- 1Laboratory of Intelligent Collaborative Computing, University of Electronic Science and Technology of China, Chengdu, Sichuan, China
- 2Trusted Cloud Computing and Big Data Key Laboratory of Sichuan Province, Chengdu, Sichuan, China
- 3School of Optoelectronic Science and Engineering, University of Electronic Science and Technology of China, Chengdu, China
- 4Department of Pulmonary and Critical Care Medicine, Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China, Chengdu, China
- 5School of Medicine, University of Electronic Science and Technology of China, Chengdu, China
- 6School of Network and Communication Engineering, Chengdu Technological University, Chengdu, China
- 7Yingcai Experimental College, University of Electronic Science and Technology of China, Chengdu, China
Cyber-Physical-Social Systems (CPSS) epitomize the modern era’s intelligent connectivity. They integrate physical devices, computer networks, and social networks, forming an innovative paradigm for intelligent systems. Utilizing CPSS to enhance intelligence, automation, and remote services in healthcare represents a primary research focus. Pulmonary embolism, a prevalent condition resulting from the blockage of the pulmonary artery and its branches by emboli, leads to a spectrum of clinical syndromes marked by impaired pulmonary circulation and right heart dysfunction, contributing to sudden and unpredictable fatalities. Nevertheless, the diagnosis of pulmonary embolism remains challenging due to non-specific clinical presentations, constrained diagnostic capabilities, delayed diagnoses, insufficient physician knowledge, and suboptimal diagnostic techniques. Consequently, we introduce the innovative LSCU-Net architecture within the CPSS framework, designed to develop an automated segmentation and intelligent assessment system for pulmonary embolism, facilitating its automated and intelligent detection. The experimental findings demonstrate that the model accurately segments pulmonary embolism, evidenced by a Jaccard index of 0.6958, a Dice coefficient of 0.8193, a Mean Pixel Accuracy (mPA) of 0.8519, and an accuracy of 0.9993. Empirical studies reveal that our proposed model substantially surpasses existing models in performance. Consequently, this model can aid physicians in the diagnosis of pulmonary embolism during clinical practice. The established pulmonary embolism automatic segmentation and assessment system also showcases the application successes of CPSS in intelligent remote healthcare. The system’s development and deployment not only streamline physicians’ diagnostic processes but also elevate public health standards and advance CPSS research within the medical domain.
1 Introduction
In recent years, the paradigm of Cyber-Physical Social Systems (CPSS [1]) has emerged. As an interdisciplinary field, CPSS integrates theories and technologies from computer science, physics, and social sciences, with a focus on exploring the interactions among sensors, physical systems, and social networks, and is dedicated to intelligent decision-making and optimization. Particularly in the field of medical image processing, the adoption of CPSS offers new opportunities to surmount the challenges of medical image data processing. Investigating the utilization of CPSS to achieve remote, automated, and intelligent medical treatment is a key area of interest. The medical treatment field is rapidly evolving from a traditional centralized treatment approach by hospitals and experts to a patient-centric distributed treatment model.
CPSS powers smart personal healthcare. In CPSS, the integration of physical devices, social networks, and networked systems enables the real-time monitoring of patient data (including blood pressure, vital signs, and activity monitoring) and facilitates the conversion of these data from the physical to the digital realm. This process not only endows devices with the ability to compute, coordinate remotely, and operate autonomously, but also advances medical treatment towards greater remoteness, automation, and intelligence. In this context, H et al. [2] introduced a doctor recommendation algorithm based on the doctor’s diagnostic and treatment efficacy and the patient’s personal preferences in 2014. In 2018, Q et al. [3] focused on e-medical services leveraging social networks and proposed an e-medical system model that utilizes the green CPSS framework to detect and predict disease transmission.
This study aims to conduct an in-depth analysis of CPSS applications within the medical field, with a particular focus on the efficacy of CPSS in supporting pulmonary embolism patients through remote, real-time, and intelligent treatment modalities.
Pulmonary embolism (PE), a prevalent medical condition, is characterized by a spectrum of diseases or clinical syndromes wherein endogenous or exogenous emboli obstruct the pulmonary artery and its branches, leading to impaired pulmonary circulation and right ventricular dysfunction [4]. Pulmonary embolism represents a critical health threat and is a primary cause of unexpected sudden death [5, 6]. Extensive data indicate that pulmonary embolism has a high global incidence. Annually, the United States sees between 650,000 and 700,000 new cases of pulmonary embolism, with a mortality rate surpassed only by cancer and coronary heart disease, making it the third leading cause of death [7]. In France, the incidence of pulmonary embolism rivals that of myocardial infarction fatalities, with over 100,000 new cases reported annually [6]. The resulting disability and morbidity from PE are significant drivers of medical expenditures and bear socioeconomic implications [8–14].
The diagnosis of pulmonary embolism remains challenging owing to the non-specific clinical manifestations of the condition, constrained diagnostic resources, delays in seeking medical attention, and the lack of physician awareness coupled with inappropriate diagnostic methods. Pulmonary embolism clinically presents as respiratory dysfunction. Numerous conditions can cause this symptom, making it challenging for physicians to directly associate this clinical feature with pulmonary embolism. Consequently, the majority of patients with pulmonary embolism do not receive an accurate diagnosis in their lifetime, with misdiagnosis rates reaching as high as 70%. An American study examining the correlation between the timing of pulmonary embolism diagnosis and mortality revealed that each hour of diagnostic advancement reduced the patient’s mortality risk by 5% [15].
Prior to the extensive adoption of artificial intelligence (AI) technology, especially deep learning, in medical image analysis, medical image segmentation was heavily dependent on traditional image processing techniques. Traditional image segmentation methods have played a pivotal role in analyzing medical images, including X-rays, MRIs, CT scans, and ultrasound images. These methods typically entail manual or semi-automatic processes and rely on a variety of mathematical and algorithmic approaches to identify and delineate the boundaries of distinct structures within the images. Traditional medical image segmentation methods can be broadly classified into several categories: 1) Threshold-based methods [16], which classify pixels within the image as target or background by predefining a characteristic feature. 2) Region-based methods [17], which extract the target area by delineating a sub-region manually and subsequently merging adjacent pixels with similar attributes. 3) Edge-based segmentation methods [18], which achieve segmentation by identifying pixels where edges undergo significant changes at the juncture between the target and background areas, effectively delineating the boundaries. 4) Atlas-based segmentation methods [19], through the incorporation of shape information and the utilization of prior knowledge, these methods can yield improved segmentation outcomes even for medical images with indistinct boundaries and substantial noise.
In practical applications, the unique shape and contour of pulmonary embolism lesions in CTPA images make it so that traditional image segmentation methods often fail to achieve optimal outcomes in segmenting pulmonary embolism.
To address the above challenges, researchers are working to develop methods that utilize Cyber-Physical-Social Systems (CPSS) to enhance medical diagnosis. In 2014, researchers such as Long [20] introduced fully convolutional neural network (FCN) by enhancing the traditional convolutional neural network architecture. FCN abandons the fully connected layer in traditional convolutional neural networks and instead uses deconvolution layers. This change brings significant advantages - the network can handle image inputs of any size and is no longer limited to a fixed input size. However, this approach is not without drawbacks. The network needs to use basic upsampling technology at the back end of the processing process to match the size of the original image. This step often leads to the loss of a large amount of spatial information, thus limiting the accuracy of the network in image segmentation tasks, resulting in segmentation results. Accuracy is compromised.
Then in 2015, Ronneberger et al. [21] proposed the U-Net architecture based on FCN, aiming to more effectively utilize the rich contextual information in images. U-Net further optimizes the performance of fully convolutional networks through carefully designed downsampling and upsampling operations, and introduces four key components: encoder, decoder, bottleneck layer, and skip connection. The proposal of U-Net is widely regarded as a milestone in the field of encoder-decoder network structure, especially in the field of medical image segmentation. U-Net not only demonstrates excellent performance, but also promotes rapid progress in this field.
Owing to U-Net’s superior performance in medical image segmentation, we aimed to enhance U-Net accordingly to better suit the pulmonary embolism segmentation task. In comparison to traditional medical image segmentation methods, the automatic segmentation of pulmonary embolism via deep learning presents multiple challenges. The pulmonary embolism region is small in comparison to the lung area, and the marked imbalance between positive and negative samples can impair the model’s predictive accuracy, potentially leading to a complete loss of predictive capability [22]. Therefore, we have incorporated an attention mechanism into the U-Net architecture, thereby directing the model’s focus more towards the pulmonary embolism region. Furthermore, considering the challenge of accessing and the limited information in medical datasets, we have endeavored to integrate the Bi-LSTM architecture into the U-Net framework to capture the inter-slice information pertinent to pulmonary embolism. Additionally, to tackle the substantial imbalance of positive and negative samples in the pulmonary embolism dataset, we employ a hybrid loss function combining Cross-Entropy Loss (CELoss) and Tversky Loss (TLoss) during the neural network’s training.
This article’s principal contributions are as follows:
(1) An enhanced LSCU-Net, derived from the U-Net architecture, was developed. This framework integrates contextual information to automatically and accurately identify and segment pulmonary embolism, thereby aiding medical professionals and reducing the rate of misdiagnosis, and actualizing the application of CPSS in medical image segmentation.
(2) During model training, a hybrid loss function combining CELoss and FTLoss addresses the challenges of small pulmonary embsolism targets and disproportionate scales between pulmonary embolism and background in segmentation tasks.
(3) Experimental results demonstrate that our proposed method achieves the following metrics on the test set: a Jaccard index (JAC) of 0.6958, a Dice similarity coefficient (DSC) of 0.8206, a Mean Pixel Accuracy (mPA) of 0.8519, and an accuracy of 0.9993, thereby substantiating the method’s feasibility.
2 Methods
2.1 LSCU-net
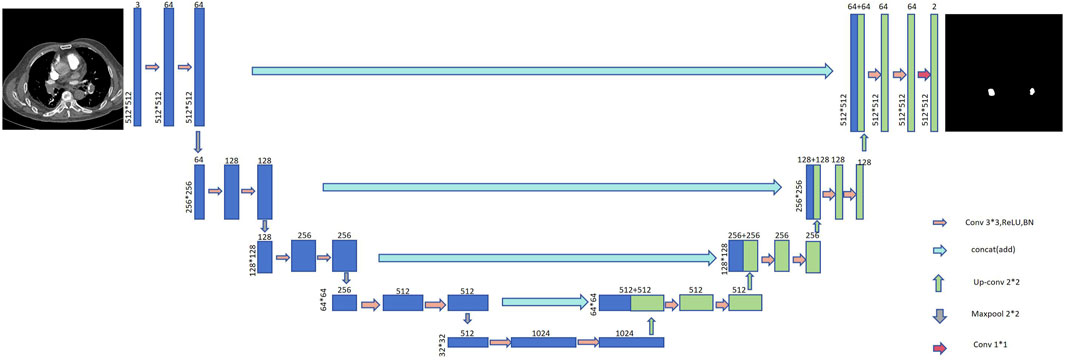
The U-Net architecture is extensively employed in medical image segmentation tasks due to its advantageous compact model parameterization, minimal data requirements, and rapid training capabilities. As depicted in Figure 1, the U-Net model is an evolution of the Fully Convolutional Network (FCN). The network’s structure resembles the letter ‘U᾽, which is the origin of its nomenclature. The U-Net framework primarily consists of four key components: the encoder, decoder, skip connections, and a bottleneck layer. The encoder module comprises convolutional and pooling layers, serving primarily to downsample and extract features. The decoder module, consisting of convolutional and upsampling layers, is chiefly responsible for localizing the target and reconstructing the image dimensions. Skip connections merge the encoder and decoder information along the channel dimension, facilitating the integration of contextual data.
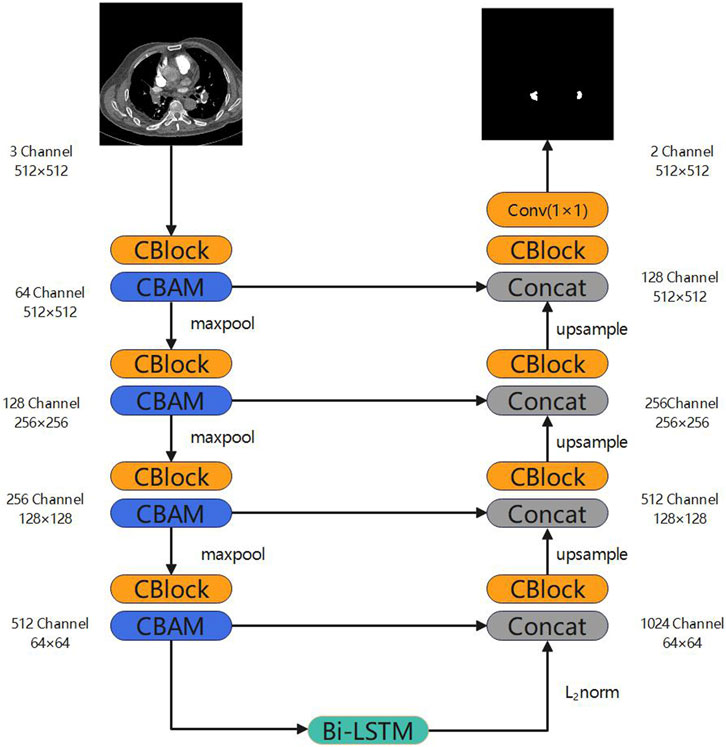
The enhanced architecture of our neural network model, termed Long Short-Term Memory and Convolutional Block Attention Module U-Net (LSCU-Net), is depicted in Figure 2. This model is a modification of the benchmark U-Net architecture. In comparison to the standard U-Net model, the principal enhancements of the LSCU-Net include:
(1) Integration of the Convolutional Block Attention Module (CBAM) into the encoder module to refine the neural network’s learning strategy, thereby focusing more acutely on pertinent areas within the channel and spatial dimensions.
The attention mechanism [23] constitutes a significant concept within the domain of deep learning. It emulates the human cognitive process of attention and concentration during information processing. This mechanism enables the neural network model to focus more intensively on pertinent information throughout the training phase, thereby enhancing the model’s capacity for representation learning and feature selection with respect to input data.
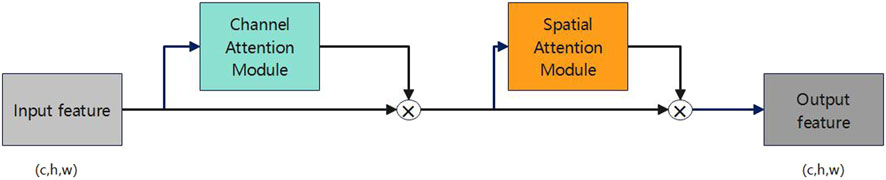
The Convolutional Block Attention Module (CBAM) [24], proposed by Sanghyun et al. in 2018, is an attention mechanism module within the realm of deep learning. Figure 3 illustrates its structure. CBAM’s central concept involves the simultaneous introduction of channel and spatial attention sub-modules, enabling the neural network model to concurrently attend to both channel and spatial information.
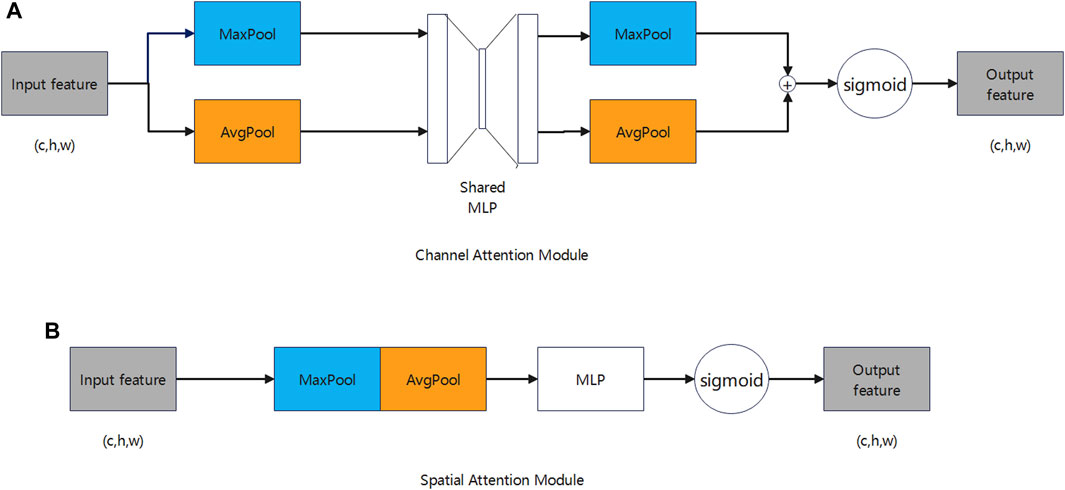
Figure 4A illustrates how the channel attention module captures the interdependencies among various channels. This module computes the significance of each channel by processing the input features, subsequently assigning weights to minimize extraneous information across channels, which enhances the feature extraction quality. The computation is described by the following formula:
Where
Figure 4B illustrates that the spatial attention module captures dependencies across various locations within the feature map. Utilizing channel attention as a basis, the spatial attention module computes the significance weights for each position, subsequently applying these weights to the feature map to attenuate information from irrelevant locations. The computation is described by the following formula:
Where
2) Incorporate a Bi-LSTM into the bottleneck layer to capture the inter-slice sequential information within the pulmonary embolism dataset.
Given that medical datasets are challenging to obtain, often contain sparse labeled samples, and limited information, the extraction of maximal information from the dataset for training purposes remains a significant challenge in current neural network research. Considering a dataset of pulmonary embolism obtained through CTPA imaging, conventional convolutional neural networks (CNNs) are limited to extracting intra-slice information from the dataset, failing to capture inter-slice correlations.
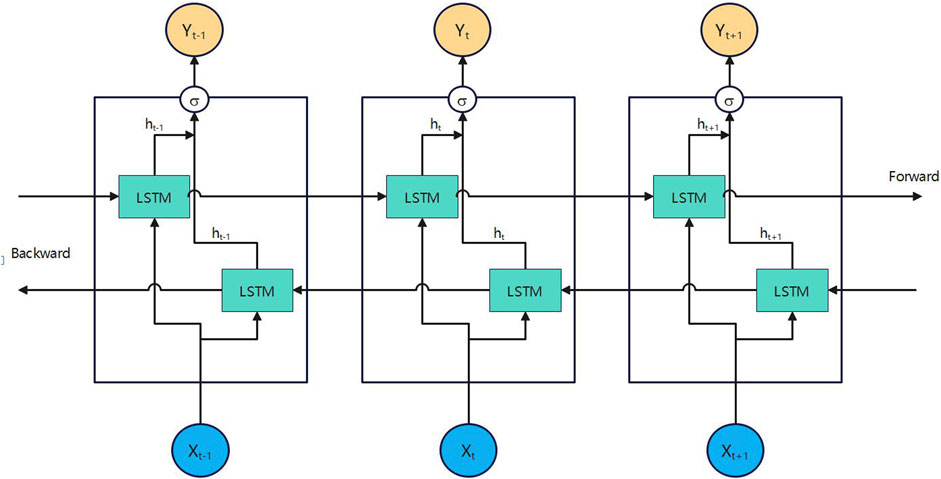
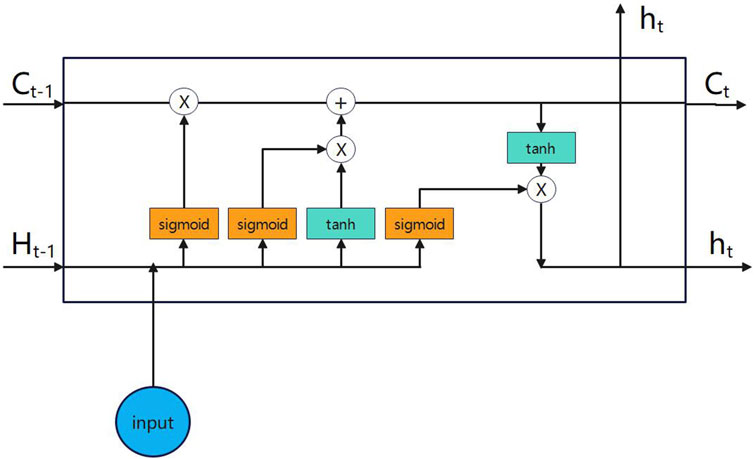
In 2018, Chen et al. applied Bi-directional Long Short-Term Memory (Bi-LSTM) [25] to Chinese word segmentation. Figures 5, 6 depict the architectures of Bi-LSTM and LSTM, respectively. This model represents an enhancement over the traditional Long Short-Term Memory (LSTM) architecture [26]. It integrates both forward and reverse LSTM networks, enabling the processing of input sequences progressively at each time step. This allows for the acquisition of information in both forward and reverse temporal directions.
2.2 Dataset
Our study utilized a single dataset: Pulmonary Embolism Dataset 1 (PEA1). This dataset was developed by the Sichuan Provincial People’s Hospital.
The PEA1 dataset comprises 15−ΔΔCT scans and 1,210 sliced JPG images. The original CT data, stored in DICOM format, undergoes slicing to produce JPG images. Typically, each CT scan encompasses 50–200 axial slices, with dimensions of 512 × 512 pixels for each slice.
The dataset was annotated by professional doctors at the Sichuan Provincial People’s Hospital using the LABEL ME software, thus rendering the PEA1 dataset suitable for training and evaluating the segmentation capabilities of our neural network. The dataset was partitioned into two subsets: a training set and a validation set, comprising 1,080 images (90%, 13 cases) and 130 images (10%, 2 cases), respectively.
2.3 Evaluation indicators
The evaluation index is derived from the confusion matrix. The confusion matrix typically comprises four elements: true positives (TP), false negatives (FN), false positives (FP), and true negatives (TN). Each column of the confusion matrix corresponds to a predicted category, with the sum of the column’s entries denoting the number of predictions for that category; each row pertains to the actual category, and the sum of the row’s entries reflects the total instances within that category.
Table 1 presents the evaluation metrics applied and the corresponding calculation methodologies.
2.4 Loss function
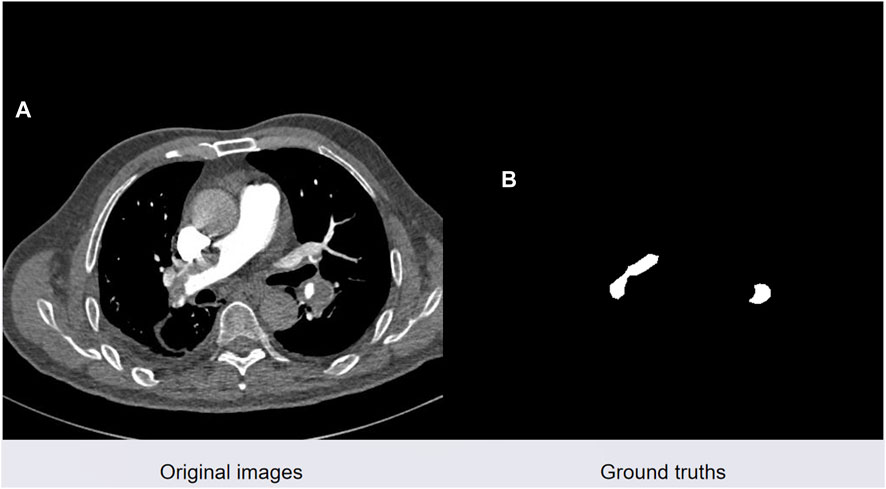
Semantic segmentation of pulmonary embolism presents challenges including severe category imbalance and the difficulty of detecting small-scale targets. Figure 7 illustrates a real CTPA image of a pulmonary embolism patient on the left, and a manually segmented pulmonary embolism mask image by a medical professional on the right. The red area represents the pulmonary embolism lesion, while the black areas denote the background.
Cross-entropy [27] is widely used as a loss function in classification tasks, defined as the measure of disparity between probability distributions for a particular random variable or set of events.
The binary cross-entropy loss function is delineated as:
Here,
The Focal Tversky Loss [28] combines Focal Loss and Tversky Loss, prioritizing challenging examples by diminishing the influence of simple, common elements, particularly in small regions of interest (ROIs), through the application of a specific coefficient as detailed subsequently:
This study is the first to integrate BCE (Binary Cross-Entropy) loss and FT (Focal Tversky) loss, aiming to enhance the performance of the neural network model while addressing the challenges of significant category imbalance and small target segmentation in the pulmonary embolism dataset.
3 Results and discussion
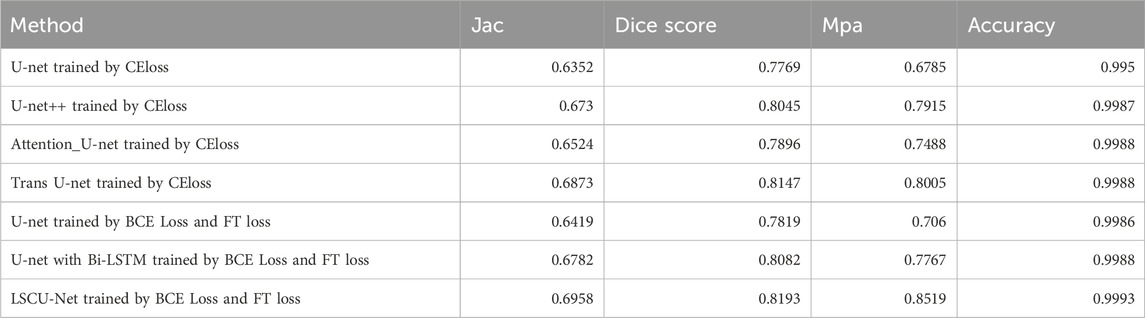
In the course of the study, we performed a series of control experiments, which included the evaluation of established neural network architectures, including U-Net, U-Net++, Attention U-Net, TransU-Net, and the integration of CBAM attention modules into the U-Net framework, as well as the incorporation of Bi-LSTM modules. Additionally, we assessed the employment of a hybrid loss function to address class imbalance and granular target segmentation in the training phase. For each experimental series, the training set was employed to retrain the model, subsequently model segmentation performance on the test set was assessed using the Jaccard index, Dice coefficient, Mean Pixel Accuracy (mPA), and accuracy.
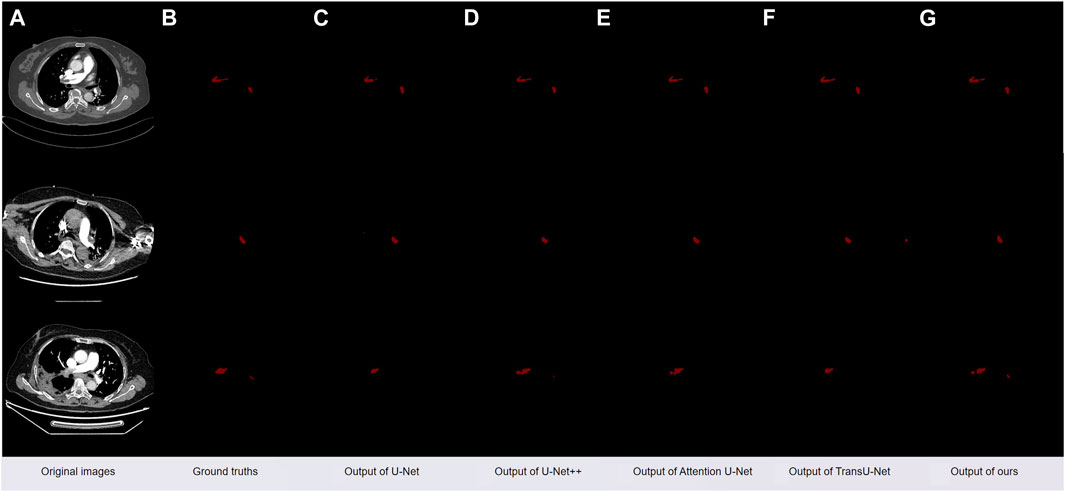
As depicted in Figure 8A, is the original CT slice image of the patient, Figure 8B is the mask image manually segmented by the doctor, Figure 8C is the segmentation image obtained using the original U-Net model, and Figure 8D is the segmentation obtained using the classic model U-Net++ Image, Figure 8E is a segmented image obtained using the classic model Attention U-Net, Figure 8F is a segmented image obtained using the classic model TransU-Net model, Figure 8G is obtained by our method after adding CBAM and Bi-LSTM modules and using a hybrid loss function segmented image.
As Table 2 demonstrates, the integration of the CBAM attention mechanism into the U-Net’s backbone, coupled with the replacement of the bottleneck layer by a Bi-LSTM and the adoption of a hybrid loss function, yields the most superior outcomes. When applied to the test set, it yielded a Jaccard index of 0.6958, a Dice score of 0.8193, an MPA of 0.8519, and an accuracy of 0.9993.
Upon implementing a hybrid loss function tailored for small object segmentation and addressing class imbalance to train the model, the Jaccard index (Jac) exhibited an increase from 0.6352 to 0.6419, the Dice Score improved from 0.7769 to 0.7819, the Mean Pixel Accuracy (MPA) rose from 0.6785 to 0.7060, and the Accuracy enhanced from 0.9950 to 0.9986. This resulted in an overall performance enhancement of the model by approximately 0.6%, demonstrating that the hybrid loss function effectively guides the model in addressing the significant class imbalance issue inherent in the dataset, and in improving the model’s performance in segmenting small targets.
Upon integrating the Bi-LSTM module into the original U-Net architecture, the Jaccard index improved from 0.6419 to 0.6782, the Dice coefficient from 0.7819 to 0.8082, the Mean Pixel Accuracy from 0.7060 to 0.7767, and the overall accuracy from 0.9986 to 0.9988. This enhancement bolstered the model’s overall performance by approximately 3.6%, demonstrating that replacing the bottleneck layer with the Bi-LSTM module enables the U-Net model to more effectively learn inter-sequential information within the pulmonary embolism dataset, thereby enhancing its feature segmentation capabilities.
Upon integrating the Convolutional Block Attention Module (CBAM) into the U-Net architecture, which already includes the Bi-LSTM module, the Jaccard Index improved from 0.6782 to 0.6958, Dice score from 0.8082 to 0.8193, Mean Pixel Accuracy (MPA) from 0.7767 to 0.8519, and accuracy from 0.9988 to 0.9993. This enhancement resulted in an overall performance improvement of approximately 1.8%, demonstrating that the incorporation of the CBAM module into the U-Net’s backbone enables the U-Net model to focus more on critical regions within pulmonary embolism images throughout the training process, thereby enhancing the model’s performance and feature learning capabilities.
4 Conclusion
This study integrates computer networking, Internet of Things (IoT), and social networking technologies and utilizes CPSS technology in the medical management of pulmonary embolism patients. We have developed an innovative LSCU-Net model and established a specialized automated segmentation system for pulmonary embolism. The key contributions are summarized as follows:
(1) We integrate the CBAM attention mechanism into the U-net model’s backbone to refine the learning strategy, enhancing focus on salient features in both channel and spatial dimensions.
(2) We employ a Bi-LSTM module to supplant the bottleneck layer, enabling the extraction of inter-slice sequential information from the pulmonary embolism dataset.
(3) Throughout the training phase, we utilize a hybrid loss function that merges BCEloss with Focal Tversky Loss, significantly enhancing the model’s ability to extract features from highly imbalanced categories and diminutive target datasets.
In future research, we will concentrate our efforts on three primary areas. First, we aim to explore the latest advancements in research on Cyber-Physical-Social Systems (CPSS), which seeks to foster deeper integration among computer networks, the Internet of Things, and social networks, and apply these insights to pulmonary embolism treatment research, with the expectation of achieving significant breakthroughs. Second, considering the limitations of existing pulmonary embolism datasets, particularly in terms of case numbers and significant individual variability, we aim to construct an extensive, detailed, and comprehensive dataset to address these challenges. Finally, we are dedicated to developing a broad spectrum of applications and advanced architectures for medical image segmentation models, while investigating sophisticated and efficient search algorithms, aiming to enhance the models’ learning capabilities, and then to promote the integrated application of the Cyber-Physical-Social Systems in the field of medical treatment.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Author contributions
SZ: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing–review and editing. XL: Data curation, Methodology, Software, Validation, Visualization, Writing–original draft. LG: Funding acquisition, Project administration, Resources, Writing–review and editing, Methodology. MX: Data curation, Software, Supervision, Writing–review and editing. TL: Formal Analysis, Funding acquisition, Project administration, Resources, Supervision, Writing–review and editing. SL: Data curation, Formal Analysis, Funding acquisition, Methodology, Project administration, Resources, Writing–review and editing. HY: Data curation, Project administration, Software, Supervision, Writing–review and editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work has been supported by the Medico-Engineering Cooperation Funds from University of Electronic Science and Technology of China “(No. ZYGX2021YGLH211)” and Sichuan Science and Technology Program “(No. 2022YFG0176)”.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wang FY. The emergence of intelligent enterprises: from CPS to CPSS. IEEE Intell Syst (2010) 25:85–8. doi:10.1109/mis.2010.104
2. Jiang H, Xu W. How to find your appropriate doctor: an integrated recommendation framework in big data context. In: IEEE Symposium on Computational Intelligence in Healthcare and e-health (CICARE); December, 2014; Orlando, FL, USA (2014). p. 92–5.
3. Xu Q, Su ZS, Shui Y. Green social CPS based e-healthcare systems to control the spread of infectious diseases. In: Proc. IEEE International Conference on Communications (ICC); 20-24 May 2018; Kansas City, MO (2018). p. 1–5.
4. Cheng X. Pulmonary vascular disease. Beijing: Beijing Medical University and Union Medical College of China Press (1993).
5. Byrnes JR, Wolberg AS. New findings on venous thrombogenesis. Hamostaseologie (2017) 37(1):25–35. doi:10.5482/HAMO-16-09-0034
6. Aggarwal A, Fullam L, Brownstein AP, Maynard GA, Ansell J, Varga EA, et al. Deep vein thrombosis (DVT) and pulmonary embolism (PE): awareness and prophylaxis practices reported by patients with cancer. Cancer Invest (2015) 33(9):405–10. doi:10.3109/07357907.2015.1048871
7. Samkoff JS, Comstock GW. Epidemiology of pulmonary embolism: mortality in a general population. Am J Epidemiol (1981) 114:488–96. doi:10.1093/oxfordjournals.aje.a113214
8. Ferrari E, Baudouy M, Morand P. Epidemiology of pulmonary embolism. Arch Mal Coeur Vaiss (1995) 88:1687–91. 11 Suppl.
9. Rathbun S. Cardiology patient pages. The Surgeon General's call to action to prevent deep vein thrombosis and pulmonary embolism. Circulation (2009) 119(15):480–2. doi:10.1161/circulationaha.108.841403
10. Nisio D, van Es N, Büller N. Deep vein thrombosis and pulmonary embolism. Lancet (2016) 388(10063):3060–3073. doi:10.1016/S0140-6736(16)30514-1
11. Grosse SD, Nelson RE, NyarkoRichardson KALC, Raskob GE. The economic burden of incident venous thromboembolism in the United States: a review of estimated attributable healthcare costs. Thromb Res (2016) 137:3–10. doi:10.1016/j.thromres.2015.11.033
13. Goldhaber SZ, Visani L, Rosa MD. Acute pulmonary embolism: clinical outcomes in the international cooperative pulmonary embolism registry (icoper). Lancet (1999) 353(9162):1386–9. doi:10.1016/s0140-6736(98)07534-5
14. Nikulina N, Terekhovskaya YV. Epidemiology of pulmonary embolism in today's context: analysis of incidence, mortality and problems of their study. Russ J Cardiol (2019)(6) 103–8. doi:10.15829/1560-4071-2019-6-103-108
15. Colin W, Ilan G, Susan S, Scott M, Logr G, Ayman E, et al. Effect of a multidisciplinary pulmonary embolism response team on patient mortality. Am J Cardiol (2021) 161:102–7. doi:10.1016/j.amjcard.2021.08.066
16. Sezgin M, Sankur B. Survey over image thresholding techniques and quantitative performance evaluation. J Electron Imaging (2004) 13(1):146–68. doi:10.1117/1.1631315
17. Xu J, Tu L, Zhang Z, et al. Tongue quality and tongue coating recognition based on image area segmentation method. Shanghai:Journal Shanghai Univ Traditional Chin Med (2009) 23:42–5.
18. Cumani A. Edge detection in multispectral images. Graphical Models Image Process (1991) 53(1):40–51. doi:10.1016/1049-9652(91)90018-f
19. Piotr Z. Atlas-based segmentation in extraction of knee joint bone structures from CT and MR. Sensors (2022) 22(22):8960. doi:10.3390/s22228960
20. Long J, Shelhamer E, Darrell T. Fully convolutional networks for semantic segmentation. In: Proceedings of the IEEE conference on computer vision and pattern recognition; June 7 2015 to June 12 2015; Boston, MA, USA (2015). p. 3431–40.
21. Ronneberger O, Fischer P, Brox T. U-net: convolutional networks for biomedical image segmentation. In: International Conference on Medical image computing and computer-assisted intervention; October 5-9, 2015; Munich, Germany. Cham: Springer (2015). 234–41.
22. Shrivastava A, Gupta A, Girshick R. Training region-based object detectors with online hard example mining. In: IEEE Conference on Computer Vision and Pattern Recognition; June 26 2016 to July 1 2016; Las Vegas, NV, USA. IEEE Computer Society (2016). 761–9.
23. Tian M, Wang W, Chen Y. Attention is all you need: an interpretable transformer-based asset allocation approach. Int Rev Financial Anal (2023) 90:102876. doi:10.1016/J.IRFA.2023.102876
24. Woo S, Park J, Lee J-Y, Kweon IS. Cbam: convolutional block attention module. In: Proceedings of theEuropean conference on computer vision (ECCV); September 8-14, 2018; Munich, Germany (2018). 3–19.
25. Chen J, Weihua LI, Chen JI, Xuze J, Yanbu G. Bi-directional long short-term memory neural networks for Chinese word segmentation. J Chin Inf Process (2018).
27. Yi-De M, Qing L, Zhi-Bai Q. Automated image segmentation using improved PCNN model based on cross-entropy. In: Proceedings of 2004 International Symposium on Intelligent Multimedia, Video and Speech Processing; October 20-22, 2004; Hong Kong. IEEE (2005). p. 743–6.
Keywords: Cyber-physical-social systems, U-Net, pulmonary embolism, CBAM, Bi-LSTM
Citation: Zhan S, Lei X, Guo L, Xiong M, Liu T, Liu S and Yu H (2024) A Cyber-physical-social systems approach to the semantic segmentation of pulmonary embolism. Front. Phys. 12:1354482. doi: 10.3389/fphy.2024.1354482
Received: 12 December 2023; Accepted: 26 January 2024;
Published: 14 February 2024.
Edited by:
Yuanyuan Huang, Chengdu University of Information Technology, ChinaCopyright © 2024 Zhan, Lei, Guo, Xiong, Liu, Liu and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tingyu Liu, bGl1dGluZ3l1MjNAMTYzLmNvbQ==
 Siyu Zhan1,2
Siyu Zhan1,2 Xin Lei
Xin Lei