
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Physiol. , 23 June 2015
Sec. Gastrointestinal Sciences
Volume 6 - 2015 | https://doi.org/10.3389/fphys.2015.00173
This article is part of the Research Topic Liver myofibroblasts View all 10 articles
Myofibroblasts are stromal cells mainly involved in tissue repair. These cells present contractile properties and play a major role in extracellular matrix deposition and remodeling. In liver, myofibroblasts are found in two critical situations. First, during fetal liver development, especially in portal tracts, myofibroblasts surround vessels and bile ducts during their maturation. After complete development of the liver, myofibroblasts disappear and are replaced in portal tracts by portal fibroblasts. Second, during liver injury, myofibroblasts re-appear principally deriving from the activation of local stromal cells such as portal fibroblasts and hepatic stellate cells or can sometimes emerge by an epithelial-mesenchymal transition process. After acute injury, myofibroblasts play also a major role during liver regeneration. While myofibroblastic precursor cells are well known, the spectrum of activation and the fate of myofibroblasts during disease evolution are not fully understood. Some data are in accordance with a possible deactivation, at least partial, or a disappearance by apoptosis. Despite these shadows, liver is definitively a pertinent model showing that myofibroblasts are pivotal cells for extracellular matrix control during morphogenesis, repair and fibrous scarring.
In homeostatic state, myofibroblasts are absent from the normal adult liver. Myofibroblasts are stromal cells showing myoid features and involved in production or remodeling of the extracellular matrix (ECM) scaffold. Myofibroblasts are recruited from the transdifferentiation of local stromal cells. Historically, in the liver, it has been postulated that the hepatic stellate cell, also named Ito cell or lipocyte, was the provider of myofibroblasts: in this logic, myofibroblasts were called also transitional cells, activated stellate cells or myofibroblastic cells (French et al., 1988; Mak and Lieber, 1988; Bachem et al., 1989). But other studies have shown afterwards, that fibroblasts located in the connective tissue of the portal tracts are important providers of myofibroblasts (Tang et al., 1994; Tuchweber et al., 1996). This implication of portal fibroblasts as precursor cells of myofibroblasts was observed in human obstructive biliary diseases as well as in animal models (Desmoulière, 2007; Wells, 2014). Indeed, portal fibroblasts are involved as hepatic stellate cells in liver repair after injury and in tumoral reaction. Portal fibroblasts are also an important stromal cell playing a major role during the fetal liver morphogenesis.
Since their first description in granulation tissue (Gabbiani et al., 1971), numerous studies have been published leading to remarkable progresses in the understanding of myofibroblast biological characteristics and of their participation in physiological and pathological situations (Hinz et al., 2012). Myofibroblasts exert traction forces by expressing α-smooth muscle (SM) actin and are able to participate in connective tissue remodeling by synthesizing ECM components, matrix metalloproteinases and their inhibitors. When the repair process is completed, in normal situations, myofibroblasts disappear by apoptosis (Desmoulière et al., 1995). Although presenting SM cell features, myofibroblasts however do not express h-caldesmon (150 kDa caldesmon) and smoothelin which seem to be specific for SM differentiation last step (Frid et al., 1992; van der Loop et al., 1997; Ceballos et al., 2000).
Liver mesenchyme deriving from the mesoblast of the septum transversum is invaded by the epithelial part of the entodermal hepatic diverticulum during the 4th week of development (WD) of normal human embryo (Roskams and Desmet, 2008). The lobulation of the fetal liver begins near the liver hilum at the 9th WD, and continues with a centrifugal pattern in the liver until about 1 month post-partum. Mesenchymal part of the liver gives birth to the sinusoid and perisinusoidal space in the future lobules and future portal tracts at the edge of the lobules, including finally portal fibroblasts (Asahina et al., 2011). Each mesenchymal compartment shows a specific maturation pattern (Figure 1).
- The portal tract maturation follows a sequence classically divided in three stages. At the first stage, the ductal plate stage, a mesenchymal future portal tract containing a large branch of portal vein and limited stroma is surrounded by segments of double-layered cylindrical or tubular structures. At the second stage, the ductal plate remodeling stage, the future portal tract incorporates the tubular structures into the stroma and branches of hepatic artery develop. At the last stage, the remodeled stage, the portal tract is mature and is characterized by a normal connective tissue containing a branch of portal vein, two branches of the hepatic artery and two bile ducts (Crawford et al., 1998).
- The mesenchyme of the septum transversum framework gives rise to sinusoidal compartment, which comprises endothelial and mesenchymal cells entrapped in the perisinusoidal space (future Disse's space). Endothelium of the sinusoid is continuous in the beginning of development and discontinuous after the 12th WD (Enzan et al., 1997). Perisinusoidal mesenchymal cells contain the developing hepatic stellate cells (Wake, 2006), which the embryonic origin is controversial (for review, see Geerts, 2004).
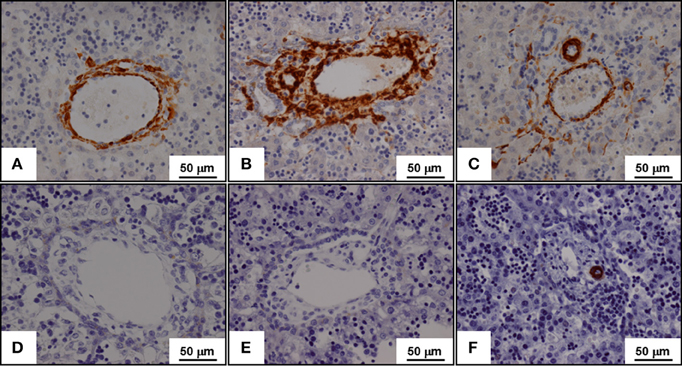
Figure 1. Expression of α-smooth muscle actin (A,B,C) and of h-caldesmon (D,E,F) in fetal liver tissues during the lobulation of the fetal liver. α-Smooth muscle (SM) actin is expressed by myofibroblasts while h-caldesmon is expressed by SM cells. The lobulation of the fetal liver begins near the liver hilum at the 9th week of development, and continues with a centrifugal pattern in the liver until about 1 month post-partum. Three stages of the portal tract maturation are described. At the ductal plate stage, the portal vein is surrounded by myofibroblasts that express α-SM actin (A); SM cells expressing h-caldesmon are not yet present (D). At the ductal plate remodeling stage, α-SM actin expressing myofibroblasts surround the biliary tubular structures from the ductal plate, which were incorporated in the portal stroma (B); again, h-caldesmon is not still present (E). At the remodeled stage, α-SM actin expressing myofibroblasts disappear; only arterial tunica media SM cells expressed both α-SM actin (C) and h-caldesmon (F).
Other mesenchymal cells in the septum transversum can differentiate into fibroblasts, which occupy the subcapsular connective tissue of the liver (Enzan et al., 1997).
Stromal cells with myoid features, called myofibroblasts by Libbrecht et al. (2002), are specially implicated in the maturation of the future portal tract (Villeneuve et al., 2009). During the first stage, future portal tract stroma contains myofibroblasts, which surround also portal vein branch. At the second stage, myofibroblasts surround developing bile ducts, developing arterial branches and portal vein. Outside these areas, portal myofibroblasts give place to fibroblastic cells, which do not express α-SM actin. During the maturation of the arterial branches, the tunica media myofibroblasts are replaced by SM cells, which express h-caldesmon. At the last stage, myofibroblasts have disappeared from the portal tract. On these morphological data, we suggest as other a potential role of the portal myofibroblasts during the maturation of biliary tree (Libbrecht et al., 2002; Villeneuve et al., 2009).
Quite the opposite, myofibroblasts are poorly implicated in perisinusoidal maturation. Numerous cellular retinol-binding protein-1 positive hepatic stellate cells extend cytoplasmic processes from the 13th WD, but only few of them also express α-SM actin (Geerts, 2001; Villeneuve et al., 2009).
Depending on the predominant tropism and the duration of the injury, different patterns of liver inflammation are described. First, two preferential tropisms of hepatitis can be separated in theory: hepatitis with lobular tropism such as viral hepatitis or hepatitis with portal tropism such as biliary obstruction diseases. On light microscopy, a liver zone was preferentially injured depending on the etiology but the other zone is often involved. Two, with the duration and the severity of the injury, the parenchyma architecture can become entirely modified.
In case of acute hepatitis, liver morphology shows inflammatory cell infiltration and hepatocellular damage. The regression is characterized by macrophage cleaning of the necrosis and regeneration. Gradually, these residual changes fade and with times, the liver architecture returns to normal (restitutio ad integrum). During this process, hepatic stellate cell play a major role (Kordes et al., 2014). In addition, after partial hepatectomy, incredible liver regeneration capacities are obvious and hepatic stellate cell-derived myofibroblasts can become progenitors, including epithelial progenitors, participating in this specific property of the liver (Swiderska-Syn et al., 2014).
In case of chronic hepatitis, the repetition and/or the persistence of the injury lead to extensive involvement of the inflammatory reaction in the organ. Then, a chronic scarring process destroys the normal architecture of the organ leading to fibrosis and finally cirrhosis. The scars are characterized by extensive fibrous septa due to accumulation of collagenous ECM. They surround regenerative nodules formed by hepatocyte hyperplasia. Profound disturbance of the liver vascular bed accompanying cirrhosis is characterized by venous thrombosis and anarchic angiogenesis within the fibrous scars, sinusoid remodeling and capillarization within the regenerative nodules (Bosch, 2007). Myofibroblasts are the producers of the ECM constituting the scars. But fibrosis is now not considered as a static state, because it can be modified in structure or remodeled in composition in regard to the extraordinary capacity of liver regeneration (Schuppan et al., 2001). Depending on the stimulus, myofibroblasts can contribute to fibrosis regression by releasing of ECM degrading proteases.
Depending on the duration of the injury, activated stromal cells in acute inflammation and myofibroblasts in sub-acute/chronic inflammation are recruited as a reaction to the lesion.
Depending on the site of injury—portal, lobular or both—, the corresponding stromal cells can be activated into myofibroblasts (Figure 2).
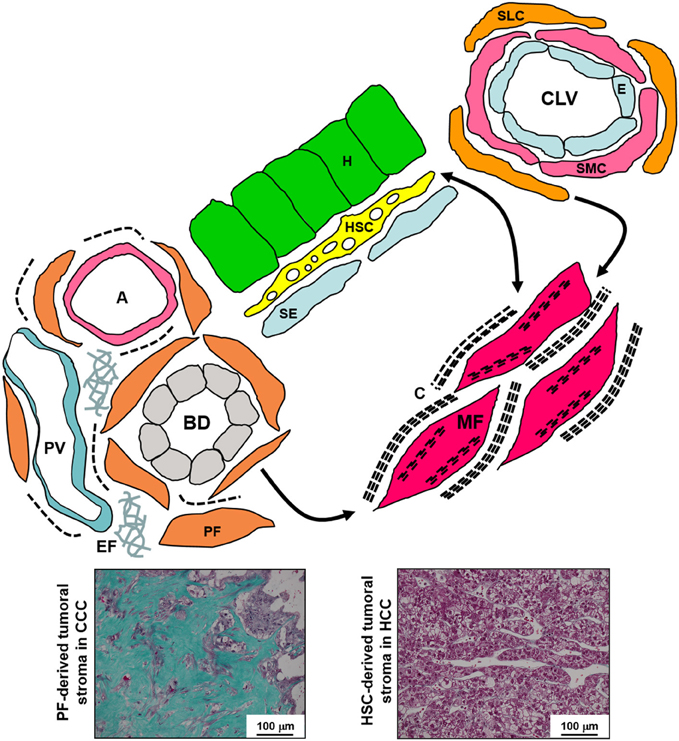
Figure 2. Schematic diagram of the various liver fibroblastic cells able to acquire a myofibroblastic phenotype and involved in fibrogenesis and tumoral stroma formation. The portal fibroblasts (PF) located in the portal tract connective tissue around bile ducts (BD), portal arteries (A), and portal veins (PV), and the second-layer cells (SLC), fibroblasts located around the smooth muscle cells (SMC) and the endothelium (E) of the centrolobular veins (CLV), can acquire a myofibroblastic phenotype, and these cells do not seem to be able to reacquire a quiescent phenotype; in contrast, the hepatic stellate cells (HSC) containing lipids droplets and located in the Disse's space between the hepatocytes (H) and the sinusoidal endothelium (SE) can modulate their myofibroblastic differentiation, and present pericyte-like features suggesting that they function as liver-specific pericytes participating in the regulation of sinusoidal blood pressure. Myofibroblasts (MF) present microfilament bundles and secrete large amounts of extracellular matrix. In addition, PF and PF-derived MF are the major, if not the only, cells that produce elastin. Numerous PF-derived MF are involved in the formation of the abundant fibrous stroma present in cholangiocarcinoma (CCC) while generally rare HSC-derived MF are present in the scanty tumoral stroma of hepatocellular carcinoma (HCC) (Masson's trichrome histochemistry). C, collagen; EF, elastic fibers.
Fibroblasts maintain the connective tissue architecture via the ECM that they secrete, but because they are a heterogeneous population of connective tissue cells, they have specific functions depending on their embryological origin and depending in fine on their organic site (Rinn et al., 2006, 2008; Tschumperlin, 2013). The fibroblasts located within the connective tissue of the portal tract—the portal fibroblasts—give rise to portal myofibroblasts, which are involved in portal fibrosis, notably in congenital biliary malformations and acquired biliary diseases in human (Ozaki et al., 2005) or after common bile duct ligation in animal models (Tuchweber et al., 1996; Kinnman et al., 2003). It is well known that hepatic stellate cells are also activated when the peripheral lobular parenchyma is invaded by the inflammatory reaction (Tuchweber et al., 1996; Kinnman and Housset, 2002). However, data concerning the origin of the myofibroblasts during portal fibrosis, i.e., hepatic stellate cells or portal fibroblasts, as well as the kinetic of this cellular contribution are controversial: in murine models, for Mederacke et al. (2013), hepatic stellate cells are the principal providers at a late time point of the injury, while it was not the case for Beaussier et al. (2007). Nevertheless, portal fibroblasts and myofibroblasts definitively have an important role in the biliary patterning. They participate in the polarity maintenance and the proliferation regulation of the cholangiocytes (Jhandier et al., 2005; He et al., 2008; Tanimizu et al., 2012). In the same way, interactions between myofibroblasts and biliary cells are also important in the ductular reaction and fibrosis development during the chronic bile duct diseases. In rat model of biliary fibrosis, reactive ductules express growth factors such as platelet-derived growth factor, connective tissue growth factor, or transforming growth factor-β2, which activate portal fibroblasts and increase matrix deposition (Milani et al., 1991; Grappone et al., 1999; Sedlaczek et al., 2001). Accompanying these epithelial-mesenchymal interactions, myofibroblasts produce tenascin and type IV collagen, which play an important role in biliary development and activation (Terada and Nakanuma, 1994; Lamireau et al., 1999). Portal fibroblasts and myofibroblasts could be also involved in portal vasculature and nerve development (for review, see Wells, 2014).
Hepatic stellate cells, which account for about 5–8% of cells in the normal liver, are characterized by a perisinusoidal distribution in the Disse's space and long processes extending along and around sinusoids, between the hepatocyte plates (Lepreux et al., 2004). The close association of hepatic stellate cells with endothelial cells resembles that of pericytes in capillaries. However, in normal liver, the endothelium is discontinuous and presents multiple fenestrations without diaphragms, allowing the rapid transport of solutes to the subendothelial space. In the normal liver, a basal lamina-like structure separates the two cell types but there is no true basement membrane. Hepatic stellate cells secrete collagens but, contrary to portal fibroblasts, they seem to not produce elastin (Lorena et al., 2004; Perepelyuk et al., 2013) even if, at least in vitro, hepatic stellate cell-derived myofibroblasts secrete tropoelastin into the culture medium (Kanta et al., 2002). On activation, the hepatic stellate cells acquire a myofibroblastic phenotype, contributing to the excessive ECM deposition observed in the pathological conditions of fibrosis and cirrhosis. Capillarization of the sinusoids also occurs, with a continuous endothelium formed, and the presence of a true basal lamina. The experimental model of carbon tetrachloride (CCl4) treatment in rats has been extensively used to study the involvement of hepatic stellate cells in liver fibrogenesis (Sakaida, 2008). Following chronic injury induced by CCl4 treatment, a large number of myofibroblastic cells accumulate around centrolobular veins; septa containing myofibroblastic cells expressing α-SM actin then develop between centrolobular areas, and large amounts of ECM are deposited (Reeves and Friedman, 2002). Elastin and α-SM actin are co-localized in septa developing after CCl4 treatment, but activated α-SM actin-positive hepatic stellate cells in the parenchyma do not contain elastin. Thus, in the CCl4 model, the typical activated hepatic stellate cells containing α-SM actin seem to play little or no part in elastin deposition (Lorena et al., 2004). These observations suggest that different liver fibroblast subpopulations are involved in deposition of the different ECM components.
Other quiescent fibrocompetent cells can be activated into myofibroblasts: vascular tunica media SM cells (Andrade et al., 1999), second layer cells around the centrilobular veins (Bhunchet and Wake, 1992), and capsular fibroblasts in the Glisson's capsule (Blanc et al., 2005). Recently, a process of mesothelial-to-mesenchymal transition has been mentioned as a novel source of myofibroblastic cells (for review, see Fausther et al., 2013).
EMT defines a process in which epithelial cells acquire mesenchymal features (Kalluri and Weinberg, 2009). EMT, as well the reverse process of mesenchymal-epithelial transition, occurs normally during the fetal development notably through Hedgehog and Notch signaling pathways. The exploration of Hedgehog signaling pathway in case of human or rat liver fibrosis secondary to biliary obstruction showed that cholangiocytes could undergo EMT (Omenetti et al., 2011). Choi and Diehl (2009) have suggested that some quiescent hepatic stellate cells are transitional cells which can differentiate into epithelial cells or myofibroblasts. But, particularly in this domain, the lack of specificity of lineage markers or tracers, the kinetic of their expression and the fact that the in vitro conditions do not reflect the in vivo situations, give rise to conflicting results (for review, see Xie and Diehl, 2013).
Some studies, particularly in advanced stages of fibrosis and cirrhosis, have shown that myofibroblasts may originate from bone marrow. For example, in a mouse model of chronic alcohol consumption, bone marrow-derived cells contribute to the development of α-SM actin expressing cells (Fujimiya et al., 2009). However, the real contribution of bone marrow-derived fibrocytes as a source of myofibroblats during liver fibrosis and cirrhosis remains a question of debates (Kisseleva and Brenner, 2012).
Hepatocellular carcinomas (liver-cell carcinoma) have numerous etiologies, notably chronic B and C virus infection or chronic alcohol abuse and often, hepatocellular carcinomas arise in livers showing cirrhosis, which is in itself a precancerous condition. Bile-duct carcinomas (cholangiocarcinomas) are less common than hepatocellular carcinomas.
During liver regeneration, myofibroblasts are involved in regenerative response, but they are also implicated in the tumoral stroma development (Lemoinne et al., 2013). These myofibroblasts derive locally from hepatic stellate cells and/or portal fibroblasts (Figure 2).
However, the nature of the tumoral stroma is totally different in hepatocellular carcinoma and in cholangiocarcinoma.
In hepatocellular carcinoma, except in rare forms of scirrhous or fibrolamellar hepatocellular carcinoma, tumoral stroma is scanty. Often, the tumoral stroma is mixed with the fibrous stroma of the surrounding cirrhosis. Interestingly, the vessels surrounding the tumoral plates are not sinusoids, but continuous capillaries with a true basement membrane. In contrast, in cholangiocarcinoma, the tumoral cells are surrounded by an abundant fibrous stroma containing numerous myofibroblasts (Darby et al., 2011). This stroma is sclerous, sometimes with calcification, may be extensive, and submerges the scanty tumoral tubules.
We suggest that in hepatocellular carcinomas, mainly hepatic stellate cells and SM cells (Lepreux et al., 2013) are involved in the formation of the discrete tumoral stroma while, in cholangiocarcinoma, essentially portal fibroblasts are responsible for the large ECM deposition. Certainly, targeting cancer-associated myofibroblats could be the key for optimal treatment in future therapies and preventing or reversing the myofibroblast activation could inhibit or at least reduce tumor growth (Rizvi et al., 2014; Heindryckx and Gerwins, 2015).
The phenomenon of stromal cell activation is related to the transdifferentiation into myofibroblast. But, depending on the intensity and the chronicity of the stimulus, stromal cells can be activated at different degrees producing a spectrum from cells showing mixed features of quiescence and activation to cells presenting typical morphological and functional characteristics of myofibroblasts. It is particularly true for the hepatic stellate cells which can express overlapping features during the progression from the quiescent state with for example, the presence of vitamin A metabolism markers (intra-cytoplasmic lipid droplets, vitamin A autofluorescence, cellular retinol-binding protein-1 expression) to the fully activated state with for example, the overexpression of α-SM actin and the overproduction of ECM components (Ramadori, 1991; Gressner and Bachem, 1995; Hautekeete and Geerts, 1997; Lepreux et al., 2001, 2004). From this point of view, hepatic stellate cells present a more malleable phenotype compared with portal fibroblasts. Indeed, differences have been reported between these two fibrogenic cell populations, concerning the mechanisms underlying myofibroblastic differentiation, activation, and deactivation (Guyot et al., 2006). After isolation from healthy rat liver and culturing under the same conditions, both hepatic stellate cells and portal fibroblasts acquire a myofibroblast phenotype. Hepatic stellate cell-derived myofibroblasts display rounded and spread morphological characteristics with an enlarged cytoplasm and, more important, a poor survival after two to three passages. In contrast, portal fibroblasts-derived myofibroblasts have more elongated morphological characteristics and proliferate over multiple passages. In vivo, during liver diseases, hepatic stellate cell- and portal fibroblast-derived myofibroblasts present different fates. By using a model of cultured precision-cut liver slices, the behavior of the myofibroblast subpopulations during remodeling differs depending on the experimental model, the pathological situation, and the disease cause (Guyot et al., 2007, 2010). Hepatic stellate cell-derived myofibroblasts can lose α-SM actin expression without undergoing cell death, whereas in similar conditions, portal fibroblast-derived myofibroblasts die by apoptosis. When liver myofibroblasts are cultured on a basement membrane-like substrate (Matrigel), they loss α-SM actin expression, reacquire cytoplasmic lipid droplets, and thus revert, at least partly, to quiescence (Sohara et al., 2002). In the mouse model of CCl4 induced liver fibrosis, although some myofibroblasts die by apoptosis (Iredale et al., 1998), other myofibroblasts revert to an inactive phenotype during regression of fibrosis (Kisseleva et al., 2012). During the fetal liver portal tract maturation, a same phenomenon of replacement of the myofibroblastic cells by fibroblastic cells was observed (Villeneuve et al., 2009). These data are in accordance with a relative plasticity of the stromal cells; however, we suggest that this plasticity is well established in hepatic stellate cell-derived myofibroblasts, knowing in addition that hepatic stellate cells clearly present pericyte-like features (Costa et al., 2001), whereas portal fibroblast differentiation in myofibroblasts is more complete and less reversible. The question of the regulation of the stromal cell activation is important to consider therapeutic strategies. For example, the blockage of Hedgehog or Notch signaling pathways in rodent models of liver fibrosis leads to partial deactivation of activated hepatic stellate cells and inhibition of fibrotic process (Chen et al., 2012a,b; Xie et al., 2013).
In the past, many studies have been performed using cells derived from explants of human liver parenchyma (Win et al., 1993). Initially, it was suggested that mainly activation of hepatic stellate cells contributes to this population. It is now assumed that these cells are rather representative of many if not all the fibrogenic cell populations present in the liver. It is also accepted that these different fibrogenic cells present different features and that their mechanisms of activation and deactivation are definitively not identical. However, to our knowledge, no reliable markers have been identified that allow unambiguous discrimination between these different cell populations and particularly, between hepatic stellate cell- and portal fibroblast-derived myofibroblasts. However, clearly, depending on the cause of the lesion (e.g., virus, alcohol) and then the primary location of the injury, the fibrogenic cells involved are different. Knowing that the deactivation mechanisms of these different cells are not similar, the question of the reversibility of the liver fibrosis/cirrhosis remains a burning issue. Certainly, portal fibroblasts are involved in many pathological situations and must be considered as a major fibrogenic cell population beside the hepatic stellate cells. Finally, the pivotal role of the portal (myo)fibroblasts in the fetal liver development, as well as in wound healing, including tumoral stroma which could be assimilated to an overhealing wound (Schäfer and Werner, 2008), notably through their interactions with the proliferative bile structures, would require more investigations in the way of liver regeneration application.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
SM, smooth muscle; ECM, extracellular matrix; WD, week of development; CCl4, carbon tetrachloride; EMT, epithelial-mesenchymal transition.
Andrade, Z. A., Guerret, S., and Fernandes, A. L. (1999). Myofibroblasts in schistosomal portal fibrosis of man. Mem. Inst. Oswaldo Cruz 94, 87–93. doi: 10.1590/S0074-02761999000100018
Asahina, K., Zhou, B., Pu, W. T., and Tsukamoto, H. (2011). Septum transversum-derived mesothelium gives rise to hepatic stellate cells and perivascular mesenchymal cells in developing mouse liver. Hepatology 53, 983–995. doi: 10.1002/hep.24119
Bachem, M. G., Riess, U., and Gressner, A. M. (1989). Liver fat storing cell proliferation is stimulated by epidermal growth factor/transforming growth factor alpha and inhibited by transforming growth factor beta. Biochem. Biophys. Res. Commun. 162, 708–714. doi: 10.1016/0006-291X(89)92368-1
Beaussier, M., Wendum, D., Schiffer, E., Dumont, S., Rey, C., Lienhart, A., et al. (2007). Prominent contribution of portal mesenchymal cells to liver fibrosis in ischemic and obstructive cholestatic injuries. Lab. Invest. 87, 292–303. doi: 10.1038/labinvest.3700513
Bhunchet, E., and Wake, K. (1992). Role of mesenchymal cell populations in porcine serum-induced rat liver fibrosis. Hepatology 16, 1452–1473. doi: 10.1002/hep.1840160623
Blanc, J. F., Bioulac-Sage, P., Balabaud, C., and Desmoulière, A. (2005). Investigation of liver fibrosis in clinical practice. Hepatol. Res. 32, 1–8. doi: 10.1016/j.hepres.2005.03.001
Bosch, J. (2007). Vascular deterioration in cirrhosis: the big picture. J. Clin. Gastroenterol. 41, S247–S253. doi: 10.1097/mcg.0b013e3181572357
Ceballos, K. M., Nielsen, G. P., Selig, M. K., and O'Connell, J. X. (2000). Is anti-h-caldesmon useful for distinguishing smooth muscle and myofibroblastic tumors? An immunohistochemical study. Am. J. Clin. Pathol. 114, 746–753. doi: 10.1309/K5JP-A9EN-UWN7-B5GG
Chen, Y., Choi, S. S., Michelotti, G. A., Chan, I. S., Swiderska-Syn, M., Kraca, G. F., et al. (2012a). Hedgehog controls hepatic stellate cell fate by regulating metabolism. Gastroenterology 143, 1319–1329. doi: 10.1053/j.gastro.2012.07.115
Chen, Y., Zheng, S., Qi, D., Zheng, S., Guo, J., Zhang, S., et al. (2012b). Inhibition of Notch signaling by a γ-secretase inhibitor attenuates hepatic fibrosis in rats. PLoS ONE 7:e46512. doi: 10.1371/journal.pone.0046512
Choi, S. S., and Diehl, A. M. (2009). Epithelial-to-mesenchymal transitions in the liver. Hepatology 50, 2007–2013. doi: 10.1002/hep.23196
Crawford, A. R., Lin, X. Z., and Crawford, J. M. (1998). The normal adult human liver biopsy: a quantitative reference standard. Hepatology 28, 323–331. doi: 10.1002/hep.510280206
Costa, A. M. A., Tuchweber, B., Rubbia-Brandt, L., Peyrol, S., Chevallier, M., Adham, M., et al. (2001). Early activation of hepatic stellate cells and perisinusoidal extracellular matrix changes during ex vivo pig liver perfusion. J. Submicrosc. Cytol. Pathol. 33, 231–240. doi: 10.1002/jgm.1223
Darby, I. A., Vuillier-Devillers, K., Pinault, E., Sarrazy, V., Lepreux, S., Balabaud, C., et al. (2011). Proteomic analysis of differentially expressed proteins in peripheral cholangiocarcinoma. Cancer Microenviron. 4, 73–91. doi: 10.1007/s12307-010-0047-2
Desmoulière, A. (2007). Hepatic stellate cells: the only cells involved in liver fibrogenesis? A dogma challenged. Gastroenterology 132, 2059–2062. doi: 10.1053/j.gastro.2007.03.075
Desmoulière, A., Redard, M., Darby, I., and Gabbiani, G. (1995). Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am. J. Pathol. 146, 56–66.
Enzan, H., Himeno, H., Hiroi, M., Kiyoku, H., Saibara, T., and Onishi, S. (1997). Development of hepatic sinusoidal structure with special reference to the Ito cells. Microsc. Res. Tech. 39, 336–349.
Fausther, M., Lavoie, E. G., and Dranoff, J. A. (2013). Contribution of myofibroblasts of different origins to liver fibrosis. Curr. Pathobiol. Rep. 1, 225–230. doi: 10.1007/s40139-013-0020-0
French, S. W., Miyamoto, K., Wong, K., Jui, L., and Briere, L. (1988). Role of the Ito cell in liver parenchymal fibrosis in rats fed alcohol and a high fat-low protein diet. Am. J. Pathol. 132, 73–85.
Frid, M. G., Shekhonin, B. V., Koteliansky, V. E., and Glukhova, M. A. (1992). Phenotypic changes of human smooth muscle cells during development: late expression of heavy caldesmon and calponin. Dev. Biol. 153, 185–193. doi: 10.1016/0012-1606(92)90104-O
Fujimiya, T., Liu, J., Kojima, H., Shirafuji, S., Kimura, H., and Fujimiya, M. (2009). Pathological roles of bone marrow-derived stellate cells in a mouse model of alcohol-induced fatty liver. Am. J. Physiol. Gastrointest. Liver Physiol. 297, G451–G460. doi: 10.1152/ajpgi.00055.2009
Gabbiani, G., Ryan, G. B., and Majno, G. (1971). Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia 27, 549–550. doi: 10.1007/BF02147594
Geerts, A. (2001). History, heterogeneity, developmental biology, and functions of quiescent hepatic stellate cells. Semin. Liver Dis. 21, 311–335. doi: 10.1055/s-2001-17550
Geerts, A. (2004). On the origin of stellate cells: mesodermal, endodermal or neuro-ectodermal? J. Hepatol. 40, 331–334. doi: 10.1016/j.jhep.2003.12.006
Grappone, C., Pinzani, M., Parola, M., Pellegrini, G., Caligiuri, A., DeFranco, R., et al. (1999). Expression of platelet-derived growth factor in newly formed cholangiocytes during experimental biliary fibrosis in rats. J. Hepatol. 31, 100–109. doi: 10.1016/S0168-8278(99)80169-X
Gressner, A. M., and Bachem, M. G. (1995). Molecular mechanisms of liver fibrogenesis–a homage to the role of activated fat-storing cells. Digestion 56, 335–346. doi: 10.1159/000201257
Guyot, C., Combe, C., Balabaud, C., Bioulac-Sage, P., and Desmoulière, A. (2007). Fibrogenic cell fate during fibrotic tissue remodelling observed in rat and human cultured liver slices. J. Hepatol. 46, 142–150. doi: 10.1016/j.jhep.2006.08.013
Guyot, C., Lepreux, S., Combe, C., Doudnikoff, E., Bioulac-Sage, P., Balabaud, C., et al. (2006). Hepatic fibrosis and cirrhosis: the (myo)fibroblastic cell subpopulations involved. Int. J. Biochem. Cell Biol. 38, 135–151. doi: 10.1016/j.biocel.2005.08.021
Guyot, C., Lepreux, S., Combe, C., Sarrazy, V., Billet, F., Balabaud, C., et al. (2010). Fibrogenic cell phenotype modifications during remodelling of normal and pathological human liver in cultured slices. Liver Int. 30, 1529–1540. doi: 10.1111/j.1478-3231.2010.02342.x
Hautekeete, M. L., and Geerts, A. (1997). The hepatic stellate (Ito) cell: its role in human liver disease. Virchows Arch. 430, 195–207. doi: 10.1007/BF01324802
He, Y., Wu, G. D., Sadahiro, T., Noh, S. I., Wang, H., Talavera, D., et al. (2008). Interaction of CD44 and hyaluronic acid enhances biliary epithelial proliferation in cholestatic livers. Am. J. Physiol. Gastrointest. Liver Physiol. 295, G305–G312. doi: 10.1152/ajpgi.90229.2008
Heindryckx, F., and Gerwins, P. (2015). Targeting the tumor stroma in hepatocellular carcinoma. World J. Hepatol. 7, 165–176. doi: 10.4254/wjh.v7.i2.165
Hinz, B., Phan, S. H., Thannickal, V. J., Prunotto, M., Desmoulière, A., Varga, J., et al. (2012). Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. Am. J. Pathol. 180, 1340–1355. doi: 10.1016/j.ajpath.2012.02.004
Iredale, J. P., Benyon, R. C., Pickering, J., McCullen, M., Northrop, M., Pawley, S., et al. (1998). Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J. Clin. Invest. 102, 538–549. doi: 10.1172/JCI1018
Jhandier, N. M., Kruglov, E. A., Lavoie, E. G., Sévigny, J., and Dranoff, J. A. (2005). Portal fibroblasts regulate the proliferation of bile duct epithelia via expression of NTPDase2. J. Biol. Chem. 280, 22986–22992. doi: 10.1074/jbc.M412371200
Kalluri, R., and Weinberg, R. A. (2009). The basics of epithelial-mesenchymal transition. J. Clin. Invest. 119, 1420–1428. doi: 10.1172/JCI39104
Kanta, J., Dooley, S., Delvoux, B., Breuer, S., D'Amico, T., and Gressner, A. M. (2002). Tropoelastin expression is up-regulated during activation of hepatic stellate cells and in the livers of CCl4-cirrhotic rats. Liver 22, 220–227. doi: 10.1046/j.0106-9543.2002.01573.x
Kinnman, N., and Housset, C. (2002). Peribiliary myofibroblasts in biliary type liver fibrosis. Front. Biosci. 7, d496–d503. doi: 10.2741/kinnman
Kinnman, N., Francoz, C., Barbu, V., Wendum, D., Rey, C., Hultcrantz, R., et al. (2003). The myofibroblastic conversion of peribiliary fibrogenic cells distinct from hepatic stellate cells is stimulated by platelet-derived growth factor during liver fibrogenesis. Lab. Invest. 83, 163–173. doi: 10.1097/01.LAB.0000054178.01162.E4
Kisseleva, T., and Brenner, D. A. (2012). The phenotypic fate and functional role for bone marrow-derived stem cells in liver fibrosis. J. Hepatol. 56, 965–972. doi: 10.1016/j.jhep.2011.09.021
Kisseleva, T., Cong, M., Paik, Y., Scholten, D., Jiang, C., Benner, C., et al. (2012). Myofibroblasts revert to an inactive phenotype during regression of liver fibrosis. Proc. Natl. Acad. Sci. U.S.A. 109, 9448–9453. doi: 10.1073/pnas.1201840109
Kordes, C., Sawitza, I., Götze, S., Herebian, D., and Häussinger, D. (2014). Hepatic stellate cells contribute to progenitor cells and liver regeneration. J. Clin. Invest. 124, 5503–5515. doi: 10.1172/JCI74119
Lamireau, T., Le Bail, B., Boussarie, L., Fabre, M., Vergnes, P., Bernard, O., et al. (1999). Expression of collagens type I and IV, osteonectin and transforming growth factor beta-1 (TGFbeta1) in biliary atresia and paucity of intrahepatic bile ducts during infancy. J. Hepatol. 31, 248–255. doi: 10.1016/S0168-8278(99)80221-9
Lemoinne, S., Cadoret, A., El Mourabit, H., Thabut, D., and Housset, C. (2013). Origins and functions of liver myofibroblasts. Biochim. Biophys. Acta. 1832, 948–954. doi: 10.1016/j.bbadis.2013.02.019
Lepreux, S., Bioulac-Sage, P., Gabbiani, G., Sapin, V., Housset, C., Rosenbaum, J., et al. (2004). Cellular retinol-binding protein-1 expression in normal and fibrotic/cirrhotic human liver: different patterns of expression in hepatic stellate cells and (myo)fibroblast subpopulations. J. Hepatol. 40, 774–780. doi: 10.1016/j.jhep.2004.01.008
Lepreux, S., Dubuisson, L., Le Bail, B., Desmoulière, A., Balabaud, C., and Bioulac-Sage, P. (2001). Can hepatic stellate cells express alpha-smooth muscle actin in normal human liver? Liver 21, 293–294. doi: 10.1034/j.1600-0676.2001.021004293.x
Lepreux, S., Guyot, C., Billet, F., Combe, C., Balabaud, C., Bioulac-Sage, P., et al. (2013). Smoothelin, a new marker to determine the origin of liver fibrogenic cells. World J. Gastroenterol. 19, 9343–9350. doi: 10.3748/wjg.v19.i48.9343
Libbrecht, L., Cassiman, D., Desmet, V., and Roskams, T. (2002). The correlation between portal myofibroblasts and development of intrahepatic bile ducts and arterial branches in human liver. Liver 22, 252–258. doi: 10.1046/j.0106-9543.2002.01674.x
Lorena, D., Darby, I. A., Reinhardt, D. P., Sapin, V., Rosenbaum, J., and Desmoulière, A. (2004). Fibrillin-1 expression in normal and fibrotic rat liver and in cultured hepatic fibroblastic cells: modulation by mechanical stress and role in cell adhesion. Lab. Invest. 84, 203–212. doi: 10.1038/labinvest.3700023
Mak, K. M., and Lieber, C. S. (1988). Lipocytes and transitional cells in alcoholic liver disease: a morphometric study. Hepatology 8, 1027–1033. doi: 10.1002/hep.1840080508
Mederacke, I., Hsu, C. C., Troeger, J. S., Huebener, P., Mu, X., Dapito, D. H., et al. (2013). Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat. Commun. 4, 2823. doi: 10.1038/ncomms3823
Milani, S., Herbst, H., Shuppan, D., Stein, H., and Surrenti, C. (1991). Transforming growth factors beta 1 and beta 2 are differentially expressed in fibrotic liver disease. Am. J. Pathol. 139, 1221–1229.
Omenetti, A., Bass, L. M., Anders, R. A., Clemente, M. G., Francis, H., Guy, C. D., et al. (2011). Hedgehog activity, epithelial-mesenchymal transitions, and biliary dysmorphogenesis in biliary atresia. Hepatology 53, 1246–1258. doi: 10.1002/hep.24156
Ozaki, S., Sato, Y., Yasoshima, M., Harada, K., and Nakanuma, Y. (2005). Diffuse expression of heparin sulfate proteoglycan and connective tissue growth factor in fibrous septa with many mast cells relate to unresolving hepatic fibrosis of congenital hepatic fibrosis. Liver Int. 25, 817–828. doi: 10.1111/j.1478-3231.2005.01067.x
Perepelyuk, M., Terajima, M., Wang, A. Y., Georges, P. C., Janmey, P. A., Yamauchi, M., et al. (2013). Hepatic stellate cells and portal fibroblasts are the major cellular sources of collagens and lysyl oxidases in normal liver and early after injury. Am. J. Physiol. Gastrointest. Liver Physiol. 304, G605–G614. doi: 10.1152/ajpgi.00222.2012
Ramadori, G. (1991). The stellate cell (Ito-cell, fat-storing cell, lipocyte, perisinusoidal cell) of the liver. New insights into pathophysiology of an intriguing cell. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 61, 147–158. doi: 10.1007/BF02890417
Reeves, H. L., and Friedman, S. L. (2002). Activation of hepatic stellate cells–a key issue in liver fibrosis. Front. Biosci. 7, d808–d826. doi: 10.2741/reeves
Rinn, J. L., Bondre, C., Gladstone, H. B., Brown, P. O., and Chang, H. Y. (2006). Anatomic demarcation by positional variation in fibroblast gene expression programs. PLoS Genet. 2:e119. doi: 10.1371/journal.pgen.0020119
Rinn, J. L., Wang, J. K., Allen, N., Brugmann, S. A., Mikels, A. J., Liu, H., et al. (2008). A dermal HOX transcriptional program regulates site-specific epidermal fate. Genes Dev. 22, 303–307. doi: 10.1101/gad.1610508
Rizvi, S., Borad, M. J., Patel, T., and Gores, G. J. (2014). Cholangiocarcinoma: molecular pathways and therapeutic opportunities. Semin. Liver Dis. 34, 456–464. doi: 10.1055/s-0034-1394144
Roskams, T., and Desmet, V. (2008). Embryology of extra- and intrahepatic bile ducts, the ductal plate. Anat. Rec. (Hoboken) 291, 628–635. doi: 10.1002/ar.20710
Sakaida, I. (2008). Autologous bone marrow cell infusion therapy for liver cirrhosis. J. Gastroenterol. Hepatol. 23, 1349–1353. doi: 10.1111/j.1440-1746.2008.05381.x
Schäfer, M., and Werner, S. (2008). Cancer as an overhealing wound: an old hypothesis revisited. Nat. Rev. Mol. Cell Biol. 9, 628–638. doi: 10.1038/nrm2455
Schuppan, D., Ruehl, M., Somasundaram, R., and Hahn, E. G. (2001). Matrix as a modulator of hepatic fibrogenesis. Semin. Liver Dis. 21, 351–372. doi: 10.1055/s-2001-17556
Sedlaczek, N., Jia, J. D., Bauer, M., Herbst, H., Ruehl, M., Hahn, E. G., et al. (2001). Proliferating bile duct epithelial cells are a major source of connective tissue growth factor in rat biliary fibrosis. Am. J. Pathol. 158, 1239–1244. doi: 10.1016/S0002-9440(10)64074-6
Sohara, N., Znoyko, I., Levy, M. T., Trojanowska, M., and Reuben, A. (2002). Reversal of activation of human myofibroblast-like cells by culture on a basement membrane-like substrate. J. Hepatol. 37, 214–221. doi: 10.1016/S0168-8278(02)00103-4
Swiderska-Syn, M., Syn, W. K., Xie, G., Krüger, L., Machado, M. V., Karaca, G., et al. (2014). Myofibroblastic cells function as progenitors to regenerate murine livers after partial hepatectomy. Gut 63, 1333–1344. doi: 10.1136/gutjnl-2013-305962
Tang, L., Tanaka, Y., Marumo, F., and Sato, C. (1994). Phenotypic change in portal fibroblasts in biliary fibrosis. Liver 14, 76–82. doi: 10.1111/j.1600-0676.1994.tb00051.x
Tanimizu, N., Kikkawa, Y., Mitaka, T., and Miyajima, A. (2012). α1- and α5-containing laminins regulate the development of bile ducts via β1 integrin signals. J. Biol. Chem. 287, 28586–28597. doi: 10.1074/jbc.M112.350488
Terada, T., and Nakanuma, Y. (1994). Expression of tenascin, type IV collagen and laminin during human intrahepatic bile duct development and in intrahepatic cholangiocarcinoma. Histopathology 25, 143–150. doi: 10.1111/j.1365-2559.1994.tb01570.x
Tschumperlin, D. J. (2013). Fibroblasts and the ground they walk on. Physiology 28, 380–390. doi: 10.1152/physiol.00024.2013
Tuchweber, B., Desmoulière, A., Bochaton-Piallat, M. L., Rubbia-Brandt, L., and Gabbiani, G. (1996). Proliferation and phenotypic modulation of portal fibroblasts in the early stages of cholestatic fibrosis in rat. Lab. Invest. 74, 265–278.
van der Loop, F. T., Gabbiani, G., Kohnen, G., Ramaekers, F. C., and van Eys, G. J. (1997). Differentiation of smooth muscle cells in human blood vessels as defined by smoothelin, a novel marker for the contractile phenotype. Arterioscler. Thromb. Vasc. Biol. 17, 665–671. doi: 10.1161/01.ATV.17.4.665
Villeneuve, J., Pelluard-Nehme, F., Combe, C., Carles, D., Chaponnier, C., Ripoche, J., et al. (2009). Immunohistochemical study of the phenotypic change of the mesenchymal cells during portal tract maturation in normal and fibrous (ductal plate malformation) fetal liver. Comp. Hepatol. 8:5. doi: 10.1186/1476-5926-8-5
Wake, K. (2006). Hepatic stellate cells: three-dimensional structure, localization, heterogeneity and development. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 82, 155–164. doi: 10.2183/pjab.82.155
Wells, R. G. (2014). The portal fibroblast: not just a poor man's stellate cell. Gastroenterology 147, 41–47. doi: 10.1053/j.gastro.2014.05.001
Win, K. M., Charlotte, F., Mallat, A., Cherqui, D., Martin, N., Mavier, P., et al. (1993). Mitogenic effect of transforming growth factor-beta 1 on human Ito cells in culture: evidence for mediation by endogenous platelet-derived growth factor. Hepatology 18, 137–145. doi: 10.1002/hep.1840180121
Xie, G., and Diehl, A. M. (2013). Evidence for and against epithelial-to-mesenchymal transition in the liver. Am. J. Physiol. Gastrointest. Liver Physiol. 305, G881–G890. doi: 10.1152/ajpgi.00289.2013
Keywords: portal fibroblast, myofibroblast, hepatic stellate cell, alpha-smooth muscle actin, liver development, fibrosis, tumoral stroma
Citation: Lepreux S and Desmoulière A (2015) Human liver myofibroblasts during development and diseases with a focus on portal (myo)fibroblasts. Front. Physiol. 6:173. doi: 10.3389/fphys.2015.00173
Received: 31 March 2015; Accepted: 21 May 2015;
Published: 23 June 2015.
Edited by:
Jiri Kanta, Charles University, Czech RepublicReviewed by:
Matthias J. Bahr, Sana Kliniken Lübeck, GermanyCopyright © 2015 Lepreux and Desmoulière. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alexis Desmoulière, Department of Physiology, Faculty of Pharmacy, University of Limoges, 2 rue du Dr. Marcland, 87025 Limoges, France,YWxleGlzLmRlc21vdWxpZXJlQHVuaWxpbS5mcg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.