- Department of Biomedical Sciences, University at Albany, State University of New York, Albany, NY, USA
Epidemiological data have demonstrated an inverse association between serum vitamin D3 levels, cancer incidence and related mortality. However, the effects of vitamin D on prostate cancer biology and its utility for prevention of prostate cancer progression are not as well-defined. The data are often conflicting: some reports suggest that vitamin D3 induces apoptosis in androgen dependent prostate cancer cell lines, while others suggest that vitamin D3 only induces cell cycle arrest. Recent molecular studies have identified an extensive synergistic crosstalk between the vitamin D- and androgen-mediated mRNA and miRNA expression, adding an additional layer of post-transcriptional regulation to the known VDR- and AR-regulated gene activation. The Warburg effect, the inefficient metabolic pathway that converts glucose to lactate for rapid energy generation, is a phenomenon common to many different types of cancer. This process supports cell proliferation and promotes cancer progression via alteration of glucose, glutamine and lipid metabolism. Prostate cancer is a notable exception to this general process since the metabolic switch that occurs early during malignancy is the reverse of the Warburg effect. This “anti-Warburg effect” is due to the unique biology of normal prostate cells that harbor a truncated TCA cycle that is required to produce and secret citrate. In prostate cancer cells, the TCA cycle activity is restored and citrate oxidation is used to produce energy for cancer cell proliferation. 1,25(OH)2D3 and androgen together modulates the TCA cycle via transcriptional regulation of zinc transporters, suggesting that 1,25(OH)2D3 and androgen maintain normal prostate metabolism by blocking citrate oxidation. These data demonstrate the importance of androgens in the anti-proliferative effect of vitamin D in prostate cancer and highlight the importance of understanding the crosstalk between these two signaling pathways.
Overview on Prostate Cancer Biology
Prostate cancer is the most commonly diagnosed non-cutaneous malignancy in males in North America (Altekruse et al., 2010). This disease is usually considered to be an androgen dependent cancer, since the normal prostate is clearly dependent on androgens for its structure and function. Paradoxically, the age-dependent incidence and associated mortality of prostate cancer between 50 and 60 years of age increase after serum testosterone levels start to decline significantly, particularly after age of 65 (Figure 1A) (Siegel et al., 2014). Prostate adenocarcinomas are slow growing tumors that are characterized by low mitotic index and a long natural history (McNeal, 1968). The progression from normal prostate to prostatic intraepithelial neoplasia (PIN), and eventually to localized adenocarcinoma takes place over several decades (Figure 1B). Autopsy studies have shown that prostatic adenocarcinoma and the pre-malignant PIN are evident in men in their early and mid-thirties. The development of advanced, locally invasive prostate cancer and metastatic disease is a relatively late process for which there are limited treatments, and hormone ablation therapy used at this late stage applies selective stress that probably is responsible for the development of castration-resistant prostate cancer (CRPC).
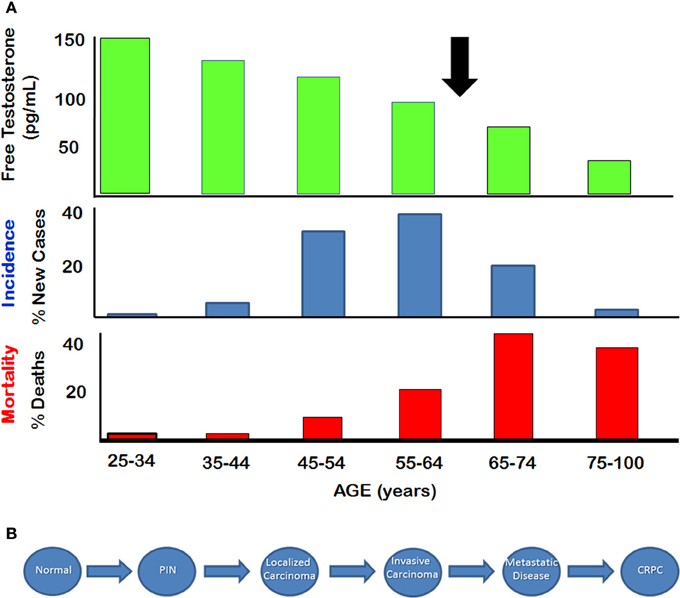
Figure 1. Natural History of Prostate Cancer (A) Relationship between serum free testosterone and incidence and mortality of prostate cancer. Arrow indicates approximate age at which free testosterone declines below 50% of the level seen in young adults. (B) Stepwise depiction of progression from normal disease to metastatic disease. Castration resistant prostate cancer (CRPC) appears to emerge after hormone therapy. (Figure adapted from SEER database and from Framington Heart Study).
Vitamin D and Prostate
There are many epidemiological studies that suggest high serum vitamin D levels, usually measured as serum 25(OH)-vitamin D3 (25(OH)D3) may be important in preventing various cancers, including breast, ovarian and colon cancer (Thorne and Campbell, 2008; Giovannucci, 2009). The risk of developing and dying of these cancers appears to be inversely correlated with sun exposure, and/or vitamin D status, suggesting that vitamin D has chemopreventive properties (Garland et al., 2009). Some studies have also suggested that vitamin D may play a role in prostate cancer prevention (Tseng et al., 2004; Schwartz and Skinner, 2007), but the data are less conclusive than in other cancers and several recent meta-analyses have found weak or no associations between 25(OH)D3 levels and prostate tumor incidence or progression (Yin et al., 2009; van der et al., 2009; Barnett et al., 2010; Park et al., 2010; Holt et al., 2013). However, a recent study of men diagnosed with prostate cancer showed that 72% of men with recurrent disease and 68% with clinically localized disease were insufficient or deficient in serum 25(OH)D3 levels, less than 20 ng/mL (desirable levels >40 ng/mL) (Trump et al., 2010). These data suggest that the majority of men with prostate cancer have low circulating androgen and low 25(OH)D3 levels at the time of diagnosis. Based on many in vitro studies (Miller, 1998; Blutt et al., 2000; Peehl et al., 2003), preclinical and clinical studies (Deeb et al., 2007), it has been suggested that vitamin D can be used either as chemopreventative or as therapeutic agent for prostate cancer. Despite extensive research, the importance of vitamin D as a chemopreventive agent for prostate cancer is still a matter of considerable controversy (van der et al., 2009; Park et al., 2010), and the results from therapeutic intervention using 1,25-dihydroxyvitamin D3 (1,25(OH)2D3), the active metabolite of vitamin D3 or its less calcemic analogs, have been generally disappointing (Vijayakumar et al., 2005; Beer and Myrthue, 2006; Wagner et al., 2013). In low-risk prostate cancer patients who enrolled in active surveillance, high dose of vitamin D3 supplementation decreases Gleason score or the number of positive cores in more than 50% of patient population (Marshall et al., 2012), whereas 1,25(OH)2D3 supplementation at adjuvant settings have provided mixed results in CRPC or recurrent diseases (Flaig et al., 2006; Chan et al., 2008; Srinivas and Feldman, 2009; Chadha et al., 2010; Scher et al., 2011; Shamseddine et al., 2013).
Various reports suggest that the action of vitamin D in prostate cancer cells is androgen dependent (Esquenet et al., 1995; Murthy et al., 2005; Weigel, 2007; Mordan-McCombs et al., 2010). In Sprague–Dawley rats, 1,25(OH)2D3 administration decreases prostatic size in intact males, but not castrated groups (Leman et al., 2003). Longitudinal studies have demonstrated a positive correlation between 25(OH)D3 levels and the production of androgen (Wehr et al., 2010; Pilz et al., 2011; Nimptsch et al., 2012), which has been further validated in vitro (Lundqvist et al., 2011). However, vitamin D also induces CYP3A4 and CYP3A5 expression, enzymes that metabolize and inactivate testosterone and androstanediol in prostate cells, suggesting that vitamin D signaling may play a role in limiting androgen levels in the prostate (Maguire et al., 2012). Previous in vitro studies have shown that 1,25(OH)2D3 also induces moderate increases in AR, PSA, and TMPRSS2 transcript levels (Hsieh et al., 1996; Zhao et al., 1999; Krishnan et al., 2004; Washington and Weigel, 2010), however this finding does not translate into clinical setting where 1,25(OH)2D3 appears to decrease the PSA velocity (Krishnan et al., 2003). Based on these findings, serum vitamin D levels appear to have a significant impact on androgen-mediated signaling and the crosstalk between androgen and vitamin D probably plays an important role in prostate cancer biology. While there have been many studies examining the effects of androgens or 1,25(OH)2D3 individually on gene expression in prostate cancer cells, there have been very few studies that explored the crosstalk between the two signaling pathways and the biological consequences of this crosstalk.
Genomic Overlay of VDR and AR Signaling
The crosstalk between VDR- and AR-mediated gene expression was first demonstrated in LNCaP cells (Qiao and Tuohimaa, 2004). Induction of FACL3 (long-chain fatty-acid CoA ligase 3) is dependent on both vitamin D and androgen levels, and treatment with bicalutamide inhibits 1,25(OH)2D3-induced FACL3 expression. This coordinated effect on gene expression has recently been validated by a comprehensive microarray study using the same in vitro model (Wang et al., 2011). 1,25(OH)2D3 and androgen share many common targets and coordinately modulate these transcript levels in the same direction (Figures 2A,B). More importantly, the combination of the two hormones regulates additional genes, including both mRNAs and miRNAs that have not been previously identified. The significance of this additional layer of transcriptional control is best illustrated by bioinformatic analysis which demonstrates the coordinated effect of 1,25(OH)2D3 and androgen on cellular processes, including cell homeostasis, proliferation, differentiation and metabolism, all of which have significant impact on prostate tumorigenesis (Figure 2C). Most of these processes are more significantly regulated by 1,25(OH)2D3 and androgen together than by either hormone alone, demonstrating the interaction between the two signaling pathways. Several genes identified from the expression microarray analysis are validated VDR and AR targets, contains functional VDRE (within 10kb upstream and 5 kb downstream of the structural gene) and ARE sites, and some genes exhibit additive induction after testosterone and 1,25(OH)2D3 stimulation. Both androgen and 1,25(OH)2D3 induces PSA mRNA levels while addition of testosterone blunts the early vitamin D dependent induction of Cyp24A1, the main enzyme involved in the catabolism of 1,25(OH)2D3. This suggests that the half-life of 1,25(OH)2D3 is extended in the presence of exogenous androgen.
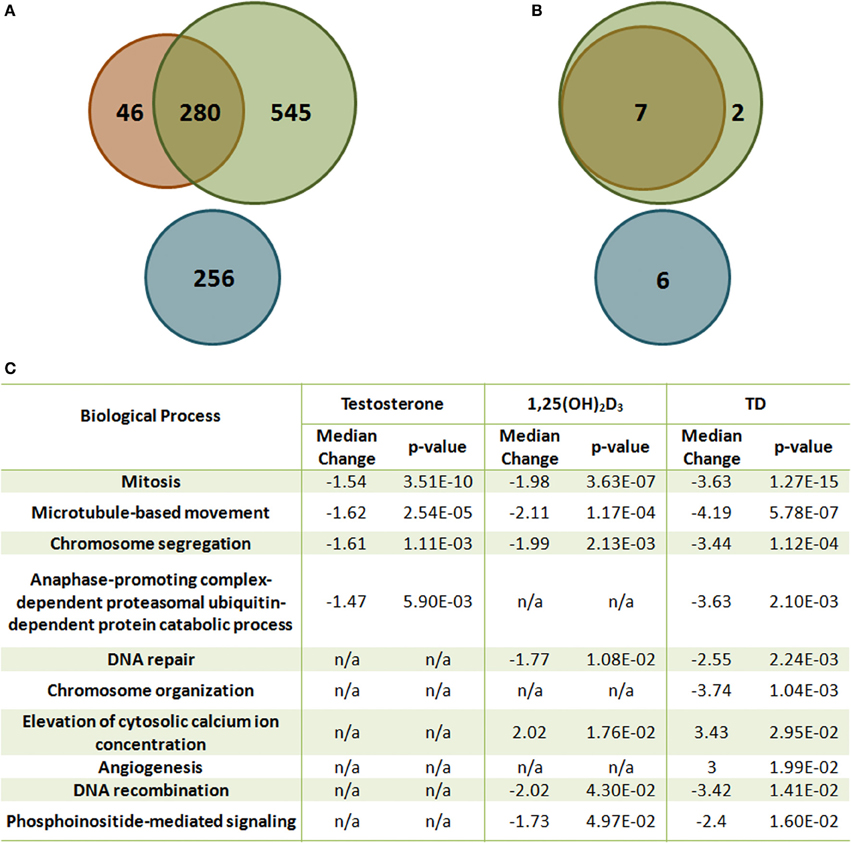
Figure 2. The effect of T and 1,25(OH)2D3 on mRNA and miRNA expression in LNCaP cells. (A) Venn diagram analysis of the gene expression microarray data [Green: 1,25(OH)2D3-moduated, Orange: androgen-modulated, Blue: synergistically modulated genes by androgen and 1,25(OH)2D3]. (B) Venn diagram analysis of the miRNA microarray data [Green: 1,25(OH)2D3-modulated, Orange: androgen-modulated, Blue: synergistically modulated genes by androgen and 1,25(OH)2D3]. (C) Gene set enrichment analysis of representative gene sets identified as significantly enriched after 1,25(OH)2D3 treatment in the presence or absence of androgen in LNCaP cells. False discovery rate <5%.
More than 50% of the responsive genes found from microarray data lack functional response elements in their promoters when comparing to existing genome-wide screens for VDREs and androgen responsive database (Wang et al., 2005; Jiang et al., 2009), raising issues regarding the regulation of these genes, particularly genes that are only expressed if both hormones are present.
The anti-neoplastic effect of vitamin D has been linked to its regulation of miRNA levels. This include the repression of miR-181ab expression (Wang et al., 2009) and the induction of miR-100, miR-125b, and miR-22 levels by 1,25(OH)2D3 (Alvarez-Diaz et al., 2012; Giangreco et al., 2013). Dysregulated miR-106b expression, which is required for the 1,25(OH)2D3-induced feed-forward loop regulating p21 expression in non-malignant RWPE-1 cells, has also been implicated in prostate cancer biology (Poliseno et al., 2010; Thorne et al., 2011). Microarray analysis that interrogates the differential miRNA expression in LNCaP cells after treatment with 1,25(OH)2D3 and testosterone, either alone or in combination suggests that VDR plays a critical role in miRNA regulation (Wang et al., 2011) and further highlights the important interactions between VDR- and AR-mediated miRNA expression. These include the additive induction of miR-22, miR-29ab, miR-134, miR-371-5p, miR-663, and miR-1207-5p and the synergistic down-regulation of the oncogenic miR-17/92 cluster by testosterone and 1,25(OH)2D3. Both miR-22 and members of the miR-29 family are candidate tumor suppressors (Alvarez-Diaz et al., 2012; Szczyrba et al., 2013; Wu et al., 2013) and their induction is consistent with the anti-proliferative effect of vitamin D in prostate cancer. In comparison, elevated miR-371-5p and miR-663 expression have been correlated with cancer progression and miR-663 expression positively associates with the Gleason score used to stage prostate cancer (Zhou et al., 2012; Liu et al., 2013; Jiao et al., 2014). In contrast, the miR-17/92 cluster is known to play an oncogenic role and its expression has been linked to more advanced prostate cancer (He et al., 2005; Volinia et al., 2006; Sylvestre et al., 2007; Yu et al., 2008; Diosdado et al., 2009; Trompeter et al., 2011; Yang et al., 2013). In addition, this cluster is a well-validated target for c-Myc, which itself is a direct target of VDR (Simpson et al., 1987; O'Donnell et al., 2005), and a recent report has proposed a regulatory role for the miR-17/92 cluster on PPARα levels, linking miR-17/92 to energy metabolism in prostate cancer cells (Wang et al., 2013). This concurrent analysis of VDR- and AR-mediated mRNA and miRNA expression reveals an extensive and complex transcription network that interconnects c-Myc, PPARα and other transcription factor-mediated signaling, which is only active when both androgen and vitamin D are present. A recent comprehensive analysis of 24 nuclear receptors and 14 transcription factors (TFs) in the MCF-7 breast cancer cell line has demonstrated a similar finding and has identified genomic regions enriched with nuclear receptors and TFs binding sites, which generates extensive regulatory networks that may modulate target gene expression (Kittler et al., 2013). Such functional interactions between nuclear receptors and TFs, including the antagonistic interaction between RARs and AR and PPARδ (Rivera-Gonzalez et al., 2012; Kittler et al., 2013), and the agonistic interaction between VDR and AR (Wang et al., 2011) provide valuable information that can be used to improve cancer prevention and therapy. The functional interactions between AR and VDR, as well as other nuclear receptors and TFs may also be important for disease management, especially now that nutritional intervention has become more widely accepted as an effective approach to prevent cancer progression. These experimental data suggest that 1,25(OH)2D3, and androgens as well as other hormones and growth factors trigger at least three mechanisms to modulate global gene expression. These include AR- and VDR-mediated gene transactivation; miRNA-mediated mRNA degradation and translational repression; and transcription factor-mediated feed-forward signaling. These mechanisms do not appear to be mutually exclusive and act together to regulate many vitamin D- and androgen-mediated cellular processes that have significant implication in prostate carcinogenesis.
Intermediate Metabolism: The Warburg Effect
A number of studies have suggested that vitamin D has a novel role in regulating energy metabolism. The vitamin D receptor knockout (VDRKO) and the Cyp27b1 knockout (Cyp27b1KO) mice exhibit elevated energy expenditure with subsequent loss of body fat over time (Narvaez et al., 2009; Wong et al., 2011). In human adipocytes, 1,25(OH)2D3 inhibits uncoupling protein-1 expression and alters Ca2+ homeostasis, suggesting a regulatory role of vitamin D in thermogenesis and provides rationale for the observed lean phenotype in VDRKO and Cyp27b1KO mice (Xue et al., 1998; Shi et al., 2001, 2002). Similarly, both 25(OH)D3 and 1,25(OH)2D3 promotes lipogenesis in primary human preadipocytes, adipocyte and adipose-derived mesenchymal progenitor cells, which is associated with increased expression of differentiation markers C/EBPα and PPARγ (Nimitphong et al., 2012; Narvaez et al., 2013). However, this effect may be cell type and lineage specific since 1,25(OH)2D3 inhibits lipid accumulation in mouse 3T3-L1 preadipocytes and prevents high fat diet-induced fatty liver syndrome in Sprague–Dawley male rats (Rayalam et al., 2008; Yin et al., 2012).
In T47D breast cancer cells, 1,25(OH)2D3 induces lipid synthesis, which has been associated with its effect on cell differentiation and reduced cell growth (Lazzaro et al., 2000). This lipogenic effect of 1,25(OH)2D3 is recapitulated in LNCaP cells and is enhanced in the presence of androgen (Esquenet et al., 1997; Wang et al., 2013), highlighting the coordinated effect of AR and VDR signaling. Increase in PPARα expression and its associated lipogenic gene signature, including the elevation of fatty acid synthase (FASN) expression, accounts for vitamin D- and androgen-induced lipid production. However, this occurs without significant changes in nuclear sterol regulatory element-binding protein (SREBP-1) levels. Nuclear activation of SREBP-1 has been implicated in de novo lipogenesis in more aggressive cancers, including prostate cancer (Menendez and Lupu, 2007; Huang et al., 2012). A recent comprehensive parallel analysis of various genomic studies using prostate cancer cell lines has uncovered a critical regulatory role of AR in the energy metabolic network, with lipid synthesis being the predominate AR-regulated process. These data suggest that altered AR signaling and its effects on the downstream targets of calcium/calmodulin-dependent protein kinase kinase 2, beta (CAMKK2), which regulates the activity of a key energy sensor AMP-activated protein kinase (AMPK), promotes the metabolic switch that provides the energy for prostate cancer growth and progression (Massie et al., 2011). These data suggest a divergent role of lipid production in prostate tumors: SREBP-1 dependent up-regulation of fatty acids production for phospholipid and membrane synthesis and signaling molecules that are essential for tumor progression (Currie et al., 2013; Soga, 2013); or SREBP-1-independent elevation of neutral and inactive lipid accumulation which restricts energy expenditure and limits tumor growth.
In addition to the modulation of lipid metabolism by vitamin D and androgen, qPCR analysis has suggested a regulatory role of these two hormones on the TCA cycle in prostate cancer cells. In most normal cells, the TCA cycle is utilized to generate energy for normal cellular functions. This process is relatively slow and ATP production does not meet the demand for highly proliferative cancer cells. As a result, cancer cells often disengage mitochondrial oxidative phosphorylation from glycolysis for rapid ATP production by employing the fermentation process, a process referred as the Warburg effect (Warburg et al., 1927; Warburg, 1956; Soga, 2013). Prostate cancer cells are a notable exception, and the metabolic switch that occurs is more appropriately regarded as an “anti-Warburg” effect. The prostate gland normally secrets high levels of citrate into the seminal fluid, a function that is supported by a truncated TCA cycle activity. The prostate has the highest levels of intracellular zinc of any tissue in the body. This high level of zinc inactivates m-aconitase 2 activity, the enzyme that converts citrate to isocitrate in the mitochondria. In prostate cancer cells, zinc transporters are down-regulated, which leads to lower intracellular zinc levels. This restores m-aconitase 2 function and the conversion of citrate to isocitrate for ATP production via the TCA cycle (Costello and Franklin, 1991a,b). This is supported by both clinical and in vitro data, demonstrating a minimal reliance of prostate cancer cells on glycolysis for proliferation especially during the early phases of tumor progression. This precludes the usage of fluorine-18-labeled 2-deoxy-2-fluoro-D-glucose (FDG-PET) for prostate cancer detection and diagnosis (Hofer et al., 1999; Jadvar, 2011). In comparison, androgen stimulates glucose usage to facilitate citrate accumulation in normal prostate epithelial cells (Harkonen, 1981; Harkonen et al., 1982) and this androgenic effect is maintained in androgen responsive prostate cancer cells, although in these cells, elevated citrate is funneled for the production of lipid (Moon et al., 2011).
In LNCaP cells, 1,25(OH)2D3 and androgen together down-regulate mitochondrial thiamine pyrophosphate (TPP) carrier (SLC25A19) and up-regulates two zinc transporters, (SLC39A1 and SLC39A11) (supplemental data to Wang et al., 2011). Low expression of SLC39A1 in adenocarcinomatous glands and PIN foci has been documented and linked to depleted zinc levels (Franklin et al., 2005). In comparison, SLC39A11 is less well-characterized, but studies have shown that it is abundantly expressed in murine testes and digestive system, and is associated with zinc import (Yu et al., 2013). This suggests that vitamin D and androgen cooperate to reset zinc levels, inhibiting m-aconitase activity in prostate cancer cells. In comparison, down-regulation of the TPP carrier, SLC25A19 (Lindhurst et al., 2006; Kang and Samuels, 2008) affects mitochondrial coenzyme TPP levels, leading to decreased activities of pyruvate dehydrogenase (PDH) and alpha-ketoglutarate dehydrogenase (OGDH) activities. In comparison, in vivo studies have shown that administration of testosterone up-regulates the expression and activities of PDH and mitochondrial aspartate aminotransferase to increase the substrate pools for citrate synthesis, acetyl-CoA and oxaloacetate (Costello and Franklin, 1993; Qian et al., 1993). This suggests that vitamin D and androgen supplementation facilitate the reversion of the metabolic switch that occurs during prostate carcinogenesis by preventing citrate oxidation, partially restoring the normal prostatic function and shunting citrate into the cytoplasm for secretion and lipid synthesis (Figure 3). This is supported by the observation that LNCaP cells retain the sensitivity to androgen-induced citrate production and accumulation (Franklin et al., 1995). This suggests that vitamin D facilitates and maintains this differentiated phenotype, rendering prostate cancer cells less aggressive. This also suggests that maintaining or restoring adequate levels of androgen, accompanied by vitamin D supplement will significantly delay prostate cancer progression in aging men.
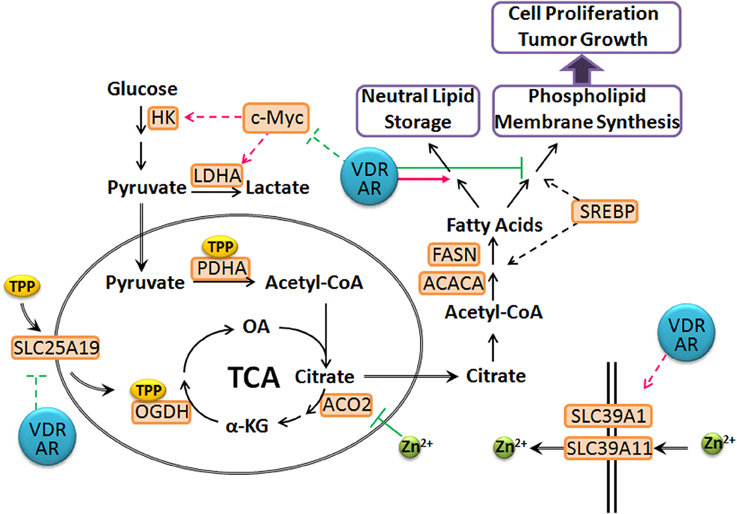
Figure 3. Proposed model of T- and 1,25(OH)2D3-mediated prostate cancer metabolism. The VDR and AR axes modulate the expression of SLC25A19 and SLC39A11, leading to elevated intracellular zinc levels and deplete mitochondrial TPP pool, resulting in a truncated TCA cycle. Citrate is shunted into lipid synthesis and storage instead of phospholipid and membrane synthesis, which prevents cancer cell proliferation. In addition, VDR and AR repress c-Myc levels and associated metabolic reprogramming to maintain prostate cancer cells in a more differentiated state. (dashed line: transcriptional regulation; green: inhibition; magenta: stimulation).
To further highlight the impact of vitamin D and androgen on resetting cancer cell metabolism, 1,25(OH)2D3 and androgen also down-regulate c-Myc levels, whose many functions include metabolic reprogramming to drive tumor progression, including the induction of glycolysis and glutaminolysis (Shim et al., 1998; Wise et al., 2008; Soga, 2013; Zirath et al., 2013). While there is good evidence suggesting a positive correlation between serum glutamate levels and more aggressive prostate cancer (Koochekpour et al., 2012), the dependence of prostate cancer on glutaminolysis for energy generation and progression is not well-studied. Nevertheless, it is reasonable to suggest that in response to vitamin D and androgen stimulation, prostate cancer cells reverse or block the metabolic switch that occurs early in the course of the disease and further blocks c-Myc-mediated metabolic reprogramming, which may occur independently of the initial metabolic switch.
Conclusion
Recent studies have shown a complex relationship between vitamin D3- and androgen-mediated signaling in the normal prostate and prostate cancer through their coordinated effect on mRNA and miRNA transcription, cell proliferation and cancer metabolism. These data suggest that the effect of vitamin D3 on global gene expression is dependent on the activity of androgen and their combined effect on miRNA transcription and other TFs. Phenotypically, the two hormones maintain normal prostatic metabolism to prevent de-differentiation of prostate cancer cells into more aggressive phenotype. These newly emerging data provide a explanation for the discrepancies observed from epidemiological and experimental studies of vitamin D3 in prostate cancer since these studies do not take the synergistic interactions between the two pathways into account. These data also suggest that maintenance of adequate levels of vitamin D3 and androgen will slow or halt prostate cancer progression especially for patients diagnosed with early stage, locally confined disease. Case-control clinical studies will be needed to fully evaluate the risk and benefit of combining these two hormones in prostate cancer patients.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Wei-Lin W. Wang would like to acknowledge the DAMD for Pre-doctoral support (PC102080).
References
Altekruse, S. F., Huang, L., Cucinelli, J. E., McNeel, T. S., Wells, K. M., and Oliver, M. N. (2010). Spatial patterns of localized-stage prostate cancer incidence among white and black men in the southeastern United States, 1999-2001. Cancer Epidemiol. Biomarkers Prev. 19, 1460–1467. doi: 10.1158/1055-9965.EPI-09-1310
Alvarez-Diaz, S., Valle, N., Ferrer-Mayorga, G., Lombardia, L., Herrera, M., Dominguez, O., et al. (2012). MicroRNA-22 is induced by vitamin D and contributes to its antiproliferative, antimigratory and gene regulatory effects in colon cancer cells. Hum. Mol. Genet. 21, 2157–2165. doi: 10.1093/hmg/dds031
Barnett, C. M., Nielson, C. M., Shannon, J., Chan, J. M., Shikany, J. M., Bauer, D. C., et al. (2010). Serum 25-OH vitamin D levels and risk of developing prostate cancer in older men. Cancer Causes Control 21, 1297–1303. doi: 10.1007/s10552-010-9557-y
Beer, T. M., and Myrthue, A. (2006). Calcitriol in the treatment of prostate cancer. Anticancer Res. 26, 2647–2651.
Blutt, S. E., Polek, T. C., Stewart, L. V., Kattan, M. W., and Weigel, N. L. (2000). A calcitriol analogue, EB1089, inhibits the growth of LNCaP tumors in nude mice. Cancer Res. 60, 779–782.
Chadha, M. K., Tian, L., Mashtare, T., Payne, V., Silliman, C., Levine, E., et al. (2010). Phase 2 trial of weekly intravenous 1,25 dihydroxy cholecalciferol (calcitriol) in combination with dexamethasone for castration-resistant prostate cancer. Cancer 116, 2132–2139. doi: 10.1002/cncr.24973
Chan, J. S., Beer, T. M., Quinn, D. I., Pinski, J. K., Garzotto, M., Sokoloff, M., et al. (2008). A phase II study of high-dose calcitriol combined with mitoxantrone and prednisone for androgen-independent prostate cancer. BJU Int. 102, 1601–1606. doi: 10.1111/j.1464-410X.2008.08017.x
Costello, L. C., and Franklin, R. B. (1991a). Concepts of citrate production and secretion by prostate. 1. metabolic relationships. Prostate 18, 25–46. doi: 10.1002/pros.2990180104
Costello, L. C., and Franklin, R. B. (1991b). Concepts of citrate production and secretion by prostate: 2. hormonal relationships in normal and neoplastic prostate. Prostate 19, 181–205. doi: 10.1002/pros.2990190302
Costello, L. C., and Franklin, R. B. (1993). Testosterone regulates pyruvate dehydrogenase activity of prostate mitochondria. Horm. Metab. Res. 25, 268–270. doi: 10.1055/s-2007-1002094
Currie, E., Schulze, A., Zechner, R., Walther, T. C., and Farese, R. V. Jr. (2013). Cellular fatty acid metabolism and cancer. Cell Metab. 18, 153–161. doi: 10.1016/j.cmet.2013.05.017
Deeb, K. K., Trump, D. L., and Johnson, C. S. (2007). Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat. Rev. Cancer 7, 684–700. doi: 10.1038/nrc2196
Diosdado, B., van de Wiel, M. A., Terhaar Sive Droste, J. S., Mongera, S., Postma, C., Meijerink, W. J., et al. (2009). MiR-17-92 cluster is associated with 13q gain and c-myc expression during colorectal adenoma to adenocarcinoma progression. Br. J. Cancer 101, 707–714. doi: 10.1038/sj.bjc.6605037
Esquenet, M., Swinnen, J. V., Heyns, W., and Verhoeven, G. (1995). Triiodothyronine modulates growth, secretory function and androgen receptor concentration in the prostatic carcinoma cell line LNCaP. Mol. Cell. Endocrinol. 109, 105–111. doi: 10.1016/0303-7207(95)03490-X
Esquenet, M., Swinnen, J. V., Van Veldhoven, P. P., Denef, C., Heyns, W., and Verhoeven, G. (1997). Retinoids stimulate lipid synthesis and accumulation in LNCaP prostatic adenocarcinoma cells. Mol. Cell. Endocrinol. 136, 37–46. doi: 10.1016/S0303-7207(97)00210-4
Flaig, T. W., Barqawi, A., Miller, G., Kane, M., Zeng, C., Crawford, E. D., et al. (2006). A phase II trial of dexamethasone, vitamin D, and carboplatin in patients with hormone-refractory prostate cancer. Cancer 107, 266–274. doi: 10.1002/cncr.21982
Franklin, R. B., Feng, P., Milon, B., Desouki, M. M., Singh, K. K., Kajdacsy-Balla, A., et al. (2005). hZIP1 zinc uptake transporter down regulation and zinc depletion in prostate cancer. Mol. Cancer 4, 32. doi: 10.1186/1476-4598-4-32
Franklin, R. B., Juang, H. H., Zou, J., and Costello, L. C. (1995). Regulation of citrate metabolism by androgen in the LNCaP human prostate carcinoma cell line. Endocrine 3, 603–607. doi: 10.1007/BF02953026
Garland, C. F., Gorham, E. D., Mohr, S. B., and Garland, F. C. (2009). Vitamin D for cancer prevention: global perspective. Ann. Epidemiol. 19, 468–483. doi: 10.1016/j.annepidem.2009.03.021
Giangreco, A. A., Vaishnav, A., Wagner, D., Finelli, A., Fleshner, N., Van der, K. T., et al. (2013). Tumor suppressor microRNAs, miR-100 and -125b, are regulated by 1,25-dihydroxyvitamin D in primary prostate cells and in patient tissue. Cancer Prev. Res. (Phila) 6, 483–494. doi: 10.1158/1940-6207.CAPR-12-0253
Giovannucci, E. (2009). Expanding roles of vitamin, D. J. Clin. Endocrinol. Metab. 94, 418–420. doi: 10.1210/jc.2008-2695
Harkonen, P. (1981). Androgenic control of glycolysis, the pentose cycle and pyruvate dehydrogenase in the rat ventral prostate. J. Steroid Biochem. 14, 1075–1084. doi: 10.1016/0022-4731(81)90219-3
Harkonen, P. L., Kostian, M. L., and Santti, R. S. (1982). Indirect androgenic control of citrate accumulation in rat ventral prostate. Arch. Androl. 8, 107–116. doi: 10.3109/01485018208987026
He, L., Thomson, J. M., Hemann, M. T., Hernando-Monge, E., Mu, D., Goodson, S., et al. (2005). A microRNA polycistron as a potential human oncogene. Nature 435, 828–833. doi: 10.1038/nature03552
Hofer, C., Laubenbacher, C., Block, T., Breul, J., Hartung, R., and Schwaiger, M. (1999). Fluorine-18-fluorodeoxyglucose positron emission tomography is useless for the detection of local recurrence after radical prostatectomy. Eur. Urol. 36, 31–35. doi: 10.1159/000019923
Holt, S. K., Kolb, S., Fu, R., Horst, R., Feng, Z., and Stanford, J. L. (2013). Circulating levels of 25-hydroxyvitamin D and prostate cancer prognosis. Cancer Epidemiol. 37, 666–670. doi: 10.1016/j.canep.2013.07.005
Hsieh, T. Y., Ng, C. Y., Mallouh, C., Tazaki, H., and Wu, J. M. (1996). Regulation of growth, PSA/PAP and androgen receptor expression by 1 alpha,25-dihydroxyvitamin D3 in the androgen-dependent LNCaP cells. Biochem. Biophys. Res. Commun. 223, 141–146. doi: 10.1006/bbrc.1996.0859
Huang, W. C., Li, X., Liu, J., Lin, J., and Chung, L. W. (2012). Activation of androgen receptor, lipogenesis, and oxidative stress converged by SREBP-1 is responsible for regulating growth and progression of prostate cancer cells. Mol. Cancer Res. 10, 133–142. doi: 10.1158/1541-7786.MCR-11-0206
Jadvar, H. (2011). Prostate cancer: PET with 18F-FDG, 18F- or 11C-acetate, and 18F- or 11C-choline. J. Nucl. Med. 52, 81–89. doi: 10.2967/jnumed.110.077941
Jiang, M., Ma, Y., Chen, C., Fu, X., Yang, S., Li, X., et al. (2009). Androgen-responsive gene database: integrated knowledge on androgen-responsive genes. Mol. Endocrinol. 23, 1927–1933. doi: 10.1210/me.2009-0103
Jiao, L., Deng, Z., Xu, C., Yu, Y., Li, Y., Yang, C., et al. (2014). MicroRNA-663 induces castration-resistant prostate cancer transformation and predicts clinical recurrence. J. Cell. Physiol. 229, 834–844. doi: 10.1002/jcp.24510
Kang, J., and Samuels, D. C. (2008). The evidence that the DNC (SLC25A19) is not the mitochondrial deoxyribonucleotide carrier. Mitochondrion 8, 103–108. doi: 10.1016/j.mito.2008.01.001
Kittler, R., Zhou, J., Hua, S., Ma, L., Liu, Y., Pendleton, E., et al. (2013). A comprehensive nuclear receptor network for breast cancer cells. Cell Rep. 3, 538–551. doi: 10.1016/j.celrep.2013.01.004
Koochekpour, S., Majumdar, S., Azabdaftari, G., Attwood, K., Scioneaux, R., Subramani, D., et al. (2012). Serum glutamate levels correlate with gleason score and glutamate blockade decreases proliferation, migration, and invasion and induces apoptosis in prostate cancer cells. Clin. Cancer Res. 18, 5888–5901. doi: 10.1158/1078-0432.CCR-12-1308
Krishnan, A. V., Peehl, D. M., and Feldman, D. (2003). Inhibition of prostate cancer growth by vitamin D: regulation of target gene expression. J. Cell. Biochem. 88, 363–371. doi: 10.1002/jcb.10334
Krishnan, A. V., Shinghal, R., Raghavachari, N., Brooks, J. D., Peehl, D. M., and Feldman, D. (2004). Analysis of vitamin D-regulated gene expression in LNCaP human prostate cancer cells using cDNA microarrays. Prostate 59, 243–251. doi: 10.1002/pros.20006
Lazzaro, G., Agadir, A., Qing, W., Poria, M., Mehta, R. R., Moriarty, R. M., et al. (2000). Induction of differentiation by 1alpha-hydroxyvitamin D(5) in T47D human breast cancer cells and its interaction with vitamin D receptors. Eur. J. Cancer 36, 780–786. doi: 10.1016/S0959-8049(00)00016-2
Leman, E. S., Arlotti, J. A., Dhir, R., and Getzenberg, R. H. (2003). Vitamin D and androgen regulation of prostatic growth. J. Cell. Biochem. 90, 138–147. doi: 10.1002/jcb.10605
Lindhurst, M. J., Fiermonte, G., Song, S., Struys, E., De Leonardis, F., Schwartzberg, P. L., et al. (2006). Knockout of Slc25a19 causes mitochondrial thiamine pyrophosphate depletion, embryonic lethality, CNS malformations, and anemia. Proc. Natl. Acad. Sci. U.S.A. 103, 15927–15932. doi: 10.1073/pnas.0607661103
Liu, R. Y., Diao, C. F., Zhang, Y., Wu, N., Wan, H. Y., Nong, X. Y., et al. (2013). miR-371-5p down-regulates pre mRNA processing factor 4 homolog B (PRPF4B) and facilitates the G1/S transition in human hepatocellular carcinoma cells. Cancer Lett. 335, 351–360. doi: 10.1016/j.canlet.2013.02.045
Lundqvist, J., Norlin, M., and Wikvall, K. (2011). 1alpha,25-Dihydroxyvitamin D3 exerts tissue-specific effects on estrogen and androgen metabolism. Biochim. Biophys. Acta 1811, 263–270. doi: 10.1016/j.bbalip.2011.01.004
Maguire, O., Pollock, C., Martin, P., Owen, A., Smyth, T., Doherty, D., et al. (2012). Regulation of CYP3A4 and CYP3A5 expression and modulation of “intracrine” metabolism of androgens in prostate cells by liganded vitamin D receptor. Mol. Cell. Endocrinol. 364, 54–64. doi: 10.1016/j.mce.2012.08.007
Marshall, D. T., Savage, S. J., Garrett-Mayer, E., Keane, T. E., Hollis, B. W., Horst, R. L., et al. (2012). Vitamin D3 supplementation at 4000 international units per day for one year results in a decrease of positive cores at repeat biopsy in subjects with low-risk prostate cancer under active surveillance. J. Clin. Endocrinol. Metab. 97, 2315–2324. doi: 10.1210/jc.2012-1451
Massie, C. E., Lynch, A., Ramos-Montoya, A., Boren, J., Stark, R., Fazli, L., et al. (2011). The androgen receptor fuels prostate cancer by regulating central metabolism and biosynthesis. EMBO J. 30, 2719–2733. doi: 10.1038/emboj.2011.158
McNeal, J. E. (1968). Regional morphology and pathology of the prostate. Am. J. Clin. Pathol. 49, 347–357.
Menendez, J. A., and Lupu, R. (2007). Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat. Rev. Cancer 7, 763–777. doi: 10.1038/nrc2222
Miller, G. J. (1998). Vitamin D and prostate cancer: biologic interactions and clinical potentials. Cancer Metastasis Rev. 17, 353–360. doi: 10.1023/A:1006102124548
Moon, J. S., Jin, W. J., Kwak, J. H., Kim, H. J., Yun, M. J., Kim, J. W., et al. (2011). Androgen stimulates glycolysis for de novo lipid synthesis by increasing the activities of hexokinase 2 and 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 2 in prostate cancer cells. Biochem. J. 433, 225–233. doi: 10.1042/BJ20101104
Mordan-McCombs, S., Brown, T., Wang, W. L., Gaupel, A. C., Welsh, J., and Tenniswood, M. (2010). Tumor progression in the LPB-Tag transgenic model of prostate cancer is altered by vitamin D receptor and serum testosterone status. J. Steroid Biochem. Mol. Biol. 121, 368–371. doi: 10.1016/j.jsbmb.2010.03.062
Murthy, S., Agoulnik, I. U., and Weigel, N. L. (2005). Androgen receptor signaling and vitamin D receptor action in prostate cancer cells. Prostate 64, 362–372. doi: 10.1002/pros.20251
Narvaez, C. J., Matthews, D., Broun, E., Chan, M., and Welsh, J. (2009). Lean phenotype and resistance to diet-induced obesity in vitamin D receptor knockout mice correlates with induction of uncoupling protein-1 in white adipose tissue. Endocrinology 150, 651–661. doi: 10.1210/en.2008-1118
Narvaez, C. J., Simmons, K. M., Brunton, J., Salinero, A., Chittur, S. V., and Welsh, J. E. (2013). Induction of STEAP4 correlates with 1,25-dihydroxyvitamin D3 stimulation of adipogenesis in mesenchymal progenitor cells derived from human adipose tissue. J. Cell. Physiol. 228, 2024–2036. doi: 10.1002/jcp.24371
Nimitphong, H., Holick, M. F., Fried, S. K., and Lee, M. J. (2012). 25-hydroxyvitamin D(3) and 1,25-dihydroxyvitamin D(3) promote the differentiation of human subcutaneous preadipocytes. PLoS ONE 7:e52171. doi: 10.1371/journal.pone.0052171
Nimptsch, K., Platz, E. A., Willett, W. C., and Giovannucci, E. (2012). Association between plasma 25-OH vitamin D and testosterone levels in men. Clin. Endocrinol. (Oxf.) 77, 106–112. doi: 10.1111/j.1365-2265.2012.04332.x
O'Donnell, K. A., Wentzel, E. A., Zeller, K. I., Dang, C. V., and Mendell, J. T. (2005). c-Myc-regulated microRNAs modulate E2F1 expression. Nature 435, 839–843. doi: 10.1038/nature03677
Park, S. Y., Cooney, R. V., Wilkens, L. R., Murphy, S. P., Henderson, B. E., and Kolonel, L. N. (2010). Plasma 25-hydroxyvitamin D and prostate cancer risk: the multiethnic cohort. Eur. J. Cancer 46, 932–936. doi: 10.1016/j.ejca.2009.12.030
Peehl, D. M., Krishnan, A. V., and Feldman, D. (2003). Pathways mediating the growth-inhibitory actions of vitamin D in prostate cancer. J. Nutr. 133, 2461S–2469S.
Pilz, S., Frisch, S., Koertke, H., Kuhn, J., Dreier, J., Obermayer-Pietsch, B., et al. (2011). Effect of vitamin D supplementation on testosterone levels in men. Horm. Metab. Res. 43, 223–225. doi: 10.1055/s-0030-1269854
Poliseno, L., Salmena, L., Riccardi, L., Fornari, A., Song, M. S., Hobbs, R. M., et al. (2010). Identification of the miR-106b~25 microRNA cluster as a proto-oncogenic PTEN-targeting intron that cooperates with its host gene MCM7 in transformation. Sci. Signal. 3, ra29. doi: 10.1126/scisignal.200059
Qian, K., Franklin, R. B., and Costello, L. C. (1993). Testosterone regulates mitochondrial aspartate aminotransferase gene expression and mRNA stability in prostate. J. Steroid Biochem. Mol. Biol. 44, 13–19. doi: 10.1016/0960-0760(93)90146-N
Qiao, S., and Tuohimaa, P. (2004). The role of long-chain fatty-acid-CoA ligase 3 in vitamin D3 and androgen control of prostate cancer LNCaP cell growth. Biochem. Biophys. Res. Commun. 319, 358–368. doi: 10.1016/j.bbrc.2004.05.014
Rayalam, S., Della-Fera, M. A., Ambati, S., Yang, J. Y., Park, H. J., and Baile, C. A. (2008). Enhanced effects of 1,25(OH)(2)D(3) plus genistein on adipogenesis and apoptosis in 3T3-L1 adipocytes. Obesity (Silver Spring) 16, 539–546. doi: 10.1038/oby.2007.90
van der, R. H., Coebergh, J. W., and de Vries, E. (2009). Sunlight, vitamin D and the prevention of cancer: a systematic review of epidemiological studies. Eur. J. Cancer Prev. 18, 458–475. doi: 10.1097/CEJ.0b013e32832f9bb1
Rivera-Gonzalez, G. C., Droop, A. P., Rippon, H. J., Tiemann, K., Pellacani, D., Georgopoulos, L. J., et al. (2012). Retinoic acid and androgen receptors combine to achieve tissue specific control of human prostatic transglutaminase expression: a novel regulatory network with broader significance. Nucleic Acids Res. 40, 4825–4840. doi: 10.1093/nar/gks143
Scher, H. I., Jia, X., Chi, K., de Wit, R., Berry, W. R., Albers, P., et al. (2011). Randomized, open-label phase III trial of docetaxel plus high-dose calcitriol versus docetaxel plus prednisone for patients with castration-resistant prostate cancer. J. Clin. Oncol. 29, 2191–2198. doi: 10.1200/JCO.2010.32.8815
Schwartz, G. G., and Skinner, H. G. (2007). Vitamin D status and cancer: new insights. Curr. Opin. Clin. Nutr. Metab. Care 10, 6–11. doi: 10.1097/MCO.0b013e328011aa60
Shamseddine, A., Farhat, F. S., Elias, E., Khauli, R. B., Saleh, A., and Bulbul, M. A. (2013). High-dose calcitriol, docetaxel and zoledronic acid in patients with castration-resistant prostate cancer: a phase II study. Urol. Int. 90, 56–61. doi: 10.1159/000343780
Shi, H., Norman, A. W., Okamura, W. H., Sen, A., and Zemel, M. B. (2001). 1alpha,25-Dihydroxyvitamin D3 modulates human adipocyte metabolism via nongenomic action. FASEB J. 15, 2751–2753. doi: 10.1096/fj.01-0584fje
Shi, H., Norman, A. W., Okamura, W. H., Sen, A., and Zemel, M. B. (2002). 1alpha,25-dihydroxyvitamin D3 inhibits uncoupling protein 2 expression in human adipocytes. FASEB J. 16, 1808–1810. doi: 10.1096/fj.02-0255fje
Shim, H., Chun, Y. S., Lewis, B. C., and Dang, C. V. (1998). A unique glucose-dependent apoptotic pathway induced by c-Myc. Proc. Natl. Acad. Sci. U.S.A. 95, 1511–1516. doi: 10.1073/pnas.95.4.1511
Siegel, R., Ma, J., Zou, Z., and Jemal, A. (2014). Cancer statistics, 2014. CA Cancer J. Clin. 64, 9–29. doi: 10.3322/caac.21208
Simpson, R. U., Hsu, T., Begley, D. A., Mitchell, B. S., and Alizadeh, B. N. (1987). Transcriptional regulation of the c-myc protooncogene by 1,25-dihydroxyvitamin D3 in HL-60 promyelocytic leukemia cells. J. Biol. Chem. 262, 4104–4108.
Soga, T. (2013). Cancer metabolism: key players in metabolic reprogramming. Cancer Sci. 104, 275–281. doi: 10.1111/cas.12085
Srinivas, S., and Feldman, D. (2009). A phase II trial of calcitriol and naproxen in recurrent prostate cancer. Anticancer Res. 29, 3605–3610.
Sylvestre, Y., De, G. V., Querido, E., Mukhopadhyay, U. K., Bourdeau, V., Major, F., et al. (2007). An E2F/miR-20a autoregulatory feedback loop. J. Biol. Chem. 282, 2135–2143. doi: 10.1074/jbc.M608939200
Szczyrba, J., Nolte, E., Hart, M., Doll, C., Wach, S., Taubert, H., et al. (2013). Identification of ZNF217, hnRNP-K, VEGF-A and IPO7 as targets for microRNAs that are downregulated in prostate carcinoma. Int. J. Cancer 132, 775–784. doi: 10.1002/ijc.27731
Thorne, J., and Campbell, M. J. (2008). The vitamin D receptor in cancer. Proc. Nutr. Soc. 67, 115–127. doi: 10.1017/S0029665108006964
Thorne, J. L., Maguire, O., Doig, C. L., Battaglia, S., Fehr, L., Sucheston, L. E., et al. (2011). Epigenetic control of a VDR-governed feed-forward loop that regulates p21(waf1/cip1) expression and function in non-malignant prostate cells. Nucleic Acids Res. 39, 2045–2056. doi: 10.1093/nar/gkq875
Trompeter, H. I., Abbad, H., Iwaniuk, K. M., Hafner, M., Renwick, N., Tuschl, T., et al. (2011). MicroRNAs MiR-17, MiR-20a, and MiR-106b act in concert to modulate E2F activity on cell cycle arrest during neuronal lineage differentiation of USSC. PLoS ONE 6:e16138. doi: 10.1371/journal.pone.0016138
Trump, D. L., Deeb, K. K., and Johnson, C. S. (2010). Vitamin D: considerations in the continued development as an agent for cancer prevention and therapy. Cancer J. 16, 1–9. doi: 10.1097/PPO.0b013e3181c51ee6
Tseng, M., Breslow, R. A., DeVellis, R. F., and Ziegler, R. G. (2004). Dietary patterns and prostate cancer risk in the national health and nutrition examination survey epidemiological follow-up study cohort. Cancer Epidemiol. Biomarkers Prev. 13, 71–77. doi: 10.1158/1055-9965.EPI-03-0076
Vijayakumar, S., Mehta, R. R., Boerner, P. S., Packianathan, S., and Mehta, R. G. (2005). Clinical trials involving vitamin D analogs in prostate cancer. Cancer J. 11, 362–373. doi: 10.1097/00130404-200509000-00002
Volinia, S., Calin, G. A., Liu, C. G., Ambs, S., Cimmino, A., Petrocca, F., et al. (2006). A microRNA expression signature of human solid tumors defines cancer gene targets. Proc. Natl. Acad. Sci. U.S.A. 103, 2257–2261. doi: 10.1073/pnas.0510565103
Wagner, D., Trudel, D., Van der, K. T., Nonn, L., Giangreco, A. A., Li, D., et al. (2013). Randomized clinical trial of vitamin D3 doses on prostatic vitamin D metabolite levels and ki67 labeling in prostate cancer patients. J. Clin. Endocrinol. Metab. 98, 1498–1507. doi: 10.1210/jc.2012-4019
Wang, T. T., Tavera-Mendoza, L. E., Laperriere, D., Libby, E., MacLeod, N. B., Nagai, Y., et al. (2005). Large-scale in silico and microarray-based identification of direct 1,25-dihydroxyvitamin D3 target genes. Mol. Endocrinol. 19, 2685–2695. doi: 10.1210/me.2005-0106
Wang, W. L., Chatterjee, N., Chittur, S. V., Welsh, J., and Tenniswood, M. P. (2011). Effects of 1alpha,25 dihydroxyvitamin D3 and testosterone on miRNA and mRNA expression in LNCaP cells. Mol. Cancer 10, 58. doi: 10.1186/1476-4598-10-58
Wang, W. L., Welsh, J., and Tenniswood, M. (2013). 1,25-Dihydroxyvitamin D3 modulates lipid metabolism in prostate cancer cells through miRNA mediated regulation of PPARA. J. Steroid Biochem. Mol. Biol. 136, 247–251. doi: 10.1016/j.jsbmb.2012.09.033
Wang, X., Gocek, E., Liu, C. G., and Studzinski, G. P. (2009). MicroRNAs181 regulate the expression of p27Kip1 in human myeloid leukemia cells induced to differentiate by 1,25-dihydroxyvitamin D3. Cell Cycle 8, 736–741. doi: 10.4161/cc.8.5.7870
Warburg, O., Wind, F., and Negelein, E. (1927). The metabolism of tumors in the body. J. Gen. Physiol. 8, 519–530. doi: 10.1085/jgp.8.6.519
Washington, M. N., and Weigel, N. L. (2010). 1{alpha},25-Dihydroxyvitamin D3 inhibits growth of VCaP prostate cancer cells despite inducing the growth-promoting TMPRSS2:ERG gene fusion. Endocrinology 151, 1409–1417. doi: 10.1210/en.2009-0991
Wehr, E., Pilz, S., Boehm, B. O., Marz, W., and Obermayer-Pietsch, B. (2010). Association of vitamin D status with serum androgen levels in men. Clin. Endocrinol. (Oxf.) 73, 243–248. doi: 10.1111/j.1365-2265.2009.03777.x
Weigel, N. L. (2007). Interactions between vitamin D and androgen receptor signaling in prostate cancer cells. Nutr. Rev. 65, S116–S117. doi: 10.1301/nr.2007.aug.S116-S117
Wise, D. R., DeBerardinis, R. J., Mancuso, A., Sayed, N., Zhang, X. Y., Pfeiffer, H. K., et al. (2008). Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc. Natl. Acad. Sci. U.S.A 105, 18782–18787. doi: 10.1073/pnas.0810199105
Wong, K. E., Kong, J., Zhang, W., Szeto, F. L., Ye, H., Deb, D. K., et al. (2011). Targeted expression of human vitamin D receptor in adipocytes decreases energy expenditure and induces obesity in mice. J. Biol. Chem. 286, 33804–33810. doi: 10.1074/jbc.M111.257568
Wu, Z., Huang, X., Huang, X., Zou, Q., and Guo, Y. (2013). The inhibitory role of Mir-29 in growth of breast cancer cells. J. Exp. Clin. Cancer Res. 32, 98. doi: 10.1186/1756-9966-32-98
Xue, B., Moustaid, N., Wilkison, W. O., and Zemel, M. B. (1998). The agouti gene product inhibits lipolysis in human adipocytes via a Ca2+-dependent mechanism. FASEB J. 12, 1391–1396.
Yang, X., Du, W. W., Li, H., Liu, F., Khorshidi, A., Rutnam, Z. J., et al. (2013). Both mature miR-17-5p and passenger strand miR-17-3p target TIMP3 and induce prostate tumor growth and invasion. Nucleic Acids Res. 41, 9688–9704. doi: 10.1093/nar/gkt680
Yin, L., Raum, E., Haug, U., Arndt, V., and Brenner, H. (2009). Meta-analysis of longitudinal studies: serum vitamin D and prostate cancer risk. Cancer Epidemiol. 33, 435–445. doi: 10.1016/j.canep.2009.10.014
Yin, Y., Yu, Z., Xia, M., Luo, X., Lu, X., and Ling, W. (2012). Vitamin D attenuates high fat diet-induced hepatic steatosis in rats by modulating lipid metabolism. Eur. J. Clin. Invest. 42, 1189–1196. doi: 10.1111/j.1365-2362.2012.02706.x
Yu, Y., Wu, A., Zhang, Z., Yan, G., Zhang, F., Zhang, L., et al. (2013). Characterization of the GufA subfamily member SLC39A11/Zip11 as a zinc transporter. J. Nutr. Biochem. 24, 1697–1708. doi: 10.1016/j.jnutbio.2013.02.010
Yu, Z., Wang, C., Wang, M., Li, Z., Casimiro, M. C., Liu, M., et al. (2008). A cyclin D1/microRNA 17/20 regulatory feedback loop in control of breast cancer cell proliferation. J. Cell Biol. 182, 509–517. doi: 10.1083/jcb.200801079
Zhao, X. Y., Ly, L. H., Peehl, D. M., and Feldman, D. (1999). Induction of androgen receptor by 1alpha,25-dihydroxyvitamin D3 and 9-cis retinoic acid in LNCaP human prostate cancer cells. Endocrinology 140, 1205–1212. doi: 10.1210/endo.140.3.6561
Zhou, A. D., Diao, L. T., Xu, H., Xiao, Z. D., Li, J. H., Zhou, H., et al. (2012). beta-Catenin/LEF1 transactivates the microRNA-371-373 cluster that modulates the Wnt/beta-catenin-signaling pathway. Oncogene 31, 2968–2978. doi: 10.1038/onc.2011.461
Keywords: vitamin D, androgen, prostate, warburg, miRNA, mRNA
Citation: Wang W-LW and Tenniswood M (2014) Vitamin D, intermediary metabolism and prostate cancer tumor progression. Front. Physiol. 5:183. doi: 10.3389/fphys.2014.00183
Received: 11 March 2014; Accepted: 22 April 2014;
Published online: 15 May 2014.
Edited by:
Carsten Carlberg, University of Eastern Finland, FinlandReviewed by:
Stephen Assinder, University of Sydney, AustraliaMoray J. Campbell, Roswell Park Cancer Institute, USA
Copyright © 2014 Wang and Tenniswood. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Martin Tenniswood, Department of Biomedical Sciences, Cancer Research Center, University at Albany, State University of New York, One Discovery Drive, Rensselaer, NY 12144, USA e-mail:bXRlbm5pc3dvb2RAYWxiYW55LmVkdQ==
 Wei-Lin W. Wang
Wei-Lin W. Wang Martin Tenniswood
Martin Tenniswood