
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Physiol. , 20 October 2015
Sec. Vascular Physiology
Volume 6 - 2015 | https://doi.org/10.3389/fphys.2015.00290
This article is part of the Research Topic The Vascular Niche in Tissue Repair: A Therapeutic Target for Regeneration View all 15 articles
Platelet degranulation allows the release of a large amount of soluble mediators, is an essential step for wound healing initiation, and stimulates clotting, and angiogenesis. The latter process is one of the most critical biological events observed during tissue repair, increasing the growth of blood vessels in the maturing wound. Angiogenesis requires the action of a variety of growth factors that act in an appropriate physiological ratio to assure functional blood vessel restoration. Platelets release main regulators of angiogenesis: Vascular Endothelial Growth Factors (VEGFs), basic fibroblast growth factor (FGF-2), and Platelet derived growth factors (PDGFs), among others. In order to stimulate tissue repair, platelet derived fractions have been used as an autologous source of growth factors and biomolecules, namely Platelet Rich Plasma (PRP), Platelet Poor Plasma (PPP), and Platelet Rich Fibrin (PRF). The continuous release of these growth factors has been proposed to promote angiogenesis both in vitro and in vivo. Considering the existence of clinical trials currently evaluating the efficacy of autologous PRP, the present review analyses fundamental questions regarding the putative role of platelet derived fractions as regulators of angiogenesis and evaluates the possible clinical implications of these formulations.
Wound healing, a natural restorative response to tissue injury, is governed by an elaborate response driven by resident and circulating cells, homing to the injury site, that release soluble mediators or signals generated from the extracellular matrix (ECM; Guo and DiPietro, 2010). In adult humans, optimal wound healing involves a cascade of complex, orderly, and predictable events that include four overlapping phases: hemostasis, inflammation, proliferation, and remodeling. The adequate timing of these phases is decisive for ultimate restoration of the vascular system (Gosain and DiPietro, 2004; Eming et al., 2007). Platelets regulate hemostasis through vascular obliteration and fibrin clot formation (Guo and DiPietro, 2010). Platelets are anucleated cell fragments that originate from megakaryocytes in the bone marrow (Speth et al., 2015). Among the three-reservoir organelles described in platelets, namely lysosomes, alpha granules, and dense granules, the biggest compartments for protein storage are alpha granules. The latter are considered key organelles with respect to platelet function. In the clot, platelets are responsible for the activation and release of important biomolecules from their alpha granules, including platelet-specific proteins, growth factors, coagulation factors, adhesion molecules, cytokines, angiogenic factors, proteoglycans, and cytokines/chemokines (Nurden, 2011). The release of cytokines, chemokines, and growth factors induces proliferation and activation of the cells that are involved in wound healing such as fibroblasts, neutrophils, monocytes, smooth muscle cells, and mesenchymal stem cells (MSC) (Thushara et al., 2015).
Biological products for wound treatment and surgical interventions have been an area of enormous growth in the last two decades, as our understanding of wound healing response has increased. In particular, the use of human plasmatic fractions has been proposed to locally deliver platelet-derived factors as an autologous source of biomolecules for tissue healing. In this review, we summarize (1) the importance of growth factors and biomolecules related to angiogenesis present in plasmatic fractions with different concentrations of platelets and (2) the clinical rationale for their use in cell therapies involved in the treatment of traumatic injuries as well as degenerative diseases.
After the inflammatory phase has been initiated, the wound healing response requires angiogenesis as a process that modulates the activation, proliferation, and migration of endothelial cells to establish new blood vessels from pre-existing vasculature (Oklu et al., 2010). Platelets play a critical role in regulating angiogenesis. Nevertheless, their contribution to blood vessel repair in the course of wound healing is still poorly understood (Eming et al., 2007; Klement et al., 2013). Alpha granules are a reservoir of biological factors for platelet physiological and pathological angiogenic responses (Peterson et al., 2010; Table 1). The release of these crucial angiogenic factors in platelet derived fraction preparations could be useful in tissue regeneration and wound healing.
The use of platelet-derived fractions in tissue repair is a developing area for clinician's and researchers. Marx et al. demonstrated a potential use of Platelet Rich Plasma (PRP) in craniofacial bone grafts in the late nineties (Marx et al., 1998), and since then, plasmatic fractions have been promoted as suitable sources of autologous growth factors. PRP may be defined as a component of plasma fraction of autologous venous blood with platelet counts in the range between 4 and 6 times above baselines considered to be of therapeutic benefit (1 million platelets/L; Chen and Liu, in press). The preparation of PRP by centrifugation was initially completed by a “two-step gradient centrifugation method.” A strong first spin was used first in order to separate the erythrocytes from the clotting factors, platelets, and leukocytes. Then, the plasma was subjected to a second centrifugation step, to harvest the PRP fraction from the platelets and leukocytes. Finally, platelets in PRP were activated to release the biomolecules, using thrombin or calcium chloride. Other authors have proposed alternative methods to liberate growth factors from platelets, such as lysing the platelets by freezing them or using sonication or ultrasound (Weed et al., 2004). Once the release of biomolecules from platelets is activated, a network is formed to establish a fibrin clot that acts as scaffold for growth factors over a limited period of time (Mautner et al., 2015).
Nowadays most of the commercially accessible kits involve a one-step method to separate the plasma into three distinct layers: the erythrocytes, the buffy coat containing PRP, and the Platelet Poor Plasma (PPP) (Bausset et al., 2012; Burnouf et al., 2013). Currently, plasmatic fractions have been classified according to at least two key parameters: the presence of leukocytes and the fibrin architecture (Dohan Ehrenfest et al., 2014). Following these criteria, we can find four family fractions:
Pure Platelet-rich Plasma (P-PRP) low or without Leukocytes: Plasmatic preparations from anticoagulated venous blood “without leukocytes and with a low- density fibrin network.” The white blood cell count of these samples is less than the whole blood percentage (Riboh et al., 2015). After its activation with calcium chloride or thrombin, this preparation can be used as a liquid solution or as a gel (Gobbi and Vitale, 2012; Dohan Ehrenfest et al., 2014; Mautner et al., 2015).
Platelet-rich Plasma (L-PRP) with Leukocytes: Plasmatic fractions from anticoagulated venous blood “with leukocytes and with a low-density fibrin network.” The leukocytes content in these preparations is at least five times compared with the base line of whole blood counting (Filardo et al., 2013). Following activation, L-PRP might be used either as a liquid solution or in an activated gel form. Among the commercial systems available are Harvest Smart- PreP (Harvest Technologies, Plymouth, MA, USA), Biomet GPS III (Biomet Inc., Warsaw, IN, USA), Plateltex (Prague, Czech Republic), and Regen PRP (RegenLab, Le Mont-sur-Lausanne, Switzerland; Gobbi and Vitale, 2012; Dohan Ehrenfest et al., 2014; Mautner et al., 2015).
Pure Platelet-rich Fibrin (P-PRF) low or without Leukocytes: These correspond to “preparations without leukocytes and with a high-density fibrin network.” Fibrin, in combination with growth factors, has been shown to effectively support cell adhesion and proliferation. P-PRF only exists in a solid activated gel form. To date, only one product of this family is commercially available, known as Fibrinet PRFM (Platelet- Rich Fibrin Matrix, Cascade Medical, Wayne, NJ, USA; Gobbi and Vitale, 2012; Dohan Ehrenfest et al., 2014; Mautner et al., 2015).
Platelet-rich Fibrin (L-PRF) with Leukocytes: Also named Choukroun's PRF. In this preparation venous blood is obtained without any anticoagulant and directly centrifuged. A cascade of calcium chloride or thrombin is used, which results in the isolation of this plasmatic fraction without any biochemical modifications. These preparations, existing only in gel form, have leukocytes and a high-density fibrin network (Dohan Ehrenfest et al., 2009, 2014; Gobbi and Vitale, 2012; Mautner et al., 2015).
For a complete and updated overview of platelet-derived fractions and the requirements for their preparation for clinical use, please refer to the following other excellent reviews: De Pascale et al. (2015), Kawase (2015), and Mautner et al. (2015).
Diverse growth factors are involved in the process of angiogenesis, of which many are secreted by platelets (Peterson et al., 2010). Given the critical role of angiogenesis in modulating wound healing and considering that platelet-derived factors are critical for vascular activation and stabilization, it is tempting to speculate whether any of the platelet-derived formulations currently used in regenerative medicine stimulate angiogenesis. Preclinical studies, using in vitro assays and animal models have suggested a positive influence of platelet-derived fractions on angiogenesis.
In order to identify the mechanisms whereby platelet-derived fractions may stimulate angiogenesis, Bertrand-Duchesne et al. (2010) evaluated the presence of angiogenic growth factors in PRP samples. They detected high levels of VEGF, PDGF-BB, EGF, and basic fibroblast growth factor (bFGF). PRP supernatants were incubated with blocking antibodies to neutralize each of these growth factors and the response to these treatments was evaluated via proliferation assays in Human Umbilical Vein Endothelial Cells (HUVEC). Notably, the use of EGF neutralizing antibodies decreased significantly the proliferation of HUVEC, while the other antibodies did not affect this response. Another mechanistic study by Mammoto et al. evaluated the role of angiopoietin-1 in driving the vascular response in mouse PRP and PRF formulations. They found that mouse platelet derived fractions containing angiopoietin-1 (Ang) were responsible for increasing proliferation, migration, and differentiation of human microvascular endothelial cells (Mammoto et al., 2013). Li et al. (2014) observed that PRP might promote vascular growth andstimulate endothelial progenitor cells to form vessel-like structures. Anitua et al. (2015) recently studied the effect of a platelet concentrate displayed within a plasma suspension that forms a fibrin matrix system on angiogenesis. This product, called PRGF (plasma rich in growth factors) (P-PRP low leukoyte content), stimulated an increase in cell proliferation and a reduction in apoptosis in primary HUVEC and skeletal myoblasts.
In a recent study, Zhou et al. (2013) investigated the effect of the application of a PRP gel in open abdominal wounds performed in rats. After inducing a peritonitis lesion they performed laparotomies and animals were treated with either PPP or PRP. After 1 week of healing, the animals treated with PRP demonstrated higher blood perfusion in the original lesion as well as a more mature granulation tissue when compared to those treated with PPP. In addition, injection of the product within the injured muscle tissue of mice induced the reperfusion of blood into the lesion. Using an innovative approach to release platelet-derived growth factors in a drug-delivery system, Notodihardjo et al. (2015) used a gelatin hydrogel compound coupled to platelet rich plasma molecules to stimulate wound healing. They observed an increase in epithelialization and vascular growth when compared to other treatments, including the gelatin hydrogel system (drug delivery system) and the PRP group. In another approach to stabilize growth factors released from platelets, a fragmine-protamine micro-nanoparticle system was used to promote healing events of skin grafts showing a positive effect on wound epithelialization and angiogenesis (Takabayashi et al., 2015). Using autologous activated platelet supernatant; Kang et al. (2014) studied their effects on vasculogenesis in peripheral blood stem cells of human origin. With this approach they observed that stem cells primed with this platelet fraction stimulated vascular growth in athymic mice (Kang et al., 2014). In skin ulcers performed in porcine, Roy et al. (2011) showed that a platelet-rich fibrin matrix was able to stimulate wound healing by enhancing angiogenesis. In order to stimulate tendon regeneration, tendon healing was studied in New Zealand rabbits in which Achilles tendons were sectioned and subsequently treated with PRP or saline. PRP treated-tendons demonstrated increased angiogenesis and better collagen fiber re-alignment when compared to saline treated specimens (Lyras et al., 2009). To this end, Mammoto et al. (2013) identified that angiopoietin-1 is highly represented in PRP. Moreover, they observed that inhibition of angiopoietin-1-Tie2 signaling was able to suppress the angiogenic induction by a platelet-rich fibrin matrix in vivo. These studies show that in general, platelet-derived fractions may exert a potent pro- angiogenic response in different organs or anatomical locations and clearly justify further research.
As stated by Vishwakarma et al. (2015): “Tissue engineering and regenerative medicine is a rapidly growing multidisciplinary field involving the life, physical, and engineering sciences that seeks to repair, regenerate, or replace biological cell, tissue, and organ substitutes that have been lost due to congenital abnormalities, injury, disease, or aging.” The development of tissue-engineered products must involve a vascular support. Cellular function and viability are highly dependent on the effective diffusive exchange of nutrients through tissue. In vivo, cells are found within 200 μm-away from the nearest capillary network, otherwise they may suffer from ischemia and necrosis. Most of the tissue engineering scaffolds and composites are typically avascular. Therefore, it is essential that revascularization strategies stimulate regeneration of vascular networks in order to obtain a successful clinical outcome of an implanted cell-construct (Upputuri et al., 2015). The scaffolds need to support cell proliferation and differentiation to replace specific tissue loss in vivo. However, they must also provide a suitable substrate that allows adequate blood vessel growth to supply nutrients and oxygen to the cells located inside this engineered composite (Cenni et al., 2011). Considering the essential role of angiogenesis during the tissue engineering process, it is important to evaluate the scaffold features and properties to predict its vascularization potential and its possible interactions with endothelial and stem cells (Cenni et al., 2011). As already mentioned, plasmatic fractions constitute a source of main angiogenic growth factors, such as PDGF, VEGF, FGF-2, and EGF, as well as other proteins involved in the angiogenic process. Platelet angiogenic potential also resides in the presence of cytokines, integrins, hepatocyte growth factor, interleukin-8 (IL-8), IL-3, αvβ3− integrin 212, and matrix metalloproteinases (MMPs), which degrade ECM facilitating endothelial cell migration (Cenni et al., 2011; Klement et al., 2013). It is also important to note that the use of platelet-derived fractions has a considerable advantage, offering multiple pro-angiogenic factors compared to the application of high doses of recombinant growth factors to the wound site. Even the use of PPP could be a good alternative to the use of autologous growth factors. Recently, our research team evidenced the presence of angiogenic factors and biomolecules related to bone differentiation on PPP (Martínez et al., 2015). These results, combined with preclinical data provided by other research teams, suggest that (1) this PPP fraction could potentially be considered a good alternative to the use of autologous growth factors and proteins in combination with tissue engineering scaffolds and (2) that it might present an opportunity to increase the effectiveness of treatments in clinical applications (Yilmaz et al., 2011; Hateyama et al., 2014; Martínez et al., 2015). Angiogenic factors found in platelet-derived fractions can participate in cellular events involved in angiogenesis (Figure 1, Agren et al., 2013; Amable et al., 2013; Martínez et al., 2015). The angiogenic process requires the proliferation, migration, and adequate differentiation of endothelial cells (EC). ECs, categorized as (1) migrating leading “tip” cells to guide the direction of new blood vessel formation and (2) trailing “stalk” cells to establish the lumen of the new vessel, are indispensable to the branching and stabilization of new blood vessels (Carmeliet and Jain, 2011, Figure 1). However, it is still unknown whether or not “leukocyte mediators” have an effect on angiogenesis in the platelet-derived fractions. Overall, the use of platelet-derived fractions is promising in the process of angiogenesis during wound healing and regeneration. However, there is no consensus regarding the protocols utilized for their extraction, their effect on target cells, the concentration of the growth factors, and the effect of inflammatory mediators. Consensus needs to be reached in order to better exploit the clinical potential of platelet-derived fractions.
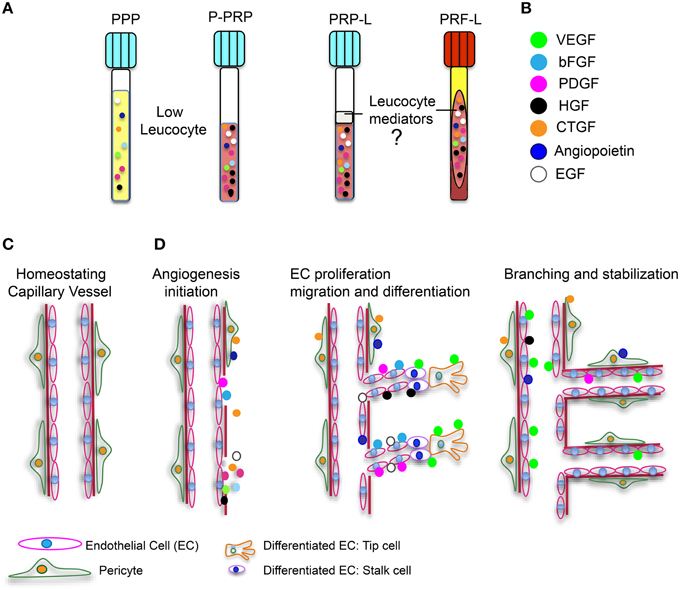
Figure 1. Platelet derived fractions and involvement of angiogenic growth factors in angionesis. (A) Four platelet-derived fractions are illustrated. Two with low leucocyte content (PPP and P-PRP), and two with high leucocyte quantities (L-PRP and L-PRF). (B) Angiogenic growth factors are represented with the indicated color code. (C) Illustration of a capillary blood vessel in physiological conditions. (D) Influence of angiogenic growth factors during angiogenesis initiation, endothelial cell (EC) proliferation, migration, and differentiation and finally, branching and stabilization of new blood vessels during a healing event.
Many preclinical and clinical studies have demonstrated the benefits of using MSC to promote tissue repair; MSC-based therapies are gaining ground in wound management. It has been shown that MSCs might repair damaged endothelium by secreting trophic factors that allow the recruitment of endogenous stem cells, which helps facilitate angiogenesis (Bronckaers et al., 2014). MSCs' activity depends on the instructive microenvironment or niche. To date, the positive influences of plasmatic fractions have been reported mainly in relation to PRP, proliferation, stemness, and preservation of the MSC immune-modulatory properties (Chieregato et al., 2011; Copland et al., 2013; for recent systematic review refer to Rubio-Azpeitia and Andia, 2014).
Additionally, translational medicine using MSC therapies requires protocols that can rule out the possibility of contamination or immunological reactions toward xenogeneic compounds (i.e., animal serum) used in traditional cell culture protocols (Lange et al., 2007; Goedecke et al., 2011). The use of fetal bovine serum in the maintenance of MSCs is undesirable because of viral/prion disease transmission risks that can initiate xenogeneic immune responses (Doucet et al., 2005; Even et al., 2006). Studies concerning bone marrow and periodontal ligament MSCs have shown that supplementing medium with autologous serum or platelet-derived fractions, instead of animal serum, is a good source of growth factors due to the fact that they provide sufficient ex vivo expansion, decrease the time required to reach confluence, increase the size of colony forming units, and maintain their osteogenic, chondrogenic, and adipogenic differentiation capability (Tonti and Mannello, 2008; Ben Azouna et al., 2012; Martínez et al., 2015). Advancement in stem cell research will help to reveal the intimate mechanisms and interactions between stem cells and platelet-derived growth factors in wounds.
From a therapeutic viewpoint, platelet concentrate seems to be quite promising. However, there is no consensus regarding their use. The application of platelet-derived fractions still demands standardization of its preparation, a more detailed characterization of their biomolecule composition and angiogenesis potential, as well as well-designed and controlled clinical trials. Several important questions regarding the timing of treatment and the actual impact of platelet fractions on restoring angiogenic activity remain to answer.
This work was supported by the National Fund for Scientific and Technological Development of The Chilean Government (FONDECYT) 11121294 (CM), (FONDECYT) 1130618 (PS), (FONDECYT) 1140697 (VP), and Fondo de Fomento al Desarrollo Científico y Tecnológico (FONDEF) D09E1047 (VP).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Agren, M. S., Rasmussen, K., Pakkenberg, B., and Jørgensen, B. (2013). Growth factor and proteinase profile of vivostat platelet-rich fibrin linked to tissue repair. Vox Sang. 107, 37–43. doi: 10.1111/vox.12120
Amable, P. R., Vierira Carias, R. B., TellesTexeira, M. V., Da Cruz Pacheco, I., Corrêa do Amaral, R. J. F., Granjeiro, J. M., et al. (2013). Platelet-rich plasma preparation for regenerative medicine: optimization and quantification of cytokines and growth factors. Stem Cell Res. Ther. 4, 67. doi: 10.1186/scrt218
Anitua, E., Pelacho, B., Prado, R., Aguirre, J. J., Sánchez, M., Padilla, S., et al. (2015). Infiltration of plasma rich in growth factors enhances in vivo angiogenesis and improves reperfusion and tissue remodeling after severe hind limb ischemia. J. Control. Release 28, 31–39. doi: 10.1016/j.jconrel.2015.01.029
Bausset, O., Giraudo, L., Veran, J., Magalon, J., Coudreuse, J. M., Magalon, G., et al. (2012). Formulation and storage of platelet-rich plasma homemade product. Biores. Open Access 1, 115–123. doi: 10.1089/biores.2012.0225
Ben Azouna, N., Jenhani, F., Regava, Z., Berraeis, L., Ben Othman, T., Ducrocq, E., et al. (2012). Phenotypical and functional characteristics of mesenchymal stem cells from bone marrow: comparison of culture using different media supplemented with human platelet lysate or fetal bovine serum. Stem Cell Res. Ther. 14, 6. doi: 10.1186/scrt97
Bertrand-Duchesne, M. P., Grenier, D., and Gagnon, G. (2010). Epidermal growth factor released from platelet-rich plasma promotes endothelial cell proliferation in vitro. J. Periodont. Res. 45, 87–93. doi: 10.1111/j.1600-0765.2009.01205.x
Bronckaers, A., Hilkens, P., Martens, W., Gervois, P., Ratajczak, J., Struys, T., et al. (2014). Mesenchymal stem/stromal cells as a pharmacological and therapeutic approach to accelerate angiogénesis. Pharmacol. Ther. 143, 181–196. doi: 10.1016/j.pharmthera.2014.02.013
Burnouf, T., Goubran, H. A., Chen, T. M., Ou, K. L., El-Kiaby, M., and Radosevic, M. (2013). Blood-derived biomaterials and platelet growth factors in regenerative medicine. Blood Rev. 27, 77–89. doi: 10.1016/j.blre.2013.02.001
Carmeliet, P., and Jain, R. (2011). Molecular mechanisms and clinical applications of angiogenesis. Nature 473, 298–307. doi: 10.1038/nature10144
Cenni, E., Peruti, F., and Baldini, N. (2011). In vitro models for the evaluation of angiogenic po-tential in bone engineering. Acta Pharmacol. Sin. 32, 21–30. doi: 10.1038/aps.2010.143
Chen, F. M., and Liu, X. (in press). Advancing biomaterials of human origin for tissue engineering. Prog. Polym. Sci. 1–83. doi: 10.1016/j.progpolymsci.2015.02.004
Chieregato, K., Castegnaro, S., Madeo, D., Astori, G., Pegoraro, M., and Rodeghiero, F. (2011). Epidermal growth factor, basic fibroblast growth factor and platelet-derived growth factor a substitute for fetal bovine serum and compete with human platelet-rich plasma in the ex vivo expansion of mesenchymal stromal cells derived from adipose tissue. Cytotherapy 13, 933–943. doi: 10.3109/14653249.2011.583232
Copland, I. B., Garcia, M. A., Waller, E. K., Roback, J. D., and Galipeau, J. (2013). The effect of platelet lysate fibrinogen on the functionality of MSCs in immunotherapy. Biomaterials 34, 7840–7850. doi: 10.1016/j.biomaterials.2013.06.050
De Falco, E., Porcelli, D., Torella, A. R., Straino, S., Iachininoto, M. G., Orlandi, A., et al. (2004). SDF-1 involvement in endothelial phenotype and ischemia-induced recruitment of bone marrow progenitor cells. Blood 104, 3472–3482. doi: 10.1182/blood-2003-12-4423
De Pascale, M. R., Sommese, L., Casamassimi, A., and Napoli, C. (2015). Platelet derivatives in regenerative medicine: an update. Transfus. Med. Rev. 29, 52–61. doi: 10.1016/j.tmrv.2014.11.001
Dimmeler, S. (2005). Platelet derived growth factor CC a Clinically useful angiogenic factor at last? N. Engl. J. Med. 352, 1815–1816. doi: 10.1056/NEJMcibr050670
Dohan Ehrenfest, D. M., Andia, I., Zumstein, M. A., Zhang, C. Q., Pinto, N., and Bielecki, T. (2014). Classification of platelet concentrates (Platelet-Rich Plasma-PRP, Platelet-Rich Fibrin-for topical and infiltrative use in orthopedic and sports medicine: current consensus, clinical implications and perspectives. Muscles Ligaments Tendons J. 4, 3–9. doi: 10.11138/mltj/2014.4.1.0013
Dohan Ehrenfest, D. M., Rasmusson, L., and Albrektsson, T. (2009). Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin Trends Biotechnol. 27, 158–167. doi: 10.1016/j.tibtech.2008.11.009
Doucet, C., Ernou, I., Zhang, Y., Llense, J. R., Begot, L., Holy, X., et al. (2005). Platelet lysates promote mesenchymal stem cell expansion: a safety substitute for animal serum in cell-based therapy applications. J. Cell. Physiol. 2, 228–236. doi: 10.1002/jcp.20391
Eming, S. A., Brachvogel, B., Odorisio, T., and Koch, M. (2007). Regulation of angiogenesis: wound healing as a model. Prog. Histochem. Cytochem. 42, 115–170. doi: 10.1016/j.proghi.2007.06.001
Even, M., Sandusky, C. B., and Barnard, N. (2006). Serum-free hybridoma culture: ethical, scientific and safety considerations. Trends Biotechnol. 3, 105–108. doi: 10.1016/j.tibtech.2006.01.001
Filardo, G., Kon, E., Di Matteo, B., Di Martino, A., Sessa, A., Merli, M. L., et al. (2013). Leukocyte-poor PRP application for the treatment of knee osteoarthritis. Joints 1, 112–120. doi: 10.11138/jts/2013.1.3.112
Gobbi, G., and Vitale, M. (2012). Platelet-rich plasma preparations for biological therapy: applications and limits. Oper. Tech. Orthop. 22, 10–15. doi: 10.1053/j.oto.2012.01.002
Goedecke, A., Wobus, M., Krech, M., Münch, N., Richter, K., Hölig, K., et al. (2011). Differential effect of platelet-rich plasma and fetal calf serum on bone marrow-derived human mesenchymal stromal cells expanded in vitro. J. Tissue Eng. Regen. Med. 8, 648–654. doi: 10.1002/term.359
Gosain, A., and DiPietro, L. A. (2004). Aging and wound healing. World J. Surg. 28, 321–326. doi: 10.1007/s00268-003-7397-6
Guo, S., and DiPietro, L. A. (2010). Factors affecting wound healing. J. Dent. Res. 89, 219–229. doi: 10.1177/0022034509359125
Hall-Gleen, F., De Young, A., Huan, B. L., Van Handel, B., Hofmann, J. J., Chen, T., et al. (2012). CCN2/connective tissue growth factor is essential for pericyte adhesion and endothelial basement membrane formation during angiogenesis. PLoS ONE 7:e30562. doi: 10.1371/journal.pone.0030562
Hateyama, I., Marukawa, E., Takahashi, Y., and Omura, K. (2014). Effects of platelet-poor plasma, platelet-rich plasma, and platelet-rich fibrin on healing of extraction sockets with buccal dehiscence in dogs. Tissue Eng. A 20, 874–882. doi: 10.1089/ten.TEA.2013.0058
Herbert, S. P., and Stainier, D. Y. R. (2011). Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nat. Rev. Mol. Cell Biol. 12, 551–564. doi: 10.1038/nrm3176
Hwang, B., Lee, S. H., Kim, J. S., Moon, J. H., Jeung, C., Lee, N. G., et al. (2015). Stimulation of angiogenesis and survival of endothelial cells by human monoclonal Tie2 receptor antibody. Biomaterials 51, 119–128. doi: 10.1016/j.biomaterials.2015.01.062
Kang, J., Hur, J., Kang, J. A., Yun, J. Y., Choi, J. I., Ko, S. B., et al. (2014). Activated platelet supernatant can augment the angiogenic potential of human peripheral blood stem cells mobilized from bone marrow by G-CSF. J. Mol. Cell. Cardiol. 75, 64–75. doi: 10.1016/j.yjmcc.2014.06.019
Kawase, T. (2015). Platelet-rich plasma and its derivatives as promising bioactive materials for regenerative medicine: basic principles and concepts underlying recent advances. Odontology 103, 126–135. doi: 10.1007/s10266-015-0209-2
Klement, G. L., Shai, E., and Varon, D. (2013). “The role of platelets in angiogenesis,” in Platelets, ed A. D. Michelson (San Diego, CA: Elsevier), 487–502.
Lange, C., Cakiroglu, F., Spies, A. N., Capallo-obermann, H., Dierlamm, J., and Zander, A. R. (2007). Accelerated and safe expansion of human mesenchymal stromal cells in animal serum-free medium for transplan- tation and regenerative medicine. J. Cell. Physiol. 213, 18–26. doi: 10.1002/jcp.21081
Li, X., Hou, J., Wu, B., Chen, T., and Luo, A. (2014). Effects of platelet-rich plasma and cell coculture on angiogenesis in human dental pulp stem cells and endothelial progenitor cells. J. Endod. 40, 1810–1814. doi: 10.1016/j.joen.2014.07.022
Litwin, M., Radwanska, A., Paprocka, M., Kieda, C., Dobosz, T., Witkiewicz, W., et al. (2015). The role of FGF2 in migration and tubulogenesis of endothelial progenitor cells in relation to pro-angiogenic growth factor production. Mol. Cell. Biochem. doi: 10.1007/s11010-015-2545-5. [Epub ahead of print].
Lyras, D. N., Kazakos, K., Verettas, D., Polychronidis, A., Tryfonidis, M., Botaitis, S., et al. (2009). The influence of platelet-rich plasma on angiogenesis during the early phase of tendon healing. Foot Ankle Int. 30, 1101–1106. doi: 10.3113/FAI.2009.1101
Mammoto, T., Jiang, A., Jiang, E., and Mammoto, A. (2013). Platelet rich plasma extract promotes angiogenesis through the angiopoietin1-Tie2 pathway. Microvasc. Res. 89, 15–24. doi: 10.1016/j.mvr.2013.04.008
Martínez, C., Gonzalez, S., Palma, V., and Smith, P. (2015). Platelet poor plasma and platelet rich plasma stimulate bone lineage differentiation in periodontal ligament stem cells. J. Periodontol. doi: 10.1902/jop.2015.150360. [Epub ahead of print].
Marx, R. E., Carlson, E. R., Eichstaedt, R. M., Schimmele, S. R., Strauss, J. E., and Georgeff, K. R. (1998). Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 85, 638–646. doi: 10.1016/S1079-2104(98)90029-4
Matsumura, A., Kubota, T., Taiyoh, H., Fujiwara, H., Okamoto, K., Ichikawa, D., et al. (2013). HGF regulates VEGF expression via the c-Met receptor downstream pathways, PI3K/Akt, MAPK and STAT3, in CT26 murine cells. Int. J. Oncol. 42, 535–542. doi: 10.3892/ijo.2012.1728
Mautner, K., Malanga, G., Smith, J., Shiple, B., Ibrahim, V., Sampson, S., et al. (2015). Call for a standard classification system for future biologic research: the rationale for new prp nomenclature. PMR 7, S53–S59. doi: 10.1016/j.pmrj.2015.02.005
Min Park, H., and Gerecht, S. (2014). Harnessing developmental processes for vascular engineering and regeneration. Development 141, 2760–2769. doi: 10.1242/dev.102194
Notodihardjo, P. V., Morimoto, N., Kakudo, N., Matsui, M., Sakamoto, M., Liem, P. H., et al. (2015). Gelatin hydrogel impregnated with platelet-rich plasma releasate promotes angiogenesis and wound healing in murine model. J. Artif. Organs 1, 64–71. doi: 10.1007/s10047-014-0795-8
Nurden, A. (2011). Platelets, inflammation and tissue regeneration. Thromb. Haemost. 105, S13–S33. doi: 10.1160/THS10-11-0720
Oklu, R., Walker, T. G., Wicky, S., and Hesketh, R. (2010). Angiogenesis and current antiangiogenic strategies for the treatment of cancer. Vasc. Interv. Radiol. 21, 1791–1805. doi: 10.1016/j.jvir.2010.08.009
Peterson, J., Zurakowski, D., Italiano, J., Michel, L., Fox, L., Klement, G. L., et al. (2010). Normal ranges of angiogenesis regulatory proteins in human platelets. Am. J. Hematol. 85, 487–493. doi: 10.1002/ajh.21732
Raz, O., Lev, D., Battler, A., and Lev, E. (2014). Pathways mediating the interaction between endothelial progenitor cells (EPCs) and platelets. PLoS ONE 9:e95156. doi: 10.1371/journal.pone.0095156
Riboh, J., Saltzman, B. M., Yanke, A. B., Fortier, L., and Cole, B. (2015). Effect of leukocyte concentration on the efficacy of platelet-rich plasma in the treatment of knee osteoarthritis. Am. J. Sports Med. doi: 10.1177/0363546515580787. [Epub ahead of print].
Roy, S., Driggs, J., Elgharably, H., Biswas, S., Findley, M., Khanna, S., et al. (2011). Platelet-rich fibrin matrix improves wound angiogenesis via inducing endothelial cell proliferation. Wound Repair Regen. 19, 753–766. doi: 10.1111/j.1524-475X.2011.00740.x
Rubio-Azpeitia, E., and Andia, I. (2014). Partnership between platelet-rich plasma and mesenchymal stem cells: in vitro experience. Muscles Ligaments Tendons J. 8, 52–62. doi: 10.11138/mltj/2014.4.1.052
Speth, C., Rambacha, G., Würzner, R., Lass-Flörl, C., Kozarcaninb, H., Hamad, O. A., et al. (2015). Complement and platelets: mutual interference in the immune network. Mol. Immunol. 67, 108–118. doi: 10.1016/j.molimm.2015.03.244
Takabayashi, Y., Ishihara, M., Sumi, Y., Takikawa, M., Nakamura, S., and Kiyosawa, T. (2015). Platelet-rich plasma-containing fragmin-protamine micro-nanoparticles promote epithelialization and angiogenesis in split-thickness skin graft donor sites. J. Surg. Res. 193, 483–491. doi: 10.1016/j.jss.2014.08.011
Thushara, R. M., Hemshekhar, M., Basappa, Kemparaju, K., Rangappa, K. S., and Girish, K. S. (2015). Biologicals, platelet apoptosis and human diseases: an outlook. Crit. Rev. Oncol. Hematol. 93, 149–158. doi: 10.1016/j.critrevonc.2014.11.002
Tonti, G. A., and Mannello, F. (2008). From bone marrow to therapeutic applications: different behaviour and genetic/epigenetic stability during mesenchymal stem cell expansion in autologous and foetal bovine sera? Int. J. Dev. Biol. 52, 1023–1032. doi: 10.1387/ijdb.082725gt
Upputuri, P. K., Sivasubramanian, K., Khoon Mark, C. S., and Pramanik, M. (2015). Recent developments in vascular imaging techniques in tissue engineering and regenerative medicine. Biomed. Res. Int. 2015:783983. doi: 10.1155/2015/783983
Vishwakarma, A., Sharpe, P., Shi, S., and Ramalingam, M. (2015). “An introduction to stem cell biology and tissue engineering,” in Stem Cell Biology and Tissue Engineering in Dental Sciences, eds A. Vishwakarma, P. Sharpe, S. Shi, and M. Ramalingam (San Diego, CA: Elsevier Academic Press), 1–13.
Weed, B., Davis, M. D. P., Felty, C. L., Liedl, D. A., Pineda, A. A., Moore, S. B., et al. (2004). Autologous platelet lysate product versus placebo in patients with chronic leg ulcerations: a pilot study using a randomized, double-blind, placebo-controlled trial. Wounds 16, 272–282. Available online at: http://www.woundsresearch.com/article/3067
Yilmaz, S., Kabadayi, C., Dirikan, S., Cakar, G., and Kuru, B. (2011). Treatment of intrabony periodontal defects with platelet-rich plasma versus platelet-poor plasma combined with a bovine-derived xenograft: a controlled clinical trial. J. Periodontol. 82, 837–844. doi: 10.1902/jop.2010.100503
Keywords: platelet poor plasma, platelet rich plasma, angiogenesis, tissue engineering, growth factors
Citation: Martínez CE, Smith PC and Palma Alvarado VA (2015) The influence of platelet-derived products on angiogenesis and tissue repair: a concise update. Front. Physiol. 6:290. doi: 10.3389/fphys.2015.00290
Received: 10 September 2015; Accepted: 02 October 2015;
Published: 20 October 2015.
Edited by:
Francisco J. Rivera, Paracelsus Medical University Salzburg, AustriaReviewed by:
Katharina Schallmoser, Federal Hospital and Paracelsus Medical University, AustriaCopyright © 2015 Martínez, Smith and Palma Alvarado. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Constanza E. Martínez, Y21hcnRpbmVyQHVjLmNs;
Verónica A. Palma Alvarado, dnBhbG1hQHVjaGlsZS5jbA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.