase of the NKA and VHA on the mRNA level is only visible in the acute low pH acclimation phase, whereas enzyme activities increase with a short delay and stay elevated until the time point of 24 h. This observation reflects the time gap between mRNA expression and translation into the functional protein, which is in general accordance with findings in other eukaryotes as well as prokaryotes (Marsh et al., 2000; Glanemann et al., 2003). Although NKA and VHA enzyme activities return back to control levels after 24 h, blood levels remain increased after 48 h in the low pH treatment. This suggests, that initial accumulation of and the associated secretion of H+ are reflected in the up regulation of NKA and VHA mRNA and activity levels. However, after 24 h the pHe compensation process is completed and routine NKA and VHA activity levels are probably sufficient to maintain increased hemolymph levels to protect pHe homeostasis. This energy saving strategy of stabilizing hemolymph pH over long exposure times can be regarded as a key adaptation of X. testudinatus to occupy an ecological niche in a highly acidified hydrothermal vent habitat.
Conclusion
Strong acid-base regulatory abilities of X. testudinatus can be regarded an essential feature of this species to successfully inhabit a highly acidic hydrothermal vent environment. Functional and histological results of the present study demonstrated that extracellular acid-base regulatory mechanisms in X. testudinatus rely on a conserved set of ion pumps that are also found in other brachyuran crabs. In accordance to phylogenetic analyses based on mitochondrial genomes (Ki et al., 2009) and morphological systematics (Ng et al., 2000), amino acid sequence comparisons of the NKA demonstrate that X. testudinatus is closely related to other varunid and grapsid species including E. sinensis and Pachygrapsus marmoratus which are characterized as powerful osmo-regulators (Gross, 1961; Onken, 1999; Figure 5). NKA homologies also confirm molecular systematics demonstrating that deep-sea hydrothermal vent crabs of the genus Bythograea spp. (Mateos et al., 2012) constitute an own phylogenetic group within the crustacea (Figure 5). Despite having weak osmo-regulating abilities, Bythograea thermydron (Martinez et al., 2001) may be able to increase ATPase activities to increase H+ efflux rates to protect from strong pH fluctuations that are a characteristic of deep-sea vent habitats (Von Damm, 1995; Tivey, 2004). However, additional studies addressing the mechanistic basis of extracellular pH regulation in crustaceans from deep-sea vent systems will be needed to support this hypothesis. Such comparative studies between deep-sea and shallow-water hydrothermal vent crabs will provide a basis for new and exciting research regarding the evolution of pH regulatory systems in crustaceans that have the ability to occupy ecological niches in highly acidic hydrothermal vent habitats.
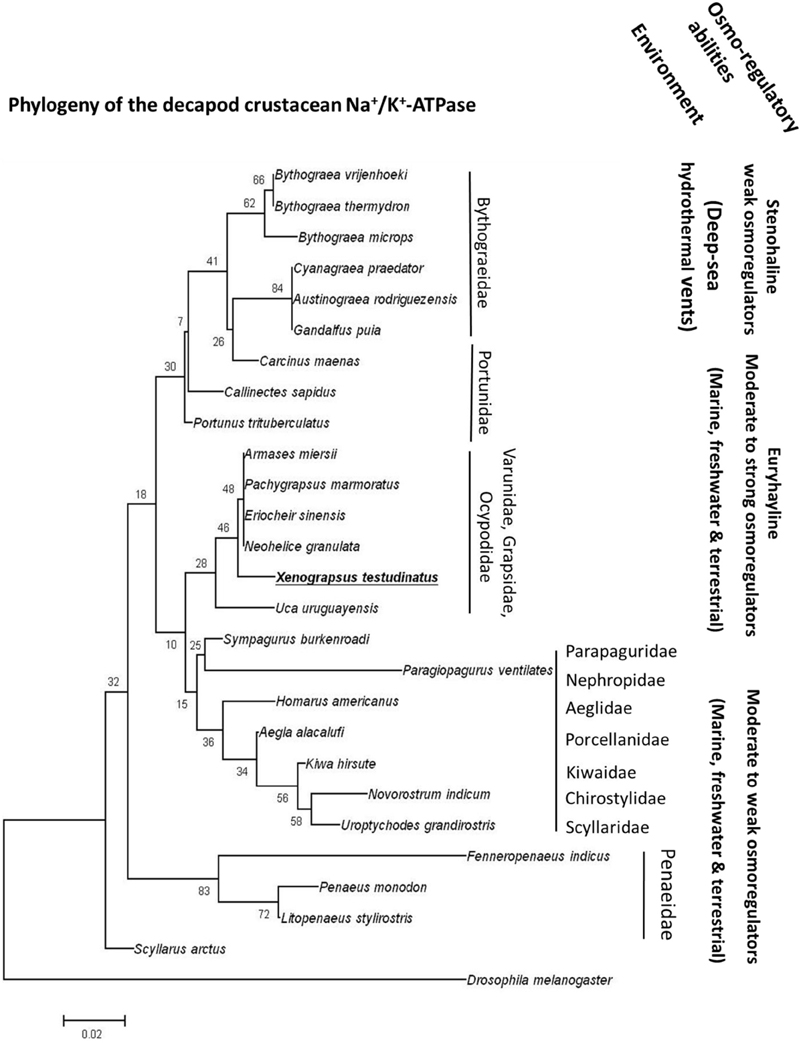
Figure 5. The phylogeny of crustacean Na+/K+-ATPases. Routed phylogenetic tree of deduced Na+/K+-ATPase amino acid sequences from different decapods crustaceans. Numbers indicate bootstrap values and accession numbers for the sequences are provided along with species names. Information regarding the habitat and osmoregulatory abilities of the different species is included in the phylogenetic tree.
Author Contributions
MH and YT designed and conducted experiment, analyzed the data, and compiled the main manuscript. MH conducted immunohistochemical experiments and evaluate enzyme activities. YS and MJ collected and acquired the animals and photos from open filed. YG and YT carried out the molecular cloning and expression studies. PK, GC, and JL conducted CO2 perturbation experiments, physiological measurements, and sample preparation. All authors reviewed and approved the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This study was financially supported by the grants to YT. YT from the Ministry of Science and Technology, Taiwan, Republic of China (MOST 104-2321-B-003-001), Alexander von Humbold/National Science Council (Taiwan) grant (NSC 102-2911-I-001-002-2), and a Cluster of Excellence “The future ocean” grant (CP1409) awarded to MH. We gratefully thank Mr. H. T. Lee (assistant in marine station of Institute of Cellular and Organismic Biology, Academia Sinica) for his assistance to maintain experimental systems.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fphys.2016.00014
References
Beyenbach, (2006). The V-type H+ ATPase: molecular structure and function, physiological roles and regulation. J. Exp. Biol. 209, 577–589. doi: 10.1242/jeb.02014
Bedford, J. J., and Leader, J. P. (1977). The composition of the hemolymph and muscle tissue of the shore crab, Hemigrapsus edwardsii, exposed to different salinities. Comp. Biochem. Physiol. A Physiol. 57A, 341–345. doi: 10.1016/0300-9629(77)90203-1
Cameron, J. N. (1978). Effects of hypercapnia on blood acid-base status, NaCl fluxes and trans-gill potential in freshwater blue crabs, Callinectes sapidus. J. Comp. Physiol. B 123, 137–141. doi: 10.1007/BF00687841
Cameron, J. N. (1985). Compensation of hypercapnic acidosis in the aquatic blue crab, Callinectes sapidus: the predominance of external sea water over carapace carbonate as the proton sink. J. Exp. Biol. 114, 197–206.
Charmantier, G., and Charmantier-Daures, M. (2001). Ontogeny of osmoregulation in crustaceans: the embryonic phase. Am. Zool 41, 1078–1089. doi: 10.1093/icb/41.5.1078
Chen, C-T. A., Wang, B. J., Huang, J., Lou, J., Kuo, F., and Tu, Y. (2005). Investigation into extremely acidic hydrothermal fluids off Kueishan Tao, Taiwan, China. Acta Oceanol. Sin. 24, 125–133.
Colina, C., Rosenthal, J. J. C., DeGiorgis, J. A., Srikumar, D., Iruku, N., and Holmgren, M. (2007). Structural basis of Na+/K+-ATPase adaptation to marine environments. Nat. Struct. Mol. Biol. 14, 427–431. doi: 10.1038/nsmb1237
Dickson, A., and Millero, F. (1987). A comparison of the equilibrium constants for the dissociation of carbonic acid in seawater media. Deep Sea Res. A 34, 1733–1743. doi: 10.1016/0198-0149(87)90021-5
Dittel, A. I., Perovich, G., and Epifanio, C. E. (2008). Biology of the vent crab Bythograea thermydron: a brief review. J. Shellfish Res. 27, 63–77. doi: 10.2983/0730-8000(2008)27[63:BOTVCB]2.0.CO;2
Dröse, S., and Altendorf, K. (1997). Bafilomycins and concanamycins as inhibitors of V-ATPases and P-ATPases. J. Exp. Biol. 200, 1–8.
Emery, A. M., Billingsley, P. F., Ready, P. D., and Djamgoz, M. B. A. (1998). Insect Na+/K+-ATPase. J. Insect. Physiol. 44, 197–209. doi: 10.1016/S0022-1910(97)00168-6
Fehsenfeld, S., Kiko, R., Appelhans, Y., Towle, D. W., Zimmer, M., and Melzner, F. (2011). Effects of elevated seawater pCO2 on gene expression patterns in the gills of the green crab, Carcinus maenas. BMC Genomics 12:488. doi: 10.1186/1471-2164-12-488
Frederich, M., Sartoris, F., and Pörtner, H.-O. (2001). Distribution patterns of decapod crustaceans in polar areas: a result of magnesium regulation? Polar Biol. 24, 719–723. doi: 10.1007/s003000100270
Freire, C. A., Onken, H., and McNamara, J. C. (2008). A structure-function analysis of ion transport in crustacean gills and excretory organs. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 151, 272–304. doi: 10.1016/j.cbpa.2007.05.008
Glanemann, C., Loos, A., Gorret, N., Willis, L. B., O'Brien, X. M., Lessard, P. A., et al. (2003). Disparity between changes in mRNA abundance and enzyme activity in Corynebacterium glutamicum: implications for DNA microarray analysis. Appl. Microbiol. Biotechnol. 61, 61–68. doi: 10.1007/s00253-002-1191-5
Goffredi, S., Childress, J., Desaulniers, N., Lallier, L. R., and Hammond, D. (1997). Inorganic carbon acquisition by the hydrothermal vent tubeworm Riftia pachyptila depends upon high P-CO2 and upon proton-equivalent ion transport by the worm. J. Exp. Biol. 200, 883–896.
Gross, W. J. (1961). Osmotic tolerance and regulation in crabs from a hypersaline lagoon. Biol. Bull. 121, 290–301. doi: 10.2307/1539433
Gutowska, M. A., Melzner, F., Langenbuch, M., Bock, C., Claireaux, G., and Pörtner, H. O. (2010). Acid-base regulatory ability of the cephalopod (Sepia officinalis) in response to environmental hypercapnia. J. Comp. Physiol. B 180, 323–335. doi: 10.1007/s00360-009-0412-y
Han, C., Ye, Y., Pan, Y., Qin, H., Wu, G., and Chen, C.-T. A. (2014). Spatial distribution pattern of seafloor hydrothermal vents to the southeastern Kueishan Tao offshore Taiwan Island. Acta Oceanol. Sin. 33, 37–44. doi: 10.1007/s13131-014-0405-x
Harms, L., Frickenhaus, S., Schiffer, M., Mark, F. C., Storch, D., Held, C., et al. (2014). Gene expression profiling in gills of the great spider crab Hyas araneus in response to ocean acidification and warming. BMC Genomics 15:789. doi: 10.1186/1471-2164-15-789
Henry, R. P., and Cameron, J. N. (1982). Acid-base balance in Callinectes sapidus during acclimation from high to low salinity. J. Exp. Biol. 101, 255–264.
Henry, R. P., Lucu, C., Onken, H., and Weihrauch, D. (2012). Multiple functions of the crustacean gill: osmotic/ionic reglation, acid-base balance, ammonia excretion, and bioaccumulation of toxic metals. Front Physiol 3:431. doi: 10.3389/fphys.2012.00431
Hicks, G. F. R. (1973). Combined effects of temperature and salinity on Hemigrapsus edwardsi (Hilgendorf) and H. crenulatus (Milne Edwards) from Wellington Harbour, New Zealand. J. Exp. Mar. Biol. Ecol. 13, 1–14. doi: 10.1016/0022-0981(73)90042-7
Horng, J.-L., Lin, L.-Y., Huang, C.-J., Katoh, F., Kaneko, T., and Hwang P.-P. (2007). Knockdown of V-ATPase subunit A (atp6v1a) impairs acid secretion and ion balance in zebrafish (Danio rerio). Am. J. Physiol. Regul. Integr. Comp. Physiol. 292, R2068–R2076. doi: 10.1152/ajpregu.00578.2006
Horng, J. L., Lin, L. Y., and Hwang, P. P. (2009). Functional regulation of H+-ATPase-rich cells in zebrafish embryos acclimated to an acidic environment. Am. J. Physiol. Cell Physiol. 296, c682–c692. doi: 10.1152/ajpcell.00576.2008
Hu, M. Y., Guh, Y.-J., Stumpp, M., Lee, J.-R., Chen, R.-D., Sung, P.-H., et al. (2014). Branchial NH-dependent acid-base transport mechanisms and energy metabolism of squid (Sepioteuthis lessoniana) affected by seawater acidification. Front. Zool. 11:55. doi: 10.1186/s12983-014-0055-z
Hu, M. Y., Lee, J.-R., Lin, L.-Y., Shih, T.-H., Stumpp, M., Lee, M.-F., et al. (2013). Development in a naturally acidified environment: Na+/H+-exchanger 3-based proton secretion leads to CO2 tolerance in cephalopod embryos. Front. Zool. 10:51. doi: 10.1186/1742-9994-10-51
Hwang, P. P. (2009). Ion uptake and acid secretion in zebrafish (Danio rerio). J. Exp. Biol. 212, 1745–1752. doi: 10.1242/jeb.026054
Hwang, P. P., Lee, T. H., and Lin, L. Y. (2011). Ion regulation in fish gills: recent progress in the cellular and molecular mechanisms. Am. J. Physiol. Regul. Integr. Comp. Physiol. 301, R28–R47. doi: 10.1152/ajpregu.00047.2011
Jeng, M. S., Ng, N. K., and Ng, P. K. L. (2004). Feeding behaviour: hydrothermal vent crabs feast on sea “snow.” Nature 432:969. doi: 10.1038/432969a
Joel, W. M., and Haney, T. A. (2005). Decapod crustaceans from hydrothermal vents and cold seeps: a review through 2005. Zool. J. Linn. Soc. 145, 445–522. doi: 10.1111/j.1096-3642.2005.00178.x
Ki, J.-S., Dahms, H.-U., Hwang, J.-S., and Lee, J.-S. (2009). The complete mitogenome of the hydrothermal vent crab Xenograpsus testudinatus (Decapoda, Brachyura) and comparison with brachyuran crabs. Comp. Biochem. Physiol. D Geonomics Proteomics 4, 290–299. doi: 10.1016/j.cbd.2009.07.002
Larsen, B. K., Pörtner, H.-O., and Jensen, F. B. (1997). Extra- and intracellular acid-base balance and ionic regulation in cod (Gadus morhua) during combined and isolated exposures to hypercapnia and copper. Mar. Biol. 128, 337–346. doi: 10.1007/s002270050099
Lewis, E., and Wallace, D. W. R. (1998). Program Developed for CO2 System Calculations. Oak Ridge, TN: Oak Ridge National Laboratory. ORNL/CDIAC-105.
Lin, C.-C., Lin, L.-Y., Hsu, H.-H., Thermes, V., Prunet, P., Horng, J.-L., et al. (2012). Acid secretion by mitochondrion-rich cells of medaka (Oryzias latipes) acclimated to acidic freshwater. Am. J. Physiol. Integr. Comp. Physiol. 15, R283–R291. doi: 10.1152/ajpregu.00483.2011
Marsh, A. G., Leong, P. K. K., and Manahan, T. (2000). Gene expression and enzyme activities of the sodium pump during sea urchin development: implications for indices of physiological state. Biol. Bull. 199, 100–107. doi: 10.2307/1542869
Martinez, A.-S., Toullec, J.-Y., Shillito, B., Charmantier-Daures, M., and Charmantier, G. (2001). Hydromineral regulation in the hydrothermal vent crab Bythograea thermydron. Biol. Bull. 201, 167–174. doi: 10.2307/1543331
Mateos, M., Hurtado, L. A., Santamaria, C. A., Leignel, V., and Guinot, D. (2012). Molecular systematics of the deep-sea hydrothermal vent endemic brachyuran family Bythograeidae: a comparison of three Bayesian species tree methods. PLoS ONE 7:e32066. doi: 10.1371/journal.pone.0032066
Mehrbach, C., Culberso, C., Hawley, J., and Pytkowic, R. (1973). Measurement of apparent dissociation constants of carbonic acid in seawter at atmospheric pressure. Limnol. Oceanogr. 18, 897–907. doi: 10.4319/lo.1973.18.6.0897
Melzner, F., Gutowska, M. A., Langenbuch, M., Dupont, S., Lucassen, M., Thorndyke, M. C., et al. (2009). Physiological basis for high CO2 tolerance in marine ectothermic animals: pre-adaptation through lifestyle and ontogeny? Biogeosciences 6, 2313–2331. doi: 10.5194/bg-6-2313-2009
Morris, J. F., Ismail-Beigi, F., Butler, V. P. Jr., Gati, I., and Lichtstein, D. (1997). Ouabain-sensitive Na+, K(+)-ATPase activity in toad brain. Comp. Biochem. Physiol. A Physiol. 118, 599–606. doi: 10.1016/S0300-9629(96)00465-3
Ng, N. K., Huang, J., and Ho, P.-H. (2000). Description of a new species of hydrothermal crab, Xenograpsus testudinatus (Crustacea: Decapoda: Brachyura: Grapsidae) from Taiwan. Natl. Taiwan Mus. Publ. Ser. 10, 191–199.
Onken, H. (1999). Active NaCl absorption across split lamellae of posterior gills of Chinese crabs (Eriocheir sinensis) adapted to different salinities. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 123, 377–384. doi: 10.1016/S1095-6433(99)00078-1
Onken, H., and Graszynski, K. (1989). Active Cl− absorption by the Chinese crab Eriocheir sinensis gill epithelium measured by potential difference. J. Comp. Physiol. B 159, 21–28. doi: 10.1007/BF00692679
Pane, E. F., and Barry, J. P. (2007). Extracellular acid-base regulation during short-term hypercapnia is effective in a shallow-water crab, but ineffective in a deep-sea crab. Mar. Ecol. Prog. Ser. 334, 1–9. doi: 10.3354/meps334001
Perry, S. F., Braun, M. H., Genz, J., Vulesevic, B., Taylor, J., Grosell, M., et al. (2010). Acid-base regulation in the plainfin midshipman (Porichthys notatus): an aglomerular marine teleost. J. Comp. Physiol. B 180, 1213–1225. doi: 10.1007/s00360-010-0492-8
Pörtner, H.-O., Webber, D. M., Boutilier, R. G., and O'Dor, R. K. (1991). Acid-base regulation in exercising squid (Illex illecebrosus, Loligo pealei). Am. J. Physiol. Regul. Integr. Comp. Physiol. 261, R239–R246.
Ramirez-Llodra, A., Shank, T. M., and German, C. R. (2007). Biodiversity and biogeography of hydrothermal vent species. Oceanography 20, 30–41. doi: 10.5670/oceanog.2007.78
Sarazin, G., Michard, G., and Prevot, F. (1999). A rapid and accurate spectroscopic method for alkalinity measurements in seawater samples. Water Res. 33, 290–294. doi: 10.1016/S0043-1354(98)00168-7
Schneider, C. A., Rasband, W. S., and Eliceiri, K. W. (2012). NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675. doi: 10.1038/nmeth.2089
Schubart, C. D., Diesel, R., and Hedges, S. B. (1997). Rapid evolution to terrestrial life in Jamaican crabs. Nature 393, 363–365. doi: 10.1038/30724
Spicer, J. I., Raffo, A., and Widdicombe, S. (2007). Influence of CO2-related seawater acidification on extracellular acid-base balance in the velvet swimming crab Necora puber. Mar. Biol. 151, 1117–1125. doi: 10.1007/s00227-006-0551-6
Tresguerres, M., Katoh, F., Fenton, H., Jasinska, E., and Goss, G. G. (2005). Regulation of branchial V-H+-ATPase, Na+/K+-ATPase and NHE2 in response to acid and base infusions in the Pacific spiny dogfish (Squalus acanthias). J. Exp. Biol. 208, 345–354. doi: 10.1242/jeb.01382
Tresguerres, M., Parks, S. K., Katoh, F., and Goss, G. G. (2006). Microtubule-dependent relocation of branchial V-H+-ATPase to the basolateral membrane in the Pacific spiny dogfish (Squalus acanthias): a role in base secretion. J. Exp. Biol. 209, 599–609. doi: 10.1242/jeb.02059
Tresguerres, M., Parks, S. K., Sabatini, S. E., Goss, G. G., and Luquet, C. M. (2008). Regulation of ion transport by pH and [HCO3-] in isolated gills of the crab Neohelice (Chasmagnathus) granulata. Am. J. Physiol. Regul. Integr. Comp. Physiol. 294, R1033–R1043. doi: 10.1152/ajpregu.00516.2007
Truchot, J. P. (1976). Carbon dioxide combining properties of the blood of the shore crab Carcinus maenas (L.): carbon dioxide solubility coefficient and carbonic acid- dissociation constants. J. Exp. Biol. 64, 45–57.
Truchot, J. P. (1981). The effect of water salinity and acid-base status on the blood balance in the euryhaline crab, Carcinus maenas (L.). Comp. Biochem. Physiol. 68, 555–561. doi: 10.1016/0300-9629(81)90361-3
Truchot, J. P. (1984). Water carbonate alkalinity as a determinant of hemolymph acid-base balance in the shore crab, Carcinus maenas - a study at two different ambient pCO2 and O2 levels. J. Comp. Physiol. 154, 601–606. doi: 10.1007/BF00684414
Truchot, J. P. (1992). Acid-base changes on transfer between sea water and freshwater in the Chinese crab, Eriocheir sinensis. Respir. Physiol. 87, 419–427. doi: 10.1016/0034-5687(92)90022-O
Tsai, J.-R., and Lin, H.-C. (2007). V-type H+-ATPase and Na+,K+-ATPase in the gills of 13 euryhaline crabs during salinity acclimation. J. Exp. Biol. 210, 620–627. doi: 10.1242/jeb.02684
Von Damm, K. L. (1995). Physical, Chemical, Biological, and Geological Interactions within Seafloow Hydrothermal Systems. Washington, DC: American Geophysical Union.
Weihrauch, D., Ziegler, A., Siebers, D., and Towle, D. W. (2001). Molecular characterization of V-type H(+)-ATPase (B-subunit) in gills of euryhaline crabs and its physiological role in osmoregulatory ion uptake. J. Exp. Biol. 204, 25–37.
Weihrauch, D., Ziegler, A., Siebers, D., and Towle, D. W. (2002). Active ammonia excretion across the gills of the green shore crab Carcinus maenas: participation of Na+/K+-ATPase, V-type H+-ATPase and functional microtubules J. Exp. Biol. 205, 2765–2775.
Keywords: hydrothermal vent, V-type H+-ATPase, Na+/K+-ATPase, hypercapnia, invertebrate physiology, gill, crustacean
Citation: Hu MY, Guh Y-J, Shao Y-T, Kuan P-L, Chen G-L, Lee J-R, Jeng M-S and Tseng Y-C (2016) Strong Ion Regulatory Abilities Enable the Crab Xenograpsus testudinatus to Inhabit Highly Acidified Marine Vent Systems. Front. Physiol. 7:14. doi: 10.3389/fphys.2016.00014
Received: 01 August 2015; Accepted: 11 January 2016;
Published: 01 February 2016.
Edited by:
Antonio L'Abbate, Scuola Superiore Sant'Anna, ItalyReviewed by:
Nia M. Whiteley, Bangor University, UKMichael B. Morris, The University of Sydney, Australia
Copyright © 2016 Hu, Guh, Shao, Kuan, Chen, Lee, Jeng and Tseng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yung-Che Tseng, eWN0QG50bnUuZWR1LnR3
 Marian Y. Hu
Marian Y. Hu Ying-Jey Guh
Ying-Jey Guh Yi-Ta Shao4
Yi-Ta Shao4 Jay-Ron Lee
Jay-Ron Lee Yung-Che Tseng
Yung-Che Tseng