- Department of Mathematics and Computer Science, Eindhoven University of Technology, Eindhoven, Netherlands
This paper proposes a statistical physics-based data assimilation model for the mobility of water-bound hydrogen nuclear spins in the brain in the context of diffusion weighted magnetic resonance imaging (DWI or DW-MRI). Point of departure is a statistical hopping model that emulates molecular motion in the presence of static and stationary microscale obstacles, statistically reflected in the apparent inhomogeneous anisotropic DWI signal profiles. Subsequently, we propose a Riemann–Finsler geometric interpretation in terms of a metric transform that simulates this molecular process as free diffusion on a vacuous manifold with all diffusion obstacles absorbed in its geometry. The geometrization procedure supports the reconstruction of neural tracts (geodesic tractography) and their quantitative characterization (tractometry). The Riemann-DTI model for geodesic tractography based on diffusion tensor imaging (DTI) arises as a limiting case. The genuine Finslerian case is a geometric representation of high-angular resolution DTI, i.e., a generalized rank-two DTI framework without the quadratic restriction implied by a simplifying Gaussianity assumption on local diffusion or a second-order harmonic approximation of local orientation distributions.
1 Introduction
1.1 The hopping model
In an attempt to gain a deeper understanding of the physics underlying the diffusion of water in the brain [1], we propose a hopping model, in which mobile, diffusion-sensitized hydrogen spins (‘particles’ henceforth) are jumping to and from any fiducial point at a stationary, position-dependent hopping rate. Frictions and orientation preferences induced by self-diffusion and collisions with static fibrous tissue barriers and DWI-insusceptible solutes are incorporated in generic statistical constraints on the jumps. These are assumed to be spatially inhomogeneous and anisotropic, with a priori unconstrained size and orientation distributions induced by the tissue’s micro-structure (graininess
To appreciate the hopping model in its simplest form, the reader is referred to the one-dimensional inhomogeneous case introduced by [4] and scrutinized by [5]. Our application to water diffusion in the brain in the context of (model-free) DWI has led us to the following generalizations:
1. Generalize this to three dimensions;
2. Account for anisotropy induced by the fibrous nature of the brain (white matter) tissue; and
3. Disentangle diffusion orientations to prevent destructive interference during signal formation.
As a result, we obtain a generic model for high-angular resolution diffusion tensor imaging (DTI) not hampered by the ambiguities of its classical counterpart (by virtue of item 3). The construction, in turn, provides the necessary insight for a geometric interpretation supporting DWI-driven geodesic tractography and tractometry [6]; [7], generalizing (and including) the familiar Riemann-DTI paradigm ([8]; [9]; [10]; [11,12]; [13]; [14]; [15]). It also offers new insights in connection to established DWI representations, such as constrained spherical deconvolution, diffusion orientation distribution functions, Q-ball imaging, HARDI, and diffusion spectrum imaging (DSI) ([16]; [17]; [18]; [19, 20]; [21]; [3]; [22–24]; [25]; [24, 26]).
1.2 The master equation
The master equation governs the instantaneous rate of change in the local hydrogen spin density at a fiducial point with Cartesian coordinates
Values at a single voxel
The scalar field
The left-hand side counts the number of particles per unit time jumping away from a fiducial point
In the quantities
2 Theory
2.1 The hopping model in configuration space
Combining Equations 1, 2 yields the following integro-differential equation:
Despite the integral, it may be interpreted as a local equation in the configuration space confined to the base point
2.2 The Fokker–Planck equation in configuration space
Assuming the pdf
Combining Equations 4, 5 and exploiting the normalization Equation 3 for the lowest-order term and, for the first-order term, imposing the symmetry assumption
implying a vanishing one-point correlation function
one ends up with the following Fokker–Planck equation:
with the configuration space diffusion tensor field
The latter integral extends over the unit sphere
Note the quadratic scaling with a typical jump size and the local (tissue-dependent) modulation by the hopping rate prior. These expressions are further analyzed in Section 2.5. The diffusion tensor, as defined in Equation 9, plays a pivotal role in DTI. An excellent introduction to DTI and its (potential) clinical ramifications is explained in the work by [28].
Without Equation 6, a nontrivial first-order term would emerge. Since its order of magnitude depends on the degree of symmetry violation, regardless of step size
As remarked by [4], due to the inhomogeneity of the hopping model, the right-hand side of Equation 8 does not take the form of Fick’s law, where the diffusion tensor field would be in-between the partial-derivative operators
2.3 Angular resolution limitations
It is well-known that the diffusion tensor field (Equation 9), the keystone of DTI, is a relatively poor descriptor of anisotropy, among others due to destructive interference caused by the
2.4 The Fokker–Planck equation in phase space
To reveal the genuine nature of anisotropy at an arbitrary angular resolution, we must refrain from
There is no unique way to extend the Fokker–Planck equation to phase space based on our hopping model in the configuration space. An intuitive way is obtained by considering the ‘vertically lifted’ particle density
Consider the particles at the base point
The function
Such a term could arise in various ways, e.g., as a
Note its particularly simple, singular form, with only a single, isotropic local degree of freedom, the local hopping rate
2.5 The Fokker–Planck equation on the sphere bundle
The phase space diffusion equation (Equation 12) and its singular diffusion tensor (Equation 14) may be natural objects of study in the context of DSI, where one probes the entire phase space (again ignoring spatial resolution ambiguities), cf. [24, 26]. However, current clinical and technical constraints preclude a sufficiently dense sampling of this six-dimensional manifold. Clinical DWI typically relies on sampling of (one or a few) spherical shell(s). Moreover, anisotropy is encoded in the distribution of jump orientations, not magnitudes. We, therefore, decouple the two by considering marginal distributions for each, setting
We may integrate Equation 12, over the jump size distribution using any spherical coordinate parametrization for
so that
Since a particular form of parametrization is irrelevant for our discussion, we focus on the notation (Equation 15), for simplicity. The five-dimensional submanifold
For the sake of definiteness, one might consider a Maxwell–Boltzmann distribution for jump sizes
in which the sphere bundle diffusion tensor field is defined in terms of the phase space diffusion tensor field (Equation 13) and jump pdf (Equation 3) as
or, in terms of the hopping rate and phenomenological diffusion scale with the help of Equation 11, as
The occurrences of
The sphere bundle diffusion tensor field (Equations 17, 18) supports a high-angular resolution DTI framework alluded to in the foregoing, generalizing the classical case defined in the configuration space. However, it inherits its singular nature from the phase space diffusion tensor field (Equation 14), with a single nonzero eigendirection spanned by the distinguished direction
In Section 2.6, we show how regularization is naturally realized in the DTI limit by virtue of vertical projection (19), albeit at the expense of what we determine to achieve, viz., an unrestricted angular resolution. In Supplementary Appendix 2, we elaborate on the classical DTI premise, for which projection does not incur the loss of angular resolution. In Section 2.7, we consider regularization in combination with geometrization of Equations 17, 18 on the sphere bundle, maintaining an unrestricted angular resolution, the novelty and main objective of this article.
2.6 Back to configuration space: classical diffusion tensor imaging
Recall that, in its most rudimentary form, the classical diffusion tensor in configuration space captures the second-order momenta of the stipulated transition density function Equation 2:
Subsequently, based on our heuristic hopping model, we have generalized it to a diffusion tensor defined on extended domains, notably on the phase space of positions and jump vectors and, ultimately, via projection over jump size, on the sphere bundle of positions and orientations, tacitly assuming antipodal symmetry. The latter, given by Equations 17, 18 and referred to as ‘high-angular resolution DTI,’ defines our main object of interest.
By construction, spherical averaging of the singular tensor (Equations 17, 18) via Equations 16 reproduces the regular, classical diffusion tensor field (10) in configuration space. This projection expresses a correspondence principle, clarifying how classical DTI emerges as a limiting case of high-angular resolution DTI. Clearly, this procedure causes irreversible loss of orientation information due to destructive interference, unless very special conditions on the nature of the medium hold. This restrictive case is considered in more detail in Supplementary Appendix 2 as an instructive hypothetical limit.
2.7 Geometrization and geodesic tractography
In the Riemann-DTI paradigm, it is stipulated that the configuration space DTI tensor defines (up to isotropic [8]; [9] or anisotropic scaling [39]; [40]) a dual Riemannian metric, in terms of which one can express the tractography inverse problem as a (constrained) geodesic or ‘shortest path’ problem ([7]; [41]; [11]; [13]; [15, 42]). This interpretation is consistent with its symmetric positive definite nature.
It is tempting to assign a similar role in a generalized, Finsler-geometric setting, to the high-angular resolution DTI tensor (Equations 17, 18) on the sphere bundle. However, there is a fundamental obstruction, viz., its singular nature. Its eigenspace is, by construction, one-dimensional, spanned by the distinguished direction
To begin with, let us stipulate a singular dual metric tensor by ‘demodulating’ the diffusion tensor on the sphere bundle, Equation 18, excluding local tissue permeability captured by the hopping rate:
with the scalar field
as a geometric counterpart of the diffusion tensor on the sphere bundle. The reason for suppressing the hopping rate in the dual metric is threefold: (i) as a heuristic toy model parameter, the exact physical significance of the hopping rate is not well-understood; (ii) the demodulated metric reflects local anisotropic hydrogen spin mobility constraints, independent of the isotropic tissue permeability captured by the hopping rate (a manifestation of non-geometric microstructural properties in a voxel); and (iii) a demodulated metric is least committed, in the sense that it can be adapted a posteriori by a conformal scale factor, defining an effective hopping rate
Figure 1 illustrates the diffusivity glyphs arising from the scalar field
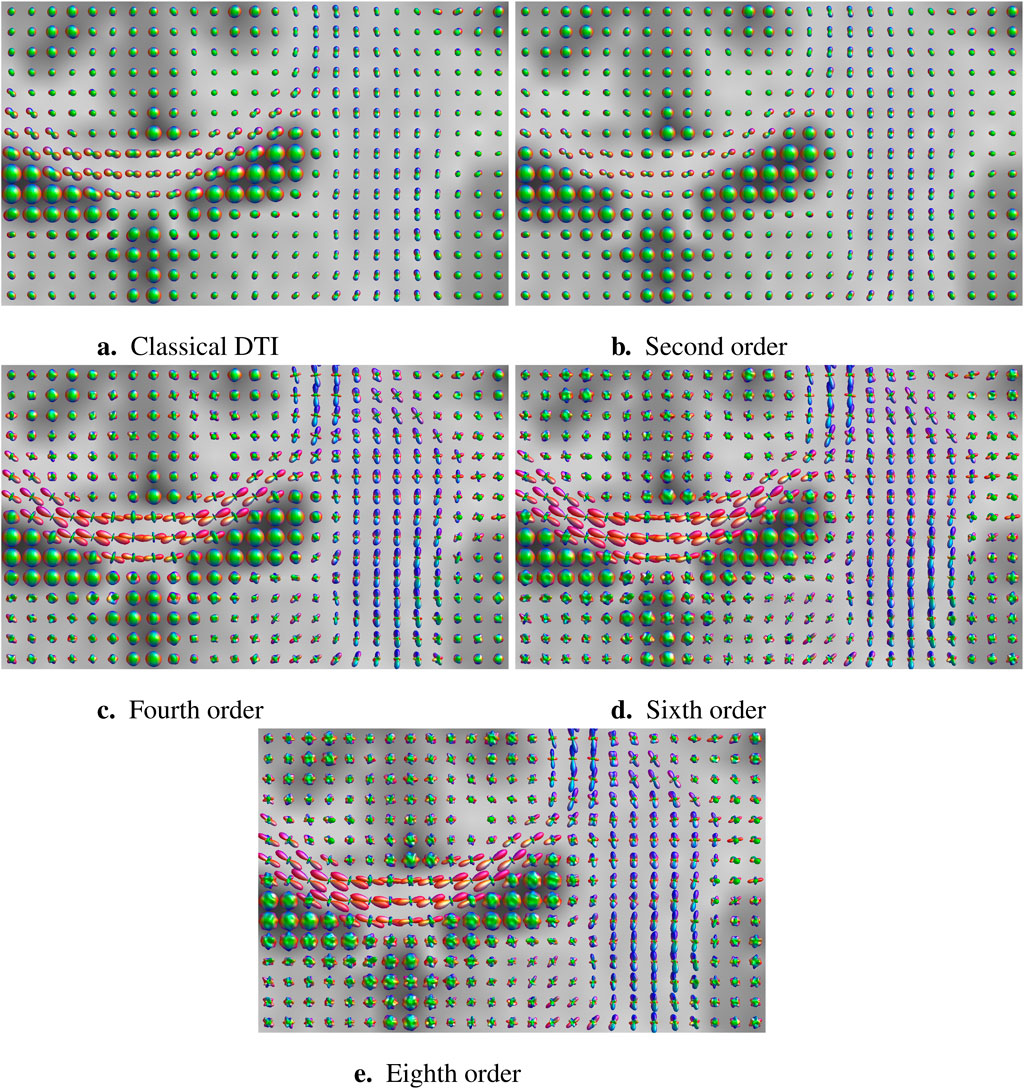
Figure 1. Classical and high-angular resolution DTI for increasing levels of spherical harmonic articulation. Glyphs represent apparent diffusivity values
Since
Such a generalized inverse is easily seen to be given by
modulo ambiguities of the form
in which
An alternative method that gracefully resolves the ambiguity proceeds via an intermediate construction of a new, generalized norm for the vector
The Hessian of this Finsler Lagrangian defines a new metric tensor in the usual manner [32]; [33]:
Since this metric exists alongside our original Euclidean metric, one must remain cautious in view of ambiguous index-raising and -lowering conventions; unless stated otherwise, index raising and lowering will henceforth pertain to the Finsler metric. Working out technicalities, setting2
and writing
If we introduce the covariant vertical derivative
with a
then we obtain
This allows us to rewrite Equation 21 as
We will refer to the regularized case (Equations 21, 24) as the Riemann–Finsler metric tensor. The vertical gauge condition (Equation 22) is the geometric ploy that allows us to sweep diffusion anisotropies under the rug. With this gauge condition, one could say that the diffusion path length
The Riemann–Finsler metric tensor is regular3 for typical media, given a physically realistic choice of Equation 20. It defines a local, orientation-dependent norm that reflects the typical diffusion path length in the local brain tissue along the corresponding orientation. This means that a vector
2.8 The Riemann–Finsler geodesic rationale
The commonly accepted tractography premise that white matter tracts follow paths of least resistance to diffusion suggests that white matter tracts are locally shortest paths, or geodesics, relative to the Riemann–Finsler metric (Equation 21). Recall that a curve between fixed end points is called locally shortest if any small perturbation of that curve increases its length. The attribute ‘local’ is necessary since two sufficiently distant points in the non-Euclidean brain space may be connected by multiple geodesics. The length of the globally shortest geodesic connection provides an unambiguous notion of distance between its endpoints.4 The Hopf–Rinow theorem [46] guarantees the existence of a geodesic curve between any two points (geodesic completeness), which makes geodesic tractography fundamentally different from mainstream streamline-inspired paradigms despite their shared underlying hypothesis.
In contrast to streamlines, geodesics are stipulated to optimize sensitivity, regardless of specificity. As a result, an arbitrarily chosen geodesic is almost surely a false positive since anatomical connections are sparse, whereas geodesic connections are dense. Data-driven tractometric analysis, ideally supported by external anatomical information, must, therefore, be a constitutional part of any unambiguous geodesic tractography paradigm. Recall that the Riemann–Finsler geometric representation of the brain is meant to hide local diffusivities such as to make things look homogeneous and isotropic, as a result of which there are no a priori preferred (such as ‘true’ versus ‘false positive’) members in a geodesic congruence. Tractometric invariants for pruning by false positive removal must necessarily incorporate explicit diffusion characteristics, defined only by virtue of a combined Euclidean/Finslerian perspective. Unlike Einstein’s general relativity theory, the Riemann–Finsler geodesic rationale, thus, relies on a metric transform connecting two metrics on a metrically amorphous manifold, which has been conveniently furnished with a global ‘Cartesian’ coordinate chart.
Tractometric analysis for the purpose of anatomical tract disambiguation is beyond the scope of this article and will be considered in future work, cf. also Colby et al., DeSantis et al., and St-Jean et al. for some inspiring ideas ([47]; [48]; [49]).
Geodesics are obtained by (locally) minimizing the length of a parametric curve
Length is then expressed by the functional
subject to Equation 25. Variational calculus produces the geodesic equations ([32]; [7]; [33]):
where the geodesic spray coefficients are given by
cf. [32] and [33] for details. Alternatively, we have
yielding
with (0-homogeneous) formal Christoffel symbols of the second kind
Figure 2 shows some (initialization-dependent) numerical solutions connecting seed and target regions associated with the corticospinal tract (CST) for both the classical Riemannian case and the genuine Finslerian case. In the Riemannian limit, the metric tensor becomes vertically constant. In that case, it is easy to see that the geodesic spray coefficients (Equation 29) reduce to quadratic forms, viz.,
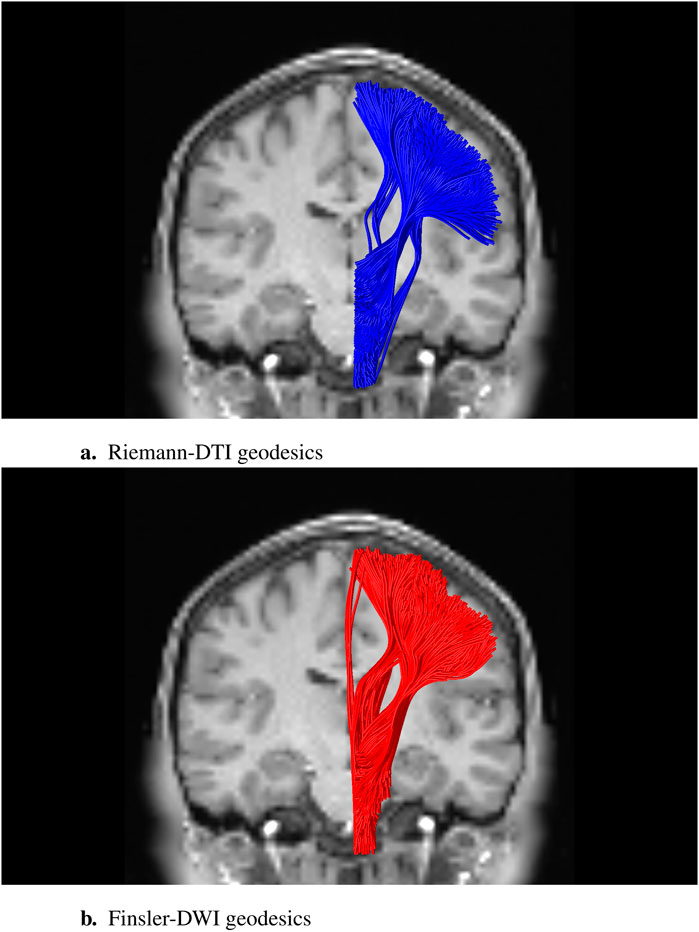
Figure 2. Sparse subset of geodesic tracts, i.e., solutions of (38), stipulated for the CST, connecting arbitrarily chosen endpoints in a seed and target region based on an anatomical atlas. These should not be mistaken for actual anatomical nerve bundles, the determination of which requires a characterization of their anatomical plausibility in terms of tractometric features and external anatomical constraints. The two-dimensional coronal background slice of the DWI image provides some qualitative anatomical context for the three-dimensional visualization of the tracts. The experiment was performed on a DWI dataset from the Human Connectome Data Project (dataset “WU-Minn HCP Data—1200 Subjects”: Subject 100,307; TE/TR/echo spacing 89.5/5520.0/0.78 ms; b = 2000 s/mm2). (A) Riemann-DTI geodesics; (B) Finsler-DWI geodesics to eighth-order spherical harmonic approximation; recall Figure 1E.
whereas the formal
In the Riemannian limit, we, thus, retrieve the established Riemann-DTI geodesic tractography paradigm. Reversely, a linear connection defines a Riemann–Finsler geometry of Berwaldian type, which is slightly more general than the Riemannian limit.5
The right-hand side of Equations 27, 30 merely contributes to a parameter-dependent ‘acceleration’ along the curve and may be set to zero (‘affine parametrization’) if one does not care about parametrization. This is automatically achieved in the variational formulation if one replaces the 1-homogeneous integrand
In the context of our extended slit tangent bundle furnished with a Finsler metric, Berwald’s horizontal derivatives are often encountered replacing partial derivatives for a geometrically consistent horizontal/vertical splitting:
The so-called nonlinear connection coefficients in this expression satisfy
Recall Equation 29, 30. Using this definition, we may define a covariant horizontal derivative:
Analogous to Equation 27, we may then set
to define the 0-homogeneous horizontal diffusion gauge field
This is the horizontal counterpart of Equation 23, rendering the scalar field
Equations 27, 30 generalize the Riemann-DTI geodesic tractography paradigm ([7]; [40]; [15]) to genuine Finsler-DWI geodesic tractography for our new high-angular resolution DTI model. The conceptually simple but mathematically profound difference lies in the nature of the DWI-induced local anisotropic norm concept. In the Riemann-DTI case, this is an inner product-induced norm, the dual Gram matrix of which matches the DTI tensor one-to-one (at the expense of residual DWI signal loss). In the genuine Finsler-DWI case, we have a general norm, the infinitely many degrees of freedom of which admit a good match with an unconstrained DWI model based on an arbitrary number of diffusion orientations, e.g., using a suitably truncated complete function basis ([2]; [3]).
3 Summary, conclusion, and discussion
Hopping models are convenient proxies for statistical models of complex diffusion phenomena, especially when corroborated by complementary empirical evidence. The brain, considered as a porous medium for water diffusion, is an epitome of such a system, for which the convoluted interplay of molecular dynamics and the physicochemical environment would be prohibitively difficult to capture if not distilled down to its quintessential components admitting ‘model-free’ data assimilation. We have proposed such a hopping model to explain inhomogeneous anisotropic diffusion in the brain with relevant model parameters for DWI data assimilation, obviating a priori constraints on anatomical complexity.
A standard statistical procedure produces a Fokker–Planck equation that is fully determined by a classical DTI tensor defined in the three-dimensional configuration space. Its form betrays a ‘vertical projection,’ or angular average, of an underlying high-angular resolution DTI tensor. The latter is a singular, dyadic tensor, defined on the sphere bundle of base points capturing both positions and orientations, with a preferred orientation aligned with that of its underlying five-dimensional base point. This enables local scalar features, notably the diffusion path length, to be defined orientation-wise, preventing loss of information due to angular superposition inherent in classical DTI.
We have subsequently provided a geometric interpretation to support an operational scheme for geodesic tractography, generalizing the restrictive Riemann-DTI framework. The generalization entails the removal of implicit constraints on either the nature of the underlying diffusion process (Gaussianity assumption), or on the angular resolution by which it is represented in classical DTI (second-order harmonic truncation). By virtue of a metrizability trick via a so-called Finsler norm on the (slit) tangent bundle, it has turned out possible to retain a simple second-order, non-singular metric structure. The generalized, Riemann–Finsler metric, defined on the sphere bundle, adds novel structure to the metrically void ‘higher-order DTI’ model defined in the configuration space, as originally proposed by [50] and [51] to overcome classical DTI limitations. This additional metric structure enables generalized Finsler-DWI geodesic tractography, fully exploiting the generic nature of such a generalized model. Thus, the paradigm shift from low- to high-angular resolution DTI reverberates in the canonical way in which Riemann geometry is embedded into the more general Finsler setting.
The classical Riemann-DTI geodesic tractography paradigm arises as a (very) special case. This suggests a quantification of the relative global volumetric contributions of complex fiber configurations versus regions with single fiber coherence, cf. [30, 31], either in terms of the difference tensor
Our heuristic approach via a hopping model defined in configuration space undergoes inherent ambiguities in the form of nontrivial kernel functions on the sphere bundle that have no manifestation in configuration space due to zero angular average. Although such ambiguities do seem harmless for our application and have been glossed over in this article, a more rigorous top–down approach, departing from a hopping model in position–velocity phase space, reflecting actual molecular motion, might be more convincing. In such a model, one would need to couple a displacement vector (or an average velocity vector over a given diffusion time interval), i.e., a jump in the ‘vertical direction’ along a tangent fiber, to a spatial displacement, i.e., a change of position in the configuration space consistent with the jump vector. In geometrical terms, phase space translations corresponding to actual molecular motions are subject to a ‘horizontality condition,’ which dictates that the displacement or instantaneous velocity vector must be consistent with the induced movement in configuration space. In such a phase space hopping model, one might stipulate a Maxwell–Boltzmann-type distribution for particle velocities with a different ‘temperature’ for each position and orientation to capture the inhomogeneous and anisotropic nature of brain tissue since this would be consistent with established physics in the limit of obstacle-free bulk water, cf. Supplementary Appendix 1.
A physically appealing but mathematically mind-boggling path integral approach may be another way to arrive at a model for high-angular resolution DTI [52]; [53]. This turns out possible, even in the mathematically closed form, for the simplified case of classical DTI. However, path integrals other than those based on quadratic forms (i.e., nearly anything beyond classical DTI) do not yield closed-form expressions and may not even be well-defined. At best, one may hope for insights via a perturbative expansion or via formal symbolic manipulation. This might nevertheless be a viable way toward theoretical underpinning.
Because it poses no a priori constraints on angular resolution, high-angular resolution DTI offers a new, generic DWI model on a par with popular existing models but, as opposed to these, is specifically geared toward a geodesic rationale. More specifically, in the operational definition of high-angular resolution DTI, a particular orientation distribution function, ‘
The operational significance of the hopping rate factor
Finsler geodesic equations are well-known from the literature. From an anatomical perspective, their solutions are imbued with the same pros and cons as their Riemann-DTI counterparts and with all validation issues common to tractography methods in general. A clear advantage is their generic and (via tractometric characterization) adaptable nature, intuitive physical appeal, and mathematical rigor, with no parameters other than boundary conditions and those instantiated by DWI measurements, cf. the observations by Schilling et al., in this respect [54]. A disadvantage, in comparison to streamline methods, is their computational complexity (which may be mitigated by dimensionality reduction, cf. the geodesic tubes exploiting the linear geodesic deviation equations by [15] in the Riemann-DTI context), and their a priori lack of anatomical significance as a result of geodesic completeness. Without complementary tractometric characterization, which is beyond the scope of this article, geodesics must not be mistaken for anatomically meaningful tracts. They are optimal diffusion pathways in an operationally well-defined sense, expressing the basic tractography premise in a least committed way, with room for anatomical specification and pruning. With regard to the latter, the flexibility offered by virtue of geodesic completeness may also be clearly seen as an advantage. Note that, by virtue of a non-vanishing Cartan tensor, high-angular resolution DTI admits a richer set of tractometric invariants for tract characterization and filtering than its classical counterpart [40]; [55].
To summarize, the main result in this paper is twofold, viz. (i) freeing classical DTI from its inherent angular resolution limitation and (ii) embedding Riemann-DTI geodesic tractography into a generic Finsler-DWI framework. This puts the geodesic tractography rationale potentially (i.e., if furnished with biologically plausible tractometric criteria) on a par with state-of-the-art tractography methods. Its operationalization, including tractometric pruning, and experimental and clinical ramifications, will be considered in future work.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Author contributions
LF: conceptualization, formal analysis, funding acquisition, methodology, project administration, supervision, writing–original draft, and writing–review and editing. RS: formal analysis, methodology, visualization, and writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This publication is part of the project Bringing Tractography into Daily Neurosurgical Practice with the project number KICH1. ST03.21.004 of the research program Key Enabling Technologies for Minimally Invasive Interventions in Healthcare, which is (partly) financed by the Dutch Research Council (NWO), Elisabeth-TweeSteden Hospital (Tilburg, Netherlands), the Erasmus Medical Center (Rotterdam, Netherlands), the Amsterdam University Medical Center (Amsterdam, Netherlands), and Medtronic are gratefully acknowledged for their support. Medtronic was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Acknowledgments
Data collection and sharing for this project was provided by the MGH-USC Human Connectome Project (HCP; Principal Investigators: Bruce Rosen, M.D., Ph.D., Arthur W. Toga, Ph.D., Van J. Weeden, MD). HCP funding was provided by the National Institute of Dental and Craniofacial Research (NIDCR), the National Institute of Mental Health (NIMH), and the National Institute of Neurological Disorders and Stroke (NINDS). HCP data are disseminated by the Laboratory of Neuro Imaging at the University of Southern California.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphy.2024.1447311/full#supplementary-material
Footnotes
1Geometrically speaking,
2The dimensionful
3In particular, in the limiting case
4One should note that the use of absolute distance as the main criterion for anatomical brain connectivity, such as implied by some fast marching schemes based on an eikonal equation, ignores non-minimal geodesics and thus incurs loss of sensitivity, which runs counter to the geodesic tractography rationale.
5By Szabó’s metrizability theorem, Berwald geometries are of little interest for parameter-invariant geodesic tractography since it claims the existence of a Riemannian metric inducing the same geodesic curves, cf. [32], [33], and [56].
References
1. Le Bihan D, Iima M. Diffusion magnetic resonance imaging: what water tells us about biological tissues. PLOS Biol (2015) 13:e1002203. doi:10.1371/journal.pbio.1002203
2. Fick R, Wassermann D, Pizzolato M, Deriche R (2015) A unified framework for spatial and temporal diffusion in diffusion MRI. In Proceedings of the twenty-fourth international conference on information processing in medical imaging–IPMI 2015 (sabhal mor ostaig, isle of skye, UK), eds. S Ourselin, DC Alexander, C-F Westin, and MJ Cardoso (New York: Springer-Verlag), vol. 9123 of Lecture Notes in Computer Science, 167–78. doi:10.1007/978-3-319-19992-4_13
3. Özarslan E, Koay CG, Shepherd TM, Komlosh ME, İrfanoğlu MO, Pierpaoli C, et al. Mean apparent propagator (MAP) MRI: a novel diffusion imaging method for mapping tissue microstructure. Neuroimage (2013) 78:16–32. doi:10.1016/j.neuroimage.2013.04.016
4. Kampen NGv. (1988) Diffusion in inhomogeneous media. J Phys Chem Sol,49:673–7. doi:10.1016/0022-3697(88)90199-0
5. Bringuier E. Particle diffusion in an inhomogeneous medium. Eur J Phys (2011) 32:975–92. doi:10.1088/.0143-0807/32/4/012
6. Dell’Acqua F, Leemans A, Descoteaux M (2024) Handbook of diffusion MR tractography: imaging methods, biophysical models, algorithms and applications. Academic Press.
8. Fuster A, Dela Haije T, Tristán-Vega A, Plantinga B, Westin C-F, Florack L. Adjugate diffusion tensors for geodesic tractography in white matter. J Math Imaging Vis (2016) 54:1–14. doi:10.1007/.s10851-015-0586-8
9. Hao X, Zygmunt K, Whitaker RT, Fletcher PT. Improved segmentation of white matter tracts with adaptive Riemannian metrics. Med Image Anal (2014) 18:161–75. doi:10.1016/j.media.2013.10.007
10. Hauberg S, Schober M, Liptrot M, Hennig P, Feragen A (2015) A random Riemannian metric for probabilistic shortest-path tractography. In: N Navab, J Hornegger, WM Wells, and A Frangi, editors. Proceedings of the 18th international conference on medical image computing and computer assisted intervention—MICCAI 2015 (munich, Germany, october 5–9 2015). Berlin: Springer-Verlag. p. 597–604. doi:10.1007/.978-3-319-24553-973
11. Lenglet C, Deriche R, Faugeras O (2004) Inferring white matter geometry from diffusion tensor MRI: application to connectivity mapping. In: T Pajdla, and J Matas, editors. Proceedings of the eighth European conference on computer vision (prague, Czech republic, may 2004). Berlin: Springer-Verlag . p. 127–40.
12. Lenglet C, Prados E, Pons J-P, Deriche R, Faugeras O. Brain connectivity mapping using Riemannian geometry, control theory and PDEs. SIAM J Imaging Sci (2009) 2:285–322. doi:10.1137/070710986
13. O’Donnell L, Haker S, Westin CF. New approaches to estimation of white matter connectivity in diffusion tensor MRI: elliptic PDEs and geodesics in a tensor-warped space. Proc Med Imaging Comput Computer Assisted Intervention (2002) 2488:459–66. doi:10.1007/3-540-45786-0_57
14. Risken H (1989) The fokker-planck equation: methods of solution and applications. In: Springer series in synergetics. vol. 18. Berlin: Springer-Verlag. doi:10.1007/978-3-642-61544-3
15. Sengers R, Florack L, Fuster A. Geodesic uncertainty in diffusion MRI. Front Computer Sci (2021) 3:718131. Invited paper. doi:10.3389/fcomp.2021.718131
16. Dell’Acqua F, Simmons A, Williams SCR, Catani M. Can spherical deconvolution provide more information than fiber orientations? hindrance modulated orientational anisotropy, a true-tract specific index to characterize white matter diffusion. Hum Brain Mapp (2013) 34:2464–83. doi:10.1002/hbm.22080
17. Dell’Acqua F, Tournier J-D. Modelling white matter with spherical deconvolution: how and why? NMR Biomed (2019) 32:e3945–18. doi:10.1002/nbm.3945
18. Descoteaux M (2015). High angular resolution diffusion imaging (HARDI). In: J Webster, editor. Wiley encyclopedia of electrical and electronics engineering. John Wiley and Sons, Inc. p. 1–25. doi:10.1002/047134608X.W8258
19. Descoteaux M, Deriche R, Knösche TR, Anwander A. Deterministic and probabilistic tractography based on complex fibre orientation distributions. IEEE Trans Med Imaging (2009) 28:269–86. doi:10.1109/tmi.2008.2004424
20. Descoteaux M, Deriche R, Le Bihan D, Mangin J-F, Poupon C. Multiple q-shell diffusion propagator imaging. Med Image Anal (2010) 15:603–21. doi:10.1016/j.media.2010.07.001
21. Jian B, Vemuri BC. A unified computational framework for reconvolution to reconstruct multiple fibers from diffusion weighted MRI. IEEE Trans Med Imaging (2007) 26:1464–71. doi:10.1109/TMI.2007.907552
22. Tournier J-D, Calamante F, Connelly A. Robust determination of the fibre orientation distribution in diffusion MRI: non-negativity constrained super-resolved spherical deconvolution. Neuroimage (2007) 35:1459–72. doi:10.1016/j.neuroimage.2007.02.016
23. Tournier J-D, Yeh C-H, Calamante F, Cho K-H, Connelly A, Lin C-P. Direct estimation of the fiber orientation density function from diffusion-weighted MRI data using spherical deconvolution. Neuroimage (2004) 23:1176–85. doi:10.1016/j.neuroimage.2004.07.037
24. Tournier J-D, Yeh C-H, Calamante F, Cho K-H, Connelly A, Lin C-P. Resolving crossing fibres using constrained spherical deconvolution: validation using diffusion-weighted imaging phantom data. Neuroimage (2008) 42:617–25. doi:10.1016/j.neuroimage.2008.05.002
26. Wedeen VJ, Hagmann P, Tseng WYI, Reese TG, Weisskoff RM. Mapping complex tissue architecture with diffusion spectrum magnetic resonance imaging. Magn Reson Med (2005) 54:1377–86. doi:10.1002/mrm.20642
27. Wedeen VJ, Wang RP, Schmahmann JD, Benner T, Tseng WYI, Dai G, et al. Diffusion spectrum magnetic resonance imaging (DSI) tractography of crossing fibers. Neuroimage (2008) 41:1267–77. doi:10.1016/j.neuroimage.2008.03.036
28. Mori S, Tournier J-D. Introduction to diffusion tensor imaging and higher order models. 2nd ed. Amsterdam: Elsevier (2014).
29. Poirier C, Descoteaux M. A unified filtering method for estimating asymmetric orientation distribution functions. Neuroimage (2024) 287:120516. doi:10.1016/j.neuroimage.2024.120516
30. Schilling KG, Gao Y, Janve V, Stepniewska I, Landman BA, Anderson AW. Can increased spatial resolution solve the crossing fiber problem for diffusion MRI? NMR Biomed (2017) 30:1–16. doi:10.1002/nbm.3787
31. Schilling KG, Tax CMW, Rheault F, Landman BA, Anderson AW, Descoteaux M, et al. Prevalence of white matter pathways coming into a single white matter voxel orientation: the bottleneck issue in tractography. Hum Brain Mapp (2021) 43:1196–213. doi:10.1002/hbm.25697
32. Bao D, Chern S-S, Shen Z (2000) An introduction to riemann-finsler geometry. In: 2000 of graduate Texts in mathematics. New York: Springer-Verlag.
34. Aganj I, Lenglet C, Sapiro G, Yacoub E, Ugurbil K, Harel N. Reconstruction of the orientation distribution function in single- and multiple-shell q-ball imaging within constant solid angle. Magn Reson Med (2010) 64:554–66. doi:10.1002/mrm.22365
35. Descoteaux M, Angelino E, Fitzgibbons S, Deriche R. Regularized, fast, and robust analytical Q-ball imaging. Magn Reson Med (2007) 58:497–510. doi:10.1002/mrm.21277
36. Hess CP, Mukherjee P, Tan ET, Xu D, Vigneron DB. Q-ball reconstruction of multimodal fiber orientations using the spherical harmonic basis. Magn Reson Med (2006) 56:104–17. doi:10.1002/mrm.20931
37. Barnett A. Theory of Q-ball imaging redux: implications for fiber tracking. Magn Reson Med (2009) 62:910–23. doi:10.1002/mrm.22073
38. Garyfallidis E, Brett M, Amirbekian B, Rokem A, van der Walt S, Descoteaux M, et al. Dipy, a library for the analysis of diffusion MRI data. Front Neuroinformatics (2014) 8:8–17. Article 8. doi:10.3389/fninf.2014.00008
39. Florack L, Sengers R, Meesters S, Smolders L, Fuster A (2021) Riemann-DTI Geodesic Tractography Revisited. In: A Fuster, E Özarslan, T Schultz, and E Zhang, editors. Anisotropy Across Fields and Scales. Mathematics and Visualization. Springer, Cham. p. 155–6.
40. Sengers HJCE (2024) Riemannian geometry in diffusion weighted imaging. Eindhoven: Eindhoven University of Technology.
41. Florack LMJ, Fuster A (2014) Riemann-Finsler geometry for diffusion weighted magnetic resonance imaging. In: C-F Westin, A Vilanova, and B Burgeth, editors. Visualization and processing of tensors and higher order descriptors for multi-valued data. Springer-Verlag. p. 189–208. doi:10.1007/978-3-642-54301-2
42. Sengers R, Fuster A, Florack L (2021) Geodesic tubes for uncertainty quantification in diffusion MRI. In: S Sommer, A Feragen, J Schnabel, and M Nielsen, editors. Proceedings of the twenty-seventh international conference on information processing in medical imaging–IPMI 2021 (bornholm, Denmark). Berlin: Springer-Verlag. p. 279–90.
43. Axler S (2024) Linear algebra done right. Springer International Publishing. doi:10.1007/978-3-031-41026-0
44. Penrose R. A generalized inverse for matrices. Math Proc Cambridge Philosophical Soc (1955) 51:406–13. doi:10.1017/S0305004100030401
45. Drazin MP. Pseudo-inverses in associative rings and semigroups. The Am Math Monthly (1958) 65:506–14. doi:10.1080/00029890.1958.11991949
46. Hopf H, Rinow W. Ueber den Begriff der vollständigen differentialgeometrischen Fläche. Commentarii Mathematici Helvetici (1931) 3:209–25. doi:10.1112/blms/7.3.261
47. Colby JB, Soderberg L, Lebel C, Dinov ID, Thompson PM, Sowell ER. Along-tract statistics allow for enhanced tractography analysis. Neuroimage (2012) 59:3227–42. doi:10.1016/j.neuroimage.2011.11.004
48. De Santis S, Drakesmith M, Bells S, Assaf Y, Jones DK. Why diffusion tensor MRI does well only some of the time: variance and covariance of white matter tissue microstructure attributes in the living human brain. Neuroimage (2014) 89:35–44. doi:10.1016/j.neuroimage.2013.12.003
49. St-Jean S, Chamberland M, Viergever MA, Leemans A. Reducing variability in along-tract analysis with diffusion profile realignment. Neuroimage (2019) 199:663–79. doi:10.1016/j.neuroimage.2019.06.016
50. Özarslan E, Mareci TH. Generalized diffusion tensor imaging and analytical relationships between diffusion tensor imaging and high angular resolution imaging. Magn Reson Med (2003) 50:955–65. doi:10.1002/mrm.10596
51. Florack L, Balmashnova E, Astola L, Brunenberg E. A new tensorial framework for single-shell high angular resolution diffusion imaging. J Math Imaging Vis (2010) 3:171–81. doi:10.1007/s10851-010-0217-3
52. Kleinert H. Path integrals in quantum mechanics, statistics, polymer physics, and financial markets. 5th ed. Singapore: World Scientific (2009).
53. Özarslan E, Westin C-F, Mareci TH. Characterizing magnetic resonance signal decay due to Gaussian diffusion: the path integral approach and a convenient computational method. Concepts Magn Reson A (2015) 44A:203–13. doi:10.1002/cmr.a.21354
54. Schilling KG, Petit L, Rheault F, Remedios S, Pierpaoli C, Anderson AW, et al. Brain connections derived from diffusion MRI tractography can be highly anatomically accurate—if we know where white matter pathways start, where they end, and where they do not go. Brain Struct Funct (2020) 225:2387–402. doi:10.1007/s00429-020-02129-z
55. Sengers R, Dela Haije T, Fuster A, Florack L. Tractometric coherence of fiber bundles in DTI. In: S Cetin-Karayumak, D Christiaens, M Figini, P Guevara, T Pieciak, E Powellet al. editors 13722. Springer-Verlag (2022). p. 137–48.
56. Vincze C. A new proof of Szabó’s theorem on the Riemann-metrizability of Berwald manifolds. Acta Mathematica Academiae Paedagogicae Nyiregyhaziensis (2005) 21:199–204.
58. Balmashnova EG, Fuster A, Florack LMJ (2012) Decomposition of higher-order homogeneous tensors and applications to HARDI. In: E Panagiotaki, L O’Donnell, T Schultz, and GH Zhang, editors. MICCAI workshop on computational diffusion MRI (october 5, 2012, nice, France). p. 79–89.
59. Einstein A (1956) Investigations on the theory of the brownian movement. New York: Dover Publications, Inc.
Keywords: inhomogeneous anisotropic diffusion, hopping model, high-angular resolution diffusion tensor imaging, diffusion weighted imaging, orientation distribution function, geodesic tractography, Riemann–Finsler geometry
Citation: Florack L and Sengers R (2024) High-angular resolution diffusion tensor imaging: physical foundation and geometric framework. Front. Phys. 12:1447311. doi: 10.3389/fphy.2024.1447311
Received: 11 June 2024; Accepted: 25 October 2024;
Published: 13 December 2024.
Edited by:
Gisela E. Hagberg, University of Tübingen, GermanyReviewed by:
Christian Herbert Ziener, German Cancer Research Center (DKFZ), GermanyKaleem Siddiqi, McGill University, Canada
Copyright © 2024 Florack and Sengers. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Luc Florack, bC5tLmouZmxvcmFja0B0dWUubmw=
 Luc Florack
Luc Florack Rick Sengers
Rick Sengers