- 1 Laboratory of Liver Diseases, National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health, Bethesda, MD, USA
- 2 Department of Medicine and Molecular Science, Gunma University Graduate School of Medicine, Gunma, Japan
Liver fibrosis, or cirrhosis, is a common end-stage condition of many chronic liver diseases after incomplete recovery from hepatocyte damage. During fibrosis progression, hepatocellular damage and inflammation trigger complex cellular events that result in collagen deposition and the disruption of the normal liver architecture. Hepatic stellate cell activation and transdifferentiation into myofibroblasts are key events in liver fibrogenesis. Research findings from cell culture and animal models have revealed that the Janus kinase-signal transducer and activator of transcription (Jak-STAT) signaling pathway, which can be activated by many cytokines, growth factors, and hormones, plays a critical role in hepatic fibrogenesis. This review summarizes the biological significance of diverse cytokines and their downstream signaling protein STATs in hepatic fibrogenesis.
Introduction
Liver fibrosis, or scarring of the liver, is induced by various types of chronic liver diseases and is a major cause of morbidity and mortality worldwide. During fibrosis progression, inflammation and liver injury trigger complex cellular events that result in collagen deposition and the disruption of the normal liver architecture. Generally, following liver injury from any causes, hepatic stellate cells (HSCs) undergo activation and transformation. HSCs are considered the most important cell type for the production of collagens (Friedman, 2008). Many other types of cells, such as bone marrow-derived progenitor cells, portal fibroblasts, cholangiocytes, and hepatocytes may also contribute to the production of collagens to a lesser extent (Kaimori et al., 2007; Higashiyama et al., 2009; Novo et al., 2009). In addition, immune cells may regulate fibrogenesis via the secretion of a wide variety of growth factors and cytokines.
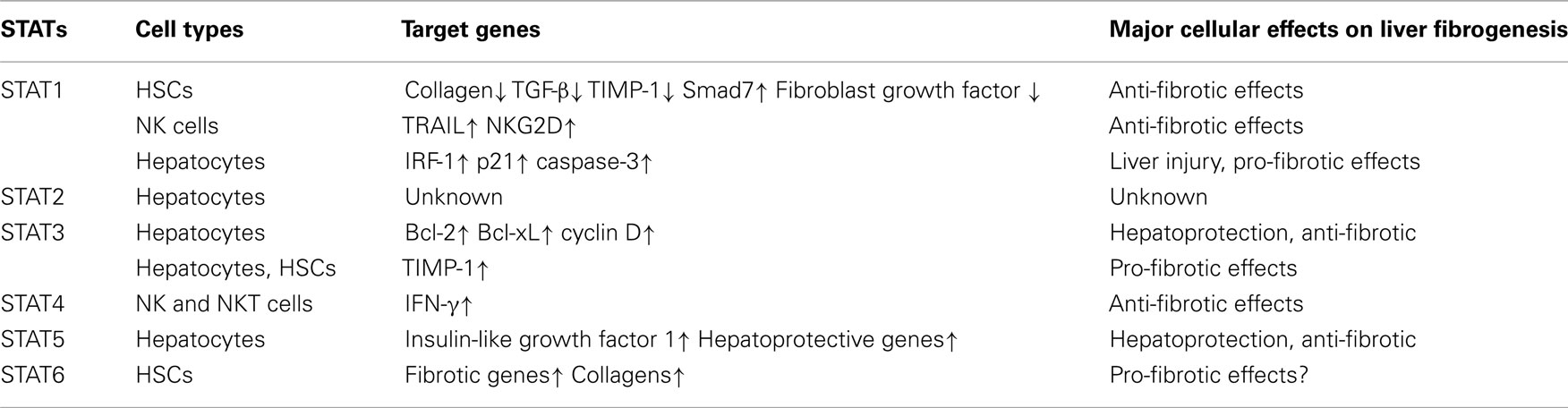
Many inflammatory cytokines have been shown to play key roles in regulating liver fibrogenesis (Bataller and Brenner, 2005; Friedman, 2008). Following liver injury, many types of cells in the liver, including Kupffer cells, hepatocytes, HSCs, natural killer cells, lymphocytes, and dendritic cells, have been shown to produce pro-inflammatory cytokines and hepatoprotective cytokines. Many of these cytokines can activate the Janus kinase–signal transducer and activator of transcription (JAK–STAT) signaling pathway in the liver, including interleukin-6 (IL-6), interferon-γ (IFN-γ), IFN-α/β, and IL-22 (Gao, 2005). Signaling through the JAK–STAT pathway is initiated when an extracellular cytokine protein binds to its corresponding transmembrane receptor complex. This leads to activation of the JAKs, including Jak1, 2, 3, and TYK2. The JAKs mediate phosphorylation at specific receptor tyrosine residues, which then serve as docking sites for the STATs (STAT1, 2, 3, 4, 5a, 5b, and 6). Studies from animal models have revealed that activation of the JAK–STAT pathway by an array of cytokines plays a variety of important functions in liver pathophysiology (Gao, 2005; Mair et al., 2011; Wang et al., 2011a; Gao et al., 2012). In this review, we update the biological significance of cytokines and their downstream STAT signaling pathways in liver fibrogenesis (Table 1).
STAT1: A Negative Regulator of Liver Fibrosis
It is well established that STAT1-deficient mice display increased sensitivity to infection by microbial pathogens. Using STAT1-deficient mice, we have previously demonstrated that STAT1 negatively regulates liver fibrosis through the inhibition of HSC proliferation and the stimulation of NK cell killing of activated HSCs (Jeong et al., 2006). Consistent with these findings, several cytokines that activate the STAT1 signaling pathway have been shown to inhibit liver fibrosis.
IFNs/STAT1 in Liver Fibrosis
STAT1 is mainly activated by IFN-α/β and IFN-γ in the liver. Treatment with IFN-α has been shown to significantly improve the serum levels of fibrotic markers and the degree of hepatic fibrosis in mice. IFN-α directly suppresses collagen gene transcription through the interaction of phosphorylated Stat1 and p300 (Inagaki et al., 2003). IFN-β treatment reduces concanavalin A-induced hepatic fibrosis via the inhibition of transforming growth factor-beta (TGF-β), basic fibroblast growth factor, collagen type I A2, and tissue inhibitor of metalloproteinase 1 (TIMP-1) mRNA expression (Tanabe et al., 2007). IFN-γ displays anti-fibrotic effects in the liver via the induction of STAT1 phosphorylation, the upregulation of Smad7 expression, and the impairment of TGF-β signaling (Weng et al., 2007).
IL-27/STAT1 in Liver Fibrosis
In addition to IFNs, IL-27 also induces STAT1 activation in the liver. IL-27 belongs to the IL-6/IL-12 cytokine family and is secreted by activated macrophages and dendritic cells. Treatment with IL-27 activates STAT1 and, to a lesser extent, STAT3 in the human HSC cell line LX2 and in primary rat HSCs (Schoenherr et al., 2010), suggesting that IL-27 may inhibit liver fibrosis. However, further studies are required to confirm the anti-fibrotic effects of the IL-27/STAT1 pathway in vivo.
STAT2
STAT2 is exclusively activated by IFN-α, IFN-β, and IFN-λ (Radaeva et al., 2002; Balagopal et al., 2010; Afdhal et al., 2011). Treatment with IFN-α induces significant STAT2 activation in primary human hepatocytes (Radaeva et al., 2002). The activation of STAT2 likely plays a key role in the antiviral effects of IFN-α in patients with viral hepatitis. Although the antiviral function of STAT2 is well documented, the role of STAT2 in liver injury and fibrosis has not yet been explored.
STAT3
A variety of cytokines have been shown to induce STAT3 activation in the liver, playing key roles in inducing the acute-phase response, protecting against hepatocellular damage, and promoting liver regeneration (Wang et al., 2011a). However, the roles of STAT3 in liver fibrogenesis remain largely unknown. Hepatocyte-specific STAT3 knockout mice displays a higher degree of liver fibrosis compared with wild-type mice in various models of liver fibrosis induced by CCl4 administration (Wang et al., 2011b), feeding with a 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC) diet (Plum et al., 2010), feeding with a choline-deficient, ethionine-supplemented (CDE) diet (Kroy et al., 2010), or upon deletion of the multidrug resistance gene 2 (Mair et al., 2010). The obvious mechanism by which hepatocyte STAT3 protects against liver fibrosis is that hepatocyte STAT3 activation prevents hepatocellular damage and subsequently reduces injury-driven liver fibrogenesis. A recent study suggests that activation of STAT3 in hepatocytes inhibits liver fibrosis by stimulating hepatocytes to produce unknown soluble factors that inhibit HSC activation (Shigekawa et al., 2011). Further studies are needed to identify these soluble factors.
Because HSC-specific STAT3 knockout mice are not available, the role of HSC STAT3 in liver fibrogenesis in vivo has not been determined. Mice or rats deficient in leptin or leptin signaling are resistant to the development of liver fibrosis (Honda et al., 2002; Ikejima et al., 2002; Saxena et al., 2002), suggesting that leptin may promote liver fibrosis via the activation of STAT3 in HSCs in vivo. In vitro experiments demonstrate that the inhibition of JAK/STAT3 activation by the specific JAK2 inhibitor AG490 prevents HSC early activation, which suggests that leptin activation of STAT3 promotes HSC survival and activation (Lakner et al., 2010). In addition, STAT3 is also involved in the leptin- and IL-6-mediated production of TIMP-1 in HSCs and hepatocytes, respectively (Cao et al., 2004; Wang et al., 2011c). TIMP-1 is a survival factor for HSCs; thus, activation of STAT3 in HSCs and hepatocytes may increase liver fibrogenesis via the upregulation of TIMP-1.
Until recently, the roles of STAT3 in other non-parenchymal cells and inflammatory cells in liver fibrogenesis remain largely unclear. Recently, Wang et al. (2009) provided evidence suggesting that leptin can promote TGF-β1 production in Kupffer cells via the activation of STAT3 and consequently augment liver fibrogenesis. This suggests that STAT3 activation in Kupffer cells by leptin exacerbates liver fibrosis. However, it is known that the activation of STAT3 by IL-10 in macrophages and Kupffer cells is a key anti-inflammatory signal for the attenuation of liver inflammation (Horiguchi et al., 2008, 2010; Lafdil et al., 2009). Thus, the activation of STAT3 by IL-10 in Kupffer cells and macrophages may prevent liver inflammation and fibrogenesis. Further studies are needed to clarify the functions of STAT3 in Kupffer cells as well as in other types of sinusoidal cells and inflammatory cells in the pathogenesis of liver fibrogenesis.
Clinical data have shown that STAT3–DNA binding is markedly suppressed in alcoholic and HCV fibrotic patients when compared with normal healthy livers, and fibrosis progression in HCV-infected patients is positively correlated with a continuous decline in STAT3–DNA binding activity (Starkel et al., 2005, 2007), indicating that STAT3 may also protect against liver fibrosis in patients.
In the liver, STAT3 is mainly activated by IL-6 and its related cytokines leptin, and IL-22. The roles of these cytokines in liver fibrogenesis are discussed below.
IL-6/STAT3 in Liver Fibrosis
IL-6 is a critical proregenerative factor and an acute-phase inducer in the liver. IL-6 stimulates hepatocytes to produce a variety of acute-phase proteins, including C-reactive protein, complement C3, and serum amyloid A (Ramadori and Christ, 1999). However, numerous studies demonstrated the hepatoprotective role of IL-6 against liver injury in spite of its pro-inflammatory effect (Blindenbacher et al., 2003; Wuestefeld et al., 2003). With respect to liver fibrosis, there are some conflicting reports. First, IL-6 knockout mice are reported to be more susceptible to CCl4-induced liver injury and fibrosis (Kovalovich et al., 2000). Another study showed that the lack of gp130/STAT3-mediated signaling in hepatocytes resulted in enhanced chronic cholestatic liver injury and fibrosis progression induced by DDC diet feeding (Plum et al., 2010). Other studies have demonstrated that liver fibrosis is reduced in IL-6-deficient mice after CCl4 treatment (Sun et al., 2004; Rio et al., 2008). The reasons for the differences observed in these experiments remain unclear. In vitro cell culture experiments showed that Kupffer cell-derived IL-6 promotes HSC survival and proliferation (Nieto, 2006). Clinical studies showed that hepatic IL-6 expression is upregulated and correlates positively with the degree of liver fibrosis in opisthorchiasis periductal fibrosis and in non-alcoholic steatohepatitis (Dogru et al., 2008; Wieckowska et al., 2008; Sripa et al., 2009). Furthermore, genetic polymorphisms of IL-6 are linked with fibrosis progression in patients with chronic HCV infection (Cussigh et al., 2011). Because IL-6 receptors are expressed ubiquitously on all types of liver cells, it is plausible to speculate that IL-6 may positively and negatively regulate liver fibrosis by targeting different types of liver cells. For example, IL-6 protects against hepatocellular damage, thereby reducing injury-driven liver fibrosis, while it may also directly promote HSC survival and proliferation, followed by enhanced liver fibrosis. The final effect of IL-6 on liver fibrosis is likely determined by the balance between these inhibitory and stimulatory effects and is dependent on the stage and etiology of liver fibrosis.
Leptin/STAT3 in Liver Fibrosis
The critical role of leptin in inducing liver fibrogenesis was first noticed from the findings that leptin-deficient ob/ob mice and leptin-receptor-deficient Zucker rats are resistant to the development of liver fibrosis (Honda et al., 2002; Ikejima et al., 2002; Saxena et al., 2002). A recent study showed that the administration of leptin increases thioacetamide-induced liver fibrosis, while the injection of mouse leptin antagonist attenuates it (Elinav et al., 2009). In vitro treatment of primary rat HSCs with a leptin antagonist, either alone or with leptin, has been shown to suppress the pro-fibrotic effects of leptin (Elinav et al., 2009). Furthermore, in vitro experiments with leptin have shown to induce STAT3 activation in HSCs, and the blockade of STAT3 activation diminishes leptin-mediated promotion of HSC survival and activation (Lakner et al., 2010). Finally, STAT3 is also involved in the leptin-mediated production of TIMP-1, an important survival factor for HSCs (Cao et al., 2004). Collectively, the pro-fibrotic effect of leptin is likely mediated via the direct promotion of HSC survival and proliferation or the indirect upregulation of TIMP-1, which subsequently promotes HSC survival.
IL-22/STAT3 in Liver Fibrosis
IL-22 has been shown to play an important role in protecting against liver injury (Radaeva et al., 2004; Zenewicz et al., 2007; Park et al., 2011), ameliorating fatty liver disease (Ki et al., 2010; Yang et al., 2010), and promoting liver cancer development (Jiang et al., 2011; Park et al., 2011). An in vitro study showed that IL-22 promotes liver cell regeneration by increasing hepatic cell proliferation and hepatocyte migration through the activation of Akt and STAT signaling, which is abrogated by SOCS-1/3 overexpression (Brand et al., 2007). Although the hepatoprotective effects of IL-22 have been extensively studied, the effects of IL-22 on liver fibrogenesis have not been investigated.
STAT4
It is well documented that IL-12 and IFN-α/β can induce STAT4 activation in several types of immune cells and subsequently generate inflammation during protective immune responses and immune-mediated diseases (Kaplan, 2005). The activation of STAT4 has not been reported in hepatocytes, and the functions of STAT4 in liver injury and fibrosis remain obscure. IL-12-deficient mice are resistant to autoimmune cholangitis in dominant negative TGF-β receptor type II mice (Yoshida et al., 2009) and to Con A-induced T cell hepatitis (Zhu et al., 2007), while the overexpression of IL-12 in the liver induces liver injury (Rodriguez-Galan et al., 2009). In addition, IL-12 treatment has been shown to induce liver inflammation and inhibit liver tumor growth in several animal models (Harada et al., 2004; Chang et al., 2007), which is likely mediated via IL-12 activation of NK and NKT cells and the subsequent production of IFN-γ (Subleski et al., 2006). This suggests that IL-12 acts as a pro-inflammatory cytokine to promote liver injury and fibrosis likely via the activation of STAT4 in immune cells. In contrast, a recent study has demonstrated that IL-10−/− IL-12/23(p40)−/− IL-13Rα2−/− triple knockout mice are more susceptible to S. mansoni-induced liver fibrosis, portal hypertension, hepatosplenomegaly, gastrointestinal bleeding, ascites, thrombocytopenia, esophageal and gastric varices, anemia, and increased levels of liver enzymes (Mentink-Kane et al., 2011), suggesting that IL-10, IL-12p40, and IL-13Rα2 act cooperatively to suppress liver fibrosis in mice following infection with S. mansoni. Collectively, IL-12 activation of STAT4 in immune cells induces inflammation in the liver, which promotes liver injury and fibrosis, but may also protect against infection, thereby ameliorating liver injury and fibrosis.
STAT5
The activation of STAT5 in hepatocytes is mainly induced by growth hormone (GH) and other cytokines to a lesser extent. Hepatocyte-specific STAT5 knockout mice were initially generated in Dr. Lothar Hennighausen’s laboratory (Cui et al., 2007). Using this strain of knockout mice, they have demonstrated that loss of STAT5 in hepatocytes results in elevated TGF-β levels and enhanced GH-induced STAT3 activity in the liver after chronic carbon tetrachloride treatment, suggesting that STAT5 inhibits liver fibrogenesis via the downregulation of TGF-β and STAT3 (Hosui et al., 2009; Baik et al., 2011). The anti-fibrotic effect of STAT5 is also demonstrated in another murine model of liver fibrosis induced by deleting the multidrug resistance gene 2 (Blaas et al., 2010). Deletion of both multidrug resistance gene 2 (global deletion) and STAT5 (hepatocyte and cholangiocyte-specific deletion) results in an early and severe liver fibrosis phenotype, accompanied by reduced expression of important hepatoprotective genes, such as epidermal growth factor receptor, hepatocyte nuclear factor 6, prolactin receptor, and leukemia inhibitory factor receptor as well as increased numbers of apoptotic hepatocytes. Furthermore, deletion of STAT5 in the liver promoted carbon tetrachloride-induced hepatic tumorigenesis in wild-type mice (Hosui et al., 2009) and induced spontaneous liver cancer development in liver-specific glucocorticoid receptor knockout mice (Mueller et al., 2011) or in GH transgenic mice (Friedbichler et al., 2012). The protective effect of STAT5 on hepatic carcinogenesis is likely mediated via the induction of a key cell cycle inhibitor–p15INK4B expression in hepatocytes (Yu et al., 2010) and protection against fatty liver disease and liver injury (Mueller et al., 2011; Friedbichler et al., 2012).
Although the functions of hepatocyte STAT5 have been extensively investigated, little is known about STAT5 activation in HSCs and other non-parenchymal cells, and how STAT5 activation in these cells affects the pathogenesis of liver fibrogenesis remains obscure.
STAT6
STAT6 is mainly activated by Th2 cytokines, including IL-4 and IL-13. The roles of both IL-4 and IL-13 in liver fibrosis have been extensively investigated, especially in a model of S. mansoni infection (Barron and Wynn, 2011), while the functions of STAT6 in liver fibrogenesis remain obscure. Disruption of the IL-13 gene or blockage of IL-13 with inhibitors leads to markedly reduced liver fibrosis after infection with S. mansoni (Chiaramonte et al., 1999a, 2001; Fallon et al., 2000), indicating that IL-13 is a pro-fibrogenic cytokine during S. mansoni infection. Expression of IL-13 is elevated and correlates with liver fibrosis in some patients with HBV or HCV infection. This suggests that IL-13 may also contribute to the pathogenesis of liver fibrosis in viral hepatitis (Weng et al., 2009). The pro-fibrogenic effect of IL-13 is likely mediated via the upregulation of a wide variety of fibrotic proteins in HSCs and the induction of HSC activation (Chiaramonte et al., 1999a; Weng et al., 2009). IL-13 may also regulate liver fibrosis via the activation of macrophages, which plays an important role in the induction and resolution of liver fibrosis (Barron and Wynn, 2011).
IL-4 is also considered a pro-fibrotic cytokine because IL-4 can directly stimulate HSC activation and the production of collagens (Aoudjehane et al., 2008; Jin et al., 2011). The potency of IL-4 stimulation of HSC activation in vitro appears to be similar to that of TGF-β (Aoudjehane et al., 2008). In addition, the expression of IL-4 is upregulated in the fibrotic liver of S. mansoni-infected baboons (Farah et al., 2000), and blockage of IL-4 significantly reduces liver fibrosis in S. mansoni-infected mice (Cheever et al., 1994). However, several other studies suggest that although both IL-4 and IL-13 are critical in the progression of the S. mansoni-induced disease, IL-13, but not IL-4, plays a key role in S. mansoni mediated granuloma formation and liver fibrosis as the reduction in fibrosis observed in IL-4-deficient mice was much less pronounced than that in sIL-13Ralpha2-Fc-treated animals (Chiaramonte et al., 1999a,b; Fallon et al., 2000)
Both IL-4 and IL-13 predominately induce STAT6 activation in HSCs. Blockage of STAT6 with siRNA attenuates HSC activation in vitro (Aoudjehane et al., 2008). After infection with S. mansoni, STAT6-deficient mice have smaller granulomas and decreased amounts of collagen deposition in the liver compared with wild-type mice (Kaplan et al., 1998). However, a recent study (Liu et al., 2011) has demonstrated that ERK1/2 pathway rather than STAT6 in HSCs contributes to IL-13 induction of connective tissue growth factor, a key mediator in stimulating extracellular matrix deposition in the liver. In addition, although IL-13 immunostaining correlates with fibrotic stage in patients with HCV infection and steatohepatitis, pSTAT6 was only detected in 5 out of 120 fibrotic tissues from these patients, suggesting that the pro-fibrotic effect of IL-13 in human liver diseases is mediated via an STAT6-independent pathway (Weng et al., 2009). Finally, in lung fibrosis, two studies showed that STAT6-deficient mice still develop into fibrosis in two murine models induced by transient ectopic overexpression of oncostatin M (Fritz et al., 2011) or by an intratracheal challenge with live A. fumigatus conidia (Blease et al., 2002). Collectively, although IL-13 plays an important role in promoting liver fibrogenesis in S. mansoni infection, the role of STAT6 in HSCs and liver fibrogenesis seems controversial, which needs to be clarified by further studies.
Conclusions and Clinical Perspectives
In summary, the activation of the JAK–STAT signaling pathways plays a complex role in controlling liver fibrogenesis. In general, as shown in Figure 1, STAT1 acts as an anti-fibrotic signaling molecule via the induction of HSC apoptosis and cell cycle arrest. STAT3 and STAT5 may also act as anti-fibrotic signaling molecules via their hepatoprotective functions, thereby preventing injury-driven liver fibrosis. However, the functions of STAT3 and STAT5 activation in HSCs remain obscure and require further studies. Although the antiviral effect of STAT2 is well documented, it is not clear whether or not STAT2 also plays a role in contributing to the IFN-α/β-mediated anti-fibrotic effects in the liver. Finally, the biological functions of STAT4 and STAT6 in the pathogenesis of liver diseases, including liver fibrogenesis, remain largely unknown.
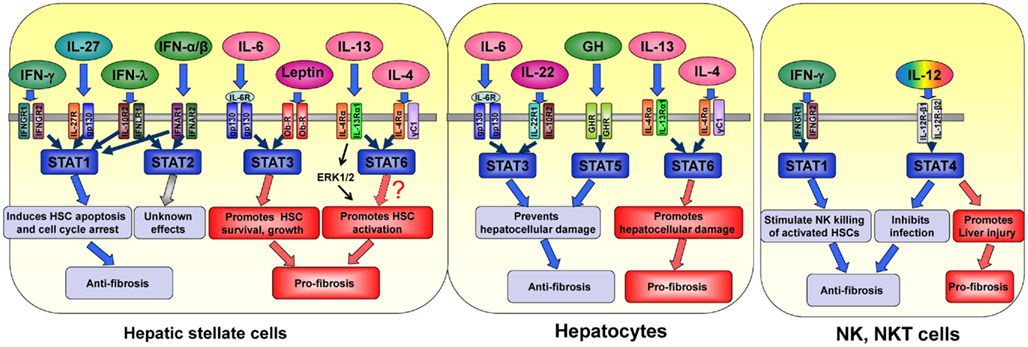
Figure 1. The effects of cytokines and their downstream STATs in different cell types in liver fibrogenesis.
Despite extensive research on the functions of STATs in liver fibrogenesis, the translation of these basic research findings into new therapies has been modest. Based on research findings that IFN-γ activation of STAT1 is effective in ameliorating liver fibrosis in animal models, IFN-γ has been tested in clinical trials for treating liver fibrosis in patients. It has been reported that IFN-γ treatment for 9 months does not reduce viral load but improves fibrosis scores in patients with chronic HBV infection (Weng et al., 2005; Wu et al., 2011); however, another large double-blind, placebo-controlled trial showed that IFN-γ therapy was not able to reverse fibrosis in patients with advanced liver disease (Pockros et al., 2007). The disappointing results from the anti-fibrotic therapy of IFN-γ in the latter study may be due to the selection of patients with end-stage of liver disease, poor efficacy, or to unwanted off-target effects. To overcome this problem, Bansal et al. (2011) recently developed an engineered, targeted IFN-γ, in which IFN-γ is conjugated to a cyclic recognizing PDGF-β, a receptor that is highly upregulated on activated HSCs. Administration of this conjugated IFN-γ markedly inhibits liver fibrosis but does not induce IFN-γ-related side effects in animal models (Bansal et al., 2011), suggesting that this conjugated IFN-γ is a promising anti-fibrotic drug for the treatment of liver fibrosis in patients. Further studies on the anti-fibrotic effect of this targeted IFN-γ in patients are warranted.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
CDE diet, choline-deficient, ethionine-supplemented diet; DDC diet, 3,5-diethoxycarbonyl-1,4-dihydrocollidine diet; HSC, hepatic stellate cell; IL, interleukin; JAK–STATs, Janus kinase–signal transducers and activators of transcription; TGF-β, transforming growth factor-beta; TIMP-1, tissue inhibitor of metalloproteinase 1.
References
Afdhal, N. H., McHutchison, J. G., Zeuzem, S., Mangia, A., Pawlotsky, J. M., Murray, J. S., Shianna, K. V., Tanaka, Y., Thomas, D. L., Booth, D. R., and Goldstein, D. B. (2011). Hepatitis C pharmacogenetics: state of the art in 2010. Hepatology 53, 336–345.
Aoudjehane, L., Pissaia, A. Jr., Scatton, O., Podevin, P., Massault, P. P., Chouzenoux, S., Soubrane, O., Calmus, Y., and Conti, F. (2008). Interleukin-4 induces the activation and collagen production of cultured human intrahepatic fibroblasts via the STAT-6 pathway. Lab. Invest. 88, 973–985.
Baik, M., Yu, J. H., and Hennighausen, L. (2011). Growth hormone-STAT5 regulation of growth, hepatocellular carcinoma, and liver metabolism. Ann. N. Y. Acad. Sci. 1229, 29–37.
Balagopal, A., Thomas, D. L., and Thio, C. L. (2010). IL28B and the control of hepatitis C virus infection. Gastroenterology 139, 1865–1876.
Bansal, R., Prakash, J., Post, E., Beljaars, L., Schuppan, D., and Poelstra, K. (2011). Novel engineered targeted interferon-gamma blocks hepatic fibrogenesis in mice. Hepatology 54, 586–596.
Barron, L., and Wynn, T. A. (2011). Fibrosis is regulated by Th2 and Th17 responses and by dynamic interactions between fibroblasts and macrophages. Am. J. Physiol. Gastrointest. Liver Physiol. 300, G723–G728.
Blaas, L., Kornfeld, J. W., Schramek, D., Musteanu, M., Zollner, G., Gumhold, J., van Zijl, F., Schneller, D., Esterbauer, H., Egger, G., Mair, M., Kenner, L., Mikulits, W., Eferl, R., Moriggl, R., Penninger, J., Trauner, M., and Casanova, E. (2010). Disruption of the growth hormone – signal transducer and activator of transcription 5 – insulin like growth factor 1 axis severely aggravates liver fibrosis in a mouse model of cholestasis. Hepatology 51, 1319–1326.
Blease, K., Schuh, J. M., Jakubzick, C., Lukacs, N. W., Kunkel, S. L., Joshi, B. H., Puri, R. K., Kaplan, M. H., and Hogaboam, C. M. (2002). Stat6-deficient mice develop airway hyperresponsiveness and peribronchial fibrosis during chronic fungal asthma. Am. J. Pathol. 160, 481–490.
Blindenbacher, A., Wang, X., Langer, I., Savino, R., Terracciano, L., and Heim, M. H. (2003). Interleukin 6 is important for survival after partial hepatectomy in mice. Hepatology 38, 674–682.
Brand, S., Dambacher, J., Beigel, F., Zitzmann, K., Heeg, M. H., Weiss, T. S., Prufer, T., Olszak, T., Steib, C. J., Storr, M., Goke, B., Diepolder, H., Bilzer, M., Thasler, W. E., and Auernhammer, C. J. (2007). IL-22-mediated liver cell regeneration is abrogated by SOCS-1/3 overexpression in vitro. Am. J. Physiol. Gastrointest. Liver Physiol. 292, G1019–G1028.
Cao, Q., Mak, K. M., Ren, C., and Lieber, C. S. (2004). Leptin stimulates tissue inhibitor of metalloproteinase-1 in human hepatic stellate cells: respective roles of the JAK/STAT and JAK-mediated H2O2-dependant MAPK pathways. J. Biol. Chem. 279, 4292–4304.
Chang, C. J., Chen, Y. H., Huang, K. W., Cheng, H. W., Chan, S. F., Tai, K. F., and Hwang, L. H. (2007). Combined GM-CSF and IL-12 gene therapy synergistically suppresses the growth of orthotopic liver tumors. Hepatology 45, 746–754.
Cheever, A. W., Williams, M. E., Wynn, T. A., Finkelman, F. D., Seder, R. A., Cox, T. M., Hieny, S., Caspar, P., and Sher, A. (1994). Anti-IL-4 treatment of Schistosoma mansoni-infected mice inhibits development of T cells and non-B, non-T cells expressing Th2 cytokines while decreasing egg-induced hepatic fibrosis. J. Immunol. 153, 753–759.
Chiaramonte, M. G., Cheever, A. W., Malley, J. D., Donaldson, D. D., and Wynn, T. A. (2001). Studies of murine schistosomiasis reveal interleukin-13 blockade as a treatment for established and progressive liver fibrosis. Hepatology 34, 273–282.
Chiaramonte, M. G., Donaldson, D. D., Cheever, A. W., and Wynn, T. A. (1999a). An IL-13 inhibitor blocks the development of hepatic fibrosis during a T-helper type 2-dominated inflammatory response. J. Clin. Invest. 104, 777–785.
Chiaramonte, M. G., Schopf, L. R., Neben, T. Y., Cheever, A. W., Donaldson, D. D., and Wynn, T. A. (1999b). IL-13 is a key regulatory cytokine for Th2 cell-mediated pulmonary granuloma formation and IgE responses induced by Schistosoma mansoni eggs. J. Immunol. 162, 920–930.
Cui, Y., Hosui, A., Sun, R., Shen, K., Gavrilova, O., Chen, W., Cam, M. C., Gao, B., Robinson, G. W., and Hennighausen, L. (2007). Loss of signal transducer and activator of transcription 5 leads to hepatosteatosis and impaired liver regeneration. Hepatology 46, 504–513.
Cussigh, A., Falleti, E., Fabris, C., Bitetto, D., Cmet, S., Fontanini, E., Bignulin, S., Fornasiere, E., Fumolo, E., Minisini, R., Pirisi, M., and Toniutto, P. (2011). Interleukin 6 promoter polymorphisms influence the outcome of chronic hepatitis C. Immunogenetics 63, 33–41.
Dogru, T., Ercin, C. N., Erdem, G., Sonmez, A., Tapan, S., and Tasci, I. (2008). Increased hepatic and circulating interleukin-6 levels in human nonalcoholic steatohepatitis. Am. J. Gastroenterol. 103, 3217–3218.
Elinav, E., Ali, M., Bruck, R., Brazowski, E., Phillips, A., Shapira, Y., Katz, M., Solomon, G., Halpern, Z., and Gertler, A. (2009). Competitive inhibition of leptin signaling results in amelioration of liver fibrosis through modulation of stellate cell function. Hepatology 49, 278–286.
Fallon, P. G., Richardson, E. J., McKenzie, G. J., and McKenzie, A. N. (2000). Schistosome infection of transgenic mice defines distinct and contrasting pathogenic roles for IL-4 and IL-13: IL-13 is a profibrotic agent. J. Immunol. 164, 2585–2591.
Farah, I. O., Mola, P. W., Kariuki, T. M., Nyindo, M., Blanton, R. E., and King, C. L. (2000). Repeated exposure induces periportal fibrosis in Schistosoma mansoni-infected baboons: role of TGF-beta and IL-4. J. Immunol. 164, 5337–5343.
Friedbichler, K., Themanns, M., Mueller, K. M., Schlederer, M., Kornfeld, J. W., Terracciano, L. M., Kozlov, A. V., Haindl, S., Kenner, L., Kolbe, T., Mueller, M., Snibson, K. J., Heim, M. H., and Moriggl, R. (2012). Growth-hormone-induced signal transducer and activator of transcription 5 signaling causes gigantism, inflammation, and premature death but protects mice from aggressive liver cancer. Hepatology 55, 941–52.
Fritz, D. K., Kerr, C., Fattouh, R., Llop-Guevara, A., Khan, W. I., Jordana, M., and Richards, C. D. (2011). A mouse model of airway disease: oncostatin M-induced pulmonary eosinophilia, goblet cell hyperplasia, and airway hyperresponsiveness are STAT6 dependent, and interstitial pulmonary fibrosis is STAT6 independent. J. Immunol. 186, 1107–1118.
Gao, B., Wang, H., Lafdil, F., and Feng, D. (2012). STAT proteins – key regulators of anti-viral responses, inflammation, and tumorigenesis in the liver. J. Hepatol. (in press).
Harada, N., Shimada, M., Okano, S., Suehiro, T., Soejima, Y., Tomita, Y., and Maehara, Y. (2004). IL-12 gene therapy is an effective therapeutic strategy for hepatocellular carcinoma in immunosuppressed mice. J. Immunol. 173, 6635–6644.
Higashiyama, R., Moro, T., Nakao, S., Mikami, K., Fukumitsu, H., Ueda, Y., Ikeda, K., Adachi, E., Bou-Gharios, G., Okazaki, I., and Inagaki, Y. (2009). Negligible contribution of bone marrow-derived cells to collagen production during hepatic fibrogenesis in mice. Gastroenterology 137, 1459–1466.
Honda, H., Ikejima, K., Hirose, M., Yoshikawa, M., Lang, T., Enomoto, N., Kitamura, T., Takei, Y., and Sato, N. (2002). Leptin is required for fibrogenic responses induced by thioacetamide in the murine liver. Hepatology 36, 12–21.
Horiguchi, N., Lafdil, F., Miller, A. M., Park, O., Wang, H., Rajesh, M., Mukhopadhyay, P., Fu, X. Y., Pacher, P., and Gao, B. (2010). Dissociation between liver inflammation and hepatocellular damage induced by carbon tetrachloride in myeloid cell-specific signal transducer and activator of transcription 3 gene knockout mice. Hepatology 51, 1724–1734.
Horiguchi, N., Wang, L., Mukhopadhyay, P., Park, O., Jeong, W. I., Lafdil, F., Osei-Hyiaman, D., Moh, A., Fu, X. Y., Pacher, P., Kunos, G., and Gao, B. (2008). Cell type-dependent pro- and anti-inflammatory role of signal transducer and activator of transcription 3 in alcoholic liver injury. Gastroenterology 134, 1148–1158.
Hosui, A., Kimura, A., Yamaji, D., Zhu, B., Na, R., and Hennighausen, L. (2009). Loss of STAT5 causes liver fibrosis and cancer development through increased TGF-β and STAT3 activation. J. Exp. Med. 206, 819–831.
Ikejima, K., Takei, Y., Honda, H., Hirose, M., Yoshikawa, M., Zhang, Y. J., Lang, T., Fukuda, T., Yamashina, S., Kitamura, T., and Sato, N. (2002). Leptin receptor-mediated signaling regulates hepatic fibrogenesis and remodeling of extracellular matrix in the rat. Gastroenterology 122, 1399–1410.
Inagaki, Y., Nemoto, T., Kushida, M., Sheng, Y., Higashi, K., Ikeda, K., Kawada, N., Shirasaki, F., Takehara, K., Sugiyama, K., Fujii, M., Yamauchi, H., Nakao, A., de Crombrugghe, B., Watanabe, T., and Okazaki, I. (2003). Interferon alfa down-regulates collagen gene transcription and suppresses experimental hepatic fibrosis in mice. Hepatology 38, 890–899.
Jeong, W. I., Park, O., Radaeva, S., and Gao, B. (2006). STAT1 inhibits liver fibrosis in mice by inhibiting stellate cell proliferation and stimulating NK cell cytotoxicity. Hepatology 44, 1441–1451.
Jiang, R. T. Z., Deng, L., Chen, Y., Xia, Y., Gao, Y., Wang, X., and Sun, B. (2011). Interleukin-22 promotes human hepatocellular carcinoma by activation of STAT3. Hepatology 54, 900–909.
Jin, Z., Sun, R., Wei, H., Gao, X., Chen, Y., and Tian, Z. (2011). Accelerated liver fibrosis in hepatitis B virus transgenic mice: involvement of natural killer T cells. Hepatology 53, 219–229.
Kaimori, A., Potter, J., Kaimori, J. Y., Wang, C., Mezey, E., and Koteish, A. (2007). Transforming growth factor-beta1 induces an epithelial-to-mesenchymal transition state in mouse hepatocytes in vitro. J. Biol. Chem. 282, 22089–22101.
Kaplan, M. H. (2005). STAT4: a critical regulator of inflammation in vivo. Immunol. Res. 31, 231–242.
Kaplan, M. H., Whitfield, J. R., Boros, D. L., and Grusby, M. J. (1998). Th2 cells are required for the Schistosoma mansoni egg-induced granulomatous response. J. Immunol. 160, 1850–1856.
Ki, S. H., Park, O., Zheng, M., Morales-Ibanez, O., Kolls, J. K., Bataller, R., and Gao, B. (2010). Interleukin-22 treatment ameliorates alcoholic liver injury in a murine model of chronic-binge ethanol feeding: role of signal transducer and activator of transcription 3. Hepatology 52, 1291–1300.
Kovalovich, K., DeAngelis, R. A., Li, W., Furth, E. E., Ciliberto, G., and Taub, R. (2000). Increased toxin-induced liver injury and fibrosis in interleukin-6-deficient mice. Hepatology 31, 149–159.
Kroy, D. C., Beraza, N., Tschaharganeh, D. F., Sander, L. E., Erschfeld, S., Giebeler, A., Liedtke, C., Wasmuth, H. E., Trautwein, C., and Streetz, K. L. (2010). Lack of interleukin-6/glycoprotein 130/signal transducers and activators of transcription-3 signaling in hepatocytes predisposes to liver steatosis and injury in mice. Hepatology 51, 463–473.
Lafdil, F., Wang, H., Park, O., Zhang, W., Moritoki, Y., Yin, S., Fu, X. Y., Gershwin, M. E., Lian, Z. X., and Gao, B. (2009). Myeloid STAT3 inhibits T cell-mediated hepatitis by regulating T helper 1 cytokine and interleukin-17 production. Gastroenterology 137, 2125–2135, e2121–e2122.
Lakner, A. M., Moore, C. C., Gulledge, A. A., and Schrum, L. W. (2010). Daily genetic profiling indicates JAK/STAT signaling promotes early hepatic stellate cell transdifferentiation. World J. Gastroenterol. 16, 5047–5056.
Liu, Y., Meyer, C., Müller, A., Herweck, F., Li, Q., Müllenbach, R., Mertens, P. R., Dooley, S., and Weng, H. L. (2011). IL-13 induces connective tissue growth factor in rat hepatic stellate cells via TGF-β-independent Smad signaling. J. Immunol. 187, 2814–2823.
Mair, M., Blaas, L., Osterreicher, C. H., Casanova, E., and Eferl, R. (2011). JAK-STAT signaling in hepatic fibrosis. Front. Biosci. 17, 2794–2811.
Mair, M., Zollner, G., Schneller, D., Musteanu, M., Fickert, P., Gumhold, J., Schuster, C., Fuchsbichler, A., Bilban, M., Tauber, S., Esterbauer, H., Kenner, L., Poli, V., Blaas, L., Kornfeld, J. W., Casanova, E., Mikulits, W., Trauner, M., and Eferl, R. (2010). Signal transducer and activator of transcription 3 protects from liver injury and fibrosis in a mouse model of sclerosing cholangitis. Gastroenterology 138, 2499–2508.
Mentink-Kane, M. M., Cheever, A. W., Wilson, M. S., Madala, S. K., Beers, L. M., Ramalingam, T. R., and Wynn, T. A. (2011). Accelerated and progressive and lethal liver fibrosis in mice that lack interleukin (IL)-10, IL-12p40, and IL-13Ralpha2. Gastroenterology 141, 2200–2209.
Mueller, K. M., Kornfeld, J. W., Friedbichler, K., Blaas, L., Egger, G., Esterbauer, H., Hasselblatt, P., Schlederer, M., Haindl, S., Wagner, K. U., Engblom, D., Haemmerle, G., Kratky, D., Sexl, V., Kenner, L., Kozlov, A. V., Terracciano, L., Zechner, R., Schuetz, G., Casanova, E., Pospisilik, J. A., Heim, M. H., and Moriggl, R. (2011). Impairment of hepatic growth hormone and glucocorticoid receptor signaling causes steatosis and hepatocellular carcinoma in mice. Hepatology 54, 1398–1409.
Nieto, N. (2006). Oxidative-stress and IL-6 mediate the fibrogenic effects of [corrected] Kupffer cells on stellate cells. Hepatology 44, 1487–1501.
Novo, E., di Bonzo, L. V., Cannito, S., Colombatto, S., and Parola, M. (2009). Hepatic myofibroblasts: a heterogeneous population of multifunctional cells in liver fibrogenesis. Int. J. Biochem. Cell Biol. 41, 2089–2093.
Park, O., Wang, H., Weng, H., Feigenbaum, L., Li, H., Yin, S., Ki, S. H., Yoo, S. H., Dooley, S., Wang, F. S., Young, H. A., and Gao, B. (2011). In vivo consequences of liver-specific interleukin-22 expression in mice: implications for human liver disease progression. Hepatology 54, 252–261.
Plum, W., Tschaharganeh, D. F., Kroy, D. C., Corsten, E., Erschfeld, S., Dierssen, U., Wasmuth, H., Trautwein, C., and Streetz, K. L. (2010). Lack of glycoprotein 130/signal transducer and activator of transcription 3-mediated signaling in hepatocytes enhances chronic liver injury and fibrosis progression in a model of sclerosing cholangitis. Am. J. Pathol. 176, 2236–2246.
Pockros, P. J., Jeffers, L., Afdhal, N., Goodman, Z. D., Nelson, D., Gish, R. G., Reddy, K. R., Reindollar, R., Rodriguez-Torres, M., Sullivan, S., Blatt, L. M., and Faris-Young, S. (2007). Final results of a double-blind, placebo-controlled trial of the antifibrotic efficacy of interferon-gamma1b in chronic hepatitis C patients with advanced fibrosis or cirrhosis. Hepatology 45, 569–578.
Radaeva, S., Jaruga, B., Hong, F., Kim, W. H., Fan, S., Cai, H., Strom, S., Liu, Y., El-Assal, O., and Gao, B. (2002). Interferon-alpha activates multiple STAT signals and down-regulates c-Met in primary human hepatocytes. Gastroenterology 122, 1020–1034.
Radaeva, S., Sun, R., Pan, H. N., Hong, F., and Gao, B. (2004). Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology 39, 1332–1342.
Ramadori, G., and Christ, B. (1999). Cytokines and the hepatic acute-phase response. Semin. Liver Dis. 19, 141–155.
Rio, A., Gassull, M. A., Aldeguer, X., Ojanguren, I., Cabre, E., and Fernandez, E. (2008). Reduced liver injury in the interleukin-6 knockout mice by chronic carbon tetrachloride administration. Eur. J. Clin. Invest. 38, 306–316.
Rodriguez-Galan, M. C., Reynolds, D., Correa, S. G., Iribarren, P., Watanabe, M., and Young, H. A. (2009). Coexpression of IL-18 strongly attenuates IL-12-induced systemic toxicity through a rapid induction of IL-10 without affecting its antitumor capacity. J. Immunol. 183, 740–748.
Saxena, N. K., Ikeda, K., Rockey, D. C., Friedman, S. L., and Anania, F. A. (2002). Leptin in hepatic fibrosis: evidence for increased collagen production in stellate cells and lean littermates of ob/ob mice. Hepatology 35, 762–771.
Schoenherr, C., Weiskirchen, R., and Haan, S. (2010). Interleukin-27 acts on hepatic stellate cells and induces signal transducer and activator of transcription 1-dependent responses. Cell Commun. Signal. 8, 19.
Shigekawa, M., Takehara, T., Kodama, T., Hikita, H., Shimizu, S., Li, W., Miyagi, T., Hosui, A., Tatsumi, T., Ishida, H., Kanto, T., Hiramatsu, N., and Hayashi, N. (2011). Involvement of STAT3-regulated hepatic soluble factors in attenuation of stellate cell activity and liver fibrogenesis in mice. Biochem. Biophys. Res. Commun. 406, 614–620.
Sripa, B., Mairiang, E., Thinkhamrop, B., Laha, T., Kaewkes, S., Sithithaworn, P., Tessana, S., Loukas, A., Brindley, P. J., and Bethony, J. M. (2009). Advanced periductal fibrosis from infection with the carcinogenic human liver fluke Opisthorchis viverrini correlates with elevated levels of interleukin-6. Hepatology 50, 1273–1281.
Starkel, P., De Saeger, C., Leclercq, I., Strain, A., and Horsmans, Y. (2005). Deficient Stat3 DNA-binding is associated with high Pias3 expression and a positive anti-apoptotic balance in human end-stage alcoholic and hepatitis C cirrhosis. J. Hepatol. 43, 687–695.
Starkel, P., Saeger, C. D., Leclercq, I., and Horsmans, Y. (2007). Role of signal transducer and activator of transcription 3 in liver fibrosis progression in chronic hepatitis C-infected patients. Lab. Invest. 87, 173–181.
Subleski, J. J., Hall, V. L., Back, T. C., Ortaldo, J. R., and Wiltrout, R. H. (2006). Enhanced antitumor response by divergent modulation of natural killer and natural killer T cells in the liver. Cancer Res. 66, 11005–11012.
Sun, R., Tian, Z., Kulkarni, S., and Gao, B. (2004). IL-6 prevents T cell-mediated hepatitis via inhibition of NKT cells in CD4+ T cell- and STAT3-dependent manners. J. Immunol. 172, 5648–5655.
Tanabe, J., Izawa, A., Takemi, N., Miyauchi, Y., Torii, Y., Tsuchiyama, H., Suzuki, T., Sone, S., and Ando, K. (2007). Interferon-beta reduces the mouse liver fibrosis induced by repeated administration of concanavalin A via the direct and indirect effects. Immunology 122, 562–570.
Wang, H., Lafdil, F., Kong, X., and Gao, B. (2011a). Signal transducer and activator of transcription 3 in liver diseases: a novel therapeutic target. Int. J. Biol. Sci. 7, 536–550.
Wang, H., Lafdil, F., Wang, L., Park, O., Yin, S., Niu, J., Miller, A. M., Sun, Z., and Gao, B. (2011b). Hepatoprotective versus oncogenic functions of STAT3 in liver tumorigenesis. Am. J. Pathol. 179, 714–724.
Wang, H., Lafdil, F., Wang, L., Yin, S., Feng, D., and Gao, B. (2011c). Tissue inhibitor of metalloproteinase 1 (TIMP-1) deficiency exacerbates carbon tetrachloride-induced liver injury and fibrosis in mice: involvement of hepatocyte STAT3 in TIMP-1 production. Cell Biosci. 1, 14.
Wang, J., Leclercq, I., Brymora, J. M., Xu, N., Ramezani-Moghadam, M., London, R. M., Brigstock, D., and George, J. (2009). Kupffer cells mediate leptin-induced liver fibrosis. Gastroenterology 137, 713–723.
Weng, H., Mertens, P. R., Gressner, A. M., and Dooley, S. (2007). IFN-gamma abrogates profibrogenic TGF-beta signaling in liver by targeting expression of inhibitory and receptor Smads. J. Hepatol. 46, 295–303.
Weng, H. L., Liu, Y., Chen, J. L., Huang, T., Xu, L. J., Godoy, P., Hu, J. H., Zhou, C., Stickel, F., Marx, A., Bohle, R. M., Zimmer, V., Lammert, F., Mueller, S., Gigou, M., Samuel, D., Mertens, P. R., Singer, M. V., Seitz, H. K., and Dooley, S. (2009). The etiology of liver damage imparts cytokines transforming growth factor beta1 or interleukin-13 as driving forces in fibrogenesis. Hepatology 50, 230–243.
Weng, H. L., Wang, B. E., Jia, J. D., Wu, W. F., Xian, J. Z., Mertens, P. R., Cai, W. M., and Dooley, S. (2005). Effect of interferon-gamma on hepatic fibrosis in chronic hepatitis B virus infection: a randomized controlled study. Clin. Gastroenterol. Hepatol. 3, 819–828.
Wieckowska, A., Papouchado, B. G., Li, Z., Lopez, R., Zein, N. N., and Feldstein, A. E. (2008). Increased hepatic and circulating interleukin-6 levels in human nonalcoholic steatohepatitis. Am. J. Gastroenterol. 103, 1372–1379.
Wu, Y. J., Cai, W. M., Li, Q., Liu, Y., Shen, H., Mertens, P. R., Dooley, S., and Weng, H. L. (2011). Long-term antifibrotic action of interferon-gamma treatment in patients with chronic hepatitis B virus infection. HBPD INT 10, 151–157.
Wuestefeld, T., Klein, C., Streetz, K. L., Betz, U., Lauber, J., Buer, J., Manns, M. P., Muller, W., and Trautwein, C. (2003). Interleukin-6/glycoprotein 130-dependent pathways are protective during liver regeneration. J. Biol. Chem. 278, 11281–11288.
Yang, L., Zhang, Y., Wang, L., Fan, F., Zhu, L., Li, Z., Ruan, X., Huang, H., Wang, Z., Huang, Z., Huang, Y., Yan, X., and Chen, Y. (2010). Amelioration of high fat diet induced liver lipogenesis and hepatic steatosis by interleukin-22. J. Hepatol. 53, 339–347.
Yoshida, K., Yang, G. X., Zhang, W., Tsuda, M., Tsuneyama, K., Moritoki, Y., Ansari, A. A., Okazaki, K., Lian, Z. X., Coppel, R. L., Mackay, I. R., and Gershwin, M. E. (2009). Deletion of interleukin-12p40 suppresses autoimmune cholangitis in dominant negative transforming growth factor beta receptor type II mice. Hepatology 50, 1494–1500.
Yu, J. H., Zhu, B. M., Wickre, M., Riedlinger, G., Chen, W., Hosui, A., Robinson, G. W., and Hennighausen, L. (2010). The transcription factors signal transducer and activator of transcription 5A (STAT5A) and STAT5B negatively regulate cell proliferation through the activation of cyclin-dependent kinase inhibitor 2b (Cdkn2b) and Cdkn1a expression. Hepatology 52, 1808–1818.
Zenewicz, L. A., Yancopoulos, G. D., Valenzuela, D. M., Murphy, A. J., Karow, M., and Flavell, R. A. (2007). Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity 27, 647–659.
Zhu, R., Diem, S., Araujo, L. M., Aumeunier, A., Denizeau, J., Philadelphe, E., Damotte, D., Samson, M., Gourdy, P., Dy, M., Schneider, E., and Herbelin, A. (2007). The Pro-Th1 cytokine IL-12 enhances IL-4 production by invariant NKT cells: relevance for T cell-mediated hepatitis. J. Immunol. 178, 5435–5442.
Keywords: stellate cells, interferon, interleukin, STATs
Citation: Kong X, Horiguchi N, Mori M and Gao B (2012) Cytokines and STATs in liver fibrosis. Front. Physio. 3:69. doi: 10.3389/fphys.2012.00069
Received: 03 January 2012; Paper pending published: 18 January 2012;
Accepted: 11 March 2012; Published online: 03 April 2012.
Edited by:
Honglei Weng, University of Heidelberg, GermanyReviewed by:
Honglei Weng, University of Heidelberg, GermanyRobert Eferl, Medical University Vienna, Austria
Copyright: © 2012 Kong, Horiguchi, Mori and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: Bin Gao, Laboratory of Liver Diseases, National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health, 5625 Fishers Lane, Room 2S-33, Bethesda, MD 20892, USA. e-mail:Ymdhb0BtYWlsLm5paC5nb3Y=